User login
PCOS: Laser, Light Therapy Helpful for Hirsutism
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
BY DEEPA VARMA
TOPLINE:
, according to the results of a systematic review.
METHODOLOGY:
- Hirsutism, which affects 70%-80% of women with PCOS, is frequently marginalized as a cosmetic issue by healthcare providers, despite its significant psychological repercussions, including diminished self-esteem, reduced quality of life, and heightened depression.
- The 2023 international evidence-based PCOS guideline considers managing hirsutism a priority in women with PCOS.
- Researchers reviewed six studies (four randomized controlled trials and two cohort studies), which included 423 patients with PCOS who underwent laser or light-based hair reduction therapies, published through 2022.
- The studies evaluated the alexandrite laser, diode laser, and intense pulsed light (IPL) therapy, with and without pharmacological treatments. The main outcomes were hirsutism severity, psychological outcome, and adverse events.
TAKEAWAY:
- Alexandrite laser (wavelength, 755 nm) showed effective hair reduction and improved patient satisfaction (one study); high-fluence treatment yielded better outcomes than low-fluence treatment (one study). Alexandrite laser 755 nm also showed longer hair-free intervals and greater hair reduction than IPL therapy at 650-1000 nm (one study).
- Combined IPL (600 nm) and metformin therapy improved hirsutism and hair count reduction compared with IPL alone, but with more side effects (one study).
- Diode laser treatments (810 nm) with combined oral contraceptives improved hirsutism and related quality of life measures compared with diode laser alone or with metformin (one study).
- Comparing two diode lasers (wavelengths, 810 nm), low-fluence, high repetition laser showed superior hair width reduction and lower pain scores than high fluence, low-repetition laser (one study).
IN PRACTICE:
Laser and light treatments alone or combined with other treatments have demonstrated “encouraging results in reducing hirsutism severity, enhancing psychological well-being, and improving overall quality of life for affected individuals,” the authors wrote, noting that additional high-quality trials evaluating these treatments, which include more patients with different skin tones, are needed.
SOURCE:
The first author of the review is Katrina Tan, MD, Monash Health, Department of Dermatology, Melbourne, Victoria, Australia, and it was published online in JAMA Dermatology.
LIMITATIONS:
Limitations include low certainty of evidence because of the observational nature of some of the studies, the small number of studies, and underrepresentation of darker skin types, limiting generalizability.
DISCLOSURES:
The review is part of an update to the PCOS guideline, which was funded by the Australian National Health and Medical Research Council through various organizations. Several authors reported receiving grants and personal fees outside this work. Dr. Tan was a member of the 2023 PCOS guideline evidence team. Other authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Consensus Statement Aims to Guide Use of Low-Dose Oral Minoxidil for Hair Loss
SAN DIEGO — .
Those are among the key recommendations that resulted from a modified eDelphi consensus of experts who convened to develop guidelines for LDOM prescribing and monitoring.
“Topical minoxidil is safe, effective, over-the-counter, and FDA-approved to treat the most common form of hair loss, androgenetic alopecia,” one of the study authors, Jennifer Fu, MD, a dermatologist who directs the Hair Disorders Clinic at the University of California, San Francisco, told this news organization following the annual meeting of the American Academy of Dermatology. The results of the expert consensus were presented during a poster session at the meeting. “It is often used off label for other types of hair loss, yet clinicians who treat hair loss know that patient compliance with topical minoxidil can be poor for a variety of reasons,” she said. “Patients report that it can be difficult to apply and complicate hair styling. For many patients, topical minoxidil can be drying or cause irritant or allergic contact reactions.”
LDOM has become a popular alternative for patients for whom topical minoxidil is logistically challenging, irritating, or ineffective, she continued. Although oral minoxidil is no longer a first-line antihypertensive agent given the risk of cardiovascular adverse effects at higher antihypertensive dosing (10-40 mg daily), a growing number of small studies have documented the use of LDOM at doses ranging from 0.25 mg to 5 mg daily as a safe, effective option for various types of hair loss.
“Given the current absence of larger trials on this topic, our research group identified a need for expert-based guidelines for prescribing and monitoring LDOM use in hair loss patients,” Dr. Fu said. “Our goal was to provide clinicians who treat hair loss patients a road map for using LDOM effectively, maximizing hair growth, and minimizing potential cardiovascular adverse effects.”
Arriving at a Consensus
The process involved 43 hair loss specialists from 12 countries with an average of 6.29 years of experience with LDOM for hair loss, who participated in a multi-round modified Delphi process. They considered questions that addressed LDOM safety, efficacy, dosing, and monitoring for hair loss, and consensus was reached if at least 70% of participants indicated “agree” or “strongly agree” on a five-point Likert scale. Round 1 consisted of 180 open-ended, multiple-choice, or Likert-scale questions, while round 2 involved 121 Likert-scale questions, round 3 consisted of 16 Likert-scale questions, and round 4 included 11 Likert-scale questions. In all, 94 items achieved Likert-scale consensus.
Specifically, experts on the panel found a direct benefit of LDOM for androgenetic alopecia, age-related patterned thinning, alopecia areata, telogen effluvium, traction alopecia, persistent chemotherapy-induced alopecia, and endocrine therapy-induced alopecia. They found a supportive benefit of LDOM for lichen planopilaris, frontal fibrosing alopecia, central centrifugal alopecia, and fibrosing alopecia in a patterned distribution.
“LDOM can be considered when topical minoxidil is more expensive, logistically challenging, has plateaued in efficacy, results in undesirable product residue/skin irritation,” or exacerbates inflammatory processes (ie eczema, psoriasis), they added.
Contraindications to LDOM listed in the consensus recommendations include hypersensitivity to minoxidil, significant drug-drug interactions with LDOM, a history of pericardial effusion/tamponade, pericarditis, heart failure, pulmonary hypertension associated with mitral stenosis, pheochromocytoma, and pregnancy/breastfeeding. Cited precautions of LDOM use include a history of tachycardia or arrhythmia, hypotension, renal impairment, and being on dialysis.
Dr. Fu and colleagues noted that the earliest time point at which LDOM should be expected to demonstrate efficacy is 3-6 months. “Baseline testing is not routine but may be considered in case of identified precautions,” they wrote. They also noted that LDOM can possibly be co-administered with beta-blockers with a specialty consultation, and with spironolactone in biologic female or transgender female patients with hirsutism, acne, polycystic ovary syndrome (PCOS), and with lower extremity and facial edema.
According to the consensus statement, the most frequently prescribed LDOM dosing regimen in adult females aged 18 years and older includes a starting dose of 1.25 mg daily, with a dosing range between 0.625 mg and 5 mg daily. For adult males, the most frequently prescribed dosing regimen is a starting dose of 2.5 daily, with a dosing range between 1.25 mg and 5 mg daily. The most frequently prescribed LDOM dosing regimen in adolescent females aged 12-17 years is a starting dose of 0.625 mg daily, with a dosing range of 0.625 to 2.5 mg daily. For adolescent males, the recommended regimen is a starting dose of 1.25 mg daily, with a dosing range of 1.25 mg to 5 mg daily.
“We hope that this consensus statement will guide our colleagues who would like to use LDOM to treat hair loss in their adult and adolescent patients,” Dr. Fu told this news organization. “These recommendations may be used to inform clinical practice until additional evidence-based data becomes available.”
She acknowledged certain limitations of the effort, including the fact that the expert panel was underrepresented in treating hair loss in pediatric patients, “and therefore failed to reach consensus on LDOM pediatric use and dosing,” she said. “We encourage our pediatric dermatology colleagues to further research LDOM in pediatric patients.”
In an interview, Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, who was asked to comment, but was not involved with the work, characterized the consensus as a “helpful, concise reference guide for dermatologists.”
The advantages of the study are the standardized methods used, “and the experience of the panel,” she said. “Study limitations include the response rate, which was less than 60%, and the risk of potential side effects are not stratified by age, sex, or comorbidities,” she added.
Dr. Fu disclosed that she is a consultant to Pfizer. Dr. Lipner reported having no relevant disclosures.
SAN DIEGO — .
Those are among the key recommendations that resulted from a modified eDelphi consensus of experts who convened to develop guidelines for LDOM prescribing and monitoring.
“Topical minoxidil is safe, effective, over-the-counter, and FDA-approved to treat the most common form of hair loss, androgenetic alopecia,” one of the study authors, Jennifer Fu, MD, a dermatologist who directs the Hair Disorders Clinic at the University of California, San Francisco, told this news organization following the annual meeting of the American Academy of Dermatology. The results of the expert consensus were presented during a poster session at the meeting. “It is often used off label for other types of hair loss, yet clinicians who treat hair loss know that patient compliance with topical minoxidil can be poor for a variety of reasons,” she said. “Patients report that it can be difficult to apply and complicate hair styling. For many patients, topical minoxidil can be drying or cause irritant or allergic contact reactions.”
LDOM has become a popular alternative for patients for whom topical minoxidil is logistically challenging, irritating, or ineffective, she continued. Although oral minoxidil is no longer a first-line antihypertensive agent given the risk of cardiovascular adverse effects at higher antihypertensive dosing (10-40 mg daily), a growing number of small studies have documented the use of LDOM at doses ranging from 0.25 mg to 5 mg daily as a safe, effective option for various types of hair loss.
“Given the current absence of larger trials on this topic, our research group identified a need for expert-based guidelines for prescribing and monitoring LDOM use in hair loss patients,” Dr. Fu said. “Our goal was to provide clinicians who treat hair loss patients a road map for using LDOM effectively, maximizing hair growth, and minimizing potential cardiovascular adverse effects.”
Arriving at a Consensus
The process involved 43 hair loss specialists from 12 countries with an average of 6.29 years of experience with LDOM for hair loss, who participated in a multi-round modified Delphi process. They considered questions that addressed LDOM safety, efficacy, dosing, and monitoring for hair loss, and consensus was reached if at least 70% of participants indicated “agree” or “strongly agree” on a five-point Likert scale. Round 1 consisted of 180 open-ended, multiple-choice, or Likert-scale questions, while round 2 involved 121 Likert-scale questions, round 3 consisted of 16 Likert-scale questions, and round 4 included 11 Likert-scale questions. In all, 94 items achieved Likert-scale consensus.
Specifically, experts on the panel found a direct benefit of LDOM for androgenetic alopecia, age-related patterned thinning, alopecia areata, telogen effluvium, traction alopecia, persistent chemotherapy-induced alopecia, and endocrine therapy-induced alopecia. They found a supportive benefit of LDOM for lichen planopilaris, frontal fibrosing alopecia, central centrifugal alopecia, and fibrosing alopecia in a patterned distribution.
“LDOM can be considered when topical minoxidil is more expensive, logistically challenging, has plateaued in efficacy, results in undesirable product residue/skin irritation,” or exacerbates inflammatory processes (ie eczema, psoriasis), they added.
Contraindications to LDOM listed in the consensus recommendations include hypersensitivity to minoxidil, significant drug-drug interactions with LDOM, a history of pericardial effusion/tamponade, pericarditis, heart failure, pulmonary hypertension associated with mitral stenosis, pheochromocytoma, and pregnancy/breastfeeding. Cited precautions of LDOM use include a history of tachycardia or arrhythmia, hypotension, renal impairment, and being on dialysis.
Dr. Fu and colleagues noted that the earliest time point at which LDOM should be expected to demonstrate efficacy is 3-6 months. “Baseline testing is not routine but may be considered in case of identified precautions,” they wrote. They also noted that LDOM can possibly be co-administered with beta-blockers with a specialty consultation, and with spironolactone in biologic female or transgender female patients with hirsutism, acne, polycystic ovary syndrome (PCOS), and with lower extremity and facial edema.
According to the consensus statement, the most frequently prescribed LDOM dosing regimen in adult females aged 18 years and older includes a starting dose of 1.25 mg daily, with a dosing range between 0.625 mg and 5 mg daily. For adult males, the most frequently prescribed dosing regimen is a starting dose of 2.5 daily, with a dosing range between 1.25 mg and 5 mg daily. The most frequently prescribed LDOM dosing regimen in adolescent females aged 12-17 years is a starting dose of 0.625 mg daily, with a dosing range of 0.625 to 2.5 mg daily. For adolescent males, the recommended regimen is a starting dose of 1.25 mg daily, with a dosing range of 1.25 mg to 5 mg daily.
“We hope that this consensus statement will guide our colleagues who would like to use LDOM to treat hair loss in their adult and adolescent patients,” Dr. Fu told this news organization. “These recommendations may be used to inform clinical practice until additional evidence-based data becomes available.”
She acknowledged certain limitations of the effort, including the fact that the expert panel was underrepresented in treating hair loss in pediatric patients, “and therefore failed to reach consensus on LDOM pediatric use and dosing,” she said. “We encourage our pediatric dermatology colleagues to further research LDOM in pediatric patients.”
In an interview, Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, who was asked to comment, but was not involved with the work, characterized the consensus as a “helpful, concise reference guide for dermatologists.”
The advantages of the study are the standardized methods used, “and the experience of the panel,” she said. “Study limitations include the response rate, which was less than 60%, and the risk of potential side effects are not stratified by age, sex, or comorbidities,” she added.
Dr. Fu disclosed that she is a consultant to Pfizer. Dr. Lipner reported having no relevant disclosures.
SAN DIEGO — .
Those are among the key recommendations that resulted from a modified eDelphi consensus of experts who convened to develop guidelines for LDOM prescribing and monitoring.
“Topical minoxidil is safe, effective, over-the-counter, and FDA-approved to treat the most common form of hair loss, androgenetic alopecia,” one of the study authors, Jennifer Fu, MD, a dermatologist who directs the Hair Disorders Clinic at the University of California, San Francisco, told this news organization following the annual meeting of the American Academy of Dermatology. The results of the expert consensus were presented during a poster session at the meeting. “It is often used off label for other types of hair loss, yet clinicians who treat hair loss know that patient compliance with topical minoxidil can be poor for a variety of reasons,” she said. “Patients report that it can be difficult to apply and complicate hair styling. For many patients, topical minoxidil can be drying or cause irritant or allergic contact reactions.”
LDOM has become a popular alternative for patients for whom topical minoxidil is logistically challenging, irritating, or ineffective, she continued. Although oral minoxidil is no longer a first-line antihypertensive agent given the risk of cardiovascular adverse effects at higher antihypertensive dosing (10-40 mg daily), a growing number of small studies have documented the use of LDOM at doses ranging from 0.25 mg to 5 mg daily as a safe, effective option for various types of hair loss.
“Given the current absence of larger trials on this topic, our research group identified a need for expert-based guidelines for prescribing and monitoring LDOM use in hair loss patients,” Dr. Fu said. “Our goal was to provide clinicians who treat hair loss patients a road map for using LDOM effectively, maximizing hair growth, and minimizing potential cardiovascular adverse effects.”
Arriving at a Consensus
The process involved 43 hair loss specialists from 12 countries with an average of 6.29 years of experience with LDOM for hair loss, who participated in a multi-round modified Delphi process. They considered questions that addressed LDOM safety, efficacy, dosing, and monitoring for hair loss, and consensus was reached if at least 70% of participants indicated “agree” or “strongly agree” on a five-point Likert scale. Round 1 consisted of 180 open-ended, multiple-choice, or Likert-scale questions, while round 2 involved 121 Likert-scale questions, round 3 consisted of 16 Likert-scale questions, and round 4 included 11 Likert-scale questions. In all, 94 items achieved Likert-scale consensus.
Specifically, experts on the panel found a direct benefit of LDOM for androgenetic alopecia, age-related patterned thinning, alopecia areata, telogen effluvium, traction alopecia, persistent chemotherapy-induced alopecia, and endocrine therapy-induced alopecia. They found a supportive benefit of LDOM for lichen planopilaris, frontal fibrosing alopecia, central centrifugal alopecia, and fibrosing alopecia in a patterned distribution.
“LDOM can be considered when topical minoxidil is more expensive, logistically challenging, has plateaued in efficacy, results in undesirable product residue/skin irritation,” or exacerbates inflammatory processes (ie eczema, psoriasis), they added.
Contraindications to LDOM listed in the consensus recommendations include hypersensitivity to minoxidil, significant drug-drug interactions with LDOM, a history of pericardial effusion/tamponade, pericarditis, heart failure, pulmonary hypertension associated with mitral stenosis, pheochromocytoma, and pregnancy/breastfeeding. Cited precautions of LDOM use include a history of tachycardia or arrhythmia, hypotension, renal impairment, and being on dialysis.
Dr. Fu and colleagues noted that the earliest time point at which LDOM should be expected to demonstrate efficacy is 3-6 months. “Baseline testing is not routine but may be considered in case of identified precautions,” they wrote. They also noted that LDOM can possibly be co-administered with beta-blockers with a specialty consultation, and with spironolactone in biologic female or transgender female patients with hirsutism, acne, polycystic ovary syndrome (PCOS), and with lower extremity and facial edema.
According to the consensus statement, the most frequently prescribed LDOM dosing regimen in adult females aged 18 years and older includes a starting dose of 1.25 mg daily, with a dosing range between 0.625 mg and 5 mg daily. For adult males, the most frequently prescribed dosing regimen is a starting dose of 2.5 daily, with a dosing range between 1.25 mg and 5 mg daily. The most frequently prescribed LDOM dosing regimen in adolescent females aged 12-17 years is a starting dose of 0.625 mg daily, with a dosing range of 0.625 to 2.5 mg daily. For adolescent males, the recommended regimen is a starting dose of 1.25 mg daily, with a dosing range of 1.25 mg to 5 mg daily.
“We hope that this consensus statement will guide our colleagues who would like to use LDOM to treat hair loss in their adult and adolescent patients,” Dr. Fu told this news organization. “These recommendations may be used to inform clinical practice until additional evidence-based data becomes available.”
She acknowledged certain limitations of the effort, including the fact that the expert panel was underrepresented in treating hair loss in pediatric patients, “and therefore failed to reach consensus on LDOM pediatric use and dosing,” she said. “We encourage our pediatric dermatology colleagues to further research LDOM in pediatric patients.”
In an interview, Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, who was asked to comment, but was not involved with the work, characterized the consensus as a “helpful, concise reference guide for dermatologists.”
The advantages of the study are the standardized methods used, “and the experience of the panel,” she said. “Study limitations include the response rate, which was less than 60%, and the risk of potential side effects are not stratified by age, sex, or comorbidities,” she added.
Dr. Fu disclosed that she is a consultant to Pfizer. Dr. Lipner reported having no relevant disclosures.
FROM AAD 2024
Alopecia Areata: Late Responses Complicate Definition of JAK Inhibitor Failure
SAN DIEGO — , according to late breaker data presented at the annual meeting of the American Academy of Dermatology.
Although the majority respond within months, response curves have so far climbed for as long as patients are followed, allowing many with disappointing early results to catch up, according to Rodney D. Sinclair, MD, professor of dermatology at the University of Melbourne, Australia.
His remarks were derived specifically from new long-term follow-up with baricitinib, the first JAK inhibitor approved for AA, but the pattern appears to be similar with ritlecitinib, the only other JAK inhibitor approved for AA, and for several if not all JAK inhibitors in phase 3 AA trials.
“We have had patients on baricitinib where not much was happening at 18 months, but now, at 4 years, they have a SALT score of zero,” Dr. Sinclair reported
A Severity of Alopecia Tool (SALT) score of 0 signifies complete hair regrowth. On a scale with a maximum score of 100 (complete hair loss), a SALT score of 20 or less, signaling clinical success, has been a primary endpoint in many JAK inhibitor trials, including those conducted with baricitinib.
Providing the most recent analysis in patients with severe AA participating in the phase 3 BRAVE-AA1 and BRAVE-AA2 trials of baricitinib, which were published together in 2022, Dr. Sinclair broke the data down into responders, mixed responders, and nonresponders at 52 weeks. The proportion of patients who responded with even longer follow-up were then tallied.
In the as-observed responses over time, the trajectory of response continued to climb through 76 weeks of follow-up in all three groups.
Relative to the 44.5% rate of overall response (SALT ≤ 20 ) at 52 weeks, there was some further growth in every group maintained on JAK inhibitor therapy over longer follow-up. In Dr. Sinclair’s late breaking analysis, this did not include nonresponders, who stopped therapy by week 52, but 78.4% of the combined responders and mixed responders who remained on treatment had reached treatment success at 76 weeks.
Response Curves Climb More Slowly With Severe Alopecia
While improvement in SALT scores was even seen in nonresponders over time as long as they remained on therapy, Dr. Sinclair reported that response curves tended to climb more slowly in those with more severe alopecia at baseline. Yet, they still climbed. For example, 28.1% of those with a baseline SALT score of 95 to 100 had reached treatment success at week 52, but the proportion had climbed to 35.4% by week 76.
The response curves climbed more quickly among those with a SALT score between 50 and 95 at baseline than among those with more severe alopecia, but the differences in SALT scores at 52 weeks and 76 weeks among patients in this range of baseline SALT scores were small.
Basically, “those with a SALT score of 94 did just as well as those with a SALT score of 51 when followed long-term,” he said, noting that this was among several findings that confounded expectations.
Duration of AA was found to be an important prognostic factor, with 4 years emerging as a general threshold separating those with a diminished likelihood of benefit relative to those with a shorter AA duration.
“When the duration of AA is more than 4 years, the response to any JAK inhibitor seems to fall off a cliff,” Dr. Sinclair said.
To clarify this observation, Dr. Sinclair made an analogy between acute and chronic urticaria. Chronicity appears to change the pathophysiology of both urticaria and AA, making durable remissions more difficult to achieve if the inflammatory response was persistently upregulated, he said.
The delayed responses in some patients “suggests that it is not enough to control inflammation for the hair to regrow. You actually have to activate the hair to grow as well as treat the inflammation,” Dr. Sinclair said.
This heterogeneity that has been observed in the speed of AA response to JAK inhibitors might be explained at least in part by the individual differences in hair growth activation. For ritlecitinib, the only other JAK inhibitor approved for AA to date, 62% were categorized as responders in the registration ALLEGRO trials, but only 44% were early responders, meaning SALT scores of ≤ 20 by week 24, according to a summary published last year. Of the remaining 16%, 11% were middle responders, meaning a SALT score of ≤ 20 reached at week 48, and 6% were late responders, meaning a SALT score of ≤ 20 reached at week 96.
In the context of late breaking 68-week data with deuruxolitinib, an oral JAK inhibitor currently under FDA review for treating moderate to severe AA, presented in the same AAD session as Dr. Sinclair’s baricitinib data, Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Connecticut, described similar long-term response curves. At 24 weeks, the SALT ≤ 20 response was achieved in 34.9% of patients, but climbed to 62.8% with continuous therapy over 68 weeks.
The difference between AA and most other inflammatory conditions treated with a JAK inhibitor is that “it takes time to treat,” Dr. King said.
Time Factor Is Important for Response
“What we are learning is that patients keep getting better over time,” Dr. Sinclair said. Asked specifically how long he would treat a patient before giving up, he acknowledged that he used to consider 6 months adequate, but that he has now changed his mind.
“It might be that even 2 years is too short,” he said, although he conceded that a trial of therapy for this long “might be an issue for third-part payers.”
Asked to comment, Melissa Piliang, MD, chair of the department of dermatology at the Cleveland Clinic, agreed with the principle that early responses are not necessarily predictive of complete response.
“In my clinical experience, 6 months is not long enough to assess response,” she told this news organization. “Some patients have hair growth after 18 months to 2 years” of treatment. Additional studies to identify the characteristics and predictors of late response, she said, “would be very helpful, as would trials allowing multiple therapies to simulate real-world practice.”
Like Dr. Sinclair, Dr. Piliang is interested in the possibility of combining a JAK inhibitor with another therapy aimed specially at promoting hair regrowth.
“Using a secondary therapy to stimulate regrowth as an addition to an anti-inflammatory medicine like a JAK inhibitor might speed up response in some patients,” she speculated. Dr. Sinclair reports financial relationships with more than 30 pharmaceutical companies, including Eli Lilly, the manufacturer of baricitinib. Dr. King reports financial relationships with multiple companies, including Concert Pharmaceuticals (consultant and investigator), the manufacturer of deuruxolitinib. Dr. Piliang reports financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble.
SAN DIEGO — , according to late breaker data presented at the annual meeting of the American Academy of Dermatology.
Although the majority respond within months, response curves have so far climbed for as long as patients are followed, allowing many with disappointing early results to catch up, according to Rodney D. Sinclair, MD, professor of dermatology at the University of Melbourne, Australia.
His remarks were derived specifically from new long-term follow-up with baricitinib, the first JAK inhibitor approved for AA, but the pattern appears to be similar with ritlecitinib, the only other JAK inhibitor approved for AA, and for several if not all JAK inhibitors in phase 3 AA trials.
“We have had patients on baricitinib where not much was happening at 18 months, but now, at 4 years, they have a SALT score of zero,” Dr. Sinclair reported
A Severity of Alopecia Tool (SALT) score of 0 signifies complete hair regrowth. On a scale with a maximum score of 100 (complete hair loss), a SALT score of 20 or less, signaling clinical success, has been a primary endpoint in many JAK inhibitor trials, including those conducted with baricitinib.
Providing the most recent analysis in patients with severe AA participating in the phase 3 BRAVE-AA1 and BRAVE-AA2 trials of baricitinib, which were published together in 2022, Dr. Sinclair broke the data down into responders, mixed responders, and nonresponders at 52 weeks. The proportion of patients who responded with even longer follow-up were then tallied.
In the as-observed responses over time, the trajectory of response continued to climb through 76 weeks of follow-up in all three groups.
Relative to the 44.5% rate of overall response (SALT ≤ 20 ) at 52 weeks, there was some further growth in every group maintained on JAK inhibitor therapy over longer follow-up. In Dr. Sinclair’s late breaking analysis, this did not include nonresponders, who stopped therapy by week 52, but 78.4% of the combined responders and mixed responders who remained on treatment had reached treatment success at 76 weeks.
Response Curves Climb More Slowly With Severe Alopecia
While improvement in SALT scores was even seen in nonresponders over time as long as they remained on therapy, Dr. Sinclair reported that response curves tended to climb more slowly in those with more severe alopecia at baseline. Yet, they still climbed. For example, 28.1% of those with a baseline SALT score of 95 to 100 had reached treatment success at week 52, but the proportion had climbed to 35.4% by week 76.
The response curves climbed more quickly among those with a SALT score between 50 and 95 at baseline than among those with more severe alopecia, but the differences in SALT scores at 52 weeks and 76 weeks among patients in this range of baseline SALT scores were small.
Basically, “those with a SALT score of 94 did just as well as those with a SALT score of 51 when followed long-term,” he said, noting that this was among several findings that confounded expectations.
Duration of AA was found to be an important prognostic factor, with 4 years emerging as a general threshold separating those with a diminished likelihood of benefit relative to those with a shorter AA duration.
“When the duration of AA is more than 4 years, the response to any JAK inhibitor seems to fall off a cliff,” Dr. Sinclair said.
To clarify this observation, Dr. Sinclair made an analogy between acute and chronic urticaria. Chronicity appears to change the pathophysiology of both urticaria and AA, making durable remissions more difficult to achieve if the inflammatory response was persistently upregulated, he said.
The delayed responses in some patients “suggests that it is not enough to control inflammation for the hair to regrow. You actually have to activate the hair to grow as well as treat the inflammation,” Dr. Sinclair said.
This heterogeneity that has been observed in the speed of AA response to JAK inhibitors might be explained at least in part by the individual differences in hair growth activation. For ritlecitinib, the only other JAK inhibitor approved for AA to date, 62% were categorized as responders in the registration ALLEGRO trials, but only 44% were early responders, meaning SALT scores of ≤ 20 by week 24, according to a summary published last year. Of the remaining 16%, 11% were middle responders, meaning a SALT score of ≤ 20 reached at week 48, and 6% were late responders, meaning a SALT score of ≤ 20 reached at week 96.
In the context of late breaking 68-week data with deuruxolitinib, an oral JAK inhibitor currently under FDA review for treating moderate to severe AA, presented in the same AAD session as Dr. Sinclair’s baricitinib data, Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Connecticut, described similar long-term response curves. At 24 weeks, the SALT ≤ 20 response was achieved in 34.9% of patients, but climbed to 62.8% with continuous therapy over 68 weeks.
The difference between AA and most other inflammatory conditions treated with a JAK inhibitor is that “it takes time to treat,” Dr. King said.
Time Factor Is Important for Response
“What we are learning is that patients keep getting better over time,” Dr. Sinclair said. Asked specifically how long he would treat a patient before giving up, he acknowledged that he used to consider 6 months adequate, but that he has now changed his mind.
“It might be that even 2 years is too short,” he said, although he conceded that a trial of therapy for this long “might be an issue for third-part payers.”
Asked to comment, Melissa Piliang, MD, chair of the department of dermatology at the Cleveland Clinic, agreed with the principle that early responses are not necessarily predictive of complete response.
“In my clinical experience, 6 months is not long enough to assess response,” she told this news organization. “Some patients have hair growth after 18 months to 2 years” of treatment. Additional studies to identify the characteristics and predictors of late response, she said, “would be very helpful, as would trials allowing multiple therapies to simulate real-world practice.”
Like Dr. Sinclair, Dr. Piliang is interested in the possibility of combining a JAK inhibitor with another therapy aimed specially at promoting hair regrowth.
“Using a secondary therapy to stimulate regrowth as an addition to an anti-inflammatory medicine like a JAK inhibitor might speed up response in some patients,” she speculated. Dr. Sinclair reports financial relationships with more than 30 pharmaceutical companies, including Eli Lilly, the manufacturer of baricitinib. Dr. King reports financial relationships with multiple companies, including Concert Pharmaceuticals (consultant and investigator), the manufacturer of deuruxolitinib. Dr. Piliang reports financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble.
SAN DIEGO — , according to late breaker data presented at the annual meeting of the American Academy of Dermatology.
Although the majority respond within months, response curves have so far climbed for as long as patients are followed, allowing many with disappointing early results to catch up, according to Rodney D. Sinclair, MD, professor of dermatology at the University of Melbourne, Australia.
His remarks were derived specifically from new long-term follow-up with baricitinib, the first JAK inhibitor approved for AA, but the pattern appears to be similar with ritlecitinib, the only other JAK inhibitor approved for AA, and for several if not all JAK inhibitors in phase 3 AA trials.
“We have had patients on baricitinib where not much was happening at 18 months, but now, at 4 years, they have a SALT score of zero,” Dr. Sinclair reported
A Severity of Alopecia Tool (SALT) score of 0 signifies complete hair regrowth. On a scale with a maximum score of 100 (complete hair loss), a SALT score of 20 or less, signaling clinical success, has been a primary endpoint in many JAK inhibitor trials, including those conducted with baricitinib.
Providing the most recent analysis in patients with severe AA participating in the phase 3 BRAVE-AA1 and BRAVE-AA2 trials of baricitinib, which were published together in 2022, Dr. Sinclair broke the data down into responders, mixed responders, and nonresponders at 52 weeks. The proportion of patients who responded with even longer follow-up were then tallied.
In the as-observed responses over time, the trajectory of response continued to climb through 76 weeks of follow-up in all three groups.
Relative to the 44.5% rate of overall response (SALT ≤ 20 ) at 52 weeks, there was some further growth in every group maintained on JAK inhibitor therapy over longer follow-up. In Dr. Sinclair’s late breaking analysis, this did not include nonresponders, who stopped therapy by week 52, but 78.4% of the combined responders and mixed responders who remained on treatment had reached treatment success at 76 weeks.
Response Curves Climb More Slowly With Severe Alopecia
While improvement in SALT scores was even seen in nonresponders over time as long as they remained on therapy, Dr. Sinclair reported that response curves tended to climb more slowly in those with more severe alopecia at baseline. Yet, they still climbed. For example, 28.1% of those with a baseline SALT score of 95 to 100 had reached treatment success at week 52, but the proportion had climbed to 35.4% by week 76.
The response curves climbed more quickly among those with a SALT score between 50 and 95 at baseline than among those with more severe alopecia, but the differences in SALT scores at 52 weeks and 76 weeks among patients in this range of baseline SALT scores were small.
Basically, “those with a SALT score of 94 did just as well as those with a SALT score of 51 when followed long-term,” he said, noting that this was among several findings that confounded expectations.
Duration of AA was found to be an important prognostic factor, with 4 years emerging as a general threshold separating those with a diminished likelihood of benefit relative to those with a shorter AA duration.
“When the duration of AA is more than 4 years, the response to any JAK inhibitor seems to fall off a cliff,” Dr. Sinclair said.
To clarify this observation, Dr. Sinclair made an analogy between acute and chronic urticaria. Chronicity appears to change the pathophysiology of both urticaria and AA, making durable remissions more difficult to achieve if the inflammatory response was persistently upregulated, he said.
The delayed responses in some patients “suggests that it is not enough to control inflammation for the hair to regrow. You actually have to activate the hair to grow as well as treat the inflammation,” Dr. Sinclair said.
This heterogeneity that has been observed in the speed of AA response to JAK inhibitors might be explained at least in part by the individual differences in hair growth activation. For ritlecitinib, the only other JAK inhibitor approved for AA to date, 62% were categorized as responders in the registration ALLEGRO trials, but only 44% were early responders, meaning SALT scores of ≤ 20 by week 24, according to a summary published last year. Of the remaining 16%, 11% were middle responders, meaning a SALT score of ≤ 20 reached at week 48, and 6% were late responders, meaning a SALT score of ≤ 20 reached at week 96.
In the context of late breaking 68-week data with deuruxolitinib, an oral JAK inhibitor currently under FDA review for treating moderate to severe AA, presented in the same AAD session as Dr. Sinclair’s baricitinib data, Brett King, MD, PhD, associate professor of dermatology, Yale University, New Haven, Connecticut, described similar long-term response curves. At 24 weeks, the SALT ≤ 20 response was achieved in 34.9% of patients, but climbed to 62.8% with continuous therapy over 68 weeks.
The difference between AA and most other inflammatory conditions treated with a JAK inhibitor is that “it takes time to treat,” Dr. King said.
Time Factor Is Important for Response
“What we are learning is that patients keep getting better over time,” Dr. Sinclair said. Asked specifically how long he would treat a patient before giving up, he acknowledged that he used to consider 6 months adequate, but that he has now changed his mind.
“It might be that even 2 years is too short,” he said, although he conceded that a trial of therapy for this long “might be an issue for third-part payers.”
Asked to comment, Melissa Piliang, MD, chair of the department of dermatology at the Cleveland Clinic, agreed with the principle that early responses are not necessarily predictive of complete response.
“In my clinical experience, 6 months is not long enough to assess response,” she told this news organization. “Some patients have hair growth after 18 months to 2 years” of treatment. Additional studies to identify the characteristics and predictors of late response, she said, “would be very helpful, as would trials allowing multiple therapies to simulate real-world practice.”
Like Dr. Sinclair, Dr. Piliang is interested in the possibility of combining a JAK inhibitor with another therapy aimed specially at promoting hair regrowth.
“Using a secondary therapy to stimulate regrowth as an addition to an anti-inflammatory medicine like a JAK inhibitor might speed up response in some patients,” she speculated. Dr. Sinclair reports financial relationships with more than 30 pharmaceutical companies, including Eli Lilly, the manufacturer of baricitinib. Dr. King reports financial relationships with multiple companies, including Concert Pharmaceuticals (consultant and investigator), the manufacturer of deuruxolitinib. Dr. Piliang reports financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble.
FROM AAD 2024
Androgenetic Alopecia: Study Finds Efficacy of Topical and Oral Minoxidil Similar
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
Oral minoxidil, 5 mg once a day, “did not demonstrate superiority” over topical minoxidil, 5%, applied twice a day, after 24 weeks, reported Mariana Alvares Penha, MD, of the department of dermatology at São Paulo State University, in Botucatu, Brazil, and coauthors. Their randomized, controlled, double-blind study was published online in JAMA Dermatology.
Topical minoxidil is approved by the US Food and Drug Administration (FDA) for androgenetic alopecia (AGA), but there has been increasing interest worldwide in the use of low-dose oral minoxidil, a vasodilator approved as an antihypertensive, as an alternative treatment.
The trial “is important information that’s never been elucidated before,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said in an interview. The data, he added, can be used to reassure patients who do not want to take the oral form of the drug that a topical is just as effective.
“This study does let us counsel patients better and really give them the evidence,” said Shari Lipner, MD, PhD, associate professor of clinical dermatology at Weill Cornell Medicine, New York, who was also asked to comment on the results.
Both Dr. Lipner and Dr. Friedman said the study was well-designed.
The investigators enrolled 90 men aged 18-55; 68 completed the trial. Most had mild to moderate AGA. Men were excluded if they had received treatment for alopecia in the previous 6 months, a history of hair transplant, cardiopathy, nephropathy, dermatoses involving the scalp, any clinical conditions causing hair loss, or hypersensitivity to minoxidil.
They were randomized to receive either 5 mg of oral minoxidil a day, plus a placebo solution to apply to the scalp, or topical minoxidil solution (5%) applied twice a day plus placebo capsules. They were told to take a capsule at bedtime and to apply 1 mL of the solution to dry hair in the morning and at night.
The final analysis included 35 men in the topical group and 33 in the oral group (mean age, 36.6 years). Seven people in the topical group and 11 in the oral group were not able to attend the final appointment at 24 weeks. Three additional patients in the topical group dropped out for insomnia, hair shedding, and scalp eczema, while one dropped out of the oral group because of headache.
At 24 weeks, the percentage increase in terminal hair density in the oral minoxidil group was 27% higher (P = .005) in the vertex and 13% higher (P = .15) in the frontal scalp, compared with the topical-treated group.
Total hair density increased by 2% in the oral group compared with topical treatment in the vertex and decreased by 0.2% in the frontal area compared with topical treatment. None of these differences were statistically significant.
Three dermatologists blinded to the treatments, who analyzed photographs, determined that 60% of the men in the oral group and 48% in the topical group had clinical improvement in the frontal area, which was not statistically significant. More orally-treated patients had improvement in the vertex area: 70% compared with 46% of those on topical treatment (P = .04).
Hypertrichosis, Headache
Of the original 90 patients in the trial, more men taking oral minoxidil had hypertrichosis: 49% compared with 25% in the topical formulation group. Headache was also more common among those on oral minoxidil: six cases (14%) vs. one case (2%) among those on topical minoxidil. There was no difference in mean arterial blood pressure or resting heart rate between the two groups. Transient hair loss was more common with topical treatment, but it was not significant.
Dr. Friedman said that the study results would not change how he practices, but that it would give him data to use to inform patients who do not want to take oral minoxidil. He generally prescribes the oral form, unless patients do not want to take it or there is a medical contraindication, which he said is rare.
“I personally think oral is superior to topical,” mainly “because the patient’s actually using it,” said Dr. Friedman. “They’re more likely to take a pill a day versus apply something topically twice a day,” he added.
Both Dr. Lipner and Dr. Friedman said that they doubted that individuals could — or would want to — follow the twice-daily topical regimen used in the trial.
“In real life, not in the clinical trial scenario, it may be very hard for patients to comply with putting on the topical minoxidil twice a day or even once a day,” Dr. Lipner said.
However, she continues to prescribe more topical minoxidil than oral, because she believes “there’s less potential for side effects.” For patients who can adhere to the topical regimen, the study shows that they will get results, said Dr. Lipner.
Dr. Friedman, however, said that for patients who are looking at a lifetime of medication, “an oral will always win out on a topical to the scalp from an adherence perspective.”
The study was supported by the Brazilian Dermatology Society Support Fund. Dr. Penha reported receiving grants from the fund; no other disclosures were reported. Dr. Friedman and Dr. Lipner reported no conflicts related to minoxidil.
FROM JAMA DERMATOLOGY
Enhancing Cosmetic and Functional Improvement of Recalcitrant Nail Lichen Planus With Resin Nail
Practice Gap
Lichen planus (LP)—a chronic inflammatory disorder affecting the nails—is prevalent in 10% to 15% of patients and is more common in the fingernails than toenails. Clinical manifestation includes longitudinal ridges, nail plate atrophy, and splitting, which all contribute to cosmetic disfigurement and difficulty with functionality. Quality of life and daily activities may be impacted profoundly.1 First-line therapies include intralesional and systemic corticosteroids; however, efficacy is limited and recurrence is common.1,2 Lichen planus is one of the few conditions that may cause permanent and debilitating nail loss.
Tools
A resin nail can be used to improve cosmetic appearance and functionality in patients with recalcitrant nail LP. The composite resin creates a flexible nonporous nail and allows the underlying natural nail to grow. Application of resin nails has been used for toenail onychodystrophies to improve cosmesis and functionality but has not been reported for fingernails. The resin typically lasts 6 to 8 weeks on toenails.
The Technique
Application of a resin nail involves several steps (see video online). First, the affected nail should be debrided and a bonding agent applied. Next, multiple layers of resin are applied until the patient’s desired thickness is achieved (typically 2 layers), followed by a sealing agent. Finally, the nail is cured with UV light. We recommend applying sunscreen to the hand(s) prior to curing with UV light. The liquid resin allows the nail to be customized to the patient’s desired length and shape. The overall procedure takes approximately 20 minutes for a single nail.
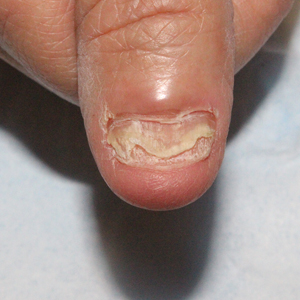
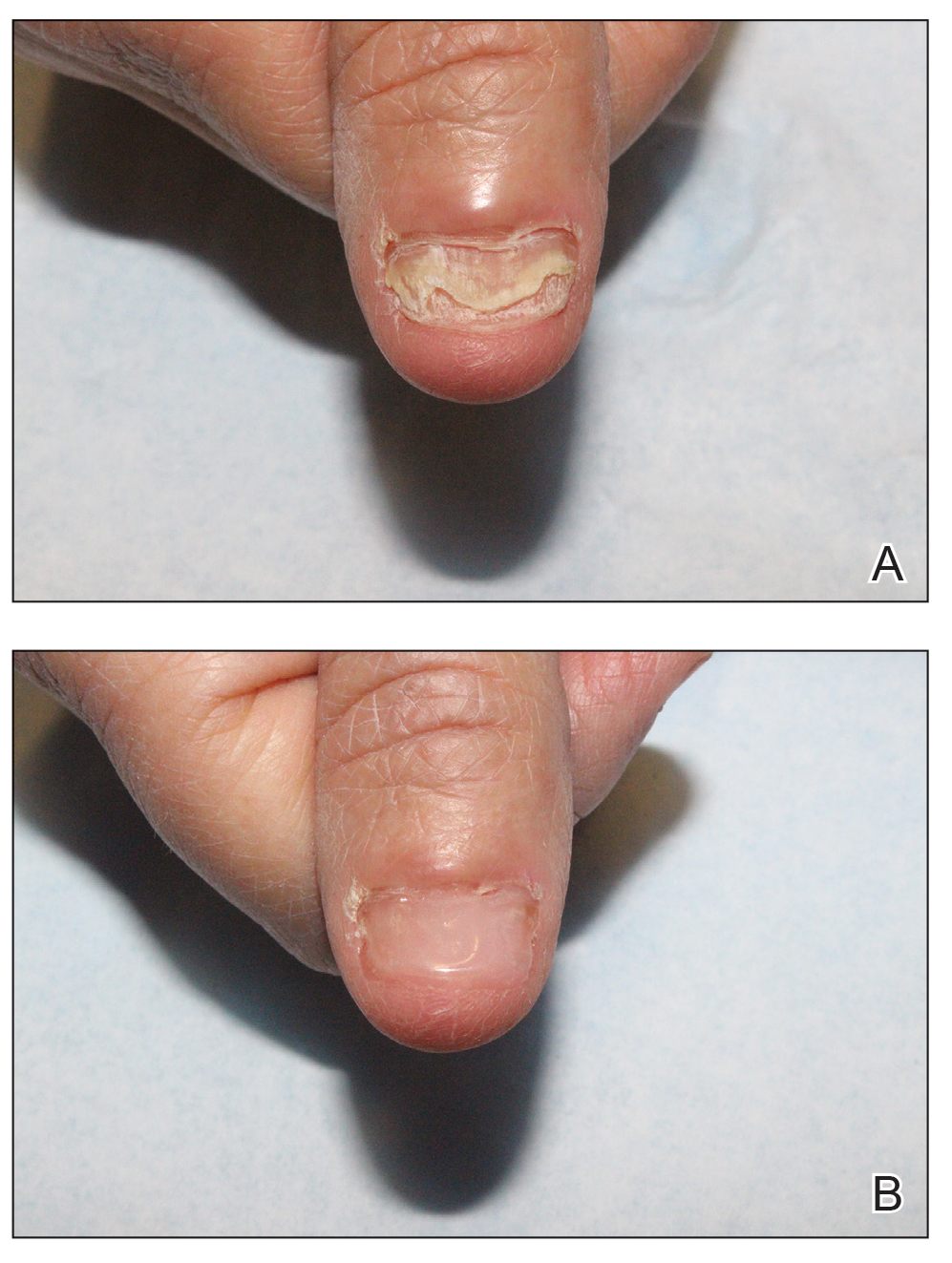
We applied resin nail to the thumbnail of a 46-year-old woman with recalcitrant isolated nail LP of 7 years’ duration (Figure). She previously had difficulties performing everyday activities, and the resin improved her functionality. She also was pleased with the cosmetic appearance. After 2 weeks, the resin started falling off with corresponding natural nail growth. The patient denied any adverse events.
Practice Implications
Resin nail application may serve as a temporary solution to improve cosmesis and functionality in patients with recalcitrant nail LP. As shown in our patient, the resin may fall off faster on the fingernails than the toenails, likely because of the faster growth rate of fingernails and more frequent exposure from daily activities. Further studies of resin nail application for the fingernails are needed to establish duration in patients with varying levels of activity (eg, washing dishes, woodworking).
Because the resin nail may be removed easily at any time, resin nail application does not interfere with treatments such as intralesional steroid injections. For patients using a topical medication regimen, the resin nail may be applied slightly distal to the cuticle so that the medication can still be applied by the proximal nail fold of the underlying natural nail.
The resin nail should be kept short and removed after 2 to 4 weeks for the fingernails and 6 to 8 weeks for the toenails to examine the underlying natural nail. Patients may go about their daily activities with the resin nail, including applying nail polish to the resin nail, bathing, and swimming. Resin nail application may complement medical treatments and improve quality of life for patients with nail LP.
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
Practice Gap
Lichen planus (LP)—a chronic inflammatory disorder affecting the nails—is prevalent in 10% to 15% of patients and is more common in the fingernails than toenails. Clinical manifestation includes longitudinal ridges, nail plate atrophy, and splitting, which all contribute to cosmetic disfigurement and difficulty with functionality. Quality of life and daily activities may be impacted profoundly.1 First-line therapies include intralesional and systemic corticosteroids; however, efficacy is limited and recurrence is common.1,2 Lichen planus is one of the few conditions that may cause permanent and debilitating nail loss.
Tools
A resin nail can be used to improve cosmetic appearance and functionality in patients with recalcitrant nail LP. The composite resin creates a flexible nonporous nail and allows the underlying natural nail to grow. Application of resin nails has been used for toenail onychodystrophies to improve cosmesis and functionality but has not been reported for fingernails. The resin typically lasts 6 to 8 weeks on toenails.
The Technique
Application of a resin nail involves several steps (see video online). First, the affected nail should be debrided and a bonding agent applied. Next, multiple layers of resin are applied until the patient’s desired thickness is achieved (typically 2 layers), followed by a sealing agent. Finally, the nail is cured with UV light. We recommend applying sunscreen to the hand(s) prior to curing with UV light. The liquid resin allows the nail to be customized to the patient’s desired length and shape. The overall procedure takes approximately 20 minutes for a single nail.

We applied resin nail to the thumbnail of a 46-year-old woman with recalcitrant isolated nail LP of 7 years’ duration (Figure). She previously had difficulties performing everyday activities, and the resin improved her functionality. She also was pleased with the cosmetic appearance. After 2 weeks, the resin started falling off with corresponding natural nail growth. The patient denied any adverse events.

Practice Implications
Resin nail application may serve as a temporary solution to improve cosmesis and functionality in patients with recalcitrant nail LP. As shown in our patient, the resin may fall off faster on the fingernails than the toenails, likely because of the faster growth rate of fingernails and more frequent exposure from daily activities. Further studies of resin nail application for the fingernails are needed to establish duration in patients with varying levels of activity (eg, washing dishes, woodworking).
Because the resin nail may be removed easily at any time, resin nail application does not interfere with treatments such as intralesional steroid injections. For patients using a topical medication regimen, the resin nail may be applied slightly distal to the cuticle so that the medication can still be applied by the proximal nail fold of the underlying natural nail.
The resin nail should be kept short and removed after 2 to 4 weeks for the fingernails and 6 to 8 weeks for the toenails to examine the underlying natural nail. Patients may go about their daily activities with the resin nail, including applying nail polish to the resin nail, bathing, and swimming. Resin nail application may complement medical treatments and improve quality of life for patients with nail LP.
Practice Gap
Lichen planus (LP)—a chronic inflammatory disorder affecting the nails—is prevalent in 10% to 15% of patients and is more common in the fingernails than toenails. Clinical manifestation includes longitudinal ridges, nail plate atrophy, and splitting, which all contribute to cosmetic disfigurement and difficulty with functionality. Quality of life and daily activities may be impacted profoundly.1 First-line therapies include intralesional and systemic corticosteroids; however, efficacy is limited and recurrence is common.1,2 Lichen planus is one of the few conditions that may cause permanent and debilitating nail loss.
Tools
A resin nail can be used to improve cosmetic appearance and functionality in patients with recalcitrant nail LP. The composite resin creates a flexible nonporous nail and allows the underlying natural nail to grow. Application of resin nails has been used for toenail onychodystrophies to improve cosmesis and functionality but has not been reported for fingernails. The resin typically lasts 6 to 8 weeks on toenails.
The Technique
Application of a resin nail involves several steps (see video online). First, the affected nail should be debrided and a bonding agent applied. Next, multiple layers of resin are applied until the patient’s desired thickness is achieved (typically 2 layers), followed by a sealing agent. Finally, the nail is cured with UV light. We recommend applying sunscreen to the hand(s) prior to curing with UV light. The liquid resin allows the nail to be customized to the patient’s desired length and shape. The overall procedure takes approximately 20 minutes for a single nail.

We applied resin nail to the thumbnail of a 46-year-old woman with recalcitrant isolated nail LP of 7 years’ duration (Figure). She previously had difficulties performing everyday activities, and the resin improved her functionality. She also was pleased with the cosmetic appearance. After 2 weeks, the resin started falling off with corresponding natural nail growth. The patient denied any adverse events.

Practice Implications
Resin nail application may serve as a temporary solution to improve cosmesis and functionality in patients with recalcitrant nail LP. As shown in our patient, the resin may fall off faster on the fingernails than the toenails, likely because of the faster growth rate of fingernails and more frequent exposure from daily activities. Further studies of resin nail application for the fingernails are needed to establish duration in patients with varying levels of activity (eg, washing dishes, woodworking).
Because the resin nail may be removed easily at any time, resin nail application does not interfere with treatments such as intralesional steroid injections. For patients using a topical medication regimen, the resin nail may be applied slightly distal to the cuticle so that the medication can still be applied by the proximal nail fold of the underlying natural nail.
The resin nail should be kept short and removed after 2 to 4 weeks for the fingernails and 6 to 8 weeks for the toenails to examine the underlying natural nail. Patients may go about their daily activities with the resin nail, including applying nail polish to the resin nail, bathing, and swimming. Resin nail application may complement medical treatments and improve quality of life for patients with nail LP.
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Gupta MK, Lipner SR. Review of nail lichen planus: epidemiology, pathogenesis, diagnosis, and treatment. Dermatol Clin. 2021;39:221-230. doi:10.1016/j.det.2020.12.002
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
Evaluating the Cost Burden of Alopecia Areata Treatment: A Comprehensive Review for Dermatologists
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.
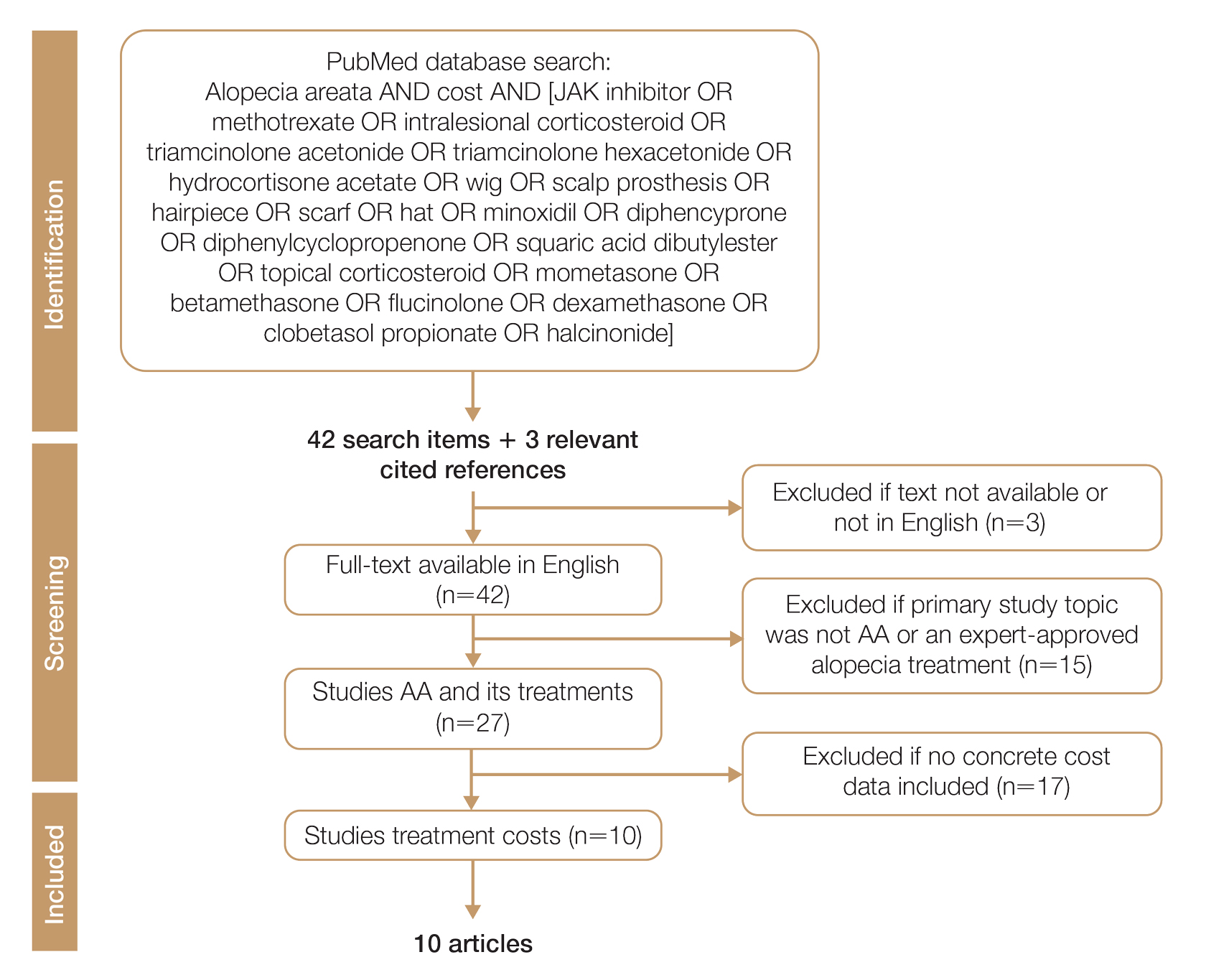
Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29
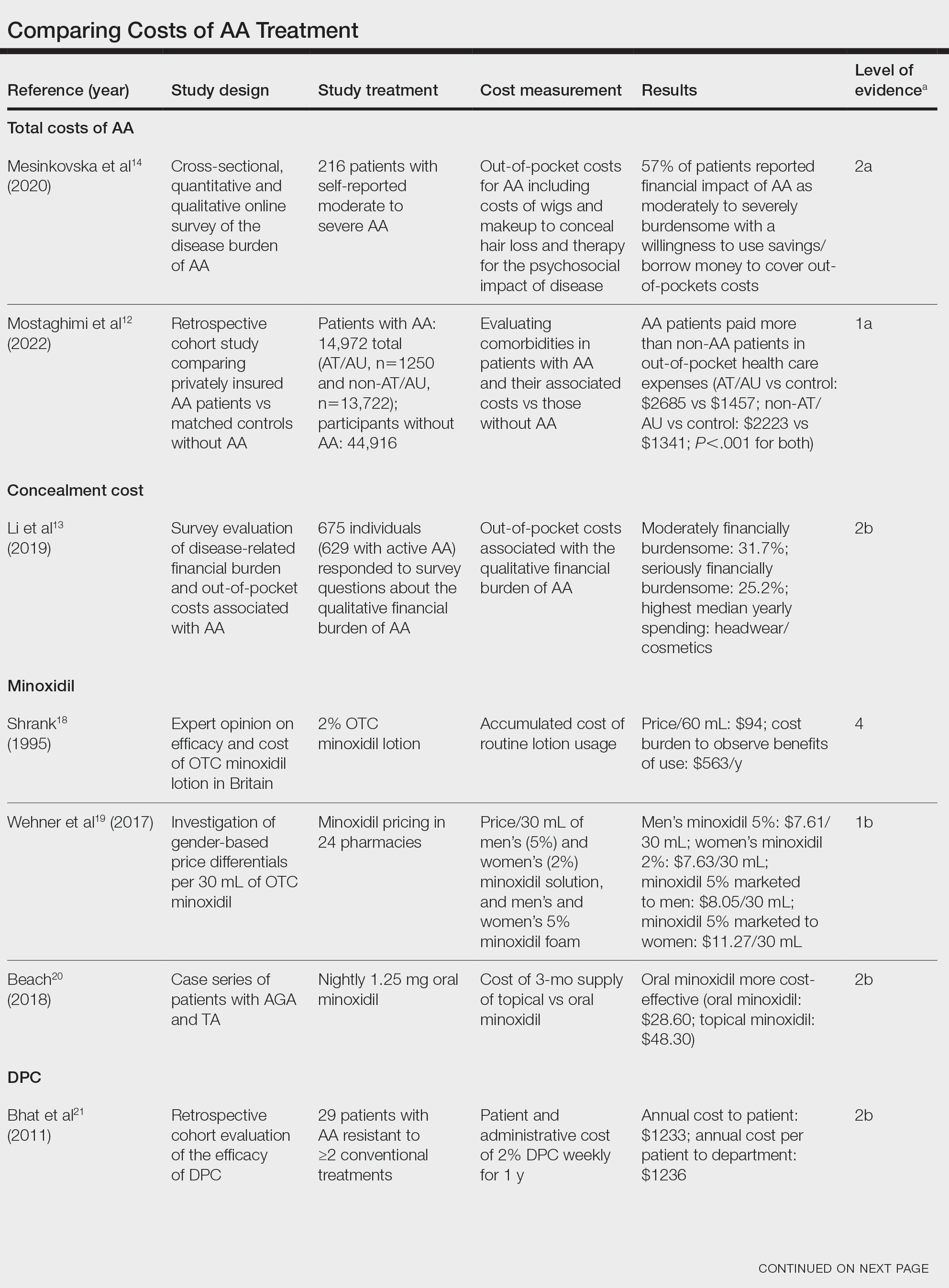
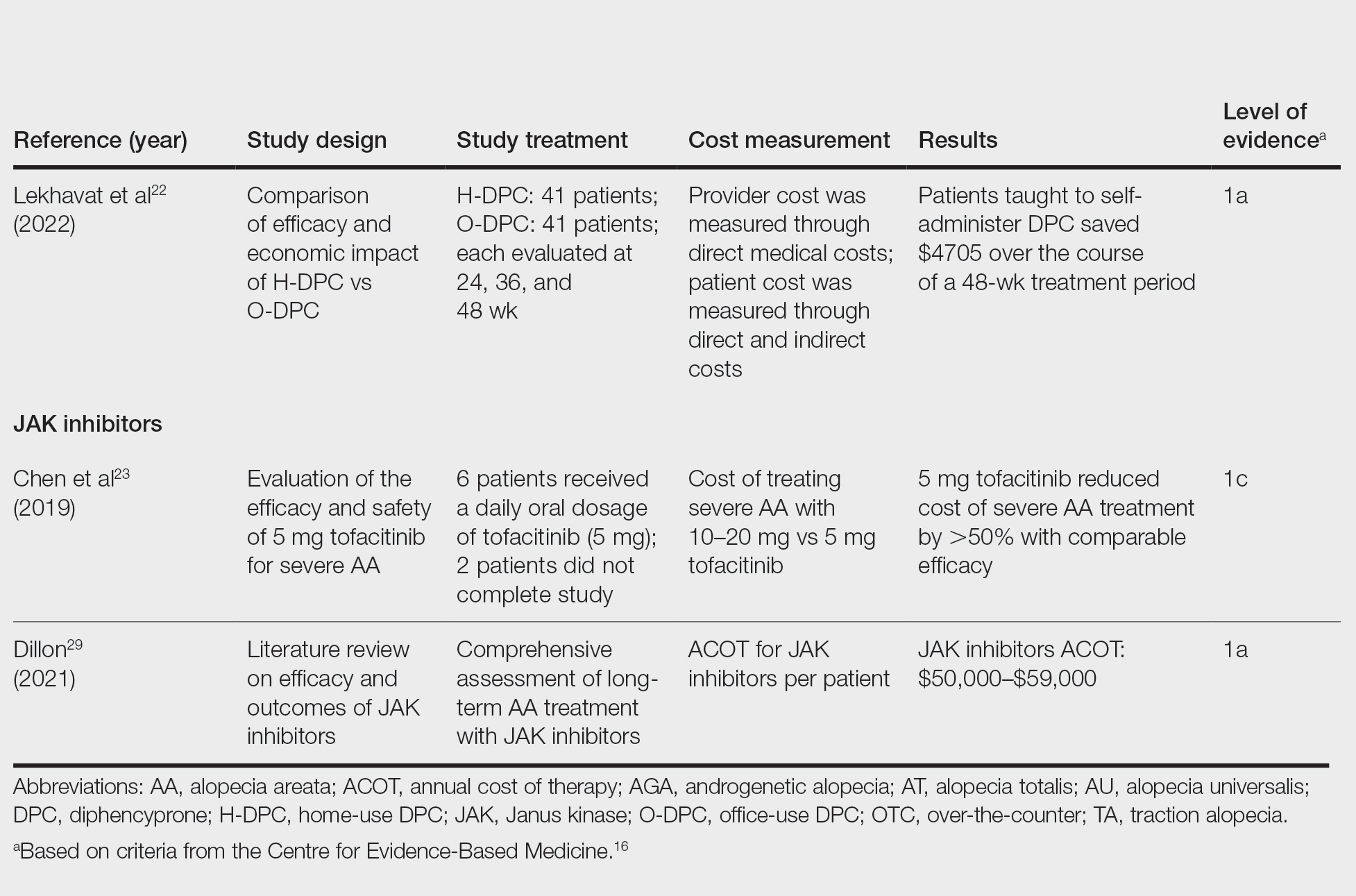
Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.

Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29


Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
Alopecia areata (AA) affects 4.5 million individuals in the United States, with 66% younger than 30 years.1,2 Inflammation causes hair loss in well-circumscribed, nonscarring patches on the body with a predilection for the scalp.3-6 The disease can devastate a patient’s self-esteem, in turn reducing quality of life.1,7 Alopecia areata is an autoimmune T-cell–mediated disease in which hair follicles lose their immune privilege.8-10 Several specific mechanisms in the cytokine interactions between T cells and the hair follicle have been discovered, revealing the Janus kinase–signal transducer and activator of transcription (JAK-STAT) pathway as pivotal in the pathogenesis of the disease and leading to the use of JAK inhibitors for treatment.11
There is no cure for AA, and the condition is managed with prolonged medical treatments and cosmetic therapies.2 Although some patients may be able to manage the annual cost, the cumulative cost of AA treatment can be burdensome.12 This cumulative cost may increase if newer, potentially expensive treatments become the standard of care. Patients with AA report dipping into their savings (41.3%) and cutting back on food or clothing expenses (33.9%) to account for the cost of alopecia treatment. Although prior estimates of the annual out-of-pocket cost of AA treatments range from $1354 to $2685, the cost burden of individual therapies is poorly understood.12-14
Patients who must juggle expensive medical bills with basic living expenses may be lost to follow-up or fall into treatment nonadherence.15 Other patients’ out-of-pocket costs may be manageable, but the costs to the health care system may compromise care in other ways. We conducted a literature review of the recommended therapies for AA based on American Academy of Dermatology (AAD) guidelines to identify the costs of alopecia treatment and consolidate the available data for the practicing dermatologist.
Methods
We conducted a PubMed search of articles indexed for MEDLINE through September 15, 2022, using the terms alopecia and cost plus one of the treatments (n=21) identified by the AAD2 for the treatment of AA (Figure). The reference lists of included articles were reviewed to identify other potentially relevant studies. Forty-five articles were identified.

Given the dearth of cost research in alopecia and the paucity of large prospective studies, we excluded articles that were not available in their full-text form or were not in English (n=3), articles whose primary study topic was not AA or an expert-approved alopecia treatment (n=15), and articles with no concrete cost data (n=17), which yielded 10 relevant articles that we studied using qualitative analysis.
Due to substantial differences in study methods and outcome measures, we did not compare the costs of alopecia among studies and did not perform statistical analysis. The quality of each study was investigated and assigned a level of evidence per the 2009 criteria from the Centre for Evidence-Based Medicine.16
All cost data were converted into US dollars ($) using the conversion rate from the time of the original article’s publication.
Results
Total and Out-of-pocket Costs of AA—Li et al13 studied out-of-pocket health care costs for AA patients (N=675). Of these participants, 56.9% said their AA was moderately to seriously financially burdensome, and 41.3% reported using their savings to manage these expenses. Participants reported median out-of-pocket spending of $1354 (interquartile range, $537–$3300) annually. The most common categories of expenses were hair appointments (81.8%) and vitamins/supplements (67.7%).13
Mesinkovska et al14 studied the qualitative and quantitative financial burdens of moderate to severe AA (N=216). Fifty-seven percent of patients reported the financial impact of AA as moderately to severely burdensome with a willingness to borrow money or use savings to cover out-of-pocket costs. Patients without insurance cited cost as a major barrier to obtaining reatment. In addition to direct treatment-related expenses, AA patients spent a mean of $1961 per year on therapy to cope with the disease’s psychological burden. Lost work hours represented another source of financial burden; 61% of patients were employed, and 45% of them reported missing time from their job because of AA.14
Mostaghimi et al12 studied health care resource utilization and all-cause direct health care costs in privately insured AA patients with or without alopecia totalis (AT) or alopecia universalis (AU)(n=14,972) matched with non-AA controls (n=44,916)(1:3 ratio). Mean total all-cause medical and pharmacy costs were higher in both AA groups compared with controls (AT/AU, $18,988 vs $11,030; non-AT/AU, $13,686 vs $9336; P<.001 for both). Out-of-pocket costs were higher for AA vs controls (AT/AU, $2685 vs $1457; non-AT/AU, $2223 vs $1341; P<.001 for both). Medical costs in the AT/AU and non-AT/AU groups largely were driven by outpatient costs (AT/AU, $10,277 vs $5713; non-AT/AU, $8078 vs $4672; P<.001 for both).12
Costs of Concealment—When studying the out-of-pocket costs of AA (N=675), Li et al13 discovered that the median yearly spending was highest on headwear or cosmetic items such as hats, wigs, and makeup ($450; interquartile range, $50–$1500). Mesinkovska et al14 reported that 49% of patients had insurance that covered AA treatment. However, 75% of patients reported that their insurance would not cover costs of concealment (eg, weave, wig, hair piece). Patients (N=112) spent a mean of $2211 per year and 10.3 hours per week on concealment.14
Minoxidil—Minoxidil solution is available over-the-counter, and its ease of access makes it a popular treatment for AA.17 Because manufacturers can sell directly to the public, minoxidil is marketed with bold claims and convincing packaging. Shrank18 noted that the product can take 4 months to work, meaning customers must incur a substantial cost burden before realizing the treatment’s benefit, which is not always obvious when purchasing minoxidil products, leaving customers—who were marketed a miracle drug—disappointed. Per Shrank,18 patients who did not experience hair regrowth after 4 months were advised to continue treatment for a year, leading them to spend hundreds of dollars for uncertain results. Those who did experience hair regrowth were advised to continue using the product twice daily 7 days per week indefinitely.18
Wehner et al19 studied the association between gender and drug cost for over-the-counter minoxidil. The price that women paid for 2% regular-strength minoxidil solutions was similar to the price that men paid for 5% extra-strength minoxidil solutions (women’s 2%, $7.63/30 mL; men’s 5%, $7.61/30 mL; P=.67). Minoxidil 5% foams with identical ingredients were priced significantly more per volume of the same product when sold as a product directed at women vs a product directed at men (men’s 5%, $8.05/30 mL; women’s 5%, $11.27/30 mL; P<.001).19
Beach20 compared the cost of oral minoxidil to topical minoxidil. At $28.60 for a 3-month supply, oral minoxidil demonstrated cost savings compared to topical minoxidil ($48.30).20
Diphencyprone—Bhat et al21 studied the cost-efficiency of diphencyprone (DPC) in patients with AA resistant to at least 2 conventional treatments (N=29). After initial sensitization with 2% DPC, patients received weekly or fortnightly treatments. Most of the annual cost burden of DPC treatment was due to staff time and overhead rather than the cost of the DPC itself: $258 for the DPC, $978 in staff time and overhead for the department, and $1233 directly charged to the patient.21
Lekhavat et al22 studied the economic impact of home-use vs office-use DPC in extensive AA (N=82). Both groups received weekly treatments in the hospital until DPC concentrations had been adjusted. Afterward, the home group was given training on self-applying DPC at home. The home group had monthly office visits for DPC concentration evaluation and refills, while the office group had weekly appointments for DPC treatment at the hospital. Calculated costs included those to the health care provider (ie, material, labor, capital costs) and the patient’s final out-of-pocket expense. The total cost to the health care provider was higher for the office group than the home group at 48 weeks (office, $683.52; home, $303.67; P<.001). Median out-of-pocket costs did not vary significantly between groups, which may have been due to small sample size affecting the range (office, $418.07; home, $189.69; P=.101). There was no significant difference between groups in the proportion of patients who responded favorably to the DPC.22
JAK Inhibitors—Chen et al23 studied the efficacy of low-dose (5 mg) tofacitinib to treat severe AA (N=6). Compared to prior studies,24-27 this analysis reported the efficacy of low-dose tofacitinib was not inferior to higher doses (10–20 mg), and low-dose tofacitinib reduced treatment costs by more than 50%.23
Per the GlobalData Healthcare database, the estimated annual cost of therapy for JAK inhibitors following US Food and Drug Administration approval was $50,000. At the time of their reporting, the next most expensive immunomodulatory drug for AA was cyclosporine, with an annual cost of therapy of $1400.28 Dillon29 reviewed the use of JAK inhibitors for the treatment of AA. The cost estimates by Dillon29 prior to FDA approval aligned with the pricing of Eli Lilly and Company for the now-approved JAK inhibitor baricitinib.30 The list price of baricitinib is $2739.99 for a 30-day supply of 2-mg tablets or $5479.98 for a 30-day supply of 4-mg tablets. This amounts to $32,879.88 for an annual supply of 2-mg tablets and $65,759.76 for an annual supply for 4-mg tablets, though the out-of-pocket costs will vary.30
Comment
We reviewed the global and treatment-specific costs of AA, consolidating the available data for the practicing dermatologist. Ten studies of approximately 16,000 patients with AA across a range of levels of evidence (1a to 4) were included (Table). Three of 10 articles studied global costs of AA, 1 studied costs of concealment, 3 studied costs of minoxidil, 2 studied costs of DPC, and 2 studied costs of JAK inhibitors. Only 2 studies achieved level of evidence 1a: the first assessed the economic impact of home-use vs office-use DPC,22 and the second researched the efficacy and outcomes of JAK inhibitors.29


Hair-loss treatments and concealment techniques cost the average patient thousands of dollars. Spending was highest on headwear or cosmetic items, which were rarely covered by insurance.13 Psychosocial sequelae further increased cost via therapy charges and lost time at work.14 Patients with AA had greater all-cause medical costs than those without AA, with most of the cost driven by outpatient visits. Patients with AA also paid nearly twice as much as non-AA patients on out-of-pocket health care expenses.14 Despite the high costs and limited efficacy of many AA therapies, patients reported willingness to incur debt or use savings to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress.13
Minoxidil solution does not require physician office visits and is available over-the-counter.17 Despite identical ingredients, minoxidil is priced more per volume when marketed to women compared with men, which reflects the larger issue of gender-based pricing that does not exist for other AAD-approved alopecia therapies but may exist for cosmetic treatments and nonapproved therapies (eg, vitamins/supplements) that are popular in the treatment of AA.19 Oral minoxidil was more cost-effective than the topical form, and gender-based pricing was a nonissue.20 However, oral minoxidil requires a prescription, mandating patients incur the cost of an office visit. Patients should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective choice.
Diphencyprone is a relatively affordable drug for AA, but the regular office visits traditionally required for its administration increase associated cost.21 Self-administration of DPC at home was more cost- and time-effective than in-office DPC administration and did not decrease efficacy. A regimen combining office visits for initial DPC titration, at-home DPC administration, and periodic office follow-up could minimize costs while preserving outcomes and safety.22
Janus kinase inhibitors are cutting-edge and expensive therapies for AA. The annual cost of these medications poses a tremendous burden on the payer (list price of annual supply ritlecitinib is $49,000),31 be that the patient or the insurance company. Low-dose tofacitinib may be similarly efficacious and could substantially reduce treatment costs.23 The true utility of these medications, specifically considering their steep costs, remains to be determined.
Conclusion
Alopecia areata poses a substantial and recurring cost burden on patients that is multifactorial including treatment, office visits, concealment, alternative therapies, psychosocial costs, and missed time at work. Although several treatment options exist, none of them are definitive. Oral minoxidil and at-home DPC administration can be cost-effective, though the cumulative cost is still high. The cost utility of JAK inhibitors remains unclear. When JAK inhibitors are prescribed, low-dose therapy may be used as maintenance to curb treatment costs. Concealment and therapy costs pose an additional, largely out-of-pocket financial burden. Despite the limited efficacy of many AA therapies, patients incur substantial expenses to manage their AA. This willingness to pay reflects AA’s impact on quality of life and puts these patients at high risk for financial distress. There are no head-to-head studies comparing the cost-effectiveness of the different AA therapies; thus, it is unclear if one treatment is most efficacious. This topic remains an avenue for future investigation. Much of the cost burden of AA treatment falls directly on patients. Increasing coverage of AA-associated expenses, such as minoxidil therapy or wigs, could decrease the cost burden on patients. Providers also can inform patients about cost-saving tactics, such as purchasing minoxidil based on concentration and vehicle rather than marketing directed at men vs women. Finally, some patients may have insurance plans that at least partially cover the costs of wigs but may not be aware of this benefit. Querying a patient’s insurance provider can further minimize costs.
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
- Tosti A, Piraccini BM, Pazzaglia M, et al. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96-98. doi:10.1067/mjd.2003.423
- Strazzulla LC, Wang EHC, Avila L, et al. Alopecia areata: an appraisal of new treatment approaches and overview of current therapies. J Am Acad Dermatol. 2018;78:15-24. doi:10.1016/j.jaad.2017.04.1142
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467-1473.
- Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73:395-399. doi:10.1016/j.jaad.2015.06.045
- Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137-145. doi:10.1111/bjd.12266
- Strober B, Buonanno M, Clark JD, et al. Effect of tofacitinib, a Janus kinase inhibitor, on haematological parameters during 12 weeks of psoriasis treatment. Br J Dermatol. 2013;169:992-999. doi:10.1111/bjd.12517
- van der Steen PH, van Baar HM, Happle R, et al. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2, pt 1):227-230. doi:10.1016/0190-9622(91)70032-w
- Strazzulla LC, Avila L, Lo Sicco K, et al. Image gallery: treatment of refractory alopecia universalis with oral tofacitinib citrate and adjunct intralesional triamcinolone injections. Br J Dermatol. 2017;176:E125. doi:10.1111/bjd.15483
- Madani S, Shapiro J. Alopecia areata update. J Am Acad Dermatol. 2000;42:549-566; quiz 567-570.
- Carnahan MC, Goldstein DA. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr Opin Ophthalmol. 2000;11:478-483. doi:10.1097/00055735-200012000-00016
- Harel S, Higgins CA, Cerise JE, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1:E1500973. doi:10.1126/sciadv.1500973
- Mostaghimi A, Gandhi K, Done N, et al. All-cause health care resource utilization and costs among adults with alopecia areata: a retrospective claims database study in the United States. J Manag Care Spec Pharm. 2022;28:426-434. doi:10.18553/jmcp.2022.28.4.426
- Li SJ, Mostaghimi A, Tkachenko E, et al. Association of out-of-pocket health care costs and financial burden for patients with alopecia areata. JAMA Dermatol. 2019;155:493-494. doi:10.1001/jamadermatol.2018.5218
- Mesinkovska N, King B, Mirmirani P, et al. Burden of illness in alopecia areata: a cross-sectional online survey study. J Investig Dermatol Symp Proc. 2020;20:S62-S68. doi:10.1016/j.jisp.2020.05.007
- Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35-44. doi:10.2147/rmhp.S19801
- Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). University of Oxford website. Accessed March 25, 2024. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009
- Klifto KM, Othman S, Kovach SJ. Minoxidil, platelet-rich plasma (PRP), or combined minoxidil and PRP for androgenetic alopecia in men: a cost-effectiveness Markov decision analysis of prospective studies. Cureus. 2021;13:E20839. doi:10.7759/cureus.20839
- Shrank AB. Minoxidil over the counter. BMJ. 1995;311:526. doi:10.1136/bmj.311.7004.526
- Wehner MR, Nead KT, Lipoff JB. Association between gender and drug cost for over-the-counter minoxidil. JAMA Dermatol. 2017;153:825-826.
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: tolerability & the five C’s of oral therapy. Dermatol Ther. 2018;31:E12707. doi:10.1111/dth.12707
- Bhat A, Sripathy K, Wahie S, et al. Efficacy and cost-efficiency of diphencyprone for alopecia areata. Br J Dermatol. 2011;165:43-44.
- Lekhavat C, Rattanaumpawan P, Juengsamranphong I. Economic impact of home-use versus office-use diphenylcyclopropenone in extensive alopecia areata. Skin Appendage Disord. 2022;8:108-117.
- Chen YY, Lin SY, Chen YC, et al. Low-dose tofacitinib for treating patients with severe alopecia areata: an efficient and cost-saving regimen. Eur J Dermatol. 2019;29:667-669. doi:10.1684/ejd.2019.3668
- Liu LY, Craiglow BG, Dai F, et al. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76:22-28. doi:10.1016/j.jaad.2016.09.007
- Kennedy Crispin M, Ko JM, Craiglow BG, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1:e89776. doi:10.1172/jci.insight.89776
- Jabbari A, Sansaricq F, Cerise J, et al. An open-label pilot study to evaluate the efficacy of tofacitinib in moderate to severe patch-type alopecia areata, totalis, and universalis. J Invest Dermatol. 2018;138:1539-1545. doi:10.1016/j.jid.2018.01.032
- Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76:29-32. doi:10.1016/j.jaad.2016.09.006
- GlobalData Healthcare. Can JAK inhibitors penetrate the alopecia areata market effectively? Pharmaceutical Technology. July 15, 2019. Accessed February 8, 2024. https://www.pharmaceutical-technology.com/analyst-comment/alopecia-areata-treatment-2019/
- Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol. 2021;14:691-714. doi:10.2147/ccid.S309215
- How much should I expect to pay for Olumiant? Accessed March 20, 2024. https://www.lillypricinginfo.com/olumiant
- McNamee A. FDA approves first-ever adolescent alopecia treatment from Pfizer. Pharmaceutical Technology. June 26, 2023. Accessed March 20, 2024. https://www.pharmaceutical-technology.com/news/fda-approves-first-ever-adolescent-alopecia-treatment-from-pfizer/?cf-view
Practice Points
- Hair loss treatments and concealment techniques cost the average patient thousands of dollars. Much of this cost burden comes from items not covered by insurance.
- Providers should be wary of gender- or marketing-related surcharges for minoxidil solutions, and oral minoxidil may be a cost-effective option.
- Self-administering diphencyprone at home is more cost- and time-effective than in-office diphencyprone administration and does not decrease efficacy.
Hair-Straightening Products Entail Acute Kidney Failure Risk
The observation was made by a team of French researchers who tested the suspected straightening product on animals. The product is believed to be the cause of several episodes of renal damage in a young woman.
“The results on mice are striking,” said study author Emmanuel Letavernier, MD, a nephrologist at Tenon Hospital in Paris. “They develop extremely severe acute kidney failure within 24 hours of applying the straightening cream. Samples show the presence of calcium oxalate crystals in all renal tubules.”
Given the potential nephrotoxicity of glyoxylic acid through topical application, products containing this compound should be avoided and ideally withdrawn from the market, the researchers suggested in a letter published in The New England Journal of Medicine. The appropriate departments of the French Agency for Food, Environmental, and Occupational Health and Safety have been alerted, Dr. Letavernier added.
Replacing Formaldehyde
Glyoxylic acid has recently been introduced into certain cosmetic products (such as shampoo, styling lotion, and straightening products), often as a replacement for formaldehyde, which is irritating and possibly carcinogenic. Glyoxylic acid is praised for its smoothing qualities. However, it is recommended to avoid contact with the scalp.
Cases of renal complications could be underdiagnosed, according to the researchers, who are preparing a nationwide survey. Renal failure can be silent. Among the signs that should raise concern are “scalp irritation accompanied by nausea or vomiting after a hair salon visit,” said Dr. Letavernier.
Similar cases have already been reported in the literature. An Israeli team recently described 26 patients treated for acute renal injuries after hair straightening in hair salons. Biopsies revealed calcium oxalate crystals in the kidneys.
The Israeli researchers suspected an effect of glycolic acid, another substance found in many cosmetic products, including straightening products. However, they could not provide evidence.
Glycolic Acid Safe?
By conducting a second animal study, which should be published soon, Dr. Letavernier and his team were able to rule out this hypothesis. “Glycolic acid does not pose a problem. Unlike glyoxylic acid, the application of glycolic acid on the skin of mice does not induce the formation of oxalate crystals in the kidneys, nor acute kidney failure.”
The French clinical case reported in the correspondence concerns a 26-year-old woman with no prior health history who had three episodes of acute renal damage 1 year apart. It turned out that each episode occurred shortly after hair straightening at a hair salon in Marseille.
The patient reported feeling a burning sensation during the hair treatment. Scalp irritations appeared. She then experienced vomiting, diarrhea, fever, and back pain. Analyses revealed high levels of plasma creatinine during each episode, indicating renal failure.
A CT scan showed no signs of urinary tract obstruction. However, the patient had a small kidney stone. Further analysis revealed the presence of blood and leukocytes in the urine. But there was no proteinuria or urinary infection.
Chronic Renal Failure
After each episode, renal function rapidly improved. “The repetition of episodes of acute renal failure is, however, a major risk factor for developing chronic renal failure in the long term,” said Dr. Letavernier.
The cream used in the hair salon to straighten hair was retrieved by the researchers. It contained a significant amount of glyoxylic acid but no glycolic acid.
To explore its potential nephrotoxic effect, they conducted a study on 10 mice. The animals were divided into two groups to test on one side topical application of the product and a gel without active product (control group) on the other.
Mice exposed to the product had oxalate crystals in their urine, unlike mice in the control group. A scan confirmed calcium oxalate deposits in the kidneys. Plasma creatinine levels increased significantly after exposure to glyoxylic acid.
“After passing through the epidermis, glyoxylic acid is rapidly converted in the blood to glyoxylate. In the liver and probably in other organs, glyoxylate is metabolized to become oxalate, which upon contact with calcium in the urine forms calcium oxalate crystals,” explained the specialist.
Excess calcium oxalate crystals causing renal failure are observed in rare conditions such as primary hyperoxaluria, a genetic disease affecting liver metabolism, or enteric hyperoxaluria, which is linked to increased intestinal permeability to oxalate: an anion naturally found in certain plants.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The observation was made by a team of French researchers who tested the suspected straightening product on animals. The product is believed to be the cause of several episodes of renal damage in a young woman.
“The results on mice are striking,” said study author Emmanuel Letavernier, MD, a nephrologist at Tenon Hospital in Paris. “They develop extremely severe acute kidney failure within 24 hours of applying the straightening cream. Samples show the presence of calcium oxalate crystals in all renal tubules.”
Given the potential nephrotoxicity of glyoxylic acid through topical application, products containing this compound should be avoided and ideally withdrawn from the market, the researchers suggested in a letter published in The New England Journal of Medicine. The appropriate departments of the French Agency for Food, Environmental, and Occupational Health and Safety have been alerted, Dr. Letavernier added.
Replacing Formaldehyde
Glyoxylic acid has recently been introduced into certain cosmetic products (such as shampoo, styling lotion, and straightening products), often as a replacement for formaldehyde, which is irritating and possibly carcinogenic. Glyoxylic acid is praised for its smoothing qualities. However, it is recommended to avoid contact with the scalp.
Cases of renal complications could be underdiagnosed, according to the researchers, who are preparing a nationwide survey. Renal failure can be silent. Among the signs that should raise concern are “scalp irritation accompanied by nausea or vomiting after a hair salon visit,” said Dr. Letavernier.
Similar cases have already been reported in the literature. An Israeli team recently described 26 patients treated for acute renal injuries after hair straightening in hair salons. Biopsies revealed calcium oxalate crystals in the kidneys.
The Israeli researchers suspected an effect of glycolic acid, another substance found in many cosmetic products, including straightening products. However, they could not provide evidence.
Glycolic Acid Safe?
By conducting a second animal study, which should be published soon, Dr. Letavernier and his team were able to rule out this hypothesis. “Glycolic acid does not pose a problem. Unlike glyoxylic acid, the application of glycolic acid on the skin of mice does not induce the formation of oxalate crystals in the kidneys, nor acute kidney failure.”
The French clinical case reported in the correspondence concerns a 26-year-old woman with no prior health history who had three episodes of acute renal damage 1 year apart. It turned out that each episode occurred shortly after hair straightening at a hair salon in Marseille.
The patient reported feeling a burning sensation during the hair treatment. Scalp irritations appeared. She then experienced vomiting, diarrhea, fever, and back pain. Analyses revealed high levels of plasma creatinine during each episode, indicating renal failure.
A CT scan showed no signs of urinary tract obstruction. However, the patient had a small kidney stone. Further analysis revealed the presence of blood and leukocytes in the urine. But there was no proteinuria or urinary infection.
Chronic Renal Failure
After each episode, renal function rapidly improved. “The repetition of episodes of acute renal failure is, however, a major risk factor for developing chronic renal failure in the long term,” said Dr. Letavernier.
The cream used in the hair salon to straighten hair was retrieved by the researchers. It contained a significant amount of glyoxylic acid but no glycolic acid.
To explore its potential nephrotoxic effect, they conducted a study on 10 mice. The animals were divided into two groups to test on one side topical application of the product and a gel without active product (control group) on the other.
Mice exposed to the product had oxalate crystals in their urine, unlike mice in the control group. A scan confirmed calcium oxalate deposits in the kidneys. Plasma creatinine levels increased significantly after exposure to glyoxylic acid.
“After passing through the epidermis, glyoxylic acid is rapidly converted in the blood to glyoxylate. In the liver and probably in other organs, glyoxylate is metabolized to become oxalate, which upon contact with calcium in the urine forms calcium oxalate crystals,” explained the specialist.
Excess calcium oxalate crystals causing renal failure are observed in rare conditions such as primary hyperoxaluria, a genetic disease affecting liver metabolism, or enteric hyperoxaluria, which is linked to increased intestinal permeability to oxalate: an anion naturally found in certain plants.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The observation was made by a team of French researchers who tested the suspected straightening product on animals. The product is believed to be the cause of several episodes of renal damage in a young woman.
“The results on mice are striking,” said study author Emmanuel Letavernier, MD, a nephrologist at Tenon Hospital in Paris. “They develop extremely severe acute kidney failure within 24 hours of applying the straightening cream. Samples show the presence of calcium oxalate crystals in all renal tubules.”
Given the potential nephrotoxicity of glyoxylic acid through topical application, products containing this compound should be avoided and ideally withdrawn from the market, the researchers suggested in a letter published in The New England Journal of Medicine. The appropriate departments of the French Agency for Food, Environmental, and Occupational Health and Safety have been alerted, Dr. Letavernier added.
Replacing Formaldehyde
Glyoxylic acid has recently been introduced into certain cosmetic products (such as shampoo, styling lotion, and straightening products), often as a replacement for formaldehyde, which is irritating and possibly carcinogenic. Glyoxylic acid is praised for its smoothing qualities. However, it is recommended to avoid contact with the scalp.
Cases of renal complications could be underdiagnosed, according to the researchers, who are preparing a nationwide survey. Renal failure can be silent. Among the signs that should raise concern are “scalp irritation accompanied by nausea or vomiting after a hair salon visit,” said Dr. Letavernier.
Similar cases have already been reported in the literature. An Israeli team recently described 26 patients treated for acute renal injuries after hair straightening in hair salons. Biopsies revealed calcium oxalate crystals in the kidneys.
The Israeli researchers suspected an effect of glycolic acid, another substance found in many cosmetic products, including straightening products. However, they could not provide evidence.
Glycolic Acid Safe?
By conducting a second animal study, which should be published soon, Dr. Letavernier and his team were able to rule out this hypothesis. “Glycolic acid does not pose a problem. Unlike glyoxylic acid, the application of glycolic acid on the skin of mice does not induce the formation of oxalate crystals in the kidneys, nor acute kidney failure.”
The French clinical case reported in the correspondence concerns a 26-year-old woman with no prior health history who had three episodes of acute renal damage 1 year apart. It turned out that each episode occurred shortly after hair straightening at a hair salon in Marseille.
The patient reported feeling a burning sensation during the hair treatment. Scalp irritations appeared. She then experienced vomiting, diarrhea, fever, and back pain. Analyses revealed high levels of plasma creatinine during each episode, indicating renal failure.
A CT scan showed no signs of urinary tract obstruction. However, the patient had a small kidney stone. Further analysis revealed the presence of blood and leukocytes in the urine. But there was no proteinuria or urinary infection.
Chronic Renal Failure
After each episode, renal function rapidly improved. “The repetition of episodes of acute renal failure is, however, a major risk factor for developing chronic renal failure in the long term,” said Dr. Letavernier.
The cream used in the hair salon to straighten hair was retrieved by the researchers. It contained a significant amount of glyoxylic acid but no glycolic acid.
To explore its potential nephrotoxic effect, they conducted a study on 10 mice. The animals were divided into two groups to test on one side topical application of the product and a gel without active product (control group) on the other.
Mice exposed to the product had oxalate crystals in their urine, unlike mice in the control group. A scan confirmed calcium oxalate deposits in the kidneys. Plasma creatinine levels increased significantly after exposure to glyoxylic acid.
“After passing through the epidermis, glyoxylic acid is rapidly converted in the blood to glyoxylate. In the liver and probably in other organs, glyoxylate is metabolized to become oxalate, which upon contact with calcium in the urine forms calcium oxalate crystals,” explained the specialist.
Excess calcium oxalate crystals causing renal failure are observed in rare conditions such as primary hyperoxaluria, a genetic disease affecting liver metabolism, or enteric hyperoxaluria, which is linked to increased intestinal permeability to oxalate: an anion naturally found in certain plants.
This story was translated from the Medscape French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Studies Reinforce JAK Inhibitor Efficacy for Most Challenging Alopecia Types
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
FROM AAD 2024
SAN DIEGO — , according to late-breaking data presented at the annual meeting of the American Academy of Dermatology.
In one study of brepocitinib, the target was cicatricial alopecia (CA), a form of hair loss for which there are no approved therapies. In the other, a subanalysis from phase 3 trials of ritlecitinib for alopecia areata (AA), hair regrowth was shown in the subset of patients who entered the study with alopecia totalis or alopecia universalis (AT/AU).
Reflecting comments from several experts, including one of the late-breaking session moderators, April W. Armstrong, MD, MPH, professor and chief of dermatology, University of California, Los Angeles, said that the CA study, which matched clinical response to changes in CA biomarkers, suggested that the results are a potential breakthrough.
“This is the first placebo-controlled study with an oral JAK inhibitor that not only shows that scarring alopecia can be reversible but also gives insights to the mechanism of action and which patients might respond best,” Emma Guttman-Yassky, MD, PhD, said in an interview. Dr. Guttman-Yassky, professor of Dermatology and Immunology, and director of the Laboratory of Inflammatory Skin Diseases, Icahn School of Medicine at Mount Sinai, New York City, was the study’s senior investigator.
Scarring Alopecia and Brepocitinib
For the study of scarring alopecia, 49 patients with CA were randomized in a 3:1 ratio to brepocitinib, a first-in-class inhibitor that targets both JAK1 and TYK2, or placebo. Because of the small size of the study, the primary endpoint was the change in CA biomarkers. The secondary outcome was clinical response, but because of a correlation between the two, these were mutually reinforcing.
Of the subtypes, nine patients enrolled in the study had frontal fibrosing alopecia (FFA), 16 had lichen planopilaris (LPP) alopecia, and 24 had central centrifugal cicatricial alopecia (CCCA). All of the forms of CA are more common in women overall and women of color specifically, particularly CCCA. For this analysis, the FFA and LPP subtypes were considered similar for assessing response and were combined.
The data included a comparison of response and safety during the 24-week randomization phase, as well as an additional follow-up conducted after another 24 weeks of open-label treatment. During the second phase, all patients on placebo were switched to active treatment.
Overall, there was a reduction in all four of the key scalp inflammatory biomarkers measured among those in the combined FFA/LLP group. In the placebo group, each of these markers — interferon gamma (IFN-gamma), CCLS, CXCL10, and STAT1 — increased over the same time period. In almost all cases, the differences were statistically significant.
In the CCCA subgroup, the same pattern (an increase among those on placebo but a decrease among those on brepocitinib) was observed for CCLS and CXCL10. For IFN-gamma and STAT1, a rise was observed among those on placebo and those on active treatment, although the rise was greater for placebo.
For clinical response, improvement on brepocitinib was observed on disease activity indexes, particularly among those in the FFA/LLP group, according to Marguerite Meariman, MD, a dermatology resident at Mount Sinai, who presented the results. She called the improvement in clinical activity scores at 48 weeks “dramatic.” Moreover, improvement was apparent within 4 weeks of starting therapy.
For CCCA, a more challenging condition to treat, Dr. Meariman said that no further progression might represent an acceptable response for many patients, but there were also cases of hair regrowth in this subset. Although improvement was not generally on the order seen among those with FFA/LLP, she suggested that there is promise even in these more difficult patients.
Further studies are planned, but Dr. Meariman said that it might be important to focus on early treatment regardless of CA subtype. She noted that patients with less than 5 years disease duration typically did better than those with longer durations.
Ritlecitinib for AT/AU
The analysis of patients with AT/AU was based on a subset analysis from the ALLEGRO phase 2b/3 study of ritlecitinib, which targets JAK3 and TEC kinases. The full results of the ALLEGRO trial were published last year in The Lancet. In the new late-breaker analysis, Severity of Alopecia Tool (SALT) scores were evaluated on an observed or last-observation-carried-forward basis. Generally, responses in the subgroup of patients with AT/AU, who had a median SALT score of 80.3 (signifying 80.3% hair loss) at baseline, were only modestly lower than those in the overall trial.
At 24 months, about 50% of patients achieved a SALT score of 20, according to Melissa Piliang, MD, chair of Dermatology at the Cleveland Clinic, Cleveland, Ohio, who presented the data. In this group, as in the non-AT/AU population, responses climbed over time, and these responses have been maintained for as long as patients have remained on therapy.
At the more rigorous threshold of SALT < 10, the proportion of responders was only slightly lower, meaning a substantial proportion of patients with AT/AU “are achieving 90% or more of hair regrowth, so really an excellent response,” Dr. Piliang said.
For the subgroup with AU, specifically, regrowth of eyebrows and eyelashes was also observed in a substantial proportion, according to Dr. Piliang. Attributed to the often-devastating psychological burden of hair loss, patient-reported assessments of these responses global were generally “even better” than those reported by the investigators.
However, Dr. Piliang advised clinicians to treat AA as early as possible. Despite the benefits seen in the AT/AU subgroup, she pointed out that starting treatment before total hair loss is associated with a higher likelihood of complete or nearly complete hair regrowth.
There are no data from the ALLEGRO trial to determine how long hair regrowth persists after discontinuation of ritlecitinib, which has been approved for the treatment of AA, but Dr. Piliang said that patients should be told that lifelong therapy should be expected in the vast majority of individuals, whether or not AA has advanced to AT/AU.
“In my experience with JAK inhibitors, you lose response when you come off these drugs,” she said.
Dr. Meariman reported a financial relationship with AbbVie. Dr. Piliang reported financial relationships with Eli Lilly, Pfizer, and Proctor & Gamble. Dr. Armstrong reported financial relationships with more than 30 pharmaceutical companies, including those that manufacture JAK inhibitors. Dr. Guttman-Yassky reported financial relationships with more than 30 companies, including those that manufacture JAK inhibitors.
A version of this article appeared on Medscape.com.
Study Finds No Increased Cancer Risk With Spironolactone
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
TOPLINE:
than that of unexposed women.
METHODOLOGY:
- Spironolactone, used off-label for several skin conditions in women, carries a warning about an increased tumor risk associated with high doses in rat models, and its antiandrogen properties have prompted hypotheses about a possible increased risk for breast or gynecologic cancers.
- The researchers reviewed data on 420 women with a history of spironolactone use for acne, hair loss, and hirsutism and 3272 women with no spironolactone use at the authors› institution. Their mean age ranged from 42 to 63 years; the majority were White, and 38% were non-White.
- Median spironolactone doses ranged from 25 mg to 225 mg; chart reviews included 5-year follow-up data from the first spironolactone exposure to allow time for tumor development.
TAKEAWAY:
- A total of 37 of the 420 women exposed to spironolactone developed any tumors, as did 546 of the 3272 with no spironolactone exposure.
- After the researchers controlled for age and race, women exposed to spironolactone were no more likely to develop a malignant tumor than a benign tumor, compared with unexposed women (odds ratio [OR], 0.48, P = .2).
- The risk for breast or uterine cancer was not significantly different in the spironolactone and non-spironolactone groups (OR, 0.95, P > .9).
IN PRACTICE:
“Women taking spironolactone for acne, hair loss, and hirsutism and who are at low risk of breast or gynecologic cancers may be counseled to have regular gynecology follow-up, but no more frequently than the general population,” but more studies are needed to evaluate risk over longer periods of time, the researchers wrote.
SOURCE:
The lead author of the study was Rachel C. Hill, BS, a student at Weill Cornell Medical College, New York City, and Shari R. Lipner, MD, PhD, of the department of dermatology at Weill Cornell Medical College, was the corresponding author. The study was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The findings were limited by the retrospective design, as well as the small number of spironolactone patients analyzed, the short follow-up period, the lack of information about spironolactone courses, and the inability to control for family history of malignancy.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences and a grant from the Clinical and Translational Science Center at Weill Cornell Medical College awarded to Ms. Hill. None of the authors had relevant disclosures; Dr. Lipner disclosed serving as a consultant for Ortho-Dermatologics, Eli Lilly, Moberg Pharmaceuticals, and BelleTorus Corporation.
A version of this article appeared on Medscape.com.
Longitudinal Melanonychia
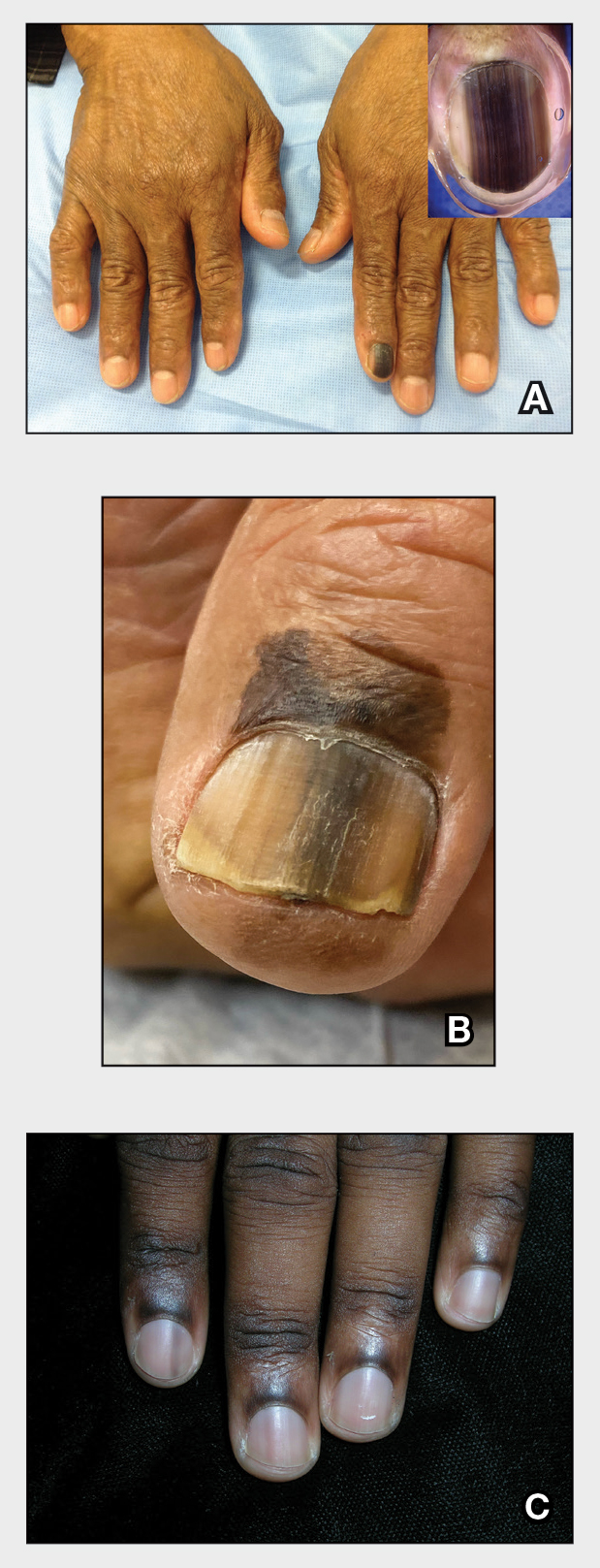
THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009

THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.

THE COMPARISON
A Melanoma in situ manifesting as longitudinal melanonychia (LM) in a single digit in a Black man. Dermoscopy showed irregular dark bands of brown pigmentation and micro-Hutchinson sign on the cuticle (inset).
B Melanoma manifesting as LM with a prominent Hutchinson sign in a Hispanic man, with variable shades of brown covering more than 50% of the nail width.
C Longitudinal melanonychia of at least 2 nails with a pseudo-Hutchinson sign (pigment on the nail folds in a benign case of LM) in a young Black man demonstrating ethnic/racial melanosis. The longitudinal bands, which were caused by benign melanocytic activation, are more gray than brown and are less than 3 mm wide.
Longitudinal melanonychia (LM) is a pigmented linear band—brown, black, or gray—spanning the length of the nail plate due to the presence of excess melanin, which may be attributed to a benign or malignant process and may warrant further investigation.1,2 The majority of patients who present with LM are diagnosed with melanocytic activation of the nail matrix due to their inherent darker skin tone or various triggers including trauma, infection, and medications. Longitudinal melanonychia secondary to melanocytic activation often occurs spontaneously in patients with skin of color.3 Less commonly, LM is caused by a nail matrix nevus or lentigo; however, LM may arise secondary to subungual melanoma, a more dangerous cause.
A thorough clinical history including duration, recent changes in LM manifestation, nail trauma, or infection is helpful in evaluating patients with LM; however, a history of nail trauma can be misleading, as nail changes attributed to the trauma may in fact be melanoma. Irregularly spaced vertical lines of pigmentation ranging from brown to black with variations in spacing and width are characteristic of subungual melanoma.4 Nail dystrophy, granular hyperpigmentation, and Hutchinson sign (extension of pigmentation to the nail folds) also are worrisome features.5 In recent years, dermoscopy has become an important tool in the clinical examination of LM, with the development of criteria based on color and pattern recognition.5,6 Dermoscopy can be useful in screening potential candidates for biopsy. Although clinical examination and dermoscopy are essential to evaluating LM, the gold-standard diagnostic test when malignancy is suspected is a nail matrix biopsy.1,2,6,7
Epidemiology
It is not unusual for patients with darker skin tones to develop LM due to melanocytic activation of multiple nails with age. This finding can be seen in approximately 80% of African American individuals, 30% of Japanese individuals, and 50% of Hispanic individuals.2 It has even been reported that approximately 100% of Black patients older than 50 years will have evidence of LM.3
In a retrospective analysis, children presenting with LM tend to have a higher prevalence of nail matrix nevi compared to adults (56.1% [60/106] vs 34.3% [23/66]; P=.005).8 Involvement of a single digit in children is most likely indicative of a nevus; however, when an adult presents with LM in a single digit, suspicion for subungual melanoma should be raised.2,3,9
Two separate single-center retrospective studies showed the prevalence of subungual melanoma in patients presenting with melanonychia in Asia. Jin et al10 reported subungual melanoma in 6.2% (17/275) of Korean patients presenting with melanonychia at a general dermatology clinic from 2002 to 2014. Lyu et al8 studied LM in 172 Chinese patients in a dermatology clinic from 2018 to 2021 and reported 9% (6/66) of adults (aged ≥18 years) with subungual melanoma, with no reported cases in childhood (aged <18 years).
Although the prevalence of subungual melanoma in patients with LM is low, it is an important diagnosis that should not be missed. In confirmed cases of subungual melanoma, two-thirds of lesions manifested as LM.3,10,11 Thus, LM arising in an adult in a single digit is more concerning for malignancy.2,3,7,9
Individuals of African and Asian descent as well as American Indian individuals are at highest risk for subungual melanoma with a poor prognosis compared to other types of melanoma, largely due to diagnosis at an advanced stage of disease.3,9 In a retrospective study of 25 patients with surgically treated subungual melanoma, the mean recurrence-free survival was 33.6 months. The recurrence-free survival was 66% at 1 year and 40% at 3 years, and the overall survival rate was 37% at 3 years.12
Key clinical features in individuals with darker skin tones
- In patients with darker skin tones, LM tends to occur on multiple nails as a result of melanocytic activation.2,13
- Several longitudinal bands may be noted on the same nail and the pigmentation of the bands may vary. With age, these longitudinal bands typically increase in number and width.13
- Pseudo-Hutchinson sign may be present due to ethnic melanosis of the proximal nail fold.13,14
- Dermoscopic findings of LM in patients with skin of color include wider bands (P=.0125), lower band brightness (P<.032), and higher frequency of changing appearance of bands (P=.0071).15
Worth noting
When patients present with LM, thorough examination of the nail plate, periungual skin, and distal pulp of all digits on all extremities with adequate lighting is important.2 Dermoscopy is useful, and a gel interface helps for examining the nail plates.7
Clinicians should be encouraged to biopsy or immediately refer patients with concerning nail unit lesions. Cases of LM most likely are benign, but if some doubt exists, the lesions should be biopsied or tracked closely with clinical and dermoscopic images, with a biopsy if changes occur.16 In conjunction with evaluation by a qualified clinician, patients also should be encouraged to take photographs, as the evolution of nail changes is a critical part of clinical decision-making on the need for a biopsy or referral.
Health disparity highlight
Despite the disproportionately high mortality rates from subungual melanoma in Black and Hispanic populations,3,9 studies often do not adequately represent these populations. Although subungual melanoma is rare, a delay in the diagnosis contributes to high morbidity and mortality rates.
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Tosti A, Piraccini BM, de Farias DC. Dealing with melanonychia. Semin Cutan Med Surg. 2009;28:49-54. doi:10.1016/j.sder.2008.12.004
- Piraccini BM, Dika E, Fanti PA. Tips for diagnosis and treatment of nail pigmentation with practical algorithm. Dermatol Clin. 2015;33:185-195. doi:10.1016/j.det.2014.12.002
- Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2016;2:156-161. doi:10.1159/000452673
- Singal A, Bisherwal K. Melanonychia: etiology, diagnosis, and treatment. Indian Dermatol J Online. 2020;11:1-11. doi:10.4103/idoj.IDOJ_167_19
- Benati E, Ribero S, Longo C, et al. Clinical and dermoscopic clues to differentiate pigmented nail bands: an International Dermoscopy Society study. J Eur Acad Dermatol Venereol. 2017; 31:732-736. doi:10.1111/jdv.13991
- Sawada M, Yokota K, Matsumoto T, et al. Proposed classification of longitudinal melanonychia based on clinical and dermoscopic criteria. Int J Dermatol. 2014;53:581-585. doi:10.1111/ijd.12001
- Starace M, Alessandrini A, Brandi N, et al. Use of nail dermoscopy in the management of melanonychia. Dermatol Pract Concept. 2019; 9:38-43. doi:10.5826/dpc.0901a10
- Lyu A, Hou Y, Wang Q. Retrospective analysis of longitudinal melanonychia: a Chinese experience. Front Pediatr. 2023;10:1065758. doi:10.3389/fped.2022.1065758
- Williams NM, Obayomi AO, Diaz-Perez, JA, et al. Monodactylous longitudinal melanonychia: a sign of Bowen’s disease in skin of color. Skin Appendage Disord. 2021;7:306-310. doi:10.1159/000514221
- Jin H, Kim JM, Kim GW, et al. Diagnostic criteria for and clinical review of melanonychia in Korean patients. J Am Acad Dermatol. 2016;74,1121-1127. doi:10.1016/j.jaad.2015.12.039
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996. doi:10.1016 /j.jaad.2016.11.053
- LaRocca CJ, Lai L, Nelson RA, et al. Subungual melanoma: a single institution experience. Med Sci (Basel). 2021;9:57. doi:10.3390 /medsci9030057
- Baran LR, Ruben BS, Kechijian P, et al. Non‐melanoma Hutchinson’s sign: a reappraisal of this important, remarkable melanoma simulant. J Eur Acad Dermatol Venereol. 2018;32:495-501. doi:10.1111/jdv.14715
- Sladden MJ, Mortimer NJ, Osborne JE. Longitudinal melanonychia and pseudo‐Hutchinson sign associated with amlodipine. Br J Dermatol. 2005;153:219-220. doi:10.1111/j.1365-2133.2005.06668.x
- Lee DK, Chang MJ, Desai AD, et al. Clinical and dermoscopic findings of benign longitudinal melanonychia due to melanocytic activation differ by skin type and predict likelihood of nail matrix biopsy. J Am Acad Dermatol. 2022;87:792-799. doi:10.1016/j.jaad.2022.06.1165
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009