User login
Tackling Inflammatory and Infectious Nail Disorders in Children
Nail disorders are common among pediatric patients but often are underdiagnosed or misdiagnosed because of their unique disease manifestations. These conditions may severely impact quality of life. There are few nail disease clinical trials that include children. Consequently, most treatment recommendations are based on case series and expert consensus recommendations. We review inflammatory and infectious nail disorders in pediatric patients. By describing characteristics, clinical manifestations, and management approaches for these conditions, we aim to provide guidance to dermatologists in their diagnosis and treatment.
INFLAMMATORY NAIL DISORDERS
Nail Psoriasis
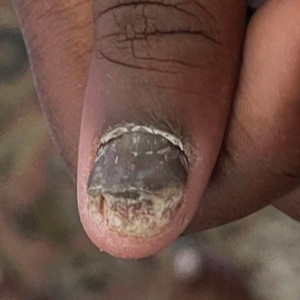
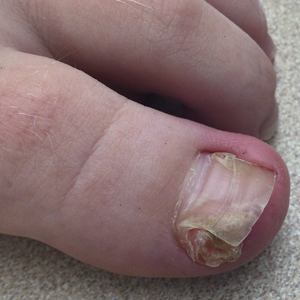
Nail involvement in children with psoriasis is common, with prevalence estimates ranging from 17% to 39.2%.1 Nail matrix psoriasis may manifest with pitting (large irregular pits) and leukonychia as well as chromonychia and nail plate crumbling. Onycholysis, oil drop spots (salmon patches), and subungual hyperkeratosis can be seen in nail bed psoriasis. Nail pitting is the most frequently observed clinical finding (Figure 1).2,3 In a cross-sectional multicenter study of 313 children with cutaneous psoriasis in France, nail findings were present in 101 patients (32.3%). There were associations between nail findings and presence of psoriatic arthritis (P=.03), palmoplantar psoriasis (P<.001), and severity of psoriatic disease, defined as use of systemic treatment with phototherapy (psoralen plus UVA, UVB), traditional systemic treatment (acitretin, methotrexate, cyclosporine), or a biologic (P=.003).4
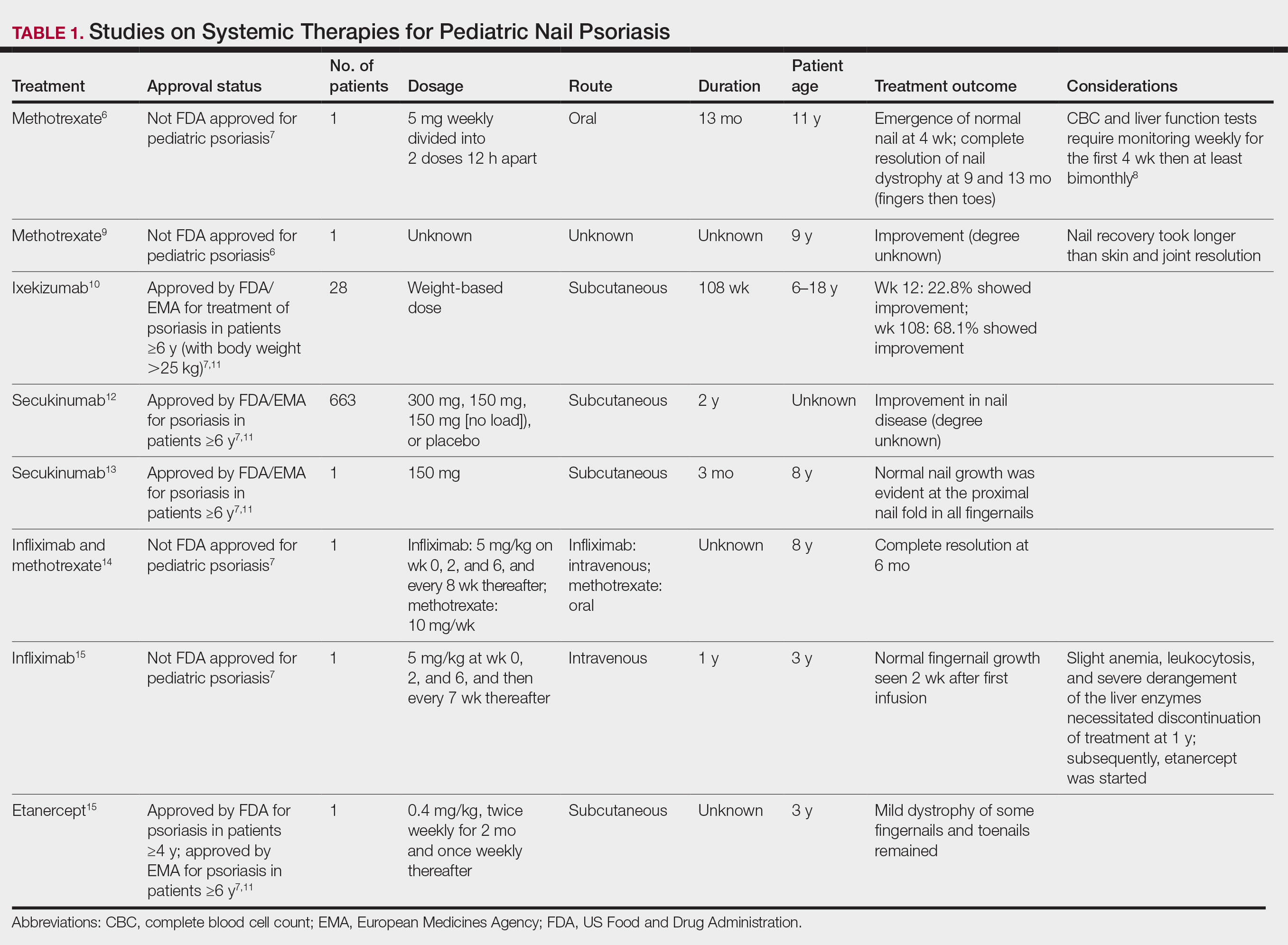
Topical steroids and vitamin D analogues may be used with or without occlusion and may be efficacious.5 Several case reports describe systemic treatments for psoriasis in children, including methotrexate, acitretin, and apremilast (approved for children 6 years and older for plaque psoriasis by the US Food and Drug Administration [FDA]).2 There are 5 biologic drugs currently approved for the treatment of pediatric psoriasis—adalimumab, etanercept, ustekinumab, secukinumab, ixekizumab—and 6 drugs currently undergoing phase 3 studies—brodalumab, guselkumab, risankizumab, tildrakizumab, certolizumab pegol, and deucravacitinib (Table 1).6-15 Adalimumab is specifically approved for moderate to severe nail psoriasis in adults 18 years and older.
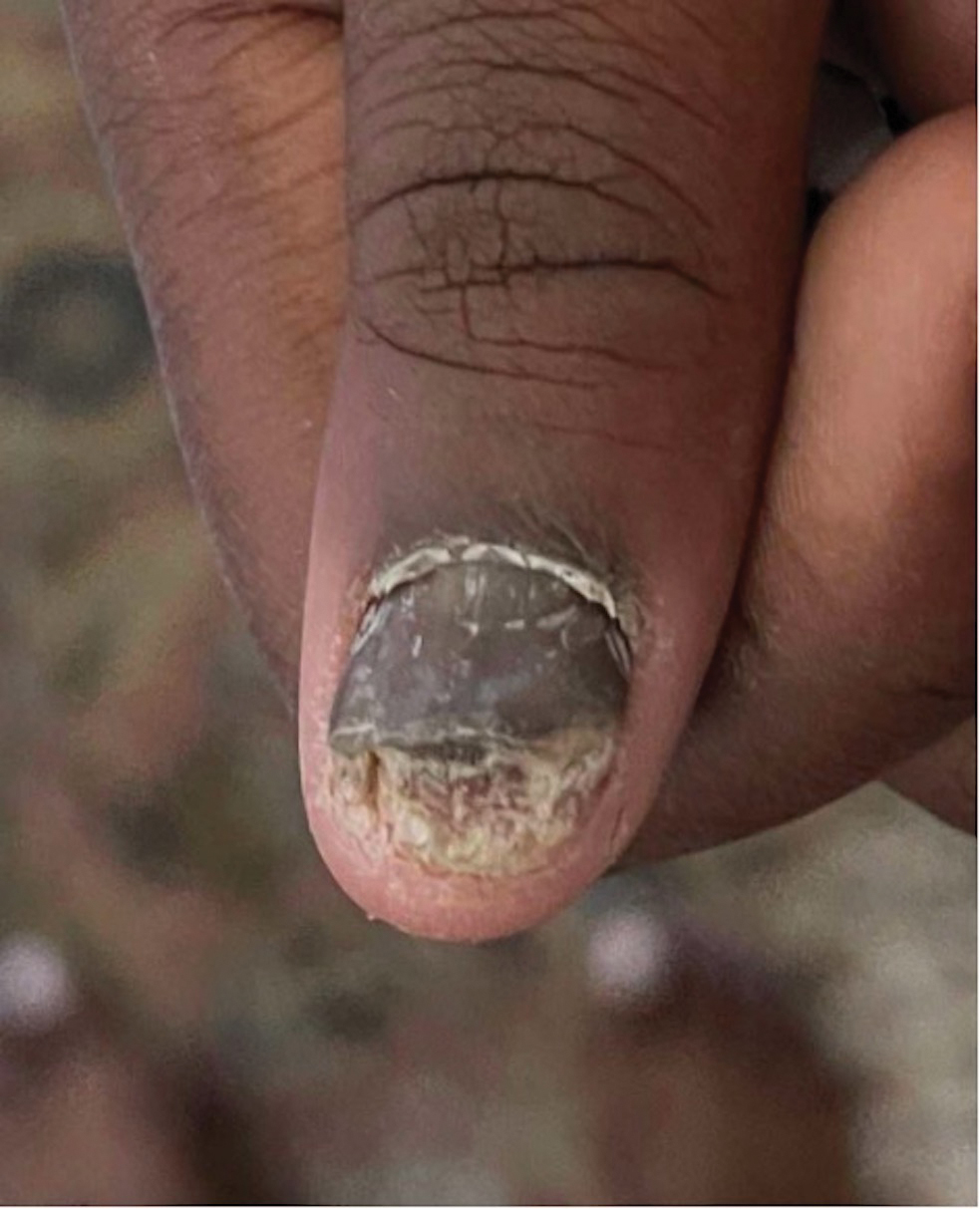
Intralesional steroid injections are sometimes useful in the management of nail matrix psoriasis; however, appropriate patient selection is critical due to the pain associated with the procedure. In a prospective study of 16 children (age range, 9–17 years) with nail psoriasis treated with intralesional triamcinolone (ILTAC) 2.5 to 5 mg/mL every 4 to 8 weeks for a minimum of 3 to 6 months, 9 patients achieved resolution and 6 had improvement of clinical findings.16 Local adverse events were mild, including injection-site pain (66%), subungual hematoma (n=1), Beau lines (n=1), proximal nail fold hypopigmentation (n=2), and proximal nail fold atrophy (n=2). Because the proximal nail fold in children is thinner than in adults, there may be an increased risk for nail fold hypopigmentation and atrophy in children. Therefore, a maximum ILTAC concentration of 2.5 mg/mL with 0.2 mL maximum volume per nail per session is recommended for children younger than 15 years.16
Nail Lichen Planus
Nail lichen planus (NLP) is uncommon in children, with few biopsy-proven cases documented in the literature.17 Common clinical findings are onychorrhexis, nail plate thinning, fissuring, splitting, and atrophy with koilonychia.5 Although pterygium development (irreversible nail matrix scarring) is uncommon in pediatric patients, NLP can be progressive and may cause irreversible destruction of the nail matrix and subsequent nail loss, warranting therapeutic intervention.18
Treatment of NLP may be difficult, as there are no options that work in all patients. Current literature supports the use of systemic corticosteroids or ILTAC for the treatment of NLP; however, recurrence rates can be high. According to an expert consensus paper on NLP treatment, ILTAC may be injected in a concentration of 2.5, 5, or 10 mg/mL according to disease severity.19 In severe or resistant cases, intramuscular (IM) triamcinolone may be considered, especially if more than 3 nails are affected. A dosage of 0.5 to 1 mg/kg/mo for at least 3 to 6 months is recommended for both children and adults, with 1 mg/kg/mo recommended in the active treatment phase (first 2–3 months).19 In a retrospective review of 5 pediatric patients with NLP treated with IM triamcinolone 0.5 mg/kg/mo, 3 patients had resolution and 2 improved with treatment.20 In a prospective study of 10 children with NLP, IM triamcinolone at a dosage of 0.5 to 1 mg/kg every 30 days for 3 to 6 months resulted in resolution of nail findings in 9 patients.17 In a prospective study of 14 pediatric patients with NLP treated with 2.5 to 5 mg/mL of ILTAC, 10 achieved resolution and 3 improved.16
Intralesional triamcinolone injections may be better suited for teenagers compared to younger children who may be more apprehensive of needles. To minimize pain, it is recommended to inject ILTAC slowly at room temperature, with use of “talkesthesia” and vibration devices, 1% lidocaine, or ethyl chloride spray.18
Trachyonychia
Trachyonychia is characterized by the presence of sandpaperlike nails. It manifests with brittle thin nails with longitudinal ridging, onychoschizia, and thickened hyperkeratotic cuticles. Trachyonychia typically involves multiple nails, with a peak age of onset between 3 and 12 years.21,22 There are 2 variants: the opaque type with rough longitudinal ridging, and the shiny variant with opalescent nails and pits that reflect light. The opaque variant is more common and is associated with psoriasis, whereas the shiny variant is less common and is associated with alopecia areata.23 Although most cases are idiopathic, some are associated with psoriasis and alopecia areata, as previously noted, as well as atopic dermatitis (AD) and lichen planus.22,24
Fortunately, trachyonychia does not lead to permanent nail damage or pterygium, making treatment primarily focused on addressing functional and cosmetic concerns.24 Spontaneous resolution occurs in approximately 50% of patients. In a prospective study of 11 patients with idiopathic trachyonychia, there was partial improvement in 5 of 9 patients treated with topical steroids, 1 with only petrolatum, and 1 with vitamin supplements. Complete resolution was reported in 1 patient treated with topical steroids.25 Because trachyonychia often is self-resolving, no treatment is required and a conservative approach is strongly recommended.26 Treatment options include topical corticosteroids, tazarotene, and 5-fluorouracil. Intralesional triamcinolone, systemic cyclosporine, retinoids, systemic corticosteroids, and tofacitinib have been described in case reports, though none of these have been shown to be 100% efficacious.24
Nail Lichen Striatus
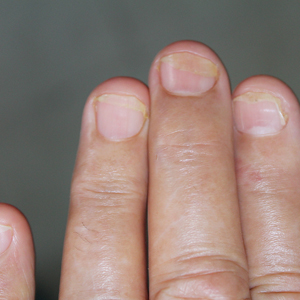
Lichen striatus involving the nail is uncommon and is characterized by onycholysis, longitudinal ridging, splitting, and fraying, as well as what appears to be a subungual tumor. It can encompass the entire nail or may be isolated to a portion of the nail (Figure 2). Usually, a Blaschko-linear array of flesh-colored papules on the more proximal digit directly adjacent to the nail dystrophy will be seen, though nail findings can occur in isolation.27-29 The underlying pathophysiology is not clear; however, one hypothesis is that a triggering event, such as trauma, induces the expression of antigens that elicit a self-limiting immune-mediated response by CD8 T lymphocytes.30
Generally, nail lichen striatus spontaneously resolves in 1 to 2 years without treatment. In a prospective study of 5 patients with nail lichen striatus, the median time to resolution was 22.6 months (range, 10–30 months).31 Topical steroids may be used for pruritus. In one case report, a 3-year-old boy with nail lichen striatus of 4 months’ duration was treated with tacrolimus ointment 0.03% daily for 3 months.28
Nail AD
Nail changes with AD may be more common in adults than children or are underreported. In a study of 777 adults with AD, nail dystrophy was present in 124 patients (16%), whereas in a study of 250 pediatric patients with AD (aged 0-2 years), nail dystrophy was present in only 4 patients.32,33
Periungual inflammation from AD causes the nail changes.34 In a cross-sectional study of 24 pediatric patients with nail dystrophy due to AD, transverse grooves (Beau lines) were present in 25% (6/24), nail pitting in 16.7% (4/24), koilonychia in 16.7% (4/24), trachyonychia in 12.5% (3/24), leukonychia in 12.5% (3/24), brachyonychia in 8.3% (2/24), melanonychia in 8.3% (2/24), onychomadesis in 8.3% (2/24), onychoschizia in 8.3% (2/24), and onycholysis in 8.3% (2/24). There was an association between disease severity and presence of toenail dystrophy (P=.03).35
Topical steroids with or without occlusion can be used to treat nail changes. Although there is limited literature describing the treatment of nail AD in children, a 61-year-old man with nail changes associated with AD achieved resolution with 3 months of treatment with dupilumab.36 Anecdotally, most patients will improve with usual cutaneous AD management.
INFECTIOUS NAIL DISORDERS
Viral Infections
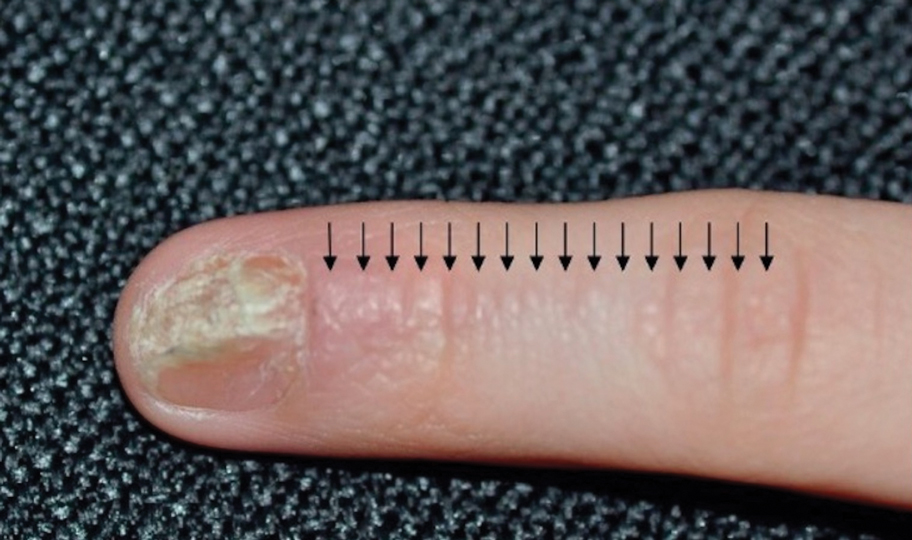
Hand, Foot, and Mouth Disease—Hand, foot, and mouth disease (HFMD) is a common childhood viral infection caused by various enteroviruses, most commonly coxsackievirus A16, with the A6 variant causing more severe disease. Fever and painful vesicles involving the oral mucosa as well as palms and soles give the disease its name. Nail changes are common. In a prospective study involving 130 patients with laboratory-confirmed coxsackievirus CA6 serotype infection, 37% developed onychomadesis vs only 5% of 145 cases with non-CA6 enterovirus infection who developed nail findings. There was an association between CA6 infection and presence of nail changes (P<.001).37
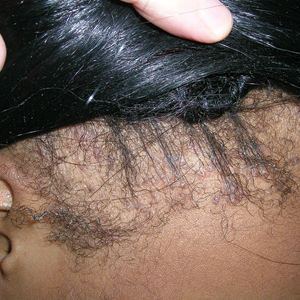
Findings ranging from transverse grooves (Beau lines) to complete nail shedding (onychomadesis)(Figure 3) may be seen.38,39 Nail findings in HFMD are due to transient inhibition of nail growth and present approximately 3 to 6 weeks after infection.40 Onychomadesis is seen in 30% to 68% of patients with HFMD.37,41,42 Nail findings in HFMD spontaneously resolve with nail growth (2–3 mm per month for fingernails and 1 mm per month for toenails) and do not require specific treatment. Although the appearance of nail changes associated with HFMD can be disturbing, dermatologists can reassure children and their parents that the nails will resolve with the next cycle of growth.
Kawasaki Disease—Kawasaki disease (KD) is a vasculitis primarily affecting children and infants. Although the specific pathogen and pathophysiology is not entirely clear, clinical observations have suggested an infectious cause, most likely a virus.43 In Japan, more than 15,000 cases of KD are documented annually, while approximately 4200 cases are seen in the United States.44 In a prospective study from 1984 to 1990, 4 of 26 (15.4%) patients with KD presented with nail manifestations during the late acute phase or early convalescent phase of disease. There were no significant associations between nail dystrophy and severity of KD, such as coronary artery aneurysm.45
Nail changes reported in children with KD include onychomadesis, onycholysis, orange-brown chromonychia, splinter hemorrhages, Beau lines, and pincer nails. In a review of nail changes associated with KD from 1980 to 2021, orange-brown transverse chromonychia, which may evolve into transverse leukonychia, was the most common nail finding reported, occurring in 17 of 31 (54.8%) patients.44 It has been hypothesized that nail changes may result from blood flow disturbance due to the underlying vasculitis.46 Nail changes appear several weeks after the onset of fever and are self-limited. Resolution occurs with nail growth, with no treatment required.
FUNGAL INFECTIONS
Onychomycosis
Onychomycosis is a fungal infection of the nails that occurs in 0.2% to 5.5% of pediatric patients, and its prevalence may be increasing, which may be due to environmental factors or increased rates of diabetes mellitus and obesity in the pediatric population.47 Onychomycosis represents 15.5% of nail dystrophies in pediatric patients.48 Some dermatologists treat presumptive onychomycosis without confirmation; however, we do not recommend that approach. Because the differential is broad and the duration of treatment is long, mycologic examination (potassium hydroxide preparation, fungal culture, polymerase chain reaction, and/or histopathology) should be obtained to confirm onychomycosis prior to initiation of antifungal management. Family members of affected individuals should be evaluated and treated, if indicated, for onychomycosis and tinea pedis, as household transmission is common.
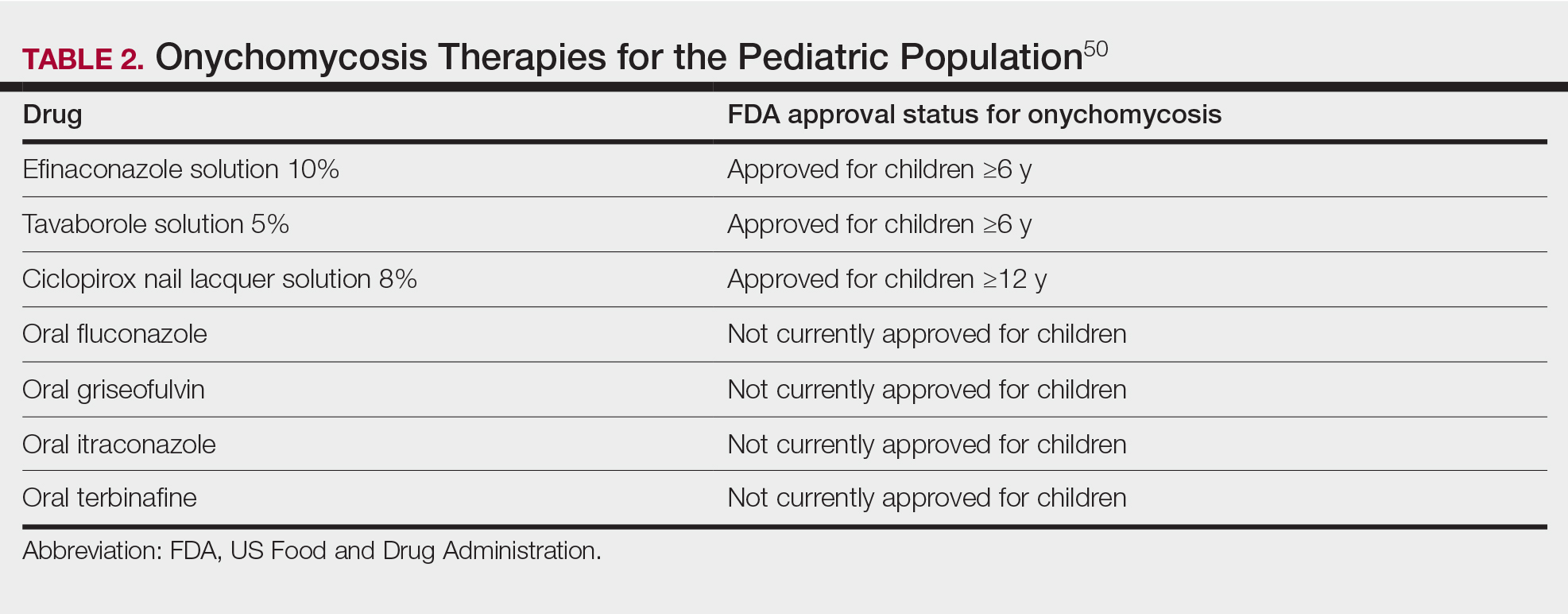
Currently, there are 2 topical FDA-approved treatments for pediatric onychomycosis in children 6 years and older (Table 2).49,50 There is a discussion of the need for confirmatory testing for onychomycosis in children, particularly when systemic treatment is prescribed. In a retrospective review of 269 pediatric patients with onychomycosis prescribed terbinafine, 53.5% (n=144) underwent laboratory monitoring of liver function and complete blood cell counts, and 12.5% had grade 1 laboratory abnormalities either prior to (12/144 [8.3%]) or during (6/144 [4.2%]) therapy.51 Baseline transaminase monitoring is recommended, though subsequent routine laboratory monitoring in healthy children may have limited utility with associated increased costs, incidental findings, and patient discomfort and likely is not needed.51
Pediatric onychomycosis responds better to topical therapy than adult disease, and pediatric patients do not always require systemic treatment.52 Ciclopirox is not FDA approved for the treatment of pediatric onychomycosis, but in a 32-week clinical trial of ciclopirox lacquer 8% use in 40 patients, 77% (27/35) of treated patients achieved mycologic cure. Overall, 71% of treated patients (25/35) vs 22% (2/9) of controls achieved efficacy (defined as investigator global assessment score of 2 or lower).52 In an open-label, single-arm clinical trial assessing tavaborole solution 5% applied once daily for 48 weeks for the treatment of toenail onychomycosis in pediatric patients (aged 6–17 years), 36.2% (20/55) of patients achieved mycologic cure, and 8.5% (5/55) achieved complete cure at week 52 with mild or minimal adverse effects.53 In an open-label, phase 4 study of the safety and efficacy of efinaconazole solution 10% applied once daily for 48 weeks in pediatric patients (aged 6 to 16 years) (n=60), 65% (35/60) achieved mycologic cure, 42% (25/60) achieved clinical cure, and 40% (24/60) achieved complete cure at 52 weeks. The most common adverse effects of efinaconazole were local and included ingrown toenail (1/60), application-site dermatitis (1/60), application-site vesicles (1/60), and application-site pain (1/60).54
In a systematic review of systemic antifungals for onychomycosis in 151 pediatric patients, itraconazole, fluconazole, griseofulvin, and terbinafine resulted in complete cure rates similar to those of the adult population, with excellent safety profiles.55 Depending on the situation, initiation of treatment with topical medications followed by addition of systemic antifungal agents only if needed may be an appropriate course of action.
BACTERIAL INFECTIONS
Acute Paronychia
Acute paronychia is a nail-fold infection that develops after the protective nail barrier has been compromised.56 In children, thumb-sucking, nail-biting, frequent oral manipulation of the digits, and poor skin hygiene are risk factors. Acute paronychia also may develop in association with congenital malalignment of the great toenails.57
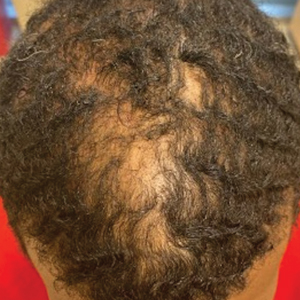
Clinical manifestations include localized pain, erythema, and nail fold edema (Figure 4). Purulent material and abscess formation may ensue. Staphylococcus aureus as well as methicillin-resistant S aureus and Streptococcus pyogenes are classically the most common causes of acute paronychia. Treatment of paronychia is based on severity. In mild cases, warm soaks with topical antibiotics are indicated. Oral antibiotics should be prescribed for more severe presentations. If there is no improvement after 48 hours, surgical drainage is required to facilitate healing.56
FINAL THOUGHTS
Inflammatory and infectious nail disorders in children are relatively common and may impact the physical and emotional well-being of young patients. By understanding the distinctive features of these nail disorders in pediatric patients, dermatologists can provide anticipatory guidance and informed treatment options to children and their parents. Further research is needed to expand our understanding of pediatric nail disorders and create targeted therapeutic interventions, particularly for NLP and psoriasis.
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207. doi:10.1111/pde.13402
- Plachouri KM, Mulita F, Georgiou S. Management of pediatric nail psoriasis. Cutis. 2021;108:292-294. doi:10.12788/cutis.0386
- Smith RJ, Rubin AI. Pediatric nail disorders: a review. Curr Opin Pediatr. 2020;32:506-515. doi:10.1097/mop.0000000000000921
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63. doi:10.1111/pde.13028
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112. doi:10.2165/11537110-000000000-00000
- Lee JYY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Nogueira M, Paller AS, Torres T. Targeted therapy for pediatric psoriasis. Paediatr Drugs. May 2021;23:203-212. doi:10.1007/s40272-021-00443-5
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. Updated August 16, 2023. Accessed July 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179. doi:10.1186/1752-1947-4-179
- Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158:533-541. doi:10.1001/jamadermatol.2022.0655
- Diotallevi F, Simonetti O, Rizzetto G, et al. Biological treatments for pediatric psoriasis: state of the art and future perspectives. Int J Mol Sci. 2022;23:11128. doi:10.3390/ijms231911128
- Nash P, Mease PJ, Kirkham B, et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clin Exp Rheumatol. 2022;40:952-959. doi:10.55563/clinexprheumatol/3nuz51
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385. doi:10.1111/pde.13767
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriaticonycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508. doi:10.1684/ejd.2015.2616
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352. doi:10.1159/000096202
- Iorizzo M, Gioia Di Chiacchio N, Di Chiacchio N, et al. Intralesional steroid injections for inflammatory nail dystrophies in the pediatric population. Pediatr Dermatol. 2023;40:759-761. doi:10.1111/pde.15295
- Tosti A, Piraccini BM, Cambiaghi S, et al. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. 2001;137:1027-1032.
- Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80:e177-e178. doi:10.1016/j.jaad.2018.11.065
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496. doi:10.1684/ejd.2010.0952
- Mahajan R, Kaushik A, De D, et al. Pediatric trachyonychia- a retrospective study of 17 cases. Indian J Dermatol. 2021;66:689-690. doi:10.4103/ijd.ijd_42_21
- Leung AKC, Leong KF, Barankin B. Trachyonychia. J Pediatr. 2020;216:239-239.e1. doi:10.1016/j.jpeds.2019.08.034
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Jacobsen AA, Tosti A. Trachyonychia and twenty-nail dystrophy: a comprehensive review and discussion of diagnostic accuracy. Skin Appendage Disord. 2016;2:7-13. doi:10.1159/000445544
- Kumar MG, Ciliberto H, Bayliss SJ. Long-term follow-up of pediatric trachyonychia. Pediatr Dermatol. 2015;32:198-200. doi:10.1111/pde.12427
- Tosti A, Piraccini BM, Iorizzo M. Trachyonychia and related disorders: evaluation and treatment plans. Dermatolog Ther. 2002;15:121-125. doi:10.1046/j.1529-8019.2002.01511.x
- Leung AKC, Leong KF, Barankin B. Lichen striatus with nail involvement in a 6-year-old boy. Case Rep Pediatr. 2020;2020:1494760. doi:10.1155/2020/1494760
- Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617. doi:10.1111/j.1346-8138.2009.00720.x
- Iorizzo M, Rubin AI, Starace M. Nail lichen striatus: is dermoscopy useful for the diagnosis? Pediatr Dermatol. 2019;36:859-863. doi:10.1111/pde.13916
- Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361. doi:10.1111/j.1525-1470.1993.tb00399.x
- Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36(6, pt 1):908-913. doi:10.1016/s0190-9622(97)80270-8
- Simpson EL, Thompson MM, Hanifin JM. Prevalence and morphology of hand eczema in patients with atopic dermatitis. Dermatitis. 2006;17:123-127. doi:10.2310/6620.2006.06005
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C. Nail alterations in 250 infant patients: a clinical study. J Eur Acad Dermatol Venereol. 2008;22:741-744. doi:10.1111/j.1468-3083.2008.02592.x
- Milanesi N, D’Erme AM, Gola M. Nail improvement during alitretinoin treatment: three case reports and review of the literature. Clin Exp Dermatol. 2015;40:533-536. doi:10.1111/ced.12584
- Chung BY, Choi YW, Kim HO, et al. Nail dystrophy in patients with atopic dermatitis and its association with disease severity. Ann Dermatol. 2019;31:121-126. doi:10.5021/ad.2019.31.2.121
- Navarro-Triviño FJ, Vega-Castillo JJ, Ruiz-Villaverde R. Nail changes successfully treated with dupilumab in a patient with severe atopic dermatitis. Australas J Dermatol. 2021;62:e468-e469. doi:10.1111/ajd.13633
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. doi:10.1186/1471-2334-11-346
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283. doi:10.5021/ad.2014.26.2.280
- Verma S, Singal A. Nail changes in hand-foot-and-mouth disease (HFMD). Indian Dermatol Online J. 2021;12:656-657. doi:10.4103 /idoj.IDOJ_271_20
- Giordano LMC, de la Fuente LA, Lorca JMB, et al. Onychomadesis secondary to hand-foot-mouth disease: a frequent manifestation and cause of concern for parents. Article in Spanish. Rev Chil Pediatr. 2018;89:380-383. doi:10.4067/s0370-41062018005000203
- Justino MCA, da SMD, Souza MF, et al. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol. 2020;126:104307. doi:10.1016/j.jcv.2020.104307
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr. 2022;11:1502-1509. doi:10.21037/tp-22-352
- Nagata S. Causes of Kawasaki disease-from past to present. Front Pediatr. 2019;7:18. doi:10.3389/fped.2019.00018
- Mitsuishi T, Miyata K, Ando A, et al. Characteristic nail lesions in Kawasaki disease: case series and literature review. J Dermatol. 2022;49:232-238. doi:10.1111/1346-8138.16276
- Lindsley CB. Nail-bed lines in Kawasaki disease. Am J Dis Child. 1992;146:659-660. doi:10.1001/archpedi.1992.02160180017005
- Matsumura O, Nakagishi Y. Pincer nails upon convalescence from Kawasaki disease. J Pediatr. 2022;246:279. doi:10.1016/j.jpeds.2022.03.002
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Gupta AK, Mays RR, Versteeg SG, et al. Onychomycosis in children: safety and efficacy of antifungal agents. Pediatr Dermatol. 2018;35:552-559. doi:10.1111/pde.13561
- 49. Gupta AK, Venkataraman M, Shear NH, et al. Labeled use of efinaconazole topical solution 10% in treating onychomycosis in children and a review of the management of pediatric onychomycosis. Dermatol Ther. 2020;33:e13613. doi:10.1111/dth.13613
- Falotico JM, Lipner SR. Updated perspectives on the diagnosis and management of onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933-1957. doi:10.2147/ccid.S362635
- Patel D, Castelo-Soccio LA, Rubin AI, et al. Laboratory monitoring during systemic terbinafine therapy for pediatric onychomycosis. JAMA Dermatol. 2017;153:1326-1327. doi:10.1001/jamadermatol.2017.4483
- Friedlander SF, Chan YC, Chan YH, et al. Onychomycosis does not always require systemic treatment for cure: a trial using topical therapy. Pediatr Dermatol. 2013;30:316-322. doi:10.1111/pde.12064
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Gupta AK, Venkataraman M, Abramovits W, et al. JUBLIA (efinaconazole 10% solution) in the treatment of pediatric onychomycosis. Skinmed. 2021;19:206-210.
- Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013;30:294-302. doi:10.1111/pde.12048
- Leggit JC. Acute and chronic paronychia. Am Fam Physician. 2017;96:44-51.
- Lipner SR, Scher RK. Congenital malalignment of the great toenails with acute paronychia. Pediatr Dermatol. 2016;33:e288-e289.doi:10.1111/pde.12924
Nail disorders are common among pediatric patients but often are underdiagnosed or misdiagnosed because of their unique disease manifestations. These conditions may severely impact quality of life. There are few nail disease clinical trials that include children. Consequently, most treatment recommendations are based on case series and expert consensus recommendations. We review inflammatory and infectious nail disorders in pediatric patients. By describing characteristics, clinical manifestations, and management approaches for these conditions, we aim to provide guidance to dermatologists in their diagnosis and treatment.
INFLAMMATORY NAIL DISORDERS
Nail Psoriasis
Nail involvement in children with psoriasis is common, with prevalence estimates ranging from 17% to 39.2%.1 Nail matrix psoriasis may manifest with pitting (large irregular pits) and leukonychia as well as chromonychia and nail plate crumbling. Onycholysis, oil drop spots (salmon patches), and subungual hyperkeratosis can be seen in nail bed psoriasis. Nail pitting is the most frequently observed clinical finding (Figure 1).2,3 In a cross-sectional multicenter study of 313 children with cutaneous psoriasis in France, nail findings were present in 101 patients (32.3%). There were associations between nail findings and presence of psoriatic arthritis (P=.03), palmoplantar psoriasis (P<.001), and severity of psoriatic disease, defined as use of systemic treatment with phototherapy (psoralen plus UVA, UVB), traditional systemic treatment (acitretin, methotrexate, cyclosporine), or a biologic (P=.003).4
Topical steroids and vitamin D analogues may be used with or without occlusion and may be efficacious.5 Several case reports describe systemic treatments for psoriasis in children, including methotrexate, acitretin, and apremilast (approved for children 6 years and older for plaque psoriasis by the US Food and Drug Administration [FDA]).2 There are 5 biologic drugs currently approved for the treatment of pediatric psoriasis—adalimumab, etanercept, ustekinumab, secukinumab, ixekizumab—and 6 drugs currently undergoing phase 3 studies—brodalumab, guselkumab, risankizumab, tildrakizumab, certolizumab pegol, and deucravacitinib (Table 1).6-15 Adalimumab is specifically approved for moderate to severe nail psoriasis in adults 18 years and older.


Intralesional steroid injections are sometimes useful in the management of nail matrix psoriasis; however, appropriate patient selection is critical due to the pain associated with the procedure. In a prospective study of 16 children (age range, 9–17 years) with nail psoriasis treated with intralesional triamcinolone (ILTAC) 2.5 to 5 mg/mL every 4 to 8 weeks for a minimum of 3 to 6 months, 9 patients achieved resolution and 6 had improvement of clinical findings.16 Local adverse events were mild, including injection-site pain (66%), subungual hematoma (n=1), Beau lines (n=1), proximal nail fold hypopigmentation (n=2), and proximal nail fold atrophy (n=2). Because the proximal nail fold in children is thinner than in adults, there may be an increased risk for nail fold hypopigmentation and atrophy in children. Therefore, a maximum ILTAC concentration of 2.5 mg/mL with 0.2 mL maximum volume per nail per session is recommended for children younger than 15 years.16
Nail Lichen Planus
Nail lichen planus (NLP) is uncommon in children, with few biopsy-proven cases documented in the literature.17 Common clinical findings are onychorrhexis, nail plate thinning, fissuring, splitting, and atrophy with koilonychia.5 Although pterygium development (irreversible nail matrix scarring) is uncommon in pediatric patients, NLP can be progressive and may cause irreversible destruction of the nail matrix and subsequent nail loss, warranting therapeutic intervention.18
Treatment of NLP may be difficult, as there are no options that work in all patients. Current literature supports the use of systemic corticosteroids or ILTAC for the treatment of NLP; however, recurrence rates can be high. According to an expert consensus paper on NLP treatment, ILTAC may be injected in a concentration of 2.5, 5, or 10 mg/mL according to disease severity.19 In severe or resistant cases, intramuscular (IM) triamcinolone may be considered, especially if more than 3 nails are affected. A dosage of 0.5 to 1 mg/kg/mo for at least 3 to 6 months is recommended for both children and adults, with 1 mg/kg/mo recommended in the active treatment phase (first 2–3 months).19 In a retrospective review of 5 pediatric patients with NLP treated with IM triamcinolone 0.5 mg/kg/mo, 3 patients had resolution and 2 improved with treatment.20 In a prospective study of 10 children with NLP, IM triamcinolone at a dosage of 0.5 to 1 mg/kg every 30 days for 3 to 6 months resulted in resolution of nail findings in 9 patients.17 In a prospective study of 14 pediatric patients with NLP treated with 2.5 to 5 mg/mL of ILTAC, 10 achieved resolution and 3 improved.16
Intralesional triamcinolone injections may be better suited for teenagers compared to younger children who may be more apprehensive of needles. To minimize pain, it is recommended to inject ILTAC slowly at room temperature, with use of “talkesthesia” and vibration devices, 1% lidocaine, or ethyl chloride spray.18
Trachyonychia
Trachyonychia is characterized by the presence of sandpaperlike nails. It manifests with brittle thin nails with longitudinal ridging, onychoschizia, and thickened hyperkeratotic cuticles. Trachyonychia typically involves multiple nails, with a peak age of onset between 3 and 12 years.21,22 There are 2 variants: the opaque type with rough longitudinal ridging, and the shiny variant with opalescent nails and pits that reflect light. The opaque variant is more common and is associated with psoriasis, whereas the shiny variant is less common and is associated with alopecia areata.23 Although most cases are idiopathic, some are associated with psoriasis and alopecia areata, as previously noted, as well as atopic dermatitis (AD) and lichen planus.22,24
Fortunately, trachyonychia does not lead to permanent nail damage or pterygium, making treatment primarily focused on addressing functional and cosmetic concerns.24 Spontaneous resolution occurs in approximately 50% of patients. In a prospective study of 11 patients with idiopathic trachyonychia, there was partial improvement in 5 of 9 patients treated with topical steroids, 1 with only petrolatum, and 1 with vitamin supplements. Complete resolution was reported in 1 patient treated with topical steroids.25 Because trachyonychia often is self-resolving, no treatment is required and a conservative approach is strongly recommended.26 Treatment options include topical corticosteroids, tazarotene, and 5-fluorouracil. Intralesional triamcinolone, systemic cyclosporine, retinoids, systemic corticosteroids, and tofacitinib have been described in case reports, though none of these have been shown to be 100% efficacious.24
Nail Lichen Striatus
Lichen striatus involving the nail is uncommon and is characterized by onycholysis, longitudinal ridging, splitting, and fraying, as well as what appears to be a subungual tumor. It can encompass the entire nail or may be isolated to a portion of the nail (Figure 2). Usually, a Blaschko-linear array of flesh-colored papules on the more proximal digit directly adjacent to the nail dystrophy will be seen, though nail findings can occur in isolation.27-29 The underlying pathophysiology is not clear; however, one hypothesis is that a triggering event, such as trauma, induces the expression of antigens that elicit a self-limiting immune-mediated response by CD8 T lymphocytes.30

Generally, nail lichen striatus spontaneously resolves in 1 to 2 years without treatment. In a prospective study of 5 patients with nail lichen striatus, the median time to resolution was 22.6 months (range, 10–30 months).31 Topical steroids may be used for pruritus. In one case report, a 3-year-old boy with nail lichen striatus of 4 months’ duration was treated with tacrolimus ointment 0.03% daily for 3 months.28
Nail AD
Nail changes with AD may be more common in adults than children or are underreported. In a study of 777 adults with AD, nail dystrophy was present in 124 patients (16%), whereas in a study of 250 pediatric patients with AD (aged 0-2 years), nail dystrophy was present in only 4 patients.32,33
Periungual inflammation from AD causes the nail changes.34 In a cross-sectional study of 24 pediatric patients with nail dystrophy due to AD, transverse grooves (Beau lines) were present in 25% (6/24), nail pitting in 16.7% (4/24), koilonychia in 16.7% (4/24), trachyonychia in 12.5% (3/24), leukonychia in 12.5% (3/24), brachyonychia in 8.3% (2/24), melanonychia in 8.3% (2/24), onychomadesis in 8.3% (2/24), onychoschizia in 8.3% (2/24), and onycholysis in 8.3% (2/24). There was an association between disease severity and presence of toenail dystrophy (P=.03).35
Topical steroids with or without occlusion can be used to treat nail changes. Although there is limited literature describing the treatment of nail AD in children, a 61-year-old man with nail changes associated with AD achieved resolution with 3 months of treatment with dupilumab.36 Anecdotally, most patients will improve with usual cutaneous AD management.
INFECTIOUS NAIL DISORDERS
Viral Infections
Hand, Foot, and Mouth Disease—Hand, foot, and mouth disease (HFMD) is a common childhood viral infection caused by various enteroviruses, most commonly coxsackievirus A16, with the A6 variant causing more severe disease. Fever and painful vesicles involving the oral mucosa as well as palms and soles give the disease its name. Nail changes are common. In a prospective study involving 130 patients with laboratory-confirmed coxsackievirus CA6 serotype infection, 37% developed onychomadesis vs only 5% of 145 cases with non-CA6 enterovirus infection who developed nail findings. There was an association between CA6 infection and presence of nail changes (P<.001).37
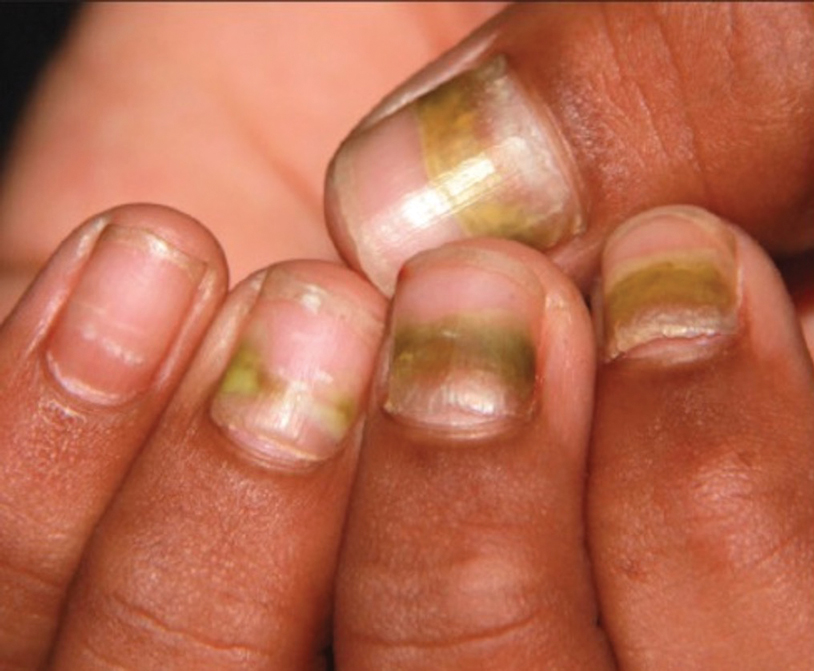
Findings ranging from transverse grooves (Beau lines) to complete nail shedding (onychomadesis)(Figure 3) may be seen.38,39 Nail findings in HFMD are due to transient inhibition of nail growth and present approximately 3 to 6 weeks after infection.40 Onychomadesis is seen in 30% to 68% of patients with HFMD.37,41,42 Nail findings in HFMD spontaneously resolve with nail growth (2–3 mm per month for fingernails and 1 mm per month for toenails) and do not require specific treatment. Although the appearance of nail changes associated with HFMD can be disturbing, dermatologists can reassure children and their parents that the nails will resolve with the next cycle of growth.
Kawasaki Disease—Kawasaki disease (KD) is a vasculitis primarily affecting children and infants. Although the specific pathogen and pathophysiology is not entirely clear, clinical observations have suggested an infectious cause, most likely a virus.43 In Japan, more than 15,000 cases of KD are documented annually, while approximately 4200 cases are seen in the United States.44 In a prospective study from 1984 to 1990, 4 of 26 (15.4%) patients with KD presented with nail manifestations during the late acute phase or early convalescent phase of disease. There were no significant associations between nail dystrophy and severity of KD, such as coronary artery aneurysm.45
Nail changes reported in children with KD include onychomadesis, onycholysis, orange-brown chromonychia, splinter hemorrhages, Beau lines, and pincer nails. In a review of nail changes associated with KD from 1980 to 2021, orange-brown transverse chromonychia, which may evolve into transverse leukonychia, was the most common nail finding reported, occurring in 17 of 31 (54.8%) patients.44 It has been hypothesized that nail changes may result from blood flow disturbance due to the underlying vasculitis.46 Nail changes appear several weeks after the onset of fever and are self-limited. Resolution occurs with nail growth, with no treatment required.

FUNGAL INFECTIONS
Onychomycosis
Onychomycosis is a fungal infection of the nails that occurs in 0.2% to 5.5% of pediatric patients, and its prevalence may be increasing, which may be due to environmental factors or increased rates of diabetes mellitus and obesity in the pediatric population.47 Onychomycosis represents 15.5% of nail dystrophies in pediatric patients.48 Some dermatologists treat presumptive onychomycosis without confirmation; however, we do not recommend that approach. Because the differential is broad and the duration of treatment is long, mycologic examination (potassium hydroxide preparation, fungal culture, polymerase chain reaction, and/or histopathology) should be obtained to confirm onychomycosis prior to initiation of antifungal management. Family members of affected individuals should be evaluated and treated, if indicated, for onychomycosis and tinea pedis, as household transmission is common.
Currently, there are 2 topical FDA-approved treatments for pediatric onychomycosis in children 6 years and older (Table 2).49,50 There is a discussion of the need for confirmatory testing for onychomycosis in children, particularly when systemic treatment is prescribed. In a retrospective review of 269 pediatric patients with onychomycosis prescribed terbinafine, 53.5% (n=144) underwent laboratory monitoring of liver function and complete blood cell counts, and 12.5% had grade 1 laboratory abnormalities either prior to (12/144 [8.3%]) or during (6/144 [4.2%]) therapy.51 Baseline transaminase monitoring is recommended, though subsequent routine laboratory monitoring in healthy children may have limited utility with associated increased costs, incidental findings, and patient discomfort and likely is not needed.51
Pediatric onychomycosis responds better to topical therapy than adult disease, and pediatric patients do not always require systemic treatment.52 Ciclopirox is not FDA approved for the treatment of pediatric onychomycosis, but in a 32-week clinical trial of ciclopirox lacquer 8% use in 40 patients, 77% (27/35) of treated patients achieved mycologic cure. Overall, 71% of treated patients (25/35) vs 22% (2/9) of controls achieved efficacy (defined as investigator global assessment score of 2 or lower).52 In an open-label, single-arm clinical trial assessing tavaborole solution 5% applied once daily for 48 weeks for the treatment of toenail onychomycosis in pediatric patients (aged 6–17 years), 36.2% (20/55) of patients achieved mycologic cure, and 8.5% (5/55) achieved complete cure at week 52 with mild or minimal adverse effects.53 In an open-label, phase 4 study of the safety and efficacy of efinaconazole solution 10% applied once daily for 48 weeks in pediatric patients (aged 6 to 16 years) (n=60), 65% (35/60) achieved mycologic cure, 42% (25/60) achieved clinical cure, and 40% (24/60) achieved complete cure at 52 weeks. The most common adverse effects of efinaconazole were local and included ingrown toenail (1/60), application-site dermatitis (1/60), application-site vesicles (1/60), and application-site pain (1/60).54
In a systematic review of systemic antifungals for onychomycosis in 151 pediatric patients, itraconazole, fluconazole, griseofulvin, and terbinafine resulted in complete cure rates similar to those of the adult population, with excellent safety profiles.55 Depending on the situation, initiation of treatment with topical medications followed by addition of systemic antifungal agents only if needed may be an appropriate course of action.
BACTERIAL INFECTIONS
Acute Paronychia
Acute paronychia is a nail-fold infection that develops after the protective nail barrier has been compromised.56 In children, thumb-sucking, nail-biting, frequent oral manipulation of the digits, and poor skin hygiene are risk factors. Acute paronychia also may develop in association with congenital malalignment of the great toenails.57
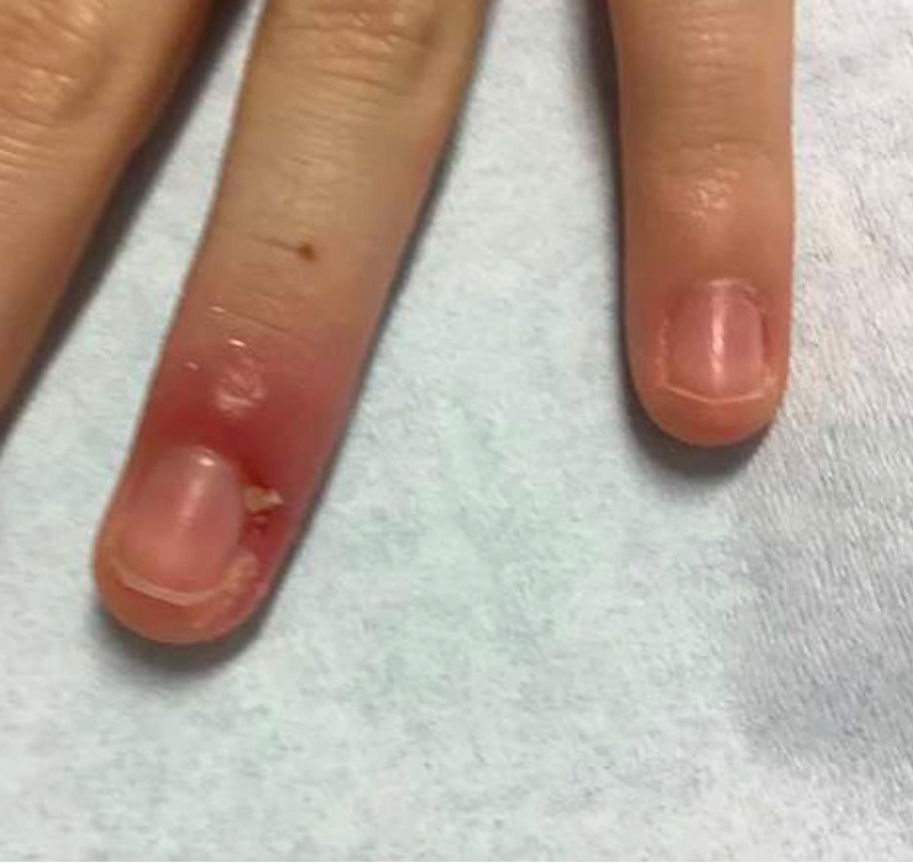
Clinical manifestations include localized pain, erythema, and nail fold edema (Figure 4). Purulent material and abscess formation may ensue. Staphylococcus aureus as well as methicillin-resistant S aureus and Streptococcus pyogenes are classically the most common causes of acute paronychia. Treatment of paronychia is based on severity. In mild cases, warm soaks with topical antibiotics are indicated. Oral antibiotics should be prescribed for more severe presentations. If there is no improvement after 48 hours, surgical drainage is required to facilitate healing.56
FINAL THOUGHTS
Inflammatory and infectious nail disorders in children are relatively common and may impact the physical and emotional well-being of young patients. By understanding the distinctive features of these nail disorders in pediatric patients, dermatologists can provide anticipatory guidance and informed treatment options to children and their parents. Further research is needed to expand our understanding of pediatric nail disorders and create targeted therapeutic interventions, particularly for NLP and psoriasis.


Nail disorders are common among pediatric patients but often are underdiagnosed or misdiagnosed because of their unique disease manifestations. These conditions may severely impact quality of life. There are few nail disease clinical trials that include children. Consequently, most treatment recommendations are based on case series and expert consensus recommendations. We review inflammatory and infectious nail disorders in pediatric patients. By describing characteristics, clinical manifestations, and management approaches for these conditions, we aim to provide guidance to dermatologists in their diagnosis and treatment.
INFLAMMATORY NAIL DISORDERS
Nail Psoriasis
Nail involvement in children with psoriasis is common, with prevalence estimates ranging from 17% to 39.2%.1 Nail matrix psoriasis may manifest with pitting (large irregular pits) and leukonychia as well as chromonychia and nail plate crumbling. Onycholysis, oil drop spots (salmon patches), and subungual hyperkeratosis can be seen in nail bed psoriasis. Nail pitting is the most frequently observed clinical finding (Figure 1).2,3 In a cross-sectional multicenter study of 313 children with cutaneous psoriasis in France, nail findings were present in 101 patients (32.3%). There were associations between nail findings and presence of psoriatic arthritis (P=.03), palmoplantar psoriasis (P<.001), and severity of psoriatic disease, defined as use of systemic treatment with phototherapy (psoralen plus UVA, UVB), traditional systemic treatment (acitretin, methotrexate, cyclosporine), or a biologic (P=.003).4
Topical steroids and vitamin D analogues may be used with or without occlusion and may be efficacious.5 Several case reports describe systemic treatments for psoriasis in children, including methotrexate, acitretin, and apremilast (approved for children 6 years and older for plaque psoriasis by the US Food and Drug Administration [FDA]).2 There are 5 biologic drugs currently approved for the treatment of pediatric psoriasis—adalimumab, etanercept, ustekinumab, secukinumab, ixekizumab—and 6 drugs currently undergoing phase 3 studies—brodalumab, guselkumab, risankizumab, tildrakizumab, certolizumab pegol, and deucravacitinib (Table 1).6-15 Adalimumab is specifically approved for moderate to severe nail psoriasis in adults 18 years and older.


Intralesional steroid injections are sometimes useful in the management of nail matrix psoriasis; however, appropriate patient selection is critical due to the pain associated with the procedure. In a prospective study of 16 children (age range, 9–17 years) with nail psoriasis treated with intralesional triamcinolone (ILTAC) 2.5 to 5 mg/mL every 4 to 8 weeks for a minimum of 3 to 6 months, 9 patients achieved resolution and 6 had improvement of clinical findings.16 Local adverse events were mild, including injection-site pain (66%), subungual hematoma (n=1), Beau lines (n=1), proximal nail fold hypopigmentation (n=2), and proximal nail fold atrophy (n=2). Because the proximal nail fold in children is thinner than in adults, there may be an increased risk for nail fold hypopigmentation and atrophy in children. Therefore, a maximum ILTAC concentration of 2.5 mg/mL with 0.2 mL maximum volume per nail per session is recommended for children younger than 15 years.16
Nail Lichen Planus
Nail lichen planus (NLP) is uncommon in children, with few biopsy-proven cases documented in the literature.17 Common clinical findings are onychorrhexis, nail plate thinning, fissuring, splitting, and atrophy with koilonychia.5 Although pterygium development (irreversible nail matrix scarring) is uncommon in pediatric patients, NLP can be progressive and may cause irreversible destruction of the nail matrix and subsequent nail loss, warranting therapeutic intervention.18
Treatment of NLP may be difficult, as there are no options that work in all patients. Current literature supports the use of systemic corticosteroids or ILTAC for the treatment of NLP; however, recurrence rates can be high. According to an expert consensus paper on NLP treatment, ILTAC may be injected in a concentration of 2.5, 5, or 10 mg/mL according to disease severity.19 In severe or resistant cases, intramuscular (IM) triamcinolone may be considered, especially if more than 3 nails are affected. A dosage of 0.5 to 1 mg/kg/mo for at least 3 to 6 months is recommended for both children and adults, with 1 mg/kg/mo recommended in the active treatment phase (first 2–3 months).19 In a retrospective review of 5 pediatric patients with NLP treated with IM triamcinolone 0.5 mg/kg/mo, 3 patients had resolution and 2 improved with treatment.20 In a prospective study of 10 children with NLP, IM triamcinolone at a dosage of 0.5 to 1 mg/kg every 30 days for 3 to 6 months resulted in resolution of nail findings in 9 patients.17 In a prospective study of 14 pediatric patients with NLP treated with 2.5 to 5 mg/mL of ILTAC, 10 achieved resolution and 3 improved.16
Intralesional triamcinolone injections may be better suited for teenagers compared to younger children who may be more apprehensive of needles. To minimize pain, it is recommended to inject ILTAC slowly at room temperature, with use of “talkesthesia” and vibration devices, 1% lidocaine, or ethyl chloride spray.18
Trachyonychia
Trachyonychia is characterized by the presence of sandpaperlike nails. It manifests with brittle thin nails with longitudinal ridging, onychoschizia, and thickened hyperkeratotic cuticles. Trachyonychia typically involves multiple nails, with a peak age of onset between 3 and 12 years.21,22 There are 2 variants: the opaque type with rough longitudinal ridging, and the shiny variant with opalescent nails and pits that reflect light. The opaque variant is more common and is associated with psoriasis, whereas the shiny variant is less common and is associated with alopecia areata.23 Although most cases are idiopathic, some are associated with psoriasis and alopecia areata, as previously noted, as well as atopic dermatitis (AD) and lichen planus.22,24
Fortunately, trachyonychia does not lead to permanent nail damage or pterygium, making treatment primarily focused on addressing functional and cosmetic concerns.24 Spontaneous resolution occurs in approximately 50% of patients. In a prospective study of 11 patients with idiopathic trachyonychia, there was partial improvement in 5 of 9 patients treated with topical steroids, 1 with only petrolatum, and 1 with vitamin supplements. Complete resolution was reported in 1 patient treated with topical steroids.25 Because trachyonychia often is self-resolving, no treatment is required and a conservative approach is strongly recommended.26 Treatment options include topical corticosteroids, tazarotene, and 5-fluorouracil. Intralesional triamcinolone, systemic cyclosporine, retinoids, systemic corticosteroids, and tofacitinib have been described in case reports, though none of these have been shown to be 100% efficacious.24
Nail Lichen Striatus
Lichen striatus involving the nail is uncommon and is characterized by onycholysis, longitudinal ridging, splitting, and fraying, as well as what appears to be a subungual tumor. It can encompass the entire nail or may be isolated to a portion of the nail (Figure 2). Usually, a Blaschko-linear array of flesh-colored papules on the more proximal digit directly adjacent to the nail dystrophy will be seen, though nail findings can occur in isolation.27-29 The underlying pathophysiology is not clear; however, one hypothesis is that a triggering event, such as trauma, induces the expression of antigens that elicit a self-limiting immune-mediated response by CD8 T lymphocytes.30

Generally, nail lichen striatus spontaneously resolves in 1 to 2 years without treatment. In a prospective study of 5 patients with nail lichen striatus, the median time to resolution was 22.6 months (range, 10–30 months).31 Topical steroids may be used for pruritus. In one case report, a 3-year-old boy with nail lichen striatus of 4 months’ duration was treated with tacrolimus ointment 0.03% daily for 3 months.28
Nail AD
Nail changes with AD may be more common in adults than children or are underreported. In a study of 777 adults with AD, nail dystrophy was present in 124 patients (16%), whereas in a study of 250 pediatric patients with AD (aged 0-2 years), nail dystrophy was present in only 4 patients.32,33
Periungual inflammation from AD causes the nail changes.34 In a cross-sectional study of 24 pediatric patients with nail dystrophy due to AD, transverse grooves (Beau lines) were present in 25% (6/24), nail pitting in 16.7% (4/24), koilonychia in 16.7% (4/24), trachyonychia in 12.5% (3/24), leukonychia in 12.5% (3/24), brachyonychia in 8.3% (2/24), melanonychia in 8.3% (2/24), onychomadesis in 8.3% (2/24), onychoschizia in 8.3% (2/24), and onycholysis in 8.3% (2/24). There was an association between disease severity and presence of toenail dystrophy (P=.03).35
Topical steroids with or without occlusion can be used to treat nail changes. Although there is limited literature describing the treatment of nail AD in children, a 61-year-old man with nail changes associated with AD achieved resolution with 3 months of treatment with dupilumab.36 Anecdotally, most patients will improve with usual cutaneous AD management.
INFECTIOUS NAIL DISORDERS
Viral Infections
Hand, Foot, and Mouth Disease—Hand, foot, and mouth disease (HFMD) is a common childhood viral infection caused by various enteroviruses, most commonly coxsackievirus A16, with the A6 variant causing more severe disease. Fever and painful vesicles involving the oral mucosa as well as palms and soles give the disease its name. Nail changes are common. In a prospective study involving 130 patients with laboratory-confirmed coxsackievirus CA6 serotype infection, 37% developed onychomadesis vs only 5% of 145 cases with non-CA6 enterovirus infection who developed nail findings. There was an association between CA6 infection and presence of nail changes (P<.001).37
Findings ranging from transverse grooves (Beau lines) to complete nail shedding (onychomadesis)(Figure 3) may be seen.38,39 Nail findings in HFMD are due to transient inhibition of nail growth and present approximately 3 to 6 weeks after infection.40 Onychomadesis is seen in 30% to 68% of patients with HFMD.37,41,42 Nail findings in HFMD spontaneously resolve with nail growth (2–3 mm per month for fingernails and 1 mm per month for toenails) and do not require specific treatment. Although the appearance of nail changes associated with HFMD can be disturbing, dermatologists can reassure children and their parents that the nails will resolve with the next cycle of growth.
Kawasaki Disease—Kawasaki disease (KD) is a vasculitis primarily affecting children and infants. Although the specific pathogen and pathophysiology is not entirely clear, clinical observations have suggested an infectious cause, most likely a virus.43 In Japan, more than 15,000 cases of KD are documented annually, while approximately 4200 cases are seen in the United States.44 In a prospective study from 1984 to 1990, 4 of 26 (15.4%) patients with KD presented with nail manifestations during the late acute phase or early convalescent phase of disease. There were no significant associations between nail dystrophy and severity of KD, such as coronary artery aneurysm.45
Nail changes reported in children with KD include onychomadesis, onycholysis, orange-brown chromonychia, splinter hemorrhages, Beau lines, and pincer nails. In a review of nail changes associated with KD from 1980 to 2021, orange-brown transverse chromonychia, which may evolve into transverse leukonychia, was the most common nail finding reported, occurring in 17 of 31 (54.8%) patients.44 It has been hypothesized that nail changes may result from blood flow disturbance due to the underlying vasculitis.46 Nail changes appear several weeks after the onset of fever and are self-limited. Resolution occurs with nail growth, with no treatment required.

FUNGAL INFECTIONS
Onychomycosis
Onychomycosis is a fungal infection of the nails that occurs in 0.2% to 5.5% of pediatric patients, and its prevalence may be increasing, which may be due to environmental factors or increased rates of diabetes mellitus and obesity in the pediatric population.47 Onychomycosis represents 15.5% of nail dystrophies in pediatric patients.48 Some dermatologists treat presumptive onychomycosis without confirmation; however, we do not recommend that approach. Because the differential is broad and the duration of treatment is long, mycologic examination (potassium hydroxide preparation, fungal culture, polymerase chain reaction, and/or histopathology) should be obtained to confirm onychomycosis prior to initiation of antifungal management. Family members of affected individuals should be evaluated and treated, if indicated, for onychomycosis and tinea pedis, as household transmission is common.
Currently, there are 2 topical FDA-approved treatments for pediatric onychomycosis in children 6 years and older (Table 2).49,50 There is a discussion of the need for confirmatory testing for onychomycosis in children, particularly when systemic treatment is prescribed. In a retrospective review of 269 pediatric patients with onychomycosis prescribed terbinafine, 53.5% (n=144) underwent laboratory monitoring of liver function and complete blood cell counts, and 12.5% had grade 1 laboratory abnormalities either prior to (12/144 [8.3%]) or during (6/144 [4.2%]) therapy.51 Baseline transaminase monitoring is recommended, though subsequent routine laboratory monitoring in healthy children may have limited utility with associated increased costs, incidental findings, and patient discomfort and likely is not needed.51
Pediatric onychomycosis responds better to topical therapy than adult disease, and pediatric patients do not always require systemic treatment.52 Ciclopirox is not FDA approved for the treatment of pediatric onychomycosis, but in a 32-week clinical trial of ciclopirox lacquer 8% use in 40 patients, 77% (27/35) of treated patients achieved mycologic cure. Overall, 71% of treated patients (25/35) vs 22% (2/9) of controls achieved efficacy (defined as investigator global assessment score of 2 or lower).52 In an open-label, single-arm clinical trial assessing tavaborole solution 5% applied once daily for 48 weeks for the treatment of toenail onychomycosis in pediatric patients (aged 6–17 years), 36.2% (20/55) of patients achieved mycologic cure, and 8.5% (5/55) achieved complete cure at week 52 with mild or minimal adverse effects.53 In an open-label, phase 4 study of the safety and efficacy of efinaconazole solution 10% applied once daily for 48 weeks in pediatric patients (aged 6 to 16 years) (n=60), 65% (35/60) achieved mycologic cure, 42% (25/60) achieved clinical cure, and 40% (24/60) achieved complete cure at 52 weeks. The most common adverse effects of efinaconazole were local and included ingrown toenail (1/60), application-site dermatitis (1/60), application-site vesicles (1/60), and application-site pain (1/60).54
In a systematic review of systemic antifungals for onychomycosis in 151 pediatric patients, itraconazole, fluconazole, griseofulvin, and terbinafine resulted in complete cure rates similar to those of the adult population, with excellent safety profiles.55 Depending on the situation, initiation of treatment with topical medications followed by addition of systemic antifungal agents only if needed may be an appropriate course of action.
BACTERIAL INFECTIONS
Acute Paronychia
Acute paronychia is a nail-fold infection that develops after the protective nail barrier has been compromised.56 In children, thumb-sucking, nail-biting, frequent oral manipulation of the digits, and poor skin hygiene are risk factors. Acute paronychia also may develop in association with congenital malalignment of the great toenails.57
Clinical manifestations include localized pain, erythema, and nail fold edema (Figure 4). Purulent material and abscess formation may ensue. Staphylococcus aureus as well as methicillin-resistant S aureus and Streptococcus pyogenes are classically the most common causes of acute paronychia. Treatment of paronychia is based on severity. In mild cases, warm soaks with topical antibiotics are indicated. Oral antibiotics should be prescribed for more severe presentations. If there is no improvement after 48 hours, surgical drainage is required to facilitate healing.56
FINAL THOUGHTS
Inflammatory and infectious nail disorders in children are relatively common and may impact the physical and emotional well-being of young patients. By understanding the distinctive features of these nail disorders in pediatric patients, dermatologists can provide anticipatory guidance and informed treatment options to children and their parents. Further research is needed to expand our understanding of pediatric nail disorders and create targeted therapeutic interventions, particularly for NLP and psoriasis.


- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207. doi:10.1111/pde.13402
- Plachouri KM, Mulita F, Georgiou S. Management of pediatric nail psoriasis. Cutis. 2021;108:292-294. doi:10.12788/cutis.0386
- Smith RJ, Rubin AI. Pediatric nail disorders: a review. Curr Opin Pediatr. 2020;32:506-515. doi:10.1097/mop.0000000000000921
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63. doi:10.1111/pde.13028
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112. doi:10.2165/11537110-000000000-00000
- Lee JYY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Nogueira M, Paller AS, Torres T. Targeted therapy for pediatric psoriasis. Paediatr Drugs. May 2021;23:203-212. doi:10.1007/s40272-021-00443-5
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. Updated August 16, 2023. Accessed July 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179. doi:10.1186/1752-1947-4-179
- Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158:533-541. doi:10.1001/jamadermatol.2022.0655
- Diotallevi F, Simonetti O, Rizzetto G, et al. Biological treatments for pediatric psoriasis: state of the art and future perspectives. Int J Mol Sci. 2022;23:11128. doi:10.3390/ijms231911128
- Nash P, Mease PJ, Kirkham B, et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clin Exp Rheumatol. 2022;40:952-959. doi:10.55563/clinexprheumatol/3nuz51
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385. doi:10.1111/pde.13767
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriaticonycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508. doi:10.1684/ejd.2015.2616
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352. doi:10.1159/000096202
- Iorizzo M, Gioia Di Chiacchio N, Di Chiacchio N, et al. Intralesional steroid injections for inflammatory nail dystrophies in the pediatric population. Pediatr Dermatol. 2023;40:759-761. doi:10.1111/pde.15295
- Tosti A, Piraccini BM, Cambiaghi S, et al. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. 2001;137:1027-1032.
- Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80:e177-e178. doi:10.1016/j.jaad.2018.11.065
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496. doi:10.1684/ejd.2010.0952
- Mahajan R, Kaushik A, De D, et al. Pediatric trachyonychia- a retrospective study of 17 cases. Indian J Dermatol. 2021;66:689-690. doi:10.4103/ijd.ijd_42_21
- Leung AKC, Leong KF, Barankin B. Trachyonychia. J Pediatr. 2020;216:239-239.e1. doi:10.1016/j.jpeds.2019.08.034
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Jacobsen AA, Tosti A. Trachyonychia and twenty-nail dystrophy: a comprehensive review and discussion of diagnostic accuracy. Skin Appendage Disord. 2016;2:7-13. doi:10.1159/000445544
- Kumar MG, Ciliberto H, Bayliss SJ. Long-term follow-up of pediatric trachyonychia. Pediatr Dermatol. 2015;32:198-200. doi:10.1111/pde.12427
- Tosti A, Piraccini BM, Iorizzo M. Trachyonychia and related disorders: evaluation and treatment plans. Dermatolog Ther. 2002;15:121-125. doi:10.1046/j.1529-8019.2002.01511.x
- Leung AKC, Leong KF, Barankin B. Lichen striatus with nail involvement in a 6-year-old boy. Case Rep Pediatr. 2020;2020:1494760. doi:10.1155/2020/1494760
- Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617. doi:10.1111/j.1346-8138.2009.00720.x
- Iorizzo M, Rubin AI, Starace M. Nail lichen striatus: is dermoscopy useful for the diagnosis? Pediatr Dermatol. 2019;36:859-863. doi:10.1111/pde.13916
- Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361. doi:10.1111/j.1525-1470.1993.tb00399.x
- Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36(6, pt 1):908-913. doi:10.1016/s0190-9622(97)80270-8
- Simpson EL, Thompson MM, Hanifin JM. Prevalence and morphology of hand eczema in patients with atopic dermatitis. Dermatitis. 2006;17:123-127. doi:10.2310/6620.2006.06005
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C. Nail alterations in 250 infant patients: a clinical study. J Eur Acad Dermatol Venereol. 2008;22:741-744. doi:10.1111/j.1468-3083.2008.02592.x
- Milanesi N, D’Erme AM, Gola M. Nail improvement during alitretinoin treatment: three case reports and review of the literature. Clin Exp Dermatol. 2015;40:533-536. doi:10.1111/ced.12584
- Chung BY, Choi YW, Kim HO, et al. Nail dystrophy in patients with atopic dermatitis and its association with disease severity. Ann Dermatol. 2019;31:121-126. doi:10.5021/ad.2019.31.2.121
- Navarro-Triviño FJ, Vega-Castillo JJ, Ruiz-Villaverde R. Nail changes successfully treated with dupilumab in a patient with severe atopic dermatitis. Australas J Dermatol. 2021;62:e468-e469. doi:10.1111/ajd.13633
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. doi:10.1186/1471-2334-11-346
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283. doi:10.5021/ad.2014.26.2.280
- Verma S, Singal A. Nail changes in hand-foot-and-mouth disease (HFMD). Indian Dermatol Online J. 2021;12:656-657. doi:10.4103 /idoj.IDOJ_271_20
- Giordano LMC, de la Fuente LA, Lorca JMB, et al. Onychomadesis secondary to hand-foot-mouth disease: a frequent manifestation and cause of concern for parents. Article in Spanish. Rev Chil Pediatr. 2018;89:380-383. doi:10.4067/s0370-41062018005000203
- Justino MCA, da SMD, Souza MF, et al. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol. 2020;126:104307. doi:10.1016/j.jcv.2020.104307
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr. 2022;11:1502-1509. doi:10.21037/tp-22-352
- Nagata S. Causes of Kawasaki disease-from past to present. Front Pediatr. 2019;7:18. doi:10.3389/fped.2019.00018
- Mitsuishi T, Miyata K, Ando A, et al. Characteristic nail lesions in Kawasaki disease: case series and literature review. J Dermatol. 2022;49:232-238. doi:10.1111/1346-8138.16276
- Lindsley CB. Nail-bed lines in Kawasaki disease. Am J Dis Child. 1992;146:659-660. doi:10.1001/archpedi.1992.02160180017005
- Matsumura O, Nakagishi Y. Pincer nails upon convalescence from Kawasaki disease. J Pediatr. 2022;246:279. doi:10.1016/j.jpeds.2022.03.002
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Gupta AK, Mays RR, Versteeg SG, et al. Onychomycosis in children: safety and efficacy of antifungal agents. Pediatr Dermatol. 2018;35:552-559. doi:10.1111/pde.13561
- 49. Gupta AK, Venkataraman M, Shear NH, et al. Labeled use of efinaconazole topical solution 10% in treating onychomycosis in children and a review of the management of pediatric onychomycosis. Dermatol Ther. 2020;33:e13613. doi:10.1111/dth.13613
- Falotico JM, Lipner SR. Updated perspectives on the diagnosis and management of onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933-1957. doi:10.2147/ccid.S362635
- Patel D, Castelo-Soccio LA, Rubin AI, et al. Laboratory monitoring during systemic terbinafine therapy for pediatric onychomycosis. JAMA Dermatol. 2017;153:1326-1327. doi:10.1001/jamadermatol.2017.4483
- Friedlander SF, Chan YC, Chan YH, et al. Onychomycosis does not always require systemic treatment for cure: a trial using topical therapy. Pediatr Dermatol. 2013;30:316-322. doi:10.1111/pde.12064
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Gupta AK, Venkataraman M, Abramovits W, et al. JUBLIA (efinaconazole 10% solution) in the treatment of pediatric onychomycosis. Skinmed. 2021;19:206-210.
- Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013;30:294-302. doi:10.1111/pde.12048
- Leggit JC. Acute and chronic paronychia. Am Fam Physician. 2017;96:44-51.
- Lipner SR, Scher RK. Congenital malalignment of the great toenails with acute paronychia. Pediatr Dermatol. 2016;33:e288-e289.doi:10.1111/pde.12924
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207. doi:10.1111/pde.13402
- Plachouri KM, Mulita F, Georgiou S. Management of pediatric nail psoriasis. Cutis. 2021;108:292-294. doi:10.12788/cutis.0386
- Smith RJ, Rubin AI. Pediatric nail disorders: a review. Curr Opin Pediatr. 2020;32:506-515. doi:10.1097/mop.0000000000000921
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63. doi:10.1111/pde.13028
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112. doi:10.2165/11537110-000000000-00000
- Lee JYY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Nogueira M, Paller AS, Torres T. Targeted therapy for pediatric psoriasis. Paediatr Drugs. May 2021;23:203-212. doi:10.1007/s40272-021-00443-5
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. Updated August 16, 2023. Accessed July 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179. doi:10.1186/1752-1947-4-179
- Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158:533-541. doi:10.1001/jamadermatol.2022.0655
- Diotallevi F, Simonetti O, Rizzetto G, et al. Biological treatments for pediatric psoriasis: state of the art and future perspectives. Int J Mol Sci. 2022;23:11128. doi:10.3390/ijms231911128
- Nash P, Mease PJ, Kirkham B, et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clin Exp Rheumatol. 2022;40:952-959. doi:10.55563/clinexprheumatol/3nuz51
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385. doi:10.1111/pde.13767
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriaticonycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508. doi:10.1684/ejd.2015.2616
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352. doi:10.1159/000096202
- Iorizzo M, Gioia Di Chiacchio N, Di Chiacchio N, et al. Intralesional steroid injections for inflammatory nail dystrophies in the pediatric population. Pediatr Dermatol. 2023;40:759-761. doi:10.1111/pde.15295
- Tosti A, Piraccini BM, Cambiaghi S, et al. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. 2001;137:1027-1032.
- Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80:e177-e178. doi:10.1016/j.jaad.2018.11.065
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496. doi:10.1684/ejd.2010.0952
- Mahajan R, Kaushik A, De D, et al. Pediatric trachyonychia- a retrospective study of 17 cases. Indian J Dermatol. 2021;66:689-690. doi:10.4103/ijd.ijd_42_21
- Leung AKC, Leong KF, Barankin B. Trachyonychia. J Pediatr. 2020;216:239-239.e1. doi:10.1016/j.jpeds.2019.08.034
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Jacobsen AA, Tosti A. Trachyonychia and twenty-nail dystrophy: a comprehensive review and discussion of diagnostic accuracy. Skin Appendage Disord. 2016;2:7-13. doi:10.1159/000445544
- Kumar MG, Ciliberto H, Bayliss SJ. Long-term follow-up of pediatric trachyonychia. Pediatr Dermatol. 2015;32:198-200. doi:10.1111/pde.12427
- Tosti A, Piraccini BM, Iorizzo M. Trachyonychia and related disorders: evaluation and treatment plans. Dermatolog Ther. 2002;15:121-125. doi:10.1046/j.1529-8019.2002.01511.x
- Leung AKC, Leong KF, Barankin B. Lichen striatus with nail involvement in a 6-year-old boy. Case Rep Pediatr. 2020;2020:1494760. doi:10.1155/2020/1494760
- Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617. doi:10.1111/j.1346-8138.2009.00720.x
- Iorizzo M, Rubin AI, Starace M. Nail lichen striatus: is dermoscopy useful for the diagnosis? Pediatr Dermatol. 2019;36:859-863. doi:10.1111/pde.13916
- Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361. doi:10.1111/j.1525-1470.1993.tb00399.x
- Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36(6, pt 1):908-913. doi:10.1016/s0190-9622(97)80270-8
- Simpson EL, Thompson MM, Hanifin JM. Prevalence and morphology of hand eczema in patients with atopic dermatitis. Dermatitis. 2006;17:123-127. doi:10.2310/6620.2006.06005
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C. Nail alterations in 250 infant patients: a clinical study. J Eur Acad Dermatol Venereol. 2008;22:741-744. doi:10.1111/j.1468-3083.2008.02592.x
- Milanesi N, D’Erme AM, Gola M. Nail improvement during alitretinoin treatment: three case reports and review of the literature. Clin Exp Dermatol. 2015;40:533-536. doi:10.1111/ced.12584
- Chung BY, Choi YW, Kim HO, et al. Nail dystrophy in patients with atopic dermatitis and its association with disease severity. Ann Dermatol. 2019;31:121-126. doi:10.5021/ad.2019.31.2.121
- Navarro-Triviño FJ, Vega-Castillo JJ, Ruiz-Villaverde R. Nail changes successfully treated with dupilumab in a patient with severe atopic dermatitis. Australas J Dermatol. 2021;62:e468-e469. doi:10.1111/ajd.13633
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. doi:10.1186/1471-2334-11-346
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283. doi:10.5021/ad.2014.26.2.280
- Verma S, Singal A. Nail changes in hand-foot-and-mouth disease (HFMD). Indian Dermatol Online J. 2021;12:656-657. doi:10.4103 /idoj.IDOJ_271_20
- Giordano LMC, de la Fuente LA, Lorca JMB, et al. Onychomadesis secondary to hand-foot-mouth disease: a frequent manifestation and cause of concern for parents. Article in Spanish. Rev Chil Pediatr. 2018;89:380-383. doi:10.4067/s0370-41062018005000203
- Justino MCA, da SMD, Souza MF, et al. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol. 2020;126:104307. doi:10.1016/j.jcv.2020.104307
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr. 2022;11:1502-1509. doi:10.21037/tp-22-352
- Nagata S. Causes of Kawasaki disease-from past to present. Front Pediatr. 2019;7:18. doi:10.3389/fped.2019.00018
- Mitsuishi T, Miyata K, Ando A, et al. Characteristic nail lesions in Kawasaki disease: case series and literature review. J Dermatol. 2022;49:232-238. doi:10.1111/1346-8138.16276
- Lindsley CB. Nail-bed lines in Kawasaki disease. Am J Dis Child. 1992;146:659-660. doi:10.1001/archpedi.1992.02160180017005
- Matsumura O, Nakagishi Y. Pincer nails upon convalescence from Kawasaki disease. J Pediatr. 2022;246:279. doi:10.1016/j.jpeds.2022.03.002
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Gupta AK, Mays RR, Versteeg SG, et al. Onychomycosis in children: safety and efficacy of antifungal agents. Pediatr Dermatol. 2018;35:552-559. doi:10.1111/pde.13561
- 49. Gupta AK, Venkataraman M, Shear NH, et al. Labeled use of efinaconazole topical solution 10% in treating onychomycosis in children and a review of the management of pediatric onychomycosis. Dermatol Ther. 2020;33:e13613. doi:10.1111/dth.13613
- Falotico JM, Lipner SR. Updated perspectives on the diagnosis and management of onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933-1957. doi:10.2147/ccid.S362635
- Patel D, Castelo-Soccio LA, Rubin AI, et al. Laboratory monitoring during systemic terbinafine therapy for pediatric onychomycosis. JAMA Dermatol. 2017;153:1326-1327. doi:10.1001/jamadermatol.2017.4483
- Friedlander SF, Chan YC, Chan YH, et al. Onychomycosis does not always require systemic treatment for cure: a trial using topical therapy. Pediatr Dermatol. 2013;30:316-322. doi:10.1111/pde.12064
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Gupta AK, Venkataraman M, Abramovits W, et al. JUBLIA (efinaconazole 10% solution) in the treatment of pediatric onychomycosis. Skinmed. 2021;19:206-210.
- Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013;30:294-302. doi:10.1111/pde.12048
- Leggit JC. Acute and chronic paronychia. Am Fam Physician. 2017;96:44-51.
- Lipner SR, Scher RK. Congenital malalignment of the great toenails with acute paronychia. Pediatr Dermatol. 2016;33:e288-e289.doi:10.1111/pde.12924
Practice Points
- Nail plate pitting is the most common clinical sign of nail psoriasis in children.
- Nail changes are common in hand, foot, and mouth disease, with the most frequent being onychomadesis.
- Because onychomycosis may resemble other nail disorders, mycologic confirmation is recommended to avoid misdiagnosis.
- Many nail conditions in children self-resolve but recognizing these manifestations is important in providing anticipatory guidance to patients and caregivers.
Nail Alterations From Musical Instruments: Insights for Dermatologists Treating Musicians
A variety of skin problems can occur in musicians due to the repetitive movements of playing instruments.1,2 Musicians’ nails are continuously exposed to the mechanical forces and chemical substances characteristic of their instruments.3 Occupational nail alterations in musicians caused by repetitive physical trauma, allergic contact dermatitis, and/or infection may lead to disability and compromise their professional career.
We conducted a systematic review of the literature on the clinical features of musical instrument–related nail alterations to optimize the management and prevention of these conditions.
Methods
We conducted a systematic review of PubMed, Scopus, and Google Scholar databases for eligible publications on instrument-related nail alterations in musicians using the search terms musicians with nail, onychopathy, and Raynaud. No time or language criteria were applied. Reviews, editorials, and articles not related to the topic were excluded. Bibliographies/reference lists were checked to find any additional relevant publications. Relevant articles in English and French were screened by 2 independent reviewers (A.G. and N.L.), and the following data were extracted for qualitative synthesis: sex, age, musical instrument, clinical features, number of years practicing the instrument, laboratory investigations, and disease course.
Results
The literature search yielded 11 publications. Sixteen additional articles were identified by other methods (ie, references, related publications). Overall, 3 full-text articles described general nail alterations but did not describe the clinical data, and 11 publications were editorials, commentaries, reviews, or not relevant. Thirteen contributions fulfilled the inclusion criteria and were eligible for qualitative synthesis. The flow diagram illustrates the screening process (Figure 1).
Twenty-three patients were included. The instruments identified were divided into 2 groups: string instruments (ie, guitar, violin, harp) and percussion instruments (ie, drums, piano, slap bass). Nail alterations were clinically expressed as: (1) modifications of the nail surface; (2) nail bed, soft-tissue, and bone abnormalities; and (3) periungual tissue and distal pulp disorders. All cases are summarized in the Table.4-16 Three articles described occupational Raynaud phenomenon.12-14
Comment
Modifications of the Nail Surface—Onychodystrophy, such as deformity or discoloration of the nail plate, was described in 6 patients among a cohort of 295 musicians and an additional 6 patients among 199 musicians with induced skin lesions. This condition was most common in string instrument players and pianists due to injury and irritation.
One patient, who had been a professional violist for 27 years, presented with lamellar onychoschizia, which corresponds to a horizontal splitting of the nail toward its distal portion (Figure 2). The 3 fingernails of the dominant hand were involved with a V-shaped incision of the distal margin of the nail due to the repetitive friction of the nails with the strings.6
Striations of the nail plate were reported in a guitarist who played for 10 years.7 Physical examination revealed linear transverse ridges alternating with depressions on the central aspect of the nail plate of the right thumbnail, as the patient was right-handed. This condition, attributed to sustained pressure on the string applied by the thumb, also has been called habit tic deformity.7
Nail Bed, Soft-Tissue, and Bone Lesions—Purpura (or hemorrhage) of the nail bed was associated with a percussion instrument (ie, piano) in 1 patient, affecting the second, third, and fourth fingernails of the right hand.8 Especially when performing ascending glissando passages, the pianist applies pressure that may damage the finger and cause fingernail purpura. This condition improved after the patient stopping practicing glissandi.8
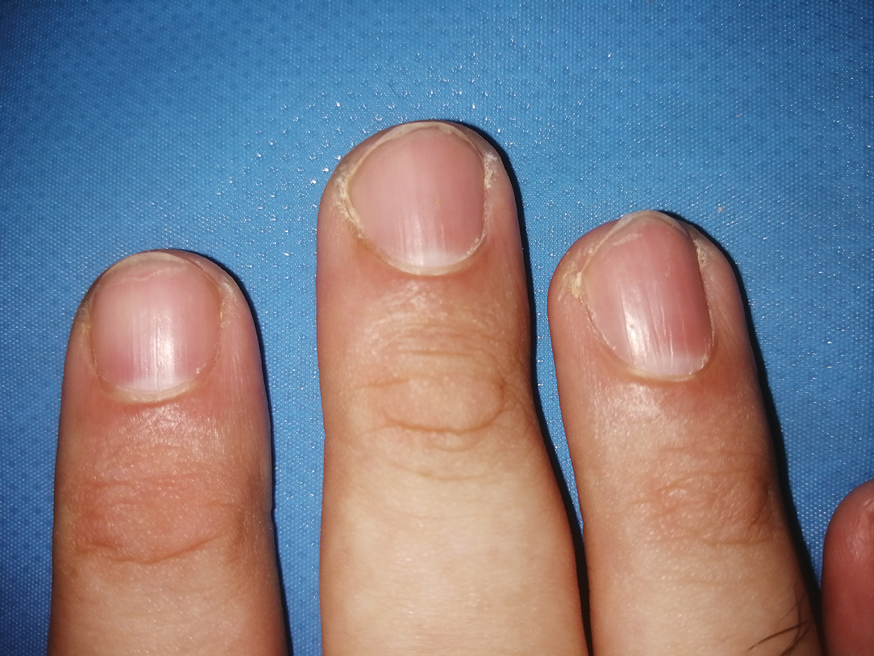
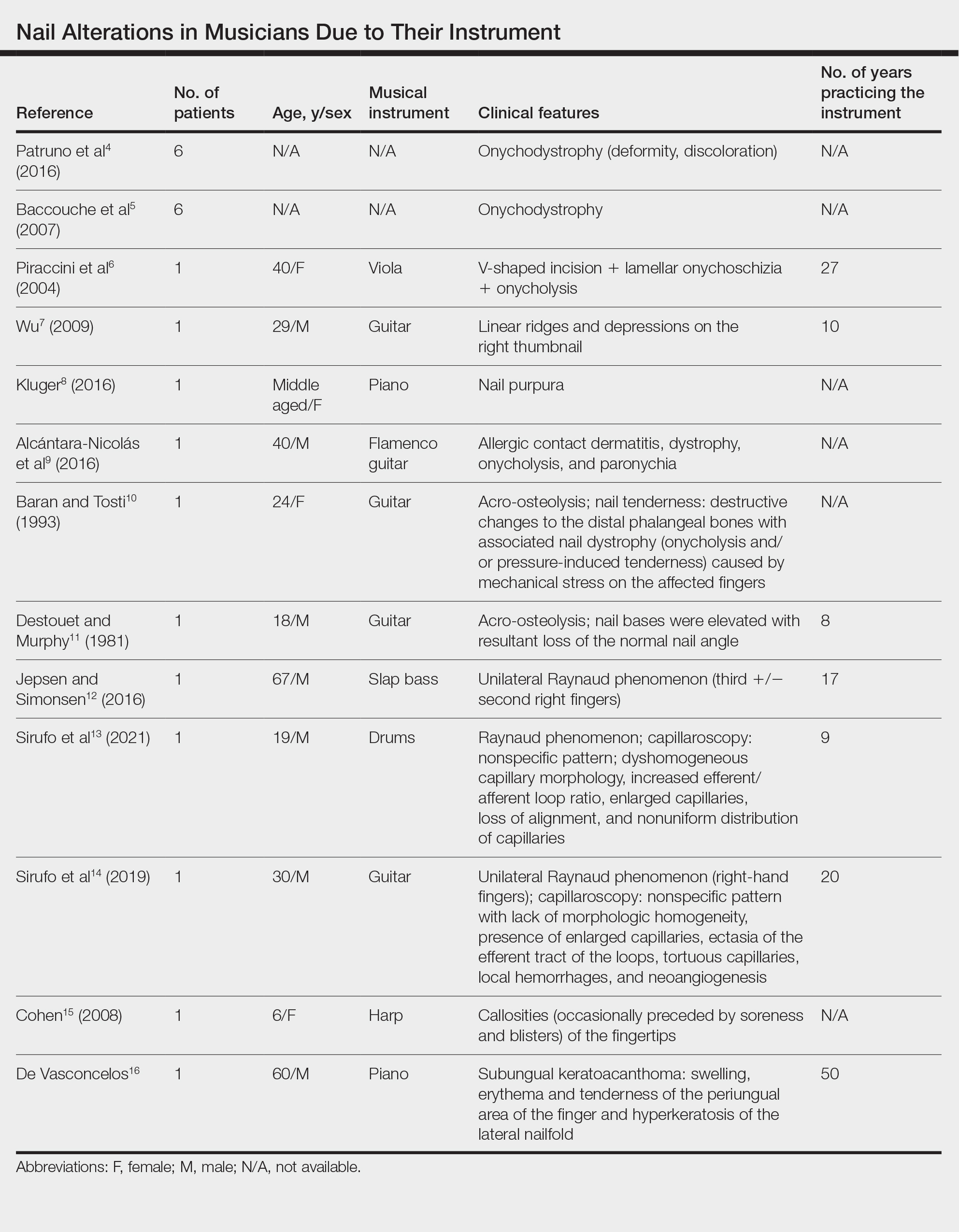
Three patients—2 guitarists and 1 violist—had onycholysis, defined by a loss of the attachment between the nail bed and the nail plate (Figure 3). It may result from repetitive trauma when strings are plucked.6,9,10
Acro-osteolysis associated with pain was reported in 2 guitarists.10,11 This condition is defined as transverse lytic bands in the distal phalanges (Figure 4). Acro-osteolysis may be secondary to multiple causes, such as vinyl chloride exposure, connective tissue diseases, thermal injuries, neuropathic diseases, hyperparathyroidism, nutritional deficiencies, psoriasis, and biomechanical stress.10 In musicians playing instruments, the mechanical stress to the guitar-playing fingers is the causative factor.17
Periungual Tissue and Distal Pulp Disorders—Paronychia is an important occupational hazard of harpists, violists, and pianists.2 It represents an inflammatory condition involving the folds of tissue surrounding fingernails. Pizzicato paronychia is related to infection in the nail fold in string players and secondary to pizzicato playing, whereby the musician plucks the instrument strings with the nails and fingertips.3
Acrylates in artificial nails frequently are used among guitarists to strengthen their nails. A case of occupational allergic contact dermatitis induced by acrylic gel nails in a flamenco guitarist was described.9 The patient developed dystrophy, onycholysis, and paronychia involving the nails of the right hand where acrylic materials were used, which resolved following the removal of the artificial nails. Patch tests were performed and were positive for 2-hydroxyethyl methacrylate, 2-hydroxyethyl acrylate, ethylene glycol dimethacrylate, and 2-hydroxypropyl methacrylate, supporting the diagnosis of allergic contact dermatitis to acrylates.9 Therefore, musicians should be aware of the sensitizing potential of acrylates and adopt preventive measures.
Unilateral Raynaud phenomenon of the dominant hand was noted in 3 cases of musicians who played string instruments due to the increased tendency to vasospasm in the digital capillaries from the direct transmission of vibrations of the strings (>100 Hz).12-14 Consequently, the disruption of the digital blood circulation leads to an abnormal reaction to cold, which is called vibration-induced white fingers or vasospastic white finger disease.19 In these 3 patients, capillaroscopy showed a nonspecific pattern with a lack of morphologic homogeneity of capillaries, the presence of enlarged capillaries, ectasia of the efferent tract of the loops, tortuous capillaries, local hemorrhages, and neoangiogenesis.13,14
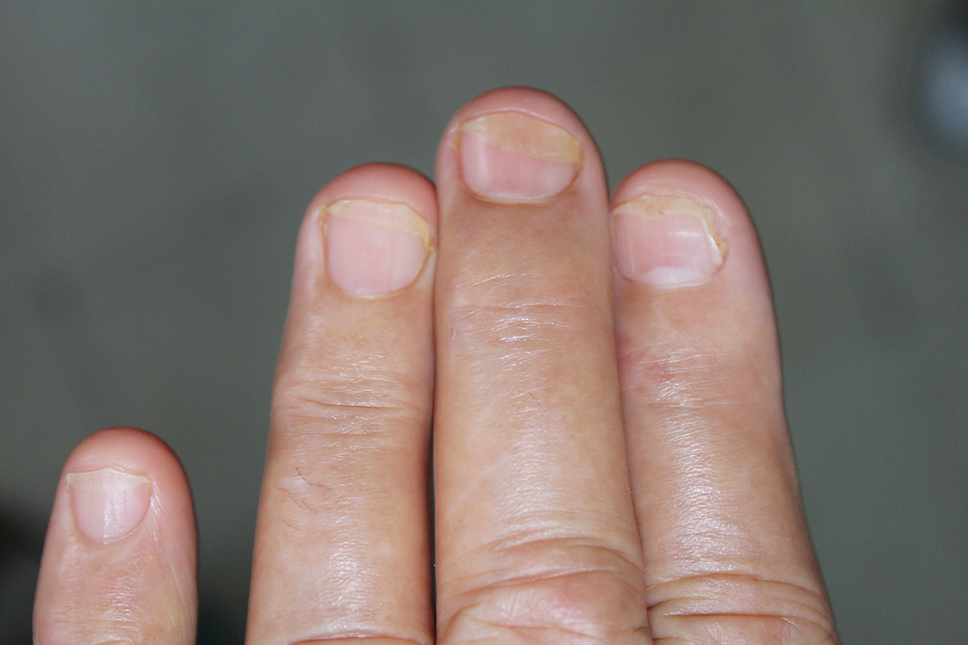
A middle-aged professional concert pianist presented with paronychia with hyperkeratosis of the lateral nail fold. Histopathology revealed a subungual keratoacanthoma eroding the distal phalanx tip, which was removed by surgical excision. The repeated fingertip trauma associated with pianistic activity was suspected to be the causative event.16
Callosities also are common on the fingertips of musicians, including 18.4% of patients in a cohort of 628 musicians, and involving fingers in 64.6% of these patients.4 These callosities are explained by the chronic mechanical forces and characterize the way musicians grasp and hold their instruments. Callosities could be preceded by soreness and blisters of the fingertips in a harpist (harpist’s finger).1,15 Calluses were located on the lateral fourth fingertip of a drummer corresponding to the friction with the drumsticks (drummer’s digit) and on the thumb of a bassoon player. Trumpet calluses generally overlie the proximal interphalangeal joint of the left index finger.
Conclusion
Healthy nails are essential for playing a musical instrument. This review highlights the occurrence of fingertip callosities, paronychia, onycholysis, and subungual hemorrhages among musicians who play instruments. Additionally, the transmission of string-vibratory movements can produce microvascular damage and occupational Raynaud phenomenon in some musicians. These occupational nail disorders are underrecognized and may be underdiagnosed. Thus, musicians and clinicians must be aware of these alterations to adopt preventive measures and to provide adequate treatment.
- Rimmer S, Spielvogel RL. Dermatologic problems of musicians. J Am Acad Dermatol. 1990;22:657-663.
- Adams RM. Skin conditions of musicians. Cutis. 2000;65:37-38.
- Vine K, DeLeo V. Dermatologic manifestations of musicians: a case report and review of skin conditions in musicians. Cutis. 2011;87:117-121.
- Patruno C, Napolitano M, La Bella S, et al. Instrument-related skin disorders in musicians. Dermatitis. 2016;27:26-29.
- Baccouche D, Mokni M, Ben Abdelaziz A, et al. Dermatological problems of musicians: a prospective study in musical students . Article in French. Ann Dermatol Venereol. 2007;134(5 Pt 1):445-449.
- Piraccini BM, Antonucci A, Iorizzo M, et al. Occupational nail fragility in a professional violist. Contact Dermatitis. 2004;51:35-36.
- Wu JJ. Habit tic deformity secondary to guitar playing. Dermatol Online J. 2009;15:16.
- Kluger N. Piano glissando purpura: another cutaneous curiosity in musicians. J Eur Acad Dermatol Venereol. 2016;30:683.
- Alcántara-Nicolás FA, Pastor-Nieto MA, Sánchez-Herreros C, et al. Allergic contact dermatitis from acrylic nails in a flamenco guitarist. Occup Med (Lond). 2016;66:751-753.
- Baran R, Tosti A. Occupational acroosteolysis in a guitar player. Acta Derm Venereol. 1993;73:64-65.
- Destouet JM, Murphy WA. Guitar player acro-osteolysis. Skeletal Radiol. 1981;6:275-277.
- Jepsen JR, Simonsen JA. Raynaud’s phenomenon in a slap bass player: a case report. Med Probl Perform Art. 2016;31:51-53.
- Sirufo MM, Catalogna A, De Pietro F, et al. Raynaud’s phenomenon in a drummer player: microvascular disorder and nailfold video capillaroscopic findings. EXCLI J. 2021;20:1526-1531.
- Sirufo MM, Ginaldi L, De Martinis M. Raynaud’s phenomenon and the nailfold capillaroscopic findings in a guitar player. QJM. 2019;112:531-533.
- Cohen PR. Harpist’s finger: case report of a trauma-induced blister in a beginner harpist and review of string instrument-associated skin problems in musicians. Cutis. 2008;82:329-334.
- De Vasconcelos P, Soares-Almeida L, Filipe P. Subungual keratoacanthoma in a pianist. G Ital Dermatol Venereol. 2016;151:455-456.
- Young RS, Bryk D, Ratner H. Selective phalangeal tuft fractures in a guitar player. Br J Radiol. 1977;50:147-148.
- Vázquez-Osorio I, Espasandín-Arias M, García-Gavín J, et al. Allergic contact dermatitis due to acrylates in acrylic gel nails: a report of 3 cases. Actas Dermosifiliogr. 2014;105:430-432.
- Atashpaz S, Ghabili K. Color triad in guitarist’s fingers: a probable case of Raynaud’s phenomenon due to string vibration phenomenon. Med Probl Perform Art. 2008;23:143.
A variety of skin problems can occur in musicians due to the repetitive movements of playing instruments.1,2 Musicians’ nails are continuously exposed to the mechanical forces and chemical substances characteristic of their instruments.3 Occupational nail alterations in musicians caused by repetitive physical trauma, allergic contact dermatitis, and/or infection may lead to disability and compromise their professional career.
We conducted a systematic review of the literature on the clinical features of musical instrument–related nail alterations to optimize the management and prevention of these conditions.
Methods
We conducted a systematic review of PubMed, Scopus, and Google Scholar databases for eligible publications on instrument-related nail alterations in musicians using the search terms musicians with nail, onychopathy, and Raynaud. No time or language criteria were applied. Reviews, editorials, and articles not related to the topic were excluded. Bibliographies/reference lists were checked to find any additional relevant publications. Relevant articles in English and French were screened by 2 independent reviewers (A.G. and N.L.), and the following data were extracted for qualitative synthesis: sex, age, musical instrument, clinical features, number of years practicing the instrument, laboratory investigations, and disease course.
Results
The literature search yielded 11 publications. Sixteen additional articles were identified by other methods (ie, references, related publications). Overall, 3 full-text articles described general nail alterations but did not describe the clinical data, and 11 publications were editorials, commentaries, reviews, or not relevant. Thirteen contributions fulfilled the inclusion criteria and were eligible for qualitative synthesis. The flow diagram illustrates the screening process (Figure 1).

Twenty-three patients were included. The instruments identified were divided into 2 groups: string instruments (ie, guitar, violin, harp) and percussion instruments (ie, drums, piano, slap bass). Nail alterations were clinically expressed as: (1) modifications of the nail surface; (2) nail bed, soft-tissue, and bone abnormalities; and (3) periungual tissue and distal pulp disorders. All cases are summarized in the Table.4-16 Three articles described occupational Raynaud phenomenon.12-14
Comment
Modifications of the Nail Surface—Onychodystrophy, such as deformity or discoloration of the nail plate, was described in 6 patients among a cohort of 295 musicians and an additional 6 patients among 199 musicians with induced skin lesions. This condition was most common in string instrument players and pianists due to injury and irritation.
One patient, who had been a professional violist for 27 years, presented with lamellar onychoschizia, which corresponds to a horizontal splitting of the nail toward its distal portion (Figure 2). The 3 fingernails of the dominant hand were involved with a V-shaped incision of the distal margin of the nail due to the repetitive friction of the nails with the strings.6
Striations of the nail plate were reported in a guitarist who played for 10 years.7 Physical examination revealed linear transverse ridges alternating with depressions on the central aspect of the nail plate of the right thumbnail, as the patient was right-handed. This condition, attributed to sustained pressure on the string applied by the thumb, also has been called habit tic deformity.7
Nail Bed, Soft-Tissue, and Bone Lesions—Purpura (or hemorrhage) of the nail bed was associated with a percussion instrument (ie, piano) in 1 patient, affecting the second, third, and fourth fingernails of the right hand.8 Especially when performing ascending glissando passages, the pianist applies pressure that may damage the finger and cause fingernail purpura. This condition improved after the patient stopping practicing glissandi.8


Three patients—2 guitarists and 1 violist—had onycholysis, defined by a loss of the attachment between the nail bed and the nail plate (Figure 3). It may result from repetitive trauma when strings are plucked.6,9,10
Acro-osteolysis associated with pain was reported in 2 guitarists.10,11 This condition is defined as transverse lytic bands in the distal phalanges (Figure 4). Acro-osteolysis may be secondary to multiple causes, such as vinyl chloride exposure, connective tissue diseases, thermal injuries, neuropathic diseases, hyperparathyroidism, nutritional deficiencies, psoriasis, and biomechanical stress.10 In musicians playing instruments, the mechanical stress to the guitar-playing fingers is the causative factor.17
Periungual Tissue and Distal Pulp Disorders—Paronychia is an important occupational hazard of harpists, violists, and pianists.2 It represents an inflammatory condition involving the folds of tissue surrounding fingernails. Pizzicato paronychia is related to infection in the nail fold in string players and secondary to pizzicato playing, whereby the musician plucks the instrument strings with the nails and fingertips.3
Acrylates in artificial nails frequently are used among guitarists to strengthen their nails. A case of occupational allergic contact dermatitis induced by acrylic gel nails in a flamenco guitarist was described.9 The patient developed dystrophy, onycholysis, and paronychia involving the nails of the right hand where acrylic materials were used, which resolved following the removal of the artificial nails. Patch tests were performed and were positive for 2-hydroxyethyl methacrylate, 2-hydroxyethyl acrylate, ethylene glycol dimethacrylate, and 2-hydroxypropyl methacrylate, supporting the diagnosis of allergic contact dermatitis to acrylates.9 Therefore, musicians should be aware of the sensitizing potential of acrylates and adopt preventive measures.
Unilateral Raynaud phenomenon of the dominant hand was noted in 3 cases of musicians who played string instruments due to the increased tendency to vasospasm in the digital capillaries from the direct transmission of vibrations of the strings (>100 Hz).12-14 Consequently, the disruption of the digital blood circulation leads to an abnormal reaction to cold, which is called vibration-induced white fingers or vasospastic white finger disease.19 In these 3 patients, capillaroscopy showed a nonspecific pattern with a lack of morphologic homogeneity of capillaries, the presence of enlarged capillaries, ectasia of the efferent tract of the loops, tortuous capillaries, local hemorrhages, and neoangiogenesis.13,14


A middle-aged professional concert pianist presented with paronychia with hyperkeratosis of the lateral nail fold. Histopathology revealed a subungual keratoacanthoma eroding the distal phalanx tip, which was removed by surgical excision. The repeated fingertip trauma associated with pianistic activity was suspected to be the causative event.16
Callosities also are common on the fingertips of musicians, including 18.4% of patients in a cohort of 628 musicians, and involving fingers in 64.6% of these patients.4 These callosities are explained by the chronic mechanical forces and characterize the way musicians grasp and hold their instruments. Callosities could be preceded by soreness and blisters of the fingertips in a harpist (harpist’s finger).1,15 Calluses were located on the lateral fourth fingertip of a drummer corresponding to the friction with the drumsticks (drummer’s digit) and on the thumb of a bassoon player. Trumpet calluses generally overlie the proximal interphalangeal joint of the left index finger.
Conclusion
Healthy nails are essential for playing a musical instrument. This review highlights the occurrence of fingertip callosities, paronychia, onycholysis, and subungual hemorrhages among musicians who play instruments. Additionally, the transmission of string-vibratory movements can produce microvascular damage and occupational Raynaud phenomenon in some musicians. These occupational nail disorders are underrecognized and may be underdiagnosed. Thus, musicians and clinicians must be aware of these alterations to adopt preventive measures and to provide adequate treatment.
A variety of skin problems can occur in musicians due to the repetitive movements of playing instruments.1,2 Musicians’ nails are continuously exposed to the mechanical forces and chemical substances characteristic of their instruments.3 Occupational nail alterations in musicians caused by repetitive physical trauma, allergic contact dermatitis, and/or infection may lead to disability and compromise their professional career.
We conducted a systematic review of the literature on the clinical features of musical instrument–related nail alterations to optimize the management and prevention of these conditions.
Methods
We conducted a systematic review of PubMed, Scopus, and Google Scholar databases for eligible publications on instrument-related nail alterations in musicians using the search terms musicians with nail, onychopathy, and Raynaud. No time or language criteria were applied. Reviews, editorials, and articles not related to the topic were excluded. Bibliographies/reference lists were checked to find any additional relevant publications. Relevant articles in English and French were screened by 2 independent reviewers (A.G. and N.L.), and the following data were extracted for qualitative synthesis: sex, age, musical instrument, clinical features, number of years practicing the instrument, laboratory investigations, and disease course.
Results
The literature search yielded 11 publications. Sixteen additional articles were identified by other methods (ie, references, related publications). Overall, 3 full-text articles described general nail alterations but did not describe the clinical data, and 11 publications were editorials, commentaries, reviews, or not relevant. Thirteen contributions fulfilled the inclusion criteria and were eligible for qualitative synthesis. The flow diagram illustrates the screening process (Figure 1).

Twenty-three patients were included. The instruments identified were divided into 2 groups: string instruments (ie, guitar, violin, harp) and percussion instruments (ie, drums, piano, slap bass). Nail alterations were clinically expressed as: (1) modifications of the nail surface; (2) nail bed, soft-tissue, and bone abnormalities; and (3) periungual tissue and distal pulp disorders. All cases are summarized in the Table.4-16 Three articles described occupational Raynaud phenomenon.12-14
Comment
Modifications of the Nail Surface—Onychodystrophy, such as deformity or discoloration of the nail plate, was described in 6 patients among a cohort of 295 musicians and an additional 6 patients among 199 musicians with induced skin lesions. This condition was most common in string instrument players and pianists due to injury and irritation.
One patient, who had been a professional violist for 27 years, presented with lamellar onychoschizia, which corresponds to a horizontal splitting of the nail toward its distal portion (Figure 2). The 3 fingernails of the dominant hand were involved with a V-shaped incision of the distal margin of the nail due to the repetitive friction of the nails with the strings.6
Striations of the nail plate were reported in a guitarist who played for 10 years.7 Physical examination revealed linear transverse ridges alternating with depressions on the central aspect of the nail plate of the right thumbnail, as the patient was right-handed. This condition, attributed to sustained pressure on the string applied by the thumb, also has been called habit tic deformity.7
Nail Bed, Soft-Tissue, and Bone Lesions—Purpura (or hemorrhage) of the nail bed was associated with a percussion instrument (ie, piano) in 1 patient, affecting the second, third, and fourth fingernails of the right hand.8 Especially when performing ascending glissando passages, the pianist applies pressure that may damage the finger and cause fingernail purpura. This condition improved after the patient stopping practicing glissandi.8


Three patients—2 guitarists and 1 violist—had onycholysis, defined by a loss of the attachment between the nail bed and the nail plate (Figure 3). It may result from repetitive trauma when strings are plucked.6,9,10
Acro-osteolysis associated with pain was reported in 2 guitarists.10,11 This condition is defined as transverse lytic bands in the distal phalanges (Figure 4). Acro-osteolysis may be secondary to multiple causes, such as vinyl chloride exposure, connective tissue diseases, thermal injuries, neuropathic diseases, hyperparathyroidism, nutritional deficiencies, psoriasis, and biomechanical stress.10 In musicians playing instruments, the mechanical stress to the guitar-playing fingers is the causative factor.17
Periungual Tissue and Distal Pulp Disorders—Paronychia is an important occupational hazard of harpists, violists, and pianists.2 It represents an inflammatory condition involving the folds of tissue surrounding fingernails. Pizzicato paronychia is related to infection in the nail fold in string players and secondary to pizzicato playing, whereby the musician plucks the instrument strings with the nails and fingertips.3
Acrylates in artificial nails frequently are used among guitarists to strengthen their nails. A case of occupational allergic contact dermatitis induced by acrylic gel nails in a flamenco guitarist was described.9 The patient developed dystrophy, onycholysis, and paronychia involving the nails of the right hand where acrylic materials were used, which resolved following the removal of the artificial nails. Patch tests were performed and were positive for 2-hydroxyethyl methacrylate, 2-hydroxyethyl acrylate, ethylene glycol dimethacrylate, and 2-hydroxypropyl methacrylate, supporting the diagnosis of allergic contact dermatitis to acrylates.9 Therefore, musicians should be aware of the sensitizing potential of acrylates and adopt preventive measures.
Unilateral Raynaud phenomenon of the dominant hand was noted in 3 cases of musicians who played string instruments due to the increased tendency to vasospasm in the digital capillaries from the direct transmission of vibrations of the strings (>100 Hz).12-14 Consequently, the disruption of the digital blood circulation leads to an abnormal reaction to cold, which is called vibration-induced white fingers or vasospastic white finger disease.19 In these 3 patients, capillaroscopy showed a nonspecific pattern with a lack of morphologic homogeneity of capillaries, the presence of enlarged capillaries, ectasia of the efferent tract of the loops, tortuous capillaries, local hemorrhages, and neoangiogenesis.13,14


A middle-aged professional concert pianist presented with paronychia with hyperkeratosis of the lateral nail fold. Histopathology revealed a subungual keratoacanthoma eroding the distal phalanx tip, which was removed by surgical excision. The repeated fingertip trauma associated with pianistic activity was suspected to be the causative event.16
Callosities also are common on the fingertips of musicians, including 18.4% of patients in a cohort of 628 musicians, and involving fingers in 64.6% of these patients.4 These callosities are explained by the chronic mechanical forces and characterize the way musicians grasp and hold their instruments. Callosities could be preceded by soreness and blisters of the fingertips in a harpist (harpist’s finger).1,15 Calluses were located on the lateral fourth fingertip of a drummer corresponding to the friction with the drumsticks (drummer’s digit) and on the thumb of a bassoon player. Trumpet calluses generally overlie the proximal interphalangeal joint of the left index finger.
Conclusion
Healthy nails are essential for playing a musical instrument. This review highlights the occurrence of fingertip callosities, paronychia, onycholysis, and subungual hemorrhages among musicians who play instruments. Additionally, the transmission of string-vibratory movements can produce microvascular damage and occupational Raynaud phenomenon in some musicians. These occupational nail disorders are underrecognized and may be underdiagnosed. Thus, musicians and clinicians must be aware of these alterations to adopt preventive measures and to provide adequate treatment.
- Rimmer S, Spielvogel RL. Dermatologic problems of musicians. J Am Acad Dermatol. 1990;22:657-663.
- Adams RM. Skin conditions of musicians. Cutis. 2000;65:37-38.
- Vine K, DeLeo V. Dermatologic manifestations of musicians: a case report and review of skin conditions in musicians. Cutis. 2011;87:117-121.
- Patruno C, Napolitano M, La Bella S, et al. Instrument-related skin disorders in musicians. Dermatitis. 2016;27:26-29.
- Baccouche D, Mokni M, Ben Abdelaziz A, et al. Dermatological problems of musicians: a prospective study in musical students . Article in French. Ann Dermatol Venereol. 2007;134(5 Pt 1):445-449.
- Piraccini BM, Antonucci A, Iorizzo M, et al. Occupational nail fragility in a professional violist. Contact Dermatitis. 2004;51:35-36.
- Wu JJ. Habit tic deformity secondary to guitar playing. Dermatol Online J. 2009;15:16.
- Kluger N. Piano glissando purpura: another cutaneous curiosity in musicians. J Eur Acad Dermatol Venereol. 2016;30:683.
- Alcántara-Nicolás FA, Pastor-Nieto MA, Sánchez-Herreros C, et al. Allergic contact dermatitis from acrylic nails in a flamenco guitarist. Occup Med (Lond). 2016;66:751-753.
- Baran R, Tosti A. Occupational acroosteolysis in a guitar player. Acta Derm Venereol. 1993;73:64-65.
- Destouet JM, Murphy WA. Guitar player acro-osteolysis. Skeletal Radiol. 1981;6:275-277.
- Jepsen JR, Simonsen JA. Raynaud’s phenomenon in a slap bass player: a case report. Med Probl Perform Art. 2016;31:51-53.
- Sirufo MM, Catalogna A, De Pietro F, et al. Raynaud’s phenomenon in a drummer player: microvascular disorder and nailfold video capillaroscopic findings. EXCLI J. 2021;20:1526-1531.
- Sirufo MM, Ginaldi L, De Martinis M. Raynaud’s phenomenon and the nailfold capillaroscopic findings in a guitar player. QJM. 2019;112:531-533.
- Cohen PR. Harpist’s finger: case report of a trauma-induced blister in a beginner harpist and review of string instrument-associated skin problems in musicians. Cutis. 2008;82:329-334.
- De Vasconcelos P, Soares-Almeida L, Filipe P. Subungual keratoacanthoma in a pianist. G Ital Dermatol Venereol. 2016;151:455-456.
- Young RS, Bryk D, Ratner H. Selective phalangeal tuft fractures in a guitar player. Br J Radiol. 1977;50:147-148.
- Vázquez-Osorio I, Espasandín-Arias M, García-Gavín J, et al. Allergic contact dermatitis due to acrylates in acrylic gel nails: a report of 3 cases. Actas Dermosifiliogr. 2014;105:430-432.
- Atashpaz S, Ghabili K. Color triad in guitarist’s fingers: a probable case of Raynaud’s phenomenon due to string vibration phenomenon. Med Probl Perform Art. 2008;23:143.
- Rimmer S, Spielvogel RL. Dermatologic problems of musicians. J Am Acad Dermatol. 1990;22:657-663.
- Adams RM. Skin conditions of musicians. Cutis. 2000;65:37-38.
- Vine K, DeLeo V. Dermatologic manifestations of musicians: a case report and review of skin conditions in musicians. Cutis. 2011;87:117-121.
- Patruno C, Napolitano M, La Bella S, et al. Instrument-related skin disorders in musicians. Dermatitis. 2016;27:26-29.
- Baccouche D, Mokni M, Ben Abdelaziz A, et al. Dermatological problems of musicians: a prospective study in musical students . Article in French. Ann Dermatol Venereol. 2007;134(5 Pt 1):445-449.
- Piraccini BM, Antonucci A, Iorizzo M, et al. Occupational nail fragility in a professional violist. Contact Dermatitis. 2004;51:35-36.
- Wu JJ. Habit tic deformity secondary to guitar playing. Dermatol Online J. 2009;15:16.
- Kluger N. Piano glissando purpura: another cutaneous curiosity in musicians. J Eur Acad Dermatol Venereol. 2016;30:683.
- Alcántara-Nicolás FA, Pastor-Nieto MA, Sánchez-Herreros C, et al. Allergic contact dermatitis from acrylic nails in a flamenco guitarist. Occup Med (Lond). 2016;66:751-753.
- Baran R, Tosti A. Occupational acroosteolysis in a guitar player. Acta Derm Venereol. 1993;73:64-65.
- Destouet JM, Murphy WA. Guitar player acro-osteolysis. Skeletal Radiol. 1981;6:275-277.
- Jepsen JR, Simonsen JA. Raynaud’s phenomenon in a slap bass player: a case report. Med Probl Perform Art. 2016;31:51-53.
- Sirufo MM, Catalogna A, De Pietro F, et al. Raynaud’s phenomenon in a drummer player: microvascular disorder and nailfold video capillaroscopic findings. EXCLI J. 2021;20:1526-1531.
- Sirufo MM, Ginaldi L, De Martinis M. Raynaud’s phenomenon and the nailfold capillaroscopic findings in a guitar player. QJM. 2019;112:531-533.
- Cohen PR. Harpist’s finger: case report of a trauma-induced blister in a beginner harpist and review of string instrument-associated skin problems in musicians. Cutis. 2008;82:329-334.
- De Vasconcelos P, Soares-Almeida L, Filipe P. Subungual keratoacanthoma in a pianist. G Ital Dermatol Venereol. 2016;151:455-456.
- Young RS, Bryk D, Ratner H. Selective phalangeal tuft fractures in a guitar player. Br J Radiol. 1977;50:147-148.
- Vázquez-Osorio I, Espasandín-Arias M, García-Gavín J, et al. Allergic contact dermatitis due to acrylates in acrylic gel nails: a report of 3 cases. Actas Dermosifiliogr. 2014;105:430-432.
- Atashpaz S, Ghabili K. Color triad in guitarist’s fingers: a probable case of Raynaud’s phenomenon due to string vibration phenomenon. Med Probl Perform Art. 2008;23:143.
Practice Points
- Long-term practice and performance with a musical instrument predispose musicians to several skin conditions and nail disorders.
- Nail alterations in musicians include onychodystrophy, callosities of the fingertips, paronychia, distal onycholysis, lamellar onychoschizia, striations, subungual hemorrhage, and occupational Raynaud phenomenon.
- Nail lesions in musicians may be caused by localized pressure, friction-induced mechanical forces, allergic or irritant contact dermatitis, or infections.
Act Fast With Traction Alopecia to Avoid Permanent Hair Loss
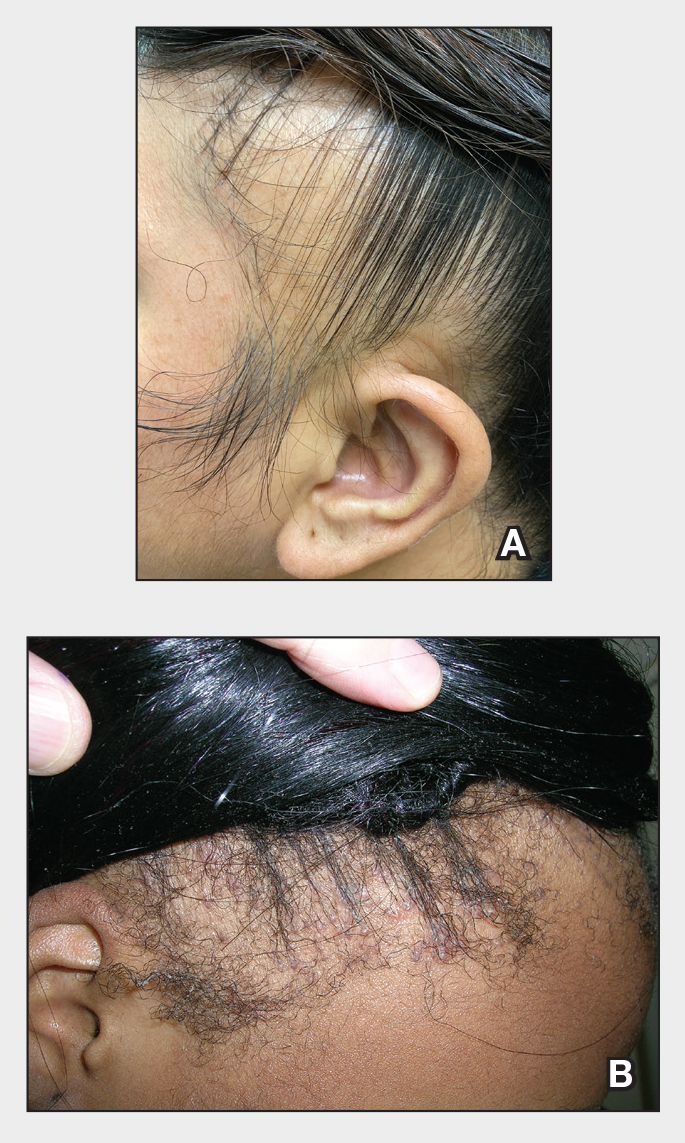
The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296

The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.

The Comparison
Traction alopecia (TA) is a common type of alopecia that ultimately can result in permanent hair loss. It often is caused or worsened by repetitive and prolonged hairstyling practices such as tight ponytails, braids, or locs, or use of wigs or weaves.1 Use of headwear, as in certain religious or ethnic groups, also can be contributory.2 Individuals participating in or training for occupations involving military service or ballet are at risk for TA due to hairstyling-specific policies. Early stages of TA are reversible with proper treatment and avoidance of exacerbating factors, emphasizing the importance of prompt recognition.3
Epidemiology
Data on the true prevalence of TA are lacking. It can occur in individuals of any race or any hair type. However, it is most common in women of African descent, affecting approximately one-third of this population.4 Other commonly affected groups include ballerinas and active-duty service members due to tight ponytails and buns, as well as the Sikh population due to the use of turbans as a part of their religious practice.2,5,6
Traction alopecia also impacts children, particularly those of African descent. A 2007 study of schoolchildren in South Africa determined that more than 17% of young African girls had evidence of TA—even some as young as 6 years of age.7
Traction alopecia can be caused or exacerbated by the use of hair clips and bobby pins that aid holding styles in place.8
Hair shaft morphology may contribute to the risk for TA, with more tightly coiled hair types being more susceptible.8 Variables such as use of chemical relaxers also increase the risk for disease, especially when combined with high-tension styling methods such as braids.9
Key clinical features
Patients with TA clinically present with hair loss and breakage in areas with tension, most commonly the marginal areas of the scalp as well as the frontal hairline and temporal scalp. Hair loss can result in a “fringe sign,” in which a patient may have preservation of a thin line of hairs at the frontal aspect of the hairline with a band of hair loss behind.10 This presentation may be used to differentiate TA from other forms of alopecia, including frontal fibrosing alopecia and female pattern hair loss. When the hair loss is not marginal, it may mimic other forms of patchy hair loss including alopecia areata and trichotillomania. Other clinical findings in TA may include broken hairs, pustules, and follicular papules.10 Patients also may describe symptoms such as scalp tenderness with specific hairstyles or headaches,11 or they may be completely asymptomatic.
Trichoscopy can be helpful in guiding diagnosis and treatment. Patients with TA often have perifollicular erythema and hair casts (cylindrical structures that encircle the proximal hair shafts) in the earlier stages of the disease, with eventual loss of follicular ostia in the later stages.10,12 Hair casts also may indicate ongoing traction.12 The flambeau sign—white tracks seen on trichoscopy in the direction the hair is pulled—resembles a lit torch.13
Worth noting
Early-stage TA can be reversed by avoiding hair tension. However, patients may not be amenable to this due to personal hairstyling preferences, job duties, or religious practices. Treatment with topical or intralesional steroids or even oral antibiotics such as doxycycline for its anti-inflammatory ability may result in regrowth of lost hair if the follicles are not permanently lost and exacerbating factors are avoided.3,14 Both topical and oral minoxidil have been used with success, with minoxidil thought to increase hair density by extending the anagen (growth) phase of hair follicles.3,15 Culturally sensitive patient counseling on the condition and potential exacerbating factors is critical.16
At later stages of the disease—after loss of follicular ostia has occurred—surgical interventions should be considered,17 such as hair transplantation, which can be successful but remains a technical challenge due to variability in hair shaft curvature.18 Additionally, the cost of the procedure can limit use, and some patients may not be optimal candidates due to the extent of their hair loss. Traction alopecia may not be the only hair loss condition present. Examining the scalp is important even if the chief area of concern is the marginal scalp.
Health disparity highlight
Prevention, early identification, and treatment initiated in a timely fashion are crucial to prevent permanent hair loss. There are added societal and cultural pressures that impact hairstyle and hair care practices, especially for those with tightly coiled hair.19 Historically, tightly coiled hair has been unfairly viewed as “unprofessional,” “unkempt,” and a challenge to “manage” by some. Thus, heat, chemical relaxers, and tight hairstyles holding hair in one position have been used to straighten the hair permanently or temporarily or to keep it maintained in a style that did not necessitate excessive manipulation—often contributing to further tension on the hair.
Military service branches have evaluated and changed some hair-related policies to reflect the diverse hair types of military personnel.20 The CROWN Act (www.thecrownact.com/about)—“Creating a Respectful and Open World for Natural Hair”—is a model law passed by 26 states that prohibits race-based hair discrimination, which is the denial of employment and educational opportunities because of hair texture. Although the law has not been passed in every state, it may help individuals with tightly coiled hair to embrace natural hairstyles. However, even hairstyles with one’s own natural curl pattern can contribute to tension and thus potential development of TA.
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
- Larrondo J, McMichael AJ. Traction alopecia. JAMA Dermatol. 2023;159:676. doi:10.1001/jamadermatol.2022.6298
- James J, Saladi RN, Fox JL. Traction alopecia in Sikh male patients. J Am Board Fam Med. 2007;20:497-498. doi:10.3122/jabfm.2007.05.070076
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176.
- Loussouarn G, El Rawadi C, Genain G. Diversity of hair growth profiles. Int J Dermatol. 2005;44(suppl 1):6-9.
- Samrao AChen CZedek Det al. Traction alopecia in a ballerina: clinicopathologic features. Arch Dermatol. 2010;146:918-935. doi:10.1001/archdermatol.2010.183
- Korona-Bailey J, Banaag A, Nguyen DR, et al. Free the bun: prevalence of alopecia among active duty service women, fiscal years 2010-2019. Mil Med. 2023;188:e492-e496. doi:10.1093/milmed/usab274
- Khumalo NP, Jessop S, Gumedze F, et al. Hairdressing is associated with scalp disease in African schoolchildren. Br J Dermatol. 2007;157:106-110. doi:10.1111/j.1365-2133.2007.07987.x
- Billero V, Miteva M. Traction alopecia: the root of the problem. Clin Cosmet Investig Dermatol. 2018;11:149-159. doi:10.2147/CCID.S137296
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
- Kararizou E, Bougea AM, Giotopoulou D, et al. An update on the less-known group of other primary headaches—a review. Eur Neurol Rev. 2014;9:71-77. doi:10.17925/ENR.2014.09.01.71
- Tosti A, Miteva M, Torres F, et al. Hair casts are a dermoscopic clue for the diagnosis of traction alopecia. Br J Dermatol. 2010;163:1353-1355.
- Agrawal S, Daruwalla SB, Dhurat RS. The flambeau sign—a new dermoscopy finding in a case of marginal traction alopecia. Australas J Dermatol. 2020;61:49-50. doi:10. 1111/ajd.13187
- Lawson CN, Hollinger J, Sethi S, et al. Updates in the understanding and treatments of skin & hair disorders in women of color. Int J Womens Dermatol. 2017;3:S21-S37.
- Awad A, Chim I, Sharma P, et al. Low-dose oral minoxidil improves hair density in traction alopecia. J Am Acad Dermatol. 2023;89:157-159. doi:10.1016/j.jaad.2023.02.024
- Grayson C, Heath CR. Counseling about traction alopecia: a “compliment, discuss, and suggest” method. Cutis. 2021;108:20-22.
- Ozçelik D. Extensive traction alopecia attributable to ponytail hairstyle and its treatment with hair transplantation. Aesthetic Plast Surg. 2005;29:325-327. doi:10.1007/s00266-005-0004-5
- Singh MK, Avram MR. Technical considerations for follicular unit extraction in African-American hair. Dermatol Surg. 2013;39:1282-1284. doi:10.1111/dsu.12229
- Jones NL, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships. Pediatr Dermatol. 2021;38(suppl 2):158-160.
- Franklin JMM, Wohltmann WE, Wong EB. From buns to braids and ponytails: entering a new era of female military hair-grooming standards. Cutis. 2021;108:31-35. doi:10.12788/cutis.0296
Transgender and Gender Diverse Health Care in the US Military: What Dermatologists Need to Know
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
People whose gender identity differs from the sex assigned at birth are referred to as transgender. For some, gender identity may not fit into the binary constructs of male and female but rather falls between, within, or outside this construct. These people often consider themselves nonbinary or gender diverse. As the terminology continues to evolve, current recommendations include referring to this patient population as transgender and gender diverse (TGD) to ensure the broadest inclusivity.1 In this article, the following terms are used as defined below:
- The terms transgender woman and trans feminine describe persons who were assigned male gender at birth but their affirmed gender is female or nonmasculine.
- The terms transgender man and trans masculine describe persons who were assigned female gender at birth but their affirmed gender is male or nonfeminine.
The US Military’s policies on the service of TGD persons have evolved considerably over the past decade. Initial military policies barred TGD service members (TSMs) from service all together, leading to challenges in accessing necessary health care. The first official memorandum explicitly allowing military service by TGD persons was released on June 30, 2016.2 The intention of this memorandum was 2-fold: (1) to allow TGD persons to serve in the military so long as they meet “the rigorous standards for military service and readiness” by fulfilling the same standards and procedures as other military service members, including medical fitness for duty, physical fitness, uniform and grooming, deployability, and retention, and (2) to direct the establishment of new or updated policies to specific departments and prescribe procedures for retention standards, separation from service, in-service transition, and medical coverage.2 Several other official policies were released following this initial memorandum that provided more specific guidance on how to implement these policies at the level of the force, unit, and individual service member.
Modifications to the original 2016 policies had varying impacts on transgender health care provision and access.3 At the time of publication of this article, the current policy—the Department of Defense Instruction 1300.284—among others, establishes standards and procedures for the process by which active and reserve TSMs may medically, socially, and legally transition genders within the military. The current policy applies to all military branches and serves as the framework by which each branch currently organizes their gender-affirmation processes (GAP).4
There currently are several different GAP models among the military branches.5 Each branch has a different model or approach to implementing the current policy, with varying service-specific processes in place for TSMs to access gender-affirming care; however, this may be changing. The Defense Health Agency is in the process of consolidating and streamlining the GAP across the Department of Defense branches in an effort to optimize costs and ensure uniformity of care. Per the Defense Health Agency Procedural Instruction Number 6025.21 published in May 2023, the proposed consolidated model likely will entail a single central transgender health center that provides oversight and guidance for several regional joint-service gender-affirming medical hubs. Patients would either be managed at the level of the hub or be referred to the central site.5
Herein, we discuss the importance of gender-affirming care and how military and civilian dermatologists can contribute. We also review disparities in health care and identify areas of improvement.
Benefits of Gender-Affirming Care
Gender-affirming procedures are critical for aligning physical appearance with gender identity. Physical appearance is essential for psychological well-being, operational readiness, and the safety of TSMs.6 It is well documented that TGD persons experience suicidal ideation, depression, stigma, discrimination and violence at higher rates than their cisgender peers.7,8 It is important to recognize that transgender identity is not a mental illness, and these elevated rates have been linked to complex trauma, societal stigma, violence, and discrimination.1 Other studies have suggested that increased access to gender-affirming interventions may ameliorate these mental health concerns.1,7-9
The major components of gender-affirming care include hormone therapy, gender confirmation surgery, and mental health care, if needed. These are covered by TRICARE, the health care program for military service members; however, at the time of publication, many of the dermatologic gender-affirming procedures are not covered by TRICARE because they are considered “cosmetic procedures,” which is a term used by insurance companies but does not accurately indicate whether a procedure is medically necessary or not. Newer literature has demonstrated that gender-affirming care positively affects the lives of TGD patients, strengthening the argument that gender-affirming care is a medical necessity and not just cosmetic.1
Aesthetic Procedures in Gender-Affirming Care
Surgeons, including those within the specialties of oto-laryngology, oral and maxillofacial surgery, urology, gynecology, and plastic surgery, provide major gender-affirming interventions; however, dermatologists may offer less invasive solutions that can serve as a temporary experience prior to undergoing more permanent procedures.Hormonally driven disorders including acne, hair loss, and melasma also are managed by dermatologists, along with scar treatment following surgeries.
Because human variation is expansive and subjective, what is considered feminine or masculine may vary by person, group, culture, and country; therefore, it is imperative to ask patients about their individual aesthetic goals and tailor their treatment accordingly. Feminine and masculine are terms that will be used to describe prototypical appearances and are not meant to define a patient’s current state or ultimate goals. The following procedures and medical interventions are where dermatologists can play an important role in TGD persons’ GAPs.
Botulinum Toxin Injections—Botulinum toxin injection is the most common nonsurgical aesthetic procedure performed around the world.10 The selective paralysis afforded by botulinum toxin has several uses for people undergoing transition. Aesthetically, the feminine eyebrow tends to be positioned above the orbital rim and is arched with its apex between the lateral limbus and lateral canthus,11 while the masculine eyebrow tends to be flatter and fuller and runs over the orbital rim without a peak. For people seeking a more feminine appearance, an eyebrow lift with botulinum toxin can help reshape the typical flatter masculine eyebrow to give it lateral lift that often is considered more feminine. The targeted muscle is the superolateral orbicularis oculi, which serves as a depressor on the eyebrow. This can be combined with purposefully avoiding total lateral frontalis paralysis, which leads to a “Spock” brow for extra lift. Conversely, a naturally arched and higher eyebrow can be flattened and lowered by selectively targeting areas of the frontalis muscle.
Broad square jawlines typically are considered a masculine feature and are another area where botulinum toxin can be used to feminize a patient’s facial features. Targeting the masseter muscle induces muscle weakness, which ultimately may result in atrophy after one or more treatment sessions. This atrophy may lead to narrowing of the lower face and thus may lead to a fuller-appearing midface or overall more heart-shaped face. Every individual’s aesthetic goals are unique and therefore should be discussed prior to any treatment.
Dermal Fillers—Dermal fillers are gel-like substances injected under the skin for subtle contouring of the face. Fillers also can be used to help promote a more masculine or feminine appearance. Filler can be placed in the lips to create a fuller, more projected, feminine-appearing lip. Malar cheek and central lower chin filler can be used to help define a heart-shaped face by accentuating the upper portion of the face and creating a more pointed chin, respectively. Alternatively, filler can be used to masculinize the chin by placing it where it can increase jawline squareness and increase anterior jaw projection. Additionally, filler at the angle of the jaw can help accentuate a square facial shape and a more defined jawline. Although not as widely practiced, lateral brow filler can create a heavier-appearing and broader forehead for a more masculine appearance. These procedures can be combined with the previously mentioned botulinum toxin procedures for a synergistic effect.
Deoxycholic Acid—Deoxycholic acid is an injectable product used to selectively remove unwanted fat. It currently is approved by the US Food and Drug Administration for submental fat, but some providers are experimenting with off-label uses. Buccal fat pad removal—or in this case reduction by dissolution—tends to give a thinner, more feminine facial appearance.12 Reducing fat around the axillae also can help promote a more masculine upper torso.13 The safety of deoxycholic acid in these areas has not been adequately tested; thus, caution should be used when discussing these off-label uses with patients.
Hair and Tattoo Removal—Hair removal may be desired by TGD persons for a variety of reasons. Because cisgender females tend to have less body hair overall, transgender people in pursuit of a more feminine appearance often desire removal of facial, neck, and body hair. Although shaving and other modalities such as waxing and chemical depilatories are readily available at-home options, they are not permanent and may lead to folliculitis or pseudofolliculitis barbae. Laser hair removal (LHR) and electrolysis are modalities provided by dermatologists that tend to be more permanent and lead to better outcomes, including less irritation and better aesthetic appearance. It is important to keep in mind that not every person and not every body site can be safely treated with LHR. Patients with lighter skin types and darker hair tend to have the most effective response with a higher margin of safety, as these features allow the laser energy to be selectively absorbed by the melanin in the hair bulb and not by the background skin pigmentation.14,15 Inappropriate patient selection or improper settings for wavelength, pulse width, or fluences can lead to burns and permanent scarring.14,15 Electrolysis is an alternative to hair removal within tattoos and is more effective for those individuals with blonde, red, or white hair.16
Another novel treatment for unwanted hair is eflornithine hydrochloride cream, which works by blocking ornithine decarboxylase, the enzyme that stimulates hair growth. It currently is approved to reduce unwanted hair on the face and adjacent areas under the chin; however the effects of this medication are modest and the medication can be expensive.17
Cosmetic hair and tattoo removal are not currently covered by TRICARE, except in cases of surgical and donor-site preparation for some GAPs. Individuals may desire removal of tattoos at surgery sites to obtain more natural-appearing skin. Currently, GAPs such as vaginoplasty, phalloplasty, and metoidioplasty—often referred to by patients as “bottom surgeries”—include insurance coverage for tattoo removal, LHR, and/or electrolysis.
Management of Hormonal Adverse Effects
Acne—Individuals on testosterone supplementation tend to develop acne for the first several years of treatment, but it may improve with time.18 Acne is treated in individuals receiving testosterone the same way as it is treated in cisgender men, with numerous options for topical and oral medications. In trans masculine persons, spironolactone therapy typically is avoided because it may interfere with the actions of exogenous testosterone administered as part of gender-affirming medical treatment and may lead to other undesired adverse effects such as impotence and gynecomastia.1
Although acne typically improves after starting estrogen therapy, patients receiving estrogens may still develop acne. Most trans feminine patients will already be on an estrogen and an antiandrogen, often spironolactone.1 Spironolactone often is used as monotherapy for acne control in cisgender women. Additionally, an important factor to consider with spironolactone is the possible adverse effect of increased micturition. Currently, the military rarely has gender-inclusive restroom options, which can create a challenge for TSMs who find themselves needing to use the restroom more frequently in the workplace.
If planning therapy with isotretinoin, dermatologists should discuss several important factors with all patients, including TGD patients. One consideration is the patient’s planned future surgeries. Although new literature shows that isotretinoin does not adversely affect wound healing,19 some surgeons still adhere to an isotretinoin washout period of 6 to 12 months prior to performing any elective procedures due to concerns about wound healing.20,21 Second, be sure to properly assess and document pregnancy potential in TGD persons. Providers should not assume that a patient is not pregnant or is not trying to become pregnant just because they are trans masculine. It also is important to note that testosterone is not a reliable birth control method.1 If a patient still has ovaries, fallopian tubes, and a uterus, they are considered medically capable of pregnancy, and providers should keep this in mind regarding all procedures in the TGD population.
Another newer acne treatment modality is the 1762-nm laser, which targets sebaceous glands.22 This device allows for targeted treatment of acne-prone areas without systemic therapy such as retinoids or antiandrogens. The 1762-nm laser is not widely available but may become a regular treatment option once its benefits are proven over time.
Alopecia and Hyperpigmentation—Androgens, whether endogenously or exogenously derived, can lead to androgenetic alopecia (AGA) in genetically susceptible individuals. Trans masculine persons and others receiving androgen therapy are at higher risk for AGA, which often is undesirable and may be considered gender affirming by some TGD persons. Standard AGA treatments for cisgender men also can be used in trans masculine persons. Some of the most common anti-AGA medications are topical minoxidil, oral finasteride, and oral minoxidil. Although Coleman et al1 recently reported that finasteride may be an appropriate treatment option in trans masculine persons experiencing alopecia, treatment with 5α-reductase inhibitors may impair clitoral growth and the development of facial and body hair. Further studies are needed to assess the efficacy and safety of 5α-reductase inhibitors in transgender populations.1 Dutasteride may be used off-label and comes with a similar potential adverse-event profile as finasteride, which includes depression, decreased libido, erectile dysfunction, ejaculation disorders, and gynecomastia.
Conversely, AGA tends to improve in trans feminine persons and others receiving estrogen and antiandrogen therapy. Natural testosterone production is suppressed by estrogens and spironolactone as well as in patients who undergo orchiectomy.1 Although spironolactone is not approved for acne, AGA, or hirsutism, it is a standard treatment of AGA in cisgender women because it functions to block the effects of androgens, including at the hair follicle. Finasteride may be used for AGA in cisgender women but it is not recommended for trans feminine persons.1
There are many other modalities available for the treatment of AGA that are less commonly used—some may be cost prohibitive or do not have robust supporting evidence, or both. One example is hair
Melasma is a hyperpigmentation disorder related to estrogens, UV light exposure, and sometimes medication use (eg, hormonal birth control, spironolactone).24 The mainstay of treatment is prevention, including sun avoidance as well as use of sun-protective clothing and broad-spectrum sunscreens. Dermatologists tend to recommend physical sunscreens containing zinc oxide, titanium dioxide, and/or iron oxide, as they cover a wider UV spectrum and also provide some protection from visible light. Once melasma is present, dermatologists still have several treatment options. Topical hydroquinone is a proven treatment; however, it must be used with caution to avoid ochronosis. With careful patient selection, chemical peels also are effective treatment options for dyspigmentation and hyperpigmentation. Energy devices such as intense pulsed light and tattoo removal lasers—Q-switched lasers and picosecond pulse widths—also can be used to treat hyperpigmentation. Oral, intralesional, and topical tranexamic acid are newer treatment options for melasma that still are being studied and have shown promising results. Further studies are needed to determine long-term safety and optimal treatment regimens.24,25
Many insurance carriers, including TRICARE, do not routinely cover medical management of AGA or melasma. Patients should be advised that they likely will have to pay for any medications prescribed and procedures undertaken for these purposes; however, some medication costs can be offset by ordering larger prescription quantities, such as a 90-day supply vs a 30-day supply, as well as utilizing pharmacy discount programs.
Scar Management Following Surgery
In TSMs who undergo gender-affirming surgeries, dermatologists play an important role when scar symptoms develop, including pruritus, tenderness, and/or paresthesia. In the military, some common treatment modalities for symptomatic scars include intralesional steroids with or without 5-fluouroruacil and the fractionated CO2 laser. There also are numerous experimental treatment options for scars, including intralesional or perilesional botulinum toxin, the pulsed dye laser, or nonablative fractionated lasers. These modalities also may be used on hypertrophic scars or keloids. Another option for keloids is scar excision followed by superficial radiation therapy.26
Mental Health Considerations
Providers must take psychological adverse effects into consideration when considering medical therapies for dermatologic conditions in TGD patients. In particular, it is important to consider the risks for increased rates of depression and suicidal ideation formerly associated with the use of isotretinoin and finasteride, though much of the evidence regarding these risks has been called into question in recent years.27,28 Nonetheless, it remains prominent in lay media and may be a more important consideration in patients at higher baseline risk.27 Although there are no known studies that have expressly assessed rates of depression or suicidal ideation in TGD patients taking isotretinoin or finasteride, it is well established that TGD persons are at higher baseline risk for depression and suicidality.1,7,8 All patients should be carefully assessed for depression and suicidal ideation as well as counseled regarding these risks prior to initiating these therapies. If concerns for untreated mental health issues arise during screening and counseling, patients should be referred for assessment by a behavioral health specialist prior to starting therapy.
Future Directions
The future of TGD health care in the military could see an expansion of covered benefits and the development of new dermatologic procedures or medications. Research and policy evolution are necessary to bridge the current gaps in care; however, it is unlikely that all procedures currently considered to be cosmetic will become covered benefits.
Facial LHR is a promising candidate for future coverage for trans feminine persons. When cisgender men develop adverse effects from mandatory daily shaving, LHR is already a covered benefit. Two arguments in support of adding LHR for TGD patients revolve around achieving and maintaining an appearance congruent with their gender along with avoiding unwanted adverse effects related to daily shaving. Visual conformity with one’s affirmed gender has been associated with improvements in well-being, quality of life, and some mental health conditions.29
Scar prevention, treatment, and reduction are additional areas under active research in which dermatologists likely will play a crucial role.30,31 As more dermatologic procedures are performed on TGD persons, the published data and collective knowledge regarding best practices in this population will continue to grow, which will lead to improved cosmetic and safety outcomes.
Final Thoughts
Although dermatologists do not directly perform gender-affirming surgeries or hormone management, they do play an important role in enhancing a TGD person’s desired appearance and managing possible adverse effects resulting from gender-affirming interventions. There have been considerable advancements in TGD health care over the past decade, but there likely are more changes on the way. As policies and understanding of TGD health care needs evolve, it is crucial that the military health care system adapts to provide comprehensive, accessible, and equitable care, which includes expanding the range of covered dermatologic treatments to fully support the health and readiness of TSMs.
Acknowledgment—We would like to extend our sincere appreciation to the invaluable contributions and editorial support provided by Allison Higgins, JD (San Antonio, Texas), throughout the writing of this article.
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
- Coleman E, Radix AE, Bouman WP, et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgend Health. 2022;23(suppl 1):S1-S260. doi:10.1080/26895269.2022.2100644
- Secretary of Defense. DTM 16-005—military service of transgender service members. June 30, 2016. Accessed June 17, 2024. https://dod.defense.gov/Portals/1/features/2016/0616_policy/DTM-16-005.pdf
- Office of the Deputy Secretary of Defense. DTM 19-004—military service by transgender persons and persons with gender dysphoria. March 17, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/03/17/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Office of the Under Secretary of Defense for Personnel and Readiness. Department of Defense Instruction (DODI) 1300.28. in-service transition for transgender service members. September 4, 2020. Accessed June 17, 2024. https://health.mil/Reference-Center/Policies/2020/09/04/Military-Service-by-Transgender-Persons-and-Persons-with-Gender-Dysphoria
- Defense Health Agency Procedural Instruction Number 6025.21, Guidance for Gender-Affirming Health Care of Transgender and Gender-Diverse Active and Reserve Component Service Members, May 12, 2023. https://www.health.mil/Reference-Center/DHA-Publications/2023/05/12/DHA-PI-6015-21
- Elders MJ, Brown GR, Coleman E, et al. Medical aspects of transgender military service. Armed Forces Soc. 2015;41:199-220. doi:10.1177/0095327X14545625.
- Almazan AN, Keuroghlian AS. Association between gender-affirming surgeries and mental health outcomes. JAMA Surg. 2021;156:611-618.
- Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:E220978. doi:10.1001/jamanetworkopen.2022.0978
- Olson-Kennedy J, Warus J, Okonta V, et al. Chest reconstruction and chest dysphoria in transmasculine minors and young adults: comparisons of nonsurgical and postsurgical cohorts. JAMA Pediatr. 2018;172:431-436. doi:10.1001/jamapediatrics.2017.5440
- Top non-invasive cosmetic procedures worldwide 2022. Statista website. February 8, 2024. Accessed June 13, 2024. https://www.statista.com/statistics/293449/leading-nonsurgical-cosmetic-procedures/
- Kashkouli MB, Abdolalizadeh P, Abolfathzadeh N, et al. Periorbital facial rejuvenation; applied anatomy and pre-operative assessment. J Curr Ophthalmol. 2017;29:154-168. doi:10.1016/j.joco.2017.04.001
- Thomas MK, D’Silva JA, Borole AJ. Injection lipolysis: a systematic review of literature and our experience with a combination of phosphatidylcholine and deoxycholate over a period of 14 years in 1269 patients of Indian and South East Asian origin. J Cutan Aesthet Surg. 2018;11:222-228. doi:10.4103/JCAS.JCAS_117_18
- Jegasothy SM. Deoxycholic acid injections for bra-line lipolysis. Dermatol Surg. 2018;44:757-760. doi:10.1097/DSS.0000000000001311
- Dierickx CC. Hair removal by lasers and intense pulsed light sources. Dermatol Clin. 2002;20:135-146. doi:10.1016/s0733-8635(03)00052-4
- Lepselter J, Elman M. Biological and clinical aspects in laser hair removal. J Dermatolog Treat. 2004;15:72-83. doi:10.1080/09546630310023152
- Yuan N, Feldman AT, Chin P, et al. Comparison of permanent hair removal procedures before gender-affirming vaginoplasty: why we should consider laser hair removal as a first-line treatment for patients who meet criteria. Sex Med. 2022;10:100545. doi:10.1016/j.esxm.2022.100545
- Kumar A, Naguib YW, Shi YC, et al. A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Deliv. 2016;23:1495-1501. doi:10.3109/10717544.2014.951746
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metabol. 2017;102:3869-3903.
- Hatami P, Balighi K, Asl HN, et al. Isotretinoin and timing of procedural interventions: clinical implications and practical points. J Cosmet Dermatol. 2023;22:2146-2149. doi:10.1111/jocd.15874
- Rubenstein R, Roenigk HH Jr, Stegman SJ, et al. Atypical keloids after dermabrasion of patients taking isotretinoin. J Am Acad Dermatol. 1986;15(2 pt 1):280-285.
- Zachariae H. Delayed wound healing and keloid formation following argon laser treatment or dermabrasion during isotretinoin treatment. Br J Dermatol. 1988;118:703-706.
- Goldberg D, Kothare A, Doucette M, et al. Selective photothermolysis with a novel 1726 nm laser beam: a safe and effective solution for acne vulgaris. J Cosmet Dermatol. 2023;22:486-496. doi:10.1111/jocd.15602
- Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45:913-914. doi:10.1111/ced.14292
- Bolognia JL, Schaffer JV, Cerroni L. Dermatology: 2-Volume Set. Elsevier; 2024:1130.
- Konisky H, Balazic E, Jaller JA, et al. Tranexamic acid in melasma: a focused review on drug administration routes. J Cosmet Dermatol. 2023;22:1197-1206. doi:10.1111/jocd.15589
- Walsh LA, Wu E, Pontes D, et al. Keloid treatments: an evidence-based systematic review of recent advances. Syst Rev. 2023;12:42. doi:10.1186/s13643-023-02192-7
- Kridin K, Ludwig RJ. Isotretinoin and the risk of psychiatric disturbances: a global study shedding new light on a debatable story. J Am Acad Dermatol. 2023;88:388-394. doi:10.1016/j.jaad.2022.10.031
- Dyson TE, Cantrell MA, Lund BC. Lack of association between 5α-reductase inhibitors and depression. J Urol. 2020;204:793-798. doi:10.1097/JU.0000000000001079
- To M, Zhang Q, Bradlyn A, et al. Visual conformity with affirmed gender or “passing”: its distribution and association with depression and anxiety in a cohort of transgender people. J Sex Med. 2020;17:2084-2092. doi:10.1016/j.jsxm.2020.07.019
- Fernandes MG, da Silva LP, Cerqueira MT, et al. Mechanomodulatory biomaterials prospects in scar prevention and treatment. Acta Biomater. 2022;150:22-33. doi:10.1016/j.actbio.2022.07.042
- Kolli H, Moy RL. Prevention of scarring with intraoperative erbium:YAG laser treatment. J Drugs Dermatol. 2020;19:1040-1043. doi:10.36849/JDD.2020.5244
Practice Points
- Transgender and gender diverse (TGD) health care is multidisciplinary, and both military and civilian dermatologists can serve an important role.
- Although dermatologists do not directly perform gender-affirming surgeries or hormone management, there are a number of dermatologic procedures and medical interventions that can enhance a TGD person’s desired appearance.
- Dermatologists also can help manage possible adverse effects from gender-affirming interventions.
How Media Coverage of Oral Minoxidil for Hair Loss Has Impacted Prescribing Habits
Minoxidil, a potent vasodilator, was approved by the US Food and Drug Administration (FDA) in 1963 to treat high blood pressure. Its application as a hair loss treatment was discovered by accident—patients taking oral minoxidil for blood pressure noticed hair growth on their bodies as a side effect of the medication. In 1988, topical minoxidil (Rogaine [Johnson & Johnson Consumer Inc]) was approved by the FDA for the treatment of androgenetic alopecia in men, and then it was approved for the same indication in women in 1991. The mechanism of action by which minoxidil increases hair growth still has not been fully elucidated. When applied topically, it is thought to extend the anagen phase (or growth phase) of the hair cycle and increase hair follicle size. It also increases oxygen to the hair follicle through vasodilation and stimulates the production of vascular endothelial growth factor, which is thought to promote hair growth.1 Since its approval, topical minoxidil has become a first-line treatment of androgenetic alopecia in men and women.
In August 2022, The New York Times (NYT) published an article on dermatologists’ use of oral minoxidil at a fraction of the dose prescribed for blood pressure with profound results in hair regrowth.2 Several dermatologists quoted in the article endorsed that the decreased dose minimizes unwanted side effects such as hypertrichosis, hypotension, and other cardiac issues while still being effective for hair loss. Also, compared to topical minoxidil, low-dose oral minoxidil (LDOM) is relatively cheaper and easier to use; topicals are more cumbersome to apply and often leave the hair and scalp sticky, leading to noncompliance among patients.2 Currently, oral minoxidil is not approved by the FDA for use in hair loss, making it an off-label use.
Since the NYT article was published, we have observed an increase in patient questions and requests for LDOM as well as heightened use by fellow dermatologists in our community. As of November 2022, the NYT had approximately 9,330,000 total subscribers, solidifying its place as a newspaper of record in the United States and across the world.3 In April 2023, we conducted a survey of US-based board-certified dermatologists to investigate the impact of the NYT article on prescribing practices of LDOM for alopecia. The survey was conducted as a poll in a Facebook group for board-certified dermatologists and asked, “How did the NYT article on oral minoxidil for alopecia change your utilization of LDOM (low-dose oral minoxidil) for alopecia?” Three answer choices were given: (1) I started Rx’ing LDOM or increased the number of patients I manage with LDOM; (2) No change. I never Rx’d LDOM and/or no increase in utilization; and (3) I was already prescribing LDOM.
Of the 65 total respondents, 27 (42%) reported that the NYT article influenced their decision to start prescribing LDOM for alopecia. Nine respondents (14%) reported that the article did not influence their prescribing habits, and 27 (42%) responded that they were already prescribing the medication prior to the article’s publication.
Data from Epiphany Dermatology, a practice with more than 70 locations throughout the United States, showed that oral minoxidil was prescribed for alopecia 107 times in 2020 and 672 times in 2021 (Amy Hadley, Epiphany Dermatology, written communication, March 24, 2023). In 2022, prescriptions increased exponentially to 1626, and in the period of January 2023 to March 2023 alone, oral minoxidil was prescribed 510 times. Following publication of the NYT article in August 2022, LDOM was prescribed a total of 1377 times in the next 8 months.
Moreover, data from Summit Pharmacy, a retail pharmacy in Centennial, Colorado, showed an 1800% increase in LDOM prescriptions in the 7 months following the NYT article’s publication (August 2022 to March 2023) compared with the 7 months prior (January 2022 to August 2022)(Brandon Johnson, Summit Pharmacy, written communication, March 30, 2023). These data provide evidence for the influence of the NYT article on prescribing habits of dermatology providers in the United States.
The safety of oral minoxidil for use in hair loss has been established through several studies in the literature.4,5 These results show that LDOM may be a safe, readily accessible, and revolutionary treatment for hair loss. A retrospective multicenter study of 1404 patients treated with LDOM for any type of alopecia found that side effects were infrequent, and only 1.7% of patients discontinued treatment due to adverse effects. The most frequent adverse effect was hypertrichosis, occurring in 15.1% of patients but leading to treatment withdrawal in only 0.5% of patients.4 Similarly, Randolph and Tosti5 found that hypertrichosis of the face and body was the most common adverse effect observed, though it rarely resulted in discontinuation and likely was dose dependent: less than 10% of patients receiving 0.25 mg/d experienced hypertrichosis compared with more than 50% of those receiving 5 mg/d (N=634). They also described patients in whom topical minoxidil, though effective, posed major barriers to compliance due to the twice-daily application, changes to hair texture from the medication, and scalp irritation. A literature review of 17 studies with 634 patients on LDOM as a primary treatment for hair loss found that it was an effective, well-tolerated treatment and should be considered for healthy patients who have difficulty with topical formulations.5
In the age of media with data constantly at users’ fingertips, the art of practicing medicine also has changed. Although physicians pride themselves on evidence-based medicine, it appears that an NYT article had an impact on how physicians, particularly dermatologists, prescribe oral minoxidil. However, it is difficult to know if the article exposed dermatologists to another treatment in their armamentarium for hair loss or if it influenced patients to ask their health care provider about LDOM for hair loss. One thing is clear—since the article’s publication, the off-label use of LDOM for alopecia has produced what many may call “miracles” for patients with hair loss.5
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j.1365-2133.2004.05785.x
- Kolata G. An old medicine grows new hair for pennies a day, doctors say. The New York Times. August 18, 2022. Accessed May 20, 2024. https://www.nytimes.com/2022/08/18/health/minoxidil-hair-loss-pills.html
- The New York Times Company reports third-quarter 2022 results. Press release. The New York Times Company; November 2, 2022. Accessed May 20, 2024. https://nytco-assets.nytimes.com/2022/11/NYT-Press-Release-Q3-2022-Final-nM7GzWGr.pdf
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84:1644-1651. doi:10.1016/j.jaad.2021.02.054
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746. doi:10.1016/j.jaad.2020.06.1009
Minoxidil, a potent vasodilator, was approved by the US Food and Drug Administration (FDA) in 1963 to treat high blood pressure. Its application as a hair loss treatment was discovered by accident—patients taking oral minoxidil for blood pressure noticed hair growth on their bodies as a side effect of the medication. In 1988, topical minoxidil (Rogaine [Johnson & Johnson Consumer Inc]) was approved by the FDA for the treatment of androgenetic alopecia in men, and then it was approved for the same indication in women in 1991. The mechanism of action by which minoxidil increases hair growth still has not been fully elucidated. When applied topically, it is thought to extend the anagen phase (or growth phase) of the hair cycle and increase hair follicle size. It also increases oxygen to the hair follicle through vasodilation and stimulates the production of vascular endothelial growth factor, which is thought to promote hair growth.1 Since its approval, topical minoxidil has become a first-line treatment of androgenetic alopecia in men and women.
In August 2022, The New York Times (NYT) published an article on dermatologists’ use of oral minoxidil at a fraction of the dose prescribed for blood pressure with profound results in hair regrowth.2 Several dermatologists quoted in the article endorsed that the decreased dose minimizes unwanted side effects such as hypertrichosis, hypotension, and other cardiac issues while still being effective for hair loss. Also, compared to topical minoxidil, low-dose oral minoxidil (LDOM) is relatively cheaper and easier to use; topicals are more cumbersome to apply and often leave the hair and scalp sticky, leading to noncompliance among patients.2 Currently, oral minoxidil is not approved by the FDA for use in hair loss, making it an off-label use.
Since the NYT article was published, we have observed an increase in patient questions and requests for LDOM as well as heightened use by fellow dermatologists in our community. As of November 2022, the NYT had approximately 9,330,000 total subscribers, solidifying its place as a newspaper of record in the United States and across the world.3 In April 2023, we conducted a survey of US-based board-certified dermatologists to investigate the impact of the NYT article on prescribing practices of LDOM for alopecia. The survey was conducted as a poll in a Facebook group for board-certified dermatologists and asked, “How did the NYT article on oral minoxidil for alopecia change your utilization of LDOM (low-dose oral minoxidil) for alopecia?” Three answer choices were given: (1) I started Rx’ing LDOM or increased the number of patients I manage with LDOM; (2) No change. I never Rx’d LDOM and/or no increase in utilization; and (3) I was already prescribing LDOM.
Of the 65 total respondents, 27 (42%) reported that the NYT article influenced their decision to start prescribing LDOM for alopecia. Nine respondents (14%) reported that the article did not influence their prescribing habits, and 27 (42%) responded that they were already prescribing the medication prior to the article’s publication.
Data from Epiphany Dermatology, a practice with more than 70 locations throughout the United States, showed that oral minoxidil was prescribed for alopecia 107 times in 2020 and 672 times in 2021 (Amy Hadley, Epiphany Dermatology, written communication, March 24, 2023). In 2022, prescriptions increased exponentially to 1626, and in the period of January 2023 to March 2023 alone, oral minoxidil was prescribed 510 times. Following publication of the NYT article in August 2022, LDOM was prescribed a total of 1377 times in the next 8 months.
Moreover, data from Summit Pharmacy, a retail pharmacy in Centennial, Colorado, showed an 1800% increase in LDOM prescriptions in the 7 months following the NYT article’s publication (August 2022 to March 2023) compared with the 7 months prior (January 2022 to August 2022)(Brandon Johnson, Summit Pharmacy, written communication, March 30, 2023). These data provide evidence for the influence of the NYT article on prescribing habits of dermatology providers in the United States.
The safety of oral minoxidil for use in hair loss has been established through several studies in the literature.4,5 These results show that LDOM may be a safe, readily accessible, and revolutionary treatment for hair loss. A retrospective multicenter study of 1404 patients treated with LDOM for any type of alopecia found that side effects were infrequent, and only 1.7% of patients discontinued treatment due to adverse effects. The most frequent adverse effect was hypertrichosis, occurring in 15.1% of patients but leading to treatment withdrawal in only 0.5% of patients.4 Similarly, Randolph and Tosti5 found that hypertrichosis of the face and body was the most common adverse effect observed, though it rarely resulted in discontinuation and likely was dose dependent: less than 10% of patients receiving 0.25 mg/d experienced hypertrichosis compared with more than 50% of those receiving 5 mg/d (N=634). They also described patients in whom topical minoxidil, though effective, posed major barriers to compliance due to the twice-daily application, changes to hair texture from the medication, and scalp irritation. A literature review of 17 studies with 634 patients on LDOM as a primary treatment for hair loss found that it was an effective, well-tolerated treatment and should be considered for healthy patients who have difficulty with topical formulations.5
In the age of media with data constantly at users’ fingertips, the art of practicing medicine also has changed. Although physicians pride themselves on evidence-based medicine, it appears that an NYT article had an impact on how physicians, particularly dermatologists, prescribe oral minoxidil. However, it is difficult to know if the article exposed dermatologists to another treatment in their armamentarium for hair loss or if it influenced patients to ask their health care provider about LDOM for hair loss. One thing is clear—since the article’s publication, the off-label use of LDOM for alopecia has produced what many may call “miracles” for patients with hair loss.5
Minoxidil, a potent vasodilator, was approved by the US Food and Drug Administration (FDA) in 1963 to treat high blood pressure. Its application as a hair loss treatment was discovered by accident—patients taking oral minoxidil for blood pressure noticed hair growth on their bodies as a side effect of the medication. In 1988, topical minoxidil (Rogaine [Johnson & Johnson Consumer Inc]) was approved by the FDA for the treatment of androgenetic alopecia in men, and then it was approved for the same indication in women in 1991. The mechanism of action by which minoxidil increases hair growth still has not been fully elucidated. When applied topically, it is thought to extend the anagen phase (or growth phase) of the hair cycle and increase hair follicle size. It also increases oxygen to the hair follicle through vasodilation and stimulates the production of vascular endothelial growth factor, which is thought to promote hair growth.1 Since its approval, topical minoxidil has become a first-line treatment of androgenetic alopecia in men and women.
In August 2022, The New York Times (NYT) published an article on dermatologists’ use of oral minoxidil at a fraction of the dose prescribed for blood pressure with profound results in hair regrowth.2 Several dermatologists quoted in the article endorsed that the decreased dose minimizes unwanted side effects such as hypertrichosis, hypotension, and other cardiac issues while still being effective for hair loss. Also, compared to topical minoxidil, low-dose oral minoxidil (LDOM) is relatively cheaper and easier to use; topicals are more cumbersome to apply and often leave the hair and scalp sticky, leading to noncompliance among patients.2 Currently, oral minoxidil is not approved by the FDA for use in hair loss, making it an off-label use.
Since the NYT article was published, we have observed an increase in patient questions and requests for LDOM as well as heightened use by fellow dermatologists in our community. As of November 2022, the NYT had approximately 9,330,000 total subscribers, solidifying its place as a newspaper of record in the United States and across the world.3 In April 2023, we conducted a survey of US-based board-certified dermatologists to investigate the impact of the NYT article on prescribing practices of LDOM for alopecia. The survey was conducted as a poll in a Facebook group for board-certified dermatologists and asked, “How did the NYT article on oral minoxidil for alopecia change your utilization of LDOM (low-dose oral minoxidil) for alopecia?” Three answer choices were given: (1) I started Rx’ing LDOM or increased the number of patients I manage with LDOM; (2) No change. I never Rx’d LDOM and/or no increase in utilization; and (3) I was already prescribing LDOM.
Of the 65 total respondents, 27 (42%) reported that the NYT article influenced their decision to start prescribing LDOM for alopecia. Nine respondents (14%) reported that the article did not influence their prescribing habits, and 27 (42%) responded that they were already prescribing the medication prior to the article’s publication.
Data from Epiphany Dermatology, a practice with more than 70 locations throughout the United States, showed that oral minoxidil was prescribed for alopecia 107 times in 2020 and 672 times in 2021 (Amy Hadley, Epiphany Dermatology, written communication, March 24, 2023). In 2022, prescriptions increased exponentially to 1626, and in the period of January 2023 to March 2023 alone, oral minoxidil was prescribed 510 times. Following publication of the NYT article in August 2022, LDOM was prescribed a total of 1377 times in the next 8 months.
Moreover, data from Summit Pharmacy, a retail pharmacy in Centennial, Colorado, showed an 1800% increase in LDOM prescriptions in the 7 months following the NYT article’s publication (August 2022 to March 2023) compared with the 7 months prior (January 2022 to August 2022)(Brandon Johnson, Summit Pharmacy, written communication, March 30, 2023). These data provide evidence for the influence of the NYT article on prescribing habits of dermatology providers in the United States.
The safety of oral minoxidil for use in hair loss has been established through several studies in the literature.4,5 These results show that LDOM may be a safe, readily accessible, and revolutionary treatment for hair loss. A retrospective multicenter study of 1404 patients treated with LDOM for any type of alopecia found that side effects were infrequent, and only 1.7% of patients discontinued treatment due to adverse effects. The most frequent adverse effect was hypertrichosis, occurring in 15.1% of patients but leading to treatment withdrawal in only 0.5% of patients.4 Similarly, Randolph and Tosti5 found that hypertrichosis of the face and body was the most common adverse effect observed, though it rarely resulted in discontinuation and likely was dose dependent: less than 10% of patients receiving 0.25 mg/d experienced hypertrichosis compared with more than 50% of those receiving 5 mg/d (N=634). They also described patients in whom topical minoxidil, though effective, posed major barriers to compliance due to the twice-daily application, changes to hair texture from the medication, and scalp irritation. A literature review of 17 studies with 634 patients on LDOM as a primary treatment for hair loss found that it was an effective, well-tolerated treatment and should be considered for healthy patients who have difficulty with topical formulations.5
In the age of media with data constantly at users’ fingertips, the art of practicing medicine also has changed. Although physicians pride themselves on evidence-based medicine, it appears that an NYT article had an impact on how physicians, particularly dermatologists, prescribe oral minoxidil. However, it is difficult to know if the article exposed dermatologists to another treatment in their armamentarium for hair loss or if it influenced patients to ask their health care provider about LDOM for hair loss. One thing is clear—since the article’s publication, the off-label use of LDOM for alopecia has produced what many may call “miracles” for patients with hair loss.5
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j.1365-2133.2004.05785.x
- Kolata G. An old medicine grows new hair for pennies a day, doctors say. The New York Times. August 18, 2022. Accessed May 20, 2024. https://www.nytimes.com/2022/08/18/health/minoxidil-hair-loss-pills.html
- The New York Times Company reports third-quarter 2022 results. Press release. The New York Times Company; November 2, 2022. Accessed May 20, 2024. https://nytco-assets.nytimes.com/2022/11/NYT-Press-Release-Q3-2022-Final-nM7GzWGr.pdf
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84:1644-1651. doi:10.1016/j.jaad.2021.02.054
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746. doi:10.1016/j.jaad.2020.06.1009
- Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186-194. doi:10.1111/j.1365-2133.2004.05785.x
- Kolata G. An old medicine grows new hair for pennies a day, doctors say. The New York Times. August 18, 2022. Accessed May 20, 2024. https://www.nytimes.com/2022/08/18/health/minoxidil-hair-loss-pills.html
- The New York Times Company reports third-quarter 2022 results. Press release. The New York Times Company; November 2, 2022. Accessed May 20, 2024. https://nytco-assets.nytimes.com/2022/11/NYT-Press-Release-Q3-2022-Final-nM7GzWGr.pdf
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, et al. Safety of low-dose oral minoxidil for hair loss: a multicenter study of 1404 patients. J Am Acad Dermatol. 2021;84:1644-1651. doi:10.1016/j.jaad.2021.02.054
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2021;84:737-746. doi:10.1016/j.jaad.2020.06.1009
Practice Points
- Low-dose oral minoxidil (LDOM) prescriptions have increased due to rising attention to its efficacy and safety.
- Media outlets can have a powerful effect on prescribing habits of physicians.
- Physicians should be aware of media trends to help direct patient education.
Central Centrifugal Cicatricial Alopecia in Males: Analysis of Time to Diagnosis and Disease Severity
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.
Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.


Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
To the Editor:
Central centrifugal cicatricial alopecia (CCCA) is a chronic progressive type of scarring alopecia that primarily affects women of African descent.1 The disorder rarely is reported in men, which may be due to misdiagnosis or delayed diagnosis. Early diagnosis and treatment are the cornerstones to slow or halt disease progression and prevent permanent damage to hair follicles. This study aimed to investigate the time to diagnosis and disease severity among males with CCCA.
We conducted a retrospective chart review of male patients older than 18 years seen in outpatient clinics at an academic dermatology department (Philadelphia, Pennsylvania) between January 2012 and December 2022. An electronic query using the International Classification of Diseases, Ninth and Tenth Revisions, code L66.9 (cicatricial alopecia, unspecified) was performed. Patients were included if they had a clinical diagnosis of CCCA, histologic evidence of CCCA, and scalp photographs from the initial dermatology visit. Patients with folliculitis decalvans, scalp biopsy features that limited characterization, or no scalp biopsy were excluded from the study. Onset of CCCA was defined as the patient-reported start time of hair loss and/or scalp symptoms. To determine alopecia severity, the degree of central scalp hair loss was independently assessed by 2 dermatologists (S.C.T., T.O.) using the central scalp alopecia photographic scale in African American women.2,3 This 6-point photographic scale displays images with grades ranging from 0 (normal) to 5 (bald scalp); higher grades indicate probable and more severe CCCA. The scale also divides the central hair loss in a frontal-accentuation or vertex-predominant pattern, which corresponds to the A or B designations, respectively; thus, a score of 5A indicates probable severe CCCA with a frontal accentuation pattern, while 5B indicates probable severe CCCA with hair loss focused on the vertex scalp. This study was approved by the University of Pennsylvania institutional review board (approval #850730).
Of 108 male patients, 12 met the eligibility criteria. Nearly all patients (91.7% [11/12]) had a CCCA severity grade of 3 or higher at the initial dermatology visit, indicating extensive hair loss (Table). The clinical appearance of severity grades 2 through 5 is demonstrated in the Figure. Among patients with a known disease duration prior to diagnosis, 72.7% (8/11) were diagnosed more than 1 year after onset of CCCA, and 45.4% (5/11) were diagnosed more than 5 years after onset. On average (SD), it took 6.4 (5.9) years for patients to receive a diagnosis of CCCA after the onset of scalp symptoms and/or hair loss.
Randomized controlled trials evaluating treatment of CCCA are lacking, and anecdotal evidence posits a better treatment response in early CCCA; however, our results suggest that most male patients present with advanced CCCA and receive a diagnosis years after disease onset. Similar research in alopecia areata has shown that 72.4% (105/145) of patients received their diagnosis within a year after onset of symptoms, and the mean time from onset of symptoms to diagnosis was 1 year.4 In contrast, male patients with CCCA experience considerable diagnostic delays. This disparity indicates the need for clinicians to increase recognition of CCCA in men and quickly refer them to a dermatologist for prompt treatment.


Androgenetic alopecia (AGA) commonly is at the top of the differential diagnosis for hair loss on the vertex of the scalp in males, but clinicians should maintain a high index of suspicion for CCCA, especially when scalp symptoms or atypical features of AGA are present.5 Androgenetic alopecia typically is asymptomatic, whereas the symptoms of CCCA may include itching, tenderness, and/or burning.6,7 Trichoscopy is useful to evaluate for scarring, and a scalp biopsy may reveal other features to lower AGA on the differential. Educating patients, barbers, and hairstylists about the importance of early intervention also may encourage earlier visits before the scarring process is advanced. Further exploration into factors impacting diagnosis and CCCA severity may uncover implications for prognosis and treatment.
This study was limited by a small sample size, retrospective design, and single-center analysis. Some patients had comorbid hair loss conditions, which could affect disease severity. Moreover, the central scalp alopecia photographic scale2 was not validated in men or designed for assessment of the nonclassical hair loss distributions noted in some of our patients. Nonetheless, we hope these data will support clinicians in efforts to advocate for early diagnosis and treatment in patients with CCCA to ultimately help improve outcomes.
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
- Ogunleye TA, McMichael A, Olsen EA. Central centrifugal cicatricial alopecia: what has been achieved, current clues for future research. Dermatol Clin. 2014;32:173-181. doi:10.1016/j.det.2013.12.005
- Olsen EA, Callender V, McMichael A, et al. Central hair loss in African American women: incidence and potential risk factors. J Am Acad Dermatol. 2011;64:245-252. doi:10.1016/j.jaad.2009.11.693
- Olsen EA, Callendar V, Sperling L, et al. Central scalp alopecia photographic scale in African American women. Dermatol Ther. 2008;21:264-267. doi:10.1111/j.1529-8019.2008.00208.x
- Andersen YMF, Nymand L, DeLozier AM, et al. Patient characteristics and disease burden of alopecia areata in the Danish Skin Cohort. BMJ Open. 2022;12:E053137. doi:10.1136/bmjopen-2021-053137
- Davis EC, Reid SD, Callender VD, et al. Differentiating central centrifugal cicatricial alopecia and androgenetic alopecia in African American men. J Clin Aesthetic Dermatol. 2012;5:37-40.
- Jackson TK, Sow Y, Ayoade KO, et al. Central centrifugal cicatricial alopecia in males. J Am Acad Dermatol. 2023;89:1136-1140. doi:10.1016/j.jaad.2023.07.1011
- Lawson CN, Bakayoko A, Callender VD. Central centrifugal cicatricial alopecia: challenges and treatments. Dermatol Clin. 2021;39:389-405. doi:10.1016/j.det.2021.03.004
Practice Points
- Most males with central centrifugal cicatricial alopecia (CCCA) experience considerable diagnostic delays and typically present to dermatology with late-stage disease.
- Dermatologists should consider CCCA in the differential diagnosis for adult Black males with alopecia.
- More research is needed to explore advanced CCCA in males, including factors limiting timely diagnosis and the impact on quality of life in this population.
Subungual Nodule in a Pediatric Patient
The Diagnosis: Subungual Exostosis
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5
Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2
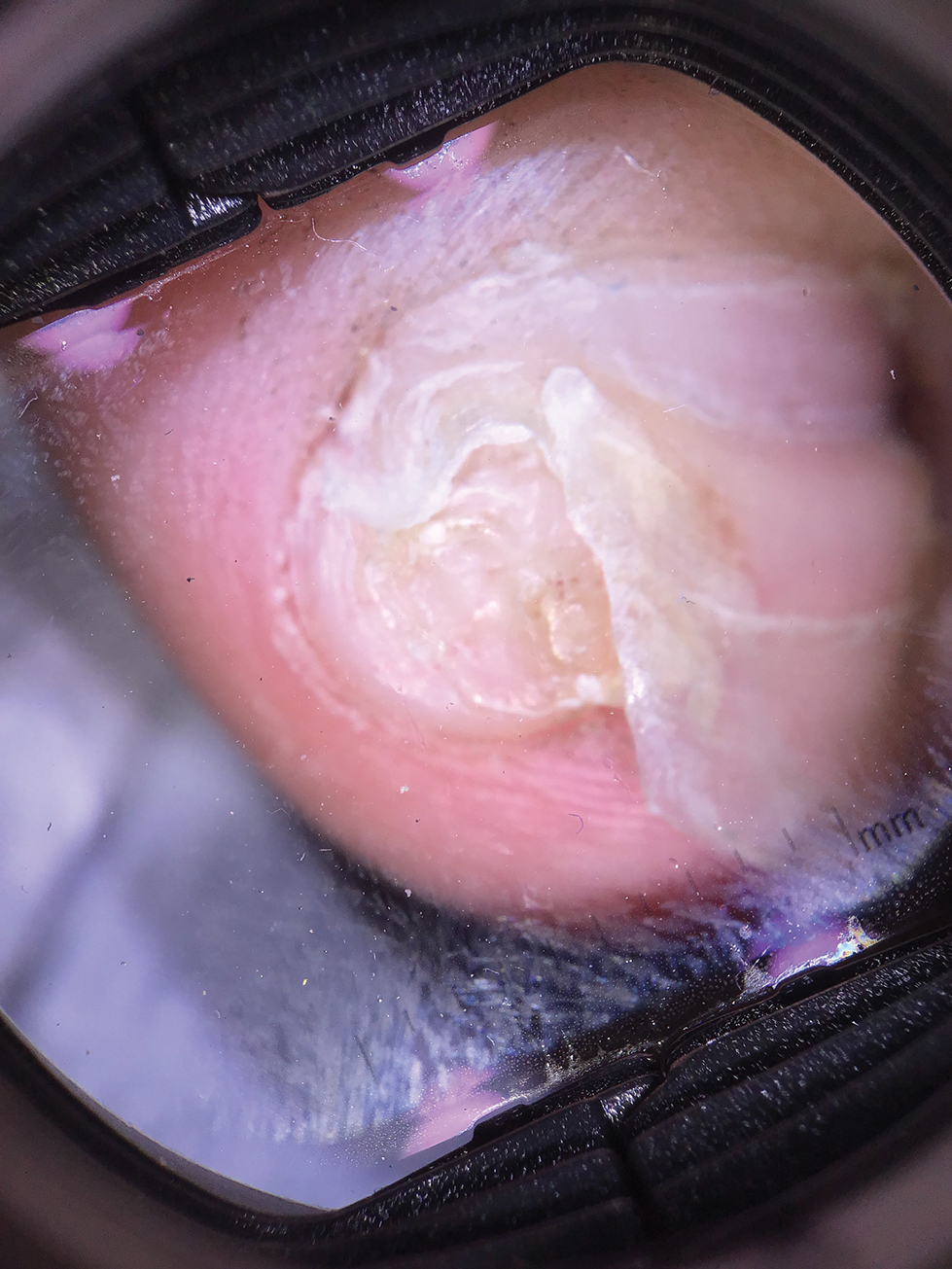
Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
The Diagnosis: Subungual Exostosis
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5

Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2

Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
The Diagnosis: Subungual Exostosis
Subungual exostosis should be considered as a possible cause of an exophytic subungual nodule in a young active female. In our patient, the involvement of the great toe was a clue, as the hallux is the most common location of subungual exostosis. The patient’s age and sex also were supportive, as subungual exostosis is most common in female children and adolescents— particularly those who are active, as trauma is thought to play a possible role in development of this benign tumor.1-3 Radiography is the preferred modality for diagnosis; in our case, it showed a trabecular bony overgrowth (Figure 1), which confirmed the diagnosis. Subungual exostosis is a rare, benign, osteocartilaginous tumor of trabecular bone. The etiology is unknown but is hypothesized to be related to trauma, infection, or activation of a cartilaginous cyst.1,3 The subungual nodule may be asymptomatic or painful. Disruption and elevation of the nail plate is common.4 The differential diagnosis includes amelanotic melanoma, fibroma, fibrokeratoma, osteochondroma, pyogenic granuloma, squamous cell carcinoma, glomus tumor, and verruca vulgaris, among others.5

Physical examination demonstrates a firm, fixed, subungual nodule, often with an accompanying nail deformity. Further workup is required to confirm the benign nature of the lesion and exclude nail tumors such as melanoma or squamous cell carcinoma. Radiography is the gold standard for diagnosis, demonstrating a trabecular bony overgrowth.6 Performing a radiograph as the initial diagnostic test spares the patient from unnecessary procedures such as biopsy or expensive imaging techniques such as magnetic resonance imaging. Early lesions may not demonstrate sufficient bone formation shown on radiography. In these situations, a combination of dermoscopy and histopathologic examination may aid in diagnosis (Figure 2).4 Vascular ectasia, hyperkeratosis, onycholysis, and ulceration are the most common findings on dermoscopy (in ascending order).7 Histopathology typically demonstrates a base or stalk of normal-appearing trabecular bone with a fibrocartilage cap.8 However, initial clinical workup via radiography allows for the least-invasive and highest-yield intervention. Clinical suspicion for this condition is important, as it can be diagnosed with noninvasive inexpensive imaging rather than biopsy or more specialized imaging modalities. Appropriate recognition can save young patients from unnecessary and expensive procedures. Treatment typically involves surgical excision; to prevent regrowth, removal of the lesion at the base of the bone is recommended.2

Although amelanotic melanoma also can manifest as a subungual nail tumor, it would be unusual in a young child and would not be expected to show characteristic changes on radiography. A glomus tumor would be painful, is more common on the fingers than on the toes, and typically has a bluish hue.9 Verruca vulgaris can occur subungually but is more common around the nailfold and often has the characteristic dermoscopic finding of thrombosed capillaries. It also would not be expected to show characteristic radiographic findings. Osteochondroma can occur in young patients and can appear clinically similar to subungual exostosis; however, it typically is painful.10
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
- Pascoal D, Balaco I, Alves C, et al. Subungual exostosis—treatment results with preservation of the nail bed. J Pediatr Orthop B. 2020;29:382-386.
- Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257.
- Chiheb S, Slimani Y, Karam R, et al. Subungual exostosis: a case series of 48 patients. Skin Appendage Disord. 2021;7:475-479.
- Zhang W, Gu L, Fan H, et al. Subungual exostosis with an unusual dermoscopic feature. JAAD Case Rep. 2020;6:725-726.
- Demirdag HG, Tugrul Ayanoglu B, Akay BN. Dermoscopic features of subungual exostosis. Australas J Dermatol. 2019;60:E138-E141.
- Tritto M, Mirkin G, Hao X. Subungual exostosis on the right hallux. J Am Podiatr Med Assoc. 2021;111.
- Piccolo V, Argenziano G, Alessandrini AM, et al. Dermoscopy of subungual exostosis: a retrospective study of 10 patients. Dermatology. 2017;233:80-85.
- Lee SK, Jung MS, Lee YH, et al. Two distinctive subungual pathologies: subungual exostosis and subungual osteochondroma. Foot Ankle Int. 2007;28:595-601. doi:10.3113/FAI.2007.0595
- Samaniego E, Crespo A, Sanz A. Key diagnostic features and treatment of subungual glomus tumor. Actas Dermosifiliogr. 2009;100:875-882.
- Glick S. Subungual osteochondroma of the third toe. Consult.360. 2013;12.
A 13-year-old girl presented to her pediatrician with a small pink bump under the left great toenail of 8 months’ duration that was slowly growing. Months later, she developed an ingrown nail on the same toe, which was treated with partial nail avulsion by the pediatrician. Given continued nail dystrophy and a visible bump under the nail, the patient was referred to dermatology. Physical examination revealed a subungual, flesh-colored, sessile nodule causing distortion of the nail plate on the left great toe with associated intermittent redness and swelling. She denied wearing new shoes or experiencing any pain, pruritus, or purulent drainage or bleeding from the lesion. She reported being physically active and playing tennis.
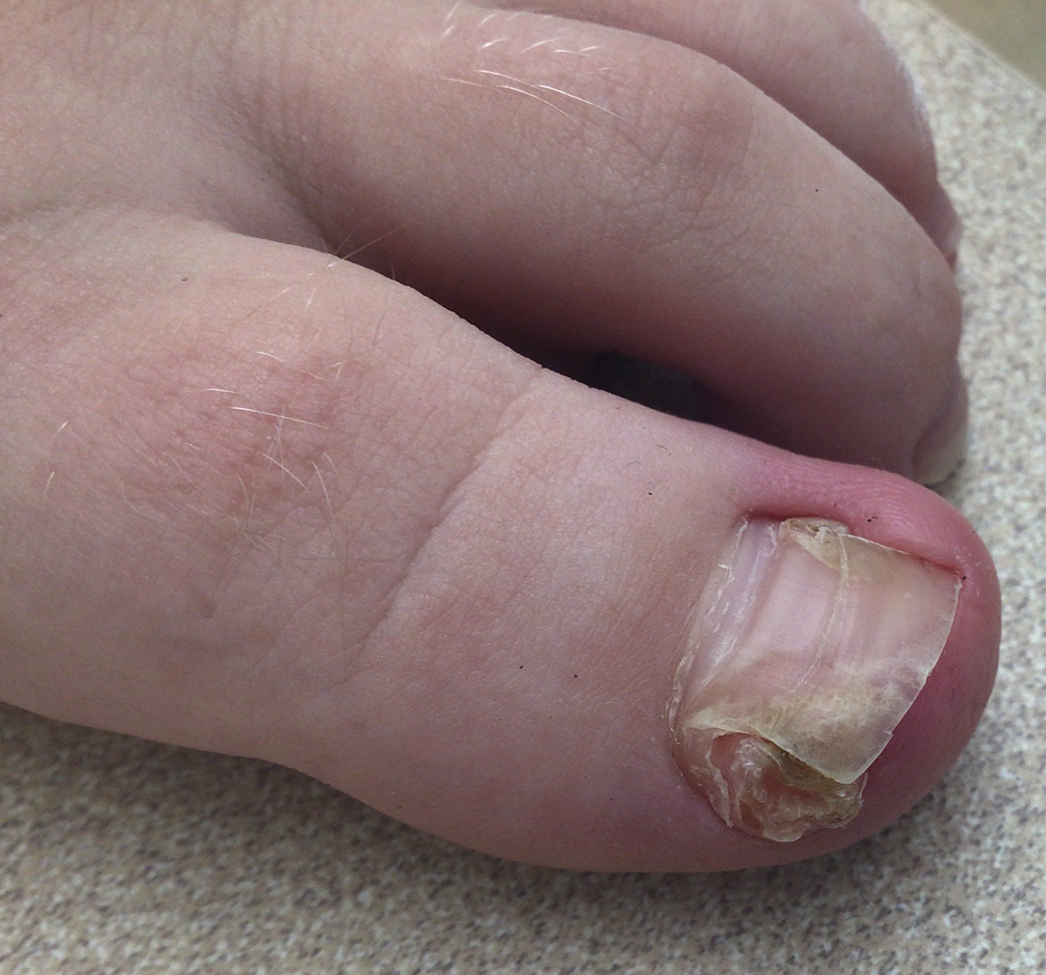
Dupilumab Evaluated as Treatment for Pediatric Alopecia Areata
showed.
“We might be opening a new avenue for a safe, long-term treatment for our children with AA,” the study’s lead investigator, Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York City, said in an interview during the annual meeting of the Society for Investigative Dermatology (SID), where the results were presented during a poster session. “I think AA is likely joining the atopic march, which may allow us to adapt some treatments from the atopy world to AA.”
When the original phase 2 and phase 3 trials of dupilumab for patients with moderate to severe AD were being conducted, Dr. Guttman-Yassky, one of the investigators, recalled observing that some patients who also had patch alopecia experienced hair regrowth. “I was scratching my head because, at the time, AA was considered to be only a Th1-driven disease,” she said. “I asked myself, ‘How can this happen?’ I looked in the literature and found many publications linking atopy in general to alopecia areata. The largest of the dermatologic publications showed that eczema and atopy in general are the highest comorbidities in alopecia areata.”
“This and other findings such as IL [interleukin]-13 genetic linkage with AA and high IgE in patients with AA link AA with Th2 immune skewing, particularly in the setting of atopy,” she continued. In addition, she said, in a large biomarker study involving the scalp and blood of patients with AA, “we found increases in Th2 biomarkers that were associated with alopecia severity.”
Case Series of 20 Pediatric Patients
As part of a case series of children with both AD and AA, Dr. Guttman-Yassky and colleagues evaluated hair regrowth using the Severity of Alopecia Tool (SALT) in 20 pediatric patients (mean age, 10.8 years) who were being treated at Mount Sinai. They collected patient demographics, atopic history, immunoglobulin E (IgE) levels, and SALT scores at follow-up visits every 12-16 weeks for more than 72 weeks and performed Spearman correlations between clinical scores, demographics, and IgE levels.
At baseline, the mean SALT score was 54.4, the mean IgE level was 1567.7 IU/mL, and 75% of patients also had a family history of atopy. The mean follow-up was 67.6 weeks. The researchers observed a significant reduction in SALT scores at week 48 compared with baseline (a mean score of 20.4; P < .01) and continued improvement up to at least 72 weeks (P < .01 vs baseline). They also noted that patients who achieved a treatment response at week 24 had baseline IgE levels > 200 IU/mL.
In other findings, baseline IgE positively correlated with improvement in SALT scores at week 36 (P < .05), while baseline SALT scores positively correlated with disease duration (P < .01) and negatively correlated with improvement in SALT scores at weeks 24, 36, and 48 (P < .005). “The robustness of the response surprised me,” Dr. Guttman-Yassky said in the interview. “Dupilumab for AA takes time to work, but once it kicks in, it kicks in. It takes anywhere from 6 to 12 months to see hair regrowth.”
She acknowledged certain limitations of the analysis, including its small sample size and the fact that it was not a standardized trial. “But, based on our data and the adult data, we are very encouraged about the potential of using dupilumab for children with AA,” she said.
Mount Sinai recently announced that the National Institutes of Health awarded a $6.6 million, 5-year grant to Dr. Guttman-Yassky to further investigate dupilumab as a treatment for children with AA. She will lead a multicenter controlled trial of 76 children with alopecia affecting at least 30% of the scalp, who will be randomized 2:1 (dupilumab:placebo) for 48 weeks, followed by 48 weeks of open-label dupilumab for all participants, with 16 weeks of follow-up, for a total of 112 weeks. Participating sites include Mount Sinai, Yale University, Northwestern University, and the University of California, Irvine.
Dr. Guttman-Yassky disclosed that she is a consultant to many pharmaceutical companies, including dupilumab manufacturers Sanofi and Regeneron.
A version of this article appeared on Medscape.com.
showed.
“We might be opening a new avenue for a safe, long-term treatment for our children with AA,” the study’s lead investigator, Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York City, said in an interview during the annual meeting of the Society for Investigative Dermatology (SID), where the results were presented during a poster session. “I think AA is likely joining the atopic march, which may allow us to adapt some treatments from the atopy world to AA.”
When the original phase 2 and phase 3 trials of dupilumab for patients with moderate to severe AD were being conducted, Dr. Guttman-Yassky, one of the investigators, recalled observing that some patients who also had patch alopecia experienced hair regrowth. “I was scratching my head because, at the time, AA was considered to be only a Th1-driven disease,” she said. “I asked myself, ‘How can this happen?’ I looked in the literature and found many publications linking atopy in general to alopecia areata. The largest of the dermatologic publications showed that eczema and atopy in general are the highest comorbidities in alopecia areata.”
“This and other findings such as IL [interleukin]-13 genetic linkage with AA and high IgE in patients with AA link AA with Th2 immune skewing, particularly in the setting of atopy,” she continued. In addition, she said, in a large biomarker study involving the scalp and blood of patients with AA, “we found increases in Th2 biomarkers that were associated with alopecia severity.”
Case Series of 20 Pediatric Patients
As part of a case series of children with both AD and AA, Dr. Guttman-Yassky and colleagues evaluated hair regrowth using the Severity of Alopecia Tool (SALT) in 20 pediatric patients (mean age, 10.8 years) who were being treated at Mount Sinai. They collected patient demographics, atopic history, immunoglobulin E (IgE) levels, and SALT scores at follow-up visits every 12-16 weeks for more than 72 weeks and performed Spearman correlations between clinical scores, demographics, and IgE levels.
At baseline, the mean SALT score was 54.4, the mean IgE level was 1567.7 IU/mL, and 75% of patients also had a family history of atopy. The mean follow-up was 67.6 weeks. The researchers observed a significant reduction in SALT scores at week 48 compared with baseline (a mean score of 20.4; P < .01) and continued improvement up to at least 72 weeks (P < .01 vs baseline). They also noted that patients who achieved a treatment response at week 24 had baseline IgE levels > 200 IU/mL.
In other findings, baseline IgE positively correlated with improvement in SALT scores at week 36 (P < .05), while baseline SALT scores positively correlated with disease duration (P < .01) and negatively correlated with improvement in SALT scores at weeks 24, 36, and 48 (P < .005). “The robustness of the response surprised me,” Dr. Guttman-Yassky said in the interview. “Dupilumab for AA takes time to work, but once it kicks in, it kicks in. It takes anywhere from 6 to 12 months to see hair regrowth.”
She acknowledged certain limitations of the analysis, including its small sample size and the fact that it was not a standardized trial. “But, based on our data and the adult data, we are very encouraged about the potential of using dupilumab for children with AA,” she said.
Mount Sinai recently announced that the National Institutes of Health awarded a $6.6 million, 5-year grant to Dr. Guttman-Yassky to further investigate dupilumab as a treatment for children with AA. She will lead a multicenter controlled trial of 76 children with alopecia affecting at least 30% of the scalp, who will be randomized 2:1 (dupilumab:placebo) for 48 weeks, followed by 48 weeks of open-label dupilumab for all participants, with 16 weeks of follow-up, for a total of 112 weeks. Participating sites include Mount Sinai, Yale University, Northwestern University, and the University of California, Irvine.
Dr. Guttman-Yassky disclosed that she is a consultant to many pharmaceutical companies, including dupilumab manufacturers Sanofi and Regeneron.
A version of this article appeared on Medscape.com.
showed.
“We might be opening a new avenue for a safe, long-term treatment for our children with AA,” the study’s lead investigator, Emma Guttman-Yassky, MD, PhD, professor and chair of dermatology at the Icahn School of Medicine at Mount Sinai, New York City, said in an interview during the annual meeting of the Society for Investigative Dermatology (SID), where the results were presented during a poster session. “I think AA is likely joining the atopic march, which may allow us to adapt some treatments from the atopy world to AA.”
When the original phase 2 and phase 3 trials of dupilumab for patients with moderate to severe AD were being conducted, Dr. Guttman-Yassky, one of the investigators, recalled observing that some patients who also had patch alopecia experienced hair regrowth. “I was scratching my head because, at the time, AA was considered to be only a Th1-driven disease,” she said. “I asked myself, ‘How can this happen?’ I looked in the literature and found many publications linking atopy in general to alopecia areata. The largest of the dermatologic publications showed that eczema and atopy in general are the highest comorbidities in alopecia areata.”
“This and other findings such as IL [interleukin]-13 genetic linkage with AA and high IgE in patients with AA link AA with Th2 immune skewing, particularly in the setting of atopy,” she continued. In addition, she said, in a large biomarker study involving the scalp and blood of patients with AA, “we found increases in Th2 biomarkers that were associated with alopecia severity.”
Case Series of 20 Pediatric Patients
As part of a case series of children with both AD and AA, Dr. Guttman-Yassky and colleagues evaluated hair regrowth using the Severity of Alopecia Tool (SALT) in 20 pediatric patients (mean age, 10.8 years) who were being treated at Mount Sinai. They collected patient demographics, atopic history, immunoglobulin E (IgE) levels, and SALT scores at follow-up visits every 12-16 weeks for more than 72 weeks and performed Spearman correlations between clinical scores, demographics, and IgE levels.
At baseline, the mean SALT score was 54.4, the mean IgE level was 1567.7 IU/mL, and 75% of patients also had a family history of atopy. The mean follow-up was 67.6 weeks. The researchers observed a significant reduction in SALT scores at week 48 compared with baseline (a mean score of 20.4; P < .01) and continued improvement up to at least 72 weeks (P < .01 vs baseline). They also noted that patients who achieved a treatment response at week 24 had baseline IgE levels > 200 IU/mL.
In other findings, baseline IgE positively correlated with improvement in SALT scores at week 36 (P < .05), while baseline SALT scores positively correlated with disease duration (P < .01) and negatively correlated with improvement in SALT scores at weeks 24, 36, and 48 (P < .005). “The robustness of the response surprised me,” Dr. Guttman-Yassky said in the interview. “Dupilumab for AA takes time to work, but once it kicks in, it kicks in. It takes anywhere from 6 to 12 months to see hair regrowth.”
She acknowledged certain limitations of the analysis, including its small sample size and the fact that it was not a standardized trial. “But, based on our data and the adult data, we are very encouraged about the potential of using dupilumab for children with AA,” she said.
Mount Sinai recently announced that the National Institutes of Health awarded a $6.6 million, 5-year grant to Dr. Guttman-Yassky to further investigate dupilumab as a treatment for children with AA. She will lead a multicenter controlled trial of 76 children with alopecia affecting at least 30% of the scalp, who will be randomized 2:1 (dupilumab:placebo) for 48 weeks, followed by 48 weeks of open-label dupilumab for all participants, with 16 weeks of follow-up, for a total of 112 weeks. Participating sites include Mount Sinai, Yale University, Northwestern University, and the University of California, Irvine.
Dr. Guttman-Yassky disclosed that she is a consultant to many pharmaceutical companies, including dupilumab manufacturers Sanofi and Regeneron.
A version of this article appeared on Medscape.com.
FROM SID 2024
Frontal Fibrosing Alopecia: Study Finds Oral Contraceptive Use Modulates Risk In Women with Genetic Variant
TOPLINE:
Investigators found that .
METHODOLOGY:
- OC use has been considered a possible factor behind the increased incidence of FFA because it was first documented in 1994, and a recent genome-wide association study of FFA identified a signal for an association with a variant in CYP1B1.
- The same researchers conducted a gene-environment interaction study with a case-control design involving 489 White female patients (mean age, 65.8 years) with FFA and 34,254 controls, matched for age and genetic ancestry.
- Data were collected from July 2015 to September 2017 and analyzed from October 2022 to December 2023.
- The study aimed to investigate the modulatory effect of OC use on the CYP1B1 variant’s impact on FFA risk, using logistic regression models for analysis.
TAKEAWAY:
- The use of OCs was associated with a 1.9 times greater risk for FFA in individuals with the specific CYP1B1 genetic variant, but there was no association among those with no history of OC use.
- The study suggests a significant gene-environment interaction, indicating that OC use may influence FFA risk in genetically predisposed individuals.
IN PRACTICE:
“This gene-environment interaction analysis suggests that the protective effect of the CYPIB1 missense variant on FFA risk might be mediated by exposure” to OCs, the authors wrote. The study, they added, “underscores the importance of considering genetic predispositions and environmental factors, such as oral contraceptive use, in understanding and managing frontal fibrosing alopecia.”
SOURCE:
Tuntas Rayinda, MD, MSc, PhD, of St. John’s Institute of Dermatology, King’s College London, led the study, which was published online May 29, 2024, in JAMA Dermatology.
LIMITATIONS:
The study’s reliance on self-reported OC use may have introduced recall and differences in ascertainment of OC use between patient and control groups and could have affected the study’s findings. The study also did not collect information on the type of OC used, which could have influenced the observed interaction.
DISCLOSURES:
The study was supported by the British Skin Foundation Young Investigator Award. One investigator reported being a subinvestigator on an alopecia areata study funded by Pfizer. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Investigators found that .
METHODOLOGY:
- OC use has been considered a possible factor behind the increased incidence of FFA because it was first documented in 1994, and a recent genome-wide association study of FFA identified a signal for an association with a variant in CYP1B1.
- The same researchers conducted a gene-environment interaction study with a case-control design involving 489 White female patients (mean age, 65.8 years) with FFA and 34,254 controls, matched for age and genetic ancestry.
- Data were collected from July 2015 to September 2017 and analyzed from October 2022 to December 2023.
- The study aimed to investigate the modulatory effect of OC use on the CYP1B1 variant’s impact on FFA risk, using logistic regression models for analysis.
TAKEAWAY:
- The use of OCs was associated with a 1.9 times greater risk for FFA in individuals with the specific CYP1B1 genetic variant, but there was no association among those with no history of OC use.
- The study suggests a significant gene-environment interaction, indicating that OC use may influence FFA risk in genetically predisposed individuals.
IN PRACTICE:
“This gene-environment interaction analysis suggests that the protective effect of the CYPIB1 missense variant on FFA risk might be mediated by exposure” to OCs, the authors wrote. The study, they added, “underscores the importance of considering genetic predispositions and environmental factors, such as oral contraceptive use, in understanding and managing frontal fibrosing alopecia.”
SOURCE:
Tuntas Rayinda, MD, MSc, PhD, of St. John’s Institute of Dermatology, King’s College London, led the study, which was published online May 29, 2024, in JAMA Dermatology.
LIMITATIONS:
The study’s reliance on self-reported OC use may have introduced recall and differences in ascertainment of OC use between patient and control groups and could have affected the study’s findings. The study also did not collect information on the type of OC used, which could have influenced the observed interaction.
DISCLOSURES:
The study was supported by the British Skin Foundation Young Investigator Award. One investigator reported being a subinvestigator on an alopecia areata study funded by Pfizer. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Investigators found that .
METHODOLOGY:
- OC use has been considered a possible factor behind the increased incidence of FFA because it was first documented in 1994, and a recent genome-wide association study of FFA identified a signal for an association with a variant in CYP1B1.
- The same researchers conducted a gene-environment interaction study with a case-control design involving 489 White female patients (mean age, 65.8 years) with FFA and 34,254 controls, matched for age and genetic ancestry.
- Data were collected from July 2015 to September 2017 and analyzed from October 2022 to December 2023.
- The study aimed to investigate the modulatory effect of OC use on the CYP1B1 variant’s impact on FFA risk, using logistic regression models for analysis.
TAKEAWAY:
- The use of OCs was associated with a 1.9 times greater risk for FFA in individuals with the specific CYP1B1 genetic variant, but there was no association among those with no history of OC use.
- The study suggests a significant gene-environment interaction, indicating that OC use may influence FFA risk in genetically predisposed individuals.
IN PRACTICE:
“This gene-environment interaction analysis suggests that the protective effect of the CYPIB1 missense variant on FFA risk might be mediated by exposure” to OCs, the authors wrote. The study, they added, “underscores the importance of considering genetic predispositions and environmental factors, such as oral contraceptive use, in understanding and managing frontal fibrosing alopecia.”
SOURCE:
Tuntas Rayinda, MD, MSc, PhD, of St. John’s Institute of Dermatology, King’s College London, led the study, which was published online May 29, 2024, in JAMA Dermatology.
LIMITATIONS:
The study’s reliance on self-reported OC use may have introduced recall and differences in ascertainment of OC use between patient and control groups and could have affected the study’s findings. The study also did not collect information on the type of OC used, which could have influenced the observed interaction.
DISCLOSURES:
The study was supported by the British Skin Foundation Young Investigator Award. One investigator reported being a subinvestigator on an alopecia areata study funded by Pfizer. No other disclosures were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
Recalcitrant Folliculitis Decalvans Treatment Outcomes With Biologics and Small Molecule Inhibitors
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).
The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).

The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
Folliculitis decalvans (FD) is classified as a rare primary neutrophilic cicatricial alopecia occurring predominantly in middle-aged adults. Although the true etiology is still unknown, the pathogenesis behind the inflammatory follicular lesions stems from possible Staphylococcus aureus infection and an impaired host immune system in response to released superantigens. 1 The clinical severity of this inflammatory scalp disorder can range from mild to severe and debilitating. Multiple treatment regimens have been developed with the goal of maintaining full remission. We provide a summary of tumor necrosis factor (TNF) inhibitors, Janus kinase (JAK) inhibitors, phosphodiesterase 4 (PDE4) inhibitors, and monoclonal antibodies being utilized for patients with therapy-recalcitrant FD.
Methods
We conducted a PubMed, Medline, and Google Scholar search for the terms refractory FD, recalcitrant FD, or therapy-resistant FD to identify articles published in English from 1998 to 2022. Articles that reported recalcitrant cases and subsequent therapy with TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies were included. Articles were excluded if recalcitrant cases were not clearly defined. Remission was defined as no recurrence in lesions or pustules or as a reduction in the inflammatory process with stabilization upon continuation or discontinuation of the therapy regimen. Two reviewers (T.F. and K.U.) independently searched for and screened each report.
Results
Treatment of recalcitrant FD with biologics or small molecule inhibitors was discussed in 9 studies with a combined total of 35 patients.2-10 The treatment regimens included TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies (Table).

The TNF inhibitors were utilized in 6 reports with a combined total of 29 patients. Treatments included adalimumab or biosimilar adalimumab (27/29 patients), infliximab (1/29 patients), and certolizumab pegol (1/29 patients). Remission was reported in 26 of 29 cases. There were 2 nonresponders to adalimumab and marked improvement with certolizumab pegol without complete resolution. The use of the JAK inhibitor baricitinib in 4 patients resulted in remission. In all 4 patients, baricitinib was used with concurrent treatments, and remission was achieved in an average of 2.25 months. The use of a PDE4 inhibitor, apremilast, was reported in 1 case; remission was achieved in 3 weeks. Secukinumab, a monoclonal antibody that targets IL-17, was utilized in 1 patient. Marked improvement was seen after 2 months, with complete remission in 7 months.
Comment
Traditional treatment regimens for FD most often include a combination of topical and oral antibiotics; isotretinoin; and oral, topical, or intralesional corticosteroids. In the past, interventions typically were suppressive as opposed to curative; however, recent treatment advancements have shown promise in achieving lasting remission.
Most reports targeting treatment-resistant FD involved the use of TNF inhibitors, including adalimumab, biosimilar adalimumab, infliximab, and certolizumab pegol. Adalimumab was the most frequently used TNF inhibitor, with 24 of 26 treated patients achieving remission. Adalimumab may have been used the most in the treatment of FD because TNF is pronounced in other neutrophilic dermatoses that have been successfully treated with TNF inhibitors. It has been reported that adalimumab needs to be continued, as stoppage or interruption led to relapse.3
Although there are few reports of the use of JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies for FD, these treatment modalities show promise, as their use led to marked improvement or lasting remission with ongoing treatment. The use of the PDE4 inhibitor apremilast displayed the most rapid improvement of any of the reviewed treatments, with remission achieved in just 3 weeks.9 The rapid success of apremilast may be attributed to the inhibitory effect on neutrophils.
Miguel-Gómez et al11 provided a therapeutic protocol for FD based on the severity of disease (N=60). The protocol included rifampicin plus clindamycin for the treatment of severe disease, as 90.5% (19/21) of resistant cases showed clinical response, with remission of 5 months’ duration. Although this may be acceptable for some patients, others may require an alternative approach. Tietze et al12 showed that rifampicin and clindamycin had the lowest success rate for long-term remission, with 8 of 10 patients relapsing within 2 to 4 months. In addition, the emergence of antimicrobial resistance remains a major concern in the treatment of FD. Upon the review of the most recent reports of successful treatment of therapy-resistant FD, biologics and small molecule inhibitors have shown remission extending through a 12-month follow-up period. We suggest considering the addition of biologics and small molecule inhibitors to the treatment protocol for severe or resistant disease.
Limitations—In the articles reviewed, the definition of remission was inconsistent among authors—some characterized it as no recurrence in lesions or pustules and some as a reduction in the inflammatory process. True duration of remission was difficult to assess from case reports, as follow-up periods varied prior to publication. The studies included in this review consisted mainly of small sample sizes owing to the rarity of FD, and consequently, strength of evidence is lacking. Inherent to the nature of systematic reviews, publication bias may have occurred. Lastly, several studies were impacted by difficulty in obtaining optimal treatment due to financial hardship, and regimens were adjusted accordingly.
Conclusion
The relapsing nature of FD leads to frustration and poor quality of life for patients. There is a paucity of data to guide treatment when FD remains recalcitrant to traditional therapy. Therapies such as TNF inhibitors, JAK inhibitors, PDE4 inhibitors, and monoclonal antibodies have shown success in the treatment of this often difficult-to-treat disease. Small sample sizes in reports discussing treatment for resistant cases as well as conflicting results make it challenging to draw conclusions about treatment efficacy. Larger studies are needed to understand the long-term outcomes of treatment options. Regardless, disease severity, patient history, patient preferences, and treatment goals can guide the selection of therapeutic options.
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
- Otberg N, Kang H, Alzolibani AA, et al. Folliculitis decalvans. Dermatol Ther. 2008;21:238-244. doi:10.1111/j.1529-8019.2008.00204.x
- Shireen F, Sudhakar A. A case of isotretinoin therapy-refractory folliculitis decalvans treated successfully with biosimilar adalimumab (Exemptia). Int J Trichology. 2018;10:240-241.
- Iorizzo M, Starace M, Vano-Galvan S, et al. Refractory folliculitis decalvans treated with adalimumab: a case series of 23 patients. J Am Acad Dermatol. 2022;87:666-669. doi:10.1016/j.jaad.2022.02.044
- Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74-76. doi:10.1111/ddg.12224
- Alhameedy MM, Alsantali AM. Therapy-recalcitrant folliculitis decalvans controlled successfully with adalimumab. Int J Trichology. 2019;11:241-243. doi:10.4103/ijt.ijt_92_19
- Mihaljevic´ N, von den Driesch P. Successful use of infliximab in a patient with recalcitrant folliculitis decalvans. J Dtsch Dermatol Ges. 2012;10:589-590. doi:10.1111/j.1610-0387.2012.07972.x
- Hoy M, Böhm M. Therapy-refractory folliculitis decalvans treated with certolizumab pegol. Int J Dermatol. 2022;61:e26-e28. doi:10.1111/ijd.15914
- Moussa A, Asfour L, Eisman S, et al. Successful treatment of folliculitis decalvans with baricitinib: a case series. Australas J Dermatol. 2022;63:279-281. doi:10.1111/ajd.13786
- Fässler M, Radonjic-Hoesli S, Feldmeyer L, et al. Successful treatment of refractory folliculitis decalvans with apremilast. JAAD Case Rep. 2020;6:1079-1081. doi:10.1016/j.jdcr.2020.08.019
- Ismail FF, Sinclair R. Successful treatment of refractory folliculitis decalvans with secukinumab. Australas J Dermatol. 2020;61:165-166. doi:10.1111/ajd.13190
- Miguel-Gómez L, Rodrigues-Barata AR, Molina-Ruiz A, et al. Folliculitis decalvans: effectiveness of therapies and prognostic factors in a multicenter series of 60 patients with long-term follow-up. J Am Acad Dermatol. 2018;79:878-883. doi:10.1016/j.jaad.2018.05.1240
- Tietze JK, Heppt MV, von Preußen A, et al. Oral isotretinoin as the most effective treatment in folliculitis decalvans: a retrospective comparison of different treatment regimens in 28 patients. J Eur Acad Dermatol Venereol. 2015;29:1816-1821. doi:10.1111/jdv.13052
Practice Points
- Tumor necrosis factor inhibitors, Janus kinase inhibitors, phosphodiesterase 4 inhibitors, and monoclonal antibodies have shown success in the treatment of folliculitis decalvans resistant to traditional therapies.
- The true etiology of folliculitis decalvans is still unknown, but possible factors include Staphylococcus aureus infection and an impaired host immune system, which may benefit from treatment with biologics and small molecule inhibitors.