User login
Sacral nerve stimulation may aid female sexual dysfunction
Sacral nerve stimulation (SNS) is a therapeutic procedure that could be used to help women with sexual dysfunction. However, the benefits of this method in this indication should still be reviewed in high-quality studies with sexual function as the primary endpoint, Erik Allemeyer, MD, PhD, a proctologist at the Niels Stensen Clinics in Georgsmarienhütte, Germany, and colleagues wrote in a recent journal article.
The World Health Organization defines sexual health as physical, emotional, mental, and social well-being in relation to sexuality. There are extensive investigations that verify the considerable importance of sexual function on a person’s quality of life. It therefore follows that therapy may be required if an individual is experiencing sexual dysfunction.
According to the authors, there are diverse data on the frequency of sexual dysfunction in women, in part because of heterogeneous definitions. The prevalence ranges between 26% and 91%. The estimated prevalence of orgasm difficulties in particular ranges from 16% to 25%. Sexual dysfunction can therefore be said to be a clinically significant problem.
It was recently discovered that SNS, which has only been used for other conditions so far, could also be an option for women with sexual dysfunction. According to Dr. Allemeyer and coauthors, SNS was first described in 1988 as a therapeutic alternative for patients with neurogenic bladder and has been approved in Europe since 1994. As a minimally invasive therapy for urge incontinence, idiopathic pelvic pain, and for nonobstructive urinary retention, SNS can now be used to treat a wide spectrum of conditions in urology and urogynecology. After the successful stimulation treatment of fecal incontinence was first described in 1995, the procedure has also been used in coloproctology.
Tested before implantation
In SNS, sacral nerve roots (S3 and S4) are permanently stimulated via a percutaneously implanted electrode. At first, the effect is reviewed using a test electrode and an external impulse generator over a period of a few weeks. Only if the test stimulation significantly alleviates symptoms can the indication for full implantation be issued, wrote the authors.
The positive effects on sexual function could be seen, even in the early years of stimulation therapy, when it was used for urinary and fecal incontinence as well as for idiopathic pelvic pain, they added. They have now summarized and discussed the current state of research on the potential effects of SNS on women’s sexual function in a literature review.
Systematic study analysis
To do this, they analyzed 16 studies, which included a total of 662 women, that reviewed the effect of SNS on sexual function when the treatment was being used in other indications. The overwhelming majority of data relates to urologic indications for SNS (such as overactive bladder, chronic retention, and idiopathic pelvic pain). In contrast, the SNS indication was rarely issued for fecal incontinence (9.1% of SNS indications or 61 patients). The most often used tool to assess the effect is the validated Female Sexual Function Index. The indicators covered in this index are “desire,” “arousal,” “lubrication,” “orgasm,” and “satisfaction.”
According to Dr. Allemeyer and coauthors, the analysis revealed evidence of significantly improved sexual function. It was unclear, however, whether this improvement was a primary or secondary effect of the SNS. All the original works and reviews expressly indicated that there was no proof of a primary effect of SNS on sexual function.
The mode of action of SNS and the immediate anatomic and physiologic link between the functions of urination, urinary incontinence, pelvic pain, fecal incontinence, and sexual function suggest a possible primary effect of SNS on sexual function, wrote the authors. However, no investigations use sexual function as the primary outcome parameter of SNS. This outcome should be reviewed in high-quality studies with sexual function as the primary endpoint.
An experimental therapy
According to Dr. Allemeyer and colleagues, two practical conclusions can be drawn from the study data available to date:
A possible primary effect of SNS on sexual function should be reviewed in high-quality, prospective studies that include detailed analyses of the different aspects of sexual dysfunction in both sexes.
An offer for trial-based SNS for sexual dysfunction should be made only at experienced sites with a multidisciplinary team of sex therapists and medical specialists and only after available therapy options have been exhausted and initially only within systematic studies.
This article was translated from Univadis Germany and a version appeared on Medscape.com.
Sacral nerve stimulation (SNS) is a therapeutic procedure that could be used to help women with sexual dysfunction. However, the benefits of this method in this indication should still be reviewed in high-quality studies with sexual function as the primary endpoint, Erik Allemeyer, MD, PhD, a proctologist at the Niels Stensen Clinics in Georgsmarienhütte, Germany, and colleagues wrote in a recent journal article.
The World Health Organization defines sexual health as physical, emotional, mental, and social well-being in relation to sexuality. There are extensive investigations that verify the considerable importance of sexual function on a person’s quality of life. It therefore follows that therapy may be required if an individual is experiencing sexual dysfunction.
According to the authors, there are diverse data on the frequency of sexual dysfunction in women, in part because of heterogeneous definitions. The prevalence ranges between 26% and 91%. The estimated prevalence of orgasm difficulties in particular ranges from 16% to 25%. Sexual dysfunction can therefore be said to be a clinically significant problem.
It was recently discovered that SNS, which has only been used for other conditions so far, could also be an option for women with sexual dysfunction. According to Dr. Allemeyer and coauthors, SNS was first described in 1988 as a therapeutic alternative for patients with neurogenic bladder and has been approved in Europe since 1994. As a minimally invasive therapy for urge incontinence, idiopathic pelvic pain, and for nonobstructive urinary retention, SNS can now be used to treat a wide spectrum of conditions in urology and urogynecology. After the successful stimulation treatment of fecal incontinence was first described in 1995, the procedure has also been used in coloproctology.
Tested before implantation
In SNS, sacral nerve roots (S3 and S4) are permanently stimulated via a percutaneously implanted electrode. At first, the effect is reviewed using a test electrode and an external impulse generator over a period of a few weeks. Only if the test stimulation significantly alleviates symptoms can the indication for full implantation be issued, wrote the authors.
The positive effects on sexual function could be seen, even in the early years of stimulation therapy, when it was used for urinary and fecal incontinence as well as for idiopathic pelvic pain, they added. They have now summarized and discussed the current state of research on the potential effects of SNS on women’s sexual function in a literature review.
Systematic study analysis
To do this, they analyzed 16 studies, which included a total of 662 women, that reviewed the effect of SNS on sexual function when the treatment was being used in other indications. The overwhelming majority of data relates to urologic indications for SNS (such as overactive bladder, chronic retention, and idiopathic pelvic pain). In contrast, the SNS indication was rarely issued for fecal incontinence (9.1% of SNS indications or 61 patients). The most often used tool to assess the effect is the validated Female Sexual Function Index. The indicators covered in this index are “desire,” “arousal,” “lubrication,” “orgasm,” and “satisfaction.”
According to Dr. Allemeyer and coauthors, the analysis revealed evidence of significantly improved sexual function. It was unclear, however, whether this improvement was a primary or secondary effect of the SNS. All the original works and reviews expressly indicated that there was no proof of a primary effect of SNS on sexual function.
The mode of action of SNS and the immediate anatomic and physiologic link between the functions of urination, urinary incontinence, pelvic pain, fecal incontinence, and sexual function suggest a possible primary effect of SNS on sexual function, wrote the authors. However, no investigations use sexual function as the primary outcome parameter of SNS. This outcome should be reviewed in high-quality studies with sexual function as the primary endpoint.
An experimental therapy
According to Dr. Allemeyer and colleagues, two practical conclusions can be drawn from the study data available to date:
A possible primary effect of SNS on sexual function should be reviewed in high-quality, prospective studies that include detailed analyses of the different aspects of sexual dysfunction in both sexes.
An offer for trial-based SNS for sexual dysfunction should be made only at experienced sites with a multidisciplinary team of sex therapists and medical specialists and only after available therapy options have been exhausted and initially only within systematic studies.
This article was translated from Univadis Germany and a version appeared on Medscape.com.
Sacral nerve stimulation (SNS) is a therapeutic procedure that could be used to help women with sexual dysfunction. However, the benefits of this method in this indication should still be reviewed in high-quality studies with sexual function as the primary endpoint, Erik Allemeyer, MD, PhD, a proctologist at the Niels Stensen Clinics in Georgsmarienhütte, Germany, and colleagues wrote in a recent journal article.
The World Health Organization defines sexual health as physical, emotional, mental, and social well-being in relation to sexuality. There are extensive investigations that verify the considerable importance of sexual function on a person’s quality of life. It therefore follows that therapy may be required if an individual is experiencing sexual dysfunction.
According to the authors, there are diverse data on the frequency of sexual dysfunction in women, in part because of heterogeneous definitions. The prevalence ranges between 26% and 91%. The estimated prevalence of orgasm difficulties in particular ranges from 16% to 25%. Sexual dysfunction can therefore be said to be a clinically significant problem.
It was recently discovered that SNS, which has only been used for other conditions so far, could also be an option for women with sexual dysfunction. According to Dr. Allemeyer and coauthors, SNS was first described in 1988 as a therapeutic alternative for patients with neurogenic bladder and has been approved in Europe since 1994. As a minimally invasive therapy for urge incontinence, idiopathic pelvic pain, and for nonobstructive urinary retention, SNS can now be used to treat a wide spectrum of conditions in urology and urogynecology. After the successful stimulation treatment of fecal incontinence was first described in 1995, the procedure has also been used in coloproctology.
Tested before implantation
In SNS, sacral nerve roots (S3 and S4) are permanently stimulated via a percutaneously implanted electrode. At first, the effect is reviewed using a test electrode and an external impulse generator over a period of a few weeks. Only if the test stimulation significantly alleviates symptoms can the indication for full implantation be issued, wrote the authors.
The positive effects on sexual function could be seen, even in the early years of stimulation therapy, when it was used for urinary and fecal incontinence as well as for idiopathic pelvic pain, they added. They have now summarized and discussed the current state of research on the potential effects of SNS on women’s sexual function in a literature review.
Systematic study analysis
To do this, they analyzed 16 studies, which included a total of 662 women, that reviewed the effect of SNS on sexual function when the treatment was being used in other indications. The overwhelming majority of data relates to urologic indications for SNS (such as overactive bladder, chronic retention, and idiopathic pelvic pain). In contrast, the SNS indication was rarely issued for fecal incontinence (9.1% of SNS indications or 61 patients). The most often used tool to assess the effect is the validated Female Sexual Function Index. The indicators covered in this index are “desire,” “arousal,” “lubrication,” “orgasm,” and “satisfaction.”
According to Dr. Allemeyer and coauthors, the analysis revealed evidence of significantly improved sexual function. It was unclear, however, whether this improvement was a primary or secondary effect of the SNS. All the original works and reviews expressly indicated that there was no proof of a primary effect of SNS on sexual function.
The mode of action of SNS and the immediate anatomic and physiologic link between the functions of urination, urinary incontinence, pelvic pain, fecal incontinence, and sexual function suggest a possible primary effect of SNS on sexual function, wrote the authors. However, no investigations use sexual function as the primary outcome parameter of SNS. This outcome should be reviewed in high-quality studies with sexual function as the primary endpoint.
An experimental therapy
According to Dr. Allemeyer and colleagues, two practical conclusions can be drawn from the study data available to date:
A possible primary effect of SNS on sexual function should be reviewed in high-quality, prospective studies that include detailed analyses of the different aspects of sexual dysfunction in both sexes.
An offer for trial-based SNS for sexual dysfunction should be made only at experienced sites with a multidisciplinary team of sex therapists and medical specialists and only after available therapy options have been exhausted and initially only within systematic studies.
This article was translated from Univadis Germany and a version appeared on Medscape.com.
FROM DIE GYNÄKOLOGIE
Postpartum sexual enjoyment: Does mode of delivery matter?
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
For some parents, resuming sexual intimacy after having a baby is a top priority. For others, not so much – and late-night feedings and diaper changes may not be the only hang-ups.
Dyspareunia – pain during sex – occurs in a substantial number of women after childbirth, and recent research sheds light on how psychological and biomedical factors relate to this condition.
Mode of delivery, for instance, may have less of an effect on sexual well-being than some people suspect.
Despite a perception that cesarean delivery might affect sexual function less than vaginal delivery does, how mothers delivered did not affect how often they had sex postpartum or the amount of enjoyment they got from it, according to research published in BJOG.
Eleven years after delivery, however, cesarean delivery was associated with a 74% increased likelihood of pain in the vagina during sex, compared with vaginal delivery, the researchers found (odds ratio, 1.74; 95% confidence interval, 1.46-2.08).
The results suggest that cesarean delivery “may not help protect against sexual dysfunction, as previously thought,” Flo Martin, a PhD student in epidemiology at the University of Bristol, United Kingdom, and lead author of the study, said in a news release.
For their study, Ms. Martin and her colleagues analyzed data from more than 10,300 participants in the Avon Longitudinal Study of Parents and Children, which recruited women in the United Kingdom who were pregnant in 1991 and 1992.
The researchers had data about pain during sex at 11 years. They had data about sexual enjoyment and frequency at 33 months, 5 years, 12 years, and 18 years after delivery.
If women experienced pain during sex years after cesarean delivery, uterine scarring might have been a cause, Ms. Martin and colleagues suggested. Alternatively, women with dyspareunia before delivery may be more likely to have cesarean surgery, which also could explain the association.
Other studies have likewise found that different modes of delivery generally lead to similar outcomes of sexual well-being after birth.
“Several of my own longitudinal studies have shown limited associations between mode of delivery and various aspects of sexual well-being, including sexual satisfaction, sexual function, and sexual desire,” said Natalie O. Rosen, PhD, director of the Couples and Sexual Health Laboratory at Dalhousie University, Halifax, N.S.
Nevertheless, other published studies have yielded conflicting results, so the question warrants further study, she said.
Pain catastrophizing
One study by Dr. Rosen’s group, published in Obstetrics & Gynecology, tracked sexual pain in 582 people from mid-pregnancy to 2 years postpartum.
About 21% of participants experienced moderate pain during sex, as determined by an average pain score greater than 4 on scale of 0-10 points. The rest were classified as having “minimal dyspareunia.”
Pain tended to peak at 3 months postpartum and then steadily decrease in both the moderate and minimal pain groups.
Mode of delivery did not affect the odds that a participant would have moderate dyspareunia. Neither did breastfeeding or prior chronic pain.
“But we did find one key thing to look out for: Those who reported a lot of negative thoughts and feelings about pain, something called pain catastrophizing, were more likely to experience moderate persistent pain during sex,” the researchers said in a video about their findings.
Pain catastrophizing 3 months after delivery was associated with significantly increased odds of following a moderate pain trajectory (odds ratio, 1.09; 95% confidence interval, 1.04-1.15).
Let’s talk about #postbabyhankypanky
Caring for a newborn while maintaining a romantic relationship can be challenging, and “there is a lack of evidence-based research aimed at helping couples prevent and navigate changes to their sexual well-being postpartum,” Dr. Rosen said.
During the 2-year study, a growing number of participants reported having sex less often over time. The percentage of women who had engaged in sexual activity in the past 4 weeks was 99% at baseline (20-24 weeks of gestation), 83.5% at 32 weeks of gestation, 73.9% at 3 months postpartum, and 69.6% at 2 years postpartum.
“One crucial way that couples sustain their connection is through their sexuality,” Dr. Rosen said. “Unfortunately, most new parents experience significant disruptions to their sexual function,” such as lower sexual desire or more pain during intercourse.
Dr. Rosen’s group has created a series of videos related to this topic dubbed #postbabyhankypanky to facilitate communication about sex postpartum. She encourages women with dyspareunia to talk with a health care provider because treatments such as cognitive-behavioral therapy, pelvic floor physical therapy, and topical medications can help manage pain.
‘Reassuring’ data
Veronica Gillispie-Bell, MD, MAS, director of quality for women’s services at the Ochsner Health System, New Orleans, said that she sees patients with postpartum sexual pain frequently.
Patients typically are instructed to have pelvic rest from delivery until 6 weeks after.
At the 6-week appointment, she tells patients to make sure that they are using lots of lubrication, because vaginal dryness related to hormonal changes during pregnancy and breastfeeding can make sex more painful, regardless of mode of delivery.
For many patients, she also recommends pelvic floor physical therapy.
As the medical director for the Louisiana Perinatal Quality Collaborative – a network of care providers, public health officials, and advocates that aims to improve outcomes for birthing persons, families, and newborns – Dr. Gillispie-Bell also is focused on reducing the rate of cesarean deliveries in the state. The BJOG study showing an increased risk for dyspareunia after a cesarean surgery serves as a reminder that there may be “long-term effects of having a C-section that may not be as obvious,” she said.
“C-sections are life-saving procedures, but they are not without risk,” Dr. Gillispie-Bell said.
Leila Frodsham, MBChB, a spokesperson for the Royal College of Obstetricians and Gynaecologists, told Medscape UK that it was “reassuring” to see “no difference in sexual enjoyment or sexual frequency at any time point postpartum between women who gave birth via cesarean section and those who delivered vaginally.”
“Women should be supported to make informed decisions about how they plan to give birth, and it is vital that health care professionals respect their preferences,” Dr. Frodsham added.
Clinicians should also remain aware that sexual pain is also common during periods of subfertility, perimenopause, and initiation of sexual activity.
Combinations of biological, psychological, and social factors can influence pain during sex, and there is an interpersonal element to keep in mind as well, Dr. Rosen noted.
“Pain during sex is typically elicited in the context of a partnered relationship,” Dr. Rosen said. “This means that this is an inherently interpersonal issue – let’s not forget about the partner who is both impacted by and can impact the pain through their own responses.”
A version of this article first appeared on Medscape.com.
Below the belt: sexual dysfunction overlooked in women with diabetes
Among patients with diabetes, women are just as likely as men to suffer from sexual dysfunction, but their issues are overlooked, with the narrative focusing mainly on the impact of this issue on men, say experts.
Women with diabetes can experience reduced sexual desire, painful sex, reduced lubrication, and sexual distress, increasing the risk of depression, and such issues often go unnoticed despite treatments being available, said Kirsty Winkley, PhD, diabetes nurse and health psychologist, King’s College London.
There is also the “embarrassment factor” on the side of both the health care professional and the patient, she said in a session she chaired at the Diabetes UK Professional Conference 2022. Many women with diabetes “wouldn’t necessarily know” that their sexual dysfunction “is related to their diabetes,” she told this news organization.
For women, sexual health conversations are “often about contraception and pregnancy,” as well as menstrual disorders, genital infections, and hormone replacement therapy. “As health care professionals, you’re trained to focus on those things, and you’re not really considering there might be sexual dysfunction. If women aren’t aware that it’s related to diabetes, you’ve got the perfect situation where it goes under the radar.”
However, cochair Debbie Cooke, PhD, health psychologist at the University of Surrey in Guildford, explained that having psychotherapy embedded within the diabetes team and “integrated throughout the whole service” means that the problem can be identified and treatment offered.
The issue is that such integration is “very uncommon” and access needs to be improved, Dr. Cooke said in an interview.
Sexual dysfunction major predictor of depression in women
Jacqueline Fosbury, psychotherapy lead at Diabetes Care for You, Sussex Community NHS Foundation Trust, said that “intimate activity is clearly beneficial for emotional and physical health,” as it is associated with increased oxytocin release, the burning of calories, better immunity, and improved sleep.
Sexual dysfunction is common in people with diabetes, she noted. Poor glycemic control can “damage” blood vessels and nerves, causing reduced blood flow and loss of sensation in sexual organs.
A recent study led by Belgian researchers found that among more than 750 adults with diabetes, 36% of men and 33% of women reported sexual dysfunction.
Sexual dysfunction was more common in women with type 1 diabetes, at 36%, compared with 26% for those with type 2 diabetes. The most commonly reported issues were decreased sexual desire, lubrication problems, orgasmic dysfunction, and pain. Body image problems and fear of hypoglycemia also affect sexuality and intimacy, leading to “sexual distress.”
Moreover, Ms. Fosbury said female sexual dysfunction has been identified as a “major predictor” of depression, which in turn reduces libido.
Treatments for women can include lubricants, local estrogen, and medications that are prescribed off-label, such as sildenafil. The same is true of testosterone therapy, which can be used to boost libido.
Couples therapy?
Next, Trudy Hannington, a psychosexual therapist with Leger Clinic, Doncaster, U.K., talked about how to use an integrated approach to address sexuality overall in people with diabetes.
She said this should be seen in a biopsychosocial context, with emphasis on the couple, on sensation and communication, and sexual growth, as well as changes in daily routines.
There should be a move away from “penetrative sex,” Ms. Hannington said, with the goal being “enjoyment, not orgasm.” Pleasure should be facilitated and the opportunities for “performance pressure and/or anxiety” reduced.
She discussed the case of Marie, a 27-year-old woman with type 1 diabetes who had been referred with painful sex and vaginal dryness. Marie had “never experienced orgasm,” despite being in a same-sex relationship with Emily.
Marie’s treatment involved a sexual growth program, to which Emily was invited, as well as recommendations to use lubricants, vibrators, and to try sildenafil.
Prioritize women
Ms. Fosbury reiterated that, in men, sexual dysfunction is “readily identified as a complication of diabetes” and is described as “traumatic” and “crucial to well-being.” It is also seen as “easy to treat” with medication, such as that for erectile dysfunction.
It is therefore crucial to talk to women with diabetes about possible sexual dysfunction, and the scene must be set before the appointment to explain that the subject will be broached. In addition, handouts and leaflets should be available for patients in the clinic so they can read about female sexual health and to lower the stigma around discussing it.
“Cultural stereotypes diminish the importance of female sexuality and prevent us from providing equal consideration to the sexual difficulties of our patients,” she concluded.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
Among patients with diabetes, women are just as likely as men to suffer from sexual dysfunction, but their issues are overlooked, with the narrative focusing mainly on the impact of this issue on men, say experts.
Women with diabetes can experience reduced sexual desire, painful sex, reduced lubrication, and sexual distress, increasing the risk of depression, and such issues often go unnoticed despite treatments being available, said Kirsty Winkley, PhD, diabetes nurse and health psychologist, King’s College London.
There is also the “embarrassment factor” on the side of both the health care professional and the patient, she said in a session she chaired at the Diabetes UK Professional Conference 2022. Many women with diabetes “wouldn’t necessarily know” that their sexual dysfunction “is related to their diabetes,” she told this news organization.
For women, sexual health conversations are “often about contraception and pregnancy,” as well as menstrual disorders, genital infections, and hormone replacement therapy. “As health care professionals, you’re trained to focus on those things, and you’re not really considering there might be sexual dysfunction. If women aren’t aware that it’s related to diabetes, you’ve got the perfect situation where it goes under the radar.”
However, cochair Debbie Cooke, PhD, health psychologist at the University of Surrey in Guildford, explained that having psychotherapy embedded within the diabetes team and “integrated throughout the whole service” means that the problem can be identified and treatment offered.
The issue is that such integration is “very uncommon” and access needs to be improved, Dr. Cooke said in an interview.
Sexual dysfunction major predictor of depression in women
Jacqueline Fosbury, psychotherapy lead at Diabetes Care for You, Sussex Community NHS Foundation Trust, said that “intimate activity is clearly beneficial for emotional and physical health,” as it is associated with increased oxytocin release, the burning of calories, better immunity, and improved sleep.
Sexual dysfunction is common in people with diabetes, she noted. Poor glycemic control can “damage” blood vessels and nerves, causing reduced blood flow and loss of sensation in sexual organs.
A recent study led by Belgian researchers found that among more than 750 adults with diabetes, 36% of men and 33% of women reported sexual dysfunction.
Sexual dysfunction was more common in women with type 1 diabetes, at 36%, compared with 26% for those with type 2 diabetes. The most commonly reported issues were decreased sexual desire, lubrication problems, orgasmic dysfunction, and pain. Body image problems and fear of hypoglycemia also affect sexuality and intimacy, leading to “sexual distress.”
Moreover, Ms. Fosbury said female sexual dysfunction has been identified as a “major predictor” of depression, which in turn reduces libido.
Treatments for women can include lubricants, local estrogen, and medications that are prescribed off-label, such as sildenafil. The same is true of testosterone therapy, which can be used to boost libido.
Couples therapy?
Next, Trudy Hannington, a psychosexual therapist with Leger Clinic, Doncaster, U.K., talked about how to use an integrated approach to address sexuality overall in people with diabetes.
She said this should be seen in a biopsychosocial context, with emphasis on the couple, on sensation and communication, and sexual growth, as well as changes in daily routines.
There should be a move away from “penetrative sex,” Ms. Hannington said, with the goal being “enjoyment, not orgasm.” Pleasure should be facilitated and the opportunities for “performance pressure and/or anxiety” reduced.
She discussed the case of Marie, a 27-year-old woman with type 1 diabetes who had been referred with painful sex and vaginal dryness. Marie had “never experienced orgasm,” despite being in a same-sex relationship with Emily.
Marie’s treatment involved a sexual growth program, to which Emily was invited, as well as recommendations to use lubricants, vibrators, and to try sildenafil.
Prioritize women
Ms. Fosbury reiterated that, in men, sexual dysfunction is “readily identified as a complication of diabetes” and is described as “traumatic” and “crucial to well-being.” It is also seen as “easy to treat” with medication, such as that for erectile dysfunction.
It is therefore crucial to talk to women with diabetes about possible sexual dysfunction, and the scene must be set before the appointment to explain that the subject will be broached. In addition, handouts and leaflets should be available for patients in the clinic so they can read about female sexual health and to lower the stigma around discussing it.
“Cultural stereotypes diminish the importance of female sexuality and prevent us from providing equal consideration to the sexual difficulties of our patients,” she concluded.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
Among patients with diabetes, women are just as likely as men to suffer from sexual dysfunction, but their issues are overlooked, with the narrative focusing mainly on the impact of this issue on men, say experts.
Women with diabetes can experience reduced sexual desire, painful sex, reduced lubrication, and sexual distress, increasing the risk of depression, and such issues often go unnoticed despite treatments being available, said Kirsty Winkley, PhD, diabetes nurse and health psychologist, King’s College London.
There is also the “embarrassment factor” on the side of both the health care professional and the patient, she said in a session she chaired at the Diabetes UK Professional Conference 2022. Many women with diabetes “wouldn’t necessarily know” that their sexual dysfunction “is related to their diabetes,” she told this news organization.
For women, sexual health conversations are “often about contraception and pregnancy,” as well as menstrual disorders, genital infections, and hormone replacement therapy. “As health care professionals, you’re trained to focus on those things, and you’re not really considering there might be sexual dysfunction. If women aren’t aware that it’s related to diabetes, you’ve got the perfect situation where it goes under the radar.”
However, cochair Debbie Cooke, PhD, health psychologist at the University of Surrey in Guildford, explained that having psychotherapy embedded within the diabetes team and “integrated throughout the whole service” means that the problem can be identified and treatment offered.
The issue is that such integration is “very uncommon” and access needs to be improved, Dr. Cooke said in an interview.
Sexual dysfunction major predictor of depression in women
Jacqueline Fosbury, psychotherapy lead at Diabetes Care for You, Sussex Community NHS Foundation Trust, said that “intimate activity is clearly beneficial for emotional and physical health,” as it is associated with increased oxytocin release, the burning of calories, better immunity, and improved sleep.
Sexual dysfunction is common in people with diabetes, she noted. Poor glycemic control can “damage” blood vessels and nerves, causing reduced blood flow and loss of sensation in sexual organs.
A recent study led by Belgian researchers found that among more than 750 adults with diabetes, 36% of men and 33% of women reported sexual dysfunction.
Sexual dysfunction was more common in women with type 1 diabetes, at 36%, compared with 26% for those with type 2 diabetes. The most commonly reported issues were decreased sexual desire, lubrication problems, orgasmic dysfunction, and pain. Body image problems and fear of hypoglycemia also affect sexuality and intimacy, leading to “sexual distress.”
Moreover, Ms. Fosbury said female sexual dysfunction has been identified as a “major predictor” of depression, which in turn reduces libido.
Treatments for women can include lubricants, local estrogen, and medications that are prescribed off-label, such as sildenafil. The same is true of testosterone therapy, which can be used to boost libido.
Couples therapy?
Next, Trudy Hannington, a psychosexual therapist with Leger Clinic, Doncaster, U.K., talked about how to use an integrated approach to address sexuality overall in people with diabetes.
She said this should be seen in a biopsychosocial context, with emphasis on the couple, on sensation and communication, and sexual growth, as well as changes in daily routines.
There should be a move away from “penetrative sex,” Ms. Hannington said, with the goal being “enjoyment, not orgasm.” Pleasure should be facilitated and the opportunities for “performance pressure and/or anxiety” reduced.
She discussed the case of Marie, a 27-year-old woman with type 1 diabetes who had been referred with painful sex and vaginal dryness. Marie had “never experienced orgasm,” despite being in a same-sex relationship with Emily.
Marie’s treatment involved a sexual growth program, to which Emily was invited, as well as recommendations to use lubricants, vibrators, and to try sildenafil.
Prioritize women
Ms. Fosbury reiterated that, in men, sexual dysfunction is “readily identified as a complication of diabetes” and is described as “traumatic” and “crucial to well-being.” It is also seen as “easy to treat” with medication, such as that for erectile dysfunction.
It is therefore crucial to talk to women with diabetes about possible sexual dysfunction, and the scene must be set before the appointment to explain that the subject will be broached. In addition, handouts and leaflets should be available for patients in the clinic so they can read about female sexual health and to lower the stigma around discussing it.
“Cultural stereotypes diminish the importance of female sexuality and prevent us from providing equal consideration to the sexual difficulties of our patients,” she concluded.
No funding declared. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
Pill not enough for ‘sexual problems’ female cancer patients face
The antidepressant bupropion failed to improve sexual dysfunction in female cancer survivors, according to new findings published online in the Journal of Clinical Oncology.
Using the Female Sexual Function Index (FSFI) as a measurement tool, investigators found that desire scores were not significantly different for participants who received bupropion versus a placebo over the 9-week study period.
“Sexual health is a complex phenomenon and [our results suggest that] no one intervention is going to solve the broader issue,” lead author Debra Barton, RN, PhD, FAAN, professor in the School of Nursing at the University of Michigan, Ann Arbor, told this news organization.
Sexual dysfunction is common among cancer survivors and experienced across multiple cancer types and stages of disease. Research shows that as many as 70% of female cancer survivors report loss of desire, compared with up to one-third of the general population.
Common sexual concerns among female cancer survivors include low desire, arousal issues, lack of appropriate lubrication, difficulty in achieving orgasm, and pain with penetrative sexual activity. Additionally, these women may experience significant overlap of symptoms, and often encounter multiple sexual issues that are exacerbated by a range of cancer treatments.
“It’s a huge problem,” Maryam B. Lustberg, MD, MPH, from Yale Cancer Center, New Haven, Conn., and colleagues wrote in an accompanying editorial.
Despite the prevalence of sexual dysfunction among cancer survivors, effective treatments remain elusive. Preliminary evidence suggests that bupropion, already approved for seasonal affective disorder, major depressive disorder, and smoking cessation, may also enhance libido.
Dr. Barton and colleagues conducted this phase 2 trial to determine whether bupropion can improve sexual desire in female cancer survivors without undesirable side effects.
In the study, Dr. Barton and colleagues compared two dose levels of extended-release bupropion in a cohort of 230 postmenopausal women diagnosed with breast or gynecologic cancer and low baseline FSFI desire scores (<3.3), who had completed definitive cancer therapy.
Participants were randomized to receive either 150 mg (79 patients) or 300 mg (74 patients) once daily of extended-release bupropion, or placebo (77 patients).
Barton and colleagues then evaluated whether sexual desire significantly improved over the 9-week study period comparing the bupropion arms and the placebo group.
Overall, the authors found no significant differences (mean between-arm change for 150 mg once daily and placebo of 0.02; P = .93; mean between-arm change for 300 mg once daily and placebo of –0.02; P = .92). Mean scores at 9 weeks on the desire subscale were 2.17, 2.27, and 2.30 for 150 mg, 300 mg, and the placebo group, respectively.
In addition, none of the subscales – which included arousal, lubrication, and orgasm – or the total score showed a significant difference between arms at either 5 or 9 weeks.
Bupropion did, however, appear to be well tolerated. No grade 4-5 treatment-related adverse events occurred. In the 150-mg bupropion arm, two patients (2.6%) experienced a grade 3 event (insomnia and headache) and one patient in the 300-mg bupropion arm (1.4%) and placebo arm (1.3%) experienced a grade 3 event related to treatment (hypertension and headache, respectively).
In the accompanying editorial, Dr. Lustberg and colleagues “applaud the authors for conducting a study in this population of cancer survivors,” noting that “evidenced-based approaches have not been extensively studied.”
Dr. Lustberg and colleagues also commented that other randomized controlled clinical trials evaluating sexual desire disorder assessed outcomes using additional metrics, such as the Female Sexual Distress Scale–Revised questionnaire, which measures distress related to sexual dysfunction and low desire, in particular.
“The use of specific validated instruments for libido in place of the FSFI might have helped determine the effect of the study intervention in this reported trial,” they wrote.
Overall, according to Dr. Lustberg and colleagues, the negative results of this study indicate that a multidisciplinary clinical approach may be needed.
“As much as we would like to have one intervention that addresses this prominent issue, the evidence strongly suggests that cancer-related sexual problems may need an integrative biopsychosocial model that intervenes on biologic, psychologic, interpersonal, and social-cultural factors, not just on one factor, such as libido,” they wrote. “Such work may require access to multidisciplinary care with specialists in women’s health, pelvic floor rehabilitation, and psychosocial oncology.”
Dr. Barton said she has been developing a multicomponent approach to addressing sexual health in female cancer survivors.
However, she noted, “there is still much we do not fully understand about the broader impact of the degree of hormone deprivation in the population of female cancer survivors. A better understanding would provide clearer targets for interventions.”
The study was supported by the National Cancer Institute and Breast Cancer Research Foundation. Dr. Barton has disclosed research funding from Merck. Dr. Lustberg reported receiving honoraria from Novartis and Biotheranostics; consulting or advising with PledPharma, Disarm Therapeutics, Pfizer; and other relationships with Cynosure/Hologic.
A version of this article first appeared on Medscape.com.
The antidepressant bupropion failed to improve sexual dysfunction in female cancer survivors, according to new findings published online in the Journal of Clinical Oncology.
Using the Female Sexual Function Index (FSFI) as a measurement tool, investigators found that desire scores were not significantly different for participants who received bupropion versus a placebo over the 9-week study period.
“Sexual health is a complex phenomenon and [our results suggest that] no one intervention is going to solve the broader issue,” lead author Debra Barton, RN, PhD, FAAN, professor in the School of Nursing at the University of Michigan, Ann Arbor, told this news organization.
Sexual dysfunction is common among cancer survivors and experienced across multiple cancer types and stages of disease. Research shows that as many as 70% of female cancer survivors report loss of desire, compared with up to one-third of the general population.
Common sexual concerns among female cancer survivors include low desire, arousal issues, lack of appropriate lubrication, difficulty in achieving orgasm, and pain with penetrative sexual activity. Additionally, these women may experience significant overlap of symptoms, and often encounter multiple sexual issues that are exacerbated by a range of cancer treatments.
“It’s a huge problem,” Maryam B. Lustberg, MD, MPH, from Yale Cancer Center, New Haven, Conn., and colleagues wrote in an accompanying editorial.
Despite the prevalence of sexual dysfunction among cancer survivors, effective treatments remain elusive. Preliminary evidence suggests that bupropion, already approved for seasonal affective disorder, major depressive disorder, and smoking cessation, may also enhance libido.
Dr. Barton and colleagues conducted this phase 2 trial to determine whether bupropion can improve sexual desire in female cancer survivors without undesirable side effects.
In the study, Dr. Barton and colleagues compared two dose levels of extended-release bupropion in a cohort of 230 postmenopausal women diagnosed with breast or gynecologic cancer and low baseline FSFI desire scores (<3.3), who had completed definitive cancer therapy.
Participants were randomized to receive either 150 mg (79 patients) or 300 mg (74 patients) once daily of extended-release bupropion, or placebo (77 patients).
Barton and colleagues then evaluated whether sexual desire significantly improved over the 9-week study period comparing the bupropion arms and the placebo group.
Overall, the authors found no significant differences (mean between-arm change for 150 mg once daily and placebo of 0.02; P = .93; mean between-arm change for 300 mg once daily and placebo of –0.02; P = .92). Mean scores at 9 weeks on the desire subscale were 2.17, 2.27, and 2.30 for 150 mg, 300 mg, and the placebo group, respectively.
In addition, none of the subscales – which included arousal, lubrication, and orgasm – or the total score showed a significant difference between arms at either 5 or 9 weeks.
Bupropion did, however, appear to be well tolerated. No grade 4-5 treatment-related adverse events occurred. In the 150-mg bupropion arm, two patients (2.6%) experienced a grade 3 event (insomnia and headache) and one patient in the 300-mg bupropion arm (1.4%) and placebo arm (1.3%) experienced a grade 3 event related to treatment (hypertension and headache, respectively).
In the accompanying editorial, Dr. Lustberg and colleagues “applaud the authors for conducting a study in this population of cancer survivors,” noting that “evidenced-based approaches have not been extensively studied.”
Dr. Lustberg and colleagues also commented that other randomized controlled clinical trials evaluating sexual desire disorder assessed outcomes using additional metrics, such as the Female Sexual Distress Scale–Revised questionnaire, which measures distress related to sexual dysfunction and low desire, in particular.
“The use of specific validated instruments for libido in place of the FSFI might have helped determine the effect of the study intervention in this reported trial,” they wrote.
Overall, according to Dr. Lustberg and colleagues, the negative results of this study indicate that a multidisciplinary clinical approach may be needed.
“As much as we would like to have one intervention that addresses this prominent issue, the evidence strongly suggests that cancer-related sexual problems may need an integrative biopsychosocial model that intervenes on biologic, psychologic, interpersonal, and social-cultural factors, not just on one factor, such as libido,” they wrote. “Such work may require access to multidisciplinary care with specialists in women’s health, pelvic floor rehabilitation, and psychosocial oncology.”
Dr. Barton said she has been developing a multicomponent approach to addressing sexual health in female cancer survivors.
However, she noted, “there is still much we do not fully understand about the broader impact of the degree of hormone deprivation in the population of female cancer survivors. A better understanding would provide clearer targets for interventions.”
The study was supported by the National Cancer Institute and Breast Cancer Research Foundation. Dr. Barton has disclosed research funding from Merck. Dr. Lustberg reported receiving honoraria from Novartis and Biotheranostics; consulting or advising with PledPharma, Disarm Therapeutics, Pfizer; and other relationships with Cynosure/Hologic.
A version of this article first appeared on Medscape.com.
The antidepressant bupropion failed to improve sexual dysfunction in female cancer survivors, according to new findings published online in the Journal of Clinical Oncology.
Using the Female Sexual Function Index (FSFI) as a measurement tool, investigators found that desire scores were not significantly different for participants who received bupropion versus a placebo over the 9-week study period.
“Sexual health is a complex phenomenon and [our results suggest that] no one intervention is going to solve the broader issue,” lead author Debra Barton, RN, PhD, FAAN, professor in the School of Nursing at the University of Michigan, Ann Arbor, told this news organization.
Sexual dysfunction is common among cancer survivors and experienced across multiple cancer types and stages of disease. Research shows that as many as 70% of female cancer survivors report loss of desire, compared with up to one-third of the general population.
Common sexual concerns among female cancer survivors include low desire, arousal issues, lack of appropriate lubrication, difficulty in achieving orgasm, and pain with penetrative sexual activity. Additionally, these women may experience significant overlap of symptoms, and often encounter multiple sexual issues that are exacerbated by a range of cancer treatments.
“It’s a huge problem,” Maryam B. Lustberg, MD, MPH, from Yale Cancer Center, New Haven, Conn., and colleagues wrote in an accompanying editorial.
Despite the prevalence of sexual dysfunction among cancer survivors, effective treatments remain elusive. Preliminary evidence suggests that bupropion, already approved for seasonal affective disorder, major depressive disorder, and smoking cessation, may also enhance libido.
Dr. Barton and colleagues conducted this phase 2 trial to determine whether bupropion can improve sexual desire in female cancer survivors without undesirable side effects.
In the study, Dr. Barton and colleagues compared two dose levels of extended-release bupropion in a cohort of 230 postmenopausal women diagnosed with breast or gynecologic cancer and low baseline FSFI desire scores (<3.3), who had completed definitive cancer therapy.
Participants were randomized to receive either 150 mg (79 patients) or 300 mg (74 patients) once daily of extended-release bupropion, or placebo (77 patients).
Barton and colleagues then evaluated whether sexual desire significantly improved over the 9-week study period comparing the bupropion arms and the placebo group.
Overall, the authors found no significant differences (mean between-arm change for 150 mg once daily and placebo of 0.02; P = .93; mean between-arm change for 300 mg once daily and placebo of –0.02; P = .92). Mean scores at 9 weeks on the desire subscale were 2.17, 2.27, and 2.30 for 150 mg, 300 mg, and the placebo group, respectively.
In addition, none of the subscales – which included arousal, lubrication, and orgasm – or the total score showed a significant difference between arms at either 5 or 9 weeks.
Bupropion did, however, appear to be well tolerated. No grade 4-5 treatment-related adverse events occurred. In the 150-mg bupropion arm, two patients (2.6%) experienced a grade 3 event (insomnia and headache) and one patient in the 300-mg bupropion arm (1.4%) and placebo arm (1.3%) experienced a grade 3 event related to treatment (hypertension and headache, respectively).
In the accompanying editorial, Dr. Lustberg and colleagues “applaud the authors for conducting a study in this population of cancer survivors,” noting that “evidenced-based approaches have not been extensively studied.”
Dr. Lustberg and colleagues also commented that other randomized controlled clinical trials evaluating sexual desire disorder assessed outcomes using additional metrics, such as the Female Sexual Distress Scale–Revised questionnaire, which measures distress related to sexual dysfunction and low desire, in particular.
“The use of specific validated instruments for libido in place of the FSFI might have helped determine the effect of the study intervention in this reported trial,” they wrote.
Overall, according to Dr. Lustberg and colleagues, the negative results of this study indicate that a multidisciplinary clinical approach may be needed.
“As much as we would like to have one intervention that addresses this prominent issue, the evidence strongly suggests that cancer-related sexual problems may need an integrative biopsychosocial model that intervenes on biologic, psychologic, interpersonal, and social-cultural factors, not just on one factor, such as libido,” they wrote. “Such work may require access to multidisciplinary care with specialists in women’s health, pelvic floor rehabilitation, and psychosocial oncology.”
Dr. Barton said she has been developing a multicomponent approach to addressing sexual health in female cancer survivors.
However, she noted, “there is still much we do not fully understand about the broader impact of the degree of hormone deprivation in the population of female cancer survivors. A better understanding would provide clearer targets for interventions.”
The study was supported by the National Cancer Institute and Breast Cancer Research Foundation. Dr. Barton has disclosed research funding from Merck. Dr. Lustberg reported receiving honoraria from Novartis and Biotheranostics; consulting or advising with PledPharma, Disarm Therapeutics, Pfizer; and other relationships with Cynosure/Hologic.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
2021 Update on female sexual health
The approach to diagnosis and treatment of female sexual function continues to be a challenge for women’s health professionals. The search for a female “little blue pill” remains elusive as researchers struggle to understand the mechanisms that underlie the complex aspects of female sexual health. This Update will review the recent literature on the use of fractional CO2 laser for treatment of female sexual dysfunction and vulvovaginal symptoms. Bottom line: While the quality of the studies is poor overall, fractional CO2 laser treatment seems to temporarily improve symptoms of genitourinary syndrome of menopause (GSM). The duration of response, cost, and the overall long-term impact on sexual health remain in question.
A retrospective look at CO2 laser and postmenopausal GSM
Filippini M, Luvero D, Salvatore S, et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause. 2019;27:43-49. doi: 10.1097/GME. 0000000000001428.
Researchers conducted a retrospective, multicenter study of postmenopausal women with at least one symptom of GSM, including itching, burning, dyspareunia with penetration, and dryness.
Study details
A total of 171 of the 645 women (26.5%) were oncology patients. Women were excluded from analysis if they used any form of topical therapy within 15 days; had prolapse stage 2 or greater; or had any infection, abscess, or anatomical deformity precluding treatment with the laser.
Patients underwent gynecologic examination and were given a questionnaire to assess vulvovaginal symptoms. Exams occurred monthly during treatment (average, 6.5 months), at 6- and 12-months posttreatment, and then annually. No topical therapy was advised during or after treatment.
Patients received either 3 or 4 fractional CO2 laser treatments to the vulva and/or vagina depending on symptom location and type. Higher power settings of the same laser were used to treat vaginal symptoms (40W; 1,000 microseconds) versus vulvar symptoms (25W; 500 microseconds). Treatment sessions were 5 to 6 minutes. The study authors used a visual analog rating scale (VAS) for “atrophy and related symptoms,” tested vaginal pH, and completed the Vaginal Health Index Score. VAS scores were obtained from the patients prior to the initial laser intervention and 1 month after the final treatment.
Results
There were statistically significant improvements in dryness, vaginal orifice pain, dyspareunia, itching, and burning for both the 3-treatment and 4-treatment cohorts. The delta of improvement was then compared for the 2 subgroups; curiously, there was greater improvement of symptoms such as dryness (65% vs 61%), itching (78% vs 72%), burning (72% vs 67%), and vaginal orifice pain (67% vs 60%) in the group that received 3 cycles than in the group that received 4 cycles.
With regard to vaginal pH improvement, the 4-cycle group performed better than the 3-cycle group (1% improvement in the 4-cycle group vs 6% in the 3-cycle group). Although vaginal pH reduction was somewhat better in the group that received 4 treatments, and the pre versus posttreatment percentages were statistically significantly different, the clinical significance of a pH difference between 5.72 and 5.53 is questionable, especially since there was a greater difference in baseline pH between the two cohorts (6.08 in the 4-cycle group vs 5.59 in the 3-cycle group).
There were no reported adverse events related to the fractional laser treatments, and 6% of the patients underwent additional laser treatments during the followup timeframe of 8 to 20 months.
This was a retrospective study with no control or comparison group and short-term follow-up. The VAS scores were obtained 1 month after the final treatment. Failure to request additional treatment at 8 to 20 months cannot be used to infer that the therapeutic improvements recorded at 1 month were enduring. In addition, although the large number of patients in this study may lead to statistical significance, clinical significance is still questionable. Given the lack of a comparison group and the very short follow-up, it is hard to draw any scientifically valid conclusions from this study.
Continue to: Randomized data on CO2 laser vs Kegels for sexual dysfunction...
Randomized data on CO2 laser vs Kegels for sexual dysfunction
Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
In a small randomized controlled trial (RCT) conducted in China, Lou and colleagues identified premenopausal women at “high risk” for sexual dysfunction as determined by the Chinese version of the Female Sexual Function Index (CFSFI).
Details of the study
A total of 84 women (mean age, 36.5 years) were included in the study. All the participants were heterosexual and married or with a long-term partner. The domain of sexual dysfunction was not considered. Women were excluded if they had no current heterosexual partner; had genital malformation, urinary incontinence, or prolapse stage 2 or higher; a history of pelvic floor mesh treatment; current gynecologic malignancy; abnormal cervical cytology; or were currently pregnant or postpartum. In addition, women were excluded if they had been treated previously for sexual dysfunction or mental “disease.” The cohort was randomized to receive fractional CO2 laser treatments (three 15-minute treatments 1 month apart at 60W, 1,000 microseconds) or coached Kegel exercises (10 exercises repeated twice daily at least 3 times/week and monitored by physical therapists at biweekly clinic visits). Sexual distress was evaluated by using the Female Sexual Distress Scale-Revised (FSDSR). Outcomes measured were pelvic floor muscle strength and scores on the CFSFI and FSDSR. Data were obtained at 3, 6, 9, and 12 months after initiation of therapy.
Both groups showed improvement
The laser cohort showed slightly more improvement in scale scores at 6 and 12 months. Specifically, the laser group had better scores on lubrication and overall satisfaction, with moderate effect size; neither group had improvements in arousal, desire, or orgasm. The Kegel group showed a significant improvement in pelvic floor strength and orgasm at 12 months, an improvement not seen in the laser cohort. Both groups showed gradual improvement in the FSDSR, with the laser group reporting a lower score (10.0) at 12 months posttreatment relative to the Kegel group (11.1). Again, these were modest effects as baseline scores for both cohorts were around 12.5. There were minimal safety signals in the laser group, with 22.5% of women reporting scant bloody discharge posttreatment and 72.5% describing mild discomfort (1 on a 1–10 VAS scale) during the procedure.
This study is problematic in several areas. Although it was a prospective, randomized trial, it was not blinded, and the therapeutic interventions were markedly different in nature and requirement for individual patient motivation. The experiences of sexual dysfunction among the participants were not stratified by type—arousal, desire, lubrication, orgasm, or pain. All patients had regular cyclic menses; however, the authors do not report on contraceptive methods, hormonal therapy, or other comorbid conditions that could impact sexual health. The cohorts may or may not have been similar in baseline types of sexual dissatisfaction.
CO2 laser for lichen sclerosus: Is it effective?
Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422. doi: 10.1097 /GME.0000000000001482.
Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi: 10.1097 /AOG.0000000000004332.
Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097 /AOG.0000000000004409.
High potency corticosteroid ointment is the current standard treatment for lichen sclerosus. Alternative options for disease that is refractory to steroids are limited. Three studies published in the past year explored the CO2 laser’s ability to treat lichen sclerosus symptoms and resultant sexual dysfunction—Pagano and colleagues conducted a small prospective study and Burkett and colleagues and Mitchell et al conducted small RCTs.
Details of the Pagano study
Three premenopausal and 37 postmenopausal women with refractory lichen sclerosus (defined as no improvement after 4 cycles of ultra-high potency steroids) were included in the study. Lichen sclerosus was uniformly biopsy confirmed. Women using topical or systemic hormones were excluded. VAS was administered prior to initial treatment and after each of 2 fractional CO2 treatments (25–30 W; 1,000 microseconds) 30 to 40 days apart to determine severity of vulvar itching, dyspareunia with penetration, vulvar dryness, sexual dysfunction, and procedure discomfort. Follow-up was conducted at 1 month after the final treatment. VAS score for the primary outcome of vulvar itching declined from 8 pretreatment to 6 after the first treatment and to 3 after the second. There was no significant treatment-related pain reported.
The authors acknowledged the limitations of their study; it was a relatively small sample size, nonrandomized and had short-term follow-up of a mixed patient population and no sham or control group. The short-term improvements reported in the study patients may not be sustained without ongoing treatment for a lifelong chronic disease, and the long-term potential for development of squamous cell carcinoma may or may not be ameliorated.
Continue to: Burkett et al: RCT study 1...
Burkett et al: RCT study 1
A total of 52 postmenopausal patients with biopsy-proven lichen sclerosus were randomly assigned to clobetasol or CO2 laser; 51 women completed 6-month follow-up. The outcomes were stratified by prior high-potency steroid use. The steroid cohort used clobetasol 0.05% nightly for 1 month, 3 times per week for 2 months, then as needed. The laser cohort received 3 treatments (26 W; 800 microseconds) 4 to 6 weeks apart. Overall adherence was only 75% in the clobetasol group, compared with 96% in the laser group. The authors found treatment efficacy of CO2 laser therapy only in the group of patients who had prior treatment with high potency topical corticosteroids. They conclude that, …“Despite previously optimistic results in well designed clinical trials of fractionated CO2 for genitourinary syndrome of menopause, and in noncontrolled case series for vulvar lichen sclerosus, our study failed to show any significant benefit of monotherapy of fractionated CO2 for vulvar lichen sclerosus. There may be a role for fractionated CO2 as an adjuvant therapy along with topical ultrapotent corticosteroids in vulvar lichen sclerosus.”
Mitchell et al: RCT study 2
This was a double blind, placebo-controlled, and histologically validated study of fractional CO2 for treatment of lichen sclerosus in 35 women; 17 in the treatment arm and 18 in the sham laser encounters. At least a 4-week no treatment period of topical steroids was required before monotherapy with CO2 laser was initiated.
The authors found no difference in their primary outcome—histopathology scale scores—after 5 treatments over 24 weeks. Secondary endpoints were changes in the CSS (Clinical Scoring System for Vulvar Lichen Sclerosus), a validated instrument that includes both a clinician’s examination of the severity of disease and a patient’s report of the severity of her symptoms. The patient score is the total of 4 domains: itching, soreness, burning, and dyspareunia. The clinician objective examination documents fissures, erosions, hyperkeratosis, agglutination, stenosis, and atrophy. At the conclusion of treatment there were no significant differences in the patient reported symptoms or the clinical findings between the active treatment and sham groups.
As a monotherapy, CO2 laser therapy is not effective in treating lichen sclerosus, although it may help improve symptoms as an adjunct to high potency steroid therapy when topical treatment alone has failed to provide adequate response.
Conclusion
The quality of evidence to support the use of the CO2 laser for improvement in sexual dysfunction is poor. Although patient satisfaction scores improved overall, and most specifically for symptoms related to GSM, the lack of blinding; inappropriate or no control groups; the very short-term outcomes; and for one of the studies, the lack of a clear definition of sexual dysfunction, make it difficult to draw meaningful conclusions for clinical care.
For GSM, we know that topical estrogen therapy works—and with little to no systemic absorption. The CO2 laser should be studied in comparison to this gold standard, with consideration of costs and potential long-term harms in addition to patient satisfaction and short-term measures of improvement. In addition, and very importantly, it is our professional responsibility to present the evidence for safety of topical estrogens to our professional colleagues as well as to our patients with estrogen-dependent cancers so that they understand the value of estrogen as a safe and appropriate alternative to expensive and potentially short-term interventions such as CO2 laser treatment. ●

Cheryl Iglesia, MD
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She is a member of the OBG Management Board of Editors.
Barbara Levy, MD: Cheryl, you have more experience with use of the energy-based cosmetic laser than most ObGyns, and I thought that speaking with you about this technology would be of benefit, not only to me in learning more about the hands-on experience of a lead researcher and practitioner but also readers who are hearing more and more about the growth of cosmetic gynecology in general. Thank you for taking the time today.
Cheryl Iglesia, MD: I’m happy to speak about this with you, Barbara.
Dr. Levy: Specifically, I would like to talk about use of these technologies for sexual dysfunction. In the last few years some of the available data have been on the CO2 laser versus physical therapy, which is not an appropriate comparison.1
Dr. Iglesia: There have been limited data, and less randomized, controlled data, on laser and radiofrequency energies for cosmetic gynecology, and in fact these devices remain unapproved for any gynecologic indication. In 2018 the US Food and Drug Administration (FDA) issued a Safety Communication about the use of energy-based devices to perform vaginal rejuvenation or cosmetic procedures. The International Urogynecological Association (IUGA) issued a consensus statement echoing concerns about the devices, and an International Continence Society/International Society for the Study of Vulvovaginal Disease Best Practice Consensus Statement did not recommend the laser for “routine treatment of vaginal atrophy or urinary incontinence unless treatment is part of a well-designed trial or with special arrangements for clinical governance, consent, and audit.”2
In May 2020, as evidence remains limited (although 522 studies are ongoing in coordination with the FDA), the American Urogynecologic Society (AUGS) published a clinical consensus statement from a panel of experts in female pelvic medicine and reconstructive surgery. The panel had about 90% consensus that there is short-term efficacy for the laser with GSM and dyspareunia. But we only have outcomes data that lasts a maximum of 1 year.2
A problem with our VeLVET trial,3 which was published in Menopause, and the Cruz and colleagues’ trial from South America,4 both of which compared the CO2 laser to estrogen and had randomized groups, was that they were limited by the outcome measures used, none of which were consistently validated. But these studies also had small numbers of participants and short-term follow-up. So I don’t think there are much existing data that are promising for supporting energy-based treatment for GSM.
We also have just-published data on the laser for lichen sclerosus.5 For the AUGS panel, there was about 80% consensus for energy-based-device use and lichen sclerosus.2 According to Mitchell et al, who conducted a small, randomized, sham-controlled trial, CO2 laser resulted in no significant difference in histopathology scale score between active and sham arms.5
Future trials may want to assess laser as a mechanism for improved local drug delivery (eg, use of combined laser plus local estrogen for GSM or combined laser plus topical steroid for lichen sclerosus). I am also aware that properly designed laser versus sham studies are underway.
Dr. Levy: What about for stress urinary incontinence (SUI)? I don’t think these technologies are going to work.
Dr. Iglesia: For the AUGS panel, there was only about 70% consensus for energy-based-device use and SUI,2 and I’m one of the naysayers. The pathophysiology of SUI is so multifactorial that it’s hard to believe that laser or radiofrequency wand therapy could have sustained improvements, especially since prior radiofrequency therapy from the last decade (for instance, Renessa, Novasys Medical) did not show long-term efficacy.
Understanding lasers and coordinating care
Dr. Levy: We don’t know what the long-term outcomes are for the CO2 laser and GSM.
Dr. Iglesia: I agree with you, and I think there needs to be an understanding of the mechanism of how lasers work, whether it be erbium (Er:YAG), which is the most common, or CO2. Erbium and CO2 lasers, which are on the far-infrared spectrum, target the chromophore, water. My feeling is that, when you look at results from the Cruz trial,4 or even our trial that compared vaginal estrogen with laser,3 when there is severe GSM and high pH with virtually no water present in the tissues, that laser is not going to properly function. But I don’t think we know exactly what optimal pretreatment is necessary, and that is one of the problems. Furthermore, when intravaginal lasers are done and no adequate speculum exam is conducted prior to introducing the laser, there could be discharge or old creams present that block the mirrors necessary to adequately fire the fractionated laser beams.
Unfortunately, oftentimes these devices are marketed to women with breast cancer, who may be taking aromatase inhibitors, which cause the no-water problem; they dry out everything. They are effective for preventing breast cancer recurrence, but they cause severe atrophy (perhaps worse than many of the other selective estrogen-receptor modulators), with a resultant high vaginal pH. If we can bring that pH level down, closer to the normal 4.5 range so that we could have some level of moisture, and add estrogen first, the overall treatment approach will probably be more effective. We still do not know what happens after 1 year, though, and how often touch-ups need to be performed.
In fact, when working with a patient with breast cancer, I will speak with her oncologist; I will collaborate to put in place a treatment plan that may include initial pretreatment with low-dose vaginal estrogen followed by laser treatment for vaginal atrophy. But I will make sure I use the lowest dose. Sometimes when the patient comes back, the estrogen’s worked so well she’ll say, “Oh, I’m happy, so I don’t need the laser anymore.” A balanced conversation is necessary, especially with cancer survivors.
Informing patients and colleagues
Dr. Levy: I completely agree, and I think one of the key points here is that our purpose is to serve our patients. The data demonstrate that low doses of vaginal estrogen are not harmful for women who are being treated for or who have recovered from breast cancer. It is our ethical obligation to convince these women and their oncologists that ongoing treatment with vaginal estrogen not only will help their GSM but also their overactive bladder and their risk of urinary tract infections and other things. We could be exploiting patients who are really fearful of using any estrogen because of a perceived cancer risk. We could actually be validating their fear by telling them we have an alternative treatment for which they have to pay cash.
Treatment access
Dr. Iglesia: Yes, these are not cosmetic conditions that we are treating. So my goal when evaluating treatment for refractory GSM or lichen sclerosus is to find optimal energy-based therapies with the hope that one day these will be approved gynecologic conditions by the US FDA for laser and wand therapies and that they will ultimately not be out-of-pocket expenses but rather therapies covered by insurance.
Dr. Levy: Great. I understand that AUGS/IUGA have been working on a terminology algorithm to help distinguish between procedures being performed to resolve a medical problem such as prolapse or incontinence versus those designed to be cosmetic.
Dr. Iglesia: Yes, there is a big document from experts in both societies out for public comment right now. It will hopefully be published soon.
Outstanding questions remain
Dr. Levy: Really, we as ObGyns shouldn’t be quick to incorporate these things into our practices without high-quality studies demonstrating value. I have a major concern about these devices in the long term. When you look at fractional CO2 use on the face, for instance, which is a much different type of skin than the vagina, the laser builds collagen—but we don’t have long-term outcome results. The vagina is supposed to be an elastic tissue, so what is the risk of long-term scarring there? Yes, the laser builds collagen in the vaginal epithelium, but what does it do to scarring in the rest of the tissue? We don’t have answers to that.
Dr. Iglesia: And that is the question—how does histology equate with function? Well, I would go with what the patients are reporting.
Dr. Levy: Absolutely. But the thing about vaginal low-dose estrogen is that it is something that the oncologists or the ObGyns could be implementing with patients while they are undergoing cancer therapy, while in their menopausal transition, to preserve vulvovaginal function as opposed to trying to regain it.
Dr. Iglesia: Certainly, although it still needs to be determined when that type of approach would actually be contraindicated.
Dr. Levy: Thank you, Cheryl, for your valuable insights.
Dr. Iglesia: Of course. Thank you. ●
References
1. Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
2. Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices. Female Pelvic Med Reconstr Surg. 2020;26:287-298. doi: 10.1097 /SPV.0000000000000872.
3. Paraiso MF, Ferrando CA, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET Trial. Menopause. 2020;27:50-56. doi: 10.1097/GME.0000000000001416.
4. Cruz VL, Steiner ML, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28. doi: 10.1097 /GME.0000000000000955.
5. Mitchell L, Goldstein A, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097/AOG.0000000000004409.

The approach to diagnosis and treatment of female sexual function continues to be a challenge for women’s health professionals. The search for a female “little blue pill” remains elusive as researchers struggle to understand the mechanisms that underlie the complex aspects of female sexual health. This Update will review the recent literature on the use of fractional CO2 laser for treatment of female sexual dysfunction and vulvovaginal symptoms. Bottom line: While the quality of the studies is poor overall, fractional CO2 laser treatment seems to temporarily improve symptoms of genitourinary syndrome of menopause (GSM). The duration of response, cost, and the overall long-term impact on sexual health remain in question.
A retrospective look at CO2 laser and postmenopausal GSM
Filippini M, Luvero D, Salvatore S, et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause. 2019;27:43-49. doi: 10.1097/GME. 0000000000001428.
Researchers conducted a retrospective, multicenter study of postmenopausal women with at least one symptom of GSM, including itching, burning, dyspareunia with penetration, and dryness.
Study details
A total of 171 of the 645 women (26.5%) were oncology patients. Women were excluded from analysis if they used any form of topical therapy within 15 days; had prolapse stage 2 or greater; or had any infection, abscess, or anatomical deformity precluding treatment with the laser.
Patients underwent gynecologic examination and were given a questionnaire to assess vulvovaginal symptoms. Exams occurred monthly during treatment (average, 6.5 months), at 6- and 12-months posttreatment, and then annually. No topical therapy was advised during or after treatment.
Patients received either 3 or 4 fractional CO2 laser treatments to the vulva and/or vagina depending on symptom location and type. Higher power settings of the same laser were used to treat vaginal symptoms (40W; 1,000 microseconds) versus vulvar symptoms (25W; 500 microseconds). Treatment sessions were 5 to 6 minutes. The study authors used a visual analog rating scale (VAS) for “atrophy and related symptoms,” tested vaginal pH, and completed the Vaginal Health Index Score. VAS scores were obtained from the patients prior to the initial laser intervention and 1 month after the final treatment.
Results
There were statistically significant improvements in dryness, vaginal orifice pain, dyspareunia, itching, and burning for both the 3-treatment and 4-treatment cohorts. The delta of improvement was then compared for the 2 subgroups; curiously, there was greater improvement of symptoms such as dryness (65% vs 61%), itching (78% vs 72%), burning (72% vs 67%), and vaginal orifice pain (67% vs 60%) in the group that received 3 cycles than in the group that received 4 cycles.
With regard to vaginal pH improvement, the 4-cycle group performed better than the 3-cycle group (1% improvement in the 4-cycle group vs 6% in the 3-cycle group). Although vaginal pH reduction was somewhat better in the group that received 4 treatments, and the pre versus posttreatment percentages were statistically significantly different, the clinical significance of a pH difference between 5.72 and 5.53 is questionable, especially since there was a greater difference in baseline pH between the two cohorts (6.08 in the 4-cycle group vs 5.59 in the 3-cycle group).
There were no reported adverse events related to the fractional laser treatments, and 6% of the patients underwent additional laser treatments during the followup timeframe of 8 to 20 months.
This was a retrospective study with no control or comparison group and short-term follow-up. The VAS scores were obtained 1 month after the final treatment. Failure to request additional treatment at 8 to 20 months cannot be used to infer that the therapeutic improvements recorded at 1 month were enduring. In addition, although the large number of patients in this study may lead to statistical significance, clinical significance is still questionable. Given the lack of a comparison group and the very short follow-up, it is hard to draw any scientifically valid conclusions from this study.
Continue to: Randomized data on CO2 laser vs Kegels for sexual dysfunction...
Randomized data on CO2 laser vs Kegels for sexual dysfunction
Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
In a small randomized controlled trial (RCT) conducted in China, Lou and colleagues identified premenopausal women at “high risk” for sexual dysfunction as determined by the Chinese version of the Female Sexual Function Index (CFSFI).
Details of the study
A total of 84 women (mean age, 36.5 years) were included in the study. All the participants were heterosexual and married or with a long-term partner. The domain of sexual dysfunction was not considered. Women were excluded if they had no current heterosexual partner; had genital malformation, urinary incontinence, or prolapse stage 2 or higher; a history of pelvic floor mesh treatment; current gynecologic malignancy; abnormal cervical cytology; or were currently pregnant or postpartum. In addition, women were excluded if they had been treated previously for sexual dysfunction or mental “disease.” The cohort was randomized to receive fractional CO2 laser treatments (three 15-minute treatments 1 month apart at 60W, 1,000 microseconds) or coached Kegel exercises (10 exercises repeated twice daily at least 3 times/week and monitored by physical therapists at biweekly clinic visits). Sexual distress was evaluated by using the Female Sexual Distress Scale-Revised (FSDSR). Outcomes measured were pelvic floor muscle strength and scores on the CFSFI and FSDSR. Data were obtained at 3, 6, 9, and 12 months after initiation of therapy.
Both groups showed improvement
The laser cohort showed slightly more improvement in scale scores at 6 and 12 months. Specifically, the laser group had better scores on lubrication and overall satisfaction, with moderate effect size; neither group had improvements in arousal, desire, or orgasm. The Kegel group showed a significant improvement in pelvic floor strength and orgasm at 12 months, an improvement not seen in the laser cohort. Both groups showed gradual improvement in the FSDSR, with the laser group reporting a lower score (10.0) at 12 months posttreatment relative to the Kegel group (11.1). Again, these were modest effects as baseline scores for both cohorts were around 12.5. There were minimal safety signals in the laser group, with 22.5% of women reporting scant bloody discharge posttreatment and 72.5% describing mild discomfort (1 on a 1–10 VAS scale) during the procedure.
This study is problematic in several areas. Although it was a prospective, randomized trial, it was not blinded, and the therapeutic interventions were markedly different in nature and requirement for individual patient motivation. The experiences of sexual dysfunction among the participants were not stratified by type—arousal, desire, lubrication, orgasm, or pain. All patients had regular cyclic menses; however, the authors do not report on contraceptive methods, hormonal therapy, or other comorbid conditions that could impact sexual health. The cohorts may or may not have been similar in baseline types of sexual dissatisfaction.
CO2 laser for lichen sclerosus: Is it effective?
Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422. doi: 10.1097 /GME.0000000000001482.
Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi: 10.1097 /AOG.0000000000004332.
Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097 /AOG.0000000000004409.
High potency corticosteroid ointment is the current standard treatment for lichen sclerosus. Alternative options for disease that is refractory to steroids are limited. Three studies published in the past year explored the CO2 laser’s ability to treat lichen sclerosus symptoms and resultant sexual dysfunction—Pagano and colleagues conducted a small prospective study and Burkett and colleagues and Mitchell et al conducted small RCTs.
Details of the Pagano study
Three premenopausal and 37 postmenopausal women with refractory lichen sclerosus (defined as no improvement after 4 cycles of ultra-high potency steroids) were included in the study. Lichen sclerosus was uniformly biopsy confirmed. Women using topical or systemic hormones were excluded. VAS was administered prior to initial treatment and after each of 2 fractional CO2 treatments (25–30 W; 1,000 microseconds) 30 to 40 days apart to determine severity of vulvar itching, dyspareunia with penetration, vulvar dryness, sexual dysfunction, and procedure discomfort. Follow-up was conducted at 1 month after the final treatment. VAS score for the primary outcome of vulvar itching declined from 8 pretreatment to 6 after the first treatment and to 3 after the second. There was no significant treatment-related pain reported.
The authors acknowledged the limitations of their study; it was a relatively small sample size, nonrandomized and had short-term follow-up of a mixed patient population and no sham or control group. The short-term improvements reported in the study patients may not be sustained without ongoing treatment for a lifelong chronic disease, and the long-term potential for development of squamous cell carcinoma may or may not be ameliorated.
Continue to: Burkett et al: RCT study 1...
Burkett et al: RCT study 1
A total of 52 postmenopausal patients with biopsy-proven lichen sclerosus were randomly assigned to clobetasol or CO2 laser; 51 women completed 6-month follow-up. The outcomes were stratified by prior high-potency steroid use. The steroid cohort used clobetasol 0.05% nightly for 1 month, 3 times per week for 2 months, then as needed. The laser cohort received 3 treatments (26 W; 800 microseconds) 4 to 6 weeks apart. Overall adherence was only 75% in the clobetasol group, compared with 96% in the laser group. The authors found treatment efficacy of CO2 laser therapy only in the group of patients who had prior treatment with high potency topical corticosteroids. They conclude that, …“Despite previously optimistic results in well designed clinical trials of fractionated CO2 for genitourinary syndrome of menopause, and in noncontrolled case series for vulvar lichen sclerosus, our study failed to show any significant benefit of monotherapy of fractionated CO2 for vulvar lichen sclerosus. There may be a role for fractionated CO2 as an adjuvant therapy along with topical ultrapotent corticosteroids in vulvar lichen sclerosus.”
Mitchell et al: RCT study 2
This was a double blind, placebo-controlled, and histologically validated study of fractional CO2 for treatment of lichen sclerosus in 35 women; 17 in the treatment arm and 18 in the sham laser encounters. At least a 4-week no treatment period of topical steroids was required before monotherapy with CO2 laser was initiated.
The authors found no difference in their primary outcome—histopathology scale scores—after 5 treatments over 24 weeks. Secondary endpoints were changes in the CSS (Clinical Scoring System for Vulvar Lichen Sclerosus), a validated instrument that includes both a clinician’s examination of the severity of disease and a patient’s report of the severity of her symptoms. The patient score is the total of 4 domains: itching, soreness, burning, and dyspareunia. The clinician objective examination documents fissures, erosions, hyperkeratosis, agglutination, stenosis, and atrophy. At the conclusion of treatment there were no significant differences in the patient reported symptoms or the clinical findings between the active treatment and sham groups.
As a monotherapy, CO2 laser therapy is not effective in treating lichen sclerosus, although it may help improve symptoms as an adjunct to high potency steroid therapy when topical treatment alone has failed to provide adequate response.
Conclusion
The quality of evidence to support the use of the CO2 laser for improvement in sexual dysfunction is poor. Although patient satisfaction scores improved overall, and most specifically for symptoms related to GSM, the lack of blinding; inappropriate or no control groups; the very short-term outcomes; and for one of the studies, the lack of a clear definition of sexual dysfunction, make it difficult to draw meaningful conclusions for clinical care.
For GSM, we know that topical estrogen therapy works—and with little to no systemic absorption. The CO2 laser should be studied in comparison to this gold standard, with consideration of costs and potential long-term harms in addition to patient satisfaction and short-term measures of improvement. In addition, and very importantly, it is our professional responsibility to present the evidence for safety of topical estrogens to our professional colleagues as well as to our patients with estrogen-dependent cancers so that they understand the value of estrogen as a safe and appropriate alternative to expensive and potentially short-term interventions such as CO2 laser treatment. ●

Cheryl Iglesia, MD
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She is a member of the OBG Management Board of Editors.
Barbara Levy, MD: Cheryl, you have more experience with use of the energy-based cosmetic laser than most ObGyns, and I thought that speaking with you about this technology would be of benefit, not only to me in learning more about the hands-on experience of a lead researcher and practitioner but also readers who are hearing more and more about the growth of cosmetic gynecology in general. Thank you for taking the time today.
Cheryl Iglesia, MD: I’m happy to speak about this with you, Barbara.
Dr. Levy: Specifically, I would like to talk about use of these technologies for sexual dysfunction. In the last few years some of the available data have been on the CO2 laser versus physical therapy, which is not an appropriate comparison.1
Dr. Iglesia: There have been limited data, and less randomized, controlled data, on laser and radiofrequency energies for cosmetic gynecology, and in fact these devices remain unapproved for any gynecologic indication. In 2018 the US Food and Drug Administration (FDA) issued a Safety Communication about the use of energy-based devices to perform vaginal rejuvenation or cosmetic procedures. The International Urogynecological Association (IUGA) issued a consensus statement echoing concerns about the devices, and an International Continence Society/International Society for the Study of Vulvovaginal Disease Best Practice Consensus Statement did not recommend the laser for “routine treatment of vaginal atrophy or urinary incontinence unless treatment is part of a well-designed trial or with special arrangements for clinical governance, consent, and audit.”2
In May 2020, as evidence remains limited (although 522 studies are ongoing in coordination with the FDA), the American Urogynecologic Society (AUGS) published a clinical consensus statement from a panel of experts in female pelvic medicine and reconstructive surgery. The panel had about 90% consensus that there is short-term efficacy for the laser with GSM and dyspareunia. But we only have outcomes data that lasts a maximum of 1 year.2
A problem with our VeLVET trial,3 which was published in Menopause, and the Cruz and colleagues’ trial from South America,4 both of which compared the CO2 laser to estrogen and had randomized groups, was that they were limited by the outcome measures used, none of which were consistently validated. But these studies also had small numbers of participants and short-term follow-up. So I don’t think there are much existing data that are promising for supporting energy-based treatment for GSM.
We also have just-published data on the laser for lichen sclerosus.5 For the AUGS panel, there was about 80% consensus for energy-based-device use and lichen sclerosus.2 According to Mitchell et al, who conducted a small, randomized, sham-controlled trial, CO2 laser resulted in no significant difference in histopathology scale score between active and sham arms.5
Future trials may want to assess laser as a mechanism for improved local drug delivery (eg, use of combined laser plus local estrogen for GSM or combined laser plus topical steroid for lichen sclerosus). I am also aware that properly designed laser versus sham studies are underway.
Dr. Levy: What about for stress urinary incontinence (SUI)? I don’t think these technologies are going to work.
Dr. Iglesia: For the AUGS panel, there was only about 70% consensus for energy-based-device use and SUI,2 and I’m one of the naysayers. The pathophysiology of SUI is so multifactorial that it’s hard to believe that laser or radiofrequency wand therapy could have sustained improvements, especially since prior radiofrequency therapy from the last decade (for instance, Renessa, Novasys Medical) did not show long-term efficacy.
Understanding lasers and coordinating care
Dr. Levy: We don’t know what the long-term outcomes are for the CO2 laser and GSM.
Dr. Iglesia: I agree with you, and I think there needs to be an understanding of the mechanism of how lasers work, whether it be erbium (Er:YAG), which is the most common, or CO2. Erbium and CO2 lasers, which are on the far-infrared spectrum, target the chromophore, water. My feeling is that, when you look at results from the Cruz trial,4 or even our trial that compared vaginal estrogen with laser,3 when there is severe GSM and high pH with virtually no water present in the tissues, that laser is not going to properly function. But I don’t think we know exactly what optimal pretreatment is necessary, and that is one of the problems. Furthermore, when intravaginal lasers are done and no adequate speculum exam is conducted prior to introducing the laser, there could be discharge or old creams present that block the mirrors necessary to adequately fire the fractionated laser beams.
Unfortunately, oftentimes these devices are marketed to women with breast cancer, who may be taking aromatase inhibitors, which cause the no-water problem; they dry out everything. They are effective for preventing breast cancer recurrence, but they cause severe atrophy (perhaps worse than many of the other selective estrogen-receptor modulators), with a resultant high vaginal pH. If we can bring that pH level down, closer to the normal 4.5 range so that we could have some level of moisture, and add estrogen first, the overall treatment approach will probably be more effective. We still do not know what happens after 1 year, though, and how often touch-ups need to be performed.
In fact, when working with a patient with breast cancer, I will speak with her oncologist; I will collaborate to put in place a treatment plan that may include initial pretreatment with low-dose vaginal estrogen followed by laser treatment for vaginal atrophy. But I will make sure I use the lowest dose. Sometimes when the patient comes back, the estrogen’s worked so well she’ll say, “Oh, I’m happy, so I don’t need the laser anymore.” A balanced conversation is necessary, especially with cancer survivors.
Informing patients and colleagues
Dr. Levy: I completely agree, and I think one of the key points here is that our purpose is to serve our patients. The data demonstrate that low doses of vaginal estrogen are not harmful for women who are being treated for or who have recovered from breast cancer. It is our ethical obligation to convince these women and their oncologists that ongoing treatment with vaginal estrogen not only will help their GSM but also their overactive bladder and their risk of urinary tract infections and other things. We could be exploiting patients who are really fearful of using any estrogen because of a perceived cancer risk. We could actually be validating their fear by telling them we have an alternative treatment for which they have to pay cash.
Treatment access
Dr. Iglesia: Yes, these are not cosmetic conditions that we are treating. So my goal when evaluating treatment for refractory GSM or lichen sclerosus is to find optimal energy-based therapies with the hope that one day these will be approved gynecologic conditions by the US FDA for laser and wand therapies and that they will ultimately not be out-of-pocket expenses but rather therapies covered by insurance.
Dr. Levy: Great. I understand that AUGS/IUGA have been working on a terminology algorithm to help distinguish between procedures being performed to resolve a medical problem such as prolapse or incontinence versus those designed to be cosmetic.
Dr. Iglesia: Yes, there is a big document from experts in both societies out for public comment right now. It will hopefully be published soon.
Outstanding questions remain
Dr. Levy: Really, we as ObGyns shouldn’t be quick to incorporate these things into our practices without high-quality studies demonstrating value. I have a major concern about these devices in the long term. When you look at fractional CO2 use on the face, for instance, which is a much different type of skin than the vagina, the laser builds collagen—but we don’t have long-term outcome results. The vagina is supposed to be an elastic tissue, so what is the risk of long-term scarring there? Yes, the laser builds collagen in the vaginal epithelium, but what does it do to scarring in the rest of the tissue? We don’t have answers to that.
Dr. Iglesia: And that is the question—how does histology equate with function? Well, I would go with what the patients are reporting.
Dr. Levy: Absolutely. But the thing about vaginal low-dose estrogen is that it is something that the oncologists or the ObGyns could be implementing with patients while they are undergoing cancer therapy, while in their menopausal transition, to preserve vulvovaginal function as opposed to trying to regain it.
Dr. Iglesia: Certainly, although it still needs to be determined when that type of approach would actually be contraindicated.
Dr. Levy: Thank you, Cheryl, for your valuable insights.
Dr. Iglesia: Of course. Thank you. ●
References
1. Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
2. Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices. Female Pelvic Med Reconstr Surg. 2020;26:287-298. doi: 10.1097 /SPV.0000000000000872.
3. Paraiso MF, Ferrando CA, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET Trial. Menopause. 2020;27:50-56. doi: 10.1097/GME.0000000000001416.
4. Cruz VL, Steiner ML, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28. doi: 10.1097 /GME.0000000000000955.
5. Mitchell L, Goldstein A, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097/AOG.0000000000004409.

The approach to diagnosis and treatment of female sexual function continues to be a challenge for women’s health professionals. The search for a female “little blue pill” remains elusive as researchers struggle to understand the mechanisms that underlie the complex aspects of female sexual health. This Update will review the recent literature on the use of fractional CO2 laser for treatment of female sexual dysfunction and vulvovaginal symptoms. Bottom line: While the quality of the studies is poor overall, fractional CO2 laser treatment seems to temporarily improve symptoms of genitourinary syndrome of menopause (GSM). The duration of response, cost, and the overall long-term impact on sexual health remain in question.
A retrospective look at CO2 laser and postmenopausal GSM
Filippini M, Luvero D, Salvatore S, et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause. 2019;27:43-49. doi: 10.1097/GME. 0000000000001428.
Researchers conducted a retrospective, multicenter study of postmenopausal women with at least one symptom of GSM, including itching, burning, dyspareunia with penetration, and dryness.
Study details
A total of 171 of the 645 women (26.5%) were oncology patients. Women were excluded from analysis if they used any form of topical therapy within 15 days; had prolapse stage 2 or greater; or had any infection, abscess, or anatomical deformity precluding treatment with the laser.
Patients underwent gynecologic examination and were given a questionnaire to assess vulvovaginal symptoms. Exams occurred monthly during treatment (average, 6.5 months), at 6- and 12-months posttreatment, and then annually. No topical therapy was advised during or after treatment.
Patients received either 3 or 4 fractional CO2 laser treatments to the vulva and/or vagina depending on symptom location and type. Higher power settings of the same laser were used to treat vaginal symptoms (40W; 1,000 microseconds) versus vulvar symptoms (25W; 500 microseconds). Treatment sessions were 5 to 6 minutes. The study authors used a visual analog rating scale (VAS) for “atrophy and related symptoms,” tested vaginal pH, and completed the Vaginal Health Index Score. VAS scores were obtained from the patients prior to the initial laser intervention and 1 month after the final treatment.
Results
There were statistically significant improvements in dryness, vaginal orifice pain, dyspareunia, itching, and burning for both the 3-treatment and 4-treatment cohorts. The delta of improvement was then compared for the 2 subgroups; curiously, there was greater improvement of symptoms such as dryness (65% vs 61%), itching (78% vs 72%), burning (72% vs 67%), and vaginal orifice pain (67% vs 60%) in the group that received 3 cycles than in the group that received 4 cycles.
With regard to vaginal pH improvement, the 4-cycle group performed better than the 3-cycle group (1% improvement in the 4-cycle group vs 6% in the 3-cycle group). Although vaginal pH reduction was somewhat better in the group that received 4 treatments, and the pre versus posttreatment percentages were statistically significantly different, the clinical significance of a pH difference between 5.72 and 5.53 is questionable, especially since there was a greater difference in baseline pH between the two cohorts (6.08 in the 4-cycle group vs 5.59 in the 3-cycle group).
There were no reported adverse events related to the fractional laser treatments, and 6% of the patients underwent additional laser treatments during the followup timeframe of 8 to 20 months.
This was a retrospective study with no control or comparison group and short-term follow-up. The VAS scores were obtained 1 month after the final treatment. Failure to request additional treatment at 8 to 20 months cannot be used to infer that the therapeutic improvements recorded at 1 month were enduring. In addition, although the large number of patients in this study may lead to statistical significance, clinical significance is still questionable. Given the lack of a comparison group and the very short follow-up, it is hard to draw any scientifically valid conclusions from this study.
Continue to: Randomized data on CO2 laser vs Kegels for sexual dysfunction...
Randomized data on CO2 laser vs Kegels for sexual dysfunction
Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
In a small randomized controlled trial (RCT) conducted in China, Lou and colleagues identified premenopausal women at “high risk” for sexual dysfunction as determined by the Chinese version of the Female Sexual Function Index (CFSFI).
Details of the study
A total of 84 women (mean age, 36.5 years) were included in the study. All the participants were heterosexual and married or with a long-term partner. The domain of sexual dysfunction was not considered. Women were excluded if they had no current heterosexual partner; had genital malformation, urinary incontinence, or prolapse stage 2 or higher; a history of pelvic floor mesh treatment; current gynecologic malignancy; abnormal cervical cytology; or were currently pregnant or postpartum. In addition, women were excluded if they had been treated previously for sexual dysfunction or mental “disease.” The cohort was randomized to receive fractional CO2 laser treatments (three 15-minute treatments 1 month apart at 60W, 1,000 microseconds) or coached Kegel exercises (10 exercises repeated twice daily at least 3 times/week and monitored by physical therapists at biweekly clinic visits). Sexual distress was evaluated by using the Female Sexual Distress Scale-Revised (FSDSR). Outcomes measured were pelvic floor muscle strength and scores on the CFSFI and FSDSR. Data were obtained at 3, 6, 9, and 12 months after initiation of therapy.
Both groups showed improvement
The laser cohort showed slightly more improvement in scale scores at 6 and 12 months. Specifically, the laser group had better scores on lubrication and overall satisfaction, with moderate effect size; neither group had improvements in arousal, desire, or orgasm. The Kegel group showed a significant improvement in pelvic floor strength and orgasm at 12 months, an improvement not seen in the laser cohort. Both groups showed gradual improvement in the FSDSR, with the laser group reporting a lower score (10.0) at 12 months posttreatment relative to the Kegel group (11.1). Again, these were modest effects as baseline scores for both cohorts were around 12.5. There were minimal safety signals in the laser group, with 22.5% of women reporting scant bloody discharge posttreatment and 72.5% describing mild discomfort (1 on a 1–10 VAS scale) during the procedure.
This study is problematic in several areas. Although it was a prospective, randomized trial, it was not blinded, and the therapeutic interventions were markedly different in nature and requirement for individual patient motivation. The experiences of sexual dysfunction among the participants were not stratified by type—arousal, desire, lubrication, orgasm, or pain. All patients had regular cyclic menses; however, the authors do not report on contraceptive methods, hormonal therapy, or other comorbid conditions that could impact sexual health. The cohorts may or may not have been similar in baseline types of sexual dissatisfaction.
CO2 laser for lichen sclerosus: Is it effective?
Pagano T, Conforti A, Buonfantino C, et al. Effect of rescue fractional microablative CO2 laser on symptoms and sexual dysfunction in women affected by vulvar lichen sclerosus resistant to long-term use of topic corticosteroid: a prospective longitudinal study. Menopause. 2020;27:418-422. doi: 10.1097 /GME.0000000000001482.
Burkett LS, Siddique M, Zeymo A, et al. Clobetasol compared with fractionated carbon dioxide laser for lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:968-978. doi: 10.1097 /AOG.0000000000004332.
Mitchell L, Goldstein AT, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097 /AOG.0000000000004409.
High potency corticosteroid ointment is the current standard treatment for lichen sclerosus. Alternative options for disease that is refractory to steroids are limited. Three studies published in the past year explored the CO2 laser’s ability to treat lichen sclerosus symptoms and resultant sexual dysfunction—Pagano and colleagues conducted a small prospective study and Burkett and colleagues and Mitchell et al conducted small RCTs.
Details of the Pagano study
Three premenopausal and 37 postmenopausal women with refractory lichen sclerosus (defined as no improvement after 4 cycles of ultra-high potency steroids) were included in the study. Lichen sclerosus was uniformly biopsy confirmed. Women using topical or systemic hormones were excluded. VAS was administered prior to initial treatment and after each of 2 fractional CO2 treatments (25–30 W; 1,000 microseconds) 30 to 40 days apart to determine severity of vulvar itching, dyspareunia with penetration, vulvar dryness, sexual dysfunction, and procedure discomfort. Follow-up was conducted at 1 month after the final treatment. VAS score for the primary outcome of vulvar itching declined from 8 pretreatment to 6 after the first treatment and to 3 after the second. There was no significant treatment-related pain reported.
The authors acknowledged the limitations of their study; it was a relatively small sample size, nonrandomized and had short-term follow-up of a mixed patient population and no sham or control group. The short-term improvements reported in the study patients may not be sustained without ongoing treatment for a lifelong chronic disease, and the long-term potential for development of squamous cell carcinoma may or may not be ameliorated.
Continue to: Burkett et al: RCT study 1...
Burkett et al: RCT study 1
A total of 52 postmenopausal patients with biopsy-proven lichen sclerosus were randomly assigned to clobetasol or CO2 laser; 51 women completed 6-month follow-up. The outcomes were stratified by prior high-potency steroid use. The steroid cohort used clobetasol 0.05% nightly for 1 month, 3 times per week for 2 months, then as needed. The laser cohort received 3 treatments (26 W; 800 microseconds) 4 to 6 weeks apart. Overall adherence was only 75% in the clobetasol group, compared with 96% in the laser group. The authors found treatment efficacy of CO2 laser therapy only in the group of patients who had prior treatment with high potency topical corticosteroids. They conclude that, …“Despite previously optimistic results in well designed clinical trials of fractionated CO2 for genitourinary syndrome of menopause, and in noncontrolled case series for vulvar lichen sclerosus, our study failed to show any significant benefit of monotherapy of fractionated CO2 for vulvar lichen sclerosus. There may be a role for fractionated CO2 as an adjuvant therapy along with topical ultrapotent corticosteroids in vulvar lichen sclerosus.”
Mitchell et al: RCT study 2
This was a double blind, placebo-controlled, and histologically validated study of fractional CO2 for treatment of lichen sclerosus in 35 women; 17 in the treatment arm and 18 in the sham laser encounters. At least a 4-week no treatment period of topical steroids was required before monotherapy with CO2 laser was initiated.
The authors found no difference in their primary outcome—histopathology scale scores—after 5 treatments over 24 weeks. Secondary endpoints were changes in the CSS (Clinical Scoring System for Vulvar Lichen Sclerosus), a validated instrument that includes both a clinician’s examination of the severity of disease and a patient’s report of the severity of her symptoms. The patient score is the total of 4 domains: itching, soreness, burning, and dyspareunia. The clinician objective examination documents fissures, erosions, hyperkeratosis, agglutination, stenosis, and atrophy. At the conclusion of treatment there were no significant differences in the patient reported symptoms or the clinical findings between the active treatment and sham groups.
As a monotherapy, CO2 laser therapy is not effective in treating lichen sclerosus, although it may help improve symptoms as an adjunct to high potency steroid therapy when topical treatment alone has failed to provide adequate response.
Conclusion
The quality of evidence to support the use of the CO2 laser for improvement in sexual dysfunction is poor. Although patient satisfaction scores improved overall, and most specifically for symptoms related to GSM, the lack of blinding; inappropriate or no control groups; the very short-term outcomes; and for one of the studies, the lack of a clear definition of sexual dysfunction, make it difficult to draw meaningful conclusions for clinical care.
For GSM, we know that topical estrogen therapy works—and with little to no systemic absorption. The CO2 laser should be studied in comparison to this gold standard, with consideration of costs and potential long-term harms in addition to patient satisfaction and short-term measures of improvement. In addition, and very importantly, it is our professional responsibility to present the evidence for safety of topical estrogens to our professional colleagues as well as to our patients with estrogen-dependent cancers so that they understand the value of estrogen as a safe and appropriate alternative to expensive and potentially short-term interventions such as CO2 laser treatment. ●

Cheryl Iglesia, MD
Dr. Iglesia is Director, Section of Female Pelvic Medicine and Reconstructive Surgery, MedStar Washington Hospital Center, and Professor, Departments of ObGyn and Urology, Georgetown University School of Medicine, Washington, DC. She is a member of the OBG Management Board of Editors.
Barbara Levy, MD: Cheryl, you have more experience with use of the energy-based cosmetic laser than most ObGyns, and I thought that speaking with you about this technology would be of benefit, not only to me in learning more about the hands-on experience of a lead researcher and practitioner but also readers who are hearing more and more about the growth of cosmetic gynecology in general. Thank you for taking the time today.
Cheryl Iglesia, MD: I’m happy to speak about this with you, Barbara.
Dr. Levy: Specifically, I would like to talk about use of these technologies for sexual dysfunction. In the last few years some of the available data have been on the CO2 laser versus physical therapy, which is not an appropriate comparison.1
Dr. Iglesia: There have been limited data, and less randomized, controlled data, on laser and radiofrequency energies for cosmetic gynecology, and in fact these devices remain unapproved for any gynecologic indication. In 2018 the US Food and Drug Administration (FDA) issued a Safety Communication about the use of energy-based devices to perform vaginal rejuvenation or cosmetic procedures. The International Urogynecological Association (IUGA) issued a consensus statement echoing concerns about the devices, and an International Continence Society/International Society for the Study of Vulvovaginal Disease Best Practice Consensus Statement did not recommend the laser for “routine treatment of vaginal atrophy or urinary incontinence unless treatment is part of a well-designed trial or with special arrangements for clinical governance, consent, and audit.”2
In May 2020, as evidence remains limited (although 522 studies are ongoing in coordination with the FDA), the American Urogynecologic Society (AUGS) published a clinical consensus statement from a panel of experts in female pelvic medicine and reconstructive surgery. The panel had about 90% consensus that there is short-term efficacy for the laser with GSM and dyspareunia. But we only have outcomes data that lasts a maximum of 1 year.2
A problem with our VeLVET trial,3 which was published in Menopause, and the Cruz and colleagues’ trial from South America,4 both of which compared the CO2 laser to estrogen and had randomized groups, was that they were limited by the outcome measures used, none of which were consistently validated. But these studies also had small numbers of participants and short-term follow-up. So I don’t think there are much existing data that are promising for supporting energy-based treatment for GSM.
We also have just-published data on the laser for lichen sclerosus.5 For the AUGS panel, there was about 80% consensus for energy-based-device use and lichen sclerosus.2 According to Mitchell et al, who conducted a small, randomized, sham-controlled trial, CO2 laser resulted in no significant difference in histopathology scale score between active and sham arms.5
Future trials may want to assess laser as a mechanism for improved local drug delivery (eg, use of combined laser plus local estrogen for GSM or combined laser plus topical steroid for lichen sclerosus). I am also aware that properly designed laser versus sham studies are underway.
Dr. Levy: What about for stress urinary incontinence (SUI)? I don’t think these technologies are going to work.
Dr. Iglesia: For the AUGS panel, there was only about 70% consensus for energy-based-device use and SUI,2 and I’m one of the naysayers. The pathophysiology of SUI is so multifactorial that it’s hard to believe that laser or radiofrequency wand therapy could have sustained improvements, especially since prior radiofrequency therapy from the last decade (for instance, Renessa, Novasys Medical) did not show long-term efficacy.
Understanding lasers and coordinating care
Dr. Levy: We don’t know what the long-term outcomes are for the CO2 laser and GSM.
Dr. Iglesia: I agree with you, and I think there needs to be an understanding of the mechanism of how lasers work, whether it be erbium (Er:YAG), which is the most common, or CO2. Erbium and CO2 lasers, which are on the far-infrared spectrum, target the chromophore, water. My feeling is that, when you look at results from the Cruz trial,4 or even our trial that compared vaginal estrogen with laser,3 when there is severe GSM and high pH with virtually no water present in the tissues, that laser is not going to properly function. But I don’t think we know exactly what optimal pretreatment is necessary, and that is one of the problems. Furthermore, when intravaginal lasers are done and no adequate speculum exam is conducted prior to introducing the laser, there could be discharge or old creams present that block the mirrors necessary to adequately fire the fractionated laser beams.
Unfortunately, oftentimes these devices are marketed to women with breast cancer, who may be taking aromatase inhibitors, which cause the no-water problem; they dry out everything. They are effective for preventing breast cancer recurrence, but they cause severe atrophy (perhaps worse than many of the other selective estrogen-receptor modulators), with a resultant high vaginal pH. If we can bring that pH level down, closer to the normal 4.5 range so that we could have some level of moisture, and add estrogen first, the overall treatment approach will probably be more effective. We still do not know what happens after 1 year, though, and how often touch-ups need to be performed.
In fact, when working with a patient with breast cancer, I will speak with her oncologist; I will collaborate to put in place a treatment plan that may include initial pretreatment with low-dose vaginal estrogen followed by laser treatment for vaginal atrophy. But I will make sure I use the lowest dose. Sometimes when the patient comes back, the estrogen’s worked so well she’ll say, “Oh, I’m happy, so I don’t need the laser anymore.” A balanced conversation is necessary, especially with cancer survivors.
Informing patients and colleagues
Dr. Levy: I completely agree, and I think one of the key points here is that our purpose is to serve our patients. The data demonstrate that low doses of vaginal estrogen are not harmful for women who are being treated for or who have recovered from breast cancer. It is our ethical obligation to convince these women and their oncologists that ongoing treatment with vaginal estrogen not only will help their GSM but also their overactive bladder and their risk of urinary tract infections and other things. We could be exploiting patients who are really fearful of using any estrogen because of a perceived cancer risk. We could actually be validating their fear by telling them we have an alternative treatment for which they have to pay cash.
Treatment access
Dr. Iglesia: Yes, these are not cosmetic conditions that we are treating. So my goal when evaluating treatment for refractory GSM or lichen sclerosus is to find optimal energy-based therapies with the hope that one day these will be approved gynecologic conditions by the US FDA for laser and wand therapies and that they will ultimately not be out-of-pocket expenses but rather therapies covered by insurance.
Dr. Levy: Great. I understand that AUGS/IUGA have been working on a terminology algorithm to help distinguish between procedures being performed to resolve a medical problem such as prolapse or incontinence versus those designed to be cosmetic.
Dr. Iglesia: Yes, there is a big document from experts in both societies out for public comment right now. It will hopefully be published soon.
Outstanding questions remain
Dr. Levy: Really, we as ObGyns shouldn’t be quick to incorporate these things into our practices without high-quality studies demonstrating value. I have a major concern about these devices in the long term. When you look at fractional CO2 use on the face, for instance, which is a much different type of skin than the vagina, the laser builds collagen—but we don’t have long-term outcome results. The vagina is supposed to be an elastic tissue, so what is the risk of long-term scarring there? Yes, the laser builds collagen in the vaginal epithelium, but what does it do to scarring in the rest of the tissue? We don’t have answers to that.
Dr. Iglesia: And that is the question—how does histology equate with function? Well, I would go with what the patients are reporting.
Dr. Levy: Absolutely. But the thing about vaginal low-dose estrogen is that it is something that the oncologists or the ObGyns could be implementing with patients while they are undergoing cancer therapy, while in their menopausal transition, to preserve vulvovaginal function as opposed to trying to regain it.
Dr. Iglesia: Certainly, although it still needs to be determined when that type of approach would actually be contraindicated.
Dr. Levy: Thank you, Cheryl, for your valuable insights.
Dr. Iglesia: Of course. Thank you. ●
References
1. Lou W, Chen F, Xu T, et al. A randomized controlled study of vaginal fractional CO2 laser therapy for female sexual dysfunction. Lasers Med Sci. March 15, 2021. doi: 10.1007/s10103-021-03260-x.
2. Alshiek J, Garcia B, Minassian V, et al. Vaginal energy-based devices. Female Pelvic Med Reconstr Surg. 2020;26:287-298. doi: 10.1097 /SPV.0000000000000872.
3. Paraiso MF, Ferrando CA, et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: the VeLVET Trial. Menopause. 2020;27:50-56. doi: 10.1097/GME.0000000000001416.
4. Cruz VL, Steiner ML, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause. 2018;25:21-28. doi: 10.1097 /GME.0000000000000955.
5. Mitchell L, Goldstein A, Heller D, et al. Fractionated carbon dioxide laser for the treatment of vulvar lichen sclerosus: a randomized controlled trial. Obstet Gynecol. 2021;137:979-987. doi: 10.1097/AOG.0000000000004409.
Patients’ sexual problems: Be proactive, make discussions routine
If the goal of a clinical encounter is to identify issues that adversely affect health, well-being, and life satisfaction, open-ended questions on sexual problems are essential, according to an expert who provided tips during a session presented by Current Psychiatry and the American Academy of Clinical Psychiatrists about how to begin a productive dialogue.
For identifying and treating the obstacles to sexual health, “the onus is on the provider,” said Anita H. Clayton, MD, chair of psychiatry and neurobehavioral sciences at the University of Virginia, Charlottesville.
In a poll published more than 20 years ago, 91% of men and 84% of women reported that a satisfying sex life is important, while 90% agreed that sexual difficulties cause emotional problems, said Dr. Clayton, who sees no reason to think that those percentages have changed. Yet, patients are traditionally reluctant to raise their concerns about sexual issues to a physician.
In the same poll, about 50% of the respondents characterized themselves as “very concerned” that a clinician would simply dismiss a sexual complaint or that there would be no treatment. Of the other respondents, 40% were somewhat concerned. Dr. Clayton assumes that those numbers are still valid and that they provide the rationale for asking routinely about sexual health, she said at the virtual meeting, presented by MedscapeLive.
Raising sexual health issues
“The clinician has to initiate the discussion and make it part of the routine examination,” said Dr. Clayton, also a professor of obstetrics and gynecology at the university. She indicated that unresolved sexual issues are a common and important but treatable problem, whether the underlying issue has a medical or psychological origin.
Yet, language is critical. Many physicians might have no difficulty discussing sexual problems, but patients often do. Dr. Clayton recommended developing strategies that might it easy if not seamless to elicit information about sexual health in the context of inquiring about other clinical issues.
“Use bridging statements,” Dr. Clayton suggested.
Bridging statements allow an easy transition into a discussion of sexual function from another clinical issue, Dr. Clayton said. As examples, she suggested moving to questions about sex from inquiries about conditions, such as diabetes, or medications, such as antidepressants, that are known to have an impact on sexual dysfunction.
Avoid yes-no questions.
To prompt a dialogue, Dr. Clayton advised against using yes-no questions that allow the patient to quickly dismiss the topic with a negative response. She tries to frame a question that requires a complete thought. In an inquiry addressed to a patient with diabetes, for example, she might first inform the patient that sexual issues are common with this disorder and then ask what types of sexual issues the patient is experiencing.
Once the topic is raised, a checklist approach is appropriate. Patients might be more or less willing to talk any one of the range of issues that influence sexual health, ranging from issues of desire and arousal to discomfort or pain. The door should be opened to a discussion of specific sexual organ function, such as ability to achieve an erection or adequate lubrication.
“Do not assume the patient is heterosexual,” Dr. Clayton cautioned.
It is reasonable and appropriate to bring up sexual health during the intake history. A discussion of sexual health can be initiated by simply posing the question: “Are you sexually active?” Importantly, Dr. Clayton strongly recommended a follow-up question when adults reply that they are not sexually active.
In the ELIXIR study, which evaluated sexual function in patients with depression, more than twice as many patients reported impairments when asked by the physician than who volunteered this information spontaneously, according to Dr. Clayton, citing a study that found sexual issues in more than 70% of the 4,557 participants.
Prioritize choice of language.
Once sexual impairments are uncovered, clinicians will need to determine how to intervene, but Dr. Clayton recommended using clear and frank language to define the problem even if the language is tailored to the patient’s comfort level. Patients should be encouraged to recognize that there are solutions for most problems, but clinicians should recognize and respect cultural issues in directing patients toward solutions.
Dr. Clayton is not alone in recommending that patients be asked routinely about sexual health. Margot Savoy, MD, MPH, chair of family and community medicine, Temple University, Philadelphia, has also advocated for a proactive approach.
“Patients deserve whole-patient care that includes sexual health,” said Dr. Savoy, who was coauthor of a recent article that also outlined techniques for eliciting a sexual history.
She suggested that the need to inquire should not be considered age specific.
“Asking patients about their sexual history and concerns is a critical part of routine primary care across the lifespan,” she said.
“We also need to intentionally create a safe environment where it is as normal to talk about sexual questions or concerns as it is about how to care for a cold or manage a backache,” she added.
MedscapeLive and this news organization are owned by the same company. Dr. Clayton disclosed financial relationships with Acadia, Alkermes, Allergan, AMAG, Astellas, Fabre-Kramer, Janssen, Ovoca Bio, PureTech Health, Relmada, S1 Biopharma, Safe Therapeutics, Takeda, and WCG MedAd-vante-Prophase. Dr. Savoy reported no conflicts of interest.
If the goal of a clinical encounter is to identify issues that adversely affect health, well-being, and life satisfaction, open-ended questions on sexual problems are essential, according to an expert who provided tips during a session presented by Current Psychiatry and the American Academy of Clinical Psychiatrists about how to begin a productive dialogue.
For identifying and treating the obstacles to sexual health, “the onus is on the provider,” said Anita H. Clayton, MD, chair of psychiatry and neurobehavioral sciences at the University of Virginia, Charlottesville.
In a poll published more than 20 years ago, 91% of men and 84% of women reported that a satisfying sex life is important, while 90% agreed that sexual difficulties cause emotional problems, said Dr. Clayton, who sees no reason to think that those percentages have changed. Yet, patients are traditionally reluctant to raise their concerns about sexual issues to a physician.
In the same poll, about 50% of the respondents characterized themselves as “very concerned” that a clinician would simply dismiss a sexual complaint or that there would be no treatment. Of the other respondents, 40% were somewhat concerned. Dr. Clayton assumes that those numbers are still valid and that they provide the rationale for asking routinely about sexual health, she said at the virtual meeting, presented by MedscapeLive.
Raising sexual health issues
“The clinician has to initiate the discussion and make it part of the routine examination,” said Dr. Clayton, also a professor of obstetrics and gynecology at the university. She indicated that unresolved sexual issues are a common and important but treatable problem, whether the underlying issue has a medical or psychological origin.
Yet, language is critical. Many physicians might have no difficulty discussing sexual problems, but patients often do. Dr. Clayton recommended developing strategies that might it easy if not seamless to elicit information about sexual health in the context of inquiring about other clinical issues.
“Use bridging statements,” Dr. Clayton suggested.
Bridging statements allow an easy transition into a discussion of sexual function from another clinical issue, Dr. Clayton said. As examples, she suggested moving to questions about sex from inquiries about conditions, such as diabetes, or medications, such as antidepressants, that are known to have an impact on sexual dysfunction.
Avoid yes-no questions.
To prompt a dialogue, Dr. Clayton advised against using yes-no questions that allow the patient to quickly dismiss the topic with a negative response. She tries to frame a question that requires a complete thought. In an inquiry addressed to a patient with diabetes, for example, she might first inform the patient that sexual issues are common with this disorder and then ask what types of sexual issues the patient is experiencing.
Once the topic is raised, a checklist approach is appropriate. Patients might be more or less willing to talk any one of the range of issues that influence sexual health, ranging from issues of desire and arousal to discomfort or pain. The door should be opened to a discussion of specific sexual organ function, such as ability to achieve an erection or adequate lubrication.
“Do not assume the patient is heterosexual,” Dr. Clayton cautioned.
It is reasonable and appropriate to bring up sexual health during the intake history. A discussion of sexual health can be initiated by simply posing the question: “Are you sexually active?” Importantly, Dr. Clayton strongly recommended a follow-up question when adults reply that they are not sexually active.
In the ELIXIR study, which evaluated sexual function in patients with depression, more than twice as many patients reported impairments when asked by the physician than who volunteered this information spontaneously, according to Dr. Clayton, citing a study that found sexual issues in more than 70% of the 4,557 participants.
Prioritize choice of language.
Once sexual impairments are uncovered, clinicians will need to determine how to intervene, but Dr. Clayton recommended using clear and frank language to define the problem even if the language is tailored to the patient’s comfort level. Patients should be encouraged to recognize that there are solutions for most problems, but clinicians should recognize and respect cultural issues in directing patients toward solutions.
Dr. Clayton is not alone in recommending that patients be asked routinely about sexual health. Margot Savoy, MD, MPH, chair of family and community medicine, Temple University, Philadelphia, has also advocated for a proactive approach.
“Patients deserve whole-patient care that includes sexual health,” said Dr. Savoy, who was coauthor of a recent article that also outlined techniques for eliciting a sexual history.
She suggested that the need to inquire should not be considered age specific.
“Asking patients about their sexual history and concerns is a critical part of routine primary care across the lifespan,” she said.
“We also need to intentionally create a safe environment where it is as normal to talk about sexual questions or concerns as it is about how to care for a cold or manage a backache,” she added.
MedscapeLive and this news organization are owned by the same company. Dr. Clayton disclosed financial relationships with Acadia, Alkermes, Allergan, AMAG, Astellas, Fabre-Kramer, Janssen, Ovoca Bio, PureTech Health, Relmada, S1 Biopharma, Safe Therapeutics, Takeda, and WCG MedAd-vante-Prophase. Dr. Savoy reported no conflicts of interest.
If the goal of a clinical encounter is to identify issues that adversely affect health, well-being, and life satisfaction, open-ended questions on sexual problems are essential, according to an expert who provided tips during a session presented by Current Psychiatry and the American Academy of Clinical Psychiatrists about how to begin a productive dialogue.
For identifying and treating the obstacles to sexual health, “the onus is on the provider,” said Anita H. Clayton, MD, chair of psychiatry and neurobehavioral sciences at the University of Virginia, Charlottesville.
In a poll published more than 20 years ago, 91% of men and 84% of women reported that a satisfying sex life is important, while 90% agreed that sexual difficulties cause emotional problems, said Dr. Clayton, who sees no reason to think that those percentages have changed. Yet, patients are traditionally reluctant to raise their concerns about sexual issues to a physician.
In the same poll, about 50% of the respondents characterized themselves as “very concerned” that a clinician would simply dismiss a sexual complaint or that there would be no treatment. Of the other respondents, 40% were somewhat concerned. Dr. Clayton assumes that those numbers are still valid and that they provide the rationale for asking routinely about sexual health, she said at the virtual meeting, presented by MedscapeLive.
Raising sexual health issues
“The clinician has to initiate the discussion and make it part of the routine examination,” said Dr. Clayton, also a professor of obstetrics and gynecology at the university. She indicated that unresolved sexual issues are a common and important but treatable problem, whether the underlying issue has a medical or psychological origin.
Yet, language is critical. Many physicians might have no difficulty discussing sexual problems, but patients often do. Dr. Clayton recommended developing strategies that might it easy if not seamless to elicit information about sexual health in the context of inquiring about other clinical issues.
“Use bridging statements,” Dr. Clayton suggested.
Bridging statements allow an easy transition into a discussion of sexual function from another clinical issue, Dr. Clayton said. As examples, she suggested moving to questions about sex from inquiries about conditions, such as diabetes, or medications, such as antidepressants, that are known to have an impact on sexual dysfunction.
Avoid yes-no questions.
To prompt a dialogue, Dr. Clayton advised against using yes-no questions that allow the patient to quickly dismiss the topic with a negative response. She tries to frame a question that requires a complete thought. In an inquiry addressed to a patient with diabetes, for example, she might first inform the patient that sexual issues are common with this disorder and then ask what types of sexual issues the patient is experiencing.
Once the topic is raised, a checklist approach is appropriate. Patients might be more or less willing to talk any one of the range of issues that influence sexual health, ranging from issues of desire and arousal to discomfort or pain. The door should be opened to a discussion of specific sexual organ function, such as ability to achieve an erection or adequate lubrication.
“Do not assume the patient is heterosexual,” Dr. Clayton cautioned.
It is reasonable and appropriate to bring up sexual health during the intake history. A discussion of sexual health can be initiated by simply posing the question: “Are you sexually active?” Importantly, Dr. Clayton strongly recommended a follow-up question when adults reply that they are not sexually active.
In the ELIXIR study, which evaluated sexual function in patients with depression, more than twice as many patients reported impairments when asked by the physician than who volunteered this information spontaneously, according to Dr. Clayton, citing a study that found sexual issues in more than 70% of the 4,557 participants.
Prioritize choice of language.
Once sexual impairments are uncovered, clinicians will need to determine how to intervene, but Dr. Clayton recommended using clear and frank language to define the problem even if the language is tailored to the patient’s comfort level. Patients should be encouraged to recognize that there are solutions for most problems, but clinicians should recognize and respect cultural issues in directing patients toward solutions.
Dr. Clayton is not alone in recommending that patients be asked routinely about sexual health. Margot Savoy, MD, MPH, chair of family and community medicine, Temple University, Philadelphia, has also advocated for a proactive approach.
“Patients deserve whole-patient care that includes sexual health,” said Dr. Savoy, who was coauthor of a recent article that also outlined techniques for eliciting a sexual history.
She suggested that the need to inquire should not be considered age specific.
“Asking patients about their sexual history and concerns is a critical part of routine primary care across the lifespan,” she said.
“We also need to intentionally create a safe environment where it is as normal to talk about sexual questions or concerns as it is about how to care for a cold or manage a backache,” she added.
MedscapeLive and this news organization are owned by the same company. Dr. Clayton disclosed financial relationships with Acadia, Alkermes, Allergan, AMAG, Astellas, Fabre-Kramer, Janssen, Ovoca Bio, PureTech Health, Relmada, S1 Biopharma, Safe Therapeutics, Takeda, and WCG MedAd-vante-Prophase. Dr. Savoy reported no conflicts of interest.
FROM CP/AACP PSYCHIATRY UPDATE
HIFEM procedure helped to improve UI and female sexual function
Using high-intensity focused electromagnetic (HIFEM) technology to strengthen pelvic floor muscles for the improvement of urinary incontinence (UI) and female sexual function was safe and effective at 9 months follow-up, results from a multicenter study showed.
“The pelvic floor consists of three pairs of muscles: the pubococcygeus, the iliococcygeus, and the puborectalis,” lead study author Joseph Berenholz, MD, and a diplomate of the American Board of Obstetics & Gynecology, said during the annual conference of the American Society for Laser Medicine and Surgery. “They control continence through support of pelvic organs. The urethra, the vagina, and the rectum pass through that diaphragm. It also contributes to sexual sensation and arousal. A deconditioning of the pelvic floor is usually the result of child-bearing years or aging, which usually results in urinary incontinence and impairment of sexual function. The noninvasive strengthening of the pelvic floor muscles helps to regain muscle tone and strength.”
In a prospective, open-label, single-arm study conducted at four sites, Dr. Berenholz, medical director of the Michigan Center for Women’s Health in Farmington Hills, and colleagues investigated the long-term effectiveness of HIFEM-induced pelvic floor muscle (PFM) strengthening for improvement of UI and sexual function. HIFEM selectively targets neuromuscular tissue and induces supramaximal PFM contractions that cannot be achieved voluntarily, he said, causing muscle strengthening due to muscle fiber hypertrophy, which helps patients to better isolate and command their muscles.
The study population consisted of 33 females with a mean age of 49 years who had UI and UI-related problems in sexual life. They received six 28-minute HIFEM treatments of the pelvic floor with the BTL Emsella, which is FDA cleared for both stress and urge incontinence. The frequency of visits was two treatments per week and the intensity of HIFEM was adjusted between 0% and 100% based on the patient’s tolerance threshold. Evaluations were conducted at baseline, after the last treatment, at 1, 3, 6, and 9 months. The primary outcomes were change in urine leakage based on the International Consultation on Incontinence Questionnaire–Short Form (ICIQ-UI-SF) and change in sexual function based on the Female Sexual Function Index (FSFI) and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Secondary endpoints were adverse events and the comfort of therapy based on a 7-point Likert scale.
Dr. Berenholz reported that from baseline the severity of UI based on the ICIQ-SF significantly decreased 60% by a mean of 8.1 points between baseline and 9 months (P < .001). At 1 month, the FSFI score improved 32% by a mean of 7.1 points (P < .001) and was sustained throughout the study. The most prominent changes were seen in the subdomains of desire, arousal, lubrication, and orgasm response.
The PISQ-12 score incrementally increased 25% to a mean improvement of 8.2 points at 9 months (P < .001). Subjects improved most in the emotive subdomain, reporting more frequent orgasms, increased desire, and sexual excitement. The minimal important difference was 6 points.
“This is a true paradigm shift in the treatment of incontinence and sexual dysfunction,” Dr. Berenholz said. “The therapy was safe, comfortable, no adverse events emerged, and 31 subjects (94%) described the therapy as comfortable. Interim data suggest that treatment effect was maintained for 9 months, and there were no significant declines in scores in the long term. The upcoming 12-month follow-up data will let us know if more maintenance therapy is needed.”
During a question-and-answer session, one of the abstract section chairs, Albert Wolkerstorfer, MD, PhD, wondered about the potential for combination treatments in this patient population. “I can imagine that something that is working on the muscle tone has a totally different mechanism than something that is working on the mucosa and the underlying tissue without really affecting the muscle,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam. “Would a combination be the way to go?”
Dr. Berenholz said that he sometimes combines HIFEM with the ULTRA Femme 360, a radiofrequency thermal energy device. “We thought this addresses two issues,” he said. “One is fascial muscle, which is the underlying structural issue for incontinence. The other is thermal energy to aid in incontinence prevention by inducing production of elastin and collagen in the midurethra, but also to promote lubrication and heightened sensitivity in the patient who’s either menopausal or has undergone chemotherapy for breast cancer.”
Dr. Berenholz reported having no financial disclosures. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
Using high-intensity focused electromagnetic (HIFEM) technology to strengthen pelvic floor muscles for the improvement of urinary incontinence (UI) and female sexual function was safe and effective at 9 months follow-up, results from a multicenter study showed.
“The pelvic floor consists of three pairs of muscles: the pubococcygeus, the iliococcygeus, and the puborectalis,” lead study author Joseph Berenholz, MD, and a diplomate of the American Board of Obstetics & Gynecology, said during the annual conference of the American Society for Laser Medicine and Surgery. “They control continence through support of pelvic organs. The urethra, the vagina, and the rectum pass through that diaphragm. It also contributes to sexual sensation and arousal. A deconditioning of the pelvic floor is usually the result of child-bearing years or aging, which usually results in urinary incontinence and impairment of sexual function. The noninvasive strengthening of the pelvic floor muscles helps to regain muscle tone and strength.”
In a prospective, open-label, single-arm study conducted at four sites, Dr. Berenholz, medical director of the Michigan Center for Women’s Health in Farmington Hills, and colleagues investigated the long-term effectiveness of HIFEM-induced pelvic floor muscle (PFM) strengthening for improvement of UI and sexual function. HIFEM selectively targets neuromuscular tissue and induces supramaximal PFM contractions that cannot be achieved voluntarily, he said, causing muscle strengthening due to muscle fiber hypertrophy, which helps patients to better isolate and command their muscles.
The study population consisted of 33 females with a mean age of 49 years who had UI and UI-related problems in sexual life. They received six 28-minute HIFEM treatments of the pelvic floor with the BTL Emsella, which is FDA cleared for both stress and urge incontinence. The frequency of visits was two treatments per week and the intensity of HIFEM was adjusted between 0% and 100% based on the patient’s tolerance threshold. Evaluations were conducted at baseline, after the last treatment, at 1, 3, 6, and 9 months. The primary outcomes were change in urine leakage based on the International Consultation on Incontinence Questionnaire–Short Form (ICIQ-UI-SF) and change in sexual function based on the Female Sexual Function Index (FSFI) and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Secondary endpoints were adverse events and the comfort of therapy based on a 7-point Likert scale.
Dr. Berenholz reported that from baseline the severity of UI based on the ICIQ-SF significantly decreased 60% by a mean of 8.1 points between baseline and 9 months (P < .001). At 1 month, the FSFI score improved 32% by a mean of 7.1 points (P < .001) and was sustained throughout the study. The most prominent changes were seen in the subdomains of desire, arousal, lubrication, and orgasm response.
The PISQ-12 score incrementally increased 25% to a mean improvement of 8.2 points at 9 months (P < .001). Subjects improved most in the emotive subdomain, reporting more frequent orgasms, increased desire, and sexual excitement. The minimal important difference was 6 points.
“This is a true paradigm shift in the treatment of incontinence and sexual dysfunction,” Dr. Berenholz said. “The therapy was safe, comfortable, no adverse events emerged, and 31 subjects (94%) described the therapy as comfortable. Interim data suggest that treatment effect was maintained for 9 months, and there were no significant declines in scores in the long term. The upcoming 12-month follow-up data will let us know if more maintenance therapy is needed.”
During a question-and-answer session, one of the abstract section chairs, Albert Wolkerstorfer, MD, PhD, wondered about the potential for combination treatments in this patient population. “I can imagine that something that is working on the muscle tone has a totally different mechanism than something that is working on the mucosa and the underlying tissue without really affecting the muscle,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam. “Would a combination be the way to go?”
Dr. Berenholz said that he sometimes combines HIFEM with the ULTRA Femme 360, a radiofrequency thermal energy device. “We thought this addresses two issues,” he said. “One is fascial muscle, which is the underlying structural issue for incontinence. The other is thermal energy to aid in incontinence prevention by inducing production of elastin and collagen in the midurethra, but also to promote lubrication and heightened sensitivity in the patient who’s either menopausal or has undergone chemotherapy for breast cancer.”
Dr. Berenholz reported having no financial disclosures. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
Using high-intensity focused electromagnetic (HIFEM) technology to strengthen pelvic floor muscles for the improvement of urinary incontinence (UI) and female sexual function was safe and effective at 9 months follow-up, results from a multicenter study showed.
“The pelvic floor consists of three pairs of muscles: the pubococcygeus, the iliococcygeus, and the puborectalis,” lead study author Joseph Berenholz, MD, and a diplomate of the American Board of Obstetics & Gynecology, said during the annual conference of the American Society for Laser Medicine and Surgery. “They control continence through support of pelvic organs. The urethra, the vagina, and the rectum pass through that diaphragm. It also contributes to sexual sensation and arousal. A deconditioning of the pelvic floor is usually the result of child-bearing years or aging, which usually results in urinary incontinence and impairment of sexual function. The noninvasive strengthening of the pelvic floor muscles helps to regain muscle tone and strength.”
In a prospective, open-label, single-arm study conducted at four sites, Dr. Berenholz, medical director of the Michigan Center for Women’s Health in Farmington Hills, and colleagues investigated the long-term effectiveness of HIFEM-induced pelvic floor muscle (PFM) strengthening for improvement of UI and sexual function. HIFEM selectively targets neuromuscular tissue and induces supramaximal PFM contractions that cannot be achieved voluntarily, he said, causing muscle strengthening due to muscle fiber hypertrophy, which helps patients to better isolate and command their muscles.
The study population consisted of 33 females with a mean age of 49 years who had UI and UI-related problems in sexual life. They received six 28-minute HIFEM treatments of the pelvic floor with the BTL Emsella, which is FDA cleared for both stress and urge incontinence. The frequency of visits was two treatments per week and the intensity of HIFEM was adjusted between 0% and 100% based on the patient’s tolerance threshold. Evaluations were conducted at baseline, after the last treatment, at 1, 3, 6, and 9 months. The primary outcomes were change in urine leakage based on the International Consultation on Incontinence Questionnaire–Short Form (ICIQ-UI-SF) and change in sexual function based on the Female Sexual Function Index (FSFI) and the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12). Secondary endpoints were adverse events and the comfort of therapy based on a 7-point Likert scale.
Dr. Berenholz reported that from baseline the severity of UI based on the ICIQ-SF significantly decreased 60% by a mean of 8.1 points between baseline and 9 months (P < .001). At 1 month, the FSFI score improved 32% by a mean of 7.1 points (P < .001) and was sustained throughout the study. The most prominent changes were seen in the subdomains of desire, arousal, lubrication, and orgasm response.
The PISQ-12 score incrementally increased 25% to a mean improvement of 8.2 points at 9 months (P < .001). Subjects improved most in the emotive subdomain, reporting more frequent orgasms, increased desire, and sexual excitement. The minimal important difference was 6 points.
“This is a true paradigm shift in the treatment of incontinence and sexual dysfunction,” Dr. Berenholz said. “The therapy was safe, comfortable, no adverse events emerged, and 31 subjects (94%) described the therapy as comfortable. Interim data suggest that treatment effect was maintained for 9 months, and there were no significant declines in scores in the long term. The upcoming 12-month follow-up data will let us know if more maintenance therapy is needed.”
During a question-and-answer session, one of the abstract section chairs, Albert Wolkerstorfer, MD, PhD, wondered about the potential for combination treatments in this patient population. “I can imagine that something that is working on the muscle tone has a totally different mechanism than something that is working on the mucosa and the underlying tissue without really affecting the muscle,” said Dr. Wolkerstorfer, a dermatologist at the Netherlands Institute for Pigment Disorders, department of dermatology, University of Amsterdam. “Would a combination be the way to go?”
Dr. Berenholz said that he sometimes combines HIFEM with the ULTRA Femme 360, a radiofrequency thermal energy device. “We thought this addresses two issues,” he said. “One is fascial muscle, which is the underlying structural issue for incontinence. The other is thermal energy to aid in incontinence prevention by inducing production of elastin and collagen in the midurethra, but also to promote lubrication and heightened sensitivity in the patient who’s either menopausal or has undergone chemotherapy for breast cancer.”
Dr. Berenholz reported having no financial disclosures. Dr. Wolkerstorfer disclosed that he has received consulting fees from Lumenis and InCyte and equipment from Humeca and PerfAction Technologies. He has also received grant funding from Novartis and InCyte and he is a member of InCyte’s advisory board.
FROM ASLMS 2021
How to choose the right vaginal moisturizer or lubricant for your patient
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.
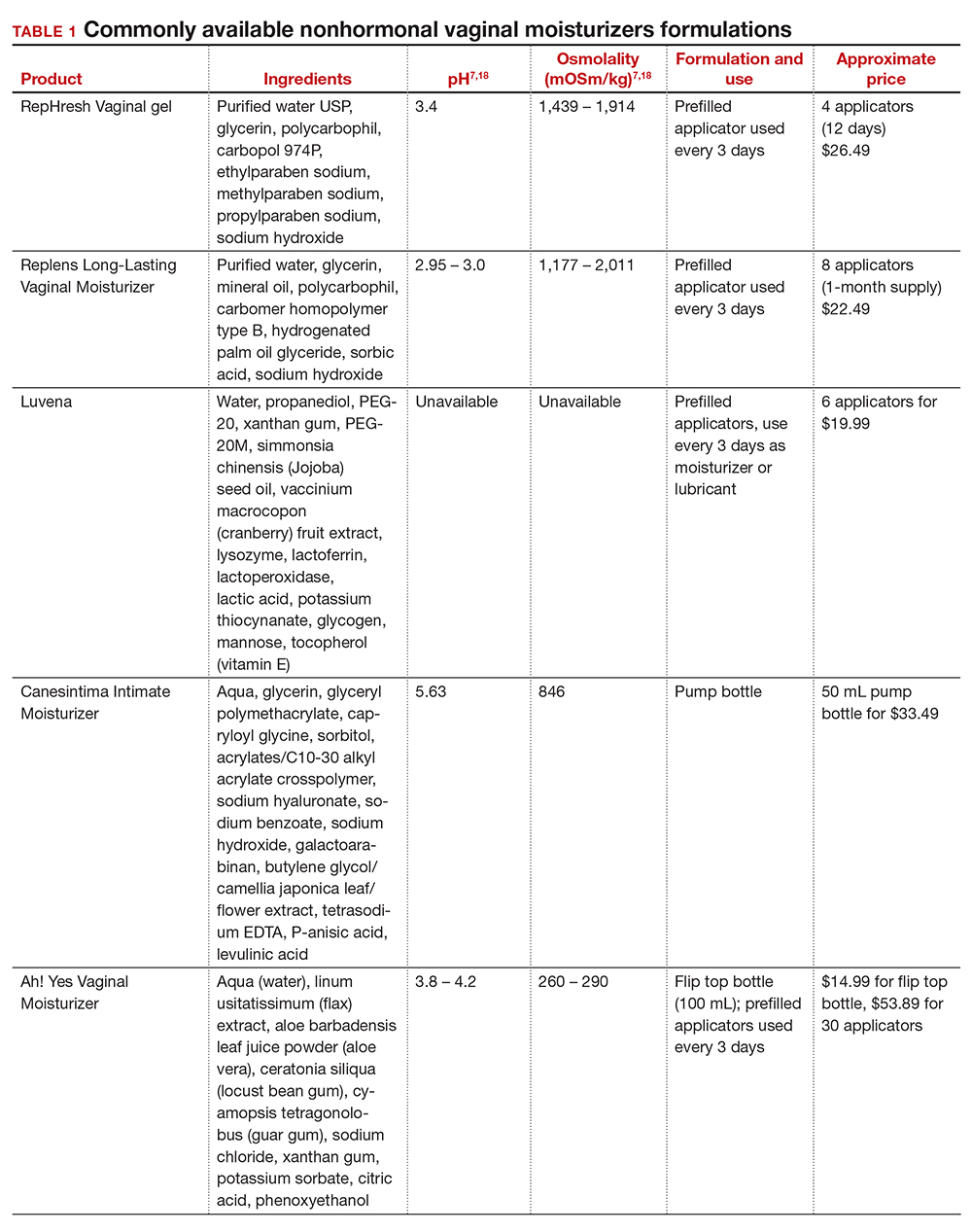
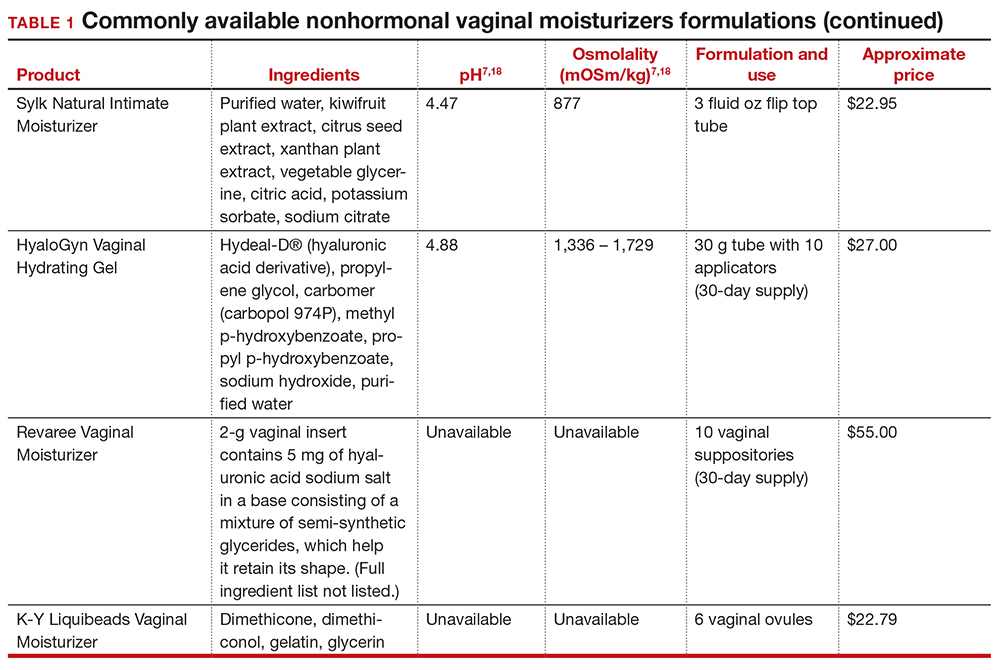
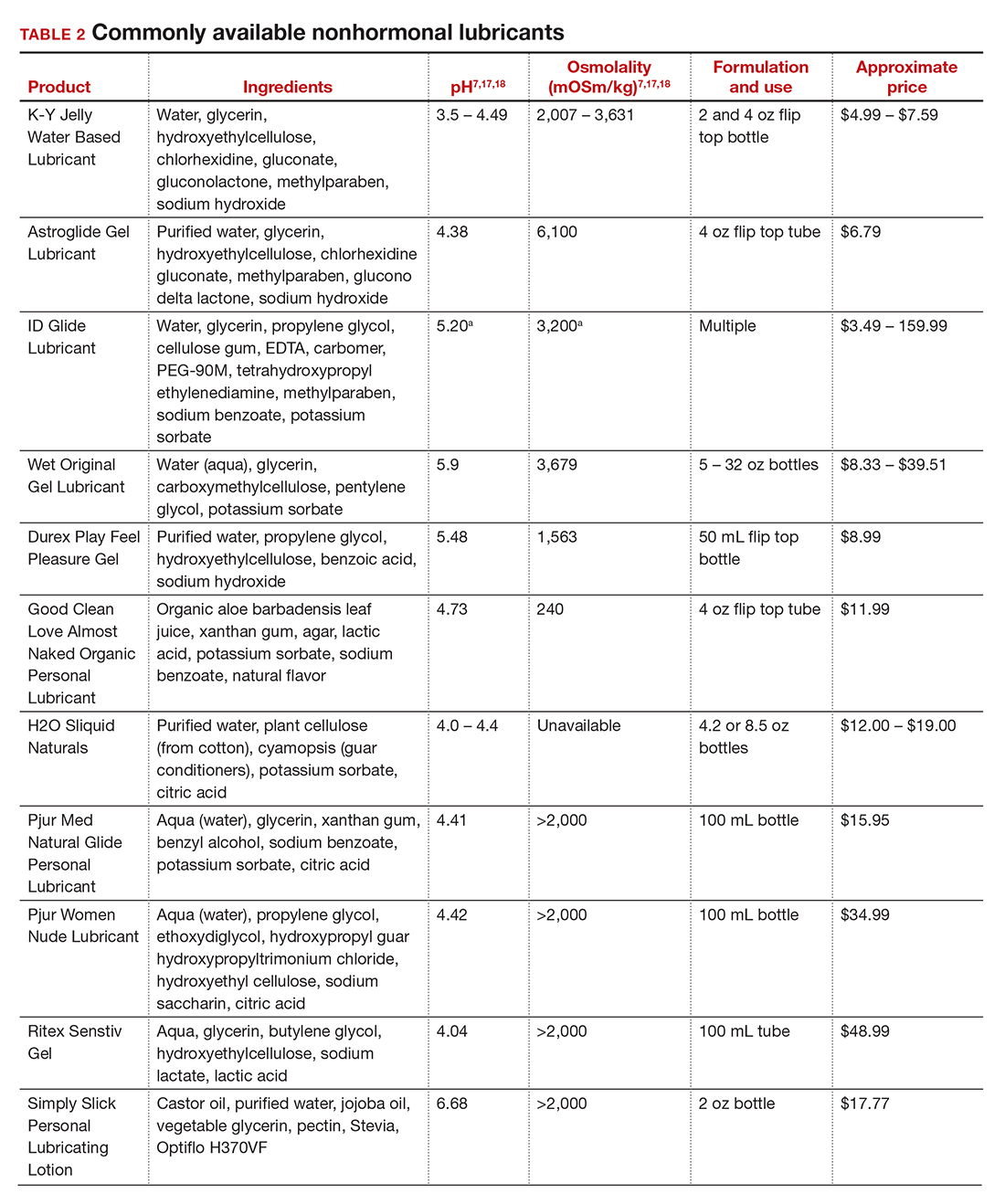
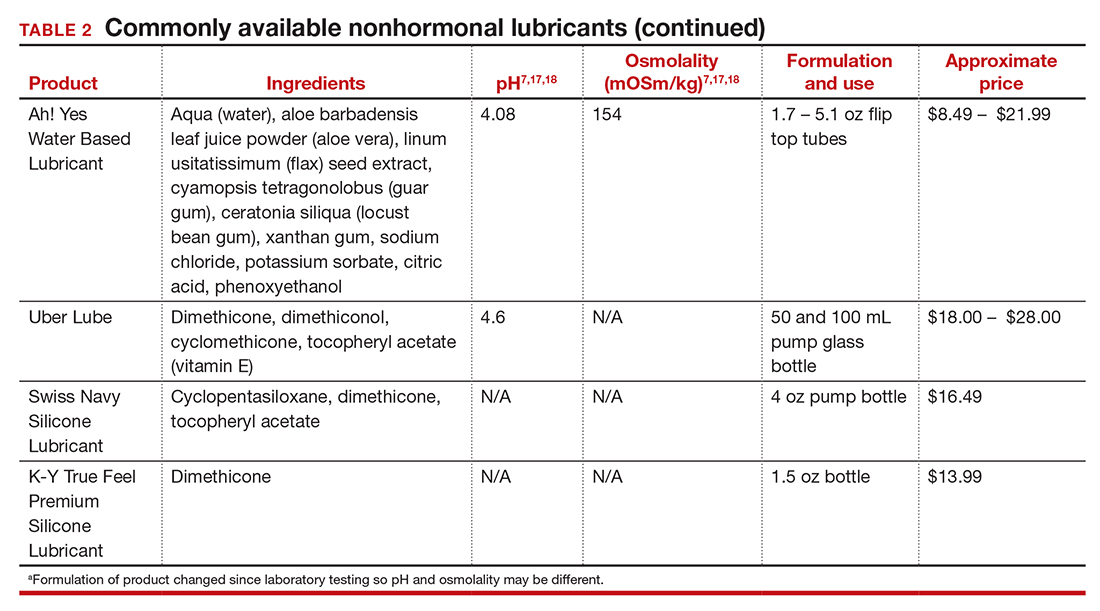
The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
Vaginal dryness, encompassed in the modern term genitourinary syndrome of menopause (GSM) affects up to 40% of menopausal women and up to 60% of postmenopausal breast cancer survivors.1,2 Premenopausal women also can have vulvovaginal dryness while breastfeeding (lactational amenorrhea) and while taking low-dose contraceptives.3 Vaginal moisturizers and lubricants are the first-line treatment options for vaginal dryness, dyspareunia, and GSM.4,5 In fact, approximately two-thirds of women have reported using a vaginal lubricant in their lifetime.6 Despite such ubiquitous use, many health care providers and patients have questions about the difference between vaginal moisturizers and lubricants and how to best choose a product.
Vaginal moisturizers
Vaginal moisturizers are designed to rehydrate the vaginal epithelium. Much like facial or skin moisturizers, they are intended to be applied regularly, every 2 to 3 days, but may be applied more often depending on the severity of symptoms. Vaginal moisturizers work by increasing the fluid content of the vaginal tissue and by lowering the vaginal pH to mimic that of natural vaginal secretions. Vaginal moisturizers are typically water based and use polymers to hydrate tissues.7 They change cell morphology but do not change vaginal maturation, indicating that they bring water to the tissue but do not shift the balance between superficial and basal cells and do not increase vaginal epithelial thickness as seen with vaginal estrogen.8 Vaginal moisturizers also have been found to be a safe alternative to vaginal estrogen therapy and may improve markers of vaginal health, including vaginal moisture, vaginal fluid volume, vaginal elasticity, and premenopausal pH.9 Commercially available vaginal moisturizers have been shown to be as effective as vaginal estrogens in reducing vaginal symptoms such as itching, irritation, and dyspareunia, but some caution should be taken when interpreting these results as neither vaginal moisturizer nor vaginal estrogen tablet were more effective than placebo in a recent randomized controlled trial.10,11 Small studies on hyaluronic acid have shown efficacy for the treatment of vaginal dryness.12,13 Hyaluronic acid is commercially available as a vaginal suppository ovule and as a liquid. It may also be obtained from a reliable compounding pharmacy. Vaginal suppository ovules may be a preferable formulation for women who find the liquids messy or cumbersome to apply.
Lubricants
Lubricants differ from vaginal moisturizers because they are specifically designed to be used during intercourse to provide short-term relief from vaginal dryness. They may be water-, silicone-, mineral oil-, or plant oil-based. The use of water- and silicone-based lubricants is associated with high satisfaction for intercourse as well as masturbation.14 These products may be particularly beneficial to women whose chief complaint is dyspareunia. In fact, women with dyspareunia report more lubricant use than women without dyspareunia, and the most common reason for lubricant use among these women was to reduce or alleviate pain.15 Overall, women both with and without dyspareunia have a positive perception regarding lubricant use and prefer sexual intercourse that feels more “wet,” and women in their forties have the most positive perception about lubricant use at the time of intercourse compared with other age groups.16 Furthermore, the World Health Organization (WHO) recommends that condom-compatible lubricants be used with condoms for menopausal and postmenopausal women.17 Both water-based and silicone-based lubricants may be used with latex condoms, while oil-based lubricants should be avoided as they can degrade the latex condom. While vaginal moisturizers and lubricants technically differ based on use, patients may use one product for both purposes, and some products are marketed as both a moisturizer and lubricant.
Continue to: Providing counsel to patients...
Providing counsel to patients
Patients often seek advice on how to choose vaginal moisturizers and lubricants. Understanding the compositions of these products and their scientific evidence is useful when helping patients make informed decisions regarding their pelvic health. Most commercially available lubricants are either water- or silicone- based. In one study comparing these two types of lubricants, water-based lubricants were associated with fewer genital symptoms than silicone-based products.14 Women may want to use a natural or organic product and may prefer plant-based oils such as coconut oil or olive oil. Patients should be counseled that latex condoms are not compatible with petroleum-, mineral oil- or plant oil-based lubricants.
In our practice, we generally recommend silicone-based lubricants, as they are readily available and compatible with latex condoms and generally require a smaller amount than water-based lubricants. They tend to be more expensive than water-based lubricants. For vaginal moisturizers, we often recommend commercially available formulations that can be purchased at local pharmacies or drug stores. However, a patient may need to try different lubricants and moisturizers in order to find a preferred product. We have included in TABLES 1 and 27,17,18 a list of commercially available vaginal moisturizers and lubricants with ingredient list, pH, osmolality, common formulation, and cost when available, which has been compiled from WHO and published research data to help guide patient counseling.




The effects of additives
Water-based moisturizers and lubricants may contain many ingredients, such as glycerols, fragrance, flavors, sweeteners, warming or cooling agents, buffering solutions, parabens and other preservatives, and numbing agents. These substances are added to water-based products to prolong water content, alter viscosity, alter pH, achieve certain sensations, and prevent bacterial contamination.7 The addition of these substances, however, will alter osmolality and pH balance of the product, which may be of clinical consequence. Silicone- or oil-based products do not contain water and therefore do not have a pH or an osmolality value.
Hyperosmolar formulations can theoretically injure epithelial tissue. In vitro studies have shown that hyperosmotic vaginal products can induce mild to moderate irritation, while very hyperosmolar formulations can induce severe irritation and tissue damage to vaginal epithelial and cervical cells.19,20 The WHO recommends that the osmolality of a vaginal product not exceed 380 mOsm/kg, but very few commercially available products meet these criteria so, clinically, the threshold is 1,200 mOsm/kg.17 It should be noted that most commercially available products exceed the 1,200 mOsm/kg threshold. Vaginal products may be a cause for vaginal irritation and should be considered in the differential diagnosis.
The normal vaginal pH is 3.8–4.5, and vaginal products should be pH balanced to this range. The exact role of pH in these products remains poorly understood. Nonetheless, products with a pH of 3 or lower are not recommended.18 Concerns about osmolality and pH remain theoretical, as a study of 12 commercially available lubricants of varying osmolality and pH found no cytotoxic effect in vivo.18
Vaginal moisturizers and lubricants contain many inactive ingredients, the most controversial of which are parabens. These substances are used in many cosmetic products as preservatives and are weakly estrogenic. These substances have been found in breast cancer tissue, but their possible role as a carcinogen remains uncertain.21,22 Nonetheless, the use of paraben-containing products is not recommended for women who have a history of hormonally-driven cancer or who are at high risk for developing cancer.7 Many lubricants contain glycerols (glycerol, glycerine, and propylene glycol) to alter viscosity or alter the water properties. The WHO recommends limits on the content of glycerols in these products.17 Glycerols have been associated with increased risk of bacterial vaginosis (adjusted odds ratio [aOR], 11.75; 95% confidence interval [CI], 1.96–70.27), and can serve as a food source for candida species, possibly increasing risk of yeast infections.7,23 Additionally, vaginal moisturizers and lubricants may contain preservatives such as chlorhexidine, which can disrupt normal vaginal flora and may cause tissue irritation.7
Continue to: Common concerns to be aware of...
Common concerns to be aware of
Women using vaginal products may be concerned about adverse effects, such as worsening vaginal irritation or infection. Vaginal moisturizers have not been shown to have increased risk of adverse effects compared with vaginal estrogens.9,10 In vitro studies have shown that vaginal moisturizers and lubricants inhibit the growth of Escherichia coli but may also inhibit Lactobacillus crispatus.24 Clinically, vaginal moisturizers have been shown to improve signs of bacterial vaginosis and have even been used to treat bacterial vaginosis.25,26 A study of commercially available vaginal lubricants inhibited the growth of L crispatus, which may predispose to irritation and infection.27 Nonetheless, the effect of the vaginal products on the vaginal microbiome and vaginal tissue remains poorly studied. Vaginal moisturizers and lubricants, while often helpful for patients, also can potentially cause irritation or predispose to infections. Providers should consider this when evaluating patients for new onset vaginal symptoms after starting vaginal products.
Bottom line
Vaginal products such as moisturizers and lubricants are often effective treatment options for women suffering from genitourinary syndrome of menopause and may be first-line treatment options, especially for women who may wish to avoid estrogen-containing products. Vaginal moisturizers can be recommended to any women experiencing vaginal irritation due to vaginal dryness while vaginal lubricants should be recommended to sexually active women who experience dyspareunia. Clinicians need to be aware of the formulations of these products and possible side effects in order to appropriately counsel patients. ●
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
- Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46-S52. doi: 10.1016/j.maturitas.2005.06.014.
- Crandall C, Peterson L, Ganz PA, et al. Association of breast cancer and its therapy with menopause-related symptoms. Menopause. 2004;11:519-530. doi: 10.1097/01.gme.0000117061.40493.ab.
- Bornstein J, Goldstein AT, Stockdale CK, et al. 2015 ISSVD, ISSWSH, and IPPS Consensus Terminology and Classification of Persistant Vulvar Pain and Vulvodynia. J Sex Med. 2016;13:607-612. doi: 10.1016/j.jsxm.2016.02.167.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Faubion S, Larkin L, Stuenkel C, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendation from The North American Menopause Society and the International Society for the Study for Women’s Sexual Health. Menopause. 2018;25:596-608. doi: 10.1097/GME.0000000000001121.
- Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014;11:642-652. doi: 10.1111/jsm.12427.
- Edwards D, Panay N. Treating vulvovaginal atrophy/genitourinary syndrome of menopause: how important is vaginal lubricant and moisturizer composition? Climacteric. 2016;19:151-116. doi: 10.3109/13697137.2015.1124259.
- Van der Lakk JAWN, de Bie LMT, de Leeuw H, et al. The effect of Replens on vaginal cytology in the treatment of postmenopausal atrophy: cytomorphology versus computerized cytometry. J Clin Pathol. 2002;55:446-451. doi: 10.1136/jcp.55.6.446.
- Nachtigall LE. Comparitive study: Replens versus local estrogen in menopausal women. Fertil Steril. 1994;61:178-180. doi: 10.1016/s0015-0282(16)56474-7.
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996;23:259-263. doi: 10.1016/0378-5122(95)00955-8.
- Mitchell CM, Reed SD, Diem S, et al. Efficacy of vaginal estradiol or vaginal moisturizer vs placebo for treating postmenopausal vulvovaginal symptoms. JAMA Intern Med. 2018;178:681-690. doi: 10.1001/jamainternmed.2018.0116.
- Chen J, Geng L, Song X, et al. Evaluation of the efficacy and safety of hyaluronic acid vaginal gel to ease vaginal dryness: a multicenter, randomized, controlled, open-label, parallel-group, clinical trial. J Sex Med. 2013;10:1575-1584. doi: 10.1111/jsm.12125.
- Jokar A, Davari T, Asadi N, et al. Comparison of the hyaluronic acid vaginal cream and conjugated estrogen used in treatment of vaginal atrophy of menopause women: a randomized controlled clinical trial. IJCBNM. 2016;4:69-78.
- Herbenick D, Reece M, Hensel D, et al. Association of lubricant use with women’s sexual pleasure, sexual satisfaction, and genital symptoms: a prospective daily diary study. J Sex Med. 2011;8:202-212. doi: 10.1111/j.1743-6109.2010.02067.x.
- Sutton KS, Boyer SC, Goldfinger C, et al. To lube or not to lube: experiences and perceptions of lubricant use in women with and without dyspareunia. J Sex Med. 2012;9:240-250. doi: 10.1111/j.1743-6109.2011.02543.x.
- Jozkowski KN, Herbenick D, Schick V, et al. Women’s perceptions about lubricant use and vaginal wetness during sexual activity. J Sex Med. 2013;10:484-492. doi: 10.1111/jsm.12022.
- World Health Organization. Use and procurement of additional lubricants for male and female condoms: WHO /UNFPA/FHI360 advisory note. 2012. https://www.who. int/reproductivehealth/publications/rtis/rhr12_33/en/. Accessed February 13, 2021.
- Cunha AR, Machado RM, Palmeira de Oliveira A, et al. Characterization of commercially available vaginal lubricants: a safety perspective. Pharmaceuticals. 2014;6:530-542. doi: 10.3390/pharmaceutics6030530.
- Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512-516. doi: 10.1097/OLQ.0b013e3181644669.
- Dezzuti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal.pone.0048328.
- Harvey PW, Everett DJ. Significance of the detection of esters of p-hydroxybenzoic acid (parabens) in human breast tumours. J Appl Toxicol. 2004:24:1-4. doi: 10.1002/jat.957.
- Darbre PD, Alijarrah A, Miller WR, et al. Concentrations of parabens in human breast tumous. J Appl Toxicol. 2004;24:5-13. doi: 10.1002/jat.958.
- Brotman RM, Ravel J, Cone RA, et al. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297-302. doi: 10.1136/sti.2009.040592.
- Hung KJ, Hudson P, Bergerat A, et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci Rep. 2020;10:7625.
- Wu JP, Fielding SL, Fiscell K. The effect of the polycarbophil gel (Replens) on bacterial vaginosis: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2007;130:132-136. doi: 10.1016/j.ejogrb.2006.01.007.
- Fiorelli A, Molteni B, Milani M. Successful treatment of bacterial vaginosis with a polycarbophil-carbopol acidic vaginal gel: results from a randomized double-bling, placebo controlled trial. Eur J Obstet Gynecol Reprod Biol. 2005;120:202-205. doi: 10.1016/j.ejogrb.2004.10.011.
- Fashemi B, Delaney ML, Onderdonk AB, et al. Effects of feminine hygiene products on the vaginal mucosal biome. Microb Ecol Health Dis. 2013;24. doi: 10.3402/mehd.v24i0.19703.
Pilot study: Hybrid laser found effective for treating genitourinary syndrome of menopause
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
, results from a pilot trial showed.
“The genitourinary syndrome of menopause causes suffering in breast cancer survivors and postmenopausal women,” Jill S. Waibel, MD, said during the annual conference of the American Society for Laser Medicine and Surgery. A common side effect for breast cancer survivors is early onset of menopause that is brought on by treatment, specifically aromatase-inhibitor therapies, she noted.
The symptoms of GSM include discomfort during sex, impaired sexual function, burning or sensation or irritation of the genital area, vaginal constriction, frequent urinary tract infections, urinary incontinence, and vaginal laxity, said Dr. Waibel, owner and medical director of the Miami Dermatology and Laser Institute. Nonhormonal treatments have included OTC vaginal lubricants, OTC moisturizers, low-dose vaginal estrogen – which increases the risk of breast cancer – and systemic estrogen therapy, which also can increase the risk of breast and endometrial cancer. “So, we need a healthy, nondrug option,” she said.
The objective of the pilot study was to determine the safety and efficacy of the diVa hybrid fractional laser as a treatment for symptoms of genitourinary syndrome of menopause, early menopause after breast cancer, or vaginal atrophy. The laser applies tunable nonablative (1,470-nm) and ablative (2,940-nm) wavelengths to the same microscopic treatment zone to maximize results and reduce downtime. The device features a motorized precision guidance system and calibrated rotation for homogeneous pulsing.
“The 2,940-nm wavelength is used to ablate to a depth of 0-800 micrometers while the 1,470-nm wavelength is used to coagulate the epithelium and the lamina propria at a depth of 100-700 micrometers,” said Dr. Waibel, who is also subsection chief of dermatology at Baptist Hospital of Miami. “This combination is used for epithelial tissue to heal quickly and the lamina propria to remodel slowly over time, laying down more collagen in tissue.” Each procedure is delivered via a single-use dilator, which expands the vaginal canal for increased treatment area. “The tip length is 5.5 cm and the diameter is 1 cm,” she said. “The clear tip acts as a hygienic barrier between the tip and the handpiece.”
Study participants included 25 women between the ages of 40 and 70 with early menopause after breast cancer or vaginal atrophy: 20 in the treatment arm and 5 in the sham-treatment arm. Dr. Waibel performed three procedures 2 weeks apart. An ob.gyn. assessed the primary endpoints, which included the Vaginal Health Index Scale (VHIS), the Vaginal Maturation Index (VMI), the Female Sexual Function Index (FSFI) questionnaire, and the Day-to-Day Impact of Vaginal Aging (DIVA) questionnaire. Secondary endpoints were histology and a satisfaction questionnaire.
Of the women in the treated group, there were data available for 19 at 3 months follow-up and 17 at 6 months follow-up. Based on the results in these patients, there were statistically significant improvements in nearly all domains of the FSFI treatment arm at 3 and 6 months when compared to baseline, especially arousal (P values of .05 at 3 months and .01 at 6 months) and lubrication (P values of .009 at three months and .001 at 6 months).
Between 3 and 6 months, patients in the treatment arm experienced improvements in four dimensions of the DIVA questionnaire: daily activities (P value of .01 at 3 months to .010 at 6 months), emotional well-being (P value of .06 at 3 months to .014 at 6 months), sexual function (P value of .30 at 3 months to .003 at 6 months), and self-concept/body image (P value of .002 at 3 months to .001 at 6 months).
As for satisfaction, a majority of those in the treatment arm were “somewhat satisfied” with the treatment and would “somewhat likely” repeat and recommend the treatment to friends and family, Dr. Waibel said. Results among the women in the control arm, who were also surveyed, were in the similar range, she noted. (No other results for women in the control arm were available.)
Following treatments, histology revealed that the collagen was denser, fibroblasts were more dense, and vascularity was more notable. No adverse events were observed. “The hybrid fractional laser is safe and effective for treating GSM, early menopause after breast cancer, or vaginal atrophy,” Dr. Waibel concluded. Further studies are important to improve the understanding of “laser dosimetry, frequency of treatments, and longevity of effect. Collaboration between ob.gyns. and dermatologists is important as we learn about laser therapy in GSM.”
Dr. Waibel disclosed that she is a member of the advisory board of Sciton, which manufactures the diVa laser. She has also conducted clinical trials for many other device and pharmaceutical companies.
FROM ASLMS 2021
Obesity pegged as source of marked increased risk of diabetes in PCOS
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
The increased risk of type 2 diabetes in women with polycystic ovary syndrome is well established, but a new analysis has shown that obesity is the major mediator and a target for preventing or reversing this comorbidity.
“Most women with PCOS are obese, complicating the effort to understand whether high rates of diabetes in this population are due to PCOS or excess weight, but our study now suggest that obesity isa targetable risk factor,” reported Panagiotis Anagnostis, MD, PhD, a reproductive endocrinologist at the Medical School of Aristotle University, Thessaloniki, Greece.
Obesity is also a known risk factor for type 2 diabetes (T2D), but there is reason to suspect that PCOS, which is associated with abnormal carbohydrate metabolism, has a direct impact on the risk of developing T2D, according to Dr. Anagnostis. It is also reasonable to expect “a synergistic deleterious effect” from PCOS and obesity on adverse changes in glucose metabolism that lead to T2D.
Even though rates of obesity among women with PCOS reach 80% in some studies, Dr. Anagnostis attempted to disentangle the relationship between obesity, PCOS, and risk of T2D using a large set of data drawn from a comprehensive search of published studies.
After screening with predefined criteria, 12 studies provided data on 224,284 women, of whom 45,361 had PCOS and 5,717 had T2D. Not least of the criteria for inclusion in this analysis, all studies stratified women as obese, defined as a body mass index (BMI) greater than 30 kg/m2, or nonobese, he reported at the annual meeting of the Endocrine Society.
Diabetes risk tripled in PCOS
When compared without regard to BMI, the relative risk of having T2D among those with PCOS relative to those without this condition was more than three times greater (RR 3.13; P < .001). When women with PCOS were stratified for BMI, obesity was associated with a more than fourfold increased risk relative to controls without PCOS (RR, 4.06; P < .001).
In women who were nonobese, the risk of T2D was numerically higher for those with PCOS than those without (RR, 2.68), but it was only a trend with a large confidence interval (95% confidence interval, 0.97-7.49).
Among women with PCOS, those who were obese also had a more than fourfold and highly significant increased risk of T2D relative to those who were not obese (RR, 4.20; P < .001).
The message from these data is that obesity is a major and potentially modifiable risk factor for diabetes in women with PCOS, according to Dr. Anagnostis.
He said these data provide the basis for recommending weight loss specifically for managing this common PCOS comorbidity.
Almost the same relative risk of diabetes was derived from an analysis of a women’s health database published 2 years ago in Diabetes Care. In that study with 1,916 person-years of follow-up, the hazard ratio for T2D was also more than three times greater (HR, 3.23; P < .001) for those with PCOS relative to those without the syndrome.
However, normal BMI did not eliminate risk of developing diabetes in this study. Rather, the relative risk of T2D in women with PCOS was higher in those of normal weight, compared with those who were obese (HR, 4.68 vs. 2.36; P < .005). The investigators recommend screening all women with PCOS at least every 3 years with more frequent screening in those with risk factors.
PCOS complexity challenges simple conclusions
The complexity of disturbed metabolic pathways in patients with PCOS and obesity might explain some of the difficulty in unraveling the relationship between these two disease states and diabetes risk. In one recent review, it was suggested that obesity and PCOS share interrelated adverse effects on glucose metabolism. As a result, these associations are “more complex than a simple cause-and-effect process.” the authors of that article concluded.
Furthermore, in their examination of metabolic pathways, genetic susceptibility, and behavioral factors that might link PCOS, weight gain, and T2D, the authors did not ignore the psychological impact of PCOS in causing obesity and, as a byproduct, diabetes. These psychological factors might be relevant to treatment.
For example, depression and stress “might hamper ongoing attempts at lifestyle change and therefore effective weight loss” in at least some women, they cautioned.
However, in encouraging weight loss in overweight women with PCOS, the debate about cause of T2D might be moot in practical terms, according to Michael Dansinger, MD, founding director of the diabetes reversal program at Tufts Medical Center, Boston.
“Reducing excess body fat reduces the risk of type 2 diabetes,” Dr. Dansinger said in an interview. “Since women with obesity and PCOS are clearly at risk for future type 2 diabetes, that’s another reason to lose excess body fat through healthy eating and exercise.”
Dr. Anagnostis and Dr. Dansinger reported no relevant conflicts of interest.
FROM ENDO 2021