User login
Patients With Stable Lupus May Be Safely Weaned Off MMF
Patients with quiescent systemic lupus erythematosus (SLE) who are on maintenance therapy with mycophenolate mofetil (MMF) may be able to be safely weaned off the drug with the understanding that disease flare may occur and may require restarting immunosuppressive therapy.
That’s the conclusion of investigators in a multicenter randomized trial conducted at 19 US centers and published in The Lancet Rheumatology. They found that among 100 patients with stable SLE who were on MMF for at least 2 years for renal indications or at least 1 year for nonrenal indications, MMF withdrawal was not significantly inferior to MMF maintenance in terms of clinically significant disease reactivation within at least 1 year.
“Our findings suggest that mycophenolate mofetil could be safely withdrawn in patients with stable SLE. However, larger studies with a longer follow-up are still needed,” wrote Eliza F. Chakravarty, MD, MS, from the University of Oklahoma College of Medicine in Oklahoma City, and colleagues.
“Our study was only for 60 weeks, so we don’t have long-term data on what happens when patients taper off, but my recommendation — and I think the data support this — is that even if you do have a history of lupus nephritis, if you had stable disease or very little to no activity for a year or 2, then I think it’s worth stopping the medication and following for any signs of disease flare,” Dr. Chakravarty said in an interview with this news organization.
She added that “in clinical practice, we would follow patients regularly no matter what they’re on, even if they’re in remission, looking for clinical signs or laboratory evidence of flare, and then if they look like they might be having flare, treat them accordingly.”
Toxicities a Concern
Although MMF is effective for inducing prolonged disease quiescence, it is a known teratogen and has significant toxicities, and it’s desirable to wean patients off the drug if it can be done safely, Dr. Chakravarty said.
The optimal duration of maintenance therapy with MMF is not known, however, which prompted the researchers to conduct the open-label study.
Patients aged 18-70 years who met the American College of Rheumatology (ACR) 1997 SLE criteria and had a clinical SLE Disease Activity Index (SLEDAI) score ≤ 4 at screening and who also had been on stable or tapering MMF therapy for 2 or more years for renal indications or 1 or more year for nonrenal indications were eligible. All patients were on a background regimen of hydroxychloroquine.
Patients were randomly assigned on an equal basis to either withdrawal with a 12-week taper or to continued maintenance at their baseline dose, ranging from 1 to 3 g/day for 60 weeks.
The investigators used an adaptive random-allocation strategy to ensure that the groups were balanced for study site, renal vs nonrenal disease, and baseline MMF dose (≥ 2 g/day vs < 2 g/day).
A total of 100 patients with an average age of 42 years were included in a modified intention-to-treat analysis: 49 were randomly assigned to maintenance and 51 to withdrawal.
Overall, 84% of patients were women, 40% were White, and 41% were Black. Most patients, 76%, had a history of lupus nephritis.
Significant disease reactivation, the primary endpoints, was defined as the need to increase prednisone to ≥ 15 mg/day for 4 weeks, the need for two or more short steroid bursts, or the need to resume MMF or start patients on another immunosuppressive therapy.
By week 60, 18% of patients in the withdrawal group had clinically significant disease reactivation compared with 10% of patients in the maintenance group.
“Although the differences were not significant, this study used an estimation-based design to determine estimated increases in clinically significant disease reactivation risk with 75%, 85%, and 95% confidence limits to assist clinicians and patients in making informed treatment decisions. We found a 6%-8% increase with upper 85% confidence limits of 11%-19% in clinically significant disease reactivation and flare risk following mycophenolate mofetil withdrawal,” the investigators wrote.
Rates of adverse events were similar between the groups, occurring in 90% of patients in the maintenance arm and 88% of those in the withdrawal arm. Infections occurred more frequently among patients in the maintenance group, at 64% vs 46%.
Encouraging Data
In an accompanying editorial, Noémie Jourde-Chiche, MD, PhD, from Aix-Marseille University in Marseille, France, and Laurent Chiche, MD, from Hopital Europeen de Marseille, wrote that the study data “were clearly encouraging.” They noted that the results show that it’s feasible to wean select patients off immunosuppressive therapy and keep SLE in check and that the quantified risk assessment strategy will allow shared decision-making for each patient.
“Overall, the prospect of a time-limited (versus lifelong) treatment may favor compliance, as observed in other disease fields, which might consolidate remission and reduce the risk of subsequent relapse, using sequentially treat-to-target and think-to-untreat strategies for a win-wean era in SLE,” they wrote.
“We’ve been awaiting the results of this trial for quite a while, and so it is nice to see it out,” commented Karen H. Costenbader, MD, MPH, professor of medicine at Harvard Medical School, and chair of the division of rheumatology and director of the Lupus Program at Brigham and Women’s Hospital in Boston, Massachusetts.
“It does provide some data to address a question that comes up in discussions with patients all the time: A person with lupus has been doing really well, in what we call low disease activity state or remission, but on mycophenolate, possibly for several years,” she said in a reply to a request for objective commentary.
“The question is how and when to taper and can MMF be safely discontinued,” she said. “Personally, I always review the severity of the underlying disease and indication for the MMF in the first place. Really active SLE with rapidly progressing kidney or other organ damage has to be treated with tremendous respect and no one wants to go back there. I also think about how long it has been, which other medications are still being taken (hydroxychloroquine, belimumab [Benlysta], etc.) and whether the labs and symptoms have really returned to completely normal. Then I have discussions about all this with my patient and we often try a long, slow, gingerly taper with a lot of interim monitoring.”
The study was funded by the National Institute of Allergy and Infectious Diseases and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Chakravarty and Dr. Costenbader report no relevant financial relationships. Dr. Jourde-Chiche declares personal consulting fees from Otsuka and AstraZeneca, personal speaking fees from GlaxoSmithKline and Otsuka, and personal payment for expert testimony from Otsuka. Dr. Chiche declares research grants paid to his institution from AstraZeneca and GlaxoSmithKline, personal consulting fees from Novartis and AstraZeneca, and personal speaking fees from GlaxoSmithKline and Novartis.
A version of this article appeared on Medscape.com .
Patients with quiescent systemic lupus erythematosus (SLE) who are on maintenance therapy with mycophenolate mofetil (MMF) may be able to be safely weaned off the drug with the understanding that disease flare may occur and may require restarting immunosuppressive therapy.
That’s the conclusion of investigators in a multicenter randomized trial conducted at 19 US centers and published in The Lancet Rheumatology. They found that among 100 patients with stable SLE who were on MMF for at least 2 years for renal indications or at least 1 year for nonrenal indications, MMF withdrawal was not significantly inferior to MMF maintenance in terms of clinically significant disease reactivation within at least 1 year.
“Our findings suggest that mycophenolate mofetil could be safely withdrawn in patients with stable SLE. However, larger studies with a longer follow-up are still needed,” wrote Eliza F. Chakravarty, MD, MS, from the University of Oklahoma College of Medicine in Oklahoma City, and colleagues.
“Our study was only for 60 weeks, so we don’t have long-term data on what happens when patients taper off, but my recommendation — and I think the data support this — is that even if you do have a history of lupus nephritis, if you had stable disease or very little to no activity for a year or 2, then I think it’s worth stopping the medication and following for any signs of disease flare,” Dr. Chakravarty said in an interview with this news organization.
She added that “in clinical practice, we would follow patients regularly no matter what they’re on, even if they’re in remission, looking for clinical signs or laboratory evidence of flare, and then if they look like they might be having flare, treat them accordingly.”
Toxicities a Concern
Although MMF is effective for inducing prolonged disease quiescence, it is a known teratogen and has significant toxicities, and it’s desirable to wean patients off the drug if it can be done safely, Dr. Chakravarty said.
The optimal duration of maintenance therapy with MMF is not known, however, which prompted the researchers to conduct the open-label study.
Patients aged 18-70 years who met the American College of Rheumatology (ACR) 1997 SLE criteria and had a clinical SLE Disease Activity Index (SLEDAI) score ≤ 4 at screening and who also had been on stable or tapering MMF therapy for 2 or more years for renal indications or 1 or more year for nonrenal indications were eligible. All patients were on a background regimen of hydroxychloroquine.
Patients were randomly assigned on an equal basis to either withdrawal with a 12-week taper or to continued maintenance at their baseline dose, ranging from 1 to 3 g/day for 60 weeks.
The investigators used an adaptive random-allocation strategy to ensure that the groups were balanced for study site, renal vs nonrenal disease, and baseline MMF dose (≥ 2 g/day vs < 2 g/day).
A total of 100 patients with an average age of 42 years were included in a modified intention-to-treat analysis: 49 were randomly assigned to maintenance and 51 to withdrawal.
Overall, 84% of patients were women, 40% were White, and 41% were Black. Most patients, 76%, had a history of lupus nephritis.
Significant disease reactivation, the primary endpoints, was defined as the need to increase prednisone to ≥ 15 mg/day for 4 weeks, the need for two or more short steroid bursts, or the need to resume MMF or start patients on another immunosuppressive therapy.
By week 60, 18% of patients in the withdrawal group had clinically significant disease reactivation compared with 10% of patients in the maintenance group.
“Although the differences were not significant, this study used an estimation-based design to determine estimated increases in clinically significant disease reactivation risk with 75%, 85%, and 95% confidence limits to assist clinicians and patients in making informed treatment decisions. We found a 6%-8% increase with upper 85% confidence limits of 11%-19% in clinically significant disease reactivation and flare risk following mycophenolate mofetil withdrawal,” the investigators wrote.
Rates of adverse events were similar between the groups, occurring in 90% of patients in the maintenance arm and 88% of those in the withdrawal arm. Infections occurred more frequently among patients in the maintenance group, at 64% vs 46%.
Encouraging Data
In an accompanying editorial, Noémie Jourde-Chiche, MD, PhD, from Aix-Marseille University in Marseille, France, and Laurent Chiche, MD, from Hopital Europeen de Marseille, wrote that the study data “were clearly encouraging.” They noted that the results show that it’s feasible to wean select patients off immunosuppressive therapy and keep SLE in check and that the quantified risk assessment strategy will allow shared decision-making for each patient.
“Overall, the prospect of a time-limited (versus lifelong) treatment may favor compliance, as observed in other disease fields, which might consolidate remission and reduce the risk of subsequent relapse, using sequentially treat-to-target and think-to-untreat strategies for a win-wean era in SLE,” they wrote.
“We’ve been awaiting the results of this trial for quite a while, and so it is nice to see it out,” commented Karen H. Costenbader, MD, MPH, professor of medicine at Harvard Medical School, and chair of the division of rheumatology and director of the Lupus Program at Brigham and Women’s Hospital in Boston, Massachusetts.
“It does provide some data to address a question that comes up in discussions with patients all the time: A person with lupus has been doing really well, in what we call low disease activity state or remission, but on mycophenolate, possibly for several years,” she said in a reply to a request for objective commentary.
“The question is how and when to taper and can MMF be safely discontinued,” she said. “Personally, I always review the severity of the underlying disease and indication for the MMF in the first place. Really active SLE with rapidly progressing kidney or other organ damage has to be treated with tremendous respect and no one wants to go back there. I also think about how long it has been, which other medications are still being taken (hydroxychloroquine, belimumab [Benlysta], etc.) and whether the labs and symptoms have really returned to completely normal. Then I have discussions about all this with my patient and we often try a long, slow, gingerly taper with a lot of interim monitoring.”
The study was funded by the National Institute of Allergy and Infectious Diseases and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Chakravarty and Dr. Costenbader report no relevant financial relationships. Dr. Jourde-Chiche declares personal consulting fees from Otsuka and AstraZeneca, personal speaking fees from GlaxoSmithKline and Otsuka, and personal payment for expert testimony from Otsuka. Dr. Chiche declares research grants paid to his institution from AstraZeneca and GlaxoSmithKline, personal consulting fees from Novartis and AstraZeneca, and personal speaking fees from GlaxoSmithKline and Novartis.
A version of this article appeared on Medscape.com .
Patients with quiescent systemic lupus erythematosus (SLE) who are on maintenance therapy with mycophenolate mofetil (MMF) may be able to be safely weaned off the drug with the understanding that disease flare may occur and may require restarting immunosuppressive therapy.
That’s the conclusion of investigators in a multicenter randomized trial conducted at 19 US centers and published in The Lancet Rheumatology. They found that among 100 patients with stable SLE who were on MMF for at least 2 years for renal indications or at least 1 year for nonrenal indications, MMF withdrawal was not significantly inferior to MMF maintenance in terms of clinically significant disease reactivation within at least 1 year.
“Our findings suggest that mycophenolate mofetil could be safely withdrawn in patients with stable SLE. However, larger studies with a longer follow-up are still needed,” wrote Eliza F. Chakravarty, MD, MS, from the University of Oklahoma College of Medicine in Oklahoma City, and colleagues.
“Our study was only for 60 weeks, so we don’t have long-term data on what happens when patients taper off, but my recommendation — and I think the data support this — is that even if you do have a history of lupus nephritis, if you had stable disease or very little to no activity for a year or 2, then I think it’s worth stopping the medication and following for any signs of disease flare,” Dr. Chakravarty said in an interview with this news organization.
She added that “in clinical practice, we would follow patients regularly no matter what they’re on, even if they’re in remission, looking for clinical signs or laboratory evidence of flare, and then if they look like they might be having flare, treat them accordingly.”
Toxicities a Concern
Although MMF is effective for inducing prolonged disease quiescence, it is a known teratogen and has significant toxicities, and it’s desirable to wean patients off the drug if it can be done safely, Dr. Chakravarty said.
The optimal duration of maintenance therapy with MMF is not known, however, which prompted the researchers to conduct the open-label study.
Patients aged 18-70 years who met the American College of Rheumatology (ACR) 1997 SLE criteria and had a clinical SLE Disease Activity Index (SLEDAI) score ≤ 4 at screening and who also had been on stable or tapering MMF therapy for 2 or more years for renal indications or 1 or more year for nonrenal indications were eligible. All patients were on a background regimen of hydroxychloroquine.
Patients were randomly assigned on an equal basis to either withdrawal with a 12-week taper or to continued maintenance at their baseline dose, ranging from 1 to 3 g/day for 60 weeks.
The investigators used an adaptive random-allocation strategy to ensure that the groups were balanced for study site, renal vs nonrenal disease, and baseline MMF dose (≥ 2 g/day vs < 2 g/day).
A total of 100 patients with an average age of 42 years were included in a modified intention-to-treat analysis: 49 were randomly assigned to maintenance and 51 to withdrawal.
Overall, 84% of patients were women, 40% were White, and 41% were Black. Most patients, 76%, had a history of lupus nephritis.
Significant disease reactivation, the primary endpoints, was defined as the need to increase prednisone to ≥ 15 mg/day for 4 weeks, the need for two or more short steroid bursts, or the need to resume MMF or start patients on another immunosuppressive therapy.
By week 60, 18% of patients in the withdrawal group had clinically significant disease reactivation compared with 10% of patients in the maintenance group.
“Although the differences were not significant, this study used an estimation-based design to determine estimated increases in clinically significant disease reactivation risk with 75%, 85%, and 95% confidence limits to assist clinicians and patients in making informed treatment decisions. We found a 6%-8% increase with upper 85% confidence limits of 11%-19% in clinically significant disease reactivation and flare risk following mycophenolate mofetil withdrawal,” the investigators wrote.
Rates of adverse events were similar between the groups, occurring in 90% of patients in the maintenance arm and 88% of those in the withdrawal arm. Infections occurred more frequently among patients in the maintenance group, at 64% vs 46%.
Encouraging Data
In an accompanying editorial, Noémie Jourde-Chiche, MD, PhD, from Aix-Marseille University in Marseille, France, and Laurent Chiche, MD, from Hopital Europeen de Marseille, wrote that the study data “were clearly encouraging.” They noted that the results show that it’s feasible to wean select patients off immunosuppressive therapy and keep SLE in check and that the quantified risk assessment strategy will allow shared decision-making for each patient.
“Overall, the prospect of a time-limited (versus lifelong) treatment may favor compliance, as observed in other disease fields, which might consolidate remission and reduce the risk of subsequent relapse, using sequentially treat-to-target and think-to-untreat strategies for a win-wean era in SLE,” they wrote.
“We’ve been awaiting the results of this trial for quite a while, and so it is nice to see it out,” commented Karen H. Costenbader, MD, MPH, professor of medicine at Harvard Medical School, and chair of the division of rheumatology and director of the Lupus Program at Brigham and Women’s Hospital in Boston, Massachusetts.
“It does provide some data to address a question that comes up in discussions with patients all the time: A person with lupus has been doing really well, in what we call low disease activity state or remission, but on mycophenolate, possibly for several years,” she said in a reply to a request for objective commentary.
“The question is how and when to taper and can MMF be safely discontinued,” she said. “Personally, I always review the severity of the underlying disease and indication for the MMF in the first place. Really active SLE with rapidly progressing kidney or other organ damage has to be treated with tremendous respect and no one wants to go back there. I also think about how long it has been, which other medications are still being taken (hydroxychloroquine, belimumab [Benlysta], etc.) and whether the labs and symptoms have really returned to completely normal. Then I have discussions about all this with my patient and we often try a long, slow, gingerly taper with a lot of interim monitoring.”
The study was funded by the National Institute of Allergy and Infectious Diseases and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Chakravarty and Dr. Costenbader report no relevant financial relationships. Dr. Jourde-Chiche declares personal consulting fees from Otsuka and AstraZeneca, personal speaking fees from GlaxoSmithKline and Otsuka, and personal payment for expert testimony from Otsuka. Dr. Chiche declares research grants paid to his institution from AstraZeneca and GlaxoSmithKline, personal consulting fees from Novartis and AstraZeneca, and personal speaking fees from GlaxoSmithKline and Novartis.
A version of this article appeared on Medscape.com .
FROM THE LANCET RHEUMATOLOGY
New Findings on Vitamin D, Omega-3 Supplements for Preventing Autoimmune Diseases
Two years after the end of a randomized trial that showed a benefit of daily vitamin D and omega-3 fatty acid (n-3 FA) supplementation for reducing risk for autoimmune diseases, the salubrious effects of daily vitamin D appear to have waned after the supplement was discontinued, while the protection from n-3 lived on for at least 2 additional years.
As previously reported, the randomized VITAL, which was designed primarily to study the effects of vitamin D and n-3 supplementation on incident cancer and cardiovascular disease, also showed that 5 years of vitamin D supplementation was associated with a 22% reduction in risk for confirmed autoimmune diseases, and 5 years of n-3 FA supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases.
Now, investigators Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston, Massachusetts, and colleagues reported that among 21,592 participants in VITAL who agreed to be followed for an additional 2 years after discontinuation, the protection against autoimmune diseases from daily vitamin D (cholecalciferol; 2000 IU/d) was no longer statistically significant, but the benefits of daily marine n-3 FAs (1 g/d as a fish-oil capsule containing 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid) remained significant.
“VITAL observational extension results suggest that vitamin D supplementation should be given on a continuous basis for long-term prevention of [autoimmune diseases]. The beneficial effects of n-3 fatty acids, however, may be prolonged for at least 2 years after discontinuation,” they wrote in an article published in Arthritis & Rheumatology.
Dr. Costenbader told this news organization that the results of the observational extension study suggest that the benefits of vitamin D “wear off more quickly, and it should be continued for a longer period of time or indefinitely, rather than only for 5 years.”
In addition to the disparity in the duration of the protective effect, the investigators also saw differences in the effects across different autoimmune diseases.
“The protective effect of vitamin D seemed strongest for psoriasis, while for omega-3 fatty acids, the protective effects were strongest for rheumatoid arthritis and inflammatory bowel disease,” she said.
Mixed Effects
In an interview with this news organization, Janet Funk, MD, MS, vice chair of research in the Department of Medicine and professor in the School of Nutritional Science and Wellness at the University of Arizona, Tucson, Arizona, who was not involved in the study, saidthat the results suggest that while each supplement may offer protection against autoimmune diseases, the effects are inconsistent and may not apply to all patients.
“I think the VITAL extension results suggest that either supplement (or both together) may have benefits in reducing risk of autoimmune diseases, including possible persistent effects posttreatment, but that these effects are nuanced (ie, only in normal weight post-vitamin D treatment) and possibly not uniform across all autoimmune diseases (including possible adverse effects for some — eg, inverse association between prior omega-3 and psoriasis and tendency for increased autoimmune thyroid disease for vitamin D), although the study was not powered sufficiently to draw disease-specific conclusions,” she said.
In an editorial accompanying the study, rheumatologist Joel M. Kremer, MD, of Albany Medical College and the Corrona Research Foundation in Delray Beach, Florida, wrote that “[T]he studies by Dr. Costenbader, et al. have shed new light on the possibility that dietary supplements of n-3 FA [fatty acid] may prevent the onset of [autoimmune disease]. The sustained benefits they describe for as long as 2 years after the supplements are discontinued are consistent with the chronicity of FA species in cellular plasma membranes where they serve as substrates for a diverse array of salient metabolic and inflammatory pathways.”
VITAL Then
To test whether vitamin D or marine-derived long-chain n-3 FA supplementation could protect against autoimmune disease over time, Dr. Costenbader and colleagues piggybacked an ancillary study onto the VITAL trial, which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older and 13,085 women aged 55 and older. The study had a 2 × 2 factorial design, with patients randomly assigned to vitamin D 2000 IU/d or placebo and then further randomized to either 1 g/d n-3 FAs or placebo in both the vitamin D and placebo primary randomization arms.
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with a hazard ratio (HR) of 0.68 (P = .02) for incident autoimmune disease, n-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03). However, when probable incident autoimmune disease cases were included, the effect of n-3 became significant, with an HR of 0.82.
VITAL Now
In the current analysis, Dr. Costenbader and colleagues reported observational data on 21,592 VITAL participants, a sample representing 83.5% of those who were initially randomized, and 87.9% of those who were alive and could be contacted at the end of the study.
As in the initial trial, the investigators used annual questionnaires to assess incident autoimmune diseases during the randomized follow-up. Participants were asked about new-onset, doctor-diagnosed rheumatoid arthritis, polymyalgia rheumatica, psoriasis, autoimmune thyroid disease, and inflammatory bowel disease. Participants could also write in any other new autoimmune disease diagnoses.
There were 236 new cases of confirmed autoimmune disease that occurred since the initial publication of the trial results, as well as 65 probable cases identified during the median 5.3 years of the randomized portion, and 42 probable cases diagnosed during the 2-year observational phase.
The investigators found that after the 2-year observation period, 255 participants initially randomized to receive vitamin D had a newly developed confirmed autoimmune disease, compared with 259 of those initially randomized to a vitamin D placebo. This translated into a nonsignificant HR of 0.98.
Adding probable autoimmune cases to the confirmed cases made little difference, resulting in a nonsignificant adjusted HR of 0.95.
In contrast, there were 234 confirmed autoimmune disease cases among patients initially assigned to n-3, compared with 280 among patients randomized to the n-3 placebo, translating into a statistically significant HR of 0.83 for new-onset autoimmune disease with n-3.
Dr. Costenbader and colleagues acknowledged that the study was limited by the use of doses intended to prevent cancer or cardiovascular disease and that higher doses intended for high-risk or nutritionally deficient populations might reveal larger effects of supplementation. In addition, they noted the difficulty of identifying the timing and onset of incident disease, and that the small number of cases that occurred during the 2-year observational period precluded detailed analyses of individual autoimmune diseases.
The study was funded by grants from the National Institutes of Health. Dr. Costenbader, Dr. Funk, and Dr. Kremer reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Two years after the end of a randomized trial that showed a benefit of daily vitamin D and omega-3 fatty acid (n-3 FA) supplementation for reducing risk for autoimmune diseases, the salubrious effects of daily vitamin D appear to have waned after the supplement was discontinued, while the protection from n-3 lived on for at least 2 additional years.
As previously reported, the randomized VITAL, which was designed primarily to study the effects of vitamin D and n-3 supplementation on incident cancer and cardiovascular disease, also showed that 5 years of vitamin D supplementation was associated with a 22% reduction in risk for confirmed autoimmune diseases, and 5 years of n-3 FA supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases.
Now, investigators Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston, Massachusetts, and colleagues reported that among 21,592 participants in VITAL who agreed to be followed for an additional 2 years after discontinuation, the protection against autoimmune diseases from daily vitamin D (cholecalciferol; 2000 IU/d) was no longer statistically significant, but the benefits of daily marine n-3 FAs (1 g/d as a fish-oil capsule containing 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid) remained significant.
“VITAL observational extension results suggest that vitamin D supplementation should be given on a continuous basis for long-term prevention of [autoimmune diseases]. The beneficial effects of n-3 fatty acids, however, may be prolonged for at least 2 years after discontinuation,” they wrote in an article published in Arthritis & Rheumatology.
Dr. Costenbader told this news organization that the results of the observational extension study suggest that the benefits of vitamin D “wear off more quickly, and it should be continued for a longer period of time or indefinitely, rather than only for 5 years.”
In addition to the disparity in the duration of the protective effect, the investigators also saw differences in the effects across different autoimmune diseases.
“The protective effect of vitamin D seemed strongest for psoriasis, while for omega-3 fatty acids, the protective effects were strongest for rheumatoid arthritis and inflammatory bowel disease,” she said.
Mixed Effects
In an interview with this news organization, Janet Funk, MD, MS, vice chair of research in the Department of Medicine and professor in the School of Nutritional Science and Wellness at the University of Arizona, Tucson, Arizona, who was not involved in the study, saidthat the results suggest that while each supplement may offer protection against autoimmune diseases, the effects are inconsistent and may not apply to all patients.
“I think the VITAL extension results suggest that either supplement (or both together) may have benefits in reducing risk of autoimmune diseases, including possible persistent effects posttreatment, but that these effects are nuanced (ie, only in normal weight post-vitamin D treatment) and possibly not uniform across all autoimmune diseases (including possible adverse effects for some — eg, inverse association between prior omega-3 and psoriasis and tendency for increased autoimmune thyroid disease for vitamin D), although the study was not powered sufficiently to draw disease-specific conclusions,” she said.
In an editorial accompanying the study, rheumatologist Joel M. Kremer, MD, of Albany Medical College and the Corrona Research Foundation in Delray Beach, Florida, wrote that “[T]he studies by Dr. Costenbader, et al. have shed new light on the possibility that dietary supplements of n-3 FA [fatty acid] may prevent the onset of [autoimmune disease]. The sustained benefits they describe for as long as 2 years after the supplements are discontinued are consistent with the chronicity of FA species in cellular plasma membranes where they serve as substrates for a diverse array of salient metabolic and inflammatory pathways.”
VITAL Then
To test whether vitamin D or marine-derived long-chain n-3 FA supplementation could protect against autoimmune disease over time, Dr. Costenbader and colleagues piggybacked an ancillary study onto the VITAL trial, which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older and 13,085 women aged 55 and older. The study had a 2 × 2 factorial design, with patients randomly assigned to vitamin D 2000 IU/d or placebo and then further randomized to either 1 g/d n-3 FAs or placebo in both the vitamin D and placebo primary randomization arms.
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with a hazard ratio (HR) of 0.68 (P = .02) for incident autoimmune disease, n-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03). However, when probable incident autoimmune disease cases were included, the effect of n-3 became significant, with an HR of 0.82.
VITAL Now
In the current analysis, Dr. Costenbader and colleagues reported observational data on 21,592 VITAL participants, a sample representing 83.5% of those who were initially randomized, and 87.9% of those who were alive and could be contacted at the end of the study.
As in the initial trial, the investigators used annual questionnaires to assess incident autoimmune diseases during the randomized follow-up. Participants were asked about new-onset, doctor-diagnosed rheumatoid arthritis, polymyalgia rheumatica, psoriasis, autoimmune thyroid disease, and inflammatory bowel disease. Participants could also write in any other new autoimmune disease diagnoses.
There were 236 new cases of confirmed autoimmune disease that occurred since the initial publication of the trial results, as well as 65 probable cases identified during the median 5.3 years of the randomized portion, and 42 probable cases diagnosed during the 2-year observational phase.
The investigators found that after the 2-year observation period, 255 participants initially randomized to receive vitamin D had a newly developed confirmed autoimmune disease, compared with 259 of those initially randomized to a vitamin D placebo. This translated into a nonsignificant HR of 0.98.
Adding probable autoimmune cases to the confirmed cases made little difference, resulting in a nonsignificant adjusted HR of 0.95.
In contrast, there were 234 confirmed autoimmune disease cases among patients initially assigned to n-3, compared with 280 among patients randomized to the n-3 placebo, translating into a statistically significant HR of 0.83 for new-onset autoimmune disease with n-3.
Dr. Costenbader and colleagues acknowledged that the study was limited by the use of doses intended to prevent cancer or cardiovascular disease and that higher doses intended for high-risk or nutritionally deficient populations might reveal larger effects of supplementation. In addition, they noted the difficulty of identifying the timing and onset of incident disease, and that the small number of cases that occurred during the 2-year observational period precluded detailed analyses of individual autoimmune diseases.
The study was funded by grants from the National Institutes of Health. Dr. Costenbader, Dr. Funk, and Dr. Kremer reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Two years after the end of a randomized trial that showed a benefit of daily vitamin D and omega-3 fatty acid (n-3 FA) supplementation for reducing risk for autoimmune diseases, the salubrious effects of daily vitamin D appear to have waned after the supplement was discontinued, while the protection from n-3 lived on for at least 2 additional years.
As previously reported, the randomized VITAL, which was designed primarily to study the effects of vitamin D and n-3 supplementation on incident cancer and cardiovascular disease, also showed that 5 years of vitamin D supplementation was associated with a 22% reduction in risk for confirmed autoimmune diseases, and 5 years of n-3 FA supplementation was associated with an 18% reduction in confirmed and probable incident autoimmune diseases.
Now, investigators Karen H. Costenbader, MD, MPH, of Brigham & Women’s Hospital in Boston, Massachusetts, and colleagues reported that among 21,592 participants in VITAL who agreed to be followed for an additional 2 years after discontinuation, the protection against autoimmune diseases from daily vitamin D (cholecalciferol; 2000 IU/d) was no longer statistically significant, but the benefits of daily marine n-3 FAs (1 g/d as a fish-oil capsule containing 460 mg of eicosapentaenoic acid and 380 mg of docosahexaenoic acid) remained significant.
“VITAL observational extension results suggest that vitamin D supplementation should be given on a continuous basis for long-term prevention of [autoimmune diseases]. The beneficial effects of n-3 fatty acids, however, may be prolonged for at least 2 years after discontinuation,” they wrote in an article published in Arthritis & Rheumatology.
Dr. Costenbader told this news organization that the results of the observational extension study suggest that the benefits of vitamin D “wear off more quickly, and it should be continued for a longer period of time or indefinitely, rather than only for 5 years.”
In addition to the disparity in the duration of the protective effect, the investigators also saw differences in the effects across different autoimmune diseases.
“The protective effect of vitamin D seemed strongest for psoriasis, while for omega-3 fatty acids, the protective effects were strongest for rheumatoid arthritis and inflammatory bowel disease,” she said.
Mixed Effects
In an interview with this news organization, Janet Funk, MD, MS, vice chair of research in the Department of Medicine and professor in the School of Nutritional Science and Wellness at the University of Arizona, Tucson, Arizona, who was not involved in the study, saidthat the results suggest that while each supplement may offer protection against autoimmune diseases, the effects are inconsistent and may not apply to all patients.
“I think the VITAL extension results suggest that either supplement (or both together) may have benefits in reducing risk of autoimmune diseases, including possible persistent effects posttreatment, but that these effects are nuanced (ie, only in normal weight post-vitamin D treatment) and possibly not uniform across all autoimmune diseases (including possible adverse effects for some — eg, inverse association between prior omega-3 and psoriasis and tendency for increased autoimmune thyroid disease for vitamin D), although the study was not powered sufficiently to draw disease-specific conclusions,” she said.
In an editorial accompanying the study, rheumatologist Joel M. Kremer, MD, of Albany Medical College and the Corrona Research Foundation in Delray Beach, Florida, wrote that “[T]he studies by Dr. Costenbader, et al. have shed new light on the possibility that dietary supplements of n-3 FA [fatty acid] may prevent the onset of [autoimmune disease]. The sustained benefits they describe for as long as 2 years after the supplements are discontinued are consistent with the chronicity of FA species in cellular plasma membranes where they serve as substrates for a diverse array of salient metabolic and inflammatory pathways.”
VITAL Then
To test whether vitamin D or marine-derived long-chain n-3 FA supplementation could protect against autoimmune disease over time, Dr. Costenbader and colleagues piggybacked an ancillary study onto the VITAL trial, which had primary outcomes of cancer and cardiovascular disease incidence.
A total of 25,871 participants were enrolled, including 12,786 men aged 50 and older and 13,085 women aged 55 and older. The study had a 2 × 2 factorial design, with patients randomly assigned to vitamin D 2000 IU/d or placebo and then further randomized to either 1 g/d n-3 FAs or placebo in both the vitamin D and placebo primary randomization arms.
In multivariate analysis adjusted for age, sex, race, and other supplement arm, vitamin D alone was associated with a hazard ratio (HR) of 0.68 (P = .02) for incident autoimmune disease, n-3 alone was associated with a nonsignificant HR of 0.74, and the combination was associated with an HR of 0.69 (P = .03). However, when probable incident autoimmune disease cases were included, the effect of n-3 became significant, with an HR of 0.82.
VITAL Now
In the current analysis, Dr. Costenbader and colleagues reported observational data on 21,592 VITAL participants, a sample representing 83.5% of those who were initially randomized, and 87.9% of those who were alive and could be contacted at the end of the study.
As in the initial trial, the investigators used annual questionnaires to assess incident autoimmune diseases during the randomized follow-up. Participants were asked about new-onset, doctor-diagnosed rheumatoid arthritis, polymyalgia rheumatica, psoriasis, autoimmune thyroid disease, and inflammatory bowel disease. Participants could also write in any other new autoimmune disease diagnoses.
There were 236 new cases of confirmed autoimmune disease that occurred since the initial publication of the trial results, as well as 65 probable cases identified during the median 5.3 years of the randomized portion, and 42 probable cases diagnosed during the 2-year observational phase.
The investigators found that after the 2-year observation period, 255 participants initially randomized to receive vitamin D had a newly developed confirmed autoimmune disease, compared with 259 of those initially randomized to a vitamin D placebo. This translated into a nonsignificant HR of 0.98.
Adding probable autoimmune cases to the confirmed cases made little difference, resulting in a nonsignificant adjusted HR of 0.95.
In contrast, there were 234 confirmed autoimmune disease cases among patients initially assigned to n-3, compared with 280 among patients randomized to the n-3 placebo, translating into a statistically significant HR of 0.83 for new-onset autoimmune disease with n-3.
Dr. Costenbader and colleagues acknowledged that the study was limited by the use of doses intended to prevent cancer or cardiovascular disease and that higher doses intended for high-risk or nutritionally deficient populations might reveal larger effects of supplementation. In addition, they noted the difficulty of identifying the timing and onset of incident disease, and that the small number of cases that occurred during the 2-year observational period precluded detailed analyses of individual autoimmune diseases.
The study was funded by grants from the National Institutes of Health. Dr. Costenbader, Dr. Funk, and Dr. Kremer reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Success with Sirolimus in Treating Skin Sarcoidosis Could Spur Studies in Other Organs
Sirolimus may be an effective treatment for patients with persistent cutaneous sarcoidosis.
In a small clinical trial, 7 of 10 patients treated with sirolimus via oral solution had improvements in skin lesions after 4 months, which was sustained for up to 2 years after the study concluded.
The results suggested that mechanistic target of rapamycin (mTOR) inhibition is a potential therapeutic avenue for sarcoidosis, which the authors said should be explored in larger clinical trials.
In the past decade, there has been a growing amount of evidence suggesting mTOR’s role in sarcoidosis. In 2017, researchers showed that activation of mTOR in macrophages could cause progressive sarcoidosis in mice. In additional studies, high levels of mTOR activity were detected in human sarcoidosis granulomas in various organs, including the skin, lung, and heart.
Three case reports also documented using the mTOR inhibitor sirolimus to effectively treat systemic sarcoidosis.
“Although all reports observed improvement of the disease following the treatment, no clinical trial investigating the efficacy and safety of sirolimus in patients with sarcoidosis had been published” prior to this study, wrote senior author Georg Stary, MD, of the Medical University of Vienna and the Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, Austria, and colleagues.
The findings were published in the The Lancet Rheumatology.
For the study, researchers recruited 16 individuals with persistent and glucocorticoid-refractory cutaneous sarcoidosis between September 2019 and June 2021. A total of 14 participants were randomly assigned to the topical phase of the study, whereas two immediately received systemic treatment. All treatment was conducted at Vienna General Hospital.
In the placebo-controlled, double-blinded topical treatment arm, patients received either 0.1% topical sirolimus in Vaseline or Vaseline alone (placebo) twice daily for 2 months. After a 1-month washout period, participants were switched to the alternate treatment arm for an additional 2 months.
Following this topical phase and an additional 1-month washout period, all remaining participants received systemic sirolimus via a 1-mg/mL solution, starting with a 6-mg loading dose and continuing with 2 mg once daily for 4 months. The primary outcome was change in Cutaneous Sarcoidosis Activity and Morphology Index (CSAMI) from baseline, with decrease of more than five points representing a response to treatment.
A total of 10 patients completed the trial.
There was no change in CSAMI in either topical treatment groups. In the systemic group, 70% of patients had clinical improvement in skin lesions, with three responders in this group having complete resolution of skin lesions. The median change in CSAMI was −7.0 points (P = .018).
This improvement persisted for 2 months following study conclusion, with more pronounced improvement from baseline after 2 years of drug-free follow-up (−11.5 points).
There were no serious adverse events reported during the study, but 42% of patients treated with systemic sirolimus reported mild skin reactions, such as acne and eczema. Other related adverse events were hypertriglyceridemia (17%), hyperglycemia (17%), and proteinuria (8%).
Compared with clinical outcomes with tofacitinib and tumor necrosis factor (TNF) inhibitors, “the strength of our study lies in the sustained treatment effect after drug withdrawal among all responders. This prolonged effect has not yet been explored with tofacitinib, whereas with TNF inhibitors disease relapse was seen in more than 50% of patients at 3-8 months,” the authors wrote.
The researchers also analyzed participants’ skin biopsies to gain a better understanding of how mTOR inhibition affected granuloma structures. They found that, at baseline, mTOR activity was significantly lower in the fibroblasts of treatment nonresponders than in responders. They speculated that lower expression of mTOR could make these granuloma-associated cells resistant to systemic sirolimus.
These promising findings combine “clinical response with a molecular analysis,” Avrom Caplan, MD, co-director of the Sarcoidosis Program at NYU Langone in New York City, told this news organization. He was not involved with the research. Adding molecular information to clinical outcome data “helps solidify that [the mTOR] pathway has relevance in the sarcoid granuloma formation.”
The study had a limited sample size — a challenge for many clinical trials of rare diseases, Dr. Caplan said. Larger clinical trials are necessary to explore mTOR inhibition in sarcoidosis, both he and the authors agreed. A larger trial could also include greater heterogeneity of patients, including varied sarcoid presentation and demographics, Dr. Caplan noted. In this study, all but one participants were White individuals, and 63% of participants were female.
Larger studies could also address important questions on ideal length of therapy, dosing, and where this therapy “would fall within the therapeutic step ladder,” Dr. Caplan continued.
Whether mTOR inhibition could be effective at treating individuals with sarcoidosis in other organs beyond the skin is also unknown.
“If the pathogenesis of sarcoid granuloma formation does include mTOR upregulation, which they are showing here…then you could hypothesize that, yes, using this therapy could benefit other organs,” he said. “But that has to be investigated in larger trials.”
The study was funded in part by a Vienna Science and Technology Fund project. Several authors report receiving grants from the Austrian Science Fund and one from the Ann Theodore Foundation Breakthrough Sarcoidosis Initiative. Dr. Caplan reported no relevant financial relationships.
A version of this article appeared on Medscape.com .
Sirolimus may be an effective treatment for patients with persistent cutaneous sarcoidosis.
In a small clinical trial, 7 of 10 patients treated with sirolimus via oral solution had improvements in skin lesions after 4 months, which was sustained for up to 2 years after the study concluded.
The results suggested that mechanistic target of rapamycin (mTOR) inhibition is a potential therapeutic avenue for sarcoidosis, which the authors said should be explored in larger clinical trials.
In the past decade, there has been a growing amount of evidence suggesting mTOR’s role in sarcoidosis. In 2017, researchers showed that activation of mTOR in macrophages could cause progressive sarcoidosis in mice. In additional studies, high levels of mTOR activity were detected in human sarcoidosis granulomas in various organs, including the skin, lung, and heart.
Three case reports also documented using the mTOR inhibitor sirolimus to effectively treat systemic sarcoidosis.
“Although all reports observed improvement of the disease following the treatment, no clinical trial investigating the efficacy and safety of sirolimus in patients with sarcoidosis had been published” prior to this study, wrote senior author Georg Stary, MD, of the Medical University of Vienna and the Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, Austria, and colleagues.
The findings were published in the The Lancet Rheumatology.
For the study, researchers recruited 16 individuals with persistent and glucocorticoid-refractory cutaneous sarcoidosis between September 2019 and June 2021. A total of 14 participants were randomly assigned to the topical phase of the study, whereas two immediately received systemic treatment. All treatment was conducted at Vienna General Hospital.
In the placebo-controlled, double-blinded topical treatment arm, patients received either 0.1% topical sirolimus in Vaseline or Vaseline alone (placebo) twice daily for 2 months. After a 1-month washout period, participants were switched to the alternate treatment arm for an additional 2 months.
Following this topical phase and an additional 1-month washout period, all remaining participants received systemic sirolimus via a 1-mg/mL solution, starting with a 6-mg loading dose and continuing with 2 mg once daily for 4 months. The primary outcome was change in Cutaneous Sarcoidosis Activity and Morphology Index (CSAMI) from baseline, with decrease of more than five points representing a response to treatment.
A total of 10 patients completed the trial.
There was no change in CSAMI in either topical treatment groups. In the systemic group, 70% of patients had clinical improvement in skin lesions, with three responders in this group having complete resolution of skin lesions. The median change in CSAMI was −7.0 points (P = .018).
This improvement persisted for 2 months following study conclusion, with more pronounced improvement from baseline after 2 years of drug-free follow-up (−11.5 points).
There were no serious adverse events reported during the study, but 42% of patients treated with systemic sirolimus reported mild skin reactions, such as acne and eczema. Other related adverse events were hypertriglyceridemia (17%), hyperglycemia (17%), and proteinuria (8%).
Compared with clinical outcomes with tofacitinib and tumor necrosis factor (TNF) inhibitors, “the strength of our study lies in the sustained treatment effect after drug withdrawal among all responders. This prolonged effect has not yet been explored with tofacitinib, whereas with TNF inhibitors disease relapse was seen in more than 50% of patients at 3-8 months,” the authors wrote.
The researchers also analyzed participants’ skin biopsies to gain a better understanding of how mTOR inhibition affected granuloma structures. They found that, at baseline, mTOR activity was significantly lower in the fibroblasts of treatment nonresponders than in responders. They speculated that lower expression of mTOR could make these granuloma-associated cells resistant to systemic sirolimus.
These promising findings combine “clinical response with a molecular analysis,” Avrom Caplan, MD, co-director of the Sarcoidosis Program at NYU Langone in New York City, told this news organization. He was not involved with the research. Adding molecular information to clinical outcome data “helps solidify that [the mTOR] pathway has relevance in the sarcoid granuloma formation.”
The study had a limited sample size — a challenge for many clinical trials of rare diseases, Dr. Caplan said. Larger clinical trials are necessary to explore mTOR inhibition in sarcoidosis, both he and the authors agreed. A larger trial could also include greater heterogeneity of patients, including varied sarcoid presentation and demographics, Dr. Caplan noted. In this study, all but one participants were White individuals, and 63% of participants were female.
Larger studies could also address important questions on ideal length of therapy, dosing, and where this therapy “would fall within the therapeutic step ladder,” Dr. Caplan continued.
Whether mTOR inhibition could be effective at treating individuals with sarcoidosis in other organs beyond the skin is also unknown.
“If the pathogenesis of sarcoid granuloma formation does include mTOR upregulation, which they are showing here…then you could hypothesize that, yes, using this therapy could benefit other organs,” he said. “But that has to be investigated in larger trials.”
The study was funded in part by a Vienna Science and Technology Fund project. Several authors report receiving grants from the Austrian Science Fund and one from the Ann Theodore Foundation Breakthrough Sarcoidosis Initiative. Dr. Caplan reported no relevant financial relationships.
A version of this article appeared on Medscape.com .
Sirolimus may be an effective treatment for patients with persistent cutaneous sarcoidosis.
In a small clinical trial, 7 of 10 patients treated with sirolimus via oral solution had improvements in skin lesions after 4 months, which was sustained for up to 2 years after the study concluded.
The results suggested that mechanistic target of rapamycin (mTOR) inhibition is a potential therapeutic avenue for sarcoidosis, which the authors said should be explored in larger clinical trials.
In the past decade, there has been a growing amount of evidence suggesting mTOR’s role in sarcoidosis. In 2017, researchers showed that activation of mTOR in macrophages could cause progressive sarcoidosis in mice. In additional studies, high levels of mTOR activity were detected in human sarcoidosis granulomas in various organs, including the skin, lung, and heart.
Three case reports also documented using the mTOR inhibitor sirolimus to effectively treat systemic sarcoidosis.
“Although all reports observed improvement of the disease following the treatment, no clinical trial investigating the efficacy and safety of sirolimus in patients with sarcoidosis had been published” prior to this study, wrote senior author Georg Stary, MD, of the Medical University of Vienna and the Research Center for Molecular Medicine of the Austrian Academy of Sciences, Vienna, Austria, and colleagues.
The findings were published in the The Lancet Rheumatology.
For the study, researchers recruited 16 individuals with persistent and glucocorticoid-refractory cutaneous sarcoidosis between September 2019 and June 2021. A total of 14 participants were randomly assigned to the topical phase of the study, whereas two immediately received systemic treatment. All treatment was conducted at Vienna General Hospital.
In the placebo-controlled, double-blinded topical treatment arm, patients received either 0.1% topical sirolimus in Vaseline or Vaseline alone (placebo) twice daily for 2 months. After a 1-month washout period, participants were switched to the alternate treatment arm for an additional 2 months.
Following this topical phase and an additional 1-month washout period, all remaining participants received systemic sirolimus via a 1-mg/mL solution, starting with a 6-mg loading dose and continuing with 2 mg once daily for 4 months. The primary outcome was change in Cutaneous Sarcoidosis Activity and Morphology Index (CSAMI) from baseline, with decrease of more than five points representing a response to treatment.
A total of 10 patients completed the trial.
There was no change in CSAMI in either topical treatment groups. In the systemic group, 70% of patients had clinical improvement in skin lesions, with three responders in this group having complete resolution of skin lesions. The median change in CSAMI was −7.0 points (P = .018).
This improvement persisted for 2 months following study conclusion, with more pronounced improvement from baseline after 2 years of drug-free follow-up (−11.5 points).
There were no serious adverse events reported during the study, but 42% of patients treated with systemic sirolimus reported mild skin reactions, such as acne and eczema. Other related adverse events were hypertriglyceridemia (17%), hyperglycemia (17%), and proteinuria (8%).
Compared with clinical outcomes with tofacitinib and tumor necrosis factor (TNF) inhibitors, “the strength of our study lies in the sustained treatment effect after drug withdrawal among all responders. This prolonged effect has not yet been explored with tofacitinib, whereas with TNF inhibitors disease relapse was seen in more than 50% of patients at 3-8 months,” the authors wrote.
The researchers also analyzed participants’ skin biopsies to gain a better understanding of how mTOR inhibition affected granuloma structures. They found that, at baseline, mTOR activity was significantly lower in the fibroblasts of treatment nonresponders than in responders. They speculated that lower expression of mTOR could make these granuloma-associated cells resistant to systemic sirolimus.
These promising findings combine “clinical response with a molecular analysis,” Avrom Caplan, MD, co-director of the Sarcoidosis Program at NYU Langone in New York City, told this news organization. He was not involved with the research. Adding molecular information to clinical outcome data “helps solidify that [the mTOR] pathway has relevance in the sarcoid granuloma formation.”
The study had a limited sample size — a challenge for many clinical trials of rare diseases, Dr. Caplan said. Larger clinical trials are necessary to explore mTOR inhibition in sarcoidosis, both he and the authors agreed. A larger trial could also include greater heterogeneity of patients, including varied sarcoid presentation and demographics, Dr. Caplan noted. In this study, all but one participants were White individuals, and 63% of participants were female.
Larger studies could also address important questions on ideal length of therapy, dosing, and where this therapy “would fall within the therapeutic step ladder,” Dr. Caplan continued.
Whether mTOR inhibition could be effective at treating individuals with sarcoidosis in other organs beyond the skin is also unknown.
“If the pathogenesis of sarcoid granuloma formation does include mTOR upregulation, which they are showing here…then you could hypothesize that, yes, using this therapy could benefit other organs,” he said. “But that has to be investigated in larger trials.”
The study was funded in part by a Vienna Science and Technology Fund project. Several authors report receiving grants from the Austrian Science Fund and one from the Ann Theodore Foundation Breakthrough Sarcoidosis Initiative. Dr. Caplan reported no relevant financial relationships.
A version of this article appeared on Medscape.com .
FROM THE LANCET RHEUMATOLOGY
Impact of Ketogenic and Low-Glycemic Diets on Inflammatory Skin Conditions
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17
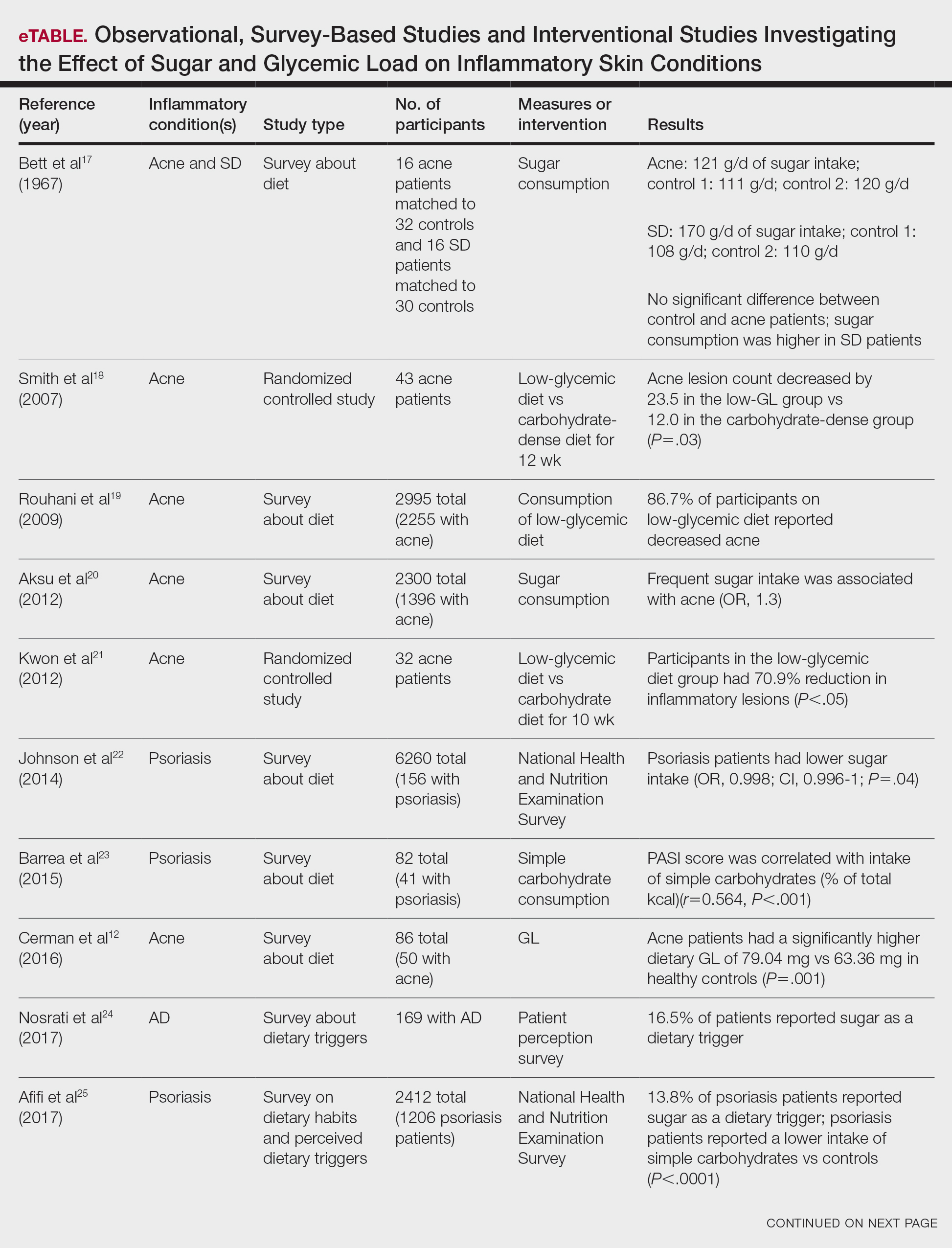

Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
Inflammatory skin conditions often have a relapsing and remitting course and represent a large proportion of chronic skin diseases. Common inflammatory skin disorders include acne, psoriasis, hidradenitis suppurativa (HS), atopic dermatitis (AD), and seborrheic dermatitis (SD).1 Although each of these conditions has a unique pathogenesis, they all are driven by a background of chronic inflammation. It has been reported that diets with high levels of refined carbohydrates and saturated or trans-fatty acids may exacerbate existing inflammation.2 Consequently, dietary interventions, such as the ketogenic and low-glycemic diets, have potential anti-inflammatory and metabolic effects that are being assessed as stand-alone or adjunctive therapies for dermatologic diseases.
Diet may partially influence systemic inflammation through its effect on weight. Higher body mass index and obesity are linked to a low-grade inflammatory state and higher levels of circulating inflammatory markers. Therefore, weight loss leads to decreases in inflammatory cytokines, including C-reactive protein, tumor necrosis factor α, and IL-6.3 These cytokines and metabolic effects overlap with inflammatory skin condition pathways. It also is posited that decreased insulin release associated with weight loss results in decreased sebaceous lipogenesis and androgens, which drive keratinocyte proliferation and acne development.4,5 For instance, in a 2015 meta-analysis of 5 randomized controlled trials on psoriasis, patients in the weight loss intervention group had more substantial reductions in psoriasis area and severity index (PASI) scores compared with controls receiving usual care (P=.004).6 However, in a systematic review of 35 studies on acne vulgaris, overweight and obese patients (defined by a body mass index of ≥23 kg/m2) had similar odds of having acne compared with normal-weight individuals (P=.671).7
Similar to weight loss, ketogenesis acts as a negative feedback mechanism to reduce insulin release, leading to decreased inflammation and androgens that often exacerbate inflammatory skin diseases.8 Ketogenesis ensues when daily carbohydrate intake is limited to less than 50 g, and long-term adherence to a ketogenic diet results in metabolic reliance on ketone bodies such as acetoacetate, β-hydroxybutyrate, and acetone.9 These metabolites may decrease free radical damage and consequently improve signs and symptoms of acne, psoriasis, and other inflammatory skin diseases.10-12 Similarly, increased ketones also may decrease activation of the NLRP3 (NOD-, LRR-, and Pyrin domain-containing protein 3) inflammasome and therefore reduce inflammatory markers such as IL-1β and IL-1.4,13 Several proposed mechanisms are outlined in the Table.

Collectively, low-glycemic and ketogenic diets have been proposed as potential interventions for reducing inflammatory skin conditions. These dietary approaches are hypothesized to exert their effects by facilitating weight loss, elevating ketone levels, and reducing systemic inflammation. The current review summarizes the existing evidence on ketogenic and low-glycemic diets as treatments for inflammatory skin conditions and evaluates the potential benefits of these dietary interventions in managing and improving outcomes for individuals with inflammatory skin conditions.
Methods
Using PubMed for articles indexed for MEDLINE and Google Scholar, a review of the literature was conducted with a combination of the following search terms: low-glycemic diet, inflammatory, dermatologic, ketogenic diet, inflammation, dermatology, acne, psoriasis, eczema, seborrheic dermatitis, and hidradenitis suppurativa. Reference citations in identified works also were reviewed. Interventional (experimental studies or clinical trials), survey-based, and observational studies that investigated the effects of low-glycemic or ketogenic diets for the treatment of inflammatory skin conditions were included. Inclusion criteria were studies assessing acne, psoriasis, SD, AD, and HS. Exclusion criteria were studies published before 1965; those written in languages other than English; and those analyzing other diets, such as the Mediterranean or low-fat diets. The search yielded a total of 11 observational studies and 4 controlled studies published between 1966 and January 2023. Because this analysis utilized publicly available data and did not qualify as human subject research, institutional review board approval was not required.
Results
Acne Vulgaris—Acne vulgaris is a disease of chronic pilosebaceous inflammation and follicular epithelial proliferation associated with Propionibacterium acnes. The association between acne and low-glycemic diets has been examined in several studies. Diet quality is measured and assessed using the glycemic index (GI), which is the effect of a single food on postprandial blood glucose, and the glycemic load, which is the GI adjusted for carbohydrates per serving.14 High levels of GI and glycemic load are associated with hyperinsulinemia and an increase in insulinlike growth factor 1 concentration that promotes
Six survey-based studies evaluated sugar intake in patients with acne compared to healthy matched controls (eTable). Among these studies, 5 reported higher glycemic loads or daily sugar intake in acne patients compared to individuals without acne.12,19,20,26,28 The remaining study was conducted in 1967 and enrolled 16 acne patients and 32 matched controls. It reported no significant difference in sugar intake between the groups (P>.05).17


Smith et al18 randomized 43 male patients aged 15 to 25 years with facial acne into 2 cohorts for 12 weeks, each consuming either a low-glycemic diet (25% protein, 45% low-glycemic food [fruits, whole grains], and 30% fat) or a carbohydrate-dense diet of foods with medium to high GI based on prior documentation of the original diet. Patients were instructed to use a noncomedogenic cleanser as their only acne treatment. At 12 weeks, patients consuming the low-glycemic diet had an average of 23.5 fewer inflammatory lesions, while those in the intervention group had 12.0 fewer lesions (P=.03).18
In another controlled study by Kwon et al,21 32 male and female acne patients were randomized to a low-glycemic diet (25% protein, 45% low-glycemic food, and 30% fat) or a standard diet for 10 weeks. Patients on the low-glycemic diet experienced a 70.9% reduction in inflammatory lesions (P<.05). Hematoxylin and eosin staining and image analysis were performed to measure sebaceous gland surface area in the low-glycemic diet group, which decreased from 0.32 to 0.24 mm2 (P=.03). The sebaceous gland surface area in the control group was not reported. Moreover, patients on the low-glycemic diet had reduced IL-8 immunohistochemical staining (decreasing from 2.9 to 1.7 [P=.03]) and sterol regulatory element-binding protein 1 levels (decreasing from 2.6 to 1.3 [P=.03]), suggesting suppression of ongoing inflammation. Patients on the low-glycemic diet had no significant difference in transforming growth factor β1(P=.83). In the control group, there was no difference in IL-8, sterol regulatory element binding protein 1, or transforming growth factor β1 (P>.05) on immunohistochemical staining.21
Psoriasis—Psoriasis is a systemic inflammatory disease characterized by hyperproliferation and aberrant keratinocyte plaque formation. The innate immune response of keratinocytes in response to epidermal damage or infection begins with neutrophil recruitment and dendritic cell activation. Dendritic cell secretion of IL-23 promotes T-cell differentiation into helper T cells (TH1) that subsequently secrete IL-17 and IL-22, thereby stimulating keratinocyte proliferation and eventual plaque formation. The relationship between diet and psoriasis is poorly understood; however, hyperinsulinemia is associated with greater severity of psoriasis.31
Four observational studies examined sugar intake in psoriasis patients. Barrea et al23 conducted a survey-based study of 82 male participants (41 with psoriasis and 41 healthy controls), reporting that PASI score was correlated with intake of simple carbohydrates (percentage of total kilocalorie)(r=0.564, P<.001). Another study by Yamashita et al27 found higher sugar intake in psoriasis patients than controls (P=.003) based on surveys from 70 patients with psoriasis and 70 matched healthy controls.
These findings contrast with 2 survey-based studies by Johnson et al22 and Afifi et al25 of sugar intake in psoriasis patients using the National Health and Nutrition Examination Survey. Johnson et al22 reported reduced sugar intake among 156 psoriasis patients compared with 6104 unmatched controls (odds ratio, 0.998; CI, 0.996-1 [P=.04]) from 2003 to 2006. Similarly, Afifi et al25 reported decreased sugar intake in 1206 psoriasis patients compared with sex- and age-matched controls (P<.0001) in 2009 and 2010. When patients were asked about dietary triggers, 13.8% of psoriasis patients reported sugar as the most common trigger, which was more frequent than alcohol (13.6%), gluten (7.2%), and dairy (6%).25
Castaldo et al29,30 published 2 nonrandomized clinical intervention studies in 2020 and 2021 evaluating the impact of the ketogenic diet on psoriasis. In the first study, 37 psoriasis patients followed a 10-week diet consisting of 4 weeks on a ketogenic diet (500 kcal/d) followed by 6 weeks on a low-caloric Mediterranean diet.29 At the end of the intervention, there was a 17.4% reduction in PASI score, a 33.2-point reduction in itch severity score, and a 13.4-point reduction in the dermatology life quality index score; however, this study did not include a control diet group for comparison.29 The second study included 30 psoriasis patients on a ketogenic diet and 30 control patients without psoriasis on a regular diet.30 The ketogenic diet consisted of 400 to 500 g of vegetables, 20 to 30 g of fat, and a proportion of protein based on body weight with at least 12 g of whey protein and various amino acids. Patients on the ketogenic diet had significant reduction in PASI scores (value relative to clinical features, 1.4916 [P=.007]). Furthermore, concentrations of cytokines IL-2 (P=.04) and IL-1β (P=.006) decreased following the ketogenic diet but were not measured in the control group.30
Seborrheic Dermatitis—Seborrheic dermatitis is associated with overcolonization of Malassezia species near lipid-rich sebaceous glands. Malassezia hydrolyzes free fatty acids, yielding oleic acids and leading to T-cell release of IL-8 and IL-17.32 Literature is sparse regarding how dietary modifications may play a role in disease severity. In a survey study, Bett et al17 compared 16 SD patients to 1:2 matched controls (N=29) to investigate the relationship between sugar consumption and presence of disease. Two control cohorts were selected, 1 from clinic patients diagnosed with verruca and 1 matched by age and sex from a survey-based study at a facility in London, England. Sugar intake was measured both in total grams per day and in “beverage sugar” per day, defined as sugar taken in tea and coffee. There was higher total sugar and higher beverage sugar intake among the SD group compared with both control groups (P<.05).17
Atopic Dermatitis—Atopic dermatitis is a disease of epidermal barrier dysfunction and IgE-mediated allergic sensitization.33 There are several mechanisms by which skin structure may be disrupted. It is well established that filaggrin mutations inhibit stratum corneum maturation and lamellar matrix deposition.34 Upregulation of IL-4–, IL-13–, and IL-17–secreting TH2 cells also is associated with disruption of tight junctions and reduction of filaggrin.35,36 Given that a T cell–mediated inflammatory response is involved in disease pathogenesis, glycemic control is hypothesized to have therapeutic potential.
Nosrati et al24 surveyed 169 AD patients about their perceived dietary triggers through a 61-question survey based on the National Health and Nutrition Examination Survey. Respondents were queried about their perceptions and dietary changes, such as removal or addition of specific food groups and trial of specific diets. Overall, 16.5% of patients reported sugar being a trigger, making it the fourth most common among those surveyed and less common than dairy (24.8%), gluten (18.3%), and alcohol (17.1%).24
Hidradenitis Suppurativa—Hidradenitis suppurativa is driven by hyperkeratosis, dilatation, and occlusion of pilosebaceous follicular ducts, whose eventual rupture evokes a local acute inflammatory response.37 The inciting event for both acne and HS involves mTOR complex–mediated follicular hyperproliferation andinsulinlike growth factor 1 stimulation of androgen receptors in pilosebaceous glands. Given the similarities between the pathogenesis of acne and HS, it is hypothesized that lifestyle changes, including diet modification, may have a beneficial effect on HS.38-40
Comment
Acne—Overall, there is strong evidence supporting the efficacy of a low-glycemic diet in the treatment of acne. Notably, among the 6 observational studies identified, there was 1 conflicting study by Bett et al17 that did not find a statistically significant difference in glucose intake between acne and control patients. However, this study included only 16 acne patients, whereas the other 5 observational studies included 32 to 2255 patients.17 The strongest evidence supporting low-glycemic dietary interventions in acne treatment is from 2 rigorous randomized clinical trials by Kwon et al21 and Smith et al.18 These trials used intention-to-treat models and maintained consistency in gender, age, and acne treatment protocols across both control and treatment groups. To ensure compliance with dietary interventions, daily telephone calls, food logs, and 24-hour urea sampling were utilized. Acne outcomes were assessed by a dermatologist who remained blinded with well-defined outcome measures. An important limitation of these studies is the difficulty in attributing the observed results solely to reduced glucose intake, as low-glycemic diets often lead to other dietary changes, including reduced fat intake and increased nutrient consumption.18,21
A 2022 systematic review of acne by Meixiong et al41 further reinforced the beneficial effects of low-glycemic diets in the management of acne patients. The group reviewed 6 interventional studies and 28 observational studies to investigate the relationship among acne, dairy, and glycemic content and found an association between decreased glucose and dairy on reduction of acne.41
It is likely that the ketogenic diet, which limits glucose, would be beneficial for acne patients. There may be added benefit through elevated ketone bodies and substantially reduced insulin secretion. However, because there are no observational or interventional studies, further research is needed to draw firm conclusions regarding diet for acne treatment. A randomized clinical trial investigating the effects of the ketogenic diet compared to the low-glycemic diet compared to a regular diet would be valuable.
Psoriasis—Among psoriasis studies, there was a lack of consensus regarding glucose intake and correlation with disease. Among the 4 observational studies, 2 reported increased glucose intake among psoriasis patients and 2 reported decreased glucose intake. It is plausible that the variability in studies is due to differences in sample size and diet heterogeneity among study populations. More specifically, Johnson et al22 and Afifi et al25 analyzed large sample sizes of 6260 and 2412 US participants, respectively, and found decreased sugar intake among psoriasis patients compared to controls. In comparison, Barrea et al23 and Yamashita et al27 analyzed substantially smaller and more specific populations consisting of 82 Italian and 140 Japanese participants, respectively; both reported increased glucose intake among psoriasis patients compared to controls. These seemingly antithetical results may be explained by regional dietary differences, with varying proportions of meats, vegetables, antioxidants, and vitamins.
Moreover, the variation among studies may be further explained by the high prevalence of comorbidities among psoriasis patients. In the study by Barrea et al,23 psoriasis patients had higher fasting glucose (P=.004) and insulin (P=.022) levels than healthy patients. After adjusting for body mass index and metabolic syndrome, the correlation coefficient measuring the relationship between the PASI score and intake of simple carbohydrates changed from r=0.564 (P<.001) to r=0.352 (P=.028). The confounding impact of these comorbidities was further highlighted by Yamashita et al,27 who found statistically significant differences in glucose intake between psoriasis and healthy patients (P=.003). However, they reported diminished significance on additional subgroup analysis accounting for potential comorbidities (P=.994).27 Johnson et al22 and Afifi et al25 did not account for comorbidities; therefore, the 4 observational study results must be interpreted cautiously.
The 2 randomized clinical trials by Castaldo et al29,30 weakly suggest that a ketogenic diet may be beneficial for psoriasis patients. The studies have several notable limitations, including insufficient sample sizes and control groups. Thus, the decreased PASI scores reported in psoriasis patients on the ketogenic diets are challenging to interpret. Additionally, both studies placed patients on highly restrictive diets of 500 kcal/d for 4 weeks. The feasibility of recommending such a diet to patients in clinical practice is questionable. Diets of less than 500 kcal/d may be dangerous for patients with underlying comorbidities and are unlikely to serve as long-term solutions.23 To contextualize our findings, a 2022 review by Chung et al42 examined the impact of various diets—low-caloric, gluten-free, Mediterranean, Western, and ketogenic—on psoriasis and reported insufficient evidence to suggest a benefit to the ketogenic diet for psoriasis patients, though the Mediterranean diet may be well suited for psoriasis patients because of improved cardiovascular health and reduced mortality.
Seborrheic Dermatitis—Sanders et al43 found that patients with a high-fruit diet had lower odds of having SD, while those on a Western diet had higher odds of having SD. Although the study did not measure glycemic load, it is conceivable that the high glycemic load characteristic of the Western diet contributed to these findings.43 However, no studies have investigated the direct link between low-glycemic or ketogenic diets and SD, leaving this area open for further study.
Atopic Dermatitis—It has been hypothesized that mitigating T cell–mediated inflammation via glucose control may contribute to the improvement in AD.35,36 However, in one study, 16.5% of AD patients self-identified sugar as a dietary trigger, ranking fourth among other dietary triggers.24 Thus, the connection between glucose levels and AD warrants further exploration.
Hidradenitis Suppurativa—Given the role of metabolic and hormonal influence in HS as well as the overlapping pathophysiology with acne, it is possible that low-glycemic and ketogenic diets may have a role in improving HS.38-40 However, there is a gap in observation and controlled studies investigating the link between low-glycemic or ketogenic diets and HS.
Conclusion
Our analysis focused on interventional and observational research exploring the effects of low-glycemic and ketogenic diets on associations and treatment of inflammatory skin conditions. There is sufficient evidence to counsel acne patients on the benefits of a low-glycemic diet as an adjunctive treatment for acne. Currently, there is insufficient evidence to recommend a low-glycemic or ketogenic diet as a treatment for patients with any other inflammatory skin disease. Prospective and controlled clinical trials are needed to clarify the utility of dietary interventions for treating inflammatory skin conditions.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
- Pickett K, Loveman E, Kalita N, et al. Educational interventions to improve quality of life in people with chronic inflammatory skin diseases: systematic reviews of clinical effectiveness and cost-effectiveness. Health Technol Assess. 2015;19:1-176, v-vi.
- Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol. 2006;48:677-685.
- Dowlatshahi EA, van der Voort EA, Arends LR, et al. Markers of systemic inflammation in psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2013;169:266-282.
- Youm YH, Nguyen KY, Grant RW, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263-269.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018;36:29-40.
- Upala S, Sanguankeo A. Effect of lifestyle weight loss intervention on disease severity in patients with psoriasis: a systematic review and meta-analysis. Int J Obes (Lond). 2015;39:1197-1202.
- Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. 2020;10:5754.
- Paoli A, Grimaldi K, Toniolo L, et al. Nutrition and acne: therapeutic potential of ketogenic diets. Skin Pharmacol Physiol. 2012;25:111-117.
- Masood W, Annamaraju P, Khan Suheb MZ, et al. Ketogenic diet. StatPearls. StatPearls Publishing; 2023.
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28:484-487.
- Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 2019;51:671-681.e5.
- Cerman AA, Aktas E, Altunay IK, et al. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016;75:155-162.
- Ferrere G, Tidjani Alou M, Liu P, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6:e145207.
- Burris J, Shikany JM, Rietkerk W, et al. A Low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Tan JKL, Stein Gold LF, Alexis AF, et al. Current concepts in acne pathogenesis: pathways to inflammation. Semin Cutan Med Surg. 2018;37(3S):S60-S62.
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535-1541.
- Bett DG, Morland J, Yudkin J. Sugar consumption in acne vulgaris and seborrhoeic dermatitis. Br Med J. 1967;3:153-155.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Rouhani P, Berman B, Rouhani G. Acne improves with a popular, low glycemic diet from South Beach. J Am Acad Dermatol. 2009;60(Suppl 1):AB14.
- Aksu AE, Metintas S, Saracoglu ZN, et al. Acne: prevalence and relationship with dietary habits in Eskisehir, Turkey. J Eur Acad Dermatol Venereol. 2012;26:1503-1509.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Johnson JA, Ma C, Kanada KN, et al. Diet and nutrition in psoriasis: analysis of the National Health and Nutrition Examination Survey (NHANES) in the United States. J Eur Acad Dermatol Venereol. 2014;28:327-332.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Nosrati A, Afifi L, Danesh MJ, et al. Dietary modifications in atopic dermatitis: patient-reported outcomes. J Dermatolog Treat. 2017;28:523-538.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Burris J, Rietkerk W, Shikany JM, et al. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Yamashita H, Morita T, Ito M, et al. Dietary habits in Japanese patients with psoriasis and psoriatic arthritis: low intake of meat in psoriasis and high intake of vitamin A in psoriatic arthritis. J Dermatol. 2019;46:759-769.
- Marson J, Baldwin HE. 12761 Acne, twins, and glycemic index: a sweet pilot study of diet and dietary beliefs. J Am Acad Dermatol. 2020;83(Suppl):AB110.
- Castaldo G, Rastrelli L, Galdo G, et al. Aggressive weight-loss program with a ketogenic induction phase for the treatment of chronic plaque psoriasis: a proof-of-concept, single-arm, open-label clinical trial. Nutrition. 2020;74:110757.
- Castaldo G, Pagano I, Grimaldi M, et al. Effect of very-low-calorie ketogenic diet on psoriasis patients: a nuclear magnetic resonance-based metabolomic study. J Proteome Res. 2021;20:1509-1521.
- Ip W, Kirchhof MG. Glycemic control in the treatment of psoriasis. Dermatology. 2017;233:23-29.
- Vijaya Chandra SH, Srinivas R, Dawson TL Jr, et al. Cutaneous Malassezia: commensal, pathogen, or protector? Front Cell Infect Microbiol. 2020;10:614446.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. 2017;1027:21-37.
- Guttman-Yassky E, Hanifin JM, Boguniewicz M, et al. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol. 2019;28:3-10.
- Furue K, Ito T, Tsuji G, et al. The IL-13–OVOL1–FLG axis in atopic dermatitis. Immunology. 2019;158:281-286.
- Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28-35.
- Sellheyer K, Krahl D. “Hidradenitis suppurativa” is acne inversa! An appeal to (finally) abandon a misnomer. Int J Dermatol. 2005;44:535-540.
- Danby FW, Margesson LJ. Hidradenitis suppurativa. Dermatol Clin. 2010;28:779-793.
- Fernandez JM, Marr KD, Hendricks AJ, et al. Alleviating and exacerbating foods in hidradenitis suppurativa. Dermatol Ther. 2020;33:E14246.
- Yamanaka-Takaichi M, Revankar R, Shih T, et al. Expert consensus on priority research gaps in dietary and lifestyle factors in hidradenitis suppurativa: a Delphi consensus study. Arch Dermatol Res. 2023;315:2129-2136.
- Meixiong J, Ricco C, Vasavda C, et al. Diet and acne: a systematic review. JAAD Int. 2022;7:95-112.
- Chung M, Bartholomew E, Yeroushalmi S, et al. Dietary intervention and supplements in the management of psoriasis: current perspectives. Psoriasis (Auckland). 2022;12:151-176. doi:10.2147/PTT.S328581
- Sanders MGH, Pardo LM, Ginger RS, et al. Association between diet and seborrheic dermatitis: a cross-sectional study. J Invest Dermatol. 2019;139:108-114.
Practice Points
- As the ketogenic diet gains in popularity, dermatologists may inform patients that there is emerging evidence supporting the idea that low-glycemic diets may contribute to improvement in inflammatory skin conditions.
- Dermatologists may educate patients about the potential benefits of a low-glycemic diet as a supplementary treatment for acne based on existing evidence.
- Current evidence is insufficient to endorse a ketogenic diet as superior to other dietary approaches in treating inflammatory skin conditions.
Autoimmune Diseases and Perinatal Depression May Share Two-Way Link
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Women with autoimmune disease are more likely to have perinatal depression (PND), according to findings from a new study that also suggested the reverse relationship is true: Women with a history of PND have a higher risk of developing autoimmune disease.
The research, published online on January 9, 2024, in Molecular Psychiatry, was led by Emma Bränn, PhD, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
The researchers used data from the Swedish Medical Birth Register and identified all women who had given birth in Sweden between 2001 and 2013. Out of the group of approximately 815,000 women and 1.3 million pregnancies, just more than 55,000 women had been diagnosed with depression during their pregnancy or within a year after delivery.
The researchers then compared the incidence of 41 autoimmune diseases in women who had and did not have PND. They controlled for factors including genetic makeup and childhood environment.
Results indicated that women with autoimmune disease were 30% more likely to have PND (odds ratio, 1.30; 95% CI, 1.25-1.35). Conversely, women with PND were 30% more likely than women with no PND to develop an autoimmune disease (hazard ratio, 1.30; 95% CI, 1.25-1.36).
A sibling comparison helped confirm the results by controlling for some shared genetic and early life environmental factors related to the household in which sisters grew up.
Potential Shared Biological Mechanisms
The association was independent of psychiatric comorbidities, suggesting there may be shared biological mechanisms.
Dr. Bränn told this news organization that the research team wanted to do the study because previous research has shown involvement of the immune system in depression, with similarities in both the symptoms of immune system–activated diseases and depression and the molecular pathways activated by the immune system.
“Adding on top of the tremendous changes in the immune system that we see in the body of the woman during the perinatal period, we hypothesized that autoimmune diseases could be associated to perinatal depression,” she said. “This had also been shown in some previous literature but not to the extent as what we have investigated in this paper.”
She said their results help make a case for counseling women at several points in healthcare interactions — before and after conception and childbirth — and in rheumatology visits to inform women with autoimmune diseases who are contemplating motherhood of the association with developing PND. The results may also demonstrate a need for monitoring women in these groups for depression or autoimmune disease.
Fred Miller, MD, PhD, retired Scientist Emeritus of the Environmental Autoimmunity Group at the National Institute of Environmental Health Sciences, who was not part of the study, said the results seem plausible as they build on early work that demonstrated selected associations between autoimmune conditions and mental illness.
“These associations may be the result of shared genetic and environmental risk factors, including stress, hormonal changes, medications, and the proinflammatory states that can lead to both,” he said.
The novelty, he said, is in the relatively strong associations of PND with autoimmune disease overall and with specific autoimmune diseases.
Strong Link Found With Multiple Sclerosis (MS)
According to the paper, a significant positive bidirectional link was found for autoimmune thyroid disease, psoriasis, MS, ulcerative colitis, and celiac disease.
Researchers found a particularly strong association — double the risk in both directions — between PND and MS.
Dr. Miller said though it is unclear from this study why the association of PND with MS was stronger than with other autoimmune diseases, people with MS are known to be at a high risk for depression in general. That may come from greater shared genetic and environmental risk factors, he added.
Additionally, MS is one of the more common autoimmune diseases, he noted, so the population is larger for study.
He said he was surprised the researchers didn’t investigate medication use because medications used in depression have immunologic effects and medications used in autoimmune diseases could have effects on mental conditions.
The study has implications for clinicians in a wide variety of specialties, Dr. Miller noted.
“It suggests that caregivers be more alert to the signs of developing autoimmune disease in women with perinatal depression and to the signs of developing perinatal depression in those with autoimmune disease,” Dr. Miller said, “so that appropriate screening, diagnostics, and interventions may be undertaken.”
The researchers say they will continue to examine the long-term effects of depression during pregnancy and in the year after childbirth.
“Depression during this sensitive period can have serious consequences for both the mother and the baby,” Dr. Bränn said. “We hope that our results will help decision-makers to steer funding toward maternal healthcare so that more women can get help and support in time.”
The study was financed by Karolinska Institute, Forte (the Swedish Research Council for Health, Working Life and Welfare), the Swedish Research Council, and the Icelandic Research Fund.
The researchers and Dr. Miller reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM MOLECULAR PSYCHIATRY
US Dermatologic Drug Approvals Rose Between 2012 and 2022
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
An 18-month-old male presents with a red mark on the forehead and nose
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
Following the initial presentation, the lesion was initially considered an acquired port wine stain and the child was referred for laser treatment. Upon reassessment during laser treatment a few months later, the lesion had progressed to hyper- and hypopigmented plaques with associated tissue sclerosis and bone atrophy on the mid forehead, nose, and scalp. Patches of alopecia and atrophy were observed on the frontal scalp. The diagnosis was revised to linear morphea en coup de sabre and the child was referred to pediatric rheumatology and commenced treatment with methotrexate and oral corticosteroids.
Linear morphea, a rare connective tissue disorder, primarily affects girls in the first 2 decades of life. Lesions can initially present in many ways. Usually, they present as hypo- or hyperpigmented patches, but may also present as lichenoid uncolored or pink plaques resembling lichen striatus. There may also be erythematous patches mimicking a capillary malformation, as seen in our patient. A recent article reviewing the progression of the lesions from erythematous patches to sclerosis suggests it occurs between 3 and 7 months of age. Subsequent stages manifest as significant atrophy, hypo- and hyperpigmentation, and in severe cases, bone atrophy and deformity, often causing substantial cosmetic disfigurement and functional impairment.
Pathophysiologically, linear morphea involves a complex interplay of immunologic, vascular, and fibrotic processes. While the initial triggers remain elusive, dysregulated immune responses leading to endothelial injury, subsequent activation of fibroblasts and myofibroblasts, and excessive collagen deposition are implicated. Angiogenic disturbances exacerbate tissue ischemia, perpetuating the fibrotic cascade. Alterations in cytokine signaling pathways, particularly TGF-beta and interleukin-6, play pivotal roles in promoting fibrosis and modulating the inflammatory milieu.
Diagnosis of linear morphea en coup de sabre relies on clinical examination, imaging (ultrasonography, MRI, CT scan), and skin biopsy for histopathological analysis. Imaging helps evaluate tissue involvement, while histology reveals characteristic dermal sclerosis, collagen deposition, and inflammation. Early-stage histology may show telangiectatic changes, complicating its differentiation from capillary malformation.
Treatment aims to mitigate symptoms, halt disease progression, and improve cosmesis and functionality. This involves a multidisciplinary approach with systemic medications, phototherapy, physical therapy, and surgical interventions in severe cases. Early identification is crucial for systemic treatments such as methotrexate and systemic corticosteroids to arrest disease progression. Other adjunctive therapies include topical corticosteroids, calcineurin inhibitors, and phototherapy. Surgical procedures like tissue expansion or autologous fat grafting may address tissue atrophy and deformities.
Linear morphea en coup de sabre presents diagnostic and therapeutic challenges because of its rarity and variable clinical course. Collaborative efforts among dermatologists, rheumatologists, radiologists, and surgeons are essential for accurate diagnosis, evaluation, and tailored management. Continued research into pathogenesis and novel therapeutic agents is pivotal to enhance understanding and improve outcomes for those affected by this enigmatic dermatologic condition.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Gomez-Garcia LA et al. Pediatr Dermatol. 2022 Mar;39(2):275-80.
Ng SS, Tay YK. J Cosmet Laser Ther. 2015;17(5):277-80.
Nijhawan RI et al. J Am Acad Dermatol. 2011 Apr;64(4):779-82.
On examination, a faint pink patch was observed on the right forehead, frontal scalp, and nose. The lesion paled under pressure, with small areas of hair loss on the scalp. No atrophy was noted.
CAR T-Cell Therapy: Cure for Systemic Autoimmune Diseases?
A single infusion of autologous CD19-directed CAR T-cell therapy led to persistent, drug-free remission in 15 patients with life-threatening systemic lupus erythematosus, idiopathic inflammatory myositis, or systemic sclerosis, according to research presented at the American Society of Hematology annual meeting.
The responses persisted at 15 months median follow-up, with all patients achieving complete remission, reported Fabian Mueller, MD, of the Bavarian Cancer Research Center and Friedrich-Alexander University of Erlangen-Nuremberg, Bavaria, Germany.
The CAR T-cell treatment appears to provide an “entire reset of B cells,” possibly even a cure, for these 15 patients who had run out of treatment options and had short life expectancies, Dr. Mueller said. “It’s impressive that we have treated these patients.”
Some of the cases have been described previously — including in Annals of the Rheumatic Diseases earlier this year, Nature Medicine in 2022, and the New England Journal of Medicine in 2021.
Now with substantially longer follow-up, the investigators have gained a greater understanding of “the B-cell biology behind our treatment,” Dr. Mueller said. However, “we need longer follow-up to establish how effective the treatment is going to be in the long run.”
All 15 patients included in the analysis were heavily pretreated and had multi-organ involvement. Prior to CAR T-cell therapy, patients had a median disease duration of 3 years, ranging from 1 to as many as 20 years, and had failed a median of five previous treatments. Patients were young — a median age of 36 years — which is much younger than most oncology patients who undergo CAR T-cell therapy, Dr. Mueller said.
The 15 patients underwent typical lymphodepletion and were apheresed and treated with a single infusion of 1 x 106 CD19 CAR T cells per kg of body weight — an established safe dose used in a phase 1 trial of B cell malignancies.
The CAR T cells, manufactured in-house, expanded rapidly, peaking around day 9. B cells disappeared within 7 days and began to reoccur in peripheral blood in all patients between 60 and 180 days. However, no disease flares occurred, Dr. Mueller said.
After 3 months, eight patients with systemic lupus erythematosus showed no sign of disease activity and dramatic improvement in symptoms. Three patients with idiopathic inflammatory myositis experienced major improvements in symptoms and normalization of creatinine kinase levels, the most clinically relevant marker for muscle inflammation. And three of four patients with systemic sclerosis demonstrated major improvements in symptoms and no new disease activity. These responses lasted for a median of 15 months, and all patients stopped taking immunosuppressive drugs.
Patients also tolerated the CAR T-cell treatment well, especially compared with the adverse event profile in oncology patients. Only low-grade inflammatory CAR T-related side effects occurred, and few patients required support for B-cell-derived immune deficiency.
However, infectious complications occurred in 14 patients, including urinary tract and respiratory infections, over the 12-month follow-up. One patient was hospitalized for severe pneumonia a few weeks after CAR T therapy, and two patients experienced herpes zoster reactivations, including one at 6 months and one at 12 months following treatment.
During a press briefing at the ASH conference, Dr. Mueller addressed the “critical question” of patient selection for CAR T-cell therapy, especially in light of the recently announced US Food and Drug Administration investigation exploring whether CAR T cells can cause secondary blood cancers.
Although the T-cell malignancy risk complicates matters, CAR T cells appear to behave differently in patients with autoimmune diseases than those with cancer, he said.
“We don’t understand the biology” related to the malignancy risk yet, Dr. Mueller said, but the benefit for end-of-life patients with no other treatment option likely outweighs the risk. That risk-benefit assessment, however, is more uncertain for those with less severe autoimmune diseases.
For now, it’s important to conduct individual assessments and inform patients about the risk, Dr. Mueller said.
Dr. Mueller disclosed relationships with BMS, AstraZeneca, Gilead, Janssen, Miltenyi Biomedicine, Novartis, Incyte, Abbvie, Sobi, and BeiGene.
A version of this article appeared on Medscape.com.
A single infusion of autologous CD19-directed CAR T-cell therapy led to persistent, drug-free remission in 15 patients with life-threatening systemic lupus erythematosus, idiopathic inflammatory myositis, or systemic sclerosis, according to research presented at the American Society of Hematology annual meeting.
The responses persisted at 15 months median follow-up, with all patients achieving complete remission, reported Fabian Mueller, MD, of the Bavarian Cancer Research Center and Friedrich-Alexander University of Erlangen-Nuremberg, Bavaria, Germany.
The CAR T-cell treatment appears to provide an “entire reset of B cells,” possibly even a cure, for these 15 patients who had run out of treatment options and had short life expectancies, Dr. Mueller said. “It’s impressive that we have treated these patients.”
Some of the cases have been described previously — including in Annals of the Rheumatic Diseases earlier this year, Nature Medicine in 2022, and the New England Journal of Medicine in 2021.
Now with substantially longer follow-up, the investigators have gained a greater understanding of “the B-cell biology behind our treatment,” Dr. Mueller said. However, “we need longer follow-up to establish how effective the treatment is going to be in the long run.”
All 15 patients included in the analysis were heavily pretreated and had multi-organ involvement. Prior to CAR T-cell therapy, patients had a median disease duration of 3 years, ranging from 1 to as many as 20 years, and had failed a median of five previous treatments. Patients were young — a median age of 36 years — which is much younger than most oncology patients who undergo CAR T-cell therapy, Dr. Mueller said.
The 15 patients underwent typical lymphodepletion and were apheresed and treated with a single infusion of 1 x 106 CD19 CAR T cells per kg of body weight — an established safe dose used in a phase 1 trial of B cell malignancies.
The CAR T cells, manufactured in-house, expanded rapidly, peaking around day 9. B cells disappeared within 7 days and began to reoccur in peripheral blood in all patients between 60 and 180 days. However, no disease flares occurred, Dr. Mueller said.
After 3 months, eight patients with systemic lupus erythematosus showed no sign of disease activity and dramatic improvement in symptoms. Three patients with idiopathic inflammatory myositis experienced major improvements in symptoms and normalization of creatinine kinase levels, the most clinically relevant marker for muscle inflammation. And three of four patients with systemic sclerosis demonstrated major improvements in symptoms and no new disease activity. These responses lasted for a median of 15 months, and all patients stopped taking immunosuppressive drugs.
Patients also tolerated the CAR T-cell treatment well, especially compared with the adverse event profile in oncology patients. Only low-grade inflammatory CAR T-related side effects occurred, and few patients required support for B-cell-derived immune deficiency.
However, infectious complications occurred in 14 patients, including urinary tract and respiratory infections, over the 12-month follow-up. One patient was hospitalized for severe pneumonia a few weeks after CAR T therapy, and two patients experienced herpes zoster reactivations, including one at 6 months and one at 12 months following treatment.
During a press briefing at the ASH conference, Dr. Mueller addressed the “critical question” of patient selection for CAR T-cell therapy, especially in light of the recently announced US Food and Drug Administration investigation exploring whether CAR T cells can cause secondary blood cancers.
Although the T-cell malignancy risk complicates matters, CAR T cells appear to behave differently in patients with autoimmune diseases than those with cancer, he said.
“We don’t understand the biology” related to the malignancy risk yet, Dr. Mueller said, but the benefit for end-of-life patients with no other treatment option likely outweighs the risk. That risk-benefit assessment, however, is more uncertain for those with less severe autoimmune diseases.
For now, it’s important to conduct individual assessments and inform patients about the risk, Dr. Mueller said.
Dr. Mueller disclosed relationships with BMS, AstraZeneca, Gilead, Janssen, Miltenyi Biomedicine, Novartis, Incyte, Abbvie, Sobi, and BeiGene.
A version of this article appeared on Medscape.com.
A single infusion of autologous CD19-directed CAR T-cell therapy led to persistent, drug-free remission in 15 patients with life-threatening systemic lupus erythematosus, idiopathic inflammatory myositis, or systemic sclerosis, according to research presented at the American Society of Hematology annual meeting.
The responses persisted at 15 months median follow-up, with all patients achieving complete remission, reported Fabian Mueller, MD, of the Bavarian Cancer Research Center and Friedrich-Alexander University of Erlangen-Nuremberg, Bavaria, Germany.
The CAR T-cell treatment appears to provide an “entire reset of B cells,” possibly even a cure, for these 15 patients who had run out of treatment options and had short life expectancies, Dr. Mueller said. “It’s impressive that we have treated these patients.”
Some of the cases have been described previously — including in Annals of the Rheumatic Diseases earlier this year, Nature Medicine in 2022, and the New England Journal of Medicine in 2021.
Now with substantially longer follow-up, the investigators have gained a greater understanding of “the B-cell biology behind our treatment,” Dr. Mueller said. However, “we need longer follow-up to establish how effective the treatment is going to be in the long run.”
All 15 patients included in the analysis were heavily pretreated and had multi-organ involvement. Prior to CAR T-cell therapy, patients had a median disease duration of 3 years, ranging from 1 to as many as 20 years, and had failed a median of five previous treatments. Patients were young — a median age of 36 years — which is much younger than most oncology patients who undergo CAR T-cell therapy, Dr. Mueller said.
The 15 patients underwent typical lymphodepletion and were apheresed and treated with a single infusion of 1 x 106 CD19 CAR T cells per kg of body weight — an established safe dose used in a phase 1 trial of B cell malignancies.
The CAR T cells, manufactured in-house, expanded rapidly, peaking around day 9. B cells disappeared within 7 days and began to reoccur in peripheral blood in all patients between 60 and 180 days. However, no disease flares occurred, Dr. Mueller said.
After 3 months, eight patients with systemic lupus erythematosus showed no sign of disease activity and dramatic improvement in symptoms. Three patients with idiopathic inflammatory myositis experienced major improvements in symptoms and normalization of creatinine kinase levels, the most clinically relevant marker for muscle inflammation. And three of four patients with systemic sclerosis demonstrated major improvements in symptoms and no new disease activity. These responses lasted for a median of 15 months, and all patients stopped taking immunosuppressive drugs.
Patients also tolerated the CAR T-cell treatment well, especially compared with the adverse event profile in oncology patients. Only low-grade inflammatory CAR T-related side effects occurred, and few patients required support for B-cell-derived immune deficiency.
However, infectious complications occurred in 14 patients, including urinary tract and respiratory infections, over the 12-month follow-up. One patient was hospitalized for severe pneumonia a few weeks after CAR T therapy, and two patients experienced herpes zoster reactivations, including one at 6 months and one at 12 months following treatment.
During a press briefing at the ASH conference, Dr. Mueller addressed the “critical question” of patient selection for CAR T-cell therapy, especially in light of the recently announced US Food and Drug Administration investigation exploring whether CAR T cells can cause secondary blood cancers.
Although the T-cell malignancy risk complicates matters, CAR T cells appear to behave differently in patients with autoimmune diseases than those with cancer, he said.
“We don’t understand the biology” related to the malignancy risk yet, Dr. Mueller said, but the benefit for end-of-life patients with no other treatment option likely outweighs the risk. That risk-benefit assessment, however, is more uncertain for those with less severe autoimmune diseases.
For now, it’s important to conduct individual assessments and inform patients about the risk, Dr. Mueller said.
Dr. Mueller disclosed relationships with BMS, AstraZeneca, Gilead, Janssen, Miltenyi Biomedicine, Novartis, Incyte, Abbvie, Sobi, and BeiGene.
A version of this article appeared on Medscape.com.
FROM ASH 2023
Autoimmune Skin Diseases Linked To Risk Of Adverse Pregnancy Outcomes
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
FROM ACR 2023
Paradoxical Reaction to TNF-α Inhibitor Therapy in a Patient With Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.
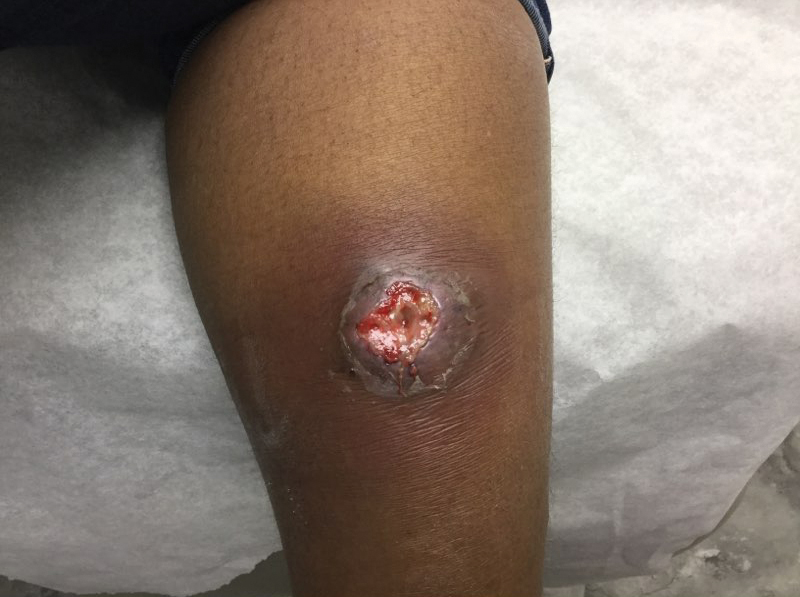
Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.
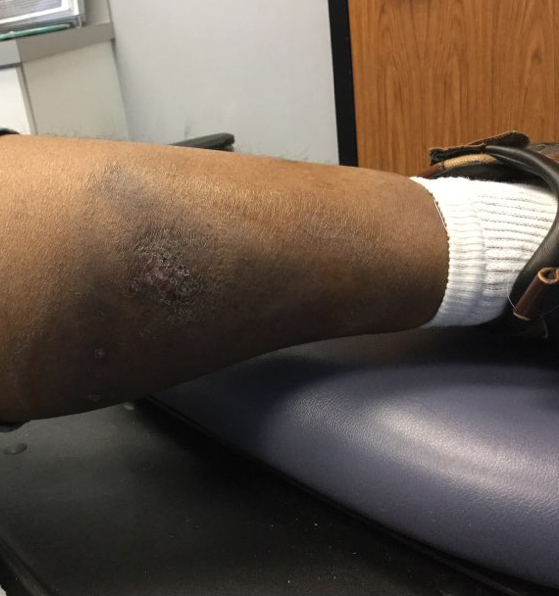
Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
To the Editor:
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the pilosebaceous unit that occurs in concert with elevations of various cytokines, including tumor necrosis factor α (TNF-α), IL-1β, IL-10, and IL-17.1,2 Adalimumab is a TNF-α inhibitor approved by the US Food and Drug Administration for the treatment of HS. Although TNF-α inhibitors are effective for many immune-mediated inflammatory disorders, paradoxical drug reactions have been reported following treatment with these agents.3-6 True paradoxical drug reactions likely are immune mediated and directly lead to new onset of a pathologic condition that would otherwise respond to that drug. For example, there are reports of rheumatoid arthritis patients who were treated with a TNF-α inhibitor and developed psoriatic skin lesions.3,6 Paradoxical drug reactions also have been reported with acute-onset inflammatory bowel disease and HS or less commonly pyoderma gangrenosum (PG), uveitis, granulomatous reactions, and vasculitis.4,5 We present the case of a patient with HS who was treated with a TNF-α inhibitor and developed 2 distinct paradoxical drug reactions. We also provide an overview of paradoxical drug reactions associated with TNF-α inhibitors.
A 38-year-old woman developed a painful “boil” on the right leg that was previously treated in the emergency department with incision and drainage as well as oral clindamycin for 7 days, but the lesion spread and continued to worsen. She had a history of HS in the axillae and groin region that had been present since 12 years of age. The condition was poorly controlled despite multiple courses of oral antibiotics and surgical resections. An oral contraceptive also was attempted, but the patient discontinued treatment when liver enzyme levels became elevated. The patient had no other notable medical history, including skin disease. There was a family history of HS in her father and a sibling. Seeking more effective treatment, the patient was offered adalimumab approximately 4 months prior to clinical presentation and agreed to start a course of the drug. She received a loading dose of 160 mg on day 1 and 80 mg on day 15 followed by a maintenance dosage of 40 mg weekly. She experienced improvement in HS symptoms after 3 months on adalimumab; however, she developed scaly pruritic patches on the scalp, arms, and legs that were consistent with psoriasis. Because of the absence of a personal or family history of psoriasis, the patient was informed of the probability of paradoxical psoriasis resulting from adalimumab. She elected to continue adalimumab because of the improvement in HS symptoms, and the psoriatic lesions were mild and adequately controlled with a topical steroid.
At the current presentation 1 month later, physical examination revealed a large indurated and ulcerated area with jagged edges at the incision and drainage site (Figure 1). Pyoderma gangrenosum was clinically suspected; a biopsy was performed, and the patient was started on oral prednisone. At 2-week follow-up, the ulcer was found to be rapidly resolving with prednisone and healing with cribriform scarring (Figure 2). Histopathology revealed an undermining neutrophilic inflammatory process that was consistent with PG. A diagnosis of PG was made based on previously published criteria7 and the following major/minor criteria in the patient: pathology; absence of infection on histologic analysis; history of pathergy related to worsening ulceration at the site of incision and drainage of the initial boil; clinical findings of an ulcer with peripheral violaceous erythema; undermined borders and tenderness at the site; and rapid resolution of the ulcer with prednisone.

Cessation of adalimumab gradually led to clearance of both psoriasiform lesions and PG; however, HS lesions persisted.

Although the precise pathogenesis of HS is unclear, both genetic abnormalities of the pilosebaceous unit and a dysregulated immune reaction appear to lead to the clinical characteristics of chronic inflammation and scarring seen in HS. A key effector appears to be helper T-cell (TH17) lymphocyte activation, with increased secretion of TNF-α, IL-1β, and IL-17.1,2 In turn, IL-17 induces higher expression of TNF-α, leading to a persistent cycle of inflammation. Peripheral recruitment of IL-17–producing neutrophils also may contribute to chronic inflammation.8
Adalimumab is the only US Food and Drug Administration–approved biologic indicated for the treatment of HS. Our patient initially responded to adalimumab with improvement of HS; however, treatment had to be discontinued because of the unusual occurrence of 2 distinct paradoxical reactions in a short span of time. Psoriasis and PG are both considered true paradoxical reactions because primary occurrences of both diseases usually are responsive to treatment with adalimumab.
Tumor necrosis factor α inhibitor–induced psoriasis arises de novo and is estimated to occur in approximately 5% of patients with rheumatoid arthritis.3,6 Palmoplantar pustular psoriasiform reactions are the most common form of paradoxical psoriasis. Topical medications can be used to treat skin lesions, but systemic treatment is required in many cases. Switching to an alternate class of a biologic, such as an IL-17, IL-12/23, or IL-23 inhibitor, can improve the skin reaction; however, such treatment is inconsistently successful, and paradoxical drug reactions also have been seen with these other classes of biologics.4,9
Recent studies support distinct immune causes for classical and paradoxical psoriasis. In classical psoriasis, plasmacytoid dendritic cells (pDCs) produce IFN-α, which stimulates conventional dendritic cells to produce TNF-α. However, TNF-α matures both pDCs and conventional dendritic cells; upon maturation, both types of dendritic cells lose the ability to produce IFN-α, thus allowing TNF-α to become dominant.10 The blockade of TNF-α prevents pDC maturation, leading to uninhibited IFN-α, which appears to drive inflammation in paradoxical psoriasis. In classical psoriasis, oligoclonal dermal CD4+ T cells and epidermal CD8+ T cells remain, even in resolved skin lesions, and can cause disease recurrence through reactivation of skin-resident memory T cells.11 No relapse of paradoxical psoriasis occurs with discontinuation of anti-TNF-α therapy, which supports the notion of an absence of memory T cells.
The incidence of paradoxical psoriasis in patients receiving a TNF-α inhibitor for HS is unclear.12 There are case series in which patients who had concurrent psoriasis and HS were successfully treated with a TNF-α inhibitor.13 A recently recognized condition—PASH syndrome—encompasses the clinical triad of PG, acne, and HS.10
Our patient had no history of acne or PG, only a long-standing history of HS. New-onset PG occurred only after a TNF-α inhibitor was initiated. Notably, PASH syndrome has been successfully treated with TNF-α inhibitors, highlighting the shared inflammatory etiology of HS and PG.14 In patients with concurrent PG and HS, TNF-α inhibitors were more effective for treating PG than for HS.
Pyoderma gangrenosum is an inflammatory disorder that often occurs concomitantly with other conditions, such as inflammatory bowel disease. The exact underlying cause of PG is unclear, but there appears to be both neutrophil and T-cell dysfunction in PG, with excess inflammatory cytokine production (eg, IL-1β, TNF-α, IL-17).15
The mainstay of treatment of PG is systemic corticosteroids and immunosuppressives, such as cyclosporine. Tumor necrosis factor α inhibitors as well as other interleukin inhibitors are increasingly utilized as potential therapeutic alternatives for PG.16,17
Unlike paradoxical psoriasis, the underlying cause of paradoxical PG is unclear.18,19 A similar mechanism may be postulated whereby inhibition of TNF-α leads to excessive activation of alternative inflammatory pathways that result in paradoxical PG. In one study, the prevalence of PG among 68,232 patients with HS was 0.18% compared with 0.01% among those without HS; therefore, patients with HS appear to be more predisposed to PG.20
This case illustrates the complex, often conflicting effects of cytokine inhibition in the paradoxical elicitation of alternative inflammatory disorders as an unintended consequence of the initial cytokine blockade. It is likely that genetic predisposition allows for paradoxical reactions in some patients when there is predominant inhibition of one cytokine in the inflammatory pathway. In rare cases, multiple paradoxical reactions are possible.
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
1. Vossen ARJV, van der Zee HH, Prens EP. Hidradenitis suppurativa: a systematic review integrating inflammatory pathways into a cohesive pathogenic model. Front Immunol. 2018;9:2965. doi:10.3389/fimmu.2018.02965
2. Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation and pathogenesis. J Am Acad Dermatol. 2020; 82:1045-1058. doi:10.1016/j.jaad.2019.08.090
3. Brown G, Wang E, Leon A, et al. Tumor necrosis factor-α inhibitor-induced psoriasis: systematic review of clinical features, histopathological findings, and management experience. J Am Acad Dermatol. 2017;76:334-341. doi:10.1016/j.jaad.2016.08.012
4. Puig L. Paradoxical reactions: anti-tumor necrosis factor alpha agents, ustekinumab, secukinumab, ixekizumab and others. Curr Prob Dermatol. 2018;53:49-63. doi:10.1159/000479475
5. Faivre C, Villani AP, Aubin F, et al; . Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
6. Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20:100-108. doi:10.1080/09546630802441234
7. Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a delphi consensus of international experts. JAMA Dermatol. 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
8. Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174:514-521. doi:10.1111/bjd.14214
9. Li SJ, Perez-Chada LM, Merola JF. TNF inhibitor-induced psoriasis: proposed algorithm for treatment and management. J Psoriasis Psoriatic Arthritis. 2019;4:70-80. doi:10.1177/2475530318810851
10. Conrad C, Di Domizio J, Mylonas A, et al. TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis. Nat Commun. 2018;9:25. doi:10.1038/s41467-017-02466-4
11. Matos TR, O’Malley JT, Lowry EL, et al. Clinically resolved psoriatic lesions contain psoriasis-specific IL-17-producing αβ T cell clones. J Clin Invest. 2017;127:4031-4041. doi:10.1172/JCI93396
12. Faivre C, Villani AP, Aubin F, et al. Hidradenitis suppurativa (HS): an unrecognized paradoxical effect of biologic agents (BA) used in chronic inflammatory diseases. J Am Acad Dermatol. 2016;74:1153-1159. doi:10.1016/j.jaad.2016.01.018
13. Marzano AV, Damiani G, Ceccherini I, et al. Autoinflammation in pyoderma gangrenosum and its syndromic form (pyoderma gangrenosum, acne and suppurative hidradenitis). Br J Dermatol. 2017;176:1588-1598. doi:10.1111/bjd.15226
14. Cugno M, Borghi A, Marzano AV. PAPA, PASH, PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562. doi:10.1007/s40257-017-0265-1
15. Wang EA, Steel A, Luxardi G, et al. Classic ulcerative pyoderma gangrenosum is a T cell-mediated disease targeting follicular adnexal structures: a hypothesis based on molecular and clinicopathologic studies. Front Immunol. 2018;8:1980. doi:10.3389/fimmu.2017.01980
16. Patel F, Fitzmaurice S, Duong C, et al. Effective strategies for the management of pyoderma gangrenosum: a comprehensive review. Acta Derm Venereol. 2015;95:525-531. doi:10.2340/00015555-2008
17. Partridge ACR, Bai JW, Rosen CF, et al. Effectiveness of systemic treatments for pyoderma gangrenosum: a systematic review of observational studies and clinical trials. Br J Dermatol. 2018;179:290-295. doi:10.1111/bjd.16485
18. Benzaquen M, Monnier J, Beaussault Y, et al. Pyoderma gangrenosum arising during treatment of psoriasis with adalimumab: effectiveness of ustekinumab. Australas J Dermatol. 2017;58:e270-e271. doi:10.1111/ajd.12545
19. Fujimoto N, Yamasaki Y, Watanabe RJ. Paradoxical uveitis and pyoderma gangrenosum in a patient with psoriatic arthritis under infliximab treatment. J Dtsch Dermatol Ges. 2018;16:1139-1140. doi:10.1111/ddg.13632
20. Tannenbaum R, Strunk A, Garg A. Overall and subgroup prevalence of pyoderma gangrenosum among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol. 2019;80:1533-1537. doi:10.1016/j.jaad.2019.02.004
Practice Points
- Clinicians need to be aware of the potential risk for a paradoxical reaction in patients receiving a tumor necrosis factor α (TNF-α) inhibitor for hidradenitis suppurativa.
- Although uncommon, developing more than 1 type of paradoxical skin reaction is possible with a TNF-α inhibitor.
- Early recognition and appropriate management of these paradoxical reactions are critical.