User login
SLE linked to subsequent risk of malignant melanoma
PORTLAND, ORE. – A diagnosis of systemic lupus erythematosus (SLE) significantly increases the risk of a subsequent diagnosis of malignant melanoma, according to the results of a large, first-in-kind, single-center longitudinal analysis of electronic medical records.
This finding expands the list of known associations between SLE and cancer, and highlights the need for careful surveillance of this population, Solomiya Grushchak, of the department of dermatology at Northwestern University, Chicago, and her associates, reported in a poster presented at the annual meeting of the Society for Investigative Dermatology.
SLE is increasingly being treated with immune checkpoint inhibitors, which can aggressively disrupt immune reactivity and trigger uncontrolled cellular responses in patients with SLE, Ms. Grushchak noted. “The findings in this large population warrant further exploration of the association between malignant melanoma and SLE to promote optimal patient management, especially in light of recent advances [in the use of] checkpoint inhibitors,” she added.
Past work has linked SLE with several other malignancies, including nonmelanoma skin cancers, non-Hodgkin and Hodgkin lymphomas, and cancers of the larynx, lungs, liver, vulva, vagina, and thyroid gland. Even when patients are not receiving checkpoint inhibitors, SLE causes chronic inflammation and is known to increase cellular dysplasia, which can ultimately trigger uncontrolled proliferation of tumor cells. In 2015, a meta-analysis showed that SLE was associated with a decreased risk of melanoma, but no studies had conclusively evaluated this relationship (PLoS One. 2015;10[4]:e0122964).
Therefore, Ms. Grushchak and her associates analyzed medical records from 2,351 patients from the urban Midwest with SLE diagnosed by a dermatologist or rheumatologist between 2000 and 2016. The data source was the Northwestern Enterprise Data Warehouse, which integrates clinical and research information from more than 50 health data systems used by the Northwestern University Feinberg School of Medicine and its health care partners. To avoid detection bias, the researchers constructed a comparison group from the same database of 1,676 patients diagnosed with systemic sclerosis.
Ten patients (0.4%) with a diagnostic code for SLE were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), the investigators reported. A Fisher’s exact test confirmed a statistically significant difference between these rates (P = .03). Among the 10 SLE patients with melanoma, 7 were white, 2 were black, and 1 was of Asian ancestry. Nine were females, and one was male. The patient with systemic sclerosis and melanoma was a white male.
The study had several limitations. The investigators did not report how much time elapsed between the diagnoses of SLE and melanoma, or the rates or cumulative exposure to checkpoint inhibitors.
The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
PORTLAND, ORE. – A diagnosis of systemic lupus erythematosus (SLE) significantly increases the risk of a subsequent diagnosis of malignant melanoma, according to the results of a large, first-in-kind, single-center longitudinal analysis of electronic medical records.
This finding expands the list of known associations between SLE and cancer, and highlights the need for careful surveillance of this population, Solomiya Grushchak, of the department of dermatology at Northwestern University, Chicago, and her associates, reported in a poster presented at the annual meeting of the Society for Investigative Dermatology.
SLE is increasingly being treated with immune checkpoint inhibitors, which can aggressively disrupt immune reactivity and trigger uncontrolled cellular responses in patients with SLE, Ms. Grushchak noted. “The findings in this large population warrant further exploration of the association between malignant melanoma and SLE to promote optimal patient management, especially in light of recent advances [in the use of] checkpoint inhibitors,” she added.
Past work has linked SLE with several other malignancies, including nonmelanoma skin cancers, non-Hodgkin and Hodgkin lymphomas, and cancers of the larynx, lungs, liver, vulva, vagina, and thyroid gland. Even when patients are not receiving checkpoint inhibitors, SLE causes chronic inflammation and is known to increase cellular dysplasia, which can ultimately trigger uncontrolled proliferation of tumor cells. In 2015, a meta-analysis showed that SLE was associated with a decreased risk of melanoma, but no studies had conclusively evaluated this relationship (PLoS One. 2015;10[4]:e0122964).
Therefore, Ms. Grushchak and her associates analyzed medical records from 2,351 patients from the urban Midwest with SLE diagnosed by a dermatologist or rheumatologist between 2000 and 2016. The data source was the Northwestern Enterprise Data Warehouse, which integrates clinical and research information from more than 50 health data systems used by the Northwestern University Feinberg School of Medicine and its health care partners. To avoid detection bias, the researchers constructed a comparison group from the same database of 1,676 patients diagnosed with systemic sclerosis.
Ten patients (0.4%) with a diagnostic code for SLE were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), the investigators reported. A Fisher’s exact test confirmed a statistically significant difference between these rates (P = .03). Among the 10 SLE patients with melanoma, 7 were white, 2 were black, and 1 was of Asian ancestry. Nine were females, and one was male. The patient with systemic sclerosis and melanoma was a white male.
The study had several limitations. The investigators did not report how much time elapsed between the diagnoses of SLE and melanoma, or the rates or cumulative exposure to checkpoint inhibitors.
The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
PORTLAND, ORE. – A diagnosis of systemic lupus erythematosus (SLE) significantly increases the risk of a subsequent diagnosis of malignant melanoma, according to the results of a large, first-in-kind, single-center longitudinal analysis of electronic medical records.
This finding expands the list of known associations between SLE and cancer, and highlights the need for careful surveillance of this population, Solomiya Grushchak, of the department of dermatology at Northwestern University, Chicago, and her associates, reported in a poster presented at the annual meeting of the Society for Investigative Dermatology.
SLE is increasingly being treated with immune checkpoint inhibitors, which can aggressively disrupt immune reactivity and trigger uncontrolled cellular responses in patients with SLE, Ms. Grushchak noted. “The findings in this large population warrant further exploration of the association between malignant melanoma and SLE to promote optimal patient management, especially in light of recent advances [in the use of] checkpoint inhibitors,” she added.
Past work has linked SLE with several other malignancies, including nonmelanoma skin cancers, non-Hodgkin and Hodgkin lymphomas, and cancers of the larynx, lungs, liver, vulva, vagina, and thyroid gland. Even when patients are not receiving checkpoint inhibitors, SLE causes chronic inflammation and is known to increase cellular dysplasia, which can ultimately trigger uncontrolled proliferation of tumor cells. In 2015, a meta-analysis showed that SLE was associated with a decreased risk of melanoma, but no studies had conclusively evaluated this relationship (PLoS One. 2015;10[4]:e0122964).
Therefore, Ms. Grushchak and her associates analyzed medical records from 2,351 patients from the urban Midwest with SLE diagnosed by a dermatologist or rheumatologist between 2000 and 2016. The data source was the Northwestern Enterprise Data Warehouse, which integrates clinical and research information from more than 50 health data systems used by the Northwestern University Feinberg School of Medicine and its health care partners. To avoid detection bias, the researchers constructed a comparison group from the same database of 1,676 patients diagnosed with systemic sclerosis.
Ten patients (0.4%) with a diagnostic code for SLE were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), the investigators reported. A Fisher’s exact test confirmed a statistically significant difference between these rates (P = .03). Among the 10 SLE patients with melanoma, 7 were white, 2 were black, and 1 was of Asian ancestry. Nine were females, and one was male. The patient with systemic sclerosis and melanoma was a white male.
The study had several limitations. The investigators did not report how much time elapsed between the diagnoses of SLE and melanoma, or the rates or cumulative exposure to checkpoint inhibitors.
The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
AT SID 2017
Key clinical point: Compared with controls, patients with systemic lupus erythematosus (SLE) were at significantly increased risk of later being diagnosed with malignant melanoma.
Major finding: Ten patients with SLE (0.4%) were later diagnosed with malignant melanoma, compared with one patient with systemic sclerosis (0.06%), a statistically significant difference (P = .03).
Data source: Electronic medical record reviews of 2,351 patients with SLE and 1,676 patients with systemic sclerosis (controls) between 2000 and 2016.
Disclosures: The National Institutes of Health provides support to the Northwestern Enterprise Data Warehouse. The investigators had no relevant financial conflicts.
Cutaneous manifestations can signify severe systemic disease in ANCA-associated vasculitis
PORTLAND, ORE. – Clinicians who treat or diagnose ANCA-associated vasculitis should watch for a variety of skin lesions, which can signify severe systemic manifestations of disease, according to the results of a cross-sectional study of 1,184 patients from 130 centers worldwide.
Among patients with granulomatosis with polyangiitis (GPA) or eosinophilic granulomatosis with polyangiitis (EGPA), the presence of skin lesions approximately doubled the likelihood of renal, pulmonary, neurologic, or other severe systemic manifestations of ANCA-associated vasculitis (hazard ratios, 2.0; P less than .03).
This cohort is part of the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS), which aims to develop classification and diagnostic criteria for primary systemic vasculitis. Fully 35% of patients had cutaneous manifestations of ANCA-associated vasculitis, including 47% of those with EGPA, 34% of those with GPA, and 28% of those with microscopic polyangiitis (MPA).
Petechiae/purpura were the most common cutaneous manifestations of all three subtypes, affecting 15% of the overall cohort, 21% of patients with EGPA, 16% of those with GPA, and 9% of those with MPA (P less than .01 for differences among groups). Petechiae/purpura did not more accurately predict systemic disease than other cutaneous findings, and skin lesions were not significantly associated with severe systemic disease in patients with MPA (HR, 0.63; 95% confidence interval, 0.35-1.14; P = .13), the investigators reported.
Besides petechiae/purpura, patients with EGPA most often presented with allergic and nonspecific cutaneous manifestations, such as pruritus (13% of patients), urticaria (8%), and maculopapular rash (8%), they said. In contrast, patients with GPA most often had painful skin lesions (10%) or maculopapular rash (7%), while those with MPA were more likely to have livedo reticularis or racemosa (7%).
Study participants tended to be in their mid-50s to mid-60s at diagnosis, about 48% were male, and most were Northern European, Southern European, or American whites, while 28% of those with MPA were Han Chinese, of another Chinese ethnicity, or Japanese.
“This study demonstrates that skin lesions are quite common and varied in granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis,” the investigators concluded.
Funders included the American College of Rheumatology, the European League Against Rheumatism, the Vasculitis Foundation, and the Dermatology Foundation. Dr. Micheletti had no conflicts of interest.
PORTLAND, ORE. – Clinicians who treat or diagnose ANCA-associated vasculitis should watch for a variety of skin lesions, which can signify severe systemic manifestations of disease, according to the results of a cross-sectional study of 1,184 patients from 130 centers worldwide.
Among patients with granulomatosis with polyangiitis (GPA) or eosinophilic granulomatosis with polyangiitis (EGPA), the presence of skin lesions approximately doubled the likelihood of renal, pulmonary, neurologic, or other severe systemic manifestations of ANCA-associated vasculitis (hazard ratios, 2.0; P less than .03).
This cohort is part of the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS), which aims to develop classification and diagnostic criteria for primary systemic vasculitis. Fully 35% of patients had cutaneous manifestations of ANCA-associated vasculitis, including 47% of those with EGPA, 34% of those with GPA, and 28% of those with microscopic polyangiitis (MPA).
Petechiae/purpura were the most common cutaneous manifestations of all three subtypes, affecting 15% of the overall cohort, 21% of patients with EGPA, 16% of those with GPA, and 9% of those with MPA (P less than .01 for differences among groups). Petechiae/purpura did not more accurately predict systemic disease than other cutaneous findings, and skin lesions were not significantly associated with severe systemic disease in patients with MPA (HR, 0.63; 95% confidence interval, 0.35-1.14; P = .13), the investigators reported.
Besides petechiae/purpura, patients with EGPA most often presented with allergic and nonspecific cutaneous manifestations, such as pruritus (13% of patients), urticaria (8%), and maculopapular rash (8%), they said. In contrast, patients with GPA most often had painful skin lesions (10%) or maculopapular rash (7%), while those with MPA were more likely to have livedo reticularis or racemosa (7%).
Study participants tended to be in their mid-50s to mid-60s at diagnosis, about 48% were male, and most were Northern European, Southern European, or American whites, while 28% of those with MPA were Han Chinese, of another Chinese ethnicity, or Japanese.
“This study demonstrates that skin lesions are quite common and varied in granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis,” the investigators concluded.
Funders included the American College of Rheumatology, the European League Against Rheumatism, the Vasculitis Foundation, and the Dermatology Foundation. Dr. Micheletti had no conflicts of interest.
PORTLAND, ORE. – Clinicians who treat or diagnose ANCA-associated vasculitis should watch for a variety of skin lesions, which can signify severe systemic manifestations of disease, according to the results of a cross-sectional study of 1,184 patients from 130 centers worldwide.
Among patients with granulomatosis with polyangiitis (GPA) or eosinophilic granulomatosis with polyangiitis (EGPA), the presence of skin lesions approximately doubled the likelihood of renal, pulmonary, neurologic, or other severe systemic manifestations of ANCA-associated vasculitis (hazard ratios, 2.0; P less than .03).
This cohort is part of the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS), which aims to develop classification and diagnostic criteria for primary systemic vasculitis. Fully 35% of patients had cutaneous manifestations of ANCA-associated vasculitis, including 47% of those with EGPA, 34% of those with GPA, and 28% of those with microscopic polyangiitis (MPA).
Petechiae/purpura were the most common cutaneous manifestations of all three subtypes, affecting 15% of the overall cohort, 21% of patients with EGPA, 16% of those with GPA, and 9% of those with MPA (P less than .01 for differences among groups). Petechiae/purpura did not more accurately predict systemic disease than other cutaneous findings, and skin lesions were not significantly associated with severe systemic disease in patients with MPA (HR, 0.63; 95% confidence interval, 0.35-1.14; P = .13), the investigators reported.
Besides petechiae/purpura, patients with EGPA most often presented with allergic and nonspecific cutaneous manifestations, such as pruritus (13% of patients), urticaria (8%), and maculopapular rash (8%), they said. In contrast, patients with GPA most often had painful skin lesions (10%) or maculopapular rash (7%), while those with MPA were more likely to have livedo reticularis or racemosa (7%).
Study participants tended to be in their mid-50s to mid-60s at diagnosis, about 48% were male, and most were Northern European, Southern European, or American whites, while 28% of those with MPA were Han Chinese, of another Chinese ethnicity, or Japanese.
“This study demonstrates that skin lesions are quite common and varied in granulomatosis with polyangiitis, microscopic polyangiitis, and eosinophilic granulomatosis with polyangiitis,” the investigators concluded.
Funders included the American College of Rheumatology, the European League Against Rheumatism, the Vasculitis Foundation, and the Dermatology Foundation. Dr. Micheletti had no conflicts of interest.
AT SID 2017
Key clinical point: Skin lesions can be a red flag for severe systemic disease in patients with ANCA-associated vasculitis.
Major finding: Among patients with granulomatosis with polyangiitis or eosinophilic granulomatosis with polyangiitis, the presence of skin lesions approximately doubled the likelihood of renal, pulmonary, neurologic, or other severe systemic manifestations of ANCA-associated vasculitis (HR, 2.0, P less than .03). The hazard ratio was not elevated in patients with microscopic polyangiitis.
Data source: A cross-sectional study of 1,184 patients with ANCA-associated vasculitis from 130 centers worldwide.
Disclosures: Funders included the American College of Rheumatology, the European League Against Rheumatism, the Vasculitis Foundation, and the Dermatology Foundation. Dr. Micheletti had no conflicts of interest.
JAK inhibitors and alopecia: After positive early data, various trials now underway
PORTLAND, ORE. – Janus kinase inhibitors are relatively safe and can produce a full head of hair in patients with moderate to severe alopecia areata (AA), although patients tend to shed hair after stopping treatment, Julian Mackay-Wiggan, MD, said at the annual meeting of the Society for Investigative Dermatology.
“At this point, there are 17 publications in the literature, from clinical trials to case reports, looking at JAK [Janus kinase] inhibitors in patients with alopecia areata,” said Dr. Mackay-Wiggan of the department of dermatology, Columbia University, New York, where she specializes in hair disorders. “Pretty much all report very positive findings. It definitely appears that Janus kinase inhibitors can play a very significant role in treatment.”
In an open label, uncontrolled pilot study at Columbia, 9 of 12 (75%) patients with moderate to severe AA improved by at least 50% on the Severity of Alopecia Tool (SALT) after receiving 20 mg ruxolitinib twice daily for 3 to 6 months (JCI Insight. 2016 Sep 22;1[15]:e89790). Responses started with the first month, and all but one responder achieved at least 50% hair regrowth by week 12, said Dr. Mackay-Wiggan, who is also the director of the Dermatology Clinical Research Unit at Columbia.
By the end of treatment, seven of nine responders achieved more than 95% regrowth, one achieved 85% regrowth, and one achieved 55% regrowth. Importantly, none of these relatively healthy patients experienced serious adverse events on ruxolitinib, and none needed to stop treatment, although one patient experienced declining hemoglobin levels that resolved after dose modification.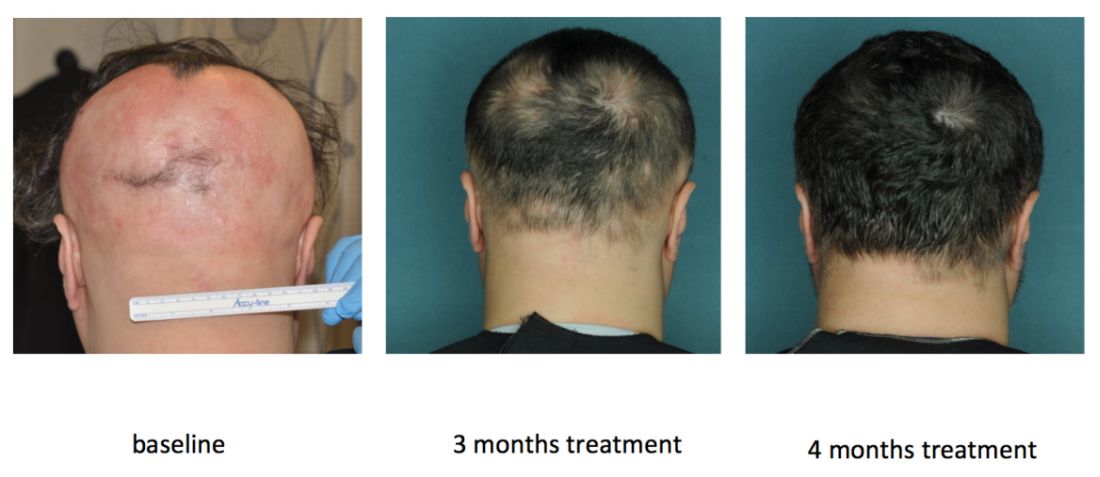
Columbia researchers are also conducting an uncontrolled, open label pilot trial of the JAK inhibitor tofacitinib (Xeljanz) in 12 patients, of whom seven have moderate to severe patchy AA and five have alopecia totalis or universalis. Tofacitinib is approved for treating rheumatoid arthritis at a dose of 5 mg twice daily, but patients have needed up to 10 mg twice daily to achieve hair regrowth, Dr. Mackay-Wiggan said. To date, 11 (92%) have achieved at least some hair regrowth, and 8 (67%) have achieved at least 50% regrowth. So far, there have been no serious adverse events over 6 to 16 months of treatment, although one patient stopped treatment after developing hypertension, a known adverse effect of tofacitinib.
In this study, heatmaps of RNA sequencing of CD8+ T cell populations clearly showed pathogenic signatures for AA and a “robust molecular response to treatment,” Dr. Mackay-Wiggan said. “These two signatures also overlapped statistically, producing 114 genes that may be targetable mediators of disease.” But as with ruxolitinib, regrowth started to decline as patients were taken off treatment.
Research indicates that inhibiting the JAK-STAT signaling pathway induces anagen and subsequent hair growth, but activating STAT 5 in the dermal papilla is also important to induce the growth phase of the hair follicle, according to Dr. Mackay-Wiggan. “Bottom line, it’s complicated,” she added. “The mode of delivery – topical versus systemic – may be important, and the timing of delivery may be crucial.”
Other studies point to a role for JAK inhibition in treating AA. In an uncontrolled, retrospective study of 90 adults with alopecia totalis, alopecia universalis, or moderate to severe AA, 58% had SALT scores of 50% or better after receiving 5 mg tofacitinib twice daily for 4 to 18 months. Patients with AA improved more than those with alopecia totalis or universalis. There were no severe adverse effects, although nearly a third of patients developed upper respiratory tract infections. In another uncontrolled study of 13 patients with AA, totalis, or universalis, 9 (70%) patients achieved full regrowth and there were no serious adverse effects, although patients experienced headaches, upper respiratory infections, and mild increases in liver transaminase levels.
JAK inhibition also has a potential role for treating some scarring alopecias, including lichen planopilaris and frontal fibrosing alopecia. These diseases are histologically “identical” and both exhibit perifollicular erythema, papules, and scale, all of which suggest active inflammation, Dr. Mackay-Wiggan said. Hair follicles from affected patients show immune markers such as interferon-inducible chemokines, cytotoxic T cell responses, and expression of major histocompatibility complexes I and II. “The important message here is that JAK/STAT signaling may play a significant role in other types of hair loss other than alopecia areata,” Dr. Mackay-Wiggan said. “These diseases may also be autoimmune diseases, and may also be treatable with JAK inhibitors.”
Studies continue to evaluate JAK inhibitors for treating alopecia and its variants. Investigators at Yale and Stanford are conducting three uncontrolled trials of oral or topical tofacitinib, while Incyte, the manufacturer of ruxolitinib, is sponsoring a multicenter, randomized, placebo-controlled trial of ruxolitinib phosphate cream for adults with AA, with topline results expected in May 2018. Concert Pharmaceuticals also is recruiting for a trial of a modified, investigational form of ruxolitinib called CTP-543 for treating moderate to severe AA. “Many more trials are in development,” Dr. Mackay-Wiggan noted.
The ruxolitinib pilot study was funded by the Locks of Love Foundation, the Alopecia Areata Initiative, NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and by an Irving Institute for Clinical and Translational Research/Columbia University Medical Center Clinical and Translational Science Award. The ongoing tofacitinib pilot study is sponsored by Dr. Mackay-Wiggan, Locks of Love, and Columbia University.
Dr. Mackay-Wiggan also acknowledged support from the Alopecia Areata Initiative – the Gates Foundation, the National Alopecia Areata Registry, and the National Alopecia Areata Foundation. She had no other relevant financial disclosures.
PORTLAND, ORE. – Janus kinase inhibitors are relatively safe and can produce a full head of hair in patients with moderate to severe alopecia areata (AA), although patients tend to shed hair after stopping treatment, Julian Mackay-Wiggan, MD, said at the annual meeting of the Society for Investigative Dermatology.
“At this point, there are 17 publications in the literature, from clinical trials to case reports, looking at JAK [Janus kinase] inhibitors in patients with alopecia areata,” said Dr. Mackay-Wiggan of the department of dermatology, Columbia University, New York, where she specializes in hair disorders. “Pretty much all report very positive findings. It definitely appears that Janus kinase inhibitors can play a very significant role in treatment.”
In an open label, uncontrolled pilot study at Columbia, 9 of 12 (75%) patients with moderate to severe AA improved by at least 50% on the Severity of Alopecia Tool (SALT) after receiving 20 mg ruxolitinib twice daily for 3 to 6 months (JCI Insight. 2016 Sep 22;1[15]:e89790). Responses started with the first month, and all but one responder achieved at least 50% hair regrowth by week 12, said Dr. Mackay-Wiggan, who is also the director of the Dermatology Clinical Research Unit at Columbia.
By the end of treatment, seven of nine responders achieved more than 95% regrowth, one achieved 85% regrowth, and one achieved 55% regrowth. Importantly, none of these relatively healthy patients experienced serious adverse events on ruxolitinib, and none needed to stop treatment, although one patient experienced declining hemoglobin levels that resolved after dose modification.
Columbia researchers are also conducting an uncontrolled, open label pilot trial of the JAK inhibitor tofacitinib (Xeljanz) in 12 patients, of whom seven have moderate to severe patchy AA and five have alopecia totalis or universalis. Tofacitinib is approved for treating rheumatoid arthritis at a dose of 5 mg twice daily, but patients have needed up to 10 mg twice daily to achieve hair regrowth, Dr. Mackay-Wiggan said. To date, 11 (92%) have achieved at least some hair regrowth, and 8 (67%) have achieved at least 50% regrowth. So far, there have been no serious adverse events over 6 to 16 months of treatment, although one patient stopped treatment after developing hypertension, a known adverse effect of tofacitinib.
In this study, heatmaps of RNA sequencing of CD8+ T cell populations clearly showed pathogenic signatures for AA and a “robust molecular response to treatment,” Dr. Mackay-Wiggan said. “These two signatures also overlapped statistically, producing 114 genes that may be targetable mediators of disease.” But as with ruxolitinib, regrowth started to decline as patients were taken off treatment.
Research indicates that inhibiting the JAK-STAT signaling pathway induces anagen and subsequent hair growth, but activating STAT 5 in the dermal papilla is also important to induce the growth phase of the hair follicle, according to Dr. Mackay-Wiggan. “Bottom line, it’s complicated,” she added. “The mode of delivery – topical versus systemic – may be important, and the timing of delivery may be crucial.”
Other studies point to a role for JAK inhibition in treating AA. In an uncontrolled, retrospective study of 90 adults with alopecia totalis, alopecia universalis, or moderate to severe AA, 58% had SALT scores of 50% or better after receiving 5 mg tofacitinib twice daily for 4 to 18 months. Patients with AA improved more than those with alopecia totalis or universalis. There were no severe adverse effects, although nearly a third of patients developed upper respiratory tract infections. In another uncontrolled study of 13 patients with AA, totalis, or universalis, 9 (70%) patients achieved full regrowth and there were no serious adverse effects, although patients experienced headaches, upper respiratory infections, and mild increases in liver transaminase levels.
JAK inhibition also has a potential role for treating some scarring alopecias, including lichen planopilaris and frontal fibrosing alopecia. These diseases are histologically “identical” and both exhibit perifollicular erythema, papules, and scale, all of which suggest active inflammation, Dr. Mackay-Wiggan said. Hair follicles from affected patients show immune markers such as interferon-inducible chemokines, cytotoxic T cell responses, and expression of major histocompatibility complexes I and II. “The important message here is that JAK/STAT signaling may play a significant role in other types of hair loss other than alopecia areata,” Dr. Mackay-Wiggan said. “These diseases may also be autoimmune diseases, and may also be treatable with JAK inhibitors.”
Studies continue to evaluate JAK inhibitors for treating alopecia and its variants. Investigators at Yale and Stanford are conducting three uncontrolled trials of oral or topical tofacitinib, while Incyte, the manufacturer of ruxolitinib, is sponsoring a multicenter, randomized, placebo-controlled trial of ruxolitinib phosphate cream for adults with AA, with topline results expected in May 2018. Concert Pharmaceuticals also is recruiting for a trial of a modified, investigational form of ruxolitinib called CTP-543 for treating moderate to severe AA. “Many more trials are in development,” Dr. Mackay-Wiggan noted.
The ruxolitinib pilot study was funded by the Locks of Love Foundation, the Alopecia Areata Initiative, NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and by an Irving Institute for Clinical and Translational Research/Columbia University Medical Center Clinical and Translational Science Award. The ongoing tofacitinib pilot study is sponsored by Dr. Mackay-Wiggan, Locks of Love, and Columbia University.
Dr. Mackay-Wiggan also acknowledged support from the Alopecia Areata Initiative – the Gates Foundation, the National Alopecia Areata Registry, and the National Alopecia Areata Foundation. She had no other relevant financial disclosures.
PORTLAND, ORE. – Janus kinase inhibitors are relatively safe and can produce a full head of hair in patients with moderate to severe alopecia areata (AA), although patients tend to shed hair after stopping treatment, Julian Mackay-Wiggan, MD, said at the annual meeting of the Society for Investigative Dermatology.
“At this point, there are 17 publications in the literature, from clinical trials to case reports, looking at JAK [Janus kinase] inhibitors in patients with alopecia areata,” said Dr. Mackay-Wiggan of the department of dermatology, Columbia University, New York, where she specializes in hair disorders. “Pretty much all report very positive findings. It definitely appears that Janus kinase inhibitors can play a very significant role in treatment.”
In an open label, uncontrolled pilot study at Columbia, 9 of 12 (75%) patients with moderate to severe AA improved by at least 50% on the Severity of Alopecia Tool (SALT) after receiving 20 mg ruxolitinib twice daily for 3 to 6 months (JCI Insight. 2016 Sep 22;1[15]:e89790). Responses started with the first month, and all but one responder achieved at least 50% hair regrowth by week 12, said Dr. Mackay-Wiggan, who is also the director of the Dermatology Clinical Research Unit at Columbia.
By the end of treatment, seven of nine responders achieved more than 95% regrowth, one achieved 85% regrowth, and one achieved 55% regrowth. Importantly, none of these relatively healthy patients experienced serious adverse events on ruxolitinib, and none needed to stop treatment, although one patient experienced declining hemoglobin levels that resolved after dose modification.
Columbia researchers are also conducting an uncontrolled, open label pilot trial of the JAK inhibitor tofacitinib (Xeljanz) in 12 patients, of whom seven have moderate to severe patchy AA and five have alopecia totalis or universalis. Tofacitinib is approved for treating rheumatoid arthritis at a dose of 5 mg twice daily, but patients have needed up to 10 mg twice daily to achieve hair regrowth, Dr. Mackay-Wiggan said. To date, 11 (92%) have achieved at least some hair regrowth, and 8 (67%) have achieved at least 50% regrowth. So far, there have been no serious adverse events over 6 to 16 months of treatment, although one patient stopped treatment after developing hypertension, a known adverse effect of tofacitinib.
In this study, heatmaps of RNA sequencing of CD8+ T cell populations clearly showed pathogenic signatures for AA and a “robust molecular response to treatment,” Dr. Mackay-Wiggan said. “These two signatures also overlapped statistically, producing 114 genes that may be targetable mediators of disease.” But as with ruxolitinib, regrowth started to decline as patients were taken off treatment.
Research indicates that inhibiting the JAK-STAT signaling pathway induces anagen and subsequent hair growth, but activating STAT 5 in the dermal papilla is also important to induce the growth phase of the hair follicle, according to Dr. Mackay-Wiggan. “Bottom line, it’s complicated,” she added. “The mode of delivery – topical versus systemic – may be important, and the timing of delivery may be crucial.”
Other studies point to a role for JAK inhibition in treating AA. In an uncontrolled, retrospective study of 90 adults with alopecia totalis, alopecia universalis, or moderate to severe AA, 58% had SALT scores of 50% or better after receiving 5 mg tofacitinib twice daily for 4 to 18 months. Patients with AA improved more than those with alopecia totalis or universalis. There were no severe adverse effects, although nearly a third of patients developed upper respiratory tract infections. In another uncontrolled study of 13 patients with AA, totalis, or universalis, 9 (70%) patients achieved full regrowth and there were no serious adverse effects, although patients experienced headaches, upper respiratory infections, and mild increases in liver transaminase levels.
JAK inhibition also has a potential role for treating some scarring alopecias, including lichen planopilaris and frontal fibrosing alopecia. These diseases are histologically “identical” and both exhibit perifollicular erythema, papules, and scale, all of which suggest active inflammation, Dr. Mackay-Wiggan said. Hair follicles from affected patients show immune markers such as interferon-inducible chemokines, cytotoxic T cell responses, and expression of major histocompatibility complexes I and II. “The important message here is that JAK/STAT signaling may play a significant role in other types of hair loss other than alopecia areata,” Dr. Mackay-Wiggan said. “These diseases may also be autoimmune diseases, and may also be treatable with JAK inhibitors.”
Studies continue to evaluate JAK inhibitors for treating alopecia and its variants. Investigators at Yale and Stanford are conducting three uncontrolled trials of oral or topical tofacitinib, while Incyte, the manufacturer of ruxolitinib, is sponsoring a multicenter, randomized, placebo-controlled trial of ruxolitinib phosphate cream for adults with AA, with topline results expected in May 2018. Concert Pharmaceuticals also is recruiting for a trial of a modified, investigational form of ruxolitinib called CTP-543 for treating moderate to severe AA. “Many more trials are in development,” Dr. Mackay-Wiggan noted.
The ruxolitinib pilot study was funded by the Locks of Love Foundation, the Alopecia Areata Initiative, NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and by an Irving Institute for Clinical and Translational Research/Columbia University Medical Center Clinical and Translational Science Award. The ongoing tofacitinib pilot study is sponsored by Dr. Mackay-Wiggan, Locks of Love, and Columbia University.
Dr. Mackay-Wiggan also acknowledged support from the Alopecia Areata Initiative – the Gates Foundation, the National Alopecia Areata Registry, and the National Alopecia Areata Foundation. She had no other relevant financial disclosures.
AT SID 2017
TNFSF13B variant linked to MS and SLE
A variant in the TNFSF13B gene has been linked to susceptibility to both multiple sclerosis and systemic lupus erythematosus, according to a report published online April 26 in the New England Journal of Medicine.
This gene encodes the cytokine B-cell activating factor (BAFF), which is essential for B-cell activation, differentiation, and survival. BAFF is targeted by agents such as belimumab that are used in the treatment of autoimmune disorders, and is primarily produced by monocytes and neutrophils, said Maristella Steri, PhD, of Istituto di Ricerca Genetica e Biomedica, Consiglio Nazionale delle Ricerche Monserrato (Italy), and her associates.
The researchers have named this TNFSF13B variation “BAFF-var.”
Previous genome-wide association studies have identified more than 110 independent signals for MS and 43 for SLE but have not yet delineated the effector mechanisms for most of these associations. To explore these mechanisms in greater detail, Dr. Steri and her associates studied the population in Sardinia, Italy, which has the highest prevalences of MS and SLE in the world.
They performed genome-wide association studies and other genetic investigations in case-control sets of 2,934 patients with MS, 411 with SLE, and 3,392 control subjects, analyzing roughly 12.2 million single-nucleotide polymorphisms (SNPs). They ruled out rs1287404 as a likely variant driving the association and identified BAFF-var as the variant most strongly associated with MS (odds ratio, 1.27).
The investigators then replicated their findings in a series of genetic studies in case-control sets from mainland Italy (2,292 patients with MS, 503 with SLE, and 2,563 controls), Sweden (4,548 patients with MS and 3,481 controls), the United Kingdom (3,176 patients with MS and 2,958 controls), and the Iberian peninsula (1,120 patients with SLE and 1,300 controls). BAFF-var was most common across Sardinia, with a frequency of 26.5%, and became progressively less common moving northward (5.7% in Italy, 4.9% in Spain, 1.8% in the United Kingdom and Sweden).
Taken together, “these findings pinpoint BAFF-var as the variant in TNFSF13B that is most strongly associated with MS,” wrote Dr. Steri and her associates (N Engl J Med. 2017 Apr 27. doi: 10.1056/NEJMoa1610528).
BAFF-var also proved to be associated with SLE in case-control sets from Sardinia (OR, 1.38), mainland Italy (OR, 1.49), and the Iberian peninsula (OR, 1.55). This indicates that “the effect of BAFF-var is not restricted to MS alone,” they noted.
Further analyses showed that BAFF-var “dramatically” increased levels of soluble BAFF and circulating B cells, especially CD24+CD27+ cells, as well as total IgG, IgA, IgM, and monocytes. In one notable analysis, preclinical blood samples taken from Sardinians participating in a longitudinal study showed elevated levels of soluble BAFF in people who did not go on to develop MS until years later.
“We infer [from this] that BAFF-var is the causal variant driving an increase in soluble BAFF and a cascade of immune effects leading to increased autoimmunity risk,” Dr. Steri and her associates said.
Further study suggested that positive selection specifically favoring BAFF-var, not random genetic drift, accounted for the high frequency of this mutation in Sardinia and its progressively lower frequency moving northward. The most likely possibility is that BAFF-var was positively selected because it provided resistance to malaria, which was “strikingly prevalent” in Sardinia until it was eradicated in the 1950s. In mouse models, BAFF overexpression confers protection against lethal malaria.
“In addition, as shown here, BAFF-var increases antibody production, and classic findings showed that antibody transfer from adults with immunity to malaria to acutely infected children reduced blood-stage parasitemia and disease severity,” the investigators said.
“The evolutionary scenario we propose is that BAFF-var was selected as an adaptive response to malaria infection, resulting in an increased present-day risk of autoimmunity,” they said.
The Italian Foundation for Multiple Sclerosis, the National Institute on Aging, the Italian Ministry of Economy and Finance, the European Union, the National Human Genome Research Institute, and other organizations supported the study. Dr. Steri reported having no relevant disclosures; some of her associates reported ties to numerous industry sources.
Perhaps the next challenge is the clinical application of the findings from Steri et al. is to determine whether BAFF-var status can be used to stratify patients for a specific therapy.
These data clearly point in that direction, but the discriminatory power of this single variant may not be sufficient for clinical decision making.
In contrast, it does seem reasonable to examine whether stratifying patients according to their BAFF-var status would be useful in clinical trials assessing B-cell–directed therapies.
Thomas Korn, MD, of the Technical University of Munich and the Munich Cluster for Systems Neurology, reported ties to Biogen, Novartis, Merck Serono, and Bayer. Mohamed Oukka, PhD, of the University of Washington, Seattle, and the Center for Immunity and Immunotherapies at Seattle Children’s Research Institute, reported having no relevant disclosures. They made these remarks in an editorial accompanying Dr. Steri’s report (N Engl J Med. 2017 Apr 27. doi: 10.1056/NEJMe1700720).
Perhaps the next challenge is the clinical application of the findings from Steri et al. is to determine whether BAFF-var status can be used to stratify patients for a specific therapy.
These data clearly point in that direction, but the discriminatory power of this single variant may not be sufficient for clinical decision making.
In contrast, it does seem reasonable to examine whether stratifying patients according to their BAFF-var status would be useful in clinical trials assessing B-cell–directed therapies.
Thomas Korn, MD, of the Technical University of Munich and the Munich Cluster for Systems Neurology, reported ties to Biogen, Novartis, Merck Serono, and Bayer. Mohamed Oukka, PhD, of the University of Washington, Seattle, and the Center for Immunity and Immunotherapies at Seattle Children’s Research Institute, reported having no relevant disclosures. They made these remarks in an editorial accompanying Dr. Steri’s report (N Engl J Med. 2017 Apr 27. doi: 10.1056/NEJMe1700720).
Perhaps the next challenge is the clinical application of the findings from Steri et al. is to determine whether BAFF-var status can be used to stratify patients for a specific therapy.
These data clearly point in that direction, but the discriminatory power of this single variant may not be sufficient for clinical decision making.
In contrast, it does seem reasonable to examine whether stratifying patients according to their BAFF-var status would be useful in clinical trials assessing B-cell–directed therapies.
Thomas Korn, MD, of the Technical University of Munich and the Munich Cluster for Systems Neurology, reported ties to Biogen, Novartis, Merck Serono, and Bayer. Mohamed Oukka, PhD, of the University of Washington, Seattle, and the Center for Immunity and Immunotherapies at Seattle Children’s Research Institute, reported having no relevant disclosures. They made these remarks in an editorial accompanying Dr. Steri’s report (N Engl J Med. 2017 Apr 27. doi: 10.1056/NEJMe1700720).
A variant in the TNFSF13B gene has been linked to susceptibility to both multiple sclerosis and systemic lupus erythematosus, according to a report published online April 26 in the New England Journal of Medicine.
This gene encodes the cytokine B-cell activating factor (BAFF), which is essential for B-cell activation, differentiation, and survival. BAFF is targeted by agents such as belimumab that are used in the treatment of autoimmune disorders, and is primarily produced by monocytes and neutrophils, said Maristella Steri, PhD, of Istituto di Ricerca Genetica e Biomedica, Consiglio Nazionale delle Ricerche Monserrato (Italy), and her associates.
The researchers have named this TNFSF13B variation “BAFF-var.”
Previous genome-wide association studies have identified more than 110 independent signals for MS and 43 for SLE but have not yet delineated the effector mechanisms for most of these associations. To explore these mechanisms in greater detail, Dr. Steri and her associates studied the population in Sardinia, Italy, which has the highest prevalences of MS and SLE in the world.
They performed genome-wide association studies and other genetic investigations in case-control sets of 2,934 patients with MS, 411 with SLE, and 3,392 control subjects, analyzing roughly 12.2 million single-nucleotide polymorphisms (SNPs). They ruled out rs1287404 as a likely variant driving the association and identified BAFF-var as the variant most strongly associated with MS (odds ratio, 1.27).
The investigators then replicated their findings in a series of genetic studies in case-control sets from mainland Italy (2,292 patients with MS, 503 with SLE, and 2,563 controls), Sweden (4,548 patients with MS and 3,481 controls), the United Kingdom (3,176 patients with MS and 2,958 controls), and the Iberian peninsula (1,120 patients with SLE and 1,300 controls). BAFF-var was most common across Sardinia, with a frequency of 26.5%, and became progressively less common moving northward (5.7% in Italy, 4.9% in Spain, 1.8% in the United Kingdom and Sweden).
Taken together, “these findings pinpoint BAFF-var as the variant in TNFSF13B that is most strongly associated with MS,” wrote Dr. Steri and her associates (N Engl J Med. 2017 Apr 27. doi: 10.1056/NEJMoa1610528).
BAFF-var also proved to be associated with SLE in case-control sets from Sardinia (OR, 1.38), mainland Italy (OR, 1.49), and the Iberian peninsula (OR, 1.55). This indicates that “the effect of BAFF-var is not restricted to MS alone,” they noted.
Further analyses showed that BAFF-var “dramatically” increased levels of soluble BAFF and circulating B cells, especially CD24+CD27+ cells, as well as total IgG, IgA, IgM, and monocytes. In one notable analysis, preclinical blood samples taken from Sardinians participating in a longitudinal study showed elevated levels of soluble BAFF in people who did not go on to develop MS until years later.
“We infer [from this] that BAFF-var is the causal variant driving an increase in soluble BAFF and a cascade of immune effects leading to increased autoimmunity risk,” Dr. Steri and her associates said.
Further study suggested that positive selection specifically favoring BAFF-var, not random genetic drift, accounted for the high frequency of this mutation in Sardinia and its progressively lower frequency moving northward. The most likely possibility is that BAFF-var was positively selected because it provided resistance to malaria, which was “strikingly prevalent” in Sardinia until it was eradicated in the 1950s. In mouse models, BAFF overexpression confers protection against lethal malaria.
“In addition, as shown here, BAFF-var increases antibody production, and classic findings showed that antibody transfer from adults with immunity to malaria to acutely infected children reduced blood-stage parasitemia and disease severity,” the investigators said.
“The evolutionary scenario we propose is that BAFF-var was selected as an adaptive response to malaria infection, resulting in an increased present-day risk of autoimmunity,” they said.
The Italian Foundation for Multiple Sclerosis, the National Institute on Aging, the Italian Ministry of Economy and Finance, the European Union, the National Human Genome Research Institute, and other organizations supported the study. Dr. Steri reported having no relevant disclosures; some of her associates reported ties to numerous industry sources.
A variant in the TNFSF13B gene has been linked to susceptibility to both multiple sclerosis and systemic lupus erythematosus, according to a report published online April 26 in the New England Journal of Medicine.
This gene encodes the cytokine B-cell activating factor (BAFF), which is essential for B-cell activation, differentiation, and survival. BAFF is targeted by agents such as belimumab that are used in the treatment of autoimmune disorders, and is primarily produced by monocytes and neutrophils, said Maristella Steri, PhD, of Istituto di Ricerca Genetica e Biomedica, Consiglio Nazionale delle Ricerche Monserrato (Italy), and her associates.
The researchers have named this TNFSF13B variation “BAFF-var.”
Previous genome-wide association studies have identified more than 110 independent signals for MS and 43 for SLE but have not yet delineated the effector mechanisms for most of these associations. To explore these mechanisms in greater detail, Dr. Steri and her associates studied the population in Sardinia, Italy, which has the highest prevalences of MS and SLE in the world.
They performed genome-wide association studies and other genetic investigations in case-control sets of 2,934 patients with MS, 411 with SLE, and 3,392 control subjects, analyzing roughly 12.2 million single-nucleotide polymorphisms (SNPs). They ruled out rs1287404 as a likely variant driving the association and identified BAFF-var as the variant most strongly associated with MS (odds ratio, 1.27).
The investigators then replicated their findings in a series of genetic studies in case-control sets from mainland Italy (2,292 patients with MS, 503 with SLE, and 2,563 controls), Sweden (4,548 patients with MS and 3,481 controls), the United Kingdom (3,176 patients with MS and 2,958 controls), and the Iberian peninsula (1,120 patients with SLE and 1,300 controls). BAFF-var was most common across Sardinia, with a frequency of 26.5%, and became progressively less common moving northward (5.7% in Italy, 4.9% in Spain, 1.8% in the United Kingdom and Sweden).
Taken together, “these findings pinpoint BAFF-var as the variant in TNFSF13B that is most strongly associated with MS,” wrote Dr. Steri and her associates (N Engl J Med. 2017 Apr 27. doi: 10.1056/NEJMoa1610528).
BAFF-var also proved to be associated with SLE in case-control sets from Sardinia (OR, 1.38), mainland Italy (OR, 1.49), and the Iberian peninsula (OR, 1.55). This indicates that “the effect of BAFF-var is not restricted to MS alone,” they noted.
Further analyses showed that BAFF-var “dramatically” increased levels of soluble BAFF and circulating B cells, especially CD24+CD27+ cells, as well as total IgG, IgA, IgM, and monocytes. In one notable analysis, preclinical blood samples taken from Sardinians participating in a longitudinal study showed elevated levels of soluble BAFF in people who did not go on to develop MS until years later.
“We infer [from this] that BAFF-var is the causal variant driving an increase in soluble BAFF and a cascade of immune effects leading to increased autoimmunity risk,” Dr. Steri and her associates said.
Further study suggested that positive selection specifically favoring BAFF-var, not random genetic drift, accounted for the high frequency of this mutation in Sardinia and its progressively lower frequency moving northward. The most likely possibility is that BAFF-var was positively selected because it provided resistance to malaria, which was “strikingly prevalent” in Sardinia until it was eradicated in the 1950s. In mouse models, BAFF overexpression confers protection against lethal malaria.
“In addition, as shown here, BAFF-var increases antibody production, and classic findings showed that antibody transfer from adults with immunity to malaria to acutely infected children reduced blood-stage parasitemia and disease severity,” the investigators said.
“The evolutionary scenario we propose is that BAFF-var was selected as an adaptive response to malaria infection, resulting in an increased present-day risk of autoimmunity,” they said.
The Italian Foundation for Multiple Sclerosis, the National Institute on Aging, the Italian Ministry of Economy and Finance, the European Union, the National Human Genome Research Institute, and other organizations supported the study. Dr. Steri reported having no relevant disclosures; some of her associates reported ties to numerous industry sources.
Key clinical point: A variant in the TNFSF13B gene has been linked to susceptibility to both multiple sclerosis and systemic lupus erythematosus.
Major finding: BAFF-var was the TNFSF13B variant most strongly associated with MS in Sardinia (OR, 1.27), and was also associated with SLE in Sardinia (OR, 1.38), mainland Italy (OR, 1.49), and the Iberian peninsula (OR, 1.55).
Data source: A series of genome-wide association studies and other genetic studies involving thousands of patients with MS or SLE in Sardinia and confirmed in thousands of patients across Italy, Spain, Sweden, and the United Kingdom.
Disclosures: The Italian Foundation for Multiple Sclerosis, the National Institute on Aging, the Italian Ministry of Economy and Finance, the European Union, the National Human Genome Research Institute, and other organizations supported the study. Dr. Steri reported having no relevant disclosures; some of her associates reported ties to numerous industry sources.
FDA grants breakthrough therapy status to rituximab for pemphigus vulgaris
The Food and Drug Administration has granted breakthrough therapy status to rituximab (Rituxan) for treating pemphigus vulgaris, according to the manufacturer.
Rituximab, a CD20-directed cytolytic antibody approved in 1997, is currently in a phase III study evaluating its efficacy for the pemphigus indication. It is approved in the United States for treating non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (with methotrexate), granulomatosis with polyangiitis (Wegener’s granulomatosis), and microscopic polyangiitis (with glucocorticoids).
The patients, who were experiencing their first episode of pemphigus vulgaris, were randomized to daily oral prednisone, tapered over a 12- to 18-month period, or rituximab administered intravenously (at days 0 and 14, and months 12 and 18), plus daily oral prednisone, tapered over 3 or 6 months. At 2 years, when they were no longer on therapy, 89% of those treated with rituximab and prednisone were in complete remission, compared with 34% of those treated with prednisone alone (P less than .0001).
The breakthrough therapy process is “designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s),” according to the FDA.
The study was supported by the French Ministry of Health, the French Society of Dermatology, and Roche, which owns Genentech. Genentech markets rituximab in the United States with Biogen and is conducting the phase III study.
The Food and Drug Administration has granted breakthrough therapy status to rituximab (Rituxan) for treating pemphigus vulgaris, according to the manufacturer.
Rituximab, a CD20-directed cytolytic antibody approved in 1997, is currently in a phase III study evaluating its efficacy for the pemphigus indication. It is approved in the United States for treating non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (with methotrexate), granulomatosis with polyangiitis (Wegener’s granulomatosis), and microscopic polyangiitis (with glucocorticoids).
The patients, who were experiencing their first episode of pemphigus vulgaris, were randomized to daily oral prednisone, tapered over a 12- to 18-month period, or rituximab administered intravenously (at days 0 and 14, and months 12 and 18), plus daily oral prednisone, tapered over 3 or 6 months. At 2 years, when they were no longer on therapy, 89% of those treated with rituximab and prednisone were in complete remission, compared with 34% of those treated with prednisone alone (P less than .0001).
The breakthrough therapy process is “designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s),” according to the FDA.
The study was supported by the French Ministry of Health, the French Society of Dermatology, and Roche, which owns Genentech. Genentech markets rituximab in the United States with Biogen and is conducting the phase III study.
The Food and Drug Administration has granted breakthrough therapy status to rituximab (Rituxan) for treating pemphigus vulgaris, according to the manufacturer.
Rituximab, a CD20-directed cytolytic antibody approved in 1997, is currently in a phase III study evaluating its efficacy for the pemphigus indication. It is approved in the United States for treating non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (with methotrexate), granulomatosis with polyangiitis (Wegener’s granulomatosis), and microscopic polyangiitis (with glucocorticoids).
The patients, who were experiencing their first episode of pemphigus vulgaris, were randomized to daily oral prednisone, tapered over a 12- to 18-month period, or rituximab administered intravenously (at days 0 and 14, and months 12 and 18), plus daily oral prednisone, tapered over 3 or 6 months. At 2 years, when they were no longer on therapy, 89% of those treated with rituximab and prednisone were in complete remission, compared with 34% of those treated with prednisone alone (P less than .0001).
The breakthrough therapy process is “designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s),” according to the FDA.
The study was supported by the French Ministry of Health, the French Society of Dermatology, and Roche, which owns Genentech. Genentech markets rituximab in the United States with Biogen and is conducting the phase III study.
Tree nut allergy responds to immunotherapy
AT 2017 AAAAI ANNUAL MEETING
ATLANTA – Long-term walnut oral immunotherapy induces clinically relevant treatment response in children with tree nut allergy, results from a small ongoing study showed.
“Tree nut allergy is a generally life-long and potentially life-threatening disorder without an active therapy,” study author Amy M. Scurlock, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Significant clinical, immunologic, and serologic cross reactivity has been described among tree nut families. Multi–tree nut allergen sensitization is common with 46% reporting allergy to more than one tree nut.”
Dr. Scurlock reported on results from 9 of 14 children with allergy to walnuts and another test tree nut (pecans, cashews, hazelnuts, or pistachios) who received open-label walnut oral immunotherapy after completing 38 weeks of blinded, placebo-controlled treatment. Walnut and test tree nut desensitization oral food challenges were performed by 142 weeks. If they passed, the subjects stopped their treatment for four weeks. Next, they underwent another oral food challenge to determine if they continued to be sensitized or if they had developed sustained unresponsiveness.
The median age of the 14 randomized subjects was 9 years, 75% were male, and 9 (64%) underwent an oral food challenge by week 142. (Two subjects dropped out after randomization, and three have yet to reach the week 142 time point.) Desensitization to both walnut and a test tree nut was observed in seven out of the nine subjects (78%). After 4 weeks off of walnut oral immunotherapy, four out of those seven patients who were desensitized (57%) also demonstrated sustained unresponsiveness to both walnuts and test tree nuts, and six out of seven subjects (86%) had sustained unresponsiveness to just walnuts.
“I am always amazed by the commitment of our food allergic subjects and their families in immunotherapy trials, and this study is no exception,” Dr. Scurlock commented. “Subjects had to undergo an increased number of oral food challenges (walnut, test tree nut, placebo) at protocol-specified time points in addition to daily home dosing. While walnut oral immunotherapy was generally well tolerated, we frequently observed oral allergy/itching associated with dosing in our cohort, which was an atopic group (75% allergic rhinitis). These symptoms can complicate assessment during dosing and oral food challenges. We observed that, with long-term therapy, there were some subjects who developed ‘dosing fatigue’ that could adversely affect adherence. Future studies will need to focus on strategies that optimize long-term sustainability/tolerability of dosing.”
She acknowledged certain limitations of the study, including its single-center design and small sample size. “While the findings are encouraging and similar to outcomes observed in other oral immunotherapy trials, further study in larger cohorts is critical before advancing toward broad clinical implementation. Specific issues regarding complexity of cross-reactivity and the efficacy of specific tree nuts to induce immunomodulation across tree nut families require future study. In addition, improving the long-term sustainability/tolerability of dosing and examining novel approaches is important.”
Dr. Scurlock disclosed that she has received funding from National Institutes of Health/National Institute of Allergy and Infectious Diseases and Food Allergy Research and Education (FARE). She is also medical director for the FARE Clinical Network Center of Excellence at Arkansas Children’s Hospital.
AT 2017 AAAAI ANNUAL MEETING
ATLANTA – Long-term walnut oral immunotherapy induces clinically relevant treatment response in children with tree nut allergy, results from a small ongoing study showed.
“Tree nut allergy is a generally life-long and potentially life-threatening disorder without an active therapy,” study author Amy M. Scurlock, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Significant clinical, immunologic, and serologic cross reactivity has been described among tree nut families. Multi–tree nut allergen sensitization is common with 46% reporting allergy to more than one tree nut.”
Dr. Scurlock reported on results from 9 of 14 children with allergy to walnuts and another test tree nut (pecans, cashews, hazelnuts, or pistachios) who received open-label walnut oral immunotherapy after completing 38 weeks of blinded, placebo-controlled treatment. Walnut and test tree nut desensitization oral food challenges were performed by 142 weeks. If they passed, the subjects stopped their treatment for four weeks. Next, they underwent another oral food challenge to determine if they continued to be sensitized or if they had developed sustained unresponsiveness.
The median age of the 14 randomized subjects was 9 years, 75% were male, and 9 (64%) underwent an oral food challenge by week 142. (Two subjects dropped out after randomization, and three have yet to reach the week 142 time point.) Desensitization to both walnut and a test tree nut was observed in seven out of the nine subjects (78%). After 4 weeks off of walnut oral immunotherapy, four out of those seven patients who were desensitized (57%) also demonstrated sustained unresponsiveness to both walnuts and test tree nuts, and six out of seven subjects (86%) had sustained unresponsiveness to just walnuts.
“I am always amazed by the commitment of our food allergic subjects and their families in immunotherapy trials, and this study is no exception,” Dr. Scurlock commented. “Subjects had to undergo an increased number of oral food challenges (walnut, test tree nut, placebo) at protocol-specified time points in addition to daily home dosing. While walnut oral immunotherapy was generally well tolerated, we frequently observed oral allergy/itching associated with dosing in our cohort, which was an atopic group (75% allergic rhinitis). These symptoms can complicate assessment during dosing and oral food challenges. We observed that, with long-term therapy, there were some subjects who developed ‘dosing fatigue’ that could adversely affect adherence. Future studies will need to focus on strategies that optimize long-term sustainability/tolerability of dosing.”
She acknowledged certain limitations of the study, including its single-center design and small sample size. “While the findings are encouraging and similar to outcomes observed in other oral immunotherapy trials, further study in larger cohorts is critical before advancing toward broad clinical implementation. Specific issues regarding complexity of cross-reactivity and the efficacy of specific tree nuts to induce immunomodulation across tree nut families require future study. In addition, improving the long-term sustainability/tolerability of dosing and examining novel approaches is important.”
Dr. Scurlock disclosed that she has received funding from National Institutes of Health/National Institute of Allergy and Infectious Diseases and Food Allergy Research and Education (FARE). She is also medical director for the FARE Clinical Network Center of Excellence at Arkansas Children’s Hospital.
AT 2017 AAAAI ANNUAL MEETING
ATLANTA – Long-term walnut oral immunotherapy induces clinically relevant treatment response in children with tree nut allergy, results from a small ongoing study showed.
“Tree nut allergy is a generally life-long and potentially life-threatening disorder without an active therapy,” study author Amy M. Scurlock, MD, said in an interview in advance of the annual meeting of the American Academy of Allergy, Asthma, and Immunology. “Significant clinical, immunologic, and serologic cross reactivity has been described among tree nut families. Multi–tree nut allergen sensitization is common with 46% reporting allergy to more than one tree nut.”
Dr. Scurlock reported on results from 9 of 14 children with allergy to walnuts and another test tree nut (pecans, cashews, hazelnuts, or pistachios) who received open-label walnut oral immunotherapy after completing 38 weeks of blinded, placebo-controlled treatment. Walnut and test tree nut desensitization oral food challenges were performed by 142 weeks. If they passed, the subjects stopped their treatment for four weeks. Next, they underwent another oral food challenge to determine if they continued to be sensitized or if they had developed sustained unresponsiveness.
The median age of the 14 randomized subjects was 9 years, 75% were male, and 9 (64%) underwent an oral food challenge by week 142. (Two subjects dropped out after randomization, and three have yet to reach the week 142 time point.) Desensitization to both walnut and a test tree nut was observed in seven out of the nine subjects (78%). After 4 weeks off of walnut oral immunotherapy, four out of those seven patients who were desensitized (57%) also demonstrated sustained unresponsiveness to both walnuts and test tree nuts, and six out of seven subjects (86%) had sustained unresponsiveness to just walnuts.
“I am always amazed by the commitment of our food allergic subjects and their families in immunotherapy trials, and this study is no exception,” Dr. Scurlock commented. “Subjects had to undergo an increased number of oral food challenges (walnut, test tree nut, placebo) at protocol-specified time points in addition to daily home dosing. While walnut oral immunotherapy was generally well tolerated, we frequently observed oral allergy/itching associated with dosing in our cohort, which was an atopic group (75% allergic rhinitis). These symptoms can complicate assessment during dosing and oral food challenges. We observed that, with long-term therapy, there were some subjects who developed ‘dosing fatigue’ that could adversely affect adherence. Future studies will need to focus on strategies that optimize long-term sustainability/tolerability of dosing.”
She acknowledged certain limitations of the study, including its single-center design and small sample size. “While the findings are encouraging and similar to outcomes observed in other oral immunotherapy trials, further study in larger cohorts is critical before advancing toward broad clinical implementation. Specific issues regarding complexity of cross-reactivity and the efficacy of specific tree nuts to induce immunomodulation across tree nut families require future study. In addition, improving the long-term sustainability/tolerability of dosing and examining novel approaches is important.”
Dr. Scurlock disclosed that she has received funding from National Institutes of Health/National Institute of Allergy and Infectious Diseases and Food Allergy Research and Education (FARE). She is also medical director for the FARE Clinical Network Center of Excellence at Arkansas Children’s Hospital.
Key clinical point:
Major finding: Of nine subjects who underwent walnut and test tree nut desensitization oral food challenges by week 142, desensitization to both was observed in seven (78%).
Data source: A review of 14 children with allergy to walnuts and another test tree nut who received open-label walnut oral immunotherapy after completing 38 weeks of blinded, placebo-controlled treatment.
Disclosures: Dr. Scurlock disclosed that she has received funding from National Institutes of Health/National Institute of Allergy and Infectious Diseases and Food Allergy Research and Education (FARE). She is also medical director for the FARE Clinical Network Center of Excellence at Arkansas Children’s Hospital.
VIDEO: Don’t overlook psychosocial concerns of vitiligo patients
ORLANDO – There are ways to assess the psychosocial needs of your patients with vitiligo, even if you don’t believe you have the necessary skills to do a complete mental health work-up, according to Seemal R. Desai, MD.
In an interview recorded at this year’s annual meeting of the American Academy of Dermatology, Dr. Desai, an assistant clinical professor of dermatology at the University of Texas, Dallas, shares his ideas for how to have casual conversations with patients that can help reveal important clues to psychosocial stress patients with this serious medical skin condition might be facing.
“There are subtle clues to look for to know that these patients are uncomfortable with others seeing their skin,” says Dr. Desai.
In the video interview, he also covers taking a multidisciplinary approach to caring for these patients, what treatments are available to those who are suffering psychosocial stress after having failed several interventions, and how patients from many Asian and African counties are especially at risk for ostracization.
“It’s important to let your patients know that you understand this is really affecting them psychosocially, and that you care,” says Dr. Desai.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
ORLANDO – There are ways to assess the psychosocial needs of your patients with vitiligo, even if you don’t believe you have the necessary skills to do a complete mental health work-up, according to Seemal R. Desai, MD.
In an interview recorded at this year’s annual meeting of the American Academy of Dermatology, Dr. Desai, an assistant clinical professor of dermatology at the University of Texas, Dallas, shares his ideas for how to have casual conversations with patients that can help reveal important clues to psychosocial stress patients with this serious medical skin condition might be facing.
“There are subtle clues to look for to know that these patients are uncomfortable with others seeing their skin,” says Dr. Desai.
In the video interview, he also covers taking a multidisciplinary approach to caring for these patients, what treatments are available to those who are suffering psychosocial stress after having failed several interventions, and how patients from many Asian and African counties are especially at risk for ostracization.
“It’s important to let your patients know that you understand this is really affecting them psychosocially, and that you care,” says Dr. Desai.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
ORLANDO – There are ways to assess the psychosocial needs of your patients with vitiligo, even if you don’t believe you have the necessary skills to do a complete mental health work-up, according to Seemal R. Desai, MD.
In an interview recorded at this year’s annual meeting of the American Academy of Dermatology, Dr. Desai, an assistant clinical professor of dermatology at the University of Texas, Dallas, shares his ideas for how to have casual conversations with patients that can help reveal important clues to psychosocial stress patients with this serious medical skin condition might be facing.
“There are subtle clues to look for to know that these patients are uncomfortable with others seeing their skin,” says Dr. Desai.
In the video interview, he also covers taking a multidisciplinary approach to caring for these patients, what treatments are available to those who are suffering psychosocial stress after having failed several interventions, and how patients from many Asian and African counties are especially at risk for ostracization.
“It’s important to let your patients know that you understand this is really affecting them psychosocially, and that you care,” says Dr. Desai.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
wmcknight@frontlinemedcom.com
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM AAD 17
For bullous pemphigoid, doxycycline noninferior to prednisolone, but safer
ORLANDO – A 6-week course of doxycycline is an effective alternative to prednisolone for initial treatment of bullous pemphigoid in elderly patients, who may be particularly vulnerable to the potentially serious side effects of steroids.
While not as effective or quick-acting as prednisolone, doxycycline effected a cure in 74% of elderly patients who received it for 6 weeks in the Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) Trial. More importantly, however, patients who took the antibiotic experienced half as many serious adverse events as those who took prednisolone (18% vs. 36%), Karen Harman, MD, said at the annual meeting of the American Academy of Dermatology.
The trial results will be published in the March 6 issue of The Lancet, she added.
BLISTER randomized 253 elderly patients with bullous pemphigoid to an initial, 6-week course of either 200 mg doxycycline/day or 0.5 mg/kg prednisolone/day. After the induction period, treatment could be adjusted as needed.
The primary efficacy outcome was blister count at 6 weeks; patients with fewer than three blisters were considered treatment successes. The primary safety outcome was the proportion who experienced an adverse event of at least grade 3 (severe, life-threatening, or fatal) related to the study medication.
The study was conducted at 54 centers in the United Kingdom and Germany, said Dr. Harman, who was also a primary investigator on the trial. It was a pragmatic noninferiority trial.
“We knew from the beginning that doxycycline would not be as effective as prednisolone. We estimated that we would see a 25% reduced efficacy, but we were prepared to accept that if we could also see an improvement in safety of at least 20%.”
Patients were a mean of age of 78 years, although about a quarter were 85 years or older. They scored a mean of 70 points on the 0-100 point Karnofsky scale of functional independence, with 100 being completely functionally independent.
A score of 70 means the patient is capable of self-care but cannot engage in normal activity or work because of disease. About 10% were unable to care for themselves and 42% unable to work.
Over the year-long trial, 33 patients dropped out. The most frequent reason for withdrawal was death – not an unexpected occurrence in such an elderly and infirm group, Dr. Harman noted. There was no significant difference in deaths between the treatment arms. Another 39 withdrew consent during the study. Adverse events caused three patients to drop out (two in the doxycycline group and one in the prednisolone group). One patient taking doxycycline was unable to tolerate the study medication and dropped out.
At 6 weeks, treatment succeeded in 74% of the doxycycline group and in 91% of the prednisolone group. This was an adjusted absolute treatment difference of 18% – well within the 25% margin of noninferiority.
Baseline disease severity didn’t interact with either of the drugs’ effectiveness. Prednisolone was more effective in every severity class. Among those with mild disease, the success rates were 76% with doxycycline and 97% with prednisolone. Among those with moderate disease, success rates were 78% and 97%. Among those with severe disease, both drugs were slightly less effective, with success rates of 65% and 75%.
Adverse events of grade 3 or greater occurred in significantly fewer patients taking doxycycline (18.5% vs. 36.6%), an adjusted difference of 19%. This was close to the 20% gain in safety the investigators hoped for.
Adverse events were common, however, with 82% of patients on doxycycline and 64% on prednisolone experiencing at least one mild-moderate event. Most of these in the doxycycline group were gastrointestinal, Dr. Harman noted.
About twice as many patients taking prednisolone had an event with a maximum of grade 3 (22% vs. 11%). The proportion with a maximum event of grade 4 was 4% in each group. Eleven in the prednisolone group and three in the doxycycline group died (9.7% vs. 2.5%).
The trial had several secondary efficacy outcomes.
Doxycycline had a slower onset of action, as evidenced by the significant difference in high blister count at week 6 (26% vs. 8.7%). At 6 weeks, 20.5% of the doxycycline group and 4% of the prednisolone group experienced treatment failure and chose to switch to the other treatment arm. Adherence was also worse in the doxycycline group, with 19% missing more than 3 consecutive days of treatment vs. 5% in the prednisolone group.
The trial also included a quality of life measure for the entire 52 weeks. Through week 26, this favored prednisolone, again reflecting the slower disease control of doxycycline. Thereafter, quality of life was similar between the two groups.
The trial was run in collaboration with the Nottingham Clinical Trials Unit, the UK Dermatology Clinical Trials Network, and the Medical Research Council Clinical Trials Unit. Dr. Harman had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – A 6-week course of doxycycline is an effective alternative to prednisolone for initial treatment of bullous pemphigoid in elderly patients, who may be particularly vulnerable to the potentially serious side effects of steroids.
While not as effective or quick-acting as prednisolone, doxycycline effected a cure in 74% of elderly patients who received it for 6 weeks in the Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) Trial. More importantly, however, patients who took the antibiotic experienced half as many serious adverse events as those who took prednisolone (18% vs. 36%), Karen Harman, MD, said at the annual meeting of the American Academy of Dermatology.
The trial results will be published in the March 6 issue of The Lancet, she added.
BLISTER randomized 253 elderly patients with bullous pemphigoid to an initial, 6-week course of either 200 mg doxycycline/day or 0.5 mg/kg prednisolone/day. After the induction period, treatment could be adjusted as needed.
The primary efficacy outcome was blister count at 6 weeks; patients with fewer than three blisters were considered treatment successes. The primary safety outcome was the proportion who experienced an adverse event of at least grade 3 (severe, life-threatening, or fatal) related to the study medication.
The study was conducted at 54 centers in the United Kingdom and Germany, said Dr. Harman, who was also a primary investigator on the trial. It was a pragmatic noninferiority trial.
“We knew from the beginning that doxycycline would not be as effective as prednisolone. We estimated that we would see a 25% reduced efficacy, but we were prepared to accept that if we could also see an improvement in safety of at least 20%.”
Patients were a mean of age of 78 years, although about a quarter were 85 years or older. They scored a mean of 70 points on the 0-100 point Karnofsky scale of functional independence, with 100 being completely functionally independent.
A score of 70 means the patient is capable of self-care but cannot engage in normal activity or work because of disease. About 10% were unable to care for themselves and 42% unable to work.
Over the year-long trial, 33 patients dropped out. The most frequent reason for withdrawal was death – not an unexpected occurrence in such an elderly and infirm group, Dr. Harman noted. There was no significant difference in deaths between the treatment arms. Another 39 withdrew consent during the study. Adverse events caused three patients to drop out (two in the doxycycline group and one in the prednisolone group). One patient taking doxycycline was unable to tolerate the study medication and dropped out.
At 6 weeks, treatment succeeded in 74% of the doxycycline group and in 91% of the prednisolone group. This was an adjusted absolute treatment difference of 18% – well within the 25% margin of noninferiority.
Baseline disease severity didn’t interact with either of the drugs’ effectiveness. Prednisolone was more effective in every severity class. Among those with mild disease, the success rates were 76% with doxycycline and 97% with prednisolone. Among those with moderate disease, success rates were 78% and 97%. Among those with severe disease, both drugs were slightly less effective, with success rates of 65% and 75%.
Adverse events of grade 3 or greater occurred in significantly fewer patients taking doxycycline (18.5% vs. 36.6%), an adjusted difference of 19%. This was close to the 20% gain in safety the investigators hoped for.
Adverse events were common, however, with 82% of patients on doxycycline and 64% on prednisolone experiencing at least one mild-moderate event. Most of these in the doxycycline group were gastrointestinal, Dr. Harman noted.
About twice as many patients taking prednisolone had an event with a maximum of grade 3 (22% vs. 11%). The proportion with a maximum event of grade 4 was 4% in each group. Eleven in the prednisolone group and three in the doxycycline group died (9.7% vs. 2.5%).
The trial had several secondary efficacy outcomes.
Doxycycline had a slower onset of action, as evidenced by the significant difference in high blister count at week 6 (26% vs. 8.7%). At 6 weeks, 20.5% of the doxycycline group and 4% of the prednisolone group experienced treatment failure and chose to switch to the other treatment arm. Adherence was also worse in the doxycycline group, with 19% missing more than 3 consecutive days of treatment vs. 5% in the prednisolone group.
The trial also included a quality of life measure for the entire 52 weeks. Through week 26, this favored prednisolone, again reflecting the slower disease control of doxycycline. Thereafter, quality of life was similar between the two groups.
The trial was run in collaboration with the Nottingham Clinical Trials Unit, the UK Dermatology Clinical Trials Network, and the Medical Research Council Clinical Trials Unit. Dr. Harman had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
ORLANDO – A 6-week course of doxycycline is an effective alternative to prednisolone for initial treatment of bullous pemphigoid in elderly patients, who may be particularly vulnerable to the potentially serious side effects of steroids.
While not as effective or quick-acting as prednisolone, doxycycline effected a cure in 74% of elderly patients who received it for 6 weeks in the Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) Trial. More importantly, however, patients who took the antibiotic experienced half as many serious adverse events as those who took prednisolone (18% vs. 36%), Karen Harman, MD, said at the annual meeting of the American Academy of Dermatology.
The trial results will be published in the March 6 issue of The Lancet, she added.
BLISTER randomized 253 elderly patients with bullous pemphigoid to an initial, 6-week course of either 200 mg doxycycline/day or 0.5 mg/kg prednisolone/day. After the induction period, treatment could be adjusted as needed.
The primary efficacy outcome was blister count at 6 weeks; patients with fewer than three blisters were considered treatment successes. The primary safety outcome was the proportion who experienced an adverse event of at least grade 3 (severe, life-threatening, or fatal) related to the study medication.
The study was conducted at 54 centers in the United Kingdom and Germany, said Dr. Harman, who was also a primary investigator on the trial. It was a pragmatic noninferiority trial.
“We knew from the beginning that doxycycline would not be as effective as prednisolone. We estimated that we would see a 25% reduced efficacy, but we were prepared to accept that if we could also see an improvement in safety of at least 20%.”
Patients were a mean of age of 78 years, although about a quarter were 85 years or older. They scored a mean of 70 points on the 0-100 point Karnofsky scale of functional independence, with 100 being completely functionally independent.
A score of 70 means the patient is capable of self-care but cannot engage in normal activity or work because of disease. About 10% were unable to care for themselves and 42% unable to work.
Over the year-long trial, 33 patients dropped out. The most frequent reason for withdrawal was death – not an unexpected occurrence in such an elderly and infirm group, Dr. Harman noted. There was no significant difference in deaths between the treatment arms. Another 39 withdrew consent during the study. Adverse events caused three patients to drop out (two in the doxycycline group and one in the prednisolone group). One patient taking doxycycline was unable to tolerate the study medication and dropped out.
At 6 weeks, treatment succeeded in 74% of the doxycycline group and in 91% of the prednisolone group. This was an adjusted absolute treatment difference of 18% – well within the 25% margin of noninferiority.
Baseline disease severity didn’t interact with either of the drugs’ effectiveness. Prednisolone was more effective in every severity class. Among those with mild disease, the success rates were 76% with doxycycline and 97% with prednisolone. Among those with moderate disease, success rates were 78% and 97%. Among those with severe disease, both drugs were slightly less effective, with success rates of 65% and 75%.
Adverse events of grade 3 or greater occurred in significantly fewer patients taking doxycycline (18.5% vs. 36.6%), an adjusted difference of 19%. This was close to the 20% gain in safety the investigators hoped for.
Adverse events were common, however, with 82% of patients on doxycycline and 64% on prednisolone experiencing at least one mild-moderate event. Most of these in the doxycycline group were gastrointestinal, Dr. Harman noted.
About twice as many patients taking prednisolone had an event with a maximum of grade 3 (22% vs. 11%). The proportion with a maximum event of grade 4 was 4% in each group. Eleven in the prednisolone group and three in the doxycycline group died (9.7% vs. 2.5%).
The trial had several secondary efficacy outcomes.
Doxycycline had a slower onset of action, as evidenced by the significant difference in high blister count at week 6 (26% vs. 8.7%). At 6 weeks, 20.5% of the doxycycline group and 4% of the prednisolone group experienced treatment failure and chose to switch to the other treatment arm. Adherence was also worse in the doxycycline group, with 19% missing more than 3 consecutive days of treatment vs. 5% in the prednisolone group.
The trial also included a quality of life measure for the entire 52 weeks. Through week 26, this favored prednisolone, again reflecting the slower disease control of doxycycline. Thereafter, quality of life was similar between the two groups.
The trial was run in collaboration with the Nottingham Clinical Trials Unit, the UK Dermatology Clinical Trials Network, and the Medical Research Council Clinical Trials Unit. Dr. Harman had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @Alz_Gal
Key clinical point:
Major finding: At 6 weeks, treatment succeeded in 74% of the doxycycline group and 91% of the prednisolone group; severe adverse events occurred in 18% and 36%, respectively.
Data source: The BLISTER trial randomized 253 patients.
Disclosures: The trial was run in collaboration with the Nottingham Clinical Trials Unit, the UK Dermatology Clinical Trials Network, and the Medical Research Council Clinical Trials Unit. Dr. Harman had no financial disclosures.
Connective tissue diseases reported in patients receiving immune checkpoint inhibitors
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
For the first time, new-onset connective tissue disease has been reported in patients who were treated with anti-PD1/PDL-1 agents, according to findings published in the Annals of the Rheumatic Diseases.
In a cohort of 447 cancer patients who received therapy with immune checkpoint inhibitors (ICIs), Sébastien Le Burel, MD, of the Bicêtre Hospital in Le Kremlin-Bicêtre, France, and his colleagues described four patients who developed a connective tissue disease (CTD). There were two cases of Sjögren’s syndrome in patients taking an anti–programmed cell death 1 (anti-PD1) drug, one case of cryoglobulinemic vasculitis as a complication of suspected Sjögren’s syndrome in a patient taking an anti–programmed cell death ligand 1 (PDL-1) agent, and a case of a patient with antinuclear antibody positive myositis who was taking an anti-PDL-1 drug (Ann Rheum Dis. 2017 Feb 27. doi: 10.1136/annrheumdis-2016-210820).
“While the onset of systemic autoimmune disease after ICI treatment remains uncommon, greater awareness of these conditions should enable physicians to provide more effective patient care,” the investigators wrote. “This underlines the need for close collaboration within a network of oncologists and other specialist physicians in the new era of immunotherapy.”
The investigators discovered the cases by screening the French prospective, multicenter, academic REISAMIC registry for reports of CTD among patients being treated with anti-PD1 or anti-PDL-1 agents.
All four of the patients who developed a CTD had metastatic cancer, and their mean age was 62 years. Two patients had been treated with anti-PD1 agents and two with anti-PDL-1 agents. None of the four patients had presented with symptoms of CTD before they began treatment.
The mean time interval between the first treatment dose and the first symptom of CTD was 60 days (range, 24-72), and the mean time interval between the first symptom and subsequent diagnosis of CTD was 40 days (range, 10-74).
Three patients discontinued the ICI agent, and two patients were treated with steroids (1 mg/kg/day).
The estimated prevalence of CTD was 0.7% in the REISAMIC registry, and the authors emphasize that the high proportion of cases of Sjögren’s syndrome is noteworthy, with two of the patients fulfilling the recent American College of Rheumatology/European League Against Rheumatism criteria for Sjögren’s syndrome.
A limitation of the study is that some patients presenting with milder symptoms might not have been investigated by their oncologist.
The findings raise the question of whether asymptomatic patients taking ICIs who are at risk for immune-related adverse events should be screened and monitored closely, the authors explained.
One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
Key clinical point:
Major finding: In a cohort of 447 patients, 4 with metastatic cancer developed connective tissue disease following anti-PD-1/PDL-1 treatment.
Data source: A prospective, multicenter, academic registry was screened for reports of CTD among patients being treated with anti-PD1/PDL-1 agents.
Disclosures: One of the study authors received research funding from Novartis and Pfizer for the current paper. Several authors report relationships with industry.
Pediatric lupus patients face large burden of serious infection
The burden of serious infection is quite high among children who have systemic lupus erythematosus, with a “striking” preponderance of bacterial pneumonia, according to a report published in Arthritis Care & Research.
Infections are known to be commonplace among systemic lupus erythematosus (SLE) patients in general, and the increased risk is attributed both to the disease and to immunosuppressant therapies. However, most information on this topic comes from studies of adult patients seen at individual academic medical centers, said Linda T. Hiraki, MD, ScD, of the division of rheumatology at The Hospital for Sick Children, Toronto, and her associates.
To examine the nationwide prevalence of serious infections among children with SLE, they analyzed administrative data from a Medicaid database. They focused on 3,500 patients aged 5-18 years, including 1,297 who also had lupus nephritis, who were enrolled in Medicaid during 2000-2006 and followed for a mean of 2.6 years. This yielded a cumulative follow-up of more than 10,100 person-years (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23219).
The overall incidence was 10.4 serious infections per 100 person-years, and it was 17.65 per 100 person-years in the subset of patients who had lupus nephritis. By comparison, this overall rate is nearly four times higher than that reported for children with juvenile idiopathic arthritis, and the incidence among children with concomitant lupus nephritis is more than six times higher.
Infection rates were markedly higher among African American (incidence rate ratio [IRR], 1.83) and Native American (IRR, 1.81) children, compared with white children. They also were higher in early adolescence (ages 9-12 years) than earlier in childhood (ages 5-8 years), the investigators said.
Most of the infections (87%) were bacterial, whereas 11% were viral and 1.3% were fungal. (The remaining amount was unknown in the data because of too few numbers for federal reporting.) The most frequent bacterial infections were pneumonia (438 cases), followed by bacteremia (274 cases) and cellulitis (272 cases). Herpes zoster was the most frequent viral infection, accounting for 81 cases. The investigators noted that the low rate of fungal infections may be an artifact of the study protocol, which excluded, for technical reasons, cases of systemic candidiasis.
Not surprisingly, the rate of serious infection was higher among children with a high comorbidity burden than among healthier children.
Overall, the risk of serious infection was 59% higher for SLE patients who took corticosteroids during the study’s 6-month baseline period, in which 67% of patients took them (minimum of 20 mg/day of prednisone equivalent). However, the risk of serious infection was no different between those who used immunosuppressants (31%) or didn’t use them during that period.
A total of 26 children died within 30 days of hospital admission for a serious infection, for an overall mortality of 4.4% among children who developed serious infections. In comparison, 1.6% in the total cohort of 3,500 died. More than half of the children who died had concomitant lupus nephritis. In addition, 77% of those who died were taking corticosteroids when they developed the infections.
It is difficult to distinguish whether the high infection rate could be attributed to SLE itself or to its treatments. More studies are needed to further investigate this, as well as to address the disproportionate incidence among nonwhite children and any potential benefits from prophylactic use of antibiotics and vaccinations, Dr. Hiraki and her associates said.
The Canadian Institutes of Health Research, the Lupus Foundation of America, the Rheumatology Research Foundation, and the National Institutes of Health supported the study. Dr. Hiraki and her associates reported having no relevant disclosures.
The study by Hiraki et al. is important because very little is known about the risks of infection in childhood SLE, and there are few sources of data involving large numbers of affected children.
The overall rate of 10.4 serious infections necessitating hospitalization per 100 person-years reported in the study is approximately 10 times higher than the rate in the general Medicaid population. The findings should prompt further study of infection in childhood SLE so we can work toward decreasing this excessive risk.
The investigators unfortunately did not assess medication use throughout the study or try to find factors besides a high SLE risk adjustment index that were associated with infection, and these missed opportunities are the most significant weaknesses of an otherwise well-conducted study because added information about the role of disease activity and medication use would have a greater impact on clinical care than the nonetheless useful knowledge that childhood SLE is associated with a markedly increased infection rate.
Timothy Beukelman, MD, and his associates are with the University of Alabama at Birmingham. They made these remarks in an editorial accompanying Dr. Hiraki and colleagues’ report (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23221). No disclosure information was available with their editorial manuscript.
The study by Hiraki et al. is important because very little is known about the risks of infection in childhood SLE, and there are few sources of data involving large numbers of affected children.
The overall rate of 10.4 serious infections necessitating hospitalization per 100 person-years reported in the study is approximately 10 times higher than the rate in the general Medicaid population. The findings should prompt further study of infection in childhood SLE so we can work toward decreasing this excessive risk.
The investigators unfortunately did not assess medication use throughout the study or try to find factors besides a high SLE risk adjustment index that were associated with infection, and these missed opportunities are the most significant weaknesses of an otherwise well-conducted study because added information about the role of disease activity and medication use would have a greater impact on clinical care than the nonetheless useful knowledge that childhood SLE is associated with a markedly increased infection rate.
Timothy Beukelman, MD, and his associates are with the University of Alabama at Birmingham. They made these remarks in an editorial accompanying Dr. Hiraki and colleagues’ report (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23221). No disclosure information was available with their editorial manuscript.
The study by Hiraki et al. is important because very little is known about the risks of infection in childhood SLE, and there are few sources of data involving large numbers of affected children.
The overall rate of 10.4 serious infections necessitating hospitalization per 100 person-years reported in the study is approximately 10 times higher than the rate in the general Medicaid population. The findings should prompt further study of infection in childhood SLE so we can work toward decreasing this excessive risk.
The investigators unfortunately did not assess medication use throughout the study or try to find factors besides a high SLE risk adjustment index that were associated with infection, and these missed opportunities are the most significant weaknesses of an otherwise well-conducted study because added information about the role of disease activity and medication use would have a greater impact on clinical care than the nonetheless useful knowledge that childhood SLE is associated with a markedly increased infection rate.
Timothy Beukelman, MD, and his associates are with the University of Alabama at Birmingham. They made these remarks in an editorial accompanying Dr. Hiraki and colleagues’ report (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23221). No disclosure information was available with their editorial manuscript.
The burden of serious infection is quite high among children who have systemic lupus erythematosus, with a “striking” preponderance of bacterial pneumonia, according to a report published in Arthritis Care & Research.
Infections are known to be commonplace among systemic lupus erythematosus (SLE) patients in general, and the increased risk is attributed both to the disease and to immunosuppressant therapies. However, most information on this topic comes from studies of adult patients seen at individual academic medical centers, said Linda T. Hiraki, MD, ScD, of the division of rheumatology at The Hospital for Sick Children, Toronto, and her associates.
To examine the nationwide prevalence of serious infections among children with SLE, they analyzed administrative data from a Medicaid database. They focused on 3,500 patients aged 5-18 years, including 1,297 who also had lupus nephritis, who were enrolled in Medicaid during 2000-2006 and followed for a mean of 2.6 years. This yielded a cumulative follow-up of more than 10,100 person-years (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23219).
The overall incidence was 10.4 serious infections per 100 person-years, and it was 17.65 per 100 person-years in the subset of patients who had lupus nephritis. By comparison, this overall rate is nearly four times higher than that reported for children with juvenile idiopathic arthritis, and the incidence among children with concomitant lupus nephritis is more than six times higher.
Infection rates were markedly higher among African American (incidence rate ratio [IRR], 1.83) and Native American (IRR, 1.81) children, compared with white children. They also were higher in early adolescence (ages 9-12 years) than earlier in childhood (ages 5-8 years), the investigators said.
Most of the infections (87%) were bacterial, whereas 11% were viral and 1.3% were fungal. (The remaining amount was unknown in the data because of too few numbers for federal reporting.) The most frequent bacterial infections were pneumonia (438 cases), followed by bacteremia (274 cases) and cellulitis (272 cases). Herpes zoster was the most frequent viral infection, accounting for 81 cases. The investigators noted that the low rate of fungal infections may be an artifact of the study protocol, which excluded, for technical reasons, cases of systemic candidiasis.
Not surprisingly, the rate of serious infection was higher among children with a high comorbidity burden than among healthier children.
Overall, the risk of serious infection was 59% higher for SLE patients who took corticosteroids during the study’s 6-month baseline period, in which 67% of patients took them (minimum of 20 mg/day of prednisone equivalent). However, the risk of serious infection was no different between those who used immunosuppressants (31%) or didn’t use them during that period.
A total of 26 children died within 30 days of hospital admission for a serious infection, for an overall mortality of 4.4% among children who developed serious infections. In comparison, 1.6% in the total cohort of 3,500 died. More than half of the children who died had concomitant lupus nephritis. In addition, 77% of those who died were taking corticosteroids when they developed the infections.
It is difficult to distinguish whether the high infection rate could be attributed to SLE itself or to its treatments. More studies are needed to further investigate this, as well as to address the disproportionate incidence among nonwhite children and any potential benefits from prophylactic use of antibiotics and vaccinations, Dr. Hiraki and her associates said.
The Canadian Institutes of Health Research, the Lupus Foundation of America, the Rheumatology Research Foundation, and the National Institutes of Health supported the study. Dr. Hiraki and her associates reported having no relevant disclosures.
The burden of serious infection is quite high among children who have systemic lupus erythematosus, with a “striking” preponderance of bacterial pneumonia, according to a report published in Arthritis Care & Research.
Infections are known to be commonplace among systemic lupus erythematosus (SLE) patients in general, and the increased risk is attributed both to the disease and to immunosuppressant therapies. However, most information on this topic comes from studies of adult patients seen at individual academic medical centers, said Linda T. Hiraki, MD, ScD, of the division of rheumatology at The Hospital for Sick Children, Toronto, and her associates.
To examine the nationwide prevalence of serious infections among children with SLE, they analyzed administrative data from a Medicaid database. They focused on 3,500 patients aged 5-18 years, including 1,297 who also had lupus nephritis, who were enrolled in Medicaid during 2000-2006 and followed for a mean of 2.6 years. This yielded a cumulative follow-up of more than 10,100 person-years (Arthritis Care Res. 2017 Feb 19. doi: 10.1002/acr.23219).
The overall incidence was 10.4 serious infections per 100 person-years, and it was 17.65 per 100 person-years in the subset of patients who had lupus nephritis. By comparison, this overall rate is nearly four times higher than that reported for children with juvenile idiopathic arthritis, and the incidence among children with concomitant lupus nephritis is more than six times higher.
Infection rates were markedly higher among African American (incidence rate ratio [IRR], 1.83) and Native American (IRR, 1.81) children, compared with white children. They also were higher in early adolescence (ages 9-12 years) than earlier in childhood (ages 5-8 years), the investigators said.
Most of the infections (87%) were bacterial, whereas 11% were viral and 1.3% were fungal. (The remaining amount was unknown in the data because of too few numbers for federal reporting.) The most frequent bacterial infections were pneumonia (438 cases), followed by bacteremia (274 cases) and cellulitis (272 cases). Herpes zoster was the most frequent viral infection, accounting for 81 cases. The investigators noted that the low rate of fungal infections may be an artifact of the study protocol, which excluded, for technical reasons, cases of systemic candidiasis.
Not surprisingly, the rate of serious infection was higher among children with a high comorbidity burden than among healthier children.
Overall, the risk of serious infection was 59% higher for SLE patients who took corticosteroids during the study’s 6-month baseline period, in which 67% of patients took them (minimum of 20 mg/day of prednisone equivalent). However, the risk of serious infection was no different between those who used immunosuppressants (31%) or didn’t use them during that period.
A total of 26 children died within 30 days of hospital admission for a serious infection, for an overall mortality of 4.4% among children who developed serious infections. In comparison, 1.6% in the total cohort of 3,500 died. More than half of the children who died had concomitant lupus nephritis. In addition, 77% of those who died were taking corticosteroids when they developed the infections.
It is difficult to distinguish whether the high infection rate could be attributed to SLE itself or to its treatments. More studies are needed to further investigate this, as well as to address the disproportionate incidence among nonwhite children and any potential benefits from prophylactic use of antibiotics and vaccinations, Dr. Hiraki and her associates said.
The Canadian Institutes of Health Research, the Lupus Foundation of America, the Rheumatology Research Foundation, and the National Institutes of Health supported the study. Dr. Hiraki and her associates reported having no relevant disclosures.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: The burden of serious infection is quite high among children who have SLE, with a “striking” preponderance of bacterial pneumonia.
Major finding: The overall incidence was 10.4 serious infections per 100 person-years, and it was 17.65 per 100 person-years in the subset of patients who had lupus nephritis.
Data source: A retrospective cohort study using administrative Medicaid data for 3,500 affected U.S. children aged 5-18 years.
Disclosures: The Canadian Institutes of Health Research, the Lupus Foundation of America, the Rheumatology Research Foundation, and the National Institutes of Health supported the study. Dr. Hiraki and her associates reported having no relevant disclosures.