User login
Rituximab is dramatically effective in IgG4-related disease
SNOWMASS, COLO. – Glucocorticoids remain the first-line therapy in immunoglobulin G4-related disease, but it’s essential to bear in mind that their long-term efficacy in this immune-mediated fibroinflammatory disease is the exception rather than the rule, John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Dr. Stone, professor of medicine at Harvard Medical School, Boston, was a coauthor of an international expert consensus statement on the treatment of IgG4-related disease (IgG4-RD) which emphasized that point (Arthritis Rheumatol. 2015 Jul;67[7]:1688-99).
“I typically start with prednisone at 40 mg/day, and there’s a dramatic response in these patients. Then I taper them off after 2-3 months. If 2-3 months doesn’t put them into a long-term sustained remission, it’s time to go to something else,” said Dr. Stone, who also serves as director of clinical rheumatology at Massachusetts General Hospital, Boston.
“Glucocorticoids are rapidly effective, but initial reports were overoptimistic about their long-term efficacy. They don’t cure this disease any more than they cure giant cell arteritis in most of our patients, or ANCA-associated vasculitis. And since patients with IgG4-related disease are often older and may already have disease-induced damage to the pancreas and other organs, the morbidity from steroids in this population is formidable,” the rheumatologist explained.
In his series of 125 patients with biopsy-proven IgG4-RD, 83% responded to steroids initially, but 77% of steroid-treated patients failed to achieve a stable steroid-free remission after treatment discontinuation (Arthritis Rheumatol. 2015 Sep;67[9]:2466-75).
There is no evidence at all to indicate that conventional steroid-sparing drugs such as methotrexate, azathioprine, and mycophenolate mofetil are effective in IgG4-RD, the rheumatologist noted.
So what’s the next move, then, after steroids fail? Dr. Stone was a pioneer in the strikingly successful use of B cell depletion via rituximab (Rituxan) in patients with IgG4-RD. First he and his coinvestigators demonstrated that this off-label use of rituximab led to rapid clinical and histologic improvement (Ann Rheum Dis. 2015 Jun; 74[6]:1171-7), then they showed it also causes levels of circulating plasmablasts, serum IgG4, and biomarkers of fibrosis to plunge, suggesting B cell depletion may halt the destructive process of collagen deposition that characterizes this immune-related disease (Ann Rheum Dis. 2015 Dec;74[12]:2236-43). They have also reported that patients with very elevated baseline serum IgG4 levels are at more than sixfold increased risk of relapse at a median of 244 days from their first rituximab infusion (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
The success with rituximab is just one example of how improved understanding of the pathophysiology of IgG4-RD has opened the door to novel treatments. Dr. Stone is the lead investigator in an ongoing phase II, open-label study in which 15 patients with active IgG4-RD will receive intravenous XmAb5871 every 2 weeks for 6 months to evaluate the effect on the IgG4-RD Responder Index. XmAb5871 is a monoclonal antibody that binds to CD19 and FCgammaRIIb in order to downregulate activated B cells and plasmablasts. It is also being developed for treatment of systemic lupus erythematosus.
Dr. Stone and his coinvestigators are working on a therapeutic approach to IgG4-RD that targets antigen presentation by activated B cells to CD4+ cytotoxic T cells at sites of disease. These CD4+ cytotoxic T cells contain signaling lymphocyte activation molecule F7 (SLAMF7) as a surface marker. Elotuzumab (Empliciti), an immunostimulatory humanized monoclonal antibody targeting SLAMF7, is already on the market for treatment of multiple myeloma, he noted.
Dr. Stone reported receiving IgG4-RD-related research funding from and serving as a consultant to Genentech and Xencor, which is developing XmAb5871.
SNOWMASS, COLO. – Glucocorticoids remain the first-line therapy in immunoglobulin G4-related disease, but it’s essential to bear in mind that their long-term efficacy in this immune-mediated fibroinflammatory disease is the exception rather than the rule, John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Dr. Stone, professor of medicine at Harvard Medical School, Boston, was a coauthor of an international expert consensus statement on the treatment of IgG4-related disease (IgG4-RD) which emphasized that point (Arthritis Rheumatol. 2015 Jul;67[7]:1688-99).
“I typically start with prednisone at 40 mg/day, and there’s a dramatic response in these patients. Then I taper them off after 2-3 months. If 2-3 months doesn’t put them into a long-term sustained remission, it’s time to go to something else,” said Dr. Stone, who also serves as director of clinical rheumatology at Massachusetts General Hospital, Boston.
“Glucocorticoids are rapidly effective, but initial reports were overoptimistic about their long-term efficacy. They don’t cure this disease any more than they cure giant cell arteritis in most of our patients, or ANCA-associated vasculitis. And since patients with IgG4-related disease are often older and may already have disease-induced damage to the pancreas and other organs, the morbidity from steroids in this population is formidable,” the rheumatologist explained.
In his series of 125 patients with biopsy-proven IgG4-RD, 83% responded to steroids initially, but 77% of steroid-treated patients failed to achieve a stable steroid-free remission after treatment discontinuation (Arthritis Rheumatol. 2015 Sep;67[9]:2466-75).
There is no evidence at all to indicate that conventional steroid-sparing drugs such as methotrexate, azathioprine, and mycophenolate mofetil are effective in IgG4-RD, the rheumatologist noted.
So what’s the next move, then, after steroids fail? Dr. Stone was a pioneer in the strikingly successful use of B cell depletion via rituximab (Rituxan) in patients with IgG4-RD. First he and his coinvestigators demonstrated that this off-label use of rituximab led to rapid clinical and histologic improvement (Ann Rheum Dis. 2015 Jun; 74[6]:1171-7), then they showed it also causes levels of circulating plasmablasts, serum IgG4, and biomarkers of fibrosis to plunge, suggesting B cell depletion may halt the destructive process of collagen deposition that characterizes this immune-related disease (Ann Rheum Dis. 2015 Dec;74[12]:2236-43). They have also reported that patients with very elevated baseline serum IgG4 levels are at more than sixfold increased risk of relapse at a median of 244 days from their first rituximab infusion (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
The success with rituximab is just one example of how improved understanding of the pathophysiology of IgG4-RD has opened the door to novel treatments. Dr. Stone is the lead investigator in an ongoing phase II, open-label study in which 15 patients with active IgG4-RD will receive intravenous XmAb5871 every 2 weeks for 6 months to evaluate the effect on the IgG4-RD Responder Index. XmAb5871 is a monoclonal antibody that binds to CD19 and FCgammaRIIb in order to downregulate activated B cells and plasmablasts. It is also being developed for treatment of systemic lupus erythematosus.
Dr. Stone and his coinvestigators are working on a therapeutic approach to IgG4-RD that targets antigen presentation by activated B cells to CD4+ cytotoxic T cells at sites of disease. These CD4+ cytotoxic T cells contain signaling lymphocyte activation molecule F7 (SLAMF7) as a surface marker. Elotuzumab (Empliciti), an immunostimulatory humanized monoclonal antibody targeting SLAMF7, is already on the market for treatment of multiple myeloma, he noted.
Dr. Stone reported receiving IgG4-RD-related research funding from and serving as a consultant to Genentech and Xencor, which is developing XmAb5871.
SNOWMASS, COLO. – Glucocorticoids remain the first-line therapy in immunoglobulin G4-related disease, but it’s essential to bear in mind that their long-term efficacy in this immune-mediated fibroinflammatory disease is the exception rather than the rule, John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Dr. Stone, professor of medicine at Harvard Medical School, Boston, was a coauthor of an international expert consensus statement on the treatment of IgG4-related disease (IgG4-RD) which emphasized that point (Arthritis Rheumatol. 2015 Jul;67[7]:1688-99).
“I typically start with prednisone at 40 mg/day, and there’s a dramatic response in these patients. Then I taper them off after 2-3 months. If 2-3 months doesn’t put them into a long-term sustained remission, it’s time to go to something else,” said Dr. Stone, who also serves as director of clinical rheumatology at Massachusetts General Hospital, Boston.
“Glucocorticoids are rapidly effective, but initial reports were overoptimistic about their long-term efficacy. They don’t cure this disease any more than they cure giant cell arteritis in most of our patients, or ANCA-associated vasculitis. And since patients with IgG4-related disease are often older and may already have disease-induced damage to the pancreas and other organs, the morbidity from steroids in this population is formidable,” the rheumatologist explained.
In his series of 125 patients with biopsy-proven IgG4-RD, 83% responded to steroids initially, but 77% of steroid-treated patients failed to achieve a stable steroid-free remission after treatment discontinuation (Arthritis Rheumatol. 2015 Sep;67[9]:2466-75).
There is no evidence at all to indicate that conventional steroid-sparing drugs such as methotrexate, azathioprine, and mycophenolate mofetil are effective in IgG4-RD, the rheumatologist noted.
So what’s the next move, then, after steroids fail? Dr. Stone was a pioneer in the strikingly successful use of B cell depletion via rituximab (Rituxan) in patients with IgG4-RD. First he and his coinvestigators demonstrated that this off-label use of rituximab led to rapid clinical and histologic improvement (Ann Rheum Dis. 2015 Jun; 74[6]:1171-7), then they showed it also causes levels of circulating plasmablasts, serum IgG4, and biomarkers of fibrosis to plunge, suggesting B cell depletion may halt the destructive process of collagen deposition that characterizes this immune-related disease (Ann Rheum Dis. 2015 Dec;74[12]:2236-43). They have also reported that patients with very elevated baseline serum IgG4 levels are at more than sixfold increased risk of relapse at a median of 244 days from their first rituximab infusion (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
The success with rituximab is just one example of how improved understanding of the pathophysiology of IgG4-RD has opened the door to novel treatments. Dr. Stone is the lead investigator in an ongoing phase II, open-label study in which 15 patients with active IgG4-RD will receive intravenous XmAb5871 every 2 weeks for 6 months to evaluate the effect on the IgG4-RD Responder Index. XmAb5871 is a monoclonal antibody that binds to CD19 and FCgammaRIIb in order to downregulate activated B cells and plasmablasts. It is also being developed for treatment of systemic lupus erythematosus.
Dr. Stone and his coinvestigators are working on a therapeutic approach to IgG4-RD that targets antigen presentation by activated B cells to CD4+ cytotoxic T cells at sites of disease. These CD4+ cytotoxic T cells contain signaling lymphocyte activation molecule F7 (SLAMF7) as a surface marker. Elotuzumab (Empliciti), an immunostimulatory humanized monoclonal antibody targeting SLAMF7, is already on the market for treatment of multiple myeloma, he noted.
Dr. Stone reported receiving IgG4-RD-related research funding from and serving as a consultant to Genentech and Xencor, which is developing XmAb5871.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
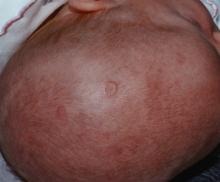
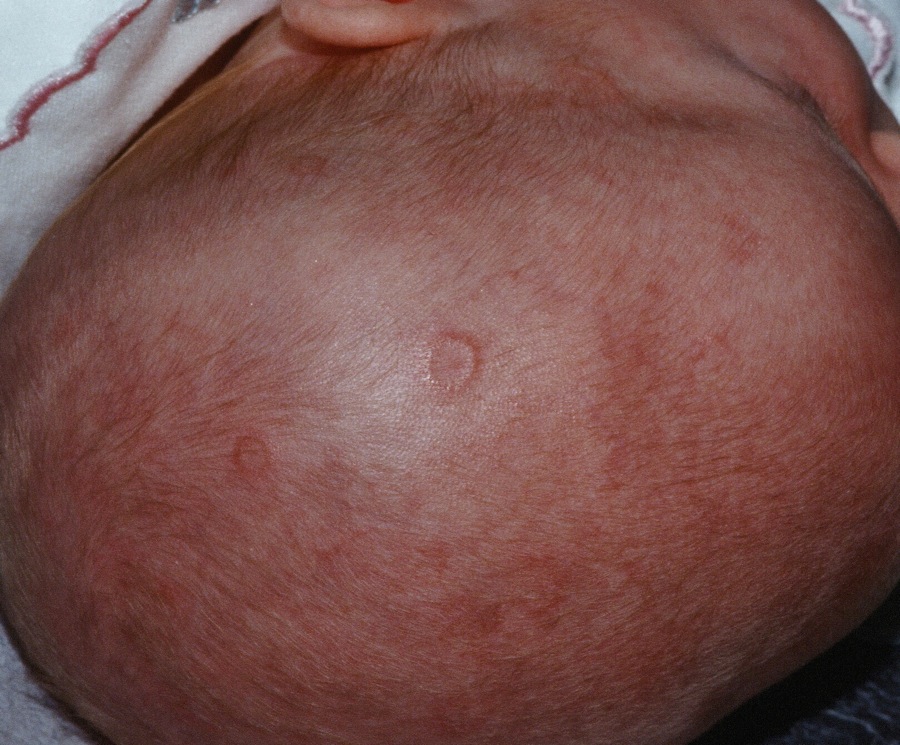
IgG4-related disease can strike any organ system
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swathe the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
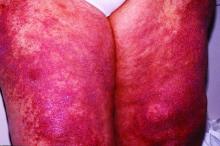
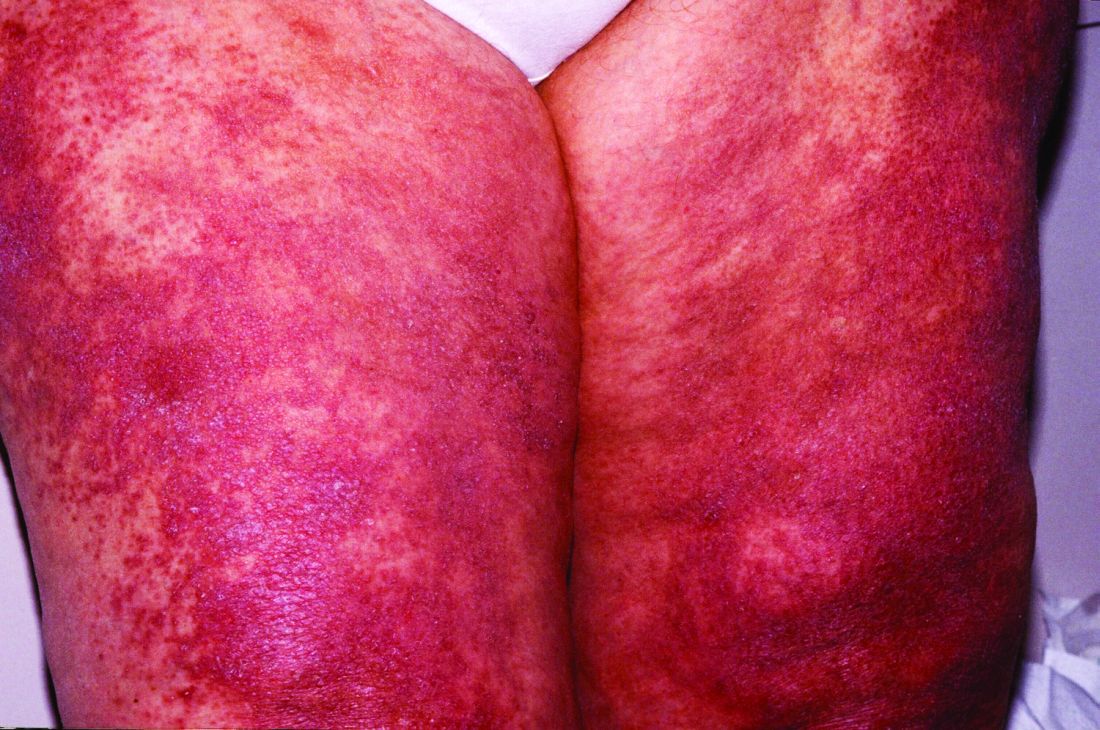
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Dr. Stone reported receiving IgG4-RD–related research funding from and serving as a consultant to Genentech and Xencor.
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swathe the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Dr. Stone reported receiving IgG4-RD–related research funding from and serving as a consultant to Genentech and Xencor.
SNOWMASS, COLO. – Progress in the understanding and treatment of immunoglobulin G4–related disease is occurring “at lightning speed,” John H. Stone, MD, said at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
Eight or nine years ago no one had heard of immunoglobulin G4–related disease (IgG4-RD). Today, because of the broad swathe the disease cuts, it’s a hot research topic in every subspecialty of medicine as well as surgery, pathology, and radiology.
This new understanding of IgG4-RD, he added, is opening the door to novel treatments.
“This is not a new disease. It was there when we were all in medical school, and for hundreds of years before that. But it’s really only in the last decade that we have come to understand that the disease can affect literally every organ system in the body with syndromes that we once thought were isolated organ-specific syndromes but we now recognize are part of a multiorgan disease currently called IgG4-related disease,” the rheumatologist said.
IgG4-RD is an immune-mediated fibroinflammatory condition characterized histopathologically by three hallmark features in involved tissue: obliterative phlebitis, storiform fibrosis, and a dense lymphoplasmacytic infiltrate.
Clinically, IgG4-RD often presents as a mass lesion that can affect any organ.
“I have many patients who’ve undergone modified Whipple procedures because they were thought to have adenocarcinoma of the pancreas,” according to Dr. Stone.
Other common presentations include Riedel’s thyroiditis, autoimmune pancreatitis, sclerosing cholangitis, sialadenitis, dacryoadenitis, periaortitis, an eosinophilic rash, and pseudotumor of the lung, lymph nodes, or orbits.
“Retroperitoneal fibrosis is a common and underappreciated manifestation. It may be the most common subsyndrome associated with IgG4-related disease,” he observed.
Another common presentation involves atopic disease – asthma, allergic rhinitis, eczema, eosinophilia, nasal polyps – developing out of the blue in middle age or later life. This observation led some other investigators to posit that IgG4-RD is a T-helper type 2–driven disease, an assertion debunked by Dr. Stone and coworkers (Allergy. 2014 Feb;69[2]:269-72).
Dr. Stone and his coinvestigators have published the largest series of patients with biopsy-proven IgG4-RD reported to date (Arthritis Rheumatol. 2015 Sep; 67[9]:2466-75). The average age at disease onset was 50 years. Of note, multiorgan involvement was the norm: 24% of patients had two organs involved, and 38% had three or more.
Analysis of this large patient series has led Dr. Stone to a surprising conclusion about the nature of IgG4-RD: “We have greatly overemphasized the importance of IgG4 in this condition,” he asserted.
Indeed, a mere 51% of the patients with clinically active untreated IgG4-RD in his series had an elevated serum IgG level. Dr. Stone characterized IgG4 as “kind of a wimpy antibody” incapable of driving the disease process because it is a noninflammatory immunoglobulin. This has led to speculation that IgG4 functions as what he termed an “antigen sink,” attempting to bind antigen at sites of inflammation.
But while an elevated serum IgG4 is of limited utility for diagnostic purposes, Dr. Stone and coworkers have demonstrated that it is of value as a predictor of relapse. Among patients with a treatment-induced remission, those in the top quartile in terms of baseline pretreatment serum IgG4 were 6.2-fold more likely to relapse (Rheumatology [Oxford]. 2016 Jun;55[6]:1000-8).
“This is a very useful marker for patients who are going to need chronic ongoing therapy. The notion of putting such patients on steroids for months and years is not appealing,” he said.
Levels of circulating plasmablasts as measured by peripheral blood flow cytometry, especially IgG4-positive plasmablasts, have proven much more helpful than serum IgG4 levels as a diagnostic tool, a reliable biomarker of disease activity, and a therapeutic target. Levels of these short-lived CD19+CD38+CD27+ plasmablasts are enormously elevated independent of serum IgG4 in patients with active IgG4-RD.
“One of the questions I’m most often asked is whether IgG4-related disease is a premalignant condition. My answer is no. The plasmablast expansion is oligoclonal, not polyclonal,” Dr. Stone continued.
He described IgG4-RD as “a continuous dance between T cells and B cells.” The latest thinking regarding pathogenesis is that type 2 T follicular helper cells activate B cells, which become memory B cells or plasmablasts. These activated B cells and plasmablasts present antigen to CD4+ cytotoxic T cells at sites of disease. Dr. Stone and his coinvestigators recently identified these CD4+ cytotoxic T cells as a novel population of clonally expanded T cells with SLAMF7 as a surface marker. The cells secrete interferon-gamma, interleukin-1, and transforming growth factor-beta, all of which are capable of driving the intense fibrosis characteristic of IgG4-RD. In addition, these CD4+ cytotoxic T cells secrete granzyme B and perforin, previously thought to be released mainly by natural killer T cells.
Joint American College of Rheumatology/European League Against Rheumatism classification criteria for the disease are expected to be finalized this winter at the Third International Symposium on IgG4-Related Diseases.
Dr. Stone reported receiving IgG4-RD–related research funding from and serving as a consultant to Genentech and Xencor.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Intermediate nature of ‘incomplete’ lupus shown in large study
People with incomplete lupus erythematosus exhibit nearly as many disease-related symptoms as do those with systemic lupus erythematosus and often receive many of the same treatments, according to findings reported from a cohort study of 3,837 patients registered with the Lupus Family Registry and Repository.
The study found that while individuals with incomplete lupus erythematosus (ILE) generally have fewer biomarkers of lupus, a subset are at risk of permanent organ damage and may transition to systemic lupus erythematosus (SLE).
“No risk stratification protocols or treatment recommendations for ILE currently exist, and clinical care is largely derived from SLE experience, in part due to the lack of consensus on what constitutes ILE,” wrote Teresa Aberle of the Oklahoma Medical Research Foundation, Oklahoma City, and her coauthors.
The treatments that ILE patients received also ran the gamut from hydroxychloroquine and steroids to immunosuppressants of varying potency.
The investigators classified participants as having ILE if they met three of the 1997 American College of Rheumatology SLE classification criteria, and were classified as having SLE if they met four or more of those criteria. Overall, 440 individuals had ILE and 3,397 had SLE, with ILE patients on average older than SLE patients and less likely to be African American (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23201).
Among those with ILE, 97.3% were ANA positive, compared with 99.3% of patients with SLE, but fewer ILE patients had ANA titers of 1:1,080 or higher (10.5% vs. 19.5%; odds ratio, 2.03; P less than .0001). ILE patients were also more than three times less likely to show anti-dsDNA antibodies than were SLE patients, but a similar percentage – approximately 42% – of both groups had anti-cardiolipin antibodies.
The researchers also examined plasma levels of B-lymphocyte stimulator, hypothesizing that lower antibody levels and fewer autoantibody specificities in ILE patients might connect with autoantibody production. In a subset of 72 ILE patients, 100 SLE patients, and 172 healthy controls, they observed that median B-lymphocyte stimulator levels were significantly lower in ILE patients, compared with SLE patients, although they were still higher than those in healthy controls. Around one-third of ILE patients had B-lymphocyte stimulator levels that exceeded the positive cutoff value, compared with 54% of SLE patients and 9% of healthy controls.
When it came to symptoms, the researchers found that each lupus classification criterion was seen in at least some ILE patients, although SLE patients were significantly more likely to have protein in urine (65.1% vs. 50%; OR, 1.9; P less than .0001), low blood counts (76.6% vs. 66%; OR, 1.7; P less than .0001), and experience seizures (19.4% vs. 14.4%; OR, 1.4; P = .012). Patients with SLE were also more likely to have pleurisy (58.2% vs. 51.9%; P = .012) and exhibit rapid hair loss (52.2% vs. 47.2%; P = .049) than were those with ILE.
Nearly one in five patients with ILE were affected by at least one clinical criterion of the disease linked to multiple indicators of disease severity, which included criteria such as serositis, renal symptoms, neurologic symptoms, hemolytic anemia, and thrombocytopenia. The investigators also noted that African Americans with ILE showed a higher rate of major clinical manifestations than did European Americans with ILE (25.7% vs. 14.9%).
“Given the association between renal disease and future transition to classified SLE, these observations suggest that patients of non-European American ancestry would likely benefit from careful monitoring and inclusion in future longitudinal studies of early or incomplete lupus,” they wrote.
While ILE patients used fewer medication types and were less likely to have used each type of medication, 65.2% of them had been treated with hydroxychloroquine and 70.7% had been treated with steroids. More than a quarter of ILE patients had used immunosuppressants and 14.8% had used major immunosuppressants, such as mycophenolate mofetil and cyclophosphamide.
“These results show that in clinical practice, ILE is often treated with hydroxychloroquine and/or steroids and occasionally may warrant treatment with potent immunosuppressants,” the authors wrote.
The authors also noted that while some patients with ILE have more severe clinical presentations and require more aggressive treatments, others with less severe disease who are at low risk of transitioning to SLE may be at risk of overtreatment.
“These results reinforce the need for new strategies to personalize disease management in ILE based on individual presentation and risk factors.”
The National Institutes of Health and the U.S. Department of Veterans Affairs supported the study. One author reported grants from pharmaceutical companies outside of the submitted work, and there were no other conflicts of interest declared.
Where the study from Ms. Aberle and her colleagues most advances knowledge of these intermediate phenotype ILE patients is in the more sophisticated interrogation of the immunologic features of ILE vs. SLE patients than has been performed in past studies. These findings are consistent with the conception of ILE as an intermediate state between health and “complete lupus” with similar, but less immune dysregulation than patients classified with SLE.
Karen H. Costenbader, MD, is with the division of rheumatology, immunology and allergy at Brigham and Women’s Hospital, Boston, and David I. Daikh, MD, is with the division of rheumatology at the University of California, San Francisco. These comments are adapted from an accompanying editorial (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23196). No conflicts of interest were declared.
Where the study from Ms. Aberle and her colleagues most advances knowledge of these intermediate phenotype ILE patients is in the more sophisticated interrogation of the immunologic features of ILE vs. SLE patients than has been performed in past studies. These findings are consistent with the conception of ILE as an intermediate state between health and “complete lupus” with similar, but less immune dysregulation than patients classified with SLE.
Karen H. Costenbader, MD, is with the division of rheumatology, immunology and allergy at Brigham and Women’s Hospital, Boston, and David I. Daikh, MD, is with the division of rheumatology at the University of California, San Francisco. These comments are adapted from an accompanying editorial (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23196). No conflicts of interest were declared.
Where the study from Ms. Aberle and her colleagues most advances knowledge of these intermediate phenotype ILE patients is in the more sophisticated interrogation of the immunologic features of ILE vs. SLE patients than has been performed in past studies. These findings are consistent with the conception of ILE as an intermediate state between health and “complete lupus” with similar, but less immune dysregulation than patients classified with SLE.
Karen H. Costenbader, MD, is with the division of rheumatology, immunology and allergy at Brigham and Women’s Hospital, Boston, and David I. Daikh, MD, is with the division of rheumatology at the University of California, San Francisco. These comments are adapted from an accompanying editorial (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23196). No conflicts of interest were declared.
People with incomplete lupus erythematosus exhibit nearly as many disease-related symptoms as do those with systemic lupus erythematosus and often receive many of the same treatments, according to findings reported from a cohort study of 3,837 patients registered with the Lupus Family Registry and Repository.
The study found that while individuals with incomplete lupus erythematosus (ILE) generally have fewer biomarkers of lupus, a subset are at risk of permanent organ damage and may transition to systemic lupus erythematosus (SLE).
“No risk stratification protocols or treatment recommendations for ILE currently exist, and clinical care is largely derived from SLE experience, in part due to the lack of consensus on what constitutes ILE,” wrote Teresa Aberle of the Oklahoma Medical Research Foundation, Oklahoma City, and her coauthors.
The treatments that ILE patients received also ran the gamut from hydroxychloroquine and steroids to immunosuppressants of varying potency.
The investigators classified participants as having ILE if they met three of the 1997 American College of Rheumatology SLE classification criteria, and were classified as having SLE if they met four or more of those criteria. Overall, 440 individuals had ILE and 3,397 had SLE, with ILE patients on average older than SLE patients and less likely to be African American (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23201).
Among those with ILE, 97.3% were ANA positive, compared with 99.3% of patients with SLE, but fewer ILE patients had ANA titers of 1:1,080 or higher (10.5% vs. 19.5%; odds ratio, 2.03; P less than .0001). ILE patients were also more than three times less likely to show anti-dsDNA antibodies than were SLE patients, but a similar percentage – approximately 42% – of both groups had anti-cardiolipin antibodies.
The researchers also examined plasma levels of B-lymphocyte stimulator, hypothesizing that lower antibody levels and fewer autoantibody specificities in ILE patients might connect with autoantibody production. In a subset of 72 ILE patients, 100 SLE patients, and 172 healthy controls, they observed that median B-lymphocyte stimulator levels were significantly lower in ILE patients, compared with SLE patients, although they were still higher than those in healthy controls. Around one-third of ILE patients had B-lymphocyte stimulator levels that exceeded the positive cutoff value, compared with 54% of SLE patients and 9% of healthy controls.
When it came to symptoms, the researchers found that each lupus classification criterion was seen in at least some ILE patients, although SLE patients were significantly more likely to have protein in urine (65.1% vs. 50%; OR, 1.9; P less than .0001), low blood counts (76.6% vs. 66%; OR, 1.7; P less than .0001), and experience seizures (19.4% vs. 14.4%; OR, 1.4; P = .012). Patients with SLE were also more likely to have pleurisy (58.2% vs. 51.9%; P = .012) and exhibit rapid hair loss (52.2% vs. 47.2%; P = .049) than were those with ILE.
Nearly one in five patients with ILE were affected by at least one clinical criterion of the disease linked to multiple indicators of disease severity, which included criteria such as serositis, renal symptoms, neurologic symptoms, hemolytic anemia, and thrombocytopenia. The investigators also noted that African Americans with ILE showed a higher rate of major clinical manifestations than did European Americans with ILE (25.7% vs. 14.9%).
“Given the association between renal disease and future transition to classified SLE, these observations suggest that patients of non-European American ancestry would likely benefit from careful monitoring and inclusion in future longitudinal studies of early or incomplete lupus,” they wrote.
While ILE patients used fewer medication types and were less likely to have used each type of medication, 65.2% of them had been treated with hydroxychloroquine and 70.7% had been treated with steroids. More than a quarter of ILE patients had used immunosuppressants and 14.8% had used major immunosuppressants, such as mycophenolate mofetil and cyclophosphamide.
“These results show that in clinical practice, ILE is often treated with hydroxychloroquine and/or steroids and occasionally may warrant treatment with potent immunosuppressants,” the authors wrote.
The authors also noted that while some patients with ILE have more severe clinical presentations and require more aggressive treatments, others with less severe disease who are at low risk of transitioning to SLE may be at risk of overtreatment.
“These results reinforce the need for new strategies to personalize disease management in ILE based on individual presentation and risk factors.”
The National Institutes of Health and the U.S. Department of Veterans Affairs supported the study. One author reported grants from pharmaceutical companies outside of the submitted work, and there were no other conflicts of interest declared.
People with incomplete lupus erythematosus exhibit nearly as many disease-related symptoms as do those with systemic lupus erythematosus and often receive many of the same treatments, according to findings reported from a cohort study of 3,837 patients registered with the Lupus Family Registry and Repository.
The study found that while individuals with incomplete lupus erythematosus (ILE) generally have fewer biomarkers of lupus, a subset are at risk of permanent organ damage and may transition to systemic lupus erythematosus (SLE).
“No risk stratification protocols or treatment recommendations for ILE currently exist, and clinical care is largely derived from SLE experience, in part due to the lack of consensus on what constitutes ILE,” wrote Teresa Aberle of the Oklahoma Medical Research Foundation, Oklahoma City, and her coauthors.
The treatments that ILE patients received also ran the gamut from hydroxychloroquine and steroids to immunosuppressants of varying potency.
The investigators classified participants as having ILE if they met three of the 1997 American College of Rheumatology SLE classification criteria, and were classified as having SLE if they met four or more of those criteria. Overall, 440 individuals had ILE and 3,397 had SLE, with ILE patients on average older than SLE patients and less likely to be African American (Arthritis Care Res. 2017 Jan 24. doi: 10.1002/acr.23201).
Among those with ILE, 97.3% were ANA positive, compared with 99.3% of patients with SLE, but fewer ILE patients had ANA titers of 1:1,080 or higher (10.5% vs. 19.5%; odds ratio, 2.03; P less than .0001). ILE patients were also more than three times less likely to show anti-dsDNA antibodies than were SLE patients, but a similar percentage – approximately 42% – of both groups had anti-cardiolipin antibodies.
The researchers also examined plasma levels of B-lymphocyte stimulator, hypothesizing that lower antibody levels and fewer autoantibody specificities in ILE patients might connect with autoantibody production. In a subset of 72 ILE patients, 100 SLE patients, and 172 healthy controls, they observed that median B-lymphocyte stimulator levels were significantly lower in ILE patients, compared with SLE patients, although they were still higher than those in healthy controls. Around one-third of ILE patients had B-lymphocyte stimulator levels that exceeded the positive cutoff value, compared with 54% of SLE patients and 9% of healthy controls.
When it came to symptoms, the researchers found that each lupus classification criterion was seen in at least some ILE patients, although SLE patients were significantly more likely to have protein in urine (65.1% vs. 50%; OR, 1.9; P less than .0001), low blood counts (76.6% vs. 66%; OR, 1.7; P less than .0001), and experience seizures (19.4% vs. 14.4%; OR, 1.4; P = .012). Patients with SLE were also more likely to have pleurisy (58.2% vs. 51.9%; P = .012) and exhibit rapid hair loss (52.2% vs. 47.2%; P = .049) than were those with ILE.
Nearly one in five patients with ILE were affected by at least one clinical criterion of the disease linked to multiple indicators of disease severity, which included criteria such as serositis, renal symptoms, neurologic symptoms, hemolytic anemia, and thrombocytopenia. The investigators also noted that African Americans with ILE showed a higher rate of major clinical manifestations than did European Americans with ILE (25.7% vs. 14.9%).
“Given the association between renal disease and future transition to classified SLE, these observations suggest that patients of non-European American ancestry would likely benefit from careful monitoring and inclusion in future longitudinal studies of early or incomplete lupus,” they wrote.
While ILE patients used fewer medication types and were less likely to have used each type of medication, 65.2% of them had been treated with hydroxychloroquine and 70.7% had been treated with steroids. More than a quarter of ILE patients had used immunosuppressants and 14.8% had used major immunosuppressants, such as mycophenolate mofetil and cyclophosphamide.
“These results show that in clinical practice, ILE is often treated with hydroxychloroquine and/or steroids and occasionally may warrant treatment with potent immunosuppressants,” the authors wrote.
The authors also noted that while some patients with ILE have more severe clinical presentations and require more aggressive treatments, others with less severe disease who are at low risk of transitioning to SLE may be at risk of overtreatment.
“These results reinforce the need for new strategies to personalize disease management in ILE based on individual presentation and risk factors.”
The National Institutes of Health and the U.S. Department of Veterans Affairs supported the study. One author reported grants from pharmaceutical companies outside of the submitted work, and there were no other conflicts of interest declared.
Key clinical point:
Major finding: Each lupus classification criterion was seen in at least some incomplete lupus erythematosus patients, although systemic lupus erythematosus patients were significantly more likely to have protein in urine, low blood counts, and experience seizures.
Data source: A cohort study of 3,837 patients registered with the Lupus Family Registry and Repository.
Disclosures: The National Institutes of Health and the U.S. Department of Veterans Affairs supported the study. One author reported grants from pharmaceutical companies outside of the submitted work, and there were no other conflicts of interest declared.
VIDEO: Pattern recognition provides clues to rheumatologic diagnoses
WAILEA, HAWAII – Several patterns can provide clues to diagnoses of rheumatologic diseases, according to Daniel E. Furst, MD, professor of rheumatology, University of Washington, Seattle.
Dermatology and rheumatology share the use of pattern recognition when making a diagnosis, he said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. An important pattern is the idea of polyarticular versus monoarticular disease. Rheumatoid arthritis, osteoarthritis, autoimmune diseases, and gout and crystalline diseases, he said, encompass “about 80% of the cases with polyarticular arthritis.”
Another pattern to consider is symmetry versus asymmetry. Osteoarthritis and gout will be asymmetric, while autoimmune diseases and RA will be symmetrical, Dr. Furst pointed out. Also consider areas of involvement, such as upper and lower parts of the body. “Lower tends to be more gout and more OA,” while upper-extremity involvement is more likely to be autoimmune diseases and RA, he said.
“You don’t have to be Einstein to be a rheumatologist; just remember some simple stuff,” added Dr. Furst, who also discusses the use of lab tests in the interview.
Dr. Furst is also professor emeritus, University of California, Los Angeles, and is affiliated with the University of Florence (Italy) Medical School.
He disclosed financial relationships with companies including AbbVie, Actelion, Amgen, BMS, Cytori, Genentech/Roche, Novartis, and Pfizer.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Several patterns can provide clues to diagnoses of rheumatologic diseases, according to Daniel E. Furst, MD, professor of rheumatology, University of Washington, Seattle.
Dermatology and rheumatology share the use of pattern recognition when making a diagnosis, he said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. An important pattern is the idea of polyarticular versus monoarticular disease. Rheumatoid arthritis, osteoarthritis, autoimmune diseases, and gout and crystalline diseases, he said, encompass “about 80% of the cases with polyarticular arthritis.”
Another pattern to consider is symmetry versus asymmetry. Osteoarthritis and gout will be asymmetric, while autoimmune diseases and RA will be symmetrical, Dr. Furst pointed out. Also consider areas of involvement, such as upper and lower parts of the body. “Lower tends to be more gout and more OA,” while upper-extremity involvement is more likely to be autoimmune diseases and RA, he said.
“You don’t have to be Einstein to be a rheumatologist; just remember some simple stuff,” added Dr. Furst, who also discusses the use of lab tests in the interview.
Dr. Furst is also professor emeritus, University of California, Los Angeles, and is affiliated with the University of Florence (Italy) Medical School.
He disclosed financial relationships with companies including AbbVie, Actelion, Amgen, BMS, Cytori, Genentech/Roche, Novartis, and Pfizer.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Several patterns can provide clues to diagnoses of rheumatologic diseases, according to Daniel E. Furst, MD, professor of rheumatology, University of Washington, Seattle.
Dermatology and rheumatology share the use of pattern recognition when making a diagnosis, he said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. An important pattern is the idea of polyarticular versus monoarticular disease. Rheumatoid arthritis, osteoarthritis, autoimmune diseases, and gout and crystalline diseases, he said, encompass “about 80% of the cases with polyarticular arthritis.”
Another pattern to consider is symmetry versus asymmetry. Osteoarthritis and gout will be asymmetric, while autoimmune diseases and RA will be symmetrical, Dr. Furst pointed out. Also consider areas of involvement, such as upper and lower parts of the body. “Lower tends to be more gout and more OA,” while upper-extremity involvement is more likely to be autoimmune diseases and RA, he said.
“You don’t have to be Einstein to be a rheumatologist; just remember some simple stuff,” added Dr. Furst, who also discusses the use of lab tests in the interview.
Dr. Furst is also professor emeritus, University of California, Los Angeles, and is affiliated with the University of Florence (Italy) Medical School.
He disclosed financial relationships with companies including AbbVie, Actelion, Amgen, BMS, Cytori, Genentech/Roche, Novartis, and Pfizer.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
New histopathologic marker may aid dermatomyositis diagnosis
The detection of sarcoplasmic myxovirus resistance A expression in immunohistochemical analysis of muscle biopsy in patients suspected of having dermatomyositis may add greater sensitivity for the diagnosis when compared with conventional pathologic hallmarks of the disease, according to findings from a retrospective cohort study.
Myxovirus resistance A (MxA) is one of the type 1 interferon–inducible proteins whose overexpression is believed to play a role in the pathogenesis of dermatomyositis, and MxA expression has rarely been observed in other idiopathic inflammatory myopathies, said first author Akinori Uruha, MD, PhD, of the National Center of Neurology and Psychiatry, Tokyo, and his colleagues. They compared MxA expression in muscle biopsy samples from definite, probable, and possible dermatomyositis cases as well as other idiopathic inflammatory myopathies and other control conditions to assess its value against other muscle pathologic markers of dermatomyositis, such as the presence of perifascicular atrophy (PFA) and capillary membrane attack complex (MAC) deposition (Neurology. 2016 Dec 30. doi: 10.1212/WNL.0000000000003568).
The investigators studied muscle biopsy samples collected from 154 consecutive patients with idiopathic inflammatory myopathies seen from all over Japan, including 34 with dermatomyositis (10 juvenile cases), 8 with polymyositis (1 juvenile), 16 with anti–tRNA-synthetase antibody–associated myopathy (ASM); 46 with immune-mediated necrotizing myopathy (IMNM), and 50 with inclusion body myositis. The IMNM cases involved included 24 with anti–signal recognition particle (SRP) antibodies, 6 with anti–3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) antibodies, and 16 without anti-SRP, anti-HMGCR, or anti–tRNA-synthetase antibodies (3 juvenile patients). They used 51 patients with muscular dystrophy and 26 with neuropathies as controls.
Sarcoplasmic MxA expression proved to be more sensitive for a diagnosis of dermatomyositis than PFA and capillary MAC deposition (71% vs. 47% and 35%, respectively) but still had comparable specificity to those two markers (98% vs. 98% and 93%, respectively).
Of 18 cases with probable dermatomyositis, defined as typical skin rash but a lack of PFA, 8 (44%) showed sarcoplasmic MxA expression, and its sensitivity was 90% in juvenile cases overall and 63% in adult patients. Only 3 (17%) of the 18 showed capillary MAC deposition. Sarcoplasmic MxA expression occurred in all 12 patients with definite dermatomyositis, defined by the typical skin rash plus presence of PFA, whereas only 7 (58%) showed capillary MAC deposition. Among the four patients with possible dermatomyositis (PFA present but lacking typical skin rash), all showed sarcoplasmic MxA expression, compared with just two showing capillary MAC deposition.
In all other patients without definite, probable, or possible dermatomyositis, only two were positive for sarcoplasmic MxA expression (one with ASM and one with IMNM).
Dr. Uruha and his associates said that the results are “clearly demonstrating that sarcoplasmic MxA expression should be an excellent diagnostic marker of [dermatomyositis].”
The authors noted that the study was limited by the fact that they could not obtain full information about dermatomyositis-associated antibodies, and because other proteins of type 1 interferon signature are known to be upregulated in dermatomyositis, additional studies will need to determine which of the proteins is a better diagnostic marker.
The study was supported partly by an Intramural Research Grant of the National Center of Neurology and Psychiatry and grants from the Japanese Ministry of Education, Science, Sports and Culture and the Ministry of Health, Labor and Welfare of Japan. The investigators had no relevant disclosures.
The detection of sarcoplasmic myxovirus resistance A expression in immunohistochemical analysis of muscle biopsy in patients suspected of having dermatomyositis may add greater sensitivity for the diagnosis when compared with conventional pathologic hallmarks of the disease, according to findings from a retrospective cohort study.
Myxovirus resistance A (MxA) is one of the type 1 interferon–inducible proteins whose overexpression is believed to play a role in the pathogenesis of dermatomyositis, and MxA expression has rarely been observed in other idiopathic inflammatory myopathies, said first author Akinori Uruha, MD, PhD, of the National Center of Neurology and Psychiatry, Tokyo, and his colleagues. They compared MxA expression in muscle biopsy samples from definite, probable, and possible dermatomyositis cases as well as other idiopathic inflammatory myopathies and other control conditions to assess its value against other muscle pathologic markers of dermatomyositis, such as the presence of perifascicular atrophy (PFA) and capillary membrane attack complex (MAC) deposition (Neurology. 2016 Dec 30. doi: 10.1212/WNL.0000000000003568).
The investigators studied muscle biopsy samples collected from 154 consecutive patients with idiopathic inflammatory myopathies seen from all over Japan, including 34 with dermatomyositis (10 juvenile cases), 8 with polymyositis (1 juvenile), 16 with anti–tRNA-synthetase antibody–associated myopathy (ASM); 46 with immune-mediated necrotizing myopathy (IMNM), and 50 with inclusion body myositis. The IMNM cases involved included 24 with anti–signal recognition particle (SRP) antibodies, 6 with anti–3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) antibodies, and 16 without anti-SRP, anti-HMGCR, or anti–tRNA-synthetase antibodies (3 juvenile patients). They used 51 patients with muscular dystrophy and 26 with neuropathies as controls.
Sarcoplasmic MxA expression proved to be more sensitive for a diagnosis of dermatomyositis than PFA and capillary MAC deposition (71% vs. 47% and 35%, respectively) but still had comparable specificity to those two markers (98% vs. 98% and 93%, respectively).
Of 18 cases with probable dermatomyositis, defined as typical skin rash but a lack of PFA, 8 (44%) showed sarcoplasmic MxA expression, and its sensitivity was 90% in juvenile cases overall and 63% in adult patients. Only 3 (17%) of the 18 showed capillary MAC deposition. Sarcoplasmic MxA expression occurred in all 12 patients with definite dermatomyositis, defined by the typical skin rash plus presence of PFA, whereas only 7 (58%) showed capillary MAC deposition. Among the four patients with possible dermatomyositis (PFA present but lacking typical skin rash), all showed sarcoplasmic MxA expression, compared with just two showing capillary MAC deposition.
In all other patients without definite, probable, or possible dermatomyositis, only two were positive for sarcoplasmic MxA expression (one with ASM and one with IMNM).
Dr. Uruha and his associates said that the results are “clearly demonstrating that sarcoplasmic MxA expression should be an excellent diagnostic marker of [dermatomyositis].”
The authors noted that the study was limited by the fact that they could not obtain full information about dermatomyositis-associated antibodies, and because other proteins of type 1 interferon signature are known to be upregulated in dermatomyositis, additional studies will need to determine which of the proteins is a better diagnostic marker.
The study was supported partly by an Intramural Research Grant of the National Center of Neurology and Psychiatry and grants from the Japanese Ministry of Education, Science, Sports and Culture and the Ministry of Health, Labor and Welfare of Japan. The investigators had no relevant disclosures.
The detection of sarcoplasmic myxovirus resistance A expression in immunohistochemical analysis of muscle biopsy in patients suspected of having dermatomyositis may add greater sensitivity for the diagnosis when compared with conventional pathologic hallmarks of the disease, according to findings from a retrospective cohort study.
Myxovirus resistance A (MxA) is one of the type 1 interferon–inducible proteins whose overexpression is believed to play a role in the pathogenesis of dermatomyositis, and MxA expression has rarely been observed in other idiopathic inflammatory myopathies, said first author Akinori Uruha, MD, PhD, of the National Center of Neurology and Psychiatry, Tokyo, and his colleagues. They compared MxA expression in muscle biopsy samples from definite, probable, and possible dermatomyositis cases as well as other idiopathic inflammatory myopathies and other control conditions to assess its value against other muscle pathologic markers of dermatomyositis, such as the presence of perifascicular atrophy (PFA) and capillary membrane attack complex (MAC) deposition (Neurology. 2016 Dec 30. doi: 10.1212/WNL.0000000000003568).
The investigators studied muscle biopsy samples collected from 154 consecutive patients with idiopathic inflammatory myopathies seen from all over Japan, including 34 with dermatomyositis (10 juvenile cases), 8 with polymyositis (1 juvenile), 16 with anti–tRNA-synthetase antibody–associated myopathy (ASM); 46 with immune-mediated necrotizing myopathy (IMNM), and 50 with inclusion body myositis. The IMNM cases involved included 24 with anti–signal recognition particle (SRP) antibodies, 6 with anti–3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) antibodies, and 16 without anti-SRP, anti-HMGCR, or anti–tRNA-synthetase antibodies (3 juvenile patients). They used 51 patients with muscular dystrophy and 26 with neuropathies as controls.
Sarcoplasmic MxA expression proved to be more sensitive for a diagnosis of dermatomyositis than PFA and capillary MAC deposition (71% vs. 47% and 35%, respectively) but still had comparable specificity to those two markers (98% vs. 98% and 93%, respectively).
Of 18 cases with probable dermatomyositis, defined as typical skin rash but a lack of PFA, 8 (44%) showed sarcoplasmic MxA expression, and its sensitivity was 90% in juvenile cases overall and 63% in adult patients. Only 3 (17%) of the 18 showed capillary MAC deposition. Sarcoplasmic MxA expression occurred in all 12 patients with definite dermatomyositis, defined by the typical skin rash plus presence of PFA, whereas only 7 (58%) showed capillary MAC deposition. Among the four patients with possible dermatomyositis (PFA present but lacking typical skin rash), all showed sarcoplasmic MxA expression, compared with just two showing capillary MAC deposition.
In all other patients without definite, probable, or possible dermatomyositis, only two were positive for sarcoplasmic MxA expression (one with ASM and one with IMNM).
Dr. Uruha and his associates said that the results are “clearly demonstrating that sarcoplasmic MxA expression should be an excellent diagnostic marker of [dermatomyositis].”
The authors noted that the study was limited by the fact that they could not obtain full information about dermatomyositis-associated antibodies, and because other proteins of type 1 interferon signature are known to be upregulated in dermatomyositis, additional studies will need to determine which of the proteins is a better diagnostic marker.
The study was supported partly by an Intramural Research Grant of the National Center of Neurology and Psychiatry and grants from the Japanese Ministry of Education, Science, Sports and Culture and the Ministry of Health, Labor and Welfare of Japan. The investigators had no relevant disclosures.
FROM NEUROLOGY
Key clinical point:
Major finding: Sarcoplasmic MxA expression proved to be more sensitive for a diagnosis of dermatomyositis than PFA and capillary MAC deposition (71% vs. 47% and 35%, respectively) but still had comparable specificity to those two markers (98% vs. 98% and 93%, respectively).
Data source: A retrospective cohort study of 154 patients with idiopathic inflammatory myopathies, 51 with muscular dystrophy, and 26 with neuropathies.
Disclosures: The study was supported partly by an Intramural Research Grant of the National Center of Neurology and Psychiatry and grants from the Japanese Ministry of Education, Science, Sports and Culture and the Ministry of Health, Labor and Welfare of Japan. The investigators had no relevant disclosures.
VIDEO: Despite toxicities, ibrutinib is beneficial for treatment-resistant graft-vs.-host disease
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – An oral regimen of 420 mg ibrutinib achieved complete response in one-third of allogeneic stem cell recipients with chronic graft-vs.-host disease, David Miklos, MD, reported during a late-breaker session at the annual meeting of the American Society of Hematology.
Fully 79% of patients in this open-label phase II study were considered responders when first assessed, 71% of responses lasted at least 5 months, and patients whose disease involved multiple organs generally showed responses in at least two organs, said Dr. Miklos of Stanford (Calif.) University.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase (BTK) inhibitor. Cardiotoxicities have been a concern with ibrutinib, but were not observed in this cohort of 42 patients whose graft-vs.-host disease had not benefited from frontline therapy, Dr. Miklos said during a video interview. However, 52% of patients in this study developed other serious adverse events that are typical with ibrutinib, including pneumonia, septic shock, and fever, he said.
Chronic graft-vs.-host disease is the most common morbidity after allogeneic transplant. This is an “orphan disease” – there are no approved therapies for patients for whom corticosteroids are ineffective, Dr. Miklos noted. Based on these results, investigators are planning a randomized, placebo-controlled, phase III study, he added.
Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, travel and expenses reimbursements, and research funding from Pharmacyclics.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2016
Key clinical point: Ibrutinib (420 mg) led to complete responses in one-third of patients with chronic, treatment-resistant graft-vs-host disease.
Major finding: No cardiotoxicities were observed, but 52% of patients had other serious adverse effects, such as sepsis, pyrexia, and pneumonia.
Data source: An open-label phase II study of 42 patients who developed chronic, treatment-resistant graft-vs.-host disease after undergoing allogeneic stem cell transplantation.
Disclosures: Ibrutinib is jointly commercialized and developed by Janssen Biotech and by Pharmacyclics LLC, an Abbvie company. Dr. Miklos disclosed a consulting relationship, reimbursement for travel and expenses, and research funding from Pharmacyclics.
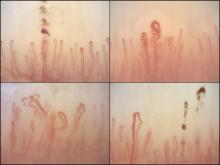
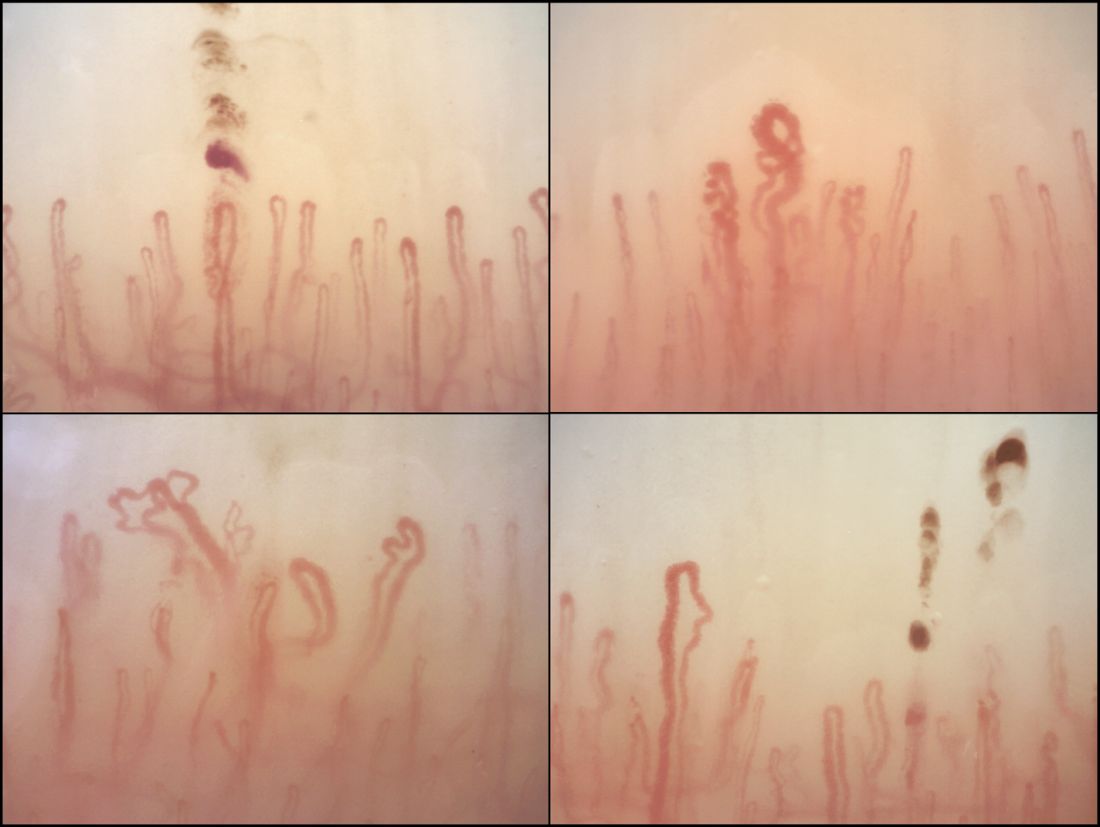
Nailfold analysis can predict cardiopulmonary complications in systemic sclerosis
Nailfold videocapillaroscopy can help to predict which patients with systemic sclerosis may develop serious cardiopulmonary complications, according to findings from a Dutch cross-sectional study.
While individual autoantibodies seen in systemic sclerosis (SSc) are known to be associated with greater or lesser risk of cardiopulmonary involvement, in this study nailfold vascularization patterns independently predicted pulmonary artery hypertension or interstitial lung disease.
All patients in the study had NVC pattern data as well as anti-extractable nuclear antigen (anti-ENA) antibodies. The mean age of the patients was 54 years; 82% were female, and median disease duration was 3 years. Just over half the cohort had interstitial lung disease, and 16% had pulmonary artery hypertension.
Among the anti-ENA autoantibody subtypes, anti-ACA was seen in 37% of patients, anti-Scl-70 in 24%, anti-RNP in 9%, and anti-RNAPIII in 5%; other subtypes were rarer. SSc-specific NVC patterns were seen in 88% of patients, with 10% of the cohort showing an early (less severe microangiopathy) pattern, 42% an active pattern, and 36% a late pattern.
One of the study’s objectives was to determine whether one or more mechanisms was responsible for both autoantibody production and the microangiopathy seen in SSc.
If a joint mechanism is implicated, “more severe NVC patterns would be determined in patients with autoantibodies (such as anti-Scl-70 and anti-RNAPIII) that are associated with more severe disease,” wrote Dr. Markusse and her colleagues. “On the other hand, if specific autoantibodies and stage of microangiopathy reflect different processes in the disease, a combination of autoantibody status and NVC could be helpful for identifying patients at highest risk for cardiopulmonary involvement.”
The investigators reported finding a similar distribution of NVC abnormalities across the major SSc autoantibody subtypes (except for anti–RNP-positive patients), suggesting that combinations of the two variables would be most predictive of cardiopulmonary involvement. More severe NVC patterns were associated with a higher risk of cardiopulmonary involvement, independent of the presence of a specific autoantibody.
Notably, the researchers wrote, “prevalence of ILD [interstitial lung disease] is generally lower among ACA-positive patients. According to our data, even among ACA-positive patients there was a trend for more ILD being associated with more severe NVC patterns (OR = 1.33).”
A similar pattern was seen for pulmonary artery hypertension. “Based on anti-RNP and anti-RNAPIII positivity, patients did not have an increased risk of a [systolic pulmonary artery pressure] greater than 35 mm Hg; however, with a severe NVC pattern, this risk was significantly increased (OR = 2.33).”
The investigators cautioned that their findings should be confirmed in larger cohorts. The study by Dr. Markusse and her colleagues was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
Systemic sclerosis is a profoundly heterogeneous disorder, with the overall prevalence of major organ-specific manifestations, such as pulmonary arterial hypertension (PAH), broadly adhering to a 15% rule. As such, the majority of patients with SSc will not develop any given organ-specific complication. The major challenge for clinicians during the early stages of the disease is predicting the future occurrence of potentially life-threatening organ-specific manifestations, such as PAH.
The complementary association of nailfold videocapillaroscopy changes and autoantibody profile in predicting cardiopulmonary involvement reported by Dr. Markusse and her colleagues is novel, but otherwise supports the findings of previous cross-sectional studies identifying associations between advanced NVC changes and SSc complications, such as digital ischemic lesions and PAH. These studies provide intriguing insight into the relationship between the evolution of microangiopathy and the emergence of organ-specific manifestations of SSc, but also represent a shift in focus from the diagnostic to the prognostic utility of NVC in SSc.
There is potential clinical utility in these observations that has yet to be unlocked fully; particularly should the predictive value and timing of NVC progression be further characterized in longitudinal studies better defining the natural history of SSc organ-specific manifestations. If evolving NVC changes (in high-risk serological subgroups) are shown to pre-date the emergence of overt organ-specific manifestations of SSc, then we might be provided with a window of opportunity for escalation of therapy with treatments targeting endothelial function (such as phosphodiesterase inhibitors and/or endothelin receptor antagonists) and/or possible immunomodulatory approaches. This could potentially usher in a new era of preventive disease-modifying therapeutic intervention in SSc.
John D. Pauling, MD, PhD, is a consultant rheumatologist at the Royal National Hospital for Rheumatic Diseases, Bath, England, and Visiting Senior Lecturer in the department of pharmacy and pharmacology at the University of Bath. His commentary is derived from an editorial accompanying the study by Dr. Markusse and her associates (Rheumatology [Oxford]. 2016 Dec 30. doi: 10.1093/rheumatology/kew461). He disclosed having received grants and consultancy income from Actelion.
Systemic sclerosis is a profoundly heterogeneous disorder, with the overall prevalence of major organ-specific manifestations, such as pulmonary arterial hypertension (PAH), broadly adhering to a 15% rule. As such, the majority of patients with SSc will not develop any given organ-specific complication. The major challenge for clinicians during the early stages of the disease is predicting the future occurrence of potentially life-threatening organ-specific manifestations, such as PAH.
The complementary association of nailfold videocapillaroscopy changes and autoantibody profile in predicting cardiopulmonary involvement reported by Dr. Markusse and her colleagues is novel, but otherwise supports the findings of previous cross-sectional studies identifying associations between advanced NVC changes and SSc complications, such as digital ischemic lesions and PAH. These studies provide intriguing insight into the relationship between the evolution of microangiopathy and the emergence of organ-specific manifestations of SSc, but also represent a shift in focus from the diagnostic to the prognostic utility of NVC in SSc.
There is potential clinical utility in these observations that has yet to be unlocked fully; particularly should the predictive value and timing of NVC progression be further characterized in longitudinal studies better defining the natural history of SSc organ-specific manifestations. If evolving NVC changes (in high-risk serological subgroups) are shown to pre-date the emergence of overt organ-specific manifestations of SSc, then we might be provided with a window of opportunity for escalation of therapy with treatments targeting endothelial function (such as phosphodiesterase inhibitors and/or endothelin receptor antagonists) and/or possible immunomodulatory approaches. This could potentially usher in a new era of preventive disease-modifying therapeutic intervention in SSc.
John D. Pauling, MD, PhD, is a consultant rheumatologist at the Royal National Hospital for Rheumatic Diseases, Bath, England, and Visiting Senior Lecturer in the department of pharmacy and pharmacology at the University of Bath. His commentary is derived from an editorial accompanying the study by Dr. Markusse and her associates (Rheumatology [Oxford]. 2016 Dec 30. doi: 10.1093/rheumatology/kew461). He disclosed having received grants and consultancy income from Actelion.
Systemic sclerosis is a profoundly heterogeneous disorder, with the overall prevalence of major organ-specific manifestations, such as pulmonary arterial hypertension (PAH), broadly adhering to a 15% rule. As such, the majority of patients with SSc will not develop any given organ-specific complication. The major challenge for clinicians during the early stages of the disease is predicting the future occurrence of potentially life-threatening organ-specific manifestations, such as PAH.
The complementary association of nailfold videocapillaroscopy changes and autoantibody profile in predicting cardiopulmonary involvement reported by Dr. Markusse and her colleagues is novel, but otherwise supports the findings of previous cross-sectional studies identifying associations between advanced NVC changes and SSc complications, such as digital ischemic lesions and PAH. These studies provide intriguing insight into the relationship between the evolution of microangiopathy and the emergence of organ-specific manifestations of SSc, but also represent a shift in focus from the diagnostic to the prognostic utility of NVC in SSc.
There is potential clinical utility in these observations that has yet to be unlocked fully; particularly should the predictive value and timing of NVC progression be further characterized in longitudinal studies better defining the natural history of SSc organ-specific manifestations. If evolving NVC changes (in high-risk serological subgroups) are shown to pre-date the emergence of overt organ-specific manifestations of SSc, then we might be provided with a window of opportunity for escalation of therapy with treatments targeting endothelial function (such as phosphodiesterase inhibitors and/or endothelin receptor antagonists) and/or possible immunomodulatory approaches. This could potentially usher in a new era of preventive disease-modifying therapeutic intervention in SSc.
John D. Pauling, MD, PhD, is a consultant rheumatologist at the Royal National Hospital for Rheumatic Diseases, Bath, England, and Visiting Senior Lecturer in the department of pharmacy and pharmacology at the University of Bath. His commentary is derived from an editorial accompanying the study by Dr. Markusse and her associates (Rheumatology [Oxford]. 2016 Dec 30. doi: 10.1093/rheumatology/kew461). He disclosed having received grants and consultancy income from Actelion.
Nailfold videocapillaroscopy can help to predict which patients with systemic sclerosis may develop serious cardiopulmonary complications, according to findings from a Dutch cross-sectional study.
While individual autoantibodies seen in systemic sclerosis (SSc) are known to be associated with greater or lesser risk of cardiopulmonary involvement, in this study nailfold vascularization patterns independently predicted pulmonary artery hypertension or interstitial lung disease.
All patients in the study had NVC pattern data as well as anti-extractable nuclear antigen (anti-ENA) antibodies. The mean age of the patients was 54 years; 82% were female, and median disease duration was 3 years. Just over half the cohort had interstitial lung disease, and 16% had pulmonary artery hypertension.
Among the anti-ENA autoantibody subtypes, anti-ACA was seen in 37% of patients, anti-Scl-70 in 24%, anti-RNP in 9%, and anti-RNAPIII in 5%; other subtypes were rarer. SSc-specific NVC patterns were seen in 88% of patients, with 10% of the cohort showing an early (less severe microangiopathy) pattern, 42% an active pattern, and 36% a late pattern.
One of the study’s objectives was to determine whether one or more mechanisms was responsible for both autoantibody production and the microangiopathy seen in SSc.
If a joint mechanism is implicated, “more severe NVC patterns would be determined in patients with autoantibodies (such as anti-Scl-70 and anti-RNAPIII) that are associated with more severe disease,” wrote Dr. Markusse and her colleagues. “On the other hand, if specific autoantibodies and stage of microangiopathy reflect different processes in the disease, a combination of autoantibody status and NVC could be helpful for identifying patients at highest risk for cardiopulmonary involvement.”
The investigators reported finding a similar distribution of NVC abnormalities across the major SSc autoantibody subtypes (except for anti–RNP-positive patients), suggesting that combinations of the two variables would be most predictive of cardiopulmonary involvement. More severe NVC patterns were associated with a higher risk of cardiopulmonary involvement, independent of the presence of a specific autoantibody.
Notably, the researchers wrote, “prevalence of ILD [interstitial lung disease] is generally lower among ACA-positive patients. According to our data, even among ACA-positive patients there was a trend for more ILD being associated with more severe NVC patterns (OR = 1.33).”
A similar pattern was seen for pulmonary artery hypertension. “Based on anti-RNP and anti-RNAPIII positivity, patients did not have an increased risk of a [systolic pulmonary artery pressure] greater than 35 mm Hg; however, with a severe NVC pattern, this risk was significantly increased (OR = 2.33).”
The investigators cautioned that their findings should be confirmed in larger cohorts. The study by Dr. Markusse and her colleagues was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
Nailfold videocapillaroscopy can help to predict which patients with systemic sclerosis may develop serious cardiopulmonary complications, according to findings from a Dutch cross-sectional study.
While individual autoantibodies seen in systemic sclerosis (SSc) are known to be associated with greater or lesser risk of cardiopulmonary involvement, in this study nailfold vascularization patterns independently predicted pulmonary artery hypertension or interstitial lung disease.
All patients in the study had NVC pattern data as well as anti-extractable nuclear antigen (anti-ENA) antibodies. The mean age of the patients was 54 years; 82% were female, and median disease duration was 3 years. Just over half the cohort had interstitial lung disease, and 16% had pulmonary artery hypertension.
Among the anti-ENA autoantibody subtypes, anti-ACA was seen in 37% of patients, anti-Scl-70 in 24%, anti-RNP in 9%, and anti-RNAPIII in 5%; other subtypes were rarer. SSc-specific NVC patterns were seen in 88% of patients, with 10% of the cohort showing an early (less severe microangiopathy) pattern, 42% an active pattern, and 36% a late pattern.
One of the study’s objectives was to determine whether one or more mechanisms was responsible for both autoantibody production and the microangiopathy seen in SSc.
If a joint mechanism is implicated, “more severe NVC patterns would be determined in patients with autoantibodies (such as anti-Scl-70 and anti-RNAPIII) that are associated with more severe disease,” wrote Dr. Markusse and her colleagues. “On the other hand, if specific autoantibodies and stage of microangiopathy reflect different processes in the disease, a combination of autoantibody status and NVC could be helpful for identifying patients at highest risk for cardiopulmonary involvement.”
The investigators reported finding a similar distribution of NVC abnormalities across the major SSc autoantibody subtypes (except for anti–RNP-positive patients), suggesting that combinations of the two variables would be most predictive of cardiopulmonary involvement. More severe NVC patterns were associated with a higher risk of cardiopulmonary involvement, independent of the presence of a specific autoantibody.
Notably, the researchers wrote, “prevalence of ILD [interstitial lung disease] is generally lower among ACA-positive patients. According to our data, even among ACA-positive patients there was a trend for more ILD being associated with more severe NVC patterns (OR = 1.33).”
A similar pattern was seen for pulmonary artery hypertension. “Based on anti-RNP and anti-RNAPIII positivity, patients did not have an increased risk of a [systolic pulmonary artery pressure] greater than 35 mm Hg; however, with a severe NVC pattern, this risk was significantly increased (OR = 2.33).”
The investigators cautioned that their findings should be confirmed in larger cohorts. The study by Dr. Markusse and her colleagues was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
FROM RHEUMATOLOGY
Key clinical point:
Major finding: Across the major autoantibody subtypes seen in an SSc cohort, NVC pattern showed a stable association with presence of interstitial lung disease (OR, 1.3-1.4) or elevated systolic pulmonary artery pressure (OR, 2.2-2.4).
Data source: A cross-section of 287 patients in a Dutch SSc cohort.
Disclosures: The study was conducted without outside funding, though manufacturers donated diagnostic antibody tests. One of the 11 study coauthors disclosed receiving financial support from Actelion.
Lupus patients may be shortchanged on lipid management
WASHINGTON – Many patients with systemic lupus erythematosus may not be getting lipid testing or statin prescriptions despite having risk factors and even frank cardiovascular conditions, according to a large Medicaid database study presented at the annual meeting of the American College of Rheumatology.
The study found that systemic lupus erythematosus (SLE) patients were half as likely to get a lipid screening and 77% less likely to get a statin prescription, compared with patients with diabetes, a group that has a similarly elevated baseline CV disease risk.
This is concerning because it has been shown in several studies that SLE patients have higher rates of CV events than do age- and sex-matched patients with diabetes, and hyperlipidemia has been associated with CV disease in SLE patients. However, no studies have directly demonstrated reduced CV disease risk with statin use in SLE patients, unlike the general population and in those with diabetes, and so no clear guidelines exist, said lead study author Sarah Chen, MD, a rheumatology fellow at Brigham and Women’s Hospital, Boston.
Analyses of 1 year of billing records for each patient showed that SLE patients visited their doctor significantly more often than did the diabetes patients during their index year (mean of 4.7 vs. 3.2 visits). They were more likely to have a renal manifestation of their disease (13% vs. 4%). Although hypertension was similar among lupus and diabetes patients (35% vs. 40%), lupus patients were less likely to have hyperlipidemia (11% vs. 24%) but more likely to have concurrent cardiovascular disease, defined as angina, heart attack, atherosclerosis, percutaneous coronary intervention, coronary artery bypass grafting, stroke, or peripheral artery disease (13% vs. 10%).
Overall, 27% of lupus patients and 42% of diabetes patients had at least one lipid screen during their study year. This proportion stayed steady among diabetes patients as they aged, but rose significantly among lupus patients from 23% in the youngest group of 18-39 years to 32% of the oldest group of 50-65 years.
“This suggests that diabetes patients are getting consistent cardiovascular assessment as they age, while lupus patients are not,” Dr. Chen said at the meeting.
Statin prescriptions were also significantly less common among lupus patients overall (12% vs. 33%). The proportion of lupus patients who got at least one statin prescription increased with age, from 7% in the youngest group to 21% in the oldest group. But it increased more among diabetes patients, from 23% among the youngest to 42% among the oldest, Dr. Chen noted.
She conducted a multivariate analysis that controlled for age, gender, race, ethnicity, region of residence, socioeconomic status, Charlson Comorbidity Index, and the presence of cardiovascular disease. This determined that lupus patients were 54% less likely to have lipid testing and 77% less likely to get a statin.
A subanalysis found some significant predictors of SLE patients’ receipt of lipid screening. Increasing age increased the odds of screening by 2% per year. Lipid screening was 6% more likely for each additional medication they took. Lupus patients with nephritis were 36% more likely to have a screening than were those without nephritis. Those with existing cardiovascular disease were 11% more likely to have a test than were those without it, although this odds ratio (OR) had a nonsignificant 95% confidence interval of 1.00-1.24.
Hispanic and Asian patients were significantly more likely to receive lipid testing than were whites.
A second subanalysis found significant predictors of lupus patients receiving a statin prescription among lupus patients. With every passing year, the chance of getting a statin increased by 5%; it also increased by 7% for every medication that a patient was taking. Being a male increased the likelihood of receiving a statin prescription by 23%. Lupus nephritis more than doubled the odds of getting a statin (OR, 2.34), and the presence of cardiovascular disease was a similarly strong predictor (OR, 1.95). Hispanics and Asians were more likely to get the medication than were whites.
One significant limitation of the study is a lack of results on lipid testing, particularly in light of the potential effect the results might have had on decisions to prescribe statins.
Dr. Chen had no financial disclosures. The study was supported by a grant from the National Institutes of Health.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Many patients with systemic lupus erythematosus may not be getting lipid testing or statin prescriptions despite having risk factors and even frank cardiovascular conditions, according to a large Medicaid database study presented at the annual meeting of the American College of Rheumatology.
The study found that systemic lupus erythematosus (SLE) patients were half as likely to get a lipid screening and 77% less likely to get a statin prescription, compared with patients with diabetes, a group that has a similarly elevated baseline CV disease risk.
This is concerning because it has been shown in several studies that SLE patients have higher rates of CV events than do age- and sex-matched patients with diabetes, and hyperlipidemia has been associated with CV disease in SLE patients. However, no studies have directly demonstrated reduced CV disease risk with statin use in SLE patients, unlike the general population and in those with diabetes, and so no clear guidelines exist, said lead study author Sarah Chen, MD, a rheumatology fellow at Brigham and Women’s Hospital, Boston.
Analyses of 1 year of billing records for each patient showed that SLE patients visited their doctor significantly more often than did the diabetes patients during their index year (mean of 4.7 vs. 3.2 visits). They were more likely to have a renal manifestation of their disease (13% vs. 4%). Although hypertension was similar among lupus and diabetes patients (35% vs. 40%), lupus patients were less likely to have hyperlipidemia (11% vs. 24%) but more likely to have concurrent cardiovascular disease, defined as angina, heart attack, atherosclerosis, percutaneous coronary intervention, coronary artery bypass grafting, stroke, or peripheral artery disease (13% vs. 10%).
Overall, 27% of lupus patients and 42% of diabetes patients had at least one lipid screen during their study year. This proportion stayed steady among diabetes patients as they aged, but rose significantly among lupus patients from 23% in the youngest group of 18-39 years to 32% of the oldest group of 50-65 years.
“This suggests that diabetes patients are getting consistent cardiovascular assessment as they age, while lupus patients are not,” Dr. Chen said at the meeting.
Statin prescriptions were also significantly less common among lupus patients overall (12% vs. 33%). The proportion of lupus patients who got at least one statin prescription increased with age, from 7% in the youngest group to 21% in the oldest group. But it increased more among diabetes patients, from 23% among the youngest to 42% among the oldest, Dr. Chen noted.
She conducted a multivariate analysis that controlled for age, gender, race, ethnicity, region of residence, socioeconomic status, Charlson Comorbidity Index, and the presence of cardiovascular disease. This determined that lupus patients were 54% less likely to have lipid testing and 77% less likely to get a statin.
A subanalysis found some significant predictors of SLE patients’ receipt of lipid screening. Increasing age increased the odds of screening by 2% per year. Lipid screening was 6% more likely for each additional medication they took. Lupus patients with nephritis were 36% more likely to have a screening than were those without nephritis. Those with existing cardiovascular disease were 11% more likely to have a test than were those without it, although this odds ratio (OR) had a nonsignificant 95% confidence interval of 1.00-1.24.
Hispanic and Asian patients were significantly more likely to receive lipid testing than were whites.
A second subanalysis found significant predictors of lupus patients receiving a statin prescription among lupus patients. With every passing year, the chance of getting a statin increased by 5%; it also increased by 7% for every medication that a patient was taking. Being a male increased the likelihood of receiving a statin prescription by 23%. Lupus nephritis more than doubled the odds of getting a statin (OR, 2.34), and the presence of cardiovascular disease was a similarly strong predictor (OR, 1.95). Hispanics and Asians were more likely to get the medication than were whites.
One significant limitation of the study is a lack of results on lipid testing, particularly in light of the potential effect the results might have had on decisions to prescribe statins.
Dr. Chen had no financial disclosures. The study was supported by a grant from the National Institutes of Health.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Many patients with systemic lupus erythematosus may not be getting lipid testing or statin prescriptions despite having risk factors and even frank cardiovascular conditions, according to a large Medicaid database study presented at the annual meeting of the American College of Rheumatology.
The study found that systemic lupus erythematosus (SLE) patients were half as likely to get a lipid screening and 77% less likely to get a statin prescription, compared with patients with diabetes, a group that has a similarly elevated baseline CV disease risk.
This is concerning because it has been shown in several studies that SLE patients have higher rates of CV events than do age- and sex-matched patients with diabetes, and hyperlipidemia has been associated with CV disease in SLE patients. However, no studies have directly demonstrated reduced CV disease risk with statin use in SLE patients, unlike the general population and in those with diabetes, and so no clear guidelines exist, said lead study author Sarah Chen, MD, a rheumatology fellow at Brigham and Women’s Hospital, Boston.
Analyses of 1 year of billing records for each patient showed that SLE patients visited their doctor significantly more often than did the diabetes patients during their index year (mean of 4.7 vs. 3.2 visits). They were more likely to have a renal manifestation of their disease (13% vs. 4%). Although hypertension was similar among lupus and diabetes patients (35% vs. 40%), lupus patients were less likely to have hyperlipidemia (11% vs. 24%) but more likely to have concurrent cardiovascular disease, defined as angina, heart attack, atherosclerosis, percutaneous coronary intervention, coronary artery bypass grafting, stroke, or peripheral artery disease (13% vs. 10%).
Overall, 27% of lupus patients and 42% of diabetes patients had at least one lipid screen during their study year. This proportion stayed steady among diabetes patients as they aged, but rose significantly among lupus patients from 23% in the youngest group of 18-39 years to 32% of the oldest group of 50-65 years.
“This suggests that diabetes patients are getting consistent cardiovascular assessment as they age, while lupus patients are not,” Dr. Chen said at the meeting.
Statin prescriptions were also significantly less common among lupus patients overall (12% vs. 33%). The proportion of lupus patients who got at least one statin prescription increased with age, from 7% in the youngest group to 21% in the oldest group. But it increased more among diabetes patients, from 23% among the youngest to 42% among the oldest, Dr. Chen noted.
She conducted a multivariate analysis that controlled for age, gender, race, ethnicity, region of residence, socioeconomic status, Charlson Comorbidity Index, and the presence of cardiovascular disease. This determined that lupus patients were 54% less likely to have lipid testing and 77% less likely to get a statin.
A subanalysis found some significant predictors of SLE patients’ receipt of lipid screening. Increasing age increased the odds of screening by 2% per year. Lipid screening was 6% more likely for each additional medication they took. Lupus patients with nephritis were 36% more likely to have a screening than were those without nephritis. Those with existing cardiovascular disease were 11% more likely to have a test than were those without it, although this odds ratio (OR) had a nonsignificant 95% confidence interval of 1.00-1.24.
Hispanic and Asian patients were significantly more likely to receive lipid testing than were whites.
A second subanalysis found significant predictors of lupus patients receiving a statin prescription among lupus patients. With every passing year, the chance of getting a statin increased by 5%; it also increased by 7% for every medication that a patient was taking. Being a male increased the likelihood of receiving a statin prescription by 23%. Lupus nephritis more than doubled the odds of getting a statin (OR, 2.34), and the presence of cardiovascular disease was a similarly strong predictor (OR, 1.95). Hispanics and Asians were more likely to get the medication than were whites.
One significant limitation of the study is a lack of results on lipid testing, particularly in light of the potential effect the results might have had on decisions to prescribe statins.
Dr. Chen had no financial disclosures. The study was supported by a grant from the National Institutes of Health.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Lupus patients were 54% less likely to have lipid level testing and 77% less likely to have a statin prescribed than were diabetes patients.
Data source: A Medicaid database review comprising nearly 20,000 lupus patients and 40,000 diabetes patients during 2007-2010.
Disclosures: Dr. Chen had no financial disclosures. The study was supported by a grant from the National Institutes of Health.
Prenatal exposure to hydroxychloroquine cuts risk of neonatal cutaneous lupus
WASHINGTON – Prenatal hydroxychloroquine reduces the risk of cutaneous neonatal lupus by 60% among the infants of women with a systemic autoimmune rheumatic disease.
The medication easily passes the placental barrier and confers significant protection to neonates born to women who have anti-Ro and anti-La antibodies, Julie Barsalou, MD, said at the annual meeting of the American College of Rheumatology.
Toll-like receptors 7 and 9 have been implicated in the initiation and maintenance of interface dermatitis, and hydroxychloroquine inhibits these receptors. In mouse studies, the drug has led to improvement of this type of dermatitis. Hydroxychloroquine also works well in treating subacute cutaneous lupus, she said, and because it can travel across the placenta, it could be an effective means of preventing this disorder in at-risk neonates.
To examine any potential benefit, Dr. Barsalou looked at three pediatric lupus databases: the SickKids NLE database from Toronto, the U.S. Research Registry for Neonatal Lupus, and the French Registry for Neonatal Lupus.
These registries include infants born to mothers with anti-Ro and/or anti-La antibodies, and a diagnosis of lupus, dermatomyositis, Sjögren’s syndrome, juvenile idiopathic arthritis, or rheumatic arthritis. No infants with cardiac neonatal lupus were included in the study.
In addition to hydroxychloroquine, Dr. Barsalou examined the use of prenatal azathioprine, nonfluorinated and fluorinated steroids, and intravenous immunoglobulin.
The cohort comprised 545 neonates born to 535 mothers. Among these, 112 developed cNLE. The remaining 433 infants were used as controls.
Mothers of both cases and controls were a mean age of 31 years. Among cases, the most common diagnosis was Sjögren’s syndrome (53%), followed by systemic lupus erythematosus (46%). Among controls, the most common maternal diagnosis was SLE (62%), followed by Sjögren’s (31%).
All mothers of cases were positive for anti-Ro antibodies; 72% were positive for anti-La antibodies. Among mothers of controls, 99% had anti-Ro antibodies and 48% had anti-La antibodies.
Mothers of cases took hydroxychloroquine (17%), fluorinated steroids (6%), and nonfluorinated steroids with or without azathioprine (28%). Mothers of controls took hydroxychloroquine (34%), fluorinated steroids (4%), and nonfluorinated steroids with or without azathioprine (44%),
There were significantly more female than male infants in the case group (65%). The median age at rash onset was 6 weeks.
Dr. Barsalou performed several multivariate analyses on the entire cohort, as well as two subgroup analyses: one on infants who developed the cNLE rash within the first 4 weeks of life and one on only the infants of mothers with SLE.
In the primary analysis, maternal anti-Ro and anti-La antibodies more than doubled the risk of an infant developing cNLE (odds ratio, 2.5). The use of hydroxychloroquine decreased this risk by 60% (OR, 0.4). Being a female infant increased the risk by 70% (OR, 1.7).
In the group of infants with early-onset rash, maternal anti-La antibodies more than tripled the risk (OR, 3.5), while the use of hydroxychloroquine decreased the risk by 80% (OR, 0.2).
Among the infants born to women with SLE, concomitant secondary Sjögren’s syndrome increased the risk of cNLE by more than threefold (OR, 3.5). Anti-La antibodies more than doubled the risk (OR, 2.5), and the use of hydroxychloroquine decreased it by 60% (OR, 0.4).
“This is the first study to address the prevention of cutaneous neonatal lupus,” Dr. Barsalou said. “We found that prenatal exposure to hydroxychloroquine is likely protective.”
She had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Prenatal hydroxychloroquine reduces the risk of cutaneous neonatal lupus by 60% among the infants of women with a systemic autoimmune rheumatic disease.
The medication easily passes the placental barrier and confers significant protection to neonates born to women who have anti-Ro and anti-La antibodies, Julie Barsalou, MD, said at the annual meeting of the American College of Rheumatology.
Toll-like receptors 7 and 9 have been implicated in the initiation and maintenance of interface dermatitis, and hydroxychloroquine inhibits these receptors. In mouse studies, the drug has led to improvement of this type of dermatitis. Hydroxychloroquine also works well in treating subacute cutaneous lupus, she said, and because it can travel across the placenta, it could be an effective means of preventing this disorder in at-risk neonates.
To examine any potential benefit, Dr. Barsalou looked at three pediatric lupus databases: the SickKids NLE database from Toronto, the U.S. Research Registry for Neonatal Lupus, and the French Registry for Neonatal Lupus.
These registries include infants born to mothers with anti-Ro and/or anti-La antibodies, and a diagnosis of lupus, dermatomyositis, Sjögren’s syndrome, juvenile idiopathic arthritis, or rheumatic arthritis. No infants with cardiac neonatal lupus were included in the study.
In addition to hydroxychloroquine, Dr. Barsalou examined the use of prenatal azathioprine, nonfluorinated and fluorinated steroids, and intravenous immunoglobulin.
The cohort comprised 545 neonates born to 535 mothers. Among these, 112 developed cNLE. The remaining 433 infants were used as controls.
Mothers of both cases and controls were a mean age of 31 years. Among cases, the most common diagnosis was Sjögren’s syndrome (53%), followed by systemic lupus erythematosus (46%). Among controls, the most common maternal diagnosis was SLE (62%), followed by Sjögren’s (31%).
All mothers of cases were positive for anti-Ro antibodies; 72% were positive for anti-La antibodies. Among mothers of controls, 99% had anti-Ro antibodies and 48% had anti-La antibodies.
Mothers of cases took hydroxychloroquine (17%), fluorinated steroids (6%), and nonfluorinated steroids with or without azathioprine (28%). Mothers of controls took hydroxychloroquine (34%), fluorinated steroids (4%), and nonfluorinated steroids with or without azathioprine (44%),
There were significantly more female than male infants in the case group (65%). The median age at rash onset was 6 weeks.
Dr. Barsalou performed several multivariate analyses on the entire cohort, as well as two subgroup analyses: one on infants who developed the cNLE rash within the first 4 weeks of life and one on only the infants of mothers with SLE.
In the primary analysis, maternal anti-Ro and anti-La antibodies more than doubled the risk of an infant developing cNLE (odds ratio, 2.5). The use of hydroxychloroquine decreased this risk by 60% (OR, 0.4). Being a female infant increased the risk by 70% (OR, 1.7).
In the group of infants with early-onset rash, maternal anti-La antibodies more than tripled the risk (OR, 3.5), while the use of hydroxychloroquine decreased the risk by 80% (OR, 0.2).
Among the infants born to women with SLE, concomitant secondary Sjögren’s syndrome increased the risk of cNLE by more than threefold (OR, 3.5). Anti-La antibodies more than doubled the risk (OR, 2.5), and the use of hydroxychloroquine decreased it by 60% (OR, 0.4).
“This is the first study to address the prevention of cutaneous neonatal lupus,” Dr. Barsalou said. “We found that prenatal exposure to hydroxychloroquine is likely protective.”
She had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Prenatal hydroxychloroquine reduces the risk of cutaneous neonatal lupus by 60% among the infants of women with a systemic autoimmune rheumatic disease.
The medication easily passes the placental barrier and confers significant protection to neonates born to women who have anti-Ro and anti-La antibodies, Julie Barsalou, MD, said at the annual meeting of the American College of Rheumatology.
Toll-like receptors 7 and 9 have been implicated in the initiation and maintenance of interface dermatitis, and hydroxychloroquine inhibits these receptors. In mouse studies, the drug has led to improvement of this type of dermatitis. Hydroxychloroquine also works well in treating subacute cutaneous lupus, she said, and because it can travel across the placenta, it could be an effective means of preventing this disorder in at-risk neonates.
To examine any potential benefit, Dr. Barsalou looked at three pediatric lupus databases: the SickKids NLE database from Toronto, the U.S. Research Registry for Neonatal Lupus, and the French Registry for Neonatal Lupus.
These registries include infants born to mothers with anti-Ro and/or anti-La antibodies, and a diagnosis of lupus, dermatomyositis, Sjögren’s syndrome, juvenile idiopathic arthritis, or rheumatic arthritis. No infants with cardiac neonatal lupus were included in the study.
In addition to hydroxychloroquine, Dr. Barsalou examined the use of prenatal azathioprine, nonfluorinated and fluorinated steroids, and intravenous immunoglobulin.
The cohort comprised 545 neonates born to 535 mothers. Among these, 112 developed cNLE. The remaining 433 infants were used as controls.
Mothers of both cases and controls were a mean age of 31 years. Among cases, the most common diagnosis was Sjögren’s syndrome (53%), followed by systemic lupus erythematosus (46%). Among controls, the most common maternal diagnosis was SLE (62%), followed by Sjögren’s (31%).
All mothers of cases were positive for anti-Ro antibodies; 72% were positive for anti-La antibodies. Among mothers of controls, 99% had anti-Ro antibodies and 48% had anti-La antibodies.
Mothers of cases took hydroxychloroquine (17%), fluorinated steroids (6%), and nonfluorinated steroids with or without azathioprine (28%). Mothers of controls took hydroxychloroquine (34%), fluorinated steroids (4%), and nonfluorinated steroids with or without azathioprine (44%),
There were significantly more female than male infants in the case group (65%). The median age at rash onset was 6 weeks.
Dr. Barsalou performed several multivariate analyses on the entire cohort, as well as two subgroup analyses: one on infants who developed the cNLE rash within the first 4 weeks of life and one on only the infants of mothers with SLE.
In the primary analysis, maternal anti-Ro and anti-La antibodies more than doubled the risk of an infant developing cNLE (odds ratio, 2.5). The use of hydroxychloroquine decreased this risk by 60% (OR, 0.4). Being a female infant increased the risk by 70% (OR, 1.7).
In the group of infants with early-onset rash, maternal anti-La antibodies more than tripled the risk (OR, 3.5), while the use of hydroxychloroquine decreased the risk by 80% (OR, 0.2).
Among the infants born to women with SLE, concomitant secondary Sjögren’s syndrome increased the risk of cNLE by more than threefold (OR, 3.5). Anti-La antibodies more than doubled the risk (OR, 2.5), and the use of hydroxychloroquine decreased it by 60% (OR, 0.4).
“This is the first study to address the prevention of cutaneous neonatal lupus,” Dr. Barsalou said. “We found that prenatal exposure to hydroxychloroquine is likely protective.”
She had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The drug was associated with a 60% decreased risk of developing the disorder.
Data source: The case-control study involved 545 infants.
Disclosures: Dr. Barsalou had no financial disclosures.
Mycophenolate puts lupus patients in remission faster than azathioprine
WASHINGTON – Mycophenolate sodium conferred complete remission sooner and more often than did azathioprine in a 24-month, open-label, randomized trial of patients with nonrenal systemic lupus erythematosus.
The enteric-coated version of mycophenolate used in the study was well tolerated, too, with just 7.5% of patients reporting gastrointestinal symptoms – a distinct advantage, Josefina Cortés-Hernández, MD, reported at the annual meeting of the American College of Rheumatology.
But azathioprine still holds one critical advantage over mycophenolate, said Dr. Cortés-Hernández of Vall d´Hebron Hospital, Barcelona: It is safe for use in pregnancy. “This is a very important advantage that we should not lose sight of,” she said.
Dr. Cortés-Hernández and her associates at 13 centers across Spain randomized 240 patients with nonrenal, active systemic lupus erythematosus (SLE) to either azathioprine (target dose, 2 mg/kg per day) or the enteric-coated mycophenolate sodium (target dose, 1,440 mg/day). Patients could add both a corticosteroid and an antimalarial as needed. No patient was allowed to take immunosuppressive therapy for at least 3 months before randomization.
The primary endpoint of the trial was complete remission at 3 months and 24 months, defined as an SLE Disease Activity Index (SLEDAI) less than 4 and/or absence of any BILAG (British Isles Lupus Assessment Group) A or B flares. Secondary endpoints were time to remission, time to first flare, and changes in prednisone use.
The patients were primarily women with a mean age of 41 years and mean disease duration of 5 years. The mean SLEDAI was 9.5; 45% had a mean SLEDAI of 10 or higher. The mean BILAG score was about 19. All the patients had autoantibodies, and about half had anti–double-stranded DNA antibodies. Half of the patients also had low complement C3 and/or C4 levels. About 80% were taking an antimalarial and 95%, a corticosteroid; the mean daily prednisone dose equivalent was about 19 mg.
In an intent-to-treat analysis, patients treated with mycophenolate had a higher remission rate at both 3 and 24 months. At 3 months, the complete remission rate was 33% for mycophenolate, compared with 19% for azathioprine. Clinical response improved over time in both groups, but mycophenolate remained significantly more effective over the entire study period. By 24 months, complete remission was present in 71% of those taking mycophenolate versus 48% of those taking azathioprine.
Both groups had significant improvements over baseline in both the SLEDAI and BILAG scores. By 24 months, the SLEDAI had dropped to a mean of 3 in the mycophenolate group and 4 in the azathioprine group. The BILAG dropped from a mean of 19 at baseline to 2 in the mycophenolate group and 5 in the azathioprine group.
BILAG A/B flares occurred in 50% of the mycophenolate group and 72% of the azathioprine group. The time to first flare was longer with mycophenolate, as was the time to a severe flare. New BILAG A flares occurred in 8% of the mycophenolate group and 22% of the azathioprine group. Azathioprine was associated with more renal flares (7% vs. 2%) and more hematologic flares (7.5% vs. 2.5%)
Steroid use declined to a prednisone equivalent of less than 7.5 mg/day in significantly more patients taking mycophenolate than azathioprine (95% vs. 85%).
Adverse events occurred in about 70% of each group; these were serious in about 10% of each group. Three patients taking mycophenolate and 10 taking azathioprine discontinued because of an adverse event, but this wasn’t statistically significant. Infections were the most common problem, occurring in about a quarter of each group; these were serious in five mycophenolate patients and seven azathioprine patients. There were two deaths, one in each group. Cancers occurred in three taking azathioprine and one taking mycophenolate. Leukopenia occurred in five patients taking azathioprine but in no one taking mycophenolate.
The study was funded by the Spanish Ministry of Health. Dr. Cortés-Hernández had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Mycophenolate sodium conferred complete remission sooner and more often than did azathioprine in a 24-month, open-label, randomized trial of patients with nonrenal systemic lupus erythematosus.
The enteric-coated version of mycophenolate used in the study was well tolerated, too, with just 7.5% of patients reporting gastrointestinal symptoms – a distinct advantage, Josefina Cortés-Hernández, MD, reported at the annual meeting of the American College of Rheumatology.
But azathioprine still holds one critical advantage over mycophenolate, said Dr. Cortés-Hernández of Vall d´Hebron Hospital, Barcelona: It is safe for use in pregnancy. “This is a very important advantage that we should not lose sight of,” she said.
Dr. Cortés-Hernández and her associates at 13 centers across Spain randomized 240 patients with nonrenal, active systemic lupus erythematosus (SLE) to either azathioprine (target dose, 2 mg/kg per day) or the enteric-coated mycophenolate sodium (target dose, 1,440 mg/day). Patients could add both a corticosteroid and an antimalarial as needed. No patient was allowed to take immunosuppressive therapy for at least 3 months before randomization.
The primary endpoint of the trial was complete remission at 3 months and 24 months, defined as an SLE Disease Activity Index (SLEDAI) less than 4 and/or absence of any BILAG (British Isles Lupus Assessment Group) A or B flares. Secondary endpoints were time to remission, time to first flare, and changes in prednisone use.
The patients were primarily women with a mean age of 41 years and mean disease duration of 5 years. The mean SLEDAI was 9.5; 45% had a mean SLEDAI of 10 or higher. The mean BILAG score was about 19. All the patients had autoantibodies, and about half had anti–double-stranded DNA antibodies. Half of the patients also had low complement C3 and/or C4 levels. About 80% were taking an antimalarial and 95%, a corticosteroid; the mean daily prednisone dose equivalent was about 19 mg.
In an intent-to-treat analysis, patients treated with mycophenolate had a higher remission rate at both 3 and 24 months. At 3 months, the complete remission rate was 33% for mycophenolate, compared with 19% for azathioprine. Clinical response improved over time in both groups, but mycophenolate remained significantly more effective over the entire study period. By 24 months, complete remission was present in 71% of those taking mycophenolate versus 48% of those taking azathioprine.
Both groups had significant improvements over baseline in both the SLEDAI and BILAG scores. By 24 months, the SLEDAI had dropped to a mean of 3 in the mycophenolate group and 4 in the azathioprine group. The BILAG dropped from a mean of 19 at baseline to 2 in the mycophenolate group and 5 in the azathioprine group.
BILAG A/B flares occurred in 50% of the mycophenolate group and 72% of the azathioprine group. The time to first flare was longer with mycophenolate, as was the time to a severe flare. New BILAG A flares occurred in 8% of the mycophenolate group and 22% of the azathioprine group. Azathioprine was associated with more renal flares (7% vs. 2%) and more hematologic flares (7.5% vs. 2.5%)
Steroid use declined to a prednisone equivalent of less than 7.5 mg/day in significantly more patients taking mycophenolate than azathioprine (95% vs. 85%).
Adverse events occurred in about 70% of each group; these were serious in about 10% of each group. Three patients taking mycophenolate and 10 taking azathioprine discontinued because of an adverse event, but this wasn’t statistically significant. Infections were the most common problem, occurring in about a quarter of each group; these were serious in five mycophenolate patients and seven azathioprine patients. There were two deaths, one in each group. Cancers occurred in three taking azathioprine and one taking mycophenolate. Leukopenia occurred in five patients taking azathioprine but in no one taking mycophenolate.
The study was funded by the Spanish Ministry of Health. Dr. Cortés-Hernández had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Mycophenolate sodium conferred complete remission sooner and more often than did azathioprine in a 24-month, open-label, randomized trial of patients with nonrenal systemic lupus erythematosus.
The enteric-coated version of mycophenolate used in the study was well tolerated, too, with just 7.5% of patients reporting gastrointestinal symptoms – a distinct advantage, Josefina Cortés-Hernández, MD, reported at the annual meeting of the American College of Rheumatology.
But azathioprine still holds one critical advantage over mycophenolate, said Dr. Cortés-Hernández of Vall d´Hebron Hospital, Barcelona: It is safe for use in pregnancy. “This is a very important advantage that we should not lose sight of,” she said.
Dr. Cortés-Hernández and her associates at 13 centers across Spain randomized 240 patients with nonrenal, active systemic lupus erythematosus (SLE) to either azathioprine (target dose, 2 mg/kg per day) or the enteric-coated mycophenolate sodium (target dose, 1,440 mg/day). Patients could add both a corticosteroid and an antimalarial as needed. No patient was allowed to take immunosuppressive therapy for at least 3 months before randomization.
The primary endpoint of the trial was complete remission at 3 months and 24 months, defined as an SLE Disease Activity Index (SLEDAI) less than 4 and/or absence of any BILAG (British Isles Lupus Assessment Group) A or B flares. Secondary endpoints were time to remission, time to first flare, and changes in prednisone use.
The patients were primarily women with a mean age of 41 years and mean disease duration of 5 years. The mean SLEDAI was 9.5; 45% had a mean SLEDAI of 10 or higher. The mean BILAG score was about 19. All the patients had autoantibodies, and about half had anti–double-stranded DNA antibodies. Half of the patients also had low complement C3 and/or C4 levels. About 80% were taking an antimalarial and 95%, a corticosteroid; the mean daily prednisone dose equivalent was about 19 mg.
In an intent-to-treat analysis, patients treated with mycophenolate had a higher remission rate at both 3 and 24 months. At 3 months, the complete remission rate was 33% for mycophenolate, compared with 19% for azathioprine. Clinical response improved over time in both groups, but mycophenolate remained significantly more effective over the entire study period. By 24 months, complete remission was present in 71% of those taking mycophenolate versus 48% of those taking azathioprine.
Both groups had significant improvements over baseline in both the SLEDAI and BILAG scores. By 24 months, the SLEDAI had dropped to a mean of 3 in the mycophenolate group and 4 in the azathioprine group. The BILAG dropped from a mean of 19 at baseline to 2 in the mycophenolate group and 5 in the azathioprine group.
BILAG A/B flares occurred in 50% of the mycophenolate group and 72% of the azathioprine group. The time to first flare was longer with mycophenolate, as was the time to a severe flare. New BILAG A flares occurred in 8% of the mycophenolate group and 22% of the azathioprine group. Azathioprine was associated with more renal flares (7% vs. 2%) and more hematologic flares (7.5% vs. 2.5%)
Steroid use declined to a prednisone equivalent of less than 7.5 mg/day in significantly more patients taking mycophenolate than azathioprine (95% vs. 85%).
Adverse events occurred in about 70% of each group; these were serious in about 10% of each group. Three patients taking mycophenolate and 10 taking azathioprine discontinued because of an adverse event, but this wasn’t statistically significant. Infections were the most common problem, occurring in about a quarter of each group; these were serious in five mycophenolate patients and seven azathioprine patients. There were two deaths, one in each group. Cancers occurred in three taking azathioprine and one taking mycophenolate. Leukopenia occurred in five patients taking azathioprine but in no one taking mycophenolate.
The study was funded by the Spanish Ministry of Health. Dr. Cortés-Hernández had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
Key clinical point:
Major finding: Complete remission rates at 3 months were 32% for mycophenolate and 19% for azathioprine.
Data source: A 24-month, open-label, randomized trial of 240 patients.
Disclosures: The Spanish Ministry of Health funded the study. Dr. Cortés-Hernández had no relevant financial disclosures.