User login
Antiseptic as good as antibiotics for preventing recurrent UTI
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
The antiseptic methenamine hippurate (MH) is known to sterilize urine and has been suggested to be of use in preventing urinary tract infections (UTIs), but firm evidence has so far been lacking. Now researchers led by clinicians and scientists from Newcastle-upon-Tyne, England, have provided the ALTAR trial (Alternative to Prophylactic Antibiotics for the Treatment of Recurrent UTIs in Women).
Daily low-dose antibiotics as recommended by current guidelines for prophylactic treatment of recurrent UTI have been linked to antibiotic resistance. Using MH as an alternative could play an important role in helping to tackle the global problem of increasing antibiotic resistance, the team said.
Study details
They recruited 240 women aged 18 or over with recurrent UTIs requiring prophylactic treatment from eight secondary care urology and urogynecology centers in the United Kingdom from June 2016 to June 2018. Women were randomized to receive MH or daily low-dose antibiotics for 12 months, with follow up for a further 6 months beyond that.
Before trial entry the women had experienced an average of more than six UTI episodes per year. During the 12-month treatment period, in the modified intention-to-treat population, there were 90 symptomatic, antibiotic-treated UTI episodes reported over 101 person-years of follow-up in the antibiotic group, and 141 episodes over 102 person-years in the MH group.
This yielded a UTI rate of 0.89 episodes per person-year in the antibiotic group, compared with 1.38 in the MH group, an absolute difference of 0.49 episodes per person-year. In the 6-month posttreatment follow-up period, the UTI incidence rate was 1.19 episodes per person-year in the antibiotic prophylaxis group versus 1.72 in the MH group, an absolute difference of 0.53.
Before the trial, a patient and public involvement group had predefined the noninferiority margin as one episode of UTI per person-year. The small difference between the two groups was less than this, confirming noninferiority of MH to antibiotic prophylaxis in this setting. This finding was consistent across the modified intention-to-treat, strict intention-to-treat, per protocol, and modified per protocol (post hoc) analyses.
Thus the ALTAR results showed that MH was no worse than antibiotics at preventing UTIs, and MH was also associated with reduced antibiotic consumption.
The vast majority of participants were over 90% adherent with the allocated treatment. Patient satisfaction was generally high and rates of adverse events and adverse reactions generally low, and both were comparable between treatment groups. Adverse reactions were reported by 34/142 (24%) in the antibiotic group and 35/127 (28%) in the MH group, and most reactions were mild. In the antibiotic group there were two serious adverse reactions (severe abdominal pain and raised alanine transaminase), whereas six participants in the MH group reported an episode of febrile UTI and four were admitted to hospital because of UTI.
Substantial global health care problem
At least 50% and up to 80% of all women have at least one acute UTI in their lifetime, most often uncomplicated acute cystitis. About a quarter of them go on to suffer recurrent infection, defined as three or more repeat infections in the past year, or two infections in the preceding 6 months. Frequent recurrences thus represent “a substantial global health care problem,” the authors say.
Guidelines from the United Kingdom, Europe, and the United States acknowledge the need for preventive strategies and strongly recommend the use of daily, low-dose antibiotics as standard prophylactic treatment. However, the United Kingdom’s antimicrobial resistance strategy recommends a “strong focus on infection prevention,” and aims to reduce antimicrobial use in humans by 15% before 2024.
“To achieve that, exploration of nonantibiotic preventive treatments in common conditions such as UTI is essential,” the team said.
MH is one such nonantibiotic treatment. It is bactericidal and works by denaturing bacterial proteins and nucleic acids. Although previous Cochrane systematic reviews had concluded that it could be effective for preventing UTI, further large trials were needed.
“This trial adds to the evidence base for the use of MH for prophylactic treatment in adult women with recurrent UTI. Although the MH group had a 55% higher rate of UTI episodes than the antibiotics group, the absolute difference was just 0.49 UTI episodes per year, which has limited clinical consequence,” the team concluded.
Results could ‘support a change in practice’
In older patients, particularly, the risks of long-term antibiotic prophylaxis might outweigh the benefits, and the authors said that their results “could support a change in practice in terms of preventive treatments for recurrent UTI and provide patients and clinicians with a credible alternative to daily antibiotics, giving them the confidence to pursue strategies that avoid long-term antibiotic use.”
They acknowledged limitations of the study, including that treatment allocation was not masked, crossover between arms was allowed, and differences in antibiotics prescribed may have affected the results. In addition, data regarding long-term safety of MH are scarce.
However, they said that the trial accurately represented the broad range of women with recurrent UTI, and that its results “might encourage patients and clinicians to consider MH as a first line treatment for UTI prevention in women.”
In a linked editorial, scientists from the Institute for Evidence-Based Healthcare at Bond University in Queensland, Australia, commented: “Although the results need cautious interpretation, they align with others, and this new research increases the confidence with which MH can be offered as an option to women needing prophylaxis against recurrent urinary tract infection.”
References
Harding C et al. Alternative to prophylactic antibiotics for the treatment of recurrent urinary tract infections in women: multicentre, open label, randomised, noninferiority trial. BMJ 2022 Mar 9;376:e068229.
Hoffmann TC et al. Methenamine hippurate for recurrent urinary tract infections. BMJ 2022 Mar 9;376:o533.
A version of this article first appeared on Medscape.co.uk.
C. difficile: New vancomycin-resistant strains raise concerns
Samples from patients in the United States and Kenya show an increasing emergence of previously undetected vancomycin-resistant strains of Clostridioides difficile, sparking concern as recurrences in the treatment of C. difficile infection (CDI) continue to rise.
“Our results may help explain a decreasing effectiveness of antibiotic-based therapy in C. difficile infection, since a significant proportion of patients harboring strains with reduced susceptibility to vancomycin may not respond to treatment,” reported the authors in research published recently in Clinical Infectious Diseases.
The spread of the resistant strains “has serious public health implications, underscoring an urgent need for a comprehensive analysis of the circulating strains to help inform clinical decisions,” they added.
Commenting on the findings, Cornelius J. Clancy, MD, professor of medicine at the University of Pittsburgh, and chief of infectious diseases at the Veterans Affairs Pittsburgh Healthcare System, echoed the concern.
“The casual belief has been that [C. difficile] strains at most centers can be assumed to be vancomycin susceptible,” he told this news organization. “This study shows that this assumption can no longer be taken as a given.”
Dr. Clancy, who was not involved with this research, noted that “based on this study, there might be need for the Infectious Diseases Society of America and other organizations to offer guidance on generating good, quality surveillance data for C. difficile resistance.”
With C. difficile showing the ability to resist multiple antibiotics, drugs in the armamentarium to treat the infection have declined in recent years, and recurrences with the infection are reported in up to 25% of cases.
Oral vancomycin is recommended as the antibiotic of choice by the IDSA and the Society for Healthcare Epidemiology of America for severe as well as nonsevere cases of CDI, and although there are reports of nine vancomycin-resistant gene clusters, most involve Enterococcus.
To take a closer look at the prevalence of vancomycin-resistant C. difficile strains, first author Charles Darkoh, PhD, with the Center for Infectious Diseases at the University of Texas Health Science Center, Houston, and colleagues analyzed stool samples from patients with CDI, including 438 patients in Houston, taken between 2012 and 2017, and 98 in Nairobi, Kenya, taken in 2017.
They found that, among samples from patients in Houston, over the time period, 26% showed vancomycin nonsusceptible C. difficile isolates and 29% had isolates that were metronidazole resistant.
And among samples from the Nairobi patients, 67% harbored vancomycin-resistant isolates and 85% had isolates resistant to metronidazole.
Of note, the proportion of samples containing vancomycin-resistant C. difficile in the Houston patients showed a marked increase over time, from «complete absence» in 2012 to approximately 35% in 2017, the authors reported.
“These nonsusceptibility rates significantly exceeded prior reports from other studies conducted in the United States and Europe from 2011 to 2014, suggesting a lower percentage of resistance to both metronidazole and vancomycin,” the authors wrote.
Further experiments on mouse models infected with one of the vancomycin-resistant isolates showed that treatment with vancomycin failed to eradicate the infection, and 5-day survival was significantly lower after vancomycin treatment in those mice (25%) versus those infected with strains known to be vancomycin sensitive (50%).
Unrecognized genetic strains
Whole-genome sequencing of 10 of the resistant isolates showed no matches with gene clusters that have been previously recognized as being vancomycin resistant, suggesting the emergence of new clusters.
“Together, these results suggest unknown genetic elements associated with vancomycin nonsusceptibility in isolates circulating in the patient population,” the authors wrote.
Dr. Darkoh told this news organization that the research team is currently working to further investigate the patterns and mechanisms.
“We are currently working on a follow-up study for the next 5 years to find out how widespread this is,” he said. “We want to make sure it’s not necessarily just occurring in the settings we studied, and we also need to establish the mechanism of resistance.”
Further commenting on the results, Dr. Clancy noted that “the extent of resistance caught many in the field a bit off guard, as they are higher than previously reported.”
“The data are also concerning because most centers do not routinely test C. difficile for drug susceptibility.”
Dr. Clancy noted that “another immediately pressing need is to understand mechanisms of resistance. It was quite striking that vancomycin-resistant strains in this study did not carry vanA genes, pointing to previously unrecognized mechanisms of resistance.”
“As is often the case, antibiotic overuse was likely a factor in the resistances, with overtesting often leading to overtreatment of C. difficile,” Dr. Clancy said. “The situation may have been compounded by failure to appreciate how entrenched C. difficile resistance may be at certain hospitals, since widespread susceptibility testing is generally not routinely performed.”
As alternative treatments, Dr. Clancy pointed to the recent IDSA update, which included a stronger endorsement of fidaxomicin.
“Of course, there is also the need to assure that data on resistance to agents like fidaxomicin are generated going forward,” he noted.
The study was supported by was supported by National Institutes of Health, the National Institute of Allergy and Infectious Diseases, the Texas Medical Center Digestive Diseases Center, and the University of Texas Health Science Center. Dr. Darkoh has disclosed no relevant financial relationships. One coauthor received grant support from Merck, Entasis Pharmaceuticals, and MeMed Diagnostics. Dr. Clancy disclosed advisory board, consulting and/or research relationships with Merck, Qpex Biopharma, Shionogi, Astellas, Cidara, Scynexis, and Needham & Associates.
A version of this article first appeared on Medscape.com.
Samples from patients in the United States and Kenya show an increasing emergence of previously undetected vancomycin-resistant strains of Clostridioides difficile, sparking concern as recurrences in the treatment of C. difficile infection (CDI) continue to rise.
“Our results may help explain a decreasing effectiveness of antibiotic-based therapy in C. difficile infection, since a significant proportion of patients harboring strains with reduced susceptibility to vancomycin may not respond to treatment,” reported the authors in research published recently in Clinical Infectious Diseases.
The spread of the resistant strains “has serious public health implications, underscoring an urgent need for a comprehensive analysis of the circulating strains to help inform clinical decisions,” they added.
Commenting on the findings, Cornelius J. Clancy, MD, professor of medicine at the University of Pittsburgh, and chief of infectious diseases at the Veterans Affairs Pittsburgh Healthcare System, echoed the concern.
“The casual belief has been that [C. difficile] strains at most centers can be assumed to be vancomycin susceptible,” he told this news organization. “This study shows that this assumption can no longer be taken as a given.”
Dr. Clancy, who was not involved with this research, noted that “based on this study, there might be need for the Infectious Diseases Society of America and other organizations to offer guidance on generating good, quality surveillance data for C. difficile resistance.”
With C. difficile showing the ability to resist multiple antibiotics, drugs in the armamentarium to treat the infection have declined in recent years, and recurrences with the infection are reported in up to 25% of cases.
Oral vancomycin is recommended as the antibiotic of choice by the IDSA and the Society for Healthcare Epidemiology of America for severe as well as nonsevere cases of CDI, and although there are reports of nine vancomycin-resistant gene clusters, most involve Enterococcus.
To take a closer look at the prevalence of vancomycin-resistant C. difficile strains, first author Charles Darkoh, PhD, with the Center for Infectious Diseases at the University of Texas Health Science Center, Houston, and colleagues analyzed stool samples from patients with CDI, including 438 patients in Houston, taken between 2012 and 2017, and 98 in Nairobi, Kenya, taken in 2017.
They found that, among samples from patients in Houston, over the time period, 26% showed vancomycin nonsusceptible C. difficile isolates and 29% had isolates that were metronidazole resistant.
And among samples from the Nairobi patients, 67% harbored vancomycin-resistant isolates and 85% had isolates resistant to metronidazole.
Of note, the proportion of samples containing vancomycin-resistant C. difficile in the Houston patients showed a marked increase over time, from «complete absence» in 2012 to approximately 35% in 2017, the authors reported.
“These nonsusceptibility rates significantly exceeded prior reports from other studies conducted in the United States and Europe from 2011 to 2014, suggesting a lower percentage of resistance to both metronidazole and vancomycin,” the authors wrote.
Further experiments on mouse models infected with one of the vancomycin-resistant isolates showed that treatment with vancomycin failed to eradicate the infection, and 5-day survival was significantly lower after vancomycin treatment in those mice (25%) versus those infected with strains known to be vancomycin sensitive (50%).
Unrecognized genetic strains
Whole-genome sequencing of 10 of the resistant isolates showed no matches with gene clusters that have been previously recognized as being vancomycin resistant, suggesting the emergence of new clusters.
“Together, these results suggest unknown genetic elements associated with vancomycin nonsusceptibility in isolates circulating in the patient population,” the authors wrote.
Dr. Darkoh told this news organization that the research team is currently working to further investigate the patterns and mechanisms.
“We are currently working on a follow-up study for the next 5 years to find out how widespread this is,” he said. “We want to make sure it’s not necessarily just occurring in the settings we studied, and we also need to establish the mechanism of resistance.”
Further commenting on the results, Dr. Clancy noted that “the extent of resistance caught many in the field a bit off guard, as they are higher than previously reported.”
“The data are also concerning because most centers do not routinely test C. difficile for drug susceptibility.”
Dr. Clancy noted that “another immediately pressing need is to understand mechanisms of resistance. It was quite striking that vancomycin-resistant strains in this study did not carry vanA genes, pointing to previously unrecognized mechanisms of resistance.”
“As is often the case, antibiotic overuse was likely a factor in the resistances, with overtesting often leading to overtreatment of C. difficile,” Dr. Clancy said. “The situation may have been compounded by failure to appreciate how entrenched C. difficile resistance may be at certain hospitals, since widespread susceptibility testing is generally not routinely performed.”
As alternative treatments, Dr. Clancy pointed to the recent IDSA update, which included a stronger endorsement of fidaxomicin.
“Of course, there is also the need to assure that data on resistance to agents like fidaxomicin are generated going forward,” he noted.
The study was supported by was supported by National Institutes of Health, the National Institute of Allergy and Infectious Diseases, the Texas Medical Center Digestive Diseases Center, and the University of Texas Health Science Center. Dr. Darkoh has disclosed no relevant financial relationships. One coauthor received grant support from Merck, Entasis Pharmaceuticals, and MeMed Diagnostics. Dr. Clancy disclosed advisory board, consulting and/or research relationships with Merck, Qpex Biopharma, Shionogi, Astellas, Cidara, Scynexis, and Needham & Associates.
A version of this article first appeared on Medscape.com.
Samples from patients in the United States and Kenya show an increasing emergence of previously undetected vancomycin-resistant strains of Clostridioides difficile, sparking concern as recurrences in the treatment of C. difficile infection (CDI) continue to rise.
“Our results may help explain a decreasing effectiveness of antibiotic-based therapy in C. difficile infection, since a significant proportion of patients harboring strains with reduced susceptibility to vancomycin may not respond to treatment,” reported the authors in research published recently in Clinical Infectious Diseases.
The spread of the resistant strains “has serious public health implications, underscoring an urgent need for a comprehensive analysis of the circulating strains to help inform clinical decisions,” they added.
Commenting on the findings, Cornelius J. Clancy, MD, professor of medicine at the University of Pittsburgh, and chief of infectious diseases at the Veterans Affairs Pittsburgh Healthcare System, echoed the concern.
“The casual belief has been that [C. difficile] strains at most centers can be assumed to be vancomycin susceptible,” he told this news organization. “This study shows that this assumption can no longer be taken as a given.”
Dr. Clancy, who was not involved with this research, noted that “based on this study, there might be need for the Infectious Diseases Society of America and other organizations to offer guidance on generating good, quality surveillance data for C. difficile resistance.”
With C. difficile showing the ability to resist multiple antibiotics, drugs in the armamentarium to treat the infection have declined in recent years, and recurrences with the infection are reported in up to 25% of cases.
Oral vancomycin is recommended as the antibiotic of choice by the IDSA and the Society for Healthcare Epidemiology of America for severe as well as nonsevere cases of CDI, and although there are reports of nine vancomycin-resistant gene clusters, most involve Enterococcus.
To take a closer look at the prevalence of vancomycin-resistant C. difficile strains, first author Charles Darkoh, PhD, with the Center for Infectious Diseases at the University of Texas Health Science Center, Houston, and colleagues analyzed stool samples from patients with CDI, including 438 patients in Houston, taken between 2012 and 2017, and 98 in Nairobi, Kenya, taken in 2017.
They found that, among samples from patients in Houston, over the time period, 26% showed vancomycin nonsusceptible C. difficile isolates and 29% had isolates that were metronidazole resistant.
And among samples from the Nairobi patients, 67% harbored vancomycin-resistant isolates and 85% had isolates resistant to metronidazole.
Of note, the proportion of samples containing vancomycin-resistant C. difficile in the Houston patients showed a marked increase over time, from «complete absence» in 2012 to approximately 35% in 2017, the authors reported.
“These nonsusceptibility rates significantly exceeded prior reports from other studies conducted in the United States and Europe from 2011 to 2014, suggesting a lower percentage of resistance to both metronidazole and vancomycin,” the authors wrote.
Further experiments on mouse models infected with one of the vancomycin-resistant isolates showed that treatment with vancomycin failed to eradicate the infection, and 5-day survival was significantly lower after vancomycin treatment in those mice (25%) versus those infected with strains known to be vancomycin sensitive (50%).
Unrecognized genetic strains
Whole-genome sequencing of 10 of the resistant isolates showed no matches with gene clusters that have been previously recognized as being vancomycin resistant, suggesting the emergence of new clusters.
“Together, these results suggest unknown genetic elements associated with vancomycin nonsusceptibility in isolates circulating in the patient population,” the authors wrote.
Dr. Darkoh told this news organization that the research team is currently working to further investigate the patterns and mechanisms.
“We are currently working on a follow-up study for the next 5 years to find out how widespread this is,” he said. “We want to make sure it’s not necessarily just occurring in the settings we studied, and we also need to establish the mechanism of resistance.”
Further commenting on the results, Dr. Clancy noted that “the extent of resistance caught many in the field a bit off guard, as they are higher than previously reported.”
“The data are also concerning because most centers do not routinely test C. difficile for drug susceptibility.”
Dr. Clancy noted that “another immediately pressing need is to understand mechanisms of resistance. It was quite striking that vancomycin-resistant strains in this study did not carry vanA genes, pointing to previously unrecognized mechanisms of resistance.”
“As is often the case, antibiotic overuse was likely a factor in the resistances, with overtesting often leading to overtreatment of C. difficile,” Dr. Clancy said. “The situation may have been compounded by failure to appreciate how entrenched C. difficile resistance may be at certain hospitals, since widespread susceptibility testing is generally not routinely performed.”
As alternative treatments, Dr. Clancy pointed to the recent IDSA update, which included a stronger endorsement of fidaxomicin.
“Of course, there is also the need to assure that data on resistance to agents like fidaxomicin are generated going forward,” he noted.
The study was supported by was supported by National Institutes of Health, the National Institute of Allergy and Infectious Diseases, the Texas Medical Center Digestive Diseases Center, and the University of Texas Health Science Center. Dr. Darkoh has disclosed no relevant financial relationships. One coauthor received grant support from Merck, Entasis Pharmaceuticals, and MeMed Diagnostics. Dr. Clancy disclosed advisory board, consulting and/or research relationships with Merck, Qpex Biopharma, Shionogi, Astellas, Cidara, Scynexis, and Needham & Associates.
A version of this article first appeared on Medscape.com.
FROM CLINICAL INFECTIOUS DISEASES
Drug-resistant malaria is emerging in Africa. Is the world ready?
In June 2017, Betty Balikagala, MD, PhD, traveled to a hospital in Gulu District, in northern Uganda. It was the rainy season: a peak time for malaria transmission. Dr. Balikagala, a researcher at Juntendo University in Japan, was back in her home country to hunt for mutations in the parasite that causes the disease.
For about 4 weeks, Dr. Balikagala and her colleagues collected blood from infected patients as they were treated with a powerful cocktail of antimalarial drugs. After initial analysis, the team then shipped their samples – glass slides smeared with blood, and filter papers with blood spots – back to Japan.
In their lab at Juntendo University, they looked for traces of malaria in the blood slides, which they had prepared by drawing blood from patients every few hours. In previous years, Dr. Balikagala and her colleagues had observed the drugs efficiently clearing the infection. This time, though, the parasite lingered in some patients. “We were very surprised when we first did the parasite reading for 2017, and we noticed that there were some patients who had delayed clearance,” recalled Dr. Balikagala. “For me, it was a shock.”
Malaria kills more than half a million people per year, most of them small children. Still, between 2000 and 2020, according to the World Health Organization, interventions prevented around 10.6 million malaria deaths, mostly in Africa. Bed nets and insecticides were responsible for most of the progress. But a fairly large number of lives were also saved by a new kind of antimalarial treatment: artemisinin-based combination therapies, or ACTs, that replaced older drugs such as chloroquine.
Used as a first-line treatment, ACTs have averted a significant number of malaria deaths since their introduction in the early 2000s. ACTs pair a derivative of the drug artemisinin with one of five partner drugs or drug combinations. Delivered together, the fast-acting artemisinin component wipes out most of the parasites within a few days, and the longer-acting partner drug clears out the stragglers.
ACTs quickly became a mainstay in malaria treatment. But in 2009, researchers observed signs of resistance to artemisinin along the Thailand-Cambodia border. The artemisinin component failed to clear the parasite quickly, which meant that the partner drug had to pick up that load, creating favorable conditions for partner drug resistance, too. The Greater Mekong Subregion now experiences high rates of multidrug resistance. Scientists have feared that the spread of such resistance to Africa, which accounts for more than 90% of global malaria cases, would be disastrous.
Now, in a pair of reports published last year, scientists have confirmed the emergence of artemisinin resistance in Africa. One study, published in April, reported that ACTs had failed to work quickly for more than 10% of participants at two sites in Rwanda. The prevalence of artemisinin resistance mutations was also higher than detected in previous reports.
In September, Dr. Balikagala’s team published the report from Uganda, which also identified mutations associated with artemisinin resistance. Alarmingly, the resistant malaria parasites had risen from 3.9% of cases in 2015 to nearly 20% in 2019. Genetic analysis shows that the resistance mutations in Rwanda and Uganda have emerged independently.
The latest malaria report from the WHO, published in December, also noted worrying signs of artemisinin resistance in the Horn of Africa, on the eastern side of the continent. No peer-reviewed studies confirming such resistance have been published.
So far, the ACTs still work. But in an experimental setting, as drug resistance sets in, it can lengthen treatment by 3 or 4 days. That may not sound like much, said Timothy Wells, PhD, chief scientific officer of the nonprofit Medicines for Malaria Venture. But “the more days of therapy you need,” he said, “then the more there is the risk that people don’t finish their course of therapy.” Dropping a treatment course midway exposes the parasites to the drug, but doesn’t clear all of them, potentially leaving behind survivors with a higher chance of being drug resistant. “That’s really bad news, because then that sets up a perfect storm for creating more resistance,” said Dr. Wells.
The reports from Uganda and Rwanda have yielded a grim consensus: “We are going to see more and more of such independent emergence,” said Pascal Ringwald, MD, PhD, coordinator at the director’s office for the WHO Global Malaria Program. “This is exactly what we saw in the Greater Mekong.” Luckily, Dr. Wells said, switching to other ACTs helped to combat resistance when it was detected there, avoiding the need for prolonged treatment.
A new malaria vaccine, which recently received the go-ahead from the WHO, may eventually help reduce the number of infections, but its rollout won’t have any significant impact on drug resistance. As for new drugs, even the most promising candidate in the pipeline would take at least 4 years to become widely available.
That leaves public health workers in Africa with only one solid option: Track and surveil resistance to artemisinin and its partner drugs. Effective surveillance systems, experts say, need to ramp up quickly and widely across the continent.
But most experts say that surveillance on the continent is patchy. Indeed, there is considerable uncertainty about how widespread antimalarial resistance already is in sub-Saharan Africa – and disagreement over how to interpret initial reports of emerging partner drug resistance in some countries.
“Our current systems are not as good as they should be,” said Philip Rosenthal, MD, a malaria researcher at the University of California, San Francisco. The new reports of artemisinin resistance, he added, “can be seen as a wake-up call to improve surveillance.”
Malaria drugs have failed before. In the early 20th century, chloroquine helped beat back the pathogen worldwide. Then, about a decade after World War II, resistance to chloroquine surfaced along the Thailand-Cambodia border.
By the 1970s, chloroquine-resistant malaria had spread across India and into Africa, where it killed millions, many of them children. “In retrospect, we know that chloroquine was used for many years after there was a huge resistance problem,” said Dr. Rosenthal. “This probably led to millions of excess deaths that could have been avoided if we were using other drugs.”
The scurry to find new drugs yielded artemisinin. Used by Chinese herbalists some 2,000 years ago to treat malaria-like symptoms, artemisinin was rediscovered in the 1970s by biomedical researchers in China, and its use became widespread in the 2000s.
Haunted by the failure of chloroquine, though, researchers have remained on the lookout for signs that the malaria parasite is evolving to resist artemisinin or its partner drugs. The gold-standard method is a therapeutic efficacy study, which involves closely monitoring infected patients as they are treated with antimalarial drugs, to see how well the drugs perform and if there are any signs of resistance.
The WHO recommends conducting these studies at several sites in a country every 2 years. But “each country interprets that with their capability,” said Philippe Guérin, MD, PhD, director of the WorldWide Antimalarial Resistance Network at the University of Oxford, England. Efficacy studies are slow, costly, and labor intensive. Also, “you don’t get a very good geographical representation,” said Dr. Guérin, because you can do a new clinical trial in only so many places at a time.
To get around the problems associated with efficacy studies, researchers also turn to molecular surveillance. Researchers draw a few drops of blood from an infected individual onto a filter paper, then scan it in the laboratory for certain genetic mutations associated with resistance. The technique is relatively easy and cheap.
With these kinds of surveillance data, policymakers can choose which drugs to use in a particular region. Moreover, early detection of resistance can prompt health authorities to take actions to limit the spread of resistance, including more aggressive screening and treatment campaigns, and expanded efforts to control the mosquitoes that spread malaria.
In practice, though, this warning system is frayed. “There is really no organized surveillance system for the continent,” said Dr. Rosenthal. “Surveillance is haphazard.”
In countries lacking a robust health care system or mired in political instability, experts say, resistance could be spreading undetected. For example, the border of South Sudan is just 60 miles from the site in northern Uganda where Dr. Balikagala and her colleagues confirmed resistance to artemisinin. “Because of the security issues and the refugee-weakened system, there is no surveillance that tells us what is happening in South Sudan,” said Dr. Guérin. The same applies in some parts of the nearby Democratic Republic of the Congo, he added.
In the past, regional antimalarial networks, such as the now defunct East African Network for Monitoring of Antimalarial Treatment, have addressed some surveillance gaps. These networks can help standardize protocols and coordinate surveillance efforts. But such networks have suffered from recent lapses in donor funding. The East African network “will be awakened,” Dr. Balikagala predicted, as concerns about artemisinin-resistant malaria grow.
In southern Africa, eight countries have come together to form the Elimination Eight Initiative, a coalition to facilitate malaria elimination efforts across national borders, which may help jump-start surveillance efforts there.
Dr. Ringwald said drug resistance is a priority for him and his WHO colleagues. At a malaria policy advisory committee meeting last fall, he said, the issue was “high on the agenda.” However, when pressed for answers on how the WHO plans to combat drug resistance in Africa, Dr. Ringwald emailed Undark an excerpt from the organization’s 2021 World Malaria Report. The report states that the WHO will “work with countries to develop a regional plan for a coordinated response,” but does not lay out any specifics on that response plan. The Africa Centers for Disease Control and Prevention, part of the African Union, did not respond to requests for comment on its plans to bolster surveillance.
“There is an ethical obligation to researchers, and to people responsible for surveillance, that if you pick up these problems, share them as quickly as possible, react to them as strongly as possible,” said Karen Barnes, a clinical pharmacologist at the University of Cape Town who cochairs the South African Malaria Elimination Committee. “And try very, very hard” to make sure “that it’s not going to be the same as when we had chloroquine resistance in Africa.”
In absence of more robust surveillance, reports have also identified worrying – but, some scientists say, inconclusive – signs of partner drug resistance.
A series of four studies conducted between 2013 and 2019 at several sites in Angola found the efficacy of artemether-lumefantrine – the most widely used ACT in Africa – had dropped below 90%, the WHO threshold for acceptable malaria treatment. Peer-reviewed studies from Burkina Faso and the Democratic Republic of the Congo have reported similar results.
The studies have not found genes associated with artemisinin resistance, suggesting that the partner drug, lumefantrine, might be faltering. But several malaria researchers told Undark they were skeptical of the studies’ methods and viewed the results as preliminary. “I would have preferred that we look at data with a standardized protocol and exclude any confounding factors like poor microscopy or analytical method,” said Dr. Ringwald.
Mateusz Plucinski, PhD, an epidemiologist at the Centers for Disease Control and Prevention’s Malaria Branch who participated in the Angola research, defended the findings. “The persistence of artemether-lumefantrine efficacy near or under 90% in Angola likely suggests that there is likely a true signal of decreased susceptibility of parasites to this drug,” he wrote in an email to this news organization. In response to the data, Angolan health officials have begun using a different ACT.
For now, it’s unclear how bad the situation is in Africa – or what the years ahead could bring. The research community and the authorities are “at the level of just watching and seeing what happens at this stage,” said Leann Tilley, PhD, a biochemist at the University of Melbourne who researches antimalarial resistance. But experts say that if artemisinin resistance does flare up and starts impinging on the partner drug, policymakers might need to consider changing to a different ACT, or even deploy triple ACTs, with two partner drugs.
Some experts are hopeful that artemisinin resistance will spread more slowly in Africa than it has in southeast Asia. But if high-grade resistance to artemisinin and partner drugs were to arise, it would put Africa in a bind. There are no immediate replacements for ACTs at the moment. The Medicines for Malaria Venture drug pipeline has about 30 molecules that show promise in preliminary testing, and about 15 molecules that are undergoing clinical trials for efficacy and safety, said Dr. Wells. But even the drugs that are at the end of the pipeline will take about 5-6 years from approval by regulatory authorities to be incorporated into WHO guidelines, he noted – if they make it through trials at all.
Dr. Wells cited one promising compound, from the drug maker Novartis, that recently performed well in early clinical trials. Still, Dr. Wells said, the drug won’t be ready to be deployed in Africa until around 2026.
Funds for malaria control and elimination programs remain limited, and scientists worry that, between COVID-19 and the malaria vaccine rollout, attention and resources for conducting surveillance and drug resistance work might dry up. “I really hope that those that do have resources available will understand that investing in Africa’s response to artemisinin resistance today, preferably yesterday, is probably one of the best places that they can put their money,” said Barnes.
The annals of malaria have shown time and again that once resistance emerges, it spreads widely and imperils progress against the deadly disease. For Africa, the writing is on the wall, she said. The bigger question, she asked, is this: “Are we capable of learning from history?”
A version of this article first appeared on Undark.com.
In June 2017, Betty Balikagala, MD, PhD, traveled to a hospital in Gulu District, in northern Uganda. It was the rainy season: a peak time for malaria transmission. Dr. Balikagala, a researcher at Juntendo University in Japan, was back in her home country to hunt for mutations in the parasite that causes the disease.
For about 4 weeks, Dr. Balikagala and her colleagues collected blood from infected patients as they were treated with a powerful cocktail of antimalarial drugs. After initial analysis, the team then shipped their samples – glass slides smeared with blood, and filter papers with blood spots – back to Japan.
In their lab at Juntendo University, they looked for traces of malaria in the blood slides, which they had prepared by drawing blood from patients every few hours. In previous years, Dr. Balikagala and her colleagues had observed the drugs efficiently clearing the infection. This time, though, the parasite lingered in some patients. “We were very surprised when we first did the parasite reading for 2017, and we noticed that there were some patients who had delayed clearance,” recalled Dr. Balikagala. “For me, it was a shock.”
Malaria kills more than half a million people per year, most of them small children. Still, between 2000 and 2020, according to the World Health Organization, interventions prevented around 10.6 million malaria deaths, mostly in Africa. Bed nets and insecticides were responsible for most of the progress. But a fairly large number of lives were also saved by a new kind of antimalarial treatment: artemisinin-based combination therapies, or ACTs, that replaced older drugs such as chloroquine.
Used as a first-line treatment, ACTs have averted a significant number of malaria deaths since their introduction in the early 2000s. ACTs pair a derivative of the drug artemisinin with one of five partner drugs or drug combinations. Delivered together, the fast-acting artemisinin component wipes out most of the parasites within a few days, and the longer-acting partner drug clears out the stragglers.
ACTs quickly became a mainstay in malaria treatment. But in 2009, researchers observed signs of resistance to artemisinin along the Thailand-Cambodia border. The artemisinin component failed to clear the parasite quickly, which meant that the partner drug had to pick up that load, creating favorable conditions for partner drug resistance, too. The Greater Mekong Subregion now experiences high rates of multidrug resistance. Scientists have feared that the spread of such resistance to Africa, which accounts for more than 90% of global malaria cases, would be disastrous.
Now, in a pair of reports published last year, scientists have confirmed the emergence of artemisinin resistance in Africa. One study, published in April, reported that ACTs had failed to work quickly for more than 10% of participants at two sites in Rwanda. The prevalence of artemisinin resistance mutations was also higher than detected in previous reports.
In September, Dr. Balikagala’s team published the report from Uganda, which also identified mutations associated with artemisinin resistance. Alarmingly, the resistant malaria parasites had risen from 3.9% of cases in 2015 to nearly 20% in 2019. Genetic analysis shows that the resistance mutations in Rwanda and Uganda have emerged independently.
The latest malaria report from the WHO, published in December, also noted worrying signs of artemisinin resistance in the Horn of Africa, on the eastern side of the continent. No peer-reviewed studies confirming such resistance have been published.
So far, the ACTs still work. But in an experimental setting, as drug resistance sets in, it can lengthen treatment by 3 or 4 days. That may not sound like much, said Timothy Wells, PhD, chief scientific officer of the nonprofit Medicines for Malaria Venture. But “the more days of therapy you need,” he said, “then the more there is the risk that people don’t finish their course of therapy.” Dropping a treatment course midway exposes the parasites to the drug, but doesn’t clear all of them, potentially leaving behind survivors with a higher chance of being drug resistant. “That’s really bad news, because then that sets up a perfect storm for creating more resistance,” said Dr. Wells.
The reports from Uganda and Rwanda have yielded a grim consensus: “We are going to see more and more of such independent emergence,” said Pascal Ringwald, MD, PhD, coordinator at the director’s office for the WHO Global Malaria Program. “This is exactly what we saw in the Greater Mekong.” Luckily, Dr. Wells said, switching to other ACTs helped to combat resistance when it was detected there, avoiding the need for prolonged treatment.
A new malaria vaccine, which recently received the go-ahead from the WHO, may eventually help reduce the number of infections, but its rollout won’t have any significant impact on drug resistance. As for new drugs, even the most promising candidate in the pipeline would take at least 4 years to become widely available.
That leaves public health workers in Africa with only one solid option: Track and surveil resistance to artemisinin and its partner drugs. Effective surveillance systems, experts say, need to ramp up quickly and widely across the continent.
But most experts say that surveillance on the continent is patchy. Indeed, there is considerable uncertainty about how widespread antimalarial resistance already is in sub-Saharan Africa – and disagreement over how to interpret initial reports of emerging partner drug resistance in some countries.
“Our current systems are not as good as they should be,” said Philip Rosenthal, MD, a malaria researcher at the University of California, San Francisco. The new reports of artemisinin resistance, he added, “can be seen as a wake-up call to improve surveillance.”
Malaria drugs have failed before. In the early 20th century, chloroquine helped beat back the pathogen worldwide. Then, about a decade after World War II, resistance to chloroquine surfaced along the Thailand-Cambodia border.
By the 1970s, chloroquine-resistant malaria had spread across India and into Africa, where it killed millions, many of them children. “In retrospect, we know that chloroquine was used for many years after there was a huge resistance problem,” said Dr. Rosenthal. “This probably led to millions of excess deaths that could have been avoided if we were using other drugs.”
The scurry to find new drugs yielded artemisinin. Used by Chinese herbalists some 2,000 years ago to treat malaria-like symptoms, artemisinin was rediscovered in the 1970s by biomedical researchers in China, and its use became widespread in the 2000s.
Haunted by the failure of chloroquine, though, researchers have remained on the lookout for signs that the malaria parasite is evolving to resist artemisinin or its partner drugs. The gold-standard method is a therapeutic efficacy study, which involves closely monitoring infected patients as they are treated with antimalarial drugs, to see how well the drugs perform and if there are any signs of resistance.
The WHO recommends conducting these studies at several sites in a country every 2 years. But “each country interprets that with their capability,” said Philippe Guérin, MD, PhD, director of the WorldWide Antimalarial Resistance Network at the University of Oxford, England. Efficacy studies are slow, costly, and labor intensive. Also, “you don’t get a very good geographical representation,” said Dr. Guérin, because you can do a new clinical trial in only so many places at a time.
To get around the problems associated with efficacy studies, researchers also turn to molecular surveillance. Researchers draw a few drops of blood from an infected individual onto a filter paper, then scan it in the laboratory for certain genetic mutations associated with resistance. The technique is relatively easy and cheap.
With these kinds of surveillance data, policymakers can choose which drugs to use in a particular region. Moreover, early detection of resistance can prompt health authorities to take actions to limit the spread of resistance, including more aggressive screening and treatment campaigns, and expanded efforts to control the mosquitoes that spread malaria.
In practice, though, this warning system is frayed. “There is really no organized surveillance system for the continent,” said Dr. Rosenthal. “Surveillance is haphazard.”
In countries lacking a robust health care system or mired in political instability, experts say, resistance could be spreading undetected. For example, the border of South Sudan is just 60 miles from the site in northern Uganda where Dr. Balikagala and her colleagues confirmed resistance to artemisinin. “Because of the security issues and the refugee-weakened system, there is no surveillance that tells us what is happening in South Sudan,” said Dr. Guérin. The same applies in some parts of the nearby Democratic Republic of the Congo, he added.
In the past, regional antimalarial networks, such as the now defunct East African Network for Monitoring of Antimalarial Treatment, have addressed some surveillance gaps. These networks can help standardize protocols and coordinate surveillance efforts. But such networks have suffered from recent lapses in donor funding. The East African network “will be awakened,” Dr. Balikagala predicted, as concerns about artemisinin-resistant malaria grow.
In southern Africa, eight countries have come together to form the Elimination Eight Initiative, a coalition to facilitate malaria elimination efforts across national borders, which may help jump-start surveillance efforts there.
Dr. Ringwald said drug resistance is a priority for him and his WHO colleagues. At a malaria policy advisory committee meeting last fall, he said, the issue was “high on the agenda.” However, when pressed for answers on how the WHO plans to combat drug resistance in Africa, Dr. Ringwald emailed Undark an excerpt from the organization’s 2021 World Malaria Report. The report states that the WHO will “work with countries to develop a regional plan for a coordinated response,” but does not lay out any specifics on that response plan. The Africa Centers for Disease Control and Prevention, part of the African Union, did not respond to requests for comment on its plans to bolster surveillance.
“There is an ethical obligation to researchers, and to people responsible for surveillance, that if you pick up these problems, share them as quickly as possible, react to them as strongly as possible,” said Karen Barnes, a clinical pharmacologist at the University of Cape Town who cochairs the South African Malaria Elimination Committee. “And try very, very hard” to make sure “that it’s not going to be the same as when we had chloroquine resistance in Africa.”
In absence of more robust surveillance, reports have also identified worrying – but, some scientists say, inconclusive – signs of partner drug resistance.
A series of four studies conducted between 2013 and 2019 at several sites in Angola found the efficacy of artemether-lumefantrine – the most widely used ACT in Africa – had dropped below 90%, the WHO threshold for acceptable malaria treatment. Peer-reviewed studies from Burkina Faso and the Democratic Republic of the Congo have reported similar results.
The studies have not found genes associated with artemisinin resistance, suggesting that the partner drug, lumefantrine, might be faltering. But several malaria researchers told Undark they were skeptical of the studies’ methods and viewed the results as preliminary. “I would have preferred that we look at data with a standardized protocol and exclude any confounding factors like poor microscopy or analytical method,” said Dr. Ringwald.
Mateusz Plucinski, PhD, an epidemiologist at the Centers for Disease Control and Prevention’s Malaria Branch who participated in the Angola research, defended the findings. “The persistence of artemether-lumefantrine efficacy near or under 90% in Angola likely suggests that there is likely a true signal of decreased susceptibility of parasites to this drug,” he wrote in an email to this news organization. In response to the data, Angolan health officials have begun using a different ACT.
For now, it’s unclear how bad the situation is in Africa – or what the years ahead could bring. The research community and the authorities are “at the level of just watching and seeing what happens at this stage,” said Leann Tilley, PhD, a biochemist at the University of Melbourne who researches antimalarial resistance. But experts say that if artemisinin resistance does flare up and starts impinging on the partner drug, policymakers might need to consider changing to a different ACT, or even deploy triple ACTs, with two partner drugs.
Some experts are hopeful that artemisinin resistance will spread more slowly in Africa than it has in southeast Asia. But if high-grade resistance to artemisinin and partner drugs were to arise, it would put Africa in a bind. There are no immediate replacements for ACTs at the moment. The Medicines for Malaria Venture drug pipeline has about 30 molecules that show promise in preliminary testing, and about 15 molecules that are undergoing clinical trials for efficacy and safety, said Dr. Wells. But even the drugs that are at the end of the pipeline will take about 5-6 years from approval by regulatory authorities to be incorporated into WHO guidelines, he noted – if they make it through trials at all.
Dr. Wells cited one promising compound, from the drug maker Novartis, that recently performed well in early clinical trials. Still, Dr. Wells said, the drug won’t be ready to be deployed in Africa until around 2026.
Funds for malaria control and elimination programs remain limited, and scientists worry that, between COVID-19 and the malaria vaccine rollout, attention and resources for conducting surveillance and drug resistance work might dry up. “I really hope that those that do have resources available will understand that investing in Africa’s response to artemisinin resistance today, preferably yesterday, is probably one of the best places that they can put their money,” said Barnes.
The annals of malaria have shown time and again that once resistance emerges, it spreads widely and imperils progress against the deadly disease. For Africa, the writing is on the wall, she said. The bigger question, she asked, is this: “Are we capable of learning from history?”
A version of this article first appeared on Undark.com.
In June 2017, Betty Balikagala, MD, PhD, traveled to a hospital in Gulu District, in northern Uganda. It was the rainy season: a peak time for malaria transmission. Dr. Balikagala, a researcher at Juntendo University in Japan, was back in her home country to hunt for mutations in the parasite that causes the disease.
For about 4 weeks, Dr. Balikagala and her colleagues collected blood from infected patients as they were treated with a powerful cocktail of antimalarial drugs. After initial analysis, the team then shipped their samples – glass slides smeared with blood, and filter papers with blood spots – back to Japan.
In their lab at Juntendo University, they looked for traces of malaria in the blood slides, which they had prepared by drawing blood from patients every few hours. In previous years, Dr. Balikagala and her colleagues had observed the drugs efficiently clearing the infection. This time, though, the parasite lingered in some patients. “We were very surprised when we first did the parasite reading for 2017, and we noticed that there were some patients who had delayed clearance,” recalled Dr. Balikagala. “For me, it was a shock.”
Malaria kills more than half a million people per year, most of them small children. Still, between 2000 and 2020, according to the World Health Organization, interventions prevented around 10.6 million malaria deaths, mostly in Africa. Bed nets and insecticides were responsible for most of the progress. But a fairly large number of lives were also saved by a new kind of antimalarial treatment: artemisinin-based combination therapies, or ACTs, that replaced older drugs such as chloroquine.
Used as a first-line treatment, ACTs have averted a significant number of malaria deaths since their introduction in the early 2000s. ACTs pair a derivative of the drug artemisinin with one of five partner drugs or drug combinations. Delivered together, the fast-acting artemisinin component wipes out most of the parasites within a few days, and the longer-acting partner drug clears out the stragglers.
ACTs quickly became a mainstay in malaria treatment. But in 2009, researchers observed signs of resistance to artemisinin along the Thailand-Cambodia border. The artemisinin component failed to clear the parasite quickly, which meant that the partner drug had to pick up that load, creating favorable conditions for partner drug resistance, too. The Greater Mekong Subregion now experiences high rates of multidrug resistance. Scientists have feared that the spread of such resistance to Africa, which accounts for more than 90% of global malaria cases, would be disastrous.
Now, in a pair of reports published last year, scientists have confirmed the emergence of artemisinin resistance in Africa. One study, published in April, reported that ACTs had failed to work quickly for more than 10% of participants at two sites in Rwanda. The prevalence of artemisinin resistance mutations was also higher than detected in previous reports.
In September, Dr. Balikagala’s team published the report from Uganda, which also identified mutations associated with artemisinin resistance. Alarmingly, the resistant malaria parasites had risen from 3.9% of cases in 2015 to nearly 20% in 2019. Genetic analysis shows that the resistance mutations in Rwanda and Uganda have emerged independently.
The latest malaria report from the WHO, published in December, also noted worrying signs of artemisinin resistance in the Horn of Africa, on the eastern side of the continent. No peer-reviewed studies confirming such resistance have been published.
So far, the ACTs still work. But in an experimental setting, as drug resistance sets in, it can lengthen treatment by 3 or 4 days. That may not sound like much, said Timothy Wells, PhD, chief scientific officer of the nonprofit Medicines for Malaria Venture. But “the more days of therapy you need,” he said, “then the more there is the risk that people don’t finish their course of therapy.” Dropping a treatment course midway exposes the parasites to the drug, but doesn’t clear all of them, potentially leaving behind survivors with a higher chance of being drug resistant. “That’s really bad news, because then that sets up a perfect storm for creating more resistance,” said Dr. Wells.
The reports from Uganda and Rwanda have yielded a grim consensus: “We are going to see more and more of such independent emergence,” said Pascal Ringwald, MD, PhD, coordinator at the director’s office for the WHO Global Malaria Program. “This is exactly what we saw in the Greater Mekong.” Luckily, Dr. Wells said, switching to other ACTs helped to combat resistance when it was detected there, avoiding the need for prolonged treatment.
A new malaria vaccine, which recently received the go-ahead from the WHO, may eventually help reduce the number of infections, but its rollout won’t have any significant impact on drug resistance. As for new drugs, even the most promising candidate in the pipeline would take at least 4 years to become widely available.
That leaves public health workers in Africa with only one solid option: Track and surveil resistance to artemisinin and its partner drugs. Effective surveillance systems, experts say, need to ramp up quickly and widely across the continent.
But most experts say that surveillance on the continent is patchy. Indeed, there is considerable uncertainty about how widespread antimalarial resistance already is in sub-Saharan Africa – and disagreement over how to interpret initial reports of emerging partner drug resistance in some countries.
“Our current systems are not as good as they should be,” said Philip Rosenthal, MD, a malaria researcher at the University of California, San Francisco. The new reports of artemisinin resistance, he added, “can be seen as a wake-up call to improve surveillance.”
Malaria drugs have failed before. In the early 20th century, chloroquine helped beat back the pathogen worldwide. Then, about a decade after World War II, resistance to chloroquine surfaced along the Thailand-Cambodia border.
By the 1970s, chloroquine-resistant malaria had spread across India and into Africa, where it killed millions, many of them children. “In retrospect, we know that chloroquine was used for many years after there was a huge resistance problem,” said Dr. Rosenthal. “This probably led to millions of excess deaths that could have been avoided if we were using other drugs.”
The scurry to find new drugs yielded artemisinin. Used by Chinese herbalists some 2,000 years ago to treat malaria-like symptoms, artemisinin was rediscovered in the 1970s by biomedical researchers in China, and its use became widespread in the 2000s.
Haunted by the failure of chloroquine, though, researchers have remained on the lookout for signs that the malaria parasite is evolving to resist artemisinin or its partner drugs. The gold-standard method is a therapeutic efficacy study, which involves closely monitoring infected patients as they are treated with antimalarial drugs, to see how well the drugs perform and if there are any signs of resistance.
The WHO recommends conducting these studies at several sites in a country every 2 years. But “each country interprets that with their capability,” said Philippe Guérin, MD, PhD, director of the WorldWide Antimalarial Resistance Network at the University of Oxford, England. Efficacy studies are slow, costly, and labor intensive. Also, “you don’t get a very good geographical representation,” said Dr. Guérin, because you can do a new clinical trial in only so many places at a time.
To get around the problems associated with efficacy studies, researchers also turn to molecular surveillance. Researchers draw a few drops of blood from an infected individual onto a filter paper, then scan it in the laboratory for certain genetic mutations associated with resistance. The technique is relatively easy and cheap.
With these kinds of surveillance data, policymakers can choose which drugs to use in a particular region. Moreover, early detection of resistance can prompt health authorities to take actions to limit the spread of resistance, including more aggressive screening and treatment campaigns, and expanded efforts to control the mosquitoes that spread malaria.
In practice, though, this warning system is frayed. “There is really no organized surveillance system for the continent,” said Dr. Rosenthal. “Surveillance is haphazard.”
In countries lacking a robust health care system or mired in political instability, experts say, resistance could be spreading undetected. For example, the border of South Sudan is just 60 miles from the site in northern Uganda where Dr. Balikagala and her colleagues confirmed resistance to artemisinin. “Because of the security issues and the refugee-weakened system, there is no surveillance that tells us what is happening in South Sudan,” said Dr. Guérin. The same applies in some parts of the nearby Democratic Republic of the Congo, he added.
In the past, regional antimalarial networks, such as the now defunct East African Network for Monitoring of Antimalarial Treatment, have addressed some surveillance gaps. These networks can help standardize protocols and coordinate surveillance efforts. But such networks have suffered from recent lapses in donor funding. The East African network “will be awakened,” Dr. Balikagala predicted, as concerns about artemisinin-resistant malaria grow.
In southern Africa, eight countries have come together to form the Elimination Eight Initiative, a coalition to facilitate malaria elimination efforts across national borders, which may help jump-start surveillance efforts there.
Dr. Ringwald said drug resistance is a priority for him and his WHO colleagues. At a malaria policy advisory committee meeting last fall, he said, the issue was “high on the agenda.” However, when pressed for answers on how the WHO plans to combat drug resistance in Africa, Dr. Ringwald emailed Undark an excerpt from the organization’s 2021 World Malaria Report. The report states that the WHO will “work with countries to develop a regional plan for a coordinated response,” but does not lay out any specifics on that response plan. The Africa Centers for Disease Control and Prevention, part of the African Union, did not respond to requests for comment on its plans to bolster surveillance.
“There is an ethical obligation to researchers, and to people responsible for surveillance, that if you pick up these problems, share them as quickly as possible, react to them as strongly as possible,” said Karen Barnes, a clinical pharmacologist at the University of Cape Town who cochairs the South African Malaria Elimination Committee. “And try very, very hard” to make sure “that it’s not going to be the same as when we had chloroquine resistance in Africa.”
In absence of more robust surveillance, reports have also identified worrying – but, some scientists say, inconclusive – signs of partner drug resistance.
A series of four studies conducted between 2013 and 2019 at several sites in Angola found the efficacy of artemether-lumefantrine – the most widely used ACT in Africa – had dropped below 90%, the WHO threshold for acceptable malaria treatment. Peer-reviewed studies from Burkina Faso and the Democratic Republic of the Congo have reported similar results.
The studies have not found genes associated with artemisinin resistance, suggesting that the partner drug, lumefantrine, might be faltering. But several malaria researchers told Undark they were skeptical of the studies’ methods and viewed the results as preliminary. “I would have preferred that we look at data with a standardized protocol and exclude any confounding factors like poor microscopy or analytical method,” said Dr. Ringwald.
Mateusz Plucinski, PhD, an epidemiologist at the Centers for Disease Control and Prevention’s Malaria Branch who participated in the Angola research, defended the findings. “The persistence of artemether-lumefantrine efficacy near or under 90% in Angola likely suggests that there is likely a true signal of decreased susceptibility of parasites to this drug,” he wrote in an email to this news organization. In response to the data, Angolan health officials have begun using a different ACT.
For now, it’s unclear how bad the situation is in Africa – or what the years ahead could bring. The research community and the authorities are “at the level of just watching and seeing what happens at this stage,” said Leann Tilley, PhD, a biochemist at the University of Melbourne who researches antimalarial resistance. But experts say that if artemisinin resistance does flare up and starts impinging on the partner drug, policymakers might need to consider changing to a different ACT, or even deploy triple ACTs, with two partner drugs.
Some experts are hopeful that artemisinin resistance will spread more slowly in Africa than it has in southeast Asia. But if high-grade resistance to artemisinin and partner drugs were to arise, it would put Africa in a bind. There are no immediate replacements for ACTs at the moment. The Medicines for Malaria Venture drug pipeline has about 30 molecules that show promise in preliminary testing, and about 15 molecules that are undergoing clinical trials for efficacy and safety, said Dr. Wells. But even the drugs that are at the end of the pipeline will take about 5-6 years from approval by regulatory authorities to be incorporated into WHO guidelines, he noted – if they make it through trials at all.
Dr. Wells cited one promising compound, from the drug maker Novartis, that recently performed well in early clinical trials. Still, Dr. Wells said, the drug won’t be ready to be deployed in Africa until around 2026.
Funds for malaria control and elimination programs remain limited, and scientists worry that, between COVID-19 and the malaria vaccine rollout, attention and resources for conducting surveillance and drug resistance work might dry up. “I really hope that those that do have resources available will understand that investing in Africa’s response to artemisinin resistance today, preferably yesterday, is probably one of the best places that they can put their money,” said Barnes.
The annals of malaria have shown time and again that once resistance emerges, it spreads widely and imperils progress against the deadly disease. For Africa, the writing is on the wall, she said. The bigger question, she asked, is this: “Are we capable of learning from history?”
A version of this article first appeared on Undark.com.
Antimicrobial resistance linked to 1.2 million global deaths in 2019
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.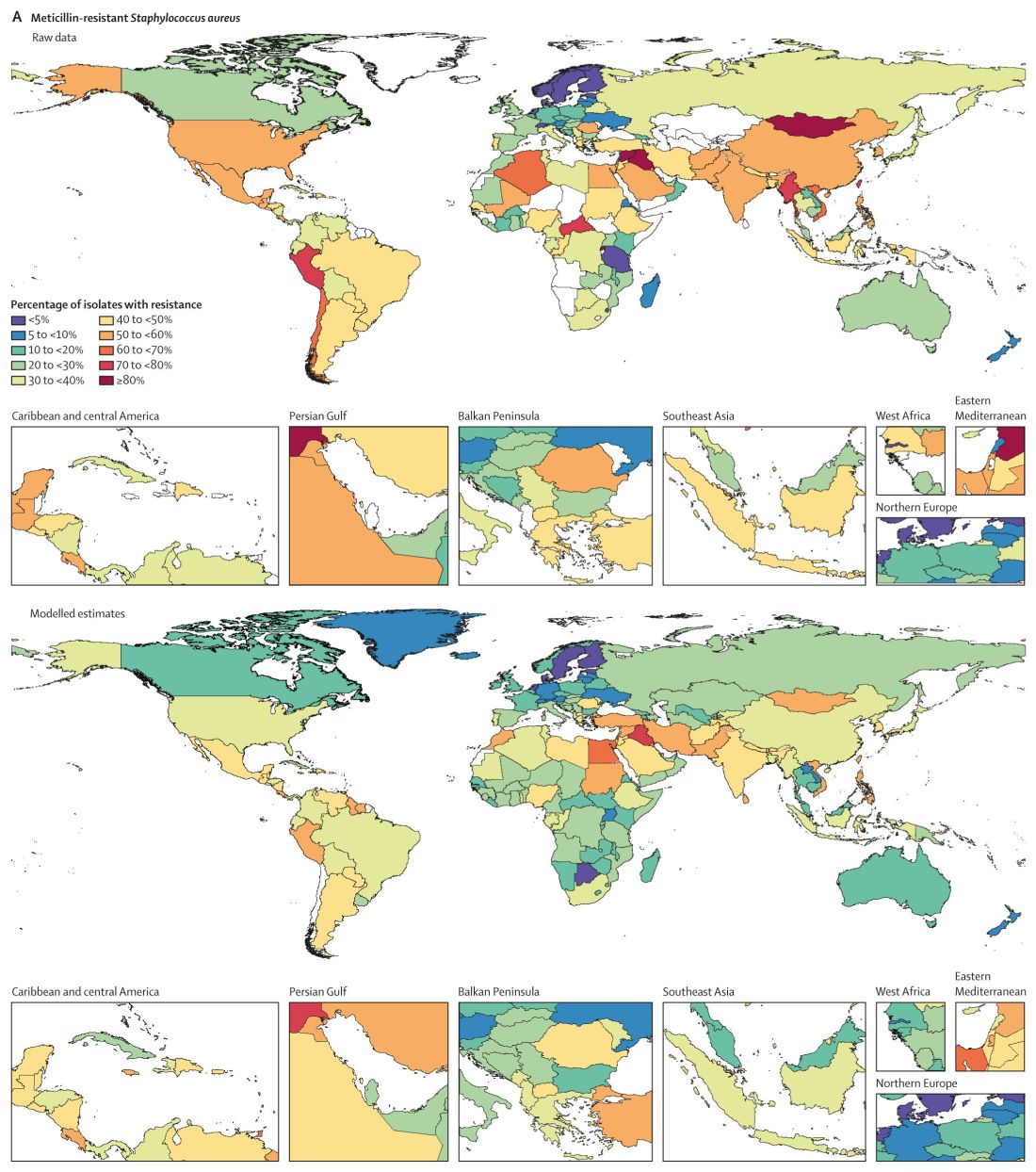
Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.
Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
More than HIV, more than malaria.
In terms of preventable deaths, 1.27 million people could have been saved if drug-resistant infections were replaced with infections susceptible to current antibiotics. Furthermore, 4.95 million fewer people would have died if drug-resistant infections were replaced by no infections, researchers estimated.
Although the COVID-19 pandemic took some focus off the AMR burden worldwide over the past 2 years, the urgency to address risk to public health did not ebb. In fact, based on the findings, the researchers noted that AMR is now a leading cause of death worldwide.
“If left unchecked, the spread of AMR could make many bacterial pathogens much more lethal in the future than they are today,” the researchers noted in the study, published online Jan. 20, 2022, in The Lancet.
“These findings are a warning signal that antibiotic resistance is placing pressure on health care systems and leading to significant health loss,” study author Kevin Ikuta, MD, MPH, told this news organization.
“We need to continue to adhere to and support infection prevention and control programs, be thoughtful about our antibiotic use, and advocate for increased funding to vaccine discovery and the antibiotic development pipeline,” added Dr. Ikuta, health sciences assistant clinical professor of medicine at the University of California, Los Angeles.
Although many investigators have studied AMR, this study is the largest in scope, covering 204 countries and territories and incorporating data on a comprehensive range of pathogens and pathogen-drug combinations.
Dr. Ikuta, lead author Christopher J.L. Murray, DPhil, and colleagues estimated the global burden of AMR using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2019. They specifically looked at rates of death directly attributed to and separately those associated with resistance.
Regional differences
Broken down by 21 regions, Australasia had 6.5 deaths per 100,000 people attributable to AMR, the lowest rate reported. This region also had 28 deaths per 100,000 associated with AMR.
Researchers found the highest rates in western sub-Saharan Africa. Deaths attributable to AMR were 27.3 per 100,000 and associated death rate was 114.8 per 100,000.
Lower- and middle-income regions had the highest AMR death rates, although resistance remains a high-priority issue for high-income countries as well.
“It’s important to take a global perspective on resistant infections because we can learn about regions and countries that are experiencing the greatest burden, information that was previously unknown,” Dr. Ikuta said. “With these estimates policy makers can prioritize regions that are hotspots and would most benefit from additional interventions.”
Furthermore, the study emphasized the global nature of AMR. “We’ve seen over the last 2 years with COVID-19 that this sort of problem doesn’t respect country borders, and high rates of resistance in one location can spread across a region or spread globally pretty quickly,” Dr. Ikuta said.
Leading resistant infections
Lower respiratory and thorax infections, bloodstream infections, and intra-abdominal infections together accounted for almost 79% of such deaths linked to AMR.
The six leading pathogens are likely household names among infectious disease specialists. The researchers found Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa, each responsible for more than 250,000 AMR-associated deaths.
The study also revealed that resistance to several first-line antibiotic agents often used empirically to treat infections accounted for more than 70% of the AMR-attributable deaths. These included fluoroquinolones and beta-lactam antibiotics such as carbapenems, cephalosporins, and penicillins.
Consistent with previous studies, MRSA stood out as a major cause of mortality. Of 88 different pathogen-drug combinations evaluated, MRSA was responsible for the most mortality: more than 100,000 deaths and 3·5 million disability-adjusted life-years.
The current study findings on MRSA “being a particularly nasty culprit” in AMR infections validates previous work that reported similar results, Vance Fowler, MD, told this news organization when asked to comment on the research. “That is reassuring.”
Potential solutions offered
Dr. Murray and colleagues outlined five strategies to address the challenge of bacterial AMR:
- Infection prevention and control remain paramount in minimizing infections in general and AMR infections in particular.
- More vaccines are needed to reduce the need for antibiotics. “Vaccines are available for only one of the six leading pathogens (S. pneumoniae), although new vaccine programs are underway for S. aureus, E. coli, and others,” the researchers wrote.
- Reduce antibiotic use unrelated to treatment of human disease.
- Avoid using antibiotics for viral infections and other unnecessary indications.
- Invest in new antibiotic development and ensure access to second-line agents in areas without widespread access.
“Identifying strategies that can work to reduce the burden of bacterial AMR – either across a wide range of settings or those that are specifically tailored to the resources available and leading pathogen-drug combinations in a particular setting – is an urgent priority,” the researchers noted.
Admirable AMR research
The results of the study are “startling, but not surprising,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C.
The authors did a “nice job” of addressing both deaths attributable and associated with AMR, Dr. Fowler added. “Those two categories unlock applications, not just in terms of how you interpret it but also what you do about it.”
The deaths attributable to AMR show that there is more work to be done regarding infection control and prevention, Dr. Fowler said, including in areas of the world like lower- and middle-income countries where infection resistance is most pronounced.
The deaths associated with AMR can be more challenging to calculate – people with infections can die for multiple reasons. However, Dr. Fowler applauded the researchers for doing “as good a job as you can” in estimating the extent of associated mortality.
‘The overlooked pandemic of antimicrobial resistance’
In an accompanying editorial in The Lancet, Ramanan Laxminarayan, PhD, MPH, wrote: “As COVID-19 rages on, the pandemic of antimicrobial resistance continues in the shadows. The toll taken by AMR on patients and their families is largely invisible but is reflected in prolonged bacterial infections that extend hospital stays and cause needless deaths.”
Dr. Laxminarayan pointed out an irony with AMR in different regions. Some of the AMR burden in sub-Saharan Africa is “probably due to inadequate access to antibiotics and high infection levels, albeit at low levels of resistance, whereas in south Asia and Latin America, it is because of high resistance even with good access to antibiotics.”
More funding to address AMR is needed, Dr. Laxminarayan noted. “Even the lower end of 911,000 deaths estimated by Murray and colleagues is higher than the number of deaths from HIV, which attracts close to U.S. $50 billion each year. However, global spending on addressing AMR is probably much lower than that.” Dr. Laxminarayan is an economist and epidemiologist affiliated with the Center for Disease Dynamics, Economics & Policy in Washington, D.C., and the Global Antibiotic Research and Development Partnership in Geneva.
An overlap with COVID-19
The Lancet report is likely “to bring more attention to AMR, especially since so many people have been distracted by COVID, and rightly so,” Dr. Fowler predicted. “The world has had its hands full with COVID.”
The two infections interact in direct ways, Dr. Fowler added. For example, some people hospitalized for COVID-19 for an extended time could develop progressively drug-resistant bacteria – leading to a superinfection.
The overlap could be illustrated by a Venn diagram, he said. A yellow circle could illustrate people with COVID-19 who are asymptomatic or who remain outpatients. Next to that would be a blue circle showing people who develop AMR infections. Where the two circles overlap would be green for those hospitalized who – because of receiving steroids, being on a ventilator, or getting a central line – develop a superinfection.
Official guidance continues
The study comes in the context of recent guidance and federal action on AMR. For example, the Infectious Diseases Society of America released new guidelines for AMR in November 2021 as part of ongoing advice on prevention and treatment of this “ongoing crisis.”
This most recent IDSA guidance addresses three pathogens in particular: AmpC beta-lactamase–producing Enterobacterales, carbapenem-resistant A. baumannii, and Stenotrophomonas maltophilia.
Also in November, the World Health Organization released an updated fact sheet on antimicrobial resistance. The WHO declared AMR one of the world’s top 10 global public health threats. The agency emphasized that misuse and overuse of antimicrobials are the main drivers in the development of drug-resistant pathogens. The WHO also pointed out that lack of clean water and sanitation in many areas of the world contribute to spread of microbes, including those resistant to current treatment options.
In September 2021, the Biden administration acknowledged the threat of AMR with allocation of more than $2 billion of the American Rescue Plan money for prevention and treatment of these infections.
Asked if there are any reasons for hope or optimism at this point, Dr. Ikuta said: “Definitely. We know what needs to be done to combat the spread of resistance. COVID-19 has demonstrated the importance of global commitment to infection control measures, such as hand washing and surveillance, and rapid investments in treatments, which can all be applied to antimicrobial resistance.”
The Bill & Melinda Gates Foundation, the Wellcome Trust, and the U.K. Department of Health and Social Care using U.K. aid funding managed by the Fleming Fund and other organizations provided funding for the study. Dr. Ikuta and Dr. Laxminarayan have disclosed no relevant financial relationships. Dr. Fowler reported receiving grants or honoraria, as well as serving as a consultant, for numerous sources. He also reported a patent pending in sepsis diagnostics and serving as chair of the V710 Scientific Advisory Committee (Merck).
A version of this article first appeared on Medscape.com.
Pediatric community-acquired pneumonia: 5 days of antibiotics better than 10 days
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
The evidence is in: and had the added benefit of a lower risk of inducing antibiotic resistance, according to the randomized, controlled SCOUT-CAP trial.
“Several studies have shown shorter antibiotic courses to be non-inferior to the standard treatment strategy, but in our study, we show that a shortened 5-day course of therapy was superior to standard therapy because the short course achieved similar outcomes with fewer days of antibiotics,” Derek Williams, MD, MPH, Vanderbilt University Medical Center, Nashville, Tenn., said in an email.
“These data are immediately applicable to frontline clinicians, and we hope this study will shift the paradigm towards more judicious treatment approaches for childhood pneumonia, resulting in care that is safer and more effective,” he added.
The study was published online Jan. 18 in JAMA Pediatrics.
Uncomplicated CAP
The study enrolled children aged 6 months to 71 months diagnosed with uncomplicated CAP who demonstrated early clinical improvement in response to 5 days of antibiotic treatment. Participants were prescribed either amoxicillin, amoxicillin and clavulanate, or cefdinir according to standard of care and were randomized on day 6 to another 5 days of their initially prescribed antibiotic course or to placebo.
“Those assessed on day 6 were eligible only if they had not yet received a dose of antibiotic therapy on that day,” the authors write. The primary endpoint was end-of-treatment response, adjusted for the duration of antibiotic risk as assessed by RADAR. As the authors explain, RADAR is a composite endpoint that ranks each child’s clinical response, resolution of symptoms, and antibiotic-associated adverse effects (AEs) in an ordinal desirability of outcome ranking, or DOOR.
“There were no differences between strategies in the DOOR or in its individual components,” Dr. Williams and colleagues point out. A total of 380 children took part in the study. The mean age of participants was 35.7 months, and half were male.
Over 90% of children randomized to active therapy were prescribed amoxicillin. “Fewer than 10% of children in either strategy had an inadequate clinical response,” the authors report.
However, the 5-day antibiotic strategy had a 69% (95% CI, 63%-75%) probability of children achieving a more desirable RADAR outcome compared with the standard, 10-day course, as assessed either on days 6 to 10 at outcome assessment visit one (OAV1) or at OAV2 on days 19 to 25.
There were also no significant differences between the two groups in the percentage of participants with persistent symptoms at either assessment point, they note. At assessment visit one, 40% of children assigned to the short-course strategy and 37% of children assigned to the 10-day strategy reported an antibiotic-related AE, most of which were mild.
Resistome analysis
Some 171 children were included in a resistome analysis in which throat swabs were collected between study days 19 and 25 to quantify antibiotic resistance genes in oropharyngeal flora. The total number of resistance genes per prokaryotic cell (RGPC) was significantly lower in children treated with antibiotics for 5 days compared with children who were treated for 10 days.
Specifically, the median number of total RGPC was 1.17 (95% CI, 0.35-2.43) for the short-course strategy and 1.33 (95% CI, 0.46-11.08) for the standard-course strategy (P = .01). Similarly, the median number of β-lactamase RGPC was 0.55 (0.18-1.24) for the short-course strategy and 0.60 (0.21-2.45) for the standard-course strategy (P = .03).
“Providing the shortest duration of antibiotics necessary to effectively treat an infection is a central tenet of antimicrobial stewardship and a convenient and cost-effective strategy for caregivers,” the authors observe. For example, reducing treatment from 10 to 5 days for outpatient CAP could reduce the number of days spent on antibiotics by up to 7.5 million days in the U.S. each year.
“If we can safely reduce antibiotic exposure, we can minimize antibiotic side effects while also helping to slow antibiotic resistance,” Dr. Williams pointed out.
Fewer days of having to give their child repeated doses of antibiotics is also more convenient for families, he added.
Asked to comment on the study, David Greenberg, MD, professor of pediatrics and infectious diseases, Ben Gurion University of the Negev, Israel, explained that the length of antibiotic therapy as recommended by various guidelines is more or less arbitrary, some infections being excepted.
“There have been no studies evaluating the recommendation for a 100-day treatment course, and it’s kind of a joke because if you look at the treatment of just about any infection, it’s either for 7 days or 14 days or even 20 days because it’s easy to calculate – it’s not that anybody proved that treatment of whatever infection it is should last this long,” he told this news organization.
Moreover, adherence to a shorter antibiotic course is much better than it is to a longer course. If, for example, physicians tell a mother to take two bottles of antibiotics for a treatment course of 10 days, she’ll finish the first bottle which is good for 5 days and, because the child is fine, “she forgets about the second bottle,” Dr. Greenberg said.
In one of the first studies to compare a short versus long course of antibiotic therapy in uncomplicated CAP in young children, Dr. Greenberg and colleagues initially compared a 3-day course of high-dose amoxicillin to a 10-day course of the same treatment, but the 3-day course was associated with an unacceptable failure rate. (At the time, the World Health Organization was recommending a 3-day course of antibiotics for the treatment of uncomplicated CAP in children.)
They stopped the study and then initiated a second study in which they compared a 5-day course of the same antibiotic to a 10-day course and found the 5-day course was comparable to the 10-day course in terms of clinical cure rates. As a result of his study, Dr. Greenberg has long since prescribed a 5-day course of antibiotics for his own patients.
“Five days is good,” he affirmed. “And if patients start a 10-day course of an antibiotic for, say, a urinary tract infection and a subsequent culture comes back negative, they don’t have to finish the antibiotics either.” Dr. Greenberg said.
Dr. Williams said he has no financial ties to industry. Dr. Greenberg said he has served as a consultant for Pfizer, Merck, Johnson & Johnson, and AstraZeneca. He is also a founder of the company Beyond Air.
A version of this article first appeared on Medscape.com.
Antibiotics used in newborns despite low risk for sepsis
Antibiotics were administered to newborns at low risk for early-onset sepsis as frequently as to newborns with EOS risk factors, based on data from approximately 7,500 infants.
EOS remains a significant cause of morbidity and mortality, and predicting which newborns are at risk remains a challenge for neonatal care that often drives high rates of antibiotic use, Dustin D. Flannery, DO, of Children’s Hospital of Philadelphia and colleagues wrote.
Antibiotic exposures are associated with short- and long-term adverse effects in both preterm and term infants, which highlights the need for improved risk assessment in this population, the researchers said.
“A robust estimate of EOS risk in relation to delivery characteristics among infants of all gestational ages at birth could significantly contribute to newborn clinical management by identifying newborns unlikely to benefit from empirical antibiotic therapy,” they emphasized.
In a study published in Pediatrics, the researchers identified 7,540 infants born between Jan. 1, 2009, and Dec. 31, 2014, at two high-risk perinatal units in Philadelphia. Gestational age ranged from 22 to 43 weeks. Criteria for low risk of EOS were determined via an algorithm that included cesarean delivery (with or without labor or membrane rupture), and no antepartum concerns for intra-amniotic infection or nonreassuring fetal status.
A total of 6,428 infants did not meet the low-risk criteria; another 1,121 infants met the low-risk criteria. The primary outcome of EOS was defined as growth of a pathogen in at least 1 blood and/or cerebrospinal fluid culture obtained at 72 hours or less after birth. Overall, 41 infants who did not meet the low-risk criteria developed EOS; none of the infants who met the low-risk criteria developed EOS. Secondary outcomes included initiation of empirical antibiotics at 72 hours or less after birth and the duration of antibiotic use.
Although fewer low-risk infants received antibiotics, compared with infants with EOS (80.4% vs. 91.0%, P < .001), the duration of antibiotic use was not significantly different between the groups, with an adjusted difference of 0.6 hours.
Among infants who did not meet low-risk criteria, 157 were started on antibiotics for each case of EOS, the researchers noted in their discussion of the findings. “Because no cases of EOS were identified in the low-risk group, this proportion could not be calculated but suggests that antibiotic exposure in this group was disproportionately higher for incidence of EOS.”
The study findings were limited by several factors including the possible lack of generalizability to other centers and the use of data from a period before more refined EOS strategies, the researchers noted. Other limitations include the inability to assess the effect of lab results on antibiotic use, a lack of data on the exact indication for delivery, and potential misclassification bias.
Risk assessment tools should not be used alone, but should be used to inform clinical decision-making, the researchers emphasized. However, the results were strengthened by the inclusion of moderately preterm infants, who are rarely studied, and the clinical utility of the risk algorithm used in the study. “The implications of our study include potential adjustments to sepsis risk assessment in term infants, and confirmation and enhancement of previous studies that identify a subset of lower-risk preterm infants,” who may be spared empirical or prolonged antibiotic exposure, they concluded.
Data inform intelligent antibiotic use
“Early-onset sepsis is predominantly caused by exposure of the fetus or neonate to ascending maternal colonization or infection by gastrointestinal or genitourinary bacteria,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “Scenarios where there is limited neonatal exposure to these organisms would decrease the risk of development of EOS, therefore it is not surprising that delivery characteristics of low-risk deliveries as defined by investigators – the absence of labor, absence of intra-amniotic infection, rupture of membranes at time of delivery, and cesarean delivery – would have resulted in decreased likelihood of EOS.”
Inappropriate antibiotic use contributes to the development of resistant and more virulent strains of bacteria. A growing body of literature also suggests that early antibiotic usage in newborns may affect the neonatal gut microbiome, which is important for development of the neonatal immune system. Early alterations of the microbiome may have long-term implications,” Dr. Krishna said.
“Understanding the delivery characteristics that increase the risk of EOS are crucial to optimizing the use of antibiotics and thereby minimize potential harm to newborns,” she said. “Studies such as the current study are needed develop EOS prediction tools to improve antibiotic utilization.” More research is needed not only to adequately predict EOS, but to explore how antibiotics affect the neonatal microbiome, and how clinicians can circumvent potential adverse implications with antibiotic use to improve long-term health, Dr. Krishna concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Krishna had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Antibiotics were administered to newborns at low risk for early-onset sepsis as frequently as to newborns with EOS risk factors, based on data from approximately 7,500 infants.
EOS remains a significant cause of morbidity and mortality, and predicting which newborns are at risk remains a challenge for neonatal care that often drives high rates of antibiotic use, Dustin D. Flannery, DO, of Children’s Hospital of Philadelphia and colleagues wrote.
Antibiotic exposures are associated with short- and long-term adverse effects in both preterm and term infants, which highlights the need for improved risk assessment in this population, the researchers said.
“A robust estimate of EOS risk in relation to delivery characteristics among infants of all gestational ages at birth could significantly contribute to newborn clinical management by identifying newborns unlikely to benefit from empirical antibiotic therapy,” they emphasized.
In a study published in Pediatrics, the researchers identified 7,540 infants born between Jan. 1, 2009, and Dec. 31, 2014, at two high-risk perinatal units in Philadelphia. Gestational age ranged from 22 to 43 weeks. Criteria for low risk of EOS were determined via an algorithm that included cesarean delivery (with or without labor or membrane rupture), and no antepartum concerns for intra-amniotic infection or nonreassuring fetal status.
A total of 6,428 infants did not meet the low-risk criteria; another 1,121 infants met the low-risk criteria. The primary outcome of EOS was defined as growth of a pathogen in at least 1 blood and/or cerebrospinal fluid culture obtained at 72 hours or less after birth. Overall, 41 infants who did not meet the low-risk criteria developed EOS; none of the infants who met the low-risk criteria developed EOS. Secondary outcomes included initiation of empirical antibiotics at 72 hours or less after birth and the duration of antibiotic use.
Although fewer low-risk infants received antibiotics, compared with infants with EOS (80.4% vs. 91.0%, P < .001), the duration of antibiotic use was not significantly different between the groups, with an adjusted difference of 0.6 hours.
Among infants who did not meet low-risk criteria, 157 were started on antibiotics for each case of EOS, the researchers noted in their discussion of the findings. “Because no cases of EOS were identified in the low-risk group, this proportion could not be calculated but suggests that antibiotic exposure in this group was disproportionately higher for incidence of EOS.”
The study findings were limited by several factors including the possible lack of generalizability to other centers and the use of data from a period before more refined EOS strategies, the researchers noted. Other limitations include the inability to assess the effect of lab results on antibiotic use, a lack of data on the exact indication for delivery, and potential misclassification bias.
Risk assessment tools should not be used alone, but should be used to inform clinical decision-making, the researchers emphasized. However, the results were strengthened by the inclusion of moderately preterm infants, who are rarely studied, and the clinical utility of the risk algorithm used in the study. “The implications of our study include potential adjustments to sepsis risk assessment in term infants, and confirmation and enhancement of previous studies that identify a subset of lower-risk preterm infants,” who may be spared empirical or prolonged antibiotic exposure, they concluded.
Data inform intelligent antibiotic use
“Early-onset sepsis is predominantly caused by exposure of the fetus or neonate to ascending maternal colonization or infection by gastrointestinal or genitourinary bacteria,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “Scenarios where there is limited neonatal exposure to these organisms would decrease the risk of development of EOS, therefore it is not surprising that delivery characteristics of low-risk deliveries as defined by investigators – the absence of labor, absence of intra-amniotic infection, rupture of membranes at time of delivery, and cesarean delivery – would have resulted in decreased likelihood of EOS.”
Inappropriate antibiotic use contributes to the development of resistant and more virulent strains of bacteria. A growing body of literature also suggests that early antibiotic usage in newborns may affect the neonatal gut microbiome, which is important for development of the neonatal immune system. Early alterations of the microbiome may have long-term implications,” Dr. Krishna said.
“Understanding the delivery characteristics that increase the risk of EOS are crucial to optimizing the use of antibiotics and thereby minimize potential harm to newborns,” she said. “Studies such as the current study are needed develop EOS prediction tools to improve antibiotic utilization.” More research is needed not only to adequately predict EOS, but to explore how antibiotics affect the neonatal microbiome, and how clinicians can circumvent potential adverse implications with antibiotic use to improve long-term health, Dr. Krishna concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Krishna had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
Antibiotics were administered to newborns at low risk for early-onset sepsis as frequently as to newborns with EOS risk factors, based on data from approximately 7,500 infants.
EOS remains a significant cause of morbidity and mortality, and predicting which newborns are at risk remains a challenge for neonatal care that often drives high rates of antibiotic use, Dustin D. Flannery, DO, of Children’s Hospital of Philadelphia and colleagues wrote.
Antibiotic exposures are associated with short- and long-term adverse effects in both preterm and term infants, which highlights the need for improved risk assessment in this population, the researchers said.
“A robust estimate of EOS risk in relation to delivery characteristics among infants of all gestational ages at birth could significantly contribute to newborn clinical management by identifying newborns unlikely to benefit from empirical antibiotic therapy,” they emphasized.
In a study published in Pediatrics, the researchers identified 7,540 infants born between Jan. 1, 2009, and Dec. 31, 2014, at two high-risk perinatal units in Philadelphia. Gestational age ranged from 22 to 43 weeks. Criteria for low risk of EOS were determined via an algorithm that included cesarean delivery (with or without labor or membrane rupture), and no antepartum concerns for intra-amniotic infection or nonreassuring fetal status.
A total of 6,428 infants did not meet the low-risk criteria; another 1,121 infants met the low-risk criteria. The primary outcome of EOS was defined as growth of a pathogen in at least 1 blood and/or cerebrospinal fluid culture obtained at 72 hours or less after birth. Overall, 41 infants who did not meet the low-risk criteria developed EOS; none of the infants who met the low-risk criteria developed EOS. Secondary outcomes included initiation of empirical antibiotics at 72 hours or less after birth and the duration of antibiotic use.
Although fewer low-risk infants received antibiotics, compared with infants with EOS (80.4% vs. 91.0%, P < .001), the duration of antibiotic use was not significantly different between the groups, with an adjusted difference of 0.6 hours.
Among infants who did not meet low-risk criteria, 157 were started on antibiotics for each case of EOS, the researchers noted in their discussion of the findings. “Because no cases of EOS were identified in the low-risk group, this proportion could not be calculated but suggests that antibiotic exposure in this group was disproportionately higher for incidence of EOS.”
The study findings were limited by several factors including the possible lack of generalizability to other centers and the use of data from a period before more refined EOS strategies, the researchers noted. Other limitations include the inability to assess the effect of lab results on antibiotic use, a lack of data on the exact indication for delivery, and potential misclassification bias.
Risk assessment tools should not be used alone, but should be used to inform clinical decision-making, the researchers emphasized. However, the results were strengthened by the inclusion of moderately preterm infants, who are rarely studied, and the clinical utility of the risk algorithm used in the study. “The implications of our study include potential adjustments to sepsis risk assessment in term infants, and confirmation and enhancement of previous studies that identify a subset of lower-risk preterm infants,” who may be spared empirical or prolonged antibiotic exposure, they concluded.
Data inform intelligent antibiotic use
“Early-onset sepsis is predominantly caused by exposure of the fetus or neonate to ascending maternal colonization or infection by gastrointestinal or genitourinary bacteria,” Iris Krishna, MD, of Emory University, Atlanta, said in an interview. “Scenarios where there is limited neonatal exposure to these organisms would decrease the risk of development of EOS, therefore it is not surprising that delivery characteristics of low-risk deliveries as defined by investigators – the absence of labor, absence of intra-amniotic infection, rupture of membranes at time of delivery, and cesarean delivery – would have resulted in decreased likelihood of EOS.”
Inappropriate antibiotic use contributes to the development of resistant and more virulent strains of bacteria. A growing body of literature also suggests that early antibiotic usage in newborns may affect the neonatal gut microbiome, which is important for development of the neonatal immune system. Early alterations of the microbiome may have long-term implications,” Dr. Krishna said.
“Understanding the delivery characteristics that increase the risk of EOS are crucial to optimizing the use of antibiotics and thereby minimize potential harm to newborns,” she said. “Studies such as the current study are needed develop EOS prediction tools to improve antibiotic utilization.” More research is needed not only to adequately predict EOS, but to explore how antibiotics affect the neonatal microbiome, and how clinicians can circumvent potential adverse implications with antibiotic use to improve long-term health, Dr. Krishna concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Krishna had no financial conflicts to disclose and serves on the editorial advisory board of Ob.Gyn. News.
FROM PEDIATRICS
The etiology of acute otitis media in young children in recent years
Since the COVID-19 pandemic began, pediatricians have been seeing fewer cases of all respiratory illnesses, including acute otitis media (AOM). However, as I prepare this column, an uptick has commenced and likely will continue in an upward trajectory as we emerge from the pandemic into an endemic coronavirus era. Our group in Rochester, N.Y., has continued prospective studies of AOM throughout the pandemic. We found that nasopharyngeal colonization by Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae, and Moraxella catarrhalis remained prevalent in our study cohort of children aged 6-36 months. However, with all the precautions of masking, social distancing, hand washing, and quick exclusion from day care when illness occurred, the frequency of detecting these common otopathogens decreased, as one might expect.1
Leading up to the pandemic, we had an abundance of data to characterize AOM etiology and found that the cause of AOM continues to change following the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13). Our most recent report on otopathogen distribution and antibiotic susceptibility covered the years 2015-2019.2 A total of 589 children were enrolled prospectively and we collected 495 middle ear fluid samples (MEF) from 319 AOM cases using tympanocentesis. The frequency of isolates was H. influenzae (34%), pneumococcus (24%), and M. catarrhalis (15%). Beta-lactamase–positive H. influenzae strains were identified among 49% of the isolates, rendering them resistant to amoxicillin. PCV13 serotypes were infrequently isolated. However, we did isolate vaccine types (VTs) in some children from MEF, notably serotypes 19F, 19A, and 3. Non-PCV13 pneumococcus serotypes 35B, 23B, and 15B/C emerged as the most common serotypes. Amoxicillin resistance was identified among 25% of pneumococcal strains. Out of 16 antibiotics tested, 9 (56%) showed a significant increase in nonsusceptibility among pneumococcal isolates. 100% of M. catarrhalis isolates were beta-lactamase producers and therefore resistant to amoxicillin.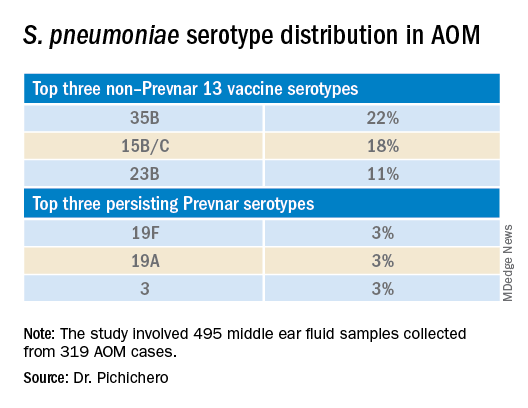
PCV13 has resulted in a decline in both invasive and noninvasive pneumococcal infections caused by strains expressing the 13 capsular serotypes included in the vaccine. However, the emergence of replacement serotypes occurred after introduction of PCV73,4 and continues to occur during the PCV13 era, as shown from the results presented here. Non-PCV13 serotypes accounted for more than 90% of MEF isolates during 2015-2019, with 35B, 21 and 23B being the most commonly isolated. Other emergent serotypes of potential importance were nonvaccine serotypes 15A, 15B, 15C, 23A and 11A. This is highly relevant because forthcoming higher-valency PCVs – PCV15 (manufactured by Merck) and PCV20 (manufactured by Pfizer) will not include many of the dominant capsular serotypes of pneumococcus strains causing AOM. Consequently, the impact of higher-valency PCVs on AOM will not be as great as was observed with the introduction of PCV7 or PCV13.
Of special interest, 22% of pneumococcus isolates from MEF were serotype 35B, making it the most prevalent. Recently we reported a significant rise in antibiotic nonsusceptibility in Spn isolates, contributed mainly by serotype 35B5 and we have been studying how 35B strains transitioned from commensal to otopathogen in children.6 Because serotype 35B strains are increasingly prevalent and often antibiotic resistant, absence of this serotype from PCV15 and PCV20 is cause for concern.
The frequency of isolation of H. influenzae and M. catarrhalis has remained stable across the PCV13 era as the No. 1 and No. 3 pathogens. Similarly, the production of beta-lactamase among strains causing AOM has remained stable at close to 50% and 100%, respectively. Use of amoxicillin, either high dose or standard dose, would not be expected to kill these bacteria.
Our study design has limitations. The population is derived from a predominantly middle-class, suburban population of children in upstate New York and may not be representative of other types of populations in the United States. The children are 6-36 months old, the age when most AOM occurs. MEF samples that were culture negative for bacteria were not further tested by polymerase chain reaction methods.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Front Pediatr. 2021;9:722483.
2. Kaur R et al. Euro J Clin Microbiol Infect Dis. 2021;41:37-44
3. Pelton SI et al. Pediatr Infect Disease J. 2004;23:1015-22.
4. Farrell DJ et al. Pediatr Infect Disease J. 2007;26:123-8..
5. Kaur R et al. Clin Infect Dis 2021;72(5):797-805.
6. Fuji N et al. Front Cell Infect Microbiol. 2021;11:744742.
Since the COVID-19 pandemic began, pediatricians have been seeing fewer cases of all respiratory illnesses, including acute otitis media (AOM). However, as I prepare this column, an uptick has commenced and likely will continue in an upward trajectory as we emerge from the pandemic into an endemic coronavirus era. Our group in Rochester, N.Y., has continued prospective studies of AOM throughout the pandemic. We found that nasopharyngeal colonization by Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae, and Moraxella catarrhalis remained prevalent in our study cohort of children aged 6-36 months. However, with all the precautions of masking, social distancing, hand washing, and quick exclusion from day care when illness occurred, the frequency of detecting these common otopathogens decreased, as one might expect.1
Leading up to the pandemic, we had an abundance of data to characterize AOM etiology and found that the cause of AOM continues to change following the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13). Our most recent report on otopathogen distribution and antibiotic susceptibility covered the years 2015-2019.2 A total of 589 children were enrolled prospectively and we collected 495 middle ear fluid samples (MEF) from 319 AOM cases using tympanocentesis. The frequency of isolates was H. influenzae (34%), pneumococcus (24%), and M. catarrhalis (15%). Beta-lactamase–positive H. influenzae strains were identified among 49% of the isolates, rendering them resistant to amoxicillin. PCV13 serotypes were infrequently isolated. However, we did isolate vaccine types (VTs) in some children from MEF, notably serotypes 19F, 19A, and 3. Non-PCV13 pneumococcus serotypes 35B, 23B, and 15B/C emerged as the most common serotypes. Amoxicillin resistance was identified among 25% of pneumococcal strains. Out of 16 antibiotics tested, 9 (56%) showed a significant increase in nonsusceptibility among pneumococcal isolates. 100% of M. catarrhalis isolates were beta-lactamase producers and therefore resistant to amoxicillin.
PCV13 has resulted in a decline in both invasive and noninvasive pneumococcal infections caused by strains expressing the 13 capsular serotypes included in the vaccine. However, the emergence of replacement serotypes occurred after introduction of PCV73,4 and continues to occur during the PCV13 era, as shown from the results presented here. Non-PCV13 serotypes accounted for more than 90% of MEF isolates during 2015-2019, with 35B, 21 and 23B being the most commonly isolated. Other emergent serotypes of potential importance were nonvaccine serotypes 15A, 15B, 15C, 23A and 11A. This is highly relevant because forthcoming higher-valency PCVs – PCV15 (manufactured by Merck) and PCV20 (manufactured by Pfizer) will not include many of the dominant capsular serotypes of pneumococcus strains causing AOM. Consequently, the impact of higher-valency PCVs on AOM will not be as great as was observed with the introduction of PCV7 or PCV13.
Of special interest, 22% of pneumococcus isolates from MEF were serotype 35B, making it the most prevalent. Recently we reported a significant rise in antibiotic nonsusceptibility in Spn isolates, contributed mainly by serotype 35B5 and we have been studying how 35B strains transitioned from commensal to otopathogen in children.6 Because serotype 35B strains are increasingly prevalent and often antibiotic resistant, absence of this serotype from PCV15 and PCV20 is cause for concern.
The frequency of isolation of H. influenzae and M. catarrhalis has remained stable across the PCV13 era as the No. 1 and No. 3 pathogens. Similarly, the production of beta-lactamase among strains causing AOM has remained stable at close to 50% and 100%, respectively. Use of amoxicillin, either high dose or standard dose, would not be expected to kill these bacteria.
Our study design has limitations. The population is derived from a predominantly middle-class, suburban population of children in upstate New York and may not be representative of other types of populations in the United States. The children are 6-36 months old, the age when most AOM occurs. MEF samples that were culture negative for bacteria were not further tested by polymerase chain reaction methods.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Front Pediatr. 2021;9:722483.
2. Kaur R et al. Euro J Clin Microbiol Infect Dis. 2021;41:37-44
3. Pelton SI et al. Pediatr Infect Disease J. 2004;23:1015-22.
4. Farrell DJ et al. Pediatr Infect Disease J. 2007;26:123-8..
5. Kaur R et al. Clin Infect Dis 2021;72(5):797-805.
6. Fuji N et al. Front Cell Infect Microbiol. 2021;11:744742.
Since the COVID-19 pandemic began, pediatricians have been seeing fewer cases of all respiratory illnesses, including acute otitis media (AOM). However, as I prepare this column, an uptick has commenced and likely will continue in an upward trajectory as we emerge from the pandemic into an endemic coronavirus era. Our group in Rochester, N.Y., has continued prospective studies of AOM throughout the pandemic. We found that nasopharyngeal colonization by Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae, and Moraxella catarrhalis remained prevalent in our study cohort of children aged 6-36 months. However, with all the precautions of masking, social distancing, hand washing, and quick exclusion from day care when illness occurred, the frequency of detecting these common otopathogens decreased, as one might expect.1
Leading up to the pandemic, we had an abundance of data to characterize AOM etiology and found that the cause of AOM continues to change following the introduction of the 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13). Our most recent report on otopathogen distribution and antibiotic susceptibility covered the years 2015-2019.2 A total of 589 children were enrolled prospectively and we collected 495 middle ear fluid samples (MEF) from 319 AOM cases using tympanocentesis. The frequency of isolates was H. influenzae (34%), pneumococcus (24%), and M. catarrhalis (15%). Beta-lactamase–positive H. influenzae strains were identified among 49% of the isolates, rendering them resistant to amoxicillin. PCV13 serotypes were infrequently isolated. However, we did isolate vaccine types (VTs) in some children from MEF, notably serotypes 19F, 19A, and 3. Non-PCV13 pneumococcus serotypes 35B, 23B, and 15B/C emerged as the most common serotypes. Amoxicillin resistance was identified among 25% of pneumococcal strains. Out of 16 antibiotics tested, 9 (56%) showed a significant increase in nonsusceptibility among pneumococcal isolates. 100% of M. catarrhalis isolates were beta-lactamase producers and therefore resistant to amoxicillin.
PCV13 has resulted in a decline in both invasive and noninvasive pneumococcal infections caused by strains expressing the 13 capsular serotypes included in the vaccine. However, the emergence of replacement serotypes occurred after introduction of PCV73,4 and continues to occur during the PCV13 era, as shown from the results presented here. Non-PCV13 serotypes accounted for more than 90% of MEF isolates during 2015-2019, with 35B, 21 and 23B being the most commonly isolated. Other emergent serotypes of potential importance were nonvaccine serotypes 15A, 15B, 15C, 23A and 11A. This is highly relevant because forthcoming higher-valency PCVs – PCV15 (manufactured by Merck) and PCV20 (manufactured by Pfizer) will not include many of the dominant capsular serotypes of pneumococcus strains causing AOM. Consequently, the impact of higher-valency PCVs on AOM will not be as great as was observed with the introduction of PCV7 or PCV13.
Of special interest, 22% of pneumococcus isolates from MEF were serotype 35B, making it the most prevalent. Recently we reported a significant rise in antibiotic nonsusceptibility in Spn isolates, contributed mainly by serotype 35B5 and we have been studying how 35B strains transitioned from commensal to otopathogen in children.6 Because serotype 35B strains are increasingly prevalent and often antibiotic resistant, absence of this serotype from PCV15 and PCV20 is cause for concern.
The frequency of isolation of H. influenzae and M. catarrhalis has remained stable across the PCV13 era as the No. 1 and No. 3 pathogens. Similarly, the production of beta-lactamase among strains causing AOM has remained stable at close to 50% and 100%, respectively. Use of amoxicillin, either high dose or standard dose, would not be expected to kill these bacteria.
Our study design has limitations. The population is derived from a predominantly middle-class, suburban population of children in upstate New York and may not be representative of other types of populations in the United States. The children are 6-36 months old, the age when most AOM occurs. MEF samples that were culture negative for bacteria were not further tested by polymerase chain reaction methods.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Kaur R et al. Front Pediatr. 2021;9:722483.
2. Kaur R et al. Euro J Clin Microbiol Infect Dis. 2021;41:37-44
3. Pelton SI et al. Pediatr Infect Disease J. 2004;23:1015-22.
4. Farrell DJ et al. Pediatr Infect Disease J. 2007;26:123-8..
5. Kaur R et al. Clin Infect Dis 2021;72(5):797-805.
6. Fuji N et al. Front Cell Infect Microbiol. 2021;11:744742.
What makes a urinary tract infection complicated?
Consider anatomical and severity risk factors
Case
A 72-year-old woman with type 2 diabetes mellitus presents with acute dysuria, fever, and flank pain. She had a urinary tract infection (UTI) 3 months prior treated with nitrofurantoin. Temperature is 102° F, heart rate 112 beats per minute, and the remainder of vital signs are normal. She has left costovertebral angle tenderness. Urine microscopy shows 70 WBCs per high power field and bacteria. Is this urinary tract infection complicated?
Background
The urinary tract is divided into the upper tract, which includes the kidneys and ureters, and the lower urinary tract, which includes the bladder, urethra, and prostate. Infection of the lower urinary tract is referred to as cystitis while infection of the upper urinary tract is pyelonephritis. A UTI is the colonization of pathogen(s) within the urinary system that causes an inflammatory response resulting in symptoms and requiring treatment. UTIs occur when there is reduced urine flow, an increase in colonization risk, and when there are factors that facilitate ascent such as catheterization or incontinence.
There are an estimated 150 million cases of UTIs worldwide per year, accounting for $6 billion in health care expenditures.1 In the inpatient setting, about 40% of nosocomial infections are associated with urinary catheters. This equates to about 1 million catheter-associated UTIs per year in the United States, and up to 40% of hospital gram-negative bacteremia per year are caused by UTIs.1
UTIs are often classified as either uncomplicated or complicated infections, which can influence the depth of management. UTIs have a wide spectrum of symptoms and can manifest anywhere from mild dysuria treated successfully with outpatient antibiotics to florid sepsis. Uncomplicated simple cystitis is often treated as an outpatient with oral nitrofurantoin or trimethoprim-sulfamethoxazole.2 Complicated UTIs are treated with broader antimicrobial coverage, and depending on severity, could require intravenous antibiotics. Many factors affect how a UTI manifests and determining whether an infection is “uncomplicated” or “complicated” is an important first step in guiding management. Unfortunately, there are differing classifications of “complicated” UTIs, making it a complicated issue itself. We outline two common approaches.
Anatomic approach
A commonly recognized definition is from the American Urological Association, which states that complicated UTIs are symptomatic cases associated with the presence of “underlying, predisposing conditions and not necessarily clinical severity, invasiveness, or complications.”3 These factors include structural or functional urinary tract abnormalities or urinary instrumentation (see Table 1). These predisposing conditions can increase microbial colonization and decrease therapy efficacy, thus increasing the frequency of infection and relapse.
This population of patients is at high risk of infections with more resistant bacteria such as extended-spectrum beta-lactamase (ESBL) producing Escherichia coli since they often lack the natural genitourinary barriers to infection. In addition, these patients more often undergo multiple antibiotic courses for their frequent infections, which also contributes to their risk of ESBL infections. Genitourinary abnormalities interfere with normal voiding, resulting in impaired flushing of bacteria. For instance, obstruction inhibits complete urinary drainage and increases the persistence of bacteria in biofilms, especially if there are stones or indwelling devices present. Biofilms usually contain a high concentration of organisms including Proteus mirabilis, Morgenella morganii, and Providencia spp.4 Keep in mind that, if there is an obstruction, the urinalysis might be without pyuria or bacteriuria.
Instrumentation increases infection risks through the direct introduction of bacteria into the genitourinary tract. Despite the efforts in maintaining sterility in urinary catheter placement, catheters provide a nidus for infection. Catheter-associated UTI (CAUTI) is defined by the Infectious Disease Society of America as UTIs that occur in patients with an indwelling catheter or who had a catheter removed for less than 48 hours who develop urinary symptoms and cultures positive for uropathogenic bacteria.4 Studies show that in general, patients with indwelling catheters will develop bacteriuria over time, with 10%-25% eventually developing symptoms.
Severity approach
There are other schools of thought that categorize uncomplicated versus complicated UTIs based on the severity of presentation (see Table 2). An uncomplicated UTI would be classified as symptoms and signs of simple cystitis limited to dysuria, frequency, urgency, and suprapubic pain. Using a symptom severity approach, systemic findings such as fever, chills, emesis, flank pain, costovertebral angle tenderness, or other findings of sepsis would be classified as a complicated UTI. These systemic findings would suggest an extension of infection beyond the bladder.
The argument for a symptomatic-based approach of classification is that the severity of symptoms should dictate the degree of management. Not all UTIs in the anatomic approach are severe. In fact, populations that are considered at risk for complicated UTIs by the AUA guidelines in Table 1 often have mild symptomatic cystitis or asymptomatic bacteriuria. Asymptomatic bacteriuria is the colonization of organisms in the urinary tract without active infection. For instance, bacteriuria is present in almost 100% of people with chronic indwelling catheters, 30%-40% of neurogenic bladder requiring intermittent catheterization, and 50% of elderly nursing home residents.4 Not all bacteriuria triggers enough of an inflammatory response to cause symptoms that require treatment.
Ultimate clinical judgment
Although there are multiple different society recommendations in distinguishing uncomplicated versus complicated UTIs, considering both anatomical and severity risk factors can better aid in clinical decision-making rather than abiding by one classification method alone.
Uncomplicated UTIs from the AUA guidelines can cause severe infections that might require longer courses of broad-spectrum antibiotics. On the other hand, people with anatomic abnormalities can present with mild symptoms that can be treated with a narrow-spectrum antibiotic for a standard time course. Recognizing the severity of the infection and using clinical judgment aids in antibiotic stewardship.
Although the existence of algorithmic approaches can help guide clinical judgment, accounting for the spectrum of host and bacterial factors should ultimately determine the complexity of the disease and management.3 Using clinical suspicion to determine when a UTI should be treated as a complicated infection can ensure effective treatment and decrease the likelihood of sepsis, renal scarring, or end-stage disease.5
Back to the case
The case presents an elderly woman with diabetes presenting with sepsis from a UTI. Because of a normal urinary tract and no prior instrumentation, by the AUA definition, she would be classified as an uncomplicated UTI; however, we would classify her as a complicated UTI based on the severity of her presentation. She has a fever, tachycardia, flank pain, and costovertebral angle tenderness that are evidence of infection extending beyond the bladder. She has sepsis warranting inpatient management. Prior urine culture results could aid in determining empiric treatment while waiting for new cultures. In her case, an intravenous antibiotic with broad gram-negative coverage such as ceftriaxone would be appropriate.
Bottom line
There are multiple interpretations of complicated UTIs including both an anatomical and severity approach. Clinical judgment regarding infection severity should determine the depth of management.
Dr. Vu is a hospitalist at the University of Kentucky, Lexington. Dr. Gray is a hospitalist at the University of Kentucky and the Lexington Veterans Affairs Medical Center.
References
1. Folk CS. AUA Core Curriculum: Urinary Tract Infection (Adult). 2021 Mar 1. https://university.auanet.org/core_topic.cfm?coreid=92.
2. Gupta K et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. doi: 10.1093/cid/ciq257.
3. Johnson JR. Definition of Complicated Urinary Tract Infection. Clin Infect Dis. 2017 February 15;64(4):529. doi: 10.1093/cid/ciw751.
4. Nicolle LE, AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16(6):349-60. doi: 10.1155/2005/385768.
5. Melekos MD and Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents. 2000;15(4):247-56. doi: 10.1016/s0924-8579(00)00168-0.
Key points
- The anatomical approach to defining complicated UTIs considers the presence of underlying, predisposing conditions such as structurally or functionally abnormal genitourinary tract or urinary instrumentation or foreign bodies.
- The severity approach to defining complicated UTIs considers the severity of presentation including the presence of systemic manifestations.
- Both approaches should consider populations that are at risk for recurrent or multidrug-resistant infections and infections that can lead to high morbidity.
- Either approach can be used as a guide, but neither should replace clinical suspicion and judgment in determining the depth of treatment.
Additional reading
Choe HS et al. Summary of the UAA‐AAUS guidelines for urinary tract infections. Int J Urol. 2018 Mar;25(3):175-85. doi:10.1111/iju.13493.
Nicolle LE et al. Infectious Diseases Society of America Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis. 2005 Mar;40(5):643-54. doi: 10.1086/427507.
Wagenlehner FME et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020 Oct;17:586-600. doi:10.1038/s41585-020-0362-4.
Wallace DW et al. Urinalysis: A simple test with complicated interpretation. J Urgent Care Med. 2020 July-Aug;14(10):11-4.
Quiz
A 68-year-old woman with type 2 diabetes mellitus presents to the emergency department with acute fever, chills, dysuria, frequency, and suprapubic pain. She has associated nausea, malaise, and fatigue. She takes metformin and denies recent antibiotic use. Her temperature is 102.8° F, heart rate 118 beats per minute, blood pressure 118/71 mm Hg, and her respiratory rate is 24 breaths per minute. She is ill-appearing and has mild suprapubic tenderness. White blood cell count is 18 k/mcL. Urinalysis is positive for leukocyte esterase, nitrites, and bacteria. Urine microscopy has 120 white blood cells per high power field. What is the most appropriate treatment?
A. Azithromycin
B. Ceftriaxone
C. Cefepime and vancomycin
D. Nitrofurantoin
The answer is B. The patient presents with sepsis secondary to a urinary tract infection. Using the anatomic approach this would be classified as uncomplicated. Using the severity approach, this would be classified as a complicated urinary tract infection. With fever, chills, and signs of sepsis, it’s likely her infection extends beyond the bladder. Given the severity of her presentation, we’d favor treating her as a complicated urinary tract infection with intravenous ceftriaxone. There is no suggestion of resistance or additional MRSA risk factors requiring intravenous vancomycin or cefepime. Nitrofurantoin, although a first-line treatment for uncomplicated cystitis, would not be appropriate if there is suspicion infection extends beyond the bladder. Azithromycin is a first-line option for chlamydia trachomatis, but not a urinary tract infection.
Consider anatomical and severity risk factors
Consider anatomical and severity risk factors
Case
A 72-year-old woman with type 2 diabetes mellitus presents with acute dysuria, fever, and flank pain. She had a urinary tract infection (UTI) 3 months prior treated with nitrofurantoin. Temperature is 102° F, heart rate 112 beats per minute, and the remainder of vital signs are normal. She has left costovertebral angle tenderness. Urine microscopy shows 70 WBCs per high power field and bacteria. Is this urinary tract infection complicated?
Background
The urinary tract is divided into the upper tract, which includes the kidneys and ureters, and the lower urinary tract, which includes the bladder, urethra, and prostate. Infection of the lower urinary tract is referred to as cystitis while infection of the upper urinary tract is pyelonephritis. A UTI is the colonization of pathogen(s) within the urinary system that causes an inflammatory response resulting in symptoms and requiring treatment. UTIs occur when there is reduced urine flow, an increase in colonization risk, and when there are factors that facilitate ascent such as catheterization or incontinence.
There are an estimated 150 million cases of UTIs worldwide per year, accounting for $6 billion in health care expenditures.1 In the inpatient setting, about 40% of nosocomial infections are associated with urinary catheters. This equates to about 1 million catheter-associated UTIs per year in the United States, and up to 40% of hospital gram-negative bacteremia per year are caused by UTIs.1
UTIs are often classified as either uncomplicated or complicated infections, which can influence the depth of management. UTIs have a wide spectrum of symptoms and can manifest anywhere from mild dysuria treated successfully with outpatient antibiotics to florid sepsis. Uncomplicated simple cystitis is often treated as an outpatient with oral nitrofurantoin or trimethoprim-sulfamethoxazole.2 Complicated UTIs are treated with broader antimicrobial coverage, and depending on severity, could require intravenous antibiotics. Many factors affect how a UTI manifests and determining whether an infection is “uncomplicated” or “complicated” is an important first step in guiding management. Unfortunately, there are differing classifications of “complicated” UTIs, making it a complicated issue itself. We outline two common approaches.
Anatomic approach
A commonly recognized definition is from the American Urological Association, which states that complicated UTIs are symptomatic cases associated with the presence of “underlying, predisposing conditions and not necessarily clinical severity, invasiveness, or complications.”3 These factors include structural or functional urinary tract abnormalities or urinary instrumentation (see Table 1). These predisposing conditions can increase microbial colonization and decrease therapy efficacy, thus increasing the frequency of infection and relapse.
This population of patients is at high risk of infections with more resistant bacteria such as extended-spectrum beta-lactamase (ESBL) producing Escherichia coli since they often lack the natural genitourinary barriers to infection. In addition, these patients more often undergo multiple antibiotic courses for their frequent infections, which also contributes to their risk of ESBL infections. Genitourinary abnormalities interfere with normal voiding, resulting in impaired flushing of bacteria. For instance, obstruction inhibits complete urinary drainage and increases the persistence of bacteria in biofilms, especially if there are stones or indwelling devices present. Biofilms usually contain a high concentration of organisms including Proteus mirabilis, Morgenella morganii, and Providencia spp.4 Keep in mind that, if there is an obstruction, the urinalysis might be without pyuria or bacteriuria.
Instrumentation increases infection risks through the direct introduction of bacteria into the genitourinary tract. Despite the efforts in maintaining sterility in urinary catheter placement, catheters provide a nidus for infection. Catheter-associated UTI (CAUTI) is defined by the Infectious Disease Society of America as UTIs that occur in patients with an indwelling catheter or who had a catheter removed for less than 48 hours who develop urinary symptoms and cultures positive for uropathogenic bacteria.4 Studies show that in general, patients with indwelling catheters will develop bacteriuria over time, with 10%-25% eventually developing symptoms.
Severity approach
There are other schools of thought that categorize uncomplicated versus complicated UTIs based on the severity of presentation (see Table 2). An uncomplicated UTI would be classified as symptoms and signs of simple cystitis limited to dysuria, frequency, urgency, and suprapubic pain. Using a symptom severity approach, systemic findings such as fever, chills, emesis, flank pain, costovertebral angle tenderness, or other findings of sepsis would be classified as a complicated UTI. These systemic findings would suggest an extension of infection beyond the bladder.
The argument for a symptomatic-based approach of classification is that the severity of symptoms should dictate the degree of management. Not all UTIs in the anatomic approach are severe. In fact, populations that are considered at risk for complicated UTIs by the AUA guidelines in Table 1 often have mild symptomatic cystitis or asymptomatic bacteriuria. Asymptomatic bacteriuria is the colonization of organisms in the urinary tract without active infection. For instance, bacteriuria is present in almost 100% of people with chronic indwelling catheters, 30%-40% of neurogenic bladder requiring intermittent catheterization, and 50% of elderly nursing home residents.4 Not all bacteriuria triggers enough of an inflammatory response to cause symptoms that require treatment.
Ultimate clinical judgment
Although there are multiple different society recommendations in distinguishing uncomplicated versus complicated UTIs, considering both anatomical and severity risk factors can better aid in clinical decision-making rather than abiding by one classification method alone.
Uncomplicated UTIs from the AUA guidelines can cause severe infections that might require longer courses of broad-spectrum antibiotics. On the other hand, people with anatomic abnormalities can present with mild symptoms that can be treated with a narrow-spectrum antibiotic for a standard time course. Recognizing the severity of the infection and using clinical judgment aids in antibiotic stewardship.
Although the existence of algorithmic approaches can help guide clinical judgment, accounting for the spectrum of host and bacterial factors should ultimately determine the complexity of the disease and management.3 Using clinical suspicion to determine when a UTI should be treated as a complicated infection can ensure effective treatment and decrease the likelihood of sepsis, renal scarring, or end-stage disease.5
Back to the case
The case presents an elderly woman with diabetes presenting with sepsis from a UTI. Because of a normal urinary tract and no prior instrumentation, by the AUA definition, she would be classified as an uncomplicated UTI; however, we would classify her as a complicated UTI based on the severity of her presentation. She has a fever, tachycardia, flank pain, and costovertebral angle tenderness that are evidence of infection extending beyond the bladder. She has sepsis warranting inpatient management. Prior urine culture results could aid in determining empiric treatment while waiting for new cultures. In her case, an intravenous antibiotic with broad gram-negative coverage such as ceftriaxone would be appropriate.
Bottom line
There are multiple interpretations of complicated UTIs including both an anatomical and severity approach. Clinical judgment regarding infection severity should determine the depth of management.
Dr. Vu is a hospitalist at the University of Kentucky, Lexington. Dr. Gray is a hospitalist at the University of Kentucky and the Lexington Veterans Affairs Medical Center.
References
1. Folk CS. AUA Core Curriculum: Urinary Tract Infection (Adult). 2021 Mar 1. https://university.auanet.org/core_topic.cfm?coreid=92.
2. Gupta K et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. doi: 10.1093/cid/ciq257.
3. Johnson JR. Definition of Complicated Urinary Tract Infection. Clin Infect Dis. 2017 February 15;64(4):529. doi: 10.1093/cid/ciw751.
4. Nicolle LE, AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16(6):349-60. doi: 10.1155/2005/385768.
5. Melekos MD and Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents. 2000;15(4):247-56. doi: 10.1016/s0924-8579(00)00168-0.
Key points
- The anatomical approach to defining complicated UTIs considers the presence of underlying, predisposing conditions such as structurally or functionally abnormal genitourinary tract or urinary instrumentation or foreign bodies.
- The severity approach to defining complicated UTIs considers the severity of presentation including the presence of systemic manifestations.
- Both approaches should consider populations that are at risk for recurrent or multidrug-resistant infections and infections that can lead to high morbidity.
- Either approach can be used as a guide, but neither should replace clinical suspicion and judgment in determining the depth of treatment.
Additional reading
Choe HS et al. Summary of the UAA‐AAUS guidelines for urinary tract infections. Int J Urol. 2018 Mar;25(3):175-85. doi:10.1111/iju.13493.
Nicolle LE et al. Infectious Diseases Society of America Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis. 2005 Mar;40(5):643-54. doi: 10.1086/427507.
Wagenlehner FME et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020 Oct;17:586-600. doi:10.1038/s41585-020-0362-4.
Wallace DW et al. Urinalysis: A simple test with complicated interpretation. J Urgent Care Med. 2020 July-Aug;14(10):11-4.
Quiz
A 68-year-old woman with type 2 diabetes mellitus presents to the emergency department with acute fever, chills, dysuria, frequency, and suprapubic pain. She has associated nausea, malaise, and fatigue. She takes metformin and denies recent antibiotic use. Her temperature is 102.8° F, heart rate 118 beats per minute, blood pressure 118/71 mm Hg, and her respiratory rate is 24 breaths per minute. She is ill-appearing and has mild suprapubic tenderness. White blood cell count is 18 k/mcL. Urinalysis is positive for leukocyte esterase, nitrites, and bacteria. Urine microscopy has 120 white blood cells per high power field. What is the most appropriate treatment?
A. Azithromycin
B. Ceftriaxone
C. Cefepime and vancomycin
D. Nitrofurantoin
The answer is B. The patient presents with sepsis secondary to a urinary tract infection. Using the anatomic approach this would be classified as uncomplicated. Using the severity approach, this would be classified as a complicated urinary tract infection. With fever, chills, and signs of sepsis, it’s likely her infection extends beyond the bladder. Given the severity of her presentation, we’d favor treating her as a complicated urinary tract infection with intravenous ceftriaxone. There is no suggestion of resistance or additional MRSA risk factors requiring intravenous vancomycin or cefepime. Nitrofurantoin, although a first-line treatment for uncomplicated cystitis, would not be appropriate if there is suspicion infection extends beyond the bladder. Azithromycin is a first-line option for chlamydia trachomatis, but not a urinary tract infection.
Case
A 72-year-old woman with type 2 diabetes mellitus presents with acute dysuria, fever, and flank pain. She had a urinary tract infection (UTI) 3 months prior treated with nitrofurantoin. Temperature is 102° F, heart rate 112 beats per minute, and the remainder of vital signs are normal. She has left costovertebral angle tenderness. Urine microscopy shows 70 WBCs per high power field and bacteria. Is this urinary tract infection complicated?
Background
The urinary tract is divided into the upper tract, which includes the kidneys and ureters, and the lower urinary tract, which includes the bladder, urethra, and prostate. Infection of the lower urinary tract is referred to as cystitis while infection of the upper urinary tract is pyelonephritis. A UTI is the colonization of pathogen(s) within the urinary system that causes an inflammatory response resulting in symptoms and requiring treatment. UTIs occur when there is reduced urine flow, an increase in colonization risk, and when there are factors that facilitate ascent such as catheterization or incontinence.
There are an estimated 150 million cases of UTIs worldwide per year, accounting for $6 billion in health care expenditures.1 In the inpatient setting, about 40% of nosocomial infections are associated with urinary catheters. This equates to about 1 million catheter-associated UTIs per year in the United States, and up to 40% of hospital gram-negative bacteremia per year are caused by UTIs.1
UTIs are often classified as either uncomplicated or complicated infections, which can influence the depth of management. UTIs have a wide spectrum of symptoms and can manifest anywhere from mild dysuria treated successfully with outpatient antibiotics to florid sepsis. Uncomplicated simple cystitis is often treated as an outpatient with oral nitrofurantoin or trimethoprim-sulfamethoxazole.2 Complicated UTIs are treated with broader antimicrobial coverage, and depending on severity, could require intravenous antibiotics. Many factors affect how a UTI manifests and determining whether an infection is “uncomplicated” or “complicated” is an important first step in guiding management. Unfortunately, there are differing classifications of “complicated” UTIs, making it a complicated issue itself. We outline two common approaches.
Anatomic approach
A commonly recognized definition is from the American Urological Association, which states that complicated UTIs are symptomatic cases associated with the presence of “underlying, predisposing conditions and not necessarily clinical severity, invasiveness, or complications.”3 These factors include structural or functional urinary tract abnormalities or urinary instrumentation (see Table 1). These predisposing conditions can increase microbial colonization and decrease therapy efficacy, thus increasing the frequency of infection and relapse.
This population of patients is at high risk of infections with more resistant bacteria such as extended-spectrum beta-lactamase (ESBL) producing Escherichia coli since they often lack the natural genitourinary barriers to infection. In addition, these patients more often undergo multiple antibiotic courses for their frequent infections, which also contributes to their risk of ESBL infections. Genitourinary abnormalities interfere with normal voiding, resulting in impaired flushing of bacteria. For instance, obstruction inhibits complete urinary drainage and increases the persistence of bacteria in biofilms, especially if there are stones or indwelling devices present. Biofilms usually contain a high concentration of organisms including Proteus mirabilis, Morgenella morganii, and Providencia spp.4 Keep in mind that, if there is an obstruction, the urinalysis might be without pyuria or bacteriuria.
Instrumentation increases infection risks through the direct introduction of bacteria into the genitourinary tract. Despite the efforts in maintaining sterility in urinary catheter placement, catheters provide a nidus for infection. Catheter-associated UTI (CAUTI) is defined by the Infectious Disease Society of America as UTIs that occur in patients with an indwelling catheter or who had a catheter removed for less than 48 hours who develop urinary symptoms and cultures positive for uropathogenic bacteria.4 Studies show that in general, patients with indwelling catheters will develop bacteriuria over time, with 10%-25% eventually developing symptoms.
Severity approach
There are other schools of thought that categorize uncomplicated versus complicated UTIs based on the severity of presentation (see Table 2). An uncomplicated UTI would be classified as symptoms and signs of simple cystitis limited to dysuria, frequency, urgency, and suprapubic pain. Using a symptom severity approach, systemic findings such as fever, chills, emesis, flank pain, costovertebral angle tenderness, or other findings of sepsis would be classified as a complicated UTI. These systemic findings would suggest an extension of infection beyond the bladder.
The argument for a symptomatic-based approach of classification is that the severity of symptoms should dictate the degree of management. Not all UTIs in the anatomic approach are severe. In fact, populations that are considered at risk for complicated UTIs by the AUA guidelines in Table 1 often have mild symptomatic cystitis or asymptomatic bacteriuria. Asymptomatic bacteriuria is the colonization of organisms in the urinary tract without active infection. For instance, bacteriuria is present in almost 100% of people with chronic indwelling catheters, 30%-40% of neurogenic bladder requiring intermittent catheterization, and 50% of elderly nursing home residents.4 Not all bacteriuria triggers enough of an inflammatory response to cause symptoms that require treatment.
Ultimate clinical judgment
Although there are multiple different society recommendations in distinguishing uncomplicated versus complicated UTIs, considering both anatomical and severity risk factors can better aid in clinical decision-making rather than abiding by one classification method alone.
Uncomplicated UTIs from the AUA guidelines can cause severe infections that might require longer courses of broad-spectrum antibiotics. On the other hand, people with anatomic abnormalities can present with mild symptoms that can be treated with a narrow-spectrum antibiotic for a standard time course. Recognizing the severity of the infection and using clinical judgment aids in antibiotic stewardship.
Although the existence of algorithmic approaches can help guide clinical judgment, accounting for the spectrum of host and bacterial factors should ultimately determine the complexity of the disease and management.3 Using clinical suspicion to determine when a UTI should be treated as a complicated infection can ensure effective treatment and decrease the likelihood of sepsis, renal scarring, or end-stage disease.5
Back to the case
The case presents an elderly woman with diabetes presenting with sepsis from a UTI. Because of a normal urinary tract and no prior instrumentation, by the AUA definition, she would be classified as an uncomplicated UTI; however, we would classify her as a complicated UTI based on the severity of her presentation. She has a fever, tachycardia, flank pain, and costovertebral angle tenderness that are evidence of infection extending beyond the bladder. She has sepsis warranting inpatient management. Prior urine culture results could aid in determining empiric treatment while waiting for new cultures. In her case, an intravenous antibiotic with broad gram-negative coverage such as ceftriaxone would be appropriate.
Bottom line
There are multiple interpretations of complicated UTIs including both an anatomical and severity approach. Clinical judgment regarding infection severity should determine the depth of management.
Dr. Vu is a hospitalist at the University of Kentucky, Lexington. Dr. Gray is a hospitalist at the University of Kentucky and the Lexington Veterans Affairs Medical Center.
References
1. Folk CS. AUA Core Curriculum: Urinary Tract Infection (Adult). 2021 Mar 1. https://university.auanet.org/core_topic.cfm?coreid=92.
2. Gupta K et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011 Mar 1;52(5):e103-20. doi: 10.1093/cid/ciq257.
3. Johnson JR. Definition of Complicated Urinary Tract Infection. Clin Infect Dis. 2017 February 15;64(4):529. doi: 10.1093/cid/ciw751.
4. Nicolle LE, AMMI Canada Guidelines Committee. Complicated urinary tract infection in adults. Can J Infect Dis Med Microbiol. 2005;16(6):349-60. doi: 10.1155/2005/385768.
5. Melekos MD and Naber KG. Complicated urinary tract infections. Int J Antimicrob Agents. 2000;15(4):247-56. doi: 10.1016/s0924-8579(00)00168-0.
Key points
- The anatomical approach to defining complicated UTIs considers the presence of underlying, predisposing conditions such as structurally or functionally abnormal genitourinary tract or urinary instrumentation or foreign bodies.
- The severity approach to defining complicated UTIs considers the severity of presentation including the presence of systemic manifestations.
- Both approaches should consider populations that are at risk for recurrent or multidrug-resistant infections and infections that can lead to high morbidity.
- Either approach can be used as a guide, but neither should replace clinical suspicion and judgment in determining the depth of treatment.
Additional reading
Choe HS et al. Summary of the UAA‐AAUS guidelines for urinary tract infections. Int J Urol. 2018 Mar;25(3):175-85. doi:10.1111/iju.13493.
Nicolle LE et al. Infectious Diseases Society of America Guidelines for the Diagnosis and Treatment of Asymptomatic Bacteriuria in Adults. Clin Infect Dis. 2005 Mar;40(5):643-54. doi: 10.1086/427507.
Wagenlehner FME et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol. 2020 Oct;17:586-600. doi:10.1038/s41585-020-0362-4.
Wallace DW et al. Urinalysis: A simple test with complicated interpretation. J Urgent Care Med. 2020 July-Aug;14(10):11-4.
Quiz
A 68-year-old woman with type 2 diabetes mellitus presents to the emergency department with acute fever, chills, dysuria, frequency, and suprapubic pain. She has associated nausea, malaise, and fatigue. She takes metformin and denies recent antibiotic use. Her temperature is 102.8° F, heart rate 118 beats per minute, blood pressure 118/71 mm Hg, and her respiratory rate is 24 breaths per minute. She is ill-appearing and has mild suprapubic tenderness. White blood cell count is 18 k/mcL. Urinalysis is positive for leukocyte esterase, nitrites, and bacteria. Urine microscopy has 120 white blood cells per high power field. What is the most appropriate treatment?
A. Azithromycin
B. Ceftriaxone
C. Cefepime and vancomycin
D. Nitrofurantoin
The answer is B. The patient presents with sepsis secondary to a urinary tract infection. Using the anatomic approach this would be classified as uncomplicated. Using the severity approach, this would be classified as a complicated urinary tract infection. With fever, chills, and signs of sepsis, it’s likely her infection extends beyond the bladder. Given the severity of her presentation, we’d favor treating her as a complicated urinary tract infection with intravenous ceftriaxone. There is no suggestion of resistance or additional MRSA risk factors requiring intravenous vancomycin or cefepime. Nitrofurantoin, although a first-line treatment for uncomplicated cystitis, would not be appropriate if there is suspicion infection extends beyond the bladder. Azithromycin is a first-line option for chlamydia trachomatis, but not a urinary tract infection.
Clinicians may overprescribe clarithromycin for H. pylori
Clinicians are prescribing clarithromycin at high rates for Helicobacter pylori infections, despite increasing resistance to this antibiotic, researchers say.
In an analysis of 1 million U.S. prescriptions for H. pylori infections, 80% contained clarithromycin, said Carol Rockett, PharmD, associate vice president of RedHill Biopharma in Raleigh, N.C.
Dr. Rockett presented the findings at the annual meeting of the American College of Gastroenterology.
“Multiple talks [at the meeting] have suggested that the use of clarithromycin in H. pylori is obsolete,” she told this news organization. “Clarithromycin is particularly ineffective in people with a genetic variant that causes rapid metabolism.”
According to the 2017 ACG clinical guideline for treating H. pylori, patients diagnosed with this infection should be asked about their previous antibiotic exposure prior to treatment.
Additionally, clinicians should prescribe clarithromycin triple therapy with a proton pump inhibitor (PPI) and amoxicillin or metronidazole as a first-line treatment only in “regions where H. pylori clarithromycin resistance is known to be less than 15%” and in patients with no previous history of macrolide exposure.
The guideline puts bismuth quadruple therapy, consisting of a PPI, bismuth, tetracycline, and a nitroimidazole, at the top of its list of six alternative first-line therapies. However, three of the six alternatives include clarithromycin.
ERADICATE Hp and ERADICATE Hp2
To understand how U.S. physicians are treating patients with H. pylori, Dr. Rockett’s colleagues analyzed data from two phase 3 clinical trials of RedHill’s RHB-105 (Talicia): ERADICATE Hp and ERADICATE Hp2.
RHB-105 is an all-in‐one combination of omeprazole (40 mg), amoxicillin (1,000 mg), and rifabutin (50 mg) that the Food and Drug Administration approved for treatment of H pylori in 2019.
The researchers followed 38 subjects from ERADICATE Hp who remained positive for H. pylori after the study’s completion. A total of 33 had received a placebo in that trial, while the other 5 had received RHB-105.
The researchers obtained data on 31 of these patients. The overall cure rate was 61.3%. Of the 31 patients, 27 received a regimen including clarithromycin. Their cure rate was 59.3%.
Turning to ERADICATE Hp2, the researchers obtained data on 94 patients whose H. pylori infections persisted after the trial. Of those, 67 had received an active comparator (amoxicillin 250 mg and omeprazole 10 mg) and 27 had received RHB-105.
The overall cure rate was 56.2%. For the 48 subjects who received therapies including clarithromycin, the cure rate was 60.4%. For the 22 subjects who received a bismuth-based quadruple regimen, the cure rate was 45.4%.
In another analysis, the researchers crunched 12 months of numbers from IQVIA PharMetrics Plus medical and prescription claim database of over 1 million prescriptions for H. pylori. They found that 80% of the prescriptions made by gastroenterologists were for regimens containing clarithromycin. That proportion increased to 84% for physician assistants and internists, 85% for nurse practitioners, 86% for family practitioners, and 89% for general practitioners.
Finally, the researchers also analyzed patients for CYP2C19 gene status. They tested 65 subjects who received RHB-105 in ERADICATE Hp and all 445 subjects in ERADICATE Hp2. They found that 58.5% in ERADICATE Hp and 48.6% in ERADICATE Hp2 were normal metabolizers.
In 20 normal metabolizers who received clarithromycin, the drug eradicated the infection in 16 (80%). Out of 11 rapid metabolizers, clarithromycin eradicated the bacterium in 2 (18.2%). The difference was statistically significant (P = .0017).
“With clarithromycin, you can see that the efficacy is reduced in those patients who are rapid metabolizers,” Dr. Rockett said. “We didn’t see that with rifabutin [one of the drugs in RHB-105].”
Jared Magee, DO, MPH, a gastroenterology fellow at the Walter Reed National Military Medical Center in Bethesda, Md., said in treating H. pylori infections, he checks the patients’ medical records to see what antibiotics they have received in the past and generally begins treatment with the bismuth quadruple therapy.
“There is education needed to get the data out there that clarithromycin-based therapies may not be the right choice for patients,” he said. “There is a subset who will do well with it, but I think where we’re at now, with the frequency of macrolide prescriptions for other conditions, that clarithromycin is going to be a difficult therapy for a lot of people.”
Clinicians who are not gastroenterologists may not be aware of the guideline promulgated by the ACG, he pointed out.
Dr. Rockett is an employee of RedHill Biopharma. Dr. Magee has disclosed no relevant financial relationships. The study was funded by RedHill Biopharma.
A version of this article first appeared on Medscape.com.
Clinicians are prescribing clarithromycin at high rates for Helicobacter pylori infections, despite increasing resistance to this antibiotic, researchers say.
In an analysis of 1 million U.S. prescriptions for H. pylori infections, 80% contained clarithromycin, said Carol Rockett, PharmD, associate vice president of RedHill Biopharma in Raleigh, N.C.
Dr. Rockett presented the findings at the annual meeting of the American College of Gastroenterology.
“Multiple talks [at the meeting] have suggested that the use of clarithromycin in H. pylori is obsolete,” she told this news organization. “Clarithromycin is particularly ineffective in people with a genetic variant that causes rapid metabolism.”
According to the 2017 ACG clinical guideline for treating H. pylori, patients diagnosed with this infection should be asked about their previous antibiotic exposure prior to treatment.
Additionally, clinicians should prescribe clarithromycin triple therapy with a proton pump inhibitor (PPI) and amoxicillin or metronidazole as a first-line treatment only in “regions where H. pylori clarithromycin resistance is known to be less than 15%” and in patients with no previous history of macrolide exposure.
The guideline puts bismuth quadruple therapy, consisting of a PPI, bismuth, tetracycline, and a nitroimidazole, at the top of its list of six alternative first-line therapies. However, three of the six alternatives include clarithromycin.
ERADICATE Hp and ERADICATE Hp2
To understand how U.S. physicians are treating patients with H. pylori, Dr. Rockett’s colleagues analyzed data from two phase 3 clinical trials of RedHill’s RHB-105 (Talicia): ERADICATE Hp and ERADICATE Hp2.
RHB-105 is an all-in‐one combination of omeprazole (40 mg), amoxicillin (1,000 mg), and rifabutin (50 mg) that the Food and Drug Administration approved for treatment of H pylori in 2019.
The researchers followed 38 subjects from ERADICATE Hp who remained positive for H. pylori after the study’s completion. A total of 33 had received a placebo in that trial, while the other 5 had received RHB-105.
The researchers obtained data on 31 of these patients. The overall cure rate was 61.3%. Of the 31 patients, 27 received a regimen including clarithromycin. Their cure rate was 59.3%.
Turning to ERADICATE Hp2, the researchers obtained data on 94 patients whose H. pylori infections persisted after the trial. Of those, 67 had received an active comparator (amoxicillin 250 mg and omeprazole 10 mg) and 27 had received RHB-105.
The overall cure rate was 56.2%. For the 48 subjects who received therapies including clarithromycin, the cure rate was 60.4%. For the 22 subjects who received a bismuth-based quadruple regimen, the cure rate was 45.4%.
In another analysis, the researchers crunched 12 months of numbers from IQVIA PharMetrics Plus medical and prescription claim database of over 1 million prescriptions for H. pylori. They found that 80% of the prescriptions made by gastroenterologists were for regimens containing clarithromycin. That proportion increased to 84% for physician assistants and internists, 85% for nurse practitioners, 86% for family practitioners, and 89% for general practitioners.
Finally, the researchers also analyzed patients for CYP2C19 gene status. They tested 65 subjects who received RHB-105 in ERADICATE Hp and all 445 subjects in ERADICATE Hp2. They found that 58.5% in ERADICATE Hp and 48.6% in ERADICATE Hp2 were normal metabolizers.
In 20 normal metabolizers who received clarithromycin, the drug eradicated the infection in 16 (80%). Out of 11 rapid metabolizers, clarithromycin eradicated the bacterium in 2 (18.2%). The difference was statistically significant (P = .0017).
“With clarithromycin, you can see that the efficacy is reduced in those patients who are rapid metabolizers,” Dr. Rockett said. “We didn’t see that with rifabutin [one of the drugs in RHB-105].”
Jared Magee, DO, MPH, a gastroenterology fellow at the Walter Reed National Military Medical Center in Bethesda, Md., said in treating H. pylori infections, he checks the patients’ medical records to see what antibiotics they have received in the past and generally begins treatment with the bismuth quadruple therapy.
“There is education needed to get the data out there that clarithromycin-based therapies may not be the right choice for patients,” he said. “There is a subset who will do well with it, but I think where we’re at now, with the frequency of macrolide prescriptions for other conditions, that clarithromycin is going to be a difficult therapy for a lot of people.”
Clinicians who are not gastroenterologists may not be aware of the guideline promulgated by the ACG, he pointed out.
Dr. Rockett is an employee of RedHill Biopharma. Dr. Magee has disclosed no relevant financial relationships. The study was funded by RedHill Biopharma.
A version of this article first appeared on Medscape.com.
Clinicians are prescribing clarithromycin at high rates for Helicobacter pylori infections, despite increasing resistance to this antibiotic, researchers say.
In an analysis of 1 million U.S. prescriptions for H. pylori infections, 80% contained clarithromycin, said Carol Rockett, PharmD, associate vice president of RedHill Biopharma in Raleigh, N.C.
Dr. Rockett presented the findings at the annual meeting of the American College of Gastroenterology.
“Multiple talks [at the meeting] have suggested that the use of clarithromycin in H. pylori is obsolete,” she told this news organization. “Clarithromycin is particularly ineffective in people with a genetic variant that causes rapid metabolism.”
According to the 2017 ACG clinical guideline for treating H. pylori, patients diagnosed with this infection should be asked about their previous antibiotic exposure prior to treatment.
Additionally, clinicians should prescribe clarithromycin triple therapy with a proton pump inhibitor (PPI) and amoxicillin or metronidazole as a first-line treatment only in “regions where H. pylori clarithromycin resistance is known to be less than 15%” and in patients with no previous history of macrolide exposure.
The guideline puts bismuth quadruple therapy, consisting of a PPI, bismuth, tetracycline, and a nitroimidazole, at the top of its list of six alternative first-line therapies. However, three of the six alternatives include clarithromycin.
ERADICATE Hp and ERADICATE Hp2
To understand how U.S. physicians are treating patients with H. pylori, Dr. Rockett’s colleagues analyzed data from two phase 3 clinical trials of RedHill’s RHB-105 (Talicia): ERADICATE Hp and ERADICATE Hp2.
RHB-105 is an all-in‐one combination of omeprazole (40 mg), amoxicillin (1,000 mg), and rifabutin (50 mg) that the Food and Drug Administration approved for treatment of H pylori in 2019.
The researchers followed 38 subjects from ERADICATE Hp who remained positive for H. pylori after the study’s completion. A total of 33 had received a placebo in that trial, while the other 5 had received RHB-105.
The researchers obtained data on 31 of these patients. The overall cure rate was 61.3%. Of the 31 patients, 27 received a regimen including clarithromycin. Their cure rate was 59.3%.
Turning to ERADICATE Hp2, the researchers obtained data on 94 patients whose H. pylori infections persisted after the trial. Of those, 67 had received an active comparator (amoxicillin 250 mg and omeprazole 10 mg) and 27 had received RHB-105.
The overall cure rate was 56.2%. For the 48 subjects who received therapies including clarithromycin, the cure rate was 60.4%. For the 22 subjects who received a bismuth-based quadruple regimen, the cure rate was 45.4%.
In another analysis, the researchers crunched 12 months of numbers from IQVIA PharMetrics Plus medical and prescription claim database of over 1 million prescriptions for H. pylori. They found that 80% of the prescriptions made by gastroenterologists were for regimens containing clarithromycin. That proportion increased to 84% for physician assistants and internists, 85% for nurse practitioners, 86% for family practitioners, and 89% for general practitioners.
Finally, the researchers also analyzed patients for CYP2C19 gene status. They tested 65 subjects who received RHB-105 in ERADICATE Hp and all 445 subjects in ERADICATE Hp2. They found that 58.5% in ERADICATE Hp and 48.6% in ERADICATE Hp2 were normal metabolizers.
In 20 normal metabolizers who received clarithromycin, the drug eradicated the infection in 16 (80%). Out of 11 rapid metabolizers, clarithromycin eradicated the bacterium in 2 (18.2%). The difference was statistically significant (P = .0017).
“With clarithromycin, you can see that the efficacy is reduced in those patients who are rapid metabolizers,” Dr. Rockett said. “We didn’t see that with rifabutin [one of the drugs in RHB-105].”
Jared Magee, DO, MPH, a gastroenterology fellow at the Walter Reed National Military Medical Center in Bethesda, Md., said in treating H. pylori infections, he checks the patients’ medical records to see what antibiotics they have received in the past and generally begins treatment with the bismuth quadruple therapy.
“There is education needed to get the data out there that clarithromycin-based therapies may not be the right choice for patients,” he said. “There is a subset who will do well with it, but I think where we’re at now, with the frequency of macrolide prescriptions for other conditions, that clarithromycin is going to be a difficult therapy for a lot of people.”
Clinicians who are not gastroenterologists may not be aware of the guideline promulgated by the ACG, he pointed out.
Dr. Rockett is an employee of RedHill Biopharma. Dr. Magee has disclosed no relevant financial relationships. The study was funded by RedHill Biopharma.
A version of this article first appeared on Medscape.com.
Managing simple febrile seizures without lumbar puncture safe: 15-year study
Most children with simple febrile seizures (SFSs) can be safely managed without lumbar puncture or other diagnostic tests without risking delayed diagnosis of bacterial meningitis, new data gathered from a 15-year span suggest.
Vidya R. Raghavan, MD, with the division of emergency medicine at Boston Children’s Hospital and Harvard Medical School, also in Boston, published their findings in Pediatrics.
In 2011, researchers published the American Academy of Pediatrics simple febrile seizure guideline, which recommends limiting lumbar puncture to non–low-risk patients. The guidelines also specified that neuroimaging and hematologic testing are not routinely recommended.
Dr. Raghavan and coauthors studied evaluation and management trends of the patients before and after the guidelines. They identified 142,121 children diagnosed with SFS who presented to 1 of 49 pediatric tertiary EDs and met other study criteria. Changes in management of SFS had started years before the guideline and positive effects continued after the guideline publication.
Researchers found a significant 95% decline in rates of lumbar puncture between 2005 and 2019 from 11.6% (95% confidence interval, 10.8%-12.4%) of children in 2005 to 0.6% (95% CI, 0.5%-0.8%; P < .001) in 2019. The most significant declines were among infants 6 months to 1 year.
“We found similar declines in rates of diagnostic laboratory and radiologic testing, intravenous antibiotic administration, hospitalization, and costs,” the authors wrote.
“Importantly,” they wrote, “the decrease in testing was not associated with a concurrent increase in delayed diagnoses of bacterial meningitis.”
The number of hospital admissions and total costs also dropped significantly over the 15-year span of the study. After adjusting for inflation, the authors wrote, costs dropped from an average $1,523 in 2005 to $605 (P < .001) in 2019.
Among first-time presentations for SFSs, 19.2% (95% CI, 18.3%-20.2%) resulted in admission in 2005. That rate dropped to 5.2% (95% CI, 4.8%-5.6%) in 2019 (P < .001), although the authors noted that trend largely plateaued after the guideline was published.
“Our findings are consistent with smaller studies published before 2011 in which researchers found declining rates of LP [lumbar puncture] in children presenting to the ED with their first SFS,” the authors wrote.
Mercedes Blackstone, MD, an emergency physician at the Children’s Hospital of Philadelphia, said in an interview that the paper offers reassurance for changed practice over the last decade.
She said there was substantial relief in pediatrics when the 2011 guidelines recognized formally that protocols were outdated, especially as bacterial meningitis had become increasingly rare with widespread use of pneumococcal and Haemophilus influenzae vaccines. Practitioners had already started to limit the spinal taps on their own.
“We were not really complying with the prior recommendation to do a spinal tap in all those children because it often felt like doing a pretty invasive procedure with a very low yield in what was often a very well child in front of you,” she said.
In 2007, the authors noted, a few years before the guidelines, rates of bacterial meningitis had decreased to 7 per 100,000 in children aged between 2 and 23 months and 0.56 per 100,000 in children aged between 2 and 10 years.
However, Dr. Blackstone said, there was still a worry among some practitioners that there could be missed cases of bacterial meningitis.
“It’s very helpful to see that in all those years, the guidelines have been very validated and there were really no missed cases,” said Dr. Blackstone, author of CHOP’s febrile seizures clinical pathway.
It was good to see the number of CT scans drop as well, she said. Dr. Raghavan’s team found they decreased from 10.6% to 1.6%; P < .001, over the study period.
“Earlier work had shown that there was still a fair amount of head CTs happening and that’s radiation to the young brain,” Dr. Blackstone noted. “This is great news.”
Dr. Blackstone said it was great to see so many children from so many children’s hospitals included in the study.
The paper confirmed that “we’ve reduced a lot of unnecessary testing, saved a lot of cost, and had no increased risk to the patients,” she said.
Dr. Blackstone pointed out that the authors include a limitation that many children are seen in nonpediatric centers in community adult ED and she said those settings tend to have more testing.
“Hopefully, these guidelines have penetrated into the whole community,” she said. “With this paper they should feel reassured that they can spare children some of these tests and procedures.”
Dr. Raghavan and Dr. Blackstone declared no relevant financial relationships.
Most children with simple febrile seizures (SFSs) can be safely managed without lumbar puncture or other diagnostic tests without risking delayed diagnosis of bacterial meningitis, new data gathered from a 15-year span suggest.
Vidya R. Raghavan, MD, with the division of emergency medicine at Boston Children’s Hospital and Harvard Medical School, also in Boston, published their findings in Pediatrics.
In 2011, researchers published the American Academy of Pediatrics simple febrile seizure guideline, which recommends limiting lumbar puncture to non–low-risk patients. The guidelines also specified that neuroimaging and hematologic testing are not routinely recommended.
Dr. Raghavan and coauthors studied evaluation and management trends of the patients before and after the guidelines. They identified 142,121 children diagnosed with SFS who presented to 1 of 49 pediatric tertiary EDs and met other study criteria. Changes in management of SFS had started years before the guideline and positive effects continued after the guideline publication.
Researchers found a significant 95% decline in rates of lumbar puncture between 2005 and 2019 from 11.6% (95% confidence interval, 10.8%-12.4%) of children in 2005 to 0.6% (95% CI, 0.5%-0.8%; P < .001) in 2019. The most significant declines were among infants 6 months to 1 year.
“We found similar declines in rates of diagnostic laboratory and radiologic testing, intravenous antibiotic administration, hospitalization, and costs,” the authors wrote.
“Importantly,” they wrote, “the decrease in testing was not associated with a concurrent increase in delayed diagnoses of bacterial meningitis.”
The number of hospital admissions and total costs also dropped significantly over the 15-year span of the study. After adjusting for inflation, the authors wrote, costs dropped from an average $1,523 in 2005 to $605 (P < .001) in 2019.
Among first-time presentations for SFSs, 19.2% (95% CI, 18.3%-20.2%) resulted in admission in 2005. That rate dropped to 5.2% (95% CI, 4.8%-5.6%) in 2019 (P < .001), although the authors noted that trend largely plateaued after the guideline was published.
“Our findings are consistent with smaller studies published before 2011 in which researchers found declining rates of LP [lumbar puncture] in children presenting to the ED with their first SFS,” the authors wrote.
Mercedes Blackstone, MD, an emergency physician at the Children’s Hospital of Philadelphia, said in an interview that the paper offers reassurance for changed practice over the last decade.
She said there was substantial relief in pediatrics when the 2011 guidelines recognized formally that protocols were outdated, especially as bacterial meningitis had become increasingly rare with widespread use of pneumococcal and Haemophilus influenzae vaccines. Practitioners had already started to limit the spinal taps on their own.
“We were not really complying with the prior recommendation to do a spinal tap in all those children because it often felt like doing a pretty invasive procedure with a very low yield in what was often a very well child in front of you,” she said.
In 2007, the authors noted, a few years before the guidelines, rates of bacterial meningitis had decreased to 7 per 100,000 in children aged between 2 and 23 months and 0.56 per 100,000 in children aged between 2 and 10 years.
However, Dr. Blackstone said, there was still a worry among some practitioners that there could be missed cases of bacterial meningitis.
“It’s very helpful to see that in all those years, the guidelines have been very validated and there were really no missed cases,” said Dr. Blackstone, author of CHOP’s febrile seizures clinical pathway.
It was good to see the number of CT scans drop as well, she said. Dr. Raghavan’s team found they decreased from 10.6% to 1.6%; P < .001, over the study period.
“Earlier work had shown that there was still a fair amount of head CTs happening and that’s radiation to the young brain,” Dr. Blackstone noted. “This is great news.”
Dr. Blackstone said it was great to see so many children from so many children’s hospitals included in the study.
The paper confirmed that “we’ve reduced a lot of unnecessary testing, saved a lot of cost, and had no increased risk to the patients,” she said.
Dr. Blackstone pointed out that the authors include a limitation that many children are seen in nonpediatric centers in community adult ED and she said those settings tend to have more testing.
“Hopefully, these guidelines have penetrated into the whole community,” she said. “With this paper they should feel reassured that they can spare children some of these tests and procedures.”
Dr. Raghavan and Dr. Blackstone declared no relevant financial relationships.
Most children with simple febrile seizures (SFSs) can be safely managed without lumbar puncture or other diagnostic tests without risking delayed diagnosis of bacterial meningitis, new data gathered from a 15-year span suggest.
Vidya R. Raghavan, MD, with the division of emergency medicine at Boston Children’s Hospital and Harvard Medical School, also in Boston, published their findings in Pediatrics.
In 2011, researchers published the American Academy of Pediatrics simple febrile seizure guideline, which recommends limiting lumbar puncture to non–low-risk patients. The guidelines also specified that neuroimaging and hematologic testing are not routinely recommended.
Dr. Raghavan and coauthors studied evaluation and management trends of the patients before and after the guidelines. They identified 142,121 children diagnosed with SFS who presented to 1 of 49 pediatric tertiary EDs and met other study criteria. Changes in management of SFS had started years before the guideline and positive effects continued after the guideline publication.
Researchers found a significant 95% decline in rates of lumbar puncture between 2005 and 2019 from 11.6% (95% confidence interval, 10.8%-12.4%) of children in 2005 to 0.6% (95% CI, 0.5%-0.8%; P < .001) in 2019. The most significant declines were among infants 6 months to 1 year.
“We found similar declines in rates of diagnostic laboratory and radiologic testing, intravenous antibiotic administration, hospitalization, and costs,” the authors wrote.
“Importantly,” they wrote, “the decrease in testing was not associated with a concurrent increase in delayed diagnoses of bacterial meningitis.”
The number of hospital admissions and total costs also dropped significantly over the 15-year span of the study. After adjusting for inflation, the authors wrote, costs dropped from an average $1,523 in 2005 to $605 (P < .001) in 2019.
Among first-time presentations for SFSs, 19.2% (95% CI, 18.3%-20.2%) resulted in admission in 2005. That rate dropped to 5.2% (95% CI, 4.8%-5.6%) in 2019 (P < .001), although the authors noted that trend largely plateaued after the guideline was published.
“Our findings are consistent with smaller studies published before 2011 in which researchers found declining rates of LP [lumbar puncture] in children presenting to the ED with their first SFS,” the authors wrote.
Mercedes Blackstone, MD, an emergency physician at the Children’s Hospital of Philadelphia, said in an interview that the paper offers reassurance for changed practice over the last decade.
She said there was substantial relief in pediatrics when the 2011 guidelines recognized formally that protocols were outdated, especially as bacterial meningitis had become increasingly rare with widespread use of pneumococcal and Haemophilus influenzae vaccines. Practitioners had already started to limit the spinal taps on their own.
“We were not really complying with the prior recommendation to do a spinal tap in all those children because it often felt like doing a pretty invasive procedure with a very low yield in what was often a very well child in front of you,” she said.
In 2007, the authors noted, a few years before the guidelines, rates of bacterial meningitis had decreased to 7 per 100,000 in children aged between 2 and 23 months and 0.56 per 100,000 in children aged between 2 and 10 years.
However, Dr. Blackstone said, there was still a worry among some practitioners that there could be missed cases of bacterial meningitis.
“It’s very helpful to see that in all those years, the guidelines have been very validated and there were really no missed cases,” said Dr. Blackstone, author of CHOP’s febrile seizures clinical pathway.
It was good to see the number of CT scans drop as well, she said. Dr. Raghavan’s team found they decreased from 10.6% to 1.6%; P < .001, over the study period.
“Earlier work had shown that there was still a fair amount of head CTs happening and that’s radiation to the young brain,” Dr. Blackstone noted. “This is great news.”
Dr. Blackstone said it was great to see so many children from so many children’s hospitals included in the study.
The paper confirmed that “we’ve reduced a lot of unnecessary testing, saved a lot of cost, and had no increased risk to the patients,” she said.
Dr. Blackstone pointed out that the authors include a limitation that many children are seen in nonpediatric centers in community adult ED and she said those settings tend to have more testing.
“Hopefully, these guidelines have penetrated into the whole community,” she said. “With this paper they should feel reassured that they can spare children some of these tests and procedures.”
Dr. Raghavan and Dr. Blackstone declared no relevant financial relationships.
FROM PEDIATRICS