User login
FDA approves new fluoroquinolone for skin, skin structure infections
The Food and Drug Administration has approved delafloxacin, a fluoroquinolone, for the treatment of acute bacterial skin and skin structure infections, according to an agency announcement June 19.
Delafloxacin, which will be marketed by Melinta Therapeutics as Baxdela, was designated a qualified infectious disease product (QIDP) and granted fast-track and priority review. These classifications are designed to speed approval of antibacterial products to treat serious or life-threatening infections, according to an FDA statement.
Common adverse events seen with use of delafloxacin included nausea, diarrhea, headache, elevated liver enzymes, and vomiting.
The approval requires Melinta Therapeutics to conduct a 5-year postmarketing surveillance study to look for resistance to delafloxacin; the final report on that study must be submitted by the end of 2022.
The drug was not subject to FDA advisory committee review because its new drug application “did not raise significant safety or efficacy issues that were unexpected” for a drug in its class, according to FDA officials.
For more information see the Drugs@FDA listing for Baxdela.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
The Food and Drug Administration has approved delafloxacin, a fluoroquinolone, for the treatment of acute bacterial skin and skin structure infections, according to an agency announcement June 19.
Delafloxacin, which will be marketed by Melinta Therapeutics as Baxdela, was designated a qualified infectious disease product (QIDP) and granted fast-track and priority review. These classifications are designed to speed approval of antibacterial products to treat serious or life-threatening infections, according to an FDA statement.
Common adverse events seen with use of delafloxacin included nausea, diarrhea, headache, elevated liver enzymes, and vomiting.
The approval requires Melinta Therapeutics to conduct a 5-year postmarketing surveillance study to look for resistance to delafloxacin; the final report on that study must be submitted by the end of 2022.
The drug was not subject to FDA advisory committee review because its new drug application “did not raise significant safety or efficacy issues that were unexpected” for a drug in its class, according to FDA officials.
For more information see the Drugs@FDA listing for Baxdela.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
The Food and Drug Administration has approved delafloxacin, a fluoroquinolone, for the treatment of acute bacterial skin and skin structure infections, according to an agency announcement June 19.
Delafloxacin, which will be marketed by Melinta Therapeutics as Baxdela, was designated a qualified infectious disease product (QIDP) and granted fast-track and priority review. These classifications are designed to speed approval of antibacterial products to treat serious or life-threatening infections, according to an FDA statement.
Common adverse events seen with use of delafloxacin included nausea, diarrhea, headache, elevated liver enzymes, and vomiting.
The approval requires Melinta Therapeutics to conduct a 5-year postmarketing surveillance study to look for resistance to delafloxacin; the final report on that study must be submitted by the end of 2022.
The drug was not subject to FDA advisory committee review because its new drug application “did not raise significant safety or efficacy issues that were unexpected” for a drug in its class, according to FDA officials.
For more information see the Drugs@FDA listing for Baxdela.
dfulton@frontlinemedcom.com
On Twitter @denisefulton
Cancer, heart disease increase MRSA mortality
Cancer, heart, and neurologic disease are associated with significantly higher 30-day mortality from methicillin-resistant Staphylococcus aureus (MRSA), according to a study that also showed mortality rates have changed little in 9 years.
A retrospective study of 1,168 patients, who were admitted to four Michigan hospitals with MRSA over a period of 9 years, showed an overall 30-day mortality of 16% (Int J Infect Dis. 2017 May 19. doi: 10.1016/j.ijid.2017.05.010).
“Notably, with time, we found no improvement in overall mortality over time despite advancement in antimicrobial treatment,” wrote Pedro Ayau, MD, of the Universidad Francisco Marroquin in Guatemala City and his coauthors. “Thus far, the role of different antimicrobial agents against MRSA infection in clinical settings is uncertain.”
Patients with cancer showed the highest 30-day mortality risk from MRSA infection (odds ratio, 2.29; P = .001). Heart disease increased the mortality risk by 78%, neurologic disease by 65%, and nursing home residence by 66%. A Charlson index score greater than 3 was associated with an 88% increase in 30-day mortality.
Age was also an independent risk factor, with each additional year of age associated with a 2.9% increase in the odds of 30-day mortality, even after accounting for other variables.
The authors found evidence of a protective effect associated with diabetes and peripheral vascular disease, with a decrease in 30-day mortality of 40% and 47%, respectively. Although this was an unexpected finding, it was likely related to the source of the infection, they said.
“Patients with [peripheral vascular disease] and diabetes are usually less acutely ill and present with skin/wound infections, which are more easily managed, and started earlier on appropriate antibiotic treatment,” the investigators wrote.
Patients who were readmitted had an 88% lower risk of 30-day mortality. The authors suggested this may be because these patients would likely have received earlier and better management of the infection.
There was also a relationship between the source of infection and mortality. Patients infected from an indwelling central venous catheter had a 61% lower 30-day mortality. Those with skin or wound infections had a 52% lower mortality, and those with genitourinary infection had a 60% lower mortality.
In contrast to other studies, the researchers did not see any significant increase in 30-day mortality with persistent bacteremia.
Two authors declared research grants from the pharmaceutical industries.
Cancer, heart, and neurologic disease are associated with significantly higher 30-day mortality from methicillin-resistant Staphylococcus aureus (MRSA), according to a study that also showed mortality rates have changed little in 9 years.
A retrospective study of 1,168 patients, who were admitted to four Michigan hospitals with MRSA over a period of 9 years, showed an overall 30-day mortality of 16% (Int J Infect Dis. 2017 May 19. doi: 10.1016/j.ijid.2017.05.010).
“Notably, with time, we found no improvement in overall mortality over time despite advancement in antimicrobial treatment,” wrote Pedro Ayau, MD, of the Universidad Francisco Marroquin in Guatemala City and his coauthors. “Thus far, the role of different antimicrobial agents against MRSA infection in clinical settings is uncertain.”
Patients with cancer showed the highest 30-day mortality risk from MRSA infection (odds ratio, 2.29; P = .001). Heart disease increased the mortality risk by 78%, neurologic disease by 65%, and nursing home residence by 66%. A Charlson index score greater than 3 was associated with an 88% increase in 30-day mortality.
Age was also an independent risk factor, with each additional year of age associated with a 2.9% increase in the odds of 30-day mortality, even after accounting for other variables.
The authors found evidence of a protective effect associated with diabetes and peripheral vascular disease, with a decrease in 30-day mortality of 40% and 47%, respectively. Although this was an unexpected finding, it was likely related to the source of the infection, they said.
“Patients with [peripheral vascular disease] and diabetes are usually less acutely ill and present with skin/wound infections, which are more easily managed, and started earlier on appropriate antibiotic treatment,” the investigators wrote.
Patients who were readmitted had an 88% lower risk of 30-day mortality. The authors suggested this may be because these patients would likely have received earlier and better management of the infection.
There was also a relationship between the source of infection and mortality. Patients infected from an indwelling central venous catheter had a 61% lower 30-day mortality. Those with skin or wound infections had a 52% lower mortality, and those with genitourinary infection had a 60% lower mortality.
In contrast to other studies, the researchers did not see any significant increase in 30-day mortality with persistent bacteremia.
Two authors declared research grants from the pharmaceutical industries.
Cancer, heart, and neurologic disease are associated with significantly higher 30-day mortality from methicillin-resistant Staphylococcus aureus (MRSA), according to a study that also showed mortality rates have changed little in 9 years.
A retrospective study of 1,168 patients, who were admitted to four Michigan hospitals with MRSA over a period of 9 years, showed an overall 30-day mortality of 16% (Int J Infect Dis. 2017 May 19. doi: 10.1016/j.ijid.2017.05.010).
“Notably, with time, we found no improvement in overall mortality over time despite advancement in antimicrobial treatment,” wrote Pedro Ayau, MD, of the Universidad Francisco Marroquin in Guatemala City and his coauthors. “Thus far, the role of different antimicrobial agents against MRSA infection in clinical settings is uncertain.”
Patients with cancer showed the highest 30-day mortality risk from MRSA infection (odds ratio, 2.29; P = .001). Heart disease increased the mortality risk by 78%, neurologic disease by 65%, and nursing home residence by 66%. A Charlson index score greater than 3 was associated with an 88% increase in 30-day mortality.
Age was also an independent risk factor, with each additional year of age associated with a 2.9% increase in the odds of 30-day mortality, even after accounting for other variables.
The authors found evidence of a protective effect associated with diabetes and peripheral vascular disease, with a decrease in 30-day mortality of 40% and 47%, respectively. Although this was an unexpected finding, it was likely related to the source of the infection, they said.
“Patients with [peripheral vascular disease] and diabetes are usually less acutely ill and present with skin/wound infections, which are more easily managed, and started earlier on appropriate antibiotic treatment,” the investigators wrote.
Patients who were readmitted had an 88% lower risk of 30-day mortality. The authors suggested this may be because these patients would likely have received earlier and better management of the infection.
There was also a relationship between the source of infection and mortality. Patients infected from an indwelling central venous catheter had a 61% lower 30-day mortality. Those with skin or wound infections had a 52% lower mortality, and those with genitourinary infection had a 60% lower mortality.
In contrast to other studies, the researchers did not see any significant increase in 30-day mortality with persistent bacteremia.
Two authors declared research grants from the pharmaceutical industries.
FROM INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Key clinical point:
Major finding: Cancer is associated with a more than twofold increase in 30-day mortality from MRSA.
Data source: A 9-year retrospective study of 1,168 patients with MRSA infection.
Disclosures: Two authors declared research grants from the pharmaceutical industry.
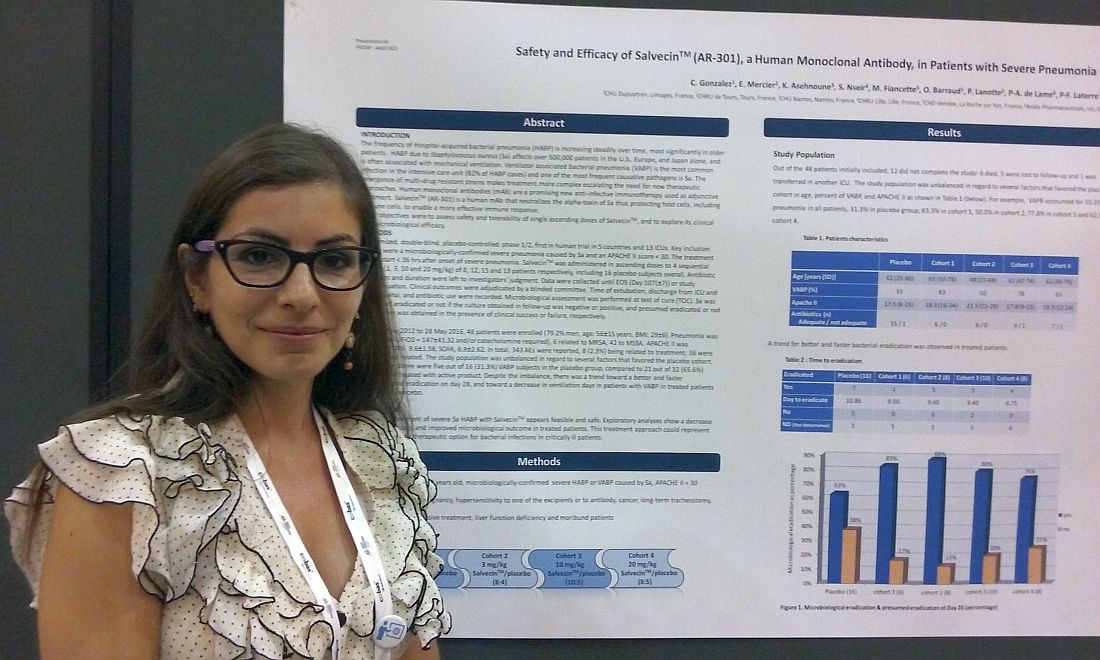
Monoclonal antibody holds promise for S. aureus pneumonia
NEW ORLEANS – Monoclonal antibody therapies have already upended treatment strategies in cancer, dermatology, and multiple inflammatory diseases, and infectious disease may be next.
That’s because , according to a new study. The monoclonal antibody attacks the alpha-toxin secreted by S. aureus, thereby helping to protect immune cells.
“We know S. aureus pneumonia is a big problem. There is a lot of antibiotic resistance, and that is why we need new treatments,” Celine Gonzalez, MD, of the Dupuytren Central University Hospital in Limoges, France, said in an interview.
“Animal studies have shown the monoclonal antibody seems to be useful. This is the first in-human study to use a monoclonal antibody to treat hospital-acquired pneumonia due to Staphylococcus aureus,” Dr. Gonzalez said in a late-breaking poster presentation at the annual meeting of the American Society for Microbiology.
Treatment started within 36 hours of onset of severe pneumonia. Severity was based on a mean PaO2/FiO2 of 147 and/or a need for catecholamine. Six cases of pneumonia were related to MRSA and the remaining 42 to methicillin-susceptible S. aureus. The mean APACHE II score was 18.7, the mean Clinical Pulmonary Infection Score was 9.6, and the mean Sequential Organ Failure Assessment score was 6.9.
Participants were recruited from 13 ICUs in four countries. About 80% of participants were men. Their mean age was 56 years, and mean body mass index was 29 kg/m2. Concurrent antibiotic treatment choice and duration were at the investigator’s discretion.
S. aureus infection was considered eradicated if a follow-up culture was negative, a result achieved by 63% of the 16 placebo patients and 75%-88% of the AR-301-dosage groups. Eradication was also based on observed clinical success in the absence of a confirmatory culture. This was achieved by 38% in the placebo group and 13%-25% of the monoclonal antibody cohorts. A total of seven placebo patients and 15 AR-301 patients met eradication by these criteria.
Side effects were primarily minor and transient, Dr. Gonzalez said. Of the 343 total adverse events reported, only 8 (2.3%) were considered treatment related, she added.
“In infectious disease, it’s the beginning” for monoclonal antibody therapy, Dr. Gonzalez said. “But, it appears to be the future because … it is a more specific treatment, and there is no resistance.”
The study suggests adjunctive treatment with AR-301 appears safe for treatment of hospital-acquired bacterial pneumonia, she noted. The next step will be to confirm the findings in a larger, follow-up study that includes more efficacy outcomes, Dr. Gonzalez added.
Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
NEW ORLEANS – Monoclonal antibody therapies have already upended treatment strategies in cancer, dermatology, and multiple inflammatory diseases, and infectious disease may be next.
That’s because , according to a new study. The monoclonal antibody attacks the alpha-toxin secreted by S. aureus, thereby helping to protect immune cells.
“We know S. aureus pneumonia is a big problem. There is a lot of antibiotic resistance, and that is why we need new treatments,” Celine Gonzalez, MD, of the Dupuytren Central University Hospital in Limoges, France, said in an interview.
“Animal studies have shown the monoclonal antibody seems to be useful. This is the first in-human study to use a monoclonal antibody to treat hospital-acquired pneumonia due to Staphylococcus aureus,” Dr. Gonzalez said in a late-breaking poster presentation at the annual meeting of the American Society for Microbiology.
Treatment started within 36 hours of onset of severe pneumonia. Severity was based on a mean PaO2/FiO2 of 147 and/or a need for catecholamine. Six cases of pneumonia were related to MRSA and the remaining 42 to methicillin-susceptible S. aureus. The mean APACHE II score was 18.7, the mean Clinical Pulmonary Infection Score was 9.6, and the mean Sequential Organ Failure Assessment score was 6.9.
Participants were recruited from 13 ICUs in four countries. About 80% of participants were men. Their mean age was 56 years, and mean body mass index was 29 kg/m2. Concurrent antibiotic treatment choice and duration were at the investigator’s discretion.
S. aureus infection was considered eradicated if a follow-up culture was negative, a result achieved by 63% of the 16 placebo patients and 75%-88% of the AR-301-dosage groups. Eradication was also based on observed clinical success in the absence of a confirmatory culture. This was achieved by 38% in the placebo group and 13%-25% of the monoclonal antibody cohorts. A total of seven placebo patients and 15 AR-301 patients met eradication by these criteria.
Side effects were primarily minor and transient, Dr. Gonzalez said. Of the 343 total adverse events reported, only 8 (2.3%) were considered treatment related, she added.
“In infectious disease, it’s the beginning” for monoclonal antibody therapy, Dr. Gonzalez said. “But, it appears to be the future because … it is a more specific treatment, and there is no resistance.”
The study suggests adjunctive treatment with AR-301 appears safe for treatment of hospital-acquired bacterial pneumonia, she noted. The next step will be to confirm the findings in a larger, follow-up study that includes more efficacy outcomes, Dr. Gonzalez added.
Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
NEW ORLEANS – Monoclonal antibody therapies have already upended treatment strategies in cancer, dermatology, and multiple inflammatory diseases, and infectious disease may be next.
That’s because , according to a new study. The monoclonal antibody attacks the alpha-toxin secreted by S. aureus, thereby helping to protect immune cells.
“We know S. aureus pneumonia is a big problem. There is a lot of antibiotic resistance, and that is why we need new treatments,” Celine Gonzalez, MD, of the Dupuytren Central University Hospital in Limoges, France, said in an interview.
“Animal studies have shown the monoclonal antibody seems to be useful. This is the first in-human study to use a monoclonal antibody to treat hospital-acquired pneumonia due to Staphylococcus aureus,” Dr. Gonzalez said in a late-breaking poster presentation at the annual meeting of the American Society for Microbiology.
Treatment started within 36 hours of onset of severe pneumonia. Severity was based on a mean PaO2/FiO2 of 147 and/or a need for catecholamine. Six cases of pneumonia were related to MRSA and the remaining 42 to methicillin-susceptible S. aureus. The mean APACHE II score was 18.7, the mean Clinical Pulmonary Infection Score was 9.6, and the mean Sequential Organ Failure Assessment score was 6.9.
Participants were recruited from 13 ICUs in four countries. About 80% of participants were men. Their mean age was 56 years, and mean body mass index was 29 kg/m2. Concurrent antibiotic treatment choice and duration were at the investigator’s discretion.
S. aureus infection was considered eradicated if a follow-up culture was negative, a result achieved by 63% of the 16 placebo patients and 75%-88% of the AR-301-dosage groups. Eradication was also based on observed clinical success in the absence of a confirmatory culture. This was achieved by 38% in the placebo group and 13%-25% of the monoclonal antibody cohorts. A total of seven placebo patients and 15 AR-301 patients met eradication by these criteria.
Side effects were primarily minor and transient, Dr. Gonzalez said. Of the 343 total adverse events reported, only 8 (2.3%) were considered treatment related, she added.
“In infectious disease, it’s the beginning” for monoclonal antibody therapy, Dr. Gonzalez said. “But, it appears to be the future because … it is a more specific treatment, and there is no resistance.”
The study suggests adjunctive treatment with AR-301 appears safe for treatment of hospital-acquired bacterial pneumonia, she noted. The next step will be to confirm the findings in a larger, follow-up study that includes more efficacy outcomes, Dr. Gonzalez added.
Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
AT ASM MICROBE 2017
Key clinical point: A monoclonal antibody that neutralizes the alpha-toxin secreted by S. aureus appeared safe and effective in an early trial.
Major finding: AR-301 appeared safe, with 8 of 343 adverse events, or 2.3%, considered treatment related.
Data source: Randomized, double-blind, placebo-controlled, international study in 48 patients from 13 ICUs.
Disclosures: Dr. Gonzalez reported having no relevant disclosures. The study’s principle investigator is a scientific advisor for Aridis Pharmaceuticals, which is developing AR-301.
Rapid culture, stewardship improve S. aureus bacteremia outcomes
NEW ORLEANS – Community hospitals could see positive outcomes using a Staphylococcus aureus bacteremia strategy that combines rapid blood cultures to speed diagnosis with antibiotic stewardship to guide treatment.
Many academic medical centers report improved outcomes with this approach. Now a study of 66 patients at a medium-sized hospital in rural North Dakota suggests this strategy translates well to the community hospital setting and can reduce mortality and 30-day readmission rates, and increase cost-effectiveness overall.
“I was pleased to see we were able to replicate the positive outcomes observed in studies from large tertiary care centers with our small cohort,” Marijo Roiko, PhD, microbiology program director for pathology and laboratory services at Altru Health System in Grand Forks, N.D., said in an interview.
Dr. Roiko and colleagues compared 33 patients diagnosed and treated prior to the strategy with 33 others following its implementation. A total of 13 patients, or 39% of each cohort, developed potentially fatal methicillin-resistant S. aureus (MRSA) bacteremia. Patients’ average age ranged from 60 to 64 years, and about two-thirds of each group were men.
The investigators reported that 30-day all-cause mortality decreased from 15.6% to 13.3% after the protocol. In addition, 30-day readmission rates decreased from 25% to 11% of the group diagnosed and managed with the new strategy. Dr. Roiko presented these and other findings from the chart review at the annual meeting of the American Society for Microbiology.
Overall, the average length of stay decreased from 13 days to 10 days among the patients with S. aureus bacteremia. Among the subgroup of patients with MRSA, the length of stay dropped from 15 to 12 days. Among those with methicillin-susceptible S. aureus infections, 11 days decreased to 9 days after institution of the protocol.
The researchers also looked at time to antibiotic deescalation, and the average time decreased from 3 days to 1 day with the new strategy.
“These results demonstrate the utility of rapid testing from positive blood cultures and antibiotic stewardship for patients with Staph. aureus bacteremia,” Dr. Roiko said.
In terms of return on investment, the estimated cost savings associated with the 3-day reduction in length of hospital stay was sufficient to cover the increased capital expenditures and reagent costs, the researchers found. They estimated these increased costs as $78,960, excluding ICU and ancillary charges. The approximate $4,290 saved per day multiplied by 33 patients means the new protocol saved a total of $141,570.
Traditionally, patients with a positive blood culture of S. aureus had a gram stain, followed by provider notification when positive. Targeted antibiotic therapy was either administered at this point or held for culture-based identification and susceptibility testing. In the new protocol, a rapid culture identification panel (FilmArray BCID) is added at the time of gram staining. Positive results are reported to both the provider and pharmacist. Targeted therapy is then either administered or held based on culture-based susceptibility testing (species identification is determined as needed).
The current study findings add to a literature already supporting use of rapid blood cultures and/or stewardship guidance to address S. aureus bacteremia in academic and tertiary care centers (J Clin Microbiol. 2016;54:2455-63; Clin Microbiol Infect. 2015;21:313-22, and Clin. Infect. Dis. 2015;61:1071-80.
Dr. Roiko had no relevant financial disclosures.
NEW ORLEANS – Community hospitals could see positive outcomes using a Staphylococcus aureus bacteremia strategy that combines rapid blood cultures to speed diagnosis with antibiotic stewardship to guide treatment.
Many academic medical centers report improved outcomes with this approach. Now a study of 66 patients at a medium-sized hospital in rural North Dakota suggests this strategy translates well to the community hospital setting and can reduce mortality and 30-day readmission rates, and increase cost-effectiveness overall.
“I was pleased to see we were able to replicate the positive outcomes observed in studies from large tertiary care centers with our small cohort,” Marijo Roiko, PhD, microbiology program director for pathology and laboratory services at Altru Health System in Grand Forks, N.D., said in an interview.
Dr. Roiko and colleagues compared 33 patients diagnosed and treated prior to the strategy with 33 others following its implementation. A total of 13 patients, or 39% of each cohort, developed potentially fatal methicillin-resistant S. aureus (MRSA) bacteremia. Patients’ average age ranged from 60 to 64 years, and about two-thirds of each group were men.
The investigators reported that 30-day all-cause mortality decreased from 15.6% to 13.3% after the protocol. In addition, 30-day readmission rates decreased from 25% to 11% of the group diagnosed and managed with the new strategy. Dr. Roiko presented these and other findings from the chart review at the annual meeting of the American Society for Microbiology.
Overall, the average length of stay decreased from 13 days to 10 days among the patients with S. aureus bacteremia. Among the subgroup of patients with MRSA, the length of stay dropped from 15 to 12 days. Among those with methicillin-susceptible S. aureus infections, 11 days decreased to 9 days after institution of the protocol.
The researchers also looked at time to antibiotic deescalation, and the average time decreased from 3 days to 1 day with the new strategy.
“These results demonstrate the utility of rapid testing from positive blood cultures and antibiotic stewardship for patients with Staph. aureus bacteremia,” Dr. Roiko said.
In terms of return on investment, the estimated cost savings associated with the 3-day reduction in length of hospital stay was sufficient to cover the increased capital expenditures and reagent costs, the researchers found. They estimated these increased costs as $78,960, excluding ICU and ancillary charges. The approximate $4,290 saved per day multiplied by 33 patients means the new protocol saved a total of $141,570.
Traditionally, patients with a positive blood culture of S. aureus had a gram stain, followed by provider notification when positive. Targeted antibiotic therapy was either administered at this point or held for culture-based identification and susceptibility testing. In the new protocol, a rapid culture identification panel (FilmArray BCID) is added at the time of gram staining. Positive results are reported to both the provider and pharmacist. Targeted therapy is then either administered or held based on culture-based susceptibility testing (species identification is determined as needed).
The current study findings add to a literature already supporting use of rapid blood cultures and/or stewardship guidance to address S. aureus bacteremia in academic and tertiary care centers (J Clin Microbiol. 2016;54:2455-63; Clin Microbiol Infect. 2015;21:313-22, and Clin. Infect. Dis. 2015;61:1071-80.
Dr. Roiko had no relevant financial disclosures.
NEW ORLEANS – Community hospitals could see positive outcomes using a Staphylococcus aureus bacteremia strategy that combines rapid blood cultures to speed diagnosis with antibiotic stewardship to guide treatment.
Many academic medical centers report improved outcomes with this approach. Now a study of 66 patients at a medium-sized hospital in rural North Dakota suggests this strategy translates well to the community hospital setting and can reduce mortality and 30-day readmission rates, and increase cost-effectiveness overall.
“I was pleased to see we were able to replicate the positive outcomes observed in studies from large tertiary care centers with our small cohort,” Marijo Roiko, PhD, microbiology program director for pathology and laboratory services at Altru Health System in Grand Forks, N.D., said in an interview.
Dr. Roiko and colleagues compared 33 patients diagnosed and treated prior to the strategy with 33 others following its implementation. A total of 13 patients, or 39% of each cohort, developed potentially fatal methicillin-resistant S. aureus (MRSA) bacteremia. Patients’ average age ranged from 60 to 64 years, and about two-thirds of each group were men.
The investigators reported that 30-day all-cause mortality decreased from 15.6% to 13.3% after the protocol. In addition, 30-day readmission rates decreased from 25% to 11% of the group diagnosed and managed with the new strategy. Dr. Roiko presented these and other findings from the chart review at the annual meeting of the American Society for Microbiology.
Overall, the average length of stay decreased from 13 days to 10 days among the patients with S. aureus bacteremia. Among the subgroup of patients with MRSA, the length of stay dropped from 15 to 12 days. Among those with methicillin-susceptible S. aureus infections, 11 days decreased to 9 days after institution of the protocol.
The researchers also looked at time to antibiotic deescalation, and the average time decreased from 3 days to 1 day with the new strategy.
“These results demonstrate the utility of rapid testing from positive blood cultures and antibiotic stewardship for patients with Staph. aureus bacteremia,” Dr. Roiko said.
In terms of return on investment, the estimated cost savings associated with the 3-day reduction in length of hospital stay was sufficient to cover the increased capital expenditures and reagent costs, the researchers found. They estimated these increased costs as $78,960, excluding ICU and ancillary charges. The approximate $4,290 saved per day multiplied by 33 patients means the new protocol saved a total of $141,570.
Traditionally, patients with a positive blood culture of S. aureus had a gram stain, followed by provider notification when positive. Targeted antibiotic therapy was either administered at this point or held for culture-based identification and susceptibility testing. In the new protocol, a rapid culture identification panel (FilmArray BCID) is added at the time of gram staining. Positive results are reported to both the provider and pharmacist. Targeted therapy is then either administered or held based on culture-based susceptibility testing (species identification is determined as needed).
The current study findings add to a literature already supporting use of rapid blood cultures and/or stewardship guidance to address S. aureus bacteremia in academic and tertiary care centers (J Clin Microbiol. 2016;54:2455-63; Clin Microbiol Infect. 2015;21:313-22, and Clin. Infect. Dis. 2015;61:1071-80.
Dr. Roiko had no relevant financial disclosures.
AT ASM MICROBE 2017
Key clinical point:
Major finding: 30-day mortality dropped from 15.6% prior to the protocol to 13.3% after implementation.
Data source: A retrospective comparison of 33 patients receiving traditional diagnosis and management versus 33 others with a new rapid blood culture and stewardship program approach.
Disclosures: Dr. Roiko had no relevant financial disclosures.
When fecal transplants for C. diff. fail, try, try again
CHICAGO – The best remedy for a failed fecal microbiota transplant for recurrent Clostridium difficile infection is most likely a second – or even a third or fourth attempt, according to Monika Fischer, MD.
Fecal microbiota transplants (FMTs) cure the large majority of those with recurrent C. difficile. But for those who don’t respond or who have an early recurrence, repeating the procedure will almost always effect cure, she said at the annual Digestive Disease Week.
“My recommendation would be to repeat FMT once you make sure the diagnosis actually is recurrent C. difficile,” said Dr. Fischer of Indiana University, Indianapolis. “There are sufficient data showing that the success rate after two FMTs significantly increases independent of the delivery route. But the effectiveness rate is highest when FMT is delivered via colonoscopy, so I recommend the second FMT be delivered that way.”
Recurrent failures can also be a sign that something else is amiss clinically, she said. So before proceeding with multiple procedures, some detective work may be in order. It’s best to start with confirmatory testing for the organism, she said.
“We have seen that about 25% of patients referred for FMT don’t actually have C. difficile at all,” Dr. Fischer said. “Be thinking about an alternative diagnosis when the stool tests negative, but the patient is still symptomatic, or if, before the FMT, there was less than a 50% improvement with vancomycin or fidaxomicin therapy.”
“When evaluating a patient for FMT failure, it should be confirmed by stool testing, preferably by toxin testing. Recent studies suggest that PCR [polymerase chain reaction]–positive but toxin-negative patients may be colonized with C. difficile but that an alternative pathology is driving the symptoms. Toxin-negative patients’ outcome is similar with or without treatment, and it is very rare that toxin-negative patients develop CDI [C. difficile infection]–related complications.”
For these patients, the problem could be any of the conditions that cause chronic diarrhea: inflammatory bowel disease, irritable bowel syndrome, celiac disease, microscopic colitis, bile salt malabsorption, chronic pancreatitis, or some other kind of infection. If C. difficile is the confirmed etiology, repeated FMTs are the way to go, Dr. Fischer said.
However, it may be worth mixing up the delivery method. The ever-expanding data on FMT continue to show that colonoscopy delivery has the lowest failure rate – about 10%. Enema is the least successful, with a 40% failure rate. In between those are nasoduodenal tube delivery, which is associated with a 20% failure rate, and oral capsules, with a failure rate varying from 12% to 30%. Fresh stool is also more effective than frozen, which, in turn, is more effective than the lyophilized preparation, Dr. Fischer said.
“Options are to repeat FMT via colonoscopy, but for patients who have had several failures, consider using the upper and lower route at the same time, and give fresh stool, especially if the first transplants used frozen.”
Although the efficacy of FMT doesn’t appear to depend on donor characteristics, patient characteristics do seem to play a role. Dr. Fischer and her colleagues have created an assessment tool to predict who may be at risk for failure. The model was developed in a 300-patient FMT cohort at two centers and validated in a third academic center FMT population. Of 24 clinical variables, three were incorporated into the failure risk model: severe disease (odds ratio, 6), inpatient status (OR, 3.8), and the number of prior C. difficile–related hospitalizations (OR, 1.4 for each one). For severe disease, patients got 5 points on the scale; for inpatient status, 4 points; and for each prior hospitalization, 1 point.
“Patients in the low-risk category [0] had up to 5% chance of failing. Patients with intermediate risk [1-2] had a 15% chance of failing, and patients in the high-risk category [3 or more points] had higher than 35% chance of not responding to single FMT,” Dr. Fischer said.
She also examined this tool in an extended cohort of nearly 500 patients at four additional sites; about 5% had failed more than two FMTs. “We identified two additional risk factors for failing multiple transplants,” Dr. Fischer said. “These were immunocompromised state, which increased the risk by 4 times, and male gender, which increased the risk by 2.5 times.”
She offered some options for the rare patient who has failed repeat FMTs and doesn’t want to try again. “There are some alternative or adjunctive therapies to repeat FMTs that may be considered, in lieu of repeating FMT for the third or fourth time or even following the first FMT failure, if dictated by patient preference. We sometimes offer these for elderly or frail patients or those with a limited life expectancy. These therapy options are from small, nonrandomized trials in multiply recurrent C. difficile infections but have not been vetted in the FMT nonresponder population.”
These include a vancomycin taper, or a vancomycin taper followed by fidaxomicin. Another option, albeit with limited applicability, is suppressive low-dose vancomycin 125 mg given every day, every other day, or every third day, indefinitely. “This can be especially good for elderly, frail patients with limited life expectancy, needing ongoing antibiotic therapy for urinary tract infections,” she said.
Finally, an 8-week vancomycin taper with daily kefir ingestion has been helpful for some patients. Although probiotics have never been proven helpful in C. difficile infections or FMT success, kefir is a different sort of supplement, she said.
“Kefir is different from yogurt. It contains bacteriocins like nisin, a protein with antibacterial properties produced by Lactococcus lactis.”
msullivan@frontlinemedcom.com
On Twitter @alz_gal
CHICAGO – The best remedy for a failed fecal microbiota transplant for recurrent Clostridium difficile infection is most likely a second – or even a third or fourth attempt, according to Monika Fischer, MD.
Fecal microbiota transplants (FMTs) cure the large majority of those with recurrent C. difficile. But for those who don’t respond or who have an early recurrence, repeating the procedure will almost always effect cure, she said at the annual Digestive Disease Week.
“My recommendation would be to repeat FMT once you make sure the diagnosis actually is recurrent C. difficile,” said Dr. Fischer of Indiana University, Indianapolis. “There are sufficient data showing that the success rate after two FMTs significantly increases independent of the delivery route. But the effectiveness rate is highest when FMT is delivered via colonoscopy, so I recommend the second FMT be delivered that way.”
Recurrent failures can also be a sign that something else is amiss clinically, she said. So before proceeding with multiple procedures, some detective work may be in order. It’s best to start with confirmatory testing for the organism, she said.
“We have seen that about 25% of patients referred for FMT don’t actually have C. difficile at all,” Dr. Fischer said. “Be thinking about an alternative diagnosis when the stool tests negative, but the patient is still symptomatic, or if, before the FMT, there was less than a 50% improvement with vancomycin or fidaxomicin therapy.”
“When evaluating a patient for FMT failure, it should be confirmed by stool testing, preferably by toxin testing. Recent studies suggest that PCR [polymerase chain reaction]–positive but toxin-negative patients may be colonized with C. difficile but that an alternative pathology is driving the symptoms. Toxin-negative patients’ outcome is similar with or without treatment, and it is very rare that toxin-negative patients develop CDI [C. difficile infection]–related complications.”
For these patients, the problem could be any of the conditions that cause chronic diarrhea: inflammatory bowel disease, irritable bowel syndrome, celiac disease, microscopic colitis, bile salt malabsorption, chronic pancreatitis, or some other kind of infection. If C. difficile is the confirmed etiology, repeated FMTs are the way to go, Dr. Fischer said.
However, it may be worth mixing up the delivery method. The ever-expanding data on FMT continue to show that colonoscopy delivery has the lowest failure rate – about 10%. Enema is the least successful, with a 40% failure rate. In between those are nasoduodenal tube delivery, which is associated with a 20% failure rate, and oral capsules, with a failure rate varying from 12% to 30%. Fresh stool is also more effective than frozen, which, in turn, is more effective than the lyophilized preparation, Dr. Fischer said.
“Options are to repeat FMT via colonoscopy, but for patients who have had several failures, consider using the upper and lower route at the same time, and give fresh stool, especially if the first transplants used frozen.”
Although the efficacy of FMT doesn’t appear to depend on donor characteristics, patient characteristics do seem to play a role. Dr. Fischer and her colleagues have created an assessment tool to predict who may be at risk for failure. The model was developed in a 300-patient FMT cohort at two centers and validated in a third academic center FMT population. Of 24 clinical variables, three were incorporated into the failure risk model: severe disease (odds ratio, 6), inpatient status (OR, 3.8), and the number of prior C. difficile–related hospitalizations (OR, 1.4 for each one). For severe disease, patients got 5 points on the scale; for inpatient status, 4 points; and for each prior hospitalization, 1 point.
“Patients in the low-risk category [0] had up to 5% chance of failing. Patients with intermediate risk [1-2] had a 15% chance of failing, and patients in the high-risk category [3 or more points] had higher than 35% chance of not responding to single FMT,” Dr. Fischer said.
She also examined this tool in an extended cohort of nearly 500 patients at four additional sites; about 5% had failed more than two FMTs. “We identified two additional risk factors for failing multiple transplants,” Dr. Fischer said. “These were immunocompromised state, which increased the risk by 4 times, and male gender, which increased the risk by 2.5 times.”
She offered some options for the rare patient who has failed repeat FMTs and doesn’t want to try again. “There are some alternative or adjunctive therapies to repeat FMTs that may be considered, in lieu of repeating FMT for the third or fourth time or even following the first FMT failure, if dictated by patient preference. We sometimes offer these for elderly or frail patients or those with a limited life expectancy. These therapy options are from small, nonrandomized trials in multiply recurrent C. difficile infections but have not been vetted in the FMT nonresponder population.”
These include a vancomycin taper, or a vancomycin taper followed by fidaxomicin. Another option, albeit with limited applicability, is suppressive low-dose vancomycin 125 mg given every day, every other day, or every third day, indefinitely. “This can be especially good for elderly, frail patients with limited life expectancy, needing ongoing antibiotic therapy for urinary tract infections,” she said.
Finally, an 8-week vancomycin taper with daily kefir ingestion has been helpful for some patients. Although probiotics have never been proven helpful in C. difficile infections or FMT success, kefir is a different sort of supplement, she said.
“Kefir is different from yogurt. It contains bacteriocins like nisin, a protein with antibacterial properties produced by Lactococcus lactis.”
msullivan@frontlinemedcom.com
On Twitter @alz_gal
CHICAGO – The best remedy for a failed fecal microbiota transplant for recurrent Clostridium difficile infection is most likely a second – or even a third or fourth attempt, according to Monika Fischer, MD.
Fecal microbiota transplants (FMTs) cure the large majority of those with recurrent C. difficile. But for those who don’t respond or who have an early recurrence, repeating the procedure will almost always effect cure, she said at the annual Digestive Disease Week.
“My recommendation would be to repeat FMT once you make sure the diagnosis actually is recurrent C. difficile,” said Dr. Fischer of Indiana University, Indianapolis. “There are sufficient data showing that the success rate after two FMTs significantly increases independent of the delivery route. But the effectiveness rate is highest when FMT is delivered via colonoscopy, so I recommend the second FMT be delivered that way.”
Recurrent failures can also be a sign that something else is amiss clinically, she said. So before proceeding with multiple procedures, some detective work may be in order. It’s best to start with confirmatory testing for the organism, she said.
“We have seen that about 25% of patients referred for FMT don’t actually have C. difficile at all,” Dr. Fischer said. “Be thinking about an alternative diagnosis when the stool tests negative, but the patient is still symptomatic, or if, before the FMT, there was less than a 50% improvement with vancomycin or fidaxomicin therapy.”
“When evaluating a patient for FMT failure, it should be confirmed by stool testing, preferably by toxin testing. Recent studies suggest that PCR [polymerase chain reaction]–positive but toxin-negative patients may be colonized with C. difficile but that an alternative pathology is driving the symptoms. Toxin-negative patients’ outcome is similar with or without treatment, and it is very rare that toxin-negative patients develop CDI [C. difficile infection]–related complications.”
For these patients, the problem could be any of the conditions that cause chronic diarrhea: inflammatory bowel disease, irritable bowel syndrome, celiac disease, microscopic colitis, bile salt malabsorption, chronic pancreatitis, or some other kind of infection. If C. difficile is the confirmed etiology, repeated FMTs are the way to go, Dr. Fischer said.
However, it may be worth mixing up the delivery method. The ever-expanding data on FMT continue to show that colonoscopy delivery has the lowest failure rate – about 10%. Enema is the least successful, with a 40% failure rate. In between those are nasoduodenal tube delivery, which is associated with a 20% failure rate, and oral capsules, with a failure rate varying from 12% to 30%. Fresh stool is also more effective than frozen, which, in turn, is more effective than the lyophilized preparation, Dr. Fischer said.
“Options are to repeat FMT via colonoscopy, but for patients who have had several failures, consider using the upper and lower route at the same time, and give fresh stool, especially if the first transplants used frozen.”
Although the efficacy of FMT doesn’t appear to depend on donor characteristics, patient characteristics do seem to play a role. Dr. Fischer and her colleagues have created an assessment tool to predict who may be at risk for failure. The model was developed in a 300-patient FMT cohort at two centers and validated in a third academic center FMT population. Of 24 clinical variables, three were incorporated into the failure risk model: severe disease (odds ratio, 6), inpatient status (OR, 3.8), and the number of prior C. difficile–related hospitalizations (OR, 1.4 for each one). For severe disease, patients got 5 points on the scale; for inpatient status, 4 points; and for each prior hospitalization, 1 point.
“Patients in the low-risk category [0] had up to 5% chance of failing. Patients with intermediate risk [1-2] had a 15% chance of failing, and patients in the high-risk category [3 or more points] had higher than 35% chance of not responding to single FMT,” Dr. Fischer said.
She also examined this tool in an extended cohort of nearly 500 patients at four additional sites; about 5% had failed more than two FMTs. “We identified two additional risk factors for failing multiple transplants,” Dr. Fischer said. “These were immunocompromised state, which increased the risk by 4 times, and male gender, which increased the risk by 2.5 times.”
She offered some options for the rare patient who has failed repeat FMTs and doesn’t want to try again. “There are some alternative or adjunctive therapies to repeat FMTs that may be considered, in lieu of repeating FMT for the third or fourth time or even following the first FMT failure, if dictated by patient preference. We sometimes offer these for elderly or frail patients or those with a limited life expectancy. These therapy options are from small, nonrandomized trials in multiply recurrent C. difficile infections but have not been vetted in the FMT nonresponder population.”
These include a vancomycin taper, or a vancomycin taper followed by fidaxomicin. Another option, albeit with limited applicability, is suppressive low-dose vancomycin 125 mg given every day, every other day, or every third day, indefinitely. “This can be especially good for elderly, frail patients with limited life expectancy, needing ongoing antibiotic therapy for urinary tract infections,” she said.
Finally, an 8-week vancomycin taper with daily kefir ingestion has been helpful for some patients. Although probiotics have never been proven helpful in C. difficile infections or FMT success, kefir is a different sort of supplement, she said.
“Kefir is different from yogurt. It contains bacteriocins like nisin, a protein with antibacterial properties produced by Lactococcus lactis.”
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT DDW 2017
E. coli, GBS account for majority of neonatal bacterial meningitis in Canada
No major shifts appear to have occurred in the bacteria that cause meningitis in Canada, said Lynda Ouchenir, MD, University of Montreal, and her associates.
“There is a paucity of information on the characteristics of neonatal meningitis in the era of infant Haemophilus influenzae type B (Hib) and pneumococcal immunization, maternal group B Streptococcus (GBS) prophylaxis, and emerging antimicrobial resistance,” the researchers said. So, they undertook a retrospective study of infants with onset of bacterial meningitis in the first 90 days of life at seven Canadian hospitals to find out the major pathogens involved and best empirical antibiotics to use.
This substitution of a carbapenem for the cephalosporin was considered prudent if the birth hospitalization was complicated and if the cerebrospinal fluid Gram-stain or the blood culture was suggestive of Gram-negative meningitis, Dr. Ouchenir and her associates said.
Read more at (Pediatrics. 2017;140[1)]:e20170476).
No major shifts appear to have occurred in the bacteria that cause meningitis in Canada, said Lynda Ouchenir, MD, University of Montreal, and her associates.
“There is a paucity of information on the characteristics of neonatal meningitis in the era of infant Haemophilus influenzae type B (Hib) and pneumococcal immunization, maternal group B Streptococcus (GBS) prophylaxis, and emerging antimicrobial resistance,” the researchers said. So, they undertook a retrospective study of infants with onset of bacterial meningitis in the first 90 days of life at seven Canadian hospitals to find out the major pathogens involved and best empirical antibiotics to use.
This substitution of a carbapenem for the cephalosporin was considered prudent if the birth hospitalization was complicated and if the cerebrospinal fluid Gram-stain or the blood culture was suggestive of Gram-negative meningitis, Dr. Ouchenir and her associates said.
Read more at (Pediatrics. 2017;140[1)]:e20170476).
No major shifts appear to have occurred in the bacteria that cause meningitis in Canada, said Lynda Ouchenir, MD, University of Montreal, and her associates.
“There is a paucity of information on the characteristics of neonatal meningitis in the era of infant Haemophilus influenzae type B (Hib) and pneumococcal immunization, maternal group B Streptococcus (GBS) prophylaxis, and emerging antimicrobial resistance,” the researchers said. So, they undertook a retrospective study of infants with onset of bacterial meningitis in the first 90 days of life at seven Canadian hospitals to find out the major pathogens involved and best empirical antibiotics to use.
This substitution of a carbapenem for the cephalosporin was considered prudent if the birth hospitalization was complicated and if the cerebrospinal fluid Gram-stain or the blood culture was suggestive of Gram-negative meningitis, Dr. Ouchenir and her associates said.
Read more at (Pediatrics. 2017;140[1)]:e20170476).
FROM PEDIATRICS
Sneak Peek: Journal of Hospital Medicine
BACKGROUND: Antimicrobial stewardship programs (ASPs) have been advocated to improve antimicrobial utilization, but program implementation is variable.
OBJECTIVE: To determine associations of ASPs with facility characteristics and inpatient antimicrobial utilization measures in the Veterans Affairs (VA) system in 2012.
SETTING: All 130 VA facilities with acute care services.
RESULTS: Variables associated with at least three favorable changes in antimicrobial utilization included presence of postgraduate physician/pharmacy training programs, number of antimicrobial-specific order sets, frequency of systematic de-escalation review, presence of pharmacists and/or infectious diseases (ID) attendings on acute care ward teams, and formal ID training of the lead ASP pharmacist. Variables associated with two unfavorable measures included bed size, the level of engagement with VA Antimicrobial Stewardship Task Force online resources, and utilization of antimicrobial stop orders.
CONCLUSIONS: Formalization of ASP processes and presence of pharmacy and ID expertise are associated with favorable utilization. Systematic de-escalation review and order set establishment may be high-yield interventions.
Also in JHM
High prevalence of inappropriate benzodiazepine and sedative hypnotic prescriptions among hospitalized older adults
AUTHORS: Elisabeth Anna Pek, MD, Andrew Remfry, MD, Ciara Pendrith, MSc, Chris Fan-Lun, BScPhm, R. Sacha Bhatia, MD, and Christine Soong, MD, MSc, SFHM
Incidence, predictors, and outcomes of hospital-acquired anemia
AUTHORS: Anil N. Makam, MD, MAS, Oanh K. Nguyen, MD, MAS, Christopher Clark, MPA, and Ethan A. Halm, MD, MPH
Association between radiologic incidental findings and resource utilization in patients admitted with chest pain in an urban medical center
AUTHORS: Venkat P. Gundareddy, MD, MPH, SFHM, Nisa M. Maruthur, MD, MHS, Abednego Chibungu, MD, Preetam Bollampally, MD, Regina Landis, MS, abd Shaker M. Eid, MD, MBA
Clinical utility of routine CBC testing in patients with community-acquired pneumonia
AUTHORS: Neelaysh Vukkadala, BS, and Andrew Auerbach, MD, MPH, SFHM
Overuse of troponin? A comprehensive evaluation of testing in a large hospital system
AUTHORS: Gibbs Wilson, MD, Kyler Barkley, MD, Kipp Slicker, DO, Robert Kowal, MD, PhD, Brandon Pope, PhD, and Jeffrey Michel, MD
BACKGROUND: Antimicrobial stewardship programs (ASPs) have been advocated to improve antimicrobial utilization, but program implementation is variable.
OBJECTIVE: To determine associations of ASPs with facility characteristics and inpatient antimicrobial utilization measures in the Veterans Affairs (VA) system in 2012.
SETTING: All 130 VA facilities with acute care services.
RESULTS: Variables associated with at least three favorable changes in antimicrobial utilization included presence of postgraduate physician/pharmacy training programs, number of antimicrobial-specific order sets, frequency of systematic de-escalation review, presence of pharmacists and/or infectious diseases (ID) attendings on acute care ward teams, and formal ID training of the lead ASP pharmacist. Variables associated with two unfavorable measures included bed size, the level of engagement with VA Antimicrobial Stewardship Task Force online resources, and utilization of antimicrobial stop orders.
CONCLUSIONS: Formalization of ASP processes and presence of pharmacy and ID expertise are associated with favorable utilization. Systematic de-escalation review and order set establishment may be high-yield interventions.
Also in JHM
High prevalence of inappropriate benzodiazepine and sedative hypnotic prescriptions among hospitalized older adults
AUTHORS: Elisabeth Anna Pek, MD, Andrew Remfry, MD, Ciara Pendrith, MSc, Chris Fan-Lun, BScPhm, R. Sacha Bhatia, MD, and Christine Soong, MD, MSc, SFHM
Incidence, predictors, and outcomes of hospital-acquired anemia
AUTHORS: Anil N. Makam, MD, MAS, Oanh K. Nguyen, MD, MAS, Christopher Clark, MPA, and Ethan A. Halm, MD, MPH
Association between radiologic incidental findings and resource utilization in patients admitted with chest pain in an urban medical center
AUTHORS: Venkat P. Gundareddy, MD, MPH, SFHM, Nisa M. Maruthur, MD, MHS, Abednego Chibungu, MD, Preetam Bollampally, MD, Regina Landis, MS, abd Shaker M. Eid, MD, MBA
Clinical utility of routine CBC testing in patients with community-acquired pneumonia
AUTHORS: Neelaysh Vukkadala, BS, and Andrew Auerbach, MD, MPH, SFHM
Overuse of troponin? A comprehensive evaluation of testing in a large hospital system
AUTHORS: Gibbs Wilson, MD, Kyler Barkley, MD, Kipp Slicker, DO, Robert Kowal, MD, PhD, Brandon Pope, PhD, and Jeffrey Michel, MD
BACKGROUND: Antimicrobial stewardship programs (ASPs) have been advocated to improve antimicrobial utilization, but program implementation is variable.
OBJECTIVE: To determine associations of ASPs with facility characteristics and inpatient antimicrobial utilization measures in the Veterans Affairs (VA) system in 2012.
SETTING: All 130 VA facilities with acute care services.
RESULTS: Variables associated with at least three favorable changes in antimicrobial utilization included presence of postgraduate physician/pharmacy training programs, number of antimicrobial-specific order sets, frequency of systematic de-escalation review, presence of pharmacists and/or infectious diseases (ID) attendings on acute care ward teams, and formal ID training of the lead ASP pharmacist. Variables associated with two unfavorable measures included bed size, the level of engagement with VA Antimicrobial Stewardship Task Force online resources, and utilization of antimicrobial stop orders.
CONCLUSIONS: Formalization of ASP processes and presence of pharmacy and ID expertise are associated with favorable utilization. Systematic de-escalation review and order set establishment may be high-yield interventions.
Also in JHM
High prevalence of inappropriate benzodiazepine and sedative hypnotic prescriptions among hospitalized older adults
AUTHORS: Elisabeth Anna Pek, MD, Andrew Remfry, MD, Ciara Pendrith, MSc, Chris Fan-Lun, BScPhm, R. Sacha Bhatia, MD, and Christine Soong, MD, MSc, SFHM
Incidence, predictors, and outcomes of hospital-acquired anemia
AUTHORS: Anil N. Makam, MD, MAS, Oanh K. Nguyen, MD, MAS, Christopher Clark, MPA, and Ethan A. Halm, MD, MPH
Association between radiologic incidental findings and resource utilization in patients admitted with chest pain in an urban medical center
AUTHORS: Venkat P. Gundareddy, MD, MPH, SFHM, Nisa M. Maruthur, MD, MHS, Abednego Chibungu, MD, Preetam Bollampally, MD, Regina Landis, MS, abd Shaker M. Eid, MD, MBA
Clinical utility of routine CBC testing in patients with community-acquired pneumonia
AUTHORS: Neelaysh Vukkadala, BS, and Andrew Auerbach, MD, MPH, SFHM
Overuse of troponin? A comprehensive evaluation of testing in a large hospital system
AUTHORS: Gibbs Wilson, MD, Kyler Barkley, MD, Kipp Slicker, DO, Robert Kowal, MD, PhD, Brandon Pope, PhD, and Jeffrey Michel, MD
Ribaxamase prevented C. difficile infections by protecting microbiome
VIENNA – An investigational beta-lactamase reduced Clostridium difficile infections by 71% in patients receiving extended antibiotic therapy for respiratory infections but not by killing the opportunistic bacteria.
Rather, ribaxamase prevented C. difficile infections (CDI) by breaking down excess therapeutic antibiotics in the gut before they could injure an otherwise healthy microbiome, John Kokai-Kun, PhD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Ribaxamase is an oral enzyme that breaks the lactam ring in penicillins and cephalosporins. It’s formulated to release at a pH of 5.5 or higher, an environment that begins to develop in the upper small intestine near the bile duct – the same place that excess antibiotics are excreted.
“The drug is intended to be administered during, and for a short time after, intravenous administration of specific beta-lactam–containing antibiotics,” Dr. Kokai-Kun said. Ribaxamase doesn’t work on carbapenem-type antibiotics, he noted, and Synthetic Biologics is working on an effective enzyme for those as well.
In early human studies, ribaxamase was well tolerated and didn’t interfere with the pharmacokinetics of therapeutic antibiotics (Antimicrob Agents Chemother. 2017 Mar;61[3]:e02197-16). It’s also effective in patients who are taking a proton pump inhibitor, he said.
Dr. Kokai-Kun reported the results of a phase IIb study of 412 patients who received IV ceftriaxone for lower respiratory infections. They were assigned 1:1 to either 150 mg ribaxamase daily or placebo throughout the IV treatment and for 3 days after.
The primary endpoint was prevention of C. difficile infection. The secondary endpoint was prevention of non–C. difficile antibiotic-associated diarrhea. An exploratory endpoint examined the drug’s ability to protect the microbiome. Patients were monitored for 6 weeks after treatment stopped.
The cohort was a mean 70 years old. One-third of patients also received a macrolide during their hospitalization, and one-third were taking proton pump inhibitors. The respiratory infection cure rate was about 99% in both groups at both 72 hours and 4 weeks.
Eight patients in the placebo group (3.8%) and two in the active group (less than 1%) developed C. difficile infection. That translated to a statistically significant 71% risk reduction, with a P value of .027, Dr. Kokai-Kun said. Ribaxamase did not hit its secondary endpoint of preventing all-cause diarrhea or antibiotic-associated diarrhea that was not caused by C. difficile infection.
Although not a primary finding, ribaxamase also inhibited colonization by vancomycin-resistant enterococci, which occurred in about 70 (40%) patients in the placebo group and 40 (20%) in the ribaxamase group at both 72 hours and 4 weeks.
All patients contributed stool samples at baseline and after treatment for microbiome analysis. That portion of the study is still ongoing, Dr. Kokai-Kun said.
Synthetic Biologics sponsored the study and is developing ribaxamase. Dr. Kokai-Kun is the company’s vice president of nonclinical affairs.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
VIENNA – An investigational beta-lactamase reduced Clostridium difficile infections by 71% in patients receiving extended antibiotic therapy for respiratory infections but not by killing the opportunistic bacteria.
Rather, ribaxamase prevented C. difficile infections (CDI) by breaking down excess therapeutic antibiotics in the gut before they could injure an otherwise healthy microbiome, John Kokai-Kun, PhD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Ribaxamase is an oral enzyme that breaks the lactam ring in penicillins and cephalosporins. It’s formulated to release at a pH of 5.5 or higher, an environment that begins to develop in the upper small intestine near the bile duct – the same place that excess antibiotics are excreted.
“The drug is intended to be administered during, and for a short time after, intravenous administration of specific beta-lactam–containing antibiotics,” Dr. Kokai-Kun said. Ribaxamase doesn’t work on carbapenem-type antibiotics, he noted, and Synthetic Biologics is working on an effective enzyme for those as well.
In early human studies, ribaxamase was well tolerated and didn’t interfere with the pharmacokinetics of therapeutic antibiotics (Antimicrob Agents Chemother. 2017 Mar;61[3]:e02197-16). It’s also effective in patients who are taking a proton pump inhibitor, he said.
Dr. Kokai-Kun reported the results of a phase IIb study of 412 patients who received IV ceftriaxone for lower respiratory infections. They were assigned 1:1 to either 150 mg ribaxamase daily or placebo throughout the IV treatment and for 3 days after.
The primary endpoint was prevention of C. difficile infection. The secondary endpoint was prevention of non–C. difficile antibiotic-associated diarrhea. An exploratory endpoint examined the drug’s ability to protect the microbiome. Patients were monitored for 6 weeks after treatment stopped.
The cohort was a mean 70 years old. One-third of patients also received a macrolide during their hospitalization, and one-third were taking proton pump inhibitors. The respiratory infection cure rate was about 99% in both groups at both 72 hours and 4 weeks.
Eight patients in the placebo group (3.8%) and two in the active group (less than 1%) developed C. difficile infection. That translated to a statistically significant 71% risk reduction, with a P value of .027, Dr. Kokai-Kun said. Ribaxamase did not hit its secondary endpoint of preventing all-cause diarrhea or antibiotic-associated diarrhea that was not caused by C. difficile infection.
Although not a primary finding, ribaxamase also inhibited colonization by vancomycin-resistant enterococci, which occurred in about 70 (40%) patients in the placebo group and 40 (20%) in the ribaxamase group at both 72 hours and 4 weeks.
All patients contributed stool samples at baseline and after treatment for microbiome analysis. That portion of the study is still ongoing, Dr. Kokai-Kun said.
Synthetic Biologics sponsored the study and is developing ribaxamase. Dr. Kokai-Kun is the company’s vice president of nonclinical affairs.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
VIENNA – An investigational beta-lactamase reduced Clostridium difficile infections by 71% in patients receiving extended antibiotic therapy for respiratory infections but not by killing the opportunistic bacteria.
Rather, ribaxamase prevented C. difficile infections (CDI) by breaking down excess therapeutic antibiotics in the gut before they could injure an otherwise healthy microbiome, John Kokai-Kun, PhD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
Ribaxamase is an oral enzyme that breaks the lactam ring in penicillins and cephalosporins. It’s formulated to release at a pH of 5.5 or higher, an environment that begins to develop in the upper small intestine near the bile duct – the same place that excess antibiotics are excreted.
“The drug is intended to be administered during, and for a short time after, intravenous administration of specific beta-lactam–containing antibiotics,” Dr. Kokai-Kun said. Ribaxamase doesn’t work on carbapenem-type antibiotics, he noted, and Synthetic Biologics is working on an effective enzyme for those as well.
In early human studies, ribaxamase was well tolerated and didn’t interfere with the pharmacokinetics of therapeutic antibiotics (Antimicrob Agents Chemother. 2017 Mar;61[3]:e02197-16). It’s also effective in patients who are taking a proton pump inhibitor, he said.
Dr. Kokai-Kun reported the results of a phase IIb study of 412 patients who received IV ceftriaxone for lower respiratory infections. They were assigned 1:1 to either 150 mg ribaxamase daily or placebo throughout the IV treatment and for 3 days after.
The primary endpoint was prevention of C. difficile infection. The secondary endpoint was prevention of non–C. difficile antibiotic-associated diarrhea. An exploratory endpoint examined the drug’s ability to protect the microbiome. Patients were monitored for 6 weeks after treatment stopped.
The cohort was a mean 70 years old. One-third of patients also received a macrolide during their hospitalization, and one-third were taking proton pump inhibitors. The respiratory infection cure rate was about 99% in both groups at both 72 hours and 4 weeks.
Eight patients in the placebo group (3.8%) and two in the active group (less than 1%) developed C. difficile infection. That translated to a statistically significant 71% risk reduction, with a P value of .027, Dr. Kokai-Kun said. Ribaxamase did not hit its secondary endpoint of preventing all-cause diarrhea or antibiotic-associated diarrhea that was not caused by C. difficile infection.
Although not a primary finding, ribaxamase also inhibited colonization by vancomycin-resistant enterococci, which occurred in about 70 (40%) patients in the placebo group and 40 (20%) in the ribaxamase group at both 72 hours and 4 weeks.
All patients contributed stool samples at baseline and after treatment for microbiome analysis. That portion of the study is still ongoing, Dr. Kokai-Kun said.
Synthetic Biologics sponsored the study and is developing ribaxamase. Dr. Kokai-Kun is the company’s vice president of nonclinical affairs.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT ECCMID 2017
Key clinical point:
Major finding: Ribaxamase reduced C. difficile infections by 71%, relative to a placebo.
Data source: The study randomized 412 patients to either placebo or ribaxamase in addition to their therapeutic antibiotics.
Disclosures: Synthetic Biologics sponsored the study and is developing ribaxamase. Dr. Kokai-Kun is the company’s vice president of nonclinical affairs.
Molecular tests for GAS pharyngitis could spur overuse of antibiotics
SAN FRANCISCO – The diagnosis of pharyngitis due to infection by group A streptococcus (GAS) based on the detection of nucleic acid is fast and accurate. But this benefit brings the possibility of overuse of antibiotics, with treatment offered to those who, in the era of growth-based detection of the bacteria, would not have received treatment, according to Robert R. Tanz, MD.
Dr. Tanz, professor of pediatrics at Northwestern University, Chicago, delivered this cautionary note at the meeting of the Pediatrics Academics Societies.
“The short turnaround time, high sensitivity, and high specificity of newer molecular tests for GAS will make their use increasingly common. Many of the additional patients identified by molecular testing may not have an illness attributable to GAS. The increase in positive tests will probably be associated with increased antibiotic prescribing. This may not be beneficial, especially in areas with low rates of acute rheumatic fever,” said Dr. Tanz, who practices at Ann & Robert Lurie Children’s Hospital of Chicago.
Nowadays, diagnosis of GAS at Ann & Robert Lurie Children’s Hospital uses the Illumigene system. Its use has been approved by the Food and Drug Administration for diagnosis without the need for backup culture of throat swabs. Results are available in about 1 hour.
Dr. Tanz and his colleagues took a retrospective look at patient records during 2013, when testing was still growth-based, and in 2014 and 2015, after the hospital had shifted to the molecular analysis of throat swabs. The aim was to determine the proportion of tests positive for GAS prior to and after the switch.
The positive detection rate of 9.6% (96 of 997 samples) in 2013 climbed to 17% (152 of 894) in 2014 and 16% (138 of 859) in 2015. The difference was highly significant (P less than .00001).
Sore throats increase in the colder months when people tend to be indoors more often, and this seasonality was evident in 2013. However, detection was more consistent throughout the 12 months in 2014 (2013 vs 2014, P less than .000001) and 2015 (2013 vs 2015, P less than .00001). The detection rates in 2014 and 2015 were similar (P equal to .59), according to Dr. Tanz.
The new era of molecular testing circumvents what is known as the spectrum effect, in which culture-based tests are more often positive in patients with symptoms that are consistent with the infection, he said. In contrast, molecular tests can increase the identification of the target bacterium, here GAS, in patients who have sore throat caused by a viral infection.
Preliminary results presented by Dr. Tanz indicated a significantly greater detection of GAS in children not displaying symptoms of infection. In decades past, these children would not have been treated.
“The increase in positive tests for group A strep is likely associated with increased antibiotic prescribing. This may or may not represent a benefit to patients, as the additional identified patients may not have an illness actually attributable to group A strep,” said Dr. Tanz.
“Our findings support rigorous selectivity in choosing which patients to test, specifically excluding those with overt viral symptoms, as recommended in the guidelines from the [Infectious Diseases Society of America], [American Academy of Pediatrics], and other groups,” he said, adding that clinician guidance on the use of molecular diagnostic tests for pharyngitis caused by group A strep is a prudent step.
Features suggestive of GAS may include sudden onset of sore throat, age 5-15 years, fever, headache, nausea, vomiting, and abdominal pain, tonsillopharyngeal inflammation and patchy tonsillopharyngeal exudates, palatal petechiae, tender nodes, and scarlatiniform rash. Viral pharyngitis features include conjunctivitis, coryza, cough, diarrhea, discrete ulcerative stomatitis, viral exanthema, and hoarseness, according to the 2012 Infectious Diseases Society of American guidelines (Clin Infect Dis. 2012. doi: 10.1093/cid/cis629).
For most people, a sore throat is more of a temporary inconvenience rather than a looming health threat, but GAS easily spreads from person to person and can lead to the more serious condition of acute rheumatic fever, Dr. Tanz said. Hence the concern with diagnosing the cause of sore throat and, when the cause is bacterial, alleviating the infection using antibiotics.
The study was conducted at Ann & Robert H. Lurie Children’s Hospital of Chicago and was not funded. Dr. Tanz reported having received support from Meridian Bioscience, manufacturer of the Illumigene Group A Streptococcus assay. Meridian Bioscience did not support this study.
SAN FRANCISCO – The diagnosis of pharyngitis due to infection by group A streptococcus (GAS) based on the detection of nucleic acid is fast and accurate. But this benefit brings the possibility of overuse of antibiotics, with treatment offered to those who, in the era of growth-based detection of the bacteria, would not have received treatment, according to Robert R. Tanz, MD.
Dr. Tanz, professor of pediatrics at Northwestern University, Chicago, delivered this cautionary note at the meeting of the Pediatrics Academics Societies.
“The short turnaround time, high sensitivity, and high specificity of newer molecular tests for GAS will make their use increasingly common. Many of the additional patients identified by molecular testing may not have an illness attributable to GAS. The increase in positive tests will probably be associated with increased antibiotic prescribing. This may not be beneficial, especially in areas with low rates of acute rheumatic fever,” said Dr. Tanz, who practices at Ann & Robert Lurie Children’s Hospital of Chicago.
Nowadays, diagnosis of GAS at Ann & Robert Lurie Children’s Hospital uses the Illumigene system. Its use has been approved by the Food and Drug Administration for diagnosis without the need for backup culture of throat swabs. Results are available in about 1 hour.
Dr. Tanz and his colleagues took a retrospective look at patient records during 2013, when testing was still growth-based, and in 2014 and 2015, after the hospital had shifted to the molecular analysis of throat swabs. The aim was to determine the proportion of tests positive for GAS prior to and after the switch.
The positive detection rate of 9.6% (96 of 997 samples) in 2013 climbed to 17% (152 of 894) in 2014 and 16% (138 of 859) in 2015. The difference was highly significant (P less than .00001).
Sore throats increase in the colder months when people tend to be indoors more often, and this seasonality was evident in 2013. However, detection was more consistent throughout the 12 months in 2014 (2013 vs 2014, P less than .000001) and 2015 (2013 vs 2015, P less than .00001). The detection rates in 2014 and 2015 were similar (P equal to .59), according to Dr. Tanz.
The new era of molecular testing circumvents what is known as the spectrum effect, in which culture-based tests are more often positive in patients with symptoms that are consistent with the infection, he said. In contrast, molecular tests can increase the identification of the target bacterium, here GAS, in patients who have sore throat caused by a viral infection.
Preliminary results presented by Dr. Tanz indicated a significantly greater detection of GAS in children not displaying symptoms of infection. In decades past, these children would not have been treated.
“The increase in positive tests for group A strep is likely associated with increased antibiotic prescribing. This may or may not represent a benefit to patients, as the additional identified patients may not have an illness actually attributable to group A strep,” said Dr. Tanz.
“Our findings support rigorous selectivity in choosing which patients to test, specifically excluding those with overt viral symptoms, as recommended in the guidelines from the [Infectious Diseases Society of America], [American Academy of Pediatrics], and other groups,” he said, adding that clinician guidance on the use of molecular diagnostic tests for pharyngitis caused by group A strep is a prudent step.
Features suggestive of GAS may include sudden onset of sore throat, age 5-15 years, fever, headache, nausea, vomiting, and abdominal pain, tonsillopharyngeal inflammation and patchy tonsillopharyngeal exudates, palatal petechiae, tender nodes, and scarlatiniform rash. Viral pharyngitis features include conjunctivitis, coryza, cough, diarrhea, discrete ulcerative stomatitis, viral exanthema, and hoarseness, according to the 2012 Infectious Diseases Society of American guidelines (Clin Infect Dis. 2012. doi: 10.1093/cid/cis629).
For most people, a sore throat is more of a temporary inconvenience rather than a looming health threat, but GAS easily spreads from person to person and can lead to the more serious condition of acute rheumatic fever, Dr. Tanz said. Hence the concern with diagnosing the cause of sore throat and, when the cause is bacterial, alleviating the infection using antibiotics.
The study was conducted at Ann & Robert H. Lurie Children’s Hospital of Chicago and was not funded. Dr. Tanz reported having received support from Meridian Bioscience, manufacturer of the Illumigene Group A Streptococcus assay. Meridian Bioscience did not support this study.
SAN FRANCISCO – The diagnosis of pharyngitis due to infection by group A streptococcus (GAS) based on the detection of nucleic acid is fast and accurate. But this benefit brings the possibility of overuse of antibiotics, with treatment offered to those who, in the era of growth-based detection of the bacteria, would not have received treatment, according to Robert R. Tanz, MD.
Dr. Tanz, professor of pediatrics at Northwestern University, Chicago, delivered this cautionary note at the meeting of the Pediatrics Academics Societies.
“The short turnaround time, high sensitivity, and high specificity of newer molecular tests for GAS will make their use increasingly common. Many of the additional patients identified by molecular testing may not have an illness attributable to GAS. The increase in positive tests will probably be associated with increased antibiotic prescribing. This may not be beneficial, especially in areas with low rates of acute rheumatic fever,” said Dr. Tanz, who practices at Ann & Robert Lurie Children’s Hospital of Chicago.
Nowadays, diagnosis of GAS at Ann & Robert Lurie Children’s Hospital uses the Illumigene system. Its use has been approved by the Food and Drug Administration for diagnosis without the need for backup culture of throat swabs. Results are available in about 1 hour.
Dr. Tanz and his colleagues took a retrospective look at patient records during 2013, when testing was still growth-based, and in 2014 and 2015, after the hospital had shifted to the molecular analysis of throat swabs. The aim was to determine the proportion of tests positive for GAS prior to and after the switch.
The positive detection rate of 9.6% (96 of 997 samples) in 2013 climbed to 17% (152 of 894) in 2014 and 16% (138 of 859) in 2015. The difference was highly significant (P less than .00001).
Sore throats increase in the colder months when people tend to be indoors more often, and this seasonality was evident in 2013. However, detection was more consistent throughout the 12 months in 2014 (2013 vs 2014, P less than .000001) and 2015 (2013 vs 2015, P less than .00001). The detection rates in 2014 and 2015 were similar (P equal to .59), according to Dr. Tanz.
The new era of molecular testing circumvents what is known as the spectrum effect, in which culture-based tests are more often positive in patients with symptoms that are consistent with the infection, he said. In contrast, molecular tests can increase the identification of the target bacterium, here GAS, in patients who have sore throat caused by a viral infection.
Preliminary results presented by Dr. Tanz indicated a significantly greater detection of GAS in children not displaying symptoms of infection. In decades past, these children would not have been treated.
“The increase in positive tests for group A strep is likely associated with increased antibiotic prescribing. This may or may not represent a benefit to patients, as the additional identified patients may not have an illness actually attributable to group A strep,” said Dr. Tanz.
“Our findings support rigorous selectivity in choosing which patients to test, specifically excluding those with overt viral symptoms, as recommended in the guidelines from the [Infectious Diseases Society of America], [American Academy of Pediatrics], and other groups,” he said, adding that clinician guidance on the use of molecular diagnostic tests for pharyngitis caused by group A strep is a prudent step.
Features suggestive of GAS may include sudden onset of sore throat, age 5-15 years, fever, headache, nausea, vomiting, and abdominal pain, tonsillopharyngeal inflammation and patchy tonsillopharyngeal exudates, palatal petechiae, tender nodes, and scarlatiniform rash. Viral pharyngitis features include conjunctivitis, coryza, cough, diarrhea, discrete ulcerative stomatitis, viral exanthema, and hoarseness, according to the 2012 Infectious Diseases Society of American guidelines (Clin Infect Dis. 2012. doi: 10.1093/cid/cis629).
For most people, a sore throat is more of a temporary inconvenience rather than a looming health threat, but GAS easily spreads from person to person and can lead to the more serious condition of acute rheumatic fever, Dr. Tanz said. Hence the concern with diagnosing the cause of sore throat and, when the cause is bacterial, alleviating the infection using antibiotics.
The study was conducted at Ann & Robert H. Lurie Children’s Hospital of Chicago and was not funded. Dr. Tanz reported having received support from Meridian Bioscience, manufacturer of the Illumigene Group A Streptococcus assay. Meridian Bioscience did not support this study.
Key clinical point: The increased molecular-based detection of group A streptococci could led to antibiotic overuse, with prescriptions for patients who do not have bacterial infections.
Major finding: In the most recent year of culture-based testing at Lurie Children’s Hospital, the detection rate of group A streptococci was 9.6%; detection rates were 17.0% and 16.1% in the next 2 years when molecular-based analysis was implemented.
Data source: Quality assessment study involving a retrospective review of hospital electronic medical records.
Disclosures: The study was conducted at Ann & Robert H. Lurie Children’s Hospital of Chicago and was not funded. Dr. Tanz reported having received support from Meridian Bioscience, manufacturer of the Illumigene Group A Streptococcus assay. Meridian Bioscience did not support this study.
VIDEO: Registry study will follow 4,000 fecal transplant patients for 10 years
CHICAGO – A 10-year registry study aims to gather clinical and patient-reported outcomes on 4,000 adult and pediatric patients who undergo fecal microbiota transplant in the United States, officials of the American Gastroenterological Association announced during Digestive Disease Week®.
The AGA Fecal Microbiota Transplantation National Registry will be the first study to assess both short- and long-term patient outcomes associated with fecal microbiota transplant (FMT) in both adults and children, Colleen Kelly, MD, said in an video interview. Most subjects will have received FMT for recurrent or refractory Clostridium difficile infections – the only indication for which Food and Drug Administration currently allows independent clinician action. But the investigational uses of FMT are expanding rapidly, and patients who undergo the procedure during any registered study will be eligible for enrollment, said Dr. Kelly, co-chair of the study’s steering committee.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study’s primary objectives are short- and long-term safety outcomes, said Dr. Kelly of Brown University, Providence, R.I. While generally considered quite safe, short-term adverse events have been reported with FMT, and some of them have been serious – including one death from aspiration pneumonia in a patient who received donor stool via nasogastric tube (Clin Infect Dis. 2015 Mar;61[1]:136-7). Other adverse events are usually self-limited but can include low-grade fever, abdominal pain, distention, bloating, and diarrhea.
Researchers seek to illuminate many of the unknowns associated with this relatively new procedure. Scientists are only now beginning to unravel the myriad ways the human microbiome promotes both health and disease. Specific alterations, for example, have been associated with obesity and other conditions; there is concern that transplanting a new microbial population could induce a disease phenotype in a recipient who might not have otherwise been at risk.
With the planned cohort size and follow-up period, the study should be able to detect any unanticipated adverse events that occur in more than 1% of the population, Dr. Kelly said. It will include a comparator group of patients with recurrent or refractory C. difficile infection from a large insurance claims database to allow comparison between patients treated with FMT and those treated with antibiotics only.
The registry study also aims to discover which method or methods of transplant material delivery are best, she said. Right now, there are a number of methods (colonoscopy/sigmoidoscopy, enema, upper gastrointestinal endoscopy, nasogastric or nasoduodenal tube, and capsules), and no consensus on which is the best. As indications for FMT expand, there may be no single best method. The approach will probably be matched to the disorder being treated, and the study may help illuminate this as well.
For the first 2 years after a transplant, clinicians will follow patients and enter data into the registry. After that, an electronic patient-reported outcomes system will automatically contact the patient annually for follow-up information by email or text message. When patients enter their data, they can access educational material that will help keep them up-to-date on potential adverse events.
The study will also include a biobank of stool samples obtained during the procedures, hosted by the American Gut Project and the Microbiome Initiative at the University of California, San Diego. This arm of the project will analyze the microbiome of 3,000 stool samples from recipients, both before and after their transplant, as well as the corresponding donors whose material was used in the fecal transplant.
The registry study, a project of the AGA Center for Gut Microbiome Research and Education, is funded by a $3.3 million grant from the National Institute of Allergy and Infectious Diseases. It will be conducted in partnership with the Crohn’s and Colitis Foundation, Infectious Diseases Society, and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
The registry study currently is accepting applications. Physicians who perform FMT for C. difficile infections, and centers that conduct FMT research for other potential indications, can fill out a short survey to indicate their interest.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT).
This article was updated June 8, 2017.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
CHICAGO – A 10-year registry study aims to gather clinical and patient-reported outcomes on 4,000 adult and pediatric patients who undergo fecal microbiota transplant in the United States, officials of the American Gastroenterological Association announced during Digestive Disease Week®.
The AGA Fecal Microbiota Transplantation National Registry will be the first study to assess both short- and long-term patient outcomes associated with fecal microbiota transplant (FMT) in both adults and children, Colleen Kelly, MD, said in an video interview. Most subjects will have received FMT for recurrent or refractory Clostridium difficile infections – the only indication for which Food and Drug Administration currently allows independent clinician action. But the investigational uses of FMT are expanding rapidly, and patients who undergo the procedure during any registered study will be eligible for enrollment, said Dr. Kelly, co-chair of the study’s steering committee.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study’s primary objectives are short- and long-term safety outcomes, said Dr. Kelly of Brown University, Providence, R.I. While generally considered quite safe, short-term adverse events have been reported with FMT, and some of them have been serious – including one death from aspiration pneumonia in a patient who received donor stool via nasogastric tube (Clin Infect Dis. 2015 Mar;61[1]:136-7). Other adverse events are usually self-limited but can include low-grade fever, abdominal pain, distention, bloating, and diarrhea.
Researchers seek to illuminate many of the unknowns associated with this relatively new procedure. Scientists are only now beginning to unravel the myriad ways the human microbiome promotes both health and disease. Specific alterations, for example, have been associated with obesity and other conditions; there is concern that transplanting a new microbial population could induce a disease phenotype in a recipient who might not have otherwise been at risk.
With the planned cohort size and follow-up period, the study should be able to detect any unanticipated adverse events that occur in more than 1% of the population, Dr. Kelly said. It will include a comparator group of patients with recurrent or refractory C. difficile infection from a large insurance claims database to allow comparison between patients treated with FMT and those treated with antibiotics only.
The registry study also aims to discover which method or methods of transplant material delivery are best, she said. Right now, there are a number of methods (colonoscopy/sigmoidoscopy, enema, upper gastrointestinal endoscopy, nasogastric or nasoduodenal tube, and capsules), and no consensus on which is the best. As indications for FMT expand, there may be no single best method. The approach will probably be matched to the disorder being treated, and the study may help illuminate this as well.
For the first 2 years after a transplant, clinicians will follow patients and enter data into the registry. After that, an electronic patient-reported outcomes system will automatically contact the patient annually for follow-up information by email or text message. When patients enter their data, they can access educational material that will help keep them up-to-date on potential adverse events.
The study will also include a biobank of stool samples obtained during the procedures, hosted by the American Gut Project and the Microbiome Initiative at the University of California, San Diego. This arm of the project will analyze the microbiome of 3,000 stool samples from recipients, both before and after their transplant, as well as the corresponding donors whose material was used in the fecal transplant.
The registry study, a project of the AGA Center for Gut Microbiome Research and Education, is funded by a $3.3 million grant from the National Institute of Allergy and Infectious Diseases. It will be conducted in partnership with the Crohn’s and Colitis Foundation, Infectious Diseases Society, and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
The registry study currently is accepting applications. Physicians who perform FMT for C. difficile infections, and centers that conduct FMT research for other potential indications, can fill out a short survey to indicate their interest.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT).
This article was updated June 8, 2017.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
CHICAGO – A 10-year registry study aims to gather clinical and patient-reported outcomes on 4,000 adult and pediatric patients who undergo fecal microbiota transplant in the United States, officials of the American Gastroenterological Association announced during Digestive Disease Week®.
The AGA Fecal Microbiota Transplantation National Registry will be the first study to assess both short- and long-term patient outcomes associated with fecal microbiota transplant (FMT) in both adults and children, Colleen Kelly, MD, said in an video interview. Most subjects will have received FMT for recurrent or refractory Clostridium difficile infections – the only indication for which Food and Drug Administration currently allows independent clinician action. But the investigational uses of FMT are expanding rapidly, and patients who undergo the procedure during any registered study will be eligible for enrollment, said Dr. Kelly, co-chair of the study’s steering committee.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The study’s primary objectives are short- and long-term safety outcomes, said Dr. Kelly of Brown University, Providence, R.I. While generally considered quite safe, short-term adverse events have been reported with FMT, and some of them have been serious – including one death from aspiration pneumonia in a patient who received donor stool via nasogastric tube (Clin Infect Dis. 2015 Mar;61[1]:136-7). Other adverse events are usually self-limited but can include low-grade fever, abdominal pain, distention, bloating, and diarrhea.
Researchers seek to illuminate many of the unknowns associated with this relatively new procedure. Scientists are only now beginning to unravel the myriad ways the human microbiome promotes both health and disease. Specific alterations, for example, have been associated with obesity and other conditions; there is concern that transplanting a new microbial population could induce a disease phenotype in a recipient who might not have otherwise been at risk.
With the planned cohort size and follow-up period, the study should be able to detect any unanticipated adverse events that occur in more than 1% of the population, Dr. Kelly said. It will include a comparator group of patients with recurrent or refractory C. difficile infection from a large insurance claims database to allow comparison between patients treated with FMT and those treated with antibiotics only.
The registry study also aims to discover which method or methods of transplant material delivery are best, she said. Right now, there are a number of methods (colonoscopy/sigmoidoscopy, enema, upper gastrointestinal endoscopy, nasogastric or nasoduodenal tube, and capsules), and no consensus on which is the best. As indications for FMT expand, there may be no single best method. The approach will probably be matched to the disorder being treated, and the study may help illuminate this as well.
For the first 2 years after a transplant, clinicians will follow patients and enter data into the registry. After that, an electronic patient-reported outcomes system will automatically contact the patient annually for follow-up information by email or text message. When patients enter their data, they can access educational material that will help keep them up-to-date on potential adverse events.
The study will also include a biobank of stool samples obtained during the procedures, hosted by the American Gut Project and the Microbiome Initiative at the University of California, San Diego. This arm of the project will analyze the microbiome of 3,000 stool samples from recipients, both before and after their transplant, as well as the corresponding donors whose material was used in the fecal transplant.
The registry study, a project of the AGA Center for Gut Microbiome Research and Education, is funded by a $3.3 million grant from the National Institute of Allergy and Infectious Diseases. It will be conducted in partnership with the Crohn’s and Colitis Foundation, Infectious Diseases Society, and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition.
The registry study currently is accepting applications. Physicians who perform FMT for C. difficile infections, and centers that conduct FMT research for other potential indications, can fill out a short survey to indicate their interest.
Digestive Disease Week® is jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT).
This article was updated June 8, 2017.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
AT DDW