User login
Should Physicians Offer Patients Medical Credit Cards?
With healthcare costs rising and payer reimbursements dwindling, many physicians are focusing even more on collecting outstanding patient balances.
Medical credit cards can be a popular choice to fill this gap because doctors get reimbursed upfront while patients receive special financing offers and the care they seek or need.
But, in recent months, federal officials have questioned whether these arrangements are genuinely win-win or if the cards prey on low-income and vulnerable individuals and warrant tighter regulatory oversight.
In July, the Consumer Financial Protection Bureau (CFPB), the US Department of Health and Human Services, and the US Department of Treasury announced an inquiry into medical credit cards. The agencies sought public comments from patients and providers to determine how much they are used.
Medical credit cards typically offer 0% or low-interest terms ranging from 6 to 24 months. Minimum monthly payments are required, often as low as $30 and not usually enough to pay the balance by the end of the promotional period.
After the introductory rate, card issuers may charge interest rates approaching 30% — not just on the remaining balance but on the original amount financed, adding considerably to total out-of-pocket costs.
Ophthalmologist Michael A. Brusco, MD, FACS, specializes in laser-assisted in situ keratomileuses and vision correction at his practice in the greater Washington, DC, area. He told this news organization that nearly all his patients are self-paying, and just under half utilize one of two medical credit cards he offers through third-party vendors, CareCredit and Alphaeon Credit.
“We are clear with our patients that it is interest-free only if they make all payments on time, and if they don’t, then the penalties and fees skyrocket,” Dr. Brusco said.
Patients pay no interest if they make the minimum monthly payments and pay the entire balance by the end of the term. Brusco said those who qualify and abide by those conditions can benefit from spreading healthcare expenses over several months and reducing the stress and financial strain associated with a larger, one-time payment.
He acknowledged that deferred interest can be problematic if patients are caught unaware but said his staff has received training from both vendors on clearly explaining the plans to patients. If someone doesn’t think they can pay off the balance in the timeframe, he suggests they pursue an alternative payment method.
Community Catalyst, a nonprofit health advocacy organization, has joined 60 other groups urging the Biden Administration to ban deferred interest medical credit cards.
They say that patients don’t understand what they are signing up for due to comments like these:
“Even though I’ve made monthly automatic payments on my account, as long as I have any balance on my account by [the end of the promotion], I’d be charged a 26.99% interest rate on the whole medical bill of [$2700].”
“I had nearly [$700] of interest that had accumulated within 4 months…based on one [$2000] charge. The employees at medical offices are selling a product they know little about without fully disclosing the terms and conditions to their patients.”
Historically, patients who apply for the cards have tended to use them to finance cosmetic or other lifestyle medicine procedures, but the CFPB said patients increasingly rely on them for routine and emergency care, which may contribute to growing medical debts and collections balances.
Federal authorities have expressed concerns that doctors may direct patients toward these financial arrangements instead of properly screening them for assistance programs or pursuing the sometimes arduous claims process to capture reimbursement from payers.
Growth of Medical Credit Card Market
One of the most widely used cards, CareCredit, is owned by Synchrony Bank and accepted at over 260,000 locations. Beyond private practices, the vendor has multiyear deals with over 300 hospitals, including Kaiser Permanente and the Cleveland Clinic.
Despite growing popularity and acceptance within the medical community, the cards may work well for some, but not all, patients.
According to a CFPB report released earlier this year, deferred interest medical credit cards were used to pay nearly $23 billion in healthcare expenses from 2018 to 2020. Individuals unable to stick to the terms paid $1 billion in deferred interest payments during that period. Three quarters of CareCredit consumers pay no interest, the organization reported.
Healthcare costs are likely driving demand for medical credit cards. In a recent survey by the Commonwealth Fund, almost half of respondents said it was very or somewhat difficult to afford care even when having insurance coverage through an employer, individual, or government plan. Consumers in the survey cited the high costs as a reason why they delayed or skipped care and prescription medication in the past year, including 29% of those with employer coverage and 42% with Medicare.
These dynamics can leave doctors between a rock and a hard place, said Alan P. Sager, PhD, a professor of health law, policy, and management at Boston University School of Public Health. He told this news organization that medical credit cards can keep cash flowing for doctors and provide elective and necessary care for patients, but the double-digit interest rates outside of the promotional periods can put patients at risk of bankruptcy. He views them as a short-term solution to a more significant problem.
“What doctors need and deserve is patients who have full coverage so that there are no medical debts and no need for medical credit cards,” said Dr. Sager.
Doctor Groups Weigh In
The Medical Group Management Association (MGMA), representing more than 15,000 medical groups, said in its public comments that Medicare cuts and staffing and inflation challenges have made running a profitable practice challenging, particularly for rural and less-resourced offices.
The organization said medical credit cards with transparent terms and conditions can help patients afford care and keep practice doors open amid rising operational costs. However, MGMA worries that the CFPB’s inquiry could “perpetuate the notion that it is acceptable for payment not to be rendered immediately after clinical services are provided, and it’s ok that payments are often subject to significant delays.”
Meanwhile, the American Society of Plastic Surgeons (ASPS) has endorsed CareCredit for over 20 years. In response to the CFPB’s request for information, the association said it supports medical credit cards that offer promotional low- or no-interest terms.
Steven Williams, MD, ASPS president, told this news organization that patients appreciate multiple payment options and the flexibility to move forward with care on short notice. Still, he said that it requires due diligence on everyone’s part.
“Lenders have a responsibility to educate their customers, and it’s critical that lending products have full disclosure in plain and clear language. And with any substantial purchase, patients need to analyze how much it adds to the bottom line,” he said.
A version of this article appeared on Medscape.com.
With healthcare costs rising and payer reimbursements dwindling, many physicians are focusing even more on collecting outstanding patient balances.
Medical credit cards can be a popular choice to fill this gap because doctors get reimbursed upfront while patients receive special financing offers and the care they seek or need.
But, in recent months, federal officials have questioned whether these arrangements are genuinely win-win or if the cards prey on low-income and vulnerable individuals and warrant tighter regulatory oversight.
In July, the Consumer Financial Protection Bureau (CFPB), the US Department of Health and Human Services, and the US Department of Treasury announced an inquiry into medical credit cards. The agencies sought public comments from patients and providers to determine how much they are used.
Medical credit cards typically offer 0% or low-interest terms ranging from 6 to 24 months. Minimum monthly payments are required, often as low as $30 and not usually enough to pay the balance by the end of the promotional period.
After the introductory rate, card issuers may charge interest rates approaching 30% — not just on the remaining balance but on the original amount financed, adding considerably to total out-of-pocket costs.
Ophthalmologist Michael A. Brusco, MD, FACS, specializes in laser-assisted in situ keratomileuses and vision correction at his practice in the greater Washington, DC, area. He told this news organization that nearly all his patients are self-paying, and just under half utilize one of two medical credit cards he offers through third-party vendors, CareCredit and Alphaeon Credit.
“We are clear with our patients that it is interest-free only if they make all payments on time, and if they don’t, then the penalties and fees skyrocket,” Dr. Brusco said.
Patients pay no interest if they make the minimum monthly payments and pay the entire balance by the end of the term. Brusco said those who qualify and abide by those conditions can benefit from spreading healthcare expenses over several months and reducing the stress and financial strain associated with a larger, one-time payment.
He acknowledged that deferred interest can be problematic if patients are caught unaware but said his staff has received training from both vendors on clearly explaining the plans to patients. If someone doesn’t think they can pay off the balance in the timeframe, he suggests they pursue an alternative payment method.
Community Catalyst, a nonprofit health advocacy organization, has joined 60 other groups urging the Biden Administration to ban deferred interest medical credit cards.
They say that patients don’t understand what they are signing up for due to comments like these:
“Even though I’ve made monthly automatic payments on my account, as long as I have any balance on my account by [the end of the promotion], I’d be charged a 26.99% interest rate on the whole medical bill of [$2700].”
“I had nearly [$700] of interest that had accumulated within 4 months…based on one [$2000] charge. The employees at medical offices are selling a product they know little about without fully disclosing the terms and conditions to their patients.”
Historically, patients who apply for the cards have tended to use them to finance cosmetic or other lifestyle medicine procedures, but the CFPB said patients increasingly rely on them for routine and emergency care, which may contribute to growing medical debts and collections balances.
Federal authorities have expressed concerns that doctors may direct patients toward these financial arrangements instead of properly screening them for assistance programs or pursuing the sometimes arduous claims process to capture reimbursement from payers.
Growth of Medical Credit Card Market
One of the most widely used cards, CareCredit, is owned by Synchrony Bank and accepted at over 260,000 locations. Beyond private practices, the vendor has multiyear deals with over 300 hospitals, including Kaiser Permanente and the Cleveland Clinic.
Despite growing popularity and acceptance within the medical community, the cards may work well for some, but not all, patients.
According to a CFPB report released earlier this year, deferred interest medical credit cards were used to pay nearly $23 billion in healthcare expenses from 2018 to 2020. Individuals unable to stick to the terms paid $1 billion in deferred interest payments during that period. Three quarters of CareCredit consumers pay no interest, the organization reported.
Healthcare costs are likely driving demand for medical credit cards. In a recent survey by the Commonwealth Fund, almost half of respondents said it was very or somewhat difficult to afford care even when having insurance coverage through an employer, individual, or government plan. Consumers in the survey cited the high costs as a reason why they delayed or skipped care and prescription medication in the past year, including 29% of those with employer coverage and 42% with Medicare.
These dynamics can leave doctors between a rock and a hard place, said Alan P. Sager, PhD, a professor of health law, policy, and management at Boston University School of Public Health. He told this news organization that medical credit cards can keep cash flowing for doctors and provide elective and necessary care for patients, but the double-digit interest rates outside of the promotional periods can put patients at risk of bankruptcy. He views them as a short-term solution to a more significant problem.
“What doctors need and deserve is patients who have full coverage so that there are no medical debts and no need for medical credit cards,” said Dr. Sager.
Doctor Groups Weigh In
The Medical Group Management Association (MGMA), representing more than 15,000 medical groups, said in its public comments that Medicare cuts and staffing and inflation challenges have made running a profitable practice challenging, particularly for rural and less-resourced offices.
The organization said medical credit cards with transparent terms and conditions can help patients afford care and keep practice doors open amid rising operational costs. However, MGMA worries that the CFPB’s inquiry could “perpetuate the notion that it is acceptable for payment not to be rendered immediately after clinical services are provided, and it’s ok that payments are often subject to significant delays.”
Meanwhile, the American Society of Plastic Surgeons (ASPS) has endorsed CareCredit for over 20 years. In response to the CFPB’s request for information, the association said it supports medical credit cards that offer promotional low- or no-interest terms.
Steven Williams, MD, ASPS president, told this news organization that patients appreciate multiple payment options and the flexibility to move forward with care on short notice. Still, he said that it requires due diligence on everyone’s part.
“Lenders have a responsibility to educate their customers, and it’s critical that lending products have full disclosure in plain and clear language. And with any substantial purchase, patients need to analyze how much it adds to the bottom line,” he said.
A version of this article appeared on Medscape.com.
With healthcare costs rising and payer reimbursements dwindling, many physicians are focusing even more on collecting outstanding patient balances.
Medical credit cards can be a popular choice to fill this gap because doctors get reimbursed upfront while patients receive special financing offers and the care they seek or need.
But, in recent months, federal officials have questioned whether these arrangements are genuinely win-win or if the cards prey on low-income and vulnerable individuals and warrant tighter regulatory oversight.
In July, the Consumer Financial Protection Bureau (CFPB), the US Department of Health and Human Services, and the US Department of Treasury announced an inquiry into medical credit cards. The agencies sought public comments from patients and providers to determine how much they are used.
Medical credit cards typically offer 0% or low-interest terms ranging from 6 to 24 months. Minimum monthly payments are required, often as low as $30 and not usually enough to pay the balance by the end of the promotional period.
After the introductory rate, card issuers may charge interest rates approaching 30% — not just on the remaining balance but on the original amount financed, adding considerably to total out-of-pocket costs.
Ophthalmologist Michael A. Brusco, MD, FACS, specializes in laser-assisted in situ keratomileuses and vision correction at his practice in the greater Washington, DC, area. He told this news organization that nearly all his patients are self-paying, and just under half utilize one of two medical credit cards he offers through third-party vendors, CareCredit and Alphaeon Credit.
“We are clear with our patients that it is interest-free only if they make all payments on time, and if they don’t, then the penalties and fees skyrocket,” Dr. Brusco said.
Patients pay no interest if they make the minimum monthly payments and pay the entire balance by the end of the term. Brusco said those who qualify and abide by those conditions can benefit from spreading healthcare expenses over several months and reducing the stress and financial strain associated with a larger, one-time payment.
He acknowledged that deferred interest can be problematic if patients are caught unaware but said his staff has received training from both vendors on clearly explaining the plans to patients. If someone doesn’t think they can pay off the balance in the timeframe, he suggests they pursue an alternative payment method.
Community Catalyst, a nonprofit health advocacy organization, has joined 60 other groups urging the Biden Administration to ban deferred interest medical credit cards.
They say that patients don’t understand what they are signing up for due to comments like these:
“Even though I’ve made monthly automatic payments on my account, as long as I have any balance on my account by [the end of the promotion], I’d be charged a 26.99% interest rate on the whole medical bill of [$2700].”
“I had nearly [$700] of interest that had accumulated within 4 months…based on one [$2000] charge. The employees at medical offices are selling a product they know little about without fully disclosing the terms and conditions to their patients.”
Historically, patients who apply for the cards have tended to use them to finance cosmetic or other lifestyle medicine procedures, but the CFPB said patients increasingly rely on them for routine and emergency care, which may contribute to growing medical debts and collections balances.
Federal authorities have expressed concerns that doctors may direct patients toward these financial arrangements instead of properly screening them for assistance programs or pursuing the sometimes arduous claims process to capture reimbursement from payers.
Growth of Medical Credit Card Market
One of the most widely used cards, CareCredit, is owned by Synchrony Bank and accepted at over 260,000 locations. Beyond private practices, the vendor has multiyear deals with over 300 hospitals, including Kaiser Permanente and the Cleveland Clinic.
Despite growing popularity and acceptance within the medical community, the cards may work well for some, but not all, patients.
According to a CFPB report released earlier this year, deferred interest medical credit cards were used to pay nearly $23 billion in healthcare expenses from 2018 to 2020. Individuals unable to stick to the terms paid $1 billion in deferred interest payments during that period. Three quarters of CareCredit consumers pay no interest, the organization reported.
Healthcare costs are likely driving demand for medical credit cards. In a recent survey by the Commonwealth Fund, almost half of respondents said it was very or somewhat difficult to afford care even when having insurance coverage through an employer, individual, or government plan. Consumers in the survey cited the high costs as a reason why they delayed or skipped care and prescription medication in the past year, including 29% of those with employer coverage and 42% with Medicare.
These dynamics can leave doctors between a rock and a hard place, said Alan P. Sager, PhD, a professor of health law, policy, and management at Boston University School of Public Health. He told this news organization that medical credit cards can keep cash flowing for doctors and provide elective and necessary care for patients, but the double-digit interest rates outside of the promotional periods can put patients at risk of bankruptcy. He views them as a short-term solution to a more significant problem.
“What doctors need and deserve is patients who have full coverage so that there are no medical debts and no need for medical credit cards,” said Dr. Sager.
Doctor Groups Weigh In
The Medical Group Management Association (MGMA), representing more than 15,000 medical groups, said in its public comments that Medicare cuts and staffing and inflation challenges have made running a profitable practice challenging, particularly for rural and less-resourced offices.
The organization said medical credit cards with transparent terms and conditions can help patients afford care and keep practice doors open amid rising operational costs. However, MGMA worries that the CFPB’s inquiry could “perpetuate the notion that it is acceptable for payment not to be rendered immediately after clinical services are provided, and it’s ok that payments are often subject to significant delays.”
Meanwhile, the American Society of Plastic Surgeons (ASPS) has endorsed CareCredit for over 20 years. In response to the CFPB’s request for information, the association said it supports medical credit cards that offer promotional low- or no-interest terms.
Steven Williams, MD, ASPS president, told this news organization that patients appreciate multiple payment options and the flexibility to move forward with care on short notice. Still, he said that it requires due diligence on everyone’s part.
“Lenders have a responsibility to educate their customers, and it’s critical that lending products have full disclosure in plain and clear language. And with any substantial purchase, patients need to analyze how much it adds to the bottom line,” he said.
A version of this article appeared on Medscape.com.
Study evaluates aesthetic concerns among Hispanic, Latinx women
CHICAGO — , according to the results of a study that involved a survey of almost 4000 women.
To date, the aesthetic needs of Hispanic/Latinx patients, the second largest ethnic group in the United States, have been poorly understood. “Most [aesthetic] marketing materials are gauged toward Caucasian patients,” Sabrina Fabi, MD, a dermatologist and dermatologic cosmetic surgeon in San Diego, California, said at the annual meeting of the American Society for Dermatologic Surgery (ASDS), where she presented the study results.
In addition, Dr. Fabi noted that current studies of facial and body aesthetics are limited in terms of representation. “When we look at studies, they are more Fitzpatrick type IIs and IIIs,” she said. Addressing this gap, she and her colleagues conducted the large multicenter study to learn more about cosmetic concerns unique to Hispanic/Latinx women, across different ethnic groups, and how they may differ by age.
In the study, an online survey was administered to aesthetically-inclined adults across different demographic groups in the United States. Specifically, respondents were surveyed regarding 41 facial and 31 body characteristics, identifying those they found bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3974 women surveyed, 748 self-identified as Hispanic/Latinx and female. Most participants (86%) were born in the United States and were interested in aesthetic treatments (93%). The majority of patients identified as Generation X (42-57 years, 40.0%), followed by older Millennials (31-41 years, 33.0%), Generation Z/young Millennials (under 30 years, 16.7%), and Baby Boomers and older (over 57 years, 10.3%). Participants most commonly reported Fitzpatrick skin types III (24%) and IV (56%), and BMIs of 18.5 kg/m2 to <25 kg/m2 (42%) and 25 to <30 kg/m2 (27%).
Among Hispanic/Latinx women, the top facial concerns were related to submental fat (36%) and under-eye hollowing (35%). This is in contrast to White counterparts, who tended to find wrinkles more bothersome, according to Dr. Fabi. Among Hispanic/Latinx women, the top body concerns were related to stubborn fat involving the stomach (50%), sides (44%), and bra or the back area (40%).
Despite the shared concern of stubborn body fat across age groups, facial concerns shifted from skin quality (50%) and under-eye issues (43%) in the younger generations to upper facial lines (52%) and jowls/sagging skin (57%) in the older generations.
Dr. Fabi stated that approximately 30% of the population she sees is Hispanic/Latinx, and the results of this study substantiate what she sees in her practice. “This magnifies the things we need to be talking to them more about specifically.” The findings from this survey may aid in the customization of treatment plans to better serve this population, she said.
The study was sponsored by Allergan Aesthetics, which participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. Dr. Fabi and three other authors are speakers, consultants, and investigators for Allergan. Other authors are on the advisory board, or are employees of Abbvie, Allergan’s parent company, and may own stock.
CHICAGO — , according to the results of a study that involved a survey of almost 4000 women.
To date, the aesthetic needs of Hispanic/Latinx patients, the second largest ethnic group in the United States, have been poorly understood. “Most [aesthetic] marketing materials are gauged toward Caucasian patients,” Sabrina Fabi, MD, a dermatologist and dermatologic cosmetic surgeon in San Diego, California, said at the annual meeting of the American Society for Dermatologic Surgery (ASDS), where she presented the study results.
In addition, Dr. Fabi noted that current studies of facial and body aesthetics are limited in terms of representation. “When we look at studies, they are more Fitzpatrick type IIs and IIIs,” she said. Addressing this gap, she and her colleagues conducted the large multicenter study to learn more about cosmetic concerns unique to Hispanic/Latinx women, across different ethnic groups, and how they may differ by age.
In the study, an online survey was administered to aesthetically-inclined adults across different demographic groups in the United States. Specifically, respondents were surveyed regarding 41 facial and 31 body characteristics, identifying those they found bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3974 women surveyed, 748 self-identified as Hispanic/Latinx and female. Most participants (86%) were born in the United States and were interested in aesthetic treatments (93%). The majority of patients identified as Generation X (42-57 years, 40.0%), followed by older Millennials (31-41 years, 33.0%), Generation Z/young Millennials (under 30 years, 16.7%), and Baby Boomers and older (over 57 years, 10.3%). Participants most commonly reported Fitzpatrick skin types III (24%) and IV (56%), and BMIs of 18.5 kg/m2 to <25 kg/m2 (42%) and 25 to <30 kg/m2 (27%).
Among Hispanic/Latinx women, the top facial concerns were related to submental fat (36%) and under-eye hollowing (35%). This is in contrast to White counterparts, who tended to find wrinkles more bothersome, according to Dr. Fabi. Among Hispanic/Latinx women, the top body concerns were related to stubborn fat involving the stomach (50%), sides (44%), and bra or the back area (40%).
Despite the shared concern of stubborn body fat across age groups, facial concerns shifted from skin quality (50%) and under-eye issues (43%) in the younger generations to upper facial lines (52%) and jowls/sagging skin (57%) in the older generations.
Dr. Fabi stated that approximately 30% of the population she sees is Hispanic/Latinx, and the results of this study substantiate what she sees in her practice. “This magnifies the things we need to be talking to them more about specifically.” The findings from this survey may aid in the customization of treatment plans to better serve this population, she said.
The study was sponsored by Allergan Aesthetics, which participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. Dr. Fabi and three other authors are speakers, consultants, and investigators for Allergan. Other authors are on the advisory board, or are employees of Abbvie, Allergan’s parent company, and may own stock.
CHICAGO — , according to the results of a study that involved a survey of almost 4000 women.
To date, the aesthetic needs of Hispanic/Latinx patients, the second largest ethnic group in the United States, have been poorly understood. “Most [aesthetic] marketing materials are gauged toward Caucasian patients,” Sabrina Fabi, MD, a dermatologist and dermatologic cosmetic surgeon in San Diego, California, said at the annual meeting of the American Society for Dermatologic Surgery (ASDS), where she presented the study results.
In addition, Dr. Fabi noted that current studies of facial and body aesthetics are limited in terms of representation. “When we look at studies, they are more Fitzpatrick type IIs and IIIs,” she said. Addressing this gap, she and her colleagues conducted the large multicenter study to learn more about cosmetic concerns unique to Hispanic/Latinx women, across different ethnic groups, and how they may differ by age.
In the study, an online survey was administered to aesthetically-inclined adults across different demographic groups in the United States. Specifically, respondents were surveyed regarding 41 facial and 31 body characteristics, identifying those they found bothersome. Maximum difference scaling was used to generate their most and least bothersome characteristics in each respective category.
Of the 3974 women surveyed, 748 self-identified as Hispanic/Latinx and female. Most participants (86%) were born in the United States and were interested in aesthetic treatments (93%). The majority of patients identified as Generation X (42-57 years, 40.0%), followed by older Millennials (31-41 years, 33.0%), Generation Z/young Millennials (under 30 years, 16.7%), and Baby Boomers and older (over 57 years, 10.3%). Participants most commonly reported Fitzpatrick skin types III (24%) and IV (56%), and BMIs of 18.5 kg/m2 to <25 kg/m2 (42%) and 25 to <30 kg/m2 (27%).
Among Hispanic/Latinx women, the top facial concerns were related to submental fat (36%) and under-eye hollowing (35%). This is in contrast to White counterparts, who tended to find wrinkles more bothersome, according to Dr. Fabi. Among Hispanic/Latinx women, the top body concerns were related to stubborn fat involving the stomach (50%), sides (44%), and bra or the back area (40%).
Despite the shared concern of stubborn body fat across age groups, facial concerns shifted from skin quality (50%) and under-eye issues (43%) in the younger generations to upper facial lines (52%) and jowls/sagging skin (57%) in the older generations.
Dr. Fabi stated that approximately 30% of the population she sees is Hispanic/Latinx, and the results of this study substantiate what she sees in her practice. “This magnifies the things we need to be talking to them more about specifically.” The findings from this survey may aid in the customization of treatment plans to better serve this population, she said.
The study was sponsored by Allergan Aesthetics, which participated in the trial design, research, analysis, data collection, interpretation of data, and the review and approval of the publication. Dr. Fabi and three other authors are speakers, consultants, and investigators for Allergan. Other authors are on the advisory board, or are employees of Abbvie, Allergan’s parent company, and may own stock.
AT ASDS 2023
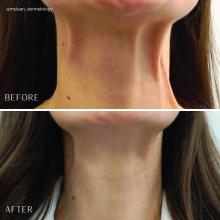
Sodium deoxycholate and triamcinolone: A good mix?
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at dermnews@mdedge.com. She was an investigator in clinical trials of Kybella.
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at dermnews@mdedge.com. She was an investigator in clinical trials of Kybella.
In September 2023, Goldman et al. published a communication in Dermatologic Surgery describing their use of subcutaneous sodium deoxycholate injection (SDOC), with or without triamcinolone acetonide, for reduction of submental fat..
As they note, “patients experience a variable degree of edema and discomfort following subcutaneous injection,” of SDOC, something that I and others have also observed in our practices.
In their double-blind study of 20 patients with a baseline Clinician-Reported Submental Fat Rating Scale of 2 or 3 out of 4, 5 patients were randomized to receive SDOC as recommended in the label, while 15 received SDOC plus triamcinolone. In the latter group, 2 mL of SDOC was mixed with 0.5 mL of 40 mg/mL of triamcinolone acetate, then administered in up to 50 injections in the submentum spaced 1.0 cm apart at 0.25 mL per injection. Three treatments were administered 1 month apart.
For both groups, volumes between 5 mL and 8 mL per treatment were delivered. There were no significant differences in efficacy 30, 60, and 90 days after the final injection between the two groups. However, at day 180, the group that received only SDOC had a significantly greater reduction in submental fat, which the authors wrote indicated that the addition of triamcinolone “may mildly diminish the fat reduction effects” at that time point.
Subcutaneous SDOC (deoxycholic acid) injections for reduction of submental fullness was approved by the Food and Drug Administration in 2015 for improving the appearance of moderate to severe convexity or fullness associated with submental fat in adults. (I was involved in the clinical trials.) We found that in the trial, for optimal efficacy, most patients require two to four treatments spread at least a month apart, with patients who had larger treatment areas requiring up to six treatments.
While the clinical trial treatments were spaced 4 weeks apart, post approval, we found that patients would sometimes report further efficacy even 2-3 months post injection. Since not everyone wants to go around with edema every month for 2-4 consecutive months, spacing the treatments farther apart allows patients more time to heal and coordinate the recovery appearance around their work and social schedules.
In my practice, very rarely have we seen minimal to moderate prolonged edema, particularly in younger patients, beyond 1 month post injection. Most people have the most noticeable edema — the “bull-frog” appearance — for the first 1-3 days, with some minor fullness that appears to be almost back to baseline at 1 week. In some of these patients with prolonged submental fullness, it looks fuller than it appeared pretreatment even months afterwards.
While rare, like the study authors, I have found intralesional triamcinolone to be helpful at reducing this persistent fullness should it occur. It is likely to be reducing any persistent inflammation or posttreatment fibrosis in these patients.
Unlike the study authors, I do not combine SDOC and triamcinolone injections at the time of treatment. Rather, I consider injecting triamcinolone if submental fullness is greater than at baseline or edema persists after SDOC treatment. It is rare that I’ve had to do this, as most cases self-resolve, but I have used triamcinolone 10 mg/mL, up to 1cc total, injected 6-8 weeks apart one to three times to the affected area and found it to be effective if fullness has persisted beyond 6 months. Liposuction may also be an option, if needed, if fullness/edema persists.
Overall, SDOC is an effective treatment for small pockets of subcutaneous fat. Approved for submental fullness, it is now sometimes used off-label for other parts of the body, such as bra fat, small pockets of the abdomen, and lipomas. While some inflammation after treatment is expected — and desired — to achieve an effective outcome of fat apoptosis, intralesional triamcinolone is an interesting tool to utilize should inflammation or posttreatment fullness persist.
Dr. Wesley practices dermatology in Beverly Hills, California. Write to her at dermnews@mdedge.com. She was an investigator in clinical trials of Kybella.
Body dysmorphic disorder diagnosis guidelines completed in Europe
BERLIN – were outlined in a late-breaker presentation at the annual Congress of the European Academy of Dermatology and Venereology.
The development of guidelines for BDD, a disorder familiar to many clinical dermatologists, is intended as a practical tool, according to Maria-Angeliki Gkini, MD, who has appointments at both Bart’s Health NHS Trust in London and the 401 General Army Hospital in Athens.
“BDD is a relatively common disorder in which the patients are preoccupied with a perceived defect or defects,” Dr. Gkini explained. “This affects them so intensely that it affects their mental health and their quality of life.”
In the DSM-5, published by the American Psychiatric Association, BDD is specifically defined as a preoccupation with “one or more perceived defects or flaws in physical appearance that are not observable or appear slight to others.” But Dr. Gkini said that BDD can also develop as a comorbidity of dermatological disorders that are visible.
These patients are challenging because they are difficult to please, added Dr. Gkini, who said they commonly become involved in doctor shopping, leaving negative reviews on social media for the clinicians they have cycled through. The problem is that the defects they seek to resolve typically stem from distorted perceptions.
BDD is related to obsessive-compulsive disorder by the frequency with which patients pursue repetitive behaviors related to their preoccupation, such as intensive grooming, frequent trips to the mirror, or difficulty in focusing on topics other than their own appearance.
The process to develop the soon-to-be-published guidelines began with a literature search. Of the approximately 3,200 articles identified on BDD, only 10 involved randomized controlled trials. Moreover, even the quality of these trials was considered “low to very low” by the experts who reviewed them, Dr. Gkini said.
One explanation is that psychodermatology has only recently started to attract more research interest, and better studies are now underway, she noted.
However, because of the dearth of high quality evidence now available, the guideline development relied on a Delphi method to reach consensus based on expert opinion in discussion of the available data.
Consensus reached by 17 experts
Specifically, 17 experts, all of whom were members of the European Society for Dermatology and Psychiatry proceeded to systematically address a series of clinical questions and recommendations. Consensus was defined as at least 75% of the participants strongly agreeing or agreeing. Several rounds of discussion were often required.
Among the conclusions, the guidelines support uniform screening for BDD in all patients prior to cosmetic procedures. In identifying depression, anxiety, and distorted perceptions, simple tools, such as the Patient Health Questionnaire might be adequate for an initial evaluation, but Dr. Gkini also recommended routinely inquiring about suicidal ideation, which has been reported in up to 80% of individuals with BDD.
Other instruments for screening that can be considered include DSM-5 criteria for BDD and the Body Dysmorphic Disorder Questionnaire–Dermatology Version, which might be particularly useful and appropriate for dermatologists.
One of the reasons to screen for BDD is that these patients often convince themselves that some specific procedure is needed to resolve the source of their obsession. The goal of screening is to verify that it is the dermatologic concern, not an underlying psychiatric disorder that is driving their search for relief. The risk of dermatologic interventions is not only that expectations are not met, but the patient’s perception of a failed intervention “sometimes makes these worse,” Dr. Gkini explained.
Collaboration with psychiatrists recommended
The guidelines include suggestions for treatment of BDD. Of these, SSRIs are recommended at high relative doses, according to Dr. Gkini. Consistent with the consensus recommendation of collaborating with mental health specialists, she said that the recommendations acknowledge evidence of greater benefits when SSRIs are combined with psychotherapy.
Katharine A. Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, has been conducting BDD research for several years and has written numerous books and articles about this topic, including a review in the journal Focus. She cautioned that, because of a normal concern for appearance, BDD is easily missed by dermatologists.
“For BDD to be diagnosed, the preoccupation with a nonexistent or slight defect in appearance must cause clinically significant distress or impairment in functioning,” she said in an interview. “This is necessary to differentiate BDD from more normal and common appearance concerns that do not qualify for the diagnosis”
She specified that patients should be considered for cognitive-behavioral therapy rather than psychotherapy, a generic term that covers many forms of treatment. She said that most other types of psychotherapy “are probably not effective” for BDD.
Dr. Phillips highly endorsed the development of BDD guidelines for dermatologists because of the frequency with which physicians in this specialty encounter BDD – and believes that more attention to this diagnosis is needed.
“I recommend that dermatologists who have a patient with BDD collaborate with a psychiatrist in delivering care with an SSRI,” she said. “High doses of these medications are often needed to effectively treat BDD.”
Dr. Gkini reported financial relationships with AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO, Novartis, Sanofi, and Regenlab. Dr. Phillips reported no relevant financial relationships.
BERLIN – were outlined in a late-breaker presentation at the annual Congress of the European Academy of Dermatology and Venereology.
The development of guidelines for BDD, a disorder familiar to many clinical dermatologists, is intended as a practical tool, according to Maria-Angeliki Gkini, MD, who has appointments at both Bart’s Health NHS Trust in London and the 401 General Army Hospital in Athens.
“BDD is a relatively common disorder in which the patients are preoccupied with a perceived defect or defects,” Dr. Gkini explained. “This affects them so intensely that it affects their mental health and their quality of life.”
In the DSM-5, published by the American Psychiatric Association, BDD is specifically defined as a preoccupation with “one or more perceived defects or flaws in physical appearance that are not observable or appear slight to others.” But Dr. Gkini said that BDD can also develop as a comorbidity of dermatological disorders that are visible.
These patients are challenging because they are difficult to please, added Dr. Gkini, who said they commonly become involved in doctor shopping, leaving negative reviews on social media for the clinicians they have cycled through. The problem is that the defects they seek to resolve typically stem from distorted perceptions.
BDD is related to obsessive-compulsive disorder by the frequency with which patients pursue repetitive behaviors related to their preoccupation, such as intensive grooming, frequent trips to the mirror, or difficulty in focusing on topics other than their own appearance.
The process to develop the soon-to-be-published guidelines began with a literature search. Of the approximately 3,200 articles identified on BDD, only 10 involved randomized controlled trials. Moreover, even the quality of these trials was considered “low to very low” by the experts who reviewed them, Dr. Gkini said.
One explanation is that psychodermatology has only recently started to attract more research interest, and better studies are now underway, she noted.
However, because of the dearth of high quality evidence now available, the guideline development relied on a Delphi method to reach consensus based on expert opinion in discussion of the available data.
Consensus reached by 17 experts
Specifically, 17 experts, all of whom were members of the European Society for Dermatology and Psychiatry proceeded to systematically address a series of clinical questions and recommendations. Consensus was defined as at least 75% of the participants strongly agreeing or agreeing. Several rounds of discussion were often required.
Among the conclusions, the guidelines support uniform screening for BDD in all patients prior to cosmetic procedures. In identifying depression, anxiety, and distorted perceptions, simple tools, such as the Patient Health Questionnaire might be adequate for an initial evaluation, but Dr. Gkini also recommended routinely inquiring about suicidal ideation, which has been reported in up to 80% of individuals with BDD.
Other instruments for screening that can be considered include DSM-5 criteria for BDD and the Body Dysmorphic Disorder Questionnaire–Dermatology Version, which might be particularly useful and appropriate for dermatologists.
One of the reasons to screen for BDD is that these patients often convince themselves that some specific procedure is needed to resolve the source of their obsession. The goal of screening is to verify that it is the dermatologic concern, not an underlying psychiatric disorder that is driving their search for relief. The risk of dermatologic interventions is not only that expectations are not met, but the patient’s perception of a failed intervention “sometimes makes these worse,” Dr. Gkini explained.
Collaboration with psychiatrists recommended
The guidelines include suggestions for treatment of BDD. Of these, SSRIs are recommended at high relative doses, according to Dr. Gkini. Consistent with the consensus recommendation of collaborating with mental health specialists, she said that the recommendations acknowledge evidence of greater benefits when SSRIs are combined with psychotherapy.
Katharine A. Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, has been conducting BDD research for several years and has written numerous books and articles about this topic, including a review in the journal Focus. She cautioned that, because of a normal concern for appearance, BDD is easily missed by dermatologists.
“For BDD to be diagnosed, the preoccupation with a nonexistent or slight defect in appearance must cause clinically significant distress or impairment in functioning,” she said in an interview. “This is necessary to differentiate BDD from more normal and common appearance concerns that do not qualify for the diagnosis”
She specified that patients should be considered for cognitive-behavioral therapy rather than psychotherapy, a generic term that covers many forms of treatment. She said that most other types of psychotherapy “are probably not effective” for BDD.
Dr. Phillips highly endorsed the development of BDD guidelines for dermatologists because of the frequency with which physicians in this specialty encounter BDD – and believes that more attention to this diagnosis is needed.
“I recommend that dermatologists who have a patient with BDD collaborate with a psychiatrist in delivering care with an SSRI,” she said. “High doses of these medications are often needed to effectively treat BDD.”
Dr. Gkini reported financial relationships with AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO, Novartis, Sanofi, and Regenlab. Dr. Phillips reported no relevant financial relationships.
BERLIN – were outlined in a late-breaker presentation at the annual Congress of the European Academy of Dermatology and Venereology.
The development of guidelines for BDD, a disorder familiar to many clinical dermatologists, is intended as a practical tool, according to Maria-Angeliki Gkini, MD, who has appointments at both Bart’s Health NHS Trust in London and the 401 General Army Hospital in Athens.
“BDD is a relatively common disorder in which the patients are preoccupied with a perceived defect or defects,” Dr. Gkini explained. “This affects them so intensely that it affects their mental health and their quality of life.”
In the DSM-5, published by the American Psychiatric Association, BDD is specifically defined as a preoccupation with “one or more perceived defects or flaws in physical appearance that are not observable or appear slight to others.” But Dr. Gkini said that BDD can also develop as a comorbidity of dermatological disorders that are visible.
These patients are challenging because they are difficult to please, added Dr. Gkini, who said they commonly become involved in doctor shopping, leaving negative reviews on social media for the clinicians they have cycled through. The problem is that the defects they seek to resolve typically stem from distorted perceptions.
BDD is related to obsessive-compulsive disorder by the frequency with which patients pursue repetitive behaviors related to their preoccupation, such as intensive grooming, frequent trips to the mirror, or difficulty in focusing on topics other than their own appearance.
The process to develop the soon-to-be-published guidelines began with a literature search. Of the approximately 3,200 articles identified on BDD, only 10 involved randomized controlled trials. Moreover, even the quality of these trials was considered “low to very low” by the experts who reviewed them, Dr. Gkini said.
One explanation is that psychodermatology has only recently started to attract more research interest, and better studies are now underway, she noted.
However, because of the dearth of high quality evidence now available, the guideline development relied on a Delphi method to reach consensus based on expert opinion in discussion of the available data.
Consensus reached by 17 experts
Specifically, 17 experts, all of whom were members of the European Society for Dermatology and Psychiatry proceeded to systematically address a series of clinical questions and recommendations. Consensus was defined as at least 75% of the participants strongly agreeing or agreeing. Several rounds of discussion were often required.
Among the conclusions, the guidelines support uniform screening for BDD in all patients prior to cosmetic procedures. In identifying depression, anxiety, and distorted perceptions, simple tools, such as the Patient Health Questionnaire might be adequate for an initial evaluation, but Dr. Gkini also recommended routinely inquiring about suicidal ideation, which has been reported in up to 80% of individuals with BDD.
Other instruments for screening that can be considered include DSM-5 criteria for BDD and the Body Dysmorphic Disorder Questionnaire–Dermatology Version, which might be particularly useful and appropriate for dermatologists.
One of the reasons to screen for BDD is that these patients often convince themselves that some specific procedure is needed to resolve the source of their obsession. The goal of screening is to verify that it is the dermatologic concern, not an underlying psychiatric disorder that is driving their search for relief. The risk of dermatologic interventions is not only that expectations are not met, but the patient’s perception of a failed intervention “sometimes makes these worse,” Dr. Gkini explained.
Collaboration with psychiatrists recommended
The guidelines include suggestions for treatment of BDD. Of these, SSRIs are recommended at high relative doses, according to Dr. Gkini. Consistent with the consensus recommendation of collaborating with mental health specialists, she said that the recommendations acknowledge evidence of greater benefits when SSRIs are combined with psychotherapy.
Katharine A. Phillips, MD, professor of psychiatry at Weill Cornell Medicine, New York, has been conducting BDD research for several years and has written numerous books and articles about this topic, including a review in the journal Focus. She cautioned that, because of a normal concern for appearance, BDD is easily missed by dermatologists.
“For BDD to be diagnosed, the preoccupation with a nonexistent or slight defect in appearance must cause clinically significant distress or impairment in functioning,” she said in an interview. “This is necessary to differentiate BDD from more normal and common appearance concerns that do not qualify for the diagnosis”
She specified that patients should be considered for cognitive-behavioral therapy rather than psychotherapy, a generic term that covers many forms of treatment. She said that most other types of psychotherapy “are probably not effective” for BDD.
Dr. Phillips highly endorsed the development of BDD guidelines for dermatologists because of the frequency with which physicians in this specialty encounter BDD – and believes that more attention to this diagnosis is needed.
“I recommend that dermatologists who have a patient with BDD collaborate with a psychiatrist in delivering care with an SSRI,” she said. “High doses of these medications are often needed to effectively treat BDD.”
Dr. Gkini reported financial relationships with AbbVie, Almirall, Celgene, Eli Lilly, Janssen, LEO, Novartis, Sanofi, and Regenlab. Dr. Phillips reported no relevant financial relationships.
AT THE EADV CONGRESS
Survey finds oral minoxidil shortage in Washington-area pharmacies
A .
Patients are not finding out until they go to pick up their prescription, which can result in an interruption of treatment – and, potentially a loss of hard-earned hair gain, said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was the lead author of the survey, published online on Oct. 26 as a research letter in the Journal of Drugs in Dermatology.
Going off low-dose oral minoxidil may spark a telogen effluvium event, and that is very disappointing to patients, Dr. Friedman told this news organization.
“There needs to be some system that alerts us,” he said. “Even if it’s a minor shortage, just so we’re aware. We can then prepare patients,” he added, noting that it would be better for someone to be taking a lower-than-normal dose rather than no medication at all while they wait for a refill.
Minoxidil has long been approved in a topical formulation to treat androgenetic alopecia, but a low-dose oral form has gained currency in the wake of findings that it might more effectively treat hair loss, and is without side effects. A New York Times article in August 2022 touting low-dose oral minoxidil as a cheap and effective hair loss drug appeared to ignite interest in this option. In May, 2023, researchers reporting in JAMA Network Open demonstrated a significant uptick in prescriptions for oral minoxidil in the wake of the article’s publication.
Oral minoxidil is approved by the Food and Drug Administration only for hypertension, but dermatologists are prescribing it off-label at a lower dose for hair loss. Dr. Friedman said it’s not clear whether the shortages his team found are national in scope, or whether they are a result of increased demand, or other factors.
After several patients told him they were having trouble filling minoxidil prescriptions, and colleagues said they’d had patients with similar experiences, Dr. Friedman and his colleagues undertook the survey. In the first week of October 2023, they contacted 277 pharmacies by phone in Washington and surrounding Virginia and Maryland counties. The pharmacies were CVS, Giant, Walgreens, and Harris Teeter.
Of the 277 pharmacies they contacted, 40% (111) reported availability of 2.5-mg tablets for a 30-day supply, and just under 30% (82) reported having 10-mg tablets for a 30-day supply.
For treating hair loss, most patients are prescribed 2.5-mg pills, with starting doses ranging from 0.625 mg to 5 mg twice a day, Dr. Friedman said. The 10-mg dose is more frequently prescribed for hypertension.
Only 28% (19 of 67) of the Maryland pharmacies had 30-day supplies of 2.5-mg tablets on hand, and just 22% (15) of the Maryland pharmacies had 30-day supplies of 10-mg tablets. In Northern Virginia, 44% (63 of 143) of the pharmacies had 30-day supplies of the 2.5 mg tablets, as did just 43% (29 of 67) of the Washington pharmacies.
Dr. Friedman said he has started giving patients paper prescriptions they can use to shop around, rather than electronically sending a prescription to a particular pharmacy.
Neither the Food and Drug Administration nor the American Society of Health System Pharmacists lists oral minoxidil as a drug in shortage.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, said the organization received a report from wholesalers in mid-September showing spotty oral minoxidil availability, with the drug on backorder with some manufacturers. ASHP's shortages list is compiled from reports from physicians, manufacturers and wholesalers, he said.
Under what he calls "blue sky conditions," pharmacies using a just-in-time inventory model should be able to fill prescriptions within hours or days, which might explain why some pharmacies in the Washington, DC area survey did not have a 30-day supply on hand, he said. However, Dr. Ganio noted that the causes of drug shortages are complex and multi-factorial. For now, he said there have been no oral minoxidil shortage reports since mid-September.
But Dr. Friedman said some of his patients have waited weeks for a new supply – and that no one is aware of the problem until the last moment.
The lack of alerts or transparency “also erodes the physician-patient relationship because there’s this expectation of the patient that we should have known this,” said Dr. Friedman.
Dr. Friedman reports no relevant financial relationships.
This story was updated on 11/2/2023.
A .
Patients are not finding out until they go to pick up their prescription, which can result in an interruption of treatment – and, potentially a loss of hard-earned hair gain, said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was the lead author of the survey, published online on Oct. 26 as a research letter in the Journal of Drugs in Dermatology.
Going off low-dose oral minoxidil may spark a telogen effluvium event, and that is very disappointing to patients, Dr. Friedman told this news organization.
“There needs to be some system that alerts us,” he said. “Even if it’s a minor shortage, just so we’re aware. We can then prepare patients,” he added, noting that it would be better for someone to be taking a lower-than-normal dose rather than no medication at all while they wait for a refill.
Minoxidil has long been approved in a topical formulation to treat androgenetic alopecia, but a low-dose oral form has gained currency in the wake of findings that it might more effectively treat hair loss, and is without side effects. A New York Times article in August 2022 touting low-dose oral minoxidil as a cheap and effective hair loss drug appeared to ignite interest in this option. In May, 2023, researchers reporting in JAMA Network Open demonstrated a significant uptick in prescriptions for oral minoxidil in the wake of the article’s publication.
Oral minoxidil is approved by the Food and Drug Administration only for hypertension, but dermatologists are prescribing it off-label at a lower dose for hair loss. Dr. Friedman said it’s not clear whether the shortages his team found are national in scope, or whether they are a result of increased demand, or other factors.
After several patients told him they were having trouble filling minoxidil prescriptions, and colleagues said they’d had patients with similar experiences, Dr. Friedman and his colleagues undertook the survey. In the first week of October 2023, they contacted 277 pharmacies by phone in Washington and surrounding Virginia and Maryland counties. The pharmacies were CVS, Giant, Walgreens, and Harris Teeter.
Of the 277 pharmacies they contacted, 40% (111) reported availability of 2.5-mg tablets for a 30-day supply, and just under 30% (82) reported having 10-mg tablets for a 30-day supply.
For treating hair loss, most patients are prescribed 2.5-mg pills, with starting doses ranging from 0.625 mg to 5 mg twice a day, Dr. Friedman said. The 10-mg dose is more frequently prescribed for hypertension.
Only 28% (19 of 67) of the Maryland pharmacies had 30-day supplies of 2.5-mg tablets on hand, and just 22% (15) of the Maryland pharmacies had 30-day supplies of 10-mg tablets. In Northern Virginia, 44% (63 of 143) of the pharmacies had 30-day supplies of the 2.5 mg tablets, as did just 43% (29 of 67) of the Washington pharmacies.
Dr. Friedman said he has started giving patients paper prescriptions they can use to shop around, rather than electronically sending a prescription to a particular pharmacy.
Neither the Food and Drug Administration nor the American Society of Health System Pharmacists lists oral minoxidil as a drug in shortage.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, said the organization received a report from wholesalers in mid-September showing spotty oral minoxidil availability, with the drug on backorder with some manufacturers. ASHP's shortages list is compiled from reports from physicians, manufacturers and wholesalers, he said.
Under what he calls "blue sky conditions," pharmacies using a just-in-time inventory model should be able to fill prescriptions within hours or days, which might explain why some pharmacies in the Washington, DC area survey did not have a 30-day supply on hand, he said. However, Dr. Ganio noted that the causes of drug shortages are complex and multi-factorial. For now, he said there have been no oral minoxidil shortage reports since mid-September.
But Dr. Friedman said some of his patients have waited weeks for a new supply – and that no one is aware of the problem until the last moment.
The lack of alerts or transparency “also erodes the physician-patient relationship because there’s this expectation of the patient that we should have known this,” said Dr. Friedman.
Dr. Friedman reports no relevant financial relationships.
This story was updated on 11/2/2023.
A .
Patients are not finding out until they go to pick up their prescription, which can result in an interruption of treatment – and, potentially a loss of hard-earned hair gain, said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was the lead author of the survey, published online on Oct. 26 as a research letter in the Journal of Drugs in Dermatology.
Going off low-dose oral minoxidil may spark a telogen effluvium event, and that is very disappointing to patients, Dr. Friedman told this news organization.
“There needs to be some system that alerts us,” he said. “Even if it’s a minor shortage, just so we’re aware. We can then prepare patients,” he added, noting that it would be better for someone to be taking a lower-than-normal dose rather than no medication at all while they wait for a refill.
Minoxidil has long been approved in a topical formulation to treat androgenetic alopecia, but a low-dose oral form has gained currency in the wake of findings that it might more effectively treat hair loss, and is without side effects. A New York Times article in August 2022 touting low-dose oral minoxidil as a cheap and effective hair loss drug appeared to ignite interest in this option. In May, 2023, researchers reporting in JAMA Network Open demonstrated a significant uptick in prescriptions for oral minoxidil in the wake of the article’s publication.
Oral minoxidil is approved by the Food and Drug Administration only for hypertension, but dermatologists are prescribing it off-label at a lower dose for hair loss. Dr. Friedman said it’s not clear whether the shortages his team found are national in scope, or whether they are a result of increased demand, or other factors.
After several patients told him they were having trouble filling minoxidil prescriptions, and colleagues said they’d had patients with similar experiences, Dr. Friedman and his colleagues undertook the survey. In the first week of October 2023, they contacted 277 pharmacies by phone in Washington and surrounding Virginia and Maryland counties. The pharmacies were CVS, Giant, Walgreens, and Harris Teeter.
Of the 277 pharmacies they contacted, 40% (111) reported availability of 2.5-mg tablets for a 30-day supply, and just under 30% (82) reported having 10-mg tablets for a 30-day supply.
For treating hair loss, most patients are prescribed 2.5-mg pills, with starting doses ranging from 0.625 mg to 5 mg twice a day, Dr. Friedman said. The 10-mg dose is more frequently prescribed for hypertension.
Only 28% (19 of 67) of the Maryland pharmacies had 30-day supplies of 2.5-mg tablets on hand, and just 22% (15) of the Maryland pharmacies had 30-day supplies of 10-mg tablets. In Northern Virginia, 44% (63 of 143) of the pharmacies had 30-day supplies of the 2.5 mg tablets, as did just 43% (29 of 67) of the Washington pharmacies.
Dr. Friedman said he has started giving patients paper prescriptions they can use to shop around, rather than electronically sending a prescription to a particular pharmacy.
Neither the Food and Drug Administration nor the American Society of Health System Pharmacists lists oral minoxidil as a drug in shortage.
Michael Ganio, PharmD, senior director of pharmacy practice and quality for ASHP, said the organization received a report from wholesalers in mid-September showing spotty oral minoxidil availability, with the drug on backorder with some manufacturers. ASHP's shortages list is compiled from reports from physicians, manufacturers and wholesalers, he said.
Under what he calls "blue sky conditions," pharmacies using a just-in-time inventory model should be able to fill prescriptions within hours or days, which might explain why some pharmacies in the Washington, DC area survey did not have a 30-day supply on hand, he said. However, Dr. Ganio noted that the causes of drug shortages are complex and multi-factorial. For now, he said there have been no oral minoxidil shortage reports since mid-September.
But Dr. Friedman said some of his patients have waited weeks for a new supply – and that no one is aware of the problem until the last moment.
The lack of alerts or transparency “also erodes the physician-patient relationship because there’s this expectation of the patient that we should have known this,” said Dr. Friedman.
Dr. Friedman reports no relevant financial relationships.
This story was updated on 11/2/2023.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY
Cysteamine and melasma
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4.
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4.
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Most subjects covered in this column are botanical ingredients used for multiple conditions in topical skin care. The focus this month, though, is a natural agent garnering attention primarily for one indication. Present in many mammals and in various cells in the human body (and particularly highly concentrated in human milk), cysteamine is a stable aminothiol that acts as an antioxidant as a result of the degradation of coenzyme A and is known to play a protective function.1 Melasma, an acquired recurrent, chronic hyperpigmentary disorder, continues to be a treatment challenge and is often psychologically troublesome for those affected, approximately 90% of whom are women.2 Individuals with Fitzpatrick skin types IV and V who reside in regions where UV exposure is likely are particularly prominent among those with melasma.2 While triple combination therapy (also known as Kligman’s formula) continues to be the modern gold standard of care for melasma (over the last 30 years),3 cysteamine, a nonmelanocytotoxic molecule, is considered viable for long-term use and safer than the long-time skin-lightening gold standard over several decades, hydroquinone (HQ), which is associated with safety concerns.4.
Recent history and the 2015 study
Prior to 2015, the quick oxidation and malodorous nature of cysteamine rendered it unsuitable for use as a topical agent. However, stabilization efforts resulted in a product that first began to show efficacy that year.5
Mansouri et al. conducted a randomized, double-blind, placebo-controlled trial to assess the efficacy of topical cysteamine 5% to treat epidermal melasma in 2015. Over 4 months, 50 volunteers (25 in each group) applied either cysteamine cream or placebo on lesions once nightly. The mean differences at baseline between pigmented and normal skin were 75.2 ± 37 in the cysteamine group and 68.9 ± 31 in the placebo group. Statistically significant differences between the groups were identified at the 2- and 4-month points. At 2 months, the mean differences were 39.7 ± 16.6 in the cysteamine group and 63.8 ± 28.6 in the placebo group; at 4 months, the respective differences were 26.2 ± 16 and 60.7 ± 27.3. Melasma area severity index (MASI) scores were significantly lower in the cysteamine group compared with the placebo group at the end of the study, and investigator global assessment scores and patient questionnaire results revealed substantial comparative efficacy of cysteamine cream.6 Topical cysteamine has also demonstrated notable efficacy in treating senile lentigines, which typically do not respond to topical depigmenting products.5
Farshi et al. used Dermacatch as a novel measurement tool to ascertain the efficacy of cysteamine cream for treating epidermal melasma in a 2018 report of a randomized, double-blind, placebo-controlled study with 40 patients. During the 4-month trial, cysteamine cream or placebo was applied nightly before sleep. Investigators measured treatment efficacy through Dermacatch, and Mexameter skin colorimetry, MASI scores, investigator global assessments, and patient questionnaires at baseline, 2 months, and 4 months. Through all measurement methods, cysteamine was found to reduce melanin content of melasma lesions, with Dermacatch performing reliably and comparably to Mexameter.7 Since then, cysteamine has been compared to several first-line melasma therapies.
Reviews
A 2019 systematic review by Austin et al. of randomized controlled trials (RCTs) on topical treatments for melasma identified 35 original RCTs evaluating a wide range of approximately 20 agents. They identified cysteamine, triple combination therapy, and tranexamic acid as the products netting the most robust recommendations. The researchers characterized cysteamine as conferring strong efficacy and reported anticancer activity while triple combination therapy poses the potential risk of ochronosis and tranexamic acid may present the risk for thrombosis. They concluded that more research is necessary, though, to establish the proper concentration and optimal formulation of cysteamine as a frontline therapy.8
More reviews have since been published to further clarify where cysteamine stands among the optimal treatments for melasma. In a May 2022 systematic PubMed review of topical agents used to treat melasma, González-Molina et al. identified 80 papers meeting inclusion criteria (double or single blinded, prospective, controlled or RCTs, reviews of literature, and meta-analysis studies), with tranexamic acid and cysteamine among the novel well-tolerated agents. Cysteamine was not associated with any severe adverse effects and is recommended as an adjuvant and maintenance therapy.3
A September 2022 review by Niazi et al. found that while the signaling mechanisms through which cysteamine suppresses melasma are not well understood, the topical application of cysteamine cream is seen as safe and effective alone or in combination with other products to treat melasma.2
A systematic review and meta-analysis reported by Gomes dos Santos-Neto et al. at the end of 2022 considered the efficacy of depigmenting formulations containing 5% cysteamine for treating melasma. The meta-analysis covered six studies, with 120 melasma patients treated. The conclusion was that 5% cysteamine was effective with adverse effects unlikely.9
Cysteamine vs. hydroquinone
In 2020, Lima et al. reported the results of a quasi-randomized, multicenter, evaluator-blinded comparative study of topical 0.56% cysteamine and 4% HQ in 40 women with facial melasma. (Note that this study originally claimed a 5% cysteamine concentration, but a letter to the editor of the International Journal of Dermatology in 2020 disputed this and proved it was 0.56%) For 120 days, volunteers applied either 0.56% cysteamine or 4% HQ nightly. Tinted sunscreen (SPF 50; PPD 19) use was required for all participants. There were no differences in colorimetric evaluations between the groups, both of which showed progressive depigmenting, or in photographic assessments. The HQ group demonstrated greater mean decreases in modified melasma area severity index (mMASI) scores (41% for HQ and 24% for cysteamine at 60 days; 53% for HQ and 38% for cysteamine at 120 days). The investigators observed that while cysteamine was safe, well tolerated, and effective, it was outperformed by HQ in terms of mMASI and melasma quality of life (MELASQoL) scores.10
Early the next year, results of a randomized, double-blind, single-center study in 20 women, conducted by Nguyen et al. comparing the efficacy of cysteamine cream with HQ for melasma treatment were published. Participants were given either treatment over 16 weeks. Ultimately, five volunteers in the cysteamine group and nine in the HQ group completed the study. There was no statistically significant difference in mMASI scores between the groups. In this notably small study, HQ was tolerated better. The researchers concluded that their findings supported the argument of comparable efficacy between cysteamine and HQ, with further studies needed to establish whether cysteamine would be an appropriate alternative to HQ.11 Notably, HQ was banned by the Food and Drug Administration in 2020 in over-the-counter products.
Cysteamine vs. Kligman’s formula
Early in 2021, Karrabi et al. published the results of a randomized, double-blind clinical trial of 50 subjects with epidermal melasma to compare cysteamine 5% with Modified Kligman’s formula. Over 4 months, participants applied once daily either cysteamine cream 5% (15 minutes exposure) or the Modified Kligman’s formula (4% hydroquinone, 0.05% retinoic acid and 0.1% betamethasone) for whole night exposure. At 2 and 4 months, a statistically significant difference in mMASI score was noted, with the percentage decline in mMASI score nearly 9% higher in the cysteamine group. The investigators concluded that cysteamine 5% demonstrated greater efficacy than the Modified Kligman’s formula and was also better tolerated.12
Cysteamine vs. tranexamic acid
Later that year, Karrabi et al. published the results of a single-blind, randomized clinical trial assessing the efficacy of tranexamic acid mesotherapy compared with cysteamine 5% cream in 54 melasma patients. For 4 consecutive months, the cysteamine 5% cream group applied the cream on lesions 30 minutes before going to sleep. Every 4 weeks until 2 months, a physician performed tranexamic acid mesotherapy (0.05 mL; 4 mg/mL) on individuals in the tranexamic acid group. The researchers concluded, after measurements using both a Dermacatch device and the mMASI, that neither treatment was significantly better than the other but fewer complications were observed in the cysteamine group.13
Safety
In 2022, Sepaskhah et al. assessed the effects of a cysteamine 5% cream and compared it with HQ 4%/ascorbic acid 3% cream for epidermal melasma in a single-blind, randomized controlled trial. Sixty-five of 80 patients completed the study. The difference in mMASI scores after 4 months was not significant between the groups nor was the improvement in quality of life, but the melanin index was significantly lower in the HQ/ascorbic acid group compared with the less substantial reduction for the cysteamine group. Nevertheless, the researchers concluded that cysteamine is a safe and suitable substitute for HQ/ascorbic acid.4
Conclusion
In the last decade, cysteamine has been established as a potent depigmenting agent. Its suitability and desirability as a top consideration for melasma treatment also appears to be compelling. More RCTs comparing cysteamine and other topline therapies are warranted, but current evidence shows that cysteamine is an effective and safe therapy for melasma.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at dermnews@mdedge.com.
References
1. Konar MC et al. J Trop Pediatr. 2020 Apr 1;66(2):129-35.
2. Niazi S et al. J Cosmet Dermatol. 2022 Sep;21(9):3867-75.
3. González-Molina V et al. J Clin Aesthet Dermatol. 2022 May;15(5):19-28.
4. Sepaskhah M et al. J Cosmet Dermatol. 2022 Jul;21(7):2871-8.
5. Desai S et al. J Drugs Dermatol. 2021 Dec 1;20(12):1276-9.
6. Mansouri P et al. Br J Dermatol. 2015 Jul;173(1):209-17.
7. Farshi S et al. J Dermatolog Treat. 2018 Mar;29(2):182-9.
8. Austin E et al. J Drugs Dermatol. 2019 Nov 1;18(11):S1545961619P1156X.
9. Gomes dos Santos-Neto A et al. Dermatol Ther. 2022 Dec;35(12):e15961.
10. Lima PB et al. Int J Dermatol. 2020 Dec;59(12):1531-6.
11. Nguyen J et al. Australas J Dermatol. 2021 Feb;62(1):e41-e46.
12. Karrabi M et al. Skin Res Technol. 2021 Jan;27(1):24-31.
13. Karrabi M et al. Arch Dermatol Res. 2021 Sep;313(7):539-47.
Attitudes Toward Utilization of Minimally Invasive Cosmetic Procedures in Black Women: Results of a Cross-sectional Survey
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.
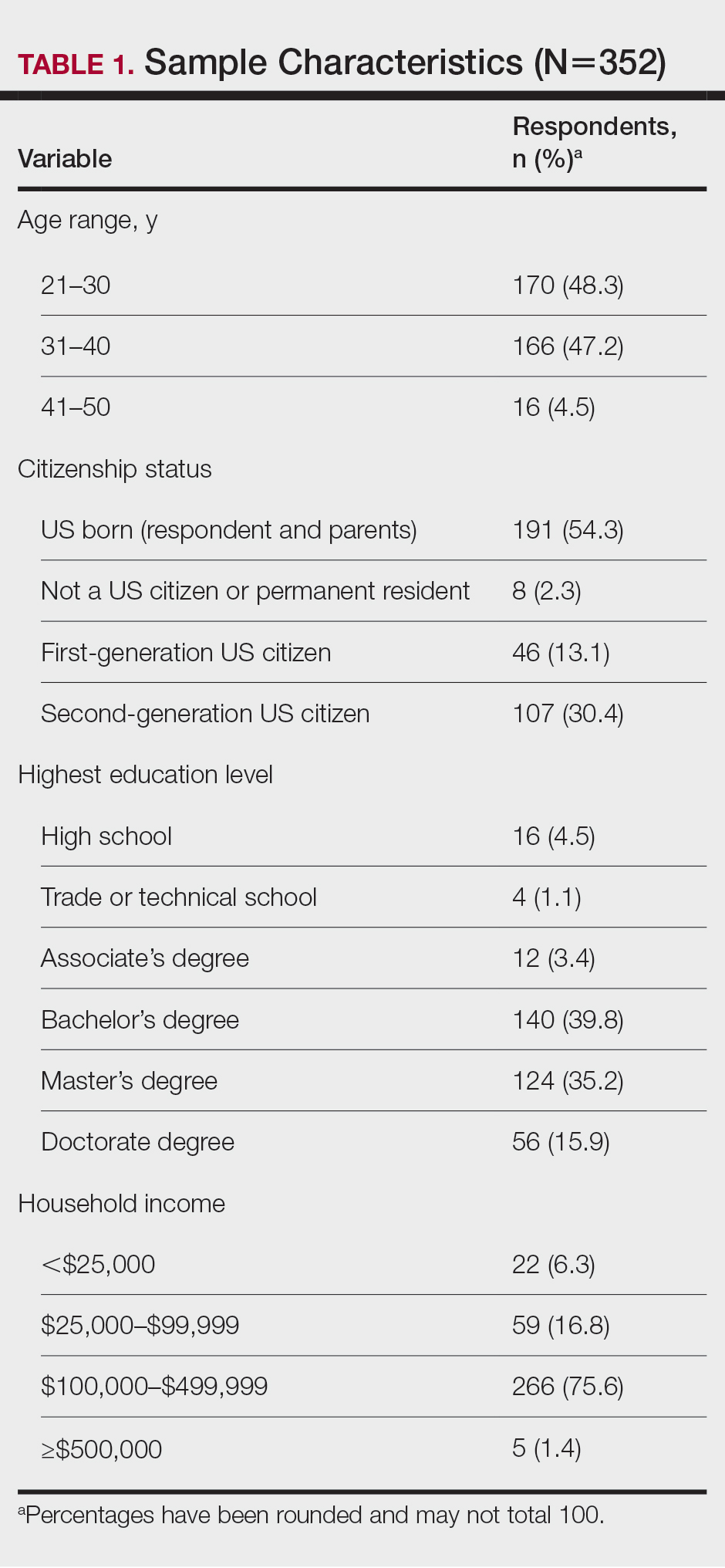
Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.
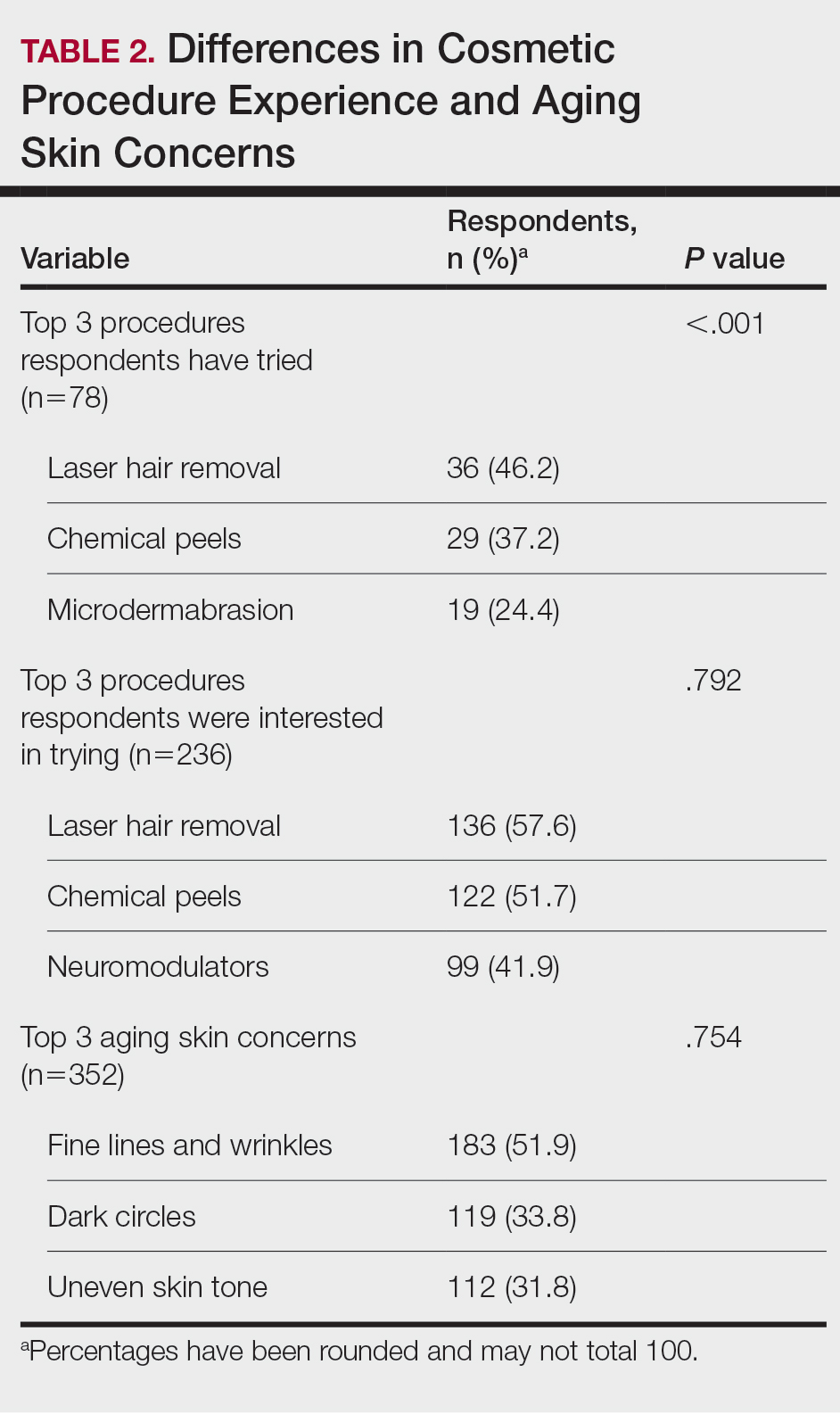
Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).
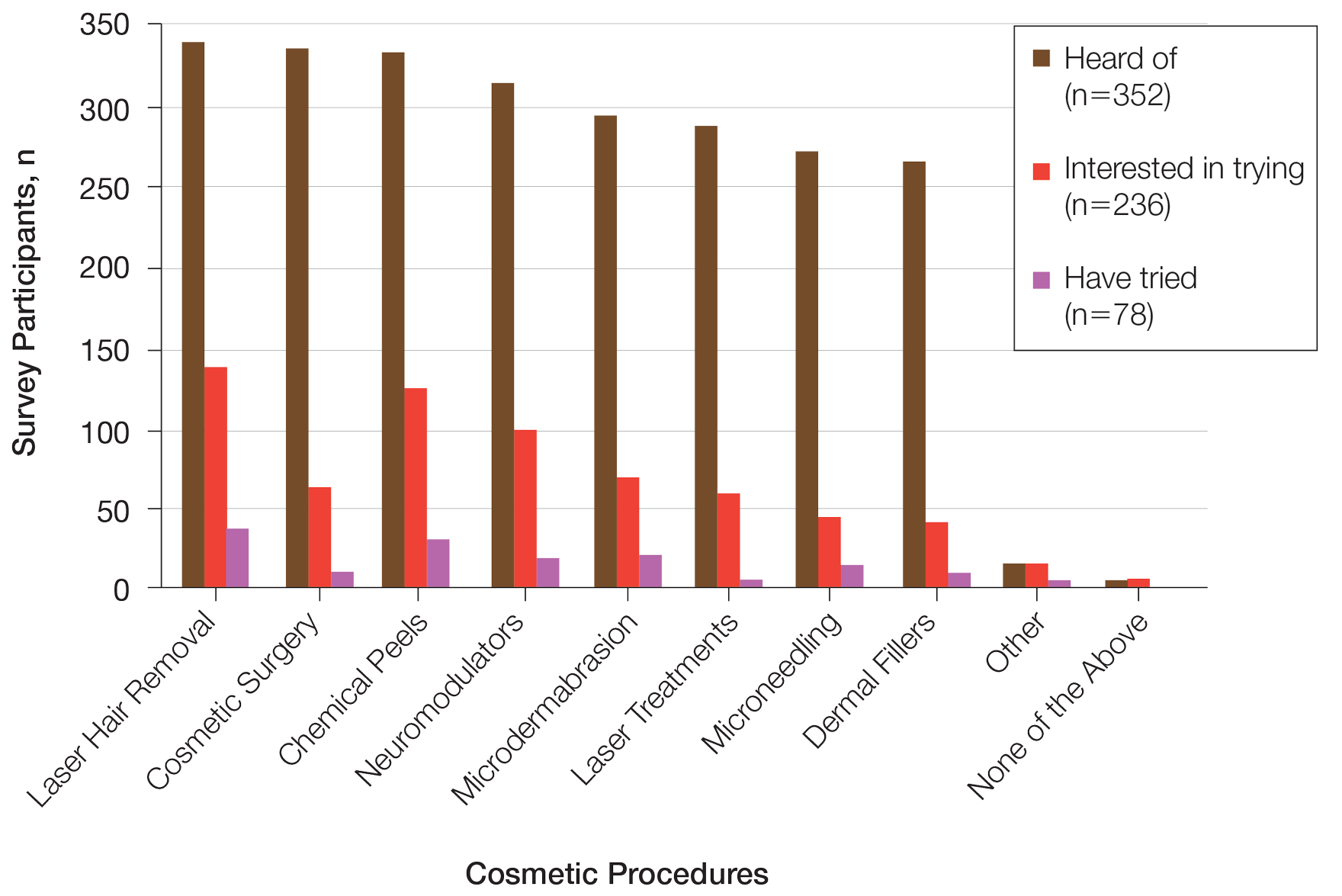
Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.

Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.

Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).

Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
Beauty has been a topic of interest for centuries. Treatments and technologies have advanced, and more women are utilizing cosmetic procedures than ever before, especially neuromodulators, minimally invasive procedures, and topical treatments.1 Over the last decade, there was a 99% increase in minimally invasive cosmetic procedures in the United States.2 There also has been an observable increase in the utilization of cosmetic procedures by Black patients in recent years; the American Society of Plastic Surgeons reported that the number of cosmetic plastic surgery procedures performed on “ethnic patients” (referring to Asian, Black, or Hispanic patients) increased 243% from 2000 to 2013,3 possibly attributed to increased accessibility, awareness of procedures due to social media, cultural acceptance, and affordability. Minimally invasive procedures are considerably less expensive than major surgical procedures and are becoming progressively more affordable, with numerous financing options available.2 Additionally, neuromodulators and fillers are now commonly administered by nonaesthetic health professionals including dentists and nurses, which has increased accessibility of these procedures among patients who typically may not seek out a consultation with a plastic surgeon or dermatologist.4
When examining the most common cosmetic procedures collectively sought out by patients with skin of color (SOC), it has been found that an even skin tone is a highly desirable feature that impacts the selection of products and procedures in this particular patient population.5 Black, Hispanic, and Asian women report fewer signs of facial aging compared to White women in the glabellar lines, crow’s-feet, oral commissures, perioral lines, and lips.6 Increased melanocytes in darker skin types help prevent photoaging but also increase susceptibility to dyschromia. Prior studies have reported the most common concerns by patients with SOC are dyschromic disorders such as postinflammatory hyperpigmentation, postinflammatory hypopigmentation, and melasma.7 Common minimally invasive cosmetic procedures utilized by the SOC population include chemical peels, laser treatments, and injectables. Fillers are utilized more for volume loss in SOC patients rather than for the deep furrows and rhytides commonly seen in the lower face of White patients.8
We conducted a survey among Black women currently residing in the United States to better understand attitudes toward beauty and aging as well as the utilization of minimally invasive cosmetic procedures in this patient population.
Methods
An in-depth questionnaire comprised of 17 questions was created for this cross-sectional observational study. The study was submitted to and deemed exempt by the institutional review board at the University of Miami (Miami, Florida)(IRB #20211184). Survey participants primarily were recruited via social media posts on personal profiles of Black dermatologists, medical residents, and medicalstudents, including the authors, targeting Black women in the United States. Utilizing a method called snowball sampling, whereby study participants are used to recruit future participants, individuals were instructed to share the survey with their social network to assist with survey distribution. After participants provided informed consent, data were captured using the REDCap secure online data collection software. The questionnaire was structured to include a sociodemographic profile of respondents, attitudes toward beauty and aging, current usage of beauty products, prior utilization of cosmetic procedures, and intentions to use cosmetic procedures in the future. Surveys with incomplete consent forms, incomplete responses, and duplicate responses, as well as surveys from participants who were not residing in the United States at the time of survey completion, were excluded.
Data characteristics were summarized by frequency and percentage. A χ2 test was performed to compare participants’ age demographics with their attitudes toward beauty and aging, utilization of cosmetic procedures, and intention to try cosmetic procedures in the future. The Fisher exact test was used instead of the χ2 test when the expected cell count was less than 5. For all tests, P<.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 28.
Results
General Characteristics of Participants—A sample of 475 self-identified Black women aged 21 to 70 years participated in the study, and 352 eligible participants were included in the final analysis. Of the 352 eligible participants, 48.3% were aged 21 to 30 years, 47.2% were aged 31 to 40 years, and 4.5% were aged 41 to 50 years. All survey participants identified their race as Black; among them, 4% specified Hispanic or Latino ethnicity, and 9% indicated that they held multiracial identities including White/Caucasian, Asian, and Native American backgrounds. Regarding the participants’ citizenship status, 54.3% reported that both they and their parents were born in the United States; 2.3% were not US citizens or permanent residents, 13.1% identified as first-generation Americans (born outside of the United States), and 30.4% identified as second-generation Americans (one or both parents born outside of the United States). Participant education levels (based on highest level) varied greatly: 4.5% were high school graduates, 1.1% attended trade or technical schools, 3.4% had associate’s degrees, 39.8% had bachelor’s degrees, 35.2% had master’s degrees, and 15.9% had doctorate degrees. Regarding household income, 6.3% earned less than $25,000 per year, 16.8% earned from $25,000 to $99,999, 75.6% earned from $100,000 to $499,999, and 1.4% earned $500,000 or more. Patient demographics are provided in Table 1.

Cosmetic Skin Concerns—The top 3 aging skin concerns among participants were fine lines and wrinkles (51.9%), dark circles (33.8%), and uneven skin tone (31.8%) (Table 2). Approximately 5.4% of participants reported no desire to avoid the natural aging process. Among age groups, fine lines and wrinkles were a major concern for 51.7% of 21- to 30-year-olds, 47.6% of 31- to 40-year-olds, and 43.5% of 41- to 50-year-olds. Dark circles were a major concern for 61.3% of 21- to 30-year-olds, 44.4% of 31- to 40-year-olds, and 46.8% of 41- to 50-year-olds. Uneven skin tone was a major concern for 56.2% of 21- to 30-year-olds, 46.5% of 31- to 40-year-olds, and 31.2% of 41- to 50-year-olds. There was no statistically significant association between participants’ age and their concern with aging skin concerns.

Differences in Experience and Acceptance of Cosmetic Procedures—Regarding participants’ prior experience with cosmetic procedures, 22.3% had tried 1 or more procedures. Additionally, 67.0% reported having intentions of trying cosmetic procedures in the future, while 10.8% reported no intentions. Of those who were uninterested in trying cosmetic procedures, 78.9% (30/38) believed it unnecessary while 47.3% (18/38) reported a fear of looking unnatural. Other perceived deterrents to cosmetic procedures among this subset of participants were the need to repeat treatment for lasting results (28.9% [11/38]), too expensive (31.6% [12/38]), and fear of side effects (39.5% [15/38]). A significant difference was found between participants’ age and their experience with cosmetic procedures (P=.020). Participants aged 21 to 30 years reported they were more likely to want to try cosmetic procedures in the future. Participants aged 31 to 40 years were more likely to have already tried a cosmetic procedure. Participants aged 41 to 50 years were more likely to report no desire to try cosmetic procedures in the future. There was no significant difference in cosmetic procedure acceptance according to citizenship status, education level, or household income.
Differences in Cosmetic Procedure Experience—Study participants indicated awareness of typically practiced cosmetic procedures. Of the 78 participants who have tried cosmetic procedures (Figure 1), the most common were laser hair removal (46.2% [36/78]), chemical peels (37.2% [29/78]), and microdermabrasion (24.4% [19/78])(Table 2). Age significantly influenced the type of cosmetic procedures utilized by participants (P<.001). Laser hair removal was the most common cosmetic procedure utilized by participants aged 21 to 30 years (64.7%) and chemical peels in participants aged 31 to 40 years (47.8%); participants aged 41 to 50 years reported equal use of chemical peels (50.0%) and microdermabrasion (50.0%).

Two hundred thirty-six participants reported interest in trying cosmetic procedures, specifically laser hair removal (57.6%), chemical peels (51.7%), and neuromodulators (41.9%)(Table 2). Although not statistically significant, age appeared to influence interest levels in cosmetic procedures. Participants aged 21 to 30 years and 31 to 40 years were most interested in trying laser hair removal (60.7% and 58.3%, respectively). Participants aged 41 to 50 years were most interested in trying neuromodulators (36.4%). There was no significant association between age and intention to try neuromodulators, chemical peels, or laser hair removal.
Attitudes Toward Beauty—Approximately 40.6% of participants believed that peak beauty occurs when women reach their 20s, and 38.6% believed that peak beauty occurs when women reach their 30s. Participants’ strategies for maintaining beauty were assessed through their regular use of certain skin care products. The most frequently used skin care products were face wash or cleanser (92.6%), moisturizer (90.1%), lip balm (76.1%), and facial sunscreen (62.2%). Other commonly used items were serum (34.7%), toner (34.9%), topical vitamin C (33.2%), and retinol/retinoid products (33.0%). Only 2.3% of participants reported not using any skin care products regularly.
Perceptions of Aging—Concerning perceived external age, most respondents believed they looked younger than their true age (69.9%); 24.4% believed they looked their true age, and 5.7% believed they looked older. Perception of age also varied considerably by age group, though most believed they looked younger than their true age.
Comment
This survey helped to identify trends in cosmetic procedure acceptance and utilization in Black women. As expected, younger Black women were more receptive to cosmetic procedures, which was consistent with a recent finding that cosmetic procedures tend to be more widely accepted among younger generations overall.8 Participants aged 21 to 30 years had greater intentions to try a cosmetic procedure, while those aged 31 to 40 years were more likely to have tried 1 or more cosmetic procedures already, which may be because they are just beginning to see the signs of aging and are motivated to address these concerns. Additionally, women in this age group may be more likely to have a stable source of income and be able to afford these procedures. It is important to note that the population surveyed had a much higher reported household income than the average Black household income, with most respondents reporting an average annual income of $100,000 to $499,000. Our data also showed a trend toward greater acceptance and utilization of cosmetic procedures in those with higher levels of income, though the results were not statistically significant.
Respondents were most concerned about fine lines and wrinkles, followed by dark circles and uneven skin tone. One report in the literature (N=2000) indicated that the most common cosmetic concerns in women with SOC were hyperpigmentation/dark spots (86%) and blotchy or uneven skin (80%).9 Interestingly, sunscreen was one of the more commonly used products in our survey, which historically has not been the case among individuals with SOC10 and suggests that the attitudes and perceptions of SOC patients are changing to favor more frequent sunscreen use, at least among the younger generations. Because we did not specify moisturizer vs moisturizer with sun protection factor, the use of facial sunscreen may even be underestimated in our survey.
Compared to cosmetic surgery or dermal fillers, the procedures found to be most frequently utilized in our study population—microdermabrasion, chemical peels, and laser hair removal—are less invasive and fairly accessible with minimal downtime. An interesting topic for further research would be to investigate how the willingness of women to openly share their cosmetic procedure usage has changed over time. The rise of social media and influencer culture has undoubtedly had an impact on the sharing of such information. It also would have been interesting to ask participants where they receive the majority of their health/beauty information.
All skin types are susceptible to photoaging; however, melanin is known to have a natural photoprotective effect, resulting in a lesser degree and later onset of photoaging in patients with darker vs lighter skin.11 It has been reported that individuals with SOC show signs of facial aging on average a decade later than those with lighter skin tones,12 which may be why the majority of participants believed they look younger than they truly are. As expected, dyspigmentation was among the top skin concerns in our study population. Although melanin does offer some degree of protection against UVA and UVB, melanocyte lability with inflammation may make darker skin types more susceptible to pigmentary issues.13
Study Limitations—The income levels of our study population were not representative of typical Black American households, which is a limitation. Seventy-seven percent of our study population earned more than $100,000 annually, while only 18% of Black American households earned more than $100,000 in 2019.14 Another major limitation of our study was the lack of representation from older generations, as most participants were aged 21 to 40 years, which was expected, as it is the younger generation who typically is targeted by a snowball sampling method primarily shared through social media. Additionally, because participants were recruited from the social media profiles of medical professionals, followers of these accounts may be more interested in cosmetic procedures, skewing the study results. Finally, because geographic location was not captured in our initial data collection, we were unable to determine if results from a particular location within the United States were overrepresented in the data set.
Conclusion
Although the discourse around beauty and antiaging is constantly evolving, data about Black women frequently are underrepresented in the literature. The results of this study highlight the changing attitudes and perceptions of Black women regarding beauty, aging, and minimally invasive cosmetic procedures. Dermatologists should stay abreast of current trends in this population to be able to make appropriate, culturally sensitive recommendations to their Black patients—for example, pointing them to sunscreen brands that are best suited for darker skin.
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
- Ahn CS, Suchonwanit P, Foy CG, et al. Hair and scalp care in African American women who exercise. JAMA Dermatol. 2016;152:579-580.
- Prendergast TI, Ong’uti SK, Ortega G, et al. Differential trends in racial preferences for cosmetic surgery procedures. Am Surg. 2011;77:1081-1085.
- American Society of Plastic Surgeons. Briefing paper: plastic surgeryfor ethnic patients. Accessed October 20, 2023. https://www.plasticsurgery.org/news/briefing-papers/briefing-paper-plastic-surgery-for-ethnic-patients
- Small K, Kelly KM, Spinelli HM. Are nurse injectors the new norm? Aesthetic Plast Surg. 2014;38:946-955.
- Quiñonez RL, Agbai ON, Burgess CM, et al. An update on cosmetic procedures in people of color. part 1: scientific background, assessment, preprocedure preparation. J Am Acad Dermatol. 2022;86:715-725.
- Alexis AF, Grimes P, Boyd C, et al. Racial and ethnic differences in self-assessed facial aging in women: results from a multinational study. Dermatol Surg. 2019;45:1635-1648.
- Talakoub L, Wesley NO. Differences in perceptions of beauty and cosmetic procedures performed in ethnic patients. Semin Cutan Med Surg. 2009;28:115-129.
- Alotaibi AS. Demographic and cultural differences in the acceptance and pursuit of cosmetic surgery: a systematic literature review. Plast Reconstr Surg Glob Open. 2021;9:E3501.
- Grimes PE. Skin and hair cosmetic issues in women of color. Dermatol Clin. 2000;18:659-665.
- Buchanan Lunsford N, Berktold J, Holman DM, et al. Skin cancer knowledge, awareness, beliefs and preventive behaviors among black and Hispanic men and women. Prev Med Rep. 2018;12:203-209.
- Alexis AF, Rossi, A. Photoaging in skin of color. Cosmet Dermatol. 2011;24:367-370.
- Vashi NA, de Castro Maymone MB, Kundu RV. Aging differences in ethnic skin. J Clin Aesthet Dermatol. 2016;9:31-38.
- Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
- Tamir C, Budiman A, Noe-Bustamante L, et al. Facts about the U.S. Black population. Pew Research Center website. Published March 2, 2023. Accessed October 20, 2023. https://www.pewresearch.org/social-trends/fact-sheet/facts-about-the-us-black-population/
Practice Points
- Cosmetic procedures may be more widely accepted among younger Black women than older Black women.
- Age has a considerable influence on the types of cosmetic procedures that Black women are interested in trying.
- Microdermabrasion, chemical peels, and laser hair removal were the most frequently utilized procedures in this study population.
- As attitudes and perceptions of young Black women are changing and favoring more frequent sunscreen use, dermatologists should remain on top of current trends to provide culturally sensitive and relevant recommendations to patients with darker skin tones.
FDA proposes ban on hair straightener ingredients
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
The
The proposal specifies that formaldehyde would be banned, as well as other chemicals that release formaldehyde, such as methylene glycol. Using hair smoothing products containing formaldehyde and formaldehyde-releasing chemicals “is linked to short-term adverse health effects, such as sensitization reactions and breathing problems, and long-term adverse health effects, including an increased risk of certain cancers,” the proposal states.
One study published last year showed that repeated use of hair straightening products, also called relaxers, could more than double the risk of uterine cancer. Although that study didn’t find that the uterine cancer risk varied based on a person’s race, the researchers noted that women who are Black are among the most likely to use the products and tend to start using them at younger ages, compared with people of other races and ethnicities.
Hair straightening products have also been linked to elevated risks of hormone-sensitive cancers, such as breast cancer and ovarian cancer.
Rep. Ayanna Pressley (D-Mass.) and Rep. Shontel Brown (D-Ohio) applauded the proposed rule in a statement issued jointly on Oct. 6. “The FDA’s proposal to ban these harmful chemicals in hair straighteners and relaxers is a win for public health – especially the health of Black women who are disproportionately put at risk by these products as a result of systemic racism and anti–Black hair sentiment,” Rep. Pressley said The two congresswomen wrote a letter to the FDA earlier this year requesting the topic be investigated.
“Regardless of how we wear our hair, we should be allowed to show up in the world without putting our health at risk. I applaud the FDA for being responsive to our calls and advancing a rule that will help prevent manufacturers from making a profit at the expense of our health,” Rep. Pressley said in the statement. “The administration should finalize this rule without delay.”
A version of this article appeared on WebMD.com
Treatment of the neck and lower face with botulinum toxin
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
.
The neck and the lower face are covered by thin layers of a vertical muscle, the anterior and posterior platysma muscle that is innervated by the cervical branch of the facial nerve. This muscle superficially blends with the muscles of the lower face, including the depressor anguli oris, depressor labii inferioris, mentalis, risorius, and orbicularis oris muscles. The inferior portion blends with the pectoralis and anterior deltoid muscles and lifts the skin of the neck.
Treatment of the platysma muscle and bands with botulinum toxin is an effective treatment for aging and sagging of the lower face and neck. Although treatment techniques differ and there are currently no standardized guidelines, the treatment starts by having the patient contract the neck muscles (I have them sit upright, with their head completely straight and say “E” with force). After evaluating the tension of the muscle, the muscle should be grasped and pulled away from the neck. Botulinum toxin is injected perpendicular to the muscle, with a dose of approximately 2 units, 2 cm apart along the vertical muscle. Approximately 20-40 units are used for the anterior and lateral bands.
To balance the opposing forces of the depressors of the lower face and improve jowling and downturning of the mouth, 10-20 units are also injected subdermally 1 cm above and 1 cm below the mandibular border.
Understanding the anatomy of the face and neck is crucial to proper injection. Side effects from improper injection include dysphagia, dysphonia, asymmetric smile, and weakness of the neck muscles. It is also important to set realistic expectations and address other components of neck aging, including actinic damage, as well as submental and jowl fat. The manufacturer of onabotulinumtoxinA (Botox Cosmetic) recently announced positive results of a second phase 3 clinical trial evaluating onabotulinumtoxinA for the treatment of moderate to severe platysma prominence. Results of the multicenter, randomized, double blind, placebo-controlled study evaluated the safety and efficacy of one treatment versus placebo in 426 adults with moderate to severe platysmal prominence. The results showed statistically significant improvement of platysma prominence from baseline, based on investigator and patient assessments, with no new safety signals, according to the company. The company expects to submit phase 3 data to the Food and Drug Administration by the end of this year and if approved, it will be the first neurotoxin approved for the treatment of platysmal bands.
Dr. Talakoub is in private practice in McLean, Va. Write to her at dermnews@mdedge.com. She had no relevant disclosures.
References
Brandt FS, Bellman B. Dermatol Surg. 1998 Nov;24(11):1232-4.
Matarasso A et al. Plast Reconstr Surg. 1999 Feb;103(2):645-52.
Rohrich RJ et al. Plast Reconstr Surg Glob Open. 2020 Jun 23;8(6):e2812.
A glimpse at devices designed to tackle cellulite
SAN DIEGO –
A noninvasive treatment, rapid acoustic pulse (RAP) technology (RESONIC), was cleared by the Food and Drug Administration in 2021 for short-term improvement in the appearance of cellulite. The device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or shear the fibrotic septa; release of the septa results in the smoothing of skin dimples, Arisa E. Ortiz, MD, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. RAP, however, was “taken off the market temporarily to refine the design.”
According to Dr. Ortiz, RAP’s repetition rate and short rise time provides microscopic mechanical disruption to the targeted cellular level structures and vacuoles, while high peak pressure and the fast repetition rate exploit the viscoelastic nature of tissue. Compressed pulses from electronic filtering and the reflector shape eliminate cavitation, heat, and pain. Researchers have postulated that the procedure stimulates collagenesis and angiogenesis.
“There’s no heat to this; it just uses sound,” Dr. Ortiz explained. “It’s also time dependent. The longer you do the treatment, the more disruption of the septa you see. The procedure takes 20-30 minutes. Unlike other treatments, it’s not just for discreet dimples. You can treat entire areas like the buttock or posterior leg,” said Dr. Ortiz, who did not use the RAP device in her practice.
Another device, targeted verifiable subcision (TVS, marketed as Avéli), is FDA cleared for temporary reduction in the appearance of cellulite in the buttock and thigh areas of adult women. “But studies show lasting results, at least through a year,” Dr. Ortiz said. The device features a light-guided probe and a hook. The light enables clinicians to navigate under the skin, while the hook releases a tiny blade that severs the septa. “Once you find the septa, then you activate the blade and release the septa. You go right to left because the direction of the blade is on the left of the probe. Then you go back to verify that you got everything that was creating that dimple.”
Previous devices, she said, would “blindly shear the area, so you would find the dimple and blindly cut, so there was no way to verify that you got the target dimple. The results were sometimes mediocre because you didn’t really know if you effectively treated the area.”
She emphasized that TVS is only useful for discreet dimples. “Many patients who come in asking for cellulite treatment have a lot of laxity and rippled texture,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. “This is not going to be appropriate for those cases. Setting expectations is important. If patients have laxity and discreet dimples and it’s just the dimples that bother them, that’s fine. They just need to understand the difference,” she said, noting that this is “safe for all skin types.”
Tumescent anesthesia is used to control pain during the procedure. The most common adverse events are bruising and soreness. Results from a pivotal trial showed that clinically significant improvements in the primary endpoint, Cellulite Severity Scale scores, were sustained 1 year after treatment. “Hemosiderin staining can occur, but it eventually dissipates on its own,” Dr. Ortiz added. “You can use laser to speed up healing but sometimes that can make it worse, so you want to be careful with that. Most of the time I have patients wait it out; it does go away on its own.”
She noted that the development of RAP and TVS have helped clinicians better understand the makeup of septa. “We used to think of septa as singular bands that are vertically oriented in cellulite, but what we’ve realized is that it’s more like a network of septa,” she said.
Another noninvasive technology, synchronous parallel ultrasound beam technology from Sofwave (marketed as SUPERB), was FDA cleared in December 2022 for the short-term improvement in the appearance of cellulite. The device has seven parallel beam transducers that increase tissue temperatures of the treatment area to 60-70° C, inducing collagen remodeling and collagen denaturation, she said.
In the pivotal study of 68 women, two blinded reviewers reported an 89% improvement rate for both cellulite and skin laxity, after two treatments 2-4 weeks apart, according to data she presented at the meeting. The mean pain score during treatment was 4.55 on a scale of 1-10. No safety issues were observed and immediate responses were limited to erythema and edema.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies, including receipt of speaker fees and honoraria from Sofwave. She is also cochair of MOAS.
SAN DIEGO –
A noninvasive treatment, rapid acoustic pulse (RAP) technology (RESONIC), was cleared by the Food and Drug Administration in 2021 for short-term improvement in the appearance of cellulite. The device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or shear the fibrotic septa; release of the septa results in the smoothing of skin dimples, Arisa E. Ortiz, MD, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. RAP, however, was “taken off the market temporarily to refine the design.”
According to Dr. Ortiz, RAP’s repetition rate and short rise time provides microscopic mechanical disruption to the targeted cellular level structures and vacuoles, while high peak pressure and the fast repetition rate exploit the viscoelastic nature of tissue. Compressed pulses from electronic filtering and the reflector shape eliminate cavitation, heat, and pain. Researchers have postulated that the procedure stimulates collagenesis and angiogenesis.
“There’s no heat to this; it just uses sound,” Dr. Ortiz explained. “It’s also time dependent. The longer you do the treatment, the more disruption of the septa you see. The procedure takes 20-30 minutes. Unlike other treatments, it’s not just for discreet dimples. You can treat entire areas like the buttock or posterior leg,” said Dr. Ortiz, who did not use the RAP device in her practice.
Another device, targeted verifiable subcision (TVS, marketed as Avéli), is FDA cleared for temporary reduction in the appearance of cellulite in the buttock and thigh areas of adult women. “But studies show lasting results, at least through a year,” Dr. Ortiz said. The device features a light-guided probe and a hook. The light enables clinicians to navigate under the skin, while the hook releases a tiny blade that severs the septa. “Once you find the septa, then you activate the blade and release the septa. You go right to left because the direction of the blade is on the left of the probe. Then you go back to verify that you got everything that was creating that dimple.”
Previous devices, she said, would “blindly shear the area, so you would find the dimple and blindly cut, so there was no way to verify that you got the target dimple. The results were sometimes mediocre because you didn’t really know if you effectively treated the area.”
She emphasized that TVS is only useful for discreet dimples. “Many patients who come in asking for cellulite treatment have a lot of laxity and rippled texture,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. “This is not going to be appropriate for those cases. Setting expectations is important. If patients have laxity and discreet dimples and it’s just the dimples that bother them, that’s fine. They just need to understand the difference,” she said, noting that this is “safe for all skin types.”
Tumescent anesthesia is used to control pain during the procedure. The most common adverse events are bruising and soreness. Results from a pivotal trial showed that clinically significant improvements in the primary endpoint, Cellulite Severity Scale scores, were sustained 1 year after treatment. “Hemosiderin staining can occur, but it eventually dissipates on its own,” Dr. Ortiz added. “You can use laser to speed up healing but sometimes that can make it worse, so you want to be careful with that. Most of the time I have patients wait it out; it does go away on its own.”
She noted that the development of RAP and TVS have helped clinicians better understand the makeup of septa. “We used to think of septa as singular bands that are vertically oriented in cellulite, but what we’ve realized is that it’s more like a network of septa,” she said.
Another noninvasive technology, synchronous parallel ultrasound beam technology from Sofwave (marketed as SUPERB), was FDA cleared in December 2022 for the short-term improvement in the appearance of cellulite. The device has seven parallel beam transducers that increase tissue temperatures of the treatment area to 60-70° C, inducing collagen remodeling and collagen denaturation, she said.
In the pivotal study of 68 women, two blinded reviewers reported an 89% improvement rate for both cellulite and skin laxity, after two treatments 2-4 weeks apart, according to data she presented at the meeting. The mean pain score during treatment was 4.55 on a scale of 1-10. No safety issues were observed and immediate responses were limited to erythema and edema.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies, including receipt of speaker fees and honoraria from Sofwave. She is also cochair of MOAS.
SAN DIEGO –
A noninvasive treatment, rapid acoustic pulse (RAP) technology (RESONIC), was cleared by the Food and Drug Administration in 2021 for short-term improvement in the appearance of cellulite. The device emits rapid acoustic pulses (shock waves) that are transmitted through the skin to rupture or shear the fibrotic septa; release of the septa results in the smoothing of skin dimples, Arisa E. Ortiz, MD, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. RAP, however, was “taken off the market temporarily to refine the design.”
According to Dr. Ortiz, RAP’s repetition rate and short rise time provides microscopic mechanical disruption to the targeted cellular level structures and vacuoles, while high peak pressure and the fast repetition rate exploit the viscoelastic nature of tissue. Compressed pulses from electronic filtering and the reflector shape eliminate cavitation, heat, and pain. Researchers have postulated that the procedure stimulates collagenesis and angiogenesis.
“There’s no heat to this; it just uses sound,” Dr. Ortiz explained. “It’s also time dependent. The longer you do the treatment, the more disruption of the septa you see. The procedure takes 20-30 minutes. Unlike other treatments, it’s not just for discreet dimples. You can treat entire areas like the buttock or posterior leg,” said Dr. Ortiz, who did not use the RAP device in her practice.
Another device, targeted verifiable subcision (TVS, marketed as Avéli), is FDA cleared for temporary reduction in the appearance of cellulite in the buttock and thigh areas of adult women. “But studies show lasting results, at least through a year,” Dr. Ortiz said. The device features a light-guided probe and a hook. The light enables clinicians to navigate under the skin, while the hook releases a tiny blade that severs the septa. “Once you find the septa, then you activate the blade and release the septa. You go right to left because the direction of the blade is on the left of the probe. Then you go back to verify that you got everything that was creating that dimple.”
Previous devices, she said, would “blindly shear the area, so you would find the dimple and blindly cut, so there was no way to verify that you got the target dimple. The results were sometimes mediocre because you didn’t really know if you effectively treated the area.”
She emphasized that TVS is only useful for discreet dimples. “Many patients who come in asking for cellulite treatment have a lot of laxity and rippled texture,” said Dr. Ortiz, who is also president-elect of the American Society for Laser Medicine and Surgery. “This is not going to be appropriate for those cases. Setting expectations is important. If patients have laxity and discreet dimples and it’s just the dimples that bother them, that’s fine. They just need to understand the difference,” she said, noting that this is “safe for all skin types.”
Tumescent anesthesia is used to control pain during the procedure. The most common adverse events are bruising and soreness. Results from a pivotal trial showed that clinically significant improvements in the primary endpoint, Cellulite Severity Scale scores, were sustained 1 year after treatment. “Hemosiderin staining can occur, but it eventually dissipates on its own,” Dr. Ortiz added. “You can use laser to speed up healing but sometimes that can make it worse, so you want to be careful with that. Most of the time I have patients wait it out; it does go away on its own.”
She noted that the development of RAP and TVS have helped clinicians better understand the makeup of septa. “We used to think of septa as singular bands that are vertically oriented in cellulite, but what we’ve realized is that it’s more like a network of septa,” she said.
Another noninvasive technology, synchronous parallel ultrasound beam technology from Sofwave (marketed as SUPERB), was FDA cleared in December 2022 for the short-term improvement in the appearance of cellulite. The device has seven parallel beam transducers that increase tissue temperatures of the treatment area to 60-70° C, inducing collagen remodeling and collagen denaturation, she said.
In the pivotal study of 68 women, two blinded reviewers reported an 89% improvement rate for both cellulite and skin laxity, after two treatments 2-4 weeks apart, according to data she presented at the meeting. The mean pain score during treatment was 4.55 on a scale of 1-10. No safety issues were observed and immediate responses were limited to erythema and edema.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies, including receipt of speaker fees and honoraria from Sofwave. She is also cochair of MOAS.
AT MOAS 2023