User login
‘Dr. Pimple Popper’ offers tips for building a social media presence
SAN DIEGO – In the fall of 2014, Sandra Lee, MD, posted a blackhead extraction video on her Instagram account, a decision that changed her professional life forever.
“I got these crazy comments,” Dr. Lee, a dermatologist who practices in Upland, Calif., recalled at the annual Masters of Aesthetics Symposium. “Either people loved it – they were obsessed – or they thought it was the most disgusting thing they’d ever seen. It created a strong reaction. Either way, they shared it with their friends.”
Soon after she started posting videos, she discovered Reddit, which has a subreddit for “popping addicts” and the “pop-curious.” “I thought, ‘These videos are so amateur. They’re culling them from the Internet. Or, they’re pinning down their son at the beach and trying to squeeze out a blackhead,’ ” Dr. Lee said. “I thought, ‘I could give them pristine videos,’ ” and that is exactly what she did.
Turning to YouTube as a platform, she began to post videos showing everything from Mohs surgery and Botox injections to keloid removals and ear lobe repair surgeries. With this, . She also grew 16.2 million subscribers on TikTok, 4.5 million followers on Instagram, 2.9 million on Facebook, and 136,700 on Twitter.
About 80% of her followers are women who range between 18 and 40 years of age. “I have over 5 billion views on YouTube, which is mind-blowing,” she said. “That tells you something about the content. It’s not something people watch once. They watch it over and over again.” These include videos compiled as a “bedtime story.”
Dr. Lee offered the following pearls of advice for dermatologists looking to build and maintain a presence on social media:
Use it to showcase what makes you unique. Post what you do on social media, and people will find you. “It’s an opportunity to freely advertise,” Dr. Lee said. “I’m super nitpicky about posting good before-and-after photos. You can also show off how nice and warm and inviting your office is. People come to see me because they know my voice. They know how I interact with patients. That is reason for them enough to travel from far away to see me. It doesn’t mean that I’m the person who is best at treating whatever condition they have.”
Make it interesting. “I say that the special sauce is entertainment and education,” said Dr. Lee, who is in the fifth season of “Dr. Pimple Popper,” her TV show that airs internationally. “The only way you can draw people in is by entertaining them, catching their interest. But I try to trick them into educating them. Five-year-old kids come up to me now and know what a lipoma is. I’m proud of that.”
Be authentic. You may be using social media to promote your dermatology practice, but it’s important for followers to get a glimpse of your nonwork personality as well. Maybe that means posting a photo of yourself at a concert, baseball game, or dinner with family and friends. “Show that you have a sense of humor, because you want them to like you,” Dr. Lee added. “That’s why someone follows you, because they want to be your friend. They enjoy spending time with you on the Internet. It’s like gambling. In order to win, you have to play. So, you have to post.”
Avoid hot-button topics. “I don’t post about my kids, and I try to choose sponsorships wisely,” she said. “I do very few branding deals. Be careful about your brand and how you present yourself. Present yourself in an authentic way, but not in a way that hurts yourself or the dermatology profession.”
Be mindful of the time investment. “It’s like running a whole other business,” Dr. Lee said. “There are also trolls out there, so you have to have thick skin.”
Don’t sweat it if you don’t want to engage. “Not everybody wants to do it, and not everybody will be good at it, but that’s okay,” she said.
Dr. Lee reported having no relevant disclosures.
SAN DIEGO – In the fall of 2014, Sandra Lee, MD, posted a blackhead extraction video on her Instagram account, a decision that changed her professional life forever.
“I got these crazy comments,” Dr. Lee, a dermatologist who practices in Upland, Calif., recalled at the annual Masters of Aesthetics Symposium. “Either people loved it – they were obsessed – or they thought it was the most disgusting thing they’d ever seen. It created a strong reaction. Either way, they shared it with their friends.”
Soon after she started posting videos, she discovered Reddit, which has a subreddit for “popping addicts” and the “pop-curious.” “I thought, ‘These videos are so amateur. They’re culling them from the Internet. Or, they’re pinning down their son at the beach and trying to squeeze out a blackhead,’ ” Dr. Lee said. “I thought, ‘I could give them pristine videos,’ ” and that is exactly what she did.
Turning to YouTube as a platform, she began to post videos showing everything from Mohs surgery and Botox injections to keloid removals and ear lobe repair surgeries. With this, . She also grew 16.2 million subscribers on TikTok, 4.5 million followers on Instagram, 2.9 million on Facebook, and 136,700 on Twitter.
About 80% of her followers are women who range between 18 and 40 years of age. “I have over 5 billion views on YouTube, which is mind-blowing,” she said. “That tells you something about the content. It’s not something people watch once. They watch it over and over again.” These include videos compiled as a “bedtime story.”
Dr. Lee offered the following pearls of advice for dermatologists looking to build and maintain a presence on social media:
Use it to showcase what makes you unique. Post what you do on social media, and people will find you. “It’s an opportunity to freely advertise,” Dr. Lee said. “I’m super nitpicky about posting good before-and-after photos. You can also show off how nice and warm and inviting your office is. People come to see me because they know my voice. They know how I interact with patients. That is reason for them enough to travel from far away to see me. It doesn’t mean that I’m the person who is best at treating whatever condition they have.”
Make it interesting. “I say that the special sauce is entertainment and education,” said Dr. Lee, who is in the fifth season of “Dr. Pimple Popper,” her TV show that airs internationally. “The only way you can draw people in is by entertaining them, catching their interest. But I try to trick them into educating them. Five-year-old kids come up to me now and know what a lipoma is. I’m proud of that.”
Be authentic. You may be using social media to promote your dermatology practice, but it’s important for followers to get a glimpse of your nonwork personality as well. Maybe that means posting a photo of yourself at a concert, baseball game, or dinner with family and friends. “Show that you have a sense of humor, because you want them to like you,” Dr. Lee added. “That’s why someone follows you, because they want to be your friend. They enjoy spending time with you on the Internet. It’s like gambling. In order to win, you have to play. So, you have to post.”
Avoid hot-button topics. “I don’t post about my kids, and I try to choose sponsorships wisely,” she said. “I do very few branding deals. Be careful about your brand and how you present yourself. Present yourself in an authentic way, but not in a way that hurts yourself or the dermatology profession.”
Be mindful of the time investment. “It’s like running a whole other business,” Dr. Lee said. “There are also trolls out there, so you have to have thick skin.”
Don’t sweat it if you don’t want to engage. “Not everybody wants to do it, and not everybody will be good at it, but that’s okay,” she said.
Dr. Lee reported having no relevant disclosures.
SAN DIEGO – In the fall of 2014, Sandra Lee, MD, posted a blackhead extraction video on her Instagram account, a decision that changed her professional life forever.
“I got these crazy comments,” Dr. Lee, a dermatologist who practices in Upland, Calif., recalled at the annual Masters of Aesthetics Symposium. “Either people loved it – they were obsessed – or they thought it was the most disgusting thing they’d ever seen. It created a strong reaction. Either way, they shared it with their friends.”
Soon after she started posting videos, she discovered Reddit, which has a subreddit for “popping addicts” and the “pop-curious.” “I thought, ‘These videos are so amateur. They’re culling them from the Internet. Or, they’re pinning down their son at the beach and trying to squeeze out a blackhead,’ ” Dr. Lee said. “I thought, ‘I could give them pristine videos,’ ” and that is exactly what she did.
Turning to YouTube as a platform, she began to post videos showing everything from Mohs surgery and Botox injections to keloid removals and ear lobe repair surgeries. With this, . She also grew 16.2 million subscribers on TikTok, 4.5 million followers on Instagram, 2.9 million on Facebook, and 136,700 on Twitter.
About 80% of her followers are women who range between 18 and 40 years of age. “I have over 5 billion views on YouTube, which is mind-blowing,” she said. “That tells you something about the content. It’s not something people watch once. They watch it over and over again.” These include videos compiled as a “bedtime story.”
Dr. Lee offered the following pearls of advice for dermatologists looking to build and maintain a presence on social media:
Use it to showcase what makes you unique. Post what you do on social media, and people will find you. “It’s an opportunity to freely advertise,” Dr. Lee said. “I’m super nitpicky about posting good before-and-after photos. You can also show off how nice and warm and inviting your office is. People come to see me because they know my voice. They know how I interact with patients. That is reason for them enough to travel from far away to see me. It doesn’t mean that I’m the person who is best at treating whatever condition they have.”
Make it interesting. “I say that the special sauce is entertainment and education,” said Dr. Lee, who is in the fifth season of “Dr. Pimple Popper,” her TV show that airs internationally. “The only way you can draw people in is by entertaining them, catching their interest. But I try to trick them into educating them. Five-year-old kids come up to me now and know what a lipoma is. I’m proud of that.”
Be authentic. You may be using social media to promote your dermatology practice, but it’s important for followers to get a glimpse of your nonwork personality as well. Maybe that means posting a photo of yourself at a concert, baseball game, or dinner with family and friends. “Show that you have a sense of humor, because you want them to like you,” Dr. Lee added. “That’s why someone follows you, because they want to be your friend. They enjoy spending time with you on the Internet. It’s like gambling. In order to win, you have to play. So, you have to post.”
Avoid hot-button topics. “I don’t post about my kids, and I try to choose sponsorships wisely,” she said. “I do very few branding deals. Be careful about your brand and how you present yourself. Present yourself in an authentic way, but not in a way that hurts yourself or the dermatology profession.”
Be mindful of the time investment. “It’s like running a whole other business,” Dr. Lee said. “There are also trolls out there, so you have to have thick skin.”
Don’t sweat it if you don’t want to engage. “Not everybody wants to do it, and not everybody will be good at it, but that’s okay,” she said.
Dr. Lee reported having no relevant disclosures.
AT MOAS 2022
Experts dispel incorrect dogmas in aesthetic medicine
At least once a week,
Those images may help Dr. Stankiewicz understand patient preferences in terms of lip size and proportion, but she points out that shape is unique to each person. “I tell them: ‘All we can do is enhance that lip shape with filler. We can’t give you somebody else’s lip shape with an injection of filler.’ ”
During a virtual course on laser and aesthetic skin therapy, she and Omar A. Ibrahimi, MD, PhD, dispelled this and other false dogmas that they hear from some clinicians who practice aesthetic medicine and the patients who see them.
Wait 1 year before treating traumatic and surgical scars with vascular and fractional CO2 lasers. “I don’t think this is controversial anymore, because there is a boatload of data, which has shown that early treatment can prevent hypertrophic scarring and promote scar maturation,” said Dr. Stankiewicz, who practices dermatology in Park City, Utah. “Histology has also shown more organized dermal collagen from early treatment. Of course, there will be situations where you may want to hold off, like doing an ablative fractional [laser treatment] over the scar of a joint replacement ... where you may risk infection.” In her clinic, she routinely treats scars on the same day as suture removal, “as long as the healing looks appropriate.”
Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, Stamford, also jumps on treating scars early. For a patient with postacne erythema, for example, he will use a pulsed-dye laser, which he believes will prevent scars from becoming atrophic.
Used equipment is a better investment than new equipment. While purchasing used laser and light devices can save money, especially when starting out, be wary of potential pitfalls, including the fact that many devices have disposable tips. “If your laser isn’t certified or you’re not the authorized owner of the device, you won’t be able to buy the disposables,” Dr. Stankiewicz noted. “So, before you buy a used device, ensure that you can buy them.”
Also, consider the cost of service if the device breaks down, she advised. Some lasers are complicated to service and others have codes set by the manufacturer so that only contracted engineers can work on them. “Otherwise, third-party engineers and service providers have to figure out how to crack the code to get into the machine,” she said. “If you’re in the situation where you have to ask the manufacturer to service your device, you have to pay a lot of money to recertify your device. Then you’ve lost all the savings you thought you made by buying a used machine.” She prefers to negotiate a good deal on a new device. “Often, a very good deal on a new device can rival the offer of a used one.”
Dr. Ibrahimi recalled buying a used fractional laser that came with a 30-day guarantee, but it stopped working around day 45. “I didn’t have much recourse there,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “You can’t go back to the company [for repair] unless you pay a recertification fee.”
Avoid exercise after Botox treatment. Although inverted yoga poses and lying down should be avoided for several hours after receiving Botox, there are no other limits to other forms of exercise post treatment, Dr. Stankiewicz said. If she suspects that a patient will develop bruising on one or more injection sites, she treats the areas with a laser. “Doing this on the same day as Botox treatment doesn’t always stop or treat bruising, many times it does.”
Another myth she hears is that it is not safe to fly in an airplane after Botox treatment. “That recommendation comes from the fact that the atmospheric pressure is lower in an airplane, so we worry about the risk of Botox spread,” Dr. Stankiewicz said. “But I practice at 7,000 feet above sea level, which is the same atmospheric pressure as that in an airplane,” she added, noting Botox is administered throughout the day in her practice and she does not see increased complications or worry about spread.
Clinician self-treatment is okay. In the opinion of Dr. Stankiewicz, aesthetic clinicians who treat themselves “have a fool for a patient.” She added: “Although no one is going to blame you and may not even know if you give yourself a little Botox touch-up at home, glorifying self-treatment on social media must stop. It’s dangerous and it can be ineffective.”
Self-treatment can also impair judgment and the objectivity of cosmetic therapies. “Also, when you’re pointing a laser at your own face and posting it on social media, it gives viewers the impression that this is not a serious medical treatment when it really is,” she emphasized. In addition, “when you treat yourself, you lose the ability to see the proper clinical endpoint. You also lose the ability to see the angle and the appropriate position for injection to avoid intervascular occlusion.”
Neither Dr. Stankiewicz nor Dr. Ibrahimi reported having relevant financial disclosures.
At least once a week,
Those images may help Dr. Stankiewicz understand patient preferences in terms of lip size and proportion, but she points out that shape is unique to each person. “I tell them: ‘All we can do is enhance that lip shape with filler. We can’t give you somebody else’s lip shape with an injection of filler.’ ”
During a virtual course on laser and aesthetic skin therapy, she and Omar A. Ibrahimi, MD, PhD, dispelled this and other false dogmas that they hear from some clinicians who practice aesthetic medicine and the patients who see them.
Wait 1 year before treating traumatic and surgical scars with vascular and fractional CO2 lasers. “I don’t think this is controversial anymore, because there is a boatload of data, which has shown that early treatment can prevent hypertrophic scarring and promote scar maturation,” said Dr. Stankiewicz, who practices dermatology in Park City, Utah. “Histology has also shown more organized dermal collagen from early treatment. Of course, there will be situations where you may want to hold off, like doing an ablative fractional [laser treatment] over the scar of a joint replacement ... where you may risk infection.” In her clinic, she routinely treats scars on the same day as suture removal, “as long as the healing looks appropriate.”
Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, Stamford, also jumps on treating scars early. For a patient with postacne erythema, for example, he will use a pulsed-dye laser, which he believes will prevent scars from becoming atrophic.
Used equipment is a better investment than new equipment. While purchasing used laser and light devices can save money, especially when starting out, be wary of potential pitfalls, including the fact that many devices have disposable tips. “If your laser isn’t certified or you’re not the authorized owner of the device, you won’t be able to buy the disposables,” Dr. Stankiewicz noted. “So, before you buy a used device, ensure that you can buy them.”
Also, consider the cost of service if the device breaks down, she advised. Some lasers are complicated to service and others have codes set by the manufacturer so that only contracted engineers can work on them. “Otherwise, third-party engineers and service providers have to figure out how to crack the code to get into the machine,” she said. “If you’re in the situation where you have to ask the manufacturer to service your device, you have to pay a lot of money to recertify your device. Then you’ve lost all the savings you thought you made by buying a used machine.” She prefers to negotiate a good deal on a new device. “Often, a very good deal on a new device can rival the offer of a used one.”
Dr. Ibrahimi recalled buying a used fractional laser that came with a 30-day guarantee, but it stopped working around day 45. “I didn’t have much recourse there,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “You can’t go back to the company [for repair] unless you pay a recertification fee.”
Avoid exercise after Botox treatment. Although inverted yoga poses and lying down should be avoided for several hours after receiving Botox, there are no other limits to other forms of exercise post treatment, Dr. Stankiewicz said. If she suspects that a patient will develop bruising on one or more injection sites, she treats the areas with a laser. “Doing this on the same day as Botox treatment doesn’t always stop or treat bruising, many times it does.”
Another myth she hears is that it is not safe to fly in an airplane after Botox treatment. “That recommendation comes from the fact that the atmospheric pressure is lower in an airplane, so we worry about the risk of Botox spread,” Dr. Stankiewicz said. “But I practice at 7,000 feet above sea level, which is the same atmospheric pressure as that in an airplane,” she added, noting Botox is administered throughout the day in her practice and she does not see increased complications or worry about spread.
Clinician self-treatment is okay. In the opinion of Dr. Stankiewicz, aesthetic clinicians who treat themselves “have a fool for a patient.” She added: “Although no one is going to blame you and may not even know if you give yourself a little Botox touch-up at home, glorifying self-treatment on social media must stop. It’s dangerous and it can be ineffective.”
Self-treatment can also impair judgment and the objectivity of cosmetic therapies. “Also, when you’re pointing a laser at your own face and posting it on social media, it gives viewers the impression that this is not a serious medical treatment when it really is,” she emphasized. In addition, “when you treat yourself, you lose the ability to see the proper clinical endpoint. You also lose the ability to see the angle and the appropriate position for injection to avoid intervascular occlusion.”
Neither Dr. Stankiewicz nor Dr. Ibrahimi reported having relevant financial disclosures.
At least once a week,
Those images may help Dr. Stankiewicz understand patient preferences in terms of lip size and proportion, but she points out that shape is unique to each person. “I tell them: ‘All we can do is enhance that lip shape with filler. We can’t give you somebody else’s lip shape with an injection of filler.’ ”
During a virtual course on laser and aesthetic skin therapy, she and Omar A. Ibrahimi, MD, PhD, dispelled this and other false dogmas that they hear from some clinicians who practice aesthetic medicine and the patients who see them.
Wait 1 year before treating traumatic and surgical scars with vascular and fractional CO2 lasers. “I don’t think this is controversial anymore, because there is a boatload of data, which has shown that early treatment can prevent hypertrophic scarring and promote scar maturation,” said Dr. Stankiewicz, who practices dermatology in Park City, Utah. “Histology has also shown more organized dermal collagen from early treatment. Of course, there will be situations where you may want to hold off, like doing an ablative fractional [laser treatment] over the scar of a joint replacement ... where you may risk infection.” In her clinic, she routinely treats scars on the same day as suture removal, “as long as the healing looks appropriate.”
Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, Stamford, also jumps on treating scars early. For a patient with postacne erythema, for example, he will use a pulsed-dye laser, which he believes will prevent scars from becoming atrophic.
Used equipment is a better investment than new equipment. While purchasing used laser and light devices can save money, especially when starting out, be wary of potential pitfalls, including the fact that many devices have disposable tips. “If your laser isn’t certified or you’re not the authorized owner of the device, you won’t be able to buy the disposables,” Dr. Stankiewicz noted. “So, before you buy a used device, ensure that you can buy them.”
Also, consider the cost of service if the device breaks down, she advised. Some lasers are complicated to service and others have codes set by the manufacturer so that only contracted engineers can work on them. “Otherwise, third-party engineers and service providers have to figure out how to crack the code to get into the machine,” she said. “If you’re in the situation where you have to ask the manufacturer to service your device, you have to pay a lot of money to recertify your device. Then you’ve lost all the savings you thought you made by buying a used machine.” She prefers to negotiate a good deal on a new device. “Often, a very good deal on a new device can rival the offer of a used one.”
Dr. Ibrahimi recalled buying a used fractional laser that came with a 30-day guarantee, but it stopped working around day 45. “I didn’t have much recourse there,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “You can’t go back to the company [for repair] unless you pay a recertification fee.”
Avoid exercise after Botox treatment. Although inverted yoga poses and lying down should be avoided for several hours after receiving Botox, there are no other limits to other forms of exercise post treatment, Dr. Stankiewicz said. If she suspects that a patient will develop bruising on one or more injection sites, she treats the areas with a laser. “Doing this on the same day as Botox treatment doesn’t always stop or treat bruising, many times it does.”
Another myth she hears is that it is not safe to fly in an airplane after Botox treatment. “That recommendation comes from the fact that the atmospheric pressure is lower in an airplane, so we worry about the risk of Botox spread,” Dr. Stankiewicz said. “But I practice at 7,000 feet above sea level, which is the same atmospheric pressure as that in an airplane,” she added, noting Botox is administered throughout the day in her practice and she does not see increased complications or worry about spread.
Clinician self-treatment is okay. In the opinion of Dr. Stankiewicz, aesthetic clinicians who treat themselves “have a fool for a patient.” She added: “Although no one is going to blame you and may not even know if you give yourself a little Botox touch-up at home, glorifying self-treatment on social media must stop. It’s dangerous and it can be ineffective.”
Self-treatment can also impair judgment and the objectivity of cosmetic therapies. “Also, when you’re pointing a laser at your own face and posting it on social media, it gives viewers the impression that this is not a serious medical treatment when it really is,” she emphasized. In addition, “when you treat yourself, you lose the ability to see the proper clinical endpoint. You also lose the ability to see the angle and the appropriate position for injection to avoid intervascular occlusion.”
Neither Dr. Stankiewicz nor Dr. Ibrahimi reported having relevant financial disclosures.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Saururus chinensis
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at dermnews@mdedge.com.
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
Novel platform harnesses 3D laser technology for skin treatments
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
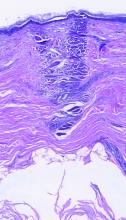
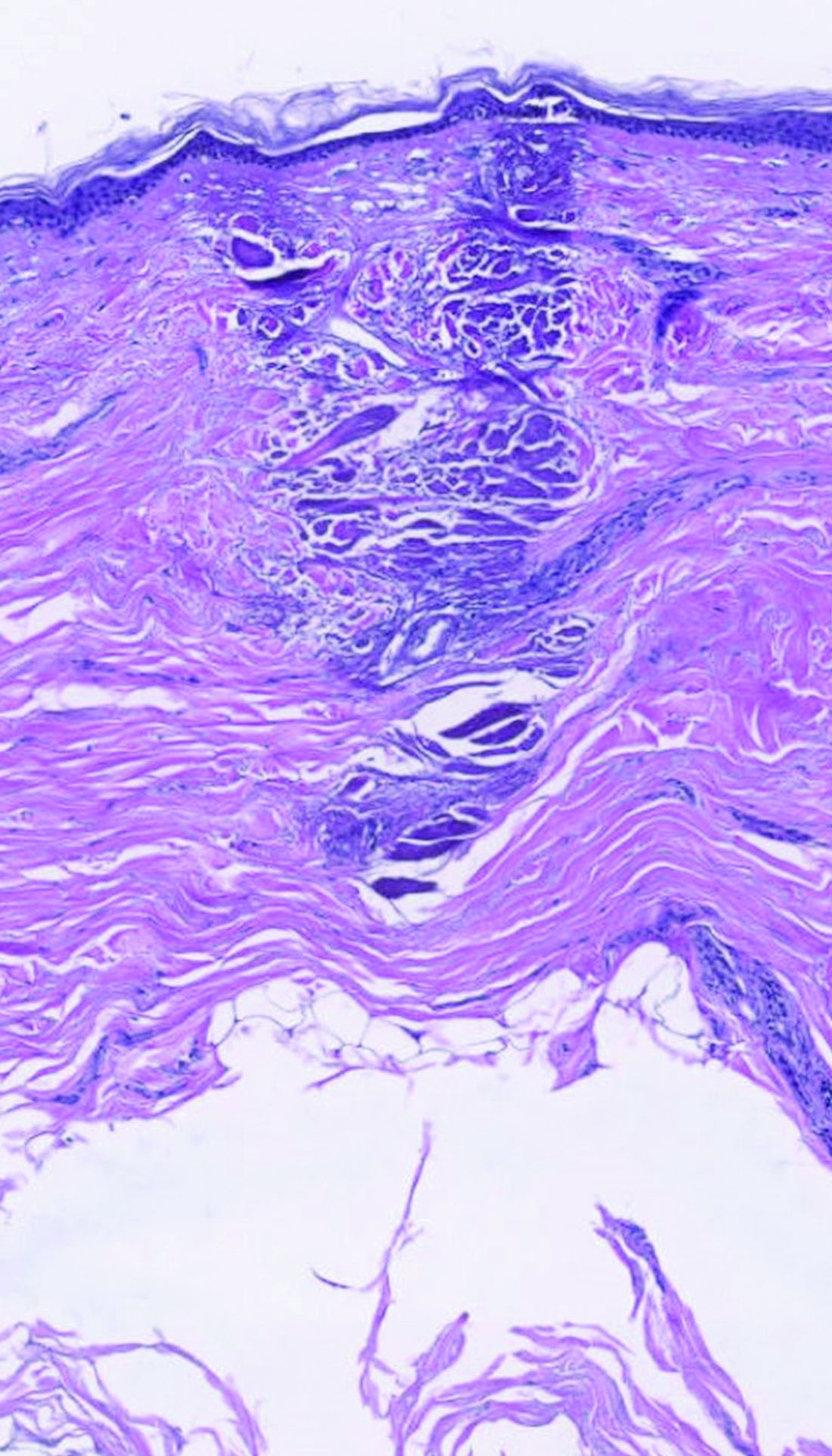
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Applications for laser-assisted drug delivery on the horizon, expert says
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
‘Slugging’: A TikTok skin trend that has some merit
They’ve been around for a while and show no signs of going away: videos on TikTok of people, often teens, slathering their face with petroleum jelly and claiming that it’s transformed their skin, cured their acne, or given them an amazing “glow up.”
TikTok videos mentioning petrolatum increased by 46% and Instagram videos by 93% from 2021 to 2022, reported Gabriel Santos Malave, BA, of the Icahn School of Medicine at Mount Sinai, New York, and William D. James, MD, professor of dermatology, University of Pennsylvania, Philadelphia, in a review of petroleum jelly’s uses recently published in Cutis.
The authors said that after application of a moisturizer.
In a typical demonstration, a dermatologist in the United Kingdom showed how she incorporates slugging into her routine in a TikTok video that’s had more than 1 million views.
Unlike many TikTok trends, slugging may not be entirely bad, say dermatologists.
“I think it’s a great way to keep your skin protected and moisturized, especially in those dry, cold winter months,” said dermatologist Mamina Turegano, MD, in a video posted in February 2022. That TikTok video has had more than 6 million views.
Dr. Turegano, who is in private practice in the New Orleans suburb of Metairie, La., told this news organization that she decided to post about slugging after she’d noticed that the topic was trending. Also, she had tried the technique herself when she was a resident in Washington more than a decade ago.
At the time, Dr. Turegano said that she was aware that “putting petroleum jelly on your face was not a normal thing.” But, given its history of being used in dermatology, she gave it a try and found that it worked well for her dry skin, she said.
Dr. Turegano is one among many dermatologists who have joined TikTok to dispel myths, educate, and inform. It’s important for them to be there “to engage and empower the public to become a better consumer of information out there and take ownership of their skin health,” said Jean McGee, MD, PhD, a dermatologist at Beth Israel Deaconess Medical Center, Boston, and assistant professor of dermatology at Harvard Medical School, also in Boston.
Dr. McGee and colleagues studied TikTok content on slugging and found that by far, videos that were created by health care providers were more educational. Dermatologists who posted were more likely to discuss the risks and benefits, whereas so-called “influencers” rarely posted on the risks, according to the study, published in Clinics in Dermatology.
Slugging is generally safe and effective for those who have a compromised skin barrier or “for those who have sensitive skin and can’t tolerate other products but need some form of moisturization,” said Dr. Turegano.
“Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL),” write Mr. Malave and Dr. James in Cutis. They note that petrolatum reduces TEWL by 98%, compared with only 20% to 30% for other oil-based moisturizers.
Dermatologists have often recommended a “seal and trap” regimen for dry skin or eczema. It involves a short, lukewarm shower, followed by immediately moisturizing with a petrolatum-based ointment, said Dr. McGee.
This could be safe for the face, but “other variables need to be considered,” including use of other topical medications and other skin care practices, she added.
The concept of double-layering a moisturizer and an occlusive agent can be beneficial but more typically for the hands and feet, where the skin can be severely dry and cracked, said Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington. “I would not recommend that on the face,” Dr. Friedman told this news organization.
He and other dermatologists warned about the potential for slugging – given petroleum jelly’s occlusive nature – to enhance the action of any topical steroid, retinol, or exfoliating agent.
Muneeb Shah, MD, who practices in Mooresville, N.C., is one of the most popular dermatologists on TikTok, with more than 17 million followers. He also warned in a February 2022 video about potential downsides. “Be careful after using retinol or exfoliating acids because it may actually irritate your skin more,” he says in the video.
“Slugging is awesome for some people but not for others, and not for every night,” said Whitney Bowe, MD, on a TikTok video she posted in July. She recommended it for eczema or really dry skin. Dr. Bowe, who practices with Advanced Dermatology in New York, advised those with acne-prone skin to “skip this trend.”
On a web page aimed at the general public, the American Academy of Dermatology similarly cautioned, “Avoid putting petroleum jelly on your face if you are acne-prone, as this may cause breakouts in some people.”
Acne cure or pore clogger?
And yet, plenty of TikTok users claim that it has improved their acne.
One such user posted a before and after video purporting to show that slugging had almost completely eliminated her acne and prior scarring. Not surprisingly, it has been viewed some 9 million times and got 1.5 million “likes.”
Dr. Friedman notes that it’s theoretically possible – but not likely – that acne could improve by slugging, given that acne basically is a disease of barrier disruption. “The idea here is you have disrupted skin barrier throughout the face regardless of whether you have a pimple in that spot or not, so you need to repair it,” he said. “That’s where I think slugging is somewhat on the right track, because by putting an occlusive agent on the skin, you are restoring the barrier element,” he said.
However, applying a thick, greasy ointment on the face could block pores and cause a backup of sebum and dead skin cells, and it could trap bacteria, he said. “Skin barrier protection and repair is central to acne management, but you need to do it in a safe way,” he said. He noted that that means applying an oil-free moisturizer to damp skin.
Dr. Turegano said she has seen slugging improve acne, but it’s hard to say which people with acne-prone skin would be the best candidates. Those who have used harsh products to treat acne and subsequently experienced worsening acne could potentially benefit, she said.
Even so, she said, “I’d be very cautious in anyone with acne.”
Dr. Friedman, Dr. McGee, and Dr. Turegano reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
They’ve been around for a while and show no signs of going away: videos on TikTok of people, often teens, slathering their face with petroleum jelly and claiming that it’s transformed their skin, cured their acne, or given them an amazing “glow up.”
TikTok videos mentioning petrolatum increased by 46% and Instagram videos by 93% from 2021 to 2022, reported Gabriel Santos Malave, BA, of the Icahn School of Medicine at Mount Sinai, New York, and William D. James, MD, professor of dermatology, University of Pennsylvania, Philadelphia, in a review of petroleum jelly’s uses recently published in Cutis.
The authors said that after application of a moisturizer.
In a typical demonstration, a dermatologist in the United Kingdom showed how she incorporates slugging into her routine in a TikTok video that’s had more than 1 million views.
Unlike many TikTok trends, slugging may not be entirely bad, say dermatologists.
“I think it’s a great way to keep your skin protected and moisturized, especially in those dry, cold winter months,” said dermatologist Mamina Turegano, MD, in a video posted in February 2022. That TikTok video has had more than 6 million views.
Dr. Turegano, who is in private practice in the New Orleans suburb of Metairie, La., told this news organization that she decided to post about slugging after she’d noticed that the topic was trending. Also, she had tried the technique herself when she was a resident in Washington more than a decade ago.
At the time, Dr. Turegano said that she was aware that “putting petroleum jelly on your face was not a normal thing.” But, given its history of being used in dermatology, she gave it a try and found that it worked well for her dry skin, she said.
Dr. Turegano is one among many dermatologists who have joined TikTok to dispel myths, educate, and inform. It’s important for them to be there “to engage and empower the public to become a better consumer of information out there and take ownership of their skin health,” said Jean McGee, MD, PhD, a dermatologist at Beth Israel Deaconess Medical Center, Boston, and assistant professor of dermatology at Harvard Medical School, also in Boston.
Dr. McGee and colleagues studied TikTok content on slugging and found that by far, videos that were created by health care providers were more educational. Dermatologists who posted were more likely to discuss the risks and benefits, whereas so-called “influencers” rarely posted on the risks, according to the study, published in Clinics in Dermatology.
Slugging is generally safe and effective for those who have a compromised skin barrier or “for those who have sensitive skin and can’t tolerate other products but need some form of moisturization,” said Dr. Turegano.
“Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL),” write Mr. Malave and Dr. James in Cutis. They note that petrolatum reduces TEWL by 98%, compared with only 20% to 30% for other oil-based moisturizers.
Dermatologists have often recommended a “seal and trap” regimen for dry skin or eczema. It involves a short, lukewarm shower, followed by immediately moisturizing with a petrolatum-based ointment, said Dr. McGee.
This could be safe for the face, but “other variables need to be considered,” including use of other topical medications and other skin care practices, she added.
The concept of double-layering a moisturizer and an occlusive agent can be beneficial but more typically for the hands and feet, where the skin can be severely dry and cracked, said Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington. “I would not recommend that on the face,” Dr. Friedman told this news organization.
He and other dermatologists warned about the potential for slugging – given petroleum jelly’s occlusive nature – to enhance the action of any topical steroid, retinol, or exfoliating agent.
Muneeb Shah, MD, who practices in Mooresville, N.C., is one of the most popular dermatologists on TikTok, with more than 17 million followers. He also warned in a February 2022 video about potential downsides. “Be careful after using retinol or exfoliating acids because it may actually irritate your skin more,” he says in the video.
“Slugging is awesome for some people but not for others, and not for every night,” said Whitney Bowe, MD, on a TikTok video she posted in July. She recommended it for eczema or really dry skin. Dr. Bowe, who practices with Advanced Dermatology in New York, advised those with acne-prone skin to “skip this trend.”
On a web page aimed at the general public, the American Academy of Dermatology similarly cautioned, “Avoid putting petroleum jelly on your face if you are acne-prone, as this may cause breakouts in some people.”
Acne cure or pore clogger?
And yet, plenty of TikTok users claim that it has improved their acne.
One such user posted a before and after video purporting to show that slugging had almost completely eliminated her acne and prior scarring. Not surprisingly, it has been viewed some 9 million times and got 1.5 million “likes.”
Dr. Friedman notes that it’s theoretically possible – but not likely – that acne could improve by slugging, given that acne basically is a disease of barrier disruption. “The idea here is you have disrupted skin barrier throughout the face regardless of whether you have a pimple in that spot or not, so you need to repair it,” he said. “That’s where I think slugging is somewhat on the right track, because by putting an occlusive agent on the skin, you are restoring the barrier element,” he said.
However, applying a thick, greasy ointment on the face could block pores and cause a backup of sebum and dead skin cells, and it could trap bacteria, he said. “Skin barrier protection and repair is central to acne management, but you need to do it in a safe way,” he said. He noted that that means applying an oil-free moisturizer to damp skin.
Dr. Turegano said she has seen slugging improve acne, but it’s hard to say which people with acne-prone skin would be the best candidates. Those who have used harsh products to treat acne and subsequently experienced worsening acne could potentially benefit, she said.
Even so, she said, “I’d be very cautious in anyone with acne.”
Dr. Friedman, Dr. McGee, and Dr. Turegano reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
They’ve been around for a while and show no signs of going away: videos on TikTok of people, often teens, slathering their face with petroleum jelly and claiming that it’s transformed their skin, cured their acne, or given them an amazing “glow up.”
TikTok videos mentioning petrolatum increased by 46% and Instagram videos by 93% from 2021 to 2022, reported Gabriel Santos Malave, BA, of the Icahn School of Medicine at Mount Sinai, New York, and William D. James, MD, professor of dermatology, University of Pennsylvania, Philadelphia, in a review of petroleum jelly’s uses recently published in Cutis.
The authors said that after application of a moisturizer.
In a typical demonstration, a dermatologist in the United Kingdom showed how she incorporates slugging into her routine in a TikTok video that’s had more than 1 million views.
Unlike many TikTok trends, slugging may not be entirely bad, say dermatologists.
“I think it’s a great way to keep your skin protected and moisturized, especially in those dry, cold winter months,” said dermatologist Mamina Turegano, MD, in a video posted in February 2022. That TikTok video has had more than 6 million views.
Dr. Turegano, who is in private practice in the New Orleans suburb of Metairie, La., told this news organization that she decided to post about slugging after she’d noticed that the topic was trending. Also, she had tried the technique herself when she was a resident in Washington more than a decade ago.
At the time, Dr. Turegano said that she was aware that “putting petroleum jelly on your face was not a normal thing.” But, given its history of being used in dermatology, she gave it a try and found that it worked well for her dry skin, she said.
Dr. Turegano is one among many dermatologists who have joined TikTok to dispel myths, educate, and inform. It’s important for them to be there “to engage and empower the public to become a better consumer of information out there and take ownership of their skin health,” said Jean McGee, MD, PhD, a dermatologist at Beth Israel Deaconess Medical Center, Boston, and assistant professor of dermatology at Harvard Medical School, also in Boston.
Dr. McGee and colleagues studied TikTok content on slugging and found that by far, videos that were created by health care providers were more educational. Dermatologists who posted were more likely to discuss the risks and benefits, whereas so-called “influencers” rarely posted on the risks, according to the study, published in Clinics in Dermatology.
Slugging is generally safe and effective for those who have a compromised skin barrier or “for those who have sensitive skin and can’t tolerate other products but need some form of moisturization,” said Dr. Turegano.
“Its oil-based nature allows it to seal water in the skin by creating a hydrophobic barrier that decreases transepidermal water loss (TEWL),” write Mr. Malave and Dr. James in Cutis. They note that petrolatum reduces TEWL by 98%, compared with only 20% to 30% for other oil-based moisturizers.
Dermatologists have often recommended a “seal and trap” regimen for dry skin or eczema. It involves a short, lukewarm shower, followed by immediately moisturizing with a petrolatum-based ointment, said Dr. McGee.
This could be safe for the face, but “other variables need to be considered,” including use of other topical medications and other skin care practices, she added.
The concept of double-layering a moisturizer and an occlusive agent can be beneficial but more typically for the hands and feet, where the skin can be severely dry and cracked, said Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington. “I would not recommend that on the face,” Dr. Friedman told this news organization.
He and other dermatologists warned about the potential for slugging – given petroleum jelly’s occlusive nature – to enhance the action of any topical steroid, retinol, or exfoliating agent.
Muneeb Shah, MD, who practices in Mooresville, N.C., is one of the most popular dermatologists on TikTok, with more than 17 million followers. He also warned in a February 2022 video about potential downsides. “Be careful after using retinol or exfoliating acids because it may actually irritate your skin more,” he says in the video.
“Slugging is awesome for some people but not for others, and not for every night,” said Whitney Bowe, MD, on a TikTok video she posted in July. She recommended it for eczema or really dry skin. Dr. Bowe, who practices with Advanced Dermatology in New York, advised those with acne-prone skin to “skip this trend.”
On a web page aimed at the general public, the American Academy of Dermatology similarly cautioned, “Avoid putting petroleum jelly on your face if you are acne-prone, as this may cause breakouts in some people.”
Acne cure or pore clogger?
And yet, plenty of TikTok users claim that it has improved their acne.
One such user posted a before and after video purporting to show that slugging had almost completely eliminated her acne and prior scarring. Not surprisingly, it has been viewed some 9 million times and got 1.5 million “likes.”
Dr. Friedman notes that it’s theoretically possible – but not likely – that acne could improve by slugging, given that acne basically is a disease of barrier disruption. “The idea here is you have disrupted skin barrier throughout the face regardless of whether you have a pimple in that spot or not, so you need to repair it,” he said. “That’s where I think slugging is somewhat on the right track, because by putting an occlusive agent on the skin, you are restoring the barrier element,” he said.
However, applying a thick, greasy ointment on the face could block pores and cause a backup of sebum and dead skin cells, and it could trap bacteria, he said. “Skin barrier protection and repair is central to acne management, but you need to do it in a safe way,” he said. He noted that that means applying an oil-free moisturizer to damp skin.
Dr. Turegano said she has seen slugging improve acne, but it’s hard to say which people with acne-prone skin would be the best candidates. Those who have used harsh products to treat acne and subsequently experienced worsening acne could potentially benefit, she said.
Even so, she said, “I’d be very cautious in anyone with acne.”
Dr. Friedman, Dr. McGee, and Dr. Turegano reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Review gives weight to supplements for hair loss
because of small sample sizes, heterogeneity of hair loss types in study subjects, or other limitations.
The review, published online in JAMA Dermatology, notes that “Twelve of the 20 nutritional interventions had high-quality studies suggesting objectively evaluated effectiveness.”
It is “ground breaking,” in part because of its breadth and depth, said Eva Simmons-O’Brien, MD, a dermatologist in Towson, Md., who often recommends supplements for her patients with hair loss. “It basically kind of vindicates what some of us have been doing for a number of years in terms of treating hair loss,” she told this news organization. “It should hopefully make it more commonplace for dermatologists to consider using nutritional supplements as an adjuvant to treating hair loss,” added Dr. Simmons-O’Brien.
The review “is very helpful,” agreed Lynne J. Goldberg, MD, professor of dermatology and pathology and laboratory medicine at Boston University. Dr. Goldberg noted that many patients are already taking supplements and want to know whether they are safe and effective. The review “points out what the problems are; it talks about what the individual ingredients are and what they do, what the problems are; and it concluded that some people may find these helpful. Which is exactly what I tell my patients,” said Dr. Goldberg, who is also director of the Hair Clinic at Boston Medical Center.
“For patients who are highly motivated and eager to try this, we’re hoping that this systematic review serves as a foundation to have a conversation,” study coauthor Arash Mostaghimi, MD, MPA, MPH, of the department of dermatology at Harvard Medical School, told this news organization. “When there’s medical uncertainty and the question is how much risk is one willing to take, the most important thing to do is to present the data and engage in shared decision-making with the patient,” noted Dr. Mostaghimi, who is also director of the inpatient dermatology consult service at Brigham and Women’s Hospital, Boston.
Surprising effectiveness
Going into the study, “we felt it would be likely that majority of nutritional supplements would either not be effective or not studied,” he said.
Dr. Mostaghimi and his coauthors conducted the study because so many patients take nutritional supplements to address hair loss, he said. An initial literature survey yielded more than 6,300 citations, but after screening and reviews, the authors included 30 articles for evaluation.
The review begins with a look at studies of saw palmetto (Serenoa repens), a botanical compound thought to inhibit the enzyme 5-alpha reductase (5AR), which converts testosterone to dihydroxytestosterone (DHT). DHT is a mediator of androgenic alopecia (AGA). The studies suggest that the compound might stabilize hair loss, “although its effect is likely less than that of finasteride,” write the authors. They also note that side effects associated with finasteride, such as sexual dysfunction, were also observed with saw palmetto “but to a lesser extent.”
For AGA, pumpkin seed oil may also be effective and a “potential alternative” to finasteride for AGA, and Forti5, a nutritional supplement that includes botanical 5AR inhibitors and other ingredients, had favorable effects in one study, the authors write. But neither has been compared to finasteride, and the Forti5 study lacked a control group.
The review also examines the micronutrients vitamin D, zinc, B vitamins, and antioxidants. Low levels of vitamin D have been associated with alopecia areata (AA), AGA, and telogen effluvium (TE) in some studies, and zinc deficiencies have been associated with TE, hair breakage, and thinning, according to the review. A single-arm vitamin D study showed improved results at 6 months for women with TE, but there was no control group and TE is self-resolving, the authors add. Studies in patients with normal zinc levels at baseline who had AA or hair loss showed significant hair regrowth and increased hair thickness and density, but the trials were a mishmash of controls and no controls and relied on self-perceived hair-loss data.
Larger more rigorous studies should be done to evaluate zinc’s effectiveness with AA, the authors comment.
Many patients take vitamin B7 (biotin) for hair loss. It has not been studied on its own but was an ingredient in some supplements in the review. Dr. Simmons-O’Brien said that biotin won’t result in new hair growth but that it can help strengthen the new hairs that grow as a result of other therapies. Both she and the study authors note that the Food and Drug Administration has warned against biotin supplementation because it can interfere with troponin and other test results.
The review also finds that immunomodulators –such as Chinese herbal extracts from paeony and glycyrrhizin – were effective in severe AA. Growth hormone modulators targeting deficiencies in insulin growth factor 1 or growth hormone are also promising. Studies of the modulators capsaicin and isoflavones – used topically – spurred hair growth, the authors write.
Products containing marine protein supplements, including Viviscal and Nourkrin, appeared effective in increasing hair counts in men and women, but the studies were funded by the manufacturer and were not well controlled. Side effects with Viviscal included bloating, according to the review.
The multi-ingredient supplements Nutrafol, Omni-Three, Apple Nutraceutical, and Lambdapil were also included in the review. Only Omni-Three showed no effectiveness, but studies of the other supplements had various limitations, including lack of controls and small sample sizes.
Complicated problem, multiple solutions
Given the many reasons for hair loss, multiple solutions are needed, the dermatologists note.
Dr. Mostaghimi said that he’s still a bit skeptical that supplements work as consistently as described or as well as described, given that he and his coauthors were unable to find any negative studies. In talking with patients who are taking supplements, he said that his first aim is to make sure they are safe. At least the supplements in the review have been studied for safety, he added.
He will encourage replacement of vitamin D or zinc or other vitamins or minerals if patients are deficient but said that he does not “actively encourage supplementation.”
Dr. Simmons-O’Brien said that, when evaluating patients with hair loss, she orders lab tests to determine whether the patient has anemia or a thyroid issue or deficiencies in vitamins or minerals or other nutritional deficiencies, asks about diet and styling practices, and takes a scalp biopsy. It is not uncommon to recommend supplementation on the basis of those findings, she added.
“As a hair-loss specialist, my job is to treat the patient at their level, in their framework, in their comfort zone,” said Dr. Goldberg. Some patients don’t want to take medications for hair loss, so she might recommend supplements in those cases but tells patients that they aren’t well studied.
She added that it can be hard to tell whether a supplement is working, particularly if it has multiple ingredients.
Dr. Mostaghimi reported consulting fees from Pfizer, Concert, Lilly, Hims and Hers, Equillium, AbbVie, Digital Diagnostics, and Bioniz and grants from Pfizer, all outside the submitted work. In addition, Dr. Mostaghimi disclosed that he is an associate editor of JAMA Dermatology but was not involved in any of the decisions regarding the review of the manuscript or its acceptance. No other disclosures were reported by the other study authors. Dr. Goldberg reported no disclosures. Dr. Simmons-O›Brien is a medical consultant for Isdin, but not for hair products.
A version of this article first appeared on Medscape.com.
because of small sample sizes, heterogeneity of hair loss types in study subjects, or other limitations.
The review, published online in JAMA Dermatology, notes that “Twelve of the 20 nutritional interventions had high-quality studies suggesting objectively evaluated effectiveness.”
It is “ground breaking,” in part because of its breadth and depth, said Eva Simmons-O’Brien, MD, a dermatologist in Towson, Md., who often recommends supplements for her patients with hair loss. “It basically kind of vindicates what some of us have been doing for a number of years in terms of treating hair loss,” she told this news organization. “It should hopefully make it more commonplace for dermatologists to consider using nutritional supplements as an adjuvant to treating hair loss,” added Dr. Simmons-O’Brien.
The review “is very helpful,” agreed Lynne J. Goldberg, MD, professor of dermatology and pathology and laboratory medicine at Boston University. Dr. Goldberg noted that many patients are already taking supplements and want to know whether they are safe and effective. The review “points out what the problems are; it talks about what the individual ingredients are and what they do, what the problems are; and it concluded that some people may find these helpful. Which is exactly what I tell my patients,” said Dr. Goldberg, who is also director of the Hair Clinic at Boston Medical Center.
“For patients who are highly motivated and eager to try this, we’re hoping that this systematic review serves as a foundation to have a conversation,” study coauthor Arash Mostaghimi, MD, MPA, MPH, of the department of dermatology at Harvard Medical School, told this news organization. “When there’s medical uncertainty and the question is how much risk is one willing to take, the most important thing to do is to present the data and engage in shared decision-making with the patient,” noted Dr. Mostaghimi, who is also director of the inpatient dermatology consult service at Brigham and Women’s Hospital, Boston.
Surprising effectiveness
Going into the study, “we felt it would be likely that majority of nutritional supplements would either not be effective or not studied,” he said.
Dr. Mostaghimi and his coauthors conducted the study because so many patients take nutritional supplements to address hair loss, he said. An initial literature survey yielded more than 6,300 citations, but after screening and reviews, the authors included 30 articles for evaluation.
The review begins with a look at studies of saw palmetto (Serenoa repens), a botanical compound thought to inhibit the enzyme 5-alpha reductase (5AR), which converts testosterone to dihydroxytestosterone (DHT). DHT is a mediator of androgenic alopecia (AGA). The studies suggest that the compound might stabilize hair loss, “although its effect is likely less than that of finasteride,” write the authors. They also note that side effects associated with finasteride, such as sexual dysfunction, were also observed with saw palmetto “but to a lesser extent.”
For AGA, pumpkin seed oil may also be effective and a “potential alternative” to finasteride for AGA, and Forti5, a nutritional supplement that includes botanical 5AR inhibitors and other ingredients, had favorable effects in one study, the authors write. But neither has been compared to finasteride, and the Forti5 study lacked a control group.
The review also examines the micronutrients vitamin D, zinc, B vitamins, and antioxidants. Low levels of vitamin D have been associated with alopecia areata (AA), AGA, and telogen effluvium (TE) in some studies, and zinc deficiencies have been associated with TE, hair breakage, and thinning, according to the review. A single-arm vitamin D study showed improved results at 6 months for women with TE, but there was no control group and TE is self-resolving, the authors add. Studies in patients with normal zinc levels at baseline who had AA or hair loss showed significant hair regrowth and increased hair thickness and density, but the trials were a mishmash of controls and no controls and relied on self-perceived hair-loss data.
Larger more rigorous studies should be done to evaluate zinc’s effectiveness with AA, the authors comment.
Many patients take vitamin B7 (biotin) for hair loss. It has not been studied on its own but was an ingredient in some supplements in the review. Dr. Simmons-O’Brien said that biotin won’t result in new hair growth but that it can help strengthen the new hairs that grow as a result of other therapies. Both she and the study authors note that the Food and Drug Administration has warned against biotin supplementation because it can interfere with troponin and other test results.
The review also finds that immunomodulators –such as Chinese herbal extracts from paeony and glycyrrhizin – were effective in severe AA. Growth hormone modulators targeting deficiencies in insulin growth factor 1 or growth hormone are also promising. Studies of the modulators capsaicin and isoflavones – used topically – spurred hair growth, the authors write.
Products containing marine protein supplements, including Viviscal and Nourkrin, appeared effective in increasing hair counts in men and women, but the studies were funded by the manufacturer and were not well controlled. Side effects with Viviscal included bloating, according to the review.
The multi-ingredient supplements Nutrafol, Omni-Three, Apple Nutraceutical, and Lambdapil were also included in the review. Only Omni-Three showed no effectiveness, but studies of the other supplements had various limitations, including lack of controls and small sample sizes.
Complicated problem, multiple solutions
Given the many reasons for hair loss, multiple solutions are needed, the dermatologists note.
Dr. Mostaghimi said that he’s still a bit skeptical that supplements work as consistently as described or as well as described, given that he and his coauthors were unable to find any negative studies. In talking with patients who are taking supplements, he said that his first aim is to make sure they are safe. At least the supplements in the review have been studied for safety, he added.
He will encourage replacement of vitamin D or zinc or other vitamins or minerals if patients are deficient but said that he does not “actively encourage supplementation.”
Dr. Simmons-O’Brien said that, when evaluating patients with hair loss, she orders lab tests to determine whether the patient has anemia or a thyroid issue or deficiencies in vitamins or minerals or other nutritional deficiencies, asks about diet and styling practices, and takes a scalp biopsy. It is not uncommon to recommend supplementation on the basis of those findings, she added.
“As a hair-loss specialist, my job is to treat the patient at their level, in their framework, in their comfort zone,” said Dr. Goldberg. Some patients don’t want to take medications for hair loss, so she might recommend supplements in those cases but tells patients that they aren’t well studied.
She added that it can be hard to tell whether a supplement is working, particularly if it has multiple ingredients.
Dr. Mostaghimi reported consulting fees from Pfizer, Concert, Lilly, Hims and Hers, Equillium, AbbVie, Digital Diagnostics, and Bioniz and grants from Pfizer, all outside the submitted work. In addition, Dr. Mostaghimi disclosed that he is an associate editor of JAMA Dermatology but was not involved in any of the decisions regarding the review of the manuscript or its acceptance. No other disclosures were reported by the other study authors. Dr. Goldberg reported no disclosures. Dr. Simmons-O›Brien is a medical consultant for Isdin, but not for hair products.
A version of this article first appeared on Medscape.com.
because of small sample sizes, heterogeneity of hair loss types in study subjects, or other limitations.
The review, published online in JAMA Dermatology, notes that “Twelve of the 20 nutritional interventions had high-quality studies suggesting objectively evaluated effectiveness.”
It is “ground breaking,” in part because of its breadth and depth, said Eva Simmons-O’Brien, MD, a dermatologist in Towson, Md., who often recommends supplements for her patients with hair loss. “It basically kind of vindicates what some of us have been doing for a number of years in terms of treating hair loss,” she told this news organization. “It should hopefully make it more commonplace for dermatologists to consider using nutritional supplements as an adjuvant to treating hair loss,” added Dr. Simmons-O’Brien.
The review “is very helpful,” agreed Lynne J. Goldberg, MD, professor of dermatology and pathology and laboratory medicine at Boston University. Dr. Goldberg noted that many patients are already taking supplements and want to know whether they are safe and effective. The review “points out what the problems are; it talks about what the individual ingredients are and what they do, what the problems are; and it concluded that some people may find these helpful. Which is exactly what I tell my patients,” said Dr. Goldberg, who is also director of the Hair Clinic at Boston Medical Center.
“For patients who are highly motivated and eager to try this, we’re hoping that this systematic review serves as a foundation to have a conversation,” study coauthor Arash Mostaghimi, MD, MPA, MPH, of the department of dermatology at Harvard Medical School, told this news organization. “When there’s medical uncertainty and the question is how much risk is one willing to take, the most important thing to do is to present the data and engage in shared decision-making with the patient,” noted Dr. Mostaghimi, who is also director of the inpatient dermatology consult service at Brigham and Women’s Hospital, Boston.
Surprising effectiveness
Going into the study, “we felt it would be likely that majority of nutritional supplements would either not be effective or not studied,” he said.
Dr. Mostaghimi and his coauthors conducted the study because so many patients take nutritional supplements to address hair loss, he said. An initial literature survey yielded more than 6,300 citations, but after screening and reviews, the authors included 30 articles for evaluation.
The review begins with a look at studies of saw palmetto (Serenoa repens), a botanical compound thought to inhibit the enzyme 5-alpha reductase (5AR), which converts testosterone to dihydroxytestosterone (DHT). DHT is a mediator of androgenic alopecia (AGA). The studies suggest that the compound might stabilize hair loss, “although its effect is likely less than that of finasteride,” write the authors. They also note that side effects associated with finasteride, such as sexual dysfunction, were also observed with saw palmetto “but to a lesser extent.”
For AGA, pumpkin seed oil may also be effective and a “potential alternative” to finasteride for AGA, and Forti5, a nutritional supplement that includes botanical 5AR inhibitors and other ingredients, had favorable effects in one study, the authors write. But neither has been compared to finasteride, and the Forti5 study lacked a control group.
The review also examines the micronutrients vitamin D, zinc, B vitamins, and antioxidants. Low levels of vitamin D have been associated with alopecia areata (AA), AGA, and telogen effluvium (TE) in some studies, and zinc deficiencies have been associated with TE, hair breakage, and thinning, according to the review. A single-arm vitamin D study showed improved results at 6 months for women with TE, but there was no control group and TE is self-resolving, the authors add. Studies in patients with normal zinc levels at baseline who had AA or hair loss showed significant hair regrowth and increased hair thickness and density, but the trials were a mishmash of controls and no controls and relied on self-perceived hair-loss data.
Larger more rigorous studies should be done to evaluate zinc’s effectiveness with AA, the authors comment.
Many patients take vitamin B7 (biotin) for hair loss. It has not been studied on its own but was an ingredient in some supplements in the review. Dr. Simmons-O’Brien said that biotin won’t result in new hair growth but that it can help strengthen the new hairs that grow as a result of other therapies. Both she and the study authors note that the Food and Drug Administration has warned against biotin supplementation because it can interfere with troponin and other test results.
The review also finds that immunomodulators –such as Chinese herbal extracts from paeony and glycyrrhizin – were effective in severe AA. Growth hormone modulators targeting deficiencies in insulin growth factor 1 or growth hormone are also promising. Studies of the modulators capsaicin and isoflavones – used topically – spurred hair growth, the authors write.
Products containing marine protein supplements, including Viviscal and Nourkrin, appeared effective in increasing hair counts in men and women, but the studies were funded by the manufacturer and were not well controlled. Side effects with Viviscal included bloating, according to the review.
The multi-ingredient supplements Nutrafol, Omni-Three, Apple Nutraceutical, and Lambdapil were also included in the review. Only Omni-Three showed no effectiveness, but studies of the other supplements had various limitations, including lack of controls and small sample sizes.
Complicated problem, multiple solutions
Given the many reasons for hair loss, multiple solutions are needed, the dermatologists note.
Dr. Mostaghimi said that he’s still a bit skeptical that supplements work as consistently as described or as well as described, given that he and his coauthors were unable to find any negative studies. In talking with patients who are taking supplements, he said that his first aim is to make sure they are safe. At least the supplements in the review have been studied for safety, he added.
He will encourage replacement of vitamin D or zinc or other vitamins or minerals if patients are deficient but said that he does not “actively encourage supplementation.”
Dr. Simmons-O’Brien said that, when evaluating patients with hair loss, she orders lab tests to determine whether the patient has anemia or a thyroid issue or deficiencies in vitamins or minerals or other nutritional deficiencies, asks about diet and styling practices, and takes a scalp biopsy. It is not uncommon to recommend supplementation on the basis of those findings, she added.
“As a hair-loss specialist, my job is to treat the patient at their level, in their framework, in their comfort zone,” said Dr. Goldberg. Some patients don’t want to take medications for hair loss, so she might recommend supplements in those cases but tells patients that they aren’t well studied.
She added that it can be hard to tell whether a supplement is working, particularly if it has multiple ingredients.
Dr. Mostaghimi reported consulting fees from Pfizer, Concert, Lilly, Hims and Hers, Equillium, AbbVie, Digital Diagnostics, and Bioniz and grants from Pfizer, all outside the submitted work. In addition, Dr. Mostaghimi disclosed that he is an associate editor of JAMA Dermatology but was not involved in any of the decisions regarding the review of the manuscript or its acceptance. No other disclosures were reported by the other study authors. Dr. Goldberg reported no disclosures. Dr. Simmons-O›Brien is a medical consultant for Isdin, but not for hair products.
A version of this article first appeared on Medscape.com.
Applications for nano-pulse stimulation continue to evolve
During a virtual course on laser and aesthetic skin therapy, Yakir Levin, MD, PhD, likened nano-pulse stimulation to microneedling or radiofrequency microneedling “in that you have an array of microneedles that go into the skin,” he said. “However, it is actually completely different.”
The CellFX System uses nano-pulse stimulation to deliver ultrashort electrical energy pulses into the skin of target lesions via a console-based handheld applicator. In September 2022, the Food and Drug Administration cleared the CellFX system for treatment of sebaceous hyperplasia in patients with Fitzpatrick skin types I-II. This followed a general clearance of the device in 2021 for dermatologic procedures requiring ablation and resurfacing of the skin.
Pulses from the device deliver a “constant electrical potential gradient across cell membranes and organelle membranes, causing them to break down,” explained Dr. Levin, a dermatologist and physician scientist at Massachusetts General Hospital, Boston, where he practices cosmetic dermatology and conducts research on birthmarks in children. This creates pores in those membranes “and leads to a controlled form of cell death,” he said. “As a result, this treatment is limited to cells, so you can do it in the dermis without damaging the collagen network. It spares tissue that’s outside of the field, and it’s nonthermal.”
Images from electron microscopy have demonstrated swelling of the mitochondria and breakdown of nuclei within 2 hours of treatment in a rat study. “Within 1 day of treatment you have death of the cells and the beginning of involution of the lesion,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “This presents us with the opportunity to treat dermal lesions without causing damage to the epidermis or to the acellular portion of the dermis.”
In published studies, nano-pulse stimulation has been shown to be effective for treating sebaceous hyperplasia and warts. According to Dr. Levin, clinicians typically treat sebaceous hyperplasia with an radiofrequency microneedle or electrodesiccation, “where we shave off the top but do not try to hit the bottom because we don’t want to cause scarring of the dermis,” he said. “Using the nano-pulse stimulation technology, however, you end up with involution of the sebaceous lesion without damaging the surrounding dermis.”
In a prospective, randomized study, 72 individuals with sebaceous gland hyperplasia received nano-pulse stimulation to 222 lesions and they returned for three to four follow-up evaluations with photographs. At the final study visit, investigators rated 99.6% of the sebaceous gland lesions as clear or mostly clear, while 79% of the study participants said they were “satisfied” or “mostly satisfied” with the outcome.
At posttreatment day 60, 55% of the lesions were judged to have no hyperpigmentation and 31% exhibited mild posttreatment hyperpigmentation.
In a more recent study, researchers used the CellFX System to treat 195 cutaneous warts up to 10 mm wide in 62 individuals enrolled at one of five sites. They found that 75% of common warts, 73% of flat warts, and 44% of plantar warts were completely clear 60 days following the last nano-pulse stimulation treatment and did not recur within the 120-day observation period.
The most common reactions at the treatment sites were erythema (51%) and eschar formation (23%) on day 30.
According to Dr. Levin, promising future applications of nano-pulse stimulation include treatment of syringomas, dermatofibromas, and basal cell carcinomas.
Dr. Levin reported financial interest in Accure Acne, Avava Medical, and Soltego. The CellFX system was developed and is marketed by Pulse Biosciences.
During a virtual course on laser and aesthetic skin therapy, Yakir Levin, MD, PhD, likened nano-pulse stimulation to microneedling or radiofrequency microneedling “in that you have an array of microneedles that go into the skin,” he said. “However, it is actually completely different.”
The CellFX System uses nano-pulse stimulation to deliver ultrashort electrical energy pulses into the skin of target lesions via a console-based handheld applicator. In September 2022, the Food and Drug Administration cleared the CellFX system for treatment of sebaceous hyperplasia in patients with Fitzpatrick skin types I-II. This followed a general clearance of the device in 2021 for dermatologic procedures requiring ablation and resurfacing of the skin.
Pulses from the device deliver a “constant electrical potential gradient across cell membranes and organelle membranes, causing them to break down,” explained Dr. Levin, a dermatologist and physician scientist at Massachusetts General Hospital, Boston, where he practices cosmetic dermatology and conducts research on birthmarks in children. This creates pores in those membranes “and leads to a controlled form of cell death,” he said. “As a result, this treatment is limited to cells, so you can do it in the dermis without damaging the collagen network. It spares tissue that’s outside of the field, and it’s nonthermal.”
Images from electron microscopy have demonstrated swelling of the mitochondria and breakdown of nuclei within 2 hours of treatment in a rat study. “Within 1 day of treatment you have death of the cells and the beginning of involution of the lesion,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “This presents us with the opportunity to treat dermal lesions without causing damage to the epidermis or to the acellular portion of the dermis.”
In published studies, nano-pulse stimulation has been shown to be effective for treating sebaceous hyperplasia and warts. According to Dr. Levin, clinicians typically treat sebaceous hyperplasia with an radiofrequency microneedle or electrodesiccation, “where we shave off the top but do not try to hit the bottom because we don’t want to cause scarring of the dermis,” he said. “Using the nano-pulse stimulation technology, however, you end up with involution of the sebaceous lesion without damaging the surrounding dermis.”
In a prospective, randomized study, 72 individuals with sebaceous gland hyperplasia received nano-pulse stimulation to 222 lesions and they returned for three to four follow-up evaluations with photographs. At the final study visit, investigators rated 99.6% of the sebaceous gland lesions as clear or mostly clear, while 79% of the study participants said they were “satisfied” or “mostly satisfied” with the outcome.
At posttreatment day 60, 55% of the lesions were judged to have no hyperpigmentation and 31% exhibited mild posttreatment hyperpigmentation.
In a more recent study, researchers used the CellFX System to treat 195 cutaneous warts up to 10 mm wide in 62 individuals enrolled at one of five sites. They found that 75% of common warts, 73% of flat warts, and 44% of plantar warts were completely clear 60 days following the last nano-pulse stimulation treatment and did not recur within the 120-day observation period.
The most common reactions at the treatment sites were erythema (51%) and eschar formation (23%) on day 30.
According to Dr. Levin, promising future applications of nano-pulse stimulation include treatment of syringomas, dermatofibromas, and basal cell carcinomas.
Dr. Levin reported financial interest in Accure Acne, Avava Medical, and Soltego. The CellFX system was developed and is marketed by Pulse Biosciences.
During a virtual course on laser and aesthetic skin therapy, Yakir Levin, MD, PhD, likened nano-pulse stimulation to microneedling or radiofrequency microneedling “in that you have an array of microneedles that go into the skin,” he said. “However, it is actually completely different.”
The CellFX System uses nano-pulse stimulation to deliver ultrashort electrical energy pulses into the skin of target lesions via a console-based handheld applicator. In September 2022, the Food and Drug Administration cleared the CellFX system for treatment of sebaceous hyperplasia in patients with Fitzpatrick skin types I-II. This followed a general clearance of the device in 2021 for dermatologic procedures requiring ablation and resurfacing of the skin.
Pulses from the device deliver a “constant electrical potential gradient across cell membranes and organelle membranes, causing them to break down,” explained Dr. Levin, a dermatologist and physician scientist at Massachusetts General Hospital, Boston, where he practices cosmetic dermatology and conducts research on birthmarks in children. This creates pores in those membranes “and leads to a controlled form of cell death,” he said. “As a result, this treatment is limited to cells, so you can do it in the dermis without damaging the collagen network. It spares tissue that’s outside of the field, and it’s nonthermal.”
Images from electron microscopy have demonstrated swelling of the mitochondria and breakdown of nuclei within 2 hours of treatment in a rat study. “Within 1 day of treatment you have death of the cells and the beginning of involution of the lesion,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “This presents us with the opportunity to treat dermal lesions without causing damage to the epidermis or to the acellular portion of the dermis.”
In published studies, nano-pulse stimulation has been shown to be effective for treating sebaceous hyperplasia and warts. According to Dr. Levin, clinicians typically treat sebaceous hyperplasia with an radiofrequency microneedle or electrodesiccation, “where we shave off the top but do not try to hit the bottom because we don’t want to cause scarring of the dermis,” he said. “Using the nano-pulse stimulation technology, however, you end up with involution of the sebaceous lesion without damaging the surrounding dermis.”
In a prospective, randomized study, 72 individuals with sebaceous gland hyperplasia received nano-pulse stimulation to 222 lesions and they returned for three to four follow-up evaluations with photographs. At the final study visit, investigators rated 99.6% of the sebaceous gland lesions as clear or mostly clear, while 79% of the study participants said they were “satisfied” or “mostly satisfied” with the outcome.
At posttreatment day 60, 55% of the lesions were judged to have no hyperpigmentation and 31% exhibited mild posttreatment hyperpigmentation.
In a more recent study, researchers used the CellFX System to treat 195 cutaneous warts up to 10 mm wide in 62 individuals enrolled at one of five sites. They found that 75% of common warts, 73% of flat warts, and 44% of plantar warts were completely clear 60 days following the last nano-pulse stimulation treatment and did not recur within the 120-day observation period.
The most common reactions at the treatment sites were erythema (51%) and eschar formation (23%) on day 30.
According to Dr. Levin, promising future applications of nano-pulse stimulation include treatment of syringomas, dermatofibromas, and basal cell carcinomas.
Dr. Levin reported financial interest in Accure Acne, Avava Medical, and Soltego. The CellFX system was developed and is marketed by Pulse Biosciences.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Laser and light devices for acne treatment continue to advance
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Laser pioneer reflects on the future of robots in dermatology
In the opinion of R. Rox Anderson, MD, it’s only a matter of time before true robots make further inroads in dermatology.
“We humans just can’t do everything perfectly,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “We have limited speed and special accuracy and are not good at repetitive tasks. We can’t see in the UV or infrared, and we’re qualitative, not quantitative. ... We’re good at high-level visual assessment.”
During a presentation at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center, he distinguished between robotics and true robots. A prime example of robotics in medicine is the Da Vinci Surgical System in which a human user “is controlling every movement of this device with capabilities that humans don’t have, such as fine movement and high magnification of imaging,” said Dr. Anderson, who conceived and developed many of the nonscarring laser treatments now widely used in dermatology. “In the military, we have drone aircraft. The pilot is perhaps thousands of miles away; it’s still run by a human being in every way.”
By contrast, true robots are devices in which a human being programs the rules for action but the action itself is not exactly predictable. Artificial intelligence enables robots to perform certain tasks. “If you look at an Amazon warehouse, there’s barely anyone there; robots are packing and unpacking the shelves,” Dr. Anderson said.
Currently, he said, one true robot exists in dermatology: the Food and Drug Administration–cleared ARTAS Robotic Hair Restoration System, which precisely dissects follicular units from the donor area and eliminates the potential for human error. The device “extracts single follicular units from the occipital scalp and makes them available to the surgeon to do an artistic human job of implanting them in the frontal scalp,” Dr. Anderson said.
He predicts that a Mohs surgery robot with image-guided laser ablation would “launch a sea change in the whole field of surgical oncology, and I believe we are in a good position to do it. Everything for this is now sitting on the shelf and it’s unbelievable to me that a company hasn’t accomplished it yet.”
He would also like to see a true laser robot for surgery of tumors that would enable clinicians to download an app for their existing laser instead of having to buy a new device. Currently, “it takes about a half second to make a good optical coherence tomography image of basal cell carcinoma,” he said. “That image could be used for real-time robotic human control of, say, a laser to extirpate the tumor.”
Dr. Anderson’s “wish list” of applications for treatment with a robotic fractional laser includes those that target the sweat glands, sebaceous glands, nerves, inflammatory cells, white hair, blood vessels, lymphatics, hair, tumors, nevi, cysts, and surface contour. “It might be possible to have one software-programmable laser robot for many different applications in dermatology,” he added.
Dr. Anderson reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
In the opinion of R. Rox Anderson, MD, it’s only a matter of time before true robots make further inroads in dermatology.
“We humans just can’t do everything perfectly,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “We have limited speed and special accuracy and are not good at repetitive tasks. We can’t see in the UV or infrared, and we’re qualitative, not quantitative. ... We’re good at high-level visual assessment.”
During a presentation at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center, he distinguished between robotics and true robots. A prime example of robotics in medicine is the Da Vinci Surgical System in which a human user “is controlling every movement of this device with capabilities that humans don’t have, such as fine movement and high magnification of imaging,” said Dr. Anderson, who conceived and developed many of the nonscarring laser treatments now widely used in dermatology. “In the military, we have drone aircraft. The pilot is perhaps thousands of miles away; it’s still run by a human being in every way.”
By contrast, true robots are devices in which a human being programs the rules for action but the action itself is not exactly predictable. Artificial intelligence enables robots to perform certain tasks. “If you look at an Amazon warehouse, there’s barely anyone there; robots are packing and unpacking the shelves,” Dr. Anderson said.
Currently, he said, one true robot exists in dermatology: the Food and Drug Administration–cleared ARTAS Robotic Hair Restoration System, which precisely dissects follicular units from the donor area and eliminates the potential for human error. The device “extracts single follicular units from the occipital scalp and makes them available to the surgeon to do an artistic human job of implanting them in the frontal scalp,” Dr. Anderson said.
He predicts that a Mohs surgery robot with image-guided laser ablation would “launch a sea change in the whole field of surgical oncology, and I believe we are in a good position to do it. Everything for this is now sitting on the shelf and it’s unbelievable to me that a company hasn’t accomplished it yet.”
He would also like to see a true laser robot for surgery of tumors that would enable clinicians to download an app for their existing laser instead of having to buy a new device. Currently, “it takes about a half second to make a good optical coherence tomography image of basal cell carcinoma,” he said. “That image could be used for real-time robotic human control of, say, a laser to extirpate the tumor.”
Dr. Anderson’s “wish list” of applications for treatment with a robotic fractional laser includes those that target the sweat glands, sebaceous glands, nerves, inflammatory cells, white hair, blood vessels, lymphatics, hair, tumors, nevi, cysts, and surface contour. “It might be possible to have one software-programmable laser robot for many different applications in dermatology,” he added.
Dr. Anderson reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
In the opinion of R. Rox Anderson, MD, it’s only a matter of time before true robots make further inroads in dermatology.
“We humans just can’t do everything perfectly,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “We have limited speed and special accuracy and are not good at repetitive tasks. We can’t see in the UV or infrared, and we’re qualitative, not quantitative. ... We’re good at high-level visual assessment.”
During a presentation at the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center, he distinguished between robotics and true robots. A prime example of robotics in medicine is the Da Vinci Surgical System in which a human user “is controlling every movement of this device with capabilities that humans don’t have, such as fine movement and high magnification of imaging,” said Dr. Anderson, who conceived and developed many of the nonscarring laser treatments now widely used in dermatology. “In the military, we have drone aircraft. The pilot is perhaps thousands of miles away; it’s still run by a human being in every way.”
By contrast, true robots are devices in which a human being programs the rules for action but the action itself is not exactly predictable. Artificial intelligence enables robots to perform certain tasks. “If you look at an Amazon warehouse, there’s barely anyone there; robots are packing and unpacking the shelves,” Dr. Anderson said.
Currently, he said, one true robot exists in dermatology: the Food and Drug Administration–cleared ARTAS Robotic Hair Restoration System, which precisely dissects follicular units from the donor area and eliminates the potential for human error. The device “extracts single follicular units from the occipital scalp and makes them available to the surgeon to do an artistic human job of implanting them in the frontal scalp,” Dr. Anderson said.
He predicts that a Mohs surgery robot with image-guided laser ablation would “launch a sea change in the whole field of surgical oncology, and I believe we are in a good position to do it. Everything for this is now sitting on the shelf and it’s unbelievable to me that a company hasn’t accomplished it yet.”
He would also like to see a true laser robot for surgery of tumors that would enable clinicians to download an app for their existing laser instead of having to buy a new device. Currently, “it takes about a half second to make a good optical coherence tomography image of basal cell carcinoma,” he said. “That image could be used for real-time robotic human control of, say, a laser to extirpate the tumor.”
Dr. Anderson’s “wish list” of applications for treatment with a robotic fractional laser includes those that target the sweat glands, sebaceous glands, nerves, inflammatory cells, white hair, blood vessels, lymphatics, hair, tumors, nevi, cysts, and surface contour. “It might be possible to have one software-programmable laser robot for many different applications in dermatology,” he added.
Dr. Anderson reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE