User login
Buprenorphine update: Looser rules and a helpful injectable
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
SAN FRANCISCO – As the opioid epidemic continues to grow and evolve, the federal government is trying to make it easier for clinicians to treat abusers with the drug buprenorphine, psychiatrists told colleagues at the annual meeting of the American Psychiatric Association. And an injectable version of the drug is making a big difference.
John A. Renner Jr., MD, of Boston University, said in a presentation at the APA meeting.
As Dr. Renner explained, the United States is now in the fourth wave of nearly a quarter-century of opioid overdose-related deaths. The outbreak began in 1999 as prescription opioids spurred deaths, and heroin overdoses began to rise in 2010. The third wave brought rises in deaths from synthetic opioids such as fentanyl in 2013. In 2015, the fourth wave – driven by deaths from combinations of synthetic opioids and psychostimulants like methamphetamines – started in 2015.
COVID-19 seems to have played a role too: In 2020, opioid overdose deaths spiked during the early months of the pandemic. In 2021, drug-related overdose deaths overall hit a high of 106,889, including 80,411 linked to opioids. In contrast, fewer than 20,000 drug-related overdose deaths were reported in 1999.
On the other hand, deaths from prescription drug overdoses are falling, Dr. Renner said, suggesting “improvement in terms of how clinicians are handling medications and our prescribing practices. But that’s being masked by what’s happened with fentanyl and methamphetamine.”
Buprenorphine (Subutex), used to treat opioid use withdrawal, is itself an opioid and can cause addiction and death in some cases. However, Dr. Renner highlighted a 2023 study that determined that efforts to increase its use from 2019 to 2021 didn’t appear to boost buprenorphine-related overdose deaths in the United States.
New federal regulations aim to make it easier to prescribe buprenorphine. Thanks to Congressional legislation, the Drug Enforcement Administration in January 2023 eliminated regulations requiring clinicians to undergo special training to get an “X-waiver” to be able to prescribe buprenorphine. But they’re not off the hook entirely: As of June 27, 2023, providers must have undergone training in order to apply for – or renew – a DEA license to prescribe certain controlled substances like buprenorphine.
“I’m afraid that people will be able to meet that requirement easily, and they’re not going to get good coordinated teaching,” Dr. Renner said. “I’m not sure that’s really going to improve the quality of care that we’re delivering.”
In regard to treatment, psychiatrist Dong Chan Park, MD, of Boston University, touted a long-acting injectable form of buprenorphine known by the brand name Sublocade. The FDA approved Sublocade in 2017 for patients who’ve been taking sublingual buprenorphine for at least 7 days, although Dr. Park said research suggests the 7-day period may not be necessary.
“We’ve utilized this about 2.5-plus years in my hospital, and it’s really been a game changer for some of our sickest, most challenging patients,” he said at the APA presentation. As he explained, one benefit is that patients can’t repeatedly avoid doses depending on how they feel, as they may do with the sublingual version. “On the first day of injection, you can actually stop the sublingual buprenorphine.”
Dr. Renner emphasized the importance of getting users on buprenorphine as fast as possible. If the treatment begins in the ED, he said, “they need to have a system that is going to be able to pick them up and continue the care.”
Otherwise, the risk is high. “We’re in a very dangerous era,” he said, “where the patient walks out the door, and then they die.”
Dr. Park had no disclosures, and Dr. Renner disclosed royalties from the APA.
AT APA 2023
Serious complications due to ‘huffing’
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
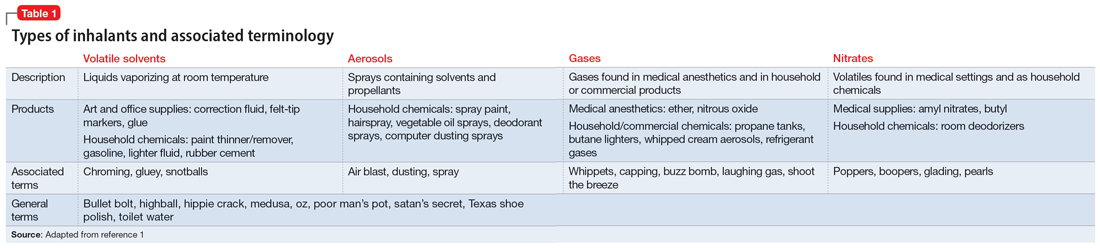
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).
The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
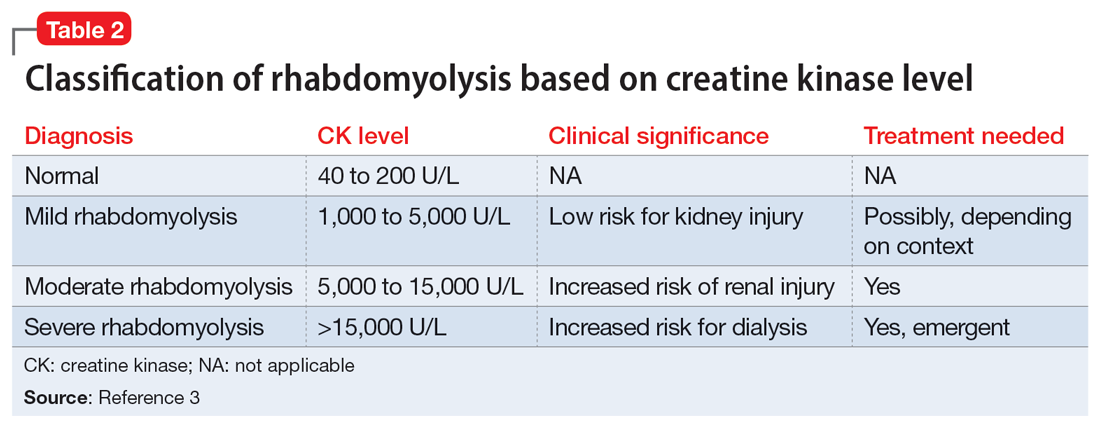
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).
Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).

The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).

Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
CASE A relapse and crisis
Ms. G, age 32, is brought to the emergency department (ED) by police after being found in a stupor-like state in a public restroom. The consultation-liaison (CL) psychiatry team assesses her for concerns of self-harm and suicide behavior. Ms. G discloses that she “huffs” an average of 4 canisters of air dusters daily to cope with psychosocial stressors and achieve a euphoric state. She recently lost her job, which led to homelessness, financial difficulties, a relapse to aerosol use after 2 years of abstinence, and stealing aerosol cans. The latest incident follows 2 prior arrests, which led officers to bring her to the ED for medical evaluation. Ms. G has a history of bipolar disorder (BD), generalized anxiety disorder (GAD), insomnia, and inhalant use disorder.
HISTORY Inhalant abuse and suicide attempt
Ms. G reports a longstanding history of severe inhalant abuse, primarily with air dusters due to their accessibility and low cost. She previously underwent inpatient rehab for inhalant abuse, and received inpatient psychiatry treatment 5 years ago for a suicide attempt by overdose linked to psychosocial stressors. In addition to BD, GAD, insomnia, and inhalant use disorder, Ms. G has a history of neuropathy, seizures, and recurrent hypokalemia. She is single and does not have insurance.
[polldaddy:12318871]
The authors’ observations
Inhalant abuse is the intentional inhalation of volatile substances to achieve an altered mental state. Inhalants are commercially available products that can produce intoxication if inhaled, such as glue, toluene, spray paint, gasoline, and lighter fluid (Table 11).

The epidemiology of inhalant abuse is difficult to accurately report due to a lack of recognition and social stigma. Due to inhalants’ ease of access and low cost, this form of substance abuse is popular among adolescents, adults of low socioeconomic status, individuals who live in rural areas, and those living in institutions. Inhalants act as reinforcers, producing a euphoric state. Rapid pulmonary absorption and lipid solubility of the substance rapidly alters the brain. Inhalant abuse can result in chemical and thermal burns, withdrawal symptoms, persistent mental illness, and catastrophic medical emergencies such as ventricular arrhythmias leading to disruptive myocardial electrical propagation. Chronic abuse can cause irreversible neurological and neuropsychological effects, cardiomyopathy, rapid airway compromise, pulmonary debilitations, renal tubular acidosis, bone marrow toxicity, reduced immunity, and peripheral neuropathy.2 Ms. G’s diagnosis of inhalant use disorder was based on her mental state and history of severe inhalant misuse, specifically with air dusters. Several additional factors further support this diagnosis, including the fact she survived a suicide attempt by overdose 5 years ago, had an inpatient rehabilitation placement for inhalant abuse, experiences insomnia, and was attempting to self-treat a depressive episode relapse with inhalants.
EVALUATION Depressed but cooperative
After being monitored in the ED for several hours, Ms. G is no longer in a stupor-like state. She has poor body habitus, appears older than her stated age, and is unkempt in appearance/attire. She is mildly distressed but relatively cooperative and engaged during the interview. Ms. G has a depressed mood and is anxious, with mood-congruent affect, and is tearful at times, especially when discussing recent stressors. She denies suicidality, homicidality, paranoia, delusions, and hallucinations. Her thought process is linear, goal-directed, and logical. She has fair insight, but relatively poor and impulsive judgment. The nursing staff expresses concerns that Ms. G was possibly responding to internal stimuli and behaving bizarrely during her initial presentation; this was not evident upon examination.
Ms. G reports having acute-on-chronic headaches, intermittent myalgias and weakness in her lower extremities (acute), and polyneuropathy (chronic). She denies a history of manic episodes or psychosis but reports previous relative hypomanic episodes that vacillated with periods of recurrent depressive episodes. Ms. G denies using illicit substances other than tobacco and inhalants. She says she had adhered to her outpatient psychiatric management services and medication regimen (duloxetine 60 mg/d at bedtime for mood/migraines, trazodone 150 mg/d at bedtime for insomnia, ziprasidone 40 mg/d at bedtime for BD, carbamazepine 200 mg twice daily for neuropathy/migraines, gabapentin 400 mg 3 times daily for neuropathy migraines/anxiety, and propranolol 10 mg 3 times daily for anxiety/tremors/migraine prophylaxis) until 4 days before her current presentation to the ED, when she used inhalants and was arrested.
Ms. G’s vitals are mostly unremarkable, but her heart rate is 116 beats per minute. There are no acute findings on physical examination. She is not pregnant, and her creatinine, glomerular filtration rate, complete blood count, and thyroid-stimulating hormone are all within normal limits. Her blood sugar is high (120 mg/dL; reference range 70 to 100 mg/dL). She has slight transaminitis with high aspartate aminotransferase (93 U/L; reference range 17 to 59 U/L) and high alanine aminotransferase (69 U/L; reference range 20 to 35 U/L); chronic hypokalemia (2.4 mmol/L; reference range 3.5 to 5.2 mmol/L), which leads the primary team to initiate a potassium replacement protocol; lactic acidosis (2.2 mmol/L; normal levels <2 mmol/L); and creatine kinase (CK) 5,930 U/L.
[polldaddy:12318873]
Continue to: The authors' observations
The authors’ observations
Efforts to improve the laboratory diagnosis of inhalant abuse are ongoing, but they have not yet been widely implemented. Systemic screening and assessment of inhalant use can help prevent and treat complications. For Ms. G, we considered several possible complications, including hypoglycemia. Although the classic triad of myalgia, weakness, and myoglobinuria (tea-colored urine) was not present, elevated CK levels in the context of Ms. G’s intermittent myalgia and lower extremity weakness led us to suspect she was experiencing moderate rhabdomyolysis (Table 23).

Rhabdomyolysis can be caused by several factors, including drug abuse, trauma, neuromuscular syndrome, and immobility. Treatment is mainly supportive, with a focus on preserving the ABCs (airway, breathing, circulation) and renal function through vigorous rehydration.4 We postulated Ms. G’s rhabdomyolysis was caused by muscle damage directly resulting from inhalant abuse and compounded by her remaining in prolonged fixed position on the ground after overdosing on inhalants.
TREATMENT Rehydration and psychotropics
The treatment team initiates IV fluid hydration of chloride 0.9% 150 mL/h and monitors Ms. G until she is stable and the trajectory of her CK levels begins to decline. On hospital Day 2, Ms. G’s CK decreases to 2,475 U/L and her lactic acid levels normalize. Ms. G restarts her regimen of duloxetine 60 mg/d, trazodone 150 mg/d, ziprasidone 40 mg/d, carbamazepine 200 mg twice daily, gabapentin 400 mg 3 times daily, and propranolol 10 mg 3 times daily. The team adds quetiapine 25 mg as needed for hallucinations, paranoia, and/or anxiety. Ms. G is closely monitored due to the potential risk of toxicity-induced or withdrawal-induced psychotic symptoms.
[polldaddy:12318869]
The authors’ observations
Presently, there are no effective treatments for acute inhalant intoxication or withdrawal, which makes supportive care and vigilant monitoring the only options.5 Although clinical research has not led to any FDA-approved treatments for chronic inhalant use disorder, a multipronged biopsychosocial treatment approach is critical in light of the negative consequences of inhalant abuse, including poor academic performance, criminal behavior, abuse of other substances, social maladjustment, low self-esteem, and suicidality.6
Ms. G had a moderate form of rhabdomyolysis, which was managed with IV fluid rehydration. Education and counseling were crucial to help Ms. G understand the unintended complications and potentially life-threatening consequences of inhalant abuse, with rehabilitation services to encourage abstinence. Ms. G had previously undergone successful inpatient rehabilitation and was willing to start such services again. She reported success with gabapentin for her polyneuropathy and migraines, which may be long-term consequences of prolonged inhalant abuse with neurological lesions. Ziprasidone may have mitigated some of the impulsivity and hypomanic symptoms of her BD that could make her more likely to engage in risky self-harm behaviors.
Continue to: After extensive discussion...
After extensive discussion on the long-term complications of inhalant abuse, Ms. G was motivated, cooperative, and sought care to return to rehabilitation services. The CL psychiatry team collaborated with the social work team to address the psychosocial components of Ms. G’s homelessness and facilitated an application for a local resource to obtain rehabilitation placement and living assistance. Her years of abstinence from inhalant use and success with rehabilitation demonstrate the need for a multimodal approach to manage and treat inhalant use disorder. Outpatient follow-up arrangements were made with local mental health resources.
OUTCOME Improved outlook and discharge
Ms. G reports improved mood and willingness to change her substance use habits. The treatment team counsels her on the acute risk of fatal arrhythmias and end-organ complications of inhalant abuse. They warn her about the potential long-term effects of mood alterations, neurological lesions, and polyneuropathy that could possibly worsen with substance abuse. Ms. G expresses appreciation for this counseling, the help associated with her aftercare, and the referral to restart the 30-day inpatient rehabilitation services. The team arranges follow-up with outpatient psychiatry and outpatient therapy services to enhance Ms. G’s coping skills and mitigate her reliance on inhalants to regulate her mood.
Bottom Line
Inhalant use is a poorly understood form of substance abuse that disproportionately affects vulnerable populations. It can lead to life-threatening medical emergencies such as rhabdomyolysis. Clinicians need to be able to identify and manage inhalant abuse and associated complications, as well as provide appropriate education and counseling to prevent further misuse.
Related Resources
- Gude J, Bisen V, Fujii K. Medication-induced rhabdomyolysis. Current Psychiatry. 2023;22(2):39-40. doi:10.12788/cp.0332
- Waldman W, Kabata PM, Dines AM, et al. Rhabdomyolysis related to acute recreational drug toxicity--a Euro-DEN study. PLoS One. 2021;16(3):e0246297. doi:10.1371/journal. pone.0246297
Drug Brand Names
Carbamazepine • Tegretol
Duloxetine • Cymbalta
Gabapentin • Neurontin
Propranolol • Inderal
Quetiapine • Seroquel
Trazodone • Oleptro
Ziprasidone • Geodon
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
1. Ahern NR, Falsafi N. Inhalant abuse: youth at risk. J Psychosoc Nurs Ment Health Serv. 2013;51(8):19-24. doi:10.3928/02793695-20130612-02
2. Howard MO, Bowen SE, Garland EL, et al. Inhalant use and inhalant use disorders in the United States. Addict Sci Clin Prac. 2011;6(1):18-31.
3. Farkas J. Rhabdomyolysis. Internet Book of Critical Care. June 25, 2021. Accessed February 24, 2023. https://emcrit.org/ibcc/rhabdo/
4. Torres PA, Helmstetter JA, Kaye AM, et al. Rhabdomyolysis: pathogenesis, diagnosis, and treatment. Ochsner J. 2015;15(1):58-69.
5. Muller AA, Muller GF. Inhalant abuse. J Emerg Nurs. 2006;32(5):447-448. doi:10.1016/j.jen.2006.05.018
6. Kozel N, Sloboda Z, De La Rosa M, eds. Epidemiology of Inhalant Abuse: An International Perspective; Nida Research Monograph 148. National Institute on Drug Abuse Research, US Dept of Health and Human Services; 1995. Accessed April 20, 2023. https://archives.nida.nih.gov/sites/default/files/monograph148.pdf
Exercise and empathy can help back pain patients in primary care
Treatment of chronic back pain remains a challenge for primary care physicians, and a new Cochrane Review confirms previous studies suggesting that analgesics and antidepressants fall short in terms of relief.
Data from another Cochrane Review support the value of exercise for chronic low back pain, although it is often underused, and the Food and Drug Administration’s recent approval of a spinal cord stimulation device for chronic back pain opens the door for another alternative.
Regardless of treatment type, however, patients report that empathy and clear communication from their doctors go a long way in their satisfaction with pain management, according to another recent study.
Exercise helps when patients adhere
The objective of the Cochrane Review on “Exercise therapy for chronic low back pain” was to determine whether exercise improves pain and functioning for people with chronic low back pain, compared with no treatment, usual care, or other common treatments, corresponding author Jill Hayden, PhD, of Dalhousie University, Halifax, N.S., said in an interview.
When back pain is chronic, it is expensive in terms of health care costs and lost work hours, said Dr. Hayden. “Exercise is promoted in many guidelines and is often recommended for, and used by, people with chronic low back pain.” However, “systematic reviews have found only small treatment effects, with considerable variation across individual trials.”
The 2021 review is one of the largest in the Cochrane Library, and included 249 trials and 24,486 study participants. However, Dr. Hayden said she had been disappointed by the methodological limitations of many of the trials. “The field is saturated with small exercise trials, many of which suffer from poor planning, conduct, and reporting due to limited resources.”
In the current review, “we found that exercise is likely to be effective for chronic low back pain. Overall, 3 months after the start of treatment, people receiving exercise treatment rated their pain an average of 15 points better on a scale of 0-100, and functional limitations were 7 points better, compared to people who had no treatment or usual care,” said Dr. Hayden.
Barriers to the use of exercise to treat pain may include fear of movement on the part of patients, she noted.
“Although our related network meta-analysis found some differences between specific types of exercise, we found all exercise types are more effective than minimal treatment,” she said. “People with chronic low back pain should be encouraged to do exercises that they enjoy and will do consistently to promote adherence.”
Limitations of medications
Both the safety and effectiveness of analgesics and antidepressants for pain in general and back pain in particular have come under scrutiny in recent research. A study published online in the British Medical Journal of patients with acute low back pain found that, although some medications were associated with large reductions in pain intensity, compared with placebo, the quality of the studies was “low or very low confidence,” according to a Medscape report on the findings.
This conclusion was supported in a large-scale analysis of the safety and effectiveness of antidepressants in chronic pain conditions, including back pain.
A new Cochrane Review led by a team of researchers in the United Kingdom found inadequate evidence to support the effectiveness of most antidepressants used for chronic pain, including amitriptyline, fluoxetine, citalopram, paroxetine, sertraline, and duloxetine.
“While chronic pain remains one of the top causes of daily disability worldwide, clinicians’ choices at offering interventions are getting fewer, especially if they tend toward a medical model and want a pharmacological solution,” corresponding author Tamar Pincus, PhD, of the University of Southampton (England), said in an interview. “We now know that opioids harm patients, and the evidence for common analgesics such as paracetamol and ibuprofen, for some conditions such as back pain, suggest they are not effective and might cause harm. This leaves clinicians with few options, and the most common prescription, supported by guidelines, is antidepressants.”
The study found moderate evidence that duloxetine can reduce pain in the short term and improve physical activity and some evidence that milnacipran might also be effective, Dr. Pincus said. “For all other antidepressants, including the commonly prescribed amitriptyline, the evidence was poor. Of importance, the average length of trials was 10 weeks, so long-term effects for all antidepressants remain unknown, and side effects and adverse events were reported poorly, so we also don’t know if any antidepressants are harmful.”
The takeaway message for the management of back pain in particular? “If a clinician and a patient decide together that it would be a good idea to try an antidepressant to reduce pain, they should consider starting with duloxetine, the drug with supporting evidence,” she said.
Physician attitude matters
Antidepressants may not have much impact on chronic pain, but a physician’s empathy and support do, according to data from a registry study of more than 1,300 individuals.
Despite efforts and guidelines from multiple medical organizations to promote optimal pain management, “much remains unknown regarding how the patient-physician interaction affects the process of delivering medical care for chronic low back pain and, ultimately, patient satisfaction,” John C. Licciardone, DO, of the University of North Texas Health Science Center, Fort Worth, and colleagues wrote in Annals of Family Medicine.
Previous studies have examined the relationship between clinical outcomes and patient satisfaction, but data on patient satisfaction with medical care for chronic low back pain specifically are limited, they said.
The researchers reviewed data from a national pain registry of adults aged 21-79 years that included self-reported measures of physician communication and empathy, prescribing data for opioids, and outcomes data for pain intensity, physical function, and health-related quality of life.
In a multivariate analysis, physician empathy and physician communication showed the strongest associations with patient satisfaction (P < .001).
The researchers found a negligible correlation between opioid prescription and perceived physician empathy and communication, “although current physician prescribing of opioids was also associated with patient satisfaction,” they wrote.
“Our findings pertaining to physician empathy are intriguing because they do not necessarily involve a therapeutic alliance with the patient based on collaborative communication or the expectation of a therapeutic effect via pharmacotherapy,” the researchers wrote .
The findings were limited by several factors including the cross-sectional design that prevented conclusions about cause and effect, the researchers noted. “It is possible that prior improvements in pain intensity, physical function, or [health-related quality of life] might have prompted participants to report more favorable ratings for physician empathy, physician communication, or patient satisfaction at registry enrollment.” However, the study supports the view that patients with low back pain in particular value physicians who validate their concerns and symptoms, and who make an effort to communicate treatment plans clearly.
Back pain patients continue to challenge primary care
“Back pain is a major issue in U.S. health care, in part because too many people have tough physical jobs or longstanding injuries that become chronic,” William Golden, MD, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
“There are no magic bullets for a lot of back pain patients, so empathy and support are key drivers,” he noted. “Helping patients maximize functionality as opposed to seeking mythical cures is the stronger line of visit discussions, but that takes a bit of time and skill in interviewing.
“It is fairly well established that duloxetine is useful in pain management, especially when present with mood disorders, either primary or secondary to the back-related disability,” said Dr. Golden. “Greater dissemination of its utility is probably useful, as is the side effect profile of the drug as well,” given the “nasty discontinuation syndrome when the treatment is reduced or stopped.”
Looking ahead, “more research is needed about microsurgery, namely for whom and for what anatomic presentations,” said Dr. Golden. Other topics for further research include a better understanding about medical marijuana and pain management and its interactions and side effects with other opioids and muscle relaxants. “Polypharmacy is still an issue in this class of patient,” and many of these patients are frustrated and angry “so the psychosocial skills of the PCP can be greatly tested as well,” he said.
Empathy promotes patient adherence to treatment
The new opioid prescription guidelines have increased interest among clinicians in how to improve patient satisfaction with the care for back pain provided, Noel Deep, MD, said in an interview. “These studies address this concern and bring forth an important aspect of the physician-patient relationship, namely the human touch and empathy.”
“I have been a strong proponent of the trust and relationship between a physician and patient; displaying empathy and increased and transparent communication between the physician and the patient has always resulted in better relationships and better outcomes for patients, especially those dealing with chronic health concerns,” said Dr. Deep, who is a general internist in a multispecialty group practice with Aspirus Antigo (Wisc.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital, also in Antigo.
Potential barriers to effective pain management include beliefs and attitudes on the part of patients, Dr. Deep noted. “Physicians lacking adequate time to communicate effectively with the patient and describe nonopioid and nonsurgical interventions would be another potential barrier.” Other issues include the time and effort, as well as cost, associated with interventions such as physical therapy and other nondrug and nonsurgical interventions. Issues with family and social support and health literacy are also potential barriers to pain management.
Clinical takeaways
Low back pain is one of the most common reasons for a visit in primary care and can be “chronic and debilitating,” Grace Lin, MD, an internal medicine physician and primary care provider at the University of California, San Francisco, said in an interview.
“One issue with the Cochrane Review on exercise is that the studies on exercise were heterogeneous, so it’s difficult to know whether there is a particular kind of exercise that would be most effective and should be recommended to patients,” she said.
Furthermore, she said, “there is a physical therapist shortage in the U.S. I practice in a major city with a large health care system, and it can still take months to get an appointment with a physical therapist.” Also, insurance coverage may limit which therapists a patient can see and how many visits they can have.
“On the clinician side, I think physicians need to be better informed about the evidence base for back pain treatment, namely that exercise is effective and that, long term, analgesics are not,” Dr. Lin said. “This might decrease overprescription of ineffective analgesics and encourage more education about and referrals to physical therapy.”
“Physicians should continue to educate patients that physical therapy is the first-line treatment for back pain and that pain medications are secondary,” she said. “I think that analgesics can be effective for the short term to get people to a point where they feel well enough to do physical therapy. Duloxetine also appears to be moderately effective for chronic low back pain, in part because it may also help address coexisting depression and anxiety,” but these options should be reserved for adjuncts to physical therapy for back pain.
The findings from the study on empathy and communication suggest that the main challenges to these behaviors are systemic, said Dr. Lin.
“Our health care system is not conducive to treating chronic back pain,” she said. Primary care visits that last for 15 or 20 minutes are not long enough to diagnose and counsel patients on such a complex problem as chronic low back pain. Since back pain is usually not the only issue the primary care physician is dealing with during that visit, this can lead to patients feeling like their doctor isn’t listening to them and doesn’t care about their pain.
“We need to better understand the mechanisms by which people develop chronic, debilitating back pain,” Dr. Lin said. “I think if we understood this better, more effective and targeted treatments, both pharmacological and nonpharmacological, could be developed.”
The Annals of Family Medicine study received no outside funding, and the researchers had no financial conflicts to disclose. The Cochrane Reviews was supported by the National Institute for Health and Care Research’s Health Technology Assessment program, and the authors had no financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose and serve on the editorial advisory board of Internal Medicine News. Dr. Lin disclosed receiving research funding from the Institute for Clinical and Economic Review and the National Institutes of Health.
Treatment of chronic back pain remains a challenge for primary care physicians, and a new Cochrane Review confirms previous studies suggesting that analgesics and antidepressants fall short in terms of relief.
Data from another Cochrane Review support the value of exercise for chronic low back pain, although it is often underused, and the Food and Drug Administration’s recent approval of a spinal cord stimulation device for chronic back pain opens the door for another alternative.
Regardless of treatment type, however, patients report that empathy and clear communication from their doctors go a long way in their satisfaction with pain management, according to another recent study.
Exercise helps when patients adhere
The objective of the Cochrane Review on “Exercise therapy for chronic low back pain” was to determine whether exercise improves pain and functioning for people with chronic low back pain, compared with no treatment, usual care, or other common treatments, corresponding author Jill Hayden, PhD, of Dalhousie University, Halifax, N.S., said in an interview.
When back pain is chronic, it is expensive in terms of health care costs and lost work hours, said Dr. Hayden. “Exercise is promoted in many guidelines and is often recommended for, and used by, people with chronic low back pain.” However, “systematic reviews have found only small treatment effects, with considerable variation across individual trials.”
The 2021 review is one of the largest in the Cochrane Library, and included 249 trials and 24,486 study participants. However, Dr. Hayden said she had been disappointed by the methodological limitations of many of the trials. “The field is saturated with small exercise trials, many of which suffer from poor planning, conduct, and reporting due to limited resources.”
In the current review, “we found that exercise is likely to be effective for chronic low back pain. Overall, 3 months after the start of treatment, people receiving exercise treatment rated their pain an average of 15 points better on a scale of 0-100, and functional limitations were 7 points better, compared to people who had no treatment or usual care,” said Dr. Hayden.
Barriers to the use of exercise to treat pain may include fear of movement on the part of patients, she noted.
“Although our related network meta-analysis found some differences between specific types of exercise, we found all exercise types are more effective than minimal treatment,” she said. “People with chronic low back pain should be encouraged to do exercises that they enjoy and will do consistently to promote adherence.”
Limitations of medications
Both the safety and effectiveness of analgesics and antidepressants for pain in general and back pain in particular have come under scrutiny in recent research. A study published online in the British Medical Journal of patients with acute low back pain found that, although some medications were associated with large reductions in pain intensity, compared with placebo, the quality of the studies was “low or very low confidence,” according to a Medscape report on the findings.
This conclusion was supported in a large-scale analysis of the safety and effectiveness of antidepressants in chronic pain conditions, including back pain.
A new Cochrane Review led by a team of researchers in the United Kingdom found inadequate evidence to support the effectiveness of most antidepressants used for chronic pain, including amitriptyline, fluoxetine, citalopram, paroxetine, sertraline, and duloxetine.
“While chronic pain remains one of the top causes of daily disability worldwide, clinicians’ choices at offering interventions are getting fewer, especially if they tend toward a medical model and want a pharmacological solution,” corresponding author Tamar Pincus, PhD, of the University of Southampton (England), said in an interview. “We now know that opioids harm patients, and the evidence for common analgesics such as paracetamol and ibuprofen, for some conditions such as back pain, suggest they are not effective and might cause harm. This leaves clinicians with few options, and the most common prescription, supported by guidelines, is antidepressants.”
The study found moderate evidence that duloxetine can reduce pain in the short term and improve physical activity and some evidence that milnacipran might also be effective, Dr. Pincus said. “For all other antidepressants, including the commonly prescribed amitriptyline, the evidence was poor. Of importance, the average length of trials was 10 weeks, so long-term effects for all antidepressants remain unknown, and side effects and adverse events were reported poorly, so we also don’t know if any antidepressants are harmful.”
The takeaway message for the management of back pain in particular? “If a clinician and a patient decide together that it would be a good idea to try an antidepressant to reduce pain, they should consider starting with duloxetine, the drug with supporting evidence,” she said.
Physician attitude matters
Antidepressants may not have much impact on chronic pain, but a physician’s empathy and support do, according to data from a registry study of more than 1,300 individuals.
Despite efforts and guidelines from multiple medical organizations to promote optimal pain management, “much remains unknown regarding how the patient-physician interaction affects the process of delivering medical care for chronic low back pain and, ultimately, patient satisfaction,” John C. Licciardone, DO, of the University of North Texas Health Science Center, Fort Worth, and colleagues wrote in Annals of Family Medicine.
Previous studies have examined the relationship between clinical outcomes and patient satisfaction, but data on patient satisfaction with medical care for chronic low back pain specifically are limited, they said.
The researchers reviewed data from a national pain registry of adults aged 21-79 years that included self-reported measures of physician communication and empathy, prescribing data for opioids, and outcomes data for pain intensity, physical function, and health-related quality of life.
In a multivariate analysis, physician empathy and physician communication showed the strongest associations with patient satisfaction (P < .001).
The researchers found a negligible correlation between opioid prescription and perceived physician empathy and communication, “although current physician prescribing of opioids was also associated with patient satisfaction,” they wrote.
“Our findings pertaining to physician empathy are intriguing because they do not necessarily involve a therapeutic alliance with the patient based on collaborative communication or the expectation of a therapeutic effect via pharmacotherapy,” the researchers wrote .
The findings were limited by several factors including the cross-sectional design that prevented conclusions about cause and effect, the researchers noted. “It is possible that prior improvements in pain intensity, physical function, or [health-related quality of life] might have prompted participants to report more favorable ratings for physician empathy, physician communication, or patient satisfaction at registry enrollment.” However, the study supports the view that patients with low back pain in particular value physicians who validate their concerns and symptoms, and who make an effort to communicate treatment plans clearly.
Back pain patients continue to challenge primary care
“Back pain is a major issue in U.S. health care, in part because too many people have tough physical jobs or longstanding injuries that become chronic,” William Golden, MD, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
“There are no magic bullets for a lot of back pain patients, so empathy and support are key drivers,” he noted. “Helping patients maximize functionality as opposed to seeking mythical cures is the stronger line of visit discussions, but that takes a bit of time and skill in interviewing.
“It is fairly well established that duloxetine is useful in pain management, especially when present with mood disorders, either primary or secondary to the back-related disability,” said Dr. Golden. “Greater dissemination of its utility is probably useful, as is the side effect profile of the drug as well,” given the “nasty discontinuation syndrome when the treatment is reduced or stopped.”
Looking ahead, “more research is needed about microsurgery, namely for whom and for what anatomic presentations,” said Dr. Golden. Other topics for further research include a better understanding about medical marijuana and pain management and its interactions and side effects with other opioids and muscle relaxants. “Polypharmacy is still an issue in this class of patient,” and many of these patients are frustrated and angry “so the psychosocial skills of the PCP can be greatly tested as well,” he said.
Empathy promotes patient adherence to treatment
The new opioid prescription guidelines have increased interest among clinicians in how to improve patient satisfaction with the care for back pain provided, Noel Deep, MD, said in an interview. “These studies address this concern and bring forth an important aspect of the physician-patient relationship, namely the human touch and empathy.”
“I have been a strong proponent of the trust and relationship between a physician and patient; displaying empathy and increased and transparent communication between the physician and the patient has always resulted in better relationships and better outcomes for patients, especially those dealing with chronic health concerns,” said Dr. Deep, who is a general internist in a multispecialty group practice with Aspirus Antigo (Wisc.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital, also in Antigo.
Potential barriers to effective pain management include beliefs and attitudes on the part of patients, Dr. Deep noted. “Physicians lacking adequate time to communicate effectively with the patient and describe nonopioid and nonsurgical interventions would be another potential barrier.” Other issues include the time and effort, as well as cost, associated with interventions such as physical therapy and other nondrug and nonsurgical interventions. Issues with family and social support and health literacy are also potential barriers to pain management.
Clinical takeaways
Low back pain is one of the most common reasons for a visit in primary care and can be “chronic and debilitating,” Grace Lin, MD, an internal medicine physician and primary care provider at the University of California, San Francisco, said in an interview.
“One issue with the Cochrane Review on exercise is that the studies on exercise were heterogeneous, so it’s difficult to know whether there is a particular kind of exercise that would be most effective and should be recommended to patients,” she said.
Furthermore, she said, “there is a physical therapist shortage in the U.S. I practice in a major city with a large health care system, and it can still take months to get an appointment with a physical therapist.” Also, insurance coverage may limit which therapists a patient can see and how many visits they can have.
“On the clinician side, I think physicians need to be better informed about the evidence base for back pain treatment, namely that exercise is effective and that, long term, analgesics are not,” Dr. Lin said. “This might decrease overprescription of ineffective analgesics and encourage more education about and referrals to physical therapy.”
“Physicians should continue to educate patients that physical therapy is the first-line treatment for back pain and that pain medications are secondary,” she said. “I think that analgesics can be effective for the short term to get people to a point where they feel well enough to do physical therapy. Duloxetine also appears to be moderately effective for chronic low back pain, in part because it may also help address coexisting depression and anxiety,” but these options should be reserved for adjuncts to physical therapy for back pain.
The findings from the study on empathy and communication suggest that the main challenges to these behaviors are systemic, said Dr. Lin.
“Our health care system is not conducive to treating chronic back pain,” she said. Primary care visits that last for 15 or 20 minutes are not long enough to diagnose and counsel patients on such a complex problem as chronic low back pain. Since back pain is usually not the only issue the primary care physician is dealing with during that visit, this can lead to patients feeling like their doctor isn’t listening to them and doesn’t care about their pain.
“We need to better understand the mechanisms by which people develop chronic, debilitating back pain,” Dr. Lin said. “I think if we understood this better, more effective and targeted treatments, both pharmacological and nonpharmacological, could be developed.”
The Annals of Family Medicine study received no outside funding, and the researchers had no financial conflicts to disclose. The Cochrane Reviews was supported by the National Institute for Health and Care Research’s Health Technology Assessment program, and the authors had no financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose and serve on the editorial advisory board of Internal Medicine News. Dr. Lin disclosed receiving research funding from the Institute for Clinical and Economic Review and the National Institutes of Health.
Treatment of chronic back pain remains a challenge for primary care physicians, and a new Cochrane Review confirms previous studies suggesting that analgesics and antidepressants fall short in terms of relief.
Data from another Cochrane Review support the value of exercise for chronic low back pain, although it is often underused, and the Food and Drug Administration’s recent approval of a spinal cord stimulation device for chronic back pain opens the door for another alternative.
Regardless of treatment type, however, patients report that empathy and clear communication from their doctors go a long way in their satisfaction with pain management, according to another recent study.
Exercise helps when patients adhere
The objective of the Cochrane Review on “Exercise therapy for chronic low back pain” was to determine whether exercise improves pain and functioning for people with chronic low back pain, compared with no treatment, usual care, or other common treatments, corresponding author Jill Hayden, PhD, of Dalhousie University, Halifax, N.S., said in an interview.
When back pain is chronic, it is expensive in terms of health care costs and lost work hours, said Dr. Hayden. “Exercise is promoted in many guidelines and is often recommended for, and used by, people with chronic low back pain.” However, “systematic reviews have found only small treatment effects, with considerable variation across individual trials.”
The 2021 review is one of the largest in the Cochrane Library, and included 249 trials and 24,486 study participants. However, Dr. Hayden said she had been disappointed by the methodological limitations of many of the trials. “The field is saturated with small exercise trials, many of which suffer from poor planning, conduct, and reporting due to limited resources.”
In the current review, “we found that exercise is likely to be effective for chronic low back pain. Overall, 3 months after the start of treatment, people receiving exercise treatment rated their pain an average of 15 points better on a scale of 0-100, and functional limitations were 7 points better, compared to people who had no treatment or usual care,” said Dr. Hayden.
Barriers to the use of exercise to treat pain may include fear of movement on the part of patients, she noted.
“Although our related network meta-analysis found some differences between specific types of exercise, we found all exercise types are more effective than minimal treatment,” she said. “People with chronic low back pain should be encouraged to do exercises that they enjoy and will do consistently to promote adherence.”
Limitations of medications
Both the safety and effectiveness of analgesics and antidepressants for pain in general and back pain in particular have come under scrutiny in recent research. A study published online in the British Medical Journal of patients with acute low back pain found that, although some medications were associated with large reductions in pain intensity, compared with placebo, the quality of the studies was “low or very low confidence,” according to a Medscape report on the findings.
This conclusion was supported in a large-scale analysis of the safety and effectiveness of antidepressants in chronic pain conditions, including back pain.
A new Cochrane Review led by a team of researchers in the United Kingdom found inadequate evidence to support the effectiveness of most antidepressants used for chronic pain, including amitriptyline, fluoxetine, citalopram, paroxetine, sertraline, and duloxetine.
“While chronic pain remains one of the top causes of daily disability worldwide, clinicians’ choices at offering interventions are getting fewer, especially if they tend toward a medical model and want a pharmacological solution,” corresponding author Tamar Pincus, PhD, of the University of Southampton (England), said in an interview. “We now know that opioids harm patients, and the evidence for common analgesics such as paracetamol and ibuprofen, for some conditions such as back pain, suggest they are not effective and might cause harm. This leaves clinicians with few options, and the most common prescription, supported by guidelines, is antidepressants.”
The study found moderate evidence that duloxetine can reduce pain in the short term and improve physical activity and some evidence that milnacipran might also be effective, Dr. Pincus said. “For all other antidepressants, including the commonly prescribed amitriptyline, the evidence was poor. Of importance, the average length of trials was 10 weeks, so long-term effects for all antidepressants remain unknown, and side effects and adverse events were reported poorly, so we also don’t know if any antidepressants are harmful.”
The takeaway message for the management of back pain in particular? “If a clinician and a patient decide together that it would be a good idea to try an antidepressant to reduce pain, they should consider starting with duloxetine, the drug with supporting evidence,” she said.
Physician attitude matters
Antidepressants may not have much impact on chronic pain, but a physician’s empathy and support do, according to data from a registry study of more than 1,300 individuals.
Despite efforts and guidelines from multiple medical organizations to promote optimal pain management, “much remains unknown regarding how the patient-physician interaction affects the process of delivering medical care for chronic low back pain and, ultimately, patient satisfaction,” John C. Licciardone, DO, of the University of North Texas Health Science Center, Fort Worth, and colleagues wrote in Annals of Family Medicine.
Previous studies have examined the relationship between clinical outcomes and patient satisfaction, but data on patient satisfaction with medical care for chronic low back pain specifically are limited, they said.
The researchers reviewed data from a national pain registry of adults aged 21-79 years that included self-reported measures of physician communication and empathy, prescribing data for opioids, and outcomes data for pain intensity, physical function, and health-related quality of life.
In a multivariate analysis, physician empathy and physician communication showed the strongest associations with patient satisfaction (P < .001).
The researchers found a negligible correlation between opioid prescription and perceived physician empathy and communication, “although current physician prescribing of opioids was also associated with patient satisfaction,” they wrote.
“Our findings pertaining to physician empathy are intriguing because they do not necessarily involve a therapeutic alliance with the patient based on collaborative communication or the expectation of a therapeutic effect via pharmacotherapy,” the researchers wrote .
The findings were limited by several factors including the cross-sectional design that prevented conclusions about cause and effect, the researchers noted. “It is possible that prior improvements in pain intensity, physical function, or [health-related quality of life] might have prompted participants to report more favorable ratings for physician empathy, physician communication, or patient satisfaction at registry enrollment.” However, the study supports the view that patients with low back pain in particular value physicians who validate their concerns and symptoms, and who make an effort to communicate treatment plans clearly.
Back pain patients continue to challenge primary care
“Back pain is a major issue in U.S. health care, in part because too many people have tough physical jobs or longstanding injuries that become chronic,” William Golden, MD, professor of medicine and public health at the University of Arkansas for Medical Sciences, Little Rock, said in an interview.
“There are no magic bullets for a lot of back pain patients, so empathy and support are key drivers,” he noted. “Helping patients maximize functionality as opposed to seeking mythical cures is the stronger line of visit discussions, but that takes a bit of time and skill in interviewing.
“It is fairly well established that duloxetine is useful in pain management, especially when present with mood disorders, either primary or secondary to the back-related disability,” said Dr. Golden. “Greater dissemination of its utility is probably useful, as is the side effect profile of the drug as well,” given the “nasty discontinuation syndrome when the treatment is reduced or stopped.”
Looking ahead, “more research is needed about microsurgery, namely for whom and for what anatomic presentations,” said Dr. Golden. Other topics for further research include a better understanding about medical marijuana and pain management and its interactions and side effects with other opioids and muscle relaxants. “Polypharmacy is still an issue in this class of patient,” and many of these patients are frustrated and angry “so the psychosocial skills of the PCP can be greatly tested as well,” he said.
Empathy promotes patient adherence to treatment
The new opioid prescription guidelines have increased interest among clinicians in how to improve patient satisfaction with the care for back pain provided, Noel Deep, MD, said in an interview. “These studies address this concern and bring forth an important aspect of the physician-patient relationship, namely the human touch and empathy.”
“I have been a strong proponent of the trust and relationship between a physician and patient; displaying empathy and increased and transparent communication between the physician and the patient has always resulted in better relationships and better outcomes for patients, especially those dealing with chronic health concerns,” said Dr. Deep, who is a general internist in a multispecialty group practice with Aspirus Antigo (Wisc.) Clinic and the chief medical officer and a staff physician at Aspirus Langlade Hospital, also in Antigo.
Potential barriers to effective pain management include beliefs and attitudes on the part of patients, Dr. Deep noted. “Physicians lacking adequate time to communicate effectively with the patient and describe nonopioid and nonsurgical interventions would be another potential barrier.” Other issues include the time and effort, as well as cost, associated with interventions such as physical therapy and other nondrug and nonsurgical interventions. Issues with family and social support and health literacy are also potential barriers to pain management.
Clinical takeaways
Low back pain is one of the most common reasons for a visit in primary care and can be “chronic and debilitating,” Grace Lin, MD, an internal medicine physician and primary care provider at the University of California, San Francisco, said in an interview.
“One issue with the Cochrane Review on exercise is that the studies on exercise were heterogeneous, so it’s difficult to know whether there is a particular kind of exercise that would be most effective and should be recommended to patients,” she said.
Furthermore, she said, “there is a physical therapist shortage in the U.S. I practice in a major city with a large health care system, and it can still take months to get an appointment with a physical therapist.” Also, insurance coverage may limit which therapists a patient can see and how many visits they can have.
“On the clinician side, I think physicians need to be better informed about the evidence base for back pain treatment, namely that exercise is effective and that, long term, analgesics are not,” Dr. Lin said. “This might decrease overprescription of ineffective analgesics and encourage more education about and referrals to physical therapy.”
“Physicians should continue to educate patients that physical therapy is the first-line treatment for back pain and that pain medications are secondary,” she said. “I think that analgesics can be effective for the short term to get people to a point where they feel well enough to do physical therapy. Duloxetine also appears to be moderately effective for chronic low back pain, in part because it may also help address coexisting depression and anxiety,” but these options should be reserved for adjuncts to physical therapy for back pain.
The findings from the study on empathy and communication suggest that the main challenges to these behaviors are systemic, said Dr. Lin.
“Our health care system is not conducive to treating chronic back pain,” she said. Primary care visits that last for 15 or 20 minutes are not long enough to diagnose and counsel patients on such a complex problem as chronic low back pain. Since back pain is usually not the only issue the primary care physician is dealing with during that visit, this can lead to patients feeling like their doctor isn’t listening to them and doesn’t care about their pain.
“We need to better understand the mechanisms by which people develop chronic, debilitating back pain,” Dr. Lin said. “I think if we understood this better, more effective and targeted treatments, both pharmacological and nonpharmacological, could be developed.”
The Annals of Family Medicine study received no outside funding, and the researchers had no financial conflicts to disclose. The Cochrane Reviews was supported by the National Institute for Health and Care Research’s Health Technology Assessment program, and the authors had no financial conflicts to disclose. Dr. Golden and Dr. Deep had no financial conflicts to disclose and serve on the editorial advisory board of Internal Medicine News. Dr. Lin disclosed receiving research funding from the Institute for Clinical and Economic Review and the National Institutes of Health.
Talking tobacco with youth? Ask the right questions
There is good news and bad news regarding the use of tobacco products by young people in the United States, according to the recently released findings from the 2021 Youth Risk Behavior Survey (YRBS).1 The use of cigarettes among high school students declined from 36.4% in 1997 to 6.0% in 2019.2 However, young people have replaced cigarettes with other tobacco products, including electronic vapor products (EVPs). So we need to ask specifically about these products.
Known by many names. EVPs are referred to as e-cigarettes, vapes, hookah pens, and mods. They usually contain nicotine, which is highly addictive, can affect brain development, and may lead to smoking of cigarettes.3 The most common reasons young people say they use EVPs are feelings of anxiety, stress, and depression, as well as the “high” associated with nicotine use.4
Use of EVPs among youth. The YRBS, which includes a representative sample of public and private school students in grades 9 to 12 in the 50 states, categorizes the use of EVPs as
- ever use
- current use (≥ 1 use during the 30 days before the survey), and
- daily use (during the 30 days before the survey).
In 2021, 36.2% of young people reported ever use of EVPs (40.9% of females; 32.1% of males), 18% reported current use (21.4% of females; 14.9% of males), and 5% reported daily use (5.6% of females; 4.5% of males). Differences between racial and ethnic groups were minor, except for markedly lower rates in Asian youth (19.5% ever use, 5.5% current use, and 1.2% daily use).5
Current recommendations. The US Preventive Services Task Force (USPSTF) recommends education and brief counseling for school-age children and adolescents to prevent them from starting to use tobacco (including use of EVPs).6 The USPSTF also recommends tobacco cessation using behavioral interventions and/or pharmacotherapy for those ages 18 years and older.7
The USPSTF makes no recommendation on cessation for those younger than 18 years, citing weak evidence. However, it would be reasonable to offer behavioral interventions to younger current users. (Pharmacotherapy is not approved for use in children and adolescents.)
The take-home message. When we ask children and adolescents about use of tobacco products, we need to specifically mention EVPs and advise against their use.
1. CDC. Youth Risk Behavior Surveillance—United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):1-93. Accessed May 24, 2023. www.cdc.gov/mmwr/volumes/72/su/pdfs/su7201-h.pdf
2. Creamer MR, Everett Jones S, Gentzke AS, et al. Tobacco product use among high school students—Youth Risk Behavior Survey, United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(suppl 1):56-63. doi: 10.15585/mmwr.su6901a7
3. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. Accessed May 24, 2023. https://nap.nationalacademies.org/catalog/24952/public-health-consequences-of-e-cigarettes
4. Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ. 2022;71(no. SS-5):1-29. doi: 10.15585/mmwr.ss7105a1
5. Oliver BE, Jones SE, Hops ED, et al. Electronic vapor product use among high school students—Youth Risk Behavior Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):93-99. doi: 10.15585/mmwr.su7201a11
6. USPSTF. Tobacco use in children and adolescents: primary care interventions. Final recommendation statement. Published April 28, 2020. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
7. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Final recommendation statement. Published January 19, 2021. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
There is good news and bad news regarding the use of tobacco products by young people in the United States, according to the recently released findings from the 2021 Youth Risk Behavior Survey (YRBS).1 The use of cigarettes among high school students declined from 36.4% in 1997 to 6.0% in 2019.2 However, young people have replaced cigarettes with other tobacco products, including electronic vapor products (EVPs). So we need to ask specifically about these products.
Known by many names. EVPs are referred to as e-cigarettes, vapes, hookah pens, and mods. They usually contain nicotine, which is highly addictive, can affect brain development, and may lead to smoking of cigarettes.3 The most common reasons young people say they use EVPs are feelings of anxiety, stress, and depression, as well as the “high” associated with nicotine use.4
Use of EVPs among youth. The YRBS, which includes a representative sample of public and private school students in grades 9 to 12 in the 50 states, categorizes the use of EVPs as
- ever use
- current use (≥ 1 use during the 30 days before the survey), and
- daily use (during the 30 days before the survey).
In 2021, 36.2% of young people reported ever use of EVPs (40.9% of females; 32.1% of males), 18% reported current use (21.4% of females; 14.9% of males), and 5% reported daily use (5.6% of females; 4.5% of males). Differences between racial and ethnic groups were minor, except for markedly lower rates in Asian youth (19.5% ever use, 5.5% current use, and 1.2% daily use).5
Current recommendations. The US Preventive Services Task Force (USPSTF) recommends education and brief counseling for school-age children and adolescents to prevent them from starting to use tobacco (including use of EVPs).6 The USPSTF also recommends tobacco cessation using behavioral interventions and/or pharmacotherapy for those ages 18 years and older.7
The USPSTF makes no recommendation on cessation for those younger than 18 years, citing weak evidence. However, it would be reasonable to offer behavioral interventions to younger current users. (Pharmacotherapy is not approved for use in children and adolescents.)
The take-home message. When we ask children and adolescents about use of tobacco products, we need to specifically mention EVPs and advise against their use.
There is good news and bad news regarding the use of tobacco products by young people in the United States, according to the recently released findings from the 2021 Youth Risk Behavior Survey (YRBS).1 The use of cigarettes among high school students declined from 36.4% in 1997 to 6.0% in 2019.2 However, young people have replaced cigarettes with other tobacco products, including electronic vapor products (EVPs). So we need to ask specifically about these products.
Known by many names. EVPs are referred to as e-cigarettes, vapes, hookah pens, and mods. They usually contain nicotine, which is highly addictive, can affect brain development, and may lead to smoking of cigarettes.3 The most common reasons young people say they use EVPs are feelings of anxiety, stress, and depression, as well as the “high” associated with nicotine use.4
Use of EVPs among youth. The YRBS, which includes a representative sample of public and private school students in grades 9 to 12 in the 50 states, categorizes the use of EVPs as
- ever use
- current use (≥ 1 use during the 30 days before the survey), and
- daily use (during the 30 days before the survey).
In 2021, 36.2% of young people reported ever use of EVPs (40.9% of females; 32.1% of males), 18% reported current use (21.4% of females; 14.9% of males), and 5% reported daily use (5.6% of females; 4.5% of males). Differences between racial and ethnic groups were minor, except for markedly lower rates in Asian youth (19.5% ever use, 5.5% current use, and 1.2% daily use).5
Current recommendations. The US Preventive Services Task Force (USPSTF) recommends education and brief counseling for school-age children and adolescents to prevent them from starting to use tobacco (including use of EVPs).6 The USPSTF also recommends tobacco cessation using behavioral interventions and/or pharmacotherapy for those ages 18 years and older.7
The USPSTF makes no recommendation on cessation for those younger than 18 years, citing weak evidence. However, it would be reasonable to offer behavioral interventions to younger current users. (Pharmacotherapy is not approved for use in children and adolescents.)
The take-home message. When we ask children and adolescents about use of tobacco products, we need to specifically mention EVPs and advise against their use.
1. CDC. Youth Risk Behavior Surveillance—United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):1-93. Accessed May 24, 2023. www.cdc.gov/mmwr/volumes/72/su/pdfs/su7201-h.pdf
2. Creamer MR, Everett Jones S, Gentzke AS, et al. Tobacco product use among high school students—Youth Risk Behavior Survey, United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(suppl 1):56-63. doi: 10.15585/mmwr.su6901a7
3. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. Accessed May 24, 2023. https://nap.nationalacademies.org/catalog/24952/public-health-consequences-of-e-cigarettes
4. Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ. 2022;71(no. SS-5):1-29. doi: 10.15585/mmwr.ss7105a1
5. Oliver BE, Jones SE, Hops ED, et al. Electronic vapor product use among high school students—Youth Risk Behavior Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):93-99. doi: 10.15585/mmwr.su7201a11
6. USPSTF. Tobacco use in children and adolescents: primary care interventions. Final recommendation statement. Published April 28, 2020. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
7. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Final recommendation statement. Published January 19, 2021. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
1. CDC. Youth Risk Behavior Surveillance—United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):1-93. Accessed May 24, 2023. www.cdc.gov/mmwr/volumes/72/su/pdfs/su7201-h.pdf
2. Creamer MR, Everett Jones S, Gentzke AS, et al. Tobacco product use among high school students—Youth Risk Behavior Survey, United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(suppl 1):56-63. doi: 10.15585/mmwr.su6901a7
3. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. Washington, DC: National Academies Press; 2018. Accessed May 24, 2023. https://nap.nationalacademies.org/catalog/24952/public-health-consequences-of-e-cigarettes
4. Gentzke AS, Wang TW, Cornelius M, et al. Tobacco product use and associated factors among middle and high school students—National Youth Tobacco Survey, United States, 2021. MMWR Surveill Summ. 2022;71(no. SS-5):1-29. doi: 10.15585/mmwr.ss7105a1
5. Oliver BE, Jones SE, Hops ED, et al. Electronic vapor product use among high school students—Youth Risk Behavior Survey, United States, 2021. MMWR Morb Mortal Wkly Rep. 2023;72(suppl 1):93-99. doi: 10.15585/mmwr.su7201a11
6. USPSTF. Tobacco use in children and adolescents: primary care interventions. Final recommendation statement. Published April 28, 2020. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
7. USPSTF. Tobacco smoking cessation in adults, including pregnant persons: interventions. Final recommendation statement. Published January 19, 2021. Accessed May 24, 2023. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
FDA approves buprenorphine injection for opioid use disorder
The medication comes in two formulations: a weekly and a monthly version. The weekly treatment is indicated in patients who have initiated treatment with a single dose of transmucosal buprenorphine or who are already being treated with the drug. The monthly version is for patients already receiving buprenorphine.
“Buprenorphine is an important treatment option for opioid use disorder. Today’s approval expands dosing options and provides people with opioid use disorder a greater opportunity to sustain long-term recovery,” said FDA Commissioner Robert M. Califf, MD, in a release. “The FDA will continue to take the critical steps necessary to pursue efforts that advance evidence-based treatments for substance use disorders, which is a strategic priority under the FDA’s Overdose Prevention Framework,” Dr. Califf added.
The Substance Abuse and Mental Health Services Administration reports that patients receiving medication for OUD have their risk for all-cause mortality cut by 50%.
In its release, the FDA said that it remains committed to increasing treatment options for OUD. Earlier this month, the agency issued a joint letter with SAMHSA to underscore the importance of counseling and other services as part of a comprehensive treatment plan the disorder. It also emphasized that receiving buprenorphine should not be contingent on participating in such services.
Brixadi is approved in both weekly and monthly subcutaneous injectable formulations at varying doses, including lower doses that may be appropriate for patients who do not tolerate higher doses of extended-release buprenorphine that are currently available.
The drug will be available through a Risk Evaluation and Mitigation Strategy program and administered only by health care providers in a health care setting.
The most common adverse reactions associated with the drug include injection-site pain, headache, constipation, nausea, injection-site erythema, injection-site pruritus, insomnia, and urinary tract infections. The FDA reports that such side effects occur in at least 5% of patients.
A version of this article originally appeared on Medscape.com.
The medication comes in two formulations: a weekly and a monthly version. The weekly treatment is indicated in patients who have initiated treatment with a single dose of transmucosal buprenorphine or who are already being treated with the drug. The monthly version is for patients already receiving buprenorphine.
“Buprenorphine is an important treatment option for opioid use disorder. Today’s approval expands dosing options and provides people with opioid use disorder a greater opportunity to sustain long-term recovery,” said FDA Commissioner Robert M. Califf, MD, in a release. “The FDA will continue to take the critical steps necessary to pursue efforts that advance evidence-based treatments for substance use disorders, which is a strategic priority under the FDA’s Overdose Prevention Framework,” Dr. Califf added.
The Substance Abuse and Mental Health Services Administration reports that patients receiving medication for OUD have their risk for all-cause mortality cut by 50%.
In its release, the FDA said that it remains committed to increasing treatment options for OUD. Earlier this month, the agency issued a joint letter with SAMHSA to underscore the importance of counseling and other services as part of a comprehensive treatment plan the disorder. It also emphasized that receiving buprenorphine should not be contingent on participating in such services.
Brixadi is approved in both weekly and monthly subcutaneous injectable formulations at varying doses, including lower doses that may be appropriate for patients who do not tolerate higher doses of extended-release buprenorphine that are currently available.
The drug will be available through a Risk Evaluation and Mitigation Strategy program and administered only by health care providers in a health care setting.
The most common adverse reactions associated with the drug include injection-site pain, headache, constipation, nausea, injection-site erythema, injection-site pruritus, insomnia, and urinary tract infections. The FDA reports that such side effects occur in at least 5% of patients.
A version of this article originally appeared on Medscape.com.
The medication comes in two formulations: a weekly and a monthly version. The weekly treatment is indicated in patients who have initiated treatment with a single dose of transmucosal buprenorphine or who are already being treated with the drug. The monthly version is for patients already receiving buprenorphine.
“Buprenorphine is an important treatment option for opioid use disorder. Today’s approval expands dosing options and provides people with opioid use disorder a greater opportunity to sustain long-term recovery,” said FDA Commissioner Robert M. Califf, MD, in a release. “The FDA will continue to take the critical steps necessary to pursue efforts that advance evidence-based treatments for substance use disorders, which is a strategic priority under the FDA’s Overdose Prevention Framework,” Dr. Califf added.
The Substance Abuse and Mental Health Services Administration reports that patients receiving medication for OUD have their risk for all-cause mortality cut by 50%.
In its release, the FDA said that it remains committed to increasing treatment options for OUD. Earlier this month, the agency issued a joint letter with SAMHSA to underscore the importance of counseling and other services as part of a comprehensive treatment plan the disorder. It also emphasized that receiving buprenorphine should not be contingent on participating in such services.
Brixadi is approved in both weekly and monthly subcutaneous injectable formulations at varying doses, including lower doses that may be appropriate for patients who do not tolerate higher doses of extended-release buprenorphine that are currently available.
The drug will be available through a Risk Evaluation and Mitigation Strategy program and administered only by health care providers in a health care setting.
The most common adverse reactions associated with the drug include injection-site pain, headache, constipation, nausea, injection-site erythema, injection-site pruritus, insomnia, and urinary tract infections. The FDA reports that such side effects occur in at least 5% of patients.
A version of this article originally appeared on Medscape.com.
Overdose deaths mark another record year, but experts hopeful
, according to newly released figures from the Centers for Disease Control and Prevention.
Overdose deaths in 2022 totaled an estimated 109,680 people, which is 2% more than the 107,573 deaths in 2021, according to the figures. But the 2022 total is still a record for the third straight year.
Public health officials are now in a hopeful position. If the 2022 data represents a peak, then the country will see deaths decline toward pre-pandemic levels. If overdose deaths instead have reached a plateau, it means that the United States will sustain the nearly 20% leap that came amid a deadly increase in drug use in 2020 and 2021.
“The fact that it does seem to be flattening out, at least at a national level, is encouraging,” Columbia University epidemiologist Katherine Keyes, PhD, MPH, told The Associated Press. “But these numbers are still extraordinarily high. We shouldn’t suggest the crisis is in any way over.”
The newly released figures from the CDC are considered estimates because some states may still send updated 2022 information later this year.
Although the number of deaths from 2021 to 2022 was stable on a national level, the picture varied more widely at the state level. More than half of U.S. states saw increases, while deaths in 23 states decreased, and just one – Iowa – had the same number of overdose deaths in 2021 and 2022.
The states with the highest counts in 2022 were:
- California: 11,978 deaths
- Florida: 8,032 deaths
- Texas: 5,607 deaths
- Pennsylvania: 5,222 deaths
- Ohio: 5,103 deaths
Synthetic opioids, such as fentanyl and tramadol, account for most drug overdose deaths, according to a December 2022 report from the CDC.
State officials told The AP that they believe the plateau in overdose deaths is in part due to educational campaigns to warn the public about the dangers of drug use, as well as from expanded addiction treatment and increased access to the overdose-reversal medicine naloxone.
A version of this article originally appeared on WebMD.com.
, according to newly released figures from the Centers for Disease Control and Prevention.
Overdose deaths in 2022 totaled an estimated 109,680 people, which is 2% more than the 107,573 deaths in 2021, according to the figures. But the 2022 total is still a record for the third straight year.
Public health officials are now in a hopeful position. If the 2022 data represents a peak, then the country will see deaths decline toward pre-pandemic levels. If overdose deaths instead have reached a plateau, it means that the United States will sustain the nearly 20% leap that came amid a deadly increase in drug use in 2020 and 2021.
“The fact that it does seem to be flattening out, at least at a national level, is encouraging,” Columbia University epidemiologist Katherine Keyes, PhD, MPH, told The Associated Press. “But these numbers are still extraordinarily high. We shouldn’t suggest the crisis is in any way over.”
The newly released figures from the CDC are considered estimates because some states may still send updated 2022 information later this year.
Although the number of deaths from 2021 to 2022 was stable on a national level, the picture varied more widely at the state level. More than half of U.S. states saw increases, while deaths in 23 states decreased, and just one – Iowa – had the same number of overdose deaths in 2021 and 2022.
The states with the highest counts in 2022 were:
- California: 11,978 deaths
- Florida: 8,032 deaths
- Texas: 5,607 deaths
- Pennsylvania: 5,222 deaths
- Ohio: 5,103 deaths
Synthetic opioids, such as fentanyl and tramadol, account for most drug overdose deaths, according to a December 2022 report from the CDC.
State officials told The AP that they believe the plateau in overdose deaths is in part due to educational campaigns to warn the public about the dangers of drug use, as well as from expanded addiction treatment and increased access to the overdose-reversal medicine naloxone.
A version of this article originally appeared on WebMD.com.
, according to newly released figures from the Centers for Disease Control and Prevention.
Overdose deaths in 2022 totaled an estimated 109,680 people, which is 2% more than the 107,573 deaths in 2021, according to the figures. But the 2022 total is still a record for the third straight year.
Public health officials are now in a hopeful position. If the 2022 data represents a peak, then the country will see deaths decline toward pre-pandemic levels. If overdose deaths instead have reached a plateau, it means that the United States will sustain the nearly 20% leap that came amid a deadly increase in drug use in 2020 and 2021.
“The fact that it does seem to be flattening out, at least at a national level, is encouraging,” Columbia University epidemiologist Katherine Keyes, PhD, MPH, told The Associated Press. “But these numbers are still extraordinarily high. We shouldn’t suggest the crisis is in any way over.”
The newly released figures from the CDC are considered estimates because some states may still send updated 2022 information later this year.
Although the number of deaths from 2021 to 2022 was stable on a national level, the picture varied more widely at the state level. More than half of U.S. states saw increases, while deaths in 23 states decreased, and just one – Iowa – had the same number of overdose deaths in 2021 and 2022.
The states with the highest counts in 2022 were:
- California: 11,978 deaths
- Florida: 8,032 deaths
- Texas: 5,607 deaths
- Pennsylvania: 5,222 deaths
- Ohio: 5,103 deaths
Synthetic opioids, such as fentanyl and tramadol, account for most drug overdose deaths, according to a December 2022 report from the CDC.
State officials told The AP that they believe the plateau in overdose deaths is in part due to educational campaigns to warn the public about the dangers of drug use, as well as from expanded addiction treatment and increased access to the overdose-reversal medicine naloxone.
A version of this article originally appeared on WebMD.com.
Balancing needs and risks as the opioid pendulum swings
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
FDA moves to curb misuse of ADHD meds
“The current prescribing information for some prescription stimulants does not provide up-to-date warnings about the harms of misuse and abuse, and particularly that most individuals who misuse prescription stimulants get their drugs from other family members or peers,” the FDA said in a drug safety communication.
Going forward, updated drug labels will clearly state that patients should never share their prescription stimulants with anyone, and the boxed warning will describe the risks of misuse, abuse, addiction, and overdose consistently for all medicines in the class, the FDA said.
The boxed warning will also advise heath care professionals to monitor patients closely for signs and symptoms of misuse, abuse, and addiction.
Patient medication guides will be updated to educate patients and caregivers about these risks.
The FDA encourages prescribers to assess patient risk of misuse, abuse, and addiction before prescribing a stimulant and to counsel patients not to share the medication.
Friends and family
A recent literature review by the FDA found that friends and family members are the most common source of prescription stimulant misuse and abuse (nonmedical use). Estimates of such use range from 56% to 80%.
Misuse/abuse of a patient’s own prescription make up 10%-20% of people who report nonmedical stimulant use.
Less commonly reported sources include drug dealers or strangers (4%-7% of people who report nonmedical use) and the Internet (1%-2%).
The groups at highest risk for misuse/abuse of prescription stimulants are young adults aged 18-25 years, college students, and adolescents and young adults who have been diagnosed with ADHD, the FDA said.
Recent data from the Centers for Disease Control and Prevention show that during the first year of the COVID-19 pandemic, prescriptions for stimulants increased 10% among older children and adults.
A version of this article first appeared on Medscape.com.
“The current prescribing information for some prescription stimulants does not provide up-to-date warnings about the harms of misuse and abuse, and particularly that most individuals who misuse prescription stimulants get their drugs from other family members or peers,” the FDA said in a drug safety communication.
Going forward, updated drug labels will clearly state that patients should never share their prescription stimulants with anyone, and the boxed warning will describe the risks of misuse, abuse, addiction, and overdose consistently for all medicines in the class, the FDA said.
The boxed warning will also advise heath care professionals to monitor patients closely for signs and symptoms of misuse, abuse, and addiction.
Patient medication guides will be updated to educate patients and caregivers about these risks.
The FDA encourages prescribers to assess patient risk of misuse, abuse, and addiction before prescribing a stimulant and to counsel patients not to share the medication.
Friends and family
A recent literature review by the FDA found that friends and family members are the most common source of prescription stimulant misuse and abuse (nonmedical use). Estimates of such use range from 56% to 80%.
Misuse/abuse of a patient’s own prescription make up 10%-20% of people who report nonmedical stimulant use.
Less commonly reported sources include drug dealers or strangers (4%-7% of people who report nonmedical use) and the Internet (1%-2%).
The groups at highest risk for misuse/abuse of prescription stimulants are young adults aged 18-25 years, college students, and adolescents and young adults who have been diagnosed with ADHD, the FDA said.
Recent data from the Centers for Disease Control and Prevention show that during the first year of the COVID-19 pandemic, prescriptions for stimulants increased 10% among older children and adults.
A version of this article first appeared on Medscape.com.
“The current prescribing information for some prescription stimulants does not provide up-to-date warnings about the harms of misuse and abuse, and particularly that most individuals who misuse prescription stimulants get their drugs from other family members or peers,” the FDA said in a drug safety communication.
Going forward, updated drug labels will clearly state that patients should never share their prescription stimulants with anyone, and the boxed warning will describe the risks of misuse, abuse, addiction, and overdose consistently for all medicines in the class, the FDA said.
The boxed warning will also advise heath care professionals to monitor patients closely for signs and symptoms of misuse, abuse, and addiction.
Patient medication guides will be updated to educate patients and caregivers about these risks.
The FDA encourages prescribers to assess patient risk of misuse, abuse, and addiction before prescribing a stimulant and to counsel patients not to share the medication.
Friends and family
A recent literature review by the FDA found that friends and family members are the most common source of prescription stimulant misuse and abuse (nonmedical use). Estimates of such use range from 56% to 80%.
Misuse/abuse of a patient’s own prescription make up 10%-20% of people who report nonmedical stimulant use.
Less commonly reported sources include drug dealers or strangers (4%-7% of people who report nonmedical use) and the Internet (1%-2%).
The groups at highest risk for misuse/abuse of prescription stimulants are young adults aged 18-25 years, college students, and adolescents and young adults who have been diagnosed with ADHD, the FDA said.
Recent data from the Centers for Disease Control and Prevention show that during the first year of the COVID-19 pandemic, prescriptions for stimulants increased 10% among older children and adults.
A version of this article first appeared on Medscape.com.
Medication-assisted recovery for opioid use disorder: A guide
Medication-assisted recovery (MAR)—the preferred terminology for the service formerly known as medication-assisted treatment—entails a comprehensive set of interventions for managing opioid use disorder (OUD), including medications for opioid use disorder (MOUD). Despite the benefits of MAR—reducing opioid use, opioid-related mortality, and health care costs1-3—only 11% of patients with a diagnosis of OUD received MOUD in 2020.3
Primary care physicians, including family physicians, are well positioned to provide MAR across the patient’s lifespan. However, many family medicine clinicians do not possess the logistical knowledge or resources to implement this service.4 In this article, we describe options for, and barriers to, MAR and societal issues that have an impact on the care of these patients.
Pathophysiology of OUD
Opioids relieve pain by stimulating μ-opioid receptors and activating the brain’s reward system. These pleasurable effects motivate repeated use.5 Frequent opioid exposure causes neuroadaptation, tolerance, and dependence. For patients with OUD who are misusing illicit or prescription opioids, periods of abstinence following neuroadaptation lead to withdrawal symptoms that vary in intensity, depending on the drug, dose, and duration of use. Upregulated noradrenergic tone and dopamine deficiency manifest as numerous signs and symptoms of withdrawal, including5:
- Physiologic: secretory (diaphoresis, rhinorrhea, lacrimation, vomiting, diarrhea) and stimulatory (mydriasis, piloerection, hypertension, tachycardia, insomnia)
- Psychological: pain, cravings, dysphoria, anxiety.
A single episode of opioid withdrawal is not directly life-threatening, but untreated episodes can progressively amplify negative feedback and reinforce continued opioid use.6 Left untreated, withdrawal can be terminal.
Medication-assisted recovery: Effective intervention
MAR services that integrate medical, behavioral, and psychosocial programs can reduce mortality from OUD 2-fold.7,8 A meta-analysis found that, when MAR services are rendered in primary care, treatment retention improves by 25% (number needed to treat [NNT] = 6) and ongoing illicit opioid use is reduced by 50% (NNT = 6), relative to care at a specialty clinic9—highlighting a role for family medicine clinicians in treating OUD.
All 3 US Food and Drug Administration (FDA)–approved MOUD (methadone, buprenorphine, and naltrexone) reduce cravings; 2 (methadone and buprenorphine) mitigate withdrawal symptoms by activating the μ-opioid receptor; and naltrexone diminishes the reinforcing effects of use (TABLE10-12). It is crucial to recognize the pharmacologic distinctions among MOUD because untreated withdrawal syndromes increase dropout from treatment programs and subsequent relapse.13
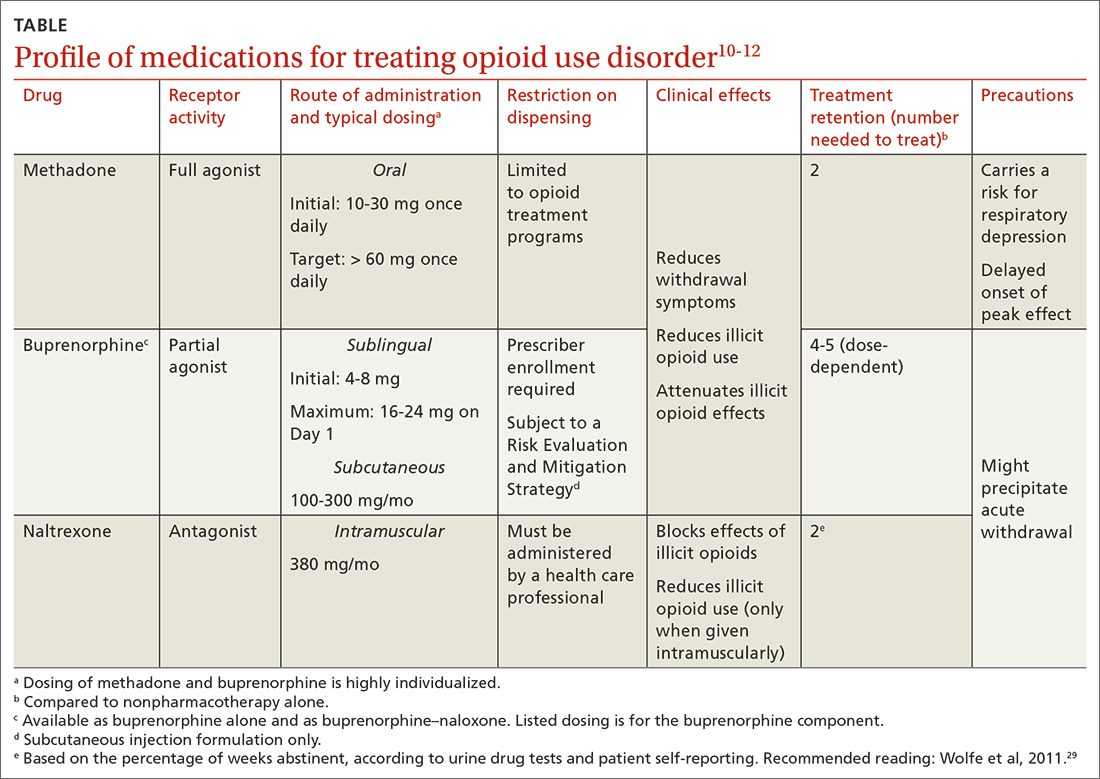
The Hx of medication-assisted recovery
To understand the landscape of MAR, it is important to understand the history of opioid treatment in the United States. In 1966, Congress passed the Narcotic Addiction Rehabilitation Act (NARA), which secured federal assistance by which state and local governments could develop drug treatment programs.14 NARA permitted legal offenders with OUD to be civilly committed to treatment programs, rather than prosecuted. However, limited resources and a burgeoning population led, instead, to low-cost outpatient programs saddled by strict requirements that lacked a basis for improving clinical outcomes.
Continue to: At the time NARA...
At the time NARA was passed by Congress, OUD was viewed—inaccurately—as a criminal problem, not a medical one. Subsequent legislation was crafted through that lens, which has placed a heavy burden on patients until today.14 Although medical understanding of OUD has advanced tremendously over the past 50 years, treatment remains siloed from mainstream medicine, even in primary care.
There is no one-size-fits-all approach to MAR, and relapse is common. Patient-specific factors and the availability of resources should be considered when designing the most individualized, advantageous plan for MAR.
Methadone
Background. Methadone has the most extensive history for treating OUD and consistently has demonstrated efficacy.13 A meta-analysis of randomized controlled trials comparing methadone to nonpharmacotherapy alone found that methadone improved treatment retention by an absolute 57% (NNT = 2).10
Methadone was approved by the FDA for detoxification and maintenance treatment in the early 1970s, although the Narcotic Addict Treatment Act (NATA) of 1974 restricted dispensing of maintenance treatment to highly regulated clinics known as opioid treatment programs (OTPs).14 NATA required the treating physician to register with the US Drug Enforcement Agency (DEA) and to comply with conservative dosing regimens and observed dosing.
Over time, regulations evolved to give the physician greater flexibility in developing a care plan, allowing “take-home” doses, and improving patients’ access to care. Although access to methadone for the treatment of OUD remains limited to federally certified OTPs, regulations facilitate incorporation of a whole-person approach to care, including counseling, individual and group therapy, and toxicology testing.7
Continue to: Clinical considerations
Clinical considerations. Methadone requires slow titration. For patients starting methadone as an outpatient, federal law15 limits the initial dose to 30 mg and requires physician documentation when the first-day total dosage exceeds 40 mg. This dosing constraint makes it challenging to provide care because a daily dosage ≥ 60 mg has been found to produce, first, higher program retention (relative risk = 1.36; 95% CI, 1.13-1.63) and, second, greater reduction in illicit opioid use (relative risk = 1.59; 95% CI, 1.16-2.18) than is seen in patients who receive a lower daily dosage.16
Due to a prolonged elimination half-life, methadone reaches steady-state in 3 to 5 days. Patients and their families should be educated that withdrawal symptoms might not feel fully managed in the first few days of therapy and that time is required to experience safely the regimen’s full effects.
Aggressive dose-titration during methadone induction can result in drug accumulation and respiratory depression. The risk for methadone-related mortality is highest in the first 2 weeks of therapy, mostly related to overdose potential if the drug is combined with other opioids.17
Buprenorphine
Background. The prescribing rate for buprenorphine, particularly in primary care, is accelerating.18 A meta-analysis of randomized controlled trials found that11:
- compared to placebo, buprenorphine, at any dosage, improves treatment retention by an absolute 21% to 28% (NNT = 4-5)
- patients receiving high-dose buprenorphine (≥ 16 mg/d) had fewer evident cases of illicit opioid use.
Unlike methadone, buprenorphine exerts partial agonism at the μ-opioid receptor, resulting in a so-called ceiling effect that significantly reduces the adverse effect profile, including respiratory depression and euphoria, relative to a full-agonist opioid, such as methadone.19
Continue to: Whereas accessing methadone...
Whereas accessing methadone is limited to OTPs, buprenorphine is available for office-based treatment. By hosting OUD treatment and primary care in the same place, primary care physicians can provide comprehensive medical care including and beyond OUD, thereby improving retention and managing comorbidity.20
Integrated models involving support staff—eg, nurses, behavioral health providers, and pharmacists—have produced the greatest success with office-based treatment models.21 Office-based treatment normalizes OUD as a chronic disease managed by the primary care physician, enabling concurrent harm-reduction strategies; medication reconciliation; and convenient, regular prescribing intervals (eg, every 30 days).22 Nevertheless, access to buprenorphine is limited. Because buprenorphine is a controlled substance, the Ryan Haight Online Pharmacy Consumer Protection Act of 2008 prevents initial prescribing of buprenorphine without in-person evaluation. Telehealth consultations increased access to buprenorphine through temporary exceptions during the COVID-19 pandemic. However, revised rules and regulations for telehealth visits for these controlled substances are forthcoming from the DEA as temporary exceptions for telehealth consultations come to an end. Additionally, prescribing buprenorphine for OUD requires that the treating physician undergo specific training and obtain qualifications, which have evolved over time through federal legislation.
The Drug Addiction Treatment Act of 2000 (DATA 2000) authorized what is known as an X-waiver, which allows physicians to prescribe controlled substances for office-based treatment of OUD, provided that:
- they are registered to do so with the Substance Abuse and Mental Health Services Administration and the DEA
- they have had subspecialty training in addiction or completed an 8-hour training course
- they are able to refer patients to appropriate counseling and ancillary services.
DATA 2000 restricted patient panel sizes to 30 patients in the first year, expanding thereafter upon appropriate certification.
The Comprehensive Addiction and Recovery Act of 2016 (CARA) and the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act of 2018 (the SUPPORT Act) collectively extended prescribing authority for MOUD to other qualifying practitioners (eg, advanced practice clinicians). Despite these attempts to expand access to services, the overdose death rate has continued to increase.
Continue to: To further expand access to MAR...
To further expand access to MAR, the US Department of Health and Human Services updated its practice guidelines in April 2021, allowing clinicians to bypass X-waiver training requirements by applying for a notification-of-intent (NOI) buprenorphine waiver.a However, clinicians are still limited to prescribing buprenorphine for 30 patients at a time. Clinicians who undergo complete X-waiver training may prescribe for 100 patients in the first year and, if eligible, 275 patients thereafter.
In addition, as a component of the Consolidation Appropriations Act of 2023, Congress passed the Mainstreaming Addiction Treatment Act of 2021, or MAT 2021, and Medication Access and Training Expansion Act of 2021, or MATE 2021. MAT eliminated the X-waiver, NOI, and restrictions on the number of patients for whom a provider could prescribe buprenorphine, under federal authority; however, restrictions within one’s state might limit the ability to prescribe buprenorphine. MATE 2021 is an educational requirement for licensing by the DEA (at application and renewal) that will require prescribers to complete 8 hours of training in substance use disorders starting in June 2023.
Use of the monthly injectable extended-release buprenorphine productb is limited by an FDA Risk Evaluation and Mitigation Strategy (REMS) program, which requires specialized training and certification by the prescriber, distributor, and administering clinician. REMS reduces buprenorphine accessibility due to time, cost, and regulatory barriers; although such restrictions have been instituted with the patient’s safety in mind, any limitation to buprenorphine prescribing, apart from controlled substance licensure, serves only to limit access to a primary component of MAR.
Clinical considerations. Due to the competitive nature of buprenorphine and its high affinity for the μ-opioid receptor, the drug can displace other opioid agonists and precipitate acute withdrawal. The withdrawal experience can thereby condition fear and disfavor toward buprenorphine among patients.
It is vital, therefore, that (1) patients’ expectations for treatment be managed appropriately and (2) the treating physician be prepared to provide additional buprenorphine for adequate maintenance doses and utilize adjunct comfort agents (clonidine, nonsteroidal anti-inflammatory drugs, ondansetron) to manage acute withdrawal symptoms. Newer buprenorphine dosing strategies, such as micro-induction and macro-induction, have emerged to curtail these risks.23,24 This is an evolving area of MAR; newer low-threshold initiation strategies25 (see “Low-threshold MOUD prescribing models,” in the text that follows) and evidence that supports micro-induction26 might eliminate the practice of requiring active withdrawal for treatment.
Continue to: Regardless of the strategy...
Regardless of the strategy for dosing buprenorphine, it’s critical that patients be educated on how to initiate treatment outside a clinical setting, such as at home, where they occupy a familiar haven during a potentially uncomfortable time and can be as effective at initiation as they would be in a clinical setting, with no difference in precipitation of adverse effects.
At-home induction might be more appropriate for patients who are not yet in significant enough withdrawal while in the physician's office.27 Guidance should be provided on dosing instructions, self-assessment of withdrawal symptoms, and, if applicable, patience with the slow-dissolving sublingual tablet or film formulation.
Naltrexone
Background. Naltrexone is available as an oral tablet and an extended-release, once-monthly intramuscular injection; the latter has demonstrated superiority in MAR.28 Oral naltrexone has limited supporting evidence, is inferior to other MOUD options, and should not be used to treat OUD.7 Altogether, approval of naltrexone for OUD is controversial, due to potentially unethical trials and approval processes,29 although a multicenter randomized controlled trial demonstrated the drug’s noninferiority with respect to treatment retention relative to buprenorphine.30 Used over time, naltrexone does not relieve withdrawal symptoms but can reduce cravings.
Clinical considerations. There are numerous clinical barriers that limit the use of naltrexone.
First, patients should be abstinent from opioids for 7 to 14 days prior to starting therapy; usually, this means undergoing medically supervised withdrawal in a controlled environment. This is an obvious limitation for patients who are constrained financially—those who lack, or have inadequate, health insurance or are unable to be away from their job for an extended time.
Continue to: Second, because naltrexone...
Second, because naltrexone does not address withdrawal symptoms, supportive therapies should be incorporated into the treatment plan, including:
- clonidine for hyperadrenergic symptoms (anxiety, diaphoresis, hypertension)
- nonopioid analgesics for pain
- antiemetics, such as ondansetron and metoclopramide, for nausea or vomiting
- loperamide for diarrhea
- diphenhydramine for insomnia.
Third, patients taking naltrexone have a diminished response to opioids. This complicates pain management in the event of an emergent surgical procedure.
Last, when naltrexone wears off, patients are effectively opioid-naïve, which increases the risk for overdose in those who stop therapy abruptly.29 The increased risk for overdose should be communicated to all patients with OUD who are being treated with naltrexone.
This nonopioid option is appealing to policymakers and is often prioritized in the criminal justice system; however, the decreased efficacy of naltrexone (compared to methadone and buprenorphine), potential for overdose, and challenges in initiating treatment are concerning and limit the drug’s use in many real-world settings.
Because naltrexone is not a controlled substance, regulations regarding maintaining inventory and distribution are more flexible.
Continue to: Overall, the cost-effectiveness...
Overall, the cost-effectiveness of intramuscular naltrexone is unclear. State-administered insurance programs vary in their requirements for coverage of naltrexone treatment.31
Comprehensive medication reconciliation is vital
Overall fragmentation of care within OTPs places patients at risk for adverse events, such as drug interactions.32 Under Title 42 of the US Code,33 patients must provide written consent for an OTP provider to disclose their history of a substance use disorder. Allowing the patient to decide which medical providers can access their treatment records for an OUD benefits patient confidentiality but poses numerous issues worth exploring.
All prescribed controlled substances are recorded in the prescription drug monitoring program, or PDMP, a state-level electronic database accessible to health care professionals to inform prescribing decisions and identify drug interactions. The PDMP has substantially reduced opioid overprescribing and improved identification of patients at risk for overdose or misuse of opioids.
Unlike all other controlled substances, however, prescriptions ordered by an OTP are not recorded in the PDMP (although there are recent exceptions to this scenario). Without such information, a physician might not have important information about the patient when making medical decisions—placing the patient at risk for harmful outcomes, such as drug–drug and drug–disease interactions.
For example: Methadone is associated with a prolonged QT interval,34 increasing the risk for a fatal arrhythmia. Concurrent QT-prolonging medications, such as azithromycin and citalopram, further increase this risk.35 Because methadone dispensing is isolated from the patient’s medical record, the clinician who prescribes MOUD has an incomplete patient history and could make a potentially fatal treatment decision.
Continue to: Diversion is unlikely
Diversion is unlikely
Health care providers often express concern about diversion in MOUD. However, misuse and diversion rates of methadone and buprenorphine have declined steadily since 2011, and, in fact, are actually lower than the diversion rate of prescription antibiotics.36
Regardless, diversion of buprenorphine should not be a concern for physicians prescribing MOUD. Although a prescriber might worry about manipulation of the formulation of buprenorphine for intravenous administration, addition of naloxone to buprenorphine in tablet form diminishes the potential for overdose. Additionally, the ceiling effect of buprenorphine limits the likelihood of significant respiratory depression and euphoria.
Should buprenorphine reach a patient for whom it was not prescribed, it is highly unlikely that an overdose would result. Rather, the medication would protect against the effects of illicit opioids and relieve withdrawal symptoms. Most people with OUD who have misused buprenorphine have done so to relieve withdrawal symptoms,37 not to experience intoxication.
Health care deserts
So-called health care deserts in parts of the United States are an ongoing problem that disproportionately affects lower-income and segregated Black and Hispanic communities38—communities that shoulder the highest burden of OUD and OUD-related mortality39 and whose populace is in greatest need of MAR. Even when health care is accessible in such a desert, some clinicians and pharmacies refuse to prescribe or dispense MOUD because of the accompanying stigma of OUD.
A MAR desert, like a pharmacy desert, is a geographic region—one without access to a MAR or an OTP provider, thereby preventing patients from reaching appropriate care; for some patients, having to travel to the nearest provider can render treatment inaccessible.40
Continue to: Efforts are in place to identify...
Efforts are in place to identify areas at greatest need of OUD-related medical services, such as heat maps that identify areas of increased utilization of emergency medical services for opioid overdose. State-run programs have been implemented to increase access, such as the Illinois Helpline (https://helplineil.org) that provides support and resources for patients, friends, family, and providers.
Novel solutions
Key strategies to increase access to care and slow the opioid epidemic include low-threshold prescribing of MOUD and mobile OTPs.41
Low-threshold MOUD prescribing models. Adoption of one of these models in a medical practice that provides MAR might increase absolute enrollment. A low-threshold prescribing model involves42:
- same-day treatment
- leniency with respect to abstinence periods and a concomitant substance use disorder
- enhanced accessibility to MOUD through nontraditional medical settings.
Low-threshold prescribing is flexible in regard to patients’ needs and bypasses many of the barriers discussed in this article. Impressive multicenter success has been achieved by the CA Bridge program in California (https://cabridge.org), including an increase in recognition of OUD, treatment initiations, and outpatient engagement.25
The cost-effectiveness of low-threshold MOUD prescribing programs remains to be determined.
Mobile OTPs. In July 2021, the DEA authorized a mobile component to existing OTP registrants that is permitted to dispense methadone and buprenorphine. Mobile units are physically separate from the OTP but have similar functions, depending on available space. Services that cannot be provided on the mobile unit of an OTP must be available at its brick-and-mortar location.7 Logistically, OTP registrants no longer need a separate registration to implement a mobile unit, thus expanding care to patients in underserved or remote areas who often encounter barriers to access.43
Conclusion
Understanding the distinct clinical and accessibility benefits and limitations among available MOUD is essential for prescribing clinicians. Accessing treatment is limited by federal regulation, stigma, and the existence of health care deserts that limit access to necessary care for patients with OUD. Newer harm-reduction models, such as low-threshold prescribing and mobile OTPs, represent progress, but many patients remain untreated.
a At buprenorphine.samhsa.gov/forms/select-practitioner-type.php
b Sold under the brand name Sublocade.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, Department of Pharmacy Practice, University of Illinois Chicago College of Pharmacy, 833 South Wood Street (MC 886), Chicago, IL 60612; jarrett8@uic.edu
1. Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235-S248.
2. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462-468. doi: 10.1111/j.1360-0443.2007.02090.x
3. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. HHS Publication PEP21-07-01-003, NSDUH Series H-56. 2021. Accessed March 19, 2023. www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
4. Haffajee RL, Andraka-Christou B, Attermann J, et al. A mixed-method comparison of physician-reported beliefs about and barriers to treatment with medications for opioid use disorder. Subst Abuse Treat Prev Policy. 2020;15:69. doi: 10.1186/s13011-020-00312-3
5. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20. doi: 10.1151/spp021113
6. Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44-53. doi: 10.1016/j.biopsych.2019.05.023
7. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. For Health care and Addiction Professionals, Policymakers, Patients, and Families. Treatment Improvement Protocol TIP 63. Publication No. PEP21-02-01-002. 2021. Accessed March 19, 2023. https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf
8. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550
9. Korownyk C, Perry D, Ton J, et al. Opioid use disorder in primary care: PEER umbrella systematic review of systematic reviews. Can Fam Physician. 2019;65:e194-e206.
10. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2
11. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4
12. Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513. doi: 10.1016/S0140-6736(11)60358-9
13. Soyka M, Zingg C, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641-653. doi: 10.1017/S146114570700836X
14. Institute of Medicine Committee on Federal Regulation of Methadone Treatment; Rettig R, Yarmolinsky A, eds. Federal Regulation of Methadone Treatment. National Academies Press; 1995.
15. 42 eCFR §8. Medication assisted treatment for opioid use disorders. Revised March 15, 2023. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8?toc=1
16. Faggiano F, Vigna-Taglianti F, Versino E, et al. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;(3):CD002208. doi: 10.1002/14651858.CD002208
17. Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377-386. doi: 10.1097/01.ADM.0000435321.39251.d7
18. Olfson M, Zhang VS, Schoenbaum M, et al. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323:276-277. doi: 10.1001/jama.2019.18913
19. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580. doi: 10.1038/clpt.1994.71
20. Walley AY, Palmisano J, Sorensen-Alawad A, et al. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J Subst Abuse Treat. 2015;59:59-66. doi: 10.1016/j.jsat.2015.07.007
21. Lagisetty P, Klasa K, Bush C, et al. Primary care models for treating opioid use disorders: what actually works? A systematic review. PloS One. 2017;12:e0186315. doi: 10.1371/journal.pone.0186315
22. Du CX, Shi J, Tetrault JM, et al. Primary care and medication management characteristics among patients receiving office-based opioid treatment with buprenorphine. Fam Pract. 2022;39:234-240. doi: 10.1093/fampra/cmab166
23. Herring AA, Vosooghi AA, Luftig J, et al. High-dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4:e2117128. doi: 10.1001/jamanetworkopen.2021.17128
24. Hämmig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105. doi: 10.2147/SAR.S109919
25. Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA Bridge Program. Ann Emerg Med. 2021;78:759-772. doi: 10.1016/j.annemergmed.2021.05.024
26. Wong JSH, Nikoo M, Westenberg JN, et al. Comparing rapid micro-induction and standard induction of buprenorphine/naloxone for treatment of opioid use disorder: protocol for an open-label, parallel-group, superiority, randomized controlled trial. Addict Sci Clin Pract. 2021;16:11. doi: 10.1186/s13722-021-00220-2
27. Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med. 2014;8:299-308. doi: 10.1097/ADM.0000000000000059
28. Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973-981. doi: 10.1001/archgenpsychiatry.2012.1a
29. Wolfe D, Carrieri MP, Dasgupta N, et al. Concerns about injectable naltrexone for opioid dependence. Lancet. 2011;377:1468-1470. doi: 10.1016/S0140-6736(10)62056-9
30. Tanum L, Solli KK, Latif ZEH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine–naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205. doi: 10.1001/jamapsychiatry.2017.3206
31. Murphy SM, Polsky D, Lee JD, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal justice-involved individuals with a history of opioid use disorder. Addiction. 2017;112:1440-1450. doi: 10.1111/add.13807
32. Ferrari A, Coccia CPR, Bertolini A, et al. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551-559. doi: 10.1016/j.phrs.2004.05.002
33. 42 eCFR Part 2. Confidentiality of substance use disorder patient records. January 18, 2017. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-2
34. Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015;110:1468-1475. doi: 10.1111/add.13013
35. Tisdale JE, Chung MK, Campbell KB, et al; . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214-e233. doi: 10.1161/CIR.0000000000000905
36. Leshner AI, Mancher M, eds. Barriers to broader use of medications to treat opioid use disorder. In: Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019:109-136.
37. Chilcoat HD, Amick HR, Sherwood MR, et al. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157. doi: 10.1016/j.jsat. 2019.07.005
38. Qato DM, Daviglus ML, Wilder J, et al. “Pharmacy deserts” are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33:1958-1965. doi: 10.1377/hlthaff.2013.1397
39. Mason M, Soliman R, Kim HS, et al. Disparities by sex and race and ethnicity in death rates due to opioid overdose among adults 55 years or older, 1999 to 2019. JAMA Netw Open. 2022;5:e2142982. doi: 10.1001/jamanetworkopen.2021.42982
40. Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789
41. Chan B, Hoffman KA, Bougatsos C, et al. Mobile methadone medication units: a brief history, scoping review and research opportunity. J Subst Abuse Treat. 2021;129:108483. doi: 10.1016/j.jsat.2021.108483
42. Jakubowski A, Fox A. Defining low-threshold buprenorphine treatment. J Addict Med. 2020;14:95-98. doi: 10.1097/ADM.0000000000000555
43. Messmer SE, Elmes AT, Jimenez AD, et al. Outcomes of a mobile medical unit for low-threshold buprenorphine access targeting opioid overdose hot spots in Chicago. J Subst Use Addict Treat. 2023;209054. doi: 10.1016/j.josat.2023.209054
Medication-assisted recovery (MAR)—the preferred terminology for the service formerly known as medication-assisted treatment—entails a comprehensive set of interventions for managing opioid use disorder (OUD), including medications for opioid use disorder (MOUD). Despite the benefits of MAR—reducing opioid use, opioid-related mortality, and health care costs1-3—only 11% of patients with a diagnosis of OUD received MOUD in 2020.3
Primary care physicians, including family physicians, are well positioned to provide MAR across the patient’s lifespan. However, many family medicine clinicians do not possess the logistical knowledge or resources to implement this service.4 In this article, we describe options for, and barriers to, MAR and societal issues that have an impact on the care of these patients.
Pathophysiology of OUD
Opioids relieve pain by stimulating μ-opioid receptors and activating the brain’s reward system. These pleasurable effects motivate repeated use.5 Frequent opioid exposure causes neuroadaptation, tolerance, and dependence. For patients with OUD who are misusing illicit or prescription opioids, periods of abstinence following neuroadaptation lead to withdrawal symptoms that vary in intensity, depending on the drug, dose, and duration of use. Upregulated noradrenergic tone and dopamine deficiency manifest as numerous signs and symptoms of withdrawal, including5:
- Physiologic: secretory (diaphoresis, rhinorrhea, lacrimation, vomiting, diarrhea) and stimulatory (mydriasis, piloerection, hypertension, tachycardia, insomnia)
- Psychological: pain, cravings, dysphoria, anxiety.
A single episode of opioid withdrawal is not directly life-threatening, but untreated episodes can progressively amplify negative feedback and reinforce continued opioid use.6 Left untreated, withdrawal can be terminal.

Medication-assisted recovery: Effective intervention
MAR services that integrate medical, behavioral, and psychosocial programs can reduce mortality from OUD 2-fold.7,8 A meta-analysis found that, when MAR services are rendered in primary care, treatment retention improves by 25% (number needed to treat [NNT] = 6) and ongoing illicit opioid use is reduced by 50% (NNT = 6), relative to care at a specialty clinic9—highlighting a role for family medicine clinicians in treating OUD.
All 3 US Food and Drug Administration (FDA)–approved MOUD (methadone, buprenorphine, and naltrexone) reduce cravings; 2 (methadone and buprenorphine) mitigate withdrawal symptoms by activating the μ-opioid receptor; and naltrexone diminishes the reinforcing effects of use (TABLE10-12). It is crucial to recognize the pharmacologic distinctions among MOUD because untreated withdrawal syndromes increase dropout from treatment programs and subsequent relapse.13

The Hx of medication-assisted recovery
To understand the landscape of MAR, it is important to understand the history of opioid treatment in the United States. In 1966, Congress passed the Narcotic Addiction Rehabilitation Act (NARA), which secured federal assistance by which state and local governments could develop drug treatment programs.14 NARA permitted legal offenders with OUD to be civilly committed to treatment programs, rather than prosecuted. However, limited resources and a burgeoning population led, instead, to low-cost outpatient programs saddled by strict requirements that lacked a basis for improving clinical outcomes.
Continue to: At the time NARA...
At the time NARA was passed by Congress, OUD was viewed—inaccurately—as a criminal problem, not a medical one. Subsequent legislation was crafted through that lens, which has placed a heavy burden on patients until today.14 Although medical understanding of OUD has advanced tremendously over the past 50 years, treatment remains siloed from mainstream medicine, even in primary care.
There is no one-size-fits-all approach to MAR, and relapse is common. Patient-specific factors and the availability of resources should be considered when designing the most individualized, advantageous plan for MAR.
Methadone
Background. Methadone has the most extensive history for treating OUD and consistently has demonstrated efficacy.13 A meta-analysis of randomized controlled trials comparing methadone to nonpharmacotherapy alone found that methadone improved treatment retention by an absolute 57% (NNT = 2).10
Methadone was approved by the FDA for detoxification and maintenance treatment in the early 1970s, although the Narcotic Addict Treatment Act (NATA) of 1974 restricted dispensing of maintenance treatment to highly regulated clinics known as opioid treatment programs (OTPs).14 NATA required the treating physician to register with the US Drug Enforcement Agency (DEA) and to comply with conservative dosing regimens and observed dosing.
Over time, regulations evolved to give the physician greater flexibility in developing a care plan, allowing “take-home” doses, and improving patients’ access to care. Although access to methadone for the treatment of OUD remains limited to federally certified OTPs, regulations facilitate incorporation of a whole-person approach to care, including counseling, individual and group therapy, and toxicology testing.7
Continue to: Clinical considerations
Clinical considerations. Methadone requires slow titration. For patients starting methadone as an outpatient, federal law15 limits the initial dose to 30 mg and requires physician documentation when the first-day total dosage exceeds 40 mg. This dosing constraint makes it challenging to provide care because a daily dosage ≥ 60 mg has been found to produce, first, higher program retention (relative risk = 1.36; 95% CI, 1.13-1.63) and, second, greater reduction in illicit opioid use (relative risk = 1.59; 95% CI, 1.16-2.18) than is seen in patients who receive a lower daily dosage.16
Due to a prolonged elimination half-life, methadone reaches steady-state in 3 to 5 days. Patients and their families should be educated that withdrawal symptoms might not feel fully managed in the first few days of therapy and that time is required to experience safely the regimen’s full effects.
Aggressive dose-titration during methadone induction can result in drug accumulation and respiratory depression. The risk for methadone-related mortality is highest in the first 2 weeks of therapy, mostly related to overdose potential if the drug is combined with other opioids.17
Buprenorphine
Background. The prescribing rate for buprenorphine, particularly in primary care, is accelerating.18 A meta-analysis of randomized controlled trials found that11:
- compared to placebo, buprenorphine, at any dosage, improves treatment retention by an absolute 21% to 28% (NNT = 4-5)
- patients receiving high-dose buprenorphine (≥ 16 mg/d) had fewer evident cases of illicit opioid use.
Unlike methadone, buprenorphine exerts partial agonism at the μ-opioid receptor, resulting in a so-called ceiling effect that significantly reduces the adverse effect profile, including respiratory depression and euphoria, relative to a full-agonist opioid, such as methadone.19
Continue to: Whereas accessing methadone...
Whereas accessing methadone is limited to OTPs, buprenorphine is available for office-based treatment. By hosting OUD treatment and primary care in the same place, primary care physicians can provide comprehensive medical care including and beyond OUD, thereby improving retention and managing comorbidity.20
Integrated models involving support staff—eg, nurses, behavioral health providers, and pharmacists—have produced the greatest success with office-based treatment models.21 Office-based treatment normalizes OUD as a chronic disease managed by the primary care physician, enabling concurrent harm-reduction strategies; medication reconciliation; and convenient, regular prescribing intervals (eg, every 30 days).22 Nevertheless, access to buprenorphine is limited. Because buprenorphine is a controlled substance, the Ryan Haight Online Pharmacy Consumer Protection Act of 2008 prevents initial prescribing of buprenorphine without in-person evaluation. Telehealth consultations increased access to buprenorphine through temporary exceptions during the COVID-19 pandemic. However, revised rules and regulations for telehealth visits for these controlled substances are forthcoming from the DEA as temporary exceptions for telehealth consultations come to an end. Additionally, prescribing buprenorphine for OUD requires that the treating physician undergo specific training and obtain qualifications, which have evolved over time through federal legislation.
The Drug Addiction Treatment Act of 2000 (DATA 2000) authorized what is known as an X-waiver, which allows physicians to prescribe controlled substances for office-based treatment of OUD, provided that:
- they are registered to do so with the Substance Abuse and Mental Health Services Administration and the DEA
- they have had subspecialty training in addiction or completed an 8-hour training course
- they are able to refer patients to appropriate counseling and ancillary services.
DATA 2000 restricted patient panel sizes to 30 patients in the first year, expanding thereafter upon appropriate certification.
The Comprehensive Addiction and Recovery Act of 2016 (CARA) and the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act of 2018 (the SUPPORT Act) collectively extended prescribing authority for MOUD to other qualifying practitioners (eg, advanced practice clinicians). Despite these attempts to expand access to services, the overdose death rate has continued to increase.
Continue to: To further expand access to MAR...
To further expand access to MAR, the US Department of Health and Human Services updated its practice guidelines in April 2021, allowing clinicians to bypass X-waiver training requirements by applying for a notification-of-intent (NOI) buprenorphine waiver.a However, clinicians are still limited to prescribing buprenorphine for 30 patients at a time. Clinicians who undergo complete X-waiver training may prescribe for 100 patients in the first year and, if eligible, 275 patients thereafter.
In addition, as a component of the Consolidation Appropriations Act of 2023, Congress passed the Mainstreaming Addiction Treatment Act of 2021, or MAT 2021, and Medication Access and Training Expansion Act of 2021, or MATE 2021. MAT eliminated the X-waiver, NOI, and restrictions on the number of patients for whom a provider could prescribe buprenorphine, under federal authority; however, restrictions within one’s state might limit the ability to prescribe buprenorphine. MATE 2021 is an educational requirement for licensing by the DEA (at application and renewal) that will require prescribers to complete 8 hours of training in substance use disorders starting in June 2023.
Use of the monthly injectable extended-release buprenorphine productb is limited by an FDA Risk Evaluation and Mitigation Strategy (REMS) program, which requires specialized training and certification by the prescriber, distributor, and administering clinician. REMS reduces buprenorphine accessibility due to time, cost, and regulatory barriers; although such restrictions have been instituted with the patient’s safety in mind, any limitation to buprenorphine prescribing, apart from controlled substance licensure, serves only to limit access to a primary component of MAR.
Clinical considerations. Due to the competitive nature of buprenorphine and its high affinity for the μ-opioid receptor, the drug can displace other opioid agonists and precipitate acute withdrawal. The withdrawal experience can thereby condition fear and disfavor toward buprenorphine among patients.
It is vital, therefore, that (1) patients’ expectations for treatment be managed appropriately and (2) the treating physician be prepared to provide additional buprenorphine for adequate maintenance doses and utilize adjunct comfort agents (clonidine, nonsteroidal anti-inflammatory drugs, ondansetron) to manage acute withdrawal symptoms. Newer buprenorphine dosing strategies, such as micro-induction and macro-induction, have emerged to curtail these risks.23,24 This is an evolving area of MAR; newer low-threshold initiation strategies25 (see “Low-threshold MOUD prescribing models,” in the text that follows) and evidence that supports micro-induction26 might eliminate the practice of requiring active withdrawal for treatment.
Continue to: Regardless of the strategy...
Regardless of the strategy for dosing buprenorphine, it’s critical that patients be educated on how to initiate treatment outside a clinical setting, such as at home, where they occupy a familiar haven during a potentially uncomfortable time and can be as effective at initiation as they would be in a clinical setting, with no difference in precipitation of adverse effects.
At-home induction might be more appropriate for patients who are not yet in significant enough withdrawal while in the physician's office.27 Guidance should be provided on dosing instructions, self-assessment of withdrawal symptoms, and, if applicable, patience with the slow-dissolving sublingual tablet or film formulation.
Naltrexone
Background. Naltrexone is available as an oral tablet and an extended-release, once-monthly intramuscular injection; the latter has demonstrated superiority in MAR.28 Oral naltrexone has limited supporting evidence, is inferior to other MOUD options, and should not be used to treat OUD.7 Altogether, approval of naltrexone for OUD is controversial, due to potentially unethical trials and approval processes,29 although a multicenter randomized controlled trial demonstrated the drug’s noninferiority with respect to treatment retention relative to buprenorphine.30 Used over time, naltrexone does not relieve withdrawal symptoms but can reduce cravings.
Clinical considerations. There are numerous clinical barriers that limit the use of naltrexone.
First, patients should be abstinent from opioids for 7 to 14 days prior to starting therapy; usually, this means undergoing medically supervised withdrawal in a controlled environment. This is an obvious limitation for patients who are constrained financially—those who lack, or have inadequate, health insurance or are unable to be away from their job for an extended time.
Continue to: Second, because naltrexone...
Second, because naltrexone does not address withdrawal symptoms, supportive therapies should be incorporated into the treatment plan, including:
- clonidine for hyperadrenergic symptoms (anxiety, diaphoresis, hypertension)
- nonopioid analgesics for pain
- antiemetics, such as ondansetron and metoclopramide, for nausea or vomiting
- loperamide for diarrhea
- diphenhydramine for insomnia.
Third, patients taking naltrexone have a diminished response to opioids. This complicates pain management in the event of an emergent surgical procedure.
Last, when naltrexone wears off, patients are effectively opioid-naïve, which increases the risk for overdose in those who stop therapy abruptly.29 The increased risk for overdose should be communicated to all patients with OUD who are being treated with naltrexone.
This nonopioid option is appealing to policymakers and is often prioritized in the criminal justice system; however, the decreased efficacy of naltrexone (compared to methadone and buprenorphine), potential for overdose, and challenges in initiating treatment are concerning and limit the drug’s use in many real-world settings.
Because naltrexone is not a controlled substance, regulations regarding maintaining inventory and distribution are more flexible.
Continue to: Overall, the cost-effectiveness...
Overall, the cost-effectiveness of intramuscular naltrexone is unclear. State-administered insurance programs vary in their requirements for coverage of naltrexone treatment.31
Comprehensive medication reconciliation is vital
Overall fragmentation of care within OTPs places patients at risk for adverse events, such as drug interactions.32 Under Title 42 of the US Code,33 patients must provide written consent for an OTP provider to disclose their history of a substance use disorder. Allowing the patient to decide which medical providers can access their treatment records for an OUD benefits patient confidentiality but poses numerous issues worth exploring.
All prescribed controlled substances are recorded in the prescription drug monitoring program, or PDMP, a state-level electronic database accessible to health care professionals to inform prescribing decisions and identify drug interactions. The PDMP has substantially reduced opioid overprescribing and improved identification of patients at risk for overdose or misuse of opioids.
Unlike all other controlled substances, however, prescriptions ordered by an OTP are not recorded in the PDMP (although there are recent exceptions to this scenario). Without such information, a physician might not have important information about the patient when making medical decisions—placing the patient at risk for harmful outcomes, such as drug–drug and drug–disease interactions.
For example: Methadone is associated with a prolonged QT interval,34 increasing the risk for a fatal arrhythmia. Concurrent QT-prolonging medications, such as azithromycin and citalopram, further increase this risk.35 Because methadone dispensing is isolated from the patient’s medical record, the clinician who prescribes MOUD has an incomplete patient history and could make a potentially fatal treatment decision.
Continue to: Diversion is unlikely
Diversion is unlikely
Health care providers often express concern about diversion in MOUD. However, misuse and diversion rates of methadone and buprenorphine have declined steadily since 2011, and, in fact, are actually lower than the diversion rate of prescription antibiotics.36
Regardless, diversion of buprenorphine should not be a concern for physicians prescribing MOUD. Although a prescriber might worry about manipulation of the formulation of buprenorphine for intravenous administration, addition of naloxone to buprenorphine in tablet form diminishes the potential for overdose. Additionally, the ceiling effect of buprenorphine limits the likelihood of significant respiratory depression and euphoria.
Should buprenorphine reach a patient for whom it was not prescribed, it is highly unlikely that an overdose would result. Rather, the medication would protect against the effects of illicit opioids and relieve withdrawal symptoms. Most people with OUD who have misused buprenorphine have done so to relieve withdrawal symptoms,37 not to experience intoxication.
Health care deserts
So-called health care deserts in parts of the United States are an ongoing problem that disproportionately affects lower-income and segregated Black and Hispanic communities38—communities that shoulder the highest burden of OUD and OUD-related mortality39 and whose populace is in greatest need of MAR. Even when health care is accessible in such a desert, some clinicians and pharmacies refuse to prescribe or dispense MOUD because of the accompanying stigma of OUD.
A MAR desert, like a pharmacy desert, is a geographic region—one without access to a MAR or an OTP provider, thereby preventing patients from reaching appropriate care; for some patients, having to travel to the nearest provider can render treatment inaccessible.40
Continue to: Efforts are in place to identify...
Efforts are in place to identify areas at greatest need of OUD-related medical services, such as heat maps that identify areas of increased utilization of emergency medical services for opioid overdose. State-run programs have been implemented to increase access, such as the Illinois Helpline (https://helplineil.org) that provides support and resources for patients, friends, family, and providers.
Novel solutions
Key strategies to increase access to care and slow the opioid epidemic include low-threshold prescribing of MOUD and mobile OTPs.41
Low-threshold MOUD prescribing models. Adoption of one of these models in a medical practice that provides MAR might increase absolute enrollment. A low-threshold prescribing model involves42:
- same-day treatment
- leniency with respect to abstinence periods and a concomitant substance use disorder
- enhanced accessibility to MOUD through nontraditional medical settings.
Low-threshold prescribing is flexible in regard to patients’ needs and bypasses many of the barriers discussed in this article. Impressive multicenter success has been achieved by the CA Bridge program in California (https://cabridge.org), including an increase in recognition of OUD, treatment initiations, and outpatient engagement.25
The cost-effectiveness of low-threshold MOUD prescribing programs remains to be determined.
Mobile OTPs. In July 2021, the DEA authorized a mobile component to existing OTP registrants that is permitted to dispense methadone and buprenorphine. Mobile units are physically separate from the OTP but have similar functions, depending on available space. Services that cannot be provided on the mobile unit of an OTP must be available at its brick-and-mortar location.7 Logistically, OTP registrants no longer need a separate registration to implement a mobile unit, thus expanding care to patients in underserved or remote areas who often encounter barriers to access.43
Conclusion
Understanding the distinct clinical and accessibility benefits and limitations among available MOUD is essential for prescribing clinicians. Accessing treatment is limited by federal regulation, stigma, and the existence of health care deserts that limit access to necessary care for patients with OUD. Newer harm-reduction models, such as low-threshold prescribing and mobile OTPs, represent progress, but many patients remain untreated.
a At buprenorphine.samhsa.gov/forms/select-practitioner-type.php
b Sold under the brand name Sublocade.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, Department of Pharmacy Practice, University of Illinois Chicago College of Pharmacy, 833 South Wood Street (MC 886), Chicago, IL 60612; jarrett8@uic.edu
Medication-assisted recovery (MAR)—the preferred terminology for the service formerly known as medication-assisted treatment—entails a comprehensive set of interventions for managing opioid use disorder (OUD), including medications for opioid use disorder (MOUD). Despite the benefits of MAR—reducing opioid use, opioid-related mortality, and health care costs1-3—only 11% of patients with a diagnosis of OUD received MOUD in 2020.3
Primary care physicians, including family physicians, are well positioned to provide MAR across the patient’s lifespan. However, many family medicine clinicians do not possess the logistical knowledge or resources to implement this service.4 In this article, we describe options for, and barriers to, MAR and societal issues that have an impact on the care of these patients.
Pathophysiology of OUD
Opioids relieve pain by stimulating μ-opioid receptors and activating the brain’s reward system. These pleasurable effects motivate repeated use.5 Frequent opioid exposure causes neuroadaptation, tolerance, and dependence. For patients with OUD who are misusing illicit or prescription opioids, periods of abstinence following neuroadaptation lead to withdrawal symptoms that vary in intensity, depending on the drug, dose, and duration of use. Upregulated noradrenergic tone and dopamine deficiency manifest as numerous signs and symptoms of withdrawal, including5:
- Physiologic: secretory (diaphoresis, rhinorrhea, lacrimation, vomiting, diarrhea) and stimulatory (mydriasis, piloerection, hypertension, tachycardia, insomnia)
- Psychological: pain, cravings, dysphoria, anxiety.
A single episode of opioid withdrawal is not directly life-threatening, but untreated episodes can progressively amplify negative feedback and reinforce continued opioid use.6 Left untreated, withdrawal can be terminal.

Medication-assisted recovery: Effective intervention
MAR services that integrate medical, behavioral, and psychosocial programs can reduce mortality from OUD 2-fold.7,8 A meta-analysis found that, when MAR services are rendered in primary care, treatment retention improves by 25% (number needed to treat [NNT] = 6) and ongoing illicit opioid use is reduced by 50% (NNT = 6), relative to care at a specialty clinic9—highlighting a role for family medicine clinicians in treating OUD.
All 3 US Food and Drug Administration (FDA)–approved MOUD (methadone, buprenorphine, and naltrexone) reduce cravings; 2 (methadone and buprenorphine) mitigate withdrawal symptoms by activating the μ-opioid receptor; and naltrexone diminishes the reinforcing effects of use (TABLE10-12). It is crucial to recognize the pharmacologic distinctions among MOUD because untreated withdrawal syndromes increase dropout from treatment programs and subsequent relapse.13

The Hx of medication-assisted recovery
To understand the landscape of MAR, it is important to understand the history of opioid treatment in the United States. In 1966, Congress passed the Narcotic Addiction Rehabilitation Act (NARA), which secured federal assistance by which state and local governments could develop drug treatment programs.14 NARA permitted legal offenders with OUD to be civilly committed to treatment programs, rather than prosecuted. However, limited resources and a burgeoning population led, instead, to low-cost outpatient programs saddled by strict requirements that lacked a basis for improving clinical outcomes.
Continue to: At the time NARA...
At the time NARA was passed by Congress, OUD was viewed—inaccurately—as a criminal problem, not a medical one. Subsequent legislation was crafted through that lens, which has placed a heavy burden on patients until today.14 Although medical understanding of OUD has advanced tremendously over the past 50 years, treatment remains siloed from mainstream medicine, even in primary care.
There is no one-size-fits-all approach to MAR, and relapse is common. Patient-specific factors and the availability of resources should be considered when designing the most individualized, advantageous plan for MAR.
Methadone
Background. Methadone has the most extensive history for treating OUD and consistently has demonstrated efficacy.13 A meta-analysis of randomized controlled trials comparing methadone to nonpharmacotherapy alone found that methadone improved treatment retention by an absolute 57% (NNT = 2).10
Methadone was approved by the FDA for detoxification and maintenance treatment in the early 1970s, although the Narcotic Addict Treatment Act (NATA) of 1974 restricted dispensing of maintenance treatment to highly regulated clinics known as opioid treatment programs (OTPs).14 NATA required the treating physician to register with the US Drug Enforcement Agency (DEA) and to comply with conservative dosing regimens and observed dosing.
Over time, regulations evolved to give the physician greater flexibility in developing a care plan, allowing “take-home” doses, and improving patients’ access to care. Although access to methadone for the treatment of OUD remains limited to federally certified OTPs, regulations facilitate incorporation of a whole-person approach to care, including counseling, individual and group therapy, and toxicology testing.7
Continue to: Clinical considerations
Clinical considerations. Methadone requires slow titration. For patients starting methadone as an outpatient, federal law15 limits the initial dose to 30 mg and requires physician documentation when the first-day total dosage exceeds 40 mg. This dosing constraint makes it challenging to provide care because a daily dosage ≥ 60 mg has been found to produce, first, higher program retention (relative risk = 1.36; 95% CI, 1.13-1.63) and, second, greater reduction in illicit opioid use (relative risk = 1.59; 95% CI, 1.16-2.18) than is seen in patients who receive a lower daily dosage.16
Due to a prolonged elimination half-life, methadone reaches steady-state in 3 to 5 days. Patients and their families should be educated that withdrawal symptoms might not feel fully managed in the first few days of therapy and that time is required to experience safely the regimen’s full effects.
Aggressive dose-titration during methadone induction can result in drug accumulation and respiratory depression. The risk for methadone-related mortality is highest in the first 2 weeks of therapy, mostly related to overdose potential if the drug is combined with other opioids.17
Buprenorphine
Background. The prescribing rate for buprenorphine, particularly in primary care, is accelerating.18 A meta-analysis of randomized controlled trials found that11:
- compared to placebo, buprenorphine, at any dosage, improves treatment retention by an absolute 21% to 28% (NNT = 4-5)
- patients receiving high-dose buprenorphine (≥ 16 mg/d) had fewer evident cases of illicit opioid use.
Unlike methadone, buprenorphine exerts partial agonism at the μ-opioid receptor, resulting in a so-called ceiling effect that significantly reduces the adverse effect profile, including respiratory depression and euphoria, relative to a full-agonist opioid, such as methadone.19
Continue to: Whereas accessing methadone...
Whereas accessing methadone is limited to OTPs, buprenorphine is available for office-based treatment. By hosting OUD treatment and primary care in the same place, primary care physicians can provide comprehensive medical care including and beyond OUD, thereby improving retention and managing comorbidity.20
Integrated models involving support staff—eg, nurses, behavioral health providers, and pharmacists—have produced the greatest success with office-based treatment models.21 Office-based treatment normalizes OUD as a chronic disease managed by the primary care physician, enabling concurrent harm-reduction strategies; medication reconciliation; and convenient, regular prescribing intervals (eg, every 30 days).22 Nevertheless, access to buprenorphine is limited. Because buprenorphine is a controlled substance, the Ryan Haight Online Pharmacy Consumer Protection Act of 2008 prevents initial prescribing of buprenorphine without in-person evaluation. Telehealth consultations increased access to buprenorphine through temporary exceptions during the COVID-19 pandemic. However, revised rules and regulations for telehealth visits for these controlled substances are forthcoming from the DEA as temporary exceptions for telehealth consultations come to an end. Additionally, prescribing buprenorphine for OUD requires that the treating physician undergo specific training and obtain qualifications, which have evolved over time through federal legislation.
The Drug Addiction Treatment Act of 2000 (DATA 2000) authorized what is known as an X-waiver, which allows physicians to prescribe controlled substances for office-based treatment of OUD, provided that:
- they are registered to do so with the Substance Abuse and Mental Health Services Administration and the DEA
- they have had subspecialty training in addiction or completed an 8-hour training course
- they are able to refer patients to appropriate counseling and ancillary services.
DATA 2000 restricted patient panel sizes to 30 patients in the first year, expanding thereafter upon appropriate certification.
The Comprehensive Addiction and Recovery Act of 2016 (CARA) and the Substance Use Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act of 2018 (the SUPPORT Act) collectively extended prescribing authority for MOUD to other qualifying practitioners (eg, advanced practice clinicians). Despite these attempts to expand access to services, the overdose death rate has continued to increase.
Continue to: To further expand access to MAR...
To further expand access to MAR, the US Department of Health and Human Services updated its practice guidelines in April 2021, allowing clinicians to bypass X-waiver training requirements by applying for a notification-of-intent (NOI) buprenorphine waiver.a However, clinicians are still limited to prescribing buprenorphine for 30 patients at a time. Clinicians who undergo complete X-waiver training may prescribe for 100 patients in the first year and, if eligible, 275 patients thereafter.
In addition, as a component of the Consolidation Appropriations Act of 2023, Congress passed the Mainstreaming Addiction Treatment Act of 2021, or MAT 2021, and Medication Access and Training Expansion Act of 2021, or MATE 2021. MAT eliminated the X-waiver, NOI, and restrictions on the number of patients for whom a provider could prescribe buprenorphine, under federal authority; however, restrictions within one’s state might limit the ability to prescribe buprenorphine. MATE 2021 is an educational requirement for licensing by the DEA (at application and renewal) that will require prescribers to complete 8 hours of training in substance use disorders starting in June 2023.
Use of the monthly injectable extended-release buprenorphine productb is limited by an FDA Risk Evaluation and Mitigation Strategy (REMS) program, which requires specialized training and certification by the prescriber, distributor, and administering clinician. REMS reduces buprenorphine accessibility due to time, cost, and regulatory barriers; although such restrictions have been instituted with the patient’s safety in mind, any limitation to buprenorphine prescribing, apart from controlled substance licensure, serves only to limit access to a primary component of MAR.
Clinical considerations. Due to the competitive nature of buprenorphine and its high affinity for the μ-opioid receptor, the drug can displace other opioid agonists and precipitate acute withdrawal. The withdrawal experience can thereby condition fear and disfavor toward buprenorphine among patients.
It is vital, therefore, that (1) patients’ expectations for treatment be managed appropriately and (2) the treating physician be prepared to provide additional buprenorphine for adequate maintenance doses and utilize adjunct comfort agents (clonidine, nonsteroidal anti-inflammatory drugs, ondansetron) to manage acute withdrawal symptoms. Newer buprenorphine dosing strategies, such as micro-induction and macro-induction, have emerged to curtail these risks.23,24 This is an evolving area of MAR; newer low-threshold initiation strategies25 (see “Low-threshold MOUD prescribing models,” in the text that follows) and evidence that supports micro-induction26 might eliminate the practice of requiring active withdrawal for treatment.
Continue to: Regardless of the strategy...
Regardless of the strategy for dosing buprenorphine, it’s critical that patients be educated on how to initiate treatment outside a clinical setting, such as at home, where they occupy a familiar haven during a potentially uncomfortable time and can be as effective at initiation as they would be in a clinical setting, with no difference in precipitation of adverse effects.
At-home induction might be more appropriate for patients who are not yet in significant enough withdrawal while in the physician's office.27 Guidance should be provided on dosing instructions, self-assessment of withdrawal symptoms, and, if applicable, patience with the slow-dissolving sublingual tablet or film formulation.
Naltrexone
Background. Naltrexone is available as an oral tablet and an extended-release, once-monthly intramuscular injection; the latter has demonstrated superiority in MAR.28 Oral naltrexone has limited supporting evidence, is inferior to other MOUD options, and should not be used to treat OUD.7 Altogether, approval of naltrexone for OUD is controversial, due to potentially unethical trials and approval processes,29 although a multicenter randomized controlled trial demonstrated the drug’s noninferiority with respect to treatment retention relative to buprenorphine.30 Used over time, naltrexone does not relieve withdrawal symptoms but can reduce cravings.
Clinical considerations. There are numerous clinical barriers that limit the use of naltrexone.
First, patients should be abstinent from opioids for 7 to 14 days prior to starting therapy; usually, this means undergoing medically supervised withdrawal in a controlled environment. This is an obvious limitation for patients who are constrained financially—those who lack, or have inadequate, health insurance or are unable to be away from their job for an extended time.
Continue to: Second, because naltrexone...
Second, because naltrexone does not address withdrawal symptoms, supportive therapies should be incorporated into the treatment plan, including:
- clonidine for hyperadrenergic symptoms (anxiety, diaphoresis, hypertension)
- nonopioid analgesics for pain
- antiemetics, such as ondansetron and metoclopramide, for nausea or vomiting
- loperamide for diarrhea
- diphenhydramine for insomnia.
Third, patients taking naltrexone have a diminished response to opioids. This complicates pain management in the event of an emergent surgical procedure.
Last, when naltrexone wears off, patients are effectively opioid-naïve, which increases the risk for overdose in those who stop therapy abruptly.29 The increased risk for overdose should be communicated to all patients with OUD who are being treated with naltrexone.
This nonopioid option is appealing to policymakers and is often prioritized in the criminal justice system; however, the decreased efficacy of naltrexone (compared to methadone and buprenorphine), potential for overdose, and challenges in initiating treatment are concerning and limit the drug’s use in many real-world settings.
Because naltrexone is not a controlled substance, regulations regarding maintaining inventory and distribution are more flexible.
Continue to: Overall, the cost-effectiveness...
Overall, the cost-effectiveness of intramuscular naltrexone is unclear. State-administered insurance programs vary in their requirements for coverage of naltrexone treatment.31
Comprehensive medication reconciliation is vital
Overall fragmentation of care within OTPs places patients at risk for adverse events, such as drug interactions.32 Under Title 42 of the US Code,33 patients must provide written consent for an OTP provider to disclose their history of a substance use disorder. Allowing the patient to decide which medical providers can access their treatment records for an OUD benefits patient confidentiality but poses numerous issues worth exploring.
All prescribed controlled substances are recorded in the prescription drug monitoring program, or PDMP, a state-level electronic database accessible to health care professionals to inform prescribing decisions and identify drug interactions. The PDMP has substantially reduced opioid overprescribing and improved identification of patients at risk for overdose or misuse of opioids.
Unlike all other controlled substances, however, prescriptions ordered by an OTP are not recorded in the PDMP (although there are recent exceptions to this scenario). Without such information, a physician might not have important information about the patient when making medical decisions—placing the patient at risk for harmful outcomes, such as drug–drug and drug–disease interactions.
For example: Methadone is associated with a prolonged QT interval,34 increasing the risk for a fatal arrhythmia. Concurrent QT-prolonging medications, such as azithromycin and citalopram, further increase this risk.35 Because methadone dispensing is isolated from the patient’s medical record, the clinician who prescribes MOUD has an incomplete patient history and could make a potentially fatal treatment decision.
Continue to: Diversion is unlikely
Diversion is unlikely
Health care providers often express concern about diversion in MOUD. However, misuse and diversion rates of methadone and buprenorphine have declined steadily since 2011, and, in fact, are actually lower than the diversion rate of prescription antibiotics.36
Regardless, diversion of buprenorphine should not be a concern for physicians prescribing MOUD. Although a prescriber might worry about manipulation of the formulation of buprenorphine for intravenous administration, addition of naloxone to buprenorphine in tablet form diminishes the potential for overdose. Additionally, the ceiling effect of buprenorphine limits the likelihood of significant respiratory depression and euphoria.
Should buprenorphine reach a patient for whom it was not prescribed, it is highly unlikely that an overdose would result. Rather, the medication would protect against the effects of illicit opioids and relieve withdrawal symptoms. Most people with OUD who have misused buprenorphine have done so to relieve withdrawal symptoms,37 not to experience intoxication.
Health care deserts
So-called health care deserts in parts of the United States are an ongoing problem that disproportionately affects lower-income and segregated Black and Hispanic communities38—communities that shoulder the highest burden of OUD and OUD-related mortality39 and whose populace is in greatest need of MAR. Even when health care is accessible in such a desert, some clinicians and pharmacies refuse to prescribe or dispense MOUD because of the accompanying stigma of OUD.
A MAR desert, like a pharmacy desert, is a geographic region—one without access to a MAR or an OTP provider, thereby preventing patients from reaching appropriate care; for some patients, having to travel to the nearest provider can render treatment inaccessible.40
Continue to: Efforts are in place to identify...
Efforts are in place to identify areas at greatest need of OUD-related medical services, such as heat maps that identify areas of increased utilization of emergency medical services for opioid overdose. State-run programs have been implemented to increase access, such as the Illinois Helpline (https://helplineil.org) that provides support and resources for patients, friends, family, and providers.
Novel solutions
Key strategies to increase access to care and slow the opioid epidemic include low-threshold prescribing of MOUD and mobile OTPs.41
Low-threshold MOUD prescribing models. Adoption of one of these models in a medical practice that provides MAR might increase absolute enrollment. A low-threshold prescribing model involves42:
- same-day treatment
- leniency with respect to abstinence periods and a concomitant substance use disorder
- enhanced accessibility to MOUD through nontraditional medical settings.
Low-threshold prescribing is flexible in regard to patients’ needs and bypasses many of the barriers discussed in this article. Impressive multicenter success has been achieved by the CA Bridge program in California (https://cabridge.org), including an increase in recognition of OUD, treatment initiations, and outpatient engagement.25
The cost-effectiveness of low-threshold MOUD prescribing programs remains to be determined.
Mobile OTPs. In July 2021, the DEA authorized a mobile component to existing OTP registrants that is permitted to dispense methadone and buprenorphine. Mobile units are physically separate from the OTP but have similar functions, depending on available space. Services that cannot be provided on the mobile unit of an OTP must be available at its brick-and-mortar location.7 Logistically, OTP registrants no longer need a separate registration to implement a mobile unit, thus expanding care to patients in underserved or remote areas who often encounter barriers to access.43
Conclusion
Understanding the distinct clinical and accessibility benefits and limitations among available MOUD is essential for prescribing clinicians. Accessing treatment is limited by federal regulation, stigma, and the existence of health care deserts that limit access to necessary care for patients with OUD. Newer harm-reduction models, such as low-threshold prescribing and mobile OTPs, represent progress, but many patients remain untreated.
a At buprenorphine.samhsa.gov/forms/select-practitioner-type.php
b Sold under the brand name Sublocade.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, Department of Pharmacy Practice, University of Illinois Chicago College of Pharmacy, 833 South Wood Street (MC 886), Chicago, IL 60612; jarrett8@uic.edu
1. Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235-S248.
2. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462-468. doi: 10.1111/j.1360-0443.2007.02090.x
3. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. HHS Publication PEP21-07-01-003, NSDUH Series H-56. 2021. Accessed March 19, 2023. www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
4. Haffajee RL, Andraka-Christou B, Attermann J, et al. A mixed-method comparison of physician-reported beliefs about and barriers to treatment with medications for opioid use disorder. Subst Abuse Treat Prev Policy. 2020;15:69. doi: 10.1186/s13011-020-00312-3
5. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20. doi: 10.1151/spp021113
6. Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44-53. doi: 10.1016/j.biopsych.2019.05.023
7. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. For Health care and Addiction Professionals, Policymakers, Patients, and Families. Treatment Improvement Protocol TIP 63. Publication No. PEP21-02-01-002. 2021. Accessed March 19, 2023. https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf
8. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550
9. Korownyk C, Perry D, Ton J, et al. Opioid use disorder in primary care: PEER umbrella systematic review of systematic reviews. Can Fam Physician. 2019;65:e194-e206.
10. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2
11. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4
12. Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513. doi: 10.1016/S0140-6736(11)60358-9
13. Soyka M, Zingg C, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641-653. doi: 10.1017/S146114570700836X
14. Institute of Medicine Committee on Federal Regulation of Methadone Treatment; Rettig R, Yarmolinsky A, eds. Federal Regulation of Methadone Treatment. National Academies Press; 1995.
15. 42 eCFR §8. Medication assisted treatment for opioid use disorders. Revised March 15, 2023. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8?toc=1
16. Faggiano F, Vigna-Taglianti F, Versino E, et al. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;(3):CD002208. doi: 10.1002/14651858.CD002208
17. Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377-386. doi: 10.1097/01.ADM.0000435321.39251.d7
18. Olfson M, Zhang VS, Schoenbaum M, et al. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323:276-277. doi: 10.1001/jama.2019.18913
19. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580. doi: 10.1038/clpt.1994.71
20. Walley AY, Palmisano J, Sorensen-Alawad A, et al. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J Subst Abuse Treat. 2015;59:59-66. doi: 10.1016/j.jsat.2015.07.007
21. Lagisetty P, Klasa K, Bush C, et al. Primary care models for treating opioid use disorders: what actually works? A systematic review. PloS One. 2017;12:e0186315. doi: 10.1371/journal.pone.0186315
22. Du CX, Shi J, Tetrault JM, et al. Primary care and medication management characteristics among patients receiving office-based opioid treatment with buprenorphine. Fam Pract. 2022;39:234-240. doi: 10.1093/fampra/cmab166
23. Herring AA, Vosooghi AA, Luftig J, et al. High-dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4:e2117128. doi: 10.1001/jamanetworkopen.2021.17128
24. Hämmig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105. doi: 10.2147/SAR.S109919
25. Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA Bridge Program. Ann Emerg Med. 2021;78:759-772. doi: 10.1016/j.annemergmed.2021.05.024
26. Wong JSH, Nikoo M, Westenberg JN, et al. Comparing rapid micro-induction and standard induction of buprenorphine/naloxone for treatment of opioid use disorder: protocol for an open-label, parallel-group, superiority, randomized controlled trial. Addict Sci Clin Pract. 2021;16:11. doi: 10.1186/s13722-021-00220-2
27. Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med. 2014;8:299-308. doi: 10.1097/ADM.0000000000000059
28. Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973-981. doi: 10.1001/archgenpsychiatry.2012.1a
29. Wolfe D, Carrieri MP, Dasgupta N, et al. Concerns about injectable naltrexone for opioid dependence. Lancet. 2011;377:1468-1470. doi: 10.1016/S0140-6736(10)62056-9
30. Tanum L, Solli KK, Latif ZEH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine–naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205. doi: 10.1001/jamapsychiatry.2017.3206
31. Murphy SM, Polsky D, Lee JD, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal justice-involved individuals with a history of opioid use disorder. Addiction. 2017;112:1440-1450. doi: 10.1111/add.13807
32. Ferrari A, Coccia CPR, Bertolini A, et al. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551-559. doi: 10.1016/j.phrs.2004.05.002
33. 42 eCFR Part 2. Confidentiality of substance use disorder patient records. January 18, 2017. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-2
34. Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015;110:1468-1475. doi: 10.1111/add.13013
35. Tisdale JE, Chung MK, Campbell KB, et al; . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214-e233. doi: 10.1161/CIR.0000000000000905
36. Leshner AI, Mancher M, eds. Barriers to broader use of medications to treat opioid use disorder. In: Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019:109-136.
37. Chilcoat HD, Amick HR, Sherwood MR, et al. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157. doi: 10.1016/j.jsat. 2019.07.005
38. Qato DM, Daviglus ML, Wilder J, et al. “Pharmacy deserts” are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33:1958-1965. doi: 10.1377/hlthaff.2013.1397
39. Mason M, Soliman R, Kim HS, et al. Disparities by sex and race and ethnicity in death rates due to opioid overdose among adults 55 years or older, 1999 to 2019. JAMA Netw Open. 2022;5:e2142982. doi: 10.1001/jamanetworkopen.2021.42982
40. Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789
41. Chan B, Hoffman KA, Bougatsos C, et al. Mobile methadone medication units: a brief history, scoping review and research opportunity. J Subst Abuse Treat. 2021;129:108483. doi: 10.1016/j.jsat.2021.108483
42. Jakubowski A, Fox A. Defining low-threshold buprenorphine treatment. J Addict Med. 2020;14:95-98. doi: 10.1097/ADM.0000000000000555
43. Messmer SE, Elmes AT, Jimenez AD, et al. Outcomes of a mobile medical unit for low-threshold buprenorphine access targeting opioid overdose hot spots in Chicago. J Subst Use Addict Treat. 2023;209054. doi: 10.1016/j.josat.2023.209054
1. Baser O, Chalk M, Fiellin DA, et al. Cost and utilization outcomes of opioid-dependence treatments. Am J Manag Care. 2011;17(suppl 8):S235-S248.
2. Gibson A, Degenhardt L, Mattick RP, et al. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103:462-468. doi: 10.1111/j.1360-0443.2007.02090.x
3. Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results From the 2020 National Survey on Drug Use and Health. HHS Publication PEP21-07-01-003, NSDUH Series H-56. 2021. Accessed March 19, 2023. www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
4. Haffajee RL, Andraka-Christou B, Attermann J, et al. A mixed-method comparison of physician-reported beliefs about and barriers to treatment with medications for opioid use disorder. Subst Abuse Treat Prev Policy. 2020;15:69. doi: 10.1186/s13011-020-00312-3
5. Kosten TR, George TP. The neurobiology of opioid dependence: implications for treatment. Sci Pract Perspect. 2002;1:13-20. doi: 10.1151/spp021113
6. Koob GF. Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry. 2020;87:44-53. doi: 10.1016/j.biopsych.2019.05.023
7. Substance Abuse and Mental Health Services Administration. Medications for Opioid Use Disorder. For Health care and Addiction Professionals, Policymakers, Patients, and Families. Treatment Improvement Protocol TIP 63. Publication No. PEP21-02-01-002. 2021. Accessed March 19, 2023. https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf
8. Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550
9. Korownyk C, Perry D, Ton J, et al. Opioid use disorder in primary care: PEER umbrella systematic review of systematic reviews. Can Fam Physician. 2019;65:e194-e206.
10. Mattick RP, Breen C, Kimber J, et al. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;(3):CD002209. doi: 10.1002/14651858.CD002209.pub2
11. Mattick RP, Breen C, Kimber J, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(2):CD002207. doi: 10.1002/14651858.CD002207.pub4
12. Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506-1513. doi: 10.1016/S0140-6736(11)60358-9
13. Soyka M, Zingg C, Koller G, et al. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11:641-653. doi: 10.1017/S146114570700836X
14. Institute of Medicine Committee on Federal Regulation of Methadone Treatment; Rettig R, Yarmolinsky A, eds. Federal Regulation of Methadone Treatment. National Academies Press; 1995.
15. 42 eCFR §8. Medication assisted treatment for opioid use disorders. Revised March 15, 2023. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-8?toc=1
16. Faggiano F, Vigna-Taglianti F, Versino E, et al. Methadone maintenance at different dosages for opioid dependence. Cochrane Database Syst Rev. 2003;(3):CD002208. doi: 10.1002/14651858.CD002208
17. Baxter LE Sr, Campbell A, Deshields M, et al. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377-386. doi: 10.1097/01.ADM.0000435321.39251.d7
18. Olfson M, Zhang VS, Schoenbaum M, et al. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323:276-277. doi: 10.1001/jama.2019.18913
19. Walsh SL, Preston KL, Stitzer ML, et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569-580. doi: 10.1038/clpt.1994.71
20. Walley AY, Palmisano J, Sorensen-Alawad A, et al. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J Subst Abuse Treat. 2015;59:59-66. doi: 10.1016/j.jsat.2015.07.007
21. Lagisetty P, Klasa K, Bush C, et al. Primary care models for treating opioid use disorders: what actually works? A systematic review. PloS One. 2017;12:e0186315. doi: 10.1371/journal.pone.0186315
22. Du CX, Shi J, Tetrault JM, et al. Primary care and medication management characteristics among patients receiving office-based opioid treatment with buprenorphine. Fam Pract. 2022;39:234-240. doi: 10.1093/fampra/cmab166
23. Herring AA, Vosooghi AA, Luftig J, et al. High-dose buprenorphine induction in the emergency department for treatment of opioid use disorder. JAMA Netw Open. 2021;4:e2117128. doi: 10.1001/jamanetworkopen.2021.17128
24. Hämmig R, Kemter A, Strasser J, et al. Use of microdoses for induction of buprenorphine treatment with overlapping full opioid agonist use: the Bernese method. Subst Abuse Rehabil. 2016;7:99-105. doi: 10.2147/SAR.S109919
25. Snyder H, Kalmin MM, Moulin A, et al. Rapid adoption of low-threshold buprenorphine treatment at California emergency departments participating in the CA Bridge Program. Ann Emerg Med. 2021;78:759-772. doi: 10.1016/j.annemergmed.2021.05.024
26. Wong JSH, Nikoo M, Westenberg JN, et al. Comparing rapid micro-induction and standard induction of buprenorphine/naloxone for treatment of opioid use disorder: protocol for an open-label, parallel-group, superiority, randomized controlled trial. Addict Sci Clin Pract. 2021;16:11. doi: 10.1186/s13722-021-00220-2
27. Lee JD, Vocci F, Fiellin DA. Unobserved “home” induction onto buprenorphine. J Addict Med. 2014;8:299-308. doi: 10.1097/ADM.0000000000000059
28. Krupitsky E, Zvartau E, Blokhina E, et al. Randomized trial of long-acting sustained-release naltrexone implant vs oral naltrexone or placebo for preventing relapse to opioid dependence. Arch Gen Psychiatry. 2012;69:973-981. doi: 10.1001/archgenpsychiatry.2012.1a
29. Wolfe D, Carrieri MP, Dasgupta N, et al. Concerns about injectable naltrexone for opioid dependence. Lancet. 2011;377:1468-1470. doi: 10.1016/S0140-6736(10)62056-9
30. Tanum L, Solli KK, Latif ZEH, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine–naloxone for opioid dependence: a randomized clinical noninferiority trial. JAMA Psychiatry. 2017;74:1197-1205. doi: 10.1001/jamapsychiatry.2017.3206
31. Murphy SM, Polsky D, Lee JD, et al. Cost-effectiveness of extended release naltrexone to prevent relapse among criminal justice-involved individuals with a history of opioid use disorder. Addiction. 2017;112:1440-1450. doi: 10.1111/add.13807
32. Ferrari A, Coccia CPR, Bertolini A, et al. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50:551-559. doi: 10.1016/j.phrs.2004.05.002
33. 42 eCFR Part 2. Confidentiality of substance use disorder patient records. January 18, 2017. Accessed March 23, 2023. www.ecfr.gov/current/title-42/chapter-I/subchapter-A/part-2
34. Kao DP, Haigney MCP, Mehler PS, et al. Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction. 2015;110:1468-1475. doi: 10.1111/add.13013
35. Tisdale JE, Chung MK, Campbell KB, et al; . Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214-e233. doi: 10.1161/CIR.0000000000000905
36. Leshner AI, Mancher M, eds. Barriers to broader use of medications to treat opioid use disorder. In: Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019:109-136.
37. Chilcoat HD, Amick HR, Sherwood MR, et al. Buprenorphine in the United States: Motives for abuse, misuse, and diversion. J Subst Abuse Treat. 2019;104:148-157. doi: 10.1016/j.jsat. 2019.07.005
38. Qato DM, Daviglus ML, Wilder J, et al. “Pharmacy deserts” are prevalent in Chicago’s predominantly minority communities, raising medication access concerns. Health Aff (Millwood). 2014;33:1958-1965. doi: 10.1377/hlthaff.2013.1397
39. Mason M, Soliman R, Kim HS, et al. Disparities by sex and race and ethnicity in death rates due to opioid overdose among adults 55 years or older, 1999 to 2019. JAMA Netw Open. 2022;5:e2142982. doi: 10.1001/jamanetworkopen.2021.42982
40. Rosenblum A, Cleland CM, Fong C, et al. Distance traveled and cross-state commuting to opioid treatment programs in the United States. J Environ Public Health. 2011;2011:948789. doi: 10.1155/2011/948789
41. Chan B, Hoffman KA, Bougatsos C, et al. Mobile methadone medication units: a brief history, scoping review and research opportunity. J Subst Abuse Treat. 2021;129:108483. doi: 10.1016/j.jsat.2021.108483
42. Jakubowski A, Fox A. Defining low-threshold buprenorphine treatment. J Addict Med. 2020;14:95-98. doi: 10.1097/ADM.0000000000000555
43. Messmer SE, Elmes AT, Jimenez AD, et al. Outcomes of a mobile medical unit for low-threshold buprenorphine access targeting opioid overdose hot spots in Chicago. J Subst Use Addict Treat. 2023;209054. doi: 10.1016/j.josat.2023.209054
PRACTICE RECOMMENDATIONS
› Consider resource availability (eg, treatment programs and regulatory barriers), in addition to patient- and medicationspecific factors, when designing the most individualized, advantageous medication-assisted recovery plan, to reduce the risk for mortality. B
› Schedule early (< 2 weeks) and frequent follow-up with patients who are starting medications for opioid use disorder (particularly methadone), to manage risk when mortality is highest and to support recovery. C
› Set and manage patient expectations for control of withdrawal symptoms when initiating medications for opioid use disorder (particularly buprenorphine). B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Thoughts on the CDC update on opioid prescribing guidelines
The media is filled with stories about the opioid crisis. We have all heard the horror stories of addiction and overdose, as well as “pill mill” doctors. In fact, more than 932,000 people have died of drug overdose since 1999 and, in recent years, approximately 75% of drug overdoses involved opioids.
Yet, they still have their place in the treatment of pain.
The CDC updated the 2016 guidelines for prescribing opioids for pain in 2022. They cover when to initiate prescribing of opioids, selecting appropriate opioids and doses, and deciding the duration of therapy. The guidelines do a great job providing evidence-based recommendations while at the same time keeping the problems with opioids in the picture.
For primary care doctors, pain is one of the most common complaints we see – from broken bones to low back pain to cancer pain. It is important to note that the current guidelines exclude pain from sickle cell disease, cancer-related pain, palliative care, and end-of-life care. The guidelines apply to acute, subacute, and chronic pain. Pain is a complex symptom and often needs a multipronged approach. We make a mistake if we just prescribe a pain medication without understanding the root cause of the pain.
The guidelines suggest starting with nonopioid medications and incorporating nonmedicinal modes of treatments, such as physical therapy, as well. Opioids should be started at the lowest dose and for the shortest duration. Immediate-release medications are preferred over long-acting or extended-release ones. The patient should always be informed of the risks and benefits.
While the guidelines do a great job recommending how to prescribe opioids, they do not go into any depth discussing other treatment options. Perhaps knowledge of other treatment modalities would help primary care physicians avoid opioid prescribing. When treating our patients, it is important to educate them on how to manage their own symptoms.
The guidelines also advise tapering patients who may have been on high-dose opioids for long periods of time. Doctors know this is a very difficult task. However, resources to help with this are often lacking. For example, rehab may not be covered under a patient’s insurance, or it may be cheaper to take an opioid than to go to physical therapy. Although the recommendation is to taper, community assets may not support this. Guidelines are one thing, but the rest of the health care system needs to catch up to them and make them practical.
Primary care doctors often utilize our physical medicine, rehabilitation, and pain management specialists to assist in managing our patients’ pain. Here too, access to this resource is often difficult to come by. Depending on a patient’s insurance, it can take months to get an appointment.
In general, the current guidelines offer 12 key recommendations when prescribing opioids. They are a great reference; however, we need more real-life tools. For many of us in primary care, these guidelines support what we’ve been doing all along.
Primary care doctors will surely play a huge role in addressing the opioid crisis. We can prescribe opioids appropriately, but it doesn’t erase the problems of those patients who were overprescribed in the past. Many still seek out these medications whether for monetary reasons or just for the high. It is often easy to blame the patient but the one in control is the one with the prescription pad. Yet, it is important to remember that many of these patients are in real pain and need help.
Often, it is simpler to just prescribe a pain medication than it is to explain why one is not appropriate. As primary care doctors, we need to be effective ambassadors of appropriate opioid prescribing and often that means doing the hard thing and saying no to a patient.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
fpnews@mdedge.com
The media is filled with stories about the opioid crisis. We have all heard the horror stories of addiction and overdose, as well as “pill mill” doctors. In fact, more than 932,000 people have died of drug overdose since 1999 and, in recent years, approximately 75% of drug overdoses involved opioids.
Yet, they still have their place in the treatment of pain.
The CDC updated the 2016 guidelines for prescribing opioids for pain in 2022. They cover when to initiate prescribing of opioids, selecting appropriate opioids and doses, and deciding the duration of therapy. The guidelines do a great job providing evidence-based recommendations while at the same time keeping the problems with opioids in the picture.
For primary care doctors, pain is one of the most common complaints we see – from broken bones to low back pain to cancer pain. It is important to note that the current guidelines exclude pain from sickle cell disease, cancer-related pain, palliative care, and end-of-life care. The guidelines apply to acute, subacute, and chronic pain. Pain is a complex symptom and often needs a multipronged approach. We make a mistake if we just prescribe a pain medication without understanding the root cause of the pain.
The guidelines suggest starting with nonopioid medications and incorporating nonmedicinal modes of treatments, such as physical therapy, as well. Opioids should be started at the lowest dose and for the shortest duration. Immediate-release medications are preferred over long-acting or extended-release ones. The patient should always be informed of the risks and benefits.
While the guidelines do a great job recommending how to prescribe opioids, they do not go into any depth discussing other treatment options. Perhaps knowledge of other treatment modalities would help primary care physicians avoid opioid prescribing. When treating our patients, it is important to educate them on how to manage their own symptoms.
The guidelines also advise tapering patients who may have been on high-dose opioids for long periods of time. Doctors know this is a very difficult task. However, resources to help with this are often lacking. For example, rehab may not be covered under a patient’s insurance, or it may be cheaper to take an opioid than to go to physical therapy. Although the recommendation is to taper, community assets may not support this. Guidelines are one thing, but the rest of the health care system needs to catch up to them and make them practical.
Primary care doctors often utilize our physical medicine, rehabilitation, and pain management specialists to assist in managing our patients’ pain. Here too, access to this resource is often difficult to come by. Depending on a patient’s insurance, it can take months to get an appointment.
In general, the current guidelines offer 12 key recommendations when prescribing opioids. They are a great reference; however, we need more real-life tools. For many of us in primary care, these guidelines support what we’ve been doing all along.
Primary care doctors will surely play a huge role in addressing the opioid crisis. We can prescribe opioids appropriately, but it doesn’t erase the problems of those patients who were overprescribed in the past. Many still seek out these medications whether for monetary reasons or just for the high. It is often easy to blame the patient but the one in control is the one with the prescription pad. Yet, it is important to remember that many of these patients are in real pain and need help.
Often, it is simpler to just prescribe a pain medication than it is to explain why one is not appropriate. As primary care doctors, we need to be effective ambassadors of appropriate opioid prescribing and often that means doing the hard thing and saying no to a patient.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
fpnews@mdedge.com
The media is filled with stories about the opioid crisis. We have all heard the horror stories of addiction and overdose, as well as “pill mill” doctors. In fact, more than 932,000 people have died of drug overdose since 1999 and, in recent years, approximately 75% of drug overdoses involved opioids.
Yet, they still have their place in the treatment of pain.
The CDC updated the 2016 guidelines for prescribing opioids for pain in 2022. They cover when to initiate prescribing of opioids, selecting appropriate opioids and doses, and deciding the duration of therapy. The guidelines do a great job providing evidence-based recommendations while at the same time keeping the problems with opioids in the picture.
For primary care doctors, pain is one of the most common complaints we see – from broken bones to low back pain to cancer pain. It is important to note that the current guidelines exclude pain from sickle cell disease, cancer-related pain, palliative care, and end-of-life care. The guidelines apply to acute, subacute, and chronic pain. Pain is a complex symptom and often needs a multipronged approach. We make a mistake if we just prescribe a pain medication without understanding the root cause of the pain.
The guidelines suggest starting with nonopioid medications and incorporating nonmedicinal modes of treatments, such as physical therapy, as well. Opioids should be started at the lowest dose and for the shortest duration. Immediate-release medications are preferred over long-acting or extended-release ones. The patient should always be informed of the risks and benefits.
While the guidelines do a great job recommending how to prescribe opioids, they do not go into any depth discussing other treatment options. Perhaps knowledge of other treatment modalities would help primary care physicians avoid opioid prescribing. When treating our patients, it is important to educate them on how to manage their own symptoms.
The guidelines also advise tapering patients who may have been on high-dose opioids for long periods of time. Doctors know this is a very difficult task. However, resources to help with this are often lacking. For example, rehab may not be covered under a patient’s insurance, or it may be cheaper to take an opioid than to go to physical therapy. Although the recommendation is to taper, community assets may not support this. Guidelines are one thing, but the rest of the health care system needs to catch up to them and make them practical.
Primary care doctors often utilize our physical medicine, rehabilitation, and pain management specialists to assist in managing our patients’ pain. Here too, access to this resource is often difficult to come by. Depending on a patient’s insurance, it can take months to get an appointment.
In general, the current guidelines offer 12 key recommendations when prescribing opioids. They are a great reference; however, we need more real-life tools. For many of us in primary care, these guidelines support what we’ve been doing all along.
Primary care doctors will surely play a huge role in addressing the opioid crisis. We can prescribe opioids appropriately, but it doesn’t erase the problems of those patients who were overprescribed in the past. Many still seek out these medications whether for monetary reasons or just for the high. It is often easy to blame the patient but the one in control is the one with the prescription pad. Yet, it is important to remember that many of these patients are in real pain and need help.
Often, it is simpler to just prescribe a pain medication than it is to explain why one is not appropriate. As primary care doctors, we need to be effective ambassadors of appropriate opioid prescribing and often that means doing the hard thing and saying no to a patient.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J.
fpnews@mdedge.com