User login
Veterans’ keratinocyte carcinoma, actinic keratosis care cost $356 million in 2012
Almost 4% of the 5.9 million veterans treated by the Veterans Health Administration in 2012 had a diagnosis of keratinocyte carcinoma (KC) or actinic keratosis (AK), and their treatment cost $356 million, according to an analysis of VHA-provided or -contracted outpatient encounters.
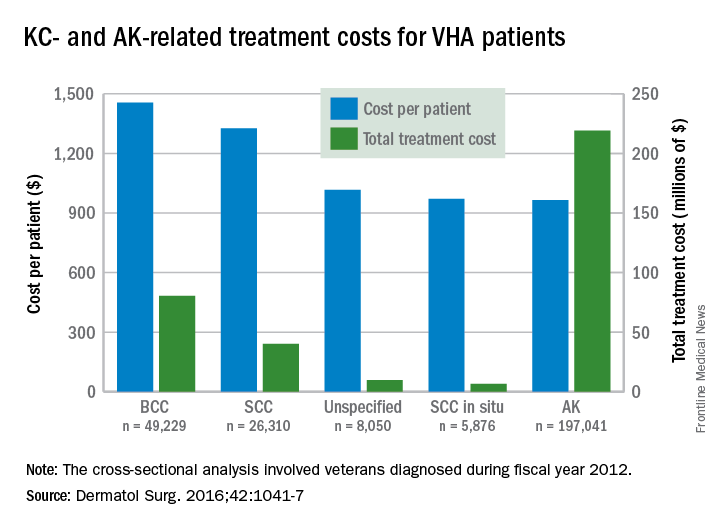
Treatment costs per patient for KCs, also known as nonmelanoma skin cancers, were $1,456 for basal cell carcinoma (BCC), $1,326 for squamous cell carcinoma (SCC), $1,016 for unspecified, nongenital invasive KCs, $971 for squamous cell carcinoma in situ, and $906 for genital skin cancer (not shown on graph), reported Jean Yoon, PhD, of Veterans Affairs Palo Alto Health Care System, Menlo Park, Calif., and her associates (Dermatol Surg. 2016;42:1041-7).
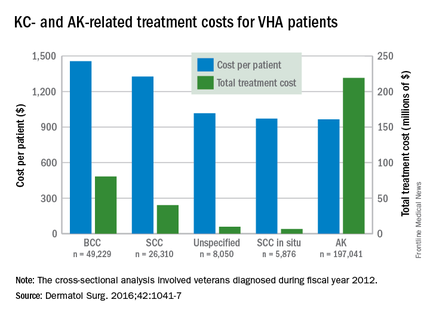
The VHA’s cost per patient for AK was relatively low – $965 per patient in 2012 – but the number of patients – 197,041 – was more than four times higher than any of the KCs. There were 49,229 veterans with BCC, 26,310 veterans with SCC, 8,050 veterans with unspecified KC, 5,876 veterans with SCC in situ, and 512 veterans with genital skin cancer, according to the analysis of administrative data on outpatient care and prescription drugs provided or paid by the VHA in fiscal year 2012. The total number of patients was 227,601, as some patients had more than one of the study diagnoses.
As a result of the high number of patients, actinic keratosis care totaled $219 million, compared with $80 million for BCC, $40 million for SCC, $9.6 million for nonspecified KC, $6.6 million for SCC in situ, and $582,000 for genital skin cancer, the investigators wrote.
The study was supported by a grant from the Department of Veterans Affairs. One of Dr. Yoon’s associates served as a consultant to several companies, but the remaining investigators had no conflicts to report.
Almost 4% of the 5.9 million veterans treated by the Veterans Health Administration in 2012 had a diagnosis of keratinocyte carcinoma (KC) or actinic keratosis (AK), and their treatment cost $356 million, according to an analysis of VHA-provided or -contracted outpatient encounters.
Treatment costs per patient for KCs, also known as nonmelanoma skin cancers, were $1,456 for basal cell carcinoma (BCC), $1,326 for squamous cell carcinoma (SCC), $1,016 for unspecified, nongenital invasive KCs, $971 for squamous cell carcinoma in situ, and $906 for genital skin cancer (not shown on graph), reported Jean Yoon, PhD, of Veterans Affairs Palo Alto Health Care System, Menlo Park, Calif., and her associates (Dermatol Surg. 2016;42:1041-7).

The VHA’s cost per patient for AK was relatively low – $965 per patient in 2012 – but the number of patients – 197,041 – was more than four times higher than any of the KCs. There were 49,229 veterans with BCC, 26,310 veterans with SCC, 8,050 veterans with unspecified KC, 5,876 veterans with SCC in situ, and 512 veterans with genital skin cancer, according to the analysis of administrative data on outpatient care and prescription drugs provided or paid by the VHA in fiscal year 2012. The total number of patients was 227,601, as some patients had more than one of the study diagnoses.
As a result of the high number of patients, actinic keratosis care totaled $219 million, compared with $80 million for BCC, $40 million for SCC, $9.6 million for nonspecified KC, $6.6 million for SCC in situ, and $582,000 for genital skin cancer, the investigators wrote.
The study was supported by a grant from the Department of Veterans Affairs. One of Dr. Yoon’s associates served as a consultant to several companies, but the remaining investigators had no conflicts to report.
Almost 4% of the 5.9 million veterans treated by the Veterans Health Administration in 2012 had a diagnosis of keratinocyte carcinoma (KC) or actinic keratosis (AK), and their treatment cost $356 million, according to an analysis of VHA-provided or -contracted outpatient encounters.
Treatment costs per patient for KCs, also known as nonmelanoma skin cancers, were $1,456 for basal cell carcinoma (BCC), $1,326 for squamous cell carcinoma (SCC), $1,016 for unspecified, nongenital invasive KCs, $971 for squamous cell carcinoma in situ, and $906 for genital skin cancer (not shown on graph), reported Jean Yoon, PhD, of Veterans Affairs Palo Alto Health Care System, Menlo Park, Calif., and her associates (Dermatol Surg. 2016;42:1041-7).

The VHA’s cost per patient for AK was relatively low – $965 per patient in 2012 – but the number of patients – 197,041 – was more than four times higher than any of the KCs. There were 49,229 veterans with BCC, 26,310 veterans with SCC, 8,050 veterans with unspecified KC, 5,876 veterans with SCC in situ, and 512 veterans with genital skin cancer, according to the analysis of administrative data on outpatient care and prescription drugs provided or paid by the VHA in fiscal year 2012. The total number of patients was 227,601, as some patients had more than one of the study diagnoses.
As a result of the high number of patients, actinic keratosis care totaled $219 million, compared with $80 million for BCC, $40 million for SCC, $9.6 million for nonspecified KC, $6.6 million for SCC in situ, and $582,000 for genital skin cancer, the investigators wrote.
The study was supported by a grant from the Department of Veterans Affairs. One of Dr. Yoon’s associates served as a consultant to several companies, but the remaining investigators had no conflicts to report.
FROM DERMATOLOGIC SURGERY
Expert shares photodynamic therapy pearls
NEWPORT BEACH, CALIF. – Offering photodynamic therapy is a smart move for any dermatology practice, but also it requires common sense. That starts with making sure you treat only appropriate candidates.
“They can’t be intimidated by the thought of light or looking red, and you have to go into depth ahead of time as far as managing reactions, as well as long-term expectations,” Neal Bhatia, MD, said at the annual meeting of the Pacific Dermatologic Association. That includes reviewing any oral medications they’re taking, as well as topical products they may be using on their face or scalp.
“I like to do this treatment on Fridays so it gives patients the weekend to stay indoors for the next 48 hours, which is really what they need to optimize the response rate,” said Dr. Bhatia, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “The other part of this is managing your staff. Make sure they have enough time to check in on the patients while they’re incubating away from light, as well as to review the post-treatment plan. And make sure they have a comfortable chair. Some patients might feel claustrophobic if you lay them down and put the light above them. Others might like to lay down for the procedure versus sitting down.”
His checklist for treatment day includes a reminder for patients to bring a wide-brimmed hat to shield the treated lesions from ambient light, as well as a book, computer, or paperwork to pass the time. He also advises putting together a take-home bag for patients that includes topical anesthetics, moisturizers, and sunscreen. In his clinical experience, prior to photodynamic therapy (PDT), there is no reason for patients to discontinue medications that are sensitizing in the UV spectrum such as antibiotics, diuretics, anti-hypertensives, since PDT works in the 410-417 nm range. “If you are concerned, you can hold the drugs the day before [the procedure], the day of, and maybe the day after, but that’s really all you need,” he said.
Dr. Bhatia discussed combinations with 5-fluorouracil (5-FU) before and after PDT, imiquimod plus aminolevulinic acid–PDT (ALA-PDT), and ingenol mebutate plus ALA-PDT. Antihistamines may play a role in helping to reduce the anticipated ALA-PDT response (J Invest Dermatol. 2006;126[10]:2296-301). Dr. Bhatia explained that the edema component is generated by mast cell release and can be uncoupled using selected H1-antihistamines, which can provide symptom relief without compromising efficacy. “Some studies talk about reducing erythema and edema based on the presence of mast cells and the surge of mast cells within first 48 to 72 hours,” he said. “Symptomatic relief is going to mean a lot to that patient who’s getting red, feeling itchy, and feeling swollen, but we don’t want to stop the reaction pattern and the efficacy by undoing what we’ve created as a response.”
It may seem counterintuitive, but direct tissue cooling after PDT appears to have a negative impact on perforin conversion, “to the point where it actually reduces the efficacy and treatment when you do direct tissue cooling,” Dr. Bhatia said. One study showed found significantly less resolution of actinic keratosis (AK) lesions treated with direct tissue cooling, compared with those who did not receive tissue cooling (J Photochem Photobiol B. 2011;103:1-7). In addition, there was a failure of conversion and optimization of the accumulated protoporphyrin IX when direct tissue cooling was used.
“That’s not to say a handheld fan or a fan in the room or aerating, making sure they have enough oxygen around the treatment area [isn’t useful],” Dr. Bhatia said. “I’m talking about the direct tissue cooling that we use with the pulsed laser device, or the Zimmer cooler. You get better conversion when you have a warming device, compared with contact cooling.”
Dr. Bhatia predicted that in the future, microneedle pre-treatment of human skin is going to gain popularity as a PDT adjunct. On early study of microneedling found that it improved 5-ALA and 5-MAL–induced protoporphyrin IX production for topical photodynamic therapy without increasing pain or erythema (Pharm Res. 2010;27[10]:2213-20). “I think that’s very important,” he said.
Dr. Bhatia disclosed having affiliations with Biofrontera and Dusa, the two main manufacturers of PDT in the United States.
NEWPORT BEACH, CALIF. – Offering photodynamic therapy is a smart move for any dermatology practice, but also it requires common sense. That starts with making sure you treat only appropriate candidates.
“They can’t be intimidated by the thought of light or looking red, and you have to go into depth ahead of time as far as managing reactions, as well as long-term expectations,” Neal Bhatia, MD, said at the annual meeting of the Pacific Dermatologic Association. That includes reviewing any oral medications they’re taking, as well as topical products they may be using on their face or scalp.
“I like to do this treatment on Fridays so it gives patients the weekend to stay indoors for the next 48 hours, which is really what they need to optimize the response rate,” said Dr. Bhatia, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “The other part of this is managing your staff. Make sure they have enough time to check in on the patients while they’re incubating away from light, as well as to review the post-treatment plan. And make sure they have a comfortable chair. Some patients might feel claustrophobic if you lay them down and put the light above them. Others might like to lay down for the procedure versus sitting down.”
His checklist for treatment day includes a reminder for patients to bring a wide-brimmed hat to shield the treated lesions from ambient light, as well as a book, computer, or paperwork to pass the time. He also advises putting together a take-home bag for patients that includes topical anesthetics, moisturizers, and sunscreen. In his clinical experience, prior to photodynamic therapy (PDT), there is no reason for patients to discontinue medications that are sensitizing in the UV spectrum such as antibiotics, diuretics, anti-hypertensives, since PDT works in the 410-417 nm range. “If you are concerned, you can hold the drugs the day before [the procedure], the day of, and maybe the day after, but that’s really all you need,” he said.
Dr. Bhatia discussed combinations with 5-fluorouracil (5-FU) before and after PDT, imiquimod plus aminolevulinic acid–PDT (ALA-PDT), and ingenol mebutate plus ALA-PDT. Antihistamines may play a role in helping to reduce the anticipated ALA-PDT response (J Invest Dermatol. 2006;126[10]:2296-301). Dr. Bhatia explained that the edema component is generated by mast cell release and can be uncoupled using selected H1-antihistamines, which can provide symptom relief without compromising efficacy. “Some studies talk about reducing erythema and edema based on the presence of mast cells and the surge of mast cells within first 48 to 72 hours,” he said. “Symptomatic relief is going to mean a lot to that patient who’s getting red, feeling itchy, and feeling swollen, but we don’t want to stop the reaction pattern and the efficacy by undoing what we’ve created as a response.”
It may seem counterintuitive, but direct tissue cooling after PDT appears to have a negative impact on perforin conversion, “to the point where it actually reduces the efficacy and treatment when you do direct tissue cooling,” Dr. Bhatia said. One study showed found significantly less resolution of actinic keratosis (AK) lesions treated with direct tissue cooling, compared with those who did not receive tissue cooling (J Photochem Photobiol B. 2011;103:1-7). In addition, there was a failure of conversion and optimization of the accumulated protoporphyrin IX when direct tissue cooling was used.
“That’s not to say a handheld fan or a fan in the room or aerating, making sure they have enough oxygen around the treatment area [isn’t useful],” Dr. Bhatia said. “I’m talking about the direct tissue cooling that we use with the pulsed laser device, or the Zimmer cooler. You get better conversion when you have a warming device, compared with contact cooling.”
Dr. Bhatia predicted that in the future, microneedle pre-treatment of human skin is going to gain popularity as a PDT adjunct. On early study of microneedling found that it improved 5-ALA and 5-MAL–induced protoporphyrin IX production for topical photodynamic therapy without increasing pain or erythema (Pharm Res. 2010;27[10]:2213-20). “I think that’s very important,” he said.
Dr. Bhatia disclosed having affiliations with Biofrontera and Dusa, the two main manufacturers of PDT in the United States.
NEWPORT BEACH, CALIF. – Offering photodynamic therapy is a smart move for any dermatology practice, but also it requires common sense. That starts with making sure you treat only appropriate candidates.
“They can’t be intimidated by the thought of light or looking red, and you have to go into depth ahead of time as far as managing reactions, as well as long-term expectations,” Neal Bhatia, MD, said at the annual meeting of the Pacific Dermatologic Association. That includes reviewing any oral medications they’re taking, as well as topical products they may be using on their face or scalp.
“I like to do this treatment on Fridays so it gives patients the weekend to stay indoors for the next 48 hours, which is really what they need to optimize the response rate,” said Dr. Bhatia, director of clinical dermatology at Therapeutics Clinical Research in San Diego. “The other part of this is managing your staff. Make sure they have enough time to check in on the patients while they’re incubating away from light, as well as to review the post-treatment plan. And make sure they have a comfortable chair. Some patients might feel claustrophobic if you lay them down and put the light above them. Others might like to lay down for the procedure versus sitting down.”
His checklist for treatment day includes a reminder for patients to bring a wide-brimmed hat to shield the treated lesions from ambient light, as well as a book, computer, or paperwork to pass the time. He also advises putting together a take-home bag for patients that includes topical anesthetics, moisturizers, and sunscreen. In his clinical experience, prior to photodynamic therapy (PDT), there is no reason for patients to discontinue medications that are sensitizing in the UV spectrum such as antibiotics, diuretics, anti-hypertensives, since PDT works in the 410-417 nm range. “If you are concerned, you can hold the drugs the day before [the procedure], the day of, and maybe the day after, but that’s really all you need,” he said.
Dr. Bhatia discussed combinations with 5-fluorouracil (5-FU) before and after PDT, imiquimod plus aminolevulinic acid–PDT (ALA-PDT), and ingenol mebutate plus ALA-PDT. Antihistamines may play a role in helping to reduce the anticipated ALA-PDT response (J Invest Dermatol. 2006;126[10]:2296-301). Dr. Bhatia explained that the edema component is generated by mast cell release and can be uncoupled using selected H1-antihistamines, which can provide symptom relief without compromising efficacy. “Some studies talk about reducing erythema and edema based on the presence of mast cells and the surge of mast cells within first 48 to 72 hours,” he said. “Symptomatic relief is going to mean a lot to that patient who’s getting red, feeling itchy, and feeling swollen, but we don’t want to stop the reaction pattern and the efficacy by undoing what we’ve created as a response.”
It may seem counterintuitive, but direct tissue cooling after PDT appears to have a negative impact on perforin conversion, “to the point where it actually reduces the efficacy and treatment when you do direct tissue cooling,” Dr. Bhatia said. One study showed found significantly less resolution of actinic keratosis (AK) lesions treated with direct tissue cooling, compared with those who did not receive tissue cooling (J Photochem Photobiol B. 2011;103:1-7). In addition, there was a failure of conversion and optimization of the accumulated protoporphyrin IX when direct tissue cooling was used.
“That’s not to say a handheld fan or a fan in the room or aerating, making sure they have enough oxygen around the treatment area [isn’t useful],” Dr. Bhatia said. “I’m talking about the direct tissue cooling that we use with the pulsed laser device, or the Zimmer cooler. You get better conversion when you have a warming device, compared with contact cooling.”
Dr. Bhatia predicted that in the future, microneedle pre-treatment of human skin is going to gain popularity as a PDT adjunct. On early study of microneedling found that it improved 5-ALA and 5-MAL–induced protoporphyrin IX production for topical photodynamic therapy without increasing pain or erythema (Pharm Res. 2010;27[10]:2213-20). “I think that’s very important,” he said.
Dr. Bhatia disclosed having affiliations with Biofrontera and Dusa, the two main manufacturers of PDT in the United States.
EXPERT ANALYSIS AT PDA 2016
Adding calcipotriene to 5-FU dramatically reduced AKs
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
SCOTTSDALE, ARIZ. – A four-day topical combination regimen of 5-fluorouracil (5-FU) and calcipotriene removed almost 90% of facial actinic keratoses – significantly more than with 5-FU monotherapy, in a randomized, double-blind controlled study.
Calcipotriene (Dovonex) is a synthetic vitamin D3 derivative approved by the Food and Drug Association for treatment of scalp psoriasis. But calcipotriene is also an immunomodulator that induces thymic stromal lymphopoietin (TSLP), which suppresses the growth of early stage skin cancers, said Dr. Shawn Demehri, of Harvard Medical School, Boston.
To determine whether short-term TSLP induction could reduce AKs, he and his coinvestigators randomly assigned 131 men and women who were at least 50 years old and who had at least four AKs on the face, scalp, and/or upper arms to apply 5% 5-FU cream mixed with either 0.005% calcipotriene or Vaseline to affected areas twice daily for four days. The researchers counted and photographed the AKs at baseline and at subsequent follow-up visits.
The average age of the patients was 70 years, and 82% were men, said Dr. Demehri, who reported the results at the annual meeting of the Society for Investigative Dermatology. The combination of 5-FU and calcipotriene was associated with an 86% reduction in the number of facial AKs, compared with a 26% reduction among patients who used 5-FU monotherapy (P less than .0001).
The investigators observed equally dramatic differences in efficacy at other body sites. On the scalp, combination therapy reduced the number of AKs by 76%, while 5-FU alone reduced the number by only 6%. On the right upper arm, the dual regimen removed 70% of AKs compared with 10% for monotherapy, and on the left upper arm, combination treatment removed 80% of AKs, while 5-FU alone removed only 16% (all P values for these differences were less than .0001).
Notably, patients did not experience pain or crusting after using the combination cream, said Dr. Demehri, who is also a principal investigator in the department of dermatology and MGH Cancer Center, Massachusetts General Hospital, Boston. The combination of 5-FU and 0.005% calcipotriene “acts as a potent topical immunotherapeutic agent against actinic keratosis,” he concluded.
Dr. Demehri had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: A combination of 5% 5-fluorouracil cream and 0.005% calcipotriene was significantly more effective at removing actinic keratoses at different anatomic sites as 5-FU monotherapy.
Major finding: At week 8, the combination group had an average 86% reduction in the number of facial AKs, compared with 26% with 5-FU monotherapy.
Data source: A randomized, double-blind, controlled study of 131 patients with at least four AKs on the face, scalp, and/or upper arms.
Disclosures: Dr. Demehri had no disclosures.
Actinic Keratosis as a Marker of Field Cancerization in Excision Specimens of Cutaneous Malignancies
The concept of field cancerization was first proposed in 1953 by Slaughter et al1 in their study of oral squamous carcinomas. Their findings of multifocal patches of premalignant disease, abnormal tissue surrounding tumors, multiple localized primary tumors, and tumor recurrence following surgical resection was suggestive of a field of dysplastic cells with malignant potential.1 Since then, modern molecular techniques have been used to establish a genetic basis for this model in many different types of cancer, including cutaneous malignancies.2-4 The field begins from a singular stem cell, which undergoes one or more genetic changes that allow for a growth advantage compared to surrounding cells. The stem cell then divides, forming a patch of clonal daughter cells that displace the surrounding normal epithelium. Growth of this patch eventually leads to a dysplastic field of monoclonal cells, which notably does not yet show invasive growth or metastatic behavior. Over time, continued carcinogenic exposure results in additional genetic alterations among different cells in the field, which leads to new subclonal proliferations that share common clonal origin but exhibit unique genetic changes. Eventually, transformative events may occur, resulting in cells with invasive and metastatic properties, thus forming a carcinoma.5
In the case of cutaneous malignancies, UV radiation in the form of UVA and UVB rays is the most common source of carcinogenesis. It is well established that UV radiation has numerous effects on the body, including but not limited to local and systemic immunosuppression, alteration of signal transduction pathways, and the development of mutations in DNA via direct damage by UVB or indirect damage by free radical formation with UVA.6,7 Normally, DNA is protected from UV radiation–induced genetic alteration by the p53 gene, TP53. As such, damage to this gene is highly associated with cancer induction. One study found that more than 90% of squamous cell carcinomas (SCCs) and more than 50% of basal cell carcinomas (BCCs) contain UV-like mutations in TP53.8 The concept of field cancerization suggests that because the skin surrounding cutaneous malignancies has been exposed to the same chronic UV light as the initial lesion, it is at an increased risk for genetic abnormalities and thus possible malignant transformation.
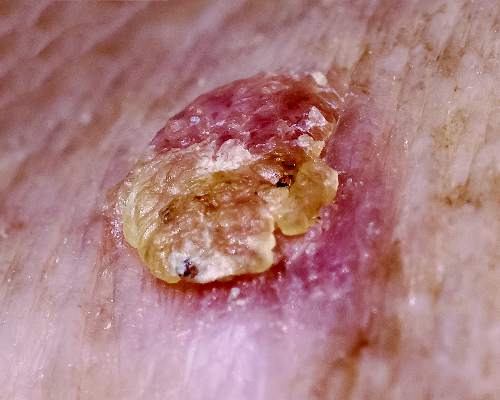
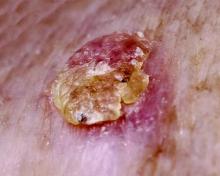
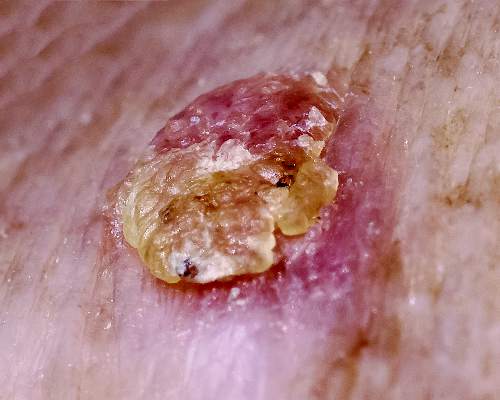
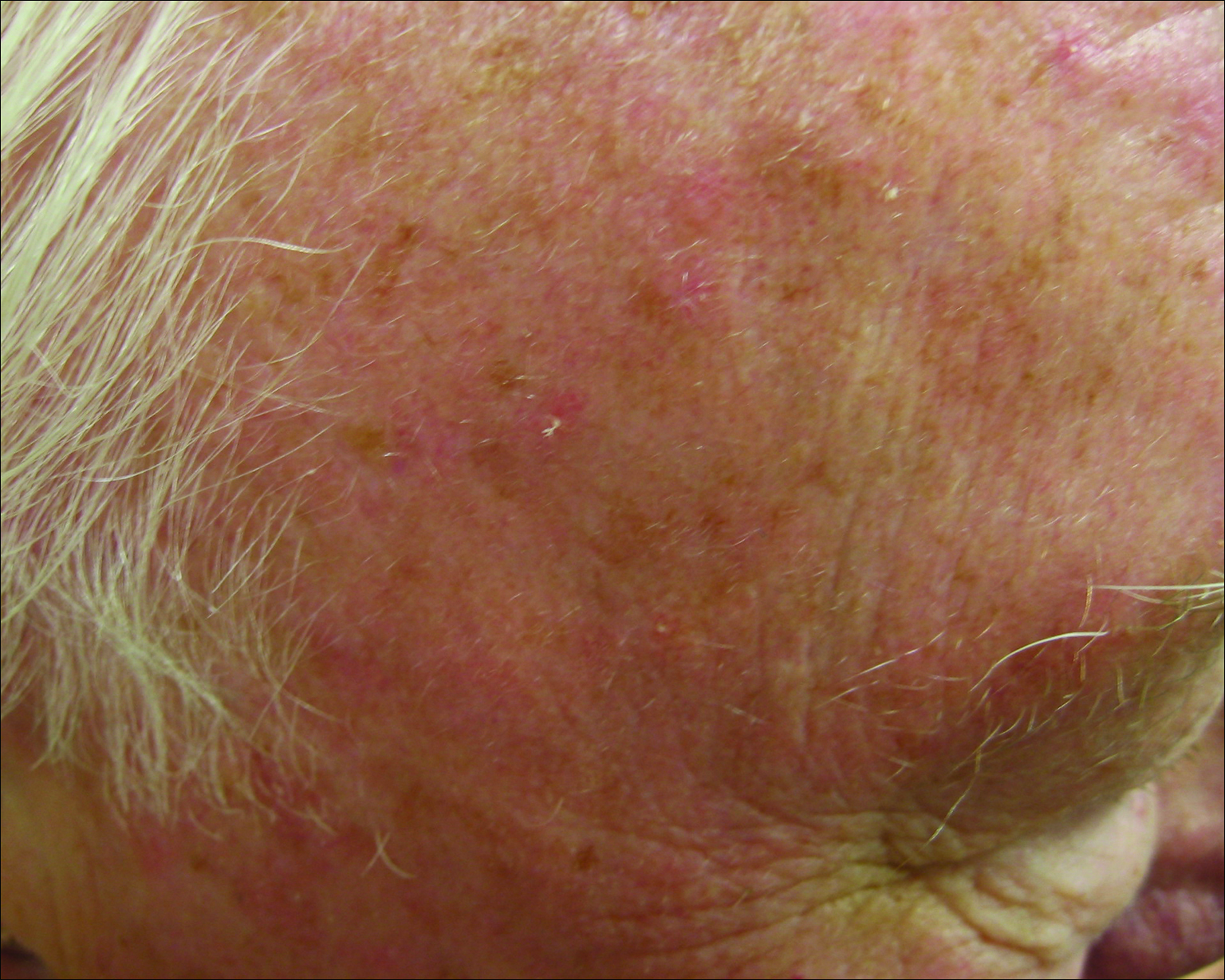
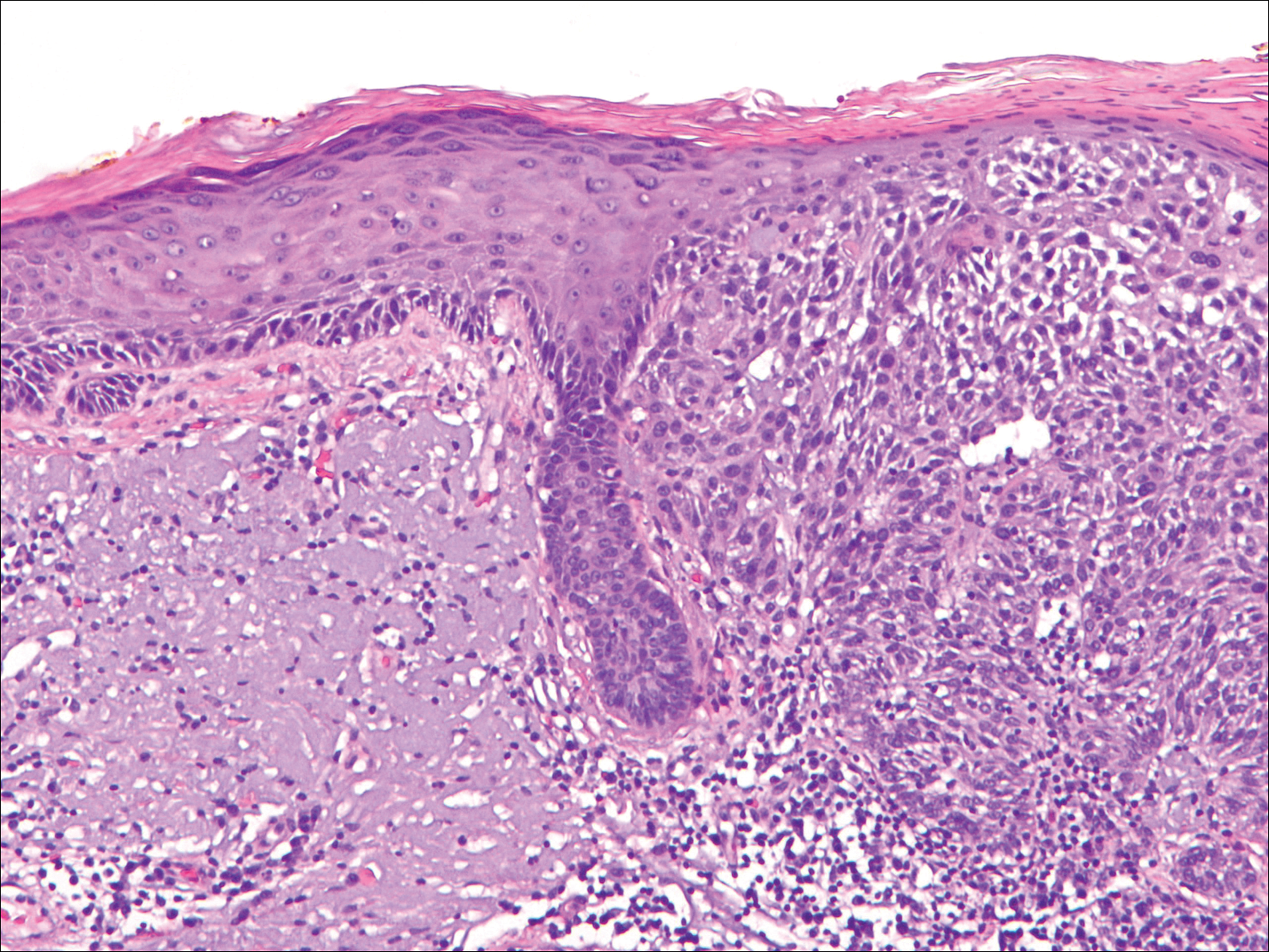
Actinic keratoses (AKs) are common neoplasms of the skin that generally are regarded as precancerous lesions or may be considered to be the earliest stage of SCC in situ.9 Actinic keratoses usually develop as a consequence of chronic exposure to UV radiation and often are clinically apparent as erythematous scaly papules in sun-exposed areas (Figure 1).10 They also are identified histologically as atypical keratinocytes along the basal layer of the epidermis with possible enlargement, hyperchromatic nuclei, lack of maturation, mitotic figures, inflammatory infiltrate, and/or hyperkeratosis.10 Furthermore, the genetic changes associated with AKs are well documented and are strongly associated with changes to p53.11 Given these characteristics, AKs serve as good markers of genetic damage with potential for malignancy. In this study, we used histologically identified AKs to assess the presence of field damage in the tissue immediately surrounding excision specimens of SCCs, BCCs, and malignant melanomas (MMs).
Methods
This study was approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai (New York, New York) prior to initiation. All cutaneous specimens submitted to the dermatopathology service for consultation between April 2013 and June 2013 were reviewed for inclusion in this study. Data collection was extended for MMs to include all specimens from January 2013 to June 2013 given the limited number of cases in the original data collection period.
Initial screening for this study was done electronically and assessed for a diagnosis of SCC (Figure 2), BCC (Figure 3), or MM (Figure 4) as determined by a board-certified dermatopathologist (G.G.). The resulting pool of specimens was then screened to include only excision specimens and to exclude curettage specimens and superficial specimens that lacked dermis. In this study, we chose to look at reexcisions rather than initial biopsies so that there was a greater likelihood of having an intact epidermis surrounding a malignancy that could be assessed for the presence of AKs as markers for field cancerization. Specimens were examined in full via serial transverse cross-sections at 3-mm intervals. Additional step sections were obtained at smaller intervals when margins were close or unclear.
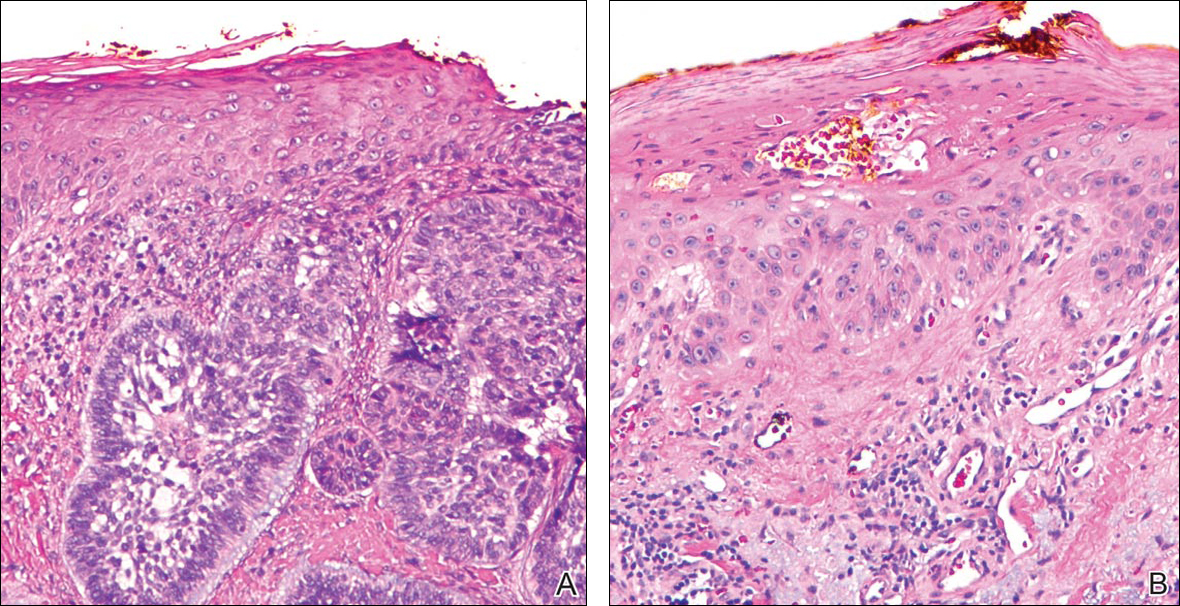
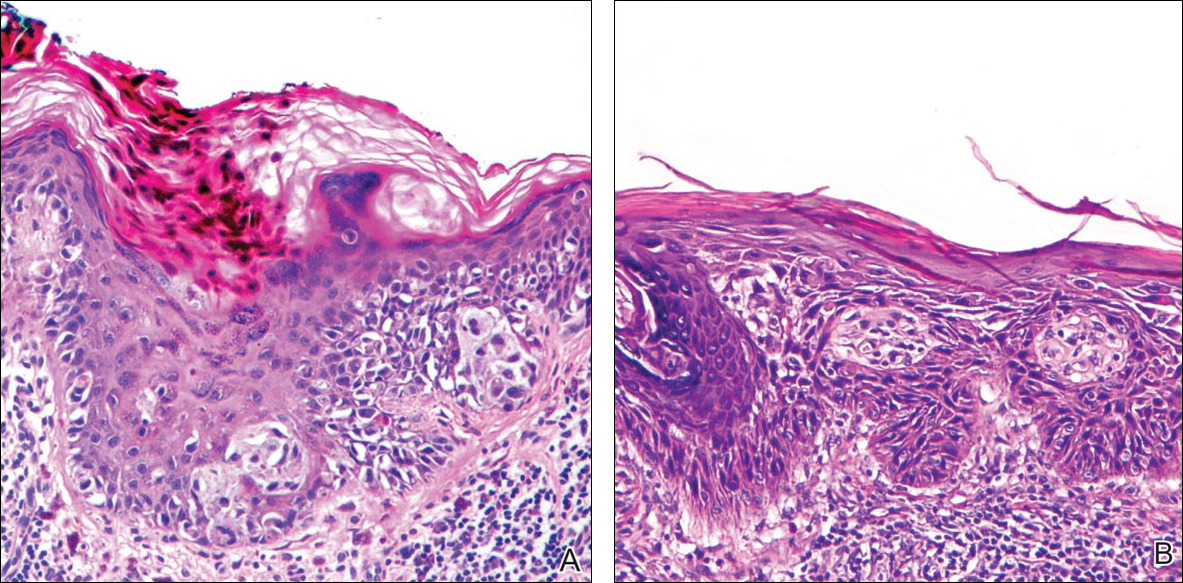
Selected cases were reassessed by a board-certified dermatopathologist (G.G.) to confirm the diagnosis and to assess for the presence of at least 1 AK within the specimen sample that was separated from the original malignancy by histologically normal-appearing cells. Samples were also assessed for the presence of an AK within 0.1 mm of the distal lateral margins of the tissue sample. Information regarding patient age, gender, lesion location, lesion type, and specimen size was collected for each sample. In accordance with institutional review board protocol, research data were collected without any protected health information. All analyses and results were deidentified and stored on a password-protected computer database. Statistical analysis was performed using SPSS software. When applicable, P<.05 was considered to indicate statistical significance.
Results
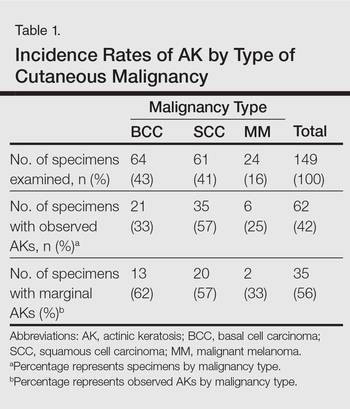
There were 205 cases that passed the initial screening filters, of which 56 were excluded due to the presence of curettage or lack of a sufficient tissue sample. Of the remaining 149 cases, the distribution by malignancy type was tabulated along with the percentage of observed AKs. If an AK was observed, the percentage that had an AK at the lateral margins (marginal AK) was determined (Table 1). A χ2 analysis determined that AKs were observed significantly more often in SCC specimens (57% [35/61]) than BCC (33% [21/64]) or malignant melanoma (25% [6/24]) specimens (P=.0125).
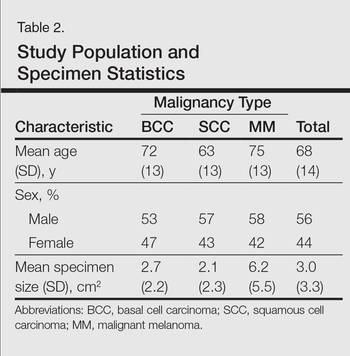
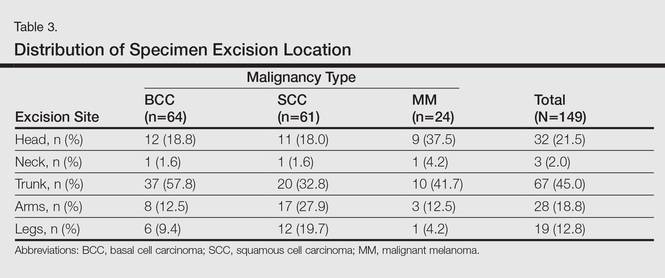
Statistics regarding patient age and gender as well as specimen size were stratified by malignancy type (Table 2). Using a receiver operating characteristic curve and the Youden index, an optimal cutoff of older than 67 years was determined to increase probability of observing an AK (P=.077) with sensitivity of 0.531 and specificity of 0.529. The distribution of specimen excision location for each malignancy type is shown in Table 3.
A multivariate analysis was performed to determine if the variables of patient age, gender, biopsy size, malignancy type (SCC, BCC, or MM), or cancer location (head, neck, trunk, arms, or legs) were independently useful in predicting whether an AK would be observed in the excision specimen. The significance of variables in the logistic regression model was assessed using a backward stepwise regression selection procedure entering variables if P<.15 and excluding variables if P>.25. Significant variables in predicting the occurrence of AK were SCC malignancy type (P=.007; odds ratio [OR], 2.61) and location on the head (P=.044; OR, 2.39) and arms (P=.042; OR, 2.55).
Comment
The χ2 analysis of our data showed that SCC specimens were significantly more likely to have an associated AK than either BCCs or MMs (P=.0125), which is not surprising given that AKs are considered by many to be early-stage SCCs.12 It is important to note, however, that BCCs and MMs both had nonnegligible rates of associated AKs. Although BCC and MM do not arise from the same background of genetic changes as SCC, this finding is noteworthy because it demonstrates definitive field damage with malignant potential in the area surrounding these cutaneous malignancies.
Our data also showed that there was a significantly greater association of AKs in malignancies located on the head (P=.044) and arms (P=.042), possibly because these 2 areas tend to be the most sun exposed and thus are more likely to have sustained field damage as evidenced by the higher percentage of AKs. A study by Jonason et al13 described a similar finding in which sun-exposed skin exhibited significantly more frequent (P=.04) and larger (P=.02) clonal patches of mutated p53 keratinocytes than sun-protected skin.
It is likely that the field damage surrounding the cutaneous lesions in our study is actually greater than what we reported because the AK was present at the margin of the excision specimens the majority of the time (56%), which suggests that there likely may have been more AKs found if a wider area surrounding the malignancy had been studied given that AKs often are at the periphery of the lesion and may be missed by a small excision. Fewer marginal AKs were observed with MM cases, possibly because the excision specimens were more than double the size of SCC or BCC excisions. Furthermore, there likely is to be more damage than what can be appreciated by visual changes alone.
Kanjilal et al14 used polymerase chain reaction and DNA sequencing to demonstrate numerous p53 mutations in nonmalignant-appearing skin surrounding BCCs and SCCs. Brennan et al15 found p53 mutations in surgical margins of excised SCCs considered to be tumor free by histopathologic analysis in more than half of the specimens studied. Notably, tumor recurrence was significantly more likely in areas where mutations were found and no tumor recurrence was seen in areas free of p53 mutations (P=.02).15 Tabor et al4 similarly found genetically altered fields in histologically clear surgical margins of SCCs but also showed that local tumor recurrence following excision had more molecular markers in common with the nonresected premalignant field than it did with the primary tumor. Thus, these studies provide a genetic basis for field damage that can exist even in histologically benign-appearing cells.
We believe our findings are clinically relevant, as they provide additional evidence for the theory of field cancerization as demonstrated by the nonnegligible rates of AKs and thus field damage with malignant potential in the skin immediately surrounding cutaneous malignancies. The limitations of our study, however, include a small sample size; no consideration of the effects of prior topical, field, or systemic treatments; and lack of a control group. Nevertheless, our findings emphasize the importance of assessing the extent of field damage when determining treatment strategies. Clinicians treating cutaneous malignancies should consider the need for field therapy, especially in sun-exposed regions, to avoid additional primary tumors.16 Further research is needed, however, to identify optimal methods for quantifying field damage clinically and determining the most effective treatment strategies.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Braakhuis B, Tabor M, Kummer J, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Stern R, Bolshakov S, Nataraj A, et al. p53 Mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol. 2002;119:522-526.
- Tabor M, Brakenhoff R, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523-1532.
- Torezan L. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Ullrich S, Kripke M, Ananthaswamy H. Mechanisms underlying UV-induced immune suppression: implications for sunscreen design. Exp Dermatol. 2002;11:1-4.
- de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359-366.
- Brash DE, Ziegler A, Jonason AS, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136-142.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895-904.
- Ziegler A, Jonason AS, Leffel DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773-776.
- Cockerell C. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J Am Acad Dermatol. 2000;42:11-17.
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci. 1996;93:14025-14029.
- Kanjilal S, Strom SS, Clayman GL, et al. p53 Mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604-3609.
- Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066.
The concept of field cancerization was first proposed in 1953 by Slaughter et al1 in their study of oral squamous carcinomas. Their findings of multifocal patches of premalignant disease, abnormal tissue surrounding tumors, multiple localized primary tumors, and tumor recurrence following surgical resection was suggestive of a field of dysplastic cells with malignant potential.1 Since then, modern molecular techniques have been used to establish a genetic basis for this model in many different types of cancer, including cutaneous malignancies.2-4 The field begins from a singular stem cell, which undergoes one or more genetic changes that allow for a growth advantage compared to surrounding cells. The stem cell then divides, forming a patch of clonal daughter cells that displace the surrounding normal epithelium. Growth of this patch eventually leads to a dysplastic field of monoclonal cells, which notably does not yet show invasive growth or metastatic behavior. Over time, continued carcinogenic exposure results in additional genetic alterations among different cells in the field, which leads to new subclonal proliferations that share common clonal origin but exhibit unique genetic changes. Eventually, transformative events may occur, resulting in cells with invasive and metastatic properties, thus forming a carcinoma.5
In the case of cutaneous malignancies, UV radiation in the form of UVA and UVB rays is the most common source of carcinogenesis. It is well established that UV radiation has numerous effects on the body, including but not limited to local and systemic immunosuppression, alteration of signal transduction pathways, and the development of mutations in DNA via direct damage by UVB or indirect damage by free radical formation with UVA.6,7 Normally, DNA is protected from UV radiation–induced genetic alteration by the p53 gene, TP53. As such, damage to this gene is highly associated with cancer induction. One study found that more than 90% of squamous cell carcinomas (SCCs) and more than 50% of basal cell carcinomas (BCCs) contain UV-like mutations in TP53.8 The concept of field cancerization suggests that because the skin surrounding cutaneous malignancies has been exposed to the same chronic UV light as the initial lesion, it is at an increased risk for genetic abnormalities and thus possible malignant transformation.
Actinic keratoses (AKs) are common neoplasms of the skin that generally are regarded as precancerous lesions or may be considered to be the earliest stage of SCC in situ.9 Actinic keratoses usually develop as a consequence of chronic exposure to UV radiation and often are clinically apparent as erythematous scaly papules in sun-exposed areas (Figure 1).10 They also are identified histologically as atypical keratinocytes along the basal layer of the epidermis with possible enlargement, hyperchromatic nuclei, lack of maturation, mitotic figures, inflammatory infiltrate, and/or hyperkeratosis.10 Furthermore, the genetic changes associated with AKs are well documented and are strongly associated with changes to p53.11 Given these characteristics, AKs serve as good markers of genetic damage with potential for malignancy. In this study, we used histologically identified AKs to assess the presence of field damage in the tissue immediately surrounding excision specimens of SCCs, BCCs, and malignant melanomas (MMs).

Methods
This study was approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai (New York, New York) prior to initiation. All cutaneous specimens submitted to the dermatopathology service for consultation between April 2013 and June 2013 were reviewed for inclusion in this study. Data collection was extended for MMs to include all specimens from January 2013 to June 2013 given the limited number of cases in the original data collection period.
Initial screening for this study was done electronically and assessed for a diagnosis of SCC (Figure 2), BCC (Figure 3), or MM (Figure 4) as determined by a board-certified dermatopathologist (G.G.). The resulting pool of specimens was then screened to include only excision specimens and to exclude curettage specimens and superficial specimens that lacked dermis. In this study, we chose to look at reexcisions rather than initial biopsies so that there was a greater likelihood of having an intact epidermis surrounding a malignancy that could be assessed for the presence of AKs as markers for field cancerization. Specimens were examined in full via serial transverse cross-sections at 3-mm intervals. Additional step sections were obtained at smaller intervals when margins were close or unclear.



Selected cases were reassessed by a board-certified dermatopathologist (G.G.) to confirm the diagnosis and to assess for the presence of at least 1 AK within the specimen sample that was separated from the original malignancy by histologically normal-appearing cells. Samples were also assessed for the presence of an AK within 0.1 mm of the distal lateral margins of the tissue sample. Information regarding patient age, gender, lesion location, lesion type, and specimen size was collected for each sample. In accordance with institutional review board protocol, research data were collected without any protected health information. All analyses and results were deidentified and stored on a password-protected computer database. Statistical analysis was performed using SPSS software. When applicable, P<.05 was considered to indicate statistical significance.
Results
There were 205 cases that passed the initial screening filters, of which 56 were excluded due to the presence of curettage or lack of a sufficient tissue sample. Of the remaining 149 cases, the distribution by malignancy type was tabulated along with the percentage of observed AKs. If an AK was observed, the percentage that had an AK at the lateral margins (marginal AK) was determined (Table 1). A χ2 analysis determined that AKs were observed significantly more often in SCC specimens (57% [35/61]) than BCC (33% [21/64]) or malignant melanoma (25% [6/24]) specimens (P=.0125).

Statistics regarding patient age and gender as well as specimen size were stratified by malignancy type (Table 2). Using a receiver operating characteristic curve and the Youden index, an optimal cutoff of older than 67 years was determined to increase probability of observing an AK (P=.077) with sensitivity of 0.531 and specificity of 0.529. The distribution of specimen excision location for each malignancy type is shown in Table 3.


A multivariate analysis was performed to determine if the variables of patient age, gender, biopsy size, malignancy type (SCC, BCC, or MM), or cancer location (head, neck, trunk, arms, or legs) were independently useful in predicting whether an AK would be observed in the excision specimen. The significance of variables in the logistic regression model was assessed using a backward stepwise regression selection procedure entering variables if P<.15 and excluding variables if P>.25. Significant variables in predicting the occurrence of AK were SCC malignancy type (P=.007; odds ratio [OR], 2.61) and location on the head (P=.044; OR, 2.39) and arms (P=.042; OR, 2.55).
Comment
The χ2 analysis of our data showed that SCC specimens were significantly more likely to have an associated AK than either BCCs or MMs (P=.0125), which is not surprising given that AKs are considered by many to be early-stage SCCs.12 It is important to note, however, that BCCs and MMs both had nonnegligible rates of associated AKs. Although BCC and MM do not arise from the same background of genetic changes as SCC, this finding is noteworthy because it demonstrates definitive field damage with malignant potential in the area surrounding these cutaneous malignancies.
Our data also showed that there was a significantly greater association of AKs in malignancies located on the head (P=.044) and arms (P=.042), possibly because these 2 areas tend to be the most sun exposed and thus are more likely to have sustained field damage as evidenced by the higher percentage of AKs. A study by Jonason et al13 described a similar finding in which sun-exposed skin exhibited significantly more frequent (P=.04) and larger (P=.02) clonal patches of mutated p53 keratinocytes than sun-protected skin.
It is likely that the field damage surrounding the cutaneous lesions in our study is actually greater than what we reported because the AK was present at the margin of the excision specimens the majority of the time (56%), which suggests that there likely may have been more AKs found if a wider area surrounding the malignancy had been studied given that AKs often are at the periphery of the lesion and may be missed by a small excision. Fewer marginal AKs were observed with MM cases, possibly because the excision specimens were more than double the size of SCC or BCC excisions. Furthermore, there likely is to be more damage than what can be appreciated by visual changes alone.
Kanjilal et al14 used polymerase chain reaction and DNA sequencing to demonstrate numerous p53 mutations in nonmalignant-appearing skin surrounding BCCs and SCCs. Brennan et al15 found p53 mutations in surgical margins of excised SCCs considered to be tumor free by histopathologic analysis in more than half of the specimens studied. Notably, tumor recurrence was significantly more likely in areas where mutations were found and no tumor recurrence was seen in areas free of p53 mutations (P=.02).15 Tabor et al4 similarly found genetically altered fields in histologically clear surgical margins of SCCs but also showed that local tumor recurrence following excision had more molecular markers in common with the nonresected premalignant field than it did with the primary tumor. Thus, these studies provide a genetic basis for field damage that can exist even in histologically benign-appearing cells.
We believe our findings are clinically relevant, as they provide additional evidence for the theory of field cancerization as demonstrated by the nonnegligible rates of AKs and thus field damage with malignant potential in the skin immediately surrounding cutaneous malignancies. The limitations of our study, however, include a small sample size; no consideration of the effects of prior topical, field, or systemic treatments; and lack of a control group. Nevertheless, our findings emphasize the importance of assessing the extent of field damage when determining treatment strategies. Clinicians treating cutaneous malignancies should consider the need for field therapy, especially in sun-exposed regions, to avoid additional primary tumors.16 Further research is needed, however, to identify optimal methods for quantifying field damage clinically and determining the most effective treatment strategies.
The concept of field cancerization was first proposed in 1953 by Slaughter et al1 in their study of oral squamous carcinomas. Their findings of multifocal patches of premalignant disease, abnormal tissue surrounding tumors, multiple localized primary tumors, and tumor recurrence following surgical resection was suggestive of a field of dysplastic cells with malignant potential.1 Since then, modern molecular techniques have been used to establish a genetic basis for this model in many different types of cancer, including cutaneous malignancies.2-4 The field begins from a singular stem cell, which undergoes one or more genetic changes that allow for a growth advantage compared to surrounding cells. The stem cell then divides, forming a patch of clonal daughter cells that displace the surrounding normal epithelium. Growth of this patch eventually leads to a dysplastic field of monoclonal cells, which notably does not yet show invasive growth or metastatic behavior. Over time, continued carcinogenic exposure results in additional genetic alterations among different cells in the field, which leads to new subclonal proliferations that share common clonal origin but exhibit unique genetic changes. Eventually, transformative events may occur, resulting in cells with invasive and metastatic properties, thus forming a carcinoma.5
In the case of cutaneous malignancies, UV radiation in the form of UVA and UVB rays is the most common source of carcinogenesis. It is well established that UV radiation has numerous effects on the body, including but not limited to local and systemic immunosuppression, alteration of signal transduction pathways, and the development of mutations in DNA via direct damage by UVB or indirect damage by free radical formation with UVA.6,7 Normally, DNA is protected from UV radiation–induced genetic alteration by the p53 gene, TP53. As such, damage to this gene is highly associated with cancer induction. One study found that more than 90% of squamous cell carcinomas (SCCs) and more than 50% of basal cell carcinomas (BCCs) contain UV-like mutations in TP53.8 The concept of field cancerization suggests that because the skin surrounding cutaneous malignancies has been exposed to the same chronic UV light as the initial lesion, it is at an increased risk for genetic abnormalities and thus possible malignant transformation.
Actinic keratoses (AKs) are common neoplasms of the skin that generally are regarded as precancerous lesions or may be considered to be the earliest stage of SCC in situ.9 Actinic keratoses usually develop as a consequence of chronic exposure to UV radiation and often are clinically apparent as erythematous scaly papules in sun-exposed areas (Figure 1).10 They also are identified histologically as atypical keratinocytes along the basal layer of the epidermis with possible enlargement, hyperchromatic nuclei, lack of maturation, mitotic figures, inflammatory infiltrate, and/or hyperkeratosis.10 Furthermore, the genetic changes associated with AKs are well documented and are strongly associated with changes to p53.11 Given these characteristics, AKs serve as good markers of genetic damage with potential for malignancy. In this study, we used histologically identified AKs to assess the presence of field damage in the tissue immediately surrounding excision specimens of SCCs, BCCs, and malignant melanomas (MMs).

Methods
This study was approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai (New York, New York) prior to initiation. All cutaneous specimens submitted to the dermatopathology service for consultation between April 2013 and June 2013 were reviewed for inclusion in this study. Data collection was extended for MMs to include all specimens from January 2013 to June 2013 given the limited number of cases in the original data collection period.
Initial screening for this study was done electronically and assessed for a diagnosis of SCC (Figure 2), BCC (Figure 3), or MM (Figure 4) as determined by a board-certified dermatopathologist (G.G.). The resulting pool of specimens was then screened to include only excision specimens and to exclude curettage specimens and superficial specimens that lacked dermis. In this study, we chose to look at reexcisions rather than initial biopsies so that there was a greater likelihood of having an intact epidermis surrounding a malignancy that could be assessed for the presence of AKs as markers for field cancerization. Specimens were examined in full via serial transverse cross-sections at 3-mm intervals. Additional step sections were obtained at smaller intervals when margins were close or unclear.



Selected cases were reassessed by a board-certified dermatopathologist (G.G.) to confirm the diagnosis and to assess for the presence of at least 1 AK within the specimen sample that was separated from the original malignancy by histologically normal-appearing cells. Samples were also assessed for the presence of an AK within 0.1 mm of the distal lateral margins of the tissue sample. Information regarding patient age, gender, lesion location, lesion type, and specimen size was collected for each sample. In accordance with institutional review board protocol, research data were collected without any protected health information. All analyses and results were deidentified and stored on a password-protected computer database. Statistical analysis was performed using SPSS software. When applicable, P<.05 was considered to indicate statistical significance.
Results
There were 205 cases that passed the initial screening filters, of which 56 were excluded due to the presence of curettage or lack of a sufficient tissue sample. Of the remaining 149 cases, the distribution by malignancy type was tabulated along with the percentage of observed AKs. If an AK was observed, the percentage that had an AK at the lateral margins (marginal AK) was determined (Table 1). A χ2 analysis determined that AKs were observed significantly more often in SCC specimens (57% [35/61]) than BCC (33% [21/64]) or malignant melanoma (25% [6/24]) specimens (P=.0125).

Statistics regarding patient age and gender as well as specimen size were stratified by malignancy type (Table 2). Using a receiver operating characteristic curve and the Youden index, an optimal cutoff of older than 67 years was determined to increase probability of observing an AK (P=.077) with sensitivity of 0.531 and specificity of 0.529. The distribution of specimen excision location for each malignancy type is shown in Table 3.


A multivariate analysis was performed to determine if the variables of patient age, gender, biopsy size, malignancy type (SCC, BCC, or MM), or cancer location (head, neck, trunk, arms, or legs) were independently useful in predicting whether an AK would be observed in the excision specimen. The significance of variables in the logistic regression model was assessed using a backward stepwise regression selection procedure entering variables if P<.15 and excluding variables if P>.25. Significant variables in predicting the occurrence of AK were SCC malignancy type (P=.007; odds ratio [OR], 2.61) and location on the head (P=.044; OR, 2.39) and arms (P=.042; OR, 2.55).
Comment
The χ2 analysis of our data showed that SCC specimens were significantly more likely to have an associated AK than either BCCs or MMs (P=.0125), which is not surprising given that AKs are considered by many to be early-stage SCCs.12 It is important to note, however, that BCCs and MMs both had nonnegligible rates of associated AKs. Although BCC and MM do not arise from the same background of genetic changes as SCC, this finding is noteworthy because it demonstrates definitive field damage with malignant potential in the area surrounding these cutaneous malignancies.
Our data also showed that there was a significantly greater association of AKs in malignancies located on the head (P=.044) and arms (P=.042), possibly because these 2 areas tend to be the most sun exposed and thus are more likely to have sustained field damage as evidenced by the higher percentage of AKs. A study by Jonason et al13 described a similar finding in which sun-exposed skin exhibited significantly more frequent (P=.04) and larger (P=.02) clonal patches of mutated p53 keratinocytes than sun-protected skin.
It is likely that the field damage surrounding the cutaneous lesions in our study is actually greater than what we reported because the AK was present at the margin of the excision specimens the majority of the time (56%), which suggests that there likely may have been more AKs found if a wider area surrounding the malignancy had been studied given that AKs often are at the periphery of the lesion and may be missed by a small excision. Fewer marginal AKs were observed with MM cases, possibly because the excision specimens were more than double the size of SCC or BCC excisions. Furthermore, there likely is to be more damage than what can be appreciated by visual changes alone.
Kanjilal et al14 used polymerase chain reaction and DNA sequencing to demonstrate numerous p53 mutations in nonmalignant-appearing skin surrounding BCCs and SCCs. Brennan et al15 found p53 mutations in surgical margins of excised SCCs considered to be tumor free by histopathologic analysis in more than half of the specimens studied. Notably, tumor recurrence was significantly more likely in areas where mutations were found and no tumor recurrence was seen in areas free of p53 mutations (P=.02).15 Tabor et al4 similarly found genetically altered fields in histologically clear surgical margins of SCCs but also showed that local tumor recurrence following excision had more molecular markers in common with the nonresected premalignant field than it did with the primary tumor. Thus, these studies provide a genetic basis for field damage that can exist even in histologically benign-appearing cells.
We believe our findings are clinically relevant, as they provide additional evidence for the theory of field cancerization as demonstrated by the nonnegligible rates of AKs and thus field damage with malignant potential in the skin immediately surrounding cutaneous malignancies. The limitations of our study, however, include a small sample size; no consideration of the effects of prior topical, field, or systemic treatments; and lack of a control group. Nevertheless, our findings emphasize the importance of assessing the extent of field damage when determining treatment strategies. Clinicians treating cutaneous malignancies should consider the need for field therapy, especially in sun-exposed regions, to avoid additional primary tumors.16 Further research is needed, however, to identify optimal methods for quantifying field damage clinically and determining the most effective treatment strategies.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Braakhuis B, Tabor M, Kummer J, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Stern R, Bolshakov S, Nataraj A, et al. p53 Mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol. 2002;119:522-526.
- Tabor M, Brakenhoff R, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523-1532.
- Torezan L. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Ullrich S, Kripke M, Ananthaswamy H. Mechanisms underlying UV-induced immune suppression: implications for sunscreen design. Exp Dermatol. 2002;11:1-4.
- de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359-366.
- Brash DE, Ziegler A, Jonason AS, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136-142.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895-904.
- Ziegler A, Jonason AS, Leffel DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773-776.
- Cockerell C. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J Am Acad Dermatol. 2000;42:11-17.
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci. 1996;93:14025-14029.
- Kanjilal S, Strom SS, Clayman GL, et al. p53 Mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604-3609.
- Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968.
- Braakhuis B, Tabor M, Kummer J, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Stern R, Bolshakov S, Nataraj A, et al. p53 Mutation in nonmelanoma skin cancers occurring in psoralen ultraviolet A-treated patients: evidence for heterogeneity and field cancerization. J Invest Dermatol. 2002;119:522-526.
- Tabor M, Brakenhoff R, van Houten VM, et al. Persistence of genetically altered fields in head and neck cancer patients: biological and clinical implications. Clin Cancer Res. 2001;7:1523-1532.
- Torezan L. Cutaneous field cancerization: clinical, histopathological and therapeutic aspects. An Bras Dermatol. 2013;88:775-786.
- Ullrich S, Kripke M, Ananthaswamy H. Mechanisms underlying UV-induced immune suppression: implications for sunscreen design. Exp Dermatol. 2002;11:1-4.
- de Gruijl FR. Photocarcinogenesis: UVA vs UVB. Methods Enzymol. 2000;319:359-366.
- Brash DE, Ziegler A, Jonason AS, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis, and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136-142.
- Ackerman AB, Mones JM. Solar (actinic) keratosis is squamous cell carcinoma. Br J Dermatol. 2006;155:9-22.
- Rossi R, Mori M, Lotti T. Actinic keratosis. Int J Dermatol. 2007;46:895-904.
- Ziegler A, Jonason AS, Leffel DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773-776.
- Cockerell C. Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J Am Acad Dermatol. 2000;42:11-17.
- Jonason AS, Kunala S, Price GJ, et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc Natl Acad Sci. 1996;93:14025-14029.
- Kanjilal S, Strom SS, Clayman GL, et al. p53 Mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604-3609.
- Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous cell carcinoma of the head and neck. N Engl J Med. 1995;332:429-435.
- Braathen LR, Morton CA, Basset-Seguin N, et al. Photodynamic therapy for skin field cancerization: an international consensus. International Society for Photodynamic Therapy in Dermatology. J Eur Acad Dermatol Venereol. 2012;26:1063-1066.
Practice Points
- Clinically apparent and subclinical actinic keratoses usually are present in patients, a concept known as field cancerization, and it is important to treat both types of lesions.
- Actinic keratoses are present in the field of cutaneous malignancies, including basal cell carcinoma, squamous cell carcinoma, and melanoma.
For preventing AKs, 5-FU beats placebo for up to 3 years
SCOTTSDALE – A single course of topical 5-fluorouracil (5-FU) prevented 62% more actinic keratoses than placebo, and this chemopreventive effect persisted for up to 3 years, according to an analysis of the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCCT) trial.
Other studies have shown that 5-FU effectively treats precancerous AKs, but have not examined whether 5-FU can prevent AKs, Dr. Joanna Walker said in an interview at the annual meeting of the Society for Investigative Dermatology.
Clinicians should consider preventive 5-FU in patients who are at high risk for basal cell and squamous cell carcinomas, especially if a skin check reveals multiple AKs, said Dr. Walker of the department of dermatology, Brown University, Providence, RI.
The VAKCCT was a randomized, double-blind, placebo-controlled study conducted at 12 Veterans Affairs dermatology clinics. The 319 patients in the analysis were nearly all elderly men with extensive sun damage, with a total of 2,386 AKs at baseline, for an average of five lesions per patient. Patients also had a history of at least two keratinocyte carcinomas in the past 5 years, including at least one lesion on the face or ears, and no recent history of 5-FU exposure.
The clinically and demographically similar study arms were randomized to either 5% topical 5-FU cream or a vehicle control cream, applied twice daily for 2-4 weeks. Both groups received cryotherapy for existing AKs, and were given free SPF 30 sunscreen. At each 6-month follow-up visit, the researchers counted existing AKs and new lesions.
At month 6, the treatment group had 62% fewer new AKs than the placebo group (average per patient, 1.78 and 4.73, respectively), a statistically significant difference. At months 12, 18, 24, 30, and 36, respectively, the treatment group had 50%, 40%, 41%, 25%, and 35% fewer new AKs than the placebo group, and these differences all were statistically significant. Furthermore, at month 6, only 56% of treated patients had at least one new AK, compared with 78% of the control group (incidence rate ratio, 0.72; 95% confidence interval, 0.54-0.95).
This chemopreventive effect remained significant for 24 months, the investigators reported. “Individuals with at least five AKs at the time of 5-FU treatment had an even more dramatic reduction in new AKs,” Dr. Walker noted. “There is now high-quality evidence supporting the use of topical 5-FU for AK chemoprevention. I think this is important information that we can take back to the clinic when we are trying to convince our patients to go through a course of 5-FU.”
The rate of new AKs in the placebo group fell during the first 2.5 years of the study and then stabilized. “For both groups, there was a dramatic increase in the use of sunscreen during the trial, and we hypothesized that the decrease in AKs in the control group was due to increased use of sun-protective measures,” Dr. Walker said.
The research was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
SCOTTSDALE – A single course of topical 5-fluorouracil (5-FU) prevented 62% more actinic keratoses than placebo, and this chemopreventive effect persisted for up to 3 years, according to an analysis of the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCCT) trial.
Other studies have shown that 5-FU effectively treats precancerous AKs, but have not examined whether 5-FU can prevent AKs, Dr. Joanna Walker said in an interview at the annual meeting of the Society for Investigative Dermatology.
Clinicians should consider preventive 5-FU in patients who are at high risk for basal cell and squamous cell carcinomas, especially if a skin check reveals multiple AKs, said Dr. Walker of the department of dermatology, Brown University, Providence, RI.
The VAKCCT was a randomized, double-blind, placebo-controlled study conducted at 12 Veterans Affairs dermatology clinics. The 319 patients in the analysis were nearly all elderly men with extensive sun damage, with a total of 2,386 AKs at baseline, for an average of five lesions per patient. Patients also had a history of at least two keratinocyte carcinomas in the past 5 years, including at least one lesion on the face or ears, and no recent history of 5-FU exposure.
The clinically and demographically similar study arms were randomized to either 5% topical 5-FU cream or a vehicle control cream, applied twice daily for 2-4 weeks. Both groups received cryotherapy for existing AKs, and were given free SPF 30 sunscreen. At each 6-month follow-up visit, the researchers counted existing AKs and new lesions.
At month 6, the treatment group had 62% fewer new AKs than the placebo group (average per patient, 1.78 and 4.73, respectively), a statistically significant difference. At months 12, 18, 24, 30, and 36, respectively, the treatment group had 50%, 40%, 41%, 25%, and 35% fewer new AKs than the placebo group, and these differences all were statistically significant. Furthermore, at month 6, only 56% of treated patients had at least one new AK, compared with 78% of the control group (incidence rate ratio, 0.72; 95% confidence interval, 0.54-0.95).
This chemopreventive effect remained significant for 24 months, the investigators reported. “Individuals with at least five AKs at the time of 5-FU treatment had an even more dramatic reduction in new AKs,” Dr. Walker noted. “There is now high-quality evidence supporting the use of topical 5-FU for AK chemoprevention. I think this is important information that we can take back to the clinic when we are trying to convince our patients to go through a course of 5-FU.”
The rate of new AKs in the placebo group fell during the first 2.5 years of the study and then stabilized. “For both groups, there was a dramatic increase in the use of sunscreen during the trial, and we hypothesized that the decrease in AKs in the control group was due to increased use of sun-protective measures,” Dr. Walker said.
The research was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
SCOTTSDALE – A single course of topical 5-fluorouracil (5-FU) prevented 62% more actinic keratoses than placebo, and this chemopreventive effect persisted for up to 3 years, according to an analysis of the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial (VAKCCT) trial.
Other studies have shown that 5-FU effectively treats precancerous AKs, but have not examined whether 5-FU can prevent AKs, Dr. Joanna Walker said in an interview at the annual meeting of the Society for Investigative Dermatology.
Clinicians should consider preventive 5-FU in patients who are at high risk for basal cell and squamous cell carcinomas, especially if a skin check reveals multiple AKs, said Dr. Walker of the department of dermatology, Brown University, Providence, RI.
The VAKCCT was a randomized, double-blind, placebo-controlled study conducted at 12 Veterans Affairs dermatology clinics. The 319 patients in the analysis were nearly all elderly men with extensive sun damage, with a total of 2,386 AKs at baseline, for an average of five lesions per patient. Patients also had a history of at least two keratinocyte carcinomas in the past 5 years, including at least one lesion on the face or ears, and no recent history of 5-FU exposure.
The clinically and demographically similar study arms were randomized to either 5% topical 5-FU cream or a vehicle control cream, applied twice daily for 2-4 weeks. Both groups received cryotherapy for existing AKs, and were given free SPF 30 sunscreen. At each 6-month follow-up visit, the researchers counted existing AKs and new lesions.
At month 6, the treatment group had 62% fewer new AKs than the placebo group (average per patient, 1.78 and 4.73, respectively), a statistically significant difference. At months 12, 18, 24, 30, and 36, respectively, the treatment group had 50%, 40%, 41%, 25%, and 35% fewer new AKs than the placebo group, and these differences all were statistically significant. Furthermore, at month 6, only 56% of treated patients had at least one new AK, compared with 78% of the control group (incidence rate ratio, 0.72; 95% confidence interval, 0.54-0.95).
This chemopreventive effect remained significant for 24 months, the investigators reported. “Individuals with at least five AKs at the time of 5-FU treatment had an even more dramatic reduction in new AKs,” Dr. Walker noted. “There is now high-quality evidence supporting the use of topical 5-FU for AK chemoprevention. I think this is important information that we can take back to the clinic when we are trying to convince our patients to go through a course of 5-FU.”
The rate of new AKs in the placebo group fell during the first 2.5 years of the study and then stabilized. “For both groups, there was a dramatic increase in the use of sunscreen during the trial, and we hypothesized that the decrease in AKs in the control group was due to increased use of sun-protective measures,” Dr. Walker said.
The research was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
AT THE 2016 SID ANNUAL MEETING
Key clinical point: One course of topical 5-fluorouracil was effective and durable in preventing new actinic keratoses in high-risk patients.
Major finding: At month 6, the treatment group had 62% fewer new AKs than the placebo group, and the difference remained significant at month 36.
Data source: The double-blind controlled study evaluated 5-FU vs. a vehicle cream in 319 veterans, most of whom were elderly men.
Disclosures: The study was funded by the Cooperative Studies Program of the U.S. Department of Veterans Affairs. Dr. Walker had no disclosures.
Average person with atopic dermatitis has no increased risk of actinic keratosis or nonmelanoma skin cancer
People with atopic dermatitis do not appear to be at greater risk for actinic keratosis or basal cell and squamous cell cancer, according to a recent population-based, cross-sectional study.
“This is the first study to examine the association between atopic dermatitis and actinic keratosis [AK]. Our findings suggest that within a population-based sample, atopic dermatitis patients do not have more AKs than the rest of the population. Patients with atopic dermatitis were not found to have more AKs or keratotic cancers [basal or squamous cell cancers]. Moreover, individuals with atopic dermatitis seem to be less likely to develop multiple AKs,” said Dr. Enes Hajdarbegovic and his associates of the Erasmus Medical Centre, Rotterdam, the Netherlands.
The study is part of an ongoing, prospective, Dutch population-based cohort study that follows people in a district of Rotterdam since 1990. There are now 14,926 participants in the database. The current study included 4,375 participants who had undergone full body skin examinations; 56% of patients were female, and the mean age was 68 years (Br J Dermatol. 2016 Jan 29. doi: 10.1111/bjd.14423).
Twenty-four percent had 1 or more AKs; 57% had 1-3 of these lesions; 23% had 4-9, and 20% had more than 10. The mean age of participants with AK was significantly higher, compared with those without AK (73 years vs. 66 years; P less than .01).
Of the 4,375 participants screened, 6.3% met the diagnostic criteria for atopic dermatitis. A lower proportion of those with atopic dermatitis had AK: 16% vs. 24%, respectively (P = .002). In a multinomial model, atopic dermatitis patients were 78% less likely to have 10 or more AKs than were those without atopic dermatitis. No effect of atopic dermatitis was found on basal cell cancer (adjusted odds ratio, 0.71) and squamous cell cancer (adjusted OR, 1.54).
The authors explained that it is already known that patients with severe atopic dermatitis exposed to ultraviolet light and immunosuppressants are at increased risk of keratinocyte malignancies. This study shows that a community-dwelling person with moderate atopic dermatitis does not develop more AKs or keratinocyte cancers.
The investigators said they had no relevant financial disclosures.
People with atopic dermatitis do not appear to be at greater risk for actinic keratosis or basal cell and squamous cell cancer, according to a recent population-based, cross-sectional study.
“This is the first study to examine the association between atopic dermatitis and actinic keratosis [AK]. Our findings suggest that within a population-based sample, atopic dermatitis patients do not have more AKs than the rest of the population. Patients with atopic dermatitis were not found to have more AKs or keratotic cancers [basal or squamous cell cancers]. Moreover, individuals with atopic dermatitis seem to be less likely to develop multiple AKs,” said Dr. Enes Hajdarbegovic and his associates of the Erasmus Medical Centre, Rotterdam, the Netherlands.
The study is part of an ongoing, prospective, Dutch population-based cohort study that follows people in a district of Rotterdam since 1990. There are now 14,926 participants in the database. The current study included 4,375 participants who had undergone full body skin examinations; 56% of patients were female, and the mean age was 68 years (Br J Dermatol. 2016 Jan 29. doi: 10.1111/bjd.14423).
Twenty-four percent had 1 or more AKs; 57% had 1-3 of these lesions; 23% had 4-9, and 20% had more than 10. The mean age of participants with AK was significantly higher, compared with those without AK (73 years vs. 66 years; P less than .01).
Of the 4,375 participants screened, 6.3% met the diagnostic criteria for atopic dermatitis. A lower proportion of those with atopic dermatitis had AK: 16% vs. 24%, respectively (P = .002). In a multinomial model, atopic dermatitis patients were 78% less likely to have 10 or more AKs than were those without atopic dermatitis. No effect of atopic dermatitis was found on basal cell cancer (adjusted odds ratio, 0.71) and squamous cell cancer (adjusted OR, 1.54).
The authors explained that it is already known that patients with severe atopic dermatitis exposed to ultraviolet light and immunosuppressants are at increased risk of keratinocyte malignancies. This study shows that a community-dwelling person with moderate atopic dermatitis does not develop more AKs or keratinocyte cancers.
The investigators said they had no relevant financial disclosures.
People with atopic dermatitis do not appear to be at greater risk for actinic keratosis or basal cell and squamous cell cancer, according to a recent population-based, cross-sectional study.
“This is the first study to examine the association between atopic dermatitis and actinic keratosis [AK]. Our findings suggest that within a population-based sample, atopic dermatitis patients do not have more AKs than the rest of the population. Patients with atopic dermatitis were not found to have more AKs or keratotic cancers [basal or squamous cell cancers]. Moreover, individuals with atopic dermatitis seem to be less likely to develop multiple AKs,” said Dr. Enes Hajdarbegovic and his associates of the Erasmus Medical Centre, Rotterdam, the Netherlands.
The study is part of an ongoing, prospective, Dutch population-based cohort study that follows people in a district of Rotterdam since 1990. There are now 14,926 participants in the database. The current study included 4,375 participants who had undergone full body skin examinations; 56% of patients were female, and the mean age was 68 years (Br J Dermatol. 2016 Jan 29. doi: 10.1111/bjd.14423).
Twenty-four percent had 1 or more AKs; 57% had 1-3 of these lesions; 23% had 4-9, and 20% had more than 10. The mean age of participants with AK was significantly higher, compared with those without AK (73 years vs. 66 years; P less than .01).
Of the 4,375 participants screened, 6.3% met the diagnostic criteria for atopic dermatitis. A lower proportion of those with atopic dermatitis had AK: 16% vs. 24%, respectively (P = .002). In a multinomial model, atopic dermatitis patients were 78% less likely to have 10 or more AKs than were those without atopic dermatitis. No effect of atopic dermatitis was found on basal cell cancer (adjusted odds ratio, 0.71) and squamous cell cancer (adjusted OR, 1.54).
The authors explained that it is already known that patients with severe atopic dermatitis exposed to ultraviolet light and immunosuppressants are at increased risk of keratinocyte malignancies. This study shows that a community-dwelling person with moderate atopic dermatitis does not develop more AKs or keratinocyte cancers.
The investigators said they had no relevant financial disclosures.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: People with atopic dermatitis do not appear to be at greater risk for actinic keratosis or basal cell and squamous cell cancer.
Major finding: In a multinomial model, atopic dermatitis patients were 78% less likely to have 10 or more actinic keratoses than were those without atopic dermatitis. No effect of atopic dermatitis was found on basal cell cancer (adjusted OR, 0.71) and squamous cell cancer (adjusted OR, 1.54).
Data source: A prospective, Dutch population-based cohort study of 4,375 participants who had undergone full body skin examinations.
Disclosures: The investigators said they had no relevant financial disclosures.
VIDEO: Consider adding photodynamic therapy to your practice
ORLANDO – Dermatologists with the right patient population should consider incorporating photodynamic therapy (PDT) into their practices, as it is becoming an increasingly popular and effective.
Although most dermatologists know about PDT, “They’re not as comfortable setting up the office” and often don’t know the best ways to use PDT to achieve optimal treatment outcomes, explained Dr. Neal Bhatia, director of clinical dermatology at Therapeutics Clinical Research in San Diego, during his session at the Orlando Dermatology Aesthetic and Clinical annual meeting.
In this video interview, Dr. Bhatia, explains the benefits of PDT, what type of patient would be best suited for this treatment, and why it’s increasingly important for dermatologists to offer this treatment to their patients – even if they’ve never considered doing so before.
Dr. Bhatia is a clinical investigator and consultant with Dusa Pharmaceuticals and Biofrontera. He did not report any other relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Dermatologists with the right patient population should consider incorporating photodynamic therapy (PDT) into their practices, as it is becoming an increasingly popular and effective.
Although most dermatologists know about PDT, “They’re not as comfortable setting up the office” and often don’t know the best ways to use PDT to achieve optimal treatment outcomes, explained Dr. Neal Bhatia, director of clinical dermatology at Therapeutics Clinical Research in San Diego, during his session at the Orlando Dermatology Aesthetic and Clinical annual meeting.
In this video interview, Dr. Bhatia, explains the benefits of PDT, what type of patient would be best suited for this treatment, and why it’s increasingly important for dermatologists to offer this treatment to their patients – even if they’ve never considered doing so before.
Dr. Bhatia is a clinical investigator and consultant with Dusa Pharmaceuticals and Biofrontera. He did not report any other relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Dermatologists with the right patient population should consider incorporating photodynamic therapy (PDT) into their practices, as it is becoming an increasingly popular and effective.
Although most dermatologists know about PDT, “They’re not as comfortable setting up the office” and often don’t know the best ways to use PDT to achieve optimal treatment outcomes, explained Dr. Neal Bhatia, director of clinical dermatology at Therapeutics Clinical Research in San Diego, during his session at the Orlando Dermatology Aesthetic and Clinical annual meeting.
In this video interview, Dr. Bhatia, explains the benefits of PDT, what type of patient would be best suited for this treatment, and why it’s increasingly important for dermatologists to offer this treatment to their patients – even if they’ve never considered doing so before.
Dr. Bhatia is a clinical investigator and consultant with Dusa Pharmaceuticals and Biofrontera. He did not report any other relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM ODAC 2016
FDA approves treatment for chemotherapy ODs, life-threatening toxicities
Uridine triacetate, a pyrimidine analogue, has been approved for the emergency treatment of fluorouracil or capecitabine overdoses in adults and children, and for patients who develop “certain severe or life-threatening toxicities within 4 days of receiving” these treatments, the Food and Drug Administration announced on Dec. 11.
“Today’s approval is a first-of-its-kind therapy that can potentially save lives following overdose or life-threatening toxicity from these chemotherapy agents,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. It will be marketed as Vistogard by Wellstat Therapeutics.
Uridine comes in an oral granule formulation that can be mixed into soft foods or, when necessary, administered via a nasogastric or gastrostomy tube, the prescribing information states. The indication is for use after an overdose “regardless of the presence of symptoms,” and for treating “early-onset, severe, or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration,” according to the prescribing information.
Uridine blocks cell damage and cell death caused by fluorouracil chemotherapy, according to the statement, which adds that it is up to the patient’s health care provider to “determine when he or she should return to the prescribed chemotherapy after treatment with Vistogard.”
Uridine was evaluated in two studies of 135 adults and children with cancer, treated with uridine for a fluorouracil or capecitabine overdose, or for early-onset, unusually severe or life-threatening toxicities within 96 hours after receiving fluorouracil (not because of an overdose). Among those treated for an overdose, 97% were alive 30 days after treatment, and among those treated for early-onset severe or life-threatening toxicity, 89% were alive 30 days after treatment. In addition, 33% of the patients resumed chemotherapy within 30 days, according to the FDA statement. Diarrhea, vomiting, and nausea were the most common adverse events associated with treatment.
Uridine was granted orphan drug, priority review, and fast track designations.
Uridine triacetate, a pyrimidine analogue, has been approved for the emergency treatment of fluorouracil or capecitabine overdoses in adults and children, and for patients who develop “certain severe or life-threatening toxicities within 4 days of receiving” these treatments, the Food and Drug Administration announced on Dec. 11.
“Today’s approval is a first-of-its-kind therapy that can potentially save lives following overdose or life-threatening toxicity from these chemotherapy agents,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. It will be marketed as Vistogard by Wellstat Therapeutics.
Uridine comes in an oral granule formulation that can be mixed into soft foods or, when necessary, administered via a nasogastric or gastrostomy tube, the prescribing information states. The indication is for use after an overdose “regardless of the presence of symptoms,” and for treating “early-onset, severe, or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration,” according to the prescribing information.
Uridine blocks cell damage and cell death caused by fluorouracil chemotherapy, according to the statement, which adds that it is up to the patient’s health care provider to “determine when he or she should return to the prescribed chemotherapy after treatment with Vistogard.”
Uridine was evaluated in two studies of 135 adults and children with cancer, treated with uridine for a fluorouracil or capecitabine overdose, or for early-onset, unusually severe or life-threatening toxicities within 96 hours after receiving fluorouracil (not because of an overdose). Among those treated for an overdose, 97% were alive 30 days after treatment, and among those treated for early-onset severe or life-threatening toxicity, 89% were alive 30 days after treatment. In addition, 33% of the patients resumed chemotherapy within 30 days, according to the FDA statement. Diarrhea, vomiting, and nausea were the most common adverse events associated with treatment.
Uridine was granted orphan drug, priority review, and fast track designations.
Uridine triacetate, a pyrimidine analogue, has been approved for the emergency treatment of fluorouracil or capecitabine overdoses in adults and children, and for patients who develop “certain severe or life-threatening toxicities within 4 days of receiving” these treatments, the Food and Drug Administration announced on Dec. 11.
“Today’s approval is a first-of-its-kind therapy that can potentially save lives following overdose or life-threatening toxicity from these chemotherapy agents,” Dr. Richard Pazdur, director of the office of hematology and oncology products in the FDA’s Center for Drug Evaluation and Research, said in the FDA statement. It will be marketed as Vistogard by Wellstat Therapeutics.
Uridine comes in an oral granule formulation that can be mixed into soft foods or, when necessary, administered via a nasogastric or gastrostomy tube, the prescribing information states. The indication is for use after an overdose “regardless of the presence of symptoms,” and for treating “early-onset, severe, or life-threatening toxicity affecting the cardiac or central nervous system, and/or early-onset, unusually severe adverse reactions (e.g., gastrointestinal toxicity and/or neutropenia) within 96 hours following the end of fluorouracil or capecitabine administration,” according to the prescribing information.
Uridine blocks cell damage and cell death caused by fluorouracil chemotherapy, according to the statement, which adds that it is up to the patient’s health care provider to “determine when he or she should return to the prescribed chemotherapy after treatment with Vistogard.”
Uridine was evaluated in two studies of 135 adults and children with cancer, treated with uridine for a fluorouracil or capecitabine overdose, or for early-onset, unusually severe or life-threatening toxicities within 96 hours after receiving fluorouracil (not because of an overdose). Among those treated for an overdose, 97% were alive 30 days after treatment, and among those treated for early-onset severe or life-threatening toxicity, 89% were alive 30 days after treatment. In addition, 33% of the patients resumed chemotherapy within 30 days, according to the FDA statement. Diarrhea, vomiting, and nausea were the most common adverse events associated with treatment.
Uridine was granted orphan drug, priority review, and fast track designations.
AK clearance rates maintained 1 year after thermally modulated PDT
Clearance rates for actinic keratoses (AKs) on the extremities were prolonged by adding thermal modulation to photodynamic therapy (PDT), a study has shown.
The single-site study of 17 adults with 10 or more AKs on their arms or legs evaluated thermally modulated PDT: 20% 5-aminolevulinic acid (ALA) applied to skin, occluded with plastic wrap and warmed with an electric heating pad to a median 41.2° C for one hour, then exposed to blue light. Treatment resulted in 90% clearance rates at 3 months and 12 months, reported Dr. Andrea Willey, a dermatologist in Sacramento, Calif., and her associates (Dermatol Surg. 2015 Nov;41[11]:1290-5).
They were inspired to conduct the study as a follow-on to a recently published pilot study of three patients, which found that clearance rates of AKs on the arm treated with thermally modulated 5-ALA blue light PDT persisted up to 14 months after treatment, when compared with the control arm, which was not heated.
In their study, there were 724 grade 1 and 2 AKs at baseline – a median of 15 per extremity; patients were followed up at 3, 6, 9, and 12 months. The total lesion count across the entire cohort was 70 at 3 months, 69 at 6 months, 64 at 9 months, and 72 at 1 year. No new AKs were seen in the treated areas during the 1-year follow-up.
There were 13 grade 3 hypertrophic AK lesions among all study participants. Although these lesions persisted, Dr. Willey and her coauthors noted that they did shrink initially after treatment.
Adverse events associated with the combination therapy were low, and included mild erythema and crusting up to 1 week post treatment, indicating the therapy was well tolerated, and patient satisfaction was high.
The results “suggest that mild skin warming may both improve efficacy and reduce variability of response to PDT in practice,” the authors wrote.
On Twitter @whitneymcknight
Clearance rates for actinic keratoses (AKs) on the extremities were prolonged by adding thermal modulation to photodynamic therapy (PDT), a study has shown.
The single-site study of 17 adults with 10 or more AKs on their arms or legs evaluated thermally modulated PDT: 20% 5-aminolevulinic acid (ALA) applied to skin, occluded with plastic wrap and warmed with an electric heating pad to a median 41.2° C for one hour, then exposed to blue light. Treatment resulted in 90% clearance rates at 3 months and 12 months, reported Dr. Andrea Willey, a dermatologist in Sacramento, Calif., and her associates (Dermatol Surg. 2015 Nov;41[11]:1290-5).
They were inspired to conduct the study as a follow-on to a recently published pilot study of three patients, which found that clearance rates of AKs on the arm treated with thermally modulated 5-ALA blue light PDT persisted up to 14 months after treatment, when compared with the control arm, which was not heated.
In their study, there were 724 grade 1 and 2 AKs at baseline – a median of 15 per extremity; patients were followed up at 3, 6, 9, and 12 months. The total lesion count across the entire cohort was 70 at 3 months, 69 at 6 months, 64 at 9 months, and 72 at 1 year. No new AKs were seen in the treated areas during the 1-year follow-up.
There were 13 grade 3 hypertrophic AK lesions among all study participants. Although these lesions persisted, Dr. Willey and her coauthors noted that they did shrink initially after treatment.
Adverse events associated with the combination therapy were low, and included mild erythema and crusting up to 1 week post treatment, indicating the therapy was well tolerated, and patient satisfaction was high.
The results “suggest that mild skin warming may both improve efficacy and reduce variability of response to PDT in practice,” the authors wrote.
On Twitter @whitneymcknight
Clearance rates for actinic keratoses (AKs) on the extremities were prolonged by adding thermal modulation to photodynamic therapy (PDT), a study has shown.
The single-site study of 17 adults with 10 or more AKs on their arms or legs evaluated thermally modulated PDT: 20% 5-aminolevulinic acid (ALA) applied to skin, occluded with plastic wrap and warmed with an electric heating pad to a median 41.2° C for one hour, then exposed to blue light. Treatment resulted in 90% clearance rates at 3 months and 12 months, reported Dr. Andrea Willey, a dermatologist in Sacramento, Calif., and her associates (Dermatol Surg. 2015 Nov;41[11]:1290-5).
They were inspired to conduct the study as a follow-on to a recently published pilot study of three patients, which found that clearance rates of AKs on the arm treated with thermally modulated 5-ALA blue light PDT persisted up to 14 months after treatment, when compared with the control arm, which was not heated.
In their study, there were 724 grade 1 and 2 AKs at baseline – a median of 15 per extremity; patients were followed up at 3, 6, 9, and 12 months. The total lesion count across the entire cohort was 70 at 3 months, 69 at 6 months, 64 at 9 months, and 72 at 1 year. No new AKs were seen in the treated areas during the 1-year follow-up.
There were 13 grade 3 hypertrophic AK lesions among all study participants. Although these lesions persisted, Dr. Willey and her coauthors noted that they did shrink initially after treatment.
Adverse events associated with the combination therapy were low, and included mild erythema and crusting up to 1 week post treatment, indicating the therapy was well tolerated, and patient satisfaction was high.
The results “suggest that mild skin warming may both improve efficacy and reduce variability of response to PDT in practice,” the authors wrote.
On Twitter @whitneymcknight
FROM DERMATOLOGIC SURGERY
Key clinical point: Mildly warming affected skin sites during photodynamic therapy can prolong the clearance rates for AKs on the extremities.
Major finding: AK clearance rates on the extremities remained at about 90% during the 1 year after treatment with thermally modulated PDT.
Data source: A single site, prospective 1-year study evaluating treatment with thermally modulated PDT for 17 adults with 10 or more AKs on their extremities.
Disclosures: Dr. Willey is a member of the scientific advisory board of DUSA Pharmaceuticals; she received a research grant, equipment loan, and study drug from the company. Her two coauthors had no relevant disclosures.
Topicals, PDT offer field treatment options for actinic keratoses
LAS VEGAS – The addition of field treatment to targeted lesion treatment may offer patients the best outcomes when addressing actinic keratoses (AKs), said Dr. David M. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, Virginia.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser said that the rationale for treating AKs stems from the fact that not all AKs will transform to squamous cell carcinoma (SCC). “We don’t know which ones will and which ones won’t.” Though treatment can be guided by a shared decision-making approach, a personal history of multiple skin cancers is an obvious reason for concern, as is any drug-related or disease-related immunosuppression, he added.
Available treatments for AKs include cryosurgery, curettage, and electrodessication; photodynamic therapy (PDT); and topical agents, including 5-fluorouracil (5-FU), diclofenac, imiquimod, and ingenol mebutate.
“Cryosurgery is what most of us do for most AKs,” said Dr. Pariser, observing that this is a bread-and-butter procedure that is part of the backbone of most dermatology practices. Cryosurgery, curettage, and electrodessication are all used to treat discrete lesions, especially for higher-grade AKs.
However, Dr. Pariser noted that many AKs occur in a “field of cancerization.” This is a larger area of photodamaged skin; the skin may not appear grossly abnormal, but deranged areas will be diffusely evident with fluorescence detection, “indicating that all of this area was damaged,” he said.
PDT and topical agents effectively provide field treatment, and do not just address discrete lesions, although field treatment is technically an off-label use for these modalities, he added.
Lower concentrations and shorter courses of topical agents may be effective and better tolerated by patients, Dr. Pariser said. In clinical trials, for example, the efficacy of imiquimod 2.5% and 3.75% has been comparable to the stronger 5% formulation. Treatment cycles lasting 2 rather than 3 weeks have also shown similar efficacy. The greater palatability of these regimens to patients may increase real-world adherence as well.
Cure rates for PDT range from 67% to 92% for both the blue light–aminolevulinic acid modality and the red light–methyl aminolevulinate more commonly used in Europe. These modalities also treat a broad field, offer cure rates comparable to other topical modalities, and result in less scarring than destructive modalities, Dr. Pariser said.
The pain of PDT may be understandably off-putting for some patients, he commented. In the United States, investigators are experimenting with shorter incubation times for the ALA topical solutions. One study found comparable reductions in the number of AKs by the end of the study period for 1 versus 2 or 3 hours of incubation, and a 2-hour incubation period with spot treatment only was also similarly effective. Clearance rates ranged from about 70%-80%, and all were highly statistically significant, compared with the vehicle-only arm. However, “a second treatment at week 8 seems to be necessary for improved efficacy,” Dr. Pariser said.
Another treatment strategy currently being investigated is “painless PDT,” where the agent is applied and the field is illuminated immediately. Photo-bleaching degrades the protoporphyrins as they are produced, dramatically reducing patient discomfort. Efficacy is promising, he added.
In Europe, “daylight PDT” is being explored – the photosensitizing agent is applied, and the patient simply goes about his or her daily business in normal daylight. This is a nearly painless therapy and shows promise for efficacy. However, said Dr. Pariser, the realities of practice economics make it very unlikely that daylight PDT will catch on in the United States, since the daylight exposure does not represent a billable procedure.
Dr. Pariser reiterated that regardless of the modality, “field therapy has better long-term efficacy rates and better sustained clearance no matter which one of the agents we use, and combination therapy [of lesional and field treatment] seems to be the most effective.”
Dr. Pariser disclosed that he is an investigator and consultant for DUSA Pharmaceuticals, Photocure, and LEO Pharma, and that he discussed off-label uses of drugs and devices.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – The addition of field treatment to targeted lesion treatment may offer patients the best outcomes when addressing actinic keratoses (AKs), said Dr. David M. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, Virginia.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser said that the rationale for treating AKs stems from the fact that not all AKs will transform to squamous cell carcinoma (SCC). “We don’t know which ones will and which ones won’t.” Though treatment can be guided by a shared decision-making approach, a personal history of multiple skin cancers is an obvious reason for concern, as is any drug-related or disease-related immunosuppression, he added.
Available treatments for AKs include cryosurgery, curettage, and electrodessication; photodynamic therapy (PDT); and topical agents, including 5-fluorouracil (5-FU), diclofenac, imiquimod, and ingenol mebutate.
“Cryosurgery is what most of us do for most AKs,” said Dr. Pariser, observing that this is a bread-and-butter procedure that is part of the backbone of most dermatology practices. Cryosurgery, curettage, and electrodessication are all used to treat discrete lesions, especially for higher-grade AKs.
However, Dr. Pariser noted that many AKs occur in a “field of cancerization.” This is a larger area of photodamaged skin; the skin may not appear grossly abnormal, but deranged areas will be diffusely evident with fluorescence detection, “indicating that all of this area was damaged,” he said.
PDT and topical agents effectively provide field treatment, and do not just address discrete lesions, although field treatment is technically an off-label use for these modalities, he added.
Lower concentrations and shorter courses of topical agents may be effective and better tolerated by patients, Dr. Pariser said. In clinical trials, for example, the efficacy of imiquimod 2.5% and 3.75% has been comparable to the stronger 5% formulation. Treatment cycles lasting 2 rather than 3 weeks have also shown similar efficacy. The greater palatability of these regimens to patients may increase real-world adherence as well.
Cure rates for PDT range from 67% to 92% for both the blue light–aminolevulinic acid modality and the red light–methyl aminolevulinate more commonly used in Europe. These modalities also treat a broad field, offer cure rates comparable to other topical modalities, and result in less scarring than destructive modalities, Dr. Pariser said.
The pain of PDT may be understandably off-putting for some patients, he commented. In the United States, investigators are experimenting with shorter incubation times for the ALA topical solutions. One study found comparable reductions in the number of AKs by the end of the study period for 1 versus 2 or 3 hours of incubation, and a 2-hour incubation period with spot treatment only was also similarly effective. Clearance rates ranged from about 70%-80%, and all were highly statistically significant, compared with the vehicle-only arm. However, “a second treatment at week 8 seems to be necessary for improved efficacy,” Dr. Pariser said.
Another treatment strategy currently being investigated is “painless PDT,” where the agent is applied and the field is illuminated immediately. Photo-bleaching degrades the protoporphyrins as they are produced, dramatically reducing patient discomfort. Efficacy is promising, he added.
In Europe, “daylight PDT” is being explored – the photosensitizing agent is applied, and the patient simply goes about his or her daily business in normal daylight. This is a nearly painless therapy and shows promise for efficacy. However, said Dr. Pariser, the realities of practice economics make it very unlikely that daylight PDT will catch on in the United States, since the daylight exposure does not represent a billable procedure.
Dr. Pariser reiterated that regardless of the modality, “field therapy has better long-term efficacy rates and better sustained clearance no matter which one of the agents we use, and combination therapy [of lesional and field treatment] seems to be the most effective.”
Dr. Pariser disclosed that he is an investigator and consultant for DUSA Pharmaceuticals, Photocure, and LEO Pharma, and that he discussed off-label uses of drugs and devices.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – The addition of field treatment to targeted lesion treatment may offer patients the best outcomes when addressing actinic keratoses (AKs), said Dr. David M. Pariser, professor of dermatology at Eastern Virginia Medical School, Norfolk, Virginia.
Speaking at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser said that the rationale for treating AKs stems from the fact that not all AKs will transform to squamous cell carcinoma (SCC). “We don’t know which ones will and which ones won’t.” Though treatment can be guided by a shared decision-making approach, a personal history of multiple skin cancers is an obvious reason for concern, as is any drug-related or disease-related immunosuppression, he added.
Available treatments for AKs include cryosurgery, curettage, and electrodessication; photodynamic therapy (PDT); and topical agents, including 5-fluorouracil (5-FU), diclofenac, imiquimod, and ingenol mebutate.
“Cryosurgery is what most of us do for most AKs,” said Dr. Pariser, observing that this is a bread-and-butter procedure that is part of the backbone of most dermatology practices. Cryosurgery, curettage, and electrodessication are all used to treat discrete lesions, especially for higher-grade AKs.
However, Dr. Pariser noted that many AKs occur in a “field of cancerization.” This is a larger area of photodamaged skin; the skin may not appear grossly abnormal, but deranged areas will be diffusely evident with fluorescence detection, “indicating that all of this area was damaged,” he said.
PDT and topical agents effectively provide field treatment, and do not just address discrete lesions, although field treatment is technically an off-label use for these modalities, he added.
Lower concentrations and shorter courses of topical agents may be effective and better tolerated by patients, Dr. Pariser said. In clinical trials, for example, the efficacy of imiquimod 2.5% and 3.75% has been comparable to the stronger 5% formulation. Treatment cycles lasting 2 rather than 3 weeks have also shown similar efficacy. The greater palatability of these regimens to patients may increase real-world adherence as well.
Cure rates for PDT range from 67% to 92% for both the blue light–aminolevulinic acid modality and the red light–methyl aminolevulinate more commonly used in Europe. These modalities also treat a broad field, offer cure rates comparable to other topical modalities, and result in less scarring than destructive modalities, Dr. Pariser said.
The pain of PDT may be understandably off-putting for some patients, he commented. In the United States, investigators are experimenting with shorter incubation times for the ALA topical solutions. One study found comparable reductions in the number of AKs by the end of the study period for 1 versus 2 or 3 hours of incubation, and a 2-hour incubation period with spot treatment only was also similarly effective. Clearance rates ranged from about 70%-80%, and all were highly statistically significant, compared with the vehicle-only arm. However, “a second treatment at week 8 seems to be necessary for improved efficacy,” Dr. Pariser said.
Another treatment strategy currently being investigated is “painless PDT,” where the agent is applied and the field is illuminated immediately. Photo-bleaching degrades the protoporphyrins as they are produced, dramatically reducing patient discomfort. Efficacy is promising, he added.
In Europe, “daylight PDT” is being explored – the photosensitizing agent is applied, and the patient simply goes about his or her daily business in normal daylight. This is a nearly painless therapy and shows promise for efficacy. However, said Dr. Pariser, the realities of practice economics make it very unlikely that daylight PDT will catch on in the United States, since the daylight exposure does not represent a billable procedure.
Dr. Pariser reiterated that regardless of the modality, “field therapy has better long-term efficacy rates and better sustained clearance no matter which one of the agents we use, and combination therapy [of lesional and field treatment] seems to be the most effective.”
Dr. Pariser disclosed that he is an investigator and consultant for DUSA Pharmaceuticals, Photocure, and LEO Pharma, and that he discussed off-label uses of drugs and devices.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR