User login
All-Inside Meniscal Repair Devices
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
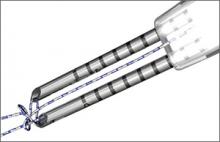
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
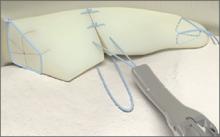
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Tools of the Trade features reviews of the hottest new products, with surgical pearls written by surgeons who know these products best.
Arthrex, Inc. (http://www.arthrex.com)
Knee Scorpion
The Arthrex Knee Scorpion allows for simple passage of suture at the root to repair the tissue. The mechanism of the Knee Scorpion self-retrieving the suture after passage eliminates the need for another step in the procedure, which saves time. There are various types of suture configurations that can be incorporated into meniscus repairs with the Knee Scorpion. Depending on tissue quality and location, I will either pass the center of the suture to create a cinch or luggage tag type of stitch, or sometimes a simple stitch.
A challenging meniscal tear pattern that repair is particularly made easier by use of the Knee Scorpion is the radial tear of the lateral meniscus. Here it is easy to place a side-to-side “spanning” circumferential suture pattern, which is the strongest suture configuration, and it reduces the tissue very well. This approach also is ideal for variant root type tears, those that are 3 to 4 mm from the root commonly seen with the lateral meniscus associated with anterior cruciate ligament tears.
Benefits: The Knee Scorpion has a very low profile to facilitate placement in the joint with a 5º upcurve to avoid injury to the femoral condyle. The needle captures the suture after passage, which saves time and an additional surgical step. It can be used with both 0 FiberWire and 2-0 FiberWire.
Surgical pearl: Using a cannula through the working portal prevents any tissue bridge when passing and tying sutures. I prefer a Passport button cannula since its flexibility allows more access in different planes compared to a rigid type cannula.
Patrick A. Smith, MD, Columbia Orthopaedic Group, Head Section of Sports Medicine University of Missouri, Head Team Physician University of Missouri
Arthrex, Inc. (http://www.arthrex.com)
SpeedCinch
The SpeedCinch’s pistol grip design permits ergonomic, one-handed all-inside meniscal repair. It is best suited for meniscal tears of the posterior horn and body. The posterior horn of the meniscus can be repaired with the SpeedCinch inserted through either the ipsilateral or contralateral portal. Meniscal body tears, however, are best approached via a contralateral portal. The end of the device contains a 15g needle that should be advanced across the meniscus. After passage of the needle, the first implant is pushed through the needle when the trigger is fully deployed.
Next, the SpeedCinch needle should be moved at least 1 cm for placement of the second implant in either a horizontal or vertical mattress fashion. After the needle is brought to the second insertion site, the implant selector button is moved to 2 and the trigger is depressed until the first click is felt and heard. Next, the trigger is held in place after the first click and the needle is advanced across the meniscus before fully depressing the trigger, which will advance the second implant. The device is then removed and the pre-tied knot is secured with a knot pusher and then cut.
Vic Goradia, MD, G2 Orthopedics and Sports Medicine, Glen Allen, VA
Cayenne Medical, Inc. (www.cayennemedical.com)
The CrossFix II System
The CrossFix II System offers a unique, all-arthroscopic, suture-only meniscal repair that reduces the risk of chondral injury. Its “all-inside” technique uses 2 parallel suture delivery needles, available in both curved and straight designs, inserted through a single incision to provide an all-inside meniscus repair. This simple procedure can be performed in minutes via the device’s pre-tied, sliding knot, creating a “suture only” mattress stitch that replicates the repair of standard open suturing techniques. The speed and reproducibility of the repair dramatically improves operating room efficiency. Even complex tears requiring multiple sutures can be repaired in minutes.
Benefits: All-suture, no implants; single insertion; pre-tied, sliding knot. Parallel needles may be difficult to use in very tight knees.
Surgical pearl: Avoid torqueing the needles upon insertion into the meniscus.
Kenneth Montgomery, MD, Tri-County Orthopedics, Cedar Knolls, NJ.
Ceterix Orthopaedics, Inc. (www.ceterix.com)
NovoStitch Plus
The Ceterix NovoStitch Plus enables surgeons to place circumferential compression stitches around meniscus tears. These stitch patterns are designed to provide anatomical reduction and uniform compression of the tear edges, and repair the femoral surface (top) and tibial surface (bottom) of the tear with each stitch. It is also designed to eliminate neurovascular risk and avoid excessive entrapment or extrusion of the meniscus to capsule. The NovoStitch Plus passes circumferential stitches via an all-inside arthroscopic technique. In addition to anatomically repairing vertical tears, circumferential stitches also enable repair of tear types that were previously considered difficult to adequately sew.
Benefits: Passes both sides of the stitch with 1 insertion (avoids tissue bridges and girth hitches). Retractable lower jaw (allows access to tight knees, and allows removal from knees with mobile menisci). Needle extends into the posterior gutter through the tip of the lower jaw (does not touch chondral surfaces). Smaller lower jaw tooth (easier insertion of the lower jaw under the meniscus). Upper jaw curved to follow the shape contour of the femoral side of the meniscus and femoral condyle. Compared to hybrid all-inside devices, inside-out and outside-in repairs. Enables anatomical reduction and uniform compression. Each stitch repairs the top and bottom of the tear. Designed to eliminate neurovascular risk. Avoids excessive capsular entrapment. Repairs effectively in front of the popliteal hiatus. Enables side-to-side radial tear repair, and top-to-bottom horizontal tear repair. Significantly smaller needle than used by hybrid all-inside devices.
Surgical pearl: A spinal needle should be used to establish the skin incision location of the working portal. The optimal skin incision location is one where a spinal needle can be inserted such that the distal end of the needle is parallel to the region of the tibial plateau under the meniscus.
Justin Saliman, MD, Cedars-Sinai Orthopaedic Center, Los Angeles CA
Mitek Sports Medicine (www.depuysynthes.com)
Mitek Omnispan
The Mitek Omnispan Meniscal Repair System is an all-inside arthroscopic meniscal repair device. It consists of a low profile needle, pre-loaded with 2 PEEK backstops and No. 2/0 Orthocord High Strength Orthopaedic Suture, which is delivered using the Omnispan System Applier. The needles come in 3 angles (0°, 12°, and 27°) and the Applier is a single-patient, multi-use design, meaning the same Applier can be used for multiple implants with a single patient. The needles also have a safety sleeve over each set of implants to prevent delivery failure. Once the needle is attached to the Applier, the device is inserted into the knee. Using the gray trigger, the first implant is delivered into the meniscocapsular region. The red trigger is then pulled, advancing the second implant. Once appropriate positioning of the second implant is determined, the gray trigger is fired again. The surgeon pulls on the sliding knot advancing the suture through the implants creating a suture bridge over the tear. The final repair consists of Orthocord Suture spanning the meniscus, while the PEEK backstops are embedded in the meniscocapsular area only.
Surgical pearls:
An adequate portal opening is paramount for successful implementation. I widen my portal with an instrument clamp to allow easy passage of the Applier. It is usually necessary to switch the camera back and forth between both portals to gain access to the angles needed to create the repair. I also always use a skid when inserting the Applier to prevent soft tissue impingement.
The surgeon should always check to make sure the implant is properly loaded on the back table, ensuring it is fully inserted into the locking mechanism and loaded in the correct direction.
The surgeon should hold the Applier firmly when firing the gray trigger, as some kick-back does occur.
The Applier should remain in the joint throughout the advancing and deployment of implants, so the surgeon can maintain visualization with the scope. Once the implants are in position, I then remove the Applier from the joint space.
Use a probe to place counter-pressure on the meniscus when tightening the suture. The sliding suture system is a double-loop design. In order to smoothly advance the suture, I recommend that a probe be placed on the smaller loop closest to the meniscus, so that when the suture is tightened, the probe is under the smaller loop. A gentle back-and-forth pulling maneuver between the free suture and the probe creates a smooth transition during tightening.
Scott A. Sigman, MD, Chief of Orthopedics, Lowell General Hospital; Team Physician, UMass Lowell
Smith & Nephew Inc. (www.smith-nephew.com)
FAST-FIX 360
The FAST-FIX360 design enables you to deploy implants in any hand position—vertically, horizontally on either side of the meniscus—with a fast, smooth advancing motion. This spring-action design facilitates the advancement of each implant into the capsule. Smaller implants and pre-tied, self-sliding knots made of ULTRABRAID 2-0 Suture create smaller needle insertions, reducing disruption to the meniscus. Low-profile, stiffer needle shaft improves control while enabling access and visibility to hard-to-reach areas of the meniscus. Set needle depth penetration from 10 mm to 18 mm with the push of a button. The FAST-FIX 360 System has biomechanical properties that best reproduce the vertical mattress suture technique.
Surgical pearl: Assess tear pattern and reparability, then reduce and template repair construct and suture points. Precisely select portal insertion sites, ensuring perpendicular vector needle delivery at the repair site. Measure the meniscal fragment size and rim width, setting the depth penetration limiter (usually set to 15 to 18 mm). Insert first anchor posteriorly and superiorly or away from the insertion portal and then after deployment, insert the second anchor anteriorly and inferiorly or into the “near” tear fragment. Tension the suture using the knot pusher/cutter as a “suture stent” to manually “pull and push” the suture, compressing the repair construct and coapting the tear. Avoid “over-repairing” the tear by spacing out sutures at 3 mm to 5 mm and alternating femoral and tibial undersurface placement.
Nicholas A. Sgaglione, MD (pictured), and Ryan A. Harrell, DO
Product News: 02 2016
Cosentyx
Novartis Pharmaceuticals Corporation announces US Food and Drug Administration approval of 2 new indications for Cosentyx (secukinumab): to treat patients with active ankylosing spondylitis and active psoriatic arthritis. Cosentyx is a human monoclonal antibody that selectively binds to IL-17A and inhibits its interaction with the IL-17 receptor. Research suggests that IL-17A may play an important role in driving the body’s immune response in psoriasis, psoriatic arthritis, and ankylosing spondylitis. Cosentyx was approved in January 2015 for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. For more information, visit www.cosentyx.com.
Emverm
Impax Laboratories, Inc, receives US Food and Drug Administration approval for the supplemental new drug application of Emverm (mebendazole) 100-mg chewable tablets for the treatment of pinworm and certain worm infections. Emverm is indicated for treatment of pinworm, whipworm, common roundworm, common hookworm, and American hookworm in single or mixed infections. Emverm is expected to become available early in the second quarter of 2016. For more information, visit www.impaxlabs.com.
Keytruda
Merck & Co, Inc, announces US Food and Drug Administration approval of an expanded indication for Keytruda (pembrolizumab) that includes the first-line treatment of patients with unresectable or metastatic melanoma. Keytruda is indicated in the United States at a dose of 2 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. Keytruda is an anti–programmed death receptor-1 therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. For more information, visit www.keytruda.com.
Opdivo + Yervoy Regimen
The US Food and Drug Administration has granted accelerated approval of nivolumab (Opdivo) in combination with ipilimumab (Yervoy) for the treatment of patients with BRAF V600 wild-type unresectable or metastatic melanoma. An international, multicenter, double-blind, randomized, active-controlled trial in patients who were previously untreated for unresectable or metastatic BRAF V600 wild-type melanoma demonstrated an increase in the objective response rate, prolonged response durations, and improvement in progression-free survival. When used in combination with ipilimumab, the recommended dose and schedule is nivolumab 1 mg/kg administered as an intravenous infusion over 60 minutes, followed by ipilimumab on the same day every 3 weeks for 4 doses. The recommended subsequent dose of nivolumab, as a single agent, is 3 mg/kg as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity. For more information, visit www.opdivoyervoyhcp.com.
TriAcnéal Day Mattifying Lotion and Night Smoothing Lotion
Pierre Fabre Dermo-Cosmetique USA introduces 2 TriAcnéal lotions in the Avène line for the treatment and prevention of acne. TriAcnéal Day Mattifying Lotion provides hydrating and mattifying care. It is gentle enough for daily use and can be used alone or in combination with topical acne prescriptions. A trio of ingredients target acne: PCC enzyme (consisting of papain, sodium alginate, caprylyl glycol, and hexanediol) for exfoliation to counteract the formation of new comedones, Diolényl (consisting of caprylyl glycol linseedate and potassium sorbate)to treat existing blemishes and prevent new lesions, and glyceryl laurate to reduce oil production. TriAcnéal Night Smoothing Lotion works to reduce the appearance of acne scars and provides moisturization and redness-reduction benefits. The nighttime formula contains PCC enzyme and Diolényl as well as retinaldehyde to diminish visible signs of aging. For more information, visit www.aveneusa.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Cosentyx
Novartis Pharmaceuticals Corporation announces US Food and Drug Administration approval of 2 new indications for Cosentyx (secukinumab): to treat patients with active ankylosing spondylitis and active psoriatic arthritis. Cosentyx is a human monoclonal antibody that selectively binds to IL-17A and inhibits its interaction with the IL-17 receptor. Research suggests that IL-17A may play an important role in driving the body’s immune response in psoriasis, psoriatic arthritis, and ankylosing spondylitis. Cosentyx was approved in January 2015 for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. For more information, visit www.cosentyx.com.
Emverm
Impax Laboratories, Inc, receives US Food and Drug Administration approval for the supplemental new drug application of Emverm (mebendazole) 100-mg chewable tablets for the treatment of pinworm and certain worm infections. Emverm is indicated for treatment of pinworm, whipworm, common roundworm, common hookworm, and American hookworm in single or mixed infections. Emverm is expected to become available early in the second quarter of 2016. For more information, visit www.impaxlabs.com.
Keytruda
Merck & Co, Inc, announces US Food and Drug Administration approval of an expanded indication for Keytruda (pembrolizumab) that includes the first-line treatment of patients with unresectable or metastatic melanoma. Keytruda is indicated in the United States at a dose of 2 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. Keytruda is an anti–programmed death receptor-1 therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. For more information, visit www.keytruda.com.
Opdivo + Yervoy Regimen
The US Food and Drug Administration has granted accelerated approval of nivolumab (Opdivo) in combination with ipilimumab (Yervoy) for the treatment of patients with BRAF V600 wild-type unresectable or metastatic melanoma. An international, multicenter, double-blind, randomized, active-controlled trial in patients who were previously untreated for unresectable or metastatic BRAF V600 wild-type melanoma demonstrated an increase in the objective response rate, prolonged response durations, and improvement in progression-free survival. When used in combination with ipilimumab, the recommended dose and schedule is nivolumab 1 mg/kg administered as an intravenous infusion over 60 minutes, followed by ipilimumab on the same day every 3 weeks for 4 doses. The recommended subsequent dose of nivolumab, as a single agent, is 3 mg/kg as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity. For more information, visit www.opdivoyervoyhcp.com.
TriAcnéal Day Mattifying Lotion and Night Smoothing Lotion
Pierre Fabre Dermo-Cosmetique USA introduces 2 TriAcnéal lotions in the Avène line for the treatment and prevention of acne. TriAcnéal Day Mattifying Lotion provides hydrating and mattifying care. It is gentle enough for daily use and can be used alone or in combination with topical acne prescriptions. A trio of ingredients target acne: PCC enzyme (consisting of papain, sodium alginate, caprylyl glycol, and hexanediol) for exfoliation to counteract the formation of new comedones, Diolényl (consisting of caprylyl glycol linseedate and potassium sorbate)to treat existing blemishes and prevent new lesions, and glyceryl laurate to reduce oil production. TriAcnéal Night Smoothing Lotion works to reduce the appearance of acne scars and provides moisturization and redness-reduction benefits. The nighttime formula contains PCC enzyme and Diolényl as well as retinaldehyde to diminish visible signs of aging. For more information, visit www.aveneusa.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Cosentyx
Novartis Pharmaceuticals Corporation announces US Food and Drug Administration approval of 2 new indications for Cosentyx (secukinumab): to treat patients with active ankylosing spondylitis and active psoriatic arthritis. Cosentyx is a human monoclonal antibody that selectively binds to IL-17A and inhibits its interaction with the IL-17 receptor. Research suggests that IL-17A may play an important role in driving the body’s immune response in psoriasis, psoriatic arthritis, and ankylosing spondylitis. Cosentyx was approved in January 2015 for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. For more information, visit www.cosentyx.com.
Emverm
Impax Laboratories, Inc, receives US Food and Drug Administration approval for the supplemental new drug application of Emverm (mebendazole) 100-mg chewable tablets for the treatment of pinworm and certain worm infections. Emverm is indicated for treatment of pinworm, whipworm, common roundworm, common hookworm, and American hookworm in single or mixed infections. Emverm is expected to become available early in the second quarter of 2016. For more information, visit www.impaxlabs.com.
Keytruda
Merck & Co, Inc, announces US Food and Drug Administration approval of an expanded indication for Keytruda (pembrolizumab) that includes the first-line treatment of patients with unresectable or metastatic melanoma. Keytruda is indicated in the United States at a dose of 2 mg/kg administered as an intravenous infusion over 30 minutes every 3 weeks. Keytruda is an anti–programmed death receptor-1 therapy that works by increasing the ability of the body’s immune system to help detect and fight tumor cells. For more information, visit www.keytruda.com.
Opdivo + Yervoy Regimen
The US Food and Drug Administration has granted accelerated approval of nivolumab (Opdivo) in combination with ipilimumab (Yervoy) for the treatment of patients with BRAF V600 wild-type unresectable or metastatic melanoma. An international, multicenter, double-blind, randomized, active-controlled trial in patients who were previously untreated for unresectable or metastatic BRAF V600 wild-type melanoma demonstrated an increase in the objective response rate, prolonged response durations, and improvement in progression-free survival. When used in combination with ipilimumab, the recommended dose and schedule is nivolumab 1 mg/kg administered as an intravenous infusion over 60 minutes, followed by ipilimumab on the same day every 3 weeks for 4 doses. The recommended subsequent dose of nivolumab, as a single agent, is 3 mg/kg as an intravenous infusion over 60 minutes every 2 weeks until disease progression or unacceptable toxicity. For more information, visit www.opdivoyervoyhcp.com.
TriAcnéal Day Mattifying Lotion and Night Smoothing Lotion
Pierre Fabre Dermo-Cosmetique USA introduces 2 TriAcnéal lotions in the Avène line for the treatment and prevention of acne. TriAcnéal Day Mattifying Lotion provides hydrating and mattifying care. It is gentle enough for daily use and can be used alone or in combination with topical acne prescriptions. A trio of ingredients target acne: PCC enzyme (consisting of papain, sodium alginate, caprylyl glycol, and hexanediol) for exfoliation to counteract the formation of new comedones, Diolényl (consisting of caprylyl glycol linseedate and potassium sorbate)to treat existing blemishes and prevent new lesions, and glyceryl laurate to reduce oil production. TriAcnéal Night Smoothing Lotion works to reduce the appearance of acne scars and provides moisturization and redness-reduction benefits. The nighttime formula contains PCC enzyme and Diolényl as well as retinaldehyde to diminish visible signs of aging. For more information, visit www.aveneusa.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Product Update: FemTouch, Lunette Menstrual Cups, ROCA Test, NextGen Home Sperm Banking Kit

CO2 Laser To Treat Vaginal Conditions FemTouch™ FemTouch™ is a fractional CO2 laser that addresses vaginal health-related conditions such as stress urinary incontinence, vaginal laxity, and vaginal atrophy. Lumenis® says that FemTouch promotes the restoration of the vaginal mucous epithelium layer. The laser is applied along the vaginal wall, resulting in gentle, controlled ablation and coagulation of the vaginal lining. Lumenis states that the FemTouch in-office procedure takes only a few minutes, is efficient (with 2 to 4 treatments needed), and requires no anesthesia or special postprocedural care. The treatment is associated with minimal discomfort for patients, according to Lumenis, and some women being treated with FemTouch report improvement in their condition after 1 or 2 procedures.
FemTouch is designed for use with the Lumenis AcuPulse™ MultiMode™ SuperPulse™ CO2 Laser System, offering 10 built-in treatment modes.
FOR MORE INFORMATION, VISIT: www.lumenis.com
According to the manufacturer’s instructions, a woman folds and inserts the cup in the lower vagina above the pelvic bone. She empties the cup 2 to 4 times per day and can use it overnight. Between uses, she rinses the cup with water and washes it with warm soapy water before reinsertion. When water is not available, toilet paper can be used for cleaning. For thorough cleaning, a woman can place the cup in boiling water then disinfect it with alcohol. Lunette should be cleaned after menses and stored in its own bag. The FDA recommends replacing the cup every 2 to 3 years.
Lunette is designed for all women, including those who have not had sexual intercourse and those who use an IUD or contraceptive ring (consult a physician). Lunette is available in 2 sizes: Model 1 is generally for those with light to moderate flow; Model 2 is recommended for normal or heavier flow. A detailed sizing guide is found on the website.
FOR MORE INFORMATION, VISIT: www.lunette.com/us

Detect Ovarian Cancer EarlyThe ROCA® Test from Abcodia offers early detection of ovarian cancer. Data reported from a large prospective randomized controlled study recently published in The Lancet demonstrate that a screening strategy using ROCA (Risk of Ovarian Cancer Algorithm) may reduce ovarian cancer mortality by an estimated 20%.
The ROCA Test uses a woman’s age, menopausal status, risk status, and serial blood measurements of CA 125 to produce a score that indicates current likelihood of having ovarian cancer. The initial risk assessment is then modified based on how closely a patient’s CA 125 profile matches the profiles seen in healthy women and women with ovarian cancer.
The test is intended for postmenopausal women between the ages of 50 and 85 years with no known risk factors other than age and for women between the ages of 35 and 85 years who are considered high risk due to family history of ovarian or breast cancer, have BRCA1 or BRCA2 gene mutations, or have Lynch Syndrome. The ROCA Test is now available in 15 states and the District of Columbia, and will be offered to additional areas of the United States in 2016.
After purchasing a ROCA Test online, the patient will receive an instructional kit, which she will take to her physician to review her medical history and sign a consent form. A blood sample will be drawn and sent to the ROCA Test laboratory. The physician will receive and share the test results with the patient. High-risk patients are encouraged to complete 3 tests per year.
FOR MORE INFORMATION, VISIT: www.rocatest.com
A semen sample can be collected at home and then shipped to the sperm banking facilities at Cleveland Clinic’s Andrology Center in Cleveland,Ohio. There, the sperm will be cryopreserved for as long as desired; an annual storage fee will be charged.
FOR MORE INFORMATION, VISIT: www.fairhavenhealth.com/nextgen-home-sperm-banking-kit.html

CO2 Laser To Treat Vaginal Conditions FemTouch™ FemTouch™ is a fractional CO2 laser that addresses vaginal health-related conditions such as stress urinary incontinence, vaginal laxity, and vaginal atrophy. Lumenis® says that FemTouch promotes the restoration of the vaginal mucous epithelium layer. The laser is applied along the vaginal wall, resulting in gentle, controlled ablation and coagulation of the vaginal lining. Lumenis states that the FemTouch in-office procedure takes only a few minutes, is efficient (with 2 to 4 treatments needed), and requires no anesthesia or special postprocedural care. The treatment is associated with minimal discomfort for patients, according to Lumenis, and some women being treated with FemTouch report improvement in their condition after 1 or 2 procedures.
FemTouch is designed for use with the Lumenis AcuPulse™ MultiMode™ SuperPulse™ CO2 Laser System, offering 10 built-in treatment modes.
FOR MORE INFORMATION, VISIT: www.lumenis.com
According to the manufacturer’s instructions, a woman folds and inserts the cup in the lower vagina above the pelvic bone. She empties the cup 2 to 4 times per day and can use it overnight. Between uses, she rinses the cup with water and washes it with warm soapy water before reinsertion. When water is not available, toilet paper can be used for cleaning. For thorough cleaning, a woman can place the cup in boiling water then disinfect it with alcohol. Lunette should be cleaned after menses and stored in its own bag. The FDA recommends replacing the cup every 2 to 3 years.
Lunette is designed for all women, including those who have not had sexual intercourse and those who use an IUD or contraceptive ring (consult a physician). Lunette is available in 2 sizes: Model 1 is generally for those with light to moderate flow; Model 2 is recommended for normal or heavier flow. A detailed sizing guide is found on the website.
FOR MORE INFORMATION, VISIT: www.lunette.com/us

Detect Ovarian Cancer EarlyThe ROCA® Test from Abcodia offers early detection of ovarian cancer. Data reported from a large prospective randomized controlled study recently published in The Lancet demonstrate that a screening strategy using ROCA (Risk of Ovarian Cancer Algorithm) may reduce ovarian cancer mortality by an estimated 20%.
The ROCA Test uses a woman’s age, menopausal status, risk status, and serial blood measurements of CA 125 to produce a score that indicates current likelihood of having ovarian cancer. The initial risk assessment is then modified based on how closely a patient’s CA 125 profile matches the profiles seen in healthy women and women with ovarian cancer.
The test is intended for postmenopausal women between the ages of 50 and 85 years with no known risk factors other than age and for women between the ages of 35 and 85 years who are considered high risk due to family history of ovarian or breast cancer, have BRCA1 or BRCA2 gene mutations, or have Lynch Syndrome. The ROCA Test is now available in 15 states and the District of Columbia, and will be offered to additional areas of the United States in 2016.
After purchasing a ROCA Test online, the patient will receive an instructional kit, which she will take to her physician to review her medical history and sign a consent form. A blood sample will be drawn and sent to the ROCA Test laboratory. The physician will receive and share the test results with the patient. High-risk patients are encouraged to complete 3 tests per year.
FOR MORE INFORMATION, VISIT: www.rocatest.com

A semen sample can be collected at home and then shipped to the sperm banking facilities at Cleveland Clinic’s Andrology Center in Cleveland,Ohio. There, the sperm will be cryopreserved for as long as desired; an annual storage fee will be charged.
FOR MORE INFORMATION, VISIT: www.fairhavenhealth.com/nextgen-home-sperm-banking-kit.html

CO2 Laser To Treat Vaginal Conditions FemTouch™ FemTouch™ is a fractional CO2 laser that addresses vaginal health-related conditions such as stress urinary incontinence, vaginal laxity, and vaginal atrophy. Lumenis® says that FemTouch promotes the restoration of the vaginal mucous epithelium layer. The laser is applied along the vaginal wall, resulting in gentle, controlled ablation and coagulation of the vaginal lining. Lumenis states that the FemTouch in-office procedure takes only a few minutes, is efficient (with 2 to 4 treatments needed), and requires no anesthesia or special postprocedural care. The treatment is associated with minimal discomfort for patients, according to Lumenis, and some women being treated with FemTouch report improvement in their condition after 1 or 2 procedures.
FemTouch is designed for use with the Lumenis AcuPulse™ MultiMode™ SuperPulse™ CO2 Laser System, offering 10 built-in treatment modes.
FOR MORE INFORMATION, VISIT: www.lumenis.com
According to the manufacturer’s instructions, a woman folds and inserts the cup in the lower vagina above the pelvic bone. She empties the cup 2 to 4 times per day and can use it overnight. Between uses, she rinses the cup with water and washes it with warm soapy water before reinsertion. When water is not available, toilet paper can be used for cleaning. For thorough cleaning, a woman can place the cup in boiling water then disinfect it with alcohol. Lunette should be cleaned after menses and stored in its own bag. The FDA recommends replacing the cup every 2 to 3 years.
Lunette is designed for all women, including those who have not had sexual intercourse and those who use an IUD or contraceptive ring (consult a physician). Lunette is available in 2 sizes: Model 1 is generally for those with light to moderate flow; Model 2 is recommended for normal or heavier flow. A detailed sizing guide is found on the website.
FOR MORE INFORMATION, VISIT: www.lunette.com/us

Detect Ovarian Cancer EarlyThe ROCA® Test from Abcodia offers early detection of ovarian cancer. Data reported from a large prospective randomized controlled study recently published in The Lancet demonstrate that a screening strategy using ROCA (Risk of Ovarian Cancer Algorithm) may reduce ovarian cancer mortality by an estimated 20%.
The ROCA Test uses a woman’s age, menopausal status, risk status, and serial blood measurements of CA 125 to produce a score that indicates current likelihood of having ovarian cancer. The initial risk assessment is then modified based on how closely a patient’s CA 125 profile matches the profiles seen in healthy women and women with ovarian cancer.
The test is intended for postmenopausal women between the ages of 50 and 85 years with no known risk factors other than age and for women between the ages of 35 and 85 years who are considered high risk due to family history of ovarian or breast cancer, have BRCA1 or BRCA2 gene mutations, or have Lynch Syndrome. The ROCA Test is now available in 15 states and the District of Columbia, and will be offered to additional areas of the United States in 2016.
After purchasing a ROCA Test online, the patient will receive an instructional kit, which she will take to her physician to review her medical history and sign a consent form. A blood sample will be drawn and sent to the ROCA Test laboratory. The physician will receive and share the test results with the patient. High-risk patients are encouraged to complete 3 tests per year.
FOR MORE INFORMATION, VISIT: www.rocatest.com

A semen sample can be collected at home and then shipped to the sperm banking facilities at Cleveland Clinic’s Andrology Center in Cleveland,Ohio. There, the sperm will be cryopreserved for as long as desired; an annual storage fee will be charged.
FOR MORE INFORMATION, VISIT: www.fairhavenhealth.com/nextgen-home-sperm-banking-kit.html
Product News: 11 2015
Carmex Winter Mint Lip Balm Click Stick
Carma Laboratories, Inc, introduces limited-edition Carmex Winter Mint Lip Balm Click Sticks. The new seasonal flavor, which comes in convenient click sticks that glide on lips easily, is available to consumers as part of specially marked 2-pack and 4-pack promotions. Both offers combine the Carmex Original Lip Balm Click Stick or Carmex Original Lip Balm Tube with a bonus Winter Mint seasonal stick and will be available at select retailers while supplies last. For more information, visit www.mycarmex.com.
Daytrana Patch
The US Food and Drug Administration (FDA) has added a new warning of chemical leukoderma to the drug label for the Daytrana patch (methylphenidate transdermal system)(Noven Therapeutics, LLC), which treats attention deficit hyperactivity disorder in children and adolescents. Chemical leukoderma is a condition that causes loss of skin color due to repeated exposure to specific chemical compounds. The areas of skin color loss described with the Daytrana patch ranged up to 8 inches in diameter. The FDA recommends that patients or their caregivers should watch for new areas of lighter skin, especially under the drug patch, and immediately report these changes to a health care professional. For more information, visit www.fda.gov/MedWatch.
Enstilar Foam
LEO Pharma Inc announces US Food and Drug Administration approval of Enstilar (calcipotriene and metamethasone dipropionate) Foam 0.005%/0.064% for topical treatment of plaque psoriasis in patients aged 18 years and older. Enstilar is an alcohol-free foam formulation in a pressurized spray can that allows application across large body areas of plaque psoriasis and should be applied to affected areas once daily for up to 4 weeks. The approval was based on pivotal clinical trial data at week 4 that also showed patients using Enstilar achieved efficacy as early as week 2. For more information, visit www.enstilar.com.
IMLYGIC
Amgen Inc announces that the US Food and Drug Administration has approved the Biologics License Application for IMLYGIC (talimogene laherparepvec), a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery. IMLYGIC is the first oncolytic viral therapy approved based on therapeutic benefit demonstrated in a pivotal study, and variability of dosing from patient to patient is expected. IMLYGIC has not been shown to improve overall survival or have an effect on visceral metastases, and treatment is contraindicated in immunocompromised patients. For more information, visit www.imlygic.com.
Juvéderm Ultra XC
Allergan, Inc announces US Food and Drug Administration approval to market Juvéderm Ultra XC gel for injection into the lips and perioral area for lip augmentation in patients over the age of 21 years. Juvéderm Ultra XC is a smooth gel formulation made up of a modified form of hyaluronic acid, a naturally occurring sugar found in the human body whose role is to deliver nutrients and help the skin retain its natural moisture and softness. The gel formulation also contains a small amount of lidocaine, which improves the comfort of the injection. For more information, visit www.juvederm.com.
Opdivo + Yervoy Regimen
Bristol-Myers Squibb Company obtains US Food and Drug Administration approval for the Opdivo (nivolumab) + Yervoy (ipilimumab) regimen in BRAF V600 wild-type unresectable or metastatic melanoma. The approval, which is based on data from a pivotal study, marks the first approval of a regimen of 2 immuno-oncology agents in cancer. This indication is approved under accelerated approval based on tumor response rate and durability of response. Opdivo and Yervoy are immune checkpoint inhibitors that target separate, distinct, and complementary checkpoint pathways (PD-1 and CTLA-4). The mechanism of action involves dual immune checkpoint inhibition resulting in increased antitumor activity. For more information, visit www.bms.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Carmex Winter Mint Lip Balm Click Stick
Carma Laboratories, Inc, introduces limited-edition Carmex Winter Mint Lip Balm Click Sticks. The new seasonal flavor, which comes in convenient click sticks that glide on lips easily, is available to consumers as part of specially marked 2-pack and 4-pack promotions. Both offers combine the Carmex Original Lip Balm Click Stick or Carmex Original Lip Balm Tube with a bonus Winter Mint seasonal stick and will be available at select retailers while supplies last. For more information, visit www.mycarmex.com.
Daytrana Patch
The US Food and Drug Administration (FDA) has added a new warning of chemical leukoderma to the drug label for the Daytrana patch (methylphenidate transdermal system)(Noven Therapeutics, LLC), which treats attention deficit hyperactivity disorder in children and adolescents. Chemical leukoderma is a condition that causes loss of skin color due to repeated exposure to specific chemical compounds. The areas of skin color loss described with the Daytrana patch ranged up to 8 inches in diameter. The FDA recommends that patients or their caregivers should watch for new areas of lighter skin, especially under the drug patch, and immediately report these changes to a health care professional. For more information, visit www.fda.gov/MedWatch.
Enstilar Foam
LEO Pharma Inc announces US Food and Drug Administration approval of Enstilar (calcipotriene and metamethasone dipropionate) Foam 0.005%/0.064% for topical treatment of plaque psoriasis in patients aged 18 years and older. Enstilar is an alcohol-free foam formulation in a pressurized spray can that allows application across large body areas of plaque psoriasis and should be applied to affected areas once daily for up to 4 weeks. The approval was based on pivotal clinical trial data at week 4 that also showed patients using Enstilar achieved efficacy as early as week 2. For more information, visit www.enstilar.com.
IMLYGIC
Amgen Inc announces that the US Food and Drug Administration has approved the Biologics License Application for IMLYGIC (talimogene laherparepvec), a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery. IMLYGIC is the first oncolytic viral therapy approved based on therapeutic benefit demonstrated in a pivotal study, and variability of dosing from patient to patient is expected. IMLYGIC has not been shown to improve overall survival or have an effect on visceral metastases, and treatment is contraindicated in immunocompromised patients. For more information, visit www.imlygic.com.
Juvéderm Ultra XC
Allergan, Inc announces US Food and Drug Administration approval to market Juvéderm Ultra XC gel for injection into the lips and perioral area for lip augmentation in patients over the age of 21 years. Juvéderm Ultra XC is a smooth gel formulation made up of a modified form of hyaluronic acid, a naturally occurring sugar found in the human body whose role is to deliver nutrients and help the skin retain its natural moisture and softness. The gel formulation also contains a small amount of lidocaine, which improves the comfort of the injection. For more information, visit www.juvederm.com.
Opdivo + Yervoy Regimen
Bristol-Myers Squibb Company obtains US Food and Drug Administration approval for the Opdivo (nivolumab) + Yervoy (ipilimumab) regimen in BRAF V600 wild-type unresectable or metastatic melanoma. The approval, which is based on data from a pivotal study, marks the first approval of a regimen of 2 immuno-oncology agents in cancer. This indication is approved under accelerated approval based on tumor response rate and durability of response. Opdivo and Yervoy are immune checkpoint inhibitors that target separate, distinct, and complementary checkpoint pathways (PD-1 and CTLA-4). The mechanism of action involves dual immune checkpoint inhibition resulting in increased antitumor activity. For more information, visit www.bms.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Carmex Winter Mint Lip Balm Click Stick
Carma Laboratories, Inc, introduces limited-edition Carmex Winter Mint Lip Balm Click Sticks. The new seasonal flavor, which comes in convenient click sticks that glide on lips easily, is available to consumers as part of specially marked 2-pack and 4-pack promotions. Both offers combine the Carmex Original Lip Balm Click Stick or Carmex Original Lip Balm Tube with a bonus Winter Mint seasonal stick and will be available at select retailers while supplies last. For more information, visit www.mycarmex.com.
Daytrana Patch
The US Food and Drug Administration (FDA) has added a new warning of chemical leukoderma to the drug label for the Daytrana patch (methylphenidate transdermal system)(Noven Therapeutics, LLC), which treats attention deficit hyperactivity disorder in children and adolescents. Chemical leukoderma is a condition that causes loss of skin color due to repeated exposure to specific chemical compounds. The areas of skin color loss described with the Daytrana patch ranged up to 8 inches in diameter. The FDA recommends that patients or their caregivers should watch for new areas of lighter skin, especially under the drug patch, and immediately report these changes to a health care professional. For more information, visit www.fda.gov/MedWatch.
Enstilar Foam
LEO Pharma Inc announces US Food and Drug Administration approval of Enstilar (calcipotriene and metamethasone dipropionate) Foam 0.005%/0.064% for topical treatment of plaque psoriasis in patients aged 18 years and older. Enstilar is an alcohol-free foam formulation in a pressurized spray can that allows application across large body areas of plaque psoriasis and should be applied to affected areas once daily for up to 4 weeks. The approval was based on pivotal clinical trial data at week 4 that also showed patients using Enstilar achieved efficacy as early as week 2. For more information, visit www.enstilar.com.
IMLYGIC
Amgen Inc announces that the US Food and Drug Administration has approved the Biologics License Application for IMLYGIC (talimogene laherparepvec), a genetically modified oncolytic viral therapy indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with melanoma recurrent after initial surgery. IMLYGIC is the first oncolytic viral therapy approved based on therapeutic benefit demonstrated in a pivotal study, and variability of dosing from patient to patient is expected. IMLYGIC has not been shown to improve overall survival or have an effect on visceral metastases, and treatment is contraindicated in immunocompromised patients. For more information, visit www.imlygic.com.
Juvéderm Ultra XC
Allergan, Inc announces US Food and Drug Administration approval to market Juvéderm Ultra XC gel for injection into the lips and perioral area for lip augmentation in patients over the age of 21 years. Juvéderm Ultra XC is a smooth gel formulation made up of a modified form of hyaluronic acid, a naturally occurring sugar found in the human body whose role is to deliver nutrients and help the skin retain its natural moisture and softness. The gel formulation also contains a small amount of lidocaine, which improves the comfort of the injection. For more information, visit www.juvederm.com.
Opdivo + Yervoy Regimen
Bristol-Myers Squibb Company obtains US Food and Drug Administration approval for the Opdivo (nivolumab) + Yervoy (ipilimumab) regimen in BRAF V600 wild-type unresectable or metastatic melanoma. The approval, which is based on data from a pivotal study, marks the first approval of a regimen of 2 immuno-oncology agents in cancer. This indication is approved under accelerated approval based on tumor response rate and durability of response. Opdivo and Yervoy are immune checkpoint inhibitors that target separate, distinct, and complementary checkpoint pathways (PD-1 and CTLA-4). The mechanism of action involves dual immune checkpoint inhibition resulting in increased antitumor activity. For more information, visit www.bms.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
10 Apps for Veterans to Improve Health and Wellness
These days you can do anything from a phone application; buy theater tickets, order groceries, and watch television. So why not do something better for your health? Here is a list of apps to assist veterans and active-duty service men and women in improving their overall wellness via smart phones.
1. CBT-i Coach
Created through a collaboration of the VA National Center for PTSD, the DoD National Center for Telehealth and Technology, and Stanford School of Medicine, the cognitive behavioral therapy for insomnia (CBT-i) application assists users with symptoms of insomnia by improving their sleep habits for a better daytime experience.
2. ACT Coach
The Acceptance and Commitment Therapy (ACT) Coach app is a tool for use while receiving face-to-face ACT treatment. It assists the user in coping with emotions and symptoms related to mental health conditions by providing mindfulness and acceptance strategies.
Breathe2Relax is a stress management tool designed by the National Center for Telehealth and Technology (T2) to help the user focus on “diaphragmatic breathing.” Through exercises in the app, the user learns to concentrate on breathing to provide deep relaxation and a stable mood that could be strained from PTSD or traumatic brain injury.
T2 MoodTracker allows the user to record their daily mood and psychological health. Within the app users can rate their mood, set daily reminders to keep track of their daily health, record new events such as starting therapy, and share information with health care providers.
5. Parenting2Go
As part of the DoD/VA Integrated Mental Health Strategy the Parenting2Go app was designed to assist service members and veterans build closer and healthier relationships with their families after a deployment. The app provides tips and tools on how to be more “present,” how to deal with being overwhelmed by the demands of parenting, and how to get support for their parenting efforts.
Developed by psychologists the Mindfulness Coach app aids the user in learning mindfulness to help focus their attention on the present experience to reduce worrying and stress. The app provides walkthroughs of various forms of mindfulness activities, a log for tracking practice, and educational materials.
7. CEMM Virtual Medical Center
The Center of Excellence for Medical Multimedia (CEMM) app provides TRICARE customer service to service members and their families. By providing an educational resource tool, information on the nearest medical treatment facility, news, and information on various TRICARE plans users can access TRICARE services anywhere.
The MOVE! Coach App helps veterans, service members, and their families in their weight loss journeys. The app provides self-management guides on a 19-week program, weight and diet diaries, a progress tracker, and other resources on how to breakthrough personal barriers to a healthier lifestyle.
In collaboration between the American Heart Association and the American College of Cardiology this app calculates the patient risk for developing atherosclerotic cardiovascular disease (ASCVD). Although primarily for health care providers veterans can assess their cardiovascular risk by indicating lifestyle choices, cholesterol levels, and body weight to determine the 10-year and lifetime risk for developing ASCVD.
10. 311VET
311VET is a tool designed for veterans who have questions and need answers about VA Benefits anytime and anywhere. The app allows veterans to receive information on survivors benefits, pensions, life insurance and more from the convenience of their smart phone or through text messaging if the user doesn’t have a smart phone.
Visit your Android or Apple iOS app store to download these apps.
These days you can do anything from a phone application; buy theater tickets, order groceries, and watch television. So why not do something better for your health? Here is a list of apps to assist veterans and active-duty service men and women in improving their overall wellness via smart phones.
1. CBT-i Coach
Created through a collaboration of the VA National Center for PTSD, the DoD National Center for Telehealth and Technology, and Stanford School of Medicine, the cognitive behavioral therapy for insomnia (CBT-i) application assists users with symptoms of insomnia by improving their sleep habits for a better daytime experience.
2. ACT Coach
The Acceptance and Commitment Therapy (ACT) Coach app is a tool for use while receiving face-to-face ACT treatment. It assists the user in coping with emotions and symptoms related to mental health conditions by providing mindfulness and acceptance strategies.
Breathe2Relax is a stress management tool designed by the National Center for Telehealth and Technology (T2) to help the user focus on “diaphragmatic breathing.” Through exercises in the app, the user learns to concentrate on breathing to provide deep relaxation and a stable mood that could be strained from PTSD or traumatic brain injury.
T2 MoodTracker allows the user to record their daily mood and psychological health. Within the app users can rate their mood, set daily reminders to keep track of their daily health, record new events such as starting therapy, and share information with health care providers.
5. Parenting2Go
As part of the DoD/VA Integrated Mental Health Strategy the Parenting2Go app was designed to assist service members and veterans build closer and healthier relationships with their families after a deployment. The app provides tips and tools on how to be more “present,” how to deal with being overwhelmed by the demands of parenting, and how to get support for their parenting efforts.
Developed by psychologists the Mindfulness Coach app aids the user in learning mindfulness to help focus their attention on the present experience to reduce worrying and stress. The app provides walkthroughs of various forms of mindfulness activities, a log for tracking practice, and educational materials.
7. CEMM Virtual Medical Center
The Center of Excellence for Medical Multimedia (CEMM) app provides TRICARE customer service to service members and their families. By providing an educational resource tool, information on the nearest medical treatment facility, news, and information on various TRICARE plans users can access TRICARE services anywhere.
The MOVE! Coach App helps veterans, service members, and their families in their weight loss journeys. The app provides self-management guides on a 19-week program, weight and diet diaries, a progress tracker, and other resources on how to breakthrough personal barriers to a healthier lifestyle.
In collaboration between the American Heart Association and the American College of Cardiology this app calculates the patient risk for developing atherosclerotic cardiovascular disease (ASCVD). Although primarily for health care providers veterans can assess their cardiovascular risk by indicating lifestyle choices, cholesterol levels, and body weight to determine the 10-year and lifetime risk for developing ASCVD.
10. 311VET
311VET is a tool designed for veterans who have questions and need answers about VA Benefits anytime and anywhere. The app allows veterans to receive information on survivors benefits, pensions, life insurance and more from the convenience of their smart phone or through text messaging if the user doesn’t have a smart phone.
Visit your Android or Apple iOS app store to download these apps.
These days you can do anything from a phone application; buy theater tickets, order groceries, and watch television. So why not do something better for your health? Here is a list of apps to assist veterans and active-duty service men and women in improving their overall wellness via smart phones.
1. CBT-i Coach
Created through a collaboration of the VA National Center for PTSD, the DoD National Center for Telehealth and Technology, and Stanford School of Medicine, the cognitive behavioral therapy for insomnia (CBT-i) application assists users with symptoms of insomnia by improving their sleep habits for a better daytime experience.
2. ACT Coach
The Acceptance and Commitment Therapy (ACT) Coach app is a tool for use while receiving face-to-face ACT treatment. It assists the user in coping with emotions and symptoms related to mental health conditions by providing mindfulness and acceptance strategies.
Breathe2Relax is a stress management tool designed by the National Center for Telehealth and Technology (T2) to help the user focus on “diaphragmatic breathing.” Through exercises in the app, the user learns to concentrate on breathing to provide deep relaxation and a stable mood that could be strained from PTSD or traumatic brain injury.
T2 MoodTracker allows the user to record their daily mood and psychological health. Within the app users can rate their mood, set daily reminders to keep track of their daily health, record new events such as starting therapy, and share information with health care providers.
5. Parenting2Go
As part of the DoD/VA Integrated Mental Health Strategy the Parenting2Go app was designed to assist service members and veterans build closer and healthier relationships with their families after a deployment. The app provides tips and tools on how to be more “present,” how to deal with being overwhelmed by the demands of parenting, and how to get support for their parenting efforts.
Developed by psychologists the Mindfulness Coach app aids the user in learning mindfulness to help focus their attention on the present experience to reduce worrying and stress. The app provides walkthroughs of various forms of mindfulness activities, a log for tracking practice, and educational materials.
7. CEMM Virtual Medical Center
The Center of Excellence for Medical Multimedia (CEMM) app provides TRICARE customer service to service members and their families. By providing an educational resource tool, information on the nearest medical treatment facility, news, and information on various TRICARE plans users can access TRICARE services anywhere.
The MOVE! Coach App helps veterans, service members, and their families in their weight loss journeys. The app provides self-management guides on a 19-week program, weight and diet diaries, a progress tracker, and other resources on how to breakthrough personal barriers to a healthier lifestyle.
In collaboration between the American Heart Association and the American College of Cardiology this app calculates the patient risk for developing atherosclerotic cardiovascular disease (ASCVD). Although primarily for health care providers veterans can assess their cardiovascular risk by indicating lifestyle choices, cholesterol levels, and body weight to determine the 10-year and lifetime risk for developing ASCVD.
10. 311VET
311VET is a tool designed for veterans who have questions and need answers about VA Benefits anytime and anywhere. The app allows veterans to receive information on survivors benefits, pensions, life insurance and more from the convenience of their smart phone or through text messaging if the user doesn’t have a smart phone.
Visit your Android or Apple iOS app store to download these apps.
Product News: 10 2015
Bellafill
Suneva Medical, Inc, recognized the treatment of acne scars was an unmet need, which led to research supporting a new indication for the dermal filler Bellafill for the treatment of moderate to severe, atrophic, distensible facial acne scars on the cheek in patients older than 21 years. Bellafill is a smooth, collagen-based dermal filler with polymethylmethacrylate (PMMA) microspheres. The collagen gel provides immediate volume and lift to correct the scar, and the PMMA microspheres remain in place and provide structural support for smoother-looking skin. Bellafill is not indicated for ice-pick scars. Although Bellafill can be used in all skin types, the patient’s acne cannot be active. Results have been observed to last 12 months. Patients may continue with ongoing topical treatments but should discontinue any topical treatment the night after injection. For more information, visit www.bellafill.com.
Cutanea Life Sciences
Cutanea Life Sciences renews its commitment to focusing on the unmet needs of patients to develop innovative technologies and therapeutic applications. In 2012, Maruho Co, Ltd, acquired Cutanea Life Sciences, solidifying the financial resources needed to create market-leading products to treat diseases and disorders of the skin and subcutaneous tissue. Cutaneous Life Sciences corporate headquarters are located in Wayne, Pennsylvania. Robert J. Bitterman Sr has served as president and chief executive officer since 2005, following executive leadership roles for other dermatology companies. For more information, visit www.cutanealife.com.
Humira
AbbVie Inc receives US Food and Drug Administration approval of Humira (adalimumab) for the treatment of moderate to severe hidradenitis suppurativa (HS), offering patients with HS a much-needed treatment for this chronic debilitating disease. The HS indication follows approvals for rheumatoid arthritis, plaque psoriasis, Crohn disease, ulcerative colitis, psoriatic arthritis, and ankylosing spondylitis. For more information, visit www.humira.com.
Teflaro
Actavis, Inc, announces US Food and Drug Administration approval of the supplemental new drug application to update the label for Teflaro (ceftaroline fosamil) for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP). With this updated label, Teflaro also is now approved to be administered by intravenous infusion over 5 minutes to 1 hour in adult patients 18 years and older, providing increased flexibility in dosing. Teflaro was first approved in 2010 for the treatment of adults with CABP and ABSSSI due to designated susceptible pathogens. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Bellafill
Suneva Medical, Inc, recognized the treatment of acne scars was an unmet need, which led to research supporting a new indication for the dermal filler Bellafill for the treatment of moderate to severe, atrophic, distensible facial acne scars on the cheek in patients older than 21 years. Bellafill is a smooth, collagen-based dermal filler with polymethylmethacrylate (PMMA) microspheres. The collagen gel provides immediate volume and lift to correct the scar, and the PMMA microspheres remain in place and provide structural support for smoother-looking skin. Bellafill is not indicated for ice-pick scars. Although Bellafill can be used in all skin types, the patient’s acne cannot be active. Results have been observed to last 12 months. Patients may continue with ongoing topical treatments but should discontinue any topical treatment the night after injection. For more information, visit www.bellafill.com.
Cutanea Life Sciences
Cutanea Life Sciences renews its commitment to focusing on the unmet needs of patients to develop innovative technologies and therapeutic applications. In 2012, Maruho Co, Ltd, acquired Cutanea Life Sciences, solidifying the financial resources needed to create market-leading products to treat diseases and disorders of the skin and subcutaneous tissue. Cutaneous Life Sciences corporate headquarters are located in Wayne, Pennsylvania. Robert J. Bitterman Sr has served as president and chief executive officer since 2005, following executive leadership roles for other dermatology companies. For more information, visit www.cutanealife.com.
Humira
AbbVie Inc receives US Food and Drug Administration approval of Humira (adalimumab) for the treatment of moderate to severe hidradenitis suppurativa (HS), offering patients with HS a much-needed treatment for this chronic debilitating disease. The HS indication follows approvals for rheumatoid arthritis, plaque psoriasis, Crohn disease, ulcerative colitis, psoriatic arthritis, and ankylosing spondylitis. For more information, visit www.humira.com.
Teflaro
Actavis, Inc, announces US Food and Drug Administration approval of the supplemental new drug application to update the label for Teflaro (ceftaroline fosamil) for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP). With this updated label, Teflaro also is now approved to be administered by intravenous infusion over 5 minutes to 1 hour in adult patients 18 years and older, providing increased flexibility in dosing. Teflaro was first approved in 2010 for the treatment of adults with CABP and ABSSSI due to designated susceptible pathogens. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Bellafill
Suneva Medical, Inc, recognized the treatment of acne scars was an unmet need, which led to research supporting a new indication for the dermal filler Bellafill for the treatment of moderate to severe, atrophic, distensible facial acne scars on the cheek in patients older than 21 years. Bellafill is a smooth, collagen-based dermal filler with polymethylmethacrylate (PMMA) microspheres. The collagen gel provides immediate volume and lift to correct the scar, and the PMMA microspheres remain in place and provide structural support for smoother-looking skin. Bellafill is not indicated for ice-pick scars. Although Bellafill can be used in all skin types, the patient’s acne cannot be active. Results have been observed to last 12 months. Patients may continue with ongoing topical treatments but should discontinue any topical treatment the night after injection. For more information, visit www.bellafill.com.
Cutanea Life Sciences
Cutanea Life Sciences renews its commitment to focusing on the unmet needs of patients to develop innovative technologies and therapeutic applications. In 2012, Maruho Co, Ltd, acquired Cutanea Life Sciences, solidifying the financial resources needed to create market-leading products to treat diseases and disorders of the skin and subcutaneous tissue. Cutaneous Life Sciences corporate headquarters are located in Wayne, Pennsylvania. Robert J. Bitterman Sr has served as president and chief executive officer since 2005, following executive leadership roles for other dermatology companies. For more information, visit www.cutanealife.com.
Humira
AbbVie Inc receives US Food and Drug Administration approval of Humira (adalimumab) for the treatment of moderate to severe hidradenitis suppurativa (HS), offering patients with HS a much-needed treatment for this chronic debilitating disease. The HS indication follows approvals for rheumatoid arthritis, plaque psoriasis, Crohn disease, ulcerative colitis, psoriatic arthritis, and ankylosing spondylitis. For more information, visit www.humira.com.
Teflaro
Actavis, Inc, announces US Food and Drug Administration approval of the supplemental new drug application to update the label for Teflaro (ceftaroline fosamil) for the treatment of adult patients with acute bacterial skin and skin structure infections (ABSSSI) and community-acquired bacterial pneumonia (CABP). With this updated label, Teflaro also is now approved to be administered by intravenous infusion over 5 minutes to 1 hour in adult patients 18 years and older, providing increased flexibility in dosing. Teflaro was first approved in 2010 for the treatment of adults with CABP and ABSSSI due to designated susceptible pathogens. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Product News: 09 2015
Cyclosporine A
Immune Pharmaceuticals acquires a nanoparticle topical formulation of cyclosporine A for the treatment of psoriasis, atopic dermatitis, pemphigus vulgaris, and other severe inflammatory dermatoses. Researchers have incorporated cyclosporine A into biodegradable nanocapsules and have developed stable topical formulations that are able to achieve therapeutic cyclosporine A levels in the targeted skin layers. This topical treatment provides a potential alternative to oral cyclosporine A and other topical immunosuppressive drugs. For more information, visit www.immunepharmaceuticals.com.
Picato Gel Warning
The US Food and Drug Administration (FDA) issues a warning about reports of severe allergic reactions and herpes zoster associated with the use of Picato Gel (ingenol mebutate) for the treatment of actinic keratosis. The allergic reaction may include throat tightness, difficulty breathing, feeling faint, or swelling of the lips or tongue. The FDA received reports of cases involving severe eye injuries associated with Picato Gel not being used according to the instructions for use on the label. Changes to the labeling have been requested by the FDA to warn about these new safety risks. Patients should not use Picato Gel for a longer period or on an area of skin larger than instructed on the drug label. Patients also should avoid application in, near, and around the mouth, lips, and eye area. For more information, visit www.fda.gov/MedWatch.
Ximino Extended-Release Capsules
Sun Pharmaceutical Industries Ltd announces US Food and Drug Administration approval of the Supplemental New Drug Application for Ximino (minocycline hydrochloride) extended-release capsules (45, 90, and 135 mg). Ximino extended-release capsules are indicated for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 12 years and older. Ximino extended-release capsules are expected to be available for patients during the fourth quarter of 2015. For more information, visit www.sunpharma.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Cyclosporine A
Immune Pharmaceuticals acquires a nanoparticle topical formulation of cyclosporine A for the treatment of psoriasis, atopic dermatitis, pemphigus vulgaris, and other severe inflammatory dermatoses. Researchers have incorporated cyclosporine A into biodegradable nanocapsules and have developed stable topical formulations that are able to achieve therapeutic cyclosporine A levels in the targeted skin layers. This topical treatment provides a potential alternative to oral cyclosporine A and other topical immunosuppressive drugs. For more information, visit www.immunepharmaceuticals.com.
Picato Gel Warning
The US Food and Drug Administration (FDA) issues a warning about reports of severe allergic reactions and herpes zoster associated with the use of Picato Gel (ingenol mebutate) for the treatment of actinic keratosis. The allergic reaction may include throat tightness, difficulty breathing, feeling faint, or swelling of the lips or tongue. The FDA received reports of cases involving severe eye injuries associated with Picato Gel not being used according to the instructions for use on the label. Changes to the labeling have been requested by the FDA to warn about these new safety risks. Patients should not use Picato Gel for a longer period or on an area of skin larger than instructed on the drug label. Patients also should avoid application in, near, and around the mouth, lips, and eye area. For more information, visit www.fda.gov/MedWatch.
Ximino Extended-Release Capsules
Sun Pharmaceutical Industries Ltd announces US Food and Drug Administration approval of the Supplemental New Drug Application for Ximino (minocycline hydrochloride) extended-release capsules (45, 90, and 135 mg). Ximino extended-release capsules are indicated for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 12 years and older. Ximino extended-release capsules are expected to be available for patients during the fourth quarter of 2015. For more information, visit www.sunpharma.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Cyclosporine A
Immune Pharmaceuticals acquires a nanoparticle topical formulation of cyclosporine A for the treatment of psoriasis, atopic dermatitis, pemphigus vulgaris, and other severe inflammatory dermatoses. Researchers have incorporated cyclosporine A into biodegradable nanocapsules and have developed stable topical formulations that are able to achieve therapeutic cyclosporine A levels in the targeted skin layers. This topical treatment provides a potential alternative to oral cyclosporine A and other topical immunosuppressive drugs. For more information, visit www.immunepharmaceuticals.com.
Picato Gel Warning
The US Food and Drug Administration (FDA) issues a warning about reports of severe allergic reactions and herpes zoster associated with the use of Picato Gel (ingenol mebutate) for the treatment of actinic keratosis. The allergic reaction may include throat tightness, difficulty breathing, feeling faint, or swelling of the lips or tongue. The FDA received reports of cases involving severe eye injuries associated with Picato Gel not being used according to the instructions for use on the label. Changes to the labeling have been requested by the FDA to warn about these new safety risks. Patients should not use Picato Gel for a longer period or on an area of skin larger than instructed on the drug label. Patients also should avoid application in, near, and around the mouth, lips, and eye area. For more information, visit www.fda.gov/MedWatch.
Ximino Extended-Release Capsules
Sun Pharmaceutical Industries Ltd announces US Food and Drug Administration approval of the Supplemental New Drug Application for Ximino (minocycline hydrochloride) extended-release capsules (45, 90, and 135 mg). Ximino extended-release capsules are indicated for the treatment of inflammatory lesions of nonnodular moderate to severe acne vulgaris in patients 12 years and older. Ximino extended-release capsules are expected to be available for patients during the fourth quarter of 2015. For more information, visit www.sunpharma.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Product News: 08 2015
Epiduo Forte Gel
Galderma Laboratories, LP, announces US Food and Drug Administration approval of antibiotic-free Epiduo Forte (adapalene 0.3% and benzoyl peroxide 2.5%) Gel for the once-daily topical treatment of acne vulgaris. Epiduo Forte Gel contains a high concentration of adapalene, which works to unclog blocked pores and inhibits the release of proinflammatory mediators. Benzoyl peroxide offers antimicrobial properties. Epiduo Forte Gel can be considered for long-term use in patients with moderate to severe acne. The formulation is offered in a pump to provide convenient once-daily dosing. Epiduo Forte Gel will be available by prescription in early September 2015. For more information, visit www.galdermausa.com.
Odomzo
Novartis Pharmaceuticals Corporation obtains US Food and Drug Administration approval of Odomzo (sonidegib) 200-mg capsules for the treatment of adult patients with locally advanced basal cell carcinoma that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy. Odomzo is an oral selective smoothened (SMO) inhibitor. SMO is a molecule that regulates the hedgehog signaling pathway, which plays a critical role in stem cell maintenance and tissue repair as well as in advanced basal cell carcinoma. For more information, visit www.novartis.com.
Physical Matte UV Defense SPF 50
SkinCeuticals introduces Physical Matte UV Defense SPF 50 sunscreen. This 100% mineral sunscreen is formulated with oil-absorbing powders to help minimize the appearance of sweat and oil to ensure a long-lasting matte finish while also providing broad-spectrum UVA/UVB protection. Physical Matte UV DefenseSPF 50 sunscreen is formulated for those with normal, combination, or oily skin with an uneven texture that is prone to shine. The mousse texture enhances application. It can be used alone or under makeup. For more information, visit www.skinceuticals.com.
Silagen Scar Refinement System
NewMedical Technology, Inc, introduces the Silagen Scar Refinement System, a line of 100% medical-grade silicone scar therapies. Silagen 100% Pure Silicone Gel is available in 15- or 30-g airless pumps. The Silagen line also includes a wide variety of silicone gel sheets, strips, and shapes with advanced adhesion technology and optimal silicone thickness. The gel has a silky feel and fast drying time. Silagen is physician dispensed. For more information, visit www.silagen.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Epiduo Forte Gel
Galderma Laboratories, LP, announces US Food and Drug Administration approval of antibiotic-free Epiduo Forte (adapalene 0.3% and benzoyl peroxide 2.5%) Gel for the once-daily topical treatment of acne vulgaris. Epiduo Forte Gel contains a high concentration of adapalene, which works to unclog blocked pores and inhibits the release of proinflammatory mediators. Benzoyl peroxide offers antimicrobial properties. Epiduo Forte Gel can be considered for long-term use in patients with moderate to severe acne. The formulation is offered in a pump to provide convenient once-daily dosing. Epiduo Forte Gel will be available by prescription in early September 2015. For more information, visit www.galdermausa.com.
Odomzo
Novartis Pharmaceuticals Corporation obtains US Food and Drug Administration approval of Odomzo (sonidegib) 200-mg capsules for the treatment of adult patients with locally advanced basal cell carcinoma that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy. Odomzo is an oral selective smoothened (SMO) inhibitor. SMO is a molecule that regulates the hedgehog signaling pathway, which plays a critical role in stem cell maintenance and tissue repair as well as in advanced basal cell carcinoma. For more information, visit www.novartis.com.
Physical Matte UV Defense SPF 50
SkinCeuticals introduces Physical Matte UV Defense SPF 50 sunscreen. This 100% mineral sunscreen is formulated with oil-absorbing powders to help minimize the appearance of sweat and oil to ensure a long-lasting matte finish while also providing broad-spectrum UVA/UVB protection. Physical Matte UV DefenseSPF 50 sunscreen is formulated for those with normal, combination, or oily skin with an uneven texture that is prone to shine. The mousse texture enhances application. It can be used alone or under makeup. For more information, visit www.skinceuticals.com.
Silagen Scar Refinement System
NewMedical Technology, Inc, introduces the Silagen Scar Refinement System, a line of 100% medical-grade silicone scar therapies. Silagen 100% Pure Silicone Gel is available in 15- or 30-g airless pumps. The Silagen line also includes a wide variety of silicone gel sheets, strips, and shapes with advanced adhesion technology and optimal silicone thickness. The gel has a silky feel and fast drying time. Silagen is physician dispensed. For more information, visit www.silagen.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Epiduo Forte Gel
Galderma Laboratories, LP, announces US Food and Drug Administration approval of antibiotic-free Epiduo Forte (adapalene 0.3% and benzoyl peroxide 2.5%) Gel for the once-daily topical treatment of acne vulgaris. Epiduo Forte Gel contains a high concentration of adapalene, which works to unclog blocked pores and inhibits the release of proinflammatory mediators. Benzoyl peroxide offers antimicrobial properties. Epiduo Forte Gel can be considered for long-term use in patients with moderate to severe acne. The formulation is offered in a pump to provide convenient once-daily dosing. Epiduo Forte Gel will be available by prescription in early September 2015. For more information, visit www.galdermausa.com.
Odomzo
Novartis Pharmaceuticals Corporation obtains US Food and Drug Administration approval of Odomzo (sonidegib) 200-mg capsules for the treatment of adult patients with locally advanced basal cell carcinoma that has recurred following surgery or radiation therapy, or those who are not candidates for surgery or radiation therapy. Odomzo is an oral selective smoothened (SMO) inhibitor. SMO is a molecule that regulates the hedgehog signaling pathway, which plays a critical role in stem cell maintenance and tissue repair as well as in advanced basal cell carcinoma. For more information, visit www.novartis.com.
Physical Matte UV Defense SPF 50
SkinCeuticals introduces Physical Matte UV Defense SPF 50 sunscreen. This 100% mineral sunscreen is formulated with oil-absorbing powders to help minimize the appearance of sweat and oil to ensure a long-lasting matte finish while also providing broad-spectrum UVA/UVB protection. Physical Matte UV DefenseSPF 50 sunscreen is formulated for those with normal, combination, or oily skin with an uneven texture that is prone to shine. The mousse texture enhances application. It can be used alone or under makeup. For more information, visit www.skinceuticals.com.
Silagen Scar Refinement System
NewMedical Technology, Inc, introduces the Silagen Scar Refinement System, a line of 100% medical-grade silicone scar therapies. Silagen 100% Pure Silicone Gel is available in 15- or 30-g airless pumps. The Silagen line also includes a wide variety of silicone gel sheets, strips, and shapes with advanced adhesion technology and optimal silicone thickness. The gel has a silky feel and fast drying time. Silagen is physician dispensed. For more information, visit www.silagen.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
PTSD Campaign: Caring and Sharing
“Learn, connect, and share”—that’s the theme of this year’s VA PTSD awareness campaign. The campaign is focused on online materials about how posttraumatic stress disorder (PTSD) treatment can help and encourages the general public to reach out to someone and share the information. The VA National Center for PTSD website (http://www.ptsd.va.gov/about/PTSD-awareness) offers a number of resources to help with that mission:
- PTSD Coach Online and the PTSD Coach mobile app, providing strategies to manage symptoms
- AboutFace, a video gallery of veterans talking about how treatment can turn lives around
- Whiteboards, short animated videos to learn about PTSD and effective treatments
Providers will also find continuing education and CME opportunities, such as PTSD 101 courses and the PTSD Monthly Update, offering the latest information about PTSD and trauma.
“Learn, connect, and share”—that’s the theme of this year’s VA PTSD awareness campaign. The campaign is focused on online materials about how posttraumatic stress disorder (PTSD) treatment can help and encourages the general public to reach out to someone and share the information. The VA National Center for PTSD website (http://www.ptsd.va.gov/about/PTSD-awareness) offers a number of resources to help with that mission:
- PTSD Coach Online and the PTSD Coach mobile app, providing strategies to manage symptoms
- AboutFace, a video gallery of veterans talking about how treatment can turn lives around
- Whiteboards, short animated videos to learn about PTSD and effective treatments
Providers will also find continuing education and CME opportunities, such as PTSD 101 courses and the PTSD Monthly Update, offering the latest information about PTSD and trauma.
“Learn, connect, and share”—that’s the theme of this year’s VA PTSD awareness campaign. The campaign is focused on online materials about how posttraumatic stress disorder (PTSD) treatment can help and encourages the general public to reach out to someone and share the information. The VA National Center for PTSD website (http://www.ptsd.va.gov/about/PTSD-awareness) offers a number of resources to help with that mission:
- PTSD Coach Online and the PTSD Coach mobile app, providing strategies to manage symptoms
- AboutFace, a video gallery of veterans talking about how treatment can turn lives around
- Whiteboards, short animated videos to learn about PTSD and effective treatments
Providers will also find continuing education and CME opportunities, such as PTSD 101 courses and the PTSD Monthly Update, offering the latest information about PTSD and trauma.
Product Update: Premama, SynDaver, ScribeAmerica, Xoft eBX System
SUPPLEMENTS FOR EXPECTANT/NEW MOMS
Premama®, a line of natural powdered drink mixes formulated to support the nutritional needs of expectant and new mothers, has introduced 2 products for preconception and postpartum health.
Fertility delivers a supplement formulation that includes myo-Inositol, which is clinically shown to help improve ovulatory function and healthy egg production, and folic acid to support prenatal health, according to Premama. Fertility is an unflavored drink mix that comes in packets of 2.2 g to be mixed with at least 12 oz of water or other noncarbonated flavored liquids such as juices or smoothies and taken daily.
Lactation is a berry-flavored drink mix daily supplement that is formulated with fenugreek, fennel seed, and blessed thistle to help support healthy milk production, according to Premama. Also included in Lactation are folic acid, Vitamin D3, calcium, and other essential nutrients for both mother and baby. A Lactation 2.5-mg packet mixes with at least 12 oz of water until blended well, or with noncarbonated, flavored liquids such as juices or smoothies.
All Premama products are physician approved, vegetarian, gluten free, and made in the United States. Premama products are available at retailers nationwide or online.
FOR MORE INFORMATION, VISIT www.drinkpremama.com
FREE EKG TRAINING APP FROM SYNDAVER
SynDaver™ Labs has released a free medical training electrocardiogram (EKG)simulator app for android devices on Google Play. The SynDaver EKG Simulator is a digital platform that can function with any medical training manikin, according to SynDaver. Currently available variables include heartbeats per minute, systolic pressure, diastolic pressure, respiration rate, SpO2, and temperature. Values are displayed both numerically and by color coordinated dynamic waveform with mutable audio indicators for heart rate.
The EKG Simulator app allows for 2 android devices to be paired using Bluetooth, which, says the manufacturer, is ideal for a classroom setting because it allows the instructor to update the display remotely to modify the training scenario. Download the free EKG Simulator at http://syndaver.com/shop/new/ekg-simulator/.
FOR MORE INFORMATION, VISIT www.syndaver.com
MEDICAL SCRIBES AND CODING TOOLS
ScribeAmerica was established in 2004 as a clinical documentation solution for providers transitioning to electronic health records (EHRs). ScribeAmerica says its focus on improving the accuracy and quality of patient documentation has resulted in higher patient satisfaction scores, improved revenue cycle, and better continuity of care.
ScribeAmerica recruits, trains, and manages over 7,300 scribes in nearly 900 locations nationwide. Certified Medical Scribes, current with all American College of Medical Scribe Specialists guidelines and testing, specialize in collecting medical data and entering it into a clinician’s EHR, resulting in improved operational workflow, claims ScribeAmerica. ScribeAmerica’s medical scribe programs are found in rural and urban hospitals, teaching facilities, private practices, and political organizations.
LiveCode Point of Service Coding is a real-time coding solution that reduces the latency in feedback and improves overall efficacy of the revenue cycle.
The Individualized Clinical Documentation Advisor (ICD-Advisor) provides custom reports tailored to the codes used most often in a specific practice. ScribeAmerica says its ICD-Advisor is fast, individualized to a practice’s needs, and affordable.
FOR MORE INFORMATION, VISIT www.scribeamerica.com
XOFT CERVICAL APPLICATOR FOR EBX
iCAD, Inc. has added a cervical applicator to the Xoft® Axxent® Electronic Brachytherapy (eBX) System® for intracavitary treatment of multiple gynecologic cancers in a minimally shielded setting. The cervical applicator is used to deliver a precise dose of radiation to larger target areas of the cervix while minimizing exposure to healthy tissue, according to iCAD.
Using proprietary miniaturized x-ray as the radiation source, the Xoft eBX System delivers isotope-free radiation treatment in a targeted prescribed dose directly to the site where cancer recurrence is most likely, designed to minimize exposure to healthy tissue such as the bladder and rectum. The system requires minimal shielding and therefore does not require room redesign or construction investment and also allows medical personnel to remain in the room with the patient during treatment, says iCAD.
FOR MORE INFORMATION, VISIT www.xoftinc.com
SUPPLEMENTS FOR EXPECTANT/NEW MOMS
Premama®, a line of natural powdered drink mixes formulated to support the nutritional needs of expectant and new mothers, has introduced 2 products for preconception and postpartum health.
Fertility delivers a supplement formulation that includes myo-Inositol, which is clinically shown to help improve ovulatory function and healthy egg production, and folic acid to support prenatal health, according to Premama. Fertility is an unflavored drink mix that comes in packets of 2.2 g to be mixed with at least 12 oz of water or other noncarbonated flavored liquids such as juices or smoothies and taken daily.
Lactation is a berry-flavored drink mix daily supplement that is formulated with fenugreek, fennel seed, and blessed thistle to help support healthy milk production, according to Premama. Also included in Lactation are folic acid, Vitamin D3, calcium, and other essential nutrients for both mother and baby. A Lactation 2.5-mg packet mixes with at least 12 oz of water until blended well, or with noncarbonated, flavored liquids such as juices or smoothies.
All Premama products are physician approved, vegetarian, gluten free, and made in the United States. Premama products are available at retailers nationwide or online.
FOR MORE INFORMATION, VISIT www.drinkpremama.com
FREE EKG TRAINING APP FROM SYNDAVER
SynDaver™ Labs has released a free medical training electrocardiogram (EKG)simulator app for android devices on Google Play. The SynDaver EKG Simulator is a digital platform that can function with any medical training manikin, according to SynDaver. Currently available variables include heartbeats per minute, systolic pressure, diastolic pressure, respiration rate, SpO2, and temperature. Values are displayed both numerically and by color coordinated dynamic waveform with mutable audio indicators for heart rate.
The EKG Simulator app allows for 2 android devices to be paired using Bluetooth, which, says the manufacturer, is ideal for a classroom setting because it allows the instructor to update the display remotely to modify the training scenario. Download the free EKG Simulator at http://syndaver.com/shop/new/ekg-simulator/.
FOR MORE INFORMATION, VISIT www.syndaver.com
MEDICAL SCRIBES AND CODING TOOLS
ScribeAmerica was established in 2004 as a clinical documentation solution for providers transitioning to electronic health records (EHRs). ScribeAmerica says its focus on improving the accuracy and quality of patient documentation has resulted in higher patient satisfaction scores, improved revenue cycle, and better continuity of care.
ScribeAmerica recruits, trains, and manages over 7,300 scribes in nearly 900 locations nationwide. Certified Medical Scribes, current with all American College of Medical Scribe Specialists guidelines and testing, specialize in collecting medical data and entering it into a clinician’s EHR, resulting in improved operational workflow, claims ScribeAmerica. ScribeAmerica’s medical scribe programs are found in rural and urban hospitals, teaching facilities, private practices, and political organizations.
LiveCode Point of Service Coding is a real-time coding solution that reduces the latency in feedback and improves overall efficacy of the revenue cycle.
The Individualized Clinical Documentation Advisor (ICD-Advisor) provides custom reports tailored to the codes used most often in a specific practice. ScribeAmerica says its ICD-Advisor is fast, individualized to a practice’s needs, and affordable.
FOR MORE INFORMATION, VISIT www.scribeamerica.com
XOFT CERVICAL APPLICATOR FOR EBX
iCAD, Inc. has added a cervical applicator to the Xoft® Axxent® Electronic Brachytherapy (eBX) System® for intracavitary treatment of multiple gynecologic cancers in a minimally shielded setting. The cervical applicator is used to deliver a precise dose of radiation to larger target areas of the cervix while minimizing exposure to healthy tissue, according to iCAD.
Using proprietary miniaturized x-ray as the radiation source, the Xoft eBX System delivers isotope-free radiation treatment in a targeted prescribed dose directly to the site where cancer recurrence is most likely, designed to minimize exposure to healthy tissue such as the bladder and rectum. The system requires minimal shielding and therefore does not require room redesign or construction investment and also allows medical personnel to remain in the room with the patient during treatment, says iCAD.
FOR MORE INFORMATION, VISIT www.xoftinc.com
SUPPLEMENTS FOR EXPECTANT/NEW MOMS
Premama®, a line of natural powdered drink mixes formulated to support the nutritional needs of expectant and new mothers, has introduced 2 products for preconception and postpartum health.
Fertility delivers a supplement formulation that includes myo-Inositol, which is clinically shown to help improve ovulatory function and healthy egg production, and folic acid to support prenatal health, according to Premama. Fertility is an unflavored drink mix that comes in packets of 2.2 g to be mixed with at least 12 oz of water or other noncarbonated flavored liquids such as juices or smoothies and taken daily.
Lactation is a berry-flavored drink mix daily supplement that is formulated with fenugreek, fennel seed, and blessed thistle to help support healthy milk production, according to Premama. Also included in Lactation are folic acid, Vitamin D3, calcium, and other essential nutrients for both mother and baby. A Lactation 2.5-mg packet mixes with at least 12 oz of water until blended well, or with noncarbonated, flavored liquids such as juices or smoothies.
All Premama products are physician approved, vegetarian, gluten free, and made in the United States. Premama products are available at retailers nationwide or online.
FOR MORE INFORMATION, VISIT www.drinkpremama.com
FREE EKG TRAINING APP FROM SYNDAVER
SynDaver™ Labs has released a free medical training electrocardiogram (EKG)simulator app for android devices on Google Play. The SynDaver EKG Simulator is a digital platform that can function with any medical training manikin, according to SynDaver. Currently available variables include heartbeats per minute, systolic pressure, diastolic pressure, respiration rate, SpO2, and temperature. Values are displayed both numerically and by color coordinated dynamic waveform with mutable audio indicators for heart rate.
The EKG Simulator app allows for 2 android devices to be paired using Bluetooth, which, says the manufacturer, is ideal for a classroom setting because it allows the instructor to update the display remotely to modify the training scenario. Download the free EKG Simulator at http://syndaver.com/shop/new/ekg-simulator/.
FOR MORE INFORMATION, VISIT www.syndaver.com
MEDICAL SCRIBES AND CODING TOOLS
ScribeAmerica was established in 2004 as a clinical documentation solution for providers transitioning to electronic health records (EHRs). ScribeAmerica says its focus on improving the accuracy and quality of patient documentation has resulted in higher patient satisfaction scores, improved revenue cycle, and better continuity of care.
ScribeAmerica recruits, trains, and manages over 7,300 scribes in nearly 900 locations nationwide. Certified Medical Scribes, current with all American College of Medical Scribe Specialists guidelines and testing, specialize in collecting medical data and entering it into a clinician’s EHR, resulting in improved operational workflow, claims ScribeAmerica. ScribeAmerica’s medical scribe programs are found in rural and urban hospitals, teaching facilities, private practices, and political organizations.
LiveCode Point of Service Coding is a real-time coding solution that reduces the latency in feedback and improves overall efficacy of the revenue cycle.
The Individualized Clinical Documentation Advisor (ICD-Advisor) provides custom reports tailored to the codes used most often in a specific practice. ScribeAmerica says its ICD-Advisor is fast, individualized to a practice’s needs, and affordable.
FOR MORE INFORMATION, VISIT www.scribeamerica.com
XOFT CERVICAL APPLICATOR FOR EBX
iCAD, Inc. has added a cervical applicator to the Xoft® Axxent® Electronic Brachytherapy (eBX) System® for intracavitary treatment of multiple gynecologic cancers in a minimally shielded setting. The cervical applicator is used to deliver a precise dose of radiation to larger target areas of the cervix while minimizing exposure to healthy tissue, according to iCAD.
Using proprietary miniaturized x-ray as the radiation source, the Xoft eBX System delivers isotope-free radiation treatment in a targeted prescribed dose directly to the site where cancer recurrence is most likely, designed to minimize exposure to healthy tissue such as the bladder and rectum. The system requires minimal shielding and therefore does not require room redesign or construction investment and also allows medical personnel to remain in the room with the patient during treatment, says iCAD.
FOR MORE INFORMATION, VISIT www.xoftinc.com