User login
Painful and Pruritic Eruptions on the Entire Body
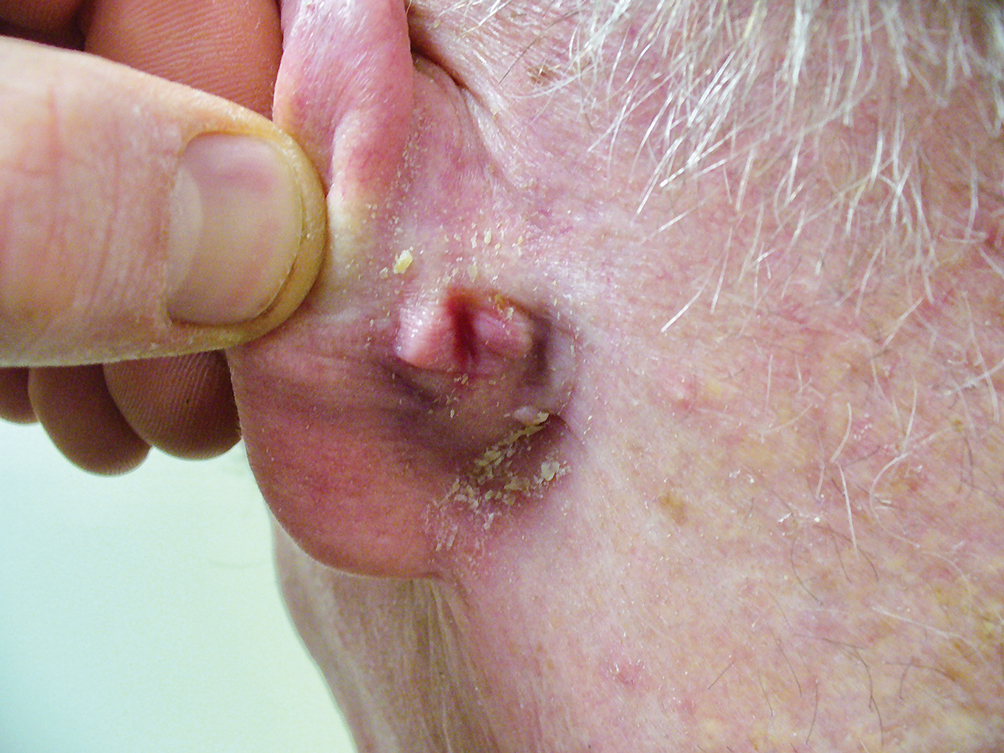
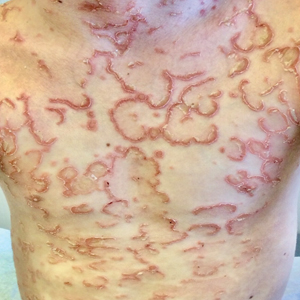
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
The Diagnosis: IgA Pemphigus
Histopathology revealed a neutrophilic pustule and vesicle formation underlying the corneal layer (Figure). Direct immunofluorescence (DIF) showed weak positive staining for IgA within the intercellular keratinocyte in the epithelial compartment and a negative pattern with IgG, IgM, C3, and fibrinogen. The patient received a 40-mg intralesional triamcinolone injection and was placed on an oral prednisone 50-mg taper within 5 days. The plaques, bullae, and pustules began to resolve, but the lesions returned 1 day later. Oral prednisone 10 mg daily was initiated for 1 month, which resulted in full resolution of the lesions.

IgA pemphigus is a rare autoimmune disorder characterized by the occurrence of painful pruritic blisters caused by circulating IgA antibodies, which react against keratinocyte cellular components responsible for mediating cell-to-cell adherence.1 The etiology of IgA pemphigus presently remains elusive, though it has been reported to occur concomitantly with several chronic malignancies and inflammatory conditions. Although its etiology is unknown, IgA pemphigus most commonly is treated with oral dapsone and corticosteroids.2
IgA pemphigus can be divided into 2 primary subtypes: subcorneal pustular dermatosis and intraepidermal neutrophilic dermatosis.1,3 The former is characterized by intercellular deposition of IgA that reacts to the glycoprotein desmocollin-1 in the upper layer of the epidermis. Intraepidermal neutrophilic dermatosis is distinguished by the presence of autoantibodies against the desmoglein members of the cadherin superfamily of proteins. Additionally, unlike subcorneal pustular dermatosis, intraepidermal neutrophilic dermatosis autoantibody reactivity occurs in the lower epidermis.4
The differential includes dermatitis herpetiformis, which is commonly seen on the elbows, knees, and buttocks, with DIF showing IgA deposition at the dermal papillae. Pemphigus foliaceus is distributed on the scalp, face, and trunk, with DIF showing IgG intercellular deposition. Pustular psoriasis presents as erythematous sterile pustules in a more localized annular pattern. Subcorneal pustular dermatosis (Sneddon-Wilkinson disease) has similar clinical and histological findings to IgA pemphigus; however, DIF is negative.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
- Kridin K, Patel PM, Jones VA, et al. IgA pemphigus: a systematic review. J Am Acad Dermatol. 2020;82:1386-1392.
- Moreno ACL, Santi CG, Gabbi TVB, et al. IgA pemphigus: case series with emphasis on therapeutic response. J Am Acad Dermatol. 2014;70:200-201.
- Niimi Y, Kawana S, Kusunoki T. IgA pemphigus: a case report and its characteristic clinical features compared with subcorneal pustular dermatosis. J Am Acad Dermatol. 2000;43:546-549.
- Aslanova M, Yarrarapu SNS, Zito PM. IgA pemphigus. StatPearls. StatPearls Publishing; 2021.
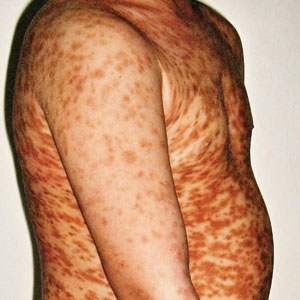
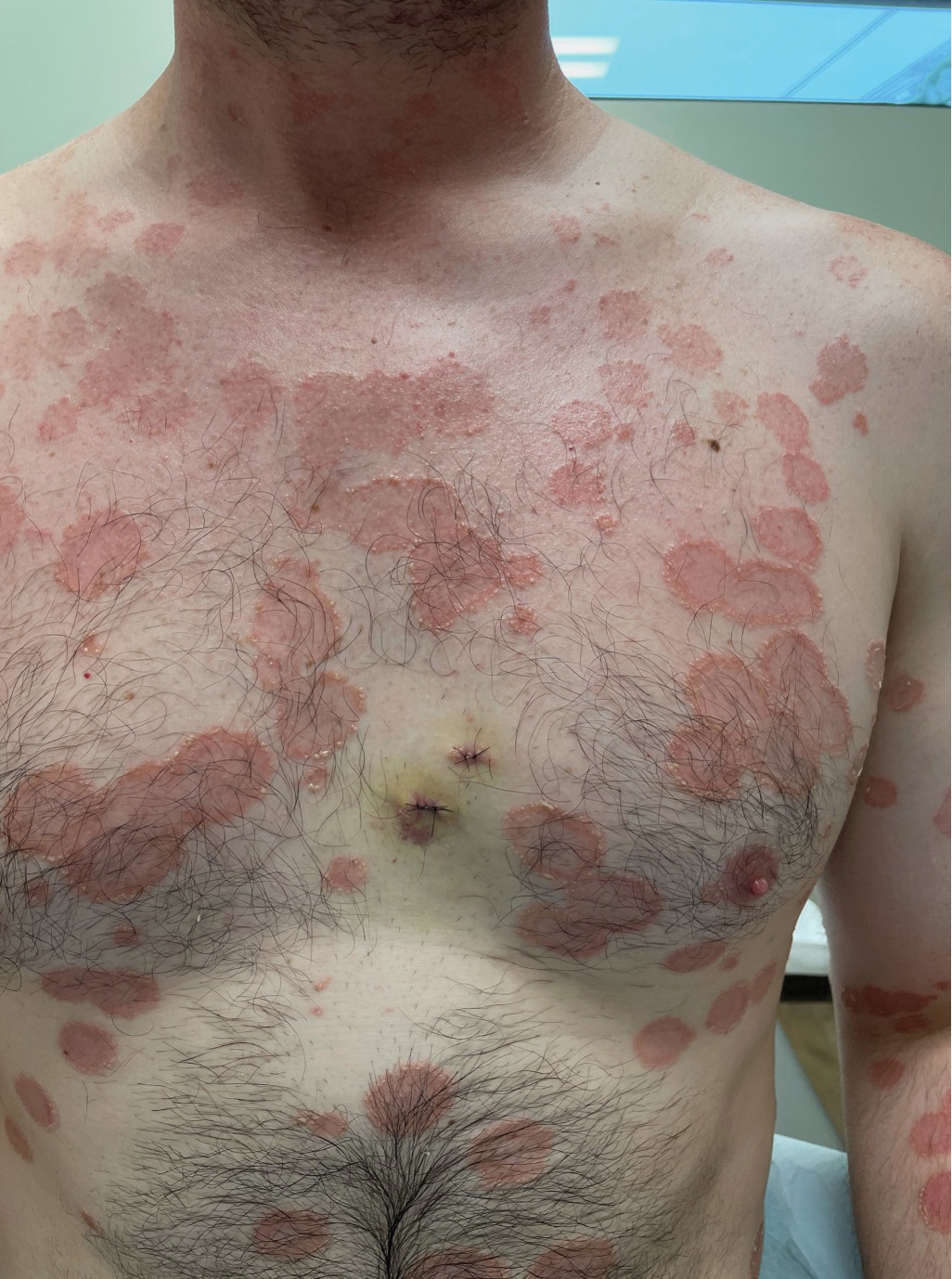
A 36-year-old man presented with painful tender blisters and rashes on the entire body, including the ears and tongue. The rash began as a few pinpointed red dots on the abdomen, which subsequently increased in size and spread over the last week. He initially felt red and flushed and noticed new lesions appearing throughout the day. He did not attempt any specific treatment for these lesions. The patient tested positive for COVID-19 four months prior to the skin eruption. He denied systemic symptoms, smoking, or recent travel. He had no history of skin cancer, skin disorders, HIV, or hepatitis. He had no known medication allergies. Physical examination revealed multiple disseminated pustules on the ears, superficial ulcerations on the tongue, and blisters on the right lip. Few lesions were tender to the touch and drained clear fluid. Bacterial, viral, HIV, herpes, and rapid plasma reagin culture and laboratory screenings were negative. He was started on valaciclovir and cephalexin; however, no improvement was noticed. Punch biopsies were taken from the blisters on the chest and perilesional area.
Itchy Red-Brown Spots on a Child
The Diagnosis: Maculopapular Cutaneous Mastocytosis (Urticaria Pigmentosa)
A stroke test revealed urtication at the exact traumatized site (Figure). A skin biopsy performed 2 years prior by another physician in the same hospital had revealed mast cell infiltration of virtually the entire dermis. The diagnosis was then firmly established as maculopapular cutaneous mastocytosis (CM)(also known as urticaria pigmentosa) with both the pathology results and a confirmative stroke test, and no additional biopsy was attempted. Serum IgE and tryptase levels were within the reference range. General recommendations about the avoidance of trigger factors were given to the family, and a new-generation H1 blocker antihistaminic syrup was prescribed for flushing, itching, and urtication.
Mastocytosis is a canopy term for a heterogeneous group of disorders caused by clonal proliferation and accumulation of abnormal mast cells within the skin and visceral organs (ie, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract). Cutaneous mastocytosis, the skin-restricted variant, is by far the most common form of childhood mastocytosis (90% of mastocytosis cases in children)1 and generally appears within the first 2 years of life.1-7 Pediatric CM usually is a benign and transient disease with an excellent prognosis and a negligible risk for systemic involvement.2,3,5
The pathogenesis of CM in children is obscure1; however, somatic or germline gain-of-function mutations of the c-KIT proto-oncogene, which encodes KIT (ie, a tyrosine kinase membrane receptor for stem cell factor), may account for most pediatric CM phenotypes.1,3,6 Activating c-KIT mutations leads to constitutive activation of the KIT receptor (expressed on the surface membrane of mast cells) and instigates autonomous (stem cell factor– independent) clonal proliferation, enhanced survival, and accumulation of mast cells.2
Maculopapular CM is the most common clinical form of CM.2,4,5 In children, maculopapular CM usually presents with polymorphous red-brown lesions of varying sizes and types—macule, papule, plaque, or nodule—on the torso and extremities.1-5 The distribution may be widespread and rarely is almost universal, as in our patient.2 Darier sign typically is positive, with a wheal and flare developing upon stroking or rubbing 1 or several lesions.1-6 The lesions gradually involute and often spontaneously regress at the time of puberty.1-3,5-7
The clinical signs and symptoms of mastocytosis are not only related to mast cell infiltration but also to mast cell activation within the tissues. The release of intracellular mediators from activated mast cells may have local and/or systemic consequences.4,7 Erythema, edema, flushing, pruritus, urticaria, blistering, and dermatographism are among the local cutaneous symptoms of mast cell activation.2-4,7 Systemic symptoms are rare in childhood CM and consist of wheezing, shortness of breath, nausea, vomiting, reflux, abdominal cramping, diarrhea, tachycardia, hypotension, syncope, anaphylaxis, and cyanotic spells.1-7 An elevated serum tryptase level is an indicator of both mast cell burden and risk for mast cell activation in the skin.4,7
Treatment of pediatric CM is conservative and symptomatic.3 Prevention of mediator release may be accomplished through avoidance of trigger factors.1 Alleviation of mediator-related symptoms might be attained using H1 and H2 histamine receptor blockers, oral cromolyn sodium, leukotriene antagonists, and epinephrine autoinjectors.1-3,5 Short-term topical or oral corticosteroids; calcineurin inhibitors (eg, pimecrolimus, tacrolimus); phototherapy; psoralen plus UVA; omalizumab; and innovative agents such as topical miltefosine, nemolizumab (an IL-31 antagonist), kinase inhibitors such as midostaurin, and tyrosine kinase inhibitors such as imatinib and masitinib may be tried in refractory or extensive pediatric CM.1,2,5,6
Although several disorders in childhood may present with red-brown macules and papules, Darier sign is unique to cutaneous mastocytosis. A biopsy also will be helpful in establishing the definitive diagnosis.
Histiocytosis X (also referred to as Langerhans cell histiocytosis) is the most common proliferative histiocytic disorder. Cutaneous lesions are polymorphic and consist of seborrheic involvement of the scalp with yellow, scaly or crusted papules; eroded patches; pustules; vesicles; petechiae; purpura; or red to purplish papules on the groin, abdomen, back, or chest.8
LEOPARD syndrome (also known as Noonan syndrome with multiple lentigines) is an acronym denoting lentigines (multiple), electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retarded growth, and deafness (sensorineural). The disorder is caused by a genetic mutation involving the PTPN11 gene and currently is categorized under the canopy of RASopathies. Cutaneous findings consist of lentiginous and café-au-lait macules and patches.9
Neurofibromatosis is a genetic disorder with a plethora of cutaneous and systemic manifestations. The type 1 variant that constitutes more than 95% of cases is caused by mutations in the neurofibromin gene. The main cutaneous findings include café-au-lait macules, freckling in axillary and inguinal locations (Crowe sign), and neurofibromas. These lesions may present as macules, patches, papules, or nodules.10
Xanthoma disseminatum is a rare sporadic proliferative histiocyte disorder involving the skin and mucosa. The disorder may be a harbinger of diabetes insipidus. Cutaneous lesions consist of asymptomatic, symmetrical, discrete, erythematous to yellow-brown papules and nodules.11
- Sandru F, Petca RC, Costescu M, et al. Cutaneous mastocytosis in childhood: update from the literature. J Clin Med. 2021;10:1474. doi:10.3390/jcm10071474
- Lange M, Hartmann K, Carter MC, et al. Molecular background, clinical features and management of pediatric mastocytosis: status 2021. Int J Mol Sci. 2021;22:2586. doi:10.3390/ijms22052586
- Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12:259-270. doi:10.2165/11588890-000000000-00000
- Nedoszytko B, Arock M, Lyons JJ, et al. Clinical impact of inherited and acquired genetic variants in mastocytosis. Int J Mol Sci. 2021;22:411. doi:10.3390/ijms22010411
- Nemat K, Abraham S. Cutaneous mastocytosis in childhood. Allergol Select. 2022;6:1-10. doi:10.5414/ALX02304E
- Giona F. Pediatric mastocytosis: an update. Mediterr J Hematol Infect Dis. 2021;13:E2021069. doi:10.4084/MJHID.2021.069
- Brockow K, Plata-Nazar K, Lange M, et al. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int J Mol Sci. 2021;22:2684. doi:10.3390/ijms22052684
- Grana N. Langerhans cell histiocytosis. Cancer Control. 2014;21: 328-334.
- García-Gil MF, Álvarez-Salafranca M, Valero-Torres A, et al. Melanoma in Noonan syndrome with multiple lentigines (LEOPARD syndrome): a new case. Actas Dermosifiliogr (Engl Ed). 2020;111:619-621.
- Ozarslan B, Russo T, Argenziano G, et al. Cutaneous findings in neurofibromatosis type 1. Cancers (Basel). 2021;13:463.
- Behra A, Sa DK, Naik R, et al. A rare case of persistent xanthoma disseminatum without any systemic involvement. Indian J Dermatol. 2020;65:239-241.
The Diagnosis: Maculopapular Cutaneous Mastocytosis (Urticaria Pigmentosa)
A stroke test revealed urtication at the exact traumatized site (Figure). A skin biopsy performed 2 years prior by another physician in the same hospital had revealed mast cell infiltration of virtually the entire dermis. The diagnosis was then firmly established as maculopapular cutaneous mastocytosis (CM)(also known as urticaria pigmentosa) with both the pathology results and a confirmative stroke test, and no additional biopsy was attempted. Serum IgE and tryptase levels were within the reference range. General recommendations about the avoidance of trigger factors were given to the family, and a new-generation H1 blocker antihistaminic syrup was prescribed for flushing, itching, and urtication.

Mastocytosis is a canopy term for a heterogeneous group of disorders caused by clonal proliferation and accumulation of abnormal mast cells within the skin and visceral organs (ie, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract). Cutaneous mastocytosis, the skin-restricted variant, is by far the most common form of childhood mastocytosis (90% of mastocytosis cases in children)1 and generally appears within the first 2 years of life.1-7 Pediatric CM usually is a benign and transient disease with an excellent prognosis and a negligible risk for systemic involvement.2,3,5
The pathogenesis of CM in children is obscure1; however, somatic or germline gain-of-function mutations of the c-KIT proto-oncogene, which encodes KIT (ie, a tyrosine kinase membrane receptor for stem cell factor), may account for most pediatric CM phenotypes.1,3,6 Activating c-KIT mutations leads to constitutive activation of the KIT receptor (expressed on the surface membrane of mast cells) and instigates autonomous (stem cell factor– independent) clonal proliferation, enhanced survival, and accumulation of mast cells.2
Maculopapular CM is the most common clinical form of CM.2,4,5 In children, maculopapular CM usually presents with polymorphous red-brown lesions of varying sizes and types—macule, papule, plaque, or nodule—on the torso and extremities.1-5 The distribution may be widespread and rarely is almost universal, as in our patient.2 Darier sign typically is positive, with a wheal and flare developing upon stroking or rubbing 1 or several lesions.1-6 The lesions gradually involute and often spontaneously regress at the time of puberty.1-3,5-7
The clinical signs and symptoms of mastocytosis are not only related to mast cell infiltration but also to mast cell activation within the tissues. The release of intracellular mediators from activated mast cells may have local and/or systemic consequences.4,7 Erythema, edema, flushing, pruritus, urticaria, blistering, and dermatographism are among the local cutaneous symptoms of mast cell activation.2-4,7 Systemic symptoms are rare in childhood CM and consist of wheezing, shortness of breath, nausea, vomiting, reflux, abdominal cramping, diarrhea, tachycardia, hypotension, syncope, anaphylaxis, and cyanotic spells.1-7 An elevated serum tryptase level is an indicator of both mast cell burden and risk for mast cell activation in the skin.4,7
Treatment of pediatric CM is conservative and symptomatic.3 Prevention of mediator release may be accomplished through avoidance of trigger factors.1 Alleviation of mediator-related symptoms might be attained using H1 and H2 histamine receptor blockers, oral cromolyn sodium, leukotriene antagonists, and epinephrine autoinjectors.1-3,5 Short-term topical or oral corticosteroids; calcineurin inhibitors (eg, pimecrolimus, tacrolimus); phototherapy; psoralen plus UVA; omalizumab; and innovative agents such as topical miltefosine, nemolizumab (an IL-31 antagonist), kinase inhibitors such as midostaurin, and tyrosine kinase inhibitors such as imatinib and masitinib may be tried in refractory or extensive pediatric CM.1,2,5,6
Although several disorders in childhood may present with red-brown macules and papules, Darier sign is unique to cutaneous mastocytosis. A biopsy also will be helpful in establishing the definitive diagnosis.
Histiocytosis X (also referred to as Langerhans cell histiocytosis) is the most common proliferative histiocytic disorder. Cutaneous lesions are polymorphic and consist of seborrheic involvement of the scalp with yellow, scaly or crusted papules; eroded patches; pustules; vesicles; petechiae; purpura; or red to purplish papules on the groin, abdomen, back, or chest.8
LEOPARD syndrome (also known as Noonan syndrome with multiple lentigines) is an acronym denoting lentigines (multiple), electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retarded growth, and deafness (sensorineural). The disorder is caused by a genetic mutation involving the PTPN11 gene and currently is categorized under the canopy of RASopathies. Cutaneous findings consist of lentiginous and café-au-lait macules and patches.9
Neurofibromatosis is a genetic disorder with a plethora of cutaneous and systemic manifestations. The type 1 variant that constitutes more than 95% of cases is caused by mutations in the neurofibromin gene. The main cutaneous findings include café-au-lait macules, freckling in axillary and inguinal locations (Crowe sign), and neurofibromas. These lesions may present as macules, patches, papules, or nodules.10
Xanthoma disseminatum is a rare sporadic proliferative histiocyte disorder involving the skin and mucosa. The disorder may be a harbinger of diabetes insipidus. Cutaneous lesions consist of asymptomatic, symmetrical, discrete, erythematous to yellow-brown papules and nodules.11
The Diagnosis: Maculopapular Cutaneous Mastocytosis (Urticaria Pigmentosa)
A stroke test revealed urtication at the exact traumatized site (Figure). A skin biopsy performed 2 years prior by another physician in the same hospital had revealed mast cell infiltration of virtually the entire dermis. The diagnosis was then firmly established as maculopapular cutaneous mastocytosis (CM)(also known as urticaria pigmentosa) with both the pathology results and a confirmative stroke test, and no additional biopsy was attempted. Serum IgE and tryptase levels were within the reference range. General recommendations about the avoidance of trigger factors were given to the family, and a new-generation H1 blocker antihistaminic syrup was prescribed for flushing, itching, and urtication.

Mastocytosis is a canopy term for a heterogeneous group of disorders caused by clonal proliferation and accumulation of abnormal mast cells within the skin and visceral organs (ie, bone marrow, liver, spleen, lymph nodes, gastrointestinal tract). Cutaneous mastocytosis, the skin-restricted variant, is by far the most common form of childhood mastocytosis (90% of mastocytosis cases in children)1 and generally appears within the first 2 years of life.1-7 Pediatric CM usually is a benign and transient disease with an excellent prognosis and a negligible risk for systemic involvement.2,3,5
The pathogenesis of CM in children is obscure1; however, somatic or germline gain-of-function mutations of the c-KIT proto-oncogene, which encodes KIT (ie, a tyrosine kinase membrane receptor for stem cell factor), may account for most pediatric CM phenotypes.1,3,6 Activating c-KIT mutations leads to constitutive activation of the KIT receptor (expressed on the surface membrane of mast cells) and instigates autonomous (stem cell factor– independent) clonal proliferation, enhanced survival, and accumulation of mast cells.2
Maculopapular CM is the most common clinical form of CM.2,4,5 In children, maculopapular CM usually presents with polymorphous red-brown lesions of varying sizes and types—macule, papule, plaque, or nodule—on the torso and extremities.1-5 The distribution may be widespread and rarely is almost universal, as in our patient.2 Darier sign typically is positive, with a wheal and flare developing upon stroking or rubbing 1 or several lesions.1-6 The lesions gradually involute and often spontaneously regress at the time of puberty.1-3,5-7
The clinical signs and symptoms of mastocytosis are not only related to mast cell infiltration but also to mast cell activation within the tissues. The release of intracellular mediators from activated mast cells may have local and/or systemic consequences.4,7 Erythema, edema, flushing, pruritus, urticaria, blistering, and dermatographism are among the local cutaneous symptoms of mast cell activation.2-4,7 Systemic symptoms are rare in childhood CM and consist of wheezing, shortness of breath, nausea, vomiting, reflux, abdominal cramping, diarrhea, tachycardia, hypotension, syncope, anaphylaxis, and cyanotic spells.1-7 An elevated serum tryptase level is an indicator of both mast cell burden and risk for mast cell activation in the skin.4,7
Treatment of pediatric CM is conservative and symptomatic.3 Prevention of mediator release may be accomplished through avoidance of trigger factors.1 Alleviation of mediator-related symptoms might be attained using H1 and H2 histamine receptor blockers, oral cromolyn sodium, leukotriene antagonists, and epinephrine autoinjectors.1-3,5 Short-term topical or oral corticosteroids; calcineurin inhibitors (eg, pimecrolimus, tacrolimus); phototherapy; psoralen plus UVA; omalizumab; and innovative agents such as topical miltefosine, nemolizumab (an IL-31 antagonist), kinase inhibitors such as midostaurin, and tyrosine kinase inhibitors such as imatinib and masitinib may be tried in refractory or extensive pediatric CM.1,2,5,6
Although several disorders in childhood may present with red-brown macules and papules, Darier sign is unique to cutaneous mastocytosis. A biopsy also will be helpful in establishing the definitive diagnosis.
Histiocytosis X (also referred to as Langerhans cell histiocytosis) is the most common proliferative histiocytic disorder. Cutaneous lesions are polymorphic and consist of seborrheic involvement of the scalp with yellow, scaly or crusted papules; eroded patches; pustules; vesicles; petechiae; purpura; or red to purplish papules on the groin, abdomen, back, or chest.8
LEOPARD syndrome (also known as Noonan syndrome with multiple lentigines) is an acronym denoting lentigines (multiple), electrocardiographic conduction abnormalities, ocular hypertelorism, pulmonary stenosis, abnormalities of the genitalia, retarded growth, and deafness (sensorineural). The disorder is caused by a genetic mutation involving the PTPN11 gene and currently is categorized under the canopy of RASopathies. Cutaneous findings consist of lentiginous and café-au-lait macules and patches.9
Neurofibromatosis is a genetic disorder with a plethora of cutaneous and systemic manifestations. The type 1 variant that constitutes more than 95% of cases is caused by mutations in the neurofibromin gene. The main cutaneous findings include café-au-lait macules, freckling in axillary and inguinal locations (Crowe sign), and neurofibromas. These lesions may present as macules, patches, papules, or nodules.10
Xanthoma disseminatum is a rare sporadic proliferative histiocyte disorder involving the skin and mucosa. The disorder may be a harbinger of diabetes insipidus. Cutaneous lesions consist of asymptomatic, symmetrical, discrete, erythematous to yellow-brown papules and nodules.11
- Sandru F, Petca RC, Costescu M, et al. Cutaneous mastocytosis in childhood: update from the literature. J Clin Med. 2021;10:1474. doi:10.3390/jcm10071474
- Lange M, Hartmann K, Carter MC, et al. Molecular background, clinical features and management of pediatric mastocytosis: status 2021. Int J Mol Sci. 2021;22:2586. doi:10.3390/ijms22052586
- Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12:259-270. doi:10.2165/11588890-000000000-00000
- Nedoszytko B, Arock M, Lyons JJ, et al. Clinical impact of inherited and acquired genetic variants in mastocytosis. Int J Mol Sci. 2021;22:411. doi:10.3390/ijms22010411
- Nemat K, Abraham S. Cutaneous mastocytosis in childhood. Allergol Select. 2022;6:1-10. doi:10.5414/ALX02304E
- Giona F. Pediatric mastocytosis: an update. Mediterr J Hematol Infect Dis. 2021;13:E2021069. doi:10.4084/MJHID.2021.069
- Brockow K, Plata-Nazar K, Lange M, et al. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int J Mol Sci. 2021;22:2684. doi:10.3390/ijms22052684
- Grana N. Langerhans cell histiocytosis. Cancer Control. 2014;21: 328-334.
- García-Gil MF, Álvarez-Salafranca M, Valero-Torres A, et al. Melanoma in Noonan syndrome with multiple lentigines (LEOPARD syndrome): a new case. Actas Dermosifiliogr (Engl Ed). 2020;111:619-621.
- Ozarslan B, Russo T, Argenziano G, et al. Cutaneous findings in neurofibromatosis type 1. Cancers (Basel). 2021;13:463.
- Behra A, Sa DK, Naik R, et al. A rare case of persistent xanthoma disseminatum without any systemic involvement. Indian J Dermatol. 2020;65:239-241.
- Sandru F, Petca RC, Costescu M, et al. Cutaneous mastocytosis in childhood: update from the literature. J Clin Med. 2021;10:1474. doi:10.3390/jcm10071474
- Lange M, Hartmann K, Carter MC, et al. Molecular background, clinical features and management of pediatric mastocytosis: status 2021. Int J Mol Sci. 2021;22:2586. doi:10.3390/ijms22052586
- Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol. 2011;12:259-270. doi:10.2165/11588890-000000000-00000
- Nedoszytko B, Arock M, Lyons JJ, et al. Clinical impact of inherited and acquired genetic variants in mastocytosis. Int J Mol Sci. 2021;22:411. doi:10.3390/ijms22010411
- Nemat K, Abraham S. Cutaneous mastocytosis in childhood. Allergol Select. 2022;6:1-10. doi:10.5414/ALX02304E
- Giona F. Pediatric mastocytosis: an update. Mediterr J Hematol Infect Dis. 2021;13:E2021069. doi:10.4084/MJHID.2021.069
- Brockow K, Plata-Nazar K, Lange M, et al. Mediator-related symptoms and anaphylaxis in children with mastocytosis. Int J Mol Sci. 2021;22:2684. doi:10.3390/ijms22052684
- Grana N. Langerhans cell histiocytosis. Cancer Control. 2014;21: 328-334.
- García-Gil MF, Álvarez-Salafranca M, Valero-Torres A, et al. Melanoma in Noonan syndrome with multiple lentigines (LEOPARD syndrome): a new case. Actas Dermosifiliogr (Engl Ed). 2020;111:619-621.
- Ozarslan B, Russo T, Argenziano G, et al. Cutaneous findings in neurofibromatosis type 1. Cancers (Basel). 2021;13:463.
- Behra A, Sa DK, Naik R, et al. A rare case of persistent xanthoma disseminatum without any systemic involvement. Indian J Dermatol. 2020;65:239-241.
A 5-year-old boy presented with red-brown spots diffusely spread over the body that were present since birth. There were no subjective symptoms, except for rare instances of flushing, itching, and urtication following hot baths and abrasive scrubs. Dermatologic examination revealed widespread brown polymorphic macules and papules of varying sizes on the forehead, neck, torso, and extremities. Physical examination was otherwise normal.
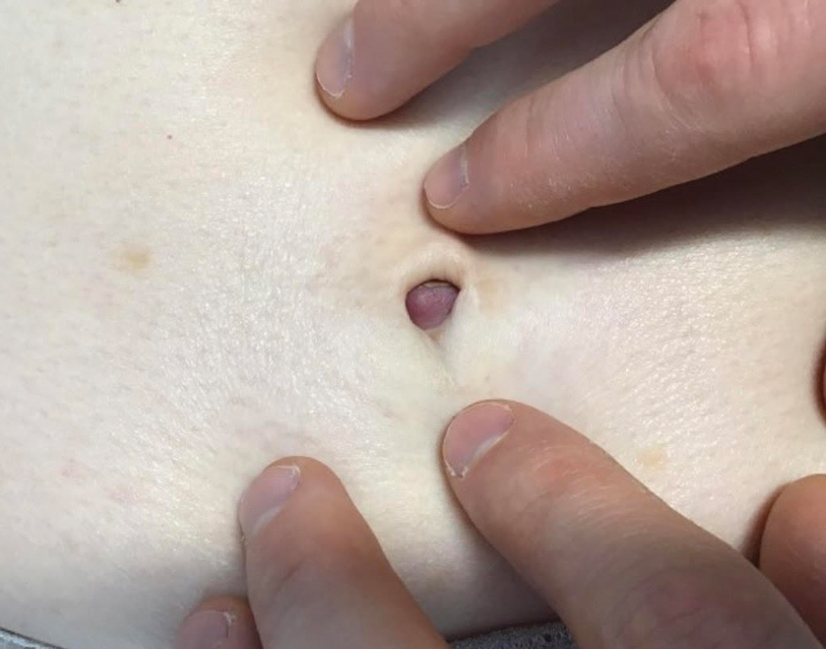
Asymptomatic Umbilical Nodule
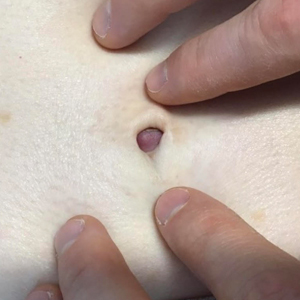
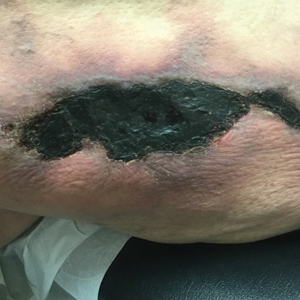
The Diagnosis: Sister Mary Joseph Nodule
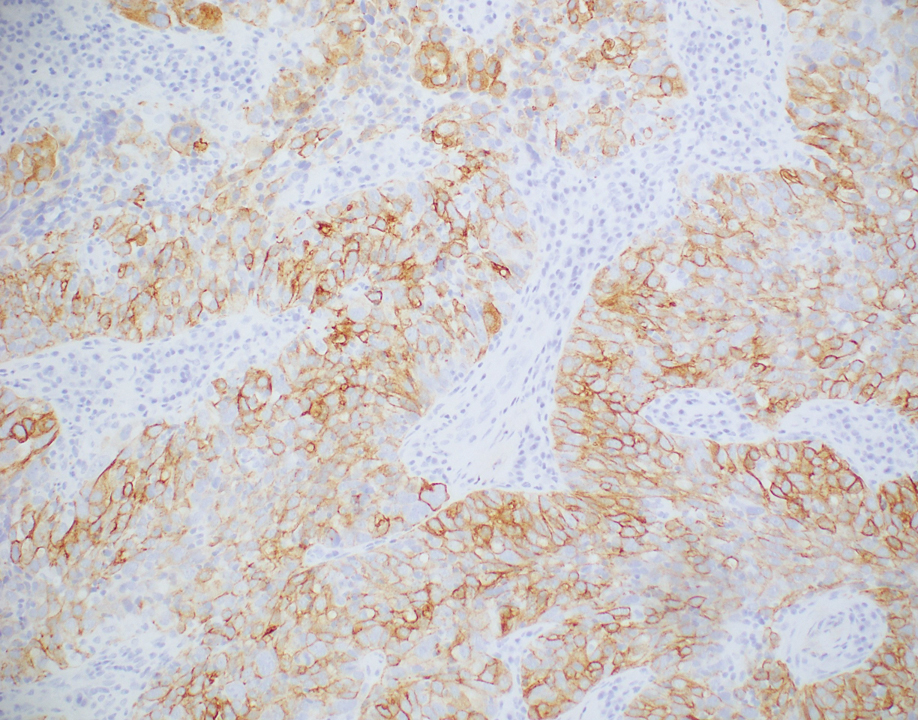
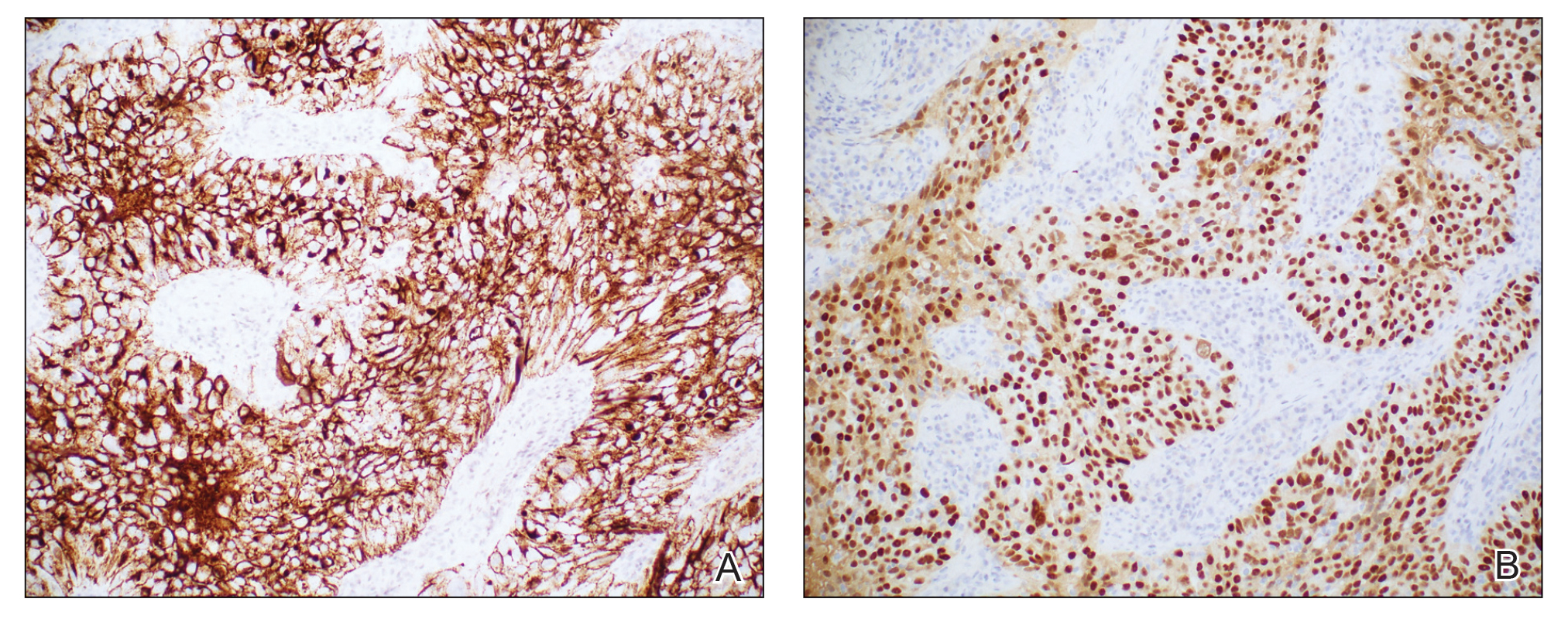
Histopathologic analysis of the biopsy specimen revealed a dense infiltrate of large, hyperchromatic, mucin-producing cells exhibiting varying degrees of nuclear pleomorphism (Figure 1). Immunohistochemical (IHC) staining was negative for cytokeratin (CK) 20; however, CK7 was found positive (Figure 2), which confirmed the presence of a metastatic adenocarcinoma, consistent with the clinical diagnosis of a Sister Mary Joseph nodule (SMJN). Subsequent IHC workup to determine the site of origin revealed densely positive expression of both cancer antigen 125 and paired homeobox gene 8 (PAX-8)(Figure 3), consistent with primary ovarian disease. Furthermore, expression of estrogen receptor and p53 both were positive within the nuclei, illustrating an aberrant expression pattern. On the other hand, cancer antigen 19-9, caudal-type homeobox 2, gross cystic disease fluid protein 15, and mammaglobin were all determined negative, thus leading to the pathologic diagnosis of a metastatic ovarian adenocarcinoma. Additional workup with computed tomography of the abdomen and pelvis highlighted a large left ovarian mass with multiple omental nodules as well as enlarged retroperitoneal and pelvic lymph nodes.

The SMJN is a rare presentation of internal malignancy that appears as a nodule that metastasizes to the umbilicus. It may be ulcerated or necrotic and is seen in up to 10% of patients with cutaneous metastases from internal malignancy.1 These nodules are named after Sister Mary Joseph, the surgical assistant of Dr. William Mayo who first described the relationship between umbilical nodules seen in patients with gastrointestinal and genitourinary cancer. The most common underlying malignancies include primary gastrointestinal and gynecologic adenocarcinomas. In a retrospective study of 34 patients by Chalya et al,2 the stomach was found to be the most common primary site (41.1%). The presence of an SMJN affords a poor prognosis, with a mean overall survival of 11 months from the time of diagnosis.3 The mechanism of disease dissemination remains unknown but is thought to occur through lymphovascular invasion of tumor cells and spread via the umbilical ligament.1,4
Merkel cell carcinoma is a cutaneous neuroendocrine tumor that most commonly presents in elderly patients as red-violet nodules or plaques. Although Merkel cell carcinoma most frequently is encountered on sun-exposed skin, they also can arise on the trunk and abdomen. Positive IHC staining for CK20 would be expected; however, it was negative in our case.5
Cutaneous endometriosis is a rare disease presentation and most commonly occurs as a secondary process due to surgical inoculation of the abdominal wall. Primary cutaneous endometriosis in which there is no history of abdominal surgery less frequently is encountered. Patients typically will report pain and cyclical bleeding with menses. Pathology demonstrates ectopic endometrial tissue with glands and uterine myxoid stroma.6
Amelanotic melanoma is an uncommon subtype of malignant melanoma that presents as nonpigmented nodules that have a propensity to ulcerate and bleed. Furthermore, the umbilicus is an exceedingly rare location for primary melanoma. However, one report does exist, and amelanotic melanoma should be considered in the differential for patients with umbilical nodules.7
Dermoid cysts are benign congenital lesions that typically present as a painless, slow-growing, and wellcircumscribed nodule, as similarly experienced by our patient. They most commonly are found on the testicles and ovaries but also are known to arise in embryologic fusion planes, and reports of umbilical lesions exist.8 Dermoid cysts are diagnosed based on histopathology, supporting the need for a biopsy to distinguish a malignant process from benign lesions.9
- Gabriele R, Conte M, Egidi F, et al. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph’s nodule at a university teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Leyrat B, Bernadach M, Ginzac A, et al. Sister Mary Joseph nodules: a case report about a rare location of skin metastasis. Case Rep Oncol. 2021;14:664-670.
- Yendluri V, Centeno B, Springett GM. Pancreatic cancer presenting as a Sister Mary Joseph’s nodule: case report and update of the literature. Pancreas. 2007;34:161-164.
- Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48.
- Bittar PG, Hryneewycz KT, Bryant EA. Primary cutaneous endometriosis presenting as an umbilical nodule. JAMA Dermatol. 2021;157:1227.
- Kovitwanichkanont T, Joseph S, Yip L. Hidden in plain sight: umbilical melanoma [published online January 28, 2020]. Med J Aust. 2020;212:154-155.e1.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Akinci O, Turker C, Erturk MS, et al. Umbilical dermoid cyst: a rare case. Cerrahpasa Med J. 2020;44:51-53.
The Diagnosis: Sister Mary Joseph Nodule
Histopathologic analysis of the biopsy specimen revealed a dense infiltrate of large, hyperchromatic, mucin-producing cells exhibiting varying degrees of nuclear pleomorphism (Figure 1). Immunohistochemical (IHC) staining was negative for cytokeratin (CK) 20; however, CK7 was found positive (Figure 2), which confirmed the presence of a metastatic adenocarcinoma, consistent with the clinical diagnosis of a Sister Mary Joseph nodule (SMJN). Subsequent IHC workup to determine the site of origin revealed densely positive expression of both cancer antigen 125 and paired homeobox gene 8 (PAX-8)(Figure 3), consistent with primary ovarian disease. Furthermore, expression of estrogen receptor and p53 both were positive within the nuclei, illustrating an aberrant expression pattern. On the other hand, cancer antigen 19-9, caudal-type homeobox 2, gross cystic disease fluid protein 15, and mammaglobin were all determined negative, thus leading to the pathologic diagnosis of a metastatic ovarian adenocarcinoma. Additional workup with computed tomography of the abdomen and pelvis highlighted a large left ovarian mass with multiple omental nodules as well as enlarged retroperitoneal and pelvic lymph nodes.

The SMJN is a rare presentation of internal malignancy that appears as a nodule that metastasizes to the umbilicus. It may be ulcerated or necrotic and is seen in up to 10% of patients with cutaneous metastases from internal malignancy.1 These nodules are named after Sister Mary Joseph, the surgical assistant of Dr. William Mayo who first described the relationship between umbilical nodules seen in patients with gastrointestinal and genitourinary cancer. The most common underlying malignancies include primary gastrointestinal and gynecologic adenocarcinomas. In a retrospective study of 34 patients by Chalya et al,2 the stomach was found to be the most common primary site (41.1%). The presence of an SMJN affords a poor prognosis, with a mean overall survival of 11 months from the time of diagnosis.3 The mechanism of disease dissemination remains unknown but is thought to occur through lymphovascular invasion of tumor cells and spread via the umbilical ligament.1,4

Merkel cell carcinoma is a cutaneous neuroendocrine tumor that most commonly presents in elderly patients as red-violet nodules or plaques. Although Merkel cell carcinoma most frequently is encountered on sun-exposed skin, they also can arise on the trunk and abdomen. Positive IHC staining for CK20 would be expected; however, it was negative in our case.5

Cutaneous endometriosis is a rare disease presentation and most commonly occurs as a secondary process due to surgical inoculation of the abdominal wall. Primary cutaneous endometriosis in which there is no history of abdominal surgery less frequently is encountered. Patients typically will report pain and cyclical bleeding with menses. Pathology demonstrates ectopic endometrial tissue with glands and uterine myxoid stroma.6
Amelanotic melanoma is an uncommon subtype of malignant melanoma that presents as nonpigmented nodules that have a propensity to ulcerate and bleed. Furthermore, the umbilicus is an exceedingly rare location for primary melanoma. However, one report does exist, and amelanotic melanoma should be considered in the differential for patients with umbilical nodules.7
Dermoid cysts are benign congenital lesions that typically present as a painless, slow-growing, and wellcircumscribed nodule, as similarly experienced by our patient. They most commonly are found on the testicles and ovaries but also are known to arise in embryologic fusion planes, and reports of umbilical lesions exist.8 Dermoid cysts are diagnosed based on histopathology, supporting the need for a biopsy to distinguish a malignant process from benign lesions.9
The Diagnosis: Sister Mary Joseph Nodule
Histopathologic analysis of the biopsy specimen revealed a dense infiltrate of large, hyperchromatic, mucin-producing cells exhibiting varying degrees of nuclear pleomorphism (Figure 1). Immunohistochemical (IHC) staining was negative for cytokeratin (CK) 20; however, CK7 was found positive (Figure 2), which confirmed the presence of a metastatic adenocarcinoma, consistent with the clinical diagnosis of a Sister Mary Joseph nodule (SMJN). Subsequent IHC workup to determine the site of origin revealed densely positive expression of both cancer antigen 125 and paired homeobox gene 8 (PAX-8)(Figure 3), consistent with primary ovarian disease. Furthermore, expression of estrogen receptor and p53 both were positive within the nuclei, illustrating an aberrant expression pattern. On the other hand, cancer antigen 19-9, caudal-type homeobox 2, gross cystic disease fluid protein 15, and mammaglobin were all determined negative, thus leading to the pathologic diagnosis of a metastatic ovarian adenocarcinoma. Additional workup with computed tomography of the abdomen and pelvis highlighted a large left ovarian mass with multiple omental nodules as well as enlarged retroperitoneal and pelvic lymph nodes.

The SMJN is a rare presentation of internal malignancy that appears as a nodule that metastasizes to the umbilicus. It may be ulcerated or necrotic and is seen in up to 10% of patients with cutaneous metastases from internal malignancy.1 These nodules are named after Sister Mary Joseph, the surgical assistant of Dr. William Mayo who first described the relationship between umbilical nodules seen in patients with gastrointestinal and genitourinary cancer. The most common underlying malignancies include primary gastrointestinal and gynecologic adenocarcinomas. In a retrospective study of 34 patients by Chalya et al,2 the stomach was found to be the most common primary site (41.1%). The presence of an SMJN affords a poor prognosis, with a mean overall survival of 11 months from the time of diagnosis.3 The mechanism of disease dissemination remains unknown but is thought to occur through lymphovascular invasion of tumor cells and spread via the umbilical ligament.1,4

Merkel cell carcinoma is a cutaneous neuroendocrine tumor that most commonly presents in elderly patients as red-violet nodules or plaques. Although Merkel cell carcinoma most frequently is encountered on sun-exposed skin, they also can arise on the trunk and abdomen. Positive IHC staining for CK20 would be expected; however, it was negative in our case.5

Cutaneous endometriosis is a rare disease presentation and most commonly occurs as a secondary process due to surgical inoculation of the abdominal wall. Primary cutaneous endometriosis in which there is no history of abdominal surgery less frequently is encountered. Patients typically will report pain and cyclical bleeding with menses. Pathology demonstrates ectopic endometrial tissue with glands and uterine myxoid stroma.6
Amelanotic melanoma is an uncommon subtype of malignant melanoma that presents as nonpigmented nodules that have a propensity to ulcerate and bleed. Furthermore, the umbilicus is an exceedingly rare location for primary melanoma. However, one report does exist, and amelanotic melanoma should be considered in the differential for patients with umbilical nodules.7
Dermoid cysts are benign congenital lesions that typically present as a painless, slow-growing, and wellcircumscribed nodule, as similarly experienced by our patient. They most commonly are found on the testicles and ovaries but also are known to arise in embryologic fusion planes, and reports of umbilical lesions exist.8 Dermoid cysts are diagnosed based on histopathology, supporting the need for a biopsy to distinguish a malignant process from benign lesions.9
- Gabriele R, Conte M, Egidi F, et al. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph’s nodule at a university teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Leyrat B, Bernadach M, Ginzac A, et al. Sister Mary Joseph nodules: a case report about a rare location of skin metastasis. Case Rep Oncol. 2021;14:664-670.
- Yendluri V, Centeno B, Springett GM. Pancreatic cancer presenting as a Sister Mary Joseph’s nodule: case report and update of the literature. Pancreas. 2007;34:161-164.
- Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48.
- Bittar PG, Hryneewycz KT, Bryant EA. Primary cutaneous endometriosis presenting as an umbilical nodule. JAMA Dermatol. 2021;157:1227.
- Kovitwanichkanont T, Joseph S, Yip L. Hidden in plain sight: umbilical melanoma [published online January 28, 2020]. Med J Aust. 2020;212:154-155.e1.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Akinci O, Turker C, Erturk MS, et al. Umbilical dermoid cyst: a rare case. Cerrahpasa Med J. 2020;44:51-53.
- Gabriele R, Conte M, Egidi F, et al. Umbilical metastases: current viewpoint. World J Surg Oncol. 2005;3:13.
- Chalya PL, Mabula JB, Rambau PF, et al. Sister Mary Joseph’s nodule at a university teaching hospital in northwestern Tanzania: a retrospective review of 34 cases. World J Surg Oncol. 2013;11:151.
- Leyrat B, Bernadach M, Ginzac A, et al. Sister Mary Joseph nodules: a case report about a rare location of skin metastasis. Case Rep Oncol. 2021;14:664-670.
- Yendluri V, Centeno B, Springett GM. Pancreatic cancer presenting as a Sister Mary Joseph’s nodule: case report and update of the literature. Pancreas. 2007;34:161-164.
- Uchi H. Merkel cell carcinoma: an update and immunotherapy. Front Oncol. 2018;8:48.
- Bittar PG, Hryneewycz KT, Bryant EA. Primary cutaneous endometriosis presenting as an umbilical nodule. JAMA Dermatol. 2021;157:1227.
- Kovitwanichkanont T, Joseph S, Yip L. Hidden in plain sight: umbilical melanoma [published online January 28, 2020]. Med J Aust. 2020;212:154-155.e1.
- Prior A, Anania P, Pacetti M, et al. Dermoid and epidermoid cysts of scalp: case series of 234 consecutive patients. World Neurosurg. 2018;120:119-124.
- Akinci O, Turker C, Erturk MS, et al. Umbilical dermoid cyst: a rare case. Cerrahpasa Med J. 2020;44:51-53.
A 64-year-old woman with no notable medical history was referred to our dermatology clinic with an intermittent eczematous rash around the eyelids of 3 months’ duration. While performing a total-body skin examination, a firm pink nodule with a smooth surface incidentally was discovered on the umbilicus. The patient was uncertain when the lesion first appeared and denied any associated symptoms including pain and bleeding. Additionally, a lymph node examination revealed right inguinal lymphadenopathy. Upon further questioning, she reported worsening muscle weakness, fatigue, night sweats, and an unintentional weight loss of 10 pounds. A 6-mm punch biopsy of the umbilical lesion was obtained for routine histopathology.
Nonblanching Rash on the Legs and Chest
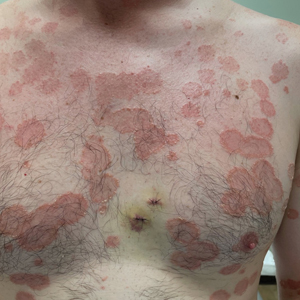
The Diagnosis: Leukemia Cutis
Hematoxylin and eosin staining revealed an infiltration of monomorphic atypical myeloid cells with cleaved nuclei within the dermis, with a relatively uninvolved epidermis (Figure, A). The cells formed aggregates in single-file lines along dermal collagen bundles. Occasional Auer rods, which are crystal aggregates of the enzyme myeloperoxidase, a marker unique to cells of the myeloid lineage (Figure, B) were appreciated.
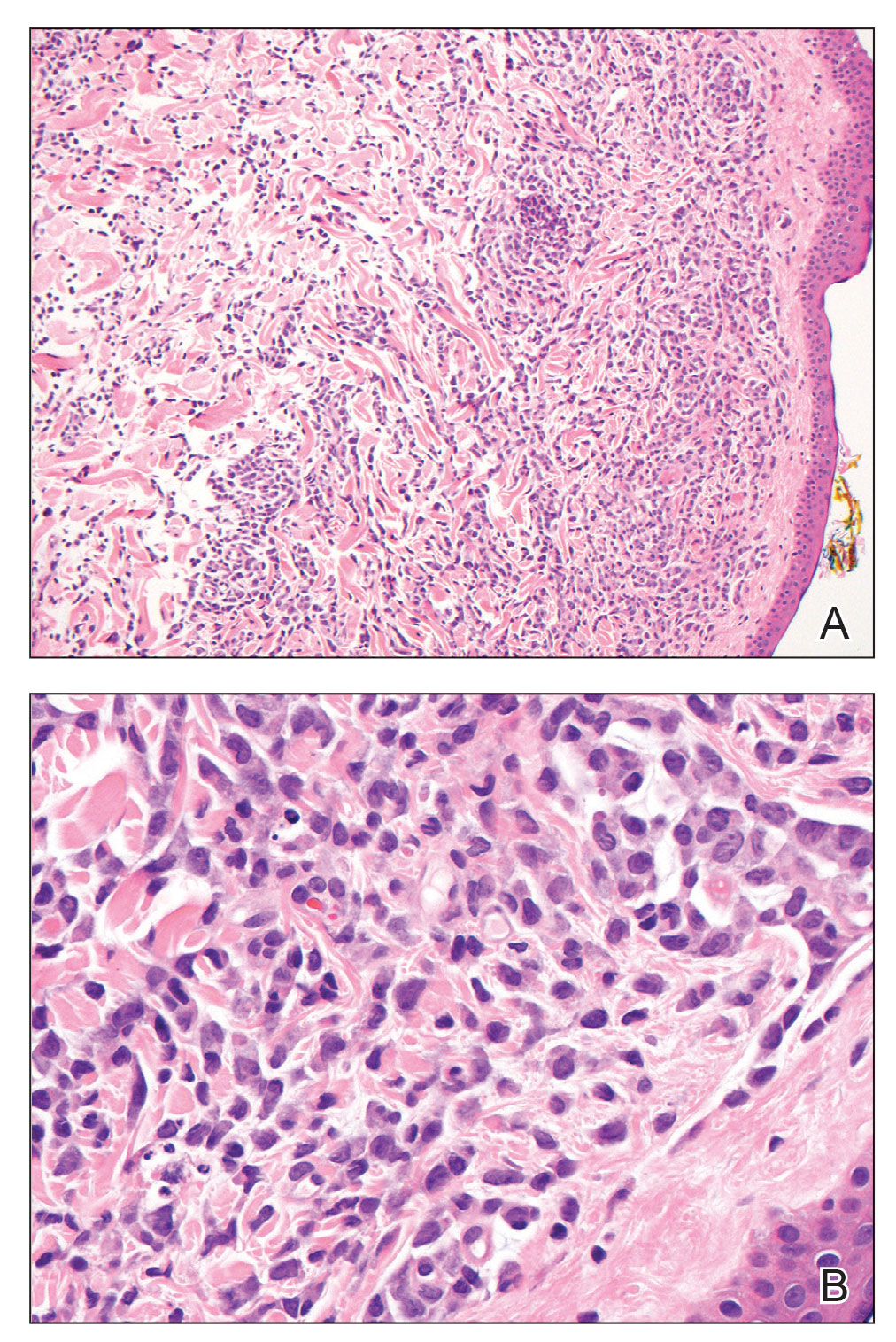
Immunohistochemical staining for myeloperoxidase was weakly positive; however, flow cytometric evaluation of the bone marrow aspirate revealed that approximately 20% of all CD45+ cells were myeloid blasts. These findings confirmed the diagnosis of recurrent acute myeloid leukemia (AML). The diagnosis of AML can be confirmed with a bone marrow biopsy demonstrating more than 20% of the total cells in blast form as well as evidence that the cells are of myeloid origin, which can be inferred by the presence of Auer rods, positive myeloperoxidase staining, or immunophenotyping. In our patient, the Auer rods, myeloperoxidase staining, and atypical myeloid cells on skin biopsy, in conjunction with the bone marrow biopsy results, confirmed leukemia cutis.
Leukemia cutis is the infiltration of neoplastic proliferating leukocytes in the epidermis, dermis, or subcutis from a primary or more commonly metastatic malignancy. Leukemic cutaneous involvement is seen in up to 13% of leukemia patients and most commonly is seen in monocytic or myelomonocytic forms of AML.1 It may present anywhere on the body but mostly is found on the back, trunk, and head. It also may have a predilection for areas with a history of trauma or inflammation. The lesions most often are firm, erythematous to violaceous papules and nodules, though leukemia cutis can present with hemorrhagic ulcers, purpura, or other cutaneous manifestations of concomitant thrombocytopenia such as petechiae and ecchymoses.2 Involvement of the lower extremities mimicking venous stasis dermatitis has been described.3,4
Treatment of leukemia cutis requires targeting the underlying leukemia2 under the guidance of hematology and oncology as well as the use of chemotherapeutic agents.5 The presence of leukemia cutis is a poor prognostic sign, and a discussion regarding goals of care often is appropriate. Our patient initially responded to FLAG (fludarabine, cytarabine, filgrastim) chemotherapy induction and consolidation, which was followed by midostaurin maintenance. However, she ultimately regressed, requiring decitabine and gilteritinib treatment, and died 9 months later from the course of the disease.
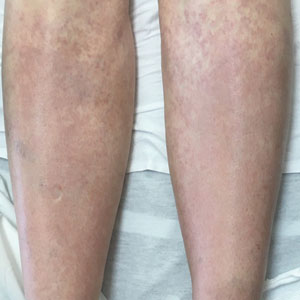
Although typically asymptomatic and presenting on the lower limbs, capillaritis (also known as the pigmented purpuric dermatoses) consists of a set of cutaneous conditions that often are chronic and relapsing in nature, as opposed to our patient’s subacute presentation. These benign conditions have several distinct morphologies; some are characterized by pigmented macules or pinpoint red-brown petechiae that most often are found on the legs but also are seen on the trunk and upper extremities.6 Of the various clinical presentations of capillaritis, our patient’s skin findings may be most consistent with pigmented purpuric lichenoid dermatitis of Gougerot and Blum, in which purpuric red-brown papules coalesce into plaques, though her lesions were not raised. The other pigmented purpuric dermatoses can present with cayenne pepper–colored petechiae, golden-brown macules, pruritic purpuric patches, or red-brown annular patches,6 which were not seen in our patient.
Venous stasis dermatitis also favors the lower extremities7; however, it classically includes the medial malleolus and often presents with scaling and hyperpigmentation from hemosiderin deposition.8 It often is associated with pruritus, as opposed to the nonpruritic nonpainful lesions in leukemia cutis. Other signs of venous insufficiency also may be appreciated, including edema or varicose veins,7 which were not evident in our patient.
Leukocytoclastic vasculitis, a small vessel vasculitis, also appears as palpable or macular purpura, which classically is asymptomatic and erupts on the shins approximately 1 week after an inciting exposure,9 such as medications, pathogens, or autoimmune diseases. One of the least distinctive vasculitides is polyarteritis nodosa, a form of medium vessel vasculitis, which presents most often with palpable purpura or painful nodules on the lower extremities and may be accompanied by livedo reticularis or digital necrosis.9 Acute leukemia may be accompanied by inflammatory paraneoplastic conditions including vasculitis, which is thought to be due to leukemic cells infiltrating and damaging blood vessels.10
Pretibial myxedema is closely associated with Graves disease and shares some features seen in the presentation of our patient’s leukemia cutis. It is asymptomatic, classically affects the pretibial regions, and most commonly affects older adults and women.11,12 Pretibial myxedema presents with thick indurated plaques rather than patches. Our patient did not demonstrate ophthalmopathy, which nearly always precedes pretibial myxedema.12 The most common form of pretibial myxedema is nonpitting, though nodular, plaquelike, polypoid, and elephantiasic forms also exist.11 Pretibial myxedema classically favors the shins; however, it also can affect the ankles, dorsal aspects of the feet, and toes. The characteristic induration of the skin is believed to be the result of excess fibroblast production of glycosaminoglycans in the dermis and subcutis likely triggered by stimulation of fibroblast thyroid stimulating hormone receptors.11
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:973-977.
- Papadavid E, Panayiotides I, Katoulis A, et al. Stasis dermatitis-like leukaemic infiltration in a patient with myelodysplastic syndrome. Clin Exp Dermatol. 2008;33:298-300.
- Chang HY, Wong KM, Bosenberg M, et al. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128-129.
- Aguilera SB, Zarraga M, Rosen L. Leukemia cutis in a patient with acute myelogenous leukemia: a case report and review of the literature. Cutis. 2010;85:31-36.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other eczematous eruptions. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:103-108.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Lorenzo C, eds. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Jones D, Dorfman DM, Barnhill RL, et al. Leukemic vasculitis: a feature of leukemia cutis in some patients. Am J Clin Pathol. 1997;107:637-642.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
The Diagnosis: Leukemia Cutis
Hematoxylin and eosin staining revealed an infiltration of monomorphic atypical myeloid cells with cleaved nuclei within the dermis, with a relatively uninvolved epidermis (Figure, A). The cells formed aggregates in single-file lines along dermal collagen bundles. Occasional Auer rods, which are crystal aggregates of the enzyme myeloperoxidase, a marker unique to cells of the myeloid lineage (Figure, B) were appreciated.

Immunohistochemical staining for myeloperoxidase was weakly positive; however, flow cytometric evaluation of the bone marrow aspirate revealed that approximately 20% of all CD45+ cells were myeloid blasts. These findings confirmed the diagnosis of recurrent acute myeloid leukemia (AML). The diagnosis of AML can be confirmed with a bone marrow biopsy demonstrating more than 20% of the total cells in blast form as well as evidence that the cells are of myeloid origin, which can be inferred by the presence of Auer rods, positive myeloperoxidase staining, or immunophenotyping. In our patient, the Auer rods, myeloperoxidase staining, and atypical myeloid cells on skin biopsy, in conjunction with the bone marrow biopsy results, confirmed leukemia cutis.
Leukemia cutis is the infiltration of neoplastic proliferating leukocytes in the epidermis, dermis, or subcutis from a primary or more commonly metastatic malignancy. Leukemic cutaneous involvement is seen in up to 13% of leukemia patients and most commonly is seen in monocytic or myelomonocytic forms of AML.1 It may present anywhere on the body but mostly is found on the back, trunk, and head. It also may have a predilection for areas with a history of trauma or inflammation. The lesions most often are firm, erythematous to violaceous papules and nodules, though leukemia cutis can present with hemorrhagic ulcers, purpura, or other cutaneous manifestations of concomitant thrombocytopenia such as petechiae and ecchymoses.2 Involvement of the lower extremities mimicking venous stasis dermatitis has been described.3,4
Treatment of leukemia cutis requires targeting the underlying leukemia2 under the guidance of hematology and oncology as well as the use of chemotherapeutic agents.5 The presence of leukemia cutis is a poor prognostic sign, and a discussion regarding goals of care often is appropriate. Our patient initially responded to FLAG (fludarabine, cytarabine, filgrastim) chemotherapy induction and consolidation, which was followed by midostaurin maintenance. However, she ultimately regressed, requiring decitabine and gilteritinib treatment, and died 9 months later from the course of the disease.
Although typically asymptomatic and presenting on the lower limbs, capillaritis (also known as the pigmented purpuric dermatoses) consists of a set of cutaneous conditions that often are chronic and relapsing in nature, as opposed to our patient’s subacute presentation. These benign conditions have several distinct morphologies; some are characterized by pigmented macules or pinpoint red-brown petechiae that most often are found on the legs but also are seen on the trunk and upper extremities.6 Of the various clinical presentations of capillaritis, our patient’s skin findings may be most consistent with pigmented purpuric lichenoid dermatitis of Gougerot and Blum, in which purpuric red-brown papules coalesce into plaques, though her lesions were not raised. The other pigmented purpuric dermatoses can present with cayenne pepper–colored petechiae, golden-brown macules, pruritic purpuric patches, or red-brown annular patches,6 which were not seen in our patient.
Venous stasis dermatitis also favors the lower extremities7; however, it classically includes the medial malleolus and often presents with scaling and hyperpigmentation from hemosiderin deposition.8 It often is associated with pruritus, as opposed to the nonpruritic nonpainful lesions in leukemia cutis. Other signs of venous insufficiency also may be appreciated, including edema or varicose veins,7 which were not evident in our patient.
Leukocytoclastic vasculitis, a small vessel vasculitis, also appears as palpable or macular purpura, which classically is asymptomatic and erupts on the shins approximately 1 week after an inciting exposure,9 such as medications, pathogens, or autoimmune diseases. One of the least distinctive vasculitides is polyarteritis nodosa, a form of medium vessel vasculitis, which presents most often with palpable purpura or painful nodules on the lower extremities and may be accompanied by livedo reticularis or digital necrosis.9 Acute leukemia may be accompanied by inflammatory paraneoplastic conditions including vasculitis, which is thought to be due to leukemic cells infiltrating and damaging blood vessels.10
Pretibial myxedema is closely associated with Graves disease and shares some features seen in the presentation of our patient’s leukemia cutis. It is asymptomatic, classically affects the pretibial regions, and most commonly affects older adults and women.11,12 Pretibial myxedema presents with thick indurated plaques rather than patches. Our patient did not demonstrate ophthalmopathy, which nearly always precedes pretibial myxedema.12 The most common form of pretibial myxedema is nonpitting, though nodular, plaquelike, polypoid, and elephantiasic forms also exist.11 Pretibial myxedema classically favors the shins; however, it also can affect the ankles, dorsal aspects of the feet, and toes. The characteristic induration of the skin is believed to be the result of excess fibroblast production of glycosaminoglycans in the dermis and subcutis likely triggered by stimulation of fibroblast thyroid stimulating hormone receptors.11
The Diagnosis: Leukemia Cutis
Hematoxylin and eosin staining revealed an infiltration of monomorphic atypical myeloid cells with cleaved nuclei within the dermis, with a relatively uninvolved epidermis (Figure, A). The cells formed aggregates in single-file lines along dermal collagen bundles. Occasional Auer rods, which are crystal aggregates of the enzyme myeloperoxidase, a marker unique to cells of the myeloid lineage (Figure, B) were appreciated.

Immunohistochemical staining for myeloperoxidase was weakly positive; however, flow cytometric evaluation of the bone marrow aspirate revealed that approximately 20% of all CD45+ cells were myeloid blasts. These findings confirmed the diagnosis of recurrent acute myeloid leukemia (AML). The diagnosis of AML can be confirmed with a bone marrow biopsy demonstrating more than 20% of the total cells in blast form as well as evidence that the cells are of myeloid origin, which can be inferred by the presence of Auer rods, positive myeloperoxidase staining, or immunophenotyping. In our patient, the Auer rods, myeloperoxidase staining, and atypical myeloid cells on skin biopsy, in conjunction with the bone marrow biopsy results, confirmed leukemia cutis.
Leukemia cutis is the infiltration of neoplastic proliferating leukocytes in the epidermis, dermis, or subcutis from a primary or more commonly metastatic malignancy. Leukemic cutaneous involvement is seen in up to 13% of leukemia patients and most commonly is seen in monocytic or myelomonocytic forms of AML.1 It may present anywhere on the body but mostly is found on the back, trunk, and head. It also may have a predilection for areas with a history of trauma or inflammation. The lesions most often are firm, erythematous to violaceous papules and nodules, though leukemia cutis can present with hemorrhagic ulcers, purpura, or other cutaneous manifestations of concomitant thrombocytopenia such as petechiae and ecchymoses.2 Involvement of the lower extremities mimicking venous stasis dermatitis has been described.3,4
Treatment of leukemia cutis requires targeting the underlying leukemia2 under the guidance of hematology and oncology as well as the use of chemotherapeutic agents.5 The presence of leukemia cutis is a poor prognostic sign, and a discussion regarding goals of care often is appropriate. Our patient initially responded to FLAG (fludarabine, cytarabine, filgrastim) chemotherapy induction and consolidation, which was followed by midostaurin maintenance. However, she ultimately regressed, requiring decitabine and gilteritinib treatment, and died 9 months later from the course of the disease.
Although typically asymptomatic and presenting on the lower limbs, capillaritis (also known as the pigmented purpuric dermatoses) consists of a set of cutaneous conditions that often are chronic and relapsing in nature, as opposed to our patient’s subacute presentation. These benign conditions have several distinct morphologies; some are characterized by pigmented macules or pinpoint red-brown petechiae that most often are found on the legs but also are seen on the trunk and upper extremities.6 Of the various clinical presentations of capillaritis, our patient’s skin findings may be most consistent with pigmented purpuric lichenoid dermatitis of Gougerot and Blum, in which purpuric red-brown papules coalesce into plaques, though her lesions were not raised. The other pigmented purpuric dermatoses can present with cayenne pepper–colored petechiae, golden-brown macules, pruritic purpuric patches, or red-brown annular patches,6 which were not seen in our patient.
Venous stasis dermatitis also favors the lower extremities7; however, it classically includes the medial malleolus and often presents with scaling and hyperpigmentation from hemosiderin deposition.8 It often is associated with pruritus, as opposed to the nonpruritic nonpainful lesions in leukemia cutis. Other signs of venous insufficiency also may be appreciated, including edema or varicose veins,7 which were not evident in our patient.
Leukocytoclastic vasculitis, a small vessel vasculitis, also appears as palpable or macular purpura, which classically is asymptomatic and erupts on the shins approximately 1 week after an inciting exposure,9 such as medications, pathogens, or autoimmune diseases. One of the least distinctive vasculitides is polyarteritis nodosa, a form of medium vessel vasculitis, which presents most often with palpable purpura or painful nodules on the lower extremities and may be accompanied by livedo reticularis or digital necrosis.9 Acute leukemia may be accompanied by inflammatory paraneoplastic conditions including vasculitis, which is thought to be due to leukemic cells infiltrating and damaging blood vessels.10
Pretibial myxedema is closely associated with Graves disease and shares some features seen in the presentation of our patient’s leukemia cutis. It is asymptomatic, classically affects the pretibial regions, and most commonly affects older adults and women.11,12 Pretibial myxedema presents with thick indurated plaques rather than patches. Our patient did not demonstrate ophthalmopathy, which nearly always precedes pretibial myxedema.12 The most common form of pretibial myxedema is nonpitting, though nodular, plaquelike, polypoid, and elephantiasic forms also exist.11 Pretibial myxedema classically favors the shins; however, it also can affect the ankles, dorsal aspects of the feet, and toes. The characteristic induration of the skin is believed to be the result of excess fibroblast production of glycosaminoglycans in the dermis and subcutis likely triggered by stimulation of fibroblast thyroid stimulating hormone receptors.11
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:973-977.
- Papadavid E, Panayiotides I, Katoulis A, et al. Stasis dermatitis-like leukaemic infiltration in a patient with myelodysplastic syndrome. Clin Exp Dermatol. 2008;33:298-300.
- Chang HY, Wong KM, Bosenberg M, et al. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128-129.
- Aguilera SB, Zarraga M, Rosen L. Leukemia cutis in a patient with acute myelogenous leukemia: a case report and review of the literature. Cutis. 2010;85:31-36.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other eczematous eruptions. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:103-108.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Lorenzo C, eds. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Jones D, Dorfman DM, Barnhill RL, et al. Leukemic vasculitis: a feature of leukemia cutis in some patients. Am J Clin Pathol. 1997;107:637-642.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Bakst RL, Tallman MS, Douer D, et al. How I treat extramedullary acute myeloid leukemia. Blood. 2011;118:3785-3793.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other lymphoproliferative and myeloproliferative diseases. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:973-977.
- Papadavid E, Panayiotides I, Katoulis A, et al. Stasis dermatitis-like leukaemic infiltration in a patient with myelodysplastic syndrome. Clin Exp Dermatol. 2008;33:298-300.
- Chang HY, Wong KM, Bosenberg M, et al. Myelogenous leukemia cutis resembling stasis dermatitis. J Am Acad Dermatol. 2003;49:128-129.
- Aguilera SB, Zarraga M, Rosen L. Leukemia cutis in a patient with acute myelogenous leukemia: a case report and review of the literature. Cutis. 2010;85:31-36.
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410.
- Bolognia JL, Schaffer JV, Duncan KO, et al. Other eczematous eruptions. In: Bolognia JL, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2014:103-108.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Wetter DA, Dutz JP, Shinkai K, et al. Cutaneous vasculitis. In: Bolognia JL, Schaffer JV, Lorenzo C, eds. Dermatology. 4th ed. Elsevier; 2018:409-439.
- Jones D, Dorfman DM, Barnhill RL, et al. Leukemic vasculitis: a feature of leukemia cutis in some patients. Am J Clin Pathol. 1997;107:637-642.
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
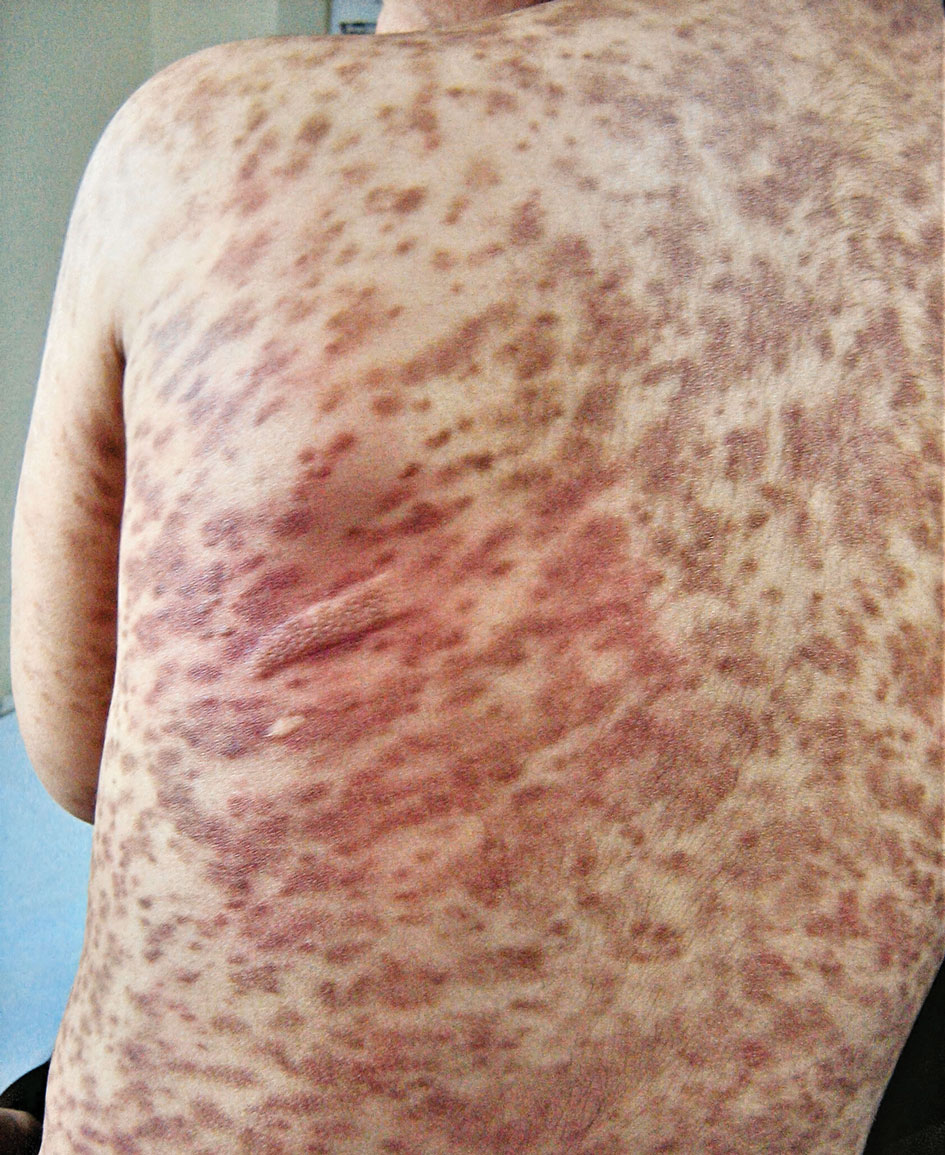
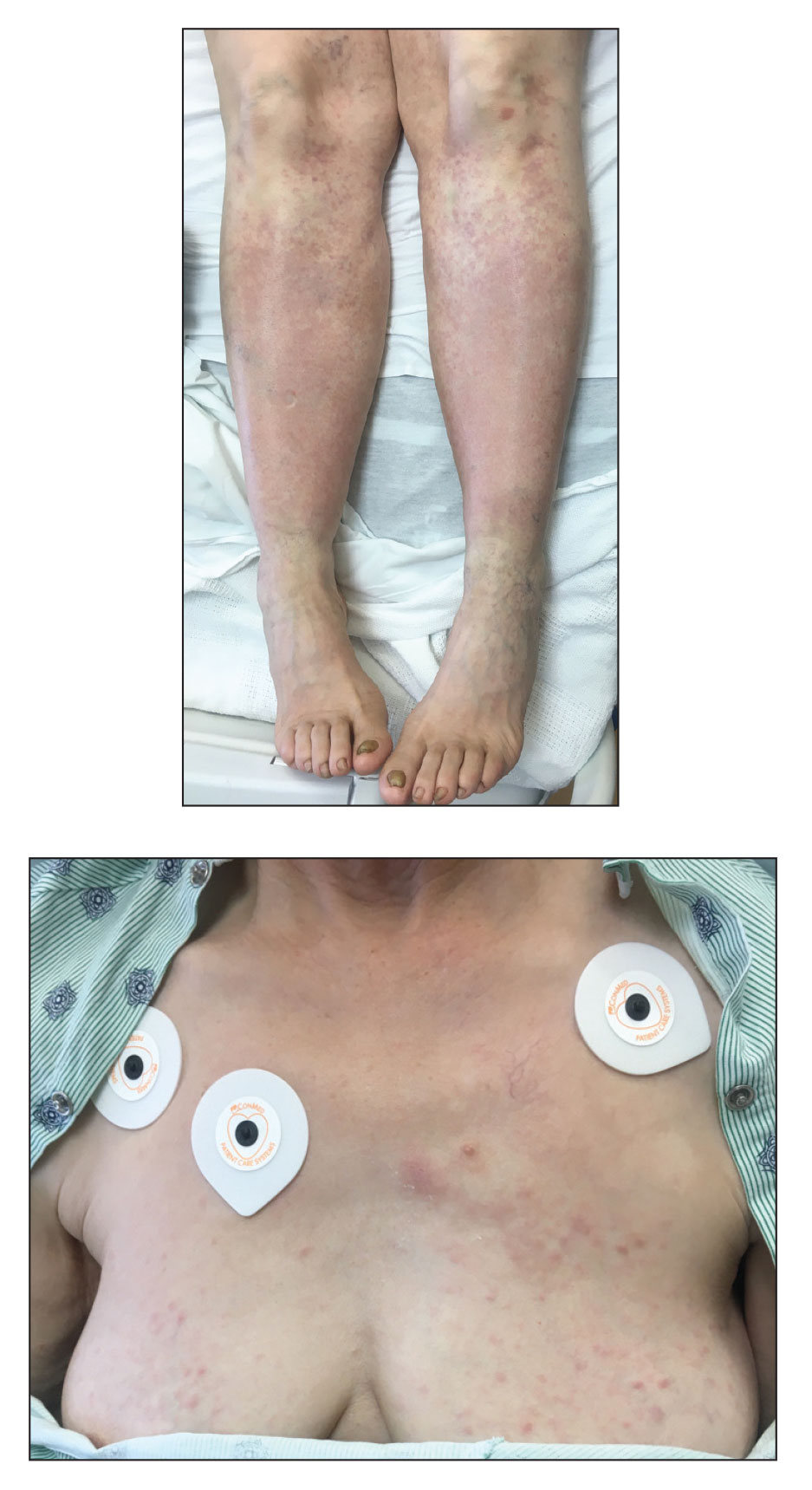
A 67-year-old woman with history of atrial fibrillation and leukemia presented with a nonpruritic nonpainful rash of 10 days' duration that began on the distal lower extremities (top) and then spread superiorly. She reported having a sore throat and mouth, cough, night sweats, unintentional weight loss, and lymphadenopathy. Physical examination revealed pink-purple nonblanching macules and patches on the lower extremities extending from the ankles to the knees. She also had firm pink papules on the chest (bottom) and back. Punch biopsies of the skin on the chest and leg were obtained for histologic examination and immunohistochemical staining.
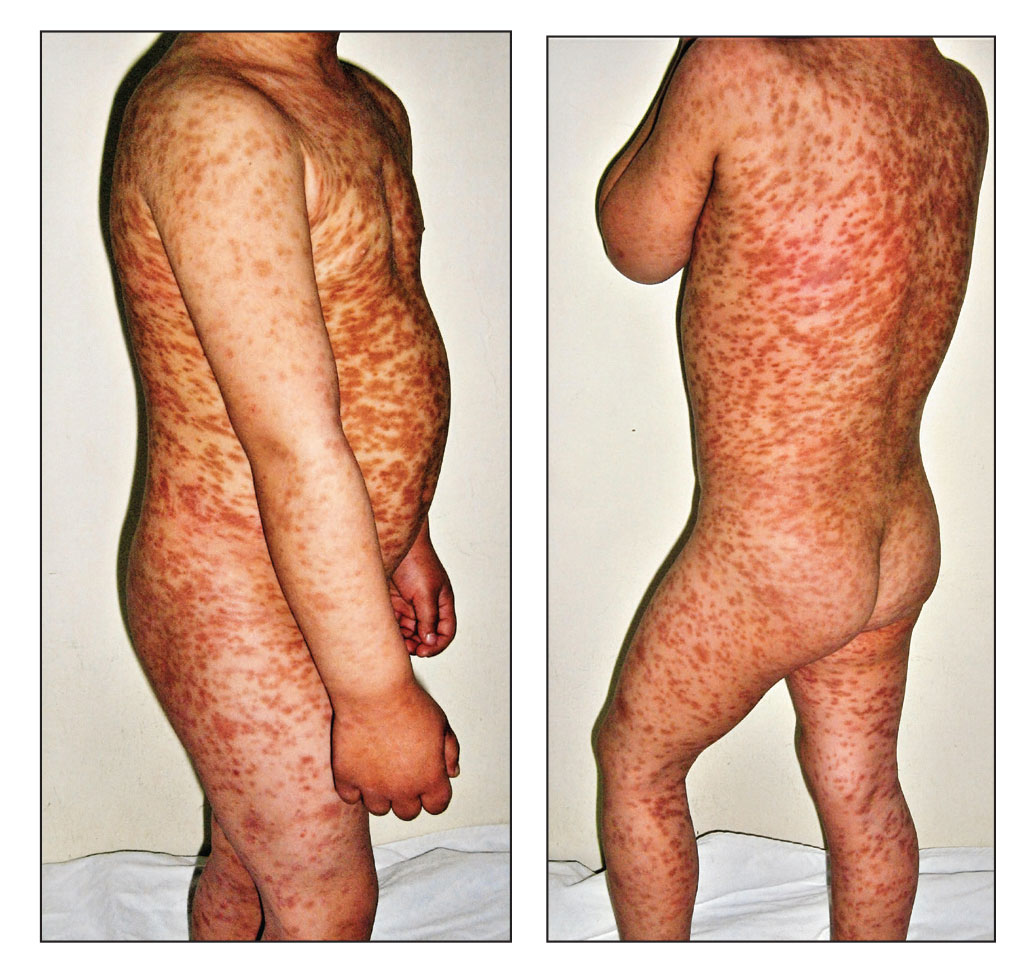
Cutaneous Eruption in an Immunocompromised Patient
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.
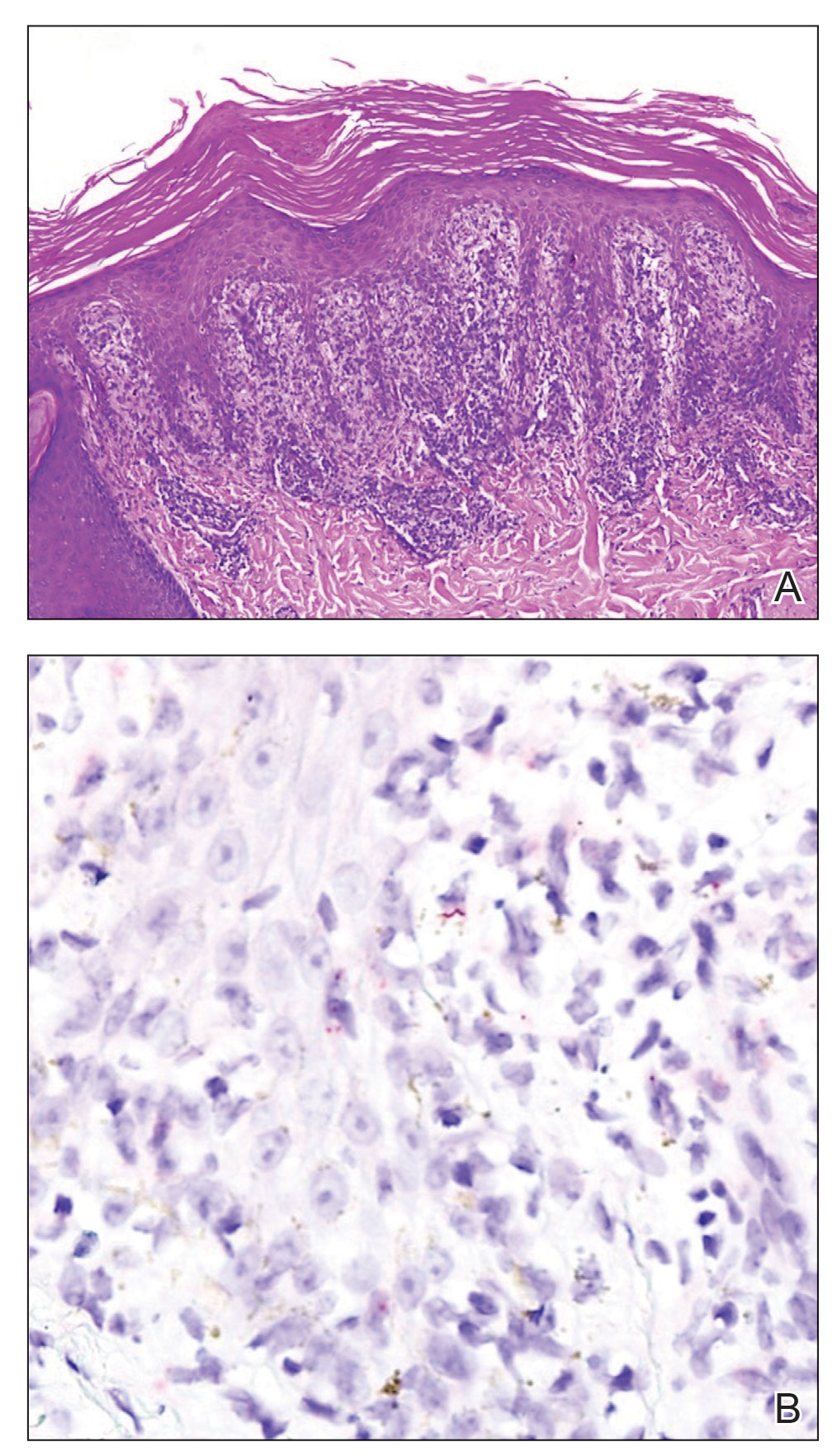
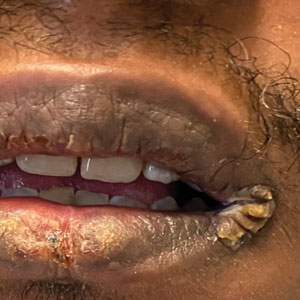
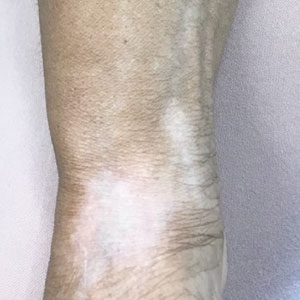
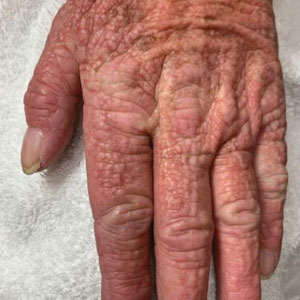
Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.

Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
The Diagnosis: Secondary Syphilis
Histopathology revealed a lichenoid interface dermatitis with psoriasiform hyperplasia (Figure 1A). A single spirochete was identified using immunohistochemical staining (Figure 1B). Laboratory workup revealed positive IgG and IgM treponemal antibodies and reactive rapid plasma reagin titer of 1:2048. A VDRL test performed on a cerebrospinal fluid specimen also was reactive at 1:8. A diagnosis of secondary syphilis with neurologic involvement was made, and the patient was treated with intravenous penicillin G for 14 days. Following treatment, his rapid plasma reagin decreased 4-fold with an improvement in his ocular and cutaneous symptoms.

Mucocutaneus manifestations of secondary syphilis are multitudinous. As in our patient, the classic presentation is a generalized morbilliform and papulosquamous eruption involving the palms (Figure 2) and soles. Split papules at the oral commissures, mucosal patches, and condyloma lata are the characteristic mucosal lesions of secondary syphilis.1 Patchy nonscarring alopecia is not uncommon and can be the only manifestation of secondary syphilis.2 The histopathologic features of secondary syphilis vary depending on the location and type of the skin eruption. Psoriasiform or lichenoid changes commonly occur in the epidermis and dermoepidermal junction.3 The dermal inflammatory patterns that have been described include granulomatous, nodular, and superficial and deep perivascular inflammation. The infiltrate often is composed of lymphocytes, plasma cells, and histocytes. Reactive endothelial cells and perineural plasma cell infiltrates also are common histologic features.3,4 Spirochetes can be identified in most cases using immunohistochemical staining; however, the absence of spirochetes does not exclude syphilis.3 The sensitivity of immunohistochemical staining in secondary syphilis is reported to be 71% to 100% with a very high specificity.5 The treatment for all stages of syphilis is benzathine penicillin G, and the route of administration and duration of treatment depend on the stage of disease.6

A broad differential diagnosis must be considered when encountering skin eruptions in patients with HIV. Psoriasis usually presents as circumscribed erythematous plaques with dry and silvery scaling and a predilection for the extensor surfaces of the limbs, sacrum, scalp, and nails. Nail manifestations include distal onycholysis, irregular pitting, oil spots, salmon patches, and subungual hyperkeratosis. Alopecia occasionally may be seen within scalp lesions7; however, the constellation of alopecia with a moth-eaten appearance, subungual hyperkeratosis, papulosquamous eruption, and split papules was more suggestive of secondary syphilis in our patient. In immunocompromised patients, crusted scabies can be considered for the diagnosis of papulosquamous eruptions involving the palms and soles. It often presents with symmetric, mildly pruritic, psoriasiform dermatitis that favors acral sites, but widespread involvement can be observed.8 Areas of the scalp and face can be affected in infants, elderly patients, and immunocompromised individuals. Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in scabies.
Sarcoidosis is common in Black individuals, and similar to syphilis, it is considered a great imitator of other dermatologic diseases. Frequently, it presents as redviolaceous papules, nodules, or plaques; however, rare variants including psoriasiform, ichthyosiform, verrucous, and lichenoid skin eruptions can occur. Nail dystrophy, split papules, and alopecia also have been observed.9 Ocular involvement is common and frequently presents as uveitis.10 The pathologic hallmark of sarcoidosis is noncaseating granulomatous inflammation, which also may occur in syphilitic lesions9; however, a papulosquamous eruption involving the palms and soles, positive serology, and the finding of interface lichenoid dermatitis with psoriasiform hyperplasia confirmed the diagnosis of secondary syphilis in our patient. Pityriasis rubra pilaris is a rare papulosquamous disorder that can be associated with HIV (type VI/HIVassociated follicular syndrome). It presents with generalized red-orange keratotic papules and often is associated with acne conglobata, hidradenitis suppurativa, and lichen spinulosus.11 Unlike in secondary syphilis, patchy alopecia, split papules, and ocular symptoms typically are not observed in pityriasis rubra pilaris.
This case highlights many classical findings of secondary syphilis and demonstrates that, while helpful, routine skin biopsy may not be required. Treatment should be guided by clinical presentation and serologic testing while reserving skin biopsy for equivocal cases.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004; 31:595-599.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention [published online February 8, 2020]. J Am Acad Dermatol. 2020;82:17-28.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386:983-994.
- Karthikeyan K. Crusted scabies. Indian J Dermatol Venereol Leprol. 2009;75:340-347.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part I. cutaneous disease. J Am Acad Dermatol. 2012;66:699.e1-718.
- Haimovic A, Sanchez M, Judson MA, et al. Sarcoidosis: a comprehensive review and update for the dermatologist: part II. extracutaneous disease. J Am Acad Dermatol. 2012;66:719.e1-730.
- Miralles E, Núñez M, De Las Heras M, et al. Pityriasis rubra pilaris and human immunodeficiency virus infection. Br J Dermatol. 1995;133:990-993.
A 29-year-old Black man with long-standing untreated HIV presented with mildly pruritic, scaly plaques on the palms and soles of 2 weeks’ duration. His medical history was notable for primary syphilis treated approximately 1 year prior. A review of symptoms was positive for blurry vision and floaters but negative for constitutional symptoms. Physical examination revealed well-defined scaly plaques over the palms, soles, and elbows with subungual hyperkeratosis. Patches of nonscarring alopecia over the scalp and split papules at the oral commissures also were noted. There were no palpable lymph nodes or genital involvement. Eye examination showed conjunctival injection and 20 cells per field in the vitreous humor. Laboratory evaluation revealed an HIV viral load of 31,623 copies/mL and a CD4 count of 47 cells/μL (reference range, 362–1531 cells/μL). A shave biopsy of the left elbow was performed for histopathologic evaluation.
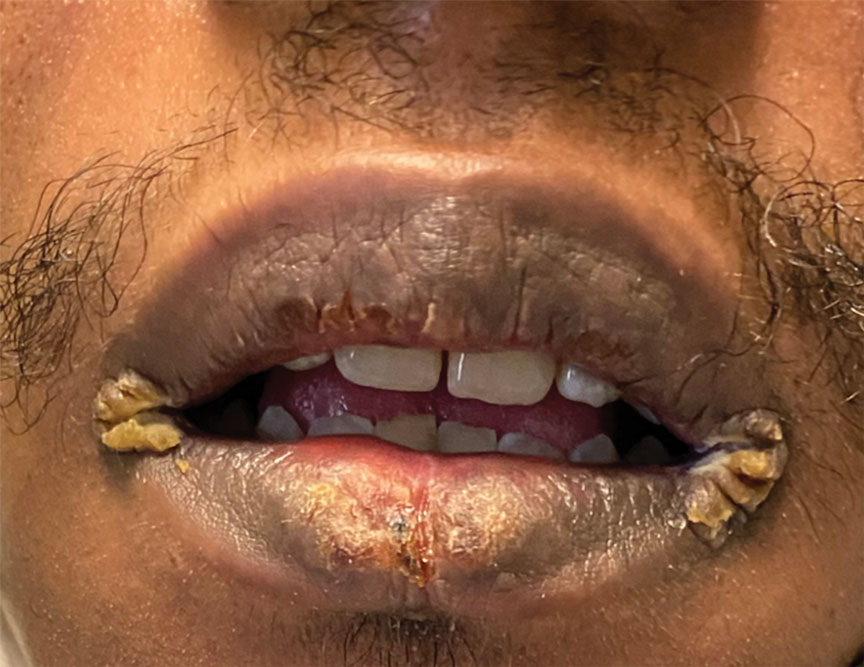
Tender Nonhealing Lesion on the Leg
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare life-threatening condition that most often is seen in patients with end-stage renal disease at a rate of 35 per 10,000 chronic dialysis patients.1 It less commonly has been described in nonuremic patients. The exact incidence of nonuremic calciphylaxis is unknown, but multiple risk factors have been identified, such as alcoholic liver disease, primary hyperparathyroidism, connective tissue diseases, and underlying malignancies. Other less common risk factors include type 2 diabetes mellitus, hypercoagulable disorders, obesity, hypoalbuminemia, and warfarin/ corticosteroid use.2 However, most often no obvious triggers are identified.1
Regardless of the etiology, calciphylaxis is characterized by the calcification of blood vessels and connective tissues, leading to vessel injury, intimal fibrosis, and thrombosis, followed by ischemic necrosis of the skin and soft tissue. It is postulated that microvascular calcification occurs as an active cell-mediated process that depends on the balance between the promoters and inhibitors of calcification.1 In our patient, liver disease likely predisposed formation of calcification through the creation of an environment susceptible to vascular injury via decreased synthesis of proteins C and S.3 Synthesis of fetuin-A, a protein that acts as a circulating inhibitor of vascular ossification/calcification, also is decreased in calcification. Another inhibitor of calcification, matrix Gla protein, is unable to undergo activation through vitamin K–dependent carboxylation secondary to liver disease–induced vitamin K deficiency.3 Microvascular calcification without calciphylaxis may occur in other conditions such as type 2 diabetes mellitus. Therefore, clinicopathologic correlation is important in determining the diagnosis.
Calciphylaxis has a variety of clinical presentations depending on the stage of disease. It begins as a fixed, indurated, livedo reticularis–like plaque. The lesions become increasingly violaceous with intermixed areas of light blanched skin secondary to ischemia and then develop retiform pupura.4 Eventually, affected sites can become bullous and ulcerate or form a necrotic eschar. Severe pain is a cardinal feature throughout all stages.4 Lesions in nonuremic calciphylaxis most commonly are located in the central and/or proximal areas of the body.2
Clinical suspicion is essential for diagnosis. Skin biopsy is the standard method for confirmation in unclear cases. The classic histologic features include intravascular and extravascular calcification, microthrombosis, and fibrointimal hyperplasia of the small dermal and subcutaneous arteries and arterioles, leading to ischemia and intense septal panniculitis.1 Von Kossa immunostaining is used to increase the detection of calcium deposits (Figure 1).1 In addition to the classic changes, our case demonstrated a rare histologic variant with pseudoxanthoma elasticum (PXE)–like changes (Figure 2), which are thought to occur secondary to pathologic elastin fibrogenesis or increased proteolytic activity resulting in abnormal remodeling of the extracellular matrix in the setting of increased calcification of elastin fibers.5 Detection of PXE-like changes may be a helpful clue when specimens lack other characteristic signs.

Wound care, pain control, and addressing underlying causes are mainstays of therapy. Sodium thiosulfate, an antioxidant with vasodilatory properties that also inhibits adipocyte calcification and blocks the ability of adipocytes to induce calcification of vascular smooth-muscle cells, also is useful. Antibiotic prophylaxis is not indicated.1

Even with treatment, both uremic and nonuremic calciphylaxis have a dismal prognosis; 1-year mortality is approximately 50% to 60% and rises to 80% at 2 years.4 Lesion location affects prognosis, and more proximal lesions portend worse outcomes. In patients with both proximal and distal lesions, there is a 90% mortality rate within 1 year. Ulceration also portends worse outcomes, as the wounds often are resistant to healing and act as nidi for infection.4 Septicemia is the most common cause of death.1
Ecthyma gangrenosum is a cutaneous manifestation secondary to an infection most commonly associated with Pseudomonas aeruginosa.6 It often presents in immunocompromised patients with an underlying gramnegative septicemia.7 The clinical presentation initially begins with painless macules that rapidly progress into necrotic ulcers, usually accompanied by associated systemic symptoms such as fever, chills, and hypotension. Histopathology reveals numerous gram-negative rods around necrotic vessels.7
Idiopathic purpura fulminans is the rarest form of purpura fulminans. It is caused by autoantibody formation against protein S, resulting in protein S depletion and subsequent hypercoagulability.8 It usually occurs 7 to 10 days after the onset of a precipitating infection. Lesions begin as erythematous macules that progress within hours to painful, sharply defined areas of purpura and hemorrhagic cutaneous necrosis that may extend to deeper tissues.8 Secondary infection of gangrenous tissue may occur. Distribution usually is diffuse and signs of septic shock and disseminated intravascular coagulation usually are present.
Hughes syndrome, also known as antiphospholipid syndrome, is an acquired autoimmune disorder that manifests clinically as recurrent arterial or venous thrombosis.9 Cutaneous manifestations consist of livedo reticularis, arterial and venous ulcers, and superficial thrombophlebitis.10 Laboratory testing for antiphospholipid antibodies and obtaining a detailed history of the patient’s cardiovascular health are crucial for diagnosis.9
Necrotizing fasciitis typically begins as an inconspicuous superficial cutaneous infection that rapidly is transmitted to the fascia. Infection can spread along fascial planes for several days without affecting the overlying skin, leading to delayed diagnosis.11 The first signs to appear are disproportionate pain and a change in skin color to reddish-purple or bluish-gray. Next, the skin will become indurated, swollen, shiny, and more painful.11 Skin breakdown will begin in 3 to 5 days and is accompanied by bullae and cutaneous gangrene. The involved area becomes painless due to thrombosis of the small vessels that supply the superficial nerves.12 Septic shock ultimately will develop if untreated.
We present a rare case of nonuremic calciphylaxis. We encourage dermatologists to include calciphylaxis in the differential when evaluating any patient with a painful retiform rash or ulcerated eschar, even in the absence of renal disease.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714.
- Nigwekar SU, Wolf M, Sterns RH, et al. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139-1143.
- Sammour YM, Saleh HM, Gad MM, et al. Non-uremic calciphylaxis associated with alcoholic hepatitis: a case report. World J Hepatol. 2019;11:127-132.
- James WD, Elston DM, Treat J, et al, eds. Cutaneous vascular diseases. Andrews’ Diseases of the Skin: Clinical Dermatology. Elsevier; 2020:813-861.
- Nathoo RK, Harb JN, Auerbach J, et al. Pseudoxanthoma elasticum-like changes in nonuremic calciphylaxis: case series and brief review of a helpful diagnostic clue. J Cutan Pathol. 2017;44:1064-1069.
- Vaiman M, Lazarovitch T, Heller L, et al. Ecthyma gangrenosum and ecthyma-like lesions: review article. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34:633-639.
- Greene SL, Su WP, Muller SA. Ecthyma gangrenosum: report of clinical, histopathologic, and bacteriologic aspects of eight cases. J Am Acad Dermatol. 1984;11(5 pt 1):781-787.
- Levin M, Eley BS, Louis J, et al. Postinfectious purpura fulminans caused by an autoantibody directed against protein S. J Pediatr. 1995;127:355-363.
- Hughes G. Hughes syndrome: the antiphospholipid syndrome—a clinical overview. Clin Rev Allergy Immunol. 2007;32:3-12.
- Chang Y, Dabiri G, Damstetter E, et al. Coagulation disorders and their cutaneous presentations: pathophysiology. J Am Acad Dermatol. 2016;74:783-792; quiz 793-794.
- Fais P, Viero A, Viel G, et al. Necrotizing fasciitis: case series and review of the literature on clinical and medico-legal diagnostic challenges. Int J Legal Med. 2018;132:1357-1366.
- Brook I. Microbiology and management of soft tissue and muscle infections. Int J Surg Lond Engl. 2008;6:328-338.
A 50-year-old woman presented to our dermatology clinic with an exquisitely tender, nonhealing lesion on the left leg of 2 weeks’ duration that began as a small red-purplish spot. She applied a triple antibiotic ointment and wrapped the area with gauze daily but reported that it continued to enlarge and darken in color before forming a “scab.” She noted occasional seropurulent discharge and denied any trauma or new exposures to the area. She was seen at a local emergency department 3 days prior to presentation and was prescribed oral clindamycin for suspected cellulitis, but she denied any improvement with the initiation of antibiotics. Her medical history was notable for obesity, depression, hypothyroidism, and liver disease secondary to alcohol use disorder. She reported that she drank a pint of vodka daily. Her medications included pantoprazole, spironolactone, bumetanide, citalopram, levothyroxine, naltrexone, tramadol, and a multivitamin. Physical examination revealed violaceous mottling with areas of superficial erythema and ulceration with necrotic eschars on the proximal left thigh that were extremely painful. A biopsy was obtained for confirmation of diagnosis, but the patient died before the results were returned.
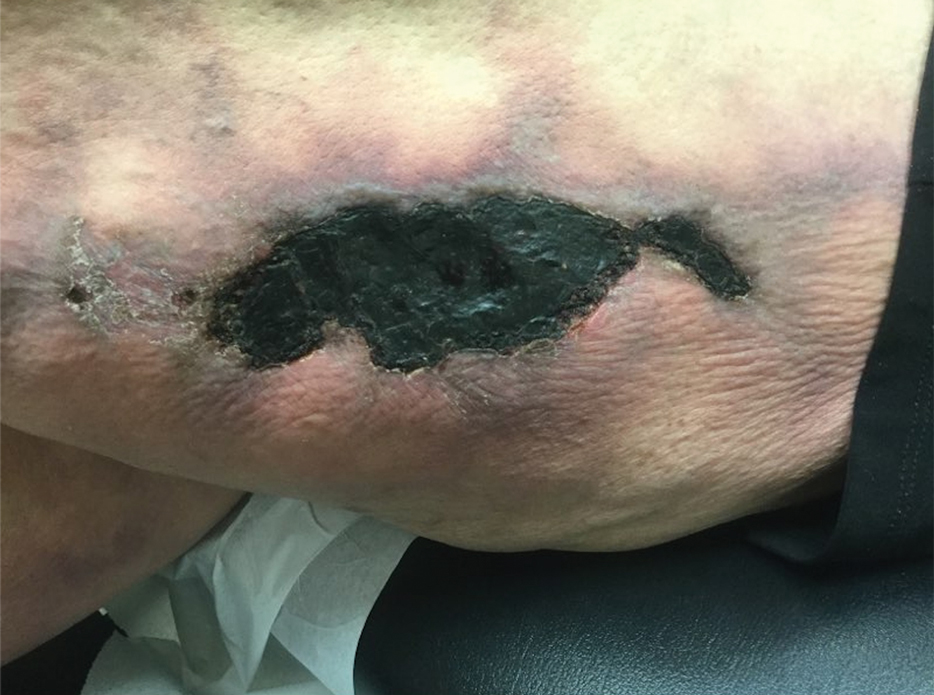
Polycyclic Scaly Eruption
The Diagnosis: Netherton Syndrome
A punch biopsy from the right lower back supported the clinical diagnosis of ichthyosis linearis circumflexa. The patient underwent genetic testing and was found to have a heterozygous mutation in the serine protease inhibitor Kazal type 5 gene, SPINK5, that was consistent with a diagnosis of Netherton syndrome.
Netherton syndrome is an autosomal-recessive genodermatosis characterized by a triad of congenital ichthyosis, hair shaft abnormalities, and atopic diatheses.1,2 It affects approximately 1 in 200,000 live births2,3; however, it is considered by many to be underdiagnosed due to the variability in the clinical appearance. Therefore, the incidence of Netherton syndrome may actually be closer 1 in 50,000 live births.1 The manifestations of the disease are caused by a germline mutation in the SPINK5 gene, which encodes the serine protease inhibitor LEKTI.1,2 Dysfunctional LEKTI results in increased proteolytic activity of the lipid-processing enzymes in the stratum corneum, resulting in a disruption in the lipid bilayer.1 Dysfunctional LEKTI also results in a loss of the antiinflammatory and antimicrobial function of the stratum corneum. Clinical features of Netherton syndrome usually present at birth or shortly thereafter.1 Congenital ichthyosiform erythroderma, or the continuous peeling of the skin, is a common presentation seen at birth and in the neonatal period.2 As the patient ages, the dermatologic manifestations evolve into serpiginous and circinate, erythematous plaques with a characteristic peripheral, double-edged scaling.1,2 This distinctive finding is termed ichthyosis linearis circumflexa and is pathognomonic for the syndrome.2 Lesions often affect the trunk and extremities and demonstrate an undulating course.1 Because eczematous and lichenified plaques in flexural areas as well as pruritus are common clinical features, this disease often is misdiagnosed as atopic dermatitis,1,3 as was the case in our patient.
Patients with Netherton syndrome can present with various hair abnormalities. Trichorrhexis invaginata, known as bamboo hair, is the intussusception of the hair shaft and is characteristic of the disease.3 It develops from a reduced number of disulfide bonds, which results in cortical softening.1 Trichorrhexis invaginata may not be present at birth and often improves with age.1,3 Other hair shaft abnormalities such as pili torti, trichorrhexis nodosa, and helical hair also may be observed in Netherton syndrome.1 Extracutaneous manifestations also are typical. There is immune dysregulation of memory B cells and natural killer cells, which manifests as frequent respiratory and skin infections as well as sepsis.1,2 Patients also may have increased levels of serum IgE and eosinophilia resulting in atopy and allergic reactions to various triggers such as foods.1 The neonatal period also may be complicated by dehydration, electrolyte imbalances, inability to regulate body temperature, and failure to thrive.1,3
When there is an extensive disruption of the skin barrier during the neonatal period, there may be severe electrolyte imbalances and thermoregulatory challenges necessitating treatment in the neonatal intensive care unit. Cutaneous disease can be treated with topical therapies with variable success.1 Topical therapies for symptom management include emollients, corticosteroids, calcineurin inhibitors, calcipotriene, and retinoids; however, utmost caution must be employed with these therapies due to the increased risk for systemic absorption resulting from the disturbance of the skin barrier. When therapy with topical tacrolimus is implemented, monitoring of serum drug levels is required.1 Pruritus may be treated symptomatically with oral antihistamines. Intravenous immunoglobulin has been shown to decrease the frequency of infections and improve skin inflammation. Systemic retinoids have unpredictable effects and result in improvement of disease in some patients but exacerbation in others. Phototherapy with narrowband UVB, psoralen plus UVA, UVA1, and balneophototherapy also are effective treatments for cutaneous disease.1 Dupilumab has been shown to decrease pruritus, improve hair abnormalities, and improve skin disease, thereby demonstrating its effectiveness in treating the atopy and ichthyosis in Netherton syndrome.4
The differential diagnosis includes other figurate erythemas including erythema marginatum and erythrokeratodermia variabilis. Erythema marginatum is a cutaneous manifestation of acute rheumatic fever and is characterized by migratory polycyclic erythematous plaques without overlying scale, usually on the trunk and proximal extremities.5 Erythrokeratodermia variabilis is caused by heterozygous mutations in gap junction protein beta 3, GJB3, and gap junction protein beta 4, GJB4, and is characterized by transient geographic and erythematous patches and stable scaly plaques; however, double-edged scaling is not a feature.1 Acrodermatitis enteropathica is an autosomal-recessive disorder caused by mutations in the zinc transporter SLC39A4. Cutaneous manifestations occur after weaning from breast milk and are characterized by erythematous plaques with erosions, vesicles, and scaling, which characteristically occur in the perioral and perianal locations.6 Neonatal lupus is a form of subacute cutaneous lupus erythematosus. Typical skin lesions are erythematous annular plaques with overlying scaling, which may be present at birth and have a predilection for the face and other sun-exposed areas. Lesions generally resolve after clearance of the pathogenic maternal antibodies.7
- Richard G, Ringpfeil F. Ichthyoses, erythrokeratodermas, and related disorders. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:888-923.
- Garza JI, Herz-Ruelas ME, Guerrero-González GA, et al. Netherton syndrome: a diagnostic and therapeutic challenge. J Am Acad Dermatol. 2016;74(suppl 1):AB129.
- Heymann W. Appending the appendages: new perspectives on Netherton syndrome and green nail syndrome. J Am Acad Dermatol. 2020;83:735-736.
- Murase C, Takeichi T, Taki T, et al. Successful dupilumab treatment for ichthyotic and atopic features of Netherton syndrome. J Dermatol Sci. 2021;102:126-129.
- España A. Figurate erythemas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:320-331.
- Noguera-Morel L, McLeish Schaefer S, Hivnor C. Nutritional diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:793-809.
- Lee L, Werth V. Lupus erythematosus. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:662-680.
The Diagnosis: Netherton Syndrome
A punch biopsy from the right lower back supported the clinical diagnosis of ichthyosis linearis circumflexa. The patient underwent genetic testing and was found to have a heterozygous mutation in the serine protease inhibitor Kazal type 5 gene, SPINK5, that was consistent with a diagnosis of Netherton syndrome.
Netherton syndrome is an autosomal-recessive genodermatosis characterized by a triad of congenital ichthyosis, hair shaft abnormalities, and atopic diatheses.1,2 It affects approximately 1 in 200,000 live births2,3; however, it is considered by many to be underdiagnosed due to the variability in the clinical appearance. Therefore, the incidence of Netherton syndrome may actually be closer 1 in 50,000 live births.1 The manifestations of the disease are caused by a germline mutation in the SPINK5 gene, which encodes the serine protease inhibitor LEKTI.1,2 Dysfunctional LEKTI results in increased proteolytic activity of the lipid-processing enzymes in the stratum corneum, resulting in a disruption in the lipid bilayer.1 Dysfunctional LEKTI also results in a loss of the antiinflammatory and antimicrobial function of the stratum corneum. Clinical features of Netherton syndrome usually present at birth or shortly thereafter.1 Congenital ichthyosiform erythroderma, or the continuous peeling of the skin, is a common presentation seen at birth and in the neonatal period.2 As the patient ages, the dermatologic manifestations evolve into serpiginous and circinate, erythematous plaques with a characteristic peripheral, double-edged scaling.1,2 This distinctive finding is termed ichthyosis linearis circumflexa and is pathognomonic for the syndrome.2 Lesions often affect the trunk and extremities and demonstrate an undulating course.1 Because eczematous and lichenified plaques in flexural areas as well as pruritus are common clinical features, this disease often is misdiagnosed as atopic dermatitis,1,3 as was the case in our patient.
Patients with Netherton syndrome can present with various hair abnormalities. Trichorrhexis invaginata, known as bamboo hair, is the intussusception of the hair shaft and is characteristic of the disease.3 It develops from a reduced number of disulfide bonds, which results in cortical softening.1 Trichorrhexis invaginata may not be present at birth and often improves with age.1,3 Other hair shaft abnormalities such as pili torti, trichorrhexis nodosa, and helical hair also may be observed in Netherton syndrome.1 Extracutaneous manifestations also are typical. There is immune dysregulation of memory B cells and natural killer cells, which manifests as frequent respiratory and skin infections as well as sepsis.1,2 Patients also may have increased levels of serum IgE and eosinophilia resulting in atopy and allergic reactions to various triggers such as foods.1 The neonatal period also may be complicated by dehydration, electrolyte imbalances, inability to regulate body temperature, and failure to thrive.1,3
When there is an extensive disruption of the skin barrier during the neonatal period, there may be severe electrolyte imbalances and thermoregulatory challenges necessitating treatment in the neonatal intensive care unit. Cutaneous disease can be treated with topical therapies with variable success.1 Topical therapies for symptom management include emollients, corticosteroids, calcineurin inhibitors, calcipotriene, and retinoids; however, utmost caution must be employed with these therapies due to the increased risk for systemic absorption resulting from the disturbance of the skin barrier. When therapy with topical tacrolimus is implemented, monitoring of serum drug levels is required.1 Pruritus may be treated symptomatically with oral antihistamines. Intravenous immunoglobulin has been shown to decrease the frequency of infections and improve skin inflammation. Systemic retinoids have unpredictable effects and result in improvement of disease in some patients but exacerbation in others. Phototherapy with narrowband UVB, psoralen plus UVA, UVA1, and balneophototherapy also are effective treatments for cutaneous disease.1 Dupilumab has been shown to decrease pruritus, improve hair abnormalities, and improve skin disease, thereby demonstrating its effectiveness in treating the atopy and ichthyosis in Netherton syndrome.4
The differential diagnosis includes other figurate erythemas including erythema marginatum and erythrokeratodermia variabilis. Erythema marginatum is a cutaneous manifestation of acute rheumatic fever and is characterized by migratory polycyclic erythematous plaques without overlying scale, usually on the trunk and proximal extremities.5 Erythrokeratodermia variabilis is caused by heterozygous mutations in gap junction protein beta 3, GJB3, and gap junction protein beta 4, GJB4, and is characterized by transient geographic and erythematous patches and stable scaly plaques; however, double-edged scaling is not a feature.1 Acrodermatitis enteropathica is an autosomal-recessive disorder caused by mutations in the zinc transporter SLC39A4. Cutaneous manifestations occur after weaning from breast milk and are characterized by erythematous plaques with erosions, vesicles, and scaling, which characteristically occur in the perioral and perianal locations.6 Neonatal lupus is a form of subacute cutaneous lupus erythematosus. Typical skin lesions are erythematous annular plaques with overlying scaling, which may be present at birth and have a predilection for the face and other sun-exposed areas. Lesions generally resolve after clearance of the pathogenic maternal antibodies.7
The Diagnosis: Netherton Syndrome
A punch biopsy from the right lower back supported the clinical diagnosis of ichthyosis linearis circumflexa. The patient underwent genetic testing and was found to have a heterozygous mutation in the serine protease inhibitor Kazal type 5 gene, SPINK5, that was consistent with a diagnosis of Netherton syndrome.
Netherton syndrome is an autosomal-recessive genodermatosis characterized by a triad of congenital ichthyosis, hair shaft abnormalities, and atopic diatheses.1,2 It affects approximately 1 in 200,000 live births2,3; however, it is considered by many to be underdiagnosed due to the variability in the clinical appearance. Therefore, the incidence of Netherton syndrome may actually be closer 1 in 50,000 live births.1 The manifestations of the disease are caused by a germline mutation in the SPINK5 gene, which encodes the serine protease inhibitor LEKTI.1,2 Dysfunctional LEKTI results in increased proteolytic activity of the lipid-processing enzymes in the stratum corneum, resulting in a disruption in the lipid bilayer.1 Dysfunctional LEKTI also results in a loss of the antiinflammatory and antimicrobial function of the stratum corneum. Clinical features of Netherton syndrome usually present at birth or shortly thereafter.1 Congenital ichthyosiform erythroderma, or the continuous peeling of the skin, is a common presentation seen at birth and in the neonatal period.2 As the patient ages, the dermatologic manifestations evolve into serpiginous and circinate, erythematous plaques with a characteristic peripheral, double-edged scaling.1,2 This distinctive finding is termed ichthyosis linearis circumflexa and is pathognomonic for the syndrome.2 Lesions often affect the trunk and extremities and demonstrate an undulating course.1 Because eczematous and lichenified plaques in flexural areas as well as pruritus are common clinical features, this disease often is misdiagnosed as atopic dermatitis,1,3 as was the case in our patient.
Patients with Netherton syndrome can present with various hair abnormalities. Trichorrhexis invaginata, known as bamboo hair, is the intussusception of the hair shaft and is characteristic of the disease.3 It develops from a reduced number of disulfide bonds, which results in cortical softening.1 Trichorrhexis invaginata may not be present at birth and often improves with age.1,3 Other hair shaft abnormalities such as pili torti, trichorrhexis nodosa, and helical hair also may be observed in Netherton syndrome.1 Extracutaneous manifestations also are typical. There is immune dysregulation of memory B cells and natural killer cells, which manifests as frequent respiratory and skin infections as well as sepsis.1,2 Patients also may have increased levels of serum IgE and eosinophilia resulting in atopy and allergic reactions to various triggers such as foods.1 The neonatal period also may be complicated by dehydration, electrolyte imbalances, inability to regulate body temperature, and failure to thrive.1,3
When there is an extensive disruption of the skin barrier during the neonatal period, there may be severe electrolyte imbalances and thermoregulatory challenges necessitating treatment in the neonatal intensive care unit. Cutaneous disease can be treated with topical therapies with variable success.1 Topical therapies for symptom management include emollients, corticosteroids, calcineurin inhibitors, calcipotriene, and retinoids; however, utmost caution must be employed with these therapies due to the increased risk for systemic absorption resulting from the disturbance of the skin barrier. When therapy with topical tacrolimus is implemented, monitoring of serum drug levels is required.1 Pruritus may be treated symptomatically with oral antihistamines. Intravenous immunoglobulin has been shown to decrease the frequency of infections and improve skin inflammation. Systemic retinoids have unpredictable effects and result in improvement of disease in some patients but exacerbation in others. Phototherapy with narrowband UVB, psoralen plus UVA, UVA1, and balneophototherapy also are effective treatments for cutaneous disease.1 Dupilumab has been shown to decrease pruritus, improve hair abnormalities, and improve skin disease, thereby demonstrating its effectiveness in treating the atopy and ichthyosis in Netherton syndrome.4
The differential diagnosis includes other figurate erythemas including erythema marginatum and erythrokeratodermia variabilis. Erythema marginatum is a cutaneous manifestation of acute rheumatic fever and is characterized by migratory polycyclic erythematous plaques without overlying scale, usually on the trunk and proximal extremities.5 Erythrokeratodermia variabilis is caused by heterozygous mutations in gap junction protein beta 3, GJB3, and gap junction protein beta 4, GJB4, and is characterized by transient geographic and erythematous patches and stable scaly plaques; however, double-edged scaling is not a feature.1 Acrodermatitis enteropathica is an autosomal-recessive disorder caused by mutations in the zinc transporter SLC39A4. Cutaneous manifestations occur after weaning from breast milk and are characterized by erythematous plaques with erosions, vesicles, and scaling, which characteristically occur in the perioral and perianal locations.6 Neonatal lupus is a form of subacute cutaneous lupus erythematosus. Typical skin lesions are erythematous annular plaques with overlying scaling, which may be present at birth and have a predilection for the face and other sun-exposed areas. Lesions generally resolve after clearance of the pathogenic maternal antibodies.7
- Richard G, Ringpfeil F. Ichthyoses, erythrokeratodermas, and related disorders. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:888-923.
- Garza JI, Herz-Ruelas ME, Guerrero-González GA, et al. Netherton syndrome: a diagnostic and therapeutic challenge. J Am Acad Dermatol. 2016;74(suppl 1):AB129.
- Heymann W. Appending the appendages: new perspectives on Netherton syndrome and green nail syndrome. J Am Acad Dermatol. 2020;83:735-736.
- Murase C, Takeichi T, Taki T, et al. Successful dupilumab treatment for ichthyotic and atopic features of Netherton syndrome. J Dermatol Sci. 2021;102:126-129.
- España A. Figurate erythemas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:320-331.
- Noguera-Morel L, McLeish Schaefer S, Hivnor C. Nutritional diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:793-809.
- Lee L, Werth V. Lupus erythematosus. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:662-680.
- Richard G, Ringpfeil F. Ichthyoses, erythrokeratodermas, and related disorders. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:888-923.
- Garza JI, Herz-Ruelas ME, Guerrero-González GA, et al. Netherton syndrome: a diagnostic and therapeutic challenge. J Am Acad Dermatol. 2016;74(suppl 1):AB129.
- Heymann W. Appending the appendages: new perspectives on Netherton syndrome and green nail syndrome. J Am Acad Dermatol. 2020;83:735-736.
- Murase C, Takeichi T, Taki T, et al. Successful dupilumab treatment for ichthyotic and atopic features of Netherton syndrome. J Dermatol Sci. 2021;102:126-129.
- España A. Figurate erythemas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:320-331.
- Noguera-Morel L, McLeish Schaefer S, Hivnor C. Nutritional diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:793-809.
- Lee L, Werth V. Lupus erythematosus. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:662-680.
A 9-year-old boy presented to the dermatology clinic with a scaly eruption distributed throughout the body that had been present since birth. He had been diagnosed with atopic dermatitis by multiple dermatologists prior to the current presentation and had been treated with various topical steroids with minimal improvement. He had no family history of similar eruptions and no personal history of asthma or allergies. Physical examination revealed erythematous, serpiginous, polycyclic plaques with peripheral, double-edged scaling. Decreased hair density of the lateral eyebrows also was observed.
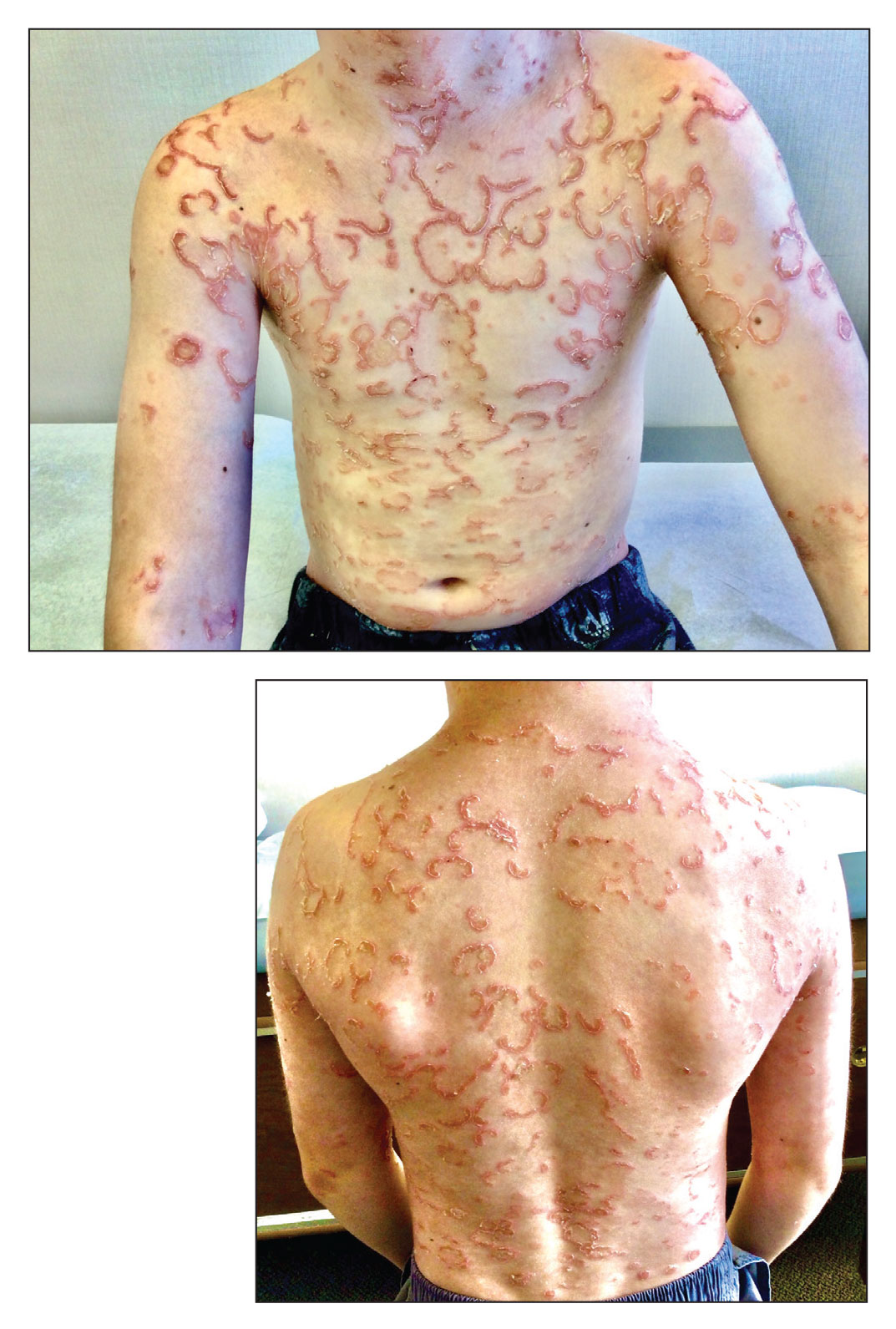
Linear Hypopigmentation on the Right Arm
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
The Diagnosis: Chemical Leukoderma
A clinical diagnosis of chemical leukoderma was made. In our patient, the observed linear hypopigmentation likely resulted from the prior treatment for De Quervain tenosynovitis in which an intralesional corticosteroid entered the lymphatic channel causing a linear distribution of chemical leukoderma. The hypopigmentation self-resolved at 6-month follow-up, and the patient was counseled to continue steroid injections if indicated.
Chemical leukoderma is an acquired depigmenting dermatosis that displays vitiligolike patterning. Detailed personal and family history in addition to complete physical examination are crucial given the inability to distinguish chemical leukoderma from vitiligo on histopathology. A set of clinical criteria proposed by Ghosh and Mukhopadhyay1 includes the presence of acquired depigmented macules and patches resembling vitiligo, history of repeat exposure to certain chemical substances, hypopigmentation at the site of exposure, and/ or confettilike white macules. Three of these 4 clinical findings must be present to establish a diagnosis of chemical leukoderma. The extent of disease involvement may be graded as follows: Stage I is defined as leukoderma only at the site of contact to the offending agent. Stage II involvement is characterized by local spread beyond the exposure site via the lymphatic system. Stages IIIA and IIIB leukoderma entail hematogenous spread distant to the site of chemical exposure. Although stage IIIA leukoderma is limited to cutaneous involvement, stage IIIB findings are marked by systemic organ involvement. Stage IV disease is defined by the distant spread of hypopigmented macules and patches that continues following 1 year of strict avoidance of the causative agent.1
The pathogenesis behind chemical leukoderma is not completely understood. Studies have suggested that individuals with certain genetic susceptibilities are predisposed to developing the condition after being exposed to chemicals with melanocytotoxic properties.2,3 It has been proposed that the chemicals accelerate pre-existing cellular stress cascades within melanocytes to levels higher than what healthy cells can tolerate. Genetic factors can increase an individual’s total melanocytic stress or establish a lower cellular threshold for stress than what the immune system can manage.4 These influences culminate in an inflammatory response that results in melanocytic destruction and subsequent cutaneous hypopigmentation.
The most well-known offending chemical agents are phenol and catechol derivatives, such as hydroquinone, which is used in topical bleaching agents to treat diseases of hyperpigmentation, including melasma.2 Potent topical or intralesional corticosteroids also may precipitate chemical leukoderma, most notably in individuals with darker skin tones. Hypomelanosis induced by intralesional steroids frequently occurs weeks to months after administration and commonly is observed in a stellate or linear pattern with an irregular outline.5 Other offending chemical agents include sulfhydryls, mercurials, arsenic, benzoyl peroxide, azelaic acid, imiquimod, chloroquine, and tyrosine kinase inhibitors.2,5
Segmental vitiligo is characterized by unilateral hypopigmentation in a linear or blocklike distribution that does not cross the midline. However, onset of segmental vitiligo classically occurs prior to 30 years of age and frequently is related with early leukotrichia.6 Additionally, the hypomelanosis associated with segmental vitiligo more often presents as broad bands or patches that occasionally have a blaschkoid distribution and most commonly appear on the face.5 Lichen striatus is a lichenoid dermatosis that presents as asymptomatic pink or hypopigmented papules that follow the Blaschko lines, often favoring the extremities. Postinflammatory hypopigmentation also may occur as an associated sequela of resolved lichen striatus. Although the disease onset of lichen striatus may occur in adulthood, it typically appears in childhood and is triggered by factors such as trauma, hypersensitivity reactions, viral infections, and medications. Physical injuries such as trauma following surgical procedures also can lead to hypomelanosis; however, our patient denied any relevant surgical history. Progressive macular hypomelanosis is a skin condition presenting as ill-defined, nummular, hypopigmented macules or patches that commonly affects women with darker skin tones with an ethnic background from a tropical location or residing in a tropical environment.5 Lesions frequently appear on the trunk and rarely progress to the proximal extremities, making it an unlikely diagnosis for our patient.
In most cases of chemical leukoderma, spontaneous repigmentation often occurs within 12 months after the elimination of the offending substance; however, hypopigmented lesions may persist or continue to develop at sites distant from the initial site despite discontinuing the causative agent.1 Therapies for vitiligo, such as topical corticosteroids, topical immunosuppressants, narrowband UVB phototherapy, and psoralen plus UVA photochemotherapy, may be utilized for chemical leukoderma that does not self-resolve.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
- Ghosh S, Mukhopadhyay S. Chemical leucoderma: a clinicoaetiological study of 864 cases in the perspective of a developing country [published online September 6, 2008]. Br J Dermatol. 2009;160:40-47.
- Ghosh S. Chemical leukoderma: what’s new on etiopathological and clinical aspects? Indian J Dermatol. 2010;55:255.
- Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208-214.
- Harris J. Chemical-induced vitiligo. Dermatol Clin. 2017; 35:151-161.
- Bolognia JL, Schaffer JV, Cerroni L, et al. Vitiligo and other disorders of hypopigmentation. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Mosby/Elsevier; 2018:1087-1114.
- Rodrigues M, Ezzedine K, Hamzavi I, et al. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77:1-13.
A 73-year-old woman presented to the dermatology clinic with hypopigmentation along the right arm. Her medical history was notable for prior treatment with intralesional triamcinolone injections for De Quervain tenosynovitis. Two months after receiving the steroid injections she noted progressive spreading of an asymptomatic white discoloration originating on the right wrist. Physical examination revealed a hypopigmented atrophic patch on the medial aspect of the right wrist (left) with linear hypopigmented patches extending proximally up the forearm (right).
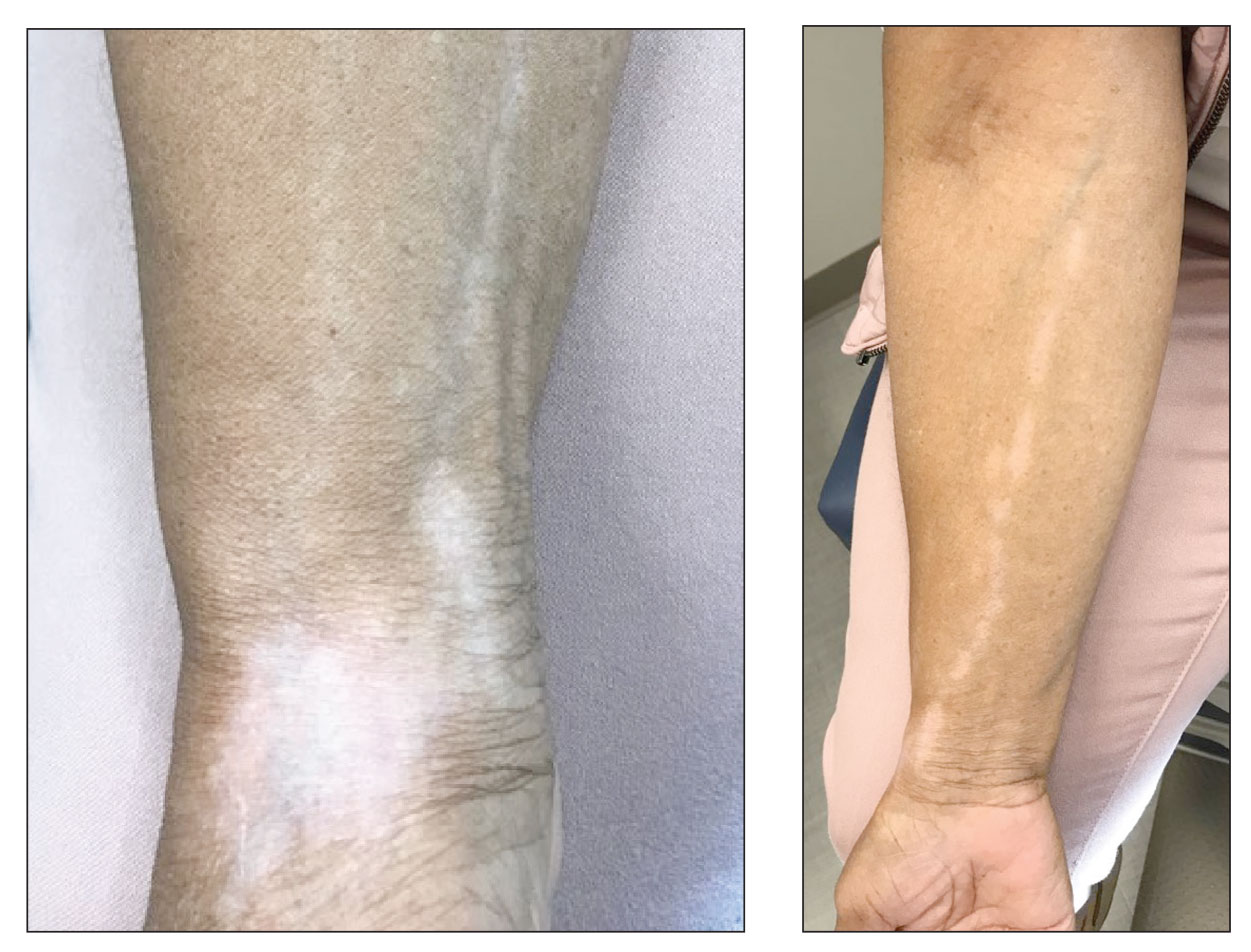
Scattered Flesh-Colored Papules in a Linear Array in the Setting of Diffuse Skin Thickening
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.
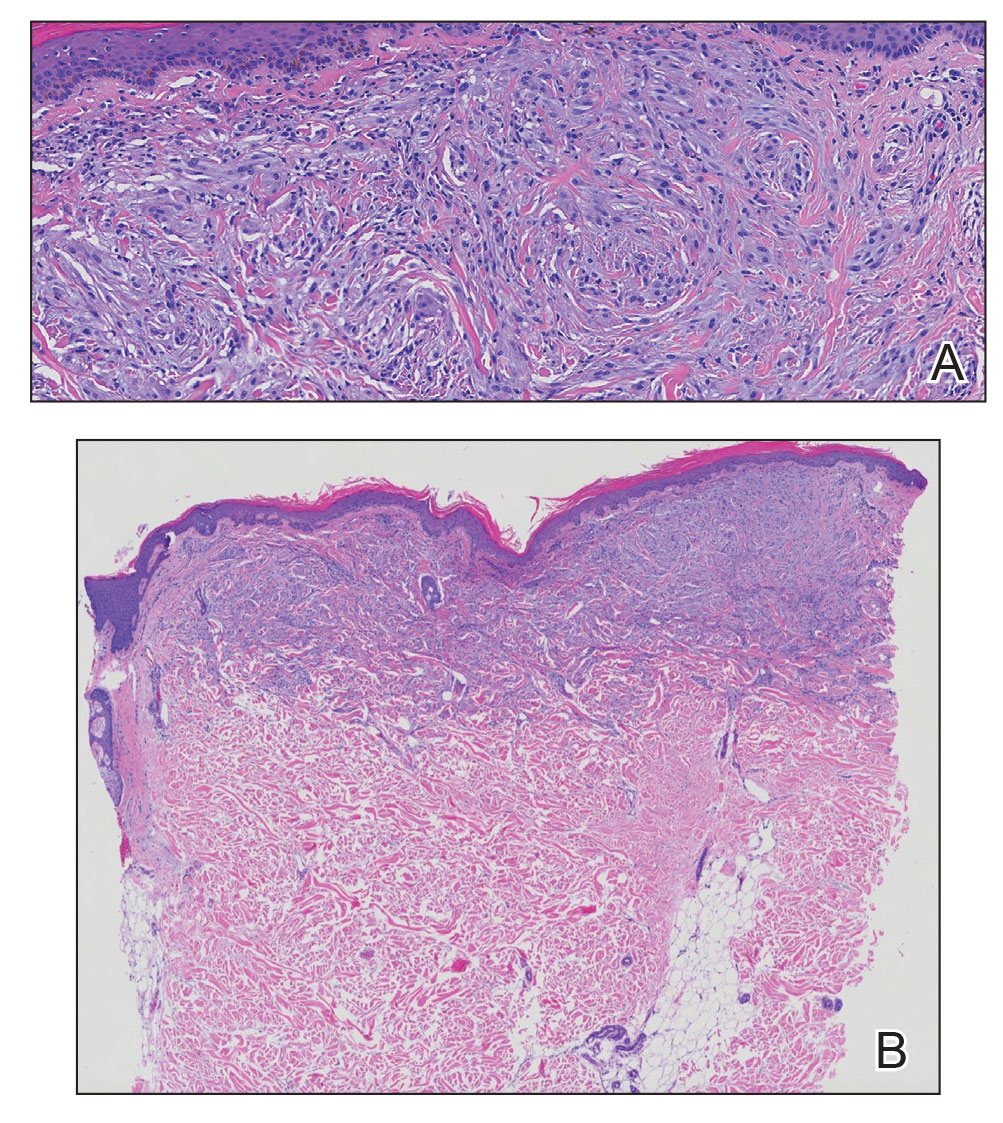
Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2
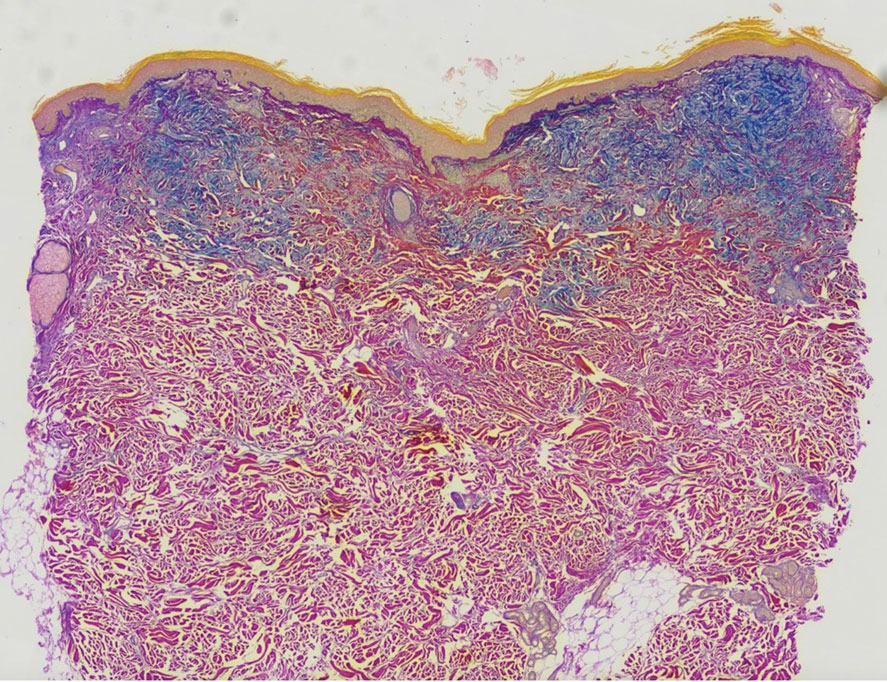
The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.

Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2

The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
The Diagnosis: Scleromyxedema
A punch biopsy of the upper back performed at an outside institution revealed increased histiocytes and abundant interstitial mucin confined to the papillary dermis (Figures 1 and 2), consistent with the lichen myxedematosus (LM) papules that may be seen in scleromyxedema. Serum protein electrophoresis revealed the presence of a protein of restricted mobility on the gamma region that occupied 5.3% of the total protein (0.3 g/dL). Urine protein electrophoresis showed free kappa light chain monoclonal protein in the gamma region. Immunofixation electrophoresis revealed the presence of IgG kappa monoclonal protein in the gamma region with 10% monotype kappa cells. The presence of Raynaud phenomenon and positive antinuclear antibody (1:320, speckled) was noted. Laboratory studies for thyroid-stimulating hormone, C-reactive protein, Scl-70 antibody, myositis panel, ribonucleoprotein antibody, Smith antibody, Sjögren syndrome–related antigens A and B antibodies, rheumatoid factor, and RNA polymerase III antibody all were within reference range. Our patient was treated with monthly intravenous immunoglobulin (IVIG), and he noted substantial improvement in skin findings after 3 months of IVIG.

Localized lichen myxedematosus is a rare idiopathic cutaneous disease that clinically is characterized by waxy indurated papules and histologically is characterized by diffuse mucin deposition and fibroblast proliferation in the upper dermis.1 Scleromyxedema is a diffuse variant of LM in which the papules and plaques of LM are associated with skin thickening involving almost the entire body and associated systemic disease. The exact mechanism of this disease is unknown, but the most widely accepted hypothesis is that immunoglobulins and cytokines contribute to the synthesis of glycosaminoglycans and thereby the deposition of mucin in the dermis.2 Scleromyxedema has a chronic course and generally responds poorly to existing treatments.1 Partial improvement has been demonstrated in treatment with topical calcineurin inhibitors and topical steroids.2

The differential diagnosis in our patient included scleromyxedema, scleredema, scleroderma, LM, and reticular erythematosus mucinosis. He was diagnosed with scleromyxedema with kappa monoclonal gammopathy. Scleromyxedema is a rare disorder involving the deposition of mucinous material in the papillary dermis that causes the formation of infiltrative skin lesions.3 The etiology is unknown, but the presence of a monoclonal protein is an important characteristic of this disorder. It is important to rule out thyroid disease as a possible etiology before concluding that the disease process is driven by the monoclonal gammopathy; this will help determine appropriate therapies.4,5 Usually the monoclonal protein is associated with the IgG lambda subtype. Intravenous immunoglobulin often is considered as a first-line treatment of scleromyxedema and usually is administered at a dosage of 2 g/kg divided over 2 to 5 consecutive days per month.3 Previously, our patient had been treated with IVIG for 3 years for chronic inflammatory demyelinating polyneuropathy and had stopped 1 to 2 years before his cutaneous symptoms started. Generally, scleromyxedema patients must stay on IVIG long-term to prevent relapse, typically every 6 to 8 weeks. Second-line treatments for scleromyxedema include systemic corticosteroids and thalidomide.6 Scleromyxedema and LM have several clinical and histopathologic features in common. Our patient’s biopsy revealed increased mucin deposition associated with fibroblast proliferation confined to the superficial dermis. These histologic changes can be seen in the setting of either LM or scleromyxedema. Our patient’s diffuse skin thickening and monoclonal gammopathy were more characteristic of scleromyxedema. In contrast, LM is a localized eruption with no internal organ manifestations and no associated systemic disease, such as monoclonal gammopathy and thyroid disease.
Scleredema adultorum of Buschke (also referred to as scleredema) is a rare idiopathic dermatologic condition characterized by thickening and tightening of the skin that leads to firm, nonpitting, woody edema that initially involves the upper back and neck but can spread to the face, scalp, and shoulders; importantly, scleredema spares the hands and feet.7 Scleredema has been associated with type 2 diabetes mellitus, streptococcal upper respiratory tract infections, and monoclonal gammopathy.8 Although our patient did have a monoclonal gammopathy, he also experienced prominent hand involvement with diffuse skin thickening, which is not typical of scleredema. Additionally, biopsy of scleredema would show increased mucin but would not show the proliferation of fibroblasts that was seen in our patient’s biopsy. Furthermore, scleredema has more profound diffuse superficial and deep mucin deposition compared to scleromyxedema. Scleroderma is an autoimmune cutaneous condition that is divided into 2 categories: localized scleroderma and systemic sclerosis (SSc).9 Localized scleroderma (also called morphea) often is characterized by indurated hyperpigmented or hypopigmented lesions. There is an absence of Raynaud phenomenon, telangiectasia, and systemic disease.9 Systemic sclerosis is further divided into 2 categories—limited cutaneous and diffuse cutaneous—which are differentiated by the extent of organ system involvement. Limited cutaneous SSc involves calcinosis, Raynaud phenomenon, esophageal dysmotility, skin sclerosis distal to the elbows and knees, and telangiectasia.9 Diffuse cutaneous SSc is characterized by Raynaud phenomenon; cutaneous sclerosis proximal to the elbows and knees; and fibrosis of the gastrointestinal, pulmonary, renal, and cardiac systems.9 Scl-70 antibodies are specific for diffuse cutaneous SSc, and centromere antibodies are specific for limited cutaneous SSc. Scleromyxedema shares many of the same clinical symptoms as scleroderma; therefore, histopathologic examination is important for differentiating these disorders. Histologically, scleroderma is characterized by thickened collagen bundles associated with a variable degree of perivascular and interstitial lymphoplasmacytic inflammation. No increased dermal mucin is present.9 Our patient did not have the clinical cutaneous features of localized scleroderma and lacked the signs of internal organ involvement that typically are found in SSc. He did have Raynaud phenomenon but did not have matlike telangiectases or Scl-70 or centromere antibodies.
Reticular erythematosus mucinosis (REM) is a rare inflammatory cutaneous disease that is characterized by diffuse reticular erythematous macules or papules that may be asymptomatic or associated with pruritus.10 Reticular erythematosus mucinosis most frequently affects middle-aged women and appears on the trunk.9 Our patient was not part of the demographic group most frequently affected by REM. More importantly, our patient’s lesions were not erythematous or reticular in appearance, making the diagnosis of REM unlikely. Furthermore, REM has no associated cutaneous sclerosis or induration.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
- Nofal A, Amer H, Alakad R, et al. Lichen myxedematosus: diagnostic criteria, classification, and severity grading. Int J Dermatol. 2017;56:284-290.
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:8.
- Haber R, Bachour J, El Gemayel M. Scleromyxedema treatment: a systematic review and update. Int J Dermatol. 2020;59:1191-1201.
- Hazan E, Griffin TD Jr, Jabbour SA, et al. Scleromyxedema in a patient with thyroid disease: an atypical case or a case for revised criteria? Cutis. 2020;105:E6-E10.
- Shenoy A, Steixner J, Beltrani V, et al. Discrete papular lichen myxedematosus and scleromyxedema with hypothyroidism: a report of two cases. Case Rep Dermatol. 2019;11:64-70.
- Hoffman JHO, Enk AH. Scleromyxedema. J Dtsch Dermatol Ges. 2020;18:1449-1467.
- Beers WH, Ince AI, Moore TL. Scleredema adultorum of Buschke: a case report and review of the literature. Semin Arthritis Rheum. 2006;35:355-359.
- Miguel D, Schliemann S, Elsner P. Treatment of scleroderma adultorum Buschke: a systematic review. Acta Derm Venereol. 2018;98:305-309.
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinicopathological correlation. G Ital Dermatol Venereol. 2018;153:208-215.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335.
A 76-year-old man presented to our clinic with diffusely thickened and tightened skin that worsened over the course of 1 year, as well as numerous scattered small, firm, flesh-colored papules arranged in a linear pattern over the face, ears, neck, chest, abdomen, arms, hands, and knees. His symptoms progressed to include substantial skin thickening initially over the thighs followed by the arms, chest, back (top), and face. He developed confluent cobblestonelike plaques over the elbows and hands (bottom) and eventually developed decreased oral aperture limiting oral intake as well as decreased range of motion in the hands. The patient had a deep furrowed appearance of the brow accompanied by discrete, scattered, flesh-colored papules on the forehead and behind the ears. Deep furrows also were present on the back. When the proximal interphalangeal joints of the hands were extended, elevated rings with central depression were seen instead of horizontal folds.
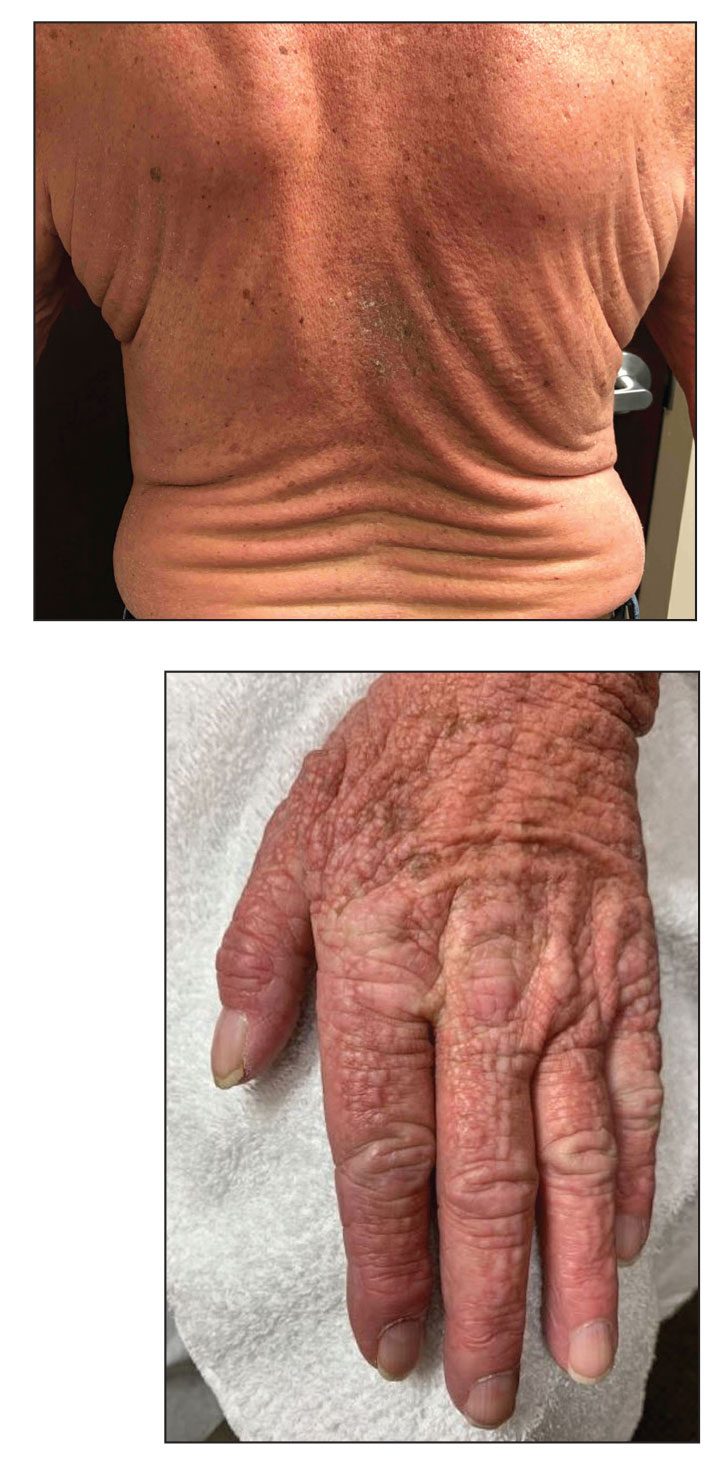
Pink Nodule Behind the Ear
The Diagnosis: Acanthoma Fissuratum
Acanthoma fissuratum is a skin lesion that results from consistent pressure, typically from ill-fitting eyeglass frames.1 The chronic irritation leads to collagen deposition and inflammation that gradually creates the lesion. Many patients never seek care, making incidence figures undeterminable.2 It usually presents as a firm, tender, flesh-colored or pink nodule or plaque with a central indentation from where the frame rests. This indentation splits the lesion in half and classically gives the appearance of a coffee bean.1 The repeated minor trauma at this point of contact also may lead to centralized ulceration, which further blurs the diagnosis to include basal cell carcinoma (BCC).3,4 Although the postauricular groove is the most cited location, lesions also may occur at other contact points of the glasses, such as the lateral aspect of the bridge of the nose and the superior auricular sulcus.5 Acanthoma fissuratum is not limited to the external head. Other etiologies of local trauma and pressure have led to its diagnosis in the upper labioalveolar fold, posterior fourchette of the vulva, penis, and external auditory canal.6-9
The diagnosis of acanthoma fissuratum mainly is clinical; however, due to its similar appearance to BCC and other lesions, a biopsy can be taken to support the diagnosis; a biopsy was not performed in our patient. The main features seen on histopathology include acanthosis, hyperkeratosis, variable parakeratosis, and perivascular nonspecific inflammatory infiltration. The epidermis may reflect the macroscopic frame indentation with central attenuation of the epidermis, which potentially is filled with inflammatory cells or keratin.5
Treatment normally encompasses removing the illfitting frames or fixing the fit, which gradually leads to reduction of the lesion.4,5 This occurred in our patient, who changed eyeglasses and saw an 80% resolution of the lesion in 8 months. Such improvement after removal of a trauma-inducing stimulus would not be seen in malignancies (eg, BCC, squamous cell carcinoma [SCC]), keloids, or cylindromas. If the granulation tissue does not regress or recurs, other potential treatments include excision, intralesional corticosteroids, and electrosurgery.5
Basal cell carcinoma is a common nonmelanoma skin cancer that most often presents on the sun-exposed areas of the head and neck, especially the cheeks, nasolabial folds, and forehead. Although the nodular subtype may clinically appear similar to acanthoma fissuratum, it more typically presents as a pearly papule or nodule with a sharp border, small telangiectases, and potential ulceration.10 Squamous cell carcinoma is another common nonmelanoma skin cancer that often arises in sun-exposed areas, which can include the postauricular area. Although the lesion can be associated with chronic wounds and also can grow vertically, SCC typically has a scalier and more hyperkeratotic surface that can ulcerate.1 A cylindroma is a benign sweat gland tumor that most commonly presents on the head and neck (also known as the turban tumor), though it can develop on the ear. It appears as solitary or multiple nodules that often are flesh colored, red, or blue with a shiny surface.1 Cylindromas are not known to be associated with chronic local trauma or irritation,11 such as wearing ill-fitting eyeglasses. Unlike acanthoma fissuratum, the treatment of cylindromas, BCC, and SCC most often involves excision.1 A keloid presents as a flesh-colored, red, or purple exophytic plaque that is composed of dense dermal tissue and progressively forms after local trauma. Although keloids can spontaneously develop, they commonly form on the ears in susceptible individuals after skin excisions including prior keloid removal, piercings, repairment of auricular traumas, or infections.1 The patient’s coffee bean–like lesion that coincided with wearing new eyeglasses better fits the diagnosis of acanthoma fissuratum than a keloid. Additionally, keloids typically do not regress without treatment. Keloid treatment consists of intralesional steroid injections, occlusive silicone dressings, compression, cryotherapy, radiation, and excisional surgery.1
- Sand M, Sand D, Brors D, et al. Cutaneous lesions of the external ear. Head Face Med. 2008;4. doi:10.1186/1746-160X-4-2
- Orengo I, Robbins K, Marsch A. Pathology of the ear. Semin Plast Surg. 2011;25:279-287. doi:10.1055/s-0031-1288920
- Ramroop S. Successful treatment of acanthoma fissuratum with intralesional triamcinolone acetonide. Clin Case Rep. 2020;8:702-703. doi:10.1002/ccr3.2708
- Delaney TJ, Stewart TW. Granuloma fissuratum. Br J Dermatol. 1971;84:373-375. doi:10.1111/j.1365-2133.1971.tb14235.x
- Deshpande NS, Sen A, Vasudevan B, et al. Acanthoma fissuratum: lest we forget. Indian Dermatol Online J. 2017;8:141-143. doi:10.4103/2229- 5178.202267
- Surron RL Jr. A fissured granulomatous lesion of the upper labioalveolar fold. Arch Dermatol Syph. 1932;26:425. doi:10.1001 /archderm.1932.01450030423004
- Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol. 2005;105:1018-1023. doi:10.1097/01. AOG.0000158863.70819.53
- Lee JL, Lee YB, Cho BK, et al. Acanthoma fissuratum on the penis. Int J Dermatol. 2013;52:382-384. doi:10.1111/j.1365-4632.2011.04903.x
- Gonzalez SA, Moore AGN. Acanthoma fissuratum of the outer auditory canal from a hearing aid. J Cutan Pathol. 1989;16:304.
- Fania L, Didona D, Morese R, et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8:449. doi:10.3390/biomedicines8110449
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61. doi:10.4103/0975-5950.102163
The Diagnosis: Acanthoma Fissuratum
Acanthoma fissuratum is a skin lesion that results from consistent pressure, typically from ill-fitting eyeglass frames.1 The chronic irritation leads to collagen deposition and inflammation that gradually creates the lesion. Many patients never seek care, making incidence figures undeterminable.2 It usually presents as a firm, tender, flesh-colored or pink nodule or plaque with a central indentation from where the frame rests. This indentation splits the lesion in half and classically gives the appearance of a coffee bean.1 The repeated minor trauma at this point of contact also may lead to centralized ulceration, which further blurs the diagnosis to include basal cell carcinoma (BCC).3,4 Although the postauricular groove is the most cited location, lesions also may occur at other contact points of the glasses, such as the lateral aspect of the bridge of the nose and the superior auricular sulcus.5 Acanthoma fissuratum is not limited to the external head. Other etiologies of local trauma and pressure have led to its diagnosis in the upper labioalveolar fold, posterior fourchette of the vulva, penis, and external auditory canal.6-9
The diagnosis of acanthoma fissuratum mainly is clinical; however, due to its similar appearance to BCC and other lesions, a biopsy can be taken to support the diagnosis; a biopsy was not performed in our patient. The main features seen on histopathology include acanthosis, hyperkeratosis, variable parakeratosis, and perivascular nonspecific inflammatory infiltration. The epidermis may reflect the macroscopic frame indentation with central attenuation of the epidermis, which potentially is filled with inflammatory cells or keratin.5
Treatment normally encompasses removing the illfitting frames or fixing the fit, which gradually leads to reduction of the lesion.4,5 This occurred in our patient, who changed eyeglasses and saw an 80% resolution of the lesion in 8 months. Such improvement after removal of a trauma-inducing stimulus would not be seen in malignancies (eg, BCC, squamous cell carcinoma [SCC]), keloids, or cylindromas. If the granulation tissue does not regress or recurs, other potential treatments include excision, intralesional corticosteroids, and electrosurgery.5
Basal cell carcinoma is a common nonmelanoma skin cancer that most often presents on the sun-exposed areas of the head and neck, especially the cheeks, nasolabial folds, and forehead. Although the nodular subtype may clinically appear similar to acanthoma fissuratum, it more typically presents as a pearly papule or nodule with a sharp border, small telangiectases, and potential ulceration.10 Squamous cell carcinoma is another common nonmelanoma skin cancer that often arises in sun-exposed areas, which can include the postauricular area. Although the lesion can be associated with chronic wounds and also can grow vertically, SCC typically has a scalier and more hyperkeratotic surface that can ulcerate.1 A cylindroma is a benign sweat gland tumor that most commonly presents on the head and neck (also known as the turban tumor), though it can develop on the ear. It appears as solitary or multiple nodules that often are flesh colored, red, or blue with a shiny surface.1 Cylindromas are not known to be associated with chronic local trauma or irritation,11 such as wearing ill-fitting eyeglasses. Unlike acanthoma fissuratum, the treatment of cylindromas, BCC, and SCC most often involves excision.1 A keloid presents as a flesh-colored, red, or purple exophytic plaque that is composed of dense dermal tissue and progressively forms after local trauma. Although keloids can spontaneously develop, they commonly form on the ears in susceptible individuals after skin excisions including prior keloid removal, piercings, repairment of auricular traumas, or infections.1 The patient’s coffee bean–like lesion that coincided with wearing new eyeglasses better fits the diagnosis of acanthoma fissuratum than a keloid. Additionally, keloids typically do not regress without treatment. Keloid treatment consists of intralesional steroid injections, occlusive silicone dressings, compression, cryotherapy, radiation, and excisional surgery.1
The Diagnosis: Acanthoma Fissuratum
Acanthoma fissuratum is a skin lesion that results from consistent pressure, typically from ill-fitting eyeglass frames.1 The chronic irritation leads to collagen deposition and inflammation that gradually creates the lesion. Many patients never seek care, making incidence figures undeterminable.2 It usually presents as a firm, tender, flesh-colored or pink nodule or plaque with a central indentation from where the frame rests. This indentation splits the lesion in half and classically gives the appearance of a coffee bean.1 The repeated minor trauma at this point of contact also may lead to centralized ulceration, which further blurs the diagnosis to include basal cell carcinoma (BCC).3,4 Although the postauricular groove is the most cited location, lesions also may occur at other contact points of the glasses, such as the lateral aspect of the bridge of the nose and the superior auricular sulcus.5 Acanthoma fissuratum is not limited to the external head. Other etiologies of local trauma and pressure have led to its diagnosis in the upper labioalveolar fold, posterior fourchette of the vulva, penis, and external auditory canal.6-9
The diagnosis of acanthoma fissuratum mainly is clinical; however, due to its similar appearance to BCC and other lesions, a biopsy can be taken to support the diagnosis; a biopsy was not performed in our patient. The main features seen on histopathology include acanthosis, hyperkeratosis, variable parakeratosis, and perivascular nonspecific inflammatory infiltration. The epidermis may reflect the macroscopic frame indentation with central attenuation of the epidermis, which potentially is filled with inflammatory cells or keratin.5
Treatment normally encompasses removing the illfitting frames or fixing the fit, which gradually leads to reduction of the lesion.4,5 This occurred in our patient, who changed eyeglasses and saw an 80% resolution of the lesion in 8 months. Such improvement after removal of a trauma-inducing stimulus would not be seen in malignancies (eg, BCC, squamous cell carcinoma [SCC]), keloids, or cylindromas. If the granulation tissue does not regress or recurs, other potential treatments include excision, intralesional corticosteroids, and electrosurgery.5
Basal cell carcinoma is a common nonmelanoma skin cancer that most often presents on the sun-exposed areas of the head and neck, especially the cheeks, nasolabial folds, and forehead. Although the nodular subtype may clinically appear similar to acanthoma fissuratum, it more typically presents as a pearly papule or nodule with a sharp border, small telangiectases, and potential ulceration.10 Squamous cell carcinoma is another common nonmelanoma skin cancer that often arises in sun-exposed areas, which can include the postauricular area. Although the lesion can be associated with chronic wounds and also can grow vertically, SCC typically has a scalier and more hyperkeratotic surface that can ulcerate.1 A cylindroma is a benign sweat gland tumor that most commonly presents on the head and neck (also known as the turban tumor), though it can develop on the ear. It appears as solitary or multiple nodules that often are flesh colored, red, or blue with a shiny surface.1 Cylindromas are not known to be associated with chronic local trauma or irritation,11 such as wearing ill-fitting eyeglasses. Unlike acanthoma fissuratum, the treatment of cylindromas, BCC, and SCC most often involves excision.1 A keloid presents as a flesh-colored, red, or purple exophytic plaque that is composed of dense dermal tissue and progressively forms after local trauma. Although keloids can spontaneously develop, they commonly form on the ears in susceptible individuals after skin excisions including prior keloid removal, piercings, repairment of auricular traumas, or infections.1 The patient’s coffee bean–like lesion that coincided with wearing new eyeglasses better fits the diagnosis of acanthoma fissuratum than a keloid. Additionally, keloids typically do not regress without treatment. Keloid treatment consists of intralesional steroid injections, occlusive silicone dressings, compression, cryotherapy, radiation, and excisional surgery.1
- Sand M, Sand D, Brors D, et al. Cutaneous lesions of the external ear. Head Face Med. 2008;4. doi:10.1186/1746-160X-4-2
- Orengo I, Robbins K, Marsch A. Pathology of the ear. Semin Plast Surg. 2011;25:279-287. doi:10.1055/s-0031-1288920
- Ramroop S. Successful treatment of acanthoma fissuratum with intralesional triamcinolone acetonide. Clin Case Rep. 2020;8:702-703. doi:10.1002/ccr3.2708
- Delaney TJ, Stewart TW. Granuloma fissuratum. Br J Dermatol. 1971;84:373-375. doi:10.1111/j.1365-2133.1971.tb14235.x
- Deshpande NS, Sen A, Vasudevan B, et al. Acanthoma fissuratum: lest we forget. Indian Dermatol Online J. 2017;8:141-143. doi:10.4103/2229- 5178.202267
- Surron RL Jr. A fissured granulomatous lesion of the upper labioalveolar fold. Arch Dermatol Syph. 1932;26:425. doi:10.1001 /archderm.1932.01450030423004
- Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol. 2005;105:1018-1023. doi:10.1097/01. AOG.0000158863.70819.53
- Lee JL, Lee YB, Cho BK, et al. Acanthoma fissuratum on the penis. Int J Dermatol. 2013;52:382-384. doi:10.1111/j.1365-4632.2011.04903.x
- Gonzalez SA, Moore AGN. Acanthoma fissuratum of the outer auditory canal from a hearing aid. J Cutan Pathol. 1989;16:304.
- Fania L, Didona D, Morese R, et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8:449. doi:10.3390/biomedicines8110449
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61. doi:10.4103/0975-5950.102163
- Sand M, Sand D, Brors D, et al. Cutaneous lesions of the external ear. Head Face Med. 2008;4. doi:10.1186/1746-160X-4-2
- Orengo I, Robbins K, Marsch A. Pathology of the ear. Semin Plast Surg. 2011;25:279-287. doi:10.1055/s-0031-1288920
- Ramroop S. Successful treatment of acanthoma fissuratum with intralesional triamcinolone acetonide. Clin Case Rep. 2020;8:702-703. doi:10.1002/ccr3.2708
- Delaney TJ, Stewart TW. Granuloma fissuratum. Br J Dermatol. 1971;84:373-375. doi:10.1111/j.1365-2133.1971.tb14235.x
- Deshpande NS, Sen A, Vasudevan B, et al. Acanthoma fissuratum: lest we forget. Indian Dermatol Online J. 2017;8:141-143. doi:10.4103/2229- 5178.202267
- Surron RL Jr. A fissured granulomatous lesion of the upper labioalveolar fold. Arch Dermatol Syph. 1932;26:425. doi:10.1001 /archderm.1932.01450030423004
- Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol. 2005;105:1018-1023. doi:10.1097/01. AOG.0000158863.70819.53
- Lee JL, Lee YB, Cho BK, et al. Acanthoma fissuratum on the penis. Int J Dermatol. 2013;52:382-384. doi:10.1111/j.1365-4632.2011.04903.x
- Gonzalez SA, Moore AGN. Acanthoma fissuratum of the outer auditory canal from a hearing aid. J Cutan Pathol. 1989;16:304.
- Fania L, Didona D, Morese R, et al. Basal cell carcinoma: from pathophysiology to novel therapeutic approaches. Biomedicines. 2020;8:449. doi:10.3390/biomedicines8110449
- Chauhan DS, Guruprasad Y. Dermal cylindroma of the scalp. Natl J Maxillofac Surg. 2012;3:59-61. doi:10.4103/0975-5950.102163
A 62-year-old man presented to the dermatology office with a 1.5-cm, pink, rubbery nodule behind the left ear that sometimes was tender. He stated that the lesion gradually grew in size over the last 2 years, and it developed after he was fitted for new glasses.