User login
Exaggerated Facial Lines on the Forehead and Cheeks
The Diagnosis: Pachydermoperiostosis
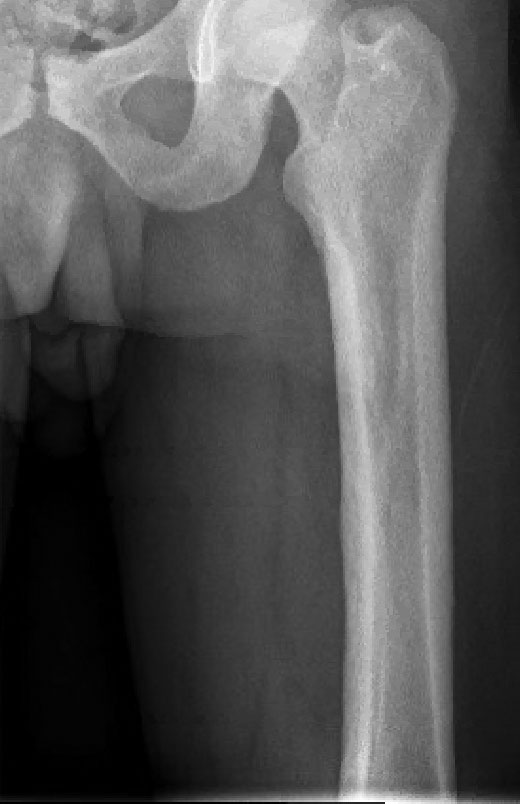
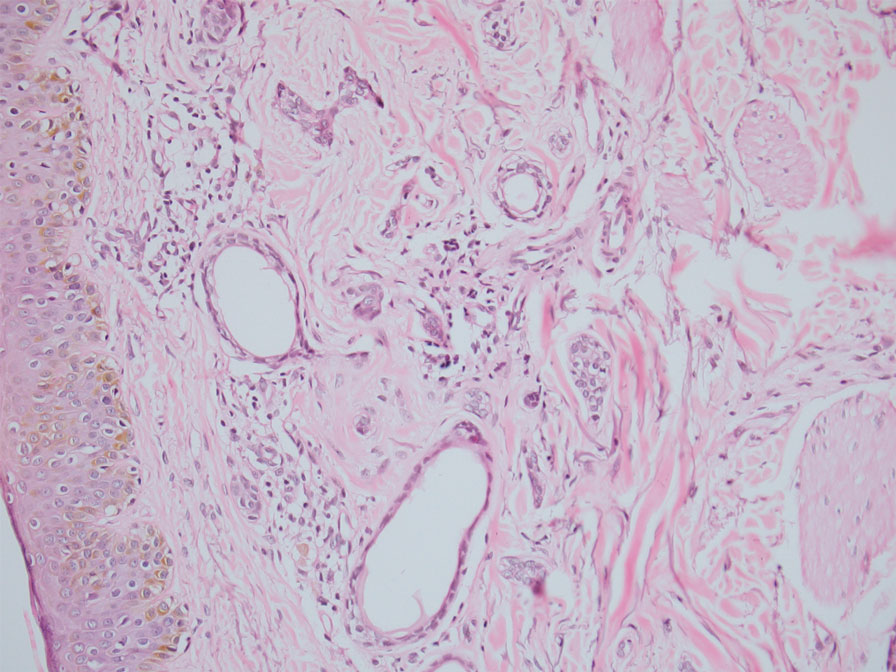
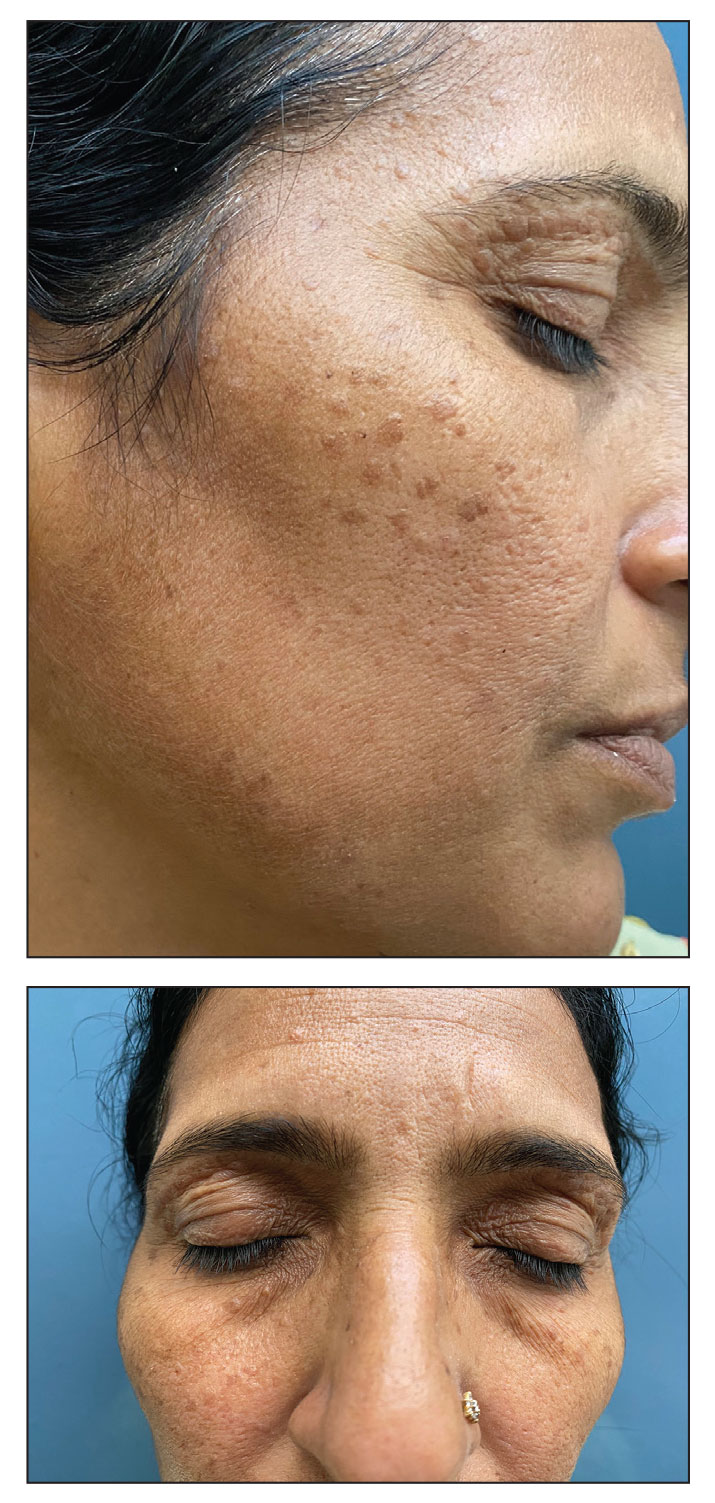
Histopathology of the forehead punch biopsy demonstrated sebaceous hyperplasia with an occupation rate of greater than 40%, increased mucin, elastic fiber degeneration, and fibrosis. Pachydermia is graded from 0 to 3 depending on the degree of these changes; our patient met criteria for grade 3 pachydermia (Figure 1). Radiography revealed diffuse cortical thickening of the long bones that was most marked in the left femur (Figure 2); however, no other findings were demonstrative of Paget disease.
Pachydermoperiostosis (PDP)(also known as Touraine-Solente-Golé syndrome or primary hypertrophic osteoarthropathy) is a rare genetic condition that affects both the dermatologic and skeletal systems. Clinical features of the disease include progressive thickening and furrowing of the skin on the scalp and face (known as pachydermia), digital clubbing, and periostosis. Other potential cutaneous features include seborrhea, acne, hyperhidrosis of the palms and soles, cutis verticis gyrata, eczema, and a burning sensation of the hands and feet. Myelofibrosis and gastrointestinal abnormalities also have been reported.1
The disease typically affects males (7:1 ratio); also, men typically display a more severe phenotype of the disease.2 It most commonly begins during puberty and follows a generally progressive course of 5 to 20 years before eventually stabilizing. Both autosomal-dominant with incomplete penetrance and recessive inheritance versions of PDP can occur. Prostaglandin E2 (PGE2) has been implicated in the pathogenesis of PDP; PGE2 is important in the inflammatory response and may evolve from disrupted protein degradation pathways.3 Sasaki et al4 additionally reported that the severity of pachydermia clinically and histologically appeared to correlate with the serum PGE2 levels in affected patients. Prostaglandin E2 causes a vasodilatory effect, perhaps explaining the clubbing observed in PDP, and also modifies the activity of osteoblasts and osteoclasts, causing the bone remodeling observed in the disease.4
In our patient, the initial differential diagnosis included PDP, as well as lepromatous leprosy, acromegaly, Paget disease of the bone, amyloidosis, scleromyxedema, and cutaneous T-cell lymphoma. However, the time course of the disease, lack of numerous symmetric thickened plaques and madarosis, and pathology argued against lepromatous leprosy. Acromegaly was ruled out due to lack of macroglossia as well as laboratory analysis within reference range including IGF-1 levels and thyroid function tests. Biopsy findings ultimately ruled out amyloidosis and cutaneous T-cell lymphoma. The bone scan revealed diffuse cortical thickening consistent with PDP, and there were no other radiologic findings suggestive of Paget disease. Pachydermoperiostosis is diagnosed using the Borochowitz criteria, which entails that 2 of the following 4 fulfillment criteria must be met: familial transmission, pachydermia, digital clubbing, and/or bony involvement with evidence of radiologic alterations or pain. Our patient met all 4 criteria. The clinical manifestations of PDP are variable with respect to skin and bone changes. The various clinical expressions include the complete form (ie, pachydermia, cutis verticis gyrata, periostosis), the incomplete form (ie, absence of cutis verticis gyrata), and forme fruste (ie, pachydermia with minimal or absent periostosis).5
Management for PDP involves surgical correction for cosmesis as well as for functional concerns if present. Symptoms secondary to periostosis should be managed with symptomatic treatment such as nonsteroidal antiinflammatory drugs. Patients managed with etoricoxib, a COX-2–selective nonsteroidal anti-inflammatory drug, have had normalized inflammatory markers that resulted in the lessening of forehead skin folds. Oral aescin has been shown to relieve joint pain due to its antiedematous effect.6 Our patient received treatment with nonsteroidal anti-inflammatory drugs for symptomatic management of the associated joint pain but unfortunately was lost to follow-up.
- Castori M, Sinibaldi L, Mingarelli R, et al. Pachydermoperiostosis: an update. Clin Genet. 2005;68:477-486.
- Reginato AJ, Shipachasse V, Guerrero R. Familial idiopathic hypertrophic osteoarthropathy and cranial suture defects in children. Skel Radiol. 1982;8:105-109.
- Coggins KG, Coffman TM, Koller BH. The Hippocratic finger points the blame at PGE2. Nat Genet. 2008;40:691-692.
- Sasaki T, Niizeki H, Shimizu A, et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68:36-44.
- Bhaskaranand K, Shetty RR, Bhat AK. Pachydermoperiostosis: three case reports. J Orthop Surg (Hong Kong). 2001;9:61-66.
- Zhang H, Yang B. Successful treatment of pachydermoperiostosis patients with etoricoxib, aescin, and arthroscopic synovectomy: two case reports. Medicine (Baltimore). 2017;96:E8865.
The Diagnosis: Pachydermoperiostosis
Histopathology of the forehead punch biopsy demonstrated sebaceous hyperplasia with an occupation rate of greater than 40%, increased mucin, elastic fiber degeneration, and fibrosis. Pachydermia is graded from 0 to 3 depending on the degree of these changes; our patient met criteria for grade 3 pachydermia (Figure 1). Radiography revealed diffuse cortical thickening of the long bones that was most marked in the left femur (Figure 2); however, no other findings were demonstrative of Paget disease.

Pachydermoperiostosis (PDP)(also known as Touraine-Solente-Golé syndrome or primary hypertrophic osteoarthropathy) is a rare genetic condition that affects both the dermatologic and skeletal systems. Clinical features of the disease include progressive thickening and furrowing of the skin on the scalp and face (known as pachydermia), digital clubbing, and periostosis. Other potential cutaneous features include seborrhea, acne, hyperhidrosis of the palms and soles, cutis verticis gyrata, eczema, and a burning sensation of the hands and feet. Myelofibrosis and gastrointestinal abnormalities also have been reported.1

The disease typically affects males (7:1 ratio); also, men typically display a more severe phenotype of the disease.2 It most commonly begins during puberty and follows a generally progressive course of 5 to 20 years before eventually stabilizing. Both autosomal-dominant with incomplete penetrance and recessive inheritance versions of PDP can occur. Prostaglandin E2 (PGE2) has been implicated in the pathogenesis of PDP; PGE2 is important in the inflammatory response and may evolve from disrupted protein degradation pathways.3 Sasaki et al4 additionally reported that the severity of pachydermia clinically and histologically appeared to correlate with the serum PGE2 levels in affected patients. Prostaglandin E2 causes a vasodilatory effect, perhaps explaining the clubbing observed in PDP, and also modifies the activity of osteoblasts and osteoclasts, causing the bone remodeling observed in the disease.4
In our patient, the initial differential diagnosis included PDP, as well as lepromatous leprosy, acromegaly, Paget disease of the bone, amyloidosis, scleromyxedema, and cutaneous T-cell lymphoma. However, the time course of the disease, lack of numerous symmetric thickened plaques and madarosis, and pathology argued against lepromatous leprosy. Acromegaly was ruled out due to lack of macroglossia as well as laboratory analysis within reference range including IGF-1 levels and thyroid function tests. Biopsy findings ultimately ruled out amyloidosis and cutaneous T-cell lymphoma. The bone scan revealed diffuse cortical thickening consistent with PDP, and there were no other radiologic findings suggestive of Paget disease. Pachydermoperiostosis is diagnosed using the Borochowitz criteria, which entails that 2 of the following 4 fulfillment criteria must be met: familial transmission, pachydermia, digital clubbing, and/or bony involvement with evidence of radiologic alterations or pain. Our patient met all 4 criteria. The clinical manifestations of PDP are variable with respect to skin and bone changes. The various clinical expressions include the complete form (ie, pachydermia, cutis verticis gyrata, periostosis), the incomplete form (ie, absence of cutis verticis gyrata), and forme fruste (ie, pachydermia with minimal or absent periostosis).5
Management for PDP involves surgical correction for cosmesis as well as for functional concerns if present. Symptoms secondary to periostosis should be managed with symptomatic treatment such as nonsteroidal antiinflammatory drugs. Patients managed with etoricoxib, a COX-2–selective nonsteroidal anti-inflammatory drug, have had normalized inflammatory markers that resulted in the lessening of forehead skin folds. Oral aescin has been shown to relieve joint pain due to its antiedematous effect.6 Our patient received treatment with nonsteroidal anti-inflammatory drugs for symptomatic management of the associated joint pain but unfortunately was lost to follow-up.
The Diagnosis: Pachydermoperiostosis
Histopathology of the forehead punch biopsy demonstrated sebaceous hyperplasia with an occupation rate of greater than 40%, increased mucin, elastic fiber degeneration, and fibrosis. Pachydermia is graded from 0 to 3 depending on the degree of these changes; our patient met criteria for grade 3 pachydermia (Figure 1). Radiography revealed diffuse cortical thickening of the long bones that was most marked in the left femur (Figure 2); however, no other findings were demonstrative of Paget disease.

Pachydermoperiostosis (PDP)(also known as Touraine-Solente-Golé syndrome or primary hypertrophic osteoarthropathy) is a rare genetic condition that affects both the dermatologic and skeletal systems. Clinical features of the disease include progressive thickening and furrowing of the skin on the scalp and face (known as pachydermia), digital clubbing, and periostosis. Other potential cutaneous features include seborrhea, acne, hyperhidrosis of the palms and soles, cutis verticis gyrata, eczema, and a burning sensation of the hands and feet. Myelofibrosis and gastrointestinal abnormalities also have been reported.1

The disease typically affects males (7:1 ratio); also, men typically display a more severe phenotype of the disease.2 It most commonly begins during puberty and follows a generally progressive course of 5 to 20 years before eventually stabilizing. Both autosomal-dominant with incomplete penetrance and recessive inheritance versions of PDP can occur. Prostaglandin E2 (PGE2) has been implicated in the pathogenesis of PDP; PGE2 is important in the inflammatory response and may evolve from disrupted protein degradation pathways.3 Sasaki et al4 additionally reported that the severity of pachydermia clinically and histologically appeared to correlate with the serum PGE2 levels in affected patients. Prostaglandin E2 causes a vasodilatory effect, perhaps explaining the clubbing observed in PDP, and also modifies the activity of osteoblasts and osteoclasts, causing the bone remodeling observed in the disease.4
In our patient, the initial differential diagnosis included PDP, as well as lepromatous leprosy, acromegaly, Paget disease of the bone, amyloidosis, scleromyxedema, and cutaneous T-cell lymphoma. However, the time course of the disease, lack of numerous symmetric thickened plaques and madarosis, and pathology argued against lepromatous leprosy. Acromegaly was ruled out due to lack of macroglossia as well as laboratory analysis within reference range including IGF-1 levels and thyroid function tests. Biopsy findings ultimately ruled out amyloidosis and cutaneous T-cell lymphoma. The bone scan revealed diffuse cortical thickening consistent with PDP, and there were no other radiologic findings suggestive of Paget disease. Pachydermoperiostosis is diagnosed using the Borochowitz criteria, which entails that 2 of the following 4 fulfillment criteria must be met: familial transmission, pachydermia, digital clubbing, and/or bony involvement with evidence of radiologic alterations or pain. Our patient met all 4 criteria. The clinical manifestations of PDP are variable with respect to skin and bone changes. The various clinical expressions include the complete form (ie, pachydermia, cutis verticis gyrata, periostosis), the incomplete form (ie, absence of cutis verticis gyrata), and forme fruste (ie, pachydermia with minimal or absent periostosis).5
Management for PDP involves surgical correction for cosmesis as well as for functional concerns if present. Symptoms secondary to periostosis should be managed with symptomatic treatment such as nonsteroidal antiinflammatory drugs. Patients managed with etoricoxib, a COX-2–selective nonsteroidal anti-inflammatory drug, have had normalized inflammatory markers that resulted in the lessening of forehead skin folds. Oral aescin has been shown to relieve joint pain due to its antiedematous effect.6 Our patient received treatment with nonsteroidal anti-inflammatory drugs for symptomatic management of the associated joint pain but unfortunately was lost to follow-up.
- Castori M, Sinibaldi L, Mingarelli R, et al. Pachydermoperiostosis: an update. Clin Genet. 2005;68:477-486.
- Reginato AJ, Shipachasse V, Guerrero R. Familial idiopathic hypertrophic osteoarthropathy and cranial suture defects in children. Skel Radiol. 1982;8:105-109.
- Coggins KG, Coffman TM, Koller BH. The Hippocratic finger points the blame at PGE2. Nat Genet. 2008;40:691-692.
- Sasaki T, Niizeki H, Shimizu A, et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68:36-44.
- Bhaskaranand K, Shetty RR, Bhat AK. Pachydermoperiostosis: three case reports. J Orthop Surg (Hong Kong). 2001;9:61-66.
- Zhang H, Yang B. Successful treatment of pachydermoperiostosis patients with etoricoxib, aescin, and arthroscopic synovectomy: two case reports. Medicine (Baltimore). 2017;96:E8865.
- Castori M, Sinibaldi L, Mingarelli R, et al. Pachydermoperiostosis: an update. Clin Genet. 2005;68:477-486.
- Reginato AJ, Shipachasse V, Guerrero R. Familial idiopathic hypertrophic osteoarthropathy and cranial suture defects in children. Skel Radiol. 1982;8:105-109.
- Coggins KG, Coffman TM, Koller BH. The Hippocratic finger points the blame at PGE2. Nat Genet. 2008;40:691-692.
- Sasaki T, Niizeki H, Shimizu A, et al. Identification of mutations in the prostaglandin transporter gene SLCO2A1 and its phenotype-genotype correlation in Japanese patients with pachydermoperiostosis. J Dermatol Sci. 2012;68:36-44.
- Bhaskaranand K, Shetty RR, Bhat AK. Pachydermoperiostosis: three case reports. J Orthop Surg (Hong Kong). 2001;9:61-66.
- Zhang H, Yang B. Successful treatment of pachydermoperiostosis patients with etoricoxib, aescin, and arthroscopic synovectomy: two case reports. Medicine (Baltimore). 2017;96:E8865.
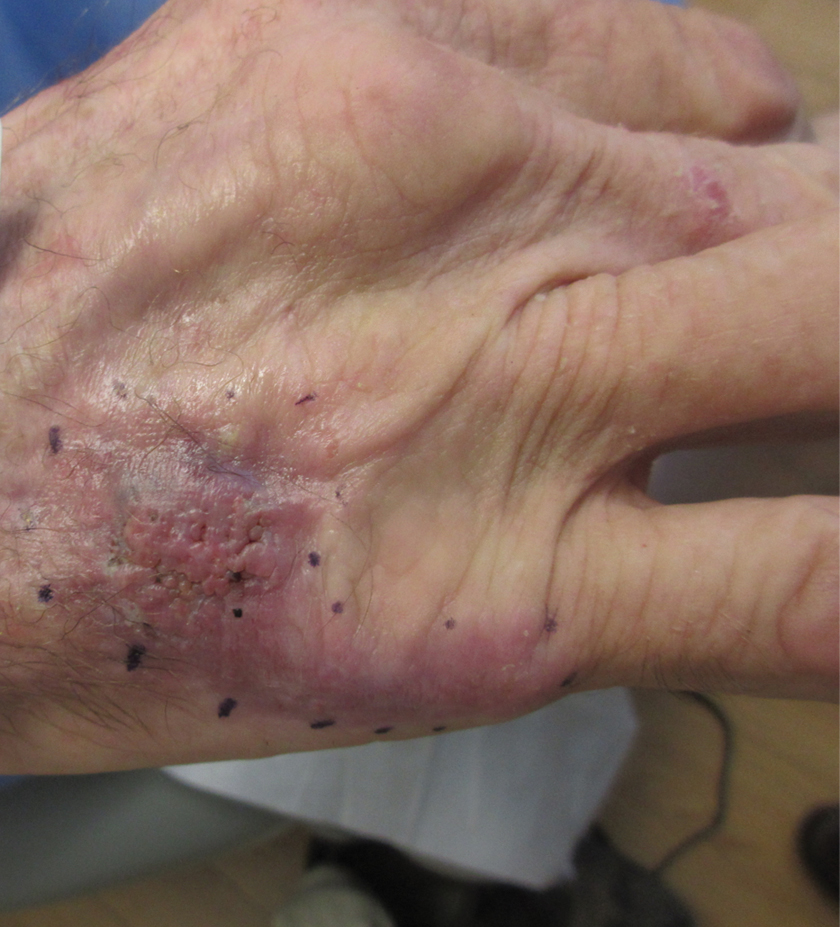
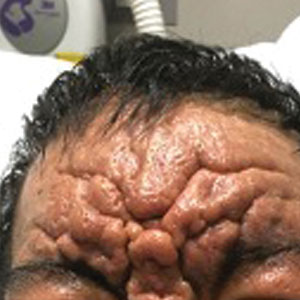
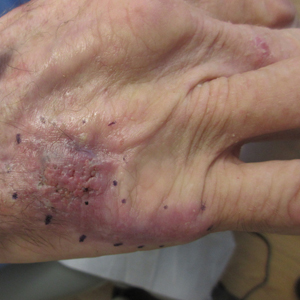
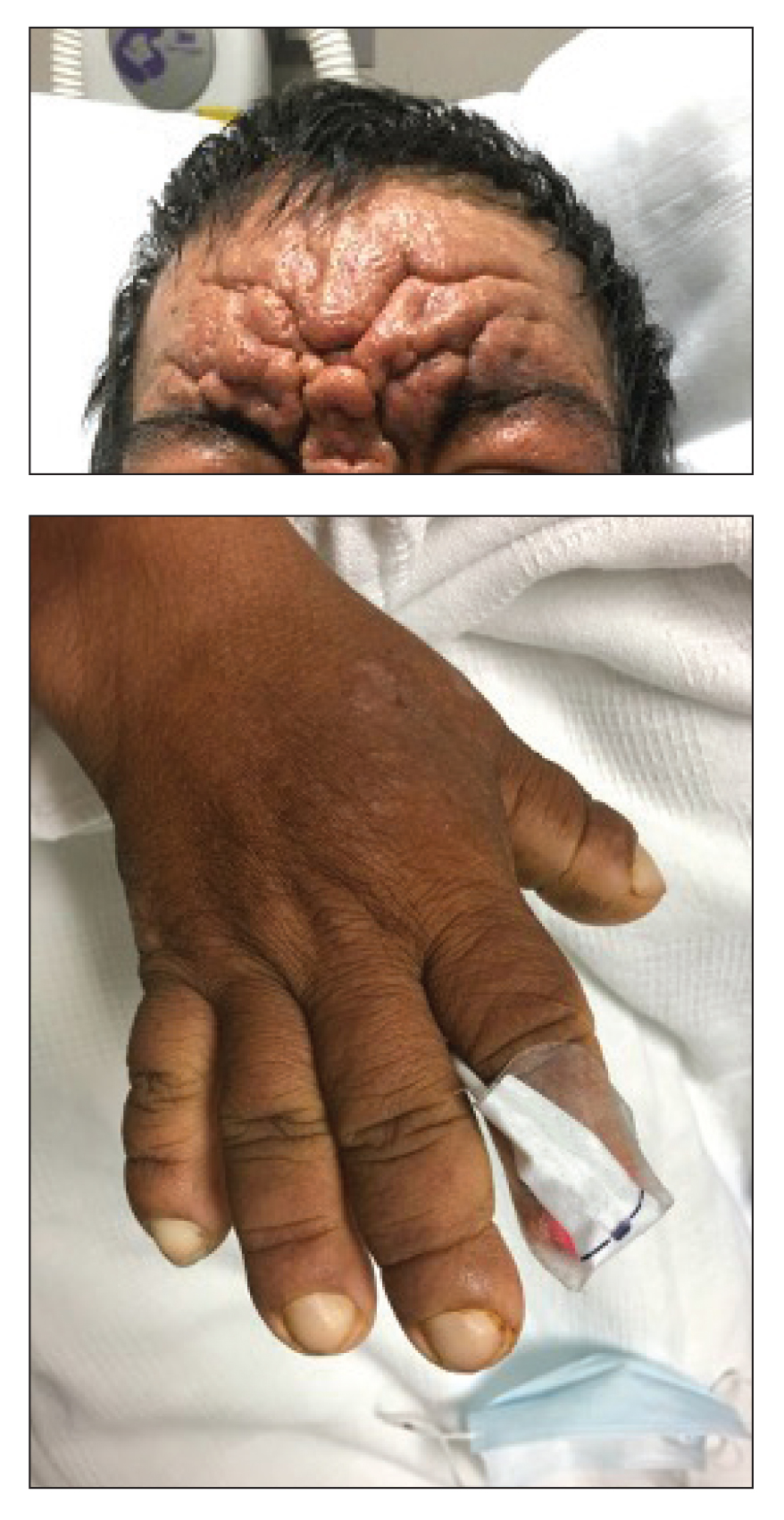
A 36-year-old man presented to the emergency department with an olecranon fracture after falling from a tree. The patient had a medical history of type 2 diabetes mellitus and a surgical history of facial cosmetic surgery. He underwent internal fixation with orthopedic surgery for the olecranon fracture, and dermatology subsequently was consulted due to diffuse skin changes on the face. He reported that these dermatologic changes began around 17 years of age and had progressed to the current presentation. He denied itching, burning, pain, or contact with armadillos. A family history revealed the patient’s brother also had a similar appearance. Physical examination revealed exaggerated facial lines on the forehead (top) and cheeks. Digital clubbing and skin thickening were noted on the hands (bottom) and feet; examination of the back revealed multiple hypopigmented patches. Observation of the scalp showed multiple symmetric ridges and grooves with sparse overlying hair consistent with cutis verticis gyrata. A punch biopsy of the forehead was obtained as well as bone radiography taken previously by the primary team.
Pigmented Papules on the Face, Neck, and Chest
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.
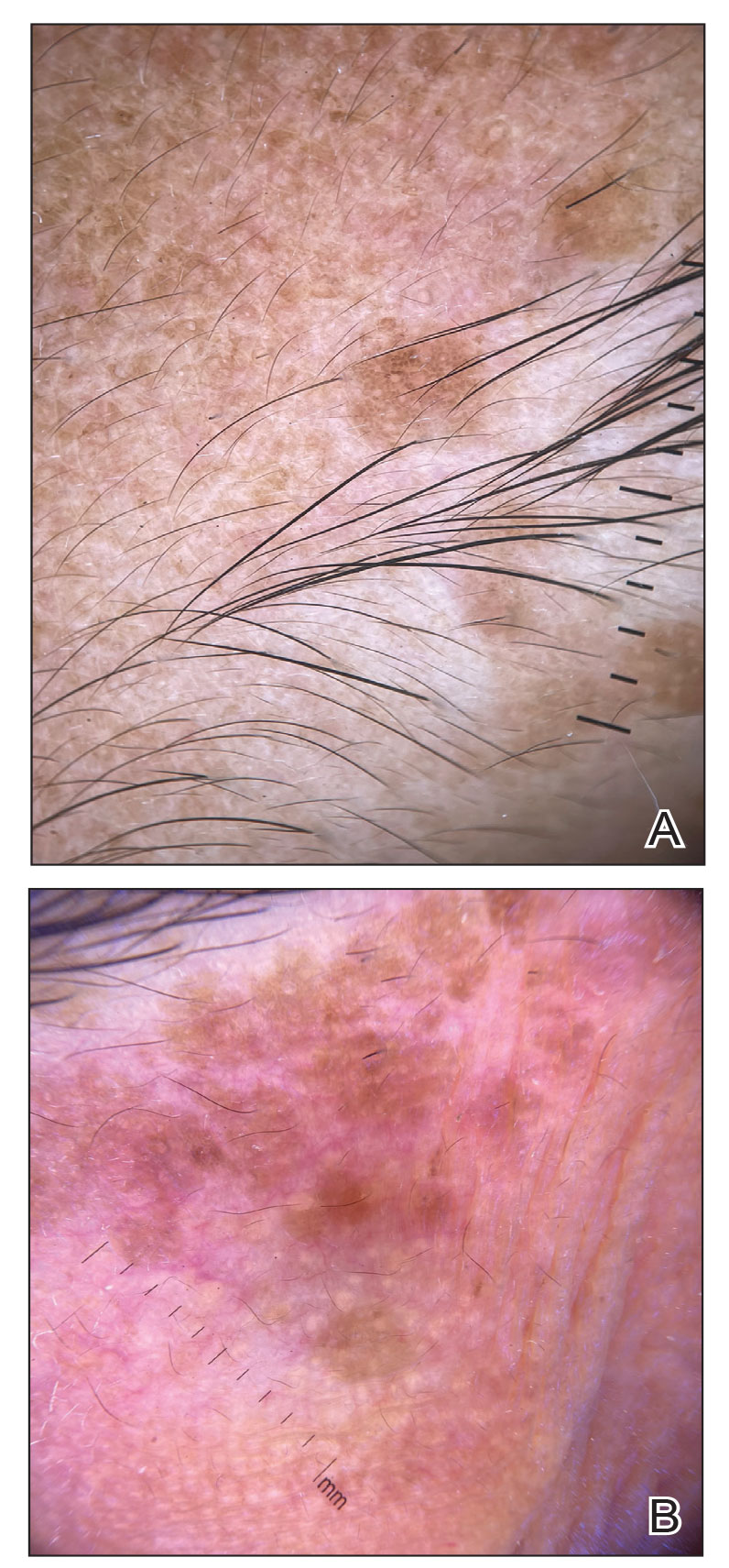
In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).
Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.

In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).

Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.

In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).

Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
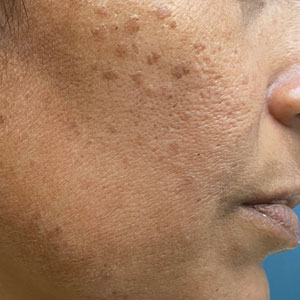
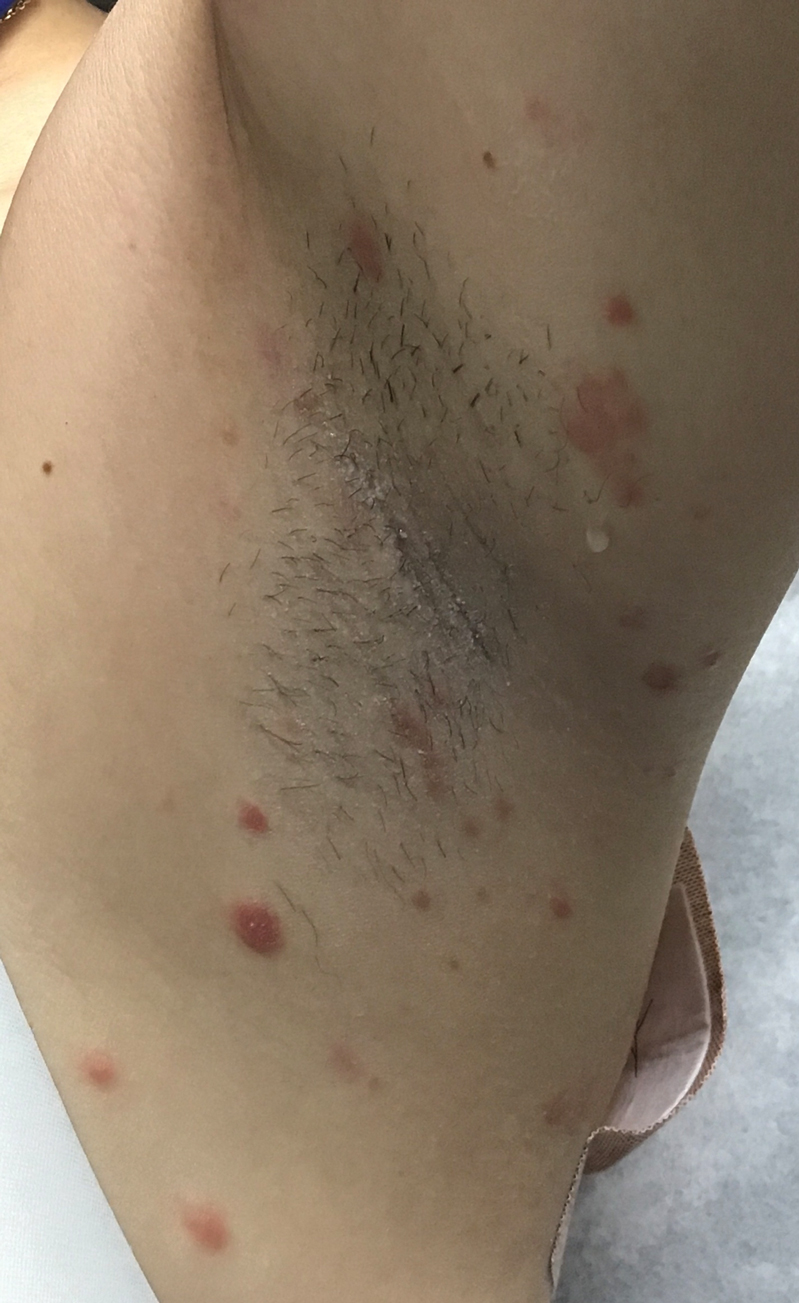
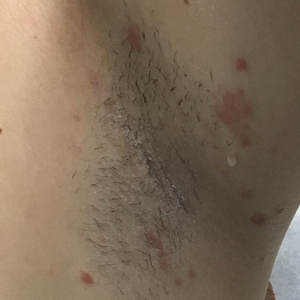
A 46-year-old woman presented with multiple asymptomatic, flesh-colored, hyperpigmented papules on the face of 5 to 6 months’ duration that were progressively increasing in number. The lesions first appeared near the eyebrows and cheeks (top) and subsequently spread to involve the neck. She had no notable medical history. Cutaneous examination revealed multiple tan to brown papules over the periorbital, malar, and neck regions ranging in size from 1 to 5 mm. The lesions over the periorbital region were arranged in a linear pattern (bottom). Similar lesions also were present on the chest and arms. No other sites were involved, and systemic examination was normal.
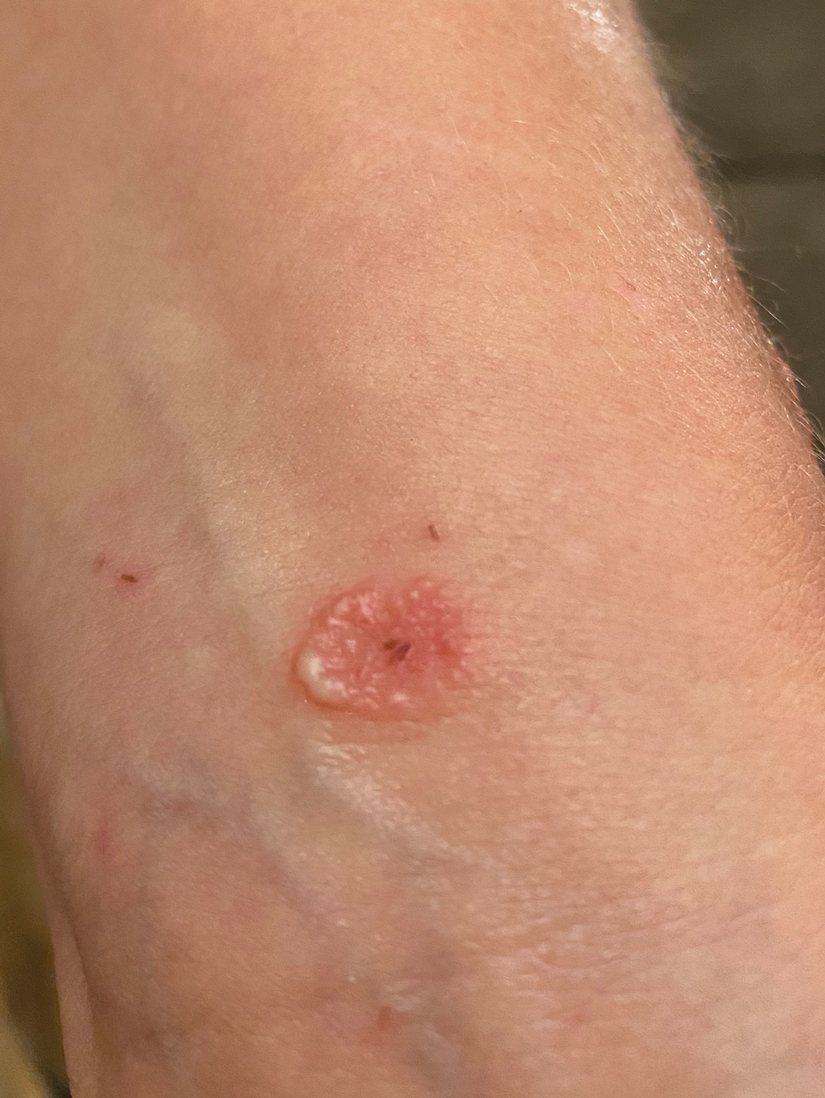
Solitary Pink Plaque on the Neck
The Diagnosis: Plaque-type Syringoma
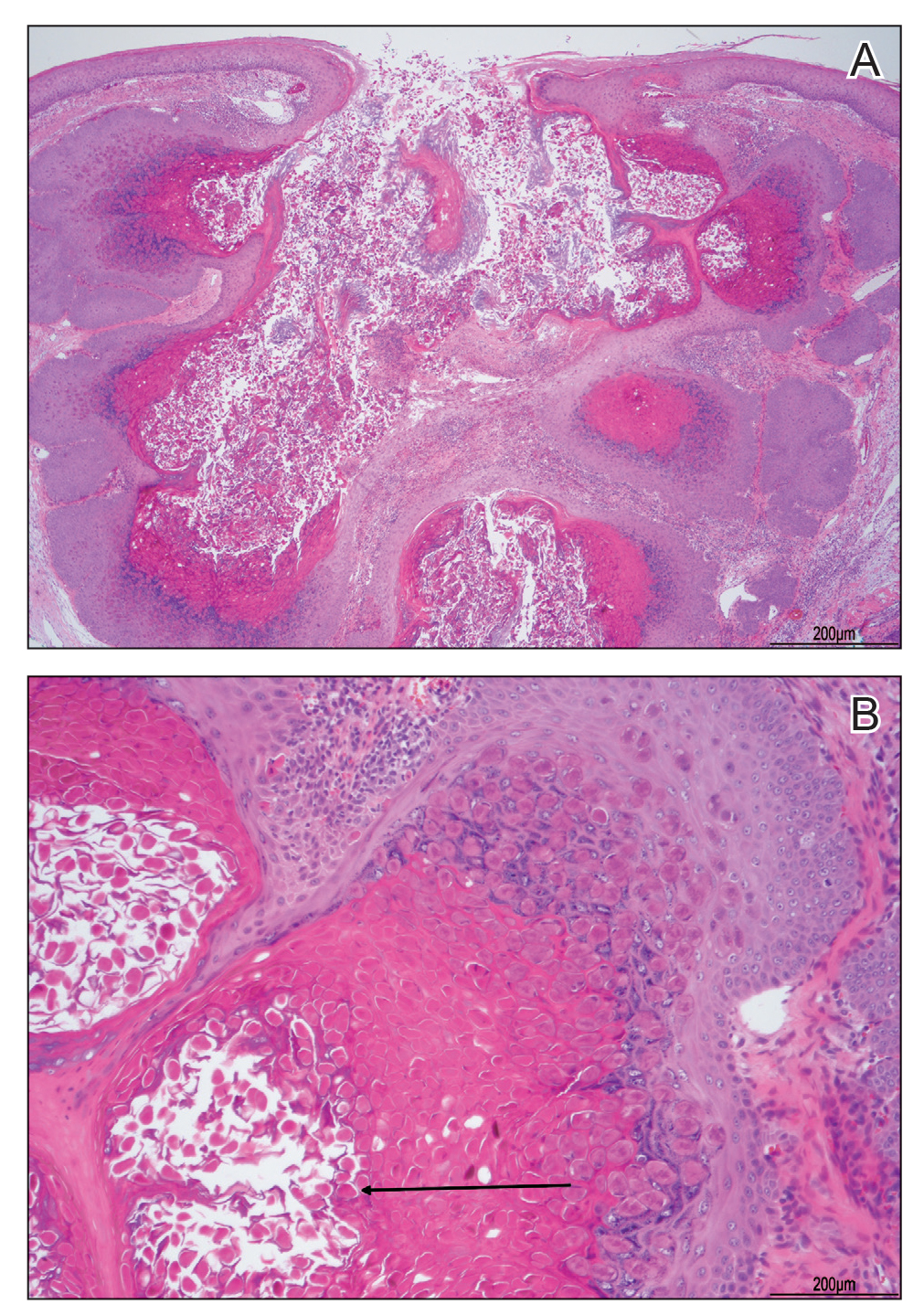
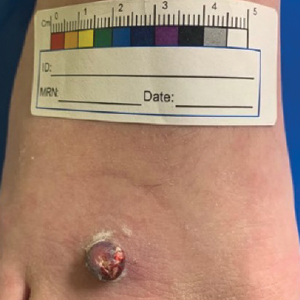
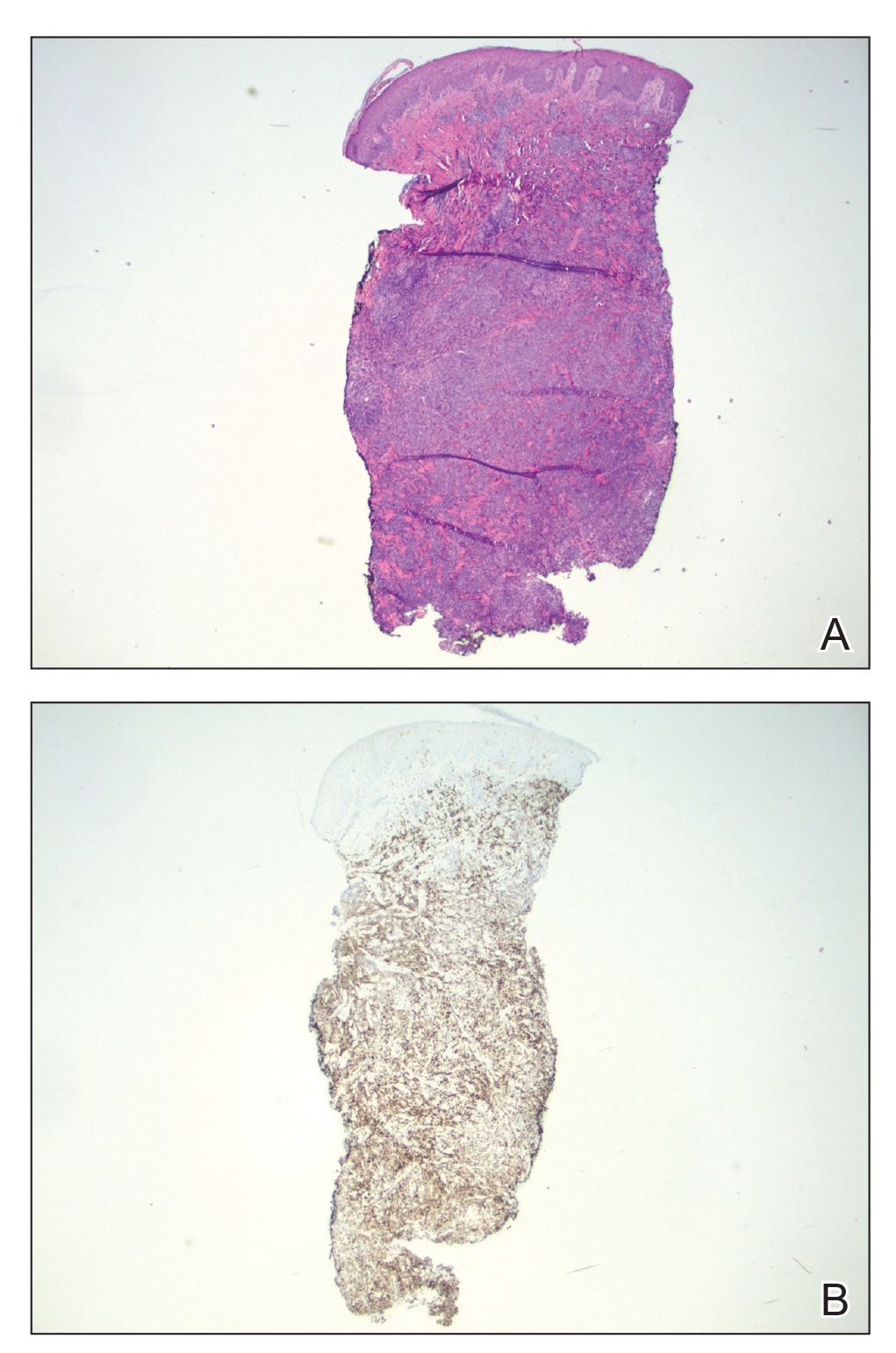
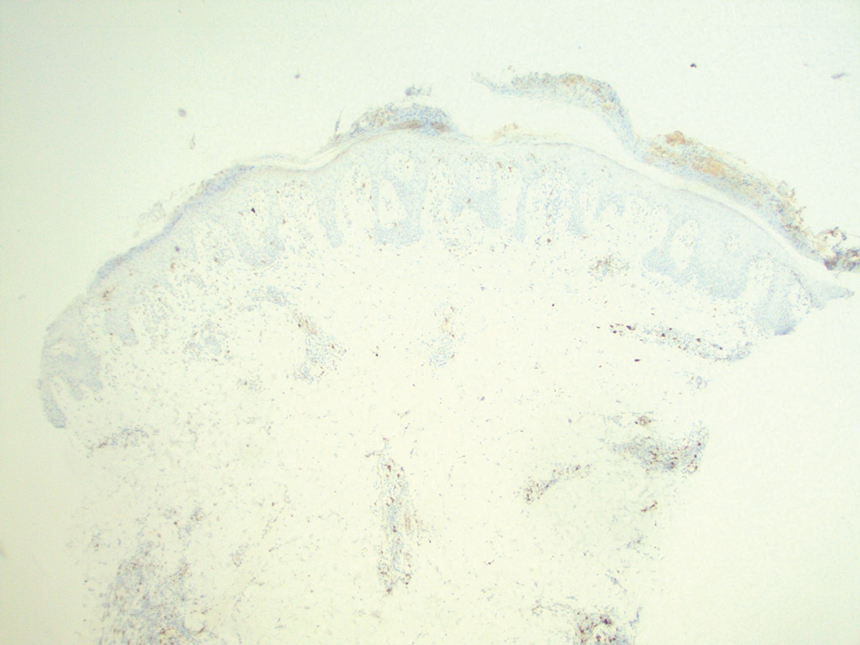
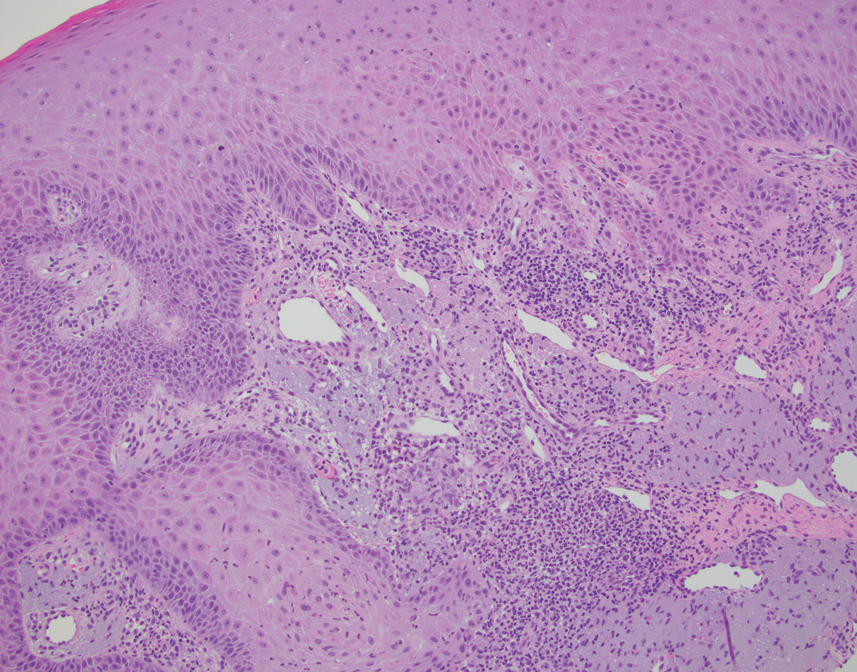
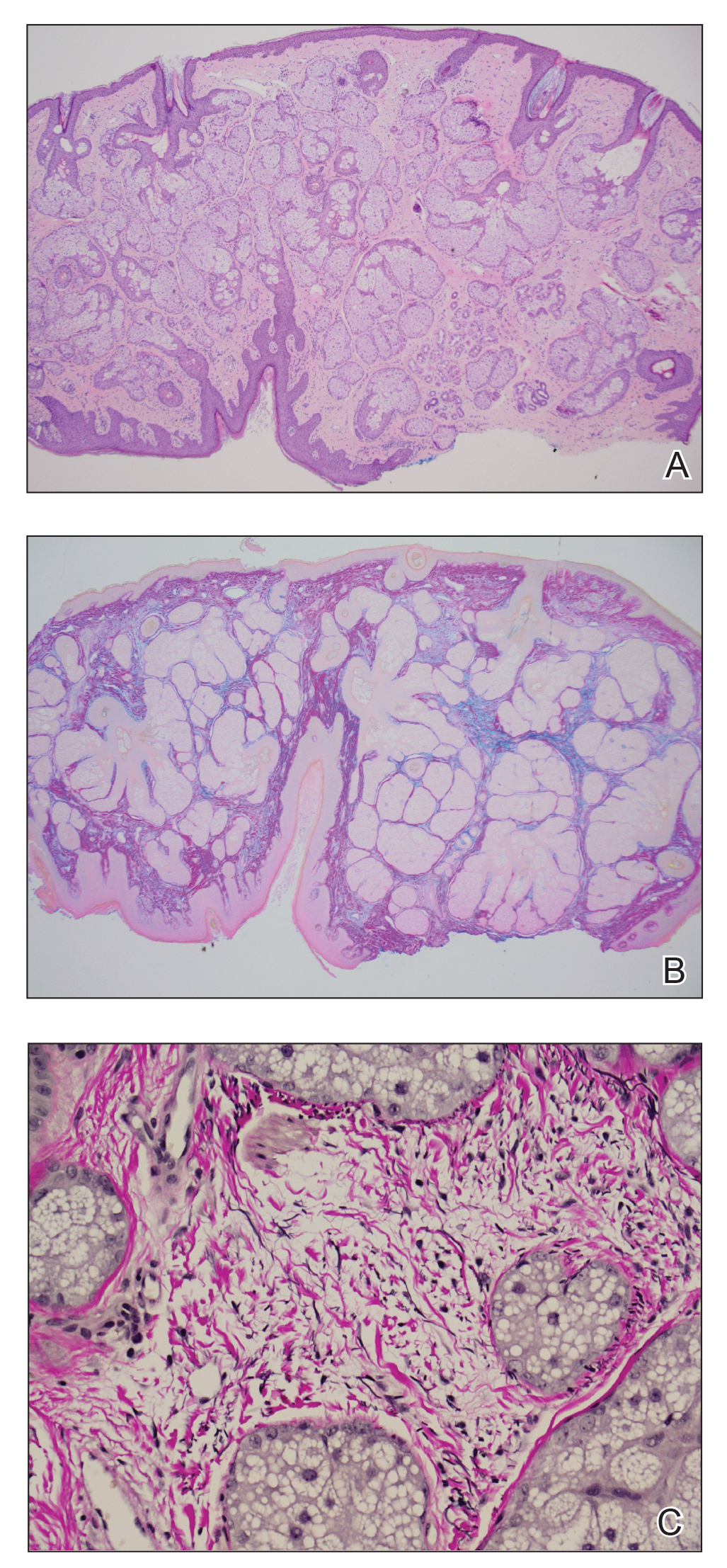
A biopsy demonstrated multiple basaloid islands of tumor cells in the reticular dermis with ductal differentiation, some with a commalike tail. The ducts were lined by 2 to 3 layers of small uniform cuboidal cells without atypia and contained inspissated secretions within the lumina of scattered ducts. There was an associated fibrotic collagenous stroma. There was no evidence of perineural invasion and no deep dermal or subcutaneous extension (Figure 1). Additional cytokeratin immunohistochemical staining highlighted the adnexal proliferation (Figure 2). A diagnosis of plaque-type syringoma (PTS) was made.

Syringomas are benign dermal sweat gland tumors that typically present as flesh-colored papules on the cheeks or periorbital area of young females. Plaque-type tumors as well as papulonodular, eruptive, disseminated, urticaria pigmentosa–like, lichen planus–like, or milialike syringomas also have been reported. Syringomas may be associated with certain medical conditions such as Down syndrome, Nicolau-Balus syndrome, and both scarring and nonscarring alopecias.1 The clear cell variant of syringoma often is associated with diabetes mellitus.2 Kikuchi et al3 first described PTS in 1979. Plaque-type syringomas rarely are reported in the literature, and sites of involvement include the head and neck region, upper lip, chest, upper extremities, vulva, penis, and scrotum.4-6

Histologically, syringomatous lesions are composed of multiple small ducts lined by 2 to 3 layers of cuboidal epithelium. The ducts may be arranged in nests or strands of basaloid cells surrounded by a dense fibrotic stroma. Occasionally, the ducts will form a comma- or teardropshaped tail; however, this also may be observed in desmoplastic trichoepithelioma (DTE).7 Perineural invasion is absent in syringomas. Syringomas exhibit a lateral growth pattern that typically is limited to the upper half of the reticular dermis and spares the underlying subcutis, muscle, and bone. The growth pattern may be discontinuous with proliferations juxtaposed by normal-appearing skin.8 Syringomas usually express progesterone receptors and are known to proliferate at puberty, suggesting that these neoplasms are under hormonal control.9 Although syringomas are benign, various treatment options that may be pursued for cosmetic purposes include radiofrequency, staged excision, laser ablation, and oral isotretinoin.8,10 If only a superficial biopsy is obtained, syringomas may display features of other adnexal neoplasms, including microcystic adnexal carcinoma (MAC), DTE, morpheaform basal cell carcinoma (BCC), and inflammatory linear verrucous epidermal nevus (ILVEN).
Microcystic adnexal carcinoma is a locally aggressive neoplasm first described by Goldstein et al11 in 1982 an indurated, ill-defined plaque or nodule on the face with a predilection for the upper and lower lip. Prior radiation therapy and immunosuppression are risk factors for the development of MAC.12 Histologically, the superficial portion displays small cornifying cysts interspersed with islands of basaloid cells and may mimic a syringoma. However, the deeper portions demonstrate ducts lined by a single layer of cells with a background of hyalinized and sclerotic stroma. The tumor cells may occupy the deep dermis and underlying subcutis, muscle, or bone and demonstrate an infiltrative growth pattern and perineural invasion. Treatment includes Mohs micrographic surgery.
Desmoplastic trichoepitheliomas most commonly present as solitary white to yellowish annular papules or plaques with a central dell located on sun-exposed areas of the face, cheeks, or chin. This benign neoplasm has a bimodal age distribution, primarily affecting females either in childhood or adulthood.13 Histologically, strands and nests of basaloid epithelial cells proliferate in a dense eosinophilic desmoplastic stroma. The basaloid islands are narrow and cordlike with growth parallel to the surface epidermis and do not dive deeply into the deep dermis or subcutis. Ductal differentiation with associated secretions typically is not seen in DTE.1 Calcifications and foreign body granulomatous infiltrates may be present. Merkel cells also are present in this tumor and may be highlighted by immunohistochemistry with cytokeratin 20.14 Rarely, desmoplastic trichoepitheliomas may transform into trichoblastic carcinomas. Treatment may consist of surgical excision or Mohs micrographic surgery.
Morpheaform BCC also is included in the clinical and histopathologic differential diagnosis of infiltrative basaloid neoplasms. It is one of the more aggressive variants of BCC. The use of immunohistochemical staining may aid in differentiating between these sclerosing adnexal neoplasms.15 For example, pleckstrin homologylike domain family A member 1 (PHLDA1) is a stem cell marker that is heavily expressed in DTE as a specific follicular bulge marker but is not present in a morpheaform BCC. This highlights the follicular nature of DTEs at the molecular level. BerEP4 is a monoclonal antibody that serves as an epithelial marker for 2 glycopolypeptides: 34 and 39 kDa. This antibody may demonstrate positivity in morpheaform BCC but does not stain cells of interest in MAC.
Inflammatory linear verrucous epidermal nevus clinically presents with erythematous and warty papules in a linear distribution following the Blaschko lines. The papules often are reported to be intensely pruritic and usually are localized to one extremity.16 Although adultonset forms of ILVEN have been described,17 it most commonly is diagnosed in young children. Histologically, ILVEN consists of psoriasiform epidermal hyperplasia with alternating areas of parakeratosis and orthokeratosis with underlying agranulosis and hypergranulosis, respectively.18 The upper dermis contains a perivascular lymphocytic infiltrate. Treatment with laser therapy and surgical excision has led to both symptomatic and clinical improvement of ILVEN.16
Plaque-type syringomas are a rare variant of syringomas that clinically may mimic other common inflammatory and neoplastic conditions. An adequate biopsy is imperative to differentiate between adnexal neoplasms, as a small superficial biopsy of a syringoma may demonstrate features observed in other malignant or locally aggressive neoplasms. In our patient, the small ducts lined by cuboidal epithelium with no cellular atypia and no deep dermal growth or perineural invasion allowed for the diagnosis of PTS. Therapeutic options were reviewed with our patient, including oral isotretinoin, laser therapy, and staged excision. Ultimately, our patient elected not to pursue treatment, and she is being monitored clinically for any changes in appearance or symptoms.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma [published online November 12, 2007]. J Cutan Pathol. 2008;35:570-574.
- Furue M, Hori Y, Nakabayashi Y. Clear-cell syringoma. association with diabetes mellitus. Am J Dermatopathol. 1984;6:131-138.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Okuda H, Tei N, Shimizu K, et al. Chondroid syringoma of the scrotum. Int J Urol. 2008;15:944-945.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaquetype syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Mainitz M, Schmidt JB, Gebhart W. Response of multiple syringomas to isotretinoin. Acta Derm Venereol. 1986;66:51-55.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Pujol RM, LeBoit PE, Su WP. Microcystic adnexal carcinoma with extensive sebaceous differentiation. Am J Dermatopathol. 1997;19:358-362.
- Rahman J, Tahir M, Arekemase H, et al. Desmoplastic trichoepithelioma: histopathologic and immunohistochemical criteria for differentiation of a rare benign hair follicle tumor from other cutaneous adnexal tumors. Cureus. 2020;12:E9703.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Gianfaldoni S, Tchernev G, Gianfaldoni R, et al. A case of “inflammatory linear verrucous epidermal nevus” (ILVEN) treated with CO2 laser ablation. Open Access Maced J Med Sci. 2017;5:454-457.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus [published online October 27, 1999]. J Dermatol. 1999;26:599-602.
- Patterson JW, Hosler GA, Prenshaw KL, et al. The psoriasiform reaction pattern. In: Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:99-120.
The Diagnosis: Plaque-type Syringoma
A biopsy demonstrated multiple basaloid islands of tumor cells in the reticular dermis with ductal differentiation, some with a commalike tail. The ducts were lined by 2 to 3 layers of small uniform cuboidal cells without atypia and contained inspissated secretions within the lumina of scattered ducts. There was an associated fibrotic collagenous stroma. There was no evidence of perineural invasion and no deep dermal or subcutaneous extension (Figure 1). Additional cytokeratin immunohistochemical staining highlighted the adnexal proliferation (Figure 2). A diagnosis of plaque-type syringoma (PTS) was made.

Syringomas are benign dermal sweat gland tumors that typically present as flesh-colored papules on the cheeks or periorbital area of young females. Plaque-type tumors as well as papulonodular, eruptive, disseminated, urticaria pigmentosa–like, lichen planus–like, or milialike syringomas also have been reported. Syringomas may be associated with certain medical conditions such as Down syndrome, Nicolau-Balus syndrome, and both scarring and nonscarring alopecias.1 The clear cell variant of syringoma often is associated with diabetes mellitus.2 Kikuchi et al3 first described PTS in 1979. Plaque-type syringomas rarely are reported in the literature, and sites of involvement include the head and neck region, upper lip, chest, upper extremities, vulva, penis, and scrotum.4-6

Histologically, syringomatous lesions are composed of multiple small ducts lined by 2 to 3 layers of cuboidal epithelium. The ducts may be arranged in nests or strands of basaloid cells surrounded by a dense fibrotic stroma. Occasionally, the ducts will form a comma- or teardropshaped tail; however, this also may be observed in desmoplastic trichoepithelioma (DTE).7 Perineural invasion is absent in syringomas. Syringomas exhibit a lateral growth pattern that typically is limited to the upper half of the reticular dermis and spares the underlying subcutis, muscle, and bone. The growth pattern may be discontinuous with proliferations juxtaposed by normal-appearing skin.8 Syringomas usually express progesterone receptors and are known to proliferate at puberty, suggesting that these neoplasms are under hormonal control.9 Although syringomas are benign, various treatment options that may be pursued for cosmetic purposes include radiofrequency, staged excision, laser ablation, and oral isotretinoin.8,10 If only a superficial biopsy is obtained, syringomas may display features of other adnexal neoplasms, including microcystic adnexal carcinoma (MAC), DTE, morpheaform basal cell carcinoma (BCC), and inflammatory linear verrucous epidermal nevus (ILVEN).
Microcystic adnexal carcinoma is a locally aggressive neoplasm first described by Goldstein et al11 in 1982 an indurated, ill-defined plaque or nodule on the face with a predilection for the upper and lower lip. Prior radiation therapy and immunosuppression are risk factors for the development of MAC.12 Histologically, the superficial portion displays small cornifying cysts interspersed with islands of basaloid cells and may mimic a syringoma. However, the deeper portions demonstrate ducts lined by a single layer of cells with a background of hyalinized and sclerotic stroma. The tumor cells may occupy the deep dermis and underlying subcutis, muscle, or bone and demonstrate an infiltrative growth pattern and perineural invasion. Treatment includes Mohs micrographic surgery.
Desmoplastic trichoepitheliomas most commonly present as solitary white to yellowish annular papules or plaques with a central dell located on sun-exposed areas of the face, cheeks, or chin. This benign neoplasm has a bimodal age distribution, primarily affecting females either in childhood or adulthood.13 Histologically, strands and nests of basaloid epithelial cells proliferate in a dense eosinophilic desmoplastic stroma. The basaloid islands are narrow and cordlike with growth parallel to the surface epidermis and do not dive deeply into the deep dermis or subcutis. Ductal differentiation with associated secretions typically is not seen in DTE.1 Calcifications and foreign body granulomatous infiltrates may be present. Merkel cells also are present in this tumor and may be highlighted by immunohistochemistry with cytokeratin 20.14 Rarely, desmoplastic trichoepitheliomas may transform into trichoblastic carcinomas. Treatment may consist of surgical excision or Mohs micrographic surgery.
Morpheaform BCC also is included in the clinical and histopathologic differential diagnosis of infiltrative basaloid neoplasms. It is one of the more aggressive variants of BCC. The use of immunohistochemical staining may aid in differentiating between these sclerosing adnexal neoplasms.15 For example, pleckstrin homologylike domain family A member 1 (PHLDA1) is a stem cell marker that is heavily expressed in DTE as a specific follicular bulge marker but is not present in a morpheaform BCC. This highlights the follicular nature of DTEs at the molecular level. BerEP4 is a monoclonal antibody that serves as an epithelial marker for 2 glycopolypeptides: 34 and 39 kDa. This antibody may demonstrate positivity in morpheaform BCC but does not stain cells of interest in MAC.
Inflammatory linear verrucous epidermal nevus clinically presents with erythematous and warty papules in a linear distribution following the Blaschko lines. The papules often are reported to be intensely pruritic and usually are localized to one extremity.16 Although adultonset forms of ILVEN have been described,17 it most commonly is diagnosed in young children. Histologically, ILVEN consists of psoriasiform epidermal hyperplasia with alternating areas of parakeratosis and orthokeratosis with underlying agranulosis and hypergranulosis, respectively.18 The upper dermis contains a perivascular lymphocytic infiltrate. Treatment with laser therapy and surgical excision has led to both symptomatic and clinical improvement of ILVEN.16
Plaque-type syringomas are a rare variant of syringomas that clinically may mimic other common inflammatory and neoplastic conditions. An adequate biopsy is imperative to differentiate between adnexal neoplasms, as a small superficial biopsy of a syringoma may demonstrate features observed in other malignant or locally aggressive neoplasms. In our patient, the small ducts lined by cuboidal epithelium with no cellular atypia and no deep dermal growth or perineural invasion allowed for the diagnosis of PTS. Therapeutic options were reviewed with our patient, including oral isotretinoin, laser therapy, and staged excision. Ultimately, our patient elected not to pursue treatment, and she is being monitored clinically for any changes in appearance or symptoms.
The Diagnosis: Plaque-type Syringoma
A biopsy demonstrated multiple basaloid islands of tumor cells in the reticular dermis with ductal differentiation, some with a commalike tail. The ducts were lined by 2 to 3 layers of small uniform cuboidal cells without atypia and contained inspissated secretions within the lumina of scattered ducts. There was an associated fibrotic collagenous stroma. There was no evidence of perineural invasion and no deep dermal or subcutaneous extension (Figure 1). Additional cytokeratin immunohistochemical staining highlighted the adnexal proliferation (Figure 2). A diagnosis of plaque-type syringoma (PTS) was made.

Syringomas are benign dermal sweat gland tumors that typically present as flesh-colored papules on the cheeks or periorbital area of young females. Plaque-type tumors as well as papulonodular, eruptive, disseminated, urticaria pigmentosa–like, lichen planus–like, or milialike syringomas also have been reported. Syringomas may be associated with certain medical conditions such as Down syndrome, Nicolau-Balus syndrome, and both scarring and nonscarring alopecias.1 The clear cell variant of syringoma often is associated with diabetes mellitus.2 Kikuchi et al3 first described PTS in 1979. Plaque-type syringomas rarely are reported in the literature, and sites of involvement include the head and neck region, upper lip, chest, upper extremities, vulva, penis, and scrotum.4-6

Histologically, syringomatous lesions are composed of multiple small ducts lined by 2 to 3 layers of cuboidal epithelium. The ducts may be arranged in nests or strands of basaloid cells surrounded by a dense fibrotic stroma. Occasionally, the ducts will form a comma- or teardropshaped tail; however, this also may be observed in desmoplastic trichoepithelioma (DTE).7 Perineural invasion is absent in syringomas. Syringomas exhibit a lateral growth pattern that typically is limited to the upper half of the reticular dermis and spares the underlying subcutis, muscle, and bone. The growth pattern may be discontinuous with proliferations juxtaposed by normal-appearing skin.8 Syringomas usually express progesterone receptors and are known to proliferate at puberty, suggesting that these neoplasms are under hormonal control.9 Although syringomas are benign, various treatment options that may be pursued for cosmetic purposes include radiofrequency, staged excision, laser ablation, and oral isotretinoin.8,10 If only a superficial biopsy is obtained, syringomas may display features of other adnexal neoplasms, including microcystic adnexal carcinoma (MAC), DTE, morpheaform basal cell carcinoma (BCC), and inflammatory linear verrucous epidermal nevus (ILVEN).
Microcystic adnexal carcinoma is a locally aggressive neoplasm first described by Goldstein et al11 in 1982 an indurated, ill-defined plaque or nodule on the face with a predilection for the upper and lower lip. Prior radiation therapy and immunosuppression are risk factors for the development of MAC.12 Histologically, the superficial portion displays small cornifying cysts interspersed with islands of basaloid cells and may mimic a syringoma. However, the deeper portions demonstrate ducts lined by a single layer of cells with a background of hyalinized and sclerotic stroma. The tumor cells may occupy the deep dermis and underlying subcutis, muscle, or bone and demonstrate an infiltrative growth pattern and perineural invasion. Treatment includes Mohs micrographic surgery.
Desmoplastic trichoepitheliomas most commonly present as solitary white to yellowish annular papules or plaques with a central dell located on sun-exposed areas of the face, cheeks, or chin. This benign neoplasm has a bimodal age distribution, primarily affecting females either in childhood or adulthood.13 Histologically, strands and nests of basaloid epithelial cells proliferate in a dense eosinophilic desmoplastic stroma. The basaloid islands are narrow and cordlike with growth parallel to the surface epidermis and do not dive deeply into the deep dermis or subcutis. Ductal differentiation with associated secretions typically is not seen in DTE.1 Calcifications and foreign body granulomatous infiltrates may be present. Merkel cells also are present in this tumor and may be highlighted by immunohistochemistry with cytokeratin 20.14 Rarely, desmoplastic trichoepitheliomas may transform into trichoblastic carcinomas. Treatment may consist of surgical excision or Mohs micrographic surgery.
Morpheaform BCC also is included in the clinical and histopathologic differential diagnosis of infiltrative basaloid neoplasms. It is one of the more aggressive variants of BCC. The use of immunohistochemical staining may aid in differentiating between these sclerosing adnexal neoplasms.15 For example, pleckstrin homologylike domain family A member 1 (PHLDA1) is a stem cell marker that is heavily expressed in DTE as a specific follicular bulge marker but is not present in a morpheaform BCC. This highlights the follicular nature of DTEs at the molecular level. BerEP4 is a monoclonal antibody that serves as an epithelial marker for 2 glycopolypeptides: 34 and 39 kDa. This antibody may demonstrate positivity in morpheaform BCC but does not stain cells of interest in MAC.
Inflammatory linear verrucous epidermal nevus clinically presents with erythematous and warty papules in a linear distribution following the Blaschko lines. The papules often are reported to be intensely pruritic and usually are localized to one extremity.16 Although adultonset forms of ILVEN have been described,17 it most commonly is diagnosed in young children. Histologically, ILVEN consists of psoriasiform epidermal hyperplasia with alternating areas of parakeratosis and orthokeratosis with underlying agranulosis and hypergranulosis, respectively.18 The upper dermis contains a perivascular lymphocytic infiltrate. Treatment with laser therapy and surgical excision has led to both symptomatic and clinical improvement of ILVEN.16
Plaque-type syringomas are a rare variant of syringomas that clinically may mimic other common inflammatory and neoplastic conditions. An adequate biopsy is imperative to differentiate between adnexal neoplasms, as a small superficial biopsy of a syringoma may demonstrate features observed in other malignant or locally aggressive neoplasms. In our patient, the small ducts lined by cuboidal epithelium with no cellular atypia and no deep dermal growth or perineural invasion allowed for the diagnosis of PTS. Therapeutic options were reviewed with our patient, including oral isotretinoin, laser therapy, and staged excision. Ultimately, our patient elected not to pursue treatment, and she is being monitored clinically for any changes in appearance or symptoms.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma [published online November 12, 2007]. J Cutan Pathol. 2008;35:570-574.
- Furue M, Hori Y, Nakabayashi Y. Clear-cell syringoma. association with diabetes mellitus. Am J Dermatopathol. 1984;6:131-138.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Okuda H, Tei N, Shimizu K, et al. Chondroid syringoma of the scrotum. Int J Urol. 2008;15:944-945.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaquetype syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Mainitz M, Schmidt JB, Gebhart W. Response of multiple syringomas to isotretinoin. Acta Derm Venereol. 1986;66:51-55.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Pujol RM, LeBoit PE, Su WP. Microcystic adnexal carcinoma with extensive sebaceous differentiation. Am J Dermatopathol. 1997;19:358-362.
- Rahman J, Tahir M, Arekemase H, et al. Desmoplastic trichoepithelioma: histopathologic and immunohistochemical criteria for differentiation of a rare benign hair follicle tumor from other cutaneous adnexal tumors. Cureus. 2020;12:E9703.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Gianfaldoni S, Tchernev G, Gianfaldoni R, et al. A case of “inflammatory linear verrucous epidermal nevus” (ILVEN) treated with CO2 laser ablation. Open Access Maced J Med Sci. 2017;5:454-457.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus [published online October 27, 1999]. J Dermatol. 1999;26:599-602.
- Patterson JW, Hosler GA, Prenshaw KL, et al. The psoriasiform reaction pattern. In: Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:99-120.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma [published online November 12, 2007]. J Cutan Pathol. 2008;35:570-574.
- Furue M, Hori Y, Nakabayashi Y. Clear-cell syringoma. association with diabetes mellitus. Am J Dermatopathol. 1984;6:131-138.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Okuda H, Tei N, Shimizu K, et al. Chondroid syringoma of the scrotum. Int J Urol. 2008;15:944-945.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaquetype syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Mainitz M, Schmidt JB, Gebhart W. Response of multiple syringomas to isotretinoin. Acta Derm Venereol. 1986;66:51-55.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Pujol RM, LeBoit PE, Su WP. Microcystic adnexal carcinoma with extensive sebaceous differentiation. Am J Dermatopathol. 1997;19:358-362.
- Rahman J, Tahir M, Arekemase H, et al. Desmoplastic trichoepithelioma: histopathologic and immunohistochemical criteria for differentiation of a rare benign hair follicle tumor from other cutaneous adnexal tumors. Cureus. 2020;12:E9703.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Gianfaldoni S, Tchernev G, Gianfaldoni R, et al. A case of “inflammatory linear verrucous epidermal nevus” (ILVEN) treated with CO2 laser ablation. Open Access Maced J Med Sci. 2017;5:454-457.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus [published online October 27, 1999]. J Dermatol. 1999;26:599-602.
- Patterson JW, Hosler GA, Prenshaw KL, et al. The psoriasiform reaction pattern. In: Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:99-120.
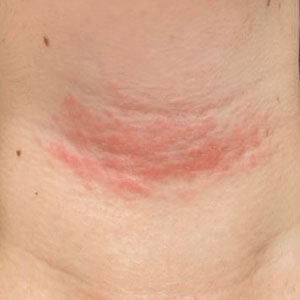
A 17-year-old adolescent girl presented with a solitary, 8-cm, pink plaque on the anterior aspect of the neck of 5 years’ duration. No similar skin findings were present elsewhere on the body. The rash was not painful or pruritic, and she denied prior trauma to the site. The patient previously had tried a salicylic acid bodywash as well as mupirocin cream 2% and mometasone ointment with no improvement. Her medical history was unremarkable, and she had no known allergies. There was no family history of a similar rash. Physical examination revealed no palpable subcutaneous lumps or masses and no lymphadenopathy of the head or neck. An incisional biopsy was performed.
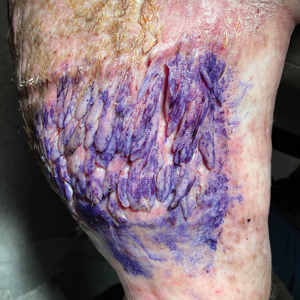
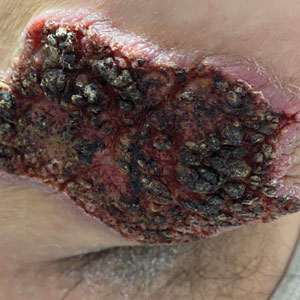
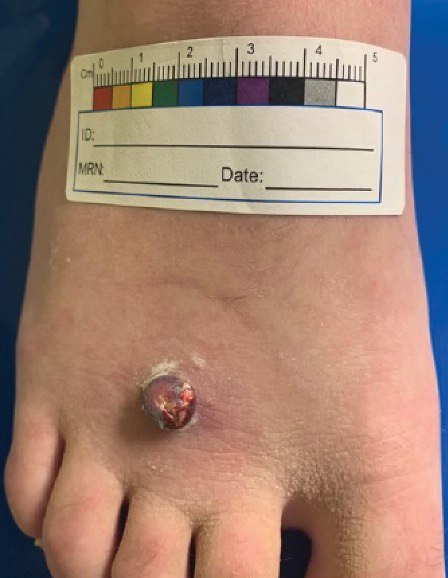
Multiple Fingerlike Projections on the Leg
The Diagnosis: Elephantiasis Nostras Verrucosa
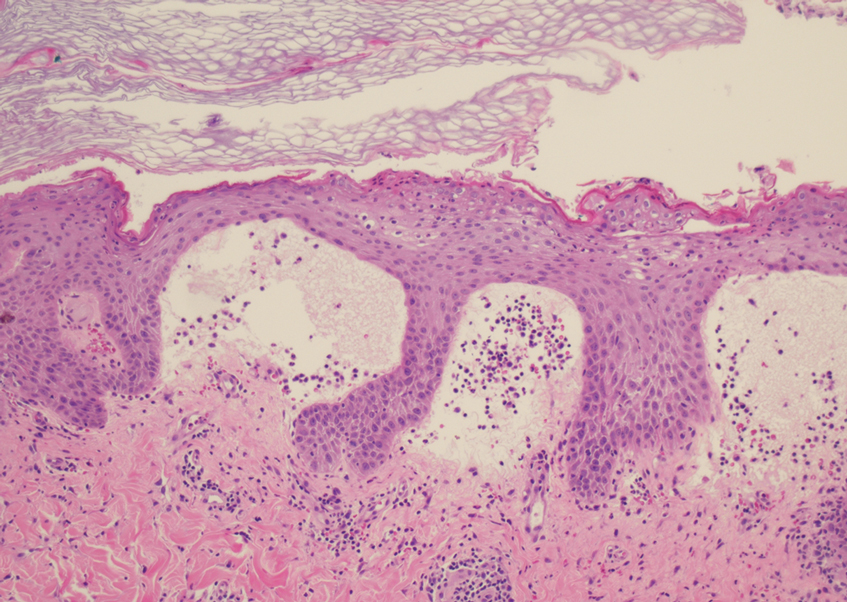
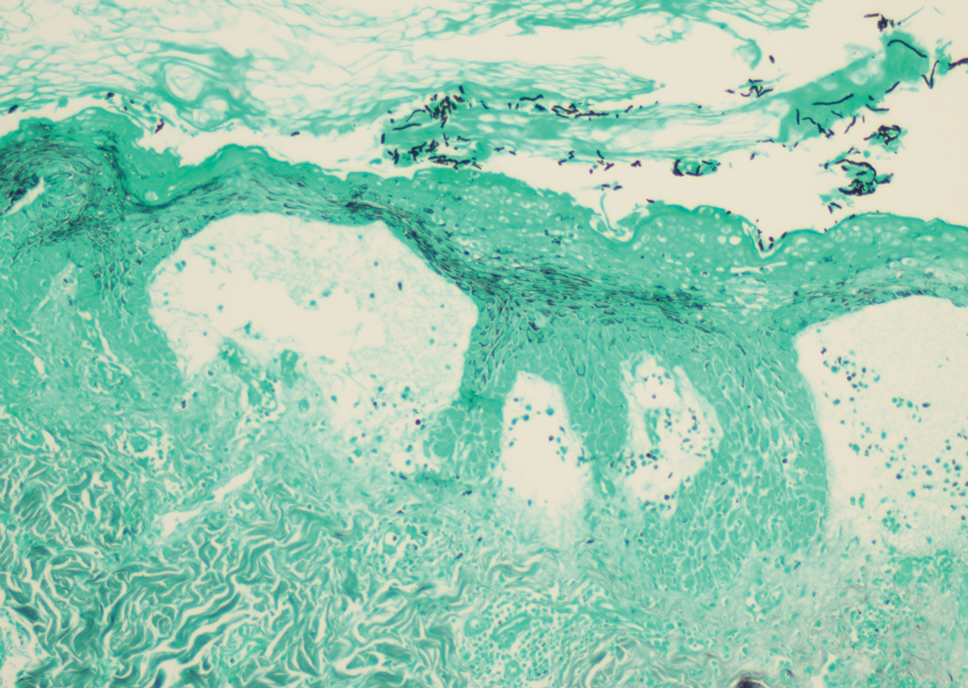
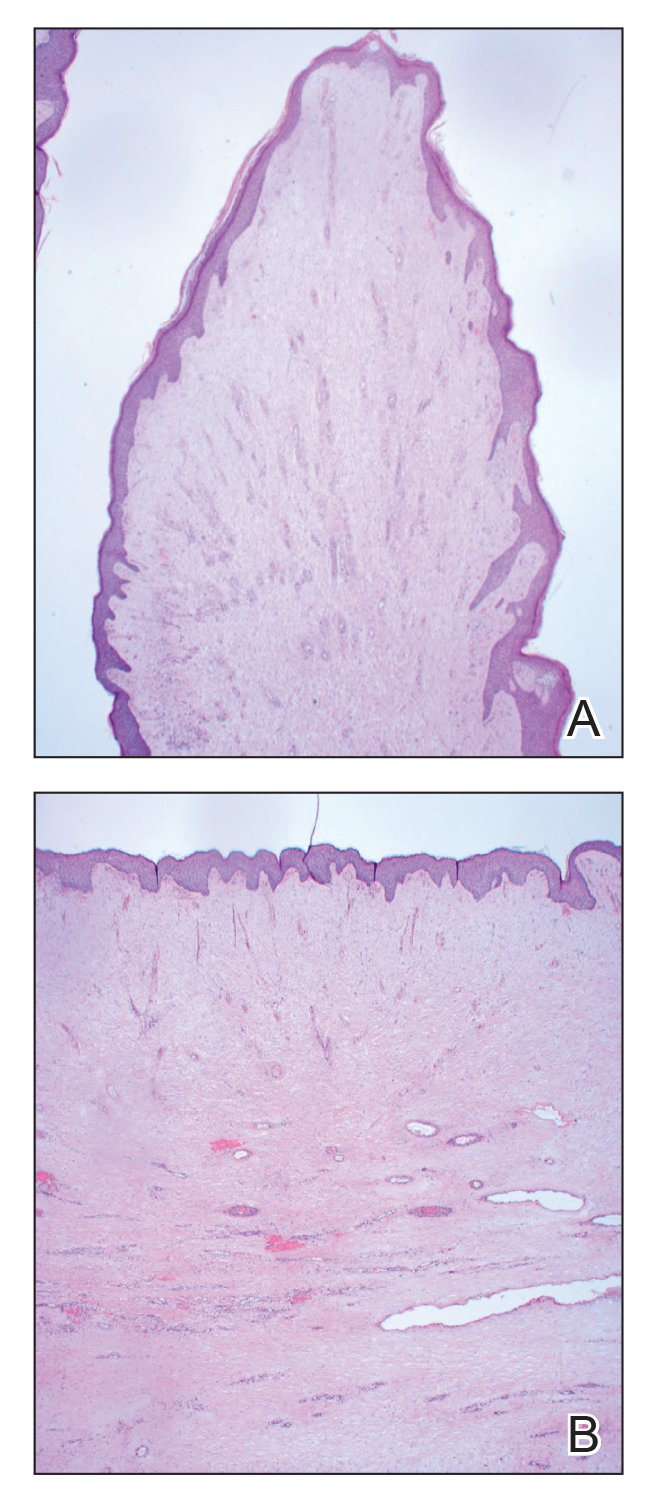
Histopathology revealed a benign fibroepithelial polyp demonstrating areas of hyperkeratosis, acanthosis, and focal papillomatosis (Figure, A). Increased superficial vessels with dilated lymphatics, stellate fibroblasts, edematous stroma, and plasmolymphocytosis also were noted (Figure, B). Clinical and histopathological findings led to a diagnosis of lymphedema papules in the setting of elephantiasis nostra verrucosa (ENV).
Elephantiasis nostras verrucosa is a complication of long-standing nonfilarial obstruction of lymphatic drainage leading to grotesque enlargement of the affected areas. Common cutaneous manifestations of ENV include nonpitting edema, dermal fibrosis, and extensive hyperkeratosis with verrucous and papillomatous lesions.1 In the beginning stages of ENV, the skin has a cobblestonelike appearance. As the disease progresses, the verrucous lesions continue to enlarge, giving the affected area a mossy appearance. Although less common, groupings of large papillomas similar to our patient’s presentation also can form.2 Ulcer formation is more likely to occur in advanced disease states, increasing the risk for bacterial and fungal colonization. Elephantiasis nostras verrucosa classically affects the legs; however, this condition can develop in any area with chronic lymphedema. Cases of ENV involving the arms, abdomen, scrotum, and ear have been documented.3-5
The pathogenesis of ENV involves the proliferation of fibroblasts and fibrosis secondary to lymphostasis and inflammation.6 When interstitial fluid builds up in the affected region, the protein-rich fluid is believed to trigger fibrogenesis and increase macrophage, keratinocyte, and adipocyte activity.7 Because of this inflammatory process, dilation and fibrosis of the lymphatic channels develop. Lymphatic obstruction can have several etiologies, most notably infection and malignancy. Staphylococcal lymphangitis and erysipelas create fibrosis of the lymphatic system and are the main infectious causes of ENV.6 Large tumors or lymphomas are insidious causes of lymphatic obstruction and should be ruled out when investigating for ENV. Other risk factors include obesity, chronic venous insufficiency, surgery, trauma, radiation, and uncontrolled congestive heart failure.1,6,8
An ENV diagnosis is clinicopathologic, involving a comprehensive metabolic panel and complete blood cell count with differential. A biopsy is needed for pathologic confirmation and to rule out malignancy. Histologically, ENV is characterized by pseudoepitheliomatous hyperplasia, dermal fibrosis, hyperkeratosis of the epidermis, and dilated lymphatic vessels.6,8 Additional studies for diagnosis include wound and lymph node culture, Wood lamp examination, and lymphoscintigraphy.
Given the chronic and progressive nature of the disease, ENV is difficult to treat. There currently is no standard of treatment, but the mainstay of management involves reducing peripheral edema. Lifestyle changes including weight loss, extremity elevation, and increased ambulation are helpful first-line therapies.3 Compression of the affected extremity using stockings or intermittent pneumatic compression devices has proven to be beneficial with long-term use.7 Patients should be followed for wound care to prevent the infection of ulcers.2 Pharmacologic treatments include systemic retinoids, which have been shown to reduce the appearance of hyperkeratosis, verrucous lesions, and papillomatous nodules.6 Prophylactic antibiotics are reserved for advanced stages of disease or in patients with recurrent infections.2,7 In severe cases of ENV that are unresponsive to medical management, surgical intervention such as lymphatic anastomosis and debulking may be considered.9,10
Other diagnoses to consider for ENV include pretibial myxedema, lymphatic filariasis, Stewart-Treves syndrome, and papillomatosis cutis carcinoides. Pretibial myxedema is an uncommon dermatologic manifestation of Graves disease. It is a local autoimmune reaction in the cutaneous tissue characterized by hyperpigmentation, nonpitting edema, and nodules on the anterior leg. Histopathology shows increased hyaluronic acid and chondroitin as well as compression of dermal lymphatics.11
Filariasis is a parasitic infection caused by Wuchereria bancrofti, Brugia malayi or Brugia timori, and Onchocerca volvulus.6 This condition presents with elephantiasis of the affected extremities but should be considered in areas endemic for filarial parasites such as tropical and subtropical countries.12 Eosinophilia and identification of microfilaria in a peripheral blood smear would indicate parasitic infection. Stewart-Treves syndrome is a rare angiosarcoma that arises in areas of chronic lymphedema. This condition classically is seen on the upper extremities following a mastectomy with lymphadenectomy, lymph node irradiation, or both.
Stewart-Treves syndrome presents with coalescing purpuric macules and nodules that eventually coalesce into cutaneous masses. Histopathology reveals proliferating vascular channels that split apart dermal collagen with hyperchromatism and pleomorphism in the tumor endothelial cells that line these channels.13
Papillomatosis cutis carcinoides is a low-grade squamous cell carcinoma that occurs secondary to human papillomavirus commonly affecting the mouth, anogenital area, and the plantar surfaces of the feet. It presents with exophytic growths and ulcerated tumors that are unilateral and asymmetrical. The presence of blunt-shaped tumor projections extending deep into the dermis to form sinuses and keratin-filled cysts is characteristic of papillomatosis cutis carcinoides.14
- Dean SM, Zirwas MJ, Horst AV. Elephantiasis nostras verrucosa: an institutional analysis of 21 cases. J Am Acad Dermatol. 2011;64: 1104-1110. doi:10.1016/j.jaad.2010.04.047
- Fife CE, Farrow W, Hebert AA, et al. Skin and wound care in lymphedema patients: a taxonomy, primer, and literature review. Adv Skin Wound Care. 2017;30:305-318. doi:10.1097/01.ASW.0000520501.23702.82
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004; 3:446-448.
- Nakai K, Taoka R, Sugimoto M, et al. Genital elephantiasis possibly caused by chronic inguinal eczema with streptococcal infection. J Dermatol. 2019;46:E196-E198. doi:10.1111/1346-8138.14746
- Carlson JA, Mazza J, Kircher K, et al. Otophyma: a case report and review of the literature of lymphedema (elephantiasis) of the ear. Am J Dermatopathol. 2008;30:67-72. doi:10.1097/DAD.0b013e31815cd937
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146. doi:10.2165/00128071-200809030-00001
- Yoho RM, Budny AM, Pea AS. Elephantiasis nostras verrucosa. J Am Podiatr Med Assoc. 2006;96:442-444. doi:10.7547/0960442
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56:901-920. doi:10.1016/j.jaad.2006.12.004
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941. doi:10.1111/j.1524-4725.2004.30267.x
- Tiwari A, Cheng KS, Button M, et al. Differential diagnosis, investigation, and current treatment of lower limb lymphedema. Arch Surg. 2003;138:152-161. doi:10.1001/archsurg.138.2.152
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309. doi:10.2165 /00128071-200506050-00003
- Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J. 2007;6:2. doi:10.1186/1475-2883-6-2
- Bernia E, Rios-Viñuela E, Requena C. Stewart-Treves syndrome. JAMA Dermatol. 2021;157:721. doi:10.1001/jamadermatol.2021.0341
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-24. doi:10.1016/0190-9622(95)90177-9
The Diagnosis: Elephantiasis Nostras Verrucosa
Histopathology revealed a benign fibroepithelial polyp demonstrating areas of hyperkeratosis, acanthosis, and focal papillomatosis (Figure, A). Increased superficial vessels with dilated lymphatics, stellate fibroblasts, edematous stroma, and plasmolymphocytosis also were noted (Figure, B). Clinical and histopathological findings led to a diagnosis of lymphedema papules in the setting of elephantiasis nostra verrucosa (ENV).

Elephantiasis nostras verrucosa is a complication of long-standing nonfilarial obstruction of lymphatic drainage leading to grotesque enlargement of the affected areas. Common cutaneous manifestations of ENV include nonpitting edema, dermal fibrosis, and extensive hyperkeratosis with verrucous and papillomatous lesions.1 In the beginning stages of ENV, the skin has a cobblestonelike appearance. As the disease progresses, the verrucous lesions continue to enlarge, giving the affected area a mossy appearance. Although less common, groupings of large papillomas similar to our patient’s presentation also can form.2 Ulcer formation is more likely to occur in advanced disease states, increasing the risk for bacterial and fungal colonization. Elephantiasis nostras verrucosa classically affects the legs; however, this condition can develop in any area with chronic lymphedema. Cases of ENV involving the arms, abdomen, scrotum, and ear have been documented.3-5
The pathogenesis of ENV involves the proliferation of fibroblasts and fibrosis secondary to lymphostasis and inflammation.6 When interstitial fluid builds up in the affected region, the protein-rich fluid is believed to trigger fibrogenesis and increase macrophage, keratinocyte, and adipocyte activity.7 Because of this inflammatory process, dilation and fibrosis of the lymphatic channels develop. Lymphatic obstruction can have several etiologies, most notably infection and malignancy. Staphylococcal lymphangitis and erysipelas create fibrosis of the lymphatic system and are the main infectious causes of ENV.6 Large tumors or lymphomas are insidious causes of lymphatic obstruction and should be ruled out when investigating for ENV. Other risk factors include obesity, chronic venous insufficiency, surgery, trauma, radiation, and uncontrolled congestive heart failure.1,6,8
An ENV diagnosis is clinicopathologic, involving a comprehensive metabolic panel and complete blood cell count with differential. A biopsy is needed for pathologic confirmation and to rule out malignancy. Histologically, ENV is characterized by pseudoepitheliomatous hyperplasia, dermal fibrosis, hyperkeratosis of the epidermis, and dilated lymphatic vessels.6,8 Additional studies for diagnosis include wound and lymph node culture, Wood lamp examination, and lymphoscintigraphy.
Given the chronic and progressive nature of the disease, ENV is difficult to treat. There currently is no standard of treatment, but the mainstay of management involves reducing peripheral edema. Lifestyle changes including weight loss, extremity elevation, and increased ambulation are helpful first-line therapies.3 Compression of the affected extremity using stockings or intermittent pneumatic compression devices has proven to be beneficial with long-term use.7 Patients should be followed for wound care to prevent the infection of ulcers.2 Pharmacologic treatments include systemic retinoids, which have been shown to reduce the appearance of hyperkeratosis, verrucous lesions, and papillomatous nodules.6 Prophylactic antibiotics are reserved for advanced stages of disease or in patients with recurrent infections.2,7 In severe cases of ENV that are unresponsive to medical management, surgical intervention such as lymphatic anastomosis and debulking may be considered.9,10
Other diagnoses to consider for ENV include pretibial myxedema, lymphatic filariasis, Stewart-Treves syndrome, and papillomatosis cutis carcinoides. Pretibial myxedema is an uncommon dermatologic manifestation of Graves disease. It is a local autoimmune reaction in the cutaneous tissue characterized by hyperpigmentation, nonpitting edema, and nodules on the anterior leg. Histopathology shows increased hyaluronic acid and chondroitin as well as compression of dermal lymphatics.11
Filariasis is a parasitic infection caused by Wuchereria bancrofti, Brugia malayi or Brugia timori, and Onchocerca volvulus.6 This condition presents with elephantiasis of the affected extremities but should be considered in areas endemic for filarial parasites such as tropical and subtropical countries.12 Eosinophilia and identification of microfilaria in a peripheral blood smear would indicate parasitic infection. Stewart-Treves syndrome is a rare angiosarcoma that arises in areas of chronic lymphedema. This condition classically is seen on the upper extremities following a mastectomy with lymphadenectomy, lymph node irradiation, or both.
Stewart-Treves syndrome presents with coalescing purpuric macules and nodules that eventually coalesce into cutaneous masses. Histopathology reveals proliferating vascular channels that split apart dermal collagen with hyperchromatism and pleomorphism in the tumor endothelial cells that line these channels.13
Papillomatosis cutis carcinoides is a low-grade squamous cell carcinoma that occurs secondary to human papillomavirus commonly affecting the mouth, anogenital area, and the plantar surfaces of the feet. It presents with exophytic growths and ulcerated tumors that are unilateral and asymmetrical. The presence of blunt-shaped tumor projections extending deep into the dermis to form sinuses and keratin-filled cysts is characteristic of papillomatosis cutis carcinoides.14
The Diagnosis: Elephantiasis Nostras Verrucosa
Histopathology revealed a benign fibroepithelial polyp demonstrating areas of hyperkeratosis, acanthosis, and focal papillomatosis (Figure, A). Increased superficial vessels with dilated lymphatics, stellate fibroblasts, edematous stroma, and plasmolymphocytosis also were noted (Figure, B). Clinical and histopathological findings led to a diagnosis of lymphedema papules in the setting of elephantiasis nostra verrucosa (ENV).

Elephantiasis nostras verrucosa is a complication of long-standing nonfilarial obstruction of lymphatic drainage leading to grotesque enlargement of the affected areas. Common cutaneous manifestations of ENV include nonpitting edema, dermal fibrosis, and extensive hyperkeratosis with verrucous and papillomatous lesions.1 In the beginning stages of ENV, the skin has a cobblestonelike appearance. As the disease progresses, the verrucous lesions continue to enlarge, giving the affected area a mossy appearance. Although less common, groupings of large papillomas similar to our patient’s presentation also can form.2 Ulcer formation is more likely to occur in advanced disease states, increasing the risk for bacterial and fungal colonization. Elephantiasis nostras verrucosa classically affects the legs; however, this condition can develop in any area with chronic lymphedema. Cases of ENV involving the arms, abdomen, scrotum, and ear have been documented.3-5
The pathogenesis of ENV involves the proliferation of fibroblasts and fibrosis secondary to lymphostasis and inflammation.6 When interstitial fluid builds up in the affected region, the protein-rich fluid is believed to trigger fibrogenesis and increase macrophage, keratinocyte, and adipocyte activity.7 Because of this inflammatory process, dilation and fibrosis of the lymphatic channels develop. Lymphatic obstruction can have several etiologies, most notably infection and malignancy. Staphylococcal lymphangitis and erysipelas create fibrosis of the lymphatic system and are the main infectious causes of ENV.6 Large tumors or lymphomas are insidious causes of lymphatic obstruction and should be ruled out when investigating for ENV. Other risk factors include obesity, chronic venous insufficiency, surgery, trauma, radiation, and uncontrolled congestive heart failure.1,6,8
An ENV diagnosis is clinicopathologic, involving a comprehensive metabolic panel and complete blood cell count with differential. A biopsy is needed for pathologic confirmation and to rule out malignancy. Histologically, ENV is characterized by pseudoepitheliomatous hyperplasia, dermal fibrosis, hyperkeratosis of the epidermis, and dilated lymphatic vessels.6,8 Additional studies for diagnosis include wound and lymph node culture, Wood lamp examination, and lymphoscintigraphy.
Given the chronic and progressive nature of the disease, ENV is difficult to treat. There currently is no standard of treatment, but the mainstay of management involves reducing peripheral edema. Lifestyle changes including weight loss, extremity elevation, and increased ambulation are helpful first-line therapies.3 Compression of the affected extremity using stockings or intermittent pneumatic compression devices has proven to be beneficial with long-term use.7 Patients should be followed for wound care to prevent the infection of ulcers.2 Pharmacologic treatments include systemic retinoids, which have been shown to reduce the appearance of hyperkeratosis, verrucous lesions, and papillomatous nodules.6 Prophylactic antibiotics are reserved for advanced stages of disease or in patients with recurrent infections.2,7 In severe cases of ENV that are unresponsive to medical management, surgical intervention such as lymphatic anastomosis and debulking may be considered.9,10
Other diagnoses to consider for ENV include pretibial myxedema, lymphatic filariasis, Stewart-Treves syndrome, and papillomatosis cutis carcinoides. Pretibial myxedema is an uncommon dermatologic manifestation of Graves disease. It is a local autoimmune reaction in the cutaneous tissue characterized by hyperpigmentation, nonpitting edema, and nodules on the anterior leg. Histopathology shows increased hyaluronic acid and chondroitin as well as compression of dermal lymphatics.11
Filariasis is a parasitic infection caused by Wuchereria bancrofti, Brugia malayi or Brugia timori, and Onchocerca volvulus.6 This condition presents with elephantiasis of the affected extremities but should be considered in areas endemic for filarial parasites such as tropical and subtropical countries.12 Eosinophilia and identification of microfilaria in a peripheral blood smear would indicate parasitic infection. Stewart-Treves syndrome is a rare angiosarcoma that arises in areas of chronic lymphedema. This condition classically is seen on the upper extremities following a mastectomy with lymphadenectomy, lymph node irradiation, or both.
Stewart-Treves syndrome presents with coalescing purpuric macules and nodules that eventually coalesce into cutaneous masses. Histopathology reveals proliferating vascular channels that split apart dermal collagen with hyperchromatism and pleomorphism in the tumor endothelial cells that line these channels.13
Papillomatosis cutis carcinoides is a low-grade squamous cell carcinoma that occurs secondary to human papillomavirus commonly affecting the mouth, anogenital area, and the plantar surfaces of the feet. It presents with exophytic growths and ulcerated tumors that are unilateral and asymmetrical. The presence of blunt-shaped tumor projections extending deep into the dermis to form sinuses and keratin-filled cysts is characteristic of papillomatosis cutis carcinoides.14
- Dean SM, Zirwas MJ, Horst AV. Elephantiasis nostras verrucosa: an institutional analysis of 21 cases. J Am Acad Dermatol. 2011;64: 1104-1110. doi:10.1016/j.jaad.2010.04.047
- Fife CE, Farrow W, Hebert AA, et al. Skin and wound care in lymphedema patients: a taxonomy, primer, and literature review. Adv Skin Wound Care. 2017;30:305-318. doi:10.1097/01.ASW.0000520501.23702.82
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004; 3:446-448.
- Nakai K, Taoka R, Sugimoto M, et al. Genital elephantiasis possibly caused by chronic inguinal eczema with streptococcal infection. J Dermatol. 2019;46:E196-E198. doi:10.1111/1346-8138.14746
- Carlson JA, Mazza J, Kircher K, et al. Otophyma: a case report and review of the literature of lymphedema (elephantiasis) of the ear. Am J Dermatopathol. 2008;30:67-72. doi:10.1097/DAD.0b013e31815cd937
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146. doi:10.2165/00128071-200809030-00001
- Yoho RM, Budny AM, Pea AS. Elephantiasis nostras verrucosa. J Am Podiatr Med Assoc. 2006;96:442-444. doi:10.7547/0960442
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56:901-920. doi:10.1016/j.jaad.2006.12.004
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941. doi:10.1111/j.1524-4725.2004.30267.x
- Tiwari A, Cheng KS, Button M, et al. Differential diagnosis, investigation, and current treatment of lower limb lymphedema. Arch Surg. 2003;138:152-161. doi:10.1001/archsurg.138.2.152
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309. doi:10.2165 /00128071-200506050-00003
- Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J. 2007;6:2. doi:10.1186/1475-2883-6-2
- Bernia E, Rios-Viñuela E, Requena C. Stewart-Treves syndrome. JAMA Dermatol. 2021;157:721. doi:10.1001/jamadermatol.2021.0341
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-24. doi:10.1016/0190-9622(95)90177-9
- Dean SM, Zirwas MJ, Horst AV. Elephantiasis nostras verrucosa: an institutional analysis of 21 cases. J Am Acad Dermatol. 2011;64: 1104-1110. doi:10.1016/j.jaad.2010.04.047
- Fife CE, Farrow W, Hebert AA, et al. Skin and wound care in lymphedema patients: a taxonomy, primer, and literature review. Adv Skin Wound Care. 2017;30:305-318. doi:10.1097/01.ASW.0000520501.23702.82
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004; 3:446-448.
- Nakai K, Taoka R, Sugimoto M, et al. Genital elephantiasis possibly caused by chronic inguinal eczema with streptococcal infection. J Dermatol. 2019;46:E196-E198. doi:10.1111/1346-8138.14746
- Carlson JA, Mazza J, Kircher K, et al. Otophyma: a case report and review of the literature of lymphedema (elephantiasis) of the ear. Am J Dermatopathol. 2008;30:67-72. doi:10.1097/DAD.0b013e31815cd937
- Sisto K, Khachemoune A. Elephantiasis nostras verrucosa: a review. Am J Clin Dermatol. 2008;9:141-146. doi:10.2165/00128071-200809030-00001
- Yoho RM, Budny AM, Pea AS. Elephantiasis nostras verrucosa. J Am Podiatr Med Assoc. 2006;96:442-444. doi:10.7547/0960442
- Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. J Am Acad Dermatol. 2007;56:901-920. doi:10.1016/j.jaad.2006.12.004
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-941. doi:10.1111/j.1524-4725.2004.30267.x
- Tiwari A, Cheng KS, Button M, et al. Differential diagnosis, investigation, and current treatment of lower limb lymphedema. Arch Surg. 2003;138:152-161. doi:10.1001/archsurg.138.2.152
- Fatourechi V. Pretibial myxedema: pathophysiology and treatment options. Am J Clin Dermatol. 2005;6:295-309. doi:10.2165 /00128071-200506050-00003
- Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: a review of the scientific literature. Filaria J. 2007;6:2. doi:10.1186/1475-2883-6-2
- Bernia E, Rios-Viñuela E, Requena C. Stewart-Treves syndrome. JAMA Dermatol. 2021;157:721. doi:10.1001/jamadermatol.2021.0341
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-24. doi:10.1016/0190-9622(95)90177-9
A 61-year-old man presented with painful skin growths on the right pretibial region of several months’ duration. The patient reported pain due to friction between the lesions and underlying skin, leading to erosions. His medical history was remarkable for morbid obesity (body mass index of 62), chronic venous stasis, and chronic lymphedema. The patient was followed for wound care of venous stasis ulcers. Dermatologic examination revealed multiple 5- to 30-mm, flesh-colored, fingerlike projections on the right tibial region. A biopsy was obtained and submitted for histopathologic analysis.
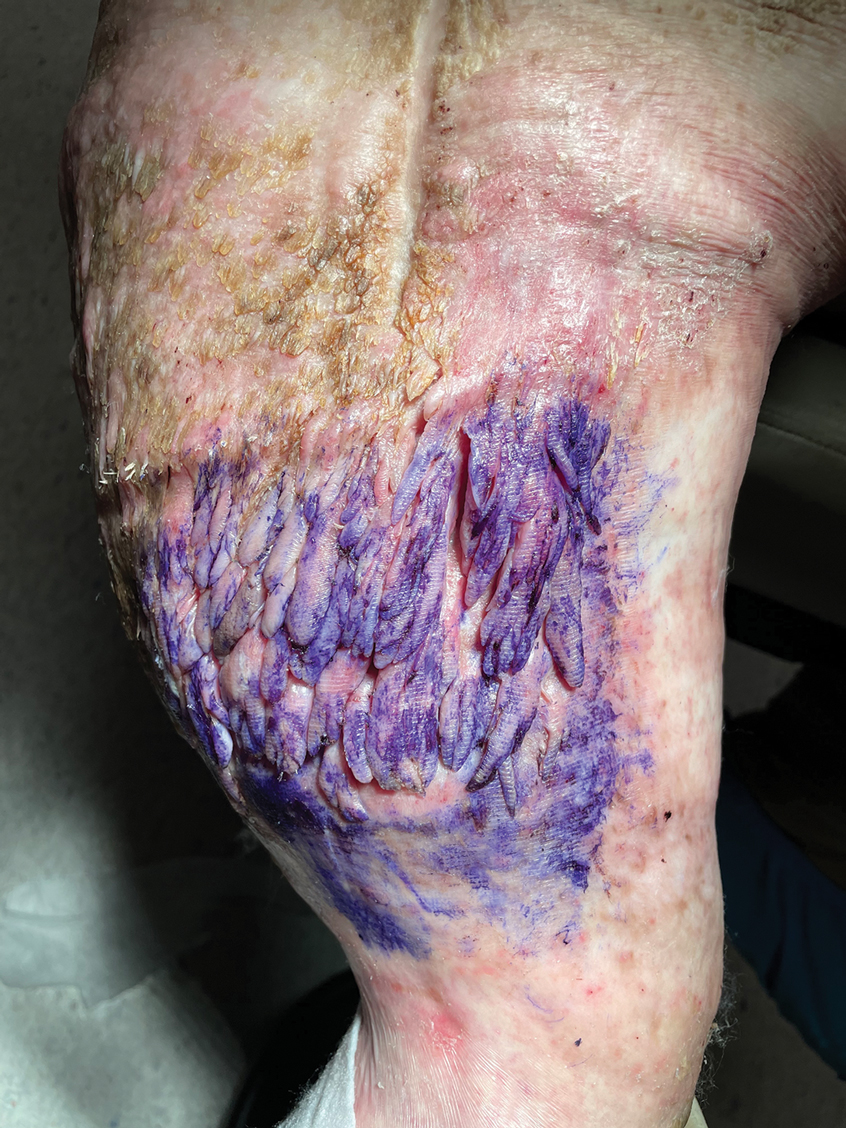
Fungated Eroded Plaque on the Arm
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
The Diagnosis: Cutaneous Blastomycosis
A skin biopsy and fungal cultures confirmed the diagnosis of cutaneous blastomycosis. Grocott- Gomori methenamine-silver staining highlighted fungal organisms with refractile walls and broad-based budding consistent with cutaneous blastomycosis (Figure 1). The histopathologic specimen also demonstrated marked pseudoepitheliomatous hyperplasia (Figure 2A) with neutrophilic microabscesses (Figure 2B). Acid-fast bacillus and Fite staining were negative for bacterial organisms. A fungal culture was positive for Blastomyces dermatitidis. Urine and serum blastomycosis antigen were positive. Although Histoplasma serum antigen also was positive, this likely was from cross-reactivity. Chest radiography was negative for lung involvement, and the patient displayed no neurologic symptoms. He was started on oral itraconazole therapy for the treatment of cutaneous blastomycosis.

Blastomyces dermatitidis, the causative organism of blastomycosis, is endemic to the Ohio and Mississippi River valleys, Great Lakes region, and southeastern United States. It is a thermally dimorphic fungus found in soils that grows as a mold at 25 °C and yeast at 37 °C. Primary infection of the lungs—blastomycosis pneumonia—often is the only clinical manifestation1; however, subsequent hematogenous dissemination to extrapulmonary sites such as the skin, bones, and genitourinary system can occur. Cutaneous blastomycosis, the most common extrapulmonary manifestation, typically follows pulmonary infection. In rare cases, it can occur from direct inoculation.2,3 Skin lesions can occur anywhere but frequently are found on exposed surfaces of the head, neck, and extremities. Lesions classically present as verrucous crusting plaques with draining microabscesses. Violaceous nodules, ulcers, and pustules also may occur.1

Diagnosis involves obtaining a thorough history of possible environmental exposures such as the patient’s geographic area of residence, occupation, and outdoor activities involving soil or decaying wood. Because blastomycosis can remain latent, remote exposures are relevant. Definitive diagnosis of cutaneous blastomycosis involves skin biopsy of the lesion with fungal culture, but the yeast’s distinctive thick wall and broad-based budding seen with periodic acid–Schiff or Grocott-Gomori methenamine-silver staining provides a rapid presumptive diagnosis.3 Pseudoepitheliomatous hyperplasia and microabscesses also are characteristic features.2 Urine antigen testing for a component of the polysaccharide cell wall has a sensitivity of 93% but a lower specificity of 79% due to cross-reactivity with histoplasmosis.4 Treatment consists of itraconazole for mild to moderate blastomycosis or amphotericin B for those with severe disease or central nervous system involvement or those who are immunosuppressed.1
The differential diagnosis for our patient’s lesion included infectious vs neoplastic etiologies. Histoplasma capsulatum, the dimorphic fungus that causes histoplasmosis, also is endemic to the Ohio and Mississippi River valleys. It is found in soil and droppings of some bats and birds such as chickens and pigeons. Similar to blastomycosis, the primary infection site most commonly is the lungs. It subsequently may disseminate to the skin or less commonly via direct inoculation of injured skin. It can present as papules, plaques, ulcers, purpura, or abscesses. Unlike blastomycosis, tissue biopsy of a cutaneous lesion reveals granuloma formation and distinctive oval, narrow-based budding yeast.5 Atypical mycobacteria are another source of infection to consider. For example, cutaneous Mycobacterium kansasii may present as papules and pustules forming verrucous or granulomatous plaques and ulceration. Histopathologic findings distinguishing mycobacterial infection from blastomycosis include granulomas and acid-fast bacilli in histiocytes.6
Noninfectious etiologies in the differential may include cutaneous squamous cell carcinoma or pemphigus vegetans. Squamous cell carcinoma may present with a broad range of clinical features—papules, plaques, or nodules with smooth, scaly, verrucous, or ulcerative secondary features all are possible presentations.7 Fairskinned individuals, such as our patient, would be at a higher risk in sun-damaged skin. Histologically, cutaneous squamous cell carcinoma is defined as an invasion of the dermis by neoplastic squamous epithelial cells in the form of cords, sheets, individual cells, nodules, or cystic structures.7 Pemphigus vegetans is the rarest variant of a group of autoimmune vesiculobullous diseases known as pemphigus. It can be differentiated from the most common variant—pemphigus vulgaris—by the presence of vegetative plaques in intertriginous areas. However, these verrucous vegetations can be misleading and make clinical diagnosis difficult. Histopathologic findings of hyperkeratosis, pseudoepitheliomatous hyperplasia, papillomatosis, and acantholysis with a suprabasal cleft would confirm the diagnosis.8
In summary, cutaneous blastomycosis classically presents as verrucous crusting plaques, as seen in our patient. It is important to conduct a thorough history for environmental exposures, but definitive diagnosis of cutaneous blastomycosis involves skin biopsy with fungal culture. Treatment depends on the severity of disease and organ involvement. Itraconazole would be appropriate for mild to moderate blastomycosis.
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
- Miceli A, Krishnamurthy K. Blastomycosis. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK441987/
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Schwartz IS, Kauffman CA. Blastomycosis. Semin Respir Crit Care Med. 2020;41:31-41. doi:10.1055/s-0039-3400281
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247-264. doi:10.1016/j.idc.2015.10.002
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348.
- Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27:63-73. doi:10.1016/j.det.2008.07.009
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll.2017.06.003
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022. Accessed June 21, 2022. https://www.ncbi.nlm.nih.gov/books/NBK545229/
A 39-year-old man from Ohio presented with a tender, 10×6-cm, fungated, eroded plaque on the right medial upper arm that developed over the last 4 years. He initially noticed a firm lump under the right arm 4 years prior that was diagnosed as possible cellulitis at an outside clinic and treated with trimethoprim-sulfamethoxazole. The lesion then began to erode and became a chronic nonhealing wound. Approximately 1 year prior to the current presentation, the patient recalled unloading a truckload of soil around the same time the wound began to enlarge in diameter and depth. He denied any prior or current respiratory or systemic symptoms including fevers, chills, or weight loss.
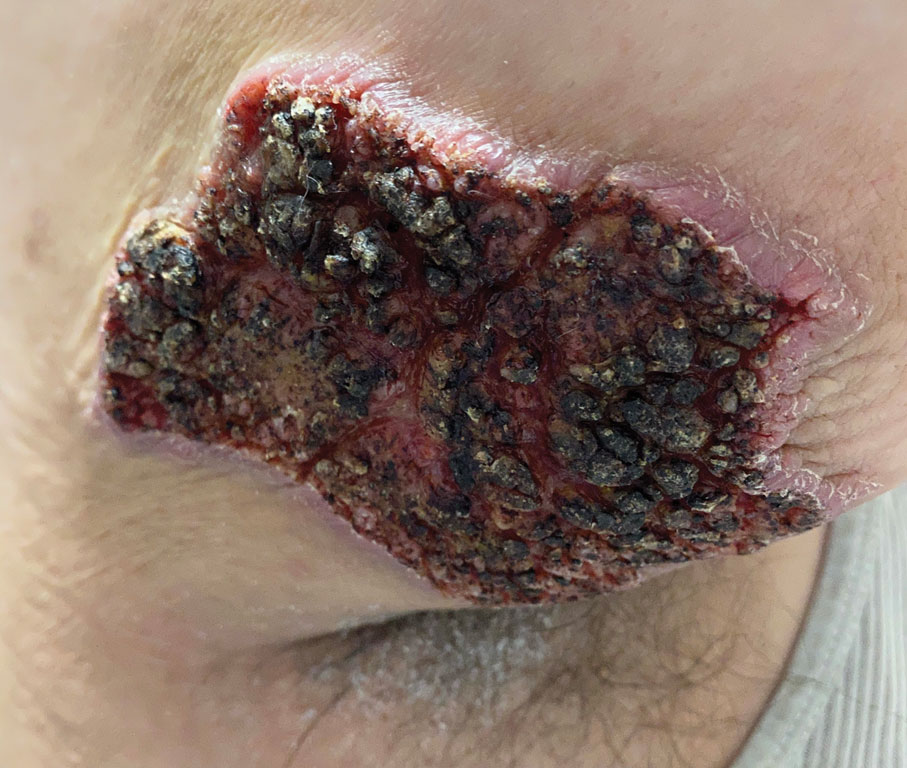
Itchy Vesicular Rash
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
A 38-year-old woman presented with a rash of 5 days’ duration that initially appeared on the wrists after playing with her kitten, with subsequent involvement of the chest, back, abdomen, and upper and lower extremities. Physical examination revealed multiple annular plaques with raised erythematous borders, rare peripheral vesicles, and superficial central scaling. Extreme pruritus accompanied the plaques, both of which developed after playing with her kitten. The patient noted that all lesions on the upper extremities evolved in areas subject to deep puncture while more superficially excoriated areas were unaffected. She denied any other prior skin conditions and had received a 5-day course of azithromycin without improvement prior to presentation; triamcinolone ointment 0.1% had provided only temporary relief. Primary care providers prescribed a short course of oral prednisone; however, she did not start it prior to presentation.
Erythematous Pedunculated Plaque on the Dorsal Aspect of the Foot
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
A 13-year-old adolescent girl presented for evaluation of a lesion on the dorsal aspect of the right foot of 1 week’s duration. She had a history of acne vulgaris and seasonal allergic rhinitis. She previously had noticed a persistent, small, flesh-colored bump of unknown chronicity in the same location, which had been diagnosed as a skin tag at an outside clinic. She denied any prior treatment in this area. Approximately a week prior to presentation, the lesion became painful, larger, and darkened in color before draining yellowish fluid. Due to concern for superinfection, the patient was prescribed cephalexin by her pediatrician. Dermatologic examination revealed a 1-cm, violaceous, pedunculated plaque with hemorrhagic crust on the dorsal aspect of the right foot with surrounding erythema and tenderness.
Rapidly Evolving Papulonodular Eruption in the Axilla
The Diagnosis: Lymphomatoid Papulosis
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.
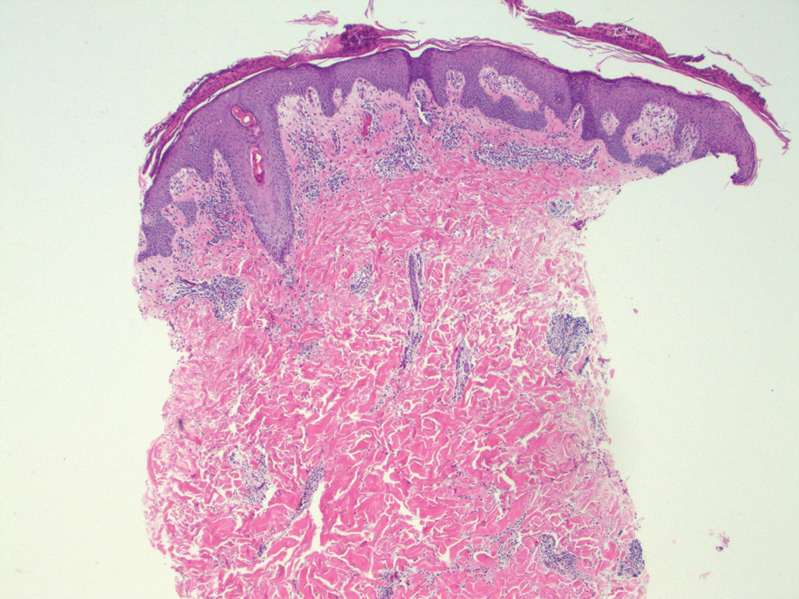
Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.
Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5
Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6
The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
The Diagnosis: Lymphomatoid Papulosis
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.

Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.
Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5
Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6
The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
The Diagnosis: Lymphomatoid Papulosis
At the time of the initial visit, a punch biopsy was performed on the posterior shoulder girdle. Histopathology revealed mild epidermal spongiosis and acanthosis with associated parakeratosis and a dermal lymphocytic infiltrate with extravasated erythrocytes consistent with pityriasis rosea (Figure 1). Two weeks after the biopsy, the patient returned for suture removal and to discuss the biopsy results. The patient reported more evolving lesions despite completing the prescribed course of dicloxacillin. At this time, physical examination revealed the persistence of several reddishbrown papules along with new nodular lesions on the arms and thighs, some with central ulceration and crusting (Figure 2). A second biopsy of a nodular lesion on the right distal forearm was performed at this visit along with a superficial tissue culture, which was negative for bacterial or fungal elements. The biopsy revealed an atypical CD30+ lymphoid proliferation (Figure 3). These cells were strongly PD-L1 positive and also positive for CD3, CD4, and granzyme-B. Ki67 showed a high proliferation rate, and T-cell gene rearrangement studies were positive. Given these histologic findings and the clinical context of rapidly evolving skin lesions from small papules to nodular skin tumors, a diagnosis of lymphomatoid papulosis (LyP) was established.

Because of the notable pathologic discordance between the 2 biopsy specimens, re-evaluation of the initial specimen was requested. The initial biopsy was subsequently found to be CD30+ with an identical peak on gene rearrangement studies as the second biopsy, further validating the diagnosis of LyP (Figure 4). Our patient was offered low-dose methotrexate therapy but declined the treatment plan, as the skin lesions had begun to resolve.
Lymphomatoid papulosis is a chronic CD30+ lymphoproliferative disorder with a characteristic recurrent and self-remitting disease course.1,2 Although it typically has a benign clinical course, it is histologically malignant and considered a low-grade variant of cutaneous T-cell lymphoma. 2,3 The classic clinical presentation of LyP involves the presence of reddish-brown papules and nodules typically measuring less than 2.0 cm, which may show evidence of central ulceration, hemorrhage, necrosis, and/or crust formation.1-5 It is characteristic that a patient may present with these skin lesions in different stages of evolution and that biopsies of these lesions may reflect different histologic features depending on the age of the lesion, making a definitive diagnosis more difficult to obtain if not clinically correlated.1,2 Any part of the body may be involved; however, there appears to be a predilection for the trunk and extremities in most cases.1-3,5 The skin eruptions usually are asymptomatic, but pruritus is a commonly associated concern.1,2,4,5
Lymphomatoid papulosis can have a localized, clustered, or generalized distribution pattern and typically will spontaneously regress without treatment within 3 to 12 weeks of symptom onset.2,3 Lymphomatoid papulosis has a slight male predominance with a male to female ratio of 1.5:1. It occurs most commonly between 35 and 45 years of age, though it can present at any age. The overall duration of the disease can range from months to decades.2,3 Lymphomatoid papulosis makes up approximately 15% of all cutaneous T-cell lymphomas.2,3 Although the overall prognosis is excellent, patients with LyP are at an increased risk of developing cutaneous or systemic lymphoma, most commonly mycosis fungoides, anaplastic large cell lymphoma, or Hodgkin lymphoma.1-3 This increased lifelong risk is the reason that patients with LyP must be followed long-term every 6 to 12 months for surveillance of emerging malignancy.1,2,6
The pathogenesis of LyP remains unknown. Some have hypothesized a possible viral trigger; however, there is insufficient data to support this theory.2,6 A diagnostic hallmark of LyP is its CD30 positivity, which is a known marker for T-cell activation.6 The spontaneous regression of skin lesions that is characteristic of LyP is believed to involve the interactions between CD30 and its ligand (CD30L), which may contribute to apoptosis of neoplastic T cells.2,3,6 With regards to the possible mechanisms contributing to tumor progression in LyP, a mutation in the transforming growth factor β receptor gene on CD30+ tumor cells within LyP lesions may allow for these cells to evade growth regulation and progress to lymphoma.2,6 A large percentage of LyP biopsy specimens show evidence of T-cell receptor gene monoclonal rearrangement, which can aid in establishing a diagnosis.1,2
The histologic features of LyP can vary greatly depending on the age of the lesion sampled.1,2 Histologic subtypes of LyP have been established, with type A being the most common (approximately 75% of cases), displaying a wedge-shaped infiltrate of scattered or clustered, large, atypical CD30+ T cells.1,2 Types B through E vary in histologic features, with the exception that all subtypes contain a CD30+ lymphocytic infiltrate.2,3
Treatment of LyP depends on the symptom/disease burden that the patient is experiencing. For patients with a limited number of nonscarring skin lesions in areas that are not cosmetically sensitive, observation is recommended. 1-3 For symptomatic patients with an extensive number of lesions, particularly those that may be scarring and/or in cosmetically sensitive areas, low-dose oral methotrexate therapy is considered first-line treatment.1-4 A methotrexate dose of 5 to 20 mg weekly can be effective in reducing the number and severity of lesions, with duration of treatment depending on clinical response.1,2 For patients who have contraindications to or who cannot tolerate oral methotrexate, phototherapy using psoralen plus UVA twice weekly for 6 to 8 weeks is another treatment option.1,2 Topical corticosteroids also can be used in children or for patients experiencing substantial pruritus.1,2,4 Oral or topical retinoids, topical carmustine or mechlorethamine, and brentuximab (an anti-CD30 monoclonal antibody) are all alternative therapies that have shown some beneficial effects.1,2 In the event that any of the skin lesions do not spontaneously regress within a 3- to 12-week time frame, surgical excision or radiotherapy can be performed on those lesions.2
Primary cutaneous anaplastic large cell lymphoma (C-ALCL) is another CD30+ lymphoproliferative disorder with overlapping clinical and histopathological features of LyP. Recurrent crops of multiple lesions favor a diagnosis of LyP, whereas solitary lesions favor C-ALCL; however, multifocal C-ALCL cases may occur.2 Mycosis fungoides is the most common type of cutaneous T-cell lymphoma that characteristically presents in a patch, plaque, tumor progression. Although mycosis fungoides eventually may transform into a CD30+ lymphoma, our patient did not display the characteristic clinical progression to suggest this diagnosis. Pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronica also fall into the spectrum of clonal T-cell cutaneous disorders that more commonly affect the pediatric population. Pityriasis lichenoides et varioliformis acuta has a marked CD8+ lymphocyte infiltrate, whereas pityriasis lichenoides chronica has more CD4+ lymphocytes. These disorders typically do not stain positive for CD30.2
All patients with a diagnosis of LyP should maintain lifelong, regular, 6- to 12-month follow-up visits to monitor disease status and screen for any evidence of developing malignancy.1,2,6 A thorough review of clinical history, complete skin examination, and physical examination with a particular focus on detection of lymphadenopathy and hepatosplenomegaly should be included at every followup visit.1 Systemic symptoms such as fever, night sweats, or weight loss are not typical features of LyP; therefore, patients who begin to develop these symptoms should be promptly evaluated for systemic lymphoma.1
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
- Kadin ME. Lymphomatoid papulosis. UpToDate website. Accessed June 4, 2022. https://www.uptodate.com/contents/lymphomatoid-papulosis
- Willemze R. Cutaneous T-cell lymphoma. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 4th ed. Elsevier Saunders; 2017:2141-2143.
- Wiznia LE, Cohen JM, Beasley JM, et al. Lymphomatoid papulosis. Dermatol Online J. 2018;24:13030/qt4xt046c9.
- Wieser I, Oh CW, Talpur R, et al. Lymphomatoid papulosis: treatment response and associated lymphomas in a study of 180 patients. J Am Acad Dermatol. 2016;74:59-67. doi:10.1016/j.jaad.2015.09.013
- Wolff K, Johnson RA, Saavedra AP, et al. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017.
- Kunishige JH, McDonald H, Alvarez G, et al. Lymphomatoid papulosis and associated lymphomas: a retrospective case series of 84 patients. Clin Exp Dermatol. 2009;34:576-581. doi:10.1111 /j.1365-2230.2008.03024.x
A 37-year-old woman presented to our dermatology clinic with a pruritic erythematous eruption involving the trunk, axillae, and proximal extremities of 10 days’ duration. Her medical history was notable only for eczema, and she denied taking any medications. Physical examination revealed scattered erythematous papules and crusts involving the trunk bilaterally and the extremities. We initially made a clinical diagnosis of bullous impetigo, and the patient was prescribed mupirocin ointment and dicloxacillin. At 1-week follow-up, the patient reported persistent skin lesions that were evolving despite therapy. Physical examination at this visit revealed an evolving eruption of multiple reddish-brown scaly papules involving the axillae, arms, forearms, and thighs, as depicted here.
Postherpetic Pink, Smooth, Annular Convalescing Plaques
The Diagnosis: Granuloma Annulare
A biopsy of a lesion on the right flank demonstrated granulomatous inflammation and interstitial mucin (Figure), characteristic of granuloma annulare (GA).1,2 Granuloma annulare is a relatively common skin disorder with an unknown etiology. It typically presents as smooth, annular, erythematous plaques.1 The most common variants of GA are localized, generalized, and subcutaneous. Our case demonstrated Wolf isotopic response, an unrelated skin disease that forms at the same location as a previously healed skin lesion.2 It is important to be aware of this phenomenon so that it is not confused with a recurrence of herpes zoster virus (HZV).
Although relatively infrequent, GA is the most common isotopic response following HZV infections.3-5 Other postherpetic isotopic eruptions include cutaneous malignancies, lichen planus, sarcoidosis, morphea, reactive perforating collagenosis, psoriasis, and infections, among others.3,5,6 The time between HZV infection and GA can be variable, ranging from a few weeks to many years apart.3
Oftentimes GA will spontaneously resolve within 2 years; however, recurrence is common.7-9 There currently are no standard treatment guidelines. The most promising treatment options include intralesional or topical glucocorticoids for localized GA as well as phototherapy or hydroxychloroquine for widespread disease.8,10

Annular elastolytic giant cell granuloma (also called actinic granuloma) is a rare idiopathic inflammatory skin disease. It is characterized by erythematous annular papules or plaques mainly found on sun-exposed skin, such as the backs of the hands, forearms, or face.11,12 Therefore, based on the distribution of our patient’s lesions, annular elastolytic giant cell granuloma was an unlikely diagnosis. Furthermore, it is not a known postherpetic isotopic reaction. Annular elastolytic giant cell granuloma can appear histologically similar to GA. Differentiating histologic features include a nonpalisading granuloma as well as the absence of mucin and necrobiosis.12
Annular lichen planus is a long-recognized but uncommon clinical variant of lichen planus that typically presents as pruritic, purple, annular plaques on the penis, scrotum, or intertriginous areas.13 The violaceous coloring is more characteristic of lichen planus. Histology is helpful in differentiating from GA.
Nummular eczema presents as scattered, welldefined, pruritic, erythematous, coin-shaped, coin-sized plaques in patients with diffusely dry skin.14 The scaling and serous crusting as well as more prominent pruritus help distinguish it from GA. The appearance of nummular eczema is quite characteristic; therefore, a biopsy typically is unnecessary for diagnosis. However, a potassium hydroxide wet mount examination of a skin scraping should be performed if tinea corporis also is suspected.
Superficial erythema annulare centrifugum classically presents as an annular or arciform pruritic lesion with an advancing outer erythematous edge with an inner rim of scale that most commonly occurs on the lower extremities. 15 The presence of pruritus and trailing scale helps distinguish this lesion from GA. Histologically, there are epidermal changes of hyperplasia, spongiosis, and parakeratosis, as well as lymphohistiocytic infiltrate surrounding the superficial dermal vessels.16
We report this case to highlight GA as the most common postherpetic isotopic response. It should be on the differential diagnosis when a patient presents with erythematous, smooth, annular plaques occurring in the distribution of a resolved case of HZV.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- . Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Kapoor R, Piris A, Saavedra AP, et al. Wolf isotopic response manifesting as postherpetic granuloma annulare: a case series. Arch Pathol Lab Med. 2013;137:255-258.
- Ezra N, Ahdout J, Haley JC, et al. Granuloma annulare in a zoster scar of a patient with multiple myeloma. Cutis. 2011;87:240-244.
- Noh TW, Park SH, Kang YS, et al. Morphea developing at the site of healed herpes zoster. Ann Dermatol. 2011;23:242-245.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Sparrow G, Abell E. Granuloma annulare and necrobiosis lipoidica treated by jet injector. Br J Dermatol. 1975;93:85-89.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479.
- Thornsberry LA, English JC. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Rubin CB, Rosenbach M. Granuloma annulare: a retrospective series of 133 patients. Cutis. 2019;103:102-106.
- Stein JA, Fangman B, Strober B. Actinic granuloma. Dermatol Online J. 2007;13:19.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456.
- Reich HL, Nguyen JT, James WD. Annular lichen planus: a case series of 20 patients. J Am Acad Dermatol. 2004;50:595-599.
- Leung AKC, Lam JM, Leong KF, et al. Nummular eczema: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:146-155.
- Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462.
- Coronel-Pérez IM, Morillo-Andújar M. Erythema annulare centrifugum responding to natural ultraviolet light [in Spanish]. Actas Dermosifiliogr. 2010;101:177-178.
The Diagnosis: Granuloma Annulare
A biopsy of a lesion on the right flank demonstrated granulomatous inflammation and interstitial mucin (Figure), characteristic of granuloma annulare (GA).1,2 Granuloma annulare is a relatively common skin disorder with an unknown etiology. It typically presents as smooth, annular, erythematous plaques.1 The most common variants of GA are localized, generalized, and subcutaneous. Our case demonstrated Wolf isotopic response, an unrelated skin disease that forms at the same location as a previously healed skin lesion.2 It is important to be aware of this phenomenon so that it is not confused with a recurrence of herpes zoster virus (HZV).
Although relatively infrequent, GA is the most common isotopic response following HZV infections.3-5 Other postherpetic isotopic eruptions include cutaneous malignancies, lichen planus, sarcoidosis, morphea, reactive perforating collagenosis, psoriasis, and infections, among others.3,5,6 The time between HZV infection and GA can be variable, ranging from a few weeks to many years apart.3
Oftentimes GA will spontaneously resolve within 2 years; however, recurrence is common.7-9 There currently are no standard treatment guidelines. The most promising treatment options include intralesional or topical glucocorticoids for localized GA as well as phototherapy or hydroxychloroquine for widespread disease.8,10

Annular elastolytic giant cell granuloma (also called actinic granuloma) is a rare idiopathic inflammatory skin disease. It is characterized by erythematous annular papules or plaques mainly found on sun-exposed skin, such as the backs of the hands, forearms, or face.11,12 Therefore, based on the distribution of our patient’s lesions, annular elastolytic giant cell granuloma was an unlikely diagnosis. Furthermore, it is not a known postherpetic isotopic reaction. Annular elastolytic giant cell granuloma can appear histologically similar to GA. Differentiating histologic features include a nonpalisading granuloma as well as the absence of mucin and necrobiosis.12
Annular lichen planus is a long-recognized but uncommon clinical variant of lichen planus that typically presents as pruritic, purple, annular plaques on the penis, scrotum, or intertriginous areas.13 The violaceous coloring is more characteristic of lichen planus. Histology is helpful in differentiating from GA.
Nummular eczema presents as scattered, welldefined, pruritic, erythematous, coin-shaped, coin-sized plaques in patients with diffusely dry skin.14 The scaling and serous crusting as well as more prominent pruritus help distinguish it from GA. The appearance of nummular eczema is quite characteristic; therefore, a biopsy typically is unnecessary for diagnosis. However, a potassium hydroxide wet mount examination of a skin scraping should be performed if tinea corporis also is suspected.
Superficial erythema annulare centrifugum classically presents as an annular or arciform pruritic lesion with an advancing outer erythematous edge with an inner rim of scale that most commonly occurs on the lower extremities. 15 The presence of pruritus and trailing scale helps distinguish this lesion from GA. Histologically, there are epidermal changes of hyperplasia, spongiosis, and parakeratosis, as well as lymphohistiocytic infiltrate surrounding the superficial dermal vessels.16
We report this case to highlight GA as the most common postherpetic isotopic response. It should be on the differential diagnosis when a patient presents with erythematous, smooth, annular plaques occurring in the distribution of a resolved case of HZV.
The Diagnosis: Granuloma Annulare
A biopsy of a lesion on the right flank demonstrated granulomatous inflammation and interstitial mucin (Figure), characteristic of granuloma annulare (GA).1,2 Granuloma annulare is a relatively common skin disorder with an unknown etiology. It typically presents as smooth, annular, erythematous plaques.1 The most common variants of GA are localized, generalized, and subcutaneous. Our case demonstrated Wolf isotopic response, an unrelated skin disease that forms at the same location as a previously healed skin lesion.2 It is important to be aware of this phenomenon so that it is not confused with a recurrence of herpes zoster virus (HZV).
Although relatively infrequent, GA is the most common isotopic response following HZV infections.3-5 Other postherpetic isotopic eruptions include cutaneous malignancies, lichen planus, sarcoidosis, morphea, reactive perforating collagenosis, psoriasis, and infections, among others.3,5,6 The time between HZV infection and GA can be variable, ranging from a few weeks to many years apart.3
Oftentimes GA will spontaneously resolve within 2 years; however, recurrence is common.7-9 There currently are no standard treatment guidelines. The most promising treatment options include intralesional or topical glucocorticoids for localized GA as well as phototherapy or hydroxychloroquine for widespread disease.8,10

Annular elastolytic giant cell granuloma (also called actinic granuloma) is a rare idiopathic inflammatory skin disease. It is characterized by erythematous annular papules or plaques mainly found on sun-exposed skin, such as the backs of the hands, forearms, or face.11,12 Therefore, based on the distribution of our patient’s lesions, annular elastolytic giant cell granuloma was an unlikely diagnosis. Furthermore, it is not a known postherpetic isotopic reaction. Annular elastolytic giant cell granuloma can appear histologically similar to GA. Differentiating histologic features include a nonpalisading granuloma as well as the absence of mucin and necrobiosis.12
Annular lichen planus is a long-recognized but uncommon clinical variant of lichen planus that typically presents as pruritic, purple, annular plaques on the penis, scrotum, or intertriginous areas.13 The violaceous coloring is more characteristic of lichen planus. Histology is helpful in differentiating from GA.
Nummular eczema presents as scattered, welldefined, pruritic, erythematous, coin-shaped, coin-sized plaques in patients with diffusely dry skin.14 The scaling and serous crusting as well as more prominent pruritus help distinguish it from GA. The appearance of nummular eczema is quite characteristic; therefore, a biopsy typically is unnecessary for diagnosis. However, a potassium hydroxide wet mount examination of a skin scraping should be performed if tinea corporis also is suspected.
Superficial erythema annulare centrifugum classically presents as an annular or arciform pruritic lesion with an advancing outer erythematous edge with an inner rim of scale that most commonly occurs on the lower extremities. 15 The presence of pruritus and trailing scale helps distinguish this lesion from GA. Histologically, there are epidermal changes of hyperplasia, spongiosis, and parakeratosis, as well as lymphohistiocytic infiltrate surrounding the superficial dermal vessels.16
We report this case to highlight GA as the most common postherpetic isotopic response. It should be on the differential diagnosis when a patient presents with erythematous, smooth, annular plaques occurring in the distribution of a resolved case of HZV.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- . Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Kapoor R, Piris A, Saavedra AP, et al. Wolf isotopic response manifesting as postherpetic granuloma annulare: a case series. Arch Pathol Lab Med. 2013;137:255-258.
- Ezra N, Ahdout J, Haley JC, et al. Granuloma annulare in a zoster scar of a patient with multiple myeloma. Cutis. 2011;87:240-244.
- Noh TW, Park SH, Kang YS, et al. Morphea developing at the site of healed herpes zoster. Ann Dermatol. 2011;23:242-245.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Sparrow G, Abell E. Granuloma annulare and necrobiosis lipoidica treated by jet injector. Br J Dermatol. 1975;93:85-89.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479.
- Thornsberry LA, English JC. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Rubin CB, Rosenbach M. Granuloma annulare: a retrospective series of 133 patients. Cutis. 2019;103:102-106.
- Stein JA, Fangman B, Strober B. Actinic granuloma. Dermatol Online J. 2007;13:19.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456.
- Reich HL, Nguyen JT, James WD. Annular lichen planus: a case series of 20 patients. J Am Acad Dermatol. 2004;50:595-599.
- Leung AKC, Lam JM, Leong KF, et al. Nummular eczema: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:146-155.
- Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462.
- Coronel-Pérez IM, Morillo-Andújar M. Erythema annulare centrifugum responding to natural ultraviolet light [in Spanish]. Actas Dermosifiliogr. 2010;101:177-178.
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465.
- . Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Kapoor R, Piris A, Saavedra AP, et al. Wolf isotopic response manifesting as postherpetic granuloma annulare: a case series. Arch Pathol Lab Med. 2013;137:255-258.
- Ezra N, Ahdout J, Haley JC, et al. Granuloma annulare in a zoster scar of a patient with multiple myeloma. Cutis. 2011;87:240-244.
- Noh TW, Park SH, Kang YS, et al. Morphea developing at the site of healed herpes zoster. Ann Dermatol. 2011;23:242-245.
- Ruocco V, Ruocco E, Ghersetich I, et al. Isotopic response after herpesvirus infection: an update. J Am Acad Dermatol. 2002;46:90-94.
- Sparrow G, Abell E. Granuloma annulare and necrobiosis lipoidica treated by jet injector. Br J Dermatol. 1975;93:85-89.
- Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479.
- Thornsberry LA, English JC. Etiology, diagnosis, and therapeutic management of granuloma annulare: an update. Am J Clin Dermatol. 2013;14:279-290.
- Rubin CB, Rosenbach M. Granuloma annulare: a retrospective series of 133 patients. Cutis. 2019;103:102-106.
- Stein JA, Fangman B, Strober B. Actinic granuloma. Dermatol Online J. 2007;13:19.
- Mistry AM, Patel R, Mistry M, et al. Annular elastolytic giant cell granuloma. Cureus. 2020;12:E11456.
- Reich HL, Nguyen JT, James WD. Annular lichen planus: a case series of 20 patients. J Am Acad Dermatol. 2004;50:595-599.
- Leung AKC, Lam JM, Leong KF, et al. Nummular eczema: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:146-155.
- Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462.
- Coronel-Pérez IM, Morillo-Andújar M. Erythema annulare centrifugum responding to natural ultraviolet light [in Spanish]. Actas Dermosifiliogr. 2010;101:177-178.
An 82-year-old man presented with painful, pink, smooth, annular convalescing plaques on the right back, flank, and abdomen in a zosteriform distribution involving the T10/11 dermatome. He had a history of hypertension and type 2 diabetes mellitus, and 12 months prior to presentation he had an outbreak of herpes zoster virus in the same distribution that was treated with valacyclovir 1000 mg 3 times daily for 7 days. Over the following month he noticed a resolution of blisters and crusting as they morphed into the current lesions.

Nonhealing Violaceous Plaque of the Hand Following a Splinter Injury
The Diagnosis: Chromoblastomycosis
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.
Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
The Diagnosis: Chromoblastomycosis
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.
Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
The Diagnosis: Chromoblastomycosis
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.
Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
A 70-year-old immunocompetent man presented to the dermatology department with a progressive asymptomatic hand wound of 2 years’ duration following a splinter injury in Belize. Prior treatment included oral antibiotics without improvement. Physical examination revealed a 5.1×3.0 cm, pink to violaceous, nonpurulent plaque with a cobblestonelike appearance on the dorsal aspect of the right hand. Both the initial and a repeat skin biopsy revealed nonspecific changes, including hyperkeratosis, hypergranulosis, acute and chronic inflammation, and vascular ectasia. Grocott-Gomori methenamine-silver staining was negative for fungal organisms. One month after the repeat biopsy, a tissue culture returned positive for the rare Fonsecaea pedrosoi.