User login
Genetic testing and the future of cerebral palsy malpractice cases
CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.)
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
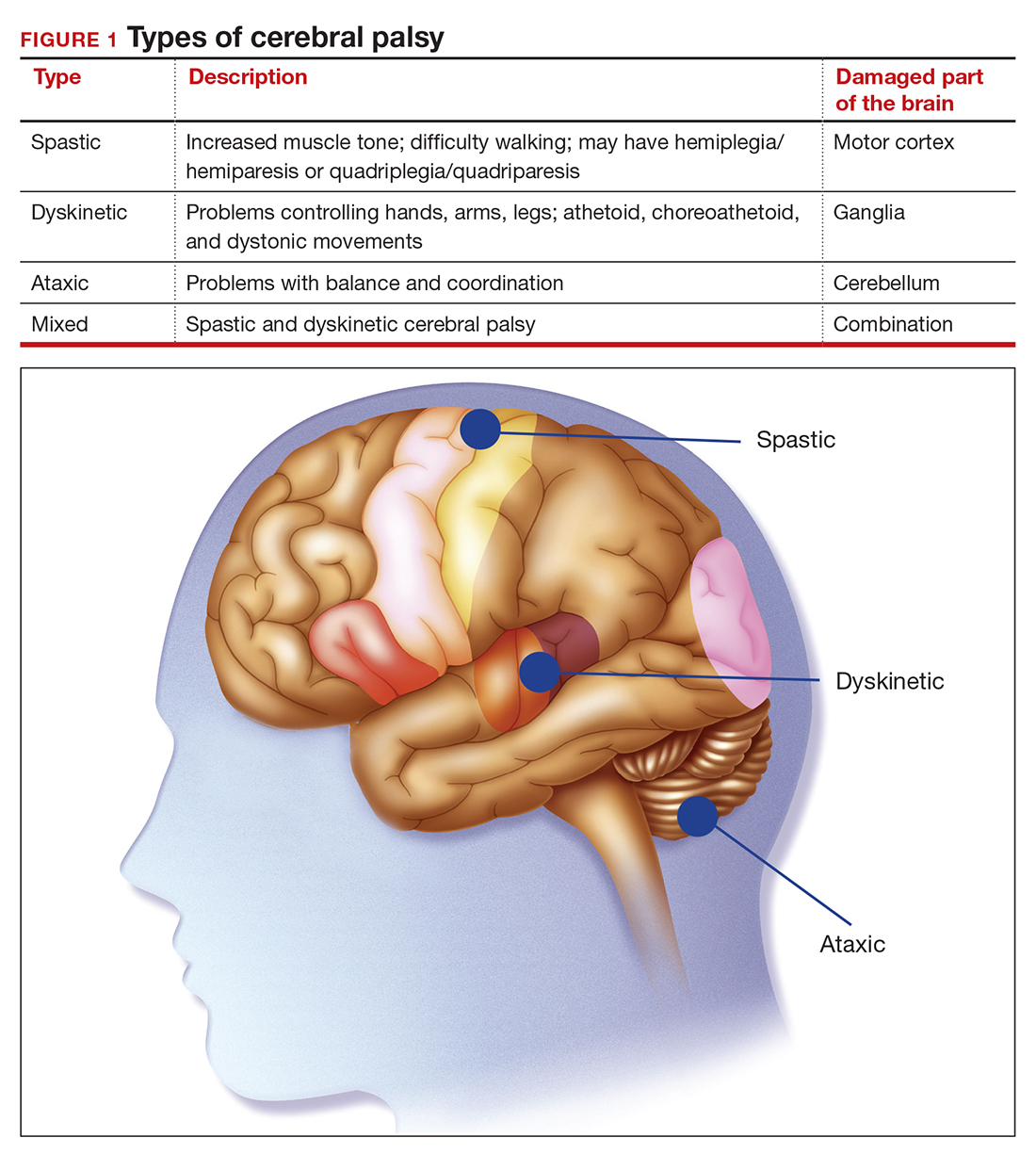
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.
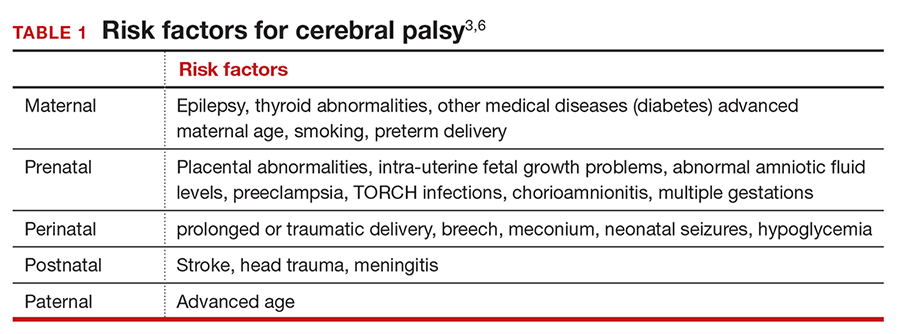
A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7
DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).
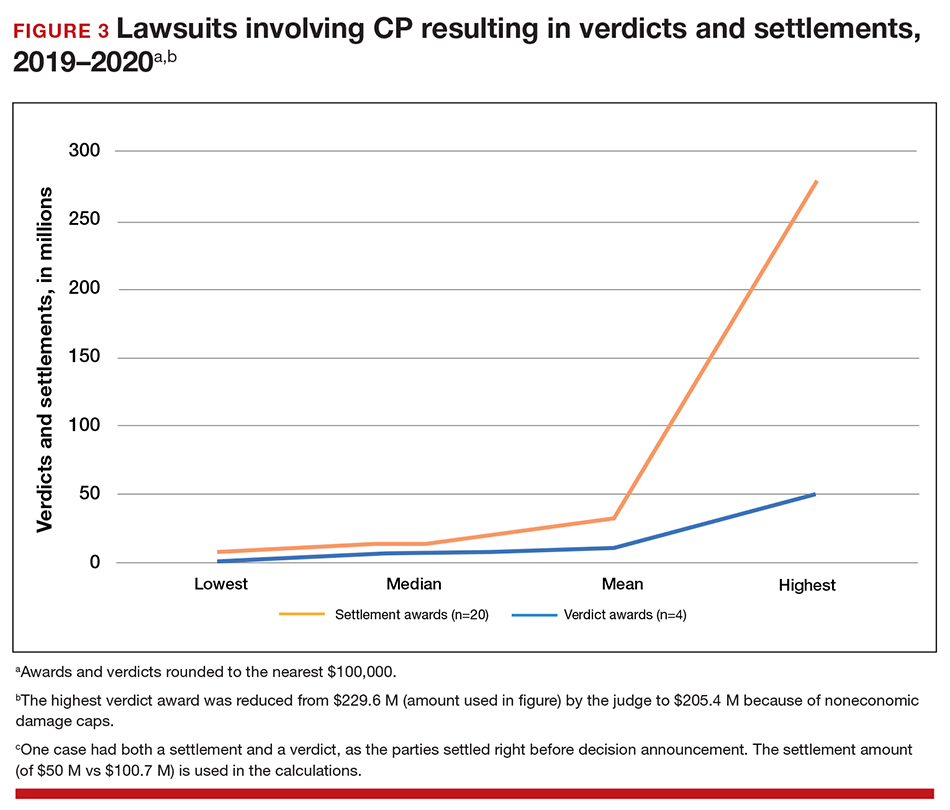
These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.

CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.)
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.

A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7

DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).

These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.

CASE Mixed CP diagnosed at age 6 months
After learning that the statute of limitations was to run out in the near future, the parents of a 17-year-old with cerebral palsy (CP) initiated a lawsuit. At the time of her pregnancy, the mother (G2P2002) was age 39 and first sought prenatal care at 14 weeks.
Her past medical history was largely noncontributory to her current pregnancy, except for that she had hypothyroidism that was being treated with levothyroxine. She also had a history of asthma, but had had no acute episodes for years. During the course of the pregnancy there was evidence of polyhydramnios; her initial thyroid studies were abnormal (thyroid-stimulating hormone levels, 7.1 mIU/L), in part due to lack of adherence with prescribed medications. She was noted to have elevated blood pressure (BP) 150/100 mm Hg but no proteinuria, with BP monitoring during her last trimester.
The patient went into labor at 40 3/7 weeks, after spontaneous rupture of membranes. In labor and delivery she was placed on a monitor, and irregular contractions were noted. The initial vaginal examination was noted as 1-cm cervical dilation, 90% effaced, and station zero. The obstetrician evaluated the patient and ordered Pitocin augmentation. The next vaginal exam several hours later noted 3-cm dilation and 100% effacement. The Pitocin was continued. Several early decelerations, moderate variability, and better contraction pattern was noted. Eight hours into the Pitocin, there were repetitive late decelerations; the obstetrician was not notified. The nursing staff proceeded with vaginal examination, and the patient was fully dilated at station +1. Again, the doctor was not informed of the patient’s status. At 10 hours post-Pitocin initiation, the patient felt the urge to push. The obstetrician was notified, and he promptly arrived to the unit and patient’s bedside. His decision was to use forceps for the delivery, feeling this would be the most expedient way to proceed, although cesarean delivery (CD) was a definite consideration. Forceps were applied, and as the nursing staff noted,” the doctor really had to pull to deliver the head.” A male baby, 8 lb 8 oz, was delivered. A second-degree tear was noted and easily repaired following delivery of the placenta. Apgar scores were 5 and 7 at 1 and 5 minutes after birth, respectively.
The patient’s postpartum course was uneventful. The patient and baby were discharged on the third day postpartum.
As the child was evaluated by the pediatrician, the mother noted at 6 months that the child’s head lagged behind when he was picked up. He appeared stiff at times and floppy at other times according to the parents. As the child progressed he had problems with hand-to-mouth coordination, and when he would crawl he seemed to “scoot his butt,” as they stated.
The child was tested and a diagnosis of mixed cerebral palsy was made, implying a combination of spastic CP and dyskinetic CP. He is wheelchair bound. The parents filed a lawsuit against the obstetrician and the hospital, focused on hypoxic-ischemic encephalopathy (HIE) due to labor and delivery management being below the standard of care. They claimed that the obstetrician should have been informed by the hospital staff during the course of labor, and the obstetrician should have been more proactive in monitoring the deteriorating circumstances. This included performing a CD based on “the Category III fetal heart tracing.”
At trial, the plaintiff expert argued that failure of nursing staff to properly communicate with the obstetrician led to mismanagement. Furthermore, the obstetrician used poor judgement (ie, below the standard of care) in not performing a CD. The defense expert argued that, overall, the fetal heart tracing was Category II, and the events occurred in utero, in part reflected by the mother having polyhydramnios and hypothyroidism that was not well controlled due to lack of adherence with prescribed medications. The child in his wheelchair was brought into the courtroom. The trial went on for more than 1 week, and the jury deliberated for several hours. (Note: This case is a composite of several different events and claims.)
Continue to: WHAT’S THE VERDICT?
WHAT’S THE VERDICT?
The jury returns a verdict for the defense.
Should anything have been done differently in this trial?
Medical considerations
Cerebral palsy is a neurodevelopmental disorder affecting 1 in 500 children.1 Other prevalence data (from a European study) indicate an incidence of 1.3–1.9 cases per 1,000 livebirths.1 The controversy continues with respect to the disorder’s etiology, especially when the infant’s magnetic resonance imaging (MRI) does not identify specific pathology. The finger is then pointed at HIE and thus the fault of the obstetrician and labor and delivery staff. In reality, HIE accounts for less than 10% of all cases of CP.2 Overall, CP is a condition focused on progressive motor impairments, many times associated with specific MRI findings.3 In addition, “MRI-negative” CP is a more vague diagnosis as discussed among neurologists.
The International Consensus Definition of CP is “a group of permanent disorders of the development of movement and posture, causing activity limitations, that are attributed to nonprogressive disturbances that occurred in the developing fetal or infant brain.”4 The International Cerebral Palsy Genomics Consortium have provided a consensus statement that defines CP based upon clinical type as opposed to etiology.5 Many times, however, ascribing an HIE cause to CP is “barking up the wrong tree,” in that we now know there are clear cut genetic causes of CP, and etiology attributed to perinatal causes, in reality, are genetic in up to 80% of cases.3 Types of CP are addressed in FIGURE 1. Overall, the pathophysiology of the disorder remains unknown. Some affected children have intellectual disabilities, as well as visual, hearing, and/or speech impairment.

A number of risk factors have been associated with CP (TABLE 1),3,6 which contribute to cell death in the brain or altered maturation of neurons and glia, resulting in abnormal white matter tracts and smaller central nervous system (CNS) volume or cerebellar hypoxia.6 One very important aspect of assessment for CP is specific gene mutations, which may vary in part dependent upon the presence or absence of environmental factors (insults).1 Mutations can lead to profound adverse effects with resultant CNS ischemia and neuromotor disability. In fact, genetics play a major role in determining the etiology of CP.1 Of interest, animal models who are subject to HIE induction have CNS effects resulting in permanent motor impairment.7

DNA sequencing
The DNA story continues to unfold with the concept that DNA variants alter susceptibility to environmental influences. These insults are, for example, thrombosis or hemorrhage, all of which affect motor function.1 Duplications or deletions of portions of a chromosome, related to copy number variants (CNVs) as well as advances in human-genome sequencing, can identify a single gene mutation leading to CP.1 Microdeletions, microduplications, and single nucleotide variants (SNVs) are to be included in genetic-related problems causing CP.3
A number of candidate genes have been considered and include “de novo heterozygous mutations in known Online Mendelian Inheritance (OMIM).” TIBA1A and SCN8A genes are highly associated with CP.8 Genetic assessment, as it evolves and more recently with the advent of exome sequencing, appears to provide a new and unprecedented level of understanding of CP. Specifically, exome sequencing provides a diagnostic tool with which to identify the prevalence of pathogenic and pathogenic variants (the latter encompassing genomic variants) with CP.9 A retrospective study assessed a cohort of patients with CP and noted that 32.7% of the pediatric-aged patients who underwent exome sequencing had pathogenic and pathogenic variants in the sequencing.9 Thus, we have a tool to identify underlying genetic pathogenesis with CP. This theoretically can change the outcome of lawsuits initiated for CP that ascribe an HIE etiology. Clinicians need to stay tuned as the genetic repertoire continues to unfold.
Continue to: Legal considerations...
Legal considerations
Although CP is not a common event, it has been a major factor in the total malpractice payments for ObGyns, neonatologists, and related medical disciplines. That is because the per-event liability can be staggering. Some law firms provide a “checklist” for plaintiffs early on in assessing a potential case (FIGURE 2).10

The financial risks and incentives
To understand what the current settlements and verdicts are in birth-related CP cases, a search of Lexis files revealed the reported outcomes of cases in 2019 and 2020 (FIGURE 3). Taking into account that the pandemic limited legal activity, 23 unduplicated cases were described with a reported settlement or verdict. Four cases resulted in verdicts for the injured patients, with the mean of these awards substantially higher than the settlements ($88.3 million vs $11.1 million, respectively).

These numbers are a glimpse at some of the very high settlements and verdicts that are common in CP cases. Notably, these are not a random sample of CP cases, but only those with the amount of the verdict or settlement reported. Potentially tried cases that may have been simply abandoned or dismissed are not reported. Furthermore, most settlements include confidentiality clauses, which may preclude the release of the financial value of the settlement. Cases in which the defense won (for example, a jury verdict in favor of the physician) are not included.
The high monetary awards in some CP cases are indirectly backed by Google search results for “cerebral palsy and liability” or “cerebral palsy and malpractice.” A very large number of results for law firms seeking clients with CP injuries is produced. Some of the websites note that only 10% (or 20% on some sites) of CP cases are caused by medical negligence, offering a “free legal case review” and a phone number for callers to “ask a legal question.” In the fine print one site notes that, “if you request any information you may receive a phone call or email from a partner law firm.”11 US physicians may be interested to note that a recent study of CP-based malpractice cases in China found that, although nearly 90% of the claims resulted in compensation, the mean damage award was $73,500.12 This was compared with a mean actual loss to the family of $128,200.
The interest by law firms in CP cases may be generated in part by the opportunity to assist a settlement or judgement that may be in the tens of millions of dollars. It is financially sensible to take a substantial risk on a contingency fee in a CP case compared with many other malpractice areas or claims where the likely damages are much lower. In addition, the vast majority of the damages in CP cases are for economic damages (cost of care and treatment and lost earning capacity), not noneconomic damages (pain and suffering). Therefore, the cap on noneconomic damages available in many states would not reduce the damages by a significant percentage.
CP cases are a significant part of the malpractice costs for ObGyns. Nearly one-third of obstetric claims are for neurologic injuries, including CP.13,14 These cases are often very complex and difficult, meaning that, in addition to the payments to the injured, there are considerable litigation costs associated with defending the cases. Perhaps as much as 60% of malpractice costs in obstetrics are in some way related to CP claims.15,16
Continue to: Negligence...
Negligence
Malpractice cases require not only damages (which clearly there are with CP) but also negligence and causation. (A more complete discussion of the elements of professional liability are included in a recent “What’s the Verdict?” column within
Several areas of negligence are common in CP related to delivery, including failure to monitor properly or ignoring, or not responding to, fetal heart rate (FHR) monitoring.18,19 For FHR monitoring, the claim is that problems can lead to asphyxia, resulting in HIE. Electronic fetal monitoring (EFM) has been an especially contentious matter. On one hand, the evidence of its efficacy is doubtful, but it has remained a standard practice, and it is often a centerpiece of delivery.20 Attorney Thomas Sartwelle has been prolific in suggesting that it not only has created legal problems for physicians but also results in unnecessary cesarean deliveries (CDs), which carry attendant risks for mother and infant.21 (It should be noted that other attorneys have expressed quite different views.22) He has argued that experts relying on EFM should be excluded from testifying because the technology is not based on sufficient science to meet the standard criteria used to determine the admissibility of expert witness (the Daubert standard).23 This argument is a difficult one so long as EFM is standard practice. Other claims of negligence include improper use of instruments at delivery, resulting in physical damage to the baby’s head, neck, or shoulders or internal hemorrhage. In addition, failure to deal with neonatal infection may be the basis for negligence.24
Causation
The question of whether or not the negligence (no matter how bad it was) caused the CP still needs to be addressed. Because a number of factors may cause CP, it has often been difficult to determine for any individual what the cause, or contributing causes, were. This fact would ordinarily work to the advantage of defendant-physicians and hospitals because the plaintiff in a malpractice case must prove by a preponderance of the evidence that the defendant’s negligence caused the CP. “Caused” is a term of art in the law; at the most basic level it means that the harm would not have occurred (or would have been less severe) but for the negligence.
In most CP cases the real cause is unknowable. It is, therefore, important to understand the difference between the certainty required in negligence cases and the certainty required in scientific studies (eg, 95% confidence). Negligence and causation in civil cases (including malpractice) must only be demonstrated by a preponderance of the evidence, which means “more likely than not.” For recovery in malpractice cases, states may require only that negligence be a “substantial factor.”
The theory that this lack of knowledge means that the plaintiff cannot prove causation, however, does not always hold.25 The following is what a jury might see: a child who will have a lifetime of medical, social, and financial burdens. Clear negligent practice by the physician coupled with severe injury can create considerable sympathy for the family. Then there are experts on both sides claiming that it is reasonably certain, in their opinions, that the injury was/was not caused by the negligence of the physician and health care team. The plaintiff’s witnesses will start eliminating other causes of CP in a form of differential diagnosis, stating that the remaining possibilities of causation clearly point to malpractice as the cause of CP. At some point, the elimination of alternative explanations for CP makes malpractice more likely than not to be a substantial factor in causing CP. On the other hand, the defense witnesses will stress that CP occurs most often without any negligence, and that, in this case, there are remaining, perhaps unknown, possible causes that are more likely than malpractice.
In this trial mix, it is not unthinkable that a jury or judge might find the plaintiff’s opinions more appealing. As a practical matter, and contrary to the technical rules, the burden of proof can seem to shift. The defendant clinician may, in effect, have to prove that the CP was caused by something other than the clinician’s negligence.
The role of insurance in award amounts
One reason that malpractice insurance companies settle CP cases for millions of dollars is that they face the possibility of judgements in the tens of millions. We saw even more than $100 million, in the 2019-2020 CP cases reported above. Another risk for malpractice insurance companies is that, if they do not settle, they may have liability beyond the policy limits. (Policy limits are the maximum an insurance policy is obligated to pay for any occurrence, or the total for all claims for the time covered by the premium.) For example, assume a malpractice policy has a $5 million policy limit covering Dr. Defendant, who has been sued for CP resulting from malpractice. There was apparently negligence during delivery in monitoring the fetus, but on the issue of causation the best estimate is that there is a 75% probability a jury would find no causal link between the negligence and the CP. If there is liability, damages would likely range from $5 to $25 million. Assume that the plaintiff has signaled it would settle for the policy limits ($5 million). Based purely on the odds and the policy limits, the insurance company should go to trial as opposed to settling for $5 million. That is because the physician personally (as opposed to the insurance company) is responsible for that part of a verdict that exceeds $5 million.
To prevent just such abuse (or bad faith), in most states, if the insurance company declines to settle the case for $5 million, it may become liable for the excess verdict above the policy limits. One reason that the cases that result in a verdict on damages—the 4 cases reported above for 2019‒2020—are interesting is that they help establish the risk of failing to settle a CP case.
Genetic understanding of causation
Given the importance of defendant-clinicians to be able to find a cause other than negligence to explain CP, the recent research of Moreno-De-Luca and colleagues may be especially meaningful.9 Using exome sequencing, the researchers found that 32.7% of pediatric-aged CP patients had pathogenic variance in the sequencing. In theory, this might mean that for about one-third of the CP plaintiffs, there may be genomic (rather than malpractice) explanations for CP, which might ultimately result in fewer cases of CP.
As significant as these findings are, caution is warranted. As the authors note, “this was an observational study and a causal relationship between detected gene variants and phenotypes in participants was not definitively established.”9 Until the causal relationship is established, it is not clear how much influence such a study would have in CP malpractice cases. Another caveat is that, at most, the genetic variants accounted for less than a third of CP cases studied, leaving many cases in which the cause remains unknown. In those cases in which a genomic association was not found, the case may be stronger for the “malpractice was the cause” claim. The follow-up research will likely shed light on some of these issues. Of course, if the genetic research demonstrates that in some proportion of cases there are genetic factors that contribute to the probability of CP, then the search will be for other triggering elements, which could possibly include poor care (that might well be a substantial factor for malpractice). Therefore, the preliminary genetic research likely represents only a part of the CP puzzle in malpractice cases.
Continue to: Why the opening case outcome was for the defense...
Why the opening case outcome was for the defense
Juries, of course, do not write opinions, so the basis for the jury’s decision in the example case is somewhat speculative. It seems most likely that causation had not been established. That is, the plaintiff-patient did not demonstrate that any malpractice was the likely, or substantial contributing, cause of the CP. The case illustrates several important issues.
Statute of limitations. This issue is common in CP cases because the condition may not be diagnosed for some time after birth. The statute of limitations can vary by state for medical malpractice cases “from 2 years to 22 years.”26 Many states begin with a 2-year statute but extend it if the injury or harm is not discovered. The extension is sometimes referred to as a statute of repose because, after that time, there is no extension even if the harm is discovered only later. In some states the statute does not run until the plaintiff is at or near the time of majority (usually age 18).27
Establishing negligence. The information provided about the presented case is mixed on the question of negligence, both regarding the hospital (through its nursing staff) for not properly contacting the obstetrician over the 10 hours, or the physician for inadequate monitoring. In addition, the reference to “really had to pull to deliver the head” may be the basis for claiming excessive, and potentially harmful use of force, which may have caused injury. In addition, the question remains whether the combination of these factors, including the Category III fetal heart tracing, made a cesarean delivery the appropriate standard of care.
Addressing causation. Assuming negligence, there is still a question of causation. It is far from clear that what the clinician did, or did not do, in terms of monitoring caused the CP injury. There is, however, no alternative causation that appeared in the case record, and this may be because of dueling expert witnesses.
The plaintiff sued both the obstetrician and the hospital, which is common among CP cases. While the legal interest of the two parties are aligned in some areas (causation), they may be in conflict in others (the failure of the hospital staff to keep the obstetrician informed). These potential conflicts are not for the clinicians to try to work out on their own. There is the potential for their actions to be misunderstood. When such a case is filed or threatened, the obstetrician should immediately discuss these matters with their attorney. In malpractice cases, malpractice insurance companies often select the attorneys who are experienced in such conflicts. If clinicians are not entirely comfortable that the appointed attorney is representing their interest and preserving a relationship with the hospital or other institution, however, they may engage their own legal counsel to protect their interests.
Practical considerations for avoiding malpractice claims
Good practices for avoiding malpractice claims apply with special force as it relates to CP.28,29
Uphold practice standards and good patient records. The causation element of these legal cases will remain problematic in the foreseeable future. But causation does not matter if negligent practice is not demonstrated. Therefore, maintaining best practices and continuous efforts at quality assurance and following all relevant professional practice guidelines is a good start. More than good intentions, it is essential that policies are implemented and reviewed. Among the areas of ongoing concern is the failure to monitor patients sufficiently. The long period of labor—where perhaps no physician is present for many hours—can introduce problems, as laypersons may have the impression that medical personnel were not on top of the situation.
Maintaining excellent records is also key for clinicians. The more complete the record, the fewer opportunities there are for faulty memories of parties and caregivers to fill in the gaps (especially when causation is so difficult to establish). Under absolutely no circumstances should records be changed or modified to eliminate damaging or an otherwise unfortunate notation. Few things are as harmful to credibility as discovered record tampering.
Inform patients of what is to come. Expectations are an important part of patient satisfaction. While not unduly frightening pregnant patients or eliminating reassurance, the informed consent process and patient counseling should be opportunities to avoid unreasonable expectations.
Stay alert to early genetic counseling, which is becoming increasingly available and important. Maintaining currency with what early testing can be done will become a critical part of ObGyn practice. For CP cases, in the near future, genetic testing may become part of determining causation. In the longer term, it will be part of counseling women and couples in deciding whether to have children, or potentially to end a pregnancy.
Expect the unexpected, and plan for it. Sometimes things just go wrong—there is a bad outcome, mistakes are made, patients are upset. It is important that any practice or institution have a clear plan for when such things happen. Some organizations have used apologies when appropriate,30 others have more complex plans for dealing with bad outcomes.31 Implement developed plans when they are needed. Individual practitioners also should consult with their attorney, who is familiar with their practice and who can help them maintain adherence to legal requirements and good legal problem prevention. ●
During a trial, all parties generally present evidence on negligence, causation, and damages. They do so without knowing whether a jury will find negligence and causation. The question of what the damages should be in cerebral palsy (CP) cases is also quite complex and expensive, but neither the defense nor the plaintiff can afford to ignore it. Past economic damages are relatively easy to calculate. Damages, for instance, includes medical care (pharmaceuticals and supplies, tests and procedures) and personal care (physical, occupational, and psychological therapy; long-term care; special educational costs; assistive equipment; and home modifications) that would have been avoided if it were not for CP. Future and personal care costs are more speculative, and must be estimated with the help of experts. In addition to future costs for the medical and personal care suggested above, depending on the state, the cost of lost future earnings (or earning capacity) may be additional economic damages. The cost of such intensive care, over a lifetime, accounts for many of the large verdicts and settlements.
Noneconomic damages are also available for such things as pain and suffering and diminished quality of life, both past and future. A number of states cap these noneconomic damages.
The wide range of damages correctly suggests that experts from several disciplines must be engaged to cover the damages landscape. This fact accounts for some of the costs of litigating these cases, and also for why damage calculations can be so complex.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.
- Fahey M, Macleenan A, Kretzschmar D, et al. The genetic basis of cerebral palsy. Dev Med Child Neurol. 2017;59:462-469. doi: 10.1111/dmcn.13363.
- Ellenberg J, Nelson, K. The association of cerebral palsy with birth asphyxia: a definitional quagmire. Dev Med Child Neurol. 2013;55:210-216. doi: 10.1111/dmcn.12016.
- Emrick L, DiCarlo S. The expanding role of genetics in cerebral palsy. Phys Med Rehabil Clin N Am. 2020;31:15-24. doi: 10.1016/j.pmr.2019.09.006.
- Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy [published correction appears in Dev Med Child Neurol. 2007;49:480]. Dev Med Child Neuro. 2007;109(suppl):8-14.
- MacLenan A, Lewis S, Moreno-DeLuca A, et al. Genetic or other causation should not change the clinical diagnosis of cerebral palsy. J Child Neurol. 2019;34:472-476. doi: 10.1177/0883073819840449.
- Lewis S, Shetty S, Wilson B, et al. Insights from genetic studies of cerebral palsy. Front Neurol. 2021;11:1-10. doi: 10.3389/fneur.2020.625428.
- Derick M, Drobyshevsky A, Ji X. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731-735. doi: 10.1161/01.STR.0000251445.94697.64.
- McMichael G, Bainbridge M, Haan E, et al. Whole exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20:176-182. doi: 10.1038/mp.2014.189.
- Moreno-DeLuca A, Milan F, Pesacreta D, et al. Molecular diagnostic yield of exome sequencing in patients with cerebral palsy. JAMA. 2021;325:467-475. doi: 10.1001/jama.2020.26148.
- Helping disabled children across Maryland & throughout the U.S. The Law Firm of Michael H. Bereston, Inc. website. https://www.berestonlaw.com/birth-injury/. Accessed April 26, 2021.
- Cerebral palsy lawsuits explained. Cerebral Palsy Guide website. https://www.cerebralpalsyguide.com/legal/. Accessed March 22, 2021.
- Zhou L, Li H, Li C, et al. Risk management and provider liabilities in infantile cerebral palsy based on malpractice litigation cases. J Forensic Leg Med. 2019;61:82-88. doi: 10.1016/j.jflm.2018.11.010.
- Cavanaugh MA. Bad cures for bad babies: policy challenges to the statutory removal of the common law claim for birth-related neurological injuries. Case West Res L Rev. 1992;43:1299-1346.
- Kain ZN, Caldwell-Andrews AA. What pediatricians should know about child-related malpractice payments in the United States. Pediatrics. 2006;118:464-468. doi: 10.1542/peds.2005-3112.
- Tabarrok A, Agan A. Medical malpractice awards, insurance, and negligence: which are related? Manhattan Institute Policy Research. Civil Justice Report; 2006. https://media4.manhattan-institute.org/pdf/cjr_10.pdf. Accessed April 27, 2021.
- Freeman AD, Freeman JM. No-fault cerebral palsy insurance: an alternative to the obstetrical malpractice lottery. J Health Politics Policy Law. 1989;14:707-718. doi: 10.1215/03616878-14-4-707.
- Sanfilippo JS, Smith SR. Is there liability if you don’t test for BRCA? OBG Manag. 2021;33:39-46. doi: 10.12788/obgm.0077.
- Fanaroff JM, Goldsmith JP. The most common patient safety issues resulting in legal action against neonatologists. Semin Perinatol. 2019;43:151181-1-9. doi: 10.1053/j.semperi.2019.08.010.
- Sartwelle TP, Johnston, JC. Cerebral palsy litigation: change course or abandon ship. J Child Neurol. 2015;30:828-841. doi: 10.1177/0883073814543306.
- Roth LM. The Business of Birth. NYU Press: New York, NY; 2021.
- Sartwelle TP. Electronic fetal monitoring: a bridge too far. J Legal Med. 2012;33:313-379. doi: 10.1080/01947648.2012.714321.
- Reiter JM, Walsh RS, Thomas EG. Best practices in birth injury litigation: timing hypoxic-ischemic fetal brain injury. Michigan Bar J. 2018;97:42-44.
- Sartwelle TP. Defending a neurologic birth injury: asphyxia neonatorum redux. J Legal Med. 2009;30:181-247. doi: 10.1080/01947640902936522.
- Daubert v Merrell Dow Pharm, Inc. 509 U.S. 579 (1993).
- Jha S. The factors making Americans litigious. J Am College Radiology. 2019;17:551-553. doi: 10.1016/j.jacr.2019.10.011.
- Salvi S, Pritchard PC. Statute of limitations on cerebral palsy cases. Personal Injury Lawyers website. https://www.salvilaw.com/birth-injury-lawyers/cerebral-palsy/time-limits/. Accessed March 24, 2021.
- Wharton R. Cerebral palsy statute of limitations. Cerebral Palsy Guidance website. October 16, 2020. https://www.cerebralpalsyguidance.com/cerebral-palsy-lawyer/statute-of-limitations/. Accessed March 24, 2021.
- Kassim PJ, Ushiro S, Najid KM. Compensating cerebral palsy cases: problems in court litigation and the no-fault alternative. Med Law. 2015;34:335-355.
- Williams D. Practice patterns to decrease the risk of malpractice suit. Clin Obstet Gynecol. 2008;51:680-687. doi: 10.1097/GRF.0b013e3181899bc7.
- McMichael BJ, Van Horn RL, Viscusi WK. “Sorry” is never enough: how state apology laws fail to reduce medical malpractice liability risk. Stanford Law Rev. 2019;71:341-409.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153:213-221. doi: 10.7326/0003-4819-153-4-201008170-00002.
Is there liability if you don’t test for BRCA?
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6
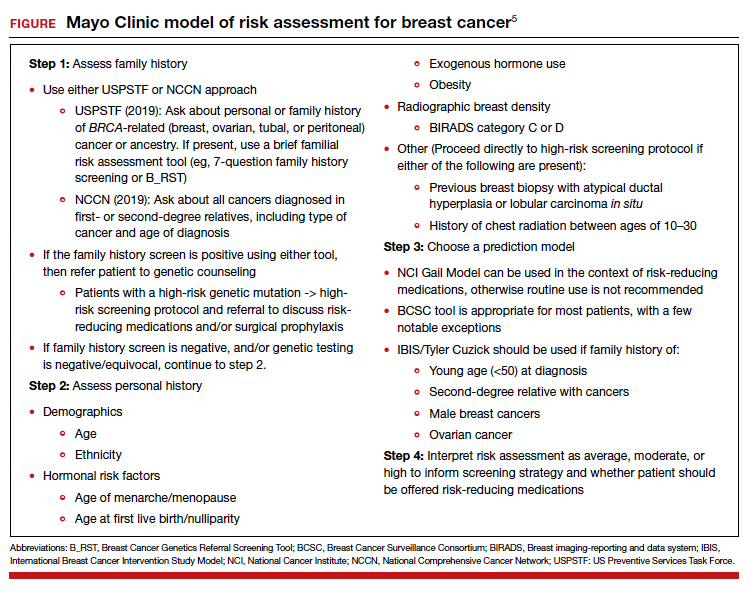
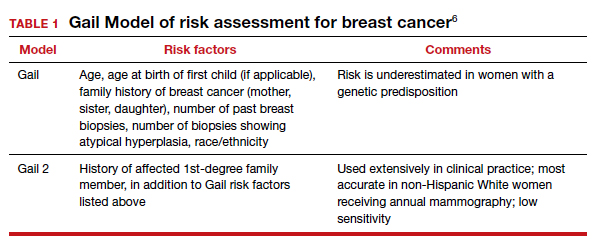
Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7
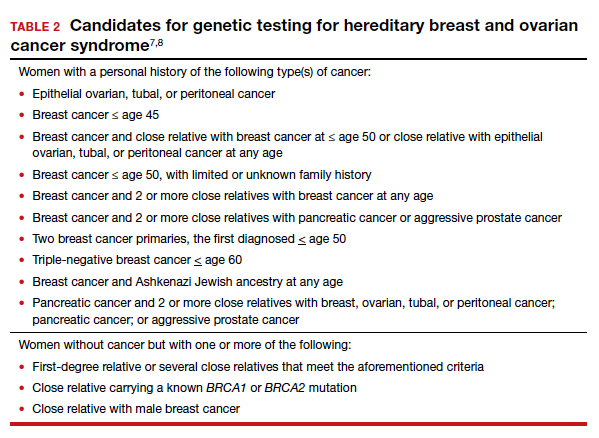
The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
CASE Young woman with family history of breast cancer detects lump
Two weeks after noting a lump on her breast when her cat happened to jump on her in that spot, a 28-year-old woman (G0) went to her primary care provider. She was referred to her gynecologist; breast imaging, ultrasonography, and mammography were obtained, with microcalcifications noted. A fine needle aspiration diagnosed intraductal malignancy. The surgical breast tissue specimen was estrogen receptor (ER)- and progestogen receptor (PR)-positive and HER2-negative. Other tumor markers were obtained, including carcinoembryonic antigen, and tissue polypeptide specific antigen, p53, cathepsin D, cyclin E, and nestin, but results were not available.
With regard to family history, the woman’s mother and maternal grandmother had a history of breast cancer. The patient and her family underwent gene testing. The patient was found to be BRCA1- and BRCA2-positive; her mother was BRCA1-positive, an older sister was BRCA2-positive, and her grandmother was not tested.
The question arose in light of her family history as to why she was not tested for BRCA and appropriately counseled by her gynecologist prior to the cancer diagnosis. Litigation was initiated. While the case did not go forward regarding litigation, it is indeed a case in point. (Please note that this is a hypothetical case. It is based on a composite of several cases.)
Medical considerations
Breast cancer is the most common type of cancer affecting women in the Western world.1 Advances in clinical testing for gene mutations have escalated and allowed for identification of patients at increased risk for breast and ovarian cancer. Along with these advances come professional liability risk. After looking at the medical considerations for BRCA1 and 2 testing, we will consider a number of important legal issues. In the view of some commentators, the failure to diagnose genetic mutations in patients predisposed to cancer is “poised to become the next wave of medical professional liability lawsuits.”2
BRCA1 and BRCA2 genes provide tumor suppressor proteins, and assessment for mutations is recommended for individuals at high risk for breast and/or ovarian cancer; mutations in BRCA genes cause DNA damage, which increases the chance of developing cancer. The other way to look at it is, BRCA1 and 2 are tumor suppressor genes that are integrally involved with DNA damage control. Once there is a mutation, it adversely affects the beneficial effects of the gene. Mutations in these genes account for 5% to 10% of all hereditary breast cancers.3 Of note, men with BRCA2 are at increased risk for prostate cancer.
A patient who presents to her gynecologist stating that there is a family history of breast cancer, without knowledge of genetic components, presents a challenge (and a medicolegal risk) for the provider to assess. Prediction models have been used to determine specific patient risk for carrying a genetic mutation with resultant breast cancer development.4 Risk prediction models do not appear to be a good answer to predicting who is more likely to develop breast or ovarian cancer, however. A Mayo model may assist (FIGURE).5 Clinicians should also be aware of other models of risk assessment, including the Gail Model (TABLE 1).6


Continue to: Guidelines for genetic testing...
Guidelines for genetic testing
The American College of Obstetricians and Gynecologists states that patient medical history and family history are paramount in obtaining information regarding risk for breast and ovarian cancer. First- and second-degree relatives are allocated to this category. Information regarding age of diagnosis, maternal and paternal lineage, and ethnic background can imply a need for genetic testing (TABLE 2).7,8 A number of genetics national organizations have participated in recommendations and include the American College of Medical Genetics and Genomics, the National Society for Genetic Counselors, and the Society of Gynecologic Oncology.7

The question always surfaces, could the clinical outcome of the cancer when diagnosed have been changed if screening were undertaken, with earlier diagnosis, or prevented with prophylactic mastectomy, and changed the end result. In addition, it is well known that breast augmentation mammoplasty alters the ability to accurately evaluate mammograms. Patients considering this type of plastic surgery, ideally, should be counselled accordingly.9
Bottom line, we as clinicians must be cognizant of both ACOG and United States Preventive Services Task Force (USPSTF) recommendations regarding screening and gene testing for women considered high risk for breast cancer based on family history.7
Legal considerations
The case presented demonstrates that the discovery of the BRCA1 and BRCA2 genes, and reliable tests for determining the existence of the genes, brought with them legal issues as well as medical advantages. We look at professional liability (malpractice) questions this technology raises, and then consider the outcome of the hypothetical case. (BRCA is used here to apply broadly—not only to BRCA1 and 2 but also to PALB2, CHEK2, and similar genetic abnormalities.)
To date, the most visible BRCA legal issues covered in cases and law reviews have focused more on patent law than malpractice. The most important of these was a decision of the US Supreme Court in Association for Molecular Pathology v Myriad Genetics.10 The US Patent Office was granting patents to companies finding useful, naturally occurring segments of human DNA, and had granted Myriad several patents on BRCA1 and BRCA2 genes. This patent policy had the potential to seriously interfere with broad scientific use of these genes.11 Fortunately, the Supreme Court stepped in and unanimously invalidated such patents. It held that a “naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated.” The Court noted, “Finding the location of the BRCA1 and BRCA2 genes does not render the genes patent eligible ‘new . . . composition[s] of matter.’”8 The Court did allow the patenting of tests for specific gene structures, and artificial changes in naturally occurring genes.
Malpractice and BRCA
While the BRCA patent wars have lingered, the potential for a significant increase in BRCA-related malpractice cases is of increasing concern. Like most malpractice liability, these new claims are based on very old principles of negligence.12 To prevail, the plaintiff (ordinarily, an injured patient) must demonstrate 4 things:
- A duty. That is, the physician owed a duty to the injured party. Usually (but not always) that requires a professional relationship between the physician and the person injured.
- A breach of that duty. Malpractice liability is based on the fact that the physician did something that a reasonably careful physician (generally, of the same specialty) would not have done, or that the physician failed to do something that a reasonable physician would have done. This usually means that the profession itself sees what the physician did (or did not do) as medically inappropriate. In medical malpractice cases, that is ordinarily measured by what the usual or common practice is among prudent physicians. In rare circumstances, courts have found the standard practice of a profession to be negligent. Where, for example, it was custom for a professional not to give an eye pressure test to anyone under age 40, a court found that common standard to be inappropriate.13 In the words of Judge Learned Hand (speaking about a different case), “a whole calling may have unduly lagged in the adoption of new and available devices. It never may set its own tests.”14 Underlying negligence is a cost-benefit analysis (discussed below).
- Damages. There must have been some damage that courts recognize, usually loss of money or opportunity to work, the cost of care, pain and suffering, or loss of enjoyment/quality of life. In malpractice, many states now recognize the “loss of chance” or the “loss of a chance.” That means, if a “physician negligently fails to diagnose a curable disease, and the patient is harmed by the disease, the physician should be liable for causing the ‘loss of a chance of a cure.’”15 (Delay in diagnosis is the most common reason for claims in breast cancer care.)16
- Causation. The breach of duty (negligence) must have caused the damages. The causation must have been reasonably close. If a driver drives through a stop sign, or a physician misreads a test, and someone is injured but there is no connection between the negligence and the injury, there is not tort liability.
The 4 elements of malpractice just described are raised in some way in the possible liability associated with BRCA testing. We next look at the ways in which liability may arise from that testing (or lack of it).
Underlying much of the following discussion is the “cost-benefit” consideration noted above. This concept is that the total cost (financial and health) of testing should be compared with the value of the benefits of testing, taking into account the probabilities that the testing will result in better health outcomes. BRCA testing, for example, is essentially cost-free in terms of physical risk. Its financial cost, while not trivial, is not great, and it is commonly covered by health insurance.17 In terms of benefits, the testing has the potential for providing critical information in making treatment decisions for a meaningful percentage of patients and their families. There are many ways of analyzing the liability risks of genetic malpractice,7,18 and the following is intended to discuss some of the greatest risks related to BRCA testing.
Continue to: Areas of liability...
Areas of liability
The failure to recommend a test. The circumstances in which BRCA testing should be undertaken are set out by professional organizations (noted above). These recommendations are not static, however. They change from time to time. Given the potential harm caused by the failure to test in relevant circumstances, malpractice liability is certainly a possibility when the failure to recommend a test to a patient results in a cancer that might have been prevented had the genetic problem been identified in a timely manner. The circumstances in which testing should be considered continue to change, placing an obligation on clinicians to stay well informed of changing genetic understandings. Another risk is that one specialist may assume that it is the job of another specialist to order the test. Whatever the cause of the failure to test, or unnecessary delay in testing, it appears to be the primary basis for BRCA liability.
The failure to properly interpret a test. Any test that is misinterpreted may lead to harm for the patient. A false negative, of course, may mean that preventive treatment that could have been undertaken will be foregone, as a “loss of a chance.” On the other hand, a false positive can lead to radical, unnecessary surgery or treatment. If a misinterpretation occurred because of carelessness by the testing organization, or confusion by a practitioner, there is a likelihood of negligence.19
A different form of “misinterpretation” could be reasonable—and not negligent. Advances in scientific-medical understanding may result in the outcome of tests being reconsidered and changed. That has been the case with genetic testing and breast cancer. The availability of multiple breast cancer SNPs (single nucleotide polymorphisms), and combining this information with other risk factors for example, results in a polygenic risk score that may be at odds with the level of risk from earlier testing.20,21 This naturally leads to the question of when later, updated testing should be recommended to look for a better current interpretation.22,23
The failure to act on BRCA test results. Testing is of no value, of course, if the results are not used properly. Test results or analyses that are not sent to the proper physicians, or are somehow ignored when properly directed, is a “never” event—it should never happen. It almost always would be considered negligence, and if the patient were injured, could lead to liability. Amazingly, one study found that, in genetic testing liability cases, nearly 20% of the claims arose from failure to return test results to patients.24 In addition, when a patient is found to be BRCA-positive, there is an obligation to discuss the options for dealing with the increased risk associated with the gene mutation(s), as well as to recommend the prudent course of action or to refer the patient to someone who will have that discussion.
Informed consent to the patient. BRCA testing requires informed consent. The physical risks of the testing process are minimal, of course, but it carries a number of other emotional and family risks. The informed consent process is an invitation to an honest discussion between clinicians and patients. It should be an opportunity to discuss what the testing is, and is not, and what the test may mean for treatment. It may also be an opportunity to discuss the implications for other members of the patient’s family (noted below).
One element of informed consent is a discussion of the consequences of failure to consent, or to undertake one of the alternatives. In the case of BRCA testing, this is especially important in cases in which a patient expresses a hesitancy to be tested with an “I’d rather not know philosophy.” Although clinicians should not practice law, some patient concerns about discrimination may be addressed by the protection that the federal Genetic Information Nondiscrimination Act (GINA) and other laws provide (which prohibit insurance and employment discrimination based on genetic information). A good source of information about GINA and related nondiscrimination laws is provided by the National Human Genome Research Institute.25 In addition, the National Institutes of Health has a website that may be helpful to many patients26 (and a much more complex site for health professionals).27 At the same time, courts have resisted plaintiffs/patients who have tried to use informed consent as a way of suing for failure to offer genetic testing.28,29
The failure to refer. In some cases, a patient should be formally referred for genetics consultation. The considerations here are similar to other circumstances in modern, fast developing medical practice that require special sensitivity to those occasions in which a patient will benefit from additional expertise. It is a principle that the AMA Council on Ethical and Judicial Affairs has expressed this way: “In the absence of adequate expertise in pretest and posttest counseling, a physician should refer the patient to an appropriate specialist.”30 The failure to refer, when that deviates from acceptable practice, may result in liability.
Informing others. BRCA testing is an area of medicine in which results may be of great significance not only to the patient but also to the patient’s family.31 Physicians should counsel patients on the importance of informing relatives about relevant results and “should make themselves available to assist patients in communicating with relatives to discuss opportunities for counseling and testing, as appropriate.”30 The question may arise, however, of whether in some circumstances physicians should go a step further in ensuring relatives receive important information regarding their loved one’s health.32 The law has been reluctant to impose liability to “third parties” (someone not a patient). Duties usually arise through the physician-patient relationship. There are exceptions. Perhaps the best known has been the obligation of mental health professionals to take action to protect third parties from patients who have made believable threats against identifiable victims.33 There are indications that some courts could find, in extreme circumstances, a “duty to warn” nonpatients in some instances where it is essential to inform third parties that they should receive a specific form of genetic testing.34,35 Such a duty would, of course, have to protect the privacy rights of the patient to the maximum extent possible. A general duty of this type has not been established widely, but may be part of the future.
Continue to: Was there liability in our example case?...
Was there liability in our example case?
The hypothetical case provided above suggests that there could be liability. Routine medical history by the primary care physician would have produced the fact that the patient’s mother, sister, and maternal grandmother had breast cancer. That would clearly have put her in a category of those who should have received genetic testing. Yet, she was not tested until after her cancer was found. From the limited facts we have, it appears that this timeline of events would have been outside accepted practice—and negligent. The case was not pursued by the patient, however, and this may represent the current state of liability for BRCA issues.
The extent of liability seems to be significant
Our discussion of liability suggests that there is significant potential for BRCA testing negligence within practice, and that the damages in these cases could be substantial. Yet the predicted “tsunami” of malpractice lawsuits related to genetic testing has not appeared.36,37 One study of cases in the United States (through 2016) found a “slowly rising tide” of liability cases instead of a tsunami,24 as the number of claims made was low. On the other hand, the payments where damages were awarded were an order of magnitude larger than other malpractice cases—a mean of $5.3 million and median of $2 million. This is compared with mean values in the range of $275,000 to $600,000 in other areas of malpractice.
The majority of the genetic malpractice cases involve prenatal and newborn testing, and diagnosis/susceptibility/pharmacogenomic accounting for about 25% of cases. In terms of type of errors claimed, approximately 50% were diagnostic-interpretation errors, 30% failure to offer testing, nearly 20% failure to return test results to the patients, and a few remaining cases of failure to properly treat in light of genetic testing.24
Despite a few very large payments, however, the fact remains that there is a surprisingly low number of genetics malpractice cases. Gary Marchant and colleagues suggest that several reasons may account for this:
- the clinical implementation of genetic science has been slower than expected
- the lack of expertise of many physicians in genetic science
- expert witnesses have sometimes been hard to find
- the lack of understanding by plaintiffs’ attorneys of genetic malpractice
- potential plaintiffs’ lack of understanding of the nature of genetic testing and the harms resulting from genetic negligence.17,24,37
The tide is slowly coming in
By all appearances, there is every reason to think that genetic malpractice will be increasing, and that the recent past of much higher damages per claim paid in the genetics area will be part of that tide. The National Human Genome Research LawSeq project has suggested a number of useful ways of dealing with the liability issues.18 In addition to the BRCA issues that we have considered in this article for ObGyns, there are other critical issues of prenatal and newborn genetic testing.38 But those are topics for another day. ●
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
- Sevilla C, Moatti JP, Reynier CJ, et al. Testing for BRCA1 mutations: a cost-effective analysis. Europ J Human Genetics. 2002;10:599-606.
- Cotton V, Kirkpatrick D. Failure to recommend genetic counseling in breast cancer: is the next wave of medical professional liability lawsuits? Contemp OB/GYN. June 1, 2017.
- Suryavanshi M, Kumar D, Panigrahi M, et al. Detection of false positive mutations in BRCA gene by next generation sequencing. Fam Cancer. 2017;16:311-317.
- Black L, Knoppers B, Avard D, et al. Legal liability and the uncertain nature of risk prediction: the case of breast cancer risk prediction models. Public Health Genomics. 2012;15:335-340.
- McClintock A, Gollab A, Laya M. Breast cancer risk assessment, a step-wise approach for primary care physicians on the front lines of shared decision making. Mayo Clin Proc. 2020;95:1268-1275.
- National Cancer Institute. The Breast Cancer Risk Assessment Tool. https://bcrisktool.cancer.gov/. Accessed February 25, 2021.
- Neff J, Richardson G, Phelps J. Legal liabilities associated with hereditary breast and ovarian cancers. J Reprod Med. 2020;65:227-230.
- American College of Obstetricians and Gynecologists. Practice Bulletin No 182: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110-e126.
- Sá dos Reis C, Gremion I, and Meystre NR. Study of breast implants mammography examinations for identification of suitable image quality criteria. Insights Imaging. 2020;11:3.
- Association for Molecular Pathology v Myriad Genetics, 569 U.S. 576 (2013).
- Smith SR. The Supreme Court 2012-2013: dogs, DNA, and DOMA. Register Rep. 2013;39(Fall):26-33.
- Bal BS. An introduction to medical malpractice in the United States. Clin Orthop Relat Res. 2009;467:339-347.
- Helling v Carey, 83 Wn.2d 514, 519 P.2d 981 (1974).
- The T.J. Hooper, 60 F.2d 737, 740 (2d Cir.1932), cert. denied 287 U.S. 662 (1932).
- Fischer DA. Tort recovery for loss of a chance. Wake Forest L Rev. 2001;36:605-655.
- Murphy BL, Ray-Zack MD, Reddy PN, et al. Breast cancer litigation in the 21st century. Ann Surg Oncol. 2018;25:2939- 2947.
- Prince AE. Prevention for those who can pay: insurance reimbursement of genetic-based preventive interventions in the liminal state between health and disease. J Law Biosci. 2015;2:365-395.
- Marchant G, Barnes M, Evans JP, et al; LawSeq Liability Task Force. From genetics to genomics: facing the liability implications in clinical care. J Law Med Ethics. 2020;48:11-43.
- Complaint, Held v Ambry Genetics Corp., No. 15-CV-8683, 2015 WL 6750024 (S.D.N.Y. Nov. 4, 2015); Order of Dismissal, Held v Ambry Genetics Corp., No. 15-CV-8683, (S.D.N.Y. Dec. 6, 2016).
- Pederson HJ. Breast cancer risk assessment and treatment: current concepts in genetics and genomics. Contemp OB/ GYN. 2017; 62:A1-A4.
- Pederson HJ. Who needs breast cancer genetics testing? OBG Manag. 2018;30:34-39.
- Roberts JL, Foulkes A. Genetic duties. William Mary L Rev. 2020;62:143-212.
- Thorogood A, Cook-Deegan R, Knoppers B. Public variant databases: liability? Genet Med. 2017;19:838–841.
- Marchant G, Lindor R. Genomic malpractice: an emerging tide or gentle ripple? Food Drug Law J. 2018;73:1-37.
- National Human Genome Research Institute. Genetic discrimination. https://www.genome.gov/about-genomics /policy-issues/Genetic-Discrimination. Updated September 16, 2020. Accessed February 25, 2021.
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. https://www.cancer.gov/about-cancer /causes-prevention/genetics/brca-fact-sheet. Reviewed November 19, 2020. Accessed February 25, 2021.
- National Cancer Institute. Genetics of breast and gynecologic cancers (PDQ®)–Health Professional Version. https://www .cancer.gov/types/breast/hp/breast-ovarian-genetics-pdq. Updated February 12, 2021. Accessed February 25, 2021.
- Reed v Campagnolo, 630 A.2d 1145, 1152–54 (Md. 1993).
- Munro v Regents of Univ. of Cal.,263 Cal. Rptr. 878, 885, 988 (1989).
- AMA Council on Ethical and Judicial Affairs. AMA Code of Medical Ethics’ opinions on genetic testing. Opinion 2.131. 2009;11:683-685. https://journalofethics.ama-assn .org/article/ama-code-medical-ethics-opinions-genetictesting/2009-09.
- Gilbar R, Barnoy S. Disclosing genetic test results to the patient’ relatives: how does the law influence clinical practice? J Law Technol Policy. 2019;125-168.
- Song K. Warning third parties of genetic risks in the era of personalized medicine. U.C. Davis L Rev. 2016;49:1987-2018.
- Tarasoff v Regents of the University of California, 551 P.2d 334, 131 Cal. Rptr. 14 (Cal. 1976).
- Safer v Estate of Pack, 677 A.2d 1188 (N.J. App. 1996), cert. denied, 683 A.2d 1163 (N.J. 1996).
- Pate v Threlkel, 661 So.2d 278 (Fla. 1995).
- Rothstein MA. Liability issues in pharmacogenomics. Louisiana L Rev. 2005;66:117-124.
- Marchant G, Lindor R. Personalized medicine and genetic malpractice. Genet Med. 2013;15:921-922.
- Westbrook M. Transforming the physician’s standard of care in the context of whole genome sequencing technologies: finding guidance in best practice standards. Saint Louis U J Health Law Policy. 2015;9:111-148.
Metadata, malpractice claims, and making changes to the EHR
In 2009, the Health Information Technology for Economic and Clinical Health Act (HITECH Act), which is part of the American Recovery and Reinvestment Act, provided several billion dollars of grants and incentives to stimulate the implementation of electronic health records (EHRs) and supporting technology in the United States.1 Since then, almost all health care organizations have employed EHRs and supporting technologies. Unfortunately, this has created new liability risks. One potential risk is that in malpractice claims, there is more discoverable evidence, including metadata, with which to prove the claims.2 In this article, I explain what metadata is and how it can be used in medical malpractice cases. In addition, because we cannot change metadata, I provide guidance on making corrections in your EHR do
What is metadata?
Metadata—commonly described as data about data—lurk behind the words and images we can see on our computer screens. Metadata can be conceptualized as data that provides details about the information we enter into a computer system, creating a permanent electronic footprint that can be used to track our activity.2,3 Examples of metadata include (but are not limited to) the user’s name, date and time of a record entry, changes or deletions made to the record, the date an entry was created or modified, annotations that the user added over a period of time, and any other data that the software captures without the user manually entering the information.3 Metadata is typically stored on a server or file that users cannot access, which ensures data integrity because a user cannot alter a patient’s medical record without those changes being captured.3
How metadata is used in malpractice claims
When a psychiatrist is sued for medical negligence, the integrity of the EHR is an important aspect of defending against the lawsuit. A plaintiff’s (patient’s) attorney can more readily discover changes to the patient’s medical record by requesting the metadata and having it analyzed by an information technology specialist. Because the computer system captures everything a user does, it is difficult to alter a patient’s record without being detected. Consequently, plaintiff attorneys frequently request metadata during discovery in the hopes of learning whether the defendant psychiatrist altered or attempted to hide information that was contained or missing from the original version of the medical record.3 If the medical record was revised at a time unrelated to the treatment, metadata can raise suspicion of deception, even in the absence of wrongdoing.2 Alternatively, metadata can be used to validate that the EHR was changed when treatment occurred, which can bolster a defendant psychiatrist’s ability to rely on the EHR against a claim of medical negligence.2
Depending on the jurisdiction, metadata may or may not be discoverable. The Federal Rules of Civil Procedure emphasize producing documents in their original format.4 For federal cases, these rules suggest that the parties discuss discovery of this material when they are initially conferring; however, the rules do not specify whether a party must produce metadata, which leaves the courts to refine these rules through case law.4,5 In one case, a federal court ruled that a party had to produce documents with metadata intact.5 Without an agreement between both parties to exclude metadata from produced documents, the parties must produce the metadata.5 State laws differ in regards to the discoverability of metadata.
Corrections vs alterations
A patient’s medical record is the best evidence of the care we provided, should that care ever be challenged in court. We can preserve the medical record’s effectiveness through appropriate changes to it. Appropriately executed corrections are a normal part of documentation, whereas alterations to the medical record can cast doubt on our credibility and lead an otherwise defensible case to require a settlement.6
Corrections are changes to a patient’s medical record during the normal course of treatment.6 These are acceptable, provided the changes are made appropriately. Health care facilities and practices have their own policies for making appropriate corrections and addendums to the medical record. Once a correction and/or addendum is made, do not remove or delete the erroneous entry, because health care colleagues may have relied on it, and deleting an erroneous entry also would alter the integrity of the medical record.6 When done appropriately, corrections will not be misconstrued as alterations.
Alterations are changes to a patient’s medical record after a psychiatrist receives notice of a lawsuit and “clarifies” certain points in the medical record to aid the defense against the claim.6 Alterations are considered deliberate misrepresentations of facts and, if discovered during litigation, can significantly impact the ability to defend against a claim.6 In addition, many medical liability policies exclude coverage for claims in which the medical record was altered, which might result in a psychiatrist having to pay for the judgment and defense costs out of pocket.6 Psychiatrists facing litigation who have a legitimate need to change an EHR entry after a claim is filed should consult with legal counsel or a risk management professional for guidance before making any changes.3 If they concur with updating the patient’s record to correct an error (including an addendum or a late entry; see below), the original entry, date, and time stamp must be accessible.3 This should also include the current date/time of the amended entry, the name of the person making the change, and the reasons for the change.3
Continue to: How to handle corrections and late entries
How to handle corrections and late entries
Sometimes situations occur that require us to make late entries, enter addendums, or add clarification notes to patient information in the EHRs. Regardless of your work environment (ie, hospital, your own practice), there should be clear procedures in place for correcting patients’ EHRs that are in accordance with applicable federal and state laws. Correcting an error in the EHR should follow the same basic principles of correcting paper records: do not obscure the original entry, make timely corrections, sign all entries, ensure the person making the change is identified, and document the reason(s) for the correction.7 The EHR must be able to track corrections or changes to an entry once they are entered or authenticated. Any physical copies of documentation must also have the same corrections or changes if they have been previously printed from the EHR.
You may need to make an entry that is late (out of sequence) or provides additional documentation to supplement previously written entries.7 A late entry should be used to record information when a pertinent entry was missed or not written in a timely manner.7 Label the new entry as a “late entry,” enter the current date and time (do not give the appearance that the entry was made on a previous date or at an earlier time), and identify or refer to the date and incident for which the late entry is written.7 If the late entry is used to document an omission, validate the source of additional information as best you can (ie, details of where you obtained the information to write the late entry).7 Make late entries as soon as possible after the original entry; although there is no time limit on writing a late entry, delays in corrections might diminish the credibility of the changes.
Addendums are used to provide additional information in conjunction with a previous entry.7 They also provide additional information to address a specific situation or incident referenced in a previous note. Addendums should not be used to document information that was forgotten or written in error.7 A clarification note is used to avoid incorrect interpretation of previously documented information.7 When writing an addendum or a clarification note, you should label it as an “addendum” or a “clarification note”; document the current date and time; state the reason for the addendum (referring back to the original entry) or clarification note (referring back to the entry being clarified); and identify any sources of information used to support an addendum or a clarification note.7
1. American Recovery and Reinvestment Act of 2009. Pub L No. 111-5, 123 Stat 115 (2009).
2. Paterick ZR, Patel NJ, Ngo E, et al. Medical liability in the electronic medical records era. Proc (Bayl Univ Med Cent). 2018;31(4):558-561.
3. Funicelli A. ‘Hidden’ information in your EHRs could increase your liability risk. Psychiatric News. 2019;54(18):12-13.
4. Federal Rules of Civil Procedure, 26(f), 115th Cong, 1st Sess (2017).
5. Williams v Sprint/United Mgmt Co, 230 FRD 640 (D Kan 2005).
6. Ryan ML. Making changes to a medical record: corrections vs. alterations. NORCAL Mutual Insurance Company. Accessed February 3, 2021. http://www.sccma.org/Portals/19/Making%20Changes%20to%20a%20Medical%20Record.pdf
7. AHIMA’s long-term care health information practice and documentation guidelines. The American Health Information Management Association. Published 2014. Accessed February 3, 2021. http://bok.ahima.org/Pages/Long%20Term%20Care%20Guidelines%20TOC/Legal%20Documentation%20Standards/Legal%20Guidelines
In 2009, the Health Information Technology for Economic and Clinical Health Act (HITECH Act), which is part of the American Recovery and Reinvestment Act, provided several billion dollars of grants and incentives to stimulate the implementation of electronic health records (EHRs) and supporting technology in the United States.1 Since then, almost all health care organizations have employed EHRs and supporting technologies. Unfortunately, this has created new liability risks. One potential risk is that in malpractice claims, there is more discoverable evidence, including metadata, with which to prove the claims.2 In this article, I explain what metadata is and how it can be used in medical malpractice cases. In addition, because we cannot change metadata, I provide guidance on making corrections in your EHR do
What is metadata?
Metadata—commonly described as data about data—lurk behind the words and images we can see on our computer screens. Metadata can be conceptualized as data that provides details about the information we enter into a computer system, creating a permanent electronic footprint that can be used to track our activity.2,3 Examples of metadata include (but are not limited to) the user’s name, date and time of a record entry, changes or deletions made to the record, the date an entry was created or modified, annotations that the user added over a period of time, and any other data that the software captures without the user manually entering the information.3 Metadata is typically stored on a server or file that users cannot access, which ensures data integrity because a user cannot alter a patient’s medical record without those changes being captured.3
How metadata is used in malpractice claims
When a psychiatrist is sued for medical negligence, the integrity of the EHR is an important aspect of defending against the lawsuit. A plaintiff’s (patient’s) attorney can more readily discover changes to the patient’s medical record by requesting the metadata and having it analyzed by an information technology specialist. Because the computer system captures everything a user does, it is difficult to alter a patient’s record without being detected. Consequently, plaintiff attorneys frequently request metadata during discovery in the hopes of learning whether the defendant psychiatrist altered or attempted to hide information that was contained or missing from the original version of the medical record.3 If the medical record was revised at a time unrelated to the treatment, metadata can raise suspicion of deception, even in the absence of wrongdoing.2 Alternatively, metadata can be used to validate that the EHR was changed when treatment occurred, which can bolster a defendant psychiatrist’s ability to rely on the EHR against a claim of medical negligence.2
Depending on the jurisdiction, metadata may or may not be discoverable. The Federal Rules of Civil Procedure emphasize producing documents in their original format.4 For federal cases, these rules suggest that the parties discuss discovery of this material when they are initially conferring; however, the rules do not specify whether a party must produce metadata, which leaves the courts to refine these rules through case law.4,5 In one case, a federal court ruled that a party had to produce documents with metadata intact.5 Without an agreement between both parties to exclude metadata from produced documents, the parties must produce the metadata.5 State laws differ in regards to the discoverability of metadata.
Corrections vs alterations
A patient’s medical record is the best evidence of the care we provided, should that care ever be challenged in court. We can preserve the medical record’s effectiveness through appropriate changes to it. Appropriately executed corrections are a normal part of documentation, whereas alterations to the medical record can cast doubt on our credibility and lead an otherwise defensible case to require a settlement.6
Corrections are changes to a patient’s medical record during the normal course of treatment.6 These are acceptable, provided the changes are made appropriately. Health care facilities and practices have their own policies for making appropriate corrections and addendums to the medical record. Once a correction and/or addendum is made, do not remove or delete the erroneous entry, because health care colleagues may have relied on it, and deleting an erroneous entry also would alter the integrity of the medical record.6 When done appropriately, corrections will not be misconstrued as alterations.
Alterations are changes to a patient’s medical record after a psychiatrist receives notice of a lawsuit and “clarifies” certain points in the medical record to aid the defense against the claim.6 Alterations are considered deliberate misrepresentations of facts and, if discovered during litigation, can significantly impact the ability to defend against a claim.6 In addition, many medical liability policies exclude coverage for claims in which the medical record was altered, which might result in a psychiatrist having to pay for the judgment and defense costs out of pocket.6 Psychiatrists facing litigation who have a legitimate need to change an EHR entry after a claim is filed should consult with legal counsel or a risk management professional for guidance before making any changes.3 If they concur with updating the patient’s record to correct an error (including an addendum or a late entry; see below), the original entry, date, and time stamp must be accessible.3 This should also include the current date/time of the amended entry, the name of the person making the change, and the reasons for the change.3
Continue to: How to handle corrections and late entries
How to handle corrections and late entries
Sometimes situations occur that require us to make late entries, enter addendums, or add clarification notes to patient information in the EHRs. Regardless of your work environment (ie, hospital, your own practice), there should be clear procedures in place for correcting patients’ EHRs that are in accordance with applicable federal and state laws. Correcting an error in the EHR should follow the same basic principles of correcting paper records: do not obscure the original entry, make timely corrections, sign all entries, ensure the person making the change is identified, and document the reason(s) for the correction.7 The EHR must be able to track corrections or changes to an entry once they are entered or authenticated. Any physical copies of documentation must also have the same corrections or changes if they have been previously printed from the EHR.
You may need to make an entry that is late (out of sequence) or provides additional documentation to supplement previously written entries.7 A late entry should be used to record information when a pertinent entry was missed or not written in a timely manner.7 Label the new entry as a “late entry,” enter the current date and time (do not give the appearance that the entry was made on a previous date or at an earlier time), and identify or refer to the date and incident for which the late entry is written.7 If the late entry is used to document an omission, validate the source of additional information as best you can (ie, details of where you obtained the information to write the late entry).7 Make late entries as soon as possible after the original entry; although there is no time limit on writing a late entry, delays in corrections might diminish the credibility of the changes.
Addendums are used to provide additional information in conjunction with a previous entry.7 They also provide additional information to address a specific situation or incident referenced in a previous note. Addendums should not be used to document information that was forgotten or written in error.7 A clarification note is used to avoid incorrect interpretation of previously documented information.7 When writing an addendum or a clarification note, you should label it as an “addendum” or a “clarification note”; document the current date and time; state the reason for the addendum (referring back to the original entry) or clarification note (referring back to the entry being clarified); and identify any sources of information used to support an addendum or a clarification note.7
In 2009, the Health Information Technology for Economic and Clinical Health Act (HITECH Act), which is part of the American Recovery and Reinvestment Act, provided several billion dollars of grants and incentives to stimulate the implementation of electronic health records (EHRs) and supporting technology in the United States.1 Since then, almost all health care organizations have employed EHRs and supporting technologies. Unfortunately, this has created new liability risks. One potential risk is that in malpractice claims, there is more discoverable evidence, including metadata, with which to prove the claims.2 In this article, I explain what metadata is and how it can be used in medical malpractice cases. In addition, because we cannot change metadata, I provide guidance on making corrections in your EHR do
What is metadata?
Metadata—commonly described as data about data—lurk behind the words and images we can see on our computer screens. Metadata can be conceptualized as data that provides details about the information we enter into a computer system, creating a permanent electronic footprint that can be used to track our activity.2,3 Examples of metadata include (but are not limited to) the user’s name, date and time of a record entry, changes or deletions made to the record, the date an entry was created or modified, annotations that the user added over a period of time, and any other data that the software captures without the user manually entering the information.3 Metadata is typically stored on a server or file that users cannot access, which ensures data integrity because a user cannot alter a patient’s medical record without those changes being captured.3
How metadata is used in malpractice claims
When a psychiatrist is sued for medical negligence, the integrity of the EHR is an important aspect of defending against the lawsuit. A plaintiff’s (patient’s) attorney can more readily discover changes to the patient’s medical record by requesting the metadata and having it analyzed by an information technology specialist. Because the computer system captures everything a user does, it is difficult to alter a patient’s record without being detected. Consequently, plaintiff attorneys frequently request metadata during discovery in the hopes of learning whether the defendant psychiatrist altered or attempted to hide information that was contained or missing from the original version of the medical record.3 If the medical record was revised at a time unrelated to the treatment, metadata can raise suspicion of deception, even in the absence of wrongdoing.2 Alternatively, metadata can be used to validate that the EHR was changed when treatment occurred, which can bolster a defendant psychiatrist’s ability to rely on the EHR against a claim of medical negligence.2
Depending on the jurisdiction, metadata may or may not be discoverable. The Federal Rules of Civil Procedure emphasize producing documents in their original format.4 For federal cases, these rules suggest that the parties discuss discovery of this material when they are initially conferring; however, the rules do not specify whether a party must produce metadata, which leaves the courts to refine these rules through case law.4,5 In one case, a federal court ruled that a party had to produce documents with metadata intact.5 Without an agreement between both parties to exclude metadata from produced documents, the parties must produce the metadata.5 State laws differ in regards to the discoverability of metadata.
Corrections vs alterations
A patient’s medical record is the best evidence of the care we provided, should that care ever be challenged in court. We can preserve the medical record’s effectiveness through appropriate changes to it. Appropriately executed corrections are a normal part of documentation, whereas alterations to the medical record can cast doubt on our credibility and lead an otherwise defensible case to require a settlement.6
Corrections are changes to a patient’s medical record during the normal course of treatment.6 These are acceptable, provided the changes are made appropriately. Health care facilities and practices have their own policies for making appropriate corrections and addendums to the medical record. Once a correction and/or addendum is made, do not remove or delete the erroneous entry, because health care colleagues may have relied on it, and deleting an erroneous entry also would alter the integrity of the medical record.6 When done appropriately, corrections will not be misconstrued as alterations.
Alterations are changes to a patient’s medical record after a psychiatrist receives notice of a lawsuit and “clarifies” certain points in the medical record to aid the defense against the claim.6 Alterations are considered deliberate misrepresentations of facts and, if discovered during litigation, can significantly impact the ability to defend against a claim.6 In addition, many medical liability policies exclude coverage for claims in which the medical record was altered, which might result in a psychiatrist having to pay for the judgment and defense costs out of pocket.6 Psychiatrists facing litigation who have a legitimate need to change an EHR entry after a claim is filed should consult with legal counsel or a risk management professional for guidance before making any changes.3 If they concur with updating the patient’s record to correct an error (including an addendum or a late entry; see below), the original entry, date, and time stamp must be accessible.3 This should also include the current date/time of the amended entry, the name of the person making the change, and the reasons for the change.3
Continue to: How to handle corrections and late entries
How to handle corrections and late entries
Sometimes situations occur that require us to make late entries, enter addendums, or add clarification notes to patient information in the EHRs. Regardless of your work environment (ie, hospital, your own practice), there should be clear procedures in place for correcting patients’ EHRs that are in accordance with applicable federal and state laws. Correcting an error in the EHR should follow the same basic principles of correcting paper records: do not obscure the original entry, make timely corrections, sign all entries, ensure the person making the change is identified, and document the reason(s) for the correction.7 The EHR must be able to track corrections or changes to an entry once they are entered or authenticated. Any physical copies of documentation must also have the same corrections or changes if they have been previously printed from the EHR.
You may need to make an entry that is late (out of sequence) or provides additional documentation to supplement previously written entries.7 A late entry should be used to record information when a pertinent entry was missed or not written in a timely manner.7 Label the new entry as a “late entry,” enter the current date and time (do not give the appearance that the entry was made on a previous date or at an earlier time), and identify or refer to the date and incident for which the late entry is written.7 If the late entry is used to document an omission, validate the source of additional information as best you can (ie, details of where you obtained the information to write the late entry).7 Make late entries as soon as possible after the original entry; although there is no time limit on writing a late entry, delays in corrections might diminish the credibility of the changes.
Addendums are used to provide additional information in conjunction with a previous entry.7 They also provide additional information to address a specific situation or incident referenced in a previous note. Addendums should not be used to document information that was forgotten or written in error.7 A clarification note is used to avoid incorrect interpretation of previously documented information.7 When writing an addendum or a clarification note, you should label it as an “addendum” or a “clarification note”; document the current date and time; state the reason for the addendum (referring back to the original entry) or clarification note (referring back to the entry being clarified); and identify any sources of information used to support an addendum or a clarification note.7
1. American Recovery and Reinvestment Act of 2009. Pub L No. 111-5, 123 Stat 115 (2009).
2. Paterick ZR, Patel NJ, Ngo E, et al. Medical liability in the electronic medical records era. Proc (Bayl Univ Med Cent). 2018;31(4):558-561.
3. Funicelli A. ‘Hidden’ information in your EHRs could increase your liability risk. Psychiatric News. 2019;54(18):12-13.
4. Federal Rules of Civil Procedure, 26(f), 115th Cong, 1st Sess (2017).
5. Williams v Sprint/United Mgmt Co, 230 FRD 640 (D Kan 2005).
6. Ryan ML. Making changes to a medical record: corrections vs. alterations. NORCAL Mutual Insurance Company. Accessed February 3, 2021. http://www.sccma.org/Portals/19/Making%20Changes%20to%20a%20Medical%20Record.pdf
7. AHIMA’s long-term care health information practice and documentation guidelines. The American Health Information Management Association. Published 2014. Accessed February 3, 2021. http://bok.ahima.org/Pages/Long%20Term%20Care%20Guidelines%20TOC/Legal%20Documentation%20Standards/Legal%20Guidelines
1. American Recovery and Reinvestment Act of 2009. Pub L No. 111-5, 123 Stat 115 (2009).
2. Paterick ZR, Patel NJ, Ngo E, et al. Medical liability in the electronic medical records era. Proc (Bayl Univ Med Cent). 2018;31(4):558-561.
3. Funicelli A. ‘Hidden’ information in your EHRs could increase your liability risk. Psychiatric News. 2019;54(18):12-13.
4. Federal Rules of Civil Procedure, 26(f), 115th Cong, 1st Sess (2017).
5. Williams v Sprint/United Mgmt Co, 230 FRD 640 (D Kan 2005).
6. Ryan ML. Making changes to a medical record: corrections vs. alterations. NORCAL Mutual Insurance Company. Accessed February 3, 2021. http://www.sccma.org/Portals/19/Making%20Changes%20to%20a%20Medical%20Record.pdf
7. AHIMA’s long-term care health information practice and documentation guidelines. The American Health Information Management Association. Published 2014. Accessed February 3, 2021. http://bok.ahima.org/Pages/Long%20Term%20Care%20Guidelines%20TOC/Legal%20Documentation%20Standards/Legal%20Guidelines
Home pregnancy tests—Is ectopic always on your mind?
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
CASE Unidentified ectopic pregnancy leads to rupture*
A 33-year-old woman (G1 P0010) with 2 positive home pregnancy tests presents to the emergency department (ED) reporting intermittent vaginal bleeding for 3 days. Her last menstrual period was 10 weeks ago, but she reports that her menses are always irregular. She has a history of asymptomatic chlamydia, as well as spontaneous abortion 2 years prior. At present, she denies abdominal pain or vaginal discharge.
Upon examination her vital signs are: temperature, 98.3 °F; pulse, 112 bpm, with a resting rate of 16 bpm; blood pressure (BP), 142/91 mm Hg; pulse O2, 99%; height, 4’ 3”; weight, 115 lb. Her labs are: hemoglobin, 12.1 g/dL; hematocrit, 38%; serum human chorionic gonadotropin (hCG) 236 mIU/mL. Upon pelvic examination, no active bleeding is noted. She agrees to be followed up by her gynecologist and is given a prescription for serum hCG in 2 days. She is instructed to return to the ED should she have pain or increased vaginal bleeding.
Three days later, the patient follows up with her gynecologist reporting mild cramping. She notes having had an episode of heavy vaginal bleeding and a “weakly positive” home pregnancy test. Transvaginal ultrasonography notes endometrial thickness 0.59 mm and unremarkable adnexa. A urine pregnancy test performed in the office is positive; urinalysis is positive for nitrites. With the bleeding slowed, the gynecologist’s overall impression is that the patient has undergone complete spontaneous abortion. She prescribes Macrobid for the urinary tract infection. She does not obtain the ED-prescribed serum HCG levels, as she feels, since complete spontaneous abortion has occurred there is no need to obtain a follow-up serum HCG.
Five days later, the patient returns to the ED reporting abdominal pain after eating. Fever and productive cough of 2 days are noted. The patient states that she had a recent miscarriage. The overall impression of the patient’s condition is bronchitis, and it is noted on the patient’s record, “unlikely ectopic pregnancy and pregnancy test may be false positive,” hence a pregnancy test is not ordered. Examination reveals mild suprapubic tenderness with no rebound; no pelvic exam is performed. The patient is instructed to follow up with a health care clinic within a week, and to return to the ED with severe abdominal pain, higher fever, or any new concerning symptoms. A Zithromax Z-pak is prescribed.
Four days later, the patient is brought by ambulance to the ED of the local major medical center with severe abdominal pain involving the right lower quadrant. She states that she had a miscarriage 3 weeks prior and was recently treated for bronchitis. She has dizziness when standing. Her vital signs are: temperature, 97.8 °F; heart rate, 95 bpm; BP, 72/48 mm Hg; pulse O2, 100%. She reports her abdominal pain to be 6/10.
The patient is given a Lactated Ringer’s bolus of 1,000 mL for a hypotensive episode. Computed tomography is obtained and notes, “low attenuation in the left adnexa with a dilated fallopian tube.” A large heterogeneous collection of fluid in the pelvis is noted with active extravasation, consistent with an “acute bleed.”
The patient is brought to the operating room with a diagnosis of probable ruptured ectopic pregnancy. Intraoperatively she is noted to have a right ruptured ectopic and left tubo-ovarian abscess. The surgeon proceeds with right salpingectomy and left salpingo-oophorectomy. Three liters of hemoperitoneum is found.
She is followed postoperatively with serum hCG until levels are negative. Her postoperative course is uneventful. Her only future option for pregnancy is through assisted reproductive technology (ART) with in vitro fertilization (IVF). The patient sues the gynecologist and second ED physician for presumed inappropriate assessment for ectopic pregnancy.
*The “facts” of this case are a composite, drawn from several cases to illustrate medical and legal issues. The statement of facts should be considered hypothetical.
Continue to: WHAT’S THE VERDICT?...
WHAT’S THE VERDICT?
A defense verdict is returned.
Medical considerations
The incidence of ectopic pregnancy is 2% of all pregnancies, with a higher incidence (about 4%) among infertility patients.1 Up to 10% of ectopic pregnancies have no symptoms.2
Clinical presentations. Classic signs of ectopic pregnancy include:
- abdominal pain
- vaginal bleeding
- late menses (often noted).
A recent case of ectopic pregnancy presenting with chest pain was reported.3 Clinicians must never lose site of the fact that ectopic pregnancy is the most common cause of maternal mortality in the first trimester, with an incidence of 1% to 10% of all first-trimester deaths.4
Risk factors include pelvic inflammatory disease, as demonstrated in the opening case. “The silent epidemic of chlamydia” comes to mind, and tobacco smoking can adversely affect tubal cilia, as can pelvic adhesions and/or prior tubal surgery. All of these factors can predispose a patient to ectopic pregnancy; in addition, intrauterine devices, endometriosis, tubal ligation (or ligation reversal), all can set the stage for an ectopic pregnancy.5 Appropriate serum hCG monitoring during early pregnancy can assist in sorting out pregnancies of unknown location (PUL; FIGURE). First trimester ultrasonography, at 5 weeks gestation, usually identifies early intrauterine gestation.

Imaging. With regard to pelvic sonography, the earliest sign of an intrauterine pregnancy (IUP) is a sac eccentrically located in the decidua.6 As the IUP progresses, it becomes equated with a “double decidual sign,” with double rings of tissue around the sac.6 If the pregnancy is located in an adnexal mass, it is frequently inhomogeneous or noncystic in appearance (ie, “the blob” sign); the positive predictive value (PPV) is 96%.2 The PPV of transvaginal ultrasound is 80%, as paratubal, paraovarian, ovarian cyst, and hydrosalpinx can affect the interpretation.7
Heterotopic pregnancy includes an intrauterine gestation and an ectopic pregnancy. This presentation includes the presence of a “pseudosac” in the endometrial cavity plus an extrauterine gestation. Heterotopic pregnancies have become somewhat more common as ART/IVF has unfolded, especially prior to the predominance of single embryo transfer.
Managing ectopic pregnancy
For cases of early pregnancy complicated by intermittent bleeding and/or pain, monitoring with serum hCG levels at 48-hour intervals to distinguish a viable IUP from an abnormal IUP or an ectopic is appropriate. The “discriminatory zone” collates serum hCG levels with findings on ultrasonography. Specific lower limits of serum hCG levels are not clear cut, with recommendations of 3,500 mIU/mL to provide sonographic evidence of an intrauterine gestation “to avoid misdiagnosis and possible interruption of intrauterine pregnancy,” as conveyed in the American College of Obstetricians and Gynecologists 2018 practice bulletin.8 Serum progesterone levels also have been suggested to complement hCG levels; a progesterone level of <20 nmol/L is consistent with an abnormal pregnancy, whereas levels >25 nmol/L are suggestive of a viable pregnancy.2 Inhibin A levels also have been suggested to be helpful, but they are not an ideal monitoring tool.
While most ectopic pregnancies are located in the fallopian tube, other locations also can be abdominal or ovarian. In addition, cesarean scar ectopic pregnancy can occur and often is associated with delay in diagnosis and greater morbidity due to such delay.9 With regard to ovarian ectopic, Spiegelberg criteria are established for diagnosis (TABLE 1).10
Appropriate management of an ectopic pregnancy is dependent upon the gestational age, serum hCG levels, and imaging findings, as well as the patient’s symptoms and exam findings. Treatment is established in large part on a case-by-case basis and includes, for early pregnancy, expectant management and use of methotrexate (TABLE 2).11 Dilation and curettage may be used to identify the pregnancy’s location when the serum hCG level is below 2,000 mIU/mL and there is no evidence of an IUP on ultrasound. Surgical treatment can include minimally invasive salpingostomy or salpingectomy and, depending on circumstance, laparotomy may be indicated.

Fertility following ectopic pregnancy varies and is affected by location, treatment, predisposing factors, total number of ectopic pregnancies, and other factors. Ectopic pregnancy, although rare, also can occur with use of IVF. Humans are not unique with regard to ectopic pregnancies, as they also occur in sheep.12
Continue to: Legal perspective...
Legal perspective
Lawsuits related to ectopic pregnancy are not a new phenomenon. In fact, in 1897, a physician in Ohio who misdiagnosed an “extrauterine pregnancy” as appendicitis was the center of a malpractice lawsuit.13 Unrecognized or mishandled ectopic pregnancy can result in serious injuries—in the range of 1% to 10% (see above) of maternal deaths are related to ectopic pregnancy.14 Ectopic pregnancy cases, therefore, have been the subject of substantial litigation over the years. An informal, noncomprehensive review of malpractice lawsuits brought from 2000 to 2019, found more than 300 ectopic pregnancy cases. Given the large number of malpractice claims against ObGyns,15 ectopic pregnancy cases are only a small portion of all ObGyn malpractice cases.16
A common claim: negligent diagnosis or treatment
The most common basis for lawsuits in cases of ectopic pregnancy is the clinician’s negligent failure to properly diagnose the ectopic nature of the pregnancy. There are also a number of cases claiming negligent treatment of an identified ectopic pregnancy. Not every missed diagnosis, or unsuccessful treatment, leads to liability, of course. It is only when a diagnosis or treatment fails to meet the standard of care within the profession that there should be liability. That standard of care is generally defined by what a reasonably prudent physician would do under the circumstances. Expert witnesses, who are familiar with the standard of practice within the specialty, are usually necessary to establish what that practice is. Both the plaintiff and the defense obtain experts, the former to prove what the standard of care is and that the standard was not met in the case at hand. The defense experts are usually arguing that the standard of care was met.17 Inadequate diagnosis of ectopic pregnancy or other condition may arise from a failure to take a sufficient history, conduct an appropriately thorough physical examination, recognize any of the symptoms that would suggest it is present, use and conduct ultrasound correctly, or follow-up appropriately with additional testing.18
A malpractice claim of negligent treatment can involve any the following circumstances19:
- failure to establish an appropriate treatment plan
- prescribing inappropriate medications for the patient (eg, methotrexate, when it is contraindicated)
- delivering the wrong medication or the wrong amount of the right medication
- performing a procedure badly
- undertaking a new treatment without adequate instruction and preparation.
Given the nature and risks of ectopic pregnancy, ongoing, frequent contact with the patient is essential from the point at which the condition is suspected. The greater the risk of harm (probability or consequence), the more careful any professional ought to be. Because ectopic pregnancy is not an uncommon occurrence, and because it can have devastating effects, including death, a reasonably prudent practitioner would be especially aware of the clinical presentations discussed above.20 In the opening case, the treatment plan was not well documented.
Negligence must lead to patient harm. In addition to negligence (proving that the physician did not act in accordance with the standard of care), to prevail in a malpractice case, the plaintiff-patient must prove that the negligence caused the injury, or worsened it. If the failure to make a diagnosis would not have made any difference in a harm the patient suffered, there are no damages and no liability. Suppose, for example, that a physician negligently failed to diagnose ectopic pregnancy, but performed surgery expecting to find the misdiagnosed condition. In the course of the surgery, however, the surgeon discovered and appropriately treated the ectopic pregnancy. (A version of this happened in the old 19th century case mentioned above.) The negligence of the physician did not cause harm, so there are no damages and no liability.
Continue to: Informed consent is vital...
Informed consent is vital
A part of malpractice is informed consent (or the absence of it)—issues that can arise in any medical care.21 It is wise to pay particular attention in cases where the nature of the illness is unknown, and where there are significant uncertainties and the nature of testing and treatment may change substantially over a period of a few days or few weeks. As always, informed consent should include a discussion of what process or procedure is proposed, its risks and benefits, alternative approaches that might be available, and the risk of doing nothing. Frequently, the uncertainty of ectopic pregnancy complicates the informed consent process.22
Because communication with the patient is an essential function of informed consent, the consent process should productively be used in PUL and similar cases to inform the patient about the uncertainty, and the testing and (nonsurgical) treatment that will occur. This is an opportunity to reinforce the message that the patient must maintain ongoing communication with the physician’s office about changes in her condition, and appear for each appointment scheduled. If more invasive procedures—notably surgery—become required, a separate consent process should be completed, because the risks and considerations are now meaningfully different than when treatment began. As a general matter, any possible treatment that may result in infertility or reduced reproductive capacity should specifically be included in the consent process.
In the hypothetical case, the gynecologist failed to obtain a follow-up serum hCG level. In addition, the record did not reflect ectopic pregnancy in the differential diagnosis. As noted above, the patient had predisposing factors for an ectopic pregnancy. The physician should have acknowledged the history of sexually transmitted disease predisposing her to an ectopic pregnancy. Monitoring of serum hCG levels until they are negative is appropriate with ectopic, or presumed ectopic, pregnancy management. Appropriate monitoring did not occur in this case. Each of these errors (following up on serum hCG levels and the inadequacy of notations about the possibility of ectopic pregnancy) seem inconsistent with the usual standard of care. Furthermore, as a result of the outcome, the only future option for the patient to pursue pregnancy was IVF.
Other legal issues
There are a number of other legal issues that are associated with the topic of ectopic pregnancy. There is evidence, for example, that Catholic and non-Catholic hospitals treat ectopic pregnancies differently,23 which may reflect different views on taking a life or the use of methotrexate and its association with abortion.24 In addition, the possibility of an increase in future ectopic pregnancies is one of the “risks” of abortion that pro-life organizations have pushed to see included in abortion informed consent.25 This has led some commentators to conclude that some Catholic hospitals violate federal law in managing ectopic pregnancy. There is also evidence of “overwhelming rates of medical misinformation on pregnancy center websites, including a link between abortion and ectopic pregnancy.”26
The fact that cesarean deliveries are related to an increased risk for ectopic pregnancy (because of the risk of cesarean scar ectopic pregnancy) also has been cited as information that should play a role in the consent process for cesarean delivery.27 In terms of liability, failed tubal ligation leads to a 33% risk of ectopic pregnancy.28 The risk of ectopic pregnancy is also commonly included in surrogacy contracts.29
Why the outcome was for the defense
The opening hypothetical case illustrates some of the uncertainties of medical malpractice cases. As noted, there appeared a deviation from the usual standard of care, particularly the failure to follow up on the serum hCG level. The weakness in the medical record, failing to note the possibility of ectopic pregnancy, also was probably an error but, apparently, the court felt that this did not result in any harm to the patient.
The question arises of how there would be a defense verdict in light of the failure to track consecutive serum hCG levels. A speculative explanation is that there are many uncertainties in most lawsuits. Procedural problems may result in a case being limited, expert witnesses are essential to both the plaintiff and defense, with the quality of their review and testimony possibly uneven. Judges and juries may rely on one expert witness rather than another, juries vary, and the quality of advocacy differs. Any of these situations can contribute to the unpredictability of the outcome of a case. In the case above, the liability was somewhat uncertain, and the various other factors tipped in favor of a defense verdict. ●
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.
- Centers for Disease Control and Prevention. Ectopic pregnancy—United States, 1990‒1992. MMWR Morb Mortal Wkly Rep. 1995;44:46-48.
- Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2012;20:250-261.
- Dichter E, Espinosa J, Baird J, Lucerna A. An unusual emergency department case: ruptured ectopic pregnancy presenting as chest pain. World J Emerg Med. 2017;8:71-73.
- Cecchino GN, Araujo E, Elito J. Methotrexate for ectopic pregnancy: when and how. Arch Gynecol Obstet. 2014;290:417- 423.
- Barnhart KT, Sammel MD, Cracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic firsttrimester pregnancies. Fertil Steril. 2006;86:36-43.
- Carusi D. Pregnancy of unknown location: evaluation and management. Semin Perinatol. 2019;43:95-100.
- Barnhart KT, Fay CA, Suescum M, et al. Clinical factors affecting the accuracy of ultrasonography in symptomatic first-trimester pregnancy. Obstet Gynecol. 2011;117:299-306.
- American College of Obstetricians and Gynecologists Practice Bulletin No. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- Bouyer J, Coste J, Fernandez H, et al. Sites of ectopic pregnancy: a 10-year population-based study of 1800 cases. Hum Reprod. 2002;17:3224-3230.
- Spiegelberg O. Zur casuistic der ovarial schwangerschaft. Arch Gynecol. 1978;13:73.
- OB Hospitalist Group. Methotrexate use for ectopic pregnancies guidelines. https://www.obhg.com/wp-content /uploads/2020/01/Methotrexate-Use-for-EctopicPregnancies_2016-updates.pdf. Accessed December 10, 2020.
- Brozos C, Kargiannis I, Kiossis E, et al. Ectopic pregnancy through a caesarean scar in a ewe. N Z Vet J. 2013;61:373-375.
- Tucker v. Gillette, 12 Ohio Cir. Dec. 401 (Cir. Ct. 1901).
- Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366-373.
- Matthews LR, Alvi FA, Milad MP. Reproductive surgery malpractice patterns. Fertil Steril. 2016;106:e42-e43.
- Kim B. The impact of malpractice risk on the use of obstetrics procedures. J Legal Studies. 2006;36:S79-S120.
- Abinader R, Warsof S. Complications involving obstetrical ultrasound. In: Warsof S, Shwayder JM, eds. Legal Concepts and Best Practices in Obstetrics: The Nuts and Bolts Guide to Mitigating Risk. 2019;45-48.
- Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980-2007. Obstet Gynecol. 2011;117:837-843.
- Shwayder JM. IUP diagnosed and treated as ectopic: How bad can it get? Contemporary OB/GYN. 2019;64:49-46.
- Kaplan AI. Should this ectopic pregnancy have been diagnosed earlier? Contemporary OB/GYN. 2017;62:53.
- American College of Obstetricians and Gynecologists Committee on Ethics. Committee opinion 439: informed consent. Reaffirmed 2015. https://www.acog.org/clinical /clinical-guidance/committee-opinion/articles/2009/08 /informed-consent. Accessed December 9, 2020.
- Shwayder JM. Liability in ob/gyn ultrasound. Contemporary OB/GYN. 2017;62:32-49.
- Fisher LN. Institutional religious exemptions: a balancing approach. BYU Law Review. 2014;415-444.
- Makdisi J. Aquinas’s prohibition of killing reconsidered. J Catholic Legal Stud. 2019:57:67-128.
- Franzonello A. Remarks of Anna Franzonello. Alb Law J Sci Tech. 2012;23:519-530.
- Malcolm HE. Pregnancy centers and the limits of mandated disclosure. Columbia Law Rev. 2019;119:1133-1168.
- Kukura E. Contested care: the limitations of evidencebased maternity care reform. Berkeley J Gender Law Justice. 2016;31:241-298.
- Donley G. Contraceptive equity: curing the sex discrimination in the ACA’s mandate. Alabama Law Rev. 2019;71:499-560.
- Berk H. Savvy surrogates and rock star parents: compensation provisions, contracting practices, and the value of womb work. Law Social Inquiry. 2020;45:398-431.