User login
Pityriasis Amiantacea Following Bone Marrow Transplant
Pityriasis amiantacea (PA) is characterized by adherence of hair shafts proximally.1 It has been associated with dermatologic conditions and rarely with medications. We describe a woman who developed PA following a bone marrow transplant with melphalan conditioning. We also review drug-induced PA and disorders that have been linked to this condition.
Case Report
A 67-year-old woman with a history of multiple myeloma was treated with 7 courses of chemotherapy (cyclophosphamide, bortezomib, prednisone). One month later, the patient underwent a bone marrow transplant with melphalan conditioning due to residual plasma cell myeloma. Following the transplant, she developed complete scalp alopecia. Prior to and following transplant, the patient’s hair care regimen included washing her hair and scalp every other day with over-the-counter “natural” shampoos. During drug-induced alopecia, the hair washing became less frequent.
The patient left the hospital 4 weeks posttransplant; her hair had started to regrow, but its appearance was altered. Posttransplant, the patient was maintained on bortezomib every other week and zoledronate once per month. She continued to develop multiple lesions in the scalp hairs during the following 4 months.
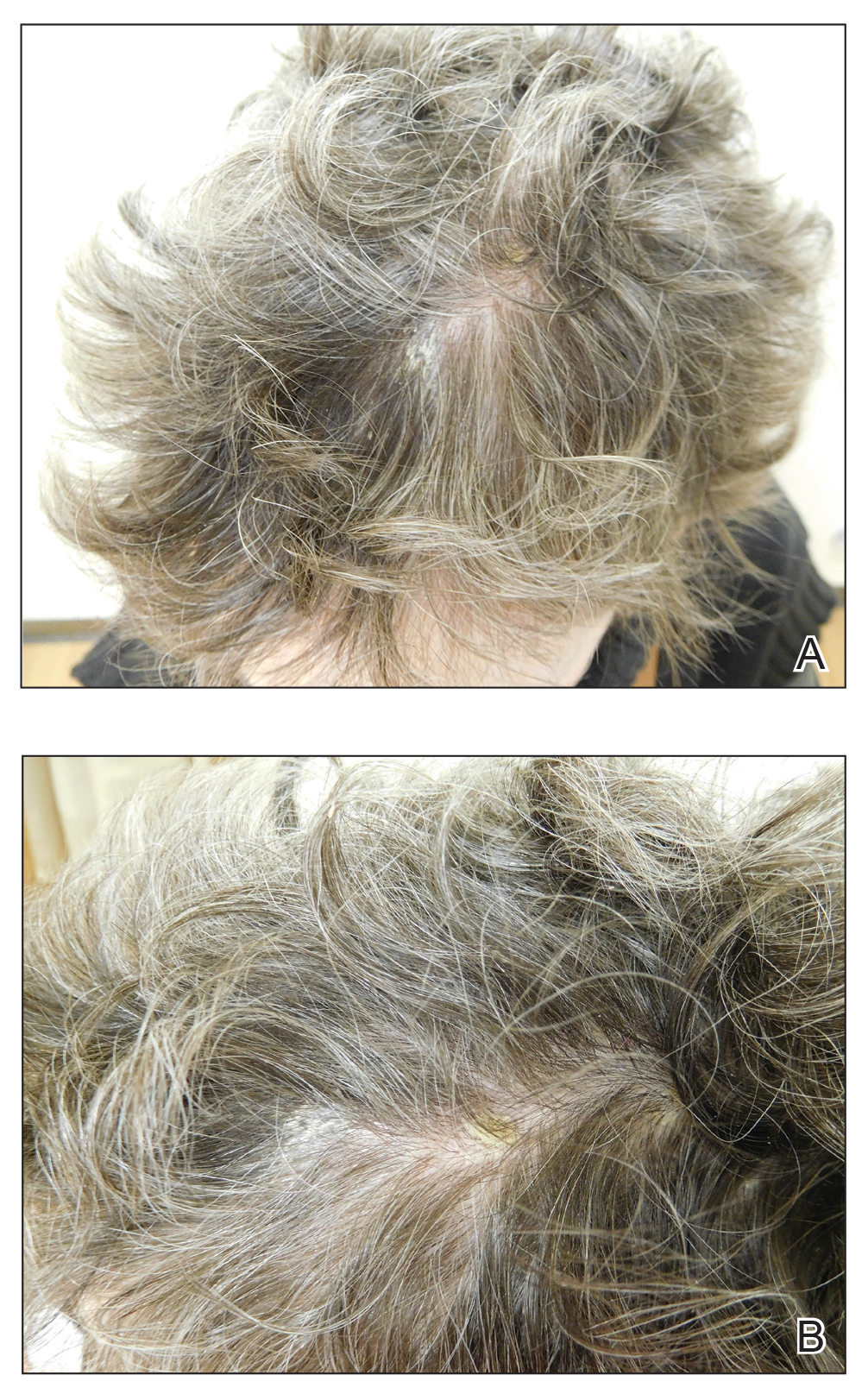
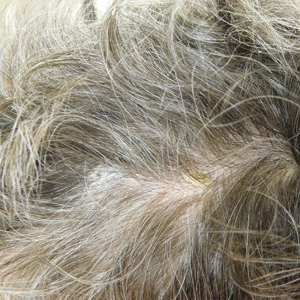
Eight months posttransplant she presented for evaluation of the scalp hair. Clinical examination showed hairs that were entwined together proximally, resulting in matting of the hair (Figure 1). A diagnosis of PA was established based on the clinical examination.
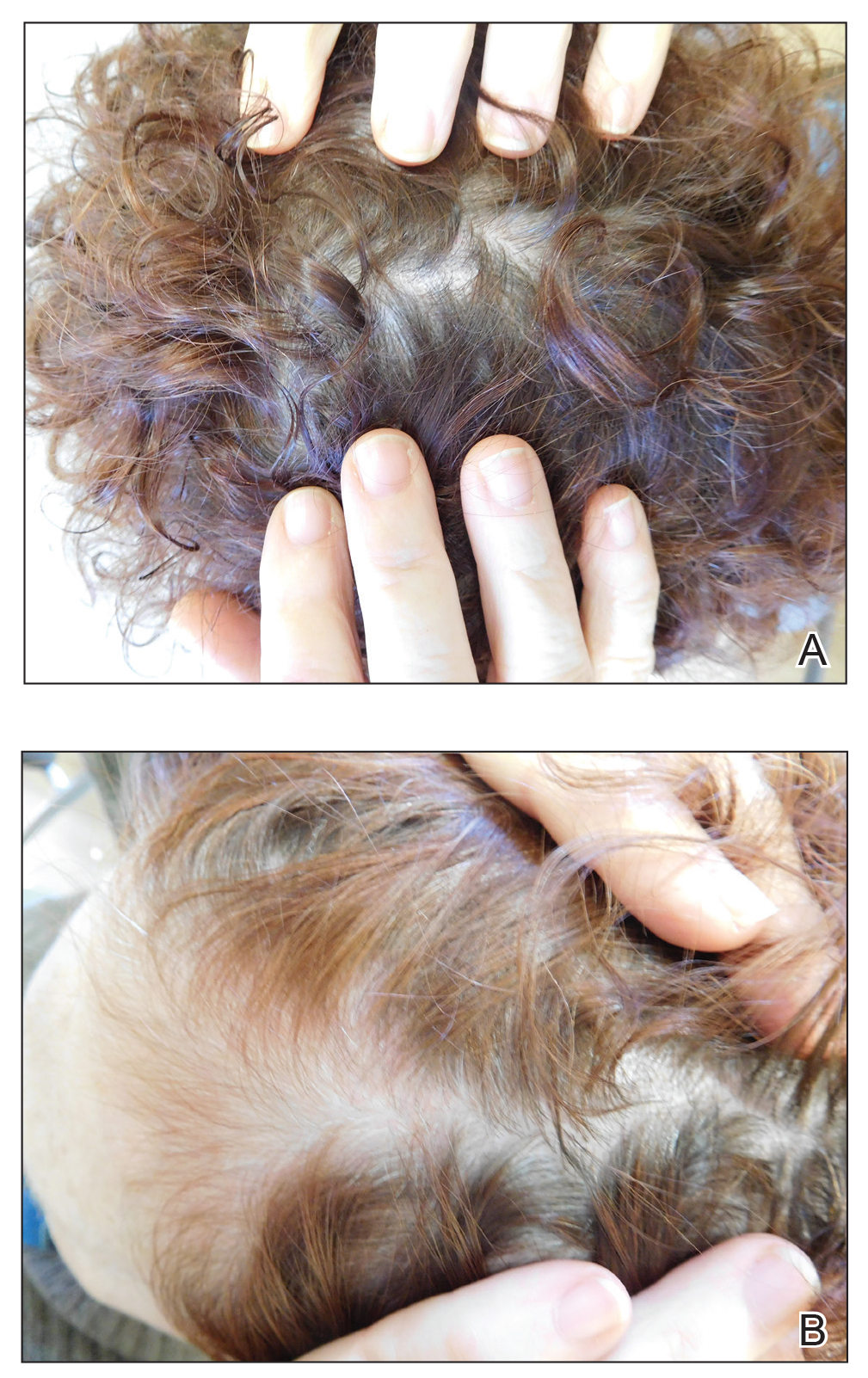
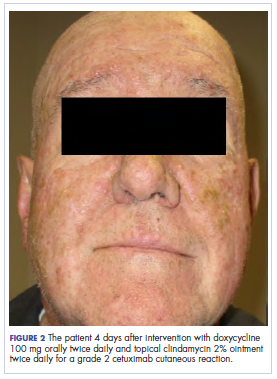
Treatment included mineral oil application to the scalp under occlusion each evening, followed by morning washing with coal tar 0.5%, salicylic acid 6%, or ketoconazole 2% shampoo in a repeating sequential manner. Within 1 month there was complete resolution of the scalp condition (Figure 2).
Comment
Clinical Presentation
Pityriasis amiantacea is characterized by thick excessive scale of the scalp1; it was initially described by Alibert2 in 1832. He described the gross appearance of the scales as resembling the feathers of young birds, which naturalists dub “amiante” or asbestoslike.1,2 In 1917, Gougerot3 explored infectious etiologies of this condition by describing cases of impetigo that transitioned into PA.1 Later, in 1929, Photinos4 described fungal origins of PA, giving credence to “tinea amiantacea.”1 However, more recent analyses failed to isolate fungus.5-7 As such, pityriasis (scaling) amiantacea is the more appropriate term, as emphasized by Brown8 in 1948. The cause of PA remains unclear; it is hypothesized that the condition is a reaction to underlying inflammatory dermatoses, though concurrent bacterial or fungal infection may be present.5,9
Prevalence
Pityriasis amiantacea is considered to be most prevalent in pediatric patients and young adults; it is more common in females.1,9,10 In a review of 85 PA patients, more than 80% were women (n=69), and the mean age at presentation was 23.8 years. Approximately half of these patients had widespread scalp lesions (n=42); however, focal localized lesions were common.9 No hereditary patterns have been described, though 3 pairs of the 10 patients with PA in Ring and Kaplan’s7 review were siblings.
Clinical Findings
Clinically, lesions of PA present as matted hairs.1 Thick scales encompass multiple hair shafts, binding down tufts of hair.1,6,11 Patients are asymptomatic, though the lesions may be accompanied by pruritus. The hairs enclosed by the scales in some cases may be easily pulled out.6 Notably, alopecia often accompanies PA; it often is reversible, but in some cases, it is permanent and can lead to scarring.9,12
Histopathology
Submission of hair specimens to histopathology usually is not performed since the diagnosis often is established based on the clinical presentation.5 However, submitted specimens have demonstrated spongiosis and parakeratosis along with reduction in the size of the sebaceous glands.1,9 Additionally, follicular keratosis that surrounds the hair shafts with a sheath of horn is present.9 Acanthosis and migration of lymphocytes into the epidermis also have been found.1 Often, Staphylococcus aureus isolates are detected.9,13
Differential Diagnosis
The clinical differential diagnosis of PA includes hair casts,11 pediculosis,14 and tinea capitis.12 In PA, thick scales surround hair shafts and thus bind down tufts of hair.9 In patients with pediculosis, nits are attached to the hair shaft at an angle and do not entirely envelop the hair shaft.14 In addition, PA may be complicated by impetiginization; bacteria often are found in the keratin surrounding the hair shaft and represent either normal flora or secondary infection.1,15 It has been speculated that microbial biofilms from S aureus and Staphylococcus epidermidis promote agglomeration of hair shafts and adherent scale.16 Bona fide dermatophyte infection of the scalp also may be concurrently present.12
Treatment
Our treatment included occlusion with mineral oil to loosen the scales from the scalp in tandem with shampoos traditionally used in patients with seborrheic dermatitis or psoriasis. Timely treatment is important to prevent scarring alopecia.13,17 Pityriasis amiantacea may be treatment resistant, and there are no specific therapeutic guidelines; rather, therapy should be targeted at the suspected underlying condition.17 Treatment generally includes keratolytic agents, such as salicylic acid.18 These agents allow enhanced penetration of other topical agents.19 Topical antifungal shampoos such as ketoconazole and ciclopirox are recommended,18 though other topical agents, such as coal tar and zinc pyrithione, also may benefit patients.13 Topical corticosteroids may be used if the condition is linked with psoriasis.13 Systemic antibiotics are added if S aureus superinfection is suspected.9
A single report described successful management of a patient with severe refractory PA who was treated with the tumor necrosis factor (TNF) α inhibitor infliximab.13 A 47-year-old woman presented with thick adherent scale on the scalp. She was treated with coal tar for 18 months but showed no improvement; the patient was subsequently prescribed salicylic acid 10%, clobetasol solution, and coal tar shampoo. After 3 months, when no improvement was observed, the patient was offered infliximab but declined. For 6 years the patient was treated with salicylic acid 20%, clobetasol (foam, lotion, shampoo, and solution), and coal tar shampoo without improvement. She then consented to infliximab therapy; after 3 infusions at weeks 0, 2, and 6, she demonstrated notable improvement. The patient was maintained on infliximab every 8 weeks.13
Pathogenesis
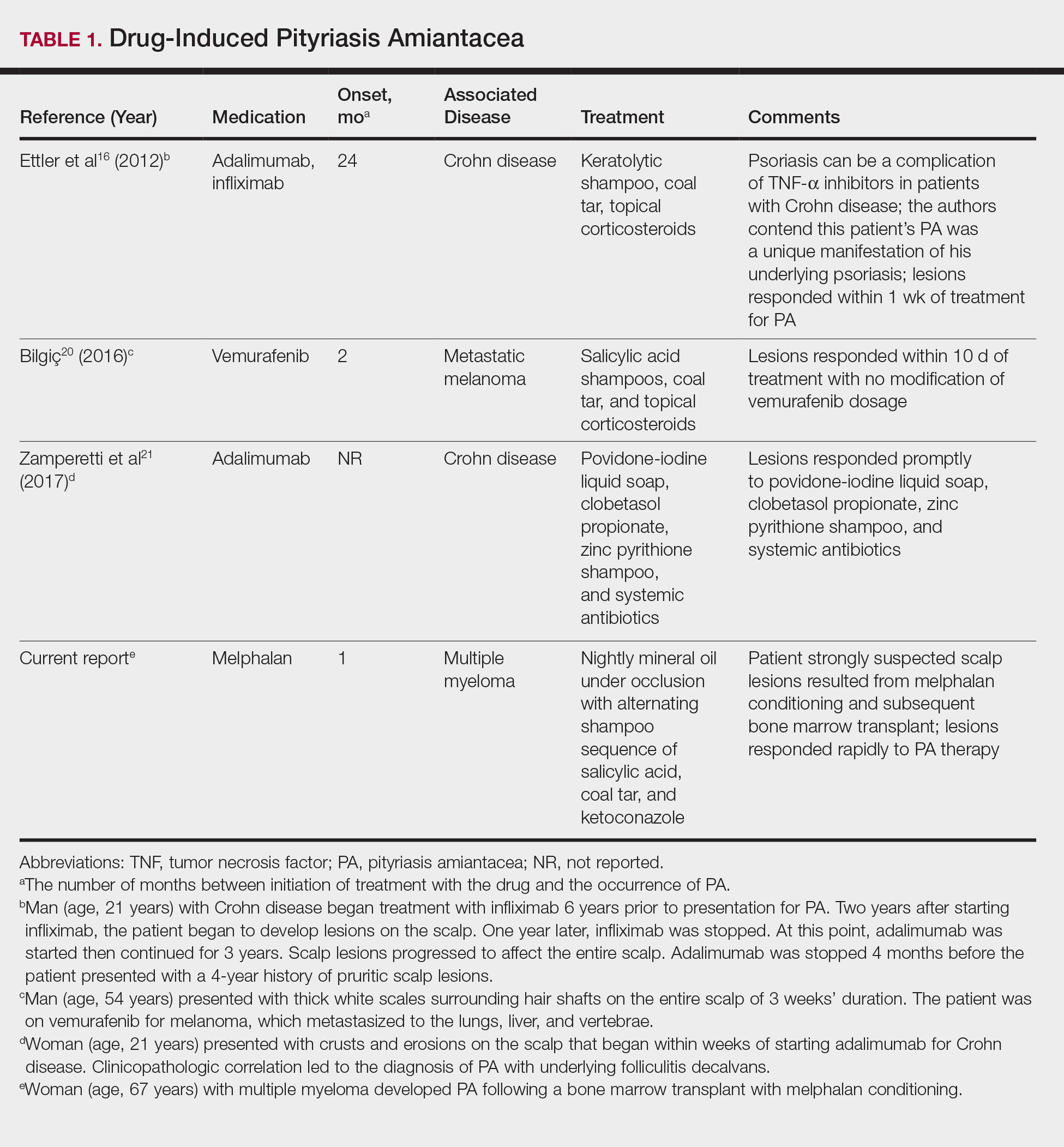
The pathogenesis of PA has yet to be definitively established, and the condition is usually idiopathic. In addition to bacterial or fungal etiologies,3,4 PA has been linked to medications (Table 1)16,20,21 and systemic conditions (Table 2).1,3,5,7-10,12,22-25
A PubMed search of articles indexed for MEDLINE using the search terms amiantacea, bone, drug, hair marrow, malignancy, melphalan, pityriasis, tinea, and transplant yielded 4 patients—2 men and 2 women (including our patient)—with possible drug-induced PA (Table 1)16,20,21; however, the onset after 2 years of medication (TNF-α inhibitors) or resolution while still receiving the agent (vemurafenib) makes the drug-induced linkage weak. The patients ranged in age from 21 to 67 years, with the median age being 37.5 years. Medications included melphalan, TNF-α inhibitors (adalimumab, infliximab),16,21 and vemurafenib20; it is interesting that infliximab was the medication associated with eliciting PA in 1 patient yet was an effective therapy in another patient with treatment-resistant PA. The onset of PA occurred between 1 month (melphalan) and 24 months (TNF-α inhibitors) after drug initiation. The patients’ associated diseases included Crohn disease,16,21 metastatic melanoma,20 and multiple myeloma.
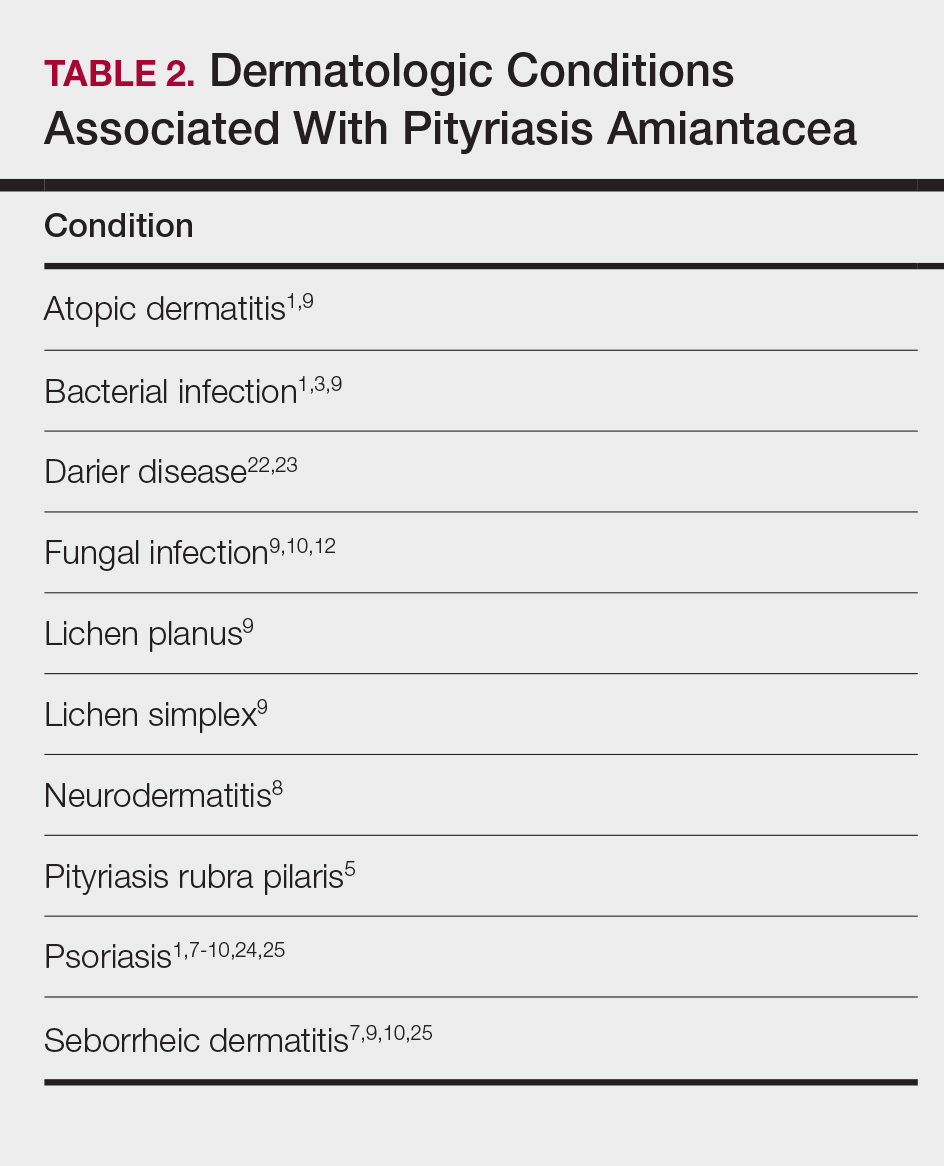
Other conditions have been described in patients with PA (Table 2). Indeed, PA may be a manifestation of an underlying inflammatory skin disease.9 In addition to dermatologic conditions, procedures or malignancy may be associated with the disease, as demonstrated in our patient. Most commonly, PA is seen in association with psoriasis and seborrheic dermatitis; atopic dermatitis, bacterial infection, fungal infection, lichen planus, and neurodermatitis also have been associated with PA.1,3,5,7-10,12,18,22-25
Conclusion
Pityriasis amiantacea is a benign condition affecting the scalp hair. Albeit uncommon, it may appear in patients treated with medications such as melphalan, TNF-α inhibitors, and vemurafenib. In addition, it has been described in individuals with dermatologic conditions, systemic procedures, or underlying malignancy. Our patient developed PA following a bone marrow transplant after receiving conditioning with melphalan.
- Knight AG. Pityriasis amiantacea: a clinical and histopathological investigation. Clin Exp Dermatol. 1977;2:137-143.
- Alibert JL. De la porrigine amiantacée. In: Monographie des Dermatoses. Paris, France: Baillère; 1832:293-295.
- Gougerot H. La teigne amiantacee D’Alibert. Progres Medical. 1917;13:101-104.
- Photinos P. Recherches sur la fausse teigne amiantacée. Ann Dermatol Syphiligr. 1929;10:743-758.
- Verardino GC, Azulay-Abulafia L, Macedo PM, et al. Pityriasis amiantacea: clinical-dermatoscopic features and microscopy of hair tufts. An Bras Dermatol. 2012;87:142-145.
- Keipert JA. Greasy scaling pityriasis amiantacea and alopecia: a syndrome in search of a cause. Australas J Dermatol. 1985;26:41-44.
- Ring DS, Kaplan DL. Pityriasis amiantacea: a report of 10 cases. Arch Dermatol. 1993;129:913-914.
- Brown WH. Some observations on neurodermatitis of the scalp, with particular reference to tinea amiantacea. Br J Dermatol Syph. 1948;60:81-90.
- Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
- Becker SW, Muir KB. Tinea amiantacea. Arch Dermatol Syphil. 1929;20:45-53.
- Dawber RP. Hair casts. Br J Dermatol. 1979;100:417-421.
- Ginarte M, Pereiro M, Fernández-Redondo V, et al. Case reports. pityriasis amiantacea as manifestation of tinea capitis due to Microsporum canis. Mycoses. 2000;43:93-96.
- Pham RK, Chan CS, Hsu S. Treatment of pityriasis amiantacea with infliximab. Dermatol Online J. 2009;15:13.
- Roberts RJ. Clinical practice. Head lice. N Engl J Med. 2002;346:1645-1650.
- Mcginley KJ, Leyden JJ, Marples RR, et al. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975;64:401-405.
- Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641.
- Mannino G, McCaughey C, Vanness E. A case of pityriasis amiantacea with rapid response to treatment. WMJ. 2014;113:119-120.
- Jamil A, Muthupalaniappen L. Scales on the scalp. Malays Fam Physician. 2013;8:48-49.
- Gupta LK, Khare AK, Masatkar V, et al. Pityriasis amiantacea. Indian Dermatol Online J. 2014;5(suppl 1):S63-S64.
- Bilgiç Ö. Vemurafenib-induced pityriasis amiantacea: a case report. Cutan Ocul Toxicol. 2016;35:329-331.
- Zamperetti M, Zelger B, Höpfl R. Pityriasis amiantacea and folliculitis decalvans: an unusual manifestation associated with antitumor necrosis factor-α therapy. Hautarzt. 2017;68:1007-1010.
- Udayashankar C, Nath AK, Anuradha P. Extensive Darier’s disease with pityriasis amiantacea, alopecia and congenital facial nerve palsy. Dermatol Online J. 2013;19:18574.
- Hussain W, Coulson IH, Salman WD. Pityriasis amiantacea as the sole manifestation of Darier’s disease. Clin Exp Dermatol. 2009;34:554-556.
- Hansted B, Lindskov R. Pityriasis amiantacea and psoriasis. a follow-up study. Dermatologica. 1983;166:314-315.
- Hersle K, Lindholm A, Mobacken H, et al. Relationship of pityriasis amiantacea to psoriasis. a follow-up study. Dermatologica. 1979;159:245-250.
Pityriasis amiantacea (PA) is characterized by adherence of hair shafts proximally.1 It has been associated with dermatologic conditions and rarely with medications. We describe a woman who developed PA following a bone marrow transplant with melphalan conditioning. We also review drug-induced PA and disorders that have been linked to this condition.
Case Report
A 67-year-old woman with a history of multiple myeloma was treated with 7 courses of chemotherapy (cyclophosphamide, bortezomib, prednisone). One month later, the patient underwent a bone marrow transplant with melphalan conditioning due to residual plasma cell myeloma. Following the transplant, she developed complete scalp alopecia. Prior to and following transplant, the patient’s hair care regimen included washing her hair and scalp every other day with over-the-counter “natural” shampoos. During drug-induced alopecia, the hair washing became less frequent.
The patient left the hospital 4 weeks posttransplant; her hair had started to regrow, but its appearance was altered. Posttransplant, the patient was maintained on bortezomib every other week and zoledronate once per month. She continued to develop multiple lesions in the scalp hairs during the following 4 months.
Eight months posttransplant she presented for evaluation of the scalp hair. Clinical examination showed hairs that were entwined together proximally, resulting in matting of the hair (Figure 1). A diagnosis of PA was established based on the clinical examination.
Treatment included mineral oil application to the scalp under occlusion each evening, followed by morning washing with coal tar 0.5%, salicylic acid 6%, or ketoconazole 2% shampoo in a repeating sequential manner. Within 1 month there was complete resolution of the scalp condition (Figure 2).
Comment
Clinical Presentation
Pityriasis amiantacea is characterized by thick excessive scale of the scalp1; it was initially described by Alibert2 in 1832. He described the gross appearance of the scales as resembling the feathers of young birds, which naturalists dub “amiante” or asbestoslike.1,2 In 1917, Gougerot3 explored infectious etiologies of this condition by describing cases of impetigo that transitioned into PA.1 Later, in 1929, Photinos4 described fungal origins of PA, giving credence to “tinea amiantacea.”1 However, more recent analyses failed to isolate fungus.5-7 As such, pityriasis (scaling) amiantacea is the more appropriate term, as emphasized by Brown8 in 1948. The cause of PA remains unclear; it is hypothesized that the condition is a reaction to underlying inflammatory dermatoses, though concurrent bacterial or fungal infection may be present.5,9
Prevalence
Pityriasis amiantacea is considered to be most prevalent in pediatric patients and young adults; it is more common in females.1,9,10 In a review of 85 PA patients, more than 80% were women (n=69), and the mean age at presentation was 23.8 years. Approximately half of these patients had widespread scalp lesions (n=42); however, focal localized lesions were common.9 No hereditary patterns have been described, though 3 pairs of the 10 patients with PA in Ring and Kaplan’s7 review were siblings.
Clinical Findings
Clinically, lesions of PA present as matted hairs.1 Thick scales encompass multiple hair shafts, binding down tufts of hair.1,6,11 Patients are asymptomatic, though the lesions may be accompanied by pruritus. The hairs enclosed by the scales in some cases may be easily pulled out.6 Notably, alopecia often accompanies PA; it often is reversible, but in some cases, it is permanent and can lead to scarring.9,12
Histopathology
Submission of hair specimens to histopathology usually is not performed since the diagnosis often is established based on the clinical presentation.5 However, submitted specimens have demonstrated spongiosis and parakeratosis along with reduction in the size of the sebaceous glands.1,9 Additionally, follicular keratosis that surrounds the hair shafts with a sheath of horn is present.9 Acanthosis and migration of lymphocytes into the epidermis also have been found.1 Often, Staphylococcus aureus isolates are detected.9,13
Differential Diagnosis
The clinical differential diagnosis of PA includes hair casts,11 pediculosis,14 and tinea capitis.12 In PA, thick scales surround hair shafts and thus bind down tufts of hair.9 In patients with pediculosis, nits are attached to the hair shaft at an angle and do not entirely envelop the hair shaft.14 In addition, PA may be complicated by impetiginization; bacteria often are found in the keratin surrounding the hair shaft and represent either normal flora or secondary infection.1,15 It has been speculated that microbial biofilms from S aureus and Staphylococcus epidermidis promote agglomeration of hair shafts and adherent scale.16 Bona fide dermatophyte infection of the scalp also may be concurrently present.12
Treatment
Our treatment included occlusion with mineral oil to loosen the scales from the scalp in tandem with shampoos traditionally used in patients with seborrheic dermatitis or psoriasis. Timely treatment is important to prevent scarring alopecia.13,17 Pityriasis amiantacea may be treatment resistant, and there are no specific therapeutic guidelines; rather, therapy should be targeted at the suspected underlying condition.17 Treatment generally includes keratolytic agents, such as salicylic acid.18 These agents allow enhanced penetration of other topical agents.19 Topical antifungal shampoos such as ketoconazole and ciclopirox are recommended,18 though other topical agents, such as coal tar and zinc pyrithione, also may benefit patients.13 Topical corticosteroids may be used if the condition is linked with psoriasis.13 Systemic antibiotics are added if S aureus superinfection is suspected.9
A single report described successful management of a patient with severe refractory PA who was treated with the tumor necrosis factor (TNF) α inhibitor infliximab.13 A 47-year-old woman presented with thick adherent scale on the scalp. She was treated with coal tar for 18 months but showed no improvement; the patient was subsequently prescribed salicylic acid 10%, clobetasol solution, and coal tar shampoo. After 3 months, when no improvement was observed, the patient was offered infliximab but declined. For 6 years the patient was treated with salicylic acid 20%, clobetasol (foam, lotion, shampoo, and solution), and coal tar shampoo without improvement. She then consented to infliximab therapy; after 3 infusions at weeks 0, 2, and 6, she demonstrated notable improvement. The patient was maintained on infliximab every 8 weeks.13
Pathogenesis
The pathogenesis of PA has yet to be definitively established, and the condition is usually idiopathic. In addition to bacterial or fungal etiologies,3,4 PA has been linked to medications (Table 1)16,20,21 and systemic conditions (Table 2).1,3,5,7-10,12,22-25
A PubMed search of articles indexed for MEDLINE using the search terms amiantacea, bone, drug, hair marrow, malignancy, melphalan, pityriasis, tinea, and transplant yielded 4 patients—2 men and 2 women (including our patient)—with possible drug-induced PA (Table 1)16,20,21; however, the onset after 2 years of medication (TNF-α inhibitors) or resolution while still receiving the agent (vemurafenib) makes the drug-induced linkage weak. The patients ranged in age from 21 to 67 years, with the median age being 37.5 years. Medications included melphalan, TNF-α inhibitors (adalimumab, infliximab),16,21 and vemurafenib20; it is interesting that infliximab was the medication associated with eliciting PA in 1 patient yet was an effective therapy in another patient with treatment-resistant PA. The onset of PA occurred between 1 month (melphalan) and 24 months (TNF-α inhibitors) after drug initiation. The patients’ associated diseases included Crohn disease,16,21 metastatic melanoma,20 and multiple myeloma.
Other conditions have been described in patients with PA (Table 2). Indeed, PA may be a manifestation of an underlying inflammatory skin disease.9 In addition to dermatologic conditions, procedures or malignancy may be associated with the disease, as demonstrated in our patient. Most commonly, PA is seen in association with psoriasis and seborrheic dermatitis; atopic dermatitis, bacterial infection, fungal infection, lichen planus, and neurodermatitis also have been associated with PA.1,3,5,7-10,12,18,22-25
Conclusion
Pityriasis amiantacea is a benign condition affecting the scalp hair. Albeit uncommon, it may appear in patients treated with medications such as melphalan, TNF-α inhibitors, and vemurafenib. In addition, it has been described in individuals with dermatologic conditions, systemic procedures, or underlying malignancy. Our patient developed PA following a bone marrow transplant after receiving conditioning with melphalan.
Pityriasis amiantacea (PA) is characterized by adherence of hair shafts proximally.1 It has been associated with dermatologic conditions and rarely with medications. We describe a woman who developed PA following a bone marrow transplant with melphalan conditioning. We also review drug-induced PA and disorders that have been linked to this condition.
Case Report
A 67-year-old woman with a history of multiple myeloma was treated with 7 courses of chemotherapy (cyclophosphamide, bortezomib, prednisone). One month later, the patient underwent a bone marrow transplant with melphalan conditioning due to residual plasma cell myeloma. Following the transplant, she developed complete scalp alopecia. Prior to and following transplant, the patient’s hair care regimen included washing her hair and scalp every other day with over-the-counter “natural” shampoos. During drug-induced alopecia, the hair washing became less frequent.
The patient left the hospital 4 weeks posttransplant; her hair had started to regrow, but its appearance was altered. Posttransplant, the patient was maintained on bortezomib every other week and zoledronate once per month. She continued to develop multiple lesions in the scalp hairs during the following 4 months.
Eight months posttransplant she presented for evaluation of the scalp hair. Clinical examination showed hairs that were entwined together proximally, resulting in matting of the hair (Figure 1). A diagnosis of PA was established based on the clinical examination.
Treatment included mineral oil application to the scalp under occlusion each evening, followed by morning washing with coal tar 0.5%, salicylic acid 6%, or ketoconazole 2% shampoo in a repeating sequential manner. Within 1 month there was complete resolution of the scalp condition (Figure 2).
Comment
Clinical Presentation
Pityriasis amiantacea is characterized by thick excessive scale of the scalp1; it was initially described by Alibert2 in 1832. He described the gross appearance of the scales as resembling the feathers of young birds, which naturalists dub “amiante” or asbestoslike.1,2 In 1917, Gougerot3 explored infectious etiologies of this condition by describing cases of impetigo that transitioned into PA.1 Later, in 1929, Photinos4 described fungal origins of PA, giving credence to “tinea amiantacea.”1 However, more recent analyses failed to isolate fungus.5-7 As such, pityriasis (scaling) amiantacea is the more appropriate term, as emphasized by Brown8 in 1948. The cause of PA remains unclear; it is hypothesized that the condition is a reaction to underlying inflammatory dermatoses, though concurrent bacterial or fungal infection may be present.5,9
Prevalence
Pityriasis amiantacea is considered to be most prevalent in pediatric patients and young adults; it is more common in females.1,9,10 In a review of 85 PA patients, more than 80% were women (n=69), and the mean age at presentation was 23.8 years. Approximately half of these patients had widespread scalp lesions (n=42); however, focal localized lesions were common.9 No hereditary patterns have been described, though 3 pairs of the 10 patients with PA in Ring and Kaplan’s7 review were siblings.
Clinical Findings
Clinically, lesions of PA present as matted hairs.1 Thick scales encompass multiple hair shafts, binding down tufts of hair.1,6,11 Patients are asymptomatic, though the lesions may be accompanied by pruritus. The hairs enclosed by the scales in some cases may be easily pulled out.6 Notably, alopecia often accompanies PA; it often is reversible, but in some cases, it is permanent and can lead to scarring.9,12
Histopathology
Submission of hair specimens to histopathology usually is not performed since the diagnosis often is established based on the clinical presentation.5 However, submitted specimens have demonstrated spongiosis and parakeratosis along with reduction in the size of the sebaceous glands.1,9 Additionally, follicular keratosis that surrounds the hair shafts with a sheath of horn is present.9 Acanthosis and migration of lymphocytes into the epidermis also have been found.1 Often, Staphylococcus aureus isolates are detected.9,13
Differential Diagnosis
The clinical differential diagnosis of PA includes hair casts,11 pediculosis,14 and tinea capitis.12 In PA, thick scales surround hair shafts and thus bind down tufts of hair.9 In patients with pediculosis, nits are attached to the hair shaft at an angle and do not entirely envelop the hair shaft.14 In addition, PA may be complicated by impetiginization; bacteria often are found in the keratin surrounding the hair shaft and represent either normal flora or secondary infection.1,15 It has been speculated that microbial biofilms from S aureus and Staphylococcus epidermidis promote agglomeration of hair shafts and adherent scale.16 Bona fide dermatophyte infection of the scalp also may be concurrently present.12
Treatment
Our treatment included occlusion with mineral oil to loosen the scales from the scalp in tandem with shampoos traditionally used in patients with seborrheic dermatitis or psoriasis. Timely treatment is important to prevent scarring alopecia.13,17 Pityriasis amiantacea may be treatment resistant, and there are no specific therapeutic guidelines; rather, therapy should be targeted at the suspected underlying condition.17 Treatment generally includes keratolytic agents, such as salicylic acid.18 These agents allow enhanced penetration of other topical agents.19 Topical antifungal shampoos such as ketoconazole and ciclopirox are recommended,18 though other topical agents, such as coal tar and zinc pyrithione, also may benefit patients.13 Topical corticosteroids may be used if the condition is linked with psoriasis.13 Systemic antibiotics are added if S aureus superinfection is suspected.9
A single report described successful management of a patient with severe refractory PA who was treated with the tumor necrosis factor (TNF) α inhibitor infliximab.13 A 47-year-old woman presented with thick adherent scale on the scalp. She was treated with coal tar for 18 months but showed no improvement; the patient was subsequently prescribed salicylic acid 10%, clobetasol solution, and coal tar shampoo. After 3 months, when no improvement was observed, the patient was offered infliximab but declined. For 6 years the patient was treated with salicylic acid 20%, clobetasol (foam, lotion, shampoo, and solution), and coal tar shampoo without improvement. She then consented to infliximab therapy; after 3 infusions at weeks 0, 2, and 6, she demonstrated notable improvement. The patient was maintained on infliximab every 8 weeks.13
Pathogenesis
The pathogenesis of PA has yet to be definitively established, and the condition is usually idiopathic. In addition to bacterial or fungal etiologies,3,4 PA has been linked to medications (Table 1)16,20,21 and systemic conditions (Table 2).1,3,5,7-10,12,22-25
A PubMed search of articles indexed for MEDLINE using the search terms amiantacea, bone, drug, hair marrow, malignancy, melphalan, pityriasis, tinea, and transplant yielded 4 patients—2 men and 2 women (including our patient)—with possible drug-induced PA (Table 1)16,20,21; however, the onset after 2 years of medication (TNF-α inhibitors) or resolution while still receiving the agent (vemurafenib) makes the drug-induced linkage weak. The patients ranged in age from 21 to 67 years, with the median age being 37.5 years. Medications included melphalan, TNF-α inhibitors (adalimumab, infliximab),16,21 and vemurafenib20; it is interesting that infliximab was the medication associated with eliciting PA in 1 patient yet was an effective therapy in another patient with treatment-resistant PA. The onset of PA occurred between 1 month (melphalan) and 24 months (TNF-α inhibitors) after drug initiation. The patients’ associated diseases included Crohn disease,16,21 metastatic melanoma,20 and multiple myeloma.
Other conditions have been described in patients with PA (Table 2). Indeed, PA may be a manifestation of an underlying inflammatory skin disease.9 In addition to dermatologic conditions, procedures or malignancy may be associated with the disease, as demonstrated in our patient. Most commonly, PA is seen in association with psoriasis and seborrheic dermatitis; atopic dermatitis, bacterial infection, fungal infection, lichen planus, and neurodermatitis also have been associated with PA.1,3,5,7-10,12,18,22-25
Conclusion
Pityriasis amiantacea is a benign condition affecting the scalp hair. Albeit uncommon, it may appear in patients treated with medications such as melphalan, TNF-α inhibitors, and vemurafenib. In addition, it has been described in individuals with dermatologic conditions, systemic procedures, or underlying malignancy. Our patient developed PA following a bone marrow transplant after receiving conditioning with melphalan.
- Knight AG. Pityriasis amiantacea: a clinical and histopathological investigation. Clin Exp Dermatol. 1977;2:137-143.
- Alibert JL. De la porrigine amiantacée. In: Monographie des Dermatoses. Paris, France: Baillère; 1832:293-295.
- Gougerot H. La teigne amiantacee D’Alibert. Progres Medical. 1917;13:101-104.
- Photinos P. Recherches sur la fausse teigne amiantacée. Ann Dermatol Syphiligr. 1929;10:743-758.
- Verardino GC, Azulay-Abulafia L, Macedo PM, et al. Pityriasis amiantacea: clinical-dermatoscopic features and microscopy of hair tufts. An Bras Dermatol. 2012;87:142-145.
- Keipert JA. Greasy scaling pityriasis amiantacea and alopecia: a syndrome in search of a cause. Australas J Dermatol. 1985;26:41-44.
- Ring DS, Kaplan DL. Pityriasis amiantacea: a report of 10 cases. Arch Dermatol. 1993;129:913-914.
- Brown WH. Some observations on neurodermatitis of the scalp, with particular reference to tinea amiantacea. Br J Dermatol Syph. 1948;60:81-90.
- Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
- Becker SW, Muir KB. Tinea amiantacea. Arch Dermatol Syphil. 1929;20:45-53.
- Dawber RP. Hair casts. Br J Dermatol. 1979;100:417-421.
- Ginarte M, Pereiro M, Fernández-Redondo V, et al. Case reports. pityriasis amiantacea as manifestation of tinea capitis due to Microsporum canis. Mycoses. 2000;43:93-96.
- Pham RK, Chan CS, Hsu S. Treatment of pityriasis amiantacea with infliximab. Dermatol Online J. 2009;15:13.
- Roberts RJ. Clinical practice. Head lice. N Engl J Med. 2002;346:1645-1650.
- Mcginley KJ, Leyden JJ, Marples RR, et al. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975;64:401-405.
- Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641.
- Mannino G, McCaughey C, Vanness E. A case of pityriasis amiantacea with rapid response to treatment. WMJ. 2014;113:119-120.
- Jamil A, Muthupalaniappen L. Scales on the scalp. Malays Fam Physician. 2013;8:48-49.
- Gupta LK, Khare AK, Masatkar V, et al. Pityriasis amiantacea. Indian Dermatol Online J. 2014;5(suppl 1):S63-S64.
- Bilgiç Ö. Vemurafenib-induced pityriasis amiantacea: a case report. Cutan Ocul Toxicol. 2016;35:329-331.
- Zamperetti M, Zelger B, Höpfl R. Pityriasis amiantacea and folliculitis decalvans: an unusual manifestation associated with antitumor necrosis factor-α therapy. Hautarzt. 2017;68:1007-1010.
- Udayashankar C, Nath AK, Anuradha P. Extensive Darier’s disease with pityriasis amiantacea, alopecia and congenital facial nerve palsy. Dermatol Online J. 2013;19:18574.
- Hussain W, Coulson IH, Salman WD. Pityriasis amiantacea as the sole manifestation of Darier’s disease. Clin Exp Dermatol. 2009;34:554-556.
- Hansted B, Lindskov R. Pityriasis amiantacea and psoriasis. a follow-up study. Dermatologica. 1983;166:314-315.
- Hersle K, Lindholm A, Mobacken H, et al. Relationship of pityriasis amiantacea to psoriasis. a follow-up study. Dermatologica. 1979;159:245-250.
- Knight AG. Pityriasis amiantacea: a clinical and histopathological investigation. Clin Exp Dermatol. 1977;2:137-143.
- Alibert JL. De la porrigine amiantacée. In: Monographie des Dermatoses. Paris, France: Baillère; 1832:293-295.
- Gougerot H. La teigne amiantacee D’Alibert. Progres Medical. 1917;13:101-104.
- Photinos P. Recherches sur la fausse teigne amiantacée. Ann Dermatol Syphiligr. 1929;10:743-758.
- Verardino GC, Azulay-Abulafia L, Macedo PM, et al. Pityriasis amiantacea: clinical-dermatoscopic features and microscopy of hair tufts. An Bras Dermatol. 2012;87:142-145.
- Keipert JA. Greasy scaling pityriasis amiantacea and alopecia: a syndrome in search of a cause. Australas J Dermatol. 1985;26:41-44.
- Ring DS, Kaplan DL. Pityriasis amiantacea: a report of 10 cases. Arch Dermatol. 1993;129:913-914.
- Brown WH. Some observations on neurodermatitis of the scalp, with particular reference to tinea amiantacea. Br J Dermatol Syph. 1948;60:81-90.
- Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
- Becker SW, Muir KB. Tinea amiantacea. Arch Dermatol Syphil. 1929;20:45-53.
- Dawber RP. Hair casts. Br J Dermatol. 1979;100:417-421.
- Ginarte M, Pereiro M, Fernández-Redondo V, et al. Case reports. pityriasis amiantacea as manifestation of tinea capitis due to Microsporum canis. Mycoses. 2000;43:93-96.
- Pham RK, Chan CS, Hsu S. Treatment of pityriasis amiantacea with infliximab. Dermatol Online J. 2009;15:13.
- Roberts RJ. Clinical practice. Head lice. N Engl J Med. 2002;346:1645-1650.
- Mcginley KJ, Leyden JJ, Marples RR, et al. Quantitative microbiology of the scalp in non-dandruff, dandruff, and seborrheic dermatitis. J Invest Dermatol. 1975;64:401-405.
- Ettler J, Wetter DA, Pittelkow MR. Pityriasis amiantacea: a distinctive presentation of psoriasis associated with tumour necrosis factor-α inhibitor therapy. Clin Exp Dermatol. 2012;37:639-641.
- Mannino G, McCaughey C, Vanness E. A case of pityriasis amiantacea with rapid response to treatment. WMJ. 2014;113:119-120.
- Jamil A, Muthupalaniappen L. Scales on the scalp. Malays Fam Physician. 2013;8:48-49.
- Gupta LK, Khare AK, Masatkar V, et al. Pityriasis amiantacea. Indian Dermatol Online J. 2014;5(suppl 1):S63-S64.
- Bilgiç Ö. Vemurafenib-induced pityriasis amiantacea: a case report. Cutan Ocul Toxicol. 2016;35:329-331.
- Zamperetti M, Zelger B, Höpfl R. Pityriasis amiantacea and folliculitis decalvans: an unusual manifestation associated with antitumor necrosis factor-α therapy. Hautarzt. 2017;68:1007-1010.
- Udayashankar C, Nath AK, Anuradha P. Extensive Darier’s disease with pityriasis amiantacea, alopecia and congenital facial nerve palsy. Dermatol Online J. 2013;19:18574.
- Hussain W, Coulson IH, Salman WD. Pityriasis amiantacea as the sole manifestation of Darier’s disease. Clin Exp Dermatol. 2009;34:554-556.
- Hansted B, Lindskov R. Pityriasis amiantacea and psoriasis. a follow-up study. Dermatologica. 1983;166:314-315.
- Hersle K, Lindholm A, Mobacken H, et al. Relationship of pityriasis amiantacea to psoriasis. a follow-up study. Dermatologica. 1979;159:245-250.
Practice Points
- Pityriasis amiantacea (PA) is associated with several dermatologic conditions, including atopic dermatitis, bacterial and fungal infections, psoriasis, and seborrheic dermatitis.
- Drug-induced PA is rare, but the condition has been reported in the context of treatment with tumor necrosis factor Symbol Stdα inhibitors and vemurafenib.
- Our report suggests that PA may be associated with either melphalan conditioning, bone marrow transplant, or both.
Cardiac failure due to left atrial angiosarcoma
Abstract
Primary heart sarcomas are rare and represent 20% of all primary cardiac tumors. Symptoms depend on which chambers and cardiac structures are involved. Angiosarcoma is one of the most common and the most aggressive types of primary heart sarcomas. Typically, these tumors are found in the right atrium, however, cardiac angiosarcomas may involve any part of the heart. Most of these tumors are diagnosed in advanced stages and the patient prognosis is poor. Most tumors are diagnosed using echocardiography. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful information on tumor size and location for planning surgery, which is the only treatment shown to increase survival. We present the case of a 69-year-old woman who presented to the emergency department with hypotension, dyspnea and progressive shortness of breath. After adequate resuscitation, a cardiac mass was identified and surgery was successfully performed. Pathology confirmed a grade 2 primary heart angiosarcoma. Following surgery, the patient was admitted to the intensive care unit and later died secondary to multi-organ system failure.
Introduction
Primary heart angiosarcoma is an aggressive and usually fatal cardiac neoplasm (1). Angiosarcomas can originate at any location in the heart (2, 3), but these tumors typically reside in the right atrium and frequently cause nonspecific symptoms such as dyspnea, cough, heart failure, and arrhythmias. (2) Surgery followed by chemotherapy is the typical approach to these tumors. (4)
We present the case of a 69-year-old woman who presented to the emergency department with hypotension and severe dyspnea.
Case Report
The patient was a 69-year-old woman with a medical history of diabetes. A week before seeking care in the emergency department, she experienced a general feeling of unwellness, dyspnea, and mild respiratory distress. She reported these symptoms had become more and more severe in the last 24 hours and were accompanied by acute chest pain and progressive shortness of breath.
On clinical examination, the patient was hypotensive, had tachypnea and tachycardia, and was hypoxic. Cardiac auscultation detected a systolic murmur in the apex, and auscultation of the lungs revealed crackles and rales, especially at the bases of the lungs. The remainder of her clinical examination was unremarkable. She had sinus tachycardia on an electrocardiogram. A chest X-ray showed a left atrial enlargement along with some patchy opacities in the middle and lower zones of the lungs, along with Kerley B lines suggestive of pulmonary edema.
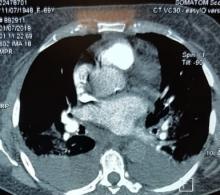
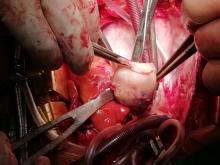
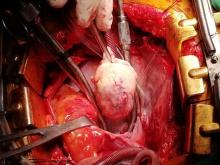
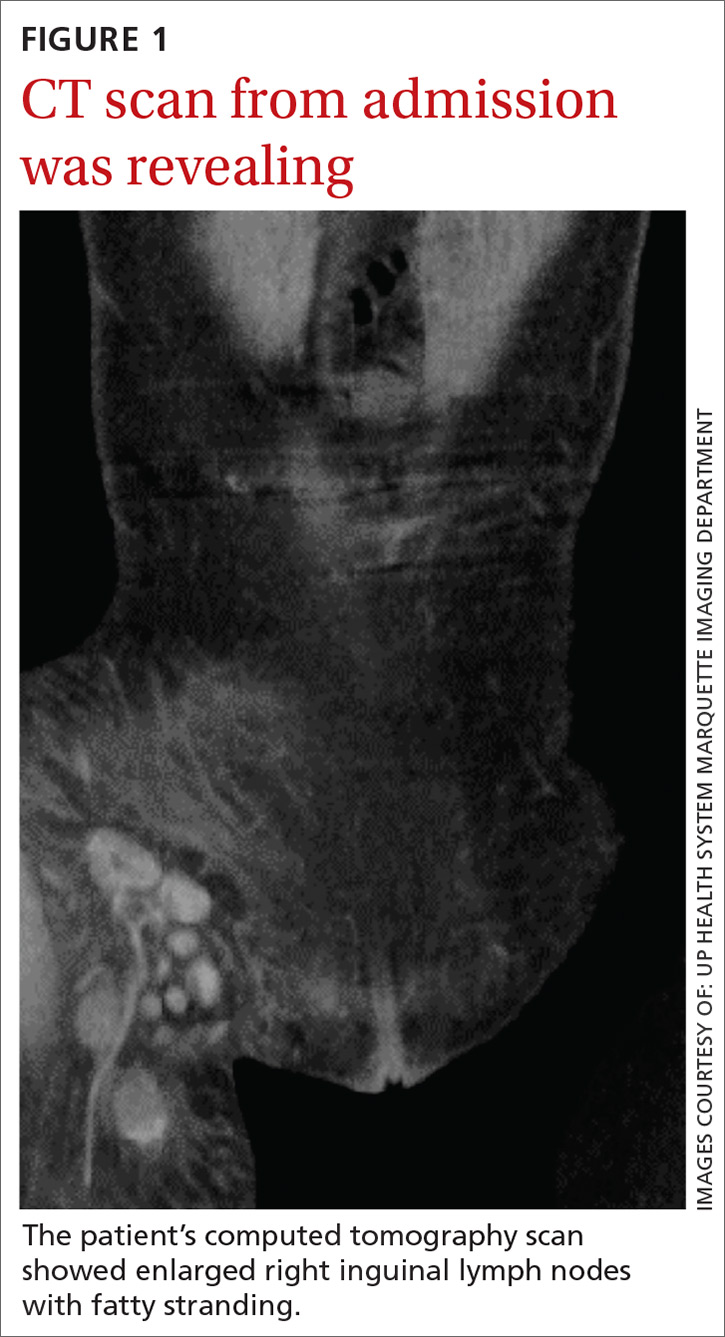
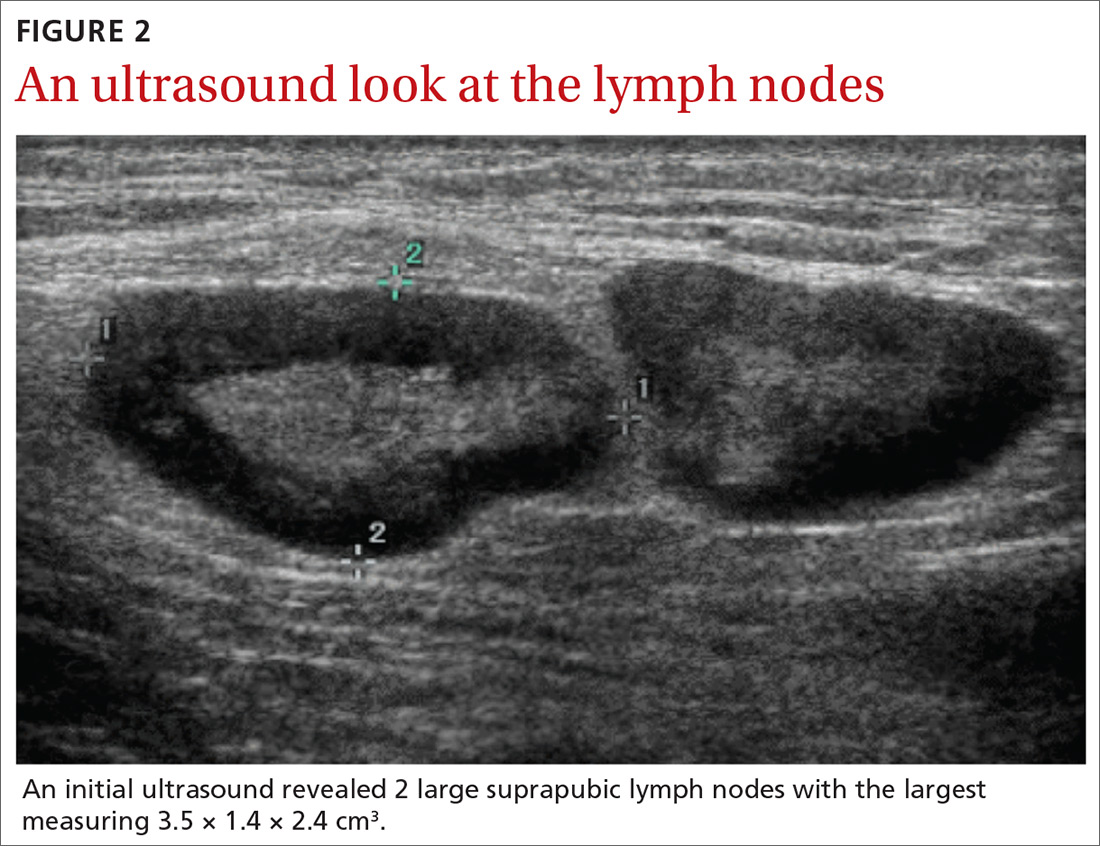
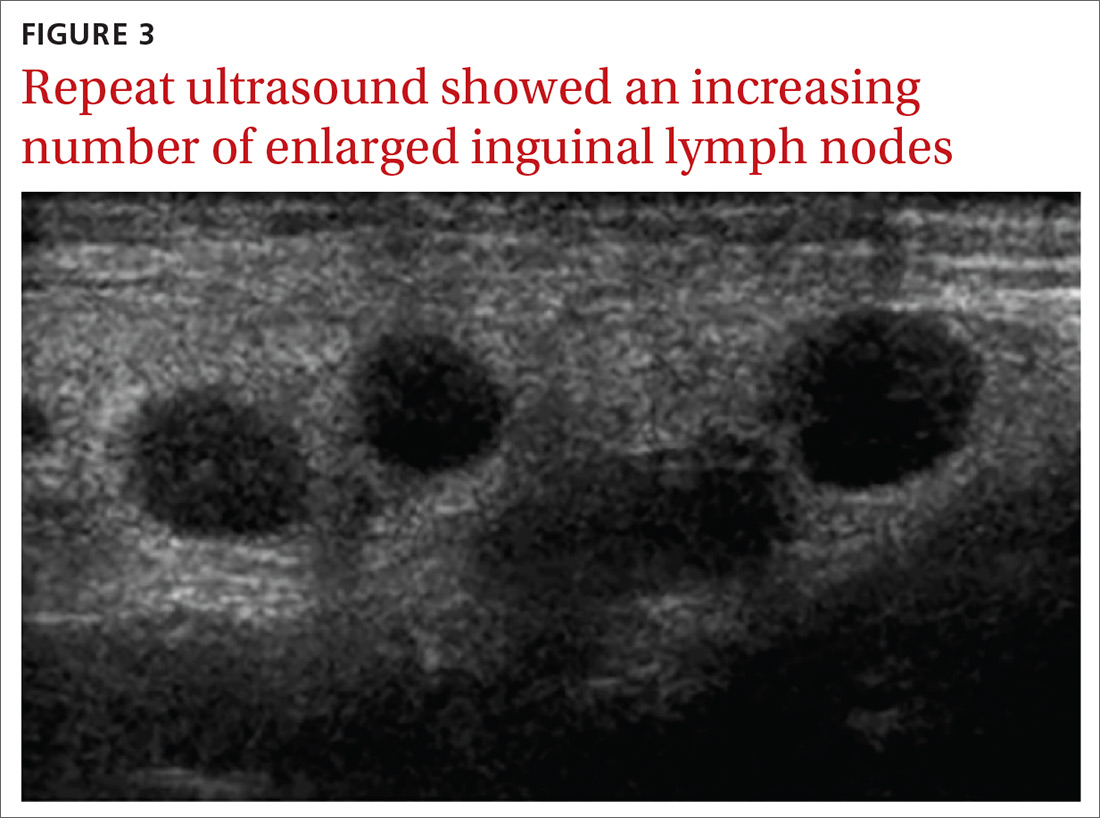
With these findings, and after adequate resuscitation, a contrast-enhanced computed tomography (CT) scan detected a filling defect in the left atrium suggestive of a large intra-cardiac mass with a thick and hyper-enhanced interatrial septum. Bilateral pleural effusions also were evident, (Figure 1A) hence an echocardiogram was requested and it confirmed the presence of a 30 x 29 x 40 mm lobulated highly mobile mass in the left atrium.
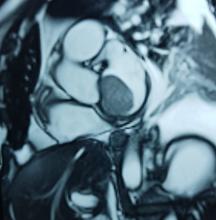
After a cardiothoracic consultation, cardiac magnetic resonance imaging (MRI) was performed. The findings showed the presence of a 58 x 45 x 6 mm well-circumscribed hyperemic mass on the anterior leaflet of the mitral valve and a second 10 x 10 x 6 mm smaller mass firmly adhered to the posterior leaflet of the mitral valve.
The patient, who was hypotensive and hypoxic, was admitted to the hospital for surgical treatment.
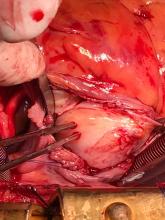
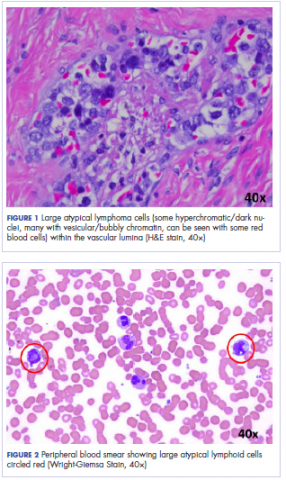
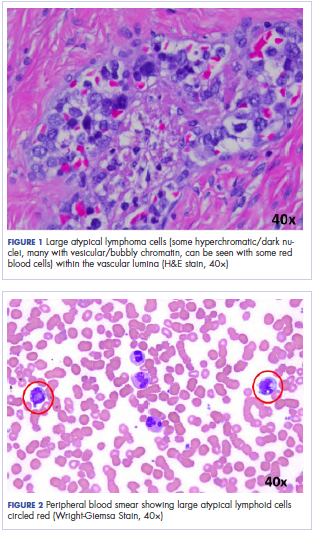
Following sternotomy and cardiopulmonary bypass, a right atriotomy was performed using a trans-septal approach. The large left atrial mass was firmly adhered to the endocardium at the level of the anterior leaflet of the mitral valve and the interatrial septum. The mass had a grey and whitish appearance with some bluish necrotic patches, (Figure 1B, 2B, 3B).
The patient had a complicated postoperative course in the Intensive Care Unit (ICU) and needed inotropic support and vasoactive agents. A postop echocardiogram indicated appropriate left ventricle systolic function, nonetheless, the patient persisted in a hypotensive status that caused refractory shock and ultimately provoked severe organ dysfunction that led to the patient’s death.
Discussion
Primary heart sarcomas are extremely rare malignant neoplasms derived from mesenchymal cells, (1) with an incidence ranging from 0.001% to 0.28% at autopsy.
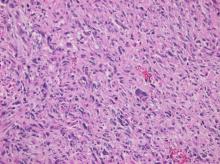
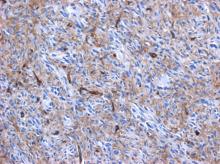
Cardiac angiosarcomas (CA) account for one-third of all primary heart sarcomas (4) and usually develop as gray-brown masses with hemorrhagic patches in the right atrium of male patients. The tumors are filled with vascular channels and their cells are positive for CD34 and factor VIII. (5) Left-sided cardiac angiosarcoma can cause heart failure early in the disease process, but the tumors tend to be more circumscribed, less infiltrative, and associated with better overall survival. (6, 7) Most patients are asymptomatic early in their disease, (2) making the diagnosis even more difficult and worsening its already poor prognosis. (1) The preference of cardiac angiosarcomas for the right heart often leads to a presentation with right-sided congestive heart failure. (2) At later stages, symptoms depend on the structures compromised and range from mild dyspnea on exertion to cardiogenic shock. (8) Cardiac angiosarcomas tend to have a notable intracavitary element, and in some cases may intermittently compromise a cardiac valve, thereby simulating a stenosis or regurgitation. (2, 7)
Our patient presented with acute cardiac failure, pulmonary edema and severe valve dysfunction due to a mass in the left atrium. The tumor had a vascular supply and showed positivity for CD34.
Most patients with cardiac angiosarcoma have metastases, typically to the lung, at diagnosis. (1) Several decades ago, cardiac angiosarcoma was mainly diagnosed postmortem. (1) Now, it can be suspected when cardiomegaly or pleural effusions are seen on chest x-rays (8). Echocardiography is the most useful diagnostic tool, (2) however, CT and MRI can provide useful information on tumor size, invasion and localization. (2, 9) This imaging combination generally provides an excellent anatomic description for preoperative planning. (1, 9)
In our patient, progressive dyspnea was the main symptom and after a prompt evaluation an intracardiac mass was identified as the cause of severe cardiac dysfunction. Because of this finding and the clinical condition of the patient, surgery was planned.
Complete resection of the tumor is the treatment of choice, and is the only therapy currently seen to influence survival. (8) But because of the highly aggressive behavior and a high incidence of systemic metastases with cardiac angiosarcomas, a complete surgical resection is often hampered. (1) Cardiac angiosarcoma carries a grim prognosis as these tumors are universally fatal with a mean survival time of several months after initial presentation even after successful surgery. (2) Chemotherapy is recommended after surgery, even when clear surgical margins are obtained because of the high probability of missed microscopic disease. (1, 2)
High clinical suspicion together with an appropriate history, a thorough physical examination, and precise complementary tests are vital for timely diagnosis and proper treatment.
Authors and Affiliations
Santiago A. Endara: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Gerardo A. Dávalos: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Patricia M. Pontón: Hospital Metropolitano, Quito, Ecuador. Department of Internal Medicine Division of Pathology, MD
Gabriel A. Molina: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY4 General Surgery Resident, MD
Daniel L. Mogrovejo: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY1 General Surgery Resident, MD
Corresponding Author Info:
Santiago A. Endara, Hospital Metropolitano, Av. Mariana de Jesus Oe 7/47 y Conclina, Edificio Diagnostico 2000 tercer piso 3/3, Quito, Ecuador, + 593 9 98416157
Email: drsantiagoendara@gmail.com
1. Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli L. Cardiac Sarcomas: An Update. Journal of Thoracic Oncology. 2010;5(9):1483-1489.
2. Brandt R, Arnold R, Bohle R, Dill T, Hamm C. Cardiac angiosarcoma: case report and review of the literature. Zeitschrift für Kardiologie. 2005;94(12):824-828.
3. Kurian K, Weisshaar D, Parekh H, Berry G, Reitz B. Primary cardiac angiosarcoma: case report and review of the literature. Cardiovascular Pathology. 2006;15(2):110-112.
4. Habibi R, Faramarzi N, Altamirano A, Dadkhah S. A Patient Presenting with Cardiac Tamponade and the Challenges of Finding Its Cause: A Cardiac Angiosarcoma. Case Reports in Cardiology. 2018;2018:1-3.
5. Leduc C, Jenkins S, Sukov W, Rustin J, Maleszewski J. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Human Pathology. 2017;60:199-207.
6. Ramlawi B, Leja M, Abu Saleh W, Al Jabbari O, Benjamin R, Ravi V et al. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. The Annals of Thoracic Surgery. 2016;101(2):698-702.
7.Engelen M. Primary left atrial angiosarcoma mimicking severe mitral valve stenosis. Heart. 2005;91(4):e27-e27.
8. Chenier M, Johnson D, Ohman M, Pavlisko E. Cardiac angiosarcoma presenting as progressive dyspnea on exertion. Journal of Cardiovascular Medicine. 2011;12(12):904-907.
9. Lindsey J, Stacey R. Cardiac magnetic resonance in cardiac angiosarcoma. Echocardiography. 2017;34(7):1077-1081.
Abstract
Primary heart sarcomas are rare and represent 20% of all primary cardiac tumors. Symptoms depend on which chambers and cardiac structures are involved. Angiosarcoma is one of the most common and the most aggressive types of primary heart sarcomas. Typically, these tumors are found in the right atrium, however, cardiac angiosarcomas may involve any part of the heart. Most of these tumors are diagnosed in advanced stages and the patient prognosis is poor. Most tumors are diagnosed using echocardiography. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful information on tumor size and location for planning surgery, which is the only treatment shown to increase survival. We present the case of a 69-year-old woman who presented to the emergency department with hypotension, dyspnea and progressive shortness of breath. After adequate resuscitation, a cardiac mass was identified and surgery was successfully performed. Pathology confirmed a grade 2 primary heart angiosarcoma. Following surgery, the patient was admitted to the intensive care unit and later died secondary to multi-organ system failure.
Introduction
Primary heart angiosarcoma is an aggressive and usually fatal cardiac neoplasm (1). Angiosarcomas can originate at any location in the heart (2, 3), but these tumors typically reside in the right atrium and frequently cause nonspecific symptoms such as dyspnea, cough, heart failure, and arrhythmias. (2) Surgery followed by chemotherapy is the typical approach to these tumors. (4)
We present the case of a 69-year-old woman who presented to the emergency department with hypotension and severe dyspnea.
Case Report
The patient was a 69-year-old woman with a medical history of diabetes. A week before seeking care in the emergency department, she experienced a general feeling of unwellness, dyspnea, and mild respiratory distress. She reported these symptoms had become more and more severe in the last 24 hours and were accompanied by acute chest pain and progressive shortness of breath.
On clinical examination, the patient was hypotensive, had tachypnea and tachycardia, and was hypoxic. Cardiac auscultation detected a systolic murmur in the apex, and auscultation of the lungs revealed crackles and rales, especially at the bases of the lungs. The remainder of her clinical examination was unremarkable. She had sinus tachycardia on an electrocardiogram. A chest X-ray showed a left atrial enlargement along with some patchy opacities in the middle and lower zones of the lungs, along with Kerley B lines suggestive of pulmonary edema.
With these findings, and after adequate resuscitation, a contrast-enhanced computed tomography (CT) scan detected a filling defect in the left atrium suggestive of a large intra-cardiac mass with a thick and hyper-enhanced interatrial septum. Bilateral pleural effusions also were evident, (Figure 1A) hence an echocardiogram was requested and it confirmed the presence of a 30 x 29 x 40 mm lobulated highly mobile mass in the left atrium.
After a cardiothoracic consultation, cardiac magnetic resonance imaging (MRI) was performed. The findings showed the presence of a 58 x 45 x 6 mm well-circumscribed hyperemic mass on the anterior leaflet of the mitral valve and a second 10 x 10 x 6 mm smaller mass firmly adhered to the posterior leaflet of the mitral valve.
The patient, who was hypotensive and hypoxic, was admitted to the hospital for surgical treatment.
Following sternotomy and cardiopulmonary bypass, a right atriotomy was performed using a trans-septal approach. The large left atrial mass was firmly adhered to the endocardium at the level of the anterior leaflet of the mitral valve and the interatrial septum. The mass had a grey and whitish appearance with some bluish necrotic patches, (Figure 1B, 2B, 3B).
The patient had a complicated postoperative course in the Intensive Care Unit (ICU) and needed inotropic support and vasoactive agents. A postop echocardiogram indicated appropriate left ventricle systolic function, nonetheless, the patient persisted in a hypotensive status that caused refractory shock and ultimately provoked severe organ dysfunction that led to the patient’s death.
Discussion
Primary heart sarcomas are extremely rare malignant neoplasms derived from mesenchymal cells, (1) with an incidence ranging from 0.001% to 0.28% at autopsy.
Cardiac angiosarcomas (CA) account for one-third of all primary heart sarcomas (4) and usually develop as gray-brown masses with hemorrhagic patches in the right atrium of male patients. The tumors are filled with vascular channels and their cells are positive for CD34 and factor VIII. (5) Left-sided cardiac angiosarcoma can cause heart failure early in the disease process, but the tumors tend to be more circumscribed, less infiltrative, and associated with better overall survival. (6, 7) Most patients are asymptomatic early in their disease, (2) making the diagnosis even more difficult and worsening its already poor prognosis. (1) The preference of cardiac angiosarcomas for the right heart often leads to a presentation with right-sided congestive heart failure. (2) At later stages, symptoms depend on the structures compromised and range from mild dyspnea on exertion to cardiogenic shock. (8) Cardiac angiosarcomas tend to have a notable intracavitary element, and in some cases may intermittently compromise a cardiac valve, thereby simulating a stenosis or regurgitation. (2, 7)
Our patient presented with acute cardiac failure, pulmonary edema and severe valve dysfunction due to a mass in the left atrium. The tumor had a vascular supply and showed positivity for CD34.
Most patients with cardiac angiosarcoma have metastases, typically to the lung, at diagnosis. (1) Several decades ago, cardiac angiosarcoma was mainly diagnosed postmortem. (1) Now, it can be suspected when cardiomegaly or pleural effusions are seen on chest x-rays (8). Echocardiography is the most useful diagnostic tool, (2) however, CT and MRI can provide useful information on tumor size, invasion and localization. (2, 9) This imaging combination generally provides an excellent anatomic description for preoperative planning. (1, 9)
In our patient, progressive dyspnea was the main symptom and after a prompt evaluation an intracardiac mass was identified as the cause of severe cardiac dysfunction. Because of this finding and the clinical condition of the patient, surgery was planned.
Complete resection of the tumor is the treatment of choice, and is the only therapy currently seen to influence survival. (8) But because of the highly aggressive behavior and a high incidence of systemic metastases with cardiac angiosarcomas, a complete surgical resection is often hampered. (1) Cardiac angiosarcoma carries a grim prognosis as these tumors are universally fatal with a mean survival time of several months after initial presentation even after successful surgery. (2) Chemotherapy is recommended after surgery, even when clear surgical margins are obtained because of the high probability of missed microscopic disease. (1, 2)
High clinical suspicion together with an appropriate history, a thorough physical examination, and precise complementary tests are vital for timely diagnosis and proper treatment.
Authors and Affiliations
Santiago A. Endara: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Gerardo A. Dávalos: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Patricia M. Pontón: Hospital Metropolitano, Quito, Ecuador. Department of Internal Medicine Division of Pathology, MD
Gabriel A. Molina: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY4 General Surgery Resident, MD
Daniel L. Mogrovejo: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY1 General Surgery Resident, MD
Corresponding Author Info:
Santiago A. Endara, Hospital Metropolitano, Av. Mariana de Jesus Oe 7/47 y Conclina, Edificio Diagnostico 2000 tercer piso 3/3, Quito, Ecuador, + 593 9 98416157
Email: drsantiagoendara@gmail.com
Abstract
Primary heart sarcomas are rare and represent 20% of all primary cardiac tumors. Symptoms depend on which chambers and cardiac structures are involved. Angiosarcoma is one of the most common and the most aggressive types of primary heart sarcomas. Typically, these tumors are found in the right atrium, however, cardiac angiosarcomas may involve any part of the heart. Most of these tumors are diagnosed in advanced stages and the patient prognosis is poor. Most tumors are diagnosed using echocardiography. Computed tomography (CT) and magnetic resonance imaging (MRI) provide useful information on tumor size and location for planning surgery, which is the only treatment shown to increase survival. We present the case of a 69-year-old woman who presented to the emergency department with hypotension, dyspnea and progressive shortness of breath. After adequate resuscitation, a cardiac mass was identified and surgery was successfully performed. Pathology confirmed a grade 2 primary heart angiosarcoma. Following surgery, the patient was admitted to the intensive care unit and later died secondary to multi-organ system failure.
Introduction
Primary heart angiosarcoma is an aggressive and usually fatal cardiac neoplasm (1). Angiosarcomas can originate at any location in the heart (2, 3), but these tumors typically reside in the right atrium and frequently cause nonspecific symptoms such as dyspnea, cough, heart failure, and arrhythmias. (2) Surgery followed by chemotherapy is the typical approach to these tumors. (4)
We present the case of a 69-year-old woman who presented to the emergency department with hypotension and severe dyspnea.
Case Report
The patient was a 69-year-old woman with a medical history of diabetes. A week before seeking care in the emergency department, she experienced a general feeling of unwellness, dyspnea, and mild respiratory distress. She reported these symptoms had become more and more severe in the last 24 hours and were accompanied by acute chest pain and progressive shortness of breath.
On clinical examination, the patient was hypotensive, had tachypnea and tachycardia, and was hypoxic. Cardiac auscultation detected a systolic murmur in the apex, and auscultation of the lungs revealed crackles and rales, especially at the bases of the lungs. The remainder of her clinical examination was unremarkable. She had sinus tachycardia on an electrocardiogram. A chest X-ray showed a left atrial enlargement along with some patchy opacities in the middle and lower zones of the lungs, along with Kerley B lines suggestive of pulmonary edema.
With these findings, and after adequate resuscitation, a contrast-enhanced computed tomography (CT) scan detected a filling defect in the left atrium suggestive of a large intra-cardiac mass with a thick and hyper-enhanced interatrial septum. Bilateral pleural effusions also were evident, (Figure 1A) hence an echocardiogram was requested and it confirmed the presence of a 30 x 29 x 40 mm lobulated highly mobile mass in the left atrium.
After a cardiothoracic consultation, cardiac magnetic resonance imaging (MRI) was performed. The findings showed the presence of a 58 x 45 x 6 mm well-circumscribed hyperemic mass on the anterior leaflet of the mitral valve and a second 10 x 10 x 6 mm smaller mass firmly adhered to the posterior leaflet of the mitral valve.
The patient, who was hypotensive and hypoxic, was admitted to the hospital for surgical treatment.
Following sternotomy and cardiopulmonary bypass, a right atriotomy was performed using a trans-septal approach. The large left atrial mass was firmly adhered to the endocardium at the level of the anterior leaflet of the mitral valve and the interatrial septum. The mass had a grey and whitish appearance with some bluish necrotic patches, (Figure 1B, 2B, 3B).
The patient had a complicated postoperative course in the Intensive Care Unit (ICU) and needed inotropic support and vasoactive agents. A postop echocardiogram indicated appropriate left ventricle systolic function, nonetheless, the patient persisted in a hypotensive status that caused refractory shock and ultimately provoked severe organ dysfunction that led to the patient’s death.
Discussion
Primary heart sarcomas are extremely rare malignant neoplasms derived from mesenchymal cells, (1) with an incidence ranging from 0.001% to 0.28% at autopsy.
Cardiac angiosarcomas (CA) account for one-third of all primary heart sarcomas (4) and usually develop as gray-brown masses with hemorrhagic patches in the right atrium of male patients. The tumors are filled with vascular channels and their cells are positive for CD34 and factor VIII. (5) Left-sided cardiac angiosarcoma can cause heart failure early in the disease process, but the tumors tend to be more circumscribed, less infiltrative, and associated with better overall survival. (6, 7) Most patients are asymptomatic early in their disease, (2) making the diagnosis even more difficult and worsening its already poor prognosis. (1) The preference of cardiac angiosarcomas for the right heart often leads to a presentation with right-sided congestive heart failure. (2) At later stages, symptoms depend on the structures compromised and range from mild dyspnea on exertion to cardiogenic shock. (8) Cardiac angiosarcomas tend to have a notable intracavitary element, and in some cases may intermittently compromise a cardiac valve, thereby simulating a stenosis or regurgitation. (2, 7)
Our patient presented with acute cardiac failure, pulmonary edema and severe valve dysfunction due to a mass in the left atrium. The tumor had a vascular supply and showed positivity for CD34.
Most patients with cardiac angiosarcoma have metastases, typically to the lung, at diagnosis. (1) Several decades ago, cardiac angiosarcoma was mainly diagnosed postmortem. (1) Now, it can be suspected when cardiomegaly or pleural effusions are seen on chest x-rays (8). Echocardiography is the most useful diagnostic tool, (2) however, CT and MRI can provide useful information on tumor size, invasion and localization. (2, 9) This imaging combination generally provides an excellent anatomic description for preoperative planning. (1, 9)
In our patient, progressive dyspnea was the main symptom and after a prompt evaluation an intracardiac mass was identified as the cause of severe cardiac dysfunction. Because of this finding and the clinical condition of the patient, surgery was planned.
Complete resection of the tumor is the treatment of choice, and is the only therapy currently seen to influence survival. (8) But because of the highly aggressive behavior and a high incidence of systemic metastases with cardiac angiosarcomas, a complete surgical resection is often hampered. (1) Cardiac angiosarcoma carries a grim prognosis as these tumors are universally fatal with a mean survival time of several months after initial presentation even after successful surgery. (2) Chemotherapy is recommended after surgery, even when clear surgical margins are obtained because of the high probability of missed microscopic disease. (1, 2)
High clinical suspicion together with an appropriate history, a thorough physical examination, and precise complementary tests are vital for timely diagnosis and proper treatment.
Authors and Affiliations
Santiago A. Endara: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Gerardo A. Dávalos: Department of General Surgery, Division of Cardiothoracic Surgery, Hospital Metropolitano, Quito, Ecuador, MD
Patricia M. Pontón: Hospital Metropolitano, Quito, Ecuador. Department of Internal Medicine Division of Pathology, MD
Gabriel A. Molina: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY4 General Surgery Resident, MD
Daniel L. Mogrovejo: Pontificia Universidad Católica del Ecuador (PUCE), Quito, Ecuador. PGY1 General Surgery Resident, MD
Corresponding Author Info:
Santiago A. Endara, Hospital Metropolitano, Av. Mariana de Jesus Oe 7/47 y Conclina, Edificio Diagnostico 2000 tercer piso 3/3, Quito, Ecuador, + 593 9 98416157
Email: drsantiagoendara@gmail.com
1. Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli L. Cardiac Sarcomas: An Update. Journal of Thoracic Oncology. 2010;5(9):1483-1489.
2. Brandt R, Arnold R, Bohle R, Dill T, Hamm C. Cardiac angiosarcoma: case report and review of the literature. Zeitschrift für Kardiologie. 2005;94(12):824-828.
3. Kurian K, Weisshaar D, Parekh H, Berry G, Reitz B. Primary cardiac angiosarcoma: case report and review of the literature. Cardiovascular Pathology. 2006;15(2):110-112.
4. Habibi R, Faramarzi N, Altamirano A, Dadkhah S. A Patient Presenting with Cardiac Tamponade and the Challenges of Finding Its Cause: A Cardiac Angiosarcoma. Case Reports in Cardiology. 2018;2018:1-3.
5. Leduc C, Jenkins S, Sukov W, Rustin J, Maleszewski J. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Human Pathology. 2017;60:199-207.
6. Ramlawi B, Leja M, Abu Saleh W, Al Jabbari O, Benjamin R, Ravi V et al. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. The Annals of Thoracic Surgery. 2016;101(2):698-702.
7.Engelen M. Primary left atrial angiosarcoma mimicking severe mitral valve stenosis. Heart. 2005;91(4):e27-e27.
8. Chenier M, Johnson D, Ohman M, Pavlisko E. Cardiac angiosarcoma presenting as progressive dyspnea on exertion. Journal of Cardiovascular Medicine. 2011;12(12):904-907.
9. Lindsey J, Stacey R. Cardiac magnetic resonance in cardiac angiosarcoma. Echocardiography. 2017;34(7):1077-1081.
1. Orlandi A, Ferlosio A, Roselli M, Chiariello L, Spagnoli L. Cardiac Sarcomas: An Update. Journal of Thoracic Oncology. 2010;5(9):1483-1489.
2. Brandt R, Arnold R, Bohle R, Dill T, Hamm C. Cardiac angiosarcoma: case report and review of the literature. Zeitschrift für Kardiologie. 2005;94(12):824-828.
3. Kurian K, Weisshaar D, Parekh H, Berry G, Reitz B. Primary cardiac angiosarcoma: case report and review of the literature. Cardiovascular Pathology. 2006;15(2):110-112.
4. Habibi R, Faramarzi N, Altamirano A, Dadkhah S. A Patient Presenting with Cardiac Tamponade and the Challenges of Finding Its Cause: A Cardiac Angiosarcoma. Case Reports in Cardiology. 2018;2018:1-3.
5. Leduc C, Jenkins S, Sukov W, Rustin J, Maleszewski J. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Human Pathology. 2017;60:199-207.
6. Ramlawi B, Leja M, Abu Saleh W, Al Jabbari O, Benjamin R, Ravi V et al. Surgical Treatment of Primary Cardiac Sarcomas: Review of a Single-Institution Experience. The Annals of Thoracic Surgery. 2016;101(2):698-702.
7.Engelen M. Primary left atrial angiosarcoma mimicking severe mitral valve stenosis. Heart. 2005;91(4):e27-e27.
8. Chenier M, Johnson D, Ohman M, Pavlisko E. Cardiac angiosarcoma presenting as progressive dyspnea on exertion. Journal of Cardiovascular Medicine. 2011;12(12):904-907.
9. Lindsey J, Stacey R. Cardiac magnetic resonance in cardiac angiosarcoma. Echocardiography. 2017;34(7):1077-1081.
Id Reaction Associated With Red Tattoo Ink
To the Editor:
Although relatively uncommon, hypersensitivity reactions to tattoo pigment are on the rise due to the increasing popularity and prevalence of tattoos.1 Multiple adverse events have been described in association with tattoos, including inflammatory, infectious, and neoplastic responses.2 An id reaction (also known as autoeczematization or autosensitization) develops distant to an initial site of infection or sensitization. We describe a unique case of an id reaction and subsequent development of prurigo nodules associated with contact allergy to red tattoo ink.
A 40-year-old woman was referred to the New York University Skin and Cancer Unit (New York, New York) for evaluation of a pruritic eruption arising on and near sites of tattooed skin on the right foot and right upper arm of 8 months’ duration. The patient reported that she had obtained a polychromatic tattoo on the right dorsal foot 9 months prior to the current presentation. Approximately 1 month later, she developed pruritic papulonodular lesions localized to the red-pigmented areas of the tattoo. Concomitantly, the patient developed a similar eruption confined to areas of red pigment in a polychromatic tattoo on the right upper arm that she had obtained 10 years prior. She was treated with intralesional triamcinolone to several of the lesions on the right dorsal foot with some benefit; however, a few days later she developed a generalized, erythematous, pruritic eruption on the back, abdomen, arms, and legs. Her medical history was remarkable only for mild iron-deficiency anemia. She had no known drug allergies or history of atopy and was not taking any medications prior to the onset of the eruption.
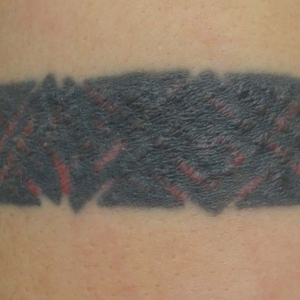
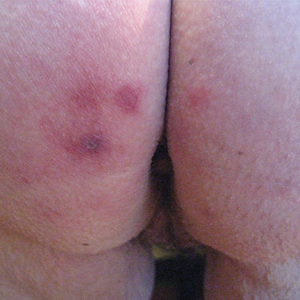
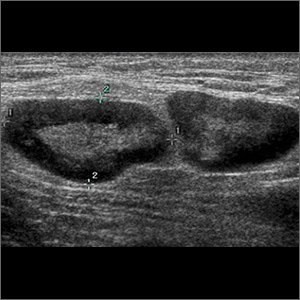
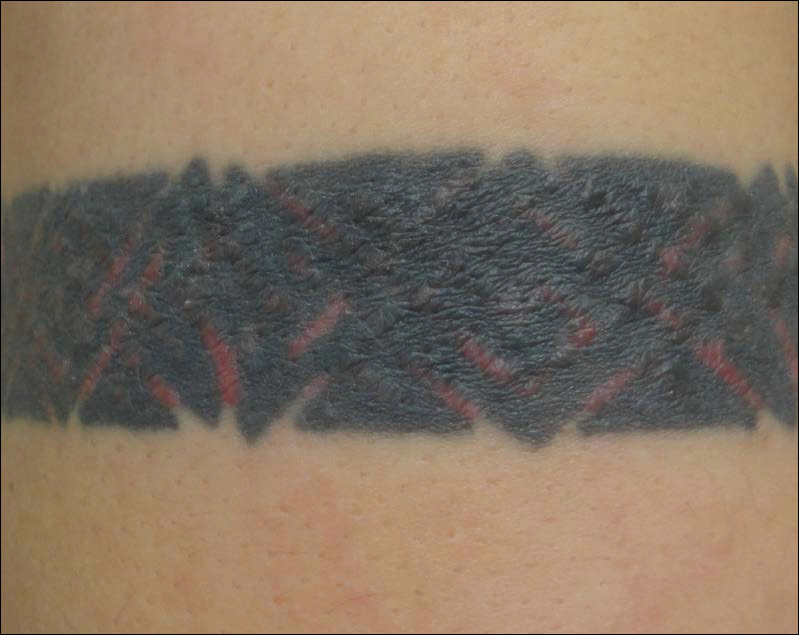
Skin examination revealed multiple, well-demarcated, eczematous papulonodules with surrounding erythema confined to the red-pigmented areas of the tattoo on the right dorsal foot, with several similar lesions on the surrounding nontattooed skin (Figure 1). Linear, well-demarcated, eczematous, hyperpigmented plaques also were noted on the red-pigmented areas of the tattoo on the patient’s right upper arm (Figure 2). Eczematous plaques and scattered excoriations were noted on the back, abdomen, flanks, arms, and legs.

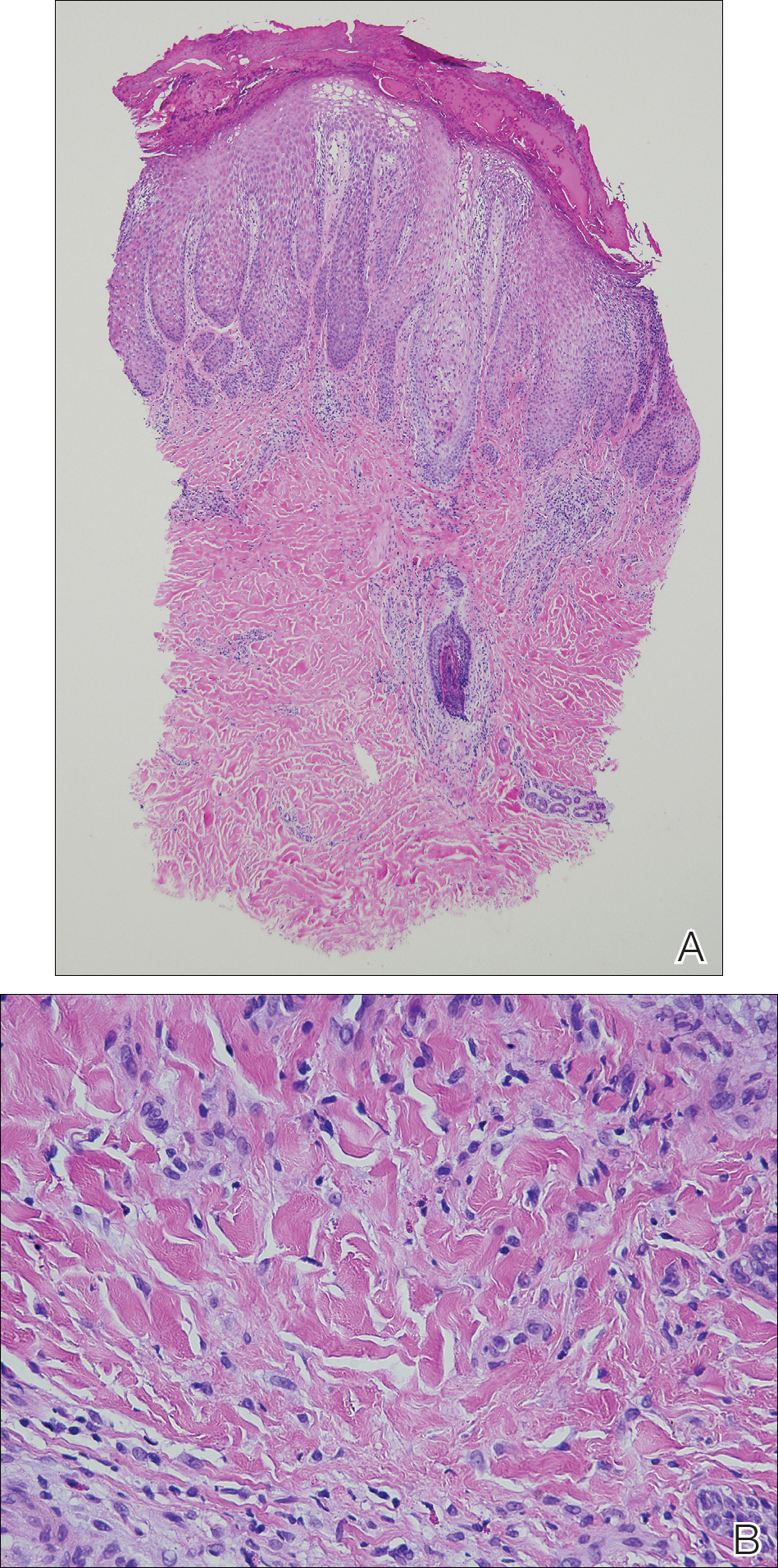
Patch testing with the North American Standard Series, metal series, and samples of the red pigments used in the tattoo on the foot were negative. A punch biopsy of a lesion on the dorsal right foot showed a psoriasiform spongiotic dermatitis with eosinophils (Figure 3). Periodic acid–Schiff staining with diastase failed to reveal fungal hyphae. The histologic findings were consistent with allergic contact dermatitis. A punch biopsy of the eczematous reaction on nontattooed skin on the trunk demonstrated a perivascular dermatitis with eosinophils and subtle spongiosis consistent with an id reaction.
The patient was treated with fluocinonide ointment for several months with no effect. Subsequently, she received several short courses of oral prednisone, after which the affected areas of the tattoo on the arm and foot flattened and the id reaction resolved; however, after several months, the red-pigmented areas of the tattoo on the foot again became elevated and pruritic, and the patient developed widespread prurigo nodules on nontattooed skin on the trunk, arms, and legs. She was subsequently referred to a laser specialist for a trial of fractional laser treatment to cautiously remove the red tattoo pigment. After 2 treatments, the pruritus improved and the papular lesions appeared slightly flatter; however, the prurigo nodules remained. The tattoo on the patient’s foot was surgically removed; however, the prurigo nodules remained. Ultimately, the lesions cleared with a several-month course of mycophenolate mofetil.
Systemic allergic reactions to tattoo ink are rare but can cause considerable morbidity. An id reaction, also known as autoeczematization or autosensitization, is a reaction that develops distant to an initial site of infection or sensitization. Although the pathogenesis of this reaction is not certain, it has been hypothesized that autoimmunity to skin antigens might play a role.3 Autologous epidermal cells are thought to become antigenic in the presence of acute inflammation at the primary cutaneous site. These antigenic autologous epidermal cells are postulated to enter the circulation and cause secondary eczematous lesions at distant sites. This proposed mechanism is supported by the development of positive skin reactions to autologous extracts of epidermal scaling in patients with active id reaction.3
Hematogenous dissemination of cytokines has been implicated in id reactions.4 Keratinocytes produce cytokines in response to conditions that are known to trigger id reactions.5 Epidermal cytokines released from the primary site of sensitization are thought to heighten sensitivity at distant skin areas.4 These cytokines regulate both cell-mediated and humoral cutaneous immune responses. Increased levels of activated HLA-DR isotype–positive T cells in patients with active autoeczemization favors a cellular-mediated immune mechanism. The presence of activated antigen-specific T cells also supports the role of allergic contact dermatitis in triggering id reactions.6
Allergic contact dermatitis is the most common hypersensitivity reaction to tattoo ink, with red pigments representing the most common cause of tattoo-related allergic contact dermatitis. Historically, cinnabar (mercuric sulfide) has been the most common red pigment to cause allergic contact dermatitis.7 More recently, mercury-free organic pigments (eg, azo dyes) have been used in polychromatic tattoos due to their ability to retain color over long periods of time8; however, these organic red tattoo pigments also have been implicated in allergic reactions.8-11 The composition of these new organic red tattoo pigments varies, but chemical analysis has revealed a mixture of aromatic azo compounds (eg, quinacridone),10 heavy metals (eg, aluminum, lead, cadmium, chromium, cobalt, iron, titanium),9,12 and intermediate reactive compounds (eg, naphthalene, 2-naphthol, chlorobenzene, benzene).8 Allergic contact dermatitis to red tattoo ink is well documented8,13; however, a PubMed search of articles indexed for MEDLINE using the terms tattoo and dermatitis, tattoo and allergy, tattoo and autosensitization, tattoo and id reaction, and tattoo and autoeczematization yielded only 3 other reports of a concomitant id reaction.11,14,15
The diagnosis of id reaction associated with allergic contact dermatitis is made on the basis of clinical history, physical examination, and histopathology. Patch testing usually is not positive in cases of tattoo allergy; it is thought that the allergen is a tattoo ink byproduct possibly caused by photoinduced or metabolic change of the tattoo pigment and a haptenization process.1,8,16 Histologically, variable reaction patterns, including eczematous, lichenoid, granulomatous, and pseudolymphomatous reactions have been reported in association with delayed-type inflammatory reactions to tattoo pigments, but the lichenoid pattern is most commonly observed.8
Treatment options for allergic contact dermatitis to tattoo ink include topical, intralesional, and oral steroids; topical calcineurin inhibitors; and surgical excision of the tattoo. Q-switched lasers—ruby, Nd:YAG, and alexandrite—are the gold standard for removing tattoo pigments17; however, these lasers remove tattoo pigment by selective photothermolysis, resulting in extracellular extravasation of pigment, which can precipitate a heightened immune response that can lead to localized and generalized allergic reactions.18 Therefore, Q-switched lasers should be avoided in the setting of an allergic reaction to tattoo ink. Fractional ablative laser resurfacing may be a safer alternative for removal of tattoos in the setting of an allergic reaction.17 Further studies are needed to confirm the safety and efficacy of this modality for allergic tattoo ink removal.17,18
Our case illustrates a rare cause of id reaction and the subsequent development of prurigo nodules associated with contact allergy to red tattoo ink. We present this case to raise awareness of the potential health and iatrogenic risks associated with tattoo placement. Further investigation of these color additives is warranted to better elucidate ink components responsible for these cutaneous allergic reactions.
Acknowledgments
We would like to thank Vitaly Terushkin, MD (West Orange, New Jersey, and New York, New York), and Arielle Kauvar, MD (New York, New York), for their contributions to the patient’s clinical care.
- Vasold R, Engel E, Konig B, et al. Health risks of tattoo colors. Anal Bioanal Chem. 2008;391:9-13.
- Swigost AJ, Peltola J, Jacobson-Dunlop E, et al. Tattoo-related squamous proliferations: a specturm of reactive hyperplasia. Clin Exp Dermatol. 2018;43:728-732.
- Cormia FE, Esplin BM. Autoeczematization; preliminary report. Arch Derm Syphilol. 1950;61:931-945.
- Goldsmith LA, Katz SI, Gilchrest BA, et al. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Garcovich S, Carbone T, Avitabile S, et al. Lichenoid red tattoo reaction: histological and immunological perspectives. Eur J Dermatol. 2012;22:93-96.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Bendsoe N, Hansson C, Sterner O. Inflammatory reactions from organic pigments in red tattoos. Acta Derm Venereol. 1991;71:70-73.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Cristaudo A, Forte G, Bocca B, et al. Permanent tattoos: evidence of pseudolymphoma in three patients and metal composition of the dyes. Eur J Dermatol. 2012;22:776-780.
- Wenzel SM, Welzel J, Hafner C, et al. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis. 2010;63:223-227.
- Goldberg HM. Tattoo allergy. Plast Reconstr Surg. 1996;98:1315-1316.
- Gamba CS, Smith FL, Wisell J, et al. Tattoo reactions in an HIV patient: autoeczematization and progressive allergic reaction to red ink after antiretroviral therapy initiation. JAAD Case Rep. 2015;1:395-398.
- Serup J, Hutton Carlsen K. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
- Ibrahimi OA, Syed Z, Sakamoto FH, et al. Treatment of tattoo allergy with ablative fractional resurfacing: a novel paradigm for tattoo removal. J Am Acad Dermatol. 2011;64:1111-1114.
- Harper J, Losch AE, Otto SG, et al. New insight into the pathophysiology of tattoo reactions following laser tattoo removal. Plast Reconstr Surg. 2010;126:313e-314e.
To the Editor:
Although relatively uncommon, hypersensitivity reactions to tattoo pigment are on the rise due to the increasing popularity and prevalence of tattoos.1 Multiple adverse events have been described in association with tattoos, including inflammatory, infectious, and neoplastic responses.2 An id reaction (also known as autoeczematization or autosensitization) develops distant to an initial site of infection or sensitization. We describe a unique case of an id reaction and subsequent development of prurigo nodules associated with contact allergy to red tattoo ink.
A 40-year-old woman was referred to the New York University Skin and Cancer Unit (New York, New York) for evaluation of a pruritic eruption arising on and near sites of tattooed skin on the right foot and right upper arm of 8 months’ duration. The patient reported that she had obtained a polychromatic tattoo on the right dorsal foot 9 months prior to the current presentation. Approximately 1 month later, she developed pruritic papulonodular lesions localized to the red-pigmented areas of the tattoo. Concomitantly, the patient developed a similar eruption confined to areas of red pigment in a polychromatic tattoo on the right upper arm that she had obtained 10 years prior. She was treated with intralesional triamcinolone to several of the lesions on the right dorsal foot with some benefit; however, a few days later she developed a generalized, erythematous, pruritic eruption on the back, abdomen, arms, and legs. Her medical history was remarkable only for mild iron-deficiency anemia. She had no known drug allergies or history of atopy and was not taking any medications prior to the onset of the eruption.
Skin examination revealed multiple, well-demarcated, eczematous papulonodules with surrounding erythema confined to the red-pigmented areas of the tattoo on the right dorsal foot, with several similar lesions on the surrounding nontattooed skin (Figure 1). Linear, well-demarcated, eczematous, hyperpigmented plaques also were noted on the red-pigmented areas of the tattoo on the patient’s right upper arm (Figure 2). Eczematous plaques and scattered excoriations were noted on the back, abdomen, flanks, arms, and legs.


Patch testing with the North American Standard Series, metal series, and samples of the red pigments used in the tattoo on the foot were negative. A punch biopsy of a lesion on the dorsal right foot showed a psoriasiform spongiotic dermatitis with eosinophils (Figure 3). Periodic acid–Schiff staining with diastase failed to reveal fungal hyphae. The histologic findings were consistent with allergic contact dermatitis. A punch biopsy of the eczematous reaction on nontattooed skin on the trunk demonstrated a perivascular dermatitis with eosinophils and subtle spongiosis consistent with an id reaction.

The patient was treated with fluocinonide ointment for several months with no effect. Subsequently, she received several short courses of oral prednisone, after which the affected areas of the tattoo on the arm and foot flattened and the id reaction resolved; however, after several months, the red-pigmented areas of the tattoo on the foot again became elevated and pruritic, and the patient developed widespread prurigo nodules on nontattooed skin on the trunk, arms, and legs. She was subsequently referred to a laser specialist for a trial of fractional laser treatment to cautiously remove the red tattoo pigment. After 2 treatments, the pruritus improved and the papular lesions appeared slightly flatter; however, the prurigo nodules remained. The tattoo on the patient’s foot was surgically removed; however, the prurigo nodules remained. Ultimately, the lesions cleared with a several-month course of mycophenolate mofetil.
Systemic allergic reactions to tattoo ink are rare but can cause considerable morbidity. An id reaction, also known as autoeczematization or autosensitization, is a reaction that develops distant to an initial site of infection or sensitization. Although the pathogenesis of this reaction is not certain, it has been hypothesized that autoimmunity to skin antigens might play a role.3 Autologous epidermal cells are thought to become antigenic in the presence of acute inflammation at the primary cutaneous site. These antigenic autologous epidermal cells are postulated to enter the circulation and cause secondary eczematous lesions at distant sites. This proposed mechanism is supported by the development of positive skin reactions to autologous extracts of epidermal scaling in patients with active id reaction.3
Hematogenous dissemination of cytokines has been implicated in id reactions.4 Keratinocytes produce cytokines in response to conditions that are known to trigger id reactions.5 Epidermal cytokines released from the primary site of sensitization are thought to heighten sensitivity at distant skin areas.4 These cytokines regulate both cell-mediated and humoral cutaneous immune responses. Increased levels of activated HLA-DR isotype–positive T cells in patients with active autoeczemization favors a cellular-mediated immune mechanism. The presence of activated antigen-specific T cells also supports the role of allergic contact dermatitis in triggering id reactions.6
Allergic contact dermatitis is the most common hypersensitivity reaction to tattoo ink, with red pigments representing the most common cause of tattoo-related allergic contact dermatitis. Historically, cinnabar (mercuric sulfide) has been the most common red pigment to cause allergic contact dermatitis.7 More recently, mercury-free organic pigments (eg, azo dyes) have been used in polychromatic tattoos due to their ability to retain color over long periods of time8; however, these organic red tattoo pigments also have been implicated in allergic reactions.8-11 The composition of these new organic red tattoo pigments varies, but chemical analysis has revealed a mixture of aromatic azo compounds (eg, quinacridone),10 heavy metals (eg, aluminum, lead, cadmium, chromium, cobalt, iron, titanium),9,12 and intermediate reactive compounds (eg, naphthalene, 2-naphthol, chlorobenzene, benzene).8 Allergic contact dermatitis to red tattoo ink is well documented8,13; however, a PubMed search of articles indexed for MEDLINE using the terms tattoo and dermatitis, tattoo and allergy, tattoo and autosensitization, tattoo and id reaction, and tattoo and autoeczematization yielded only 3 other reports of a concomitant id reaction.11,14,15
The diagnosis of id reaction associated with allergic contact dermatitis is made on the basis of clinical history, physical examination, and histopathology. Patch testing usually is not positive in cases of tattoo allergy; it is thought that the allergen is a tattoo ink byproduct possibly caused by photoinduced or metabolic change of the tattoo pigment and a haptenization process.1,8,16 Histologically, variable reaction patterns, including eczematous, lichenoid, granulomatous, and pseudolymphomatous reactions have been reported in association with delayed-type inflammatory reactions to tattoo pigments, but the lichenoid pattern is most commonly observed.8
Treatment options for allergic contact dermatitis to tattoo ink include topical, intralesional, and oral steroids; topical calcineurin inhibitors; and surgical excision of the tattoo. Q-switched lasers—ruby, Nd:YAG, and alexandrite—are the gold standard for removing tattoo pigments17; however, these lasers remove tattoo pigment by selective photothermolysis, resulting in extracellular extravasation of pigment, which can precipitate a heightened immune response that can lead to localized and generalized allergic reactions.18 Therefore, Q-switched lasers should be avoided in the setting of an allergic reaction to tattoo ink. Fractional ablative laser resurfacing may be a safer alternative for removal of tattoos in the setting of an allergic reaction.17 Further studies are needed to confirm the safety and efficacy of this modality for allergic tattoo ink removal.17,18
Our case illustrates a rare cause of id reaction and the subsequent development of prurigo nodules associated with contact allergy to red tattoo ink. We present this case to raise awareness of the potential health and iatrogenic risks associated with tattoo placement. Further investigation of these color additives is warranted to better elucidate ink components responsible for these cutaneous allergic reactions.
Acknowledgments
We would like to thank Vitaly Terushkin, MD (West Orange, New Jersey, and New York, New York), and Arielle Kauvar, MD (New York, New York), for their contributions to the patient’s clinical care.
To the Editor:
Although relatively uncommon, hypersensitivity reactions to tattoo pigment are on the rise due to the increasing popularity and prevalence of tattoos.1 Multiple adverse events have been described in association with tattoos, including inflammatory, infectious, and neoplastic responses.2 An id reaction (also known as autoeczematization or autosensitization) develops distant to an initial site of infection or sensitization. We describe a unique case of an id reaction and subsequent development of prurigo nodules associated with contact allergy to red tattoo ink.
A 40-year-old woman was referred to the New York University Skin and Cancer Unit (New York, New York) for evaluation of a pruritic eruption arising on and near sites of tattooed skin on the right foot and right upper arm of 8 months’ duration. The patient reported that she had obtained a polychromatic tattoo on the right dorsal foot 9 months prior to the current presentation. Approximately 1 month later, she developed pruritic papulonodular lesions localized to the red-pigmented areas of the tattoo. Concomitantly, the patient developed a similar eruption confined to areas of red pigment in a polychromatic tattoo on the right upper arm that she had obtained 10 years prior. She was treated with intralesional triamcinolone to several of the lesions on the right dorsal foot with some benefit; however, a few days later she developed a generalized, erythematous, pruritic eruption on the back, abdomen, arms, and legs. Her medical history was remarkable only for mild iron-deficiency anemia. She had no known drug allergies or history of atopy and was not taking any medications prior to the onset of the eruption.
Skin examination revealed multiple, well-demarcated, eczematous papulonodules with surrounding erythema confined to the red-pigmented areas of the tattoo on the right dorsal foot, with several similar lesions on the surrounding nontattooed skin (Figure 1). Linear, well-demarcated, eczematous, hyperpigmented plaques also were noted on the red-pigmented areas of the tattoo on the patient’s right upper arm (Figure 2). Eczematous plaques and scattered excoriations were noted on the back, abdomen, flanks, arms, and legs.


Patch testing with the North American Standard Series, metal series, and samples of the red pigments used in the tattoo on the foot were negative. A punch biopsy of a lesion on the dorsal right foot showed a psoriasiform spongiotic dermatitis with eosinophils (Figure 3). Periodic acid–Schiff staining with diastase failed to reveal fungal hyphae. The histologic findings were consistent with allergic contact dermatitis. A punch biopsy of the eczematous reaction on nontattooed skin on the trunk demonstrated a perivascular dermatitis with eosinophils and subtle spongiosis consistent with an id reaction.

The patient was treated with fluocinonide ointment for several months with no effect. Subsequently, she received several short courses of oral prednisone, after which the affected areas of the tattoo on the arm and foot flattened and the id reaction resolved; however, after several months, the red-pigmented areas of the tattoo on the foot again became elevated and pruritic, and the patient developed widespread prurigo nodules on nontattooed skin on the trunk, arms, and legs. She was subsequently referred to a laser specialist for a trial of fractional laser treatment to cautiously remove the red tattoo pigment. After 2 treatments, the pruritus improved and the papular lesions appeared slightly flatter; however, the prurigo nodules remained. The tattoo on the patient’s foot was surgically removed; however, the prurigo nodules remained. Ultimately, the lesions cleared with a several-month course of mycophenolate mofetil.
Systemic allergic reactions to tattoo ink are rare but can cause considerable morbidity. An id reaction, also known as autoeczematization or autosensitization, is a reaction that develops distant to an initial site of infection or sensitization. Although the pathogenesis of this reaction is not certain, it has been hypothesized that autoimmunity to skin antigens might play a role.3 Autologous epidermal cells are thought to become antigenic in the presence of acute inflammation at the primary cutaneous site. These antigenic autologous epidermal cells are postulated to enter the circulation and cause secondary eczematous lesions at distant sites. This proposed mechanism is supported by the development of positive skin reactions to autologous extracts of epidermal scaling in patients with active id reaction.3
Hematogenous dissemination of cytokines has been implicated in id reactions.4 Keratinocytes produce cytokines in response to conditions that are known to trigger id reactions.5 Epidermal cytokines released from the primary site of sensitization are thought to heighten sensitivity at distant skin areas.4 These cytokines regulate both cell-mediated and humoral cutaneous immune responses. Increased levels of activated HLA-DR isotype–positive T cells in patients with active autoeczemization favors a cellular-mediated immune mechanism. The presence of activated antigen-specific T cells also supports the role of allergic contact dermatitis in triggering id reactions.6
Allergic contact dermatitis is the most common hypersensitivity reaction to tattoo ink, with red pigments representing the most common cause of tattoo-related allergic contact dermatitis. Historically, cinnabar (mercuric sulfide) has been the most common red pigment to cause allergic contact dermatitis.7 More recently, mercury-free organic pigments (eg, azo dyes) have been used in polychromatic tattoos due to their ability to retain color over long periods of time8; however, these organic red tattoo pigments also have been implicated in allergic reactions.8-11 The composition of these new organic red tattoo pigments varies, but chemical analysis has revealed a mixture of aromatic azo compounds (eg, quinacridone),10 heavy metals (eg, aluminum, lead, cadmium, chromium, cobalt, iron, titanium),9,12 and intermediate reactive compounds (eg, naphthalene, 2-naphthol, chlorobenzene, benzene).8 Allergic contact dermatitis to red tattoo ink is well documented8,13; however, a PubMed search of articles indexed for MEDLINE using the terms tattoo and dermatitis, tattoo and allergy, tattoo and autosensitization, tattoo and id reaction, and tattoo and autoeczematization yielded only 3 other reports of a concomitant id reaction.11,14,15
The diagnosis of id reaction associated with allergic contact dermatitis is made on the basis of clinical history, physical examination, and histopathology. Patch testing usually is not positive in cases of tattoo allergy; it is thought that the allergen is a tattoo ink byproduct possibly caused by photoinduced or metabolic change of the tattoo pigment and a haptenization process.1,8,16 Histologically, variable reaction patterns, including eczematous, lichenoid, granulomatous, and pseudolymphomatous reactions have been reported in association with delayed-type inflammatory reactions to tattoo pigments, but the lichenoid pattern is most commonly observed.8
Treatment options for allergic contact dermatitis to tattoo ink include topical, intralesional, and oral steroids; topical calcineurin inhibitors; and surgical excision of the tattoo. Q-switched lasers—ruby, Nd:YAG, and alexandrite—are the gold standard for removing tattoo pigments17; however, these lasers remove tattoo pigment by selective photothermolysis, resulting in extracellular extravasation of pigment, which can precipitate a heightened immune response that can lead to localized and generalized allergic reactions.18 Therefore, Q-switched lasers should be avoided in the setting of an allergic reaction to tattoo ink. Fractional ablative laser resurfacing may be a safer alternative for removal of tattoos in the setting of an allergic reaction.17 Further studies are needed to confirm the safety and efficacy of this modality for allergic tattoo ink removal.17,18
Our case illustrates a rare cause of id reaction and the subsequent development of prurigo nodules associated with contact allergy to red tattoo ink. We present this case to raise awareness of the potential health and iatrogenic risks associated with tattoo placement. Further investigation of these color additives is warranted to better elucidate ink components responsible for these cutaneous allergic reactions.
Acknowledgments
We would like to thank Vitaly Terushkin, MD (West Orange, New Jersey, and New York, New York), and Arielle Kauvar, MD (New York, New York), for their contributions to the patient’s clinical care.
- Vasold R, Engel E, Konig B, et al. Health risks of tattoo colors. Anal Bioanal Chem. 2008;391:9-13.
- Swigost AJ, Peltola J, Jacobson-Dunlop E, et al. Tattoo-related squamous proliferations: a specturm of reactive hyperplasia. Clin Exp Dermatol. 2018;43:728-732.
- Cormia FE, Esplin BM. Autoeczematization; preliminary report. Arch Derm Syphilol. 1950;61:931-945.
- Goldsmith LA, Katz SI, Gilchrest BA, et al. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Garcovich S, Carbone T, Avitabile S, et al. Lichenoid red tattoo reaction: histological and immunological perspectives. Eur J Dermatol. 2012;22:93-96.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Bendsoe N, Hansson C, Sterner O. Inflammatory reactions from organic pigments in red tattoos. Acta Derm Venereol. 1991;71:70-73.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Cristaudo A, Forte G, Bocca B, et al. Permanent tattoos: evidence of pseudolymphoma in three patients and metal composition of the dyes. Eur J Dermatol. 2012;22:776-780.
- Wenzel SM, Welzel J, Hafner C, et al. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis. 2010;63:223-227.
- Goldberg HM. Tattoo allergy. Plast Reconstr Surg. 1996;98:1315-1316.
- Gamba CS, Smith FL, Wisell J, et al. Tattoo reactions in an HIV patient: autoeczematization and progressive allergic reaction to red ink after antiretroviral therapy initiation. JAAD Case Rep. 2015;1:395-398.
- Serup J, Hutton Carlsen K. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
- Ibrahimi OA, Syed Z, Sakamoto FH, et al. Treatment of tattoo allergy with ablative fractional resurfacing: a novel paradigm for tattoo removal. J Am Acad Dermatol. 2011;64:1111-1114.
- Harper J, Losch AE, Otto SG, et al. New insight into the pathophysiology of tattoo reactions following laser tattoo removal. Plast Reconstr Surg. 2010;126:313e-314e.
- Vasold R, Engel E, Konig B, et al. Health risks of tattoo colors. Anal Bioanal Chem. 2008;391:9-13.
- Swigost AJ, Peltola J, Jacobson-Dunlop E, et al. Tattoo-related squamous proliferations: a specturm of reactive hyperplasia. Clin Exp Dermatol. 2018;43:728-732.
- Cormia FE, Esplin BM. Autoeczematization; preliminary report. Arch Derm Syphilol. 1950;61:931-945.
- Goldsmith LA, Katz SI, Gilchrest BA, et al. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- Mortimer NJ, Chave TA, Johnston GA. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Garcovich S, Carbone T, Avitabile S, et al. Lichenoid red tattoo reaction: histological and immunological perspectives. Eur J Dermatol. 2012;22:93-96.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Bendsoe N, Hansson C, Sterner O. Inflammatory reactions from organic pigments in red tattoos. Acta Derm Venereol. 1991;71:70-73.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Cristaudo A, Forte G, Bocca B, et al. Permanent tattoos: evidence of pseudolymphoma in three patients and metal composition of the dyes. Eur J Dermatol. 2012;22:776-780.
- Wenzel SM, Welzel J, Hafner C, et al. Permanent make-up colorants may cause severe skin reactions. Contact Dermatitis. 2010;63:223-227.
- Goldberg HM. Tattoo allergy. Plast Reconstr Surg. 1996;98:1315-1316.
- Gamba CS, Smith FL, Wisell J, et al. Tattoo reactions in an HIV patient: autoeczematization and progressive allergic reaction to red ink after antiretroviral therapy initiation. JAAD Case Rep. 2015;1:395-398.
- Serup J, Hutton Carlsen K. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
- Ibrahimi OA, Syed Z, Sakamoto FH, et al. Treatment of tattoo allergy with ablative fractional resurfacing: a novel paradigm for tattoo removal. J Am Acad Dermatol. 2011;64:1111-1114.
- Harper J, Losch AE, Otto SG, et al. New insight into the pathophysiology of tattoo reactions following laser tattoo removal. Plast Reconstr Surg. 2010;126:313e-314e.
Practice Points
- Hypersensitivity reactions to tattoo pigment are on the rise due to the increasing popularity and prevalence of tattoos. Systemic allergic reactions to tattoo ink are rare but can cause considerable morbidity.
- Id reaction, also known as autoeczematization or autosensitization, is a reaction that develops distant to an initial site of infection or sensitization.
- Further investigation of color additives in tattoo pigments is warranted to better elucidate the components responsible for cutaneous allergic reactions associated with tattoo ink.
Intravascular large B-cell lymphoma: an elusive diagnosis with challenging management
Intravascular large B-cell lymphoma (IVBCL) is an aggressive and systemically disseminated disease that affects the elderly, with a median age of diagnosis around 70 years and no gender predilection. It is a rare subtype of extranodal diffuse large B-cell lymphoma (DLBCL) characterized by selective growth of neoplastic cells within blood vessel lumen without any obvious extravascular tumor mass. Hence, an absence of marked lymphadenopathy and heterogeneous clinical presentation make it difficult to diagnose accurately and timely, with roughly half of the cases found postmortem in previous case reports.1,2 The exact incidence of this disease is not known, but more recently, the accuracy of diagnosis of this type of lymphoma has improved with random skin and bone marrow biopsy.1,2 We present here a clinical case of this disease with an atypical presentation followed by a detailed review of its clinical aspects.
Case presentation and summary
A 43-year-old white woman with a history of hypothyroidism and recurrent ovarian cysts presented to clinic with 3 months of loss of appetite, abdominal distension, pelvic pain, and progressive lower-extremity swelling. A physical examination was notable for marked abdominal distension, diffuse lower abdominal tenderness, and pitting lower-extremity edema. No skin rash or any other cutaneous abnormality was noted on exam. Laboratory test results revealed a lactate dehydrogenase (LDH) level of 1652 U/L and a CA-125 level of 50 U/mL (reference range, 0-35 U/mL). No significant beta-human chorionic gonadotropin and alpha-fetoprotein levels were detected. Computed-tomographic (CT) imaging revealed small bilateral pleural effusions and gallbladder wall thickening with abdominal wall edema, but it was otherwise unrevealing. An echocardiogram showed normal cardiac structure and function, with a left ventricular ejection fraction of 60%. No protein was detected in the patient’s urine, and thyroid function tests were unrevealing. Doppler ultrasound studies of her lower extremities and abdomen revealed no thrombosis. Given the patient’s continued pelvic pain, history of ovarian cysts, and elevation in CA-125, she underwent a laparoscopic total abdominal hysterectomy and bilateral salpingoopherectomy.
Histologic examination revealed neoplastic cells involving only the vascular lumina of the cervix, endomyometrium, bilateral fallopian tubes, and bilateral ovaries (Figure 1). Immunohistochemistry stains were positive for CD5, CD20, PAX-5, CD45, BCL-2, and BCL-6 and focally positive for CD10. Peripheral smear showed pseudo-Pelger–Huet cells with 5% atypical lymphoma cells (Figure 2). Complete staging with positron-emission and CT (PET–CT) imaging revealed no metabolic activity, and a bone marrow biopsy showed trilineage hematopoiesis with adequate maturation and less than 5% of the marrow involved with large B-cell lymphoma cells. A diagnosis of IVBCL was made.
Further work-up to rule out involvement of the central nervous system (CNS) included magnetic-resonance imaging (MRI) of the brain and cerebrospinal fluid (CSF) cytology and flow cytometry, which were negative.
Our patient underwent treatment with 6 cycles of infusional, dose-adjusted R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) and 6 doses of prophylactic intrathecal chemotherapy with alternating methotrexate and cytarabine (Ara-C), and initial and subsequent CSF sampling showed no disease involvement. Consolidation with high-dose chemotherapy with R-BEAM (rituximab, carmustine, etoposide, Ara-C [cytarabine], melphalan) followed by rescue autologous stem cell transplantation (ASCT) was performed, and the patient has remained in clinical and hematologic remission for the past 24 months.
Discussion
Clinical presentation
The clinical manifestation of this disease is highly variable, and virtually any organ can be involved. Besides causing constitutional symptoms, including fatigue, B symptoms, and decline in performance status, heterogeneity of the clinical presentation depends on the organ system involved. One of the exceptional features of this disease is the difference in clinical presentation based on the geographical origin of the patient.2-4
Western-variant IVBCL has a higher frequency of CNS and skin involvement, whereas Asian-variant IVBCL shows preferential involvement of bone marrow with hemophagocytosis, hepatosplenomegaly, and thrombocytopenia. However, these 2 clinical variants have no difference in clinical outcome, except with the cutaneous-variant kind.24 A retrospective case series of 38 Western-variant IVBCL cases showed that 55% of patients had B symptoms with poor performance status.3 Brain and skin were the organs that were most frequently involved, with 68% of patients having involvement of at least 1 of those organs. Ten patients in this case series had disease that was exclusively limited to the skin and described as a “cutaneous variant” of IVBCL.3
Similarly, a retrospective case series of 96 cases of Asian-variant IVBCL showed B symptoms in 76% of patients, with predominant bone marrow involvement in 75% of patients, accompanied by hemophagocytosis in 66% and hepatosplenomegaly and anemia/thrombocytopenia in 77% and 84% of the patients, respectively.4 This difference in clinical presentation might have existed as a result of ethnic difference associated with production of inflammatory cytokines, including interferon gamma, tumor necrosis factor-alpha, interlukin-1 beta, and soluble interlukin-2 receptor, with levels of soluble interlukin-2 receptor found to be significantly higher in Asian patients than non-Asian patients.2
Diagnosis
Involved organ biopsy is mandatory for establishing the diagnosis of IVBCL. Laboratory findings are nonspecific, with the most common abnormality being increased serum LDH and beta-2 microglobulin levels observed in 80% to 90% or more of patients. Despite its intravascular growth pattern, IVBCL was associated with peripheral blood involvement in only 5% to 9% of patients.1
Staging
Clinical staging work-up suggested for IVBCL patients by International Extranodal lymphoma study group in 2005 included physical examination (with emphasis on nervous system and skin), routine blood studies, peripheral blood smear, total body CT scan with contrast or PET–CT scan, MRI brain with contrast, CSF cytology, and bone marrow or organ biopsy.1 The role of fluorodeoxyglucose-PET scan is controversial but can be helpful to detect unexpected locations for biopsy and to assess treatment response.5,6
Morphology and immunophenotyping
In general, IVBCL histopathology shows large neoplastic lymphoid cells with large nuclei along with one or more nucleoli and scant cytoplasm within blood vessel lumen. Immunophenotypically, IVBCL cells mostly express nongerminal B-cell–associated markers with CD79a (100%), CD20 (96%), MUM-IRF4 (95%), CD5 (38%), and CD10 (12%) expressions. IVBCL cells have been demonstrated to lack cell surface protein CD29 and CD54 critical to transvascular migration. Similarly, aberrant expression of proteins such as CD11a and CXCR3 allows lymphoma cells to be attracted to endothelial cells, which might explain their intravascular confinement.7
Genetics
No pathognomic cytogenetic abnormalities have been reported in IVBCL to date, and the genetic features of this disease are not yet completely understood.2,7
Management
IVBCL is considered a stage IV disseminated disease with an International Prognostic Index score of high-intermediate to high in most cases. Half of the patients with IVBCL who were treated with anthracycline-based chemotherapy relapsed and died within 18 months of diagnosis. One third of the relapses involved the CNS, thereby highlighting the importance of prophylactic CNS-directed Intrathecal therapy in an induction treatment regimen.2-4 Ferreri and colleagues reported in their case series response rates of about 60%, with an overall survival (OS) of 3 years of 30% in patients who were treated with anthracycline-based chemotherapy. A multivariate analysis of the entire series showed cutaneous variant of the disease to be an independent favorable prognostic factor for OS.3
In the Murase and colleagues case series, the authors reported 67% response rates and a median OS of 13 months with CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) or CHOP-like regimens. Multivariate analysis showed older age, thrombocytopenia, and absence of anthracycline-based chemotherapy to be an independent negative prognostic factor for OS.4 Another retrospective analysis by Shimada and colleagues of 106 patients with IVBCL showed improved outcome with the addition of rituximab to CHOP-based chemotherapy (R-CHOP). Complete response rate (CR), 2-year progression-free survival, and OS were significantly higher for patients in rituximab-chemotherapy group than for those in the chemotherapy-alone group (CR, 82% vs 51%, respectively, P = .001; PFS, 56% vs 27%; OS, 66% vs 46%, P = .001), thereby establishing rituximab with CHOP-based therapy as induction therapy for IVBCL patients.8
The role of high-dose chemotherapy followed by ASCT could also be used as consolidation therapy to improve clinical outcomes as reported in 7 patients, showing durable remission after transplant in these 2 case series.3,4 Another retrospective analysis of 6 patients with IVBCL who were treated with 6 cycles of R-CHOP as induction therapy and consolidated with ASCT reported all patients to be alive and in complete remission after a median follow-up of 56 months.9 Based on the retrospective case series data by Kato and colleagues and considering that more than 80% of the patients with IVBCL were in the high-risk International Prognostic Index group, ASCT in first remission might be a useful treatment option for durable remission; however, because the median age for the diagnosis of IVBCL is about 70 years, ASCT may not be a realistic option for all patients.
Conclusions
IVBCL is a rare, aggressive, and distinct type of DLBCL with complex constellations of symptoms requiring strong clinical suspicion to establish this challenging diagnosis. Rituximab with anthracycline-based therapy along with prophylactic CNS-directed therapy followed by consolidative ASCT may lead to long-term remission. More research is needed into the genetic features of this disease to better understand its pathogenesis and potential targets for treatment.
1. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25(21):3168-3173.
2. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10(9):895-902.
3. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127(2):173-183.
4. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478-485.
5. Miura Y, Tsudo M. Fluorodeoxyglucose-PET/CT for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2010;85(8):e56-e57.
6. Shimada K, Kosugi H, Shimada S, et al. Evaluation of organ involvement in intravascular large B-cell lymphoma by 18F-fluorodeoxyglucose positron emission tomography. Int J Hematol. 2008;88(2):149-153.
7. Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136(3):333-338.
8. Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189-3195.
9. Kato K, Ohno Y, Kamimura T, et al. Long-term remission after high-dose chemotherapy followed by auto-SCT as consolidation for intravascular large B-cell lymphoma. Bone Marrow Transplant. 2014;49(12):1543-1544.
Intravascular large B-cell lymphoma (IVBCL) is an aggressive and systemically disseminated disease that affects the elderly, with a median age of diagnosis around 70 years and no gender predilection. It is a rare subtype of extranodal diffuse large B-cell lymphoma (DLBCL) characterized by selective growth of neoplastic cells within blood vessel lumen without any obvious extravascular tumor mass. Hence, an absence of marked lymphadenopathy and heterogeneous clinical presentation make it difficult to diagnose accurately and timely, with roughly half of the cases found postmortem in previous case reports.1,2 The exact incidence of this disease is not known, but more recently, the accuracy of diagnosis of this type of lymphoma has improved with random skin and bone marrow biopsy.1,2 We present here a clinical case of this disease with an atypical presentation followed by a detailed review of its clinical aspects.
Case presentation and summary
A 43-year-old white woman with a history of hypothyroidism and recurrent ovarian cysts presented to clinic with 3 months of loss of appetite, abdominal distension, pelvic pain, and progressive lower-extremity swelling. A physical examination was notable for marked abdominal distension, diffuse lower abdominal tenderness, and pitting lower-extremity edema. No skin rash or any other cutaneous abnormality was noted on exam. Laboratory test results revealed a lactate dehydrogenase (LDH) level of 1652 U/L and a CA-125 level of 50 U/mL (reference range, 0-35 U/mL). No significant beta-human chorionic gonadotropin and alpha-fetoprotein levels were detected. Computed-tomographic (CT) imaging revealed small bilateral pleural effusions and gallbladder wall thickening with abdominal wall edema, but it was otherwise unrevealing. An echocardiogram showed normal cardiac structure and function, with a left ventricular ejection fraction of 60%. No protein was detected in the patient’s urine, and thyroid function tests were unrevealing. Doppler ultrasound studies of her lower extremities and abdomen revealed no thrombosis. Given the patient’s continued pelvic pain, history of ovarian cysts, and elevation in CA-125, she underwent a laparoscopic total abdominal hysterectomy and bilateral salpingoopherectomy.
Histologic examination revealed neoplastic cells involving only the vascular lumina of the cervix, endomyometrium, bilateral fallopian tubes, and bilateral ovaries (Figure 1). Immunohistochemistry stains were positive for CD5, CD20, PAX-5, CD45, BCL-2, and BCL-6 and focally positive for CD10. Peripheral smear showed pseudo-Pelger–Huet cells with 5% atypical lymphoma cells (Figure 2). Complete staging with positron-emission and CT (PET–CT) imaging revealed no metabolic activity, and a bone marrow biopsy showed trilineage hematopoiesis with adequate maturation and less than 5% of the marrow involved with large B-cell lymphoma cells. A diagnosis of IVBCL was made.
Further work-up to rule out involvement of the central nervous system (CNS) included magnetic-resonance imaging (MRI) of the brain and cerebrospinal fluid (CSF) cytology and flow cytometry, which were negative.
Our patient underwent treatment with 6 cycles of infusional, dose-adjusted R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) and 6 doses of prophylactic intrathecal chemotherapy with alternating methotrexate and cytarabine (Ara-C), and initial and subsequent CSF sampling showed no disease involvement. Consolidation with high-dose chemotherapy with R-BEAM (rituximab, carmustine, etoposide, Ara-C [cytarabine], melphalan) followed by rescue autologous stem cell transplantation (ASCT) was performed, and the patient has remained in clinical and hematologic remission for the past 24 months.
Discussion
Clinical presentation
The clinical manifestation of this disease is highly variable, and virtually any organ can be involved. Besides causing constitutional symptoms, including fatigue, B symptoms, and decline in performance status, heterogeneity of the clinical presentation depends on the organ system involved. One of the exceptional features of this disease is the difference in clinical presentation based on the geographical origin of the patient.2-4
Western-variant IVBCL has a higher frequency of CNS and skin involvement, whereas Asian-variant IVBCL shows preferential involvement of bone marrow with hemophagocytosis, hepatosplenomegaly, and thrombocytopenia. However, these 2 clinical variants have no difference in clinical outcome, except with the cutaneous-variant kind.24 A retrospective case series of 38 Western-variant IVBCL cases showed that 55% of patients had B symptoms with poor performance status.3 Brain and skin were the organs that were most frequently involved, with 68% of patients having involvement of at least 1 of those organs. Ten patients in this case series had disease that was exclusively limited to the skin and described as a “cutaneous variant” of IVBCL.3
Similarly, a retrospective case series of 96 cases of Asian-variant IVBCL showed B symptoms in 76% of patients, with predominant bone marrow involvement in 75% of patients, accompanied by hemophagocytosis in 66% and hepatosplenomegaly and anemia/thrombocytopenia in 77% and 84% of the patients, respectively.4 This difference in clinical presentation might have existed as a result of ethnic difference associated with production of inflammatory cytokines, including interferon gamma, tumor necrosis factor-alpha, interlukin-1 beta, and soluble interlukin-2 receptor, with levels of soluble interlukin-2 receptor found to be significantly higher in Asian patients than non-Asian patients.2
Diagnosis
Involved organ biopsy is mandatory for establishing the diagnosis of IVBCL. Laboratory findings are nonspecific, with the most common abnormality being increased serum LDH and beta-2 microglobulin levels observed in 80% to 90% or more of patients. Despite its intravascular growth pattern, IVBCL was associated with peripheral blood involvement in only 5% to 9% of patients.1
Staging
Clinical staging work-up suggested for IVBCL patients by International Extranodal lymphoma study group in 2005 included physical examination (with emphasis on nervous system and skin), routine blood studies, peripheral blood smear, total body CT scan with contrast or PET–CT scan, MRI brain with contrast, CSF cytology, and bone marrow or organ biopsy.1 The role of fluorodeoxyglucose-PET scan is controversial but can be helpful to detect unexpected locations for biopsy and to assess treatment response.5,6
Morphology and immunophenotyping
In general, IVBCL histopathology shows large neoplastic lymphoid cells with large nuclei along with one or more nucleoli and scant cytoplasm within blood vessel lumen. Immunophenotypically, IVBCL cells mostly express nongerminal B-cell–associated markers with CD79a (100%), CD20 (96%), MUM-IRF4 (95%), CD5 (38%), and CD10 (12%) expressions. IVBCL cells have been demonstrated to lack cell surface protein CD29 and CD54 critical to transvascular migration. Similarly, aberrant expression of proteins such as CD11a and CXCR3 allows lymphoma cells to be attracted to endothelial cells, which might explain their intravascular confinement.7
Genetics
No pathognomic cytogenetic abnormalities have been reported in IVBCL to date, and the genetic features of this disease are not yet completely understood.2,7
Management
IVBCL is considered a stage IV disseminated disease with an International Prognostic Index score of high-intermediate to high in most cases. Half of the patients with IVBCL who were treated with anthracycline-based chemotherapy relapsed and died within 18 months of diagnosis. One third of the relapses involved the CNS, thereby highlighting the importance of prophylactic CNS-directed Intrathecal therapy in an induction treatment regimen.2-4 Ferreri and colleagues reported in their case series response rates of about 60%, with an overall survival (OS) of 3 years of 30% in patients who were treated with anthracycline-based chemotherapy. A multivariate analysis of the entire series showed cutaneous variant of the disease to be an independent favorable prognostic factor for OS.3
In the Murase and colleagues case series, the authors reported 67% response rates and a median OS of 13 months with CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) or CHOP-like regimens. Multivariate analysis showed older age, thrombocytopenia, and absence of anthracycline-based chemotherapy to be an independent negative prognostic factor for OS.4 Another retrospective analysis by Shimada and colleagues of 106 patients with IVBCL showed improved outcome with the addition of rituximab to CHOP-based chemotherapy (R-CHOP). Complete response rate (CR), 2-year progression-free survival, and OS were significantly higher for patients in rituximab-chemotherapy group than for those in the chemotherapy-alone group (CR, 82% vs 51%, respectively, P = .001; PFS, 56% vs 27%; OS, 66% vs 46%, P = .001), thereby establishing rituximab with CHOP-based therapy as induction therapy for IVBCL patients.8
The role of high-dose chemotherapy followed by ASCT could also be used as consolidation therapy to improve clinical outcomes as reported in 7 patients, showing durable remission after transplant in these 2 case series.3,4 Another retrospective analysis of 6 patients with IVBCL who were treated with 6 cycles of R-CHOP as induction therapy and consolidated with ASCT reported all patients to be alive and in complete remission after a median follow-up of 56 months.9 Based on the retrospective case series data by Kato and colleagues and considering that more than 80% of the patients with IVBCL were in the high-risk International Prognostic Index group, ASCT in first remission might be a useful treatment option for durable remission; however, because the median age for the diagnosis of IVBCL is about 70 years, ASCT may not be a realistic option for all patients.
Conclusions
IVBCL is a rare, aggressive, and distinct type of DLBCL with complex constellations of symptoms requiring strong clinical suspicion to establish this challenging diagnosis. Rituximab with anthracycline-based therapy along with prophylactic CNS-directed therapy followed by consolidative ASCT may lead to long-term remission. More research is needed into the genetic features of this disease to better understand its pathogenesis and potential targets for treatment.
Intravascular large B-cell lymphoma (IVBCL) is an aggressive and systemically disseminated disease that affects the elderly, with a median age of diagnosis around 70 years and no gender predilection. It is a rare subtype of extranodal diffuse large B-cell lymphoma (DLBCL) characterized by selective growth of neoplastic cells within blood vessel lumen without any obvious extravascular tumor mass. Hence, an absence of marked lymphadenopathy and heterogeneous clinical presentation make it difficult to diagnose accurately and timely, with roughly half of the cases found postmortem in previous case reports.1,2 The exact incidence of this disease is not known, but more recently, the accuracy of diagnosis of this type of lymphoma has improved with random skin and bone marrow biopsy.1,2 We present here a clinical case of this disease with an atypical presentation followed by a detailed review of its clinical aspects.
Case presentation and summary
A 43-year-old white woman with a history of hypothyroidism and recurrent ovarian cysts presented to clinic with 3 months of loss of appetite, abdominal distension, pelvic pain, and progressive lower-extremity swelling. A physical examination was notable for marked abdominal distension, diffuse lower abdominal tenderness, and pitting lower-extremity edema. No skin rash or any other cutaneous abnormality was noted on exam. Laboratory test results revealed a lactate dehydrogenase (LDH) level of 1652 U/L and a CA-125 level of 50 U/mL (reference range, 0-35 U/mL). No significant beta-human chorionic gonadotropin and alpha-fetoprotein levels were detected. Computed-tomographic (CT) imaging revealed small bilateral pleural effusions and gallbladder wall thickening with abdominal wall edema, but it was otherwise unrevealing. An echocardiogram showed normal cardiac structure and function, with a left ventricular ejection fraction of 60%. No protein was detected in the patient’s urine, and thyroid function tests were unrevealing. Doppler ultrasound studies of her lower extremities and abdomen revealed no thrombosis. Given the patient’s continued pelvic pain, history of ovarian cysts, and elevation in CA-125, she underwent a laparoscopic total abdominal hysterectomy and bilateral salpingoopherectomy.
Histologic examination revealed neoplastic cells involving only the vascular lumina of the cervix, endomyometrium, bilateral fallopian tubes, and bilateral ovaries (Figure 1). Immunohistochemistry stains were positive for CD5, CD20, PAX-5, CD45, BCL-2, and BCL-6 and focally positive for CD10. Peripheral smear showed pseudo-Pelger–Huet cells with 5% atypical lymphoma cells (Figure 2). Complete staging with positron-emission and CT (PET–CT) imaging revealed no metabolic activity, and a bone marrow biopsy showed trilineage hematopoiesis with adequate maturation and less than 5% of the marrow involved with large B-cell lymphoma cells. A diagnosis of IVBCL was made.
Further work-up to rule out involvement of the central nervous system (CNS) included magnetic-resonance imaging (MRI) of the brain and cerebrospinal fluid (CSF) cytology and flow cytometry, which were negative.
Our patient underwent treatment with 6 cycles of infusional, dose-adjusted R-EPOCH (rituximab, etoposide phosphate, prednisone, vincristine sulfate, cyclophosphamide, doxorubicin hydrochloride) and 6 doses of prophylactic intrathecal chemotherapy with alternating methotrexate and cytarabine (Ara-C), and initial and subsequent CSF sampling showed no disease involvement. Consolidation with high-dose chemotherapy with R-BEAM (rituximab, carmustine, etoposide, Ara-C [cytarabine], melphalan) followed by rescue autologous stem cell transplantation (ASCT) was performed, and the patient has remained in clinical and hematologic remission for the past 24 months.
Discussion
Clinical presentation
The clinical manifestation of this disease is highly variable, and virtually any organ can be involved. Besides causing constitutional symptoms, including fatigue, B symptoms, and decline in performance status, heterogeneity of the clinical presentation depends on the organ system involved. One of the exceptional features of this disease is the difference in clinical presentation based on the geographical origin of the patient.2-4
Western-variant IVBCL has a higher frequency of CNS and skin involvement, whereas Asian-variant IVBCL shows preferential involvement of bone marrow with hemophagocytosis, hepatosplenomegaly, and thrombocytopenia. However, these 2 clinical variants have no difference in clinical outcome, except with the cutaneous-variant kind.24 A retrospective case series of 38 Western-variant IVBCL cases showed that 55% of patients had B symptoms with poor performance status.3 Brain and skin were the organs that were most frequently involved, with 68% of patients having involvement of at least 1 of those organs. Ten patients in this case series had disease that was exclusively limited to the skin and described as a “cutaneous variant” of IVBCL.3
Similarly, a retrospective case series of 96 cases of Asian-variant IVBCL showed B symptoms in 76% of patients, with predominant bone marrow involvement in 75% of patients, accompanied by hemophagocytosis in 66% and hepatosplenomegaly and anemia/thrombocytopenia in 77% and 84% of the patients, respectively.4 This difference in clinical presentation might have existed as a result of ethnic difference associated with production of inflammatory cytokines, including interferon gamma, tumor necrosis factor-alpha, interlukin-1 beta, and soluble interlukin-2 receptor, with levels of soluble interlukin-2 receptor found to be significantly higher in Asian patients than non-Asian patients.2
Diagnosis
Involved organ biopsy is mandatory for establishing the diagnosis of IVBCL. Laboratory findings are nonspecific, with the most common abnormality being increased serum LDH and beta-2 microglobulin levels observed in 80% to 90% or more of patients. Despite its intravascular growth pattern, IVBCL was associated with peripheral blood involvement in only 5% to 9% of patients.1
Staging
Clinical staging work-up suggested for IVBCL patients by International Extranodal lymphoma study group in 2005 included physical examination (with emphasis on nervous system and skin), routine blood studies, peripheral blood smear, total body CT scan with contrast or PET–CT scan, MRI brain with contrast, CSF cytology, and bone marrow or organ biopsy.1 The role of fluorodeoxyglucose-PET scan is controversial but can be helpful to detect unexpected locations for biopsy and to assess treatment response.5,6
Morphology and immunophenotyping
In general, IVBCL histopathology shows large neoplastic lymphoid cells with large nuclei along with one or more nucleoli and scant cytoplasm within blood vessel lumen. Immunophenotypically, IVBCL cells mostly express nongerminal B-cell–associated markers with CD79a (100%), CD20 (96%), MUM-IRF4 (95%), CD5 (38%), and CD10 (12%) expressions. IVBCL cells have been demonstrated to lack cell surface protein CD29 and CD54 critical to transvascular migration. Similarly, aberrant expression of proteins such as CD11a and CXCR3 allows lymphoma cells to be attracted to endothelial cells, which might explain their intravascular confinement.7
Genetics
No pathognomic cytogenetic abnormalities have been reported in IVBCL to date, and the genetic features of this disease are not yet completely understood.2,7
Management
IVBCL is considered a stage IV disseminated disease with an International Prognostic Index score of high-intermediate to high in most cases. Half of the patients with IVBCL who were treated with anthracycline-based chemotherapy relapsed and died within 18 months of diagnosis. One third of the relapses involved the CNS, thereby highlighting the importance of prophylactic CNS-directed Intrathecal therapy in an induction treatment regimen.2-4 Ferreri and colleagues reported in their case series response rates of about 60%, with an overall survival (OS) of 3 years of 30% in patients who were treated with anthracycline-based chemotherapy. A multivariate analysis of the entire series showed cutaneous variant of the disease to be an independent favorable prognostic factor for OS.3
In the Murase and colleagues case series, the authors reported 67% response rates and a median OS of 13 months with CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, prednisone) or CHOP-like regimens. Multivariate analysis showed older age, thrombocytopenia, and absence of anthracycline-based chemotherapy to be an independent negative prognostic factor for OS.4 Another retrospective analysis by Shimada and colleagues of 106 patients with IVBCL showed improved outcome with the addition of rituximab to CHOP-based chemotherapy (R-CHOP). Complete response rate (CR), 2-year progression-free survival, and OS were significantly higher for patients in rituximab-chemotherapy group than for those in the chemotherapy-alone group (CR, 82% vs 51%, respectively, P = .001; PFS, 56% vs 27%; OS, 66% vs 46%, P = .001), thereby establishing rituximab with CHOP-based therapy as induction therapy for IVBCL patients.8
The role of high-dose chemotherapy followed by ASCT could also be used as consolidation therapy to improve clinical outcomes as reported in 7 patients, showing durable remission after transplant in these 2 case series.3,4 Another retrospective analysis of 6 patients with IVBCL who were treated with 6 cycles of R-CHOP as induction therapy and consolidated with ASCT reported all patients to be alive and in complete remission after a median follow-up of 56 months.9 Based on the retrospective case series data by Kato and colleagues and considering that more than 80% of the patients with IVBCL were in the high-risk International Prognostic Index group, ASCT in first remission might be a useful treatment option for durable remission; however, because the median age for the diagnosis of IVBCL is about 70 years, ASCT may not be a realistic option for all patients.
Conclusions
IVBCL is a rare, aggressive, and distinct type of DLBCL with complex constellations of symptoms requiring strong clinical suspicion to establish this challenging diagnosis. Rituximab with anthracycline-based therapy along with prophylactic CNS-directed therapy followed by consolidative ASCT may lead to long-term remission. More research is needed into the genetic features of this disease to better understand its pathogenesis and potential targets for treatment.
1. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25(21):3168-3173.
2. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10(9):895-902.
3. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127(2):173-183.
4. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478-485.
5. Miura Y, Tsudo M. Fluorodeoxyglucose-PET/CT for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2010;85(8):e56-e57.
6. Shimada K, Kosugi H, Shimada S, et al. Evaluation of organ involvement in intravascular large B-cell lymphoma by 18F-fluorodeoxyglucose positron emission tomography. Int J Hematol. 2008;88(2):149-153.
7. Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136(3):333-338.
8. Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189-3195.
9. Kato K, Ohno Y, Kamimura T, et al. Long-term remission after high-dose chemotherapy followed by auto-SCT as consolidation for intravascular large B-cell lymphoma. Bone Marrow Transplant. 2014;49(12):1543-1544.
1. Ponzoni M, Ferreri AJ, Campo E, et al. Definition, diagnosis, and management of intravascular large B-cell lymphoma: proposals and perspectives from an international consensus meeting. J Clin Oncol. 2007;25(21):3168-3173.
2. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10(9):895-902.
3. Ferreri AJ, Campo E, Seymour JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127(2):173-183.
4. Murase T, Yamaguchi M, Suzuki R, et al. Intravascular large B-cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood. 2007;109(2):478-485.
5. Miura Y, Tsudo M. Fluorodeoxyglucose-PET/CT for diagnosis of intravascular large B-cell lymphoma. Mayo Clin Proc. 2010;85(8):e56-e57.
6. Shimada K, Kosugi H, Shimada S, et al. Evaluation of organ involvement in intravascular large B-cell lymphoma by 18F-fluorodeoxyglucose positron emission tomography. Int J Hematol. 2008;88(2):149-153.
7. Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136(3):333-338.
8. Shimada K, Matsue K, Yamamoto K, et al. Retrospective analysis of intravascular large B-cell lymphoma treated with rituximab-containing chemotherapy as reported by the IVL study group in Japan. J Clin Oncol. 2008;26(19):3189-3195.
9. Kato K, Ohno Y, Kamimura T, et al. Long-term remission after high-dose chemotherapy followed by auto-SCT as consolidation for intravascular large B-cell lymphoma. Bone Marrow Transplant. 2014;49(12):1543-1544.
Elevated liver function tests in a patient on palbociclib and fulvestrant
About 12.4% of women in the United States will be diagnosed with breast cancer at some point in their lifetime.1 A percentage of these women will develop metastatic disease and are estimated to have a 5-year survival rate of 22%.2 There have been meaningful improvements in su
However, endocrine resistance inevitably occurs, and a great deal of research has been focused on developing strategies to combat resistance. One mechanism of endocrine resistance is though the Cyclin-dependent kinases 4 and 6 (CDK4/6) complexes. Among the most promising of the strategies to prevent resistance are the CDK4/6 inhibitors. There are now 3 approved CDK4/6 inhibitor drugs that can be used in combination with endocrine therapy, 1 of which can also be used as a single agent. When used in combination with endocrine therapy, the use of CDK 4/6 inhibitors has significantly improved progression-free survival (PFS) in patients with hormone-sensitive HER2-negative metastatic breast cancer by inhibiting cellular division and growth.3 In postmenopausal women, endocrine therapy plus CDK4/6 inhibitors are the preferred first-line regimen for metastatic disease.
Since the approval of palbociclib by the US Food and Drug Administration in 2015, the most common hematologic lab abnormalities are anemia, leukopenia, neutropenia, and thrombocytopenia. The most common nonhematologic adverse events (AEs) are fatigue, infection, nausea, and stomatitis. Hepatic toxicity has not been commonly observed. We report here the case of a 57-year-old woman on palbociclib and fulvestrant who developed significant elevation of liver function tests after starting palbociclib, suggesting a possible drug-induced liver injury from palbociclib.
Case presentation and summary
A 57-year-old woman with history of hypothyroidism and hypertension presented in May 2016 with a lump in her right breast and back pain. The lump was biopsied and revealed invasive ductal carcinoma, moderately differentiated, estrogen receptor (ER) positive 100%, progesterone receptor (PR) positive 95%, and HER2 negative. A positron emission tomography (PET)–computed tomography (CT) scan and magnetic resonance imaging showed bone metastasis at several vertebral levels, and the results of a bone biopsy confirmed metastatic adenocarcinoma of breast origin, ER positive 60%, PR positive 40%, and HER2 negative. No liver lesions were seen on imaging, but there was suggestion of fatty liver. She was started on letrozole 2.5 mg daily in July 2016 while undergoing kyphoplasty and subsequent radiation. A restaging PET scan revealed progression of disease on letrozole, with possible new rib lesion and progression in the breast. No liver disease was noted. Therapy was changed to fulvestrant and palbociclib. Fulvestrant was started in March 2017 with standard dosing of 500 mg intramuscular on days 1, 15, and 29, and then once a month thereafter. Her first cycle of palbociclib was started on April 5, dosed at 125 mg by mouth daily for 21 days, followed by 7 days off, repeated every 28 days (all dates hereinafter fell within 2017, unless otherwise stipulated).
Labs checked on April 28 and May 26 were unremarkable. A restaging CT scan of the chest, abdomen, and pelvis was done on June 21 after completion of 3 cycles of fulvestrant and palbociclib. There was no evidence of liver metastases, only the fatty infiltration of the liver that had been seen previously. On June 23, 2017, lab results showed a transaminitis with an alanine aminotransferase (ALT) level of 446 IU/L (reference range 10-33 IU/L) and aspartate aminotransferase (AST) level of 183 IU/L (reference range 0-32 IU/L).
The patient’s liver enzyme levels continued to increase and peaked on July 3 at ALT >700 IU/L and AST at 421 IU/L. Her total bilirubin and alkaline phosphatase levels remained within normal limits. She had received her final dose of fulvestrant on May 31 and had taken her last dose of palbociclib on June 20, 2017. She had no history of elevated liver enzymes or liver disease, although the initial PET scan done at diagnosis had suggested hepatic steatosis. She said she had not recently used antibiotics, alcohol, or over-the-counter medications or supplements. There was no family history of liver problems, inflammatory bowel disease, or gastrointestinal malignancy. The only other medications she had taken recently were denosumab, levothyroxine for hypothyroidism, and amlodipine for hypertension. She was seen by hepatology for evaluation of acute hepatitis. Other etiologies for her elevated liver enzymes were ruled out, and she was diagnosed with a drug-induced liver injury from one of her anticancer medications. Her treatments with fulvestrant and palbociclib were held, and the results of her liver function tests normalized by September 2017.
Fulvestrant was restarted on August 24, and her lab results remained normal through November of that year, when restaging scans showed progression with new axillary adenopathy suspicious for metastasis. Imaging also showed a 1.6-cm hepatic lesion suggestive of a focal area of fat deposition or atypical hemangioma without definitive evidence of metastasis. Follow-up imaging was recommended. She was therefore rechallenged with palbociclib at a reduced dose of 100 mg by mouth daily and received the first dose on November 30. On December 8, repeat labs again showed elevated liver function tests (ALT, 285 IU/L; AST, 112 IU/L). Treatment with palbociclib was discontinued on December 10. Because the patient was not able to tolerate palbociclib, and fulvestrant alone was not controlling the disease, she was started on an alternate endocrine therapy with tamoxifen on December 26. The patient’s liver function tests normalized again by January 2018.
Discussion
The use of targeted therapies has changed the landscape of oncologic treatments. Several studies have evaluated the safety and efficacy of palbociclib in combination with endocrine therapy. The Palbociclib Ongoing Trials in the Management of Breast Cancer (PALOMA)-1 study, an open-label, randomized, phase-2 trial involving patients with newly diagnosed metastatic hormone sensitive HER2-negative breast cancer, demonstrated that palbociclib in combination with letrozole was associated with significantly longer PFS than letrozole alone.4 These results were later confirmed in the larger PALOMA-2 study, a randomized, double-blind, phase-3 trial that evaluated 666 postmenopausal patients with no prior systemic therapy. In that study, median PFS for the palbociclib–letrozole group was 24.8 months, compared with 14.5 months for the letrozole-alone group (hazard ratio [HR] for disease progression or death, 0.58 [0.46–0.72], P < .001).5 The most recent PALOMA-3 study, a phase-3 trial involving 521 patients with advanced hormone receptor–positive, HER2-negative breast cancer that had progressed during initial endocrine therapy, evaluated the efficacy of combined palbociclib and fulvestrant in a randomized, double-blind, placebo-controlled, parallel-group trial. The result was that the palbociclib–fulvestrant combination resulted in longer median PFS of 9.2 months, compared with 3.8 months with fulvestrant alone (P < .001).6
These trials also monitored the number of AEs as secondary aims. The most commonly reported AEs in the PALOMA trials for those patients in the palbociclib group were hematologic, with neutropenia being the most common, followed by leukopenia, anemia, and thrombocytopenia. The most common nonhematologic AEs reported in the palbociclib-fulvestrant group were fatigue, nausea, and headache. Elevated liver function tests were a rare but reported AE in 7.2% of the palbociclib-treated patients in the PALOMA-1 study.7 In the PALOMA-2 study, ALT and AST elevations were reported as AEs (all grades) in 9.9% and 9.7% of palbociclib-treated patients, respectively.5 In the PALOMA-3 study, there was 1 fatal serious AE of hepatic failure with grade 5 disease progression in the palbociclib group; however, the patient’s medical history included progressive liver metastasis and disease progression.6 A pooled safety analysis conducted across all PALOMA studies demonstrated that grade 3/4 AST and ALT elevations occurred in 3.3% and 2.3% of palbociclib-treated patients, respectively, again highlighting a reported but rare occurrence.8
The patient described in the present case report started on combination fulvestrant and palbociclib after her disease showed progression on letrozole. She developed an increase in transaminases after completing 3 cycles of palbociclib. Liver function tests increased nearly 12 weeks after beginning her first cycle of the CDK 4/6 inhibitor. Staging scans of the patient demonstrated fatty liver. It is not known if her fatty liver contributed to her transminitis; however, her baseline labs showed normal liver function tests, and they did not increase until after therapy with fulvestrant–palbociclib was started. It might have been that her fatty liver caused her to be at higher risk of transaminitis with administration of palbociclib, although we cannot be certain. Her lab results remained normal while she was on fulvestrant alone, and the liver function test results increased only after palbociclib was started, making this drug the more likely culprit.
Both events of increased liver enzymes occurred within a week of the last palbociclib dose; however, we note that hepatotoxicity developed at a faster rate when the patient was rechallenged with palbociclib at a lower dose, with elevated liver function tests increasing 1 week after restarting treatment as opposed to the first episode that occurred after 3 cycles of the palbociclib. After discontinuation of the medication, liver function tests again normalized, suggesting that palbociclib was most likely the causative agent. In addition, the degree of elevated liver enzymes was less severe on re-exposure at the lower dose of 100 mg, which raises the possibility that there could be a dose-dependent association between palbociclib and hepatotoxicity. There have been few case reports of increased liver enzymes associated with palbociclib, and it is only recently that this association has been more recognized. A meta-analysis by Zaw and colleagues has demonstrated that CDK 4/6 inhibitor–based regimens are associated with a higher risk of elevated AST and ALT; however, their relation with dose dependence was not described. In particular, they found that CDK 4/6 inhibitors increased the risk of high-grade, elevated ALT with a relative risk of 4.33 (95% confidence interval, 2.15-8.71; P < .0001). The meta-analysis also included other CDK 4/6 inhibitors such as abemaciclib and ribociclib, which have been more commonly associated with liver toxicity than palbociclib has.9 Our case report highlights the specific association between palbociclib and elevated liver enzymes.
In conclusion, this case report illustrates that our patient’s elevated liver enzymes were likely related to palbociclib. This is further supported by the fact that this AE occurred twice, both times after palbociclib exposure. In each instance, liver enzymes normalized after discontinuation of palbociclib. One cannot entirely rule out that fulvestrant might have been the culprit medication, but the patient’s normal hepatic panel for several months after starting fulvestrant suggests that is less likely. This case report is indicative of an uncommon complication in the treatment of metastatic breast cancer, one that is starting to gain more recognition, and we must think of palbociclib as a possible cause of drug-induced liver injury when targeted CDK 4/6–based regimens are used.
1. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/csr/1975_2014/. Accessed April 3, 2018.
2. American Cancer Society. Breast cancer survival rates. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html. Accessed April 3, 2018.
3. Wolff AC. CDK4 and CDK6 inhibition in breast cancer - a new standard. N Engl J Med. 2016; 375(20):1993-1994.
4. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomized phase 2 study. Lancet Oncol. 2015;16(1):25-35.
5. Finn RS, Martin M, Rugo, HS et. al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med. 2016;375:1925-1936
6. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomized controlled trial. Lancet Oncol. 2016;17(4):425-439.
7. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209-219.
8. Dieras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HR- advanced breast cancer [published online July 18, 2018]. Natl Cancer Inst. 2018;111.
9. Zaw M, Thein KZ, Tun A, et al. A systematic review and meta-analysis of randomized controlled trials to evaluate the risk of gastrointestinal and hepatic toxicities in patients with hormone receptor positive HER2-negative breast cancer treated with CKD 4/6 inhibitors. J Clin Oncol. 2017;35(suppl 31):209.
About 12.4% of women in the United States will be diagnosed with breast cancer at some point in their lifetime.1 A percentage of these women will develop metastatic disease and are estimated to have a 5-year survival rate of 22%.2 There have been meaningful improvements in su
However, endocrine resistance inevitably occurs, and a great deal of research has been focused on developing strategies to combat resistance. One mechanism of endocrine resistance is though the Cyclin-dependent kinases 4 and 6 (CDK4/6) complexes. Among the most promising of the strategies to prevent resistance are the CDK4/6 inhibitors. There are now 3 approved CDK4/6 inhibitor drugs that can be used in combination with endocrine therapy, 1 of which can also be used as a single agent. When used in combination with endocrine therapy, the use of CDK 4/6 inhibitors has significantly improved progression-free survival (PFS) in patients with hormone-sensitive HER2-negative metastatic breast cancer by inhibiting cellular division and growth.3 In postmenopausal women, endocrine therapy plus CDK4/6 inhibitors are the preferred first-line regimen for metastatic disease.
Since the approval of palbociclib by the US Food and Drug Administration in 2015, the most common hematologic lab abnormalities are anemia, leukopenia, neutropenia, and thrombocytopenia. The most common nonhematologic adverse events (AEs) are fatigue, infection, nausea, and stomatitis. Hepatic toxicity has not been commonly observed. We report here the case of a 57-year-old woman on palbociclib and fulvestrant who developed significant elevation of liver function tests after starting palbociclib, suggesting a possible drug-induced liver injury from palbociclib.
Case presentation and summary
A 57-year-old woman with history of hypothyroidism and hypertension presented in May 2016 with a lump in her right breast and back pain. The lump was biopsied and revealed invasive ductal carcinoma, moderately differentiated, estrogen receptor (ER) positive 100%, progesterone receptor (PR) positive 95%, and HER2 negative. A positron emission tomography (PET)–computed tomography (CT) scan and magnetic resonance imaging showed bone metastasis at several vertebral levels, and the results of a bone biopsy confirmed metastatic adenocarcinoma of breast origin, ER positive 60%, PR positive 40%, and HER2 negative. No liver lesions were seen on imaging, but there was suggestion of fatty liver. She was started on letrozole 2.5 mg daily in July 2016 while undergoing kyphoplasty and subsequent radiation. A restaging PET scan revealed progression of disease on letrozole, with possible new rib lesion and progression in the breast. No liver disease was noted. Therapy was changed to fulvestrant and palbociclib. Fulvestrant was started in March 2017 with standard dosing of 500 mg intramuscular on days 1, 15, and 29, and then once a month thereafter. Her first cycle of palbociclib was started on April 5, dosed at 125 mg by mouth daily for 21 days, followed by 7 days off, repeated every 28 days (all dates hereinafter fell within 2017, unless otherwise stipulated).
Labs checked on April 28 and May 26 were unremarkable. A restaging CT scan of the chest, abdomen, and pelvis was done on June 21 after completion of 3 cycles of fulvestrant and palbociclib. There was no evidence of liver metastases, only the fatty infiltration of the liver that had been seen previously. On June 23, 2017, lab results showed a transaminitis with an alanine aminotransferase (ALT) level of 446 IU/L (reference range 10-33 IU/L) and aspartate aminotransferase (AST) level of 183 IU/L (reference range 0-32 IU/L).
The patient’s liver enzyme levels continued to increase and peaked on July 3 at ALT >700 IU/L and AST at 421 IU/L. Her total bilirubin and alkaline phosphatase levels remained within normal limits. She had received her final dose of fulvestrant on May 31 and had taken her last dose of palbociclib on June 20, 2017. She had no history of elevated liver enzymes or liver disease, although the initial PET scan done at diagnosis had suggested hepatic steatosis. She said she had not recently used antibiotics, alcohol, or over-the-counter medications or supplements. There was no family history of liver problems, inflammatory bowel disease, or gastrointestinal malignancy. The only other medications she had taken recently were denosumab, levothyroxine for hypothyroidism, and amlodipine for hypertension. She was seen by hepatology for evaluation of acute hepatitis. Other etiologies for her elevated liver enzymes were ruled out, and she was diagnosed with a drug-induced liver injury from one of her anticancer medications. Her treatments with fulvestrant and palbociclib were held, and the results of her liver function tests normalized by September 2017.
Fulvestrant was restarted on August 24, and her lab results remained normal through November of that year, when restaging scans showed progression with new axillary adenopathy suspicious for metastasis. Imaging also showed a 1.6-cm hepatic lesion suggestive of a focal area of fat deposition or atypical hemangioma without definitive evidence of metastasis. Follow-up imaging was recommended. She was therefore rechallenged with palbociclib at a reduced dose of 100 mg by mouth daily and received the first dose on November 30. On December 8, repeat labs again showed elevated liver function tests (ALT, 285 IU/L; AST, 112 IU/L). Treatment with palbociclib was discontinued on December 10. Because the patient was not able to tolerate palbociclib, and fulvestrant alone was not controlling the disease, she was started on an alternate endocrine therapy with tamoxifen on December 26. The patient’s liver function tests normalized again by January 2018.
Discussion
The use of targeted therapies has changed the landscape of oncologic treatments. Several studies have evaluated the safety and efficacy of palbociclib in combination with endocrine therapy. The Palbociclib Ongoing Trials in the Management of Breast Cancer (PALOMA)-1 study, an open-label, randomized, phase-2 trial involving patients with newly diagnosed metastatic hormone sensitive HER2-negative breast cancer, demonstrated that palbociclib in combination with letrozole was associated with significantly longer PFS than letrozole alone.4 These results were later confirmed in the larger PALOMA-2 study, a randomized, double-blind, phase-3 trial that evaluated 666 postmenopausal patients with no prior systemic therapy. In that study, median PFS for the palbociclib–letrozole group was 24.8 months, compared with 14.5 months for the letrozole-alone group (hazard ratio [HR] for disease progression or death, 0.58 [0.46–0.72], P < .001).5 The most recent PALOMA-3 study, a phase-3 trial involving 521 patients with advanced hormone receptor–positive, HER2-negative breast cancer that had progressed during initial endocrine therapy, evaluated the efficacy of combined palbociclib and fulvestrant in a randomized, double-blind, placebo-controlled, parallel-group trial. The result was that the palbociclib–fulvestrant combination resulted in longer median PFS of 9.2 months, compared with 3.8 months with fulvestrant alone (P < .001).6
These trials also monitored the number of AEs as secondary aims. The most commonly reported AEs in the PALOMA trials for those patients in the palbociclib group were hematologic, with neutropenia being the most common, followed by leukopenia, anemia, and thrombocytopenia. The most common nonhematologic AEs reported in the palbociclib-fulvestrant group were fatigue, nausea, and headache. Elevated liver function tests were a rare but reported AE in 7.2% of the palbociclib-treated patients in the PALOMA-1 study.7 In the PALOMA-2 study, ALT and AST elevations were reported as AEs (all grades) in 9.9% and 9.7% of palbociclib-treated patients, respectively.5 In the PALOMA-3 study, there was 1 fatal serious AE of hepatic failure with grade 5 disease progression in the palbociclib group; however, the patient’s medical history included progressive liver metastasis and disease progression.6 A pooled safety analysis conducted across all PALOMA studies demonstrated that grade 3/4 AST and ALT elevations occurred in 3.3% and 2.3% of palbociclib-treated patients, respectively, again highlighting a reported but rare occurrence.8
The patient described in the present case report started on combination fulvestrant and palbociclib after her disease showed progression on letrozole. She developed an increase in transaminases after completing 3 cycles of palbociclib. Liver function tests increased nearly 12 weeks after beginning her first cycle of the CDK 4/6 inhibitor. Staging scans of the patient demonstrated fatty liver. It is not known if her fatty liver contributed to her transminitis; however, her baseline labs showed normal liver function tests, and they did not increase until after therapy with fulvestrant–palbociclib was started. It might have been that her fatty liver caused her to be at higher risk of transaminitis with administration of palbociclib, although we cannot be certain. Her lab results remained normal while she was on fulvestrant alone, and the liver function test results increased only after palbociclib was started, making this drug the more likely culprit.
Both events of increased liver enzymes occurred within a week of the last palbociclib dose; however, we note that hepatotoxicity developed at a faster rate when the patient was rechallenged with palbociclib at a lower dose, with elevated liver function tests increasing 1 week after restarting treatment as opposed to the first episode that occurred after 3 cycles of the palbociclib. After discontinuation of the medication, liver function tests again normalized, suggesting that palbociclib was most likely the causative agent. In addition, the degree of elevated liver enzymes was less severe on re-exposure at the lower dose of 100 mg, which raises the possibility that there could be a dose-dependent association between palbociclib and hepatotoxicity. There have been few case reports of increased liver enzymes associated with palbociclib, and it is only recently that this association has been more recognized. A meta-analysis by Zaw and colleagues has demonstrated that CDK 4/6 inhibitor–based regimens are associated with a higher risk of elevated AST and ALT; however, their relation with dose dependence was not described. In particular, they found that CDK 4/6 inhibitors increased the risk of high-grade, elevated ALT with a relative risk of 4.33 (95% confidence interval, 2.15-8.71; P < .0001). The meta-analysis also included other CDK 4/6 inhibitors such as abemaciclib and ribociclib, which have been more commonly associated with liver toxicity than palbociclib has.9 Our case report highlights the specific association between palbociclib and elevated liver enzymes.
In conclusion, this case report illustrates that our patient’s elevated liver enzymes were likely related to palbociclib. This is further supported by the fact that this AE occurred twice, both times after palbociclib exposure. In each instance, liver enzymes normalized after discontinuation of palbociclib. One cannot entirely rule out that fulvestrant might have been the culprit medication, but the patient’s normal hepatic panel for several months after starting fulvestrant suggests that is less likely. This case report is indicative of an uncommon complication in the treatment of metastatic breast cancer, one that is starting to gain more recognition, and we must think of palbociclib as a possible cause of drug-induced liver injury when targeted CDK 4/6–based regimens are used.
About 12.4% of women in the United States will be diagnosed with breast cancer at some point in their lifetime.1 A percentage of these women will develop metastatic disease and are estimated to have a 5-year survival rate of 22%.2 There have been meaningful improvements in su
However, endocrine resistance inevitably occurs, and a great deal of research has been focused on developing strategies to combat resistance. One mechanism of endocrine resistance is though the Cyclin-dependent kinases 4 and 6 (CDK4/6) complexes. Among the most promising of the strategies to prevent resistance are the CDK4/6 inhibitors. There are now 3 approved CDK4/6 inhibitor drugs that can be used in combination with endocrine therapy, 1 of which can also be used as a single agent. When used in combination with endocrine therapy, the use of CDK 4/6 inhibitors has significantly improved progression-free survival (PFS) in patients with hormone-sensitive HER2-negative metastatic breast cancer by inhibiting cellular division and growth.3 In postmenopausal women, endocrine therapy plus CDK4/6 inhibitors are the preferred first-line regimen for metastatic disease.
Since the approval of palbociclib by the US Food and Drug Administration in 2015, the most common hematologic lab abnormalities are anemia, leukopenia, neutropenia, and thrombocytopenia. The most common nonhematologic adverse events (AEs) are fatigue, infection, nausea, and stomatitis. Hepatic toxicity has not been commonly observed. We report here the case of a 57-year-old woman on palbociclib and fulvestrant who developed significant elevation of liver function tests after starting palbociclib, suggesting a possible drug-induced liver injury from palbociclib.
Case presentation and summary
A 57-year-old woman with history of hypothyroidism and hypertension presented in May 2016 with a lump in her right breast and back pain. The lump was biopsied and revealed invasive ductal carcinoma, moderately differentiated, estrogen receptor (ER) positive 100%, progesterone receptor (PR) positive 95%, and HER2 negative. A positron emission tomography (PET)–computed tomography (CT) scan and magnetic resonance imaging showed bone metastasis at several vertebral levels, and the results of a bone biopsy confirmed metastatic adenocarcinoma of breast origin, ER positive 60%, PR positive 40%, and HER2 negative. No liver lesions were seen on imaging, but there was suggestion of fatty liver. She was started on letrozole 2.5 mg daily in July 2016 while undergoing kyphoplasty and subsequent radiation. A restaging PET scan revealed progression of disease on letrozole, with possible new rib lesion and progression in the breast. No liver disease was noted. Therapy was changed to fulvestrant and palbociclib. Fulvestrant was started in March 2017 with standard dosing of 500 mg intramuscular on days 1, 15, and 29, and then once a month thereafter. Her first cycle of palbociclib was started on April 5, dosed at 125 mg by mouth daily for 21 days, followed by 7 days off, repeated every 28 days (all dates hereinafter fell within 2017, unless otherwise stipulated).
Labs checked on April 28 and May 26 were unremarkable. A restaging CT scan of the chest, abdomen, and pelvis was done on June 21 after completion of 3 cycles of fulvestrant and palbociclib. There was no evidence of liver metastases, only the fatty infiltration of the liver that had been seen previously. On June 23, 2017, lab results showed a transaminitis with an alanine aminotransferase (ALT) level of 446 IU/L (reference range 10-33 IU/L) and aspartate aminotransferase (AST) level of 183 IU/L (reference range 0-32 IU/L).
The patient’s liver enzyme levels continued to increase and peaked on July 3 at ALT >700 IU/L and AST at 421 IU/L. Her total bilirubin and alkaline phosphatase levels remained within normal limits. She had received her final dose of fulvestrant on May 31 and had taken her last dose of palbociclib on June 20, 2017. She had no history of elevated liver enzymes or liver disease, although the initial PET scan done at diagnosis had suggested hepatic steatosis. She said she had not recently used antibiotics, alcohol, or over-the-counter medications or supplements. There was no family history of liver problems, inflammatory bowel disease, or gastrointestinal malignancy. The only other medications she had taken recently were denosumab, levothyroxine for hypothyroidism, and amlodipine for hypertension. She was seen by hepatology for evaluation of acute hepatitis. Other etiologies for her elevated liver enzymes were ruled out, and she was diagnosed with a drug-induced liver injury from one of her anticancer medications. Her treatments with fulvestrant and palbociclib were held, and the results of her liver function tests normalized by September 2017.
Fulvestrant was restarted on August 24, and her lab results remained normal through November of that year, when restaging scans showed progression with new axillary adenopathy suspicious for metastasis. Imaging also showed a 1.6-cm hepatic lesion suggestive of a focal area of fat deposition or atypical hemangioma without definitive evidence of metastasis. Follow-up imaging was recommended. She was therefore rechallenged with palbociclib at a reduced dose of 100 mg by mouth daily and received the first dose on November 30. On December 8, repeat labs again showed elevated liver function tests (ALT, 285 IU/L; AST, 112 IU/L). Treatment with palbociclib was discontinued on December 10. Because the patient was not able to tolerate palbociclib, and fulvestrant alone was not controlling the disease, she was started on an alternate endocrine therapy with tamoxifen on December 26. The patient’s liver function tests normalized again by January 2018.
Discussion
The use of targeted therapies has changed the landscape of oncologic treatments. Several studies have evaluated the safety and efficacy of palbociclib in combination with endocrine therapy. The Palbociclib Ongoing Trials in the Management of Breast Cancer (PALOMA)-1 study, an open-label, randomized, phase-2 trial involving patients with newly diagnosed metastatic hormone sensitive HER2-negative breast cancer, demonstrated that palbociclib in combination with letrozole was associated with significantly longer PFS than letrozole alone.4 These results were later confirmed in the larger PALOMA-2 study, a randomized, double-blind, phase-3 trial that evaluated 666 postmenopausal patients with no prior systemic therapy. In that study, median PFS for the palbociclib–letrozole group was 24.8 months, compared with 14.5 months for the letrozole-alone group (hazard ratio [HR] for disease progression or death, 0.58 [0.46–0.72], P < .001).5 The most recent PALOMA-3 study, a phase-3 trial involving 521 patients with advanced hormone receptor–positive, HER2-negative breast cancer that had progressed during initial endocrine therapy, evaluated the efficacy of combined palbociclib and fulvestrant in a randomized, double-blind, placebo-controlled, parallel-group trial. The result was that the palbociclib–fulvestrant combination resulted in longer median PFS of 9.2 months, compared with 3.8 months with fulvestrant alone (P < .001).6
These trials also monitored the number of AEs as secondary aims. The most commonly reported AEs in the PALOMA trials for those patients in the palbociclib group were hematologic, with neutropenia being the most common, followed by leukopenia, anemia, and thrombocytopenia. The most common nonhematologic AEs reported in the palbociclib-fulvestrant group were fatigue, nausea, and headache. Elevated liver function tests were a rare but reported AE in 7.2% of the palbociclib-treated patients in the PALOMA-1 study.7 In the PALOMA-2 study, ALT and AST elevations were reported as AEs (all grades) in 9.9% and 9.7% of palbociclib-treated patients, respectively.5 In the PALOMA-3 study, there was 1 fatal serious AE of hepatic failure with grade 5 disease progression in the palbociclib group; however, the patient’s medical history included progressive liver metastasis and disease progression.6 A pooled safety analysis conducted across all PALOMA studies demonstrated that grade 3/4 AST and ALT elevations occurred in 3.3% and 2.3% of palbociclib-treated patients, respectively, again highlighting a reported but rare occurrence.8
The patient described in the present case report started on combination fulvestrant and palbociclib after her disease showed progression on letrozole. She developed an increase in transaminases after completing 3 cycles of palbociclib. Liver function tests increased nearly 12 weeks after beginning her first cycle of the CDK 4/6 inhibitor. Staging scans of the patient demonstrated fatty liver. It is not known if her fatty liver contributed to her transminitis; however, her baseline labs showed normal liver function tests, and they did not increase until after therapy with fulvestrant–palbociclib was started. It might have been that her fatty liver caused her to be at higher risk of transaminitis with administration of palbociclib, although we cannot be certain. Her lab results remained normal while she was on fulvestrant alone, and the liver function test results increased only after palbociclib was started, making this drug the more likely culprit.
Both events of increased liver enzymes occurred within a week of the last palbociclib dose; however, we note that hepatotoxicity developed at a faster rate when the patient was rechallenged with palbociclib at a lower dose, with elevated liver function tests increasing 1 week after restarting treatment as opposed to the first episode that occurred after 3 cycles of the palbociclib. After discontinuation of the medication, liver function tests again normalized, suggesting that palbociclib was most likely the causative agent. In addition, the degree of elevated liver enzymes was less severe on re-exposure at the lower dose of 100 mg, which raises the possibility that there could be a dose-dependent association between palbociclib and hepatotoxicity. There have been few case reports of increased liver enzymes associated with palbociclib, and it is only recently that this association has been more recognized. A meta-analysis by Zaw and colleagues has demonstrated that CDK 4/6 inhibitor–based regimens are associated with a higher risk of elevated AST and ALT; however, their relation with dose dependence was not described. In particular, they found that CDK 4/6 inhibitors increased the risk of high-grade, elevated ALT with a relative risk of 4.33 (95% confidence interval, 2.15-8.71; P < .0001). The meta-analysis also included other CDK 4/6 inhibitors such as abemaciclib and ribociclib, which have been more commonly associated with liver toxicity than palbociclib has.9 Our case report highlights the specific association between palbociclib and elevated liver enzymes.
In conclusion, this case report illustrates that our patient’s elevated liver enzymes were likely related to palbociclib. This is further supported by the fact that this AE occurred twice, both times after palbociclib exposure. In each instance, liver enzymes normalized after discontinuation of palbociclib. One cannot entirely rule out that fulvestrant might have been the culprit medication, but the patient’s normal hepatic panel for several months after starting fulvestrant suggests that is less likely. This case report is indicative of an uncommon complication in the treatment of metastatic breast cancer, one that is starting to gain more recognition, and we must think of palbociclib as a possible cause of drug-induced liver injury when targeted CDK 4/6–based regimens are used.
1. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/csr/1975_2014/. Accessed April 3, 2018.
2. American Cancer Society. Breast cancer survival rates. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html. Accessed April 3, 2018.
3. Wolff AC. CDK4 and CDK6 inhibition in breast cancer - a new standard. N Engl J Med. 2016; 375(20):1993-1994.
4. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomized phase 2 study. Lancet Oncol. 2015;16(1):25-35.
5. Finn RS, Martin M, Rugo, HS et. al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med. 2016;375:1925-1936
6. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomized controlled trial. Lancet Oncol. 2016;17(4):425-439.
7. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209-219.
8. Dieras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HR- advanced breast cancer [published online July 18, 2018]. Natl Cancer Inst. 2018;111.
9. Zaw M, Thein KZ, Tun A, et al. A systematic review and meta-analysis of randomized controlled trials to evaluate the risk of gastrointestinal and hepatic toxicities in patients with hormone receptor positive HER2-negative breast cancer treated with CKD 4/6 inhibitors. J Clin Oncol. 2017;35(suppl 31):209.
1. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2014. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/csr/1975_2014/. Accessed April 3, 2018.
2. American Cancer Society. Breast cancer survival rates. https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-survival-rates.html. Accessed April 3, 2018.
3. Wolff AC. CDK4 and CDK6 inhibition in breast cancer - a new standard. N Engl J Med. 2016; 375(20):1993-1994.
4. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomized phase 2 study. Lancet Oncol. 2015;16(1):25-35.
5. Finn RS, Martin M, Rugo, HS et. al. Palbociclib and letrozole in advanced breast cancer. New Engl J Med. 2016;375:1925-1936
6. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomized controlled trial. Lancet Oncol. 2016;17(4):425-439.
7. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373(3):209-219.
8. Dieras V, Rugo HS, Schnell P, et al. Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HR- advanced breast cancer [published online July 18, 2018]. Natl Cancer Inst. 2018;111.
9. Zaw M, Thein KZ, Tun A, et al. A systematic review and meta-analysis of randomized controlled trials to evaluate the risk of gastrointestinal and hepatic toxicities in patients with hormone receptor positive HER2-negative breast cancer treated with CKD 4/6 inhibitors. J Clin Oncol. 2017;35(suppl 31):209.
The challenge of managing a cetuximab rash
Epidermal growth factor receptor antibodies (EGFR) such as cetuximab have been approved for use as first-line management as well as salvage therapy for head and neck and colorectal cancers. Among the most common expected toxicity is a cutaneous eruption described as acneiform. The presence of a rash has been postulated to predict a more favorable treatment outcome for cancers of the head and neck1 but not for colorectum.2 With more severe drug reactions, patients may require a treatment break, which has been shown to reduce locoregional control and survival, particularly in patients with head and neck cancer.3 This has prompted clinicians to affect rapid therapy to reverse the drug eruption. Given the controversy around rapid and effective reversal of this drug reaction, this report aims to address the current status of clinical management using an actual patient vignette.
Case presentation and summary
The patient was a 57-year-old white man who had been diagnosed with stage 4 T4N0M1 grade 3 cutaneous squamous cell carcinoma (SCC) of the right postauricular soft tissues, with erosion into the right mastoid and biopsy-proven metastatic disease involving the contralateral left supraclavicular fossa and bilateral lungs. His disease became chemotherapy-refractory, and he was referred for palliative local therapy to the base of skull. Because of the size of the tumor (4 cm × 5 cm), he was considered for sensitizing chemotherapy, but cisplatin was not appropriate because of chronic hearing loss.4 The patient was recommended sensitizing doses of cetuximab. This EGFR antibody has been shown to offer similar benefits to those seen with cisplatin in the definitive management of head and neck SCC.5
The standard loading dose of cetuximab was given at 400 mg/m2 intravenously (IV). The following week, the sensitizing dose of 250 mg/m2 IV was given along with daily radiotherapy to the target volumes. The weekly dose of cetuximab continued at 250 mg/m2. The radiotherapy prescription was for 6,000 cGy in 200 cGy daily fractions, encompassing the gross tumor volume as identified on a computed-tomographic scan with 3-mm cuts. We used a noncoplanar arc radiotherapy beam arrangement because it inherently spreads the dose over a larger volume of normal tissue while conformally delivering its largest dose to the gross tumor volume. As such, a volume of the patient’s oropharynx and oral cavity was included within the radiotherapy dose penumbra. After receiving 3 weekly doses of cetuximab (1 loading dose and 2 weekly sensitizing doses) and 2,000 cGy of radiotherapy, the patient developed a robust grade 2 cutaneous eruption delimited to the face, with few scattered lesions on the upper anterior chest. He was seen in the medical oncology department and was prescribed doxycycline 100 mg orally twice daily and topical clindamycin 2% ointment twice daily.
In the radiation oncology clinic, his drug therapy was manipulated. His cetuximab cutaneous reaction was a grade 2, manifested by moderate erythema with nonconfluent moist desquamation. Because of concern that the patient would develop oral candida, which would further delay his therapy, the oral and topical antibiotics were discontinued, as was the oral prednisone. He was prescribed triamcinolone cream 0.1% to be applied to the facial and few chest wall areas twice daily and an oncology mouth rinse to address early nonconfluent mucositis. The accompanying images show the extent of the patient’s cetuximab cutaneous reaction at baseline before treatment initiation (Figure 1), at 4 days after the intervention (Figure 2), and again at 6 days after the intervention (Figure 3). The patient consented to having his photographs taken and understood that they would be used for educational and research publication purposes.
As can be seen from the photographs, the patient’s rash began to dry and peel by day 4 after the intervention, and there were no new eruptions. The pruritus that accompanied the rash had entirely resolved. By day 6, the rash had completely subsided. Because of the response to the topical steroid, the patient continued cetuximab without a dose modification. He was recommended to continue with the triamcinolone cream until the chemoradiotherapy course concluded.
Discussion
A cetuximab-induced rash is common. In a 2011 meta-analysis quantifying grades 1 to 4 in severity, about 75% of patients treated with an EGFR inhibitor experienced a rash. Most of the rashes were lower than grade 3, and the drug was either dose-reduced or temporarily held, but it was not generally discontinued.6 Of note is that in a nonselected survey of medical oncologists who were prescribing cetuximab, 76% reported holding the drug owing to rash severity, 60% reported dose reductions for a drug rash, and 32% reported changing the drug because of rash severity.7
In the initial pharmaceutical registration trial, 76% to 88% of patients who received cetuximab developed a rash, 17% of which were at least grade 3. The pharma recommendations for managing the drug rash include a drug delay for up to 2 weeks for a rash of grade 3 or less and to terminate use of the drug if there is no clinical improvement after 2 weeks.8 Biopsies of the rash confirm a suppurative inflammatory reaction separate from an infectious acne reaction,9 resulting in a recommendation to treat with topical steroid therapy. In some circumstances, the drug reaction can become infected or involve the paronychia, often related to Staphylococcus aureus.10 Despite what would otherwise be a problem addressed by anti-inflammatory medical therapy, the clinical appearance of the rash marked by pustules, coupled with the relative immunosuppressed state of a cancer patient, has prompted medical oncologists to prescribe antibiotic therapy.
To address the many single-institutional reports on management of the EGFR rash, several guidelines have been published. The earliest guideline – after a report that concurrent cetuximab and radiotherapy was superior to radiotherapy alone in locally advanced head and neck cancer, which documented a 23% incidence of at least grade 3 cutaneous toxicity in the cetuximab arm1 – attempted to score the severity of the rash according to the National Cancer Institute’s (NCI) Common Terminology Criteria for Adverse Events (CTCAE). Under those criteria, the authors defined grade 2 toxicity as moderate to brisk erythema with patchy moist desquamation, mostly confined to skin folds and creases. Grade 3 toxicity was described as moist desquamation other than skin folds and creases with bleeding induced by minor trauma, and grade 4 skin toxicity was defined as skin necrosis or ulceration of full thickness dermis with spontaneous bleeding from the involved site. The authors went on to describe a grade-related treatment algorithm that included gently washing the skin, keeping it dry, and using topical anti-inflammatory agents, including steroids. Antibiotics should be used in the presence of a suspected infection after culturing the area, and grade 4 toxicity should be referred to a wound care center.11
In a consensus statement from the National Comprehensive Cancer Network, the authors noted that most management recommendations were anecdotal. They recommended against the use of astringents and other drying agents because they exacerbate pain. The ultimate choice of topical steroids or antibiotics was based entirely on subjective judgement given the absence of prospective data.12
A Spanish consensus conference report argued against any prophylaxis against a skin reaction, other than keeping the skin clean and dry.13 The authors of the report recommended against washing the affected skin more than twice a day to avoid excess drying, and they advocated for moisturizers and debridement of skin crusting with hydrogels to reduce superinfection and bleeding.13 The authors also noted that some guidelines have suggested that topical steroids might exacerbate a skin rash,14 but they concluded that topical steroids are beneficial as long as they are used for less than 2 weeks. Any use of antibiotics should be based on clear evidence of an infection.13
In the first modification of the NCI’s CTCAE rash grading scale, an international panel addressed the increasing number of reports in the literature suggesting that the previous toxicity scale was possibly inadequate in its recommendations for appropriate treatment. The initial scale had defined only the skin reaction and not what therapy should be administered; therefore, in the update, the descriptions for grades 1 and 2 toxicity remained unchanged, but oral antibiotics were recommended for grade 3 lesion, and parenteral antibiotics with skin grafting were required with grade 4 toxicity.15
An Asian expert panel suggested modifying the bioradiation dermatitis scale, defining a grade 3 dermatitis as >50% moist desquamation of the involved field with formation of confluent lesions because of treatment. They recommended both topical and oral therapy, wound care, and possible hospitalization in severe cases. The panel suggested topical and systemic steroids and antibiotics.16
Finally, in an Italian consensus report, the members again modified the skin toxicity grading and were notably more aggressive in terms of their management recommendations. They defined grade 2 toxicity as pustules or papules covering 10% to 30% of the body surface area, with potential pruritus or tenderness. They also noted the psychosocial impact of skin toxicities on patients and the limits to their activities of daily living. They recommended vitamin K1 (menadione) cream, topical antibiotics, topical intermediate potency steroids, and oral antibiotic therapy for up to 4 weeks for grade 2 toxicity. Despite this aggressive treatment course, the authors admitted that the utility of topical steroids and antibiotics was unknown. They defined grade 3 toxicity as pustules or papules covering more than 30% of the body surface area, with signs of possible pruritus and tenderness. Activities of daily living and self-care were affected, and there was evidence of a superinfection. The panel suggested use of antibiotics pending culture results, oral prednisone, antihistamines, and oral analgesics. Topical therapy was not included.17 It is noteworthy that only the Italian panel recommended the use of vitamin K1 cream. In a prospective randomized, double-blinded, placebo-controlled phase 2 trial of 30 patients, menadione exhibited no clinical benefit in terms of reducing the severity of cetuximab skin lesions.18
Figure 4 illustrates our institutional approach to treating cetuximab rash based on a combination of the Spanish and NCI approaches.
The ultimate choice of therapy to manage a cetuximab rash must be patient and treatment specific. Our institutional approach, like that of the Spanish series,13 is to avoid chemoprophylaxis against a rash; rather, we recommend daily washing of the skin with a gentle soap followed by thorough rinsing and adequate, nonaggressive drying. Moisturizing the intact skin has been shown to reduce exfoliation, and we have incorporated that approach into our regimen.19
In our patient, whose head and neck radiotherapy tumor volume included a portion of the oral cavity and oropharynx, systemic antibiotic and steroid therapy would likely lead to further complications with the development of oral candidiasis. Therefore, while the severity of the reaction remained a grade 2, it seemed appropriate to treat with topical intermediate potency steroids and skin cleansing only. If the reaction had become more severe, then cultures would have been obtained to guide our decision on antibiotic therapy. Our patient’s response to topical steroids was predictable and effective, and he was able to proceed with his course of cancer therapy.
1. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21-28.
2. Sommeijer DW, Karapetis CS, Zalcberg JR, et al. The relationship between rash, tumor mutation KRAS status and clinical and quality of life outcomes in patients with advanced colorectal cancer treated with cetuximab in the NCIC CTG/AGITG CO.17. Acta Oncol. 2014;53(7):877-884.
3. Vahabzadeh-Hagh AM, Rwigema JM, Nabili V, Wang MB, Lorentz WC. Predictors of prolongation in radiation treatment time in a veteran population treated with chemoradiation for oropharyngeal cancer. Acta Otolaryngol. 2018;138(1):80-84.
4. Waissbluth S, Peleva E, Daniel SJ. Platinum-induced ototoxicity: a prevailing ototoxicity criteria. Eur Arch Otorhinlaryngol. 2017;274(3):1187-1196.
5. Huang J, Zhang J, Shi C, Liu L, Wei Y. Survival, recurrence and toxicity of HNSCC in comparison of a radiotherapy combination with cisplatin versus cetuximab: a meta-analysis. BMC cancer. 2016;16(1):689-713.
6. Mittman N, Seung SJ. Rash rates with EGFR inhibitors: meta-analysis. Curr Oncol. 2011;18(2):e54-e63.
7. Boone SL, Rademaker A, Liu D, Pfeiffer C, Mauro DJ, Lacouture ME. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology. 2007;72(3-4):152-159.
8. Erbitux (cetuximab). Ask Lilly website. www.erbitux.com/hcp/index.html. Updated July 3, 2018. Accessed November 27.
9. Busam KJ, Capodieci P, Motzer R, Kiehn T, Phelan D, Halpern AC. Cutaneous side-effects in cancer patients treated with antiepidermal growth factor receptor antibody C225. Br J Dermatol. 2001;144(6):1169-1176.
10. Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670, 2006.
11. Bernier J, Bonner J, Vermorken JB, et al. Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol. 2008;19(1):142-149.
12. Burtness B, Anadkat M, Basti S, et al. NCCN task force report: management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(suppl 1):S5-S21.
13.
14. Li T, Perez-Soler R. Skin toxicity associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4(2):107-119.
15. Bernier J, Russi EG, Homey B, et al. Management of radiation dermatitis in patients receiving cetuximab and radiotherapy for locally advanced squamous cell carcinoma of the head and neck: proposals for a revised grading system and consensus management guidelines. Ann Oncol. 2011;22(10):2191-2200.
16. Zhu G, Lin JC, Kim SB, Bernier J, et al. Asian expert recommendation on management of skin and mucosal effects of radiation, with or without the addition of cetuximab or chemotherapy, in treatment of head and neck squamous cell carcinoma. BMC Cancer. 2016;16:42-62.
17. Pinto C, Barone CA, Girolomoni G, et al. Management of skin reactions during cetuximab treatment in association with chemotherapy or radiotherapy: update of the Italian expert recommendations. Am J Clin Oncol. 2016;39(4):407-415.
18. Eriksen JG, Kaalund I, Clemmensen O, Overgaard J, Pfeiffer P. Placebo-controlled phase II study of vitamin K3 cream for the treatment of cetuximab-induced rash. Support Care Cancer. 2017;25(7):2179-2185.
19. Watanabe S, Nakamura M, Takahashi H, et al. Dermopathy associated with cetuximab and panitumumab: investigation of the usefulness of moisturizers in its management. Clin Cosmet Investig Dermatol. 2017;10:353-361.
Epidermal growth factor receptor antibodies (EGFR) such as cetuximab have been approved for use as first-line management as well as salvage therapy for head and neck and colorectal cancers. Among the most common expected toxicity is a cutaneous eruption described as acneiform. The presence of a rash has been postulated to predict a more favorable treatment outcome for cancers of the head and neck1 but not for colorectum.2 With more severe drug reactions, patients may require a treatment break, which has been shown to reduce locoregional control and survival, particularly in patients with head and neck cancer.3 This has prompted clinicians to affect rapid therapy to reverse the drug eruption. Given the controversy around rapid and effective reversal of this drug reaction, this report aims to address the current status of clinical management using an actual patient vignette.
Case presentation and summary
The patient was a 57-year-old white man who had been diagnosed with stage 4 T4N0M1 grade 3 cutaneous squamous cell carcinoma (SCC) of the right postauricular soft tissues, with erosion into the right mastoid and biopsy-proven metastatic disease involving the contralateral left supraclavicular fossa and bilateral lungs. His disease became chemotherapy-refractory, and he was referred for palliative local therapy to the base of skull. Because of the size of the tumor (4 cm × 5 cm), he was considered for sensitizing chemotherapy, but cisplatin was not appropriate because of chronic hearing loss.4 The patient was recommended sensitizing doses of cetuximab. This EGFR antibody has been shown to offer similar benefits to those seen with cisplatin in the definitive management of head and neck SCC.5
The standard loading dose of cetuximab was given at 400 mg/m2 intravenously (IV). The following week, the sensitizing dose of 250 mg/m2 IV was given along with daily radiotherapy to the target volumes. The weekly dose of cetuximab continued at 250 mg/m2. The radiotherapy prescription was for 6,000 cGy in 200 cGy daily fractions, encompassing the gross tumor volume as identified on a computed-tomographic scan with 3-mm cuts. We used a noncoplanar arc radiotherapy beam arrangement because it inherently spreads the dose over a larger volume of normal tissue while conformally delivering its largest dose to the gross tumor volume. As such, a volume of the patient’s oropharynx and oral cavity was included within the radiotherapy dose penumbra. After receiving 3 weekly doses of cetuximab (1 loading dose and 2 weekly sensitizing doses) and 2,000 cGy of radiotherapy, the patient developed a robust grade 2 cutaneous eruption delimited to the face, with few scattered lesions on the upper anterior chest. He was seen in the medical oncology department and was prescribed doxycycline 100 mg orally twice daily and topical clindamycin 2% ointment twice daily.
In the radiation oncology clinic, his drug therapy was manipulated. His cetuximab cutaneous reaction was a grade 2, manifested by moderate erythema with nonconfluent moist desquamation. Because of concern that the patient would develop oral candida, which would further delay his therapy, the oral and topical antibiotics were discontinued, as was the oral prednisone. He was prescribed triamcinolone cream 0.1% to be applied to the facial and few chest wall areas twice daily and an oncology mouth rinse to address early nonconfluent mucositis. The accompanying images show the extent of the patient’s cetuximab cutaneous reaction at baseline before treatment initiation (Figure 1), at 4 days after the intervention (Figure 2), and again at 6 days after the intervention (Figure 3). The patient consented to having his photographs taken and understood that they would be used for educational and research publication purposes.
As can be seen from the photographs, the patient’s rash began to dry and peel by day 4 after the intervention, and there were no new eruptions. The pruritus that accompanied the rash had entirely resolved. By day 6, the rash had completely subsided. Because of the response to the topical steroid, the patient continued cetuximab without a dose modification. He was recommended to continue with the triamcinolone cream until the chemoradiotherapy course concluded.
Discussion
A cetuximab-induced rash is common. In a 2011 meta-analysis quantifying grades 1 to 4 in severity, about 75% of patients treated with an EGFR inhibitor experienced a rash. Most of the rashes were lower than grade 3, and the drug was either dose-reduced or temporarily held, but it was not generally discontinued.6 Of note is that in a nonselected survey of medical oncologists who were prescribing cetuximab, 76% reported holding the drug owing to rash severity, 60% reported dose reductions for a drug rash, and 32% reported changing the drug because of rash severity.7
In the initial pharmaceutical registration trial, 76% to 88% of patients who received cetuximab developed a rash, 17% of which were at least grade 3. The pharma recommendations for managing the drug rash include a drug delay for up to 2 weeks for a rash of grade 3 or less and to terminate use of the drug if there is no clinical improvement after 2 weeks.8 Biopsies of the rash confirm a suppurative inflammatory reaction separate from an infectious acne reaction,9 resulting in a recommendation to treat with topical steroid therapy. In some circumstances, the drug reaction can become infected or involve the paronychia, often related to Staphylococcus aureus.10 Despite what would otherwise be a problem addressed by anti-inflammatory medical therapy, the clinical appearance of the rash marked by pustules, coupled with the relative immunosuppressed state of a cancer patient, has prompted medical oncologists to prescribe antibiotic therapy.
To address the many single-institutional reports on management of the EGFR rash, several guidelines have been published. The earliest guideline – after a report that concurrent cetuximab and radiotherapy was superior to radiotherapy alone in locally advanced head and neck cancer, which documented a 23% incidence of at least grade 3 cutaneous toxicity in the cetuximab arm1 – attempted to score the severity of the rash according to the National Cancer Institute’s (NCI) Common Terminology Criteria for Adverse Events (CTCAE). Under those criteria, the authors defined grade 2 toxicity as moderate to brisk erythema with patchy moist desquamation, mostly confined to skin folds and creases. Grade 3 toxicity was described as moist desquamation other than skin folds and creases with bleeding induced by minor trauma, and grade 4 skin toxicity was defined as skin necrosis or ulceration of full thickness dermis with spontaneous bleeding from the involved site. The authors went on to describe a grade-related treatment algorithm that included gently washing the skin, keeping it dry, and using topical anti-inflammatory agents, including steroids. Antibiotics should be used in the presence of a suspected infection after culturing the area, and grade 4 toxicity should be referred to a wound care center.11
In a consensus statement from the National Comprehensive Cancer Network, the authors noted that most management recommendations were anecdotal. They recommended against the use of astringents and other drying agents because they exacerbate pain. The ultimate choice of topical steroids or antibiotics was based entirely on subjective judgement given the absence of prospective data.12
A Spanish consensus conference report argued against any prophylaxis against a skin reaction, other than keeping the skin clean and dry.13 The authors of the report recommended against washing the affected skin more than twice a day to avoid excess drying, and they advocated for moisturizers and debridement of skin crusting with hydrogels to reduce superinfection and bleeding.13 The authors also noted that some guidelines have suggested that topical steroids might exacerbate a skin rash,14 but they concluded that topical steroids are beneficial as long as they are used for less than 2 weeks. Any use of antibiotics should be based on clear evidence of an infection.13
In the first modification of the NCI’s CTCAE rash grading scale, an international panel addressed the increasing number of reports in the literature suggesting that the previous toxicity scale was possibly inadequate in its recommendations for appropriate treatment. The initial scale had defined only the skin reaction and not what therapy should be administered; therefore, in the update, the descriptions for grades 1 and 2 toxicity remained unchanged, but oral antibiotics were recommended for grade 3 lesion, and parenteral antibiotics with skin grafting were required with grade 4 toxicity.15
An Asian expert panel suggested modifying the bioradiation dermatitis scale, defining a grade 3 dermatitis as >50% moist desquamation of the involved field with formation of confluent lesions because of treatment. They recommended both topical and oral therapy, wound care, and possible hospitalization in severe cases. The panel suggested topical and systemic steroids and antibiotics.16
Finally, in an Italian consensus report, the members again modified the skin toxicity grading and were notably more aggressive in terms of their management recommendations. They defined grade 2 toxicity as pustules or papules covering 10% to 30% of the body surface area, with potential pruritus or tenderness. They also noted the psychosocial impact of skin toxicities on patients and the limits to their activities of daily living. They recommended vitamin K1 (menadione) cream, topical antibiotics, topical intermediate potency steroids, and oral antibiotic therapy for up to 4 weeks for grade 2 toxicity. Despite this aggressive treatment course, the authors admitted that the utility of topical steroids and antibiotics was unknown. They defined grade 3 toxicity as pustules or papules covering more than 30% of the body surface area, with signs of possible pruritus and tenderness. Activities of daily living and self-care were affected, and there was evidence of a superinfection. The panel suggested use of antibiotics pending culture results, oral prednisone, antihistamines, and oral analgesics. Topical therapy was not included.17 It is noteworthy that only the Italian panel recommended the use of vitamin K1 cream. In a prospective randomized, double-blinded, placebo-controlled phase 2 trial of 30 patients, menadione exhibited no clinical benefit in terms of reducing the severity of cetuximab skin lesions.18
Figure 4 illustrates our institutional approach to treating cetuximab rash based on a combination of the Spanish and NCI approaches.
The ultimate choice of therapy to manage a cetuximab rash must be patient and treatment specific. Our institutional approach, like that of the Spanish series,13 is to avoid chemoprophylaxis against a rash; rather, we recommend daily washing of the skin with a gentle soap followed by thorough rinsing and adequate, nonaggressive drying. Moisturizing the intact skin has been shown to reduce exfoliation, and we have incorporated that approach into our regimen.19
In our patient, whose head and neck radiotherapy tumor volume included a portion of the oral cavity and oropharynx, systemic antibiotic and steroid therapy would likely lead to further complications with the development of oral candidiasis. Therefore, while the severity of the reaction remained a grade 2, it seemed appropriate to treat with topical intermediate potency steroids and skin cleansing only. If the reaction had become more severe, then cultures would have been obtained to guide our decision on antibiotic therapy. Our patient’s response to topical steroids was predictable and effective, and he was able to proceed with his course of cancer therapy.
Epidermal growth factor receptor antibodies (EGFR) such as cetuximab have been approved for use as first-line management as well as salvage therapy for head and neck and colorectal cancers. Among the most common expected toxicity is a cutaneous eruption described as acneiform. The presence of a rash has been postulated to predict a more favorable treatment outcome for cancers of the head and neck1 but not for colorectum.2 With more severe drug reactions, patients may require a treatment break, which has been shown to reduce locoregional control and survival, particularly in patients with head and neck cancer.3 This has prompted clinicians to affect rapid therapy to reverse the drug eruption. Given the controversy around rapid and effective reversal of this drug reaction, this report aims to address the current status of clinical management using an actual patient vignette.
Case presentation and summary
The patient was a 57-year-old white man who had been diagnosed with stage 4 T4N0M1 grade 3 cutaneous squamous cell carcinoma (SCC) of the right postauricular soft tissues, with erosion into the right mastoid and biopsy-proven metastatic disease involving the contralateral left supraclavicular fossa and bilateral lungs. His disease became chemotherapy-refractory, and he was referred for palliative local therapy to the base of skull. Because of the size of the tumor (4 cm × 5 cm), he was considered for sensitizing chemotherapy, but cisplatin was not appropriate because of chronic hearing loss.4 The patient was recommended sensitizing doses of cetuximab. This EGFR antibody has been shown to offer similar benefits to those seen with cisplatin in the definitive management of head and neck SCC.5
The standard loading dose of cetuximab was given at 400 mg/m2 intravenously (IV). The following week, the sensitizing dose of 250 mg/m2 IV was given along with daily radiotherapy to the target volumes. The weekly dose of cetuximab continued at 250 mg/m2. The radiotherapy prescription was for 6,000 cGy in 200 cGy daily fractions, encompassing the gross tumor volume as identified on a computed-tomographic scan with 3-mm cuts. We used a noncoplanar arc radiotherapy beam arrangement because it inherently spreads the dose over a larger volume of normal tissue while conformally delivering its largest dose to the gross tumor volume. As such, a volume of the patient’s oropharynx and oral cavity was included within the radiotherapy dose penumbra. After receiving 3 weekly doses of cetuximab (1 loading dose and 2 weekly sensitizing doses) and 2,000 cGy of radiotherapy, the patient developed a robust grade 2 cutaneous eruption delimited to the face, with few scattered lesions on the upper anterior chest. He was seen in the medical oncology department and was prescribed doxycycline 100 mg orally twice daily and topical clindamycin 2% ointment twice daily.
In the radiation oncology clinic, his drug therapy was manipulated. His cetuximab cutaneous reaction was a grade 2, manifested by moderate erythema with nonconfluent moist desquamation. Because of concern that the patient would develop oral candida, which would further delay his therapy, the oral and topical antibiotics were discontinued, as was the oral prednisone. He was prescribed triamcinolone cream 0.1% to be applied to the facial and few chest wall areas twice daily and an oncology mouth rinse to address early nonconfluent mucositis. The accompanying images show the extent of the patient’s cetuximab cutaneous reaction at baseline before treatment initiation (Figure 1), at 4 days after the intervention (Figure 2), and again at 6 days after the intervention (Figure 3). The patient consented to having his photographs taken and understood that they would be used for educational and research publication purposes.
As can be seen from the photographs, the patient’s rash began to dry and peel by day 4 after the intervention, and there were no new eruptions. The pruritus that accompanied the rash had entirely resolved. By day 6, the rash had completely subsided. Because of the response to the topical steroid, the patient continued cetuximab without a dose modification. He was recommended to continue with the triamcinolone cream until the chemoradiotherapy course concluded.
Discussion
A cetuximab-induced rash is common. In a 2011 meta-analysis quantifying grades 1 to 4 in severity, about 75% of patients treated with an EGFR inhibitor experienced a rash. Most of the rashes were lower than grade 3, and the drug was either dose-reduced or temporarily held, but it was not generally discontinued.6 Of note is that in a nonselected survey of medical oncologists who were prescribing cetuximab, 76% reported holding the drug owing to rash severity, 60% reported dose reductions for a drug rash, and 32% reported changing the drug because of rash severity.7
In the initial pharmaceutical registration trial, 76% to 88% of patients who received cetuximab developed a rash, 17% of which were at least grade 3. The pharma recommendations for managing the drug rash include a drug delay for up to 2 weeks for a rash of grade 3 or less and to terminate use of the drug if there is no clinical improvement after 2 weeks.8 Biopsies of the rash confirm a suppurative inflammatory reaction separate from an infectious acne reaction,9 resulting in a recommendation to treat with topical steroid therapy. In some circumstances, the drug reaction can become infected or involve the paronychia, often related to Staphylococcus aureus.10 Despite what would otherwise be a problem addressed by anti-inflammatory medical therapy, the clinical appearance of the rash marked by pustules, coupled with the relative immunosuppressed state of a cancer patient, has prompted medical oncologists to prescribe antibiotic therapy.
To address the many single-institutional reports on management of the EGFR rash, several guidelines have been published. The earliest guideline – after a report that concurrent cetuximab and radiotherapy was superior to radiotherapy alone in locally advanced head and neck cancer, which documented a 23% incidence of at least grade 3 cutaneous toxicity in the cetuximab arm1 – attempted to score the severity of the rash according to the National Cancer Institute’s (NCI) Common Terminology Criteria for Adverse Events (CTCAE). Under those criteria, the authors defined grade 2 toxicity as moderate to brisk erythema with patchy moist desquamation, mostly confined to skin folds and creases. Grade 3 toxicity was described as moist desquamation other than skin folds and creases with bleeding induced by minor trauma, and grade 4 skin toxicity was defined as skin necrosis or ulceration of full thickness dermis with spontaneous bleeding from the involved site. The authors went on to describe a grade-related treatment algorithm that included gently washing the skin, keeping it dry, and using topical anti-inflammatory agents, including steroids. Antibiotics should be used in the presence of a suspected infection after culturing the area, and grade 4 toxicity should be referred to a wound care center.11
In a consensus statement from the National Comprehensive Cancer Network, the authors noted that most management recommendations were anecdotal. They recommended against the use of astringents and other drying agents because they exacerbate pain. The ultimate choice of topical steroids or antibiotics was based entirely on subjective judgement given the absence of prospective data.12
A Spanish consensus conference report argued against any prophylaxis against a skin reaction, other than keeping the skin clean and dry.13 The authors of the report recommended against washing the affected skin more than twice a day to avoid excess drying, and they advocated for moisturizers and debridement of skin crusting with hydrogels to reduce superinfection and bleeding.13 The authors also noted that some guidelines have suggested that topical steroids might exacerbate a skin rash,14 but they concluded that topical steroids are beneficial as long as they are used for less than 2 weeks. Any use of antibiotics should be based on clear evidence of an infection.13
In the first modification of the NCI’s CTCAE rash grading scale, an international panel addressed the increasing number of reports in the literature suggesting that the previous toxicity scale was possibly inadequate in its recommendations for appropriate treatment. The initial scale had defined only the skin reaction and not what therapy should be administered; therefore, in the update, the descriptions for grades 1 and 2 toxicity remained unchanged, but oral antibiotics were recommended for grade 3 lesion, and parenteral antibiotics with skin grafting were required with grade 4 toxicity.15
An Asian expert panel suggested modifying the bioradiation dermatitis scale, defining a grade 3 dermatitis as >50% moist desquamation of the involved field with formation of confluent lesions because of treatment. They recommended both topical and oral therapy, wound care, and possible hospitalization in severe cases. The panel suggested topical and systemic steroids and antibiotics.16
Finally, in an Italian consensus report, the members again modified the skin toxicity grading and were notably more aggressive in terms of their management recommendations. They defined grade 2 toxicity as pustules or papules covering 10% to 30% of the body surface area, with potential pruritus or tenderness. They also noted the psychosocial impact of skin toxicities on patients and the limits to their activities of daily living. They recommended vitamin K1 (menadione) cream, topical antibiotics, topical intermediate potency steroids, and oral antibiotic therapy for up to 4 weeks for grade 2 toxicity. Despite this aggressive treatment course, the authors admitted that the utility of topical steroids and antibiotics was unknown. They defined grade 3 toxicity as pustules or papules covering more than 30% of the body surface area, with signs of possible pruritus and tenderness. Activities of daily living and self-care were affected, and there was evidence of a superinfection. The panel suggested use of antibiotics pending culture results, oral prednisone, antihistamines, and oral analgesics. Topical therapy was not included.17 It is noteworthy that only the Italian panel recommended the use of vitamin K1 cream. In a prospective randomized, double-blinded, placebo-controlled phase 2 trial of 30 patients, menadione exhibited no clinical benefit in terms of reducing the severity of cetuximab skin lesions.18
Figure 4 illustrates our institutional approach to treating cetuximab rash based on a combination of the Spanish and NCI approaches.
The ultimate choice of therapy to manage a cetuximab rash must be patient and treatment specific. Our institutional approach, like that of the Spanish series,13 is to avoid chemoprophylaxis against a rash; rather, we recommend daily washing of the skin with a gentle soap followed by thorough rinsing and adequate, nonaggressive drying. Moisturizing the intact skin has been shown to reduce exfoliation, and we have incorporated that approach into our regimen.19
In our patient, whose head and neck radiotherapy tumor volume included a portion of the oral cavity and oropharynx, systemic antibiotic and steroid therapy would likely lead to further complications with the development of oral candidiasis. Therefore, while the severity of the reaction remained a grade 2, it seemed appropriate to treat with topical intermediate potency steroids and skin cleansing only. If the reaction had become more severe, then cultures would have been obtained to guide our decision on antibiotic therapy. Our patient’s response to topical steroids was predictable and effective, and he was able to proceed with his course of cancer therapy.
1. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21-28.
2. Sommeijer DW, Karapetis CS, Zalcberg JR, et al. The relationship between rash, tumor mutation KRAS status and clinical and quality of life outcomes in patients with advanced colorectal cancer treated with cetuximab in the NCIC CTG/AGITG CO.17. Acta Oncol. 2014;53(7):877-884.
3. Vahabzadeh-Hagh AM, Rwigema JM, Nabili V, Wang MB, Lorentz WC. Predictors of prolongation in radiation treatment time in a veteran population treated with chemoradiation for oropharyngeal cancer. Acta Otolaryngol. 2018;138(1):80-84.
4. Waissbluth S, Peleva E, Daniel SJ. Platinum-induced ototoxicity: a prevailing ototoxicity criteria. Eur Arch Otorhinlaryngol. 2017;274(3):1187-1196.
5. Huang J, Zhang J, Shi C, Liu L, Wei Y. Survival, recurrence and toxicity of HNSCC in comparison of a radiotherapy combination with cisplatin versus cetuximab: a meta-analysis. BMC cancer. 2016;16(1):689-713.
6. Mittman N, Seung SJ. Rash rates with EGFR inhibitors: meta-analysis. Curr Oncol. 2011;18(2):e54-e63.
7. Boone SL, Rademaker A, Liu D, Pfeiffer C, Mauro DJ, Lacouture ME. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology. 2007;72(3-4):152-159.
8. Erbitux (cetuximab). Ask Lilly website. www.erbitux.com/hcp/index.html. Updated July 3, 2018. Accessed November 27.
9. Busam KJ, Capodieci P, Motzer R, Kiehn T, Phelan D, Halpern AC. Cutaneous side-effects in cancer patients treated with antiepidermal growth factor receptor antibody C225. Br J Dermatol. 2001;144(6):1169-1176.
10. Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670, 2006.
11. Bernier J, Bonner J, Vermorken JB, et al. Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol. 2008;19(1):142-149.
12. Burtness B, Anadkat M, Basti S, et al. NCCN task force report: management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(suppl 1):S5-S21.
13.
14. Li T, Perez-Soler R. Skin toxicity associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4(2):107-119.
15. Bernier J, Russi EG, Homey B, et al. Management of radiation dermatitis in patients receiving cetuximab and radiotherapy for locally advanced squamous cell carcinoma of the head and neck: proposals for a revised grading system and consensus management guidelines. Ann Oncol. 2011;22(10):2191-2200.
16. Zhu G, Lin JC, Kim SB, Bernier J, et al. Asian expert recommendation on management of skin and mucosal effects of radiation, with or without the addition of cetuximab or chemotherapy, in treatment of head and neck squamous cell carcinoma. BMC Cancer. 2016;16:42-62.
17. Pinto C, Barone CA, Girolomoni G, et al. Management of skin reactions during cetuximab treatment in association with chemotherapy or radiotherapy: update of the Italian expert recommendations. Am J Clin Oncol. 2016;39(4):407-415.
18. Eriksen JG, Kaalund I, Clemmensen O, Overgaard J, Pfeiffer P. Placebo-controlled phase II study of vitamin K3 cream for the treatment of cetuximab-induced rash. Support Care Cancer. 2017;25(7):2179-2185.
19. Watanabe S, Nakamura M, Takahashi H, et al. Dermopathy associated with cetuximab and panitumumab: investigation of the usefulness of moisturizers in its management. Clin Cosmet Investig Dermatol. 2017;10:353-361.
1. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11(1):21-28.
2. Sommeijer DW, Karapetis CS, Zalcberg JR, et al. The relationship between rash, tumor mutation KRAS status and clinical and quality of life outcomes in patients with advanced colorectal cancer treated with cetuximab in the NCIC CTG/AGITG CO.17. Acta Oncol. 2014;53(7):877-884.
3. Vahabzadeh-Hagh AM, Rwigema JM, Nabili V, Wang MB, Lorentz WC. Predictors of prolongation in radiation treatment time in a veteran population treated with chemoradiation for oropharyngeal cancer. Acta Otolaryngol. 2018;138(1):80-84.
4. Waissbluth S, Peleva E, Daniel SJ. Platinum-induced ototoxicity: a prevailing ototoxicity criteria. Eur Arch Otorhinlaryngol. 2017;274(3):1187-1196.
5. Huang J, Zhang J, Shi C, Liu L, Wei Y. Survival, recurrence and toxicity of HNSCC in comparison of a radiotherapy combination with cisplatin versus cetuximab: a meta-analysis. BMC cancer. 2016;16(1):689-713.
6. Mittman N, Seung SJ. Rash rates with EGFR inhibitors: meta-analysis. Curr Oncol. 2011;18(2):e54-e63.
7. Boone SL, Rademaker A, Liu D, Pfeiffer C, Mauro DJ, Lacouture ME. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology. 2007;72(3-4):152-159.
8. Erbitux (cetuximab). Ask Lilly website. www.erbitux.com/hcp/index.html. Updated July 3, 2018. Accessed November 27.
9. Busam KJ, Capodieci P, Motzer R, Kiehn T, Phelan D, Halpern AC. Cutaneous side-effects in cancer patients treated with antiepidermal growth factor receptor antibody C225. Br J Dermatol. 2001;144(6):1169-1176.
10. Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55:657-670, 2006.
11. Bernier J, Bonner J, Vermorken JB, et al. Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol. 2008;19(1):142-149.
12. Burtness B, Anadkat M, Basti S, et al. NCCN task force report: management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(suppl 1):S5-S21.
13.
14. Li T, Perez-Soler R. Skin toxicity associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4(2):107-119.
15. Bernier J, Russi EG, Homey B, et al. Management of radiation dermatitis in patients receiving cetuximab and radiotherapy for locally advanced squamous cell carcinoma of the head and neck: proposals for a revised grading system and consensus management guidelines. Ann Oncol. 2011;22(10):2191-2200.
16. Zhu G, Lin JC, Kim SB, Bernier J, et al. Asian expert recommendation on management of skin and mucosal effects of radiation, with or without the addition of cetuximab or chemotherapy, in treatment of head and neck squamous cell carcinoma. BMC Cancer. 2016;16:42-62.
17. Pinto C, Barone CA, Girolomoni G, et al. Management of skin reactions during cetuximab treatment in association with chemotherapy or radiotherapy: update of the Italian expert recommendations. Am J Clin Oncol. 2016;39(4):407-415.
18. Eriksen JG, Kaalund I, Clemmensen O, Overgaard J, Pfeiffer P. Placebo-controlled phase II study of vitamin K3 cream for the treatment of cetuximab-induced rash. Support Care Cancer. 2017;25(7):2179-2185.
19. Watanabe S, Nakamura M, Takahashi H, et al. Dermopathy associated with cetuximab and panitumumab: investigation of the usefulness of moisturizers in its management. Clin Cosmet Investig Dermatol. 2017;10:353-361.
Incidental Asymptomatic Fibular Stress Fractures Presenting as Varus Knee Osteoarthritis: A Case Report
ABSTRACT
Stress fractures are often missed, especially in unusual clinical settings. We report on 2 patients who presented to our orthopedic surgery clinic with incidental findings of asymptomatic proximal fibular tension side stress fractures in severe longstanding varus osteoarthritic knees. Initial plain films demonstrated an expansile deformity of the proximal fibular shaft, and differential diagnosis included a healed or healing fracture versus possible neoplasm. Magnetic resonance imaging with and without gadolinium was utilized to rule out the latter prior to planned total knee arthroplasty.
Continue to: The proximal fibula...
The proximal fibula is a rare site for stress fractures, with most of these fractures occurring in military recruits.1 To the authors’ knowledge, there has been only 1 documented case of a proximal fibular stress fracture in patients with severe osteoarthritis (OA) and fixed varus deformity, which mimicked L5 radiculopathy.2 We are not aware of any reports of asymptomatic tension-side fibular stress fractures in varus knees. In our 2 cases, the patients were indicated for total knee arthroplasty (TKA) for varus degenerative joint disease after failing nonoperative treatment; however, further work-up was justified to rule out neoplasm after plain films revealed expansile deformities of the proximal fibular shaft. Each patient subsequently underwent magnetic resonance imaging (MRI) with and without gadolinium contrast, which demonstrated a healed and healing proximal fibular stress fracture. Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease, and stress fractures about a varus knee generally occur on the compression side of the tibia and are symptomatic.3-7 The patients provided informed written consent for print and electronic publication of this case report.
CASE REPORT
The first patient was a 77-year-old male who presented with longstanding knee pain, left greater than right, exacerbated by weight-bearing activities. The patient had no improvement with physical therapy or anti-inflammatory medication. He denied any history of trauma, weakness, paresthesias, or a recent increase in activity. The patient also denied any fevers, chills, night sweats, or other constitutional symptoms. On physical examination, the patient had an antalgic gait and limited range of motion bilaterally. Examination of his right lower extremity demonstrated a fixed 5° varus deformity. No distinct point tenderness was noted.
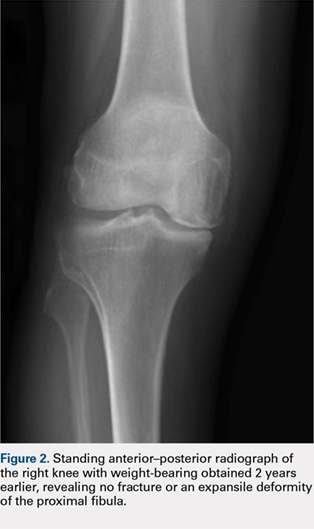
Radiographs of the right knee demonstrated varus deformity and tricompartmental degenerative changes with severe medial joint space narrowing. An expansile deformity of the proximal right fibular shaft was also noted (Figure 1), which was not present on the films 2 years earlier (Figure 2). The absence of this deformity on previous imaging raised the suspicion of a tumor. An MRI with and without gadolinium, which was obtained to rule out a neoplastic process, showed an old, healed proximal fibular shaft fracture with chronic periosteal reaction (Figure 3). There was no marrow edema to suggest acute injury and no neoplastic lesion. He was reassured regarding the benign findings and was scheduled for a left TKA, as his pain was more severe on the left knee. The patient’s stress fracture healed without complications, and he underwent a successful left TKA. He returned approximately 6 months after his procedure with worsening right knee pain and underwent a successful TKA on the right knee as well.
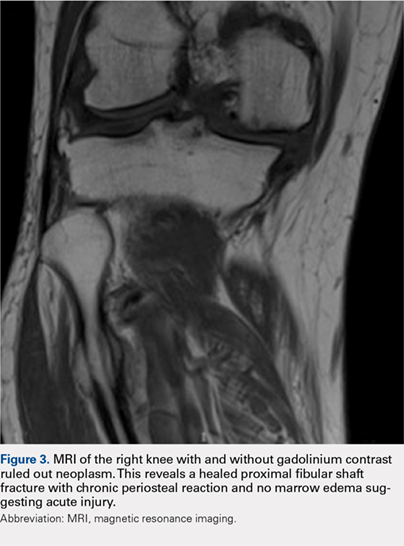
The second patient was a 67-year-old male with longstanding bilateral knee pain, right greater than left, with no antecedent trauma. He denied a history of increased activity, or weakness or paresthesias. He denied any fevers, chills, night sweats, or other constitutional symptoms. One year prior to presentation at our clinic, he had received corticosteroid injections and hyaluronic acid, without relief. The patient also had a history with another surgeon of arthroscopy 1 year earlier and subchondroplasty 3 years before presentation to our clinic. On physical examination, the patient’s right knee displayed a fixed 7° varus deformity with decreased range of motion, effusion, and diffuse crepitus. Further examination revealed tenderness to palpation of the proximal fibula.
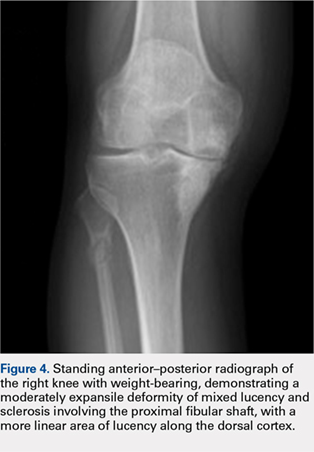
Radiographs of the right knee showed degenerative joint disease with varus deformity and medial compartment joint space narrowing. They also demonstrated an expansile deformity of mixed lucency and sclerosis involving the proximal right fibular shaft (Figure 4). Although these findings appeared to be consistent with a stress fracture, their appearance was also suspicious for a neoplasm. To rule out malignancy, an MRI with and without gadolinium was obtained that revealed a healing stress fracture of the proximal fibula (Figure 5). The patient was reassured, and plans were made to proceed with a TKA. The patient’s stress fracture healed without complications, and he underwent successful right TKA. Radiographs from the patient’s 8-week follow-up showed a healed fibular stress fracture (Figure 6).
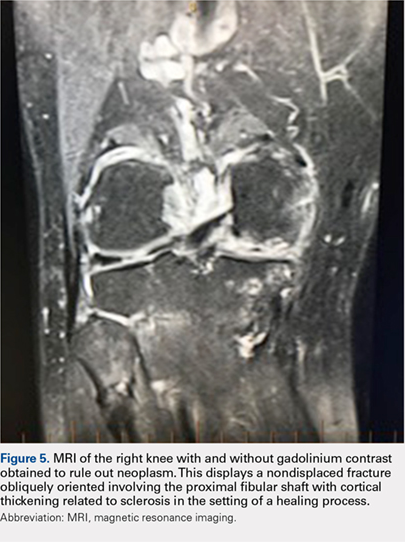

Continue to: DISCUSSION
DISCUSSION
To our knowledge, this is the first report of incidental tension-side stress fractures in varus osteoarthritic knees. Stress fractures have been classified into 2 groups, fatigue fractures and insufficiency fractures. Fatigue fractures occur when abnormal stress is applied to normal bones, and insufficiency fractures result when normal stress is applied to abnormal bones.8 Stress fractures can also be classified into risk categories based on which bone is involved and the loading of the bone.9 Sites loaded in tension have increased risk of nonunion, progression to complete fracture, and reoccurrence compared with sites loaded in compression.9 Stress fractures of the fibula occur rarely, and when present, they are more commonly observed in the distal fibula in athletes and military recruits.1 Stress fractures occur rarely in patients with primary OA, and when present in this setting, obesity and malalignment are the contributing factors.3 Neither patient was obese in our case (body mass index of 27 and 28, respectively), but significant varus deformity was present in both patients. Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.3-7
Regarding malalignment, Cheung and colleagues10 reported about a case of an elderly female with OA of the knee with valgus deformity that initially developed a proximal fibular stress fracture followed by a proximal tibial stress fracture. However, both of our patients had varus deformities. Mullaji and Shetty3 documented stress fractures in 34 patients with OA, a majority with varus deformities, but did not report any isolated proximal fibular stress fractures. Manish and colleagues2 reported the only documented case of an isolated proximal fibular stress fracture in a patient with osteoarthritic varus deformity. The patient presented initially with pain and paresthesias of the lower thigh and leg consistent with an L5 radiculopathy. They believed that the varus deformity and the repetitive contraction of the lateral knee muscles put increased shear forces on the fibula leading to the stress fracture. Our patients did not present with any radicular symptoms, a history of acute worsening pain, or an increased activity concerning for a stress fracture. Instead, our patients presented with progressively worsening knee pain typical of severe OA and incidental findings on imaging of tension-side fibular stress fractures. An MRI with and without gadolinium confirmed the diagnosis of a healed fracture in our first patient and a healing fracture in our second patient.
CONCLUSION
Although exceedingly rare in osteoarthritic varus knees, we presented 2 cases of MRI-confirmed proximal fibular stress fractures in this report. As demonstrated, patients may present with symptoms of OA or radicular symptoms as described by Manish and colleagues.2 Presentation may also include an expansile lesion on imaging, prompting a differential diagnosis that includes a neoplasm. If present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and not affect the plan or outcome of TKA.
- Devas MB, Sweetnam R. Stress fractures of the fibula; a review of fifty cases in athletes. J Bone Joint Surg Br. 1956;38-B(4):818-829.
- Manish KK, Agnivesh T, Pramod PS, Samir SD. Isolated proximal fibular stress fracture in osteoarthritis knee presenting as L5 radiculopathy. J Orthop Case Reports. 2015;5(3):75-77. doi:10.13107/jocr.2250-0685.315.
- Mullaji A, Shetty G. Total knee arthroplasty for arthritic knees with tibiofibular stress fractures: classification and treatment guidelines. J Arthroplasty. 2010;25(2):295-301. doi:10.1016/j.arth.2008.11.012.
- Sourlas I, Papachristou G, Pilichou A, Giannoudis PV, Efstathopoulos N, Nikolaou VS. Proximal tibial stress fractures associated with primary degenerative knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2009;38(3):120-124
- Demir B, Gursu S, Oke R, Ozturk K, Sahin V. Proximal tibia stress fracture caused by severe arthrosis of the knee with varus deformity. Am J Orthop (Belle Mead NJ). 2009;38(9):457-459.
- Satku K, Kumar VP, Pho RW. Stress fractures of the tibia in osteoarthritis of the knee. J Bone Joint Surg Br. 1987;69(2):309-311. doi:10.1302/0301-620X.69B2.3818767.
- Martin LM, Bourne RB, Rorabeck CH. Stress fractures associated with osteoarthritis of the knee. A report of three cases. J Bone Joint Surg Am. 1988;70(5):771-774.
- Hong SH, Chu IT. Stress fracture of the proximal fibula in military recruits. Clin Orthop Surg. 2009;1(3):161-164. doi:10.4055/cios.2009.1.3.161
- Knapik JJ, Reynolds K, Hoedebecke KL. Stress fractures: Etiology, epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 17(2):120-130.
- Cheung MHS, Lee M-F, Lui TH. Insufficiency fracture of the proximal fibula and then tibia: A case report. J Orthop Surg. 2013;21(1):103-105. doi:10.1177/230949901302100126
ABSTRACT
Stress fractures are often missed, especially in unusual clinical settings. We report on 2 patients who presented to our orthopedic surgery clinic with incidental findings of asymptomatic proximal fibular tension side stress fractures in severe longstanding varus osteoarthritic knees. Initial plain films demonstrated an expansile deformity of the proximal fibular shaft, and differential diagnosis included a healed or healing fracture versus possible neoplasm. Magnetic resonance imaging with and without gadolinium was utilized to rule out the latter prior to planned total knee arthroplasty.
Continue to: The proximal fibula...
The proximal fibula is a rare site for stress fractures, with most of these fractures occurring in military recruits.1 To the authors’ knowledge, there has been only 1 documented case of a proximal fibular stress fracture in patients with severe osteoarthritis (OA) and fixed varus deformity, which mimicked L5 radiculopathy.2 We are not aware of any reports of asymptomatic tension-side fibular stress fractures in varus knees. In our 2 cases, the patients were indicated for total knee arthroplasty (TKA) for varus degenerative joint disease after failing nonoperative treatment; however, further work-up was justified to rule out neoplasm after plain films revealed expansile deformities of the proximal fibular shaft. Each patient subsequently underwent magnetic resonance imaging (MRI) with and without gadolinium contrast, which demonstrated a healed and healing proximal fibular stress fracture. Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease, and stress fractures about a varus knee generally occur on the compression side of the tibia and are symptomatic.3-7 The patients provided informed written consent for print and electronic publication of this case report.

CASE REPORT
The first patient was a 77-year-old male who presented with longstanding knee pain, left greater than right, exacerbated by weight-bearing activities. The patient had no improvement with physical therapy or anti-inflammatory medication. He denied any history of trauma, weakness, paresthesias, or a recent increase in activity. The patient also denied any fevers, chills, night sweats, or other constitutional symptoms. On physical examination, the patient had an antalgic gait and limited range of motion bilaterally. Examination of his right lower extremity demonstrated a fixed 5° varus deformity. No distinct point tenderness was noted.

Radiographs of the right knee demonstrated varus deformity and tricompartmental degenerative changes with severe medial joint space narrowing. An expansile deformity of the proximal right fibular shaft was also noted (Figure 1), which was not present on the films 2 years earlier (Figure 2). The absence of this deformity on previous imaging raised the suspicion of a tumor. An MRI with and without gadolinium, which was obtained to rule out a neoplastic process, showed an old, healed proximal fibular shaft fracture with chronic periosteal reaction (Figure 3). There was no marrow edema to suggest acute injury and no neoplastic lesion. He was reassured regarding the benign findings and was scheduled for a left TKA, as his pain was more severe on the left knee. The patient’s stress fracture healed without complications, and he underwent a successful left TKA. He returned approximately 6 months after his procedure with worsening right knee pain and underwent a successful TKA on the right knee as well.

The second patient was a 67-year-old male with longstanding bilateral knee pain, right greater than left, with no antecedent trauma. He denied a history of increased activity, or weakness or paresthesias. He denied any fevers, chills, night sweats, or other constitutional symptoms. One year prior to presentation at our clinic, he had received corticosteroid injections and hyaluronic acid, without relief. The patient also had a history with another surgeon of arthroscopy 1 year earlier and subchondroplasty 3 years before presentation to our clinic. On physical examination, the patient’s right knee displayed a fixed 7° varus deformity with decreased range of motion, effusion, and diffuse crepitus. Further examination revealed tenderness to palpation of the proximal fibula.

Radiographs of the right knee showed degenerative joint disease with varus deformity and medial compartment joint space narrowing. They also demonstrated an expansile deformity of mixed lucency and sclerosis involving the proximal right fibular shaft (Figure 4). Although these findings appeared to be consistent with a stress fracture, their appearance was also suspicious for a neoplasm. To rule out malignancy, an MRI with and without gadolinium was obtained that revealed a healing stress fracture of the proximal fibula (Figure 5). The patient was reassured, and plans were made to proceed with a TKA. The patient’s stress fracture healed without complications, and he underwent successful right TKA. Radiographs from the patient’s 8-week follow-up showed a healed fibular stress fracture (Figure 6).


Continue to: DISCUSSION
DISCUSSION
To our knowledge, this is the first report of incidental tension-side stress fractures in varus osteoarthritic knees. Stress fractures have been classified into 2 groups, fatigue fractures and insufficiency fractures. Fatigue fractures occur when abnormal stress is applied to normal bones, and insufficiency fractures result when normal stress is applied to abnormal bones.8 Stress fractures can also be classified into risk categories based on which bone is involved and the loading of the bone.9 Sites loaded in tension have increased risk of nonunion, progression to complete fracture, and reoccurrence compared with sites loaded in compression.9 Stress fractures of the fibula occur rarely, and when present, they are more commonly observed in the distal fibula in athletes and military recruits.1 Stress fractures occur rarely in patients with primary OA, and when present in this setting, obesity and malalignment are the contributing factors.3 Neither patient was obese in our case (body mass index of 27 and 28, respectively), but significant varus deformity was present in both patients. Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.3-7
Regarding malalignment, Cheung and colleagues10 reported about a case of an elderly female with OA of the knee with valgus deformity that initially developed a proximal fibular stress fracture followed by a proximal tibial stress fracture. However, both of our patients had varus deformities. Mullaji and Shetty3 documented stress fractures in 34 patients with OA, a majority with varus deformities, but did not report any isolated proximal fibular stress fractures. Manish and colleagues2 reported the only documented case of an isolated proximal fibular stress fracture in a patient with osteoarthritic varus deformity. The patient presented initially with pain and paresthesias of the lower thigh and leg consistent with an L5 radiculopathy. They believed that the varus deformity and the repetitive contraction of the lateral knee muscles put increased shear forces on the fibula leading to the stress fracture. Our patients did not present with any radicular symptoms, a history of acute worsening pain, or an increased activity concerning for a stress fracture. Instead, our patients presented with progressively worsening knee pain typical of severe OA and incidental findings on imaging of tension-side fibular stress fractures. An MRI with and without gadolinium confirmed the diagnosis of a healed fracture in our first patient and a healing fracture in our second patient.
CONCLUSION
Although exceedingly rare in osteoarthritic varus knees, we presented 2 cases of MRI-confirmed proximal fibular stress fractures in this report. As demonstrated, patients may present with symptoms of OA or radicular symptoms as described by Manish and colleagues.2 Presentation may also include an expansile lesion on imaging, prompting a differential diagnosis that includes a neoplasm. If present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and not affect the plan or outcome of TKA.
ABSTRACT
Stress fractures are often missed, especially in unusual clinical settings. We report on 2 patients who presented to our orthopedic surgery clinic with incidental findings of asymptomatic proximal fibular tension side stress fractures in severe longstanding varus osteoarthritic knees. Initial plain films demonstrated an expansile deformity of the proximal fibular shaft, and differential diagnosis included a healed or healing fracture versus possible neoplasm. Magnetic resonance imaging with and without gadolinium was utilized to rule out the latter prior to planned total knee arthroplasty.
Continue to: The proximal fibula...
The proximal fibula is a rare site for stress fractures, with most of these fractures occurring in military recruits.1 To the authors’ knowledge, there has been only 1 documented case of a proximal fibular stress fracture in patients with severe osteoarthritis (OA) and fixed varus deformity, which mimicked L5 radiculopathy.2 We are not aware of any reports of asymptomatic tension-side fibular stress fractures in varus knees. In our 2 cases, the patients were indicated for total knee arthroplasty (TKA) for varus degenerative joint disease after failing nonoperative treatment; however, further work-up was justified to rule out neoplasm after plain films revealed expansile deformities of the proximal fibular shaft. Each patient subsequently underwent magnetic resonance imaging (MRI) with and without gadolinium contrast, which demonstrated a healed and healing proximal fibular stress fracture. Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease, and stress fractures about a varus knee generally occur on the compression side of the tibia and are symptomatic.3-7 The patients provided informed written consent for print and electronic publication of this case report.

CASE REPORT
The first patient was a 77-year-old male who presented with longstanding knee pain, left greater than right, exacerbated by weight-bearing activities. The patient had no improvement with physical therapy or anti-inflammatory medication. He denied any history of trauma, weakness, paresthesias, or a recent increase in activity. The patient also denied any fevers, chills, night sweats, or other constitutional symptoms. On physical examination, the patient had an antalgic gait and limited range of motion bilaterally. Examination of his right lower extremity demonstrated a fixed 5° varus deformity. No distinct point tenderness was noted.

Radiographs of the right knee demonstrated varus deformity and tricompartmental degenerative changes with severe medial joint space narrowing. An expansile deformity of the proximal right fibular shaft was also noted (Figure 1), which was not present on the films 2 years earlier (Figure 2). The absence of this deformity on previous imaging raised the suspicion of a tumor. An MRI with and without gadolinium, which was obtained to rule out a neoplastic process, showed an old, healed proximal fibular shaft fracture with chronic periosteal reaction (Figure 3). There was no marrow edema to suggest acute injury and no neoplastic lesion. He was reassured regarding the benign findings and was scheduled for a left TKA, as his pain was more severe on the left knee. The patient’s stress fracture healed without complications, and he underwent a successful left TKA. He returned approximately 6 months after his procedure with worsening right knee pain and underwent a successful TKA on the right knee as well.

The second patient was a 67-year-old male with longstanding bilateral knee pain, right greater than left, with no antecedent trauma. He denied a history of increased activity, or weakness or paresthesias. He denied any fevers, chills, night sweats, or other constitutional symptoms. One year prior to presentation at our clinic, he had received corticosteroid injections and hyaluronic acid, without relief. The patient also had a history with another surgeon of arthroscopy 1 year earlier and subchondroplasty 3 years before presentation to our clinic. On physical examination, the patient’s right knee displayed a fixed 7° varus deformity with decreased range of motion, effusion, and diffuse crepitus. Further examination revealed tenderness to palpation of the proximal fibula.

Radiographs of the right knee showed degenerative joint disease with varus deformity and medial compartment joint space narrowing. They also demonstrated an expansile deformity of mixed lucency and sclerosis involving the proximal right fibular shaft (Figure 4). Although these findings appeared to be consistent with a stress fracture, their appearance was also suspicious for a neoplasm. To rule out malignancy, an MRI with and without gadolinium was obtained that revealed a healing stress fracture of the proximal fibula (Figure 5). The patient was reassured, and plans were made to proceed with a TKA. The patient’s stress fracture healed without complications, and he underwent successful right TKA. Radiographs from the patient’s 8-week follow-up showed a healed fibular stress fracture (Figure 6).


Continue to: DISCUSSION
DISCUSSION
To our knowledge, this is the first report of incidental tension-side stress fractures in varus osteoarthritic knees. Stress fractures have been classified into 2 groups, fatigue fractures and insufficiency fractures. Fatigue fractures occur when abnormal stress is applied to normal bones, and insufficiency fractures result when normal stress is applied to abnormal bones.8 Stress fractures can also be classified into risk categories based on which bone is involved and the loading of the bone.9 Sites loaded in tension have increased risk of nonunion, progression to complete fracture, and reoccurrence compared with sites loaded in compression.9 Stress fractures of the fibula occur rarely, and when present, they are more commonly observed in the distal fibula in athletes and military recruits.1 Stress fractures occur rarely in patients with primary OA, and when present in this setting, obesity and malalignment are the contributing factors.3 Neither patient was obese in our case (body mass index of 27 and 28, respectively), but significant varus deformity was present in both patients. Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.3-7
Regarding malalignment, Cheung and colleagues10 reported about a case of an elderly female with OA of the knee with valgus deformity that initially developed a proximal fibular stress fracture followed by a proximal tibial stress fracture. However, both of our patients had varus deformities. Mullaji and Shetty3 documented stress fractures in 34 patients with OA, a majority with varus deformities, but did not report any isolated proximal fibular stress fractures. Manish and colleagues2 reported the only documented case of an isolated proximal fibular stress fracture in a patient with osteoarthritic varus deformity. The patient presented initially with pain and paresthesias of the lower thigh and leg consistent with an L5 radiculopathy. They believed that the varus deformity and the repetitive contraction of the lateral knee muscles put increased shear forces on the fibula leading to the stress fracture. Our patients did not present with any radicular symptoms, a history of acute worsening pain, or an increased activity concerning for a stress fracture. Instead, our patients presented with progressively worsening knee pain typical of severe OA and incidental findings on imaging of tension-side fibular stress fractures. An MRI with and without gadolinium confirmed the diagnosis of a healed fracture in our first patient and a healing fracture in our second patient.
CONCLUSION
Although exceedingly rare in osteoarthritic varus knees, we presented 2 cases of MRI-confirmed proximal fibular stress fractures in this report. As demonstrated, patients may present with symptoms of OA or radicular symptoms as described by Manish and colleagues.2 Presentation may also include an expansile lesion on imaging, prompting a differential diagnosis that includes a neoplasm. If present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and not affect the plan or outcome of TKA.
- Devas MB, Sweetnam R. Stress fractures of the fibula; a review of fifty cases in athletes. J Bone Joint Surg Br. 1956;38-B(4):818-829.
- Manish KK, Agnivesh T, Pramod PS, Samir SD. Isolated proximal fibular stress fracture in osteoarthritis knee presenting as L5 radiculopathy. J Orthop Case Reports. 2015;5(3):75-77. doi:10.13107/jocr.2250-0685.315.
- Mullaji A, Shetty G. Total knee arthroplasty for arthritic knees with tibiofibular stress fractures: classification and treatment guidelines. J Arthroplasty. 2010;25(2):295-301. doi:10.1016/j.arth.2008.11.012.
- Sourlas I, Papachristou G, Pilichou A, Giannoudis PV, Efstathopoulos N, Nikolaou VS. Proximal tibial stress fractures associated with primary degenerative knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2009;38(3):120-124
- Demir B, Gursu S, Oke R, Ozturk K, Sahin V. Proximal tibia stress fracture caused by severe arthrosis of the knee with varus deformity. Am J Orthop (Belle Mead NJ). 2009;38(9):457-459.
- Satku K, Kumar VP, Pho RW. Stress fractures of the tibia in osteoarthritis of the knee. J Bone Joint Surg Br. 1987;69(2):309-311. doi:10.1302/0301-620X.69B2.3818767.
- Martin LM, Bourne RB, Rorabeck CH. Stress fractures associated with osteoarthritis of the knee. A report of three cases. J Bone Joint Surg Am. 1988;70(5):771-774.
- Hong SH, Chu IT. Stress fracture of the proximal fibula in military recruits. Clin Orthop Surg. 2009;1(3):161-164. doi:10.4055/cios.2009.1.3.161
- Knapik JJ, Reynolds K, Hoedebecke KL. Stress fractures: Etiology, epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 17(2):120-130.
- Cheung MHS, Lee M-F, Lui TH. Insufficiency fracture of the proximal fibula and then tibia: A case report. J Orthop Surg. 2013;21(1):103-105. doi:10.1177/230949901302100126
- Devas MB, Sweetnam R. Stress fractures of the fibula; a review of fifty cases in athletes. J Bone Joint Surg Br. 1956;38-B(4):818-829.
- Manish KK, Agnivesh T, Pramod PS, Samir SD. Isolated proximal fibular stress fracture in osteoarthritis knee presenting as L5 radiculopathy. J Orthop Case Reports. 2015;5(3):75-77. doi:10.13107/jocr.2250-0685.315.
- Mullaji A, Shetty G. Total knee arthroplasty for arthritic knees with tibiofibular stress fractures: classification and treatment guidelines. J Arthroplasty. 2010;25(2):295-301. doi:10.1016/j.arth.2008.11.012.
- Sourlas I, Papachristou G, Pilichou A, Giannoudis PV, Efstathopoulos N, Nikolaou VS. Proximal tibial stress fractures associated with primary degenerative knee osteoarthritis. Am J Orthop (Belle Mead NJ). 2009;38(3):120-124
- Demir B, Gursu S, Oke R, Ozturk K, Sahin V. Proximal tibia stress fracture caused by severe arthrosis of the knee with varus deformity. Am J Orthop (Belle Mead NJ). 2009;38(9):457-459.
- Satku K, Kumar VP, Pho RW. Stress fractures of the tibia in osteoarthritis of the knee. J Bone Joint Surg Br. 1987;69(2):309-311. doi:10.1302/0301-620X.69B2.3818767.
- Martin LM, Bourne RB, Rorabeck CH. Stress fractures associated with osteoarthritis of the knee. A report of three cases. J Bone Joint Surg Am. 1988;70(5):771-774.
- Hong SH, Chu IT. Stress fracture of the proximal fibula in military recruits. Clin Orthop Surg. 2009;1(3):161-164. doi:10.4055/cios.2009.1.3.161
- Knapik JJ, Reynolds K, Hoedebecke KL. Stress fractures: Etiology, epidemiology, diagnosis, treatment, and prevention. J Spec Oper Med. 17(2):120-130.
- Cheung MHS, Lee M-F, Lui TH. Insufficiency fracture of the proximal fibula and then tibia: A case report. J Orthop Surg. 2013;21(1):103-105. doi:10.1177/230949901302100126
TAKE-HOME POINTS
- Proximal fibular stress fractures in patients with primary osteoarthritis and fixed varus deformity have rarely been reported.
- Stress fractures occurring near the knee in the setting of a varus deformity generally occur on the compression side of the tibia and are symptomatic.
- Proximal fibular stress fractures may present as an incidental finding of an expansile deformity on plain films in patients with varus osteoarthritic knees.
- Magnetic resonance imaging is rarely indicated in the evaluation of degenerative joint disease; however, it was justified in our case to rule out neoplasm.
- When present in the setting of an osteoarthritic varus knee, stress fractures of the proximal fibula should heal with conservative treatment and should not affect the plan or outcome of TKA.
Ice Pack–Induced Perniosis: A Rare and Underrecognized Association
Perniosis, or chilblain, is characterized by localized, tender, erythematous skin lesions that occur as an abnormal reaction to exposure to cold and damp conditions. Although the lesions favor the distal extremities, perniosis may present anywhere on the body. Lesions can develop within hours to days following exposure to temperature less than 10°C or damp environments with greater than 60% humidity.1 Acute cases may lead to pruritus and tenderness, whereas chronic cases may involve lesions that blister or ulcerate and can take weeks to heal. We report an unusual case of erythematous plaques arising on the buttocks of a 73-year-old woman using ice pack treatments for chronic low back pain.
Case Report
A 73-year-old woman presented with recurrent tender lesions on the buttocks of 5 years’ duration. Her medical history was remarkable for hypertension, hypothyroidism, and lumbar spinal fusion surgery 5 years prior. Physical examination revealed indurated erythematous plaques with areas of erosions on the left buttock with some involvement of the right buttock (Figure 1).
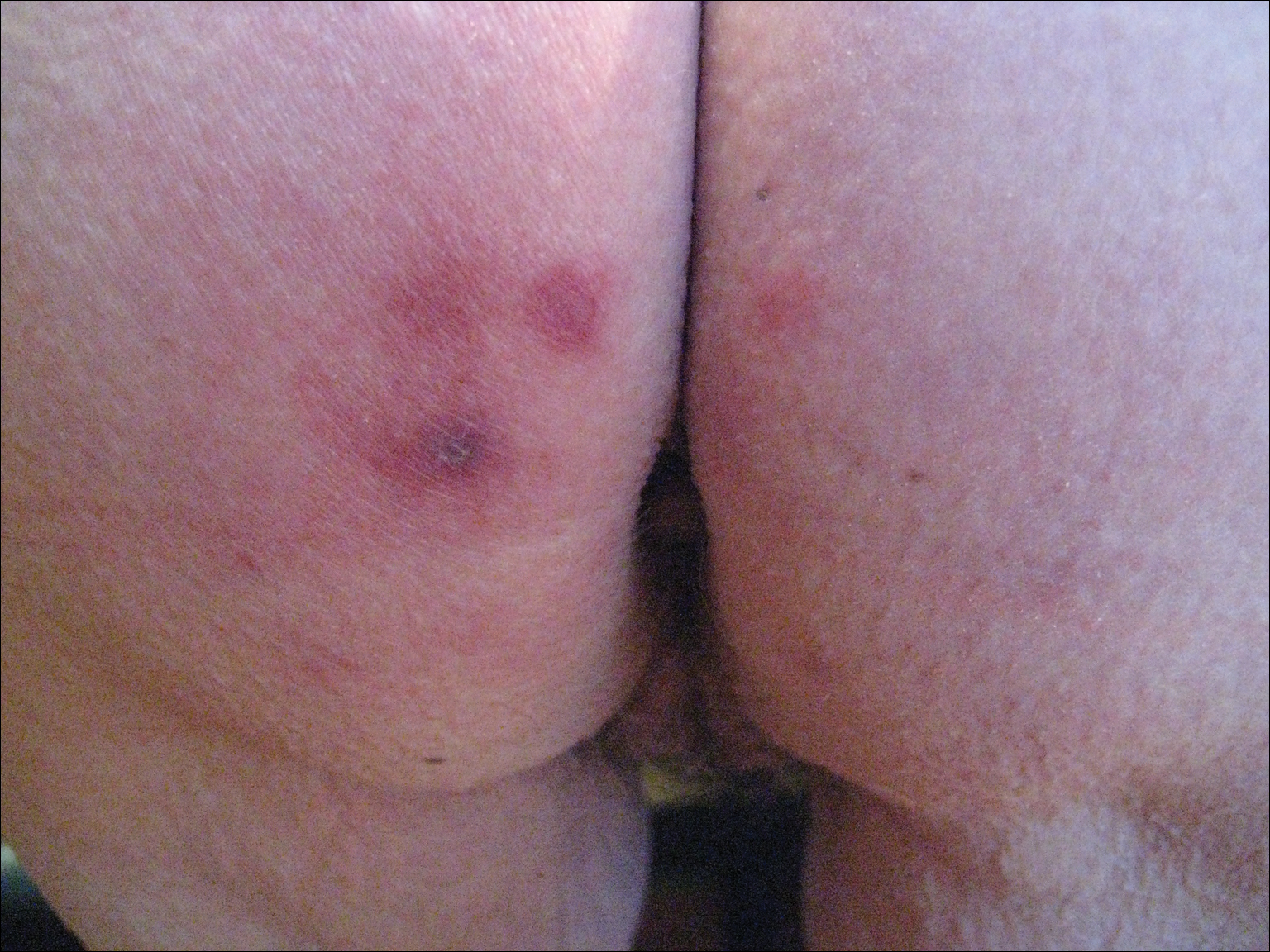
After a trial of oral valacyclovir for presumed herpes simplex infection provided no relief, a punch biopsy of the left buttock was performed, which revealed a cell-poor interface dermatitis with superficial and deep perivascular and periadnexal lymphocytic infiltrates (Figure 2). Perieccrine lymphocytes were present in a small portion of the reticular dermis (Figure 3). The patient revealed she had been sitting on ice packs for several hours daily since the lumbar spinal fusion surgery 5 years prior to alleviate chronic low back pain.
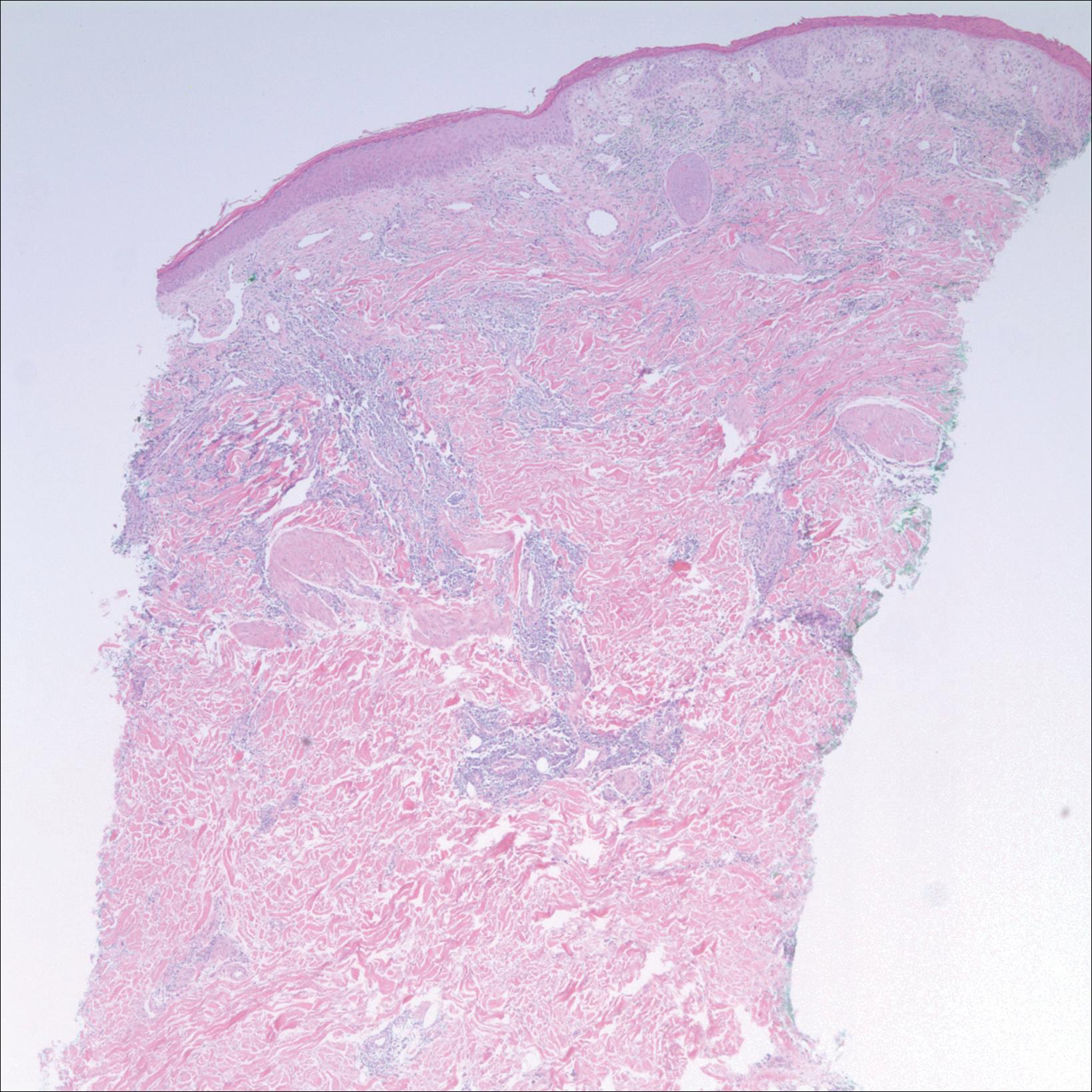
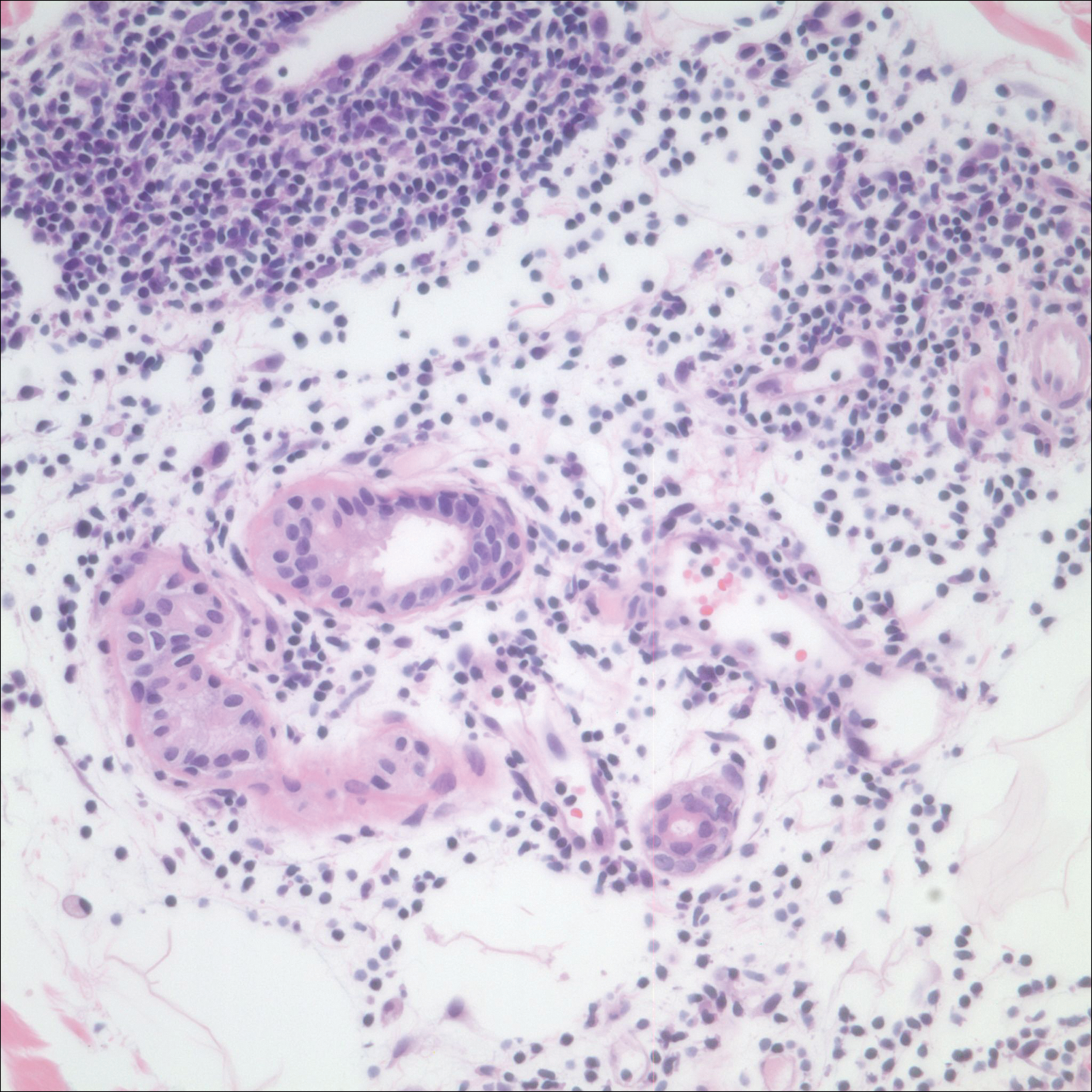
Based on the clinicopathologic correlation, a diagnosis of perniosis secondary to ice pack therapy was made. An evaluation for concomitant or underlying connective tissue disease (CTD) including a complete blood cell count with sedimentation rate, antinuclear antibodies (ANAs), serum protein electrophoresis, and serum levels of cryoglobulins and complement components was unremarkable. Our patient was treated with simple analgesia and was encouraged to avoid direct contact with ice packs for extended periods of time. Because of her low back pain, she continued to use ice packs but readjusted them sporadically and decreased frequency of use. She had complete resolution of the lesions at 6-month follow-up.
Comment
Perniosis is a self-limited condition, manifesting as erythematous plaques or nodules following exposure to cold and damp conditions. It was first reported in 1902 by Hochsinger2 as tender submental plaques occurring in children after exposure to cold weather. Since then, reports of perniosis have been described in equestrians and long-distance cyclists as well as in the context of other outdoor activities.3-5 In all cases, patients developed perniosis at sites of exposure to cold or damp conditions.
Perniosis arising in patients using ice pack therapy is a rare and recent phenomenon, with only 3 other known reported cases.6,7 In all cases, including ours, patients reported treating chronic low back pain with ice packs for more than 2 hours per day. Clinical presentations included erythematous to purpuric plaques with ulceration on the lower back or buttocks that reoccurred with subsequent use of ice packs. No concomitant CTD was reported.6
Much controversy exists as to whether idiopathic perniosis (IP) increases susceptibility to acquiring an autoimmune disease or if IP is a form of CTD that follows a more indolent course.8 In a prospective study of 33 patients with underlying IP, no patients developed lupus erythematosus (LE), with a median follow-up of 38 months.9 A study by Crowson and Magro8 revealed that 18 of 39 patients with perniotic lesions had an associated systemic disease including LE, human immunodeficiency virus, viral hepatitis, rheumatoid arthritis, cryofibrinogenemia, hypergammaglobulinemia, iritis, or Crohn disease. Of the 21 other patients who had no underlying CTD or systemic disease, 10 had a positive ANA test but no systemic symptoms; therefore, all 21 of these patients were classified as cases of IP.8
Cutaneous biopsy to distinguish between IP and autoimmune perniosis remains controversial; perniotic lesions and discoid LE share histopathologic features,9 as was evident with our case, which demonstrated overlapping findings of vacuolar change with superficial and deep perivascular and periadnexal lymphoid infiltrates. Typical features of IP include thrombosed capillaries in the papillary dermis and lymphocytic exocytosis localized to the acrosyringia, whereas secondary perniosis has superficial and deep perivascular and perieccrine lymphocytic infiltrates with vascular thrombosis in the reticular dermis. Vascular ectasia, dermal mucinosis, basement membrane zone thickening, and erythrocyte extravasation are not reliable and may be seen in both cases.8 One study revealed the only significant difference between both entities was the perieccrine distribution of lymphocytic infiltrate in cases of IP (P=.007), whereas an absence of perieccrine involvement was noted in autoimmune cases.9
Direct immunofluorescence (DIF) may help differentiate IP from autoimmune perniosis. In a prospective study by Viguier et al,9 6 of 9 patients with IP had negative DIF and 3 had slight nonspecific C3 immunoreactivity of dermal vessels. Conversely, in patients with autoimmune perniosis, positive DIF with the lupus band test was seen in 3 of 7 patients, all who had a positive ANA test9; however, positive ANA levels also were reported in patients with autoimmune perniosis but negative DIF, suggesting that DIF lacks specificity in diagnosing autoimmune perniosis.
Although histopathologic findings bear similarities to LE, there are no guidelines to suggest for or against laboratory testing for CTD in patients presenting with perniosis. Some investigators have suggested that any patient with clinical features suggestive of perniosis should undergo laboratory evaluation including a complete blood cell count and assessment for antibodies to Ro, ANA, rheumatoid factor, cryofibrinogens, and antiphospholipid antibodies.9 Serum protein electrophoresis and immunofixation electrophoresis may be done to exclude monoclonal gammopathy.
For idiopathic cases, treatment is aimed at limiting or removing cold exposure. Patients should be advised regarding the use of long-term ice pack use and the potential development of perniosis. For chronic perniosis lasting beyond several weeks, a combination of a slow taper of oral prednisone, hydroxychloroquine, and quinacrine has been successful in patients with persistent lesions despite making environmental modifications.3 Intralesional triamcinolone acetonide and nifedipine also have been effective in perniotic hand lesions.10
Conclusion
We report a rare case of perniosis on the buttocks that arose in a patient who utilized ice packs for treatment of chronic low back pain. Ice pack–induced perniosis may be an underreported entity. Histopathologic examination is nondescript, as overlapping features of perniosis and LE have been observed with no underlying CTD present. Correlation with patient history and clinical examination is paramount in diagnosis and management.
- Praminik T, Jha AK, Ghimire A. A retrospective study of cases with chilblains (perniosis) in Out Patient Department of Dermatology, Nepal Medical College and Teaching Hospital (NMCTH). Nepal Med Coll J. 2011;13:190-192.
- Hochsinger C. Acute perniosis in submental region of child [in German]. Monatsschr Kinderheilkd. 1902;1:323-327.
- Stewart CL, Adler DJ, Jacobson A, et al. Equestrian perniosis: a report of 2 cases and a review of the literature. Am J Dermatopathol. 2013;35:237-240.
- Neal AJ, Jarman AM, Bennett TG. Perniosis in a long-distance cyclist crossing Mongolia. J Travel Med. 2012;19:66-68.
- Price RD, Murdoch DR. Perniosis (chilblains) of the thigh: report of five cases including four following river crossings. High Alt Met Biol. 2001;2:535-538.
- West SA, McCalmont TH, North JP. Ice-pack dermatosis: a cold-induced dermatitis with similarities to cold panniculitis and perniosis that histopathologically resembles lupus. JAMA Dermatol. 2013;149:1314-1318.
- Haber JS, Ker KJ, Werth VP, et al. Ice‐pack dermatosis: a diagnositic pitfall for dermatopathologists that mimics lupus erythematosus. J Cutan Pathol. 2016;43:1-4.
- Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28:478-484.
- Viguier M, Pinguier L, Cavelier-Balloy B, et al. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. a study of the relationship to lupus erythematosus. Medicine. 2001;80:180-188.
- Patra AK, Das AL, Ramadasan P. Diltiazem vs. nifedipine in chilblains: a clinical trial. Indian J Dermatol Venereol Leprol. 2003;69:209-211.
Perniosis, or chilblain, is characterized by localized, tender, erythematous skin lesions that occur as an abnormal reaction to exposure to cold and damp conditions. Although the lesions favor the distal extremities, perniosis may present anywhere on the body. Lesions can develop within hours to days following exposure to temperature less than 10°C or damp environments with greater than 60% humidity.1 Acute cases may lead to pruritus and tenderness, whereas chronic cases may involve lesions that blister or ulcerate and can take weeks to heal. We report an unusual case of erythematous plaques arising on the buttocks of a 73-year-old woman using ice pack treatments for chronic low back pain.
Case Report
A 73-year-old woman presented with recurrent tender lesions on the buttocks of 5 years’ duration. Her medical history was remarkable for hypertension, hypothyroidism, and lumbar spinal fusion surgery 5 years prior. Physical examination revealed indurated erythematous plaques with areas of erosions on the left buttock with some involvement of the right buttock (Figure 1).

After a trial of oral valacyclovir for presumed herpes simplex infection provided no relief, a punch biopsy of the left buttock was performed, which revealed a cell-poor interface dermatitis with superficial and deep perivascular and periadnexal lymphocytic infiltrates (Figure 2). Perieccrine lymphocytes were present in a small portion of the reticular dermis (Figure 3). The patient revealed she had been sitting on ice packs for several hours daily since the lumbar spinal fusion surgery 5 years prior to alleviate chronic low back pain.


Based on the clinicopathologic correlation, a diagnosis of perniosis secondary to ice pack therapy was made. An evaluation for concomitant or underlying connective tissue disease (CTD) including a complete blood cell count with sedimentation rate, antinuclear antibodies (ANAs), serum protein electrophoresis, and serum levels of cryoglobulins and complement components was unremarkable. Our patient was treated with simple analgesia and was encouraged to avoid direct contact with ice packs for extended periods of time. Because of her low back pain, she continued to use ice packs but readjusted them sporadically and decreased frequency of use. She had complete resolution of the lesions at 6-month follow-up.
Comment
Perniosis is a self-limited condition, manifesting as erythematous plaques or nodules following exposure to cold and damp conditions. It was first reported in 1902 by Hochsinger2 as tender submental plaques occurring in children after exposure to cold weather. Since then, reports of perniosis have been described in equestrians and long-distance cyclists as well as in the context of other outdoor activities.3-5 In all cases, patients developed perniosis at sites of exposure to cold or damp conditions.
Perniosis arising in patients using ice pack therapy is a rare and recent phenomenon, with only 3 other known reported cases.6,7 In all cases, including ours, patients reported treating chronic low back pain with ice packs for more than 2 hours per day. Clinical presentations included erythematous to purpuric plaques with ulceration on the lower back or buttocks that reoccurred with subsequent use of ice packs. No concomitant CTD was reported.6
Much controversy exists as to whether idiopathic perniosis (IP) increases susceptibility to acquiring an autoimmune disease or if IP is a form of CTD that follows a more indolent course.8 In a prospective study of 33 patients with underlying IP, no patients developed lupus erythematosus (LE), with a median follow-up of 38 months.9 A study by Crowson and Magro8 revealed that 18 of 39 patients with perniotic lesions had an associated systemic disease including LE, human immunodeficiency virus, viral hepatitis, rheumatoid arthritis, cryofibrinogenemia, hypergammaglobulinemia, iritis, or Crohn disease. Of the 21 other patients who had no underlying CTD or systemic disease, 10 had a positive ANA test but no systemic symptoms; therefore, all 21 of these patients were classified as cases of IP.8
Cutaneous biopsy to distinguish between IP and autoimmune perniosis remains controversial; perniotic lesions and discoid LE share histopathologic features,9 as was evident with our case, which demonstrated overlapping findings of vacuolar change with superficial and deep perivascular and periadnexal lymphoid infiltrates. Typical features of IP include thrombosed capillaries in the papillary dermis and lymphocytic exocytosis localized to the acrosyringia, whereas secondary perniosis has superficial and deep perivascular and perieccrine lymphocytic infiltrates with vascular thrombosis in the reticular dermis. Vascular ectasia, dermal mucinosis, basement membrane zone thickening, and erythrocyte extravasation are not reliable and may be seen in both cases.8 One study revealed the only significant difference between both entities was the perieccrine distribution of lymphocytic infiltrate in cases of IP (P=.007), whereas an absence of perieccrine involvement was noted in autoimmune cases.9
Direct immunofluorescence (DIF) may help differentiate IP from autoimmune perniosis. In a prospective study by Viguier et al,9 6 of 9 patients with IP had negative DIF and 3 had slight nonspecific C3 immunoreactivity of dermal vessels. Conversely, in patients with autoimmune perniosis, positive DIF with the lupus band test was seen in 3 of 7 patients, all who had a positive ANA test9; however, positive ANA levels also were reported in patients with autoimmune perniosis but negative DIF, suggesting that DIF lacks specificity in diagnosing autoimmune perniosis.
Although histopathologic findings bear similarities to LE, there are no guidelines to suggest for or against laboratory testing for CTD in patients presenting with perniosis. Some investigators have suggested that any patient with clinical features suggestive of perniosis should undergo laboratory evaluation including a complete blood cell count and assessment for antibodies to Ro, ANA, rheumatoid factor, cryofibrinogens, and antiphospholipid antibodies.9 Serum protein electrophoresis and immunofixation electrophoresis may be done to exclude monoclonal gammopathy.
For idiopathic cases, treatment is aimed at limiting or removing cold exposure. Patients should be advised regarding the use of long-term ice pack use and the potential development of perniosis. For chronic perniosis lasting beyond several weeks, a combination of a slow taper of oral prednisone, hydroxychloroquine, and quinacrine has been successful in patients with persistent lesions despite making environmental modifications.3 Intralesional triamcinolone acetonide and nifedipine also have been effective in perniotic hand lesions.10
Conclusion
We report a rare case of perniosis on the buttocks that arose in a patient who utilized ice packs for treatment of chronic low back pain. Ice pack–induced perniosis may be an underreported entity. Histopathologic examination is nondescript, as overlapping features of perniosis and LE have been observed with no underlying CTD present. Correlation with patient history and clinical examination is paramount in diagnosis and management.
Perniosis, or chilblain, is characterized by localized, tender, erythematous skin lesions that occur as an abnormal reaction to exposure to cold and damp conditions. Although the lesions favor the distal extremities, perniosis may present anywhere on the body. Lesions can develop within hours to days following exposure to temperature less than 10°C or damp environments with greater than 60% humidity.1 Acute cases may lead to pruritus and tenderness, whereas chronic cases may involve lesions that blister or ulcerate and can take weeks to heal. We report an unusual case of erythematous plaques arising on the buttocks of a 73-year-old woman using ice pack treatments for chronic low back pain.
Case Report
A 73-year-old woman presented with recurrent tender lesions on the buttocks of 5 years’ duration. Her medical history was remarkable for hypertension, hypothyroidism, and lumbar spinal fusion surgery 5 years prior. Physical examination revealed indurated erythematous plaques with areas of erosions on the left buttock with some involvement of the right buttock (Figure 1).

After a trial of oral valacyclovir for presumed herpes simplex infection provided no relief, a punch biopsy of the left buttock was performed, which revealed a cell-poor interface dermatitis with superficial and deep perivascular and periadnexal lymphocytic infiltrates (Figure 2). Perieccrine lymphocytes were present in a small portion of the reticular dermis (Figure 3). The patient revealed she had been sitting on ice packs for several hours daily since the lumbar spinal fusion surgery 5 years prior to alleviate chronic low back pain.


Based on the clinicopathologic correlation, a diagnosis of perniosis secondary to ice pack therapy was made. An evaluation for concomitant or underlying connective tissue disease (CTD) including a complete blood cell count with sedimentation rate, antinuclear antibodies (ANAs), serum protein electrophoresis, and serum levels of cryoglobulins and complement components was unremarkable. Our patient was treated with simple analgesia and was encouraged to avoid direct contact with ice packs for extended periods of time. Because of her low back pain, she continued to use ice packs but readjusted them sporadically and decreased frequency of use. She had complete resolution of the lesions at 6-month follow-up.
Comment
Perniosis is a self-limited condition, manifesting as erythematous plaques or nodules following exposure to cold and damp conditions. It was first reported in 1902 by Hochsinger2 as tender submental plaques occurring in children after exposure to cold weather. Since then, reports of perniosis have been described in equestrians and long-distance cyclists as well as in the context of other outdoor activities.3-5 In all cases, patients developed perniosis at sites of exposure to cold or damp conditions.
Perniosis arising in patients using ice pack therapy is a rare and recent phenomenon, with only 3 other known reported cases.6,7 In all cases, including ours, patients reported treating chronic low back pain with ice packs for more than 2 hours per day. Clinical presentations included erythematous to purpuric plaques with ulceration on the lower back or buttocks that reoccurred with subsequent use of ice packs. No concomitant CTD was reported.6
Much controversy exists as to whether idiopathic perniosis (IP) increases susceptibility to acquiring an autoimmune disease or if IP is a form of CTD that follows a more indolent course.8 In a prospective study of 33 patients with underlying IP, no patients developed lupus erythematosus (LE), with a median follow-up of 38 months.9 A study by Crowson and Magro8 revealed that 18 of 39 patients with perniotic lesions had an associated systemic disease including LE, human immunodeficiency virus, viral hepatitis, rheumatoid arthritis, cryofibrinogenemia, hypergammaglobulinemia, iritis, or Crohn disease. Of the 21 other patients who had no underlying CTD or systemic disease, 10 had a positive ANA test but no systemic symptoms; therefore, all 21 of these patients were classified as cases of IP.8
Cutaneous biopsy to distinguish between IP and autoimmune perniosis remains controversial; perniotic lesions and discoid LE share histopathologic features,9 as was evident with our case, which demonstrated overlapping findings of vacuolar change with superficial and deep perivascular and periadnexal lymphoid infiltrates. Typical features of IP include thrombosed capillaries in the papillary dermis and lymphocytic exocytosis localized to the acrosyringia, whereas secondary perniosis has superficial and deep perivascular and perieccrine lymphocytic infiltrates with vascular thrombosis in the reticular dermis. Vascular ectasia, dermal mucinosis, basement membrane zone thickening, and erythrocyte extravasation are not reliable and may be seen in both cases.8 One study revealed the only significant difference between both entities was the perieccrine distribution of lymphocytic infiltrate in cases of IP (P=.007), whereas an absence of perieccrine involvement was noted in autoimmune cases.9
Direct immunofluorescence (DIF) may help differentiate IP from autoimmune perniosis. In a prospective study by Viguier et al,9 6 of 9 patients with IP had negative DIF and 3 had slight nonspecific C3 immunoreactivity of dermal vessels. Conversely, in patients with autoimmune perniosis, positive DIF with the lupus band test was seen in 3 of 7 patients, all who had a positive ANA test9; however, positive ANA levels also were reported in patients with autoimmune perniosis but negative DIF, suggesting that DIF lacks specificity in diagnosing autoimmune perniosis.
Although histopathologic findings bear similarities to LE, there are no guidelines to suggest for or against laboratory testing for CTD in patients presenting with perniosis. Some investigators have suggested that any patient with clinical features suggestive of perniosis should undergo laboratory evaluation including a complete blood cell count and assessment for antibodies to Ro, ANA, rheumatoid factor, cryofibrinogens, and antiphospholipid antibodies.9 Serum protein electrophoresis and immunofixation electrophoresis may be done to exclude monoclonal gammopathy.
For idiopathic cases, treatment is aimed at limiting or removing cold exposure. Patients should be advised regarding the use of long-term ice pack use and the potential development of perniosis. For chronic perniosis lasting beyond several weeks, a combination of a slow taper of oral prednisone, hydroxychloroquine, and quinacrine has been successful in patients with persistent lesions despite making environmental modifications.3 Intralesional triamcinolone acetonide and nifedipine also have been effective in perniotic hand lesions.10
Conclusion
We report a rare case of perniosis on the buttocks that arose in a patient who utilized ice packs for treatment of chronic low back pain. Ice pack–induced perniosis may be an underreported entity. Histopathologic examination is nondescript, as overlapping features of perniosis and LE have been observed with no underlying CTD present. Correlation with patient history and clinical examination is paramount in diagnosis and management.
- Praminik T, Jha AK, Ghimire A. A retrospective study of cases with chilblains (perniosis) in Out Patient Department of Dermatology, Nepal Medical College and Teaching Hospital (NMCTH). Nepal Med Coll J. 2011;13:190-192.
- Hochsinger C. Acute perniosis in submental region of child [in German]. Monatsschr Kinderheilkd. 1902;1:323-327.
- Stewart CL, Adler DJ, Jacobson A, et al. Equestrian perniosis: a report of 2 cases and a review of the literature. Am J Dermatopathol. 2013;35:237-240.
- Neal AJ, Jarman AM, Bennett TG. Perniosis in a long-distance cyclist crossing Mongolia. J Travel Med. 2012;19:66-68.
- Price RD, Murdoch DR. Perniosis (chilblains) of the thigh: report of five cases including four following river crossings. High Alt Met Biol. 2001;2:535-538.
- West SA, McCalmont TH, North JP. Ice-pack dermatosis: a cold-induced dermatitis with similarities to cold panniculitis and perniosis that histopathologically resembles lupus. JAMA Dermatol. 2013;149:1314-1318.
- Haber JS, Ker KJ, Werth VP, et al. Ice‐pack dermatosis: a diagnositic pitfall for dermatopathologists that mimics lupus erythematosus. J Cutan Pathol. 2016;43:1-4.
- Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28:478-484.
- Viguier M, Pinguier L, Cavelier-Balloy B, et al. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. a study of the relationship to lupus erythematosus. Medicine. 2001;80:180-188.
- Patra AK, Das AL, Ramadasan P. Diltiazem vs. nifedipine in chilblains: a clinical trial. Indian J Dermatol Venereol Leprol. 2003;69:209-211.
- Praminik T, Jha AK, Ghimire A. A retrospective study of cases with chilblains (perniosis) in Out Patient Department of Dermatology, Nepal Medical College and Teaching Hospital (NMCTH). Nepal Med Coll J. 2011;13:190-192.
- Hochsinger C. Acute perniosis in submental region of child [in German]. Monatsschr Kinderheilkd. 1902;1:323-327.
- Stewart CL, Adler DJ, Jacobson A, et al. Equestrian perniosis: a report of 2 cases and a review of the literature. Am J Dermatopathol. 2013;35:237-240.
- Neal AJ, Jarman AM, Bennett TG. Perniosis in a long-distance cyclist crossing Mongolia. J Travel Med. 2012;19:66-68.
- Price RD, Murdoch DR. Perniosis (chilblains) of the thigh: report of five cases including four following river crossings. High Alt Met Biol. 2001;2:535-538.
- West SA, McCalmont TH, North JP. Ice-pack dermatosis: a cold-induced dermatitis with similarities to cold panniculitis and perniosis that histopathologically resembles lupus. JAMA Dermatol. 2013;149:1314-1318.
- Haber JS, Ker KJ, Werth VP, et al. Ice‐pack dermatosis: a diagnositic pitfall for dermatopathologists that mimics lupus erythematosus. J Cutan Pathol. 2016;43:1-4.
- Crowson AN, Magro CM. Idiopathic perniosis and its mimics: a clinical and histological study of 38 cases. Hum Pathol. 1997;28:478-484.
- Viguier M, Pinguier L, Cavelier-Balloy B, et al. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. a study of the relationship to lupus erythematosus. Medicine. 2001;80:180-188.
- Patra AK, Das AL, Ramadasan P. Diltiazem vs. nifedipine in chilblains: a clinical trial. Indian J Dermatol Venereol Leprol. 2003;69:209-211.
Practice Points
- Ice pack-induced perniosis is a rare condition that can occur in patients using long-term ice pack therapy.
- This entity histopathologically mimics cutaneous lupus erythematosus and can present a diagnostic challenge.
- A thorough clinical history and awareness of this diagnosis is essential for diagnostic accuracy.
6-day history of fever • groin pain and swelling • recent hiking trip in Colorado • Dx?
THE CASE
A 33-year-old Caucasian woman presented to the emergency department with a 6-day history of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado.
Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high.
The patient’s work-up included a negative pregnancy test and urinalysis. A complete blood count demonstrated a white blood cell count of 8.6 (4.3-10.5) × 103/UL with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). Blood cultures were negative for any bacterial or fungal growth after 48 hours; stool cultures were negative for Salmonella, Shigella, Campylobacter, Giardia, generalized Yersinia, and Escherichia coli O157:H7. Swabs for Gardnerella vaginalis, Trichomonas vaginalis, Candida, Chlamydia trachomatis, and Neisseria gonorrhea also were negative. Lyme, Bartonella henselae, and heterophile antibodies were also negative. Francisella tularensis was not cultured due to low suspicion.
Imaging included a normal chest x-ray and a computed tomography scan of the abdomen and pelvis that showed enlarged right inguinal lymph nodes with fatty stranding, a thicker distal right iliopsoas, hepatosplenomegaly, and an enlarged right adnexa (FIGURE 1). Initial ultrasound of the bubo showed 2 enlarged suprapubic lymph nodes, the largest measuring 3.5 × 1.4 × 2.4 cm3 (FIGURE 2), and 8 enlarged inguinal nodes.
The patient continued to have a low-grade fever, diarrhea, and inguinal lymphadenopathy throughout her first 2 hospitalized days. The cefoxitin was discontinued by Day 3, and the consulting infectious disease physician started oral metronidazole 500 mg every 12 hours due to the patient’s failure to improve. Later that night, the patient experienced increasing erythema and pain in her right inguinal region. A repeat ultrasound showed increased inguinal lymphadenopathy with the largest nodes measuring 2.9 × 1.5 × 2.5 cm3 and 2.7 × 1.3 × 2 cm3 (FIGURE 3).
Although doxycycline is considered an acceptable regimen for Y pestis infection, the infectious disease physician added oral ciprofloxacin 750 mg every 12 hours the following morning, as the patient had not improved.
THE DIAGNOSIS
Although the initial gram stain was negative for Yersinia, clinical suspicion pointed to a diagnosis of bubonic plague. Serology was considered; however, it was not available through the hospital. A definitive diagnosis required bubo aspiration and culture, which was performed but required 48 hours before results would be available.
Continue to: By Day 5, the patient was clinically improved and...
By Day 5, the patient was clinically improved and deemed safe for discharge on empiric treatment with ciprofloxacin 750 mg twice daily and doxycycline 100 mg twice daily to complete a 14-day course of antibiotic therapy for bubonic plague. The bubo culture subsequently grew Y pestis, confirming the diagnosis. The patient made a full recovery and was greatly improved when seen in the outpatient setting by the treating infectious disease physician. Outpatient ultrasound repeated 3 weeks after discharge showed borderline lymphadenopathy, no greater than 1 cm.
DISCUSSION
Between 2000 and 2009, there were 57 cases of Y pestis in the United States; in early 2015, 11 cases were found in 6 Western states.1 The plague presents in the bubonic form 80% to 95% of the time, and it has never been reported in Michigan (where we treated this patient); however, there was a laboratory case in Illinois. Although rats were traditionally the host for Y pestis, the prairie dog, Cynomys gunnisoni, is a host in the United States.2 Rodents are the most important hosts, but more than 200 mammalian species, including domestic pets, have had reported infections. Transmission is primarily via flea bites, but Y pestis also may be transmitted via respiratory secretion, inhalation, or direct handling of contaminated animal tissues. Due to the risk of respiratory spread, the Centers for Disease Control and Prevention must be notified of a diagnosis.3,4
Y pestis travels from the site of the flea bite to regional lymph nodes, where it reproduces, and the resultant inflammatory reaction creates buboes. The bacteria then circulate in the blood to other organs, although Y pestis bacteria are primarily removed by the liver and spleen. Patients often develop symptoms such as headache, fevers, chills, and gastrointestinal distress. Diagnosis is reached by bubo culture or rapid testing for the F1 antigen. Early intervention with antibiotics is crucial as untreated bubonic plague has a mortality rate of 50% to 90%.3,4
The differential diagnosis for unilateral inguinal lymphadenopathy with associated constitutional symptoms was broad, in this case, and included pelvic inflammatory disease, bubonic plague, iliopsoas abscess, lymphogranuloma venereum, bartonellosis, infectious mononucleosis, and tick-borne diseases, such as ehrlichiosis, tularemia, Lyme disease, Rocky Mountain spotted fever, and Colorado tick fever.
Treatment. Food and Drug Administration–approved treatments include streptomycin (gentamicin 5 mg/kg/day IM or IV for 14 days is more widely utilized), doxycycline 200 mg PO once daily for 10 to 14 days, and fluoroquinolones (ciprofloxacin 500-750 mg every 12 hours for 10-14 days). Trimethoprim-sulfamethoxazole may be used as an alternative, but limitations include potentially incomplete or slowed responses.
Continue to: THE TAKEAWAY
THE TAKEAWAY
This case points to the importance of a complete, systematic approach to each patient. While bubonic plague is not a diagnosis that would immediately come to mind in a patient visiting an emergency department in Michigan, a thorough history revealed a recent trip to a bubonic plague–endemic area. A thorough physical exam demonstrated unilateral painful inguinal adenopathy—which, when paired with the patient’s history—was consistent with the uncommon diagnosis of bubonic plague.
The authors thank Brian Waite, MD, and James Addison, MD, for critically revising this report for important intellectual content.
CORRESPONDENCE
Katherine Lazet, DO, 3838 N First Avenue, Evansville, IN 47710; lazetkat@gmail.com
1. Kwit N, Nelson C, Kugeler K, et al. Human Plague – United States, 2015. MMWR Morb Mortal Wkly Rep. 2015,64:918-919.
2. Friggens MM, Parmenter RR, Boyden M, et al. Flea abundance, diversity, and plague in Gunnison’s prairie dog (Cynomys gunnisoni) and their burrows in Montane grasslands in northern New Mexico. J Wildl Dis. 2010;46:356-367.
3. Mandell G, Bennett J, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:2943-2953.
4. Perry RD, Featherston JD. Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev. 1997;10:35-66.
THE CASE
A 33-year-old Caucasian woman presented to the emergency department with a 6-day history of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado.
Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high.
The patient’s work-up included a negative pregnancy test and urinalysis. A complete blood count demonstrated a white blood cell count of 8.6 (4.3-10.5) × 103/UL with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). Blood cultures were negative for any bacterial or fungal growth after 48 hours; stool cultures were negative for Salmonella, Shigella, Campylobacter, Giardia, generalized Yersinia, and Escherichia coli O157:H7. Swabs for Gardnerella vaginalis, Trichomonas vaginalis, Candida, Chlamydia trachomatis, and Neisseria gonorrhea also were negative. Lyme, Bartonella henselae, and heterophile antibodies were also negative. Francisella tularensis was not cultured due to low suspicion.
Imaging included a normal chest x-ray and a computed tomography scan of the abdomen and pelvis that showed enlarged right inguinal lymph nodes with fatty stranding, a thicker distal right iliopsoas, hepatosplenomegaly, and an enlarged right adnexa (FIGURE 1). Initial ultrasound of the bubo showed 2 enlarged suprapubic lymph nodes, the largest measuring 3.5 × 1.4 × 2.4 cm3 (FIGURE 2), and 8 enlarged inguinal nodes.
The patient continued to have a low-grade fever, diarrhea, and inguinal lymphadenopathy throughout her first 2 hospitalized days. The cefoxitin was discontinued by Day 3, and the consulting infectious disease physician started oral metronidazole 500 mg every 12 hours due to the patient’s failure to improve. Later that night, the patient experienced increasing erythema and pain in her right inguinal region. A repeat ultrasound showed increased inguinal lymphadenopathy with the largest nodes measuring 2.9 × 1.5 × 2.5 cm3 and 2.7 × 1.3 × 2 cm3 (FIGURE 3).
Although doxycycline is considered an acceptable regimen for Y pestis infection, the infectious disease physician added oral ciprofloxacin 750 mg every 12 hours the following morning, as the patient had not improved.
THE DIAGNOSIS
Although the initial gram stain was negative for Yersinia, clinical suspicion pointed to a diagnosis of bubonic plague. Serology was considered; however, it was not available through the hospital. A definitive diagnosis required bubo aspiration and culture, which was performed but required 48 hours before results would be available.
Continue to: By Day 5, the patient was clinically improved and...
By Day 5, the patient was clinically improved and deemed safe for discharge on empiric treatment with ciprofloxacin 750 mg twice daily and doxycycline 100 mg twice daily to complete a 14-day course of antibiotic therapy for bubonic plague. The bubo culture subsequently grew Y pestis, confirming the diagnosis. The patient made a full recovery and was greatly improved when seen in the outpatient setting by the treating infectious disease physician. Outpatient ultrasound repeated 3 weeks after discharge showed borderline lymphadenopathy, no greater than 1 cm.
DISCUSSION
Between 2000 and 2009, there were 57 cases of Y pestis in the United States; in early 2015, 11 cases were found in 6 Western states.1 The plague presents in the bubonic form 80% to 95% of the time, and it has never been reported in Michigan (where we treated this patient); however, there was a laboratory case in Illinois. Although rats were traditionally the host for Y pestis, the prairie dog, Cynomys gunnisoni, is a host in the United States.2 Rodents are the most important hosts, but more than 200 mammalian species, including domestic pets, have had reported infections. Transmission is primarily via flea bites, but Y pestis also may be transmitted via respiratory secretion, inhalation, or direct handling of contaminated animal tissues. Due to the risk of respiratory spread, the Centers for Disease Control and Prevention must be notified of a diagnosis.3,4
Y pestis travels from the site of the flea bite to regional lymph nodes, where it reproduces, and the resultant inflammatory reaction creates buboes. The bacteria then circulate in the blood to other organs, although Y pestis bacteria are primarily removed by the liver and spleen. Patients often develop symptoms such as headache, fevers, chills, and gastrointestinal distress. Diagnosis is reached by bubo culture or rapid testing for the F1 antigen. Early intervention with antibiotics is crucial as untreated bubonic plague has a mortality rate of 50% to 90%.3,4
The differential diagnosis for unilateral inguinal lymphadenopathy with associated constitutional symptoms was broad, in this case, and included pelvic inflammatory disease, bubonic plague, iliopsoas abscess, lymphogranuloma venereum, bartonellosis, infectious mononucleosis, and tick-borne diseases, such as ehrlichiosis, tularemia, Lyme disease, Rocky Mountain spotted fever, and Colorado tick fever.
Treatment. Food and Drug Administration–approved treatments include streptomycin (gentamicin 5 mg/kg/day IM or IV for 14 days is more widely utilized), doxycycline 200 mg PO once daily for 10 to 14 days, and fluoroquinolones (ciprofloxacin 500-750 mg every 12 hours for 10-14 days). Trimethoprim-sulfamethoxazole may be used as an alternative, but limitations include potentially incomplete or slowed responses.
Continue to: THE TAKEAWAY
THE TAKEAWAY
This case points to the importance of a complete, systematic approach to each patient. While bubonic plague is not a diagnosis that would immediately come to mind in a patient visiting an emergency department in Michigan, a thorough history revealed a recent trip to a bubonic plague–endemic area. A thorough physical exam demonstrated unilateral painful inguinal adenopathy—which, when paired with the patient’s history—was consistent with the uncommon diagnosis of bubonic plague.
The authors thank Brian Waite, MD, and James Addison, MD, for critically revising this report for important intellectual content.
CORRESPONDENCE
Katherine Lazet, DO, 3838 N First Avenue, Evansville, IN 47710; lazetkat@gmail.com
THE CASE
A 33-year-old Caucasian woman presented to the emergency department with a 6-day history of fever (103°-104°F) and right groin pain and swelling. Associated symptoms included headache, diarrhea, malaise, weakness, nausea, cough, and anorexia. Upon presentation, she admitted to a recent hike on a bubonic plague–endemic trail in Colorado.
Her vital signs were unremarkable, and the physical examination demonstrated normal findings except for tender, erythematous, nonfluctuant right inguinal lymphadenopathy. The patient was admitted for intractable pain and fever and started on intravenous cefoxitin 2 g IV every 8 hours and oral doxycycline 100 mg every 12 hours for pelvic inflammatory disease vs tick- or flea-borne illness. Due to the patient’s recent trip to a plague-infested area, our suspicion for Yersinia pestis infection was high.
The patient’s work-up included a negative pregnancy test and urinalysis. A complete blood count demonstrated a white blood cell count of 8.6 (4.3-10.5) × 103/UL with a 3+ left shift and a platelet count of 112 (180-500) × 103/UL. A complete metabolic panel showed hypokalemia and hyponatremia (potassium 2.8 [3.5-5.1] mmol/L and sodium 134 [137-145] mmol/L). Blood cultures were negative for any bacterial or fungal growth after 48 hours; stool cultures were negative for Salmonella, Shigella, Campylobacter, Giardia, generalized Yersinia, and Escherichia coli O157:H7. Swabs for Gardnerella vaginalis, Trichomonas vaginalis, Candida, Chlamydia trachomatis, and Neisseria gonorrhea also were negative. Lyme, Bartonella henselae, and heterophile antibodies were also negative. Francisella tularensis was not cultured due to low suspicion.
Imaging included a normal chest x-ray and a computed tomography scan of the abdomen and pelvis that showed enlarged right inguinal lymph nodes with fatty stranding, a thicker distal right iliopsoas, hepatosplenomegaly, and an enlarged right adnexa (FIGURE 1). Initial ultrasound of the bubo showed 2 enlarged suprapubic lymph nodes, the largest measuring 3.5 × 1.4 × 2.4 cm3 (FIGURE 2), and 8 enlarged inguinal nodes.
The patient continued to have a low-grade fever, diarrhea, and inguinal lymphadenopathy throughout her first 2 hospitalized days. The cefoxitin was discontinued by Day 3, and the consulting infectious disease physician started oral metronidazole 500 mg every 12 hours due to the patient’s failure to improve. Later that night, the patient experienced increasing erythema and pain in her right inguinal region. A repeat ultrasound showed increased inguinal lymphadenopathy with the largest nodes measuring 2.9 × 1.5 × 2.5 cm3 and 2.7 × 1.3 × 2 cm3 (FIGURE 3).
Although doxycycline is considered an acceptable regimen for Y pestis infection, the infectious disease physician added oral ciprofloxacin 750 mg every 12 hours the following morning, as the patient had not improved.
THE DIAGNOSIS
Although the initial gram stain was negative for Yersinia, clinical suspicion pointed to a diagnosis of bubonic plague. Serology was considered; however, it was not available through the hospital. A definitive diagnosis required bubo aspiration and culture, which was performed but required 48 hours before results would be available.
Continue to: By Day 5, the patient was clinically improved and...
By Day 5, the patient was clinically improved and deemed safe for discharge on empiric treatment with ciprofloxacin 750 mg twice daily and doxycycline 100 mg twice daily to complete a 14-day course of antibiotic therapy for bubonic plague. The bubo culture subsequently grew Y pestis, confirming the diagnosis. The patient made a full recovery and was greatly improved when seen in the outpatient setting by the treating infectious disease physician. Outpatient ultrasound repeated 3 weeks after discharge showed borderline lymphadenopathy, no greater than 1 cm.
DISCUSSION
Between 2000 and 2009, there were 57 cases of Y pestis in the United States; in early 2015, 11 cases were found in 6 Western states.1 The plague presents in the bubonic form 80% to 95% of the time, and it has never been reported in Michigan (where we treated this patient); however, there was a laboratory case in Illinois. Although rats were traditionally the host for Y pestis, the prairie dog, Cynomys gunnisoni, is a host in the United States.2 Rodents are the most important hosts, but more than 200 mammalian species, including domestic pets, have had reported infections. Transmission is primarily via flea bites, but Y pestis also may be transmitted via respiratory secretion, inhalation, or direct handling of contaminated animal tissues. Due to the risk of respiratory spread, the Centers for Disease Control and Prevention must be notified of a diagnosis.3,4
Y pestis travels from the site of the flea bite to regional lymph nodes, where it reproduces, and the resultant inflammatory reaction creates buboes. The bacteria then circulate in the blood to other organs, although Y pestis bacteria are primarily removed by the liver and spleen. Patients often develop symptoms such as headache, fevers, chills, and gastrointestinal distress. Diagnosis is reached by bubo culture or rapid testing for the F1 antigen. Early intervention with antibiotics is crucial as untreated bubonic plague has a mortality rate of 50% to 90%.3,4
The differential diagnosis for unilateral inguinal lymphadenopathy with associated constitutional symptoms was broad, in this case, and included pelvic inflammatory disease, bubonic plague, iliopsoas abscess, lymphogranuloma venereum, bartonellosis, infectious mononucleosis, and tick-borne diseases, such as ehrlichiosis, tularemia, Lyme disease, Rocky Mountain spotted fever, and Colorado tick fever.
Treatment. Food and Drug Administration–approved treatments include streptomycin (gentamicin 5 mg/kg/day IM or IV for 14 days is more widely utilized), doxycycline 200 mg PO once daily for 10 to 14 days, and fluoroquinolones (ciprofloxacin 500-750 mg every 12 hours for 10-14 days). Trimethoprim-sulfamethoxazole may be used as an alternative, but limitations include potentially incomplete or slowed responses.
Continue to: THE TAKEAWAY
THE TAKEAWAY
This case points to the importance of a complete, systematic approach to each patient. While bubonic plague is not a diagnosis that would immediately come to mind in a patient visiting an emergency department in Michigan, a thorough history revealed a recent trip to a bubonic plague–endemic area. A thorough physical exam demonstrated unilateral painful inguinal adenopathy—which, when paired with the patient’s history—was consistent with the uncommon diagnosis of bubonic plague.
The authors thank Brian Waite, MD, and James Addison, MD, for critically revising this report for important intellectual content.
CORRESPONDENCE
Katherine Lazet, DO, 3838 N First Avenue, Evansville, IN 47710; lazetkat@gmail.com
1. Kwit N, Nelson C, Kugeler K, et al. Human Plague – United States, 2015. MMWR Morb Mortal Wkly Rep. 2015,64:918-919.
2. Friggens MM, Parmenter RR, Boyden M, et al. Flea abundance, diversity, and plague in Gunnison’s prairie dog (Cynomys gunnisoni) and their burrows in Montane grasslands in northern New Mexico. J Wildl Dis. 2010;46:356-367.
3. Mandell G, Bennett J, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:2943-2953.
4. Perry RD, Featherston JD. Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev. 1997;10:35-66.
1. Kwit N, Nelson C, Kugeler K, et al. Human Plague – United States, 2015. MMWR Morb Mortal Wkly Rep. 2015,64:918-919.
2. Friggens MM, Parmenter RR, Boyden M, et al. Flea abundance, diversity, and plague in Gunnison’s prairie dog (Cynomys gunnisoni) and their burrows in Montane grasslands in northern New Mexico. J Wildl Dis. 2010;46:356-367.
3. Mandell G, Bennett J, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone Elsevier; 2010:2943-2953.
4. Perry RD, Featherston JD. Yersinia pestis - etiologic agent of plague. Clin Microbiol Rev. 1997;10:35-66.