User login
Poppy Seed Brew Triggers Morphine Overdose, Drawing Attention of Lawmakers
It sounds like a joke: poppy seeds infused with opioids.
Indeed, it was a plotline on the sitcom Seinfeld. But for some it has been a tragedy.
People have died after drinking tea brewed from unwashed poppy seeds.
And after eating lemon poppy seed bread or an everything bagel, mothers reportedly have been separated from newborns because the women failed drug tests.
Poppy seeds come from the plant that produces opium and from which narcotics such as morphine and codeine are derived.
Members of the House and Senate have proposed legislation “to prohibit the distribution and sale of contaminated poppy seeds in order to prevent harm, addiction, and further deaths from morphine-contaminated poppy seeds.” The bill was one of several on the agenda for a September 10 House hearing.
The day before the hearing, The Marshall Project and Reveal reported on a woman who ate a salad with poppy seed dressing before giving birth, tested positive at the hospital for opiates, was reported to child welfare, and saw her baby taken into protective custody. Almost 2 weeks passed before she was allowed to bring her baby home.
“It’s not an urban legend: Eating poppy seeds can cause diners to test positive for codeine on a urinalysis,” the Defense Department warned military personnel in 2023.
The US Anti-Doping Agency long ago issued a similar warning to athletes.
The Center for Science in the Public Interest, a watchdog group, petitioned the Food and Drug Administration (FDA) in 2021 to limit the opiate content of poppy seeds. In May, after more than three years with no response, it sued the agency to force action.
“So far the FDA has been negligent in protecting consumers,” said Steve Hacala, whose son died after consuming poppy seed tea and who has joined forces with CSPI.
The lawsuit was put on hold in July, after the FDA said it would respond to the group’s petition by the end of February 2025.
The FDA did not answer questions for this article. The agency generally does not comment on litigation, spokesperson Courtney Rhodes said.
A 2021 study coauthored by CSPI personnel found more than 100 reports to poison control centers between 2000 and 2018 resulting from intentional abuse or misuse of poppy seeds, said CSPI scientist Eva Greenthal, one of the study’s authors.
Only rarely would baked goods or other food items containing washed poppy seeds trigger positive drug tests, doctors who have studied the issue said.
It’s “exquisitely doubtful” that the “relatively trivial” amount of morphine in an everything bagel or the like would cause anyone harm, said Irving Haber, a doctor who has written about poppy seeds, specializes in pain medicine, and signed the CSPI petition to the FDA.
On the other hand, tea made from large quantities of unwashed poppy seeds could lead to addiction and overdose, doctors said. The risks are heightened if the person drinking the brew is also consuming other opioids, such as prescription pain relievers.
Benjamin Lai, a physician who chairs a program on opioids at the Mayo Clinic in Rochester, Minnesota, said he has been treating a patient who developed long-term opioid addiction from consuming poppy seed tea. The patient, a man in his 30s, found it at a health food store and was under the impression it would help him relax and recover from gym workouts. After a few months, he tried to stop and experienced withdrawal symptoms, Lai said.
Another patient, an older woman, developed withdrawal symptoms under similar circumstances but responded well to treatment, Lai said.
Some websites tout poppy seed tea as offering health benefits. And some sellers “may use specific language such as ‘raw,’ ‘unprocessed,’ or ‘unwashed’ to signal that their products contain higher concentrations of opiates than properly processed seeds,” the CSPI lawsuit said.
Steve Hacala’s son, Stephen Hacala, a music teacher, had been experiencing anxiety and insomnia, for which poppy seed tea is promoted as a natural remedy, the lawsuit said. In 2016, at age 24, he ordered a bag of poppy seeds online, rinsed them with water, and consumed the rinse. He died of morphine poisoning.
The only source of morphine found in Stephen’s home, where he died, was commercially available poppy seeds, a medical examiner at the Arkansas State Crime Lab said in a letter to the father. The medical examiner wrote that poppy seeds “very likely” caused Stephen’s death.
Steve Hacala estimated that the quantity of poppy seeds found in a 1-liter plastic water bottle in his son’s home could have delivered more than 10 times a lethal dose.
Steve Hacala and his wife, Betty, have funded CSPI’s efforts to call attention to the issue. (The publisher of KFF Health News, David Rousseau, is on the CSPI board.)
The lawsuit also cited mothers who, like those in the investigation by The Marshall Project and Reveal, ran afoul of rules meant to protect newborns. For example, though Jamie Silakowski had not used opioids while pregnant, she was initially prevented from leaving the hospital with her baby, the suit said.
Silakowski recalled that, before going to the hospital, she had eaten lemon poppy seed bread at Tim Hortons, a fast-food chain, CSPI said in its petition. “No one in the hospital believed Ms. Silakowski or appeared to be aware that the test results could occur from poppy seeds.”
People from child protective services made unannounced visits to her home, interviewed her other children, and questioned teachers at their school, she said in an interview.
While on maternity leave, she had to undergo drug testing, Silakowski said. “Peeing in front of someone like I’m a criminal — it was just mortifying.”
Even family members were questioning her, and there was nothing she could do to dispel doubts, she said. “Relationships were torn apart,” she said.
The parent company of Tim Hortons, Restaurant Brands International, which also owns Burger King and Popeyes, did not respond to questions from KFF Health News.
In July, The Washington Post reported that Trader Joe’s Everything but the Bagel seasoning was banned and being confiscated in South Korea because it contains poppy seeds. Trader Joe’s did not respond to inquiries for this article. The seasoning is listed for sale on the company’s website.
The US Drug Enforcement Agency says unwashed poppy seeds can kill when used alone or in combination with other drugs. While poppy seeds are exempt from drug control under the Controlled Substances Act, opium contaminants on the seeds are not, the agency says. The Justice Department has brought criminal prosecutions over the sale of unwashed poppy seeds.
Meanwhile, the legislation to control poppy seed contamination has not gained much traction.
The Senate bill, introduced by Sen. Tom Cotton (R-Ark.), has two cosponsors.
The House bill, introduced by Rep. Steve Womack (R-Ark.), has none. Though it was on the agenda, it didn’t come up at the recent hearing.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
It sounds like a joke: poppy seeds infused with opioids.
Indeed, it was a plotline on the sitcom Seinfeld. But for some it has been a tragedy.
People have died after drinking tea brewed from unwashed poppy seeds.
And after eating lemon poppy seed bread or an everything bagel, mothers reportedly have been separated from newborns because the women failed drug tests.
Poppy seeds come from the plant that produces opium and from which narcotics such as morphine and codeine are derived.
Members of the House and Senate have proposed legislation “to prohibit the distribution and sale of contaminated poppy seeds in order to prevent harm, addiction, and further deaths from morphine-contaminated poppy seeds.” The bill was one of several on the agenda for a September 10 House hearing.
The day before the hearing, The Marshall Project and Reveal reported on a woman who ate a salad with poppy seed dressing before giving birth, tested positive at the hospital for opiates, was reported to child welfare, and saw her baby taken into protective custody. Almost 2 weeks passed before she was allowed to bring her baby home.
“It’s not an urban legend: Eating poppy seeds can cause diners to test positive for codeine on a urinalysis,” the Defense Department warned military personnel in 2023.
The US Anti-Doping Agency long ago issued a similar warning to athletes.
The Center for Science in the Public Interest, a watchdog group, petitioned the Food and Drug Administration (FDA) in 2021 to limit the opiate content of poppy seeds. In May, after more than three years with no response, it sued the agency to force action.
“So far the FDA has been negligent in protecting consumers,” said Steve Hacala, whose son died after consuming poppy seed tea and who has joined forces with CSPI.
The lawsuit was put on hold in July, after the FDA said it would respond to the group’s petition by the end of February 2025.
The FDA did not answer questions for this article. The agency generally does not comment on litigation, spokesperson Courtney Rhodes said.
A 2021 study coauthored by CSPI personnel found more than 100 reports to poison control centers between 2000 and 2018 resulting from intentional abuse or misuse of poppy seeds, said CSPI scientist Eva Greenthal, one of the study’s authors.
Only rarely would baked goods or other food items containing washed poppy seeds trigger positive drug tests, doctors who have studied the issue said.
It’s “exquisitely doubtful” that the “relatively trivial” amount of morphine in an everything bagel or the like would cause anyone harm, said Irving Haber, a doctor who has written about poppy seeds, specializes in pain medicine, and signed the CSPI petition to the FDA.
On the other hand, tea made from large quantities of unwashed poppy seeds could lead to addiction and overdose, doctors said. The risks are heightened if the person drinking the brew is also consuming other opioids, such as prescription pain relievers.
Benjamin Lai, a physician who chairs a program on opioids at the Mayo Clinic in Rochester, Minnesota, said he has been treating a patient who developed long-term opioid addiction from consuming poppy seed tea. The patient, a man in his 30s, found it at a health food store and was under the impression it would help him relax and recover from gym workouts. After a few months, he tried to stop and experienced withdrawal symptoms, Lai said.
Another patient, an older woman, developed withdrawal symptoms under similar circumstances but responded well to treatment, Lai said.
Some websites tout poppy seed tea as offering health benefits. And some sellers “may use specific language such as ‘raw,’ ‘unprocessed,’ or ‘unwashed’ to signal that their products contain higher concentrations of opiates than properly processed seeds,” the CSPI lawsuit said.
Steve Hacala’s son, Stephen Hacala, a music teacher, had been experiencing anxiety and insomnia, for which poppy seed tea is promoted as a natural remedy, the lawsuit said. In 2016, at age 24, he ordered a bag of poppy seeds online, rinsed them with water, and consumed the rinse. He died of morphine poisoning.
The only source of morphine found in Stephen’s home, where he died, was commercially available poppy seeds, a medical examiner at the Arkansas State Crime Lab said in a letter to the father. The medical examiner wrote that poppy seeds “very likely” caused Stephen’s death.
Steve Hacala estimated that the quantity of poppy seeds found in a 1-liter plastic water bottle in his son’s home could have delivered more than 10 times a lethal dose.
Steve Hacala and his wife, Betty, have funded CSPI’s efforts to call attention to the issue. (The publisher of KFF Health News, David Rousseau, is on the CSPI board.)
The lawsuit also cited mothers who, like those in the investigation by The Marshall Project and Reveal, ran afoul of rules meant to protect newborns. For example, though Jamie Silakowski had not used opioids while pregnant, she was initially prevented from leaving the hospital with her baby, the suit said.
Silakowski recalled that, before going to the hospital, she had eaten lemon poppy seed bread at Tim Hortons, a fast-food chain, CSPI said in its petition. “No one in the hospital believed Ms. Silakowski or appeared to be aware that the test results could occur from poppy seeds.”
People from child protective services made unannounced visits to her home, interviewed her other children, and questioned teachers at their school, she said in an interview.
While on maternity leave, she had to undergo drug testing, Silakowski said. “Peeing in front of someone like I’m a criminal — it was just mortifying.”
Even family members were questioning her, and there was nothing she could do to dispel doubts, she said. “Relationships were torn apart,” she said.
The parent company of Tim Hortons, Restaurant Brands International, which also owns Burger King and Popeyes, did not respond to questions from KFF Health News.
In July, The Washington Post reported that Trader Joe’s Everything but the Bagel seasoning was banned and being confiscated in South Korea because it contains poppy seeds. Trader Joe’s did not respond to inquiries for this article. The seasoning is listed for sale on the company’s website.
The US Drug Enforcement Agency says unwashed poppy seeds can kill when used alone or in combination with other drugs. While poppy seeds are exempt from drug control under the Controlled Substances Act, opium contaminants on the seeds are not, the agency says. The Justice Department has brought criminal prosecutions over the sale of unwashed poppy seeds.
Meanwhile, the legislation to control poppy seed contamination has not gained much traction.
The Senate bill, introduced by Sen. Tom Cotton (R-Ark.), has two cosponsors.
The House bill, introduced by Rep. Steve Womack (R-Ark.), has none. Though it was on the agenda, it didn’t come up at the recent hearing.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
It sounds like a joke: poppy seeds infused with opioids.
Indeed, it was a plotline on the sitcom Seinfeld. But for some it has been a tragedy.
People have died after drinking tea brewed from unwashed poppy seeds.
And after eating lemon poppy seed bread or an everything bagel, mothers reportedly have been separated from newborns because the women failed drug tests.
Poppy seeds come from the plant that produces opium and from which narcotics such as morphine and codeine are derived.
Members of the House and Senate have proposed legislation “to prohibit the distribution and sale of contaminated poppy seeds in order to prevent harm, addiction, and further deaths from morphine-contaminated poppy seeds.” The bill was one of several on the agenda for a September 10 House hearing.
The day before the hearing, The Marshall Project and Reveal reported on a woman who ate a salad with poppy seed dressing before giving birth, tested positive at the hospital for opiates, was reported to child welfare, and saw her baby taken into protective custody. Almost 2 weeks passed before she was allowed to bring her baby home.
“It’s not an urban legend: Eating poppy seeds can cause diners to test positive for codeine on a urinalysis,” the Defense Department warned military personnel in 2023.
The US Anti-Doping Agency long ago issued a similar warning to athletes.
The Center for Science in the Public Interest, a watchdog group, petitioned the Food and Drug Administration (FDA) in 2021 to limit the opiate content of poppy seeds. In May, after more than three years with no response, it sued the agency to force action.
“So far the FDA has been negligent in protecting consumers,” said Steve Hacala, whose son died after consuming poppy seed tea and who has joined forces with CSPI.
The lawsuit was put on hold in July, after the FDA said it would respond to the group’s petition by the end of February 2025.
The FDA did not answer questions for this article. The agency generally does not comment on litigation, spokesperson Courtney Rhodes said.
A 2021 study coauthored by CSPI personnel found more than 100 reports to poison control centers between 2000 and 2018 resulting from intentional abuse or misuse of poppy seeds, said CSPI scientist Eva Greenthal, one of the study’s authors.
Only rarely would baked goods or other food items containing washed poppy seeds trigger positive drug tests, doctors who have studied the issue said.
It’s “exquisitely doubtful” that the “relatively trivial” amount of morphine in an everything bagel or the like would cause anyone harm, said Irving Haber, a doctor who has written about poppy seeds, specializes in pain medicine, and signed the CSPI petition to the FDA.
On the other hand, tea made from large quantities of unwashed poppy seeds could lead to addiction and overdose, doctors said. The risks are heightened if the person drinking the brew is also consuming other opioids, such as prescription pain relievers.
Benjamin Lai, a physician who chairs a program on opioids at the Mayo Clinic in Rochester, Minnesota, said he has been treating a patient who developed long-term opioid addiction from consuming poppy seed tea. The patient, a man in his 30s, found it at a health food store and was under the impression it would help him relax and recover from gym workouts. After a few months, he tried to stop and experienced withdrawal symptoms, Lai said.
Another patient, an older woman, developed withdrawal symptoms under similar circumstances but responded well to treatment, Lai said.
Some websites tout poppy seed tea as offering health benefits. And some sellers “may use specific language such as ‘raw,’ ‘unprocessed,’ or ‘unwashed’ to signal that their products contain higher concentrations of opiates than properly processed seeds,” the CSPI lawsuit said.
Steve Hacala’s son, Stephen Hacala, a music teacher, had been experiencing anxiety and insomnia, for which poppy seed tea is promoted as a natural remedy, the lawsuit said. In 2016, at age 24, he ordered a bag of poppy seeds online, rinsed them with water, and consumed the rinse. He died of morphine poisoning.
The only source of morphine found in Stephen’s home, where he died, was commercially available poppy seeds, a medical examiner at the Arkansas State Crime Lab said in a letter to the father. The medical examiner wrote that poppy seeds “very likely” caused Stephen’s death.
Steve Hacala estimated that the quantity of poppy seeds found in a 1-liter plastic water bottle in his son’s home could have delivered more than 10 times a lethal dose.
Steve Hacala and his wife, Betty, have funded CSPI’s efforts to call attention to the issue. (The publisher of KFF Health News, David Rousseau, is on the CSPI board.)
The lawsuit also cited mothers who, like those in the investigation by The Marshall Project and Reveal, ran afoul of rules meant to protect newborns. For example, though Jamie Silakowski had not used opioids while pregnant, she was initially prevented from leaving the hospital with her baby, the suit said.
Silakowski recalled that, before going to the hospital, she had eaten lemon poppy seed bread at Tim Hortons, a fast-food chain, CSPI said in its petition. “No one in the hospital believed Ms. Silakowski or appeared to be aware that the test results could occur from poppy seeds.”
People from child protective services made unannounced visits to her home, interviewed her other children, and questioned teachers at their school, she said in an interview.
While on maternity leave, she had to undergo drug testing, Silakowski said. “Peeing in front of someone like I’m a criminal — it was just mortifying.”
Even family members were questioning her, and there was nothing she could do to dispel doubts, she said. “Relationships were torn apart,” she said.
The parent company of Tim Hortons, Restaurant Brands International, which also owns Burger King and Popeyes, did not respond to questions from KFF Health News.
In July, The Washington Post reported that Trader Joe’s Everything but the Bagel seasoning was banned and being confiscated in South Korea because it contains poppy seeds. Trader Joe’s did not respond to inquiries for this article. The seasoning is listed for sale on the company’s website.
The US Drug Enforcement Agency says unwashed poppy seeds can kill when used alone or in combination with other drugs. While poppy seeds are exempt from drug control under the Controlled Substances Act, opium contaminants on the seeds are not, the agency says. The Justice Department has brought criminal prosecutions over the sale of unwashed poppy seeds.
Meanwhile, the legislation to control poppy seed contamination has not gained much traction.
The Senate bill, introduced by Sen. Tom Cotton (R-Ark.), has two cosponsors.
The House bill, introduced by Rep. Steve Womack (R-Ark.), has none. Though it was on the agenda, it didn’t come up at the recent hearing.
KFF Health News is a national newsroom that produces in-depth journalism about health issues and is one of the core operating programs at KFF — the independent source for health policy research, polling, and journalism.
True Benefit of Screening Colonoscopy for CRC Underestimated in NordICC
TOPLINE:
a new analysis found.
METHODOLOGY:
- The NordICC trial randomly assigned 85,179 adults aged 55-64 years in a 1:2 ratio to receive or not receive an invitation for a single screening colonoscopy and determined the risk for CRC diagnosis and death over 10-15 years of follow-up using cancer registries. After randomization, the trial excluded 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization.
- The trial found that CRC risk and associated mortality were lower in adults who had colonoscopy, though only modestly so, which generated considerable controversy.
- Because registration delays are a known concern with population-based cancer registries but the trial did not account for them, researchers on the current study postulated that delays might have led to an underestimation of the impact of colonoscopy on CRC risk.
- They estimated the magnitude of delayed reporting of CRC diagnosis to cancer registries by comparing the 221 exclusions with expected CRC diagnoses per year. They explored the impact that delays may have had on the results of the trial’s intention-to-screen analysis and adjusted per-protocol analysis.
TAKEAWAY:
- The trial’s post hoc exclusion of 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization suggests delays of 2-3 years in registration.
- With no assumed delay in cancer registration, the 10-year reported CRC risk difference was 0.22% in intention-to-screen and 0.38% in adjusted per-protocol analyses. With a mean delay in cancer registration of 2 years, the risk difference rose to 0.44% in the intention-to-screen analysis and 0.76% in the adjusted per-protocol analysis.
- Assuming no delay in cancer registration, the number needed to invite for screening colonoscopy and number needed to undergo the procedure to prevent 1 CRC diagnosis/death were 455 and 263, respectively. These numbers decreased to 227 and 132, respectively, with a 2-year reporting delay.
- Registration delays of 1, 2, or 3 years led to an underestimated risk for CRC by 25%, 50%, and 75%, respectively.
IN PRACTICE:
“Updated analyses ensuring complete 10- and 15-year follow-up will be crucial to derive the true reductions of CRC risk and mortality in the trial’s predefined interim and primary analysis. In the meantime, available estimates are to be interpreted with caution, as they likely severely underestimate true screening colonoscopy effects,” the authors concluded.
The lag in reporting found by the study raises questions about the time interval needed beyond the end of a study to assure its completeness, which varies across registries, wrote Chyke A. Doubeni, MD, MPH, Ohio State University Wexner Medical Center, Columbus, and colleagues in an invited commentary. “Publication guidelines should be strengthened to ensure affirmation of completeness and quality of cancer registries used for outcomes ascertainment to minimize uncertainties.”
SOURCE:
The study, with first author Hermann Brenner, MD, MPH, German Cancer Research Center, Heidelberg, was published online in JAMA Network Open, as was the invited commentary.
LIMITATIONS:
Exact quantification of and correction for registration delays were not possible.
DISCLOSURES:
The study was partially funded by the German Federal Ministry of Education and Research and German Cancer Aid. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a new analysis found.
METHODOLOGY:
- The NordICC trial randomly assigned 85,179 adults aged 55-64 years in a 1:2 ratio to receive or not receive an invitation for a single screening colonoscopy and determined the risk for CRC diagnosis and death over 10-15 years of follow-up using cancer registries. After randomization, the trial excluded 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization.
- The trial found that CRC risk and associated mortality were lower in adults who had colonoscopy, though only modestly so, which generated considerable controversy.
- Because registration delays are a known concern with population-based cancer registries but the trial did not account for them, researchers on the current study postulated that delays might have led to an underestimation of the impact of colonoscopy on CRC risk.
- They estimated the magnitude of delayed reporting of CRC diagnosis to cancer registries by comparing the 221 exclusions with expected CRC diagnoses per year. They explored the impact that delays may have had on the results of the trial’s intention-to-screen analysis and adjusted per-protocol analysis.
TAKEAWAY:
- The trial’s post hoc exclusion of 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization suggests delays of 2-3 years in registration.
- With no assumed delay in cancer registration, the 10-year reported CRC risk difference was 0.22% in intention-to-screen and 0.38% in adjusted per-protocol analyses. With a mean delay in cancer registration of 2 years, the risk difference rose to 0.44% in the intention-to-screen analysis and 0.76% in the adjusted per-protocol analysis.
- Assuming no delay in cancer registration, the number needed to invite for screening colonoscopy and number needed to undergo the procedure to prevent 1 CRC diagnosis/death were 455 and 263, respectively. These numbers decreased to 227 and 132, respectively, with a 2-year reporting delay.
- Registration delays of 1, 2, or 3 years led to an underestimated risk for CRC by 25%, 50%, and 75%, respectively.
IN PRACTICE:
“Updated analyses ensuring complete 10- and 15-year follow-up will be crucial to derive the true reductions of CRC risk and mortality in the trial’s predefined interim and primary analysis. In the meantime, available estimates are to be interpreted with caution, as they likely severely underestimate true screening colonoscopy effects,” the authors concluded.
The lag in reporting found by the study raises questions about the time interval needed beyond the end of a study to assure its completeness, which varies across registries, wrote Chyke A. Doubeni, MD, MPH, Ohio State University Wexner Medical Center, Columbus, and colleagues in an invited commentary. “Publication guidelines should be strengthened to ensure affirmation of completeness and quality of cancer registries used for outcomes ascertainment to minimize uncertainties.”
SOURCE:
The study, with first author Hermann Brenner, MD, MPH, German Cancer Research Center, Heidelberg, was published online in JAMA Network Open, as was the invited commentary.
LIMITATIONS:
Exact quantification of and correction for registration delays were not possible.
DISCLOSURES:
The study was partially funded by the German Federal Ministry of Education and Research and German Cancer Aid. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a new analysis found.
METHODOLOGY:
- The NordICC trial randomly assigned 85,179 adults aged 55-64 years in a 1:2 ratio to receive or not receive an invitation for a single screening colonoscopy and determined the risk for CRC diagnosis and death over 10-15 years of follow-up using cancer registries. After randomization, the trial excluded 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization.
- The trial found that CRC risk and associated mortality were lower in adults who had colonoscopy, though only modestly so, which generated considerable controversy.
- Because registration delays are a known concern with population-based cancer registries but the trial did not account for them, researchers on the current study postulated that delays might have led to an underestimation of the impact of colonoscopy on CRC risk.
- They estimated the magnitude of delayed reporting of CRC diagnosis to cancer registries by comparing the 221 exclusions with expected CRC diagnoses per year. They explored the impact that delays may have had on the results of the trial’s intention-to-screen analysis and adjusted per-protocol analysis.
TAKEAWAY:
- The trial’s post hoc exclusion of 221 adults who had CRC at baseline but who did not yet appear in a cancer registry at the time of randomization suggests delays of 2-3 years in registration.
- With no assumed delay in cancer registration, the 10-year reported CRC risk difference was 0.22% in intention-to-screen and 0.38% in adjusted per-protocol analyses. With a mean delay in cancer registration of 2 years, the risk difference rose to 0.44% in the intention-to-screen analysis and 0.76% in the adjusted per-protocol analysis.
- Assuming no delay in cancer registration, the number needed to invite for screening colonoscopy and number needed to undergo the procedure to prevent 1 CRC diagnosis/death were 455 and 263, respectively. These numbers decreased to 227 and 132, respectively, with a 2-year reporting delay.
- Registration delays of 1, 2, or 3 years led to an underestimated risk for CRC by 25%, 50%, and 75%, respectively.
IN PRACTICE:
“Updated analyses ensuring complete 10- and 15-year follow-up will be crucial to derive the true reductions of CRC risk and mortality in the trial’s predefined interim and primary analysis. In the meantime, available estimates are to be interpreted with caution, as they likely severely underestimate true screening colonoscopy effects,” the authors concluded.
The lag in reporting found by the study raises questions about the time interval needed beyond the end of a study to assure its completeness, which varies across registries, wrote Chyke A. Doubeni, MD, MPH, Ohio State University Wexner Medical Center, Columbus, and colleagues in an invited commentary. “Publication guidelines should be strengthened to ensure affirmation of completeness and quality of cancer registries used for outcomes ascertainment to minimize uncertainties.”
SOURCE:
The study, with first author Hermann Brenner, MD, MPH, German Cancer Research Center, Heidelberg, was published online in JAMA Network Open, as was the invited commentary.
LIMITATIONS:
Exact quantification of and correction for registration delays were not possible.
DISCLOSURES:
The study was partially funded by the German Federal Ministry of Education and Research and German Cancer Aid. The authors reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Eggs: A Weighty Matter for Postmenopausal Women?
TOPLINE:
such as processed and red meat, French fries, sweets, and deserts. Genetic predisposition for a high body mass index (BMI) also influences weight gain with higher egg intake.
METHODOLOGY:
- Egg consumption and elevated body weight are each linked to an increased risk for serious chronic diseases; however, whether elevated body weight mediates the association between egg intake and an elevated risk for serious chronic diseases needs further assessment.
- To investigate the association between eating eggs and weight gain, as well as the role of genetic susceptibility to an elevated BMI, researchers conducted a prospective study including 4439 postmenopausal women of European descent from the Women’s Health Initiative (WHI).
- They measured the participants’ consumption of egg and egg nutrients using a self-administered food frequency questionnaire.
- Changes in the consumption of eggs and egg nutrients such as cholesterol, choline, and betaine were assessed between baseline and follow-up visits at 1, 3, 6, and 9 years.
- The primary outcome was the change in body weight between baseline and each follow-up visit. Furthermore, an exploratory analysis evaluated how eating Western foods and genetic predisposition for a high BMI (assessed through a polygenic score) influenced weight change.
TAKEAWAY:
- An increased consumption of eggs was associated with weight gain, showing a positive linear trend at 1, 2, 3, and 6 years. By the third year, women whose egg consumption had increased by two eggs per week gained 0.70 kg more weight (P = .0002) than women whose egg consumption was reduced by 2.4 eggs per week (P-linear < .0001).
- An increase in the consumption of nutrients obtained from eggs, including cholesterol (P-linear < .0001) and choline (P-linear < .02), was positively associated with weight gain.
- Women with a higher consumption of Western foods showed significant associations between changes in egg, cholesterol, and choline intake and weight gain.
- A significant association was found between the BMI polygenic score and changes in body weight, with women most genetically predisposed to a higher BMI showing greater weight gain when their egg consumption increased by an average of 3.5 eggs per week.
IN PRACTICE:
“These results suggest that even relatively small increases or decreases in egg consumption could cause increases or decreases, respectively, in body weight among postmenopausal women, unless there are adequate compensating changes in factors such as dietary energy intake or physical activity,” the authors wrote. “Our results require confirmation,” they added.
SOURCE:
This study, led by James A. Greenberg, Department of Health and Nutrition Sciences, Brooklyn College, The City University of New York, was published online in Clinical Nutrition.
LIMITATIONS:
This observational study was susceptible to residual confounding, which suggests that the observed associations may not have established causality. Additionally, the results were according to data from a group of postmenopausal American women of European descent, which limited the generalizability to other populations.
DISCLOSURES:
The WHI program was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health & Human Services. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
such as processed and red meat, French fries, sweets, and deserts. Genetic predisposition for a high body mass index (BMI) also influences weight gain with higher egg intake.
METHODOLOGY:
- Egg consumption and elevated body weight are each linked to an increased risk for serious chronic diseases; however, whether elevated body weight mediates the association between egg intake and an elevated risk for serious chronic diseases needs further assessment.
- To investigate the association between eating eggs and weight gain, as well as the role of genetic susceptibility to an elevated BMI, researchers conducted a prospective study including 4439 postmenopausal women of European descent from the Women’s Health Initiative (WHI).
- They measured the participants’ consumption of egg and egg nutrients using a self-administered food frequency questionnaire.
- Changes in the consumption of eggs and egg nutrients such as cholesterol, choline, and betaine were assessed between baseline and follow-up visits at 1, 3, 6, and 9 years.
- The primary outcome was the change in body weight between baseline and each follow-up visit. Furthermore, an exploratory analysis evaluated how eating Western foods and genetic predisposition for a high BMI (assessed through a polygenic score) influenced weight change.
TAKEAWAY:
- An increased consumption of eggs was associated with weight gain, showing a positive linear trend at 1, 2, 3, and 6 years. By the third year, women whose egg consumption had increased by two eggs per week gained 0.70 kg more weight (P = .0002) than women whose egg consumption was reduced by 2.4 eggs per week (P-linear < .0001).
- An increase in the consumption of nutrients obtained from eggs, including cholesterol (P-linear < .0001) and choline (P-linear < .02), was positively associated with weight gain.
- Women with a higher consumption of Western foods showed significant associations between changes in egg, cholesterol, and choline intake and weight gain.
- A significant association was found between the BMI polygenic score and changes in body weight, with women most genetically predisposed to a higher BMI showing greater weight gain when their egg consumption increased by an average of 3.5 eggs per week.
IN PRACTICE:
“These results suggest that even relatively small increases or decreases in egg consumption could cause increases or decreases, respectively, in body weight among postmenopausal women, unless there are adequate compensating changes in factors such as dietary energy intake or physical activity,” the authors wrote. “Our results require confirmation,” they added.
SOURCE:
This study, led by James A. Greenberg, Department of Health and Nutrition Sciences, Brooklyn College, The City University of New York, was published online in Clinical Nutrition.
LIMITATIONS:
This observational study was susceptible to residual confounding, which suggests that the observed associations may not have established causality. Additionally, the results were according to data from a group of postmenopausal American women of European descent, which limited the generalizability to other populations.
DISCLOSURES:
The WHI program was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health & Human Services. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
such as processed and red meat, French fries, sweets, and deserts. Genetic predisposition for a high body mass index (BMI) also influences weight gain with higher egg intake.
METHODOLOGY:
- Egg consumption and elevated body weight are each linked to an increased risk for serious chronic diseases; however, whether elevated body weight mediates the association between egg intake and an elevated risk for serious chronic diseases needs further assessment.
- To investigate the association between eating eggs and weight gain, as well as the role of genetic susceptibility to an elevated BMI, researchers conducted a prospective study including 4439 postmenopausal women of European descent from the Women’s Health Initiative (WHI).
- They measured the participants’ consumption of egg and egg nutrients using a self-administered food frequency questionnaire.
- Changes in the consumption of eggs and egg nutrients such as cholesterol, choline, and betaine were assessed between baseline and follow-up visits at 1, 3, 6, and 9 years.
- The primary outcome was the change in body weight between baseline and each follow-up visit. Furthermore, an exploratory analysis evaluated how eating Western foods and genetic predisposition for a high BMI (assessed through a polygenic score) influenced weight change.
TAKEAWAY:
- An increased consumption of eggs was associated with weight gain, showing a positive linear trend at 1, 2, 3, and 6 years. By the third year, women whose egg consumption had increased by two eggs per week gained 0.70 kg more weight (P = .0002) than women whose egg consumption was reduced by 2.4 eggs per week (P-linear < .0001).
- An increase in the consumption of nutrients obtained from eggs, including cholesterol (P-linear < .0001) and choline (P-linear < .02), was positively associated with weight gain.
- Women with a higher consumption of Western foods showed significant associations between changes in egg, cholesterol, and choline intake and weight gain.
- A significant association was found between the BMI polygenic score and changes in body weight, with women most genetically predisposed to a higher BMI showing greater weight gain when their egg consumption increased by an average of 3.5 eggs per week.
IN PRACTICE:
“These results suggest that even relatively small increases or decreases in egg consumption could cause increases or decreases, respectively, in body weight among postmenopausal women, unless there are adequate compensating changes in factors such as dietary energy intake or physical activity,” the authors wrote. “Our results require confirmation,” they added.
SOURCE:
This study, led by James A. Greenberg, Department of Health and Nutrition Sciences, Brooklyn College, The City University of New York, was published online in Clinical Nutrition.
LIMITATIONS:
This observational study was susceptible to residual confounding, which suggests that the observed associations may not have established causality. Additionally, the results were according to data from a group of postmenopausal American women of European descent, which limited the generalizability to other populations.
DISCLOSURES:
The WHI program was funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health & Human Services. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Study Evaluates Safety of Benzoyl Peroxide Products for Acne
according to results from an analysis that used gas chromatography–mass spectrometry and other methods.
The analysis, which was published in the Journal of Investigative Dermatology and expands on a similar study released more than 6 months ago, also found that encapsulated BPO products break down into benzene at room temperature but that refrigerating them may mitigate this effect.
“Our research provides the first experimental evidence that cold storage can help reduce the rate of benzoyl peroxide breakdown into benzene,” said one of the study authors, Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Connecticut. “Therefore, cold storage throughout the entire supply chain — from manufacturing to patient use — is a reasonable and proportional measure at this time for those continuing to use benzoyl peroxide medicine.” One acne product, the newer prescription triple-combination therapy (adapalene-clindamycin-BPO) “already has a cold shipping process in place; the patient just needs to continue that at home,” he noted.
For the study — which was funded by an independent lab, Valisure — researchers led by Valisure CEO and founder David Light, used gas chromatography-mass spectrometry to detect benzene levels in 111 BPO drug products from major US retailers and selected ion flow tube mass-spectrometry to quantify the release of benzene in real time. Benzene levels ranged from 0.16 ppm to 35.30 ppm, and 38 of the products (34%) had levels above the FDA limit of 2 ppm for drug products. “The results of the products sampled in this study suggest that formulation is likely the strongest contributor to benzene concentrations in BPO drug products that are commercially available, since the magnitude of benzene detected correlates most closely with specific brands or product types within certain brands,” the study authors wrote.
When the researchers tested the stability of a prescription encapsulated BPO drug product at cold (2 °C) and elevated temperature (50 °C), no apparent benzene formation was observed at 2 °C, whereas high levels of benzene formed at 50 °C, “suggesting that encapsulation technology may not stabilize BPO drug products, but cold storage may greatly reduce benzene formation,” they wrote.
In another component of the study, researchers exposed a BP drug product to a UVA/UVB lamp for 2 hours and found detectable benzene through evaporation and substantial benzene formation when exposed to UV light at levels below peak sunlight. The experiment “strongly justifies the package label warnings to avoid sun exposure when using BPO drug products,” the authors wrote. “Further evaluation to determine the influence of sun exposure on BPO drug product degradation and benzene formation is warranted.”
In an interview, John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, Massachusetts characterized the findings as “an important issue that we should take seriously.” However, “we also must not overreact.”
BPO is a foundational acne treatment without any clear alternative, he said, pointing out that no evidence currently exists “to support that routine use of benzoyl peroxide–containing products for acne is associated with a meaningful risk of benzene in the blood or an increased risk of cancer.”
And although it is prudent to minimize benzene exposure as much as possible, Barbieri continued, “it is not clear that these levels are a clinically meaningful incremental risk in the setting of an acne cream or wash. There is minimal cutaneous absorption of benzene, and it is uncertain how much benzene aerosolizes with routine use, particularly for washes which are not left on the skin.”
Bunick said that the combined data from this and the study published in March 2024 affected which BPO products he recommends for patients with acne. “I am using exclusively the triple combination therapy (adapalene-clindamycin-benzoyl peroxide) because I know it has the necessary cold supply chain in place to protect the product’s stability. I further encourage patients to place all their benzoyl peroxide–containing products in the refrigerator at home to reduce benzene formation and exposure.”
Bunick reported having served as an investigator and/or a consultant/speaker for many pharmaceutical companies, including as a consultant for Ortho-Dermatologics; but none related to this study. Barbieri reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to results from an analysis that used gas chromatography–mass spectrometry and other methods.
The analysis, which was published in the Journal of Investigative Dermatology and expands on a similar study released more than 6 months ago, also found that encapsulated BPO products break down into benzene at room temperature but that refrigerating them may mitigate this effect.
“Our research provides the first experimental evidence that cold storage can help reduce the rate of benzoyl peroxide breakdown into benzene,” said one of the study authors, Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Connecticut. “Therefore, cold storage throughout the entire supply chain — from manufacturing to patient use — is a reasonable and proportional measure at this time for those continuing to use benzoyl peroxide medicine.” One acne product, the newer prescription triple-combination therapy (adapalene-clindamycin-BPO) “already has a cold shipping process in place; the patient just needs to continue that at home,” he noted.
For the study — which was funded by an independent lab, Valisure — researchers led by Valisure CEO and founder David Light, used gas chromatography-mass spectrometry to detect benzene levels in 111 BPO drug products from major US retailers and selected ion flow tube mass-spectrometry to quantify the release of benzene in real time. Benzene levels ranged from 0.16 ppm to 35.30 ppm, and 38 of the products (34%) had levels above the FDA limit of 2 ppm for drug products. “The results of the products sampled in this study suggest that formulation is likely the strongest contributor to benzene concentrations in BPO drug products that are commercially available, since the magnitude of benzene detected correlates most closely with specific brands or product types within certain brands,” the study authors wrote.
When the researchers tested the stability of a prescription encapsulated BPO drug product at cold (2 °C) and elevated temperature (50 °C), no apparent benzene formation was observed at 2 °C, whereas high levels of benzene formed at 50 °C, “suggesting that encapsulation technology may not stabilize BPO drug products, but cold storage may greatly reduce benzene formation,” they wrote.
In another component of the study, researchers exposed a BP drug product to a UVA/UVB lamp for 2 hours and found detectable benzene through evaporation and substantial benzene formation when exposed to UV light at levels below peak sunlight. The experiment “strongly justifies the package label warnings to avoid sun exposure when using BPO drug products,” the authors wrote. “Further evaluation to determine the influence of sun exposure on BPO drug product degradation and benzene formation is warranted.”
In an interview, John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, Massachusetts characterized the findings as “an important issue that we should take seriously.” However, “we also must not overreact.”
BPO is a foundational acne treatment without any clear alternative, he said, pointing out that no evidence currently exists “to support that routine use of benzoyl peroxide–containing products for acne is associated with a meaningful risk of benzene in the blood or an increased risk of cancer.”
And although it is prudent to minimize benzene exposure as much as possible, Barbieri continued, “it is not clear that these levels are a clinically meaningful incremental risk in the setting of an acne cream or wash. There is minimal cutaneous absorption of benzene, and it is uncertain how much benzene aerosolizes with routine use, particularly for washes which are not left on the skin.”
Bunick said that the combined data from this and the study published in March 2024 affected which BPO products he recommends for patients with acne. “I am using exclusively the triple combination therapy (adapalene-clindamycin-benzoyl peroxide) because I know it has the necessary cold supply chain in place to protect the product’s stability. I further encourage patients to place all their benzoyl peroxide–containing products in the refrigerator at home to reduce benzene formation and exposure.”
Bunick reported having served as an investigator and/or a consultant/speaker for many pharmaceutical companies, including as a consultant for Ortho-Dermatologics; but none related to this study. Barbieri reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
according to results from an analysis that used gas chromatography–mass spectrometry and other methods.
The analysis, which was published in the Journal of Investigative Dermatology and expands on a similar study released more than 6 months ago, also found that encapsulated BPO products break down into benzene at room temperature but that refrigerating them may mitigate this effect.
“Our research provides the first experimental evidence that cold storage can help reduce the rate of benzoyl peroxide breakdown into benzene,” said one of the study authors, Christopher G. Bunick, MD, PhD, associate professor of dermatology at Yale University, New Haven, Connecticut. “Therefore, cold storage throughout the entire supply chain — from manufacturing to patient use — is a reasonable and proportional measure at this time for those continuing to use benzoyl peroxide medicine.” One acne product, the newer prescription triple-combination therapy (adapalene-clindamycin-BPO) “already has a cold shipping process in place; the patient just needs to continue that at home,” he noted.
For the study — which was funded by an independent lab, Valisure — researchers led by Valisure CEO and founder David Light, used gas chromatography-mass spectrometry to detect benzene levels in 111 BPO drug products from major US retailers and selected ion flow tube mass-spectrometry to quantify the release of benzene in real time. Benzene levels ranged from 0.16 ppm to 35.30 ppm, and 38 of the products (34%) had levels above the FDA limit of 2 ppm for drug products. “The results of the products sampled in this study suggest that formulation is likely the strongest contributor to benzene concentrations in BPO drug products that are commercially available, since the magnitude of benzene detected correlates most closely with specific brands or product types within certain brands,” the study authors wrote.
When the researchers tested the stability of a prescription encapsulated BPO drug product at cold (2 °C) and elevated temperature (50 °C), no apparent benzene formation was observed at 2 °C, whereas high levels of benzene formed at 50 °C, “suggesting that encapsulation technology may not stabilize BPO drug products, but cold storage may greatly reduce benzene formation,” they wrote.
In another component of the study, researchers exposed a BP drug product to a UVA/UVB lamp for 2 hours and found detectable benzene through evaporation and substantial benzene formation when exposed to UV light at levels below peak sunlight. The experiment “strongly justifies the package label warnings to avoid sun exposure when using BPO drug products,” the authors wrote. “Further evaluation to determine the influence of sun exposure on BPO drug product degradation and benzene formation is warranted.”
In an interview, John Barbieri, MD, MBA, assistant professor of dermatology at Harvard Medical School and director of the Advanced Acne Therapeutics Clinic at Brigham and Women’s Hospital, Boston, Massachusetts characterized the findings as “an important issue that we should take seriously.” However, “we also must not overreact.”
BPO is a foundational acne treatment without any clear alternative, he said, pointing out that no evidence currently exists “to support that routine use of benzoyl peroxide–containing products for acne is associated with a meaningful risk of benzene in the blood or an increased risk of cancer.”
And although it is prudent to minimize benzene exposure as much as possible, Barbieri continued, “it is not clear that these levels are a clinically meaningful incremental risk in the setting of an acne cream or wash. There is minimal cutaneous absorption of benzene, and it is uncertain how much benzene aerosolizes with routine use, particularly for washes which are not left on the skin.”
Bunick said that the combined data from this and the study published in March 2024 affected which BPO products he recommends for patients with acne. “I am using exclusively the triple combination therapy (adapalene-clindamycin-benzoyl peroxide) because I know it has the necessary cold supply chain in place to protect the product’s stability. I further encourage patients to place all their benzoyl peroxide–containing products in the refrigerator at home to reduce benzene formation and exposure.”
Bunick reported having served as an investigator and/or a consultant/speaker for many pharmaceutical companies, including as a consultant for Ortho-Dermatologics; but none related to this study. Barbieri reported having no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Physician Empathy Mitigates Patients’ Chronic Pain
Physicians who treat patients are potentially exposed to two opposing psychological processes: A positive feeling related to the experience of helping someone in need and, on the other hand, the adverse experience of seeing someone’s suffering and being frustrated about their inability to help. The ability to share the feelings of others is often referred to as empathy, while the ability to care for and show interest in others is the key aspect of compassion. Empathy makes it possible to share the positive and negative feelings of others in the same way: We can therefore feel happy when we indirectly share others’ joy and sad when we indirectly share others’ suffering.
Empathy in healthcare professionals is associated with patient satisfaction, diagnostic accuracy, adherence to treatment recommendations, clinical outcomes, clinical expertise, and physician retention. However, evidence indicates a tendency for empathy to decline during physicians’ training and specialization.
Estimating Empathy
Empathy studies are primarily based on observational data that include physician self-assessment or patient-perceived empathy. External evaluation of empathy by the recipient or observer is not the dominant approach, and a systematic review of the topic showed that, in 331 of the 470 studies examined (70.4%), individuals self-reported their level of empathy. The self-assessment system, particularly for doctors, is more likely to measure the doctor’s attitudes about empathy than empathy itself. The lack of correlation between physician and patient empathy assessments made it clear that patients cannot be disregarded when assessing physician empathy.
Consultation and Relational Empathy (CARE) is the primary assessment tool available to patients to measure physician empathy. It is a reliable and consistent system, particularly in primary care scenarios.
The CARE measure captures even small nuances of patient interactions with the physician and has been confirmed as a valuable tool in assessing the relational components of empathy.
Doctor-Patient Relationship
Communication with the physician is generally considered an important element of chronic pain care because it affects patient engagement and decision-making. A collaborative approach involving the patient and clinician in clinical decisions was associated with adherence to pain treatment and improved outcomes among patients with chronic lower back pain. The study conducted in a primary care setting of 1352 participants showed findings regarding physician empathy that did not necessarily involve a therapeutic alliance with the patient based on collaborative communication or expectation of a therapeutic effect of pharmacotherapy. Physician empathy remained the strongest factor associated with patient satisfaction, even after considering various potential confounders, including communication with the physician. In addition, ongoing empathy, especially when reported by patients with a long-term relationship with the physician, supported the hypothesis of a possible lasting effect on patient satisfaction.
Treating Chronic Pain
Empathy is an aspect of the doctor-patient relationship that may be particularly important in patients with chronic pain. A cohort study of 1470 patients with chronic low back pain analyzed whether and how it correlated with chronic pain outcomes. Patients reported their physician’s empathy at the time of enrollment using the CARE measure, which included 10 items on physician’s empathy characteristics during meetings. Physicians whose scores were 30 or higher (ie, rated as good, very good, or excellent in most items) were classified as very empathetic physicians (VEPs), while those whose scores were 29 or lower (ie, rated as poor or passable in most items) were classified as slightly empathetic physicians (SEPs).
Pain intensity was measured with a numerical rating scale (0-10) for the typical pain level within 7 days before each encounter. The long-term stability of CARE scores was assessed in patients who maintained the same physician for more than 24 months. The study showed the following results:
- The CARE score was inversely associated with pain intensity (P < .001).
- Pain intensity was lower in patients in the VEP group than those in the SEP group (6.3 vs 6.7; P < .001).
- The likelihood of having a more empathetic physician generally increased with the decrease in the cut point of the CARE score for greater or less empathy of the physician.
- The extent of the physician’s empathy effects exceeded that reported for nonpharmacological treatments, current opioid use, and lumbar spine surgery.
- The effects of the interaction of empathy with time tended to favor the VEP group with regard to pain but were not statistically significant.
Empathy is an essential aspect of the patient-physician relationship (particularly in delivering care), and these findings demonstrate its relevance in pain therapy. Empathy has high therapeutic value, compared with many pain treatments that are often recommended in clinical practice.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Physicians who treat patients are potentially exposed to two opposing psychological processes: A positive feeling related to the experience of helping someone in need and, on the other hand, the adverse experience of seeing someone’s suffering and being frustrated about their inability to help. The ability to share the feelings of others is often referred to as empathy, while the ability to care for and show interest in others is the key aspect of compassion. Empathy makes it possible to share the positive and negative feelings of others in the same way: We can therefore feel happy when we indirectly share others’ joy and sad when we indirectly share others’ suffering.
Empathy in healthcare professionals is associated with patient satisfaction, diagnostic accuracy, adherence to treatment recommendations, clinical outcomes, clinical expertise, and physician retention. However, evidence indicates a tendency for empathy to decline during physicians’ training and specialization.
Estimating Empathy
Empathy studies are primarily based on observational data that include physician self-assessment or patient-perceived empathy. External evaluation of empathy by the recipient or observer is not the dominant approach, and a systematic review of the topic showed that, in 331 of the 470 studies examined (70.4%), individuals self-reported their level of empathy. The self-assessment system, particularly for doctors, is more likely to measure the doctor’s attitudes about empathy than empathy itself. The lack of correlation between physician and patient empathy assessments made it clear that patients cannot be disregarded when assessing physician empathy.
Consultation and Relational Empathy (CARE) is the primary assessment tool available to patients to measure physician empathy. It is a reliable and consistent system, particularly in primary care scenarios.
The CARE measure captures even small nuances of patient interactions with the physician and has been confirmed as a valuable tool in assessing the relational components of empathy.
Doctor-Patient Relationship
Communication with the physician is generally considered an important element of chronic pain care because it affects patient engagement and decision-making. A collaborative approach involving the patient and clinician in clinical decisions was associated with adherence to pain treatment and improved outcomes among patients with chronic lower back pain. The study conducted in a primary care setting of 1352 participants showed findings regarding physician empathy that did not necessarily involve a therapeutic alliance with the patient based on collaborative communication or expectation of a therapeutic effect of pharmacotherapy. Physician empathy remained the strongest factor associated with patient satisfaction, even after considering various potential confounders, including communication with the physician. In addition, ongoing empathy, especially when reported by patients with a long-term relationship with the physician, supported the hypothesis of a possible lasting effect on patient satisfaction.
Treating Chronic Pain
Empathy is an aspect of the doctor-patient relationship that may be particularly important in patients with chronic pain. A cohort study of 1470 patients with chronic low back pain analyzed whether and how it correlated with chronic pain outcomes. Patients reported their physician’s empathy at the time of enrollment using the CARE measure, which included 10 items on physician’s empathy characteristics during meetings. Physicians whose scores were 30 or higher (ie, rated as good, very good, or excellent in most items) were classified as very empathetic physicians (VEPs), while those whose scores were 29 or lower (ie, rated as poor or passable in most items) were classified as slightly empathetic physicians (SEPs).
Pain intensity was measured with a numerical rating scale (0-10) for the typical pain level within 7 days before each encounter. The long-term stability of CARE scores was assessed in patients who maintained the same physician for more than 24 months. The study showed the following results:
- The CARE score was inversely associated with pain intensity (P < .001).
- Pain intensity was lower in patients in the VEP group than those in the SEP group (6.3 vs 6.7; P < .001).
- The likelihood of having a more empathetic physician generally increased with the decrease in the cut point of the CARE score for greater or less empathy of the physician.
- The extent of the physician’s empathy effects exceeded that reported for nonpharmacological treatments, current opioid use, and lumbar spine surgery.
- The effects of the interaction of empathy with time tended to favor the VEP group with regard to pain but were not statistically significant.
Empathy is an essential aspect of the patient-physician relationship (particularly in delivering care), and these findings demonstrate its relevance in pain therapy. Empathy has high therapeutic value, compared with many pain treatments that are often recommended in clinical practice.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Physicians who treat patients are potentially exposed to two opposing psychological processes: A positive feeling related to the experience of helping someone in need and, on the other hand, the adverse experience of seeing someone’s suffering and being frustrated about their inability to help. The ability to share the feelings of others is often referred to as empathy, while the ability to care for and show interest in others is the key aspect of compassion. Empathy makes it possible to share the positive and negative feelings of others in the same way: We can therefore feel happy when we indirectly share others’ joy and sad when we indirectly share others’ suffering.
Empathy in healthcare professionals is associated with patient satisfaction, diagnostic accuracy, adherence to treatment recommendations, clinical outcomes, clinical expertise, and physician retention. However, evidence indicates a tendency for empathy to decline during physicians’ training and specialization.
Estimating Empathy
Empathy studies are primarily based on observational data that include physician self-assessment or patient-perceived empathy. External evaluation of empathy by the recipient or observer is not the dominant approach, and a systematic review of the topic showed that, in 331 of the 470 studies examined (70.4%), individuals self-reported their level of empathy. The self-assessment system, particularly for doctors, is more likely to measure the doctor’s attitudes about empathy than empathy itself. The lack of correlation between physician and patient empathy assessments made it clear that patients cannot be disregarded when assessing physician empathy.
Consultation and Relational Empathy (CARE) is the primary assessment tool available to patients to measure physician empathy. It is a reliable and consistent system, particularly in primary care scenarios.
The CARE measure captures even small nuances of patient interactions with the physician and has been confirmed as a valuable tool in assessing the relational components of empathy.
Doctor-Patient Relationship
Communication with the physician is generally considered an important element of chronic pain care because it affects patient engagement and decision-making. A collaborative approach involving the patient and clinician in clinical decisions was associated with adherence to pain treatment and improved outcomes among patients with chronic lower back pain. The study conducted in a primary care setting of 1352 participants showed findings regarding physician empathy that did not necessarily involve a therapeutic alliance with the patient based on collaborative communication or expectation of a therapeutic effect of pharmacotherapy. Physician empathy remained the strongest factor associated with patient satisfaction, even after considering various potential confounders, including communication with the physician. In addition, ongoing empathy, especially when reported by patients with a long-term relationship with the physician, supported the hypothesis of a possible lasting effect on patient satisfaction.
Treating Chronic Pain
Empathy is an aspect of the doctor-patient relationship that may be particularly important in patients with chronic pain. A cohort study of 1470 patients with chronic low back pain analyzed whether and how it correlated with chronic pain outcomes. Patients reported their physician’s empathy at the time of enrollment using the CARE measure, which included 10 items on physician’s empathy characteristics during meetings. Physicians whose scores were 30 or higher (ie, rated as good, very good, or excellent in most items) were classified as very empathetic physicians (VEPs), while those whose scores were 29 or lower (ie, rated as poor or passable in most items) were classified as slightly empathetic physicians (SEPs).
Pain intensity was measured with a numerical rating scale (0-10) for the typical pain level within 7 days before each encounter. The long-term stability of CARE scores was assessed in patients who maintained the same physician for more than 24 months. The study showed the following results:
- The CARE score was inversely associated with pain intensity (P < .001).
- Pain intensity was lower in patients in the VEP group than those in the SEP group (6.3 vs 6.7; P < .001).
- The likelihood of having a more empathetic physician generally increased with the decrease in the cut point of the CARE score for greater or less empathy of the physician.
- The extent of the physician’s empathy effects exceeded that reported for nonpharmacological treatments, current opioid use, and lumbar spine surgery.
- The effects of the interaction of empathy with time tended to favor the VEP group with regard to pain but were not statistically significant.
Empathy is an essential aspect of the patient-physician relationship (particularly in delivering care), and these findings demonstrate its relevance in pain therapy. Empathy has high therapeutic value, compared with many pain treatments that are often recommended in clinical practice.
This story was translated from Univadis Italy, which is part of the Medscape professional network, using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
GPs Urged to Embed Lifestyle Medicine into Primary Care
LIVERPOOL — “Healthy doctors make healthy patients”, stated a GP during a workshop at the Royal College of General Practitioners (RCGP) annual meeting. The session aimed to encourage GPs to embed lifestyle medicine into primary care through collaborative action.
Callum Leese from Aberfeldy Medical Practice in Scotland, who is also a lecturer at the University of Dundee for the Scottish Clinical Research Excellence Development Scheme (SCREDS), discussed the benefits of lifestyle medicine services in addressing lifestyle-related diseases, reducing their contribution towards the prevalence of chronic conditions, and helping prevent premature mortality.
Leese is leading a project to make Aberfeldy the healthiest town in Scotland by promoting physical activities, such as the 2-km, 5-km, and 7-km Santa Stride walking group in November, and a recent food festival to encourage healthy cooking and eating. “There’s loads of things that can be done to try and inspire change,” he said. “The research is fairly unequivocal in that healthy doctors make healthy patients,” Leese asserted. “The most important thing we can do is target our doctors and our nurses and make them advocates for what we want to see with our patients.”
Speaking to this news organization, he emphasized that, “if the doctors are moving, they’re much more likely to promote it, and if they’re eating well, they’re much more likely to be able to be evangelistic.”
Physical Activity Advice Shows High Return
About one-third of the population in the United Kingdom are physically inactive, which costs the economy £7.2 billion, with £1 billion attributed directly to the NHS, he informed the workshop.
As an honorary support fellow in physical activity and lifestyle medicine at the RCGP, Leese specializes in integrating physical activity into primary care settings. “We know it’s cost effective. If we compare it to smoking cessation advice, we know that we need to give advice to one person about 50 times for one person to stop smoking in primary care. But for physical activity, you need to give advice to 12 people for one person to increase their physical activity levels to meet the guidance,” he noted.
Leese stressed the importance of short but effective discussions between GPs and patients. He gave examples of online resources to recommend to patients, such as Moving Medicine, which aims to help healthcare professionals integrate physical activity into routine clinical conversations, or the RCGP toolkit (the Physical Activity Hub). “It really takes 1 minute of asking if the patient has ever considered being more active, and briefly explaining that being more active might have really significant outcomes for their condition,” he said.
In primary care, most patients who need to be more physically activity are directed toward 12-week exercise referral schemes, and sometimes we use social prescribing, for example, inviting patients to walk in groups, Leese explained. “However, despite the best intentions, about 78% of GPs aren’t doing it [advising on physical activity] regularly,” he noted. He cited four main challenges: lack of time, knowledge, resources, and financial support.
Geographical Variation in Social Prescribing
Social prescribing, which links patients with non–medical community support, also varies widely across the United Kingdom. “Social prescribing is a real example of that because it’s really well established in some places and not in others,” Leese remarked. He noted that inner-city and rural areas often have different needs. Contrary to some expectations, city dwellers are sometimes more active than those living in rural areas because despite having lots of green space for physical activity, “they tend to park the car outside the front door and park again right outside their place of work, whereas in London, for example, you can persuade people to get off a stop early on the Tube or a stop early in the bus.”
MAN v FAT 5-a-side Football
Leese also emphasized the importance of innovation in implementing lifestyle medicine, pointing out that nonmedical personnel, social prescribers, and health coaches can alleviate time pressures on GPs.
Citing an example of a physical activity-related intervention, he described a UK-wide organization developed for men in the 40s-50s age group, called MAN v FAT, which involves a novel weight-related way of playing five-a-side football. Players have a weigh-in before each game and teams are rewarded with points on the pitch for every pound lost as a team since their last match.
However, Leese acknowledged the need to tailor physical activity advice to different age groups. For example, “in an 80-year-old, physical activity might improve their balance and they’re less likely to fall and break something.”
Lifestyle Clinics
Leese cited the PCN Lifestyle Clinics, originating from the Leamington Primary Care Network (PCN), as an example of successful lifestyle medicine integration to help address the needs of people living with chronic conditions. “We don’t want to prescribe a model, but we can draw on a program run by the Leamington Spa PCN, that involves four group sessions of 6-10 people focused on lifestyle,” he said.
The weekly group-based sessions are run by a GP, a health and wellbeing coach, a dietitian, and a psychiatrist. Together, they cover four aspects of lifestyle and health comprising individual challenges, how community influences behavior and vice versa, food and nutrition, and physical activity for health and wellbeing.
“We try to debunk some of those myths around nutrition, compared with diet, and physical activity, compared with exercise. So, for example, the idea that exercise is usually considered to be using an elliptical cross-trainer whereas physical activity, which might be just dancing in your kitchen while you’re making dinner, is something that can be done more easily,” explained Leese.
Physical activities include running and swimming in collaboration with a leisure center. “It’s an amazing program,” he remarked.
Outcomes from 142 patients who attended the Lifestyle Clinic at a North Leamington GP practice over 14 months showed that 53% gained confidence in making lifestyle changes, 60% noticed a positive impact on their physical health, and 77% reported positive impacts on their mental health.
GP Embraces Lifestyle Medicine
Rachel Burnett, a GP from Park Medical Practice in Derby, a delegate who attended the session, commented on the central idea of incorporating lifestyle medicine into primary care practice. She told this news organization that, “I think it could prevent a lot of ill health and therefore a lot of health inequalities just by embedding lifestyle medicine into our work. To hear about the Leamington Spa project and how it›s been a success was really inspiring.”
Referring to her own practice, Burnett said: “My patients are familiar with the way I go on and on about lifestyle measures, but I believe the way forward is with group sessions because we need to give the same advice to a large number of patients, for example, with prediabetes. This could save time and resource, and I think patients who are more likely to make the changes will actually attend the sessions so we’re not wasting our breath.”
Neither Leese nor Burnett declared any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
LIVERPOOL — “Healthy doctors make healthy patients”, stated a GP during a workshop at the Royal College of General Practitioners (RCGP) annual meeting. The session aimed to encourage GPs to embed lifestyle medicine into primary care through collaborative action.
Callum Leese from Aberfeldy Medical Practice in Scotland, who is also a lecturer at the University of Dundee for the Scottish Clinical Research Excellence Development Scheme (SCREDS), discussed the benefits of lifestyle medicine services in addressing lifestyle-related diseases, reducing their contribution towards the prevalence of chronic conditions, and helping prevent premature mortality.
Leese is leading a project to make Aberfeldy the healthiest town in Scotland by promoting physical activities, such as the 2-km, 5-km, and 7-km Santa Stride walking group in November, and a recent food festival to encourage healthy cooking and eating. “There’s loads of things that can be done to try and inspire change,” he said. “The research is fairly unequivocal in that healthy doctors make healthy patients,” Leese asserted. “The most important thing we can do is target our doctors and our nurses and make them advocates for what we want to see with our patients.”
Speaking to this news organization, he emphasized that, “if the doctors are moving, they’re much more likely to promote it, and if they’re eating well, they’re much more likely to be able to be evangelistic.”
Physical Activity Advice Shows High Return
About one-third of the population in the United Kingdom are physically inactive, which costs the economy £7.2 billion, with £1 billion attributed directly to the NHS, he informed the workshop.
As an honorary support fellow in physical activity and lifestyle medicine at the RCGP, Leese specializes in integrating physical activity into primary care settings. “We know it’s cost effective. If we compare it to smoking cessation advice, we know that we need to give advice to one person about 50 times for one person to stop smoking in primary care. But for physical activity, you need to give advice to 12 people for one person to increase their physical activity levels to meet the guidance,” he noted.
Leese stressed the importance of short but effective discussions between GPs and patients. He gave examples of online resources to recommend to patients, such as Moving Medicine, which aims to help healthcare professionals integrate physical activity into routine clinical conversations, or the RCGP toolkit (the Physical Activity Hub). “It really takes 1 minute of asking if the patient has ever considered being more active, and briefly explaining that being more active might have really significant outcomes for their condition,” he said.
In primary care, most patients who need to be more physically activity are directed toward 12-week exercise referral schemes, and sometimes we use social prescribing, for example, inviting patients to walk in groups, Leese explained. “However, despite the best intentions, about 78% of GPs aren’t doing it [advising on physical activity] regularly,” he noted. He cited four main challenges: lack of time, knowledge, resources, and financial support.
Geographical Variation in Social Prescribing
Social prescribing, which links patients with non–medical community support, also varies widely across the United Kingdom. “Social prescribing is a real example of that because it’s really well established in some places and not in others,” Leese remarked. He noted that inner-city and rural areas often have different needs. Contrary to some expectations, city dwellers are sometimes more active than those living in rural areas because despite having lots of green space for physical activity, “they tend to park the car outside the front door and park again right outside their place of work, whereas in London, for example, you can persuade people to get off a stop early on the Tube or a stop early in the bus.”
MAN v FAT 5-a-side Football
Leese also emphasized the importance of innovation in implementing lifestyle medicine, pointing out that nonmedical personnel, social prescribers, and health coaches can alleviate time pressures on GPs.
Citing an example of a physical activity-related intervention, he described a UK-wide organization developed for men in the 40s-50s age group, called MAN v FAT, which involves a novel weight-related way of playing five-a-side football. Players have a weigh-in before each game and teams are rewarded with points on the pitch for every pound lost as a team since their last match.
However, Leese acknowledged the need to tailor physical activity advice to different age groups. For example, “in an 80-year-old, physical activity might improve their balance and they’re less likely to fall and break something.”
Lifestyle Clinics
Leese cited the PCN Lifestyle Clinics, originating from the Leamington Primary Care Network (PCN), as an example of successful lifestyle medicine integration to help address the needs of people living with chronic conditions. “We don’t want to prescribe a model, but we can draw on a program run by the Leamington Spa PCN, that involves four group sessions of 6-10 people focused on lifestyle,” he said.
The weekly group-based sessions are run by a GP, a health and wellbeing coach, a dietitian, and a psychiatrist. Together, they cover four aspects of lifestyle and health comprising individual challenges, how community influences behavior and vice versa, food and nutrition, and physical activity for health and wellbeing.
“We try to debunk some of those myths around nutrition, compared with diet, and physical activity, compared with exercise. So, for example, the idea that exercise is usually considered to be using an elliptical cross-trainer whereas physical activity, which might be just dancing in your kitchen while you’re making dinner, is something that can be done more easily,” explained Leese.
Physical activities include running and swimming in collaboration with a leisure center. “It’s an amazing program,” he remarked.
Outcomes from 142 patients who attended the Lifestyle Clinic at a North Leamington GP practice over 14 months showed that 53% gained confidence in making lifestyle changes, 60% noticed a positive impact on their physical health, and 77% reported positive impacts on their mental health.
GP Embraces Lifestyle Medicine
Rachel Burnett, a GP from Park Medical Practice in Derby, a delegate who attended the session, commented on the central idea of incorporating lifestyle medicine into primary care practice. She told this news organization that, “I think it could prevent a lot of ill health and therefore a lot of health inequalities just by embedding lifestyle medicine into our work. To hear about the Leamington Spa project and how it›s been a success was really inspiring.”
Referring to her own practice, Burnett said: “My patients are familiar with the way I go on and on about lifestyle measures, but I believe the way forward is with group sessions because we need to give the same advice to a large number of patients, for example, with prediabetes. This could save time and resource, and I think patients who are more likely to make the changes will actually attend the sessions so we’re not wasting our breath.”
Neither Leese nor Burnett declared any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
LIVERPOOL — “Healthy doctors make healthy patients”, stated a GP during a workshop at the Royal College of General Practitioners (RCGP) annual meeting. The session aimed to encourage GPs to embed lifestyle medicine into primary care through collaborative action.
Callum Leese from Aberfeldy Medical Practice in Scotland, who is also a lecturer at the University of Dundee for the Scottish Clinical Research Excellence Development Scheme (SCREDS), discussed the benefits of lifestyle medicine services in addressing lifestyle-related diseases, reducing their contribution towards the prevalence of chronic conditions, and helping prevent premature mortality.
Leese is leading a project to make Aberfeldy the healthiest town in Scotland by promoting physical activities, such as the 2-km, 5-km, and 7-km Santa Stride walking group in November, and a recent food festival to encourage healthy cooking and eating. “There’s loads of things that can be done to try and inspire change,” he said. “The research is fairly unequivocal in that healthy doctors make healthy patients,” Leese asserted. “The most important thing we can do is target our doctors and our nurses and make them advocates for what we want to see with our patients.”
Speaking to this news organization, he emphasized that, “if the doctors are moving, they’re much more likely to promote it, and if they’re eating well, they’re much more likely to be able to be evangelistic.”
Physical Activity Advice Shows High Return
About one-third of the population in the United Kingdom are physically inactive, which costs the economy £7.2 billion, with £1 billion attributed directly to the NHS, he informed the workshop.
As an honorary support fellow in physical activity and lifestyle medicine at the RCGP, Leese specializes in integrating physical activity into primary care settings. “We know it’s cost effective. If we compare it to smoking cessation advice, we know that we need to give advice to one person about 50 times for one person to stop smoking in primary care. But for physical activity, you need to give advice to 12 people for one person to increase their physical activity levels to meet the guidance,” he noted.
Leese stressed the importance of short but effective discussions between GPs and patients. He gave examples of online resources to recommend to patients, such as Moving Medicine, which aims to help healthcare professionals integrate physical activity into routine clinical conversations, or the RCGP toolkit (the Physical Activity Hub). “It really takes 1 minute of asking if the patient has ever considered being more active, and briefly explaining that being more active might have really significant outcomes for their condition,” he said.
In primary care, most patients who need to be more physically activity are directed toward 12-week exercise referral schemes, and sometimes we use social prescribing, for example, inviting patients to walk in groups, Leese explained. “However, despite the best intentions, about 78% of GPs aren’t doing it [advising on physical activity] regularly,” he noted. He cited four main challenges: lack of time, knowledge, resources, and financial support.
Geographical Variation in Social Prescribing
Social prescribing, which links patients with non–medical community support, also varies widely across the United Kingdom. “Social prescribing is a real example of that because it’s really well established in some places and not in others,” Leese remarked. He noted that inner-city and rural areas often have different needs. Contrary to some expectations, city dwellers are sometimes more active than those living in rural areas because despite having lots of green space for physical activity, “they tend to park the car outside the front door and park again right outside their place of work, whereas in London, for example, you can persuade people to get off a stop early on the Tube or a stop early in the bus.”
MAN v FAT 5-a-side Football
Leese also emphasized the importance of innovation in implementing lifestyle medicine, pointing out that nonmedical personnel, social prescribers, and health coaches can alleviate time pressures on GPs.
Citing an example of a physical activity-related intervention, he described a UK-wide organization developed for men in the 40s-50s age group, called MAN v FAT, which involves a novel weight-related way of playing five-a-side football. Players have a weigh-in before each game and teams are rewarded with points on the pitch for every pound lost as a team since their last match.
However, Leese acknowledged the need to tailor physical activity advice to different age groups. For example, “in an 80-year-old, physical activity might improve their balance and they’re less likely to fall and break something.”
Lifestyle Clinics
Leese cited the PCN Lifestyle Clinics, originating from the Leamington Primary Care Network (PCN), as an example of successful lifestyle medicine integration to help address the needs of people living with chronic conditions. “We don’t want to prescribe a model, but we can draw on a program run by the Leamington Spa PCN, that involves four group sessions of 6-10 people focused on lifestyle,” he said.
The weekly group-based sessions are run by a GP, a health and wellbeing coach, a dietitian, and a psychiatrist. Together, they cover four aspects of lifestyle and health comprising individual challenges, how community influences behavior and vice versa, food and nutrition, and physical activity for health and wellbeing.
“We try to debunk some of those myths around nutrition, compared with diet, and physical activity, compared with exercise. So, for example, the idea that exercise is usually considered to be using an elliptical cross-trainer whereas physical activity, which might be just dancing in your kitchen while you’re making dinner, is something that can be done more easily,” explained Leese.
Physical activities include running and swimming in collaboration with a leisure center. “It’s an amazing program,” he remarked.
Outcomes from 142 patients who attended the Lifestyle Clinic at a North Leamington GP practice over 14 months showed that 53% gained confidence in making lifestyle changes, 60% noticed a positive impact on their physical health, and 77% reported positive impacts on their mental health.
GP Embraces Lifestyle Medicine
Rachel Burnett, a GP from Park Medical Practice in Derby, a delegate who attended the session, commented on the central idea of incorporating lifestyle medicine into primary care practice. She told this news organization that, “I think it could prevent a lot of ill health and therefore a lot of health inequalities just by embedding lifestyle medicine into our work. To hear about the Leamington Spa project and how it›s been a success was really inspiring.”
Referring to her own practice, Burnett said: “My patients are familiar with the way I go on and on about lifestyle measures, but I believe the way forward is with group sessions because we need to give the same advice to a large number of patients, for example, with prediabetes. This could save time and resource, and I think patients who are more likely to make the changes will actually attend the sessions so we’re not wasting our breath.”
Neither Leese nor Burnett declared any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Smartphone Data Flag Early Dementia Risk in Older Adults
a novel real-world study suggested.
During a smartphone-assisted scavenger hunt on a university campus, researchers observed that older adults with subjective cognitive decline (SCD) paused more frequently, likely to reorient themselves, than those without SCD. This behavior served as an identifier of individuals with SCD.
“Deficits in spatial navigation are one of the first signs of Alzheimer’s disease,” said study investigator Nadine Diersch, PhD, guest researcher with the German Center for Neurodegenerative Diseases (DZNE), Tübingen.
This study, said Diersch, provides “first evidence of how a digital footprint for early dementia-related cognitive decline might look like in real-world settings during a short (less than 30 minutes) and remotely performed wayfinding task.”
The study was published online in PLOS Digital Health.
Trouble With Orientation
A total of 72 men and women in their mid-20s to mid-60s participated in the study; 23 of the 48 older adults had SCD but still scored normally on neuropsychological assessments.
All study participants were instructed to independently find five buildings on the medical campus of the Otto-von-Guericke-University Magdeburg in Germany, guided by a smartphone app developed by the study team. Their patterns of movement were tracked by GPS.
All participants had similar knowledge of the campus, and all were experienced in using smartphones. They also practiced using the app beforehand.
In most cases, participants reached the five destinations in less than half an hour. The younger participants performed better than the older ones; on average, the younger adults walked shorter distances and generally did not use the help function on the app as often as the older ones.
In the older adults, the number of orientation stops was predictive of SCD status. The adults with SCD tended to hesitate more at intersections. A decline in executive functioning might explain this finding, Diersch said.
“Intact executive functioning is an important component of efficient navigation, for example, when switching between different navigation strategies or planning a route. However, since this was the first study on that subject, more research is needed to determine the precise contribution of different cognitive processes on digital wayfinding data,” said Diersch.
With more study, “we think that such a smartphone-assisted wayfinding task, performed in the immediate surroundings, could be used as a low-threshold screening tool — for example, to stratify subjects with regard to the need of extended cognitive and clinical diagnostics in specialized care,” she added.
‘A Game Changer’
Commenting on the research, Shaheen Lakhan, MD, PhD, neurologist and researcher based in Miami, Florida, who wasn’t involved in the research, said the findings have the potential to “revolutionize” dementia care.
“We’ve seen smartphones transform everything from banking to dating — now they’re set to reshape brain health monitoring. This ingenious digital scavenger hunt detects cognitive decline in real-world scenarios, bypassing costly, complex tests. It’s a game changer,” said Lakhan.
“Just as we track our steps and calories, we could soon track our cognitive health with a tap. This isn’t just innovation; it’s the future of dementia prevention and care unfolding on our smartphone screens. We’re not just talking about convenience. We’re talking about catching Alzheimer’s before it catches us,” he added.
The next phase, Lakhan noted, would be to develop smartphone apps as digital therapeutics, not just to detect cognitive decline but to treat or even prevent it.
“Imagine your phone not only flagging potential issues but also providing personalized brain training exercises to keep your mind sharp and resilient against dementia,” Lakhan said.
This work was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation) within the Collaborative Research Center “Neural Resources of Cognition” and a DZNE Innovation-2-Application Award. Diersch is now a full-time employee of neotiv. Lakhan had no relevant disclosures.
A version of this article first appeared on Medscape.com.
a novel real-world study suggested.
During a smartphone-assisted scavenger hunt on a university campus, researchers observed that older adults with subjective cognitive decline (SCD) paused more frequently, likely to reorient themselves, than those without SCD. This behavior served as an identifier of individuals with SCD.
“Deficits in spatial navigation are one of the first signs of Alzheimer’s disease,” said study investigator Nadine Diersch, PhD, guest researcher with the German Center for Neurodegenerative Diseases (DZNE), Tübingen.
This study, said Diersch, provides “first evidence of how a digital footprint for early dementia-related cognitive decline might look like in real-world settings during a short (less than 30 minutes) and remotely performed wayfinding task.”
The study was published online in PLOS Digital Health.
Trouble With Orientation
A total of 72 men and women in their mid-20s to mid-60s participated in the study; 23 of the 48 older adults had SCD but still scored normally on neuropsychological assessments.
All study participants were instructed to independently find five buildings on the medical campus of the Otto-von-Guericke-University Magdeburg in Germany, guided by a smartphone app developed by the study team. Their patterns of movement were tracked by GPS.
All participants had similar knowledge of the campus, and all were experienced in using smartphones. They also practiced using the app beforehand.
In most cases, participants reached the five destinations in less than half an hour. The younger participants performed better than the older ones; on average, the younger adults walked shorter distances and generally did not use the help function on the app as often as the older ones.
In the older adults, the number of orientation stops was predictive of SCD status. The adults with SCD tended to hesitate more at intersections. A decline in executive functioning might explain this finding, Diersch said.
“Intact executive functioning is an important component of efficient navigation, for example, when switching between different navigation strategies or planning a route. However, since this was the first study on that subject, more research is needed to determine the precise contribution of different cognitive processes on digital wayfinding data,” said Diersch.
With more study, “we think that such a smartphone-assisted wayfinding task, performed in the immediate surroundings, could be used as a low-threshold screening tool — for example, to stratify subjects with regard to the need of extended cognitive and clinical diagnostics in specialized care,” she added.
‘A Game Changer’
Commenting on the research, Shaheen Lakhan, MD, PhD, neurologist and researcher based in Miami, Florida, who wasn’t involved in the research, said the findings have the potential to “revolutionize” dementia care.
“We’ve seen smartphones transform everything from banking to dating — now they’re set to reshape brain health monitoring. This ingenious digital scavenger hunt detects cognitive decline in real-world scenarios, bypassing costly, complex tests. It’s a game changer,” said Lakhan.
“Just as we track our steps and calories, we could soon track our cognitive health with a tap. This isn’t just innovation; it’s the future of dementia prevention and care unfolding on our smartphone screens. We’re not just talking about convenience. We’re talking about catching Alzheimer’s before it catches us,” he added.
The next phase, Lakhan noted, would be to develop smartphone apps as digital therapeutics, not just to detect cognitive decline but to treat or even prevent it.
“Imagine your phone not only flagging potential issues but also providing personalized brain training exercises to keep your mind sharp and resilient against dementia,” Lakhan said.
This work was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation) within the Collaborative Research Center “Neural Resources of Cognition” and a DZNE Innovation-2-Application Award. Diersch is now a full-time employee of neotiv. Lakhan had no relevant disclosures.
A version of this article first appeared on Medscape.com.
a novel real-world study suggested.
During a smartphone-assisted scavenger hunt on a university campus, researchers observed that older adults with subjective cognitive decline (SCD) paused more frequently, likely to reorient themselves, than those without SCD. This behavior served as an identifier of individuals with SCD.
“Deficits in spatial navigation are one of the first signs of Alzheimer’s disease,” said study investigator Nadine Diersch, PhD, guest researcher with the German Center for Neurodegenerative Diseases (DZNE), Tübingen.
This study, said Diersch, provides “first evidence of how a digital footprint for early dementia-related cognitive decline might look like in real-world settings during a short (less than 30 minutes) and remotely performed wayfinding task.”
The study was published online in PLOS Digital Health.
Trouble With Orientation
A total of 72 men and women in their mid-20s to mid-60s participated in the study; 23 of the 48 older adults had SCD but still scored normally on neuropsychological assessments.
All study participants were instructed to independently find five buildings on the medical campus of the Otto-von-Guericke-University Magdeburg in Germany, guided by a smartphone app developed by the study team. Their patterns of movement were tracked by GPS.
All participants had similar knowledge of the campus, and all were experienced in using smartphones. They also practiced using the app beforehand.
In most cases, participants reached the five destinations in less than half an hour. The younger participants performed better than the older ones; on average, the younger adults walked shorter distances and generally did not use the help function on the app as often as the older ones.
In the older adults, the number of orientation stops was predictive of SCD status. The adults with SCD tended to hesitate more at intersections. A decline in executive functioning might explain this finding, Diersch said.
“Intact executive functioning is an important component of efficient navigation, for example, when switching between different navigation strategies or planning a route. However, since this was the first study on that subject, more research is needed to determine the precise contribution of different cognitive processes on digital wayfinding data,” said Diersch.
With more study, “we think that such a smartphone-assisted wayfinding task, performed in the immediate surroundings, could be used as a low-threshold screening tool — for example, to stratify subjects with regard to the need of extended cognitive and clinical diagnostics in specialized care,” she added.
‘A Game Changer’
Commenting on the research, Shaheen Lakhan, MD, PhD, neurologist and researcher based in Miami, Florida, who wasn’t involved in the research, said the findings have the potential to “revolutionize” dementia care.
“We’ve seen smartphones transform everything from banking to dating — now they’re set to reshape brain health monitoring. This ingenious digital scavenger hunt detects cognitive decline in real-world scenarios, bypassing costly, complex tests. It’s a game changer,” said Lakhan.
“Just as we track our steps and calories, we could soon track our cognitive health with a tap. This isn’t just innovation; it’s the future of dementia prevention and care unfolding on our smartphone screens. We’re not just talking about convenience. We’re talking about catching Alzheimer’s before it catches us,” he added.
The next phase, Lakhan noted, would be to develop smartphone apps as digital therapeutics, not just to detect cognitive decline but to treat or even prevent it.
“Imagine your phone not only flagging potential issues but also providing personalized brain training exercises to keep your mind sharp and resilient against dementia,” Lakhan said.
This work was funded by the Deutsche Forschungsgemeinschaft (German Research Foundation) within the Collaborative Research Center “Neural Resources of Cognition” and a DZNE Innovation-2-Application Award. Diersch is now a full-time employee of neotiv. Lakhan had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM PLOS DIGITAL HEALTH
Do PFAs Cause Kidney Cancer? VA to Investigate
The US Department of Veterans Affairs (VA) will conduct a scientific assessment to find out in whether kidney cancer should be considered a presumptive service-connected condition for veterans exposed to per- and polyfluoroalkyl substances (PFAs). This assessment is the first step in the VA presumptive condition investigative process, which could allow exposed veterans who were exposed to PFAs during their service to access more VA services.
A class of more than 12,000 chemicals, PFAs have been used in the military since the early 1970s in many items, including military-grade firefighting foam. Studies have already suggested links between the so-called forever chemicals and cancer, particularly kidney cancer.
The US Department of Defense (DoD) is assessing contamination at hundreds of sites, while the National Defense Authorization Act in Fiscal Year 2020 mandated that DoD stop using those foams starting in October and remove all stocks from active and former installations and equipment. That may not happen until next year, though, because the DoD has requested a waiver through October 2025 and may extend it through 2026.
When a condition is considered presumptive, eligible veterans do not need to prove their service caused their disease to receive benefits. As part of the Biden Administration’s efforts to expand benefits and services for toxin-exposed veterans and their families, the VA expedited health care and benefits eligibility under the PACT Act by several years—including extending presumptions for head cancer, neck cancer, gastrointestinal cancer, reproductive cancer, lymphoma, pancreatic cancer, kidney cancer, melanoma, and hypertension for Vietnam era veterans. The VA has also extended presumptions for > 300 new conditions, most recently for male breast cancer, urethral cancer, and cancer of the paraurethral glands.
Whether a condition is an established presumptive condition or not, the VA will consider claims on a case-by-case basis and can grant disability compensation benefits if sufficient evidence of service connection is found. “[M]ake no mistake: Veterans should not wait for the outcome of this review to apply for the benefits and care they deserve,” VA Secretary Denis McDonough said in a release. “If you’re a veteran and believe your military service has negatively impacted your health, we encourage you to apply for VA care and benefits today.”
The public has 30 days to comment on the proposed scientific assessment between PFAs exposure and kidney cancer via the Federal Register. The VA is set to host a listening session on Nov. 19, 2024, to allow individuals to share research and input. Interested individuals may register to participate. The public may also comment via either forum on other conditions that would benefit from review for potential service-connection.
The US Department of Veterans Affairs (VA) will conduct a scientific assessment to find out in whether kidney cancer should be considered a presumptive service-connected condition for veterans exposed to per- and polyfluoroalkyl substances (PFAs). This assessment is the first step in the VA presumptive condition investigative process, which could allow exposed veterans who were exposed to PFAs during their service to access more VA services.
A class of more than 12,000 chemicals, PFAs have been used in the military since the early 1970s in many items, including military-grade firefighting foam. Studies have already suggested links between the so-called forever chemicals and cancer, particularly kidney cancer.
The US Department of Defense (DoD) is assessing contamination at hundreds of sites, while the National Defense Authorization Act in Fiscal Year 2020 mandated that DoD stop using those foams starting in October and remove all stocks from active and former installations and equipment. That may not happen until next year, though, because the DoD has requested a waiver through October 2025 and may extend it through 2026.
When a condition is considered presumptive, eligible veterans do not need to prove their service caused their disease to receive benefits. As part of the Biden Administration’s efforts to expand benefits and services for toxin-exposed veterans and their families, the VA expedited health care and benefits eligibility under the PACT Act by several years—including extending presumptions for head cancer, neck cancer, gastrointestinal cancer, reproductive cancer, lymphoma, pancreatic cancer, kidney cancer, melanoma, and hypertension for Vietnam era veterans. The VA has also extended presumptions for > 300 new conditions, most recently for male breast cancer, urethral cancer, and cancer of the paraurethral glands.
Whether a condition is an established presumptive condition or not, the VA will consider claims on a case-by-case basis and can grant disability compensation benefits if sufficient evidence of service connection is found. “[M]ake no mistake: Veterans should not wait for the outcome of this review to apply for the benefits and care they deserve,” VA Secretary Denis McDonough said in a release. “If you’re a veteran and believe your military service has negatively impacted your health, we encourage you to apply for VA care and benefits today.”
The public has 30 days to comment on the proposed scientific assessment between PFAs exposure and kidney cancer via the Federal Register. The VA is set to host a listening session on Nov. 19, 2024, to allow individuals to share research and input. Interested individuals may register to participate. The public may also comment via either forum on other conditions that would benefit from review for potential service-connection.
The US Department of Veterans Affairs (VA) will conduct a scientific assessment to find out in whether kidney cancer should be considered a presumptive service-connected condition for veterans exposed to per- and polyfluoroalkyl substances (PFAs). This assessment is the first step in the VA presumptive condition investigative process, which could allow exposed veterans who were exposed to PFAs during their service to access more VA services.
A class of more than 12,000 chemicals, PFAs have been used in the military since the early 1970s in many items, including military-grade firefighting foam. Studies have already suggested links between the so-called forever chemicals and cancer, particularly kidney cancer.
The US Department of Defense (DoD) is assessing contamination at hundreds of sites, while the National Defense Authorization Act in Fiscal Year 2020 mandated that DoD stop using those foams starting in October and remove all stocks from active and former installations and equipment. That may not happen until next year, though, because the DoD has requested a waiver through October 2025 and may extend it through 2026.
When a condition is considered presumptive, eligible veterans do not need to prove their service caused their disease to receive benefits. As part of the Biden Administration’s efforts to expand benefits and services for toxin-exposed veterans and their families, the VA expedited health care and benefits eligibility under the PACT Act by several years—including extending presumptions for head cancer, neck cancer, gastrointestinal cancer, reproductive cancer, lymphoma, pancreatic cancer, kidney cancer, melanoma, and hypertension for Vietnam era veterans. The VA has also extended presumptions for > 300 new conditions, most recently for male breast cancer, urethral cancer, and cancer of the paraurethral glands.
Whether a condition is an established presumptive condition or not, the VA will consider claims on a case-by-case basis and can grant disability compensation benefits if sufficient evidence of service connection is found. “[M]ake no mistake: Veterans should not wait for the outcome of this review to apply for the benefits and care they deserve,” VA Secretary Denis McDonough said in a release. “If you’re a veteran and believe your military service has negatively impacted your health, we encourage you to apply for VA care and benefits today.”
The public has 30 days to comment on the proposed scientific assessment between PFAs exposure and kidney cancer via the Federal Register. The VA is set to host a listening session on Nov. 19, 2024, to allow individuals to share research and input. Interested individuals may register to participate. The public may also comment via either forum on other conditions that would benefit from review for potential service-connection.
VA Tele-Emergency Care Program Expanded Nationwide
The US Department of Veterans Affairs (VA) has announced that tele-emergency care (tele-EC) is now available nationwide. According to the VA, the expansion has already helped > 61,000 callers with a 59.4% case resolution rate, meaning veterans’ needs were resolved without them having to travel to urgent care or an emergency department.
Tele-EC does not replace the need for in-person emergency evaluation, but offers quick, virtual triage assessments for veterans in rural areas or those with mobility and transportation challenges when in-person immediate care can be difficult to access. The program is a part of VA Health Connect, which connects the caller to a clinical triage nurse, who connects the veteran to tele-emergency care when clinically appropriate. Tele-EC practitioners evaluate the veteran over the phone or on video and recommend treatment or follow-up, including in-person care if needed. In life-threatening emergencies, the clinical triage nurse will call 911 and stay on the phone with the veteran until help arrives. The VA however, says the best step for a veteran experiencing a life-threatening emergency is to immediately contact 911 as opposed to seeking support via tele-EC.
The program can save time not only through on-the-spot evaluation, but by avoiding drive and wait times. “Sometimes, you’re not sure whether what you’re experiencing is a minor emergency or not — and tele-emergency care can help you resolve those questions,” VA Under Secretary for Health Shereef Elnahal, MD, says. “Veterans can get immediate, virtual triage with a VA medical provider who has direct access to their medical records. This avoids having to potentially drive to the nearest emergency department and wait to be evaluated, if appropriate.”
Veterans enrolled in VA health care can now access tele-EC nationwide by calling VA Health Connect and through the VA Health Chat app. Veterans can find their local VA Health Connect number by searching for their facility.
The US Department of Veterans Affairs (VA) has announced that tele-emergency care (tele-EC) is now available nationwide. According to the VA, the expansion has already helped > 61,000 callers with a 59.4% case resolution rate, meaning veterans’ needs were resolved without them having to travel to urgent care or an emergency department.
Tele-EC does not replace the need for in-person emergency evaluation, but offers quick, virtual triage assessments for veterans in rural areas or those with mobility and transportation challenges when in-person immediate care can be difficult to access. The program is a part of VA Health Connect, which connects the caller to a clinical triage nurse, who connects the veteran to tele-emergency care when clinically appropriate. Tele-EC practitioners evaluate the veteran over the phone or on video and recommend treatment or follow-up, including in-person care if needed. In life-threatening emergencies, the clinical triage nurse will call 911 and stay on the phone with the veteran until help arrives. The VA however, says the best step for a veteran experiencing a life-threatening emergency is to immediately contact 911 as opposed to seeking support via tele-EC.
The program can save time not only through on-the-spot evaluation, but by avoiding drive and wait times. “Sometimes, you’re not sure whether what you’re experiencing is a minor emergency or not — and tele-emergency care can help you resolve those questions,” VA Under Secretary for Health Shereef Elnahal, MD, says. “Veterans can get immediate, virtual triage with a VA medical provider who has direct access to their medical records. This avoids having to potentially drive to the nearest emergency department and wait to be evaluated, if appropriate.”
Veterans enrolled in VA health care can now access tele-EC nationwide by calling VA Health Connect and through the VA Health Chat app. Veterans can find their local VA Health Connect number by searching for their facility.
The US Department of Veterans Affairs (VA) has announced that tele-emergency care (tele-EC) is now available nationwide. According to the VA, the expansion has already helped > 61,000 callers with a 59.4% case resolution rate, meaning veterans’ needs were resolved without them having to travel to urgent care or an emergency department.
Tele-EC does not replace the need for in-person emergency evaluation, but offers quick, virtual triage assessments for veterans in rural areas or those with mobility and transportation challenges when in-person immediate care can be difficult to access. The program is a part of VA Health Connect, which connects the caller to a clinical triage nurse, who connects the veteran to tele-emergency care when clinically appropriate. Tele-EC practitioners evaluate the veteran over the phone or on video and recommend treatment or follow-up, including in-person care if needed. In life-threatening emergencies, the clinical triage nurse will call 911 and stay on the phone with the veteran until help arrives. The VA however, says the best step for a veteran experiencing a life-threatening emergency is to immediately contact 911 as opposed to seeking support via tele-EC.
The program can save time not only through on-the-spot evaluation, but by avoiding drive and wait times. “Sometimes, you’re not sure whether what you’re experiencing is a minor emergency or not — and tele-emergency care can help you resolve those questions,” VA Under Secretary for Health Shereef Elnahal, MD, says. “Veterans can get immediate, virtual triage with a VA medical provider who has direct access to their medical records. This avoids having to potentially drive to the nearest emergency department and wait to be evaluated, if appropriate.”
Veterans enrolled in VA health care can now access tele-EC nationwide by calling VA Health Connect and through the VA Health Chat app. Veterans can find their local VA Health Connect number by searching for their facility.
Persistent headaches and nightmares
The correct diagnosis is adolescent posttraumatic stress disorder (PTSD), as the patient's symptoms — recurrent nightmares, flashbacks, hypervigilance, and avoidance behaviors — are closely linked to her recent traumatic experience, fitting the clinical profile of PTSD. The MRI finding, although abnormal, does not correlate with a neurologic cause for her symptoms and may be incidental.
Temporal lobe epilepsy can cause behavioral changes but does not explain the specific PTSD symptoms like flashbacks and nightmares.
Chronic migraine could explain the headaches but not the full spectrum of PTSD symptoms.
Major depressive disorder could account for some of the emotional and social symptoms but lacks the characteristic re-experiencing and avoidance behaviors typical of PTSD.
Adolescent PTSD is a significant public health concern, causing significant distress to a small portion of the youth population. By late adolescence, approximately two thirds of youths have been exposed to trauma, and 8% of these individuals meet the criteria for PTSD by age 18. The incidence is exceptionally high in cases of sexual abuse and assault, with rates reaching up to 40%. PTSD in adolescents is associated with severe psychological distress, reduced academic performance, and a high rate of comorbidities, including anxiety and depression. There are specific populations (including children who are evacuated from home, asylum seekers, etc.) that show higher rates of PTSD.
PTSD can lead to chronic impairments, comorbid psychiatric disorders, and an increased risk for suicide, with cases documented in toddlers as young as 1 year old. Thus, it is important to consider the individual's background and social history, as older children with PTSD may present with symptoms from early childhood trauma, often distant from the time of clinical evaluation.
Intrusion symptoms are a hallmark of PTSD, characterized by persistent and uncontrollable thoughts, dreams, and emotional reactions related to the traumatic event. These symptoms distinguish PTSD from other anxiety and mood disorders. Children with PTSD often experience involuntary, distressing thoughts and memories triggered by trauma cues, such as sights, sounds, or smells associated with the traumatic event. In younger children, these intrusive thoughts may manifest through repetitive play that re-enacts aspects of the trauma.
Nightmares are also common, although in children the content may not always directly relate to the traumatic event. Chronic nightmares contribute to sleep disturbances, exacerbating PTSD symptoms. Trauma reminders, which can be both internal (thoughts, memories) and external (places, sensory experiences), can provoke severe distress and physiologic reactions.
Avoidance symptoms often develop as a coping mechanism in response to distressing re-experiencing symptoms. Children may avoid thoughts, feelings, and memories of the traumatic event or people, places, and activities associated with the trauma. In young children, avoidance may manifest as restricted play or reduced exploration of their environment.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) outlines specific criteria for diagnosing PTSD in individuals over 6 years old, which includes exposure to actual or threatened death, serious injury, or sexual violence, and the presence of symptoms such as intrusion, avoidance, negative mood alterations, and heightened arousal. The DSM-5-TR provides tailored diagnostic criteria for developmental differences in symptom expression for children under 6.
Managing PTSD in children requires a patient-specific approach, with an emphasis on obtaining consent from both the patient and guardian. The American Academy of Child and Adolescent Psychiatry (AACAP) recommends psychotherapy as the first-line treatment for pediatric PTSD. However, patients with severe symptoms or comorbidities may initially be unable to engage in meaningful therapy and may require medication to stabilize symptoms before starting psychotherapy.
Trauma-focused psychotherapy, including cognitive-behavioral therapy (CBT), exposure-based therapy, and eye movement desensitization and reprocessing (EMDR) therapy, is the preferred treatment for PTSD. Clinical studies have shown that patients receiving trauma-focused psychotherapy experience more remarkable symptom improvement than those who do not receive treatment and, in children, psychotherapy generally yields better outcomes than pharmacotherapy.
While selective serotonin reuptake inhibitors like sertraline and paroxetine are FDA-approved for PTSD treatment in adults, their efficacy in children often produces outcomes similar to those of placebo. Medications are typically reserved for severe symptoms and are used as an off-label treatment in pediatric cases. Pharmacologic management may be necessary when the severity of symptoms prevents the use of trauma-focused psychotherapy or requires immediate stabilization.
Heidi Moawad, MD, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Heidi Moawad, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The correct diagnosis is adolescent posttraumatic stress disorder (PTSD), as the patient's symptoms — recurrent nightmares, flashbacks, hypervigilance, and avoidance behaviors — are closely linked to her recent traumatic experience, fitting the clinical profile of PTSD. The MRI finding, although abnormal, does not correlate with a neurologic cause for her symptoms and may be incidental.
Temporal lobe epilepsy can cause behavioral changes but does not explain the specific PTSD symptoms like flashbacks and nightmares.
Chronic migraine could explain the headaches but not the full spectrum of PTSD symptoms.
Major depressive disorder could account for some of the emotional and social symptoms but lacks the characteristic re-experiencing and avoidance behaviors typical of PTSD.
Adolescent PTSD is a significant public health concern, causing significant distress to a small portion of the youth population. By late adolescence, approximately two thirds of youths have been exposed to trauma, and 8% of these individuals meet the criteria for PTSD by age 18. The incidence is exceptionally high in cases of sexual abuse and assault, with rates reaching up to 40%. PTSD in adolescents is associated with severe psychological distress, reduced academic performance, and a high rate of comorbidities, including anxiety and depression. There are specific populations (including children who are evacuated from home, asylum seekers, etc.) that show higher rates of PTSD.
PTSD can lead to chronic impairments, comorbid psychiatric disorders, and an increased risk for suicide, with cases documented in toddlers as young as 1 year old. Thus, it is important to consider the individual's background and social history, as older children with PTSD may present with symptoms from early childhood trauma, often distant from the time of clinical evaluation.
Intrusion symptoms are a hallmark of PTSD, characterized by persistent and uncontrollable thoughts, dreams, and emotional reactions related to the traumatic event. These symptoms distinguish PTSD from other anxiety and mood disorders. Children with PTSD often experience involuntary, distressing thoughts and memories triggered by trauma cues, such as sights, sounds, or smells associated with the traumatic event. In younger children, these intrusive thoughts may manifest through repetitive play that re-enacts aspects of the trauma.
Nightmares are also common, although in children the content may not always directly relate to the traumatic event. Chronic nightmares contribute to sleep disturbances, exacerbating PTSD symptoms. Trauma reminders, which can be both internal (thoughts, memories) and external (places, sensory experiences), can provoke severe distress and physiologic reactions.
Avoidance symptoms often develop as a coping mechanism in response to distressing re-experiencing symptoms. Children may avoid thoughts, feelings, and memories of the traumatic event or people, places, and activities associated with the trauma. In young children, avoidance may manifest as restricted play or reduced exploration of their environment.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) outlines specific criteria for diagnosing PTSD in individuals over 6 years old, which includes exposure to actual or threatened death, serious injury, or sexual violence, and the presence of symptoms such as intrusion, avoidance, negative mood alterations, and heightened arousal. The DSM-5-TR provides tailored diagnostic criteria for developmental differences in symptom expression for children under 6.
Managing PTSD in children requires a patient-specific approach, with an emphasis on obtaining consent from both the patient and guardian. The American Academy of Child and Adolescent Psychiatry (AACAP) recommends psychotherapy as the first-line treatment for pediatric PTSD. However, patients with severe symptoms or comorbidities may initially be unable to engage in meaningful therapy and may require medication to stabilize symptoms before starting psychotherapy.
Trauma-focused psychotherapy, including cognitive-behavioral therapy (CBT), exposure-based therapy, and eye movement desensitization and reprocessing (EMDR) therapy, is the preferred treatment for PTSD. Clinical studies have shown that patients receiving trauma-focused psychotherapy experience more remarkable symptom improvement than those who do not receive treatment and, in children, psychotherapy generally yields better outcomes than pharmacotherapy.
While selective serotonin reuptake inhibitors like sertraline and paroxetine are FDA-approved for PTSD treatment in adults, their efficacy in children often produces outcomes similar to those of placebo. Medications are typically reserved for severe symptoms and are used as an off-label treatment in pediatric cases. Pharmacologic management may be necessary when the severity of symptoms prevents the use of trauma-focused psychotherapy or requires immediate stabilization.
Heidi Moawad, MD, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Heidi Moawad, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The correct diagnosis is adolescent posttraumatic stress disorder (PTSD), as the patient's symptoms — recurrent nightmares, flashbacks, hypervigilance, and avoidance behaviors — are closely linked to her recent traumatic experience, fitting the clinical profile of PTSD. The MRI finding, although abnormal, does not correlate with a neurologic cause for her symptoms and may be incidental.
Temporal lobe epilepsy can cause behavioral changes but does not explain the specific PTSD symptoms like flashbacks and nightmares.
Chronic migraine could explain the headaches but not the full spectrum of PTSD symptoms.
Major depressive disorder could account for some of the emotional and social symptoms but lacks the characteristic re-experiencing and avoidance behaviors typical of PTSD.
Adolescent PTSD is a significant public health concern, causing significant distress to a small portion of the youth population. By late adolescence, approximately two thirds of youths have been exposed to trauma, and 8% of these individuals meet the criteria for PTSD by age 18. The incidence is exceptionally high in cases of sexual abuse and assault, with rates reaching up to 40%. PTSD in adolescents is associated with severe psychological distress, reduced academic performance, and a high rate of comorbidities, including anxiety and depression. There are specific populations (including children who are evacuated from home, asylum seekers, etc.) that show higher rates of PTSD.
PTSD can lead to chronic impairments, comorbid psychiatric disorders, and an increased risk for suicide, with cases documented in toddlers as young as 1 year old. Thus, it is important to consider the individual's background and social history, as older children with PTSD may present with symptoms from early childhood trauma, often distant from the time of clinical evaluation.
Intrusion symptoms are a hallmark of PTSD, characterized by persistent and uncontrollable thoughts, dreams, and emotional reactions related to the traumatic event. These symptoms distinguish PTSD from other anxiety and mood disorders. Children with PTSD often experience involuntary, distressing thoughts and memories triggered by trauma cues, such as sights, sounds, or smells associated with the traumatic event. In younger children, these intrusive thoughts may manifest through repetitive play that re-enacts aspects of the trauma.
Nightmares are also common, although in children the content may not always directly relate to the traumatic event. Chronic nightmares contribute to sleep disturbances, exacerbating PTSD symptoms. Trauma reminders, which can be both internal (thoughts, memories) and external (places, sensory experiences), can provoke severe distress and physiologic reactions.
Avoidance symptoms often develop as a coping mechanism in response to distressing re-experiencing symptoms. Children may avoid thoughts, feelings, and memories of the traumatic event or people, places, and activities associated with the trauma. In young children, avoidance may manifest as restricted play or reduced exploration of their environment.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) outlines specific criteria for diagnosing PTSD in individuals over 6 years old, which includes exposure to actual or threatened death, serious injury, or sexual violence, and the presence of symptoms such as intrusion, avoidance, negative mood alterations, and heightened arousal. The DSM-5-TR provides tailored diagnostic criteria for developmental differences in symptom expression for children under 6.
Managing PTSD in children requires a patient-specific approach, with an emphasis on obtaining consent from both the patient and guardian. The American Academy of Child and Adolescent Psychiatry (AACAP) recommends psychotherapy as the first-line treatment for pediatric PTSD. However, patients with severe symptoms or comorbidities may initially be unable to engage in meaningful therapy and may require medication to stabilize symptoms before starting psychotherapy.
Trauma-focused psychotherapy, including cognitive-behavioral therapy (CBT), exposure-based therapy, and eye movement desensitization and reprocessing (EMDR) therapy, is the preferred treatment for PTSD. Clinical studies have shown that patients receiving trauma-focused psychotherapy experience more remarkable symptom improvement than those who do not receive treatment and, in children, psychotherapy generally yields better outcomes than pharmacotherapy.
While selective serotonin reuptake inhibitors like sertraline and paroxetine are FDA-approved for PTSD treatment in adults, their efficacy in children often produces outcomes similar to those of placebo. Medications are typically reserved for severe symptoms and are used as an off-label treatment in pediatric cases. Pharmacologic management may be necessary when the severity of symptoms prevents the use of trauma-focused psychotherapy or requires immediate stabilization.
Heidi Moawad, MD, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Heidi Moawad, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
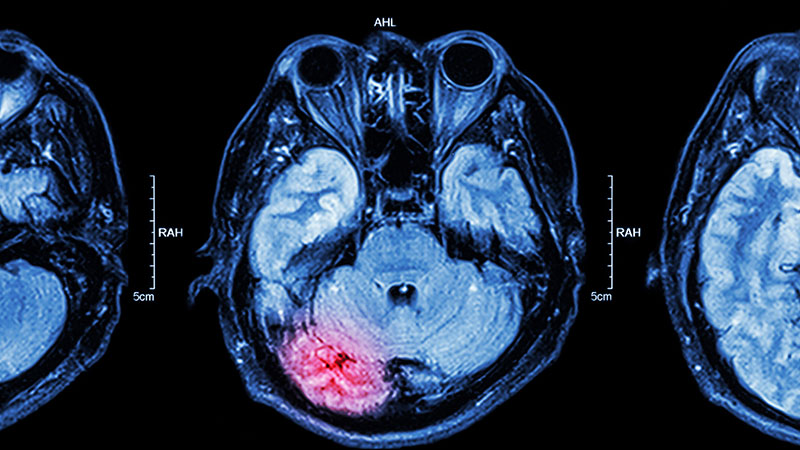
A 15-year-old girl presented to the emergency department with complaints of persistent headaches, nightmares, and difficulty concentrating in school over the past 3 months. The patient had recently experienced a traumatic event, a severe car accident in which a close friend was critically injured. Since the incident, the patient has been exhibiting increased irritability, avoidance of activities that she previously enjoyed, and a noticeable withdrawal from social interactions. Additionally, she reported recurrent flashbacks to the accident, often triggered by sounds resembling car engines. On physical examination, the patient appeared anxious and exhibited hypervigilance. An MRI of the brain was performed to rule out any organic causes of her symptoms, revealing an area of increased signal intensity in the left cerebellar hemisphere (as highlighted in the image).