User login
QI enthusiast to QI leader: Jonathan Bae, MD, SFHM
Editor’s Note: This SHM series highlights the professional pathways of quality improvement leaders. This month features the story of Jonathan Bae, MD, SFHM, associate chief medical officer for patient and clinical quality at Duke University Health System, Durham, N.C.
With a father and two sisters in medicine, Jonathan Bae was destined to become a physician – or something completely different, as he explains.
“Either outcome is common when you have a parent who is a doctor,” said Dr. Bae, who has two siblings who chose a different career path. But while Dr. Bae’s desire to be a clinician was set at an early age, his interest in quality improvement work came much later.
Twelve years ago, Dr. Bae matched in Duke’s Medicine-Pediatrics residency program because he wanted to be well equipped to treat patients across the age spectrum. Completing residency in 2009, Dr. Bae enjoyed providing clinical care as a hospitalist, but discovered that he also enjoyed teaching. To enhance his skills as a clinician educator, Dr. Bae enrolled in the Academic Hospitalist Academy, where the curriculum introduced him to quality improvement and patient safety, and some aspects of hospital administration. “Jeff Glasheen’s talk on the drivers of medicine, and how to find your unique voice and identity … brought together my interest in education and quality work,” Dr. Bae recalled.
“I left the meeting energized with new information, and then the opportunity came up to lead a QI initiative here,” he said. The project focused on improving improve care delivery to diabetic patients, specifically the completion of foot exams. “We saw our rates of screening go from less than 50% to greater than 80%,” Dr. Bae said. “I found it to be extremely gratifying to be involved in implementing changes that could lead to care improvement for patients.”
Once Dr. Bae made his interests in QI work known to colleagues and administrators, the projects came readily. Following his chief residency year, Dr. Bae remained with Duke Medicine Residency Program as an associate program director for QI, “which was a great platform for doing project work that aligned my interests in teaching and doing QI work,” he said. In addition to developing a residency curriculum in QI, Dr. Bae initiated a program to incentivize GME trainees across the health system in performance metrics such as readmissions, patient satisfaction, hand hygiene, and safety event reporting. The outcomes, Dr. Bae said, “have had an improved quality and safety impact on our organization.”
From there, Dr. Bae initiated multiple projects focused on reducing readmissions and mortality. Currently, he is standardizing the mortality review process across three hospitals in Duke’s health system. Consistent methodology and language will allow for more accurate analysis and comparison of factors contributing to patient mortality in the system, Dr. Bae said, adding, “We have already learned a lot about care delivery and operations, and measures that can be taken to reduce gaps in care delivery and keep patients safe.”
Looking back on the days when he only thought about providing care, Dr. Bae said, “my world view has changed but my desire to change the world hasn’t. I now do more quality work because I find it so gratifying. In the QI space, I’m affecting not one, but many people at a time.”
He encourages hospitalists with similar interests to seek out colleagues and leaders – internal and external to their institutions – that will help them initiate and implement projects that feed their passions. Getting to know the QI basics is the simple part, Dr. Bae said.
“There’s no magic behind PDSA cycles or models of improvement,” he said. “It’s the team and people you pull together that makes a project successful.”
His current work centers on understanding and building health care provider resiliency at Duke. “I feel this … is going to make a tremendous difference for our organization,” Dr. Bae said. “The system should be designed to promote well-being, not just prevent burnout.”
Claudia Stahl is content manager at the Society of Hospital Medicine.
Editor’s Note: This SHM series highlights the professional pathways of quality improvement leaders. This month features the story of Jonathan Bae, MD, SFHM, associate chief medical officer for patient and clinical quality at Duke University Health System, Durham, N.C.
With a father and two sisters in medicine, Jonathan Bae was destined to become a physician – or something completely different, as he explains.
“Either outcome is common when you have a parent who is a doctor,” said Dr. Bae, who has two siblings who chose a different career path. But while Dr. Bae’s desire to be a clinician was set at an early age, his interest in quality improvement work came much later.
Twelve years ago, Dr. Bae matched in Duke’s Medicine-Pediatrics residency program because he wanted to be well equipped to treat patients across the age spectrum. Completing residency in 2009, Dr. Bae enjoyed providing clinical care as a hospitalist, but discovered that he also enjoyed teaching. To enhance his skills as a clinician educator, Dr. Bae enrolled in the Academic Hospitalist Academy, where the curriculum introduced him to quality improvement and patient safety, and some aspects of hospital administration. “Jeff Glasheen’s talk on the drivers of medicine, and how to find your unique voice and identity … brought together my interest in education and quality work,” Dr. Bae recalled.
“I left the meeting energized with new information, and then the opportunity came up to lead a QI initiative here,” he said. The project focused on improving improve care delivery to diabetic patients, specifically the completion of foot exams. “We saw our rates of screening go from less than 50% to greater than 80%,” Dr. Bae said. “I found it to be extremely gratifying to be involved in implementing changes that could lead to care improvement for patients.”
Once Dr. Bae made his interests in QI work known to colleagues and administrators, the projects came readily. Following his chief residency year, Dr. Bae remained with Duke Medicine Residency Program as an associate program director for QI, “which was a great platform for doing project work that aligned my interests in teaching and doing QI work,” he said. In addition to developing a residency curriculum in QI, Dr. Bae initiated a program to incentivize GME trainees across the health system in performance metrics such as readmissions, patient satisfaction, hand hygiene, and safety event reporting. The outcomes, Dr. Bae said, “have had an improved quality and safety impact on our organization.”
From there, Dr. Bae initiated multiple projects focused on reducing readmissions and mortality. Currently, he is standardizing the mortality review process across three hospitals in Duke’s health system. Consistent methodology and language will allow for more accurate analysis and comparison of factors contributing to patient mortality in the system, Dr. Bae said, adding, “We have already learned a lot about care delivery and operations, and measures that can be taken to reduce gaps in care delivery and keep patients safe.”
Looking back on the days when he only thought about providing care, Dr. Bae said, “my world view has changed but my desire to change the world hasn’t. I now do more quality work because I find it so gratifying. In the QI space, I’m affecting not one, but many people at a time.”
He encourages hospitalists with similar interests to seek out colleagues and leaders – internal and external to their institutions – that will help them initiate and implement projects that feed their passions. Getting to know the QI basics is the simple part, Dr. Bae said.
“There’s no magic behind PDSA cycles or models of improvement,” he said. “It’s the team and people you pull together that makes a project successful.”
His current work centers on understanding and building health care provider resiliency at Duke. “I feel this … is going to make a tremendous difference for our organization,” Dr. Bae said. “The system should be designed to promote well-being, not just prevent burnout.”
Claudia Stahl is content manager at the Society of Hospital Medicine.
Editor’s Note: This SHM series highlights the professional pathways of quality improvement leaders. This month features the story of Jonathan Bae, MD, SFHM, associate chief medical officer for patient and clinical quality at Duke University Health System, Durham, N.C.
With a father and two sisters in medicine, Jonathan Bae was destined to become a physician – or something completely different, as he explains.
“Either outcome is common when you have a parent who is a doctor,” said Dr. Bae, who has two siblings who chose a different career path. But while Dr. Bae’s desire to be a clinician was set at an early age, his interest in quality improvement work came much later.
Twelve years ago, Dr. Bae matched in Duke’s Medicine-Pediatrics residency program because he wanted to be well equipped to treat patients across the age spectrum. Completing residency in 2009, Dr. Bae enjoyed providing clinical care as a hospitalist, but discovered that he also enjoyed teaching. To enhance his skills as a clinician educator, Dr. Bae enrolled in the Academic Hospitalist Academy, where the curriculum introduced him to quality improvement and patient safety, and some aspects of hospital administration. “Jeff Glasheen’s talk on the drivers of medicine, and how to find your unique voice and identity … brought together my interest in education and quality work,” Dr. Bae recalled.
“I left the meeting energized with new information, and then the opportunity came up to lead a QI initiative here,” he said. The project focused on improving improve care delivery to diabetic patients, specifically the completion of foot exams. “We saw our rates of screening go from less than 50% to greater than 80%,” Dr. Bae said. “I found it to be extremely gratifying to be involved in implementing changes that could lead to care improvement for patients.”
Once Dr. Bae made his interests in QI work known to colleagues and administrators, the projects came readily. Following his chief residency year, Dr. Bae remained with Duke Medicine Residency Program as an associate program director for QI, “which was a great platform for doing project work that aligned my interests in teaching and doing QI work,” he said. In addition to developing a residency curriculum in QI, Dr. Bae initiated a program to incentivize GME trainees across the health system in performance metrics such as readmissions, patient satisfaction, hand hygiene, and safety event reporting. The outcomes, Dr. Bae said, “have had an improved quality and safety impact on our organization.”
From there, Dr. Bae initiated multiple projects focused on reducing readmissions and mortality. Currently, he is standardizing the mortality review process across three hospitals in Duke’s health system. Consistent methodology and language will allow for more accurate analysis and comparison of factors contributing to patient mortality in the system, Dr. Bae said, adding, “We have already learned a lot about care delivery and operations, and measures that can be taken to reduce gaps in care delivery and keep patients safe.”
Looking back on the days when he only thought about providing care, Dr. Bae said, “my world view has changed but my desire to change the world hasn’t. I now do more quality work because I find it so gratifying. In the QI space, I’m affecting not one, but many people at a time.”
He encourages hospitalists with similar interests to seek out colleagues and leaders – internal and external to their institutions – that will help them initiate and implement projects that feed their passions. Getting to know the QI basics is the simple part, Dr. Bae said.
“There’s no magic behind PDSA cycles or models of improvement,” he said. “It’s the team and people you pull together that makes a project successful.”
His current work centers on understanding and building health care provider resiliency at Duke. “I feel this … is going to make a tremendous difference for our organization,” Dr. Bae said. “The system should be designed to promote well-being, not just prevent burnout.”
Claudia Stahl is content manager at the Society of Hospital Medicine.
RANGE: Small PFS edge in urothelial cancer with VEGFR-2 inhibitor
MADRID – For patients with advanced or metastatic urothelial cancer that progressed on platinum-based chemotherapy, a combination of the vascular endothelial growth factor receptor (VEGFR)-2 inhibitor ramucirumab and docetaxel offered a small but significant improvement in progression-free survival (PFS) compared with docetaxel alone.
Among 437 patients treated in the phase 3 RANGE trial, investigator-assessed PFS, the primary endpoint, was 4.07 months for patients randomized to ramucirumab and docetaxel, compared with 2.76 months for patients assigned to docetaxel and placebo, translating into a hazard ratio (HR) for ramucirumab of 0.757 (P = .0118).
“RANGE is the first phase 3 study to demonstrate a progression-free survival advantage over chemotherapy alone in platinum-refractory advanced or metastatic urothelial carcinoma,” lead author Daniel P. Petrylak, MD of Yale University, New Haven, Conn., said at a briefing at the European Society for Medical Oncology Congress.
But Richard Cathomas, Dr.med, of Kantonsspital Graubünden, in Chur, Switzerland, who was not involved in the study, said it “is too early for these results alone to change the standard of care second-line treatment, which is immune checkpoint inhibition.”
He noted that the magnitude of the benefit with ramucirumab was just 1.3 months, “and, while statistically significant, it raises the question of whether it is clinically relevant.”
He acknowledged that the improvement in response rates seen with ramucirumab in RANGE indicates a potential role for angiogenesis inhibitors in the treatment of urothelial cancers.
In the RANGE trial, results of which were published in The Lancet, investigators at 124 sites in 23 countries enrolled 530 patients with advanced or metastatic urothelial carcinoma who experienced progression during or after platinum-based chemotherapy.
Patients were randomly assigned to receive intravenous docetaxel 75 mg/m2 plus intravenous ramucirumab 10 mg/kg or matching placebo on day 1 of each repeating 21-day cycle until disease progression or discontinuation of treatment for other reasons. The primary endpoint was assessed in an intention-to-treat analysis in the first 437 patients randomized.
As noted, at a median follow-up in the ITT analysis of 5.0 months, investigator-assessed PFS favored the addition of ramucirumab, as did a blinded central review of outcomes, which determined a median PFS of 4.04 months with ramucirumab plus docetaxel compared with 2.46 months for docetaxel alone (HR 0.672, P = .0005).
Ramucirumab/docetaxel was also associated with an improvement in the objective response rate, at 24.5% compared with 14.0% for placebo/docetaxel.
Treatment toxicities were similar between the study arms, although patients in the ramucirumab arm experienced slightly less anemia compared with those in the placebo arm.
In all, 209 of 263 patients assigned to ramucirumab/docetaxel, and 229 of 267 patients assigned to placebo/docetaxel had their treatments discontinued, primarily because they experienced disease progression.
There were no significant changes in quality of life measures during the trial, and there were no differences in quality of life between the treatment arms, Dr. Petrylak reported.
Ultimately, the combination of ramucirumab and docetaxel may be best suited for use as a third-line treatment for patients with platinum-resistant metastatic urothelial cancer that has progressed after second-line therapy with an immune checkpoint inhibitor, said Yohann Loriot, MD, of Gustave-Roussy Cancer Institute in Villejuif, France, the invited discussant at the presidential symposium where the RANGE data were presented.
However, for patients who have a short response to first-line platinum-based chemotherapy, have no visceral metastases, or are ineligible to receive immunotherapy, ramucirumab plus docetaxel could be a suitable second-line option, he said.
The RANGE trial was funded by Eli Lilly. Dr. Petrylak disclosed research funding from the company. Dr. Cathomas and Dr. Loriot disclosed consulting or advisory roles with companies other than Lilly.
MADRID – For patients with advanced or metastatic urothelial cancer that progressed on platinum-based chemotherapy, a combination of the vascular endothelial growth factor receptor (VEGFR)-2 inhibitor ramucirumab and docetaxel offered a small but significant improvement in progression-free survival (PFS) compared with docetaxel alone.
Among 437 patients treated in the phase 3 RANGE trial, investigator-assessed PFS, the primary endpoint, was 4.07 months for patients randomized to ramucirumab and docetaxel, compared with 2.76 months for patients assigned to docetaxel and placebo, translating into a hazard ratio (HR) for ramucirumab of 0.757 (P = .0118).
“RANGE is the first phase 3 study to demonstrate a progression-free survival advantage over chemotherapy alone in platinum-refractory advanced or metastatic urothelial carcinoma,” lead author Daniel P. Petrylak, MD of Yale University, New Haven, Conn., said at a briefing at the European Society for Medical Oncology Congress.
But Richard Cathomas, Dr.med, of Kantonsspital Graubünden, in Chur, Switzerland, who was not involved in the study, said it “is too early for these results alone to change the standard of care second-line treatment, which is immune checkpoint inhibition.”
He noted that the magnitude of the benefit with ramucirumab was just 1.3 months, “and, while statistically significant, it raises the question of whether it is clinically relevant.”
He acknowledged that the improvement in response rates seen with ramucirumab in RANGE indicates a potential role for angiogenesis inhibitors in the treatment of urothelial cancers.
In the RANGE trial, results of which were published in The Lancet, investigators at 124 sites in 23 countries enrolled 530 patients with advanced or metastatic urothelial carcinoma who experienced progression during or after platinum-based chemotherapy.
Patients were randomly assigned to receive intravenous docetaxel 75 mg/m2 plus intravenous ramucirumab 10 mg/kg or matching placebo on day 1 of each repeating 21-day cycle until disease progression or discontinuation of treatment for other reasons. The primary endpoint was assessed in an intention-to-treat analysis in the first 437 patients randomized.
As noted, at a median follow-up in the ITT analysis of 5.0 months, investigator-assessed PFS favored the addition of ramucirumab, as did a blinded central review of outcomes, which determined a median PFS of 4.04 months with ramucirumab plus docetaxel compared with 2.46 months for docetaxel alone (HR 0.672, P = .0005).
Ramucirumab/docetaxel was also associated with an improvement in the objective response rate, at 24.5% compared with 14.0% for placebo/docetaxel.
Treatment toxicities were similar between the study arms, although patients in the ramucirumab arm experienced slightly less anemia compared with those in the placebo arm.
In all, 209 of 263 patients assigned to ramucirumab/docetaxel, and 229 of 267 patients assigned to placebo/docetaxel had their treatments discontinued, primarily because they experienced disease progression.
There were no significant changes in quality of life measures during the trial, and there were no differences in quality of life between the treatment arms, Dr. Petrylak reported.
Ultimately, the combination of ramucirumab and docetaxel may be best suited for use as a third-line treatment for patients with platinum-resistant metastatic urothelial cancer that has progressed after second-line therapy with an immune checkpoint inhibitor, said Yohann Loriot, MD, of Gustave-Roussy Cancer Institute in Villejuif, France, the invited discussant at the presidential symposium where the RANGE data were presented.
However, for patients who have a short response to first-line platinum-based chemotherapy, have no visceral metastases, or are ineligible to receive immunotherapy, ramucirumab plus docetaxel could be a suitable second-line option, he said.
The RANGE trial was funded by Eli Lilly. Dr. Petrylak disclosed research funding from the company. Dr. Cathomas and Dr. Loriot disclosed consulting or advisory roles with companies other than Lilly.
MADRID – For patients with advanced or metastatic urothelial cancer that progressed on platinum-based chemotherapy, a combination of the vascular endothelial growth factor receptor (VEGFR)-2 inhibitor ramucirumab and docetaxel offered a small but significant improvement in progression-free survival (PFS) compared with docetaxel alone.
Among 437 patients treated in the phase 3 RANGE trial, investigator-assessed PFS, the primary endpoint, was 4.07 months for patients randomized to ramucirumab and docetaxel, compared with 2.76 months for patients assigned to docetaxel and placebo, translating into a hazard ratio (HR) for ramucirumab of 0.757 (P = .0118).
“RANGE is the first phase 3 study to demonstrate a progression-free survival advantage over chemotherapy alone in platinum-refractory advanced or metastatic urothelial carcinoma,” lead author Daniel P. Petrylak, MD of Yale University, New Haven, Conn., said at a briefing at the European Society for Medical Oncology Congress.
But Richard Cathomas, Dr.med, of Kantonsspital Graubünden, in Chur, Switzerland, who was not involved in the study, said it “is too early for these results alone to change the standard of care second-line treatment, which is immune checkpoint inhibition.”
He noted that the magnitude of the benefit with ramucirumab was just 1.3 months, “and, while statistically significant, it raises the question of whether it is clinically relevant.”
He acknowledged that the improvement in response rates seen with ramucirumab in RANGE indicates a potential role for angiogenesis inhibitors in the treatment of urothelial cancers.
In the RANGE trial, results of which were published in The Lancet, investigators at 124 sites in 23 countries enrolled 530 patients with advanced or metastatic urothelial carcinoma who experienced progression during or after platinum-based chemotherapy.
Patients were randomly assigned to receive intravenous docetaxel 75 mg/m2 plus intravenous ramucirumab 10 mg/kg or matching placebo on day 1 of each repeating 21-day cycle until disease progression or discontinuation of treatment for other reasons. The primary endpoint was assessed in an intention-to-treat analysis in the first 437 patients randomized.
As noted, at a median follow-up in the ITT analysis of 5.0 months, investigator-assessed PFS favored the addition of ramucirumab, as did a blinded central review of outcomes, which determined a median PFS of 4.04 months with ramucirumab plus docetaxel compared with 2.46 months for docetaxel alone (HR 0.672, P = .0005).
Ramucirumab/docetaxel was also associated with an improvement in the objective response rate, at 24.5% compared with 14.0% for placebo/docetaxel.
Treatment toxicities were similar between the study arms, although patients in the ramucirumab arm experienced slightly less anemia compared with those in the placebo arm.
In all, 209 of 263 patients assigned to ramucirumab/docetaxel, and 229 of 267 patients assigned to placebo/docetaxel had their treatments discontinued, primarily because they experienced disease progression.
There were no significant changes in quality of life measures during the trial, and there were no differences in quality of life between the treatment arms, Dr. Petrylak reported.
Ultimately, the combination of ramucirumab and docetaxel may be best suited for use as a third-line treatment for patients with platinum-resistant metastatic urothelial cancer that has progressed after second-line therapy with an immune checkpoint inhibitor, said Yohann Loriot, MD, of Gustave-Roussy Cancer Institute in Villejuif, France, the invited discussant at the presidential symposium where the RANGE data were presented.
However, for patients who have a short response to first-line platinum-based chemotherapy, have no visceral metastases, or are ineligible to receive immunotherapy, ramucirumab plus docetaxel could be a suitable second-line option, he said.
The RANGE trial was funded by Eli Lilly. Dr. Petrylak disclosed research funding from the company. Dr. Cathomas and Dr. Loriot disclosed consulting or advisory roles with companies other than Lilly.
AT ESMO 2017
Key clinical point: Adding the VEGFR-2 inhibitor ramucirumab to docetaxel slightly improved progression-free survival of advanced/metastatic urothelial carcinoma.
Major finding: Investigator-assessed PFS was 4.07 months for patients randomized to ramucirumab and docetaxel, vs. 2.76 months for docetaxel/placebo.
Data source: Randomized phase 3 trial of 530 patients with urothelial carcinoma that progressed during or after platinum-based chemotherapy.
Disclosures: The RANGE trial was funded by Eli Lilly. Dr. Petrylak disclosed research funding from the company. Dr. Cathomas and Dr. Loriot disclosed consulting or advisory roles with companies other than Lilly.
Do not withhold opioid addiction drugs from patients taking benzodiazepines
The opioid addiction medications buprenorphine and methadone should not be withheld from patients who are taking benzodiazepines or other drugs that depress the central nervous system, the Food and Drug Administration advised in a safety alert posted Sept. 20.
The combined use of these medication-assisted treatment (MAT) drugs and central nervous system (CNS) depressants can lead to serious side effects. But the harm associated with opioid addiction that remains untreated usually outweighs those risks, the agency said. After reviewing this issue, the FDA said, it is requiring that this information be added to the drug labels of buprenorphine and methadone in addition to “recommendations for minimizing the use of [MAT] drugs and benzodiazepines together.”
Discontinuing MAT drugs is a goal, but patients might need medication-assisted treatment indefinitely. “Use should continue for as long as patients are benefiting and their use contributes to the intended treatment goals,” the alert said.
The opioid addiction medications buprenorphine and methadone should not be withheld from patients who are taking benzodiazepines or other drugs that depress the central nervous system, the Food and Drug Administration advised in a safety alert posted Sept. 20.
The combined use of these medication-assisted treatment (MAT) drugs and central nervous system (CNS) depressants can lead to serious side effects. But the harm associated with opioid addiction that remains untreated usually outweighs those risks, the agency said. After reviewing this issue, the FDA said, it is requiring that this information be added to the drug labels of buprenorphine and methadone in addition to “recommendations for minimizing the use of [MAT] drugs and benzodiazepines together.”
Discontinuing MAT drugs is a goal, but patients might need medication-assisted treatment indefinitely. “Use should continue for as long as patients are benefiting and their use contributes to the intended treatment goals,” the alert said.
The opioid addiction medications buprenorphine and methadone should not be withheld from patients who are taking benzodiazepines or other drugs that depress the central nervous system, the Food and Drug Administration advised in a safety alert posted Sept. 20.
The combined use of these medication-assisted treatment (MAT) drugs and central nervous system (CNS) depressants can lead to serious side effects. But the harm associated with opioid addiction that remains untreated usually outweighs those risks, the agency said. After reviewing this issue, the FDA said, it is requiring that this information be added to the drug labels of buprenorphine and methadone in addition to “recommendations for minimizing the use of [MAT] drugs and benzodiazepines together.”
Discontinuing MAT drugs is a goal, but patients might need medication-assisted treatment indefinitely. “Use should continue for as long as patients are benefiting and their use contributes to the intended treatment goals,” the alert said.
Preferred osteoporosis treatment order with teriparatide, denosumab reaffirmed
DENVER – A treatment regimen for postmenopausal women with osteoporosis that started with teriparatide (TPTD) for 2 years and switched to denosumab (DMAB) improved their spine trabecular microarchitecture – a predictor of fracture risk independent of areal bone mineral density – in a new analysis of the DATA trial and its extension study, DATA-Switch.
On the other hand, the converse strategy of switching from denosumab to teriparatide resulted in a temporary decline in bone mineral density. “The observed transient decrease in bone density corresponds to extremely elevated bone formation and resorptions markers, suggesting that high bone turnover is a cause of the transient loss,” Joy Tsai, MD, an instructor in medicine at Massachusetts General Hospital, Boston, said in an interview at a poster session of the annual meeting of the American Society for Bone and Mineral Research.
“Our take-home is that for patients who are at extremely high risk of fracture, combination strategy is a treatment strategy to be considered,” said Dr. Tsai, who presented the results of the study at the meeting.
The findings also reinforce the general strategy of treating with anabolic therapy followed by an antiresorptive agent, rather than the other way around.
Specifically, “we would caution against the use of teriparatide immediately following denosumab because of this transient decrease in bone density that correlates with high bone turnover,” Dr. Tsai said.
The DATA and DATA-Switch studies randomized 94 postmenopausal women with osteoporosis to 2 years of TPTD (20 mcg/day), DMAB (60 mg every 6 months), or both drugs for 2 years. In DATA-Switch, women who received TPTD in the first 2 years were switched to DMAB, and those receiving DMAB were switched to TPTD. Women in the combination group were switched to DMAB only.
The researchers used dual-energy x-ray absorptiometry (DXA) spine scans to assess spine trabecular microarchitecture by calculating trabecular bone score (TBS) at 0, 12, 24, 30, 36, and 48 months.
After 2 years, TPTD alone was associated with a 2.7% increase in TBS over baseline (P = .009), while DMAB alone was associated with a 1.8% increase (P = .118 vs. baseline). Combination treatment led to a 4.5% increase (P = .017 vs. baseline).
In the 6 months after the treatment switch at year 2, the researchers noted increases in TBS in the combination-to-DMAB group (2.1%) and the TPTD-to-DMAB group (2.0%), but the DMAB-to-TPTD group experienced a decrease of 1.1% over months 24-30 (P less than .05 compared with other groups).
The decrease in TBS was temporary: At 48 months, all groups had an overall increase in TBS (TPTD-to-DMAB group, 5.1%; DMAB-to-TPTD group, 3.6%; combination-to-DMAB group, 6.1%). There were no significant differences between the groups.
From baseline to month 48, the percentage of patients considered to be at high risk of fracture based on TBS score (1.23 or less) dropped from 24% to 8% in the TPTD-to-DMAB group, from 18% to 14% in the DMAB-to-TPTD group, and from 39% to 11% in the combination-to-DMAB group.
“Ultimately, at the 4-year mark all three groups increased trabecular bone scores, so it also supports our rationale to consider the use of these treatment strategies, specifically the ones when you’re switching to or continuing denosumab,” Dr. Tsai said.
Amgen and Eli Lilly funded the study. Dr. Tsai reported having no financial disclosures.
DENVER – A treatment regimen for postmenopausal women with osteoporosis that started with teriparatide (TPTD) for 2 years and switched to denosumab (DMAB) improved their spine trabecular microarchitecture – a predictor of fracture risk independent of areal bone mineral density – in a new analysis of the DATA trial and its extension study, DATA-Switch.
On the other hand, the converse strategy of switching from denosumab to teriparatide resulted in a temporary decline in bone mineral density. “The observed transient decrease in bone density corresponds to extremely elevated bone formation and resorptions markers, suggesting that high bone turnover is a cause of the transient loss,” Joy Tsai, MD, an instructor in medicine at Massachusetts General Hospital, Boston, said in an interview at a poster session of the annual meeting of the American Society for Bone and Mineral Research.
“Our take-home is that for patients who are at extremely high risk of fracture, combination strategy is a treatment strategy to be considered,” said Dr. Tsai, who presented the results of the study at the meeting.
The findings also reinforce the general strategy of treating with anabolic therapy followed by an antiresorptive agent, rather than the other way around.
Specifically, “we would caution against the use of teriparatide immediately following denosumab because of this transient decrease in bone density that correlates with high bone turnover,” Dr. Tsai said.
The DATA and DATA-Switch studies randomized 94 postmenopausal women with osteoporosis to 2 years of TPTD (20 mcg/day), DMAB (60 mg every 6 months), or both drugs for 2 years. In DATA-Switch, women who received TPTD in the first 2 years were switched to DMAB, and those receiving DMAB were switched to TPTD. Women in the combination group were switched to DMAB only.
The researchers used dual-energy x-ray absorptiometry (DXA) spine scans to assess spine trabecular microarchitecture by calculating trabecular bone score (TBS) at 0, 12, 24, 30, 36, and 48 months.
After 2 years, TPTD alone was associated with a 2.7% increase in TBS over baseline (P = .009), while DMAB alone was associated with a 1.8% increase (P = .118 vs. baseline). Combination treatment led to a 4.5% increase (P = .017 vs. baseline).
In the 6 months after the treatment switch at year 2, the researchers noted increases in TBS in the combination-to-DMAB group (2.1%) and the TPTD-to-DMAB group (2.0%), but the DMAB-to-TPTD group experienced a decrease of 1.1% over months 24-30 (P less than .05 compared with other groups).
The decrease in TBS was temporary: At 48 months, all groups had an overall increase in TBS (TPTD-to-DMAB group, 5.1%; DMAB-to-TPTD group, 3.6%; combination-to-DMAB group, 6.1%). There were no significant differences between the groups.
From baseline to month 48, the percentage of patients considered to be at high risk of fracture based on TBS score (1.23 or less) dropped from 24% to 8% in the TPTD-to-DMAB group, from 18% to 14% in the DMAB-to-TPTD group, and from 39% to 11% in the combination-to-DMAB group.
“Ultimately, at the 4-year mark all three groups increased trabecular bone scores, so it also supports our rationale to consider the use of these treatment strategies, specifically the ones when you’re switching to or continuing denosumab,” Dr. Tsai said.
Amgen and Eli Lilly funded the study. Dr. Tsai reported having no financial disclosures.
DENVER – A treatment regimen for postmenopausal women with osteoporosis that started with teriparatide (TPTD) for 2 years and switched to denosumab (DMAB) improved their spine trabecular microarchitecture – a predictor of fracture risk independent of areal bone mineral density – in a new analysis of the DATA trial and its extension study, DATA-Switch.
On the other hand, the converse strategy of switching from denosumab to teriparatide resulted in a temporary decline in bone mineral density. “The observed transient decrease in bone density corresponds to extremely elevated bone formation and resorptions markers, suggesting that high bone turnover is a cause of the transient loss,” Joy Tsai, MD, an instructor in medicine at Massachusetts General Hospital, Boston, said in an interview at a poster session of the annual meeting of the American Society for Bone and Mineral Research.
“Our take-home is that for patients who are at extremely high risk of fracture, combination strategy is a treatment strategy to be considered,” said Dr. Tsai, who presented the results of the study at the meeting.
The findings also reinforce the general strategy of treating with anabolic therapy followed by an antiresorptive agent, rather than the other way around.
Specifically, “we would caution against the use of teriparatide immediately following denosumab because of this transient decrease in bone density that correlates with high bone turnover,” Dr. Tsai said.
The DATA and DATA-Switch studies randomized 94 postmenopausal women with osteoporosis to 2 years of TPTD (20 mcg/day), DMAB (60 mg every 6 months), or both drugs for 2 years. In DATA-Switch, women who received TPTD in the first 2 years were switched to DMAB, and those receiving DMAB were switched to TPTD. Women in the combination group were switched to DMAB only.
The researchers used dual-energy x-ray absorptiometry (DXA) spine scans to assess spine trabecular microarchitecture by calculating trabecular bone score (TBS) at 0, 12, 24, 30, 36, and 48 months.
After 2 years, TPTD alone was associated with a 2.7% increase in TBS over baseline (P = .009), while DMAB alone was associated with a 1.8% increase (P = .118 vs. baseline). Combination treatment led to a 4.5% increase (P = .017 vs. baseline).
In the 6 months after the treatment switch at year 2, the researchers noted increases in TBS in the combination-to-DMAB group (2.1%) and the TPTD-to-DMAB group (2.0%), but the DMAB-to-TPTD group experienced a decrease of 1.1% over months 24-30 (P less than .05 compared with other groups).
The decrease in TBS was temporary: At 48 months, all groups had an overall increase in TBS (TPTD-to-DMAB group, 5.1%; DMAB-to-TPTD group, 3.6%; combination-to-DMAB group, 6.1%). There were no significant differences between the groups.
From baseline to month 48, the percentage of patients considered to be at high risk of fracture based on TBS score (1.23 or less) dropped from 24% to 8% in the TPTD-to-DMAB group, from 18% to 14% in the DMAB-to-TPTD group, and from 39% to 11% in the combination-to-DMAB group.
“Ultimately, at the 4-year mark all three groups increased trabecular bone scores, so it also supports our rationale to consider the use of these treatment strategies, specifically the ones when you’re switching to or continuing denosumab,” Dr. Tsai said.
Amgen and Eli Lilly funded the study. Dr. Tsai reported having no financial disclosures.
AT ASBMR
Key clinical point:
Major finding: After 24 months, teriparatide alone increased trabecular bone score (TBS) by 2.7%, denosumab (DMAB) alone by 1.8%, and the combination by 4.5%.
Data source: A new analysis of data from 94 postmenopausal women with osteoporosis who participated in the DATA trial and its extension study, DATA-Switch.
Disclosures: Amgen and Eli Lilly funded the study. Dr. Tsai reported having no financial disclosures.
Flotetuzumab for AML passes phase 1 test
MADRID – Flotetuzumab, a novel bispecific monoclonal antibody that employs a proprietary technology to redirect T lymphocytes to kill CD123-expressing cells, was safe and demonstrated efficacy in patients with acute myeloid leukemia in a phase 1 trial, based on data presented at the European Society for Medical Oncology (ESMO) Congress.
Flotetuzumab combines a portion of antibody recognizing CD3, which is an activating molecule expressed by T cells, and an arm that recognizes CD123 on the cancer cell, explained Norbert Vey, MD, PhD, Head, Leukemia Treatment Unit, Institut Paoli-Calmettes, Marseille, France. This bispecific quality, produced through a proprietary technology called Dual-Affinity ReTargeting, is considered promising because CD123 is expressed by more than 90% of AML cells and is highly expressed on stem cells involved in initiating myelodysplastic syndrome (MDS).
In this dose-ranging study (NCT02152956), 42 patients with AML and 5 patients with MDS were treated in the first cycle with infusions of flotetuzumab on either a continuous 7-day or a 4-days-on, 3-days-off schedule. For subsequent cycles, patients received the 4-days-on, 3-days-off schedule. Continuous infusion is employed due to the short half-life of flotetuzumab.
The most common adverse events were infusion-related reactions, which were observed in 76.6% of patients. Pyrexia, a potential sign of cytokine release syndrome (CRS), was observed in 23.4% of patients, who were given tocilizumab at the earliest sign of CRS. Two patients had grade 3 CRS, and one discontinued therapy. There was also one case of grade 3 myalgia. Dr. Vey characterized the overall level of adverse events as “acceptable.”
In addition to its relative safety, flotetuzumab was associated with “encouraging antileukemic activity,” Dr. Vey said. Six of 14 patients receiving doses that exceeded 500 ng/kg per day had objective responses and two of these patients had complete responses. Again, toxicity at these dose levels was considered manageable.
“This rate of clinical response is exciting,” commented Tim Somervaille, MD, Senior Group Leader, Leukemia Biology Laboratory, Cancer Research UK Institute, Manchester (England). An ESMO-invited discussant on this paper, Dr. Somervaille expressed enthusiasm in general about a growing role for bispecific T-cell engagers. Blinatumomab, the first of these agents, received regulatory approval for refractory AML in 2014.
“There are a number of these bispecific T cell antibodies that are in early-phase trials,” said Dr. Somervaille, citing several that also target CD123 within the context of a different partner antigen than that employed by flotetuzumab. He also mentioned ongoing efforts to develop bispecific natural killer cell engagers that target malignant cells through immune activation.
As for flotetuzumab, the phase 1 trial provided adequate data to encourage further development.
“A cohort expansion is now ongoing and enrolling patients at 11 sites in the United States and Europe,” Dr. Vey reported. “A clinical update on these results is expected by the end of the year.”
Dr. Vey reported financial relationships with Bristol-Myers Squibb, Novartis, and Servier.
MacroGenics retains full development and commercialization rights to flotetuzumab in the United States, Canada, Mexico, Japan, South Korea, and India. Servier participates in the development and has rights to flotetuzumab in all other countries. The U.S. Food and Drug Administration has granted orphan drug designation to flotetuzumab for the investigational treatment of AML, according to a press release from MacroGenics.
MADRID – Flotetuzumab, a novel bispecific monoclonal antibody that employs a proprietary technology to redirect T lymphocytes to kill CD123-expressing cells, was safe and demonstrated efficacy in patients with acute myeloid leukemia in a phase 1 trial, based on data presented at the European Society for Medical Oncology (ESMO) Congress.
Flotetuzumab combines a portion of antibody recognizing CD3, which is an activating molecule expressed by T cells, and an arm that recognizes CD123 on the cancer cell, explained Norbert Vey, MD, PhD, Head, Leukemia Treatment Unit, Institut Paoli-Calmettes, Marseille, France. This bispecific quality, produced through a proprietary technology called Dual-Affinity ReTargeting, is considered promising because CD123 is expressed by more than 90% of AML cells and is highly expressed on stem cells involved in initiating myelodysplastic syndrome (MDS).
In this dose-ranging study (NCT02152956), 42 patients with AML and 5 patients with MDS were treated in the first cycle with infusions of flotetuzumab on either a continuous 7-day or a 4-days-on, 3-days-off schedule. For subsequent cycles, patients received the 4-days-on, 3-days-off schedule. Continuous infusion is employed due to the short half-life of flotetuzumab.
The most common adverse events were infusion-related reactions, which were observed in 76.6% of patients. Pyrexia, a potential sign of cytokine release syndrome (CRS), was observed in 23.4% of patients, who were given tocilizumab at the earliest sign of CRS. Two patients had grade 3 CRS, and one discontinued therapy. There was also one case of grade 3 myalgia. Dr. Vey characterized the overall level of adverse events as “acceptable.”
In addition to its relative safety, flotetuzumab was associated with “encouraging antileukemic activity,” Dr. Vey said. Six of 14 patients receiving doses that exceeded 500 ng/kg per day had objective responses and two of these patients had complete responses. Again, toxicity at these dose levels was considered manageable.
“This rate of clinical response is exciting,” commented Tim Somervaille, MD, Senior Group Leader, Leukemia Biology Laboratory, Cancer Research UK Institute, Manchester (England). An ESMO-invited discussant on this paper, Dr. Somervaille expressed enthusiasm in general about a growing role for bispecific T-cell engagers. Blinatumomab, the first of these agents, received regulatory approval for refractory AML in 2014.
“There are a number of these bispecific T cell antibodies that are in early-phase trials,” said Dr. Somervaille, citing several that also target CD123 within the context of a different partner antigen than that employed by flotetuzumab. He also mentioned ongoing efforts to develop bispecific natural killer cell engagers that target malignant cells through immune activation.
As for flotetuzumab, the phase 1 trial provided adequate data to encourage further development.
“A cohort expansion is now ongoing and enrolling patients at 11 sites in the United States and Europe,” Dr. Vey reported. “A clinical update on these results is expected by the end of the year.”
Dr. Vey reported financial relationships with Bristol-Myers Squibb, Novartis, and Servier.
MacroGenics retains full development and commercialization rights to flotetuzumab in the United States, Canada, Mexico, Japan, South Korea, and India. Servier participates in the development and has rights to flotetuzumab in all other countries. The U.S. Food and Drug Administration has granted orphan drug designation to flotetuzumab for the investigational treatment of AML, according to a press release from MacroGenics.
MADRID – Flotetuzumab, a novel bispecific monoclonal antibody that employs a proprietary technology to redirect T lymphocytes to kill CD123-expressing cells, was safe and demonstrated efficacy in patients with acute myeloid leukemia in a phase 1 trial, based on data presented at the European Society for Medical Oncology (ESMO) Congress.
Flotetuzumab combines a portion of antibody recognizing CD3, which is an activating molecule expressed by T cells, and an arm that recognizes CD123 on the cancer cell, explained Norbert Vey, MD, PhD, Head, Leukemia Treatment Unit, Institut Paoli-Calmettes, Marseille, France. This bispecific quality, produced through a proprietary technology called Dual-Affinity ReTargeting, is considered promising because CD123 is expressed by more than 90% of AML cells and is highly expressed on stem cells involved in initiating myelodysplastic syndrome (MDS).
In this dose-ranging study (NCT02152956), 42 patients with AML and 5 patients with MDS were treated in the first cycle with infusions of flotetuzumab on either a continuous 7-day or a 4-days-on, 3-days-off schedule. For subsequent cycles, patients received the 4-days-on, 3-days-off schedule. Continuous infusion is employed due to the short half-life of flotetuzumab.
The most common adverse events were infusion-related reactions, which were observed in 76.6% of patients. Pyrexia, a potential sign of cytokine release syndrome (CRS), was observed in 23.4% of patients, who were given tocilizumab at the earliest sign of CRS. Two patients had grade 3 CRS, and one discontinued therapy. There was also one case of grade 3 myalgia. Dr. Vey characterized the overall level of adverse events as “acceptable.”
In addition to its relative safety, flotetuzumab was associated with “encouraging antileukemic activity,” Dr. Vey said. Six of 14 patients receiving doses that exceeded 500 ng/kg per day had objective responses and two of these patients had complete responses. Again, toxicity at these dose levels was considered manageable.
“This rate of clinical response is exciting,” commented Tim Somervaille, MD, Senior Group Leader, Leukemia Biology Laboratory, Cancer Research UK Institute, Manchester (England). An ESMO-invited discussant on this paper, Dr. Somervaille expressed enthusiasm in general about a growing role for bispecific T-cell engagers. Blinatumomab, the first of these agents, received regulatory approval for refractory AML in 2014.
“There are a number of these bispecific T cell antibodies that are in early-phase trials,” said Dr. Somervaille, citing several that also target CD123 within the context of a different partner antigen than that employed by flotetuzumab. He also mentioned ongoing efforts to develop bispecific natural killer cell engagers that target malignant cells through immune activation.
As for flotetuzumab, the phase 1 trial provided adequate data to encourage further development.
“A cohort expansion is now ongoing and enrolling patients at 11 sites in the United States and Europe,” Dr. Vey reported. “A clinical update on these results is expected by the end of the year.”
Dr. Vey reported financial relationships with Bristol-Myers Squibb, Novartis, and Servier.
MacroGenics retains full development and commercialization rights to flotetuzumab in the United States, Canada, Mexico, Japan, South Korea, and India. Servier participates in the development and has rights to flotetuzumab in all other countries. The U.S. Food and Drug Administration has granted orphan drug designation to flotetuzumab for the investigational treatment of AML, according to a press release from MacroGenics.
AT ESMO 2017
Key clinical point: The phase 1 trial provided adequate data to encourage further development of flotetuzumab for patients with acute myeloid leukemia.
Major finding: Six of 14 patients receiving doses that exceeded 500 ng/kg per day had objective responses and two of these patients had complete responses.
Data source: Phase 1 dose-escalation study in 42 patients with AML and 5 patients with MDS.
Disclosures: Dr. Vey reported financial relationships with Bristol-Myers Squibb, Novartis, and Servier.
AGA releases new clinical guideline on therapeutic drug monitoring in IBD
AGA has issued a new clinical guideline on the role of therapeutic drug monitoring (TDM) in the management of IBD, published in the September 2017 issue of Gastroenterology. The guideline focuses on the application of TDM for biologic therapy, specifically anti–tumor necrosis factor-alpha (TNF) agents and thiopurines, and addresses questions about the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes. AGA’s recommendations include:
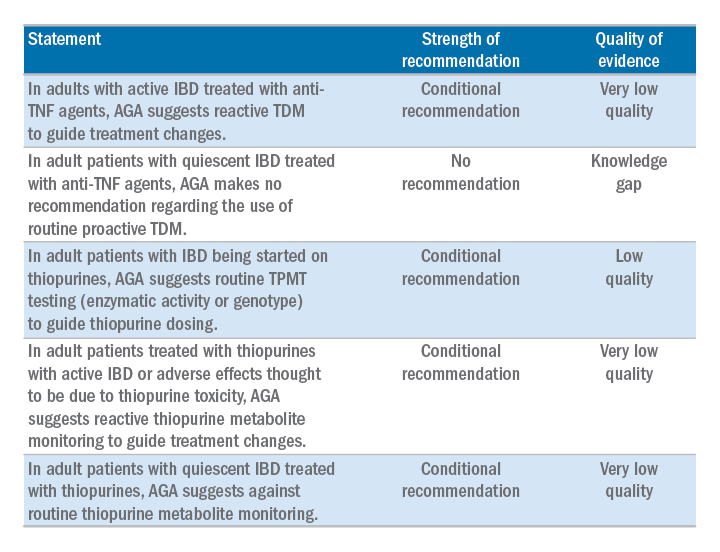
The guideline is accompanied by a technical review, Clinical Decision Support Tool, and patient companion, which provides key points and important information directly to patients about this approach, written at an appropriate reading level. Access the patient companion in the Patient Info Center, www.gastro.org/IBD.
AGA has issued a new clinical guideline on the role of therapeutic drug monitoring (TDM) in the management of IBD, published in the September 2017 issue of Gastroenterology. The guideline focuses on the application of TDM for biologic therapy, specifically anti–tumor necrosis factor-alpha (TNF) agents and thiopurines, and addresses questions about the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes. AGA’s recommendations include:

The guideline is accompanied by a technical review, Clinical Decision Support Tool, and patient companion, which provides key points and important information directly to patients about this approach, written at an appropriate reading level. Access the patient companion in the Patient Info Center, www.gastro.org/IBD.
AGA has issued a new clinical guideline on the role of therapeutic drug monitoring (TDM) in the management of IBD, published in the September 2017 issue of Gastroenterology. The guideline focuses on the application of TDM for biologic therapy, specifically anti–tumor necrosis factor-alpha (TNF) agents and thiopurines, and addresses questions about the risks and benefits of reactive TDM, routine proactive TDM, or no TDM in guiding treatment changes. AGA’s recommendations include:

The guideline is accompanied by a technical review, Clinical Decision Support Tool, and patient companion, which provides key points and important information directly to patients about this approach, written at an appropriate reading level. Access the patient companion in the Patient Info Center, www.gastro.org/IBD.
Make a difference – support AGA’s Research Awards program
Many breakthroughs have been achieved through gastroenterological and hepatological research over the past century, forming the basis of the modern medical practice. As the charitable arm of the American Gastroenterological Association (AGA), the AGA Research Foundation contributes to this tradition of discovery by providing a key source of funding at a critical juncture in a young researcher’s career.
“The Research Scholar Award will have a pivotal effect on my future career,” said Michael Dougan, MD, PhD, Massachusetts General Hospital, Boston, 2017 Research Scholar Award recipient. “This award enables me to establish my own research infrastructure, and lay the experimental foundations for my future work as a clinician‐scientist striving to understand the complex interplay between the immune system, metabolism, and cancer.”
By joining others in donating to the AGA Research Foundation, you will help to foster a new pipeline of scientists – the next generation of leaders in GI.
Make a tax-deductible donation and help us keep the best and brightest investigators working in gastroenterology and hepatology. Donate at www.gastro.org/dontateonline or by mail to 4930 Del Ray Avenue, Bethesda, MD 20814.
Many breakthroughs have been achieved through gastroenterological and hepatological research over the past century, forming the basis of the modern medical practice. As the charitable arm of the American Gastroenterological Association (AGA), the AGA Research Foundation contributes to this tradition of discovery by providing a key source of funding at a critical juncture in a young researcher’s career.
“The Research Scholar Award will have a pivotal effect on my future career,” said Michael Dougan, MD, PhD, Massachusetts General Hospital, Boston, 2017 Research Scholar Award recipient. “This award enables me to establish my own research infrastructure, and lay the experimental foundations for my future work as a clinician‐scientist striving to understand the complex interplay between the immune system, metabolism, and cancer.”
By joining others in donating to the AGA Research Foundation, you will help to foster a new pipeline of scientists – the next generation of leaders in GI.
Make a tax-deductible donation and help us keep the best and brightest investigators working in gastroenterology and hepatology. Donate at www.gastro.org/dontateonline or by mail to 4930 Del Ray Avenue, Bethesda, MD 20814.
Many breakthroughs have been achieved through gastroenterological and hepatological research over the past century, forming the basis of the modern medical practice. As the charitable arm of the American Gastroenterological Association (AGA), the AGA Research Foundation contributes to this tradition of discovery by providing a key source of funding at a critical juncture in a young researcher’s career.
“The Research Scholar Award will have a pivotal effect on my future career,” said Michael Dougan, MD, PhD, Massachusetts General Hospital, Boston, 2017 Research Scholar Award recipient. “This award enables me to establish my own research infrastructure, and lay the experimental foundations for my future work as a clinician‐scientist striving to understand the complex interplay between the immune system, metabolism, and cancer.”
By joining others in donating to the AGA Research Foundation, you will help to foster a new pipeline of scientists – the next generation of leaders in GI.
Make a tax-deductible donation and help us keep the best and brightest investigators working in gastroenterology and hepatology. Donate at www.gastro.org/dontateonline or by mail to 4930 Del Ray Avenue, Bethesda, MD 20814.
Pediatric Dermatology Consult - August 2017
BY AYAN KUSARI AND CATALINA MATIZ, MD
The patient was diagnosed with eruptive vellus hair cysts (EVHC). Treatment with a keratolytic, such as 12% lactic acid cream, was recommended. Hydrocortisone 2.5% once daily as needed also was recommended to treat the patient’s itch.
EVHC are benign middermal cysts characterized by epidermoid keratinization of the cyst wall, as well as lamellar keratin and vellus hairs within the cyst.1 The term “eruptive vellus hair cysts” was first used to describe a longstanding hyperpigmented, monomorphous papular eruption in two children by Esterly, Fretzin, and Pinkus in 1977.2 Clinically, EVHC present as 1- to 3-mm follicular, dome-shaped papules that are often skin-colored but also have been described as being brown, gray, green or black colored.3,4 They appear suddenly and sometimes are associated with mild tenderness and pruritus.1,5 EVHC most commonly present on the anterior chest but also can present on the upper and lower extremities, face, neck, axillae, and buttocks.4
Furthermore, although spontaneous resolution is possible through transepidermal elimination of cyst products, cases may persist for years in the absence of treatment.1
Accurate diagnosis of eruptive vellus hair cysts is important to guide therapy.
Keratosis pilaris consists of follicular-based papules with variable erythema.4 It may be widespread – including over the anterior chest – but is most commonly seen on the cheeks, extensor surfaces of proximal upper extremities, and the anterior thighs.4 It is related to excessive keratinization, which leads to formation of horny plugs within hair-follicle orifices.1
Steatocystoma multiplex is typically characterized by firm, yellow-to-flesh–colored dermal cysts ranging from a few millimeters to 1 cm in size.1 They are sometimes clinically hard to distinguish from EVHC, and both are associated with keratin 17 gene mutations and type 2 pachyonychia congenita.1 Nonetheless, this patient’s lesions did not have any features – such as size or drainage – that would point toward steatocystoma multiplex or other skin findings suggestive of pachyonychia congenita.
Superficial folliculitis, also known as Bockhart’s impetigo, is an infection of the follicular ostium and typically presents with perifollicular pustules on an erythematous base that may be painful or pruritic and can occur throughout the corpus, including the anterior trunk.1
Acne vulgaris is a very common disease that involves the pilosebaceous unit and occurs most frequently on the face, back, upper arms, and chest. However, it is characterized by open and closed comedones, papules, and pustules and, in severe cases, nodules and cysts that may leave postinflammatory hyperpigmentation and scarring. Tiny, hyperpigmented, dome-shaped macules occurring exclusively on the chest would not be characteristic.
Patients with hypohidrotic ectodermal dysplasia, also known as Christ-Siemens-Touraine syndrome, can present with EVHC. This condition is characterized by a triad of fair, sparse short hair; hyperthermia related to decreased sweating; and missing teeth.4 Although EVHC have been reported in association with hypohidrotic ectodermal dysplasia, this patient does not have any of the dysmorphic features associated with this syndrome.11
Patients with pachyonychia congenita (type 2) also may have EVHC as part of their presentation, but this patient does not have nail dystrophy, focal palmoplantar keratoderma, follicular keratoses, or multiple steatocysts which also are features of this condition.4
Treatment may be offered to patients who are distressed by the lesions or seek cosmesis. A 2012 review of 220 cases of EVHC found that topical retinoic acid, incision/excision, CO2 laser, erbium:yttrium-aluminum-garnet laser, needle evacuation, dermabrasion, and 10% urea cream were each associated with successful treatment in multiple cases.3
Forty years have passed since EVHC was identified as a distinct disease entity. Despite this, eruptive vellus hair cysts remains somewhat understudied, and further research is needed to determine an ideal treatment algorithm for patients with this condition. Our approach was to attempt noninvasive keratolytic therapy before considering retinoids or surgical options; we also recommended steroid treatment for symptom relief. Providers should keep EVHC in the differential for eruptions consisting of tiny papules so that appropriate treatment may be offered.
Dr. Matiz is a pediatric dermatologist at Rady Children’s Hospital, San Diego, and an assistant clinical professor in the department of pediatric and adolescent dermatology at the University of California, San Diego. Mr. Kusari is a medical student at the University of California, San Diego. Dr. Matiz and Mr. Kusari said they had no relevant financial disclosures.
Email them at pdnews@frontlinemedcom.com.
References
1. “Dermatology.” 3rd ed. (Philadelphia: Saunders, 2012).
2. Arch Dermatol. 1977 Apr;113(4):500-3.
3. Am J Clin Dermatol. 2012 Feb 1;13(1):19-28.
5. Indian Dermatol Online J. 2013 Jul;4(3):213-5.
6. J Am Acad Dermatol. 1980 Oct;3(4):425-9.
7. Am J Dermatopathol. 1997 Jun;19(3):250-3.
8. Hum Mol Genet. 1998 Jul;7(7):1143-8.
9. Dermatology. 1998;196(4):392-6.
BY AYAN KUSARI AND CATALINA MATIZ, MD
The patient was diagnosed with eruptive vellus hair cysts (EVHC). Treatment with a keratolytic, such as 12% lactic acid cream, was recommended. Hydrocortisone 2.5% once daily as needed also was recommended to treat the patient’s itch.
EVHC are benign middermal cysts characterized by epidermoid keratinization of the cyst wall, as well as lamellar keratin and vellus hairs within the cyst.1 The term “eruptive vellus hair cysts” was first used to describe a longstanding hyperpigmented, monomorphous papular eruption in two children by Esterly, Fretzin, and Pinkus in 1977.2 Clinically, EVHC present as 1- to 3-mm follicular, dome-shaped papules that are often skin-colored but also have been described as being brown, gray, green or black colored.3,4 They appear suddenly and sometimes are associated with mild tenderness and pruritus.1,5 EVHC most commonly present on the anterior chest but also can present on the upper and lower extremities, face, neck, axillae, and buttocks.4
Furthermore, although spontaneous resolution is possible through transepidermal elimination of cyst products, cases may persist for years in the absence of treatment.1
Accurate diagnosis of eruptive vellus hair cysts is important to guide therapy.
Keratosis pilaris consists of follicular-based papules with variable erythema.4 It may be widespread – including over the anterior chest – but is most commonly seen on the cheeks, extensor surfaces of proximal upper extremities, and the anterior thighs.4 It is related to excessive keratinization, which leads to formation of horny plugs within hair-follicle orifices.1
Steatocystoma multiplex is typically characterized by firm, yellow-to-flesh–colored dermal cysts ranging from a few millimeters to 1 cm in size.1 They are sometimes clinically hard to distinguish from EVHC, and both are associated with keratin 17 gene mutations and type 2 pachyonychia congenita.1 Nonetheless, this patient’s lesions did not have any features – such as size or drainage – that would point toward steatocystoma multiplex or other skin findings suggestive of pachyonychia congenita.
Superficial folliculitis, also known as Bockhart’s impetigo, is an infection of the follicular ostium and typically presents with perifollicular pustules on an erythematous base that may be painful or pruritic and can occur throughout the corpus, including the anterior trunk.1
Acne vulgaris is a very common disease that involves the pilosebaceous unit and occurs most frequently on the face, back, upper arms, and chest. However, it is characterized by open and closed comedones, papules, and pustules and, in severe cases, nodules and cysts that may leave postinflammatory hyperpigmentation and scarring. Tiny, hyperpigmented, dome-shaped macules occurring exclusively on the chest would not be characteristic.
Patients with hypohidrotic ectodermal dysplasia, also known as Christ-Siemens-Touraine syndrome, can present with EVHC. This condition is characterized by a triad of fair, sparse short hair; hyperthermia related to decreased sweating; and missing teeth.4 Although EVHC have been reported in association with hypohidrotic ectodermal dysplasia, this patient does not have any of the dysmorphic features associated with this syndrome.11
Patients with pachyonychia congenita (type 2) also may have EVHC as part of their presentation, but this patient does not have nail dystrophy, focal palmoplantar keratoderma, follicular keratoses, or multiple steatocysts which also are features of this condition.4
Treatment may be offered to patients who are distressed by the lesions or seek cosmesis. A 2012 review of 220 cases of EVHC found that topical retinoic acid, incision/excision, CO2 laser, erbium:yttrium-aluminum-garnet laser, needle evacuation, dermabrasion, and 10% urea cream were each associated with successful treatment in multiple cases.3
Forty years have passed since EVHC was identified as a distinct disease entity. Despite this, eruptive vellus hair cysts remains somewhat understudied, and further research is needed to determine an ideal treatment algorithm for patients with this condition. Our approach was to attempt noninvasive keratolytic therapy before considering retinoids or surgical options; we also recommended steroid treatment for symptom relief. Providers should keep EVHC in the differential for eruptions consisting of tiny papules so that appropriate treatment may be offered.
Dr. Matiz is a pediatric dermatologist at Rady Children’s Hospital, San Diego, and an assistant clinical professor in the department of pediatric and adolescent dermatology at the University of California, San Diego. Mr. Kusari is a medical student at the University of California, San Diego. Dr. Matiz and Mr. Kusari said they had no relevant financial disclosures.
Email them at pdnews@frontlinemedcom.com.
References
1. “Dermatology.” 3rd ed. (Philadelphia: Saunders, 2012).
2. Arch Dermatol. 1977 Apr;113(4):500-3.
3. Am J Clin Dermatol. 2012 Feb 1;13(1):19-28.
5. Indian Dermatol Online J. 2013 Jul;4(3):213-5.
6. J Am Acad Dermatol. 1980 Oct;3(4):425-9.
7. Am J Dermatopathol. 1997 Jun;19(3):250-3.
8. Hum Mol Genet. 1998 Jul;7(7):1143-8.
9. Dermatology. 1998;196(4):392-6.
BY AYAN KUSARI AND CATALINA MATIZ, MD
The patient was diagnosed with eruptive vellus hair cysts (EVHC). Treatment with a keratolytic, such as 12% lactic acid cream, was recommended. Hydrocortisone 2.5% once daily as needed also was recommended to treat the patient’s itch.
EVHC are benign middermal cysts characterized by epidermoid keratinization of the cyst wall, as well as lamellar keratin and vellus hairs within the cyst.1 The term “eruptive vellus hair cysts” was first used to describe a longstanding hyperpigmented, monomorphous papular eruption in two children by Esterly, Fretzin, and Pinkus in 1977.2 Clinically, EVHC present as 1- to 3-mm follicular, dome-shaped papules that are often skin-colored but also have been described as being brown, gray, green or black colored.3,4 They appear suddenly and sometimes are associated with mild tenderness and pruritus.1,5 EVHC most commonly present on the anterior chest but also can present on the upper and lower extremities, face, neck, axillae, and buttocks.4
Furthermore, although spontaneous resolution is possible through transepidermal elimination of cyst products, cases may persist for years in the absence of treatment.1
Accurate diagnosis of eruptive vellus hair cysts is important to guide therapy.
Keratosis pilaris consists of follicular-based papules with variable erythema.4 It may be widespread – including over the anterior chest – but is most commonly seen on the cheeks, extensor surfaces of proximal upper extremities, and the anterior thighs.4 It is related to excessive keratinization, which leads to formation of horny plugs within hair-follicle orifices.1
Steatocystoma multiplex is typically characterized by firm, yellow-to-flesh–colored dermal cysts ranging from a few millimeters to 1 cm in size.1 They are sometimes clinically hard to distinguish from EVHC, and both are associated with keratin 17 gene mutations and type 2 pachyonychia congenita.1 Nonetheless, this patient’s lesions did not have any features – such as size or drainage – that would point toward steatocystoma multiplex or other skin findings suggestive of pachyonychia congenita.
Superficial folliculitis, also known as Bockhart’s impetigo, is an infection of the follicular ostium and typically presents with perifollicular pustules on an erythematous base that may be painful or pruritic and can occur throughout the corpus, including the anterior trunk.1
Acne vulgaris is a very common disease that involves the pilosebaceous unit and occurs most frequently on the face, back, upper arms, and chest. However, it is characterized by open and closed comedones, papules, and pustules and, in severe cases, nodules and cysts that may leave postinflammatory hyperpigmentation and scarring. Tiny, hyperpigmented, dome-shaped macules occurring exclusively on the chest would not be characteristic.
Patients with hypohidrotic ectodermal dysplasia, also known as Christ-Siemens-Touraine syndrome, can present with EVHC. This condition is characterized by a triad of fair, sparse short hair; hyperthermia related to decreased sweating; and missing teeth.4 Although EVHC have been reported in association with hypohidrotic ectodermal dysplasia, this patient does not have any of the dysmorphic features associated with this syndrome.11
Patients with pachyonychia congenita (type 2) also may have EVHC as part of their presentation, but this patient does not have nail dystrophy, focal palmoplantar keratoderma, follicular keratoses, or multiple steatocysts which also are features of this condition.4
Treatment may be offered to patients who are distressed by the lesions or seek cosmesis. A 2012 review of 220 cases of EVHC found that topical retinoic acid, incision/excision, CO2 laser, erbium:yttrium-aluminum-garnet laser, needle evacuation, dermabrasion, and 10% urea cream were each associated with successful treatment in multiple cases.3
Forty years have passed since EVHC was identified as a distinct disease entity. Despite this, eruptive vellus hair cysts remains somewhat understudied, and further research is needed to determine an ideal treatment algorithm for patients with this condition. Our approach was to attempt noninvasive keratolytic therapy before considering retinoids or surgical options; we also recommended steroid treatment for symptom relief. Providers should keep EVHC in the differential for eruptions consisting of tiny papules so that appropriate treatment may be offered.
Dr. Matiz is a pediatric dermatologist at Rady Children’s Hospital, San Diego, and an assistant clinical professor in the department of pediatric and adolescent dermatology at the University of California, San Diego. Mr. Kusari is a medical student at the University of California, San Diego. Dr. Matiz and Mr. Kusari said they had no relevant financial disclosures.
Email them at pdnews@frontlinemedcom.com.
References
1. “Dermatology.” 3rd ed. (Philadelphia: Saunders, 2012).
2. Arch Dermatol. 1977 Apr;113(4):500-3.
3. Am J Clin Dermatol. 2012 Feb 1;13(1):19-28.
5. Indian Dermatol Online J. 2013 Jul;4(3):213-5.
6. J Am Acad Dermatol. 1980 Oct;3(4):425-9.
7. Am J Dermatopathol. 1997 Jun;19(3):250-3.
8. Hum Mol Genet. 1998 Jul;7(7):1143-8.
9. Dermatology. 1998;196(4):392-6.
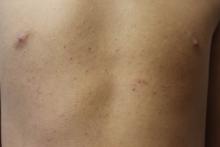
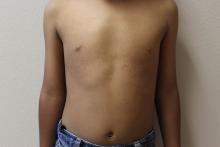
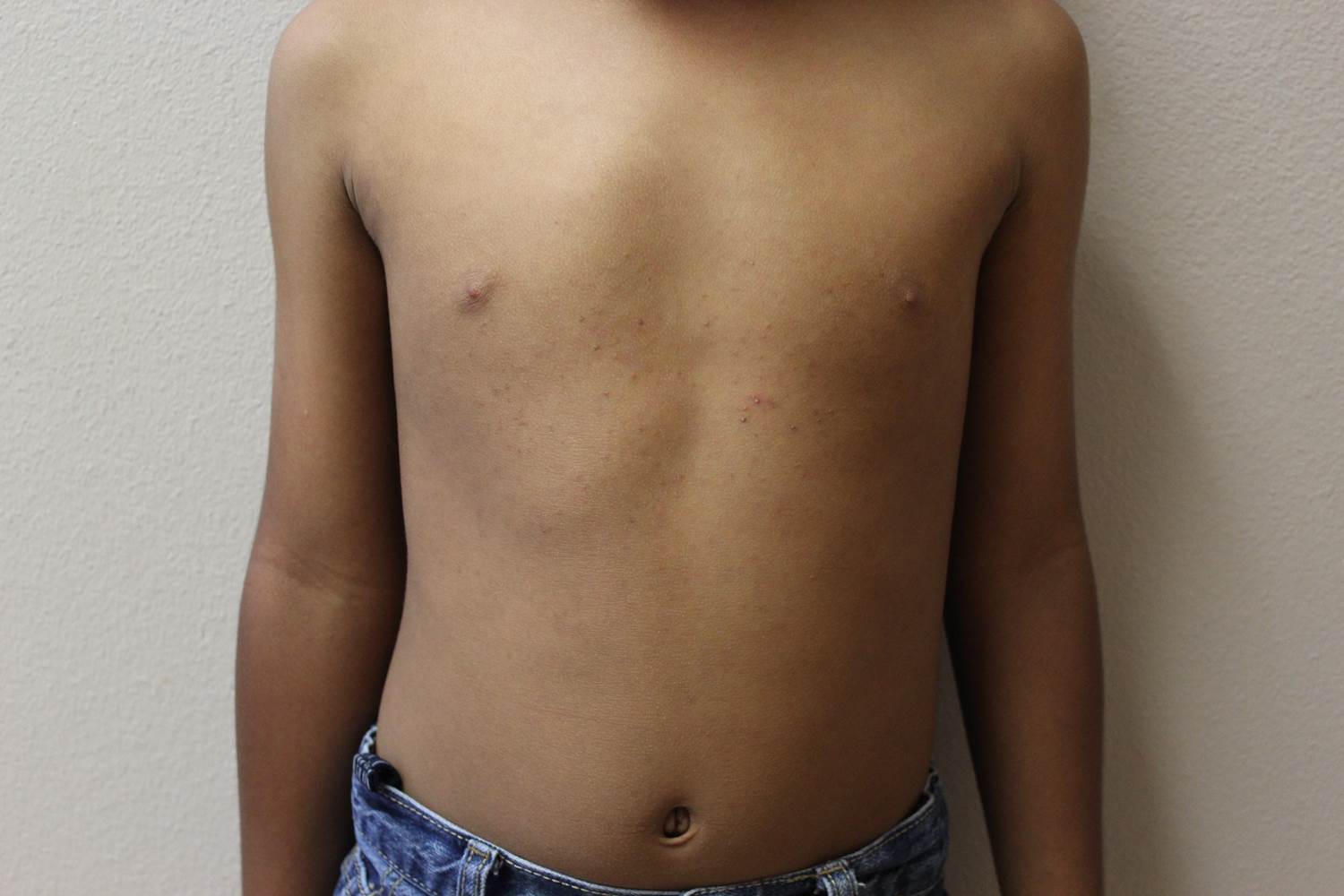
A 6-year-old boy presents with bumps on his chest and lower abdomen that have been present for 6 months. The patient’s mother states that the bumps are occasionally pruritic but not painful. She reports that the bumps first appeared on the chest and subsequently spread downward to involve the upper abdomen.
The patient is otherwise healthy. No similar lesions are present beyond the trunk. The patient’s past medical history and developmental history are unremarkable aside from bilateral amblyopia and high myopia. The patient’s mother denies any other family members with similar lesions. There is no history of teeth or nail abnormalities.
On exam, you find symmetrically distributed, firm, nontender, tiny 1- to 2-mm hyperpigmented dome-shaped papules on the anterior chest with no similar lesions elsewhere on the body. The remainder of the physical exam discloses no abnormalities.
CMS releases some good news for ASCs
CMS released the Medicare Inpatient Prospective Payment System (IPPS) final rule, which affects hospital payments and includes provisions for ambulatory surgery centers (ASCs) and physician payments.
Thanks to the AGA members who submitted comments to the proposed rule, CMS withdrew plans to publicly post facility accreditation reviews and correction plans. Below is a summary of AGA’s position and where CMS landed on each issue.
1. Public display of final accreditation surveys and plans of correction.
Summary of AGA position – AGA urged CMS to withdraw its proposal making ASC accreditation surveys open to the public. To support shared transparency objectives, AGA recommended that if CMS were to finalize its proposal, the agency should first develop standards and a framework that considers both violation severity and scope.
CMS final rule – After consideration of the public comments received, CMS will not make ASC accreditation surveys open to the public. CMS was concerned that the suggestion to have accrediting organizations post their survey reports would appear as if it was attempting to circumvent current law, which prohibits CMS from disclosing survey reports or compelling the accrediting organizations to disclose the reports themselves.
2. EHR Incentive Program certification requirements for payment year 2018.
Summary of AGA position – AGA supported increased flexibility for 2018 and urged CMS to allow use of EHR technology certified to the 2014 software edition OR the 2015 software edition for the 2018 EHR Incentive Program.
CMS final rule – CMS will allow health care providers to use either 2014 or 2015 CEHRT or a combination of 2014 and 2015 CEHRT for the 2018 EHR Incentive Program.
3. Exception for ASC-based physicians under the EHR Incentive Program for payment years 2017 and 2018.
Summary of AGA position – AGA encouraged CMS to define ASC-based as a physician or other eligible professional who provides more than 50% of Medicare billed services in an ASC. AGA was concerned that implementing a higher threshold would leave certain physicians exposed to payment penalties, because the meaningful use requirement is set at 50% or more.
CMS final rule – Unfortunately, CMS set the definition of “ASC-based” as those who provide 75% of all services in an ASC, based on previous statutory definitions.
Policy changes are effective on Oct. 1, 2017, and changes to the 2017 and 2018 EHR Incentive Program apply immediately to the 2015 and 2016 reporting period, and provide relief that will impact 2017 and 2018 payments.
CMS released the Medicare Inpatient Prospective Payment System (IPPS) final rule, which affects hospital payments and includes provisions for ambulatory surgery centers (ASCs) and physician payments.
Thanks to the AGA members who submitted comments to the proposed rule, CMS withdrew plans to publicly post facility accreditation reviews and correction plans. Below is a summary of AGA’s position and where CMS landed on each issue.
1. Public display of final accreditation surveys and plans of correction.
Summary of AGA position – AGA urged CMS to withdraw its proposal making ASC accreditation surveys open to the public. To support shared transparency objectives, AGA recommended that if CMS were to finalize its proposal, the agency should first develop standards and a framework that considers both violation severity and scope.
CMS final rule – After consideration of the public comments received, CMS will not make ASC accreditation surveys open to the public. CMS was concerned that the suggestion to have accrediting organizations post their survey reports would appear as if it was attempting to circumvent current law, which prohibits CMS from disclosing survey reports or compelling the accrediting organizations to disclose the reports themselves.
2. EHR Incentive Program certification requirements for payment year 2018.
Summary of AGA position – AGA supported increased flexibility for 2018 and urged CMS to allow use of EHR technology certified to the 2014 software edition OR the 2015 software edition for the 2018 EHR Incentive Program.
CMS final rule – CMS will allow health care providers to use either 2014 or 2015 CEHRT or a combination of 2014 and 2015 CEHRT for the 2018 EHR Incentive Program.
3. Exception for ASC-based physicians under the EHR Incentive Program for payment years 2017 and 2018.
Summary of AGA position – AGA encouraged CMS to define ASC-based as a physician or other eligible professional who provides more than 50% of Medicare billed services in an ASC. AGA was concerned that implementing a higher threshold would leave certain physicians exposed to payment penalties, because the meaningful use requirement is set at 50% or more.
CMS final rule – Unfortunately, CMS set the definition of “ASC-based” as those who provide 75% of all services in an ASC, based on previous statutory definitions.
Policy changes are effective on Oct. 1, 2017, and changes to the 2017 and 2018 EHR Incentive Program apply immediately to the 2015 and 2016 reporting period, and provide relief that will impact 2017 and 2018 payments.
CMS released the Medicare Inpatient Prospective Payment System (IPPS) final rule, which affects hospital payments and includes provisions for ambulatory surgery centers (ASCs) and physician payments.
Thanks to the AGA members who submitted comments to the proposed rule, CMS withdrew plans to publicly post facility accreditation reviews and correction plans. Below is a summary of AGA’s position and where CMS landed on each issue.
1. Public display of final accreditation surveys and plans of correction.
Summary of AGA position – AGA urged CMS to withdraw its proposal making ASC accreditation surveys open to the public. To support shared transparency objectives, AGA recommended that if CMS were to finalize its proposal, the agency should first develop standards and a framework that considers both violation severity and scope.
CMS final rule – After consideration of the public comments received, CMS will not make ASC accreditation surveys open to the public. CMS was concerned that the suggestion to have accrediting organizations post their survey reports would appear as if it was attempting to circumvent current law, which prohibits CMS from disclosing survey reports or compelling the accrediting organizations to disclose the reports themselves.
2. EHR Incentive Program certification requirements for payment year 2018.
Summary of AGA position – AGA supported increased flexibility for 2018 and urged CMS to allow use of EHR technology certified to the 2014 software edition OR the 2015 software edition for the 2018 EHR Incentive Program.
CMS final rule – CMS will allow health care providers to use either 2014 or 2015 CEHRT or a combination of 2014 and 2015 CEHRT for the 2018 EHR Incentive Program.
3. Exception for ASC-based physicians under the EHR Incentive Program for payment years 2017 and 2018.
Summary of AGA position – AGA encouraged CMS to define ASC-based as a physician or other eligible professional who provides more than 50% of Medicare billed services in an ASC. AGA was concerned that implementing a higher threshold would leave certain physicians exposed to payment penalties, because the meaningful use requirement is set at 50% or more.
CMS final rule – Unfortunately, CMS set the definition of “ASC-based” as those who provide 75% of all services in an ASC, based on previous statutory definitions.
Policy changes are effective on Oct. 1, 2017, and changes to the 2017 and 2018 EHR Incentive Program apply immediately to the 2015 and 2016 reporting period, and provide relief that will impact 2017 and 2018 payments.
She's Losing It—Her Hair, That Is
Following the stressful divorce of her parents, this 8-year-old girl’s hair began to fall out, prompting her referral to dermatology. Along with the hair loss, she has mild itching and burning in the area.
The child has a history of several atopic phenomena, including seasonal allergies, asthma, and eczema—all of which are replete in her family’s history.
EXAMINATION
The child is in no distress and is quite willing to show the affected area—a sizeable (10 x 8 cm), roughly round area of complete hair loss involving the nuchal periphery of her scalp. Fortunately, the area is covered by longer hair that drapes down.
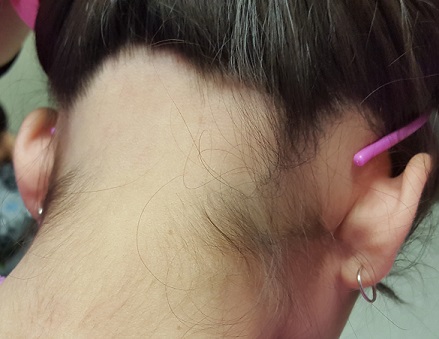
No epidermal changes (ie, redness, scaling, edema) are seen, and no nodes are palpable in the area. Her arms, brows, and lashes appear normal after careful examination.
What is the diagnosis?
Alopecia areata (AA) is quite common, especially among children, and affects both genders equally. It appears to be stress-related and can manifest in many forms. This particular type, with its distinguishing features of large size and peripheral involvement of the scalp margin, is known as ophiasis.
However, the real significance of ophiasis is its uncertain prognosis. For this patient, the extent and type of hair loss, her young age, and her atopic history all predict a poor prognosis. The hair loss is likely to be slow to resolve, if it does at all. It could progress to loss of all scalp hair (alopecia totalis) or every hair on her body (alopecia universalis).
Compounding the problem is the fact that no good treatment exists for this autoimmune disease, which affects those with genetic predisposition. Topical steroid application or intralesional steroid injections (3 to 5 mg/cc triamcinolone suspension) can promote the growth of a few hairs, but neither have an effect on the ultimate outcome. There have been reports of benefit from injectable biologics and oral antimalarials, but these medications have not been approved for use in AA.
Histopathologic studies with special stains show a lymphocytic infiltrate surrounding the hair follicle that prevents the growth of new hairs. Glucocorticoids (eg, prednisone) resolve this and allow new hair growth, but the treatment must be continued for months with unjustifiable adverse effects. Even then, full resolution must come on its own.
This patient was treated with a month-long course of topical triamcinolone 0.1% cream. But as stated above, her prognosis is somewhat guarded.
TAKE-HOME LEARNING POINTS
- Alopecia areata (AA) is an autoimmune process and a common cause of localized hair loss.
- This hair loss is usually acute, complete, and round, and is often seen in multiple patches.
- Of the many forms of AA, this one (ophiasis) has a less certain prognosis, worsened by youth, atopy, and lesion size.
- While ordinary AA resolves on its own in most cases, ophiasis can progress into total loss of scalp hair (alopecia totalis) or loss of all body hair (alopecia universalis).
Following the stressful divorce of her parents, this 8-year-old girl’s hair began to fall out, prompting her referral to dermatology. Along with the hair loss, she has mild itching and burning in the area.
The child has a history of several atopic phenomena, including seasonal allergies, asthma, and eczema—all of which are replete in her family’s history.
EXAMINATION
The child is in no distress and is quite willing to show the affected area—a sizeable (10 x 8 cm), roughly round area of complete hair loss involving the nuchal periphery of her scalp. Fortunately, the area is covered by longer hair that drapes down.

No epidermal changes (ie, redness, scaling, edema) are seen, and no nodes are palpable in the area. Her arms, brows, and lashes appear normal after careful examination.
What is the diagnosis?
Alopecia areata (AA) is quite common, especially among children, and affects both genders equally. It appears to be stress-related and can manifest in many forms. This particular type, with its distinguishing features of large size and peripheral involvement of the scalp margin, is known as ophiasis.
However, the real significance of ophiasis is its uncertain prognosis. For this patient, the extent and type of hair loss, her young age, and her atopic history all predict a poor prognosis. The hair loss is likely to be slow to resolve, if it does at all. It could progress to loss of all scalp hair (alopecia totalis) or every hair on her body (alopecia universalis).
Compounding the problem is the fact that no good treatment exists for this autoimmune disease, which affects those with genetic predisposition. Topical steroid application or intralesional steroid injections (3 to 5 mg/cc triamcinolone suspension) can promote the growth of a few hairs, but neither have an effect on the ultimate outcome. There have been reports of benefit from injectable biologics and oral antimalarials, but these medications have not been approved for use in AA.
Histopathologic studies with special stains show a lymphocytic infiltrate surrounding the hair follicle that prevents the growth of new hairs. Glucocorticoids (eg, prednisone) resolve this and allow new hair growth, but the treatment must be continued for months with unjustifiable adverse effects. Even then, full resolution must come on its own.
This patient was treated with a month-long course of topical triamcinolone 0.1% cream. But as stated above, her prognosis is somewhat guarded.
TAKE-HOME LEARNING POINTS
- Alopecia areata (AA) is an autoimmune process and a common cause of localized hair loss.
- This hair loss is usually acute, complete, and round, and is often seen in multiple patches.
- Of the many forms of AA, this one (ophiasis) has a less certain prognosis, worsened by youth, atopy, and lesion size.
- While ordinary AA resolves on its own in most cases, ophiasis can progress into total loss of scalp hair (alopecia totalis) or loss of all body hair (alopecia universalis).
Following the stressful divorce of her parents, this 8-year-old girl’s hair began to fall out, prompting her referral to dermatology. Along with the hair loss, she has mild itching and burning in the area.
The child has a history of several atopic phenomena, including seasonal allergies, asthma, and eczema—all of which are replete in her family’s history.
EXAMINATION
The child is in no distress and is quite willing to show the affected area—a sizeable (10 x 8 cm), roughly round area of complete hair loss involving the nuchal periphery of her scalp. Fortunately, the area is covered by longer hair that drapes down.

No epidermal changes (ie, redness, scaling, edema) are seen, and no nodes are palpable in the area. Her arms, brows, and lashes appear normal after careful examination.
What is the diagnosis?
Alopecia areata (AA) is quite common, especially among children, and affects both genders equally. It appears to be stress-related and can manifest in many forms. This particular type, with its distinguishing features of large size and peripheral involvement of the scalp margin, is known as ophiasis.
However, the real significance of ophiasis is its uncertain prognosis. For this patient, the extent and type of hair loss, her young age, and her atopic history all predict a poor prognosis. The hair loss is likely to be slow to resolve, if it does at all. It could progress to loss of all scalp hair (alopecia totalis) or every hair on her body (alopecia universalis).
Compounding the problem is the fact that no good treatment exists for this autoimmune disease, which affects those with genetic predisposition. Topical steroid application or intralesional steroid injections (3 to 5 mg/cc triamcinolone suspension) can promote the growth of a few hairs, but neither have an effect on the ultimate outcome. There have been reports of benefit from injectable biologics and oral antimalarials, but these medications have not been approved for use in AA.
Histopathologic studies with special stains show a lymphocytic infiltrate surrounding the hair follicle that prevents the growth of new hairs. Glucocorticoids (eg, prednisone) resolve this and allow new hair growth, but the treatment must be continued for months with unjustifiable adverse effects. Even then, full resolution must come on its own.
This patient was treated with a month-long course of topical triamcinolone 0.1% cream. But as stated above, her prognosis is somewhat guarded.
TAKE-HOME LEARNING POINTS
- Alopecia areata (AA) is an autoimmune process and a common cause of localized hair loss.
- This hair loss is usually acute, complete, and round, and is often seen in multiple patches.
- Of the many forms of AA, this one (ophiasis) has a less certain prognosis, worsened by youth, atopy, and lesion size.
- While ordinary AA resolves on its own in most cases, ophiasis can progress into total loss of scalp hair (alopecia totalis) or loss of all body hair (alopecia universalis).