User login
In the reemergence of typhus, the challenge is early diagnosis
SAN DIEGO – Typhus in many forms, particularly scrub typhus, has reemerged worldwide, but none of these rickettsial infections poses a significant public health threat if promptly diagnosed, according to an update presented at an annual scientific meeting on infectious diseases.
Scrub typhus, which is spread by several species of trombiculid mites or chiggers, poses a large threat in regard to typhus epidemics, particularly in Asia, but sporadic cases of different types of typhus are being seen everywhere, including in the United States, according to George M. Varghese, MD, department of infectious diseases, Christian Medical College, Vellore, India.
“The typhus diseases are clinically similar but epidemiologically and etiologically distinct,” reported Dr. Varghese, “but doxycycline is the drug of choice for almost all of the rickettsial infections.”
The bacteria responsible for scrub typhus is Orientia tsutsugamushi, which is no longer included in the genus Rickettsia, but Dr. Varghese, who has published frequently on the epidemiology of scrub typhus, said that it is still appropriately grouped among rickettsial infections. It shares many features with the other rickettsioses, which were considered to be fading but are now resurging after the large epidemics that occurred prior to the introduction of antibiotics.
In the World Wars, Rickettsia prowazekii – which is carried and spread by body lice – was the most well known typhus threat. According to Dr. Varghese, this bacterium may have killed more soldiers in these conflicts than did firearms. Although R. prowazekii has not disappeared as a source of typhus outbreaks, particularly in South America and Africa, there are current epidemics produced from rickettsial infections carried by fleas, such as R. typhi, or ticks, like R. rickettsii, or mice, like R. felis.
For clinical detection of these forms of typhus, there are differences. Although all are associated with a rapid onset of fever, headache, and myalgia, subtle signs can be helpful in making a diagnosis while waiting for laboratory confirmation. For example, scrub typhus, unlike Rocky Mountain Fever, which is caused by tick bites, does not generally include a rash. Rather, eschars, which are small patches of necrotic skin, are far more characteristic.
“Serological tests are the most common diagnostic tool for typhus, but serology may not allow early diagnosis. You can obtain a false positive in the early stages of disease,” Dr. Varghese warned. To speed the diagnosis, he said that looking for the clinical clues characteristic of the suspected form of typhus, such as the scrub typhus-associated eschar, “is valuable.” However, he also emphasized that even with positive serology results, “good epidemiology and history is helpful for laboratory interpretation.”
A variety of serological tests can identify typhus pathogens, but ELISA is now the most widely used, according to Dr. Varghese, noting that this test offers a sensitivity of 93% and a specificity of 91%. Both are higher than those provided by alternatives. As a result of improved sensitivity of diagnostic tests, prevalence rates of some forms of typhus have proved to be unexpectedly high. For example, in a study undertaken in his region of India, the seroprevalence of scrub typhus was 31.8% (Trop Med Int Health. 2017;22:576-82. doi: 10.1111/tmi.12853).
Of unmet needs in the clinical management of typhus, Dr. Varghese listed better strategies for point-of-care diagnosis and treatment and better data on how to manage patients who are severely ill. Advanced disease, which is common to rural areas with limited access to health care, is the source of almost all typhus mortality, according to Dr. Varghese. He described a trial now being initiated in severe disease that will compare intravenous doxycycline to IV azithromycin and to a combination of both IV doxycycline and azithromycin.
Although Dr. Varghese cautioned that reports of resistant typhus infections, particularly in Thailand, might prove to be the next big clinical challenge in typhus, he said that progress is being made toward reducing the burden of this disease in his area of the world. In a disease associated with a mortality of 50% if left untreated, he attributes gains to earlier diagnosis and prompt treatment.
At his medical center, “we have been working with this disease for a decade and a half,” he said, referring to scrub typhus. “When we started off, the mortality was around 15% after diagnosis. Today, the mortality is about 5%-7%.”
Dr. Varghese reported that he has no financial relationships relevant to this topic. The event was the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
SAN DIEGO – Typhus in many forms, particularly scrub typhus, has reemerged worldwide, but none of these rickettsial infections poses a significant public health threat if promptly diagnosed, according to an update presented at an annual scientific meeting on infectious diseases.
Scrub typhus, which is spread by several species of trombiculid mites or chiggers, poses a large threat in regard to typhus epidemics, particularly in Asia, but sporadic cases of different types of typhus are being seen everywhere, including in the United States, according to George M. Varghese, MD, department of infectious diseases, Christian Medical College, Vellore, India.
“The typhus diseases are clinically similar but epidemiologically and etiologically distinct,” reported Dr. Varghese, “but doxycycline is the drug of choice for almost all of the rickettsial infections.”
The bacteria responsible for scrub typhus is Orientia tsutsugamushi, which is no longer included in the genus Rickettsia, but Dr. Varghese, who has published frequently on the epidemiology of scrub typhus, said that it is still appropriately grouped among rickettsial infections. It shares many features with the other rickettsioses, which were considered to be fading but are now resurging after the large epidemics that occurred prior to the introduction of antibiotics.
In the World Wars, Rickettsia prowazekii – which is carried and spread by body lice – was the most well known typhus threat. According to Dr. Varghese, this bacterium may have killed more soldiers in these conflicts than did firearms. Although R. prowazekii has not disappeared as a source of typhus outbreaks, particularly in South America and Africa, there are current epidemics produced from rickettsial infections carried by fleas, such as R. typhi, or ticks, like R. rickettsii, or mice, like R. felis.
For clinical detection of these forms of typhus, there are differences. Although all are associated with a rapid onset of fever, headache, and myalgia, subtle signs can be helpful in making a diagnosis while waiting for laboratory confirmation. For example, scrub typhus, unlike Rocky Mountain Fever, which is caused by tick bites, does not generally include a rash. Rather, eschars, which are small patches of necrotic skin, are far more characteristic.
“Serological tests are the most common diagnostic tool for typhus, but serology may not allow early diagnosis. You can obtain a false positive in the early stages of disease,” Dr. Varghese warned. To speed the diagnosis, he said that looking for the clinical clues characteristic of the suspected form of typhus, such as the scrub typhus-associated eschar, “is valuable.” However, he also emphasized that even with positive serology results, “good epidemiology and history is helpful for laboratory interpretation.”
A variety of serological tests can identify typhus pathogens, but ELISA is now the most widely used, according to Dr. Varghese, noting that this test offers a sensitivity of 93% and a specificity of 91%. Both are higher than those provided by alternatives. As a result of improved sensitivity of diagnostic tests, prevalence rates of some forms of typhus have proved to be unexpectedly high. For example, in a study undertaken in his region of India, the seroprevalence of scrub typhus was 31.8% (Trop Med Int Health. 2017;22:576-82. doi: 10.1111/tmi.12853).
Of unmet needs in the clinical management of typhus, Dr. Varghese listed better strategies for point-of-care diagnosis and treatment and better data on how to manage patients who are severely ill. Advanced disease, which is common to rural areas with limited access to health care, is the source of almost all typhus mortality, according to Dr. Varghese. He described a trial now being initiated in severe disease that will compare intravenous doxycycline to IV azithromycin and to a combination of both IV doxycycline and azithromycin.
Although Dr. Varghese cautioned that reports of resistant typhus infections, particularly in Thailand, might prove to be the next big clinical challenge in typhus, he said that progress is being made toward reducing the burden of this disease in his area of the world. In a disease associated with a mortality of 50% if left untreated, he attributes gains to earlier diagnosis and prompt treatment.
At his medical center, “we have been working with this disease for a decade and a half,” he said, referring to scrub typhus. “When we started off, the mortality was around 15% after diagnosis. Today, the mortality is about 5%-7%.”
Dr. Varghese reported that he has no financial relationships relevant to this topic. The event was the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
SAN DIEGO – Typhus in many forms, particularly scrub typhus, has reemerged worldwide, but none of these rickettsial infections poses a significant public health threat if promptly diagnosed, according to an update presented at an annual scientific meeting on infectious diseases.
Scrub typhus, which is spread by several species of trombiculid mites or chiggers, poses a large threat in regard to typhus epidemics, particularly in Asia, but sporadic cases of different types of typhus are being seen everywhere, including in the United States, according to George M. Varghese, MD, department of infectious diseases, Christian Medical College, Vellore, India.
“The typhus diseases are clinically similar but epidemiologically and etiologically distinct,” reported Dr. Varghese, “but doxycycline is the drug of choice for almost all of the rickettsial infections.”
The bacteria responsible for scrub typhus is Orientia tsutsugamushi, which is no longer included in the genus Rickettsia, but Dr. Varghese, who has published frequently on the epidemiology of scrub typhus, said that it is still appropriately grouped among rickettsial infections. It shares many features with the other rickettsioses, which were considered to be fading but are now resurging after the large epidemics that occurred prior to the introduction of antibiotics.
In the World Wars, Rickettsia prowazekii – which is carried and spread by body lice – was the most well known typhus threat. According to Dr. Varghese, this bacterium may have killed more soldiers in these conflicts than did firearms. Although R. prowazekii has not disappeared as a source of typhus outbreaks, particularly in South America and Africa, there are current epidemics produced from rickettsial infections carried by fleas, such as R. typhi, or ticks, like R. rickettsii, or mice, like R. felis.
For clinical detection of these forms of typhus, there are differences. Although all are associated with a rapid onset of fever, headache, and myalgia, subtle signs can be helpful in making a diagnosis while waiting for laboratory confirmation. For example, scrub typhus, unlike Rocky Mountain Fever, which is caused by tick bites, does not generally include a rash. Rather, eschars, which are small patches of necrotic skin, are far more characteristic.
“Serological tests are the most common diagnostic tool for typhus, but serology may not allow early diagnosis. You can obtain a false positive in the early stages of disease,” Dr. Varghese warned. To speed the diagnosis, he said that looking for the clinical clues characteristic of the suspected form of typhus, such as the scrub typhus-associated eschar, “is valuable.” However, he also emphasized that even with positive serology results, “good epidemiology and history is helpful for laboratory interpretation.”
A variety of serological tests can identify typhus pathogens, but ELISA is now the most widely used, according to Dr. Varghese, noting that this test offers a sensitivity of 93% and a specificity of 91%. Both are higher than those provided by alternatives. As a result of improved sensitivity of diagnostic tests, prevalence rates of some forms of typhus have proved to be unexpectedly high. For example, in a study undertaken in his region of India, the seroprevalence of scrub typhus was 31.8% (Trop Med Int Health. 2017;22:576-82. doi: 10.1111/tmi.12853).
Of unmet needs in the clinical management of typhus, Dr. Varghese listed better strategies for point-of-care diagnosis and treatment and better data on how to manage patients who are severely ill. Advanced disease, which is common to rural areas with limited access to health care, is the source of almost all typhus mortality, according to Dr. Varghese. He described a trial now being initiated in severe disease that will compare intravenous doxycycline to IV azithromycin and to a combination of both IV doxycycline and azithromycin.
Although Dr. Varghese cautioned that reports of resistant typhus infections, particularly in Thailand, might prove to be the next big clinical challenge in typhus, he said that progress is being made toward reducing the burden of this disease in his area of the world. In a disease associated with a mortality of 50% if left untreated, he attributes gains to earlier diagnosis and prompt treatment.
At his medical center, “we have been working with this disease for a decade and a half,” he said, referring to scrub typhus. “When we started off, the mortality was around 15% after diagnosis. Today, the mortality is about 5%-7%.”
Dr. Varghese reported that he has no financial relationships relevant to this topic. The event was the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
AT IDWEEK 2017
Key clinical point:
Major finding: Almost all of the estimated 15,000 annual global deaths attributed to rickettsial infections could be eliminated with prompt doxycycline therapy.
Data source: Topic review.
Disclosures: Dr. Varghese reported that he has no relevant conflicts to disclose.
Study: Macrolide treatment lowers risk of failure in pediatric CAP patients
SAN DIEGO – Macrolide use showed lower treatment failure rates than did amoxicillin or beta-lactam treatment for pediatric community acquired pneumonia (CAP) patients, according to a study presented at an annual scientific meeting on infectious diseases.
While guidelines recommend amoxicillin as the first-line therapy against CAP, investigators have noticed an increase in macrolide prescriptions to pediatric outpatients, despite reported shortcomings in its use against atypical pneumonia.
“Macrolides are probably prescribed out of proportion to the presence of atypical pneumonia in that practice setting,” said Lori Handy, MD, of Children’s Hospital of Philadelphia. This could be an issue, according to Dr. Handy: “We also know that depending on the study, up to 40% of Streptococcus pneumoniae is resistant to macrolides, meaning there are children out there who may have S. pneumoniae who are receiving therapy not targeted at their disease pathogen.”
To examine the possible impact of an increase in macrolide prescriptions, the investigators conducted a retrospective cohort study of 10,470 CAP pediatric patients across 31 primary care practices in the Children’s Hospital of Philadelphia network who were diagnosed between January 2009 and December 2013.
The studied cohort was split into three groups based on treatment options: amoxicillin monotherapy (4,252, 40.6%), macrolide monotherapy (4,459, 42.6%), and broad-spectrum beta-lactams (1,759, 16.8%).
Patient age ranged from 3 months to 18 years, the majority were white, with a roughly equal number of each sex. Of the children studied, 634 (6.1%) experienced treatment failure, defined as a change in antibiotics, an emergency department visit for related symptoms, or hospitalization for pneumonia, all of which had to occur more than 24 hours after a pediatric visit, according to Dr. Handy.
Of the children who failed treatment, 341 (54%) were in the amoxicillin group, 145 (23%) were in the macrolide group, and 147 (23%) were in the broad-spectrum group.
Patients younger than 5 years old who received macrolide therapy were half as likely to experience treatment failure compared with those given amoxicillin (odds ratio [OR] .52 [95% confidence interval (CI), 0.34-0.78]).
“What this translates to in practice is that about 32 children would need to treated with macrolides to prevent one failure in the amoxicillin group,” said Dr. Handy.
Patients 5 years and older showed even lower odds of treatment failure, at approximately one-third the rate of amoxicillin treated patients (OR .31 [95% CI, 0.23-0.92]).
Dr. Handy stated that the retrospective nature of the study and the possibility of changes in the epidemiology of CAP occurring since 2013 should be considered when evaluating the findings.
In addition, she pointed out, CAP is a clinical diagnosis, and there is generally no microbiological data associated with it in order to determine the etiology of the infection.
Overall, in healthy children with CAP, it would be better to use macrolide antibiotics compared with amoxicillin, Dr. Handy concluded. However, without the microbiological data, a more randomized, controlled trial would be needed to determine how to best treat these patients, she added.
During discussion, members of the audience asked about the appropriateness of measuring a change in antibiotics as an endpoint, especially in children with viral pneumonia, who may have had parents request stronger medication when their children did not improve quickly enough.
The 47 patients who were hospitalized would not have provided enough control to properly test the results, Dr. Handy replied, although she did acknowledge the potential issue of viral infections.
She stated the need for further study to assess its possible impact, saying she didn’t know whether viral infections may have skewed their results. “Either they’ve done nothing because they’re equally distributed among the groups or they’ve pushed them one way or the other way,” she said.
Dr. Handy and her colleagues reported having no relevant financial disclosures. The event was the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
SAN DIEGO – Macrolide use showed lower treatment failure rates than did amoxicillin or beta-lactam treatment for pediatric community acquired pneumonia (CAP) patients, according to a study presented at an annual scientific meeting on infectious diseases.
While guidelines recommend amoxicillin as the first-line therapy against CAP, investigators have noticed an increase in macrolide prescriptions to pediatric outpatients, despite reported shortcomings in its use against atypical pneumonia.
“Macrolides are probably prescribed out of proportion to the presence of atypical pneumonia in that practice setting,” said Lori Handy, MD, of Children’s Hospital of Philadelphia. This could be an issue, according to Dr. Handy: “We also know that depending on the study, up to 40% of Streptococcus pneumoniae is resistant to macrolides, meaning there are children out there who may have S. pneumoniae who are receiving therapy not targeted at their disease pathogen.”
To examine the possible impact of an increase in macrolide prescriptions, the investigators conducted a retrospective cohort study of 10,470 CAP pediatric patients across 31 primary care practices in the Children’s Hospital of Philadelphia network who were diagnosed between January 2009 and December 2013.
The studied cohort was split into three groups based on treatment options: amoxicillin monotherapy (4,252, 40.6%), macrolide monotherapy (4,459, 42.6%), and broad-spectrum beta-lactams (1,759, 16.8%).
Patient age ranged from 3 months to 18 years, the majority were white, with a roughly equal number of each sex. Of the children studied, 634 (6.1%) experienced treatment failure, defined as a change in antibiotics, an emergency department visit for related symptoms, or hospitalization for pneumonia, all of which had to occur more than 24 hours after a pediatric visit, according to Dr. Handy.
Of the children who failed treatment, 341 (54%) were in the amoxicillin group, 145 (23%) were in the macrolide group, and 147 (23%) were in the broad-spectrum group.
Patients younger than 5 years old who received macrolide therapy were half as likely to experience treatment failure compared with those given amoxicillin (odds ratio [OR] .52 [95% confidence interval (CI), 0.34-0.78]).
“What this translates to in practice is that about 32 children would need to treated with macrolides to prevent one failure in the amoxicillin group,” said Dr. Handy.
Patients 5 years and older showed even lower odds of treatment failure, at approximately one-third the rate of amoxicillin treated patients (OR .31 [95% CI, 0.23-0.92]).
Dr. Handy stated that the retrospective nature of the study and the possibility of changes in the epidemiology of CAP occurring since 2013 should be considered when evaluating the findings.
In addition, she pointed out, CAP is a clinical diagnosis, and there is generally no microbiological data associated with it in order to determine the etiology of the infection.
Overall, in healthy children with CAP, it would be better to use macrolide antibiotics compared with amoxicillin, Dr. Handy concluded. However, without the microbiological data, a more randomized, controlled trial would be needed to determine how to best treat these patients, she added.
During discussion, members of the audience asked about the appropriateness of measuring a change in antibiotics as an endpoint, especially in children with viral pneumonia, who may have had parents request stronger medication when their children did not improve quickly enough.
The 47 patients who were hospitalized would not have provided enough control to properly test the results, Dr. Handy replied, although she did acknowledge the potential issue of viral infections.
She stated the need for further study to assess its possible impact, saying she didn’t know whether viral infections may have skewed their results. “Either they’ve done nothing because they’re equally distributed among the groups or they’ve pushed them one way or the other way,” she said.
Dr. Handy and her colleagues reported having no relevant financial disclosures. The event was the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
SAN DIEGO – Macrolide use showed lower treatment failure rates than did amoxicillin or beta-lactam treatment for pediatric community acquired pneumonia (CAP) patients, according to a study presented at an annual scientific meeting on infectious diseases.
While guidelines recommend amoxicillin as the first-line therapy against CAP, investigators have noticed an increase in macrolide prescriptions to pediatric outpatients, despite reported shortcomings in its use against atypical pneumonia.
“Macrolides are probably prescribed out of proportion to the presence of atypical pneumonia in that practice setting,” said Lori Handy, MD, of Children’s Hospital of Philadelphia. This could be an issue, according to Dr. Handy: “We also know that depending on the study, up to 40% of Streptococcus pneumoniae is resistant to macrolides, meaning there are children out there who may have S. pneumoniae who are receiving therapy not targeted at their disease pathogen.”
To examine the possible impact of an increase in macrolide prescriptions, the investigators conducted a retrospective cohort study of 10,470 CAP pediatric patients across 31 primary care practices in the Children’s Hospital of Philadelphia network who were diagnosed between January 2009 and December 2013.
The studied cohort was split into three groups based on treatment options: amoxicillin monotherapy (4,252, 40.6%), macrolide monotherapy (4,459, 42.6%), and broad-spectrum beta-lactams (1,759, 16.8%).
Patient age ranged from 3 months to 18 years, the majority were white, with a roughly equal number of each sex. Of the children studied, 634 (6.1%) experienced treatment failure, defined as a change in antibiotics, an emergency department visit for related symptoms, or hospitalization for pneumonia, all of which had to occur more than 24 hours after a pediatric visit, according to Dr. Handy.
Of the children who failed treatment, 341 (54%) were in the amoxicillin group, 145 (23%) were in the macrolide group, and 147 (23%) were in the broad-spectrum group.
Patients younger than 5 years old who received macrolide therapy were half as likely to experience treatment failure compared with those given amoxicillin (odds ratio [OR] .52 [95% confidence interval (CI), 0.34-0.78]).
“What this translates to in practice is that about 32 children would need to treated with macrolides to prevent one failure in the amoxicillin group,” said Dr. Handy.
Patients 5 years and older showed even lower odds of treatment failure, at approximately one-third the rate of amoxicillin treated patients (OR .31 [95% CI, 0.23-0.92]).
Dr. Handy stated that the retrospective nature of the study and the possibility of changes in the epidemiology of CAP occurring since 2013 should be considered when evaluating the findings.
In addition, she pointed out, CAP is a clinical diagnosis, and there is generally no microbiological data associated with it in order to determine the etiology of the infection.
Overall, in healthy children with CAP, it would be better to use macrolide antibiotics compared with amoxicillin, Dr. Handy concluded. However, without the microbiological data, a more randomized, controlled trial would be needed to determine how to best treat these patients, she added.
During discussion, members of the audience asked about the appropriateness of measuring a change in antibiotics as an endpoint, especially in children with viral pneumonia, who may have had parents request stronger medication when their children did not improve quickly enough.
The 47 patients who were hospitalized would not have provided enough control to properly test the results, Dr. Handy replied, although she did acknowledge the potential issue of viral infections.
She stated the need for further study to assess its possible impact, saying she didn’t know whether viral infections may have skewed their results. “Either they’ve done nothing because they’re equally distributed among the groups or they’ve pushed them one way or the other way,” she said.
Dr. Handy and her colleagues reported having no relevant financial disclosures. The event was the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
AT ID WEEK 2017
Key clinical point:
Major finding: Macrolide treatment was associated with treatment failure OR of .52 in patients younger than 5 years and .31 among patients older than 5 years.
Data source: Retrospective study of 10,460 pediatric patients receiving antibiotics for community acquired pneumonia during 2009-2013.
Disclosures: Dr. Handy and her colleagues reported having no relevant financial disclosures.
Rare type of MCL mimics Castleman disease
A rare type of mantle cell lymphoma (MCL) has features that are similar to those of Castleman disease, according to a recent report based on three patient cases.
Lymph node biopsies for these patients initially indicated histologic features consistent with those of plasma cell (PC)-type Castleman disease, reported Takuro Igawa, MD, PhD, of Okayama (Japan) University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, and his coauthors. Further work-up, including flow cytometric analysis and cyclin D1 immunostaining, showed features consistent with those of MCL.
“This rare type of MCL can mimic Castleman disease in the clinical setting and upon histological examination,” Dr. Igawa and his colleagues wrote (Pathol Res Pract. 2017 Sep 18. pii: S0344-0338[17]30684-2. doi: 10.1016/j.prp.2017.09.015). “These confusing characteristics can make the diagnosis challenging, and careful flow cytometric analysis is recommended when a histopathological diagnosis is made.”
The patients in the study, all male, were 51, 74, and 81 years of age. Each presented with systemic lymphadenopathy, along with abnormal laboratory findings that according to the investigators are not usually associated with B-cell lymphomas such as MCL, including anemia, polyclonal IgG hypergammaglobulinemia, and elevated levels of C-reactive protein.
In lymph node biopsy specimens, the MCL component was “masked by histological features of PC-type Castleman disease” such as interfollicular plasmacytosis and atrophic germinal centers, the researchers wrote.
However, further pathologic investigations revealed features that were “essential to distinguish these 3 cases of MCL from PC-type Castleman disease,” they added.
In particular, an abnormal B-cell population was found using flow cytometric analysis, while subsequent cyclin D1 immunostaining in all three cases showed abnormal B-cells primarily in the mantle zone that were positive for CD20 and CD5, both typically expressed by MCL, along with SOX11, which is an “excellent diagnostic marker for MCL, including atypical MCL,” the investigators wrote.
These case reports also provide some evidence that interleukin-6 (IL-6), which is thought to be a driver of Castleman disease, might also be implicated in the pathogenesis of this rare MCL variant. the researchers found that all three cases had positive IL-6 staining in the interfollicular areas.
If further studies confirm the role of IL-6 in this rare setting, “specific treatments other than chemotherapy could potentially be used for patients with MCL with features of Castleman disease, such as an anti-IL-6 receptor antibody (tocilizumab), which is already used for patients with Castleman disease,” they said.
A rare type of mantle cell lymphoma (MCL) has features that are similar to those of Castleman disease, according to a recent report based on three patient cases.
Lymph node biopsies for these patients initially indicated histologic features consistent with those of plasma cell (PC)-type Castleman disease, reported Takuro Igawa, MD, PhD, of Okayama (Japan) University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, and his coauthors. Further work-up, including flow cytometric analysis and cyclin D1 immunostaining, showed features consistent with those of MCL.
“This rare type of MCL can mimic Castleman disease in the clinical setting and upon histological examination,” Dr. Igawa and his colleagues wrote (Pathol Res Pract. 2017 Sep 18. pii: S0344-0338[17]30684-2. doi: 10.1016/j.prp.2017.09.015). “These confusing characteristics can make the diagnosis challenging, and careful flow cytometric analysis is recommended when a histopathological diagnosis is made.”
The patients in the study, all male, were 51, 74, and 81 years of age. Each presented with systemic lymphadenopathy, along with abnormal laboratory findings that according to the investigators are not usually associated with B-cell lymphomas such as MCL, including anemia, polyclonal IgG hypergammaglobulinemia, and elevated levels of C-reactive protein.
In lymph node biopsy specimens, the MCL component was “masked by histological features of PC-type Castleman disease” such as interfollicular plasmacytosis and atrophic germinal centers, the researchers wrote.
However, further pathologic investigations revealed features that were “essential to distinguish these 3 cases of MCL from PC-type Castleman disease,” they added.
In particular, an abnormal B-cell population was found using flow cytometric analysis, while subsequent cyclin D1 immunostaining in all three cases showed abnormal B-cells primarily in the mantle zone that were positive for CD20 and CD5, both typically expressed by MCL, along with SOX11, which is an “excellent diagnostic marker for MCL, including atypical MCL,” the investigators wrote.
These case reports also provide some evidence that interleukin-6 (IL-6), which is thought to be a driver of Castleman disease, might also be implicated in the pathogenesis of this rare MCL variant. the researchers found that all three cases had positive IL-6 staining in the interfollicular areas.
If further studies confirm the role of IL-6 in this rare setting, “specific treatments other than chemotherapy could potentially be used for patients with MCL with features of Castleman disease, such as an anti-IL-6 receptor antibody (tocilizumab), which is already used for patients with Castleman disease,” they said.
A rare type of mantle cell lymphoma (MCL) has features that are similar to those of Castleman disease, according to a recent report based on three patient cases.
Lymph node biopsies for these patients initially indicated histologic features consistent with those of plasma cell (PC)-type Castleman disease, reported Takuro Igawa, MD, PhD, of Okayama (Japan) University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, and his coauthors. Further work-up, including flow cytometric analysis and cyclin D1 immunostaining, showed features consistent with those of MCL.
“This rare type of MCL can mimic Castleman disease in the clinical setting and upon histological examination,” Dr. Igawa and his colleagues wrote (Pathol Res Pract. 2017 Sep 18. pii: S0344-0338[17]30684-2. doi: 10.1016/j.prp.2017.09.015). “These confusing characteristics can make the diagnosis challenging, and careful flow cytometric analysis is recommended when a histopathological diagnosis is made.”
The patients in the study, all male, were 51, 74, and 81 years of age. Each presented with systemic lymphadenopathy, along with abnormal laboratory findings that according to the investigators are not usually associated with B-cell lymphomas such as MCL, including anemia, polyclonal IgG hypergammaglobulinemia, and elevated levels of C-reactive protein.
In lymph node biopsy specimens, the MCL component was “masked by histological features of PC-type Castleman disease” such as interfollicular plasmacytosis and atrophic germinal centers, the researchers wrote.
However, further pathologic investigations revealed features that were “essential to distinguish these 3 cases of MCL from PC-type Castleman disease,” they added.
In particular, an abnormal B-cell population was found using flow cytometric analysis, while subsequent cyclin D1 immunostaining in all three cases showed abnormal B-cells primarily in the mantle zone that were positive for CD20 and CD5, both typically expressed by MCL, along with SOX11, which is an “excellent diagnostic marker for MCL, including atypical MCL,” the investigators wrote.
These case reports also provide some evidence that interleukin-6 (IL-6), which is thought to be a driver of Castleman disease, might also be implicated in the pathogenesis of this rare MCL variant. the researchers found that all three cases had positive IL-6 staining in the interfollicular areas.
If further studies confirm the role of IL-6 in this rare setting, “specific treatments other than chemotherapy could potentially be used for patients with MCL with features of Castleman disease, such as an anti-IL-6 receptor antibody (tocilizumab), which is already used for patients with Castleman disease,” they said.
FROM PATHOLOGY – RESEARCH AND PRACTICE
Key clinical point:
Major finding: Lymph node biopsy revealed histologic features consistent with plasma cell (PC)-type Castleman disease, but cyclin D1 immunostaining and flow cytometric analysis showed features consistent with a diagnosis of MCL.
Data source: A report on three patient cases of MCL with features of PC-type Castleman disease retrieved from surgical pathology consultation files.
Disclosures: The authors reported no conflicts of interest.
Plenary sessions at ANA 2017 cover wide spectrum of neurologic topics
The six plenary sessions of the annual meeting of the American Neurological Association, taking place Oct. 15-17 in San Diego, promise to cover a broad range of research areas, including neuronal circuits and behavior, global neurology, precision medicine, antisense oligonucleotide therapies, and molecular imaging.
The morning of Oct. 15 starts off with the plenary session, “Linking Circuits to Behavior: Promise & Peril,” which seeks to impart how technologies such as optogenetics enable manipulation of discrete neural populations but require careful consideration of the methods for interpreting the resulting data in order to translate it to human functional neuroimaging for potential therapeutic use.
Later, in the afternoon of Oct. 15, the traditional Derek Denny-Brown Young Neurological Scholar Symposium will showcase the presentations from the two clinical science winners and one basic science winner of the Derek Denny-Brown Young Neurological Scholar Awards, as well as the 2017 Distinguished Neurology Teacher Award, the 2017 Grass Foundation ANA Award in Neuroscience, and the 2017 Wolfe Neuropathy Research Prize. The Derek Denny-Brown Young Neurological Scholar Award recognizes neurologists and neuroscientists in the first 10 years of their career at the assistant/associate faculty (equivalent) level who have made outstanding basic and clinical scientific advances toward the prevention, diagnosis, treatment, and cure of neurologic diseases. This year, award winner Keven N. Sheth, MD, of Yale University, New Haven, Conn., will present on “Instructive, Pragmatic, and Successful Trials in Acute Brain Injury: Making Intracerebral Hemorrhage the LEAST Devastating Form of Stroke”; Leslie E. Skolarus, MD, of the University of Michigan, Ann Arbor, will present on “Reducing the Burden of Stroke in a Disadvantaged Community”; and Conrad Chris Weihl, MD, PhD, of Washington University in St. Louis will present on “Connecting Protein Quality Control Pathways in Skeletal Muscle and Muscle Disease.” The 2017 Distinguished Neurology Teacher Award goes to Zachary Nathaniel London, MD, of the University of Michigan, Ann Arbor. The winner of this year’s Grass Foundation ANA Award in Neuroscience, which goes to an outstanding young physician-scientist conducting research in basic or clinical neuroscience, is Clotilde Lagier-Tourenne, MD, PhD, of Massachusetts General Hospital, Boston, who will discuss “Modeling C9ORF72 Disease: A Crucial Step for Therapeutic Development in ALS and Frontotemporal Dementia.” The symposium’s final presentation will have Stefanie Geisler, MD, of Washington University in St. Louis, talk about “Targeting a Core Axonal Degeneration Program to Treat Vincristine and Bortezomib-Induced Axonal Degeneration.” Dr. Geisler won the Wolfe Neuropathy Research Prize, which honors outstanding investigators who identify a new cause or treatment of axonal peripheral neuropathy.
The morning plenary session on Oct. 16 will focus on translational neuroscience efforts that are paying off with discoveries and insights into neurologic disorders that have higher prevalence or greater relevance to low- and middle-income countries. Presentations on these efforts will include discussion of the causation and prevention of Konzo, a distinct upper–motor neuron disease associated with cassava cyanogenic poisoning in sub-Saharan Africa; a case-control study on the impact of multiple mycotoxins on the development of Nodding syndrome in northern Uganda; efforts to address neurologic manifestations of sexually transmitted virus infections in Peru; a longitudinal cohort study of neurologic sequelae in Ebola virus disease survivors in Liberia; efforts to protect against cerebral malaria; the epidemiology of peripheral neuropathy in urban and rural Bangladeshi type 2 diabetes patients; and the use of smartphones and teleconsultations to improve care for people with epilepsy in low- and middle-income countries.
“Precision Medicine in Neurologic Disease” is the theme of four presentations in the afternoon plenary session on Oct. 16. Huda Y. Zoghbi, MD, of Baylor University, and Texas Children’s Hospital in Houston will talk about how her work in animal models of disease has enabled new insights into the effect that certain regulator proteins have on levels of disease-driving proteins such as tau and alpha-synuclein in neurodegenerative diseases. Amy Wagers, PhD, of Harvard Medical School, Boston, will describe her lab’s use of the gene-editing potential of the CRISPR-Cas9 system to fix frame-disrupting mutations in the Duchenne muscular dystrophy gene, DMD, which encodes dystrophin, and produce functional dystrophin expression in muscle stem cells in a mouse model of the disease, which partially recovered functional deficiencies of dystrophic muscle. Donald Berry, PhD, of the University of Texas, M.D. Anderson Cancer Center in Houston plans to discuss the importance of adaptive platform trials – which match therapies to patients – from oncology to neurologic therapy trials and the lessons learned from two major ongoing oncology treatment trials. Cristina Sampaio, MD, PhD, of the CHDI Foundation, aims to inform attendees of the power of prognostic and predictive biomarker-guided trials in neurology to improve the likelihood of success of drug development. Three high-scoring abstracts in the field of precision medicine also will be presented.
The final day of the meeting brings a morning plenary session on “Antisense Oligonucleotide Treatment of Genetic Neurological Diseases” that will focus on the use of antisense oligonucleotides (ASOs) to silence specific genes or alter their pre-mRNA splicing in Duchenne muscular dystrophy, spinal muscular atrophy, Huntington’s disease, amyotrophic lateral sclerosis, and tauopathies. Additional presentations will focus on abstracts about blood and salivary biomarkers in Huntington’s disease and the early efficacy and safety results of an ASO in patients with hereditary transthyretin amyloidosis with polyneuropathy.
The expanding use and development of methods to assess brain pathology in vivo sets the scene for the meeting’s final plenary session, “Molecular Imaging in Neurologic Disease” in the afternoon of Oct. 17. The use of positron emission tomography and single-photon emission computed tomography (SPECT) tracers for glucose metabolism, the dopamine system, amyloid-beta, tau, synaptic markers, and activated microglia has grown substantially to investigate disease mechanisms, develop new therapeutics, and provide diagnostic and prognostic clinical care. Reisa Sperling, MD, of Harvard Medical School, Boston, will provide an overview of the direction of PET ligand use and development in diagnosing early Alzheimer’s disease. Nicolaas I. Bohnen, MD, PhD, of the University of Michigan, Ann Arbor, will describe a hypothesis for how hypercholinergic activity in the brain of Parkinson’s disease patients may for a time compensate for the loss of striatal dopamine and influence the appearance of a tremor-predominant motor phenotype in patients. Richard E. Carson, PhD, of Yale University will focus on the development of PET ligands to monitor synaptic density loss in neuropsychiatric disorders. Noninvasive imaging has also begun to influence research in the detection of neuroinflammation in a wide variety of conditions, with most research focusing on tracers for activated microglia and astrocytes, according to speaker Martin Pomper, MD, PhD, of Johns Hopkins University, Baltimore. The session will conclude with three molecular imaging abstract presentations.
The six plenary sessions of the annual meeting of the American Neurological Association, taking place Oct. 15-17 in San Diego, promise to cover a broad range of research areas, including neuronal circuits and behavior, global neurology, precision medicine, antisense oligonucleotide therapies, and molecular imaging.
The morning of Oct. 15 starts off with the plenary session, “Linking Circuits to Behavior: Promise & Peril,” which seeks to impart how technologies such as optogenetics enable manipulation of discrete neural populations but require careful consideration of the methods for interpreting the resulting data in order to translate it to human functional neuroimaging for potential therapeutic use.
Later, in the afternoon of Oct. 15, the traditional Derek Denny-Brown Young Neurological Scholar Symposium will showcase the presentations from the two clinical science winners and one basic science winner of the Derek Denny-Brown Young Neurological Scholar Awards, as well as the 2017 Distinguished Neurology Teacher Award, the 2017 Grass Foundation ANA Award in Neuroscience, and the 2017 Wolfe Neuropathy Research Prize. The Derek Denny-Brown Young Neurological Scholar Award recognizes neurologists and neuroscientists in the first 10 years of their career at the assistant/associate faculty (equivalent) level who have made outstanding basic and clinical scientific advances toward the prevention, diagnosis, treatment, and cure of neurologic diseases. This year, award winner Keven N. Sheth, MD, of Yale University, New Haven, Conn., will present on “Instructive, Pragmatic, and Successful Trials in Acute Brain Injury: Making Intracerebral Hemorrhage the LEAST Devastating Form of Stroke”; Leslie E. Skolarus, MD, of the University of Michigan, Ann Arbor, will present on “Reducing the Burden of Stroke in a Disadvantaged Community”; and Conrad Chris Weihl, MD, PhD, of Washington University in St. Louis will present on “Connecting Protein Quality Control Pathways in Skeletal Muscle and Muscle Disease.” The 2017 Distinguished Neurology Teacher Award goes to Zachary Nathaniel London, MD, of the University of Michigan, Ann Arbor. The winner of this year’s Grass Foundation ANA Award in Neuroscience, which goes to an outstanding young physician-scientist conducting research in basic or clinical neuroscience, is Clotilde Lagier-Tourenne, MD, PhD, of Massachusetts General Hospital, Boston, who will discuss “Modeling C9ORF72 Disease: A Crucial Step for Therapeutic Development in ALS and Frontotemporal Dementia.” The symposium’s final presentation will have Stefanie Geisler, MD, of Washington University in St. Louis, talk about “Targeting a Core Axonal Degeneration Program to Treat Vincristine and Bortezomib-Induced Axonal Degeneration.” Dr. Geisler won the Wolfe Neuropathy Research Prize, which honors outstanding investigators who identify a new cause or treatment of axonal peripheral neuropathy.
The morning plenary session on Oct. 16 will focus on translational neuroscience efforts that are paying off with discoveries and insights into neurologic disorders that have higher prevalence or greater relevance to low- and middle-income countries. Presentations on these efforts will include discussion of the causation and prevention of Konzo, a distinct upper–motor neuron disease associated with cassava cyanogenic poisoning in sub-Saharan Africa; a case-control study on the impact of multiple mycotoxins on the development of Nodding syndrome in northern Uganda; efforts to address neurologic manifestations of sexually transmitted virus infections in Peru; a longitudinal cohort study of neurologic sequelae in Ebola virus disease survivors in Liberia; efforts to protect against cerebral malaria; the epidemiology of peripheral neuropathy in urban and rural Bangladeshi type 2 diabetes patients; and the use of smartphones and teleconsultations to improve care for people with epilepsy in low- and middle-income countries.
“Precision Medicine in Neurologic Disease” is the theme of four presentations in the afternoon plenary session on Oct. 16. Huda Y. Zoghbi, MD, of Baylor University, and Texas Children’s Hospital in Houston will talk about how her work in animal models of disease has enabled new insights into the effect that certain regulator proteins have on levels of disease-driving proteins such as tau and alpha-synuclein in neurodegenerative diseases. Amy Wagers, PhD, of Harvard Medical School, Boston, will describe her lab’s use of the gene-editing potential of the CRISPR-Cas9 system to fix frame-disrupting mutations in the Duchenne muscular dystrophy gene, DMD, which encodes dystrophin, and produce functional dystrophin expression in muscle stem cells in a mouse model of the disease, which partially recovered functional deficiencies of dystrophic muscle. Donald Berry, PhD, of the University of Texas, M.D. Anderson Cancer Center in Houston plans to discuss the importance of adaptive platform trials – which match therapies to patients – from oncology to neurologic therapy trials and the lessons learned from two major ongoing oncology treatment trials. Cristina Sampaio, MD, PhD, of the CHDI Foundation, aims to inform attendees of the power of prognostic and predictive biomarker-guided trials in neurology to improve the likelihood of success of drug development. Three high-scoring abstracts in the field of precision medicine also will be presented.
The final day of the meeting brings a morning plenary session on “Antisense Oligonucleotide Treatment of Genetic Neurological Diseases” that will focus on the use of antisense oligonucleotides (ASOs) to silence specific genes or alter their pre-mRNA splicing in Duchenne muscular dystrophy, spinal muscular atrophy, Huntington’s disease, amyotrophic lateral sclerosis, and tauopathies. Additional presentations will focus on abstracts about blood and salivary biomarkers in Huntington’s disease and the early efficacy and safety results of an ASO in patients with hereditary transthyretin amyloidosis with polyneuropathy.
The expanding use and development of methods to assess brain pathology in vivo sets the scene for the meeting’s final plenary session, “Molecular Imaging in Neurologic Disease” in the afternoon of Oct. 17. The use of positron emission tomography and single-photon emission computed tomography (SPECT) tracers for glucose metabolism, the dopamine system, amyloid-beta, tau, synaptic markers, and activated microglia has grown substantially to investigate disease mechanisms, develop new therapeutics, and provide diagnostic and prognostic clinical care. Reisa Sperling, MD, of Harvard Medical School, Boston, will provide an overview of the direction of PET ligand use and development in diagnosing early Alzheimer’s disease. Nicolaas I. Bohnen, MD, PhD, of the University of Michigan, Ann Arbor, will describe a hypothesis for how hypercholinergic activity in the brain of Parkinson’s disease patients may for a time compensate for the loss of striatal dopamine and influence the appearance of a tremor-predominant motor phenotype in patients. Richard E. Carson, PhD, of Yale University will focus on the development of PET ligands to monitor synaptic density loss in neuropsychiatric disorders. Noninvasive imaging has also begun to influence research in the detection of neuroinflammation in a wide variety of conditions, with most research focusing on tracers for activated microglia and astrocytes, according to speaker Martin Pomper, MD, PhD, of Johns Hopkins University, Baltimore. The session will conclude with three molecular imaging abstract presentations.
The six plenary sessions of the annual meeting of the American Neurological Association, taking place Oct. 15-17 in San Diego, promise to cover a broad range of research areas, including neuronal circuits and behavior, global neurology, precision medicine, antisense oligonucleotide therapies, and molecular imaging.
The morning of Oct. 15 starts off with the plenary session, “Linking Circuits to Behavior: Promise & Peril,” which seeks to impart how technologies such as optogenetics enable manipulation of discrete neural populations but require careful consideration of the methods for interpreting the resulting data in order to translate it to human functional neuroimaging for potential therapeutic use.
Later, in the afternoon of Oct. 15, the traditional Derek Denny-Brown Young Neurological Scholar Symposium will showcase the presentations from the two clinical science winners and one basic science winner of the Derek Denny-Brown Young Neurological Scholar Awards, as well as the 2017 Distinguished Neurology Teacher Award, the 2017 Grass Foundation ANA Award in Neuroscience, and the 2017 Wolfe Neuropathy Research Prize. The Derek Denny-Brown Young Neurological Scholar Award recognizes neurologists and neuroscientists in the first 10 years of their career at the assistant/associate faculty (equivalent) level who have made outstanding basic and clinical scientific advances toward the prevention, diagnosis, treatment, and cure of neurologic diseases. This year, award winner Keven N. Sheth, MD, of Yale University, New Haven, Conn., will present on “Instructive, Pragmatic, and Successful Trials in Acute Brain Injury: Making Intracerebral Hemorrhage the LEAST Devastating Form of Stroke”; Leslie E. Skolarus, MD, of the University of Michigan, Ann Arbor, will present on “Reducing the Burden of Stroke in a Disadvantaged Community”; and Conrad Chris Weihl, MD, PhD, of Washington University in St. Louis will present on “Connecting Protein Quality Control Pathways in Skeletal Muscle and Muscle Disease.” The 2017 Distinguished Neurology Teacher Award goes to Zachary Nathaniel London, MD, of the University of Michigan, Ann Arbor. The winner of this year’s Grass Foundation ANA Award in Neuroscience, which goes to an outstanding young physician-scientist conducting research in basic or clinical neuroscience, is Clotilde Lagier-Tourenne, MD, PhD, of Massachusetts General Hospital, Boston, who will discuss “Modeling C9ORF72 Disease: A Crucial Step for Therapeutic Development in ALS and Frontotemporal Dementia.” The symposium’s final presentation will have Stefanie Geisler, MD, of Washington University in St. Louis, talk about “Targeting a Core Axonal Degeneration Program to Treat Vincristine and Bortezomib-Induced Axonal Degeneration.” Dr. Geisler won the Wolfe Neuropathy Research Prize, which honors outstanding investigators who identify a new cause or treatment of axonal peripheral neuropathy.
The morning plenary session on Oct. 16 will focus on translational neuroscience efforts that are paying off with discoveries and insights into neurologic disorders that have higher prevalence or greater relevance to low- and middle-income countries. Presentations on these efforts will include discussion of the causation and prevention of Konzo, a distinct upper–motor neuron disease associated with cassava cyanogenic poisoning in sub-Saharan Africa; a case-control study on the impact of multiple mycotoxins on the development of Nodding syndrome in northern Uganda; efforts to address neurologic manifestations of sexually transmitted virus infections in Peru; a longitudinal cohort study of neurologic sequelae in Ebola virus disease survivors in Liberia; efforts to protect against cerebral malaria; the epidemiology of peripheral neuropathy in urban and rural Bangladeshi type 2 diabetes patients; and the use of smartphones and teleconsultations to improve care for people with epilepsy in low- and middle-income countries.
“Precision Medicine in Neurologic Disease” is the theme of four presentations in the afternoon plenary session on Oct. 16. Huda Y. Zoghbi, MD, of Baylor University, and Texas Children’s Hospital in Houston will talk about how her work in animal models of disease has enabled new insights into the effect that certain regulator proteins have on levels of disease-driving proteins such as tau and alpha-synuclein in neurodegenerative diseases. Amy Wagers, PhD, of Harvard Medical School, Boston, will describe her lab’s use of the gene-editing potential of the CRISPR-Cas9 system to fix frame-disrupting mutations in the Duchenne muscular dystrophy gene, DMD, which encodes dystrophin, and produce functional dystrophin expression in muscle stem cells in a mouse model of the disease, which partially recovered functional deficiencies of dystrophic muscle. Donald Berry, PhD, of the University of Texas, M.D. Anderson Cancer Center in Houston plans to discuss the importance of adaptive platform trials – which match therapies to patients – from oncology to neurologic therapy trials and the lessons learned from two major ongoing oncology treatment trials. Cristina Sampaio, MD, PhD, of the CHDI Foundation, aims to inform attendees of the power of prognostic and predictive biomarker-guided trials in neurology to improve the likelihood of success of drug development. Three high-scoring abstracts in the field of precision medicine also will be presented.
The final day of the meeting brings a morning plenary session on “Antisense Oligonucleotide Treatment of Genetic Neurological Diseases” that will focus on the use of antisense oligonucleotides (ASOs) to silence specific genes or alter their pre-mRNA splicing in Duchenne muscular dystrophy, spinal muscular atrophy, Huntington’s disease, amyotrophic lateral sclerosis, and tauopathies. Additional presentations will focus on abstracts about blood and salivary biomarkers in Huntington’s disease and the early efficacy and safety results of an ASO in patients with hereditary transthyretin amyloidosis with polyneuropathy.
The expanding use and development of methods to assess brain pathology in vivo sets the scene for the meeting’s final plenary session, “Molecular Imaging in Neurologic Disease” in the afternoon of Oct. 17. The use of positron emission tomography and single-photon emission computed tomography (SPECT) tracers for glucose metabolism, the dopamine system, amyloid-beta, tau, synaptic markers, and activated microglia has grown substantially to investigate disease mechanisms, develop new therapeutics, and provide diagnostic and prognostic clinical care. Reisa Sperling, MD, of Harvard Medical School, Boston, will provide an overview of the direction of PET ligand use and development in diagnosing early Alzheimer’s disease. Nicolaas I. Bohnen, MD, PhD, of the University of Michigan, Ann Arbor, will describe a hypothesis for how hypercholinergic activity in the brain of Parkinson’s disease patients may for a time compensate for the loss of striatal dopamine and influence the appearance of a tremor-predominant motor phenotype in patients. Richard E. Carson, PhD, of Yale University will focus on the development of PET ligands to monitor synaptic density loss in neuropsychiatric disorders. Noninvasive imaging has also begun to influence research in the detection of neuroinflammation in a wide variety of conditions, with most research focusing on tracers for activated microglia and astrocytes, according to speaker Martin Pomper, MD, PhD, of Johns Hopkins University, Baltimore. The session will conclude with three molecular imaging abstract presentations.
VCR regimen showed efficacy in mantle cell and indolent lymphomas
The combination of bortezomib, cladribine, and rituximab (VCR) was an effective treatment regimen for patients with CD20-positive mantle cell lymphoma (MCL) and indolent non-Hodgkin’s lymphoma (iNHL), based on results of a recent phase 2, open-label study.
The overall response rate was 92% in the single-center, 24-patient study. The 2-year progression-free survival (PFS) was 82% and 54%, respectively, for MCL and iNHL patients; PFS was 80% for treatment-naive patients and 57% for those with refractory/recalcitrant disease, according to Soham D. Puvvada, MD, of the University of Arizona Cancer Center in Tucson, and her associates.
Two-year overall survival was 91% for MCL and 69% for iNHL patients. Median time to progression was 34.5 months, and median PFS had not been reached at 2 years, according to the researchers.
While the study (NCT00980395) was small and limited by its single-center design, the VCR combination “has encouraging activity in both MCL and iNHL and could be compared to standard therapies in future studies,” the researchers wrote. “For MCL in particular, we believe a noninferiority comparison to standard therapies would be justified by our results.”
Adverse events were most commonly hematologic, and three patients experienced febrile neutropenia, data show.
“Although hematological toxicity can be an issue, the regimen provides an alternative option in transplant ineligible relapsed/refractory MCL and iNHL,” wrote Dr. Puvvada and her colleagues. The study was published in Clinical Lymphoma, Myeloma & Leukemia (doi: 10.1016/j.clml.2017.09.001).
The researchers studied the combination of bortezomib, the proteasome inhibitor initially approved for relapsed/refractory MCL, cladribine, which has shown activity and promising response rates in patients with indolent lymphomas, and rituximab in patients with CD20-positive mantle cell or indolent lymphoma.
Patients with follicular lymphomas were eligible to be included in the study if they had received at least one previous line of therapy. All other participants could be treatment naive or have relapsed after previous treatment.
Of the 24 patients enrolled, 11 had MCL, 5 had follicular lymphoma, 4 had marginal zone lymphoma, 3 had lymphoplasmacytic lymphoma, and 1 had small lymphocytic lymphoma.
The VCR regimen, given every 28 days for no more than six cycles, included rituximab at 375 mg/m2 given intravenously on day 1 of each cycle, cladribine 4 mg/m2 given intravenously over 2 hours on days 1 through 5, and bortezomib 1.3 mg/m2 given intravenously on days 1 and 4. Patients received a median of five cycles of therapy.
Adverse events of grade 3 or greater occurred in 14 patients (58%); 8 patients had leukopenia, 6 had thrombocytopenia, 5 had fatigue, and 5 had neutropenia, which included febrile neutropenia in 3 patients.
With a median follow-up of 38.5 months, overall response rate for VCR was 96%. Complete responses occurred in 8 of 23 evaluable patients (35%) and partial responses in 14 more patients (61%).
The combination of bortezomib, cladribine, and rituximab (VCR) was an effective treatment regimen for patients with CD20-positive mantle cell lymphoma (MCL) and indolent non-Hodgkin’s lymphoma (iNHL), based on results of a recent phase 2, open-label study.
The overall response rate was 92% in the single-center, 24-patient study. The 2-year progression-free survival (PFS) was 82% and 54%, respectively, for MCL and iNHL patients; PFS was 80% for treatment-naive patients and 57% for those with refractory/recalcitrant disease, according to Soham D. Puvvada, MD, of the University of Arizona Cancer Center in Tucson, and her associates.
Two-year overall survival was 91% for MCL and 69% for iNHL patients. Median time to progression was 34.5 months, and median PFS had not been reached at 2 years, according to the researchers.
While the study (NCT00980395) was small and limited by its single-center design, the VCR combination “has encouraging activity in both MCL and iNHL and could be compared to standard therapies in future studies,” the researchers wrote. “For MCL in particular, we believe a noninferiority comparison to standard therapies would be justified by our results.”
Adverse events were most commonly hematologic, and three patients experienced febrile neutropenia, data show.
“Although hematological toxicity can be an issue, the regimen provides an alternative option in transplant ineligible relapsed/refractory MCL and iNHL,” wrote Dr. Puvvada and her colleagues. The study was published in Clinical Lymphoma, Myeloma & Leukemia (doi: 10.1016/j.clml.2017.09.001).
The researchers studied the combination of bortezomib, the proteasome inhibitor initially approved for relapsed/refractory MCL, cladribine, which has shown activity and promising response rates in patients with indolent lymphomas, and rituximab in patients with CD20-positive mantle cell or indolent lymphoma.
Patients with follicular lymphomas were eligible to be included in the study if they had received at least one previous line of therapy. All other participants could be treatment naive or have relapsed after previous treatment.
Of the 24 patients enrolled, 11 had MCL, 5 had follicular lymphoma, 4 had marginal zone lymphoma, 3 had lymphoplasmacytic lymphoma, and 1 had small lymphocytic lymphoma.
The VCR regimen, given every 28 days for no more than six cycles, included rituximab at 375 mg/m2 given intravenously on day 1 of each cycle, cladribine 4 mg/m2 given intravenously over 2 hours on days 1 through 5, and bortezomib 1.3 mg/m2 given intravenously on days 1 and 4. Patients received a median of five cycles of therapy.
Adverse events of grade 3 or greater occurred in 14 patients (58%); 8 patients had leukopenia, 6 had thrombocytopenia, 5 had fatigue, and 5 had neutropenia, which included febrile neutropenia in 3 patients.
With a median follow-up of 38.5 months, overall response rate for VCR was 96%. Complete responses occurred in 8 of 23 evaluable patients (35%) and partial responses in 14 more patients (61%).
The combination of bortezomib, cladribine, and rituximab (VCR) was an effective treatment regimen for patients with CD20-positive mantle cell lymphoma (MCL) and indolent non-Hodgkin’s lymphoma (iNHL), based on results of a recent phase 2, open-label study.
The overall response rate was 92% in the single-center, 24-patient study. The 2-year progression-free survival (PFS) was 82% and 54%, respectively, for MCL and iNHL patients; PFS was 80% for treatment-naive patients and 57% for those with refractory/recalcitrant disease, according to Soham D. Puvvada, MD, of the University of Arizona Cancer Center in Tucson, and her associates.
Two-year overall survival was 91% for MCL and 69% for iNHL patients. Median time to progression was 34.5 months, and median PFS had not been reached at 2 years, according to the researchers.
While the study (NCT00980395) was small and limited by its single-center design, the VCR combination “has encouraging activity in both MCL and iNHL and could be compared to standard therapies in future studies,” the researchers wrote. “For MCL in particular, we believe a noninferiority comparison to standard therapies would be justified by our results.”
Adverse events were most commonly hematologic, and three patients experienced febrile neutropenia, data show.
“Although hematological toxicity can be an issue, the regimen provides an alternative option in transplant ineligible relapsed/refractory MCL and iNHL,” wrote Dr. Puvvada and her colleagues. The study was published in Clinical Lymphoma, Myeloma & Leukemia (doi: 10.1016/j.clml.2017.09.001).
The researchers studied the combination of bortezomib, the proteasome inhibitor initially approved for relapsed/refractory MCL, cladribine, which has shown activity and promising response rates in patients with indolent lymphomas, and rituximab in patients with CD20-positive mantle cell or indolent lymphoma.
Patients with follicular lymphomas were eligible to be included in the study if they had received at least one previous line of therapy. All other participants could be treatment naive or have relapsed after previous treatment.
Of the 24 patients enrolled, 11 had MCL, 5 had follicular lymphoma, 4 had marginal zone lymphoma, 3 had lymphoplasmacytic lymphoma, and 1 had small lymphocytic lymphoma.
The VCR regimen, given every 28 days for no more than six cycles, included rituximab at 375 mg/m2 given intravenously on day 1 of each cycle, cladribine 4 mg/m2 given intravenously over 2 hours on days 1 through 5, and bortezomib 1.3 mg/m2 given intravenously on days 1 and 4. Patients received a median of five cycles of therapy.
Adverse events of grade 3 or greater occurred in 14 patients (58%); 8 patients had leukopenia, 6 had thrombocytopenia, 5 had fatigue, and 5 had neutropenia, which included febrile neutropenia in 3 patients.
With a median follow-up of 38.5 months, overall response rate for VCR was 96%. Complete responses occurred in 8 of 23 evaluable patients (35%) and partial responses in 14 more patients (61%).
FROM LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: The overall response rate was 92%, with a 2-year PFS of 82% and 54% for patients with mantle cell lymphoma (MCL) and indolent non-Hodgkin’s lymphoma (iNHL), respectively. Adverse events were most commonly hematologic, and three patients experienced febrile neutropenia.
Data source: A phase 2, open-label study including 24 patients with mantle cell or indolent lymphomas.
Disclosures: No disclosures were reported in the accepted manuscript.
FDA approves test to screen donated blood for Zika virus
The cobas Zika test has been approved for detecting the virus in whole blood, blood components, and donated organs, the U.S. Food and Drug Administration announced in a press release.
“Today’s action represents the first approval of a Zika virus detection test for use with screening the nation’s blood supply,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a press release. “Screening blood donations for the Zika virus is critical to preventing infected donations from entering the U.S. blood supply.”
In August 2016, the FDA issued a final guidance document recommending that all individual units of whole blood and blood components be screened with an investigational blood screening test available under an investigational new drug application. Data obtained on the cobas Zika test under its investigational new drug application and from additional studies performed by the manufacturer demonstrated that the cobas Zika test is effective. Testing individual samples from blood donations at five external laboratory sites resulted in a clinical specificity exceeding 99%.
The cobas Zika test is intended for use on the fully automated cobas 6800 and cobas 8800 systems. The cobas Zika test, cobas 6800, and cobas 8800 systems are manufactured by Roche Molecular Systems.
The cobas Zika test has been approved for detecting the virus in whole blood, blood components, and donated organs, the U.S. Food and Drug Administration announced in a press release.
“Today’s action represents the first approval of a Zika virus detection test for use with screening the nation’s blood supply,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a press release. “Screening blood donations for the Zika virus is critical to preventing infected donations from entering the U.S. blood supply.”
In August 2016, the FDA issued a final guidance document recommending that all individual units of whole blood and blood components be screened with an investigational blood screening test available under an investigational new drug application. Data obtained on the cobas Zika test under its investigational new drug application and from additional studies performed by the manufacturer demonstrated that the cobas Zika test is effective. Testing individual samples from blood donations at five external laboratory sites resulted in a clinical specificity exceeding 99%.
The cobas Zika test is intended for use on the fully automated cobas 6800 and cobas 8800 systems. The cobas Zika test, cobas 6800, and cobas 8800 systems are manufactured by Roche Molecular Systems.
The cobas Zika test has been approved for detecting the virus in whole blood, blood components, and donated organs, the U.S. Food and Drug Administration announced in a press release.
“Today’s action represents the first approval of a Zika virus detection test for use with screening the nation’s blood supply,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said in a press release. “Screening blood donations for the Zika virus is critical to preventing infected donations from entering the U.S. blood supply.”
In August 2016, the FDA issued a final guidance document recommending that all individual units of whole blood and blood components be screened with an investigational blood screening test available under an investigational new drug application. Data obtained on the cobas Zika test under its investigational new drug application and from additional studies performed by the manufacturer demonstrated that the cobas Zika test is effective. Testing individual samples from blood donations at five external laboratory sites resulted in a clinical specificity exceeding 99%.
The cobas Zika test is intended for use on the fully automated cobas 6800 and cobas 8800 systems. The cobas Zika test, cobas 6800, and cobas 8800 systems are manufactured by Roche Molecular Systems.
Emergency Imaging: Left Periorbital Swelling
Case
A 3-year-old boy was brought to the ED by his parents for evaluation of left periorbital swelling. A few days prior to presentation, the child was seen at an outpatient center where he was diagnosed with preseptal cellulitis and given an oral antibiotic. However, even after receiving three doses of the antibiotic, the periorbital swelling and redness around the child’s eye worsened, prompting this visit to the ED.
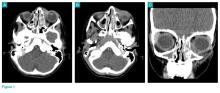
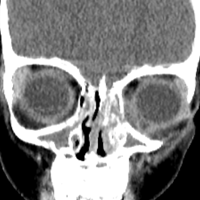
Physical examination revealed edema and erythema both above and below the left eye, with associated tenderness to palpation. A contrast-enhanced maxillofacial computed tomography (CT) scan, with special attention to the orbits, was ordered; representative images are shown (Figure 1a-1c).
What is the diagnosis?
Answer
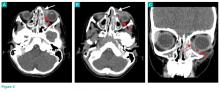
The CT images of the orbits demonstrated edema in the superficial left eyelid (white arrows, Figure 2a and 2b) and left deep orbital septum (red arrows, Figure 2a-2c). A peripherally enhancing fluid collection centered in the left nasolacrimal gland was present (red asterisks, Figure 2b and 2c) with mild mass effect on the left globe. Opacification was also noted within the paranasal sinuses (white asterisks, Figure 2a-2c). Together these findings indicated sinusitis with dacryocystitis and orbital cellulitis.
Dacryocystitis
Dacryocystitis is an infection or inflammation of the lacrimal sac, usually developing secondary to blockage of the nasolacrimal duct. Orbital cellulitis is an infection involving the contents of the orbit, including the fat and ocular muscles. Orbital cellulitis should not be confused with preseptal cellulitis, which is an infection involving the eyelid occurring posterior to the orbital septum. While both of these conditions are more common in children than in adults, preseptal cellulitis is much more common than orbital cellulitis.
Preseptal Cellulitis
Preseptal cellulitis is typically due to local trauma, local skin infection, or dacryocystitis.1 Preseptal cellulitis rarely extends into the orbit, though a minority of cases have been reported in patients with concomitant dacryocystitis.2 Orbital cellulitis most commonly results from paranasal sinus disease, particularly of the ethmoid sinus, which is only separated from the orbit by the thin lamina papyracea.3 While both preseptal cellulitis and orbital cellulitis can cause eyelid swelling and erythema, preseptal cellulitis is typically a mild condition. Orbital cellulitis, however, is a serious medical emergency that requires prompt diagnosis and treatment to avoid loss of vision and intracranial complications, such as venous thrombosis and empyema.3
Imaging Studies
Although the clinical features of orbital cellulitis (eg, proptosis, ophthalmoplegia, pain with ocular movement) can sometimes distinguish it from preseptal cellulitis, imaging studies are helpful to confirm the diagnosis.4 As previously noted, prompt recognition, diagnosis, and treatment of orbital cellulitis are essential to avoid serious complications.
Computed tomography has a high specificity and sensitivity in detecting the extension of infection into the orbit and associated complications such as subperiosteal or intracranial abscess. For patients in whom intravenous (IV) contrast is contraindicated or who wish to avoid ionizing radiation, magnetic resonance imaging is a useful alternate modality, and diffusion-weighted imaging is particularly sensitive in diagnosing abscess.5
Treatment
1. Baring DE, Hilmi OJ. An evidence based review of periorbital cellulitis. Clin Otolaryngol. 2011;36(1):57-64. doi:10.1111/j.1749-4486.2011.02258.x.
2. Kikkawa DO, Heinz GW, Martin RT, Nunery WN, Eiseman AS. Orbital cellulitis and abscess secondary to dacryocystitis. Arch Ophthalmol. 2002;120(8):1096-1099.
3. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. doi:10.1259/bjr.20130503.
4. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. doi:10.1542/peds.2009-1709.
5. Sepahdari AR, Aakalu VK, Kapur R, et al. MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol. 2009;193(3):W244-W250. doi:10.2214/AJR.08.1838.
6. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2007;40(6):518-524.
Case
A 3-year-old boy was brought to the ED by his parents for evaluation of left periorbital swelling. A few days prior to presentation, the child was seen at an outpatient center where he was diagnosed with preseptal cellulitis and given an oral antibiotic. However, even after receiving three doses of the antibiotic, the periorbital swelling and redness around the child’s eye worsened, prompting this visit to the ED.
Physical examination revealed edema and erythema both above and below the left eye, with associated tenderness to palpation. A contrast-enhanced maxillofacial computed tomography (CT) scan, with special attention to the orbits, was ordered; representative images are shown (Figure 1a-1c).
What is the diagnosis?
Answer
The CT images of the orbits demonstrated edema in the superficial left eyelid (white arrows, Figure 2a and 2b) and left deep orbital septum (red arrows, Figure 2a-2c). A peripherally enhancing fluid collection centered in the left nasolacrimal gland was present (red asterisks, Figure 2b and 2c) with mild mass effect on the left globe. Opacification was also noted within the paranasal sinuses (white asterisks, Figure 2a-2c). Together these findings indicated sinusitis with dacryocystitis and orbital cellulitis.
Dacryocystitis
Dacryocystitis is an infection or inflammation of the lacrimal sac, usually developing secondary to blockage of the nasolacrimal duct. Orbital cellulitis is an infection involving the contents of the orbit, including the fat and ocular muscles. Orbital cellulitis should not be confused with preseptal cellulitis, which is an infection involving the eyelid occurring posterior to the orbital septum. While both of these conditions are more common in children than in adults, preseptal cellulitis is much more common than orbital cellulitis.
Preseptal Cellulitis
Preseptal cellulitis is typically due to local trauma, local skin infection, or dacryocystitis.1 Preseptal cellulitis rarely extends into the orbit, though a minority of cases have been reported in patients with concomitant dacryocystitis.2 Orbital cellulitis most commonly results from paranasal sinus disease, particularly of the ethmoid sinus, which is only separated from the orbit by the thin lamina papyracea.3 While both preseptal cellulitis and orbital cellulitis can cause eyelid swelling and erythema, preseptal cellulitis is typically a mild condition. Orbital cellulitis, however, is a serious medical emergency that requires prompt diagnosis and treatment to avoid loss of vision and intracranial complications, such as venous thrombosis and empyema.3
Imaging Studies
Although the clinical features of orbital cellulitis (eg, proptosis, ophthalmoplegia, pain with ocular movement) can sometimes distinguish it from preseptal cellulitis, imaging studies are helpful to confirm the diagnosis.4 As previously noted, prompt recognition, diagnosis, and treatment of orbital cellulitis are essential to avoid serious complications.
Computed tomography has a high specificity and sensitivity in detecting the extension of infection into the orbit and associated complications such as subperiosteal or intracranial abscess. For patients in whom intravenous (IV) contrast is contraindicated or who wish to avoid ionizing radiation, magnetic resonance imaging is a useful alternate modality, and diffusion-weighted imaging is particularly sensitive in diagnosing abscess.5
Treatment
Case
A 3-year-old boy was brought to the ED by his parents for evaluation of left periorbital swelling. A few days prior to presentation, the child was seen at an outpatient center where he was diagnosed with preseptal cellulitis and given an oral antibiotic. However, even after receiving three doses of the antibiotic, the periorbital swelling and redness around the child’s eye worsened, prompting this visit to the ED.
Physical examination revealed edema and erythema both above and below the left eye, with associated tenderness to palpation. A contrast-enhanced maxillofacial computed tomography (CT) scan, with special attention to the orbits, was ordered; representative images are shown (Figure 1a-1c).
What is the diagnosis?
Answer
The CT images of the orbits demonstrated edema in the superficial left eyelid (white arrows, Figure 2a and 2b) and left deep orbital septum (red arrows, Figure 2a-2c). A peripherally enhancing fluid collection centered in the left nasolacrimal gland was present (red asterisks, Figure 2b and 2c) with mild mass effect on the left globe. Opacification was also noted within the paranasal sinuses (white asterisks, Figure 2a-2c). Together these findings indicated sinusitis with dacryocystitis and orbital cellulitis.
Dacryocystitis
Dacryocystitis is an infection or inflammation of the lacrimal sac, usually developing secondary to blockage of the nasolacrimal duct. Orbital cellulitis is an infection involving the contents of the orbit, including the fat and ocular muscles. Orbital cellulitis should not be confused with preseptal cellulitis, which is an infection involving the eyelid occurring posterior to the orbital septum. While both of these conditions are more common in children than in adults, preseptal cellulitis is much more common than orbital cellulitis.
Preseptal Cellulitis
Preseptal cellulitis is typically due to local trauma, local skin infection, or dacryocystitis.1 Preseptal cellulitis rarely extends into the orbit, though a minority of cases have been reported in patients with concomitant dacryocystitis.2 Orbital cellulitis most commonly results from paranasal sinus disease, particularly of the ethmoid sinus, which is only separated from the orbit by the thin lamina papyracea.3 While both preseptal cellulitis and orbital cellulitis can cause eyelid swelling and erythema, preseptal cellulitis is typically a mild condition. Orbital cellulitis, however, is a serious medical emergency that requires prompt diagnosis and treatment to avoid loss of vision and intracranial complications, such as venous thrombosis and empyema.3
Imaging Studies
Although the clinical features of orbital cellulitis (eg, proptosis, ophthalmoplegia, pain with ocular movement) can sometimes distinguish it from preseptal cellulitis, imaging studies are helpful to confirm the diagnosis.4 As previously noted, prompt recognition, diagnosis, and treatment of orbital cellulitis are essential to avoid serious complications.
Computed tomography has a high specificity and sensitivity in detecting the extension of infection into the orbit and associated complications such as subperiosteal or intracranial abscess. For patients in whom intravenous (IV) contrast is contraindicated or who wish to avoid ionizing radiation, magnetic resonance imaging is a useful alternate modality, and diffusion-weighted imaging is particularly sensitive in diagnosing abscess.5
Treatment
1. Baring DE, Hilmi OJ. An evidence based review of periorbital cellulitis. Clin Otolaryngol. 2011;36(1):57-64. doi:10.1111/j.1749-4486.2011.02258.x.
2. Kikkawa DO, Heinz GW, Martin RT, Nunery WN, Eiseman AS. Orbital cellulitis and abscess secondary to dacryocystitis. Arch Ophthalmol. 2002;120(8):1096-1099.
3. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. doi:10.1259/bjr.20130503.
4. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. doi:10.1542/peds.2009-1709.
5. Sepahdari AR, Aakalu VK, Kapur R, et al. MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol. 2009;193(3):W244-W250. doi:10.2214/AJR.08.1838.
6. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2007;40(6):518-524.
1. Baring DE, Hilmi OJ. An evidence based review of periorbital cellulitis. Clin Otolaryngol. 2011;36(1):57-64. doi:10.1111/j.1749-4486.2011.02258.x.
2. Kikkawa DO, Heinz GW, Martin RT, Nunery WN, Eiseman AS. Orbital cellulitis and abscess secondary to dacryocystitis. Arch Ophthalmol. 2002;120(8):1096-1099.
3. Mathew AV, Craig E, Al-Mahmoud R, et al. Paediatric post-septal and pre-septal cellulitis: 10 years’ experience at a tertiary-level children’s hospital. Br J Radiol. 2014;87(1033):20130503. doi:10.1259/bjr.20130503.
4. Rudloe TF, Harper MB, Prabhu SP, Rahbar R, Vanderveen D, Kimia AA. Acute periorbital infections: who needs emergent imaging? Pediatrics. 2010;125(4):e719-e726. doi:10.1542/peds.2009-1709.
5. Sepahdari AR, Aakalu VK, Kapur R, et al. MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol. 2009;193(3):W244-W250. doi:10.2214/AJR.08.1838.
6. Ho CF, Huang YC, Wang CJ, Chiu CH, Lin TY. Clinical analysis of computed tomography-staged orbital cellulitis in children. J Microbiol Immunol Infect. 2007;40(6):518-524.
Malpractice Counsel: A Pain in the…Scrotum
Case
A 52-year-old man presented to the ED for evaluation of right scrotal pain and swelling. The patient stated that the pain started several hours prior to presentation and had gradually worsened. He denied any trauma or inciting event to the affected area; he further denied abdominal pain, nausea, vomiting, dysuria, polyuria, or fever. The patient’s remote medical history was significant for type 2 diabetes mellitus (DM), which he managed through dietary modification-only as he had refused pharmacological therapy. The patient admitted to smoking one half-pack of cigarettes per week, but denied alcohol or illicit drug use.
At presentation, the patient’s vital signs were all within normal range. The physical examination was remarkable only for right testicular tenderness and mild scrotal swelling, and there were no hernias or lymphadenopathy present.
The emergency physician (EP) ordered a urinalysis and color-flow Doppler ultrasound study of both testes, which the radiologist interpreted as an enlarged right epididymis with hyperemia; the left testicle was normal. The urinalysis was normal.
The patient was diagnosed with epididymitis and discharged home with a prescription for oral levofloxacin 500 mg daily for 10 days. He also was instructed to take ibuprofen for pain, apply ice to the affected area, keep the scrotal area elevated, and follow-up with a urologist in 1 week.
Approximately 8 hours after discharge, the patient returned to the same ED with complaints of increasing right testicular pain and swelling. The history and physical examination at this visit were essentially unchanged from his initial presentation. No laboratory evaluation, imaging studies, or other tests were ordered at the second visit.
The patient was discharged home with a prescription for a narcotic analgesic, which he was instructed to take in addition to the ibuprofen; he was also instructed to follow-up with a urologist within the next 2 to 3 days, instead of in 1 week.
The patient returned the following morning to the same ED with complaints of increased swelling and pain of the right testicle. In addition to the worsening testicular pain and swelling, he also had right inguinal pain, nausea, vomiting, and fever. Vital signs at this third presentation were: blood pressure (BP), 124/64 mm Hg; heart rate (HR), 110 beats/min; respiratory rate, 20 breaths/min; and temperature, 99.8o F. Oxygen saturation was 98% on room air.
The patient was tachycardic on heart examination, but with regular rhythm and no murmurs, rubs, or gallops. The lung and abdominal examinations were normal. The genital examination revealed marked right scrotal swelling and tenderness, as well as tender right inguinal lymphadenopathy.
The EP ordered an intravenous (IV) bolus of 1 L normal saline and laboratory studies, which included lactic acid, blood cultures, urinalysis, and urine culture and sensitivity. The EP was concerned for a scrotal abscess and ordered a testicular Doppler color-flow ultrasound study. The laboratory studies revealed an elevated white blood count of 16.5 K/uL, elevated blood glucose of 364 mg/dL, and elevated lactate of 2.8 mg/dL. As demonstrated on the ultrasound study performed at the patient’s first presentation, the ultrasound again showed an enlarged right epididymis, but without orchitis or abscess. The scrotal wall had significant thickening, consistent with cellulitis. The EP ordered broad spectrum IV antibiotics and admitted the patient to the hospitalist with a consult request for urology services.
The patient continued to receive IV fluids and antibiotics throughout the evening. In the morning, he was seen by the same hospitalist/admitting physician from the previous evening. Upon physical examination, the hospitalist noted tenderness, swelling, and erythema in the patient’s perineal area. The patient’s BP had dropped to 100/60 mm Hg, and his HR had increased to 115 beats/min despite receiving nearly 2 L of normal saline IV throughout the previous evening and night.
The urologist examined the patient soon after the consult request and diagnosed him with Fournier’s gangrene. He started the patient on aggressive IV fluid resuscitation, after which the patient was immediately taken to the operating room for extensive surgical debridement and scrotectomy. The patient’s postoperative course was complicated by acute kidney injury, respiratory failure requiring ventilator support, and sepsis. After a lengthy hospital stay, the patient was discharged home, but required a scrotal skin graft, and experienced erectile dysfunction and depression.
The patient sued all of the EPs involved in his care, the hospital, the hospitalist/admitting physician, and the urologist for negligence. The plaintiff’s attorney argued that since the patient progressively deteriorated over the 24 to 36 hours during his three presentations to the ED, urology services should have been consulted earlier, and that the urologist should have seen the patient immediately at the time of hospital admission.
The attorneys for the defendants claimed the patient denied dysuria, penile lesions, or urethral discharge and that the history, physical examination, and testicular ultrasound were all consistent with the diagnosis of epididymitis. For this reason, they argued, there was no indication for an emergent consultation with urology services. The jury returned a defense verdict.
Discussion
It is easy for a busy EP to have a differential diagnosis of only two disorders when evaluating a patient for unilateral testicular pain and swelling—in this case, testicular torsion and epididymitis. While these are the most common causes of testicular pain and swelling, this case emphasizes the need to also consider Fournier’s gangrene in the differential. A thorough history and physical examination, coupled with appropriate testing, will usually identify the correct diagnosis. While the differential diagnosis is broader than just these three disease processes (see the Box), we will review the evaluation and management of the three most serious: epididymitis, testicular torsion, and Fournier’s gangrene.
Noninfectious and Bacterial Epididymitis
Epididymitis is the most common cause of acute scrotal pain among US adults, accounting for approximately 600,000 cases each year.1 Infectious epididymitis is typically classified as acute (symptom duration of <6 weeks) or chronic (symptom duration of ≥6 weeks).2
Cases of noninfectious epididymitis are typically due to a chronic condition, such as autoimmune disease, cancer, or vasculitis. Although not as common, noninfectious epididymitis can also occur due to testicular trauma or amiodarone therapy.3,4
Patients with acute bacterial epididymitis typically present with scrotal pain and swelling ranging from mild to marked. These patients may also exhibit fever and chills, along with dysuria, frequency, and urgency, if associated with a urinary tract infection.2 The chronic presentation is more common though, and usually not associated with voiding issues.
Chronic epididymis is frequently seen in postpubertal boys and men following sexual activity, heavy physical exertion, and bicycle/motorcycle riding.2 On physical examination, palpation reveals induration and swelling of the involved epididymis with exquisite tenderness.2 Testicular swelling and pain, along with scrotal wall erythema, may be present in more advanced cases.2 The cremasteric reflex should be intact (ie, scratching the medial proximal thigh will cause ipsilateral testicle retraction). Similarly, the lie of both testicles while the patient is standing should be equal and symmetrical—ie, both testicles descended equally. However, in the presence of moderate-to-severe scrotal swelling, both of these physical findings may be impossible to confirm.
A urinalysis and urine culture should be ordered if there is any suspicion of epididymitis; pyuria will be present in approximately 50% of cases. However, since pyuria is neither sensitive nor specific for epididymitis, in most cases, a testicular ultrasound with Doppler flow is required to exclude testicular torsion. In cases of epididymitis, ultrasound usually demonstrates increased flow on the affected side, whereas in testicular torsion, there is decreased or absent blood flow.
The treatment for epididymitis involves antibiotics and symptomatic care. If epididymitis from chlamydia and/or gonorrhea is the suspected cause, or if the patient is younger than age 35 years, he should be given ceftriaxone 250 mg intramuscularly plus oral doxycycline 100 mg twice a day for 10 days. Patients who practice insertive anal sex should be treated with ceftriaxone, plus either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.
In cases in which enteric organisms are suspected, the patient is older than age 35 years, or if patient status is posturinary tract instrumentation or vasectomy, he should be treated with either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.2
For symptomatic relief, scrotal elevation, ice application, and nonsteroidal anti-inflammatory drugs are recommended.
Patients with epididymitis, regardless of etiology, should be instructed to follow-up with a urologist within 1 week. If the patient appears ill, septic, or in significant pain, admission to the hospital with IV antibiotics, IV fluids, and an urgent consult with urology services is required.
Testicular Torsion
Testicular torsion is a time-sensitive issue, requiring early diagnosis and rapid treatment to preserve the patient’s fertility. Most clinicians recommend detorsion within 6 hours of torsion onset because salvage rates are excellent when performed within this timeframe; after 12 hours, the testis will likely suffer irreversible damage due to ischemia.5,6
Testicular torsion can occur at any age, but is most commonly seen in a bimodal distribution—ie, neonates and postpubertal boys. The prevalence of testicular torsion in adult patients hospitalized with acute scrotal pain is approximately 25% to 50%.2
Patients with testicular torsion usually describe a sudden onset of severe, acute pain. The pain frequently occurs a few hours after vigorous physical activity or minor testicular trauma.2 Occasionally, the patient may complain of lower quadrant abdominal pain rather than testicular or scrotal pain. Nausea with vomiting can also be present.
On physical examination, significant testicular swelling is usually present. Examining the patient in the standing position will often reveal an asymmetrical, high-riding testis with a transverse lie on the affected side. The cremasteric reflex is usually absent in patients with testicular torsion.
Because of the significant overlap in history and physical examination findings for epididymitis and testicular torsion, a testicular ultrasound with color Doppler should be ordered. Multiple studies have confirmed the high sensitivity and specificity of ultrasound in the diagnosis of testicular torsion.
The treatment for suspected or confirmed testicular torsion is immediate surgical exploration with intraoperative detorsion and fixation of the testes. The EP can attempt manual detorsion (ie, performed in a medial to lateral motion, similar to opening a book). However, this should not delay the EP from consulting with urology services.
Pediatric patients with testicular torsion usually have a more favorable outcome than do adults. In one retrospective study, patients younger than age 21 years had a 70% testicular salvage rate compared to only 41% of patients aged 21 years and older.7 Regardless of age, better outcomes are associated with shorter periods of torsion.
Fournier’s Gangrene
Fournier’s gangrene is a polymicrobial necrotizing fasciitis of the perineum and scrotum that typically develops initially as a benign infection or abscess but quickly spreads. Risk factors for Fournier’s gangrene include DM, alcohol abuse, and any immunocompromised state (eg, HIV, cancer).
If the patient presents early in onset, there may be only mild tenderness, erythema, or swelling of the affected area; however, this infection progresses rapidly. Later findings include marked tenderness, swelling, crepitus, blisters, and ecchymoses. Patients with Fournier’s gangrene also develop systemic signs of infection, including fever, tachycardia, tachypnea, and hypotension. The key to diagnosis is careful examination of the perineal and scrotal area in any patient presenting with acute scrotal pain.
In the majority of cases, the diagnosis of Fournier’s gangrene is made clinically. Once the diagnosis is made, patients require immediate and aggressive IV fluid resuscitation, broad-spectrum IV antibiotics (typically vancomycin and piperacillin/tazobactam), and emergent evaluation by a urologist. It is essential that these patients undergo early and aggressive surgical exploration and debridement of necrotic tissue.2 Antibiotic therapy alone is associated with a 100% mortality rate, emphasizing the need for urgent surgery.2 Even with optimal medical and surgical management, the mortality rate remains significant.
Summary
This case emphasizes several important teaching points. The EP should be mindful of the patient who keeps returning to the ED with the same complaint—despite “appropriate” treatment—as the initial diagnosis may not be the correct one. Such returning patients require greater, not less, scrutiny. As with any patient, the EP should always take a complete history and perform a thorough physical examination at each presentation—as one would with a de novo patient. Finally, the EP should consider Fournier’s gangrene in addition to testicular torsion and epididymitis in the differential diagnosis for acute scrotal pain.
1. Trojian TH, Lishnak TS, Heiman D. Epididymitis and orchitis: an overview. Am Fam Physician. 2009;79(7):583-587.
2. Eyre RC. Evaluation of acute scrotal pain in adults. UpToDate Web site. https://www.uptodate.com/contents/evaluation-of-acute-scrotal-pain-in-adults. Updated July 31, 2017. Accessed September 7, 2017.
3. Shen Y, Liu H, Cheng J, Bu P. Amiodarone-induced epididymitis: a pathologically confirmed case report and review of the literature. Cardiology. 2014;128(4):349-351. doi:10.1159/000361038.
4. Tracy CR, Steers WD, Costabile R. Diagnosis and management of epididymitis. Urol Clin North Am. 2008;35(1):101-108. doi:10.1016/j.ucl.2007.09.013.
5. Wampler SM, Llanes M. Common scrotal and testicular problems. Prim Care. 2010;37(3):613-626. doi:10.1016/j.pop.2010.04.009.
6. Dunne PJ, O’Loughlin BS. Testicular torsion: time is the enemy. Aust NZ J Surg. 2000;70(6):441-442.
7. Cummings JM, Boullier JA, Sekhon D, Bose K. Adult testicular torsion. J Urol. 2002;167(5):2109-2110.
Case
A 52-year-old man presented to the ED for evaluation of right scrotal pain and swelling. The patient stated that the pain started several hours prior to presentation and had gradually worsened. He denied any trauma or inciting event to the affected area; he further denied abdominal pain, nausea, vomiting, dysuria, polyuria, or fever. The patient’s remote medical history was significant for type 2 diabetes mellitus (DM), which he managed through dietary modification-only as he had refused pharmacological therapy. The patient admitted to smoking one half-pack of cigarettes per week, but denied alcohol or illicit drug use.
At presentation, the patient’s vital signs were all within normal range. The physical examination was remarkable only for right testicular tenderness and mild scrotal swelling, and there were no hernias or lymphadenopathy present.
The emergency physician (EP) ordered a urinalysis and color-flow Doppler ultrasound study of both testes, which the radiologist interpreted as an enlarged right epididymis with hyperemia; the left testicle was normal. The urinalysis was normal.
The patient was diagnosed with epididymitis and discharged home with a prescription for oral levofloxacin 500 mg daily for 10 days. He also was instructed to take ibuprofen for pain, apply ice to the affected area, keep the scrotal area elevated, and follow-up with a urologist in 1 week.
Approximately 8 hours after discharge, the patient returned to the same ED with complaints of increasing right testicular pain and swelling. The history and physical examination at this visit were essentially unchanged from his initial presentation. No laboratory evaluation, imaging studies, or other tests were ordered at the second visit.
The patient was discharged home with a prescription for a narcotic analgesic, which he was instructed to take in addition to the ibuprofen; he was also instructed to follow-up with a urologist within the next 2 to 3 days, instead of in 1 week.
The patient returned the following morning to the same ED with complaints of increased swelling and pain of the right testicle. In addition to the worsening testicular pain and swelling, he also had right inguinal pain, nausea, vomiting, and fever. Vital signs at this third presentation were: blood pressure (BP), 124/64 mm Hg; heart rate (HR), 110 beats/min; respiratory rate, 20 breaths/min; and temperature, 99.8o F. Oxygen saturation was 98% on room air.
The patient was tachycardic on heart examination, but with regular rhythm and no murmurs, rubs, or gallops. The lung and abdominal examinations were normal. The genital examination revealed marked right scrotal swelling and tenderness, as well as tender right inguinal lymphadenopathy.
The EP ordered an intravenous (IV) bolus of 1 L normal saline and laboratory studies, which included lactic acid, blood cultures, urinalysis, and urine culture and sensitivity. The EP was concerned for a scrotal abscess and ordered a testicular Doppler color-flow ultrasound study. The laboratory studies revealed an elevated white blood count of 16.5 K/uL, elevated blood glucose of 364 mg/dL, and elevated lactate of 2.8 mg/dL. As demonstrated on the ultrasound study performed at the patient’s first presentation, the ultrasound again showed an enlarged right epididymis, but without orchitis or abscess. The scrotal wall had significant thickening, consistent with cellulitis. The EP ordered broad spectrum IV antibiotics and admitted the patient to the hospitalist with a consult request for urology services.
The patient continued to receive IV fluids and antibiotics throughout the evening. In the morning, he was seen by the same hospitalist/admitting physician from the previous evening. Upon physical examination, the hospitalist noted tenderness, swelling, and erythema in the patient’s perineal area. The patient’s BP had dropped to 100/60 mm Hg, and his HR had increased to 115 beats/min despite receiving nearly 2 L of normal saline IV throughout the previous evening and night.
The urologist examined the patient soon after the consult request and diagnosed him with Fournier’s gangrene. He started the patient on aggressive IV fluid resuscitation, after which the patient was immediately taken to the operating room for extensive surgical debridement and scrotectomy. The patient’s postoperative course was complicated by acute kidney injury, respiratory failure requiring ventilator support, and sepsis. After a lengthy hospital stay, the patient was discharged home, but required a scrotal skin graft, and experienced erectile dysfunction and depression.
The patient sued all of the EPs involved in his care, the hospital, the hospitalist/admitting physician, and the urologist for negligence. The plaintiff’s attorney argued that since the patient progressively deteriorated over the 24 to 36 hours during his three presentations to the ED, urology services should have been consulted earlier, and that the urologist should have seen the patient immediately at the time of hospital admission.
The attorneys for the defendants claimed the patient denied dysuria, penile lesions, or urethral discharge and that the history, physical examination, and testicular ultrasound were all consistent with the diagnosis of epididymitis. For this reason, they argued, there was no indication for an emergent consultation with urology services. The jury returned a defense verdict.
Discussion
It is easy for a busy EP to have a differential diagnosis of only two disorders when evaluating a patient for unilateral testicular pain and swelling—in this case, testicular torsion and epididymitis. While these are the most common causes of testicular pain and swelling, this case emphasizes the need to also consider Fournier’s gangrene in the differential. A thorough history and physical examination, coupled with appropriate testing, will usually identify the correct diagnosis. While the differential diagnosis is broader than just these three disease processes (see the Box), we will review the evaluation and management of the three most serious: epididymitis, testicular torsion, and Fournier’s gangrene.
Noninfectious and Bacterial Epididymitis
Epididymitis is the most common cause of acute scrotal pain among US adults, accounting for approximately 600,000 cases each year.1 Infectious epididymitis is typically classified as acute (symptom duration of <6 weeks) or chronic (symptom duration of ≥6 weeks).2
Cases of noninfectious epididymitis are typically due to a chronic condition, such as autoimmune disease, cancer, or vasculitis. Although not as common, noninfectious epididymitis can also occur due to testicular trauma or amiodarone therapy.3,4
Patients with acute bacterial epididymitis typically present with scrotal pain and swelling ranging from mild to marked. These patients may also exhibit fever and chills, along with dysuria, frequency, and urgency, if associated with a urinary tract infection.2 The chronic presentation is more common though, and usually not associated with voiding issues.
Chronic epididymis is frequently seen in postpubertal boys and men following sexual activity, heavy physical exertion, and bicycle/motorcycle riding.2 On physical examination, palpation reveals induration and swelling of the involved epididymis with exquisite tenderness.2 Testicular swelling and pain, along with scrotal wall erythema, may be present in more advanced cases.2 The cremasteric reflex should be intact (ie, scratching the medial proximal thigh will cause ipsilateral testicle retraction). Similarly, the lie of both testicles while the patient is standing should be equal and symmetrical—ie, both testicles descended equally. However, in the presence of moderate-to-severe scrotal swelling, both of these physical findings may be impossible to confirm.
A urinalysis and urine culture should be ordered if there is any suspicion of epididymitis; pyuria will be present in approximately 50% of cases. However, since pyuria is neither sensitive nor specific for epididymitis, in most cases, a testicular ultrasound with Doppler flow is required to exclude testicular torsion. In cases of epididymitis, ultrasound usually demonstrates increased flow on the affected side, whereas in testicular torsion, there is decreased or absent blood flow.
The treatment for epididymitis involves antibiotics and symptomatic care. If epididymitis from chlamydia and/or gonorrhea is the suspected cause, or if the patient is younger than age 35 years, he should be given ceftriaxone 250 mg intramuscularly plus oral doxycycline 100 mg twice a day for 10 days. Patients who practice insertive anal sex should be treated with ceftriaxone, plus either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.
In cases in which enteric organisms are suspected, the patient is older than age 35 years, or if patient status is posturinary tract instrumentation or vasectomy, he should be treated with either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.2
For symptomatic relief, scrotal elevation, ice application, and nonsteroidal anti-inflammatory drugs are recommended.
Patients with epididymitis, regardless of etiology, should be instructed to follow-up with a urologist within 1 week. If the patient appears ill, septic, or in significant pain, admission to the hospital with IV antibiotics, IV fluids, and an urgent consult with urology services is required.
Testicular Torsion
Testicular torsion is a time-sensitive issue, requiring early diagnosis and rapid treatment to preserve the patient’s fertility. Most clinicians recommend detorsion within 6 hours of torsion onset because salvage rates are excellent when performed within this timeframe; after 12 hours, the testis will likely suffer irreversible damage due to ischemia.5,6
Testicular torsion can occur at any age, but is most commonly seen in a bimodal distribution—ie, neonates and postpubertal boys. The prevalence of testicular torsion in adult patients hospitalized with acute scrotal pain is approximately 25% to 50%.2
Patients with testicular torsion usually describe a sudden onset of severe, acute pain. The pain frequently occurs a few hours after vigorous physical activity or minor testicular trauma.2 Occasionally, the patient may complain of lower quadrant abdominal pain rather than testicular or scrotal pain. Nausea with vomiting can also be present.
On physical examination, significant testicular swelling is usually present. Examining the patient in the standing position will often reveal an asymmetrical, high-riding testis with a transverse lie on the affected side. The cremasteric reflex is usually absent in patients with testicular torsion.
Because of the significant overlap in history and physical examination findings for epididymitis and testicular torsion, a testicular ultrasound with color Doppler should be ordered. Multiple studies have confirmed the high sensitivity and specificity of ultrasound in the diagnosis of testicular torsion.
The treatment for suspected or confirmed testicular torsion is immediate surgical exploration with intraoperative detorsion and fixation of the testes. The EP can attempt manual detorsion (ie, performed in a medial to lateral motion, similar to opening a book). However, this should not delay the EP from consulting with urology services.
Pediatric patients with testicular torsion usually have a more favorable outcome than do adults. In one retrospective study, patients younger than age 21 years had a 70% testicular salvage rate compared to only 41% of patients aged 21 years and older.7 Regardless of age, better outcomes are associated with shorter periods of torsion.
Fournier’s Gangrene
Fournier’s gangrene is a polymicrobial necrotizing fasciitis of the perineum and scrotum that typically develops initially as a benign infection or abscess but quickly spreads. Risk factors for Fournier’s gangrene include DM, alcohol abuse, and any immunocompromised state (eg, HIV, cancer).
If the patient presents early in onset, there may be only mild tenderness, erythema, or swelling of the affected area; however, this infection progresses rapidly. Later findings include marked tenderness, swelling, crepitus, blisters, and ecchymoses. Patients with Fournier’s gangrene also develop systemic signs of infection, including fever, tachycardia, tachypnea, and hypotension. The key to diagnosis is careful examination of the perineal and scrotal area in any patient presenting with acute scrotal pain.
In the majority of cases, the diagnosis of Fournier’s gangrene is made clinically. Once the diagnosis is made, patients require immediate and aggressive IV fluid resuscitation, broad-spectrum IV antibiotics (typically vancomycin and piperacillin/tazobactam), and emergent evaluation by a urologist. It is essential that these patients undergo early and aggressive surgical exploration and debridement of necrotic tissue.2 Antibiotic therapy alone is associated with a 100% mortality rate, emphasizing the need for urgent surgery.2 Even with optimal medical and surgical management, the mortality rate remains significant.
Summary
This case emphasizes several important teaching points. The EP should be mindful of the patient who keeps returning to the ED with the same complaint—despite “appropriate” treatment—as the initial diagnosis may not be the correct one. Such returning patients require greater, not less, scrutiny. As with any patient, the EP should always take a complete history and perform a thorough physical examination at each presentation—as one would with a de novo patient. Finally, the EP should consider Fournier’s gangrene in addition to testicular torsion and epididymitis in the differential diagnosis for acute scrotal pain.
Case
A 52-year-old man presented to the ED for evaluation of right scrotal pain and swelling. The patient stated that the pain started several hours prior to presentation and had gradually worsened. He denied any trauma or inciting event to the affected area; he further denied abdominal pain, nausea, vomiting, dysuria, polyuria, or fever. The patient’s remote medical history was significant for type 2 diabetes mellitus (DM), which he managed through dietary modification-only as he had refused pharmacological therapy. The patient admitted to smoking one half-pack of cigarettes per week, but denied alcohol or illicit drug use.
At presentation, the patient’s vital signs were all within normal range. The physical examination was remarkable only for right testicular tenderness and mild scrotal swelling, and there were no hernias or lymphadenopathy present.
The emergency physician (EP) ordered a urinalysis and color-flow Doppler ultrasound study of both testes, which the radiologist interpreted as an enlarged right epididymis with hyperemia; the left testicle was normal. The urinalysis was normal.
The patient was diagnosed with epididymitis and discharged home with a prescription for oral levofloxacin 500 mg daily for 10 days. He also was instructed to take ibuprofen for pain, apply ice to the affected area, keep the scrotal area elevated, and follow-up with a urologist in 1 week.
Approximately 8 hours after discharge, the patient returned to the same ED with complaints of increasing right testicular pain and swelling. The history and physical examination at this visit were essentially unchanged from his initial presentation. No laboratory evaluation, imaging studies, or other tests were ordered at the second visit.
The patient was discharged home with a prescription for a narcotic analgesic, which he was instructed to take in addition to the ibuprofen; he was also instructed to follow-up with a urologist within the next 2 to 3 days, instead of in 1 week.
The patient returned the following morning to the same ED with complaints of increased swelling and pain of the right testicle. In addition to the worsening testicular pain and swelling, he also had right inguinal pain, nausea, vomiting, and fever. Vital signs at this third presentation were: blood pressure (BP), 124/64 mm Hg; heart rate (HR), 110 beats/min; respiratory rate, 20 breaths/min; and temperature, 99.8o F. Oxygen saturation was 98% on room air.
The patient was tachycardic on heart examination, but with regular rhythm and no murmurs, rubs, or gallops. The lung and abdominal examinations were normal. The genital examination revealed marked right scrotal swelling and tenderness, as well as tender right inguinal lymphadenopathy.
The EP ordered an intravenous (IV) bolus of 1 L normal saline and laboratory studies, which included lactic acid, blood cultures, urinalysis, and urine culture and sensitivity. The EP was concerned for a scrotal abscess and ordered a testicular Doppler color-flow ultrasound study. The laboratory studies revealed an elevated white blood count of 16.5 K/uL, elevated blood glucose of 364 mg/dL, and elevated lactate of 2.8 mg/dL. As demonstrated on the ultrasound study performed at the patient’s first presentation, the ultrasound again showed an enlarged right epididymis, but without orchitis or abscess. The scrotal wall had significant thickening, consistent with cellulitis. The EP ordered broad spectrum IV antibiotics and admitted the patient to the hospitalist with a consult request for urology services.
The patient continued to receive IV fluids and antibiotics throughout the evening. In the morning, he was seen by the same hospitalist/admitting physician from the previous evening. Upon physical examination, the hospitalist noted tenderness, swelling, and erythema in the patient’s perineal area. The patient’s BP had dropped to 100/60 mm Hg, and his HR had increased to 115 beats/min despite receiving nearly 2 L of normal saline IV throughout the previous evening and night.
The urologist examined the patient soon after the consult request and diagnosed him with Fournier’s gangrene. He started the patient on aggressive IV fluid resuscitation, after which the patient was immediately taken to the operating room for extensive surgical debridement and scrotectomy. The patient’s postoperative course was complicated by acute kidney injury, respiratory failure requiring ventilator support, and sepsis. After a lengthy hospital stay, the patient was discharged home, but required a scrotal skin graft, and experienced erectile dysfunction and depression.
The patient sued all of the EPs involved in his care, the hospital, the hospitalist/admitting physician, and the urologist for negligence. The plaintiff’s attorney argued that since the patient progressively deteriorated over the 24 to 36 hours during his three presentations to the ED, urology services should have been consulted earlier, and that the urologist should have seen the patient immediately at the time of hospital admission.
The attorneys for the defendants claimed the patient denied dysuria, penile lesions, or urethral discharge and that the history, physical examination, and testicular ultrasound were all consistent with the diagnosis of epididymitis. For this reason, they argued, there was no indication for an emergent consultation with urology services. The jury returned a defense verdict.
Discussion
It is easy for a busy EP to have a differential diagnosis of only two disorders when evaluating a patient for unilateral testicular pain and swelling—in this case, testicular torsion and epididymitis. While these are the most common causes of testicular pain and swelling, this case emphasizes the need to also consider Fournier’s gangrene in the differential. A thorough history and physical examination, coupled with appropriate testing, will usually identify the correct diagnosis. While the differential diagnosis is broader than just these three disease processes (see the Box), we will review the evaluation and management of the three most serious: epididymitis, testicular torsion, and Fournier’s gangrene.
Noninfectious and Bacterial Epididymitis
Epididymitis is the most common cause of acute scrotal pain among US adults, accounting for approximately 600,000 cases each year.1 Infectious epididymitis is typically classified as acute (symptom duration of <6 weeks) or chronic (symptom duration of ≥6 weeks).2
Cases of noninfectious epididymitis are typically due to a chronic condition, such as autoimmune disease, cancer, or vasculitis. Although not as common, noninfectious epididymitis can also occur due to testicular trauma or amiodarone therapy.3,4
Patients with acute bacterial epididymitis typically present with scrotal pain and swelling ranging from mild to marked. These patients may also exhibit fever and chills, along with dysuria, frequency, and urgency, if associated with a urinary tract infection.2 The chronic presentation is more common though, and usually not associated with voiding issues.
Chronic epididymis is frequently seen in postpubertal boys and men following sexual activity, heavy physical exertion, and bicycle/motorcycle riding.2 On physical examination, palpation reveals induration and swelling of the involved epididymis with exquisite tenderness.2 Testicular swelling and pain, along with scrotal wall erythema, may be present in more advanced cases.2 The cremasteric reflex should be intact (ie, scratching the medial proximal thigh will cause ipsilateral testicle retraction). Similarly, the lie of both testicles while the patient is standing should be equal and symmetrical—ie, both testicles descended equally. However, in the presence of moderate-to-severe scrotal swelling, both of these physical findings may be impossible to confirm.
A urinalysis and urine culture should be ordered if there is any suspicion of epididymitis; pyuria will be present in approximately 50% of cases. However, since pyuria is neither sensitive nor specific for epididymitis, in most cases, a testicular ultrasound with Doppler flow is required to exclude testicular torsion. In cases of epididymitis, ultrasound usually demonstrates increased flow on the affected side, whereas in testicular torsion, there is decreased or absent blood flow.
The treatment for epididymitis involves antibiotics and symptomatic care. If epididymitis from chlamydia and/or gonorrhea is the suspected cause, or if the patient is younger than age 35 years, he should be given ceftriaxone 250 mg intramuscularly plus oral doxycycline 100 mg twice a day for 10 days. Patients who practice insertive anal sex should be treated with ceftriaxone, plus either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.
In cases in which enteric organisms are suspected, the patient is older than age 35 years, or if patient status is posturinary tract instrumentation or vasectomy, he should be treated with either oral ofloxacin 300 mg twice a day or oral levofloxacin 500 mg daily for 10 days.2
For symptomatic relief, scrotal elevation, ice application, and nonsteroidal anti-inflammatory drugs are recommended.
Patients with epididymitis, regardless of etiology, should be instructed to follow-up with a urologist within 1 week. If the patient appears ill, septic, or in significant pain, admission to the hospital with IV antibiotics, IV fluids, and an urgent consult with urology services is required.
Testicular Torsion
Testicular torsion is a time-sensitive issue, requiring early diagnosis and rapid treatment to preserve the patient’s fertility. Most clinicians recommend detorsion within 6 hours of torsion onset because salvage rates are excellent when performed within this timeframe; after 12 hours, the testis will likely suffer irreversible damage due to ischemia.5,6
Testicular torsion can occur at any age, but is most commonly seen in a bimodal distribution—ie, neonates and postpubertal boys. The prevalence of testicular torsion in adult patients hospitalized with acute scrotal pain is approximately 25% to 50%.2
Patients with testicular torsion usually describe a sudden onset of severe, acute pain. The pain frequently occurs a few hours after vigorous physical activity or minor testicular trauma.2 Occasionally, the patient may complain of lower quadrant abdominal pain rather than testicular or scrotal pain. Nausea with vomiting can also be present.
On physical examination, significant testicular swelling is usually present. Examining the patient in the standing position will often reveal an asymmetrical, high-riding testis with a transverse lie on the affected side. The cremasteric reflex is usually absent in patients with testicular torsion.
Because of the significant overlap in history and physical examination findings for epididymitis and testicular torsion, a testicular ultrasound with color Doppler should be ordered. Multiple studies have confirmed the high sensitivity and specificity of ultrasound in the diagnosis of testicular torsion.
The treatment for suspected or confirmed testicular torsion is immediate surgical exploration with intraoperative detorsion and fixation of the testes. The EP can attempt manual detorsion (ie, performed in a medial to lateral motion, similar to opening a book). However, this should not delay the EP from consulting with urology services.
Pediatric patients with testicular torsion usually have a more favorable outcome than do adults. In one retrospective study, patients younger than age 21 years had a 70% testicular salvage rate compared to only 41% of patients aged 21 years and older.7 Regardless of age, better outcomes are associated with shorter periods of torsion.
Fournier’s Gangrene
Fournier’s gangrene is a polymicrobial necrotizing fasciitis of the perineum and scrotum that typically develops initially as a benign infection or abscess but quickly spreads. Risk factors for Fournier’s gangrene include DM, alcohol abuse, and any immunocompromised state (eg, HIV, cancer).
If the patient presents early in onset, there may be only mild tenderness, erythema, or swelling of the affected area; however, this infection progresses rapidly. Later findings include marked tenderness, swelling, crepitus, blisters, and ecchymoses. Patients with Fournier’s gangrene also develop systemic signs of infection, including fever, tachycardia, tachypnea, and hypotension. The key to diagnosis is careful examination of the perineal and scrotal area in any patient presenting with acute scrotal pain.
In the majority of cases, the diagnosis of Fournier’s gangrene is made clinically. Once the diagnosis is made, patients require immediate and aggressive IV fluid resuscitation, broad-spectrum IV antibiotics (typically vancomycin and piperacillin/tazobactam), and emergent evaluation by a urologist. It is essential that these patients undergo early and aggressive surgical exploration and debridement of necrotic tissue.2 Antibiotic therapy alone is associated with a 100% mortality rate, emphasizing the need for urgent surgery.2 Even with optimal medical and surgical management, the mortality rate remains significant.
Summary
This case emphasizes several important teaching points. The EP should be mindful of the patient who keeps returning to the ED with the same complaint—despite “appropriate” treatment—as the initial diagnosis may not be the correct one. Such returning patients require greater, not less, scrutiny. As with any patient, the EP should always take a complete history and perform a thorough physical examination at each presentation—as one would with a de novo patient. Finally, the EP should consider Fournier’s gangrene in addition to testicular torsion and epididymitis in the differential diagnosis for acute scrotal pain.
1. Trojian TH, Lishnak TS, Heiman D. Epididymitis and orchitis: an overview. Am Fam Physician. 2009;79(7):583-587.
2. Eyre RC. Evaluation of acute scrotal pain in adults. UpToDate Web site. https://www.uptodate.com/contents/evaluation-of-acute-scrotal-pain-in-adults. Updated July 31, 2017. Accessed September 7, 2017.
3. Shen Y, Liu H, Cheng J, Bu P. Amiodarone-induced epididymitis: a pathologically confirmed case report and review of the literature. Cardiology. 2014;128(4):349-351. doi:10.1159/000361038.
4. Tracy CR, Steers WD, Costabile R. Diagnosis and management of epididymitis. Urol Clin North Am. 2008;35(1):101-108. doi:10.1016/j.ucl.2007.09.013.
5. Wampler SM, Llanes M. Common scrotal and testicular problems. Prim Care. 2010;37(3):613-626. doi:10.1016/j.pop.2010.04.009.
6. Dunne PJ, O’Loughlin BS. Testicular torsion: time is the enemy. Aust NZ J Surg. 2000;70(6):441-442.
7. Cummings JM, Boullier JA, Sekhon D, Bose K. Adult testicular torsion. J Urol. 2002;167(5):2109-2110.
1. Trojian TH, Lishnak TS, Heiman D. Epididymitis and orchitis: an overview. Am Fam Physician. 2009;79(7):583-587.
2. Eyre RC. Evaluation of acute scrotal pain in adults. UpToDate Web site. https://www.uptodate.com/contents/evaluation-of-acute-scrotal-pain-in-adults. Updated July 31, 2017. Accessed September 7, 2017.
3. Shen Y, Liu H, Cheng J, Bu P. Amiodarone-induced epididymitis: a pathologically confirmed case report and review of the literature. Cardiology. 2014;128(4):349-351. doi:10.1159/000361038.
4. Tracy CR, Steers WD, Costabile R. Diagnosis and management of epididymitis. Urol Clin North Am. 2008;35(1):101-108. doi:10.1016/j.ucl.2007.09.013.
5. Wampler SM, Llanes M. Common scrotal and testicular problems. Prim Care. 2010;37(3):613-626. doi:10.1016/j.pop.2010.04.009.
6. Dunne PJ, O’Loughlin BS. Testicular torsion: time is the enemy. Aust NZ J Surg. 2000;70(6):441-442.
7. Cummings JM, Boullier JA, Sekhon D, Bose K. Adult testicular torsion. J Urol. 2002;167(5):2109-2110.
Case Studies in Toxicology: Always Cook Your Boba
Case
A 45-year-old Chinese man with no known medical history presented to the ED with right-sided facial spasm and cheek swelling, which began immediately after he bit into a piece of taro root, approximately 2 hours prior to presentation. The patient stated that the root was an ingredient in a soup that a relative had made. According to the patient, after biting into the root, he immediately experienced a burning pain on the right side of his mouth. He further noted that he swallowed less than two bites of the root and stopped eating because the act of chewing was too painful.
Initial vital signs at presentation were: blood pressure, 140/100 mm Hg; heart rate, 84 beats/min; respiratory rate, 14 beats/min; and temperature, 97.6°F. Oxygen saturation was 98% on room air. The patient’s physical examination was remarkable for pain upon opening the mouth, as well as right-sided cheek and lip swelling and tenderness. The tongue and oropharynx were not erythematous or swollen. The patient was only able to speak in short sentences, secondary to oropharyngeal pain, but he was in no respiratory distress. No urticaria, pruritus, wheezing, or stridor was present.
During the patient’s workup, his 40-year-old wife also presented to the same ED for evaluation of burning pain and spasm on the left side of her mouth, which she stated also developed immediately after she bit into a piece of taro root contained in the same soup as that ingested by the patient.
The wife’s vital signs were unremarkable, and she was in no respiratory distress. Her physical examination was remarkable only for left-sided cheek and lip swelling and tenderness, associated with an erythematous oropharynx and pain with speaking.
What is taro? What are the manifestations of taro toxicity?
Taro commonly refers to plants from the Araceae family, usually Colocasia esculenta.1 Taro is ubiquitous in Southern Asia and Southeast India. It is a widely naturalized and perennial tropical plant primarily grown as a root vegetable, and is a common flavor in boba (bubble) tea. All members of Araceae contain calcium oxalate crystals in the form of raphides, sharp needle-shaped crystals packaged in idioblasts and contained within the waxy leaf.2 Pressure on the idioblasts, such as from mastication, triggers the release of the raphides. The needles pierce the surface of any tissue with which they come into contact, creating a gateway for proteolytic enzymes to enter the consumer.3 The leaves and root of Araceae must be cooked before eating to inactivate the raphides.
Oral exposure to uncooked taro leaves or taro root can result in mouth irritation and swelling that can progress to angioedema and airway obstruction. Although the traditional method of removing taro raphides is to soak the root in cold water overnight,4,5 this does not fully remove all of the raphides. Instead, taro root should be thoroughly cooked in boiling water to draw-out oxalates from the root into the cooking water, which must then be discarded. Consuming taro with warm milk also reduces the effect of the oxalates by about 80%.6
Many other plants of the Araceae family, such as Dieffenbachia (dumbcane), share similar toxicity and are commonly kept in the home and office.
Patients with oral exposure to taro may experience a delayed (also termed biphasic) anaphylactic reaction, ie, the development of anaphylactic symptoms more than 4 hours after the inciting event. Delayed anaphylaxis is distinct from delayed hypersensitivity, though both may be immunoglobulin E-mediated. Delayed hypersensitivity presents later (2-14 days) and with less immediately life-threatening effects, most commonly dermatitis (eg, poison ivy dermatitis).
While both of the patients in this case presented with mild symptoms, life-threatening angioedema of the oropharynx, anaphylaxis, and hypocalcemia have been reported7,8 and should be considered in any symptomatic patient with exposure to taro.
What is the differential diagnosis of plant-related mouth pain?
The oral mucosa is composed of superficial layers of mucin and epithelial cells that lie over the dermis and connective tissue. Local immune cells, including mast cells and Langerhans cells, reside in the deeper layers. The differential diagnosis of plant-based mouth pain can be divided into mechanical, chemical, and thermal causes.
Mechanical Causes. Causes of mechanical plant-based oral pain include structural damage when foreign matter, such as barbs, sharp leaves, or hard seeds, pierce the layers of the oral mucosa.
Chemical Causes. Chemical-related causes of oral pain include caustic ingestion, for example from detergents or cleaning agents that contaminate the broth. Araceae, such as taro or arum, have sharp calcium oxalate crystals tipped with phospholipases and proteases that cause mechanical pain on piercing mucous membranes, and chemical pain by enzymatically degrading epithelium and mucosa. Both chemical and mechanical irritation can lead to an inflammatory response. Raw taro can cause irritant contact stomatitis as the raphides pierce the oral mucosa. It can also cause allergic stomatitis if antigens related to the phospholipases or proteases are presented to Langerhans cells.9
Thermal Causes. The hot temperature of the ingested broth could cause thermal injury, but the injury is likely to be more diffuse.
How common is taro exposure, and how is it treated?
From 1995 to 1999, 15 cases of taro poisoning were reported to the Drug and Toxicology Information service in Zimbabwe.10 From 2005 to 2009, 21 out of 31 cases reported to the Hong Kong Poison Control Center involving gastrointestinal irritation involved the consumption of Colocasia fallax, a form of taro more common in Tibet, the Himalayas, and northern Indochina.7 Of the 31 cases, six patients were treated with diphenhydramine, epinephrine, and dexamethasone for angioedema.
From 2011 to 2013, two cases of mouth irritation and swelling after eating raw taro leaves were reported to the British Columbia Poison Control Center.11 Those two patients were observed for 6 hours without specific treatment and discharged.
Case Conclusion
Due to concerns of the potential for anaphylaxis, both patients were treated intravenously with 50 mg diphenhydramine and 10 mg dexamethasone. The husband was also given 650 mg acetaminophen orally for pain relief; his wife declined pain medication. Laboratory evaluation, including a complete blood count, basic metabolic panel, liver function panel, and urinalysis were ordered for both patients; all results were within normal limits for both patients.
After an uneventful 6-hour observation period, both patients were discharged home with instructions to return to the ED if they develop any signs of allergic reaction and to call emergency medical services for any sign of anaphylaxis.
1. Rao RV, Matthews PJ, Eyzaguirre PB, Hunter D, eds. 2010. The Global Diversity of Taro: Ethnobotany and Conservation. Rome, Italy; Biouniversity International; 2010. http://www.bioversityinternational.org/fileadmin/user_upload/online_library/publications/pdfs/1402.pdf#page=11. Accessed September 15, 2017.
2. Franceschi VR, Nakata PA. Calcium oxalate in plants: formation and function. Annu Rev Plant Biol. 2005;56:41-71. doi:10.1146/annurev.arplant.56.032604.144106.
3. Herbert DA. Stinging crystals in plants. Science. 1924;60(1548):204-205. doi:10.1126/science.60.1548.204-a.
4. Njintang YN, Mbofung CMF. Effect of precooking time and drying temperature on the physico-chemical characteristics and in-vitro carbohydrate digestibility of taro flour. LWT – Food Sci and Tech. 2006;39(6):684-691. doi.org/10.1016/j.lwt.2005.03.022.
5. Savage GP, Dubois M. The effect of soaking and cooking on the oxalate content of taro leaves. Int J Food Sci Nutr. 2006;57(5-6):376-381. doi:10.1080/09637480600855239.
6. Oscarsson, KV. Savage GP. Composition and availability of soluble and insoluble oxalates in raw and cooked taro (Colocasia esculenta var. Schott) leaves. Food Chem 101. 2007;101(2):559-562. doi:10.1016/j.foodchem.2006.02.014.
7. Pang CT, Ng HW, Lau FL. Oral mucosal irritating plant ingestion in Hong Kong, epidemiology and its clinical presentation. Hong Kong J Emerg Med. 2010;17(5):477-481.
8. Yuen E. Upper airway obstruction as a presentation of Taro poisoning. Hong Kong J Emerg Med. 2001;8(3):163-165.
9. Davis CC, Squier CA, Lilly GE. Irritant contact stomatitis: a review of the condition. J Periodontol. 1998;69(6):620-631. doi:10.1902/jop.1998.69.6.620.
10 Tagwireyi D, Ball DE. The management of Elephant’s Ear poisoning. Hum Exp Toxicol. 2001;20(4):189-192. doi:10.1191/096032701678766822.
11. Omura JD, Blake C, McIntyre L, Li D, Kosatsky T. Two cases of poisoning by raw taro leaf and how a poison control centre, food safety inspectors, and a specialty supermarket chain found a solution.” Environ Health Rev. 2014;57(3):59-64. doi.org/10.5864/d2014-027.
Case
A 45-year-old Chinese man with no known medical history presented to the ED with right-sided facial spasm and cheek swelling, which began immediately after he bit into a piece of taro root, approximately 2 hours prior to presentation. The patient stated that the root was an ingredient in a soup that a relative had made. According to the patient, after biting into the root, he immediately experienced a burning pain on the right side of his mouth. He further noted that he swallowed less than two bites of the root and stopped eating because the act of chewing was too painful.
Initial vital signs at presentation were: blood pressure, 140/100 mm Hg; heart rate, 84 beats/min; respiratory rate, 14 beats/min; and temperature, 97.6°F. Oxygen saturation was 98% on room air. The patient’s physical examination was remarkable for pain upon opening the mouth, as well as right-sided cheek and lip swelling and tenderness. The tongue and oropharynx were not erythematous or swollen. The patient was only able to speak in short sentences, secondary to oropharyngeal pain, but he was in no respiratory distress. No urticaria, pruritus, wheezing, or stridor was present.
During the patient’s workup, his 40-year-old wife also presented to the same ED for evaluation of burning pain and spasm on the left side of her mouth, which she stated also developed immediately after she bit into a piece of taro root contained in the same soup as that ingested by the patient.
The wife’s vital signs were unremarkable, and she was in no respiratory distress. Her physical examination was remarkable only for left-sided cheek and lip swelling and tenderness, associated with an erythematous oropharynx and pain with speaking.
What is taro? What are the manifestations of taro toxicity?
Taro commonly refers to plants from the Araceae family, usually Colocasia esculenta.1 Taro is ubiquitous in Southern Asia and Southeast India. It is a widely naturalized and perennial tropical plant primarily grown as a root vegetable, and is a common flavor in boba (bubble) tea. All members of Araceae contain calcium oxalate crystals in the form of raphides, sharp needle-shaped crystals packaged in idioblasts and contained within the waxy leaf.2 Pressure on the idioblasts, such as from mastication, triggers the release of the raphides. The needles pierce the surface of any tissue with which they come into contact, creating a gateway for proteolytic enzymes to enter the consumer.3 The leaves and root of Araceae must be cooked before eating to inactivate the raphides.
Oral exposure to uncooked taro leaves or taro root can result in mouth irritation and swelling that can progress to angioedema and airway obstruction. Although the traditional method of removing taro raphides is to soak the root in cold water overnight,4,5 this does not fully remove all of the raphides. Instead, taro root should be thoroughly cooked in boiling water to draw-out oxalates from the root into the cooking water, which must then be discarded. Consuming taro with warm milk also reduces the effect of the oxalates by about 80%.6
Many other plants of the Araceae family, such as Dieffenbachia (dumbcane), share similar toxicity and are commonly kept in the home and office.
Patients with oral exposure to taro may experience a delayed (also termed biphasic) anaphylactic reaction, ie, the development of anaphylactic symptoms more than 4 hours after the inciting event. Delayed anaphylaxis is distinct from delayed hypersensitivity, though both may be immunoglobulin E-mediated. Delayed hypersensitivity presents later (2-14 days) and with less immediately life-threatening effects, most commonly dermatitis (eg, poison ivy dermatitis).
While both of the patients in this case presented with mild symptoms, life-threatening angioedema of the oropharynx, anaphylaxis, and hypocalcemia have been reported7,8 and should be considered in any symptomatic patient with exposure to taro.
What is the differential diagnosis of plant-related mouth pain?
The oral mucosa is composed of superficial layers of mucin and epithelial cells that lie over the dermis and connective tissue. Local immune cells, including mast cells and Langerhans cells, reside in the deeper layers. The differential diagnosis of plant-based mouth pain can be divided into mechanical, chemical, and thermal causes.
Mechanical Causes. Causes of mechanical plant-based oral pain include structural damage when foreign matter, such as barbs, sharp leaves, or hard seeds, pierce the layers of the oral mucosa.
Chemical Causes. Chemical-related causes of oral pain include caustic ingestion, for example from detergents or cleaning agents that contaminate the broth. Araceae, such as taro or arum, have sharp calcium oxalate crystals tipped with phospholipases and proteases that cause mechanical pain on piercing mucous membranes, and chemical pain by enzymatically degrading epithelium and mucosa. Both chemical and mechanical irritation can lead to an inflammatory response. Raw taro can cause irritant contact stomatitis as the raphides pierce the oral mucosa. It can also cause allergic stomatitis if antigens related to the phospholipases or proteases are presented to Langerhans cells.9
Thermal Causes. The hot temperature of the ingested broth could cause thermal injury, but the injury is likely to be more diffuse.
How common is taro exposure, and how is it treated?
From 1995 to 1999, 15 cases of taro poisoning were reported to the Drug and Toxicology Information service in Zimbabwe.10 From 2005 to 2009, 21 out of 31 cases reported to the Hong Kong Poison Control Center involving gastrointestinal irritation involved the consumption of Colocasia fallax, a form of taro more common in Tibet, the Himalayas, and northern Indochina.7 Of the 31 cases, six patients were treated with diphenhydramine, epinephrine, and dexamethasone for angioedema.
From 2011 to 2013, two cases of mouth irritation and swelling after eating raw taro leaves were reported to the British Columbia Poison Control Center.11 Those two patients were observed for 6 hours without specific treatment and discharged.
Case Conclusion
Due to concerns of the potential for anaphylaxis, both patients were treated intravenously with 50 mg diphenhydramine and 10 mg dexamethasone. The husband was also given 650 mg acetaminophen orally for pain relief; his wife declined pain medication. Laboratory evaluation, including a complete blood count, basic metabolic panel, liver function panel, and urinalysis were ordered for both patients; all results were within normal limits for both patients.
After an uneventful 6-hour observation period, both patients were discharged home with instructions to return to the ED if they develop any signs of allergic reaction and to call emergency medical services for any sign of anaphylaxis.
Case
A 45-year-old Chinese man with no known medical history presented to the ED with right-sided facial spasm and cheek swelling, which began immediately after he bit into a piece of taro root, approximately 2 hours prior to presentation. The patient stated that the root was an ingredient in a soup that a relative had made. According to the patient, after biting into the root, he immediately experienced a burning pain on the right side of his mouth. He further noted that he swallowed less than two bites of the root and stopped eating because the act of chewing was too painful.
Initial vital signs at presentation were: blood pressure, 140/100 mm Hg; heart rate, 84 beats/min; respiratory rate, 14 beats/min; and temperature, 97.6°F. Oxygen saturation was 98% on room air. The patient’s physical examination was remarkable for pain upon opening the mouth, as well as right-sided cheek and lip swelling and tenderness. The tongue and oropharynx were not erythematous or swollen. The patient was only able to speak in short sentences, secondary to oropharyngeal pain, but he was in no respiratory distress. No urticaria, pruritus, wheezing, or stridor was present.
During the patient’s workup, his 40-year-old wife also presented to the same ED for evaluation of burning pain and spasm on the left side of her mouth, which she stated also developed immediately after she bit into a piece of taro root contained in the same soup as that ingested by the patient.
The wife’s vital signs were unremarkable, and she was in no respiratory distress. Her physical examination was remarkable only for left-sided cheek and lip swelling and tenderness, associated with an erythematous oropharynx and pain with speaking.
What is taro? What are the manifestations of taro toxicity?
Taro commonly refers to plants from the Araceae family, usually Colocasia esculenta.1 Taro is ubiquitous in Southern Asia and Southeast India. It is a widely naturalized and perennial tropical plant primarily grown as a root vegetable, and is a common flavor in boba (bubble) tea. All members of Araceae contain calcium oxalate crystals in the form of raphides, sharp needle-shaped crystals packaged in idioblasts and contained within the waxy leaf.2 Pressure on the idioblasts, such as from mastication, triggers the release of the raphides. The needles pierce the surface of any tissue with which they come into contact, creating a gateway for proteolytic enzymes to enter the consumer.3 The leaves and root of Araceae must be cooked before eating to inactivate the raphides.
Oral exposure to uncooked taro leaves or taro root can result in mouth irritation and swelling that can progress to angioedema and airway obstruction. Although the traditional method of removing taro raphides is to soak the root in cold water overnight,4,5 this does not fully remove all of the raphides. Instead, taro root should be thoroughly cooked in boiling water to draw-out oxalates from the root into the cooking water, which must then be discarded. Consuming taro with warm milk also reduces the effect of the oxalates by about 80%.6
Many other plants of the Araceae family, such as Dieffenbachia (dumbcane), share similar toxicity and are commonly kept in the home and office.
Patients with oral exposure to taro may experience a delayed (also termed biphasic) anaphylactic reaction, ie, the development of anaphylactic symptoms more than 4 hours after the inciting event. Delayed anaphylaxis is distinct from delayed hypersensitivity, though both may be immunoglobulin E-mediated. Delayed hypersensitivity presents later (2-14 days) and with less immediately life-threatening effects, most commonly dermatitis (eg, poison ivy dermatitis).
While both of the patients in this case presented with mild symptoms, life-threatening angioedema of the oropharynx, anaphylaxis, and hypocalcemia have been reported7,8 and should be considered in any symptomatic patient with exposure to taro.
What is the differential diagnosis of plant-related mouth pain?
The oral mucosa is composed of superficial layers of mucin and epithelial cells that lie over the dermis and connective tissue. Local immune cells, including mast cells and Langerhans cells, reside in the deeper layers. The differential diagnosis of plant-based mouth pain can be divided into mechanical, chemical, and thermal causes.
Mechanical Causes. Causes of mechanical plant-based oral pain include structural damage when foreign matter, such as barbs, sharp leaves, or hard seeds, pierce the layers of the oral mucosa.
Chemical Causes. Chemical-related causes of oral pain include caustic ingestion, for example from detergents or cleaning agents that contaminate the broth. Araceae, such as taro or arum, have sharp calcium oxalate crystals tipped with phospholipases and proteases that cause mechanical pain on piercing mucous membranes, and chemical pain by enzymatically degrading epithelium and mucosa. Both chemical and mechanical irritation can lead to an inflammatory response. Raw taro can cause irritant contact stomatitis as the raphides pierce the oral mucosa. It can also cause allergic stomatitis if antigens related to the phospholipases or proteases are presented to Langerhans cells.9
Thermal Causes. The hot temperature of the ingested broth could cause thermal injury, but the injury is likely to be more diffuse.
How common is taro exposure, and how is it treated?
From 1995 to 1999, 15 cases of taro poisoning were reported to the Drug and Toxicology Information service in Zimbabwe.10 From 2005 to 2009, 21 out of 31 cases reported to the Hong Kong Poison Control Center involving gastrointestinal irritation involved the consumption of Colocasia fallax, a form of taro more common in Tibet, the Himalayas, and northern Indochina.7 Of the 31 cases, six patients were treated with diphenhydramine, epinephrine, and dexamethasone for angioedema.
From 2011 to 2013, two cases of mouth irritation and swelling after eating raw taro leaves were reported to the British Columbia Poison Control Center.11 Those two patients were observed for 6 hours without specific treatment and discharged.
Case Conclusion
Due to concerns of the potential for anaphylaxis, both patients were treated intravenously with 50 mg diphenhydramine and 10 mg dexamethasone. The husband was also given 650 mg acetaminophen orally for pain relief; his wife declined pain medication. Laboratory evaluation, including a complete blood count, basic metabolic panel, liver function panel, and urinalysis were ordered for both patients; all results were within normal limits for both patients.
After an uneventful 6-hour observation period, both patients were discharged home with instructions to return to the ED if they develop any signs of allergic reaction and to call emergency medical services for any sign of anaphylaxis.
1. Rao RV, Matthews PJ, Eyzaguirre PB, Hunter D, eds. 2010. The Global Diversity of Taro: Ethnobotany and Conservation. Rome, Italy; Biouniversity International; 2010. http://www.bioversityinternational.org/fileadmin/user_upload/online_library/publications/pdfs/1402.pdf#page=11. Accessed September 15, 2017.
2. Franceschi VR, Nakata PA. Calcium oxalate in plants: formation and function. Annu Rev Plant Biol. 2005;56:41-71. doi:10.1146/annurev.arplant.56.032604.144106.
3. Herbert DA. Stinging crystals in plants. Science. 1924;60(1548):204-205. doi:10.1126/science.60.1548.204-a.
4. Njintang YN, Mbofung CMF. Effect of precooking time and drying temperature on the physico-chemical characteristics and in-vitro carbohydrate digestibility of taro flour. LWT – Food Sci and Tech. 2006;39(6):684-691. doi.org/10.1016/j.lwt.2005.03.022.
5. Savage GP, Dubois M. The effect of soaking and cooking on the oxalate content of taro leaves. Int J Food Sci Nutr. 2006;57(5-6):376-381. doi:10.1080/09637480600855239.
6. Oscarsson, KV. Savage GP. Composition and availability of soluble and insoluble oxalates in raw and cooked taro (Colocasia esculenta var. Schott) leaves. Food Chem 101. 2007;101(2):559-562. doi:10.1016/j.foodchem.2006.02.014.
7. Pang CT, Ng HW, Lau FL. Oral mucosal irritating plant ingestion in Hong Kong, epidemiology and its clinical presentation. Hong Kong J Emerg Med. 2010;17(5):477-481.
8. Yuen E. Upper airway obstruction as a presentation of Taro poisoning. Hong Kong J Emerg Med. 2001;8(3):163-165.
9. Davis CC, Squier CA, Lilly GE. Irritant contact stomatitis: a review of the condition. J Periodontol. 1998;69(6):620-631. doi:10.1902/jop.1998.69.6.620.
10 Tagwireyi D, Ball DE. The management of Elephant’s Ear poisoning. Hum Exp Toxicol. 2001;20(4):189-192. doi:10.1191/096032701678766822.
11. Omura JD, Blake C, McIntyre L, Li D, Kosatsky T. Two cases of poisoning by raw taro leaf and how a poison control centre, food safety inspectors, and a specialty supermarket chain found a solution.” Environ Health Rev. 2014;57(3):59-64. doi.org/10.5864/d2014-027.
1. Rao RV, Matthews PJ, Eyzaguirre PB, Hunter D, eds. 2010. The Global Diversity of Taro: Ethnobotany and Conservation. Rome, Italy; Biouniversity International; 2010. http://www.bioversityinternational.org/fileadmin/user_upload/online_library/publications/pdfs/1402.pdf#page=11. Accessed September 15, 2017.
2. Franceschi VR, Nakata PA. Calcium oxalate in plants: formation and function. Annu Rev Plant Biol. 2005;56:41-71. doi:10.1146/annurev.arplant.56.032604.144106.
3. Herbert DA. Stinging crystals in plants. Science. 1924;60(1548):204-205. doi:10.1126/science.60.1548.204-a.
4. Njintang YN, Mbofung CMF. Effect of precooking time and drying temperature on the physico-chemical characteristics and in-vitro carbohydrate digestibility of taro flour. LWT – Food Sci and Tech. 2006;39(6):684-691. doi.org/10.1016/j.lwt.2005.03.022.
5. Savage GP, Dubois M. The effect of soaking and cooking on the oxalate content of taro leaves. Int J Food Sci Nutr. 2006;57(5-6):376-381. doi:10.1080/09637480600855239.
6. Oscarsson, KV. Savage GP. Composition and availability of soluble and insoluble oxalates in raw and cooked taro (Colocasia esculenta var. Schott) leaves. Food Chem 101. 2007;101(2):559-562. doi:10.1016/j.foodchem.2006.02.014.
7. Pang CT, Ng HW, Lau FL. Oral mucosal irritating plant ingestion in Hong Kong, epidemiology and its clinical presentation. Hong Kong J Emerg Med. 2010;17(5):477-481.
8. Yuen E. Upper airway obstruction as a presentation of Taro poisoning. Hong Kong J Emerg Med. 2001;8(3):163-165.
9. Davis CC, Squier CA, Lilly GE. Irritant contact stomatitis: a review of the condition. J Periodontol. 1998;69(6):620-631. doi:10.1902/jop.1998.69.6.620.
10 Tagwireyi D, Ball DE. The management of Elephant’s Ear poisoning. Hum Exp Toxicol. 2001;20(4):189-192. doi:10.1191/096032701678766822.
11. Omura JD, Blake C, McIntyre L, Li D, Kosatsky T. Two cases of poisoning by raw taro leaf and how a poison control centre, food safety inspectors, and a specialty supermarket chain found a solution.” Environ Health Rev. 2014;57(3):59-64. doi.org/10.5864/d2014-027.
Heart Failure in the Emergency Department
Patients with heart failure (HF) present daily to busy EDs. An estimated 6.5 million Americans are living with this diagnosis, and the number is predicted to grow to 8 million by 2023.1 Most HF patients (82.1%) who present to EDs are hospitalized, while a selected minority are either managed in the ED and discharged (11.6%) or managed in observation units (OU) (6.3%).2 The prognosis after HF is initially diagnosed is poor, with a 5-year mortality of 50%,3 and after a single HF hospitalization, 29% will die within 1 year.4
One-third of the total Medicare budget is spent on HF, despite the fact that HF represents only 10.5% of the Medicare population.2 Up to 80% of HF costs are for hospitalizations, which cost an average of $11,840 per inpatient admission.5,6 The high costs are due to an average length of stay (LOS) of 5.2 days7 (Table 1).
Adding to hospital costs is the degree of “reactivism,” with approximately 20% of patients discharged from the ED returning within 2 weeks, of whom nearly 50% will be hospitalized.11 Following HF hospitalization and discharge, the 30-day readmission rate is 26.2%,2 increasing to 36% by 90 days.12 The Centers for Medicare & Medicaid Services (CMS) has incentivized hospitals and providers to reduce admissions, but penalize hospitals that do not. Overall, CMS will reduce payments by up to 3% to hospitals with excess readmissions for select conditions, including HF.13
Causes of Heart Failure
Heart failure represents a final common pathway, which in the United States is most often due to coronary artery disease (CAD). Many types of pathology ultimately result in left ventricular (LV) dysfunction, and much of its rising prevalence is a result of the success we now have in managing historically fatal cardiovascular (CV) conditions. These include hypertension, diabetes mellitus (DM), CAD, and valvular and other CV structural conditions.
Heart failure is caused by either a dilated ventricle with a reduced ejection fraction (HFrEF) and inability to eject volume, or a stiffened ventricle with a preserved EF (HFpEF) that is unable to receive increased venous return. Both conditions acutely decompensate pulmonary congestion. A preserved EF is defined as an EF at or greater than 50%, whereas a reduced EF is at or less than 40%, with the 41% to 49% range considered as borderline preserved EF.3
While there are important differences in the treatment of chronic and subacute HF, driven by the EF, the effect of EF on early decision-making and treatment in the ED is negligible: Although the probability of HFpEF increases with increasing initial ED systolic blood pressure (SBP), clinical presentation and treatment in the ED are initially identical—regardless of the EF.
Noninvasive continuous transcutaneous hemodynamic monitoring is available for ED use, and may provide further insight into the underlying pathophysiology. A study of 127 acute heart failure (AHF) ED patients identified three hemodynamic AHF phenotypes. These include normal cardiac index (CI) and systemic vascular resistance index (SVRI), low CI and SVRI, and low CI and elevated SVRI.14 While it is attractive to suggest therapeutic interventions based on these measurements, outcome data are lacking.
Presentation
The most common ED presentation of patients suffering from AHF is dyspnea secondary to volume overload, or as the result of acute hypertension with relatively less volume overload. However, regardless of the cause of dyspnea, it is not only the most common resulting complaint, but one that requires immediate treatment. Ultimately, 59% of all HF admissions are attributed to volume overload and dyspnea (Figure 1).15
Heart failure can also present in a more protean manner, with cough, fatigue, and edema, as well as more subtle symptoms predominating and resulting in a complicated differential diagnosis (Table 2).16
Because HF is a disease that most significantly affects older patients who frequently have concomitant morbidities (eg, myocardial ischemia, chronic obstructive pulmonary disease [COPD] exacerbation, uncontrolled DM), other less clinically obvious disease presentations may actually be the cause of the AHF exacerbation.
Diagnosis
A focused history and physical examination that is part of all ED evaluations should be expedited whenever there is evidence of hemodynamic instability or respiratory compromise. An early working diagnosis is essential to avoid a delay in appropriate treatment, which is associated with increased mortality.
When HF is likely, the potential etiology and precipitants for decompensation must be considered. This list is long, but medication noncompliance and dietary indiscretion are the most common causes.
Symptoms and Prior History of HF
The classic symptoms for AHF include dyspnea at rest or exertion, and orthopnea, both of which unfortunately have poor sensitivity and specificity for AHF. As an isolated symptom, dyspnea is of marginal diagnostic utility (sensitivity and specificity for an HF diagnosis is 56% and 53%, respectively), and orthopnea is only slightly better (sensitivity and specificity 77% and 50%, respectively). A prior HF diagnosis makes repeat presentations much more likely (sensitivity and specificity 60% and 90%, respectively).17
Physical Examination
Simple observation and a directed examination can rapidly point to the diagnosis (Figure 2).
Electrocardiography
Because CAD is one of the most common underlying AHF etiologies, an electrocardiogram (ECG) should always be obtained early for a patient presenting with potential AHF. Although the ECG does not usually contribute to ED management, the identification of new ST-segment changes or a malignant arrhythmia will guide critical management decisions.
Imaging Studies
Chest X-ray Imaging. A chest X-ray (CXR) study must be considered early when a patient presents with signs and symptoms suggestive of AHF. Although the classic findings of HF (eg, Kerley B lines [short horizontal lines perpendicular to the pleural surface],18 interstitial congestion, pulmonary effusion) can lag behind the clinical presentation, and also be nondiagnostic in the setting of mild HF, the CXR is an effective aid in identifying other causes of dyspnea such as pneumonia. Ultimately, the utility of the CXR for diagnosis is similar to that of the history and physical examination in that it will be diagnostic when positive but cannot exclude AHF if normal.
Ultrasound. Because it is fast, inexpensive, noninvasive, and readily available in the ED, ultrasound is frequently used to evaluate potential HF patients. Several studies have demonstrated that the presence of B lines in two or more regions is specific for AHF (specificity 75%-100%), although the sensitivity may be limited (40%-91%).19-21 The presence of inferior vena cava (IVC) dilation is also associated with adverse outcomes.22 In 80 patients hospitalized with acute decompensated HF (ADHF), a dilated IVC (≥1.9 cm) at admission was associated with higher 90-day mortality (25.4% vs 3.4%, P = 0.009).23 These findings may be considered in groups: In an evaluation of the combination of LV EF, IVC collapsibility, and B lines for an HF diagnosis, the combination of all three had a poor sensitivity (36%) but an excellent specificity (100%), and any two of the three had a specificity of at least 93%.24
Laboratory Evaluation
Myocardial Strain: BNP/NTproBNP. Natriuretic peptides (NPs) are not AHF-specific, but rather they are synthesized and released by the myocardium in the setting of myocardial pressure or volume stress. They are manufactured as preproBNP, then enzymatically cleaved into the active BNP and the inactive fragment N-terminal proBNP (NTproBNP). The predominant hormonal effects of BNP are vasodilation and natriuresis, as well as antagonism of the hormones associated with sodium retention (aldosterone) and vasoconstriction (endothelin, norepinephrine).
As AHF results in myocardial stress, NP elevation provides diagnostic and prognostic information. Clinical judgment supported by a BNP greater than 100 pg/mL is a better predictor of AHF than clinical judgment alone (accuracy 81% vs 74%, respectively).25 While low levels (BNP <100 pg/mL or NTproBNP <300 pg/mL) reliably exclude the diagnosis of HF (sensitivities >95%), higher levels (BNP >500 pg/mL, NTproBNP >900 pg/mL) are useful as “rule-in” markers, with specificity greater than 95%. The NTproBNP also requires adjustment for patients older than age 75 years, with a higher level (>1,800 pg/mL) to rule-in HF. The NP grey zone (BNP 100-500 pg/mL, NTproBNP 300-900 pg/mL)requires additional testing for accurate diagnoses (Figure 3).25-29
There are several confounders to the interpretation of NP results: NPs are negatively confounded by the presence of obesity, resulting in a lowering of the value as compared to the clinical presentation.Thus, the measured BNP level should be doubled if the patient’s body mass index exceeds 35 kg/m2.30 Secondly, because NP metabolism is partially renal dependent, elevated levels may not reflect AHF in the presence of renal failure. If the estimated glomerular filtration rate is less than 60 mL/min, measured BNP levels should be halved.31
AHF vs Myocardial Ischemia: Troponin Levels. Large registry data using contemporary troponin assays clearly identify the association between elevated troponin levels (>99th percentile in a healthy population) and increased short-term risk. With the US Food and Drug Association (FDA) approval of a high-sensitivity troponin (hs-cTnT) assay, a greater frequency of elevated cardiac troponin T (cTnT) and cardiac troponin I (cTnl) will be identified in AHF patients in the ED.
In one retrospective study of 4,705 AHF patients in the ED, hs-cTnT were elevated in 48.4% of cases (25.3% in cTnI, 37.9% in cTnT, and 82.2% in hs-cTnT). Although 1-year mortality was higher in those with elevated troponin (adjusted heart rate [HR] 1.61; CI 95% 1.38-1.88), elevated troponin was not associated with 30-day revisits to the ED (1.01; 0.87-1.19) and high sensitive elevations less than double the reference value had no impact on outcomes.32 Thus, in terms of management of AHF in the ED, slightly elevated stable serial troponins are more consistent with underlying HF, and should be managed as such. This is not true of rising/falling troponin levels, which should still engender concern for underlying myocardial ischemia and a different management pathway.
Renal function. Comprised renal function is an important predictor of AHF outcome. Large registry data from hospitalized HF patients demonstrate that a presenting blood urea nitrogen level greater than 43 mg/dL is one of the most important predictors of increased acute mortality,33 and levels below 30 mg/dL identify a cohort likely to be successfully managed in an observation environment.34 Creatinine is a helpful lagging indicator of mortality, with higher levels (>2.75 mg/dL) associated with increased short-term adverse outcomes and decreased therapeutic responsiveness (Figure 4).
For patients presenting with ADHF, a newer test recently approved by the FDA uses the product of the urine markers tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7, to generate a score predictive of acute kidney injury.35 While promising, no studies of ED outcomes are currently available.
Volume Assessment
Objective volume assessment is useful for diagnosis and prognosis in AHF. Bioimpedance vector analysis (BIVA) is a rapid, inexpensive, noninvasive technique that measures total body water by placing a pair of electrodes on the wrist and ipsilateral ankle. The BIVA measurements have strong correlations with the gold standard volume-assessment technique of deuterium dilution (r > 0.99).36 In HF, BIVA can assess volume depletion37 and overload,38 and identifies differences in hydration status between 90-day survivors and non-survivors (P < 0.01).39
Used in combination with BNP, one prospective study of 292 dyspneic patients found that, while BIVA was a strong predictor of AHF (c-statistic 0.93, P = 0.016), the most accurate volume status determination was the combination of both (c-statistic, 0.99; P = 0.005), for which the combined accuracy exceeded either alone.40 Finally, in 166 hospitalized HF patients discharged by BNP and BIVA parameters, vs 149 discharged based on clinical impressions, those assessed with BNP and BIVA had lower 6-month readmissions (23% vs 35%, P = 0.02) and overall cost of care.41
Combination Technologies
Obviously, EPs may consider multiple technologies to arrive at an accurate diagnosis. One prospective evaluation enrolled 236 patients to determine the diagnostic accuracy for AHF in the ED and reported lung ultrasound, CXR, and NTproBNP had a sensitivity of 57.7% and 88.0%, 74.5% and 86.3%, and a specificity of 97.6% and 28.0%. The best overall combination was the CXR with lung ultrasound (sensitivity 84.7%, specificity 77.7%).42
Another prospective study evaluated IVC diameter, bioelectrical impedance analysis (BIA), and NTproBNP in 96 elderly patients. ADHF patients had higher IVC diameters and lower collapsibility index, lower resistance and reactance, and higher NTproBNP levels. While all had high and statistically similar C-statistics (range 0.8 to 0.9) for an ADHF diagnosis, they concluded that IVC ultrasonography and BIA were as useful as NT-proBNP for diagnosing ADHF. 24
Diagnostic Scoring Systems
A scoring system has been proposed to improve diagnosis in the ED. Unfortunately, the value over clinical impression has not been clearly proven, though one randomized, controlled trial did not show statistically significant improvement in diagnostic accuracy when compared to standard care (77% vs 74%, P = 0.77).43
Differential Diagnosis
The differential diagnosis for acute dyspnea is long and potentially arcane. Efforts should focus on excluding non-HF causes of dyspnea, while considering the high risk of alternative etiologies for signs and symptoms. These include asthma, COPD, pneumonia, and pulmonary embolism, which may represent the primary pathologies in a patient with a history of HF, or be the cause of a HF exacerbation. Additional causes of noncardiogenic pulmonary edema should also be considered (eg, acute respiratory distress syndrome, toxins, etc). Acute coronary syndrome and dyspnea may be angina equivalents—one important consideration.
Treatment and Management
Airway Management
Treatment of CH in the ED must always start with an immediate airway evaluation, with the possible need for endotracheal intubation preceding all diagnostic or other management considerations. Intubation is a decision most successfully based on physician clinical assessment, including oxygen (O2) selection rather than waiting for the results of objective measures such as arterial blood gas analysis.
Oxygen
Supplemental O2 should be administered to maintain an O2 saturation above 95%, but obviously is unnecessary in the absence of hypoxia.
Noninvasive Ventilation
Two kinds of noninvasive ventilation (NIV) are available, continuous positive airway pressure and bilevel positive airway pressure ventilation. The physiological differences between these types of NIV have little bearing on ED treatment.
Noninvasive ventilation has not been clearly shown to provide long-term mortality benefit. Large registry data44 report that outcomes are no worse than the alternative of endotracheal intubation, while multiple systematic reviews,45,46meta-analysis,47and Cochrane reviews48,49have established NIV as an acute pulmonary edema intervention that provides reductions in hospital mortality (numbers needed to treat [NNT] 13) and intubation (NNT 8), the prospective randomized C3PO (Congenital Cardiac Catheterization Project on Outcomes) trial50 failed to demonstrate any mortality reduction.
In patients with severe respiratory distress, NIV is a reasonable strategy during the aggressive administration of medical therapy in an attempt to avoid endotracheal intubation. However, NIV is not a stand-alone therapy and though its use may obviate the need for immediate intubation, its implementation should not be considered definitive management.
Correction of Abnormal Vital Signs: Abnormal SBP
Vital signs are an important determinant of therapy, driving treatment strategies. Interventions for HF are based on the patient’s SBP, in particular correction of symptomatic hypotension and hypertensive HF (Table 3).51
Symptomatic Hypotension. The presence of symptomatic hypotension is an extremely poor prognostic finding in AHF. Inotrope therapy may be considered, but it does not reduce mortality except as a bridge to mechanical interventions (LV assist device or transplant).52-54 Temporary inotropic support is recommended for cardiogenic shock to maintain systemic perfusion and prevent end organ damage.3 The inotropic support includes administration of dopamine, dobutamine, or milrinone, though none have been proven to be superior over the other. The lowest possible dose of the selected inotrope should be used to limit arrhythmogenic effects. Inotropic agents should not be used in the absence of severe systolic dysfunction, or low BP, or impaired perfusion, or evidence of significantly decreased cardiac output.
Hypertensive Heart Failure. Defined as the rapid onset of pulmonary congestion with an SBP greater than 140 mm Hg, and commonly greater than 160 mm Hg, these patients may have profound dyspnea, requiring endotracheal intubation. However, in this situation, aggressive vasodilation is typically rapidly effective. Overall, patients presenting with an elevated SBP have lower rates of in-hospital mortality, 30-day myocardial infarction (MI), death, or rehospitalization, and a greater likelihood of discharge within 24 hours—as long as the elevated SBP is aggressively and rapidly treated.
Pharmacological Therapy
Pharmacological management is the mainstay for treating HF. No other acute therapy (eg, NIV) has demonstrated a morality benefit (See Table 4 for specific dose and administration strategies).55 The time to initiate pharmacological therapy and whether an aggressive approach is indicated must be based on the severity of the clinical symptoms and objective risk stratification measures (eg, NP, troponin levels).
Furosemide. Except for hypertensive HF—in which case BP lowering is the most important goal—diuretics are a mainstay of AHF treatment, and consensus guidelines provide a class I recommendation for their use.3 The DOSE (Diuretic Strategies in Patients with ADHF) trial56 prospectively evaluated diuretics in 308 hospitalized AHF patients and found no outcome differences in administration route (bolus or continuous infusion) or dose (high vs low dose). This study reported trends toward greater improvement with higher furosemide dosing, as well as greater diuresis, but at a cost of transient worsening of renal function.
In general, diuretics should be administered in an intravenous (IV) dose equal to 1 to 2.5 times the patient’s usual daily oral dose. For patients who are diuretic-naïve, a dose of 40 mg IV furosemide or 1 mg IV bumetanide, with subsequent dosing titrated to urine output, is recommended.
Vasodilators. In patients with both AHF and even mildly elevated BP, vasodilators can be extremely effective in achieving symptom improvement. The choice of vasodilator, and how aggressive to increase dosing, depends upon symptom severity. The purpose of vasodilators is to lower BP and therefore, should not be used in the setting of hypotension or signs of hypoperfusion. Flow-limiting, preload-dependent CV states (eg, right ventricular infarction) increase the risk of hypotension, and are relative contraindications to the use of vasodilators. For patients who are severely dyspneic and with critical presentations, the emergency physician (EP) should preclude a detailed history and examination to initiate immediate therapy with short-acting agents that can be terminated rapidly in the case of an adverse event (eg, unexpected hypotension) are preferred.
Nitroglycerin. Nitroglycerin is the vasodilation agent of choice for hypertensive AHF. It is a short-acting, rapid-onset, venous and arterial dilator that decreases BP by preload reduction, and by afterload reduction in higher doses. Nitroglycerin has coronary vasodilatory effects associated with decreased ischemia, but should be avoided in patients taking phosphodiesterase inhibitors.55 Its most common side effect is headache, and hypotension occurs in about 3.5% of patients.57
Commonly given as a continuous infusion at IV doses up to 400 mcg/min, nitroglycerin may be associated with higher costs and longer LOS.58 Some authors suggest that bolus nitroglycerin therapy may be superior: In a retrospective study of 395 patients, an IV bolus of nitroglycerin 0.5 mg was superior to both an infusion, or a combination of bolus and infusion, as demonstrated by lower rates of ICU admission (48% vs 67% and 79%, respectively, P = 0.006) and shorter hospital stays (4.4 vs 6.3 and 7.3 days, respectively, P = 0.01). In all cohorts, adverse event rates were similar for hypotension, troponin elevation, and creatinine increase over 48 hours.59 Nitroprusside. Nitroprusside is a potent arterial and venous dilator that causes rapid decrease in BP and LV-filling pressures. It is usually considered more effective than nitroglycerin, despite a small study showing similar hemodynamic responses.60
Initial dosing of nitroprusside starts at 0.3 µg/kg/min IV, and is increased every 5 minutes to a maximum of 10 mcg/kg/min, based on BP and clinical response. The most common acute complication of nitroprusside infusions is hypotension. Cyanide toxicity may occur with prolonged use, high doses, or in patients with renal failure.55
Nesiritide. Exogenously administered, the B-type NP nesiritide is effective in lowering BP and improving dyspnea in AHF,55 although large prospective studies showed it had little long-term advantage over standard care.61 In a small, randomized, controlled trial, nesiritide reduced 30-day revisit LOS when given in an OU.62 The 22-minute half-life of nesiritide is longer than that of the nitrates, and its side effect is predominately hypotension, which occurs at rates similar to those of other vasodilators.55
Angiotensin Converting Enzyme Inhibitors. Because angiotensin converting enzyme inhibitors (ACEIs) have chronic mortality reduction benefits, their use in the acute setting is theoretically attractive, however, this has been poorly proven in AHF ED patients. In a retrospective review of 103 patients with elevated NTproBNP levels receiving bolus IV enalaprilat within 3 hours of presentation, the mean SBP decreased by 30 mm Hg, with only 2% of patients developing hypotension.63 However, with the longer half-life of ACEIs, if hypotension occurs, the potential for a prolonged BP-lowering effect exists.
Calcium Channel Blockers. Clevidipine and nicardipine are rapidly acting IV calcium channel blockers that lower BP by selective arteriolar vasodilation and increased cardiac output as vascular resistance declines.55 Because these agents have no negative inotropic or chronotropic effects, they may be beneficial in hypertensive AHF. In an open-label trial of 104 hypertensive AHF patients, clevidipine was more effective than standard care for the rapid control of BP and relief of dyspnea.64
Morphine. Large registry analyses have demonstrated potential harm with the routine use of morphine,65 as do recent propensity score matched analyses.66 Until there are studies demonstrating benefit, the use of morphine at present should be reserved for palliative care.
Time to Treatment
Although a randomized controlled trial on the importance of time to treatment of AHF is unlikely to ever be completed, data suggest that, as in the case of MI, delayed AHF therapy is associated with adverse outcomes. In a study of 499 suspected AHF patients transferred by ambulance, patients randomized to immediate therapy vs those whose therapy was not initiated until hospital arrival (mean delay of 36 minutes), had a 251% increase in survival (P < 0.01).67
Furthermore, the delayed administration of vasoactive agents, defined as medication administered to alter hemodynamics (eg, dobutamine, dopamine, nitroglycerin, nesiritide) is also associated with harm,68 and registry studies demonstrate increased death rates (n = 35,700).69 Finally, another registry (n = 14,900) study demonstrated early IV furosemide is associated with decreased mortality.70 This latter finding was also validated in a prospective observational cohort study (mortality 2.3 vs 6.0 in early vs delayed therapy groups, respectively).71
Patient Disposition
One of the unique features of emergency medicine is the need to determine, with very limited information and time, a patient’s very short-term clinical trajectory. Few physicians are required to have greater accuracy with less information or time than do EPs. Several studies report objective data points and risk scores to assist in this task, but none has been universally adopted, reflecting the challenge of applying population data to individuals.
Short-term Prognosis
In 1,638 patients evaluated for 14-day outcomes, an HR lower than 50% maximal HR (MHR), and an SBP greater than 140 mm Hg were associated with the lowest rate of serious adverse events (SAEs) (6%) and hospitalization (38%).72 An MHR over 75% was associated with the highest SAE rate, although SAEs decreased as SBP increased (30%, 24%, and 21% with SBPs < 120 mm Hg, 120-140 mm Hg, and > 140 mm Hg, respectively).72
Risk Scores
In a prospective, observational cohort study of 1,100 ED patients, the Ottawa Heart Failure Risk Scale, combined with NTproBNP values, had a sensitivity of 95.8%—at the cost of increasing the admission rate (from 60.8% to 88%)—for serious adverse events (defined as death within 30 days), admission to a monitored unit, intubation, NIV, MI, or relapse resulting in hospital admission within 14 days.73
Observation Unit
Overall, 44% of in-patient HF admissions are for less than 3 days (Table 1),2 supporting the practice of managing selected patients in shorter clinical-care environments than in inpatient units. Further, ED patients presenting with moderate dyspnea require both a diagnosis and an evaluation of their therapeutic response to determine the need for hospitalization. However, evaluating therapeutic response requires more time than is available in the typical ED. Thus, an ED OU offers the following:
(1) The OU provides the EP with a longer evaluation time, and therefore a more accurate disposition may be effected;
(2) Costs are significantly lower in patients managed in an ED OU; and
(3) Patient satisfaction may be improved, as most patients prefer home management over hospitalization.
All three of these opportunities are supported by a number of studies,74-78 with validated entry and exclusion criteria, treatment algorithms and discharge metrics. Most recently, in a registry of hospitals in Spain registry, patients presenting to hospitals that had OUs had a 2.2-day shorter LOS, lower 30-day ED revisit rate, and similar mortality rates compared to those in institutions without OUs—although these beneficial effects occurred at the cost of an 8.9% higher admission rate.79
Patient Education
Intuitively, it would be expected that patient education would reduce return visits, 30-day hospitalizations, and AHF-related mortality. Unfortunately, it has not been demonstrated that patient education results in a consistent benefit at hospital discharge, or in the outpatient environment.80-85
Although AHF education in the ED has been poorly studied, areas that have shown promise are education occurring before ED management (ie, in the ED waiting area) in underinsured patients,86 and during ED care for patients with poor health care literacy.87 As educational interventions are both inexpensive and unlikely to result in harm, their implementation should be considered.
Conclusion
The spectrum of HF is a common presentation in the ED. Because HF generally appears as dyspnea, in a cohort with multiple comorbidities, the diagnosis can be challenging. This is complicated by the fact that patients with severe presentations may require life-saving interventions long before a clinical evaluation is completed (or even initiated). The skill of the EP, and his or her ability to improve the clinical condition before intubation is required, will determine the patient’s trajectory. Conversely, as a chronic condition, HF may present with moderate symptoms for which a short diuretic “tune-up” in an observation environment may be appropriate.
How these decisions are made will depend upon the local environment, the availability of outpatient resources, and individual patient choices. There are few chronic diseases that are more complex, are seen more often in the ED, or that require more skill and finesse in management.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603.
2. Fitch KV, Engel T, Lauet J. The cost burden of worsening heart failure in the Medicare fee for service population: an actuarial analysis. Milliman Web site. 2017. http://www.milliman.com/insight/2017/The-cost-burden-of-worsening-heart-failure-in-the-Medicare-fee-for-service-population-An-actuarial-analysis/ Published April 3, 2017. Accessed June 1, 2017.
3. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013. J Am Coll Cardiol. 2013;62(16):e147-e239. doi:10.1016/j.jacc.2013.05.019.
4. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669-1678. doi:10.1001/jama.2011.1474.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a.
6. Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4(1):68-75. doi:10.1161/CIRCOUTCOMES.110.957225.
7. HCUP National Inpatient Sample (NIS); 2014 Agency for Healthcare Research and Quality (AHRQ). HCUPnet Healthcare Cost and Utilization Project. http://bit.ly/2u2ymFA. Published 2014. Accessed July 27, 2017.
8. FY 2017 Final Rule and Correction Notice Tables. CMS 2014 data based on DRGs, Table 5:List of MS-DRGs, Relative Weighting Factors and Geometric and Arithmetic Mean Length of Stay. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2017-IPPS-Final-Rule-Home-Page-Items/FY2017-IPPS-Final-Rule-Tables.html. Published 2017. Accessed August 28, 2017.
9. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. http://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed August 2, 2017
10. Ellison, A. Average cost per inpatient day across 50 states. Becker’s Hospital CFO Report. http://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states-2016.html. Published January 13, 2016. Accessed August 2, 2017
11. Claret PG, Calder LA, Stiell IG, et al. Rates and predictive factors of return to the emergency department following an initial release by the emergency department for acute heart failure. [published online ahead of print April 3, 2017]. CJEM. 1-8. doi:10.1017/cem.2017.14.
12. Yam FK, Lew T, Eraly SA, Lin HW, Hirsch JD, Devor M. Changes in medication regimen complexity and the risk for 90-day hospital readmission and/or emergency department visits in U.S. Veterans with heart failure. Res Social Adm Pharm. 2016;12(5):713-721. doi:10.1016/j.sapharm.2015.10.004.
13. Readmissions Reduction Program. CMS. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html Updated April 18, 2016. Accessed July 13, 2017.
14. Nowak RM, Reed BP, DiSomma S, et al. Presenting phenotypes of acute heart failure patients in the ED: Identification and implications. Am J Emerg Med. 2017;35(4):536-542. doi:10.1016/j.ajem.2016.12.003.
15. Bennett SJ, Huster GA, Baker SL, et al. Characterization of the precipitants of hospitalization for heart failure decompensation. Am J Crit Care. 1998;7(3):168-174.
16. Kuo DC, Peacock WF. Diagnosing and managing acute heart failure in the emergency department. Clin Exp Emerg Med. 2015;2(3):141-149. eCollection 2015 Sep. doi:10.15441/ceem.15.007.
17. Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944-1956.
18. Koga T, Fujimoto K. Images in clinical medicine. Kerley’s A, B, and C line. N Engl J Med. 2009;360(15):1539. doi:10.1056/NEJMicm0708489.
19. Glöckner E, Christ M, Geier F, et al. Accuracy of point-of-care B-Line lung ultrasound in comparison to NT-ProBNP for screening acute heart failure. Ultrasound Int Open. 2016;2(3):e90-e92. doi:10.1055/s-0042-108343.
20. Bitar Z, Maadarani O, Almerri K. Sonographic chest B-lines anticipate elevated B-type natriuretic peptide level, irrespective of ejection fraction. Ann Intensive Care. 2015;5(1): 56. doi:10.1186/s13613-015-0100-x.
21. Miglioranza MH, Gargani L, Sant’Anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6(11):1141-1151. doi:10.1016/j.jcmg.2013.08.004.
22. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31(8):1208-1214. doi:10.1016/j.ajem.2013.05.007.
23. Cubo-Romano P, Torres-Macho J, Soni NJ, et al. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med. 2016;11(11):778-784. doi:10.1002/jhm.2620.
24. Martínez PG, Martínez DM, García JC, Loidi JC. Amino-terminal pro–B-type natriuretic peptide, inferior vena cava ultrasound, and bioelectrical impedance analysis for the diagnosis of acute decompensated CHF. Am J Emerg Med. 2016;34(9): 1817–1822. doi:10.1016/j.ajem.2016.06.043.

25. Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161-167.
26. Van Kimmenade RR, Pinto YM, Bayes-Genis A, Lainchbury JG, Richards AM, Januzzi JL Jr. Usefulness of intermediate amino-terminal pro-brain natriuretic peptide concentrations for diagnosis and prognosis of acute heart failure. Am J Cardiol. 2006;98(3):386-390.
27. Moe GW, Howlett J, Januzzi JL, Zowall H; Canadian Multicenter Improved Management of Patients With Congestive Heart Failure (IMPROVE-CHF) Study Investigators. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007;115(24):3103-3110.
28. Mayo DD, Colletti JE, Kuo DC. Brain natriuretic peptide (BNP) testing in the emergency department. J Emerg Med. 2006;31(2):201-210.
29. Mueller C, Scholer A, Laule-Kilian K, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647-654.
30. Krauser DG, Lloyd-Jones DM, Chae CU, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;149(4):744-750.
31. McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
32. Jacob J, Roset A, Miró Ò, et al; ICA-SEMES Research Group. EAHFE - TROPICA2 study. Prognostic value of troponin in patients with acute heart failure treated in Spanish hospital emergency departments. Biomarkers. 2017;22(3-4):337-344. doi:10.1080/1354750X.2016.1265006.
33. Fonarow GC, Adams KF Jr, Abraham WT, et al; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580.
34. Burkhardt J, Peacock WF, Emerman CL. Predictors of emergency department observation unit outcomes. Acad Emerg Med. 2005;12(9):869-874.
35. Schanz M, Shi J, Wasser C, Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol. 2017;40(7):485-491. doi:10.1002/clc.22683.
36. Kushner RF, Schoeller DA, Fjeld CR, Danford L: Is the impedance index (ht2/R) significant in predicting total body water? Am J Clin Nutr. 1992;56(5): 835-839.
37. Ackland GL, Singh-Ranger D, Fox S, et al. Assessment of preoperative fluid depletion using bioimpedance analysis. Br J Anaesth. 2004;92(1): 134-136.
38. Uszko-Lencer NH, Bothmer F, van Pol PE, Schols AM. Measuring body composition in chronic heart failure: a comparison of methods. Eur J Heart Fail. 2006;8(2): 208-214.
39. Santarelli S, Russo V, Lalle I, et al; GREAT network. Usefulness of combining admission brain natriuretic peptide (BNP) plus hospital discharge bioelectrical impedance vector analysis (BIVA) in predicting 90 days cardiovascular mortality in patients with acute heart failure. Intern Emerg Med. 2017;12(4):445-451. doi:10.1007/s11739-016-1581-9.
40. Parrinello G, Paterna S, Di Pasquale P, et al. The usefulness of bioelectrical impedance analysis in differentiating dyspnea due to decompensated heart failure. J Card Fail. 2008;14(8): 676-686. doi:10.1016/j.cardfail.2008.04.005.
41. Valle R, Aspromonte N, Carbonieri E, et al. Fall in readmission rate for heart failure after implementation of B-type natriuretic peptide testing for discharge decision: a retrospective study. Int J Cardiol. 2008;126(3): 400-406.
42. Sartini S, Frizzi J, Borselli M, et al. Which method is best for an early accurate diagnosis of acute heart failure? Comparison between lung ultrasound, chest X-ray and NT pro-BNP performance: a prospective study. [published online ahead of print July 11, 2016]. Intern Emerg Med. doi:10.1007/s11739-016-1498-3.
43. Steinhart BD, Levy P, Vandenberghe H, et al. A randomized control trial using a validated prediction model for diagnosing acute heart failure in undifferentiated dyspneic emergency department patients-results of the GASP4Ar study. J Card Fail. 2017;23(2):145-152. doi:10.1016/j.cardfail.2016.08.007.
44. Tallman TA, Peacock WF, Emerman CL, et al; ADHERE Registry. Noninvasive ventilation outcomes in 2,430 acute decompensated heart failure patients: an ADHERE registry analysis. Acad EM. 2008;15(4):355–362. doi:10.1111/j.1553-2712.2008.00059.x.
45. Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest. 1998;114(4):1185-1192.
46. Peter JV, Moran JL, Phillips-Hughes J, Graham P, Bersten AD. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367(9517):1155-1163.
47. Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600
48. Vital FM, Saconato H, Ladeira MT, et al. Noninvasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev. 2008;(3):CD005351. doi:10.1002/14651858.CD005351.pub2.
49. Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;(5):CD005351. doi:10.1002/14651858.CD005351.pub3.
50. Gray A, Goodacre S, Seah M, Tilley S. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema: analysis from the 3CPO trial. QJM. 2010;103(8):573-581. doi:10.1093/qjmed/hcq077.
51. Collins SP, Storrow AB, Levy PD, et al. Early management of patients with acute heart failure: state of the art and future directions—a consensus document from the SAEM/HFSA acute heart failure working group. Acad Emerg Med. 2015;22(1):94-112. doi:10.1111/acem.12538.]
52. O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1999;138(1 Pt 1):78-86.
53. Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;(9):180-187.
54. Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009;2(4):320-324. doi:10.1161/CIRCHEARTFAILURE.108.839076.
55. Collins SP, Levy PD, Martindale JL, et al. Clinical and research considerations for patients with hypertensive acute heart failure: A consensus statement from the Society for Academic Emergency Medicine and the Heart Failure Society of America Acute Heart Failure Working Group. Acad Emerg Med. 2016;23(8):922-931. doi:10.1111/acem.13025.
56. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011; 364(9):797-805. doi:10.1056/NEJMoa1005419.

57. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531-1540.
58. Gradman AH, Vekeman F, Eldar-Lissai A, Trahey A, Ong SH, Duh MS. Is addition of vasodilators to loop diuretics of value in the care of hospitalized acute heart failure patients? Real-world evidence from a retrospective analysis of a large United States hospital database. J Card Fail. 2014;20(11):853-863. doi:10.1016/j.cardfail.2014.08.006.
59. Wilson SS, Kwiatkowski GM, Millis SR, Purakal JD, Mahajan AP, Levy PD. Use of nitroglycerin by bolus prevents intensive care unit admission in patients with acute hypertensive heart failure. Am J Emerg Med. 2017;35(1):126-131. doi:10.1016/j.ajem.2016.10.038.
60. Eryonucu B, Guler N, Guntekin U, Tuncer M. Comparison of the effects of nitroglycerin and nitroprusside on transmitral Doppler flow parameters in patients with hypertensive urgency. Ann Pharmacother. 2005;39(6):997–1001.
61. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32-43. doi:10.1056/NEJMoa1100171.
62. Peacock WF 4th, Holland R, Gyarmathy R, et al. Observation unit treatment of heart failure with nesiritide: results from the proaction trial. J Emerg Med. 2005;29(3):243-252.
63. Ayaz SI, Sharkey CM, Kwiatkowski GM, et al. Intravenous enalaprilat for treatment of acute hypertensive heart failure in the emergency department. Int J Emerg Med. 2016;9(1):28. doi:10.1186/s12245-016-0125-4.
64. Peacock WF 4th, Chandra A, Char D, et al. Clevidipine in acute heart failure: results of the A study of BP control in acute heart failure-a pilot study (PRONTO). Am Heart J. 2014;167(4):529-536. doi:10.1016/j.ahj.2013.12.023.
65. Peacock WF 4th, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25(4):205-209. doi:10.1136/emj.2007.050419.
66. Miró Ò, Gil V, Martín-Sánchez FJ, Herrero-Puente P, Jet al; ICA-SEMES Research Group. Morphine use in the ED and outcomes of patients with acute heart failure: a propensity score-matching analysis based on the EAHFE registry. [published ahead of print April 12, 2017] Chest. pii:S0012-3692(17)30707-9. doi:10.1016/j.chest.2017.03.037.
67. Wuerz RC, Meador SA. Effects of prehospital medications on mortality and length of stay in congestive heart failure. Ann Emerg Med. 1992;21(6):669-674.
68. Peacock WF 4th, Fonarow GC, Emerman CL, Mills RM, Wynne J; ADHERE Scientific Advisory Committee and Investigators; Adhere Study Group. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology. 2007; 107(1):44-51. doi:10.1159/000093612.
69. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009;15(6): 256-264. doi:10.1111/j.1751-7133.2009.00112.x.
70. Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol. 2008;52(7):534-540. doi:10.1016/j.jacc.2008.05.010.
71. Matsue Y, Damman K, Voors AA, et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017 Jun 27;69(25):3042-3051. doi:10.1016/j.jacc.2017.04.042.
72. Claret PG, Stiell IG, Yan JW, et al. Hemodynamic, management, and outcomes of patients admitted to emergency department with heart failure. Scand J Trauma Resusc Emerg Med. 2016;24(1):132.
73. Stiell IG, Perry JJ, Clement CM, et al. Prospective and explicit clinical validation of the Ottawa Heart Failure Risk Scale, with and without use of quantitative NT-proBNP. Acad Emerg Med. 2017;24(3):316-327. doi:10.1111/acem.13141.
74. Pang PS, Jesse R, Collins SP, Maisel A. Patients with acute heart failure in the emergency department: do they all need to be admitted? J Card Fail. 2012;18:900-903. doi:10.1016/j.cardfail.2012.10.014.
75. Peacock WF 4th, Young J, Collins S, Emerman C, Diercks D. Heart failure observation units: optimizing care. Ann Emerg Med. 2006;47(1):22-33.
76. Storrow AB, Collins SP, Lyons MS, Wagoner LE, Gibler WB, Lindsell CJ. Emergency department observation of heart failure: preliminary analysis of safety and cost. Congest Heart Fail. 2005;11(2):68-72.
77. Peacock WF 4th, Remer EE, Aponte J, Moffa DA, Emerman CE, Albert NM. Effective observation unit treatment of decompensated heart failure. Congest Heart Fail. 2002;8(2):68 -73.
78. Peacock WF 4th, Albert NM. Observation unit management of heart failure. Emerg Med Clin North Am. 2001;19(1):209-232.
79. Miró O, Carbajosa V, Peacock WF 4th, et al; ICA-SEMES group. The effect of a short-stay unit on hospital admission and length of stay in acute heart failure: REDUCE-AHF study. Eur J Intern Med. 2017;40:30-36. doi:10.1016/j.ejim.2017.01.015.
80. Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur Heart J. 1998;19(8):1254-1260.
81. Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162(6):705-712.
82. Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Arch Intern Med. 2003;163(7):809-817.
83. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med. 1998;158(10):1067-1072.
84. Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354(9184):1077-1083.
85. Weinberger M, Oddone EZ, Henderson WG; Veterans Affairs Cooperative Study Group. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on primary care and hospital readmission. N Engl J Med. 1996;334(22):1441-1447.
86. Asthana V, Sundararajan M, Karun V, et al. Educational strategy for management of heart failure markedly reduces 90-day emergency department and hospital readmissions in un- and underinsured patients. J Am Coll Cardiol. 2017;69(11Suppl): 780. doi:10.1016/S0735-1097(17)34169-4.
87. Bell SP, Schnipper JL, Goggins K, et al; Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) Study Group. Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. J Gen Intern Med. 2016;31(5):470-477. doi:10.1007/s11606-016-3596-3.
Patients with heart failure (HF) present daily to busy EDs. An estimated 6.5 million Americans are living with this diagnosis, and the number is predicted to grow to 8 million by 2023.1 Most HF patients (82.1%) who present to EDs are hospitalized, while a selected minority are either managed in the ED and discharged (11.6%) or managed in observation units (OU) (6.3%).2 The prognosis after HF is initially diagnosed is poor, with a 5-year mortality of 50%,3 and after a single HF hospitalization, 29% will die within 1 year.4
One-third of the total Medicare budget is spent on HF, despite the fact that HF represents only 10.5% of the Medicare population.2 Up to 80% of HF costs are for hospitalizations, which cost an average of $11,840 per inpatient admission.5,6 The high costs are due to an average length of stay (LOS) of 5.2 days7 (Table 1).
Adding to hospital costs is the degree of “reactivism,” with approximately 20% of patients discharged from the ED returning within 2 weeks, of whom nearly 50% will be hospitalized.11 Following HF hospitalization and discharge, the 30-day readmission rate is 26.2%,2 increasing to 36% by 90 days.12 The Centers for Medicare & Medicaid Services (CMS) has incentivized hospitals and providers to reduce admissions, but penalize hospitals that do not. Overall, CMS will reduce payments by up to 3% to hospitals with excess readmissions for select conditions, including HF.13
Causes of Heart Failure
Heart failure represents a final common pathway, which in the United States is most often due to coronary artery disease (CAD). Many types of pathology ultimately result in left ventricular (LV) dysfunction, and much of its rising prevalence is a result of the success we now have in managing historically fatal cardiovascular (CV) conditions. These include hypertension, diabetes mellitus (DM), CAD, and valvular and other CV structural conditions.
Heart failure is caused by either a dilated ventricle with a reduced ejection fraction (HFrEF) and inability to eject volume, or a stiffened ventricle with a preserved EF (HFpEF) that is unable to receive increased venous return. Both conditions acutely decompensate pulmonary congestion. A preserved EF is defined as an EF at or greater than 50%, whereas a reduced EF is at or less than 40%, with the 41% to 49% range considered as borderline preserved EF.3
While there are important differences in the treatment of chronic and subacute HF, driven by the EF, the effect of EF on early decision-making and treatment in the ED is negligible: Although the probability of HFpEF increases with increasing initial ED systolic blood pressure (SBP), clinical presentation and treatment in the ED are initially identical—regardless of the EF.
Noninvasive continuous transcutaneous hemodynamic monitoring is available for ED use, and may provide further insight into the underlying pathophysiology. A study of 127 acute heart failure (AHF) ED patients identified three hemodynamic AHF phenotypes. These include normal cardiac index (CI) and systemic vascular resistance index (SVRI), low CI and SVRI, and low CI and elevated SVRI.14 While it is attractive to suggest therapeutic interventions based on these measurements, outcome data are lacking.
Presentation
The most common ED presentation of patients suffering from AHF is dyspnea secondary to volume overload, or as the result of acute hypertension with relatively less volume overload. However, regardless of the cause of dyspnea, it is not only the most common resulting complaint, but one that requires immediate treatment. Ultimately, 59% of all HF admissions are attributed to volume overload and dyspnea (Figure 1).15
Heart failure can also present in a more protean manner, with cough, fatigue, and edema, as well as more subtle symptoms predominating and resulting in a complicated differential diagnosis (Table 2).16
Because HF is a disease that most significantly affects older patients who frequently have concomitant morbidities (eg, myocardial ischemia, chronic obstructive pulmonary disease [COPD] exacerbation, uncontrolled DM), other less clinically obvious disease presentations may actually be the cause of the AHF exacerbation.
Diagnosis
A focused history and physical examination that is part of all ED evaluations should be expedited whenever there is evidence of hemodynamic instability or respiratory compromise. An early working diagnosis is essential to avoid a delay in appropriate treatment, which is associated with increased mortality.
When HF is likely, the potential etiology and precipitants for decompensation must be considered. This list is long, but medication noncompliance and dietary indiscretion are the most common causes.
Symptoms and Prior History of HF
The classic symptoms for AHF include dyspnea at rest or exertion, and orthopnea, both of which unfortunately have poor sensitivity and specificity for AHF. As an isolated symptom, dyspnea is of marginal diagnostic utility (sensitivity and specificity for an HF diagnosis is 56% and 53%, respectively), and orthopnea is only slightly better (sensitivity and specificity 77% and 50%, respectively). A prior HF diagnosis makes repeat presentations much more likely (sensitivity and specificity 60% and 90%, respectively).17
Physical Examination
Simple observation and a directed examination can rapidly point to the diagnosis (Figure 2).
Electrocardiography
Because CAD is one of the most common underlying AHF etiologies, an electrocardiogram (ECG) should always be obtained early for a patient presenting with potential AHF. Although the ECG does not usually contribute to ED management, the identification of new ST-segment changes or a malignant arrhythmia will guide critical management decisions.
Imaging Studies
Chest X-ray Imaging. A chest X-ray (CXR) study must be considered early when a patient presents with signs and symptoms suggestive of AHF. Although the classic findings of HF (eg, Kerley B lines [short horizontal lines perpendicular to the pleural surface],18 interstitial congestion, pulmonary effusion) can lag behind the clinical presentation, and also be nondiagnostic in the setting of mild HF, the CXR is an effective aid in identifying other causes of dyspnea such as pneumonia. Ultimately, the utility of the CXR for diagnosis is similar to that of the history and physical examination in that it will be diagnostic when positive but cannot exclude AHF if normal.
Ultrasound. Because it is fast, inexpensive, noninvasive, and readily available in the ED, ultrasound is frequently used to evaluate potential HF patients. Several studies have demonstrated that the presence of B lines in two or more regions is specific for AHF (specificity 75%-100%), although the sensitivity may be limited (40%-91%).19-21 The presence of inferior vena cava (IVC) dilation is also associated with adverse outcomes.22 In 80 patients hospitalized with acute decompensated HF (ADHF), a dilated IVC (≥1.9 cm) at admission was associated with higher 90-day mortality (25.4% vs 3.4%, P = 0.009).23 These findings may be considered in groups: In an evaluation of the combination of LV EF, IVC collapsibility, and B lines for an HF diagnosis, the combination of all three had a poor sensitivity (36%) but an excellent specificity (100%), and any two of the three had a specificity of at least 93%.24
Laboratory Evaluation
Myocardial Strain: BNP/NTproBNP. Natriuretic peptides (NPs) are not AHF-specific, but rather they are synthesized and released by the myocardium in the setting of myocardial pressure or volume stress. They are manufactured as preproBNP, then enzymatically cleaved into the active BNP and the inactive fragment N-terminal proBNP (NTproBNP). The predominant hormonal effects of BNP are vasodilation and natriuresis, as well as antagonism of the hormones associated with sodium retention (aldosterone) and vasoconstriction (endothelin, norepinephrine).
As AHF results in myocardial stress, NP elevation provides diagnostic and prognostic information. Clinical judgment supported by a BNP greater than 100 pg/mL is a better predictor of AHF than clinical judgment alone (accuracy 81% vs 74%, respectively).25 While low levels (BNP <100 pg/mL or NTproBNP <300 pg/mL) reliably exclude the diagnosis of HF (sensitivities >95%), higher levels (BNP >500 pg/mL, NTproBNP >900 pg/mL) are useful as “rule-in” markers, with specificity greater than 95%. The NTproBNP also requires adjustment for patients older than age 75 years, with a higher level (>1,800 pg/mL) to rule-in HF. The NP grey zone (BNP 100-500 pg/mL, NTproBNP 300-900 pg/mL)requires additional testing for accurate diagnoses (Figure 3).25-29
There are several confounders to the interpretation of NP results: NPs are negatively confounded by the presence of obesity, resulting in a lowering of the value as compared to the clinical presentation.Thus, the measured BNP level should be doubled if the patient’s body mass index exceeds 35 kg/m2.30 Secondly, because NP metabolism is partially renal dependent, elevated levels may not reflect AHF in the presence of renal failure. If the estimated glomerular filtration rate is less than 60 mL/min, measured BNP levels should be halved.31
AHF vs Myocardial Ischemia: Troponin Levels. Large registry data using contemporary troponin assays clearly identify the association between elevated troponin levels (>99th percentile in a healthy population) and increased short-term risk. With the US Food and Drug Association (FDA) approval of a high-sensitivity troponin (hs-cTnT) assay, a greater frequency of elevated cardiac troponin T (cTnT) and cardiac troponin I (cTnl) will be identified in AHF patients in the ED.
In one retrospective study of 4,705 AHF patients in the ED, hs-cTnT were elevated in 48.4% of cases (25.3% in cTnI, 37.9% in cTnT, and 82.2% in hs-cTnT). Although 1-year mortality was higher in those with elevated troponin (adjusted heart rate [HR] 1.61; CI 95% 1.38-1.88), elevated troponin was not associated with 30-day revisits to the ED (1.01; 0.87-1.19) and high sensitive elevations less than double the reference value had no impact on outcomes.32 Thus, in terms of management of AHF in the ED, slightly elevated stable serial troponins are more consistent with underlying HF, and should be managed as such. This is not true of rising/falling troponin levels, which should still engender concern for underlying myocardial ischemia and a different management pathway.
Renal function. Comprised renal function is an important predictor of AHF outcome. Large registry data from hospitalized HF patients demonstrate that a presenting blood urea nitrogen level greater than 43 mg/dL is one of the most important predictors of increased acute mortality,33 and levels below 30 mg/dL identify a cohort likely to be successfully managed in an observation environment.34 Creatinine is a helpful lagging indicator of mortality, with higher levels (>2.75 mg/dL) associated with increased short-term adverse outcomes and decreased therapeutic responsiveness (Figure 4).
For patients presenting with ADHF, a newer test recently approved by the FDA uses the product of the urine markers tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7, to generate a score predictive of acute kidney injury.35 While promising, no studies of ED outcomes are currently available.
Volume Assessment
Objective volume assessment is useful for diagnosis and prognosis in AHF. Bioimpedance vector analysis (BIVA) is a rapid, inexpensive, noninvasive technique that measures total body water by placing a pair of electrodes on the wrist and ipsilateral ankle. The BIVA measurements have strong correlations with the gold standard volume-assessment technique of deuterium dilution (r > 0.99).36 In HF, BIVA can assess volume depletion37 and overload,38 and identifies differences in hydration status between 90-day survivors and non-survivors (P < 0.01).39
Used in combination with BNP, one prospective study of 292 dyspneic patients found that, while BIVA was a strong predictor of AHF (c-statistic 0.93, P = 0.016), the most accurate volume status determination was the combination of both (c-statistic, 0.99; P = 0.005), for which the combined accuracy exceeded either alone.40 Finally, in 166 hospitalized HF patients discharged by BNP and BIVA parameters, vs 149 discharged based on clinical impressions, those assessed with BNP and BIVA had lower 6-month readmissions (23% vs 35%, P = 0.02) and overall cost of care.41
Combination Technologies
Obviously, EPs may consider multiple technologies to arrive at an accurate diagnosis. One prospective evaluation enrolled 236 patients to determine the diagnostic accuracy for AHF in the ED and reported lung ultrasound, CXR, and NTproBNP had a sensitivity of 57.7% and 88.0%, 74.5% and 86.3%, and a specificity of 97.6% and 28.0%. The best overall combination was the CXR with lung ultrasound (sensitivity 84.7%, specificity 77.7%).42
Another prospective study evaluated IVC diameter, bioelectrical impedance analysis (BIA), and NTproBNP in 96 elderly patients. ADHF patients had higher IVC diameters and lower collapsibility index, lower resistance and reactance, and higher NTproBNP levels. While all had high and statistically similar C-statistics (range 0.8 to 0.9) for an ADHF diagnosis, they concluded that IVC ultrasonography and BIA were as useful as NT-proBNP for diagnosing ADHF. 24
Diagnostic Scoring Systems
A scoring system has been proposed to improve diagnosis in the ED. Unfortunately, the value over clinical impression has not been clearly proven, though one randomized, controlled trial did not show statistically significant improvement in diagnostic accuracy when compared to standard care (77% vs 74%, P = 0.77).43
Differential Diagnosis
The differential diagnosis for acute dyspnea is long and potentially arcane. Efforts should focus on excluding non-HF causes of dyspnea, while considering the high risk of alternative etiologies for signs and symptoms. These include asthma, COPD, pneumonia, and pulmonary embolism, which may represent the primary pathologies in a patient with a history of HF, or be the cause of a HF exacerbation. Additional causes of noncardiogenic pulmonary edema should also be considered (eg, acute respiratory distress syndrome, toxins, etc). Acute coronary syndrome and dyspnea may be angina equivalents—one important consideration.
Treatment and Management
Airway Management
Treatment of CH in the ED must always start with an immediate airway evaluation, with the possible need for endotracheal intubation preceding all diagnostic or other management considerations. Intubation is a decision most successfully based on physician clinical assessment, including oxygen (O2) selection rather than waiting for the results of objective measures such as arterial blood gas analysis.
Oxygen
Supplemental O2 should be administered to maintain an O2 saturation above 95%, but obviously is unnecessary in the absence of hypoxia.
Noninvasive Ventilation
Two kinds of noninvasive ventilation (NIV) are available, continuous positive airway pressure and bilevel positive airway pressure ventilation. The physiological differences between these types of NIV have little bearing on ED treatment.
Noninvasive ventilation has not been clearly shown to provide long-term mortality benefit. Large registry data44 report that outcomes are no worse than the alternative of endotracheal intubation, while multiple systematic reviews,45,46meta-analysis,47and Cochrane reviews48,49have established NIV as an acute pulmonary edema intervention that provides reductions in hospital mortality (numbers needed to treat [NNT] 13) and intubation (NNT 8), the prospective randomized C3PO (Congenital Cardiac Catheterization Project on Outcomes) trial50 failed to demonstrate any mortality reduction.
In patients with severe respiratory distress, NIV is a reasonable strategy during the aggressive administration of medical therapy in an attempt to avoid endotracheal intubation. However, NIV is not a stand-alone therapy and though its use may obviate the need for immediate intubation, its implementation should not be considered definitive management.
Correction of Abnormal Vital Signs: Abnormal SBP
Vital signs are an important determinant of therapy, driving treatment strategies. Interventions for HF are based on the patient’s SBP, in particular correction of symptomatic hypotension and hypertensive HF (Table 3).51
Symptomatic Hypotension. The presence of symptomatic hypotension is an extremely poor prognostic finding in AHF. Inotrope therapy may be considered, but it does not reduce mortality except as a bridge to mechanical interventions (LV assist device or transplant).52-54 Temporary inotropic support is recommended for cardiogenic shock to maintain systemic perfusion and prevent end organ damage.3 The inotropic support includes administration of dopamine, dobutamine, or milrinone, though none have been proven to be superior over the other. The lowest possible dose of the selected inotrope should be used to limit arrhythmogenic effects. Inotropic agents should not be used in the absence of severe systolic dysfunction, or low BP, or impaired perfusion, or evidence of significantly decreased cardiac output.
Hypertensive Heart Failure. Defined as the rapid onset of pulmonary congestion with an SBP greater than 140 mm Hg, and commonly greater than 160 mm Hg, these patients may have profound dyspnea, requiring endotracheal intubation. However, in this situation, aggressive vasodilation is typically rapidly effective. Overall, patients presenting with an elevated SBP have lower rates of in-hospital mortality, 30-day myocardial infarction (MI), death, or rehospitalization, and a greater likelihood of discharge within 24 hours—as long as the elevated SBP is aggressively and rapidly treated.
Pharmacological Therapy
Pharmacological management is the mainstay for treating HF. No other acute therapy (eg, NIV) has demonstrated a morality benefit (See Table 4 for specific dose and administration strategies).55 The time to initiate pharmacological therapy and whether an aggressive approach is indicated must be based on the severity of the clinical symptoms and objective risk stratification measures (eg, NP, troponin levels).
Furosemide. Except for hypertensive HF—in which case BP lowering is the most important goal—diuretics are a mainstay of AHF treatment, and consensus guidelines provide a class I recommendation for their use.3 The DOSE (Diuretic Strategies in Patients with ADHF) trial56 prospectively evaluated diuretics in 308 hospitalized AHF patients and found no outcome differences in administration route (bolus or continuous infusion) or dose (high vs low dose). This study reported trends toward greater improvement with higher furosemide dosing, as well as greater diuresis, but at a cost of transient worsening of renal function.
In general, diuretics should be administered in an intravenous (IV) dose equal to 1 to 2.5 times the patient’s usual daily oral dose. For patients who are diuretic-naïve, a dose of 40 mg IV furosemide or 1 mg IV bumetanide, with subsequent dosing titrated to urine output, is recommended.
Vasodilators. In patients with both AHF and even mildly elevated BP, vasodilators can be extremely effective in achieving symptom improvement. The choice of vasodilator, and how aggressive to increase dosing, depends upon symptom severity. The purpose of vasodilators is to lower BP and therefore, should not be used in the setting of hypotension or signs of hypoperfusion. Flow-limiting, preload-dependent CV states (eg, right ventricular infarction) increase the risk of hypotension, and are relative contraindications to the use of vasodilators. For patients who are severely dyspneic and with critical presentations, the emergency physician (EP) should preclude a detailed history and examination to initiate immediate therapy with short-acting agents that can be terminated rapidly in the case of an adverse event (eg, unexpected hypotension) are preferred.
Nitroglycerin. Nitroglycerin is the vasodilation agent of choice for hypertensive AHF. It is a short-acting, rapid-onset, venous and arterial dilator that decreases BP by preload reduction, and by afterload reduction in higher doses. Nitroglycerin has coronary vasodilatory effects associated with decreased ischemia, but should be avoided in patients taking phosphodiesterase inhibitors.55 Its most common side effect is headache, and hypotension occurs in about 3.5% of patients.57
Commonly given as a continuous infusion at IV doses up to 400 mcg/min, nitroglycerin may be associated with higher costs and longer LOS.58 Some authors suggest that bolus nitroglycerin therapy may be superior: In a retrospective study of 395 patients, an IV bolus of nitroglycerin 0.5 mg was superior to both an infusion, or a combination of bolus and infusion, as demonstrated by lower rates of ICU admission (48% vs 67% and 79%, respectively, P = 0.006) and shorter hospital stays (4.4 vs 6.3 and 7.3 days, respectively, P = 0.01). In all cohorts, adverse event rates were similar for hypotension, troponin elevation, and creatinine increase over 48 hours.59 Nitroprusside. Nitroprusside is a potent arterial and venous dilator that causes rapid decrease in BP and LV-filling pressures. It is usually considered more effective than nitroglycerin, despite a small study showing similar hemodynamic responses.60
Initial dosing of nitroprusside starts at 0.3 µg/kg/min IV, and is increased every 5 minutes to a maximum of 10 mcg/kg/min, based on BP and clinical response. The most common acute complication of nitroprusside infusions is hypotension. Cyanide toxicity may occur with prolonged use, high doses, or in patients with renal failure.55
Nesiritide. Exogenously administered, the B-type NP nesiritide is effective in lowering BP and improving dyspnea in AHF,55 although large prospective studies showed it had little long-term advantage over standard care.61 In a small, randomized, controlled trial, nesiritide reduced 30-day revisit LOS when given in an OU.62 The 22-minute half-life of nesiritide is longer than that of the nitrates, and its side effect is predominately hypotension, which occurs at rates similar to those of other vasodilators.55
Angiotensin Converting Enzyme Inhibitors. Because angiotensin converting enzyme inhibitors (ACEIs) have chronic mortality reduction benefits, their use in the acute setting is theoretically attractive, however, this has been poorly proven in AHF ED patients. In a retrospective review of 103 patients with elevated NTproBNP levels receiving bolus IV enalaprilat within 3 hours of presentation, the mean SBP decreased by 30 mm Hg, with only 2% of patients developing hypotension.63 However, with the longer half-life of ACEIs, if hypotension occurs, the potential for a prolonged BP-lowering effect exists.
Calcium Channel Blockers. Clevidipine and nicardipine are rapidly acting IV calcium channel blockers that lower BP by selective arteriolar vasodilation and increased cardiac output as vascular resistance declines.55 Because these agents have no negative inotropic or chronotropic effects, they may be beneficial in hypertensive AHF. In an open-label trial of 104 hypertensive AHF patients, clevidipine was more effective than standard care for the rapid control of BP and relief of dyspnea.64
Morphine. Large registry analyses have demonstrated potential harm with the routine use of morphine,65 as do recent propensity score matched analyses.66 Until there are studies demonstrating benefit, the use of morphine at present should be reserved for palliative care.
Time to Treatment
Although a randomized controlled trial on the importance of time to treatment of AHF is unlikely to ever be completed, data suggest that, as in the case of MI, delayed AHF therapy is associated with adverse outcomes. In a study of 499 suspected AHF patients transferred by ambulance, patients randomized to immediate therapy vs those whose therapy was not initiated until hospital arrival (mean delay of 36 minutes), had a 251% increase in survival (P < 0.01).67
Furthermore, the delayed administration of vasoactive agents, defined as medication administered to alter hemodynamics (eg, dobutamine, dopamine, nitroglycerin, nesiritide) is also associated with harm,68 and registry studies demonstrate increased death rates (n = 35,700).69 Finally, another registry (n = 14,900) study demonstrated early IV furosemide is associated with decreased mortality.70 This latter finding was also validated in a prospective observational cohort study (mortality 2.3 vs 6.0 in early vs delayed therapy groups, respectively).71
Patient Disposition
One of the unique features of emergency medicine is the need to determine, with very limited information and time, a patient’s very short-term clinical trajectory. Few physicians are required to have greater accuracy with less information or time than do EPs. Several studies report objective data points and risk scores to assist in this task, but none has been universally adopted, reflecting the challenge of applying population data to individuals.
Short-term Prognosis
In 1,638 patients evaluated for 14-day outcomes, an HR lower than 50% maximal HR (MHR), and an SBP greater than 140 mm Hg were associated with the lowest rate of serious adverse events (SAEs) (6%) and hospitalization (38%).72 An MHR over 75% was associated with the highest SAE rate, although SAEs decreased as SBP increased (30%, 24%, and 21% with SBPs < 120 mm Hg, 120-140 mm Hg, and > 140 mm Hg, respectively).72
Risk Scores
In a prospective, observational cohort study of 1,100 ED patients, the Ottawa Heart Failure Risk Scale, combined with NTproBNP values, had a sensitivity of 95.8%—at the cost of increasing the admission rate (from 60.8% to 88%)—for serious adverse events (defined as death within 30 days), admission to a monitored unit, intubation, NIV, MI, or relapse resulting in hospital admission within 14 days.73
Observation Unit
Overall, 44% of in-patient HF admissions are for less than 3 days (Table 1),2 supporting the practice of managing selected patients in shorter clinical-care environments than in inpatient units. Further, ED patients presenting with moderate dyspnea require both a diagnosis and an evaluation of their therapeutic response to determine the need for hospitalization. However, evaluating therapeutic response requires more time than is available in the typical ED. Thus, an ED OU offers the following:
(1) The OU provides the EP with a longer evaluation time, and therefore a more accurate disposition may be effected;
(2) Costs are significantly lower in patients managed in an ED OU; and
(3) Patient satisfaction may be improved, as most patients prefer home management over hospitalization.
All three of these opportunities are supported by a number of studies,74-78 with validated entry and exclusion criteria, treatment algorithms and discharge metrics. Most recently, in a registry of hospitals in Spain registry, patients presenting to hospitals that had OUs had a 2.2-day shorter LOS, lower 30-day ED revisit rate, and similar mortality rates compared to those in institutions without OUs—although these beneficial effects occurred at the cost of an 8.9% higher admission rate.79
Patient Education
Intuitively, it would be expected that patient education would reduce return visits, 30-day hospitalizations, and AHF-related mortality. Unfortunately, it has not been demonstrated that patient education results in a consistent benefit at hospital discharge, or in the outpatient environment.80-85
Although AHF education in the ED has been poorly studied, areas that have shown promise are education occurring before ED management (ie, in the ED waiting area) in underinsured patients,86 and during ED care for patients with poor health care literacy.87 As educational interventions are both inexpensive and unlikely to result in harm, their implementation should be considered.
Conclusion
The spectrum of HF is a common presentation in the ED. Because HF generally appears as dyspnea, in a cohort with multiple comorbidities, the diagnosis can be challenging. This is complicated by the fact that patients with severe presentations may require life-saving interventions long before a clinical evaluation is completed (or even initiated). The skill of the EP, and his or her ability to improve the clinical condition before intubation is required, will determine the patient’s trajectory. Conversely, as a chronic condition, HF may present with moderate symptoms for which a short diuretic “tune-up” in an observation environment may be appropriate.
How these decisions are made will depend upon the local environment, the availability of outpatient resources, and individual patient choices. There are few chronic diseases that are more complex, are seen more often in the ED, or that require more skill and finesse in management.
Patients with heart failure (HF) present daily to busy EDs. An estimated 6.5 million Americans are living with this diagnosis, and the number is predicted to grow to 8 million by 2023.1 Most HF patients (82.1%) who present to EDs are hospitalized, while a selected minority are either managed in the ED and discharged (11.6%) or managed in observation units (OU) (6.3%).2 The prognosis after HF is initially diagnosed is poor, with a 5-year mortality of 50%,3 and after a single HF hospitalization, 29% will die within 1 year.4
One-third of the total Medicare budget is spent on HF, despite the fact that HF represents only 10.5% of the Medicare population.2 Up to 80% of HF costs are for hospitalizations, which cost an average of $11,840 per inpatient admission.5,6 The high costs are due to an average length of stay (LOS) of 5.2 days7 (Table 1).
Adding to hospital costs is the degree of “reactivism,” with approximately 20% of patients discharged from the ED returning within 2 weeks, of whom nearly 50% will be hospitalized.11 Following HF hospitalization and discharge, the 30-day readmission rate is 26.2%,2 increasing to 36% by 90 days.12 The Centers for Medicare & Medicaid Services (CMS) has incentivized hospitals and providers to reduce admissions, but penalize hospitals that do not. Overall, CMS will reduce payments by up to 3% to hospitals with excess readmissions for select conditions, including HF.13
Causes of Heart Failure
Heart failure represents a final common pathway, which in the United States is most often due to coronary artery disease (CAD). Many types of pathology ultimately result in left ventricular (LV) dysfunction, and much of its rising prevalence is a result of the success we now have in managing historically fatal cardiovascular (CV) conditions. These include hypertension, diabetes mellitus (DM), CAD, and valvular and other CV structural conditions.
Heart failure is caused by either a dilated ventricle with a reduced ejection fraction (HFrEF) and inability to eject volume, or a stiffened ventricle with a preserved EF (HFpEF) that is unable to receive increased venous return. Both conditions acutely decompensate pulmonary congestion. A preserved EF is defined as an EF at or greater than 50%, whereas a reduced EF is at or less than 40%, with the 41% to 49% range considered as borderline preserved EF.3
While there are important differences in the treatment of chronic and subacute HF, driven by the EF, the effect of EF on early decision-making and treatment in the ED is negligible: Although the probability of HFpEF increases with increasing initial ED systolic blood pressure (SBP), clinical presentation and treatment in the ED are initially identical—regardless of the EF.
Noninvasive continuous transcutaneous hemodynamic monitoring is available for ED use, and may provide further insight into the underlying pathophysiology. A study of 127 acute heart failure (AHF) ED patients identified three hemodynamic AHF phenotypes. These include normal cardiac index (CI) and systemic vascular resistance index (SVRI), low CI and SVRI, and low CI and elevated SVRI.14 While it is attractive to suggest therapeutic interventions based on these measurements, outcome data are lacking.
Presentation
The most common ED presentation of patients suffering from AHF is dyspnea secondary to volume overload, or as the result of acute hypertension with relatively less volume overload. However, regardless of the cause of dyspnea, it is not only the most common resulting complaint, but one that requires immediate treatment. Ultimately, 59% of all HF admissions are attributed to volume overload and dyspnea (Figure 1).15
Heart failure can also present in a more protean manner, with cough, fatigue, and edema, as well as more subtle symptoms predominating and resulting in a complicated differential diagnosis (Table 2).16
Because HF is a disease that most significantly affects older patients who frequently have concomitant morbidities (eg, myocardial ischemia, chronic obstructive pulmonary disease [COPD] exacerbation, uncontrolled DM), other less clinically obvious disease presentations may actually be the cause of the AHF exacerbation.
Diagnosis
A focused history and physical examination that is part of all ED evaluations should be expedited whenever there is evidence of hemodynamic instability or respiratory compromise. An early working diagnosis is essential to avoid a delay in appropriate treatment, which is associated with increased mortality.
When HF is likely, the potential etiology and precipitants for decompensation must be considered. This list is long, but medication noncompliance and dietary indiscretion are the most common causes.
Symptoms and Prior History of HF
The classic symptoms for AHF include dyspnea at rest or exertion, and orthopnea, both of which unfortunately have poor sensitivity and specificity for AHF. As an isolated symptom, dyspnea is of marginal diagnostic utility (sensitivity and specificity for an HF diagnosis is 56% and 53%, respectively), and orthopnea is only slightly better (sensitivity and specificity 77% and 50%, respectively). A prior HF diagnosis makes repeat presentations much more likely (sensitivity and specificity 60% and 90%, respectively).17
Physical Examination
Simple observation and a directed examination can rapidly point to the diagnosis (Figure 2).
Electrocardiography
Because CAD is one of the most common underlying AHF etiologies, an electrocardiogram (ECG) should always be obtained early for a patient presenting with potential AHF. Although the ECG does not usually contribute to ED management, the identification of new ST-segment changes or a malignant arrhythmia will guide critical management decisions.
Imaging Studies
Chest X-ray Imaging. A chest X-ray (CXR) study must be considered early when a patient presents with signs and symptoms suggestive of AHF. Although the classic findings of HF (eg, Kerley B lines [short horizontal lines perpendicular to the pleural surface],18 interstitial congestion, pulmonary effusion) can lag behind the clinical presentation, and also be nondiagnostic in the setting of mild HF, the CXR is an effective aid in identifying other causes of dyspnea such as pneumonia. Ultimately, the utility of the CXR for diagnosis is similar to that of the history and physical examination in that it will be diagnostic when positive but cannot exclude AHF if normal.
Ultrasound. Because it is fast, inexpensive, noninvasive, and readily available in the ED, ultrasound is frequently used to evaluate potential HF patients. Several studies have demonstrated that the presence of B lines in two or more regions is specific for AHF (specificity 75%-100%), although the sensitivity may be limited (40%-91%).19-21 The presence of inferior vena cava (IVC) dilation is also associated with adverse outcomes.22 In 80 patients hospitalized with acute decompensated HF (ADHF), a dilated IVC (≥1.9 cm) at admission was associated with higher 90-day mortality (25.4% vs 3.4%, P = 0.009).23 These findings may be considered in groups: In an evaluation of the combination of LV EF, IVC collapsibility, and B lines for an HF diagnosis, the combination of all three had a poor sensitivity (36%) but an excellent specificity (100%), and any two of the three had a specificity of at least 93%.24
Laboratory Evaluation
Myocardial Strain: BNP/NTproBNP. Natriuretic peptides (NPs) are not AHF-specific, but rather they are synthesized and released by the myocardium in the setting of myocardial pressure or volume stress. They are manufactured as preproBNP, then enzymatically cleaved into the active BNP and the inactive fragment N-terminal proBNP (NTproBNP). The predominant hormonal effects of BNP are vasodilation and natriuresis, as well as antagonism of the hormones associated with sodium retention (aldosterone) and vasoconstriction (endothelin, norepinephrine).
As AHF results in myocardial stress, NP elevation provides diagnostic and prognostic information. Clinical judgment supported by a BNP greater than 100 pg/mL is a better predictor of AHF than clinical judgment alone (accuracy 81% vs 74%, respectively).25 While low levels (BNP <100 pg/mL or NTproBNP <300 pg/mL) reliably exclude the diagnosis of HF (sensitivities >95%), higher levels (BNP >500 pg/mL, NTproBNP >900 pg/mL) are useful as “rule-in” markers, with specificity greater than 95%. The NTproBNP also requires adjustment for patients older than age 75 years, with a higher level (>1,800 pg/mL) to rule-in HF. The NP grey zone (BNP 100-500 pg/mL, NTproBNP 300-900 pg/mL)requires additional testing for accurate diagnoses (Figure 3).25-29
There are several confounders to the interpretation of NP results: NPs are negatively confounded by the presence of obesity, resulting in a lowering of the value as compared to the clinical presentation.Thus, the measured BNP level should be doubled if the patient’s body mass index exceeds 35 kg/m2.30 Secondly, because NP metabolism is partially renal dependent, elevated levels may not reflect AHF in the presence of renal failure. If the estimated glomerular filtration rate is less than 60 mL/min, measured BNP levels should be halved.31
AHF vs Myocardial Ischemia: Troponin Levels. Large registry data using contemporary troponin assays clearly identify the association between elevated troponin levels (>99th percentile in a healthy population) and increased short-term risk. With the US Food and Drug Association (FDA) approval of a high-sensitivity troponin (hs-cTnT) assay, a greater frequency of elevated cardiac troponin T (cTnT) and cardiac troponin I (cTnl) will be identified in AHF patients in the ED.
In one retrospective study of 4,705 AHF patients in the ED, hs-cTnT were elevated in 48.4% of cases (25.3% in cTnI, 37.9% in cTnT, and 82.2% in hs-cTnT). Although 1-year mortality was higher in those with elevated troponin (adjusted heart rate [HR] 1.61; CI 95% 1.38-1.88), elevated troponin was not associated with 30-day revisits to the ED (1.01; 0.87-1.19) and high sensitive elevations less than double the reference value had no impact on outcomes.32 Thus, in terms of management of AHF in the ED, slightly elevated stable serial troponins are more consistent with underlying HF, and should be managed as such. This is not true of rising/falling troponin levels, which should still engender concern for underlying myocardial ischemia and a different management pathway.
Renal function. Comprised renal function is an important predictor of AHF outcome. Large registry data from hospitalized HF patients demonstrate that a presenting blood urea nitrogen level greater than 43 mg/dL is one of the most important predictors of increased acute mortality,33 and levels below 30 mg/dL identify a cohort likely to be successfully managed in an observation environment.34 Creatinine is a helpful lagging indicator of mortality, with higher levels (>2.75 mg/dL) associated with increased short-term adverse outcomes and decreased therapeutic responsiveness (Figure 4).
For patients presenting with ADHF, a newer test recently approved by the FDA uses the product of the urine markers tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7, to generate a score predictive of acute kidney injury.35 While promising, no studies of ED outcomes are currently available.
Volume Assessment
Objective volume assessment is useful for diagnosis and prognosis in AHF. Bioimpedance vector analysis (BIVA) is a rapid, inexpensive, noninvasive technique that measures total body water by placing a pair of electrodes on the wrist and ipsilateral ankle. The BIVA measurements have strong correlations with the gold standard volume-assessment technique of deuterium dilution (r > 0.99).36 In HF, BIVA can assess volume depletion37 and overload,38 and identifies differences in hydration status between 90-day survivors and non-survivors (P < 0.01).39
Used in combination with BNP, one prospective study of 292 dyspneic patients found that, while BIVA was a strong predictor of AHF (c-statistic 0.93, P = 0.016), the most accurate volume status determination was the combination of both (c-statistic, 0.99; P = 0.005), for which the combined accuracy exceeded either alone.40 Finally, in 166 hospitalized HF patients discharged by BNP and BIVA parameters, vs 149 discharged based on clinical impressions, those assessed with BNP and BIVA had lower 6-month readmissions (23% vs 35%, P = 0.02) and overall cost of care.41
Combination Technologies
Obviously, EPs may consider multiple technologies to arrive at an accurate diagnosis. One prospective evaluation enrolled 236 patients to determine the diagnostic accuracy for AHF in the ED and reported lung ultrasound, CXR, and NTproBNP had a sensitivity of 57.7% and 88.0%, 74.5% and 86.3%, and a specificity of 97.6% and 28.0%. The best overall combination was the CXR with lung ultrasound (sensitivity 84.7%, specificity 77.7%).42
Another prospective study evaluated IVC diameter, bioelectrical impedance analysis (BIA), and NTproBNP in 96 elderly patients. ADHF patients had higher IVC diameters and lower collapsibility index, lower resistance and reactance, and higher NTproBNP levels. While all had high and statistically similar C-statistics (range 0.8 to 0.9) for an ADHF diagnosis, they concluded that IVC ultrasonography and BIA were as useful as NT-proBNP for diagnosing ADHF. 24
Diagnostic Scoring Systems
A scoring system has been proposed to improve diagnosis in the ED. Unfortunately, the value over clinical impression has not been clearly proven, though one randomized, controlled trial did not show statistically significant improvement in diagnostic accuracy when compared to standard care (77% vs 74%, P = 0.77).43
Differential Diagnosis
The differential diagnosis for acute dyspnea is long and potentially arcane. Efforts should focus on excluding non-HF causes of dyspnea, while considering the high risk of alternative etiologies for signs and symptoms. These include asthma, COPD, pneumonia, and pulmonary embolism, which may represent the primary pathologies in a patient with a history of HF, or be the cause of a HF exacerbation. Additional causes of noncardiogenic pulmonary edema should also be considered (eg, acute respiratory distress syndrome, toxins, etc). Acute coronary syndrome and dyspnea may be angina equivalents—one important consideration.
Treatment and Management
Airway Management
Treatment of CH in the ED must always start with an immediate airway evaluation, with the possible need for endotracheal intubation preceding all diagnostic or other management considerations. Intubation is a decision most successfully based on physician clinical assessment, including oxygen (O2) selection rather than waiting for the results of objective measures such as arterial blood gas analysis.
Oxygen
Supplemental O2 should be administered to maintain an O2 saturation above 95%, but obviously is unnecessary in the absence of hypoxia.
Noninvasive Ventilation
Two kinds of noninvasive ventilation (NIV) are available, continuous positive airway pressure and bilevel positive airway pressure ventilation. The physiological differences between these types of NIV have little bearing on ED treatment.
Noninvasive ventilation has not been clearly shown to provide long-term mortality benefit. Large registry data44 report that outcomes are no worse than the alternative of endotracheal intubation, while multiple systematic reviews,45,46meta-analysis,47and Cochrane reviews48,49have established NIV as an acute pulmonary edema intervention that provides reductions in hospital mortality (numbers needed to treat [NNT] 13) and intubation (NNT 8), the prospective randomized C3PO (Congenital Cardiac Catheterization Project on Outcomes) trial50 failed to demonstrate any mortality reduction.
In patients with severe respiratory distress, NIV is a reasonable strategy during the aggressive administration of medical therapy in an attempt to avoid endotracheal intubation. However, NIV is not a stand-alone therapy and though its use may obviate the need for immediate intubation, its implementation should not be considered definitive management.
Correction of Abnormal Vital Signs: Abnormal SBP
Vital signs are an important determinant of therapy, driving treatment strategies. Interventions for HF are based on the patient’s SBP, in particular correction of symptomatic hypotension and hypertensive HF (Table 3).51
Symptomatic Hypotension. The presence of symptomatic hypotension is an extremely poor prognostic finding in AHF. Inotrope therapy may be considered, but it does not reduce mortality except as a bridge to mechanical interventions (LV assist device or transplant).52-54 Temporary inotropic support is recommended for cardiogenic shock to maintain systemic perfusion and prevent end organ damage.3 The inotropic support includes administration of dopamine, dobutamine, or milrinone, though none have been proven to be superior over the other. The lowest possible dose of the selected inotrope should be used to limit arrhythmogenic effects. Inotropic agents should not be used in the absence of severe systolic dysfunction, or low BP, or impaired perfusion, or evidence of significantly decreased cardiac output.
Hypertensive Heart Failure. Defined as the rapid onset of pulmonary congestion with an SBP greater than 140 mm Hg, and commonly greater than 160 mm Hg, these patients may have profound dyspnea, requiring endotracheal intubation. However, in this situation, aggressive vasodilation is typically rapidly effective. Overall, patients presenting with an elevated SBP have lower rates of in-hospital mortality, 30-day myocardial infarction (MI), death, or rehospitalization, and a greater likelihood of discharge within 24 hours—as long as the elevated SBP is aggressively and rapidly treated.
Pharmacological Therapy
Pharmacological management is the mainstay for treating HF. No other acute therapy (eg, NIV) has demonstrated a morality benefit (See Table 4 for specific dose and administration strategies).55 The time to initiate pharmacological therapy and whether an aggressive approach is indicated must be based on the severity of the clinical symptoms and objective risk stratification measures (eg, NP, troponin levels).
Furosemide. Except for hypertensive HF—in which case BP lowering is the most important goal—diuretics are a mainstay of AHF treatment, and consensus guidelines provide a class I recommendation for their use.3 The DOSE (Diuretic Strategies in Patients with ADHF) trial56 prospectively evaluated diuretics in 308 hospitalized AHF patients and found no outcome differences in administration route (bolus or continuous infusion) or dose (high vs low dose). This study reported trends toward greater improvement with higher furosemide dosing, as well as greater diuresis, but at a cost of transient worsening of renal function.
In general, diuretics should be administered in an intravenous (IV) dose equal to 1 to 2.5 times the patient’s usual daily oral dose. For patients who are diuretic-naïve, a dose of 40 mg IV furosemide or 1 mg IV bumetanide, with subsequent dosing titrated to urine output, is recommended.
Vasodilators. In patients with both AHF and even mildly elevated BP, vasodilators can be extremely effective in achieving symptom improvement. The choice of vasodilator, and how aggressive to increase dosing, depends upon symptom severity. The purpose of vasodilators is to lower BP and therefore, should not be used in the setting of hypotension or signs of hypoperfusion. Flow-limiting, preload-dependent CV states (eg, right ventricular infarction) increase the risk of hypotension, and are relative contraindications to the use of vasodilators. For patients who are severely dyspneic and with critical presentations, the emergency physician (EP) should preclude a detailed history and examination to initiate immediate therapy with short-acting agents that can be terminated rapidly in the case of an adverse event (eg, unexpected hypotension) are preferred.
Nitroglycerin. Nitroglycerin is the vasodilation agent of choice for hypertensive AHF. It is a short-acting, rapid-onset, venous and arterial dilator that decreases BP by preload reduction, and by afterload reduction in higher doses. Nitroglycerin has coronary vasodilatory effects associated with decreased ischemia, but should be avoided in patients taking phosphodiesterase inhibitors.55 Its most common side effect is headache, and hypotension occurs in about 3.5% of patients.57
Commonly given as a continuous infusion at IV doses up to 400 mcg/min, nitroglycerin may be associated with higher costs and longer LOS.58 Some authors suggest that bolus nitroglycerin therapy may be superior: In a retrospective study of 395 patients, an IV bolus of nitroglycerin 0.5 mg was superior to both an infusion, or a combination of bolus and infusion, as demonstrated by lower rates of ICU admission (48% vs 67% and 79%, respectively, P = 0.006) and shorter hospital stays (4.4 vs 6.3 and 7.3 days, respectively, P = 0.01). In all cohorts, adverse event rates were similar for hypotension, troponin elevation, and creatinine increase over 48 hours.59 Nitroprusside. Nitroprusside is a potent arterial and venous dilator that causes rapid decrease in BP and LV-filling pressures. It is usually considered more effective than nitroglycerin, despite a small study showing similar hemodynamic responses.60
Initial dosing of nitroprusside starts at 0.3 µg/kg/min IV, and is increased every 5 minutes to a maximum of 10 mcg/kg/min, based on BP and clinical response. The most common acute complication of nitroprusside infusions is hypotension. Cyanide toxicity may occur with prolonged use, high doses, or in patients with renal failure.55
Nesiritide. Exogenously administered, the B-type NP nesiritide is effective in lowering BP and improving dyspnea in AHF,55 although large prospective studies showed it had little long-term advantage over standard care.61 In a small, randomized, controlled trial, nesiritide reduced 30-day revisit LOS when given in an OU.62 The 22-minute half-life of nesiritide is longer than that of the nitrates, and its side effect is predominately hypotension, which occurs at rates similar to those of other vasodilators.55
Angiotensin Converting Enzyme Inhibitors. Because angiotensin converting enzyme inhibitors (ACEIs) have chronic mortality reduction benefits, their use in the acute setting is theoretically attractive, however, this has been poorly proven in AHF ED patients. In a retrospective review of 103 patients with elevated NTproBNP levels receiving bolus IV enalaprilat within 3 hours of presentation, the mean SBP decreased by 30 mm Hg, with only 2% of patients developing hypotension.63 However, with the longer half-life of ACEIs, if hypotension occurs, the potential for a prolonged BP-lowering effect exists.
Calcium Channel Blockers. Clevidipine and nicardipine are rapidly acting IV calcium channel blockers that lower BP by selective arteriolar vasodilation and increased cardiac output as vascular resistance declines.55 Because these agents have no negative inotropic or chronotropic effects, they may be beneficial in hypertensive AHF. In an open-label trial of 104 hypertensive AHF patients, clevidipine was more effective than standard care for the rapid control of BP and relief of dyspnea.64
Morphine. Large registry analyses have demonstrated potential harm with the routine use of morphine,65 as do recent propensity score matched analyses.66 Until there are studies demonstrating benefit, the use of morphine at present should be reserved for palliative care.
Time to Treatment
Although a randomized controlled trial on the importance of time to treatment of AHF is unlikely to ever be completed, data suggest that, as in the case of MI, delayed AHF therapy is associated with adverse outcomes. In a study of 499 suspected AHF patients transferred by ambulance, patients randomized to immediate therapy vs those whose therapy was not initiated until hospital arrival (mean delay of 36 minutes), had a 251% increase in survival (P < 0.01).67
Furthermore, the delayed administration of vasoactive agents, defined as medication administered to alter hemodynamics (eg, dobutamine, dopamine, nitroglycerin, nesiritide) is also associated with harm,68 and registry studies demonstrate increased death rates (n = 35,700).69 Finally, another registry (n = 14,900) study demonstrated early IV furosemide is associated with decreased mortality.70 This latter finding was also validated in a prospective observational cohort study (mortality 2.3 vs 6.0 in early vs delayed therapy groups, respectively).71
Patient Disposition
One of the unique features of emergency medicine is the need to determine, with very limited information and time, a patient’s very short-term clinical trajectory. Few physicians are required to have greater accuracy with less information or time than do EPs. Several studies report objective data points and risk scores to assist in this task, but none has been universally adopted, reflecting the challenge of applying population data to individuals.
Short-term Prognosis
In 1,638 patients evaluated for 14-day outcomes, an HR lower than 50% maximal HR (MHR), and an SBP greater than 140 mm Hg were associated with the lowest rate of serious adverse events (SAEs) (6%) and hospitalization (38%).72 An MHR over 75% was associated with the highest SAE rate, although SAEs decreased as SBP increased (30%, 24%, and 21% with SBPs < 120 mm Hg, 120-140 mm Hg, and > 140 mm Hg, respectively).72
Risk Scores
In a prospective, observational cohort study of 1,100 ED patients, the Ottawa Heart Failure Risk Scale, combined with NTproBNP values, had a sensitivity of 95.8%—at the cost of increasing the admission rate (from 60.8% to 88%)—for serious adverse events (defined as death within 30 days), admission to a monitored unit, intubation, NIV, MI, or relapse resulting in hospital admission within 14 days.73
Observation Unit
Overall, 44% of in-patient HF admissions are for less than 3 days (Table 1),2 supporting the practice of managing selected patients in shorter clinical-care environments than in inpatient units. Further, ED patients presenting with moderate dyspnea require both a diagnosis and an evaluation of their therapeutic response to determine the need for hospitalization. However, evaluating therapeutic response requires more time than is available in the typical ED. Thus, an ED OU offers the following:
(1) The OU provides the EP with a longer evaluation time, and therefore a more accurate disposition may be effected;
(2) Costs are significantly lower in patients managed in an ED OU; and
(3) Patient satisfaction may be improved, as most patients prefer home management over hospitalization.
All three of these opportunities are supported by a number of studies,74-78 with validated entry and exclusion criteria, treatment algorithms and discharge metrics. Most recently, in a registry of hospitals in Spain registry, patients presenting to hospitals that had OUs had a 2.2-day shorter LOS, lower 30-day ED revisit rate, and similar mortality rates compared to those in institutions without OUs—although these beneficial effects occurred at the cost of an 8.9% higher admission rate.79
Patient Education
Intuitively, it would be expected that patient education would reduce return visits, 30-day hospitalizations, and AHF-related mortality. Unfortunately, it has not been demonstrated that patient education results in a consistent benefit at hospital discharge, or in the outpatient environment.80-85
Although AHF education in the ED has been poorly studied, areas that have shown promise are education occurring before ED management (ie, in the ED waiting area) in underinsured patients,86 and during ED care for patients with poor health care literacy.87 As educational interventions are both inexpensive and unlikely to result in harm, their implementation should be considered.
Conclusion
The spectrum of HF is a common presentation in the ED. Because HF generally appears as dyspnea, in a cohort with multiple comorbidities, the diagnosis can be challenging. This is complicated by the fact that patients with severe presentations may require life-saving interventions long before a clinical evaluation is completed (or even initiated). The skill of the EP, and his or her ability to improve the clinical condition before intubation is required, will determine the patient’s trajectory. Conversely, as a chronic condition, HF may present with moderate symptoms for which a short diuretic “tune-up” in an observation environment may be appropriate.
How these decisions are made will depend upon the local environment, the availability of outpatient resources, and individual patient choices. There are few chronic diseases that are more complex, are seen more often in the ED, or that require more skill and finesse in management.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603.
2. Fitch KV, Engel T, Lauet J. The cost burden of worsening heart failure in the Medicare fee for service population: an actuarial analysis. Milliman Web site. 2017. http://www.milliman.com/insight/2017/The-cost-burden-of-worsening-heart-failure-in-the-Medicare-fee-for-service-population-An-actuarial-analysis/ Published April 3, 2017. Accessed June 1, 2017.
3. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013. J Am Coll Cardiol. 2013;62(16):e147-e239. doi:10.1016/j.jacc.2013.05.019.
4. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669-1678. doi:10.1001/jama.2011.1474.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a.
6. Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4(1):68-75. doi:10.1161/CIRCOUTCOMES.110.957225.
7. HCUP National Inpatient Sample (NIS); 2014 Agency for Healthcare Research and Quality (AHRQ). HCUPnet Healthcare Cost and Utilization Project. http://bit.ly/2u2ymFA. Published 2014. Accessed July 27, 2017.
8. FY 2017 Final Rule and Correction Notice Tables. CMS 2014 data based on DRGs, Table 5:List of MS-DRGs, Relative Weighting Factors and Geometric and Arithmetic Mean Length of Stay. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2017-IPPS-Final-Rule-Home-Page-Items/FY2017-IPPS-Final-Rule-Tables.html. Published 2017. Accessed August 28, 2017.
9. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. http://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed August 2, 2017
10. Ellison, A. Average cost per inpatient day across 50 states. Becker’s Hospital CFO Report. http://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states-2016.html. Published January 13, 2016. Accessed August 2, 2017
11. Claret PG, Calder LA, Stiell IG, et al. Rates and predictive factors of return to the emergency department following an initial release by the emergency department for acute heart failure. [published online ahead of print April 3, 2017]. CJEM. 1-8. doi:10.1017/cem.2017.14.
12. Yam FK, Lew T, Eraly SA, Lin HW, Hirsch JD, Devor M. Changes in medication regimen complexity and the risk for 90-day hospital readmission and/or emergency department visits in U.S. Veterans with heart failure. Res Social Adm Pharm. 2016;12(5):713-721. doi:10.1016/j.sapharm.2015.10.004.
13. Readmissions Reduction Program. CMS. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html Updated April 18, 2016. Accessed July 13, 2017.
14. Nowak RM, Reed BP, DiSomma S, et al. Presenting phenotypes of acute heart failure patients in the ED: Identification and implications. Am J Emerg Med. 2017;35(4):536-542. doi:10.1016/j.ajem.2016.12.003.
15. Bennett SJ, Huster GA, Baker SL, et al. Characterization of the precipitants of hospitalization for heart failure decompensation. Am J Crit Care. 1998;7(3):168-174.
16. Kuo DC, Peacock WF. Diagnosing and managing acute heart failure in the emergency department. Clin Exp Emerg Med. 2015;2(3):141-149. eCollection 2015 Sep. doi:10.15441/ceem.15.007.
17. Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944-1956.
18. Koga T, Fujimoto K. Images in clinical medicine. Kerley’s A, B, and C line. N Engl J Med. 2009;360(15):1539. doi:10.1056/NEJMicm0708489.
19. Glöckner E, Christ M, Geier F, et al. Accuracy of point-of-care B-Line lung ultrasound in comparison to NT-ProBNP for screening acute heart failure. Ultrasound Int Open. 2016;2(3):e90-e92. doi:10.1055/s-0042-108343.
20. Bitar Z, Maadarani O, Almerri K. Sonographic chest B-lines anticipate elevated B-type natriuretic peptide level, irrespective of ejection fraction. Ann Intensive Care. 2015;5(1): 56. doi:10.1186/s13613-015-0100-x.
21. Miglioranza MH, Gargani L, Sant’Anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6(11):1141-1151. doi:10.1016/j.jcmg.2013.08.004.
22. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31(8):1208-1214. doi:10.1016/j.ajem.2013.05.007.
23. Cubo-Romano P, Torres-Macho J, Soni NJ, et al. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med. 2016;11(11):778-784. doi:10.1002/jhm.2620.
24. Martínez PG, Martínez DM, García JC, Loidi JC. Amino-terminal pro–B-type natriuretic peptide, inferior vena cava ultrasound, and bioelectrical impedance analysis for the diagnosis of acute decompensated CHF. Am J Emerg Med. 2016;34(9): 1817–1822. doi:10.1016/j.ajem.2016.06.043.

25. Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161-167.
26. Van Kimmenade RR, Pinto YM, Bayes-Genis A, Lainchbury JG, Richards AM, Januzzi JL Jr. Usefulness of intermediate amino-terminal pro-brain natriuretic peptide concentrations for diagnosis and prognosis of acute heart failure. Am J Cardiol. 2006;98(3):386-390.
27. Moe GW, Howlett J, Januzzi JL, Zowall H; Canadian Multicenter Improved Management of Patients With Congestive Heart Failure (IMPROVE-CHF) Study Investigators. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007;115(24):3103-3110.
28. Mayo DD, Colletti JE, Kuo DC. Brain natriuretic peptide (BNP) testing in the emergency department. J Emerg Med. 2006;31(2):201-210.
29. Mueller C, Scholer A, Laule-Kilian K, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647-654.
30. Krauser DG, Lloyd-Jones DM, Chae CU, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;149(4):744-750.
31. McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
32. Jacob J, Roset A, Miró Ò, et al; ICA-SEMES Research Group. EAHFE - TROPICA2 study. Prognostic value of troponin in patients with acute heart failure treated in Spanish hospital emergency departments. Biomarkers. 2017;22(3-4):337-344. doi:10.1080/1354750X.2016.1265006.
33. Fonarow GC, Adams KF Jr, Abraham WT, et al; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580.
34. Burkhardt J, Peacock WF, Emerman CL. Predictors of emergency department observation unit outcomes. Acad Emerg Med. 2005;12(9):869-874.
35. Schanz M, Shi J, Wasser C, Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol. 2017;40(7):485-491. doi:10.1002/clc.22683.
36. Kushner RF, Schoeller DA, Fjeld CR, Danford L: Is the impedance index (ht2/R) significant in predicting total body water? Am J Clin Nutr. 1992;56(5): 835-839.
37. Ackland GL, Singh-Ranger D, Fox S, et al. Assessment of preoperative fluid depletion using bioimpedance analysis. Br J Anaesth. 2004;92(1): 134-136.
38. Uszko-Lencer NH, Bothmer F, van Pol PE, Schols AM. Measuring body composition in chronic heart failure: a comparison of methods. Eur J Heart Fail. 2006;8(2): 208-214.
39. Santarelli S, Russo V, Lalle I, et al; GREAT network. Usefulness of combining admission brain natriuretic peptide (BNP) plus hospital discharge bioelectrical impedance vector analysis (BIVA) in predicting 90 days cardiovascular mortality in patients with acute heart failure. Intern Emerg Med. 2017;12(4):445-451. doi:10.1007/s11739-016-1581-9.
40. Parrinello G, Paterna S, Di Pasquale P, et al. The usefulness of bioelectrical impedance analysis in differentiating dyspnea due to decompensated heart failure. J Card Fail. 2008;14(8): 676-686. doi:10.1016/j.cardfail.2008.04.005.
41. Valle R, Aspromonte N, Carbonieri E, et al. Fall in readmission rate for heart failure after implementation of B-type natriuretic peptide testing for discharge decision: a retrospective study. Int J Cardiol. 2008;126(3): 400-406.
42. Sartini S, Frizzi J, Borselli M, et al. Which method is best for an early accurate diagnosis of acute heart failure? Comparison between lung ultrasound, chest X-ray and NT pro-BNP performance: a prospective study. [published online ahead of print July 11, 2016]. Intern Emerg Med. doi:10.1007/s11739-016-1498-3.
43. Steinhart BD, Levy P, Vandenberghe H, et al. A randomized control trial using a validated prediction model for diagnosing acute heart failure in undifferentiated dyspneic emergency department patients-results of the GASP4Ar study. J Card Fail. 2017;23(2):145-152. doi:10.1016/j.cardfail.2016.08.007.
44. Tallman TA, Peacock WF, Emerman CL, et al; ADHERE Registry. Noninvasive ventilation outcomes in 2,430 acute decompensated heart failure patients: an ADHERE registry analysis. Acad EM. 2008;15(4):355–362. doi:10.1111/j.1553-2712.2008.00059.x.
45. Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest. 1998;114(4):1185-1192.
46. Peter JV, Moran JL, Phillips-Hughes J, Graham P, Bersten AD. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367(9517):1155-1163.
47. Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600
48. Vital FM, Saconato H, Ladeira MT, et al. Noninvasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev. 2008;(3):CD005351. doi:10.1002/14651858.CD005351.pub2.
49. Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;(5):CD005351. doi:10.1002/14651858.CD005351.pub3.
50. Gray A, Goodacre S, Seah M, Tilley S. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema: analysis from the 3CPO trial. QJM. 2010;103(8):573-581. doi:10.1093/qjmed/hcq077.
51. Collins SP, Storrow AB, Levy PD, et al. Early management of patients with acute heart failure: state of the art and future directions—a consensus document from the SAEM/HFSA acute heart failure working group. Acad Emerg Med. 2015;22(1):94-112. doi:10.1111/acem.12538.]
52. O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1999;138(1 Pt 1):78-86.
53. Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;(9):180-187.
54. Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009;2(4):320-324. doi:10.1161/CIRCHEARTFAILURE.108.839076.
55. Collins SP, Levy PD, Martindale JL, et al. Clinical and research considerations for patients with hypertensive acute heart failure: A consensus statement from the Society for Academic Emergency Medicine and the Heart Failure Society of America Acute Heart Failure Working Group. Acad Emerg Med. 2016;23(8):922-931. doi:10.1111/acem.13025.
56. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011; 364(9):797-805. doi:10.1056/NEJMoa1005419.

57. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531-1540.
58. Gradman AH, Vekeman F, Eldar-Lissai A, Trahey A, Ong SH, Duh MS. Is addition of vasodilators to loop diuretics of value in the care of hospitalized acute heart failure patients? Real-world evidence from a retrospective analysis of a large United States hospital database. J Card Fail. 2014;20(11):853-863. doi:10.1016/j.cardfail.2014.08.006.
59. Wilson SS, Kwiatkowski GM, Millis SR, Purakal JD, Mahajan AP, Levy PD. Use of nitroglycerin by bolus prevents intensive care unit admission in patients with acute hypertensive heart failure. Am J Emerg Med. 2017;35(1):126-131. doi:10.1016/j.ajem.2016.10.038.
60. Eryonucu B, Guler N, Guntekin U, Tuncer M. Comparison of the effects of nitroglycerin and nitroprusside on transmitral Doppler flow parameters in patients with hypertensive urgency. Ann Pharmacother. 2005;39(6):997–1001.
61. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32-43. doi:10.1056/NEJMoa1100171.
62. Peacock WF 4th, Holland R, Gyarmathy R, et al. Observation unit treatment of heart failure with nesiritide: results from the proaction trial. J Emerg Med. 2005;29(3):243-252.
63. Ayaz SI, Sharkey CM, Kwiatkowski GM, et al. Intravenous enalaprilat for treatment of acute hypertensive heart failure in the emergency department. Int J Emerg Med. 2016;9(1):28. doi:10.1186/s12245-016-0125-4.
64. Peacock WF 4th, Chandra A, Char D, et al. Clevidipine in acute heart failure: results of the A study of BP control in acute heart failure-a pilot study (PRONTO). Am Heart J. 2014;167(4):529-536. doi:10.1016/j.ahj.2013.12.023.
65. Peacock WF 4th, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25(4):205-209. doi:10.1136/emj.2007.050419.
66. Miró Ò, Gil V, Martín-Sánchez FJ, Herrero-Puente P, Jet al; ICA-SEMES Research Group. Morphine use in the ED and outcomes of patients with acute heart failure: a propensity score-matching analysis based on the EAHFE registry. [published ahead of print April 12, 2017] Chest. pii:S0012-3692(17)30707-9. doi:10.1016/j.chest.2017.03.037.
67. Wuerz RC, Meador SA. Effects of prehospital medications on mortality and length of stay in congestive heart failure. Ann Emerg Med. 1992;21(6):669-674.
68. Peacock WF 4th, Fonarow GC, Emerman CL, Mills RM, Wynne J; ADHERE Scientific Advisory Committee and Investigators; Adhere Study Group. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology. 2007; 107(1):44-51. doi:10.1159/000093612.
69. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009;15(6): 256-264. doi:10.1111/j.1751-7133.2009.00112.x.
70. Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol. 2008;52(7):534-540. doi:10.1016/j.jacc.2008.05.010.
71. Matsue Y, Damman K, Voors AA, et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017 Jun 27;69(25):3042-3051. doi:10.1016/j.jacc.2017.04.042.
72. Claret PG, Stiell IG, Yan JW, et al. Hemodynamic, management, and outcomes of patients admitted to emergency department with heart failure. Scand J Trauma Resusc Emerg Med. 2016;24(1):132.
73. Stiell IG, Perry JJ, Clement CM, et al. Prospective and explicit clinical validation of the Ottawa Heart Failure Risk Scale, with and without use of quantitative NT-proBNP. Acad Emerg Med. 2017;24(3):316-327. doi:10.1111/acem.13141.
74. Pang PS, Jesse R, Collins SP, Maisel A. Patients with acute heart failure in the emergency department: do they all need to be admitted? J Card Fail. 2012;18:900-903. doi:10.1016/j.cardfail.2012.10.014.
75. Peacock WF 4th, Young J, Collins S, Emerman C, Diercks D. Heart failure observation units: optimizing care. Ann Emerg Med. 2006;47(1):22-33.
76. Storrow AB, Collins SP, Lyons MS, Wagoner LE, Gibler WB, Lindsell CJ. Emergency department observation of heart failure: preliminary analysis of safety and cost. Congest Heart Fail. 2005;11(2):68-72.
77. Peacock WF 4th, Remer EE, Aponte J, Moffa DA, Emerman CE, Albert NM. Effective observation unit treatment of decompensated heart failure. Congest Heart Fail. 2002;8(2):68 -73.
78. Peacock WF 4th, Albert NM. Observation unit management of heart failure. Emerg Med Clin North Am. 2001;19(1):209-232.
79. Miró O, Carbajosa V, Peacock WF 4th, et al; ICA-SEMES group. The effect of a short-stay unit on hospital admission and length of stay in acute heart failure: REDUCE-AHF study. Eur J Intern Med. 2017;40:30-36. doi:10.1016/j.ejim.2017.01.015.
80. Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur Heart J. 1998;19(8):1254-1260.
81. Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162(6):705-712.
82. Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Arch Intern Med. 2003;163(7):809-817.
83. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med. 1998;158(10):1067-1072.
84. Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354(9184):1077-1083.
85. Weinberger M, Oddone EZ, Henderson WG; Veterans Affairs Cooperative Study Group. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on primary care and hospital readmission. N Engl J Med. 1996;334(22):1441-1447.
86. Asthana V, Sundararajan M, Karun V, et al. Educational strategy for management of heart failure markedly reduces 90-day emergency department and hospital readmissions in un- and underinsured patients. J Am Coll Cardiol. 2017;69(11Suppl): 780. doi:10.1016/S0735-1097(17)34169-4.
87. Bell SP, Schnipper JL, Goggins K, et al; Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) Study Group. Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. J Gen Intern Med. 2016;31(5):470-477. doi:10.1007/s11606-016-3596-3.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146-e603.
2. Fitch KV, Engel T, Lauet J. The cost burden of worsening heart failure in the Medicare fee for service population: an actuarial analysis. Milliman Web site. 2017. http://www.milliman.com/insight/2017/The-cost-burden-of-worsening-heart-failure-in-the-Medicare-fee-for-service-population-An-actuarial-analysis/ Published April 3, 2017. Accessed June 1, 2017.
3. Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013. J Am Coll Cardiol. 2013;62(16):e147-e239. doi:10.1016/j.jacc.2013.05.019.
4. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669-1678. doi:10.1001/jama.2011.1474.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619. doi:10.1161/HHF.0b013e318291329a.
6. Dunlay SM, Shah ND, Shi Q, et al. Lifetime costs of medical care after heart failure diagnosis. Circ Cardiovasc Qual Outcomes. 2011;4(1):68-75. doi:10.1161/CIRCOUTCOMES.110.957225.
7. HCUP National Inpatient Sample (NIS); 2014 Agency for Healthcare Research and Quality (AHRQ). HCUPnet Healthcare Cost and Utilization Project. http://bit.ly/2u2ymFA. Published 2014. Accessed July 27, 2017.
8. FY 2017 Final Rule and Correction Notice Tables. CMS 2014 data based on DRGs, Table 5:List of MS-DRGs, Relative Weighting Factors and Geometric and Arithmetic Mean Length of Stay. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/FY2017-IPPS-Final-Rule-Home-Page-Items/FY2017-IPPS-Final-Rule-Tables.html. Published 2017. Accessed August 28, 2017.
9. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. http://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed August 2, 2017
10. Ellison, A. Average cost per inpatient day across 50 states. Becker’s Hospital CFO Report. http://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states-2016.html. Published January 13, 2016. Accessed August 2, 2017
11. Claret PG, Calder LA, Stiell IG, et al. Rates and predictive factors of return to the emergency department following an initial release by the emergency department for acute heart failure. [published online ahead of print April 3, 2017]. CJEM. 1-8. doi:10.1017/cem.2017.14.
12. Yam FK, Lew T, Eraly SA, Lin HW, Hirsch JD, Devor M. Changes in medication regimen complexity and the risk for 90-day hospital readmission and/or emergency department visits in U.S. Veterans with heart failure. Res Social Adm Pharm. 2016;12(5):713-721. doi:10.1016/j.sapharm.2015.10.004.
13. Readmissions Reduction Program. CMS. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html Updated April 18, 2016. Accessed July 13, 2017.
14. Nowak RM, Reed BP, DiSomma S, et al. Presenting phenotypes of acute heart failure patients in the ED: Identification and implications. Am J Emerg Med. 2017;35(4):536-542. doi:10.1016/j.ajem.2016.12.003.
15. Bennett SJ, Huster GA, Baker SL, et al. Characterization of the precipitants of hospitalization for heart failure decompensation. Am J Crit Care. 1998;7(3):168-174.
16. Kuo DC, Peacock WF. Diagnosing and managing acute heart failure in the emergency department. Clin Exp Emerg Med. 2015;2(3):141-149. eCollection 2015 Sep. doi:10.15441/ceem.15.007.
17. Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944-1956.
18. Koga T, Fujimoto K. Images in clinical medicine. Kerley’s A, B, and C line. N Engl J Med. 2009;360(15):1539. doi:10.1056/NEJMicm0708489.
19. Glöckner E, Christ M, Geier F, et al. Accuracy of point-of-care B-Line lung ultrasound in comparison to NT-ProBNP for screening acute heart failure. Ultrasound Int Open. 2016;2(3):e90-e92. doi:10.1055/s-0042-108343.
20. Bitar Z, Maadarani O, Almerri K. Sonographic chest B-lines anticipate elevated B-type natriuretic peptide level, irrespective of ejection fraction. Ann Intensive Care. 2015;5(1): 56. doi:10.1186/s13613-015-0100-x.
21. Miglioranza MH, Gargani L, Sant’Anna RT, et al. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging. 2013;6(11):1141-1151. doi:10.1016/j.jcmg.2013.08.004.
22. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing heart failure among acutely dyspneic patients with cardiac, inferior vena cava, and lung ultrasonography. Am J Emerg Med. 2013;31(8):1208-1214. doi:10.1016/j.ajem.2013.05.007.
23. Cubo-Romano P, Torres-Macho J, Soni NJ, et al. Admission inferior vena cava measurements are associated with mortality after hospitalization for acute decompensated heart failure. J Hosp Med. 2016;11(11):778-784. doi:10.1002/jhm.2620.
24. Martínez PG, Martínez DM, García JC, Loidi JC. Amino-terminal pro–B-type natriuretic peptide, inferior vena cava ultrasound, and bioelectrical impedance analysis for the diagnosis of acute decompensated CHF. Am J Emerg Med. 2016;34(9): 1817–1822. doi:10.1016/j.ajem.2016.06.043.

25. Maisel AS, Krishnaswamy P, Nowak RM, et al; Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161-167.
26. Van Kimmenade RR, Pinto YM, Bayes-Genis A, Lainchbury JG, Richards AM, Januzzi JL Jr. Usefulness of intermediate amino-terminal pro-brain natriuretic peptide concentrations for diagnosis and prognosis of acute heart failure. Am J Cardiol. 2006;98(3):386-390.
27. Moe GW, Howlett J, Januzzi JL, Zowall H; Canadian Multicenter Improved Management of Patients With Congestive Heart Failure (IMPROVE-CHF) Study Investigators. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation. 2007;115(24):3103-3110.
28. Mayo DD, Colletti JE, Kuo DC. Brain natriuretic peptide (BNP) testing in the emergency department. J Emerg Med. 2006;31(2):201-210.
29. Mueller C, Scholer A, Laule-Kilian K, et al. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647-654.
30. Krauser DG, Lloyd-Jones DM, Chae CU, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Am Heart J. 2005;149(4):744-750.
31. McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
32. Jacob J, Roset A, Miró Ò, et al; ICA-SEMES Research Group. EAHFE - TROPICA2 study. Prognostic value of troponin in patients with acute heart failure treated in Spanish hospital emergency departments. Biomarkers. 2017;22(3-4):337-344. doi:10.1080/1354750X.2016.1265006.
33. Fonarow GC, Adams KF Jr, Abraham WT, et al; ADHERE Scientific Advisory Committee, Study Group, and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293(5):572-580.
34. Burkhardt J, Peacock WF, Emerman CL. Predictors of emergency department observation unit outcomes. Acad Emerg Med. 2005;12(9):869-874.
35. Schanz M, Shi J, Wasser C, Alscher MD, Kimmel M. Urinary [TIMP-2] × [IGFBP7] for risk prediction of acute kidney injury in decompensated heart failure. Clin Cardiol. 2017;40(7):485-491. doi:10.1002/clc.22683.
36. Kushner RF, Schoeller DA, Fjeld CR, Danford L: Is the impedance index (ht2/R) significant in predicting total body water? Am J Clin Nutr. 1992;56(5): 835-839.
37. Ackland GL, Singh-Ranger D, Fox S, et al. Assessment of preoperative fluid depletion using bioimpedance analysis. Br J Anaesth. 2004;92(1): 134-136.
38. Uszko-Lencer NH, Bothmer F, van Pol PE, Schols AM. Measuring body composition in chronic heart failure: a comparison of methods. Eur J Heart Fail. 2006;8(2): 208-214.
39. Santarelli S, Russo V, Lalle I, et al; GREAT network. Usefulness of combining admission brain natriuretic peptide (BNP) plus hospital discharge bioelectrical impedance vector analysis (BIVA) in predicting 90 days cardiovascular mortality in patients with acute heart failure. Intern Emerg Med. 2017;12(4):445-451. doi:10.1007/s11739-016-1581-9.
40. Parrinello G, Paterna S, Di Pasquale P, et al. The usefulness of bioelectrical impedance analysis in differentiating dyspnea due to decompensated heart failure. J Card Fail. 2008;14(8): 676-686. doi:10.1016/j.cardfail.2008.04.005.
41. Valle R, Aspromonte N, Carbonieri E, et al. Fall in readmission rate for heart failure after implementation of B-type natriuretic peptide testing for discharge decision: a retrospective study. Int J Cardiol. 2008;126(3): 400-406.
42. Sartini S, Frizzi J, Borselli M, et al. Which method is best for an early accurate diagnosis of acute heart failure? Comparison between lung ultrasound, chest X-ray and NT pro-BNP performance: a prospective study. [published online ahead of print July 11, 2016]. Intern Emerg Med. doi:10.1007/s11739-016-1498-3.
43. Steinhart BD, Levy P, Vandenberghe H, et al. A randomized control trial using a validated prediction model for diagnosing acute heart failure in undifferentiated dyspneic emergency department patients-results of the GASP4Ar study. J Card Fail. 2017;23(2):145-152. doi:10.1016/j.cardfail.2016.08.007.
44. Tallman TA, Peacock WF, Emerman CL, et al; ADHERE Registry. Noninvasive ventilation outcomes in 2,430 acute decompensated heart failure patients: an ADHERE registry analysis. Acad EM. 2008;15(4):355–362. doi:10.1111/j.1553-2712.2008.00059.x.
45. Pang D, Keenan SP, Cook DJ, Sibbald WJ. The effect of positive pressure airway support on mortality and the need for intubation in cardiogenic pulmonary edema: a systematic review. Chest. 1998;114(4):1185-1192.
46. Peter JV, Moran JL, Phillips-Hughes J, Graham P, Bersten AD. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367(9517):1155-1163.
47. Weng CL, Zhao YT, Liu QH, et al. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152(9):590-600
48. Vital FM, Saconato H, Ladeira MT, et al. Noninvasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary edema. Cochrane Database Syst Rev. 2008;(3):CD005351. doi:10.1002/14651858.CD005351.pub2.
49. Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013;(5):CD005351. doi:10.1002/14651858.CD005351.pub3.
50. Gray A, Goodacre S, Seah M, Tilley S. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema: analysis from the 3CPO trial. QJM. 2010;103(8):573-581. doi:10.1093/qjmed/hcq077.
51. Collins SP, Storrow AB, Levy PD, et al. Early management of patients with acute heart failure: state of the art and future directions—a consensus document from the SAEM/HFSA acute heart failure working group. Acad Emerg Med. 2015;22(1):94-112. doi:10.1111/acem.12538.]
52. O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST). Am Heart J. 1999;138(1 Pt 1):78-86.
53. Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;(9):180-187.
54. Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage D heart failure. Circ Heart Fail. 2009;2(4):320-324. doi:10.1161/CIRCHEARTFAILURE.108.839076.
55. Collins SP, Levy PD, Martindale JL, et al. Clinical and research considerations for patients with hypertensive acute heart failure: A consensus statement from the Society for Academic Emergency Medicine and the Heart Failure Society of America Acute Heart Failure Working Group. Acad Emerg Med. 2016;23(8):922-931. doi:10.1111/acem.13025.
56. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011; 364(9):797-805. doi:10.1056/NEJMoa1005419.

57. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531-1540.
58. Gradman AH, Vekeman F, Eldar-Lissai A, Trahey A, Ong SH, Duh MS. Is addition of vasodilators to loop diuretics of value in the care of hospitalized acute heart failure patients? Real-world evidence from a retrospective analysis of a large United States hospital database. J Card Fail. 2014;20(11):853-863. doi:10.1016/j.cardfail.2014.08.006.
59. Wilson SS, Kwiatkowski GM, Millis SR, Purakal JD, Mahajan AP, Levy PD. Use of nitroglycerin by bolus prevents intensive care unit admission in patients with acute hypertensive heart failure. Am J Emerg Med. 2017;35(1):126-131. doi:10.1016/j.ajem.2016.10.038.
60. Eryonucu B, Guler N, Guntekin U, Tuncer M. Comparison of the effects of nitroglycerin and nitroprusside on transmitral Doppler flow parameters in patients with hypertensive urgency. Ann Pharmacother. 2005;39(6):997–1001.
61. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32-43. doi:10.1056/NEJMoa1100171.
62. Peacock WF 4th, Holland R, Gyarmathy R, et al. Observation unit treatment of heart failure with nesiritide: results from the proaction trial. J Emerg Med. 2005;29(3):243-252.
63. Ayaz SI, Sharkey CM, Kwiatkowski GM, et al. Intravenous enalaprilat for treatment of acute hypertensive heart failure in the emergency department. Int J Emerg Med. 2016;9(1):28. doi:10.1186/s12245-016-0125-4.
64. Peacock WF 4th, Chandra A, Char D, et al. Clevidipine in acute heart failure: results of the A study of BP control in acute heart failure-a pilot study (PRONTO). Am Heart J. 2014;167(4):529-536. doi:10.1016/j.ahj.2013.12.023.
65. Peacock WF 4th, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J. 2008;25(4):205-209. doi:10.1136/emj.2007.050419.
66. Miró Ò, Gil V, Martín-Sánchez FJ, Herrero-Puente P, Jet al; ICA-SEMES Research Group. Morphine use in the ED and outcomes of patients with acute heart failure: a propensity score-matching analysis based on the EAHFE registry. [published ahead of print April 12, 2017] Chest. pii:S0012-3692(17)30707-9. doi:10.1016/j.chest.2017.03.037.
67. Wuerz RC, Meador SA. Effects of prehospital medications on mortality and length of stay in congestive heart failure. Ann Emerg Med. 1992;21(6):669-674.
68. Peacock WF 4th, Fonarow GC, Emerman CL, Mills RM, Wynne J; ADHERE Scientific Advisory Committee and Investigators; Adhere Study Group. Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE. Cardiology. 2007; 107(1):44-51. doi:10.1159/000093612.
69. Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009;15(6): 256-264. doi:10.1111/j.1751-7133.2009.00112.x.
70. Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. J Am Coll Cardiol. 2008;52(7):534-540. doi:10.1016/j.jacc.2008.05.010.
71. Matsue Y, Damman K, Voors AA, et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017 Jun 27;69(25):3042-3051. doi:10.1016/j.jacc.2017.04.042.
72. Claret PG, Stiell IG, Yan JW, et al. Hemodynamic, management, and outcomes of patients admitted to emergency department with heart failure. Scand J Trauma Resusc Emerg Med. 2016;24(1):132.
73. Stiell IG, Perry JJ, Clement CM, et al. Prospective and explicit clinical validation of the Ottawa Heart Failure Risk Scale, with and without use of quantitative NT-proBNP. Acad Emerg Med. 2017;24(3):316-327. doi:10.1111/acem.13141.
74. Pang PS, Jesse R, Collins SP, Maisel A. Patients with acute heart failure in the emergency department: do they all need to be admitted? J Card Fail. 2012;18:900-903. doi:10.1016/j.cardfail.2012.10.014.
75. Peacock WF 4th, Young J, Collins S, Emerman C, Diercks D. Heart failure observation units: optimizing care. Ann Emerg Med. 2006;47(1):22-33.
76. Storrow AB, Collins SP, Lyons MS, Wagoner LE, Gibler WB, Lindsell CJ. Emergency department observation of heart failure: preliminary analysis of safety and cost. Congest Heart Fail. 2005;11(2):68-72.
77. Peacock WF 4th, Remer EE, Aponte J, Moffa DA, Emerman CE, Albert NM. Effective observation unit treatment of decompensated heart failure. Congest Heart Fail. 2002;8(2):68 -73.
78. Peacock WF 4th, Albert NM. Observation unit management of heart failure. Emerg Med Clin North Am. 2001;19(1):209-232.
79. Miró O, Carbajosa V, Peacock WF 4th, et al; ICA-SEMES group. The effect of a short-stay unit on hospital admission and length of stay in acute heart failure: REDUCE-AHF study. Eur J Intern Med. 2017;40:30-36. doi:10.1016/j.ejim.2017.01.015.
80. Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur Heart J. 1998;19(8):1254-1260.
81. Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162(6):705-712.
82. Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Arch Intern Med. 2003;163(7):809-817.
83. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med. 1998;158(10):1067-1072.
84. Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354(9184):1077-1083.
85. Weinberger M, Oddone EZ, Henderson WG; Veterans Affairs Cooperative Study Group. Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on primary care and hospital readmission. N Engl J Med. 1996;334(22):1441-1447.
86. Asthana V, Sundararajan M, Karun V, et al. Educational strategy for management of heart failure markedly reduces 90-day emergency department and hospital readmissions in un- and underinsured patients. J Am Coll Cardiol. 2017;69(11Suppl): 780. doi:10.1016/S0735-1097(17)34169-4.
87. Bell SP, Schnipper JL, Goggins K, et al; Pharmacist Intervention for Low Literacy in Cardiovascular Disease (PILL-CVD) Study Group. Effect of pharmacist counseling intervention on health care utilization following hospital discharge: a randomized control trial. J Gen Intern Med. 2016;31(5):470-477. doi:10.1007/s11606-016-3596-3.