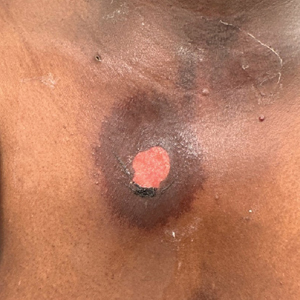
User login
Financial Hardship Common With Rheumatologic Disease: How Can Doctors Help?
Many patients struggle with healthcare costs and basic expenses, according to new research.
People with rheumatologic diseases often experience a hidden symptom: financial toxicity or significant economic strain from out-of-pocket costs. A new study of 41,502 patients published in JCR: Journal of Clinical Rheumatology showed that 20% of those with rheumatologic diseases faced financial hardship from medical expenses, with 55% of those unable to pay their bills.
Compared with patients who do not have rheumatologic diseases, and after clinical and sociodemographic factors were controlled for, patients with rheumatologic diseases were:
- 29% more likely to have high levels of financial hardship — difficulty paying; needing to pay over time; or inability to pay bills for doctors, dentists, hospitals, therapists, medication, equipment, nursing homes, or home care.
- 53% more likely to have high levels of financial distress — significant worry about having enough money for retirement, paying medical costs in the event of a serious illness or accident, maintaining their standard of living, paying their usual healthcare costs, and affording their normal monthly bills and housing costs.
- 29% more likely to experience food insecurity, defined as limited or uncertain access to adequate food.
- 58% more likely to report cost-related medication nonadherence — skipping doses, taking less medication, or delaying filling a prescription to save money.
People who were younger than 64 years, male, Black, or uninsured had higher odds of experiencing financial hardship, financial distress, food insecurity, and cost-related medication nonadherence.
This study highlights “just how costly everyday rheumatologic conditions can be for your average American,” said lead study author Troy Amen, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York City. These diseases can be disabling, limiting a patient’s ability to work at the very time when expensive medications are needed.
“It’s critical for clinicians to recognize how common the financial burden from healthcare costs can be, and only then can they take steps to better support patients,” said G. Caleb Alexander, MD, MS, professor of epidemiology at Johns Hopkins University in Baltimore, who was not involved in the study.
Here’s how healthcare providers can help.
Consider skipped medication a red flag. It’s often the first sign of a financial concern. “Sometimes with these problems, it looks like simple medication noncompliance, but it’s really a more complex form of nonadherence,” said Susan M. Goodman, MD, professor of clinical medicine at Weill Cornell Medicine in New York City and a coauthor of the study. “And I think if someone’s not taking the medication that had been very helpful, it does behoove the physician to try and figure out why that is.”
Normalize the issue to help patients open up. “I will often say, ‘You know, many, many patients don’t take their medicines exactly as prescribed. About how many days a week do you take this medicine?’” said Dr. Alexander. “If you ask in a nonconfrontational, supportive manner, I’ve found that patients are quite candid.”
Don’t assume insurance has it covered. If patients are uninsured, help them enroll in (or renew) insurance coverage. But don’t assume insurance will solve the whole problem. “There are many people who, although they do have coverage, still can’t afford their medications,” said Dr. Goodman.
For products on high formulary tiers, the patient’s monthly cost can be hundreds to thousands of dollars. “Over the past 10-20 years, we’ve seen remarkable technological innovation in the types of medicines being brought to market, and here, I’m referring primarily to biologics and medicines made from living cells,” said Dr. Alexander, “but many of these have a price tag that is simply astronomical, and insurers aren’t going to bear the brunt of these costs alone.”
Biosimilars can be a bit more affordable, but “the dirty little secret of biosimilars is that they’re not really very much less expensive,” said Dr. Goodman. “If your patient is doing well on a drug that gets dropped from their insurance plan’s formulary, or if they switch to a plan that doesn’t cover it, try calling and advocating for an exception. It’s an uphill battle, but it sometimes works,” she said.
If not? Help your patients apply for a patient assistance program. Many drug manufacturers offer copay assistance through their websites, and nonprofit patient assistance organizations such as the PAN Foundation, the Patient Advocate Foundation’s Co-Pay Relief Program, or The Assistance Fund can also help fill the gaps. One study published in the Journal of Managed Care and Specialty Pharmacy showed that in patients with rheumatoid arthritis, copay assistance was associated with 79% lower odds of prescription abandonment (failure to fill within 30 days of health plan approval).
Beware of “shiny penny syndrome.” It’s easy to get excited about new, innovative medications, especially when sales reps provide plenty of free samples. “There is a tendency to treat every new medicine as if it’s a bright shiny object in the streambed, and you know that’s not always the case,” said Dr. Alexander. “So, I think we have to be careful, especially in settings when we’re talking about ultra–high-cost medicines, that we’re aware of the burden these medicines may place on patients and that we’re navigating that with patients together, and not simply leaving that as a conversation that never happens in the exam room.”
Maybe there’s an older, time-tested drug that works just as well as the newer, more expensive one. Perhaps there is a slightly less effective medicine that costs a lot less. “These are cost–quality trade-offs that clinicians and patients should be navigating together,” said Dr. Alexander. For example, in a patient with rheumatoid arthritis, a tumor necrosis factor alpha inhibitor might work similarly to or almost as well as an interleukin inhibitor, the newer and typically more expensive choice.
“Some clinicians may find it quite unpalatable to be potentially compromising on safety or efficacy in the interest of reducing the cost of therapies, but as former Surgeon General C. Everett Koop said, ‘Drugs don’t work in patients who don’t take them,’ ” said Dr. Alexander. “So, if the choice is for someone not to be taking a treatment, or to be taking one that may be a little bit less good, I’ll take the latter.”
Consider the patient’s broader care team. Encourage patients to discuss costs with their other healthcare providers. For patients taking multiple medications, a few adjustments could make a big impact on their wallets. Primary care providers or other specialists might recommend some older and less expensive, but still effective, drugs, such as thiazides for hypertension or metformin for type 2 diabetes. Another option might be to simplify the patient’s regimen or include some fixed-dose combination pills in place of two others.
And if no one has referred the patient to a medical social worker, make the connection. A social worker can put patients in touch with local agencies that can help them with food, housing, and other nonmedical costs.
Talk about this problem with anyone who will listen. One of the best ways to help patients with rheumatologic diseases is to ensure that decision-makers don’t overlook them. Professional societies such as the American College of Rheumatology can be great resources for advocacy in Washington, DC. Political movements can make drugs more affordable — for example, insulin prices have dropped in recent years because of political pressure, said Dr. Goodman.
“A lot of our national policy now focuses on aiding patients with single high-cost events, but we hope studies like these can really get policymakers to think through how to better support patients with chronic conditions that may have been historically ignored, such as patients with rheumatologic disease,” said Dr. Amen.
The first step is raising awareness and telling your story. “As providers, we are often [at the] forefront in witnessing how chronic conditions and their associated costs can negatively affect patients’ lives and even alter clinical outcomes,” Dr. Amen added. “By publishing data and sharing meaningful patient stories and clinical vignettes, we can begin to advocate and humanize these patients to policymakers.”
Information on study funding was not available. All authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Many patients struggle with healthcare costs and basic expenses, according to new research.
People with rheumatologic diseases often experience a hidden symptom: financial toxicity or significant economic strain from out-of-pocket costs. A new study of 41,502 patients published in JCR: Journal of Clinical Rheumatology showed that 20% of those with rheumatologic diseases faced financial hardship from medical expenses, with 55% of those unable to pay their bills.
Compared with patients who do not have rheumatologic diseases, and after clinical and sociodemographic factors were controlled for, patients with rheumatologic diseases were:
- 29% more likely to have high levels of financial hardship — difficulty paying; needing to pay over time; or inability to pay bills for doctors, dentists, hospitals, therapists, medication, equipment, nursing homes, or home care.
- 53% more likely to have high levels of financial distress — significant worry about having enough money for retirement, paying medical costs in the event of a serious illness or accident, maintaining their standard of living, paying their usual healthcare costs, and affording their normal monthly bills and housing costs.
- 29% more likely to experience food insecurity, defined as limited or uncertain access to adequate food.
- 58% more likely to report cost-related medication nonadherence — skipping doses, taking less medication, or delaying filling a prescription to save money.
People who were younger than 64 years, male, Black, or uninsured had higher odds of experiencing financial hardship, financial distress, food insecurity, and cost-related medication nonadherence.
This study highlights “just how costly everyday rheumatologic conditions can be for your average American,” said lead study author Troy Amen, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York City. These diseases can be disabling, limiting a patient’s ability to work at the very time when expensive medications are needed.
“It’s critical for clinicians to recognize how common the financial burden from healthcare costs can be, and only then can they take steps to better support patients,” said G. Caleb Alexander, MD, MS, professor of epidemiology at Johns Hopkins University in Baltimore, who was not involved in the study.
Here’s how healthcare providers can help.
Consider skipped medication a red flag. It’s often the first sign of a financial concern. “Sometimes with these problems, it looks like simple medication noncompliance, but it’s really a more complex form of nonadherence,” said Susan M. Goodman, MD, professor of clinical medicine at Weill Cornell Medicine in New York City and a coauthor of the study. “And I think if someone’s not taking the medication that had been very helpful, it does behoove the physician to try and figure out why that is.”
Normalize the issue to help patients open up. “I will often say, ‘You know, many, many patients don’t take their medicines exactly as prescribed. About how many days a week do you take this medicine?’” said Dr. Alexander. “If you ask in a nonconfrontational, supportive manner, I’ve found that patients are quite candid.”
Don’t assume insurance has it covered. If patients are uninsured, help them enroll in (or renew) insurance coverage. But don’t assume insurance will solve the whole problem. “There are many people who, although they do have coverage, still can’t afford their medications,” said Dr. Goodman.
For products on high formulary tiers, the patient’s monthly cost can be hundreds to thousands of dollars. “Over the past 10-20 years, we’ve seen remarkable technological innovation in the types of medicines being brought to market, and here, I’m referring primarily to biologics and medicines made from living cells,” said Dr. Alexander, “but many of these have a price tag that is simply astronomical, and insurers aren’t going to bear the brunt of these costs alone.”
Biosimilars can be a bit more affordable, but “the dirty little secret of biosimilars is that they’re not really very much less expensive,” said Dr. Goodman. “If your patient is doing well on a drug that gets dropped from their insurance plan’s formulary, or if they switch to a plan that doesn’t cover it, try calling and advocating for an exception. It’s an uphill battle, but it sometimes works,” she said.
If not? Help your patients apply for a patient assistance program. Many drug manufacturers offer copay assistance through their websites, and nonprofit patient assistance organizations such as the PAN Foundation, the Patient Advocate Foundation’s Co-Pay Relief Program, or The Assistance Fund can also help fill the gaps. One study published in the Journal of Managed Care and Specialty Pharmacy showed that in patients with rheumatoid arthritis, copay assistance was associated with 79% lower odds of prescription abandonment (failure to fill within 30 days of health plan approval).
Beware of “shiny penny syndrome.” It’s easy to get excited about new, innovative medications, especially when sales reps provide plenty of free samples. “There is a tendency to treat every new medicine as if it’s a bright shiny object in the streambed, and you know that’s not always the case,” said Dr. Alexander. “So, I think we have to be careful, especially in settings when we’re talking about ultra–high-cost medicines, that we’re aware of the burden these medicines may place on patients and that we’re navigating that with patients together, and not simply leaving that as a conversation that never happens in the exam room.”
Maybe there’s an older, time-tested drug that works just as well as the newer, more expensive one. Perhaps there is a slightly less effective medicine that costs a lot less. “These are cost–quality trade-offs that clinicians and patients should be navigating together,” said Dr. Alexander. For example, in a patient with rheumatoid arthritis, a tumor necrosis factor alpha inhibitor might work similarly to or almost as well as an interleukin inhibitor, the newer and typically more expensive choice.
“Some clinicians may find it quite unpalatable to be potentially compromising on safety or efficacy in the interest of reducing the cost of therapies, but as former Surgeon General C. Everett Koop said, ‘Drugs don’t work in patients who don’t take them,’ ” said Dr. Alexander. “So, if the choice is for someone not to be taking a treatment, or to be taking one that may be a little bit less good, I’ll take the latter.”
Consider the patient’s broader care team. Encourage patients to discuss costs with their other healthcare providers. For patients taking multiple medications, a few adjustments could make a big impact on their wallets. Primary care providers or other specialists might recommend some older and less expensive, but still effective, drugs, such as thiazides for hypertension or metformin for type 2 diabetes. Another option might be to simplify the patient’s regimen or include some fixed-dose combination pills in place of two others.
And if no one has referred the patient to a medical social worker, make the connection. A social worker can put patients in touch with local agencies that can help them with food, housing, and other nonmedical costs.
Talk about this problem with anyone who will listen. One of the best ways to help patients with rheumatologic diseases is to ensure that decision-makers don’t overlook them. Professional societies such as the American College of Rheumatology can be great resources for advocacy in Washington, DC. Political movements can make drugs more affordable — for example, insulin prices have dropped in recent years because of political pressure, said Dr. Goodman.
“A lot of our national policy now focuses on aiding patients with single high-cost events, but we hope studies like these can really get policymakers to think through how to better support patients with chronic conditions that may have been historically ignored, such as patients with rheumatologic disease,” said Dr. Amen.
The first step is raising awareness and telling your story. “As providers, we are often [at the] forefront in witnessing how chronic conditions and their associated costs can negatively affect patients’ lives and even alter clinical outcomes,” Dr. Amen added. “By publishing data and sharing meaningful patient stories and clinical vignettes, we can begin to advocate and humanize these patients to policymakers.”
Information on study funding was not available. All authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Many patients struggle with healthcare costs and basic expenses, according to new research.
People with rheumatologic diseases often experience a hidden symptom: financial toxicity or significant economic strain from out-of-pocket costs. A new study of 41,502 patients published in JCR: Journal of Clinical Rheumatology showed that 20% of those with rheumatologic diseases faced financial hardship from medical expenses, with 55% of those unable to pay their bills.
Compared with patients who do not have rheumatologic diseases, and after clinical and sociodemographic factors were controlled for, patients with rheumatologic diseases were:
- 29% more likely to have high levels of financial hardship — difficulty paying; needing to pay over time; or inability to pay bills for doctors, dentists, hospitals, therapists, medication, equipment, nursing homes, or home care.
- 53% more likely to have high levels of financial distress — significant worry about having enough money for retirement, paying medical costs in the event of a serious illness or accident, maintaining their standard of living, paying their usual healthcare costs, and affording their normal monthly bills and housing costs.
- 29% more likely to experience food insecurity, defined as limited or uncertain access to adequate food.
- 58% more likely to report cost-related medication nonadherence — skipping doses, taking less medication, or delaying filling a prescription to save money.
People who were younger than 64 years, male, Black, or uninsured had higher odds of experiencing financial hardship, financial distress, food insecurity, and cost-related medication nonadherence.
This study highlights “just how costly everyday rheumatologic conditions can be for your average American,” said lead study author Troy Amen, MD, MBA, an orthopedic surgery resident at the Hospital for Special Surgery in New York City. These diseases can be disabling, limiting a patient’s ability to work at the very time when expensive medications are needed.
“It’s critical for clinicians to recognize how common the financial burden from healthcare costs can be, and only then can they take steps to better support patients,” said G. Caleb Alexander, MD, MS, professor of epidemiology at Johns Hopkins University in Baltimore, who was not involved in the study.
Here’s how healthcare providers can help.
Consider skipped medication a red flag. It’s often the first sign of a financial concern. “Sometimes with these problems, it looks like simple medication noncompliance, but it’s really a more complex form of nonadherence,” said Susan M. Goodman, MD, professor of clinical medicine at Weill Cornell Medicine in New York City and a coauthor of the study. “And I think if someone’s not taking the medication that had been very helpful, it does behoove the physician to try and figure out why that is.”
Normalize the issue to help patients open up. “I will often say, ‘You know, many, many patients don’t take their medicines exactly as prescribed. About how many days a week do you take this medicine?’” said Dr. Alexander. “If you ask in a nonconfrontational, supportive manner, I’ve found that patients are quite candid.”
Don’t assume insurance has it covered. If patients are uninsured, help them enroll in (or renew) insurance coverage. But don’t assume insurance will solve the whole problem. “There are many people who, although they do have coverage, still can’t afford their medications,” said Dr. Goodman.
For products on high formulary tiers, the patient’s monthly cost can be hundreds to thousands of dollars. “Over the past 10-20 years, we’ve seen remarkable technological innovation in the types of medicines being brought to market, and here, I’m referring primarily to biologics and medicines made from living cells,” said Dr. Alexander, “but many of these have a price tag that is simply astronomical, and insurers aren’t going to bear the brunt of these costs alone.”
Biosimilars can be a bit more affordable, but “the dirty little secret of biosimilars is that they’re not really very much less expensive,” said Dr. Goodman. “If your patient is doing well on a drug that gets dropped from their insurance plan’s formulary, or if they switch to a plan that doesn’t cover it, try calling and advocating for an exception. It’s an uphill battle, but it sometimes works,” she said.
If not? Help your patients apply for a patient assistance program. Many drug manufacturers offer copay assistance through their websites, and nonprofit patient assistance organizations such as the PAN Foundation, the Patient Advocate Foundation’s Co-Pay Relief Program, or The Assistance Fund can also help fill the gaps. One study published in the Journal of Managed Care and Specialty Pharmacy showed that in patients with rheumatoid arthritis, copay assistance was associated with 79% lower odds of prescription abandonment (failure to fill within 30 days of health plan approval).
Beware of “shiny penny syndrome.” It’s easy to get excited about new, innovative medications, especially when sales reps provide plenty of free samples. “There is a tendency to treat every new medicine as if it’s a bright shiny object in the streambed, and you know that’s not always the case,” said Dr. Alexander. “So, I think we have to be careful, especially in settings when we’re talking about ultra–high-cost medicines, that we’re aware of the burden these medicines may place on patients and that we’re navigating that with patients together, and not simply leaving that as a conversation that never happens in the exam room.”
Maybe there’s an older, time-tested drug that works just as well as the newer, more expensive one. Perhaps there is a slightly less effective medicine that costs a lot less. “These are cost–quality trade-offs that clinicians and patients should be navigating together,” said Dr. Alexander. For example, in a patient with rheumatoid arthritis, a tumor necrosis factor alpha inhibitor might work similarly to or almost as well as an interleukin inhibitor, the newer and typically more expensive choice.
“Some clinicians may find it quite unpalatable to be potentially compromising on safety or efficacy in the interest of reducing the cost of therapies, but as former Surgeon General C. Everett Koop said, ‘Drugs don’t work in patients who don’t take them,’ ” said Dr. Alexander. “So, if the choice is for someone not to be taking a treatment, or to be taking one that may be a little bit less good, I’ll take the latter.”
Consider the patient’s broader care team. Encourage patients to discuss costs with their other healthcare providers. For patients taking multiple medications, a few adjustments could make a big impact on their wallets. Primary care providers or other specialists might recommend some older and less expensive, but still effective, drugs, such as thiazides for hypertension or metformin for type 2 diabetes. Another option might be to simplify the patient’s regimen or include some fixed-dose combination pills in place of two others.
And if no one has referred the patient to a medical social worker, make the connection. A social worker can put patients in touch with local agencies that can help them with food, housing, and other nonmedical costs.
Talk about this problem with anyone who will listen. One of the best ways to help patients with rheumatologic diseases is to ensure that decision-makers don’t overlook them. Professional societies such as the American College of Rheumatology can be great resources for advocacy in Washington, DC. Political movements can make drugs more affordable — for example, insulin prices have dropped in recent years because of political pressure, said Dr. Goodman.
“A lot of our national policy now focuses on aiding patients with single high-cost events, but we hope studies like these can really get policymakers to think through how to better support patients with chronic conditions that may have been historically ignored, such as patients with rheumatologic disease,” said Dr. Amen.
The first step is raising awareness and telling your story. “As providers, we are often [at the] forefront in witnessing how chronic conditions and their associated costs can negatively affect patients’ lives and even alter clinical outcomes,” Dr. Amen added. “By publishing data and sharing meaningful patient stories and clinical vignettes, we can begin to advocate and humanize these patients to policymakers.”
Information on study funding was not available. All authors reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Recurrent Monoarthritis: Does Microwave Ablation Help?
TOPLINE:
Microwave ablation reduces synovial hypertrophy, the number of monoarthritis attacks, and the need for intra-articular aspiration (IAA) in patients with recurrent monoarthritis, significantly improving functional disability and pain scores.
METHODOLOGY:
- Researchers conducted a prospective study to assess the long-term effects of microwave ablation as adjunct therapy in patients with various rheumatic diseases and recurrent monoarthritis resistant to medical treatment.
- Overall, 24 knee joints of 22 patients (10 women; 12 men; median age, 37 years) with recurrent monoarthritis were included.
- Microwave ablation (15 or 20 W) was performed until microbubbles indicating coagulation necrosis were observed.
- Patients underwent clinical and radiologic follow-up at 2 weeks and at 1, 3, 6, and 12 months post procedure.
- Clinical follow-up included monitoring for remission or recurrence of monoarthritis and any complications.
TAKEAWAY:
- Microwave ablation significantly reduced the IAA count in the last 6 months before ablation from 129 (144 months total) to 7 in a total of 226 months post ablation (P < .001).
- Functional disability and pain scores improved significantly after microwave ablation, with median scores dropping from 9 to 1 (both P < .0001).
- No complications were observed during the procedure or follow-up, indicating the safety of microwave ablation.
- MRI findings showed a significant regression in synovial hypertrophy at 6 months post microwave ablation.
IN PRACTICE:
“Reducing the volume of intractable synovial hypertrophy by shrinking and inactivating with heat-based degradation by MWA [microwave ablation] makes it possible to avoid more systemic treatments or invasive approach and their possible side effects,” the authors wrote.
SOURCE:
The study was led by Rabia Deniz, MD, Department of Rheumatology, University of Health Sciences Başakşehir Çam and Sakura City Hospital in Istanbul, Turkey. It was published online in Rheumatology.
LIMITATIONS:
The relatively small sample size and enrollment of patients with heterogeneous severity and diagnosis of rheumatic diseases may limit the generalizability of the findings. Patient noncompliance with medical treatment and additional mechanical trauma to the targeted joints may have resulted in follow-up problems. The semiobjective evaluation of the ultrasonography and MRI findings with regard to the synovial hypertrophy volume was another limitation.
DISCLOSURES:
The study did not receive any specific funding. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Microwave ablation reduces synovial hypertrophy, the number of monoarthritis attacks, and the need for intra-articular aspiration (IAA) in patients with recurrent monoarthritis, significantly improving functional disability and pain scores.
METHODOLOGY:
- Researchers conducted a prospective study to assess the long-term effects of microwave ablation as adjunct therapy in patients with various rheumatic diseases and recurrent monoarthritis resistant to medical treatment.
- Overall, 24 knee joints of 22 patients (10 women; 12 men; median age, 37 years) with recurrent monoarthritis were included.
- Microwave ablation (15 or 20 W) was performed until microbubbles indicating coagulation necrosis were observed.
- Patients underwent clinical and radiologic follow-up at 2 weeks and at 1, 3, 6, and 12 months post procedure.
- Clinical follow-up included monitoring for remission or recurrence of monoarthritis and any complications.
TAKEAWAY:
- Microwave ablation significantly reduced the IAA count in the last 6 months before ablation from 129 (144 months total) to 7 in a total of 226 months post ablation (P < .001).
- Functional disability and pain scores improved significantly after microwave ablation, with median scores dropping from 9 to 1 (both P < .0001).
- No complications were observed during the procedure or follow-up, indicating the safety of microwave ablation.
- MRI findings showed a significant regression in synovial hypertrophy at 6 months post microwave ablation.
IN PRACTICE:
“Reducing the volume of intractable synovial hypertrophy by shrinking and inactivating with heat-based degradation by MWA [microwave ablation] makes it possible to avoid more systemic treatments or invasive approach and their possible side effects,” the authors wrote.
SOURCE:
The study was led by Rabia Deniz, MD, Department of Rheumatology, University of Health Sciences Başakşehir Çam and Sakura City Hospital in Istanbul, Turkey. It was published online in Rheumatology.
LIMITATIONS:
The relatively small sample size and enrollment of patients with heterogeneous severity and diagnosis of rheumatic diseases may limit the generalizability of the findings. Patient noncompliance with medical treatment and additional mechanical trauma to the targeted joints may have resulted in follow-up problems. The semiobjective evaluation of the ultrasonography and MRI findings with regard to the synovial hypertrophy volume was another limitation.
DISCLOSURES:
The study did not receive any specific funding. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Microwave ablation reduces synovial hypertrophy, the number of monoarthritis attacks, and the need for intra-articular aspiration (IAA) in patients with recurrent monoarthritis, significantly improving functional disability and pain scores.
METHODOLOGY:
- Researchers conducted a prospective study to assess the long-term effects of microwave ablation as adjunct therapy in patients with various rheumatic diseases and recurrent monoarthritis resistant to medical treatment.
- Overall, 24 knee joints of 22 patients (10 women; 12 men; median age, 37 years) with recurrent monoarthritis were included.
- Microwave ablation (15 or 20 W) was performed until microbubbles indicating coagulation necrosis were observed.
- Patients underwent clinical and radiologic follow-up at 2 weeks and at 1, 3, 6, and 12 months post procedure.
- Clinical follow-up included monitoring for remission or recurrence of monoarthritis and any complications.
TAKEAWAY:
- Microwave ablation significantly reduced the IAA count in the last 6 months before ablation from 129 (144 months total) to 7 in a total of 226 months post ablation (P < .001).
- Functional disability and pain scores improved significantly after microwave ablation, with median scores dropping from 9 to 1 (both P < .0001).
- No complications were observed during the procedure or follow-up, indicating the safety of microwave ablation.
- MRI findings showed a significant regression in synovial hypertrophy at 6 months post microwave ablation.
IN PRACTICE:
“Reducing the volume of intractable synovial hypertrophy by shrinking and inactivating with heat-based degradation by MWA [microwave ablation] makes it possible to avoid more systemic treatments or invasive approach and their possible side effects,” the authors wrote.
SOURCE:
The study was led by Rabia Deniz, MD, Department of Rheumatology, University of Health Sciences Başakşehir Çam and Sakura City Hospital in Istanbul, Turkey. It was published online in Rheumatology.
LIMITATIONS:
The relatively small sample size and enrollment of patients with heterogeneous severity and diagnosis of rheumatic diseases may limit the generalizability of the findings. Patient noncompliance with medical treatment and additional mechanical trauma to the targeted joints may have resulted in follow-up problems. The semiobjective evaluation of the ultrasonography and MRI findings with regard to the synovial hypertrophy volume was another limitation.
DISCLOSURES:
The study did not receive any specific funding. The authors declared no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Early RA Diagnosis and Treatment Lowers Treatment Costs
TOPLINE:
Early detection and treatment of rheumatoid arthritis (RA; within 12 weeks of symptom onset) results in lower treatment-related costs over 5 years compared with later diagnosis.
METHODOLOGY:
- The study enrolled 431 patients in the Leiden Early Arthritis Clinic at Leiden University Medical Center, Leiden, the Netherlands.
- Symptom duration was defined as time between symptom onset and first clinic visit.
- Early treatment was defined as a symptom duration of under 12 weeks, and later treatment defined as symptom duration over 12 weeks.
- Prescription data from patient records and 2022 disease-modifying antirheumatic drug prices (including biologics) was used to determine overall costs over 5 years.
- Autoantibody-negative and autoantibody-positive RA were studied separately because of possible differences in disease severity.
TAKEAWAY:
- For the 165 patients with autoantibody-negative RA, late treatment was associated with 316% higher costs over 5 years than early treatment (€4856/$5292 vs €1159/$1263)
- For antibody-positive RA, costs were 19% higher in the late-treatment group.
- In the 43 patients with antibody-positive RA only prescribed biologics, costs were 46% higher for those with delayed treatment.
IN PRACTICE:
“This is the first study showing the effect of early diagnosis and treatment on treatment-related costs,” wrote the authors. “When RA is detected within 12 weeks after symptom onset, treatment-related costs seem to be lower.”
SOURCE:
The study was led by Elise van Mulligen, PhD, Department of Rheumatology, Leiden University Medical Center. It was published online in Annals of the Rheumatic Diseases.
LIMITATIONS:
The division of symptom duration by 12 weeks was “arbitrary.” Baseline characteristics, though similar, showed differences for inflammatory markers in autoantibody-positive and autoantibody-negative RA. Thirty seven patients were lost to follow-up, which could induce attrition bias, though the percentage of these patients in the early- and late-treatment groups was similar.
DISCLOSURES:
This study was funded by ZonMw, a Dutch organization for healthcare research. The authors declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Early detection and treatment of rheumatoid arthritis (RA; within 12 weeks of symptom onset) results in lower treatment-related costs over 5 years compared with later diagnosis.
METHODOLOGY:
- The study enrolled 431 patients in the Leiden Early Arthritis Clinic at Leiden University Medical Center, Leiden, the Netherlands.
- Symptom duration was defined as time between symptom onset and first clinic visit.
- Early treatment was defined as a symptom duration of under 12 weeks, and later treatment defined as symptom duration over 12 weeks.
- Prescription data from patient records and 2022 disease-modifying antirheumatic drug prices (including biologics) was used to determine overall costs over 5 years.
- Autoantibody-negative and autoantibody-positive RA were studied separately because of possible differences in disease severity.
TAKEAWAY:
- For the 165 patients with autoantibody-negative RA, late treatment was associated with 316% higher costs over 5 years than early treatment (€4856/$5292 vs €1159/$1263)
- For antibody-positive RA, costs were 19% higher in the late-treatment group.
- In the 43 patients with antibody-positive RA only prescribed biologics, costs were 46% higher for those with delayed treatment.
IN PRACTICE:
“This is the first study showing the effect of early diagnosis and treatment on treatment-related costs,” wrote the authors. “When RA is detected within 12 weeks after symptom onset, treatment-related costs seem to be lower.”
SOURCE:
The study was led by Elise van Mulligen, PhD, Department of Rheumatology, Leiden University Medical Center. It was published online in Annals of the Rheumatic Diseases.
LIMITATIONS:
The division of symptom duration by 12 weeks was “arbitrary.” Baseline characteristics, though similar, showed differences for inflammatory markers in autoantibody-positive and autoantibody-negative RA. Thirty seven patients were lost to follow-up, which could induce attrition bias, though the percentage of these patients in the early- and late-treatment groups was similar.
DISCLOSURES:
This study was funded by ZonMw, a Dutch organization for healthcare research. The authors declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Early detection and treatment of rheumatoid arthritis (RA; within 12 weeks of symptom onset) results in lower treatment-related costs over 5 years compared with later diagnosis.
METHODOLOGY:
- The study enrolled 431 patients in the Leiden Early Arthritis Clinic at Leiden University Medical Center, Leiden, the Netherlands.
- Symptom duration was defined as time between symptom onset and first clinic visit.
- Early treatment was defined as a symptom duration of under 12 weeks, and later treatment defined as symptom duration over 12 weeks.
- Prescription data from patient records and 2022 disease-modifying antirheumatic drug prices (including biologics) was used to determine overall costs over 5 years.
- Autoantibody-negative and autoantibody-positive RA were studied separately because of possible differences in disease severity.
TAKEAWAY:
- For the 165 patients with autoantibody-negative RA, late treatment was associated with 316% higher costs over 5 years than early treatment (€4856/$5292 vs €1159/$1263)
- For antibody-positive RA, costs were 19% higher in the late-treatment group.
- In the 43 patients with antibody-positive RA only prescribed biologics, costs were 46% higher for those with delayed treatment.
IN PRACTICE:
“This is the first study showing the effect of early diagnosis and treatment on treatment-related costs,” wrote the authors. “When RA is detected within 12 weeks after symptom onset, treatment-related costs seem to be lower.”
SOURCE:
The study was led by Elise van Mulligen, PhD, Department of Rheumatology, Leiden University Medical Center. It was published online in Annals of the Rheumatic Diseases.
LIMITATIONS:
The division of symptom duration by 12 weeks was “arbitrary.” Baseline characteristics, though similar, showed differences for inflammatory markers in autoantibody-positive and autoantibody-negative RA. Thirty seven patients were lost to follow-up, which could induce attrition bias, though the percentage of these patients in the early- and late-treatment groups was similar.
DISCLOSURES:
This study was funded by ZonMw, a Dutch organization for healthcare research. The authors declared no relevant conflicts of interest.
A version of this article appeared on Medscape.com.
Atogepant May Prevent Rebound Headache From Medication Overuse in Chronic Migraine
The oral calcitonin gene-related peptide receptor antagonist atogepant is effective in preventing rebound headache related to medication overuse in patients with chronic migraine (CM), new research suggested.
Results of a subgroup analysis of a phase 3, 12-week randomized, double-blind, placebo-controlled trial showed up to a 62% reduction in the proportion of atogepant-treated participants who met acute medication overuse criteria.
“Based on our findings, treatment with atogepant may potentially decrease the risk of developing rebound headache by reducing the use of pain medications,” principal investigator Peter Goadsby, MD, PhD, of King’s College London, London, England, said in a news release.
The study was published online in Neurology.
Effective Prevention Needed
Acute treatments for migraine can mitigate symptoms and reduce disability but can also be ineffective and even result in increased dosing and overuse of these medications, the investigators noted.
Acute medication overuse is defined as “taking simple analgesics for ≥ 15 days per month or taking triptans, ergots, opioids, or combinations of medications for ≥ 10 days per month.”
“There is a high prevalence of pain medication overuse among people with migraine as they try to manage what are often debilitating symptoms,” Dr. Goadsby said. “However, medication overuse can lead to more headaches, called rebound headaches, so more effective preventive treatments are needed.”
Atogepant was developed for migraine prevention in adults. It had been studied in the phase 3 PROGRESS trial, which showed it significantly reduced monthly migraine days (MMDs) compared with placebo during the 12-week trial.
The new subgroup analysis of the study focused specifically on the efficacy and safety of atogepant vs placebo in participants with CM with, and without, medication overuse.
Participants (mean age, 42.1 years; 87.6% women) were randomized to receive either atogepant 30 mg twice daily (n = 253), atogepant 60 mg once daily (n = 256), or placebo (n = 240), with baseline demographics and clinical characteristics similar across all treatment arms. A total of 66.2% met baseline acute medication overuse criteria.
Participants were asked to record migraine and headache experiences in an electronic diary.
‘Effective and Safe’
Participants in both atogepant groups experienced fewer monthly headache days (MHDs) than those in the placebo group, with a least squares mean difference (LSMD) of −2.7 (95% confidence interval [CI], −4.0 to −1.4) in the atogepant 30 mg twice daily group and −1.9 (95% CI, −3.2 to −0.6) in the atogepant 60 mg once daily group.
MHDs were also reduced in both treatment groups, with LSMDs of −2.8 (95% CI, −4.0 to −1.5) and −2.1 (95% CI, −3.3 to −0.8), respectively. Mean acute medication use days were lower in both the treatment groups, with LSMDs of −2.8 (95% CI, −4.1 to −1.6) and −2.6 (95% CI, −3.9 to −1.3), respectively.
A higher proportion of participants achieved a ≥ 50% reduction in MMDs with atogepant 30 mg twice daily (odds ratio [OR], 2.5; 95% CI, 1.5-4.0) and atogepant 60 mg once daily (OR, 2.3; 95% CI, 1.4-3.7).
Notably, the researchers found a 52.1%-61.9% reduction in the proportion of atogepant-treated participants meeting acute medication overuse criteria during the study period vs 38.3% in the placebo group.
Similar results were observed in the subgroup without acute medication overuse.
Treatment-emergent adverse events were reported by 55.8% of participants treated with atogepant 30 mg twice daily, 66.1% with atogepant 60 mg once daily, and 48.5% with placebo in the acute medication overuse subgroup, with similar reports in the non-overuse subgroup.
A limitation cited by the authors was that participants’ self-report of migraines and headaches via electronic diaries might have been inaccurate.
Nevertheless, they concluded that the results showed atogepant to be an “effective and safe” preventive treatment for patients with CM with, and without, acute medication overuse.
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. No honoraria or payments were made for authorship. Dr. Goadsby received personal fees from AbbVie during the conduct of the study, and over the last 36 months, he received a research grant from Celgene; personal fees from Aeon Biopharma, Amgen, CoolTechLLC, Dr. Reddy’s, Eli Lilly and Company, Epalex, Lundbeck, Novartis, Pfizer, Praxis, Sanofi, Satsuma, ShiraTronics, Teva Pharmaceuticals, and Tremeau; personal fees for advice through Gerson Lehrman Group, Guidepoint, SAI Med Partners, and Vector Metric; fees for educational materials from CME Outfitters; and publishing royalties or fees from Massachusetts Medical Society, Oxford University Press, UpToDate, and Wolters Kluwer. The other authors’ disclosures are listed on the original paper.
A version of this article first appeared on Medscape.com.
The oral calcitonin gene-related peptide receptor antagonist atogepant is effective in preventing rebound headache related to medication overuse in patients with chronic migraine (CM), new research suggested.
Results of a subgroup analysis of a phase 3, 12-week randomized, double-blind, placebo-controlled trial showed up to a 62% reduction in the proportion of atogepant-treated participants who met acute medication overuse criteria.
“Based on our findings, treatment with atogepant may potentially decrease the risk of developing rebound headache by reducing the use of pain medications,” principal investigator Peter Goadsby, MD, PhD, of King’s College London, London, England, said in a news release.
The study was published online in Neurology.
Effective Prevention Needed
Acute treatments for migraine can mitigate symptoms and reduce disability but can also be ineffective and even result in increased dosing and overuse of these medications, the investigators noted.
Acute medication overuse is defined as “taking simple analgesics for ≥ 15 days per month or taking triptans, ergots, opioids, or combinations of medications for ≥ 10 days per month.”
“There is a high prevalence of pain medication overuse among people with migraine as they try to manage what are often debilitating symptoms,” Dr. Goadsby said. “However, medication overuse can lead to more headaches, called rebound headaches, so more effective preventive treatments are needed.”
Atogepant was developed for migraine prevention in adults. It had been studied in the phase 3 PROGRESS trial, which showed it significantly reduced monthly migraine days (MMDs) compared with placebo during the 12-week trial.
The new subgroup analysis of the study focused specifically on the efficacy and safety of atogepant vs placebo in participants with CM with, and without, medication overuse.
Participants (mean age, 42.1 years; 87.6% women) were randomized to receive either atogepant 30 mg twice daily (n = 253), atogepant 60 mg once daily (n = 256), or placebo (n = 240), with baseline demographics and clinical characteristics similar across all treatment arms. A total of 66.2% met baseline acute medication overuse criteria.
Participants were asked to record migraine and headache experiences in an electronic diary.
‘Effective and Safe’
Participants in both atogepant groups experienced fewer monthly headache days (MHDs) than those in the placebo group, with a least squares mean difference (LSMD) of −2.7 (95% confidence interval [CI], −4.0 to −1.4) in the atogepant 30 mg twice daily group and −1.9 (95% CI, −3.2 to −0.6) in the atogepant 60 mg once daily group.
MHDs were also reduced in both treatment groups, with LSMDs of −2.8 (95% CI, −4.0 to −1.5) and −2.1 (95% CI, −3.3 to −0.8), respectively. Mean acute medication use days were lower in both the treatment groups, with LSMDs of −2.8 (95% CI, −4.1 to −1.6) and −2.6 (95% CI, −3.9 to −1.3), respectively.
A higher proportion of participants achieved a ≥ 50% reduction in MMDs with atogepant 30 mg twice daily (odds ratio [OR], 2.5; 95% CI, 1.5-4.0) and atogepant 60 mg once daily (OR, 2.3; 95% CI, 1.4-3.7).
Notably, the researchers found a 52.1%-61.9% reduction in the proportion of atogepant-treated participants meeting acute medication overuse criteria during the study period vs 38.3% in the placebo group.
Similar results were observed in the subgroup without acute medication overuse.
Treatment-emergent adverse events were reported by 55.8% of participants treated with atogepant 30 mg twice daily, 66.1% with atogepant 60 mg once daily, and 48.5% with placebo in the acute medication overuse subgroup, with similar reports in the non-overuse subgroup.
A limitation cited by the authors was that participants’ self-report of migraines and headaches via electronic diaries might have been inaccurate.
Nevertheless, they concluded that the results showed atogepant to be an “effective and safe” preventive treatment for patients with CM with, and without, acute medication overuse.
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. No honoraria or payments were made for authorship. Dr. Goadsby received personal fees from AbbVie during the conduct of the study, and over the last 36 months, he received a research grant from Celgene; personal fees from Aeon Biopharma, Amgen, CoolTechLLC, Dr. Reddy’s, Eli Lilly and Company, Epalex, Lundbeck, Novartis, Pfizer, Praxis, Sanofi, Satsuma, ShiraTronics, Teva Pharmaceuticals, and Tremeau; personal fees for advice through Gerson Lehrman Group, Guidepoint, SAI Med Partners, and Vector Metric; fees for educational materials from CME Outfitters; and publishing royalties or fees from Massachusetts Medical Society, Oxford University Press, UpToDate, and Wolters Kluwer. The other authors’ disclosures are listed on the original paper.
A version of this article first appeared on Medscape.com.
The oral calcitonin gene-related peptide receptor antagonist atogepant is effective in preventing rebound headache related to medication overuse in patients with chronic migraine (CM), new research suggested.
Results of a subgroup analysis of a phase 3, 12-week randomized, double-blind, placebo-controlled trial showed up to a 62% reduction in the proportion of atogepant-treated participants who met acute medication overuse criteria.
“Based on our findings, treatment with atogepant may potentially decrease the risk of developing rebound headache by reducing the use of pain medications,” principal investigator Peter Goadsby, MD, PhD, of King’s College London, London, England, said in a news release.
The study was published online in Neurology.
Effective Prevention Needed
Acute treatments for migraine can mitigate symptoms and reduce disability but can also be ineffective and even result in increased dosing and overuse of these medications, the investigators noted.
Acute medication overuse is defined as “taking simple analgesics for ≥ 15 days per month or taking triptans, ergots, opioids, or combinations of medications for ≥ 10 days per month.”
“There is a high prevalence of pain medication overuse among people with migraine as they try to manage what are often debilitating symptoms,” Dr. Goadsby said. “However, medication overuse can lead to more headaches, called rebound headaches, so more effective preventive treatments are needed.”
Atogepant was developed for migraine prevention in adults. It had been studied in the phase 3 PROGRESS trial, which showed it significantly reduced monthly migraine days (MMDs) compared with placebo during the 12-week trial.
The new subgroup analysis of the study focused specifically on the efficacy and safety of atogepant vs placebo in participants with CM with, and without, medication overuse.
Participants (mean age, 42.1 years; 87.6% women) were randomized to receive either atogepant 30 mg twice daily (n = 253), atogepant 60 mg once daily (n = 256), or placebo (n = 240), with baseline demographics and clinical characteristics similar across all treatment arms. A total of 66.2% met baseline acute medication overuse criteria.
Participants were asked to record migraine and headache experiences in an electronic diary.
‘Effective and Safe’
Participants in both atogepant groups experienced fewer monthly headache days (MHDs) than those in the placebo group, with a least squares mean difference (LSMD) of −2.7 (95% confidence interval [CI], −4.0 to −1.4) in the atogepant 30 mg twice daily group and −1.9 (95% CI, −3.2 to −0.6) in the atogepant 60 mg once daily group.
MHDs were also reduced in both treatment groups, with LSMDs of −2.8 (95% CI, −4.0 to −1.5) and −2.1 (95% CI, −3.3 to −0.8), respectively. Mean acute medication use days were lower in both the treatment groups, with LSMDs of −2.8 (95% CI, −4.1 to −1.6) and −2.6 (95% CI, −3.9 to −1.3), respectively.
A higher proportion of participants achieved a ≥ 50% reduction in MMDs with atogepant 30 mg twice daily (odds ratio [OR], 2.5; 95% CI, 1.5-4.0) and atogepant 60 mg once daily (OR, 2.3; 95% CI, 1.4-3.7).
Notably, the researchers found a 52.1%-61.9% reduction in the proportion of atogepant-treated participants meeting acute medication overuse criteria during the study period vs 38.3% in the placebo group.
Similar results were observed in the subgroup without acute medication overuse.
Treatment-emergent adverse events were reported by 55.8% of participants treated with atogepant 30 mg twice daily, 66.1% with atogepant 60 mg once daily, and 48.5% with placebo in the acute medication overuse subgroup, with similar reports in the non-overuse subgroup.
A limitation cited by the authors was that participants’ self-report of migraines and headaches via electronic diaries might have been inaccurate.
Nevertheless, they concluded that the results showed atogepant to be an “effective and safe” preventive treatment for patients with CM with, and without, acute medication overuse.
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. No honoraria or payments were made for authorship. Dr. Goadsby received personal fees from AbbVie during the conduct of the study, and over the last 36 months, he received a research grant from Celgene; personal fees from Aeon Biopharma, Amgen, CoolTechLLC, Dr. Reddy’s, Eli Lilly and Company, Epalex, Lundbeck, Novartis, Pfizer, Praxis, Sanofi, Satsuma, ShiraTronics, Teva Pharmaceuticals, and Tremeau; personal fees for advice through Gerson Lehrman Group, Guidepoint, SAI Med Partners, and Vector Metric; fees for educational materials from CME Outfitters; and publishing royalties or fees from Massachusetts Medical Society, Oxford University Press, UpToDate, and Wolters Kluwer. The other authors’ disclosures are listed on the original paper.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Avoid These Common Mistakes in Treating Hyperkalemia
Hyperkalemia tends to cause panic in healthcare professionals, and rightfully so. On a good day, it causes weakness in the legs; on a bad day, it causes cardiac arrest.
It makes sense that a high potassium level causes clinicians to feel a bit jumpy. This anxiety tends to result in treating the issue by overly restricting potassium in the diet. The problem with this method is that it should be temporary but often isn’t. There are only a few concerns that justify long-term potassium restriction.
As a dietitian, I have seen numerous patients with varying disease states who are terrified of potassium because they were never properly educated on the situation that required restriction or were never notified that their potassium was corrected.
I’ve seen patients whose potassium level hasn’t been elevated in years refuse banana bread because they were told that they could never eat a banana again. I’ve worked with patients who continued to needlessly restrict, which eventually led to hypokalemia.
Not only does this indicate ineffective education — banana bread is actually a low-potassium food at about 80 mg per slice — but also poor follow-up.
Potassium has been designated by the United States Department of Agriculture as a nutrient of public health concern due to its underconsumption in the general population. Although there is concern in the public health community that the current guidelines for potassium intake (3500-4700 mg/d) are unattainable, with some professionals arguing for lowering the standard, there remains significant deficiency in the general population. This deficiency has also been connected to increasing rates of hypertension and cardiovascular disease.
Nondietary Causes of Hyperkalemia
There are many causes of hyperkalemia, of which excessive potassium intake is only one, and an uncommon one at that. A high potassium level should resolve during the course of treatment for metabolic acidosis, hyperglycemia, and dehydration. We may also see resolution with medication changes. But the question remains: Are we relaying this information to patients?
Renal insufficiency is a common cause of hyperkalemia, but it is also a common cause of chronic constipation that can cause hyperkalemia as well. Are we addressing bowel movements with these patients? I often work with patients who aren’t having their bowel movements addressed until the patient themselves voices discomfort.
Depending upon the urgency of treatment, potassium restriction may be the most effective and efficient way to address an acutely elevated value. However, long-term potassium restriction may not be an appropriate intervention for all patients, even those with kidney conditions.
As a dietitian, I have seen many patients who overly restrict dietary potassium because they had one elevated value. These patients tend to view potassium as the enemy because they were never educated on the actual cause of their hyperkalemia. They were simply given a list of high-potassium foods and told to avoid them. A lack of follow-up education may cause them to avoid those foods forever.
Benefits of Potassium
The problem with this perpetual avoidance of high potassium foods is that a potassium-rich diet has been shown to be exceptionally beneficial.
Potassium exists in many forms in the Western diet: as a preservative and additive, a salt substitute, and naturally occurring in both animal and plant products. My concern regarding blanket potassium restriction is that potassium-rich plant and animal products can actually be beneficial, even to those with kidney and heart conditions who are most often advised to restrict its intake.
Adequate potassium intake can:
- Decrease blood pressure by increasing urinary excretion of sodium
- Improve nephrolithiasis by decreasing urinary excretion of calcium
- Decrease incidence of metabolic acidosis by providing precursors to bicarbonate that facilitate excretion of potassium
- Increase bone density in postmenopausal women
- Decrease risk for stroke and cardiovascular disease in the general population
One study found that metabolic acidosis can be corrected in patients with stage 4 chronic kidney disease, without hyperkalemia, by increasing fruit and vegetable intake when compared with those treated with bicarbonate alone, thus preserving kidney function.
Do I suggest encouraging a patient with acute hyperkalemia to eat a banana? Of course not. But I would suggest finding ways to work with patients who have chronic hyperkalemia to increase intake of potassium-rich plant foods to maintain homeostasis while liberalizing diet and preventing progression of chronic kidney disease.
When to Refer to a Dietitian
In patients for whom a potassium-restricted diet is a necessary long-term treatment of hyperkalemia, education with a registered dietitian can be beneficial. A registered dietitian has the time and expertise to address the areas in the diet where excessive potassium exists without forfeiting other nutritional benefits that come from whole foods like fruits, vegetables, lean protein, legumes, nuts, and seeds in a way that is both realistic and helpful. A dietitian can work with patients to reduce intake of potassium-containing salt substitutes, preservatives, and other additives while still encouraging a whole-food diet rich in antioxidants, fiber, and healthy fats.
Dietitians also provide education on serving size and methods to reduce potassium content of food.
For example, tomatoes are a high-potassium food at 300+ mg per medium-sized tomato. But how often does a patient eat a whole tomato? A slice of tomato on a sandwich or a handful of cherry tomatoes in a salad are actually low in potassium per serving and can provide additional nutrients like vitamin C, beta-carotene, and antioxidants like lycopene, which is linked to a decreased incidence of prostate cancer.
By incorporating the assistance of a registered dietitian into the treatment of chronic hyperkalemia, we can develop individualized restrictions that are realistic for the patient and tailored to their nutritional needs to promote optimal health and thus encourage continued compliance.
Ms. Winfree is a renal dietitian in private practice in Mary Esther, Florida. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Hyperkalemia tends to cause panic in healthcare professionals, and rightfully so. On a good day, it causes weakness in the legs; on a bad day, it causes cardiac arrest.
It makes sense that a high potassium level causes clinicians to feel a bit jumpy. This anxiety tends to result in treating the issue by overly restricting potassium in the diet. The problem with this method is that it should be temporary but often isn’t. There are only a few concerns that justify long-term potassium restriction.
As a dietitian, I have seen numerous patients with varying disease states who are terrified of potassium because they were never properly educated on the situation that required restriction or were never notified that their potassium was corrected.
I’ve seen patients whose potassium level hasn’t been elevated in years refuse banana bread because they were told that they could never eat a banana again. I’ve worked with patients who continued to needlessly restrict, which eventually led to hypokalemia.
Not only does this indicate ineffective education — banana bread is actually a low-potassium food at about 80 mg per slice — but also poor follow-up.
Potassium has been designated by the United States Department of Agriculture as a nutrient of public health concern due to its underconsumption in the general population. Although there is concern in the public health community that the current guidelines for potassium intake (3500-4700 mg/d) are unattainable, with some professionals arguing for lowering the standard, there remains significant deficiency in the general population. This deficiency has also been connected to increasing rates of hypertension and cardiovascular disease.
Nondietary Causes of Hyperkalemia
There are many causes of hyperkalemia, of which excessive potassium intake is only one, and an uncommon one at that. A high potassium level should resolve during the course of treatment for metabolic acidosis, hyperglycemia, and dehydration. We may also see resolution with medication changes. But the question remains: Are we relaying this information to patients?
Renal insufficiency is a common cause of hyperkalemia, but it is also a common cause of chronic constipation that can cause hyperkalemia as well. Are we addressing bowel movements with these patients? I often work with patients who aren’t having their bowel movements addressed until the patient themselves voices discomfort.
Depending upon the urgency of treatment, potassium restriction may be the most effective and efficient way to address an acutely elevated value. However, long-term potassium restriction may not be an appropriate intervention for all patients, even those with kidney conditions.
As a dietitian, I have seen many patients who overly restrict dietary potassium because they had one elevated value. These patients tend to view potassium as the enemy because they were never educated on the actual cause of their hyperkalemia. They were simply given a list of high-potassium foods and told to avoid them. A lack of follow-up education may cause them to avoid those foods forever.
Benefits of Potassium
The problem with this perpetual avoidance of high potassium foods is that a potassium-rich diet has been shown to be exceptionally beneficial.
Potassium exists in many forms in the Western diet: as a preservative and additive, a salt substitute, and naturally occurring in both animal and plant products. My concern regarding blanket potassium restriction is that potassium-rich plant and animal products can actually be beneficial, even to those with kidney and heart conditions who are most often advised to restrict its intake.
Adequate potassium intake can:
- Decrease blood pressure by increasing urinary excretion of sodium
- Improve nephrolithiasis by decreasing urinary excretion of calcium
- Decrease incidence of metabolic acidosis by providing precursors to bicarbonate that facilitate excretion of potassium
- Increase bone density in postmenopausal women
- Decrease risk for stroke and cardiovascular disease in the general population
One study found that metabolic acidosis can be corrected in patients with stage 4 chronic kidney disease, without hyperkalemia, by increasing fruit and vegetable intake when compared with those treated with bicarbonate alone, thus preserving kidney function.
Do I suggest encouraging a patient with acute hyperkalemia to eat a banana? Of course not. But I would suggest finding ways to work with patients who have chronic hyperkalemia to increase intake of potassium-rich plant foods to maintain homeostasis while liberalizing diet and preventing progression of chronic kidney disease.
When to Refer to a Dietitian
In patients for whom a potassium-restricted diet is a necessary long-term treatment of hyperkalemia, education with a registered dietitian can be beneficial. A registered dietitian has the time and expertise to address the areas in the diet where excessive potassium exists without forfeiting other nutritional benefits that come from whole foods like fruits, vegetables, lean protein, legumes, nuts, and seeds in a way that is both realistic and helpful. A dietitian can work with patients to reduce intake of potassium-containing salt substitutes, preservatives, and other additives while still encouraging a whole-food diet rich in antioxidants, fiber, and healthy fats.
Dietitians also provide education on serving size and methods to reduce potassium content of food.
For example, tomatoes are a high-potassium food at 300+ mg per medium-sized tomato. But how often does a patient eat a whole tomato? A slice of tomato on a sandwich or a handful of cherry tomatoes in a salad are actually low in potassium per serving and can provide additional nutrients like vitamin C, beta-carotene, and antioxidants like lycopene, which is linked to a decreased incidence of prostate cancer.
By incorporating the assistance of a registered dietitian into the treatment of chronic hyperkalemia, we can develop individualized restrictions that are realistic for the patient and tailored to their nutritional needs to promote optimal health and thus encourage continued compliance.
Ms. Winfree is a renal dietitian in private practice in Mary Esther, Florida. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Hyperkalemia tends to cause panic in healthcare professionals, and rightfully so. On a good day, it causes weakness in the legs; on a bad day, it causes cardiac arrest.
It makes sense that a high potassium level causes clinicians to feel a bit jumpy. This anxiety tends to result in treating the issue by overly restricting potassium in the diet. The problem with this method is that it should be temporary but often isn’t. There are only a few concerns that justify long-term potassium restriction.
As a dietitian, I have seen numerous patients with varying disease states who are terrified of potassium because they were never properly educated on the situation that required restriction or were never notified that their potassium was corrected.
I’ve seen patients whose potassium level hasn’t been elevated in years refuse banana bread because they were told that they could never eat a banana again. I’ve worked with patients who continued to needlessly restrict, which eventually led to hypokalemia.
Not only does this indicate ineffective education — banana bread is actually a low-potassium food at about 80 mg per slice — but also poor follow-up.
Potassium has been designated by the United States Department of Agriculture as a nutrient of public health concern due to its underconsumption in the general population. Although there is concern in the public health community that the current guidelines for potassium intake (3500-4700 mg/d) are unattainable, with some professionals arguing for lowering the standard, there remains significant deficiency in the general population. This deficiency has also been connected to increasing rates of hypertension and cardiovascular disease.
Nondietary Causes of Hyperkalemia
There are many causes of hyperkalemia, of which excessive potassium intake is only one, and an uncommon one at that. A high potassium level should resolve during the course of treatment for metabolic acidosis, hyperglycemia, and dehydration. We may also see resolution with medication changes. But the question remains: Are we relaying this information to patients?
Renal insufficiency is a common cause of hyperkalemia, but it is also a common cause of chronic constipation that can cause hyperkalemia as well. Are we addressing bowel movements with these patients? I often work with patients who aren’t having their bowel movements addressed until the patient themselves voices discomfort.
Depending upon the urgency of treatment, potassium restriction may be the most effective and efficient way to address an acutely elevated value. However, long-term potassium restriction may not be an appropriate intervention for all patients, even those with kidney conditions.
As a dietitian, I have seen many patients who overly restrict dietary potassium because they had one elevated value. These patients tend to view potassium as the enemy because they were never educated on the actual cause of their hyperkalemia. They were simply given a list of high-potassium foods and told to avoid them. A lack of follow-up education may cause them to avoid those foods forever.
Benefits of Potassium
The problem with this perpetual avoidance of high potassium foods is that a potassium-rich diet has been shown to be exceptionally beneficial.
Potassium exists in many forms in the Western diet: as a preservative and additive, a salt substitute, and naturally occurring in both animal and plant products. My concern regarding blanket potassium restriction is that potassium-rich plant and animal products can actually be beneficial, even to those with kidney and heart conditions who are most often advised to restrict its intake.
Adequate potassium intake can:
- Decrease blood pressure by increasing urinary excretion of sodium
- Improve nephrolithiasis by decreasing urinary excretion of calcium
- Decrease incidence of metabolic acidosis by providing precursors to bicarbonate that facilitate excretion of potassium
- Increase bone density in postmenopausal women
- Decrease risk for stroke and cardiovascular disease in the general population
One study found that metabolic acidosis can be corrected in patients with stage 4 chronic kidney disease, without hyperkalemia, by increasing fruit and vegetable intake when compared with those treated with bicarbonate alone, thus preserving kidney function.
Do I suggest encouraging a patient with acute hyperkalemia to eat a banana? Of course not. But I would suggest finding ways to work with patients who have chronic hyperkalemia to increase intake of potassium-rich plant foods to maintain homeostasis while liberalizing diet and preventing progression of chronic kidney disease.
When to Refer to a Dietitian
In patients for whom a potassium-restricted diet is a necessary long-term treatment of hyperkalemia, education with a registered dietitian can be beneficial. A registered dietitian has the time and expertise to address the areas in the diet where excessive potassium exists without forfeiting other nutritional benefits that come from whole foods like fruits, vegetables, lean protein, legumes, nuts, and seeds in a way that is both realistic and helpful. A dietitian can work with patients to reduce intake of potassium-containing salt substitutes, preservatives, and other additives while still encouraging a whole-food diet rich in antioxidants, fiber, and healthy fats.
Dietitians also provide education on serving size and methods to reduce potassium content of food.
For example, tomatoes are a high-potassium food at 300+ mg per medium-sized tomato. But how often does a patient eat a whole tomato? A slice of tomato on a sandwich or a handful of cherry tomatoes in a salad are actually low in potassium per serving and can provide additional nutrients like vitamin C, beta-carotene, and antioxidants like lycopene, which is linked to a decreased incidence of prostate cancer.
By incorporating the assistance of a registered dietitian into the treatment of chronic hyperkalemia, we can develop individualized restrictions that are realistic for the patient and tailored to their nutritional needs to promote optimal health and thus encourage continued compliance.
Ms. Winfree is a renal dietitian in private practice in Mary Esther, Florida. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Experts Debate How to Best Define Obesity
in three new opinion papers.
The three statements were published on July 22, 2024, in Annals of Internal Medicine. In one, the authors expressed caution about the recent movement away from using BMI alone to define obesity, noting that the measure remains a useful population-level and clinical tool for addressing adiposity, particularly within racial and ethnic groups. But the authors of a second paper pointed out that the use of lower BMI cutoffs to define obesity in Asian populations, in place since 2004, is inadequate in part because it doesn’t account for heterogeneity among different Asian groups.
And in the third paper, an editorial, an Annals editor cautioned that the recent framing of obesity exclusively as a “disease” rather than a “broader, more inclusive construct” may inadvertently reinforce the bias it was meant to combat.
Asked to comment on the issues raised in the papers, Professor Gijs Goossens of Maastricht University Medical Center, Maastricht, the Netherlands, said, “It is important to emphasize that the management and treatment of obesity have wider objectives than weight loss alone and include the prevention, resolution, or improvement of obesity-related complications; achieving better quality of life and mental well-being; and improvement of physical and social functioning.”
Added Dr. Goossens, who was an author of a recent European Association for the Study of Obesity (EASO) framework calling for moving beyond BMI in defining obesity, “Personalized therapeutic goals should be set at the beginning of the treatment, according to the stage of obesity, taking into account available therapeutic options, possible side effects or risks, and patient preferences. The drivers of obesity and possible barriers to treatment should also be discussed with the patient.” Dr. Goossens emphasized that he was providing his personal views and not speaking for the EASO or his coauthors.
BMI: ‘Not a Perfect Measure of Adiposity but Remains Useful’
In their “Ideas and Opinions” paper, Adolfo G. Cuevas, PhD, of New York University School of Global Public Health, New York City, and Walter C. Willett, MD, DrPH, of Harvard T.H. Chan School of Public Health, Boston, argued that “BMI, although not a perfect measure of adiposity, remains a useful population-level and clinical tool for addressing adiposity, including within groups defined by race and ethnicity.”
They added that despite the criticism that BMI doesn’t distinguish between fat and lean body mass, the measure still strongly correlates with fat mass as well as cardiovascular risk and mortality, and it does so similarly across racial and ethnic groups.
Clinically, Dr. Cuevas and Dr. Willett pointed out that BMI correlates fat mass as assessed with the gold standard measure dual x-ray absorptiometry but is far simpler and less expensive. Measuring waist circumference can provide additional information about visceral fat and disease risk but is “more difficult to standardize and suffers from the same limitations as BMI when cut points are used.”
They suggest the addition of change in weight since early adulthood and over time as a “simple and sensitive variable” for assessing adiposity.
Luca Busetto, MD, associate professor of medicine at the University of Padua, Italy, and the first author of the EASO framework, said, “The paper from Cuevas and Willett sounds like a strong defense of BMI, and I can substantially agree with this defense ... We remain anchored on BMI, but we tried to move beyond it adding an estimate of high risk abdominal fat — waist to height ratio — and coupling the anthropometric assessment with a complete clinical evaluation and staging.”
Dr. Goossens said, “I agree with the authors that despite the limitations of BMI as a measure of body fatness, it remains a useful clinical screening tool. Yet the diagnosis of obesity should not be based solely on BMI” due to the stronger association of abdominal fat with cardiometabolic complications.
That link, he noted, “also applies to individuals with a BMI level below the current cutoff values for obesity, who may already have medical, functional, or psychological impairments. We should be aware of the risk of undertreatment in this particular group of patients.”
Does Calling Obesity a ‘Disease’ Have Unintended Consequences?
In her editorial, Christina C. Wee, MD, senior deputy editor, Annals of Internal Medicine, wrote, “Beyond diagnostic challenges, framing obesity exclusively as a disease rather than a broader, more inclusive construct may have unintended consequences — including reinforcing the weight bias this framing was in part intended to combat.”
Focusing solely on biological causes of obesity while ignoring psychosocial, cultural, environmental, and behavioral contexts could undermine public health and policy efforts to address those factors, Dr. Wee argued.
Moreover, she wrote, “Ironically, framing obesity as a disease to justify coverage for treatment reinforces weight bias. It conflates the need to label a condition a disease with healthcare reimbursement and raises the stakes for developing accurate diagnostic criteria ... By exclusively linking obesity as a disease to reimbursement, it sends the message that only those who manifest disease from excess adiposity warrant treatment — and, by inference, those on the continuum who have not yet manifested disease do not warrant treatment.”
Likening obesity to other risk factors such as hypertension or dyslipidemia for which treatment is typically reimbursed, Dr. Wee pointed out that Medicare still prohibits coverage of medications for obesity.
Regarding the high costs of newer obesity medications and the need for payers and clinicians to ration their use, Dr. Wee argued, “Rather than focusing on whether one’s adiposity conforms to an expert panel’s definition of ‘disease,’ we should address how to best stage obesity risk with sufficient accuracy and fairness and reach a consensus on how to prioritize and match treatments to individual patients.”
Dr. Busetto said that EASO stands by its definition of obesity as a disease, adding “we can adhere to the suggestion of a holistic approach deciding treatment modalities according to the risk and the presence of mental, functional, and medical complications of impairments. Of course, we cannot agree on any proposal that is oriented at leaving patients with obesity still in the asymptomatic phase of the disease without treatment. This would be like treating diabetes only after the occurrence of nephropathy or managing hypertension only after a stroke. Prevention of the symptomatic stage is a part of obesity management, even beyond weight loss.”
Dr. Goossens said, “indeed, it is of utmost importance to develop accurate risk stratification tools for adequately clinical staging of obesity, according to the severity of its medical, psychological and functional impairments.”
Do the Current Lower BMI Cutoffs for Defining Obesity in Asian People Make Sense?
Simar S. Bajaj, AB, of Harvard University, Cambridge, Massachusetts, and colleagues, all of Harvard Medical School, Boston, raised several concerns regarding the 2004 World Health Organization’s suggestion to use lower BMI categories for defining overweight and obesity in Asian populations, that is, 23-27.5 kg/m2 and 27.5 kg/m2 or higher for obesity, respectively, as opposed to 25-29.9 and ≥ 30, respectively, for other populations.
Different Asian countries have created their own obesity BMI cutoffs, ranging from 25 kg/m2 in India to 28 kg/m2 in China. But “Asian Americans continue to be treated as a monolith without official disaggregated cutoffs,” Mr. Bajaj and colleagues noted.
The heterogeneity translates to different risk levels across Asian subgroups. For example, in one study, age- and sex-adjusted BMI cutoffs for increased risk of developing type 2 diabetes were 23.9 kg/m2 in South Asian populations, 26.6 kg/m2 in Arab populations, 26.9 kg/m2 in Chinese populations, and 28.1 kg/m2 in Black populations.
These findings raise important questions, the researchers said. “Does it make sense for people of Chinese descent to use the same BMI threshold as the South Asian group when their ‘equivalent risk cutoff’ is closer to that of Arab and Black groups who share the standard BMI threshold?” Most data in this area are cross-sectional rather than the longitudinal data needed to answer those questions, they noted.
They suggest that professional diabetes and obesity organizations consider BMI thresholds to be “placeholders” until more sensitive and specific thresholds can be defined for Asian American populations.
Mr. Bajaj and colleagues also noted the need for disaggregated data is not unique to Asian groups but that they focused on Asian Americans for two main reasons. “First, success would create a precedent for complete disaggregation and help ensure that other groups do not stall at an intermediary level. Second, substantial research into Asian ethnic groups — and the WHO’s precedent 20 years ago — creates a solid foundation to build upon.”
Ultimately, they said, “advancing equity will require funding research that engages diverse Asian communities and developing tailored interventions for all ethnicities.”
Dr. Cuevas, Dr. Willett, Mr. Bajaj, and Dr. Wee had no disclosures. Dr. Goossens received research funding from the European Foundation for the Study of Diabetes, the Dutch Diabetes Research Foundation, and the Dutch Research Council. Dr. Busetto received personal funding from Novo Nordisk, Boehringer Ingelheim, Eli Lilly, Pfizer, and Bruno Farmaceutici as a member of advisory boards and from Rhythm Pharmaceuticals and Pronokal as a speaker.
A version of this article first appeared on Medscape.com.
in three new opinion papers.
The three statements were published on July 22, 2024, in Annals of Internal Medicine. In one, the authors expressed caution about the recent movement away from using BMI alone to define obesity, noting that the measure remains a useful population-level and clinical tool for addressing adiposity, particularly within racial and ethnic groups. But the authors of a second paper pointed out that the use of lower BMI cutoffs to define obesity in Asian populations, in place since 2004, is inadequate in part because it doesn’t account for heterogeneity among different Asian groups.
And in the third paper, an editorial, an Annals editor cautioned that the recent framing of obesity exclusively as a “disease” rather than a “broader, more inclusive construct” may inadvertently reinforce the bias it was meant to combat.
Asked to comment on the issues raised in the papers, Professor Gijs Goossens of Maastricht University Medical Center, Maastricht, the Netherlands, said, “It is important to emphasize that the management and treatment of obesity have wider objectives than weight loss alone and include the prevention, resolution, or improvement of obesity-related complications; achieving better quality of life and mental well-being; and improvement of physical and social functioning.”
Added Dr. Goossens, who was an author of a recent European Association for the Study of Obesity (EASO) framework calling for moving beyond BMI in defining obesity, “Personalized therapeutic goals should be set at the beginning of the treatment, according to the stage of obesity, taking into account available therapeutic options, possible side effects or risks, and patient preferences. The drivers of obesity and possible barriers to treatment should also be discussed with the patient.” Dr. Goossens emphasized that he was providing his personal views and not speaking for the EASO or his coauthors.
BMI: ‘Not a Perfect Measure of Adiposity but Remains Useful’
In their “Ideas and Opinions” paper, Adolfo G. Cuevas, PhD, of New York University School of Global Public Health, New York City, and Walter C. Willett, MD, DrPH, of Harvard T.H. Chan School of Public Health, Boston, argued that “BMI, although not a perfect measure of adiposity, remains a useful population-level and clinical tool for addressing adiposity, including within groups defined by race and ethnicity.”
They added that despite the criticism that BMI doesn’t distinguish between fat and lean body mass, the measure still strongly correlates with fat mass as well as cardiovascular risk and mortality, and it does so similarly across racial and ethnic groups.
Clinically, Dr. Cuevas and Dr. Willett pointed out that BMI correlates fat mass as assessed with the gold standard measure dual x-ray absorptiometry but is far simpler and less expensive. Measuring waist circumference can provide additional information about visceral fat and disease risk but is “more difficult to standardize and suffers from the same limitations as BMI when cut points are used.”
They suggest the addition of change in weight since early adulthood and over time as a “simple and sensitive variable” for assessing adiposity.
Luca Busetto, MD, associate professor of medicine at the University of Padua, Italy, and the first author of the EASO framework, said, “The paper from Cuevas and Willett sounds like a strong defense of BMI, and I can substantially agree with this defense ... We remain anchored on BMI, but we tried to move beyond it adding an estimate of high risk abdominal fat — waist to height ratio — and coupling the anthropometric assessment with a complete clinical evaluation and staging.”
Dr. Goossens said, “I agree with the authors that despite the limitations of BMI as a measure of body fatness, it remains a useful clinical screening tool. Yet the diagnosis of obesity should not be based solely on BMI” due to the stronger association of abdominal fat with cardiometabolic complications.
That link, he noted, “also applies to individuals with a BMI level below the current cutoff values for obesity, who may already have medical, functional, or psychological impairments. We should be aware of the risk of undertreatment in this particular group of patients.”
Does Calling Obesity a ‘Disease’ Have Unintended Consequences?
In her editorial, Christina C. Wee, MD, senior deputy editor, Annals of Internal Medicine, wrote, “Beyond diagnostic challenges, framing obesity exclusively as a disease rather than a broader, more inclusive construct may have unintended consequences — including reinforcing the weight bias this framing was in part intended to combat.”
Focusing solely on biological causes of obesity while ignoring psychosocial, cultural, environmental, and behavioral contexts could undermine public health and policy efforts to address those factors, Dr. Wee argued.
Moreover, she wrote, “Ironically, framing obesity as a disease to justify coverage for treatment reinforces weight bias. It conflates the need to label a condition a disease with healthcare reimbursement and raises the stakes for developing accurate diagnostic criteria ... By exclusively linking obesity as a disease to reimbursement, it sends the message that only those who manifest disease from excess adiposity warrant treatment — and, by inference, those on the continuum who have not yet manifested disease do not warrant treatment.”
Likening obesity to other risk factors such as hypertension or dyslipidemia for which treatment is typically reimbursed, Dr. Wee pointed out that Medicare still prohibits coverage of medications for obesity.
Regarding the high costs of newer obesity medications and the need for payers and clinicians to ration their use, Dr. Wee argued, “Rather than focusing on whether one’s adiposity conforms to an expert panel’s definition of ‘disease,’ we should address how to best stage obesity risk with sufficient accuracy and fairness and reach a consensus on how to prioritize and match treatments to individual patients.”
Dr. Busetto said that EASO stands by its definition of obesity as a disease, adding “we can adhere to the suggestion of a holistic approach deciding treatment modalities according to the risk and the presence of mental, functional, and medical complications of impairments. Of course, we cannot agree on any proposal that is oriented at leaving patients with obesity still in the asymptomatic phase of the disease without treatment. This would be like treating diabetes only after the occurrence of nephropathy or managing hypertension only after a stroke. Prevention of the symptomatic stage is a part of obesity management, even beyond weight loss.”
Dr. Goossens said, “indeed, it is of utmost importance to develop accurate risk stratification tools for adequately clinical staging of obesity, according to the severity of its medical, psychological and functional impairments.”
Do the Current Lower BMI Cutoffs for Defining Obesity in Asian People Make Sense?
Simar S. Bajaj, AB, of Harvard University, Cambridge, Massachusetts, and colleagues, all of Harvard Medical School, Boston, raised several concerns regarding the 2004 World Health Organization’s suggestion to use lower BMI categories for defining overweight and obesity in Asian populations, that is, 23-27.5 kg/m2 and 27.5 kg/m2 or higher for obesity, respectively, as opposed to 25-29.9 and ≥ 30, respectively, for other populations.
Different Asian countries have created their own obesity BMI cutoffs, ranging from 25 kg/m2 in India to 28 kg/m2 in China. But “Asian Americans continue to be treated as a monolith without official disaggregated cutoffs,” Mr. Bajaj and colleagues noted.
The heterogeneity translates to different risk levels across Asian subgroups. For example, in one study, age- and sex-adjusted BMI cutoffs for increased risk of developing type 2 diabetes were 23.9 kg/m2 in South Asian populations, 26.6 kg/m2 in Arab populations, 26.9 kg/m2 in Chinese populations, and 28.1 kg/m2 in Black populations.
These findings raise important questions, the researchers said. “Does it make sense for people of Chinese descent to use the same BMI threshold as the South Asian group when their ‘equivalent risk cutoff’ is closer to that of Arab and Black groups who share the standard BMI threshold?” Most data in this area are cross-sectional rather than the longitudinal data needed to answer those questions, they noted.
They suggest that professional diabetes and obesity organizations consider BMI thresholds to be “placeholders” until more sensitive and specific thresholds can be defined for Asian American populations.
Mr. Bajaj and colleagues also noted the need for disaggregated data is not unique to Asian groups but that they focused on Asian Americans for two main reasons. “First, success would create a precedent for complete disaggregation and help ensure that other groups do not stall at an intermediary level. Second, substantial research into Asian ethnic groups — and the WHO’s precedent 20 years ago — creates a solid foundation to build upon.”
Ultimately, they said, “advancing equity will require funding research that engages diverse Asian communities and developing tailored interventions for all ethnicities.”
Dr. Cuevas, Dr. Willett, Mr. Bajaj, and Dr. Wee had no disclosures. Dr. Goossens received research funding from the European Foundation for the Study of Diabetes, the Dutch Diabetes Research Foundation, and the Dutch Research Council. Dr. Busetto received personal funding from Novo Nordisk, Boehringer Ingelheim, Eli Lilly, Pfizer, and Bruno Farmaceutici as a member of advisory boards and from Rhythm Pharmaceuticals and Pronokal as a speaker.
A version of this article first appeared on Medscape.com.
in three new opinion papers.
The three statements were published on July 22, 2024, in Annals of Internal Medicine. In one, the authors expressed caution about the recent movement away from using BMI alone to define obesity, noting that the measure remains a useful population-level and clinical tool for addressing adiposity, particularly within racial and ethnic groups. But the authors of a second paper pointed out that the use of lower BMI cutoffs to define obesity in Asian populations, in place since 2004, is inadequate in part because it doesn’t account for heterogeneity among different Asian groups.
And in the third paper, an editorial, an Annals editor cautioned that the recent framing of obesity exclusively as a “disease” rather than a “broader, more inclusive construct” may inadvertently reinforce the bias it was meant to combat.
Asked to comment on the issues raised in the papers, Professor Gijs Goossens of Maastricht University Medical Center, Maastricht, the Netherlands, said, “It is important to emphasize that the management and treatment of obesity have wider objectives than weight loss alone and include the prevention, resolution, or improvement of obesity-related complications; achieving better quality of life and mental well-being; and improvement of physical and social functioning.”
Added Dr. Goossens, who was an author of a recent European Association for the Study of Obesity (EASO) framework calling for moving beyond BMI in defining obesity, “Personalized therapeutic goals should be set at the beginning of the treatment, according to the stage of obesity, taking into account available therapeutic options, possible side effects or risks, and patient preferences. The drivers of obesity and possible barriers to treatment should also be discussed with the patient.” Dr. Goossens emphasized that he was providing his personal views and not speaking for the EASO or his coauthors.
BMI: ‘Not a Perfect Measure of Adiposity but Remains Useful’
In their “Ideas and Opinions” paper, Adolfo G. Cuevas, PhD, of New York University School of Global Public Health, New York City, and Walter C. Willett, MD, DrPH, of Harvard T.H. Chan School of Public Health, Boston, argued that “BMI, although not a perfect measure of adiposity, remains a useful population-level and clinical tool for addressing adiposity, including within groups defined by race and ethnicity.”
They added that despite the criticism that BMI doesn’t distinguish between fat and lean body mass, the measure still strongly correlates with fat mass as well as cardiovascular risk and mortality, and it does so similarly across racial and ethnic groups.
Clinically, Dr. Cuevas and Dr. Willett pointed out that BMI correlates fat mass as assessed with the gold standard measure dual x-ray absorptiometry but is far simpler and less expensive. Measuring waist circumference can provide additional information about visceral fat and disease risk but is “more difficult to standardize and suffers from the same limitations as BMI when cut points are used.”
They suggest the addition of change in weight since early adulthood and over time as a “simple and sensitive variable” for assessing adiposity.
Luca Busetto, MD, associate professor of medicine at the University of Padua, Italy, and the first author of the EASO framework, said, “The paper from Cuevas and Willett sounds like a strong defense of BMI, and I can substantially agree with this defense ... We remain anchored on BMI, but we tried to move beyond it adding an estimate of high risk abdominal fat — waist to height ratio — and coupling the anthropometric assessment with a complete clinical evaluation and staging.”
Dr. Goossens said, “I agree with the authors that despite the limitations of BMI as a measure of body fatness, it remains a useful clinical screening tool. Yet the diagnosis of obesity should not be based solely on BMI” due to the stronger association of abdominal fat with cardiometabolic complications.
That link, he noted, “also applies to individuals with a BMI level below the current cutoff values for obesity, who may already have medical, functional, or psychological impairments. We should be aware of the risk of undertreatment in this particular group of patients.”
Does Calling Obesity a ‘Disease’ Have Unintended Consequences?
In her editorial, Christina C. Wee, MD, senior deputy editor, Annals of Internal Medicine, wrote, “Beyond diagnostic challenges, framing obesity exclusively as a disease rather than a broader, more inclusive construct may have unintended consequences — including reinforcing the weight bias this framing was in part intended to combat.”
Focusing solely on biological causes of obesity while ignoring psychosocial, cultural, environmental, and behavioral contexts could undermine public health and policy efforts to address those factors, Dr. Wee argued.
Moreover, she wrote, “Ironically, framing obesity as a disease to justify coverage for treatment reinforces weight bias. It conflates the need to label a condition a disease with healthcare reimbursement and raises the stakes for developing accurate diagnostic criteria ... By exclusively linking obesity as a disease to reimbursement, it sends the message that only those who manifest disease from excess adiposity warrant treatment — and, by inference, those on the continuum who have not yet manifested disease do not warrant treatment.”
Likening obesity to other risk factors such as hypertension or dyslipidemia for which treatment is typically reimbursed, Dr. Wee pointed out that Medicare still prohibits coverage of medications for obesity.
Regarding the high costs of newer obesity medications and the need for payers and clinicians to ration their use, Dr. Wee argued, “Rather than focusing on whether one’s adiposity conforms to an expert panel’s definition of ‘disease,’ we should address how to best stage obesity risk with sufficient accuracy and fairness and reach a consensus on how to prioritize and match treatments to individual patients.”
Dr. Busetto said that EASO stands by its definition of obesity as a disease, adding “we can adhere to the suggestion of a holistic approach deciding treatment modalities according to the risk and the presence of mental, functional, and medical complications of impairments. Of course, we cannot agree on any proposal that is oriented at leaving patients with obesity still in the asymptomatic phase of the disease without treatment. This would be like treating diabetes only after the occurrence of nephropathy or managing hypertension only after a stroke. Prevention of the symptomatic stage is a part of obesity management, even beyond weight loss.”
Dr. Goossens said, “indeed, it is of utmost importance to develop accurate risk stratification tools for adequately clinical staging of obesity, according to the severity of its medical, psychological and functional impairments.”
Do the Current Lower BMI Cutoffs for Defining Obesity in Asian People Make Sense?
Simar S. Bajaj, AB, of Harvard University, Cambridge, Massachusetts, and colleagues, all of Harvard Medical School, Boston, raised several concerns regarding the 2004 World Health Organization’s suggestion to use lower BMI categories for defining overweight and obesity in Asian populations, that is, 23-27.5 kg/m2 and 27.5 kg/m2 or higher for obesity, respectively, as opposed to 25-29.9 and ≥ 30, respectively, for other populations.
Different Asian countries have created their own obesity BMI cutoffs, ranging from 25 kg/m2 in India to 28 kg/m2 in China. But “Asian Americans continue to be treated as a monolith without official disaggregated cutoffs,” Mr. Bajaj and colleagues noted.
The heterogeneity translates to different risk levels across Asian subgroups. For example, in one study, age- and sex-adjusted BMI cutoffs for increased risk of developing type 2 diabetes were 23.9 kg/m2 in South Asian populations, 26.6 kg/m2 in Arab populations, 26.9 kg/m2 in Chinese populations, and 28.1 kg/m2 in Black populations.
These findings raise important questions, the researchers said. “Does it make sense for people of Chinese descent to use the same BMI threshold as the South Asian group when their ‘equivalent risk cutoff’ is closer to that of Arab and Black groups who share the standard BMI threshold?” Most data in this area are cross-sectional rather than the longitudinal data needed to answer those questions, they noted.
They suggest that professional diabetes and obesity organizations consider BMI thresholds to be “placeholders” until more sensitive and specific thresholds can be defined for Asian American populations.
Mr. Bajaj and colleagues also noted the need for disaggregated data is not unique to Asian groups but that they focused on Asian Americans for two main reasons. “First, success would create a precedent for complete disaggregation and help ensure that other groups do not stall at an intermediary level. Second, substantial research into Asian ethnic groups — and the WHO’s precedent 20 years ago — creates a solid foundation to build upon.”
Ultimately, they said, “advancing equity will require funding research that engages diverse Asian communities and developing tailored interventions for all ethnicities.”
Dr. Cuevas, Dr. Willett, Mr. Bajaj, and Dr. Wee had no disclosures. Dr. Goossens received research funding from the European Foundation for the Study of Diabetes, the Dutch Diabetes Research Foundation, and the Dutch Research Council. Dr. Busetto received personal funding from Novo Nordisk, Boehringer Ingelheim, Eli Lilly, Pfizer, and Bruno Farmaceutici as a member of advisory boards and from Rhythm Pharmaceuticals and Pronokal as a speaker.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Generalized Fixed Drug Eruptions Require Urgent Care: A Case Series
Recognizing cutaneous drug eruptions is important for treatment and prevention of recurrence. Fixed drug eruptions (FDEs) typically are harmless but can have major negative cosmetic consequences for patients. In its more severe forms, patients are at risk for widespread epithelial necrosis with accompanying complications. We report 1 patient with generalized FDE and 2 with generalized bullous FDE. We also discuss the recognition and treatment of the condition. Two patients previously had been diagnosed with systemic lupus erythematosus (SLE).
Case Series
Patient 1—A 60-year-old woman presented to dermatology with a rash on the trunk and groin folds of 4 days’ duration. She had a history of SLE and cutaneous lupus treated with hydroxychloroquine 200 mg twice daily and topical corticosteroids. She had started sulfamethoxazole-trimethoprim for a urinary tract infection with a rash appearing 1 day later. She reported burning skin pain with progression to blisters that “sloughed” off. She denied any known history of allergy to sulfa drugs. Prior to evaluation by dermatology, she visited an urgent care facility and was prescribed hydroxyzine and intramuscular corticosteroids. At presentation to dermatology 3 days after taking sulfamethoxazole-trimethoprim, she had annular flaccid bullae and superficial erosions with dusky borders on the right posterior thigh, right side of the chest, left inframammary fold, and right inguinal fold (Figure 1). She had no ocular, oral, or vaginal erosions. A diagnosis of generalized bullous FDE was favored over erythema multiforme or Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). Shave biopsies from lesions on the right posterior thigh and right inguinal fold demonstrated interface dermatitis with epidermal necrosis, pigment incontinence, and numerous eosinophils. Direct immunofluorescence of the perilesional skin was negative for immunoprotein deposition. These findings were consistent with the clinical impression of generalized bullous FDE. Prior to receiving the histopathology report, the patient was initiated on a regimen of cyclosporine 5 mg/kg/d in the setting of normal renal function and followed until the eruption resolved completely. Cyclosporine was tapered at 2 weeks and discontinued at 3 weeks.
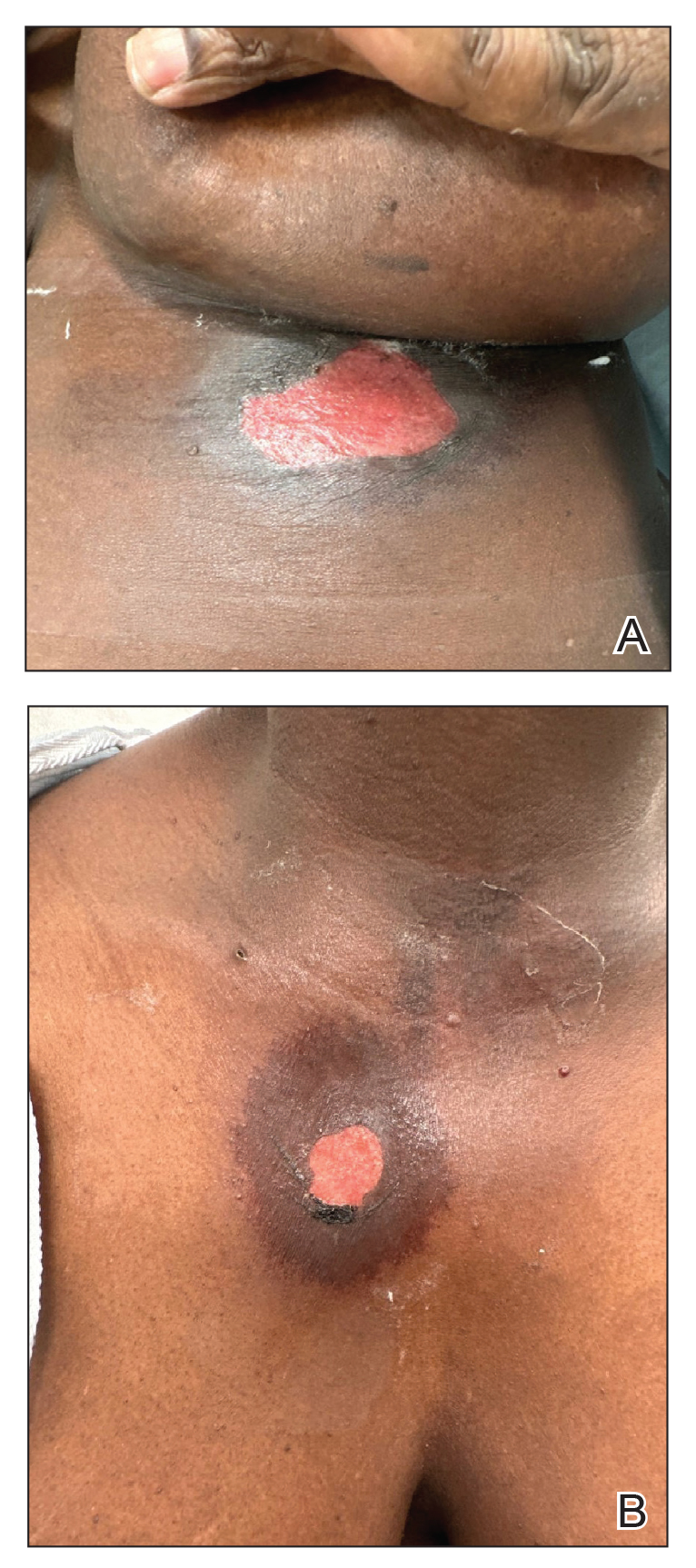
Patient 2—A 32-year-old woman presented for follow-up management of discoid lupus erythematosus. She had a history of systemic and cutaneous lupus, juvenile rheumatoid arthritis, and mixed connective tissue disease managed with prednisone, hydroxychloroquine, azathioprine, and belimumab. Physical examination revealed scarring alopecia with dyspigmentation and active inflammation consistent with uncontrolled cutaneous lupus. However, she also had oval-shaped hyperpigmented patches over the left breast, clavicle, and anterior chest consistent with a generalized FDE (Figure 2). The patient did not recall a history of similar lesions and could not identify a possible trigger. She was counseled on possible culprits and advised to avoid unnecessary medications. She had an unremarkable clinical course; therefore, no further intervention was necessary.
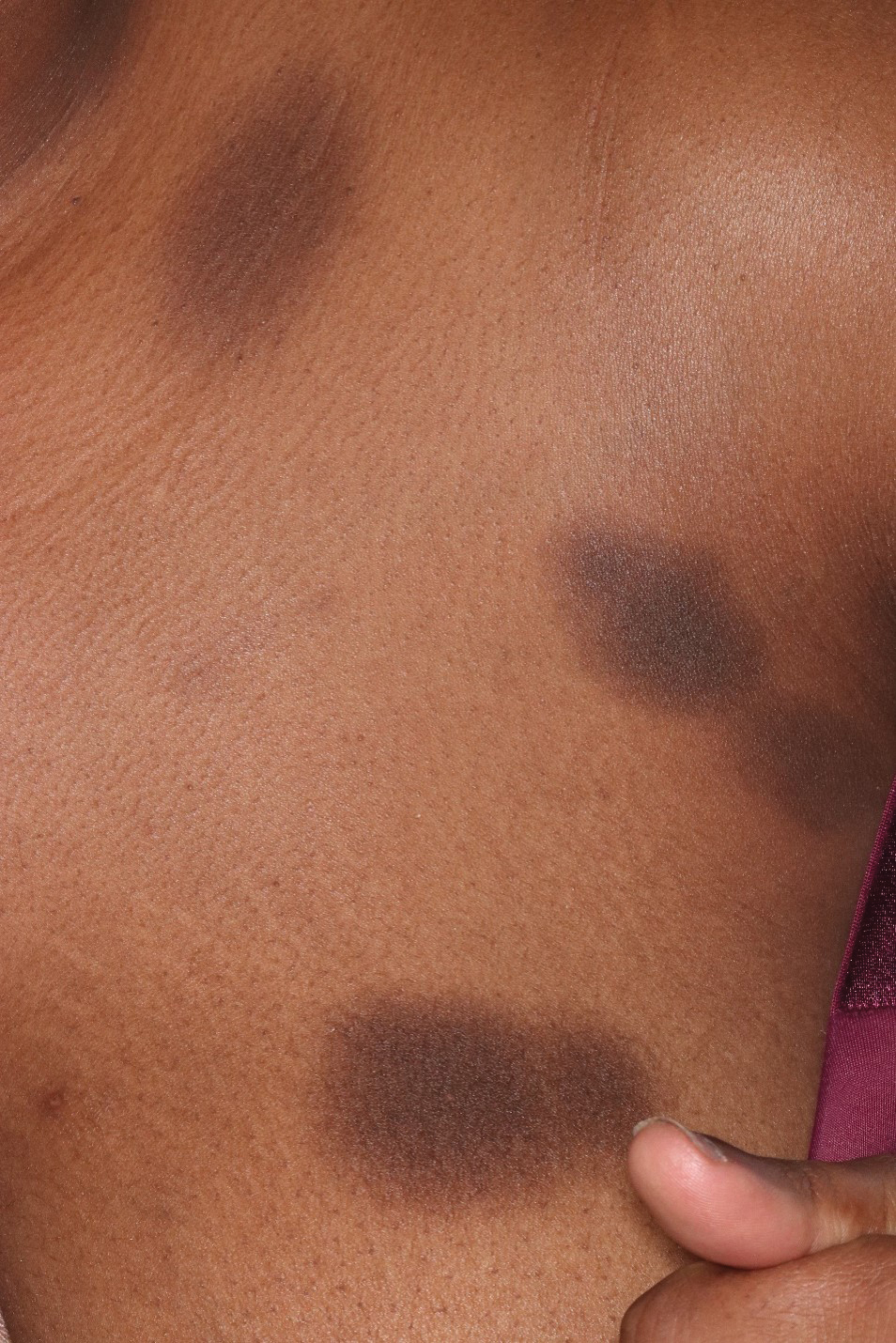
Patient 3—A 33-year-old man presented to the emergency department with a painful rash on the chest and back of 2 days’ duration that began 1 hour after taking naproxen (dosage unknown) for back pain. He had no notable medical history. The patient stated that the rash had slowly worsened and started to develop blisters. He visited an urgent care facility 1 day prior to the current presentation and was started on a 5-day course of prednisone 40 mg daily; the first 2 doses did not help. He denied any mucosal involvement apart from a tender lesion on the penis. He reported a history of an allergic reaction to penicillin. Physical examination revealed extensive dusky violaceous annular plaques with erythematous borders across the anterior and posterior trunk (Figure 3). Multiple flaccid bullae developed within these plaques, involving 15% of the body surface area. He was diagnosed with generalized bullous FDE based on the clinical history and histopathology. He was admitted to the burn intensive care unit and treated with cyclosporine 3 mg/kg/d with subsequent resolution of the eruption.
Comment
Presentation of FDEs—A fixed drug eruption manifests with 1 or more well-demarcated, red or violaceous, annular patches that resolve with postinflammatory hyperpigmentation; it occasionally may manifest with bullae. Initial eruptions may occur up to 2 weeks following medication exposure, but recurrent eruptions usually happen within minutes to hours later. They often are in the same location as prior lesions. A fixed drug eruption can be solitary, scattered, or generalized; a generalized FDE typically demonstrates multiple bilateral lesions that may itch, burn, or cause no symptoms. Patients can experience an FDE at any age, though the median age is reported as 35 to 60 years of age.1 A fixed drug eruption usually occurs after ingestion of oral medications, though there have been a few reports with iodinated contrast.2 Well-known culprits include antibiotics (eg, sulfamethoxazole-trimethoprim, tetracyclines, penicillins/cephalosporins, quinolones, dapsone), nonsteroidal anti-inflammatory drugs, acetaminophen (eg, paracetamol), barbiturates, antimalarials, and anticonvulsants. It also can occur with vaccines or with certain foods (fixed food eruption).3,4 Clinicians may try an oral drug challenge to identify the cause of an FDE, but in patients with a history of a generalized FDE, the risk for developing an increasingly severe reaction with repeated exposure to the medication is too high.5
Histopathology—Patch testing at the site of prior eruption with suspected drug culprits may be useful.6 Histopathology of FDE typically demonstrates vacuolar changes at the dermoepidermal junction with a lichenoid lymphocytic infiltrate. Early lesions often show a predominance of eosinophils. Subepidermal clefting is a feature of the bullous variant. In an active lesion, there are large numbers of CD8+ T lymphocytes expressing natural killer cell–associated molecules.7 The pathologic mechanism is not well understood, though it has been hypothesized that memory CD8+ cells are maintained in specific regions of the epidermis by IL-15 produced in the microenvironment and are activated upon rechallenge.7Considerations in Generalized Bullous FDE—Generalized FDE is defined in the literature as an FDE with involvement of 3 of 6 body areas: head, neck, trunk, upper limbs, lower limbs, and genital area. It may cover more or less than 10% of the body surface area.8-10 Although an isolated FDE frequently is asymptomatic and may not be cause for alarm, recurring drug eruptions increase the risk for development of generalized bullous FDE. Generalized bullous FDE is a rare subset. It is frequently misdiagnosed, and data on its incidence are uncertain.11 Of note, several pathologies causing bullous lesions may be in the differential diagnosis, including bullous pemphigoid; pemphigus vulgaris; bullous SLE; or bullae from cutaneous lupus, staphylococcal scalded skin syndrome, erythema multiforme, or SJS/TEN.12 When matched for body surface area involvement with SJS/TEN, generalized bullous FDE shares nearly identical mortality rates10; therefore, these patients should be treated with the same level of urgency and admitted to a critical care or burn unit, as they are at serious risk for infection and other complications.13
Clinical history and presentation along with histopathologic findings help to narrow down the differential diagnosis. Clinically, generalized bullous FDE does not affect the surrounding skin and manifests sooner after drug exposure (1–24 hours) with less mucosal involvement than SJS/TEN.9 Additionally, SJS/TEN patients frequently have generalized malaise and/or fever, while generalized bullous FDE patients do not. Finally, patients with generalized bullous FDE may report a history of a cutaneous eruption similar in morphology or in the same location.
Histopathologically, generalized bullous FDE may be similar to FDE with the addition of a subepidermal blister. Generalized bullous FDE patients have greater eosinophil infiltration and dermal melanophages than patients with SJS/TEN.9 Cellular infiltrates in generalized bullous FDE include more dermal CD41 cells, such as Foxp31 regulatory T cells; fewer intraepidermal CD561 cells; and fewer intraepidermal cells with granulysin.9 Occasionally, generalized bullous FDE causes full-thickness necrosis. In those cases, generalized bullous FDE cannot reliably be distinguished from other conditions with epidermal necrolysis on histopathology.13
FDE Diagnostics—A cytotoxin produced by
Management—Avoidance of the inciting drug often is sufficient for patients with an FDE, as demonstrated in patient 2 in our case series. Clinicians also should counsel patients on avoidance of potential cross-reacting drugs. Symptomatic treatment for itch or pain is appropriate and may include antihistamines or topical steroids. Nonsteroidal anti-inflammatory drugs may exacerbate or be causative of FDE. For generalized bullous FDE, cyclosporine is favored in the literature15,16 and was used to successfully treat both patients 1 and 3 in our case series. A short course of systemic corticosteroids or intravenous immunoglobulin also may be considered. Mild cases of generalized bullous FDE may be treated with close outpatient follow-up (patient 1), while severe cases require inpatient or even critical care monitoring with aggressive medical management to prevent the progression of skin desquamation (patient 3). Patients with severe oral lesions may require inpatient support for fluid maintenance.
Lupus History—Two patients in our case series had a history of lupus. Lupus itself can cause primary bullous lesions. Similar to FDE, bullous SLE can involve sun-exposed and nonexposed areas of the skin as well as the mucous membranes with a predilection for the lower vermilion lip.17 In bullous SLE, tense subepidermal blisters with a neutrophil-rich infiltrate form due to circulating antibodies to type VII collagen. These blisters have an erythematous or urticated base, most commonly on the face, upper trunk, and proximal extremities.18 In both SLE with skin manifestations and lupus limited to the skin, bullae may form due to extensive vacuolar degeneration. Similar to TEN, they can form rapidly in a widespread distribution.17 However, there is limited mucosal involvement, no clear drug association, and a better prognosis. Bullae caused by lupus will frequently demonstrate deposition of immunoproteins IgG, IgM, IgA, and complement component 3 at the basement membrane zone in perilesional skin on direct immunofluorescence. However, negative direct immunofluorescence does not rule out lupus.12 At the same time, patients with lupus frequently have comorbidities requiring multiple medications; the need for these medications may predispose patients to higher rates of cutaneous drug eruptions.19 To our knowledge, there is no known association between FDE and lupus.
Conclusion
Patients with acute eruptions following the initiation of a new prescription or over-the-counter medication require urgent evaluation. Generalized bullous FDE requires timely diagnosis and intervention. Patients with lupus have an increased risk for cutaneous drug eruptions due to polypharmacy. Further investigation is necessary to determine if there is a pathophysiologic mechanism responsible for the development of FDE in lupus patients.
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925.
- Gavin M, Sharp L, Walker K, et al. Contrast-induced generalized bullous fixed drug eruption resembling Stevens-Johnson syndrome. Proc (Bayl Univ Med Cent). 2019;32:601-602.
- Kabir S, Feit EJ, Heilman ER. Generalized fixed drug eruption following Pfizer-BioNtech COVID-19 vaccination. Clin Case Rep. 2022;10:E6684.
- Choi S, Kim SH, Hwang JH, et al. Rapidly progressing generalized bullous fixed drug eruption after the first dose of COVID-19 messenger RNA vaccination. J Dermatol. 2023;50:1190-1193.
- Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998;37:833-838.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
- Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol. 2008;158:1230-1238.
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15.
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ranario JS, Smith JL. Bullous lesions in a patient with systemic lupus erythematosus. J Clin Aesthet Dermatol. 2014;7:44-49.
- Perron E, Viarnaud A, Marciano L, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol. 2021;31:372-380.
- Chen CB, Kuo KL, Wang CW, et al. Detecting lesional granulysin levels for rapid diagnosis of cytotoxic T lymphocyte-mediated bullous skin disorders. J Allergy Clin Immunol Pract. 2021;9:1327-1337.e3.
- Beniwal R, Gupta LK, Khare AK, et al. Cyclosporine in generalized bullous-fixed drug eruption. Indian J Dermatol. 2018;63:432-433.
- Vargas Mora P, García S, Valenzuela F, et al. Generalized bullous fixed drug eruption successfully treated with cyclosporine. Dermatol Ther. 2020;33:E13492.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Zonzits E, Aberer W, Tappeiner G. Drug eruptions from mesna. After cyclophosphamide treatment of patients with systemic lupus erythematosus and dermatomyositis. Arch Dermatol. 1992;128:80-82.
Recognizing cutaneous drug eruptions is important for treatment and prevention of recurrence. Fixed drug eruptions (FDEs) typically are harmless but can have major negative cosmetic consequences for patients. In its more severe forms, patients are at risk for widespread epithelial necrosis with accompanying complications. We report 1 patient with generalized FDE and 2 with generalized bullous FDE. We also discuss the recognition and treatment of the condition. Two patients previously had been diagnosed with systemic lupus erythematosus (SLE).
Case Series
Patient 1—A 60-year-old woman presented to dermatology with a rash on the trunk and groin folds of 4 days’ duration. She had a history of SLE and cutaneous lupus treated with hydroxychloroquine 200 mg twice daily and topical corticosteroids. She had started sulfamethoxazole-trimethoprim for a urinary tract infection with a rash appearing 1 day later. She reported burning skin pain with progression to blisters that “sloughed” off. She denied any known history of allergy to sulfa drugs. Prior to evaluation by dermatology, she visited an urgent care facility and was prescribed hydroxyzine and intramuscular corticosteroids. At presentation to dermatology 3 days after taking sulfamethoxazole-trimethoprim, she had annular flaccid bullae and superficial erosions with dusky borders on the right posterior thigh, right side of the chest, left inframammary fold, and right inguinal fold (Figure 1). She had no ocular, oral, or vaginal erosions. A diagnosis of generalized bullous FDE was favored over erythema multiforme or Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). Shave biopsies from lesions on the right posterior thigh and right inguinal fold demonstrated interface dermatitis with epidermal necrosis, pigment incontinence, and numerous eosinophils. Direct immunofluorescence of the perilesional skin was negative for immunoprotein deposition. These findings were consistent with the clinical impression of generalized bullous FDE. Prior to receiving the histopathology report, the patient was initiated on a regimen of cyclosporine 5 mg/kg/d in the setting of normal renal function and followed until the eruption resolved completely. Cyclosporine was tapered at 2 weeks and discontinued at 3 weeks.

Patient 2—A 32-year-old woman presented for follow-up management of discoid lupus erythematosus. She had a history of systemic and cutaneous lupus, juvenile rheumatoid arthritis, and mixed connective tissue disease managed with prednisone, hydroxychloroquine, azathioprine, and belimumab. Physical examination revealed scarring alopecia with dyspigmentation and active inflammation consistent with uncontrolled cutaneous lupus. However, she also had oval-shaped hyperpigmented patches over the left breast, clavicle, and anterior chest consistent with a generalized FDE (Figure 2). The patient did not recall a history of similar lesions and could not identify a possible trigger. She was counseled on possible culprits and advised to avoid unnecessary medications. She had an unremarkable clinical course; therefore, no further intervention was necessary.

Patient 3—A 33-year-old man presented to the emergency department with a painful rash on the chest and back of 2 days’ duration that began 1 hour after taking naproxen (dosage unknown) for back pain. He had no notable medical history. The patient stated that the rash had slowly worsened and started to develop blisters. He visited an urgent care facility 1 day prior to the current presentation and was started on a 5-day course of prednisone 40 mg daily; the first 2 doses did not help. He denied any mucosal involvement apart from a tender lesion on the penis. He reported a history of an allergic reaction to penicillin. Physical examination revealed extensive dusky violaceous annular plaques with erythematous borders across the anterior and posterior trunk (Figure 3). Multiple flaccid bullae developed within these plaques, involving 15% of the body surface area. He was diagnosed with generalized bullous FDE based on the clinical history and histopathology. He was admitted to the burn intensive care unit and treated with cyclosporine 3 mg/kg/d with subsequent resolution of the eruption.

Comment
Presentation of FDEs—A fixed drug eruption manifests with 1 or more well-demarcated, red or violaceous, annular patches that resolve with postinflammatory hyperpigmentation; it occasionally may manifest with bullae. Initial eruptions may occur up to 2 weeks following medication exposure, but recurrent eruptions usually happen within minutes to hours later. They often are in the same location as prior lesions. A fixed drug eruption can be solitary, scattered, or generalized; a generalized FDE typically demonstrates multiple bilateral lesions that may itch, burn, or cause no symptoms. Patients can experience an FDE at any age, though the median age is reported as 35 to 60 years of age.1 A fixed drug eruption usually occurs after ingestion of oral medications, though there have been a few reports with iodinated contrast.2 Well-known culprits include antibiotics (eg, sulfamethoxazole-trimethoprim, tetracyclines, penicillins/cephalosporins, quinolones, dapsone), nonsteroidal anti-inflammatory drugs, acetaminophen (eg, paracetamol), barbiturates, antimalarials, and anticonvulsants. It also can occur with vaccines or with certain foods (fixed food eruption).3,4 Clinicians may try an oral drug challenge to identify the cause of an FDE, but in patients with a history of a generalized FDE, the risk for developing an increasingly severe reaction with repeated exposure to the medication is too high.5
Histopathology—Patch testing at the site of prior eruption with suspected drug culprits may be useful.6 Histopathology of FDE typically demonstrates vacuolar changes at the dermoepidermal junction with a lichenoid lymphocytic infiltrate. Early lesions often show a predominance of eosinophils. Subepidermal clefting is a feature of the bullous variant. In an active lesion, there are large numbers of CD8+ T lymphocytes expressing natural killer cell–associated molecules.7 The pathologic mechanism is not well understood, though it has been hypothesized that memory CD8+ cells are maintained in specific regions of the epidermis by IL-15 produced in the microenvironment and are activated upon rechallenge.7Considerations in Generalized Bullous FDE—Generalized FDE is defined in the literature as an FDE with involvement of 3 of 6 body areas: head, neck, trunk, upper limbs, lower limbs, and genital area. It may cover more or less than 10% of the body surface area.8-10 Although an isolated FDE frequently is asymptomatic and may not be cause for alarm, recurring drug eruptions increase the risk for development of generalized bullous FDE. Generalized bullous FDE is a rare subset. It is frequently misdiagnosed, and data on its incidence are uncertain.11 Of note, several pathologies causing bullous lesions may be in the differential diagnosis, including bullous pemphigoid; pemphigus vulgaris; bullous SLE; or bullae from cutaneous lupus, staphylococcal scalded skin syndrome, erythema multiforme, or SJS/TEN.12 When matched for body surface area involvement with SJS/TEN, generalized bullous FDE shares nearly identical mortality rates10; therefore, these patients should be treated with the same level of urgency and admitted to a critical care or burn unit, as they are at serious risk for infection and other complications.13
Clinical history and presentation along with histopathologic findings help to narrow down the differential diagnosis. Clinically, generalized bullous FDE does not affect the surrounding skin and manifests sooner after drug exposure (1–24 hours) with less mucosal involvement than SJS/TEN.9 Additionally, SJS/TEN patients frequently have generalized malaise and/or fever, while generalized bullous FDE patients do not. Finally, patients with generalized bullous FDE may report a history of a cutaneous eruption similar in morphology or in the same location.
Histopathologically, generalized bullous FDE may be similar to FDE with the addition of a subepidermal blister. Generalized bullous FDE patients have greater eosinophil infiltration and dermal melanophages than patients with SJS/TEN.9 Cellular infiltrates in generalized bullous FDE include more dermal CD41 cells, such as Foxp31 regulatory T cells; fewer intraepidermal CD561 cells; and fewer intraepidermal cells with granulysin.9 Occasionally, generalized bullous FDE causes full-thickness necrosis. In those cases, generalized bullous FDE cannot reliably be distinguished from other conditions with epidermal necrolysis on histopathology.13
FDE Diagnostics—A cytotoxin produced by
Management—Avoidance of the inciting drug often is sufficient for patients with an FDE, as demonstrated in patient 2 in our case series. Clinicians also should counsel patients on avoidance of potential cross-reacting drugs. Symptomatic treatment for itch or pain is appropriate and may include antihistamines or topical steroids. Nonsteroidal anti-inflammatory drugs may exacerbate or be causative of FDE. For generalized bullous FDE, cyclosporine is favored in the literature15,16 and was used to successfully treat both patients 1 and 3 in our case series. A short course of systemic corticosteroids or intravenous immunoglobulin also may be considered. Mild cases of generalized bullous FDE may be treated with close outpatient follow-up (patient 1), while severe cases require inpatient or even critical care monitoring with aggressive medical management to prevent the progression of skin desquamation (patient 3). Patients with severe oral lesions may require inpatient support for fluid maintenance.
Lupus History—Two patients in our case series had a history of lupus. Lupus itself can cause primary bullous lesions. Similar to FDE, bullous SLE can involve sun-exposed and nonexposed areas of the skin as well as the mucous membranes with a predilection for the lower vermilion lip.17 In bullous SLE, tense subepidermal blisters with a neutrophil-rich infiltrate form due to circulating antibodies to type VII collagen. These blisters have an erythematous or urticated base, most commonly on the face, upper trunk, and proximal extremities.18 In both SLE with skin manifestations and lupus limited to the skin, bullae may form due to extensive vacuolar degeneration. Similar to TEN, they can form rapidly in a widespread distribution.17 However, there is limited mucosal involvement, no clear drug association, and a better prognosis. Bullae caused by lupus will frequently demonstrate deposition of immunoproteins IgG, IgM, IgA, and complement component 3 at the basement membrane zone in perilesional skin on direct immunofluorescence. However, negative direct immunofluorescence does not rule out lupus.12 At the same time, patients with lupus frequently have comorbidities requiring multiple medications; the need for these medications may predispose patients to higher rates of cutaneous drug eruptions.19 To our knowledge, there is no known association between FDE and lupus.
Conclusion
Patients with acute eruptions following the initiation of a new prescription or over-the-counter medication require urgent evaluation. Generalized bullous FDE requires timely diagnosis and intervention. Patients with lupus have an increased risk for cutaneous drug eruptions due to polypharmacy. Further investigation is necessary to determine if there is a pathophysiologic mechanism responsible for the development of FDE in lupus patients.
Recognizing cutaneous drug eruptions is important for treatment and prevention of recurrence. Fixed drug eruptions (FDEs) typically are harmless but can have major negative cosmetic consequences for patients. In its more severe forms, patients are at risk for widespread epithelial necrosis with accompanying complications. We report 1 patient with generalized FDE and 2 with generalized bullous FDE. We also discuss the recognition and treatment of the condition. Two patients previously had been diagnosed with systemic lupus erythematosus (SLE).
Case Series
Patient 1—A 60-year-old woman presented to dermatology with a rash on the trunk and groin folds of 4 days’ duration. She had a history of SLE and cutaneous lupus treated with hydroxychloroquine 200 mg twice daily and topical corticosteroids. She had started sulfamethoxazole-trimethoprim for a urinary tract infection with a rash appearing 1 day later. She reported burning skin pain with progression to blisters that “sloughed” off. She denied any known history of allergy to sulfa drugs. Prior to evaluation by dermatology, she visited an urgent care facility and was prescribed hydroxyzine and intramuscular corticosteroids. At presentation to dermatology 3 days after taking sulfamethoxazole-trimethoprim, she had annular flaccid bullae and superficial erosions with dusky borders on the right posterior thigh, right side of the chest, left inframammary fold, and right inguinal fold (Figure 1). She had no ocular, oral, or vaginal erosions. A diagnosis of generalized bullous FDE was favored over erythema multiforme or Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). Shave biopsies from lesions on the right posterior thigh and right inguinal fold demonstrated interface dermatitis with epidermal necrosis, pigment incontinence, and numerous eosinophils. Direct immunofluorescence of the perilesional skin was negative for immunoprotein deposition. These findings were consistent with the clinical impression of generalized bullous FDE. Prior to receiving the histopathology report, the patient was initiated on a regimen of cyclosporine 5 mg/kg/d in the setting of normal renal function and followed until the eruption resolved completely. Cyclosporine was tapered at 2 weeks and discontinued at 3 weeks.

Patient 2—A 32-year-old woman presented for follow-up management of discoid lupus erythematosus. She had a history of systemic and cutaneous lupus, juvenile rheumatoid arthritis, and mixed connective tissue disease managed with prednisone, hydroxychloroquine, azathioprine, and belimumab. Physical examination revealed scarring alopecia with dyspigmentation and active inflammation consistent with uncontrolled cutaneous lupus. However, she also had oval-shaped hyperpigmented patches over the left breast, clavicle, and anterior chest consistent with a generalized FDE (Figure 2). The patient did not recall a history of similar lesions and could not identify a possible trigger. She was counseled on possible culprits and advised to avoid unnecessary medications. She had an unremarkable clinical course; therefore, no further intervention was necessary.

Patient 3—A 33-year-old man presented to the emergency department with a painful rash on the chest and back of 2 days’ duration that began 1 hour after taking naproxen (dosage unknown) for back pain. He had no notable medical history. The patient stated that the rash had slowly worsened and started to develop blisters. He visited an urgent care facility 1 day prior to the current presentation and was started on a 5-day course of prednisone 40 mg daily; the first 2 doses did not help. He denied any mucosal involvement apart from a tender lesion on the penis. He reported a history of an allergic reaction to penicillin. Physical examination revealed extensive dusky violaceous annular plaques with erythematous borders across the anterior and posterior trunk (Figure 3). Multiple flaccid bullae developed within these plaques, involving 15% of the body surface area. He was diagnosed with generalized bullous FDE based on the clinical history and histopathology. He was admitted to the burn intensive care unit and treated with cyclosporine 3 mg/kg/d with subsequent resolution of the eruption.

Comment
Presentation of FDEs—A fixed drug eruption manifests with 1 or more well-demarcated, red or violaceous, annular patches that resolve with postinflammatory hyperpigmentation; it occasionally may manifest with bullae. Initial eruptions may occur up to 2 weeks following medication exposure, but recurrent eruptions usually happen within minutes to hours later. They often are in the same location as prior lesions. A fixed drug eruption can be solitary, scattered, or generalized; a generalized FDE typically demonstrates multiple bilateral lesions that may itch, burn, or cause no symptoms. Patients can experience an FDE at any age, though the median age is reported as 35 to 60 years of age.1 A fixed drug eruption usually occurs after ingestion of oral medications, though there have been a few reports with iodinated contrast.2 Well-known culprits include antibiotics (eg, sulfamethoxazole-trimethoprim, tetracyclines, penicillins/cephalosporins, quinolones, dapsone), nonsteroidal anti-inflammatory drugs, acetaminophen (eg, paracetamol), barbiturates, antimalarials, and anticonvulsants. It also can occur with vaccines or with certain foods (fixed food eruption).3,4 Clinicians may try an oral drug challenge to identify the cause of an FDE, but in patients with a history of a generalized FDE, the risk for developing an increasingly severe reaction with repeated exposure to the medication is too high.5
Histopathology—Patch testing at the site of prior eruption with suspected drug culprits may be useful.6 Histopathology of FDE typically demonstrates vacuolar changes at the dermoepidermal junction with a lichenoid lymphocytic infiltrate. Early lesions often show a predominance of eosinophils. Subepidermal clefting is a feature of the bullous variant. In an active lesion, there are large numbers of CD8+ T lymphocytes expressing natural killer cell–associated molecules.7 The pathologic mechanism is not well understood, though it has been hypothesized that memory CD8+ cells are maintained in specific regions of the epidermis by IL-15 produced in the microenvironment and are activated upon rechallenge.7Considerations in Generalized Bullous FDE—Generalized FDE is defined in the literature as an FDE with involvement of 3 of 6 body areas: head, neck, trunk, upper limbs, lower limbs, and genital area. It may cover more or less than 10% of the body surface area.8-10 Although an isolated FDE frequently is asymptomatic and may not be cause for alarm, recurring drug eruptions increase the risk for development of generalized bullous FDE. Generalized bullous FDE is a rare subset. It is frequently misdiagnosed, and data on its incidence are uncertain.11 Of note, several pathologies causing bullous lesions may be in the differential diagnosis, including bullous pemphigoid; pemphigus vulgaris; bullous SLE; or bullae from cutaneous lupus, staphylococcal scalded skin syndrome, erythema multiforme, or SJS/TEN.12 When matched for body surface area involvement with SJS/TEN, generalized bullous FDE shares nearly identical mortality rates10; therefore, these patients should be treated with the same level of urgency and admitted to a critical care or burn unit, as they are at serious risk for infection and other complications.13
Clinical history and presentation along with histopathologic findings help to narrow down the differential diagnosis. Clinically, generalized bullous FDE does not affect the surrounding skin and manifests sooner after drug exposure (1–24 hours) with less mucosal involvement than SJS/TEN.9 Additionally, SJS/TEN patients frequently have generalized malaise and/or fever, while generalized bullous FDE patients do not. Finally, patients with generalized bullous FDE may report a history of a cutaneous eruption similar in morphology or in the same location.
Histopathologically, generalized bullous FDE may be similar to FDE with the addition of a subepidermal blister. Generalized bullous FDE patients have greater eosinophil infiltration and dermal melanophages than patients with SJS/TEN.9 Cellular infiltrates in generalized bullous FDE include more dermal CD41 cells, such as Foxp31 regulatory T cells; fewer intraepidermal CD561 cells; and fewer intraepidermal cells with granulysin.9 Occasionally, generalized bullous FDE causes full-thickness necrosis. In those cases, generalized bullous FDE cannot reliably be distinguished from other conditions with epidermal necrolysis on histopathology.13
FDE Diagnostics—A cytotoxin produced by
Management—Avoidance of the inciting drug often is sufficient for patients with an FDE, as demonstrated in patient 2 in our case series. Clinicians also should counsel patients on avoidance of potential cross-reacting drugs. Symptomatic treatment for itch or pain is appropriate and may include antihistamines or topical steroids. Nonsteroidal anti-inflammatory drugs may exacerbate or be causative of FDE. For generalized bullous FDE, cyclosporine is favored in the literature15,16 and was used to successfully treat both patients 1 and 3 in our case series. A short course of systemic corticosteroids or intravenous immunoglobulin also may be considered. Mild cases of generalized bullous FDE may be treated with close outpatient follow-up (patient 1), while severe cases require inpatient or even critical care monitoring with aggressive medical management to prevent the progression of skin desquamation (patient 3). Patients with severe oral lesions may require inpatient support for fluid maintenance.
Lupus History—Two patients in our case series had a history of lupus. Lupus itself can cause primary bullous lesions. Similar to FDE, bullous SLE can involve sun-exposed and nonexposed areas of the skin as well as the mucous membranes with a predilection for the lower vermilion lip.17 In bullous SLE, tense subepidermal blisters with a neutrophil-rich infiltrate form due to circulating antibodies to type VII collagen. These blisters have an erythematous or urticated base, most commonly on the face, upper trunk, and proximal extremities.18 In both SLE with skin manifestations and lupus limited to the skin, bullae may form due to extensive vacuolar degeneration. Similar to TEN, they can form rapidly in a widespread distribution.17 However, there is limited mucosal involvement, no clear drug association, and a better prognosis. Bullae caused by lupus will frequently demonstrate deposition of immunoproteins IgG, IgM, IgA, and complement component 3 at the basement membrane zone in perilesional skin on direct immunofluorescence. However, negative direct immunofluorescence does not rule out lupus.12 At the same time, patients with lupus frequently have comorbidities requiring multiple medications; the need for these medications may predispose patients to higher rates of cutaneous drug eruptions.19 To our knowledge, there is no known association between FDE and lupus.
Conclusion
Patients with acute eruptions following the initiation of a new prescription or over-the-counter medication require urgent evaluation. Generalized bullous FDE requires timely diagnosis and intervention. Patients with lupus have an increased risk for cutaneous drug eruptions due to polypharmacy. Further investigation is necessary to determine if there is a pathophysiologic mechanism responsible for the development of FDE in lupus patients.
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925.
- Gavin M, Sharp L, Walker K, et al. Contrast-induced generalized bullous fixed drug eruption resembling Stevens-Johnson syndrome. Proc (Bayl Univ Med Cent). 2019;32:601-602.
- Kabir S, Feit EJ, Heilman ER. Generalized fixed drug eruption following Pfizer-BioNtech COVID-19 vaccination. Clin Case Rep. 2022;10:E6684.
- Choi S, Kim SH, Hwang JH, et al. Rapidly progressing generalized bullous fixed drug eruption after the first dose of COVID-19 messenger RNA vaccination. J Dermatol. 2023;50:1190-1193.
- Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998;37:833-838.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
- Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol. 2008;158:1230-1238.
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15.
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ranario JS, Smith JL. Bullous lesions in a patient with systemic lupus erythematosus. J Clin Aesthet Dermatol. 2014;7:44-49.
- Perron E, Viarnaud A, Marciano L, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol. 2021;31:372-380.
- Chen CB, Kuo KL, Wang CW, et al. Detecting lesional granulysin levels for rapid diagnosis of cytotoxic T lymphocyte-mediated bullous skin disorders. J Allergy Clin Immunol Pract. 2021;9:1327-1337.e3.
- Beniwal R, Gupta LK, Khare AK, et al. Cyclosporine in generalized bullous-fixed drug eruption. Indian J Dermatol. 2018;63:432-433.
- Vargas Mora P, García S, Valenzuela F, et al. Generalized bullous fixed drug eruption successfully treated with cyclosporine. Dermatol Ther. 2020;33:E13492.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Zonzits E, Aberer W, Tappeiner G. Drug eruptions from mesna. After cyclophosphamide treatment of patients with systemic lupus erythematosus and dermatomyositis. Arch Dermatol. 1992;128:80-82.
- Anderson HJ, Lee JB. A review of fixed drug eruption with a special focus on generalized bullous fixed drug eruption. Medicina (Kaunas). 2021;57:925.
- Gavin M, Sharp L, Walker K, et al. Contrast-induced generalized bullous fixed drug eruption resembling Stevens-Johnson syndrome. Proc (Bayl Univ Med Cent). 2019;32:601-602.
- Kabir S, Feit EJ, Heilman ER. Generalized fixed drug eruption following Pfizer-BioNtech COVID-19 vaccination. Clin Case Rep. 2022;10:E6684.
- Choi S, Kim SH, Hwang JH, et al. Rapidly progressing generalized bullous fixed drug eruption after the first dose of COVID-19 messenger RNA vaccination. J Dermatol. 2023;50:1190-1193.
- Mahboob A, Haroon TS. Drugs causing fixed eruptions: a study of 450 cases. Int J Dermatol. 1998;37:833-838.
- Shiohara T. Fixed drug eruption: pathogenesis and diagnostic tests. Curr Opin Allergy Clin Immunol. 2009;9:316-321.
- Mizukawa Y, Yamazaki Y, Shiohara T. In vivo dynamics of intraepidermal CD8+ T cells and CD4+ T cells during the evolution of fixed drug eruption. Br J Dermatol. 2008;158:1230-1238.
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15.
- Cho YT, Lin JW, Chen YC, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ranario JS, Smith JL. Bullous lesions in a patient with systemic lupus erythematosus. J Clin Aesthet Dermatol. 2014;7:44-49.
- Perron E, Viarnaud A, Marciano L, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol. 2021;31:372-380.
- Chen CB, Kuo KL, Wang CW, et al. Detecting lesional granulysin levels for rapid diagnosis of cytotoxic T lymphocyte-mediated bullous skin disorders. J Allergy Clin Immunol Pract. 2021;9:1327-1337.e3.
- Beniwal R, Gupta LK, Khare AK, et al. Cyclosporine in generalized bullous-fixed drug eruption. Indian J Dermatol. 2018;63:432-433.
- Vargas Mora P, García S, Valenzuela F, et al. Generalized bullous fixed drug eruption successfully treated with cyclosporine. Dermatol Ther. 2020;33:E13492.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Zonzits E, Aberer W, Tappeiner G. Drug eruptions from mesna. After cyclophosphamide treatment of patients with systemic lupus erythematosus and dermatomyositis. Arch Dermatol. 1992;128:80-82.
Practice Points
- Although localized fixed drug eruption (FDE) is a relatively benign diagnosis, generalized bullous FDE requires urgent management and may necessitate intensive burn care.
- Patients with lupus are at increased risk for drug eruptions due to polypharmacy, and there is a wide differential for bullous eruptions in these patients.
Mycobacterium interjectum Infection in an Immunocompetent Host Following Contact With Aquarium Fish
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.
The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.
The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.
The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
Practice Points
- Mycobacterium interjectum can cause cutaneous nodules in a sporotrichoid or lymphocutaneous pattern and may affect immunocompromised and immunocompetent patients.
- This mycobacteria has been detected in water, soil, and aquarium fish. The latter could be a source of infection and should be taken into account in the anamnesis.
- There is no established therapeutic regimen for M interjectum infection. Combination therapy with rifampicin, clarithromycin, and co-trimoxazole could be an option, though it must always be adapted to an antibiogram if results are available.
Dermatoporosis in Older Adults: A Condition That Requires Holistic, Creative Management
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
WASHINGTON — and conveys the skin’s vulnerability to serious medical complications, said Adam Friedman, MD, at the ElderDerm conference on dermatology in the older patient.
Key features of dermatoporosis include atrophic skin, solar purpura, white pseudoscars, easily acquired skin lacerations and tears, bruises, and delayed healing. “We’re going to see more of this, and it will more and more be a chief complaint of patients,” said Dr. Friedman, professor and chair of dermatology at George Washington University (GWU) in Washington, and co-chair of the meeting. GWU hosted the conference, describing it as a first-of-its-kind meeting dedicated to improving dermatologic care for older adults.
Dermatoporosis was described in the literature in 2007 by dermatologists at the University of Geneva in Switzerland. “It is not only a cosmetic problem,” Dr. Friedman said. “This is a medical problem ... which can absolutely lead to comorbidities [such as deep dissecting hematomas] that are a huge strain on the healthcare system.”
Dermatologists can meet the moment with holistic, creative combination treatment and counseling approaches aimed at improving the mechanical strength of skin and preventing potential complications in older patients, Dr. Friedman said at the meeting.
He described the case of a 76-year-old woman who presented with dermatoporosis on her arms involving pronounced skin atrophy, solar purpura, and a small covered laceration. “This was a patient who was both devastated by the appearance” and impacted by the pain and burden of dressing frequent wounds, said Dr. Friedman, who is also the director of the Residency Program, of Translational Research, and of Supportive Oncodermatology, all within the Department of Dermatology at GWU.
With 11 months of topical treatment that included daily application of calcipotriene 0.05% ointment and nightly application of tazarotene 0.045% lotion and oral supplementation with 1000-mg vitamin C twice daily and 1000-mg citrus bioflavonoid complex daily, as well as no changes to the medications she took for various comorbidities, the solar purpura improved significantly and “we made a huge difference in the integrity of her skin,” he said.
Dr. Friedman also described this case in a recently published article in the Journal of Drugs in Dermatology titled “What’s Old Is New: An Emerging Focus on Dermatoporosis”.
Likely Pathophysiology
Advancing age and chronic ultraviolet (UV) radiation exposure are the chief drivers of dermatoporosis. In addition to UVA and UVB light, other secondary drivers include genetic susceptibility, topical and systematic corticosteroid use, and anticoagulant treatment.
Its pathogenesis is not well described in the literature but is easy to envision, Dr. Friedman said. For one, both advancing age and exposure to UV light lead to a reduction in hygroscopic glycosaminoglycans, including hyaluronate (HA), and the impact of this diminishment is believed to go “beyond [the loss of] buoyancy,” he noted. Researchers have “been showing these are not just water-loving molecules, they also have some biologic properties” relating to keratinocyte production and epidermal turnover that appear to be intricately linked to the pathogenesis of dermatoporosis.
HAs have been shown to interact with the cell surface receptor CD44 to stimulate keratinocyte proliferation, and low levels of CD44 have been reported in skin with dermatoporosis compared with a younger control population. (A newly characterized organelle, the hyaluronosome, serves as an HA factory and contains CD44 and heparin-binding epidermal growth factor, Dr. Friedman noted. Inadequate functioning may be involved in skin atrophy.)
Advancing age also brings an increase in matrix metalloproteinases (MMPs)–1, –2, and –3, which are “the demolition workers of the skin,” and downregulation of a tissue inhibitor of MMPs, he said.
Adding insult to injury, dermis-penetrating UVA also activates MMPs, “obliterating collagen and elastin.” UVB generates DNA photoproducts, including oxidative stress and damaging skin cell DNA. “That UV light induces breakdown [of the skin] through different mechanisms and inhibits buildup is a simple concept I think our patients can understand,” Dr. Friedman said.
Multifaceted Treatment
For an older adult, “there is never a wrong time to start sun-protective measures” to prevent or try to halt the progression of dermatoporosis, Dr. Friedman said, noting that “UV radiation is an immunosuppressant, so there are many good reasons to start” if the adult is not already taking measures on a regular basis.
Potential treatments for the syndrome of dermatoporosis are backed by few clinical studies, but dermatologists are skilled at translating the use of products from one disease state to another based on understandings of pathophysiology and mechanistic pathways, Dr. Friedman commented in an interview after the meeting.
For instance, “from decades of research, we know what retinoids will do to the skin,” he said in the interview. “We know they will turn on collagen-1 and -3 genes in the skin, and that they will increase the production of glycosaminoglycans ... By understanding the biology, we can translate this to dermatoporosis.” These changes were demonstrated, for instance, in a small study of topical retinol in older adults.
Studies of topical alpha hydroxy acid (AHA), moreover, have demonstrated epidermal thickening and firmness, and “some studies show they can limit steroid-induced atrophy,” Dr. Friedman said at the meeting. “And things like lactic acid and urea are super accessible.”
Topical dehydroepiandrosterone is backed by even less data than retinoids or AHAs are, “but it’s still something to consider” as part of a multimechanistic approach to dermatoporosis, Dr. Friedman shared, noting that a small study demonstrated beneficial effects on epidermal atrophy in aging skin.
The use of vitamin D analogues such as calcipotriene, which is approved for the treatment of psoriasis, may also be promising. “One concept is that [vitamin D analogues] increase calcium concentrations in the epidermis, and calcium is so central to keratinocyte differentiation” and epidermal function that calcipotriene in combination with topical steroid therapy has been shown to limit skin atrophy, he noted.
Nutritionally, low protein intake is a known problem in the older population and is associated with increased skin fragility and poorer healing. From a prevention and treatment standpoint, therefore, patients can be counseled to be attentive to their diets, Dr. Friedman said. Experts have recommended a higher protein intake for older adults than for younger adults; in 2013, an international group recommended a protein intake of 1-1.5 g/kg/d for healthy older adults and more for those with acute or chronic illness.
“Patients love talking about diet and skin disease ... and they love over-the-counter nutraceuticals as well because they want something natural,” Dr. Friedman said. “I like using bioflavonoids in combination with vitamin C, which can be effective especially for solar purpura.”
A 6-week randomized, placebo-controlled, double-blind trial involving 67 patients with purpura associated with aging found a 50% reduction in purpura lesions among those took a particular citrus bioflavonoid blend twice daily. “I thought this was a pretty well-done study,” he said, noting that patient self-assessment and investigator global assessment were utilized.
Skin Injury and Wound Prevention
In addition to recommending gentle skin cleansers and daily moisturizing, dermatologists should talk to their older patients with dermatoporosis about their home environments. “What is it like? Is there furniture with sharp edges?” Dr. Friedman advised. If so, could they use sleeves or protectors on their arms or legs “to protect against injury?”
In a later meeting session about lower-extremity wounds on geriatric patients, Michael Stempel, DPM, assistant professor of medicine and surgery and chief of podiatry at GWU, said that he was happy to hear the term dermatoporosis being used because like diabetes, it’s a risk factor for developing lower-extremity wounds and poor wound healing.
He shared the case of an older woman with dermatoporosis who “tripped and skinned her knee against a step and then self-treated it for over a month by pouring hydrogen peroxide over it and letting air get to it.” The wound developed into “full-thickness tissue loss,” said Dr. Stempel, also medical director of the Wound Healing and Limb Preservation Center at GWU Hospital.
Misperceptions are common among older patients about how a simple wound should be managed; for instance, the adage “just let it get air” is not uncommon. This makes anticipatory guidance about basic wound care — such as the importance of a moist and occlusive environment and the safe use of hydrogen peroxide — especially important for patients with dermatoporosis, Dr. Friedman commented after the meeting.
Dermatoporosis is quantifiable, Dr. Friedman said during the meeting, with a scoring system having been developed by the researchers in Switzerland who originally coined the term. Its use in practice is unnecessary, but its existence is “nice to share with patients who feel bothered because oftentimes, patients feel it’s been dismissed by other providers,” he said. “Telling your patients there’s an actual name for their problem, and that there are ways to quantify and measure changes over time, is validating.”
Its recognition as a medical condition, Dr. Friedman added, also enables the dermatologist to bring it up and counsel appropriately — without a patient feeling shame — when it is identified in the context of a skin excision, treatment of a primary inflammatory skin disease, or management of another dermatologic problem.
Dr. Friedman disclosed that he is a consultant/advisory board member for L’Oréal, La Roche-Posay, Galderma, and other companies; a speaker for Regeneron/Sanofi, Incyte, BMD, and Janssen; and has grants from Pfizer, Lilly, Incyte, and other companies. Dr. Stempel reported no disclosures.
A version of this article first appeared on Medscape.com.
FROM ELDERDERM 2024
It’s the Television, Stupid
As more and more of us begin to feel (or believe we are feeling) the symptoms of aging, our language has begun to incorporate new words and phrases such as “aging in place” or “healthy aging.” In fact, some scientists have created a diagnostic criteria to define “healthy aging.” If you have reached your 70th birthday without mental health issues, memory issues, physical impairments, or chronic disease, according to some researchers at T.H. Chan School of Public Health and Brigham and Women’s Hospital, you should receive a gold star for healthy aging.
I am now nearly a decade past that milestone and can’t remember where I’ve put my gold star, or even if I had ever received one. But, I get up each morning looking forward to another day of activity and feeling “pretty good.”
Healthy aging is not something you start doing when you turn 65. Aging is something that goes on from the moment you are born. For the first couple decades we call it “maturing.” If you have lived well, the odds are you will age well. And, for that reason we should take note of some recent work by Boston-based researchers.
Looking at recent data from 45,000 participants in the well-known Nurses Health Study, the investigators found that for every 2-hour increase in daily sedentary behavior, the participants cut their chances of healthy aging by 12%. On the other hand, for every 2 hours of light physical activity, they increased their odds of healthy aging by 6 %.
There are two important messages sitting just below the surface of these two observations. First, we continue to overemphasize the importance of “exercise” in our attempt to help our patients achieve wellness. The word “exercise” carries with it whole carousel full of baggage including “fitness programs,” gym memberships, pulse rate monitors, pain, sweat, and spandex, to name just a few. Exercise can conjure up bad memories of suiting up for phys ed class, group showers, and being picked last when teams were being chosen.
It turns out the we should simply be promoting activity, and light activity at that — vacuuming the living room, walking around the block, rearranging the books on your bedroom book shelf, making a pot of soup, doing the laundry. Just getting up off one’s behind and doing something instead of being a passive spectator.
This somewhat counterintuitive notion of the benefit of light activity is beginning to get more attention. Earlier this year, I reported on a study by Andre O. Abaje MD, MPH, in which he showed that light physical activity in children was superior to more vigorous activity in lowering lipids.
The more important message embedded in this paper based on the Nurses Health Study is that the researchers used television watching time as their proxy for sedentary behavior. The investigators chose TV viewing because it is ubiquitous and includes prolonged sitting. Being semi-reclined on the couch or in a lounger requires very little muscle activity, which is in turn linked to disruption of glucose metabolism, increased inflammation, and altered blood flow to the brain, to name just a few of its collateral damages. I would add that TV viewing often prompts viewers to stay up well beyond their healthy bedtime. And, we know sleep deprivation is not compatible with health aging.
A traditional warning issued to new retirees was once “Don’t let the old rocking chair get ya.” In fact, I wonder how many folks watching television even have or use wood rocking chairs anymore, which, if rocked, might qualify as a light exercise if the viewer made the effort to rock. Instead I suspect most television viewing is done cocooned in soft recliners or curled up on a couch.
I will admit that this recent paper merely supports a suspicion I have harbored for decades. Like many of you, I have wondered how our society got to the point where obesity is frequent enough to be labeled a disease, attention deficit diagnoses are becoming increasingly prevalent, and our life expectancy is shrinking. There are dozens of factors, but if I had to pick one, I would paraphrase James Carville’s advice to Bill Clinton: “It’s the television, stupid.”
At least a couple of notches above “Are you wearing your seatbelt?” It can start with a nonjudgmental question such as “What are your favorite television shows?” And then deftly move toward compiling a tally of how many hours the patient watches each day.
How you manage the situation from there is up to you and can be based on the patient’s complaints and problem list. You might suggest he or she start by eliminating 2 hours of viewing a day. Then ask if he or she thinks that new schedule is achievable. If they ask for alternatives, be ready with a list of light activities that they might be surprised are healthier than their current behavior. Follow up with another visit or a call to see how they are doing. It’s that important, and your call will underscore your concern.
Sedentism is a serious health problem in this country and our emphasis on encouraging vigorous exercise isn’t working. Selling a television diet will be a tough sell, but it needs to be done.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
As more and more of us begin to feel (or believe we are feeling) the symptoms of aging, our language has begun to incorporate new words and phrases such as “aging in place” or “healthy aging.” In fact, some scientists have created a diagnostic criteria to define “healthy aging.” If you have reached your 70th birthday without mental health issues, memory issues, physical impairments, or chronic disease, according to some researchers at T.H. Chan School of Public Health and Brigham and Women’s Hospital, you should receive a gold star for healthy aging.
I am now nearly a decade past that milestone and can’t remember where I’ve put my gold star, or even if I had ever received one. But, I get up each morning looking forward to another day of activity and feeling “pretty good.”
Healthy aging is not something you start doing when you turn 65. Aging is something that goes on from the moment you are born. For the first couple decades we call it “maturing.” If you have lived well, the odds are you will age well. And, for that reason we should take note of some recent work by Boston-based researchers.
Looking at recent data from 45,000 participants in the well-known Nurses Health Study, the investigators found that for every 2-hour increase in daily sedentary behavior, the participants cut their chances of healthy aging by 12%. On the other hand, for every 2 hours of light physical activity, they increased their odds of healthy aging by 6 %.
There are two important messages sitting just below the surface of these two observations. First, we continue to overemphasize the importance of “exercise” in our attempt to help our patients achieve wellness. The word “exercise” carries with it whole carousel full of baggage including “fitness programs,” gym memberships, pulse rate monitors, pain, sweat, and spandex, to name just a few. Exercise can conjure up bad memories of suiting up for phys ed class, group showers, and being picked last when teams were being chosen.
It turns out the we should simply be promoting activity, and light activity at that — vacuuming the living room, walking around the block, rearranging the books on your bedroom book shelf, making a pot of soup, doing the laundry. Just getting up off one’s behind and doing something instead of being a passive spectator.
This somewhat counterintuitive notion of the benefit of light activity is beginning to get more attention. Earlier this year, I reported on a study by Andre O. Abaje MD, MPH, in which he showed that light physical activity in children was superior to more vigorous activity in lowering lipids.
The more important message embedded in this paper based on the Nurses Health Study is that the researchers used television watching time as their proxy for sedentary behavior. The investigators chose TV viewing because it is ubiquitous and includes prolonged sitting. Being semi-reclined on the couch or in a lounger requires very little muscle activity, which is in turn linked to disruption of glucose metabolism, increased inflammation, and altered blood flow to the brain, to name just a few of its collateral damages. I would add that TV viewing often prompts viewers to stay up well beyond their healthy bedtime. And, we know sleep deprivation is not compatible with health aging.
A traditional warning issued to new retirees was once “Don’t let the old rocking chair get ya.” In fact, I wonder how many folks watching television even have or use wood rocking chairs anymore, which, if rocked, might qualify as a light exercise if the viewer made the effort to rock. Instead I suspect most television viewing is done cocooned in soft recliners or curled up on a couch.
I will admit that this recent paper merely supports a suspicion I have harbored for decades. Like many of you, I have wondered how our society got to the point where obesity is frequent enough to be labeled a disease, attention deficit diagnoses are becoming increasingly prevalent, and our life expectancy is shrinking. There are dozens of factors, but if I had to pick one, I would paraphrase James Carville’s advice to Bill Clinton: “It’s the television, stupid.”
At least a couple of notches above “Are you wearing your seatbelt?” It can start with a nonjudgmental question such as “What are your favorite television shows?” And then deftly move toward compiling a tally of how many hours the patient watches each day.
How you manage the situation from there is up to you and can be based on the patient’s complaints and problem list. You might suggest he or she start by eliminating 2 hours of viewing a day. Then ask if he or she thinks that new schedule is achievable. If they ask for alternatives, be ready with a list of light activities that they might be surprised are healthier than their current behavior. Follow up with another visit or a call to see how they are doing. It’s that important, and your call will underscore your concern.
Sedentism is a serious health problem in this country and our emphasis on encouraging vigorous exercise isn’t working. Selling a television diet will be a tough sell, but it needs to be done.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
As more and more of us begin to feel (or believe we are feeling) the symptoms of aging, our language has begun to incorporate new words and phrases such as “aging in place” or “healthy aging.” In fact, some scientists have created a diagnostic criteria to define “healthy aging.” If you have reached your 70th birthday without mental health issues, memory issues, physical impairments, or chronic disease, according to some researchers at T.H. Chan School of Public Health and Brigham and Women’s Hospital, you should receive a gold star for healthy aging.
I am now nearly a decade past that milestone and can’t remember where I’ve put my gold star, or even if I had ever received one. But, I get up each morning looking forward to another day of activity and feeling “pretty good.”
Healthy aging is not something you start doing when you turn 65. Aging is something that goes on from the moment you are born. For the first couple decades we call it “maturing.” If you have lived well, the odds are you will age well. And, for that reason we should take note of some recent work by Boston-based researchers.
Looking at recent data from 45,000 participants in the well-known Nurses Health Study, the investigators found that for every 2-hour increase in daily sedentary behavior, the participants cut their chances of healthy aging by 12%. On the other hand, for every 2 hours of light physical activity, they increased their odds of healthy aging by 6 %.
There are two important messages sitting just below the surface of these two observations. First, we continue to overemphasize the importance of “exercise” in our attempt to help our patients achieve wellness. The word “exercise” carries with it whole carousel full of baggage including “fitness programs,” gym memberships, pulse rate monitors, pain, sweat, and spandex, to name just a few. Exercise can conjure up bad memories of suiting up for phys ed class, group showers, and being picked last when teams were being chosen.
It turns out the we should simply be promoting activity, and light activity at that — vacuuming the living room, walking around the block, rearranging the books on your bedroom book shelf, making a pot of soup, doing the laundry. Just getting up off one’s behind and doing something instead of being a passive spectator.
This somewhat counterintuitive notion of the benefit of light activity is beginning to get more attention. Earlier this year, I reported on a study by Andre O. Abaje MD, MPH, in which he showed that light physical activity in children was superior to more vigorous activity in lowering lipids.
The more important message embedded in this paper based on the Nurses Health Study is that the researchers used television watching time as their proxy for sedentary behavior. The investigators chose TV viewing because it is ubiquitous and includes prolonged sitting. Being semi-reclined on the couch or in a lounger requires very little muscle activity, which is in turn linked to disruption of glucose metabolism, increased inflammation, and altered blood flow to the brain, to name just a few of its collateral damages. I would add that TV viewing often prompts viewers to stay up well beyond their healthy bedtime. And, we know sleep deprivation is not compatible with health aging.
A traditional warning issued to new retirees was once “Don’t let the old rocking chair get ya.” In fact, I wonder how many folks watching television even have or use wood rocking chairs anymore, which, if rocked, might qualify as a light exercise if the viewer made the effort to rock. Instead I suspect most television viewing is done cocooned in soft recliners or curled up on a couch.
I will admit that this recent paper merely supports a suspicion I have harbored for decades. Like many of you, I have wondered how our society got to the point where obesity is frequent enough to be labeled a disease, attention deficit diagnoses are becoming increasingly prevalent, and our life expectancy is shrinking. There are dozens of factors, but if I had to pick one, I would paraphrase James Carville’s advice to Bill Clinton: “It’s the television, stupid.”
At least a couple of notches above “Are you wearing your seatbelt?” It can start with a nonjudgmental question such as “What are your favorite television shows?” And then deftly move toward compiling a tally of how many hours the patient watches each day.
How you manage the situation from there is up to you and can be based on the patient’s complaints and problem list. You might suggest he or she start by eliminating 2 hours of viewing a day. Then ask if he or she thinks that new schedule is achievable. If they ask for alternatives, be ready with a list of light activities that they might be surprised are healthier than their current behavior. Follow up with another visit or a call to see how they are doing. It’s that important, and your call will underscore your concern.
Sedentism is a serious health problem in this country and our emphasis on encouraging vigorous exercise isn’t working. Selling a television diet will be a tough sell, but it needs to be done.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.