User login
Nonsurgical biomarkers show potential in chronic endometriosis
SALT LAKE CITY – and might soon spare patients from years of misdiagnosis and the costs and burden of diagnostic surgery, according to experts at the annual meeting of the American Society for Reproductive Medicine.
Most notably, expression levels of three microRNAs, 125-b-5p, 451a, and 3613-5p, correctly distinguished patients with chronic endometriosis from healthy controls, said Hugh S. Taylor, MD, of Yale University in New Haven, Conn.
Chronic endometriosis affects about 10% of women and up to half of those with infertility, Dr. Taylor noted. The disease costs the United States at least $22 billion annually and is the second most-common reason for hysterectomy (Reprod Sci. 2009 Apr;16[4]:335-46). Its complexity means that patients face many barriers to diagnosis, particularly young women, who are often told they have “routine” menstrual pain, Dr. Taylor said.
Patients may go up to 12 years and see five or more physicians before they are diagnosed. Clinicians tend to rely on surgical diagnosis, but “there is a reluctance to perform surgery unless there is severe disease,” he added. “The lack of nonsurgical biomarkers contributes significantly to delays in diagnosis and timely intervention.”
These concerns prompted Dr. Taylor and his associates to study microRNAs – the short, noncoding, functional RNAs that promote messenger RNA breakdown or repress its translation. MicroRNA expression varies by tissue type and disease status, and occurs in a variety of body fluids, giving them real potential as nonsurgical biomarkers, Dr. Taylor said. To examine their role in endometriosis, he and his associates performed microarray profiling and confirmatory quantitative real-time polymerase chain reaction testing of serum samples from 24 women with chronic endometriosis and 24 healthy women who served as controls (Fertil Steril. 2016 Aug;106[2]:402-9).
MicroRNA 125b-5p was upregulated the most in endometriosis patients and distinguished patients from controls with a “giant” area under the receiver operating characteristic curve value of 0.974, Dr. Taylor said. Remarkably, this value rose to 1 – meaning that sensitivity and specificity both were 100% – when the researchers added another upregulated microRNA (451a) and a downregulated microRNA (3613-5p) to the model. More work is underway to understand how a test for these microRNAs would perform in larger populations, Dr. Taylor said.
MicroRNAs also are likely to play functional roles in chronic endometriosis and may mediate treatment response, he noted. For example, the microRNA 125b-5p, which is upregulated in endometriosis, increases the expression of inflammatory cytokines and tumor necrosis factor alpha in macrophages, and the aromatase inhibitor letrozole, which reduces pelvic pain in the disease, increases the expression of let-7 microRNAs, with corresponding decreases in the migration of endometrial cells (Fertil Steril. 2016 Sep 1;106[3]:673-80). “Maybe these microRNAs are changing metabolism. Maybe they are changing immune cell activity,” Dr. Taylor said. “I think they are doing a lot more than sitting around waiting for us to discover them.”
None of the 24 patients with endometriosis in his study had taken oral contraceptives in the 3 months prior to serum sampling, Dr. Taylor noted. “We need to look at oral contraception as a potential confounder,” he acknowledged. “If something is independent of the menstrual cycle phase, that is much better than a marker that is dependent on cycle phase.”
Menstrual cycle phase and oral contraceptives are just two of many potential confounders of biomarkers in chronic endometriosis, according to Linda Giudice, MD, PhD, of the University of California, San Francisco. Disease severity, as well as the type, number, and location of lesions and the presence or absence of coexisting inflammatory disorders all can potentially affect the sensitivity and specificity of a marker, she said. Consequently, “there is no single biomarker for chronic endometriosis,” but there are several candidates besides microRNAs, she added. For example, studies show that menstrual blood is readily distinguishable from peripheral blood, and closely resembles the immune environment of the uterus. Another study found that urinary peptides distinguished patients with moderate to severe endometriosis from healthy controls, and mild disease from severe disease, with sensitivities and specificities ranging from 72% to 88% (Fertil Steril. 2011 Mar 15;95[4]:1261-6).
Other potential sources of diagnostic tests include the endometrial proteome, transcriptome, and methylome, as well as endometrial stem cells, Dr. Giudice said. But for now, surgical diagnosis remains the gold standard, and the World Endometriosis Research Foundation is working to homogenize recording of surgical phenotypic information and laparoscopic specimens to improve data quality, she added.
Dr. Taylor did not report funding sources. He disclosed financial ties to Pfizer, OvaScience AbbVie, Bayer, and Euroscreen. Dr. Giudice acknowledged support from the National Institutes of Health and the UCSF NIH Human Endometrial Tissue and DNA Bank. She disclosed ties to Merck, Pfizer, NextGen Jane, AbbVie, and Juniper Pharmaceuticals.
SALT LAKE CITY – and might soon spare patients from years of misdiagnosis and the costs and burden of diagnostic surgery, according to experts at the annual meeting of the American Society for Reproductive Medicine.
Most notably, expression levels of three microRNAs, 125-b-5p, 451a, and 3613-5p, correctly distinguished patients with chronic endometriosis from healthy controls, said Hugh S. Taylor, MD, of Yale University in New Haven, Conn.
Chronic endometriosis affects about 10% of women and up to half of those with infertility, Dr. Taylor noted. The disease costs the United States at least $22 billion annually and is the second most-common reason for hysterectomy (Reprod Sci. 2009 Apr;16[4]:335-46). Its complexity means that patients face many barriers to diagnosis, particularly young women, who are often told they have “routine” menstrual pain, Dr. Taylor said.
Patients may go up to 12 years and see five or more physicians before they are diagnosed. Clinicians tend to rely on surgical diagnosis, but “there is a reluctance to perform surgery unless there is severe disease,” he added. “The lack of nonsurgical biomarkers contributes significantly to delays in diagnosis and timely intervention.”
These concerns prompted Dr. Taylor and his associates to study microRNAs – the short, noncoding, functional RNAs that promote messenger RNA breakdown or repress its translation. MicroRNA expression varies by tissue type and disease status, and occurs in a variety of body fluids, giving them real potential as nonsurgical biomarkers, Dr. Taylor said. To examine their role in endometriosis, he and his associates performed microarray profiling and confirmatory quantitative real-time polymerase chain reaction testing of serum samples from 24 women with chronic endometriosis and 24 healthy women who served as controls (Fertil Steril. 2016 Aug;106[2]:402-9).
MicroRNA 125b-5p was upregulated the most in endometriosis patients and distinguished patients from controls with a “giant” area under the receiver operating characteristic curve value of 0.974, Dr. Taylor said. Remarkably, this value rose to 1 – meaning that sensitivity and specificity both were 100% – when the researchers added another upregulated microRNA (451a) and a downregulated microRNA (3613-5p) to the model. More work is underway to understand how a test for these microRNAs would perform in larger populations, Dr. Taylor said.
MicroRNAs also are likely to play functional roles in chronic endometriosis and may mediate treatment response, he noted. For example, the microRNA 125b-5p, which is upregulated in endometriosis, increases the expression of inflammatory cytokines and tumor necrosis factor alpha in macrophages, and the aromatase inhibitor letrozole, which reduces pelvic pain in the disease, increases the expression of let-7 microRNAs, with corresponding decreases in the migration of endometrial cells (Fertil Steril. 2016 Sep 1;106[3]:673-80). “Maybe these microRNAs are changing metabolism. Maybe they are changing immune cell activity,” Dr. Taylor said. “I think they are doing a lot more than sitting around waiting for us to discover them.”
None of the 24 patients with endometriosis in his study had taken oral contraceptives in the 3 months prior to serum sampling, Dr. Taylor noted. “We need to look at oral contraception as a potential confounder,” he acknowledged. “If something is independent of the menstrual cycle phase, that is much better than a marker that is dependent on cycle phase.”
Menstrual cycle phase and oral contraceptives are just two of many potential confounders of biomarkers in chronic endometriosis, according to Linda Giudice, MD, PhD, of the University of California, San Francisco. Disease severity, as well as the type, number, and location of lesions and the presence or absence of coexisting inflammatory disorders all can potentially affect the sensitivity and specificity of a marker, she said. Consequently, “there is no single biomarker for chronic endometriosis,” but there are several candidates besides microRNAs, she added. For example, studies show that menstrual blood is readily distinguishable from peripheral blood, and closely resembles the immune environment of the uterus. Another study found that urinary peptides distinguished patients with moderate to severe endometriosis from healthy controls, and mild disease from severe disease, with sensitivities and specificities ranging from 72% to 88% (Fertil Steril. 2011 Mar 15;95[4]:1261-6).
Other potential sources of diagnostic tests include the endometrial proteome, transcriptome, and methylome, as well as endometrial stem cells, Dr. Giudice said. But for now, surgical diagnosis remains the gold standard, and the World Endometriosis Research Foundation is working to homogenize recording of surgical phenotypic information and laparoscopic specimens to improve data quality, she added.
Dr. Taylor did not report funding sources. He disclosed financial ties to Pfizer, OvaScience AbbVie, Bayer, and Euroscreen. Dr. Giudice acknowledged support from the National Institutes of Health and the UCSF NIH Human Endometrial Tissue and DNA Bank. She disclosed ties to Merck, Pfizer, NextGen Jane, AbbVie, and Juniper Pharmaceuticals.
SALT LAKE CITY – and might soon spare patients from years of misdiagnosis and the costs and burden of diagnostic surgery, according to experts at the annual meeting of the American Society for Reproductive Medicine.
Most notably, expression levels of three microRNAs, 125-b-5p, 451a, and 3613-5p, correctly distinguished patients with chronic endometriosis from healthy controls, said Hugh S. Taylor, MD, of Yale University in New Haven, Conn.
Chronic endometriosis affects about 10% of women and up to half of those with infertility, Dr. Taylor noted. The disease costs the United States at least $22 billion annually and is the second most-common reason for hysterectomy (Reprod Sci. 2009 Apr;16[4]:335-46). Its complexity means that patients face many barriers to diagnosis, particularly young women, who are often told they have “routine” menstrual pain, Dr. Taylor said.
Patients may go up to 12 years and see five or more physicians before they are diagnosed. Clinicians tend to rely on surgical diagnosis, but “there is a reluctance to perform surgery unless there is severe disease,” he added. “The lack of nonsurgical biomarkers contributes significantly to delays in diagnosis and timely intervention.”
These concerns prompted Dr. Taylor and his associates to study microRNAs – the short, noncoding, functional RNAs that promote messenger RNA breakdown or repress its translation. MicroRNA expression varies by tissue type and disease status, and occurs in a variety of body fluids, giving them real potential as nonsurgical biomarkers, Dr. Taylor said. To examine their role in endometriosis, he and his associates performed microarray profiling and confirmatory quantitative real-time polymerase chain reaction testing of serum samples from 24 women with chronic endometriosis and 24 healthy women who served as controls (Fertil Steril. 2016 Aug;106[2]:402-9).
MicroRNA 125b-5p was upregulated the most in endometriosis patients and distinguished patients from controls with a “giant” area under the receiver operating characteristic curve value of 0.974, Dr. Taylor said. Remarkably, this value rose to 1 – meaning that sensitivity and specificity both were 100% – when the researchers added another upregulated microRNA (451a) and a downregulated microRNA (3613-5p) to the model. More work is underway to understand how a test for these microRNAs would perform in larger populations, Dr. Taylor said.
MicroRNAs also are likely to play functional roles in chronic endometriosis and may mediate treatment response, he noted. For example, the microRNA 125b-5p, which is upregulated in endometriosis, increases the expression of inflammatory cytokines and tumor necrosis factor alpha in macrophages, and the aromatase inhibitor letrozole, which reduces pelvic pain in the disease, increases the expression of let-7 microRNAs, with corresponding decreases in the migration of endometrial cells (Fertil Steril. 2016 Sep 1;106[3]:673-80). “Maybe these microRNAs are changing metabolism. Maybe they are changing immune cell activity,” Dr. Taylor said. “I think they are doing a lot more than sitting around waiting for us to discover them.”
None of the 24 patients with endometriosis in his study had taken oral contraceptives in the 3 months prior to serum sampling, Dr. Taylor noted. “We need to look at oral contraception as a potential confounder,” he acknowledged. “If something is independent of the menstrual cycle phase, that is much better than a marker that is dependent on cycle phase.”
Menstrual cycle phase and oral contraceptives are just two of many potential confounders of biomarkers in chronic endometriosis, according to Linda Giudice, MD, PhD, of the University of California, San Francisco. Disease severity, as well as the type, number, and location of lesions and the presence or absence of coexisting inflammatory disorders all can potentially affect the sensitivity and specificity of a marker, she said. Consequently, “there is no single biomarker for chronic endometriosis,” but there are several candidates besides microRNAs, she added. For example, studies show that menstrual blood is readily distinguishable from peripheral blood, and closely resembles the immune environment of the uterus. Another study found that urinary peptides distinguished patients with moderate to severe endometriosis from healthy controls, and mild disease from severe disease, with sensitivities and specificities ranging from 72% to 88% (Fertil Steril. 2011 Mar 15;95[4]:1261-6).
Other potential sources of diagnostic tests include the endometrial proteome, transcriptome, and methylome, as well as endometrial stem cells, Dr. Giudice said. But for now, surgical diagnosis remains the gold standard, and the World Endometriosis Research Foundation is working to homogenize recording of surgical phenotypic information and laparoscopic specimens to improve data quality, she added.
Dr. Taylor did not report funding sources. He disclosed financial ties to Pfizer, OvaScience AbbVie, Bayer, and Euroscreen. Dr. Giudice acknowledged support from the National Institutes of Health and the UCSF NIH Human Endometrial Tissue and DNA Bank. She disclosed ties to Merck, Pfizer, NextGen Jane, AbbVie, and Juniper Pharmaceuticals.
EXPERT ANALYSIS FROM 2016 ASRM
Expert tips for working with aspiring LGBT parents
SALT LAKE CITY – An aspiring mother calls the sperm bank she has arranged to work with for insemination. “We can ship sperm across state lines,” she is told – until the clinic learns she has a wife and reverses its policy.
This is just one of many experiences faced by lesbian, gay, bisexual, and transgender (LGBT) individuals on the road to parenthood, Sarah R. Holley, PhD, in the psychology department bat San Francisco (Calif.) State University, said at the annual meeting of the American Society of Reproductive Medicine.
Such experiences harm LGBT persons seeking ART and convince them that clinics only care about their money, according to Dr. Holley. So how to do better? “In order to combat heteronormative bias, we first need to get out of problem-solving mode and offer emotional understanding,” she said. For example, providers should never assume that the uteri and eggs of two female partners are interchangeable, or that a woman who is infertile should not grieve, simply because her wife can conceive.
Providers also should be ready to discuss the downsides of tandem pregnancy with lesbian couples, said Angela K. Lawson, PhD, a psychologist at Northwestern University in Chicago. While few studies have examined specific outcomes, “Each woman has a different chance of a successful pregnancy,” she said. “What if one or both women have complications, or a medically challenged child, or they don’t get pregnant at same time?” By taking turns at IVF, couples can better cope with these potential outcomes, she suggested.
Increasingly, lesbian couples are pursuing “reciprocal in vitro fertilization,” in which one woman contributes her ovum for embryo formation, and her partner undergoes implantation. The correct terms here are “genetic mother” and “gestational mother,” not “egg donor” and “gestational carrier,” which have completely different emotional and legal implications, Dr. Lawson emphasized.
That difference makes it imperative for ART clinics to use properly worded consent forms, said Colleen M. Quinn, JD, of the Adoption and Surrogacy Law Center at Locke & Quinn in Richmond, Va. The U.S. Supreme Court decision recognizing same-sex marriage did not clarify legal parental status; birth certificates do not grant legal parental status or rescind the rights of sperm donors, she said. As a result, lesbian couples who separated have won or lost custody battles based on the wording of ART consent forms, she added. Clinics are ethically obligated to advise their LGBT clients to seek legal counsel and create their own agreements about intended parenthood, she emphasized.
For their own protection, clinics also should insist that couples create a legal property disposition agreement between themselves before creating or storing embryos on their behalf, Ms. Quinn said. “An informed consent document is not sufficient,” she added. “Neither is a disposition agreement with the clinic.”
Prospective transgender parents also merit empathic consideration, said Dr. Lawson. Self-identified women who are biologically male may face “profound sadness” because they cannot carry a pregnancy. Conversely, transgender men who become pregnant in order to fulfill dreams of children may nonetheless experience intense gender dysphoria. In at least one case, a transgender male secluded himself at home throughout his pregnancy to avoid public scrutiny, Dr. Lawson said. In all cases, it helps to identify local sources of support and information and refer patients appropriately, Dr. Holley noted.
The experts also briefly covered prospective gay male fathers, who may pay upward of $100,000 to work with a gestational carrier from an agency, they said. Couples who cannot afford to do so may work with a female friend or seek a gestational carrier in another country, each of which raises questions about parental involvement and legal rights. When same-sex male partners are both donating sperm for embryo formation, “One of the biggest controversies is what to do if only one embryo implants,” Dr. Lawson said. “Will the fathers pursue DNA testing of that child? This gets us into issues of intentional unknowing and secrecy, and we know that secrecy can destroy families, whereas privacy typically doesn’t. It’s in the best interest of children to know the story of their parentage.”
None of the experts acknowledged funding sources. Ms. Quinn had no disclosures.
SALT LAKE CITY – An aspiring mother calls the sperm bank she has arranged to work with for insemination. “We can ship sperm across state lines,” she is told – until the clinic learns she has a wife and reverses its policy.
This is just one of many experiences faced by lesbian, gay, bisexual, and transgender (LGBT) individuals on the road to parenthood, Sarah R. Holley, PhD, in the psychology department bat San Francisco (Calif.) State University, said at the annual meeting of the American Society of Reproductive Medicine.
Such experiences harm LGBT persons seeking ART and convince them that clinics only care about their money, according to Dr. Holley. So how to do better? “In order to combat heteronormative bias, we first need to get out of problem-solving mode and offer emotional understanding,” she said. For example, providers should never assume that the uteri and eggs of two female partners are interchangeable, or that a woman who is infertile should not grieve, simply because her wife can conceive.
Providers also should be ready to discuss the downsides of tandem pregnancy with lesbian couples, said Angela K. Lawson, PhD, a psychologist at Northwestern University in Chicago. While few studies have examined specific outcomes, “Each woman has a different chance of a successful pregnancy,” she said. “What if one or both women have complications, or a medically challenged child, or they don’t get pregnant at same time?” By taking turns at IVF, couples can better cope with these potential outcomes, she suggested.
Increasingly, lesbian couples are pursuing “reciprocal in vitro fertilization,” in which one woman contributes her ovum for embryo formation, and her partner undergoes implantation. The correct terms here are “genetic mother” and “gestational mother,” not “egg donor” and “gestational carrier,” which have completely different emotional and legal implications, Dr. Lawson emphasized.
That difference makes it imperative for ART clinics to use properly worded consent forms, said Colleen M. Quinn, JD, of the Adoption and Surrogacy Law Center at Locke & Quinn in Richmond, Va. The U.S. Supreme Court decision recognizing same-sex marriage did not clarify legal parental status; birth certificates do not grant legal parental status or rescind the rights of sperm donors, she said. As a result, lesbian couples who separated have won or lost custody battles based on the wording of ART consent forms, she added. Clinics are ethically obligated to advise their LGBT clients to seek legal counsel and create their own agreements about intended parenthood, she emphasized.
For their own protection, clinics also should insist that couples create a legal property disposition agreement between themselves before creating or storing embryos on their behalf, Ms. Quinn said. “An informed consent document is not sufficient,” she added. “Neither is a disposition agreement with the clinic.”
Prospective transgender parents also merit empathic consideration, said Dr. Lawson. Self-identified women who are biologically male may face “profound sadness” because they cannot carry a pregnancy. Conversely, transgender men who become pregnant in order to fulfill dreams of children may nonetheless experience intense gender dysphoria. In at least one case, a transgender male secluded himself at home throughout his pregnancy to avoid public scrutiny, Dr. Lawson said. In all cases, it helps to identify local sources of support and information and refer patients appropriately, Dr. Holley noted.
The experts also briefly covered prospective gay male fathers, who may pay upward of $100,000 to work with a gestational carrier from an agency, they said. Couples who cannot afford to do so may work with a female friend or seek a gestational carrier in another country, each of which raises questions about parental involvement and legal rights. When same-sex male partners are both donating sperm for embryo formation, “One of the biggest controversies is what to do if only one embryo implants,” Dr. Lawson said. “Will the fathers pursue DNA testing of that child? This gets us into issues of intentional unknowing and secrecy, and we know that secrecy can destroy families, whereas privacy typically doesn’t. It’s in the best interest of children to know the story of their parentage.”
None of the experts acknowledged funding sources. Ms. Quinn had no disclosures.
SALT LAKE CITY – An aspiring mother calls the sperm bank she has arranged to work with for insemination. “We can ship sperm across state lines,” she is told – until the clinic learns she has a wife and reverses its policy.
This is just one of many experiences faced by lesbian, gay, bisexual, and transgender (LGBT) individuals on the road to parenthood, Sarah R. Holley, PhD, in the psychology department bat San Francisco (Calif.) State University, said at the annual meeting of the American Society of Reproductive Medicine.
Such experiences harm LGBT persons seeking ART and convince them that clinics only care about their money, according to Dr. Holley. So how to do better? “In order to combat heteronormative bias, we first need to get out of problem-solving mode and offer emotional understanding,” she said. For example, providers should never assume that the uteri and eggs of two female partners are interchangeable, or that a woman who is infertile should not grieve, simply because her wife can conceive.
Providers also should be ready to discuss the downsides of tandem pregnancy with lesbian couples, said Angela K. Lawson, PhD, a psychologist at Northwestern University in Chicago. While few studies have examined specific outcomes, “Each woman has a different chance of a successful pregnancy,” she said. “What if one or both women have complications, or a medically challenged child, or they don’t get pregnant at same time?” By taking turns at IVF, couples can better cope with these potential outcomes, she suggested.
Increasingly, lesbian couples are pursuing “reciprocal in vitro fertilization,” in which one woman contributes her ovum for embryo formation, and her partner undergoes implantation. The correct terms here are “genetic mother” and “gestational mother,” not “egg donor” and “gestational carrier,” which have completely different emotional and legal implications, Dr. Lawson emphasized.
That difference makes it imperative for ART clinics to use properly worded consent forms, said Colleen M. Quinn, JD, of the Adoption and Surrogacy Law Center at Locke & Quinn in Richmond, Va. The U.S. Supreme Court decision recognizing same-sex marriage did not clarify legal parental status; birth certificates do not grant legal parental status or rescind the rights of sperm donors, she said. As a result, lesbian couples who separated have won or lost custody battles based on the wording of ART consent forms, she added. Clinics are ethically obligated to advise their LGBT clients to seek legal counsel and create their own agreements about intended parenthood, she emphasized.
For their own protection, clinics also should insist that couples create a legal property disposition agreement between themselves before creating or storing embryos on their behalf, Ms. Quinn said. “An informed consent document is not sufficient,” she added. “Neither is a disposition agreement with the clinic.”
Prospective transgender parents also merit empathic consideration, said Dr. Lawson. Self-identified women who are biologically male may face “profound sadness” because they cannot carry a pregnancy. Conversely, transgender men who become pregnant in order to fulfill dreams of children may nonetheless experience intense gender dysphoria. In at least one case, a transgender male secluded himself at home throughout his pregnancy to avoid public scrutiny, Dr. Lawson said. In all cases, it helps to identify local sources of support and information and refer patients appropriately, Dr. Holley noted.
The experts also briefly covered prospective gay male fathers, who may pay upward of $100,000 to work with a gestational carrier from an agency, they said. Couples who cannot afford to do so may work with a female friend or seek a gestational carrier in another country, each of which raises questions about parental involvement and legal rights. When same-sex male partners are both donating sperm for embryo formation, “One of the biggest controversies is what to do if only one embryo implants,” Dr. Lawson said. “Will the fathers pursue DNA testing of that child? This gets us into issues of intentional unknowing and secrecy, and we know that secrecy can destroy families, whereas privacy typically doesn’t. It’s in the best interest of children to know the story of their parentage.”
None of the experts acknowledged funding sources. Ms. Quinn had no disclosures.
EXPERT ANALYSIS FROM ASRM 2016
Obese IVF patients need higher HCG trigger dose
SALT LAKE CITY – Women who are obese should receive more than 5,000 IU of human chorionic gonadotropin (HCG) to trigger final oocyte maturation during in vitro fertilization, findings of a retrospective cohort study suggest.
Fully 23% of obese women had low beta-HCG levels the day after receiving a 5,000 IU HCG trigger dose, compared with 1% of non-obese women (odds ratio, 21.4; 95% confidence interval, 15.0-30.4), reported Mohamad Irani, MD, and his associates from Cornell University, New York. In contrast, 10,000 IU HCG triggered adequate beta-HCG levels in 81% of obese patients with a BMI of 30-40 kg/m2, and in 90% of those whose BMI exceeded 40 kg/m2.
Low beta-HCG levels decreased the chances of oocyte maturation, fertilization, and live birth in the study. “Patients’ BMI should be taken into consideration when determining the dose of HCG trigger,” the investigators concluded in a poster presented at the annual meeting of the American Society for Reproductive Medicine.
Patients with hypothalamic amenorrhea or who are undergoing stimulation with a gonadotropin-releasing hormone (GnRH) agonist are not candidates for a GnRH-agonist trigger and therefore need to receive HCG instead, the researchers noted. To understand how the dose of HCG affects the chances of final oocyte maturation, they studied 19,084 HCG trigger recipients at their center between 2004 and 2013.
By protocol, patients received 10,000 IU HCG if their serum estradiol (E2) level was less than 1,500 pg/mL on the day of trigger; 5,000 IU if it measured 1,501-2,500 pg/mL; 4,000 IU if it was 2,501-3,000 pg/mL; and 3,300 IU if it exceeded 3,000 pg/mL.
The day after HCG trigger, 18,666 patients had beta-HCG levels of at least 50 mIU/mL, while 418 patients had low beta-HCG levels of less than 50 mIU/mL. A comparison of the two groups showed that low beta-HCG was associated with significantly lower rates of oocyte maturation (77% vs. 81%; P less than .001) and fertilization (63% vs. 72%; P less than .001). It was also associated with more than a 30% lower chance of a live birth (adjusted OR, 0.67; 95% CI, 0.5-0.8), even after accounting for age, and the stage and number of embryos transferred.
The researchers also examined response to HCG trigger among non-obese patients. Among patients who were overweight (BMI 25 to 30 kg/m2), the chances of a low beta-HCG level the day after trigger were 14% when the HCG dose was 3,300 IU, 12.5% when it was 4,000 IU, 4.3% when it was 5,000 IU, and 0.4% when it was 10,000 IU.
Among healthy-weight patients (BMI 18.5-25 kg/m2), low beta-HCG levels occurred 3.6% of the time when the HCG dose was 3,300 IU, 2.3% of the time when it was 4,000 IU, 0.6% of the time when it was 5,000 IU, and 0.08% of the time when it was 10,000 IU. Notably, these same doses triggered adequate beta-HCG levels in all 660 patients who were underweight (BMI less than 18.5 kg/m2), the researchers reported.
Dr. Irani reported having no relevant financial disclosures.
SALT LAKE CITY – Women who are obese should receive more than 5,000 IU of human chorionic gonadotropin (HCG) to trigger final oocyte maturation during in vitro fertilization, findings of a retrospective cohort study suggest.
Fully 23% of obese women had low beta-HCG levels the day after receiving a 5,000 IU HCG trigger dose, compared with 1% of non-obese women (odds ratio, 21.4; 95% confidence interval, 15.0-30.4), reported Mohamad Irani, MD, and his associates from Cornell University, New York. In contrast, 10,000 IU HCG triggered adequate beta-HCG levels in 81% of obese patients with a BMI of 30-40 kg/m2, and in 90% of those whose BMI exceeded 40 kg/m2.
Low beta-HCG levels decreased the chances of oocyte maturation, fertilization, and live birth in the study. “Patients’ BMI should be taken into consideration when determining the dose of HCG trigger,” the investigators concluded in a poster presented at the annual meeting of the American Society for Reproductive Medicine.
Patients with hypothalamic amenorrhea or who are undergoing stimulation with a gonadotropin-releasing hormone (GnRH) agonist are not candidates for a GnRH-agonist trigger and therefore need to receive HCG instead, the researchers noted. To understand how the dose of HCG affects the chances of final oocyte maturation, they studied 19,084 HCG trigger recipients at their center between 2004 and 2013.
By protocol, patients received 10,000 IU HCG if their serum estradiol (E2) level was less than 1,500 pg/mL on the day of trigger; 5,000 IU if it measured 1,501-2,500 pg/mL; 4,000 IU if it was 2,501-3,000 pg/mL; and 3,300 IU if it exceeded 3,000 pg/mL.
The day after HCG trigger, 18,666 patients had beta-HCG levels of at least 50 mIU/mL, while 418 patients had low beta-HCG levels of less than 50 mIU/mL. A comparison of the two groups showed that low beta-HCG was associated with significantly lower rates of oocyte maturation (77% vs. 81%; P less than .001) and fertilization (63% vs. 72%; P less than .001). It was also associated with more than a 30% lower chance of a live birth (adjusted OR, 0.67; 95% CI, 0.5-0.8), even after accounting for age, and the stage and number of embryos transferred.
The researchers also examined response to HCG trigger among non-obese patients. Among patients who were overweight (BMI 25 to 30 kg/m2), the chances of a low beta-HCG level the day after trigger were 14% when the HCG dose was 3,300 IU, 12.5% when it was 4,000 IU, 4.3% when it was 5,000 IU, and 0.4% when it was 10,000 IU.
Among healthy-weight patients (BMI 18.5-25 kg/m2), low beta-HCG levels occurred 3.6% of the time when the HCG dose was 3,300 IU, 2.3% of the time when it was 4,000 IU, 0.6% of the time when it was 5,000 IU, and 0.08% of the time when it was 10,000 IU. Notably, these same doses triggered adequate beta-HCG levels in all 660 patients who were underweight (BMI less than 18.5 kg/m2), the researchers reported.
Dr. Irani reported having no relevant financial disclosures.
SALT LAKE CITY – Women who are obese should receive more than 5,000 IU of human chorionic gonadotropin (HCG) to trigger final oocyte maturation during in vitro fertilization, findings of a retrospective cohort study suggest.
Fully 23% of obese women had low beta-HCG levels the day after receiving a 5,000 IU HCG trigger dose, compared with 1% of non-obese women (odds ratio, 21.4; 95% confidence interval, 15.0-30.4), reported Mohamad Irani, MD, and his associates from Cornell University, New York. In contrast, 10,000 IU HCG triggered adequate beta-HCG levels in 81% of obese patients with a BMI of 30-40 kg/m2, and in 90% of those whose BMI exceeded 40 kg/m2.
Low beta-HCG levels decreased the chances of oocyte maturation, fertilization, and live birth in the study. “Patients’ BMI should be taken into consideration when determining the dose of HCG trigger,” the investigators concluded in a poster presented at the annual meeting of the American Society for Reproductive Medicine.
Patients with hypothalamic amenorrhea or who are undergoing stimulation with a gonadotropin-releasing hormone (GnRH) agonist are not candidates for a GnRH-agonist trigger and therefore need to receive HCG instead, the researchers noted. To understand how the dose of HCG affects the chances of final oocyte maturation, they studied 19,084 HCG trigger recipients at their center between 2004 and 2013.
By protocol, patients received 10,000 IU HCG if their serum estradiol (E2) level was less than 1,500 pg/mL on the day of trigger; 5,000 IU if it measured 1,501-2,500 pg/mL; 4,000 IU if it was 2,501-3,000 pg/mL; and 3,300 IU if it exceeded 3,000 pg/mL.
The day after HCG trigger, 18,666 patients had beta-HCG levels of at least 50 mIU/mL, while 418 patients had low beta-HCG levels of less than 50 mIU/mL. A comparison of the two groups showed that low beta-HCG was associated with significantly lower rates of oocyte maturation (77% vs. 81%; P less than .001) and fertilization (63% vs. 72%; P less than .001). It was also associated with more than a 30% lower chance of a live birth (adjusted OR, 0.67; 95% CI, 0.5-0.8), even after accounting for age, and the stage and number of embryos transferred.
The researchers also examined response to HCG trigger among non-obese patients. Among patients who were overweight (BMI 25 to 30 kg/m2), the chances of a low beta-HCG level the day after trigger were 14% when the HCG dose was 3,300 IU, 12.5% when it was 4,000 IU, 4.3% when it was 5,000 IU, and 0.4% when it was 10,000 IU.
Among healthy-weight patients (BMI 18.5-25 kg/m2), low beta-HCG levels occurred 3.6% of the time when the HCG dose was 3,300 IU, 2.3% of the time when it was 4,000 IU, 0.6% of the time when it was 5,000 IU, and 0.08% of the time when it was 10,000 IU. Notably, these same doses triggered adequate beta-HCG levels in all 660 patients who were underweight (BMI less than 18.5 kg/m2), the researchers reported.
Dr. Irani reported having no relevant financial disclosures.
AT 2016 ASRM
Key clinical point:
Major finding: Fully 23% of obese individuals had low beta-HCG levels the day after receiving a 5,000 IU HCG trigger, compared with 1% of non-obese patients (OR, 21.4).
Data source: A retrospective cohort study of 18,666 patients with beta-HCG levels of at least 50 mIU/mL and 418 patients with “low” levels of less than 50 mIU/mL the day after HCG trigger.
Disclosures: Dr. Irani reported having no relevant financial disclosures.
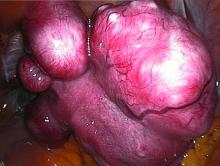
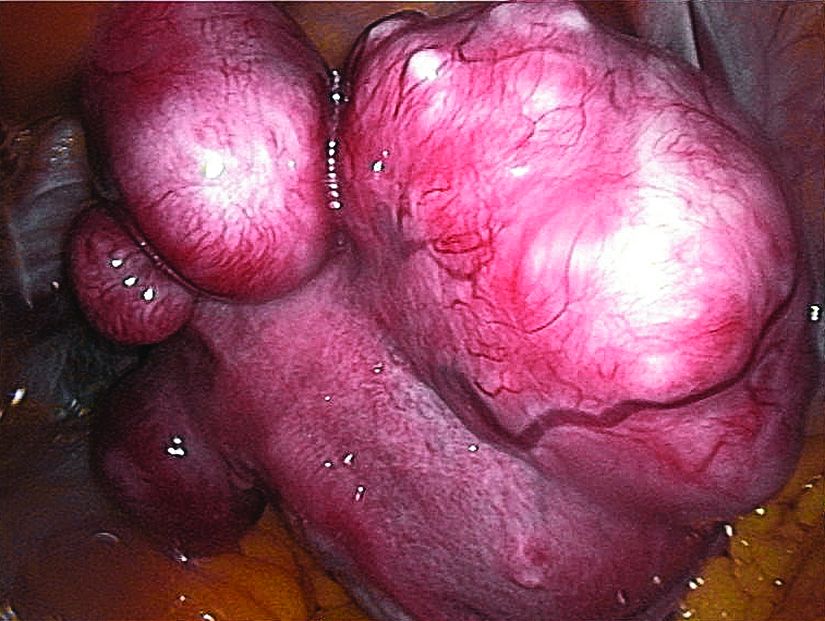
Ulipristal acetate meets primary endpoint in pivotal uterine fibroids trial
SALT LAKE CITY – The selective progesterone receptor modulator ulipristal acetate significantly reduced bleeding associated with fibroids in VENUS-I, a pivotal phase III trial of 147 premenopausal women.
A total of 58% of patients who took 10 mg ulipristal acetate per day had no bleeding except spotting during the last 35 days of treatment, compared with 2% of the placebo group (P less than .0001), James Simon, MD, said at the annual meeting of the American Society for Reproductive Medicine. Forty-seven percent of patients who took 5 mg ulipristal acetate per day also met the primary endpoint (P less than .0001, compared with placebo), he said.
The most common treatment-related adverse effect was hypertension, which affected 8% of women in the 10-mg group and 4% of women in the 5-mg group, he added during the late-breaking oral presentation.
Ulipristal acetate is currently approved in the United States as an emergency contraceptive, but is marketed for treating symptomatic fibroids in Canada and Europe. The drug reduced bleeding in about 90% of patients in the European trials (N Engl J Med. 2012;366:409-20; Fertil Steril. 2014 Jun;101[6]:1565-73).
The VENUS-I trial included premenopausal women aged 18-50 years who had experienced 22-35 days of bleeding during at least four of their last six menstrual cycles, with at least 80 mL menstrual blood loss, and at least one fibroid confirmed by transvaginal ultrasound.
“The women in our study were more severely affected, in terms of the amount of bleeding and in terms of fibroid size and uterine size, than what was reported in the European trials,” Dr. Simon said.
The average age of the patients was 41 years, and 69% were African-American, reflecting the disproportionate burden of severely symptomatic fibroids in this group, he said.
Patients were randomly assigned to oral treatment with either placebo or 10 mg or 5 mg ulipristal acetate for 12 weeks, followed by a 12-week drug-free observation period. The primary endpoint was amenorrhea, not including spotting, during the last 35 consecutive days of treatment.
The 10-mg group was 49 times more likely to reach this endpoint than was the placebo arm (P less than .0001), even after the researchers controlled for multiple potential confounders, Dr. Simon reported. The hazard ratio for the 5-mg group was also highly statistically significant at 35.5 (P less than .0001).
“Almost all patients who achieved amenorrhea in the ulipristal acetate groups did so by day 11,” Dr. Simon said.
Rates of amenorrhea from day 11 through the end of treatment were 58% in the 10-mg group, 43% in the 5-mg group, and 0% in the placebo group (P less than .0001 for differences among groups). Patients who received ulipristal acetate also reported significantly greater improvements on the Uterine Fibroid Symptom and Quality of Life (UFS-QOL) questionnaire than did the placebo group.
No patients stopped treatment because of adverse effects. Of the six patients who developed hypertension while on ulipristal acetate, five had a history of high blood pressure, and the increases were generally “slight,” Dr. Simon said. The other common treatment-emergent adverse effects included increased creatinine phosphokinase, hot flashes, acne, and nausea.
Blinded pathologists interpreted endometrial biopsies at baseline, the end of treatment, and at the end of the drug-free observation period. These revealed no cases of atypical endometrial hyperplasia or malignancy, and no baseline increases in rates of progesterone receptor modulator–associated endometrial changes, Dr. Simon said. One patient developed endometrial hyperplasia without atypia at the end of treatment, which resolved by the end of the 12-week drug-free observation period.
VENUS-I is one of two pivotal trials of ulipristal acetate for the treatment of uterine fibroids in the United States. Results from the second trial, VENUS-II, are expected in 2017.
The VENUS-I trial was supported by Allergan and Gedeon Richter. Dr. Simon reported ties to numerous other pharmaceutical companies, but not to Allergan or Gedeon Richter.
SALT LAKE CITY – The selective progesterone receptor modulator ulipristal acetate significantly reduced bleeding associated with fibroids in VENUS-I, a pivotal phase III trial of 147 premenopausal women.
A total of 58% of patients who took 10 mg ulipristal acetate per day had no bleeding except spotting during the last 35 days of treatment, compared with 2% of the placebo group (P less than .0001), James Simon, MD, said at the annual meeting of the American Society for Reproductive Medicine. Forty-seven percent of patients who took 5 mg ulipristal acetate per day also met the primary endpoint (P less than .0001, compared with placebo), he said.
The most common treatment-related adverse effect was hypertension, which affected 8% of women in the 10-mg group and 4% of women in the 5-mg group, he added during the late-breaking oral presentation.
Ulipristal acetate is currently approved in the United States as an emergency contraceptive, but is marketed for treating symptomatic fibroids in Canada and Europe. The drug reduced bleeding in about 90% of patients in the European trials (N Engl J Med. 2012;366:409-20; Fertil Steril. 2014 Jun;101[6]:1565-73).
The VENUS-I trial included premenopausal women aged 18-50 years who had experienced 22-35 days of bleeding during at least four of their last six menstrual cycles, with at least 80 mL menstrual blood loss, and at least one fibroid confirmed by transvaginal ultrasound.
“The women in our study were more severely affected, in terms of the amount of bleeding and in terms of fibroid size and uterine size, than what was reported in the European trials,” Dr. Simon said.
The average age of the patients was 41 years, and 69% were African-American, reflecting the disproportionate burden of severely symptomatic fibroids in this group, he said.
Patients were randomly assigned to oral treatment with either placebo or 10 mg or 5 mg ulipristal acetate for 12 weeks, followed by a 12-week drug-free observation period. The primary endpoint was amenorrhea, not including spotting, during the last 35 consecutive days of treatment.
The 10-mg group was 49 times more likely to reach this endpoint than was the placebo arm (P less than .0001), even after the researchers controlled for multiple potential confounders, Dr. Simon reported. The hazard ratio for the 5-mg group was also highly statistically significant at 35.5 (P less than .0001).
“Almost all patients who achieved amenorrhea in the ulipristal acetate groups did so by day 11,” Dr. Simon said.
Rates of amenorrhea from day 11 through the end of treatment were 58% in the 10-mg group, 43% in the 5-mg group, and 0% in the placebo group (P less than .0001 for differences among groups). Patients who received ulipristal acetate also reported significantly greater improvements on the Uterine Fibroid Symptom and Quality of Life (UFS-QOL) questionnaire than did the placebo group.
No patients stopped treatment because of adverse effects. Of the six patients who developed hypertension while on ulipristal acetate, five had a history of high blood pressure, and the increases were generally “slight,” Dr. Simon said. The other common treatment-emergent adverse effects included increased creatinine phosphokinase, hot flashes, acne, and nausea.
Blinded pathologists interpreted endometrial biopsies at baseline, the end of treatment, and at the end of the drug-free observation period. These revealed no cases of atypical endometrial hyperplasia or malignancy, and no baseline increases in rates of progesterone receptor modulator–associated endometrial changes, Dr. Simon said. One patient developed endometrial hyperplasia without atypia at the end of treatment, which resolved by the end of the 12-week drug-free observation period.
VENUS-I is one of two pivotal trials of ulipristal acetate for the treatment of uterine fibroids in the United States. Results from the second trial, VENUS-II, are expected in 2017.
The VENUS-I trial was supported by Allergan and Gedeon Richter. Dr. Simon reported ties to numerous other pharmaceutical companies, but not to Allergan or Gedeon Richter.
SALT LAKE CITY – The selective progesterone receptor modulator ulipristal acetate significantly reduced bleeding associated with fibroids in VENUS-I, a pivotal phase III trial of 147 premenopausal women.
A total of 58% of patients who took 10 mg ulipristal acetate per day had no bleeding except spotting during the last 35 days of treatment, compared with 2% of the placebo group (P less than .0001), James Simon, MD, said at the annual meeting of the American Society for Reproductive Medicine. Forty-seven percent of patients who took 5 mg ulipristal acetate per day also met the primary endpoint (P less than .0001, compared with placebo), he said.
The most common treatment-related adverse effect was hypertension, which affected 8% of women in the 10-mg group and 4% of women in the 5-mg group, he added during the late-breaking oral presentation.
Ulipristal acetate is currently approved in the United States as an emergency contraceptive, but is marketed for treating symptomatic fibroids in Canada and Europe. The drug reduced bleeding in about 90% of patients in the European trials (N Engl J Med. 2012;366:409-20; Fertil Steril. 2014 Jun;101[6]:1565-73).
The VENUS-I trial included premenopausal women aged 18-50 years who had experienced 22-35 days of bleeding during at least four of their last six menstrual cycles, with at least 80 mL menstrual blood loss, and at least one fibroid confirmed by transvaginal ultrasound.
“The women in our study were more severely affected, in terms of the amount of bleeding and in terms of fibroid size and uterine size, than what was reported in the European trials,” Dr. Simon said.
The average age of the patients was 41 years, and 69% were African-American, reflecting the disproportionate burden of severely symptomatic fibroids in this group, he said.
Patients were randomly assigned to oral treatment with either placebo or 10 mg or 5 mg ulipristal acetate for 12 weeks, followed by a 12-week drug-free observation period. The primary endpoint was amenorrhea, not including spotting, during the last 35 consecutive days of treatment.
The 10-mg group was 49 times more likely to reach this endpoint than was the placebo arm (P less than .0001), even after the researchers controlled for multiple potential confounders, Dr. Simon reported. The hazard ratio for the 5-mg group was also highly statistically significant at 35.5 (P less than .0001).
“Almost all patients who achieved amenorrhea in the ulipristal acetate groups did so by day 11,” Dr. Simon said.
Rates of amenorrhea from day 11 through the end of treatment were 58% in the 10-mg group, 43% in the 5-mg group, and 0% in the placebo group (P less than .0001 for differences among groups). Patients who received ulipristal acetate also reported significantly greater improvements on the Uterine Fibroid Symptom and Quality of Life (UFS-QOL) questionnaire than did the placebo group.
No patients stopped treatment because of adverse effects. Of the six patients who developed hypertension while on ulipristal acetate, five had a history of high blood pressure, and the increases were generally “slight,” Dr. Simon said. The other common treatment-emergent adverse effects included increased creatinine phosphokinase, hot flashes, acne, and nausea.
Blinded pathologists interpreted endometrial biopsies at baseline, the end of treatment, and at the end of the drug-free observation period. These revealed no cases of atypical endometrial hyperplasia or malignancy, and no baseline increases in rates of progesterone receptor modulator–associated endometrial changes, Dr. Simon said. One patient developed endometrial hyperplasia without atypia at the end of treatment, which resolved by the end of the 12-week drug-free observation period.
VENUS-I is one of two pivotal trials of ulipristal acetate for the treatment of uterine fibroids in the United States. Results from the second trial, VENUS-II, are expected in 2017.
The VENUS-I trial was supported by Allergan and Gedeon Richter. Dr. Simon reported ties to numerous other pharmaceutical companies, but not to Allergan or Gedeon Richter.
AT 2016 ASRM
Key clinical point:
Major finding: Rates of amenorrhea during the last 35 consecutive days of treatment were 58% in the 10-mg group, compared with 2% in the placebo group (P less than .0001).
Data source: A randomized, double-blind trial of 147 premenopausal women with uterine fibroids and an average of 80 mL menstrual blood loss.
Disclosures: VENUS-I was supported by Allergan and Gedeon Richter. Dr. Simon reported ties to numerous other pharmaceutical companies, but not to Allergan or Gedeon Richter.
Delaying cancer treatment for fertility preservation did not affect outcomes
SALT LAKE CITY – Delaying cancer treatment to allow women to undergo fertility preservation did not affect long-term cancer outcomes, suggest the findings from a retrospective study of more than 500 patients.
Over a median of 3.7 years of follow-up, 2.3% of patients who elected fertility preservation developed recurrent cancer, compared with 5.3% of patients who did not undergo fertility preservation (P = .09), according to Molly Moravek, MD, of the University of Michigan, Ann Arbor, and her associates.
Survival rates were 97.7% for patients who underwent fertility preservation and 94.1% for those who did not (P = .05), the investigators reported in a poster at the annual meeting of the American Society for Reproductive Medicine.
“Pursuing fertility preservation results in minimal delays to initiation of cancer treatment and is unlikely to be clinically significant,” the investigators wrote. “There is no evidence of increased recurrence or mortality in fertility preservation patients versus controls, suggesting fertility preservation is safe for eligible cancer patients.”
Progress in cancer detection and survival has sharpened the focus on quality of life issues, including fertility preservation, the researchers said. But oncologists and patients themselves have raised concerns about postponing cancer treatment for this reason, and some have recommended shortening the delay by triggering ovarian stimulation regardless of the phase of the menstrual cycle – known as the “random start” protocol.
To explore these issues, the researchers reviewed the charts of all 553 cancer patients who had used the online patient navigator for fertility preservation at Northwestern University from 2006 to 2015.
A total of 213 patients pursued fertility preservation, while 340 did not. Undergoing fertility preservation postponed treatment of breast, hematologic, gynecologic, and other cancers by an average of 10 days, but this delay did not translate to worse recurrence rates or mortality, either overall or for any cancer subtype.
Cycle outcomes were similar between the 117 patients who underwent random-start protocols and the 23 patients underwent cycle-specific protocols, the investigators reported. Both protocols were associated with similar numbers of oocytes retrieved, numbers of mature oocytes, peak serum estradiol levels, days of stimulation, and times to cancer treatment.
The Northwestern Memorial Foundation supported the work. Dr. Moravek reported having no relevant financial disclosures.
SALT LAKE CITY – Delaying cancer treatment to allow women to undergo fertility preservation did not affect long-term cancer outcomes, suggest the findings from a retrospective study of more than 500 patients.
Over a median of 3.7 years of follow-up, 2.3% of patients who elected fertility preservation developed recurrent cancer, compared with 5.3% of patients who did not undergo fertility preservation (P = .09), according to Molly Moravek, MD, of the University of Michigan, Ann Arbor, and her associates.
Survival rates were 97.7% for patients who underwent fertility preservation and 94.1% for those who did not (P = .05), the investigators reported in a poster at the annual meeting of the American Society for Reproductive Medicine.
“Pursuing fertility preservation results in minimal delays to initiation of cancer treatment and is unlikely to be clinically significant,” the investigators wrote. “There is no evidence of increased recurrence or mortality in fertility preservation patients versus controls, suggesting fertility preservation is safe for eligible cancer patients.”
Progress in cancer detection and survival has sharpened the focus on quality of life issues, including fertility preservation, the researchers said. But oncologists and patients themselves have raised concerns about postponing cancer treatment for this reason, and some have recommended shortening the delay by triggering ovarian stimulation regardless of the phase of the menstrual cycle – known as the “random start” protocol.
To explore these issues, the researchers reviewed the charts of all 553 cancer patients who had used the online patient navigator for fertility preservation at Northwestern University from 2006 to 2015.
A total of 213 patients pursued fertility preservation, while 340 did not. Undergoing fertility preservation postponed treatment of breast, hematologic, gynecologic, and other cancers by an average of 10 days, but this delay did not translate to worse recurrence rates or mortality, either overall or for any cancer subtype.
Cycle outcomes were similar between the 117 patients who underwent random-start protocols and the 23 patients underwent cycle-specific protocols, the investigators reported. Both protocols were associated with similar numbers of oocytes retrieved, numbers of mature oocytes, peak serum estradiol levels, days of stimulation, and times to cancer treatment.
The Northwestern Memorial Foundation supported the work. Dr. Moravek reported having no relevant financial disclosures.
SALT LAKE CITY – Delaying cancer treatment to allow women to undergo fertility preservation did not affect long-term cancer outcomes, suggest the findings from a retrospective study of more than 500 patients.
Over a median of 3.7 years of follow-up, 2.3% of patients who elected fertility preservation developed recurrent cancer, compared with 5.3% of patients who did not undergo fertility preservation (P = .09), according to Molly Moravek, MD, of the University of Michigan, Ann Arbor, and her associates.
Survival rates were 97.7% for patients who underwent fertility preservation and 94.1% for those who did not (P = .05), the investigators reported in a poster at the annual meeting of the American Society for Reproductive Medicine.
“Pursuing fertility preservation results in minimal delays to initiation of cancer treatment and is unlikely to be clinically significant,” the investigators wrote. “There is no evidence of increased recurrence or mortality in fertility preservation patients versus controls, suggesting fertility preservation is safe for eligible cancer patients.”
Progress in cancer detection and survival has sharpened the focus on quality of life issues, including fertility preservation, the researchers said. But oncologists and patients themselves have raised concerns about postponing cancer treatment for this reason, and some have recommended shortening the delay by triggering ovarian stimulation regardless of the phase of the menstrual cycle – known as the “random start” protocol.
To explore these issues, the researchers reviewed the charts of all 553 cancer patients who had used the online patient navigator for fertility preservation at Northwestern University from 2006 to 2015.
A total of 213 patients pursued fertility preservation, while 340 did not. Undergoing fertility preservation postponed treatment of breast, hematologic, gynecologic, and other cancers by an average of 10 days, but this delay did not translate to worse recurrence rates or mortality, either overall or for any cancer subtype.
Cycle outcomes were similar between the 117 patients who underwent random-start protocols and the 23 patients underwent cycle-specific protocols, the investigators reported. Both protocols were associated with similar numbers of oocytes retrieved, numbers of mature oocytes, peak serum estradiol levels, days of stimulation, and times to cancer treatment.
The Northwestern Memorial Foundation supported the work. Dr. Moravek reported having no relevant financial disclosures.
AT 2016 ASRM
Key clinical point:
Major finding: Fertility preservation was associated with an average 10-day delay in cancer treatment, which did not affect rates of cancer recurrence or mortality.
Data source: Retrospective chart reviews of 553 patients with breast, hematologic, ovarian, and other cancers, with a median of 3.7 years of follow-up.
Disclosures: The Northwestern Memorial Foundation supported the work. Dr. Moravek reported having no relevant financial disclosures.
Women recovered half their ovarian reserve 13 months after chemotherapy
SALT LAKE CITY – Women who resumed menstruating after undergoing chemotherapy for breast cancer usually recovered about half their ovarian reserve 13 months later, according to interim results from a prospective cohort study.
“These findings provide important references for counseling patients before chemotherapy about their expectations for ovarian recovery. Patients who were not able to freeze enough oocytes before chemotherapy can expect to be able to stimulate approximately half as many for cryopreservation post chemo,” Joseph Letourneau, MD, and his colleagues at the University of California, San Francisco wrote in a poster presented at the annual meeting of the American Society for Reproductive Medicine.
Chemotherapy increases the risk of infertility and early menopause. But in past studies, some 70% to 80% of women regained at least some level of ovarian function after completing treatment, the researchers noted. Over the course of 6-12 months, quiescent follicles mature to the antral stage, in which exposure to follicle-stimulating hormone triggers their rapid growth and maturation. Thus, antral follicle count is an accepted marker of ovarian reserve that reliably predicts how many oocytes will be retrieved during in vitro fertilization and fertility preservation, according to the investigators.
Based on these observations, they tracked antral follicle counts over time among 199 patients who were seen for fertility preservation consultations before starting cyclophosphamide-based chemotherapy for breast cancer, and who resumed menstruating afterward. Before chemotherapy, these women had an average antral follicle count of 15, with a standard deviation of 12. They averaged 35 years of age, with a standard deviation of 5 years.
A total of 66 women returned after chemotherapy for follow-up, and underwent an average of four antral follicle counts, with a standard deviation of two, the researchers said. These measurements showed that for up to 12 months after finishing chemotherapy, patients typically had only about 14% to 25% of their baseline ovarian reserve. By month 13, however, antral follicle counts rose to an average of 52% of baseline, and remained at this level through month 18 and beyond.
Treatment with leuprolide during chemotherapy appeared to increase ovarian recovery from about month 7 onward, the researchers reported. Between 7 and 9 months after chemotherapy, antral follicle counts averaged 32% of baseline among patients who had received leuprolide, but were about 8% of baseline among patients who had not received leuprolide. Between months 13 and 18, leuprolide recipients recovered about 74% of their ovarian reserve, while other patients recovered about 35% (P = .09). Beyond month 18, leuprolide recipients recovered an average of 69% of their baseline antral follicle count and other patients recovered an average of 4 (P = .07).
“Women who did not take [leuprolide] during chemotherapy represent 75% of our study population,” the researchers said. “[These women] appeared to have lower antral follicle count recovery, despite resumption of menses, than those whose menses resumed after chemotherapy with concurrent [leuprolide].”
Dr. Letourneau reported having no relevant financial disclosures.
SALT LAKE CITY – Women who resumed menstruating after undergoing chemotherapy for breast cancer usually recovered about half their ovarian reserve 13 months later, according to interim results from a prospective cohort study.
“These findings provide important references for counseling patients before chemotherapy about their expectations for ovarian recovery. Patients who were not able to freeze enough oocytes before chemotherapy can expect to be able to stimulate approximately half as many for cryopreservation post chemo,” Joseph Letourneau, MD, and his colleagues at the University of California, San Francisco wrote in a poster presented at the annual meeting of the American Society for Reproductive Medicine.
Chemotherapy increases the risk of infertility and early menopause. But in past studies, some 70% to 80% of women regained at least some level of ovarian function after completing treatment, the researchers noted. Over the course of 6-12 months, quiescent follicles mature to the antral stage, in which exposure to follicle-stimulating hormone triggers their rapid growth and maturation. Thus, antral follicle count is an accepted marker of ovarian reserve that reliably predicts how many oocytes will be retrieved during in vitro fertilization and fertility preservation, according to the investigators.
Based on these observations, they tracked antral follicle counts over time among 199 patients who were seen for fertility preservation consultations before starting cyclophosphamide-based chemotherapy for breast cancer, and who resumed menstruating afterward. Before chemotherapy, these women had an average antral follicle count of 15, with a standard deviation of 12. They averaged 35 years of age, with a standard deviation of 5 years.
A total of 66 women returned after chemotherapy for follow-up, and underwent an average of four antral follicle counts, with a standard deviation of two, the researchers said. These measurements showed that for up to 12 months after finishing chemotherapy, patients typically had only about 14% to 25% of their baseline ovarian reserve. By month 13, however, antral follicle counts rose to an average of 52% of baseline, and remained at this level through month 18 and beyond.
Treatment with leuprolide during chemotherapy appeared to increase ovarian recovery from about month 7 onward, the researchers reported. Between 7 and 9 months after chemotherapy, antral follicle counts averaged 32% of baseline among patients who had received leuprolide, but were about 8% of baseline among patients who had not received leuprolide. Between months 13 and 18, leuprolide recipients recovered about 74% of their ovarian reserve, while other patients recovered about 35% (P = .09). Beyond month 18, leuprolide recipients recovered an average of 69% of their baseline antral follicle count and other patients recovered an average of 4 (P = .07).
“Women who did not take [leuprolide] during chemotherapy represent 75% of our study population,” the researchers said. “[These women] appeared to have lower antral follicle count recovery, despite resumption of menses, than those whose menses resumed after chemotherapy with concurrent [leuprolide].”
Dr. Letourneau reported having no relevant financial disclosures.
SALT LAKE CITY – Women who resumed menstruating after undergoing chemotherapy for breast cancer usually recovered about half their ovarian reserve 13 months later, according to interim results from a prospective cohort study.
“These findings provide important references for counseling patients before chemotherapy about their expectations for ovarian recovery. Patients who were not able to freeze enough oocytes before chemotherapy can expect to be able to stimulate approximately half as many for cryopreservation post chemo,” Joseph Letourneau, MD, and his colleagues at the University of California, San Francisco wrote in a poster presented at the annual meeting of the American Society for Reproductive Medicine.
Chemotherapy increases the risk of infertility and early menopause. But in past studies, some 70% to 80% of women regained at least some level of ovarian function after completing treatment, the researchers noted. Over the course of 6-12 months, quiescent follicles mature to the antral stage, in which exposure to follicle-stimulating hormone triggers their rapid growth and maturation. Thus, antral follicle count is an accepted marker of ovarian reserve that reliably predicts how many oocytes will be retrieved during in vitro fertilization and fertility preservation, according to the investigators.
Based on these observations, they tracked antral follicle counts over time among 199 patients who were seen for fertility preservation consultations before starting cyclophosphamide-based chemotherapy for breast cancer, and who resumed menstruating afterward. Before chemotherapy, these women had an average antral follicle count of 15, with a standard deviation of 12. They averaged 35 years of age, with a standard deviation of 5 years.
A total of 66 women returned after chemotherapy for follow-up, and underwent an average of four antral follicle counts, with a standard deviation of two, the researchers said. These measurements showed that for up to 12 months after finishing chemotherapy, patients typically had only about 14% to 25% of their baseline ovarian reserve. By month 13, however, antral follicle counts rose to an average of 52% of baseline, and remained at this level through month 18 and beyond.
Treatment with leuprolide during chemotherapy appeared to increase ovarian recovery from about month 7 onward, the researchers reported. Between 7 and 9 months after chemotherapy, antral follicle counts averaged 32% of baseline among patients who had received leuprolide, but were about 8% of baseline among patients who had not received leuprolide. Between months 13 and 18, leuprolide recipients recovered about 74% of their ovarian reserve, while other patients recovered about 35% (P = .09). Beyond month 18, leuprolide recipients recovered an average of 69% of their baseline antral follicle count and other patients recovered an average of 4 (P = .07).
“Women who did not take [leuprolide] during chemotherapy represent 75% of our study population,” the researchers said. “[These women] appeared to have lower antral follicle count recovery, despite resumption of menses, than those whose menses resumed after chemotherapy with concurrent [leuprolide].”
Dr. Letourneau reported having no relevant financial disclosures.
Key clinical point:
Major finding: Average antral follicle counts rose to 52% of baseline, on average, by 13 months after patients completed chemotherapy.
Data source: A prospective cohort study of 66 patients who resumed menstruating and returned for at least one follow-up visit after chemotherapy.
Disclosures: Dr. Letourneau reported having no relevant financial disclosures.
Resveratrol cut androgen levels in small PCOS trial
SALT LAKE CITY – Resveratrol significantly decreased androgen levels and insulin resistance among women with polycystic ovary syndrome (PCOS), according to a first-in-kind randomized, double-blind placebo-controlled trial of 30 patients.
After 3 months of daily oral treatment with 1.5 g of the antioxidant polyphenol, testosterone levels fell an average of 23%, dehydroepiandrosterone sulfate (DHEAS) levels dropped 22%, fasting insulin levels decreased by 32%, and insulin sensitivity improved by 66%, Antoni Duleba, MD, said at the annual meeting of the American Society for Reproductive Medicine. No such improvements occurred in the placebo group, he and his coinvestigators reported simultaneously in the Journal of Clinical Endocrinology & Metabolism (doi: 10.1210/jc.2016-1858).
Past work has found that resveratrol inhibits mRNA expression of Cyp17a1 and reduces androgen production by ovarian theca-interstitial cells. To build on those findings, Dr. Duleba worked with researchers at Poznan (Poland) University of Medical Sciences to enroll 30 women with PCOS based on the Rotterdam criteria. Treatment and placebo groups resembled each other at baseline in terms of age, body mass index, androgen levels, lipid profiles, and levels of follicle-stimulating hormone, luteinizing hormone, prolactin, sex hormone–binding globulin, fasting glucose, and insulin, as well as scores on an insulin sensitivity index.
For the primary outcome measure – total testosterone level – the resveratrol group averaged 0.53 ng/mL at baseline and 0.41 ng/mL at 3-month follow-up, a statistically significant decrease (P = .01). In contrast, testosterone levels in the placebo group remained essentially unchanged, averaging 0.48 ng/mL at baseline and 0.49 ng/mL at follow-up. The difference in effect between the resveratrol and placebo groups was statistically significant (P = .04).
Similarly, average DHEAS levels dropped from 8.1 to 6.3 micromol/L in the resveratrol group (a 22% decline), but increased by 10% in the placebo group.
Resveratrol did not significantly affect progesterone levels, which is consistent with prior findings, Dr. Duleba said. Nor was resveratrol associated with significant changes in body mass index, lipid profile, markers of inflammation or endothelial function, ovarian volume, or gonadotropins.
“We were disappointed that we didn’t see gross changes in ovarian morphology on ultrasound,” he added. Whether those changes might occur with longer treatment is unknown, but for now, “we can only be sure of declining androgen and insulin levels, and improvements in insulin sensitivity.”
The study won a Scientific Congress Prize from ASRM.
RevGenetics provided the resveratrol for the study. Dr. Duleba reported having no relevant financial disclosures.
SALT LAKE CITY – Resveratrol significantly decreased androgen levels and insulin resistance among women with polycystic ovary syndrome (PCOS), according to a first-in-kind randomized, double-blind placebo-controlled trial of 30 patients.
After 3 months of daily oral treatment with 1.5 g of the antioxidant polyphenol, testosterone levels fell an average of 23%, dehydroepiandrosterone sulfate (DHEAS) levels dropped 22%, fasting insulin levels decreased by 32%, and insulin sensitivity improved by 66%, Antoni Duleba, MD, said at the annual meeting of the American Society for Reproductive Medicine. No such improvements occurred in the placebo group, he and his coinvestigators reported simultaneously in the Journal of Clinical Endocrinology & Metabolism (doi: 10.1210/jc.2016-1858).
Past work has found that resveratrol inhibits mRNA expression of Cyp17a1 and reduces androgen production by ovarian theca-interstitial cells. To build on those findings, Dr. Duleba worked with researchers at Poznan (Poland) University of Medical Sciences to enroll 30 women with PCOS based on the Rotterdam criteria. Treatment and placebo groups resembled each other at baseline in terms of age, body mass index, androgen levels, lipid profiles, and levels of follicle-stimulating hormone, luteinizing hormone, prolactin, sex hormone–binding globulin, fasting glucose, and insulin, as well as scores on an insulin sensitivity index.
For the primary outcome measure – total testosterone level – the resveratrol group averaged 0.53 ng/mL at baseline and 0.41 ng/mL at 3-month follow-up, a statistically significant decrease (P = .01). In contrast, testosterone levels in the placebo group remained essentially unchanged, averaging 0.48 ng/mL at baseline and 0.49 ng/mL at follow-up. The difference in effect between the resveratrol and placebo groups was statistically significant (P = .04).
Similarly, average DHEAS levels dropped from 8.1 to 6.3 micromol/L in the resveratrol group (a 22% decline), but increased by 10% in the placebo group.
Resveratrol did not significantly affect progesterone levels, which is consistent with prior findings, Dr. Duleba said. Nor was resveratrol associated with significant changes in body mass index, lipid profile, markers of inflammation or endothelial function, ovarian volume, or gonadotropins.
“We were disappointed that we didn’t see gross changes in ovarian morphology on ultrasound,” he added. Whether those changes might occur with longer treatment is unknown, but for now, “we can only be sure of declining androgen and insulin levels, and improvements in insulin sensitivity.”
The study won a Scientific Congress Prize from ASRM.
RevGenetics provided the resveratrol for the study. Dr. Duleba reported having no relevant financial disclosures.
SALT LAKE CITY – Resveratrol significantly decreased androgen levels and insulin resistance among women with polycystic ovary syndrome (PCOS), according to a first-in-kind randomized, double-blind placebo-controlled trial of 30 patients.
After 3 months of daily oral treatment with 1.5 g of the antioxidant polyphenol, testosterone levels fell an average of 23%, dehydroepiandrosterone sulfate (DHEAS) levels dropped 22%, fasting insulin levels decreased by 32%, and insulin sensitivity improved by 66%, Antoni Duleba, MD, said at the annual meeting of the American Society for Reproductive Medicine. No such improvements occurred in the placebo group, he and his coinvestigators reported simultaneously in the Journal of Clinical Endocrinology & Metabolism (doi: 10.1210/jc.2016-1858).
Past work has found that resveratrol inhibits mRNA expression of Cyp17a1 and reduces androgen production by ovarian theca-interstitial cells. To build on those findings, Dr. Duleba worked with researchers at Poznan (Poland) University of Medical Sciences to enroll 30 women with PCOS based on the Rotterdam criteria. Treatment and placebo groups resembled each other at baseline in terms of age, body mass index, androgen levels, lipid profiles, and levels of follicle-stimulating hormone, luteinizing hormone, prolactin, sex hormone–binding globulin, fasting glucose, and insulin, as well as scores on an insulin sensitivity index.
For the primary outcome measure – total testosterone level – the resveratrol group averaged 0.53 ng/mL at baseline and 0.41 ng/mL at 3-month follow-up, a statistically significant decrease (P = .01). In contrast, testosterone levels in the placebo group remained essentially unchanged, averaging 0.48 ng/mL at baseline and 0.49 ng/mL at follow-up. The difference in effect between the resveratrol and placebo groups was statistically significant (P = .04).
Similarly, average DHEAS levels dropped from 8.1 to 6.3 micromol/L in the resveratrol group (a 22% decline), but increased by 10% in the placebo group.
Resveratrol did not significantly affect progesterone levels, which is consistent with prior findings, Dr. Duleba said. Nor was resveratrol associated with significant changes in body mass index, lipid profile, markers of inflammation or endothelial function, ovarian volume, or gonadotropins.
“We were disappointed that we didn’t see gross changes in ovarian morphology on ultrasound,” he added. Whether those changes might occur with longer treatment is unknown, but for now, “we can only be sure of declining androgen and insulin levels, and improvements in insulin sensitivity.”
The study won a Scientific Congress Prize from ASRM.
RevGenetics provided the resveratrol for the study. Dr. Duleba reported having no relevant financial disclosures.
AT 2016 ASRM
Key clinical point:
Major finding: Average total testosterone levels dropped 23% in the treatment group and remained unchanged in the placebo group (P = .04).
Data source: A single-center, double-blind, randomized controlled trial of 30 women with polycystic ovary syndrome.
Disclosures: RevGenetics provided the resveratrol for the study. Dr. Duleba reported having no relevant financial disclosures.
Psychological stress did not harm IVF outcomes
SALT LAKE CITY – Self-reported psychological distress and biological markers of stress did not affect the chances of pregnancy after in vitro fertilization, according to a single-center prospective cohort study of 186 women.
Rates of ultrasound-confirmed pregnancy were 36% among first-time IVF recipients and 30% among women undergoing their third round of IVF, and did not differ based on self-reported depressive symptoms, stress, anxiety, or serum levels of adrenocorticotropic hormone (ACTH), cortisol, or interleukin-6, Maria Costantini-Ferrando, MD, PhD, said at the annual meeting of the American Society for Reproductive Medicine.
Nonetheless, IVF recipients reported more depressive symptoms than oocyte donors did, especially after a negative pregnancy test, reported Dr. Costantini-Ferrando, a reproductive endocrinologist in Englewood, N.J.
“Patients undergoing IVF do experience real psychological distress. The efficiency of IVF in controlling the hypothalamic-pituitary-adrenal and the hypothalamic-pituitary-gonadal axes may overshadow any deleterious effect of heightened stress, but it will not eliminate patients’ experience of it,” she said.
Patients need support and follow-up to help lessen the psychological burden of treatment and decrease drop-out, she said.
Women in the study were aged 22-44 years, and the IVF recipients had a mean age of 37 years. In all, 75 women (40%) were first-time IVF patients, 29% were undergoing their third cycle of IVF, and 31% were oocyte donors. Among the IVF recipients, 22% were in psychotherapy, and 4% were on psychotropic medications. None of the women had other medical comorbidities. Patients were assessed at baseline, time of oocyte retrieval, time of embryo transfer, and on the day of the pregnancy test.
Patients with infertility reported significantly worse depressive symptoms at baseline compared with oocyte donors, with average Beck Depression Inventory scores of 11, compared with 2.3 (P less than .01). This difference persisted at the time of vaginal oocyte retrieval. First-time and third-time IVF recipients tended to have similar Beck Depression Inventory scores until pregnancy testing, when the average scores of third-time recipients increased by about five additional points.
Scores on the daily stress questionnaire echoed these findings. Patients who were receiving IVF reported significantly more stress than did oocyte donors, with the highest increase at the time of the pregnancy test among patients with previous IVF failures. However, both recipients and donors reported mild to moderate anxiety based on the State-Trait Anxiety Inventory, Dr. Costantini-Ferrando said.
All groups had low interleukin-6 levels when starting IVF, but donors had the highest levels of both ACTH and cortisol. Levels of these hormones dropped over time, and then rose at the time of pregnancy testing. That trend suggests that IVF was an acute “procedural” stressor for both recipients and donors, but that the magnitude of the stress response lessened somewhat over time with repeated exposure to the stressor, Dr. Costantini-Ferrando noted.
“The complexity of this interaction may explain why it is difficult to elucidate the true impact of stress on IVF outcome,” she said.
Ultimately, these findings support a shift in focus, she added. Instead of asking if stress decreases reproductive potential, clinicians and researchers can instead explore how to address psychological distress to maximize reproductive potential. “This is important to keep patients engaged in infertility treatment and facilitate the difficult process of decision making during treatment,” she added.
The paper won a prize from the ASRM Mental Health Professional Group.
The National Institutes of Health provided funding for the study. Dr. Costantini-Ferrando reported having no relevant financial disclosures.
SALT LAKE CITY – Self-reported psychological distress and biological markers of stress did not affect the chances of pregnancy after in vitro fertilization, according to a single-center prospective cohort study of 186 women.
Rates of ultrasound-confirmed pregnancy were 36% among first-time IVF recipients and 30% among women undergoing their third round of IVF, and did not differ based on self-reported depressive symptoms, stress, anxiety, or serum levels of adrenocorticotropic hormone (ACTH), cortisol, or interleukin-6, Maria Costantini-Ferrando, MD, PhD, said at the annual meeting of the American Society for Reproductive Medicine.
Nonetheless, IVF recipients reported more depressive symptoms than oocyte donors did, especially after a negative pregnancy test, reported Dr. Costantini-Ferrando, a reproductive endocrinologist in Englewood, N.J.
“Patients undergoing IVF do experience real psychological distress. The efficiency of IVF in controlling the hypothalamic-pituitary-adrenal and the hypothalamic-pituitary-gonadal axes may overshadow any deleterious effect of heightened stress, but it will not eliminate patients’ experience of it,” she said.
Patients need support and follow-up to help lessen the psychological burden of treatment and decrease drop-out, she said.
Women in the study were aged 22-44 years, and the IVF recipients had a mean age of 37 years. In all, 75 women (40%) were first-time IVF patients, 29% were undergoing their third cycle of IVF, and 31% were oocyte donors. Among the IVF recipients, 22% were in psychotherapy, and 4% were on psychotropic medications. None of the women had other medical comorbidities. Patients were assessed at baseline, time of oocyte retrieval, time of embryo transfer, and on the day of the pregnancy test.
Patients with infertility reported significantly worse depressive symptoms at baseline compared with oocyte donors, with average Beck Depression Inventory scores of 11, compared with 2.3 (P less than .01). This difference persisted at the time of vaginal oocyte retrieval. First-time and third-time IVF recipients tended to have similar Beck Depression Inventory scores until pregnancy testing, when the average scores of third-time recipients increased by about five additional points.
Scores on the daily stress questionnaire echoed these findings. Patients who were receiving IVF reported significantly more stress than did oocyte donors, with the highest increase at the time of the pregnancy test among patients with previous IVF failures. However, both recipients and donors reported mild to moderate anxiety based on the State-Trait Anxiety Inventory, Dr. Costantini-Ferrando said.
All groups had low interleukin-6 levels when starting IVF, but donors had the highest levels of both ACTH and cortisol. Levels of these hormones dropped over time, and then rose at the time of pregnancy testing. That trend suggests that IVF was an acute “procedural” stressor for both recipients and donors, but that the magnitude of the stress response lessened somewhat over time with repeated exposure to the stressor, Dr. Costantini-Ferrando noted.
“The complexity of this interaction may explain why it is difficult to elucidate the true impact of stress on IVF outcome,” she said.
Ultimately, these findings support a shift in focus, she added. Instead of asking if stress decreases reproductive potential, clinicians and researchers can instead explore how to address psychological distress to maximize reproductive potential. “This is important to keep patients engaged in infertility treatment and facilitate the difficult process of decision making during treatment,” she added.
The paper won a prize from the ASRM Mental Health Professional Group.
The National Institutes of Health provided funding for the study. Dr. Costantini-Ferrando reported having no relevant financial disclosures.
SALT LAKE CITY – Self-reported psychological distress and biological markers of stress did not affect the chances of pregnancy after in vitro fertilization, according to a single-center prospective cohort study of 186 women.
Rates of ultrasound-confirmed pregnancy were 36% among first-time IVF recipients and 30% among women undergoing their third round of IVF, and did not differ based on self-reported depressive symptoms, stress, anxiety, or serum levels of adrenocorticotropic hormone (ACTH), cortisol, or interleukin-6, Maria Costantini-Ferrando, MD, PhD, said at the annual meeting of the American Society for Reproductive Medicine.
Nonetheless, IVF recipients reported more depressive symptoms than oocyte donors did, especially after a negative pregnancy test, reported Dr. Costantini-Ferrando, a reproductive endocrinologist in Englewood, N.J.
“Patients undergoing IVF do experience real psychological distress. The efficiency of IVF in controlling the hypothalamic-pituitary-adrenal and the hypothalamic-pituitary-gonadal axes may overshadow any deleterious effect of heightened stress, but it will not eliminate patients’ experience of it,” she said.
Patients need support and follow-up to help lessen the psychological burden of treatment and decrease drop-out, she said.
Women in the study were aged 22-44 years, and the IVF recipients had a mean age of 37 years. In all, 75 women (40%) were first-time IVF patients, 29% were undergoing their third cycle of IVF, and 31% were oocyte donors. Among the IVF recipients, 22% were in psychotherapy, and 4% were on psychotropic medications. None of the women had other medical comorbidities. Patients were assessed at baseline, time of oocyte retrieval, time of embryo transfer, and on the day of the pregnancy test.
Patients with infertility reported significantly worse depressive symptoms at baseline compared with oocyte donors, with average Beck Depression Inventory scores of 11, compared with 2.3 (P less than .01). This difference persisted at the time of vaginal oocyte retrieval. First-time and third-time IVF recipients tended to have similar Beck Depression Inventory scores until pregnancy testing, when the average scores of third-time recipients increased by about five additional points.
Scores on the daily stress questionnaire echoed these findings. Patients who were receiving IVF reported significantly more stress than did oocyte donors, with the highest increase at the time of the pregnancy test among patients with previous IVF failures. However, both recipients and donors reported mild to moderate anxiety based on the State-Trait Anxiety Inventory, Dr. Costantini-Ferrando said.
All groups had low interleukin-6 levels when starting IVF, but donors had the highest levels of both ACTH and cortisol. Levels of these hormones dropped over time, and then rose at the time of pregnancy testing. That trend suggests that IVF was an acute “procedural” stressor for both recipients and donors, but that the magnitude of the stress response lessened somewhat over time with repeated exposure to the stressor, Dr. Costantini-Ferrando noted.
“The complexity of this interaction may explain why it is difficult to elucidate the true impact of stress on IVF outcome,” she said.
Ultimately, these findings support a shift in focus, she added. Instead of asking if stress decreases reproductive potential, clinicians and researchers can instead explore how to address psychological distress to maximize reproductive potential. “This is important to keep patients engaged in infertility treatment and facilitate the difficult process of decision making during treatment,” she added.
The paper won a prize from the ASRM Mental Health Professional Group.
The National Institutes of Health provided funding for the study. Dr. Costantini-Ferrando reported having no relevant financial disclosures.
Key clinical point:
Major finding: Rates of pregnancy were 36% among first-time IVF recipients and 30% among third-time IVF recipients, and did not correlate with self-reported depressive symptoms, stress, anxiety, or serum levels of adrenocorticotropic hormone, cortisol, or interleukin-6.
Data source: A single-center prospective study of 186 IVF patients.
Disclosures: The National Institutes of Health provided funding for the study. Dr. Costantini-Ferrando reported having no financial disclosures.
Low serum AMH predicted miscarriage after IVF
SALT LAKE CITY – Low serum levels of anti-Müllerian hormone predicted miscarriage after in vitro fertilization and embryo transfer, according to a single-center prospective study of more than 2,000 women.
This relationship remained statistically significant after the study controlled for age and response to controlled ovarian hyperstimulation, which suggests that AMH is a marker of the reproductive potential of oocytes, not just of oocyte quantity, Bruno Tarasconi, MD, said at the annual meeting of the American Society for Reproductive Medicine.
“Low” serum AMH was defined as less than 1.61 ng per mL, but specific levels vary by test type, so “this is not a definite cutoff,” Dr. Tarasconi emphasized. “What we need to know is that patients with low AMH are probably experiencing some problems in the ovary that affect oocyte quality.”
The study comprised 2,365 women undergoing 2,688 IVF cycles with fresh oocytes. Levels of AMH were measured by ELISA (AMH Gen II, Beckman Coulter) within 12 months before IVF on cycle days 2 and 4, said Dr. Tarasconi of University of Paris Ouest in France.
Women younger than 34 years old, women 34-36 years old, and women 37 years and older had the highest rates of miscarriage when they had low serum AMH levels. For the two older age groups, low AMH was associated with about a 33% miscarriage rate – about twice the rate of miscarriage among women whose AMH measured at least 5.6 ng per mL. This difference reached statistical significance for both age groups (P less than .05).
Among women younger than 34 years, low serum AMH was associated with a 22% rate of miscarriage, compared with about 13% among women with higher AMH levels. This difference did not reach significance because of a lack of power, Dr. Tarasconi said. “However, binary logistic regression of the whole population confirmed the association between low serum AMH and miscarriage, independent of patient’s age and oocyte yield, with the P value less than .001.”
Low circulating AMH can reflect an above-average prevalence of oocyte abnormalities, which are linked to miscarriage, Dr. Tarasconi noted. “The fact that oocyte yield failed to alter the relationship between AMH and miscarriage suggests that altered per-follicle AMH production, not oocyte or embryo availability, fosters pregnancy loss,” he added. However, the researchers did not test either preimplantation embryos or the expelled gestational sacs to directly link miscarriages to abnormalities such as aneuploidy, he noted.
Low AMH levels also were associated with lower rates of pregnancy after IVF, regardless of age, as has been previously reported. Patients in the study were of normal karyotype, with no history of recurrent pregnancy loss, uterine abnormalities, or family history of genetic or congenital disorders, Dr. Tarasconi noted.
He reported having no financial disclosures.
SALT LAKE CITY – Low serum levels of anti-Müllerian hormone predicted miscarriage after in vitro fertilization and embryo transfer, according to a single-center prospective study of more than 2,000 women.
This relationship remained statistically significant after the study controlled for age and response to controlled ovarian hyperstimulation, which suggests that AMH is a marker of the reproductive potential of oocytes, not just of oocyte quantity, Bruno Tarasconi, MD, said at the annual meeting of the American Society for Reproductive Medicine.
“Low” serum AMH was defined as less than 1.61 ng per mL, but specific levels vary by test type, so “this is not a definite cutoff,” Dr. Tarasconi emphasized. “What we need to know is that patients with low AMH are probably experiencing some problems in the ovary that affect oocyte quality.”
The study comprised 2,365 women undergoing 2,688 IVF cycles with fresh oocytes. Levels of AMH were measured by ELISA (AMH Gen II, Beckman Coulter) within 12 months before IVF on cycle days 2 and 4, said Dr. Tarasconi of University of Paris Ouest in France.
Women younger than 34 years old, women 34-36 years old, and women 37 years and older had the highest rates of miscarriage when they had low serum AMH levels. For the two older age groups, low AMH was associated with about a 33% miscarriage rate – about twice the rate of miscarriage among women whose AMH measured at least 5.6 ng per mL. This difference reached statistical significance for both age groups (P less than .05).
Among women younger than 34 years, low serum AMH was associated with a 22% rate of miscarriage, compared with about 13% among women with higher AMH levels. This difference did not reach significance because of a lack of power, Dr. Tarasconi said. “However, binary logistic regression of the whole population confirmed the association between low serum AMH and miscarriage, independent of patient’s age and oocyte yield, with the P value less than .001.”
Low circulating AMH can reflect an above-average prevalence of oocyte abnormalities, which are linked to miscarriage, Dr. Tarasconi noted. “The fact that oocyte yield failed to alter the relationship between AMH and miscarriage suggests that altered per-follicle AMH production, not oocyte or embryo availability, fosters pregnancy loss,” he added. However, the researchers did not test either preimplantation embryos or the expelled gestational sacs to directly link miscarriages to abnormalities such as aneuploidy, he noted.
Low AMH levels also were associated with lower rates of pregnancy after IVF, regardless of age, as has been previously reported. Patients in the study were of normal karyotype, with no history of recurrent pregnancy loss, uterine abnormalities, or family history of genetic or congenital disorders, Dr. Tarasconi noted.
He reported having no financial disclosures.
SALT LAKE CITY – Low serum levels of anti-Müllerian hormone predicted miscarriage after in vitro fertilization and embryo transfer, according to a single-center prospective study of more than 2,000 women.
This relationship remained statistically significant after the study controlled for age and response to controlled ovarian hyperstimulation, which suggests that AMH is a marker of the reproductive potential of oocytes, not just of oocyte quantity, Bruno Tarasconi, MD, said at the annual meeting of the American Society for Reproductive Medicine.
“Low” serum AMH was defined as less than 1.61 ng per mL, but specific levels vary by test type, so “this is not a definite cutoff,” Dr. Tarasconi emphasized. “What we need to know is that patients with low AMH are probably experiencing some problems in the ovary that affect oocyte quality.”
The study comprised 2,365 women undergoing 2,688 IVF cycles with fresh oocytes. Levels of AMH were measured by ELISA (AMH Gen II, Beckman Coulter) within 12 months before IVF on cycle days 2 and 4, said Dr. Tarasconi of University of Paris Ouest in France.
Women younger than 34 years old, women 34-36 years old, and women 37 years and older had the highest rates of miscarriage when they had low serum AMH levels. For the two older age groups, low AMH was associated with about a 33% miscarriage rate – about twice the rate of miscarriage among women whose AMH measured at least 5.6 ng per mL. This difference reached statistical significance for both age groups (P less than .05).
Among women younger than 34 years, low serum AMH was associated with a 22% rate of miscarriage, compared with about 13% among women with higher AMH levels. This difference did not reach significance because of a lack of power, Dr. Tarasconi said. “However, binary logistic regression of the whole population confirmed the association between low serum AMH and miscarriage, independent of patient’s age and oocyte yield, with the P value less than .001.”
Low circulating AMH can reflect an above-average prevalence of oocyte abnormalities, which are linked to miscarriage, Dr. Tarasconi noted. “The fact that oocyte yield failed to alter the relationship between AMH and miscarriage suggests that altered per-follicle AMH production, not oocyte or embryo availability, fosters pregnancy loss,” he added. However, the researchers did not test either preimplantation embryos or the expelled gestational sacs to directly link miscarriages to abnormalities such as aneuploidy, he noted.
Low AMH levels also were associated with lower rates of pregnancy after IVF, regardless of age, as has been previously reported. Patients in the study were of normal karyotype, with no history of recurrent pregnancy loss, uterine abnormalities, or family history of genetic or congenital disorders, Dr. Tarasconi noted.
He reported having no financial disclosures.
Key clinical point:
Major finding: The miscarriage rate was about 33% in women with low serum AMH levels after IVF, about twice that of women with high AMH levels.
Data source: A single-center prospective study of 2,365 women undergoing 2,688 IVF cycles with fresh oocytes.
Disclosures: Dr. Tarasconi reported having no financial disclosures.