User login
SALT LAKE CITY – The selective progesterone receptor modulator ulipristal acetate significantly reduced bleeding associated with fibroids in VENUS-I, a pivotal phase III trial of 147 premenopausal women.
A total of 58% of patients who took 10 mg ulipristal acetate per day had no bleeding except spotting during the last 35 days of treatment, compared with 2% of the placebo group (P less than .0001), James Simon, MD, said at the annual meeting of the American Society for Reproductive Medicine. Forty-seven percent of patients who took 5 mg ulipristal acetate per day also met the primary endpoint (P less than .0001, compared with placebo), he said.
The most common treatment-related adverse effect was hypertension, which affected 8% of women in the 10-mg group and 4% of women in the 5-mg group, he added during the late-breaking oral presentation.
Ulipristal acetate is currently approved in the United States as an emergency contraceptive, but is marketed for treating symptomatic fibroids in Canada and Europe. The drug reduced bleeding in about 90% of patients in the European trials (N Engl J Med. 2012;366:409-20; Fertil Steril. 2014 Jun;101[6]:1565-73).
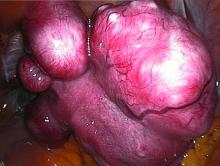
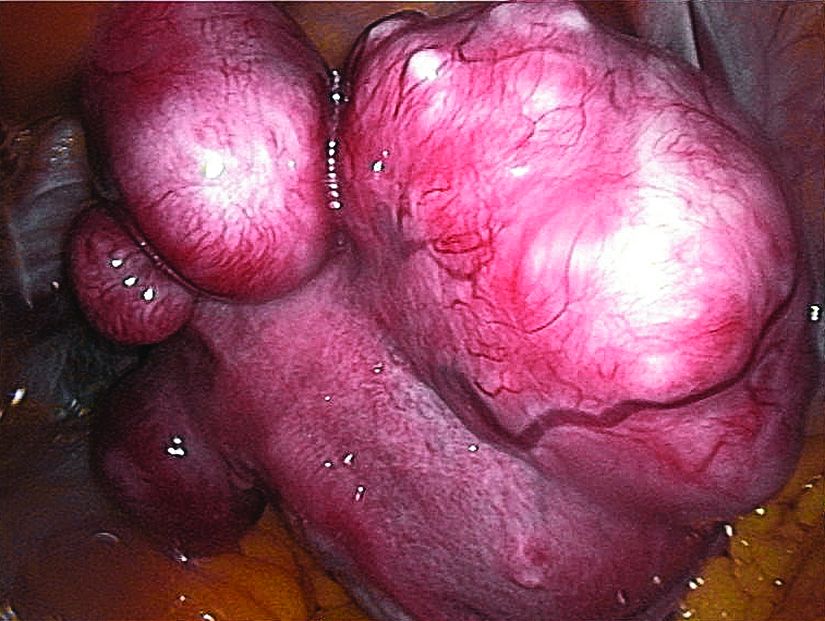
The VENUS-I trial included premenopausal women aged 18-50 years who had experienced 22-35 days of bleeding during at least four of their last six menstrual cycles, with at least 80 mL menstrual blood loss, and at least one fibroid confirmed by transvaginal ultrasound.
“The women in our study were more severely affected, in terms of the amount of bleeding and in terms of fibroid size and uterine size, than what was reported in the European trials,” Dr. Simon said.
The average age of the patients was 41 years, and 69% were African-American, reflecting the disproportionate burden of severely symptomatic fibroids in this group, he said.
Patients were randomly assigned to oral treatment with either placebo or 10 mg or 5 mg ulipristal acetate for 12 weeks, followed by a 12-week drug-free observation period. The primary endpoint was amenorrhea, not including spotting, during the last 35 consecutive days of treatment.
The 10-mg group was 49 times more likely to reach this endpoint than was the placebo arm (P less than .0001), even after the researchers controlled for multiple potential confounders, Dr. Simon reported. The hazard ratio for the 5-mg group was also highly statistically significant at 35.5 (P less than .0001).
“Almost all patients who achieved amenorrhea in the ulipristal acetate groups did so by day 11,” Dr. Simon said.
Rates of amenorrhea from day 11 through the end of treatment were 58% in the 10-mg group, 43% in the 5-mg group, and 0% in the placebo group (P less than .0001 for differences among groups). Patients who received ulipristal acetate also reported significantly greater improvements on the Uterine Fibroid Symptom and Quality of Life (UFS-QOL) questionnaire than did the placebo group.
No patients stopped treatment because of adverse effects. Of the six patients who developed hypertension while on ulipristal acetate, five had a history of high blood pressure, and the increases were generally “slight,” Dr. Simon said. The other common treatment-emergent adverse effects included increased creatinine phosphokinase, hot flashes, acne, and nausea.
Blinded pathologists interpreted endometrial biopsies at baseline, the end of treatment, and at the end of the drug-free observation period. These revealed no cases of atypical endometrial hyperplasia or malignancy, and no baseline increases in rates of progesterone receptor modulator–associated endometrial changes, Dr. Simon said. One patient developed endometrial hyperplasia without atypia at the end of treatment, which resolved by the end of the 12-week drug-free observation period.
VENUS-I is one of two pivotal trials of ulipristal acetate for the treatment of uterine fibroids in the United States. Results from the second trial, VENUS-II, are expected in 2017.
The VENUS-I trial was supported by Allergan and Gedeon Richter. Dr. Simon reported ties to numerous other pharmaceutical companies, but not to Allergan or Gedeon Richter.
SALT LAKE CITY – The selective progesterone receptor modulator ulipristal acetate significantly reduced bleeding associated with fibroids in VENUS-I, a pivotal phase III trial of 147 premenopausal women.
A total of 58% of patients who took 10 mg ulipristal acetate per day had no bleeding except spotting during the last 35 days of treatment, compared with 2% of the placebo group (P less than .0001), James Simon, MD, said at the annual meeting of the American Society for Reproductive Medicine. Forty-seven percent of patients who took 5 mg ulipristal acetate per day also met the primary endpoint (P less than .0001, compared with placebo), he said.
The most common treatment-related adverse effect was hypertension, which affected 8% of women in the 10-mg group and 4% of women in the 5-mg group, he added during the late-breaking oral presentation.
Ulipristal acetate is currently approved in the United States as an emergency contraceptive, but is marketed for treating symptomatic fibroids in Canada and Europe. The drug reduced bleeding in about 90% of patients in the European trials (N Engl J Med. 2012;366:409-20; Fertil Steril. 2014 Jun;101[6]:1565-73).
The VENUS-I trial included premenopausal women aged 18-50 years who had experienced 22-35 days of bleeding during at least four of their last six menstrual cycles, with at least 80 mL menstrual blood loss, and at least one fibroid confirmed by transvaginal ultrasound.
“The women in our study were more severely affected, in terms of the amount of bleeding and in terms of fibroid size and uterine size, than what was reported in the European trials,” Dr. Simon said.
The average age of the patients was 41 years, and 69% were African-American, reflecting the disproportionate burden of severely symptomatic fibroids in this group, he said.
Patients were randomly assigned to oral treatment with either placebo or 10 mg or 5 mg ulipristal acetate for 12 weeks, followed by a 12-week drug-free observation period. The primary endpoint was amenorrhea, not including spotting, during the last 35 consecutive days of treatment.
The 10-mg group was 49 times more likely to reach this endpoint than was the placebo arm (P less than .0001), even after the researchers controlled for multiple potential confounders, Dr. Simon reported. The hazard ratio for the 5-mg group was also highly statistically significant at 35.5 (P less than .0001).
“Almost all patients who achieved amenorrhea in the ulipristal acetate groups did so by day 11,” Dr. Simon said.
Rates of amenorrhea from day 11 through the end of treatment were 58% in the 10-mg group, 43% in the 5-mg group, and 0% in the placebo group (P less than .0001 for differences among groups). Patients who received ulipristal acetate also reported significantly greater improvements on the Uterine Fibroid Symptom and Quality of Life (UFS-QOL) questionnaire than did the placebo group.
No patients stopped treatment because of adverse effects. Of the six patients who developed hypertension while on ulipristal acetate, five had a history of high blood pressure, and the increases were generally “slight,” Dr. Simon said. The other common treatment-emergent adverse effects included increased creatinine phosphokinase, hot flashes, acne, and nausea.
Blinded pathologists interpreted endometrial biopsies at baseline, the end of treatment, and at the end of the drug-free observation period. These revealed no cases of atypical endometrial hyperplasia or malignancy, and no baseline increases in rates of progesterone receptor modulator–associated endometrial changes, Dr. Simon said. One patient developed endometrial hyperplasia without atypia at the end of treatment, which resolved by the end of the 12-week drug-free observation period.
VENUS-I is one of two pivotal trials of ulipristal acetate for the treatment of uterine fibroids in the United States. Results from the second trial, VENUS-II, are expected in 2017.
The VENUS-I trial was supported by Allergan and Gedeon Richter. Dr. Simon reported ties to numerous other pharmaceutical companies, but not to Allergan or Gedeon Richter.
SALT LAKE CITY – The selective progesterone receptor modulator ulipristal acetate significantly reduced bleeding associated with fibroids in VENUS-I, a pivotal phase III trial of 147 premenopausal women.
A total of 58% of patients who took 10 mg ulipristal acetate per day had no bleeding except spotting during the last 35 days of treatment, compared with 2% of the placebo group (P less than .0001), James Simon, MD, said at the annual meeting of the American Society for Reproductive Medicine. Forty-seven percent of patients who took 5 mg ulipristal acetate per day also met the primary endpoint (P less than .0001, compared with placebo), he said.
The most common treatment-related adverse effect was hypertension, which affected 8% of women in the 10-mg group and 4% of women in the 5-mg group, he added during the late-breaking oral presentation.
Ulipristal acetate is currently approved in the United States as an emergency contraceptive, but is marketed for treating symptomatic fibroids in Canada and Europe. The drug reduced bleeding in about 90% of patients in the European trials (N Engl J Med. 2012;366:409-20; Fertil Steril. 2014 Jun;101[6]:1565-73).
The VENUS-I trial included premenopausal women aged 18-50 years who had experienced 22-35 days of bleeding during at least four of their last six menstrual cycles, with at least 80 mL menstrual blood loss, and at least one fibroid confirmed by transvaginal ultrasound.
“The women in our study were more severely affected, in terms of the amount of bleeding and in terms of fibroid size and uterine size, than what was reported in the European trials,” Dr. Simon said.
The average age of the patients was 41 years, and 69% were African-American, reflecting the disproportionate burden of severely symptomatic fibroids in this group, he said.
Patients were randomly assigned to oral treatment with either placebo or 10 mg or 5 mg ulipristal acetate for 12 weeks, followed by a 12-week drug-free observation period. The primary endpoint was amenorrhea, not including spotting, during the last 35 consecutive days of treatment.
The 10-mg group was 49 times more likely to reach this endpoint than was the placebo arm (P less than .0001), even after the researchers controlled for multiple potential confounders, Dr. Simon reported. The hazard ratio for the 5-mg group was also highly statistically significant at 35.5 (P less than .0001).
“Almost all patients who achieved amenorrhea in the ulipristal acetate groups did so by day 11,” Dr. Simon said.
Rates of amenorrhea from day 11 through the end of treatment were 58% in the 10-mg group, 43% in the 5-mg group, and 0% in the placebo group (P less than .0001 for differences among groups). Patients who received ulipristal acetate also reported significantly greater improvements on the Uterine Fibroid Symptom and Quality of Life (UFS-QOL) questionnaire than did the placebo group.
No patients stopped treatment because of adverse effects. Of the six patients who developed hypertension while on ulipristal acetate, five had a history of high blood pressure, and the increases were generally “slight,” Dr. Simon said. The other common treatment-emergent adverse effects included increased creatinine phosphokinase, hot flashes, acne, and nausea.
Blinded pathologists interpreted endometrial biopsies at baseline, the end of treatment, and at the end of the drug-free observation period. These revealed no cases of atypical endometrial hyperplasia or malignancy, and no baseline increases in rates of progesterone receptor modulator–associated endometrial changes, Dr. Simon said. One patient developed endometrial hyperplasia without atypia at the end of treatment, which resolved by the end of the 12-week drug-free observation period.
VENUS-I is one of two pivotal trials of ulipristal acetate for the treatment of uterine fibroids in the United States. Results from the second trial, VENUS-II, are expected in 2017.
The VENUS-I trial was supported by Allergan and Gedeon Richter. Dr. Simon reported ties to numerous other pharmaceutical companies, but not to Allergan or Gedeon Richter.
AT 2016 ASRM
Key clinical point:
Major finding: Rates of amenorrhea during the last 35 consecutive days of treatment were 58% in the 10-mg group, compared with 2% in the placebo group (P less than .0001).
Data source: A randomized, double-blind trial of 147 premenopausal women with uterine fibroids and an average of 80 mL menstrual blood loss.
Disclosures: VENUS-I was supported by Allergan and Gedeon Richter. Dr. Simon reported ties to numerous other pharmaceutical companies, but not to Allergan or Gedeon Richter.