User login
Scleroderma SCOT trial findings hold similar in lung disease
CHICAGO – Changes in quantitative lung CT scores for scleroderma-related interstitial lung disease independently validate the superiority of hematopoietic stem cell transplantation versus cyclophosphamide for severe systemic sclerosis, according to findings in a subset of patients from the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial.
The recently published findings from the SCOT trial showed that myeloablation followed by autologous hematopoietic stem cell transplant (HSCT) significantly improved event-free and overall survival of systemic sclerosis patients at 54 months, compared with 12 monthly treatments with intravenous cyclophosphamide (N Engl J Med. 2018;378:35-47).
In a subset of 75 patients from the SCOT trial, the investigators analyzed changes in lung parenchymal abnormalities on high-resolution CT scans between baseline and serial follow-up exams performed yearly for up to 5 years. Follow-up scans at 14, 26, 48, and 54 months in available patients at each time point showed that whole-lung quantitative interstitial lung disease (QILD) scores – a validated measure that combines various CT texture-based characteristics to determine disease extent – decreased significantly by 7% at 54 months in patients who underwent HSCT, compared with no change in those who received cyclophosphamide (CYC; P = .024), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
Additionally, whole-lung quantitative lung fibrosis (QLF) scores were stable (–1%) in the HSCT patients, but increased 3% in the CYC patients (P = .047), said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Dr. Sullivan was the first author on the SCOT trial, and he reported the current study results on behalf of lead investigator Jonathan Goldin, MD, PhD, of the department of radiologic sciences at the University of California, Los Angeles.
“These are really kind of meaningful associations, especially since the worst of the [CYC] treatment group didn’t make it to month 54,” Dr. Sullivan said.
Quantitative scores of scleroderma-related interstitial lung disease were measured using computer-based quantitative image analysis of standardized, noncontrast, volumetric, thin-section, thoracic, high-resolution CT. The same CT machine was used for all time points (except for one subject) with careful attention to breath hold reproducibility and image quality. Baseline characteristics were not different between the HSCT and CYC groups, he noted, stressing the rigorous study design.
CT assessments were also compared for the most severe lobe in each patient and showed similar findings, with both QILD and QLF scores for that lobe improving in the HSCT patients relative to the CYC patients (P = .004 and P = .002, respectively), Dr. Sullivan said, adding that the direction of change in structural measures of QILD and QLF for both whole lung and most severe lobe CTs tracked with physiological pulmonary function tests, including forced vital capacity (FVC), forced expiratory volume in 1 second, and diffusing capacity of the lungs for carbon monoxide.
“The FVC improved while QILD decreased, and that’s what you would expect to see,” he said. “So for each of these ways of displaying data, there was an expected and sensible inverse correlation.”
Scleroderma-related interstitial lung disease is a major cause of morbidity and mortality in severe systemic sclerosis. In the wake of the SCOT trial findings, questions remained with respect to correlation between those findings and pulmonary function; if the improvements with HSCT are real and meaningful, they should have meaningful correlation with pulmonary function, and these findings demonstrate those correlates, he said.
“Changes in quantitative lung CT scoring of scleroderma lung disease provide an objective radiologic validation of the long-term benefits of transplant compared to cyclophosphamide in individuals with severe scleroderma and lung involvement. Improvement in imaging after transplant continues for up to 54 months after randomization, giving radiologic confirmation of a durable treatment benefit,” Dr. Sullivan concluded.
The investigators reported having no relevant disclosures.
SOURCE: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
CHICAGO – Changes in quantitative lung CT scores for scleroderma-related interstitial lung disease independently validate the superiority of hematopoietic stem cell transplantation versus cyclophosphamide for severe systemic sclerosis, according to findings in a subset of patients from the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial.
The recently published findings from the SCOT trial showed that myeloablation followed by autologous hematopoietic stem cell transplant (HSCT) significantly improved event-free and overall survival of systemic sclerosis patients at 54 months, compared with 12 monthly treatments with intravenous cyclophosphamide (N Engl J Med. 2018;378:35-47).
In a subset of 75 patients from the SCOT trial, the investigators analyzed changes in lung parenchymal abnormalities on high-resolution CT scans between baseline and serial follow-up exams performed yearly for up to 5 years. Follow-up scans at 14, 26, 48, and 54 months in available patients at each time point showed that whole-lung quantitative interstitial lung disease (QILD) scores – a validated measure that combines various CT texture-based characteristics to determine disease extent – decreased significantly by 7% at 54 months in patients who underwent HSCT, compared with no change in those who received cyclophosphamide (CYC; P = .024), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
Additionally, whole-lung quantitative lung fibrosis (QLF) scores were stable (–1%) in the HSCT patients, but increased 3% in the CYC patients (P = .047), said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Dr. Sullivan was the first author on the SCOT trial, and he reported the current study results on behalf of lead investigator Jonathan Goldin, MD, PhD, of the department of radiologic sciences at the University of California, Los Angeles.
“These are really kind of meaningful associations, especially since the worst of the [CYC] treatment group didn’t make it to month 54,” Dr. Sullivan said.
Quantitative scores of scleroderma-related interstitial lung disease were measured using computer-based quantitative image analysis of standardized, noncontrast, volumetric, thin-section, thoracic, high-resolution CT. The same CT machine was used for all time points (except for one subject) with careful attention to breath hold reproducibility and image quality. Baseline characteristics were not different between the HSCT and CYC groups, he noted, stressing the rigorous study design.
CT assessments were also compared for the most severe lobe in each patient and showed similar findings, with both QILD and QLF scores for that lobe improving in the HSCT patients relative to the CYC patients (P = .004 and P = .002, respectively), Dr. Sullivan said, adding that the direction of change in structural measures of QILD and QLF for both whole lung and most severe lobe CTs tracked with physiological pulmonary function tests, including forced vital capacity (FVC), forced expiratory volume in 1 second, and diffusing capacity of the lungs for carbon monoxide.
“The FVC improved while QILD decreased, and that’s what you would expect to see,” he said. “So for each of these ways of displaying data, there was an expected and sensible inverse correlation.”
Scleroderma-related interstitial lung disease is a major cause of morbidity and mortality in severe systemic sclerosis. In the wake of the SCOT trial findings, questions remained with respect to correlation between those findings and pulmonary function; if the improvements with HSCT are real and meaningful, they should have meaningful correlation with pulmonary function, and these findings demonstrate those correlates, he said.
“Changes in quantitative lung CT scoring of scleroderma lung disease provide an objective radiologic validation of the long-term benefits of transplant compared to cyclophosphamide in individuals with severe scleroderma and lung involvement. Improvement in imaging after transplant continues for up to 54 months after randomization, giving radiologic confirmation of a durable treatment benefit,” Dr. Sullivan concluded.
The investigators reported having no relevant disclosures.
SOURCE: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
CHICAGO – Changes in quantitative lung CT scores for scleroderma-related interstitial lung disease independently validate the superiority of hematopoietic stem cell transplantation versus cyclophosphamide for severe systemic sclerosis, according to findings in a subset of patients from the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial.
The recently published findings from the SCOT trial showed that myeloablation followed by autologous hematopoietic stem cell transplant (HSCT) significantly improved event-free and overall survival of systemic sclerosis patients at 54 months, compared with 12 monthly treatments with intravenous cyclophosphamide (N Engl J Med. 2018;378:35-47).
In a subset of 75 patients from the SCOT trial, the investigators analyzed changes in lung parenchymal abnormalities on high-resolution CT scans between baseline and serial follow-up exams performed yearly for up to 5 years. Follow-up scans at 14, 26, 48, and 54 months in available patients at each time point showed that whole-lung quantitative interstitial lung disease (QILD) scores – a validated measure that combines various CT texture-based characteristics to determine disease extent – decreased significantly by 7% at 54 months in patients who underwent HSCT, compared with no change in those who received cyclophosphamide (CYC; P = .024), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
Additionally, whole-lung quantitative lung fibrosis (QLF) scores were stable (–1%) in the HSCT patients, but increased 3% in the CYC patients (P = .047), said Dr. Sullivan, a professor of medicine at Duke University, Durham, N.C.
Dr. Sullivan was the first author on the SCOT trial, and he reported the current study results on behalf of lead investigator Jonathan Goldin, MD, PhD, of the department of radiologic sciences at the University of California, Los Angeles.
“These are really kind of meaningful associations, especially since the worst of the [CYC] treatment group didn’t make it to month 54,” Dr. Sullivan said.
Quantitative scores of scleroderma-related interstitial lung disease were measured using computer-based quantitative image analysis of standardized, noncontrast, volumetric, thin-section, thoracic, high-resolution CT. The same CT machine was used for all time points (except for one subject) with careful attention to breath hold reproducibility and image quality. Baseline characteristics were not different between the HSCT and CYC groups, he noted, stressing the rigorous study design.
CT assessments were also compared for the most severe lobe in each patient and showed similar findings, with both QILD and QLF scores for that lobe improving in the HSCT patients relative to the CYC patients (P = .004 and P = .002, respectively), Dr. Sullivan said, adding that the direction of change in structural measures of QILD and QLF for both whole lung and most severe lobe CTs tracked with physiological pulmonary function tests, including forced vital capacity (FVC), forced expiratory volume in 1 second, and diffusing capacity of the lungs for carbon monoxide.
“The FVC improved while QILD decreased, and that’s what you would expect to see,” he said. “So for each of these ways of displaying data, there was an expected and sensible inverse correlation.”
Scleroderma-related interstitial lung disease is a major cause of morbidity and mortality in severe systemic sclerosis. In the wake of the SCOT trial findings, questions remained with respect to correlation between those findings and pulmonary function; if the improvements with HSCT are real and meaningful, they should have meaningful correlation with pulmonary function, and these findings demonstrate those correlates, he said.
“Changes in quantitative lung CT scoring of scleroderma lung disease provide an objective radiologic validation of the long-term benefits of transplant compared to cyclophosphamide in individuals with severe scleroderma and lung involvement. Improvement in imaging after transplant continues for up to 54 months after randomization, giving radiologic confirmation of a durable treatment benefit,” Dr. Sullivan concluded.
The investigators reported having no relevant disclosures.
SOURCE: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Lung CT scores remain stable or improve following hematopoietic stem cell transplantation in patients with scleroderma-related interstitial lung disease when compared against monthly cyclophosphamide treatments.
Major finding: Quantitative interstitial lung disease scores decreased by 7% at 54 months in hematopoietic stem cell transplant patients versus no change in those who received cyclophosphamide (P = .024).
Study details: A study of 75 patients from the SCOT trial.
Disclosures: The investigators reported having no relevant disclosures.
Source: Goldin J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 901.
Rituximab shines in pediatric vasculitis
CHICAGO – Rituximab demonstrated a high degree of effectiveness with no safety surprises in the first-ever major clinical trial of the potent B-cell inhibitor conducted in pediatric patients with newly diagnosed or relapsing granulomatosis with polyangiitis or microscopic polyangiitis, Jennifer Cooper, MD, PharmD, said at the annual meeting of the American College of Rheumatology.
“This is exciting news. We know that rituximab is very effective in treating adults with GPA and MPA, both in terms of inducing remission and even for maintenance therapy for this rare and severe disease. A lot of pediatric rheumatologists would like to have access to rituximab. Some are even using it for this condition. But until now there were no data in children. I hope this study improves access to rituximab for pediatric patients with ANCA-associated vasculitis,” said Dr. Cooper, a pediatric rheumatologist at the University of Colorado, Denver.
She presented the results of the PePRS (Pediatric Polyangiitis and Rituximab Study), a phase 2a, single-arm, open-label, long-term study of 25 patients in six countries. She anticipates the results will be practice changing, given that pediatric GPA and MPA are recognized as severe systemic autoimmune disorders with a high unmet need for new therapies.
“I don’t believe there are plans for a randomized, controlled trial. Since we now have the pharmacokinetic and safety data in children, we’ll hopefully be able to use extrapolation and our exploratory efficacy endpoints to gain a pediatric indication, or at least a label update for these patients based on this study,” Dr. Cooper continued.
A total of 19 patients had GPA and 6 had MPA, with a median disease duration of 6 months at study entry. All received three pulsed doses of methylprednisolone during the screening period. Then for induction remission they got four once-weekly intravenous infusions of rituximab (Rituxan) at 375 mg/m2 as well as oral corticosteroids, which were tapered from 1 mg/kg per day to 0.2 mg/kg per day over the course of the first 6 months. After that, two-thirds of patients received additional rituximab at their provider’s discretion.
The remission rate by 6 months as defined by Pediatric Vasculitis Activity Score (PVAS) criteria was 56%. At 12 months, the rate was 92%, and at 18 months the rate of remission and sustained disease control was 100%. The mean and median durations of remission were 72 and 56 weeks, respectively.
These results in pediatric patients are comparable to those seen in adults in the landmark RAVE (Rituximab in ANCA-Associated Vasculitis) trial (N Engl J Med. 2013 Aug 1;369[5]:417-27), where 64% of patients reached remission at 6 months, Dr. Cooper noted.
All 25 patients were able to tolerate the four rituximab infusions for remission induction. The main side effect was infusion-related reactions. Overall, 32% of patients experienced such reactions in response to their first infusion, 20% with the second, 12% with the third, and only 8% with the fourth.
Eight patients withdrew from the study after 18 months, mostly because of transfer to adult care. The remaining participants were followed for as long as 4.5 years.
F. Hoffmann-La Roche and Genentech sponsored the PePRS study. Dr. Cooper was a Genentech clinical research fellow at the time.
SOURCE: Brogan P at al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L04.
CHICAGO – Rituximab demonstrated a high degree of effectiveness with no safety surprises in the first-ever major clinical trial of the potent B-cell inhibitor conducted in pediatric patients with newly diagnosed or relapsing granulomatosis with polyangiitis or microscopic polyangiitis, Jennifer Cooper, MD, PharmD, said at the annual meeting of the American College of Rheumatology.
“This is exciting news. We know that rituximab is very effective in treating adults with GPA and MPA, both in terms of inducing remission and even for maintenance therapy for this rare and severe disease. A lot of pediatric rheumatologists would like to have access to rituximab. Some are even using it for this condition. But until now there were no data in children. I hope this study improves access to rituximab for pediatric patients with ANCA-associated vasculitis,” said Dr. Cooper, a pediatric rheumatologist at the University of Colorado, Denver.
She presented the results of the PePRS (Pediatric Polyangiitis and Rituximab Study), a phase 2a, single-arm, open-label, long-term study of 25 patients in six countries. She anticipates the results will be practice changing, given that pediatric GPA and MPA are recognized as severe systemic autoimmune disorders with a high unmet need for new therapies.
“I don’t believe there are plans for a randomized, controlled trial. Since we now have the pharmacokinetic and safety data in children, we’ll hopefully be able to use extrapolation and our exploratory efficacy endpoints to gain a pediatric indication, or at least a label update for these patients based on this study,” Dr. Cooper continued.
A total of 19 patients had GPA and 6 had MPA, with a median disease duration of 6 months at study entry. All received three pulsed doses of methylprednisolone during the screening period. Then for induction remission they got four once-weekly intravenous infusions of rituximab (Rituxan) at 375 mg/m2 as well as oral corticosteroids, which were tapered from 1 mg/kg per day to 0.2 mg/kg per day over the course of the first 6 months. After that, two-thirds of patients received additional rituximab at their provider’s discretion.
The remission rate by 6 months as defined by Pediatric Vasculitis Activity Score (PVAS) criteria was 56%. At 12 months, the rate was 92%, and at 18 months the rate of remission and sustained disease control was 100%. The mean and median durations of remission were 72 and 56 weeks, respectively.
These results in pediatric patients are comparable to those seen in adults in the landmark RAVE (Rituximab in ANCA-Associated Vasculitis) trial (N Engl J Med. 2013 Aug 1;369[5]:417-27), where 64% of patients reached remission at 6 months, Dr. Cooper noted.
All 25 patients were able to tolerate the four rituximab infusions for remission induction. The main side effect was infusion-related reactions. Overall, 32% of patients experienced such reactions in response to their first infusion, 20% with the second, 12% with the third, and only 8% with the fourth.
Eight patients withdrew from the study after 18 months, mostly because of transfer to adult care. The remaining participants were followed for as long as 4.5 years.
F. Hoffmann-La Roche and Genentech sponsored the PePRS study. Dr. Cooper was a Genentech clinical research fellow at the time.
SOURCE: Brogan P at al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L04.
CHICAGO – Rituximab demonstrated a high degree of effectiveness with no safety surprises in the first-ever major clinical trial of the potent B-cell inhibitor conducted in pediatric patients with newly diagnosed or relapsing granulomatosis with polyangiitis or microscopic polyangiitis, Jennifer Cooper, MD, PharmD, said at the annual meeting of the American College of Rheumatology.
“This is exciting news. We know that rituximab is very effective in treating adults with GPA and MPA, both in terms of inducing remission and even for maintenance therapy for this rare and severe disease. A lot of pediatric rheumatologists would like to have access to rituximab. Some are even using it for this condition. But until now there were no data in children. I hope this study improves access to rituximab for pediatric patients with ANCA-associated vasculitis,” said Dr. Cooper, a pediatric rheumatologist at the University of Colorado, Denver.
She presented the results of the PePRS (Pediatric Polyangiitis and Rituximab Study), a phase 2a, single-arm, open-label, long-term study of 25 patients in six countries. She anticipates the results will be practice changing, given that pediatric GPA and MPA are recognized as severe systemic autoimmune disorders with a high unmet need for new therapies.
“I don’t believe there are plans for a randomized, controlled trial. Since we now have the pharmacokinetic and safety data in children, we’ll hopefully be able to use extrapolation and our exploratory efficacy endpoints to gain a pediatric indication, or at least a label update for these patients based on this study,” Dr. Cooper continued.
A total of 19 patients had GPA and 6 had MPA, with a median disease duration of 6 months at study entry. All received three pulsed doses of methylprednisolone during the screening period. Then for induction remission they got four once-weekly intravenous infusions of rituximab (Rituxan) at 375 mg/m2 as well as oral corticosteroids, which were tapered from 1 mg/kg per day to 0.2 mg/kg per day over the course of the first 6 months. After that, two-thirds of patients received additional rituximab at their provider’s discretion.
The remission rate by 6 months as defined by Pediatric Vasculitis Activity Score (PVAS) criteria was 56%. At 12 months, the rate was 92%, and at 18 months the rate of remission and sustained disease control was 100%. The mean and median durations of remission were 72 and 56 weeks, respectively.
These results in pediatric patients are comparable to those seen in adults in the landmark RAVE (Rituximab in ANCA-Associated Vasculitis) trial (N Engl J Med. 2013 Aug 1;369[5]:417-27), where 64% of patients reached remission at 6 months, Dr. Cooper noted.
All 25 patients were able to tolerate the four rituximab infusions for remission induction. The main side effect was infusion-related reactions. Overall, 32% of patients experienced such reactions in response to their first infusion, 20% with the second, 12% with the third, and only 8% with the fourth.
Eight patients withdrew from the study after 18 months, mostly because of transfer to adult care. The remaining participants were followed for as long as 4.5 years.
F. Hoffmann-La Roche and Genentech sponsored the PePRS study. Dr. Cooper was a Genentech clinical research fellow at the time.
SOURCE: Brogan P at al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L04.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: The first clinical trial of rituximab for newly diagnosed or relapsing GPA or MPA in pediatric patients showed remission rates of 56%, 92%, and 100% by months 6, 12, and 18, respectively.
Study details: The PePRS study was a phase 2a, single-arm, open-label, long-term study of 25 patients in six countries.
Disclosures: F. Hoffmann-La Roche and Genentech sponsored the study. The presenter was a Genentech clinical research fellow at the time.
Source: Brogan P at al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract L04.
ACR forges ahead with physician-focused APM for RA
CHICAGO – with hopes to send the fully developed model to the Physician-Focused Payment Model Technical Advisory Committee once financial data gathering and analysis is complete, followed by pilot testing once it is accepted by the Centers for Medicare & Medicaid Services, speakers said at the annual meeting of the ACR.
The “RA Care Team” APM is meant to be versatile and work across various practice settings, from rural Alaska and the Southwest to urban Chicago and Boston, and would consist of a rheumatologist or a nurse practitioner or physician assistant working with a rheumatologist in some areas, while in others a patient would be managed by a primary care physician who has a formal arrangement with a rheumatologist to provide early treatment of RA. Participation in the model would use a standard treatment approach pathway that follows ACR treatment guidelines and saves money by reducing the variability in initiation of expensive medications and would be versatile enough to allow for unique patients by requiring only 75% adherence to the pathway across a practice’s patients, said Kwas Huston, MD, cochair of the ACR’s APM work group and a rheumatologist with Kansas City (Mo.) Physician Partners.
The model covers four phases of care, including diagnosis and treatment planning for patients with potential RA, support for primary care practices in evaluating joint symptoms, the initial treatment of patients with RA, and the continued care of RA. For instance, a rheumatologist could receive payment for an e-consult with a primary care provider to determine if a patient has symptoms requiring an evaluation for RA. A rheumatologist could also receive a one-time payment for treatment and planning services when a diagnosis of RA cannot be established in a patient suspected of having RA, whereas the rheumatologist or a primary care provider with rheumatology support would receive monthly payments for 6 months for the initial treatment of an RA patient and then thereafter would receive monthly payments for continued care of RA. The payments would not be dependent on the number of visits or face-to-face time, would be stratified based on patient characteristics, and would include some lab testing and imaging.
Currently, the RA APM work group is analyzing financial data gathered from two large rheumatology practices for specific CPT codes matched to specific patients based on their clinical characteristics “to get a sense of how much revenue is coming in right now for practices taking care of patients with, let’s say, moderate disease activity and two comorbidities or low disease activity and no comorbidities,” Dr. Huston said. The work group is also surveying practices to estimate additional costs required to participate in the APM and thereby develop a financial model to adequately pay for APM services. They additionally plan to develop a tool kit that is designed to help individual practices determine the economic impact that the APM would have.
Notably, the cost of medications is not included in the APM. “That would be too much of a risk for small practices to take on,” Dr. Huston said.
“This model is a work in progress. We still have a lot of additional work to validate the data,” he added.
Providers who wish to participate in an advanced APM in 2019 need to have at least 25% of their Medicare Part B payments or have at least 20% of Medicare patients in their practice come through the APM in order to avoid having to submit data to comply with the performance criteria requirements of the Merit-Based Incentive Payment System, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a practicing rheumatologist in the Washington area.
“The ACR has advocated to reduce these thresholds over the years, but unfortunately that has not happened yet,” Dr. Worthing said.
jevans@mdedge.com
CHICAGO – with hopes to send the fully developed model to the Physician-Focused Payment Model Technical Advisory Committee once financial data gathering and analysis is complete, followed by pilot testing once it is accepted by the Centers for Medicare & Medicaid Services, speakers said at the annual meeting of the ACR.
The “RA Care Team” APM is meant to be versatile and work across various practice settings, from rural Alaska and the Southwest to urban Chicago and Boston, and would consist of a rheumatologist or a nurse practitioner or physician assistant working with a rheumatologist in some areas, while in others a patient would be managed by a primary care physician who has a formal arrangement with a rheumatologist to provide early treatment of RA. Participation in the model would use a standard treatment approach pathway that follows ACR treatment guidelines and saves money by reducing the variability in initiation of expensive medications and would be versatile enough to allow for unique patients by requiring only 75% adherence to the pathway across a practice’s patients, said Kwas Huston, MD, cochair of the ACR’s APM work group and a rheumatologist with Kansas City (Mo.) Physician Partners.
The model covers four phases of care, including diagnosis and treatment planning for patients with potential RA, support for primary care practices in evaluating joint symptoms, the initial treatment of patients with RA, and the continued care of RA. For instance, a rheumatologist could receive payment for an e-consult with a primary care provider to determine if a patient has symptoms requiring an evaluation for RA. A rheumatologist could also receive a one-time payment for treatment and planning services when a diagnosis of RA cannot be established in a patient suspected of having RA, whereas the rheumatologist or a primary care provider with rheumatology support would receive monthly payments for 6 months for the initial treatment of an RA patient and then thereafter would receive monthly payments for continued care of RA. The payments would not be dependent on the number of visits or face-to-face time, would be stratified based on patient characteristics, and would include some lab testing and imaging.
Currently, the RA APM work group is analyzing financial data gathered from two large rheumatology practices for specific CPT codes matched to specific patients based on their clinical characteristics “to get a sense of how much revenue is coming in right now for practices taking care of patients with, let’s say, moderate disease activity and two comorbidities or low disease activity and no comorbidities,” Dr. Huston said. The work group is also surveying practices to estimate additional costs required to participate in the APM and thereby develop a financial model to adequately pay for APM services. They additionally plan to develop a tool kit that is designed to help individual practices determine the economic impact that the APM would have.
Notably, the cost of medications is not included in the APM. “That would be too much of a risk for small practices to take on,” Dr. Huston said.
“This model is a work in progress. We still have a lot of additional work to validate the data,” he added.
Providers who wish to participate in an advanced APM in 2019 need to have at least 25% of their Medicare Part B payments or have at least 20% of Medicare patients in their practice come through the APM in order to avoid having to submit data to comply with the performance criteria requirements of the Merit-Based Incentive Payment System, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a practicing rheumatologist in the Washington area.
“The ACR has advocated to reduce these thresholds over the years, but unfortunately that has not happened yet,” Dr. Worthing said.
jevans@mdedge.com
CHICAGO – with hopes to send the fully developed model to the Physician-Focused Payment Model Technical Advisory Committee once financial data gathering and analysis is complete, followed by pilot testing once it is accepted by the Centers for Medicare & Medicaid Services, speakers said at the annual meeting of the ACR.
The “RA Care Team” APM is meant to be versatile and work across various practice settings, from rural Alaska and the Southwest to urban Chicago and Boston, and would consist of a rheumatologist or a nurse practitioner or physician assistant working with a rheumatologist in some areas, while in others a patient would be managed by a primary care physician who has a formal arrangement with a rheumatologist to provide early treatment of RA. Participation in the model would use a standard treatment approach pathway that follows ACR treatment guidelines and saves money by reducing the variability in initiation of expensive medications and would be versatile enough to allow for unique patients by requiring only 75% adherence to the pathway across a practice’s patients, said Kwas Huston, MD, cochair of the ACR’s APM work group and a rheumatologist with Kansas City (Mo.) Physician Partners.
The model covers four phases of care, including diagnosis and treatment planning for patients with potential RA, support for primary care practices in evaluating joint symptoms, the initial treatment of patients with RA, and the continued care of RA. For instance, a rheumatologist could receive payment for an e-consult with a primary care provider to determine if a patient has symptoms requiring an evaluation for RA. A rheumatologist could also receive a one-time payment for treatment and planning services when a diagnosis of RA cannot be established in a patient suspected of having RA, whereas the rheumatologist or a primary care provider with rheumatology support would receive monthly payments for 6 months for the initial treatment of an RA patient and then thereafter would receive monthly payments for continued care of RA. The payments would not be dependent on the number of visits or face-to-face time, would be stratified based on patient characteristics, and would include some lab testing and imaging.
Currently, the RA APM work group is analyzing financial data gathered from two large rheumatology practices for specific CPT codes matched to specific patients based on their clinical characteristics “to get a sense of how much revenue is coming in right now for practices taking care of patients with, let’s say, moderate disease activity and two comorbidities or low disease activity and no comorbidities,” Dr. Huston said. The work group is also surveying practices to estimate additional costs required to participate in the APM and thereby develop a financial model to adequately pay for APM services. They additionally plan to develop a tool kit that is designed to help individual practices determine the economic impact that the APM would have.
Notably, the cost of medications is not included in the APM. “That would be too much of a risk for small practices to take on,” Dr. Huston said.
“This model is a work in progress. We still have a lot of additional work to validate the data,” he added.
Providers who wish to participate in an advanced APM in 2019 need to have at least 25% of their Medicare Part B payments or have at least 20% of Medicare patients in their practice come through the APM in order to avoid having to submit data to comply with the performance criteria requirements of the Merit-Based Incentive Payment System, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a practicing rheumatologist in the Washington area.
“The ACR has advocated to reduce these thresholds over the years, but unfortunately that has not happened yet,” Dr. Worthing said.
jevans@mdedge.com
REPORTING FROM THE ACR ANNUAL MEETING
MBDA score predicts, tracks RA patients’ responses to tofacitinib and rituximab
CHICAGO – The commercially available multibiomarker disease activity score assay and power Doppler ultrasound at baseline in rheumatoid arthritis patients treated with tofacitinib predicted 12-week responses on some clinical, imaging, and biomarker endpoints, according to findings from an investigator-initiated, open-label study.
The blood test–based multibiomarker disease activity (MBDA) score, which is calculated using measurements of 12 inflammatory biomarkers to score RA disease activity on a 0-100 point scale (Vectra DA, Myriad Autoimmune), also appears to track RA patients’ responses to rituximab, according to a post-hoc analysis of three cohort studies.
The findings of both studies were presented in posters at the annual meeting of the American College of Rheumatology. They are the first studies to evaluate early musculoskeletal ultrasound (MSUS) and MBDA score changes as predictors of response to tofacitinib in patients with RA, and the first to assess the ability of the MBDA score to track response to rituximab treatment. They provide valuable information that can help guide patient treatment and thereby improve outcomes, Elena Hitraya, MD, PhD, chief medical officer for Crescendo Bioscience/Myriad Autoimmune, San Francisco, said in an interview.
In the tofacitinib study, 25 RA patients with a mean age of 52 years, mean disease duration of 10.4 years, baseline Disease Activity Score 28-joint count (DAS28) greater than 3.2, and power Doppler ultrasound (PDUS) scores greater than 10 were treated with the approved oral tofacitinib dose of 5 mg twice daily. Assessments at baseline, 2 weeks, and 12 weeks included MSUS to score 34 joints for PDUS and gray scale ultrasound (GSUS), MBDA score, clinical disease activity index (CDAI), and DAS28, according to Amir Razmjou, MD, of the University of California, Los Angeles, and his colleagues.
Statistically significant improvement was seen on all measures over the 12-week study period (all at P less than .0001). For example, from baseline to 12 weeks the PDUS score improved from 28.6 to 12.2, GSUS score improved from 48.4 to 37.9, MBDA score improved from 50.6 to 39.6, CDAI score improved from 39.9 to 21.6, and DAS28–erythrocyte sedimentation rate (DAS28-ESR) score improved from 6.3 to 4.6, they said, noting further that baseline PDUS and MBDA scores significantly predicted CDAI and DAS28 responses at 12 weeks (P less than .01).
In the rituximab study, the MBDA score tracked disease activity in 57 RA patients from three different cohorts with a mean age of 57 years and mean disease duration of 11.5 years. Changes in the MBDA score reflected the degree of treatment response, Nadia M.T. Roodenrijs of University Medical Center Utrecht (the Netherlands) and her colleagues reported.
All patients were treated with 1,000 mg rituximab and 100 mg methylprednisolone on days 1 and 15, and MBDA score was assessed at baseline and 6 months.
MBDA scores correlated significantly with change from baseline to 6 months in DAS28-ESR (r = 0.60), DAS28–high-sensitivity C-reactive protein, (DAS28-hsCRP; r = 0.48), ESR (r = 0.48), and hsCRP (r = 0.71), and with European League Against Rheumatism (EULAR) good or moderate response at 6 months based on DAS28-ESR (adjusted odds ratio, 0.91).
Extensive work has been done to validate the MBDA score for assessing disease activity, and it has been shown to perform well for predicting response to a variety of disease-modifying antirheumatic drugs and biologic agents, Dr. Hitraya explained.
Additionally, multiple studies have demonstrated that the MBDA score defines risk categories for radiographic progression and performs better than traditional measures of disease activity for identifying those at increased risk of radiographic progression, which can help physicians mitigate associated risks through increased surveillance and therapeutic choices, she said.
“Having data for these specific molecules [tofacitinib and rituximab] is very important for rheumatologists,” Dr. Hitraya said.
The tofacitinib study was supported by Pfizer. Dr. Razmjou and Dr. Roodenrijs each reported having no disclosures.
SOURCE: Razmjou A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 582; Roodenrijs N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1500.
CHICAGO – The commercially available multibiomarker disease activity score assay and power Doppler ultrasound at baseline in rheumatoid arthritis patients treated with tofacitinib predicted 12-week responses on some clinical, imaging, and biomarker endpoints, according to findings from an investigator-initiated, open-label study.
The blood test–based multibiomarker disease activity (MBDA) score, which is calculated using measurements of 12 inflammatory biomarkers to score RA disease activity on a 0-100 point scale (Vectra DA, Myriad Autoimmune), also appears to track RA patients’ responses to rituximab, according to a post-hoc analysis of three cohort studies.
The findings of both studies were presented in posters at the annual meeting of the American College of Rheumatology. They are the first studies to evaluate early musculoskeletal ultrasound (MSUS) and MBDA score changes as predictors of response to tofacitinib in patients with RA, and the first to assess the ability of the MBDA score to track response to rituximab treatment. They provide valuable information that can help guide patient treatment and thereby improve outcomes, Elena Hitraya, MD, PhD, chief medical officer for Crescendo Bioscience/Myriad Autoimmune, San Francisco, said in an interview.
In the tofacitinib study, 25 RA patients with a mean age of 52 years, mean disease duration of 10.4 years, baseline Disease Activity Score 28-joint count (DAS28) greater than 3.2, and power Doppler ultrasound (PDUS) scores greater than 10 were treated with the approved oral tofacitinib dose of 5 mg twice daily. Assessments at baseline, 2 weeks, and 12 weeks included MSUS to score 34 joints for PDUS and gray scale ultrasound (GSUS), MBDA score, clinical disease activity index (CDAI), and DAS28, according to Amir Razmjou, MD, of the University of California, Los Angeles, and his colleagues.
Statistically significant improvement was seen on all measures over the 12-week study period (all at P less than .0001). For example, from baseline to 12 weeks the PDUS score improved from 28.6 to 12.2, GSUS score improved from 48.4 to 37.9, MBDA score improved from 50.6 to 39.6, CDAI score improved from 39.9 to 21.6, and DAS28–erythrocyte sedimentation rate (DAS28-ESR) score improved from 6.3 to 4.6, they said, noting further that baseline PDUS and MBDA scores significantly predicted CDAI and DAS28 responses at 12 weeks (P less than .01).
In the rituximab study, the MBDA score tracked disease activity in 57 RA patients from three different cohorts with a mean age of 57 years and mean disease duration of 11.5 years. Changes in the MBDA score reflected the degree of treatment response, Nadia M.T. Roodenrijs of University Medical Center Utrecht (the Netherlands) and her colleagues reported.
All patients were treated with 1,000 mg rituximab and 100 mg methylprednisolone on days 1 and 15, and MBDA score was assessed at baseline and 6 months.
MBDA scores correlated significantly with change from baseline to 6 months in DAS28-ESR (r = 0.60), DAS28–high-sensitivity C-reactive protein, (DAS28-hsCRP; r = 0.48), ESR (r = 0.48), and hsCRP (r = 0.71), and with European League Against Rheumatism (EULAR) good or moderate response at 6 months based on DAS28-ESR (adjusted odds ratio, 0.91).
Extensive work has been done to validate the MBDA score for assessing disease activity, and it has been shown to perform well for predicting response to a variety of disease-modifying antirheumatic drugs and biologic agents, Dr. Hitraya explained.
Additionally, multiple studies have demonstrated that the MBDA score defines risk categories for radiographic progression and performs better than traditional measures of disease activity for identifying those at increased risk of radiographic progression, which can help physicians mitigate associated risks through increased surveillance and therapeutic choices, she said.
“Having data for these specific molecules [tofacitinib and rituximab] is very important for rheumatologists,” Dr. Hitraya said.
The tofacitinib study was supported by Pfizer. Dr. Razmjou and Dr. Roodenrijs each reported having no disclosures.
SOURCE: Razmjou A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 582; Roodenrijs N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1500.
CHICAGO – The commercially available multibiomarker disease activity score assay and power Doppler ultrasound at baseline in rheumatoid arthritis patients treated with tofacitinib predicted 12-week responses on some clinical, imaging, and biomarker endpoints, according to findings from an investigator-initiated, open-label study.
The blood test–based multibiomarker disease activity (MBDA) score, which is calculated using measurements of 12 inflammatory biomarkers to score RA disease activity on a 0-100 point scale (Vectra DA, Myriad Autoimmune), also appears to track RA patients’ responses to rituximab, according to a post-hoc analysis of three cohort studies.
The findings of both studies were presented in posters at the annual meeting of the American College of Rheumatology. They are the first studies to evaluate early musculoskeletal ultrasound (MSUS) and MBDA score changes as predictors of response to tofacitinib in patients with RA, and the first to assess the ability of the MBDA score to track response to rituximab treatment. They provide valuable information that can help guide patient treatment and thereby improve outcomes, Elena Hitraya, MD, PhD, chief medical officer for Crescendo Bioscience/Myriad Autoimmune, San Francisco, said in an interview.
In the tofacitinib study, 25 RA patients with a mean age of 52 years, mean disease duration of 10.4 years, baseline Disease Activity Score 28-joint count (DAS28) greater than 3.2, and power Doppler ultrasound (PDUS) scores greater than 10 were treated with the approved oral tofacitinib dose of 5 mg twice daily. Assessments at baseline, 2 weeks, and 12 weeks included MSUS to score 34 joints for PDUS and gray scale ultrasound (GSUS), MBDA score, clinical disease activity index (CDAI), and DAS28, according to Amir Razmjou, MD, of the University of California, Los Angeles, and his colleagues.
Statistically significant improvement was seen on all measures over the 12-week study period (all at P less than .0001). For example, from baseline to 12 weeks the PDUS score improved from 28.6 to 12.2, GSUS score improved from 48.4 to 37.9, MBDA score improved from 50.6 to 39.6, CDAI score improved from 39.9 to 21.6, and DAS28–erythrocyte sedimentation rate (DAS28-ESR) score improved from 6.3 to 4.6, they said, noting further that baseline PDUS and MBDA scores significantly predicted CDAI and DAS28 responses at 12 weeks (P less than .01).
In the rituximab study, the MBDA score tracked disease activity in 57 RA patients from three different cohorts with a mean age of 57 years and mean disease duration of 11.5 years. Changes in the MBDA score reflected the degree of treatment response, Nadia M.T. Roodenrijs of University Medical Center Utrecht (the Netherlands) and her colleagues reported.
All patients were treated with 1,000 mg rituximab and 100 mg methylprednisolone on days 1 and 15, and MBDA score was assessed at baseline and 6 months.
MBDA scores correlated significantly with change from baseline to 6 months in DAS28-ESR (r = 0.60), DAS28–high-sensitivity C-reactive protein, (DAS28-hsCRP; r = 0.48), ESR (r = 0.48), and hsCRP (r = 0.71), and with European League Against Rheumatism (EULAR) good or moderate response at 6 months based on DAS28-ESR (adjusted odds ratio, 0.91).
Extensive work has been done to validate the MBDA score for assessing disease activity, and it has been shown to perform well for predicting response to a variety of disease-modifying antirheumatic drugs and biologic agents, Dr. Hitraya explained.
Additionally, multiple studies have demonstrated that the MBDA score defines risk categories for radiographic progression and performs better than traditional measures of disease activity for identifying those at increased risk of radiographic progression, which can help physicians mitigate associated risks through increased surveillance and therapeutic choices, she said.
“Having data for these specific molecules [tofacitinib and rituximab] is very important for rheumatologists,” Dr. Hitraya said.
The tofacitinib study was supported by Pfizer. Dr. Razmjou and Dr. Roodenrijs each reported having no disclosures.
SOURCE: Razmjou A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 582; Roodenrijs N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1500.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Baseline PDUS and MBDA scores predicted 12-week CDAI and DAS28 responses to tofacitinib (P less than .01); MBDA scores correlated significantly with EULAR good or moderate response at 6 months (adjusted OR, 0.91).
Study details: An open-label study of 25 patients and a post-hoc analysis of three studies including 57 patients.
Disclosures: The tofacitinib study was supported by Pfizer. Dr. Razmjou and Dr. Roodenrijs each reported having no disclosures. Dr. Hitraya is an employee of Myriad Genetics.
Source: Razmjou A et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 582; Roodenrijs N et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1500.
Draft JIA recommendations from ACR seek inactive disease
CHICAGO – New draft guidelines for treating juvenile idiopathic arthritis (JIA) written by experts assembled by the American College of Rheumatology “formalize inactive disease as the goal of treatment,” Timothy G. Beukelman, MD, said at the annual meeting of the American College of Rheumatology.
“We defined low disease activity as patients with a single active joint, and the goal is to have zero active joints. Low disease activity should not be tolerated” in patients with JIA, said Dr. Beukelman, a pediatric rheumatologist at the University of Alabama at Birmingham and a member of the guideline-writing committee. “Until now, treating these patients to zero active joints has not been recommended. But clinically inactive disease is a realistic target for a majority of JIA patients,” he said in an interview.
Despite this shift in the recommended treatment goal, the writing panel was forced to rely largely on their expertise rather than reported evidence. The recommendation by the committee to escalate therapy in patients with low disease activity was “conditional,” with a level of evidence deemed “very low,” Dr. Beukelman said during a talk in which he cited selected highlights from the committee’s full list of 39 recommendations.
The paucity of evidence reflected the status of many of the recommendations: 31 of the 39 recommendations were conditional, which means that the desirable effects from treatment “probably” outweigh the undesirable effects, and they may not apply to some patients. The writing panel pegged 22 of their recommendations as having a very low evidence backing and another 13 recommendations had low evidence. The JIA committee believed that none of its recommendations had strong evidence to back them up.
Dr. Beukelman defended writing recommendations despite this absence of evidence. “We should continue to write conditional recommendations when we don’t have the evidence. These recommendations had approval from at least 70% of the writing group, a diverse committee of experts. They should not be taken lightly just because they are conditional.”
Dr. Beukelman also stressed that he was presenting draft recommendations that still awaited approval from the ACR and the Arthritis Foundation, which collaborated with the ACR on this project. Once adopted, the new document would revise the existing management recommendations that the ACR approved in 2011 (Arthritis Rheum. 2011 Apr;63[4]:465-82).
The recommendations Dr. Beukelman outlined focused on treatment of polyarthritis, sacroiliitis, and enthesitis, and Dr. Beukelman devoted the most time to detailing some of the statements on polyarthritis. The panel conditionally recommended methotrexate over leflunomide (Arava) or sulfasalazine with moderate or very low evidence and said that subcutaneous methotrexate was conditionally preferred over oral dosing for reasons of both better efficacy and tolerability. Combination of a biologic agent with a nonbiologic received a conditional recommendation over biologic monotherapy, with moderate to very low evidence, but with a strong recommendation for this combined approach when using infliximab (Remicade), based on moderate evidence.
Another strong recommendation was to avoid treating patients with a chronic course of a low-dose, systemic glucocorticoid, based on a very low level of evidence. A brief course, less than 3 months, of an oral glucocorticoid received conditional recommendation for patients with moderate or high disease activity, based on very low evidence, but also received a conditional negative recommendation for patients with low disease activity, also based on very low evidence.
Initial therapy with a disease-modifying antirheumatic drug (DMARD) instead of monotherapy with an NSAID received a strong recommendation, based on a moderate level of evidence, while initial therapy with a DMARD received conditional support over initial therapy with a biologic agent, based on low evidence.
The panel gave a conditional endorsement to the idea of switching from a tumor necrosis factor inhibitor (TNFi) to a different biologic drug class when patients remained with moderate or high disease activity, based on a very low level of evidence.
Regarding sacroiliitis, the panel strongly recommended starting a TNFi in patients with active sacroiliitis despite NSAID treatment, based on low evidence, and the committee strongly recommended against starting methotrexate treatment in these patients, based on very low evidence. For treating active enthesitis despite NSAID treatment, the panel conditionally recommended adding a TNFi over treatment with methotrexate or sulfasalazine, based on a low level of evidence. Dr. Beukelman highlighted that the new recommendations placed increased emphasis on treating sacroiliitis, compared with the 2011 statement, and that the new recommendations dealt with treating enthesitis for the first time.
Dr. Beukelman has been a consultant to Bristol-Myers Squibb, Novartis, Sobi, and UCB.
CHICAGO – New draft guidelines for treating juvenile idiopathic arthritis (JIA) written by experts assembled by the American College of Rheumatology “formalize inactive disease as the goal of treatment,” Timothy G. Beukelman, MD, said at the annual meeting of the American College of Rheumatology.
“We defined low disease activity as patients with a single active joint, and the goal is to have zero active joints. Low disease activity should not be tolerated” in patients with JIA, said Dr. Beukelman, a pediatric rheumatologist at the University of Alabama at Birmingham and a member of the guideline-writing committee. “Until now, treating these patients to zero active joints has not been recommended. But clinically inactive disease is a realistic target for a majority of JIA patients,” he said in an interview.
Despite this shift in the recommended treatment goal, the writing panel was forced to rely largely on their expertise rather than reported evidence. The recommendation by the committee to escalate therapy in patients with low disease activity was “conditional,” with a level of evidence deemed “very low,” Dr. Beukelman said during a talk in which he cited selected highlights from the committee’s full list of 39 recommendations.
The paucity of evidence reflected the status of many of the recommendations: 31 of the 39 recommendations were conditional, which means that the desirable effects from treatment “probably” outweigh the undesirable effects, and they may not apply to some patients. The writing panel pegged 22 of their recommendations as having a very low evidence backing and another 13 recommendations had low evidence. The JIA committee believed that none of its recommendations had strong evidence to back them up.
Dr. Beukelman defended writing recommendations despite this absence of evidence. “We should continue to write conditional recommendations when we don’t have the evidence. These recommendations had approval from at least 70% of the writing group, a diverse committee of experts. They should not be taken lightly just because they are conditional.”
Dr. Beukelman also stressed that he was presenting draft recommendations that still awaited approval from the ACR and the Arthritis Foundation, which collaborated with the ACR on this project. Once adopted, the new document would revise the existing management recommendations that the ACR approved in 2011 (Arthritis Rheum. 2011 Apr;63[4]:465-82).
The recommendations Dr. Beukelman outlined focused on treatment of polyarthritis, sacroiliitis, and enthesitis, and Dr. Beukelman devoted the most time to detailing some of the statements on polyarthritis. The panel conditionally recommended methotrexate over leflunomide (Arava) or sulfasalazine with moderate or very low evidence and said that subcutaneous methotrexate was conditionally preferred over oral dosing for reasons of both better efficacy and tolerability. Combination of a biologic agent with a nonbiologic received a conditional recommendation over biologic monotherapy, with moderate to very low evidence, but with a strong recommendation for this combined approach when using infliximab (Remicade), based on moderate evidence.
Another strong recommendation was to avoid treating patients with a chronic course of a low-dose, systemic glucocorticoid, based on a very low level of evidence. A brief course, less than 3 months, of an oral glucocorticoid received conditional recommendation for patients with moderate or high disease activity, based on very low evidence, but also received a conditional negative recommendation for patients with low disease activity, also based on very low evidence.
Initial therapy with a disease-modifying antirheumatic drug (DMARD) instead of monotherapy with an NSAID received a strong recommendation, based on a moderate level of evidence, while initial therapy with a DMARD received conditional support over initial therapy with a biologic agent, based on low evidence.
The panel gave a conditional endorsement to the idea of switching from a tumor necrosis factor inhibitor (TNFi) to a different biologic drug class when patients remained with moderate or high disease activity, based on a very low level of evidence.
Regarding sacroiliitis, the panel strongly recommended starting a TNFi in patients with active sacroiliitis despite NSAID treatment, based on low evidence, and the committee strongly recommended against starting methotrexate treatment in these patients, based on very low evidence. For treating active enthesitis despite NSAID treatment, the panel conditionally recommended adding a TNFi over treatment with methotrexate or sulfasalazine, based on a low level of evidence. Dr. Beukelman highlighted that the new recommendations placed increased emphasis on treating sacroiliitis, compared with the 2011 statement, and that the new recommendations dealt with treating enthesitis for the first time.
Dr. Beukelman has been a consultant to Bristol-Myers Squibb, Novartis, Sobi, and UCB.
CHICAGO – New draft guidelines for treating juvenile idiopathic arthritis (JIA) written by experts assembled by the American College of Rheumatology “formalize inactive disease as the goal of treatment,” Timothy G. Beukelman, MD, said at the annual meeting of the American College of Rheumatology.
“We defined low disease activity as patients with a single active joint, and the goal is to have zero active joints. Low disease activity should not be tolerated” in patients with JIA, said Dr. Beukelman, a pediatric rheumatologist at the University of Alabama at Birmingham and a member of the guideline-writing committee. “Until now, treating these patients to zero active joints has not been recommended. But clinically inactive disease is a realistic target for a majority of JIA patients,” he said in an interview.
Despite this shift in the recommended treatment goal, the writing panel was forced to rely largely on their expertise rather than reported evidence. The recommendation by the committee to escalate therapy in patients with low disease activity was “conditional,” with a level of evidence deemed “very low,” Dr. Beukelman said during a talk in which he cited selected highlights from the committee’s full list of 39 recommendations.
The paucity of evidence reflected the status of many of the recommendations: 31 of the 39 recommendations were conditional, which means that the desirable effects from treatment “probably” outweigh the undesirable effects, and they may not apply to some patients. The writing panel pegged 22 of their recommendations as having a very low evidence backing and another 13 recommendations had low evidence. The JIA committee believed that none of its recommendations had strong evidence to back them up.
Dr. Beukelman defended writing recommendations despite this absence of evidence. “We should continue to write conditional recommendations when we don’t have the evidence. These recommendations had approval from at least 70% of the writing group, a diverse committee of experts. They should not be taken lightly just because they are conditional.”
Dr. Beukelman also stressed that he was presenting draft recommendations that still awaited approval from the ACR and the Arthritis Foundation, which collaborated with the ACR on this project. Once adopted, the new document would revise the existing management recommendations that the ACR approved in 2011 (Arthritis Rheum. 2011 Apr;63[4]:465-82).
The recommendations Dr. Beukelman outlined focused on treatment of polyarthritis, sacroiliitis, and enthesitis, and Dr. Beukelman devoted the most time to detailing some of the statements on polyarthritis. The panel conditionally recommended methotrexate over leflunomide (Arava) or sulfasalazine with moderate or very low evidence and said that subcutaneous methotrexate was conditionally preferred over oral dosing for reasons of both better efficacy and tolerability. Combination of a biologic agent with a nonbiologic received a conditional recommendation over biologic monotherapy, with moderate to very low evidence, but with a strong recommendation for this combined approach when using infliximab (Remicade), based on moderate evidence.
Another strong recommendation was to avoid treating patients with a chronic course of a low-dose, systemic glucocorticoid, based on a very low level of evidence. A brief course, less than 3 months, of an oral glucocorticoid received conditional recommendation for patients with moderate or high disease activity, based on very low evidence, but also received a conditional negative recommendation for patients with low disease activity, also based on very low evidence.
Initial therapy with a disease-modifying antirheumatic drug (DMARD) instead of monotherapy with an NSAID received a strong recommendation, based on a moderate level of evidence, while initial therapy with a DMARD received conditional support over initial therapy with a biologic agent, based on low evidence.
The panel gave a conditional endorsement to the idea of switching from a tumor necrosis factor inhibitor (TNFi) to a different biologic drug class when patients remained with moderate or high disease activity, based on a very low level of evidence.
Regarding sacroiliitis, the panel strongly recommended starting a TNFi in patients with active sacroiliitis despite NSAID treatment, based on low evidence, and the committee strongly recommended against starting methotrexate treatment in these patients, based on very low evidence. For treating active enthesitis despite NSAID treatment, the panel conditionally recommended adding a TNFi over treatment with methotrexate or sulfasalazine, based on a low level of evidence. Dr. Beukelman highlighted that the new recommendations placed increased emphasis on treating sacroiliitis, compared with the 2011 statement, and that the new recommendations dealt with treating enthesitis for the first time.
Dr. Beukelman has been a consultant to Bristol-Myers Squibb, Novartis, Sobi, and UCB.
EXPERT ANALYSIS FROM THE ACR ANNUAL MEETING
Study confirms advice to halt methotrexate when giving flu vaccine to RA patients
CHICAGO – Discontinuing methotrexate for 2 weeks in patients with RA starting the day they receive the seasonal influenza vaccine significantly improves the vaccine’s immunogenicity without aggravating disease activity, Kevin L. Winthrop, MD, reported at the annual meeting of the American College of Rheumatology.
“I think this is potentially clinically practice changing because now there are two studies showing the same thing,” said Dr. Winthrop, a professor of public health and preventive medicine at Oregon Health & Science University, Portland.
Based upon these prospective randomized studies, which he conducted together with investigators at Seoul National University in South Korea, initiating a 2-week halt of methotrexate on the day the influenza vaccine is given to patients with RA is now his routine practice, and he recommends other physicians do the same.
The first prospective, randomized trial included 199 RA patients on stable doses of methotrexate who were assigned to one of four groups in conjunction with seasonal influenza vaccination. One group continued their methotrexate as usual, the second stopped the drug for 1 month prior to vaccination and then restarted it at the time of vaccination, the third group halted methotrexate for 2 weeks before and 2 weeks after vaccination, and the fourth suspended methotrexate for 4 weeks starting on the day they got their flu shot. Everyone received trivalent influenza vaccine containing H1N1, H3N2, and B/Yamagata.
The lowest rate of satisfactory vaccine response as defined by at least a 300% titer increase 1 month after vaccination occurred in the group that continued their methotrexate as usual. The group that halted the drug for 2 weeks before and 2 weeks after influenza vaccination had a 51% satisfactory vaccine response against all three antigens, compared with a 31.5% rate in the methotrexate-as-usual group. RA flare rates ranged from 21% to 39% across the four study arms, differences that weren’t statistically significant (Ann Rheum Dis. 2017 Sep;76[9]:1559-65).
Next Dr. Winthrop and his colleagues conducted a confirmatory prospective, multicenter, randomized trial in which they sought to nail down the optimal duration and timing of methotrexate discontinuation. A total of 320 RA patients on stable doses of methotrexate were assigned to halt the drug for 2 weeks starting at the time they received a quadrivalent seasonal influenza vaccine containing H1N1, H3N2, B/Yamagata, and B/Victoria strains, or to continue their methotrexate throughout.
A satisfactory vaccine response was achieved in 75.5% of the group that discontinued the drug, significantly better than the 54.5% rate in the methotrexate continuers. The absolute difference in seroprotection was greater in patients who halted their methotrexate for 2 weeks after vaccination for all four antigens: an absolute 11% difference for H1N1, 16% for H3N2, 12% for B/Yamagata, and 15% for B/Victoria (Ann Rheum Dis. 2018 Jun;77[6]:898-904).
“It does seem to be a nice strategy. The percentage of people who flared their RA during their 2 weeks off methotrexate was very low, so there seems to be a good reason to do this,” according to Dr. Winthrop.
Some rheumatologists he has spoken with initially balked at the plausibility of the results.
“I had the same thought about these studies: It doesn’t make sense to me in terms of how methotrexate works, and why we would see this effect acutely by stopping methotrexate for just 2 weeks?” he said.
But then a coinvestigator drilled down deeper into the data and came up with the explanation: The benefit in terms of enhanced flu vaccine immunogenicity through temporary withholding of methotrexate was confined to the subgroup of RA patients with high baseline levels of B-cell activation factor (BAFF). In contrast, withholding methotrexate didn’t affect the vaccine response in patients with low or normal baseline BAFF (Ann Rheum Dis. 2018 Oct 8. doi: 10.1136/annrheumdis-2018-214025).
“I don’t know how to check anyone’s BAFF levels. I don’t think there’s a commercial test out there. But this does help explain why we saw this observation. So I think I would still hold everyone’s methotrexate for 2 weeks. That’s how I approach it. And they may get benefit from it, and they may not,” he said.
Dr. Winthrop reported having no financial conflicts regarding the study, which was funded by GC Pharma.
CHICAGO – Discontinuing methotrexate for 2 weeks in patients with RA starting the day they receive the seasonal influenza vaccine significantly improves the vaccine’s immunogenicity without aggravating disease activity, Kevin L. Winthrop, MD, reported at the annual meeting of the American College of Rheumatology.
“I think this is potentially clinically practice changing because now there are two studies showing the same thing,” said Dr. Winthrop, a professor of public health and preventive medicine at Oregon Health & Science University, Portland.
Based upon these prospective randomized studies, which he conducted together with investigators at Seoul National University in South Korea, initiating a 2-week halt of methotrexate on the day the influenza vaccine is given to patients with RA is now his routine practice, and he recommends other physicians do the same.
The first prospective, randomized trial included 199 RA patients on stable doses of methotrexate who were assigned to one of four groups in conjunction with seasonal influenza vaccination. One group continued their methotrexate as usual, the second stopped the drug for 1 month prior to vaccination and then restarted it at the time of vaccination, the third group halted methotrexate for 2 weeks before and 2 weeks after vaccination, and the fourth suspended methotrexate for 4 weeks starting on the day they got their flu shot. Everyone received trivalent influenza vaccine containing H1N1, H3N2, and B/Yamagata.
The lowest rate of satisfactory vaccine response as defined by at least a 300% titer increase 1 month after vaccination occurred in the group that continued their methotrexate as usual. The group that halted the drug for 2 weeks before and 2 weeks after influenza vaccination had a 51% satisfactory vaccine response against all three antigens, compared with a 31.5% rate in the methotrexate-as-usual group. RA flare rates ranged from 21% to 39% across the four study arms, differences that weren’t statistically significant (Ann Rheum Dis. 2017 Sep;76[9]:1559-65).
Next Dr. Winthrop and his colleagues conducted a confirmatory prospective, multicenter, randomized trial in which they sought to nail down the optimal duration and timing of methotrexate discontinuation. A total of 320 RA patients on stable doses of methotrexate were assigned to halt the drug for 2 weeks starting at the time they received a quadrivalent seasonal influenza vaccine containing H1N1, H3N2, B/Yamagata, and B/Victoria strains, or to continue their methotrexate throughout.
A satisfactory vaccine response was achieved in 75.5% of the group that discontinued the drug, significantly better than the 54.5% rate in the methotrexate continuers. The absolute difference in seroprotection was greater in patients who halted their methotrexate for 2 weeks after vaccination for all four antigens: an absolute 11% difference for H1N1, 16% for H3N2, 12% for B/Yamagata, and 15% for B/Victoria (Ann Rheum Dis. 2018 Jun;77[6]:898-904).
“It does seem to be a nice strategy. The percentage of people who flared their RA during their 2 weeks off methotrexate was very low, so there seems to be a good reason to do this,” according to Dr. Winthrop.
Some rheumatologists he has spoken with initially balked at the plausibility of the results.
“I had the same thought about these studies: It doesn’t make sense to me in terms of how methotrexate works, and why we would see this effect acutely by stopping methotrexate for just 2 weeks?” he said.
But then a coinvestigator drilled down deeper into the data and came up with the explanation: The benefit in terms of enhanced flu vaccine immunogenicity through temporary withholding of methotrexate was confined to the subgroup of RA patients with high baseline levels of B-cell activation factor (BAFF). In contrast, withholding methotrexate didn’t affect the vaccine response in patients with low or normal baseline BAFF (Ann Rheum Dis. 2018 Oct 8. doi: 10.1136/annrheumdis-2018-214025).
“I don’t know how to check anyone’s BAFF levels. I don’t think there’s a commercial test out there. But this does help explain why we saw this observation. So I think I would still hold everyone’s methotrexate for 2 weeks. That’s how I approach it. And they may get benefit from it, and they may not,” he said.
Dr. Winthrop reported having no financial conflicts regarding the study, which was funded by GC Pharma.
CHICAGO – Discontinuing methotrexate for 2 weeks in patients with RA starting the day they receive the seasonal influenza vaccine significantly improves the vaccine’s immunogenicity without aggravating disease activity, Kevin L. Winthrop, MD, reported at the annual meeting of the American College of Rheumatology.
“I think this is potentially clinically practice changing because now there are two studies showing the same thing,” said Dr. Winthrop, a professor of public health and preventive medicine at Oregon Health & Science University, Portland.
Based upon these prospective randomized studies, which he conducted together with investigators at Seoul National University in South Korea, initiating a 2-week halt of methotrexate on the day the influenza vaccine is given to patients with RA is now his routine practice, and he recommends other physicians do the same.
The first prospective, randomized trial included 199 RA patients on stable doses of methotrexate who were assigned to one of four groups in conjunction with seasonal influenza vaccination. One group continued their methotrexate as usual, the second stopped the drug for 1 month prior to vaccination and then restarted it at the time of vaccination, the third group halted methotrexate for 2 weeks before and 2 weeks after vaccination, and the fourth suspended methotrexate for 4 weeks starting on the day they got their flu shot. Everyone received trivalent influenza vaccine containing H1N1, H3N2, and B/Yamagata.
The lowest rate of satisfactory vaccine response as defined by at least a 300% titer increase 1 month after vaccination occurred in the group that continued their methotrexate as usual. The group that halted the drug for 2 weeks before and 2 weeks after influenza vaccination had a 51% satisfactory vaccine response against all three antigens, compared with a 31.5% rate in the methotrexate-as-usual group. RA flare rates ranged from 21% to 39% across the four study arms, differences that weren’t statistically significant (Ann Rheum Dis. 2017 Sep;76[9]:1559-65).
Next Dr. Winthrop and his colleagues conducted a confirmatory prospective, multicenter, randomized trial in which they sought to nail down the optimal duration and timing of methotrexate discontinuation. A total of 320 RA patients on stable doses of methotrexate were assigned to halt the drug for 2 weeks starting at the time they received a quadrivalent seasonal influenza vaccine containing H1N1, H3N2, B/Yamagata, and B/Victoria strains, or to continue their methotrexate throughout.
A satisfactory vaccine response was achieved in 75.5% of the group that discontinued the drug, significantly better than the 54.5% rate in the methotrexate continuers. The absolute difference in seroprotection was greater in patients who halted their methotrexate for 2 weeks after vaccination for all four antigens: an absolute 11% difference for H1N1, 16% for H3N2, 12% for B/Yamagata, and 15% for B/Victoria (Ann Rheum Dis. 2018 Jun;77[6]:898-904).
“It does seem to be a nice strategy. The percentage of people who flared their RA during their 2 weeks off methotrexate was very low, so there seems to be a good reason to do this,” according to Dr. Winthrop.
Some rheumatologists he has spoken with initially balked at the plausibility of the results.
“I had the same thought about these studies: It doesn’t make sense to me in terms of how methotrexate works, and why we would see this effect acutely by stopping methotrexate for just 2 weeks?” he said.
But then a coinvestigator drilled down deeper into the data and came up with the explanation: The benefit in terms of enhanced flu vaccine immunogenicity through temporary withholding of methotrexate was confined to the subgroup of RA patients with high baseline levels of B-cell activation factor (BAFF). In contrast, withholding methotrexate didn’t affect the vaccine response in patients with low or normal baseline BAFF (Ann Rheum Dis. 2018 Oct 8. doi: 10.1136/annrheumdis-2018-214025).
“I don’t know how to check anyone’s BAFF levels. I don’t think there’s a commercial test out there. But this does help explain why we saw this observation. So I think I would still hold everyone’s methotrexate for 2 weeks. That’s how I approach it. And they may get benefit from it, and they may not,” he said.
Dr. Winthrop reported having no financial conflicts regarding the study, which was funded by GC Pharma.
REPORTING FROM THE ACR ANNUAL MEETING
Genetic profile flags scleroderma patients with best HSCT responses
CHICAGO – Subgroup categorization of patients with severe scleroderma by their gene-expression profile correlated with responses to a newly proven treatment for the disease that involves myeloablation and autologous hematopoietic stem cell transplantation (HSCT).
Patients who fell into the “fibroproliferative” scleroderma subgroup, roughly one-third of patients enrolled in the treatment study, showed a high level of benefit from myeloablation and autologous HSCT, Michael L. Whitfield, PhD, said at the annual meeting of the American College of Rheumatology.
In contrast, the roughly one-third of patients in the study with a gene-expression profile that placed them into the “normal-like” subgroup had outcomes that closely matched the normal-like patients in the control group, who were treated with cyclophosphamide, which suggests that the normal-like patients are probably not good candidates for HSCT, said Dr. Whitfield, a professor of molecular and systems biology at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
This study starts to get at the question of “How do you do personalized medicine in a disease like scleroderma?” he explained. “HSCT may be a game changer for patients with the fibroproliferative type of scleroderma,” who did relatively poorly in the control group of the trial when they received cyclophosphamide. Categorization of patients by their gene-expression profiles is a way to find order among scleroderma patients in what is otherwise “a very heterogeneous disease, where some patients improve on a treatment and others do not,” Dr Whitfield said.
The study used data collected in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial, which enrolled 75 patients with severe scleroderma at any of 26 sites in the United States and Canada. SCOT compared the safety and efficacy of myeloablation followed by autologous HSCT with that of treatment with cyclophosphamide, and it followed patients for a median of 54 months. The results showed that overall HSCT was superior for the primary endpoint and several secondary endpoints, including event-free survival, which was 79% after HSCT and 50% in control patients by the end of follow-up (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Whitfield’s group took peripheral blood cells from 30 of the patients treated by HSCT and from 33 of the patients treated with cyclophosphamide per protocol and analyzed the gene-expression profiles of the cells to categorize patients into the gene-expression subtypes of scleroderma that had been previously defined by Dr. Whitfield and his associates: fibroproliferative, inflammatory, limited, or normal-like (PLOS One. 2008 Jul 18;3[7]:e2696). The gene-expression analysis, which now looks at the activity of about 1,300 genes, showed that the 33 cyclophosphamide-treated patients included 12 with an inflammatory profile, 12 with a normal-like profile, and 9 with a fibroproliferative profile. Among the 30 patients treated with HSCT, 11 were in the fibroproliferative group, 11 were normal-like, and 8 had an inflammatory pattern.
Analysis of event-free survival out to 6 years following enrollment showed that, in the fibroproliferative subgroup, roughly 90% of patients treated with HSCT remained alive and event free, compared with about 35% of the cyclophosphamide patients, a highly statistically significant difference. In the inflammatory subgroup, event-free survival persisted in about 90% in the HSCT recipients, compared with about 50% of those in the control arm, a difference that did not reach statistical significance. Among patients with normal-like gene expression, the event-free survival rate was about the same regardless of treatment, about 60% in each treatment arm. The results suggest that patients with normal-like disease “are probably not good candidates for a treatment as intensive as HSCT,” Dr. Whitfield said in an interview.
Although the HSCT and cyclophosphamide treatment groups included relatively small numbers of patients, when the researchers subdivided the trial cohort into three different scleroderma types, the analysis remained “powered well enough to see a difference; the difference was very clearly statistically significant,” Dr. Whitfield declared.
“Now that we have a treatment [HSCT] to tie to the [gene-expression analysis], we can think about using this in routine practice,” he concluded.
Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
SOURCE: Franks J et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract 1876.
CHICAGO – Subgroup categorization of patients with severe scleroderma by their gene-expression profile correlated with responses to a newly proven treatment for the disease that involves myeloablation and autologous hematopoietic stem cell transplantation (HSCT).
Patients who fell into the “fibroproliferative” scleroderma subgroup, roughly one-third of patients enrolled in the treatment study, showed a high level of benefit from myeloablation and autologous HSCT, Michael L. Whitfield, PhD, said at the annual meeting of the American College of Rheumatology.
In contrast, the roughly one-third of patients in the study with a gene-expression profile that placed them into the “normal-like” subgroup had outcomes that closely matched the normal-like patients in the control group, who were treated with cyclophosphamide, which suggests that the normal-like patients are probably not good candidates for HSCT, said Dr. Whitfield, a professor of molecular and systems biology at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
This study starts to get at the question of “How do you do personalized medicine in a disease like scleroderma?” he explained. “HSCT may be a game changer for patients with the fibroproliferative type of scleroderma,” who did relatively poorly in the control group of the trial when they received cyclophosphamide. Categorization of patients by their gene-expression profiles is a way to find order among scleroderma patients in what is otherwise “a very heterogeneous disease, where some patients improve on a treatment and others do not,” Dr Whitfield said.
The study used data collected in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial, which enrolled 75 patients with severe scleroderma at any of 26 sites in the United States and Canada. SCOT compared the safety and efficacy of myeloablation followed by autologous HSCT with that of treatment with cyclophosphamide, and it followed patients for a median of 54 months. The results showed that overall HSCT was superior for the primary endpoint and several secondary endpoints, including event-free survival, which was 79% after HSCT and 50% in control patients by the end of follow-up (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Whitfield’s group took peripheral blood cells from 30 of the patients treated by HSCT and from 33 of the patients treated with cyclophosphamide per protocol and analyzed the gene-expression profiles of the cells to categorize patients into the gene-expression subtypes of scleroderma that had been previously defined by Dr. Whitfield and his associates: fibroproliferative, inflammatory, limited, or normal-like (PLOS One. 2008 Jul 18;3[7]:e2696). The gene-expression analysis, which now looks at the activity of about 1,300 genes, showed that the 33 cyclophosphamide-treated patients included 12 with an inflammatory profile, 12 with a normal-like profile, and 9 with a fibroproliferative profile. Among the 30 patients treated with HSCT, 11 were in the fibroproliferative group, 11 were normal-like, and 8 had an inflammatory pattern.
Analysis of event-free survival out to 6 years following enrollment showed that, in the fibroproliferative subgroup, roughly 90% of patients treated with HSCT remained alive and event free, compared with about 35% of the cyclophosphamide patients, a highly statistically significant difference. In the inflammatory subgroup, event-free survival persisted in about 90% in the HSCT recipients, compared with about 50% of those in the control arm, a difference that did not reach statistical significance. Among patients with normal-like gene expression, the event-free survival rate was about the same regardless of treatment, about 60% in each treatment arm. The results suggest that patients with normal-like disease “are probably not good candidates for a treatment as intensive as HSCT,” Dr. Whitfield said in an interview.
Although the HSCT and cyclophosphamide treatment groups included relatively small numbers of patients, when the researchers subdivided the trial cohort into three different scleroderma types, the analysis remained “powered well enough to see a difference; the difference was very clearly statistically significant,” Dr. Whitfield declared.
“Now that we have a treatment [HSCT] to tie to the [gene-expression analysis], we can think about using this in routine practice,” he concluded.
Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
SOURCE: Franks J et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract 1876.
CHICAGO – Subgroup categorization of patients with severe scleroderma by their gene-expression profile correlated with responses to a newly proven treatment for the disease that involves myeloablation and autologous hematopoietic stem cell transplantation (HSCT).
Patients who fell into the “fibroproliferative” scleroderma subgroup, roughly one-third of patients enrolled in the treatment study, showed a high level of benefit from myeloablation and autologous HSCT, Michael L. Whitfield, PhD, said at the annual meeting of the American College of Rheumatology.
In contrast, the roughly one-third of patients in the study with a gene-expression profile that placed them into the “normal-like” subgroup had outcomes that closely matched the normal-like patients in the control group, who were treated with cyclophosphamide, which suggests that the normal-like patients are probably not good candidates for HSCT, said Dr. Whitfield, a professor of molecular and systems biology at the Geisel School of Medicine at Dartmouth in Hanover, N.H.
This study starts to get at the question of “How do you do personalized medicine in a disease like scleroderma?” he explained. “HSCT may be a game changer for patients with the fibroproliferative type of scleroderma,” who did relatively poorly in the control group of the trial when they received cyclophosphamide. Categorization of patients by their gene-expression profiles is a way to find order among scleroderma patients in what is otherwise “a very heterogeneous disease, where some patients improve on a treatment and others do not,” Dr Whitfield said.
The study used data collected in the SCOT (Scleroderma: Cyclophosphamide or Transplantation) trial, which enrolled 75 patients with severe scleroderma at any of 26 sites in the United States and Canada. SCOT compared the safety and efficacy of myeloablation followed by autologous HSCT with that of treatment with cyclophosphamide, and it followed patients for a median of 54 months. The results showed that overall HSCT was superior for the primary endpoint and several secondary endpoints, including event-free survival, which was 79% after HSCT and 50% in control patients by the end of follow-up (N Engl J Med. 2018 Jan 4;378[1]:35-47).
Dr. Whitfield’s group took peripheral blood cells from 30 of the patients treated by HSCT and from 33 of the patients treated with cyclophosphamide per protocol and analyzed the gene-expression profiles of the cells to categorize patients into the gene-expression subtypes of scleroderma that had been previously defined by Dr. Whitfield and his associates: fibroproliferative, inflammatory, limited, or normal-like (PLOS One. 2008 Jul 18;3[7]:e2696). The gene-expression analysis, which now looks at the activity of about 1,300 genes, showed that the 33 cyclophosphamide-treated patients included 12 with an inflammatory profile, 12 with a normal-like profile, and 9 with a fibroproliferative profile. Among the 30 patients treated with HSCT, 11 were in the fibroproliferative group, 11 were normal-like, and 8 had an inflammatory pattern.
Analysis of event-free survival out to 6 years following enrollment showed that, in the fibroproliferative subgroup, roughly 90% of patients treated with HSCT remained alive and event free, compared with about 35% of the cyclophosphamide patients, a highly statistically significant difference. In the inflammatory subgroup, event-free survival persisted in about 90% in the HSCT recipients, compared with about 50% of those in the control arm, a difference that did not reach statistical significance. Among patients with normal-like gene expression, the event-free survival rate was about the same regardless of treatment, about 60% in each treatment arm. The results suggest that patients with normal-like disease “are probably not good candidates for a treatment as intensive as HSCT,” Dr. Whitfield said in an interview.
Although the HSCT and cyclophosphamide treatment groups included relatively small numbers of patients, when the researchers subdivided the trial cohort into three different scleroderma types, the analysis remained “powered well enough to see a difference; the difference was very clearly statistically significant,” Dr. Whitfield declared.
“Now that we have a treatment [HSCT] to tie to the [gene-expression analysis], we can think about using this in routine practice,” he concluded.
Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
SOURCE: Franks J et al. Arthritis Rheumatol. 2018;70(suppl 10): Abstract 1876.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point:
Major finding: About 90% of fibroproliferative scleroderma patients had prolonged event-free survival after stem cell transplant, compared with about 35% of controls.
Study details: The study used data collected in the SCOT trial of 75 patients with severe scleroderma.
Disclosures: Dr. Whitfield is a cofounder of Celdara, and he has been a consultant to Bristol-Myers Squibb, Corbus, UCB, and Third Rock Ventures.
Source: Franks J et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1876.
Dueling SLE classification criteria: And the winner is...
CHICAGO – Newer isn’t necessarily better – especially when it comes to the plethora of SLE classification criteria, according to Michelle A. Petri, MD, professor of medicine and director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
“We have an embarrassment of criteria for lupus right now, and everyone wants to know if one is better than the others,” the rheumatologist said at the annual meeting of the American College of Rheumatology.
She and several coinvestigators who are members of the Systemic Lupus International Collaborating Clinics (SLICC) set out to learn the answer. They developed a new, modified, weighted version of the 2012 SLICC criteria and compared its sensitivity and specificity for SLE diagnosis by 690 physicians with three other major classification systems: the 1997 update to ACR-11 criteria, the nonweighted SLICC 2012 criteria, and the proposed EULAR/ACR criteria, which uses a differentially weighted approach in which the various possible disease manifestations are each assigned a different point score. In contrast, the revised ACR-11 and SLICC 2012 criteria count each SLE manifestation equally.
Long story short: “The two newly derived weighted classification rules did not perform better than the existing list-based rules in terms of overall agreement,” according to Dr. Petri.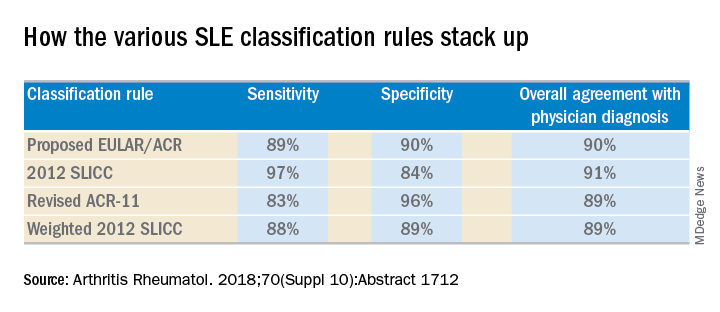
“We don’t think that weighting all the criteria, which is what the EULAR/ACR and weighted SLICC 2012 rules do, adds to the performance of the criteria set, and in fact it makes it much more difficult for clinicians to use when there’s a complicated weighting system, unless it’s web-based or there’s an app for it. And to be honest, clinicians are so busy that they’re probably not going to take time out in a clinic visit to go use the web or an app. Our criteria need to be user friendly,” she continued.
So which of the four classification systems is most user friendly? The EULAR/ACR criteria can be dismissed on that score because they are supposed to be used only for research, according to the rheumatologist.
“I think the SLICC 2012 criteria are very useful for clinicians because they have the highest sensitivity. And what a clinician wants is not to miss a diagnosis and to start treatment early,” Dr. Petri said.
To develop the weighted SLICC criteria, whose future at this point doesn’t look bright, she and her coinvestigators redeployed the same physician-rated patient scenarios used to develop the nonweighted 2012 SLICC classification criteria and assigned each of the potential manifestations of SLE a specific point score. For example, acute cutaneous manifestations received 16 points, serositis 9, oral ulcers 16, thrombocytopenia 15, and so forth. Under this system, a patient with a score of at least 56 points, or lupus nephritis, or least one clinical and one immunologic component of SLE was classified as having the disease.
Dr. Petri reported having no financial conflicts regarding her study, supported by the National Institutes of Health.
SOURCE: Petri MA et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1712.
CHICAGO – Newer isn’t necessarily better – especially when it comes to the plethora of SLE classification criteria, according to Michelle A. Petri, MD, professor of medicine and director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
“We have an embarrassment of criteria for lupus right now, and everyone wants to know if one is better than the others,” the rheumatologist said at the annual meeting of the American College of Rheumatology.
She and several coinvestigators who are members of the Systemic Lupus International Collaborating Clinics (SLICC) set out to learn the answer. They developed a new, modified, weighted version of the 2012 SLICC criteria and compared its sensitivity and specificity for SLE diagnosis by 690 physicians with three other major classification systems: the 1997 update to ACR-11 criteria, the nonweighted SLICC 2012 criteria, and the proposed EULAR/ACR criteria, which uses a differentially weighted approach in which the various possible disease manifestations are each assigned a different point score. In contrast, the revised ACR-11 and SLICC 2012 criteria count each SLE manifestation equally.
Long story short: “The two newly derived weighted classification rules did not perform better than the existing list-based rules in terms of overall agreement,” according to Dr. Petri.
“We don’t think that weighting all the criteria, which is what the EULAR/ACR and weighted SLICC 2012 rules do, adds to the performance of the criteria set, and in fact it makes it much more difficult for clinicians to use when there’s a complicated weighting system, unless it’s web-based or there’s an app for it. And to be honest, clinicians are so busy that they’re probably not going to take time out in a clinic visit to go use the web or an app. Our criteria need to be user friendly,” she continued.
So which of the four classification systems is most user friendly? The EULAR/ACR criteria can be dismissed on that score because they are supposed to be used only for research, according to the rheumatologist.
“I think the SLICC 2012 criteria are very useful for clinicians because they have the highest sensitivity. And what a clinician wants is not to miss a diagnosis and to start treatment early,” Dr. Petri said.
To develop the weighted SLICC criteria, whose future at this point doesn’t look bright, she and her coinvestigators redeployed the same physician-rated patient scenarios used to develop the nonweighted 2012 SLICC classification criteria and assigned each of the potential manifestations of SLE a specific point score. For example, acute cutaneous manifestations received 16 points, serositis 9, oral ulcers 16, thrombocytopenia 15, and so forth. Under this system, a patient with a score of at least 56 points, or lupus nephritis, or least one clinical and one immunologic component of SLE was classified as having the disease.
Dr. Petri reported having no financial conflicts regarding her study, supported by the National Institutes of Health.
SOURCE: Petri MA et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1712.
CHICAGO – Newer isn’t necessarily better – especially when it comes to the plethora of SLE classification criteria, according to Michelle A. Petri, MD, professor of medicine and director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
“We have an embarrassment of criteria for lupus right now, and everyone wants to know if one is better than the others,” the rheumatologist said at the annual meeting of the American College of Rheumatology.
She and several coinvestigators who are members of the Systemic Lupus International Collaborating Clinics (SLICC) set out to learn the answer. They developed a new, modified, weighted version of the 2012 SLICC criteria and compared its sensitivity and specificity for SLE diagnosis by 690 physicians with three other major classification systems: the 1997 update to ACR-11 criteria, the nonweighted SLICC 2012 criteria, and the proposed EULAR/ACR criteria, which uses a differentially weighted approach in which the various possible disease manifestations are each assigned a different point score. In contrast, the revised ACR-11 and SLICC 2012 criteria count each SLE manifestation equally.
Long story short: “The two newly derived weighted classification rules did not perform better than the existing list-based rules in terms of overall agreement,” according to Dr. Petri.
“We don’t think that weighting all the criteria, which is what the EULAR/ACR and weighted SLICC 2012 rules do, adds to the performance of the criteria set, and in fact it makes it much more difficult for clinicians to use when there’s a complicated weighting system, unless it’s web-based or there’s an app for it. And to be honest, clinicians are so busy that they’re probably not going to take time out in a clinic visit to go use the web or an app. Our criteria need to be user friendly,” she continued.
So which of the four classification systems is most user friendly? The EULAR/ACR criteria can be dismissed on that score because they are supposed to be used only for research, according to the rheumatologist.
“I think the SLICC 2012 criteria are very useful for clinicians because they have the highest sensitivity. And what a clinician wants is not to miss a diagnosis and to start treatment early,” Dr. Petri said.
To develop the weighted SLICC criteria, whose future at this point doesn’t look bright, she and her coinvestigators redeployed the same physician-rated patient scenarios used to develop the nonweighted 2012 SLICC classification criteria and assigned each of the potential manifestations of SLE a specific point score. For example, acute cutaneous manifestations received 16 points, serositis 9, oral ulcers 16, thrombocytopenia 15, and so forth. Under this system, a patient with a score of at least 56 points, or lupus nephritis, or least one clinical and one immunologic component of SLE was classified as having the disease.
Dr. Petri reported having no financial conflicts regarding her study, supported by the National Institutes of Health.
SOURCE: Petri MA et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1712.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: The 2012 SLICC SLE classification criteria are best suited for clinical practice.
Major finding:
Study details: This study compared the sensitivity, specificity, and overall agreement with physician diagnosis of four different sets of SLE classification criteria.
Disclosures: The study was supported by the National Institutes of Health. The presenter reported having no financial conflicts.
Source: Petri MA et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1712.
ACR and EULAR draft classification criteria for IgG4-related disease
CHICAGO – A joint American College of Rheumatology and European League Against Rheumatism panel has written the first-ever classification criteria for immunoglobulin G4-related disease (IgG4-RD), and the draft version of the criteria identified the disorder with 99.2% specificity and 85.5% sensitivity when compared with expert case opinions.
“We’ve come a long way” to write these criteria 17 years after the first case report, and about a decade after IgG4-RD first became part of routine rheumatology practice, John H. Stone, MD, said at the annual meeting of the American College of Rheumatology. He cited one estimate that about 185,000 Americans currently have IgG4-RD.
Approval of the draft criteria by both the ACR and EULAR remains pending.
The working group assembled by the American College of Rheumatology and the European League Against Rheumatism to write the classification criteria included 89 members, and the draft document they produced combined inclusion and exclusion criteria, “the first ACR and EULAR classification criteria to include specific exclusions, to my knowledge,” said Dr. Stone professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital in Boston. The exclusions reflect the many other disorders that can mimic IgG4-RD, including cancers and several rheumatologic diseases, especially granulomatosis with polyangiitis and Sjögren’s syndrome.
The writing panel used 487 case reports from 272 patients diagnosed with IgG4-RD and 215 patients diagnosed with a different, mimic disease to derive the classification criteria, and then used 908 case reports – 493 from IgG4-RD patients and 415 reports from mimic cases – to test and validate the criteria.
The first step in classifying a patient with IgG4-RD is to identify involvement of at least one organ from the list the panel compiled of 10 organs where involvement typifies the disease: pancreas, bile ducts, orbits, lacrimal glands, major salivary glands, retroperitoneum, kidney, aorta, pachymeninges, and thyroid gland (Riedel’s thyroiditis, but not Hashimoto’s disease). Patients who do not have disease involvement in at least one of these organs don’t qualify as having IgG4-RD.
The next step is to rule out patients who have at least one exclusion criterion from a list of 21 exclusions the panel cited, divided into four categories based on the test that finds each exclusion: clinical examination, serology, radiology, or pathology.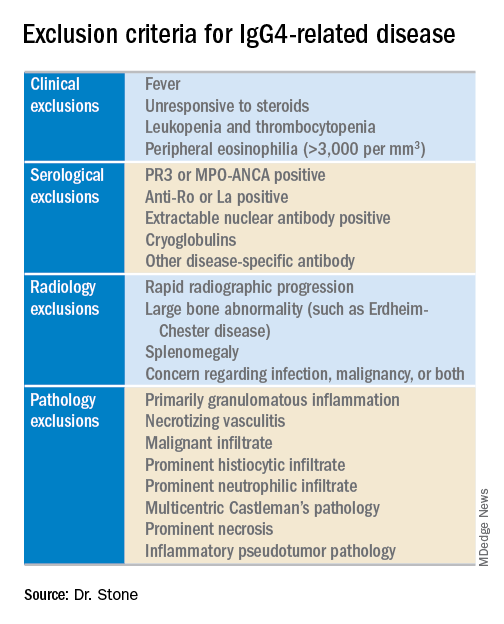
The last step is to identify enough individual classification hallmarks in the patient so that collectively they definitively identify IgG4-RD. The writing panel endorsed seven inclusion-criteria domains that each contain at least two different disease manifestations that confer points if fulfilled. To qualify for IgG4-RD classification, a patient needs to have enough manifestations to tally at least 19 points.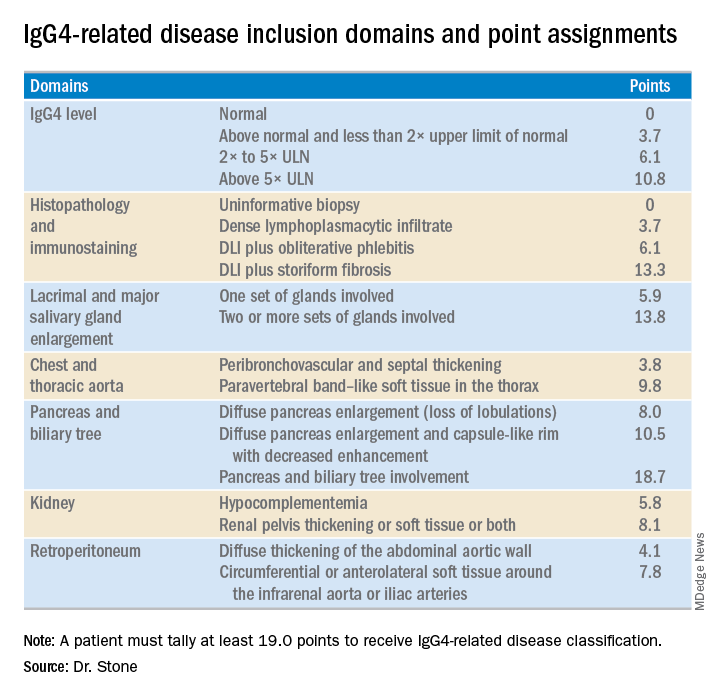
Fulfilling the inclusion criteria is the key step in classification, but the exclusion criteria also play a role in helping to rule the disease in or out, Dr. Stone noted. Without the exclusion criteria, the remaining classification criteria identified the 1,395 total cases and mimics studied with an increased sensitivity of 90% (compared with 85.5% when the exclusion criteria also apply), but with reduced specificity of 88.5% (compared with 99.2%). High specificity is a key aim. The criteria are supposed to give greater uniformity to patient selection for studies and ensure that enrolled patients actually have IgG4-RD. “Our goal was criteria that would prevent enrollment of patients without IgG4-RD,” he said.
Although IgG4 level is one of the seven inclusion domains and can give a patient as many as 10.8 points toward classification when the level exceeds five times the upper limit of normal, the criteria solidify the notion that “we have greatly overemphasized IgG4” in past considerations of the disease, said Dr. Stone. Elevation of IgG4 is one of several disease markers in most patients, but it’s not essential to classification and is missing in nearly a third of patients. While the cause of IgG4-RD remains unknown, it appears to involve an abnormal interaction between B cells and a CD4+ cytotoxic T lymphocyte, an understanding that has led to testing investigational therapies that target B cells including rituximab (Rituxan) and an agent called XmAb5871. “Rituximab works very well,” Dr. Stone said. The absence of a known cause is a reason why classification is so complex.
Dr. Stone also reminded his audience that IgG4-RD is an indolent disease that can produce symptoms for months or years before getting diagnosed. It often is accompanied by significant weight loss of 20 or more pounds, but without fever, and often features a dissociation between a high erythrocyte sedimentation rate but a relatively low level of C-reactive protein. “It’s astonishing how much weight patients lose,” he said.
Though barely more than a decade on the scene, awareness of IgG4-RD among rheumatologists has become widespread, though awareness has probably lagged among many primary care physicians, Dr. Stone said in an interview. The estimated prevalence of about 185,000 U.S. residents with IgG4-RD is probably an underestimate, he added. His group at Massachusetts General Hospital in Boston averages 3-5 patients evaluated each week as possibly having IgG4-RD, and this one group is now following more than 350 patients who have been diagnosed with the disease. “It’s probably more common than a lot of other conditions that rheumatologists treat, more common than scleroderma or ANCA-associated vasculitis,” Dr. Stone said. “The new criteria will help further raise awareness.”
Dr. Stone has been a consultant to and has received research funding from Genentech, Roche, and Xencor.
CHICAGO – A joint American College of Rheumatology and European League Against Rheumatism panel has written the first-ever classification criteria for immunoglobulin G4-related disease (IgG4-RD), and the draft version of the criteria identified the disorder with 99.2% specificity and 85.5% sensitivity when compared with expert case opinions.
“We’ve come a long way” to write these criteria 17 years after the first case report, and about a decade after IgG4-RD first became part of routine rheumatology practice, John H. Stone, MD, said at the annual meeting of the American College of Rheumatology. He cited one estimate that about 185,000 Americans currently have IgG4-RD.
Approval of the draft criteria by both the ACR and EULAR remains pending.
The working group assembled by the American College of Rheumatology and the European League Against Rheumatism to write the classification criteria included 89 members, and the draft document they produced combined inclusion and exclusion criteria, “the first ACR and EULAR classification criteria to include specific exclusions, to my knowledge,” said Dr. Stone professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital in Boston. The exclusions reflect the many other disorders that can mimic IgG4-RD, including cancers and several rheumatologic diseases, especially granulomatosis with polyangiitis and Sjögren’s syndrome.
The writing panel used 487 case reports from 272 patients diagnosed with IgG4-RD and 215 patients diagnosed with a different, mimic disease to derive the classification criteria, and then used 908 case reports – 493 from IgG4-RD patients and 415 reports from mimic cases – to test and validate the criteria.
The first step in classifying a patient with IgG4-RD is to identify involvement of at least one organ from the list the panel compiled of 10 organs where involvement typifies the disease: pancreas, bile ducts, orbits, lacrimal glands, major salivary glands, retroperitoneum, kidney, aorta, pachymeninges, and thyroid gland (Riedel’s thyroiditis, but not Hashimoto’s disease). Patients who do not have disease involvement in at least one of these organs don’t qualify as having IgG4-RD.
The next step is to rule out patients who have at least one exclusion criterion from a list of 21 exclusions the panel cited, divided into four categories based on the test that finds each exclusion: clinical examination, serology, radiology, or pathology.
The last step is to identify enough individual classification hallmarks in the patient so that collectively they definitively identify IgG4-RD. The writing panel endorsed seven inclusion-criteria domains that each contain at least two different disease manifestations that confer points if fulfilled. To qualify for IgG4-RD classification, a patient needs to have enough manifestations to tally at least 19 points.
Fulfilling the inclusion criteria is the key step in classification, but the exclusion criteria also play a role in helping to rule the disease in or out, Dr. Stone noted. Without the exclusion criteria, the remaining classification criteria identified the 1,395 total cases and mimics studied with an increased sensitivity of 90% (compared with 85.5% when the exclusion criteria also apply), but with reduced specificity of 88.5% (compared with 99.2%). High specificity is a key aim. The criteria are supposed to give greater uniformity to patient selection for studies and ensure that enrolled patients actually have IgG4-RD. “Our goal was criteria that would prevent enrollment of patients without IgG4-RD,” he said.
Although IgG4 level is one of the seven inclusion domains and can give a patient as many as 10.8 points toward classification when the level exceeds five times the upper limit of normal, the criteria solidify the notion that “we have greatly overemphasized IgG4” in past considerations of the disease, said Dr. Stone. Elevation of IgG4 is one of several disease markers in most patients, but it’s not essential to classification and is missing in nearly a third of patients. While the cause of IgG4-RD remains unknown, it appears to involve an abnormal interaction between B cells and a CD4+ cytotoxic T lymphocyte, an understanding that has led to testing investigational therapies that target B cells including rituximab (Rituxan) and an agent called XmAb5871. “Rituximab works very well,” Dr. Stone said. The absence of a known cause is a reason why classification is so complex.
Dr. Stone also reminded his audience that IgG4-RD is an indolent disease that can produce symptoms for months or years before getting diagnosed. It often is accompanied by significant weight loss of 20 or more pounds, but without fever, and often features a dissociation between a high erythrocyte sedimentation rate but a relatively low level of C-reactive protein. “It’s astonishing how much weight patients lose,” he said.
Though barely more than a decade on the scene, awareness of IgG4-RD among rheumatologists has become widespread, though awareness has probably lagged among many primary care physicians, Dr. Stone said in an interview. The estimated prevalence of about 185,000 U.S. residents with IgG4-RD is probably an underestimate, he added. His group at Massachusetts General Hospital in Boston averages 3-5 patients evaluated each week as possibly having IgG4-RD, and this one group is now following more than 350 patients who have been diagnosed with the disease. “It’s probably more common than a lot of other conditions that rheumatologists treat, more common than scleroderma or ANCA-associated vasculitis,” Dr. Stone said. “The new criteria will help further raise awareness.”
Dr. Stone has been a consultant to and has received research funding from Genentech, Roche, and Xencor.
CHICAGO – A joint American College of Rheumatology and European League Against Rheumatism panel has written the first-ever classification criteria for immunoglobulin G4-related disease (IgG4-RD), and the draft version of the criteria identified the disorder with 99.2% specificity and 85.5% sensitivity when compared with expert case opinions.
“We’ve come a long way” to write these criteria 17 years after the first case report, and about a decade after IgG4-RD first became part of routine rheumatology practice, John H. Stone, MD, said at the annual meeting of the American College of Rheumatology. He cited one estimate that about 185,000 Americans currently have IgG4-RD.
Approval of the draft criteria by both the ACR and EULAR remains pending.
The working group assembled by the American College of Rheumatology and the European League Against Rheumatism to write the classification criteria included 89 members, and the draft document they produced combined inclusion and exclusion criteria, “the first ACR and EULAR classification criteria to include specific exclusions, to my knowledge,” said Dr. Stone professor of medicine at Harvard Medical School and director of clinical rheumatology at Massachusetts General Hospital in Boston. The exclusions reflect the many other disorders that can mimic IgG4-RD, including cancers and several rheumatologic diseases, especially granulomatosis with polyangiitis and Sjögren’s syndrome.
The writing panel used 487 case reports from 272 patients diagnosed with IgG4-RD and 215 patients diagnosed with a different, mimic disease to derive the classification criteria, and then used 908 case reports – 493 from IgG4-RD patients and 415 reports from mimic cases – to test and validate the criteria.
The first step in classifying a patient with IgG4-RD is to identify involvement of at least one organ from the list the panel compiled of 10 organs where involvement typifies the disease: pancreas, bile ducts, orbits, lacrimal glands, major salivary glands, retroperitoneum, kidney, aorta, pachymeninges, and thyroid gland (Riedel’s thyroiditis, but not Hashimoto’s disease). Patients who do not have disease involvement in at least one of these organs don’t qualify as having IgG4-RD.
The next step is to rule out patients who have at least one exclusion criterion from a list of 21 exclusions the panel cited, divided into four categories based on the test that finds each exclusion: clinical examination, serology, radiology, or pathology.
The last step is to identify enough individual classification hallmarks in the patient so that collectively they definitively identify IgG4-RD. The writing panel endorsed seven inclusion-criteria domains that each contain at least two different disease manifestations that confer points if fulfilled. To qualify for IgG4-RD classification, a patient needs to have enough manifestations to tally at least 19 points.
Fulfilling the inclusion criteria is the key step in classification, but the exclusion criteria also play a role in helping to rule the disease in or out, Dr. Stone noted. Without the exclusion criteria, the remaining classification criteria identified the 1,395 total cases and mimics studied with an increased sensitivity of 90% (compared with 85.5% when the exclusion criteria also apply), but with reduced specificity of 88.5% (compared with 99.2%). High specificity is a key aim. The criteria are supposed to give greater uniformity to patient selection for studies and ensure that enrolled patients actually have IgG4-RD. “Our goal was criteria that would prevent enrollment of patients without IgG4-RD,” he said.
Although IgG4 level is one of the seven inclusion domains and can give a patient as many as 10.8 points toward classification when the level exceeds five times the upper limit of normal, the criteria solidify the notion that “we have greatly overemphasized IgG4” in past considerations of the disease, said Dr. Stone. Elevation of IgG4 is one of several disease markers in most patients, but it’s not essential to classification and is missing in nearly a third of patients. While the cause of IgG4-RD remains unknown, it appears to involve an abnormal interaction between B cells and a CD4+ cytotoxic T lymphocyte, an understanding that has led to testing investigational therapies that target B cells including rituximab (Rituxan) and an agent called XmAb5871. “Rituximab works very well,” Dr. Stone said. The absence of a known cause is a reason why classification is so complex.
Dr. Stone also reminded his audience that IgG4-RD is an indolent disease that can produce symptoms for months or years before getting diagnosed. It often is accompanied by significant weight loss of 20 or more pounds, but without fever, and often features a dissociation between a high erythrocyte sedimentation rate but a relatively low level of C-reactive protein. “It’s astonishing how much weight patients lose,” he said.
Though barely more than a decade on the scene, awareness of IgG4-RD among rheumatologists has become widespread, though awareness has probably lagged among many primary care physicians, Dr. Stone said in an interview. The estimated prevalence of about 185,000 U.S. residents with IgG4-RD is probably an underestimate, he added. His group at Massachusetts General Hospital in Boston averages 3-5 patients evaluated each week as possibly having IgG4-RD, and this one group is now following more than 350 patients who have been diagnosed with the disease. “It’s probably more common than a lot of other conditions that rheumatologists treat, more common than scleroderma or ANCA-associated vasculitis,” Dr. Stone said. “The new criteria will help further raise awareness.”
Dr. Stone has been a consultant to and has received research funding from Genentech, Roche, and Xencor.
REPORTING FROM THE ACR ANNUAL MEETING
Complications cluster in inflammatory arthritis patients after total knee replacement
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
CHICAGO – Patients with an inflammatory arthritis had significantly higher rates of infections, transfusions, and readmissions following total knee replacement than did patients without inflammatory arthritis in a study of more than 137,000 Americans who underwent this surgery.
A sampling of U.S. patients who underwent total knee arthroplasty (TKA) during 2007-2016 showed that among the small percentage of these patients who had an inflammatory arthritis (IA), the rate of periprosthetic joint or wound infection while hospitalized or out to 30 days after surgery was a statistically significant 64% higher relative to patients without inflammatory arthritis, after adjustment for several demographic and clinical confounders, including recent glucocorticoid treatment, Susan M. Goodman, MD, said at the annual meeting of the American College of Rheumatology. The analysis also showed a statistically significant 46% higher relative rate of hospital readmission for any cause during the 90 days after surgery, and a significant 39% relative increase in blood transfusions during the 30 days after TKA in the IA patients.
“These results have important implications for evolving bundled payment models” for TKA, said Dr. Goodman, a rheumatologist at the Hospital for Special Surgery in New York. “Hospitals should receive commensurate resources to maintain access to total TKA for patients with IA.”
For this analysis, Dr. Goodman and her associates classified IA as a patient with a recorded diagnosis of rheumatoid arthritis, spondyloarthritis, or systemic lupus erythematosus if the patient had also received treatment during the year before surgery with a disease-modifying antirheumatic drug, a biologic agent, or a drug that treats systemic lupus erythematosus.
Complications following TKA became a particular concern to hospitals starting in 2013 when the Centers for Medicare & Medicaid Services began a program that penalized hospitals for outcomes such as excessive readmissions following selected types of hospitalizations and also with recent steps to bundle TKA reimbursement with related 90-day outcomes.
“My concern is to ensure that patients with IA aren’t penalized and can maintain access” to TKA despite recent policy moves by the CMS. Faced with potential disincentives to treat patients with an IA, “hospitals might cherry pick patients,” Dr. Goodman said in an interview. The new findings “are a reason for administrators to argue for patients with IA to come out of the cost bundle.”
Dr. Goodman expressed hope that future policies will better reflect the higher levels of risk faced by patients with an IA undergoing TKA. CMS “is pretty responsive,” she said.
The study used data collected by Humana for about 25 million American health insurance beneficiaries during 2007-2016, which included 137,550 people who underwent a TKA. Of these, 3,067 (2%) met the study’s definition for IA, and 134,483 did not. Most of those who did not meet the definition likely had osteoarthritis, Dr. Goodman said. This low percentage of U.S. TKA patients with IA was consistent with numbers in prior reports.
The researchers calculated the relative risk of the IA patients, compared with all the others, for nine potential complications, including acute MI, pneumonia, sepsis, pulmonary embolism, and death. The complications with significantly higher rates among the IA patients after confounder adjustment were 30-day infections, 30-day transfusions, and 90-day readmissions.
Dr. Goodman had no relevant disclosures.
SOURCE: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.
REPORTING FROM THE ACR ANNUAL MEETING
Key clinical point: Complications were more common after total knee arthroplasty in patients with an inflammatory arthritis.
Major finding: Inflammatory arthritis patients had a 64% higher rate of infections after total knee arthroplasty, compared with patients without inflammatory arthritis.
Study details: Data analysis for 137,550 Americans who underwent total knee arthroplasty during 2007-2016.
Disclosures: Dr. Goodman had no relevant disclosures.
Source: Richardson S et al. Arthritis Rheumatol. 2018;70(Suppl 10): Abstract 1932.

















