User login
American College of Rheumatology (ACR): Annual Scientific Meeting
ACR: A reminder about ADHD-stimulant vasculopathy
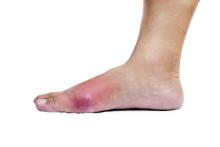
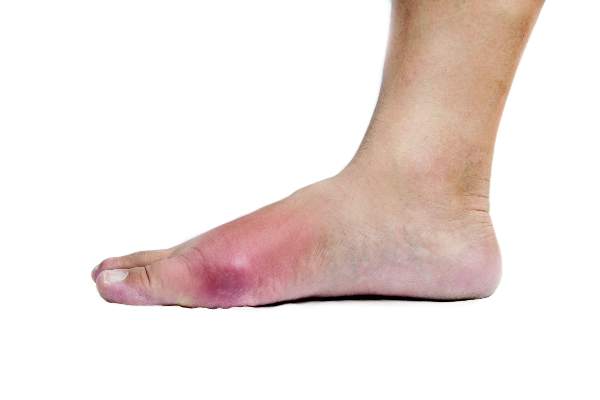
SAN FRANCISCO – Forty-seven children referred to Children’s Hospital of Georgia’s rheumatology clinic in Augusta for work-up of autoimmune vasculopathy had vasculopathy symptoms triggered by their ADHD stimulants.
They had been referred by primary care providers who told families that their children probably had an autoimmune disease; the families were relieved to find out that they did not, according to Dr. Rita Jerath, a pediatric rheumatologist in the department of rheumatology, Medical College of Georgia, Augusta.
The findings show that psychostimulant-induced peripheral vasculopathy, a known side effect included in the labeling of drugs such as methylphenidate (Ritalin or Concerta), dexmethylphenidate (Focalin), and amphetamine/dextroamphetamine (Adderall), is underrecognized in the medical community, leading to needless anxiety and unnecessary medical expenses, she noted.
Although uncommon, “there is a strong association between the use of psychostimulants and peripheral vasculopathy symptoms. Our study demonstrates the need of making prescribing physicians aware of this significant side effect so they may educate the families and also elucidate occurrence of new vascular symptoms [in] this patient population,” noted Dr. Jerath.
“A lot of rheumatologists will recognize this, but we want to get this message across to primary care physicians and families,” she added.
She had several tips for recognizing the problem. Otherwise well children seem to have Raynaud’s disease, “but their signs and symptoms are different,” she said. The color change is more extensive, on the tops of hands and feet, and more dusky and cyanotic. Hands and feet can be cold, numb, and painful, but the condition doesn’t change with cold or stress, as in Raynaud’s. Children can be positive for antinuclear antibodies, as several were in the study, Dr. Jerath said.
Children are usually on their psychostimulants from a few months or few years before an attack, and attacks seem to be triggered by a change in dose or drug.
The symptoms often improve with a drug holiday, for instance during vacation.
Although families are glad to learn their children don’t have an autoimmune disease, they are often reluctant to discontinue the stimulants. “A lot of times, the question is, ‘What else is available?’ There are a few medications that are not psychostimulants – Straterra [atomoxetine] is one – but usually physicians find they are not as helpful,” Dr. Jerath said.
The investigators had no disclosures.
SAN FRANCISCO – Forty-seven children referred to Children’s Hospital of Georgia’s rheumatology clinic in Augusta for work-up of autoimmune vasculopathy had vasculopathy symptoms triggered by their ADHD stimulants.
They had been referred by primary care providers who told families that their children probably had an autoimmune disease; the families were relieved to find out that they did not, according to Dr. Rita Jerath, a pediatric rheumatologist in the department of rheumatology, Medical College of Georgia, Augusta.
The findings show that psychostimulant-induced peripheral vasculopathy, a known side effect included in the labeling of drugs such as methylphenidate (Ritalin or Concerta), dexmethylphenidate (Focalin), and amphetamine/dextroamphetamine (Adderall), is underrecognized in the medical community, leading to needless anxiety and unnecessary medical expenses, she noted.
Although uncommon, “there is a strong association between the use of psychostimulants and peripheral vasculopathy symptoms. Our study demonstrates the need of making prescribing physicians aware of this significant side effect so they may educate the families and also elucidate occurrence of new vascular symptoms [in] this patient population,” noted Dr. Jerath.
“A lot of rheumatologists will recognize this, but we want to get this message across to primary care physicians and families,” she added.
She had several tips for recognizing the problem. Otherwise well children seem to have Raynaud’s disease, “but their signs and symptoms are different,” she said. The color change is more extensive, on the tops of hands and feet, and more dusky and cyanotic. Hands and feet can be cold, numb, and painful, but the condition doesn’t change with cold or stress, as in Raynaud’s. Children can be positive for antinuclear antibodies, as several were in the study, Dr. Jerath said.
Children are usually on their psychostimulants from a few months or few years before an attack, and attacks seem to be triggered by a change in dose or drug.
The symptoms often improve with a drug holiday, for instance during vacation.
Although families are glad to learn their children don’t have an autoimmune disease, they are often reluctant to discontinue the stimulants. “A lot of times, the question is, ‘What else is available?’ There are a few medications that are not psychostimulants – Straterra [atomoxetine] is one – but usually physicians find they are not as helpful,” Dr. Jerath said.
The investigators had no disclosures.
SAN FRANCISCO – Forty-seven children referred to Children’s Hospital of Georgia’s rheumatology clinic in Augusta for work-up of autoimmune vasculopathy had vasculopathy symptoms triggered by their ADHD stimulants.
They had been referred by primary care providers who told families that their children probably had an autoimmune disease; the families were relieved to find out that they did not, according to Dr. Rita Jerath, a pediatric rheumatologist in the department of rheumatology, Medical College of Georgia, Augusta.
The findings show that psychostimulant-induced peripheral vasculopathy, a known side effect included in the labeling of drugs such as methylphenidate (Ritalin or Concerta), dexmethylphenidate (Focalin), and amphetamine/dextroamphetamine (Adderall), is underrecognized in the medical community, leading to needless anxiety and unnecessary medical expenses, she noted.
Although uncommon, “there is a strong association between the use of psychostimulants and peripheral vasculopathy symptoms. Our study demonstrates the need of making prescribing physicians aware of this significant side effect so they may educate the families and also elucidate occurrence of new vascular symptoms [in] this patient population,” noted Dr. Jerath.
“A lot of rheumatologists will recognize this, but we want to get this message across to primary care physicians and families,” she added.
She had several tips for recognizing the problem. Otherwise well children seem to have Raynaud’s disease, “but their signs and symptoms are different,” she said. The color change is more extensive, on the tops of hands and feet, and more dusky and cyanotic. Hands and feet can be cold, numb, and painful, but the condition doesn’t change with cold or stress, as in Raynaud’s. Children can be positive for antinuclear antibodies, as several were in the study, Dr. Jerath said.
Children are usually on their psychostimulants from a few months or few years before an attack, and attacks seem to be triggered by a change in dose or drug.
The symptoms often improve with a drug holiday, for instance during vacation.
Although families are glad to learn their children don’t have an autoimmune disease, they are often reluctant to discontinue the stimulants. “A lot of times, the question is, ‘What else is available?’ There are a few medications that are not psychostimulants – Straterra [atomoxetine] is one – but usually physicians find they are not as helpful,” Dr. Jerath said.
The investigators had no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: ADHD stimulants can cause peripheral vasculopathy.
Major finding: Forty-seven children with stimulant-induced peripheral vasoconstriction were misdiagnosed with autoimmune disease.
Data source: A review of 47 pediatric ADHD cases.
Disclosures: The investigators had no disclosures.
ACR: Mycophenolate reduces need for GAVE endoscopy
SAN FRANCISCO – Mycophenolate significantly reduces the need for endoscopic interventions for gastric antral vascular ectasia in patients with systemic sclerosis, according to investigators from the Cleveland Clinic.
In a retrospective chart review of 48 systemic sclerosis patients with gastric antral vascular ectasia (GAVE), the 19 patients not on immunosuppressive agents needed 1.37 endoscopies per year. The 14 patients on mycophenolate needed 0.25 per year. Mycophenolate patients were far less likely to need endoscopy (relative risk, 0.19; 95% confidence interval, 0.05-0.70; P = .013).
Other immunosuppressives such as glucocorticoids, hydroxychloroquine, cyclophosphamide, and methotrexate also reduced the frequency of endoscopies, but not as much. The investigators found that patients on any immunosuppressive agent needed 0.51 endoscopies per year.
“Mycophenolate should be considered a potential treatment to alter the course of disease particularly in patients that need frequent therapeutic endoscopies. We are considering it more and more in our GAVE patients,” said investigator Dr. Tiffany Lin, a rheumatology fellow at the Cleveland Clinic Foundation.
“We had a suspicion that” immunosuppression might help “based on prior reports that looked at cyclophosphamide. We sought to” find other “immunosuppressive therapies that were less toxic and were surprised and excited about the results with mycophenolate,” she said at the annual meeting of the American College of Rheumatology.
The mean dose of mycophenolate in the study was 1 g twice a day. The duration of treatment varied from months to years.
In GAVE, also known as “watermelon stomach,” antral blood vessels are dilated and likely to bleed. It is an increasingly recognized cause of upper gastrointestinal bleeding in patients with systemic sclerosis.
There’s currently no defined role for immunosuppressive therapy in GAVE, which is usually treated with endoscopic argon plasma coagulation of the aberrant vessels.
Patients in the study were in their mid-60s on average, and the majority were women. There were no significant differences in hospitalization or blood transfusion frequencies between immunosuppressed and nonimmunosuppressed patients.
The investigators have no disclosures, and there was no outside funding for the project.
SAN FRANCISCO – Mycophenolate significantly reduces the need for endoscopic interventions for gastric antral vascular ectasia in patients with systemic sclerosis, according to investigators from the Cleveland Clinic.
In a retrospective chart review of 48 systemic sclerosis patients with gastric antral vascular ectasia (GAVE), the 19 patients not on immunosuppressive agents needed 1.37 endoscopies per year. The 14 patients on mycophenolate needed 0.25 per year. Mycophenolate patients were far less likely to need endoscopy (relative risk, 0.19; 95% confidence interval, 0.05-0.70; P = .013).
Other immunosuppressives such as glucocorticoids, hydroxychloroquine, cyclophosphamide, and methotrexate also reduced the frequency of endoscopies, but not as much. The investigators found that patients on any immunosuppressive agent needed 0.51 endoscopies per year.
“Mycophenolate should be considered a potential treatment to alter the course of disease particularly in patients that need frequent therapeutic endoscopies. We are considering it more and more in our GAVE patients,” said investigator Dr. Tiffany Lin, a rheumatology fellow at the Cleveland Clinic Foundation.
“We had a suspicion that” immunosuppression might help “based on prior reports that looked at cyclophosphamide. We sought to” find other “immunosuppressive therapies that were less toxic and were surprised and excited about the results with mycophenolate,” she said at the annual meeting of the American College of Rheumatology.
The mean dose of mycophenolate in the study was 1 g twice a day. The duration of treatment varied from months to years.
In GAVE, also known as “watermelon stomach,” antral blood vessels are dilated and likely to bleed. It is an increasingly recognized cause of upper gastrointestinal bleeding in patients with systemic sclerosis.
There’s currently no defined role for immunosuppressive therapy in GAVE, which is usually treated with endoscopic argon plasma coagulation of the aberrant vessels.
Patients in the study were in their mid-60s on average, and the majority were women. There were no significant differences in hospitalization or blood transfusion frequencies between immunosuppressed and nonimmunosuppressed patients.
The investigators have no disclosures, and there was no outside funding for the project.
SAN FRANCISCO – Mycophenolate significantly reduces the need for endoscopic interventions for gastric antral vascular ectasia in patients with systemic sclerosis, according to investigators from the Cleveland Clinic.
In a retrospective chart review of 48 systemic sclerosis patients with gastric antral vascular ectasia (GAVE), the 19 patients not on immunosuppressive agents needed 1.37 endoscopies per year. The 14 patients on mycophenolate needed 0.25 per year. Mycophenolate patients were far less likely to need endoscopy (relative risk, 0.19; 95% confidence interval, 0.05-0.70; P = .013).
Other immunosuppressives such as glucocorticoids, hydroxychloroquine, cyclophosphamide, and methotrexate also reduced the frequency of endoscopies, but not as much. The investigators found that patients on any immunosuppressive agent needed 0.51 endoscopies per year.
“Mycophenolate should be considered a potential treatment to alter the course of disease particularly in patients that need frequent therapeutic endoscopies. We are considering it more and more in our GAVE patients,” said investigator Dr. Tiffany Lin, a rheumatology fellow at the Cleveland Clinic Foundation.
“We had a suspicion that” immunosuppression might help “based on prior reports that looked at cyclophosphamide. We sought to” find other “immunosuppressive therapies that were less toxic and were surprised and excited about the results with mycophenolate,” she said at the annual meeting of the American College of Rheumatology.
The mean dose of mycophenolate in the study was 1 g twice a day. The duration of treatment varied from months to years.
In GAVE, also known as “watermelon stomach,” antral blood vessels are dilated and likely to bleed. It is an increasingly recognized cause of upper gastrointestinal bleeding in patients with systemic sclerosis.
There’s currently no defined role for immunosuppressive therapy in GAVE, which is usually treated with endoscopic argon plasma coagulation of the aberrant vessels.
Patients in the study were in their mid-60s on average, and the majority were women. There were no significant differences in hospitalization or blood transfusion frequencies between immunosuppressed and nonimmunosuppressed patients.
The investigators have no disclosures, and there was no outside funding for the project.
AT THE ACR ANNUAL MEETING
Key clinical point: Mycophenolate reduces the frequency of recurrent gastric antral vascular ectasia in systemic sclerosis.
Major finding: Nineteen patients not on immunosuppressive agents needed 1.37 endoscopies per year. The 14 patients on mycophenolate needed 0.25 per year.
Data source: The study was a retrospective review of 48 systemic sclerosis patients with GAVE.
Disclosures: The investigators have no disclosures, and there was no outside funding for the project.
Gout hospitalizations, costs surpass those for rheumatoid arthritis
SAN FRANCISCO – Hospitalization rates for gout doubled between 1993 and 2011, while those for rheumatoid arthritis fell by 67%, Dr. Sian Yik Lim reported at the annual meeting of the American College of Rheumatology.
The trends reveal marked progress in the treatment of rheumatoid arthritis, but “also highlight a critical need to improve gout care” and to implement measures to combat its rising prevalence, said Dr. Lim, a research fellow in medicine at Massachusetts General Hospital, Boston.
The study is “a very nice piece of work,” added Dr. James Galloway, who helped moderate the session at the meeting and is a lecturer in rheumatology at King’s College Hospital in London. “It makes us wonder whether we should spend more time teaching our primary care physician colleagues more about gout than rheumatoid arthritis.”
Gout and rheumatoid arthritis are the two most common inflammatory joint diseases, but few longitudinal studies have examined trends in related hospital admissions or costs, Dr. Lim said. Therefore, he and his associates extracted primary hospital discharge ICD-9 diagnosis codes from the National Inpatient Sample and also studied primary procedure codes for total knee replacement, total hip replacement, and other major joint surgeries for 1993 through 2011. In addition, they merged these with cost data from the Healthcare Cost and Utilization Project for 2001 to 2011.
Hospitalizations for gout and rheumatoid arthritis “crisscrossed” during the study period, Dr. Lim said. While there were more than three times as many hospitalizations for rheumatoid arthritis (26,712) as for gout (8,485) in 1993, by 2011, there were nearly twice as many admissions for gout (20,949) as for rheumatoid arthritis (11,015). Likewise, hospitalization rates for gout rose from 4.5 to 9 per 100,000 adults, while rheumatoid arthritis admissions fell by about two-thirds, from 14 to 5 per 100,000 adults. For the entire study period, hospital admissions for gout totaled 254,982, compared with 323,649 for rheumatoid arthritis, Dr. Lim added.
The investigators also documented a steady drop in admissions for total knee replacement, total hip replacement, and other major joint surgeries among rheumatoid arthritis patients, mirroring the results of recent multicenter studies in California (Ann Rheum Dis. 2009 Jul 5. doi: 10.1136/ard.2009.112474), the United Kingdom (Rheumatology [Oxford]. 2015 Apr;54[4]:666-71), and Germany (Ann Rheum Dis. 2015;74:738-45), Dr. Lim said. “This finding contrasts with the overall dramatic increase in these surgeries in the general United States population in recent years,” he added.
Inflation-adjusted hospital costs for gout and rheumatoid arthritis also converged during the study period, and by 2011, costs for gout actually exceeded those for rheumatoid arthritis, Dr. Lim said.
Overall, the findings reflect stark differences in care for gout and rheumatoid arthritis in the United States, he added. “New and potent drugs, effective combination regimens, and management strategies are increasingly being adopted for rheumatoid arthritis, but care for gout remains suboptimal, even though its prevalence and incidence are increasing,” he said. “Although the vast majority of gout patients are indicated for urate-lowering therapy, only a small proportion retrieve treatment, and the level of uric acid is not even measured in the vast majority of patients.”
Surveys also indicate that patients with gout often do not receive clear explanations about their disease, which may explain why as few as 10% follow treatment recommendations, according to Dr. Lim. He pointed to a hospital-based, retrospective cohort study reported at last year’s ACR annual meeting in which 89% of hospitalizations for gout were considered preventable. “These data support the need to improve gout prevention and care,” he said.
Dr. Lim had no disclosures. Dr. Hyon Choi, the senior author, reported having served on advisory boards for Takeda and AstraZeneca.
SAN FRANCISCO – Hospitalization rates for gout doubled between 1993 and 2011, while those for rheumatoid arthritis fell by 67%, Dr. Sian Yik Lim reported at the annual meeting of the American College of Rheumatology.
The trends reveal marked progress in the treatment of rheumatoid arthritis, but “also highlight a critical need to improve gout care” and to implement measures to combat its rising prevalence, said Dr. Lim, a research fellow in medicine at Massachusetts General Hospital, Boston.
The study is “a very nice piece of work,” added Dr. James Galloway, who helped moderate the session at the meeting and is a lecturer in rheumatology at King’s College Hospital in London. “It makes us wonder whether we should spend more time teaching our primary care physician colleagues more about gout than rheumatoid arthritis.”
Gout and rheumatoid arthritis are the two most common inflammatory joint diseases, but few longitudinal studies have examined trends in related hospital admissions or costs, Dr. Lim said. Therefore, he and his associates extracted primary hospital discharge ICD-9 diagnosis codes from the National Inpatient Sample and also studied primary procedure codes for total knee replacement, total hip replacement, and other major joint surgeries for 1993 through 2011. In addition, they merged these with cost data from the Healthcare Cost and Utilization Project for 2001 to 2011.
Hospitalizations for gout and rheumatoid arthritis “crisscrossed” during the study period, Dr. Lim said. While there were more than three times as many hospitalizations for rheumatoid arthritis (26,712) as for gout (8,485) in 1993, by 2011, there were nearly twice as many admissions for gout (20,949) as for rheumatoid arthritis (11,015). Likewise, hospitalization rates for gout rose from 4.5 to 9 per 100,000 adults, while rheumatoid arthritis admissions fell by about two-thirds, from 14 to 5 per 100,000 adults. For the entire study period, hospital admissions for gout totaled 254,982, compared with 323,649 for rheumatoid arthritis, Dr. Lim added.
The investigators also documented a steady drop in admissions for total knee replacement, total hip replacement, and other major joint surgeries among rheumatoid arthritis patients, mirroring the results of recent multicenter studies in California (Ann Rheum Dis. 2009 Jul 5. doi: 10.1136/ard.2009.112474), the United Kingdom (Rheumatology [Oxford]. 2015 Apr;54[4]:666-71), and Germany (Ann Rheum Dis. 2015;74:738-45), Dr. Lim said. “This finding contrasts with the overall dramatic increase in these surgeries in the general United States population in recent years,” he added.
Inflation-adjusted hospital costs for gout and rheumatoid arthritis also converged during the study period, and by 2011, costs for gout actually exceeded those for rheumatoid arthritis, Dr. Lim said.
Overall, the findings reflect stark differences in care for gout and rheumatoid arthritis in the United States, he added. “New and potent drugs, effective combination regimens, and management strategies are increasingly being adopted for rheumatoid arthritis, but care for gout remains suboptimal, even though its prevalence and incidence are increasing,” he said. “Although the vast majority of gout patients are indicated for urate-lowering therapy, only a small proportion retrieve treatment, and the level of uric acid is not even measured in the vast majority of patients.”
Surveys also indicate that patients with gout often do not receive clear explanations about their disease, which may explain why as few as 10% follow treatment recommendations, according to Dr. Lim. He pointed to a hospital-based, retrospective cohort study reported at last year’s ACR annual meeting in which 89% of hospitalizations for gout were considered preventable. “These data support the need to improve gout prevention and care,” he said.
Dr. Lim had no disclosures. Dr. Hyon Choi, the senior author, reported having served on advisory boards for Takeda and AstraZeneca.
SAN FRANCISCO – Hospitalization rates for gout doubled between 1993 and 2011, while those for rheumatoid arthritis fell by 67%, Dr. Sian Yik Lim reported at the annual meeting of the American College of Rheumatology.
The trends reveal marked progress in the treatment of rheumatoid arthritis, but “also highlight a critical need to improve gout care” and to implement measures to combat its rising prevalence, said Dr. Lim, a research fellow in medicine at Massachusetts General Hospital, Boston.
The study is “a very nice piece of work,” added Dr. James Galloway, who helped moderate the session at the meeting and is a lecturer in rheumatology at King’s College Hospital in London. “It makes us wonder whether we should spend more time teaching our primary care physician colleagues more about gout than rheumatoid arthritis.”
Gout and rheumatoid arthritis are the two most common inflammatory joint diseases, but few longitudinal studies have examined trends in related hospital admissions or costs, Dr. Lim said. Therefore, he and his associates extracted primary hospital discharge ICD-9 diagnosis codes from the National Inpatient Sample and also studied primary procedure codes for total knee replacement, total hip replacement, and other major joint surgeries for 1993 through 2011. In addition, they merged these with cost data from the Healthcare Cost and Utilization Project for 2001 to 2011.
Hospitalizations for gout and rheumatoid arthritis “crisscrossed” during the study period, Dr. Lim said. While there were more than three times as many hospitalizations for rheumatoid arthritis (26,712) as for gout (8,485) in 1993, by 2011, there were nearly twice as many admissions for gout (20,949) as for rheumatoid arthritis (11,015). Likewise, hospitalization rates for gout rose from 4.5 to 9 per 100,000 adults, while rheumatoid arthritis admissions fell by about two-thirds, from 14 to 5 per 100,000 adults. For the entire study period, hospital admissions for gout totaled 254,982, compared with 323,649 for rheumatoid arthritis, Dr. Lim added.
The investigators also documented a steady drop in admissions for total knee replacement, total hip replacement, and other major joint surgeries among rheumatoid arthritis patients, mirroring the results of recent multicenter studies in California (Ann Rheum Dis. 2009 Jul 5. doi: 10.1136/ard.2009.112474), the United Kingdom (Rheumatology [Oxford]. 2015 Apr;54[4]:666-71), and Germany (Ann Rheum Dis. 2015;74:738-45), Dr. Lim said. “This finding contrasts with the overall dramatic increase in these surgeries in the general United States population in recent years,” he added.
Inflation-adjusted hospital costs for gout and rheumatoid arthritis also converged during the study period, and by 2011, costs for gout actually exceeded those for rheumatoid arthritis, Dr. Lim said.
Overall, the findings reflect stark differences in care for gout and rheumatoid arthritis in the United States, he added. “New and potent drugs, effective combination regimens, and management strategies are increasingly being adopted for rheumatoid arthritis, but care for gout remains suboptimal, even though its prevalence and incidence are increasing,” he said. “Although the vast majority of gout patients are indicated for urate-lowering therapy, only a small proportion retrieve treatment, and the level of uric acid is not even measured in the vast majority of patients.”
Surveys also indicate that patients with gout often do not receive clear explanations about their disease, which may explain why as few as 10% follow treatment recommendations, according to Dr. Lim. He pointed to a hospital-based, retrospective cohort study reported at last year’s ACR annual meeting in which 89% of hospitalizations for gout were considered preventable. “These data support the need to improve gout prevention and care,” he said.
Dr. Lim had no disclosures. Dr. Hyon Choi, the senior author, reported having served on advisory boards for Takeda and AstraZeneca.
AT THE ACR ANNUAL MEETING
Key clinical point: Hospitalizations and costs for gout in the United States have surpassed those for rheumatoid arthritis, reflecting stark differences in the state of care for these diseases.
Major finding: Between 1993 and 2011, hospitalizations for gout rose from 4.5 to 9 per 100,000 adults, while rheumatoid arthritis admissions fell from 14 to 5 per 100,000 adults.
Data source: Analysis of primary discharge diagnoses from the National Inpatient Sample and cost data from the Healthcare Cost and Utilization Project.
Disclosures: Dr. Lim had no disclosures. Dr. Hyon Choi, the senior author, reported having served on advisory boards for Takeda and AstraZeneca.
Shingles vaccine protection lasted about 5 years in autoimmune disease patients
SAN FRANCISCO – Protection against shingles appeared to wane about 5 years after patients with autoimmune diseases received the live herpes zoster vaccine, according to a large retrospective cohort study presented at the annual meeting of the American College of Rheumatology.
In contrast, shingles risk remained fairly constant among unvaccinated patients during 7 years of follow-up, reported Dr. Huifeng Yun of the department of epidemiology at the University of Alabama at Birmingham. “Our findings suggest that patients might benefit from a booster vaccine at some point after initial vaccination, but further research is needed to determine when and if such a strategy is effective at preventing herpes zoster,” she said.
The long-term Shingles Prevention Study showed that the live herpes zoster vaccine is effective for about a decade among healthy older individuals. But about 90% of zoster cases occur in patients with autoimmune and inflammatory diseases, for whom the duration of vaccine protection has been unclear, Dr. Yun said. “Antiviral medication approved for herpes zoster reduces the severity and duration of symptoms, but may not prevent postherpetic neuralgia,” making vaccine protection a priority, she added.
Dr. Yun and her associates retrospectively analyzed Medicare data for nearly 179,000 patients with autoimmune and inflammatory conditions between 2006 and 2013. About one-third of patients were vaccinated against herpes zoster, and more than 40% had rheumatoid arthritis, nearly one-third had psoriasis, about 20% had inflammatory bowel disease, about 5% had psoriatic arthritis, and about 1% had ankylosing spondylitis. The researchers matched unvaccinated and vaccinated patients in a 2:1 ratio based on calendar year, age, sex, race, disease type, and use of disease-modifying antirheumatic drugs, biologics, and glucocorticoids. They followed patients from 30 days after vaccination – or the corresponding date in the unvaccinated cohort – until death, first episode of herpes zoster, or the end of 2013, whichever came first.
The rates of herpes zoster among vaccinated patients rose from 0.75/100 person-years during the first year after vaccination to 1.25/100 person-years during the seventh year of follow-up, Dr. Yun said. In contrast, zoster rates among unvaccinated patients remained steady, ranging between 1.32 and 1.35 cases per 100 person-years. In an adjusted analysis of data from 2006 through 2012, vaccinated patients had about half the risk of herpes zoster, compared with unvaccinated patients, during year 1 (relative risk, 0.52) and remained significantly less likely to develop shingles until year 6, when the gap in risk between vaccinated and unvaccinated patients essentially closed (RR, 0.92).
“The herpes zoster vaccine was effective for about 5 years, and results of subgroup analyses were consistent with the main analysis,” Dr. Yun concluded. But the Medicare database lacked details on disease severity or lifestyle factors, both of which might have affected the results, she cautioned.
Dr. Yun disclosed a financial relationship with Amgen. The senior author and two coauthors also disclosed relationships with several pharmaceutical companies. Two investigators had no disclosures.
SAN FRANCISCO – Protection against shingles appeared to wane about 5 years after patients with autoimmune diseases received the live herpes zoster vaccine, according to a large retrospective cohort study presented at the annual meeting of the American College of Rheumatology.
In contrast, shingles risk remained fairly constant among unvaccinated patients during 7 years of follow-up, reported Dr. Huifeng Yun of the department of epidemiology at the University of Alabama at Birmingham. “Our findings suggest that patients might benefit from a booster vaccine at some point after initial vaccination, but further research is needed to determine when and if such a strategy is effective at preventing herpes zoster,” she said.
The long-term Shingles Prevention Study showed that the live herpes zoster vaccine is effective for about a decade among healthy older individuals. But about 90% of zoster cases occur in patients with autoimmune and inflammatory diseases, for whom the duration of vaccine protection has been unclear, Dr. Yun said. “Antiviral medication approved for herpes zoster reduces the severity and duration of symptoms, but may not prevent postherpetic neuralgia,” making vaccine protection a priority, she added.
Dr. Yun and her associates retrospectively analyzed Medicare data for nearly 179,000 patients with autoimmune and inflammatory conditions between 2006 and 2013. About one-third of patients were vaccinated against herpes zoster, and more than 40% had rheumatoid arthritis, nearly one-third had psoriasis, about 20% had inflammatory bowel disease, about 5% had psoriatic arthritis, and about 1% had ankylosing spondylitis. The researchers matched unvaccinated and vaccinated patients in a 2:1 ratio based on calendar year, age, sex, race, disease type, and use of disease-modifying antirheumatic drugs, biologics, and glucocorticoids. They followed patients from 30 days after vaccination – or the corresponding date in the unvaccinated cohort – until death, first episode of herpes zoster, or the end of 2013, whichever came first.
The rates of herpes zoster among vaccinated patients rose from 0.75/100 person-years during the first year after vaccination to 1.25/100 person-years during the seventh year of follow-up, Dr. Yun said. In contrast, zoster rates among unvaccinated patients remained steady, ranging between 1.32 and 1.35 cases per 100 person-years. In an adjusted analysis of data from 2006 through 2012, vaccinated patients had about half the risk of herpes zoster, compared with unvaccinated patients, during year 1 (relative risk, 0.52) and remained significantly less likely to develop shingles until year 6, when the gap in risk between vaccinated and unvaccinated patients essentially closed (RR, 0.92).
“The herpes zoster vaccine was effective for about 5 years, and results of subgroup analyses were consistent with the main analysis,” Dr. Yun concluded. But the Medicare database lacked details on disease severity or lifestyle factors, both of which might have affected the results, she cautioned.
Dr. Yun disclosed a financial relationship with Amgen. The senior author and two coauthors also disclosed relationships with several pharmaceutical companies. Two investigators had no disclosures.
SAN FRANCISCO – Protection against shingles appeared to wane about 5 years after patients with autoimmune diseases received the live herpes zoster vaccine, according to a large retrospective cohort study presented at the annual meeting of the American College of Rheumatology.
In contrast, shingles risk remained fairly constant among unvaccinated patients during 7 years of follow-up, reported Dr. Huifeng Yun of the department of epidemiology at the University of Alabama at Birmingham. “Our findings suggest that patients might benefit from a booster vaccine at some point after initial vaccination, but further research is needed to determine when and if such a strategy is effective at preventing herpes zoster,” she said.
The long-term Shingles Prevention Study showed that the live herpes zoster vaccine is effective for about a decade among healthy older individuals. But about 90% of zoster cases occur in patients with autoimmune and inflammatory diseases, for whom the duration of vaccine protection has been unclear, Dr. Yun said. “Antiviral medication approved for herpes zoster reduces the severity and duration of symptoms, but may not prevent postherpetic neuralgia,” making vaccine protection a priority, she added.
Dr. Yun and her associates retrospectively analyzed Medicare data for nearly 179,000 patients with autoimmune and inflammatory conditions between 2006 and 2013. About one-third of patients were vaccinated against herpes zoster, and more than 40% had rheumatoid arthritis, nearly one-third had psoriasis, about 20% had inflammatory bowel disease, about 5% had psoriatic arthritis, and about 1% had ankylosing spondylitis. The researchers matched unvaccinated and vaccinated patients in a 2:1 ratio based on calendar year, age, sex, race, disease type, and use of disease-modifying antirheumatic drugs, biologics, and glucocorticoids. They followed patients from 30 days after vaccination – or the corresponding date in the unvaccinated cohort – until death, first episode of herpes zoster, or the end of 2013, whichever came first.
The rates of herpes zoster among vaccinated patients rose from 0.75/100 person-years during the first year after vaccination to 1.25/100 person-years during the seventh year of follow-up, Dr. Yun said. In contrast, zoster rates among unvaccinated patients remained steady, ranging between 1.32 and 1.35 cases per 100 person-years. In an adjusted analysis of data from 2006 through 2012, vaccinated patients had about half the risk of herpes zoster, compared with unvaccinated patients, during year 1 (relative risk, 0.52) and remained significantly less likely to develop shingles until year 6, when the gap in risk between vaccinated and unvaccinated patients essentially closed (RR, 0.92).
“The herpes zoster vaccine was effective for about 5 years, and results of subgroup analyses were consistent with the main analysis,” Dr. Yun concluded. But the Medicare database lacked details on disease severity or lifestyle factors, both of which might have affected the results, she cautioned.
Dr. Yun disclosed a financial relationship with Amgen. The senior author and two coauthors also disclosed relationships with several pharmaceutical companies. Two investigators had no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Protection against shingles appeared to wane 5 years after patients with autoimmune diseases received the live herpes zoster vaccine.
Major finding: The risk of herpes zoster was significantly lower among vaccinated, compared with unvaccinated, patients during each of the first 5 years after vaccination.
Data source: A retrospective analysis of 178,881 Medicare patients with autoimmune diseases between 2006 and 2013.
Disclosures: Dr. Yun disclosed a financial relationship with Amgen. The senior author and two coauthors also disclosed relationships with several pharmaceutical companies. Two investigators had no disclosures.
ACR: Epratuzumab falls flat in phase III lupus trials
SAN FRANCISCO – The anti-CD22 monoclonal antibody epratuzumab missed its primary and secondary endpoints in two phase III trials involving adults with moderate to severe systemic lupus erythematosus.
At week 48, patients who received epratuzumab and standard therapy fared no better than those who received standard care and placebo, said Dr. Megan E.B. Clowse, who is a rheumatologist and clinical researcher at Duke University in Durham, N.C. “Many additional exploratory analyses were performed, and we really did not find any difference among the groups that would reveal a set of patients that would benefit from this treatment,” she said in her presentation of the two trials at the annual meeting of the American College of Rheumatology.
The failed EMBODY 1 and EMBODY 2 trials were identically designed phase III, multicenter, double-blind, randomized, placebo-controlled studies of adults with moderate to severe systemic lupus erythematosus (SLE), based on at least four ACR criteria, who also tested positive for anti–double stranded DNA or antinuclear antibodies. Participants were required to have received corticosteroids for at least 5 days before enrollment at a stable dose equivalent to 5-60 mg/day of prednisone. Doses of immunosuppressants and antimalarials also had to be stable, and patients could not have antiphospholipid syndrome or a British Isles Lupus Activity Group (BILAG) A score involving the kidneys or central nervous system. Most patients had musculoskeletal or musculocutaneous disease activity at baseline, Dr. Clowse said.
The treatment groups received standard-of-care therapies plus intravenous epratuzumab at doses of 1,200 mg every other week or 600 mg every week for a cumulative dose of 2,400 mg per 12-week treatment cycle, followed by 8 weeks without infusion. The primary endpoint was the percentage of responders by 48 weeks, as assessed by the composite BILAG-based Combined Lupus Assessment (BICLA). The BICLA measures improvement in BILAG disease activity across all eight body systems, with no worsening in BILAG or other disease activity indexes at the same time point and no treatment failure at any time point. There were a total of 1,579 patients in both studies combined.
About two-thirds of patients completed each study, and lack of efficacy was the main reason for dropping out, Dr. Clowse said. The primary endpoint was about 40% for all groups in both trials. Secondary endpoints, including changes in corticosteroid use, physician’s global assessment of disease activity, patient’s global assessment of disease activity, and other physician-reported and patient-reported outcomes, also were similar among the groups, she said. About 40% of patients remained on their baseline dose of corticosteroids, while less than 20% were able to cut their dose by at least half.
Epratuzumab was associated with improved SLE disease activity in phase IIb trials, Dr. Clowse noted. The biologic’s phase III safety profile resembled that of phase IIb trials and other prior studies, with no new safety signals, she added. The most common adverse events were upper respiratory tract infection, urinary tract infection, headache, and nausea.
UCB Pharma funded the study. Dr. Clowse reported having received consulting fees from UCB Pharma. Twelve of 15 coinvestigators also reported financial relationships with UCB Pharma and a number of other pharmaceutical companies.
SAN FRANCISCO – The anti-CD22 monoclonal antibody epratuzumab missed its primary and secondary endpoints in two phase III trials involving adults with moderate to severe systemic lupus erythematosus.
At week 48, patients who received epratuzumab and standard therapy fared no better than those who received standard care and placebo, said Dr. Megan E.B. Clowse, who is a rheumatologist and clinical researcher at Duke University in Durham, N.C. “Many additional exploratory analyses were performed, and we really did not find any difference among the groups that would reveal a set of patients that would benefit from this treatment,” she said in her presentation of the two trials at the annual meeting of the American College of Rheumatology.
The failed EMBODY 1 and EMBODY 2 trials were identically designed phase III, multicenter, double-blind, randomized, placebo-controlled studies of adults with moderate to severe systemic lupus erythematosus (SLE), based on at least four ACR criteria, who also tested positive for anti–double stranded DNA or antinuclear antibodies. Participants were required to have received corticosteroids for at least 5 days before enrollment at a stable dose equivalent to 5-60 mg/day of prednisone. Doses of immunosuppressants and antimalarials also had to be stable, and patients could not have antiphospholipid syndrome or a British Isles Lupus Activity Group (BILAG) A score involving the kidneys or central nervous system. Most patients had musculoskeletal or musculocutaneous disease activity at baseline, Dr. Clowse said.
The treatment groups received standard-of-care therapies plus intravenous epratuzumab at doses of 1,200 mg every other week or 600 mg every week for a cumulative dose of 2,400 mg per 12-week treatment cycle, followed by 8 weeks without infusion. The primary endpoint was the percentage of responders by 48 weeks, as assessed by the composite BILAG-based Combined Lupus Assessment (BICLA). The BICLA measures improvement in BILAG disease activity across all eight body systems, with no worsening in BILAG or other disease activity indexes at the same time point and no treatment failure at any time point. There were a total of 1,579 patients in both studies combined.
About two-thirds of patients completed each study, and lack of efficacy was the main reason for dropping out, Dr. Clowse said. The primary endpoint was about 40% for all groups in both trials. Secondary endpoints, including changes in corticosteroid use, physician’s global assessment of disease activity, patient’s global assessment of disease activity, and other physician-reported and patient-reported outcomes, also were similar among the groups, she said. About 40% of patients remained on their baseline dose of corticosteroids, while less than 20% were able to cut their dose by at least half.
Epratuzumab was associated with improved SLE disease activity in phase IIb trials, Dr. Clowse noted. The biologic’s phase III safety profile resembled that of phase IIb trials and other prior studies, with no new safety signals, she added. The most common adverse events were upper respiratory tract infection, urinary tract infection, headache, and nausea.
UCB Pharma funded the study. Dr. Clowse reported having received consulting fees from UCB Pharma. Twelve of 15 coinvestigators also reported financial relationships with UCB Pharma and a number of other pharmaceutical companies.
SAN FRANCISCO – The anti-CD22 monoclonal antibody epratuzumab missed its primary and secondary endpoints in two phase III trials involving adults with moderate to severe systemic lupus erythematosus.
At week 48, patients who received epratuzumab and standard therapy fared no better than those who received standard care and placebo, said Dr. Megan E.B. Clowse, who is a rheumatologist and clinical researcher at Duke University in Durham, N.C. “Many additional exploratory analyses were performed, and we really did not find any difference among the groups that would reveal a set of patients that would benefit from this treatment,” she said in her presentation of the two trials at the annual meeting of the American College of Rheumatology.
The failed EMBODY 1 and EMBODY 2 trials were identically designed phase III, multicenter, double-blind, randomized, placebo-controlled studies of adults with moderate to severe systemic lupus erythematosus (SLE), based on at least four ACR criteria, who also tested positive for anti–double stranded DNA or antinuclear antibodies. Participants were required to have received corticosteroids for at least 5 days before enrollment at a stable dose equivalent to 5-60 mg/day of prednisone. Doses of immunosuppressants and antimalarials also had to be stable, and patients could not have antiphospholipid syndrome or a British Isles Lupus Activity Group (BILAG) A score involving the kidneys or central nervous system. Most patients had musculoskeletal or musculocutaneous disease activity at baseline, Dr. Clowse said.
The treatment groups received standard-of-care therapies plus intravenous epratuzumab at doses of 1,200 mg every other week or 600 mg every week for a cumulative dose of 2,400 mg per 12-week treatment cycle, followed by 8 weeks without infusion. The primary endpoint was the percentage of responders by 48 weeks, as assessed by the composite BILAG-based Combined Lupus Assessment (BICLA). The BICLA measures improvement in BILAG disease activity across all eight body systems, with no worsening in BILAG or other disease activity indexes at the same time point and no treatment failure at any time point. There were a total of 1,579 patients in both studies combined.
About two-thirds of patients completed each study, and lack of efficacy was the main reason for dropping out, Dr. Clowse said. The primary endpoint was about 40% for all groups in both trials. Secondary endpoints, including changes in corticosteroid use, physician’s global assessment of disease activity, patient’s global assessment of disease activity, and other physician-reported and patient-reported outcomes, also were similar among the groups, she said. About 40% of patients remained on their baseline dose of corticosteroids, while less than 20% were able to cut their dose by at least half.
Epratuzumab was associated with improved SLE disease activity in phase IIb trials, Dr. Clowse noted. The biologic’s phase III safety profile resembled that of phase IIb trials and other prior studies, with no new safety signals, she added. The most common adverse events were upper respiratory tract infection, urinary tract infection, headache, and nausea.
UCB Pharma funded the study. Dr. Clowse reported having received consulting fees from UCB Pharma. Twelve of 15 coinvestigators also reported financial relationships with UCB Pharma and a number of other pharmaceutical companies.
AT THE ACR ANNUAL MEETING
Key clinical point: Epratuzumab was no more effective than placebo in two phase III trials of adults with moderate to severe systemic lupus erythematosus.
Major finding: Rates of BICLA response at 48 weeks were similar for the epratuzumab and placebo groups, as were all secondary and exploratory endpoints.
Data source: EMBODY 1 and 2 were phase III, multicenter, randomized, double-blind, placebo-controlled studies involving 1,579 adults.
Disclosures: UCB Pharma funded the study. Dr. Clowse reported having received consulting fees from UCB Pharma. Twelve of 15 coinvestigators also reported financial relationships with UCB Pharma and a number of other pharmaceutical companies.
Tocilizumab effective in giant cell arteritis
SAN FRANCISCO – Mounting evidence supports the use of tocilizumab in giant cell arteritis (GCA), and a new study supports use of the drug for induction and maintenance therapy in patients with newly diagnosed or relapsed GCA in the context of rapidly tapered glucocorticoid therapy.
“This is the first randomized controlled trial to prove efficacy of tocilizumab in induction and maintenance of remission in GCA. Tocilizumab reached the primary and secondary endpoints in our trial compared with placebo. After 12 months of therapy, more serious adverse events were observed in the placebo group,” said lead author Dr. Sabine Adler, University Hospital, Bern, Switzerland. Dr. Adler presented results of this late-breaking abstract at a special session during the annual meeting of the American College of Rheumatology.
A recent small open-label trial showed that tocilizumab was effective in patients with refractory GCA and unacceptable side effects on glucocorticoid (GC) therapy (Sem Arthr Rheum. Dec. 26, 2014. DOI:10.1016/j.semarthrit.2014.12.005). The present study was of newly diagnosed patients or those who had relapsed.
GCA is characterized by destructive inflammation in the walls of medium and large arteries. GC treatment has been the mainstay of therapy, controlling symptoms and reducing the risk of dreaded complications such as blindness, stroke, and claudication. But many patients require prolonged treatment, and cumulative doses of GC lead to substantial toxicity and morbidity. Thus, a better treatment is needed.
IL-6, the target of tocilizumab, is one of the cytokines involved in the pathogenesis of GCA. It is elevated in the serum and tissue of affected patients, Dr. Adler explained.
Dr. Adler and colleagues conducted a single-center, randomized, placebo-controlled trial evaluating tocilizumab in 30 patients with newly diagnosed or recurrent GCA. Patients were randomized in a 2:1 ratio to receive tocilizumab 8 mg/kg IV plus oral GC or IV placebo and oral GC. Infusions were given every 4 weeks for 1 year, over which time GC dose was slowly tapered to 0 mg in all patients. Some patients underwent angiography to rule out or define aortic involvement. Most patients in the trial had new onset GCA, she said.
The primary endpoint was complete remission at week 12 with GC dose of 0.1 mg/kg/day. Complete remission was defined as the absence of clinical signs and symptoms of GCA plus negative values for C-reactive protein (CRP) and erythrocyte sedimentation rate. At 12 weeks, the complete response rate was 85% for tocilizumab versus 40% for placebo, a significant difference. At the end of the trial, 85% and 20%, respectively, had not had a relapse; this difference was significant.
Placebo patients received a higher cumulative dose of GC, she continued.
There were 7 serious adverse events reported in the tocilizumab arm and 5 in the placebo arm; 1 death occurred in the placebo arm due to myocardial infarction.
“A closer look at serious adverse events reveals few cardiovascular problems,” Dr. Adler continued. Back pain and psychosis were reported as reasons for hospitalization.
During the question and answer session, an audience member noted that it was difficult to understand how a short-term treatment is effective in a chronic disease. “Probably patients will relapse when they stop the medication, so we are not on the ‘green’ side yet,” she replied. Dr. Adler noted that it could be possible that a reduced dose of tocilizumab could be effective but have fewer side effects, but that has not been studied.
Tocilizumab was provided by Roche for this investigator-initiated trial. Dr. Adler reported no financial disclosures.
SAN FRANCISCO – Mounting evidence supports the use of tocilizumab in giant cell arteritis (GCA), and a new study supports use of the drug for induction and maintenance therapy in patients with newly diagnosed or relapsed GCA in the context of rapidly tapered glucocorticoid therapy.
“This is the first randomized controlled trial to prove efficacy of tocilizumab in induction and maintenance of remission in GCA. Tocilizumab reached the primary and secondary endpoints in our trial compared with placebo. After 12 months of therapy, more serious adverse events were observed in the placebo group,” said lead author Dr. Sabine Adler, University Hospital, Bern, Switzerland. Dr. Adler presented results of this late-breaking abstract at a special session during the annual meeting of the American College of Rheumatology.
A recent small open-label trial showed that tocilizumab was effective in patients with refractory GCA and unacceptable side effects on glucocorticoid (GC) therapy (Sem Arthr Rheum. Dec. 26, 2014. DOI:10.1016/j.semarthrit.2014.12.005). The present study was of newly diagnosed patients or those who had relapsed.
GCA is characterized by destructive inflammation in the walls of medium and large arteries. GC treatment has been the mainstay of therapy, controlling symptoms and reducing the risk of dreaded complications such as blindness, stroke, and claudication. But many patients require prolonged treatment, and cumulative doses of GC lead to substantial toxicity and morbidity. Thus, a better treatment is needed.
IL-6, the target of tocilizumab, is one of the cytokines involved in the pathogenesis of GCA. It is elevated in the serum and tissue of affected patients, Dr. Adler explained.
Dr. Adler and colleagues conducted a single-center, randomized, placebo-controlled trial evaluating tocilizumab in 30 patients with newly diagnosed or recurrent GCA. Patients were randomized in a 2:1 ratio to receive tocilizumab 8 mg/kg IV plus oral GC or IV placebo and oral GC. Infusions were given every 4 weeks for 1 year, over which time GC dose was slowly tapered to 0 mg in all patients. Some patients underwent angiography to rule out or define aortic involvement. Most patients in the trial had new onset GCA, she said.
The primary endpoint was complete remission at week 12 with GC dose of 0.1 mg/kg/day. Complete remission was defined as the absence of clinical signs and symptoms of GCA plus negative values for C-reactive protein (CRP) and erythrocyte sedimentation rate. At 12 weeks, the complete response rate was 85% for tocilizumab versus 40% for placebo, a significant difference. At the end of the trial, 85% and 20%, respectively, had not had a relapse; this difference was significant.
Placebo patients received a higher cumulative dose of GC, she continued.
There were 7 serious adverse events reported in the tocilizumab arm and 5 in the placebo arm; 1 death occurred in the placebo arm due to myocardial infarction.
“A closer look at serious adverse events reveals few cardiovascular problems,” Dr. Adler continued. Back pain and psychosis were reported as reasons for hospitalization.
During the question and answer session, an audience member noted that it was difficult to understand how a short-term treatment is effective in a chronic disease. “Probably patients will relapse when they stop the medication, so we are not on the ‘green’ side yet,” she replied. Dr. Adler noted that it could be possible that a reduced dose of tocilizumab could be effective but have fewer side effects, but that has not been studied.
Tocilizumab was provided by Roche for this investigator-initiated trial. Dr. Adler reported no financial disclosures.
SAN FRANCISCO – Mounting evidence supports the use of tocilizumab in giant cell arteritis (GCA), and a new study supports use of the drug for induction and maintenance therapy in patients with newly diagnosed or relapsed GCA in the context of rapidly tapered glucocorticoid therapy.
“This is the first randomized controlled trial to prove efficacy of tocilizumab in induction and maintenance of remission in GCA. Tocilizumab reached the primary and secondary endpoints in our trial compared with placebo. After 12 months of therapy, more serious adverse events were observed in the placebo group,” said lead author Dr. Sabine Adler, University Hospital, Bern, Switzerland. Dr. Adler presented results of this late-breaking abstract at a special session during the annual meeting of the American College of Rheumatology.
A recent small open-label trial showed that tocilizumab was effective in patients with refractory GCA and unacceptable side effects on glucocorticoid (GC) therapy (Sem Arthr Rheum. Dec. 26, 2014. DOI:10.1016/j.semarthrit.2014.12.005). The present study was of newly diagnosed patients or those who had relapsed.
GCA is characterized by destructive inflammation in the walls of medium and large arteries. GC treatment has been the mainstay of therapy, controlling symptoms and reducing the risk of dreaded complications such as blindness, stroke, and claudication. But many patients require prolonged treatment, and cumulative doses of GC lead to substantial toxicity and morbidity. Thus, a better treatment is needed.
IL-6, the target of tocilizumab, is one of the cytokines involved in the pathogenesis of GCA. It is elevated in the serum and tissue of affected patients, Dr. Adler explained.
Dr. Adler and colleagues conducted a single-center, randomized, placebo-controlled trial evaluating tocilizumab in 30 patients with newly diagnosed or recurrent GCA. Patients were randomized in a 2:1 ratio to receive tocilizumab 8 mg/kg IV plus oral GC or IV placebo and oral GC. Infusions were given every 4 weeks for 1 year, over which time GC dose was slowly tapered to 0 mg in all patients. Some patients underwent angiography to rule out or define aortic involvement. Most patients in the trial had new onset GCA, she said.
The primary endpoint was complete remission at week 12 with GC dose of 0.1 mg/kg/day. Complete remission was defined as the absence of clinical signs and symptoms of GCA plus negative values for C-reactive protein (CRP) and erythrocyte sedimentation rate. At 12 weeks, the complete response rate was 85% for tocilizumab versus 40% for placebo, a significant difference. At the end of the trial, 85% and 20%, respectively, had not had a relapse; this difference was significant.
Placebo patients received a higher cumulative dose of GC, she continued.
There were 7 serious adverse events reported in the tocilizumab arm and 5 in the placebo arm; 1 death occurred in the placebo arm due to myocardial infarction.
“A closer look at serious adverse events reveals few cardiovascular problems,” Dr. Adler continued. Back pain and psychosis were reported as reasons for hospitalization.
During the question and answer session, an audience member noted that it was difficult to understand how a short-term treatment is effective in a chronic disease. “Probably patients will relapse when they stop the medication, so we are not on the ‘green’ side yet,” she replied. Dr. Adler noted that it could be possible that a reduced dose of tocilizumab could be effective but have fewer side effects, but that has not been studied.
Tocilizumab was provided by Roche for this investigator-initiated trial. Dr. Adler reported no financial disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Tocilizumab was effective in newly diagnosed and recurrent giant cell arteritis.
Major finding: At week 12, 85% of the tocilizumab-treated patients were in complete remission versus 40% of placebo patients.
Data source: Single-center, randomized, double-blind trial of 30 patients.
Disclosures: Tocilizumab was provided by Roche for this investigator-initiated trial. Dr. Adler reported no financial disclosures.
Denosumab better at building bone than zoledronic acid
SAN FRANCISCO – Denosumab was superior to zoledronic acid (ZA) in building bone at the lumber spine, total hip, and femoral neck in postmenopausal women with osteoporosis previously treated with oral bisphosphonate therapy in a randomized phase III trial. This study is one more piece of level I evidence to support the effectiveness of transitioning to denosumab in such patients.
“In postmenopausal women with osteoporosis on prior oral bisphosphonates for 2 or more years, transitioning to denosumab resulted in significantly greater increases in bone mineral density [BMD], compared with zoledronic acid at all measured skeletal sites,” said lead author Dr. Paul D. Miller of the Colorado Center for Bone Research, Lakewood, Colo.
Speaking at the annual meeting of the American College of Rheumatology, Dr. Miller said: “This study completes a suite of trials that show transitioning from oral bisphosphonates to denosumab provides greater increases in bone mineral density and reduction in bone turnover markers, compared to maintaining therapy with another bisphosphonate.”
“Adherence rates to oral bisphosphonates are low,” he continued. “Patients who are intolerant to or fail other bisphosphonates may cycle from one bisphosphonate to another, but clinical benefits are not shown. Previous trials have shown that denosumab increased bone mineral density in women previously treated with oral bisphosphonates, whereas zoledronic acid did not, in women previously treated with alendronate.”
A prospective, double-blind, double-dummy, randomized trial was mounted to compare denosumab given subcutaneously at 60 mg every 6 months (n = 321) vs. ZA 5 mg once a year (n = 322) in postmenopausal women with osteoporosis who were intolerant to or who failed oral bisphosphonate therapy after at least 2 years of treatment.
Baseline characteristics were similar between both treatment arms. Median age was 69 years. About 38% had previous osteoporotic fracture. Bone mineral density T scores were similar between groups. Prior oral bisphosphonate exposure was a median of 6.4 years. Bone turnover marker levels were similar between the two arms.
For the primary endpoint, at 12 months, denosumab achieved a greater increase in BMD at all sites, compared with ZA: a 2.1% difference was observed at the lumbar spine and a 1.4% difference at the total hip.
Changes in bone turnover markers over time were more favorable with denosumab, he continued. Serum C-terminal cross-linking telopeptide (CTX) level was maintained in the denosumab arm and increased with ZA over 12 months. A similar pattern was observed with procollagen type 1 N-terminal propeptide (P1NP).
Adverse events were comparable between both groups. Serious adverse events were reported in 7.8% in the denosumab arm versus 9.1% in the ZA arm, but this difference was not statistically significant. Slightly more hypersensitivity adverse events were reported in the denosumab group: 3.8% versus 1.9% for ZA. The number of osteoporotic-related fractures was doubled in the ZA arm: 7 for denosumab vs. 15 for ZA.
Dr. Miller told listeners that this is the fourth study to show that denosumab is effective in osteoporotic patients previously on oral bisphosphonates, including prior risedronate, ibandronate, and alendronate.
SAN FRANCISCO – Denosumab was superior to zoledronic acid (ZA) in building bone at the lumber spine, total hip, and femoral neck in postmenopausal women with osteoporosis previously treated with oral bisphosphonate therapy in a randomized phase III trial. This study is one more piece of level I evidence to support the effectiveness of transitioning to denosumab in such patients.
“In postmenopausal women with osteoporosis on prior oral bisphosphonates for 2 or more years, transitioning to denosumab resulted in significantly greater increases in bone mineral density [BMD], compared with zoledronic acid at all measured skeletal sites,” said lead author Dr. Paul D. Miller of the Colorado Center for Bone Research, Lakewood, Colo.
Speaking at the annual meeting of the American College of Rheumatology, Dr. Miller said: “This study completes a suite of trials that show transitioning from oral bisphosphonates to denosumab provides greater increases in bone mineral density and reduction in bone turnover markers, compared to maintaining therapy with another bisphosphonate.”
“Adherence rates to oral bisphosphonates are low,” he continued. “Patients who are intolerant to or fail other bisphosphonates may cycle from one bisphosphonate to another, but clinical benefits are not shown. Previous trials have shown that denosumab increased bone mineral density in women previously treated with oral bisphosphonates, whereas zoledronic acid did not, in women previously treated with alendronate.”
A prospective, double-blind, double-dummy, randomized trial was mounted to compare denosumab given subcutaneously at 60 mg every 6 months (n = 321) vs. ZA 5 mg once a year (n = 322) in postmenopausal women with osteoporosis who were intolerant to or who failed oral bisphosphonate therapy after at least 2 years of treatment.
Baseline characteristics were similar between both treatment arms. Median age was 69 years. About 38% had previous osteoporotic fracture. Bone mineral density T scores were similar between groups. Prior oral bisphosphonate exposure was a median of 6.4 years. Bone turnover marker levels were similar between the two arms.
For the primary endpoint, at 12 months, denosumab achieved a greater increase in BMD at all sites, compared with ZA: a 2.1% difference was observed at the lumbar spine and a 1.4% difference at the total hip.
Changes in bone turnover markers over time were more favorable with denosumab, he continued. Serum C-terminal cross-linking telopeptide (CTX) level was maintained in the denosumab arm and increased with ZA over 12 months. A similar pattern was observed with procollagen type 1 N-terminal propeptide (P1NP).
Adverse events were comparable between both groups. Serious adverse events were reported in 7.8% in the denosumab arm versus 9.1% in the ZA arm, but this difference was not statistically significant. Slightly more hypersensitivity adverse events were reported in the denosumab group: 3.8% versus 1.9% for ZA. The number of osteoporotic-related fractures was doubled in the ZA arm: 7 for denosumab vs. 15 for ZA.
Dr. Miller told listeners that this is the fourth study to show that denosumab is effective in osteoporotic patients previously on oral bisphosphonates, including prior risedronate, ibandronate, and alendronate.
SAN FRANCISCO – Denosumab was superior to zoledronic acid (ZA) in building bone at the lumber spine, total hip, and femoral neck in postmenopausal women with osteoporosis previously treated with oral bisphosphonate therapy in a randomized phase III trial. This study is one more piece of level I evidence to support the effectiveness of transitioning to denosumab in such patients.
“In postmenopausal women with osteoporosis on prior oral bisphosphonates for 2 or more years, transitioning to denosumab resulted in significantly greater increases in bone mineral density [BMD], compared with zoledronic acid at all measured skeletal sites,” said lead author Dr. Paul D. Miller of the Colorado Center for Bone Research, Lakewood, Colo.
Speaking at the annual meeting of the American College of Rheumatology, Dr. Miller said: “This study completes a suite of trials that show transitioning from oral bisphosphonates to denosumab provides greater increases in bone mineral density and reduction in bone turnover markers, compared to maintaining therapy with another bisphosphonate.”
“Adherence rates to oral bisphosphonates are low,” he continued. “Patients who are intolerant to or fail other bisphosphonates may cycle from one bisphosphonate to another, but clinical benefits are not shown. Previous trials have shown that denosumab increased bone mineral density in women previously treated with oral bisphosphonates, whereas zoledronic acid did not, in women previously treated with alendronate.”
A prospective, double-blind, double-dummy, randomized trial was mounted to compare denosumab given subcutaneously at 60 mg every 6 months (n = 321) vs. ZA 5 mg once a year (n = 322) in postmenopausal women with osteoporosis who were intolerant to or who failed oral bisphosphonate therapy after at least 2 years of treatment.
Baseline characteristics were similar between both treatment arms. Median age was 69 years. About 38% had previous osteoporotic fracture. Bone mineral density T scores were similar between groups. Prior oral bisphosphonate exposure was a median of 6.4 years. Bone turnover marker levels were similar between the two arms.
For the primary endpoint, at 12 months, denosumab achieved a greater increase in BMD at all sites, compared with ZA: a 2.1% difference was observed at the lumbar spine and a 1.4% difference at the total hip.
Changes in bone turnover markers over time were more favorable with denosumab, he continued. Serum C-terminal cross-linking telopeptide (CTX) level was maintained in the denosumab arm and increased with ZA over 12 months. A similar pattern was observed with procollagen type 1 N-terminal propeptide (P1NP).
Adverse events were comparable between both groups. Serious adverse events were reported in 7.8% in the denosumab arm versus 9.1% in the ZA arm, but this difference was not statistically significant. Slightly more hypersensitivity adverse events were reported in the denosumab group: 3.8% versus 1.9% for ZA. The number of osteoporotic-related fractures was doubled in the ZA arm: 7 for denosumab vs. 15 for ZA.
Dr. Miller told listeners that this is the fourth study to show that denosumab is effective in osteoporotic patients previously on oral bisphosphonates, including prior risedronate, ibandronate, and alendronate.
AT THE ACR ANNUAL MEETING
Key clinical point: Denosumab was superior to zoledronic acid in building bone mineral density in postmenopausal women with osteoporosis who had previously been treated with oral bisphosphonates.
Major finding: Denosumab achieved a 2.1% difference in BMD at the lumbar spine and a 1.4% difference at the hip, compared with zoledronic acid.
Data source: Prospective, randomized, double-blind, double-dummy trial that included 643 patients.
Disclosures: Dr. Miller’s financial disclosures include Alexion, Amgen, Merck, Merck Serono, Boehringer Ingelheim, Regeneron, National Bone Health Alliance, Eli Lilly, Pfizer, Radius, and he is owner and director of the Colorado Center for Bone Research. The study was supported by Amgen.
ACR: Study confirms potential genetic basis for poor response to allopurinol in gout
SAN FRANCISCO – Patients with gout who fail to sufficiently lower their serum urate level despite adherence to a regimen of allopurinol 300 mg/day may have a genetic polymorphism affecting their response to the medication, according to new findings presented at the annual meeting of the American College of Rheumatology.
“ABCG2 genotyping may identify patients who will not reach target serum urate levels on standard allopurinol doses,” said study presenter and principal investigator Dr. Lisa K. Stamp of the University of Otago, Christchurch, New Zealand.
Sustained lowering of serum urate (SU) to less than 6 mg/dL – achieved most commonly by inhibition of xanthine oxidase using allopurinol – is crucial for the long-term management of gout. Yet, in clinical practice, less than half of patients treated with allopurinol achieve this SU target. This is usually because of poor adherence or an inappropriately low dose of the drug. But, in some patients, the response can be poor even despite allopurinol 300 mg daily. The reason for failure of allopurinol treatment in these poor responders has been unknown.
“It is important to consider genes that might help predict response or toxicity. To date, pharmacogenetic studies of allopurinol have focused on adverse effects. However, we were more interested in predicting response. The ability to predict poor response is important because it can influence drug choice and shorten the time to achieve target SU,” Dr. Stamp said.
The present study helps to validate the results of a previous genomewide association study in which the single nucleotide polymorphism (SNP) rs2231142 in ABCG2 was associated with poor response to allopurinol (Clin Pharmacol Ther. 2015 May;97[5]:518-25). Adherence to allopurinol was not examined in that study, so Dr. Stamp and her colleagues set out to make sure that it was not a confounder. The current study examined the SNP’s association with allopurinol response more closely in 264 gout patients who were well characterized phenotypically. All were being treated with allopurinol and all adhered to their treatment regimen. Overall, 120 were deemed good responders, with SU of 6 mg/dL or less on daily allopurinol of 300 mg or less, and 68 were poor responders, with SU of 6 mg/dL or higher despite a daily dose of allopurinol exceeding 300 mg. Another 76 patients were nonadherent/nonclassifiable. The poor responders tended to be younger and were more likely to be male, nonwhite, and heavier than their counterparts who responded well.
Genotyping for the rs2231142 SNP revealed a greater preponderance of the minor genotype in poor responders, compared with good responders (39% vs. 18%). This genotype was significantly associated with poor response after adjusting for age, sex, body-mass index, and ethnicity (odds ratio, 2.91; 95% confidence interval, 1.71-5.17; P = .00015). The mechanism – still to be proven – may involve reduced urate excretion from the gut.
There may not be a case for screening for the genotype just yet, according to Dr. Christopher M. Burns, a rheumatologist at Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not involved in the study. He said “it’s hard to see why you would screen for this variant, as it doesn’t account for all resistance, and you would still end up pushing the allopurinol dose up to achieve a SU of less than 6.0 mg/dL anyway. Since it’s not an adverse event warning signal, but simply one marker of possibly requiring a higher allopurinol dose, and allopurinol is an inexpensive drug, why would you check it? At least, that’s my sense. The findings may be more important simply for a better understanding of urate metabolism and the mechanism of action of allopurinol and oxypurinol.”
Dr. Stamp reported a financial disclosure with AstraZeneca.
SAN FRANCISCO – Patients with gout who fail to sufficiently lower their serum urate level despite adherence to a regimen of allopurinol 300 mg/day may have a genetic polymorphism affecting their response to the medication, according to new findings presented at the annual meeting of the American College of Rheumatology.
“ABCG2 genotyping may identify patients who will not reach target serum urate levels on standard allopurinol doses,” said study presenter and principal investigator Dr. Lisa K. Stamp of the University of Otago, Christchurch, New Zealand.
Sustained lowering of serum urate (SU) to less than 6 mg/dL – achieved most commonly by inhibition of xanthine oxidase using allopurinol – is crucial for the long-term management of gout. Yet, in clinical practice, less than half of patients treated with allopurinol achieve this SU target. This is usually because of poor adherence or an inappropriately low dose of the drug. But, in some patients, the response can be poor even despite allopurinol 300 mg daily. The reason for failure of allopurinol treatment in these poor responders has been unknown.
“It is important to consider genes that might help predict response or toxicity. To date, pharmacogenetic studies of allopurinol have focused on adverse effects. However, we were more interested in predicting response. The ability to predict poor response is important because it can influence drug choice and shorten the time to achieve target SU,” Dr. Stamp said.
The present study helps to validate the results of a previous genomewide association study in which the single nucleotide polymorphism (SNP) rs2231142 in ABCG2 was associated with poor response to allopurinol (Clin Pharmacol Ther. 2015 May;97[5]:518-25). Adherence to allopurinol was not examined in that study, so Dr. Stamp and her colleagues set out to make sure that it was not a confounder. The current study examined the SNP’s association with allopurinol response more closely in 264 gout patients who were well characterized phenotypically. All were being treated with allopurinol and all adhered to their treatment regimen. Overall, 120 were deemed good responders, with SU of 6 mg/dL or less on daily allopurinol of 300 mg or less, and 68 were poor responders, with SU of 6 mg/dL or higher despite a daily dose of allopurinol exceeding 300 mg. Another 76 patients were nonadherent/nonclassifiable. The poor responders tended to be younger and were more likely to be male, nonwhite, and heavier than their counterparts who responded well.
Genotyping for the rs2231142 SNP revealed a greater preponderance of the minor genotype in poor responders, compared with good responders (39% vs. 18%). This genotype was significantly associated with poor response after adjusting for age, sex, body-mass index, and ethnicity (odds ratio, 2.91; 95% confidence interval, 1.71-5.17; P = .00015). The mechanism – still to be proven – may involve reduced urate excretion from the gut.
There may not be a case for screening for the genotype just yet, according to Dr. Christopher M. Burns, a rheumatologist at Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not involved in the study. He said “it’s hard to see why you would screen for this variant, as it doesn’t account for all resistance, and you would still end up pushing the allopurinol dose up to achieve a SU of less than 6.0 mg/dL anyway. Since it’s not an adverse event warning signal, but simply one marker of possibly requiring a higher allopurinol dose, and allopurinol is an inexpensive drug, why would you check it? At least, that’s my sense. The findings may be more important simply for a better understanding of urate metabolism and the mechanism of action of allopurinol and oxypurinol.”
Dr. Stamp reported a financial disclosure with AstraZeneca.
SAN FRANCISCO – Patients with gout who fail to sufficiently lower their serum urate level despite adherence to a regimen of allopurinol 300 mg/day may have a genetic polymorphism affecting their response to the medication, according to new findings presented at the annual meeting of the American College of Rheumatology.
“ABCG2 genotyping may identify patients who will not reach target serum urate levels on standard allopurinol doses,” said study presenter and principal investigator Dr. Lisa K. Stamp of the University of Otago, Christchurch, New Zealand.
Sustained lowering of serum urate (SU) to less than 6 mg/dL – achieved most commonly by inhibition of xanthine oxidase using allopurinol – is crucial for the long-term management of gout. Yet, in clinical practice, less than half of patients treated with allopurinol achieve this SU target. This is usually because of poor adherence or an inappropriately low dose of the drug. But, in some patients, the response can be poor even despite allopurinol 300 mg daily. The reason for failure of allopurinol treatment in these poor responders has been unknown.
“It is important to consider genes that might help predict response or toxicity. To date, pharmacogenetic studies of allopurinol have focused on adverse effects. However, we were more interested in predicting response. The ability to predict poor response is important because it can influence drug choice and shorten the time to achieve target SU,” Dr. Stamp said.
The present study helps to validate the results of a previous genomewide association study in which the single nucleotide polymorphism (SNP) rs2231142 in ABCG2 was associated with poor response to allopurinol (Clin Pharmacol Ther. 2015 May;97[5]:518-25). Adherence to allopurinol was not examined in that study, so Dr. Stamp and her colleagues set out to make sure that it was not a confounder. The current study examined the SNP’s association with allopurinol response more closely in 264 gout patients who were well characterized phenotypically. All were being treated with allopurinol and all adhered to their treatment regimen. Overall, 120 were deemed good responders, with SU of 6 mg/dL or less on daily allopurinol of 300 mg or less, and 68 were poor responders, with SU of 6 mg/dL or higher despite a daily dose of allopurinol exceeding 300 mg. Another 76 patients were nonadherent/nonclassifiable. The poor responders tended to be younger and were more likely to be male, nonwhite, and heavier than their counterparts who responded well.
Genotyping for the rs2231142 SNP revealed a greater preponderance of the minor genotype in poor responders, compared with good responders (39% vs. 18%). This genotype was significantly associated with poor response after adjusting for age, sex, body-mass index, and ethnicity (odds ratio, 2.91; 95% confidence interval, 1.71-5.17; P = .00015). The mechanism – still to be proven – may involve reduced urate excretion from the gut.
There may not be a case for screening for the genotype just yet, according to Dr. Christopher M. Burns, a rheumatologist at Dartmouth Hitchcock Medical Center, Lebanon, N.H., who was not involved in the study. He said “it’s hard to see why you would screen for this variant, as it doesn’t account for all resistance, and you would still end up pushing the allopurinol dose up to achieve a SU of less than 6.0 mg/dL anyway. Since it’s not an adverse event warning signal, but simply one marker of possibly requiring a higher allopurinol dose, and allopurinol is an inexpensive drug, why would you check it? At least, that’s my sense. The findings may be more important simply for a better understanding of urate metabolism and the mechanism of action of allopurinol and oxypurinol.”
Dr. Stamp reported a financial disclosure with AstraZeneca.
AT THE ACR ANNUAL MEETING
Key clinical point: Screening could identify gout patients who require a dose adjustment of allopurinol.
Major finding: Presence of the rs2231142 SNP in the ABCG2 gene was significantly associated with poor response to allopurinol after adjusting for age, sex, body-mass index, and ethnicity (OR, 2.91; 95% CI, 1.71-5.17; P = .00015).
Data source: An analysis of 264 gout patients participating in clinical trials with allopurinol.
Disclosures: Dr. Stamp reported a financial disclosure with AstraZeneca.
ACR: Video capsule endoscopy finds Crohn’s disease best in spondyloarthropathy patients
SAN FRANCISCO – Video capsule endoscopy proved superior to ileocolonoscopy in detecting lesions consistent with Crohn’s disease in patients with known spondyloarthropathies in a study presented at the annual meeting of the American College of Rheumatology.
“The incremental yield of CE [capsule endoscopy) was 31% over IC [ileocolonoscopy]. CE changed management in two-thirds of patients in this study. CE should be the first round of tests to detect CD [Crohn’s disease] in patients with SpA [spondyloarthropathies]. This is a ‘game-changer,’ ” stated senior author Dr. Ernest Seidman of McGill University, Montreal.
Although IC can show asymptomatic inflammation of the terminal ileum, it is “ignoring the rest of the small bowel,” he said.
About 5%-10% of patients with SpA manifest with inflammatory bowel disease, whereas 40%-60% of SpA patients have microscopic biological evidence of gut inflammation that typically occurs without symptoms. These patients don’t meet the criteria for inflammatory bowel disease, Dr. Seidman explained.
The study included 64 patients with SpA or ankylosing spondylitis with or without gastrointestinal symptoms; 58% had gastrointestinal symptoms. All patients were taken off NSAIDs for 1 month prior to the study because their use may be associated with inflammation that is indistinguishable from microscopic bowel inflammation. Treatment with anti–tumor necrosis factor biologics was not allowed, with the exception of etanercept. On the day before CE, patients had a liquid diet. CE was performed on all patients first, and then IC was performed.
“The sequence was important,” Dr. Seidman noted.
The investigators used a value of greater than 350 on the Lewis score, a validated measure of inflammatory activity on small-bowel CE, to identify moderate Crohn’s disease.
None of the patients with negative CE had Crohn’s disease; 45.3% had inflammation typical for Crohn’s disease on CE, compared with 14% on IC (P =.036). All positive ileal and colonoscopic biopsies were consistent with Crohn’s disease.
The study also showed that fecal calprotectin levels were significantly correlated with mucosal inflammation observed on CE. However, the presence of gastrointestinal symptoms, C-reactive protein level, and the results from a panel of serologic, inflammatory and genetic tests (a commercial test called IBD sgi Diagnostic) were not predictive of small bowel inflammation in these patients.
AbbVie funded the study. Dr. Seidman reported financial disclosures with Abbott Immunology Pharmaceuticals and Prometheus Laboratories, which he said supplied the video capsules.
SAN FRANCISCO – Video capsule endoscopy proved superior to ileocolonoscopy in detecting lesions consistent with Crohn’s disease in patients with known spondyloarthropathies in a study presented at the annual meeting of the American College of Rheumatology.
“The incremental yield of CE [capsule endoscopy) was 31% over IC [ileocolonoscopy]. CE changed management in two-thirds of patients in this study. CE should be the first round of tests to detect CD [Crohn’s disease] in patients with SpA [spondyloarthropathies]. This is a ‘game-changer,’ ” stated senior author Dr. Ernest Seidman of McGill University, Montreal.
Although IC can show asymptomatic inflammation of the terminal ileum, it is “ignoring the rest of the small bowel,” he said.
About 5%-10% of patients with SpA manifest with inflammatory bowel disease, whereas 40%-60% of SpA patients have microscopic biological evidence of gut inflammation that typically occurs without symptoms. These patients don’t meet the criteria for inflammatory bowel disease, Dr. Seidman explained.
The study included 64 patients with SpA or ankylosing spondylitis with or without gastrointestinal symptoms; 58% had gastrointestinal symptoms. All patients were taken off NSAIDs for 1 month prior to the study because their use may be associated with inflammation that is indistinguishable from microscopic bowel inflammation. Treatment with anti–tumor necrosis factor biologics was not allowed, with the exception of etanercept. On the day before CE, patients had a liquid diet. CE was performed on all patients first, and then IC was performed.
“The sequence was important,” Dr. Seidman noted.
The investigators used a value of greater than 350 on the Lewis score, a validated measure of inflammatory activity on small-bowel CE, to identify moderate Crohn’s disease.
None of the patients with negative CE had Crohn’s disease; 45.3% had inflammation typical for Crohn’s disease on CE, compared with 14% on IC (P =.036). All positive ileal and colonoscopic biopsies were consistent with Crohn’s disease.
The study also showed that fecal calprotectin levels were significantly correlated with mucosal inflammation observed on CE. However, the presence of gastrointestinal symptoms, C-reactive protein level, and the results from a panel of serologic, inflammatory and genetic tests (a commercial test called IBD sgi Diagnostic) were not predictive of small bowel inflammation in these patients.
AbbVie funded the study. Dr. Seidman reported financial disclosures with Abbott Immunology Pharmaceuticals and Prometheus Laboratories, which he said supplied the video capsules.
SAN FRANCISCO – Video capsule endoscopy proved superior to ileocolonoscopy in detecting lesions consistent with Crohn’s disease in patients with known spondyloarthropathies in a study presented at the annual meeting of the American College of Rheumatology.
“The incremental yield of CE [capsule endoscopy) was 31% over IC [ileocolonoscopy]. CE changed management in two-thirds of patients in this study. CE should be the first round of tests to detect CD [Crohn’s disease] in patients with SpA [spondyloarthropathies]. This is a ‘game-changer,’ ” stated senior author Dr. Ernest Seidman of McGill University, Montreal.
Although IC can show asymptomatic inflammation of the terminal ileum, it is “ignoring the rest of the small bowel,” he said.
About 5%-10% of patients with SpA manifest with inflammatory bowel disease, whereas 40%-60% of SpA patients have microscopic biological evidence of gut inflammation that typically occurs without symptoms. These patients don’t meet the criteria for inflammatory bowel disease, Dr. Seidman explained.
The study included 64 patients with SpA or ankylosing spondylitis with or without gastrointestinal symptoms; 58% had gastrointestinal symptoms. All patients were taken off NSAIDs for 1 month prior to the study because their use may be associated with inflammation that is indistinguishable from microscopic bowel inflammation. Treatment with anti–tumor necrosis factor biologics was not allowed, with the exception of etanercept. On the day before CE, patients had a liquid diet. CE was performed on all patients first, and then IC was performed.
“The sequence was important,” Dr. Seidman noted.
The investigators used a value of greater than 350 on the Lewis score, a validated measure of inflammatory activity on small-bowel CE, to identify moderate Crohn’s disease.
None of the patients with negative CE had Crohn’s disease; 45.3% had inflammation typical for Crohn’s disease on CE, compared with 14% on IC (P =.036). All positive ileal and colonoscopic biopsies were consistent with Crohn’s disease.
The study also showed that fecal calprotectin levels were significantly correlated with mucosal inflammation observed on CE. However, the presence of gastrointestinal symptoms, C-reactive protein level, and the results from a panel of serologic, inflammatory and genetic tests (a commercial test called IBD sgi Diagnostic) were not predictive of small bowel inflammation in these patients.
AbbVie funded the study. Dr. Seidman reported financial disclosures with Abbott Immunology Pharmaceuticals and Prometheus Laboratories, which he said supplied the video capsules.
AT THE ACR ANNUAL MEETING
Key clinical point:Video capsule endoscopy is better than ileocolonoscopy for detection of Crohn’s disease in patients with spondyloarthropathies.
Major finding: Video capsule endoscopy detected Crohn’s disease in 45% of patients, compared with 14% detected by ileocolonoscopy (P =.036).
Data source: Prospective study of 64 patients with SpA who underwent video capsule endoscopy followed by ileocolonoscopy.
Disclosures: AbbVie funded the study. Dr. Seidman reported financial disclosures with Abbott Immunology Pharmaceuticals and Prometheus Laboratories, which he said supplied the video capsules.
ACR: Ozone injections reduce pain and improve function in knee OA
SAN FRANCISCO – Intra-articular ozone injections were effective in reducing pain, improving function, and improving quality of life in patients with knee osteoarthritis in the first randomized study to evaluate this approach.
Patients treated with a series of ozone injections achieved significant improvements on all measures, except for the Timed Up and Go Test, compared with patients given placebo, according to study results presented at the annual meeting of the American College of Rheumatology.
“After 8 weeks of treatment, ozone can give patients with knee osteoarthritis [OA] better quality of life with less pain and more independence in performing daily activities. More studies are needed to validate this option in patients with OA. Intra-articular ozone injections are safe with similar complications to placebo. In elderly people with comorbidities requiring chronic medication, this approach is a good option because it doesn’t interact with medications. The only restriction is anticoagulant therapy, as there may be bleeding at the injection site,” said Dr. Virginia Trevisani, professor at the Federal University of São Paulo.
The next series of studies Dr. Trevisani and her coauthors are planning will incorporate MRI imaging to assess the effect of the ozone injections on structural progression in knee OA.
Ozone is thought to have anti-inflammatory effects by reducing oxidative stress. Ozone is being used for medical purposes in countries such as Russia, Germany, and Spain, but it is not currently used clinically in the United States. “You can’t perform these injections in patients without experience. The only requirement is a machine to make ozone that costs about $1,000 USD,” she said.
Before this study, evidence in support of ozone injections in knee OA was anecdotal and from observational studies. The present study is the first randomized trial to evaluate intra-articular ozone injections in patients with knee OA.
The study enrolled 98 patients with documented knee OA between the ages of 60 and 85 years; 63 patients were randomized to intra-articular injections of ozone in the knee with the most pain (one injection per week for 8 consecutive weeks), and 35 were randomized to placebo injections of a small amount of air.
Patients were evaluated at baseline, after 4 and 8 injections, and 8 weeks following the last injection. Two patients in the ozone group withdrew from the study. The only adverse events were three puncture-site wounds – two in the ozone group and one in the placebo group.
Significant improvement was observed on all measures, except for the Timed Up and Go Test (getting up from a chair), at every time point for the ozone injections. Dr. Trevisani said that the ability to get up from a chair without help depends on balance and muscle strength, which may explain why the results were not significant.
Measures of pain on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the visual analog scale improved significantly with ozone, compared with placebo, and by week 16, the P value was .000 for both measures. WOMAC joint stiffness intensity was significantly improved by ozone (P = .075 at week 4; P = .002 at week 8). Quality of life on the Short Form–36 for pain and functional capacity were significantly improved by ozone, compared with placebo (P = .000 at week 16 for both measures).
Dr. Trevisani said ozone injections may be able to delay the need for total joint replacement surgery, and that they are cost effective, compared with surgery and other pharmacologic treatments.
Dr. Trevisani had no relevant financial disclosures.
SAN FRANCISCO – Intra-articular ozone injections were effective in reducing pain, improving function, and improving quality of life in patients with knee osteoarthritis in the first randomized study to evaluate this approach.
Patients treated with a series of ozone injections achieved significant improvements on all measures, except for the Timed Up and Go Test, compared with patients given placebo, according to study results presented at the annual meeting of the American College of Rheumatology.
“After 8 weeks of treatment, ozone can give patients with knee osteoarthritis [OA] better quality of life with less pain and more independence in performing daily activities. More studies are needed to validate this option in patients with OA. Intra-articular ozone injections are safe with similar complications to placebo. In elderly people with comorbidities requiring chronic medication, this approach is a good option because it doesn’t interact with medications. The only restriction is anticoagulant therapy, as there may be bleeding at the injection site,” said Dr. Virginia Trevisani, professor at the Federal University of São Paulo.
The next series of studies Dr. Trevisani and her coauthors are planning will incorporate MRI imaging to assess the effect of the ozone injections on structural progression in knee OA.
Ozone is thought to have anti-inflammatory effects by reducing oxidative stress. Ozone is being used for medical purposes in countries such as Russia, Germany, and Spain, but it is not currently used clinically in the United States. “You can’t perform these injections in patients without experience. The only requirement is a machine to make ozone that costs about $1,000 USD,” she said.
Before this study, evidence in support of ozone injections in knee OA was anecdotal and from observational studies. The present study is the first randomized trial to evaluate intra-articular ozone injections in patients with knee OA.
The study enrolled 98 patients with documented knee OA between the ages of 60 and 85 years; 63 patients were randomized to intra-articular injections of ozone in the knee with the most pain (one injection per week for 8 consecutive weeks), and 35 were randomized to placebo injections of a small amount of air.
Patients were evaluated at baseline, after 4 and 8 injections, and 8 weeks following the last injection. Two patients in the ozone group withdrew from the study. The only adverse events were three puncture-site wounds – two in the ozone group and one in the placebo group.
Significant improvement was observed on all measures, except for the Timed Up and Go Test (getting up from a chair), at every time point for the ozone injections. Dr. Trevisani said that the ability to get up from a chair without help depends on balance and muscle strength, which may explain why the results were not significant.
Measures of pain on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the visual analog scale improved significantly with ozone, compared with placebo, and by week 16, the P value was .000 for both measures. WOMAC joint stiffness intensity was significantly improved by ozone (P = .075 at week 4; P = .002 at week 8). Quality of life on the Short Form–36 for pain and functional capacity were significantly improved by ozone, compared with placebo (P = .000 at week 16 for both measures).
Dr. Trevisani said ozone injections may be able to delay the need for total joint replacement surgery, and that they are cost effective, compared with surgery and other pharmacologic treatments.
Dr. Trevisani had no relevant financial disclosures.
SAN FRANCISCO – Intra-articular ozone injections were effective in reducing pain, improving function, and improving quality of life in patients with knee osteoarthritis in the first randomized study to evaluate this approach.
Patients treated with a series of ozone injections achieved significant improvements on all measures, except for the Timed Up and Go Test, compared with patients given placebo, according to study results presented at the annual meeting of the American College of Rheumatology.
“After 8 weeks of treatment, ozone can give patients with knee osteoarthritis [OA] better quality of life with less pain and more independence in performing daily activities. More studies are needed to validate this option in patients with OA. Intra-articular ozone injections are safe with similar complications to placebo. In elderly people with comorbidities requiring chronic medication, this approach is a good option because it doesn’t interact with medications. The only restriction is anticoagulant therapy, as there may be bleeding at the injection site,” said Dr. Virginia Trevisani, professor at the Federal University of São Paulo.
The next series of studies Dr. Trevisani and her coauthors are planning will incorporate MRI imaging to assess the effect of the ozone injections on structural progression in knee OA.
Ozone is thought to have anti-inflammatory effects by reducing oxidative stress. Ozone is being used for medical purposes in countries such as Russia, Germany, and Spain, but it is not currently used clinically in the United States. “You can’t perform these injections in patients without experience. The only requirement is a machine to make ozone that costs about $1,000 USD,” she said.
Before this study, evidence in support of ozone injections in knee OA was anecdotal and from observational studies. The present study is the first randomized trial to evaluate intra-articular ozone injections in patients with knee OA.
The study enrolled 98 patients with documented knee OA between the ages of 60 and 85 years; 63 patients were randomized to intra-articular injections of ozone in the knee with the most pain (one injection per week for 8 consecutive weeks), and 35 were randomized to placebo injections of a small amount of air.
Patients were evaluated at baseline, after 4 and 8 injections, and 8 weeks following the last injection. Two patients in the ozone group withdrew from the study. The only adverse events were three puncture-site wounds – two in the ozone group and one in the placebo group.
Significant improvement was observed on all measures, except for the Timed Up and Go Test (getting up from a chair), at every time point for the ozone injections. Dr. Trevisani said that the ability to get up from a chair without help depends on balance and muscle strength, which may explain why the results were not significant.
Measures of pain on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the visual analog scale improved significantly with ozone, compared with placebo, and by week 16, the P value was .000 for both measures. WOMAC joint stiffness intensity was significantly improved by ozone (P = .075 at week 4; P = .002 at week 8). Quality of life on the Short Form–36 for pain and functional capacity were significantly improved by ozone, compared with placebo (P = .000 at week 16 for both measures).
Dr. Trevisani said ozone injections may be able to delay the need for total joint replacement surgery, and that they are cost effective, compared with surgery and other pharmacologic treatments.
Dr. Trevisani had no relevant financial disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point:Intra-articular ozone injections reduce pain, improve function, and improve quality of life in patients with knee osteoarthritis.
Major finding: On all measures of pain, function, and quality of life, ozone injections were significantly superior to placebo.
Data source: A randomized, double-blind placebo-controlled trial of 98 patients with knee OA.
Disclosures: Dr. Trevisani had no relevant financial disclosures.