User login
American Academy of Dermatology (AAD): Summer Academy 2015
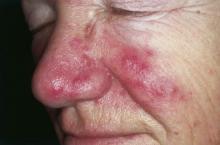
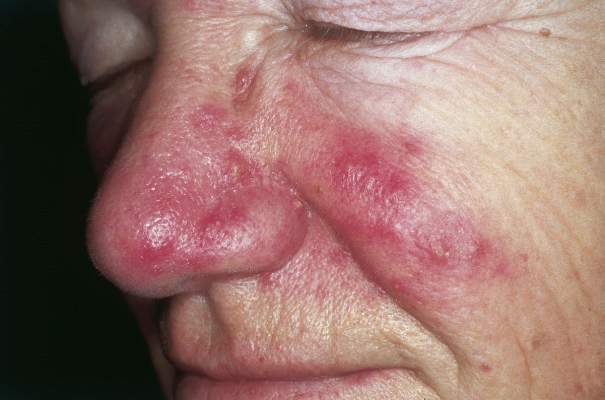
Inflammatory loop drives rosacea
NEW YORK – Rosacea seems to represent a Möbius strip of an overly enthusiastic immune response, with chemicals that recruit neutrophils, which, in turn, activate even more immunomodulators.
Researchers have been pointing the finger at trigger-happy neutrophils for more than a decade, Dr. David E. Cohen said at the summer meeting of the American Academy of Dermatology. But this group of cells is really more of an accomplice than a mastermind.
“Neutrophils were once the alpha and omega of rosacea,” said Dr. Cohen, the Charles C. and Dorothea E. Harris Professor of Dermatology, New York University. “But what are they doing on the skin and why are they there? They’re doing exactly what they’re supposed to do – defending against invasion by dumping out a whole poisonous soup of chemicals,” designed to protect and defend against microbes.
In the chronic disease of rosacea, however, these frontline soldiers are constantly recruited, even when there’s no invader to fight. They continue to carry out their preprogrammed attacks, releasing proteins that activate enzymes that stimulate immunomodulators that, in turn, call more neutrophils to the battlefield. Now, new research suggests that cathelicidins – antimicrobial peptides that are especially present in epithelial cells – are giving neutrophils their marching orders.
“Cathelicidins sit fully engaged on the skin and, the minute there’s a breach, they are turned on as the first defense,” Dr. Cohen said. “We need that kind of help right away – we can’t afford to wait around” for the second wave of defenders: neutrophils and lymphocytes.
This command seems to be bidirectional, though. Neutrophils release matrix metalloproteases (MMPs), enzymes critical for wound healing. MMPs remove dead extracellular matrix and microbes, prepare the wound bed for angiogenesis, and stimulate epidermal cell migration. But these benefits also exacerbate the things that make rosacea such a tough problem. By dissolving tissue, MMPs ramp up inflammation. By stimulating angiogenesis, they contribute to vasodilation, erythema, and telangiectasias.
And, Dr. Cohen noted, MMPs also just happen to recruit more neutrophils, which are capable of activating triptych enzymes, which turn on cathelicidins. “Now, a loop is being theorized,” he said. “The recruitment tools are kept on and this keeps cycling and cycling allowing the neutrophils to stay in the skin” for years.
The exaggerated presence of MMPs is likely the very reason that the tetracycline class of antibiotics can be so effective during rosacea exacerbations, Dr. Cohen said. The drugs aren’t necessarily fighting bacteria – instead, their anti-MMP properties function as a temporary break in this inflammatory loop. “They interfere with MMP, and you can’t turn on cathelicidin without MMP. If you lock MMP down, you can break the cycle because there is no neutrophil recruitment.”
Interestingly, he said, antibiotics that target MMP appear to suppress the enzymes at subantimicrobial doses. “This opens the possibility that we might be able to treat with these drugs and not exert antibiotic effects,” lessening the problems associated with indiscriminate antibiotic usage.
Inflammatory overdrive makes rosacea skin hypersensitive to any kind of inflammatory trigger, said Dr. Hilary Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. At the meeting, Dr. Baldwin discussed several new topical medications aimed at breaking the cycle:
• 1% Ivermectin: People with rosacea can harbor up to six times more demodex mites than can people without the disorder. The explanation behind this relationship is unclear: Is rosacea skin somehow more hospitable to the mite, or is an excess of mites a direct causative factor? Whatever the interaction, topical ivermectin breaks the link as both an antimicrobial and an anti-inflammatory agent. Ivermectin directly inhibits cellular and humoral immune response, and regulates tumor necrosis factor–alpha and interleukins 1-beta and 10.
Two randomized, double-blind vehicle-controlled studies evaluating 1% ivermectin cream in patients with papulopustular rosacea found that almost 80% of rosacea patients were clear or nearly clear on the Investigator’s Global Assessment (IGA) scale at 12 weeks – significantly more than those who were using a vehicle placebo. Inflammatory lesions significantly decreased and adverse events were similar to those associated with the vehicle (J Drugs Dermatol. 2014 Mar;13[3]:316-23). Another study found that ivermectin was significantly more effective than metronidazole, she said.
• Azelaic acid: A phase III study examined azelaic acid foam in almost 1,000 patients with moderate to severe papulopustular rosacea. IGA was clear or near-clear in 32% of the active group at 12 weeks, and 23.5% of the vehicle control group – a significant difference. There were significantly fewer inflammatory lesions among those in the active treatment group as well. Drug-related cutaneous adverse events were significantly more common among those treated with azelaic acid (7% vs. about 4%) and were mostly pain, pruritus, and dryness at application site (Cutis. 2015 Jul;96[1]:54-61).
• Oxymetazoline and brimonidine: These two alpha-adrenergic receptor agonists are under investigation for their ability to decrease erythema. Both cause a transient vasoconstriction. Two studies of patients with moderate or severe erythema of rosacea found that a topical brimonidine gel formulation significantly decreased erythema for up to 12 hours after application, compared with a vehicle gel (J Drugs Dermatol. 2013 Jun 1;12[6]:650-6). However, there have been several case reports of severe rebound, with redness not only returning, but returning at a more severe grade (J Am Acad Dermatol. 2014 Feb;70[2]:e37-8).
• Isotretinoin: New investigations are looking at topical retinoids for rosacea. They do appear effective – but not only for the reason they work in acne. While they decrease the size of sebaceous glands and the amount of sebum produced, they seem to exert their benefit through an anti-inflammatory pathway, as well. Isotretinoin significantly decreases toll-like receptor-2 expression on monocytes.
Isotretinoin has been shown to be more effective than doxycycline in clearing skin (J Dtsch Dermatol Ges. 2010 Jul;8[7]:505-15) but has also seemed helpful in preventing and slowing phyma development and improving ocular disease.
Both Dr. Cohen and Dr. Baldwin disclosed relationships with multiple pharmaceutical companies.
NEW YORK – Rosacea seems to represent a Möbius strip of an overly enthusiastic immune response, with chemicals that recruit neutrophils, which, in turn, activate even more immunomodulators.
Researchers have been pointing the finger at trigger-happy neutrophils for more than a decade, Dr. David E. Cohen said at the summer meeting of the American Academy of Dermatology. But this group of cells is really more of an accomplice than a mastermind.
“Neutrophils were once the alpha and omega of rosacea,” said Dr. Cohen, the Charles C. and Dorothea E. Harris Professor of Dermatology, New York University. “But what are they doing on the skin and why are they there? They’re doing exactly what they’re supposed to do – defending against invasion by dumping out a whole poisonous soup of chemicals,” designed to protect and defend against microbes.
In the chronic disease of rosacea, however, these frontline soldiers are constantly recruited, even when there’s no invader to fight. They continue to carry out their preprogrammed attacks, releasing proteins that activate enzymes that stimulate immunomodulators that, in turn, call more neutrophils to the battlefield. Now, new research suggests that cathelicidins – antimicrobial peptides that are especially present in epithelial cells – are giving neutrophils their marching orders.
“Cathelicidins sit fully engaged on the skin and, the minute there’s a breach, they are turned on as the first defense,” Dr. Cohen said. “We need that kind of help right away – we can’t afford to wait around” for the second wave of defenders: neutrophils and lymphocytes.
This command seems to be bidirectional, though. Neutrophils release matrix metalloproteases (MMPs), enzymes critical for wound healing. MMPs remove dead extracellular matrix and microbes, prepare the wound bed for angiogenesis, and stimulate epidermal cell migration. But these benefits also exacerbate the things that make rosacea such a tough problem. By dissolving tissue, MMPs ramp up inflammation. By stimulating angiogenesis, they contribute to vasodilation, erythema, and telangiectasias.
And, Dr. Cohen noted, MMPs also just happen to recruit more neutrophils, which are capable of activating triptych enzymes, which turn on cathelicidins. “Now, a loop is being theorized,” he said. “The recruitment tools are kept on and this keeps cycling and cycling allowing the neutrophils to stay in the skin” for years.
The exaggerated presence of MMPs is likely the very reason that the tetracycline class of antibiotics can be so effective during rosacea exacerbations, Dr. Cohen said. The drugs aren’t necessarily fighting bacteria – instead, their anti-MMP properties function as a temporary break in this inflammatory loop. “They interfere with MMP, and you can’t turn on cathelicidin without MMP. If you lock MMP down, you can break the cycle because there is no neutrophil recruitment.”
Interestingly, he said, antibiotics that target MMP appear to suppress the enzymes at subantimicrobial doses. “This opens the possibility that we might be able to treat with these drugs and not exert antibiotic effects,” lessening the problems associated with indiscriminate antibiotic usage.
Inflammatory overdrive makes rosacea skin hypersensitive to any kind of inflammatory trigger, said Dr. Hilary Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. At the meeting, Dr. Baldwin discussed several new topical medications aimed at breaking the cycle:
• 1% Ivermectin: People with rosacea can harbor up to six times more demodex mites than can people without the disorder. The explanation behind this relationship is unclear: Is rosacea skin somehow more hospitable to the mite, or is an excess of mites a direct causative factor? Whatever the interaction, topical ivermectin breaks the link as both an antimicrobial and an anti-inflammatory agent. Ivermectin directly inhibits cellular and humoral immune response, and regulates tumor necrosis factor–alpha and interleukins 1-beta and 10.
Two randomized, double-blind vehicle-controlled studies evaluating 1% ivermectin cream in patients with papulopustular rosacea found that almost 80% of rosacea patients were clear or nearly clear on the Investigator’s Global Assessment (IGA) scale at 12 weeks – significantly more than those who were using a vehicle placebo. Inflammatory lesions significantly decreased and adverse events were similar to those associated with the vehicle (J Drugs Dermatol. 2014 Mar;13[3]:316-23). Another study found that ivermectin was significantly more effective than metronidazole, she said.
• Azelaic acid: A phase III study examined azelaic acid foam in almost 1,000 patients with moderate to severe papulopustular rosacea. IGA was clear or near-clear in 32% of the active group at 12 weeks, and 23.5% of the vehicle control group – a significant difference. There were significantly fewer inflammatory lesions among those in the active treatment group as well. Drug-related cutaneous adverse events were significantly more common among those treated with azelaic acid (7% vs. about 4%) and were mostly pain, pruritus, and dryness at application site (Cutis. 2015 Jul;96[1]:54-61).
• Oxymetazoline and brimonidine: These two alpha-adrenergic receptor agonists are under investigation for their ability to decrease erythema. Both cause a transient vasoconstriction. Two studies of patients with moderate or severe erythema of rosacea found that a topical brimonidine gel formulation significantly decreased erythema for up to 12 hours after application, compared with a vehicle gel (J Drugs Dermatol. 2013 Jun 1;12[6]:650-6). However, there have been several case reports of severe rebound, with redness not only returning, but returning at a more severe grade (J Am Acad Dermatol. 2014 Feb;70[2]:e37-8).
• Isotretinoin: New investigations are looking at topical retinoids for rosacea. They do appear effective – but not only for the reason they work in acne. While they decrease the size of sebaceous glands and the amount of sebum produced, they seem to exert their benefit through an anti-inflammatory pathway, as well. Isotretinoin significantly decreases toll-like receptor-2 expression on monocytes.
Isotretinoin has been shown to be more effective than doxycycline in clearing skin (J Dtsch Dermatol Ges. 2010 Jul;8[7]:505-15) but has also seemed helpful in preventing and slowing phyma development and improving ocular disease.
Both Dr. Cohen and Dr. Baldwin disclosed relationships with multiple pharmaceutical companies.
NEW YORK – Rosacea seems to represent a Möbius strip of an overly enthusiastic immune response, with chemicals that recruit neutrophils, which, in turn, activate even more immunomodulators.
Researchers have been pointing the finger at trigger-happy neutrophils for more than a decade, Dr. David E. Cohen said at the summer meeting of the American Academy of Dermatology. But this group of cells is really more of an accomplice than a mastermind.
“Neutrophils were once the alpha and omega of rosacea,” said Dr. Cohen, the Charles C. and Dorothea E. Harris Professor of Dermatology, New York University. “But what are they doing on the skin and why are they there? They’re doing exactly what they’re supposed to do – defending against invasion by dumping out a whole poisonous soup of chemicals,” designed to protect and defend against microbes.
In the chronic disease of rosacea, however, these frontline soldiers are constantly recruited, even when there’s no invader to fight. They continue to carry out their preprogrammed attacks, releasing proteins that activate enzymes that stimulate immunomodulators that, in turn, call more neutrophils to the battlefield. Now, new research suggests that cathelicidins – antimicrobial peptides that are especially present in epithelial cells – are giving neutrophils their marching orders.
“Cathelicidins sit fully engaged on the skin and, the minute there’s a breach, they are turned on as the first defense,” Dr. Cohen said. “We need that kind of help right away – we can’t afford to wait around” for the second wave of defenders: neutrophils and lymphocytes.
This command seems to be bidirectional, though. Neutrophils release matrix metalloproteases (MMPs), enzymes critical for wound healing. MMPs remove dead extracellular matrix and microbes, prepare the wound bed for angiogenesis, and stimulate epidermal cell migration. But these benefits also exacerbate the things that make rosacea such a tough problem. By dissolving tissue, MMPs ramp up inflammation. By stimulating angiogenesis, they contribute to vasodilation, erythema, and telangiectasias.
And, Dr. Cohen noted, MMPs also just happen to recruit more neutrophils, which are capable of activating triptych enzymes, which turn on cathelicidins. “Now, a loop is being theorized,” he said. “The recruitment tools are kept on and this keeps cycling and cycling allowing the neutrophils to stay in the skin” for years.
The exaggerated presence of MMPs is likely the very reason that the tetracycline class of antibiotics can be so effective during rosacea exacerbations, Dr. Cohen said. The drugs aren’t necessarily fighting bacteria – instead, their anti-MMP properties function as a temporary break in this inflammatory loop. “They interfere with MMP, and you can’t turn on cathelicidin without MMP. If you lock MMP down, you can break the cycle because there is no neutrophil recruitment.”
Interestingly, he said, antibiotics that target MMP appear to suppress the enzymes at subantimicrobial doses. “This opens the possibility that we might be able to treat with these drugs and not exert antibiotic effects,” lessening the problems associated with indiscriminate antibiotic usage.
Inflammatory overdrive makes rosacea skin hypersensitive to any kind of inflammatory trigger, said Dr. Hilary Baldwin, medical director of the Acne Treatment and Research Center in Morristown, N.J. At the meeting, Dr. Baldwin discussed several new topical medications aimed at breaking the cycle:
• 1% Ivermectin: People with rosacea can harbor up to six times more demodex mites than can people without the disorder. The explanation behind this relationship is unclear: Is rosacea skin somehow more hospitable to the mite, or is an excess of mites a direct causative factor? Whatever the interaction, topical ivermectin breaks the link as both an antimicrobial and an anti-inflammatory agent. Ivermectin directly inhibits cellular and humoral immune response, and regulates tumor necrosis factor–alpha and interleukins 1-beta and 10.
Two randomized, double-blind vehicle-controlled studies evaluating 1% ivermectin cream in patients with papulopustular rosacea found that almost 80% of rosacea patients were clear or nearly clear on the Investigator’s Global Assessment (IGA) scale at 12 weeks – significantly more than those who were using a vehicle placebo. Inflammatory lesions significantly decreased and adverse events were similar to those associated with the vehicle (J Drugs Dermatol. 2014 Mar;13[3]:316-23). Another study found that ivermectin was significantly more effective than metronidazole, she said.
• Azelaic acid: A phase III study examined azelaic acid foam in almost 1,000 patients with moderate to severe papulopustular rosacea. IGA was clear or near-clear in 32% of the active group at 12 weeks, and 23.5% of the vehicle control group – a significant difference. There were significantly fewer inflammatory lesions among those in the active treatment group as well. Drug-related cutaneous adverse events were significantly more common among those treated with azelaic acid (7% vs. about 4%) and were mostly pain, pruritus, and dryness at application site (Cutis. 2015 Jul;96[1]:54-61).
• Oxymetazoline and brimonidine: These two alpha-adrenergic receptor agonists are under investigation for their ability to decrease erythema. Both cause a transient vasoconstriction. Two studies of patients with moderate or severe erythema of rosacea found that a topical brimonidine gel formulation significantly decreased erythema for up to 12 hours after application, compared with a vehicle gel (J Drugs Dermatol. 2013 Jun 1;12[6]:650-6). However, there have been several case reports of severe rebound, with redness not only returning, but returning at a more severe grade (J Am Acad Dermatol. 2014 Feb;70[2]:e37-8).
• Isotretinoin: New investigations are looking at topical retinoids for rosacea. They do appear effective – but not only for the reason they work in acne. While they decrease the size of sebaceous glands and the amount of sebum produced, they seem to exert their benefit through an anti-inflammatory pathway, as well. Isotretinoin significantly decreases toll-like receptor-2 expression on monocytes.
Isotretinoin has been shown to be more effective than doxycycline in clearing skin (J Dtsch Dermatol Ges. 2010 Jul;8[7]:505-15) but has also seemed helpful in preventing and slowing phyma development and improving ocular disease.
Both Dr. Cohen and Dr. Baldwin disclosed relationships with multiple pharmaceutical companies.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY
Head for Oral Contraceptives to Target Women’s Acne
NEW YORK – Almost all women with acne will have at least a fair response to therapy with oral contraceptive pills.
Most should experience at least a 50% reduction in lesions, Dr. Bethanee Schlosser said at the American Academy of Dermatology summer meeting.
“From baseline, you are generally speaking about a 50% decrease in inflammatory and noninflammatory lesions and total lesion count,” said Dr. Schlosser of Northwestern University, Chicago. “The important thing, though, is that you have to tell a patient this is not an overnight thing. You have to wait at least three cycles before you make any kind of judgment on whether it’s working.”
The improvement will be seen on all affected areas, not just the face, she said.
“This is important. It’s not just facial acne that’s hormonally sensitive. For us to say it’s just the facial distribution that’s hormonally sensitive is ridiculous. We all know as dermatologists that all acne is androgen driven, and it’s all hormonally sensitive.”
Oral contraceptives can be used alone, as Dr. Schlosser usually initiates treatment, or they can be used in conjunction with spironolactone or antibiotics. Three OCs are approved by the FDA for the treatment of acne.
“I often get asked which OC is the best,” she said. “Just because the FDA approved some for acne doesn’t mean they are better. It means the company had the studies done and basically paid for this labeling indication.”
A 2012 Cochrane review examined 31 studies that compared different OCs to placebo and to each other. The investigators found that all the OCs were consistently more effective than placebo (Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004425.pub6). The head-to-head comparisons produced conflicting results with no clear advantage of one formulation over another.
“I would say use what you are comfortable with,” Dr. Schlosser said.
Some personal and family history and health screenings are necessary before prescribing OCs, although leading women’s health associations, as well as the FDA have said there’s no need for a pelvic exam and Pap smear. “You do have to make sure they are not pregnant, hypertensive, or at risk for stroke or heart disease.”
Spironolactone is usually prescribed at 100-150 mg/day and rarely up to 200 mg/day, she noted. It can be added to an OC regimen if the patient has not adequately responded to monotherapy. It can also be combined with drospirenone, an antibiotic, or with both OCs and antibiotics.
Since spironolactone is a diuretic, women should be monitored for increased thirst and urination, and signs of hypokalemia (lethargy, muscle cramps, dizziness, and increased heart rate). In utero exposure can cause feminization of a male fetus, so reliable contraception is a must.
The drug does carry a boxed warning, as it was carcinogenic in rat studies – but only when given at 50-100 times the usual human dose.
Dr. Schlosser disclosed that she is an investigator with Galderma and Allergan.
NEW YORK – Almost all women with acne will have at least a fair response to therapy with oral contraceptive pills.
Most should experience at least a 50% reduction in lesions, Dr. Bethanee Schlosser said at the American Academy of Dermatology summer meeting.
“From baseline, you are generally speaking about a 50% decrease in inflammatory and noninflammatory lesions and total lesion count,” said Dr. Schlosser of Northwestern University, Chicago. “The important thing, though, is that you have to tell a patient this is not an overnight thing. You have to wait at least three cycles before you make any kind of judgment on whether it’s working.”
The improvement will be seen on all affected areas, not just the face, she said.
“This is important. It’s not just facial acne that’s hormonally sensitive. For us to say it’s just the facial distribution that’s hormonally sensitive is ridiculous. We all know as dermatologists that all acne is androgen driven, and it’s all hormonally sensitive.”
Oral contraceptives can be used alone, as Dr. Schlosser usually initiates treatment, or they can be used in conjunction with spironolactone or antibiotics. Three OCs are approved by the FDA for the treatment of acne.
“I often get asked which OC is the best,” she said. “Just because the FDA approved some for acne doesn’t mean they are better. It means the company had the studies done and basically paid for this labeling indication.”
A 2012 Cochrane review examined 31 studies that compared different OCs to placebo and to each other. The investigators found that all the OCs were consistently more effective than placebo (Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004425.pub6). The head-to-head comparisons produced conflicting results with no clear advantage of one formulation over another.
“I would say use what you are comfortable with,” Dr. Schlosser said.
Some personal and family history and health screenings are necessary before prescribing OCs, although leading women’s health associations, as well as the FDA have said there’s no need for a pelvic exam and Pap smear. “You do have to make sure they are not pregnant, hypertensive, or at risk for stroke or heart disease.”
Spironolactone is usually prescribed at 100-150 mg/day and rarely up to 200 mg/day, she noted. It can be added to an OC regimen if the patient has not adequately responded to monotherapy. It can also be combined with drospirenone, an antibiotic, or with both OCs and antibiotics.
Since spironolactone is a diuretic, women should be monitored for increased thirst and urination, and signs of hypokalemia (lethargy, muscle cramps, dizziness, and increased heart rate). In utero exposure can cause feminization of a male fetus, so reliable contraception is a must.
The drug does carry a boxed warning, as it was carcinogenic in rat studies – but only when given at 50-100 times the usual human dose.
Dr. Schlosser disclosed that she is an investigator with Galderma and Allergan.
NEW YORK – Almost all women with acne will have at least a fair response to therapy with oral contraceptive pills.
Most should experience at least a 50% reduction in lesions, Dr. Bethanee Schlosser said at the American Academy of Dermatology summer meeting.
“From baseline, you are generally speaking about a 50% decrease in inflammatory and noninflammatory lesions and total lesion count,” said Dr. Schlosser of Northwestern University, Chicago. “The important thing, though, is that you have to tell a patient this is not an overnight thing. You have to wait at least three cycles before you make any kind of judgment on whether it’s working.”
The improvement will be seen on all affected areas, not just the face, she said.
“This is important. It’s not just facial acne that’s hormonally sensitive. For us to say it’s just the facial distribution that’s hormonally sensitive is ridiculous. We all know as dermatologists that all acne is androgen driven, and it’s all hormonally sensitive.”
Oral contraceptives can be used alone, as Dr. Schlosser usually initiates treatment, or they can be used in conjunction with spironolactone or antibiotics. Three OCs are approved by the FDA for the treatment of acne.
“I often get asked which OC is the best,” she said. “Just because the FDA approved some for acne doesn’t mean they are better. It means the company had the studies done and basically paid for this labeling indication.”
A 2012 Cochrane review examined 31 studies that compared different OCs to placebo and to each other. The investigators found that all the OCs were consistently more effective than placebo (Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004425.pub6). The head-to-head comparisons produced conflicting results with no clear advantage of one formulation over another.
“I would say use what you are comfortable with,” Dr. Schlosser said.
Some personal and family history and health screenings are necessary before prescribing OCs, although leading women’s health associations, as well as the FDA have said there’s no need for a pelvic exam and Pap smear. “You do have to make sure they are not pregnant, hypertensive, or at risk for stroke or heart disease.”
Spironolactone is usually prescribed at 100-150 mg/day and rarely up to 200 mg/day, she noted. It can be added to an OC regimen if the patient has not adequately responded to monotherapy. It can also be combined with drospirenone, an antibiotic, or with both OCs and antibiotics.
Since spironolactone is a diuretic, women should be monitored for increased thirst and urination, and signs of hypokalemia (lethargy, muscle cramps, dizziness, and increased heart rate). In utero exposure can cause feminization of a male fetus, so reliable contraception is a must.
The drug does carry a boxed warning, as it was carcinogenic in rat studies – but only when given at 50-100 times the usual human dose.
Dr. Schlosser disclosed that she is an investigator with Galderma and Allergan.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Head for oral contraceptives to target women’s acne
NEW YORK – Almost all women with acne will have at least a fair response to therapy with oral contraceptive pills.
Most should experience at least a 50% reduction in lesions, Dr. Bethanee Schlosser said at the American Academy of Dermatology summer meeting.
“From baseline, you are generally speaking about a 50% decrease in inflammatory and noninflammatory lesions and total lesion count,” said Dr. Schlosser of Northwestern University, Chicago. “The important thing, though, is that you have to tell a patient this is not an overnight thing. You have to wait at least three cycles before you make any kind of judgment on whether it’s working.”
The improvement will be seen on all affected areas, not just the face, she said.
“This is important. It’s not just facial acne that’s hormonally sensitive. For us to say it’s just the facial distribution that’s hormonally sensitive is ridiculous. We all know as dermatologists that all acne is androgen driven, and it’s all hormonally sensitive.”
Oral contraceptives can be used alone, as Dr. Schlosser usually initiates treatment, or they can be used in conjunction with spironolactone or antibiotics. Three OCs are approved by the FDA for the treatment of acne.
“I often get asked which OC is the best,” she said. “Just because the FDA approved some for acne doesn’t mean they are better. It means the company had the studies done and basically paid for this labeling indication.”
A 2012 Cochrane review examined 31 studies that compared different OCs to placebo and to each other. The investigators found that all the OCs were consistently more effective than placebo (Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004425.pub6). The head-to-head comparisons produced conflicting results with no clear advantage of one formulation over another.
“I would say use what you are comfortable with,” Dr. Schlosser said.
Some personal and family history and health screenings are necessary before prescribing OCs, although leading women’s health associations, as well as the FDA have said there’s no need for a pelvic exam and Pap smear. “You do have to make sure they are not pregnant, hypertensive, or at risk for stroke or heart disease.”
Spironolactone is usually prescribed at 100-150 mg/day and rarely up to 200 mg/day, she noted. It can be added to an OC regimen if the patient has not adequately responded to monotherapy. It can also be combined with drospirenone, an antibiotic, or with both OCs and antibiotics.
Since spironolactone is a diuretic, women should be monitored for increased thirst and urination, and signs of hypokalemia (lethargy, muscle cramps, dizziness, and increased heart rate). In utero exposure can cause feminization of a male fetus, so reliable contraception is a must.
The drug does carry a boxed warning, as it was carcinogenic in rat studies – but only when given at 50-100 times the usual human dose.
Dr. Schlosser disclosed that she is an investigator with Galderma and Allergan.
On Twitter @Alz_Gal
NEW YORK – Almost all women with acne will have at least a fair response to therapy with oral contraceptive pills.
Most should experience at least a 50% reduction in lesions, Dr. Bethanee Schlosser said at the American Academy of Dermatology summer meeting.
“From baseline, you are generally speaking about a 50% decrease in inflammatory and noninflammatory lesions and total lesion count,” said Dr. Schlosser of Northwestern University, Chicago. “The important thing, though, is that you have to tell a patient this is not an overnight thing. You have to wait at least three cycles before you make any kind of judgment on whether it’s working.”
The improvement will be seen on all affected areas, not just the face, she said.
“This is important. It’s not just facial acne that’s hormonally sensitive. For us to say it’s just the facial distribution that’s hormonally sensitive is ridiculous. We all know as dermatologists that all acne is androgen driven, and it’s all hormonally sensitive.”
Oral contraceptives can be used alone, as Dr. Schlosser usually initiates treatment, or they can be used in conjunction with spironolactone or antibiotics. Three OCs are approved by the FDA for the treatment of acne.
“I often get asked which OC is the best,” she said. “Just because the FDA approved some for acne doesn’t mean they are better. It means the company had the studies done and basically paid for this labeling indication.”
A 2012 Cochrane review examined 31 studies that compared different OCs to placebo and to each other. The investigators found that all the OCs were consistently more effective than placebo (Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004425.pub6). The head-to-head comparisons produced conflicting results with no clear advantage of one formulation over another.
“I would say use what you are comfortable with,” Dr. Schlosser said.
Some personal and family history and health screenings are necessary before prescribing OCs, although leading women’s health associations, as well as the FDA have said there’s no need for a pelvic exam and Pap smear. “You do have to make sure they are not pregnant, hypertensive, or at risk for stroke or heart disease.”
Spironolactone is usually prescribed at 100-150 mg/day and rarely up to 200 mg/day, she noted. It can be added to an OC regimen if the patient has not adequately responded to monotherapy. It can also be combined with drospirenone, an antibiotic, or with both OCs and antibiotics.
Since spironolactone is a diuretic, women should be monitored for increased thirst and urination, and signs of hypokalemia (lethargy, muscle cramps, dizziness, and increased heart rate). In utero exposure can cause feminization of a male fetus, so reliable contraception is a must.
The drug does carry a boxed warning, as it was carcinogenic in rat studies – but only when given at 50-100 times the usual human dose.
Dr. Schlosser disclosed that she is an investigator with Galderma and Allergan.
On Twitter @Alz_Gal
NEW YORK – Almost all women with acne will have at least a fair response to therapy with oral contraceptive pills.
Most should experience at least a 50% reduction in lesions, Dr. Bethanee Schlosser said at the American Academy of Dermatology summer meeting.
“From baseline, you are generally speaking about a 50% decrease in inflammatory and noninflammatory lesions and total lesion count,” said Dr. Schlosser of Northwestern University, Chicago. “The important thing, though, is that you have to tell a patient this is not an overnight thing. You have to wait at least three cycles before you make any kind of judgment on whether it’s working.”
The improvement will be seen on all affected areas, not just the face, she said.
“This is important. It’s not just facial acne that’s hormonally sensitive. For us to say it’s just the facial distribution that’s hormonally sensitive is ridiculous. We all know as dermatologists that all acne is androgen driven, and it’s all hormonally sensitive.”
Oral contraceptives can be used alone, as Dr. Schlosser usually initiates treatment, or they can be used in conjunction with spironolactone or antibiotics. Three OCs are approved by the FDA for the treatment of acne.
“I often get asked which OC is the best,” she said. “Just because the FDA approved some for acne doesn’t mean they are better. It means the company had the studies done and basically paid for this labeling indication.”
A 2012 Cochrane review examined 31 studies that compared different OCs to placebo and to each other. The investigators found that all the OCs were consistently more effective than placebo (Cochrane Database Syst Rev. doi: 10.1002/14651858.CD004425.pub6). The head-to-head comparisons produced conflicting results with no clear advantage of one formulation over another.
“I would say use what you are comfortable with,” Dr. Schlosser said.
Some personal and family history and health screenings are necessary before prescribing OCs, although leading women’s health associations, as well as the FDA have said there’s no need for a pelvic exam and Pap smear. “You do have to make sure they are not pregnant, hypertensive, or at risk for stroke or heart disease.”
Spironolactone is usually prescribed at 100-150 mg/day and rarely up to 200 mg/day, she noted. It can be added to an OC regimen if the patient has not adequately responded to monotherapy. It can also be combined with drospirenone, an antibiotic, or with both OCs and antibiotics.
Since spironolactone is a diuretic, women should be monitored for increased thirst and urination, and signs of hypokalemia (lethargy, muscle cramps, dizziness, and increased heart rate). In utero exposure can cause feminization of a male fetus, so reliable contraception is a must.
The drug does carry a boxed warning, as it was carcinogenic in rat studies – but only when given at 50-100 times the usual human dose.
Dr. Schlosser disclosed that she is an investigator with Galderma and Allergan.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Balance Caution With Necessity When Prescribing Dermatology Drugs in Pregnancy
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Balance caution with necessity when prescribing dermatology drugs in pregnancy
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
On Twitter @Alz_Gal
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
On Twitter @Alz_Gal
NEW YORK – Disease exacerbations during pregnancy can be more dangerous for the fetus than some of the medications used to treat them..
No one wants to take unnecessary drugs during pregnancy, Dr. Jenny Murase said at the American Academy of Dermatology summer meeting. But concern about medication safety should never override the need to treat new-onset disease or control existing disorders.
“Often, patients are so worried about the effects of medicines on the baby that they don’t think about the effects of the disease. This is a thing we need to address with each patient. What would happen to you, and your baby, if this disease is not treated?” said Dr. Murase of the Palo Alto Foundation Medical Group, San Francisco.
Primary herpes simplex infections are an excellent example of this dilemma, she said. “Primary HSV has up to a 50% transmission rate to the fetus. In utero infections are associated with microcephaly, hydrocephalus, and chorioretinitis. About a third of neonates have central nervous system disease, and a quarter are born with disseminated disease,” and the neonatal mortality rate is nearly 70%.
“On the other hand, acyclovir is very, very safe,” based on data on thousands of patients, Dr. Murase said. Famciclovir and valacyclovir are also likely safe but data on those drugs are more limited, she added.
Sometimes, ideas about a drug’s safety during pregnancy are based on old or confusing studies. The worries about the teratogenicity of systemic steroids, for example, stem from a 1951 report that cortisone caused orofacial cleft in prenatally exposed mice.
“Since then, no prospective studies have found any evidence of congenital malformations associated with systemic steroids,” Dr. Murase said. However, “epidemiologic studies have suggested a threefold increase, which, interestingly, is in line with the mouse findings.”
The drugs do, however, have an important place in the armamentarium; they should be used judiciously and as part of a careful risk-benefit analysis. Both betamethasone and dexamethasone pass the placental barrier well; prednisone is not as transferable.
Dr. Murase provided a list of some medications commonly employed in dermatologic practice, with considerations for their use during pregnancy.
• Topical steroids. A 2013 study found that pregnancy-long exposure to more than 300 g of a potent or superpotent topical corticosteroid did not increase risk of orofacial cleft in the newborn, but did increase risk of low birth weight. “So if you are giving more than five tubes of 60 g, there is a potential for low birth weight – although this could also be related to the actual severity of the disease. Still, you should discuss this risk with your patient.”
• Antihistamines. These should be avoided in the last month of pregnancy as they can stimulate uterine contractions, especially if they are given intravenously or in very high doses. There have been reports of a doubling of retinopathy in premature infants (22% vs. 11%) associated with antihistamines used within 2 weeks of delivery. Some infants can have withdrawal symptoms, including seizure, if the mother has been taking high doses of the drugs.
• Antibiotics. The first-line antibiotics are penicillin, cephalosporins, and dicloxacillin; all are safe. Of the macrolides, erythromycin is preferred. However, the estolate form of the drug has been associated with reversible abnormalities in liver enzymes; the base and ethylsuccinate forms don’t have this risk. Rifampin may be used, but vitamin K should be given with it. Sulfonamides are a second-line choice up until the third trimester. They shouldn’t be used after that, as they can cause anemia, hyperbilirubinemia, and kernicterus. Tetracycline can be used up to 14 weeks’ gestation but not after that, because of the risks of bone growth inhibition and tooth discoloration in the infant, and maternal hepatitis.
• Antifungals. Topical antifungals are not too concerning. Nystatin is safe, but not as effective as the later-generation medications. Clotrimazole is the first choice, followed by miconazole and ketoconazole. Data are limited, but topical terbinafine, naftifine, and ciclopirox are also probably safe, she said.
Systemic antifungals are a different matter. None of the imidazole derivatives are advised during pregnancy, as they have been associated with craniosynostosis, congenital heart defects, and skeletal anomalies. If they are used, a diagnostic ultrasound at 20 weeks is advisable.
• Light. Light therapy is generally considered safe, although there is some evidence that narrow-band ultraviolet can deplete folic acid levels. Despite that, there are no reports of neural tube or orofacial defects associated with light therapy. Nevertheless, make sure patients are supplementing with folic acid if they receive light therapy.
Light therapy should never be combined with oxsoralen in pregnant women; the drug is a known mutagen. Data on cyclosporine are more limited, but it’s a category C drug in pregnancy, so it’s best to avoid it.
• Biologics. Etanercept, adalimumab, infliximab, and alefacept are all category B drugs in pregnancy. There are some hints that they, as well as tumor necrosis factor (TNF)-inhibitors, may be associated with some fetal anomalies in the VACTERL association cluster (vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal anomalies, and limb abnormalities). A confirmation of the syndrome requires three signs to be present. Only two infants born to mothers taking a biologic have had three qualifying signs.
However, pregnancy outcome reports for the drugs have recorded malformations. Among the adalimumab cohorts totaling 256 pregnancies, there were 14 fetal anomalies (5.5%); of these, nine were VACTERL-related. In the etanercept cohorts totaling 204 pregnancies, there were 13 anomalies (6.4%); of these, six were VACTERL-related. In the infliximab cohorts totaling 112 pregnancies, there was only one anomaly, which was in the VACTERL cluster. Among the 407 pregnancies in the TNF-inhibitors, there were three anomalies, none of which were related to VACTERL.
Dr. Murase had no financial disclosures relevant to her talk.
The current system of using letter categories to denote risk of prescription drugs during pregnancy and breastfeeding is being replaced by the Food and Drug Administration, with the addition of subsections in drug labeling.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Turn down the androgens to treat female pattern hair loss
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
NEW YORK – Antiandrogen hormones can help stabilize, and even improve, female pattern hair loss.
The pathophysiology of the disorder is unknown, but treatment is based on the assumption that women must be like men, at least when it comes to losing their hair. Intuitively, decreasing androgens should help correct the problem.
The answer, though, is a complicated mix of yes and maybe, Dr. Rochelle Torgerson said at the American Academy of Dermatology summer meeting.
“It used to be assumed that pattern hair loss in women was just the same as it is in men,” said Dr. Torgerson of the Mayo Clinic in Rochester, Minn. “Now there is some evidence that’s not true. In 2010, for example, this was seen in a woman with complete androgen insensitivity syndrome, so in her, androgens were not affecting hair follicles. There must be a place for estrogen.”
Further complicating the picture is the fact that no hormonal medications have FDA approval for hair loss in women, and their use has a history of conflicting data in clinical studies. Still, they remain the cornerstone for treating this physically and emotionally challenging problem.
The initial challenge is simply what to label it at the first visit.
“I have no problem with term ‘androgenetic alopecia,’ since that is what women are seeing when they first look on the Internet for information. But I do try to transition them to ‘female pattern hair loss.’ And I never – ever – use the term ‘male pattern baldness.’ It has a huge impact on women.”
The disease is a progressive miniaturization of the hair follicle over time. The growing cycle slows and the resting phase lengthens. There is progressive thinning over the vertex. Some women may keep most of their frontal hairline, but the vast majority do say it’s thinner than it was.
Spironolactone and oral contraceptives with spironolactone analogues are Dr. Torgerson’s go-to medications for first-line treatment. For spironolactone, she prefers a dose of 100-200 mg/day. Some women experience gastrointestinal upset, dizziness, cramps, breast tenderness, and spotting with these medications.
Her choice for an oral contraceptive is the combination of 20 mcg ethinyl estradiol plus drospirenone, but any oral contraceptive approved for acne may work.
Finasteride and dutasteride are approved for pattern hair loss in men, but not in women. Both inhibit 5 alpha-reductase type II. Dutasteride is more potent that finasteride and also inhibits type 1 alpha-reductase; both of these enzymes convert testosterone into the more potent dihydrotestosterone. The side-effect profile is more moderate than that of spironolactone, but both of the drugs have had mixed results in clinical trials.
One problem with the finasteride trials has been the variation in dosing. The least positive studies used the lowest dose of 1.25 mg. As the dosage increased to 2.5 mg and 5 mg, the benefit increased.
Despite her support for hormonal therapies, Dr. Torgerson doesn’t rely upon them alone – she supports them with the direct action of a 5% minoxidil foam. In addition to prescribing effective therapy, she urges women to actually be patient and to have realistic expectations.
Most women expect dramatic improvement in a short time. “I have no idea where that expectation comes from. This is a slow progressive condition. I agree with them that it’s completely unsexy to have the head of hair they do at that time. But if, in 3 years, they have this same head of hair, that’s going to be an amazing success. And once they have that expectation in their mind, they are usually happy with any other results that they see.”
Dr. Torgerson had no financial conflicts with regard to her presentation.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Nipple Raynaud’s can freeze out breastfeeding desire
NEW YORK – The “perfect storm” of pregnancy and lactation can throw breastfeeding moms into a painful deep freeze.
Almost 25% of women with lactation pain may actually be experiencing symptoms of Raynaud’s, Dr. Honor Fullerton Stone said at the American Academy of Dermatology summer meeting.
“Breastfeeding mothers are in a perfect storm,” of physical developments that predispose them to this vasoconstrictive phenomenon, said Dr. Fullerton Stone, who practices dermatology in Menlo Park, Ca. “Estrogen increases the alpha-adrenergic receptors on smooth muscle. Nerve irritation from constant breastfeeding upregulates those receptors. And emotional stress – crying baby, anyone? – increases epinephrine, which contributes further to vasoconstriction.”
She conducted a review of 88 of her own patients with nursing-related breast pain. Of these, about a quarter ended up with a diagnosis of nipple vasoconstriction. None of the women had a history of Raynaud’s disease.
Pain during a nursing session is the presenting complaint, but breastfeeding pain is incredibly nonspecific as a symptom. It’s the quality of the pain, Dr Stone said, that should ring a bell.
“There’s let-down pain, which occurs at latch and then comes on again later, with refill. There’s pain from candidiasis, which is dramatic at latch, like a radiating heat, but goes away rapidly after a few days of antifungals,” she noted. But with Raynaud’s, “the pain is persistent. It’s throbbing, which makes sense since it’s vascular. And it’s constant,” lasting through every nursing session, which she said is the kind of experience that makes mothers stop breastfeeding.
Since pain is such a pervasive symptom in breastfeeding complaints, women with vasoconstriction of the nipple are often misdiagnosed. They can go for months trying to improve latch technique or receiving antifungal therapy with no improvement, she said.
Dr. Stone considers a diagnosis of Raynaud’s if two of the following criteria are met:
• Color change of nipple, cold sensitivity, or color change of acral surfaces with cold exposure.
• Chronic deep breast pain for 4 or more weeks.
• Failure of oral antifungals and or antibiotics.
Treatment is both supportive and systemic and avoiding cold is key. She recalled a young mother who arrived in her office bundled up in a down parka on a warm California spring day. “I don’t know why, but this really seems to help,” she said.
“I suggest taking two hot showers a day and all the better if they can be right before nursing. Hot pads and compresses are not going to help. You need to warm up the entire body to calm this vascular reactivity.”
Women should also avoid consuming anything that can cause vasoconstriction, including caffeine and tobacco, she advised.
Nifedipine is a very effective medication, and is safe for nursing infants, Dr. Stone said. The sustained-released 30 mg/day dose is typically recommended, but she has changed her thinking on this a bit.
Her internal study showed that, although 83% responded very well to the drug, about a third of them also had a vasodilation-related side effect.
“For this reason, I usually now start with the 10 mg nonsustained form, and warn about things like headache, dizziness, postural hypotension, and fainting,” she said. Typically, patients adjust well to the drug’s effects and then the dose can be individually titrated.
Dr. Fullerton Stone had no relevant financial disclosures.
On Twitter @Alz_Gal
NEW YORK – The “perfect storm” of pregnancy and lactation can throw breastfeeding moms into a painful deep freeze.
Almost 25% of women with lactation pain may actually be experiencing symptoms of Raynaud’s, Dr. Honor Fullerton Stone said at the American Academy of Dermatology summer meeting.
“Breastfeeding mothers are in a perfect storm,” of physical developments that predispose them to this vasoconstrictive phenomenon, said Dr. Fullerton Stone, who practices dermatology in Menlo Park, Ca. “Estrogen increases the alpha-adrenergic receptors on smooth muscle. Nerve irritation from constant breastfeeding upregulates those receptors. And emotional stress – crying baby, anyone? – increases epinephrine, which contributes further to vasoconstriction.”
She conducted a review of 88 of her own patients with nursing-related breast pain. Of these, about a quarter ended up with a diagnosis of nipple vasoconstriction. None of the women had a history of Raynaud’s disease.
Pain during a nursing session is the presenting complaint, but breastfeeding pain is incredibly nonspecific as a symptom. It’s the quality of the pain, Dr Stone said, that should ring a bell.
“There’s let-down pain, which occurs at latch and then comes on again later, with refill. There’s pain from candidiasis, which is dramatic at latch, like a radiating heat, but goes away rapidly after a few days of antifungals,” she noted. But with Raynaud’s, “the pain is persistent. It’s throbbing, which makes sense since it’s vascular. And it’s constant,” lasting through every nursing session, which she said is the kind of experience that makes mothers stop breastfeeding.
Since pain is such a pervasive symptom in breastfeeding complaints, women with vasoconstriction of the nipple are often misdiagnosed. They can go for months trying to improve latch technique or receiving antifungal therapy with no improvement, she said.
Dr. Stone considers a diagnosis of Raynaud’s if two of the following criteria are met:
• Color change of nipple, cold sensitivity, or color change of acral surfaces with cold exposure.
• Chronic deep breast pain for 4 or more weeks.
• Failure of oral antifungals and or antibiotics.
Treatment is both supportive and systemic and avoiding cold is key. She recalled a young mother who arrived in her office bundled up in a down parka on a warm California spring day. “I don’t know why, but this really seems to help,” she said.
“I suggest taking two hot showers a day and all the better if they can be right before nursing. Hot pads and compresses are not going to help. You need to warm up the entire body to calm this vascular reactivity.”
Women should also avoid consuming anything that can cause vasoconstriction, including caffeine and tobacco, she advised.
Nifedipine is a very effective medication, and is safe for nursing infants, Dr. Stone said. The sustained-released 30 mg/day dose is typically recommended, but she has changed her thinking on this a bit.
Her internal study showed that, although 83% responded very well to the drug, about a third of them also had a vasodilation-related side effect.
“For this reason, I usually now start with the 10 mg nonsustained form, and warn about things like headache, dizziness, postural hypotension, and fainting,” she said. Typically, patients adjust well to the drug’s effects and then the dose can be individually titrated.
Dr. Fullerton Stone had no relevant financial disclosures.
On Twitter @Alz_Gal
NEW YORK – The “perfect storm” of pregnancy and lactation can throw breastfeeding moms into a painful deep freeze.
Almost 25% of women with lactation pain may actually be experiencing symptoms of Raynaud’s, Dr. Honor Fullerton Stone said at the American Academy of Dermatology summer meeting.
“Breastfeeding mothers are in a perfect storm,” of physical developments that predispose them to this vasoconstrictive phenomenon, said Dr. Fullerton Stone, who practices dermatology in Menlo Park, Ca. “Estrogen increases the alpha-adrenergic receptors on smooth muscle. Nerve irritation from constant breastfeeding upregulates those receptors. And emotional stress – crying baby, anyone? – increases epinephrine, which contributes further to vasoconstriction.”
She conducted a review of 88 of her own patients with nursing-related breast pain. Of these, about a quarter ended up with a diagnosis of nipple vasoconstriction. None of the women had a history of Raynaud’s disease.
Pain during a nursing session is the presenting complaint, but breastfeeding pain is incredibly nonspecific as a symptom. It’s the quality of the pain, Dr Stone said, that should ring a bell.
“There’s let-down pain, which occurs at latch and then comes on again later, with refill. There’s pain from candidiasis, which is dramatic at latch, like a radiating heat, but goes away rapidly after a few days of antifungals,” she noted. But with Raynaud’s, “the pain is persistent. It’s throbbing, which makes sense since it’s vascular. And it’s constant,” lasting through every nursing session, which she said is the kind of experience that makes mothers stop breastfeeding.
Since pain is such a pervasive symptom in breastfeeding complaints, women with vasoconstriction of the nipple are often misdiagnosed. They can go for months trying to improve latch technique or receiving antifungal therapy with no improvement, she said.
Dr. Stone considers a diagnosis of Raynaud’s if two of the following criteria are met:
• Color change of nipple, cold sensitivity, or color change of acral surfaces with cold exposure.
• Chronic deep breast pain for 4 or more weeks.
• Failure of oral antifungals and or antibiotics.
Treatment is both supportive and systemic and avoiding cold is key. She recalled a young mother who arrived in her office bundled up in a down parka on a warm California spring day. “I don’t know why, but this really seems to help,” she said.
“I suggest taking two hot showers a day and all the better if they can be right before nursing. Hot pads and compresses are not going to help. You need to warm up the entire body to calm this vascular reactivity.”
Women should also avoid consuming anything that can cause vasoconstriction, including caffeine and tobacco, she advised.
Nifedipine is a very effective medication, and is safe for nursing infants, Dr. Stone said. The sustained-released 30 mg/day dose is typically recommended, but she has changed her thinking on this a bit.
Her internal study showed that, although 83% responded very well to the drug, about a third of them also had a vasodilation-related side effect.
“For this reason, I usually now start with the 10 mg nonsustained form, and warn about things like headache, dizziness, postural hypotension, and fainting,” she said. Typically, patients adjust well to the drug’s effects and then the dose can be individually titrated.
Dr. Fullerton Stone had no relevant financial disclosures.
On Twitter @Alz_Gal
AT THE AAD SUMMER ACADEMY 2015
Vulvar lichen sclerosus often overlooked in women of reproductive age
NEW YORK – A delayed diagnosis of vulvar lichen sclerosus is common, and the risk of this delay is permanent scarring and structural genital changes, cautioned an expert who holds teaching appointments in both dermatology and obstetrics/gynecology.
Vulvar lichen sclerosus (VLS) should be considered in females of any age complaining of vulvar itching, including sexually active women for whom other diseases may be more likely, emphasized Dr. Bethanee J. Schlosser, director of the Vulvar Mucosal Specialty Clinic at Northwestern University, Chicago. She spoke at the American Academy of Dermatology summer meeting in a session jointly sponsored by the European Academy of Dermatology and Venereology.
“We need to recognize that when women in their 20s and 30s complain of vulvar pruritus, it is not always vulvovaginal candidiasis,” Dr. Schlosser emphasized. She said some clinicians do not even consider VLS in this age group, because they have been mistakenly informed that this disease has a bimodal distribution that restricts most cases to preadolescent girls and postmenopausal women. According to Dr. Schlosser, up to 40% of cases occur in women of reproductive age.
VLS is a variation on lichen sclerosus, which is a chronic inflammatory condition associated with epithelial thinning that can occur anywhere on the body. The disease is progressive. It is not an erosive process initially, but Dr. Schlosser presented several cases that demonstrated secondary erosions and fissures can eventually result in permanent structural damage to the anatomy.
The etiology remains incompletely understood, but Dr. Schlosser said that VLS is now considered an autoimmune condition that is commonly associated with other autoimmune diseases, particularly thyroiditis. Dr. Schlosser advised that screening VLS patients for additional autoimmune disorders is appropriate.
The key issue, however, is making the diagnosis in the first place. The waxy plaques and epidermal wrinkling that characterize this disease may not be immediately distinguishable from other dermatologic lesions, particularly as the severity varies. Pruritus is the most common symptom, but up to 30% of women are asymptomatic, according to Dr. Schlosser. Histologic evidence of hyperkeratosis on biopsy in the context of characteristic clinical signs confirms the diagnosis.
Whether in girls, women of reproductive age, or postmenopausal women, the substantial gap between the median age of onset and the median age of diagnosis is a source of concern. In peri- and postmenopausal women, Dr. Schlosser cited data suggesting that the mean delay to a diagnosis can be 5 or more years. According to Dr. Schlosser, one source of delay may be the well-known reluctance of many patients to disclose genital symptoms, but she also maintained that VLS, which has an incidence of 0.1%-0.2%, is not often considered in the initial evaluation of vulvar dermatologic complaints.
The standard first-line therapy for VLS is highly potent corticosteroids. In contrast, topical testosterone, once widely used when VLS was thought to be a product of hormonal imbalance, “has no role in this disease,” according to Dr. Schlosser. Second-line treatments for those who need an alternative to steroids include topical calcineurin inhibitors, such as tacrolimus or pimecrolimus, the synthetic vitamin D cream calcipotriene, and topical retinoids. Oral steroids can be used in difficult cases, but Dr. Schlosser said this is uncommon, estimating that she may have placed only 4 of the 150 VLS cases she has accumulated in her clinic on a systemic therapy.
Generally, topical therapies, which must be maintained indefinitely, suppress symptoms and slow or halt the progressive disease process, according to Dr. Schlosser, who cited a large study published more than 10 years ago. In this study, 66% of 255 VLS patients followed for a median of 66 months on topical steroids became symptom free (Arch Dermatol. 2004;140:702-6). All but 4% improved. Normal skin texture was achieved in 23%, and 68% showed partial improvement. Scarring was less common in children than adults. Squamous cell carcinoma occurred in 2.4%.
The small but clinically significant risk of squamous cell carcinoma has been documented previously, but Dr. Schlosser said there is new evidence that topical therapy may reduce the risk. In a study of 507 women with a median follow-up of 4.7 years, the risk of squamous cell carcinoma was inversely related to compliance with therapy.
“Among the women who used the assigned therapy most or all of the time, zero developed squamous cell carcinoma as opposed to seven cases in the partially compliant therapy group,” she reported. Although this does not prove that treatment reduces risk of cancer, Dr. Schlosser indicated that it is strongly suggestive.
Several questions about VLS remain unanswered, according to Dr. Schlosser. More data, for example, are needed to determine whether the current order of therapies for first-line treatment as well as maintenance regimens is optimal, particularly for reducing cancer risk. However, Dr. Schlosser emphasized that the most important challenge to better outcomes is early recognition of this entity.
Dr. Schlosser reported financial disclosures with Allergan and Galderma Laboratories.
NEW YORK – A delayed diagnosis of vulvar lichen sclerosus is common, and the risk of this delay is permanent scarring and structural genital changes, cautioned an expert who holds teaching appointments in both dermatology and obstetrics/gynecology.
Vulvar lichen sclerosus (VLS) should be considered in females of any age complaining of vulvar itching, including sexually active women for whom other diseases may be more likely, emphasized Dr. Bethanee J. Schlosser, director of the Vulvar Mucosal Specialty Clinic at Northwestern University, Chicago. She spoke at the American Academy of Dermatology summer meeting in a session jointly sponsored by the European Academy of Dermatology and Venereology.
“We need to recognize that when women in their 20s and 30s complain of vulvar pruritus, it is not always vulvovaginal candidiasis,” Dr. Schlosser emphasized. She said some clinicians do not even consider VLS in this age group, because they have been mistakenly informed that this disease has a bimodal distribution that restricts most cases to preadolescent girls and postmenopausal women. According to Dr. Schlosser, up to 40% of cases occur in women of reproductive age.
VLS is a variation on lichen sclerosus, which is a chronic inflammatory condition associated with epithelial thinning that can occur anywhere on the body. The disease is progressive. It is not an erosive process initially, but Dr. Schlosser presented several cases that demonstrated secondary erosions and fissures can eventually result in permanent structural damage to the anatomy.
The etiology remains incompletely understood, but Dr. Schlosser said that VLS is now considered an autoimmune condition that is commonly associated with other autoimmune diseases, particularly thyroiditis. Dr. Schlosser advised that screening VLS patients for additional autoimmune disorders is appropriate.
The key issue, however, is making the diagnosis in the first place. The waxy plaques and epidermal wrinkling that characterize this disease may not be immediately distinguishable from other dermatologic lesions, particularly as the severity varies. Pruritus is the most common symptom, but up to 30% of women are asymptomatic, according to Dr. Schlosser. Histologic evidence of hyperkeratosis on biopsy in the context of characteristic clinical signs confirms the diagnosis.
Whether in girls, women of reproductive age, or postmenopausal women, the substantial gap between the median age of onset and the median age of diagnosis is a source of concern. In peri- and postmenopausal women, Dr. Schlosser cited data suggesting that the mean delay to a diagnosis can be 5 or more years. According to Dr. Schlosser, one source of delay may be the well-known reluctance of many patients to disclose genital symptoms, but she also maintained that VLS, which has an incidence of 0.1%-0.2%, is not often considered in the initial evaluation of vulvar dermatologic complaints.
The standard first-line therapy for VLS is highly potent corticosteroids. In contrast, topical testosterone, once widely used when VLS was thought to be a product of hormonal imbalance, “has no role in this disease,” according to Dr. Schlosser. Second-line treatments for those who need an alternative to steroids include topical calcineurin inhibitors, such as tacrolimus or pimecrolimus, the synthetic vitamin D cream calcipotriene, and topical retinoids. Oral steroids can be used in difficult cases, but Dr. Schlosser said this is uncommon, estimating that she may have placed only 4 of the 150 VLS cases she has accumulated in her clinic on a systemic therapy.
Generally, topical therapies, which must be maintained indefinitely, suppress symptoms and slow or halt the progressive disease process, according to Dr. Schlosser, who cited a large study published more than 10 years ago. In this study, 66% of 255 VLS patients followed for a median of 66 months on topical steroids became symptom free (Arch Dermatol. 2004;140:702-6). All but 4% improved. Normal skin texture was achieved in 23%, and 68% showed partial improvement. Scarring was less common in children than adults. Squamous cell carcinoma occurred in 2.4%.
The small but clinically significant risk of squamous cell carcinoma has been documented previously, but Dr. Schlosser said there is new evidence that topical therapy may reduce the risk. In a study of 507 women with a median follow-up of 4.7 years, the risk of squamous cell carcinoma was inversely related to compliance with therapy.
“Among the women who used the assigned therapy most or all of the time, zero developed squamous cell carcinoma as opposed to seven cases in the partially compliant therapy group,” she reported. Although this does not prove that treatment reduces risk of cancer, Dr. Schlosser indicated that it is strongly suggestive.
Several questions about VLS remain unanswered, according to Dr. Schlosser. More data, for example, are needed to determine whether the current order of therapies for first-line treatment as well as maintenance regimens is optimal, particularly for reducing cancer risk. However, Dr. Schlosser emphasized that the most important challenge to better outcomes is early recognition of this entity.
Dr. Schlosser reported financial disclosures with Allergan and Galderma Laboratories.
NEW YORK – A delayed diagnosis of vulvar lichen sclerosus is common, and the risk of this delay is permanent scarring and structural genital changes, cautioned an expert who holds teaching appointments in both dermatology and obstetrics/gynecology.
Vulvar lichen sclerosus (VLS) should be considered in females of any age complaining of vulvar itching, including sexually active women for whom other diseases may be more likely, emphasized Dr. Bethanee J. Schlosser, director of the Vulvar Mucosal Specialty Clinic at Northwestern University, Chicago. She spoke at the American Academy of Dermatology summer meeting in a session jointly sponsored by the European Academy of Dermatology and Venereology.
“We need to recognize that when women in their 20s and 30s complain of vulvar pruritus, it is not always vulvovaginal candidiasis,” Dr. Schlosser emphasized. She said some clinicians do not even consider VLS in this age group, because they have been mistakenly informed that this disease has a bimodal distribution that restricts most cases to preadolescent girls and postmenopausal women. According to Dr. Schlosser, up to 40% of cases occur in women of reproductive age.
VLS is a variation on lichen sclerosus, which is a chronic inflammatory condition associated with epithelial thinning that can occur anywhere on the body. The disease is progressive. It is not an erosive process initially, but Dr. Schlosser presented several cases that demonstrated secondary erosions and fissures can eventually result in permanent structural damage to the anatomy.
The etiology remains incompletely understood, but Dr. Schlosser said that VLS is now considered an autoimmune condition that is commonly associated with other autoimmune diseases, particularly thyroiditis. Dr. Schlosser advised that screening VLS patients for additional autoimmune disorders is appropriate.
The key issue, however, is making the diagnosis in the first place. The waxy plaques and epidermal wrinkling that characterize this disease may not be immediately distinguishable from other dermatologic lesions, particularly as the severity varies. Pruritus is the most common symptom, but up to 30% of women are asymptomatic, according to Dr. Schlosser. Histologic evidence of hyperkeratosis on biopsy in the context of characteristic clinical signs confirms the diagnosis.
Whether in girls, women of reproductive age, or postmenopausal women, the substantial gap between the median age of onset and the median age of diagnosis is a source of concern. In peri- and postmenopausal women, Dr. Schlosser cited data suggesting that the mean delay to a diagnosis can be 5 or more years. According to Dr. Schlosser, one source of delay may be the well-known reluctance of many patients to disclose genital symptoms, but she also maintained that VLS, which has an incidence of 0.1%-0.2%, is not often considered in the initial evaluation of vulvar dermatologic complaints.
The standard first-line therapy for VLS is highly potent corticosteroids. In contrast, topical testosterone, once widely used when VLS was thought to be a product of hormonal imbalance, “has no role in this disease,” according to Dr. Schlosser. Second-line treatments for those who need an alternative to steroids include topical calcineurin inhibitors, such as tacrolimus or pimecrolimus, the synthetic vitamin D cream calcipotriene, and topical retinoids. Oral steroids can be used in difficult cases, but Dr. Schlosser said this is uncommon, estimating that she may have placed only 4 of the 150 VLS cases she has accumulated in her clinic on a systemic therapy.
Generally, topical therapies, which must be maintained indefinitely, suppress symptoms and slow or halt the progressive disease process, according to Dr. Schlosser, who cited a large study published more than 10 years ago. In this study, 66% of 255 VLS patients followed for a median of 66 months on topical steroids became symptom free (Arch Dermatol. 2004;140:702-6). All but 4% improved. Normal skin texture was achieved in 23%, and 68% showed partial improvement. Scarring was less common in children than adults. Squamous cell carcinoma occurred in 2.4%.
The small but clinically significant risk of squamous cell carcinoma has been documented previously, but Dr. Schlosser said there is new evidence that topical therapy may reduce the risk. In a study of 507 women with a median follow-up of 4.7 years, the risk of squamous cell carcinoma was inversely related to compliance with therapy.
“Among the women who used the assigned therapy most or all of the time, zero developed squamous cell carcinoma as opposed to seven cases in the partially compliant therapy group,” she reported. Although this does not prove that treatment reduces risk of cancer, Dr. Schlosser indicated that it is strongly suggestive.
Several questions about VLS remain unanswered, according to Dr. Schlosser. More data, for example, are needed to determine whether the current order of therapies for first-line treatment as well as maintenance regimens is optimal, particularly for reducing cancer risk. However, Dr. Schlosser emphasized that the most important challenge to better outcomes is early recognition of this entity.
Dr. Schlosser reported financial disclosures with Allergan and Galderma Laboratories.
EXPERT ANALYSIS FROM AAD SUMMER ACADEMY 2015
Early intervention may forestall menopause-related skin aging
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
NEW YORK – Evidence is mounting that early intervention in the menopausal transition could help forestall some of the skin aging associated with estrogen decline.
Estrogen supplementation and collagen stimulation both seem effective in preserving the integrity of a woman’s skin as levels of the hormone decrease, Dr. Diane Madfes said at the American Academy of Dermatology summer meeting.
Type 3 collagen decreases by up to 50% within a few years of menopause, said Dr. Madfes, a dermatologist in New York. This is directly related to a loss of estrogen receptor beta in the dermal matrix, which promotes collagen formation.
“There is a theory – the timing hypothesis – that we have a window of opportunity to intervene. If we can stimulate the collagen before the receptors go down, maybe we can have a beneficial effect on skin.”
Any method of collagen stimulation should work, she said: laser resurfacing, microneedling, or radiofrequency. “We are very good about being able to stimulate collagen. The method doesn’t matter as much as the timing. The important thing is to intervene early. If you see your patients starting to sag, see a loss of elasticity, that is the time to intervene. Get at the collagen while it’s still receptive.”
Estrogen exerts a plethora of antiaging, skin-preserving effects. “We know that a decrease in estrogen is related to telomere shortening. Estrogen protects against oxidative damage. It signals keratinocytes through IGF-1,” she said.
The hormone also protects skin’s water-binding qualities by promoting mucopolysaccharides, sebum production, barrier function, and hyaluronic acid. It may even play a role in protecting against ultraviolet light. Estrogen downregulation affects healing by inhibiting the proliferation of keratinocytes and the proliferation and migration of fibroblasts.
All these add up to rapid skin aging after estrogen levels drop.
“The visible effects of aging on women’s skin are not so much related to her chronological age as to the years after menopause,” Dr. Madfes said – a finding that is particularly illustrated in young women with surgical menopause and those with breast cancer who take tamoxifen. The observation seems to suggest that early intervention with estrogen might help prevent at least some of the signs of aging.
The ongoing KEEPS trial (Kronos Early Estrogen Prevention Study) may shed some light on the issue. KEEPS has randomized 729 women aged 42-58 years to oral or transdermal estrogen; the primary endpoint is rate of atherosclerosis. But an ancillary study is looking at the effect of estrogen on skin wrinkles and skin rigidity.
The substudy is based on positive findings of a 1996 study, which found evidence for facial application of topical estrogen designed for vulvar use. After 6 months, elasticity and firmness significantly improved. Skin moisture increased, as did type 3 collagen and collagen fibers.
Some women do use topical estrogens on their faces. “It seems to promote skin thickening and tightening,“ Dr. Madfes said, although a recent editorial suggested that using the product anywhere but on the genitals can cause estrogen-mediated side effects in both children and pets.
But recommending estrogen is fraught with controversy. Large studies have come to conflicting conclusions about its benefit and safety. And prescribing estrogen is not really within a dermatologist’s purview.
“It’s not for us to suggest that women go on hormone therapy. But we can explain these things and ask if she is taking it, or if she’s talked to her gynecologist about it.”
Dr. Madfes has no financial disclosures to report.
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015
Pruritus prophylaxis appropriate for targeted cancer therapies
NEW YORK – Due to the frequency with which patients develop pruritus while on a targeted cancer therapy, prophylaxis should be strongly considered for at least some of the drugs, such as epidermal growth factor–receptor (EGFR) inhibitors, according to an expert in oncodermatology.
“Pruritus is a frequent adverse event in cancer patients treated with targeted therapies and an early and proactive approach towards pruritus is advisable,” Dr. Mario E. Lacouture, director of the oncodermatology program at New York’s Memorial Sloan Kettering Cancer Center. The increasing use of targeted therapies, including the growing proportion of patients on long-term maintenance regimens, is expected to make these complaints more common.
Targeted cancer therapies, such as monoclonal antibodies and tyrosine kinase inhibitors (TKIs), are, in general, associated with a low risk of adverse events relative to cytotoxic chemotherapies. The exception is dermatologic adverse events, Dr. Lacouture said at the American Academy of Dermatology summer meeting. Skin rashes are characteristic adverse events with several targeted agents, such as the EGFR inhibitor cetuximab, but Dr. Lacouture warned that pruritus for many patients imposes the greatest burden.
“Since the introduction of the first targeted cancer drug, imatinib, in 2001, a long list of targeted agents have been approved, and all are associated with pruritus that significantly reduces quality of life,” he reported. Dermatologists need to fill a void.
“Oncologists are not generally familiar with strategies to treat pruritus and typically resort to antihistamines, such as diphenhydramine or hydroxyzine,” Dr. Lacouture added. He suggested such sedative agents “are not ideal” when managing an adverse event that may persist for weeks or months.
The first step may simply be to prepare patients initiating targeted therapy for the substantial risk of pruritus. Dr. Lacouture cited two studies in which cancer patients were asked about unexpected side effects. In both, dermatologic complaints were the most common.
“Patients are told that they are going to lose their hair, that they are going to have nausea and vomiting, but they are never told that they are going to have dry skin, irritated skin, or itchy skin,” Dr. Lacouture noted. He cited one study in which all three of the top side effects identified by cancer patients as unexpected were dermatologic, including nail changes and pruritus.
In fact, published studies suggest that most patients treated with EGFR inhibitors, and approximately one-third of patients treated with a variety of TKIs, such as those targeting BRAF and MEK pathways, will develop significant pruritus, according to Dr. Lacouture. However, several studies, including one of his own, suggest that this itching can be greatly mitigated not only by treatment but also with prophylaxis.
In his study of the effect of prophylaxis, 95 patients initiating the EGFR inhibitor panitumumab were randomized to a prophylactic regimen that included skin moisturizers, sunscreen, a topical steroid, and doxycycline, or to treatment adjusted for symptoms once they developed (J Clin Oncol. 2010;28:1351-7). At 6 weeks, the proportion of patients with grade 2 or higher dermatologic events, including pruritus, was reduced from 62% to 29%. More favorable quality of life scores in the prophylactic regimen group supported the advantage.
“When you looked at grade 3 or higher adverse events, you see that the incidence of acneiform rash [at the grade 3 level of severity] was lowered by almost 75% and pruritus was almost abolished,” Dr. Lacouture reported.
For refractory pruritus related to targeted therapy, aprepitant may be the best option based on Dr. Lacouture’s own experience and a prospective but nonrandomized study (Lancet Oncol. 2012;13:1020-4). In the published study, which included patients on EGFR inhibitors or TKIs, the median visual analog scores (VAS) for pruritus fell from 8 before treatment to 1, 1 week later. According to Dr. Lacouture, the treatment effects are durable. In some cases, pruritus does not recur even after a single course of aprepitant.
“The importance of pruritus in the cancer population is going to increase as targeted therapies enter the adjuvant setting,” Dr. Lacouture remarked. Also important, patients on targeted therapies are living longer, “and as patients live longer, more attention is being placed on quality of life issues of which pruritus is one of the topmost concerns.”
Dr. Lacouture reports financial relationships with Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Foamix, Galderma Laboratories, Hana Biosciences, Imclone, Merck Serono, Novartis, Novocure, OSI Pharma, Pfizer, Pierre Fabre Dermatologie, Reata Pharmaceuticals, Roche Laboratories, Threshold Pharmaceuticals, and Vertex Pharmaceuticals.
NEW YORK – Due to the frequency with which patients develop pruritus while on a targeted cancer therapy, prophylaxis should be strongly considered for at least some of the drugs, such as epidermal growth factor–receptor (EGFR) inhibitors, according to an expert in oncodermatology.
“Pruritus is a frequent adverse event in cancer patients treated with targeted therapies and an early and proactive approach towards pruritus is advisable,” Dr. Mario E. Lacouture, director of the oncodermatology program at New York’s Memorial Sloan Kettering Cancer Center. The increasing use of targeted therapies, including the growing proportion of patients on long-term maintenance regimens, is expected to make these complaints more common.
Targeted cancer therapies, such as monoclonal antibodies and tyrosine kinase inhibitors (TKIs), are, in general, associated with a low risk of adverse events relative to cytotoxic chemotherapies. The exception is dermatologic adverse events, Dr. Lacouture said at the American Academy of Dermatology summer meeting. Skin rashes are characteristic adverse events with several targeted agents, such as the EGFR inhibitor cetuximab, but Dr. Lacouture warned that pruritus for many patients imposes the greatest burden.
“Since the introduction of the first targeted cancer drug, imatinib, in 2001, a long list of targeted agents have been approved, and all are associated with pruritus that significantly reduces quality of life,” he reported. Dermatologists need to fill a void.
“Oncologists are not generally familiar with strategies to treat pruritus and typically resort to antihistamines, such as diphenhydramine or hydroxyzine,” Dr. Lacouture added. He suggested such sedative agents “are not ideal” when managing an adverse event that may persist for weeks or months.
The first step may simply be to prepare patients initiating targeted therapy for the substantial risk of pruritus. Dr. Lacouture cited two studies in which cancer patients were asked about unexpected side effects. In both, dermatologic complaints were the most common.
“Patients are told that they are going to lose their hair, that they are going to have nausea and vomiting, but they are never told that they are going to have dry skin, irritated skin, or itchy skin,” Dr. Lacouture noted. He cited one study in which all three of the top side effects identified by cancer patients as unexpected were dermatologic, including nail changes and pruritus.
In fact, published studies suggest that most patients treated with EGFR inhibitors, and approximately one-third of patients treated with a variety of TKIs, such as those targeting BRAF and MEK pathways, will develop significant pruritus, according to Dr. Lacouture. However, several studies, including one of his own, suggest that this itching can be greatly mitigated not only by treatment but also with prophylaxis.
In his study of the effect of prophylaxis, 95 patients initiating the EGFR inhibitor panitumumab were randomized to a prophylactic regimen that included skin moisturizers, sunscreen, a topical steroid, and doxycycline, or to treatment adjusted for symptoms once they developed (J Clin Oncol. 2010;28:1351-7). At 6 weeks, the proportion of patients with grade 2 or higher dermatologic events, including pruritus, was reduced from 62% to 29%. More favorable quality of life scores in the prophylactic regimen group supported the advantage.
“When you looked at grade 3 or higher adverse events, you see that the incidence of acneiform rash [at the grade 3 level of severity] was lowered by almost 75% and pruritus was almost abolished,” Dr. Lacouture reported.
For refractory pruritus related to targeted therapy, aprepitant may be the best option based on Dr. Lacouture’s own experience and a prospective but nonrandomized study (Lancet Oncol. 2012;13:1020-4). In the published study, which included patients on EGFR inhibitors or TKIs, the median visual analog scores (VAS) for pruritus fell from 8 before treatment to 1, 1 week later. According to Dr. Lacouture, the treatment effects are durable. In some cases, pruritus does not recur even after a single course of aprepitant.
“The importance of pruritus in the cancer population is going to increase as targeted therapies enter the adjuvant setting,” Dr. Lacouture remarked. Also important, patients on targeted therapies are living longer, “and as patients live longer, more attention is being placed on quality of life issues of which pruritus is one of the topmost concerns.”
Dr. Lacouture reports financial relationships with Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Foamix, Galderma Laboratories, Hana Biosciences, Imclone, Merck Serono, Novartis, Novocure, OSI Pharma, Pfizer, Pierre Fabre Dermatologie, Reata Pharmaceuticals, Roche Laboratories, Threshold Pharmaceuticals, and Vertex Pharmaceuticals.
NEW YORK – Due to the frequency with which patients develop pruritus while on a targeted cancer therapy, prophylaxis should be strongly considered for at least some of the drugs, such as epidermal growth factor–receptor (EGFR) inhibitors, according to an expert in oncodermatology.
“Pruritus is a frequent adverse event in cancer patients treated with targeted therapies and an early and proactive approach towards pruritus is advisable,” Dr. Mario E. Lacouture, director of the oncodermatology program at New York’s Memorial Sloan Kettering Cancer Center. The increasing use of targeted therapies, including the growing proportion of patients on long-term maintenance regimens, is expected to make these complaints more common.
Targeted cancer therapies, such as monoclonal antibodies and tyrosine kinase inhibitors (TKIs), are, in general, associated with a low risk of adverse events relative to cytotoxic chemotherapies. The exception is dermatologic adverse events, Dr. Lacouture said at the American Academy of Dermatology summer meeting. Skin rashes are characteristic adverse events with several targeted agents, such as the EGFR inhibitor cetuximab, but Dr. Lacouture warned that pruritus for many patients imposes the greatest burden.
“Since the introduction of the first targeted cancer drug, imatinib, in 2001, a long list of targeted agents have been approved, and all are associated with pruritus that significantly reduces quality of life,” he reported. Dermatologists need to fill a void.
“Oncologists are not generally familiar with strategies to treat pruritus and typically resort to antihistamines, such as diphenhydramine or hydroxyzine,” Dr. Lacouture added. He suggested such sedative agents “are not ideal” when managing an adverse event that may persist for weeks or months.
The first step may simply be to prepare patients initiating targeted therapy for the substantial risk of pruritus. Dr. Lacouture cited two studies in which cancer patients were asked about unexpected side effects. In both, dermatologic complaints were the most common.
“Patients are told that they are going to lose their hair, that they are going to have nausea and vomiting, but they are never told that they are going to have dry skin, irritated skin, or itchy skin,” Dr. Lacouture noted. He cited one study in which all three of the top side effects identified by cancer patients as unexpected were dermatologic, including nail changes and pruritus.
In fact, published studies suggest that most patients treated with EGFR inhibitors, and approximately one-third of patients treated with a variety of TKIs, such as those targeting BRAF and MEK pathways, will develop significant pruritus, according to Dr. Lacouture. However, several studies, including one of his own, suggest that this itching can be greatly mitigated not only by treatment but also with prophylaxis.
In his study of the effect of prophylaxis, 95 patients initiating the EGFR inhibitor panitumumab were randomized to a prophylactic regimen that included skin moisturizers, sunscreen, a topical steroid, and doxycycline, or to treatment adjusted for symptoms once they developed (J Clin Oncol. 2010;28:1351-7). At 6 weeks, the proportion of patients with grade 2 or higher dermatologic events, including pruritus, was reduced from 62% to 29%. More favorable quality of life scores in the prophylactic regimen group supported the advantage.
“When you looked at grade 3 or higher adverse events, you see that the incidence of acneiform rash [at the grade 3 level of severity] was lowered by almost 75% and pruritus was almost abolished,” Dr. Lacouture reported.
For refractory pruritus related to targeted therapy, aprepitant may be the best option based on Dr. Lacouture’s own experience and a prospective but nonrandomized study (Lancet Oncol. 2012;13:1020-4). In the published study, which included patients on EGFR inhibitors or TKIs, the median visual analog scores (VAS) for pruritus fell from 8 before treatment to 1, 1 week later. According to Dr. Lacouture, the treatment effects are durable. In some cases, pruritus does not recur even after a single course of aprepitant.
“The importance of pruritus in the cancer population is going to increase as targeted therapies enter the adjuvant setting,” Dr. Lacouture remarked. Also important, patients on targeted therapies are living longer, “and as patients live longer, more attention is being placed on quality of life issues of which pruritus is one of the topmost concerns.”
Dr. Lacouture reports financial relationships with Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Foamix, Galderma Laboratories, Hana Biosciences, Imclone, Merck Serono, Novartis, Novocure, OSI Pharma, Pfizer, Pierre Fabre Dermatologie, Reata Pharmaceuticals, Roche Laboratories, Threshold Pharmaceuticals, and Vertex Pharmaceuticals.
EXPERT ANALYSIS FROM THE AAD SUMMER ACADEMY 2015