User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Rise of ‘alarming’ subvariants of COVID ‘worrisome’ for winter
It’s a story perhaps more appropriate for Halloween than for the festive holiday season, given its scary implications.
Not too dire so far, until the researchers’ other findings are considered.
The BQ.1, BQ1.1, XBB, and XBB.1 subvariants are the most resistant to neutralizing antibodies, researcher Qian Wang, PhD, and colleagues wrote in a study published online in the journal Cell. This means people have no or “markedly reduced” protection against infection from these four strains, even if they’ve already had COVID-19 or are vaccinated and boosted multiple times, including with a bivalent vaccine.
On top of that, all available monoclonal antibody treatments are mostly or completely ineffective against these subvariants.
What does that mean for the immediate future? The findings are definitely “worrisome,” said Eric Topol, MD, founder and director of the Scripps Translational Research Institute in La Jolla, Calif.
But evidence from other countries, specifically Singapore and France, show that at least two of these variants turned out not to be as damaging as expected, likely because of high numbers of people vaccinated or who survived previous infections, he said.
Still, there is little to celebrate in the new findings, except that COVID-19 vaccinations and prior infections can still reduce the risk for serious outcomes such as hospitalization and death, the researchers wrote.
In fact, Centers for Disease Control and Prevention data released on Dec. 16 shows that people who have received four shots of the original COVID-19 vaccines as well as the bivalent booster were 57% less likely to visit an urgent care clinic or emergency room, regardless of age.
It comes at a time when BQ.1 and BQ.1.1 account for about 70% of the circulating variants, data show. In addition, hospitalizations are up 18% over the past 2 weeks and COVID-19 deaths are up 50% nationwide, The New York Times reported.
Globally, in many places, an “immunity wall” that has been built, Dr. Topol said. That may not be the case in the United States.
“The problem in the United States, making it harder to predict, is that we have a very low rate of recent boosters, in the past 6 months, especially in seniors,” he said. For example, only 36% of Americans aged 65 years and older, the group with highest risk, have received an updated bivalent booster.
An evolving virus
The subvariants are successfully replacing BA.5, which reigned as one of the most common Omicron variants over the past year. The latest CDC data show that BA.5 now accounts for only about 10% of the circulating virus. The researchers wrote: “This rapid replacement of virus strains is raising the specter of yet another wave of infections in the coming months.”
BQ.1 and BQ.1.1 evolved directly from BA.5 – adding more and some novel mutations to the SARS-CoV-2 virus. XBB and XBB.1 are the “offspring” of a combination of two other strains, known as BJ.1 and BA.2.75.
The story sounds familiar to the researchers. “The rapid rise of these subvariants and their extensive array of spike mutations are reminiscent of the appearance of the first Omicron variant last year, thus raising concerns that they may further compromise the efficacy of current COVID-19 vaccines and monoclonal antibody therapeutics,” they wrote. “We now report findings that indicate that such concerns are, sadly, justified, especially so for the XBB and XBB.1 subvariants.”
To figure out how effective existing antibodies could be against these newer subvariants, Dr. Wang and colleagues used blood samples from five groups of people. They tested serum from people who had three doses of the original COVID-19 vaccine, four doses of the original vaccine, those who received a bivalent booster, people who experienced a breakthrough infection with the BA.2 Omicron variant, and those who had a breakthrough with a BA.4 or BA.5 variant.
Adding the new subvariants to these serum samples revealed that the existing antibodies in the blood were ineffective at wiping out or neutralizing BQ.1, BQ.1.1, XBB, and XBB.1.
The BQ.1 subvariant was six times more resistant to antibodies than BA.5, its parent strain, and XBB.1 was 63 times more resistant compared with its predecessor, BA.2.
This shift in the ability of vaccines to stop the subvariants “is particularly concerning,” the researchers wrote.
Wiping out treatments too
Dr. Wang and colleagues also tested how well a panel of 23 different monoclonal antibody drugs might work against the four subvariants. The therapies all worked well against the original Omicron variant and included some approved for use through the Food and Drug Administration emergency use authorization (EUA) program at the time of the study.
They found that 19 of these 23 monoclonal antibodies lost effectiveness “greatly or completely” against XBB and XBB.1, for example.
This is not the first time that monoclonal antibody therapies have gone from effective to ineffective. Previous variants have come out that no longer responded to treatment with bamlanivimab, etesevimab, imdevimab, casirivimab, tixagevimab, cilgavimab, and sotrovimab. Bebtelovimab now joins this list and is no longer available from Eli Lilly under EUA because of this lack of effectiveness.
The lack of an effective monoclonal antibody treatment “poses a serious problem for millions of immunocompromised individuals who do not respond robustly to COVID-19 vaccines,” the researchers wrote, adding that “the urgent need to develop active monoclonal antibodies for clinical use is obvious.”
A limitation of the study is that the work is done in blood samples. The effectiveness of COVID-19 vaccination against the BQ and XBB subvariants should be evaluated in people in clinical studies, the authors noted.
Also, the current study looked at how well antibodies could neutralize the viral strains, but future research, they added, should look at how well “cellular immunity” or other aspects of the immune system might protect people.
Going forward, the challenge remains to develop vaccines and treatments that offer broad protection as the coronavirus continues to evolve.
In an alarming ending, the researchers wrote: “We have collectively chased after SARS-CoV-2 variants for over 2 years, and yet, the virus continues to evolve and evade.”
A version of this article first appeared on Medscape.com.
It’s a story perhaps more appropriate for Halloween than for the festive holiday season, given its scary implications.
Not too dire so far, until the researchers’ other findings are considered.
The BQ.1, BQ1.1, XBB, and XBB.1 subvariants are the most resistant to neutralizing antibodies, researcher Qian Wang, PhD, and colleagues wrote in a study published online in the journal Cell. This means people have no or “markedly reduced” protection against infection from these four strains, even if they’ve already had COVID-19 or are vaccinated and boosted multiple times, including with a bivalent vaccine.
On top of that, all available monoclonal antibody treatments are mostly or completely ineffective against these subvariants.
What does that mean for the immediate future? The findings are definitely “worrisome,” said Eric Topol, MD, founder and director of the Scripps Translational Research Institute in La Jolla, Calif.
But evidence from other countries, specifically Singapore and France, show that at least two of these variants turned out not to be as damaging as expected, likely because of high numbers of people vaccinated or who survived previous infections, he said.
Still, there is little to celebrate in the new findings, except that COVID-19 vaccinations and prior infections can still reduce the risk for serious outcomes such as hospitalization and death, the researchers wrote.
In fact, Centers for Disease Control and Prevention data released on Dec. 16 shows that people who have received four shots of the original COVID-19 vaccines as well as the bivalent booster were 57% less likely to visit an urgent care clinic or emergency room, regardless of age.
It comes at a time when BQ.1 and BQ.1.1 account for about 70% of the circulating variants, data show. In addition, hospitalizations are up 18% over the past 2 weeks and COVID-19 deaths are up 50% nationwide, The New York Times reported.
Globally, in many places, an “immunity wall” that has been built, Dr. Topol said. That may not be the case in the United States.
“The problem in the United States, making it harder to predict, is that we have a very low rate of recent boosters, in the past 6 months, especially in seniors,” he said. For example, only 36% of Americans aged 65 years and older, the group with highest risk, have received an updated bivalent booster.
An evolving virus
The subvariants are successfully replacing BA.5, which reigned as one of the most common Omicron variants over the past year. The latest CDC data show that BA.5 now accounts for only about 10% of the circulating virus. The researchers wrote: “This rapid replacement of virus strains is raising the specter of yet another wave of infections in the coming months.”
BQ.1 and BQ.1.1 evolved directly from BA.5 – adding more and some novel mutations to the SARS-CoV-2 virus. XBB and XBB.1 are the “offspring” of a combination of two other strains, known as BJ.1 and BA.2.75.
The story sounds familiar to the researchers. “The rapid rise of these subvariants and their extensive array of spike mutations are reminiscent of the appearance of the first Omicron variant last year, thus raising concerns that they may further compromise the efficacy of current COVID-19 vaccines and monoclonal antibody therapeutics,” they wrote. “We now report findings that indicate that such concerns are, sadly, justified, especially so for the XBB and XBB.1 subvariants.”
To figure out how effective existing antibodies could be against these newer subvariants, Dr. Wang and colleagues used blood samples from five groups of people. They tested serum from people who had three doses of the original COVID-19 vaccine, four doses of the original vaccine, those who received a bivalent booster, people who experienced a breakthrough infection with the BA.2 Omicron variant, and those who had a breakthrough with a BA.4 or BA.5 variant.
Adding the new subvariants to these serum samples revealed that the existing antibodies in the blood were ineffective at wiping out or neutralizing BQ.1, BQ.1.1, XBB, and XBB.1.
The BQ.1 subvariant was six times more resistant to antibodies than BA.5, its parent strain, and XBB.1 was 63 times more resistant compared with its predecessor, BA.2.
This shift in the ability of vaccines to stop the subvariants “is particularly concerning,” the researchers wrote.
Wiping out treatments too
Dr. Wang and colleagues also tested how well a panel of 23 different monoclonal antibody drugs might work against the four subvariants. The therapies all worked well against the original Omicron variant and included some approved for use through the Food and Drug Administration emergency use authorization (EUA) program at the time of the study.
They found that 19 of these 23 monoclonal antibodies lost effectiveness “greatly or completely” against XBB and XBB.1, for example.
This is not the first time that monoclonal antibody therapies have gone from effective to ineffective. Previous variants have come out that no longer responded to treatment with bamlanivimab, etesevimab, imdevimab, casirivimab, tixagevimab, cilgavimab, and sotrovimab. Bebtelovimab now joins this list and is no longer available from Eli Lilly under EUA because of this lack of effectiveness.
The lack of an effective monoclonal antibody treatment “poses a serious problem for millions of immunocompromised individuals who do not respond robustly to COVID-19 vaccines,” the researchers wrote, adding that “the urgent need to develop active monoclonal antibodies for clinical use is obvious.”
A limitation of the study is that the work is done in blood samples. The effectiveness of COVID-19 vaccination against the BQ and XBB subvariants should be evaluated in people in clinical studies, the authors noted.
Also, the current study looked at how well antibodies could neutralize the viral strains, but future research, they added, should look at how well “cellular immunity” or other aspects of the immune system might protect people.
Going forward, the challenge remains to develop vaccines and treatments that offer broad protection as the coronavirus continues to evolve.
In an alarming ending, the researchers wrote: “We have collectively chased after SARS-CoV-2 variants for over 2 years, and yet, the virus continues to evolve and evade.”
A version of this article first appeared on Medscape.com.
It’s a story perhaps more appropriate for Halloween than for the festive holiday season, given its scary implications.
Not too dire so far, until the researchers’ other findings are considered.
The BQ.1, BQ1.1, XBB, and XBB.1 subvariants are the most resistant to neutralizing antibodies, researcher Qian Wang, PhD, and colleagues wrote in a study published online in the journal Cell. This means people have no or “markedly reduced” protection against infection from these four strains, even if they’ve already had COVID-19 or are vaccinated and boosted multiple times, including with a bivalent vaccine.
On top of that, all available monoclonal antibody treatments are mostly or completely ineffective against these subvariants.
What does that mean for the immediate future? The findings are definitely “worrisome,” said Eric Topol, MD, founder and director of the Scripps Translational Research Institute in La Jolla, Calif.
But evidence from other countries, specifically Singapore and France, show that at least two of these variants turned out not to be as damaging as expected, likely because of high numbers of people vaccinated or who survived previous infections, he said.
Still, there is little to celebrate in the new findings, except that COVID-19 vaccinations and prior infections can still reduce the risk for serious outcomes such as hospitalization and death, the researchers wrote.
In fact, Centers for Disease Control and Prevention data released on Dec. 16 shows that people who have received four shots of the original COVID-19 vaccines as well as the bivalent booster were 57% less likely to visit an urgent care clinic or emergency room, regardless of age.
It comes at a time when BQ.1 and BQ.1.1 account for about 70% of the circulating variants, data show. In addition, hospitalizations are up 18% over the past 2 weeks and COVID-19 deaths are up 50% nationwide, The New York Times reported.
Globally, in many places, an “immunity wall” that has been built, Dr. Topol said. That may not be the case in the United States.
“The problem in the United States, making it harder to predict, is that we have a very low rate of recent boosters, in the past 6 months, especially in seniors,” he said. For example, only 36% of Americans aged 65 years and older, the group with highest risk, have received an updated bivalent booster.
An evolving virus
The subvariants are successfully replacing BA.5, which reigned as one of the most common Omicron variants over the past year. The latest CDC data show that BA.5 now accounts for only about 10% of the circulating virus. The researchers wrote: “This rapid replacement of virus strains is raising the specter of yet another wave of infections in the coming months.”
BQ.1 and BQ.1.1 evolved directly from BA.5 – adding more and some novel mutations to the SARS-CoV-2 virus. XBB and XBB.1 are the “offspring” of a combination of two other strains, known as BJ.1 and BA.2.75.
The story sounds familiar to the researchers. “The rapid rise of these subvariants and their extensive array of spike mutations are reminiscent of the appearance of the first Omicron variant last year, thus raising concerns that they may further compromise the efficacy of current COVID-19 vaccines and monoclonal antibody therapeutics,” they wrote. “We now report findings that indicate that such concerns are, sadly, justified, especially so for the XBB and XBB.1 subvariants.”
To figure out how effective existing antibodies could be against these newer subvariants, Dr. Wang and colleagues used blood samples from five groups of people. They tested serum from people who had three doses of the original COVID-19 vaccine, four doses of the original vaccine, those who received a bivalent booster, people who experienced a breakthrough infection with the BA.2 Omicron variant, and those who had a breakthrough with a BA.4 or BA.5 variant.
Adding the new subvariants to these serum samples revealed that the existing antibodies in the blood were ineffective at wiping out or neutralizing BQ.1, BQ.1.1, XBB, and XBB.1.
The BQ.1 subvariant was six times more resistant to antibodies than BA.5, its parent strain, and XBB.1 was 63 times more resistant compared with its predecessor, BA.2.
This shift in the ability of vaccines to stop the subvariants “is particularly concerning,” the researchers wrote.
Wiping out treatments too
Dr. Wang and colleagues also tested how well a panel of 23 different monoclonal antibody drugs might work against the four subvariants. The therapies all worked well against the original Omicron variant and included some approved for use through the Food and Drug Administration emergency use authorization (EUA) program at the time of the study.
They found that 19 of these 23 monoclonal antibodies lost effectiveness “greatly or completely” against XBB and XBB.1, for example.
This is not the first time that monoclonal antibody therapies have gone from effective to ineffective. Previous variants have come out that no longer responded to treatment with bamlanivimab, etesevimab, imdevimab, casirivimab, tixagevimab, cilgavimab, and sotrovimab. Bebtelovimab now joins this list and is no longer available from Eli Lilly under EUA because of this lack of effectiveness.
The lack of an effective monoclonal antibody treatment “poses a serious problem for millions of immunocompromised individuals who do not respond robustly to COVID-19 vaccines,” the researchers wrote, adding that “the urgent need to develop active monoclonal antibodies for clinical use is obvious.”
A limitation of the study is that the work is done in blood samples. The effectiveness of COVID-19 vaccination against the BQ and XBB subvariants should be evaluated in people in clinical studies, the authors noted.
Also, the current study looked at how well antibodies could neutralize the viral strains, but future research, they added, should look at how well “cellular immunity” or other aspects of the immune system might protect people.
Going forward, the challenge remains to develop vaccines and treatments that offer broad protection as the coronavirus continues to evolve.
In an alarming ending, the researchers wrote: “We have collectively chased after SARS-CoV-2 variants for over 2 years, and yet, the virus continues to evolve and evade.”
A version of this article first appeared on Medscape.com.
FROM CELL
What length antibiotic course for prostatitis?
PARIS – To date, studies of antibiotic course length for treating urinary tract infections in men have been patchy and retrospective.
Through recent randomized trials, guidelines can now be based on more solid data.
In sum, to maximize clinical and microbiologic success, a nonfebrile urinary tract infection is treated for 7 days, and a febrile urinary tract infection is treated for a minimum of 14 days.
At the 116th conference of the French urology association, Matthieu Lafaurie, MD, of the Multidisciplinary Infectious Diseases Unit U21, Saint Louis Hospital, Paris, reviewed the literature on this subject.
Guidelines for men
The European Association of Urology made its position clear in a text updated in 2022. It stated: Therefore, treatment with antimicrobial drugs that penetrate the prostate tissue is needed in men presenting with symptoms of a urinary tract infection.” In its classification of prostatitis, the National Institutes of Health distinguishes between acute prostatitis (symptoms of a urinary tract infection; stage I) and chronic prostatitis (recurrent infection with the same microorganism; stage II).
Although the French-language Society of Infectious Diseases distinguishes between febrile and nonfebrile urinary tract infections in males, the academic body does not take into account whether the patient has a fever when determining which antibiotic should be given and how long the course should be: A minimum of 14 days’ treatment is recommended when opting for fluoroquinolones, trimethoprim-sulfamethoxazole (cotrimoxazole), or injectable beta-lactam antibiotics, and at least 21 days is recommended for other drugs or in cases in which there is an underlying urologic condition that has not been treated.
Yet the EAU recommends treating cystitis with antibiotics for at least 7 days, preferably with cotrimoxazole or fluoroquinolone, depending on the results of sensitivity testing. For acute prostatitis, the length of treatment with fluoroquinolones should be at least 14 days.
Nonfebrile infections
Participation of men in studies of the treatment of complicated cystitis is variable; at most only 10% of patients in such trials are men. There are few data specific to men with nonfebrile urinary tract infections, and most studies are retrospective and involve small cohorts. One of these is a community-based study that involved 422 men aged 18-104 years who presented with nonfebrile urinary tract infection (acute dysuria, frequency of urination and/or urgency of urination, temperature < 38° C, no general symptoms). Antibiotic treatment was prescribed in 60% of cases. In more than 55% of cases, the length of the course of treatment was 1–7 days. Treatment was with cotrimoxazole, quinolones, and nitrofurantoin.
Another observational retrospective study showed benefit with nitrofurantoin (50 mg/8 h in 94% of cases; 69 patients) and pivmecillinam (200 mg/8 h in 65% of cases; 200 mg/12 h in 30% of patients; 57 patients) in treating lower urinary tract infections in men. The median treatment duration was 7 days. The failure rate was 1.4% and 12%, respectively, for these treatments. Compared to the so-called gold-standard treatment, trimethoprim (10 days/800 mg/12 h; 45 patients), the recurrence rate was 11% and 26% for nitrofurantoin and pivmecillinam versus 7% for trimethoprim. The most significant relapse rate with pivmecillinam was when treatment was given for fewer than 7 days.
This is the only risk factor for further antibiotic treatment and/or recurrence. There was no significant difference between the three drugs with regard to other parameters (urinary tract infection symptoms, benign prostatic hypertrophy, prostate cancer, gram-positive bacteria, etc).
Another retrospective, European study of nitrofurantoin that was published in 2015 included 485 patients (100 mg twice daily in 71% of cases). Clinical cure was defined as an absence of signs or symptoms of a urinary tract infection for 14 days after stopping nitrofurantoin, without use of other antibiotics. The cure rate was 77%. Better efficacy was achieved for patients with gram-negative (vs. gram-positive) bacteria. The treatment duration did not differ significantly (clinical success was achieved when the treatment was taken for 8.6 ± 3.6 days; clinical failure occurred when the treatment was taken for 9.3 ± 6.9 days; P = .28).
Regarding pivmecillinam, a retrospective 2010-2016 study involved 21,864 adults and included 2,524 men who had been treated empirically with pivmecillinam (400 mg three times daily) for significant bacteriuria (Escherichia coli) and a lower urinary tract infection. The researchers concluded that for men, the success rate was identical whether the treatment lasted 5 or 7 days.
An American community-based (urologists, primary care physicians, general medicine services) retrospective cohort study involving 573 men with nonfebrile lower urinary tract infections was conducted from 2011 to 2015. The patients received antibiotic treatment with fluoroquinolones (69.7%), cotrimoxazole (21.2%), nitrofurantoin (5.3%), trimethoprim, beta-lactam antibiotics, or aminoglycosides. No clinical advantage was seen in treating men with urinary tract infections for longer than 7 days.
There are some data on the use of fosfomycin. In an observational retrospective study, 25 men of 52 male adults with leukocyturia and E. coli greater than 105, ESBL, were treated with fosfomycin trometamol 3 g on days 1, 3, 5. Clinical and microbiologic success was achieved for 94% and 78.5%, respectively. No distinction was made between the sexes.
These results were confirmed in a retrospective, observational study involving 18 men (of a total of 75 adults) with no fever or hyperleukocytosis who received the same fosfomycin trometamol regimen. The rate of clinical cure or sterile urine microscopy and culture was 69% at 13 days. The risk failure factor was, as expected, infection with Klebsiella pneumoniae, which was slightly susceptible to fosfomycin, unlike E. coli.
The most recent study in this field was published in 2021. It was also the first randomized, double-blind, placebo-controlled study. In all, 272 men older than 18 years were prescribed either ciprofloxacin or cotrimoxazole for 7-14 days to treat a nonfebrile urinary tract infection. To be eligible for the trial, patients were required to have disease of new onset with at least one of the following symptoms: dysuria, frequency of urination, urgency of urination, hematuria, costovertebral angle tenderness, or perineal, flank, or suprapubic pain. Urine microscopy and culture were not necessary; the approach was wholly symptomatic. Treatment was prescribed for 7 days. Patients were randomly allocated on day 8 to receive treatment for the following 7 days (molecule or placebo). The primary outcome was resolution of clinical symptoms of urinary tract infection by 14 days after completion of active antibiotic treatment. In an intention-to-treat or per-protocol analysis, the difference in efficacy between the two molecules was largely below the required 10%. The treatment duration noninferiority margin was 7 days, compared with 14 days.
“In 2022, with regard to the duration of treatment of nonfebrile urinary tract infections in men, the not completely irrefutable evidence does, however, stack up in favor of the possibility of a 7-day or even 5-day course,” pointed out Dr. Lafaurie. “Fluoroquinolones [such as] ofloxacin, levofloxacin, ciprofloxacin, as well as cotrimoxazole and other antibiotics, such as pivmecillinam, nitrofurantoin, or fosfomycin trometamol, can be used, despite the fact that they pass less easily into the prostate – a not-so-obvious benefit.”
Febrile infections
In terms of febrile urinary tract infections, a single-center, prospective, open-label study from 2003 involved 72 male inpatients who were randomly to receive treatment either for 2 weeks or 4 weeks. Treatment consisted of ciprofloxacin 500 mg twice daily. This study provided most of the evidence to justify the recommended 14-day antibiotic course.
Another noninferiority, randomized, placebo-controlled study published in 2017 compared 7- and 14-day treatment with ciprofloxacin 500 mg to placebo twice per week. In men, 7 days of antibiotic therapy was inferior to 14 days during a short-term follow-up but was not inferior during a longer follow-up.
A decisive study, which is currently in the submission phase, could silence debate. “In our noninferiority, multicenter, randomized, double-blind, placebo-controlled study, we have enrolled 240 men over the age of 18 years with a febrile infection documented by a fever of 38° C or more, clinical signs of infection, and leukocyturia at least above 10/mm3 and with symptoms lasting less than 3 months,” said Dr. Lafaurie, the trial coordinator.
The primary outcome for efficacy was microbiologic and clinical success after 6 weeks. Patients received either ofloxacin, ceftriaxone, or cefotaxime (two third-generation cephalosporins in the beta-lactam family).
“We clearly show that, for a 7-day course, the clinical success rate is 55.7%, and for a 14-day course, this goes up to 77.6%, with no difference in terms of adverse effects or selection of resistant bacteria. The predictive factors for success are a 14-day treatment and being under the age of 50 years,” said Dr. Lafaurie.
“Unlike nonfebrile urinary tract infections in men, a 7-day course is insufficient for patients with febrile urinary tract infections, and a minimum of 14 days is required to achieve clinical and microbiological success,” he concluded.
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
PARIS – To date, studies of antibiotic course length for treating urinary tract infections in men have been patchy and retrospective.
Through recent randomized trials, guidelines can now be based on more solid data.
In sum, to maximize clinical and microbiologic success, a nonfebrile urinary tract infection is treated for 7 days, and a febrile urinary tract infection is treated for a minimum of 14 days.
At the 116th conference of the French urology association, Matthieu Lafaurie, MD, of the Multidisciplinary Infectious Diseases Unit U21, Saint Louis Hospital, Paris, reviewed the literature on this subject.
Guidelines for men
The European Association of Urology made its position clear in a text updated in 2022. It stated: Therefore, treatment with antimicrobial drugs that penetrate the prostate tissue is needed in men presenting with symptoms of a urinary tract infection.” In its classification of prostatitis, the National Institutes of Health distinguishes between acute prostatitis (symptoms of a urinary tract infection; stage I) and chronic prostatitis (recurrent infection with the same microorganism; stage II).
Although the French-language Society of Infectious Diseases distinguishes between febrile and nonfebrile urinary tract infections in males, the academic body does not take into account whether the patient has a fever when determining which antibiotic should be given and how long the course should be: A minimum of 14 days’ treatment is recommended when opting for fluoroquinolones, trimethoprim-sulfamethoxazole (cotrimoxazole), or injectable beta-lactam antibiotics, and at least 21 days is recommended for other drugs or in cases in which there is an underlying urologic condition that has not been treated.
Yet the EAU recommends treating cystitis with antibiotics for at least 7 days, preferably with cotrimoxazole or fluoroquinolone, depending on the results of sensitivity testing. For acute prostatitis, the length of treatment with fluoroquinolones should be at least 14 days.
Nonfebrile infections
Participation of men in studies of the treatment of complicated cystitis is variable; at most only 10% of patients in such trials are men. There are few data specific to men with nonfebrile urinary tract infections, and most studies are retrospective and involve small cohorts. One of these is a community-based study that involved 422 men aged 18-104 years who presented with nonfebrile urinary tract infection (acute dysuria, frequency of urination and/or urgency of urination, temperature < 38° C, no general symptoms). Antibiotic treatment was prescribed in 60% of cases. In more than 55% of cases, the length of the course of treatment was 1–7 days. Treatment was with cotrimoxazole, quinolones, and nitrofurantoin.
Another observational retrospective study showed benefit with nitrofurantoin (50 mg/8 h in 94% of cases; 69 patients) and pivmecillinam (200 mg/8 h in 65% of cases; 200 mg/12 h in 30% of patients; 57 patients) in treating lower urinary tract infections in men. The median treatment duration was 7 days. The failure rate was 1.4% and 12%, respectively, for these treatments. Compared to the so-called gold-standard treatment, trimethoprim (10 days/800 mg/12 h; 45 patients), the recurrence rate was 11% and 26% for nitrofurantoin and pivmecillinam versus 7% for trimethoprim. The most significant relapse rate with pivmecillinam was when treatment was given for fewer than 7 days.
This is the only risk factor for further antibiotic treatment and/or recurrence. There was no significant difference between the three drugs with regard to other parameters (urinary tract infection symptoms, benign prostatic hypertrophy, prostate cancer, gram-positive bacteria, etc).
Another retrospective, European study of nitrofurantoin that was published in 2015 included 485 patients (100 mg twice daily in 71% of cases). Clinical cure was defined as an absence of signs or symptoms of a urinary tract infection for 14 days after stopping nitrofurantoin, without use of other antibiotics. The cure rate was 77%. Better efficacy was achieved for patients with gram-negative (vs. gram-positive) bacteria. The treatment duration did not differ significantly (clinical success was achieved when the treatment was taken for 8.6 ± 3.6 days; clinical failure occurred when the treatment was taken for 9.3 ± 6.9 days; P = .28).
Regarding pivmecillinam, a retrospective 2010-2016 study involved 21,864 adults and included 2,524 men who had been treated empirically with pivmecillinam (400 mg three times daily) for significant bacteriuria (Escherichia coli) and a lower urinary tract infection. The researchers concluded that for men, the success rate was identical whether the treatment lasted 5 or 7 days.
An American community-based (urologists, primary care physicians, general medicine services) retrospective cohort study involving 573 men with nonfebrile lower urinary tract infections was conducted from 2011 to 2015. The patients received antibiotic treatment with fluoroquinolones (69.7%), cotrimoxazole (21.2%), nitrofurantoin (5.3%), trimethoprim, beta-lactam antibiotics, or aminoglycosides. No clinical advantage was seen in treating men with urinary tract infections for longer than 7 days.
There are some data on the use of fosfomycin. In an observational retrospective study, 25 men of 52 male adults with leukocyturia and E. coli greater than 105, ESBL, were treated with fosfomycin trometamol 3 g on days 1, 3, 5. Clinical and microbiologic success was achieved for 94% and 78.5%, respectively. No distinction was made between the sexes.
These results were confirmed in a retrospective, observational study involving 18 men (of a total of 75 adults) with no fever or hyperleukocytosis who received the same fosfomycin trometamol regimen. The rate of clinical cure or sterile urine microscopy and culture was 69% at 13 days. The risk failure factor was, as expected, infection with Klebsiella pneumoniae, which was slightly susceptible to fosfomycin, unlike E. coli.
The most recent study in this field was published in 2021. It was also the first randomized, double-blind, placebo-controlled study. In all, 272 men older than 18 years were prescribed either ciprofloxacin or cotrimoxazole for 7-14 days to treat a nonfebrile urinary tract infection. To be eligible for the trial, patients were required to have disease of new onset with at least one of the following symptoms: dysuria, frequency of urination, urgency of urination, hematuria, costovertebral angle tenderness, or perineal, flank, or suprapubic pain. Urine microscopy and culture were not necessary; the approach was wholly symptomatic. Treatment was prescribed for 7 days. Patients were randomly allocated on day 8 to receive treatment for the following 7 days (molecule or placebo). The primary outcome was resolution of clinical symptoms of urinary tract infection by 14 days after completion of active antibiotic treatment. In an intention-to-treat or per-protocol analysis, the difference in efficacy between the two molecules was largely below the required 10%. The treatment duration noninferiority margin was 7 days, compared with 14 days.
“In 2022, with regard to the duration of treatment of nonfebrile urinary tract infections in men, the not completely irrefutable evidence does, however, stack up in favor of the possibility of a 7-day or even 5-day course,” pointed out Dr. Lafaurie. “Fluoroquinolones [such as] ofloxacin, levofloxacin, ciprofloxacin, as well as cotrimoxazole and other antibiotics, such as pivmecillinam, nitrofurantoin, or fosfomycin trometamol, can be used, despite the fact that they pass less easily into the prostate – a not-so-obvious benefit.”
Febrile infections
In terms of febrile urinary tract infections, a single-center, prospective, open-label study from 2003 involved 72 male inpatients who were randomly to receive treatment either for 2 weeks or 4 weeks. Treatment consisted of ciprofloxacin 500 mg twice daily. This study provided most of the evidence to justify the recommended 14-day antibiotic course.
Another noninferiority, randomized, placebo-controlled study published in 2017 compared 7- and 14-day treatment with ciprofloxacin 500 mg to placebo twice per week. In men, 7 days of antibiotic therapy was inferior to 14 days during a short-term follow-up but was not inferior during a longer follow-up.
A decisive study, which is currently in the submission phase, could silence debate. “In our noninferiority, multicenter, randomized, double-blind, placebo-controlled study, we have enrolled 240 men over the age of 18 years with a febrile infection documented by a fever of 38° C or more, clinical signs of infection, and leukocyturia at least above 10/mm3 and with symptoms lasting less than 3 months,” said Dr. Lafaurie, the trial coordinator.
The primary outcome for efficacy was microbiologic and clinical success after 6 weeks. Patients received either ofloxacin, ceftriaxone, or cefotaxime (two third-generation cephalosporins in the beta-lactam family).
“We clearly show that, for a 7-day course, the clinical success rate is 55.7%, and for a 14-day course, this goes up to 77.6%, with no difference in terms of adverse effects or selection of resistant bacteria. The predictive factors for success are a 14-day treatment and being under the age of 50 years,” said Dr. Lafaurie.
“Unlike nonfebrile urinary tract infections in men, a 7-day course is insufficient for patients with febrile urinary tract infections, and a minimum of 14 days is required to achieve clinical and microbiological success,” he concluded.
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
PARIS – To date, studies of antibiotic course length for treating urinary tract infections in men have been patchy and retrospective.
Through recent randomized trials, guidelines can now be based on more solid data.
In sum, to maximize clinical and microbiologic success, a nonfebrile urinary tract infection is treated for 7 days, and a febrile urinary tract infection is treated for a minimum of 14 days.
At the 116th conference of the French urology association, Matthieu Lafaurie, MD, of the Multidisciplinary Infectious Diseases Unit U21, Saint Louis Hospital, Paris, reviewed the literature on this subject.
Guidelines for men
The European Association of Urology made its position clear in a text updated in 2022. It stated: Therefore, treatment with antimicrobial drugs that penetrate the prostate tissue is needed in men presenting with symptoms of a urinary tract infection.” In its classification of prostatitis, the National Institutes of Health distinguishes between acute prostatitis (symptoms of a urinary tract infection; stage I) and chronic prostatitis (recurrent infection with the same microorganism; stage II).
Although the French-language Society of Infectious Diseases distinguishes between febrile and nonfebrile urinary tract infections in males, the academic body does not take into account whether the patient has a fever when determining which antibiotic should be given and how long the course should be: A minimum of 14 days’ treatment is recommended when opting for fluoroquinolones, trimethoprim-sulfamethoxazole (cotrimoxazole), or injectable beta-lactam antibiotics, and at least 21 days is recommended for other drugs or in cases in which there is an underlying urologic condition that has not been treated.
Yet the EAU recommends treating cystitis with antibiotics for at least 7 days, preferably with cotrimoxazole or fluoroquinolone, depending on the results of sensitivity testing. For acute prostatitis, the length of treatment with fluoroquinolones should be at least 14 days.
Nonfebrile infections
Participation of men in studies of the treatment of complicated cystitis is variable; at most only 10% of patients in such trials are men. There are few data specific to men with nonfebrile urinary tract infections, and most studies are retrospective and involve small cohorts. One of these is a community-based study that involved 422 men aged 18-104 years who presented with nonfebrile urinary tract infection (acute dysuria, frequency of urination and/or urgency of urination, temperature < 38° C, no general symptoms). Antibiotic treatment was prescribed in 60% of cases. In more than 55% of cases, the length of the course of treatment was 1–7 days. Treatment was with cotrimoxazole, quinolones, and nitrofurantoin.
Another observational retrospective study showed benefit with nitrofurantoin (50 mg/8 h in 94% of cases; 69 patients) and pivmecillinam (200 mg/8 h in 65% of cases; 200 mg/12 h in 30% of patients; 57 patients) in treating lower urinary tract infections in men. The median treatment duration was 7 days. The failure rate was 1.4% and 12%, respectively, for these treatments. Compared to the so-called gold-standard treatment, trimethoprim (10 days/800 mg/12 h; 45 patients), the recurrence rate was 11% and 26% for nitrofurantoin and pivmecillinam versus 7% for trimethoprim. The most significant relapse rate with pivmecillinam was when treatment was given for fewer than 7 days.
This is the only risk factor for further antibiotic treatment and/or recurrence. There was no significant difference between the three drugs with regard to other parameters (urinary tract infection symptoms, benign prostatic hypertrophy, prostate cancer, gram-positive bacteria, etc).
Another retrospective, European study of nitrofurantoin that was published in 2015 included 485 patients (100 mg twice daily in 71% of cases). Clinical cure was defined as an absence of signs or symptoms of a urinary tract infection for 14 days after stopping nitrofurantoin, without use of other antibiotics. The cure rate was 77%. Better efficacy was achieved for patients with gram-negative (vs. gram-positive) bacteria. The treatment duration did not differ significantly (clinical success was achieved when the treatment was taken for 8.6 ± 3.6 days; clinical failure occurred when the treatment was taken for 9.3 ± 6.9 days; P = .28).
Regarding pivmecillinam, a retrospective 2010-2016 study involved 21,864 adults and included 2,524 men who had been treated empirically with pivmecillinam (400 mg three times daily) for significant bacteriuria (Escherichia coli) and a lower urinary tract infection. The researchers concluded that for men, the success rate was identical whether the treatment lasted 5 or 7 days.
An American community-based (urologists, primary care physicians, general medicine services) retrospective cohort study involving 573 men with nonfebrile lower urinary tract infections was conducted from 2011 to 2015. The patients received antibiotic treatment with fluoroquinolones (69.7%), cotrimoxazole (21.2%), nitrofurantoin (5.3%), trimethoprim, beta-lactam antibiotics, or aminoglycosides. No clinical advantage was seen in treating men with urinary tract infections for longer than 7 days.
There are some data on the use of fosfomycin. In an observational retrospective study, 25 men of 52 male adults with leukocyturia and E. coli greater than 105, ESBL, were treated with fosfomycin trometamol 3 g on days 1, 3, 5. Clinical and microbiologic success was achieved for 94% and 78.5%, respectively. No distinction was made between the sexes.
These results were confirmed in a retrospective, observational study involving 18 men (of a total of 75 adults) with no fever or hyperleukocytosis who received the same fosfomycin trometamol regimen. The rate of clinical cure or sterile urine microscopy and culture was 69% at 13 days. The risk failure factor was, as expected, infection with Klebsiella pneumoniae, which was slightly susceptible to fosfomycin, unlike E. coli.
The most recent study in this field was published in 2021. It was also the first randomized, double-blind, placebo-controlled study. In all, 272 men older than 18 years were prescribed either ciprofloxacin or cotrimoxazole for 7-14 days to treat a nonfebrile urinary tract infection. To be eligible for the trial, patients were required to have disease of new onset with at least one of the following symptoms: dysuria, frequency of urination, urgency of urination, hematuria, costovertebral angle tenderness, or perineal, flank, or suprapubic pain. Urine microscopy and culture were not necessary; the approach was wholly symptomatic. Treatment was prescribed for 7 days. Patients were randomly allocated on day 8 to receive treatment for the following 7 days (molecule or placebo). The primary outcome was resolution of clinical symptoms of urinary tract infection by 14 days after completion of active antibiotic treatment. In an intention-to-treat or per-protocol analysis, the difference in efficacy between the two molecules was largely below the required 10%. The treatment duration noninferiority margin was 7 days, compared with 14 days.
“In 2022, with regard to the duration of treatment of nonfebrile urinary tract infections in men, the not completely irrefutable evidence does, however, stack up in favor of the possibility of a 7-day or even 5-day course,” pointed out Dr. Lafaurie. “Fluoroquinolones [such as] ofloxacin, levofloxacin, ciprofloxacin, as well as cotrimoxazole and other antibiotics, such as pivmecillinam, nitrofurantoin, or fosfomycin trometamol, can be used, despite the fact that they pass less easily into the prostate – a not-so-obvious benefit.”
Febrile infections
In terms of febrile urinary tract infections, a single-center, prospective, open-label study from 2003 involved 72 male inpatients who were randomly to receive treatment either for 2 weeks or 4 weeks. Treatment consisted of ciprofloxacin 500 mg twice daily. This study provided most of the evidence to justify the recommended 14-day antibiotic course.
Another noninferiority, randomized, placebo-controlled study published in 2017 compared 7- and 14-day treatment with ciprofloxacin 500 mg to placebo twice per week. In men, 7 days of antibiotic therapy was inferior to 14 days during a short-term follow-up but was not inferior during a longer follow-up.
A decisive study, which is currently in the submission phase, could silence debate. “In our noninferiority, multicenter, randomized, double-blind, placebo-controlled study, we have enrolled 240 men over the age of 18 years with a febrile infection documented by a fever of 38° C or more, clinical signs of infection, and leukocyturia at least above 10/mm3 and with symptoms lasting less than 3 months,” said Dr. Lafaurie, the trial coordinator.
The primary outcome for efficacy was microbiologic and clinical success after 6 weeks. Patients received either ofloxacin, ceftriaxone, or cefotaxime (two third-generation cephalosporins in the beta-lactam family).
“We clearly show that, for a 7-day course, the clinical success rate is 55.7%, and for a 14-day course, this goes up to 77.6%, with no difference in terms of adverse effects or selection of resistant bacteria. The predictive factors for success are a 14-day treatment and being under the age of 50 years,” said Dr. Lafaurie.
“Unlike nonfebrile urinary tract infections in men, a 7-day course is insufficient for patients with febrile urinary tract infections, and a minimum of 14 days is required to achieve clinical and microbiological success,” he concluded.
This article was translated from the Medscape French edition. A version appeared on Medscape.com.
Flu hospitalizations drop amid signs of an early peak
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.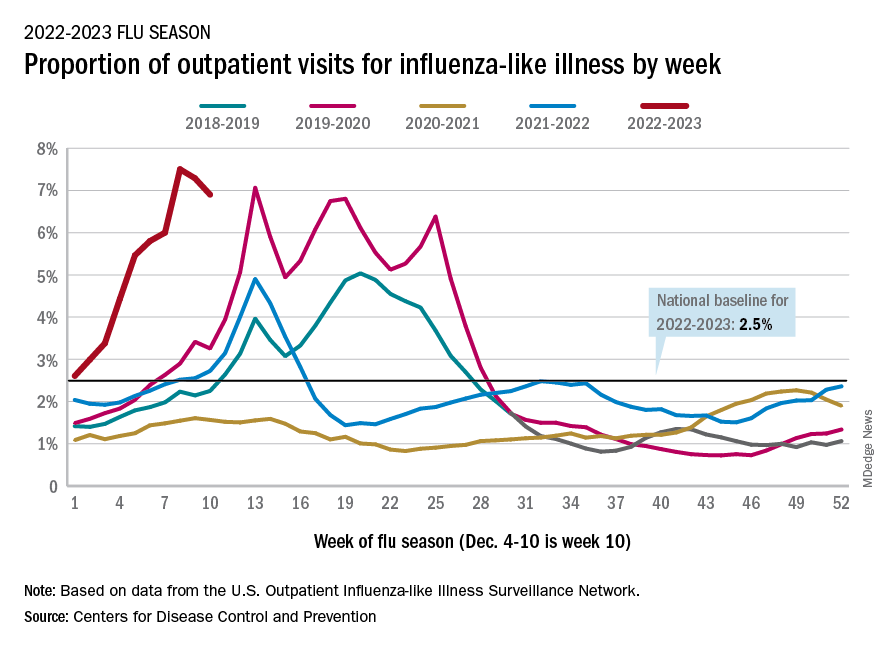
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
Infectious disease fellowship matches nose-dive after pandemic bump
Just 56% of infectious disease fellowship programs filled their 2023 slots, according to new data released by the National Resident Matching Program. Infectious disease (ID) fellowships had seen a jump in applications in the previous 2 years, but these new numbers may suggest a backward slide in a specialty that for many years has struggled to recruit residents.
There are unfilled positions across the country, including in health care hot spots. In Boston, all three slots at Boston Medical Center ID fellowship program are currently empty.
“For our program, going unfilled is a pretty rare event,” said Daniel Bourque, MD, an assistant professor of infectious disease at Boston University and director of the program. “For a program in the city of Boston that’s at a large tertiary care center, that definitely was a big surprise.”
Many other ID fellowships have joined BMC in posting about their vacancies on social media, looking for residents who may not have matched in other fellowships and for physicians who initially decided not to pursue additional training but are now reconsidering.
“If you are interested in a career in this exciting field, in the amazing city of Seattle, with incredible and friendly colleagues, please contact us,” the University of Washington’s ID fellowship program tweeted. Tulane University, Creighton University, the University of Connecticut, Washington University in St. Louis, and the University of Colorado also advertised their unfilled positions.
Other ID doctors commiserated with the disappointing match year. “I made a new riddle after yesterday’s match results: In the hospital, everyone needs me. Yet, no one wants to be me. What am I? An ID doctor,” tweeted Nathan Nolan, MD, MPH, an infectious disease specialist at the Veterans Health Administration in St. Louis.
Infectious disease positions continue to grow
One contributor to this downturn could be the growing number of infectious disease programs offered, whereas the number of applicants has generally remained stable. In 2018, there were 394 slots at 151 infectious disease fellowship programs offered. For the 2023 match year, there were 441 slots at 175 programs.
At the same time, there has not been a notable rise in applicants. From match years 2018 to 2020, about 320 applicants applied for ID fellowship positions each year. There was a rise in in interest in first 2 years of the pandemic, with 404 and 387 applicants in the 2021 and 2022 match years, respectively. The most recent round suggests a return to prepandemic numbers, with 330 residents applying to ID programs.
“I think it’s fair to question whether, as a field, we should be increasing training programs and spots at this point, and if it’s better to focus on ways to increase interest and demand,” said Daniel Diekema, MD, an ID physician at Maine Medical Center in Portland. “Otherwise, we’re just going to look worse and worse every year,” he added, and the work that goes into creating these training opportunities will not have a return on investment.
More training, less pay
The fellowship recruitment issues combined with an already short supply of infectious disease specialists can be traced back to comparatively worse pay compared with other subspecialties, experts say. Infectious disease was the fifth lowest paid specialty in the 2022 Medscape Physician Compensation Report – ranking above only primary care specialties and diabetes and endocrinology.
Pursuing this subspeciality in medicine may not translate to higher pay, Dr. Diekema noted. For example, a physician who completes an internal medicine residency and then a 2- to 3-year infectious disease fellowship can make less than a physician who pursues hospital medicine directly after completing the same residency.
“You’re in a situation where you’re doing additional training to reduce your income earning potential, and that’s a very hard sales pitch to make,” he said. It’s become more difficult as student loan debts continue to increase, he added.
Because infectious disease is a cognitive specialty and does not perform procedures, it is at a disadvantage in a typical fee-for-service pay model. ID physicians also advise on hospital policies for testing and personal protective equipment, which is not always compensated, said Wendy Armstrong, MD, a professor of infectious diseases at Emory University, Atlanta.
A reflection of pandemic burnout?
Experts also wonder if the past 2 years of the pandemic and the notable burnout in ID and other in-demand specialties may have dissuaded applicants from pursuing the ID career path.
“This residency class is the class that started their training in June or July of 2020 and represent that residency class that has trained throughout the pandemic,” Dr. Bourque said. “Does [this low match rate] reflect a negative outlook on the field of ID because of COVID? Is it a reflection of trainee burnout in the setting of the pandemic?”
Dr. Diekema wonders if increased public scrutiny and politicization of the field may have discouraged residents. “The vilification of public health and [of] infectious disease experts like Dr. Fauci by significant portions of our society can be demoralizing,” he said. “People might say, ‘Why would I want to put myself through that?’ ”
But Dr. Armstrong doubts this is the case. “I’ve never had a resident tell me that was on their radar screen,” she said, noting that while there had been recent improvements in applicants, lower match numbers for ID fellowships have been a long-standing issue.
Rethinking reimbursement
Experts agree that pay issues need to be addressed to make ID a more attractive specialty. Moving away from traditional payment plans to value-based models using quality measurements specific to infectious disease could be one way to quantify the value of ID specialists in care systems.
The Infectious Diseases Society of America recently met with the panel that sets compensation rates for Medicare to discuss ways to increase compensation for ID, said IDSA president Carlos del Rio, MD. He is also a professor of medicine at Emory University.
ID specialists need to be able to put a dollar value to their policy work that’s not related to patient reimbursement, Dr. del Rio said. IDSA’s ongoing compensation initiative advocates for value-based care and provides salary negotiation tools for ID specialists, he added.
“Salaries shouldn’t simply be defined by what reimbursement is, and that’s true for other specialties,” such as hospital medicine and palliative care at many institutions, Dr. Armstrong said. “Infectious disease needs to be held at the same level of respect and value.”
But despite issues within the specialties, ID physicians remain passionate about their field.
“It is the most fascinating specialty I can ever imagine,” Dr. Armstrong said. Dr. Bourque agreed, noting the dynamic nature of specialty, with the emergence of new diseases like COVID-19 and reemergence of diseases like mpox (formerly called monkeypox), Zika, Ebola, and chikungunya in the past decade.
“There’s nothing about the field of infectious diseases that, in my mind, isn’t fascinating or rewarding enough to bring people in,” added Dr. Diekema. “The factors that are keeping people out are primarily economic factors and aspects of our health care system that need attention.”
A version of this article first appeared on Medscape.com.
Just 56% of infectious disease fellowship programs filled their 2023 slots, according to new data released by the National Resident Matching Program. Infectious disease (ID) fellowships had seen a jump in applications in the previous 2 years, but these new numbers may suggest a backward slide in a specialty that for many years has struggled to recruit residents.
There are unfilled positions across the country, including in health care hot spots. In Boston, all three slots at Boston Medical Center ID fellowship program are currently empty.
“For our program, going unfilled is a pretty rare event,” said Daniel Bourque, MD, an assistant professor of infectious disease at Boston University and director of the program. “For a program in the city of Boston that’s at a large tertiary care center, that definitely was a big surprise.”
Many other ID fellowships have joined BMC in posting about their vacancies on social media, looking for residents who may not have matched in other fellowships and for physicians who initially decided not to pursue additional training but are now reconsidering.
“If you are interested in a career in this exciting field, in the amazing city of Seattle, with incredible and friendly colleagues, please contact us,” the University of Washington’s ID fellowship program tweeted. Tulane University, Creighton University, the University of Connecticut, Washington University in St. Louis, and the University of Colorado also advertised their unfilled positions.
Other ID doctors commiserated with the disappointing match year. “I made a new riddle after yesterday’s match results: In the hospital, everyone needs me. Yet, no one wants to be me. What am I? An ID doctor,” tweeted Nathan Nolan, MD, MPH, an infectious disease specialist at the Veterans Health Administration in St. Louis.
Infectious disease positions continue to grow
One contributor to this downturn could be the growing number of infectious disease programs offered, whereas the number of applicants has generally remained stable. In 2018, there were 394 slots at 151 infectious disease fellowship programs offered. For the 2023 match year, there were 441 slots at 175 programs.
At the same time, there has not been a notable rise in applicants. From match years 2018 to 2020, about 320 applicants applied for ID fellowship positions each year. There was a rise in in interest in first 2 years of the pandemic, with 404 and 387 applicants in the 2021 and 2022 match years, respectively. The most recent round suggests a return to prepandemic numbers, with 330 residents applying to ID programs.
“I think it’s fair to question whether, as a field, we should be increasing training programs and spots at this point, and if it’s better to focus on ways to increase interest and demand,” said Daniel Diekema, MD, an ID physician at Maine Medical Center in Portland. “Otherwise, we’re just going to look worse and worse every year,” he added, and the work that goes into creating these training opportunities will not have a return on investment.
More training, less pay
The fellowship recruitment issues combined with an already short supply of infectious disease specialists can be traced back to comparatively worse pay compared with other subspecialties, experts say. Infectious disease was the fifth lowest paid specialty in the 2022 Medscape Physician Compensation Report – ranking above only primary care specialties and diabetes and endocrinology.
Pursuing this subspeciality in medicine may not translate to higher pay, Dr. Diekema noted. For example, a physician who completes an internal medicine residency and then a 2- to 3-year infectious disease fellowship can make less than a physician who pursues hospital medicine directly after completing the same residency.
“You’re in a situation where you’re doing additional training to reduce your income earning potential, and that’s a very hard sales pitch to make,” he said. It’s become more difficult as student loan debts continue to increase, he added.
Because infectious disease is a cognitive specialty and does not perform procedures, it is at a disadvantage in a typical fee-for-service pay model. ID physicians also advise on hospital policies for testing and personal protective equipment, which is not always compensated, said Wendy Armstrong, MD, a professor of infectious diseases at Emory University, Atlanta.
A reflection of pandemic burnout?
Experts also wonder if the past 2 years of the pandemic and the notable burnout in ID and other in-demand specialties may have dissuaded applicants from pursuing the ID career path.
“This residency class is the class that started their training in June or July of 2020 and represent that residency class that has trained throughout the pandemic,” Dr. Bourque said. “Does [this low match rate] reflect a negative outlook on the field of ID because of COVID? Is it a reflection of trainee burnout in the setting of the pandemic?”
Dr. Diekema wonders if increased public scrutiny and politicization of the field may have discouraged residents. “The vilification of public health and [of] infectious disease experts like Dr. Fauci by significant portions of our society can be demoralizing,” he said. “People might say, ‘Why would I want to put myself through that?’ ”
But Dr. Armstrong doubts this is the case. “I’ve never had a resident tell me that was on their radar screen,” she said, noting that while there had been recent improvements in applicants, lower match numbers for ID fellowships have been a long-standing issue.
Rethinking reimbursement
Experts agree that pay issues need to be addressed to make ID a more attractive specialty. Moving away from traditional payment plans to value-based models using quality measurements specific to infectious disease could be one way to quantify the value of ID specialists in care systems.
The Infectious Diseases Society of America recently met with the panel that sets compensation rates for Medicare to discuss ways to increase compensation for ID, said IDSA president Carlos del Rio, MD. He is also a professor of medicine at Emory University.
ID specialists need to be able to put a dollar value to their policy work that’s not related to patient reimbursement, Dr. del Rio said. IDSA’s ongoing compensation initiative advocates for value-based care and provides salary negotiation tools for ID specialists, he added.
“Salaries shouldn’t simply be defined by what reimbursement is, and that’s true for other specialties,” such as hospital medicine and palliative care at many institutions, Dr. Armstrong said. “Infectious disease needs to be held at the same level of respect and value.”
But despite issues within the specialties, ID physicians remain passionate about their field.
“It is the most fascinating specialty I can ever imagine,” Dr. Armstrong said. Dr. Bourque agreed, noting the dynamic nature of specialty, with the emergence of new diseases like COVID-19 and reemergence of diseases like mpox (formerly called monkeypox), Zika, Ebola, and chikungunya in the past decade.
“There’s nothing about the field of infectious diseases that, in my mind, isn’t fascinating or rewarding enough to bring people in,” added Dr. Diekema. “The factors that are keeping people out are primarily economic factors and aspects of our health care system that need attention.”
A version of this article first appeared on Medscape.com.
Just 56% of infectious disease fellowship programs filled their 2023 slots, according to new data released by the National Resident Matching Program. Infectious disease (ID) fellowships had seen a jump in applications in the previous 2 years, but these new numbers may suggest a backward slide in a specialty that for many years has struggled to recruit residents.
There are unfilled positions across the country, including in health care hot spots. In Boston, all three slots at Boston Medical Center ID fellowship program are currently empty.
“For our program, going unfilled is a pretty rare event,” said Daniel Bourque, MD, an assistant professor of infectious disease at Boston University and director of the program. “For a program in the city of Boston that’s at a large tertiary care center, that definitely was a big surprise.”
Many other ID fellowships have joined BMC in posting about their vacancies on social media, looking for residents who may not have matched in other fellowships and for physicians who initially decided not to pursue additional training but are now reconsidering.
“If you are interested in a career in this exciting field, in the amazing city of Seattle, with incredible and friendly colleagues, please contact us,” the University of Washington’s ID fellowship program tweeted. Tulane University, Creighton University, the University of Connecticut, Washington University in St. Louis, and the University of Colorado also advertised their unfilled positions.
Other ID doctors commiserated with the disappointing match year. “I made a new riddle after yesterday’s match results: In the hospital, everyone needs me. Yet, no one wants to be me. What am I? An ID doctor,” tweeted Nathan Nolan, MD, MPH, an infectious disease specialist at the Veterans Health Administration in St. Louis.
Infectious disease positions continue to grow
One contributor to this downturn could be the growing number of infectious disease programs offered, whereas the number of applicants has generally remained stable. In 2018, there were 394 slots at 151 infectious disease fellowship programs offered. For the 2023 match year, there were 441 slots at 175 programs.
At the same time, there has not been a notable rise in applicants. From match years 2018 to 2020, about 320 applicants applied for ID fellowship positions each year. There was a rise in in interest in first 2 years of the pandemic, with 404 and 387 applicants in the 2021 and 2022 match years, respectively. The most recent round suggests a return to prepandemic numbers, with 330 residents applying to ID programs.
“I think it’s fair to question whether, as a field, we should be increasing training programs and spots at this point, and if it’s better to focus on ways to increase interest and demand,” said Daniel Diekema, MD, an ID physician at Maine Medical Center in Portland. “Otherwise, we’re just going to look worse and worse every year,” he added, and the work that goes into creating these training opportunities will not have a return on investment.
More training, less pay
The fellowship recruitment issues combined with an already short supply of infectious disease specialists can be traced back to comparatively worse pay compared with other subspecialties, experts say. Infectious disease was the fifth lowest paid specialty in the 2022 Medscape Physician Compensation Report – ranking above only primary care specialties and diabetes and endocrinology.
Pursuing this subspeciality in medicine may not translate to higher pay, Dr. Diekema noted. For example, a physician who completes an internal medicine residency and then a 2- to 3-year infectious disease fellowship can make less than a physician who pursues hospital medicine directly after completing the same residency.
“You’re in a situation where you’re doing additional training to reduce your income earning potential, and that’s a very hard sales pitch to make,” he said. It’s become more difficult as student loan debts continue to increase, he added.
Because infectious disease is a cognitive specialty and does not perform procedures, it is at a disadvantage in a typical fee-for-service pay model. ID physicians also advise on hospital policies for testing and personal protective equipment, which is not always compensated, said Wendy Armstrong, MD, a professor of infectious diseases at Emory University, Atlanta.
A reflection of pandemic burnout?
Experts also wonder if the past 2 years of the pandemic and the notable burnout in ID and other in-demand specialties may have dissuaded applicants from pursuing the ID career path.
“This residency class is the class that started their training in June or July of 2020 and represent that residency class that has trained throughout the pandemic,” Dr. Bourque said. “Does [this low match rate] reflect a negative outlook on the field of ID because of COVID? Is it a reflection of trainee burnout in the setting of the pandemic?”
Dr. Diekema wonders if increased public scrutiny and politicization of the field may have discouraged residents. “The vilification of public health and [of] infectious disease experts like Dr. Fauci by significant portions of our society can be demoralizing,” he said. “People might say, ‘Why would I want to put myself through that?’ ”
But Dr. Armstrong doubts this is the case. “I’ve never had a resident tell me that was on their radar screen,” she said, noting that while there had been recent improvements in applicants, lower match numbers for ID fellowships have been a long-standing issue.
Rethinking reimbursement
Experts agree that pay issues need to be addressed to make ID a more attractive specialty. Moving away from traditional payment plans to value-based models using quality measurements specific to infectious disease could be one way to quantify the value of ID specialists in care systems.
The Infectious Diseases Society of America recently met with the panel that sets compensation rates for Medicare to discuss ways to increase compensation for ID, said IDSA president Carlos del Rio, MD. He is also a professor of medicine at Emory University.
ID specialists need to be able to put a dollar value to their policy work that’s not related to patient reimbursement, Dr. del Rio said. IDSA’s ongoing compensation initiative advocates for value-based care and provides salary negotiation tools for ID specialists, he added.
“Salaries shouldn’t simply be defined by what reimbursement is, and that’s true for other specialties,” such as hospital medicine and palliative care at many institutions, Dr. Armstrong said. “Infectious disease needs to be held at the same level of respect and value.”
But despite issues within the specialties, ID physicians remain passionate about their field.
“It is the most fascinating specialty I can ever imagine,” Dr. Armstrong said. Dr. Bourque agreed, noting the dynamic nature of specialty, with the emergence of new diseases like COVID-19 and reemergence of diseases like mpox (formerly called monkeypox), Zika, Ebola, and chikungunya in the past decade.
“There’s nothing about the field of infectious diseases that, in my mind, isn’t fascinating or rewarding enough to bring people in,” added Dr. Diekema. “The factors that are keeping people out are primarily economic factors and aspects of our health care system that need attention.”
A version of this article first appeared on Medscape.com.
Have you heard the one about the cow in the doctor’s office?
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
HIV vaccine trial makes pivotal leap toward making ‘super antibodies’
The announcement comes from the journal Science, which published phase 1 results of a small clinical trial for a vaccine technology that aims to cause the body to create a rare kind of cell.
“At the most general level, the trial results show that one can design vaccines that induce antibodies with prespecified genetic features, and this may herald a new era of precision vaccines,” William Schief, PhD, a researcher at the Scripps Research Institute and study coauthor, told the American Association for the Advancement of Science.
The study was the first to test the approach in humans and was effective in 97% – or 35 of 36 – participants. The vaccine technology is called “germline targeting.” Trial results show that “one can design a vaccine that elicits made-to-order antibodies in humans,” Dr. Schief said in a news release.
In addition to possibly being a breakthrough for the treatment of HIV, the vaccine technology could also impact the development of treatments for flu, hepatitis C, and coronaviruses, study authors wrote.
There is no cure for HIV, but there are treatments to manage how the disease progresses. HIV attacks the body’s immune system, destroys white blood cells, and increases susceptibility to other infections, AAAS summarized. More than 1 million people in the United States and 38 million people worldwide have HIV.
Previous HIV vaccine attempts were not able to cause the production of specialized cells known as “broadly neutralizing antibodies,” CNN reported.
“Call them super antibodies, if you want,” University of Minnesota HIV researcher Timothy Schacker, MD, who was not involved in the research, told CNN. “The hope is that if you can induce this kind of immunity in people, you can protect them from some of these viruses that we’ve had a very hard time designing vaccines for that are effective. So this is an important step forward.”
Study authors said this is just the first step in the multiphase vaccine design, which so far is a theory. Further study is needed to see if the next steps also work in humans, and then if all the steps can be linked together and can be effective against HIV.
A version of this article first appeared on WebMD.com.
The announcement comes from the journal Science, which published phase 1 results of a small clinical trial for a vaccine technology that aims to cause the body to create a rare kind of cell.
“At the most general level, the trial results show that one can design vaccines that induce antibodies with prespecified genetic features, and this may herald a new era of precision vaccines,” William Schief, PhD, a researcher at the Scripps Research Institute and study coauthor, told the American Association for the Advancement of Science.
The study was the first to test the approach in humans and was effective in 97% – or 35 of 36 – participants. The vaccine technology is called “germline targeting.” Trial results show that “one can design a vaccine that elicits made-to-order antibodies in humans,” Dr. Schief said in a news release.
In addition to possibly being a breakthrough for the treatment of HIV, the vaccine technology could also impact the development of treatments for flu, hepatitis C, and coronaviruses, study authors wrote.
There is no cure for HIV, but there are treatments to manage how the disease progresses. HIV attacks the body’s immune system, destroys white blood cells, and increases susceptibility to other infections, AAAS summarized. More than 1 million people in the United States and 38 million people worldwide have HIV.
Previous HIV vaccine attempts were not able to cause the production of specialized cells known as “broadly neutralizing antibodies,” CNN reported.
“Call them super antibodies, if you want,” University of Minnesota HIV researcher Timothy Schacker, MD, who was not involved in the research, told CNN. “The hope is that if you can induce this kind of immunity in people, you can protect them from some of these viruses that we’ve had a very hard time designing vaccines for that are effective. So this is an important step forward.”
Study authors said this is just the first step in the multiphase vaccine design, which so far is a theory. Further study is needed to see if the next steps also work in humans, and then if all the steps can be linked together and can be effective against HIV.
A version of this article first appeared on WebMD.com.
The announcement comes from the journal Science, which published phase 1 results of a small clinical trial for a vaccine technology that aims to cause the body to create a rare kind of cell.
“At the most general level, the trial results show that one can design vaccines that induce antibodies with prespecified genetic features, and this may herald a new era of precision vaccines,” William Schief, PhD, a researcher at the Scripps Research Institute and study coauthor, told the American Association for the Advancement of Science.
The study was the first to test the approach in humans and was effective in 97% – or 35 of 36 – participants. The vaccine technology is called “germline targeting.” Trial results show that “one can design a vaccine that elicits made-to-order antibodies in humans,” Dr. Schief said in a news release.
In addition to possibly being a breakthrough for the treatment of HIV, the vaccine technology could also impact the development of treatments for flu, hepatitis C, and coronaviruses, study authors wrote.
There is no cure for HIV, but there are treatments to manage how the disease progresses. HIV attacks the body’s immune system, destroys white blood cells, and increases susceptibility to other infections, AAAS summarized. More than 1 million people in the United States and 38 million people worldwide have HIV.
Previous HIV vaccine attempts were not able to cause the production of specialized cells known as “broadly neutralizing antibodies,” CNN reported.
“Call them super antibodies, if you want,” University of Minnesota HIV researcher Timothy Schacker, MD, who was not involved in the research, told CNN. “The hope is that if you can induce this kind of immunity in people, you can protect them from some of these viruses that we’ve had a very hard time designing vaccines for that are effective. So this is an important step forward.”
Study authors said this is just the first step in the multiphase vaccine design, which so far is a theory. Further study is needed to see if the next steps also work in humans, and then if all the steps can be linked together and can be effective against HIV.
A version of this article first appeared on WebMD.com.
FROM SCIENCE
Should you quit employment to open a practice? These docs share how they did it
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
CAB-LA’s full potential for HIV prevention hits snags
, say authors of a new review article.
CAB-LA “represents the most important breakthrough in HIV prevention in recent years,” write Geoffroy Liegeon, MD, and Jade Ghosn, MD, PhD, with Université Paris Cité, in this month’s HIV Medicine.
It has been found to be safe, and more effective in phase 3 trials than oral PrEP, and is well-accepted in men who have sex with men, and transgender and cisgender women.
Reductions in stigma
Surveys show patients at high risk for HIV – especially those who see PrEP as burdensome – are highly interested in long-acting injectable drugs. Reduced stigma with the injections also appears to steer the choice toward a long-acting agent and may attract more people to HIV prevention programs.
The first two injections are given 4 weeks apart, followed by an injection every 8 weeks.
Models designed to increase uptake, adherence, and persistence when on and after discontinuing CAB-LA will be important for wider rollout, as will better patient education and demonstrated efficacy and safety in populations not included in clinical trials, Dr. Liegeon and Dr. Ghosn note.
Still, they point out that its broader integration into clinical routine is held back by factors including breakthrough infections despite timely injections, complexity of follow-up, logistical considerations, and its cost-effectiveness compared with oral PrEP.
A hefty price tag
“[T]he cost effectiveness compared with TDF-FTC [tenofovir/emtricitabine] generics may not support its use at the current price in many settings,” the authors write.
For low- and middle-income countries, the TDF/FTC price is about $55, according to the World Health Organization’s Global Price Reporting, while the current price of CAB-LA in the United States is about $22,000, according to Dr. Ghosn. He said in an interview that because the cost of generics can reach $400-$500 per year in the United States, depending on the pharmaceutical companies, the price for CAB-LA is almost 60 times higher than TDF/FTC in the Untied States.
The biggest hope for the price reduction, at least in lower-income countries, he said, is a new licensing agreement.
ViiV Healthcare signed a new voluntary licensing agreement with the Medicines Patent Pool in July to help access in low-income, lower-middle-income, and sub-Saharan African countries, he explained.
The authors summarize: “[E]stablishing the effectiveness of CAB-LA does not guarantee its uptake into clinical routine.”
Because of the combined issues, the WHO recommended CAB-LA as an additional prevention choice for PrEP in its recent guidelines, pending further studies.
Barriers frustrate providers
Lauren Fontana, DO, assistant professor at the University of Minnesota, Minneapolis, and infectious disease physician at M Health Fairview, said in an interview that “as a health care provider, cost and insurance barriers can be frustrating, especially when CAB-LA is identified as the best option for a patient.”
Lack of nonphysician-led initiatives, such as nurse- or pharmacy-led services for CAB-LA, may limit availability to marginalized and at-risk populations, she said.
“If a clinic can acquire CAB-LA, clinic protocols need to be developed and considerations of missed visits and doses must be thought about when implementing a program,” Dr. Fontana said.
Clinics need resources to engage with patients to promote retention in the program with case management and pharmacy support, she added.
“Simplification processes need to be developed to make CAB-LA an option for more clinics and patients,” she continued. “We are still learning about the incidence of breakthrough HIV infections, patterns of HIV seroconversion, and how to optimize testing so that HIV infections are detected early.”
Dr. Liegeon, Dr. Ghosn, and Dr. Fontana report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, say authors of a new review article.
CAB-LA “represents the most important breakthrough in HIV prevention in recent years,” write Geoffroy Liegeon, MD, and Jade Ghosn, MD, PhD, with Université Paris Cité, in this month’s HIV Medicine.
It has been found to be safe, and more effective in phase 3 trials than oral PrEP, and is well-accepted in men who have sex with men, and transgender and cisgender women.
Reductions in stigma
Surveys show patients at high risk for HIV – especially those who see PrEP as burdensome – are highly interested in long-acting injectable drugs. Reduced stigma with the injections also appears to steer the choice toward a long-acting agent and may attract more people to HIV prevention programs.
The first two injections are given 4 weeks apart, followed by an injection every 8 weeks.
Models designed to increase uptake, adherence, and persistence when on and after discontinuing CAB-LA will be important for wider rollout, as will better patient education and demonstrated efficacy and safety in populations not included in clinical trials, Dr. Liegeon and Dr. Ghosn note.
Still, they point out that its broader integration into clinical routine is held back by factors including breakthrough infections despite timely injections, complexity of follow-up, logistical considerations, and its cost-effectiveness compared with oral PrEP.
A hefty price tag
“[T]he cost effectiveness compared with TDF-FTC [tenofovir/emtricitabine] generics may not support its use at the current price in many settings,” the authors write.
For low- and middle-income countries, the TDF/FTC price is about $55, according to the World Health Organization’s Global Price Reporting, while the current price of CAB-LA in the United States is about $22,000, according to Dr. Ghosn. He said in an interview that because the cost of generics can reach $400-$500 per year in the United States, depending on the pharmaceutical companies, the price for CAB-LA is almost 60 times higher than TDF/FTC in the Untied States.
The biggest hope for the price reduction, at least in lower-income countries, he said, is a new licensing agreement.
ViiV Healthcare signed a new voluntary licensing agreement with the Medicines Patent Pool in July to help access in low-income, lower-middle-income, and sub-Saharan African countries, he explained.
The authors summarize: “[E]stablishing the effectiveness of CAB-LA does not guarantee its uptake into clinical routine.”
Because of the combined issues, the WHO recommended CAB-LA as an additional prevention choice for PrEP in its recent guidelines, pending further studies.
Barriers frustrate providers
Lauren Fontana, DO, assistant professor at the University of Minnesota, Minneapolis, and infectious disease physician at M Health Fairview, said in an interview that “as a health care provider, cost and insurance barriers can be frustrating, especially when CAB-LA is identified as the best option for a patient.”
Lack of nonphysician-led initiatives, such as nurse- or pharmacy-led services for CAB-LA, may limit availability to marginalized and at-risk populations, she said.
“If a clinic can acquire CAB-LA, clinic protocols need to be developed and considerations of missed visits and doses must be thought about when implementing a program,” Dr. Fontana said.
Clinics need resources to engage with patients to promote retention in the program with case management and pharmacy support, she added.
“Simplification processes need to be developed to make CAB-LA an option for more clinics and patients,” she continued. “We are still learning about the incidence of breakthrough HIV infections, patterns of HIV seroconversion, and how to optimize testing so that HIV infections are detected early.”
Dr. Liegeon, Dr. Ghosn, and Dr. Fontana report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, say authors of a new review article.
CAB-LA “represents the most important breakthrough in HIV prevention in recent years,” write Geoffroy Liegeon, MD, and Jade Ghosn, MD, PhD, with Université Paris Cité, in this month’s HIV Medicine.
It has been found to be safe, and more effective in phase 3 trials than oral PrEP, and is well-accepted in men who have sex with men, and transgender and cisgender women.
Reductions in stigma
Surveys show patients at high risk for HIV – especially those who see PrEP as burdensome – are highly interested in long-acting injectable drugs. Reduced stigma with the injections also appears to steer the choice toward a long-acting agent and may attract more people to HIV prevention programs.
The first two injections are given 4 weeks apart, followed by an injection every 8 weeks.
Models designed to increase uptake, adherence, and persistence when on and after discontinuing CAB-LA will be important for wider rollout, as will better patient education and demonstrated efficacy and safety in populations not included in clinical trials, Dr. Liegeon and Dr. Ghosn note.
Still, they point out that its broader integration into clinical routine is held back by factors including breakthrough infections despite timely injections, complexity of follow-up, logistical considerations, and its cost-effectiveness compared with oral PrEP.
A hefty price tag
“[T]he cost effectiveness compared with TDF-FTC [tenofovir/emtricitabine] generics may not support its use at the current price in many settings,” the authors write.
For low- and middle-income countries, the TDF/FTC price is about $55, according to the World Health Organization’s Global Price Reporting, while the current price of CAB-LA in the United States is about $22,000, according to Dr. Ghosn. He said in an interview that because the cost of generics can reach $400-$500 per year in the United States, depending on the pharmaceutical companies, the price for CAB-LA is almost 60 times higher than TDF/FTC in the Untied States.
The biggest hope for the price reduction, at least in lower-income countries, he said, is a new licensing agreement.
ViiV Healthcare signed a new voluntary licensing agreement with the Medicines Patent Pool in July to help access in low-income, lower-middle-income, and sub-Saharan African countries, he explained.
The authors summarize: “[E]stablishing the effectiveness of CAB-LA does not guarantee its uptake into clinical routine.”
Because of the combined issues, the WHO recommended CAB-LA as an additional prevention choice for PrEP in its recent guidelines, pending further studies.
Barriers frustrate providers
Lauren Fontana, DO, assistant professor at the University of Minnesota, Minneapolis, and infectious disease physician at M Health Fairview, said in an interview that “as a health care provider, cost and insurance barriers can be frustrating, especially when CAB-LA is identified as the best option for a patient.”
Lack of nonphysician-led initiatives, such as nurse- or pharmacy-led services for CAB-LA, may limit availability to marginalized and at-risk populations, she said.
“If a clinic can acquire CAB-LA, clinic protocols need to be developed and considerations of missed visits and doses must be thought about when implementing a program,” Dr. Fontana said.
Clinics need resources to engage with patients to promote retention in the program with case management and pharmacy support, she added.
“Simplification processes need to be developed to make CAB-LA an option for more clinics and patients,” she continued. “We are still learning about the incidence of breakthrough HIV infections, patterns of HIV seroconversion, and how to optimize testing so that HIV infections are detected early.”
Dr. Liegeon, Dr. Ghosn, and Dr. Fontana report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HIV MEDICINE
Pregnancy outcomes on long-acting antiretroviral
In a cautiously optimistic report,
Among 10 live births, there was one birth defect (congenital ptosis, or droopy eyelid), which was not attributed to the trial drugs. There were no instances of perinatal HIV transmission at delivery or during the 1-year follow-up.
“Long-acting cabotegravir-rilpivirine is the first and only complete injectable regimen potentially available for pregnant women,” first author Parul Patel, PharmD, global medical affairs director for cabotegravir at ViiV Healthcare, said in an interview. The regimen was approved by the U.S. Food and Drug Administration in January 2021 for injections every 4 weeks and in February 2022 for injections every 8 weeks.
“Importantly, it can be dosed monthly or every 2 months,” Patel said. “This could be advantageous for women who are experiencing constant change during pregnancy. This could be a consideration for women who might have problems tolerating oral pills during pregnancy or might have problems with emesis.”
The study was published in HIV Medicine.
“We are really pursuing the development of the long-acting version of cabotegravir in combination with rilpivirine,” Dr. Patel said. “It’s an industry standard during initial development that you start very conservatively and not allow a woman who is pregnant to continue dosing of a drug while still evaluating its overall safety profile. We really want to understand the use of this agent in nonpregnant adults before exposing pregnant women to active treatment.”
Pregnancies in trials excluding pregnant women
In the paper, Dr. Patel and her coauthors noted the limited data on pregnant women exposed to CAB + RPV. They analyzed pregnancies in four phase 2b/3/3b clinical trials sponsored by ViiV Healthcare and a compassionate use program. All clinical trial participants first received oral CAB + RPV daily for 4 weeks to assess individual tolerance before the experimental long-acting injection of CAB + RPV every 4 weeks or every 8 weeks.
Women participants were required to use highly effective contraception during the trials and for at least 52 weeks after the last injection. Urine pregnancy tests were given at baseline, before each injection, and when pregnancy was suspected. If a pregnancy was detected, CAB + RPV (oral or long-acting injections) was discontinued and the woman switched to an alternative oral antiretroviral, unless she and her physician decided to continue with injections in the compassionate use program.
Pregnancy outcomes
Among 25 reported pregnancies in 22 women during the trial, there were 10 live births. Nine of the mothers who delivered their babies at term had switched to an alternative antiretroviral regimen and maintained virologic suppression throughout pregnancy and post partum, or the last available viral load assessment.
The 10th participant remained on long-acting CAB + RPV during her pregnancy and had a live birth with congenital ptosis that was resolving without treatment at the 4-month ophthalmology consult, the authors wrote. The mother experienced persistent low-level viremia before and throughout her pregnancy.
Two of the pregnancies occurred after the last monthly injection, during the washout period. Other studies have reported that each long-acting drug, CAB and RPV, can be detected more than 1 year after the last injection. In the new report, plasma CAB and RPV washout concentrations during pregnancy were within the range of those in nonpregnant women, the authors wrote.
Among the 14 participants with non–live birth outcomes, 13 switched to an alternative antiretroviral regimen during pregnancy and maintained virologic suppression through pregnancy and post partum, or until their last viral assessment. The remaining participant received long-acting CAB + RPV and continued this treatment for the duration of their pregnancy.
“It’s a very limited data set, so we’re not in a position to be able to make definitive conclusions around long-acting cabotegravir-rilpivirine in pregnancy,” Dr. Patel acknowledged. “But the data that we presented among the 25 women who were exposed to cabotegravir-rilpivirine looks reassuring.”
Planned studies during pregnancy
Vani Vannappagari, MBBS, MPH, PhD, global head of epidemiology and real-world evidence at ViiV Healthcare and study coauthor, said in an interview that the initial results are spurring promising new research.
“We are working with an external IMPAACT [International Maternal Pediatric Adolescent AIDS Clinical Trials Network] group on a clinical trial ... to try to determine the appropriate dose of long-acting cabotegravir-rilpivirine during pregnancy,” Dr. Vannappagari said. “The clinical trial will give us the immediate safety, dose information, and viral suppression rates for both the mother and the infant. But long-term safety, especially birth defects and any adverse pregnancy and neonatal outcomes, will come from our antiretroviral pregnancy registry and other noninterventional studies.
“In the very small cohort studied, [in] pregnancies that were continued after exposure to long-acting cabotegravir and rilpivirine in the first trimester, there were no significant adverse fetal outcomes identified,” he said. “That’s reassuring, as is the fact that at the time these patients were switched in early pregnancy, their viral loads were all undetectable at the time that their pregnancies were diagnosed.”
Neil Silverman, MD, professor of clinical obstetrics and gynecology and director of the Infections in Pregnancy Program at the University of California, Los Angeles, Medical Center, who was not associated with the study, provided a comment to this news organization.
“The larger question still remains why pregnant women were so actively excluded from the original study design when this trial was evaluating a newer long-acting preparation of two anti-HIV medications that otherwise would be perfectly fine to use during pregnancy?”
Dr. Silverman continued, “In this case, it’s particularly frustrating since the present study was simply evaluating established medications currently being used to manage HIV infection, but in a newer longer-acting mode of administration by an injection every 2 months. If a patient had already been successfully managed on an oral antiviral regimen containing an integrase inhibitor and a non-nucleoside reverse transcriptase inhibitor, like the two drugs studied here, it would not be considered reasonable to switch that regimen simply because she was found to be pregnant.”
Dr. Patel and Dr. Vannappagari are employees of ViiV Healthcare and stockholders of GlaxoSmithKline.
This analysis was funded by ViiV Healthcare, and all studies were cofunded by ViiV Healthcare and Janssen Research & Development. Dr. Silverman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a cautiously optimistic report,
Among 10 live births, there was one birth defect (congenital ptosis, or droopy eyelid), which was not attributed to the trial drugs. There were no instances of perinatal HIV transmission at delivery or during the 1-year follow-up.
“Long-acting cabotegravir-rilpivirine is the first and only complete injectable regimen potentially available for pregnant women,” first author Parul Patel, PharmD, global medical affairs director for cabotegravir at ViiV Healthcare, said in an interview. The regimen was approved by the U.S. Food and Drug Administration in January 2021 for injections every 4 weeks and in February 2022 for injections every 8 weeks.
“Importantly, it can be dosed monthly or every 2 months,” Patel said. “This could be advantageous for women who are experiencing constant change during pregnancy. This could be a consideration for women who might have problems tolerating oral pills during pregnancy or might have problems with emesis.”
The study was published in HIV Medicine.
“We are really pursuing the development of the long-acting version of cabotegravir in combination with rilpivirine,” Dr. Patel said. “It’s an industry standard during initial development that you start very conservatively and not allow a woman who is pregnant to continue dosing of a drug while still evaluating its overall safety profile. We really want to understand the use of this agent in nonpregnant adults before exposing pregnant women to active treatment.”
Pregnancies in trials excluding pregnant women
In the paper, Dr. Patel and her coauthors noted the limited data on pregnant women exposed to CAB + RPV. They analyzed pregnancies in four phase 2b/3/3b clinical trials sponsored by ViiV Healthcare and a compassionate use program. All clinical trial participants first received oral CAB + RPV daily for 4 weeks to assess individual tolerance before the experimental long-acting injection of CAB + RPV every 4 weeks or every 8 weeks.
Women participants were required to use highly effective contraception during the trials and for at least 52 weeks after the last injection. Urine pregnancy tests were given at baseline, before each injection, and when pregnancy was suspected. If a pregnancy was detected, CAB + RPV (oral or long-acting injections) was discontinued and the woman switched to an alternative oral antiretroviral, unless she and her physician decided to continue with injections in the compassionate use program.
Pregnancy outcomes
Among 25 reported pregnancies in 22 women during the trial, there were 10 live births. Nine of the mothers who delivered their babies at term had switched to an alternative antiretroviral regimen and maintained virologic suppression throughout pregnancy and post partum, or the last available viral load assessment.
The 10th participant remained on long-acting CAB + RPV during her pregnancy and had a live birth with congenital ptosis that was resolving without treatment at the 4-month ophthalmology consult, the authors wrote. The mother experienced persistent low-level viremia before and throughout her pregnancy.
Two of the pregnancies occurred after the last monthly injection, during the washout period. Other studies have reported that each long-acting drug, CAB and RPV, can be detected more than 1 year after the last injection. In the new report, plasma CAB and RPV washout concentrations during pregnancy were within the range of those in nonpregnant women, the authors wrote.
Among the 14 participants with non–live birth outcomes, 13 switched to an alternative antiretroviral regimen during pregnancy and maintained virologic suppression through pregnancy and post partum, or until their last viral assessment. The remaining participant received long-acting CAB + RPV and continued this treatment for the duration of their pregnancy.
“It’s a very limited data set, so we’re not in a position to be able to make definitive conclusions around long-acting cabotegravir-rilpivirine in pregnancy,” Dr. Patel acknowledged. “But the data that we presented among the 25 women who were exposed to cabotegravir-rilpivirine looks reassuring.”
Planned studies during pregnancy
Vani Vannappagari, MBBS, MPH, PhD, global head of epidemiology and real-world evidence at ViiV Healthcare and study coauthor, said in an interview that the initial results are spurring promising new research.
“We are working with an external IMPAACT [International Maternal Pediatric Adolescent AIDS Clinical Trials Network] group on a clinical trial ... to try to determine the appropriate dose of long-acting cabotegravir-rilpivirine during pregnancy,” Dr. Vannappagari said. “The clinical trial will give us the immediate safety, dose information, and viral suppression rates for both the mother and the infant. But long-term safety, especially birth defects and any adverse pregnancy and neonatal outcomes, will come from our antiretroviral pregnancy registry and other noninterventional studies.
“In the very small cohort studied, [in] pregnancies that were continued after exposure to long-acting cabotegravir and rilpivirine in the first trimester, there were no significant adverse fetal outcomes identified,” he said. “That’s reassuring, as is the fact that at the time these patients were switched in early pregnancy, their viral loads were all undetectable at the time that their pregnancies were diagnosed.”
Neil Silverman, MD, professor of clinical obstetrics and gynecology and director of the Infections in Pregnancy Program at the University of California, Los Angeles, Medical Center, who was not associated with the study, provided a comment to this news organization.
“The larger question still remains why pregnant women were so actively excluded from the original study design when this trial was evaluating a newer long-acting preparation of two anti-HIV medications that otherwise would be perfectly fine to use during pregnancy?”
Dr. Silverman continued, “In this case, it’s particularly frustrating since the present study was simply evaluating established medications currently being used to manage HIV infection, but in a newer longer-acting mode of administration by an injection every 2 months. If a patient had already been successfully managed on an oral antiviral regimen containing an integrase inhibitor and a non-nucleoside reverse transcriptase inhibitor, like the two drugs studied here, it would not be considered reasonable to switch that regimen simply because she was found to be pregnant.”
Dr. Patel and Dr. Vannappagari are employees of ViiV Healthcare and stockholders of GlaxoSmithKline.
This analysis was funded by ViiV Healthcare, and all studies were cofunded by ViiV Healthcare and Janssen Research & Development. Dr. Silverman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a cautiously optimistic report,
Among 10 live births, there was one birth defect (congenital ptosis, or droopy eyelid), which was not attributed to the trial drugs. There were no instances of perinatal HIV transmission at delivery or during the 1-year follow-up.
“Long-acting cabotegravir-rilpivirine is the first and only complete injectable regimen potentially available for pregnant women,” first author Parul Patel, PharmD, global medical affairs director for cabotegravir at ViiV Healthcare, said in an interview. The regimen was approved by the U.S. Food and Drug Administration in January 2021 for injections every 4 weeks and in February 2022 for injections every 8 weeks.
“Importantly, it can be dosed monthly or every 2 months,” Patel said. “This could be advantageous for women who are experiencing constant change during pregnancy. This could be a consideration for women who might have problems tolerating oral pills during pregnancy or might have problems with emesis.”
The study was published in HIV Medicine.
“We are really pursuing the development of the long-acting version of cabotegravir in combination with rilpivirine,” Dr. Patel said. “It’s an industry standard during initial development that you start very conservatively and not allow a woman who is pregnant to continue dosing of a drug while still evaluating its overall safety profile. We really want to understand the use of this agent in nonpregnant adults before exposing pregnant women to active treatment.”
Pregnancies in trials excluding pregnant women
In the paper, Dr. Patel and her coauthors noted the limited data on pregnant women exposed to CAB + RPV. They analyzed pregnancies in four phase 2b/3/3b clinical trials sponsored by ViiV Healthcare and a compassionate use program. All clinical trial participants first received oral CAB + RPV daily for 4 weeks to assess individual tolerance before the experimental long-acting injection of CAB + RPV every 4 weeks or every 8 weeks.
Women participants were required to use highly effective contraception during the trials and for at least 52 weeks after the last injection. Urine pregnancy tests were given at baseline, before each injection, and when pregnancy was suspected. If a pregnancy was detected, CAB + RPV (oral or long-acting injections) was discontinued and the woman switched to an alternative oral antiretroviral, unless she and her physician decided to continue with injections in the compassionate use program.
Pregnancy outcomes
Among 25 reported pregnancies in 22 women during the trial, there were 10 live births. Nine of the mothers who delivered their babies at term had switched to an alternative antiretroviral regimen and maintained virologic suppression throughout pregnancy and post partum, or the last available viral load assessment.
The 10th participant remained on long-acting CAB + RPV during her pregnancy and had a live birth with congenital ptosis that was resolving without treatment at the 4-month ophthalmology consult, the authors wrote. The mother experienced persistent low-level viremia before and throughout her pregnancy.
Two of the pregnancies occurred after the last monthly injection, during the washout period. Other studies have reported that each long-acting drug, CAB and RPV, can be detected more than 1 year after the last injection. In the new report, plasma CAB and RPV washout concentrations during pregnancy were within the range of those in nonpregnant women, the authors wrote.
Among the 14 participants with non–live birth outcomes, 13 switched to an alternative antiretroviral regimen during pregnancy and maintained virologic suppression through pregnancy and post partum, or until their last viral assessment. The remaining participant received long-acting CAB + RPV and continued this treatment for the duration of their pregnancy.
“It’s a very limited data set, so we’re not in a position to be able to make definitive conclusions around long-acting cabotegravir-rilpivirine in pregnancy,” Dr. Patel acknowledged. “But the data that we presented among the 25 women who were exposed to cabotegravir-rilpivirine looks reassuring.”
Planned studies during pregnancy
Vani Vannappagari, MBBS, MPH, PhD, global head of epidemiology and real-world evidence at ViiV Healthcare and study coauthor, said in an interview that the initial results are spurring promising new research.
“We are working with an external IMPAACT [International Maternal Pediatric Adolescent AIDS Clinical Trials Network] group on a clinical trial ... to try to determine the appropriate dose of long-acting cabotegravir-rilpivirine during pregnancy,” Dr. Vannappagari said. “The clinical trial will give us the immediate safety, dose information, and viral suppression rates for both the mother and the infant. But long-term safety, especially birth defects and any adverse pregnancy and neonatal outcomes, will come from our antiretroviral pregnancy registry and other noninterventional studies.
“In the very small cohort studied, [in] pregnancies that were continued after exposure to long-acting cabotegravir and rilpivirine in the first trimester, there were no significant adverse fetal outcomes identified,” he said. “That’s reassuring, as is the fact that at the time these patients were switched in early pregnancy, their viral loads were all undetectable at the time that their pregnancies were diagnosed.”
Neil Silverman, MD, professor of clinical obstetrics and gynecology and director of the Infections in Pregnancy Program at the University of California, Los Angeles, Medical Center, who was not associated with the study, provided a comment to this news organization.
“The larger question still remains why pregnant women were so actively excluded from the original study design when this trial was evaluating a newer long-acting preparation of two anti-HIV medications that otherwise would be perfectly fine to use during pregnancy?”
Dr. Silverman continued, “In this case, it’s particularly frustrating since the present study was simply evaluating established medications currently being used to manage HIV infection, but in a newer longer-acting mode of administration by an injection every 2 months. If a patient had already been successfully managed on an oral antiviral regimen containing an integrase inhibitor and a non-nucleoside reverse transcriptase inhibitor, like the two drugs studied here, it would not be considered reasonable to switch that regimen simply because she was found to be pregnant.”
Dr. Patel and Dr. Vannappagari are employees of ViiV Healthcare and stockholders of GlaxoSmithKline.
This analysis was funded by ViiV Healthcare, and all studies were cofunded by ViiV Healthcare and Janssen Research & Development. Dr. Silverman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HIV MEDICINE
U.S. sees most flu hospitalizations in a decade
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.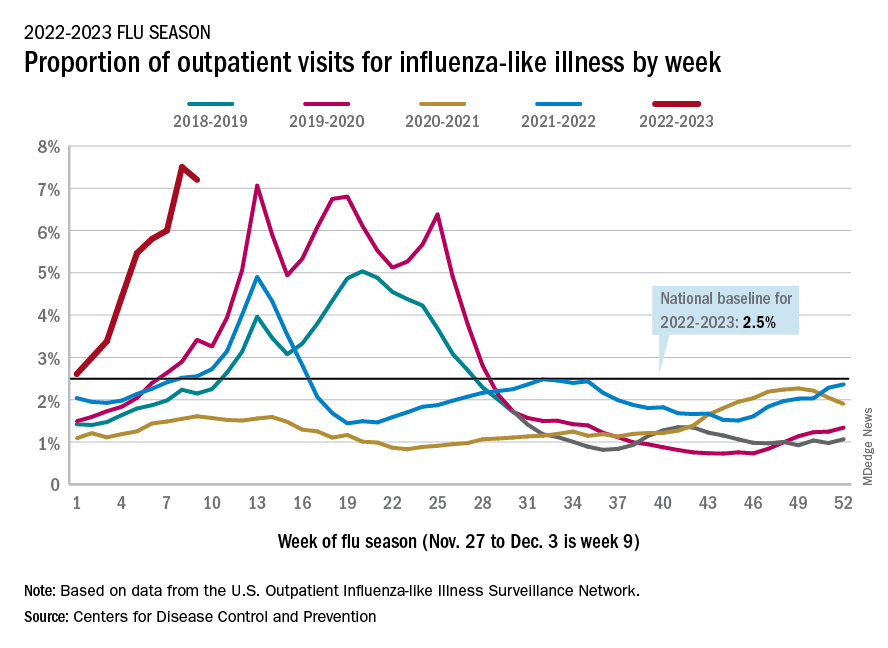
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.



