User login
-
Unexplained collapse unveils rare blood disorder
This case report was published in the New England Journal of Medicine.
Noting the patient’s confusion and aphasia, emergency medical services were alerted, and she was taken to the emergency department of Massachusetts General Hospital. Initial examination revealed aphasia and coordination difficulties. However, imaging studies, including CT angiography, showed no signs of stroke or other neurological abnormalities.
The patient’s coworkers had observed that she appeared “unwell.” Her medical history included hypertension, which was managed with amlodipine, and there was no known family history of neurologic disorders.
During the examination, her vital signs were within normal ranges.
The patient’s potassium level of 2.5 mmol/L was noteworthy, indicating hypokalemia. Additionally, the patient presented with anemia and thrombocytopenia. Additional laboratory results unveiled thrombotic thrombocytopenic purpura (TTP), a rare blood disorder characterized by microangiopathic hemolytic anemia. The microscopic examination of a peripheral blood smear confirmed the extent of thrombocytopenia and was particularly notable for the increased number of schistocytes. The patient’s peripheral blood smear revealed five or six schistocytes per high-power field, constituting approximately 5% of the red cells. This significant number of schistocytes aligned with the severity of anemia and thrombocytopenia, confirming the diagnosis of microangiopathic hemolytic anemia.
Acquired TTP is an autoimmune condition driven by antibody-mediated clearance of the plasma enzyme ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin motif 13). Confirmatory laboratory testing for ADAMTS13 takes 1-3 days; therefore, therapeutic plasma exchange with glucocorticoid therapy and rituximab was initiated, which promptly improved her condition.
In this patient, the ADAMTS13 activity level was severely reduced (< 5%; reference value > 67%), and the inhibitor was present (1.4 inhibitor units; reference value ≤ 0.4).
Rectal cancer was diagnosed in this patient 2 months after the diagnosis of acquired TTP.
After undergoing four weekly infusions of rituximab and a 2-month tapering course of glucocorticoids, the patient experienced a relapse, approximately 6 months following the acquired TTP diagnosis. In response, therapeutic plasma exchange and glucocorticoid therapy were administered. There is a possibility that the underlying cancer played a role in the relapse. To minimize the risk for recurrence, the patient also received a second round of rituximab.
While establishing a clear cause is difficult, acquired TTP often appears to arise in connection with either an immune trigger, such as a viral infection, or immune dysregulation associated with another autoimmune disease or ongoing cancer. In this case, 4 weeks before the acquired TTP diagnosis, the patient had experienced COVID-19, which was likely to be the most probable trigger. However, rectal cancer was also identified in the patient, and whether these conditions are directly linked remains unclear.
A version of this article first appeared on Medscape.com.
This case report was published in the New England Journal of Medicine.
Noting the patient’s confusion and aphasia, emergency medical services were alerted, and she was taken to the emergency department of Massachusetts General Hospital. Initial examination revealed aphasia and coordination difficulties. However, imaging studies, including CT angiography, showed no signs of stroke or other neurological abnormalities.
The patient’s coworkers had observed that she appeared “unwell.” Her medical history included hypertension, which was managed with amlodipine, and there was no known family history of neurologic disorders.
During the examination, her vital signs were within normal ranges.
The patient’s potassium level of 2.5 mmol/L was noteworthy, indicating hypokalemia. Additionally, the patient presented with anemia and thrombocytopenia. Additional laboratory results unveiled thrombotic thrombocytopenic purpura (TTP), a rare blood disorder characterized by microangiopathic hemolytic anemia. The microscopic examination of a peripheral blood smear confirmed the extent of thrombocytopenia and was particularly notable for the increased number of schistocytes. The patient’s peripheral blood smear revealed five or six schistocytes per high-power field, constituting approximately 5% of the red cells. This significant number of schistocytes aligned with the severity of anemia and thrombocytopenia, confirming the diagnosis of microangiopathic hemolytic anemia.
Acquired TTP is an autoimmune condition driven by antibody-mediated clearance of the plasma enzyme ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin motif 13). Confirmatory laboratory testing for ADAMTS13 takes 1-3 days; therefore, therapeutic plasma exchange with glucocorticoid therapy and rituximab was initiated, which promptly improved her condition.
In this patient, the ADAMTS13 activity level was severely reduced (< 5%; reference value > 67%), and the inhibitor was present (1.4 inhibitor units; reference value ≤ 0.4).
Rectal cancer was diagnosed in this patient 2 months after the diagnosis of acquired TTP.
After undergoing four weekly infusions of rituximab and a 2-month tapering course of glucocorticoids, the patient experienced a relapse, approximately 6 months following the acquired TTP diagnosis. In response, therapeutic plasma exchange and glucocorticoid therapy were administered. There is a possibility that the underlying cancer played a role in the relapse. To minimize the risk for recurrence, the patient also received a second round of rituximab.
While establishing a clear cause is difficult, acquired TTP often appears to arise in connection with either an immune trigger, such as a viral infection, or immune dysregulation associated with another autoimmune disease or ongoing cancer. In this case, 4 weeks before the acquired TTP diagnosis, the patient had experienced COVID-19, which was likely to be the most probable trigger. However, rectal cancer was also identified in the patient, and whether these conditions are directly linked remains unclear.
A version of this article first appeared on Medscape.com.
This case report was published in the New England Journal of Medicine.
Noting the patient’s confusion and aphasia, emergency medical services were alerted, and she was taken to the emergency department of Massachusetts General Hospital. Initial examination revealed aphasia and coordination difficulties. However, imaging studies, including CT angiography, showed no signs of stroke or other neurological abnormalities.
The patient’s coworkers had observed that she appeared “unwell.” Her medical history included hypertension, which was managed with amlodipine, and there was no known family history of neurologic disorders.
During the examination, her vital signs were within normal ranges.
The patient’s potassium level of 2.5 mmol/L was noteworthy, indicating hypokalemia. Additionally, the patient presented with anemia and thrombocytopenia. Additional laboratory results unveiled thrombotic thrombocytopenic purpura (TTP), a rare blood disorder characterized by microangiopathic hemolytic anemia. The microscopic examination of a peripheral blood smear confirmed the extent of thrombocytopenia and was particularly notable for the increased number of schistocytes. The patient’s peripheral blood smear revealed five or six schistocytes per high-power field, constituting approximately 5% of the red cells. This significant number of schistocytes aligned with the severity of anemia and thrombocytopenia, confirming the diagnosis of microangiopathic hemolytic anemia.
Acquired TTP is an autoimmune condition driven by antibody-mediated clearance of the plasma enzyme ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin motif 13). Confirmatory laboratory testing for ADAMTS13 takes 1-3 days; therefore, therapeutic plasma exchange with glucocorticoid therapy and rituximab was initiated, which promptly improved her condition.
In this patient, the ADAMTS13 activity level was severely reduced (< 5%; reference value > 67%), and the inhibitor was present (1.4 inhibitor units; reference value ≤ 0.4).
Rectal cancer was diagnosed in this patient 2 months after the diagnosis of acquired TTP.
After undergoing four weekly infusions of rituximab and a 2-month tapering course of glucocorticoids, the patient experienced a relapse, approximately 6 months following the acquired TTP diagnosis. In response, therapeutic plasma exchange and glucocorticoid therapy were administered. There is a possibility that the underlying cancer played a role in the relapse. To minimize the risk for recurrence, the patient also received a second round of rituximab.
While establishing a clear cause is difficult, acquired TTP often appears to arise in connection with either an immune trigger, such as a viral infection, or immune dysregulation associated with another autoimmune disease or ongoing cancer. In this case, 4 weeks before the acquired TTP diagnosis, the patient had experienced COVID-19, which was likely to be the most probable trigger. However, rectal cancer was also identified in the patient, and whether these conditions are directly linked remains unclear.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
FTC considers proposals on mergers and noncompete clauses
Changes may be in store for how physicians do business based on pending proposals from the Federal Trade Commission to ban noncompete clauses and monitor potential merger monopolies.
In January 2023, the FTC announced a rule that would ban noncompete clauses, stating that such clauses reduce workers’ wages and stifle new businesses. Simply put, the rule would ban employers from entering into noncompete clauses with workers, including independent contractors.
Aspects of the rule include whether it should pertain to franchisees, whether senior executives should be exempted, and whether low-wage and high-wage workers should be treated differently.
According to the FTC, banning noncompete clauses would increase workers’ earnings by approximately $300 billion per year, save consumers as much as $148 billion in health care costs, and double the number of companies founded by former workers in the same field.
In June 2023, the FTC and the Department of Justice proposed changes to rules governing mergers, including changes to prenotification forms that would promote more efficient screening of potential mergers. According to a press release from the FTC, the proposed changes include provision of details about investments or corporate relationships, product and services, projected revenue streams, and previous acquisitions.
The proposal also includes a waiting period during which agencies would assess the risk that a merger would lessen competition or tend to create a monopoly.
What the FTC proposals mean for physicians
FTC Chair Lina M. Khan addressed attendees at the American College of Physicians at their annual meeting in October.
In March 2023, ACEP wrote to Ms. Khan in support of the banning of noncompete clauses. The ACEP also stated that the FTC should monitor the effect of a ban on the ability to recruit and maintain a stable physician workforce in rural and underserved areas “and should examine the potential impacts should nonprofit health systems be exempt from a ban.”
However, the American Medical Group Association, a nonprofit trade organization that supports multispecialty medical groups, opposes the ban. In a press release issued in March 2023, AMGA noted that, “As employers, AMGA members rely in part on noncompete agreements to build strong, sustainable care teams that work together to coordinate care for their patients. These care teams emphasize the importance of the doctor-patient relationship, which reasonable noncompete agreements help support.”
The American Medical Association supports the ban on noncompete clauses, detailed in an official AMA policy statement as, “support[ing] policies, regulations, and legislation that prohibits covenants not-to-compete for all physicians in clinical practice who hold employment contracts with for-profit or nonprofit hospital, hospital system, or staffing company employers.”
In regard to the merger guidelines, ACEP wrote a separate letter to Ms. Khan identifying some of the unique aspects of emergency medicine practice. The ACEP stressed the need for caution as the consolidation of medical practices continues, many under the umbrella of private equity investment companies.
“Unchecked mergers that substantially lessen competition in the labor market for emergency physicians, in which the employer is the buyer and the physician is the seller, can impact physicians directly by lowering wages or slowing wage growth, worsening benefits or working conditions, or contributing to other degradations in workplace quality,” according to ACEP.
The AMA also supports the FTC’s draft merger guidelines as protective of physicians and their working environments.
In September 2023, the AMA sent a letter to the FTC commending the agency on the proposed guidelines: “It is our strong contention that the agencies must have merger guidelines that protect physicians against health insurer mergers that may substantially lessen competition for the purchase of physician services and that degrade physician working conditions,” according to the AMA letter.
According the FTC, the proposed changes represent an expansion and reorganization of information along with the addition of new document requirements and represents the first comprehensive review of the Hart-Scott-Rodino Antitrust Improvements Act since 1978.
After soliciting public comments, the FTC is reviewing the proposals, and no specific date for a final vote has been announced.
More specifics on the potential changes to premerger notification, reporting, and waiting period requirements are available on the FTC website.
A version of this article appeared on Medscape.com.
Changes may be in store for how physicians do business based on pending proposals from the Federal Trade Commission to ban noncompete clauses and monitor potential merger monopolies.
In January 2023, the FTC announced a rule that would ban noncompete clauses, stating that such clauses reduce workers’ wages and stifle new businesses. Simply put, the rule would ban employers from entering into noncompete clauses with workers, including independent contractors.
Aspects of the rule include whether it should pertain to franchisees, whether senior executives should be exempted, and whether low-wage and high-wage workers should be treated differently.
According to the FTC, banning noncompete clauses would increase workers’ earnings by approximately $300 billion per year, save consumers as much as $148 billion in health care costs, and double the number of companies founded by former workers in the same field.
In June 2023, the FTC and the Department of Justice proposed changes to rules governing mergers, including changes to prenotification forms that would promote more efficient screening of potential mergers. According to a press release from the FTC, the proposed changes include provision of details about investments or corporate relationships, product and services, projected revenue streams, and previous acquisitions.
The proposal also includes a waiting period during which agencies would assess the risk that a merger would lessen competition or tend to create a monopoly.
What the FTC proposals mean for physicians
FTC Chair Lina M. Khan addressed attendees at the American College of Physicians at their annual meeting in October.
In March 2023, ACEP wrote to Ms. Khan in support of the banning of noncompete clauses. The ACEP also stated that the FTC should monitor the effect of a ban on the ability to recruit and maintain a stable physician workforce in rural and underserved areas “and should examine the potential impacts should nonprofit health systems be exempt from a ban.”
However, the American Medical Group Association, a nonprofit trade organization that supports multispecialty medical groups, opposes the ban. In a press release issued in March 2023, AMGA noted that, “As employers, AMGA members rely in part on noncompete agreements to build strong, sustainable care teams that work together to coordinate care for their patients. These care teams emphasize the importance of the doctor-patient relationship, which reasonable noncompete agreements help support.”
The American Medical Association supports the ban on noncompete clauses, detailed in an official AMA policy statement as, “support[ing] policies, regulations, and legislation that prohibits covenants not-to-compete for all physicians in clinical practice who hold employment contracts with for-profit or nonprofit hospital, hospital system, or staffing company employers.”
In regard to the merger guidelines, ACEP wrote a separate letter to Ms. Khan identifying some of the unique aspects of emergency medicine practice. The ACEP stressed the need for caution as the consolidation of medical practices continues, many under the umbrella of private equity investment companies.
“Unchecked mergers that substantially lessen competition in the labor market for emergency physicians, in which the employer is the buyer and the physician is the seller, can impact physicians directly by lowering wages or slowing wage growth, worsening benefits or working conditions, or contributing to other degradations in workplace quality,” according to ACEP.
The AMA also supports the FTC’s draft merger guidelines as protective of physicians and their working environments.
In September 2023, the AMA sent a letter to the FTC commending the agency on the proposed guidelines: “It is our strong contention that the agencies must have merger guidelines that protect physicians against health insurer mergers that may substantially lessen competition for the purchase of physician services and that degrade physician working conditions,” according to the AMA letter.
According the FTC, the proposed changes represent an expansion and reorganization of information along with the addition of new document requirements and represents the first comprehensive review of the Hart-Scott-Rodino Antitrust Improvements Act since 1978.
After soliciting public comments, the FTC is reviewing the proposals, and no specific date for a final vote has been announced.
More specifics on the potential changes to premerger notification, reporting, and waiting period requirements are available on the FTC website.
A version of this article appeared on Medscape.com.
Changes may be in store for how physicians do business based on pending proposals from the Federal Trade Commission to ban noncompete clauses and monitor potential merger monopolies.
In January 2023, the FTC announced a rule that would ban noncompete clauses, stating that such clauses reduce workers’ wages and stifle new businesses. Simply put, the rule would ban employers from entering into noncompete clauses with workers, including independent contractors.
Aspects of the rule include whether it should pertain to franchisees, whether senior executives should be exempted, and whether low-wage and high-wage workers should be treated differently.
According to the FTC, banning noncompete clauses would increase workers’ earnings by approximately $300 billion per year, save consumers as much as $148 billion in health care costs, and double the number of companies founded by former workers in the same field.
In June 2023, the FTC and the Department of Justice proposed changes to rules governing mergers, including changes to prenotification forms that would promote more efficient screening of potential mergers. According to a press release from the FTC, the proposed changes include provision of details about investments or corporate relationships, product and services, projected revenue streams, and previous acquisitions.
The proposal also includes a waiting period during which agencies would assess the risk that a merger would lessen competition or tend to create a monopoly.
What the FTC proposals mean for physicians
FTC Chair Lina M. Khan addressed attendees at the American College of Physicians at their annual meeting in October.
In March 2023, ACEP wrote to Ms. Khan in support of the banning of noncompete clauses. The ACEP also stated that the FTC should monitor the effect of a ban on the ability to recruit and maintain a stable physician workforce in rural and underserved areas “and should examine the potential impacts should nonprofit health systems be exempt from a ban.”
However, the American Medical Group Association, a nonprofit trade organization that supports multispecialty medical groups, opposes the ban. In a press release issued in March 2023, AMGA noted that, “As employers, AMGA members rely in part on noncompete agreements to build strong, sustainable care teams that work together to coordinate care for their patients. These care teams emphasize the importance of the doctor-patient relationship, which reasonable noncompete agreements help support.”
The American Medical Association supports the ban on noncompete clauses, detailed in an official AMA policy statement as, “support[ing] policies, regulations, and legislation that prohibits covenants not-to-compete for all physicians in clinical practice who hold employment contracts with for-profit or nonprofit hospital, hospital system, or staffing company employers.”
In regard to the merger guidelines, ACEP wrote a separate letter to Ms. Khan identifying some of the unique aspects of emergency medicine practice. The ACEP stressed the need for caution as the consolidation of medical practices continues, many under the umbrella of private equity investment companies.
“Unchecked mergers that substantially lessen competition in the labor market for emergency physicians, in which the employer is the buyer and the physician is the seller, can impact physicians directly by lowering wages or slowing wage growth, worsening benefits or working conditions, or contributing to other degradations in workplace quality,” according to ACEP.
The AMA also supports the FTC’s draft merger guidelines as protective of physicians and their working environments.
In September 2023, the AMA sent a letter to the FTC commending the agency on the proposed guidelines: “It is our strong contention that the agencies must have merger guidelines that protect physicians against health insurer mergers that may substantially lessen competition for the purchase of physician services and that degrade physician working conditions,” according to the AMA letter.
According the FTC, the proposed changes represent an expansion and reorganization of information along with the addition of new document requirements and represents the first comprehensive review of the Hart-Scott-Rodino Antitrust Improvements Act since 1978.
After soliciting public comments, the FTC is reviewing the proposals, and no specific date for a final vote has been announced.
More specifics on the potential changes to premerger notification, reporting, and waiting period requirements are available on the FTC website.
A version of this article appeared on Medscape.com.
Study confirms small blood cancer risk from CT scans
The findings, published online in Nature Medicine, are based on more than 1.3 million CT scans in nearly 900,000 people younger than 22 years old when scanned.
This study makes a “significant contribution to the understanding of the effects of ionizing radiation, specifically x-rays, on the human body at the levels of radiation exposure encountered in diagnostic CT procedures,” Peter Marsden, PhD, and Jim Thurston, radiation protection experts at Dorset County (England) Hospital, NHS Foundation Trust, said in a press release from the U.K. nonprofit Science Media Centre.
These findings highlight levels of risk that “align with those currently estimated and do not suggest that the use of CT carries a greater risk than previously thought,” Dr. Marsden and Thurston said.
Exposure to moderate- (≥ 100 mGy) to high-dose (≥ 1 Gy) ionizing radiation is a well-established risk factor for leukemia in both children and adults. However, the risk associated with low-dose exposure (< 100 mGy) typically associated with diagnostic CT exams in children and teens remains unclear.
The current study, coordinated by the International Agency for Research on Cancer, aimed to improve direct estimates of cancer risk from low-dose radiation exposure from CT scans performed in childhood and adolescence. The researchers estimated radiation doses to the active bone marrow based on body part scanned, patient characteristics, time period, and inferred CT technical parameters.
A total of 790 hematologic malignancies, including lymphoid and myeloid malignancies, were identified during follow-up. More than half (51%) of the cases were diagnosed in people under age 20 and 88.5% were diagnosed in people under age 30 years.
Overall, the observational study found a nearly twofold excess risk of all hematologic malignancies per 100 mGy in children, adolescents, and young adults, with similar risk estimates observed for lymphoid and myeloid cancers. The excess relative risk for hematologic malignancies increased as the number of CT exams increased – with risk rising by 43% per exam.
The results of this study “strengthen the findings from previous low-dose studies of a consistent and robust dose-related increased risk of radiation-induced hematological malignancies” and highlight the importance of optimizing doses in this patient population, study author Elisabeth Cardis, PhD, with the Barcelona Institute for Global Health, and colleagues concluded.
Sarah McQuaid, PhD, chair of the nuclear medicine special interest group, Institute of Physics and Engineering in Medicine, York, England, agreed.
“This publication indicates that there could be a small cancer risk from CT scans in young people, but it is important for this to be viewed in the context of the substantial benefit these scans bring, due to the important diagnostic information they provide,” Dr. McQuaid said in the press release. Overall, “the number of patients whose medical care will have been improved from these CT scans will have been very high, and lives undoubtedly saved as a result.”
The study had no commercial funding. The authors and outside experts reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings, published online in Nature Medicine, are based on more than 1.3 million CT scans in nearly 900,000 people younger than 22 years old when scanned.
This study makes a “significant contribution to the understanding of the effects of ionizing radiation, specifically x-rays, on the human body at the levels of radiation exposure encountered in diagnostic CT procedures,” Peter Marsden, PhD, and Jim Thurston, radiation protection experts at Dorset County (England) Hospital, NHS Foundation Trust, said in a press release from the U.K. nonprofit Science Media Centre.
These findings highlight levels of risk that “align with those currently estimated and do not suggest that the use of CT carries a greater risk than previously thought,” Dr. Marsden and Thurston said.
Exposure to moderate- (≥ 100 mGy) to high-dose (≥ 1 Gy) ionizing radiation is a well-established risk factor for leukemia in both children and adults. However, the risk associated with low-dose exposure (< 100 mGy) typically associated with diagnostic CT exams in children and teens remains unclear.
The current study, coordinated by the International Agency for Research on Cancer, aimed to improve direct estimates of cancer risk from low-dose radiation exposure from CT scans performed in childhood and adolescence. The researchers estimated radiation doses to the active bone marrow based on body part scanned, patient characteristics, time period, and inferred CT technical parameters.
A total of 790 hematologic malignancies, including lymphoid and myeloid malignancies, were identified during follow-up. More than half (51%) of the cases were diagnosed in people under age 20 and 88.5% were diagnosed in people under age 30 years.
Overall, the observational study found a nearly twofold excess risk of all hematologic malignancies per 100 mGy in children, adolescents, and young adults, with similar risk estimates observed for lymphoid and myeloid cancers. The excess relative risk for hematologic malignancies increased as the number of CT exams increased – with risk rising by 43% per exam.
The results of this study “strengthen the findings from previous low-dose studies of a consistent and robust dose-related increased risk of radiation-induced hematological malignancies” and highlight the importance of optimizing doses in this patient population, study author Elisabeth Cardis, PhD, with the Barcelona Institute for Global Health, and colleagues concluded.
Sarah McQuaid, PhD, chair of the nuclear medicine special interest group, Institute of Physics and Engineering in Medicine, York, England, agreed.
“This publication indicates that there could be a small cancer risk from CT scans in young people, but it is important for this to be viewed in the context of the substantial benefit these scans bring, due to the important diagnostic information they provide,” Dr. McQuaid said in the press release. Overall, “the number of patients whose medical care will have been improved from these CT scans will have been very high, and lives undoubtedly saved as a result.”
The study had no commercial funding. The authors and outside experts reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings, published online in Nature Medicine, are based on more than 1.3 million CT scans in nearly 900,000 people younger than 22 years old when scanned.
This study makes a “significant contribution to the understanding of the effects of ionizing radiation, specifically x-rays, on the human body at the levels of radiation exposure encountered in diagnostic CT procedures,” Peter Marsden, PhD, and Jim Thurston, radiation protection experts at Dorset County (England) Hospital, NHS Foundation Trust, said in a press release from the U.K. nonprofit Science Media Centre.
These findings highlight levels of risk that “align with those currently estimated and do not suggest that the use of CT carries a greater risk than previously thought,” Dr. Marsden and Thurston said.
Exposure to moderate- (≥ 100 mGy) to high-dose (≥ 1 Gy) ionizing radiation is a well-established risk factor for leukemia in both children and adults. However, the risk associated with low-dose exposure (< 100 mGy) typically associated with diagnostic CT exams in children and teens remains unclear.
The current study, coordinated by the International Agency for Research on Cancer, aimed to improve direct estimates of cancer risk from low-dose radiation exposure from CT scans performed in childhood and adolescence. The researchers estimated radiation doses to the active bone marrow based on body part scanned, patient characteristics, time period, and inferred CT technical parameters.
A total of 790 hematologic malignancies, including lymphoid and myeloid malignancies, were identified during follow-up. More than half (51%) of the cases were diagnosed in people under age 20 and 88.5% were diagnosed in people under age 30 years.
Overall, the observational study found a nearly twofold excess risk of all hematologic malignancies per 100 mGy in children, adolescents, and young adults, with similar risk estimates observed for lymphoid and myeloid cancers. The excess relative risk for hematologic malignancies increased as the number of CT exams increased – with risk rising by 43% per exam.
The results of this study “strengthen the findings from previous low-dose studies of a consistent and robust dose-related increased risk of radiation-induced hematological malignancies” and highlight the importance of optimizing doses in this patient population, study author Elisabeth Cardis, PhD, with the Barcelona Institute for Global Health, and colleagues concluded.
Sarah McQuaid, PhD, chair of the nuclear medicine special interest group, Institute of Physics and Engineering in Medicine, York, England, agreed.
“This publication indicates that there could be a small cancer risk from CT scans in young people, but it is important for this to be viewed in the context of the substantial benefit these scans bring, due to the important diagnostic information they provide,” Dr. McQuaid said in the press release. Overall, “the number of patients whose medical care will have been improved from these CT scans will have been very high, and lives undoubtedly saved as a result.”
The study had no commercial funding. The authors and outside experts reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Trial shows utility of small-volume blood collection tubes
A large Canadian clinical trial has found that using small-volume tubes to collect blood samples for laboratory testing of intensive care unit patients can reduce blood transfusions without affecting lab results.
“We showed in a large pragmatic cluster trial that automatically collect less blood for laboratory testing reduced red blood cell transfusions by about 10 units of red blood cells per 100 patients in the ICU,” lead study author Deborah M. Siegal, MD, associate professor at the University of Ottawa and scientist at the Ottawa Hospital Research Institute, said.
The study was coordinated by the Population Health Research Institute, an affiliate of McMaster University in Hamilton (Ont.) Health Sciences, where Dr. Siegal worked before moving to Ottawa.
The STRATUS randomized clinical trial, published in JAMA, involved 25 adult medical-surgical ICUs across Canada, where 21,201 patients were randomized to either standard-volume or small-volume tubes for collecting blood samples. During the course of the study, each site switched to the small-volume collection tubes.
“We also showed there were no negative effects on lab testing, and by that we measured the sufficiency of the specimens,” Dr. Siegal added. “We were able to show that there wasn’t a problem with the amount of blood that was available for the tests to be done.”
The samples were collected from February 2019 through January 2021, through the period of COVID-19 restrictions. Dr. Siegal explained that 6,210 patients admitted early in the COVID-19 pandemic were excluded from the primary analysis, but were included in secondary analyses.
Study results
While the study found no significant difference in RBC units per patient per ICU stage – a relative risk of .91 (95% confidence interval, 0.79-1.05; P = .19), it did find an absolute reduction of 7.24 RBC units/100 patients per ICU stay.
Findings from the secondary analyses, which included 27,411 patients, were:
- A 12% reduction in RBC units per patient per ICU stay after switching from standard-volume to small-volume tubes (RR, 0.88; 95% CI, 0.77-1; P = .04).
- An absolute reduction of 9.84 RBC units/100 patients per ICU stay (95% CI, 0.24-20.76).
In the primary analysis population, the median transfusion-adjusted hemoglobin was not statistically different between the standard- and small-volume collection tube groups, with an average difference of 0.1 g/dL (95% CI, –0.04 to .23), but it was lower in the secondary population, with a mean difference of .17 g/dL (95% CI, 0.05-0.29).
“Those patients that we analyzed in the secondary analysis population received about 36,000 units of blood, just in 25 ICU units in Canada in less than 2 years,” Dr. Siegal said. “If we saved 10 units per 100 patients, that’s 1,500 units of blood. That really speaks to a small effect at the individual patient level but really potential for widespread effect. We are now in a period of blood product shortage not only in Canada but worldwide.”
First clinical trial for small tubes
Dr. Siegal noted this was the first clinical trial to compare standard- and small-volume blood collection tools, “and also to show there is both a benefit and a lack of harm,” Dr. Siegal said. “We thought that a randomized trial was the best way to move the needle. If we could design a trial of a large population of patients to show benefit and no harm, it would be a win, and that’s in fact what happened.”
She added, “The tubes essentially have the same cost, work the same, and go on the same equipment the same way the standard-volume tubes do, so it wasn’t a practice change for people in the hospital.”
The study also found an identical low rate of unusable specimens did not differ regardless of the type of collection tube: less than .03%.
Dr. Siegal said the study group is collaborating with hematology stakeholders in Canada, including Canadian Blood Services, which provides blood plasma to the country’s provincial and territorial health systems, and is reaching out to the American Society of Hematology.
“We’re going to target both hematologists and critical care providers and, even more broadly than the critical care community, hospitals, because anemia is big problem in hospitals,” Dr. Siegal said. “I think we can think about this more broadly.”
The study received funding from the Hamilton Academic Health Sciences Organization. Dr. Siegal disclosed relationships with Bristol-Myers Squibb-Pfizer, AstraZeneca and Roche.
A large Canadian clinical trial has found that using small-volume tubes to collect blood samples for laboratory testing of intensive care unit patients can reduce blood transfusions without affecting lab results.
“We showed in a large pragmatic cluster trial that automatically collect less blood for laboratory testing reduced red blood cell transfusions by about 10 units of red blood cells per 100 patients in the ICU,” lead study author Deborah M. Siegal, MD, associate professor at the University of Ottawa and scientist at the Ottawa Hospital Research Institute, said.
The study was coordinated by the Population Health Research Institute, an affiliate of McMaster University in Hamilton (Ont.) Health Sciences, where Dr. Siegal worked before moving to Ottawa.
The STRATUS randomized clinical trial, published in JAMA, involved 25 adult medical-surgical ICUs across Canada, where 21,201 patients were randomized to either standard-volume or small-volume tubes for collecting blood samples. During the course of the study, each site switched to the small-volume collection tubes.
“We also showed there were no negative effects on lab testing, and by that we measured the sufficiency of the specimens,” Dr. Siegal added. “We were able to show that there wasn’t a problem with the amount of blood that was available for the tests to be done.”
The samples were collected from February 2019 through January 2021, through the period of COVID-19 restrictions. Dr. Siegal explained that 6,210 patients admitted early in the COVID-19 pandemic were excluded from the primary analysis, but were included in secondary analyses.
Study results
While the study found no significant difference in RBC units per patient per ICU stage – a relative risk of .91 (95% confidence interval, 0.79-1.05; P = .19), it did find an absolute reduction of 7.24 RBC units/100 patients per ICU stay.
Findings from the secondary analyses, which included 27,411 patients, were:
- A 12% reduction in RBC units per patient per ICU stay after switching from standard-volume to small-volume tubes (RR, 0.88; 95% CI, 0.77-1; P = .04).
- An absolute reduction of 9.84 RBC units/100 patients per ICU stay (95% CI, 0.24-20.76).
In the primary analysis population, the median transfusion-adjusted hemoglobin was not statistically different between the standard- and small-volume collection tube groups, with an average difference of 0.1 g/dL (95% CI, –0.04 to .23), but it was lower in the secondary population, with a mean difference of .17 g/dL (95% CI, 0.05-0.29).
“Those patients that we analyzed in the secondary analysis population received about 36,000 units of blood, just in 25 ICU units in Canada in less than 2 years,” Dr. Siegal said. “If we saved 10 units per 100 patients, that’s 1,500 units of blood. That really speaks to a small effect at the individual patient level but really potential for widespread effect. We are now in a period of blood product shortage not only in Canada but worldwide.”
First clinical trial for small tubes
Dr. Siegal noted this was the first clinical trial to compare standard- and small-volume blood collection tools, “and also to show there is both a benefit and a lack of harm,” Dr. Siegal said. “We thought that a randomized trial was the best way to move the needle. If we could design a trial of a large population of patients to show benefit and no harm, it would be a win, and that’s in fact what happened.”
She added, “The tubes essentially have the same cost, work the same, and go on the same equipment the same way the standard-volume tubes do, so it wasn’t a practice change for people in the hospital.”
The study also found an identical low rate of unusable specimens did not differ regardless of the type of collection tube: less than .03%.
Dr. Siegal said the study group is collaborating with hematology stakeholders in Canada, including Canadian Blood Services, which provides blood plasma to the country’s provincial and territorial health systems, and is reaching out to the American Society of Hematology.
“We’re going to target both hematologists and critical care providers and, even more broadly than the critical care community, hospitals, because anemia is big problem in hospitals,” Dr. Siegal said. “I think we can think about this more broadly.”
The study received funding from the Hamilton Academic Health Sciences Organization. Dr. Siegal disclosed relationships with Bristol-Myers Squibb-Pfizer, AstraZeneca and Roche.
A large Canadian clinical trial has found that using small-volume tubes to collect blood samples for laboratory testing of intensive care unit patients can reduce blood transfusions without affecting lab results.
“We showed in a large pragmatic cluster trial that automatically collect less blood for laboratory testing reduced red blood cell transfusions by about 10 units of red blood cells per 100 patients in the ICU,” lead study author Deborah M. Siegal, MD, associate professor at the University of Ottawa and scientist at the Ottawa Hospital Research Institute, said.
The study was coordinated by the Population Health Research Institute, an affiliate of McMaster University in Hamilton (Ont.) Health Sciences, where Dr. Siegal worked before moving to Ottawa.
The STRATUS randomized clinical trial, published in JAMA, involved 25 adult medical-surgical ICUs across Canada, where 21,201 patients were randomized to either standard-volume or small-volume tubes for collecting blood samples. During the course of the study, each site switched to the small-volume collection tubes.
“We also showed there were no negative effects on lab testing, and by that we measured the sufficiency of the specimens,” Dr. Siegal added. “We were able to show that there wasn’t a problem with the amount of blood that was available for the tests to be done.”
The samples were collected from February 2019 through January 2021, through the period of COVID-19 restrictions. Dr. Siegal explained that 6,210 patients admitted early in the COVID-19 pandemic were excluded from the primary analysis, but were included in secondary analyses.
Study results
While the study found no significant difference in RBC units per patient per ICU stage – a relative risk of .91 (95% confidence interval, 0.79-1.05; P = .19), it did find an absolute reduction of 7.24 RBC units/100 patients per ICU stay.
Findings from the secondary analyses, which included 27,411 patients, were:
- A 12% reduction in RBC units per patient per ICU stay after switching from standard-volume to small-volume tubes (RR, 0.88; 95% CI, 0.77-1; P = .04).
- An absolute reduction of 9.84 RBC units/100 patients per ICU stay (95% CI, 0.24-20.76).
In the primary analysis population, the median transfusion-adjusted hemoglobin was not statistically different between the standard- and small-volume collection tube groups, with an average difference of 0.1 g/dL (95% CI, –0.04 to .23), but it was lower in the secondary population, with a mean difference of .17 g/dL (95% CI, 0.05-0.29).
“Those patients that we analyzed in the secondary analysis population received about 36,000 units of blood, just in 25 ICU units in Canada in less than 2 years,” Dr. Siegal said. “If we saved 10 units per 100 patients, that’s 1,500 units of blood. That really speaks to a small effect at the individual patient level but really potential for widespread effect. We are now in a period of blood product shortage not only in Canada but worldwide.”
First clinical trial for small tubes
Dr. Siegal noted this was the first clinical trial to compare standard- and small-volume blood collection tools, “and also to show there is both a benefit and a lack of harm,” Dr. Siegal said. “We thought that a randomized trial was the best way to move the needle. If we could design a trial of a large population of patients to show benefit and no harm, it would be a win, and that’s in fact what happened.”
She added, “The tubes essentially have the same cost, work the same, and go on the same equipment the same way the standard-volume tubes do, so it wasn’t a practice change for people in the hospital.”
The study also found an identical low rate of unusable specimens did not differ regardless of the type of collection tube: less than .03%.
Dr. Siegal said the study group is collaborating with hematology stakeholders in Canada, including Canadian Blood Services, which provides blood plasma to the country’s provincial and territorial health systems, and is reaching out to the American Society of Hematology.
“We’re going to target both hematologists and critical care providers and, even more broadly than the critical care community, hospitals, because anemia is big problem in hospitals,” Dr. Siegal said. “I think we can think about this more broadly.”
The study received funding from the Hamilton Academic Health Sciences Organization. Dr. Siegal disclosed relationships with Bristol-Myers Squibb-Pfizer, AstraZeneca and Roche.
FROM JAMA
FDA approves first tx for rare, deadly clotting disorder
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
Congenital TTP affects fewer than 1,000 people in the United States and is caused by a mutation in the ADAMTS13 gene, which makes an enzyme that regulates blood clotting. Patients with the congenital TTP typically receive prophylactic plasma-based therapy to replenish the ADAMTS13 enzyme and reduce the risk for clotting and bleeding. The condition, however, can be fatal if left untreated.
The new agent is a purified recombinant form of the ADAMTS13 enzyme that works by replacing low levels of the deficient enzyme in patients with congenital TTP. Adzynma is given prophylactically to reduce the risk for disease symptoms and on demand when a patient is experiencing an acute event, according to the FDA approval announcement.
The approval was based on a global randomized phase 3 study comparing the product with plasma-based therapies in 46 patients with congenital TTP. Patients in the trial were randomized to receive 6 months of treatment with either intravenous Adzynma — given once every other week as prophylactic enzyme replacement therapy or once daily as on-demand enzyme replacement therapy — or plasma-based therapies. The patients then crossed over to the other treatment for 6 months.
Interim findings from the study showed that Adzynma reduced the incidence of thrombocytopenia — the most common symptom of congenital TTP — by 60% compared with plasma-based therapy (rate ratio, 0.40). No patients experienced an acute TTP event during Adzynma prophylaxis, Takeda said.
Significantly more patients receiving plasma-based therapies experienced treatment-emergent adverse events compared with those receiving the biologic.
The most common side effects associated with the biologic were headache (31.3%), diarrhea (16.7%), migraine (14.6%), abdominal pain (12.5%), nausea (12.5%), upper respiratory tract infection (12.5%), dizziness (10.4%), and vomiting (10.4%). No treatment-related adverse events, including allergic reactions, were observed during administration.
“The FDA remains deeply committed in our efforts to help facilitate the development and approval of safe and effective therapies for patients with rare diseases,” Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, stated. The “approval reflects important progress in the development of much-needed treatment options for patients affected by this life-threatening disorder.”
A version of this article first appeared on Medscape.com.
In MI with anemia, results may favor liberal transfusion: MINT
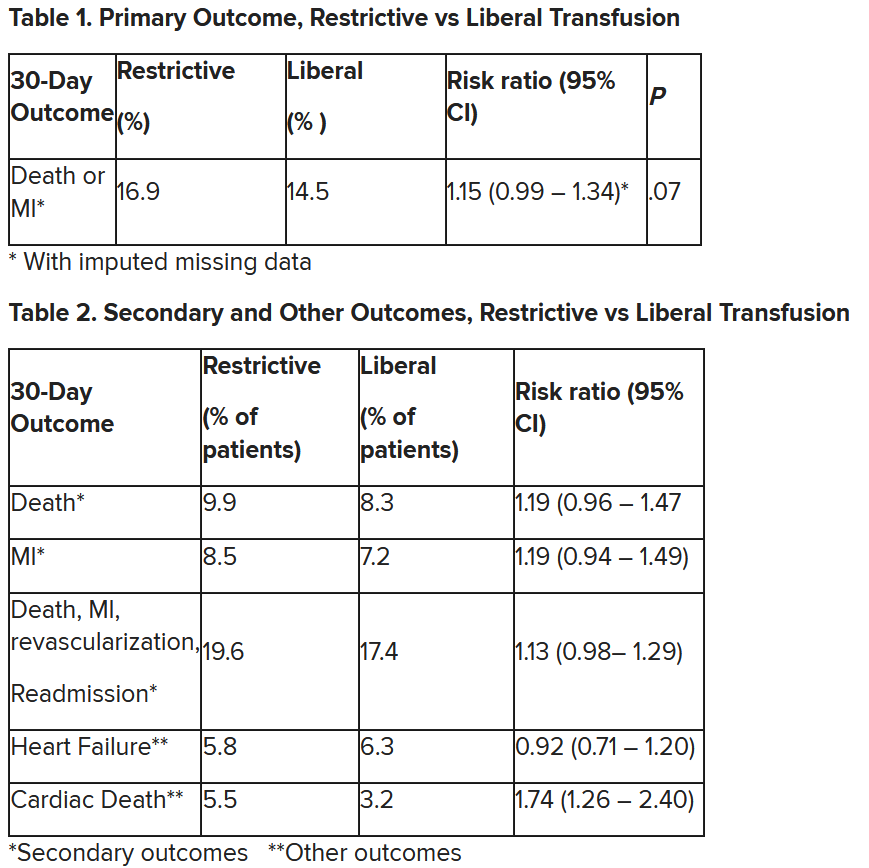
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with myocardial infarction and anemia, a “liberal” red blood cell transfusion strategy did not significantly reduce the risk of recurrent MI or death within 30 days, compared with a “restrictive” transfusion strategy, in the 3,500-patient MINT trial.
Jeffrey L. Carson, MD, from Robert Wood Johnson Medical School, New Brunswick, N.J., said in a press briefing.
He presented the study in a late-breaking trial session at the annual scientific sessions of the American Heart Association, and it was simultaneously published online in the New England Journal of Medicine.
“Whether to transfuse is an everyday decision faced by clinicians caring for patients with acute MI,” Dr. Carson said.
“We cannot claim that a liberal transfusion strategy is definitively superior based on our primary outcome,” he said, but “the 95% confidence interval is consistent with treatment effects corresponding to no difference between the two transfusion strategies and to a clinically relevant benefit with the liberal strategy.”
“In contrast to other trials in other settings,” such as anemia and cardiac surgery, Dr. Carson said, “the results suggest that a liberal transfusion strategy has the potential for clinical benefit with an acceptable risk of harm.”
“A liberal transfusion strategy may be the most prudent approach to transfusion in anemic patients with MI,” he added.
Not a home run
Others agreed with this interpretation. Martin B. Leon, MD, from Columbia University, New York, the study discussant in the press briefing, said the study “addresses a question that is common” in clinical practice. It was well conducted, and international (although most patients were in the United States and Canada), in a very broad group of patients, designed to make the results more generalizable. The 98% follow-up was extremely good, Dr. Leon added, and the trialists achieved their goal in that they did show a difference between the two transfusion strategies.
The number needed to treat was 40 to see a benefit in the combined outcome of death or recurrent MI at 30 days, Dr. Leon said. The P value for this was .07, “right on the edge” of statistical significance.
This study is “not a home run,” for the primary outcome, he noted; however, many of the outcomes tended to be in favor of a liberal transfusion strategy. Notably, cardiovascular death, which was not a specified outcome, was significantly lower in the group who received a liberal transfusion strategy.
Although a liberal transfusion strategy was “not definitely superior” in these patients with MI and anemia, Dr. Carson said, he thinks the trial will be interpreted as favoring a liberal transfusion strategy.
C. Michael Gibson, MD, professor of medicine at Harvard Medical School, Boston, and CEO of Harvard’s Baim and PERFUSE institutes for clinical research, voiced similar views.
“Given the lack of acute harm associated with liberal transfusion and the preponderance of evidence favoring liberal transfusion in the largest trial to date,” concluded Dr. Gibson, the assigned discussant at the session, “liberal transfusion appears to be a viable management strategy, particularly among patients with non-STEMI type 1 MI and as clinical judgment dictates.”
Only three small randomized controlled trials have compared transfusion thresholds in a total of 820 patients with MI and anemia, Dr. Gibson said, a point that the trial investigators also made. The results were inconsistent between trials: the CRIT trial (n = 45) favored a restrictive strategy, the MINT pilot study (n = 110) favored a liberal one, and the REALITY trial (n = 668) showed noninferiority of a restrictive strategy, compared with a liberal strategy in 30-day MACE.
The MINT trial was four times larger than all prior studies combined. However, most outcomes were negative or of borderline significance for benefit.
Cardiac death was more common in the restrictive group at 5.5% than the liberal group at 3.2% (risk ratio, 1.74, 95% CI, 1.26-2.40), but this was nonadjudicated, and not designated as a primary, secondary, or tertiary outcome – which the researchers also noted. Fewer than half of the deaths were classified as cardiac, which was “odd,” Dr. Gibson observed.
A restrictive transfusion strategy was associated with increased events among participants with type 1 MI (RR, 1.32, 95% CI, 1.04-1.67), he noted.
Study strengths included that 45.5% of participants were women, Dr. Gibson said. Limitations included that the trial was “somewhat underpowered.” Also, even in the restrictive group, participants received a mean of 0.7 units of packed red blood cells.
Adherence to the 10 g/dL threshold in the liberal transfusion group was moderate (86.3% at hospital discharge), which the researchers acknowledged. They noted that this was frequently caused by clinical discretion, such as concern about fluid overload, and to the timing of hospital discharge. In addition, long-term potential for harm (microchimerism) is not known.
“There was a consistent nonsignificant acute benefit for liberal transfusion and a nominal reduction in CV mortality and improved outcomes in patients with type 1 MI in exploratory analyses, in a trial that ended up underpowered,” Dr. Gibson summarized. “Long-term follow up would be helpful to evaluate chronic outcomes.”
This is a very well-conducted, high-quality, important study that will be considered a landmark trial, C. David Mazer, MD, University of Toronto and St. Michael’s Hospital, also in Toronto, said in an interview.
Unfortunately, “it was not as definitive as hoped for,” Dr. Mazer lamented. Nevertheless, “I think people may interpret it as providing support for a liberal transfusion strategy” in patients with anemia and MI, he said.
Dr. Mazer, who was not involved with this research, was a principal investigator on the TRICS-3 trial, which disputed a liberal RBC transfusion strategy in patients with anemia undergoing cardiac surgery, as previously reported.
The “Red Blood Cell Transfusion: 2023 AABB International Guidelines,” led by Dr. Carson and published in JAMA, recommend a restrictive strategy in stable patients, although these guidelines did not include the current study, Dr. Mazer observed.
In the REALITY trial, there were fewer major adverse cardiac events (MACE) events in the restrictive strategy, he noted.
MINT can be viewed as comparing a high versus low hemoglobin threshold. “It is possible that the best is in between,” he said.
Dr. Mazer also noted that MINT may have achieved significance if it was designed with a larger enrollment and a higher power (for example, 90% instead of 80%) to detect between-group difference for the primary outcome.
Study rationale, design, and findings
Anemia, or low RBC count, is common in patients with MI, Dr. Carson noted. A normal hemoglobin is 13 g/dL in men and 12 g/dL in women. Administering a packed RBC transfusion only when a patient’s hemoglobin falls below 7 or 8 g/dL has been widely adopted, but it is unclear if patients with acute MI may benefit from a higher hemoglobin level.
“Blood transfusion may decrease ischemic injury by improving oxygen delivery to myocardial tissues and reduce the risk of reinfarction or death,” the researchers wrote. “Alternatively, administering more blood could result in more frequent heart failure from fluid overload, infection from immunosuppression, thrombosis from higher viscosity, and inflammation.”
From 2017 to 2023, investigators enrolled 3,504 adults aged 18 and older at 144 sites in the United States (2,157 patients), Canada (885), France (323), Brazil (105), New Zealand (25), and Australia (9).
The participants had ST-elevation or non–ST-elevation MI and hemoglobin less than 10 g/dL within 24 hours. Patients with type 1 (atherosclerotic plaque disruption), type 2 (supply-demand mismatch without atherothrombotic plaque disruption), type 4b, or type 4c MI were eligible.
They were randomly assigned to receive:
- A ‘restrictive’ transfusion strategy (1,749 patients): Transfusion was permitted but not required when a patient’s hemoglobin was less than 8 g/dL and was strongly recommended when it was less than 7 g/dL or when anginal symptoms were not controlled with medications.
- A ‘liberal’ transfusion strategy (1,755 patients): One unit of RBCs was administered after randomization, and RBCs were transfused to maintain hemoglobin 10 g/dL or higher until hospital discharge or 30 days.
The patients had a mean age of 72 years and 46% were women. More than three-quarters (78%) were White and 14% were Black. They had frequent coexisting illnesses, about a third had a history of MI, percutaneous coronary intervention, or heart failure; 14% were on a ventilator and 12% had renal dialysis. The median duration of hospitalization was 5 days in the two groups.
At baseline, the mean hemoglobin was 8.6 g/dL in both groups. At days 1, 2, and 3, the mean hemoglobin was 8.8, 8.9, and 8.9 g/dL, respectively, in the restrictive transfusion group, and 10.1, 10.4, and 10.5 g/dL, respectively, in the liberal transfusion group.
The mean number of transfused blood units was 0.7 units in the restrictive strategy group and 2.5 units in the liberal strategy group, roughly a 3.5-fold difference.
After adjustment for site and incomplete follow-up in 57 patients (20 with the restrictive strategy and 37 with the liberal strategy), the estimated RR for the primary outcome in the restrictive group versus the liberal group was 1.15 (P = .07).
“We observed that the 95% confidence interval contains values that suggest a clinical benefit for the liberal transfusion strategy and does not include values that suggest a benefit for the more restrictive transfusion strategy,” the researchers wrote. Heart failure and other safety outcomes were comparable in the two groups.
The trial was supported by grants from the National Heart, Lung, and Blood Institute and by the Canadian Blood Services and Canadian Institutes of Health Research Institute of Circulatory and Respiratory Health. Dr. Carson, Dr. Leon, Dr. Gibson, and Dr. Mazer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AHA 2023
T-cell cancers: CAR T therapy to the rescue?
As Baylor College of Medicine’s Max Mamonkin, PhD, noted in a presentation, patients with conditions such as T-cell lymphoma and T-cell acute lymphoblastic leukemia (ALL) have limited treatment options and grim prognoses. “This is an area with huge unmet need,” he said. “They don’t have options that patients with B-cell malignancies have, like [CAR T-cell therapy] and bispecifics.”
One big challenge is that CAR-targeted antigens in T-cell blood cancers are shared by both normal and malignant T-cells, he said. That poses a risk during therapy that the engineered cells will target each other with “disastrous consequences.”
Research by his team and others have shown that gene editing can help the cells to stop engaging in “fratricide,” Dr. Mamonkin said.
The problem is “it’s much easier to do gene editing on the bench and much harder to translate it into the clinic,” especially in light of limitations posed by the Food and Drug administration, he said. “We started to think about alternative methods to get this approach to the clinic.”
One strategy is to use pharmacologic inhibition via the Bruton’s tyrosine kinase inhibitors ibrutinib and dasatinib to mute the tendency of CAR T toward self-destruction. When tested in mice, “the unedited cells not just persisted, they expanded with sustained anti-leukemic activity and significantly prolonged their lives even more than the knock-out [gene-edited] cells.”
The research has now moved to human subjects. In 2021, researchers at Texas Children’s Hospital and Houston Methodist Hospital launched a clinical trial to test CD7 CAR T-cell therapy with CD28 in 21 patients with CD7-positive T-cell lymphoma. The initial part of the transplant-enabling CRIMSON-NE study is expected to be completed by mid-2024, and patients will be followed for 15 years.
Early results show that CD7 CAR T-cells have persisted in the blood of patients over weeks and months, Dr. Mamonkin said. In eight patients, “we’re seeing good evidence of activity,” with two patients reaching complete remissions.
The findings suggest that CD7 can be targeted in T-cell malignancies, he said. What about CD5? A similar study known as MAGENTA is testing CD5 CAR T-cell therapy with CD28 in T-cell leukemia and lymphoma in 42 patients. The phase 1 trial began in 2017. It’s expected to be completed by 2024 and to track patients for 15 years.
Results so far have been positive with complete remission achieved in three of nine patients with T-cell lymphoma; two remained in remission for more than 4 years.
Results in T-cell ALL improved after researchers adjusted the manufacturing of the cells. As for durability in these patients, “we try to bridge them to transplantation as soon as possible.”
As for side effects overall, there wasn’t much immune effector cell-associated neurotoxicity syndrome, and the CD7 approach seems to be more inflammatory, he said.
The presentation didn’t address the potential cost of the therapies. CAR T-cell therapy can cost between $500,000 and $1 million. Medicare covers it, but Medicaid may not depending on the state, and insurers may refuse to pay for it.
Dr. Mamonkin disclosed ties with Allogene, Amgen, Fate, Galapagos, March Bio, and NKILT.
As Baylor College of Medicine’s Max Mamonkin, PhD, noted in a presentation, patients with conditions such as T-cell lymphoma and T-cell acute lymphoblastic leukemia (ALL) have limited treatment options and grim prognoses. “This is an area with huge unmet need,” he said. “They don’t have options that patients with B-cell malignancies have, like [CAR T-cell therapy] and bispecifics.”
One big challenge is that CAR-targeted antigens in T-cell blood cancers are shared by both normal and malignant T-cells, he said. That poses a risk during therapy that the engineered cells will target each other with “disastrous consequences.”
Research by his team and others have shown that gene editing can help the cells to stop engaging in “fratricide,” Dr. Mamonkin said.
The problem is “it’s much easier to do gene editing on the bench and much harder to translate it into the clinic,” especially in light of limitations posed by the Food and Drug administration, he said. “We started to think about alternative methods to get this approach to the clinic.”
One strategy is to use pharmacologic inhibition via the Bruton’s tyrosine kinase inhibitors ibrutinib and dasatinib to mute the tendency of CAR T toward self-destruction. When tested in mice, “the unedited cells not just persisted, they expanded with sustained anti-leukemic activity and significantly prolonged their lives even more than the knock-out [gene-edited] cells.”
The research has now moved to human subjects. In 2021, researchers at Texas Children’s Hospital and Houston Methodist Hospital launched a clinical trial to test CD7 CAR T-cell therapy with CD28 in 21 patients with CD7-positive T-cell lymphoma. The initial part of the transplant-enabling CRIMSON-NE study is expected to be completed by mid-2024, and patients will be followed for 15 years.
Early results show that CD7 CAR T-cells have persisted in the blood of patients over weeks and months, Dr. Mamonkin said. In eight patients, “we’re seeing good evidence of activity,” with two patients reaching complete remissions.
The findings suggest that CD7 can be targeted in T-cell malignancies, he said. What about CD5? A similar study known as MAGENTA is testing CD5 CAR T-cell therapy with CD28 in T-cell leukemia and lymphoma in 42 patients. The phase 1 trial began in 2017. It’s expected to be completed by 2024 and to track patients for 15 years.
Results so far have been positive with complete remission achieved in three of nine patients with T-cell lymphoma; two remained in remission for more than 4 years.
Results in T-cell ALL improved after researchers adjusted the manufacturing of the cells. As for durability in these patients, “we try to bridge them to transplantation as soon as possible.”
As for side effects overall, there wasn’t much immune effector cell-associated neurotoxicity syndrome, and the CD7 approach seems to be more inflammatory, he said.
The presentation didn’t address the potential cost of the therapies. CAR T-cell therapy can cost between $500,000 and $1 million. Medicare covers it, but Medicaid may not depending on the state, and insurers may refuse to pay for it.
Dr. Mamonkin disclosed ties with Allogene, Amgen, Fate, Galapagos, March Bio, and NKILT.
As Baylor College of Medicine’s Max Mamonkin, PhD, noted in a presentation, patients with conditions such as T-cell lymphoma and T-cell acute lymphoblastic leukemia (ALL) have limited treatment options and grim prognoses. “This is an area with huge unmet need,” he said. “They don’t have options that patients with B-cell malignancies have, like [CAR T-cell therapy] and bispecifics.”
One big challenge is that CAR-targeted antigens in T-cell blood cancers are shared by both normal and malignant T-cells, he said. That poses a risk during therapy that the engineered cells will target each other with “disastrous consequences.”
Research by his team and others have shown that gene editing can help the cells to stop engaging in “fratricide,” Dr. Mamonkin said.
The problem is “it’s much easier to do gene editing on the bench and much harder to translate it into the clinic,” especially in light of limitations posed by the Food and Drug administration, he said. “We started to think about alternative methods to get this approach to the clinic.”
One strategy is to use pharmacologic inhibition via the Bruton’s tyrosine kinase inhibitors ibrutinib and dasatinib to mute the tendency of CAR T toward self-destruction. When tested in mice, “the unedited cells not just persisted, they expanded with sustained anti-leukemic activity and significantly prolonged their lives even more than the knock-out [gene-edited] cells.”
The research has now moved to human subjects. In 2021, researchers at Texas Children’s Hospital and Houston Methodist Hospital launched a clinical trial to test CD7 CAR T-cell therapy with CD28 in 21 patients with CD7-positive T-cell lymphoma. The initial part of the transplant-enabling CRIMSON-NE study is expected to be completed by mid-2024, and patients will be followed for 15 years.
Early results show that CD7 CAR T-cells have persisted in the blood of patients over weeks and months, Dr. Mamonkin said. In eight patients, “we’re seeing good evidence of activity,” with two patients reaching complete remissions.
The findings suggest that CD7 can be targeted in T-cell malignancies, he said. What about CD5? A similar study known as MAGENTA is testing CD5 CAR T-cell therapy with CD28 in T-cell leukemia and lymphoma in 42 patients. The phase 1 trial began in 2017. It’s expected to be completed by 2024 and to track patients for 15 years.
Results so far have been positive with complete remission achieved in three of nine patients with T-cell lymphoma; two remained in remission for more than 4 years.
Results in T-cell ALL improved after researchers adjusted the manufacturing of the cells. As for durability in these patients, “we try to bridge them to transplantation as soon as possible.”
As for side effects overall, there wasn’t much immune effector cell-associated neurotoxicity syndrome, and the CD7 approach seems to be more inflammatory, he said.
The presentation didn’t address the potential cost of the therapies. CAR T-cell therapy can cost between $500,000 and $1 million. Medicare covers it, but Medicaid may not depending on the state, and insurers may refuse to pay for it.
Dr. Mamonkin disclosed ties with Allogene, Amgen, Fate, Galapagos, March Bio, and NKILT.
FROM SITC 2023
Children with sickle cell disease at risk for vision loss
Clinicians must monitor children with sickle cell disease for eye complications as much as they do for adults, a new research review suggests.
Earlier research indicated that older patients were more at risk for eye complications from sickle cell disease, but the new study found that a full third of young people aged 10-25 years with sickle cell disease had retinopathy, including nonproliferative retinopathy (33%) and proliferative retinopathy (6%), which can progress to vision loss.
Two patients experienced retinal detachment, while two suffered retinal artery occlusion. One patient with retinal artery occlusion lost their vision and had a final best-corrected visual acuity of 20/60, according to the researchers, who presented their findings at the annual meeting of the American Academy of Ophthalmology.
“Our data underscores the need for patients – including pediatric patients – with sickle cell disease to get routine ophthalmic screenings along with appropriate systemic and ophthalmic treatment,” Mary Ellen Hoehn, MD, a professor of ophthalmology at the University of Tennessee Health Science Center, Memphis, who led the research, said in a press release.
The review covered records for 652 patients with sickle cell disease aged 10-25 years (median age, 14 years), who underwent eye exams over a 12-year period.
Besides looking at rates of retinopathy, Dr. Hoehn’s group studied which treatments were most effective. They found that hydroxyurea and chronic transfusions best lowered retinopathy rates among all genotypes.
“We hope that people will use this information to better care for patients with sickle cell disease, and that more timely ophthalmic screen exams will be performed so that vision-threatening complications from this disease are prevented,” Dr. Hoehn said.
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians must monitor children with sickle cell disease for eye complications as much as they do for adults, a new research review suggests.
Earlier research indicated that older patients were more at risk for eye complications from sickle cell disease, but the new study found that a full third of young people aged 10-25 years with sickle cell disease had retinopathy, including nonproliferative retinopathy (33%) and proliferative retinopathy (6%), which can progress to vision loss.
Two patients experienced retinal detachment, while two suffered retinal artery occlusion. One patient with retinal artery occlusion lost their vision and had a final best-corrected visual acuity of 20/60, according to the researchers, who presented their findings at the annual meeting of the American Academy of Ophthalmology.
“Our data underscores the need for patients – including pediatric patients – with sickle cell disease to get routine ophthalmic screenings along with appropriate systemic and ophthalmic treatment,” Mary Ellen Hoehn, MD, a professor of ophthalmology at the University of Tennessee Health Science Center, Memphis, who led the research, said in a press release.
The review covered records for 652 patients with sickle cell disease aged 10-25 years (median age, 14 years), who underwent eye exams over a 12-year period.
Besides looking at rates of retinopathy, Dr. Hoehn’s group studied which treatments were most effective. They found that hydroxyurea and chronic transfusions best lowered retinopathy rates among all genotypes.
“We hope that people will use this information to better care for patients with sickle cell disease, and that more timely ophthalmic screen exams will be performed so that vision-threatening complications from this disease are prevented,” Dr. Hoehn said.
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians must monitor children with sickle cell disease for eye complications as much as they do for adults, a new research review suggests.
Earlier research indicated that older patients were more at risk for eye complications from sickle cell disease, but the new study found that a full third of young people aged 10-25 years with sickle cell disease had retinopathy, including nonproliferative retinopathy (33%) and proliferative retinopathy (6%), which can progress to vision loss.
Two patients experienced retinal detachment, while two suffered retinal artery occlusion. One patient with retinal artery occlusion lost their vision and had a final best-corrected visual acuity of 20/60, according to the researchers, who presented their findings at the annual meeting of the American Academy of Ophthalmology.
“Our data underscores the need for patients – including pediatric patients – with sickle cell disease to get routine ophthalmic screenings along with appropriate systemic and ophthalmic treatment,” Mary Ellen Hoehn, MD, a professor of ophthalmology at the University of Tennessee Health Science Center, Memphis, who led the research, said in a press release.
The review covered records for 652 patients with sickle cell disease aged 10-25 years (median age, 14 years), who underwent eye exams over a 12-year period.
Besides looking at rates of retinopathy, Dr. Hoehn’s group studied which treatments were most effective. They found that hydroxyurea and chronic transfusions best lowered retinopathy rates among all genotypes.
“We hope that people will use this information to better care for patients with sickle cell disease, and that more timely ophthalmic screen exams will be performed so that vision-threatening complications from this disease are prevented,” Dr. Hoehn said.
The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AAO 2023
Early cryoprecipitate fails to improve trauma hemorrhage outcomes
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
DLBCL treatment options: CAR T outperforms ASCT
NEW YORK – according to evidence presented at the 2023 Lymphoma, Leukemia, and Myeloma Congress.
DLBCL is characterized by the National Institutes of Health as an aggressive malignancy and the most common lymphoma. Research presented at the conference indicated that 60%70% of patients were cured with six to eight cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP).
“In the past, if a relapsed of refractory DLBCL patient couldn’t get a transplant, they were likely headed for palliative care. CAR T-cell therapy is no longer experimental. Not only can we offer it to patients who are ineligible for transplant (and manage the side effects), the treatment has proven to offer better overall survival and cure rates, even in patients who eligible for ASCT,” said presenter Jason Westin, MD, director of the lymphoma clinical research program at the University of Texas MD Anderson Cancer Center, Houston.
The ZUMA-7 phase 3trial among patients with early r/r DLBCL demonstrated the superiority of CAR T-cell therapy with the agent, axicabtagene ciloleucel (YESCARTA, Kita Pharma) versus standard of care (chemoimmunotherapy followed by high-dose chemotherapy and ASCT). Those in the axicabtagene ciloleucel (axi-cel) group had a median progression free survival (PFS) of 14.7 months and an estimated 4-year overall survival (OS) rate of 54.6% compared to 3.7 months and 46% in the control group.
Patients treated with axi-cel experienced a higher rate of adverse events (AE) grade 3 of higher, compared with the ACST group (91% vs. 83%) Furthermore, patients who received axi-cel had cytokine release syndrome (6%) and neurologic events in (21%) grade 3 or higher, compared with 0% and less than 1% in the ASCT group.
At the conference, Dr. Westin’s copanelist Jennifer Amengual, MD, of Columbia University Irving Medical Center, New York, interpreted the data on adverse events (AEs) from ZUMA-7 to mean that if a patient is especially susceptible to CAR T side effects, then ASCT could be preferred. She also outlined a second strategy that shows promise when a patient has either failed CAR T and ASCT or whose frailty demands an approach that avoids AEs.
Dr. Amengual cited a study in which patients with r/r DLBCL were treated with the bispecific antibody glofitamab (Columvi/Roche), which induced a complete response in 39% of patients at a median follow-up of 12.6 months and a 12-month PFS rate of 37%. Those treated with the agent experienced cytokine release syndrome and neurologic events grade 3 or higher, at a rate of 4% and 3% respectively.
“Efforts to make off-the-shelf CAR T therapy are ongoing. With some fine-tuning the PFS and OS with bispecific antibodies will likely approach or exceed both CAR T and ACST. The fact that they could come right off the shelf, rather than having to be tailor made for each patient, gives them a huge advantage in terms of cost and availability, while maintaining what appears to be an excellent safety profile” said Morton Colman, MD, professor of medicine at Weill Cornell Medicine, New York, and chair of the 2023 Lymphoma, Leukemia, and Myeloma Congress.
Dr. Amengual disclosed ties with Astra Zeneca and Incyte. Dr. Westin reported ties with Abbie, ADC therapeutics, AstraZeneca, Bristol-Myers Squibb, Genentech, GenMad, Hanssen, Kite/Gilead, Morphosys/Incyte, Novartis, Nurix, Regeneron, and SeaGen. Dr. Coleman had no disclosures.
NEW YORK – according to evidence presented at the 2023 Lymphoma, Leukemia, and Myeloma Congress.
DLBCL is characterized by the National Institutes of Health as an aggressive malignancy and the most common lymphoma. Research presented at the conference indicated that 60%70% of patients were cured with six to eight cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP).
“In the past, if a relapsed of refractory DLBCL patient couldn’t get a transplant, they were likely headed for palliative care. CAR T-cell therapy is no longer experimental. Not only can we offer it to patients who are ineligible for transplant (and manage the side effects), the treatment has proven to offer better overall survival and cure rates, even in patients who eligible for ASCT,” said presenter Jason Westin, MD, director of the lymphoma clinical research program at the University of Texas MD Anderson Cancer Center, Houston.
The ZUMA-7 phase 3trial among patients with early r/r DLBCL demonstrated the superiority of CAR T-cell therapy with the agent, axicabtagene ciloleucel (YESCARTA, Kita Pharma) versus standard of care (chemoimmunotherapy followed by high-dose chemotherapy and ASCT). Those in the axicabtagene ciloleucel (axi-cel) group had a median progression free survival (PFS) of 14.7 months and an estimated 4-year overall survival (OS) rate of 54.6% compared to 3.7 months and 46% in the control group.
Patients treated with axi-cel experienced a higher rate of adverse events (AE) grade 3 of higher, compared with the ACST group (91% vs. 83%) Furthermore, patients who received axi-cel had cytokine release syndrome (6%) and neurologic events in (21%) grade 3 or higher, compared with 0% and less than 1% in the ASCT group.
At the conference, Dr. Westin’s copanelist Jennifer Amengual, MD, of Columbia University Irving Medical Center, New York, interpreted the data on adverse events (AEs) from ZUMA-7 to mean that if a patient is especially susceptible to CAR T side effects, then ASCT could be preferred. She also outlined a second strategy that shows promise when a patient has either failed CAR T and ASCT or whose frailty demands an approach that avoids AEs.
Dr. Amengual cited a study in which patients with r/r DLBCL were treated with the bispecific antibody glofitamab (Columvi/Roche), which induced a complete response in 39% of patients at a median follow-up of 12.6 months and a 12-month PFS rate of 37%. Those treated with the agent experienced cytokine release syndrome and neurologic events grade 3 or higher, at a rate of 4% and 3% respectively.
“Efforts to make off-the-shelf CAR T therapy are ongoing. With some fine-tuning the PFS and OS with bispecific antibodies will likely approach or exceed both CAR T and ACST. The fact that they could come right off the shelf, rather than having to be tailor made for each patient, gives them a huge advantage in terms of cost and availability, while maintaining what appears to be an excellent safety profile” said Morton Colman, MD, professor of medicine at Weill Cornell Medicine, New York, and chair of the 2023 Lymphoma, Leukemia, and Myeloma Congress.
Dr. Amengual disclosed ties with Astra Zeneca and Incyte. Dr. Westin reported ties with Abbie, ADC therapeutics, AstraZeneca, Bristol-Myers Squibb, Genentech, GenMad, Hanssen, Kite/Gilead, Morphosys/Incyte, Novartis, Nurix, Regeneron, and SeaGen. Dr. Coleman had no disclosures.
NEW YORK – according to evidence presented at the 2023 Lymphoma, Leukemia, and Myeloma Congress.
DLBCL is characterized by the National Institutes of Health as an aggressive malignancy and the most common lymphoma. Research presented at the conference indicated that 60%70% of patients were cured with six to eight cycles of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP).
“In the past, if a relapsed of refractory DLBCL patient couldn’t get a transplant, they were likely headed for palliative care. CAR T-cell therapy is no longer experimental. Not only can we offer it to patients who are ineligible for transplant (and manage the side effects), the treatment has proven to offer better overall survival and cure rates, even in patients who eligible for ASCT,” said presenter Jason Westin, MD, director of the lymphoma clinical research program at the University of Texas MD Anderson Cancer Center, Houston.
The ZUMA-7 phase 3trial among patients with early r/r DLBCL demonstrated the superiority of CAR T-cell therapy with the agent, axicabtagene ciloleucel (YESCARTA, Kita Pharma) versus standard of care (chemoimmunotherapy followed by high-dose chemotherapy and ASCT). Those in the axicabtagene ciloleucel (axi-cel) group had a median progression free survival (PFS) of 14.7 months and an estimated 4-year overall survival (OS) rate of 54.6% compared to 3.7 months and 46% in the control group.
Patients treated with axi-cel experienced a higher rate of adverse events (AE) grade 3 of higher, compared with the ACST group (91% vs. 83%) Furthermore, patients who received axi-cel had cytokine release syndrome (6%) and neurologic events in (21%) grade 3 or higher, compared with 0% and less than 1% in the ASCT group.
At the conference, Dr. Westin’s copanelist Jennifer Amengual, MD, of Columbia University Irving Medical Center, New York, interpreted the data on adverse events (AEs) from ZUMA-7 to mean that if a patient is especially susceptible to CAR T side effects, then ASCT could be preferred. She also outlined a second strategy that shows promise when a patient has either failed CAR T and ASCT or whose frailty demands an approach that avoids AEs.
Dr. Amengual cited a study in which patients with r/r DLBCL were treated with the bispecific antibody glofitamab (Columvi/Roche), which induced a complete response in 39% of patients at a median follow-up of 12.6 months and a 12-month PFS rate of 37%. Those treated with the agent experienced cytokine release syndrome and neurologic events grade 3 or higher, at a rate of 4% and 3% respectively.
“Efforts to make off-the-shelf CAR T therapy are ongoing. With some fine-tuning the PFS and OS with bispecific antibodies will likely approach or exceed both CAR T and ACST. The fact that they could come right off the shelf, rather than having to be tailor made for each patient, gives them a huge advantage in terms of cost and availability, while maintaining what appears to be an excellent safety profile” said Morton Colman, MD, professor of medicine at Weill Cornell Medicine, New York, and chair of the 2023 Lymphoma, Leukemia, and Myeloma Congress.
Dr. Amengual disclosed ties with Astra Zeneca and Incyte. Dr. Westin reported ties with Abbie, ADC therapeutics, AstraZeneca, Bristol-Myers Squibb, Genentech, GenMad, Hanssen, Kite/Gilead, Morphosys/Incyte, Novartis, Nurix, Regeneron, and SeaGen. Dr. Coleman had no disclosures.
AT LLM CONGRESS 2023