User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
A new long COVID explanation: Low serotonin levels?
Could antidepressants hold the key to treating long COVID? The study even points to a possible treatment.
Serotonin is a neurotransmitter that has many functions in the body and is targeted by the most commonly prescribed antidepressants – the selective serotonin reuptake inhibitors.
Serotonin is widely studied for its effects on the brain – it regulates the messaging between neurons, affecting sleep, mood, and memory. It is present in the gut, is found in cells along the gastrointestinal tract, and is absorbed by blood platelets. Gut serotonin, known as circulating serotonin, is responsible for a host of other functions, including the regulation of blood flow, body temperature, and digestion.
Low levels of serotonin could result in any number of seemingly unrelated symptoms, as in the case of long COVID, experts say. The condition affects about 7% of Americans and is associated with a wide range of health problems, including fatigue, shortness of breath, neurological symptoms, joint pain, blood clots, heart palpitations, and digestive problems.
Long COVID is difficult to treat because researchers haven’t been able to pinpoint the underlying mechanisms that cause prolonged illness after a SARS-CoV-2 infection, said study author Christoph A. Thaiss, PhD, an assistant professor of microbiology at the Perelman School of Medicine at the University of Pennsylvania.
The hope is that this study could have implications for new treatments, he said.
“Long COVID can have manifestations not only in the brain but in many different parts of the body, so it’s possible that serotonin reductions are involved in many different aspects of the disease,” said Dr. Thaiss.
Dr. Thaiss’s study, published in the journal Cell, found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but who fully recovered.
His team found that reductions in serotonin were driven by low levels of circulating SARS-CoV-2 virus that caused persistent inflammation as well as an inability of the body to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactive blood platelets were also shown to play a role; they serve as the primary means of serotonin absorption.
The study doesn’t make any recommendations for treatment, but understanding the role of serotonin in long COVID opens the door to a host of novel ideas that could set the stage for clinical trials and affect care.
“The study gives us a few possible targets that could be used in future clinical studies,” Dr. Thaiss said.
Persistent circulating virus is one of the drivers of low serotonin levels, said study author Michael Peluso, MD, an assistant research professor of infectious medicine at the University of California, San Francisco, School of Medicine. This points to the need to reduce viral load using antiviral medications like nirmatrelvir/ritonavir (Paxlovid), which is approved by the U.S. Food and Drug Administration for the treatment of COVID-19, and VV116, which has not yet been approved for use against COVID.
Research published in the New England Journal of Medicine found that the oral antiviral agent VV116 was as effective as nirmatrelvir/ritonavir in reducing the body’s viral load and aiding recovery from SARS-CoV-2 infection. Paxlovid has also been shown to reduce the likelihood of getting long COVID after an acute SARS-CoV-2 infection.
Researchers are investigating ways to target serotonin levels directly, potentially using SSRIs. But first they need to study whether improvement in serotonin level makes a difference.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said.
Indeed, the research did show that the SSRI fluoxetine, as well as a glycine-tryptophan supplement, improved cognitive function in SARS-CoV-2-infected rodent models, which were used in a portion of the study.
David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City, said the research is helping “to paint a biological picture” that’s in line with other research on the mechanisms that cause long COVID symptoms.
But Dr. Putrino, who was not involved in the study, cautions against treating long COVID patients with SSRIs or any other treatment that increases serotonin before testing patients to determine whether their serotonin levels are actually lower than those of healthy persons.
“We don’t want to assume that every patient with long COVID is going to have lower serotonin levels,” said Dr. Putrino.
What’s more, researchers need to investigate whether SSRIs increase levels of circulating serotonin. It’s important to note that researchers found lower levels of circulating serotonin but that serotonin levels in the brain remained normal.
Traditionally, SSRIs are used clinically for increasing the levels of serotonin in the brain, not the body.
“Whether that’s going to contribute to an increase in systemic levels of serotonin, that’s something that needs to be tested,” said Akiko Iwasaki, PhD, co-lead investigator of the Yale School of Medicine, New Haven, Conn., COVID-19 Recovery Study, who was not involved in the research.
Thus far, investigators have not identified one unifying biomarker that seems to cause long COVID in all patients, said Dr. Iwasaki. Some research has found higher levels of certain immune cells and biomarkers: for example, monocytes and activated B lymphocytes, indicating a stronger and ongoing antibody response to the virus. Other recent research conducted by Dr. Iwasaki, Dr. Putrino, and others, published in the journal Nature, showed that long COVID patients tend to have lower levels of cortisol, which could be a factor in the extreme fatigue experienced by many who suffer from the condition.
The findings in the study in The Cell are promising, but they need to be replicated in more people, said Dr. Iwasaki. And even if they’re replicated in a larger study population, this would still be just one biomarker that is associated with one subtype of the disease. There is a need to better understand which biomarkers go with which symptoms so that the most effective treatments can be identified, she said.
Both Dr. Putrino and Dr. Iwasaki contended that there isn’t a single factor that can explain all of long COVID. It’s a complex disease caused by a host of different mechanisms.
Still, low levels of serotonin could be an important piece of the puzzle. The next step, said Dr. Iwasaki, is to uncover how many of the millions of Americans with long COVID have this biomarker.
“People working in the field of long COVID should now be considering this pathway and thinking of ways to measure serotonin in their patients.”
A version of this article first appeared on Medscape.com.
Could antidepressants hold the key to treating long COVID? The study even points to a possible treatment.
Serotonin is a neurotransmitter that has many functions in the body and is targeted by the most commonly prescribed antidepressants – the selective serotonin reuptake inhibitors.
Serotonin is widely studied for its effects on the brain – it regulates the messaging between neurons, affecting sleep, mood, and memory. It is present in the gut, is found in cells along the gastrointestinal tract, and is absorbed by blood platelets. Gut serotonin, known as circulating serotonin, is responsible for a host of other functions, including the regulation of blood flow, body temperature, and digestion.
Low levels of serotonin could result in any number of seemingly unrelated symptoms, as in the case of long COVID, experts say. The condition affects about 7% of Americans and is associated with a wide range of health problems, including fatigue, shortness of breath, neurological symptoms, joint pain, blood clots, heart palpitations, and digestive problems.
Long COVID is difficult to treat because researchers haven’t been able to pinpoint the underlying mechanisms that cause prolonged illness after a SARS-CoV-2 infection, said study author Christoph A. Thaiss, PhD, an assistant professor of microbiology at the Perelman School of Medicine at the University of Pennsylvania.
The hope is that this study could have implications for new treatments, he said.
“Long COVID can have manifestations not only in the brain but in many different parts of the body, so it’s possible that serotonin reductions are involved in many different aspects of the disease,” said Dr. Thaiss.
Dr. Thaiss’s study, published in the journal Cell, found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but who fully recovered.
His team found that reductions in serotonin were driven by low levels of circulating SARS-CoV-2 virus that caused persistent inflammation as well as an inability of the body to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactive blood platelets were also shown to play a role; they serve as the primary means of serotonin absorption.
The study doesn’t make any recommendations for treatment, but understanding the role of serotonin in long COVID opens the door to a host of novel ideas that could set the stage for clinical trials and affect care.
“The study gives us a few possible targets that could be used in future clinical studies,” Dr. Thaiss said.
Persistent circulating virus is one of the drivers of low serotonin levels, said study author Michael Peluso, MD, an assistant research professor of infectious medicine at the University of California, San Francisco, School of Medicine. This points to the need to reduce viral load using antiviral medications like nirmatrelvir/ritonavir (Paxlovid), which is approved by the U.S. Food and Drug Administration for the treatment of COVID-19, and VV116, which has not yet been approved for use against COVID.
Research published in the New England Journal of Medicine found that the oral antiviral agent VV116 was as effective as nirmatrelvir/ritonavir in reducing the body’s viral load and aiding recovery from SARS-CoV-2 infection. Paxlovid has also been shown to reduce the likelihood of getting long COVID after an acute SARS-CoV-2 infection.
Researchers are investigating ways to target serotonin levels directly, potentially using SSRIs. But first they need to study whether improvement in serotonin level makes a difference.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said.
Indeed, the research did show that the SSRI fluoxetine, as well as a glycine-tryptophan supplement, improved cognitive function in SARS-CoV-2-infected rodent models, which were used in a portion of the study.
David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City, said the research is helping “to paint a biological picture” that’s in line with other research on the mechanisms that cause long COVID symptoms.
But Dr. Putrino, who was not involved in the study, cautions against treating long COVID patients with SSRIs or any other treatment that increases serotonin before testing patients to determine whether their serotonin levels are actually lower than those of healthy persons.
“We don’t want to assume that every patient with long COVID is going to have lower serotonin levels,” said Dr. Putrino.
What’s more, researchers need to investigate whether SSRIs increase levels of circulating serotonin. It’s important to note that researchers found lower levels of circulating serotonin but that serotonin levels in the brain remained normal.
Traditionally, SSRIs are used clinically for increasing the levels of serotonin in the brain, not the body.
“Whether that’s going to contribute to an increase in systemic levels of serotonin, that’s something that needs to be tested,” said Akiko Iwasaki, PhD, co-lead investigator of the Yale School of Medicine, New Haven, Conn., COVID-19 Recovery Study, who was not involved in the research.
Thus far, investigators have not identified one unifying biomarker that seems to cause long COVID in all patients, said Dr. Iwasaki. Some research has found higher levels of certain immune cells and biomarkers: for example, monocytes and activated B lymphocytes, indicating a stronger and ongoing antibody response to the virus. Other recent research conducted by Dr. Iwasaki, Dr. Putrino, and others, published in the journal Nature, showed that long COVID patients tend to have lower levels of cortisol, which could be a factor in the extreme fatigue experienced by many who suffer from the condition.
The findings in the study in The Cell are promising, but they need to be replicated in more people, said Dr. Iwasaki. And even if they’re replicated in a larger study population, this would still be just one biomarker that is associated with one subtype of the disease. There is a need to better understand which biomarkers go with which symptoms so that the most effective treatments can be identified, she said.
Both Dr. Putrino and Dr. Iwasaki contended that there isn’t a single factor that can explain all of long COVID. It’s a complex disease caused by a host of different mechanisms.
Still, low levels of serotonin could be an important piece of the puzzle. The next step, said Dr. Iwasaki, is to uncover how many of the millions of Americans with long COVID have this biomarker.
“People working in the field of long COVID should now be considering this pathway and thinking of ways to measure serotonin in their patients.”
A version of this article first appeared on Medscape.com.
Could antidepressants hold the key to treating long COVID? The study even points to a possible treatment.
Serotonin is a neurotransmitter that has many functions in the body and is targeted by the most commonly prescribed antidepressants – the selective serotonin reuptake inhibitors.
Serotonin is widely studied for its effects on the brain – it regulates the messaging between neurons, affecting sleep, mood, and memory. It is present in the gut, is found in cells along the gastrointestinal tract, and is absorbed by blood platelets. Gut serotonin, known as circulating serotonin, is responsible for a host of other functions, including the regulation of blood flow, body temperature, and digestion.
Low levels of serotonin could result in any number of seemingly unrelated symptoms, as in the case of long COVID, experts say. The condition affects about 7% of Americans and is associated with a wide range of health problems, including fatigue, shortness of breath, neurological symptoms, joint pain, blood clots, heart palpitations, and digestive problems.
Long COVID is difficult to treat because researchers haven’t been able to pinpoint the underlying mechanisms that cause prolonged illness after a SARS-CoV-2 infection, said study author Christoph A. Thaiss, PhD, an assistant professor of microbiology at the Perelman School of Medicine at the University of Pennsylvania.
The hope is that this study could have implications for new treatments, he said.
“Long COVID can have manifestations not only in the brain but in many different parts of the body, so it’s possible that serotonin reductions are involved in many different aspects of the disease,” said Dr. Thaiss.
Dr. Thaiss’s study, published in the journal Cell, found lower serotonin levels in long COVID patients, compared with patients who were diagnosed with acute COVID-19 but who fully recovered.
His team found that reductions in serotonin were driven by low levels of circulating SARS-CoV-2 virus that caused persistent inflammation as well as an inability of the body to absorb tryptophan, an amino acid that’s a precursor to serotonin. Overactive blood platelets were also shown to play a role; they serve as the primary means of serotonin absorption.
The study doesn’t make any recommendations for treatment, but understanding the role of serotonin in long COVID opens the door to a host of novel ideas that could set the stage for clinical trials and affect care.
“The study gives us a few possible targets that could be used in future clinical studies,” Dr. Thaiss said.
Persistent circulating virus is one of the drivers of low serotonin levels, said study author Michael Peluso, MD, an assistant research professor of infectious medicine at the University of California, San Francisco, School of Medicine. This points to the need to reduce viral load using antiviral medications like nirmatrelvir/ritonavir (Paxlovid), which is approved by the U.S. Food and Drug Administration for the treatment of COVID-19, and VV116, which has not yet been approved for use against COVID.
Research published in the New England Journal of Medicine found that the oral antiviral agent VV116 was as effective as nirmatrelvir/ritonavir in reducing the body’s viral load and aiding recovery from SARS-CoV-2 infection. Paxlovid has also been shown to reduce the likelihood of getting long COVID after an acute SARS-CoV-2 infection.
Researchers are investigating ways to target serotonin levels directly, potentially using SSRIs. But first they need to study whether improvement in serotonin level makes a difference.
“What we need now is a good clinical trial to see whether altering levels of serotonin in people with long COVID will lead to symptom relief,” Dr. Peluso said.
Indeed, the research did show that the SSRI fluoxetine, as well as a glycine-tryptophan supplement, improved cognitive function in SARS-CoV-2-infected rodent models, which were used in a portion of the study.
David F. Putrino, PhD, who runs the long COVID clinic at Mount Sinai Health System in New York City, said the research is helping “to paint a biological picture” that’s in line with other research on the mechanisms that cause long COVID symptoms.
But Dr. Putrino, who was not involved in the study, cautions against treating long COVID patients with SSRIs or any other treatment that increases serotonin before testing patients to determine whether their serotonin levels are actually lower than those of healthy persons.
“We don’t want to assume that every patient with long COVID is going to have lower serotonin levels,” said Dr. Putrino.
What’s more, researchers need to investigate whether SSRIs increase levels of circulating serotonin. It’s important to note that researchers found lower levels of circulating serotonin but that serotonin levels in the brain remained normal.
Traditionally, SSRIs are used clinically for increasing the levels of serotonin in the brain, not the body.
“Whether that’s going to contribute to an increase in systemic levels of serotonin, that’s something that needs to be tested,” said Akiko Iwasaki, PhD, co-lead investigator of the Yale School of Medicine, New Haven, Conn., COVID-19 Recovery Study, who was not involved in the research.
Thus far, investigators have not identified one unifying biomarker that seems to cause long COVID in all patients, said Dr. Iwasaki. Some research has found higher levels of certain immune cells and biomarkers: for example, monocytes and activated B lymphocytes, indicating a stronger and ongoing antibody response to the virus. Other recent research conducted by Dr. Iwasaki, Dr. Putrino, and others, published in the journal Nature, showed that long COVID patients tend to have lower levels of cortisol, which could be a factor in the extreme fatigue experienced by many who suffer from the condition.
The findings in the study in The Cell are promising, but they need to be replicated in more people, said Dr. Iwasaki. And even if they’re replicated in a larger study population, this would still be just one biomarker that is associated with one subtype of the disease. There is a need to better understand which biomarkers go with which symptoms so that the most effective treatments can be identified, she said.
Both Dr. Putrino and Dr. Iwasaki contended that there isn’t a single factor that can explain all of long COVID. It’s a complex disease caused by a host of different mechanisms.
Still, low levels of serotonin could be an important piece of the puzzle. The next step, said Dr. Iwasaki, is to uncover how many of the millions of Americans with long COVID have this biomarker.
“People working in the field of long COVID should now be considering this pathway and thinking of ways to measure serotonin in their patients.”
A version of this article first appeared on Medscape.com.
FROM CELL
Even one night in the ED raises risk for death
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
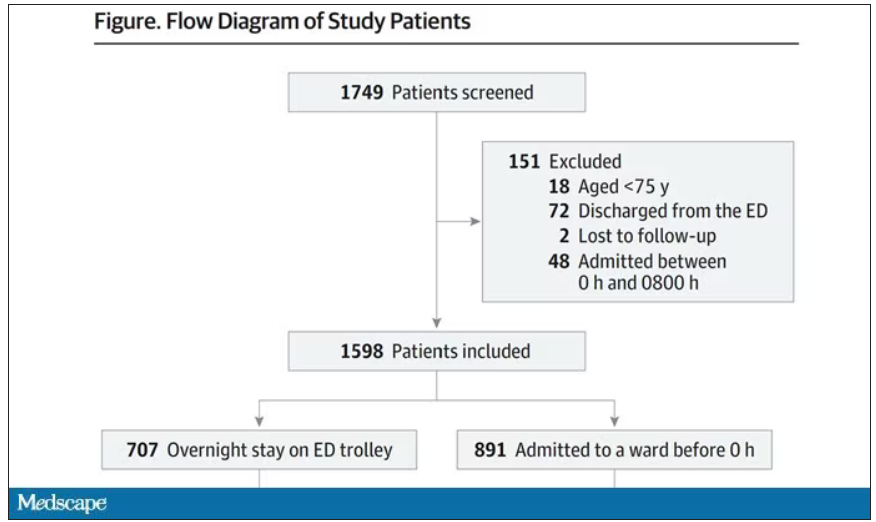
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.
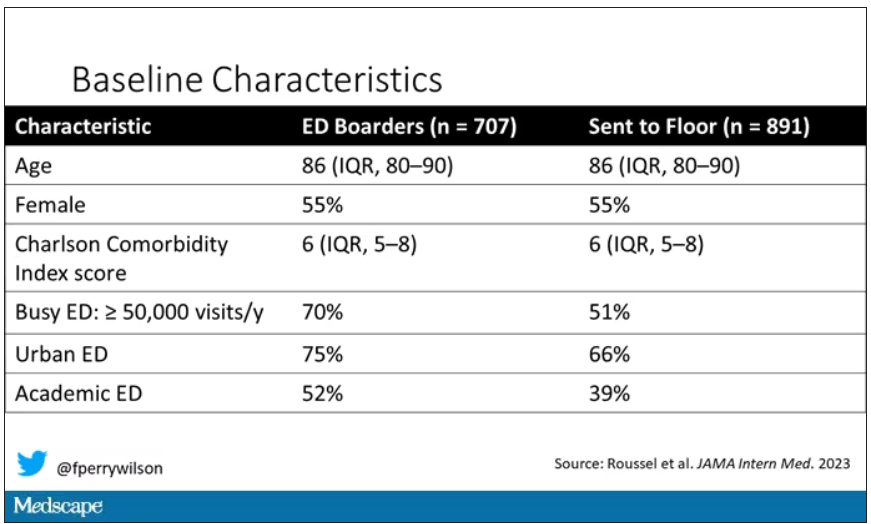
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
As a consulting nephrologist, I go all over the hospital. Medicine floors, surgical floors, the ICU – I’ve even done consults in the operating room. And more and more, I do consults in the emergency department.
The reason I am doing more consults in the ED is not because the ED docs are getting gun shy with creatinine increases; it’s because patients are staying for extended periods in the ED despite being formally admitted to the hospital. It’s a phenomenon known as boarding, because there are simply not enough beds. You know the scene if you have ever been to a busy hospital: The ED is full to breaking, with patients on stretchers in hallways. It can often feel more like a warzone than a place for healing.
This is a huge problem.
The Joint Commission specifies that admitted patients should spend no more than 4 hours in the ED waiting for a bed in the hospital.
That is, based on what I’ve seen, hugely ambitious. But I should point out that I work in a hospital that runs near capacity all the time, and studies – from some of my Yale colleagues, actually – have shown that once hospital capacity exceeds 85%, boarding rates skyrocket.
I want to discuss some of the causes of extended boarding and some solutions. But before that, I should prove to you that this really matters, and for that we are going to dig in to a new study which suggests that ED boarding kills.
To put some hard numbers to the boarding problem, we turn to this paper out of France, appearing in JAMA Internal Medicine.
This is a unique study design. Basically, on a single day – Dec. 12, 2022 – researchers fanned out across France to 97 EDs and started counting patients. The study focused on those older than age 75 who were admitted to a hospital ward from the ED. The researchers then defined two groups: those who were sent up to the hospital floor before midnight, and those who spent at least from midnight until 8 AM in the ED (basically, people forced to sleep in the ED for a night). The middle-ground people who were sent up between midnight and 8 AM were excluded.
The baseline characteristics between the two groups of patients were pretty similar: median age around 86, 55% female. There were no significant differences in comorbidities. That said, comporting with previous studies, people in an urban ED, an academic ED, or a busy ED were much more likely to board overnight.
So, what we have are two similar groups of patients treated quite differently. Not quite a randomized trial, given the hospital differences, but not bad for purposes of analysis.
Here are the most important numbers from the trial:
This difference held up even after adjustment for patient and hospital characteristics. Put another way, you’d need to send 22 patients to the floor instead of boarding in the ED to save one life. Not a bad return on investment.
It’s not entirely clear what the mechanism for the excess mortality might be, but the researchers note that patients kept in the ED overnight were about twice as likely to have a fall during their hospital stay – not surprising, given the dangers of gurneys in hallways and the sleep deprivation that trying to rest in a busy ED engenders.
I should point out that this could be worse in the United States. French ED doctors continue to care for admitted patients boarding in the ED, whereas in many hospitals in the United States, admitted patients are the responsibility of the floor team, regardless of where they are, making it more likely that these individuals may be neglected.
So, if boarding in the ED is a life-threatening situation, why do we do it? What conditions predispose to this?
You’ll hear a lot of talk, mostly from hospital administrators, saying that this is simply a problem of supply and demand. There are not enough beds for the number of patients who need beds. And staffing shortages don’t help either.
However, they never want to talk about the reasons for the staffing shortages, like poor pay, poor support, and, of course, the moral injury of treating patients in hallways.
The issue of volume is real. We could do a lot to prevent ED visits and hospital admissions by providing better access to preventive and primary care and improving our outpatient mental health infrastructure. But I think this framing passes the buck a little.
Another reason ED boarding occurs is the way our health care system is paid for. If you are building a hospital, you have little incentive to build in excess capacity. The most efficient hospital, from a profit-and-loss standpoint, is one that is 100% full as often as possible. That may be fine at times, but throw in a respiratory virus or even a pandemic, and those systems fracture under the pressure.
Let us also remember that not all hospital beds are given to patients who acutely need hospital beds. Many beds, in many hospitals, are necessary to handle postoperative patients undergoing elective procedures. Those patients having a knee replacement or abdominoplasty don’t spend the night in the ED when they leave the OR; they go to a hospital bed. And those procedures are – let’s face it – more profitable than an ED admission for a medical issue. That’s why, even when hospitals expand the number of beds they have, they do it with an eye toward increasing the rate of those profitable procedures, not decreasing the burden faced by their ED.
For now, the band-aid to the solution might be to better triage individuals boarding in the ED for floor access, prioritizing those of older age, greater frailty, or more medical complexity. But it feels like a stop-gap measure as long as the incentives are aligned to view an empty hospital bed as a sign of failure in the health system instead of success.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Second infection hikes long COVID risk: Expert Q&A
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Those are two of the most striking findings of a comprehensive new research study of 138,000 veterans.
Lead researcher Ziyad Al-Aly, MD, chief of research at Veterans Affairs St. Louis Health Care and clinical epidemiologist at Washington University in St. Louis, spoke with this news organization about his team’s findings, what we know – and don’t – about long COVID, and what it means for physicians treating patients with the condition.
Excerpts of the interview follow.
Your research concluded that for those infected early in the pandemic, some long COVID symptoms declined over 2 years, but some did not. You have also concluded that long COVID is a chronic disease. Why?
We’ve been in this journey a little bit more than three and a half years. Some patients do experience some recovery. But that’s not the norm. Most people do not really fully recover. The health trajectory for people with long COVID is really very heterogeneous. There is no one-size-fits-all. There’s really no one line that I could give you that could cover all your patients. But it is very, very, very clear that a bunch of them experienced long COVID for sure; that’s really happening.
It happened in the pre-Delta era and in the Delta era, and with Omicron subvariants, even now. There are people who think, “This is a nothing-burger anymore,” or “It’s not an issue anymore.” It’s still happening with the current variants. Vaccines do reduce risk for long COVID, but do not completely eliminate the risk for long COVID.
You work with patients with long COVID in the clinic and also analyze data from thousands more. If long COVID does not go away, what should doctors look for in everyday practice that will help them recognize and help patients with long COVID?
Long COVID is not uncommon. We see it in the clinic in large numbers. Whatever clinic you’re running – if you’re running a cardiology clinic, or a nephrology clinic, or diabetes, or primary care – probably some of your people have it. You may not know about it. They may not tell you about it. You may not recognize it.
Not all long COVID is the same, and that’s really what makes it complex and makes it really hard to deal with in the clinic. But that’s the reality that we’re all dealing with. And it’s multisystemic; it’s not like it affects the heart only, the brain only, or the autonomic nervous system only. It does not behave in the same way in different individuals – they may have different manifestations, various health trajectories, and different outcomes. It’s important for doctors to get up to speed on long COVID as a multisystem illness.
Management at this point is really managing the symptoms. We don’t have a treatment for it; we don’t have a cure for it.
Some patients experience what you’ve described as partial recovery. What does that look like?
Some individuals do experience some recovery over time, but for most individuals, the recovery is long and arduous. Long COVID can last with them for many years. Some people may come back to the clinic and say, “I’m doing better,” but if you really flesh it out and dig deeper, they didn’t do better; they adjusted to a new baseline. They used to walk the dog three to four blocks, and now they walk the dog only half a block. They used to do an activity with their partner every Saturday or Sunday, and now they do half of that.
If you’re a physician, a primary care provider, or any other provider who is dealing with a patient with long COVID, know that this is really happening. It can happen even in vaccinated individuals. The presentation is heterogeneous. Some people may present to you with and say. “Well, before I had COVID I was mentally sharp and now having I’m having difficulty with memory, etc.” It can sometimes present as fatigue or postexertional malaise.
In some instances, it can present as sleep problems. It can present as what we call postural orthostatic tachycardia syndrome (POTS). Those people get a significant increase in heart rate with postural changes.
What the most important thing we can we learn from the emergence of long COVID?
This whole thing taught us that infections can cause chronic disease. That’s really the No. 1 lesson that I take from this pandemic – that infections can cause chronic disease.
Looking at only acute illness from COVID is really only looking at the tip of the iceberg. Beneath that tip of the iceberg lies this hidden toll of disease that we don’t really talk about that much.
This pandemic shone a very, very good light on the idea that there is really an intimate connection between infections and chronic disease. It was really hardwired into our medical training as doctors that most infections, when people get over the hump of the acute phase of the disease, it’s all behind them. I think long COVID has humbled us in many, many ways, but chief among those is the realization – the stark realization – that infections can cause chronic disease.
That’s really going back to your [first] question: What does it mean that some people are not recovering? They actually have chronic illness. I’m hoping that we will find a treatment, that we’ll start finding things that would help them get back to baseline. But at this point in time, what we’re dealing with is people with chronic illness or chronic disease that may continue to affect them for many years to come in the absence of a treatment or a cure.
A version of this article first appeared on Medscape.com.
Early cryoprecipitate fails to improve trauma hemorrhage outcomes
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
TOPLINE:
(MHP).
METHODOLOGY:
- CRYOSTAT-2 was an interventional, randomized, open-label, parallel-group controlled, international, multicenter study.
- A total of 1,604 patients were enrolled from 25 major trauma centers in the United Kingdom (n = 1,555) and 1 in the United States (n = 49) between August 2017 and November 2021.
- A total of 805 patients were randomly assigned to receive the standard MHP (standard care), and 799 were randomly assigned to receive an additional three pools of cryoprecipitate.
- The primary outcome was all-cause mortality at 28 days.
TAKEAWAY:
- Addition of early cryoprecipitate versus standard care did not improve all-cause 28-day mortality in the intent-to-treat population (25.3% vs. 26.1%; P = .74).
- In patient subgroup with penetrating trauma, 28-day mortality was significantly higher in the cryoprecipitate group than in the standard care group (16.2% vs. 10.0%; odds ratio, 1.74; P = .006).
- Massive transfusion (RBC ≥ 10 U) was similar between the cryoprecipitate and standard care groups.
IN PRACTICE:
According to the authors, it is possible that certain patients may have benefited from cryoprecipitate, but they did not receive it promptly or in adequate doses to restore functional fibrinogen levels. Despite the study’s goal of early cryoprecipitate administration, the median time to the first transfusion exceeded 1 hour after the patient’s arrival, which highlights the logistical challenges of preparing and delivering a frozen blood component from a distant blood laboratory to the patient.
SOURCE:
The study, with first author Ross Davenport, PhD, of Queen Mary University of London and colleagues, was published in JAMA).
LIMITATIONS:
There was variability of timing of cryoprecipitate administration and an overlap with patients in the standard care group receiving the intervention as part of their usual MHP treatment.
DISCLOSURES:
The study was funded by the U.K. National Institute for Health and Care Research: Health Technology Assessment and Barts Charity, U.K.
A version of this article first appeared on Medscape.com.
Five times greater suicide risk for trans, gender-diverse teens in ED
WASHINGTON – , according to a study presented at the annual meeting of the American Academy of Pediatrics.
“The take-home message here is this study emphasizes the importance of universal screening to identify gender-diverse youth at risk,” Amanda Burnside, PhD, assistant professor of psychiatry and behavioral sciences at Ann and Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, told attendees. “We really need to develop robust strategies and systems to link better mental health services.”
Suicide rates in transgender and gender-diverse youth are exceptionally high among youth in the U.S., Dr. Burnside said during her presentation. For example, the 2022 LGBTQ health survey from the Trevor Project found that much higher percentages of transgender and gender nonconforming youth had considered suicide in the past year compared with cisgender youth, even within the LGBTQ umbrella. Among nearly 34,000 LGBTQ youth aged 13-24, nearly half of trans females (48%) and more than half of trans males (59%) had considered suicide, compared with 28% of cisgender males and 37% of cisgender females. The rate among nonbinary/genderqueer individuals was 53%, and it was 48% for those questioning their gender.
Current methods of identifying trans and gender-diverse (TGD) youth in the hospital, however, may not actually be capturing the entire population.
“In health care settings, research involving TGD individuals has historically been limited to specialized clinic populations or youth with gender-specific diagnostic codes documented in the electronic medical record,” an approach that “likely significantly underestimates the prevalence of TGD youth in health care settings.” While at least one study has attempted to bridge this gap by searching the EMR for keywords, that study only tried to identify trans youth and not other youth on the gender diversity spectrum, such as nonbinary youth or those questioning their gender identity. Dr. Burnside and her colleagues therefore designed a study that used keywords to identify both trans youth and other gender-diverse youth who visited the ED so they could assess the rate of positive suicide screens in this population.
Underestimating the population at risk?
The researchers conducted a retrospective cross-sectional study of EMR data for all ED visits during which the patient underwent suicide screening. For the period of November 2019 to August 2022, they collected data on the screening results and the patient’s gender identity, age, race/ethnicity, insurance status, chief complaint in the ED and child opportunity index, which assess a youth’s access to resources based on geography. The suicide screener used was the Ask Suicide–Screening Questions (ASQ) tool.
The keywords they looked for in the EMR to identify trans and gender-diverse youth included transgender, pronouns, agender, gender dysphoria, male-to-female, female-to-male, nonbinary, preferred name, and they/them (captured as a complete term, not as “they” and “them” separately).
“If a keyword was present, the surrounding text was extracted and reviewed by two members of our team,” Dr. Burnside explained in her presentation. “We categorized keywords into either indicative of gender-diverse identity or not, and if it wasn’t clear based on the text extracted, we would conduct a manual chart review,” though that only occurred in about 3% of cases, she added.
Among 15,413 ED encounters with a suicide screen, the researchers identified 1,126 of these keywords in the EMR, among which 91.2% were classified as referring to a gender-diverse patient. Nearly all of the words were at least 90% effective in identify a gender-diverse youth, Dr. Burnside said, and all of the 197 instances of “they/them” were classified as gender diverse.
The accuracy was a little lower for the two keywords that appeared most frequently: For “pronouns,” 86.3% of 306 instances were classified as gender diverse, and for “transgender,” 83.1% of 207 instances were classified as gender diverse. Since some providers ask all patients their pronouns, the presence of “pronouns” in the EMR alone did not necessarily indicate the patient was gender diverse, Dr. Burnside said. A common reason the term “transgender” occurred in the EMR of non–gender diverse patients is that the department’s list of crisis resources includes transgender hotlines.
After identifying all the keywords, the researchers determined how many of these occurred in unique ED encounters and removed those with incomplete screening. Overall, they found 565 encounters by 399 gender-diverse individuals who had a suicide screening, representing 4.6% of total visits. This percentage is slightly lower than recent population-based estimates of gender-diverse youth, the researchers noted.
This population ranged from 8 to 23 years old, and 43% were publicly insured. The chief complaint for most of the patients (77.5%) was a mental health one. They were predominantly White (43%) or Hispanic (35%), with 10% Black youth, 4% Asian youth, and 8% youth who were “other” or two or more races. About half (52%) lived in a neighborhood with a “low” or “very low” child opportunity index.
Within this population, 81% of the patients screened positive on the suicide screening, compared with 23% positive screens across all ED visits. One in ten (10%) gender-diverse youth had active suicidal ideation, compared with 3.4% of the rest of the ED patient population. The researchers calculated that gender-diverse youth had 5.35 times greater odds of screening positive than cisgender youth in the ED (95% confidence interval [CI] 8.7-15.92). Further, a quarter (25%) of the trans and gender-diverse youth who screened positive for suicide risk had come to the ED for a primary complaint unrelated to mental health.
“We had a kid who came in because he broke his arm who had active suicidal ideation,” study coauthor Jennifer A. Hoffmann, MD, assistant professor of pediatrics at the Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, mentioned after the presentation. That particular patient even had a suicide plan, but was identified as actively suicidal only because of the screening. In other cases, she said, a youth may come in with self-inflicted injuries, and while those are the primary complaint, they are linked with suicidal ideation.
Among the study’s limitations are that gender identity is not necessarily being systematically assessed during visits, misspellings might have missed some youth, and their search strategy has not yet been externally validated, though they plan to seek that.
“Overall, however, this study did demonstrate that keyword searching is a promising technique to identify and prioritize gender-diverse youth in health services research,” Dr. Burnside said. In addition to showing the feasibility of using a keyword search strategy for identifying gender-diverse youth, Dr. Burnside noted that 31% of the encounters were identified by just one of the keywords they used, “highlighting the importance of using a comprehensive list of keywords to identify gender-diverse youth.”
Uncovering valuable information
Jason Rafferty, MD, MPH, EdM, clinical assistant professor of pediatrics and of psychiatry and human behavior at Brown University, Providence, R.I., who attended the presentation, noted that the study provides information on a population that’s often difficult to get through traditional EMR research methods.
“A lot of medical record systems don’t have uniform ways of capturing [gender diversity], but what we know as providers is that kids are really struggling and that it’s not a surprise that we’re seeing these disparities with suicidality,” Dr. Rafferty said.
The study also provides more discrete estimates by age than what most other current research measures, which tends to be lifetime suicidality as opposed to suicidal thoughts or attempts within the past year, Dr. Rafferty added.
”What this shows is, for adolescents, the risk of suicide is something we need to be paying attention to. Because it’s not that it’s something that only happens in adults, this really dispels a lot of the misquoting of the data that’s out there.” That kind of information is valuable for determining resource allocation, he said. “A disparity like this really underlies the importance of mental health resources in this field,” he said.
Dr. Burnside, Dr. Hoffmann, and Dr. Rafferty had no disclosures, and no external funding sources were noted.
WASHINGTON – , according to a study presented at the annual meeting of the American Academy of Pediatrics.
“The take-home message here is this study emphasizes the importance of universal screening to identify gender-diverse youth at risk,” Amanda Burnside, PhD, assistant professor of psychiatry and behavioral sciences at Ann and Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, told attendees. “We really need to develop robust strategies and systems to link better mental health services.”
Suicide rates in transgender and gender-diverse youth are exceptionally high among youth in the U.S., Dr. Burnside said during her presentation. For example, the 2022 LGBTQ health survey from the Trevor Project found that much higher percentages of transgender and gender nonconforming youth had considered suicide in the past year compared with cisgender youth, even within the LGBTQ umbrella. Among nearly 34,000 LGBTQ youth aged 13-24, nearly half of trans females (48%) and more than half of trans males (59%) had considered suicide, compared with 28% of cisgender males and 37% of cisgender females. The rate among nonbinary/genderqueer individuals was 53%, and it was 48% for those questioning their gender.
Current methods of identifying trans and gender-diverse (TGD) youth in the hospital, however, may not actually be capturing the entire population.
“In health care settings, research involving TGD individuals has historically been limited to specialized clinic populations or youth with gender-specific diagnostic codes documented in the electronic medical record,” an approach that “likely significantly underestimates the prevalence of TGD youth in health care settings.” While at least one study has attempted to bridge this gap by searching the EMR for keywords, that study only tried to identify trans youth and not other youth on the gender diversity spectrum, such as nonbinary youth or those questioning their gender identity. Dr. Burnside and her colleagues therefore designed a study that used keywords to identify both trans youth and other gender-diverse youth who visited the ED so they could assess the rate of positive suicide screens in this population.
Underestimating the population at risk?
The researchers conducted a retrospective cross-sectional study of EMR data for all ED visits during which the patient underwent suicide screening. For the period of November 2019 to August 2022, they collected data on the screening results and the patient’s gender identity, age, race/ethnicity, insurance status, chief complaint in the ED and child opportunity index, which assess a youth’s access to resources based on geography. The suicide screener used was the Ask Suicide–Screening Questions (ASQ) tool.
The keywords they looked for in the EMR to identify trans and gender-diverse youth included transgender, pronouns, agender, gender dysphoria, male-to-female, female-to-male, nonbinary, preferred name, and they/them (captured as a complete term, not as “they” and “them” separately).
“If a keyword was present, the surrounding text was extracted and reviewed by two members of our team,” Dr. Burnside explained in her presentation. “We categorized keywords into either indicative of gender-diverse identity or not, and if it wasn’t clear based on the text extracted, we would conduct a manual chart review,” though that only occurred in about 3% of cases, she added.
Among 15,413 ED encounters with a suicide screen, the researchers identified 1,126 of these keywords in the EMR, among which 91.2% were classified as referring to a gender-diverse patient. Nearly all of the words were at least 90% effective in identify a gender-diverse youth, Dr. Burnside said, and all of the 197 instances of “they/them” were classified as gender diverse.
The accuracy was a little lower for the two keywords that appeared most frequently: For “pronouns,” 86.3% of 306 instances were classified as gender diverse, and for “transgender,” 83.1% of 207 instances were classified as gender diverse. Since some providers ask all patients their pronouns, the presence of “pronouns” in the EMR alone did not necessarily indicate the patient was gender diverse, Dr. Burnside said. A common reason the term “transgender” occurred in the EMR of non–gender diverse patients is that the department’s list of crisis resources includes transgender hotlines.
After identifying all the keywords, the researchers determined how many of these occurred in unique ED encounters and removed those with incomplete screening. Overall, they found 565 encounters by 399 gender-diverse individuals who had a suicide screening, representing 4.6% of total visits. This percentage is slightly lower than recent population-based estimates of gender-diverse youth, the researchers noted.
This population ranged from 8 to 23 years old, and 43% were publicly insured. The chief complaint for most of the patients (77.5%) was a mental health one. They were predominantly White (43%) or Hispanic (35%), with 10% Black youth, 4% Asian youth, and 8% youth who were “other” or two or more races. About half (52%) lived in a neighborhood with a “low” or “very low” child opportunity index.
Within this population, 81% of the patients screened positive on the suicide screening, compared with 23% positive screens across all ED visits. One in ten (10%) gender-diverse youth had active suicidal ideation, compared with 3.4% of the rest of the ED patient population. The researchers calculated that gender-diverse youth had 5.35 times greater odds of screening positive than cisgender youth in the ED (95% confidence interval [CI] 8.7-15.92). Further, a quarter (25%) of the trans and gender-diverse youth who screened positive for suicide risk had come to the ED for a primary complaint unrelated to mental health.
“We had a kid who came in because he broke his arm who had active suicidal ideation,” study coauthor Jennifer A. Hoffmann, MD, assistant professor of pediatrics at the Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, mentioned after the presentation. That particular patient even had a suicide plan, but was identified as actively suicidal only because of the screening. In other cases, she said, a youth may come in with self-inflicted injuries, and while those are the primary complaint, they are linked with suicidal ideation.
Among the study’s limitations are that gender identity is not necessarily being systematically assessed during visits, misspellings might have missed some youth, and their search strategy has not yet been externally validated, though they plan to seek that.
“Overall, however, this study did demonstrate that keyword searching is a promising technique to identify and prioritize gender-diverse youth in health services research,” Dr. Burnside said. In addition to showing the feasibility of using a keyword search strategy for identifying gender-diverse youth, Dr. Burnside noted that 31% of the encounters were identified by just one of the keywords they used, “highlighting the importance of using a comprehensive list of keywords to identify gender-diverse youth.”
Uncovering valuable information
Jason Rafferty, MD, MPH, EdM, clinical assistant professor of pediatrics and of psychiatry and human behavior at Brown University, Providence, R.I., who attended the presentation, noted that the study provides information on a population that’s often difficult to get through traditional EMR research methods.
“A lot of medical record systems don’t have uniform ways of capturing [gender diversity], but what we know as providers is that kids are really struggling and that it’s not a surprise that we’re seeing these disparities with suicidality,” Dr. Rafferty said.
The study also provides more discrete estimates by age than what most other current research measures, which tends to be lifetime suicidality as opposed to suicidal thoughts or attempts within the past year, Dr. Rafferty added.
”What this shows is, for adolescents, the risk of suicide is something we need to be paying attention to. Because it’s not that it’s something that only happens in adults, this really dispels a lot of the misquoting of the data that’s out there.” That kind of information is valuable for determining resource allocation, he said. “A disparity like this really underlies the importance of mental health resources in this field,” he said.
Dr. Burnside, Dr. Hoffmann, and Dr. Rafferty had no disclosures, and no external funding sources were noted.
WASHINGTON – , according to a study presented at the annual meeting of the American Academy of Pediatrics.
“The take-home message here is this study emphasizes the importance of universal screening to identify gender-diverse youth at risk,” Amanda Burnside, PhD, assistant professor of psychiatry and behavioral sciences at Ann and Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, told attendees. “We really need to develop robust strategies and systems to link better mental health services.”
Suicide rates in transgender and gender-diverse youth are exceptionally high among youth in the U.S., Dr. Burnside said during her presentation. For example, the 2022 LGBTQ health survey from the Trevor Project found that much higher percentages of transgender and gender nonconforming youth had considered suicide in the past year compared with cisgender youth, even within the LGBTQ umbrella. Among nearly 34,000 LGBTQ youth aged 13-24, nearly half of trans females (48%) and more than half of trans males (59%) had considered suicide, compared with 28% of cisgender males and 37% of cisgender females. The rate among nonbinary/genderqueer individuals was 53%, and it was 48% for those questioning their gender.
Current methods of identifying trans and gender-diverse (TGD) youth in the hospital, however, may not actually be capturing the entire population.
“In health care settings, research involving TGD individuals has historically been limited to specialized clinic populations or youth with gender-specific diagnostic codes documented in the electronic medical record,” an approach that “likely significantly underestimates the prevalence of TGD youth in health care settings.” While at least one study has attempted to bridge this gap by searching the EMR for keywords, that study only tried to identify trans youth and not other youth on the gender diversity spectrum, such as nonbinary youth or those questioning their gender identity. Dr. Burnside and her colleagues therefore designed a study that used keywords to identify both trans youth and other gender-diverse youth who visited the ED so they could assess the rate of positive suicide screens in this population.
Underestimating the population at risk?
The researchers conducted a retrospective cross-sectional study of EMR data for all ED visits during which the patient underwent suicide screening. For the period of November 2019 to August 2022, they collected data on the screening results and the patient’s gender identity, age, race/ethnicity, insurance status, chief complaint in the ED and child opportunity index, which assess a youth’s access to resources based on geography. The suicide screener used was the Ask Suicide–Screening Questions (ASQ) tool.
The keywords they looked for in the EMR to identify trans and gender-diverse youth included transgender, pronouns, agender, gender dysphoria, male-to-female, female-to-male, nonbinary, preferred name, and they/them (captured as a complete term, not as “they” and “them” separately).
“If a keyword was present, the surrounding text was extracted and reviewed by two members of our team,” Dr. Burnside explained in her presentation. “We categorized keywords into either indicative of gender-diverse identity or not, and if it wasn’t clear based on the text extracted, we would conduct a manual chart review,” though that only occurred in about 3% of cases, she added.
Among 15,413 ED encounters with a suicide screen, the researchers identified 1,126 of these keywords in the EMR, among which 91.2% were classified as referring to a gender-diverse patient. Nearly all of the words were at least 90% effective in identify a gender-diverse youth, Dr. Burnside said, and all of the 197 instances of “they/them” were classified as gender diverse.
The accuracy was a little lower for the two keywords that appeared most frequently: For “pronouns,” 86.3% of 306 instances were classified as gender diverse, and for “transgender,” 83.1% of 207 instances were classified as gender diverse. Since some providers ask all patients their pronouns, the presence of “pronouns” in the EMR alone did not necessarily indicate the patient was gender diverse, Dr. Burnside said. A common reason the term “transgender” occurred in the EMR of non–gender diverse patients is that the department’s list of crisis resources includes transgender hotlines.
After identifying all the keywords, the researchers determined how many of these occurred in unique ED encounters and removed those with incomplete screening. Overall, they found 565 encounters by 399 gender-diverse individuals who had a suicide screening, representing 4.6% of total visits. This percentage is slightly lower than recent population-based estimates of gender-diverse youth, the researchers noted.
This population ranged from 8 to 23 years old, and 43% were publicly insured. The chief complaint for most of the patients (77.5%) was a mental health one. They were predominantly White (43%) or Hispanic (35%), with 10% Black youth, 4% Asian youth, and 8% youth who were “other” or two or more races. About half (52%) lived in a neighborhood with a “low” or “very low” child opportunity index.
Within this population, 81% of the patients screened positive on the suicide screening, compared with 23% positive screens across all ED visits. One in ten (10%) gender-diverse youth had active suicidal ideation, compared with 3.4% of the rest of the ED patient population. The researchers calculated that gender-diverse youth had 5.35 times greater odds of screening positive than cisgender youth in the ED (95% confidence interval [CI] 8.7-15.92). Further, a quarter (25%) of the trans and gender-diverse youth who screened positive for suicide risk had come to the ED for a primary complaint unrelated to mental health.
“We had a kid who came in because he broke his arm who had active suicidal ideation,” study coauthor Jennifer A. Hoffmann, MD, assistant professor of pediatrics at the Ann & Robert H. Lurie Children’s Hospital of Chicago and Northwestern University, mentioned after the presentation. That particular patient even had a suicide plan, but was identified as actively suicidal only because of the screening. In other cases, she said, a youth may come in with self-inflicted injuries, and while those are the primary complaint, they are linked with suicidal ideation.
Among the study’s limitations are that gender identity is not necessarily being systematically assessed during visits, misspellings might have missed some youth, and their search strategy has not yet been externally validated, though they plan to seek that.
“Overall, however, this study did demonstrate that keyword searching is a promising technique to identify and prioritize gender-diverse youth in health services research,” Dr. Burnside said. In addition to showing the feasibility of using a keyword search strategy for identifying gender-diverse youth, Dr. Burnside noted that 31% of the encounters were identified by just one of the keywords they used, “highlighting the importance of using a comprehensive list of keywords to identify gender-diverse youth.”
Uncovering valuable information
Jason Rafferty, MD, MPH, EdM, clinical assistant professor of pediatrics and of psychiatry and human behavior at Brown University, Providence, R.I., who attended the presentation, noted that the study provides information on a population that’s often difficult to get through traditional EMR research methods.
“A lot of medical record systems don’t have uniform ways of capturing [gender diversity], but what we know as providers is that kids are really struggling and that it’s not a surprise that we’re seeing these disparities with suicidality,” Dr. Rafferty said.
The study also provides more discrete estimates by age than what most other current research measures, which tends to be lifetime suicidality as opposed to suicidal thoughts or attempts within the past year, Dr. Rafferty added.
”What this shows is, for adolescents, the risk of suicide is something we need to be paying attention to. Because it’s not that it’s something that only happens in adults, this really dispels a lot of the misquoting of the data that’s out there.” That kind of information is valuable for determining resource allocation, he said. “A disparity like this really underlies the importance of mental health resources in this field,” he said.
Dr. Burnside, Dr. Hoffmann, and Dr. Rafferty had no disclosures, and no external funding sources were noted.
AT AAP 2023
Piperacillin-tazobactam poses no renal risk in adults with sepsis
TOPLINE:
METHODOLOGY:
The coadministration of piperacillin-tazobactam and vancomycin may raise the risk for AKI, according to a warning from the Food and Drug Administration.
The ACORN trial included 2,511 adults presenting to emergency department or intensive care unit with suspected infection.
Within 12 hours of presentation, these individuals were prescribed either cefepime (n = 1,214) or piperacillin-tazobactam (n = 1,297).
The primary outcome was the risk for the highest stage of AKI or death within 14 days of randomization.
TAKEAWAY:
The highest stage of AKI or death within 14 days did not differ significantly between the cefepime and piperacillin-tazobactam groups (odds ratio, 0.95; P = .56).
The incidence of major adverse kidney events by day 14 was not significantly different between the two groups (absolute risk difference, 1.4%; 95% confidence interval, −1.0% to 3.8%).
Patients in the cefepime versus piperacillin-tazobactam group had fewer days alive and free of delirium and coma within 14 days (OR, 0.79; 95% CI, 0.65-0.95).
IN PRACTICE:
In an accompanying editorial, Steven Y. C. Tong, department of infectious diseases, University of Melbourne, and colleagues wrote: “Because institutions must make decisions about which antibiotics to position on medical wards for rapid administration in patients meeting sepsis criteria, these data should offer solace that if the choice is made to use piperacillin-tazobactam, there is not an increased risk of AKI.”
SOURCE:
The study was led by Edward T. Qian, MD, of Vanderbilt University Medical Center, Nashville, Tenn. It was published online in JAMA with an accompanying editorial.
LIMITATIONS:
The study was conducted at a single academic center, which may limit the generalizability of findings.
Both patients and clinicians were not blinded to group assignment, which may have influenced clinical assessments like Richmond Agitation-Sedation Scale and CAM-ICU or the frequency of laboratory measurements like creatinine.
DISCLOSURES:
The project was supported by the Vanderbilt Institute for Clinical and Translational Research and several other sources, including grants from the National Center for Advancing Translational Sciences. Some authors declared receiving travel grant, personal fees, honoraria, and unrelated research support from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
The coadministration of piperacillin-tazobactam and vancomycin may raise the risk for AKI, according to a warning from the Food and Drug Administration.
The ACORN trial included 2,511 adults presenting to emergency department or intensive care unit with suspected infection.
Within 12 hours of presentation, these individuals were prescribed either cefepime (n = 1,214) or piperacillin-tazobactam (n = 1,297).
The primary outcome was the risk for the highest stage of AKI or death within 14 days of randomization.
TAKEAWAY:
The highest stage of AKI or death within 14 days did not differ significantly between the cefepime and piperacillin-tazobactam groups (odds ratio, 0.95; P = .56).
The incidence of major adverse kidney events by day 14 was not significantly different between the two groups (absolute risk difference, 1.4%; 95% confidence interval, −1.0% to 3.8%).
Patients in the cefepime versus piperacillin-tazobactam group had fewer days alive and free of delirium and coma within 14 days (OR, 0.79; 95% CI, 0.65-0.95).
IN PRACTICE:
In an accompanying editorial, Steven Y. C. Tong, department of infectious diseases, University of Melbourne, and colleagues wrote: “Because institutions must make decisions about which antibiotics to position on medical wards for rapid administration in patients meeting sepsis criteria, these data should offer solace that if the choice is made to use piperacillin-tazobactam, there is not an increased risk of AKI.”
SOURCE:
The study was led by Edward T. Qian, MD, of Vanderbilt University Medical Center, Nashville, Tenn. It was published online in JAMA with an accompanying editorial.
LIMITATIONS:
The study was conducted at a single academic center, which may limit the generalizability of findings.
Both patients and clinicians were not blinded to group assignment, which may have influenced clinical assessments like Richmond Agitation-Sedation Scale and CAM-ICU or the frequency of laboratory measurements like creatinine.
DISCLOSURES:
The project was supported by the Vanderbilt Institute for Clinical and Translational Research and several other sources, including grants from the National Center for Advancing Translational Sciences. Some authors declared receiving travel grant, personal fees, honoraria, and unrelated research support from various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
The coadministration of piperacillin-tazobactam and vancomycin may raise the risk for AKI, according to a warning from the Food and Drug Administration.
The ACORN trial included 2,511 adults presenting to emergency department or intensive care unit with suspected infection.
Within 12 hours of presentation, these individuals were prescribed either cefepime (n = 1,214) or piperacillin-tazobactam (n = 1,297).
The primary outcome was the risk for the highest stage of AKI or death within 14 days of randomization.
TAKEAWAY:
The highest stage of AKI or death within 14 days did not differ significantly between the cefepime and piperacillin-tazobactam groups (odds ratio, 0.95; P = .56).
The incidence of major adverse kidney events by day 14 was not significantly different between the two groups (absolute risk difference, 1.4%; 95% confidence interval, −1.0% to 3.8%).
Patients in the cefepime versus piperacillin-tazobactam group had fewer days alive and free of delirium and coma within 14 days (OR, 0.79; 95% CI, 0.65-0.95).
IN PRACTICE:
In an accompanying editorial, Steven Y. C. Tong, department of infectious diseases, University of Melbourne, and colleagues wrote: “Because institutions must make decisions about which antibiotics to position on medical wards for rapid administration in patients meeting sepsis criteria, these data should offer solace that if the choice is made to use piperacillin-tazobactam, there is not an increased risk of AKI.”
SOURCE:
The study was led by Edward T. Qian, MD, of Vanderbilt University Medical Center, Nashville, Tenn. It was published online in JAMA with an accompanying editorial.
LIMITATIONS:
The study was conducted at a single academic center, which may limit the generalizability of findings.
Both patients and clinicians were not blinded to group assignment, which may have influenced clinical assessments like Richmond Agitation-Sedation Scale and CAM-ICU or the frequency of laboratory measurements like creatinine.
DISCLOSURES:
The project was supported by the Vanderbilt Institute for Clinical and Translational Research and several other sources, including grants from the National Center for Advancing Translational Sciences. Some authors declared receiving travel grant, personal fees, honoraria, and unrelated research support from various sources.
A version of this article appeared on Medscape.com.
Serious mental illness tied to 50% higher all-cause mortality risk after COVID
TOPLINE:
METHODOLOGY:
- Investigators analyzed data from the Clinical Practice Research Datalink database, which contains health information on 13.5 million patients receiving care from family practices in England and Northern Ireland.
- The study included participants with SMI, including schizophrenia, schizoaffective disorder, and bipolar disorder.
- Participants were aged 5 years or older with a SARS-CoV-2 infection recorded between Feb. 1, 2020, and March 31, 2021, spanning two waves of the pandemic.
- Death rates among participants with SMI and COVID-19 (n = 7,150; 56% female) were compared with those in a control group of participants without SMI who had been diagnosed with COVID-19 (n = 650,000; 55% female).
TAKEAWAY:
- Participants with SMI and COVID-19 had a 53% higher risk for death than those in the non-SMI control group (adjusted hazard ratio, 1.53; 95% confidence interval, 1.39-1.68).
- Black Caribbean/Black African participants were more likely than White participants to die of COVID-19 (aHR, 1.22; 95% CI, 1.12-1.34), although ethnicity was not recorded in 30% of participants.
- After SARS-CoV-2 infection, for every additional multimorbid condition, the aHR for death increased by 6% in the SMI group and 16% in the non-SMI group (P = .001). Some of these conditions included hypertension, heart disease, diabetes, kidney disease, depression, and anxiety.
IN PRACTICE:
“From a public health perspective, our study has emphasized the need for early and timely preventative interventions (e.g. vaccination) for the SMI population. Future studies are needed to disentangle the complex biological and psychosocial factors, and health care pathways, that have led to the greater mortality rates in the SMI population,” the authors write.
SOURCE:
Jayati Das-Munshi, MD, of Kings College London, led the study, which was published online in the British Journal of Psychiatry. The study was funded by the Health Foundation.
LIMITATIONS:
COVID-19 may have been underdiagnosed or underreported in the records studied. Also, investigators did not have information about cause of death.
DISCLOSURES:
One author received funding from Janssen, GSK, and Takeda. All other authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators analyzed data from the Clinical Practice Research Datalink database, which contains health information on 13.5 million patients receiving care from family practices in England and Northern Ireland.
- The study included participants with SMI, including schizophrenia, schizoaffective disorder, and bipolar disorder.
- Participants were aged 5 years or older with a SARS-CoV-2 infection recorded between Feb. 1, 2020, and March 31, 2021, spanning two waves of the pandemic.
- Death rates among participants with SMI and COVID-19 (n = 7,150; 56% female) were compared with those in a control group of participants without SMI who had been diagnosed with COVID-19 (n = 650,000; 55% female).
TAKEAWAY:
- Participants with SMI and COVID-19 had a 53% higher risk for death than those in the non-SMI control group (adjusted hazard ratio, 1.53; 95% confidence interval, 1.39-1.68).
- Black Caribbean/Black African participants were more likely than White participants to die of COVID-19 (aHR, 1.22; 95% CI, 1.12-1.34), although ethnicity was not recorded in 30% of participants.
- After SARS-CoV-2 infection, for every additional multimorbid condition, the aHR for death increased by 6% in the SMI group and 16% in the non-SMI group (P = .001). Some of these conditions included hypertension, heart disease, diabetes, kidney disease, depression, and anxiety.
IN PRACTICE:
“From a public health perspective, our study has emphasized the need for early and timely preventative interventions (e.g. vaccination) for the SMI population. Future studies are needed to disentangle the complex biological and psychosocial factors, and health care pathways, that have led to the greater mortality rates in the SMI population,” the authors write.
SOURCE:
Jayati Das-Munshi, MD, of Kings College London, led the study, which was published online in the British Journal of Psychiatry. The study was funded by the Health Foundation.
LIMITATIONS:
COVID-19 may have been underdiagnosed or underreported in the records studied. Also, investigators did not have information about cause of death.
DISCLOSURES:
One author received funding from Janssen, GSK, and Takeda. All other authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Investigators analyzed data from the Clinical Practice Research Datalink database, which contains health information on 13.5 million patients receiving care from family practices in England and Northern Ireland.
- The study included participants with SMI, including schizophrenia, schizoaffective disorder, and bipolar disorder.
- Participants were aged 5 years or older with a SARS-CoV-2 infection recorded between Feb. 1, 2020, and March 31, 2021, spanning two waves of the pandemic.
- Death rates among participants with SMI and COVID-19 (n = 7,150; 56% female) were compared with those in a control group of participants without SMI who had been diagnosed with COVID-19 (n = 650,000; 55% female).
TAKEAWAY:
- Participants with SMI and COVID-19 had a 53% higher risk for death than those in the non-SMI control group (adjusted hazard ratio, 1.53; 95% confidence interval, 1.39-1.68).
- Black Caribbean/Black African participants were more likely than White participants to die of COVID-19 (aHR, 1.22; 95% CI, 1.12-1.34), although ethnicity was not recorded in 30% of participants.
- After SARS-CoV-2 infection, for every additional multimorbid condition, the aHR for death increased by 6% in the SMI group and 16% in the non-SMI group (P = .001). Some of these conditions included hypertension, heart disease, diabetes, kidney disease, depression, and anxiety.
IN PRACTICE:
“From a public health perspective, our study has emphasized the need for early and timely preventative interventions (e.g. vaccination) for the SMI population. Future studies are needed to disentangle the complex biological and psychosocial factors, and health care pathways, that have led to the greater mortality rates in the SMI population,” the authors write.
SOURCE:
Jayati Das-Munshi, MD, of Kings College London, led the study, which was published online in the British Journal of Psychiatry. The study was funded by the Health Foundation.
LIMITATIONS:
COVID-19 may have been underdiagnosed or underreported in the records studied. Also, investigators did not have information about cause of death.
DISCLOSURES:
One author received funding from Janssen, GSK, and Takeda. All other authors declared no conflicts of interest.
A version of this article first appeared on Medscape.com.
Nirmatrelvir-ritonavir ineffective at reducing most post-COVID conditions
TOPLINE:
Thromboembolic events are the exception.
METHODOLOGY:
- A retrospective study of 9,593 veterans older than 65 years examined the impact of nirmatrelvir-ritonavir in comparison with no treatment on post–COVID-19 conditions (PCCs).
- Researchers coded 31 conditions, including those that fell into cardiac, pulmonary, renal, thromboembolic, gastrointestinal, neurologic, mental health, musculoskeletal, and endocrine categories.
- The incidence of PCCs was analyzed 31-180 days after treatment.
TAKEAWAY:
- The combined incidence of venous thromboembolism and pulmonary embolism was reduced among patients given nirmatrelvir-ritonavir.
- No statistically significant reduction of other conditions was found.
- Results differ from the conclusions of a smaller study that found that the incidence of 10 of 13 PCCs was lower.
IN PRACTICE:
“Our results suggest that considerations about PCCs may not be an important factor in COVID-19 treatment decisions,” the authors write.
SOURCE:
The study was funded by the Department of Veterans Affairs and was published online in Annals of Internal Medicine. George Ioannou, MD, director of hepatology at the VA Puget Sound Health Care System in Seattle, led the study.
LIMITATIONS:
A large number of outcomes were observed, so it’s possible that the association between treatment with nirmatrelvir-ritonavir and reduced incidence of thromboembolic events occurred by chance.
Data on COVID-19 treatments and PCCs may be incomplete. The long-term effects of PCCs may not have been fully captured by the ICD-10, which was used for diagnosis codes.
Electronic health records did not accurately capture the symptom burden or the date symptoms began. Patients in the treatment arm may have had more symptoms than matched control persons who were not treated.
DISCLOSURES:
The authors reported relationships with the Korean Diabetes Association, the American Diabetes Association, the International Society for the Diabetic Foot, Quality Insights, Brown University, and the Society for Women in Urology, among others.
A version of this article appeared on Medscape.com.
TOPLINE:
Thromboembolic events are the exception.
METHODOLOGY:
- A retrospective study of 9,593 veterans older than 65 years examined the impact of nirmatrelvir-ritonavir in comparison with no treatment on post–COVID-19 conditions (PCCs).
- Researchers coded 31 conditions, including those that fell into cardiac, pulmonary, renal, thromboembolic, gastrointestinal, neurologic, mental health, musculoskeletal, and endocrine categories.
- The incidence of PCCs was analyzed 31-180 days after treatment.
TAKEAWAY:
- The combined incidence of venous thromboembolism and pulmonary embolism was reduced among patients given nirmatrelvir-ritonavir.
- No statistically significant reduction of other conditions was found.
- Results differ from the conclusions of a smaller study that found that the incidence of 10 of 13 PCCs was lower.
IN PRACTICE:
“Our results suggest that considerations about PCCs may not be an important factor in COVID-19 treatment decisions,” the authors write.
SOURCE:
The study was funded by the Department of Veterans Affairs and was published online in Annals of Internal Medicine. George Ioannou, MD, director of hepatology at the VA Puget Sound Health Care System in Seattle, led the study.
LIMITATIONS:
A large number of outcomes were observed, so it’s possible that the association between treatment with nirmatrelvir-ritonavir and reduced incidence of thromboembolic events occurred by chance.
Data on COVID-19 treatments and PCCs may be incomplete. The long-term effects of PCCs may not have been fully captured by the ICD-10, which was used for diagnosis codes.
Electronic health records did not accurately capture the symptom burden or the date symptoms began. Patients in the treatment arm may have had more symptoms than matched control persons who were not treated.
DISCLOSURES:
The authors reported relationships with the Korean Diabetes Association, the American Diabetes Association, the International Society for the Diabetic Foot, Quality Insights, Brown University, and the Society for Women in Urology, among others.
A version of this article appeared on Medscape.com.
TOPLINE:
Thromboembolic events are the exception.
METHODOLOGY:
- A retrospective study of 9,593 veterans older than 65 years examined the impact of nirmatrelvir-ritonavir in comparison with no treatment on post–COVID-19 conditions (PCCs).
- Researchers coded 31 conditions, including those that fell into cardiac, pulmonary, renal, thromboembolic, gastrointestinal, neurologic, mental health, musculoskeletal, and endocrine categories.
- The incidence of PCCs was analyzed 31-180 days after treatment.
TAKEAWAY:
- The combined incidence of venous thromboembolism and pulmonary embolism was reduced among patients given nirmatrelvir-ritonavir.
- No statistically significant reduction of other conditions was found.
- Results differ from the conclusions of a smaller study that found that the incidence of 10 of 13 PCCs was lower.
IN PRACTICE:
“Our results suggest that considerations about PCCs may not be an important factor in COVID-19 treatment decisions,” the authors write.
SOURCE:
The study was funded by the Department of Veterans Affairs and was published online in Annals of Internal Medicine. George Ioannou, MD, director of hepatology at the VA Puget Sound Health Care System in Seattle, led the study.
LIMITATIONS:
A large number of outcomes were observed, so it’s possible that the association between treatment with nirmatrelvir-ritonavir and reduced incidence of thromboembolic events occurred by chance.
Data on COVID-19 treatments and PCCs may be incomplete. The long-term effects of PCCs may not have been fully captured by the ICD-10, which was used for diagnosis codes.
Electronic health records did not accurately capture the symptom burden or the date symptoms began. Patients in the treatment arm may have had more symptoms than matched control persons who were not treated.
DISCLOSURES:
The authors reported relationships with the Korean Diabetes Association, the American Diabetes Association, the International Society for the Diabetic Foot, Quality Insights, Brown University, and the Society for Women in Urology, among others.
A version of this article appeared on Medscape.com.
No benefit of colchicine after stroke, TIA: CHANCE-3
The anti-inflammatory agent in the CHANCE-3 trial.
The results were presented by Yongjun Wang, MD, Beijing Tiantan Hospital, Capital Medical University, at the annual World Stroke Congress, sponsored by the World Stroke Organization.
Dr. Wang noted that inflammation may be a key factor involved in the residual risk for recurrent stroke, with data from previous CHANCE trials suggesting a higher stroke recurrence rate in patients with higher levels of high-sensitivity C-reactive protein (hsCRP), a key marker of inflammation.
Low-dose colchicine, which acts as an anti-inflammatory agent, has recently been approved in many countries for patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease to reduce the risk for future cardiovascular events. This follows benefits seen in those populations in the LoDoCo-2 and COLCOT trials.
The CHANCE-3 study was conducted to evaluate whether similar benefits could be found in patients with acute ischemic stroke.
The trial involved 8,369 Chinese patients with minor to moderate ischemic stroke (National Institutes of Health Stroke Scale score ≤ 5) or high-risk TIA (ABCD2 score ≥ 4) who had an hsCRP level of at least 2 mg/L.
Patients were assigned within 24 hours after symptom onset, in a 1:1 ratio, to receive colchicine (1 mg daily on days 1-3, followed by 0.5 mg daily for a total of 90 days) or placebo, on a background of optimal medical therapy.
The primary outcome was any stroke within 90 days. The key secondary outcomes included a composite of stroke, TIA, myocardial infarction, and vascular death within 90 days, and Modified Rankin Scale score greater than 1 at 90 days.
Results showed that the primary outcome of any stroke at 90 days occurred in 6.3% of the colchicine group versus 6.5% of the placebo group, a nonsignificant difference (P = .79).
All secondary outcomes were also neutral, with no differences between the two groups.
Addressing the different results in CHANCE-3, compared with those of the cardiovascular trials of colchicine, Dr. Wang pointed out that the cardiovascular trials had a much longer treatment and follow-up time (an average of 22 months), compared with just 3 months in CHANCE-3.
“Clinical trials with longer treatment times are needed to further assess the effects of colchicine after cerebrovascular events, but it may be that ischemic cerebrovascular disease and ischemic heart disease respond differently to colchicine treatment,” he concluded.
Commenting on the study, cochair of the WSC session at which it was presented, Ashkan Shoamanesh, MD, associate professor of medicine at McMaster University, Hamilton, Ont., said CHANCE-3 was a well-designed large phase 3 randomized trial and the first such trial to test colchicine for secondary stroke prevention.
He agreed with Dr. Wang that the follow-up duration for this initial analysis of 3-month outcomes may have been too short to see an effect.
“So, we require randomized trials with longer follow-up prior to abandoning this potential treatment,” he added.
Dr. Shoamanesh noted that several additional trials are currently ongoing testing colchicine for secondary prevention in patients with stroke. These include the CONVINCE, CASPER, CoVasc-ICH, and RIISC-THETIS trials.
He also pointed out that, in contrast to ischemic heart disease, which results from atherosclerosis, the mechanisms underlying ischemic stroke are more heterogeneous and include various vascular and cardioembolic pathologies.
The CHANCE-3 study was funded by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences, and the Beijing Municipal Health Commission.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agent in the CHANCE-3 trial.
The results were presented by Yongjun Wang, MD, Beijing Tiantan Hospital, Capital Medical University, at the annual World Stroke Congress, sponsored by the World Stroke Organization.
Dr. Wang noted that inflammation may be a key factor involved in the residual risk for recurrent stroke, with data from previous CHANCE trials suggesting a higher stroke recurrence rate in patients with higher levels of high-sensitivity C-reactive protein (hsCRP), a key marker of inflammation.
Low-dose colchicine, which acts as an anti-inflammatory agent, has recently been approved in many countries for patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease to reduce the risk for future cardiovascular events. This follows benefits seen in those populations in the LoDoCo-2 and COLCOT trials.
The CHANCE-3 study was conducted to evaluate whether similar benefits could be found in patients with acute ischemic stroke.
The trial involved 8,369 Chinese patients with minor to moderate ischemic stroke (National Institutes of Health Stroke Scale score ≤ 5) or high-risk TIA (ABCD2 score ≥ 4) who had an hsCRP level of at least 2 mg/L.
Patients were assigned within 24 hours after symptom onset, in a 1:1 ratio, to receive colchicine (1 mg daily on days 1-3, followed by 0.5 mg daily for a total of 90 days) or placebo, on a background of optimal medical therapy.
The primary outcome was any stroke within 90 days. The key secondary outcomes included a composite of stroke, TIA, myocardial infarction, and vascular death within 90 days, and Modified Rankin Scale score greater than 1 at 90 days.
Results showed that the primary outcome of any stroke at 90 days occurred in 6.3% of the colchicine group versus 6.5% of the placebo group, a nonsignificant difference (P = .79).
All secondary outcomes were also neutral, with no differences between the two groups.
Addressing the different results in CHANCE-3, compared with those of the cardiovascular trials of colchicine, Dr. Wang pointed out that the cardiovascular trials had a much longer treatment and follow-up time (an average of 22 months), compared with just 3 months in CHANCE-3.
“Clinical trials with longer treatment times are needed to further assess the effects of colchicine after cerebrovascular events, but it may be that ischemic cerebrovascular disease and ischemic heart disease respond differently to colchicine treatment,” he concluded.
Commenting on the study, cochair of the WSC session at which it was presented, Ashkan Shoamanesh, MD, associate professor of medicine at McMaster University, Hamilton, Ont., said CHANCE-3 was a well-designed large phase 3 randomized trial and the first such trial to test colchicine for secondary stroke prevention.
He agreed with Dr. Wang that the follow-up duration for this initial analysis of 3-month outcomes may have been too short to see an effect.
“So, we require randomized trials with longer follow-up prior to abandoning this potential treatment,” he added.
Dr. Shoamanesh noted that several additional trials are currently ongoing testing colchicine for secondary prevention in patients with stroke. These include the CONVINCE, CASPER, CoVasc-ICH, and RIISC-THETIS trials.
He also pointed out that, in contrast to ischemic heart disease, which results from atherosclerosis, the mechanisms underlying ischemic stroke are more heterogeneous and include various vascular and cardioembolic pathologies.
The CHANCE-3 study was funded by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences, and the Beijing Municipal Health Commission.
A version of this article first appeared on Medscape.com.
The anti-inflammatory agent in the CHANCE-3 trial.
The results were presented by Yongjun Wang, MD, Beijing Tiantan Hospital, Capital Medical University, at the annual World Stroke Congress, sponsored by the World Stroke Organization.
Dr. Wang noted that inflammation may be a key factor involved in the residual risk for recurrent stroke, with data from previous CHANCE trials suggesting a higher stroke recurrence rate in patients with higher levels of high-sensitivity C-reactive protein (hsCRP), a key marker of inflammation.
Low-dose colchicine, which acts as an anti-inflammatory agent, has recently been approved in many countries for patients with established atherosclerotic disease or multiple risk factors for cardiovascular disease to reduce the risk for future cardiovascular events. This follows benefits seen in those populations in the LoDoCo-2 and COLCOT trials.
The CHANCE-3 study was conducted to evaluate whether similar benefits could be found in patients with acute ischemic stroke.
The trial involved 8,369 Chinese patients with minor to moderate ischemic stroke (National Institutes of Health Stroke Scale score ≤ 5) or high-risk TIA (ABCD2 score ≥ 4) who had an hsCRP level of at least 2 mg/L.
Patients were assigned within 24 hours after symptom onset, in a 1:1 ratio, to receive colchicine (1 mg daily on days 1-3, followed by 0.5 mg daily for a total of 90 days) or placebo, on a background of optimal medical therapy.
The primary outcome was any stroke within 90 days. The key secondary outcomes included a composite of stroke, TIA, myocardial infarction, and vascular death within 90 days, and Modified Rankin Scale score greater than 1 at 90 days.
Results showed that the primary outcome of any stroke at 90 days occurred in 6.3% of the colchicine group versus 6.5% of the placebo group, a nonsignificant difference (P = .79).
All secondary outcomes were also neutral, with no differences between the two groups.
Addressing the different results in CHANCE-3, compared with those of the cardiovascular trials of colchicine, Dr. Wang pointed out that the cardiovascular trials had a much longer treatment and follow-up time (an average of 22 months), compared with just 3 months in CHANCE-3.
“Clinical trials with longer treatment times are needed to further assess the effects of colchicine after cerebrovascular events, but it may be that ischemic cerebrovascular disease and ischemic heart disease respond differently to colchicine treatment,” he concluded.
Commenting on the study, cochair of the WSC session at which it was presented, Ashkan Shoamanesh, MD, associate professor of medicine at McMaster University, Hamilton, Ont., said CHANCE-3 was a well-designed large phase 3 randomized trial and the first such trial to test colchicine for secondary stroke prevention.
He agreed with Dr. Wang that the follow-up duration for this initial analysis of 3-month outcomes may have been too short to see an effect.
“So, we require randomized trials with longer follow-up prior to abandoning this potential treatment,” he added.
Dr. Shoamanesh noted that several additional trials are currently ongoing testing colchicine for secondary prevention in patients with stroke. These include the CONVINCE, CASPER, CoVasc-ICH, and RIISC-THETIS trials.
He also pointed out that, in contrast to ischemic heart disease, which results from atherosclerosis, the mechanisms underlying ischemic stroke are more heterogeneous and include various vascular and cardioembolic pathologies.
The CHANCE-3 study was funded by grants from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, the Chinese Academy of Medical Sciences, and the Beijing Municipal Health Commission.
A version of this article first appeared on Medscape.com.
FROM WSC 2023
mRNA vaccine cuts COVID-related Guillain-Barré risk
TOPLINE:
, according to a new study that also showed receipt of the Pfizer-BioNTech mRNA vaccine reduced GSB risk by 59%.
METHODOLOGY:
- The nested-case control study analyzed data from the largest healthcare provider in Israel for 3.2 million patients aged 16 years and older, with no history of GBS.
- GBS cases (n = 76) were identified based on hospital discharge data from January 2021 to June 2022.
- For every GBS case, investigators chose 10 controls at random, matched for age, gender, and follow-up duration (n = 760).
- Investigators examined the association between GBS and SARS-CoV-2 infection, established through documentation of prior positive SARS-CoV-2 test (PCR or antigen), and any COVID-19 vaccine administration.
TAKEAWAY:
- Among those diagnosed with GBS, 8 were exposed to SARS-CoV-2 infection only, 7 were exposed to COVID-19 vaccination only, and 1 patient was exposed to both SARS-CoV-2 infection and COVID-19 vaccination in the prior 6 weeks, leaving 60 GBS patients without exposure to either infection or vaccination.
- All COVID-19 vaccine doses administered in GBS cases within 6 weeks of the index date, and all but two doses administered in controls in the same timeframe, were Pfizer-BioNTech vaccines.
- Compared with people without GBS, those with the condition were more than six times as likely to have had SARS-CoV-2 infection within 6 weeks of GBS diagnosis (adjusted odds ratio, 6.30; 95% confidence interval, 2.55-15.56).
- People who received the COVID-19 vaccine were 59% less likely to develop GBS than those who did not get the vaccine (aOR, 0.41; 95% CI, 0.17-0.96).
IN PRACTICE:
“While Guillain-Barré is extremely rare, people should be aware that having a COVID infection can increase their risk of developing the disorder, and receiving an mRNA vaccine can decrease their risk,” study author Anat Arbel, MD, of Lady Davis Carmel Medical Center and the Technion-Israel Institute of Technology, Haifa, Israel, said in a press release.
SOURCE:
In addition to Dr. Arbel, the other lead author is Haya Bishara, MD, of Lady Davis Carmel Medical Center. The research was published online in the journal Neurology.
LIMITATIONS:
There is a possibility of misclassification of SARS-CoV-2 infection, which could lead to an overestimation of the magnitude of association between infection and GBS. The diagnosis of GBS relied solely on ICD-9 coding, which has been shown in prior studies to contain errors.
DISCLOSURES:
The study was unfunded. Dr. Bishara and Dr. Arbel report no relevant financial relationships. One co-author, Eitan Auriel, MD, has received lecturer fees from Novo Nordisk, Pfizer, Boehringer Ingelheim, and Medison.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study that also showed receipt of the Pfizer-BioNTech mRNA vaccine reduced GSB risk by 59%.
METHODOLOGY:
- The nested-case control study analyzed data from the largest healthcare provider in Israel for 3.2 million patients aged 16 years and older, with no history of GBS.
- GBS cases (n = 76) were identified based on hospital discharge data from January 2021 to June 2022.
- For every GBS case, investigators chose 10 controls at random, matched for age, gender, and follow-up duration (n = 760).
- Investigators examined the association between GBS and SARS-CoV-2 infection, established through documentation of prior positive SARS-CoV-2 test (PCR or antigen), and any COVID-19 vaccine administration.
TAKEAWAY:
- Among those diagnosed with GBS, 8 were exposed to SARS-CoV-2 infection only, 7 were exposed to COVID-19 vaccination only, and 1 patient was exposed to both SARS-CoV-2 infection and COVID-19 vaccination in the prior 6 weeks, leaving 60 GBS patients without exposure to either infection or vaccination.
- All COVID-19 vaccine doses administered in GBS cases within 6 weeks of the index date, and all but two doses administered in controls in the same timeframe, were Pfizer-BioNTech vaccines.
- Compared with people without GBS, those with the condition were more than six times as likely to have had SARS-CoV-2 infection within 6 weeks of GBS diagnosis (adjusted odds ratio, 6.30; 95% confidence interval, 2.55-15.56).
- People who received the COVID-19 vaccine were 59% less likely to develop GBS than those who did not get the vaccine (aOR, 0.41; 95% CI, 0.17-0.96).
IN PRACTICE:
“While Guillain-Barré is extremely rare, people should be aware that having a COVID infection can increase their risk of developing the disorder, and receiving an mRNA vaccine can decrease their risk,” study author Anat Arbel, MD, of Lady Davis Carmel Medical Center and the Technion-Israel Institute of Technology, Haifa, Israel, said in a press release.
SOURCE:
In addition to Dr. Arbel, the other lead author is Haya Bishara, MD, of Lady Davis Carmel Medical Center. The research was published online in the journal Neurology.
LIMITATIONS:
There is a possibility of misclassification of SARS-CoV-2 infection, which could lead to an overestimation of the magnitude of association between infection and GBS. The diagnosis of GBS relied solely on ICD-9 coding, which has been shown in prior studies to contain errors.
DISCLOSURES:
The study was unfunded. Dr. Bishara and Dr. Arbel report no relevant financial relationships. One co-author, Eitan Auriel, MD, has received lecturer fees from Novo Nordisk, Pfizer, Boehringer Ingelheim, and Medison.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study that also showed receipt of the Pfizer-BioNTech mRNA vaccine reduced GSB risk by 59%.
METHODOLOGY:
- The nested-case control study analyzed data from the largest healthcare provider in Israel for 3.2 million patients aged 16 years and older, with no history of GBS.
- GBS cases (n = 76) were identified based on hospital discharge data from January 2021 to June 2022.
- For every GBS case, investigators chose 10 controls at random, matched for age, gender, and follow-up duration (n = 760).
- Investigators examined the association between GBS and SARS-CoV-2 infection, established through documentation of prior positive SARS-CoV-2 test (PCR or antigen), and any COVID-19 vaccine administration.
TAKEAWAY:
- Among those diagnosed with GBS, 8 were exposed to SARS-CoV-2 infection only, 7 were exposed to COVID-19 vaccination only, and 1 patient was exposed to both SARS-CoV-2 infection and COVID-19 vaccination in the prior 6 weeks, leaving 60 GBS patients without exposure to either infection or vaccination.
- All COVID-19 vaccine doses administered in GBS cases within 6 weeks of the index date, and all but two doses administered in controls in the same timeframe, were Pfizer-BioNTech vaccines.
- Compared with people without GBS, those with the condition were more than six times as likely to have had SARS-CoV-2 infection within 6 weeks of GBS diagnosis (adjusted odds ratio, 6.30; 95% confidence interval, 2.55-15.56).
- People who received the COVID-19 vaccine were 59% less likely to develop GBS than those who did not get the vaccine (aOR, 0.41; 95% CI, 0.17-0.96).
IN PRACTICE:
“While Guillain-Barré is extremely rare, people should be aware that having a COVID infection can increase their risk of developing the disorder, and receiving an mRNA vaccine can decrease their risk,” study author Anat Arbel, MD, of Lady Davis Carmel Medical Center and the Technion-Israel Institute of Technology, Haifa, Israel, said in a press release.
SOURCE:
In addition to Dr. Arbel, the other lead author is Haya Bishara, MD, of Lady Davis Carmel Medical Center. The research was published online in the journal Neurology.
LIMITATIONS:
There is a possibility of misclassification of SARS-CoV-2 infection, which could lead to an overestimation of the magnitude of association between infection and GBS. The diagnosis of GBS relied solely on ICD-9 coding, which has been shown in prior studies to contain errors.
DISCLOSURES:
The study was unfunded. Dr. Bishara and Dr. Arbel report no relevant financial relationships. One co-author, Eitan Auriel, MD, has received lecturer fees from Novo Nordisk, Pfizer, Boehringer Ingelheim, and Medison.
A version of this article first appeared on Medscape.com.