User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
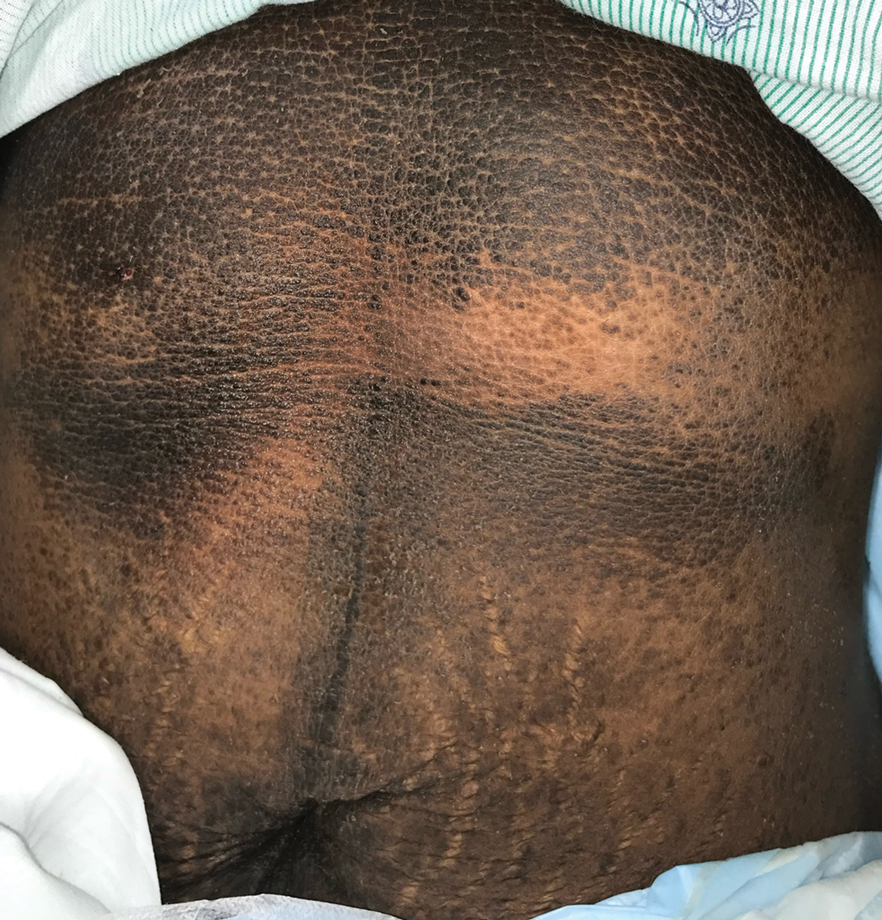
Velvety Plaques on the Abdomen and Extremities
The Diagnosis: Dermatitis Neglecta
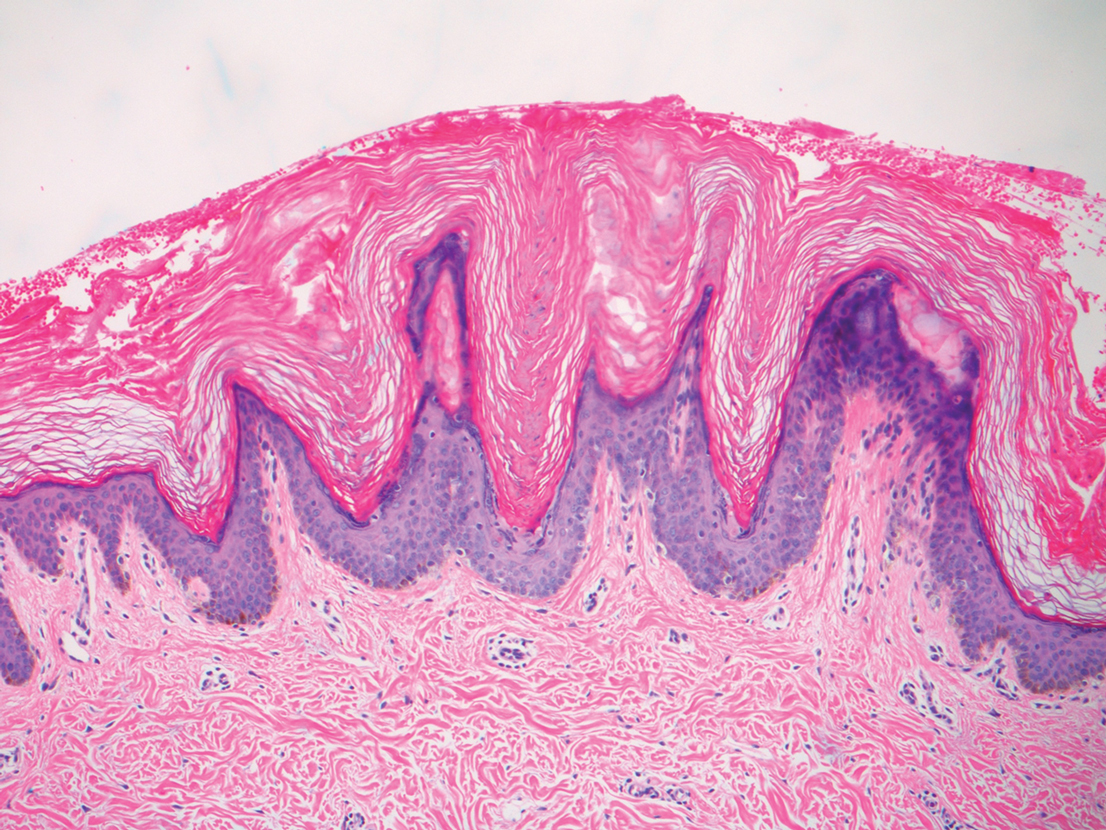
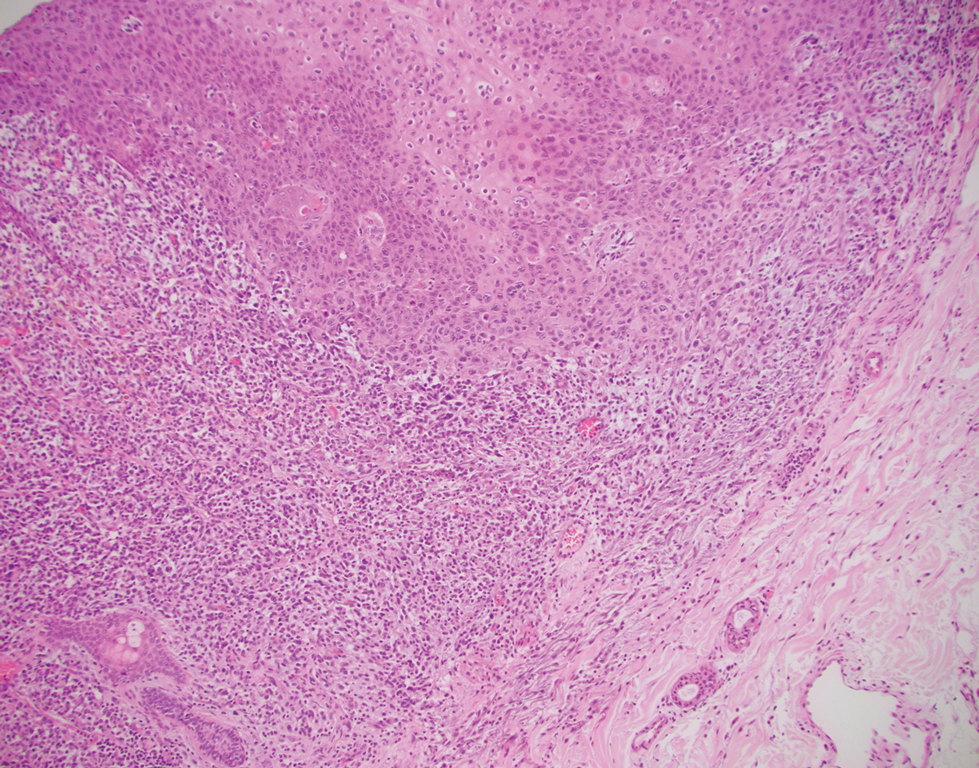
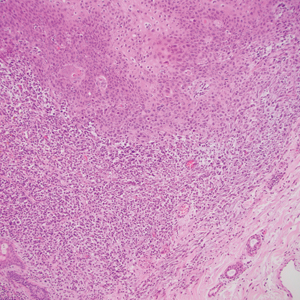
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.
Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
The Diagnosis: Dermatitis Neglecta
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.
Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
The Diagnosis: Dermatitis Neglecta
A punch biopsy of the abdomen revealed hyperkeratosis and mild papillomatosis (Figure), which can be seen in dermatitis neglecta (DN) and acanthosis nigricans (AN) as well as confluent and reticulated papillomatosis (CARP). Due to the patient’s history of mood and psychotic disorders, collateral information was obtained from the patient’s family, who reported that the patient had a depressed mood in the last few months and was not showering or caring for herself during this period. There was no additional personal or family history of skin disease. Clinical and histopathologic findings led to a diagnosis of DN. Following recommendations for daily cleansing with soap and water along with topical ammonium lactate, near-complete resolution of the rash was achieved in 3 weeks.
Dermatitis neglecta, or unwashed dermatosis, is a skin condition that occurs secondary to poor hygiene, which was first reported in 1995 by Poskitt et al.1 Avoidance of washing in affected areas can be due to physical disability, pain after injury, neurological deficit, or psychologically induced fear or neglect. Sebum, sweat, corneocytes, and bacteria combine into compact adherent crusts of dirt, which appear as hyperkeratotic plaques with cornflakelike scale.2,3 Despite its innate simplicity, DN is a diagnostic challenge, as it clinically and histologically mimics other dermatoses including AN, terra firmaforme dermatosis, and CARP.2,4 Ultimately, the diagnosis of DN can be made when a history of poor hygiene is probable or elicited, and lesions can be removed with soap and water. Treatment of DN includes daily cleansing with soap and water; however, resistant lesions or extensive disease may require keratolytic agents, as in our patient.2-4 In contrast, terra firma-forme dermatosis, which may look similar, is not due to poor hygiene, and the lesions typically are resistant to soap and water, classically requiring isopropyl alcohol for removal. Overall, maintained awareness of DN is imperative, as early diagnosis can avoid unnecessary biopsies and more complex treatment measures as well as facilitate coordination of care when additional medical or psychiatric concerns are present.
Although the diagnoses of DN and terra firma-forme dermatosis can be distinguished based on the patient’s clinical history and response to simple cleansing measures alone, the alternate diagnoses can be excluded based on different clinical distributions and response to other treatment modalities but sometimes may require clinicopathologic correlation for definitive diagnosis. Our patient had a biopsy diagnosis of psoriasiform dermatitis from an outside provider, but neither her clinical disease nor repeated histopathologic findings supported a diagnosis of psoriasis or other classic psoriasiform dermatoses such as contact dermatitis, dermatophyte/ candidal infection, seborrheic dermatitis, pityriasis rubra pilaris, pityriasis rosea, scabies, or syphilis.
It is imperative to exclude alternative diagnoses because they can have systemic implications and can misguide treatment, as was done initially with our patient. Psoriasis vulgaris in its classic form is a chronic inflammatory skin disease that manifests as sharply demarcated, erythematous plaques with overlying thick silvery scale; it has the additional histologic findings of neutrophilic spongiform pustules in the epidermis, tortuous blood vessels in the papillary dermis, and neutrophils and parakeratosis in the stratum corneum. In its benign form, AN is associated with endocrinopathies, most commonly obesity and insulin-resistant diabetes mellitus, and presents as hyperkeratotic, velvety, hyperpigmented plaques typically limited to the neck and axillae. Malignant AN spontaneously arises in association with systemic malignancy and can be extensive and generalized.5 Treatment of AN primarily focuses on resolution of the underlying systemic disease; however, cosmetic treatment with topical or oral retinoids may hasten resolution of cutaneous disease.6 Confluent and reticulated papillomatosis is characterized by reticulated hyperkeratotic plaques with a common distribution over the central and upper trunk. Unlike DN and AN, which may occur at any age, CARP typically is seen in adolescents and young adults.7 There is no evidence-based gold standard for the management of CARP; however, the successful use of various antibiotics, antifungals, and retinoids—alone or in combination—has been reported.8 Overall, compared to the other entities in the differential diagnosis, DN easily can be prevented with consistent use of soap and water and may be underreported given the asymptomatic nature of the disease and the typical patient population.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
- Poskitt L, Wayte J, Wojnarowska F, et al. ‘Dermatitis neglecta’: unwashed dermatosis. Br J Dermatol. 1995;132:827-829.
- Perez-Rodriguez IM, Munoz-Garza FZ, Ocampo-Candiani J. An unusually severe case of dermatosis neglecta: a diagnostic challenge. Case Rep Dermatol. 2014;6:194-199.
- Park JM, Roh MR, Kwon JE, et al. A case of generalized dermatitis neglecta mimicking psoriasis vulgaris. Arch Dermatol. 2010;146:1050-1051.
- Lopes S, Vide J, Antunes I, et al. Dermatitis neglecta: a challenging diagnosis in psychodermatology. Acta Dermatovenerol Alp Pannonica Adriat. 2018;27:109-110.
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189. e1-21; quiz 210.
- Patel NU, Roach C, Alinia H, et al. Current treatment options for acanthosis nigricans. Clin Cosmet Investig Dermatol. 2018; 11:407-413.
- Kurtyka DJ, Burke KT, DeKlotz CMC. Use of topical sirolimus (rapamycin) for treating confluent and reticulated papillomatosis. JAMA Dermatol. 2021;157:121-123.
- Mufti A, Sachdeva M, Maliyar K, et al. Treatment outcomes in confluent and reticulated papillomatosis: a systematic review. J Am Acad Dermatol. 2021;84:825-829.
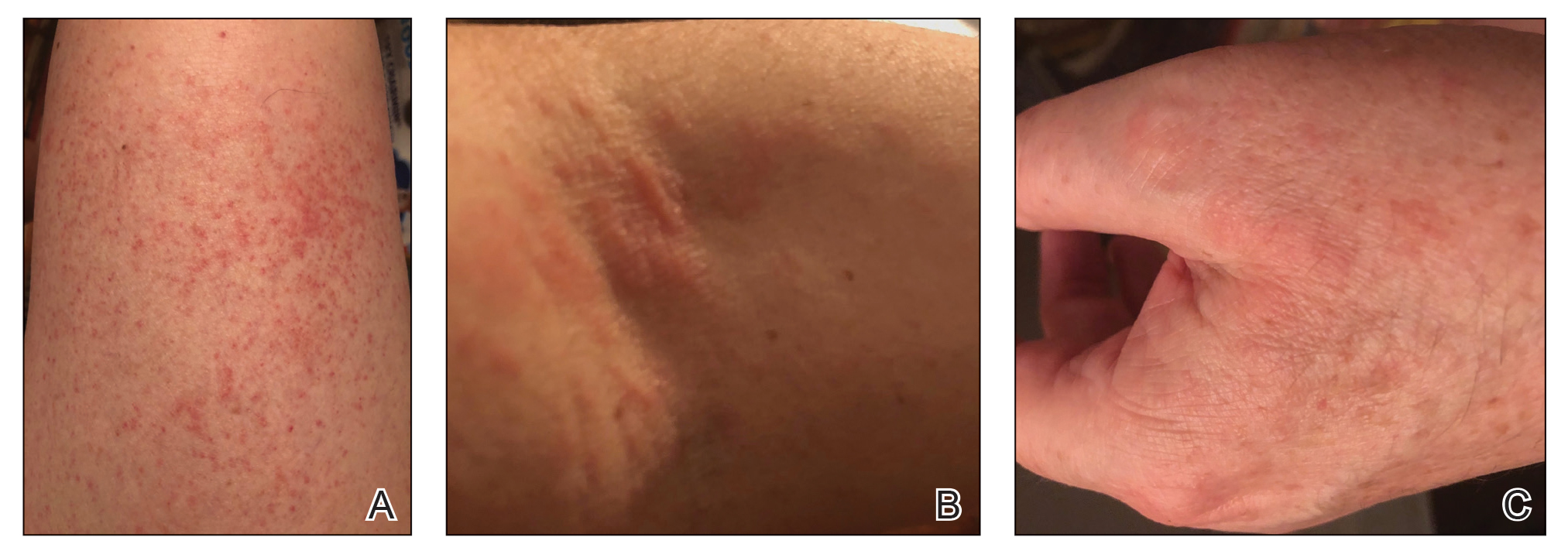
A 28-year-old woman was admitted to the medicine service with bilateral pedal numbness and ataxia, as well as an asymptomatic rash on the neck, chest, abdomen, and extremities of a few months’ duration. The patient was seen by an outside dermatologist for the same rash 1 month prior, at which time a punch biopsy of the right forearm was suggestive of psoriasiform dermatitis; however, the rash failed to improve with topical ammonium lactate and corticosteroids. During the current admission, the patient was found to have low methylmalonic acid and vitamin B1 levels; however, vitamin B12, thyroid studies, rapid plasma reagin test, and inflammatory markers, as well as central and peripheral imaging and nerve conduction studies were normal.
Dermatology was consulted. Physical examination revealed retention hyperkeratosis on the neck that was wipeable with 70% isopropyl alcohol, as well as nonwipeable, thin, reticulated plaques on the mid chest and thick velvety plaques on the abdomen and bilateral extremities. There was notable sparing of areas with natural occlusion such as the back and body folds. A punch biopsy of the abdomen was performed.
Acne Vulgaris
THE COMPARISON
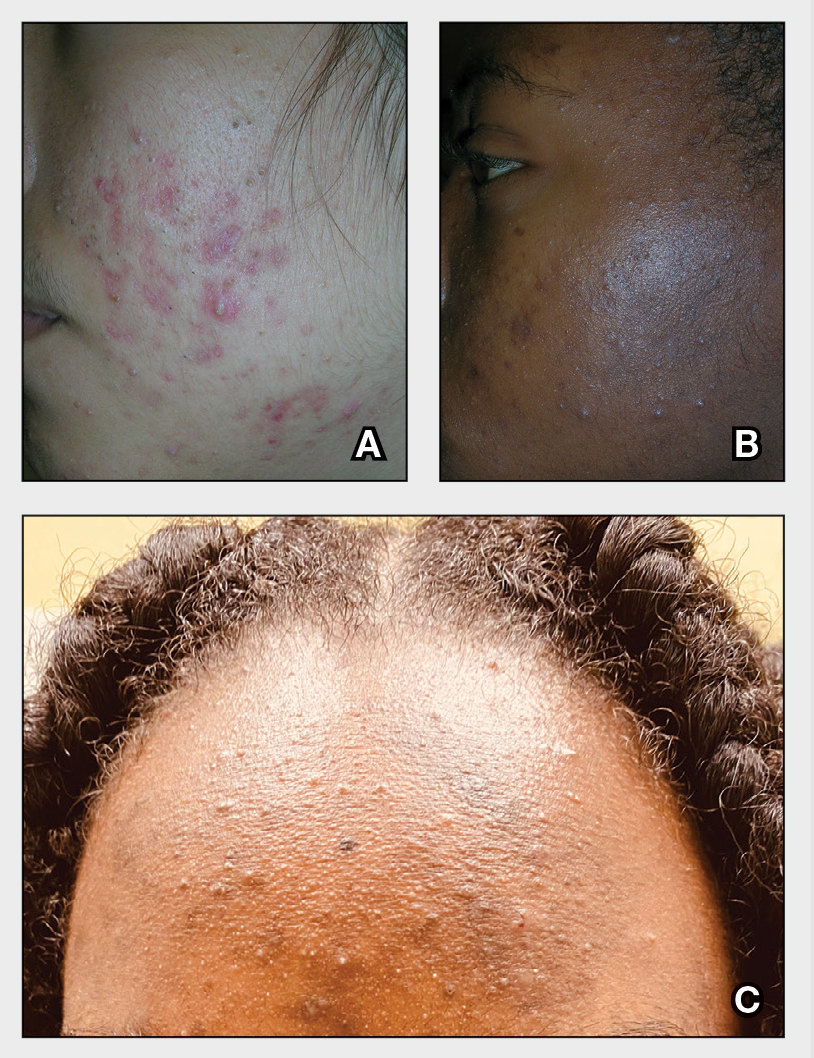
A A 27-year-old Hispanic woman with comedonal and inflammatory acne. Erythema is prominent around the inflammatory lesions. Note the pustule on the cheek surrounded by pink color.
B A teenaged Black boy with acne papules and pustules on the face. There are comedones, hyperpigmented macules, and pustules on the cheek.
C A teenaged Black girl with pomade acne. The patient used various hair care products, which obstructed the pilosebaceous units on the forehead.
Epidemiology
Acne is a leading dermatologic condition in individuals with skin of color in the United States.1
Key clinical features in people with darker skin tones include:
- erythematous or hyperpigmented papules or comedones
- hyperpigmented macules and postinflammatory hyperpigmentation (PIH)
- increased risk for keloidal scars.2
Worth noting
- Patients with darker skin tones may be more concerned with the dark marks (also referred to as scars or manchas in Spanish) than the acne itself. This PIH may be viewed by patients as the major problem.
- Acne medications such as azelaic acid and some retinoids (when applied appropriately) can treat both acne and PIH.3
- Irritation from topical acne medications, including retinoid dermatitis, may lead to more PIH. Using noncomedogenic moisturizers and applying medication appropriately (ie, a pea-sized amount of topical retinoid per application) may help limit irritation.4,5
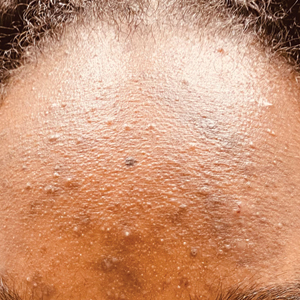
- One type of acne seen more commonly, although not exclusively, in Black patients is pomade acne, which principally appears on the forehead and is associated with use of hair care and styling products (Figure, C).
Health disparity highlight
Disparities in access to health care exist for those with dermatologic concerns. According to one study, African American (28.5%) and Hispanic patients (23.9%) were less likely to be seen by a dermatologist solely for the diagnosis of a dermatologic condition compared to Asian and Pacific Islander patients (36.7%) or White patients (43.2%).1
Noting that isotretinoin is the most potent systemic therapy for severe cystic acne vulgaris, Bell et al6 reported that Black patients had lower odds of receiving isotretinoin compared to White patients. Hispanic patients had lower odds of receiving a topical retinoid, tretinoin, than non-Hispanic patients.6
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20:716-725.
- Woolery-Lloyd HC, Keri J, Doig S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J Drugs Dermatol. 2013;12:434-437.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14525
- Alexis AD, Harper JC, Stein Gold L, et al. Treating acne in patients with skin of color. Semin Cutan Med Surg. 2018;37(suppl 3):S71-S73.
- Bell MA, Whang KA, Thomas J, et al. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112:650-653.
THE COMPARISON
A A 27-year-old Hispanic woman with comedonal and inflammatory acne. Erythema is prominent around the inflammatory lesions. Note the pustule on the cheek surrounded by pink color.
B A teenaged Black boy with acne papules and pustules on the face. There are comedones, hyperpigmented macules, and pustules on the cheek.
C A teenaged Black girl with pomade acne. The patient used various hair care products, which obstructed the pilosebaceous units on the forehead.
Epidemiology
Acne is a leading dermatologic condition in individuals with skin of color in the United States.1
Key clinical features in people with darker skin tones include:
- erythematous or hyperpigmented papules or comedones
- hyperpigmented macules and postinflammatory hyperpigmentation (PIH)
- increased risk for keloidal scars.2
Worth noting
- Patients with darker skin tones may be more concerned with the dark marks (also referred to as scars or manchas in Spanish) than the acne itself. This PIH may be viewed by patients as the major problem.
- Acne medications such as azelaic acid and some retinoids (when applied appropriately) can treat both acne and PIH.3
- Irritation from topical acne medications, including retinoid dermatitis, may lead to more PIH. Using noncomedogenic moisturizers and applying medication appropriately (ie, a pea-sized amount of topical retinoid per application) may help limit irritation.4,5
- One type of acne seen more commonly, although not exclusively, in Black patients is pomade acne, which principally appears on the forehead and is associated with use of hair care and styling products (Figure, C).
Health disparity highlight
Disparities in access to health care exist for those with dermatologic concerns. According to one study, African American (28.5%) and Hispanic patients (23.9%) were less likely to be seen by a dermatologist solely for the diagnosis of a dermatologic condition compared to Asian and Pacific Islander patients (36.7%) or White patients (43.2%).1
Noting that isotretinoin is the most potent systemic therapy for severe cystic acne vulgaris, Bell et al6 reported that Black patients had lower odds of receiving isotretinoin compared to White patients. Hispanic patients had lower odds of receiving a topical retinoid, tretinoin, than non-Hispanic patients.6
THE COMPARISON
A A 27-year-old Hispanic woman with comedonal and inflammatory acne. Erythema is prominent around the inflammatory lesions. Note the pustule on the cheek surrounded by pink color.
B A teenaged Black boy with acne papules and pustules on the face. There are comedones, hyperpigmented macules, and pustules on the cheek.
C A teenaged Black girl with pomade acne. The patient used various hair care products, which obstructed the pilosebaceous units on the forehead.
Epidemiology
Acne is a leading dermatologic condition in individuals with skin of color in the United States.1
Key clinical features in people with darker skin tones include:
- erythematous or hyperpigmented papules or comedones
- hyperpigmented macules and postinflammatory hyperpigmentation (PIH)
- increased risk for keloidal scars.2
Worth noting
- Patients with darker skin tones may be more concerned with the dark marks (also referred to as scars or manchas in Spanish) than the acne itself. This PIH may be viewed by patients as the major problem.
- Acne medications such as azelaic acid and some retinoids (when applied appropriately) can treat both acne and PIH.3
- Irritation from topical acne medications, including retinoid dermatitis, may lead to more PIH. Using noncomedogenic moisturizers and applying medication appropriately (ie, a pea-sized amount of topical retinoid per application) may help limit irritation.4,5
- One type of acne seen more commonly, although not exclusively, in Black patients is pomade acne, which principally appears on the forehead and is associated with use of hair care and styling products (Figure, C).
Health disparity highlight
Disparities in access to health care exist for those with dermatologic concerns. According to one study, African American (28.5%) and Hispanic patients (23.9%) were less likely to be seen by a dermatologist solely for the diagnosis of a dermatologic condition compared to Asian and Pacific Islander patients (36.7%) or White patients (43.2%).1
Noting that isotretinoin is the most potent systemic therapy for severe cystic acne vulgaris, Bell et al6 reported that Black patients had lower odds of receiving isotretinoin compared to White patients. Hispanic patients had lower odds of receiving a topical retinoid, tretinoin, than non-Hispanic patients.6
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20:716-725.
- Woolery-Lloyd HC, Keri J, Doig S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J Drugs Dermatol. 2013;12:434-437.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14525
- Alexis AD, Harper JC, Stein Gold L, et al. Treating acne in patients with skin of color. Semin Cutan Med Surg. 2018;37(suppl 3):S71-S73.
- Bell MA, Whang KA, Thomas J, et al. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112:650-653.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Woolery-Lloyd H, Williams K, et al. Racial/ethnic variations in acne: implications for treatment and skin care recommendations for acne patients with skin of color. J Drugs Dermatol. 2021;20:716-725.
- Woolery-Lloyd HC, Keri J, Doig S. Retinoids and azelaic acid to treat acne and hyperpigmentation in skin of color. J Drugs Dermatol. 2013;12:434-437.
- Grayson C, Heath C. Tips for addressing common conditions affecting pediatric and adolescent patients with skin of color [published online March 2, 2021]. Pediatr Dermatol. doi:10.1111/pde.14525
- Alexis AD, Harper JC, Stein Gold L, et al. Treating acne in patients with skin of color. Semin Cutan Med Surg. 2018;37(suppl 3):S71-S73.
- Bell MA, Whang KA, Thomas J, et al. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112:650-653.
Erythematous and Ulcerated Plaque on the Left Temple
The Diagnosis: Primary Cutaneous Carcinosarcoma
The immunohistochemical findings supported an epithelial component consistent with moderately differentiated squamous cell carcinoma (SCC) and a mesenchymal component with features consistent with a sarcoma. Consequently, the lesion was diagnosed as a primary cutaneous carcinosarcoma (PCCS).
Primary cutaneous carcinosarcoma is a rare biphasic neoplasm consisting of malignant epithelial (carcinoma) and mesenchymal (sarcoma) components.1 Primary cutaneous carcinosarcomas are uncommon, poorly understood, primary cutaneous tumors.2,3 Characteristic of this tumor, cytokeratins highlight the epithelial component while vimentin highlights the mesenchymal component.4 Histologically, the sarcomatous components of PCCS often are highly variable, with an absence of transitional areas within the epithelial component, which frequently resembles basal cell carcinoma and/ or SCC.5-7 Primary cutaneous carcinosarcoma favors areas of chronic UV radiation exposure, particularly on the head and neck. Most tumors present with a slowly growing, polypoid, flesh-colored to erythematous nodule due to the infiltrative mesenchymal component.7 Primary cutaneous carcinosarcoma primarily is diagnosed in elderly patients, with the majority of cases diagnosed in the eighth or ninth decades of life (range, 32–98 years).1,8 Men appear to be twice as likely to be diagnosed with a PCCS compared to women.1 Primary cutaneous carcinosarcomas are recognized as aggressive tumors with a high propensity to metastasize and recur locally, necessitating early diagnosis and treatment.4 Accurate diagnosis of PCCSs can be challenging due to the biphasic nature of the neoplasm as well as poor differentiation or unequal proportions of the epithelial and mesenchymal components.5 Additionally, overlapping diagnostic criteria coupled with vague demarcation between soft-tissue sarcomas and distinct carcinomas also may contribute to a delay in diagnosis.9 Treatment is achieved surgically by complete wide resection, with no evidence to support the use of adjuvant or neoadjuvant external beam radiation therapy. Due to the small number of reported cases, no treatment recommendations currently exist.1
Surgical management with wide local excision has been disappointing, with recurrence rates reported as high as 33%.6 Primary cutaneous carcinosarcoma has an estimated overall recurrence rate of 19% and a 5-year disease-free rate of 50%.10 Risk factors associated with poorer prognosis include tumors with adnexal subtype, age less than 65 years, rapid tumor growth, a tumor greater than 20 mm at presentation, and a long-standing tumor lasting up to 30 years.2,4 Although wide local excision and Mohs micrographic surgery (MMS) both have been utilized successfully, MMS has been shown to result in a cure rate of greater than 98%.6
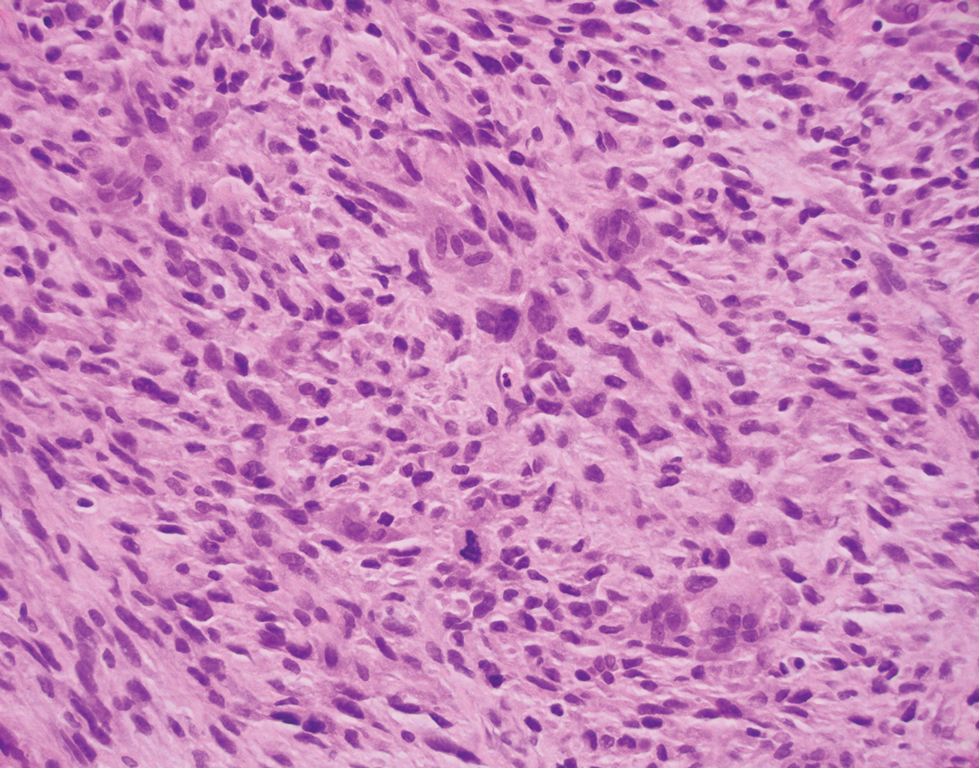
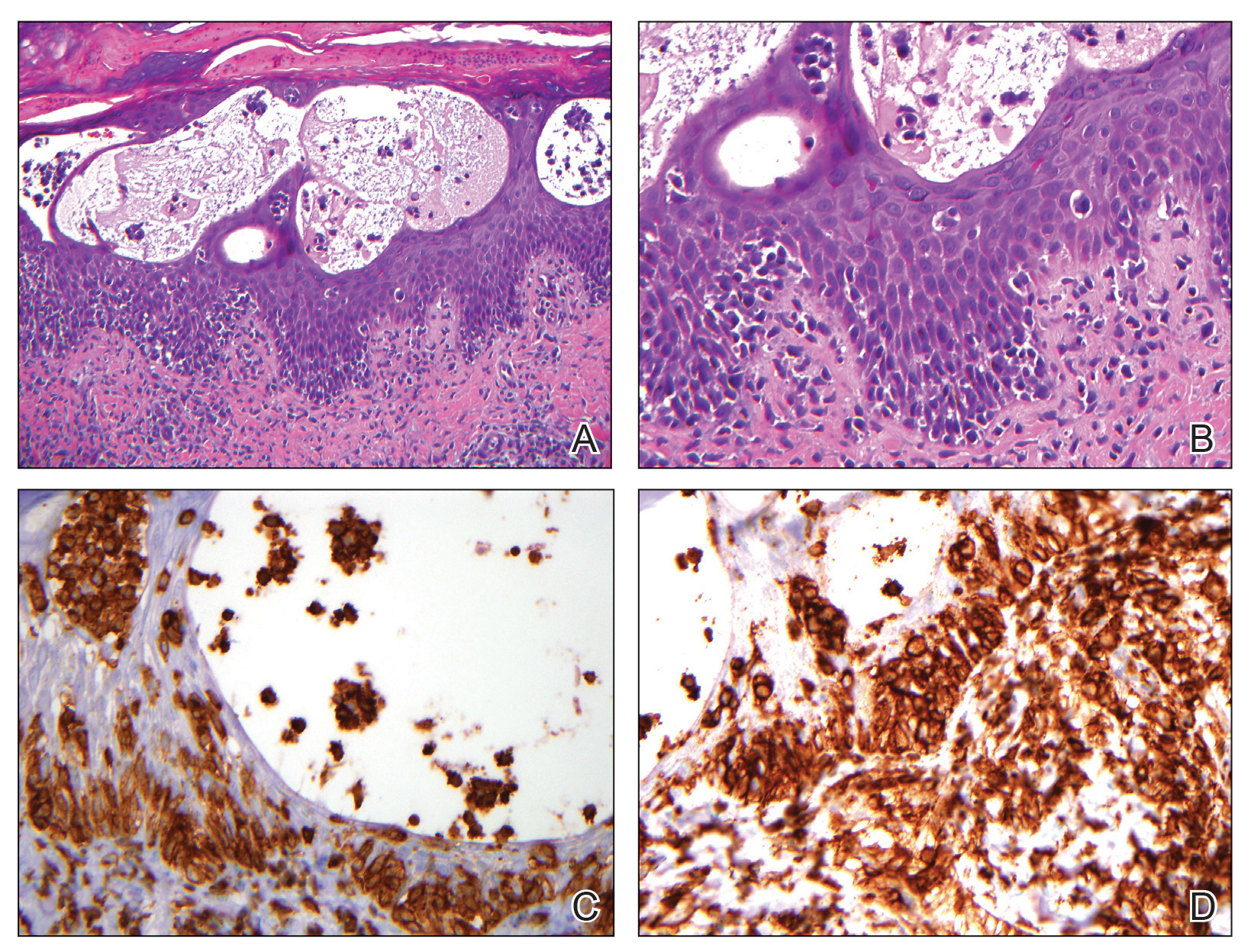
Atypical fibroxanthoma (AFX) is a cutaneous tumor of fibrohistiocytic mesenchymal origin that typically manifests on sun-damaged skin in elderly individuals. Clinically, it presents as a rapidly growing neoplasm that often ulcerates and bleeds. These heterogenous neoplasms have several distinct characteristics, including dense cellularity with disorganized, large, pleomorphic, and atypical-appearing spindle-shaped cells arising in the upper layers of the dermis, often disseminating into the reticular dermis and occasionally into the subcutaneous fat (Figure 1). The neoplastic cells often exhibit hyperchromic and irregular nuclei, multinucleated giant cells, and atypical mitotic figures. In most cases, negative immunohistochemical staining with SOX-10, S-100, cytokeratins, desmin, and caldesmon will allow pathologists to differentiate between AFX and other common tumors on the differential diagnosis, such as SCC, melanoma, and leiomyosarcoma. CD10 and procollagen type 1 are positive antigenic markers in AFX, but they are not specific. The standard treatment of AFX includes wide local excision or MMS for superior margin control.11
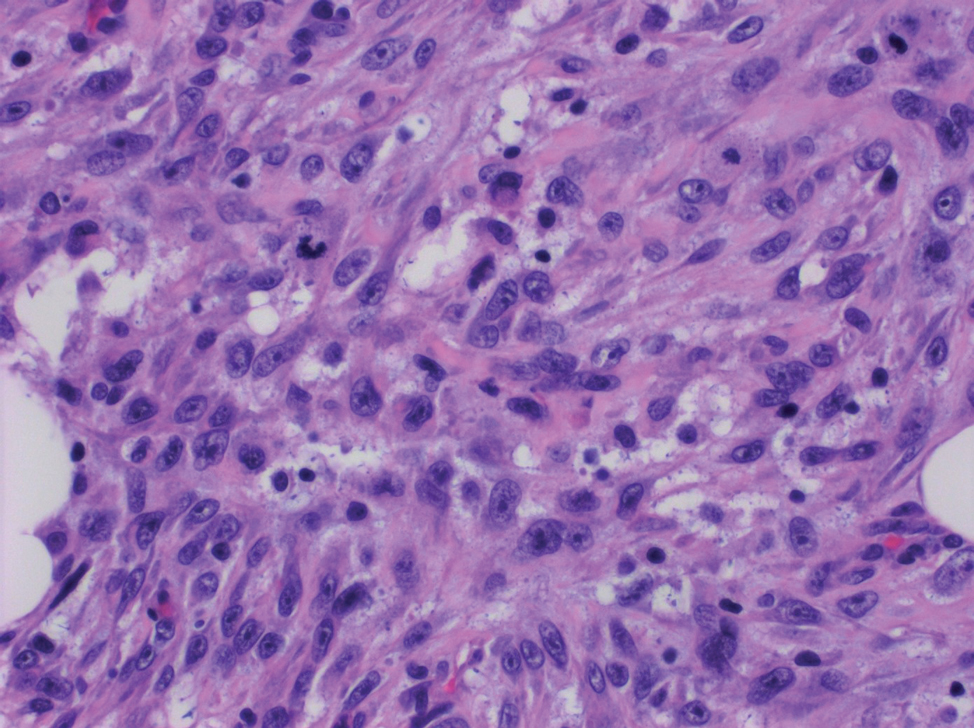
Spindle cell SCC presents as a raised or exophytic nodule, often with spontaneous bleeding and central ulceration. It usually presents on sun-damaged skin or in individuals with a history of ionizing radiation. Histologically, it is characterized by atypical spindleshaped keratinocytes in the dermis existing as single cells or cohesive nests along with keratin pearls (Figure 2). The atypical spindle cells may comprise the entire tumor or only a small portion. The use of immunohistochemical markers often is required to establish a definitive diagnosis. Spindle cell SCC stains positively, albeit frequently focally, for p63, p40, and high-molecular-weight cytokeratins such as cytokeratin 5/6, while S-100 protein, SOX-10, MART-1/Melan-A, and muscle-specific actin stains typically are negative. Wide local excision or MMS is recommended for treatment of these lesions.12
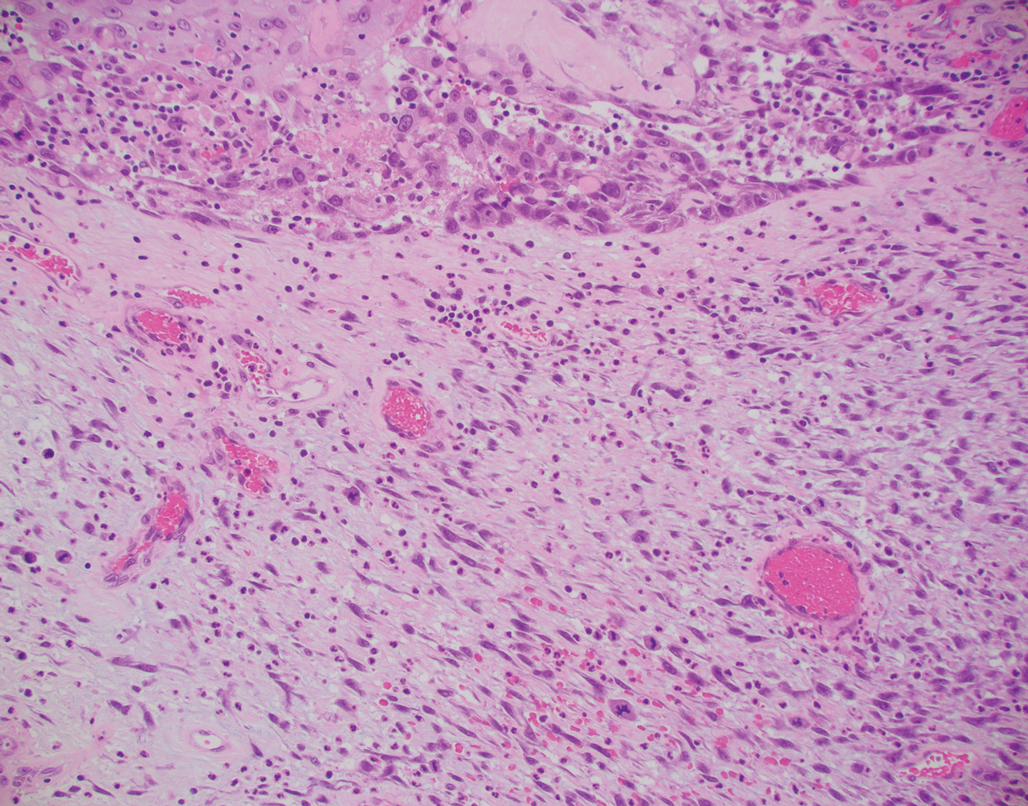
Primary cutaneous myoepithelial carcinomas are uncommon neoplasms of myoepithelial differentiation. Clinically, they often arise as soft nodular lesions on the head, neck, and lower extremities with a bimodal age distribution (50 years). Histologically cutaneous myoepithelial tumors are well-differentiated, dermal-based nodules without connection to the overlying epidermis (Figure 3). The myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show variability in cell growth patterns. One of the most common growth patterns is oval to round cells forming cords and chains in a chondromyxoid stroma. Most cases display an immunophenotyped co-expression of an epithelial cytokeratin and S-100 protein. Myoepithelial markers also may be present, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin. Surgical removal with wide local excision or MMS is essential.13
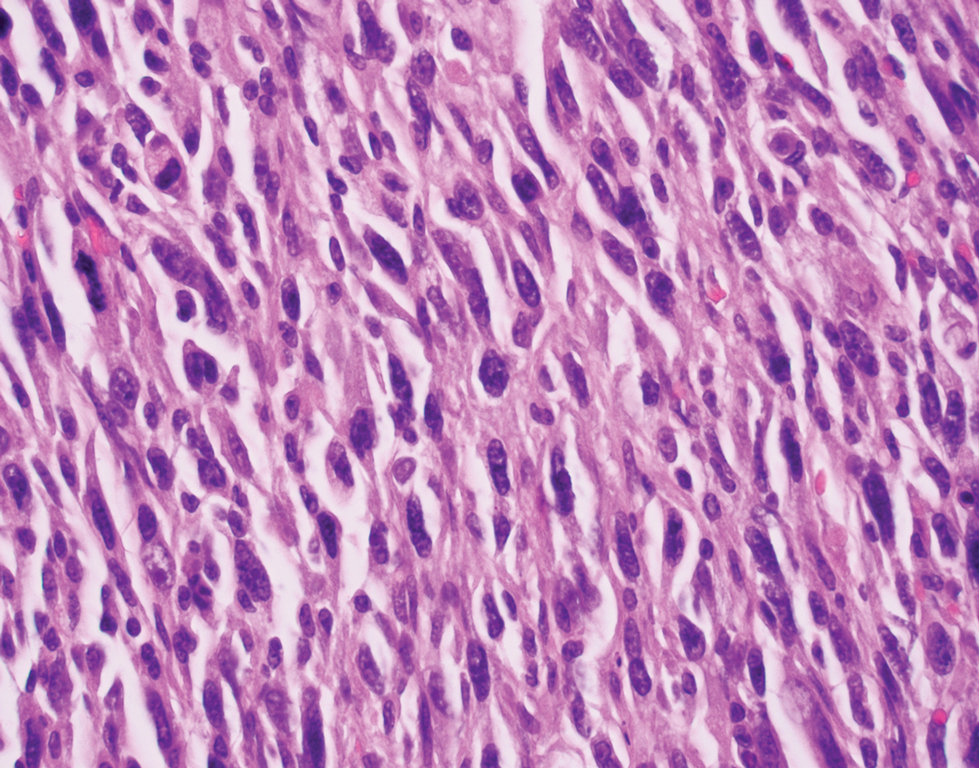
Leiomyosarcoma (LMS) is a tumor that originates from smooth muscle and rarely develops in the dermis.14 Pleomorphic LMS is a morphologic variant of LMS that has a low propensity to metastasize but commonly exhibits local recurrence.15 Leiomyosarcoma can present in any age group but most commonly manifests in individuals aged 50 to 70 years. Clinically, LMS presents as a firm solitary nodule with a smooth pink surface or a more exophytic tumor with a reddish or brown color on the extensor surface of the lower limbs; it is less common on the scalp and face.14 Histologically, most cases of pleomorphic LMS show small foci of fascicles consisting of smooth muscle tumor cells in addition to cellular pleomorphism (Figure 4).15 Many of these cells demonstrate a clear perinuclear vacuole that generally is appreciated in neoplastic smooth muscle cells.14 Pleomorphic LMS typically stains positively for at least one smooth muscle marker including desmin, h-caldesmon, muscle-specific actin, α-smooth muscle actin, or smooth muscle myosin in the leiomyosarcomatous fascicular areas.16 Complete surgical excision is the treatment of choice, and the best results are obtained with MMS.14
- Syme-Grant J, Syme-Grant NJ, Motta L, et al. Are primary cutaneous carcinosarcomas underdiagnosed? five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006;59:1402-1408.
- Bourgeault E, Alain J, Gagne E. Primary cutaneous carcinosarcoma of the basal cell subtype should be treated as a high-risk basal cell carcinoma. J Cutan Med Surg. 2015;19:407-411.
- West L, Srivastava D. Cutaneous carcinosarcoma of the medial canthus discovered on Mohs debulk analysis. Dermatol Surg. 2019;45:1700-1702.
- Kwan JM, Satter EK. Carcinosarcoma: a primary cutaneous tumor with biphasic differentiation. Cutis. 2013;92:247-249.
- Suh KY, Lacouture M, Gerami P. p63 in primary cutaneous carcinosarcoma. Am J Dermatopathol. 2007;29:374‐377.
- Ruiz-Villaverde R, Aneiros-Fernandez J. Primary cutaneous carcinosarcoma: a cutaneous neoplasm with an exceptional presentation. Sultan Qaboos Univ Med J. 2018;18:E114-E115.
- Smart CN, Pucci RA, Binder SW, et al. Cutaneous carcinosarcoma with myoepithelial differentiation: immunohistochemical and cytogenetic analysis of a case presenting in an unusual location. Am J Dermatopathol. 2009;31:715‐717.
- Clark JJ, Bowen AR, Bowen GM, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017;44:34‐44.
- Müller CS, Pföhler C, Schiekofer C, et al. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol. 2014;36:328‐339.
- Bellew S, Del Rosso JQ, Mobini N. Primary carcinosarcoma of the ear: case report and review of the literature. J Clin Aesthet Dermatol. 2009;2:33‐35.
- Hong SH, Hong SJ, Lee Y, et al. Primary cutaneous carcinosarcoma of the shoulder: case report with literature review. Dermatol Surg. 2013;39:338-340.
- Soleymani T, Aasi SZ, Novoa R, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: updates on classification and management. Dermatol Clin. 2019;37:253-259.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525.
- Johnson GE, Stevens K, Morrison AO, et al. Cutaneous myoepithelial carcinoma with disseminated metastases. Cutis. 2017;99:E19-E26.
- Llombart B, Serra-Guillén C, Requena C, et al. Leiomyosarcoma and pleomorphic dermal sarcoma: guidelines for diagnosis and treatment. Actas Dermosifiliogr. 2019;110:4-11.
- Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030-1038.
The Diagnosis: Primary Cutaneous Carcinosarcoma
The immunohistochemical findings supported an epithelial component consistent with moderately differentiated squamous cell carcinoma (SCC) and a mesenchymal component with features consistent with a sarcoma. Consequently, the lesion was diagnosed as a primary cutaneous carcinosarcoma (PCCS).
Primary cutaneous carcinosarcoma is a rare biphasic neoplasm consisting of malignant epithelial (carcinoma) and mesenchymal (sarcoma) components.1 Primary cutaneous carcinosarcomas are uncommon, poorly understood, primary cutaneous tumors.2,3 Characteristic of this tumor, cytokeratins highlight the epithelial component while vimentin highlights the mesenchymal component.4 Histologically, the sarcomatous components of PCCS often are highly variable, with an absence of transitional areas within the epithelial component, which frequently resembles basal cell carcinoma and/ or SCC.5-7 Primary cutaneous carcinosarcoma favors areas of chronic UV radiation exposure, particularly on the head and neck. Most tumors present with a slowly growing, polypoid, flesh-colored to erythematous nodule due to the infiltrative mesenchymal component.7 Primary cutaneous carcinosarcoma primarily is diagnosed in elderly patients, with the majority of cases diagnosed in the eighth or ninth decades of life (range, 32–98 years).1,8 Men appear to be twice as likely to be diagnosed with a PCCS compared to women.1 Primary cutaneous carcinosarcomas are recognized as aggressive tumors with a high propensity to metastasize and recur locally, necessitating early diagnosis and treatment.4 Accurate diagnosis of PCCSs can be challenging due to the biphasic nature of the neoplasm as well as poor differentiation or unequal proportions of the epithelial and mesenchymal components.5 Additionally, overlapping diagnostic criteria coupled with vague demarcation between soft-tissue sarcomas and distinct carcinomas also may contribute to a delay in diagnosis.9 Treatment is achieved surgically by complete wide resection, with no evidence to support the use of adjuvant or neoadjuvant external beam radiation therapy. Due to the small number of reported cases, no treatment recommendations currently exist.1
Surgical management with wide local excision has been disappointing, with recurrence rates reported as high as 33%.6 Primary cutaneous carcinosarcoma has an estimated overall recurrence rate of 19% and a 5-year disease-free rate of 50%.10 Risk factors associated with poorer prognosis include tumors with adnexal subtype, age less than 65 years, rapid tumor growth, a tumor greater than 20 mm at presentation, and a long-standing tumor lasting up to 30 years.2,4 Although wide local excision and Mohs micrographic surgery (MMS) both have been utilized successfully, MMS has been shown to result in a cure rate of greater than 98%.6
Atypical fibroxanthoma (AFX) is a cutaneous tumor of fibrohistiocytic mesenchymal origin that typically manifests on sun-damaged skin in elderly individuals. Clinically, it presents as a rapidly growing neoplasm that often ulcerates and bleeds. These heterogenous neoplasms have several distinct characteristics, including dense cellularity with disorganized, large, pleomorphic, and atypical-appearing spindle-shaped cells arising in the upper layers of the dermis, often disseminating into the reticular dermis and occasionally into the subcutaneous fat (Figure 1). The neoplastic cells often exhibit hyperchromic and irregular nuclei, multinucleated giant cells, and atypical mitotic figures. In most cases, negative immunohistochemical staining with SOX-10, S-100, cytokeratins, desmin, and caldesmon will allow pathologists to differentiate between AFX and other common tumors on the differential diagnosis, such as SCC, melanoma, and leiomyosarcoma. CD10 and procollagen type 1 are positive antigenic markers in AFX, but they are not specific. The standard treatment of AFX includes wide local excision or MMS for superior margin control.11
Spindle cell SCC presents as a raised or exophytic nodule, often with spontaneous bleeding and central ulceration. It usually presents on sun-damaged skin or in individuals with a history of ionizing radiation. Histologically, it is characterized by atypical spindleshaped keratinocytes in the dermis existing as single cells or cohesive nests along with keratin pearls (Figure 2). The atypical spindle cells may comprise the entire tumor or only a small portion. The use of immunohistochemical markers often is required to establish a definitive diagnosis. Spindle cell SCC stains positively, albeit frequently focally, for p63, p40, and high-molecular-weight cytokeratins such as cytokeratin 5/6, while S-100 protein, SOX-10, MART-1/Melan-A, and muscle-specific actin stains typically are negative. Wide local excision or MMS is recommended for treatment of these lesions.12

Primary cutaneous myoepithelial carcinomas are uncommon neoplasms of myoepithelial differentiation. Clinically, they often arise as soft nodular lesions on the head, neck, and lower extremities with a bimodal age distribution (50 years). Histologically cutaneous myoepithelial tumors are well-differentiated, dermal-based nodules without connection to the overlying epidermis (Figure 3). The myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show variability in cell growth patterns. One of the most common growth patterns is oval to round cells forming cords and chains in a chondromyxoid stroma. Most cases display an immunophenotyped co-expression of an epithelial cytokeratin and S-100 protein. Myoepithelial markers also may be present, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin. Surgical removal with wide local excision or MMS is essential.13
Leiomyosarcoma (LMS) is a tumor that originates from smooth muscle and rarely develops in the dermis.14 Pleomorphic LMS is a morphologic variant of LMS that has a low propensity to metastasize but commonly exhibits local recurrence.15 Leiomyosarcoma can present in any age group but most commonly manifests in individuals aged 50 to 70 years. Clinically, LMS presents as a firm solitary nodule with a smooth pink surface or a more exophytic tumor with a reddish or brown color on the extensor surface of the lower limbs; it is less common on the scalp and face.14 Histologically, most cases of pleomorphic LMS show small foci of fascicles consisting of smooth muscle tumor cells in addition to cellular pleomorphism (Figure 4).15 Many of these cells demonstrate a clear perinuclear vacuole that generally is appreciated in neoplastic smooth muscle cells.14 Pleomorphic LMS typically stains positively for at least one smooth muscle marker including desmin, h-caldesmon, muscle-specific actin, α-smooth muscle actin, or smooth muscle myosin in the leiomyosarcomatous fascicular areas.16 Complete surgical excision is the treatment of choice, and the best results are obtained with MMS.14
The Diagnosis: Primary Cutaneous Carcinosarcoma
The immunohistochemical findings supported an epithelial component consistent with moderately differentiated squamous cell carcinoma (SCC) and a mesenchymal component with features consistent with a sarcoma. Consequently, the lesion was diagnosed as a primary cutaneous carcinosarcoma (PCCS).
Primary cutaneous carcinosarcoma is a rare biphasic neoplasm consisting of malignant epithelial (carcinoma) and mesenchymal (sarcoma) components.1 Primary cutaneous carcinosarcomas are uncommon, poorly understood, primary cutaneous tumors.2,3 Characteristic of this tumor, cytokeratins highlight the epithelial component while vimentin highlights the mesenchymal component.4 Histologically, the sarcomatous components of PCCS often are highly variable, with an absence of transitional areas within the epithelial component, which frequently resembles basal cell carcinoma and/ or SCC.5-7 Primary cutaneous carcinosarcoma favors areas of chronic UV radiation exposure, particularly on the head and neck. Most tumors present with a slowly growing, polypoid, flesh-colored to erythematous nodule due to the infiltrative mesenchymal component.7 Primary cutaneous carcinosarcoma primarily is diagnosed in elderly patients, with the majority of cases diagnosed in the eighth or ninth decades of life (range, 32–98 years).1,8 Men appear to be twice as likely to be diagnosed with a PCCS compared to women.1 Primary cutaneous carcinosarcomas are recognized as aggressive tumors with a high propensity to metastasize and recur locally, necessitating early diagnosis and treatment.4 Accurate diagnosis of PCCSs can be challenging due to the biphasic nature of the neoplasm as well as poor differentiation or unequal proportions of the epithelial and mesenchymal components.5 Additionally, overlapping diagnostic criteria coupled with vague demarcation between soft-tissue sarcomas and distinct carcinomas also may contribute to a delay in diagnosis.9 Treatment is achieved surgically by complete wide resection, with no evidence to support the use of adjuvant or neoadjuvant external beam radiation therapy. Due to the small number of reported cases, no treatment recommendations currently exist.1
Surgical management with wide local excision has been disappointing, with recurrence rates reported as high as 33%.6 Primary cutaneous carcinosarcoma has an estimated overall recurrence rate of 19% and a 5-year disease-free rate of 50%.10 Risk factors associated with poorer prognosis include tumors with adnexal subtype, age less than 65 years, rapid tumor growth, a tumor greater than 20 mm at presentation, and a long-standing tumor lasting up to 30 years.2,4 Although wide local excision and Mohs micrographic surgery (MMS) both have been utilized successfully, MMS has been shown to result in a cure rate of greater than 98%.6
Atypical fibroxanthoma (AFX) is a cutaneous tumor of fibrohistiocytic mesenchymal origin that typically manifests on sun-damaged skin in elderly individuals. Clinically, it presents as a rapidly growing neoplasm that often ulcerates and bleeds. These heterogenous neoplasms have several distinct characteristics, including dense cellularity with disorganized, large, pleomorphic, and atypical-appearing spindle-shaped cells arising in the upper layers of the dermis, often disseminating into the reticular dermis and occasionally into the subcutaneous fat (Figure 1). The neoplastic cells often exhibit hyperchromic and irregular nuclei, multinucleated giant cells, and atypical mitotic figures. In most cases, negative immunohistochemical staining with SOX-10, S-100, cytokeratins, desmin, and caldesmon will allow pathologists to differentiate between AFX and other common tumors on the differential diagnosis, such as SCC, melanoma, and leiomyosarcoma. CD10 and procollagen type 1 are positive antigenic markers in AFX, but they are not specific. The standard treatment of AFX includes wide local excision or MMS for superior margin control.11
Spindle cell SCC presents as a raised or exophytic nodule, often with spontaneous bleeding and central ulceration. It usually presents on sun-damaged skin or in individuals with a history of ionizing radiation. Histologically, it is characterized by atypical spindleshaped keratinocytes in the dermis existing as single cells or cohesive nests along with keratin pearls (Figure 2). The atypical spindle cells may comprise the entire tumor or only a small portion. The use of immunohistochemical markers often is required to establish a definitive diagnosis. Spindle cell SCC stains positively, albeit frequently focally, for p63, p40, and high-molecular-weight cytokeratins such as cytokeratin 5/6, while S-100 protein, SOX-10, MART-1/Melan-A, and muscle-specific actin stains typically are negative. Wide local excision or MMS is recommended for treatment of these lesions.12

Primary cutaneous myoepithelial carcinomas are uncommon neoplasms of myoepithelial differentiation. Clinically, they often arise as soft nodular lesions on the head, neck, and lower extremities with a bimodal age distribution (50 years). Histologically cutaneous myoepithelial tumors are well-differentiated, dermal-based nodules without connection to the overlying epidermis (Figure 3). The myoepithelial cells can exhibit spindled, epithelioid, plasmacytoid, or clear cell morphologic features and show variability in cell growth patterns. One of the most common growth patterns is oval to round cells forming cords and chains in a chondromyxoid stroma. Most cases display an immunophenotyped co-expression of an epithelial cytokeratin and S-100 protein. Myoepithelial markers also may be present, including keratins, smooth muscle actin, calponin, glial fibrillary acidic protein, p63, and desmin. Surgical removal with wide local excision or MMS is essential.13
Leiomyosarcoma (LMS) is a tumor that originates from smooth muscle and rarely develops in the dermis.14 Pleomorphic LMS is a morphologic variant of LMS that has a low propensity to metastasize but commonly exhibits local recurrence.15 Leiomyosarcoma can present in any age group but most commonly manifests in individuals aged 50 to 70 years. Clinically, LMS presents as a firm solitary nodule with a smooth pink surface or a more exophytic tumor with a reddish or brown color on the extensor surface of the lower limbs; it is less common on the scalp and face.14 Histologically, most cases of pleomorphic LMS show small foci of fascicles consisting of smooth muscle tumor cells in addition to cellular pleomorphism (Figure 4).15 Many of these cells demonstrate a clear perinuclear vacuole that generally is appreciated in neoplastic smooth muscle cells.14 Pleomorphic LMS typically stains positively for at least one smooth muscle marker including desmin, h-caldesmon, muscle-specific actin, α-smooth muscle actin, or smooth muscle myosin in the leiomyosarcomatous fascicular areas.16 Complete surgical excision is the treatment of choice, and the best results are obtained with MMS.14
- Syme-Grant J, Syme-Grant NJ, Motta L, et al. Are primary cutaneous carcinosarcomas underdiagnosed? five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006;59:1402-1408.
- Bourgeault E, Alain J, Gagne E. Primary cutaneous carcinosarcoma of the basal cell subtype should be treated as a high-risk basal cell carcinoma. J Cutan Med Surg. 2015;19:407-411.
- West L, Srivastava D. Cutaneous carcinosarcoma of the medial canthus discovered on Mohs debulk analysis. Dermatol Surg. 2019;45:1700-1702.
- Kwan JM, Satter EK. Carcinosarcoma: a primary cutaneous tumor with biphasic differentiation. Cutis. 2013;92:247-249.
- Suh KY, Lacouture M, Gerami P. p63 in primary cutaneous carcinosarcoma. Am J Dermatopathol. 2007;29:374‐377.
- Ruiz-Villaverde R, Aneiros-Fernandez J. Primary cutaneous carcinosarcoma: a cutaneous neoplasm with an exceptional presentation. Sultan Qaboos Univ Med J. 2018;18:E114-E115.
- Smart CN, Pucci RA, Binder SW, et al. Cutaneous carcinosarcoma with myoepithelial differentiation: immunohistochemical and cytogenetic analysis of a case presenting in an unusual location. Am J Dermatopathol. 2009;31:715‐717.
- Clark JJ, Bowen AR, Bowen GM, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017;44:34‐44.
- Müller CS, Pföhler C, Schiekofer C, et al. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol. 2014;36:328‐339.
- Bellew S, Del Rosso JQ, Mobini N. Primary carcinosarcoma of the ear: case report and review of the literature. J Clin Aesthet Dermatol. 2009;2:33‐35.
- Hong SH, Hong SJ, Lee Y, et al. Primary cutaneous carcinosarcoma of the shoulder: case report with literature review. Dermatol Surg. 2013;39:338-340.
- Soleymani T, Aasi SZ, Novoa R, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: updates on classification and management. Dermatol Clin. 2019;37:253-259.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525.
- Johnson GE, Stevens K, Morrison AO, et al. Cutaneous myoepithelial carcinoma with disseminated metastases. Cutis. 2017;99:E19-E26.
- Llombart B, Serra-Guillén C, Requena C, et al. Leiomyosarcoma and pleomorphic dermal sarcoma: guidelines for diagnosis and treatment. Actas Dermosifiliogr. 2019;110:4-11.
- Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030-1038.
- Syme-Grant J, Syme-Grant NJ, Motta L, et al. Are primary cutaneous carcinosarcomas underdiagnosed? five cases and a review of the literature. J Plast Reconstr Aesthet Surg. 2006;59:1402-1408.
- Bourgeault E, Alain J, Gagne E. Primary cutaneous carcinosarcoma of the basal cell subtype should be treated as a high-risk basal cell carcinoma. J Cutan Med Surg. 2015;19:407-411.
- West L, Srivastava D. Cutaneous carcinosarcoma of the medial canthus discovered on Mohs debulk analysis. Dermatol Surg. 2019;45:1700-1702.
- Kwan JM, Satter EK. Carcinosarcoma: a primary cutaneous tumor with biphasic differentiation. Cutis. 2013;92:247-249.
- Suh KY, Lacouture M, Gerami P. p63 in primary cutaneous carcinosarcoma. Am J Dermatopathol. 2007;29:374‐377.
- Ruiz-Villaverde R, Aneiros-Fernandez J. Primary cutaneous carcinosarcoma: a cutaneous neoplasm with an exceptional presentation. Sultan Qaboos Univ Med J. 2018;18:E114-E115.
- Smart CN, Pucci RA, Binder SW, et al. Cutaneous carcinosarcoma with myoepithelial differentiation: immunohistochemical and cytogenetic analysis of a case presenting in an unusual location. Am J Dermatopathol. 2009;31:715‐717.
- Clark JJ, Bowen AR, Bowen GM, et al. Cutaneous carcinosarcoma: a series of six cases and a review of the literature. J Cutan Pathol. 2017;44:34‐44.
- Müller CS, Pföhler C, Schiekofer C, et al. Primary cutaneous carcinosarcomas: a morphological histogenetic concept revisited. Am J Dermatopathol. 2014;36:328‐339.
- Bellew S, Del Rosso JQ, Mobini N. Primary carcinosarcoma of the ear: case report and review of the literature. J Clin Aesthet Dermatol. 2009;2:33‐35.
- Hong SH, Hong SJ, Lee Y, et al. Primary cutaneous carcinosarcoma of the shoulder: case report with literature review. Dermatol Surg. 2013;39:338-340.
- Soleymani T, Aasi SZ, Novoa R, et al. Atypical fibroxanthoma and pleomorphic dermal sarcoma: updates on classification and management. Dermatol Clin. 2019;37:253-259.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525.
- Johnson GE, Stevens K, Morrison AO, et al. Cutaneous myoepithelial carcinoma with disseminated metastases. Cutis. 2017;99:E19-E26.
- Llombart B, Serra-Guillén C, Requena C, et al. Leiomyosarcoma and pleomorphic dermal sarcoma: guidelines for diagnosis and treatment. Actas Dermosifiliogr. 2019;110:4-11.
- Oda Y, Miyajima K, Kawaguchi K, et al. Pleomorphic leiomyosarcoma: clinicopathologic and immunohistochemical study with special emphasis on its distinction from ordinary leiomyosarcoma and malignant fibrous histiocytoma. Am J Surg Pathol. 2001;25:1030-1038.
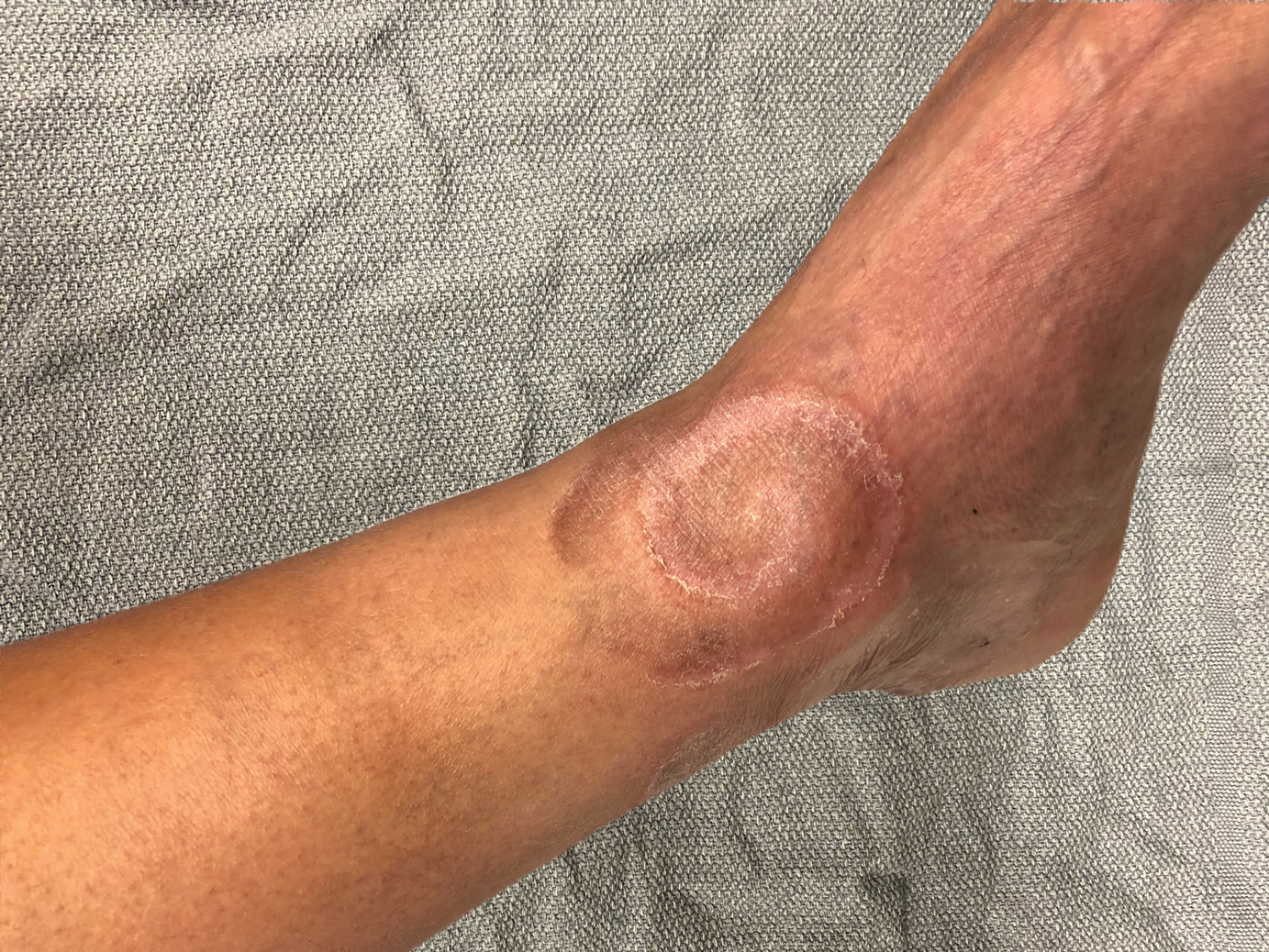
A 72-year-old man with a history of nonmelanoma skin cancer and lung transplant maintained on stable doses of prednisone and tacrolimus presented with a 1.3×1.8-cm, slow-growing, well-demarcated, ulcerated, erythematous plaque with overlying serous crust on the left temple of 6 months’ duration. No cervical or axillary lymphadenopathy was appreciated on physical examination. A biopsy was performed followed by Mohs micrographic surgery. Microscopic examination of the debulking specimen revealed atypical spindle cells in the papillary and reticular dermis radiating from a central focus of a moderately differentiated squamous cell carcinoma. The squamous cells stained positive for cytokeratin 5/6, pankeratin, and p40, while the spindle cells stained positive only for vimentin.
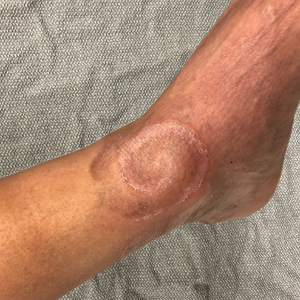
Spiral Plaque on the Left Ankle
The Diagnosis: Recurrent Cutaneous T-Cell Lymphoma
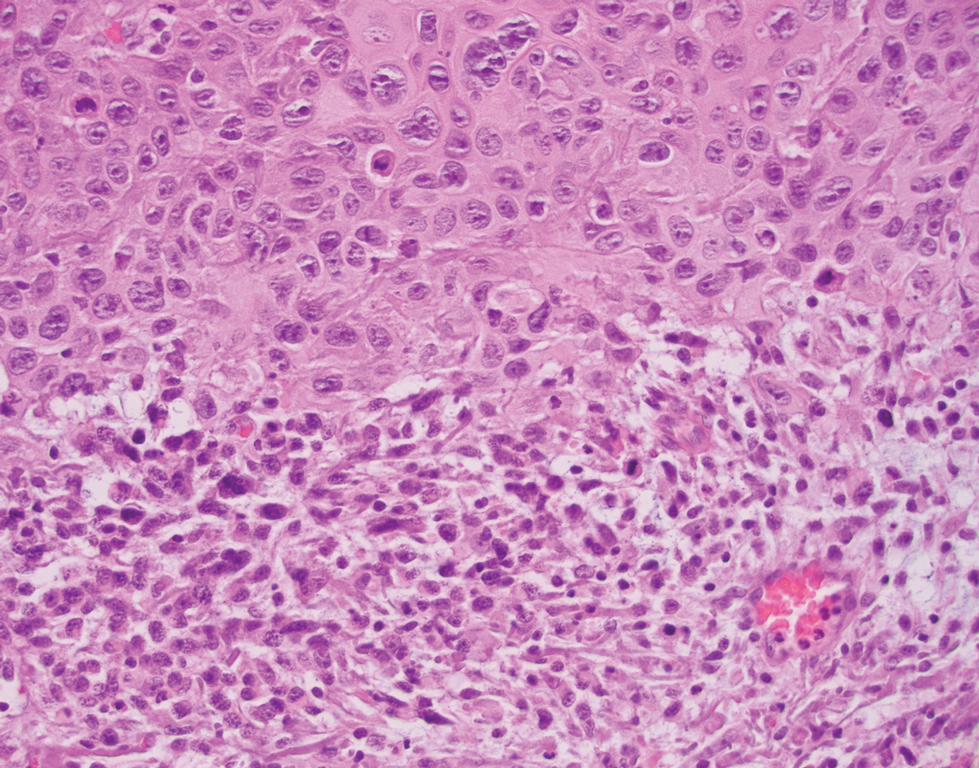
The skin biopsy revealed alternating orthokeratosis and parakeratosis with mild to moderate spongiosis and intraepidermal vesiculation as well as individual and nested atypical mononuclear cells with moderately enlarged hyperchromatic nuclei in the epidermis. There was a superficial interstitial lymphocytic infiltrate with occasional enlarged cells (Figure, A and B), and atypical cells in the epidermis and dermis stained with antibodies against CD3 and CD4 (Figure, C and D) but not against CD20 or CD8. These histopathologic findings were consistent with cutaneous T-cell lymphoma (CTCL), mycosis fungoides (MF) type. Additional application of bexarotene gel on days the patient received narrowband UVB was recommended with noted improvement of the skin.
Cutaneous T-cell lymphomas are a heterogenous group of diseases with monoclonal proliferation of T lymphocytes that largely are confined to the skin at the time of diagnosis.1 The incidence of CTCL rose steadily for more than 25 years, with an annual age-adjusted incidence of 6.4 to 9.6 cases per million individuals in the United States from 1973 to 2002.2 Mycosis fungoides is the most common classification of CTCL. It usually is characterized by patches or plaques of scaly erythema or poikiloderma; however, it also can present with annular, arcuate, concentrative, annular and linear morphologies. Mycosis fungoides tumor cells typically express a mature memory T helper cell phenotype of CD3+, CD4+, and CD8−, but there are different variants that have been discovered.3 Mycosis fungoides distributed in a spiral pattern is a distinctly unusual manifestation. Mechanisms of such dynamic morphologies are unknown but may represent an interplay between malignant cell proliferation and lost immune responses in temporospatial relationships.
The presence of keratotic gyrate lesions on acral surfaces should raise the possibility of pagetoid reticulosis. However, our patient had a history of MF involving areas of the body beyond the extremities, making this diagnosis less likely. Pagetoid reticulosis is categorized as an MF variant under the current World Health Organization– European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas.4 Pagetoid reticulosis clinically presents as a solitary psoriasiform or hyperkeratotic patch or plaque that affects the distal extremities. Variable immunophenotypes have been shown in pagetoid reticulosis, such as CD4−/CD8+ and CD4−/CD8−, while classic MF typically shows CD4+/CD8−, as in our case.5
Tinea pedis is a superficial fungal infection usually caused by anthropophilic dermatophytes, with Trichophyton rubrum being the most common organism. Four common clinical presentations of tinea pedis have been identified: interdigital, moccasin, vesicular, and acute ulcerative. Clinical presentation ranges from macerations, ulcerations, and erosions in the toe web spaces to dry hyperkeratotic scaling and fissures on the plantar foot.6 Tinea pedis primarily affects the plantar and interdigital spaces, sparing the dorsal foot and ankle. Treatment is recommended to alleviate symptoms and limit the spread of infection; topical antifungals for 4 weeks is the treatment of choice. However, recurrence is common, and maintenance therapy often is indicated. Oral antifungals or a combination of both topical and oral medications may be needed in certain cases.7
Erythema annulare centrifugum (EAC) is a rare dermatologic disease described as erythematous or urticarial papules that can enlarge centrifugally to form annular lesions that clear centrally. Thought to be a hypersensitivity reaction to an underlying condition, EAC has been associated with fungal infections, various cutaneous diseases, and even internal malignancies. Clinically, EAC can be divided into 2 forms: deep and superficial. Deep gyrate erythema is characterized by a firm indurated border with rare scaling and pruritus that histologically shows perivascular lymphocytic infiltration in the upper and deep dermis. Superficial gyrate erythema has minimally elevated lesions with an indistinct border and trailing scales and pruritus; histopathologic findings present a dense, perivascular, lymphocytic infiltration restricted to the upper dermis.8 Therapy for EAC is directed at relieving symptoms and treating the underlying condition if there is one associated.
Granuloma annulare (GA) is a common skin disorder classically characterized by ringed erythematous plaques, though many variants have been identified. Localized GA is the most common variant and presents with pink-red, nonscaly, annular patches or plaques, typically affecting the hands and feet. Generalized GA is characterized as diffuse annular patches or plaques classically affecting the trunk and extremities. Histology is notable for mucin with a palisading or interstitial pattern of granulomatous inflammation, which was not evident in our patient.9 Topical or intralesional corticosteroids are the first-line treatment of localized GA; however, localized GA generally is self-limited, and treatment often is not necessary. Treatment with cryosurgery, laser therapy, and topical dapsone and tacrolimus also has been described, but evidence of the efficacy of these agents is limited. For generalized GA, phototherapy currently is the most reliable therapy. Systemic therapies include antimalarials, fumaric acid esters, biologics, antimicrobials, and isotretinoin.10
Erythema gyratum repens (EGR) is a rare dermatologic disease described as erythematous concentric bands arranged in parallel rings that can be annular, figurate, or gyrate, with a fine scale trailing the leading edge. Histopathologic features of EGR are nonspecific but are characterized by a perivascular, superficial, mononuclear dermatitis. Diagnosis is based on its characteristic clinical presentation. Although EGR commonly is associated with internal malignancies such as bronchial carcinoma, it also may be associated with benign conditions.11 Improvement often is seen with successful therapy of the underlying associated malignancy.12
Treatment of MF is based on tumor-node-metastasisblood classification, prognostic factors, and clinical stage at the time of diagnosis. Early-stage MF (IA–IIA) commonly is treated with skin-directed therapies such as topical corticosteroids, topical mechlorethamine, topical retinoids, UV phototherapy, and localized radiotherapy. In late stages (IIB–IV), systemic therapy is indicated and includes systemic retinoids, interferon alfa, chemotherapy, monoclonal antibodies, and psoralen plus UVA.13 In many cases, patients may require combination therapy to achieve remission or better control of their condition, as in our patient.
The Diagnosis: Recurrent Cutaneous T-Cell Lymphoma
The skin biopsy revealed alternating orthokeratosis and parakeratosis with mild to moderate spongiosis and intraepidermal vesiculation as well as individual and nested atypical mononuclear cells with moderately enlarged hyperchromatic nuclei in the epidermis. There was a superficial interstitial lymphocytic infiltrate with occasional enlarged cells (Figure, A and B), and atypical cells in the epidermis and dermis stained with antibodies against CD3 and CD4 (Figure, C and D) but not against CD20 or CD8. These histopathologic findings were consistent with cutaneous T-cell lymphoma (CTCL), mycosis fungoides (MF) type. Additional application of bexarotene gel on days the patient received narrowband UVB was recommended with noted improvement of the skin.
Cutaneous T-cell lymphomas are a heterogenous group of diseases with monoclonal proliferation of T lymphocytes that largely are confined to the skin at the time of diagnosis.1 The incidence of CTCL rose steadily for more than 25 years, with an annual age-adjusted incidence of 6.4 to 9.6 cases per million individuals in the United States from 1973 to 2002.2 Mycosis fungoides is the most common classification of CTCL. It usually is characterized by patches or plaques of scaly erythema or poikiloderma; however, it also can present with annular, arcuate, concentrative, annular and linear morphologies. Mycosis fungoides tumor cells typically express a mature memory T helper cell phenotype of CD3+, CD4+, and CD8−, but there are different variants that have been discovered.3 Mycosis fungoides distributed in a spiral pattern is a distinctly unusual manifestation. Mechanisms of such dynamic morphologies are unknown but may represent an interplay between malignant cell proliferation and lost immune responses in temporospatial relationships.
The presence of keratotic gyrate lesions on acral surfaces should raise the possibility of pagetoid reticulosis. However, our patient had a history of MF involving areas of the body beyond the extremities, making this diagnosis less likely. Pagetoid reticulosis is categorized as an MF variant under the current World Health Organization– European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas.4 Pagetoid reticulosis clinically presents as a solitary psoriasiform or hyperkeratotic patch or plaque that affects the distal extremities. Variable immunophenotypes have been shown in pagetoid reticulosis, such as CD4−/CD8+ and CD4−/CD8−, while classic MF typically shows CD4+/CD8−, as in our case.5
Tinea pedis is a superficial fungal infection usually caused by anthropophilic dermatophytes, with Trichophyton rubrum being the most common organism. Four common clinical presentations of tinea pedis have been identified: interdigital, moccasin, vesicular, and acute ulcerative. Clinical presentation ranges from macerations, ulcerations, and erosions in the toe web spaces to dry hyperkeratotic scaling and fissures on the plantar foot.6 Tinea pedis primarily affects the plantar and interdigital spaces, sparing the dorsal foot and ankle. Treatment is recommended to alleviate symptoms and limit the spread of infection; topical antifungals for 4 weeks is the treatment of choice. However, recurrence is common, and maintenance therapy often is indicated. Oral antifungals or a combination of both topical and oral medications may be needed in certain cases.7
Erythema annulare centrifugum (EAC) is a rare dermatologic disease described as erythematous or urticarial papules that can enlarge centrifugally to form annular lesions that clear centrally. Thought to be a hypersensitivity reaction to an underlying condition, EAC has been associated with fungal infections, various cutaneous diseases, and even internal malignancies. Clinically, EAC can be divided into 2 forms: deep and superficial. Deep gyrate erythema is characterized by a firm indurated border with rare scaling and pruritus that histologically shows perivascular lymphocytic infiltration in the upper and deep dermis. Superficial gyrate erythema has minimally elevated lesions with an indistinct border and trailing scales and pruritus; histopathologic findings present a dense, perivascular, lymphocytic infiltration restricted to the upper dermis.8 Therapy for EAC is directed at relieving symptoms and treating the underlying condition if there is one associated.
Granuloma annulare (GA) is a common skin disorder classically characterized by ringed erythematous plaques, though many variants have been identified. Localized GA is the most common variant and presents with pink-red, nonscaly, annular patches or plaques, typically affecting the hands and feet. Generalized GA is characterized as diffuse annular patches or plaques classically affecting the trunk and extremities. Histology is notable for mucin with a palisading or interstitial pattern of granulomatous inflammation, which was not evident in our patient.9 Topical or intralesional corticosteroids are the first-line treatment of localized GA; however, localized GA generally is self-limited, and treatment often is not necessary. Treatment with cryosurgery, laser therapy, and topical dapsone and tacrolimus also has been described, but evidence of the efficacy of these agents is limited. For generalized GA, phototherapy currently is the most reliable therapy. Systemic therapies include antimalarials, fumaric acid esters, biologics, antimicrobials, and isotretinoin.10
Erythema gyratum repens (EGR) is a rare dermatologic disease described as erythematous concentric bands arranged in parallel rings that can be annular, figurate, or gyrate, with a fine scale trailing the leading edge. Histopathologic features of EGR are nonspecific but are characterized by a perivascular, superficial, mononuclear dermatitis. Diagnosis is based on its characteristic clinical presentation. Although EGR commonly is associated with internal malignancies such as bronchial carcinoma, it also may be associated with benign conditions.11 Improvement often is seen with successful therapy of the underlying associated malignancy.12
Treatment of MF is based on tumor-node-metastasisblood classification, prognostic factors, and clinical stage at the time of diagnosis. Early-stage MF (IA–IIA) commonly is treated with skin-directed therapies such as topical corticosteroids, topical mechlorethamine, topical retinoids, UV phototherapy, and localized radiotherapy. In late stages (IIB–IV), systemic therapy is indicated and includes systemic retinoids, interferon alfa, chemotherapy, monoclonal antibodies, and psoralen plus UVA.13 In many cases, patients may require combination therapy to achieve remission or better control of their condition, as in our patient.
The Diagnosis: Recurrent Cutaneous T-Cell Lymphoma
The skin biopsy revealed alternating orthokeratosis and parakeratosis with mild to moderate spongiosis and intraepidermal vesiculation as well as individual and nested atypical mononuclear cells with moderately enlarged hyperchromatic nuclei in the epidermis. There was a superficial interstitial lymphocytic infiltrate with occasional enlarged cells (Figure, A and B), and atypical cells in the epidermis and dermis stained with antibodies against CD3 and CD4 (Figure, C and D) but not against CD20 or CD8. These histopathologic findings were consistent with cutaneous T-cell lymphoma (CTCL), mycosis fungoides (MF) type. Additional application of bexarotene gel on days the patient received narrowband UVB was recommended with noted improvement of the skin.
Cutaneous T-cell lymphomas are a heterogenous group of diseases with monoclonal proliferation of T lymphocytes that largely are confined to the skin at the time of diagnosis.1 The incidence of CTCL rose steadily for more than 25 years, with an annual age-adjusted incidence of 6.4 to 9.6 cases per million individuals in the United States from 1973 to 2002.2 Mycosis fungoides is the most common classification of CTCL. It usually is characterized by patches or plaques of scaly erythema or poikiloderma; however, it also can present with annular, arcuate, concentrative, annular and linear morphologies. Mycosis fungoides tumor cells typically express a mature memory T helper cell phenotype of CD3+, CD4+, and CD8−, but there are different variants that have been discovered.3 Mycosis fungoides distributed in a spiral pattern is a distinctly unusual manifestation. Mechanisms of such dynamic morphologies are unknown but may represent an interplay between malignant cell proliferation and lost immune responses in temporospatial relationships.
The presence of keratotic gyrate lesions on acral surfaces should raise the possibility of pagetoid reticulosis. However, our patient had a history of MF involving areas of the body beyond the extremities, making this diagnosis less likely. Pagetoid reticulosis is categorized as an MF variant under the current World Health Organization– European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas.4 Pagetoid reticulosis clinically presents as a solitary psoriasiform or hyperkeratotic patch or plaque that affects the distal extremities. Variable immunophenotypes have been shown in pagetoid reticulosis, such as CD4−/CD8+ and CD4−/CD8−, while classic MF typically shows CD4+/CD8−, as in our case.5
Tinea pedis is a superficial fungal infection usually caused by anthropophilic dermatophytes, with Trichophyton rubrum being the most common organism. Four common clinical presentations of tinea pedis have been identified: interdigital, moccasin, vesicular, and acute ulcerative. Clinical presentation ranges from macerations, ulcerations, and erosions in the toe web spaces to dry hyperkeratotic scaling and fissures on the plantar foot.6 Tinea pedis primarily affects the plantar and interdigital spaces, sparing the dorsal foot and ankle. Treatment is recommended to alleviate symptoms and limit the spread of infection; topical antifungals for 4 weeks is the treatment of choice. However, recurrence is common, and maintenance therapy often is indicated. Oral antifungals or a combination of both topical and oral medications may be needed in certain cases.7
Erythema annulare centrifugum (EAC) is a rare dermatologic disease described as erythematous or urticarial papules that can enlarge centrifugally to form annular lesions that clear centrally. Thought to be a hypersensitivity reaction to an underlying condition, EAC has been associated with fungal infections, various cutaneous diseases, and even internal malignancies. Clinically, EAC can be divided into 2 forms: deep and superficial. Deep gyrate erythema is characterized by a firm indurated border with rare scaling and pruritus that histologically shows perivascular lymphocytic infiltration in the upper and deep dermis. Superficial gyrate erythema has minimally elevated lesions with an indistinct border and trailing scales and pruritus; histopathologic findings present a dense, perivascular, lymphocytic infiltration restricted to the upper dermis.8 Therapy for EAC is directed at relieving symptoms and treating the underlying condition if there is one associated.
Granuloma annulare (GA) is a common skin disorder classically characterized by ringed erythematous plaques, though many variants have been identified. Localized GA is the most common variant and presents with pink-red, nonscaly, annular patches or plaques, typically affecting the hands and feet. Generalized GA is characterized as diffuse annular patches or plaques classically affecting the trunk and extremities. Histology is notable for mucin with a palisading or interstitial pattern of granulomatous inflammation, which was not evident in our patient.9 Topical or intralesional corticosteroids are the first-line treatment of localized GA; however, localized GA generally is self-limited, and treatment often is not necessary. Treatment with cryosurgery, laser therapy, and topical dapsone and tacrolimus also has been described, but evidence of the efficacy of these agents is limited. For generalized GA, phototherapy currently is the most reliable therapy. Systemic therapies include antimalarials, fumaric acid esters, biologics, antimicrobials, and isotretinoin.10
Erythema gyratum repens (EGR) is a rare dermatologic disease described as erythematous concentric bands arranged in parallel rings that can be annular, figurate, or gyrate, with a fine scale trailing the leading edge. Histopathologic features of EGR are nonspecific but are characterized by a perivascular, superficial, mononuclear dermatitis. Diagnosis is based on its characteristic clinical presentation. Although EGR commonly is associated with internal malignancies such as bronchial carcinoma, it also may be associated with benign conditions.11 Improvement often is seen with successful therapy of the underlying associated malignancy.12
Treatment of MF is based on tumor-node-metastasisblood classification, prognostic factors, and clinical stage at the time of diagnosis. Early-stage MF (IA–IIA) commonly is treated with skin-directed therapies such as topical corticosteroids, topical mechlorethamine, topical retinoids, UV phototherapy, and localized radiotherapy. In late stages (IIB–IV), systemic therapy is indicated and includes systemic retinoids, interferon alfa, chemotherapy, monoclonal antibodies, and psoralen plus UVA.13 In many cases, patients may require combination therapy to achieve remission or better control of their condition, as in our patient.
A 60-year-old man presented with a whorl-like plaque on the left ankle that he had noticed while undergoing treatment with narrowband UVB every other week and nitrogen mustard gel daily for stage IB cutaneous T-cell lymphoma, mycosis fungoides type. He denied pain, pruritus, and any other associated symptoms at the site. He denied recent illness, new medications, or changes in diet. His medical history included multiple sclerosis, vascular disease, and stroke. Physical examination revealed an 8×6-cm, welldemarcated, slightly scaly, erythematous plaque with a spiral appearance and peripheral hyperpigmentation involving the left ankle. The remainder of the examination was notable for well-controlled mycosis fungoides with several hyperpigmented patches at sites of prior involvement on the trunk and upper and lower extremities. No cervical, axillary, or inguinal lymphadenopathy was noted. A 4-mm punch biopsy was performed and sent for histopathologic examination.
Modifier -25 and the New 2021 E/M Codes: Documentation of Separate and Distinct Just Got Easier
Insurers Target Modifier -25
Modifier -25 allows reporting of both a minor procedure (ie, one with a 0- or 10-day global period) and a separate and distinct evaluation and management (E/M) service on the same date of service.1 Because of the multicomplaint nature of dermatology, the ability to report a same-day procedure and an E/M service is critical for efficient, cost-effective, and patient-centered dermatologic care. However, it is well known that the use of modifier -25 has been under notable insurer scrutiny and is a common reason for medical record audits.2,3 Some insurers have responded to increased utilization of modifier -25 by cutting reimbursement for claims that include both a procedure and an E/M service or by denying one of the services altogether.4-6 The Centers for Medicare and Medicaid Services also have expressed concern about this coding combination with proposed cuts to reimbursement.7 Moreover, the Office of Inspector General has announced a work plan to investigate the frequent utilization of E/M codes and minor procedures by dermatologists.8 Clearly, modifier -25 is a continued target by insurers and regulators; therefore, dermatologists will want to make sure their coding and documentation meet all requirements and are updated for the new E/M codes for 2021.
The American Medical Association’s Current Procedural Terminology indicates that modifier -25 allows reporting of a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of a procedure or other service.”1 Given that dermatology patients typically present with multiple concerns, dermatologists commonly evaluate and treat numerous conditions during one visit. Understanding what constitutes a separately identifiable E/M service is critical to bill accurately and to pass insurer audits.
Global Surgical Package
To appropriately bill both a procedure and an E/M service, the physician must indicate that the patient’s condition required an E/M service above and beyond the usual work of the procedure. The compilation of evaluation and work included in the payment for a procedure is called the global surgical package.9 In general, the global surgical package includes local or topical anesthesia; the surgical service/procedure itself; immediate postoperative care, including dictating the operative note; meeting/discussing the patient’s procedure with family and other physicians; and writing orders for the patient. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M services associated with the decision to perform surgery. An appropriate history and physical examination as well as a discussion of the differential diagnosis, treatment options, and risk and benefits of treatment are all included in the payment of a minor procedure itself. Therefore, an evaluation to discuss a patient’s condition or change in condition, alternatives to treatment, or next steps after a diagnosis related to a treatment or diagnostic procedure should not be separately reported. Moreover, the fact that the patient is new to the physician is not in itself sufficient to allow reporting of an E/M service with these minor procedures. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
2021 E/M Codes Simplify Documentation
The biggest coding change of 2021 was the new E/M codes.10 Prior to this year, the descriptors of E/M services recognized 7 components to define the levels of E/M services11: history and nature of the presenting problem; physical examination; medical decision-making (MDM); counseling; coordination of care; and time. Furthermore, history, physical examination, and MDM were all broken down into more granular elements that were summed to determine the level for each component; for example, the history of the presenting problem was defined as a chronological description of the development of the patient’s present illness, including the following elements: location, quality, severity, duration, timing, context, modifying factors, and associated signs and symptoms. Each of these categories would constitute bullet points to be summed to determine the level of history. Physical examination and MDM bullet points also would be summed to determine a proper coding level.11 Understandably, this coding scheme was complicated and burdensome to medical providers.
The redefinition of the E/M codes for 2021 substantially simplified the determination of coding level and documentation.10 The revisions to the E/M office visit code descriptors and documentation standards are now centered around how physicians think and take care of patients and not on mandatory standards and checking boxes. The main changes involve MDM as the prime determinant of the coding level. Elements of MDM affecting coding for an outpatient or office visit now include only 3 components: the number and complexity of problems addressed in the encounter, the amount or complexity of data to be reviewed and analyzed, and the risk of complications or morbidity of patient management. Gone are the requirements from the earlier criteria requiring so many bullet points for the history, physical examination, and MDM.
Dermatologists may ask, “How does the new E/M coding structure affect reporting and documenting an E/M and a procedure on the same day?” The answer is that the determination of separate and distinct is basically unchanged with the new E/M codes; however, the documentation requirements for modifier -25 using the new E/M codes are simplified.
As always, the key to determining whether a separate and distinct E/M service was provided and subsequently documented is to deconstruct the medical note. All evaluation services associated with the procedure—making a clinical diagnosis or differential diagnosis, decision to perform surgery, and discussion of alternative treatments—should be removed from one’s documentation as shown in the example below. If a complete E/M service still exists, then an E/M may be billed in addition to the procedure. Physical examination of the treatment area is included in the surgical package. With the prior E/M criteria, physical examination of the procedural area could not be used again as a bullet point to count for the E/M level. However, with the new 2021 coding requirements, the documentation of a separate MDM will be sufficient to meet criteria because documentation of physical examination is not a requirement.
Modifier -25 Examples
Let’s examine a typical dermatologist medical note. An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. The patient also complains of a growing tender lesion on the left elbow of 2 months’ duration. Physical examination reveals a linear vesicular eruption on the left wrist and a tender hyperkeratotic papule on the left elbow. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed. The decision is made to perform a tangential biopsy of the lesion on the left elbow because of the suspicion for malignancy. The biopsy is performed the same day.
This case clearly illustrates performance of an E/M service in the treatment of rhus dermatitis, which is separate and distinct from the biopsy procedure; however, in evaluating whether the case meets the documentation requirements for modifier -25, the information in the medical note inclusive to the procedure’s global surgical package, including history associated with establishing the diagnosis, physical examination of the procedure area(s), and discussion of treatment options, is eliminated, leaving the following notes: An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed.
Because the physical examination of the body part (left arm) is included in the procedure’s global surgical package, the examination of the left wrist cannot be used as coding support for the E/M service. This makes a difference for coding level in the prior E/M coding requirements, which required examination bullet points. However, with the 2021 E/M codes, documentation of physical examination bullet points is irrelevant to the coding level. Therefore, qualifying for a modifier -25 claim is more straightforward in this case with the new code set. Because bullet points are not integral to the 2021 E/M codes, qualifying and properly documenting for a higher level of service will likely be more common in dermatology.
Final Thoughts
Frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of minor procedures and E/M services allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. The new E/M codes for 2021 actually make the documentation of a separate and distinct E/M service less complicated because the bullet point requirements associated with the old E/M codes have been eliminated. Understanding how the new E/M code descriptors affect modifier -25 reporting and clear documentation of separate, distinct, and medically necessary E/M services will be needed due to increased insurer scrutiny and audits.
- Current Procedural Terminology 2021, Professional Edition. American Medical Association; 2020.
- Rogers HW. Modifier −25 victory, but the battle is not over. Cutis. 2018;101:409-410.
- Rogers HW. One diagnosis and modifier −25: appropriate or audit target? Cutis. 2017;99:165-166.
- Update regarding E/M with modifier −25—professional. Anthem Blue Cross Blue Shield website. Published February 1, 2019. Accessed August 17, 2021. https://providernews.anthem.com/ohio/article/update-regarding-em-with-modifier-25-professional
- Payment policies—surgery. Harvard Pilgrim Health Care website. Updated May 2021. Accessed August 17, 2021. https://www.harvardpilgrim.org/provider/wp-content/uploads/sites/7/2020/07/H-6-Surgery-PM.pdf
- Modifier 25: frequently asked questions. Independence Blue Cross website. Updated September 25, 2017. Accessed August 17, 2021. https://provcomm.ibx.com/ibc/archive/pages/A86603B03881756B8525817E00768006.aspx
- Huang G. CMS 2019 fee schedule takes modifier 25 cuts, runs with them. Doctors Management website. Accessed August 17, 2021. https://www.doctors-management.com/cms-2019-feeschedule-modifier25/
- Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. US Department of Health and Humans Services Office of Inspector General website. Accessed August 17, 2021. https://oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- Global surgery booklet. Centers for Medicare and Medicaid Services website. Updated September 2018. Accessed August 17, 2021. https://www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnproducts/downloads/globallsurgery-icn907166.pdf
- American Medical Association. CPT® Evaluation and management (E/M)—office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Updated March 9, 2021. Accessed August 17, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- 1997 documentation guidelines for evaluation and management services. Centers for Medicare and Medicaid Services website. Accessed August 17, 2021. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNEdWebGuide/Downloads/97Docguidelines.pdf
Insurers Target Modifier -25
Modifier -25 allows reporting of both a minor procedure (ie, one with a 0- or 10-day global period) and a separate and distinct evaluation and management (E/M) service on the same date of service.1 Because of the multicomplaint nature of dermatology, the ability to report a same-day procedure and an E/M service is critical for efficient, cost-effective, and patient-centered dermatologic care. However, it is well known that the use of modifier -25 has been under notable insurer scrutiny and is a common reason for medical record audits.2,3 Some insurers have responded to increased utilization of modifier -25 by cutting reimbursement for claims that include both a procedure and an E/M service or by denying one of the services altogether.4-6 The Centers for Medicare and Medicaid Services also have expressed concern about this coding combination with proposed cuts to reimbursement.7 Moreover, the Office of Inspector General has announced a work plan to investigate the frequent utilization of E/M codes and minor procedures by dermatologists.8 Clearly, modifier -25 is a continued target by insurers and regulators; therefore, dermatologists will want to make sure their coding and documentation meet all requirements and are updated for the new E/M codes for 2021.
The American Medical Association’s Current Procedural Terminology indicates that modifier -25 allows reporting of a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of a procedure or other service.”1 Given that dermatology patients typically present with multiple concerns, dermatologists commonly evaluate and treat numerous conditions during one visit. Understanding what constitutes a separately identifiable E/M service is critical to bill accurately and to pass insurer audits.
Global Surgical Package
To appropriately bill both a procedure and an E/M service, the physician must indicate that the patient’s condition required an E/M service above and beyond the usual work of the procedure. The compilation of evaluation and work included in the payment for a procedure is called the global surgical package.9 In general, the global surgical package includes local or topical anesthesia; the surgical service/procedure itself; immediate postoperative care, including dictating the operative note; meeting/discussing the patient’s procedure with family and other physicians; and writing orders for the patient. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M services associated with the decision to perform surgery. An appropriate history and physical examination as well as a discussion of the differential diagnosis, treatment options, and risk and benefits of treatment are all included in the payment of a minor procedure itself. Therefore, an evaluation to discuss a patient’s condition or change in condition, alternatives to treatment, or next steps after a diagnosis related to a treatment or diagnostic procedure should not be separately reported. Moreover, the fact that the patient is new to the physician is not in itself sufficient to allow reporting of an E/M service with these minor procedures. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
2021 E/M Codes Simplify Documentation
The biggest coding change of 2021 was the new E/M codes.10 Prior to this year, the descriptors of E/M services recognized 7 components to define the levels of E/M services11: history and nature of the presenting problem; physical examination; medical decision-making (MDM); counseling; coordination of care; and time. Furthermore, history, physical examination, and MDM were all broken down into more granular elements that were summed to determine the level for each component; for example, the history of the presenting problem was defined as a chronological description of the development of the patient’s present illness, including the following elements: location, quality, severity, duration, timing, context, modifying factors, and associated signs and symptoms. Each of these categories would constitute bullet points to be summed to determine the level of history. Physical examination and MDM bullet points also would be summed to determine a proper coding level.11 Understandably, this coding scheme was complicated and burdensome to medical providers.
The redefinition of the E/M codes for 2021 substantially simplified the determination of coding level and documentation.10 The revisions to the E/M office visit code descriptors and documentation standards are now centered around how physicians think and take care of patients and not on mandatory standards and checking boxes. The main changes involve MDM as the prime determinant of the coding level. Elements of MDM affecting coding for an outpatient or office visit now include only 3 components: the number and complexity of problems addressed in the encounter, the amount or complexity of data to be reviewed and analyzed, and the risk of complications or morbidity of patient management. Gone are the requirements from the earlier criteria requiring so many bullet points for the history, physical examination, and MDM.
Dermatologists may ask, “How does the new E/M coding structure affect reporting and documenting an E/M and a procedure on the same day?” The answer is that the determination of separate and distinct is basically unchanged with the new E/M codes; however, the documentation requirements for modifier -25 using the new E/M codes are simplified.
As always, the key to determining whether a separate and distinct E/M service was provided and subsequently documented is to deconstruct the medical note. All evaluation services associated with the procedure—making a clinical diagnosis or differential diagnosis, decision to perform surgery, and discussion of alternative treatments—should be removed from one’s documentation as shown in the example below. If a complete E/M service still exists, then an E/M may be billed in addition to the procedure. Physical examination of the treatment area is included in the surgical package. With the prior E/M criteria, physical examination of the procedural area could not be used again as a bullet point to count for the E/M level. However, with the new 2021 coding requirements, the documentation of a separate MDM will be sufficient to meet criteria because documentation of physical examination is not a requirement.
Modifier -25 Examples
Let’s examine a typical dermatologist medical note. An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. The patient also complains of a growing tender lesion on the left elbow of 2 months’ duration. Physical examination reveals a linear vesicular eruption on the left wrist and a tender hyperkeratotic papule on the left elbow. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed. The decision is made to perform a tangential biopsy of the lesion on the left elbow because of the suspicion for malignancy. The biopsy is performed the same day.
This case clearly illustrates performance of an E/M service in the treatment of rhus dermatitis, which is separate and distinct from the biopsy procedure; however, in evaluating whether the case meets the documentation requirements for modifier -25, the information in the medical note inclusive to the procedure’s global surgical package, including history associated with establishing the diagnosis, physical examination of the procedure area(s), and discussion of treatment options, is eliminated, leaving the following notes: An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed.
Because the physical examination of the body part (left arm) is included in the procedure’s global surgical package, the examination of the left wrist cannot be used as coding support for the E/M service. This makes a difference for coding level in the prior E/M coding requirements, which required examination bullet points. However, with the 2021 E/M codes, documentation of physical examination bullet points is irrelevant to the coding level. Therefore, qualifying for a modifier -25 claim is more straightforward in this case with the new code set. Because bullet points are not integral to the 2021 E/M codes, qualifying and properly documenting for a higher level of service will likely be more common in dermatology.
Final Thoughts
Frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of minor procedures and E/M services allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. The new E/M codes for 2021 actually make the documentation of a separate and distinct E/M service less complicated because the bullet point requirements associated with the old E/M codes have been eliminated. Understanding how the new E/M code descriptors affect modifier -25 reporting and clear documentation of separate, distinct, and medically necessary E/M services will be needed due to increased insurer scrutiny and audits.
Insurers Target Modifier -25
Modifier -25 allows reporting of both a minor procedure (ie, one with a 0- or 10-day global period) and a separate and distinct evaluation and management (E/M) service on the same date of service.1 Because of the multicomplaint nature of dermatology, the ability to report a same-day procedure and an E/M service is critical for efficient, cost-effective, and patient-centered dermatologic care. However, it is well known that the use of modifier -25 has been under notable insurer scrutiny and is a common reason for medical record audits.2,3 Some insurers have responded to increased utilization of modifier -25 by cutting reimbursement for claims that include both a procedure and an E/M service or by denying one of the services altogether.4-6 The Centers for Medicare and Medicaid Services also have expressed concern about this coding combination with proposed cuts to reimbursement.7 Moreover, the Office of Inspector General has announced a work plan to investigate the frequent utilization of E/M codes and minor procedures by dermatologists.8 Clearly, modifier -25 is a continued target by insurers and regulators; therefore, dermatologists will want to make sure their coding and documentation meet all requirements and are updated for the new E/M codes for 2021.
The American Medical Association’s Current Procedural Terminology indicates that modifier -25 allows reporting of a “significant, separately identifiable evaluation and management service by the same physician or other qualified health care professional on the same day of a procedure or other service.”1 Given that dermatology patients typically present with multiple concerns, dermatologists commonly evaluate and treat numerous conditions during one visit. Understanding what constitutes a separately identifiable E/M service is critical to bill accurately and to pass insurer audits.
Global Surgical Package
To appropriately bill both a procedure and an E/M service, the physician must indicate that the patient’s condition required an E/M service above and beyond the usual work of the procedure. The compilation of evaluation and work included in the payment for a procedure is called the global surgical package.9 In general, the global surgical package includes local or topical anesthesia; the surgical service/procedure itself; immediate postoperative care, including dictating the operative note; meeting/discussing the patient’s procedure with family and other physicians; and writing orders for the patient. For minor procedures (ie, those with either 0- or 10-day global periods), the surgical package also includes same-day E/M services associated with the decision to perform surgery. An appropriate history and physical examination as well as a discussion of the differential diagnosis, treatment options, and risk and benefits of treatment are all included in the payment of a minor procedure itself. Therefore, an evaluation to discuss a patient’s condition or change in condition, alternatives to treatment, or next steps after a diagnosis related to a treatment or diagnostic procedure should not be separately reported. Moreover, the fact that the patient is new to the physician is not in itself sufficient to allow reporting of an E/M service with these minor procedures. For major procedures (ie, those with 90-day postoperative periods), the decision for surgery is excluded from the global surgical package.
2021 E/M Codes Simplify Documentation
The biggest coding change of 2021 was the new E/M codes.10 Prior to this year, the descriptors of E/M services recognized 7 components to define the levels of E/M services11: history and nature of the presenting problem; physical examination; medical decision-making (MDM); counseling; coordination of care; and time. Furthermore, history, physical examination, and MDM were all broken down into more granular elements that were summed to determine the level for each component; for example, the history of the presenting problem was defined as a chronological description of the development of the patient’s present illness, including the following elements: location, quality, severity, duration, timing, context, modifying factors, and associated signs and symptoms. Each of these categories would constitute bullet points to be summed to determine the level of history. Physical examination and MDM bullet points also would be summed to determine a proper coding level.11 Understandably, this coding scheme was complicated and burdensome to medical providers.
The redefinition of the E/M codes for 2021 substantially simplified the determination of coding level and documentation.10 The revisions to the E/M office visit code descriptors and documentation standards are now centered around how physicians think and take care of patients and not on mandatory standards and checking boxes. The main changes involve MDM as the prime determinant of the coding level. Elements of MDM affecting coding for an outpatient or office visit now include only 3 components: the number and complexity of problems addressed in the encounter, the amount or complexity of data to be reviewed and analyzed, and the risk of complications or morbidity of patient management. Gone are the requirements from the earlier criteria requiring so many bullet points for the history, physical examination, and MDM.
Dermatologists may ask, “How does the new E/M coding structure affect reporting and documenting an E/M and a procedure on the same day?” The answer is that the determination of separate and distinct is basically unchanged with the new E/M codes; however, the documentation requirements for modifier -25 using the new E/M codes are simplified.
As always, the key to determining whether a separate and distinct E/M service was provided and subsequently documented is to deconstruct the medical note. All evaluation services associated with the procedure—making a clinical diagnosis or differential diagnosis, decision to perform surgery, and discussion of alternative treatments—should be removed from one’s documentation as shown in the example below. If a complete E/M service still exists, then an E/M may be billed in addition to the procedure. Physical examination of the treatment area is included in the surgical package. With the prior E/M criteria, physical examination of the procedural area could not be used again as a bullet point to count for the E/M level. However, with the new 2021 coding requirements, the documentation of a separate MDM will be sufficient to meet criteria because documentation of physical examination is not a requirement.
Modifier -25 Examples
Let’s examine a typical dermatologist medical note. An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. The patient also complains of a growing tender lesion on the left elbow of 2 months’ duration. Physical examination reveals a linear vesicular eruption on the left wrist and a tender hyperkeratotic papule on the left elbow. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed. The decision is made to perform a tangential biopsy of the lesion on the left elbow because of the suspicion for malignancy. The biopsy is performed the same day.
This case clearly illustrates performance of an E/M service in the treatment of rhus dermatitis, which is separate and distinct from the biopsy procedure; however, in evaluating whether the case meets the documentation requirements for modifier -25, the information in the medical note inclusive to the procedure’s global surgical package, including history associated with establishing the diagnosis, physical examination of the procedure area(s), and discussion of treatment options, is eliminated, leaving the following notes: An established patient presents to the dermatologist complaining of an itchy rash on the left wrist after a hiking trip. Treatment with topical hydrocortisone 1% did not help. No data is evaluated. A diagnosis of acute rhus dermatitis of the left wrist is made, and betamethasone cream is prescribed.
Because the physical examination of the body part (left arm) is included in the procedure’s global surgical package, the examination of the left wrist cannot be used as coding support for the E/M service. This makes a difference for coding level in the prior E/M coding requirements, which required examination bullet points. However, with the 2021 E/M codes, documentation of physical examination bullet points is irrelevant to the coding level. Therefore, qualifying for a modifier -25 claim is more straightforward in this case with the new code set. Because bullet points are not integral to the 2021 E/M codes, qualifying and properly documenting for a higher level of service will likely be more common in dermatology.
Final Thoughts
Frequent use of modifier -25 is a critical part of a high-quality and cost-effective dermatology practice. Same-day performance of minor procedures and E/M services allows for more rapid and efficient diagnosis and treatment of various conditions as well as minimizing unnecessary office visits. The new E/M codes for 2021 actually make the documentation of a separate and distinct E/M service less complicated because the bullet point requirements associated with the old E/M codes have been eliminated. Understanding how the new E/M code descriptors affect modifier -25 reporting and clear documentation of separate, distinct, and medically necessary E/M services will be needed due to increased insurer scrutiny and audits.
- Current Procedural Terminology 2021, Professional Edition. American Medical Association; 2020.
- Rogers HW. Modifier −25 victory, but the battle is not over. Cutis. 2018;101:409-410.
- Rogers HW. One diagnosis and modifier −25: appropriate or audit target? Cutis. 2017;99:165-166.
- Update regarding E/M with modifier −25—professional. Anthem Blue Cross Blue Shield website. Published February 1, 2019. Accessed August 17, 2021. https://providernews.anthem.com/ohio/article/update-regarding-em-with-modifier-25-professional
- Payment policies—surgery. Harvard Pilgrim Health Care website. Updated May 2021. Accessed August 17, 2021. https://www.harvardpilgrim.org/provider/wp-content/uploads/sites/7/2020/07/H-6-Surgery-PM.pdf
- Modifier 25: frequently asked questions. Independence Blue Cross website. Updated September 25, 2017. Accessed August 17, 2021. https://provcomm.ibx.com/ibc/archive/pages/A86603B03881756B8525817E00768006.aspx
- Huang G. CMS 2019 fee schedule takes modifier 25 cuts, runs with them. Doctors Management website. Accessed August 17, 2021. https://www.doctors-management.com/cms-2019-feeschedule-modifier25/
- Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. US Department of Health and Humans Services Office of Inspector General website. Accessed August 17, 2021. https://oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- Global surgery booklet. Centers for Medicare and Medicaid Services website. Updated September 2018. Accessed August 17, 2021. https://www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnproducts/downloads/globallsurgery-icn907166.pdf
- American Medical Association. CPT® Evaluation and management (E/M)—office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Updated March 9, 2021. Accessed August 17, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- 1997 documentation guidelines for evaluation and management services. Centers for Medicare and Medicaid Services website. Accessed August 17, 2021. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNEdWebGuide/Downloads/97Docguidelines.pdf
- Current Procedural Terminology 2021, Professional Edition. American Medical Association; 2020.
- Rogers HW. Modifier −25 victory, but the battle is not over. Cutis. 2018;101:409-410.
- Rogers HW. One diagnosis and modifier −25: appropriate or audit target? Cutis. 2017;99:165-166.
- Update regarding E/M with modifier −25—professional. Anthem Blue Cross Blue Shield website. Published February 1, 2019. Accessed August 17, 2021. https://providernews.anthem.com/ohio/article/update-regarding-em-with-modifier-25-professional
- Payment policies—surgery. Harvard Pilgrim Health Care website. Updated May 2021. Accessed August 17, 2021. https://www.harvardpilgrim.org/provider/wp-content/uploads/sites/7/2020/07/H-6-Surgery-PM.pdf
- Modifier 25: frequently asked questions. Independence Blue Cross website. Updated September 25, 2017. Accessed August 17, 2021. https://provcomm.ibx.com/ibc/archive/pages/A86603B03881756B8525817E00768006.aspx
- Huang G. CMS 2019 fee schedule takes modifier 25 cuts, runs with them. Doctors Management website. Accessed August 17, 2021. https://www.doctors-management.com/cms-2019-feeschedule-modifier25/
- Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. US Department of Health and Humans Services Office of Inspector General website. Accessed August 17, 2021. https://oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- Global surgery booklet. Centers for Medicare and Medicaid Services website. Updated September 2018. Accessed August 17, 2021. https://www.cms.gov/outreach-and-education/medicare-learning-network-mln/mlnproducts/downloads/globallsurgery-icn907166.pdf
- American Medical Association. CPT® Evaluation and management (E/M)—office or other outpatient (99202-99215) and prolonged services (99354, 99355, 99356, 99417) code and guideline changes. Updated March 9, 2021. Accessed August 17, 2021. https://www.ama-assn.org/system/files/2019-06/cpt-office-prolonged-svs-code-changes.pdf
- 1997 documentation guidelines for evaluation and management services. Centers for Medicare and Medicaid Services website. Accessed August 17, 2021. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNEdWebGuide/Downloads/97Docguidelines.pdf
Practice Points
- Insurer scrutiny of same-day evaluation and management (E/M) and procedure services has increased, and dermatologists should be prepared for more frequent medical record reviews and audits.
- The new 2021 E/M codes actually reduce the hurdles for reporting a separate and distinct E/M service by eliminating the history and physical examination bullet points of the previous code set.
Increasing Skin of Color Publications in the Dermatology Literature: A Call to Action
The US population is becoming more diverse. By 2044, it is predicted that there will be a majority minority population in the United States.1 Therefore, it is imperative to continue to develop educational mechanisms for all dermatologists to increase and maintain competency in skin of color dermatology, which will contribute to the achievement of health equity for patients with all skin tones and hair types.
Not only is clinical skin of color education necessary, but diversity, equity, and inclusion (DEI) education for dermatologists also is critical. Clinical examination,2 diagnosis, and treatment of skin and hair disorders across the skin of color spectrum with cultural humility is essential to achieve health equity. If trainees, dermatologists, other specialists, and primary care clinicians are not frequently exposed to patients with darker skin tones and coily hair, the nuances in diagnosing and treating these patients must be learned in alternate ways.
To ready the nation’s physicians and clinicians to care for the growing diverse population, exposure to more images of dermatologic diseases in those with darker skin tones in journal articles, textbooks, conference lectures, and online dermatology image libraries is necessary to help close the skin of color training and practice gap.3,4 The following initiatives demonstrate how Cutis has sought to address these educational gaps and remains committed to improving DEI education in dermatology.
Collaboration With the Skin of Color Society
The Skin of Color Society (SOCS), which was founded in 2004 by Dr. Susan C. Taylor, is a dermatologic organization with more than 800 members representing 32 countries. Its mission includes promoting awareness and excellence within skin of color dermatology through research, education, and mentorship. The SOCS has utilized strategic partnerships with national and international dermatologists, as well as professional medical organizations and community, industry, and corporate groups, to ultimately ensure that patients with skin of color receive the expert care they deserve.5 In 2017, Cutis published the inaugural article in its collaboration with the SOCS,6 and more articles, which undergo regular peer review, continue to be published quarterly (https://www.mdedge.com/dermatology/skin-color).
Increase Number of Journal Articles on Skin of Color Topics
Increasing the number of journal articles on skin of color–related topics needs to be intentional, as it is a tool that has been identified as a necessary part of enhancing awareness and subsequently improving patient care. Wilson et al7 used stringent criteria to review all articles published from January 2018 to October 2020 in 52 dermatology journals for inclusion of topics on skin of color, hair in patients with skin of color, diversity and inclusion, and socioeconomic and health care disparities in the skin of color population. The journals they reviewed included publications based on continents with majority skin of color populations, such as Asia, as well as those with minority skin of color populations, such as Europe. During the study period, the percentage of articles covering skin of color ranged from 2.04% to 61.8%, with an average of 16.8%.7
The total number of Cutis articles published during the study period was 709, with 132 (18.62%) meeting the investigators’ criteria for articles on skin of color; these included case reports in which at least 1 patient with skin of color was featured.7 Overall, Cutis ranked 16th of the 52 journals for inclusion of skin of color content. Cutis was one of only a few journals based in North America, a non–skin-of-color–predominant continent, to make the top 16 in this study.7
Some of the 132 skin of color articles published in Cutis were the result of the journal’s collaboration with the SOCS. Through this collaboration, articles were published on a variety of skin of color topics, including DEI (6), alopecia and hair care (5), dermoscopy/optical coherence tomography imaging (1), atopic dermatitis (1), cosmetics (1), hidradenitis suppurativa (1), pigmentation (1), rosacea (1), and skin cancer (2). These articles also resulted in a number of podcast discussions (https://www.mdedge.com/podcasts/dermatology-weekly), including one on dealing with DEI, one on pigmentation, and one on dermoscopy/optical coherence tomography imaging. The latter featured the SOCS Scientific Symposium poster winners in 2020.
The number of articles published specifically through Cutis’s collaboration with the SOCS accounted for only a small part of the journal’s 132 skin of color articles identified in the study by Wilson et al.7 We speculate that Cutis’s display of intentional commitment to supporting the inclusion of skin of color articles in the journal may in turn encourage its broader readership to submit more skin of color–focused articles for peer review.
Wilson et al7 specifically remarked that “Cutis’s [Skin of Color] section in each issue is a promising idea.” They also highlighted Clinics in Dermatology for committing an entire issue to skin of color; however, despite this initiative, Clinics in Dermatology still ranked 35th of 52 journals with regard to the overall percentage of skin of color articles published.7 This suggests that a journal publishing one special issue on skin of color annually is a helpful addition to the literature, but increasing the number of articles related to skin of color in each journal issue, similar to Cutis, will ultimately result in a higher overall number of skin of color articles in the dermatology literature.
Both Amuzie et al4 and Wilson et al7 concluded that the higher a journal’s impact factor, the lower the number of skin of color articles published.However, skin of color articles published in high-impact journals received a higher number of citations than those in other lower-impact journals.4 High-impact journals may use Cutis as a model for increasing the number of skin of color articles they publish, which will have a notable impact on increasing skin of color knowledge and educating dermatologists.
Coverage of Diversity, Equity, and Inclusion
In another study, Bray et al8 conducted a PubMed search of articles indexed for MEDLINE from January 2008 to July 2019 to quantify the number of articles specifically focused on DEI in a variety of medical specialties. The field of dermatology had the highest number of articles published on DEI (25) compared to the other specialties, including family medicine (23), orthopedic surgery (12), internal medicine (9), general surgery (7), radiology (6), ophthalmology (2), and anesthesiology (2).8 However, Wilson et al7 found that, out of all the categories of skin of color articles published in dermatology journals during their study period, those focused on DEI made up less than 1% of the total number of articles. Dermatology is off to a great start compared to other specialties, but there is still more work to do in dermatology for DEI. Cutis’s collaboration with the SOCS has resulted in 6 DEI articles published since 2017.
Think Beyond Dermatology Education
The collaboration between Cutis and the SOCS was established to create a series of articles dedicated to increasing the skin of color dermatology knowledge base of the Cutis readership and beyond; however, increased readership and more citations are needed to amplify the reach of the articles published by these skin of color experts. Cutis’s collaboration with SOCS is one mechanism to increase the skin of color literature, but skin of color and DEI articles outside of this collaboration should continue to be published in each issue of Cutis.
The collaboration between SOCS and Cutis was and continues to be a forward-thinking step toward improving skin of color dermatology education, but there is still work to be done across the medical literature with regard to increasing intentional publication of skin of color articles. Nondermatologist clinicians in the Cutis readership benefit from knowledge of skin of color, as all specialties and primary care will see increased patient diversity in their examination rooms.
To further ensure that primary care is not left behind, Cutis has partnered with The Journal of Family Practice to produce a new column called Dx Across the Skin of Color Spectrum (https://www.mdedge.com/dermatology/dx-across-skin-color-spectrum), which is co-published in both journals.9,10 These one-page fact sheets highlight images of dermatologic conditions in skin of color as well as images of the same condition in lighter skin, a concept suggested by Cutis Associate Editor, Dr. Candrice R. Heath. The goal of this new column is to increase the accurate diagnosis of dermatologic conditions in skin of color and to highlight health disparities related to a particular condition in an easy-to-understand format. Uniquely, Dr. Heath co-authors this content with family physician Dr. Richard P. Usatine.
Final Thoughts
The entire community of medical journals should continue to develop creative ways to educate their readership. Medical professionals stay up-to-date on best practices through journal articles, textbooks, conferences, and even podcasts. Therefore, it is best to incorporate skin of color knowledge throughout all educational programming, particularly through enduring materials such as journal articles. Wilson et al7 suggested that a minimum of 16.8% of a dermatology journal’s articles in each issue should focus on skin of color in addition to special focus issues, as this will work toward more equitable dermatologic care.
Knowledge is only part of the equation; compassionate care with cultural humility is the other part. Publishing scientific facts about biology and structure, diagnosis, and treatment selection in skin of color, as well as committing to lifelong learning about the differences in our patients despite the absence of shared life or cultural experiences, may be the key to truly impacting health equity.11 We believe that together we will get there one journal article and one citation at a time.
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed August 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Amuzie AU, Jia JL, Taylor SC, et al. Skin-of-color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Learn more about SOCS. Skin of Color Society website. Accessed August 11, 2021. https://skinofcolorsociety.org/about-socs/
- Subash J, Tull R, McMichael A. Diversity in dermatology: a society devoted to skin of color. Cutis. 2017;99:322-324.
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of colorand diversity and inclusion content of dermatologic published literature: an analysis and call to action [published online April 20, 2021]. Int J Womens Dermatol. https://doi.org/10.1016/j.ijwd.2021.04.001
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:13030/qt094243gp.
- Heath CR, Usatine R. Atopic dermatitis. Cutis. 2021;107:332. doi:10.12788/cutis.0274
- Heath CR, Usatine R. Psoriasis. Cutis. 2021;108:56. doi:10.12788/cutis.0298
- Jones N, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships [published online August 3, 2021]. Pediatr Dermatol. doi:10.1111/pde.14721
The US population is becoming more diverse. By 2044, it is predicted that there will be a majority minority population in the United States.1 Therefore, it is imperative to continue to develop educational mechanisms for all dermatologists to increase and maintain competency in skin of color dermatology, which will contribute to the achievement of health equity for patients with all skin tones and hair types.
Not only is clinical skin of color education necessary, but diversity, equity, and inclusion (DEI) education for dermatologists also is critical. Clinical examination,2 diagnosis, and treatment of skin and hair disorders across the skin of color spectrum with cultural humility is essential to achieve health equity. If trainees, dermatologists, other specialists, and primary care clinicians are not frequently exposed to patients with darker skin tones and coily hair, the nuances in diagnosing and treating these patients must be learned in alternate ways.
To ready the nation’s physicians and clinicians to care for the growing diverse population, exposure to more images of dermatologic diseases in those with darker skin tones in journal articles, textbooks, conference lectures, and online dermatology image libraries is necessary to help close the skin of color training and practice gap.3,4 The following initiatives demonstrate how Cutis has sought to address these educational gaps and remains committed to improving DEI education in dermatology.
Collaboration With the Skin of Color Society
The Skin of Color Society (SOCS), which was founded in 2004 by Dr. Susan C. Taylor, is a dermatologic organization with more than 800 members representing 32 countries. Its mission includes promoting awareness and excellence within skin of color dermatology through research, education, and mentorship. The SOCS has utilized strategic partnerships with national and international dermatologists, as well as professional medical organizations and community, industry, and corporate groups, to ultimately ensure that patients with skin of color receive the expert care they deserve.5 In 2017, Cutis published the inaugural article in its collaboration with the SOCS,6 and more articles, which undergo regular peer review, continue to be published quarterly (https://www.mdedge.com/dermatology/skin-color).
Increase Number of Journal Articles on Skin of Color Topics
Increasing the number of journal articles on skin of color–related topics needs to be intentional, as it is a tool that has been identified as a necessary part of enhancing awareness and subsequently improving patient care. Wilson et al7 used stringent criteria to review all articles published from January 2018 to October 2020 in 52 dermatology journals for inclusion of topics on skin of color, hair in patients with skin of color, diversity and inclusion, and socioeconomic and health care disparities in the skin of color population. The journals they reviewed included publications based on continents with majority skin of color populations, such as Asia, as well as those with minority skin of color populations, such as Europe. During the study period, the percentage of articles covering skin of color ranged from 2.04% to 61.8%, with an average of 16.8%.7
The total number of Cutis articles published during the study period was 709, with 132 (18.62%) meeting the investigators’ criteria for articles on skin of color; these included case reports in which at least 1 patient with skin of color was featured.7 Overall, Cutis ranked 16th of the 52 journals for inclusion of skin of color content. Cutis was one of only a few journals based in North America, a non–skin-of-color–predominant continent, to make the top 16 in this study.7
Some of the 132 skin of color articles published in Cutis were the result of the journal’s collaboration with the SOCS. Through this collaboration, articles were published on a variety of skin of color topics, including DEI (6), alopecia and hair care (5), dermoscopy/optical coherence tomography imaging (1), atopic dermatitis (1), cosmetics (1), hidradenitis suppurativa (1), pigmentation (1), rosacea (1), and skin cancer (2). These articles also resulted in a number of podcast discussions (https://www.mdedge.com/podcasts/dermatology-weekly), including one on dealing with DEI, one on pigmentation, and one on dermoscopy/optical coherence tomography imaging. The latter featured the SOCS Scientific Symposium poster winners in 2020.
The number of articles published specifically through Cutis’s collaboration with the SOCS accounted for only a small part of the journal’s 132 skin of color articles identified in the study by Wilson et al.7 We speculate that Cutis’s display of intentional commitment to supporting the inclusion of skin of color articles in the journal may in turn encourage its broader readership to submit more skin of color–focused articles for peer review.
Wilson et al7 specifically remarked that “Cutis’s [Skin of Color] section in each issue is a promising idea.” They also highlighted Clinics in Dermatology for committing an entire issue to skin of color; however, despite this initiative, Clinics in Dermatology still ranked 35th of 52 journals with regard to the overall percentage of skin of color articles published.7 This suggests that a journal publishing one special issue on skin of color annually is a helpful addition to the literature, but increasing the number of articles related to skin of color in each journal issue, similar to Cutis, will ultimately result in a higher overall number of skin of color articles in the dermatology literature.
Both Amuzie et al4 and Wilson et al7 concluded that the higher a journal’s impact factor, the lower the number of skin of color articles published.However, skin of color articles published in high-impact journals received a higher number of citations than those in other lower-impact journals.4 High-impact journals may use Cutis as a model for increasing the number of skin of color articles they publish, which will have a notable impact on increasing skin of color knowledge and educating dermatologists.
Coverage of Diversity, Equity, and Inclusion
In another study, Bray et al8 conducted a PubMed search of articles indexed for MEDLINE from January 2008 to July 2019 to quantify the number of articles specifically focused on DEI in a variety of medical specialties. The field of dermatology had the highest number of articles published on DEI (25) compared to the other specialties, including family medicine (23), orthopedic surgery (12), internal medicine (9), general surgery (7), radiology (6), ophthalmology (2), and anesthesiology (2).8 However, Wilson et al7 found that, out of all the categories of skin of color articles published in dermatology journals during their study period, those focused on DEI made up less than 1% of the total number of articles. Dermatology is off to a great start compared to other specialties, but there is still more work to do in dermatology for DEI. Cutis’s collaboration with the SOCS has resulted in 6 DEI articles published since 2017.
Think Beyond Dermatology Education
The collaboration between Cutis and the SOCS was established to create a series of articles dedicated to increasing the skin of color dermatology knowledge base of the Cutis readership and beyond; however, increased readership and more citations are needed to amplify the reach of the articles published by these skin of color experts. Cutis’s collaboration with SOCS is one mechanism to increase the skin of color literature, but skin of color and DEI articles outside of this collaboration should continue to be published in each issue of Cutis.
The collaboration between SOCS and Cutis was and continues to be a forward-thinking step toward improving skin of color dermatology education, but there is still work to be done across the medical literature with regard to increasing intentional publication of skin of color articles. Nondermatologist clinicians in the Cutis readership benefit from knowledge of skin of color, as all specialties and primary care will see increased patient diversity in their examination rooms.
To further ensure that primary care is not left behind, Cutis has partnered with The Journal of Family Practice to produce a new column called Dx Across the Skin of Color Spectrum (https://www.mdedge.com/dermatology/dx-across-skin-color-spectrum), which is co-published in both journals.9,10 These one-page fact sheets highlight images of dermatologic conditions in skin of color as well as images of the same condition in lighter skin, a concept suggested by Cutis Associate Editor, Dr. Candrice R. Heath. The goal of this new column is to increase the accurate diagnosis of dermatologic conditions in skin of color and to highlight health disparities related to a particular condition in an easy-to-understand format. Uniquely, Dr. Heath co-authors this content with family physician Dr. Richard P. Usatine.
Final Thoughts
The entire community of medical journals should continue to develop creative ways to educate their readership. Medical professionals stay up-to-date on best practices through journal articles, textbooks, conferences, and even podcasts. Therefore, it is best to incorporate skin of color knowledge throughout all educational programming, particularly through enduring materials such as journal articles. Wilson et al7 suggested that a minimum of 16.8% of a dermatology journal’s articles in each issue should focus on skin of color in addition to special focus issues, as this will work toward more equitable dermatologic care.
Knowledge is only part of the equation; compassionate care with cultural humility is the other part. Publishing scientific facts about biology and structure, diagnosis, and treatment selection in skin of color, as well as committing to lifelong learning about the differences in our patients despite the absence of shared life or cultural experiences, may be the key to truly impacting health equity.11 We believe that together we will get there one journal article and one citation at a time.
The US population is becoming more diverse. By 2044, it is predicted that there will be a majority minority population in the United States.1 Therefore, it is imperative to continue to develop educational mechanisms for all dermatologists to increase and maintain competency in skin of color dermatology, which will contribute to the achievement of health equity for patients with all skin tones and hair types.
Not only is clinical skin of color education necessary, but diversity, equity, and inclusion (DEI) education for dermatologists also is critical. Clinical examination,2 diagnosis, and treatment of skin and hair disorders across the skin of color spectrum with cultural humility is essential to achieve health equity. If trainees, dermatologists, other specialists, and primary care clinicians are not frequently exposed to patients with darker skin tones and coily hair, the nuances in diagnosing and treating these patients must be learned in alternate ways.
To ready the nation’s physicians and clinicians to care for the growing diverse population, exposure to more images of dermatologic diseases in those with darker skin tones in journal articles, textbooks, conference lectures, and online dermatology image libraries is necessary to help close the skin of color training and practice gap.3,4 The following initiatives demonstrate how Cutis has sought to address these educational gaps and remains committed to improving DEI education in dermatology.
Collaboration With the Skin of Color Society
The Skin of Color Society (SOCS), which was founded in 2004 by Dr. Susan C. Taylor, is a dermatologic organization with more than 800 members representing 32 countries. Its mission includes promoting awareness and excellence within skin of color dermatology through research, education, and mentorship. The SOCS has utilized strategic partnerships with national and international dermatologists, as well as professional medical organizations and community, industry, and corporate groups, to ultimately ensure that patients with skin of color receive the expert care they deserve.5 In 2017, Cutis published the inaugural article in its collaboration with the SOCS,6 and more articles, which undergo regular peer review, continue to be published quarterly (https://www.mdedge.com/dermatology/skin-color).
Increase Number of Journal Articles on Skin of Color Topics
Increasing the number of journal articles on skin of color–related topics needs to be intentional, as it is a tool that has been identified as a necessary part of enhancing awareness and subsequently improving patient care. Wilson et al7 used stringent criteria to review all articles published from January 2018 to October 2020 in 52 dermatology journals for inclusion of topics on skin of color, hair in patients with skin of color, diversity and inclusion, and socioeconomic and health care disparities in the skin of color population. The journals they reviewed included publications based on continents with majority skin of color populations, such as Asia, as well as those with minority skin of color populations, such as Europe. During the study period, the percentage of articles covering skin of color ranged from 2.04% to 61.8%, with an average of 16.8%.7
The total number of Cutis articles published during the study period was 709, with 132 (18.62%) meeting the investigators’ criteria for articles on skin of color; these included case reports in which at least 1 patient with skin of color was featured.7 Overall, Cutis ranked 16th of the 52 journals for inclusion of skin of color content. Cutis was one of only a few journals based in North America, a non–skin-of-color–predominant continent, to make the top 16 in this study.7
Some of the 132 skin of color articles published in Cutis were the result of the journal’s collaboration with the SOCS. Through this collaboration, articles were published on a variety of skin of color topics, including DEI (6), alopecia and hair care (5), dermoscopy/optical coherence tomography imaging (1), atopic dermatitis (1), cosmetics (1), hidradenitis suppurativa (1), pigmentation (1), rosacea (1), and skin cancer (2). These articles also resulted in a number of podcast discussions (https://www.mdedge.com/podcasts/dermatology-weekly), including one on dealing with DEI, one on pigmentation, and one on dermoscopy/optical coherence tomography imaging. The latter featured the SOCS Scientific Symposium poster winners in 2020.
The number of articles published specifically through Cutis’s collaboration with the SOCS accounted for only a small part of the journal’s 132 skin of color articles identified in the study by Wilson et al.7 We speculate that Cutis’s display of intentional commitment to supporting the inclusion of skin of color articles in the journal may in turn encourage its broader readership to submit more skin of color–focused articles for peer review.
Wilson et al7 specifically remarked that “Cutis’s [Skin of Color] section in each issue is a promising idea.” They also highlighted Clinics in Dermatology for committing an entire issue to skin of color; however, despite this initiative, Clinics in Dermatology still ranked 35th of 52 journals with regard to the overall percentage of skin of color articles published.7 This suggests that a journal publishing one special issue on skin of color annually is a helpful addition to the literature, but increasing the number of articles related to skin of color in each journal issue, similar to Cutis, will ultimately result in a higher overall number of skin of color articles in the dermatology literature.
Both Amuzie et al4 and Wilson et al7 concluded that the higher a journal’s impact factor, the lower the number of skin of color articles published.However, skin of color articles published in high-impact journals received a higher number of citations than those in other lower-impact journals.4 High-impact journals may use Cutis as a model for increasing the number of skin of color articles they publish, which will have a notable impact on increasing skin of color knowledge and educating dermatologists.
Coverage of Diversity, Equity, and Inclusion
In another study, Bray et al8 conducted a PubMed search of articles indexed for MEDLINE from January 2008 to July 2019 to quantify the number of articles specifically focused on DEI in a variety of medical specialties. The field of dermatology had the highest number of articles published on DEI (25) compared to the other specialties, including family medicine (23), orthopedic surgery (12), internal medicine (9), general surgery (7), radiology (6), ophthalmology (2), and anesthesiology (2).8 However, Wilson et al7 found that, out of all the categories of skin of color articles published in dermatology journals during their study period, those focused on DEI made up less than 1% of the total number of articles. Dermatology is off to a great start compared to other specialties, but there is still more work to do in dermatology for DEI. Cutis’s collaboration with the SOCS has resulted in 6 DEI articles published since 2017.
Think Beyond Dermatology Education
The collaboration between Cutis and the SOCS was established to create a series of articles dedicated to increasing the skin of color dermatology knowledge base of the Cutis readership and beyond; however, increased readership and more citations are needed to amplify the reach of the articles published by these skin of color experts. Cutis’s collaboration with SOCS is one mechanism to increase the skin of color literature, but skin of color and DEI articles outside of this collaboration should continue to be published in each issue of Cutis.
The collaboration between SOCS and Cutis was and continues to be a forward-thinking step toward improving skin of color dermatology education, but there is still work to be done across the medical literature with regard to increasing intentional publication of skin of color articles. Nondermatologist clinicians in the Cutis readership benefit from knowledge of skin of color, as all specialties and primary care will see increased patient diversity in their examination rooms.
To further ensure that primary care is not left behind, Cutis has partnered with The Journal of Family Practice to produce a new column called Dx Across the Skin of Color Spectrum (https://www.mdedge.com/dermatology/dx-across-skin-color-spectrum), which is co-published in both journals.9,10 These one-page fact sheets highlight images of dermatologic conditions in skin of color as well as images of the same condition in lighter skin, a concept suggested by Cutis Associate Editor, Dr. Candrice R. Heath. The goal of this new column is to increase the accurate diagnosis of dermatologic conditions in skin of color and to highlight health disparities related to a particular condition in an easy-to-understand format. Uniquely, Dr. Heath co-authors this content with family physician Dr. Richard P. Usatine.
Final Thoughts
The entire community of medical journals should continue to develop creative ways to educate their readership. Medical professionals stay up-to-date on best practices through journal articles, textbooks, conferences, and even podcasts. Therefore, it is best to incorporate skin of color knowledge throughout all educational programming, particularly through enduring materials such as journal articles. Wilson et al7 suggested that a minimum of 16.8% of a dermatology journal’s articles in each issue should focus on skin of color in addition to special focus issues, as this will work toward more equitable dermatologic care.
Knowledge is only part of the equation; compassionate care with cultural humility is the other part. Publishing scientific facts about biology and structure, diagnosis, and treatment selection in skin of color, as well as committing to lifelong learning about the differences in our patients despite the absence of shared life or cultural experiences, may be the key to truly impacting health equity.11 We believe that together we will get there one journal article and one citation at a time.
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed August 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Amuzie AU, Jia JL, Taylor SC, et al. Skin-of-color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Learn more about SOCS. Skin of Color Society website. Accessed August 11, 2021. https://skinofcolorsociety.org/about-socs/
- Subash J, Tull R, McMichael A. Diversity in dermatology: a society devoted to skin of color. Cutis. 2017;99:322-324.
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of colorand diversity and inclusion content of dermatologic published literature: an analysis and call to action [published online April 20, 2021]. Int J Womens Dermatol. https://doi.org/10.1016/j.ijwd.2021.04.001
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:13030/qt094243gp.
- Heath CR, Usatine R. Atopic dermatitis. Cutis. 2021;107:332. doi:10.12788/cutis.0274
- Heath CR, Usatine R. Psoriasis. Cutis. 2021;108:56. doi:10.12788/cutis.0298
- Jones N, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships [published online August 3, 2021]. Pediatr Dermatol. doi:10.1111/pde.14721
- Colby SL, Ortman JM. Projections of the size and composition of the U.S. population: 2014 to 2060. United States Census Bureau website. Published March 2015. Accessed August 11, 2021. https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf
- Grayson C, Heath C. An approach to examining tightly coiled hair among patients with hair loss in race-discordant patient-physician interactions. JAMA Dermatol. 2021;157:505-506. doi:10.1001/jamadermatol.2021.0338
- Alvarado SM, Feng H. Representation of dark skin images of common dermatologic conditions in educational resources: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1427-1431. doi:10.1016/j.jaad.2020.06.041
- Amuzie AU, Jia JL, Taylor SC, et al. Skin-of-color article representation in dermatology literature 2009-2019: higher citation counts and opportunities for inclusion [published online March 24, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.03.063
- Learn more about SOCS. Skin of Color Society website. Accessed August 11, 2021. https://skinofcolorsociety.org/about-socs/
- Subash J, Tull R, McMichael A. Diversity in dermatology: a society devoted to skin of color. Cutis. 2017;99:322-324.
- Wilson BN, Sun M, Ashbaugh AG, et al. Assessment of skin of colorand diversity and inclusion content of dermatologic published literature: an analysis and call to action [published online April 20, 2021]. Int J Womens Dermatol. https://doi.org/10.1016/j.ijwd.2021.04.001
- Bray JK, McMichael AJ, Huang WW, et al. Publication rates on the topic of racial and ethnic diversity in dermatology versus other specialties. Dermatol Online J. 2020;26:13030/qt094243gp.
- Heath CR, Usatine R. Atopic dermatitis. Cutis. 2021;107:332. doi:10.12788/cutis.0274
- Heath CR, Usatine R. Psoriasis. Cutis. 2021;108:56. doi:10.12788/cutis.0298
- Jones N, Heath CR. Hair at the intersection of dermatology and anthropology: a conversation on race and relationships [published online August 3, 2021]. Pediatr Dermatol. doi:10.1111/pde.14721
Practice Points
- Submitting more articles related to skin of color for peer review and publication will increase educational opportunities.
- Journals that publish skin of color articles play a critical role in reducing educational gaps and ultimately help improve patient care for those with skin of color.
A Modified Anchor Taping Technique for Distal Onychocryptosis
Practice Gap
Onychocryptosis, colloquially known as an ingrown nail, most commonly affects the lateral folds of the toenails. It also can affect the fingernails and the distal aspect of the nail unit, though these presentations are not as well described in the literature. In onychocryptosis, the nail plate grows downward into the periungual skin, resulting in chronic pain and inflammation. Risk factors include overtrimming the nails with rounded edges, local trauma, nail surgery, wearing tight footwear, obesity, and onychomycosis.1
Although surgical intervention might be required for severe or refractory disease, conservative treatment options are first line and often curative. A variety of techniques have been designed to separate the ingrown portion of the nail plate from underlying skin, including placement of an intervening piece of dental floss, cotton, or plastic tubing.2
Anchor taping is another effective method of treating onychocryptosis; a strip of tape is used to gently pull and secure the affected nail fold away from the overlying nail plate. This technique has been well described for the treatment of onychocryptosis of the lateral toenail.3-5 In 2017, Arai and Haneke5 presented a modified technique for the treatment of distal disease.
We present a simplified method that was used successfully in a case of distal onychocryptosis of the thumbnail that occurred approximately 4 months after complete nail avulsion with a nail matrix biopsy (Figure 1).
The Technique
A strongly adhesive, soft cotton, elastic tape that is 1-inch wide, such as Elastikon Elastic Tape (Johnson & Johnson), is used to pull and secure the hyponychium away from the overlying nail plate. When this technique is used for lateral onychocryptosis, a single strip of tape is secured to the affected lateral nail fold, pulled obliquely and proximally, and secured to the base of the digit.3-5 In the Arai and Haneke5 method for the treatment of distal disease, a piece of tape is first placed at the distal nail fold, pulled proximally, and secured to the ventral aspect of the digit. Then, 1 or 2 additional strips of tape are applied to the lateral nail folds, pulled obliquely, and adhered to the base of the digit, as in the classic technique for lateral onychocryptosis.5
In our modification for the treatment of distal disease, only 2 strips of tape are required, each approximately 5-cm long. The first strip of tape is applied to the hyponychium parallel to the long axis of the finger, pulled away from the distal edge of the nail plate, and secured obliquely and proximally to the base of the finger on one side. The second strip of tape is applied to the hyponychium in the same manner, directly overlying the first strip, but is then pulled obliquely in the opposite direction and secured to the other side of the proximal finger (Figure 2). The 2 strips of tape are applied directly overlying each other at the distal nail fold but with opposing tension vectors to optimize pull on the distal nail fold. This modification eliminates the need to apply an initial strip of tape along the long axis of the digit, as described by Arai and Haneke.5
The patient is instructed on this method in the office and will change the tape at home daily for 2 to 6 weeks, until the nail plate has grown out over the hyponychium (Figure 3). This technique also can be combined with other modalities, such as dilute vinegar soaks performed daily after changing the tape to ease inflammation and prevent infection. Because strongly adhesive tape is used, it also is recommended that the patient soak the tape before removing it to prevent damage to underlying skin.
Practice Implications
Anchor taping is a common and effective treatment of onychocryptosis. Most techniques described in the literature are for lateral toenail cases, which often are managed by podiatry. A modification for the treatment of distal onychocryptosis has been previously described.5 We describe a similar modification using 2 tape strips pulled in opposite directions, which successfully resolved a case of distal onychocryptosis of the fingernail that developed following a nail procedure.
Because nail dystrophy is a relatively common complication of nail surgery, dermatologic surgeons should be aware of this simple, cost-effective, and noninvasive technique for the treatment of distal onychocryptosis.
- Geizhals S, Lipner SR. Review of onychocryptosis: epidemiology, pathogenesis, risk factors, diagnosis and treatment. Dermatol Online J. 2019;25:13030/qt9985w2n0
- Mayeaux EJ Jr, Carter C, Murphy TE. Ingrown toenail management. Am Fam Physician. 2019;100:158-164.
- Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555. doi:10.1370/afm.1712
- Watabe A, Yamasaki K, Hashimoto A, et al. Retrospective evaluation of conservative treatment for 140 ingrown toenails with a novel taping procedure. Acta Derm Venereol. 2015;95:822-825. doi:10.2340/00015555-2065
- Arai H, Haneke E. Noninvasive treatment for ingrown nails: anchor taping, acrylic affixed gutter splint, sculptured nail, and others. In: Baran R, Hadj-Rabia S, Silverman R, eds. Pediatric Nail Disorders. CRC Press; 2017:252-274.
Practice Gap
Onychocryptosis, colloquially known as an ingrown nail, most commonly affects the lateral folds of the toenails. It also can affect the fingernails and the distal aspect of the nail unit, though these presentations are not as well described in the literature. In onychocryptosis, the nail plate grows downward into the periungual skin, resulting in chronic pain and inflammation. Risk factors include overtrimming the nails with rounded edges, local trauma, nail surgery, wearing tight footwear, obesity, and onychomycosis.1
Although surgical intervention might be required for severe or refractory disease, conservative treatment options are first line and often curative. A variety of techniques have been designed to separate the ingrown portion of the nail plate from underlying skin, including placement of an intervening piece of dental floss, cotton, or plastic tubing.2
Anchor taping is another effective method of treating onychocryptosis; a strip of tape is used to gently pull and secure the affected nail fold away from the overlying nail plate. This technique has been well described for the treatment of onychocryptosis of the lateral toenail.3-5 In 2017, Arai and Haneke5 presented a modified technique for the treatment of distal disease.
We present a simplified method that was used successfully in a case of distal onychocryptosis of the thumbnail that occurred approximately 4 months after complete nail avulsion with a nail matrix biopsy (Figure 1).
The Technique
A strongly adhesive, soft cotton, elastic tape that is 1-inch wide, such as Elastikon Elastic Tape (Johnson & Johnson), is used to pull and secure the hyponychium away from the overlying nail plate. When this technique is used for lateral onychocryptosis, a single strip of tape is secured to the affected lateral nail fold, pulled obliquely and proximally, and secured to the base of the digit.3-5 In the Arai and Haneke5 method for the treatment of distal disease, a piece of tape is first placed at the distal nail fold, pulled proximally, and secured to the ventral aspect of the digit. Then, 1 or 2 additional strips of tape are applied to the lateral nail folds, pulled obliquely, and adhered to the base of the digit, as in the classic technique for lateral onychocryptosis.5
In our modification for the treatment of distal disease, only 2 strips of tape are required, each approximately 5-cm long. The first strip of tape is applied to the hyponychium parallel to the long axis of the finger, pulled away from the distal edge of the nail plate, and secured obliquely and proximally to the base of the finger on one side. The second strip of tape is applied to the hyponychium in the same manner, directly overlying the first strip, but is then pulled obliquely in the opposite direction and secured to the other side of the proximal finger (Figure 2). The 2 strips of tape are applied directly overlying each other at the distal nail fold but with opposing tension vectors to optimize pull on the distal nail fold. This modification eliminates the need to apply an initial strip of tape along the long axis of the digit, as described by Arai and Haneke.5
The patient is instructed on this method in the office and will change the tape at home daily for 2 to 6 weeks, until the nail plate has grown out over the hyponychium (Figure 3). This technique also can be combined with other modalities, such as dilute vinegar soaks performed daily after changing the tape to ease inflammation and prevent infection. Because strongly adhesive tape is used, it also is recommended that the patient soak the tape before removing it to prevent damage to underlying skin.
Practice Implications
Anchor taping is a common and effective treatment of onychocryptosis. Most techniques described in the literature are for lateral toenail cases, which often are managed by podiatry. A modification for the treatment of distal onychocryptosis has been previously described.5 We describe a similar modification using 2 tape strips pulled in opposite directions, which successfully resolved a case of distal onychocryptosis of the fingernail that developed following a nail procedure.
Because nail dystrophy is a relatively common complication of nail surgery, dermatologic surgeons should be aware of this simple, cost-effective, and noninvasive technique for the treatment of distal onychocryptosis.
Practice Gap
Onychocryptosis, colloquially known as an ingrown nail, most commonly affects the lateral folds of the toenails. It also can affect the fingernails and the distal aspect of the nail unit, though these presentations are not as well described in the literature. In onychocryptosis, the nail plate grows downward into the periungual skin, resulting in chronic pain and inflammation. Risk factors include overtrimming the nails with rounded edges, local trauma, nail surgery, wearing tight footwear, obesity, and onychomycosis.1
Although surgical intervention might be required for severe or refractory disease, conservative treatment options are first line and often curative. A variety of techniques have been designed to separate the ingrown portion of the nail plate from underlying skin, including placement of an intervening piece of dental floss, cotton, or plastic tubing.2
Anchor taping is another effective method of treating onychocryptosis; a strip of tape is used to gently pull and secure the affected nail fold away from the overlying nail plate. This technique has been well described for the treatment of onychocryptosis of the lateral toenail.3-5 In 2017, Arai and Haneke5 presented a modified technique for the treatment of distal disease.
We present a simplified method that was used successfully in a case of distal onychocryptosis of the thumbnail that occurred approximately 4 months after complete nail avulsion with a nail matrix biopsy (Figure 1).
The Technique
A strongly adhesive, soft cotton, elastic tape that is 1-inch wide, such as Elastikon Elastic Tape (Johnson & Johnson), is used to pull and secure the hyponychium away from the overlying nail plate. When this technique is used for lateral onychocryptosis, a single strip of tape is secured to the affected lateral nail fold, pulled obliquely and proximally, and secured to the base of the digit.3-5 In the Arai and Haneke5 method for the treatment of distal disease, a piece of tape is first placed at the distal nail fold, pulled proximally, and secured to the ventral aspect of the digit. Then, 1 or 2 additional strips of tape are applied to the lateral nail folds, pulled obliquely, and adhered to the base of the digit, as in the classic technique for lateral onychocryptosis.5
In our modification for the treatment of distal disease, only 2 strips of tape are required, each approximately 5-cm long. The first strip of tape is applied to the hyponychium parallel to the long axis of the finger, pulled away from the distal edge of the nail plate, and secured obliquely and proximally to the base of the finger on one side. The second strip of tape is applied to the hyponychium in the same manner, directly overlying the first strip, but is then pulled obliquely in the opposite direction and secured to the other side of the proximal finger (Figure 2). The 2 strips of tape are applied directly overlying each other at the distal nail fold but with opposing tension vectors to optimize pull on the distal nail fold. This modification eliminates the need to apply an initial strip of tape along the long axis of the digit, as described by Arai and Haneke.5
The patient is instructed on this method in the office and will change the tape at home daily for 2 to 6 weeks, until the nail plate has grown out over the hyponychium (Figure 3). This technique also can be combined with other modalities, such as dilute vinegar soaks performed daily after changing the tape to ease inflammation and prevent infection. Because strongly adhesive tape is used, it also is recommended that the patient soak the tape before removing it to prevent damage to underlying skin.
Practice Implications
Anchor taping is a common and effective treatment of onychocryptosis. Most techniques described in the literature are for lateral toenail cases, which often are managed by podiatry. A modification for the treatment of distal onychocryptosis has been previously described.5 We describe a similar modification using 2 tape strips pulled in opposite directions, which successfully resolved a case of distal onychocryptosis of the fingernail that developed following a nail procedure.
Because nail dystrophy is a relatively common complication of nail surgery, dermatologic surgeons should be aware of this simple, cost-effective, and noninvasive technique for the treatment of distal onychocryptosis.
- Geizhals S, Lipner SR. Review of onychocryptosis: epidemiology, pathogenesis, risk factors, diagnosis and treatment. Dermatol Online J. 2019;25:13030/qt9985w2n0
- Mayeaux EJ Jr, Carter C, Murphy TE. Ingrown toenail management. Am Fam Physician. 2019;100:158-164.
- Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555. doi:10.1370/afm.1712
- Watabe A, Yamasaki K, Hashimoto A, et al. Retrospective evaluation of conservative treatment for 140 ingrown toenails with a novel taping procedure. Acta Derm Venereol. 2015;95:822-825. doi:10.2340/00015555-2065
- Arai H, Haneke E. Noninvasive treatment for ingrown nails: anchor taping, acrylic affixed gutter splint, sculptured nail, and others. In: Baran R, Hadj-Rabia S, Silverman R, eds. Pediatric Nail Disorders. CRC Press; 2017:252-274.
- Geizhals S, Lipner SR. Review of onychocryptosis: epidemiology, pathogenesis, risk factors, diagnosis and treatment. Dermatol Online J. 2019;25:13030/qt9985w2n0
- Mayeaux EJ Jr, Carter C, Murphy TE. Ingrown toenail management. Am Fam Physician. 2019;100:158-164.
- Tsunoda M, Tsunoda K. Patient-controlled taping for the treatment of ingrown toenails. Ann Fam Med. 2014;12:553-555. doi:10.1370/afm.1712
- Watabe A, Yamasaki K, Hashimoto A, et al. Retrospective evaluation of conservative treatment for 140 ingrown toenails with a novel taping procedure. Acta Derm Venereol. 2015;95:822-825. doi:10.2340/00015555-2065
- Arai H, Haneke E. Noninvasive treatment for ingrown nails: anchor taping, acrylic affixed gutter splint, sculptured nail, and others. In: Baran R, Hadj-Rabia S, Silverman R, eds. Pediatric Nail Disorders. CRC Press; 2017:252-274.
Autoeczematization: A Strange Id Reaction of the Skin
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6
Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16
Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6
Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16
Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6
Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16
Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
Practice Points
- Autoeczematization, or id reaction, is a disseminated reaction of the skin occurring at a site distant to a primary cutaneous infection or stimulus.
- T lymphocytes and keratinocytes are postulated to be involved in the pathogenesis of id reactions.
- Therapy includes treating the underlying pathology while providing topical corticosteroids for the autoeczematous lesions.
Atopic Dermatitis Oral Therapies: What Are Patients Learning on YouTube?
To the Editor:
Oral immunosuppressive therapies are prescribed for moderate to severe atopic dermatitis. Patients often consult YouTube to make informed decisions about these therapies. In the United States, most health-related online searches are initiated through a search engine, which frequently leads to social media sites such as YouTube. Recent studies have examined the reasons why users turn to the Internet for health-related information, indicating that users typically seek specific information regarding health concerns.1,2 Furthermore, social media platforms such as YouTube are a popular means of sharing health information with the public.3-5 Currently, YouTube has more than 1 billion registered users, and 30 million health-related videos are watched each day.6 Almost one-third of US consumers use YouTube, Facebook, and Twitter to obtain medical information.7 YouTube is a versatile tool because of its video-discovery mechanisms such as a keyword-based search engine, video-recommendation system, highlight feature for videos on home pages, and the capacity to embed YouTube videos on various web pages.8 Searchers use videos that are short, fast paced, emotion evoking, from credible sources, recently uploaded, and relevant to the searcher for aiding in health decisions.9 Furthermore, studies have demonstrated YouTube’s capacity to support a change in attitude and increase users’ knowledge. In fact, YouTube had higher impact on recall, attitudes, and behaviors when compared with written materials on other social media platforms, such as Facebook and Twitter.9 We conducted a cross-sectional study to examine the quality of YouTube videos on oral therapies for atopic dermatitis, such as cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil.
On April 23, 2020, we performed 8 searches using a private browser with default filters on YouTube (Figure). Injectables were not included in the analysis, as the YouTube experience on dupilumab previously has been investigated.10 The top 40 videos from each search were screened by 3 researchers. Duplicates, non–English-language videos, and videos that did not discuss atopic dermatitis or oral therapies were excluded, resulting in 73 videos included in this analysis. Testimonials generated by patients made up 39 of 73 (53.4%) videos. Health care professionals created 23 of 73 (31.5%) videos, and educators with financial interest created 11 of 73 (15.1%) videos. The dates of production for the videos spanned from 2008 to 2020.
The major topics addressed in the videos were symptomatic changes (63 [68.8% of all topics discussed]), adverse effects (52 [67.5%]), and quality-of-life changes (37 [48.1%]). Of the videos included, the majority (42/73 [57.5%]) contained a neutral tone about the medication, citing advantages and disadvantages with therapy, while 22 of 73 (30.1%) had an encouraging tone, and 9 of 73 (12.3%) had a discouraging tone. Regarding videos with positive tones, there were 17 videos on cyclosporine, 9 on azathioprine, 7 on methotrexate, 4 on oral steroids, and 2 on mycophenolate mofetil. Regarding videos with negative tones, there were 4 on cyclosporine, 3 on azathioprine, 2 on methotrexate, and 2 on mycophenolate mofetil.
Of the videos made with financial interest, the majority (28/34 [77.8%]) were more suitable for informing health care providers rather than patients, containing jargon as well as complex information on clinical trials, dosing, and mechanisms of action. From the videos discussing clinical recommendations, there were 9 of 73 (12.3%) Grade A recommendations (eg, citing evidence-based information and clinical trials) and 64 of 73 (87.7%) Grade B recommendations (eg, anecdotal information on patient experience). Thirty-seven of 73 (50.7%) videos were evidence based, and 36 of 73 (49.3%) were non–evidence based. Six videos were patient-oriented news broadcasts.
Patient-generated testimonials had the most views (mean, 9238.4) and highest interaction ratio (the sum of likes, dislikes, and comments divided by the number of views)(mean, 0.027), while health care provider–generated videos had fewer views (mean, 9218.7) and a lower interaction ratio (mean, 0.011). Financial-based videos had 4233.4 views on average, with an average interaction ratio of 0.014. Based on these results, biased, patient-generated content comprised greater than 50% of YouTube videos about oral therapies for atopic dermatitis and was quite likely to be engaged with by users. Thus, these patient testimonials have great potential to affect decision-making.
The high number of patient-generated videos about oral therapies was consistent with prior studies of YouTube videos about therapies for numerous conditions.11-13 Dermatologists should consider utilizing YouTube for providing evidence-based, patient-oriented information about novel therapeutics. They may consider collaborating with patients to assist with their creation of YouTube videos and directing patients to credible resources by the American Academy of Dermatology and Canadian Dermatology Association for decision-making.
Importantly, this analysis is limited by its lack of quality-assessment tools for video-based resources such as JAMA score and DISCERN score.14,15 However, these metrics have limited ability to evaluate audiovisual elements, indicating the need for novel tools to score their validity.
- Fox S, Duggan M. Health online 2013. January 15, 2013. Accessed August 15, 2021. https://www.pewresearch.org/internet/2013/01/15/health-online-2013/
- Ní Ríordáin R, McCreary C. Dental patients’ use of the Internet. Br Dent J. 2009;207:583-586, 575.
- Fergie G, Hilton S, Hunt K. Young adults’ experiences of seeking online information about diabetes and mental health in the age of social media. Health Expect. 2016;19:1324-1335.
- Antheunis ML, Tates K, Nieboer TE. Patients’ and health professionals’ use of social media in health care: motives, barriers and expectations. Patient Educ Couns. 2013;92:426-431.
- McGregor F, Somner JE, Bourne RR, et al. Social media use by patients with glaucoma: what can we learn? Ophthalmic Physiol Opt. 2014;34:46-52.
- YouTube Statistics—15 Amazing Stats for 2015. Published April 30, 2015. Accessed August 27, 2021. YouTube.com/watch?v=9ZLBSPzY7GQ
- Health Research Institute. Social media “likes” healthcare: from marketing to social business. April 2012. Accessed August 15, 2021. https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf
- Zhou R, Khemmarat S, Gao L, et al. How YouTube videos are discovered and its impact on videos views. Multimed Tools Appl. 2016;75:6035-6058.
- Haslam K, Doucette H, Hachey S, et al. YouTube videos as health decision aids for the public: an integrative review. Can J Dent Hyg. 2019;53:53-66.
- Pithadia D, Reynolds K, Lee E, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube [published online ahead of print April 16,2020]? J Dermatolog Treat. doi: 10.1080/09546634.2020.1755418
- Tolu S, Yurdakul OV, Basaran B, et al. English-language videos on YouTube as a source of information on self-administer subcutaneous anti-tumour necrosis factor agent injections. Rheumatol Int. 2018;38:1285-1292.
- Reynolds KA, Pithadia DJ, Lee EB, et al. A cross-sectional study of YouTube videos about psoriasis biologics. Int J Dermatol. 2019;58:E61-E62.
- Kocyigit BF, Akaltun MS. Does YouTube provide high quality information? assessment of secukinumab videos. Rheumatol Int. 2019;39:1263-1268.
- Qi J, Trang T, Doong J, et al. Misinformation is prevalent in psoriasis-related YouTube videos. Dermatol Online J. 2016;22:13030/qt7qc9z2m5
- Gokcen HB, Gumussuyu G. A quality analysis of disc herniation videos on YouTube. World Neurosurg. 2019;124:E799-E804.
To the Editor:
Oral immunosuppressive therapies are prescribed for moderate to severe atopic dermatitis. Patients often consult YouTube to make informed decisions about these therapies. In the United States, most health-related online searches are initiated through a search engine, which frequently leads to social media sites such as YouTube. Recent studies have examined the reasons why users turn to the Internet for health-related information, indicating that users typically seek specific information regarding health concerns.1,2 Furthermore, social media platforms such as YouTube are a popular means of sharing health information with the public.3-5 Currently, YouTube has more than 1 billion registered users, and 30 million health-related videos are watched each day.6 Almost one-third of US consumers use YouTube, Facebook, and Twitter to obtain medical information.7 YouTube is a versatile tool because of its video-discovery mechanisms such as a keyword-based search engine, video-recommendation system, highlight feature for videos on home pages, and the capacity to embed YouTube videos on various web pages.8 Searchers use videos that are short, fast paced, emotion evoking, from credible sources, recently uploaded, and relevant to the searcher for aiding in health decisions.9 Furthermore, studies have demonstrated YouTube’s capacity to support a change in attitude and increase users’ knowledge. In fact, YouTube had higher impact on recall, attitudes, and behaviors when compared with written materials on other social media platforms, such as Facebook and Twitter.9 We conducted a cross-sectional study to examine the quality of YouTube videos on oral therapies for atopic dermatitis, such as cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil.
On April 23, 2020, we performed 8 searches using a private browser with default filters on YouTube (Figure). Injectables were not included in the analysis, as the YouTube experience on dupilumab previously has been investigated.10 The top 40 videos from each search were screened by 3 researchers. Duplicates, non–English-language videos, and videos that did not discuss atopic dermatitis or oral therapies were excluded, resulting in 73 videos included in this analysis. Testimonials generated by patients made up 39 of 73 (53.4%) videos. Health care professionals created 23 of 73 (31.5%) videos, and educators with financial interest created 11 of 73 (15.1%) videos. The dates of production for the videos spanned from 2008 to 2020.
The major topics addressed in the videos were symptomatic changes (63 [68.8% of all topics discussed]), adverse effects (52 [67.5%]), and quality-of-life changes (37 [48.1%]). Of the videos included, the majority (42/73 [57.5%]) contained a neutral tone about the medication, citing advantages and disadvantages with therapy, while 22 of 73 (30.1%) had an encouraging tone, and 9 of 73 (12.3%) had a discouraging tone. Regarding videos with positive tones, there were 17 videos on cyclosporine, 9 on azathioprine, 7 on methotrexate, 4 on oral steroids, and 2 on mycophenolate mofetil. Regarding videos with negative tones, there were 4 on cyclosporine, 3 on azathioprine, 2 on methotrexate, and 2 on mycophenolate mofetil.
Of the videos made with financial interest, the majority (28/34 [77.8%]) were more suitable for informing health care providers rather than patients, containing jargon as well as complex information on clinical trials, dosing, and mechanisms of action. From the videos discussing clinical recommendations, there were 9 of 73 (12.3%) Grade A recommendations (eg, citing evidence-based information and clinical trials) and 64 of 73 (87.7%) Grade B recommendations (eg, anecdotal information on patient experience). Thirty-seven of 73 (50.7%) videos were evidence based, and 36 of 73 (49.3%) were non–evidence based. Six videos were patient-oriented news broadcasts.
Patient-generated testimonials had the most views (mean, 9238.4) and highest interaction ratio (the sum of likes, dislikes, and comments divided by the number of views)(mean, 0.027), while health care provider–generated videos had fewer views (mean, 9218.7) and a lower interaction ratio (mean, 0.011). Financial-based videos had 4233.4 views on average, with an average interaction ratio of 0.014. Based on these results, biased, patient-generated content comprised greater than 50% of YouTube videos about oral therapies for atopic dermatitis and was quite likely to be engaged with by users. Thus, these patient testimonials have great potential to affect decision-making.
The high number of patient-generated videos about oral therapies was consistent with prior studies of YouTube videos about therapies for numerous conditions.11-13 Dermatologists should consider utilizing YouTube for providing evidence-based, patient-oriented information about novel therapeutics. They may consider collaborating with patients to assist with their creation of YouTube videos and directing patients to credible resources by the American Academy of Dermatology and Canadian Dermatology Association for decision-making.
Importantly, this analysis is limited by its lack of quality-assessment tools for video-based resources such as JAMA score and DISCERN score.14,15 However, these metrics have limited ability to evaluate audiovisual elements, indicating the need for novel tools to score their validity.
To the Editor:
Oral immunosuppressive therapies are prescribed for moderate to severe atopic dermatitis. Patients often consult YouTube to make informed decisions about these therapies. In the United States, most health-related online searches are initiated through a search engine, which frequently leads to social media sites such as YouTube. Recent studies have examined the reasons why users turn to the Internet for health-related information, indicating that users typically seek specific information regarding health concerns.1,2 Furthermore, social media platforms such as YouTube are a popular means of sharing health information with the public.3-5 Currently, YouTube has more than 1 billion registered users, and 30 million health-related videos are watched each day.6 Almost one-third of US consumers use YouTube, Facebook, and Twitter to obtain medical information.7 YouTube is a versatile tool because of its video-discovery mechanisms such as a keyword-based search engine, video-recommendation system, highlight feature for videos on home pages, and the capacity to embed YouTube videos on various web pages.8 Searchers use videos that are short, fast paced, emotion evoking, from credible sources, recently uploaded, and relevant to the searcher for aiding in health decisions.9 Furthermore, studies have demonstrated YouTube’s capacity to support a change in attitude and increase users’ knowledge. In fact, YouTube had higher impact on recall, attitudes, and behaviors when compared with written materials on other social media platforms, such as Facebook and Twitter.9 We conducted a cross-sectional study to examine the quality of YouTube videos on oral therapies for atopic dermatitis, such as cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil.
On April 23, 2020, we performed 8 searches using a private browser with default filters on YouTube (Figure). Injectables were not included in the analysis, as the YouTube experience on dupilumab previously has been investigated.10 The top 40 videos from each search were screened by 3 researchers. Duplicates, non–English-language videos, and videos that did not discuss atopic dermatitis or oral therapies were excluded, resulting in 73 videos included in this analysis. Testimonials generated by patients made up 39 of 73 (53.4%) videos. Health care professionals created 23 of 73 (31.5%) videos, and educators with financial interest created 11 of 73 (15.1%) videos. The dates of production for the videos spanned from 2008 to 2020.
The major topics addressed in the videos were symptomatic changes (63 [68.8% of all topics discussed]), adverse effects (52 [67.5%]), and quality-of-life changes (37 [48.1%]). Of the videos included, the majority (42/73 [57.5%]) contained a neutral tone about the medication, citing advantages and disadvantages with therapy, while 22 of 73 (30.1%) had an encouraging tone, and 9 of 73 (12.3%) had a discouraging tone. Regarding videos with positive tones, there were 17 videos on cyclosporine, 9 on azathioprine, 7 on methotrexate, 4 on oral steroids, and 2 on mycophenolate mofetil. Regarding videos with negative tones, there were 4 on cyclosporine, 3 on azathioprine, 2 on methotrexate, and 2 on mycophenolate mofetil.
Of the videos made with financial interest, the majority (28/34 [77.8%]) were more suitable for informing health care providers rather than patients, containing jargon as well as complex information on clinical trials, dosing, and mechanisms of action. From the videos discussing clinical recommendations, there were 9 of 73 (12.3%) Grade A recommendations (eg, citing evidence-based information and clinical trials) and 64 of 73 (87.7%) Grade B recommendations (eg, anecdotal information on patient experience). Thirty-seven of 73 (50.7%) videos were evidence based, and 36 of 73 (49.3%) were non–evidence based. Six videos were patient-oriented news broadcasts.
Patient-generated testimonials had the most views (mean, 9238.4) and highest interaction ratio (the sum of likes, dislikes, and comments divided by the number of views)(mean, 0.027), while health care provider–generated videos had fewer views (mean, 9218.7) and a lower interaction ratio (mean, 0.011). Financial-based videos had 4233.4 views on average, with an average interaction ratio of 0.014. Based on these results, biased, patient-generated content comprised greater than 50% of YouTube videos about oral therapies for atopic dermatitis and was quite likely to be engaged with by users. Thus, these patient testimonials have great potential to affect decision-making.
The high number of patient-generated videos about oral therapies was consistent with prior studies of YouTube videos about therapies for numerous conditions.11-13 Dermatologists should consider utilizing YouTube for providing evidence-based, patient-oriented information about novel therapeutics. They may consider collaborating with patients to assist with their creation of YouTube videos and directing patients to credible resources by the American Academy of Dermatology and Canadian Dermatology Association for decision-making.
Importantly, this analysis is limited by its lack of quality-assessment tools for video-based resources such as JAMA score and DISCERN score.14,15 However, these metrics have limited ability to evaluate audiovisual elements, indicating the need for novel tools to score their validity.
- Fox S, Duggan M. Health online 2013. January 15, 2013. Accessed August 15, 2021. https://www.pewresearch.org/internet/2013/01/15/health-online-2013/
- Ní Ríordáin R, McCreary C. Dental patients’ use of the Internet. Br Dent J. 2009;207:583-586, 575.
- Fergie G, Hilton S, Hunt K. Young adults’ experiences of seeking online information about diabetes and mental health in the age of social media. Health Expect. 2016;19:1324-1335.
- Antheunis ML, Tates K, Nieboer TE. Patients’ and health professionals’ use of social media in health care: motives, barriers and expectations. Patient Educ Couns. 2013;92:426-431.
- McGregor F, Somner JE, Bourne RR, et al. Social media use by patients with glaucoma: what can we learn? Ophthalmic Physiol Opt. 2014;34:46-52.
- YouTube Statistics—15 Amazing Stats for 2015. Published April 30, 2015. Accessed August 27, 2021. YouTube.com/watch?v=9ZLBSPzY7GQ
- Health Research Institute. Social media “likes” healthcare: from marketing to social business. April 2012. Accessed August 15, 2021. https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf
- Zhou R, Khemmarat S, Gao L, et al. How YouTube videos are discovered and its impact on videos views. Multimed Tools Appl. 2016;75:6035-6058.
- Haslam K, Doucette H, Hachey S, et al. YouTube videos as health decision aids for the public: an integrative review. Can J Dent Hyg. 2019;53:53-66.
- Pithadia D, Reynolds K, Lee E, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube [published online ahead of print April 16,2020]? J Dermatolog Treat. doi: 10.1080/09546634.2020.1755418
- Tolu S, Yurdakul OV, Basaran B, et al. English-language videos on YouTube as a source of information on self-administer subcutaneous anti-tumour necrosis factor agent injections. Rheumatol Int. 2018;38:1285-1292.
- Reynolds KA, Pithadia DJ, Lee EB, et al. A cross-sectional study of YouTube videos about psoriasis biologics. Int J Dermatol. 2019;58:E61-E62.
- Kocyigit BF, Akaltun MS. Does YouTube provide high quality information? assessment of secukinumab videos. Rheumatol Int. 2019;39:1263-1268.
- Qi J, Trang T, Doong J, et al. Misinformation is prevalent in psoriasis-related YouTube videos. Dermatol Online J. 2016;22:13030/qt7qc9z2m5
- Gokcen HB, Gumussuyu G. A quality analysis of disc herniation videos on YouTube. World Neurosurg. 2019;124:E799-E804.
- Fox S, Duggan M. Health online 2013. January 15, 2013. Accessed August 15, 2021. https://www.pewresearch.org/internet/2013/01/15/health-online-2013/
- Ní Ríordáin R, McCreary C. Dental patients’ use of the Internet. Br Dent J. 2009;207:583-586, 575.
- Fergie G, Hilton S, Hunt K. Young adults’ experiences of seeking online information about diabetes and mental health in the age of social media. Health Expect. 2016;19:1324-1335.
- Antheunis ML, Tates K, Nieboer TE. Patients’ and health professionals’ use of social media in health care: motives, barriers and expectations. Patient Educ Couns. 2013;92:426-431.
- McGregor F, Somner JE, Bourne RR, et al. Social media use by patients with glaucoma: what can we learn? Ophthalmic Physiol Opt. 2014;34:46-52.
- YouTube Statistics—15 Amazing Stats for 2015. Published April 30, 2015. Accessed August 27, 2021. YouTube.com/watch?v=9ZLBSPzY7GQ
- Health Research Institute. Social media “likes” healthcare: from marketing to social business. April 2012. Accessed August 15, 2021. https://www.pwc.com/us/en/health-industries/health-research-institute/publications/pdf/health-care-social-media-report.pdf
- Zhou R, Khemmarat S, Gao L, et al. How YouTube videos are discovered and its impact on videos views. Multimed Tools Appl. 2016;75:6035-6058.
- Haslam K, Doucette H, Hachey S, et al. YouTube videos as health decision aids for the public: an integrative review. Can J Dent Hyg. 2019;53:53-66.
- Pithadia D, Reynolds K, Lee E, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube [published online ahead of print April 16,2020]? J Dermatolog Treat. doi: 10.1080/09546634.2020.1755418
- Tolu S, Yurdakul OV, Basaran B, et al. English-language videos on YouTube as a source of information on self-administer subcutaneous anti-tumour necrosis factor agent injections. Rheumatol Int. 2018;38:1285-1292.
- Reynolds KA, Pithadia DJ, Lee EB, et al. A cross-sectional study of YouTube videos about psoriasis biologics. Int J Dermatol. 2019;58:E61-E62.
- Kocyigit BF, Akaltun MS. Does YouTube provide high quality information? assessment of secukinumab videos. Rheumatol Int. 2019;39:1263-1268.
- Qi J, Trang T, Doong J, et al. Misinformation is prevalent in psoriasis-related YouTube videos. Dermatol Online J. 2016;22:13030/qt7qc9z2m5
- Gokcen HB, Gumussuyu G. A quality analysis of disc herniation videos on YouTube. World Neurosurg. 2019;124:E799-E804.
Practice Points
- Patient-based YouTube videos comprised the majority of videos on oral therapies for atopic dermatitis, with the greatest views and interaction ratio.
- Most YouTube videos on this topic contained a neutral tone and Grade B recommendations, thus meriting production of more evidence-based videos in collaboration with patients on the YouTube platform.