User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Sunscreens Causing Cancer? The Facts
Skin cancer is the most common form of cancer in the United States and continues to rise in incidence and mortality each year.1 It is common knowledge that UV light plays a major role in the development of skin cancer.2,3 Studies have long demonstrated that using sunscreen on a daily basis can help prevent the development of skin cancer, premature aging, and exacerbation of photodermatoses.4-7 Although there are several photoprotective measures available, sunscreen remains the most popular and widely used among patients.8 Sunscreens that are on the market today contain either organic or inorganic UV filters or a combination of both based on their chemical composition and photoprotection mechanisms.9 Concerns about these ingredients causing cancer have created confusion among consumers. I will attempt to clarify these concerns by critically analyzing available evidence-based data on sunscreen use so that as dermatology residents we will be more knowledgeable about sunscreen safety topics and will be able to provide accurate and up-to-date information to our patients.
Organic UV Filters
Organic UV filters are classified as aromatic compounds that provide photoprotection by absorbing UV light.10 Aside from the photoallergic potential of organic UV filters, controversy has arisen in response to studies reporting their possible hormone disruptive effects.11-18 Although there are several US Food and Drug Administration (FDA)–approved organic UV filters in use today, one of the most commonly manufactured and controversial agents is oxybenzone.10 Claims regarding the estrogenic and antiandrogenic effects of oxybenzone have been investigated with results refuting the claims or concluding that more sensitive studies are needed to determine if these organic ingredients pose such risks.10,19,20 One study demonstrated that nearly 300 years of daily sunscreen application would be needed to reach similar exposure levels of oxybenzone used and described in prior animal studies.21 Additionally, most of the studied adverse effects of UV filters have been evaluated based on oral exposure rather than actual dermal application.11 Although these compounds are absorbed systemically, studies have reported that the amounts are insignificant and noncumulative in the body.10,22-24 Furthermore, the binding affinity of oxybenzone for estrogen receptors has been shown to be much weaker and near insignificant compared to estrogen and estradiol.24,25 Although numerous important studies examining systemic absorption have not shown a clinically significant disruption of hormonal homeostasis or acute toxicity in humans by organic UV filters, further studies are needed.
Inorganic UV Filters
Used as the main active ingredients in sunscreen for decades, titanium dioxide (TiO2) and zinc oxide (ZnO) compounds generally are more photostable and less photoallergic than their organic counterparts.10 In recent years, the safety of these long-used photoprotectors has been questioned because of the development of nanoparticle (<100 nm) formulas that are less opaque on application. Although this formula provides a thin, transparent, and cosmetically appealing medium, there is concern that the metal oxides penetrate the skin and cause local and systemic toxicities.26-28 Several recent scientific studies have shown no percutaneous permeation of these particles in normal adult human skin and reported no causal damage to mammalian cells.10,29-31 Although skin penetration of TiO2 and ZnO has been described as insignificant, focus has shifted to health risks associated with inhaling TiO2 through the use of spray or powder products following statements made by the International Agency for Research on Cancer in 2006.32 Several studies investigating increased health risks, specifically lung cancer, in factory workers who were subjected to TiO2 and ZnO inhalation concluded that exposure was unlikely to pose substantial health risks or subchronic toxicity.33,34 Despite a relatively strong safety profile, a major concern of using these metal oxides as UV filters has been potential free radical formation.35-39 For this reason, the Scientific Committee on Emerging and Newly Identified Health Risks extensively researched and delivered opinions on the use of TiO2 and ZnO in cosmetics, concluding that topical application of either compound does not result in toxicity or other adverse effects.30,40-42 Additionally, an effort has been made by manufacturers to encapsulate nanoparticles with magnesium and other materials to quench the reactive oxygen species along with the human body’s own antioxidant defense system.10 In summary, it appears that the current weight of scientific evidence suggests that percutaneous absorption and toxicity by UV filters in humans may be overestimated and that the use of nanoparticles in sunscreens poses no or negligible potential risks to human health.43,44
Concerns Beyond Organic and Inorganic UV Filters
Beyond these concerns with organic and inorganic UV filters, there are several other claims regarding sunscreen safety that have stirred up controversy, including the side-effect profile of retinyl palmitate, vitamin D deficiency, phototoxicity, environmental effects, futility of sun protection factor levels greater than 50, and increased health risks in children. Although some studies report mixed results, the majority of scientific investigations have addressed and refuted several of these claims, again confirming the relative safety of sunscreen use. It is beyond the scope of this article to further discuss these topics specifically. However, it is worth mentioning that consumer studies report that the actual use of sunscreens is 0.5 mg/cm2 or less compared to the ideal application of 2 mg/cm2, thereby confounding many of the claims made about sunscreen use, such as vitamin D deficiency.45 Sunscreens often contain a combination of several UV filters. To date, only a few existing studies have shown that mixtures of the photoprotective agents discussed might interact and exhibit toxic activity when combined, even when there is no observed adverse toxic effect when used individually in products.46-48
The current FDA ruling on sunscreen labeling does not require manufacturers to state if inorganic UV filters have been formulated into nanoparticles; however, manufacturers are now required to include a statement on all sunscreen labels warning consumers to avoid using sunscreen on damaged or broken skin49 in an effort to prevent the active ingredients from getting under the skin, potentially causing inflammation and/or health risks, because available data do not provide conclusive evidence on increased penetration of open skin.50 Additional information regarding the 2011 FDA sunscreen ruling can be found in a prior Cutis Resident Corner column.51
Final Thoughts
As health care providers, we should take advantage of opportunities to educate our patients about other sun safety practices, such as avoiding excessive sun exposure during peak hours (10 am to 2 pm), seeking shade, and wearing photoprotective clothing (eg, wide-brimmed hats, sunglasses).
The research is quite clear: Using broadband sunscreens that absorb and/or block UV radiation results in reduced damage to the skin’s DNA, a fact that should be considered when taking into account the risks and benefits of sunscreen use.2,3 Although sunscreen use is highly recommended in addition to the other sun protection methods, it is ultimately the patient’s choice. If a patient is still concerned about the active ingredients of UV filters, even given the high probability of safety, there are products available on the market that do not include organic filters or nanoparticles. Given the established benefits of UV protection, the use of sunscreens remain one of the most important photoprotective methods, and with increased usage by the public, continuous monitoring of the overall safety and benefit profile of future products is prudent.
1. Skin cancer statistics. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/cancer/skin/statistics/index.htm. Updated September 2, 2014. Accessed December 30, 2014.
2. World Health Organization, International Agency for Research on Cancer. Solar and ultraviolet radiation. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 55. Lyon, France: International Agency for Research on Cancer; 1992.
3. Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257-263.
4. Darlington S, Williams G, Neale R, et al. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol. 2003;139:451-455.
5. Van der Pols JC, Williams GM, Pandeya N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546-2548.
6. Hughes MC, Williams GM, Baker P, et al. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med. 2013;158:781-790.
7. Bissonnette R, Nigen S, Bolduc C. Influence of the quantity of sunscreen applied on the ability to protect against ultraviolet-induced polymorphous light eruption. Photodermatol Photoimmunol Photomed. 2012;28:240-243.
8. Cancer trends progress report 2011/2012 update: sun protection. National Cancer Institute Web site. http://progressreport.cancer.gov/doc_detail.asp?pid¡1&did¡2009&chid¡91&coid¡911. Accessed December 30, 2014.
9. Sunscreen Drug Products for Over-the-counter Human Use, 21 CFR §352.10. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=352.10. Updated September 1, 2014. Accessed December 30, 2014.
10. Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photoimmunol Photomed. 2011;27:58-67.
11. Krause M, Klit A, Blomberg Jensen M, et al. Sunscreens: are they beneficial for health? an overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35:424-436.
12. Schlumpf M, Cotton B, Conscience M, et al. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109:239-244.
13. Schlumpf M, Schmid P, Durrer S, et al. Endocrine activity and developmental toxicity of cosmetic UV filters–an update. Toxicol. 2004;205:113-122.
14. Schlumpf M, Kypke K, Vökt C, et al. Endocrine active UV filters: developmental toxicity and exposure through breast milk. Chimia. 2008;62:345-351.
15. Nakagawa Y, Suzuki T. Metabolism of 2-hydroxy-4-methoxybenzophenone in isolated rat hepatocytes and xenoestrogenic effects of its metabolites on MCF-7 human breast cancer cells. Chem Biol Interact. 2002;139:115-128.
16. Ma R, Cotton B, Lichtensteiger W, et al. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol Sci. 2003;74:43-50.
17. Heneweer M, Muusse M, van den Berg M, et al. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
18. Knobler E, Almeida L, Ruzkowski AM, et al. Photoallergy to benzophenone. Arch Dermatol. 1989;125:801-804.
19. Draelos ZD. Are sunscreens safe? J Cosmet Dermatol. 2010;9:1-2.
20. Gilbert E, Pirot F, Bertholle V. Commonly used UV filter toxicity on biological functions: review of last decade studies. Int J of Cosmet Sci. 2013;35:208-219.
21. Wang SQ, Burnett ME, Lim HW. Safety of oxybenzone: putting numbers into perspective. Arch Dermatol. 2011;147:865-866.
22. Mancebo SE, Hu JY, Wang SQ. Sunscreens: a review of health benefits, regulations, and controversies. Dermatol Clin. 2014;32:427-438.
23. Jansen R, Osterwalder U, Wang SQ, et al. Photoprotection: part II. sunscreen: development, efficacy, and controversies. J Am Acad Dermatol. 2013;69:867.e1-867.e14.
24. Janjua NR, Mogensen B, Andersson AM, et al. Systemic absorption of the sunscreens benzo- phenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzy-lidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol. 2004;123:57-61.
25. Kadry AM, Chukwuemeka SO, Mohamed S, et al. Pharmacokinetics of benzophenone-3 after oral exposure in male rats. J Appl Toxicol. 1995;15:97-102.
26. Gulson B, McCall M, Korsch M, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci. 2010;118:140-149.
27. Gulson B, Wong H, Korsch M, et al. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Sci Total Environ. 2012:420:313-318.
28. Benech-Kieffer F, Meuling WJ, Leclerc C, et al. Percutaneous absorption of Mexoryl SX in human volunteers: comparison with in vitro data. Skin Pharmacol Appl Skin Physiol. 2003;16:343-355.
29. Nash JF. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatol Clin. 2006;24:35-51.
30. Nohynek GJ, Lademann J, Ribaud C, et al. Grey goo on the skin? nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37:251-277.
31. Sadrieh N, Wokovich AM, Gopee NV, et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol Sci. 2010;115:156-166.
32. International Agency for Research on Cancer. Carbon black, titanium dioxide, and talc. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 93. Lyon, France: International Agency for Research on Cancer; 2006.
33. Liao CM, Chiang YH, Chio CP. Model-based assessment for human inhalation exposure risk to airborne nano/fine titanium dioxide particles. Sci Total Environ. 2008:15;407:165-177.
34. Adamcakova-Dodd A, Stebounova LV, Kim JS, et al. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 2014;11:15.
35. Wamer WG, Yin JJ, Wei RR. Oxidative damage to nucleic acids photosensitized by titanium dioxide. Free Radic Biol Med. 1997;23:851-858.
36. Nakagawa Y, Wakuri S, Sakamoto K, et al. The photogenotoxicity of titanium dioxide particles. Mutat Res. 1997;394:125-132.
37. Dunford R, Salinaro A, Cai L, et al. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418:87-90, 99.
38. Hidaka H, Kobayashi H, Koike T, et al. DNA damage photoinduced by cosmetic pigments and sunscreen agents under solar exposure and artificial UV illumination. J Oleo Sci. 2006;55:249-261.
39. Dufour EK, Kumaravel T, Nohynek GJ, et al. Clastogenicity, photo-clastogenicity or pseudo-photo-clastogenicity: genotoxic effects of zinc oxide in the dark, in pre-irradiated or simultaneously irradiated Chinese hamster ovary cells [published online ahead of print June 21, 2006]. Mutat Res. 2006;607:215-224.
40. Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers concerning titanium dioxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out135_en.pdf. Published October 24, 2000. Accessed December 30, 2014.
41. The Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers opinion concerning zinc oxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out222_en.pdf. Published June 24-25, 2003. Accessed December 30, 2014.
42. Hackenberg S, Friehs G, Kessler M, et al. Nanosized titanium dioxide particles do not induce DNA damage in human peripheral blood lymphocytes. Environ Mol Mutagen. 2010;52:264-268.
43. Bach-Thomsen M, Wulf HC. Sunbather’s application of sunscreen is probably inadequate to obtain the sun protection factor assigned to the preparation. Photodermatol Photoimmunol Photomed. 1993:9;242-244.
44. Nohynek GJ, Antignac E, Re T, et al. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol. 2010:1;243:239-259.
45. Diffey BL. Sunscreens: use and misuse. In: Giacomoni PU, ed. Sun Protection in Man. Vol 3. Amsterdam, the Netherlands: Elsevier Science BV; 2001:521-534.
46. Heneweer M, Muusse M, Van den BM, et al. Additive estrogenic effects of mixtures of frequently used UV-filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
47. Kunz PY, Galicia HF, Fent K. Comparison of in vitro and in vivo estrogenic activity of UV-filters in fish. Toxicol Sci. 2006;90:349-361.
48. Kortenkamp A, Faust M, Scholze M, et al. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect. 2007;115(suppl 1):106-114.
49. Labeling and effectiveness testing: sunscreen drug products for over-the-counter human use—small entity compliance guide. US Food and Drug Administration Web site. http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm330694.htm. Published December 2012. Updated May 13, 2014. Accessed December 30, 2014.
50. Schafer-Korting M, Korting HC, Ponce-Poschl E. Liposomal tretinoin for uncomplicated acne vulgaris. Clin Investig. 1994;72:1086-1091.
51. Bronfenbrener R. Simplifying sun safety: a guide to the new FDA sunscreen monograph. Cutis. 2014;93:e17-e19.
Skin cancer is the most common form of cancer in the United States and continues to rise in incidence and mortality each year.1 It is common knowledge that UV light plays a major role in the development of skin cancer.2,3 Studies have long demonstrated that using sunscreen on a daily basis can help prevent the development of skin cancer, premature aging, and exacerbation of photodermatoses.4-7 Although there are several photoprotective measures available, sunscreen remains the most popular and widely used among patients.8 Sunscreens that are on the market today contain either organic or inorganic UV filters or a combination of both based on their chemical composition and photoprotection mechanisms.9 Concerns about these ingredients causing cancer have created confusion among consumers. I will attempt to clarify these concerns by critically analyzing available evidence-based data on sunscreen use so that as dermatology residents we will be more knowledgeable about sunscreen safety topics and will be able to provide accurate and up-to-date information to our patients.
Organic UV Filters
Organic UV filters are classified as aromatic compounds that provide photoprotection by absorbing UV light.10 Aside from the photoallergic potential of organic UV filters, controversy has arisen in response to studies reporting their possible hormone disruptive effects.11-18 Although there are several US Food and Drug Administration (FDA)–approved organic UV filters in use today, one of the most commonly manufactured and controversial agents is oxybenzone.10 Claims regarding the estrogenic and antiandrogenic effects of oxybenzone have been investigated with results refuting the claims or concluding that more sensitive studies are needed to determine if these organic ingredients pose such risks.10,19,20 One study demonstrated that nearly 300 years of daily sunscreen application would be needed to reach similar exposure levels of oxybenzone used and described in prior animal studies.21 Additionally, most of the studied adverse effects of UV filters have been evaluated based on oral exposure rather than actual dermal application.11 Although these compounds are absorbed systemically, studies have reported that the amounts are insignificant and noncumulative in the body.10,22-24 Furthermore, the binding affinity of oxybenzone for estrogen receptors has been shown to be much weaker and near insignificant compared to estrogen and estradiol.24,25 Although numerous important studies examining systemic absorption have not shown a clinically significant disruption of hormonal homeostasis or acute toxicity in humans by organic UV filters, further studies are needed.
Inorganic UV Filters
Used as the main active ingredients in sunscreen for decades, titanium dioxide (TiO2) and zinc oxide (ZnO) compounds generally are more photostable and less photoallergic than their organic counterparts.10 In recent years, the safety of these long-used photoprotectors has been questioned because of the development of nanoparticle (<100 nm) formulas that are less opaque on application. Although this formula provides a thin, transparent, and cosmetically appealing medium, there is concern that the metal oxides penetrate the skin and cause local and systemic toxicities.26-28 Several recent scientific studies have shown no percutaneous permeation of these particles in normal adult human skin and reported no causal damage to mammalian cells.10,29-31 Although skin penetration of TiO2 and ZnO has been described as insignificant, focus has shifted to health risks associated with inhaling TiO2 through the use of spray or powder products following statements made by the International Agency for Research on Cancer in 2006.32 Several studies investigating increased health risks, specifically lung cancer, in factory workers who were subjected to TiO2 and ZnO inhalation concluded that exposure was unlikely to pose substantial health risks or subchronic toxicity.33,34 Despite a relatively strong safety profile, a major concern of using these metal oxides as UV filters has been potential free radical formation.35-39 For this reason, the Scientific Committee on Emerging and Newly Identified Health Risks extensively researched and delivered opinions on the use of TiO2 and ZnO in cosmetics, concluding that topical application of either compound does not result in toxicity or other adverse effects.30,40-42 Additionally, an effort has been made by manufacturers to encapsulate nanoparticles with magnesium and other materials to quench the reactive oxygen species along with the human body’s own antioxidant defense system.10 In summary, it appears that the current weight of scientific evidence suggests that percutaneous absorption and toxicity by UV filters in humans may be overestimated and that the use of nanoparticles in sunscreens poses no or negligible potential risks to human health.43,44
Concerns Beyond Organic and Inorganic UV Filters
Beyond these concerns with organic and inorganic UV filters, there are several other claims regarding sunscreen safety that have stirred up controversy, including the side-effect profile of retinyl palmitate, vitamin D deficiency, phototoxicity, environmental effects, futility of sun protection factor levels greater than 50, and increased health risks in children. Although some studies report mixed results, the majority of scientific investigations have addressed and refuted several of these claims, again confirming the relative safety of sunscreen use. It is beyond the scope of this article to further discuss these topics specifically. However, it is worth mentioning that consumer studies report that the actual use of sunscreens is 0.5 mg/cm2 or less compared to the ideal application of 2 mg/cm2, thereby confounding many of the claims made about sunscreen use, such as vitamin D deficiency.45 Sunscreens often contain a combination of several UV filters. To date, only a few existing studies have shown that mixtures of the photoprotective agents discussed might interact and exhibit toxic activity when combined, even when there is no observed adverse toxic effect when used individually in products.46-48
The current FDA ruling on sunscreen labeling does not require manufacturers to state if inorganic UV filters have been formulated into nanoparticles; however, manufacturers are now required to include a statement on all sunscreen labels warning consumers to avoid using sunscreen on damaged or broken skin49 in an effort to prevent the active ingredients from getting under the skin, potentially causing inflammation and/or health risks, because available data do not provide conclusive evidence on increased penetration of open skin.50 Additional information regarding the 2011 FDA sunscreen ruling can be found in a prior Cutis Resident Corner column.51
Final Thoughts
As health care providers, we should take advantage of opportunities to educate our patients about other sun safety practices, such as avoiding excessive sun exposure during peak hours (10 am to 2 pm), seeking shade, and wearing photoprotective clothing (eg, wide-brimmed hats, sunglasses).
The research is quite clear: Using broadband sunscreens that absorb and/or block UV radiation results in reduced damage to the skin’s DNA, a fact that should be considered when taking into account the risks and benefits of sunscreen use.2,3 Although sunscreen use is highly recommended in addition to the other sun protection methods, it is ultimately the patient’s choice. If a patient is still concerned about the active ingredients of UV filters, even given the high probability of safety, there are products available on the market that do not include organic filters or nanoparticles. Given the established benefits of UV protection, the use of sunscreens remain one of the most important photoprotective methods, and with increased usage by the public, continuous monitoring of the overall safety and benefit profile of future products is prudent.
Skin cancer is the most common form of cancer in the United States and continues to rise in incidence and mortality each year.1 It is common knowledge that UV light plays a major role in the development of skin cancer.2,3 Studies have long demonstrated that using sunscreen on a daily basis can help prevent the development of skin cancer, premature aging, and exacerbation of photodermatoses.4-7 Although there are several photoprotective measures available, sunscreen remains the most popular and widely used among patients.8 Sunscreens that are on the market today contain either organic or inorganic UV filters or a combination of both based on their chemical composition and photoprotection mechanisms.9 Concerns about these ingredients causing cancer have created confusion among consumers. I will attempt to clarify these concerns by critically analyzing available evidence-based data on sunscreen use so that as dermatology residents we will be more knowledgeable about sunscreen safety topics and will be able to provide accurate and up-to-date information to our patients.
Organic UV Filters
Organic UV filters are classified as aromatic compounds that provide photoprotection by absorbing UV light.10 Aside from the photoallergic potential of organic UV filters, controversy has arisen in response to studies reporting their possible hormone disruptive effects.11-18 Although there are several US Food and Drug Administration (FDA)–approved organic UV filters in use today, one of the most commonly manufactured and controversial agents is oxybenzone.10 Claims regarding the estrogenic and antiandrogenic effects of oxybenzone have been investigated with results refuting the claims or concluding that more sensitive studies are needed to determine if these organic ingredients pose such risks.10,19,20 One study demonstrated that nearly 300 years of daily sunscreen application would be needed to reach similar exposure levels of oxybenzone used and described in prior animal studies.21 Additionally, most of the studied adverse effects of UV filters have been evaluated based on oral exposure rather than actual dermal application.11 Although these compounds are absorbed systemically, studies have reported that the amounts are insignificant and noncumulative in the body.10,22-24 Furthermore, the binding affinity of oxybenzone for estrogen receptors has been shown to be much weaker and near insignificant compared to estrogen and estradiol.24,25 Although numerous important studies examining systemic absorption have not shown a clinically significant disruption of hormonal homeostasis or acute toxicity in humans by organic UV filters, further studies are needed.
Inorganic UV Filters
Used as the main active ingredients in sunscreen for decades, titanium dioxide (TiO2) and zinc oxide (ZnO) compounds generally are more photostable and less photoallergic than their organic counterparts.10 In recent years, the safety of these long-used photoprotectors has been questioned because of the development of nanoparticle (<100 nm) formulas that are less opaque on application. Although this formula provides a thin, transparent, and cosmetically appealing medium, there is concern that the metal oxides penetrate the skin and cause local and systemic toxicities.26-28 Several recent scientific studies have shown no percutaneous permeation of these particles in normal adult human skin and reported no causal damage to mammalian cells.10,29-31 Although skin penetration of TiO2 and ZnO has been described as insignificant, focus has shifted to health risks associated with inhaling TiO2 through the use of spray or powder products following statements made by the International Agency for Research on Cancer in 2006.32 Several studies investigating increased health risks, specifically lung cancer, in factory workers who were subjected to TiO2 and ZnO inhalation concluded that exposure was unlikely to pose substantial health risks or subchronic toxicity.33,34 Despite a relatively strong safety profile, a major concern of using these metal oxides as UV filters has been potential free radical formation.35-39 For this reason, the Scientific Committee on Emerging and Newly Identified Health Risks extensively researched and delivered opinions on the use of TiO2 and ZnO in cosmetics, concluding that topical application of either compound does not result in toxicity or other adverse effects.30,40-42 Additionally, an effort has been made by manufacturers to encapsulate nanoparticles with magnesium and other materials to quench the reactive oxygen species along with the human body’s own antioxidant defense system.10 In summary, it appears that the current weight of scientific evidence suggests that percutaneous absorption and toxicity by UV filters in humans may be overestimated and that the use of nanoparticles in sunscreens poses no or negligible potential risks to human health.43,44
Concerns Beyond Organic and Inorganic UV Filters
Beyond these concerns with organic and inorganic UV filters, there are several other claims regarding sunscreen safety that have stirred up controversy, including the side-effect profile of retinyl palmitate, vitamin D deficiency, phototoxicity, environmental effects, futility of sun protection factor levels greater than 50, and increased health risks in children. Although some studies report mixed results, the majority of scientific investigations have addressed and refuted several of these claims, again confirming the relative safety of sunscreen use. It is beyond the scope of this article to further discuss these topics specifically. However, it is worth mentioning that consumer studies report that the actual use of sunscreens is 0.5 mg/cm2 or less compared to the ideal application of 2 mg/cm2, thereby confounding many of the claims made about sunscreen use, such as vitamin D deficiency.45 Sunscreens often contain a combination of several UV filters. To date, only a few existing studies have shown that mixtures of the photoprotective agents discussed might interact and exhibit toxic activity when combined, even when there is no observed adverse toxic effect when used individually in products.46-48
The current FDA ruling on sunscreen labeling does not require manufacturers to state if inorganic UV filters have been formulated into nanoparticles; however, manufacturers are now required to include a statement on all sunscreen labels warning consumers to avoid using sunscreen on damaged or broken skin49 in an effort to prevent the active ingredients from getting under the skin, potentially causing inflammation and/or health risks, because available data do not provide conclusive evidence on increased penetration of open skin.50 Additional information regarding the 2011 FDA sunscreen ruling can be found in a prior Cutis Resident Corner column.51
Final Thoughts
As health care providers, we should take advantage of opportunities to educate our patients about other sun safety practices, such as avoiding excessive sun exposure during peak hours (10 am to 2 pm), seeking shade, and wearing photoprotective clothing (eg, wide-brimmed hats, sunglasses).
The research is quite clear: Using broadband sunscreens that absorb and/or block UV radiation results in reduced damage to the skin’s DNA, a fact that should be considered when taking into account the risks and benefits of sunscreen use.2,3 Although sunscreen use is highly recommended in addition to the other sun protection methods, it is ultimately the patient’s choice. If a patient is still concerned about the active ingredients of UV filters, even given the high probability of safety, there are products available on the market that do not include organic filters or nanoparticles. Given the established benefits of UV protection, the use of sunscreens remain one of the most important photoprotective methods, and with increased usage by the public, continuous monitoring of the overall safety and benefit profile of future products is prudent.
1. Skin cancer statistics. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/cancer/skin/statistics/index.htm. Updated September 2, 2014. Accessed December 30, 2014.
2. World Health Organization, International Agency for Research on Cancer. Solar and ultraviolet radiation. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 55. Lyon, France: International Agency for Research on Cancer; 1992.
3. Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257-263.
4. Darlington S, Williams G, Neale R, et al. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol. 2003;139:451-455.
5. Van der Pols JC, Williams GM, Pandeya N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546-2548.
6. Hughes MC, Williams GM, Baker P, et al. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med. 2013;158:781-790.
7. Bissonnette R, Nigen S, Bolduc C. Influence of the quantity of sunscreen applied on the ability to protect against ultraviolet-induced polymorphous light eruption. Photodermatol Photoimmunol Photomed. 2012;28:240-243.
8. Cancer trends progress report 2011/2012 update: sun protection. National Cancer Institute Web site. http://progressreport.cancer.gov/doc_detail.asp?pid¡1&did¡2009&chid¡91&coid¡911. Accessed December 30, 2014.
9. Sunscreen Drug Products for Over-the-counter Human Use, 21 CFR §352.10. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=352.10. Updated September 1, 2014. Accessed December 30, 2014.
10. Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photoimmunol Photomed. 2011;27:58-67.
11. Krause M, Klit A, Blomberg Jensen M, et al. Sunscreens: are they beneficial for health? an overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35:424-436.
12. Schlumpf M, Cotton B, Conscience M, et al. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109:239-244.
13. Schlumpf M, Schmid P, Durrer S, et al. Endocrine activity and developmental toxicity of cosmetic UV filters–an update. Toxicol. 2004;205:113-122.
14. Schlumpf M, Kypke K, Vökt C, et al. Endocrine active UV filters: developmental toxicity and exposure through breast milk. Chimia. 2008;62:345-351.
15. Nakagawa Y, Suzuki T. Metabolism of 2-hydroxy-4-methoxybenzophenone in isolated rat hepatocytes and xenoestrogenic effects of its metabolites on MCF-7 human breast cancer cells. Chem Biol Interact. 2002;139:115-128.
16. Ma R, Cotton B, Lichtensteiger W, et al. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol Sci. 2003;74:43-50.
17. Heneweer M, Muusse M, van den Berg M, et al. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
18. Knobler E, Almeida L, Ruzkowski AM, et al. Photoallergy to benzophenone. Arch Dermatol. 1989;125:801-804.
19. Draelos ZD. Are sunscreens safe? J Cosmet Dermatol. 2010;9:1-2.
20. Gilbert E, Pirot F, Bertholle V. Commonly used UV filter toxicity on biological functions: review of last decade studies. Int J of Cosmet Sci. 2013;35:208-219.
21. Wang SQ, Burnett ME, Lim HW. Safety of oxybenzone: putting numbers into perspective. Arch Dermatol. 2011;147:865-866.
22. Mancebo SE, Hu JY, Wang SQ. Sunscreens: a review of health benefits, regulations, and controversies. Dermatol Clin. 2014;32:427-438.
23. Jansen R, Osterwalder U, Wang SQ, et al. Photoprotection: part II. sunscreen: development, efficacy, and controversies. J Am Acad Dermatol. 2013;69:867.e1-867.e14.
24. Janjua NR, Mogensen B, Andersson AM, et al. Systemic absorption of the sunscreens benzo- phenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzy-lidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol. 2004;123:57-61.
25. Kadry AM, Chukwuemeka SO, Mohamed S, et al. Pharmacokinetics of benzophenone-3 after oral exposure in male rats. J Appl Toxicol. 1995;15:97-102.
26. Gulson B, McCall M, Korsch M, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci. 2010;118:140-149.
27. Gulson B, Wong H, Korsch M, et al. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Sci Total Environ. 2012:420:313-318.
28. Benech-Kieffer F, Meuling WJ, Leclerc C, et al. Percutaneous absorption of Mexoryl SX in human volunteers: comparison with in vitro data. Skin Pharmacol Appl Skin Physiol. 2003;16:343-355.
29. Nash JF. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatol Clin. 2006;24:35-51.
30. Nohynek GJ, Lademann J, Ribaud C, et al. Grey goo on the skin? nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37:251-277.
31. Sadrieh N, Wokovich AM, Gopee NV, et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol Sci. 2010;115:156-166.
32. International Agency for Research on Cancer. Carbon black, titanium dioxide, and talc. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 93. Lyon, France: International Agency for Research on Cancer; 2006.
33. Liao CM, Chiang YH, Chio CP. Model-based assessment for human inhalation exposure risk to airborne nano/fine titanium dioxide particles. Sci Total Environ. 2008:15;407:165-177.
34. Adamcakova-Dodd A, Stebounova LV, Kim JS, et al. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 2014;11:15.
35. Wamer WG, Yin JJ, Wei RR. Oxidative damage to nucleic acids photosensitized by titanium dioxide. Free Radic Biol Med. 1997;23:851-858.
36. Nakagawa Y, Wakuri S, Sakamoto K, et al. The photogenotoxicity of titanium dioxide particles. Mutat Res. 1997;394:125-132.
37. Dunford R, Salinaro A, Cai L, et al. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418:87-90, 99.
38. Hidaka H, Kobayashi H, Koike T, et al. DNA damage photoinduced by cosmetic pigments and sunscreen agents under solar exposure and artificial UV illumination. J Oleo Sci. 2006;55:249-261.
39. Dufour EK, Kumaravel T, Nohynek GJ, et al. Clastogenicity, photo-clastogenicity or pseudo-photo-clastogenicity: genotoxic effects of zinc oxide in the dark, in pre-irradiated or simultaneously irradiated Chinese hamster ovary cells [published online ahead of print June 21, 2006]. Mutat Res. 2006;607:215-224.
40. Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers concerning titanium dioxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out135_en.pdf. Published October 24, 2000. Accessed December 30, 2014.
41. The Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers opinion concerning zinc oxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out222_en.pdf. Published June 24-25, 2003. Accessed December 30, 2014.
42. Hackenberg S, Friehs G, Kessler M, et al. Nanosized titanium dioxide particles do not induce DNA damage in human peripheral blood lymphocytes. Environ Mol Mutagen. 2010;52:264-268.
43. Bach-Thomsen M, Wulf HC. Sunbather’s application of sunscreen is probably inadequate to obtain the sun protection factor assigned to the preparation. Photodermatol Photoimmunol Photomed. 1993:9;242-244.
44. Nohynek GJ, Antignac E, Re T, et al. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol. 2010:1;243:239-259.
45. Diffey BL. Sunscreens: use and misuse. In: Giacomoni PU, ed. Sun Protection in Man. Vol 3. Amsterdam, the Netherlands: Elsevier Science BV; 2001:521-534.
46. Heneweer M, Muusse M, Van den BM, et al. Additive estrogenic effects of mixtures of frequently used UV-filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
47. Kunz PY, Galicia HF, Fent K. Comparison of in vitro and in vivo estrogenic activity of UV-filters in fish. Toxicol Sci. 2006;90:349-361.
48. Kortenkamp A, Faust M, Scholze M, et al. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect. 2007;115(suppl 1):106-114.
49. Labeling and effectiveness testing: sunscreen drug products for over-the-counter human use—small entity compliance guide. US Food and Drug Administration Web site. http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm330694.htm. Published December 2012. Updated May 13, 2014. Accessed December 30, 2014.
50. Schafer-Korting M, Korting HC, Ponce-Poschl E. Liposomal tretinoin for uncomplicated acne vulgaris. Clin Investig. 1994;72:1086-1091.
51. Bronfenbrener R. Simplifying sun safety: a guide to the new FDA sunscreen monograph. Cutis. 2014;93:e17-e19.
1. Skin cancer statistics. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/cancer/skin/statistics/index.htm. Updated September 2, 2014. Accessed December 30, 2014.
2. World Health Organization, International Agency for Research on Cancer. Solar and ultraviolet radiation. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 55. Lyon, France: International Agency for Research on Cancer; 1992.
3. Green AC, Williams GM, Logan V, et al. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257-263.
4. Darlington S, Williams G, Neale R, et al. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol. 2003;139:451-455.
5. Van der Pols JC, Williams GM, Pandeya N, et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev. 2006;15:2546-2548.
6. Hughes MC, Williams GM, Baker P, et al. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med. 2013;158:781-790.
7. Bissonnette R, Nigen S, Bolduc C. Influence of the quantity of sunscreen applied on the ability to protect against ultraviolet-induced polymorphous light eruption. Photodermatol Photoimmunol Photomed. 2012;28:240-243.
8. Cancer trends progress report 2011/2012 update: sun protection. National Cancer Institute Web site. http://progressreport.cancer.gov/doc_detail.asp?pid¡1&did¡2009&chid¡91&coid¡911. Accessed December 30, 2014.
9. Sunscreen Drug Products for Over-the-counter Human Use, 21 CFR §352.10. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=352.10. Updated September 1, 2014. Accessed December 30, 2014.
10. Burnett ME, Wang SQ. Current sunscreen controversies: a critical review. Photodermatol Photoimmunol Photomed. 2011;27:58-67.
11. Krause M, Klit A, Blomberg Jensen M, et al. Sunscreens: are they beneficial for health? an overview of endocrine disrupting properties of UV-filters. Int J Androl. 2012;35:424-436.
12. Schlumpf M, Cotton B, Conscience M, et al. In vitro and in vivo estrogenicity of UV screens. Environ Health Perspect. 2001;109:239-244.
13. Schlumpf M, Schmid P, Durrer S, et al. Endocrine activity and developmental toxicity of cosmetic UV filters–an update. Toxicol. 2004;205:113-122.
14. Schlumpf M, Kypke K, Vökt C, et al. Endocrine active UV filters: developmental toxicity and exposure through breast milk. Chimia. 2008;62:345-351.
15. Nakagawa Y, Suzuki T. Metabolism of 2-hydroxy-4-methoxybenzophenone in isolated rat hepatocytes and xenoestrogenic effects of its metabolites on MCF-7 human breast cancer cells. Chem Biol Interact. 2002;139:115-128.
16. Ma R, Cotton B, Lichtensteiger W, et al. UV filters with antagonistic action at androgen receptors in the MDA-kb2 cell transcriptional-activation assay. Toxicol Sci. 2003;74:43-50.
17. Heneweer M, Muusse M, van den Berg M, et al. Additive estrogenic effects of mixtures of frequently used UV filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
18. Knobler E, Almeida L, Ruzkowski AM, et al. Photoallergy to benzophenone. Arch Dermatol. 1989;125:801-804.
19. Draelos ZD. Are sunscreens safe? J Cosmet Dermatol. 2010;9:1-2.
20. Gilbert E, Pirot F, Bertholle V. Commonly used UV filter toxicity on biological functions: review of last decade studies. Int J of Cosmet Sci. 2013;35:208-219.
21. Wang SQ, Burnett ME, Lim HW. Safety of oxybenzone: putting numbers into perspective. Arch Dermatol. 2011;147:865-866.
22. Mancebo SE, Hu JY, Wang SQ. Sunscreens: a review of health benefits, regulations, and controversies. Dermatol Clin. 2014;32:427-438.
23. Jansen R, Osterwalder U, Wang SQ, et al. Photoprotection: part II. sunscreen: development, efficacy, and controversies. J Am Acad Dermatol. 2013;69:867.e1-867.e14.
24. Janjua NR, Mogensen B, Andersson AM, et al. Systemic absorption of the sunscreens benzo- phenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzy-lidene) camphor after whole-body topical application and reproductive hormone levels in humans. J Invest Dermatol. 2004;123:57-61.
25. Kadry AM, Chukwuemeka SO, Mohamed S, et al. Pharmacokinetics of benzophenone-3 after oral exposure in male rats. J Appl Toxicol. 1995;15:97-102.
26. Gulson B, McCall M, Korsch M, et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol Sci. 2010;118:140-149.
27. Gulson B, Wong H, Korsch M, et al. Comparison of dermal absorption of zinc from different sunscreen formulations and differing UV exposure based on stable isotope tracing. Sci Total Environ. 2012:420:313-318.
28. Benech-Kieffer F, Meuling WJ, Leclerc C, et al. Percutaneous absorption of Mexoryl SX in human volunteers: comparison with in vitro data. Skin Pharmacol Appl Skin Physiol. 2003;16:343-355.
29. Nash JF. Human safety and efficacy of ultraviolet filters and sunscreen products. Dermatol Clin. 2006;24:35-51.
30. Nohynek GJ, Lademann J, Ribaud C, et al. Grey goo on the skin? nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol. 2007;37:251-277.
31. Sadrieh N, Wokovich AM, Gopee NV, et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol Sci. 2010;115:156-166.
32. International Agency for Research on Cancer. Carbon black, titanium dioxide, and talc. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 93. Lyon, France: International Agency for Research on Cancer; 2006.
33. Liao CM, Chiang YH, Chio CP. Model-based assessment for human inhalation exposure risk to airborne nano/fine titanium dioxide particles. Sci Total Environ. 2008:15;407:165-177.
34. Adamcakova-Dodd A, Stebounova LV, Kim JS, et al. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 2014;11:15.
35. Wamer WG, Yin JJ, Wei RR. Oxidative damage to nucleic acids photosensitized by titanium dioxide. Free Radic Biol Med. 1997;23:851-858.
36. Nakagawa Y, Wakuri S, Sakamoto K, et al. The photogenotoxicity of titanium dioxide particles. Mutat Res. 1997;394:125-132.
37. Dunford R, Salinaro A, Cai L, et al. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997;418:87-90, 99.
38. Hidaka H, Kobayashi H, Koike T, et al. DNA damage photoinduced by cosmetic pigments and sunscreen agents under solar exposure and artificial UV illumination. J Oleo Sci. 2006;55:249-261.
39. Dufour EK, Kumaravel T, Nohynek GJ, et al. Clastogenicity, photo-clastogenicity or pseudo-photo-clastogenicity: genotoxic effects of zinc oxide in the dark, in pre-irradiated or simultaneously irradiated Chinese hamster ovary cells [published online ahead of print June 21, 2006]. Mutat Res. 2006;607:215-224.
40. Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers concerning titanium dioxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out135_en.pdf. Published October 24, 2000. Accessed December 30, 2014.
41. The Scientific Committee on Cosmetic Products and Non-Food Products intended for Consumers opinion concerning zinc oxide. http://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out222_en.pdf. Published June 24-25, 2003. Accessed December 30, 2014.
42. Hackenberg S, Friehs G, Kessler M, et al. Nanosized titanium dioxide particles do not induce DNA damage in human peripheral blood lymphocytes. Environ Mol Mutagen. 2010;52:264-268.
43. Bach-Thomsen M, Wulf HC. Sunbather’s application of sunscreen is probably inadequate to obtain the sun protection factor assigned to the preparation. Photodermatol Photoimmunol Photomed. 1993:9;242-244.
44. Nohynek GJ, Antignac E, Re T, et al. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol Appl Pharmacol. 2010:1;243:239-259.
45. Diffey BL. Sunscreens: use and misuse. In: Giacomoni PU, ed. Sun Protection in Man. Vol 3. Amsterdam, the Netherlands: Elsevier Science BV; 2001:521-534.
46. Heneweer M, Muusse M, Van den BM, et al. Additive estrogenic effects of mixtures of frequently used UV-filters on pS2-gene transcription in MCF-7 cells. Toxicol Appl Pharmacol. 2005;208:170-177.
47. Kunz PY, Galicia HF, Fent K. Comparison of in vitro and in vivo estrogenic activity of UV-filters in fish. Toxicol Sci. 2006;90:349-361.
48. Kortenkamp A, Faust M, Scholze M, et al. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect. 2007;115(suppl 1):106-114.
49. Labeling and effectiveness testing: sunscreen drug products for over-the-counter human use—small entity compliance guide. US Food and Drug Administration Web site. http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm330694.htm. Published December 2012. Updated May 13, 2014. Accessed December 30, 2014.
50. Schafer-Korting M, Korting HC, Ponce-Poschl E. Liposomal tretinoin for uncomplicated acne vulgaris. Clin Investig. 1994;72:1086-1091.
51. Bronfenbrener R. Simplifying sun safety: a guide to the new FDA sunscreen monograph. Cutis. 2014;93:e17-e19.
Does Your Dermatology Center Need a Dermatoscenter?

There are anecdotal reports of dogs detecting melanoma and studies of canines being able to not only detect but also distinguish cancer from noncancer. Analysis of volatile compounds or metabolites from exhaled human breath and excreted urine also has been shown to differentiate between patients with certain cancers and healthy individuals. In addition, investigators have demonstrated that melanoma tissue has a volatile profile that is distinct from healthy nonneoplastic skin and nevi.
Abaffy et al (Metabolomics. 2013;9:998-1008) conducted a study that gives further support to the potential for analyzing volatile organic compounds as biomarkers of melanoma. They used the headspace solid phase microextraction method followed by gas chromatography and mass spectrometry to compare the volatile metabolic profiles of melanoma and nonneoplastic healthy-appearing adjacent skin from the same patient. They discovered increased levels of lauric acid (C12:0) and palmitic acid (C16:0) in melanoma and they postulated that the increased levels of these fatty acids were due to cancer-associated upregulation of de novo lipid synthesis.
What’s the issue?
In the 1980s, nail fold capillary microscopy using an ophthalmoscope was occasionally performed to evaluate for disease-associated vascular changes in patients who were being evaluated for connective tissue disorders. Within 2 decades, a dermoscope to assist in the evaluation of not only nail folds but also pigmented and other lesions replaced the ophthalmoscope. The US Food and Drug Administration recently approved a software-driven optical imaging and data analysis device that can be used to obtain additional information to assist the clinician in making a decision whether to biopsy a pigmented lesion.
As our ability to develop more sensitive and specific methods to diagnose melanoma and differentiate it from benign lesions advances, our approach to the evaluation of patients with pigmented lesions shall continue to be modified. Based on the detection of melanoma-associated volatile organic compounds coupled with their potential use as readily accessible tumor-related biomarkers, it is reasonable to speculate: (1) that a handheld office-based device, a dermatoscenter, that can identify melanoma-induced volatile tumor markers shall be developed to evaluate whether pigmented lesions are malignant or benign, and (2) that this device will eventually become an integral component of the dermatologist’s diagnostic armamentarium. Does your dermatology center need a dermatoscenter?

There are anecdotal reports of dogs detecting melanoma and studies of canines being able to not only detect but also distinguish cancer from noncancer. Analysis of volatile compounds or metabolites from exhaled human breath and excreted urine also has been shown to differentiate between patients with certain cancers and healthy individuals. In addition, investigators have demonstrated that melanoma tissue has a volatile profile that is distinct from healthy nonneoplastic skin and nevi.
Abaffy et al (Metabolomics. 2013;9:998-1008) conducted a study that gives further support to the potential for analyzing volatile organic compounds as biomarkers of melanoma. They used the headspace solid phase microextraction method followed by gas chromatography and mass spectrometry to compare the volatile metabolic profiles of melanoma and nonneoplastic healthy-appearing adjacent skin from the same patient. They discovered increased levels of lauric acid (C12:0) and palmitic acid (C16:0) in melanoma and they postulated that the increased levels of these fatty acids were due to cancer-associated upregulation of de novo lipid synthesis.
What’s the issue?
In the 1980s, nail fold capillary microscopy using an ophthalmoscope was occasionally performed to evaluate for disease-associated vascular changes in patients who were being evaluated for connective tissue disorders. Within 2 decades, a dermoscope to assist in the evaluation of not only nail folds but also pigmented and other lesions replaced the ophthalmoscope. The US Food and Drug Administration recently approved a software-driven optical imaging and data analysis device that can be used to obtain additional information to assist the clinician in making a decision whether to biopsy a pigmented lesion.
As our ability to develop more sensitive and specific methods to diagnose melanoma and differentiate it from benign lesions advances, our approach to the evaluation of patients with pigmented lesions shall continue to be modified. Based on the detection of melanoma-associated volatile organic compounds coupled with their potential use as readily accessible tumor-related biomarkers, it is reasonable to speculate: (1) that a handheld office-based device, a dermatoscenter, that can identify melanoma-induced volatile tumor markers shall be developed to evaluate whether pigmented lesions are malignant or benign, and (2) that this device will eventually become an integral component of the dermatologist’s diagnostic armamentarium. Does your dermatology center need a dermatoscenter?

There are anecdotal reports of dogs detecting melanoma and studies of canines being able to not only detect but also distinguish cancer from noncancer. Analysis of volatile compounds or metabolites from exhaled human breath and excreted urine also has been shown to differentiate between patients with certain cancers and healthy individuals. In addition, investigators have demonstrated that melanoma tissue has a volatile profile that is distinct from healthy nonneoplastic skin and nevi.
Abaffy et al (Metabolomics. 2013;9:998-1008) conducted a study that gives further support to the potential for analyzing volatile organic compounds as biomarkers of melanoma. They used the headspace solid phase microextraction method followed by gas chromatography and mass spectrometry to compare the volatile metabolic profiles of melanoma and nonneoplastic healthy-appearing adjacent skin from the same patient. They discovered increased levels of lauric acid (C12:0) and palmitic acid (C16:0) in melanoma and they postulated that the increased levels of these fatty acids were due to cancer-associated upregulation of de novo lipid synthesis.
What’s the issue?
In the 1980s, nail fold capillary microscopy using an ophthalmoscope was occasionally performed to evaluate for disease-associated vascular changes in patients who were being evaluated for connective tissue disorders. Within 2 decades, a dermoscope to assist in the evaluation of not only nail folds but also pigmented and other lesions replaced the ophthalmoscope. The US Food and Drug Administration recently approved a software-driven optical imaging and data analysis device that can be used to obtain additional information to assist the clinician in making a decision whether to biopsy a pigmented lesion.
As our ability to develop more sensitive and specific methods to diagnose melanoma and differentiate it from benign lesions advances, our approach to the evaluation of patients with pigmented lesions shall continue to be modified. Based on the detection of melanoma-associated volatile organic compounds coupled with their potential use as readily accessible tumor-related biomarkers, it is reasonable to speculate: (1) that a handheld office-based device, a dermatoscenter, that can identify melanoma-induced volatile tumor markers shall be developed to evaluate whether pigmented lesions are malignant or benign, and (2) that this device will eventually become an integral component of the dermatologist’s diagnostic armamentarium. Does your dermatology center need a dermatoscenter?
Product News: 01 2015
Onexton
Valeant Pharmaceuticals International, Inc, an-nounces US Food and Drug Administration approval of Onexton Gel (clindamycin phosphate 1.2% and benzoyl peroxide 3.75%) for the once-daily treatment of comedonal and inflammatory acne in patients 12 years and older. This dual-action topical therapy has a favorable cutaneous tolerability profile and contains no surfactants, alcohol, or preservatives. Onexton is expected to launch in early 2015. For more information, visit www.valeant.com.
Physical Eye UV Defense Sunscreen
SkinCeuticals presents Physical Eye UV Defense Sunscreen that provides broad-spectrum SPF 50 protection without migrating into or irritating the eyes. Physical Eye UV Defense unifies natural skin tone around the eye and provides a translucent universal tint. It should be applied around the entire eye area and is optimized for application under makeup. It also can be used following hyaluronic acid filler and botulinum toxin injections. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
Refining Mineral Mask
Revision Skincare introduces the limited edition Refining Mineral Mask, a warming mask to reduce the appearance of pores and leave skin looking refined. It contains kaolin to purify the complexion, pumpkin enzymes to gently exfoliate skin, zeolite to provide an extra boost of radiance with its warming effect, and vitamin E microspheres to condition skin. Revision Skincare products are available exclusively through dermatologists, plastic surgeons, and medical spas. For more information, visit www.revisionskincare.com.
Resveratrol B E
SkinCeuticals launches Resveratrol B E, an intensive antioxidant night concentrate that boosts the skin’s endogenous antioxidant defense system, which loses efficiency with age and accumulated damage. It works by neutralizing age-accelerating internal free radicals, strength-ening functionality to resist new damage, and promoting skin’s natural repair to diminish the signs of accumulated damage. Resveratrol B E corrects signs of photodamage, loss of firmness and radiance, poor elasticity, and fine lines and wrinkles. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Onexton
Valeant Pharmaceuticals International, Inc, an-nounces US Food and Drug Administration approval of Onexton Gel (clindamycin phosphate 1.2% and benzoyl peroxide 3.75%) for the once-daily treatment of comedonal and inflammatory acne in patients 12 years and older. This dual-action topical therapy has a favorable cutaneous tolerability profile and contains no surfactants, alcohol, or preservatives. Onexton is expected to launch in early 2015. For more information, visit www.valeant.com.
Physical Eye UV Defense Sunscreen
SkinCeuticals presents Physical Eye UV Defense Sunscreen that provides broad-spectrum SPF 50 protection without migrating into or irritating the eyes. Physical Eye UV Defense unifies natural skin tone around the eye and provides a translucent universal tint. It should be applied around the entire eye area and is optimized for application under makeup. It also can be used following hyaluronic acid filler and botulinum toxin injections. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
Refining Mineral Mask
Revision Skincare introduces the limited edition Refining Mineral Mask, a warming mask to reduce the appearance of pores and leave skin looking refined. It contains kaolin to purify the complexion, pumpkin enzymes to gently exfoliate skin, zeolite to provide an extra boost of radiance with its warming effect, and vitamin E microspheres to condition skin. Revision Skincare products are available exclusively through dermatologists, plastic surgeons, and medical spas. For more information, visit www.revisionskincare.com.
Resveratrol B E
SkinCeuticals launches Resveratrol B E, an intensive antioxidant night concentrate that boosts the skin’s endogenous antioxidant defense system, which loses efficiency with age and accumulated damage. It works by neutralizing age-accelerating internal free radicals, strength-ening functionality to resist new damage, and promoting skin’s natural repair to diminish the signs of accumulated damage. Resveratrol B E corrects signs of photodamage, loss of firmness and radiance, poor elasticity, and fine lines and wrinkles. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
Onexton
Valeant Pharmaceuticals International, Inc, an-nounces US Food and Drug Administration approval of Onexton Gel (clindamycin phosphate 1.2% and benzoyl peroxide 3.75%) for the once-daily treatment of comedonal and inflammatory acne in patients 12 years and older. This dual-action topical therapy has a favorable cutaneous tolerability profile and contains no surfactants, alcohol, or preservatives. Onexton is expected to launch in early 2015. For more information, visit www.valeant.com.
Physical Eye UV Defense Sunscreen
SkinCeuticals presents Physical Eye UV Defense Sunscreen that provides broad-spectrum SPF 50 protection without migrating into or irritating the eyes. Physical Eye UV Defense unifies natural skin tone around the eye and provides a translucent universal tint. It should be applied around the entire eye area and is optimized for application under makeup. It also can be used following hyaluronic acid filler and botulinum toxin injections. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
Refining Mineral Mask
Revision Skincare introduces the limited edition Refining Mineral Mask, a warming mask to reduce the appearance of pores and leave skin looking refined. It contains kaolin to purify the complexion, pumpkin enzymes to gently exfoliate skin, zeolite to provide an extra boost of radiance with its warming effect, and vitamin E microspheres to condition skin. Revision Skincare products are available exclusively through dermatologists, plastic surgeons, and medical spas. For more information, visit www.revisionskincare.com.
Resveratrol B E
SkinCeuticals launches Resveratrol B E, an intensive antioxidant night concentrate that boosts the skin’s endogenous antioxidant defense system, which loses efficiency with age and accumulated damage. It works by neutralizing age-accelerating internal free radicals, strength-ening functionality to resist new damage, and promoting skin’s natural repair to diminish the signs of accumulated damage. Resveratrol B E corrects signs of photodamage, loss of firmness and radiance, poor elasticity, and fine lines and wrinkles. SkinCeuticals products are physician dispensed. For more information, visit www.skinceuticals.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at cutis@frontlinemedcom.com.
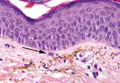
Lesions With a Distinct Fingerprint Presentation
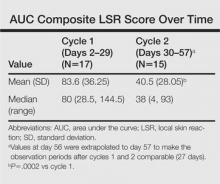
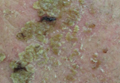
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
The Diagnosis: Phytophotodermatitis
Phytophotodermatitis (PPD) is a nonimmunologic cutaneous phototoxic inflammatory reaction resulting from the activation of photosensitizing botanical agents such as furanocoumarins in contact with the skin by exposure to UVA light.1,2 Furanocoumarins, including psoralens and angelicins, become photoexcited and covalently bind to pyrimidine bases on DNA strands, resulting in acute damage to epidermal, dermal, and endothelial cells.1,3
Vegetation most commonly implicated in this plant solar dermatitis are celery, fennel, parsnip, parsley, and hogweed (Apiaceae [formerly known as the Umbelliferae family]), as well as oranges, lemons, limes, and grapefruits (Rutaceae or citrus family).1,3 Psoralens found in the Persian lime have been noted to cause phototoxic eruptions in the United States, with the rind containing higher concentrations than the pulp.4
Clinical features of PPD include erythema, edema, and vesicle or bullae formation 12 to 36 hours after psoralen and UV light exposure. Burning and pain may be present, but pruritus is not a common characteristic of the eruptions, distinguishing PPD from allergic phytodermatitis.
Hyperpigmentation appears on resolution of the lesions and slowly fades over months to years.1,3,5 Mild exposure may lead to hyperpigmentation without a vesicular or erythematous eruption.1 Phytophotodermatitis follows a benign course and often spontaneously resolves; however, prolonged hyperpigmentation may cause concern for these patients.
Phytophotodermatitis is common among patients preparing drinks and foods with citrus juices or after gardening. Our patient had prepared limeade 3 weeks prior to presentation. The distribution of cutaneous exposure to furanocoumarins influences clinical presentation and may range from blotches and streaks to distinct fingerprint smudges and handprints, as seen in our patient. The distinct full handprint on the right arm was striking. The bullous lesions and resulting hyperpigmentation may mimic burns and healing bruises. In children, PPD often is mistaken for child abuse.1,6,7 In adults, it often is misdiagnosed as poison oak dermatitis, erythema multiforme, and thrombocytopenic purpura.1,3 It is important to recognize PPD to avoid delay in or misdiagnosis and to better counsel patients on how to avoid recurrent episodes of PPD.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.
1. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Maryland Heights, MO: Mosby; 2008.
2. Pomeranz MK, Karen JK. Phytophotodermatitis and limes. N Engl J Med. 2007;357:e1.
3. Sassiville D. Clinical patterns of phytophotodermatitis. Dermatol Clin. 2009;27:299-308.
4. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
5. Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
6. Mill J, Wallis B, Cuttle L, et al. Phytophotodermatitis: case reports of children presenting with blistering after preparing lime juice. Burns. 2008;34:731-733.
7. Carlsen K, Weismann K. Phytophotodermatitis in 19 children admitted to hospital and their differential diagnoses: child abuse and herpes simplex virus infection. J Am Acad Dermatol. 2007;57(suppl):S88-S91.

A 17-year-old adolescent girl presented with scattered brown macules over the dorsal aspect of the hands bilaterally and a brown patch in the shape of a hand on the right upper arm of 3 weeks’ duration.
Reduced Degree of Irritation During a Second Cycle of Ingenol Mebutate Gel 0.015% for the Treatment of Actinic Keratosis
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.
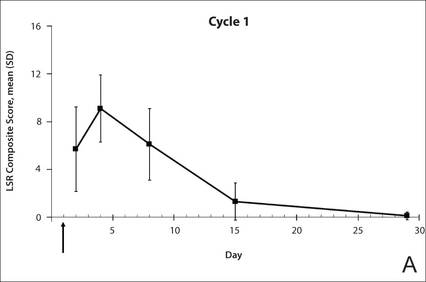 |

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.
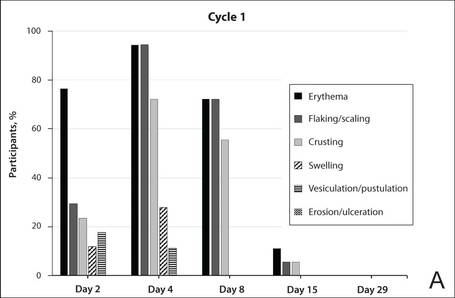 |
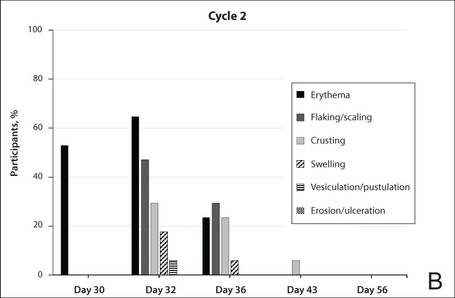 |
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes
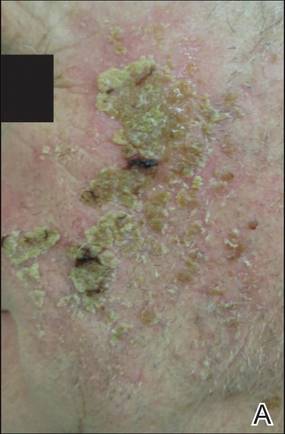 |
 |
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.
 |

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.
 |
 |
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes
 |
 |
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
Actinic keratoses (AKs) are common skin lesions resulting from cumulative exposure to UV radiation and are associated with an increased risk for invasive squamous cell carcinoma1; therefore, diagnosis and treatment are important.2 Individual AKs are most frequently treated with cryosurgery, while topical agents including ingenol mebutate gel are used as field treatments on areas of confluent AKs of sun-damaged skin.2,3 Studies have shown that rates of complete clearance with topical therapy can be improved with more than a single treatment course.4-6
Although the mechanisms of action of ingenol mebutate on AKs are not fully understood, studies indicate that it induces cell death in proliferating keratinocytes, which suggests that it may act preferentially on AKs and not on healthy skin.7 The field treatment of AKs of the face and scalp using ingenol mebutate gel 0.015% involves a 3-day regimen,8 and clearance rates are similar to those observed with topical agents that are used for longer periods of time.3,9,10 Local skin reactions (LSRs) associated with application of ingenol mebutate gel 0.015% on the face and scalp generally are mild to moderate in intensity and resolve after 2 weeks without sequelae.3
The presumption that the cytotoxic actions of ingenol mebutate affect proliferating keratinocytes preferentially was the basis for this study. We hypothesized that application of a second sequential cycle of ingenol mebutate during AK treatment should produce lower LSR scores than the first application cycle due to the specific elimination of transformed keratinocytes from the treatment area. This open-label study compared the intensity of LSRs during 2 sequential cycles of treatment on the same site of the face or scalp using ingenol mebutate gel 0.015%.
Methods
Study Population
Eligible participants were adults with 4 to 8 clinically typical, visible, nonhypertrophic AKs in a 25-cm2 contiguous area of the face or scalp. Inclusion and exclusion criteria were the same as in the pivotal studies.3 The study was approved by the institutional review board at the Icahn School of Medicine at Mount Sinai (New York, New York). Enrollment took place from March 2013 to August 2013.
Study Design and Assessments
All participants were treated with 2 sequential 4-week cycles of ingenol mebutate gel 0.015% applied once daily for 3 consecutive days starting on the first day of each cycle (day 1 and day 29). Participants were evaluated at 11 visits (days 1, 2, 4, 8, 15, 29, 30, 32, 36, 43, and 56) during the 56-day study period (Figure 1). Eligibility, demographics, and medical history were assessed at day 1, and concomitant medications and adverse events (AEs) were evaluated at all visits. Using standardized photographic guides, 6 individual LSRs—erythema, flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were assessed on a scale of 0 (none) to 4 (severe), with higher numbers indicating more severe reactions. For each participant, a composite score was calculated as the sum of the individual LSR scores.3 Throughout the study, 3 qualified evaluators assessed AK lesion count and graded the LSRs. The same evaluator assessed both treatment courses for each participant for the majority of assessments.
 |

|
The primary end point of the study was to evaluate the degree of irritation in each of the 2 sequential cycles of ingenol mebutate treatment by assessing the mean area under the curve (AUC) of the composite LSR score over time following each of the 2 applications. Actinic keratoses were counted at baseline and at the end of each treatment cycle. The paired t test was used to compare AUCs of the composite LSR scores of the 2 cycles and to compare the changes in lesion counts from baseline to day 29 and from baseline to day 56. The complete clearance rates (number of participants with no AKs) at the end of cycles 1 and 2 were compared using a logistic regression model. Participant-perceived irritation and treatment satisfaction were evaluated using a 0 to 100 visual analog scale (VAS), with higher numbers indicating greater irritation and higher satisfaction. Participant-reported scores were summarized.
Results
Participant Characteristics
A total of 20 participants were enrolled in the study. At the completion of the study, 2 participants withdrew consent but allowed use of data from their completed assessments. Consequently, a total of 18 patients completed the entire study. The mean age was 75.35 years (median, 77.5 years; age range, 49–87 years). Most of the participants (15/20 [75%]) were men. All participants were white, and 2 were of Hispanic ethnicity. Of the 20 participants, 19 (95%) were Fitzpatrick skin type II, and 1 (5%) was Fitzpatrick skin type I. Most of the participants (16/20 [80%]) received treatment of lesions on the face. With the exception of 2 (10%) participants, all had received prior treatment of AKs, including cryosurgery (16/20 [80%]), imiquimod (5/20 [25%]), fluorouracil (2/20 [10%]), diclofenac (2/20 [10%]), and photodynamic therapy (2/20 [10%]); 8 (40%) participants had received more than 1 type of treatment.
LSRs in Cycles 1 and 2
The time course for the development and resolution of LSRs during both treatment cycles was similar. Local skin reactions were evident on day 2 in each cycle, peaked at 3 days after the application of the first dose, declined rapidly by the 15th day of the cycle, and returned to baseline by the end of each 4-week cycle (Figure 1). The mean (standard deviation [SD]) composite LSR score at 3 days after application of the first dose was higher in cycle 1 than in cycle 2 (9.1 [2.83] vs 5.0 [3.24])(Figure 1). The composite LSR score assessed over time based on the mean (SD) AUC was significantly lower in cycle 2 than in cycle 1 (40.5 [28.05] vs 83.6 [36.25])(P=.0002)(Table). Statistical differences in scores for individual reactions between the 2 cycles were not determined because of the risk for a spurious indication of significance from multiple comparisons in such a limited patient sample.
The percentage of participants who had a score greater than 1 for any of the 6 components of the LSR assessment was lower in cycle 2 than in cycle 1 at all of the assessed time points (Figure 2). In both cycles, the percentage of participants with an LSR score greater than 1 was highest 3 days after the application of the first dose in the cycle (day 4 or day 32, respectively). Erythema, flaking/scaling, and crusting were the most freq-uently observed reactions. At day 29, there were no participants with an LSR score greater than 1 in any of the 6 components. At day 29 and day 56, 94% (17/18) and 100% (18/18) of participants, respectively, had a score of 0 for all reactions.
 |
 |
The photographs in Figure 3, taken 7 days after the application of the first dose of ingenol mebutate gel 0.015% in each cycle of treatment of AK lesions on the face, show that there was less flaking/scaling and crusting in cycle 2 than in cycle 1. A review of participant photographs from the third treatment day of each cycle showed that the areas of erythema were the same in both cycles. The other 5 LSRs—flaking/scaling, crusting, swelling, vesiculation/pustulation, and erosion/ulceration—were observed in different areas of the treated field in the 2 cycles when applicable.
Adverse Events
The few AEs that were reported were considered to be mild in severity. The AEs included application-site pain (n=5), application-site pruritus (n=3), and nasopharyngitis (n=1). No serious AEs were reported. After the first treatment cycle, 1 participant experienced hypopigmentation at the treatment site that persisted as faint hypopigmentation at the last study visit (day 56).
AK Lesion Count
The lesion count in all participants at baseline ranged from 4 to 8, with a mean (SD) of 5.9 (1.55). Mean lesion count was substantially reduced at the end of cycle 1 (0.9 [1.39]) and cycle 2 (0.3 [0.57]). The change in lesion count from baseline to day 56 was greater than the change from baseline to day 29 (-5.7 [1.61] vs -5.0 [1.57])(P=.0137). Complete clearance at day 29 and day 56 was achieved in 55.6% (10/18) and 77.8% (14/18) of participants, respectively. The difference in the clearance rate between day 29 and day 56 did not reach statistical significance, most likely due to the small sample size.
Participant-Reported Outcomes
 |
 |
Visual analog scale scores for participant-perceived irritation were less than 50 on a scale of 0 to 100 during both application cycles. At 1 day and 3 days after application of the first dose of ingenol mebutate gel 0.015% in cycle 1, the mean (SD) VAS scores for irritation were 31.8 (37.06) and 37.9 (30.77), respectively. At the same time points in cycle 2, VAS scores were 44.2 (32.45) and 49.6 (26.90), respectively. No information was available regarding resolution of participant-perceived irritation, as irritation data were not collected after day 4 of each treatment cycle; therefore, P values were not determined. Participant satisfaction with treatment was high and nearly the same at the end of cycles 1 and 2 (VAS scores: 83.7 [12.73] and 83.8 [20.46], respectively).
Comment
Our findings show that a second course of treatment with ingenol mebutate gel 0.015% on the same site on the face or scalp produced a less intense inflammatory reaction than the first course of treatment. Composite LSR scores at each time point after the start of treatment were lower in cycle 2 than in cycle 1. The percentage of participants who demonstrated a severity score greater than 1 for any of the 6 components of the LSR assessment also was lower at time points in cycle 2 than in cycle 1. These results are consistent with the hypothesis that the activity of ingenol mebutate includes a mechanism that specifically targets transformed keratinocytes, which are reduced by the start of a second cycle of treatment.
The mechanism for the clinical efficacy of ingenol mebutate has not been fully described. Studies in preclinical models suggest at least 2 components, including direct cytotoxic effects on tumor cells and a localized inflammatory reaction that includes protein kinase C activation.11 Ingenol mebutate preferentially induces death in tumor cells and in proliferating undifferentiated keratinocytes.7,12 Cell death and protein kinase C activation lead to an inflammatory response dominated by neutrophils and other immunocompetent cells that add to the destruction of transformed cells.11
The reduced inflammatory response observed in participants during the second cycle of treatment in this study is consistent with the theory of a preferential action on transformed keratinocytes by ingenol mebutate. Once transformed keratinocytes are substantially cleared in cycle 1, fewer target cells remain, and therefore the inflammatory response is less intense in cycle 2. If ingenol mebutate were uniformly cytotoxic and inflammatory to all cells, the LSR scores in both cycles would be expected to be similar.
Assessment of participant-perceived irritation supplemented the measurement of the 6 visible manifestations of inflammation over each 4-week cycle. Participant-perceived irritation was recorded early in the cycles at 1 and 3 days after the first dose. Although it is difficult to standardize patient perceptions, VAS scores for irritation in cycle 2 were higher than those reported in cycle 1, which suggests an increased perception of irritation. The clinical relevance of this perception is not certain and may be due to the small number of participants and/or the time interval between the 2 treatment courses.
The results of this study were limited by the small patient sample. Additionally, LSR assessments were limited by the quality of the photographs. However, LSRs and AK clearance rates were similar to the pooled findings seen in the phase 3 studies of ingenol mebutate.3 Adverse events were predominantly conditions that occurred at the application site, as in phase 3 studies.3 Similarly, the time course of LSR development and resolution followed the same pattern as in those trials. The peak composite LSR score for the face and scalp was approximately 9 in both the present study (cycle 1) and in the pooled phase 3 studies.3
Conclusion
Ingenol mebutate gel 0.015% may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over the course of 2 treatment cycles. Patients may experience fewer LSRs on reapplication of ingenol mebutate to a previously treated site.
Acknowledgment
Editorial support was provided by Tanya MacNeil, PhD, of p-value communications, LLC, Cedar Knolls, New Jersey.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
1. Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
2. Berman B, Cohen DE, Amini S. What is the role of field-directed therapy in the treatment of actinic keratosis? part 1: overview and investigational topical agents. Cutis. 2012;89:241-250.
3. Lebwohl M, Swanson N, Anderson LL, et al. Ingenol mebutate gel for actinic keratosis. N Engl J Med. 2012;366:1010-1019.
4. Alomar A, Bichel J, McRae S. Vehicle-controlled, randomized, double-blind study to assess safety and efficacy of imiquimod 5% cream applied once daily 3 days per week in one or two courses of treatment of actinic keratoses on the head. Br J Dermatol. 2007;157:133-141.
5. Jorizzo J, Dinehart S, Matheson R, et al. Vehicle-controlled, double-blind, randomized study of imiquimod 5% cream applied 3 days per week in one or two courses of treatment for actinic keratoses on the head. J Am Acad Dermatol. 2007;57:265-268.
6. Del Rosso JQ, Sofen H, Leshin B, et al. Safety and efficacy of multiple 16-week courses of topical imiquimod for the treatment of large areas of skin involved with actinic keratoses. J Clin Aesthet Dermatol. 2009;2:20-28.
7. Stahlhut M, Bertelsen M, Hoyer-Hansen M, et al. Ingenol mebutate: induced cell death patterns in normal and cancer epithelial cells. J Drugs Dermatol. 2012;11:1181-1192.
8. Picato gel 0.015%, 0.05% [package insert]. Parsippany, NJ: LEO Pharma; 2013.
9. Rivers JK, Arlette J, Shear N, et al. Topical treatment of actinic keratoses with 3.0% diclofenac in 2.5% hyaluronan gel. Br J Dermatol. 2002;146:94-100.
10. Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62:582-590.
11. Challacombe JM, Suhrbier A, Parsons PG, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123-8132.
12. Ogbourne SM, Suhrbier A, Jones B, et al. Antitumor activity of 3-ingenyl angelate: plasma membrane and mitochondrial disruption and necrotic cell death. Cancer Res. 2004;64:2833-2839.
Practice Points
- Reapplication of ingenol mebutate gel 0.015% to the same treatment area on the face or scalp produced a less intense inflammatory reaction than the first treatment course.
- Ingenol mebutate may specifically target and remove transformed proliferating keratinocytes, cumulatively reducing the burden of sun-damaged skin over 2 treatment cycles.
- Almost all patients were either clear or almost clear of actinic keratosis lesions by 4 weeks following the second application of ingenol mebutate.
Localized Argyria With Pseudo-ochronosis
Localized cutaneous argyria often presents as asymptomatic black or blue-gray pigmented macules in areas of the skin exposed to silver-containing compounds.1 Silver may enter the skin by traumatic implantation or absorption via eccrine sweat glands.2 Our patient witnessed a gun fight several years ago while on a mission trip and sustained multiple shrapnel wounds.
As in our patient, hyperpigmentation may appear years following initial exposure. Over time, incident light reduces colorless silver salts and compounds to black elemental silver.3 It also has been suggested that metallic silver granules stimulate tyrosine kinase activity, leading to locally increased melanin production.4 Together, these processes result in the clinical appearance of a blue-black macule. Despite its long-standing association with silver, this appearance also has been noted with deposition of other metals.5 Histologically, metal deposits can be seen as black granules surrounding eccrine glands, blood vessels, and elastic fibers on higher magnification.6 Granules also may be found in sebaceous glands and arrector pili muscle fibers. These findings do not distinguish from generalized argyria due to increased serum silver levels; however, some cases of localized cutaneous argyria have demonstrated spheroid black globules with surrounding collagen necrosis,1 which have not been reported with generalized disease. Localized cutaneous argyria also may be associated with ocher pigmentation of thickened collagen fibers, resembling changes typically found in alkaptonuria, an inherited deficiency of homogentisic acid oxidase (an enzyme involved in tyrosine metabolism).7 The resulting buildup of metabolic intermediates leads to ochronosis, a deposition of ocher-pigmented intermediates in connective tissue throughout the body. In the skin, ocher pigmentation occurs in elastic fibers of the reticular dermis.1 Grossly, these changes result in a blue-gray discoloration of the skin due to a light-scattering phenomenon known as the Tyndall effect. Exogenous ochronosis also can occur, most commonly from the topical application of hydroquinone or other skin-lightening compounds.1,5 Ocher pigmentation occurring in the setting of localized cutaneous argyria is referred to as pseudo-ochronosis, a finding first described by Robinson-Bostom et al.1 The etiology of this condition is poorly understood, but Robinson-Bostom et al1 noted the appearance of dark metal granules surrounding collagen bundles and hypothesized that metal aggregates surrounding collagen bundles in pseudo-ochronosis cause a homogenized appearance under light microscopy. Yellow-brown, swollen, homogenized collagen bundles can be visualized in the reticular dermis with surrounding deposition of metal granules (Figures 1 and 2).1 Typical patterns of granule deposition in localized argyria also are present.
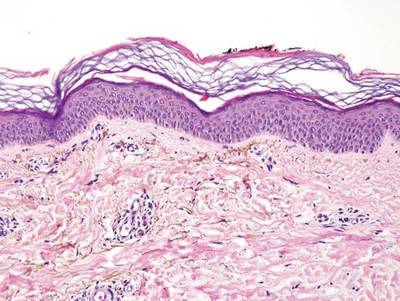

A blue nevus is a collection of proliferating dermal melanocytes. Many histologic subtypes exist and there may be extensive variability in the extent of sclerosis, cellular architecture, and tissue cellularity between each variant.8 Blue nevi commonly present as blue-black hyperpigmentation in the dermis and subcutaneous tissue.9 Histologically, they are characterized by slender, bipolar, dendritic melanocytes in a sclerotic stroma (Figure 3).8 Melanocytes are highly pigmented and contain small monomorphic nuclei. Lesions are relatively homogenous and typically are restricted to the dermis with epidermal sparing.9 Dark granules and ocher fibers are absent.
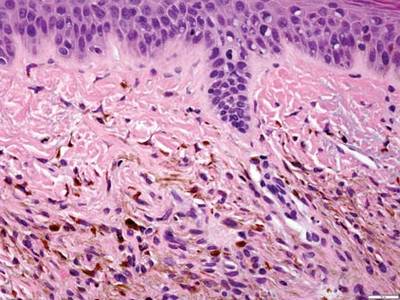
Long-term use of hydroxychloroquine or other antimalarials may cause a macular pattern of blue-gray hyperpigmentation.10 Biopsy specimens typically reveal coarse, yellow-brown pigment granules primarily affecting the superficial dermis (Figure 4). Granules are found both extracellularly and within macrophages. Fontana-Masson silver staining may identify melanin, as hydroxychloroquine-melanin binding may contribute to patterns of hyperpigmentation.10 Hemosiderin often is present in cases of hydroxychloroquine pigmentation. Preceding ecchymosis appears to favor the deposition of hydroxychloroquine in the skin.11 The absence of dark metal granules helps distinguish hydroxychloroquine pigmentation from argyria.
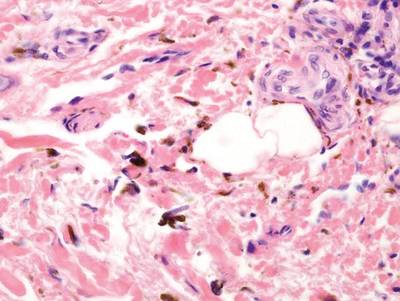
Regressed melanomas may appear clinically as gray macules. These lesions arise in cases of malignant melanoma that spontaneously regress without treatment. Spontaneous regression occurs in 10% to 35% of cases depending on tumor subtype.12 Lesions can have a variable appearance based on the degree of regression. Partial regression is demonstrated by mixed melanosis and fibrosis in the dermis (Figure 5).13,14 Melanin is housed within melanophages present in a variably expanded papillary dermis. Tumors in early stages of regression can be surrounded by an inflammatory infiltrate, which becomes diminished at later stages. However, a few exceptional cases have been noted with extensive inflammatory infiltrate and no residual tumor.14 Completely regressed lesions typically appear as a band of dermal melanophages in the absence of inflammation or melanocytic atypia.15 The finding of regressed melanoma should prompt further investigation including sentinel lymph node biopsy, as it may be associated with metastasis.
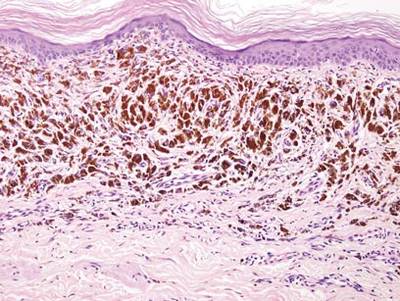
Tattooing occurs following traumatic penetration of the skin with impregnation of pigmented foreign material into deep dermal layers.16 Histologic examination usually reveals clumps of fine particulate material in the dermis (Figure 6). The color of the pigment depends on the agent used. For example, graphite appears as black particles that may be confused with localized cutaneous argyria. Distinction can be made using elemental identification techniques such as energy-dispersive X-ray spectroscopy.1 The intensity of the pigment in granules found in tattoos or localized cutaneous argyria will fail to diminish with the application of melanin bleach.6
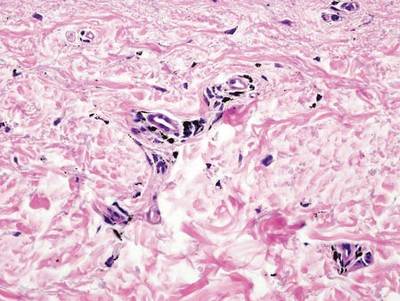
- Robinson-Bostom L, Pomerantz D, Wilkel C, et al. Localized argyria with pseudo-ochronosis. J Am Acad Dermatol. 2002;46:222-227.
- Tajirian AL, Campbell RM, Robinson-Bostom L. Localized argyria after exposure to aerosolized solder. Cutis. 2006;78:305-308.
- Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal photograph, a manifestation of passive photosensitivity. J Am Acad Dermatol. 1987;16:211-217.
- Buckley WR, Terhaar CJ. The skin as an excretory organ in argyria. Trans St Johns Hosp Dermatol Soc. 1973;59:39-44.
- Shimizu I, Dill SW, McBean J, et al. Metal-induced granule deposition with pseudo-ochronosis. J Am Acad Dermatol. 2010;63:357-359.
- Rackoff EMJ, Benbenisty KM, Maize JC, et al. Localized cutaneous argyria from an acupuncture needle clini-cally concerning for metastatic melanoma. Cutis. 2007;80:423-426.
- Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D, et al. The molecular basis of alkaptonuria. Nat Genet. 1996;14:5-6.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Granter SR, McKee PH, Calonje E, et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus.’ Am J Surg Pathol. 2001;25:316.
- Puri PK, Lountzis NI, Tyler W, et al. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35:1134-1137.
- Jallouli M, Francès C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149:935-940.
- Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20:315-322.
- LeBoit PE. Melanosis and its meanings. Am J Dermatopathol. 2002;24:369-372.
- Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
- Yang CH, Yeh JT, Shen SC, et al. Regressed subungual melanoma simulating cellular blue nevus: managed with sentinel lymph node biopsy. Dermatol Surg. 2006;32:577-581.
- Apfelberg DB, Manchester GH. Decorative and traumatic tattoo biophysics and removal. Clin Plast Surg. 1987;14:243-251.
Localized cutaneous argyria often presents as asymptomatic black or blue-gray pigmented macules in areas of the skin exposed to silver-containing compounds.1 Silver may enter the skin by traumatic implantation or absorption via eccrine sweat glands.2 Our patient witnessed a gun fight several years ago while on a mission trip and sustained multiple shrapnel wounds.
As in our patient, hyperpigmentation may appear years following initial exposure. Over time, incident light reduces colorless silver salts and compounds to black elemental silver.3 It also has been suggested that metallic silver granules stimulate tyrosine kinase activity, leading to locally increased melanin production.4 Together, these processes result in the clinical appearance of a blue-black macule. Despite its long-standing association with silver, this appearance also has been noted with deposition of other metals.5 Histologically, metal deposits can be seen as black granules surrounding eccrine glands, blood vessels, and elastic fibers on higher magnification.6 Granules also may be found in sebaceous glands and arrector pili muscle fibers. These findings do not distinguish from generalized argyria due to increased serum silver levels; however, some cases of localized cutaneous argyria have demonstrated spheroid black globules with surrounding collagen necrosis,1 which have not been reported with generalized disease. Localized cutaneous argyria also may be associated with ocher pigmentation of thickened collagen fibers, resembling changes typically found in alkaptonuria, an inherited deficiency of homogentisic acid oxidase (an enzyme involved in tyrosine metabolism).7 The resulting buildup of metabolic intermediates leads to ochronosis, a deposition of ocher-pigmented intermediates in connective tissue throughout the body. In the skin, ocher pigmentation occurs in elastic fibers of the reticular dermis.1 Grossly, these changes result in a blue-gray discoloration of the skin due to a light-scattering phenomenon known as the Tyndall effect. Exogenous ochronosis also can occur, most commonly from the topical application of hydroquinone or other skin-lightening compounds.1,5 Ocher pigmentation occurring in the setting of localized cutaneous argyria is referred to as pseudo-ochronosis, a finding first described by Robinson-Bostom et al.1 The etiology of this condition is poorly understood, but Robinson-Bostom et al1 noted the appearance of dark metal granules surrounding collagen bundles and hypothesized that metal aggregates surrounding collagen bundles in pseudo-ochronosis cause a homogenized appearance under light microscopy. Yellow-brown, swollen, homogenized collagen bundles can be visualized in the reticular dermis with surrounding deposition of metal granules (Figures 1 and 2).1 Typical patterns of granule deposition in localized argyria also are present.


A blue nevus is a collection of proliferating dermal melanocytes. Many histologic subtypes exist and there may be extensive variability in the extent of sclerosis, cellular architecture, and tissue cellularity between each variant.8 Blue nevi commonly present as blue-black hyperpigmentation in the dermis and subcutaneous tissue.9 Histologically, they are characterized by slender, bipolar, dendritic melanocytes in a sclerotic stroma (Figure 3).8 Melanocytes are highly pigmented and contain small monomorphic nuclei. Lesions are relatively homogenous and typically are restricted to the dermis with epidermal sparing.9 Dark granules and ocher fibers are absent.

Long-term use of hydroxychloroquine or other antimalarials may cause a macular pattern of blue-gray hyperpigmentation.10 Biopsy specimens typically reveal coarse, yellow-brown pigment granules primarily affecting the superficial dermis (Figure 4). Granules are found both extracellularly and within macrophages. Fontana-Masson silver staining may identify melanin, as hydroxychloroquine-melanin binding may contribute to patterns of hyperpigmentation.10 Hemosiderin often is present in cases of hydroxychloroquine pigmentation. Preceding ecchymosis appears to favor the deposition of hydroxychloroquine in the skin.11 The absence of dark metal granules helps distinguish hydroxychloroquine pigmentation from argyria.

Regressed melanomas may appear clinically as gray macules. These lesions arise in cases of malignant melanoma that spontaneously regress without treatment. Spontaneous regression occurs in 10% to 35% of cases depending on tumor subtype.12 Lesions can have a variable appearance based on the degree of regression. Partial regression is demonstrated by mixed melanosis and fibrosis in the dermis (Figure 5).13,14 Melanin is housed within melanophages present in a variably expanded papillary dermis. Tumors in early stages of regression can be surrounded by an inflammatory infiltrate, which becomes diminished at later stages. However, a few exceptional cases have been noted with extensive inflammatory infiltrate and no residual tumor.14 Completely regressed lesions typically appear as a band of dermal melanophages in the absence of inflammation or melanocytic atypia.15 The finding of regressed melanoma should prompt further investigation including sentinel lymph node biopsy, as it may be associated with metastasis.

Tattooing occurs following traumatic penetration of the skin with impregnation of pigmented foreign material into deep dermal layers.16 Histologic examination usually reveals clumps of fine particulate material in the dermis (Figure 6). The color of the pigment depends on the agent used. For example, graphite appears as black particles that may be confused with localized cutaneous argyria. Distinction can be made using elemental identification techniques such as energy-dispersive X-ray spectroscopy.1 The intensity of the pigment in granules found in tattoos or localized cutaneous argyria will fail to diminish with the application of melanin bleach.6

Localized cutaneous argyria often presents as asymptomatic black or blue-gray pigmented macules in areas of the skin exposed to silver-containing compounds.1 Silver may enter the skin by traumatic implantation or absorption via eccrine sweat glands.2 Our patient witnessed a gun fight several years ago while on a mission trip and sustained multiple shrapnel wounds.
As in our patient, hyperpigmentation may appear years following initial exposure. Over time, incident light reduces colorless silver salts and compounds to black elemental silver.3 It also has been suggested that metallic silver granules stimulate tyrosine kinase activity, leading to locally increased melanin production.4 Together, these processes result in the clinical appearance of a blue-black macule. Despite its long-standing association with silver, this appearance also has been noted with deposition of other metals.5 Histologically, metal deposits can be seen as black granules surrounding eccrine glands, blood vessels, and elastic fibers on higher magnification.6 Granules also may be found in sebaceous glands and arrector pili muscle fibers. These findings do not distinguish from generalized argyria due to increased serum silver levels; however, some cases of localized cutaneous argyria have demonstrated spheroid black globules with surrounding collagen necrosis,1 which have not been reported with generalized disease. Localized cutaneous argyria also may be associated with ocher pigmentation of thickened collagen fibers, resembling changes typically found in alkaptonuria, an inherited deficiency of homogentisic acid oxidase (an enzyme involved in tyrosine metabolism).7 The resulting buildup of metabolic intermediates leads to ochronosis, a deposition of ocher-pigmented intermediates in connective tissue throughout the body. In the skin, ocher pigmentation occurs in elastic fibers of the reticular dermis.1 Grossly, these changes result in a blue-gray discoloration of the skin due to a light-scattering phenomenon known as the Tyndall effect. Exogenous ochronosis also can occur, most commonly from the topical application of hydroquinone or other skin-lightening compounds.1,5 Ocher pigmentation occurring in the setting of localized cutaneous argyria is referred to as pseudo-ochronosis, a finding first described by Robinson-Bostom et al.1 The etiology of this condition is poorly understood, but Robinson-Bostom et al1 noted the appearance of dark metal granules surrounding collagen bundles and hypothesized that metal aggregates surrounding collagen bundles in pseudo-ochronosis cause a homogenized appearance under light microscopy. Yellow-brown, swollen, homogenized collagen bundles can be visualized in the reticular dermis with surrounding deposition of metal granules (Figures 1 and 2).1 Typical patterns of granule deposition in localized argyria also are present.


A blue nevus is a collection of proliferating dermal melanocytes. Many histologic subtypes exist and there may be extensive variability in the extent of sclerosis, cellular architecture, and tissue cellularity between each variant.8 Blue nevi commonly present as blue-black hyperpigmentation in the dermis and subcutaneous tissue.9 Histologically, they are characterized by slender, bipolar, dendritic melanocytes in a sclerotic stroma (Figure 3).8 Melanocytes are highly pigmented and contain small monomorphic nuclei. Lesions are relatively homogenous and typically are restricted to the dermis with epidermal sparing.9 Dark granules and ocher fibers are absent.

Long-term use of hydroxychloroquine or other antimalarials may cause a macular pattern of blue-gray hyperpigmentation.10 Biopsy specimens typically reveal coarse, yellow-brown pigment granules primarily affecting the superficial dermis (Figure 4). Granules are found both extracellularly and within macrophages. Fontana-Masson silver staining may identify melanin, as hydroxychloroquine-melanin binding may contribute to patterns of hyperpigmentation.10 Hemosiderin often is present in cases of hydroxychloroquine pigmentation. Preceding ecchymosis appears to favor the deposition of hydroxychloroquine in the skin.11 The absence of dark metal granules helps distinguish hydroxychloroquine pigmentation from argyria.

Regressed melanomas may appear clinically as gray macules. These lesions arise in cases of malignant melanoma that spontaneously regress without treatment. Spontaneous regression occurs in 10% to 35% of cases depending on tumor subtype.12 Lesions can have a variable appearance based on the degree of regression. Partial regression is demonstrated by mixed melanosis and fibrosis in the dermis (Figure 5).13,14 Melanin is housed within melanophages present in a variably expanded papillary dermis. Tumors in early stages of regression can be surrounded by an inflammatory infiltrate, which becomes diminished at later stages. However, a few exceptional cases have been noted with extensive inflammatory infiltrate and no residual tumor.14 Completely regressed lesions typically appear as a band of dermal melanophages in the absence of inflammation or melanocytic atypia.15 The finding of regressed melanoma should prompt further investigation including sentinel lymph node biopsy, as it may be associated with metastasis.

Tattooing occurs following traumatic penetration of the skin with impregnation of pigmented foreign material into deep dermal layers.16 Histologic examination usually reveals clumps of fine particulate material in the dermis (Figure 6). The color of the pigment depends on the agent used. For example, graphite appears as black particles that may be confused with localized cutaneous argyria. Distinction can be made using elemental identification techniques such as energy-dispersive X-ray spectroscopy.1 The intensity of the pigment in granules found in tattoos or localized cutaneous argyria will fail to diminish with the application of melanin bleach.6

- Robinson-Bostom L, Pomerantz D, Wilkel C, et al. Localized argyria with pseudo-ochronosis. J Am Acad Dermatol. 2002;46:222-227.
- Tajirian AL, Campbell RM, Robinson-Bostom L. Localized argyria after exposure to aerosolized solder. Cutis. 2006;78:305-308.
- Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal photograph, a manifestation of passive photosensitivity. J Am Acad Dermatol. 1987;16:211-217.
- Buckley WR, Terhaar CJ. The skin as an excretory organ in argyria. Trans St Johns Hosp Dermatol Soc. 1973;59:39-44.
- Shimizu I, Dill SW, McBean J, et al. Metal-induced granule deposition with pseudo-ochronosis. J Am Acad Dermatol. 2010;63:357-359.
- Rackoff EMJ, Benbenisty KM, Maize JC, et al. Localized cutaneous argyria from an acupuncture needle clini-cally concerning for metastatic melanoma. Cutis. 2007;80:423-426.
- Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D, et al. The molecular basis of alkaptonuria. Nat Genet. 1996;14:5-6.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Granter SR, McKee PH, Calonje E, et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus.’ Am J Surg Pathol. 2001;25:316.
- Puri PK, Lountzis NI, Tyler W, et al. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35:1134-1137.
- Jallouli M, Francès C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149:935-940.
- Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20:315-322.
- LeBoit PE. Melanosis and its meanings. Am J Dermatopathol. 2002;24:369-372.
- Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
- Yang CH, Yeh JT, Shen SC, et al. Regressed subungual melanoma simulating cellular blue nevus: managed with sentinel lymph node biopsy. Dermatol Surg. 2006;32:577-581.
- Apfelberg DB, Manchester GH. Decorative and traumatic tattoo biophysics and removal. Clin Plast Surg. 1987;14:243-251.
- Robinson-Bostom L, Pomerantz D, Wilkel C, et al. Localized argyria with pseudo-ochronosis. J Am Acad Dermatol. 2002;46:222-227.
- Tajirian AL, Campbell RM, Robinson-Bostom L. Localized argyria after exposure to aerosolized solder. Cutis. 2006;78:305-308.
- Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal photograph, a manifestation of passive photosensitivity. J Am Acad Dermatol. 1987;16:211-217.
- Buckley WR, Terhaar CJ. The skin as an excretory organ in argyria. Trans St Johns Hosp Dermatol Soc. 1973;59:39-44.
- Shimizu I, Dill SW, McBean J, et al. Metal-induced granule deposition with pseudo-ochronosis. J Am Acad Dermatol. 2010;63:357-359.
- Rackoff EMJ, Benbenisty KM, Maize JC, et al. Localized cutaneous argyria from an acupuncture needle clini-cally concerning for metastatic melanoma. Cutis. 2007;80:423-426.
- Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D, et al. The molecular basis of alkaptonuria. Nat Genet. 1996;14:5-6.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Granter SR, McKee PH, Calonje E, et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus.’ Am J Surg Pathol. 2001;25:316.
- Puri PK, Lountzis NI, Tyler W, et al. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35:1134-1137.
- Jallouli M, Francès C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149:935-940.
- Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20:315-322.
- LeBoit PE. Melanosis and its meanings. Am J Dermatopathol. 2002;24:369-372.
- Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
- Yang CH, Yeh JT, Shen SC, et al. Regressed subungual melanoma simulating cellular blue nevus: managed with sentinel lymph node biopsy. Dermatol Surg. 2006;32:577-581.
- Apfelberg DB, Manchester GH. Decorative and traumatic tattoo biophysics and removal. Clin Plast Surg. 1987;14:243-251.
What Is Your Diagnosis? Lepromatous Leprosy
The Diagnosis: Lepromatous Leprosy
Histopathologic examination of a punch biopsy specimen (Figures 1 and 2) disclosed a grenz zone and a diffuse infiltrative process beneath a normal-appearing epidermis. Higher-power examination revealed areas containing macrophages (Virchow cells) with cloudy regions devoid of nuclei (globi). Fite stain demonstrated numerous intracytoplasmic acid-fast bacilli (Figure 3). Laboratory test results for rapid plasma reagin and human immunodeficiency virus were negative, and a complete blood cell count was normal.



On further questioning the patient revealed he was an immigrant from Micronesia, and he described decreased sensation and numbness in the lesions that had been present from onset. Physical examination was consistent with this history and revealed hypoesthesia of the lesions, particularly over the central aspect of the depigmented macules. Based on the clinical examination and histopathologic findings, a diagnosis of lepromatous leprosy was made.
Therapy with rifampin, clofazimine, and dapsone was initiated. Unfortunately, compliance was poor, and at clinic follow-up 10 months later the patient demonstrated formation of new indurated lesions as well as mild eyelid swelling and edema of the hands thought to be consistent with erythema nodosum leprosum. Prednisone was then initiated and the dose of clofazimine was increased from 50 mg daily to 100 mg daily with excellent clinical response.
Mycobacterium leprae is a small, slightly curved rod that is an acid-fast, obligate, intracellular organism. It remains endemic in Brazil and Southeast Asia but may present outside of these areas secondary to immigration.1
Hallmarks of the disease are anesthetic skin or mucous membrane lesions with thickened peripheral nerves.2 It grows best at 27°C to 33°C, thereby affecting cooler areas of the human body such as earlobes, knees, and distal extremities.3 It is most likely spread by aerosolized respiratory droplets and less commonly by direct contact. There have been reports suggesting transmission via armadillos.4
Genetic susceptibility influences the development of leprosy, while HLA type influences the immune response and hence the type of leprosy.5 Ridley and Jopling6 devised a classification system based on the immunologic response to M leprae. Highly reactive hosts with a vigorous cell-mediated response to M leprae develop tuberculoid leprosy and exhibit few skin lesions containing rare organisms. In contrast, anergic hosts develop lepromatous leprosy, characterized by multiple skin lesions, abundant organisms, and diffuse disease. Borderline tuberculoid, borderline, and borderline lepromatous make up the middle of the spectrum.6-9 Skin lesions can present with poorly defined, hypopigmented macules of indeterminate leprosy on one end and diffuse skin involvement of lepromatous leprosy on the opposite end. Diffuse involvement includes facial skin thickening, classic leonine facies, loss of eyebrows and eyelashes, anesthetic lesions, and anhidrosis.
Erythema nodosum leprosum occurs with chronic infection from M leprae, most commonly lepromatous leprosy. Immune complex deposition results in vasculitis and inflammatory foci. This phenomenon is thought to be secondary to high antigen load released by dying mycobacteria, causing secretion of tumor necrosis factor a from macrophages.1 Erythema nodosum leprosum demonstrates rapid onset of tender erythematous plaques or nodules, most commonly on the face and extensor surfaces of the extremities, with fever, malaise, iritis, arthralgia, and orchitis. Clofazimine therapy probably decreases the occurence.1 Treatment includes systemic corticosteroids and/or thalidomide.
- Moschella S, Ooi W. Update on leprosy in immigrants in the United States: status in the year 2000. Clin Infect Dis. 2001;32:930-937.
- Abraham S, Job C, Joseph G, et al. Epidemiological significance of first skin lesion in leprosy. Int J Lep Other Mycobact Dis. 1998;66:131-139.
- Shepard C. The experimental disease that follows the injection of human bacilli into footpads of mice. J Exp Med. 1960;112:445-454.
- Leprosy: global target attained. Wkly Epidemiol Rec. 2001;20:155-156.
- World Health Organization. Global leprosy situation, 2005. Wkly Epidemiol Rec. 2005;80:289-295.
- Ridley DS, Jopling WH. A classification of leprosy for research purposes. Lepr Rev. 1962;33:119-128.
- Lane J. Borderline tuberculoid leprosy in a woman from the state of Georgia with armadillo exposure. J Am Acad Dermatol. 2006;55:714-716.
- Fitness J, Tosh K, Hill AV. Genetics of susceptibility to leprosy. Genes Immun. 2002;3:441-453.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
The Diagnosis: Lepromatous Leprosy
Histopathologic examination of a punch biopsy specimen (Figures 1 and 2) disclosed a grenz zone and a diffuse infiltrative process beneath a normal-appearing epidermis. Higher-power examination revealed areas containing macrophages (Virchow cells) with cloudy regions devoid of nuclei (globi). Fite stain demonstrated numerous intracytoplasmic acid-fast bacilli (Figure 3). Laboratory test results for rapid plasma reagin and human immunodeficiency virus were negative, and a complete blood cell count was normal.



On further questioning the patient revealed he was an immigrant from Micronesia, and he described decreased sensation and numbness in the lesions that had been present from onset. Physical examination was consistent with this history and revealed hypoesthesia of the lesions, particularly over the central aspect of the depigmented macules. Based on the clinical examination and histopathologic findings, a diagnosis of lepromatous leprosy was made.
Therapy with rifampin, clofazimine, and dapsone was initiated. Unfortunately, compliance was poor, and at clinic follow-up 10 months later the patient demonstrated formation of new indurated lesions as well as mild eyelid swelling and edema of the hands thought to be consistent with erythema nodosum leprosum. Prednisone was then initiated and the dose of clofazimine was increased from 50 mg daily to 100 mg daily with excellent clinical response.
Mycobacterium leprae is a small, slightly curved rod that is an acid-fast, obligate, intracellular organism. It remains endemic in Brazil and Southeast Asia but may present outside of these areas secondary to immigration.1
Hallmarks of the disease are anesthetic skin or mucous membrane lesions with thickened peripheral nerves.2 It grows best at 27°C to 33°C, thereby affecting cooler areas of the human body such as earlobes, knees, and distal extremities.3 It is most likely spread by aerosolized respiratory droplets and less commonly by direct contact. There have been reports suggesting transmission via armadillos.4
Genetic susceptibility influences the development of leprosy, while HLA type influences the immune response and hence the type of leprosy.5 Ridley and Jopling6 devised a classification system based on the immunologic response to M leprae. Highly reactive hosts with a vigorous cell-mediated response to M leprae develop tuberculoid leprosy and exhibit few skin lesions containing rare organisms. In contrast, anergic hosts develop lepromatous leprosy, characterized by multiple skin lesions, abundant organisms, and diffuse disease. Borderline tuberculoid, borderline, and borderline lepromatous make up the middle of the spectrum.6-9 Skin lesions can present with poorly defined, hypopigmented macules of indeterminate leprosy on one end and diffuse skin involvement of lepromatous leprosy on the opposite end. Diffuse involvement includes facial skin thickening, classic leonine facies, loss of eyebrows and eyelashes, anesthetic lesions, and anhidrosis.
Erythema nodosum leprosum occurs with chronic infection from M leprae, most commonly lepromatous leprosy. Immune complex deposition results in vasculitis and inflammatory foci. This phenomenon is thought to be secondary to high antigen load released by dying mycobacteria, causing secretion of tumor necrosis factor a from macrophages.1 Erythema nodosum leprosum demonstrates rapid onset of tender erythematous plaques or nodules, most commonly on the face and extensor surfaces of the extremities, with fever, malaise, iritis, arthralgia, and orchitis. Clofazimine therapy probably decreases the occurence.1 Treatment includes systemic corticosteroids and/or thalidomide.
The Diagnosis: Lepromatous Leprosy
Histopathologic examination of a punch biopsy specimen (Figures 1 and 2) disclosed a grenz zone and a diffuse infiltrative process beneath a normal-appearing epidermis. Higher-power examination revealed areas containing macrophages (Virchow cells) with cloudy regions devoid of nuclei (globi). Fite stain demonstrated numerous intracytoplasmic acid-fast bacilli (Figure 3). Laboratory test results for rapid plasma reagin and human immunodeficiency virus were negative, and a complete blood cell count was normal.



On further questioning the patient revealed he was an immigrant from Micronesia, and he described decreased sensation and numbness in the lesions that had been present from onset. Physical examination was consistent with this history and revealed hypoesthesia of the lesions, particularly over the central aspect of the depigmented macules. Based on the clinical examination and histopathologic findings, a diagnosis of lepromatous leprosy was made.
Therapy with rifampin, clofazimine, and dapsone was initiated. Unfortunately, compliance was poor, and at clinic follow-up 10 months later the patient demonstrated formation of new indurated lesions as well as mild eyelid swelling and edema of the hands thought to be consistent with erythema nodosum leprosum. Prednisone was then initiated and the dose of clofazimine was increased from 50 mg daily to 100 mg daily with excellent clinical response.
Mycobacterium leprae is a small, slightly curved rod that is an acid-fast, obligate, intracellular organism. It remains endemic in Brazil and Southeast Asia but may present outside of these areas secondary to immigration.1
Hallmarks of the disease are anesthetic skin or mucous membrane lesions with thickened peripheral nerves.2 It grows best at 27°C to 33°C, thereby affecting cooler areas of the human body such as earlobes, knees, and distal extremities.3 It is most likely spread by aerosolized respiratory droplets and less commonly by direct contact. There have been reports suggesting transmission via armadillos.4
Genetic susceptibility influences the development of leprosy, while HLA type influences the immune response and hence the type of leprosy.5 Ridley and Jopling6 devised a classification system based on the immunologic response to M leprae. Highly reactive hosts with a vigorous cell-mediated response to M leprae develop tuberculoid leprosy and exhibit few skin lesions containing rare organisms. In contrast, anergic hosts develop lepromatous leprosy, characterized by multiple skin lesions, abundant organisms, and diffuse disease. Borderline tuberculoid, borderline, and borderline lepromatous make up the middle of the spectrum.6-9 Skin lesions can present with poorly defined, hypopigmented macules of indeterminate leprosy on one end and diffuse skin involvement of lepromatous leprosy on the opposite end. Diffuse involvement includes facial skin thickening, classic leonine facies, loss of eyebrows and eyelashes, anesthetic lesions, and anhidrosis.
Erythema nodosum leprosum occurs with chronic infection from M leprae, most commonly lepromatous leprosy. Immune complex deposition results in vasculitis and inflammatory foci. This phenomenon is thought to be secondary to high antigen load released by dying mycobacteria, causing secretion of tumor necrosis factor a from macrophages.1 Erythema nodosum leprosum demonstrates rapid onset of tender erythematous plaques or nodules, most commonly on the face and extensor surfaces of the extremities, with fever, malaise, iritis, arthralgia, and orchitis. Clofazimine therapy probably decreases the occurence.1 Treatment includes systemic corticosteroids and/or thalidomide.
- Moschella S, Ooi W. Update on leprosy in immigrants in the United States: status in the year 2000. Clin Infect Dis. 2001;32:930-937.
- Abraham S, Job C, Joseph G, et al. Epidemiological significance of first skin lesion in leprosy. Int J Lep Other Mycobact Dis. 1998;66:131-139.
- Shepard C. The experimental disease that follows the injection of human bacilli into footpads of mice. J Exp Med. 1960;112:445-454.
- Leprosy: global target attained. Wkly Epidemiol Rec. 2001;20:155-156.
- World Health Organization. Global leprosy situation, 2005. Wkly Epidemiol Rec. 2005;80:289-295.
- Ridley DS, Jopling WH. A classification of leprosy for research purposes. Lepr Rev. 1962;33:119-128.
- Lane J. Borderline tuberculoid leprosy in a woman from the state of Georgia with armadillo exposure. J Am Acad Dermatol. 2006;55:714-716.
- Fitness J, Tosh K, Hill AV. Genetics of susceptibility to leprosy. Genes Immun. 2002;3:441-453.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
- Moschella S, Ooi W. Update on leprosy in immigrants in the United States: status in the year 2000. Clin Infect Dis. 2001;32:930-937.
- Abraham S, Job C, Joseph G, et al. Epidemiological significance of first skin lesion in leprosy. Int J Lep Other Mycobact Dis. 1998;66:131-139.
- Shepard C. The experimental disease that follows the injection of human bacilli into footpads of mice. J Exp Med. 1960;112:445-454.
- Leprosy: global target attained. Wkly Epidemiol Rec. 2001;20:155-156.
- World Health Organization. Global leprosy situation, 2005. Wkly Epidemiol Rec. 2005;80:289-295.
- Ridley DS, Jopling WH. A classification of leprosy for research purposes. Lepr Rev. 1962;33:119-128.
- Lane J. Borderline tuberculoid leprosy in a woman from the state of Georgia with armadillo exposure. J Am Acad Dermatol. 2006;55:714-716.
- Fitness J, Tosh K, Hill AV. Genetics of susceptibility to leprosy. Genes Immun. 2002;3:441-453.
- Moschella SL. An update on the diagnosis and treatment of leprosy. J Am Acad Dermatol. 2004;51:417-426.
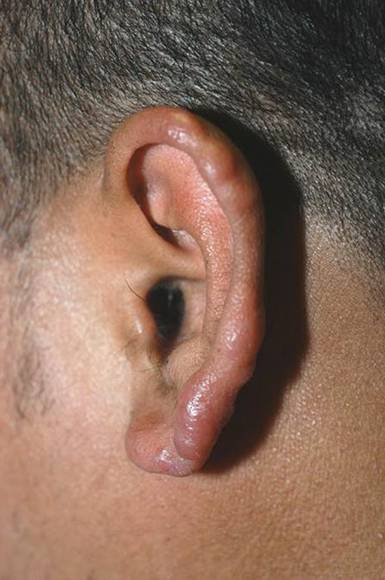
A 37-year-old man presented with pruritic lesions over the arms, legs, face, and back of 4 months’ duration that had been refractory to topical steroid treatment. He reported a 15-lb weight loss that he attributed to recent intranasal cocaine use. His medical history revealed obesity. There was no known history of sexually transmitted diseases, human immunodeficiency virus infection, tuberculosis, diabetes mellitus, or intravenous drug use. Physical examination revealed small nodules over the pinnae, plaques on the forehead, and large plaques with depigmented macules of variable sizes over the extremities and back. Some lesions on the extremities were violaceous in appearance, while others on the upper extremities had raised borders.
Cutaneous Side Effects of Chemotherapy in Pediatric Oncology Patients
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.
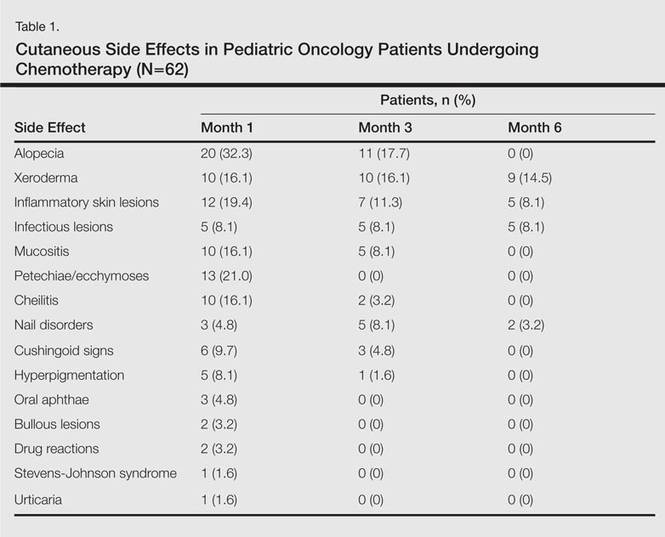
The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).
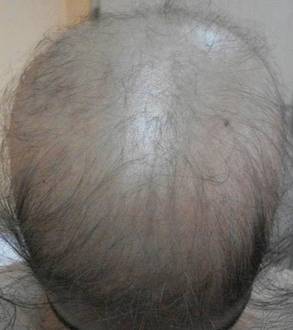
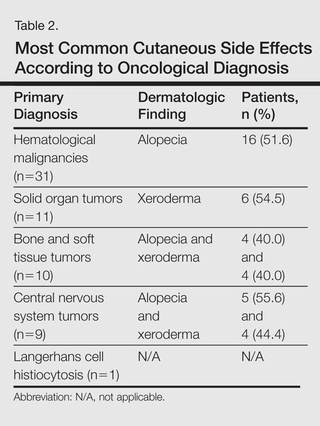
The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).
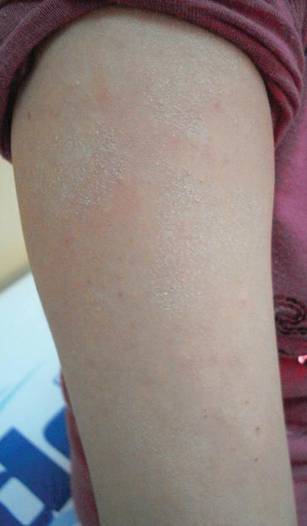
Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.
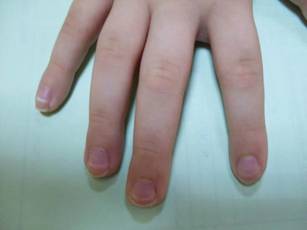
Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.

The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).


The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).

Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.

Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
Pediatric oncology patients can present with various skin lesions related to both their primary disease and immunosuppressive treatments. In the majority of cases, cutaneous findings are associated with the use of chemotherapeutic agents. The toxic effects of chemotherapeutic agents, which generally are associated with treatment of solid organ malignancies (eg, liver, kidneys), can be detected by oncologists using clinical signs and laboratory tests.1-3 However, it also is important for dermatologists to recognize and evaluate cutaneous side effects associated with chemotherapeutic agents. Reports in the literature of cutaneous side effects of chemotherapy in pediatric patients generally are limited to case studies. This study aimed to evaluate the characteristics of cutaneous side effects of chemotherapy in pediatric oncology patients.
Materials and Methods
The study was performed through the collaboration of the departments of dermatology and venereology and pediatric oncology in the Faculty of Medicine at Ege University, Izmir, Turkey. Sixty-five pediatric oncology patients who were scheduled to undergo chemotherapy from May 2011 to May 2013 were included in the study. Clinical examination of dermatologic findings was conducted at baseline (prior to beginning chemotherapy) and at months 1, 3, and 6 of treatment. Patients were examined a total of 4 times during the study. Patients with a history of skin disease prior to diagnosis of their malignancy were excluded, as the study aimed to evaluate cutaneous side effects of chemotherapy. Patients who developed cutaneous side effects during the study period were photographed. Skin biopsy was performed to confirm clinical diagnosis. Patients were split into 5 groups according to oncological diagnoses, including hematological malignancies, solid organ tumors, bone and soft tissue tumors, central nervous system tumors, and Langerhans cell histiocytosis. Data regarding age, gender, treatments administered (ie, chemotherapeutics, antibiotics, antifungals, antivirals), and dermatologic signs were recorded. Mucocutaneous findings were classified as infectious (viral, bacterial, fungal) lesions, bullous lesions, inflammatory dermatoses (eg, diaper dermatitis, asteatotic eczema, contact dermatitis, seborrheic dermatitis), xeroderma, petechiae/ecchymoses, nail signs, alopecia, mucositis, cheilitis, oral aphthae, drug reactions confirmed by histopathology, cushingoid signs (eg, striae, acneform eruption, hypertrichosis), and cutaneous hyperpigmentation.
Statistical analysis was performed using SPSS version 15.0 and χ2 test was applied to the analysis.
Results
Of 65 patients, 62 completed the study and were included in the analysis. Three patients were excluded from the results, as 2 patients died during treatment and 1 patient withdrew from the study prior to completion. Twenty-seven (43.5%) patients were female and 35 (56.5%) were male ranging in age from 1 to 17 years (mean age, 8.14 years; median age [standard deviation], 7.25 [5.42] years). There were 31 (50%) patients in the hematological malignancies group, 11 (17.7%) in the solid organ tumors group, 10 (16.1%) in the bone and soft tissue tumors group, and 9 (14.5%) in the central nervous system tumors group; Langerhans cell histiocytosis was diagnosed in 1 (1.6%) patient. Hodgkin lymphoma made up 29.0% (n=9) of hematological malignancies. Other hematological malignancies included acute myeloblastic leukemia (n=7 [22.5%]), acute lymphoblastic leukemia (n=7 [22.5%]), T-cell lymphoma (n=5 [16.1%]), non-Hodgkin lym-phoma (n=1 [3.2%]), anaplastic giant cell lymphoma (n=1 [3.2%]), and diffuse giant cell lymphoma (n=1 [3.2%]).
In addition to chemotherapeutic agents, 7 (11.3%) patients in this study also received antibiotics and 3 (4.8%) received antivirals. The most frequently employed chemotherapeutic agents were vincristine, methotrexate, cytarabine, etoposide, and dexamethasone. Cyclophosphamide, doxorubicin, ifosfamide, asparaginase, carboplatin, procarbazine, daunorubicin, actinomycin D, vinblastine, cisplatin, bleomycin, idarubicin, 6-mercaptopurine, temozolamide, and cyclosporine also were administered. The most commonly encountered dermatological side effects were alopecia, xeroderma, inflammatory skin lesions, infectious lesions, and mucositis, respectively (Table 1). Cutaneous side effects were frequently seen at months 1 and 3 of treatment.

The most commonly encountered dermatologic side effect was alopecia (31/62 [50%]). Anagen effluvium (Figure 1) was detected in half of the cases, while complete scalp hair loss was noted in the rest. Alopecia was encountered more commonly in cases with central nervous system tumors (5/9 [55.6%]) and hematological malignancies (16/31 [51.6%])(Table 2).


The second most commonly encountered side effect was xeroderma (29/62 [46.8%])(Figure 2). This side effect was most commonly encountered in patients with solid organ tumors (6/11 [54.5%]) and central nervous system tumors (4/9 [44.4%]), and occurred less frequently with bone and soft tissue tumors (4/10 [40.0%]).

Findings of eczema accounted for the majority of inflammatory lesions, which were the third most commonly encountered side effects. Among 24 cases of inflammatory skin lesions, 8 patients (33.3%) had diaper dermatitis, 7 (29.2%) had asteatotic eczema, 6 (25.0%) had contact dermatitis, and 3 (12.5%) had seborrheic dermatitis. Although inflammatory skin lesions were commonly encountered in patients with hematological malignancies (14/31 [45.2%]), the difference was not statistically significant.
Mucositis and oral aphthous lesions were observed in 15 (24.2%) and 3 (4.8%) patients, respectively. Nail signs were noted in 10 (16.1%) patients; 4 patients had transverse streaks on the nail plates, 3 had linear streaks, 2 had nail plate fragility, and 1 had increased pigmentation at the nail bed and periungual area. Figure 3 shows linear streaks on the nail plate. These side effects were most commonly encountered in patients with solid organ tumors (5/11 [45.5%]); however, the difference was not statistically significant when compared with the other diagnostic groups.

Dermatologic signs with infectious origins were detected in 15 (24.2%) patients; 2 patients had herpes labialis, 2 had verruca vulgaris, 3 had bacterial folliculitis, 1 had acute paronychia, 1 had soft tissue infection, 2 had tinea versicolor, and 4 had mucocutaneous candidiasis. Dermatologic side effects due to infectious causes were more commonly encountered in patients with bone and soft tissue tumors (4/11 [36.4%]), and the difference was statistically significant when compared with the other diagnostic groups (P=.04).
Petechiae and ecchymotic lesions were present in 13 (21.0%) patients. These side effects occurred mainly in the first month of chemotherapy, namely when patients were in the pancytopenic phase.
Comment
Variability among the oncological diagnosis and drugs used in treatment as well as increased numbers of chemotherapeutic agents available have led to many side effects and complications in pediatric oncology patients undergoing chemotherapy.1,2 Comprehensive studies regarding the cutaneous side effects of chemotherapeuticagents in cancer treatment have been conducted in adult patients. Side effects in pediatric patients have only been documented in case reports in the literature. In our study of pediatric oncology patients undergoing treatment with chemotherapy, the most commonly observed dermatologic side effect was alopecia, followed by xeroderma, inflammatory lesions, infectious lesions, mucositis, petechiae/ecchymoses, cheilitis, nail disorders, cushingoid signs, oral aphthae, bullous lesions, and drug reactions confirmed histopathologically (Table 1).
Because the common effects of chemotherapeutic agents used in cancer treatment are greatest in areas of rapidly dividing cells, the skin and skin appendages frequently are affected by these drugs.1-3 Cutaneous signs are frequently observed, especially in regions with increased mitotic activity such as the hair, mucosa, and nails.
Kamil et al1 reported that the incidence of alopecia was 64.3% (74/115) in a study of adult cancer patients who underwent chemotherapy. Chemotherapeutic agents that have commonly caused alopecia are vincristine, daunorubicin, doxorubicin, cyclophosphamide, etoposide, cytarabine, and carboplatin.1,2 In our study, alopecia was noted in 31 (50.0%) patients, especially with the use of vincristine (7/31 [22.6%]), daunorubicin (8/31 [25.8%]), doxorubicin (6/31 [19.4%]), and cyclophosphamide (10/31 [32.3%]).
Darkening of the skin and paleness accompanied the majority of cases of xeroderma in our study. Skin dryness was in an ichthyosiform appearance and was severe in 1 patient who was diagnosed with osteosarcoma. Asteatotic eczema and cheilitis were related to skin dryness. It has been reported that acquired paraneoplastic ichthyosis can develop in hematological malignancies, primarily in patients with Hodgkin lymphoma.4
The incidence of mucositis has been related to the doses of chemotherapeutic agents. Although it is a commonly encountered side effect, there is no standard treatment of mucositis; therefore, preventive care in patients undergoing chemotherapy is important. It has been reported that practicing good oral hygiene before the treatment period can decrease the incidence of mucositis.5-9 The lower incidence of mucositis in our study compared to the literature (55.6%)5 can be attributed to the lower doses of chemotherapy drugs administered to children due to their weights; they also had active oral mucosa care during chemotherapy.
Another common complication observed in our study was nail disorders. Transverse streaks commonly are encountered due to damage in the nail matrix. Other signs are increased linear streaks, longitudinal melanonychia, nail plate fragility, and onycholysis.10
Cancer patients acquire infections more frequently because of immunosuppression from chemotherapy and malignancy.11,12 In our study, cutaneous side effects with infectious causes were noted in 15 patients. Steroids, which are included in the majority of chemotherapeutic protocols, can cause cushingoid changes. Striae from rapid weight gain, acneform eruptions, hypertrichosis, and atrophy of the skin also have been observed among secondary changes to chemotherapy.1,11
Other skin signs observed in the study were acute urticaria in 1 patient (1.6%) following administration of intrathecal methotrexate; Stevens-Johnson syndrome related to voriconazole was noted in 1 (1.6%) patient.
Hyperpigmentation is a common side effect observed in oncology patients.13-15 It can be observed locally in the skin as well as the mucosa, teeth, hair, and nails, and it generally develops secondary to alkylating agents.16 Moreover, hyperpigmentation may develop in regions with occlusions (eg, electrocardiogram pads, adhesion sites of plasters), and commonly is associated with ifosfamide, etoposide, carboplatin, and cyclosporine. Although the development mechanism of hyperpigmentation related to chemotherapy drugs is not clearly known, it is thought to be due to direct toxicity, melanocyte stimulation, or postinflammatory changes.1,6,17 In our study, xeroderma was noted in some patients with hyperpigmentation; all of them had received cyclosporine and systemic steroid treatments. The other chemotherapeutics were defined as etoposide, cytarabine, dacarbazine, and ifosfamide.1 Our patients with hyperpigmentation were not taking these therapies.
Increased skin malignancies have been reported in adult cases with hematological malignancies.18 None of the patients in our study had a secondary skin malignancy, likely because we evaluated a pediatric population and the follow-up period (6 months) was too short for the development of a secondary malignancy.
Conclusion
A wide range of cutaneous side effects can be observed in pediatric oncology patients undergoing chemotherapy based on oncological diagnosis and treatment protocol. Although these side effects are not fatal, they may negatively affect morbidity and can lead to emotional distress. Knowing the possible cutaneous side effects of chemotherapy in pediatric patients and their causes is important for early diagnosis and minimal treatment.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
- Kamil N, Kamil S, Ahmed SP, et al. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7-14.
- Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212-216.
- Ozkan A, Apak H, Celkan T, et al. Toxic epidermal necrolysis after the use of high-dose cytosine arabinoside. Pediatr Dermatol. 2001;18:38-40.
- Rizos E, Milionis HJ, Pavlidis N, et al. Acquired ichthyosis: a paraneoplastic skin manifestation of Hodgkin’s disease. Lancet Oncol. 2002;3:727.
- Otmani N, Alami R, Hessissen L, et al. Determinants of severe oral mucositis in pediatric cancer patients: a prospective study. Int J Pediatr Dent. 2011;21:210-216.
- Mateus C, Robert C. New drugs in oncology and skin toxicity [in French]. Rev Med Interne. 2009;30:401-410.
- Manji A, Tomlinson D, Ethier MC, et al. Psychometric properties of the Oral Mucositis Daily Questionnaire for child self-report and importance of mucositis in children treated with chemotherapy. Support Care Cancer. 2012;20:1251-1258.
- Keefe DM. Mucositis management in patients with cancer. Support Cancer Ther. 2006;3:154-157.
- Raber-Durlacher JE, Elad S, Barasch A. Oral mucositis. Oral Oncol. 2010;46:452-456.
- Utas S, Kulluk P. A case of hydroxyurea-induced longitudinal melanonychia. Int J Dermatol. 2010;49:466-474.
- Ott H, Höger PH. Dermatologic manifestations of infections in pediatric cancer patients [in German]. Klin Padiatr. 2005;217(suppl 1):110-119.
- Ramphal R, Grant RM, Dzolganovski B, et al. Herpes simplex virus in febrile neutropenic children undergoing chemotherapy for cancer: a prospective cohort study. Pediatr Infect Dis J. 2007;26:700-704.
- Yaris N, Cakir M, Kalyoncu M, et al. Bleomycin induced hyperpigmentation with yolk sac tumor. Indian J Pediatr. 2007;74:505-506.
- Kleynberg RL, Sofi AA, Chaudhary RT. Hand-foot hyperpigmentation skin lesions associated with combination gemcitabine-carboplatin (GemCarbo) therapy. Am J Ther. 2011;18:261-263.
- Blaya M, Saba N. Chemotherapy-induced hyperpigmentation of the tongue. N Engl J Med. 2011;365:e20.
- Anandajeya WV, Corrêa ZM, Augsburger JJ. Primary acquired melanosis with atypia treated with mitomycin C. Int Ophthalmol. 2009;29:285-288.
- Torres C, Wong L, Welsh O, et al. Skin manifestations associated with chemotherapy in children with hematologic malignancies. Pediatr Dermatol. 2011;2:123-147.
- Mays SR, Cohen PR. Emerging dermatologic issues in the oncology patient. Semin Cutan Med Surg. 2006;25:179-189.
Practice Points
- Chemotherapeutic agents can cause a variety of cutaneous side effects.
- Pediatric oncology patients should be examined regularly for cutaneous side effects of chemotherapeutics.
50 Years of Helping Dermatologists Improve Patient Care
Fifty years! It might not have been easy to imagine that a journal that is not supported by a medical group or society and is supplied free of charge to its audience could survive for half a century. But here we are, thanks to the continued interest of our readers and the hundreds of clinicians and scientists who have committed to the task of composing articles to educate their fellow physicians in the field of dermatology.
In the first issue of Cutis® (Figure), Chief Editor Eugene F. Traub, MD, outlined what were, and still are, the goals of the journal: to provide articles “dealing with common dermatoses or those rarer diseases of great interest to all practitioners.”1 Dr. Traub chose John T. McCarthy, MD, to conduct the day-to-day business of the journal as the Assistant Chief Editor. Dr. McCarthy then became Editor of Cutis in 1983 following Dr. Traub’s retirement and led the journal until his death in 2000. Dr. McCarthy loved his job and the journal, serving for an amazing 35 years, and could rightly be called “the father of Cutis.” In his 25th anniversary editorial entitled “Thank You,” he emphasized both the struggles and successes he experienced during his leadership and concluded by thanking the readers of Cutis for their support.2 During this time, his great friend and colleague Joseph W. Burnett, MD, served as Senior Associate Editor.
 |
 |
In 2001, along with my colleagues Jeffrey M. Weinberg, MD, and Nanette B. Silverberg, MD, I was honored to join the staff of Cutis as the new Editor-in-Chief. On that occasion, we laid out what we hoped would be some changes in the journal’s structure, aesthetics, and content, but we also stated our intention to maintain what we considered to be the most important aspect of the journal: “to publish ORIGINAL and PRACTICAL articles.”3 We have continued to emphasize the publication of articles that describe the “clinical presentation, diagnosis, histopathology, therapy, and management of the more common entities.”3
Medicine and the specialty of dermatology have changed in our 13 years at the helm of Cutis. With the changes brought by the digital revolution, the ways physicians, both young and old, can access information have been broadened. Our editorial staff has expanded our reach with online exclusives that comprise the digital component of the journal (http://www.cutis.com). Our digital archive dates back to 2000. We have greatly increased our outreach to our young colleagues in training with a Resident Resources section on our Web site, and we also have expanded our online presence with our popular Photo Challenge as well as audio and video commentaries.
Because our specialty has become more and more complex, Cutis will be taking a new route in 2015, focusing solely on practicing dermatologists, dermatopathologists, dermatologic surgeons, dermatology nurse practitioners and physician assistants, and our resident colleagues. Loyal readers from other areas of medicine will still have full online access to the journal.
At this juncture, we look forward to a reinvigoration of our efforts and another 50 years! We wish to thank all of our Editorial Board members for their continued dedication and support. Finally, my colleagues and I would like to thank all of the behind-the-scenes professionals that make the publication of this journal possible, with special thanks to the tireless efforts of our Senior Vice President/Group Publisher Sharon Finch and our Group Editor Melissa Steiger Sears. I would be remiss if I did not also thank our advertisers in the pharmaceutical industry; without their support publication would not be possible. And finally, in the words of Dr. McCarthy, “Most important of all is you, the reader.”2
1. Traub EF. Our editorial objectives. Cutis. 1965;1:9.
2. McCarthy JT. Thank you. 1990;45:80.
3. DeLeo VA, Weinberg JM, Silverberg NB. Original and practical. 2001;67:191.
Fifty years! It might not have been easy to imagine that a journal that is not supported by a medical group or society and is supplied free of charge to its audience could survive for half a century. But here we are, thanks to the continued interest of our readers and the hundreds of clinicians and scientists who have committed to the task of composing articles to educate their fellow physicians in the field of dermatology.
In the first issue of Cutis® (Figure), Chief Editor Eugene F. Traub, MD, outlined what were, and still are, the goals of the journal: to provide articles “dealing with common dermatoses or those rarer diseases of great interest to all practitioners.”1 Dr. Traub chose John T. McCarthy, MD, to conduct the day-to-day business of the journal as the Assistant Chief Editor. Dr. McCarthy then became Editor of Cutis in 1983 following Dr. Traub’s retirement and led the journal until his death in 2000. Dr. McCarthy loved his job and the journal, serving for an amazing 35 years, and could rightly be called “the father of Cutis.” In his 25th anniversary editorial entitled “Thank You,” he emphasized both the struggles and successes he experienced during his leadership and concluded by thanking the readers of Cutis for their support.2 During this time, his great friend and colleague Joseph W. Burnett, MD, served as Senior Associate Editor.
 |
 |
In 2001, along with my colleagues Jeffrey M. Weinberg, MD, and Nanette B. Silverberg, MD, I was honored to join the staff of Cutis as the new Editor-in-Chief. On that occasion, we laid out what we hoped would be some changes in the journal’s structure, aesthetics, and content, but we also stated our intention to maintain what we considered to be the most important aspect of the journal: “to publish ORIGINAL and PRACTICAL articles.”3 We have continued to emphasize the publication of articles that describe the “clinical presentation, diagnosis, histopathology, therapy, and management of the more common entities.”3
Medicine and the specialty of dermatology have changed in our 13 years at the helm of Cutis. With the changes brought by the digital revolution, the ways physicians, both young and old, can access information have been broadened. Our editorial staff has expanded our reach with online exclusives that comprise the digital component of the journal (http://www.cutis.com). Our digital archive dates back to 2000. We have greatly increased our outreach to our young colleagues in training with a Resident Resources section on our Web site, and we also have expanded our online presence with our popular Photo Challenge as well as audio and video commentaries.
Because our specialty has become more and more complex, Cutis will be taking a new route in 2015, focusing solely on practicing dermatologists, dermatopathologists, dermatologic surgeons, dermatology nurse practitioners and physician assistants, and our resident colleagues. Loyal readers from other areas of medicine will still have full online access to the journal.
At this juncture, we look forward to a reinvigoration of our efforts and another 50 years! We wish to thank all of our Editorial Board members for their continued dedication and support. Finally, my colleagues and I would like to thank all of the behind-the-scenes professionals that make the publication of this journal possible, with special thanks to the tireless efforts of our Senior Vice President/Group Publisher Sharon Finch and our Group Editor Melissa Steiger Sears. I would be remiss if I did not also thank our advertisers in the pharmaceutical industry; without their support publication would not be possible. And finally, in the words of Dr. McCarthy, “Most important of all is you, the reader.”2
Fifty years! It might not have been easy to imagine that a journal that is not supported by a medical group or society and is supplied free of charge to its audience could survive for half a century. But here we are, thanks to the continued interest of our readers and the hundreds of clinicians and scientists who have committed to the task of composing articles to educate their fellow physicians in the field of dermatology.
In the first issue of Cutis® (Figure), Chief Editor Eugene F. Traub, MD, outlined what were, and still are, the goals of the journal: to provide articles “dealing with common dermatoses or those rarer diseases of great interest to all practitioners.”1 Dr. Traub chose John T. McCarthy, MD, to conduct the day-to-day business of the journal as the Assistant Chief Editor. Dr. McCarthy then became Editor of Cutis in 1983 following Dr. Traub’s retirement and led the journal until his death in 2000. Dr. McCarthy loved his job and the journal, serving for an amazing 35 years, and could rightly be called “the father of Cutis.” In his 25th anniversary editorial entitled “Thank You,” he emphasized both the struggles and successes he experienced during his leadership and concluded by thanking the readers of Cutis for their support.2 During this time, his great friend and colleague Joseph W. Burnett, MD, served as Senior Associate Editor.
 |
 |
In 2001, along with my colleagues Jeffrey M. Weinberg, MD, and Nanette B. Silverberg, MD, I was honored to join the staff of Cutis as the new Editor-in-Chief. On that occasion, we laid out what we hoped would be some changes in the journal’s structure, aesthetics, and content, but we also stated our intention to maintain what we considered to be the most important aspect of the journal: “to publish ORIGINAL and PRACTICAL articles.”3 We have continued to emphasize the publication of articles that describe the “clinical presentation, diagnosis, histopathology, therapy, and management of the more common entities.”3
Medicine and the specialty of dermatology have changed in our 13 years at the helm of Cutis. With the changes brought by the digital revolution, the ways physicians, both young and old, can access information have been broadened. Our editorial staff has expanded our reach with online exclusives that comprise the digital component of the journal (http://www.cutis.com). Our digital archive dates back to 2000. We have greatly increased our outreach to our young colleagues in training with a Resident Resources section on our Web site, and we also have expanded our online presence with our popular Photo Challenge as well as audio and video commentaries.
Because our specialty has become more and more complex, Cutis will be taking a new route in 2015, focusing solely on practicing dermatologists, dermatopathologists, dermatologic surgeons, dermatology nurse practitioners and physician assistants, and our resident colleagues. Loyal readers from other areas of medicine will still have full online access to the journal.
At this juncture, we look forward to a reinvigoration of our efforts and another 50 years! We wish to thank all of our Editorial Board members for their continued dedication and support. Finally, my colleagues and I would like to thank all of the behind-the-scenes professionals that make the publication of this journal possible, with special thanks to the tireless efforts of our Senior Vice President/Group Publisher Sharon Finch and our Group Editor Melissa Steiger Sears. I would be remiss if I did not also thank our advertisers in the pharmaceutical industry; without their support publication would not be possible. And finally, in the words of Dr. McCarthy, “Most important of all is you, the reader.”2
1. Traub EF. Our editorial objectives. Cutis. 1965;1:9.
2. McCarthy JT. Thank you. 1990;45:80.
3. DeLeo VA, Weinberg JM, Silverberg NB. Original and practical. 2001;67:191.
1. Traub EF. Our editorial objectives. Cutis. 1965;1:9.
2. McCarthy JT. Thank you. 1990;45:80.
3. DeLeo VA, Weinberg JM, Silverberg NB. Original and practical. 2001;67:191.
Herpes Esophagitis in the Setting of Immunosuppression From Pemphigus Vulgaris Therapy
Pemphigus vulgaris (PV) is a chronic autoimmune intraepithelial bullous disease caused by pathogenic IgG antibodies at the intraepidermal cell-surface proteins desmoglein 1 (DSG1) and desmoglein 3 (DSG3), which are members of the cadherin superfamily of desmosomal proteins and are involved in keratinocyte adhesion. Autoantibody binding to these molecules leads to the loss of cell-cell adhesion in the epithelial suprabasilar layer, producing flaccid blisters on an erythematous base with a positive Nikolsky sign.1 The blisters frequently rupture, leaving painful nonscarring erosions with the potential for secondary infection.
The clinical phenotype of PV is directly related to the autoantibody profile. Clinically, PV often is mucosal dominant on presentation with painful oropharyngeal involvement and associated IgG antibodies against DSG3. Progression to cutaneous disease, such as on the scalp or axillae, is accompanied by a shift in IgG antibodies against both DSG1 and DSG3.2,3
Combination therapy with prednisone and mycophenolate mofetil (MMF) has proven to be an effective method of controlling the signs and symptoms of PV4; however, the immunosuppressive effects of these medications put the patient at risk for a host of opportunistic infections. Herpes simplex virus (HSV) has been associated with PV lesions of the oral mucosa, though a clear-cut relationship between these 2 entities has yet to be established.5 Herpes simplex virus has likewise been confirmed in therapy-resistant exacerbations of PV.6 Herpes esophagitis is a rare consequence of treatment with prednisone and MMF that is primarily encountered in patients with a history of solid organ transplantation7 and rarely has been reported in PV patients undergoing therapeutic immunosuppression.
Acute odynophagia in patients undergoing systemic treatment of active PV warrants prompt endoscopic evaluation to rule out esophageal pemphigus or superinfection. We report the case of a 35-year-old man with stable but poorly controlled PV who was undergoing systemic treatment and experienced rapid deterioration due to herpes esophagitis from immunosuppression.
Case Report
A 35-year-old man was referred to our clinic for evaluation of blisters on the scalp, oral mucosa, and proximal upper and lower extremities of 4 months’ duration. A biopsy performed by his primary care physician within a month of onset of symptoms was reportedly suggestive of PV; although no direct immunofluorescence had been performed, serum indirect immunofluorescence was highly positive for IgG antibodies toward DSG3 and to a lesser extent DSG1. The blisters failed to improve with a 2-week prednisone taper completed 1 month prior to presentation. The patient was not currently taking any other medications. He had a remote history of fever blisters but no other dermatologic issues.
Initial examination revealed flaccid bullae on an erythematous base involving the posterior scalp as well as tender white erosions to shallow ulcers on the tongue and hard and soft palates. A Tzanck smear (modified Wright-Giemsa stain) of these erosions confirmed acantholytic mucosal cells. Punch biopsies of lesional and perilesional skin from the scalp were obtained for histopathologic confirmation and immunofluorescence. An acantholytic dermatosis with a tombstone pattern along the basement membrane was present on hematoxylin and eosin staining, and direct immunofluorescence was positive for IgG and C3 in an intraepidermal lacelike pattern, confirming a diagnosis of PV.
Despite starting an oral regimen of high-dose corticosteroids (prednisone 80 mg once daily), no improvement was noted at 2-week follow-up. He had developed flaccid blisters on the left axillae and mildly worsened oral erosions. He also reported moderate difficulty eating due to pain with swallowing. Mycophenolate mofetil (500 mg twice daily) was added as combination therapy with the prednisone.
One week later, the patient was unable to eat or drink due to worsening odynophagia. He was admitted as an inpatient for treatment with intravenous methylprednisolone (120 mg every 8 hours) and MMF (1000 mg daily). The gastroenterology department was consulted and an esophagogastroduodenoscopy revealed diffuse areas of denuded and friable mucosa with an overlay of white exudate (Figure 1). Cytology performed on esophageal brushings revealed viral cytopathic changes confirming herpes esophagitis (Figure 2). No esophageal viral cultures were taken. The patient was started on intravenous acyclovir (800 mg 4 times daily), leading to rapid resolution of the odynophagia. He was discharged after 4 days with a course of oral acyclovir (400 mg 4 times daily for 14 days). Tzanck smears and HSV cultures of oral lesions performed immediately following discharge were negative. Combination therapy with MMF (500 mg twice daily) and a slow taper of prednisone (down to 5 mg once daily) was continued past 1 year without flare of his cutaneous disease.
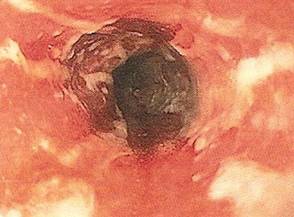
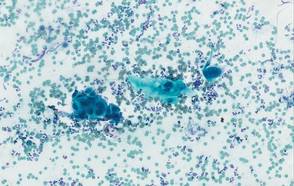
Comment
Although PV may have been considered a fatal disease at one time, treatment with systemic steroids has made it a manageable, albeit relapsing, condition. The development of corticosteroid-sparing, adjuvant immunosuppressives such as MMF has allowed for the more aggressive treatment of this disease with fewer steroid-related side effects.4,8,9 As seen in solid organ transplant recipients who often utilize combination therapy, the use of adjuvant immunosuppressives is associated with potential complications including bone marrow suppression and an increased risk for infections.7,10
Odynophagia is among the potential complications in patients with PV and has a wide differential diagnosis. Mucosal lesions of PV previously have been associated with HSV colonization, though a causal relationship has not been corroborated.5 Herpes simplex virus is more often detected in PV patients being treated with immunosuppressive agents than in nontreated patient groups.11 Recalcitrant or suddenly exacerbated oral mucosal lesions of PV under appropriate therapy may therefore be the result of HSV superinfection, which has been deferentially referred to as pemphigus herpeticum.12 Esophageal mucosal involvement by PV also may be more common than previously thought and should be suspected in patients with active oral disease.13 Esophagitis secondary to medications or various opportunistic organisms such as Candida, cytomegalovirus, or HSV also should be ruled out in patients taking immunosuppressives.5,10
Herpes esophagitis primarily occurs in immunocompromised hosts and is well documented in the literature regarding treatment with MMF and prednisone following renal and cardiac transplantation.10 Prednisone therapy in patients with chronic obstructive pulmonary disease also has been implicated.14 Reactivation of latent HSV resulting from immunosuppression is most often described, though primary infection also is possible.15 Patients typically present with acute odynophagia progressing to dysphagia, with complications ranging from sequelae of poor oral intake to esophageal perforation and hemorrhage, but the course generally is self-limited if immune function is promptly restored. Intravenous acyclovir has been known to hasten the recovery process and improve symptoms.16 Characteristic findings on esophagogastroduodenoscopy in combination with tissue biopsy, viral culture, and/or polymerase chain reaction aid in the diagnosis of herpes esophagitis.15,16 Our patient had a grossly abnormal esophagogastroduodenoscopy with positive cytology; however, no further diagnostic workup was performed. The cytologic findings and the rapid symptomatic improvement following the initiation of acyclovir helped support HSV as the etiology.
Conclusion
We present a case of herpes esophagitis that complicated the treatment of PV with MMF and prednisone. A diagnosis of herpes esophagitis must be ruled out in patients with PV who are undergoing therapeutic immunosuppression and present with an acute episode of odynophagia that is resistant to upscaling of therapy.
- Mustasim DF, Bilic M, Hawayek LH, et al. Immunobullous diseases. J Am Acad Dermatol. 2005;52:1029-1043.
- Amagai M, Tsunoda K, Zillikens D, et al. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2, pt 1):167-170.
- Sirois DA, Fatahzadeh M, Roth R, et al. Diagnostic patterns and delays in pemphigus vulgaris: experience from 99 patients. Arch Dermatol. 2000;136:1569-1570.
- Strowd LC, Taylor SL, Jorizzo JL, et al. Therapeutic ladder for pemphigus vulgaris: emphasis on achieving complete remission. J Am Acad Dermatol. 2011;64:490-494.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Hale EK, Bystryn JC. Atypical herpes simplex can mimic a flare of disease activity in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol. 1999;13:221-223.
- Smak Gregoor PJ, van Gelder T, van Riemsdijk-van Overbeeke IC, et al. Unusual presentation of herpes virus infections in renal transplant recipients exposed to high mycophenolic acid plasma concentrations. Transpl Infect Dis. 2003;5:79-83.
- Beissert S, Mimouni D, Kanwar AJ, et al. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: a multicenter, randomized, placebo-controlled trial. J Invest Dermatol. 2010;130:2041-2048.
- Yeh SW, Sami N, Ahmed RA. Treatment of pemphigus vulgaris: current and emerging options. Am J Clin Dermatol. 2005;6:327-342.
- Eisen HJ, Kobashigawa J, Keogh A, et al. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant. 2005;24:517-525.
- Marzano AV, Tourlaki A, Merlo V, et al. Herpes simplex virus infection and pemphigus. Int J Immunopathol Pharmacol. 2009;22:781-786.
- Feldmeyer L, Trüeb RM, French LE, et al. Pitfall: pemphigus herpeticatus should not be confounded with resistant pemphigus vulgaris. J Dermatolog Treat. 2010;21:311-313.
- Rao PN, Samarth A, Aurangabadkar SJ, et al. Study of upper gastrointestinal tract involvement in pemphigus by esophago-gastro-duodenoscopy. Indian J Dermatol Venereol Leprol. 2006;72:421-424.
- Wiest PM, Flanigan T, Salata RA, et al. Serious infectious complications of corticosteroid therapy for COPD. Chest. 1989;95:1180-1184.
- Lee B, Caddy G. A rare cause of dysphagia: herpes simplex esophagitis. World J Gastroenterol. 2007;13:2756-2757.
- Robertson AG, Dunn LJ, Immanuel A, et al. An unusual presentation of herpes simplex esophagitis: a nonhealing “peptic” ulcer. Endoscopy. 2009;41(suppl 2):E213.
Pemphigus vulgaris (PV) is a chronic autoimmune intraepithelial bullous disease caused by pathogenic IgG antibodies at the intraepidermal cell-surface proteins desmoglein 1 (DSG1) and desmoglein 3 (DSG3), which are members of the cadherin superfamily of desmosomal proteins and are involved in keratinocyte adhesion. Autoantibody binding to these molecules leads to the loss of cell-cell adhesion in the epithelial suprabasilar layer, producing flaccid blisters on an erythematous base with a positive Nikolsky sign.1 The blisters frequently rupture, leaving painful nonscarring erosions with the potential for secondary infection.
The clinical phenotype of PV is directly related to the autoantibody profile. Clinically, PV often is mucosal dominant on presentation with painful oropharyngeal involvement and associated IgG antibodies against DSG3. Progression to cutaneous disease, such as on the scalp or axillae, is accompanied by a shift in IgG antibodies against both DSG1 and DSG3.2,3
Combination therapy with prednisone and mycophenolate mofetil (MMF) has proven to be an effective method of controlling the signs and symptoms of PV4; however, the immunosuppressive effects of these medications put the patient at risk for a host of opportunistic infections. Herpes simplex virus (HSV) has been associated with PV lesions of the oral mucosa, though a clear-cut relationship between these 2 entities has yet to be established.5 Herpes simplex virus has likewise been confirmed in therapy-resistant exacerbations of PV.6 Herpes esophagitis is a rare consequence of treatment with prednisone and MMF that is primarily encountered in patients with a history of solid organ transplantation7 and rarely has been reported in PV patients undergoing therapeutic immunosuppression.
Acute odynophagia in patients undergoing systemic treatment of active PV warrants prompt endoscopic evaluation to rule out esophageal pemphigus or superinfection. We report the case of a 35-year-old man with stable but poorly controlled PV who was undergoing systemic treatment and experienced rapid deterioration due to herpes esophagitis from immunosuppression.
Case Report
A 35-year-old man was referred to our clinic for evaluation of blisters on the scalp, oral mucosa, and proximal upper and lower extremities of 4 months’ duration. A biopsy performed by his primary care physician within a month of onset of symptoms was reportedly suggestive of PV; although no direct immunofluorescence had been performed, serum indirect immunofluorescence was highly positive for IgG antibodies toward DSG3 and to a lesser extent DSG1. The blisters failed to improve with a 2-week prednisone taper completed 1 month prior to presentation. The patient was not currently taking any other medications. He had a remote history of fever blisters but no other dermatologic issues.
Initial examination revealed flaccid bullae on an erythematous base involving the posterior scalp as well as tender white erosions to shallow ulcers on the tongue and hard and soft palates. A Tzanck smear (modified Wright-Giemsa stain) of these erosions confirmed acantholytic mucosal cells. Punch biopsies of lesional and perilesional skin from the scalp were obtained for histopathologic confirmation and immunofluorescence. An acantholytic dermatosis with a tombstone pattern along the basement membrane was present on hematoxylin and eosin staining, and direct immunofluorescence was positive for IgG and C3 in an intraepidermal lacelike pattern, confirming a diagnosis of PV.
Despite starting an oral regimen of high-dose corticosteroids (prednisone 80 mg once daily), no improvement was noted at 2-week follow-up. He had developed flaccid blisters on the left axillae and mildly worsened oral erosions. He also reported moderate difficulty eating due to pain with swallowing. Mycophenolate mofetil (500 mg twice daily) was added as combination therapy with the prednisone.
One week later, the patient was unable to eat or drink due to worsening odynophagia. He was admitted as an inpatient for treatment with intravenous methylprednisolone (120 mg every 8 hours) and MMF (1000 mg daily). The gastroenterology department was consulted and an esophagogastroduodenoscopy revealed diffuse areas of denuded and friable mucosa with an overlay of white exudate (Figure 1). Cytology performed on esophageal brushings revealed viral cytopathic changes confirming herpes esophagitis (Figure 2). No esophageal viral cultures were taken. The patient was started on intravenous acyclovir (800 mg 4 times daily), leading to rapid resolution of the odynophagia. He was discharged after 4 days with a course of oral acyclovir (400 mg 4 times daily for 14 days). Tzanck smears and HSV cultures of oral lesions performed immediately following discharge were negative. Combination therapy with MMF (500 mg twice daily) and a slow taper of prednisone (down to 5 mg once daily) was continued past 1 year without flare of his cutaneous disease.


Comment
Although PV may have been considered a fatal disease at one time, treatment with systemic steroids has made it a manageable, albeit relapsing, condition. The development of corticosteroid-sparing, adjuvant immunosuppressives such as MMF has allowed for the more aggressive treatment of this disease with fewer steroid-related side effects.4,8,9 As seen in solid organ transplant recipients who often utilize combination therapy, the use of adjuvant immunosuppressives is associated with potential complications including bone marrow suppression and an increased risk for infections.7,10
Odynophagia is among the potential complications in patients with PV and has a wide differential diagnosis. Mucosal lesions of PV previously have been associated with HSV colonization, though a causal relationship has not been corroborated.5 Herpes simplex virus is more often detected in PV patients being treated with immunosuppressive agents than in nontreated patient groups.11 Recalcitrant or suddenly exacerbated oral mucosal lesions of PV under appropriate therapy may therefore be the result of HSV superinfection, which has been deferentially referred to as pemphigus herpeticum.12 Esophageal mucosal involvement by PV also may be more common than previously thought and should be suspected in patients with active oral disease.13 Esophagitis secondary to medications or various opportunistic organisms such as Candida, cytomegalovirus, or HSV also should be ruled out in patients taking immunosuppressives.5,10
Herpes esophagitis primarily occurs in immunocompromised hosts and is well documented in the literature regarding treatment with MMF and prednisone following renal and cardiac transplantation.10 Prednisone therapy in patients with chronic obstructive pulmonary disease also has been implicated.14 Reactivation of latent HSV resulting from immunosuppression is most often described, though primary infection also is possible.15 Patients typically present with acute odynophagia progressing to dysphagia, with complications ranging from sequelae of poor oral intake to esophageal perforation and hemorrhage, but the course generally is self-limited if immune function is promptly restored. Intravenous acyclovir has been known to hasten the recovery process and improve symptoms.16 Characteristic findings on esophagogastroduodenoscopy in combination with tissue biopsy, viral culture, and/or polymerase chain reaction aid in the diagnosis of herpes esophagitis.15,16 Our patient had a grossly abnormal esophagogastroduodenoscopy with positive cytology; however, no further diagnostic workup was performed. The cytologic findings and the rapid symptomatic improvement following the initiation of acyclovir helped support HSV as the etiology.
Conclusion
We present a case of herpes esophagitis that complicated the treatment of PV with MMF and prednisone. A diagnosis of herpes esophagitis must be ruled out in patients with PV who are undergoing therapeutic immunosuppression and present with an acute episode of odynophagia that is resistant to upscaling of therapy.
Pemphigus vulgaris (PV) is a chronic autoimmune intraepithelial bullous disease caused by pathogenic IgG antibodies at the intraepidermal cell-surface proteins desmoglein 1 (DSG1) and desmoglein 3 (DSG3), which are members of the cadherin superfamily of desmosomal proteins and are involved in keratinocyte adhesion. Autoantibody binding to these molecules leads to the loss of cell-cell adhesion in the epithelial suprabasilar layer, producing flaccid blisters on an erythematous base with a positive Nikolsky sign.1 The blisters frequently rupture, leaving painful nonscarring erosions with the potential for secondary infection.
The clinical phenotype of PV is directly related to the autoantibody profile. Clinically, PV often is mucosal dominant on presentation with painful oropharyngeal involvement and associated IgG antibodies against DSG3. Progression to cutaneous disease, such as on the scalp or axillae, is accompanied by a shift in IgG antibodies against both DSG1 and DSG3.2,3
Combination therapy with prednisone and mycophenolate mofetil (MMF) has proven to be an effective method of controlling the signs and symptoms of PV4; however, the immunosuppressive effects of these medications put the patient at risk for a host of opportunistic infections. Herpes simplex virus (HSV) has been associated with PV lesions of the oral mucosa, though a clear-cut relationship between these 2 entities has yet to be established.5 Herpes simplex virus has likewise been confirmed in therapy-resistant exacerbations of PV.6 Herpes esophagitis is a rare consequence of treatment with prednisone and MMF that is primarily encountered in patients with a history of solid organ transplantation7 and rarely has been reported in PV patients undergoing therapeutic immunosuppression.
Acute odynophagia in patients undergoing systemic treatment of active PV warrants prompt endoscopic evaluation to rule out esophageal pemphigus or superinfection. We report the case of a 35-year-old man with stable but poorly controlled PV who was undergoing systemic treatment and experienced rapid deterioration due to herpes esophagitis from immunosuppression.
Case Report
A 35-year-old man was referred to our clinic for evaluation of blisters on the scalp, oral mucosa, and proximal upper and lower extremities of 4 months’ duration. A biopsy performed by his primary care physician within a month of onset of symptoms was reportedly suggestive of PV; although no direct immunofluorescence had been performed, serum indirect immunofluorescence was highly positive for IgG antibodies toward DSG3 and to a lesser extent DSG1. The blisters failed to improve with a 2-week prednisone taper completed 1 month prior to presentation. The patient was not currently taking any other medications. He had a remote history of fever blisters but no other dermatologic issues.
Initial examination revealed flaccid bullae on an erythematous base involving the posterior scalp as well as tender white erosions to shallow ulcers on the tongue and hard and soft palates. A Tzanck smear (modified Wright-Giemsa stain) of these erosions confirmed acantholytic mucosal cells. Punch biopsies of lesional and perilesional skin from the scalp were obtained for histopathologic confirmation and immunofluorescence. An acantholytic dermatosis with a tombstone pattern along the basement membrane was present on hematoxylin and eosin staining, and direct immunofluorescence was positive for IgG and C3 in an intraepidermal lacelike pattern, confirming a diagnosis of PV.
Despite starting an oral regimen of high-dose corticosteroids (prednisone 80 mg once daily), no improvement was noted at 2-week follow-up. He had developed flaccid blisters on the left axillae and mildly worsened oral erosions. He also reported moderate difficulty eating due to pain with swallowing. Mycophenolate mofetil (500 mg twice daily) was added as combination therapy with the prednisone.
One week later, the patient was unable to eat or drink due to worsening odynophagia. He was admitted as an inpatient for treatment with intravenous methylprednisolone (120 mg every 8 hours) and MMF (1000 mg daily). The gastroenterology department was consulted and an esophagogastroduodenoscopy revealed diffuse areas of denuded and friable mucosa with an overlay of white exudate (Figure 1). Cytology performed on esophageal brushings revealed viral cytopathic changes confirming herpes esophagitis (Figure 2). No esophageal viral cultures were taken. The patient was started on intravenous acyclovir (800 mg 4 times daily), leading to rapid resolution of the odynophagia. He was discharged after 4 days with a course of oral acyclovir (400 mg 4 times daily for 14 days). Tzanck smears and HSV cultures of oral lesions performed immediately following discharge were negative. Combination therapy with MMF (500 mg twice daily) and a slow taper of prednisone (down to 5 mg once daily) was continued past 1 year without flare of his cutaneous disease.


Comment
Although PV may have been considered a fatal disease at one time, treatment with systemic steroids has made it a manageable, albeit relapsing, condition. The development of corticosteroid-sparing, adjuvant immunosuppressives such as MMF has allowed for the more aggressive treatment of this disease with fewer steroid-related side effects.4,8,9 As seen in solid organ transplant recipients who often utilize combination therapy, the use of adjuvant immunosuppressives is associated with potential complications including bone marrow suppression and an increased risk for infections.7,10
Odynophagia is among the potential complications in patients with PV and has a wide differential diagnosis. Mucosal lesions of PV previously have been associated with HSV colonization, though a causal relationship has not been corroborated.5 Herpes simplex virus is more often detected in PV patients being treated with immunosuppressive agents than in nontreated patient groups.11 Recalcitrant or suddenly exacerbated oral mucosal lesions of PV under appropriate therapy may therefore be the result of HSV superinfection, which has been deferentially referred to as pemphigus herpeticum.12 Esophageal mucosal involvement by PV also may be more common than previously thought and should be suspected in patients with active oral disease.13 Esophagitis secondary to medications or various opportunistic organisms such as Candida, cytomegalovirus, or HSV also should be ruled out in patients taking immunosuppressives.5,10
Herpes esophagitis primarily occurs in immunocompromised hosts and is well documented in the literature regarding treatment with MMF and prednisone following renal and cardiac transplantation.10 Prednisone therapy in patients with chronic obstructive pulmonary disease also has been implicated.14 Reactivation of latent HSV resulting from immunosuppression is most often described, though primary infection also is possible.15 Patients typically present with acute odynophagia progressing to dysphagia, with complications ranging from sequelae of poor oral intake to esophageal perforation and hemorrhage, but the course generally is self-limited if immune function is promptly restored. Intravenous acyclovir has been known to hasten the recovery process and improve symptoms.16 Characteristic findings on esophagogastroduodenoscopy in combination with tissue biopsy, viral culture, and/or polymerase chain reaction aid in the diagnosis of herpes esophagitis.15,16 Our patient had a grossly abnormal esophagogastroduodenoscopy with positive cytology; however, no further diagnostic workup was performed. The cytologic findings and the rapid symptomatic improvement following the initiation of acyclovir helped support HSV as the etiology.
Conclusion
We present a case of herpes esophagitis that complicated the treatment of PV with MMF and prednisone. A diagnosis of herpes esophagitis must be ruled out in patients with PV who are undergoing therapeutic immunosuppression and present with an acute episode of odynophagia that is resistant to upscaling of therapy.
- Mustasim DF, Bilic M, Hawayek LH, et al. Immunobullous diseases. J Am Acad Dermatol. 2005;52:1029-1043.
- Amagai M, Tsunoda K, Zillikens D, et al. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2, pt 1):167-170.
- Sirois DA, Fatahzadeh M, Roth R, et al. Diagnostic patterns and delays in pemphigus vulgaris: experience from 99 patients. Arch Dermatol. 2000;136:1569-1570.
- Strowd LC, Taylor SL, Jorizzo JL, et al. Therapeutic ladder for pemphigus vulgaris: emphasis on achieving complete remission. J Am Acad Dermatol. 2011;64:490-494.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Hale EK, Bystryn JC. Atypical herpes simplex can mimic a flare of disease activity in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol. 1999;13:221-223.
- Smak Gregoor PJ, van Gelder T, van Riemsdijk-van Overbeeke IC, et al. Unusual presentation of herpes virus infections in renal transplant recipients exposed to high mycophenolic acid plasma concentrations. Transpl Infect Dis. 2003;5:79-83.
- Beissert S, Mimouni D, Kanwar AJ, et al. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: a multicenter, randomized, placebo-controlled trial. J Invest Dermatol. 2010;130:2041-2048.
- Yeh SW, Sami N, Ahmed RA. Treatment of pemphigus vulgaris: current and emerging options. Am J Clin Dermatol. 2005;6:327-342.
- Eisen HJ, Kobashigawa J, Keogh A, et al. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant. 2005;24:517-525.
- Marzano AV, Tourlaki A, Merlo V, et al. Herpes simplex virus infection and pemphigus. Int J Immunopathol Pharmacol. 2009;22:781-786.
- Feldmeyer L, Trüeb RM, French LE, et al. Pitfall: pemphigus herpeticatus should not be confounded with resistant pemphigus vulgaris. J Dermatolog Treat. 2010;21:311-313.
- Rao PN, Samarth A, Aurangabadkar SJ, et al. Study of upper gastrointestinal tract involvement in pemphigus by esophago-gastro-duodenoscopy. Indian J Dermatol Venereol Leprol. 2006;72:421-424.
- Wiest PM, Flanigan T, Salata RA, et al. Serious infectious complications of corticosteroid therapy for COPD. Chest. 1989;95:1180-1184.
- Lee B, Caddy G. A rare cause of dysphagia: herpes simplex esophagitis. World J Gastroenterol. 2007;13:2756-2757.
- Robertson AG, Dunn LJ, Immanuel A, et al. An unusual presentation of herpes simplex esophagitis: a nonhealing “peptic” ulcer. Endoscopy. 2009;41(suppl 2):E213.
- Mustasim DF, Bilic M, Hawayek LH, et al. Immunobullous diseases. J Am Acad Dermatol. 2005;52:1029-1043.
- Amagai M, Tsunoda K, Zillikens D, et al. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. 1999;40(2, pt 1):167-170.
- Sirois DA, Fatahzadeh M, Roth R, et al. Diagnostic patterns and delays in pemphigus vulgaris: experience from 99 patients. Arch Dermatol. 2000;136:1569-1570.
- Strowd LC, Taylor SL, Jorizzo JL, et al. Therapeutic ladder for pemphigus vulgaris: emphasis on achieving complete remission. J Am Acad Dermatol. 2011;64:490-494.
- Nikkels AF, Delvenne P, Herfs M, et al. Occult herpes simplex virus colonization of bullous dermatitides. Am J Clin Dermatol. 2008;9:163-168.
- Hale EK, Bystryn JC. Atypical herpes simplex can mimic a flare of disease activity in patients with pemphigus vulgaris. J Eur Acad Dermatol Venereol. 1999;13:221-223.
- Smak Gregoor PJ, van Gelder T, van Riemsdijk-van Overbeeke IC, et al. Unusual presentation of herpes virus infections in renal transplant recipients exposed to high mycophenolic acid plasma concentrations. Transpl Infect Dis. 2003;5:79-83.
- Beissert S, Mimouni D, Kanwar AJ, et al. Treating pemphigus vulgaris with prednisone and mycophenolate mofetil: a multicenter, randomized, placebo-controlled trial. J Invest Dermatol. 2010;130:2041-2048.
- Yeh SW, Sami N, Ahmed RA. Treatment of pemphigus vulgaris: current and emerging options. Am J Clin Dermatol. 2005;6:327-342.
- Eisen HJ, Kobashigawa J, Keogh A, et al. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant. 2005;24:517-525.
- Marzano AV, Tourlaki A, Merlo V, et al. Herpes simplex virus infection and pemphigus. Int J Immunopathol Pharmacol. 2009;22:781-786.
- Feldmeyer L, Trüeb RM, French LE, et al. Pitfall: pemphigus herpeticatus should not be confounded with resistant pemphigus vulgaris. J Dermatolog Treat. 2010;21:311-313.
- Rao PN, Samarth A, Aurangabadkar SJ, et al. Study of upper gastrointestinal tract involvement in pemphigus by esophago-gastro-duodenoscopy. Indian J Dermatol Venereol Leprol. 2006;72:421-424.
- Wiest PM, Flanigan T, Salata RA, et al. Serious infectious complications of corticosteroid therapy for COPD. Chest. 1989;95:1180-1184.
- Lee B, Caddy G. A rare cause of dysphagia: herpes simplex esophagitis. World J Gastroenterol. 2007;13:2756-2757.
- Robertson AG, Dunn LJ, Immanuel A, et al. An unusual presentation of herpes simplex esophagitis: a nonhealing “peptic” ulcer. Endoscopy. 2009;41(suppl 2):E213.
Practice Points
- Pemphigus vulgaris (PV) often requires therapeutic immunosuppression for disease control.
- Acute odynophagia in the setting of systemic immunosuppression for PV requires endoscopic evaluation.