User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Dupilumab-Induced Facial Flushing After Alcohol Consumption
Dupilumab is a fully humanized monoclonal antibody to the α subunit of the IL-4 receptor that inhibits the action of helper T cell (TH2)–type cytokines IL-4 and IL-13. Dupilumab was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe atopic dermatitis (AD). We report 2 patients with AD who were treated with dupilumab and subsequently developed facial flushing after consuming alcohol.
Case Report
Patient 1
A 24-year-old woman presented to the dermatology clinic with a lifelong history of moderate to severe AD. She had a medical history of asthma and seasonal allergies, which were treated with fexofenadine and an inhaler, as needed. The patient had an affected body surface area of approximately 70% and had achieved only partial relief with topical corticosteroids and topical calcineurin inhibitors.
Because her disease was severe, the patient was started on dupilumab at FDA-approved dosing for AD: a 600-mg subcutaneous (SC) loading dose, followed by 300 mg SC every 2 weeks. She reported rapid skin clearance within 2 weeks of the start of treatment. Her course was complicated by mild head and neck dermatitis.
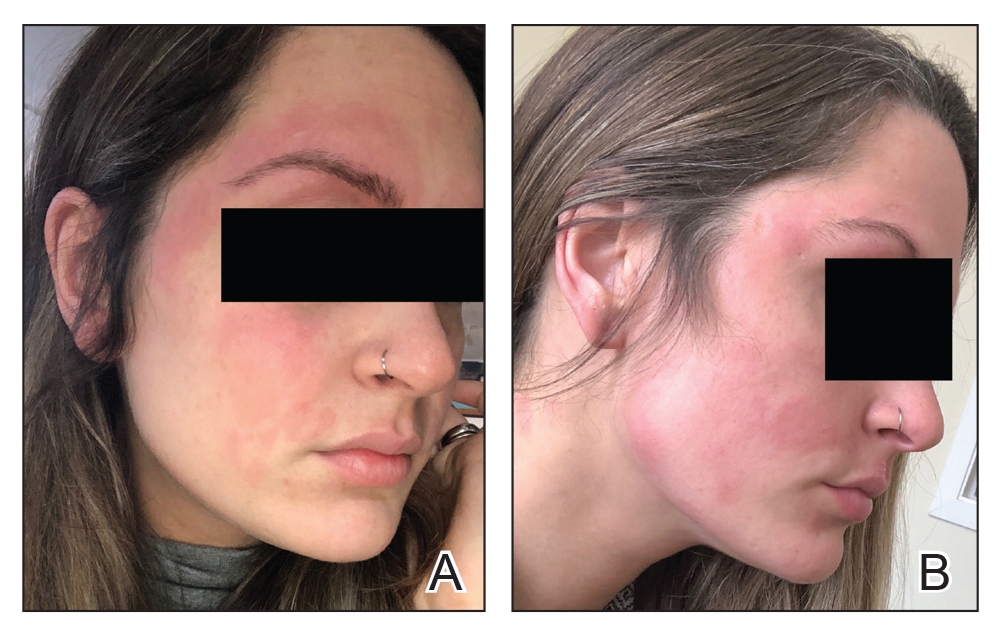
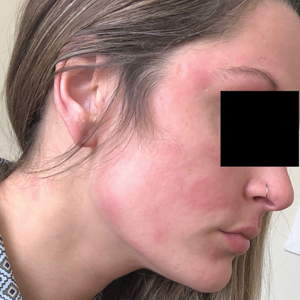
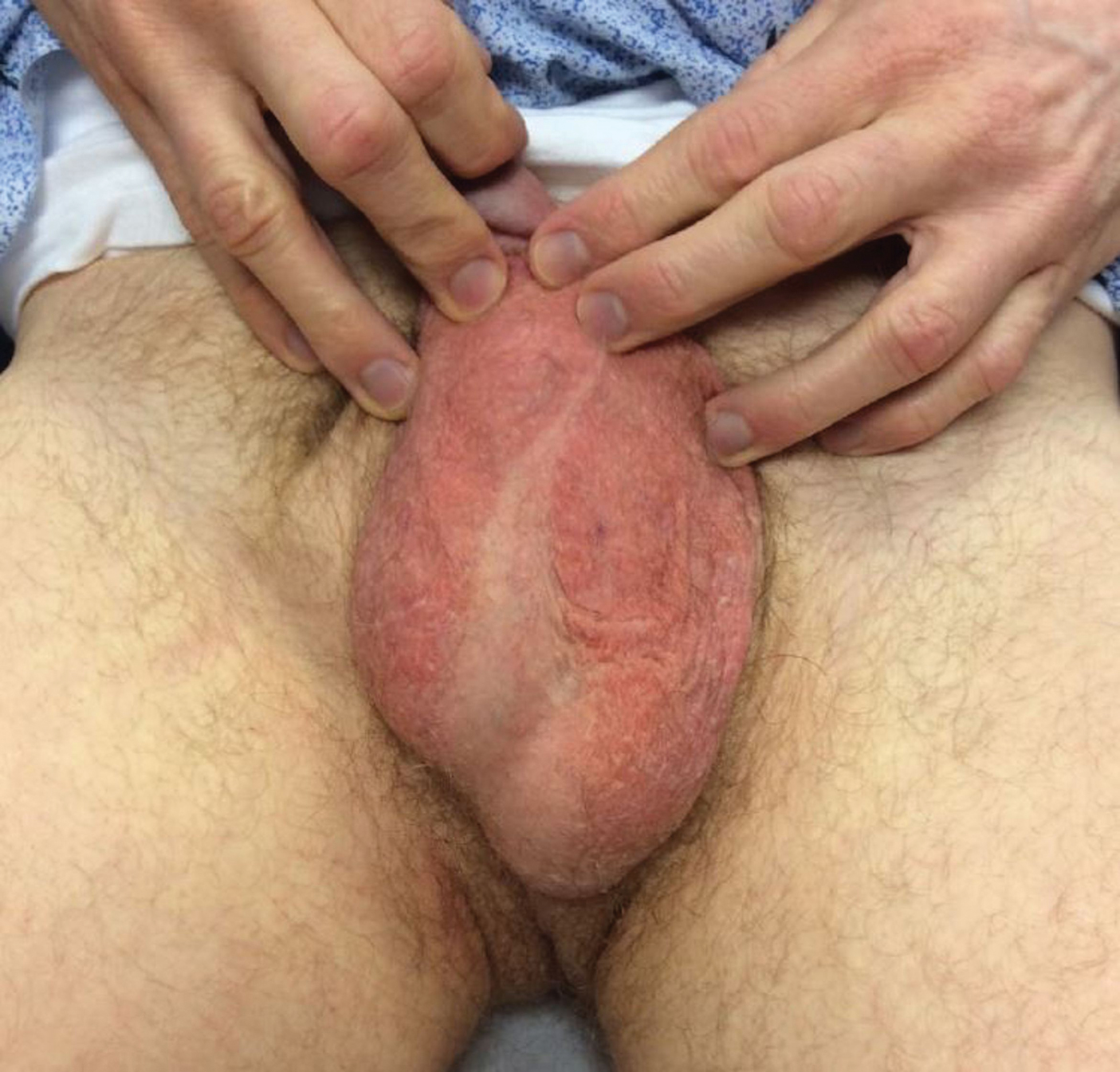
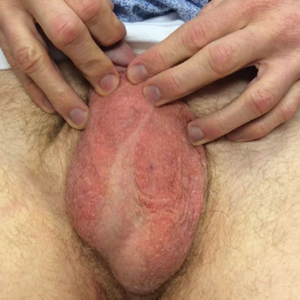
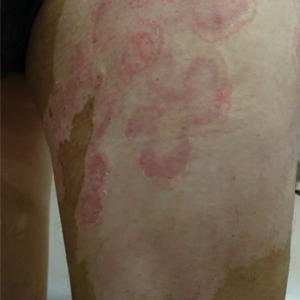
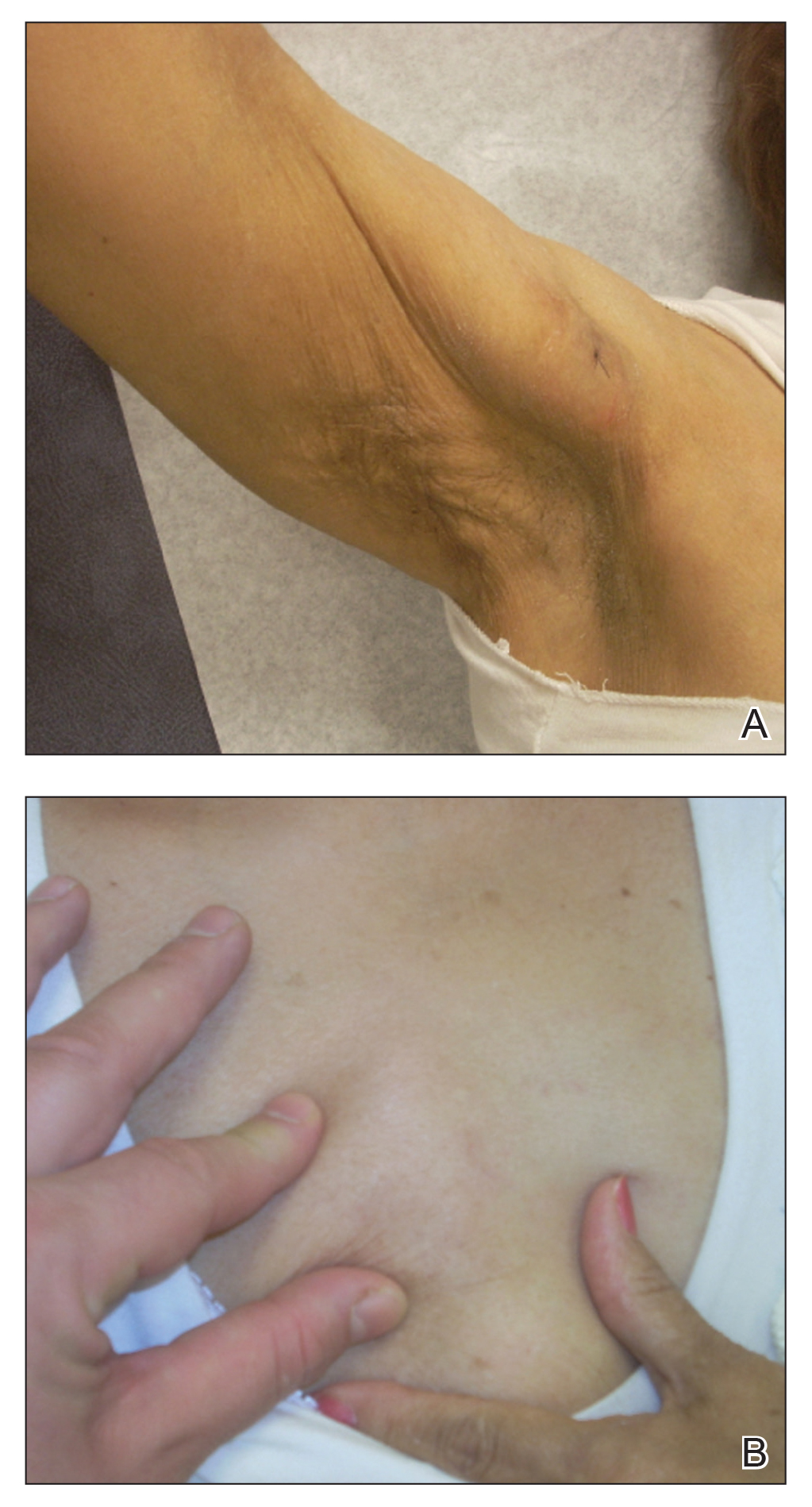
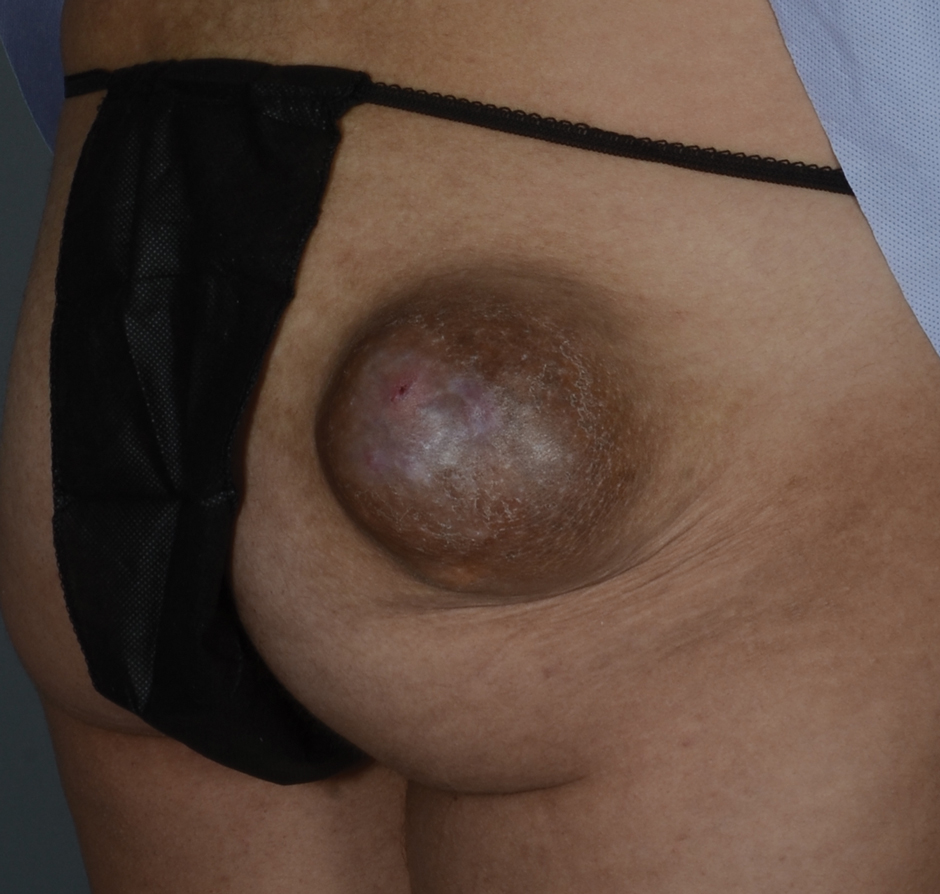
Seven months after starting treatment, the patient began to acutely experience erythema and warmth over the entire face that was triggered by drinking alcohol (Figure). Before starting dupilumab, she had consumed alcohol on multiple occasions without a flushing effect. This new finding was distinguishable from her facial dermatitis. Onset was within a few minutes after drinking alcohol; flushing self-resolved in 15 to 30 minutes. Although diffuse, erythema and warmth were concentrated around the jawline, eyebrows, and ears and occurred every time the patient drank alcohol. Moreover, she reported that consumption of hard (ie, distilled) liquor, specifically tequila, caused a more severe presentation. She denied other symptoms associated with dupilumab.
Patient 2
A 32-year-old man presented to the dermatology clinic with a 10-year history of moderate to severe AD. He had a medical history of asthma (treated with albuterol, montelukast, and fluticasone); allergic rhinitis; and severe environmental allergies, including sensitivity to dust mites, dogs, trees, and grass.
For AD, the patient had been treated with topical corticosteroids and the Goeckerman regimen (a combination of phototherapy and crude coal tar). He experienced only partial relief with topical corticosteroids; the Goeckerman regimen cleared his skin, but he had quick recurrence after approximately 1 month. Given his work schedule, the patient was unable to resume phototherapy.
Because of symptoms related to the patient’s severe allergies, his allergist prescribed dupilumab: a 600-mg SC loading dose, followed by 300 mg SC every 2 weeks. The patient reported near-complete resolution of AD symptoms approximately 2 months after initiating treatment. He reported a few episodes of mild conjunctivitis that self-resolved after the first month of treatment.
Three weeks after initiating dupilumab, the patient noticed new-onset facial flushing in response to consuming alcohol. He described flushing as sudden immediate redness and warmth concentrated around the forehead, eyes, and cheeks. He reported that flushing was worse with hard liquor than with beer. Flushing would slowly subside over approximately 30 minutes despite continued alcohol consumption.
Comment
Two other single-patient case reports have discussed similar findings of alcohol-induced flushing associated with dupilumab.1,2 Both of those patients—a 19-year-old woman and a 26-year-old woman—had not experienced flushing before beginning treatment with dupilumab for AD. Both experienced onset of facial flushing months after beginning dupilumab even though both had consumed alcohol before starting dupilumab, similar to the cases presented here. One patient had a history of asthma; the other had a history of seasonal and environmental allergies.
Possible Mechanism of Action
Acute alcohol ingestion causes dermal vasodilation of the skin (ie, flushing).3 A proposed mechanism is that flushing results from direct action on central vascular-control mechanisms. This theory results from observations that individuals with quadriplegia lack notable ethanol-induced vasodilation, suggesting that ethanol has a central neural site of action.Although some research has indicated that ethanol might induce these effects by altering the action of certain hormones (eg, angiotensin, vasopressin, and catecholamines), the precise mechanism by which ethanol alters vascular function in humans remains unexplained.3
Deficiencies in alcohol dehydrogenase (ADH), aldehyde dehydrogenase 2, and certain cytochrome P450 enzymes also might contribute to facial flushing. People of Asian, especially East Asian, descent often respond to an acute dose of ethanol with symptoms of facial flushing—predominantly the result of an elevated blood level of acetaldehyde caused by an inherited deficiency of aldehyde dehydrogenase 2,4 which is downstream from ADH in the metabolic pathway of alcohol. The major enzyme system responsible for metabolism of ethanol is ADH; however, the cytochrome P450–dependent ethanol-oxidizing system—including major CYP450 isoforms CYP3A, CYP2C19, CYP2C9, CYP1A2, and CYP2D6, as well as minor CYP450 isoforms, such as CYP2E1— also are involved, to a lesser extent.5
A Role for Dupilumab?
A recent pharmacokinetic study found that dupilumab appears to have little effect on the activity of the major CYP450 isoforms. However, the drug’s effect on ADH and minor CYP450 minor isoforms is unknown. Prior drug-drug interaction studies have shown that certain cytokines and cytokine modulators can markedly influence the expression, stability, and activity of specific CYP450 enzymes.6 For example, IL-6 causes a reduction in messenger RNA for CYP3A4 and, to a lesser extent, for other isoforms.7 Whether dupilumab influences enzymes involved in processing alcohol requires further study.
Conclusion
We describe 2 cases of dupilumab-induced facial flushing after alcohol consumption. The mechanism of this dupilumab-associated flushing is unknown and requires further research.
- Herz S, Petri M, Sondermann W. New alcohol flushing in a patient with atopic dermatitis under therapy with dupilumab. Dermatol Ther. 2019;32:e12762. doi:10.1111/dth.12762
- Igelman SJ, Na C, Simpson EL. Alcohol-induced facial flushing in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020;6:139-140. doi:10.1016/j.jdcr.2019.12.002
- Malpas SC, Robinson BJ, Maling TJ. Mechanism of ethanol-induced vasodilation. J Appl Physiol (1985). 1990;68:731-734. doi:10.1152/jappl.1990.68.2.731
- Brooks PJ, Enoch M-A, Goldman D, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi:10.1371/journal.pmed.1000050
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. doi:10.1016/j.cld.2012.08.002
- Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146-1154. doi:10.1002/cpt.1058
- Mimura H, Kobayashi K, Xu L, et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet. 2015;30:105-110. doi:10.1016/j.dmpk.2014.09.004
Dupilumab is a fully humanized monoclonal antibody to the α subunit of the IL-4 receptor that inhibits the action of helper T cell (TH2)–type cytokines IL-4 and IL-13. Dupilumab was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe atopic dermatitis (AD). We report 2 patients with AD who were treated with dupilumab and subsequently developed facial flushing after consuming alcohol.
Case Report
Patient 1
A 24-year-old woman presented to the dermatology clinic with a lifelong history of moderate to severe AD. She had a medical history of asthma and seasonal allergies, which were treated with fexofenadine and an inhaler, as needed. The patient had an affected body surface area of approximately 70% and had achieved only partial relief with topical corticosteroids and topical calcineurin inhibitors.
Because her disease was severe, the patient was started on dupilumab at FDA-approved dosing for AD: a 600-mg subcutaneous (SC) loading dose, followed by 300 mg SC every 2 weeks. She reported rapid skin clearance within 2 weeks of the start of treatment. Her course was complicated by mild head and neck dermatitis.
Seven months after starting treatment, the patient began to acutely experience erythema and warmth over the entire face that was triggered by drinking alcohol (Figure). Before starting dupilumab, she had consumed alcohol on multiple occasions without a flushing effect. This new finding was distinguishable from her facial dermatitis. Onset was within a few minutes after drinking alcohol; flushing self-resolved in 15 to 30 minutes. Although diffuse, erythema and warmth were concentrated around the jawline, eyebrows, and ears and occurred every time the patient drank alcohol. Moreover, she reported that consumption of hard (ie, distilled) liquor, specifically tequila, caused a more severe presentation. She denied other symptoms associated with dupilumab.
Patient 2
A 32-year-old man presented to the dermatology clinic with a 10-year history of moderate to severe AD. He had a medical history of asthma (treated with albuterol, montelukast, and fluticasone); allergic rhinitis; and severe environmental allergies, including sensitivity to dust mites, dogs, trees, and grass.
For AD, the patient had been treated with topical corticosteroids and the Goeckerman regimen (a combination of phototherapy and crude coal tar). He experienced only partial relief with topical corticosteroids; the Goeckerman regimen cleared his skin, but he had quick recurrence after approximately 1 month. Given his work schedule, the patient was unable to resume phototherapy.
Because of symptoms related to the patient’s severe allergies, his allergist prescribed dupilumab: a 600-mg SC loading dose, followed by 300 mg SC every 2 weeks. The patient reported near-complete resolution of AD symptoms approximately 2 months after initiating treatment. He reported a few episodes of mild conjunctivitis that self-resolved after the first month of treatment.
Three weeks after initiating dupilumab, the patient noticed new-onset facial flushing in response to consuming alcohol. He described flushing as sudden immediate redness and warmth concentrated around the forehead, eyes, and cheeks. He reported that flushing was worse with hard liquor than with beer. Flushing would slowly subside over approximately 30 minutes despite continued alcohol consumption.
Comment
Two other single-patient case reports have discussed similar findings of alcohol-induced flushing associated with dupilumab.1,2 Both of those patients—a 19-year-old woman and a 26-year-old woman—had not experienced flushing before beginning treatment with dupilumab for AD. Both experienced onset of facial flushing months after beginning dupilumab even though both had consumed alcohol before starting dupilumab, similar to the cases presented here. One patient had a history of asthma; the other had a history of seasonal and environmental allergies.
Possible Mechanism of Action
Acute alcohol ingestion causes dermal vasodilation of the skin (ie, flushing).3 A proposed mechanism is that flushing results from direct action on central vascular-control mechanisms. This theory results from observations that individuals with quadriplegia lack notable ethanol-induced vasodilation, suggesting that ethanol has a central neural site of action.Although some research has indicated that ethanol might induce these effects by altering the action of certain hormones (eg, angiotensin, vasopressin, and catecholamines), the precise mechanism by which ethanol alters vascular function in humans remains unexplained.3
Deficiencies in alcohol dehydrogenase (ADH), aldehyde dehydrogenase 2, and certain cytochrome P450 enzymes also might contribute to facial flushing. People of Asian, especially East Asian, descent often respond to an acute dose of ethanol with symptoms of facial flushing—predominantly the result of an elevated blood level of acetaldehyde caused by an inherited deficiency of aldehyde dehydrogenase 2,4 which is downstream from ADH in the metabolic pathway of alcohol. The major enzyme system responsible for metabolism of ethanol is ADH; however, the cytochrome P450–dependent ethanol-oxidizing system—including major CYP450 isoforms CYP3A, CYP2C19, CYP2C9, CYP1A2, and CYP2D6, as well as minor CYP450 isoforms, such as CYP2E1— also are involved, to a lesser extent.5
A Role for Dupilumab?
A recent pharmacokinetic study found that dupilumab appears to have little effect on the activity of the major CYP450 isoforms. However, the drug’s effect on ADH and minor CYP450 minor isoforms is unknown. Prior drug-drug interaction studies have shown that certain cytokines and cytokine modulators can markedly influence the expression, stability, and activity of specific CYP450 enzymes.6 For example, IL-6 causes a reduction in messenger RNA for CYP3A4 and, to a lesser extent, for other isoforms.7 Whether dupilumab influences enzymes involved in processing alcohol requires further study.
Conclusion
We describe 2 cases of dupilumab-induced facial flushing after alcohol consumption. The mechanism of this dupilumab-associated flushing is unknown and requires further research.
Dupilumab is a fully humanized monoclonal antibody to the α subunit of the IL-4 receptor that inhibits the action of helper T cell (TH2)–type cytokines IL-4 and IL-13. Dupilumab was approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of moderate to severe atopic dermatitis (AD). We report 2 patients with AD who were treated with dupilumab and subsequently developed facial flushing after consuming alcohol.
Case Report
Patient 1
A 24-year-old woman presented to the dermatology clinic with a lifelong history of moderate to severe AD. She had a medical history of asthma and seasonal allergies, which were treated with fexofenadine and an inhaler, as needed. The patient had an affected body surface area of approximately 70% and had achieved only partial relief with topical corticosteroids and topical calcineurin inhibitors.
Because her disease was severe, the patient was started on dupilumab at FDA-approved dosing for AD: a 600-mg subcutaneous (SC) loading dose, followed by 300 mg SC every 2 weeks. She reported rapid skin clearance within 2 weeks of the start of treatment. Her course was complicated by mild head and neck dermatitis.
Seven months after starting treatment, the patient began to acutely experience erythema and warmth over the entire face that was triggered by drinking alcohol (Figure). Before starting dupilumab, she had consumed alcohol on multiple occasions without a flushing effect. This new finding was distinguishable from her facial dermatitis. Onset was within a few minutes after drinking alcohol; flushing self-resolved in 15 to 30 minutes. Although diffuse, erythema and warmth were concentrated around the jawline, eyebrows, and ears and occurred every time the patient drank alcohol. Moreover, she reported that consumption of hard (ie, distilled) liquor, specifically tequila, caused a more severe presentation. She denied other symptoms associated with dupilumab.
Patient 2
A 32-year-old man presented to the dermatology clinic with a 10-year history of moderate to severe AD. He had a medical history of asthma (treated with albuterol, montelukast, and fluticasone); allergic rhinitis; and severe environmental allergies, including sensitivity to dust mites, dogs, trees, and grass.
For AD, the patient had been treated with topical corticosteroids and the Goeckerman regimen (a combination of phototherapy and crude coal tar). He experienced only partial relief with topical corticosteroids; the Goeckerman regimen cleared his skin, but he had quick recurrence after approximately 1 month. Given his work schedule, the patient was unable to resume phototherapy.
Because of symptoms related to the patient’s severe allergies, his allergist prescribed dupilumab: a 600-mg SC loading dose, followed by 300 mg SC every 2 weeks. The patient reported near-complete resolution of AD symptoms approximately 2 months after initiating treatment. He reported a few episodes of mild conjunctivitis that self-resolved after the first month of treatment.
Three weeks after initiating dupilumab, the patient noticed new-onset facial flushing in response to consuming alcohol. He described flushing as sudden immediate redness and warmth concentrated around the forehead, eyes, and cheeks. He reported that flushing was worse with hard liquor than with beer. Flushing would slowly subside over approximately 30 minutes despite continued alcohol consumption.
Comment
Two other single-patient case reports have discussed similar findings of alcohol-induced flushing associated with dupilumab.1,2 Both of those patients—a 19-year-old woman and a 26-year-old woman—had not experienced flushing before beginning treatment with dupilumab for AD. Both experienced onset of facial flushing months after beginning dupilumab even though both had consumed alcohol before starting dupilumab, similar to the cases presented here. One patient had a history of asthma; the other had a history of seasonal and environmental allergies.
Possible Mechanism of Action
Acute alcohol ingestion causes dermal vasodilation of the skin (ie, flushing).3 A proposed mechanism is that flushing results from direct action on central vascular-control mechanisms. This theory results from observations that individuals with quadriplegia lack notable ethanol-induced vasodilation, suggesting that ethanol has a central neural site of action.Although some research has indicated that ethanol might induce these effects by altering the action of certain hormones (eg, angiotensin, vasopressin, and catecholamines), the precise mechanism by which ethanol alters vascular function in humans remains unexplained.3
Deficiencies in alcohol dehydrogenase (ADH), aldehyde dehydrogenase 2, and certain cytochrome P450 enzymes also might contribute to facial flushing. People of Asian, especially East Asian, descent often respond to an acute dose of ethanol with symptoms of facial flushing—predominantly the result of an elevated blood level of acetaldehyde caused by an inherited deficiency of aldehyde dehydrogenase 2,4 which is downstream from ADH in the metabolic pathway of alcohol. The major enzyme system responsible for metabolism of ethanol is ADH; however, the cytochrome P450–dependent ethanol-oxidizing system—including major CYP450 isoforms CYP3A, CYP2C19, CYP2C9, CYP1A2, and CYP2D6, as well as minor CYP450 isoforms, such as CYP2E1— also are involved, to a lesser extent.5
A Role for Dupilumab?
A recent pharmacokinetic study found that dupilumab appears to have little effect on the activity of the major CYP450 isoforms. However, the drug’s effect on ADH and minor CYP450 minor isoforms is unknown. Prior drug-drug interaction studies have shown that certain cytokines and cytokine modulators can markedly influence the expression, stability, and activity of specific CYP450 enzymes.6 For example, IL-6 causes a reduction in messenger RNA for CYP3A4 and, to a lesser extent, for other isoforms.7 Whether dupilumab influences enzymes involved in processing alcohol requires further study.
Conclusion
We describe 2 cases of dupilumab-induced facial flushing after alcohol consumption. The mechanism of this dupilumab-associated flushing is unknown and requires further research.
- Herz S, Petri M, Sondermann W. New alcohol flushing in a patient with atopic dermatitis under therapy with dupilumab. Dermatol Ther. 2019;32:e12762. doi:10.1111/dth.12762
- Igelman SJ, Na C, Simpson EL. Alcohol-induced facial flushing in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020;6:139-140. doi:10.1016/j.jdcr.2019.12.002
- Malpas SC, Robinson BJ, Maling TJ. Mechanism of ethanol-induced vasodilation. J Appl Physiol (1985). 1990;68:731-734. doi:10.1152/jappl.1990.68.2.731
- Brooks PJ, Enoch M-A, Goldman D, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi:10.1371/journal.pmed.1000050
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. doi:10.1016/j.cld.2012.08.002
- Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146-1154. doi:10.1002/cpt.1058
- Mimura H, Kobayashi K, Xu L, et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet. 2015;30:105-110. doi:10.1016/j.dmpk.2014.09.004
- Herz S, Petri M, Sondermann W. New alcohol flushing in a patient with atopic dermatitis under therapy with dupilumab. Dermatol Ther. 2019;32:e12762. doi:10.1111/dth.12762
- Igelman SJ, Na C, Simpson EL. Alcohol-induced facial flushing in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. 2020;6:139-140. doi:10.1016/j.jdcr.2019.12.002
- Malpas SC, Robinson BJ, Maling TJ. Mechanism of ethanol-induced vasodilation. J Appl Physiol (1985). 1990;68:731-734. doi:10.1152/jappl.1990.68.2.731
- Brooks PJ, Enoch M-A, Goldman D, et al. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi:10.1371/journal.pmed.1000050
- Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. doi:10.1016/j.cld.2012.08.002
- Davis JD, Bansal A, Hassman D, et al. Evaluation of potential disease-mediated drug-drug interaction in patients with moderate-to-severe atopic dermatitis receiving dupilumab. Clin Pharmacol Ther. 2018;104:1146-1154. doi:10.1002/cpt.1058
- Mimura H, Kobayashi K, Xu L, et al. Effects of cytokines on CYP3A4 expression and reversal of the effects by anti-cytokine agents in the three-dimensionally cultured human hepatoma cell line FLC-4. Drug Metab Pharmacokinet. 2015;30:105-110. doi:10.1016/j.dmpk.2014.09.004
Practice Points
- Dupilumab is a fully humanized monoclonal antibody that inhibits the action of IL-4 and IL-13. It was approved by the US Food and Drug Administration in 2017 for treatment of moderate to severe atopic dermatitis.
- Facial flushing after alcohol consumption may be an emerging side effect of dupilumab.
- Whether dupilumab influences enzymes involved in processing alcohol requires further study.
An Algorithm for Managing Spitting Sutures
Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
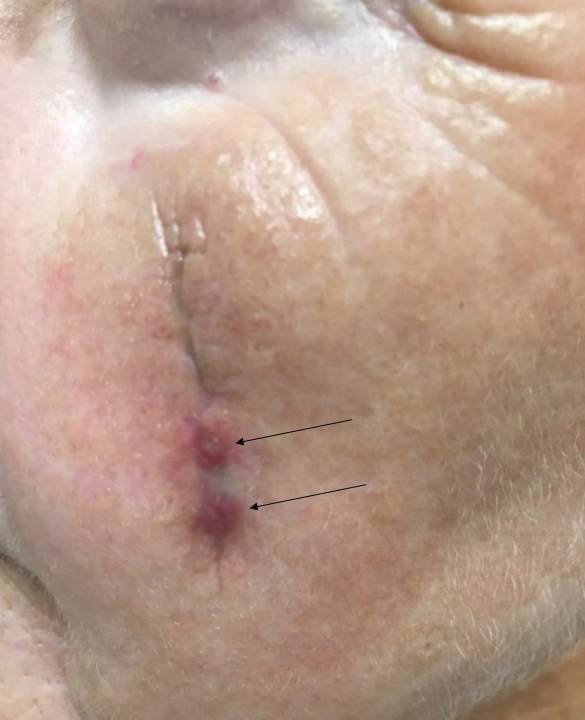
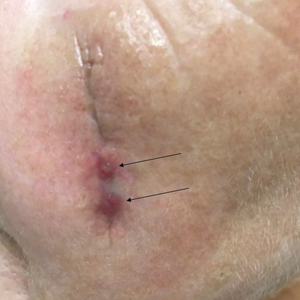
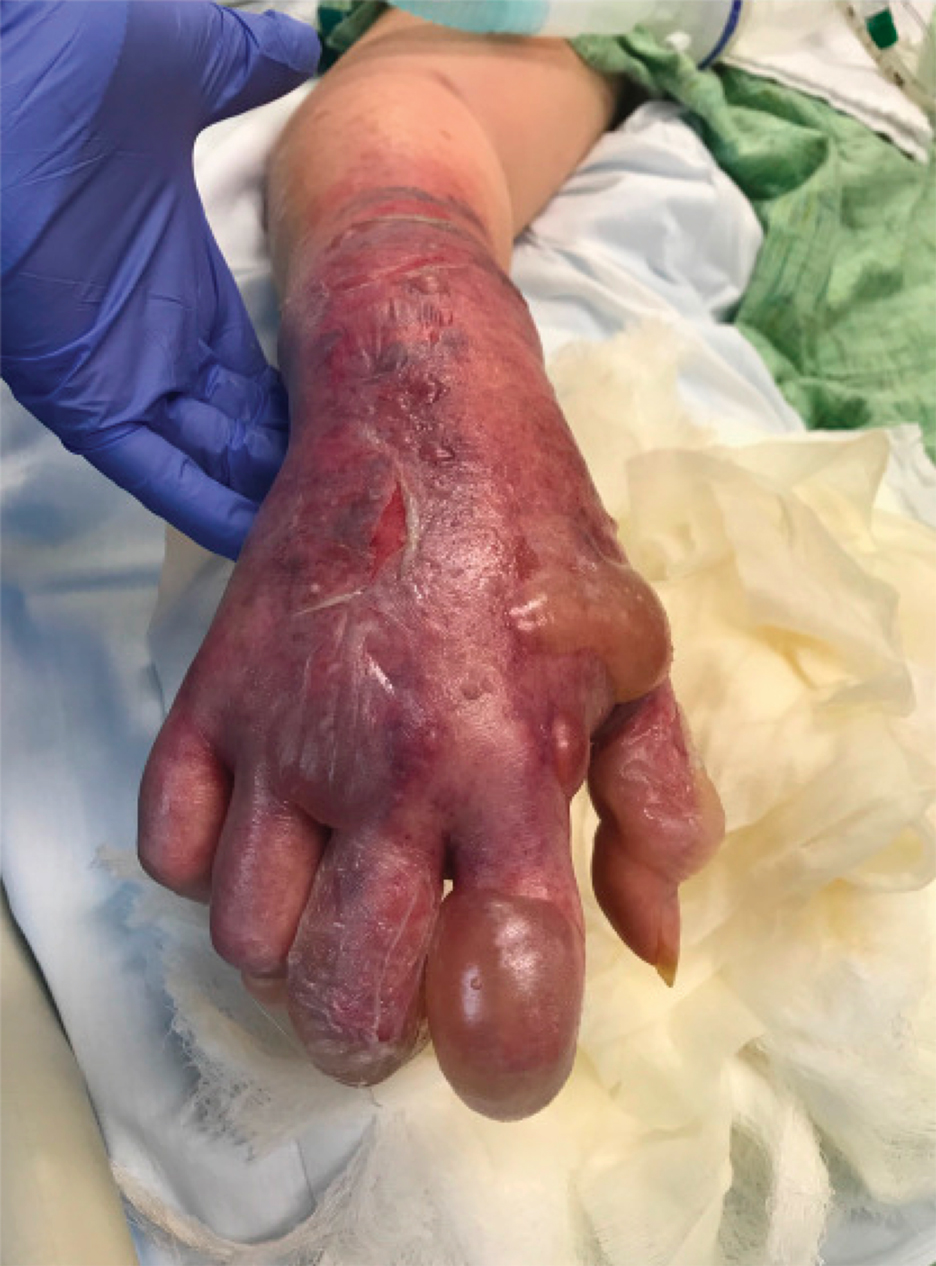
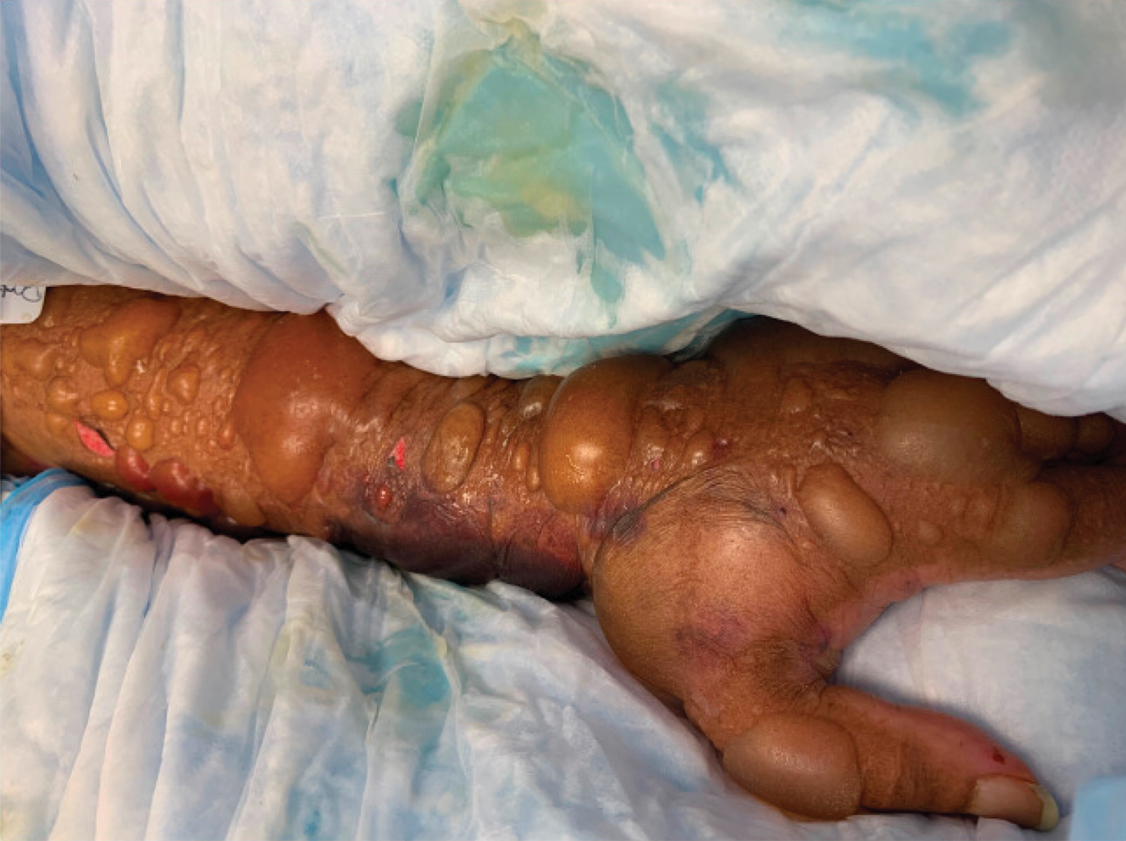
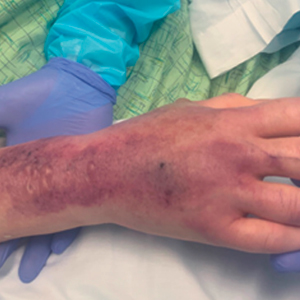
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).
Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
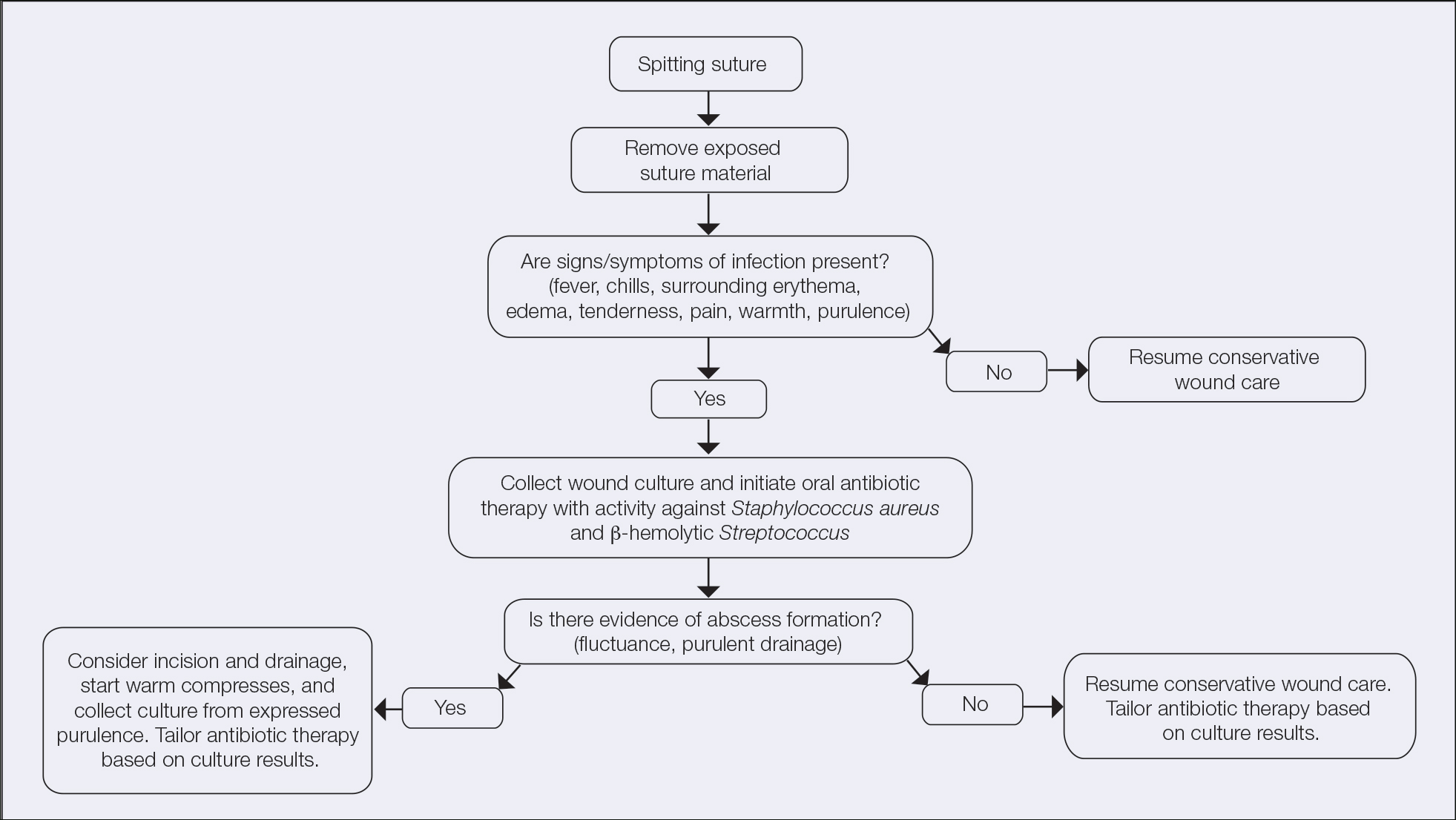
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.
- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).
Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.
Practice Gap
It is well established that surgical complications and a poor scar outcome can have a remarkable impact on patient satisfaction.1 A common complication following dermatologic surgery is suture spitting, in which a buried suture is extruded through the skin surface. When repairing a cutaneous defect following dermatologic surgery, absorbable or nonabsorbable sutures are placed under the skin surface to approximate wound edges, eliminate dead space, and reduce tension on the edges of the wound, improving the cosmetic outcomes.
Absorbable sutures constitute most buried sutures in cutaneous surgery and can be made of natural or synthetic fibers.2 Absorbable sutures made from synthetic fibers are degraded by hydrolysis, in which water breaks down polymer chains of the suture filament. Natural absorbable sutures are composed of mammalian collagen; they are broken down by the enzymatic process of proteolysis.
Tensile strength is lost long before a suture is fully absorbed. Although synthetic fibers have, in general, higher tensile strength and generate less tissue inflammation, they take much longer to absorb.2 During absorption, in some cases, a buried suture is pushed to the surface and extrudes along the wound edge or scar, which is known as spitting3 (Figure 1).
Suture spitting typically occurs in the 2-week to 3-month postoperative period. However, with the use of long-lasting absorbable or nonabsorbable sutures, spitting can occur several months or years postoperatively. Spitting sutures often are associated with surrounding erythema, edema, discharge, and a foreign-body sensation4—symptoms that can be highly distressing to the patient and can lead to postoperative infection or stitch abscess.3
Herein, we review techniques that can decrease the risk for suture spitting, and we present a stepwise approach to managing this common problem.
The Technique
Choice of suture material for buried sutures can influence the risk of spitting.
Factors Impacting Increased Spitting
The 3 most common absorbable sutures in dermatologic surgery include poliglecaprone 25, polyglactin 910, and polydioxanone; of them, polyglactin 910 has been found to have a higher rate of spitting than poliglecaprone 25 and polydioxanone.2 However, because complete absorption of polydioxanone can take as long as 8 months, this suture might “spit” much later than polyglactin 910 or poliglecaprone 25, which typically are fully hydrolyzed by 3 and 4 months, respectively.2 Placing sutures superficially in the dermis has been found to increase the rate of spitting.5 Throwing more knots per closure also has been found to increase the rate of spitting.5
How to Decrease Spitting
Careful choice of suture material and proper depth of suture placement might decrease the risk for spitting in dermatologic surgery. Furthermore, if polyglactin 910 or a long-lasting suture is to be used, sutures should be placed deeply.
What to Do If Sutures Spit
When a suture has begun to spit, the extruding foreign material needs to be removed and the surgical site assessed for infection or abscess. Exposed suture material typically can be removed with forceps without local anesthesia. In some cases, fine-tipped Bishop-Harmon tissue forceps or jewelers forceps might be required.
If the suture cannot be removed completely, it should be trimmed as short as possible. This can be accomplished by pulling on the exposed end of the suture, tenting the skin, and trimming it as close as possible to the surface. Once the foreign material is removed, assessment for signs of infection is paramount.
How to Manage Infection—Postoperative infection associated with a spitting suture can take the form of a periwound cellulitis or stitch abscess.3 A stitch abscess can reflect a sterile inflammatory response to the buried suture or a true infection4; the former is more common.3 In the event of an infected stitch abscess, provide warm compresses, obtain specimens for culture, and prescribe antibiotics after the spitting suture has been removed. Incision and drainage also might be required if notable fluctuance is present.
It is crucial for dermatologic surgeons to identify and manage these complications. Figure 2 illustrates an algorithmic approach to managing spitting sutures.
Practical Implications
Spitting sutures are a common occurrence following dermatologic surgery that can lead to remarkable patient distress. Fortunately, in the absence of superimposed infection, spitting sutures have not been shown to worsen outcomes of healing and scarring.5 Nevertheless, it is important to identify and appropriately treat this common complication. The simple algorithm we provide (Figure 2) aids in cutaneous surgery by providing a straightforward approach to managing spitting sutures and their complications.
- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
- Balaraman B, Geddes ER, Friedman PM. Best reconstructive techniques: improving the final scar. Dermatol Surg. 2015;41(suppl 10):S265-S275. doi:10.1097/DSS.0000000000000496
- Yag-Howard C. Sutures, needles, and tissue adhesives: a review for dermatologic surgery. Dermatol Surg. 2014;40(suppl 9):S3-S15. doi:10.1097/01.DSS.0000452738.23278.2d
- Gloster HM. Complications in Cutaneous Surgery. Springer; 2011.
- Slutsky JB, Fosko ST. Complications in Mohs surgery. In: Berlin A, ed. Mohs and Cutaneous Surgery: Maximizing Aesthetic Outcomes. CRC Press; 2015:55-89.
- Kim B, Sgarioto M, Hewitt D, et al. Scar outcomes in dermatological surgery. Australas J Dermatol. 2018;59:48-51. doi:10.1111/ajd.12570
Patch Test–Directed Dietary Avoidance in the Management of Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is one of the most common disorders managed by primary care physicians and gastroenterologists.1 Characterized by abdominal pain coinciding with altered stool form and/or frequency as defined by the Rome IV diagnostic criteria,2 symptoms range from mild to debilitating and may remarkably impair quality of life and work productivity.1
The cause of IBS is poorly understood. Proposed pathophysiologic factors include impaired mucosal function, microbial imbalance, visceral hypersensitivity, psychologic dysfunction, genetic factors, neurotransmitter imbalance, postinfectious gastroenteritis, inflammation, and food intolerance, any or all of which may lead to the development and maintenance of IBS symptoms.3 More recent observations of inflammation in the intestinal lining4,5 and proinflammatory peripherally circulating cytokines6 challenge its traditional classification as a functional disorder.
The cause of this inflammation is of intense interest, with speculation that the bacterial microbiota, bile acids, association with postinfectious gastroenteritis and inflammatory bowel disease cases, and/or foods may contribute. Although approximately 50% of individuals with IBS report that foods aggravate their symptoms,7 studies investigating type I antibody–mediated immediate hypersensitivity have largely failed to demonstrate a substantial link, prompting many authorities to regard these associations as food “intolerances” rather than true allergies. Based on this body of literature, a large 2010 consensus report on all aspects of food allergies advises against food allergy testing for IBS.8
In contrast, by utilizing type IV food allergen skin patch testing, 2 proof-of-concept studies9,10 investigated a different allergic mechanism in IBS, namely cell-mediated delayed-type hypersensitivity. Because many foods and food additives are known to cause allergic contact dermatitis,11 it was hypothesized that these foods may elicit a similar delayed-type hypersensitivity response in the intestinal lining in previously sensitized individuals. By following a patch test–guided food avoidance diet, a large subpopulation of patients with IBS experienced partial or complete IBS symptom relief.9,10 Our study further investigates a role for food-related delayed-type hypersensitivities in the pathogenesis of IBS.
Methods
Patient Selection
This study was conducted in a secondary care community-based setting. All patients were self-referred over an 18-month period ending in October 2019, had physician-diagnosed IBS, and/or met the Rome IV criteria for IBS and presented expressly for the food patch testing on a fee-for-service basis. Subtype of IBS was determined on presentation by the self-reported historically predominant symptom. Duration of IBS symptoms was self-reported and was rounded to the nearest year for purposes of data collection.
Exclusion criteria included pregnancy, known allergy to adhesive tape or any of the food allergens used in the study, severe skin rash, symptoms that had a known cause other than IBS, or active treatment with systemic immunosuppressive medications.
Patch Testing
Skin patch testing was initiated using an extensive panel of 117 type IV food allergens (eTable)11 identified in the literature,12 most of which utilized standard compounded formulations13 or were available from reputable patch test manufacturers (Brial Allergen GmbH; Chemotechnique Diagnostics). This panel was not approved by the US Food and Drug Administration. The freeze-dried vegetable formulations were taken from the 2018 report.9 Standard skin patch test procedure protocols12 were used, affixing the patches to the upper aspect of the back.
Following patch test application on day 1, two follow-up visits occurred on day 3 and either day 4 or day 5. On day 3, patches were removed, and the initial results were read by a board-certified dermatologist according to a standard grading system.14 Interpretation of patch tests included no reaction, questionable reaction consisting of macular erythema, weak reaction consisting of erythema and slight edema, or strong reaction consisting of erythema and marked edema. On day 4 or day 5, the final patch test reading was performed, and patients were informed of their results. Patients were advised to avoid ingestion of all foods that elicited a questionable or positive patch test response for at least 3 months, and information about the foods and their avoidance also was distributed and reviewed.
Food Avoidance Questionnaire
Patients with questionable or positive patch tests at 72 or 96 hours were advised of their eligibility to participate in an institutional review board–approved food avoidance questionnaire study investigating the utility of patch test–guided food avoidance on IBS symptoms. The questionnaire assessed the following: (1) baseline average abdominal pain prior to patch test–guided avoidance diet (0=no symptoms; 10=very severe); (2) average abdominal pain since initiation of patch test–guided avoidance diet (0=no symptoms; 10=very severe); (3) degree of improvement in overall IBS symptoms by the end of the food avoidance period (0=no improvement; 10=great improvement); (4) compliance with the avoidance diet for the duration of the avoidance period (completely, partially, not at all, or not sure).
Questionnaires and informed consent were mailed to patients via the US Postal Service 3 months after completing the patch testing. The questionnaire and consent were to be completed and returned after dietary avoidance of the identified allergens for at least 3 months. Patients were not compensated for participation in the study.
Statistical Analysis
Statistical analysis of data collected from study questionnaires was performed with Microsoft Excel. Mean abdominal pain and mean global improvement scores were reported along with 1 SD of the mean. For comparison of mean abdominal pain and improvement in global IBS symptoms from baseline to after 3 months of identified allergen avoidance, a Mann-Whitney U test was performed, with P<.05 being considered statistically significant.
Results
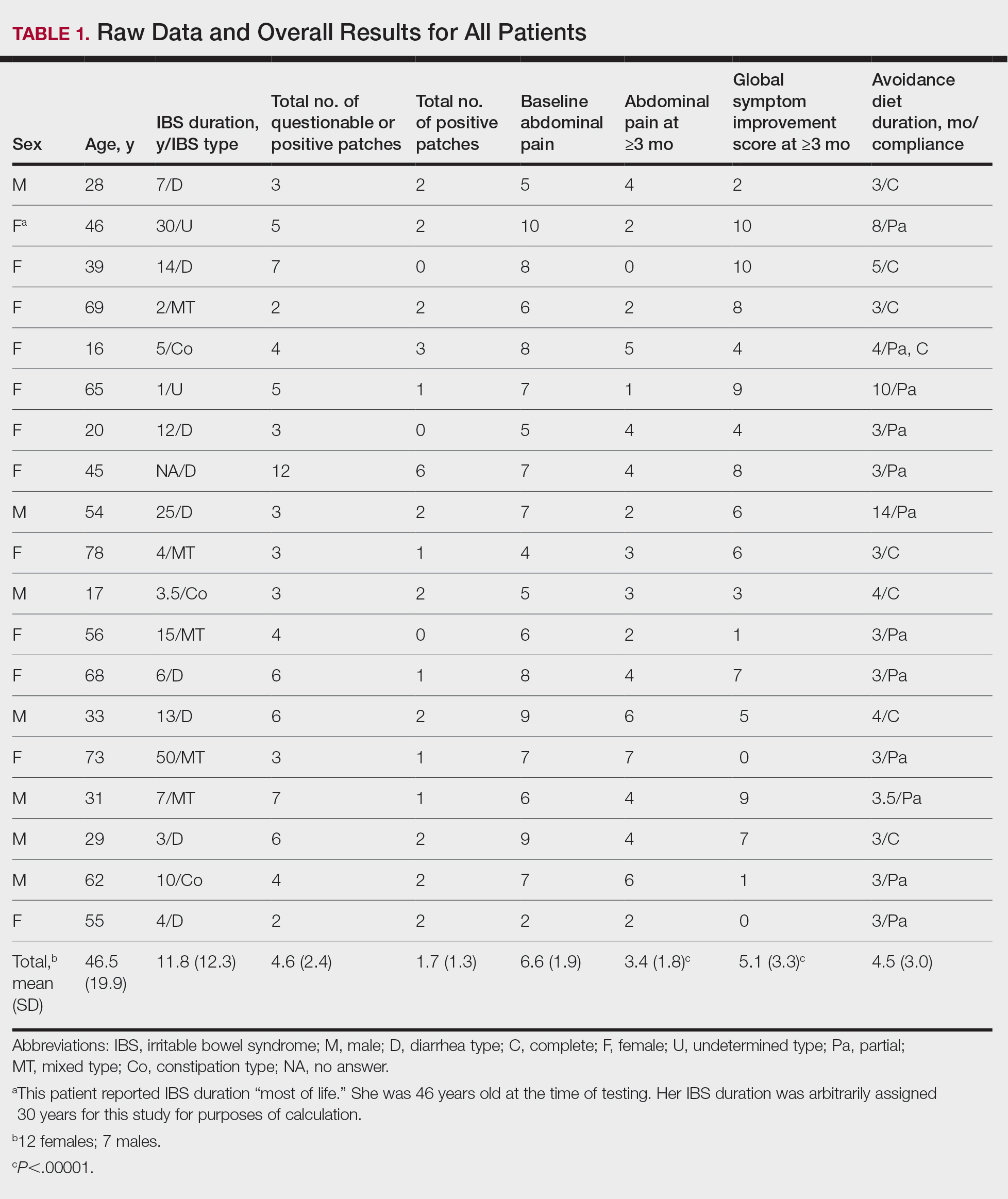
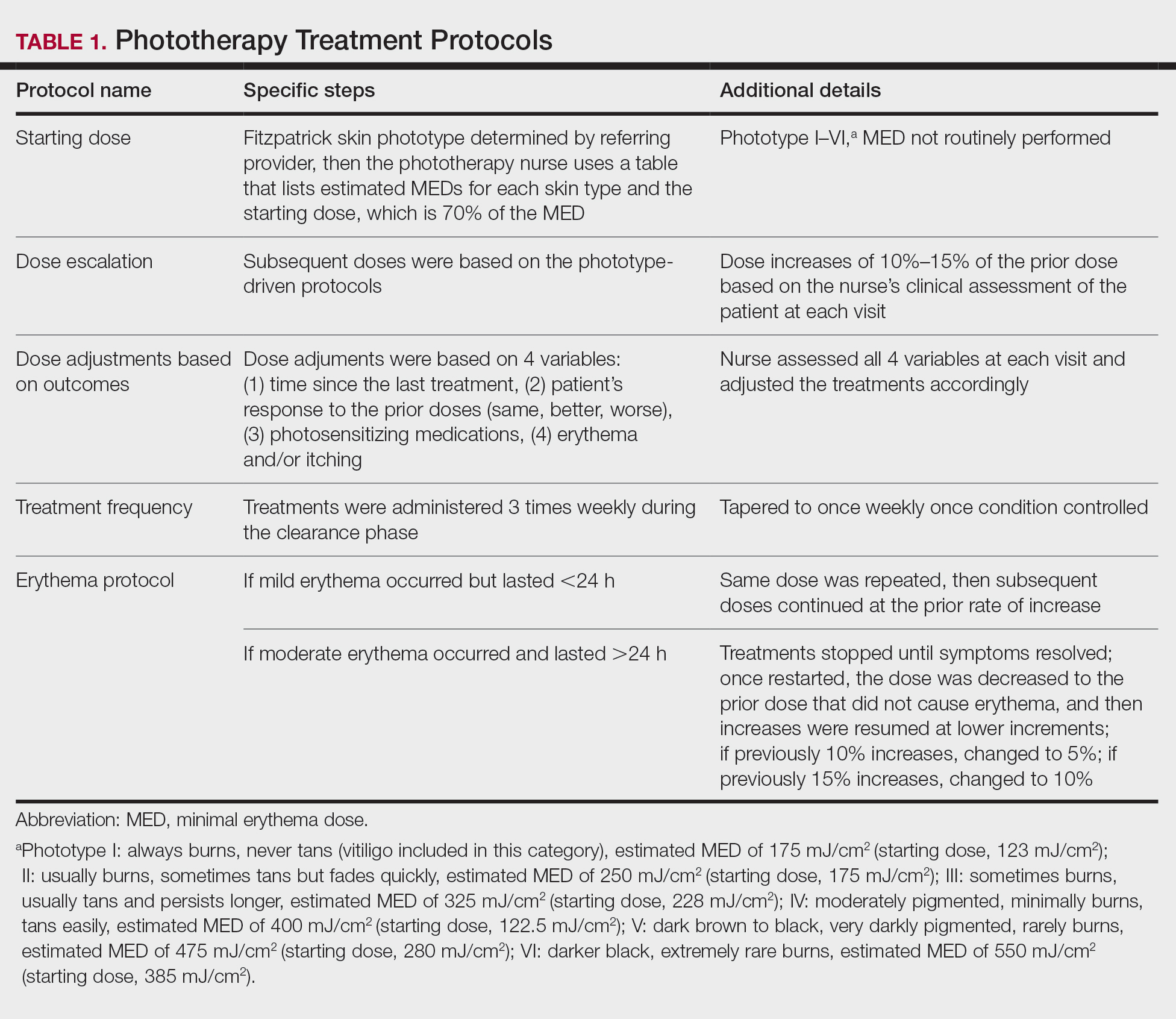
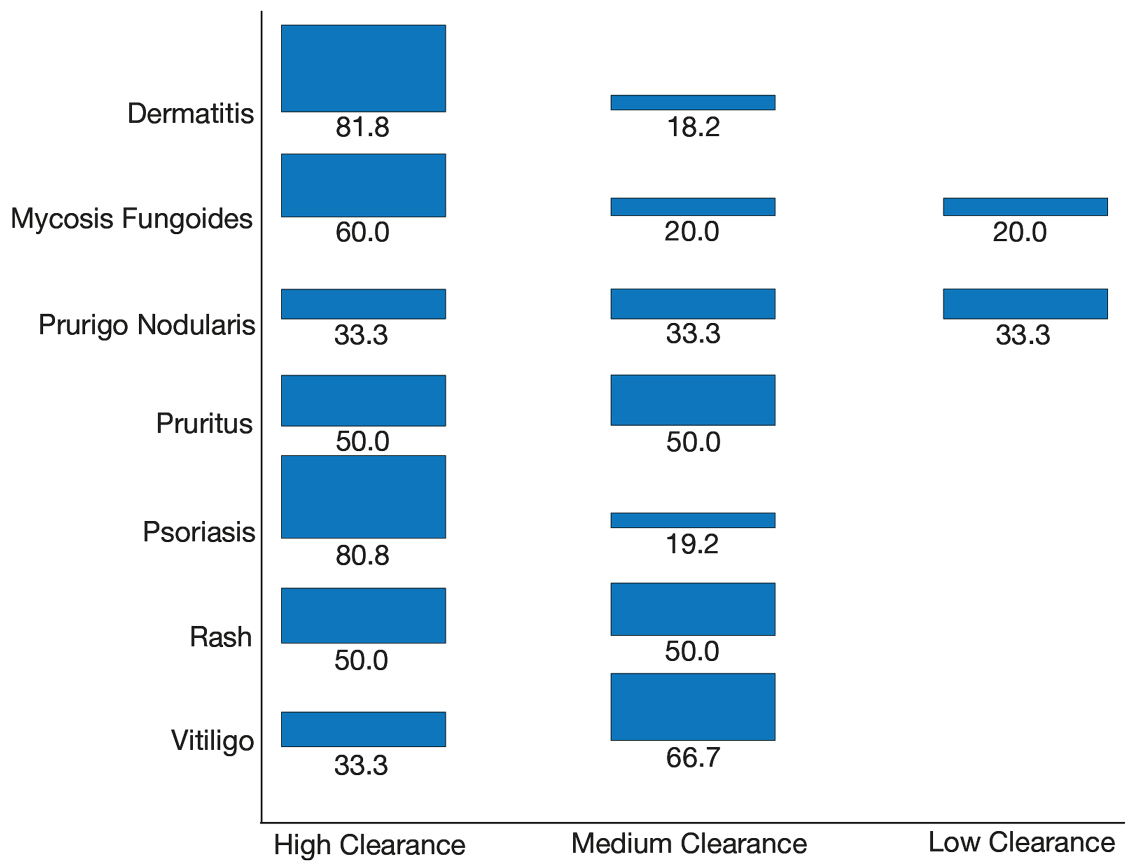
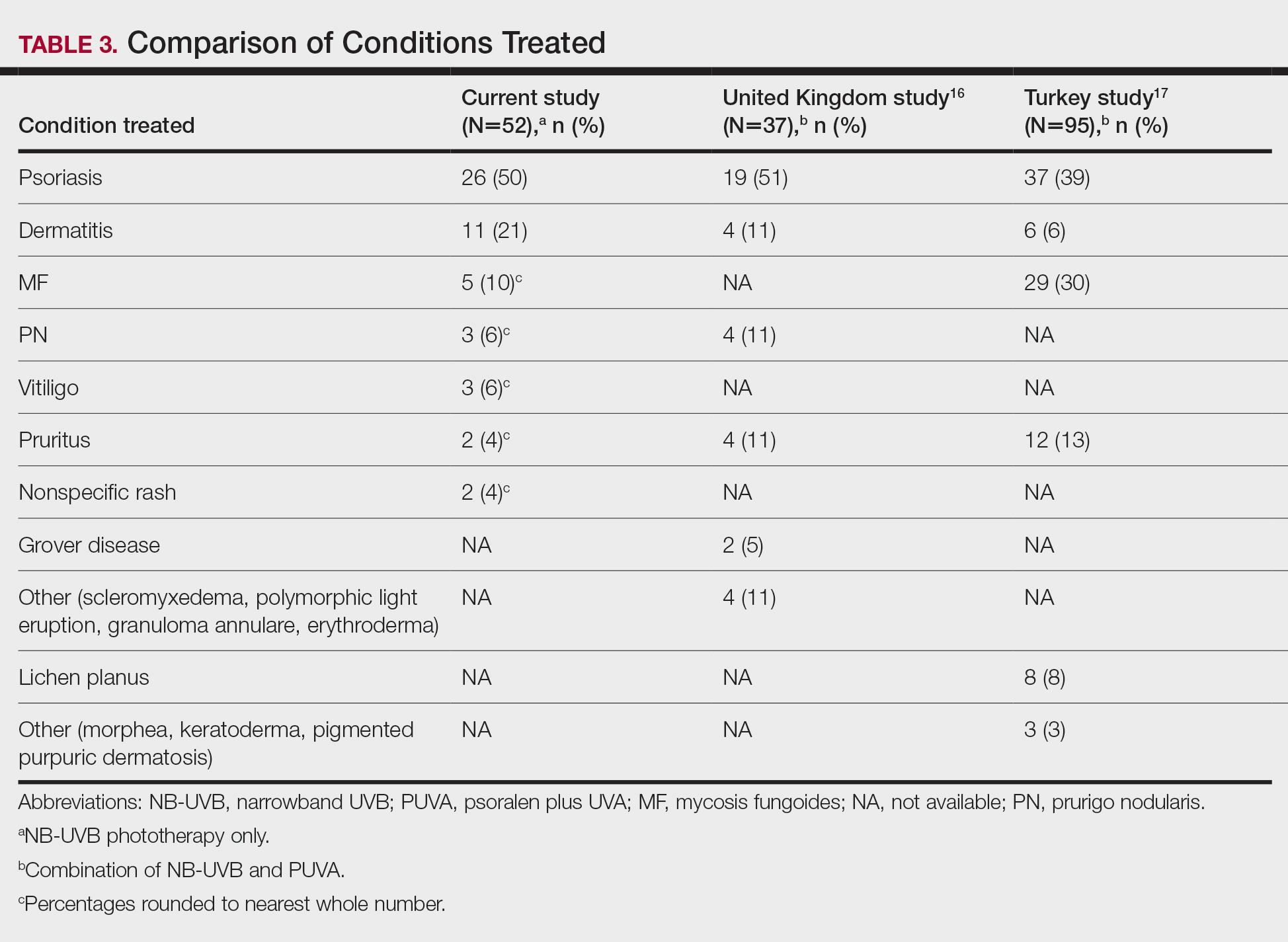
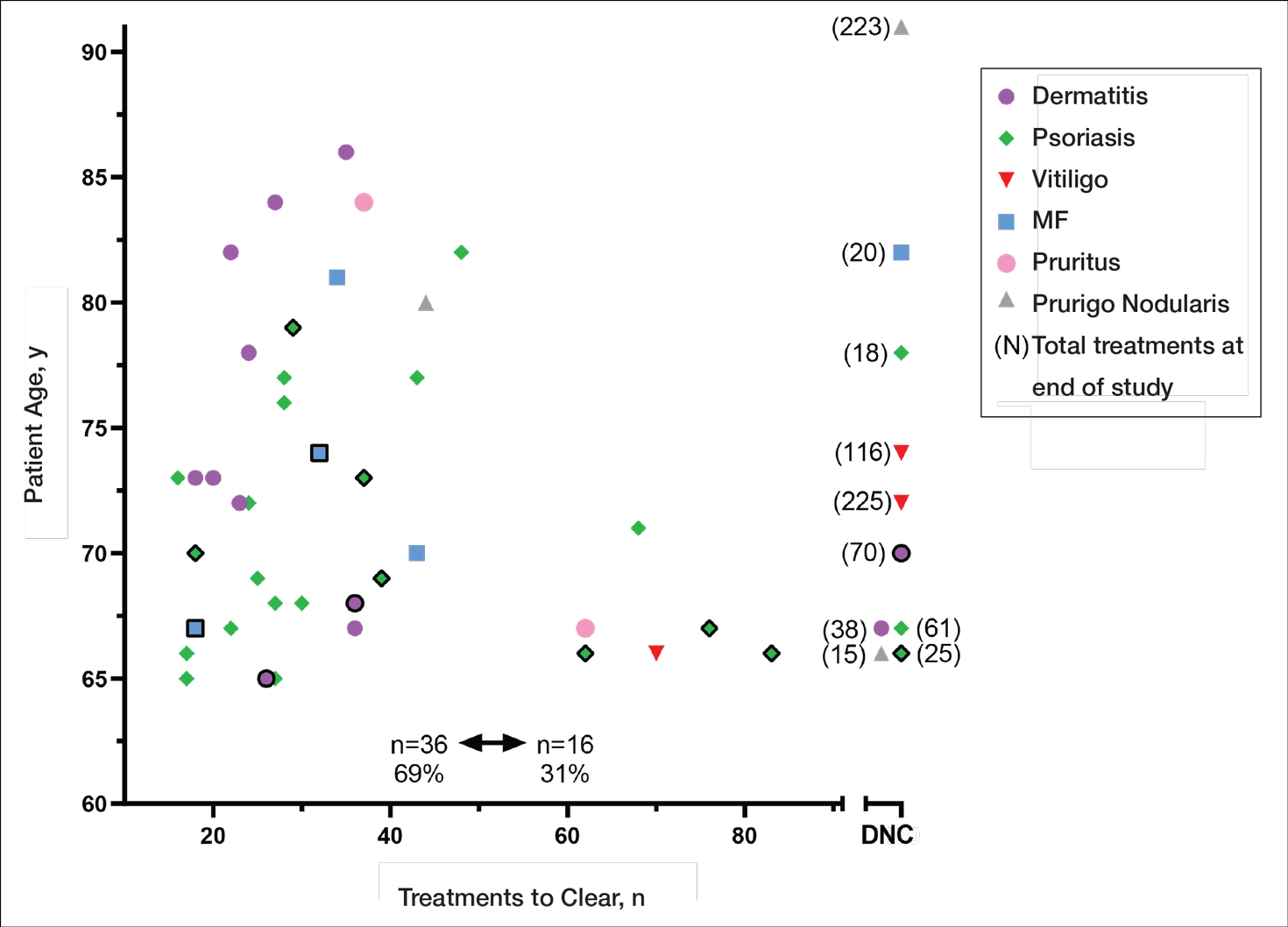
Thirty-seven consecutive patients underwent the testing and were eligible for the study. Nineteen patients were included in the study by virtue of completing and returning their posttest food avoidance questionnaire and informed consent. Eighteen patients were White and 1 was Asian. Subcategories of IBS were diarrhea predominant (9 [47.4%]), constipation predominant (3 [15.8%]), mixed type (5 [26.3%]), and undetermined type (2 [10.5%]). Questionnaire answers were reported after a mean (SD) duration of patch test–directed food avoidance of 4.5 (3.0) months (Table 1).
Overall Improvement
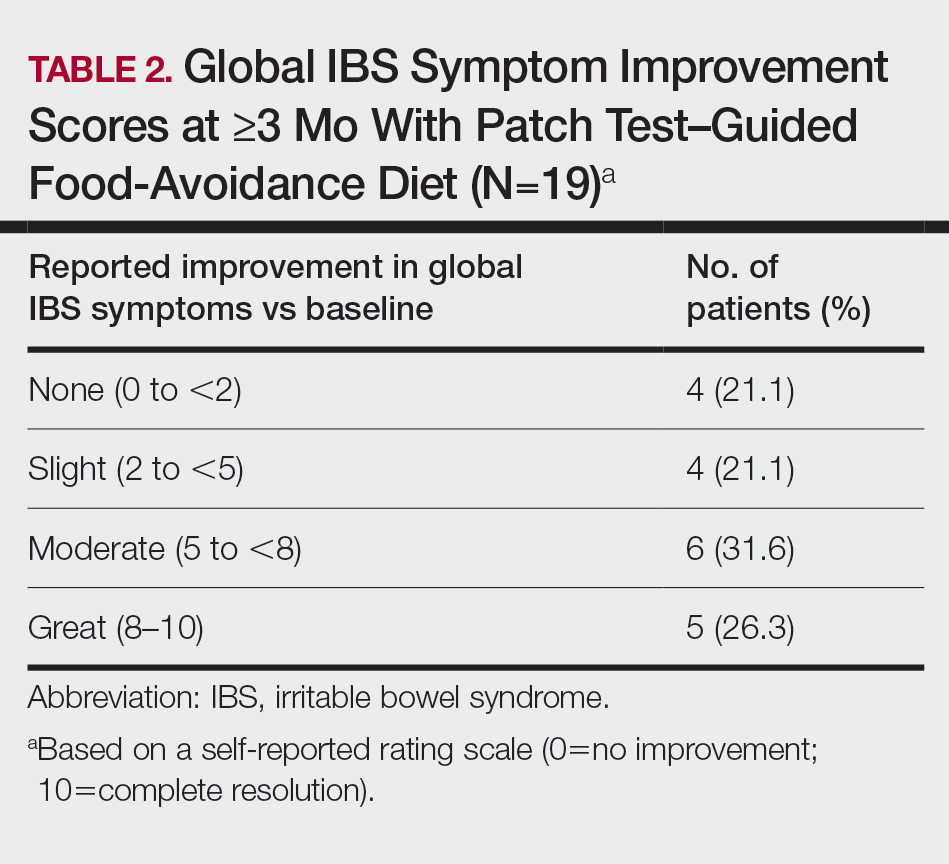
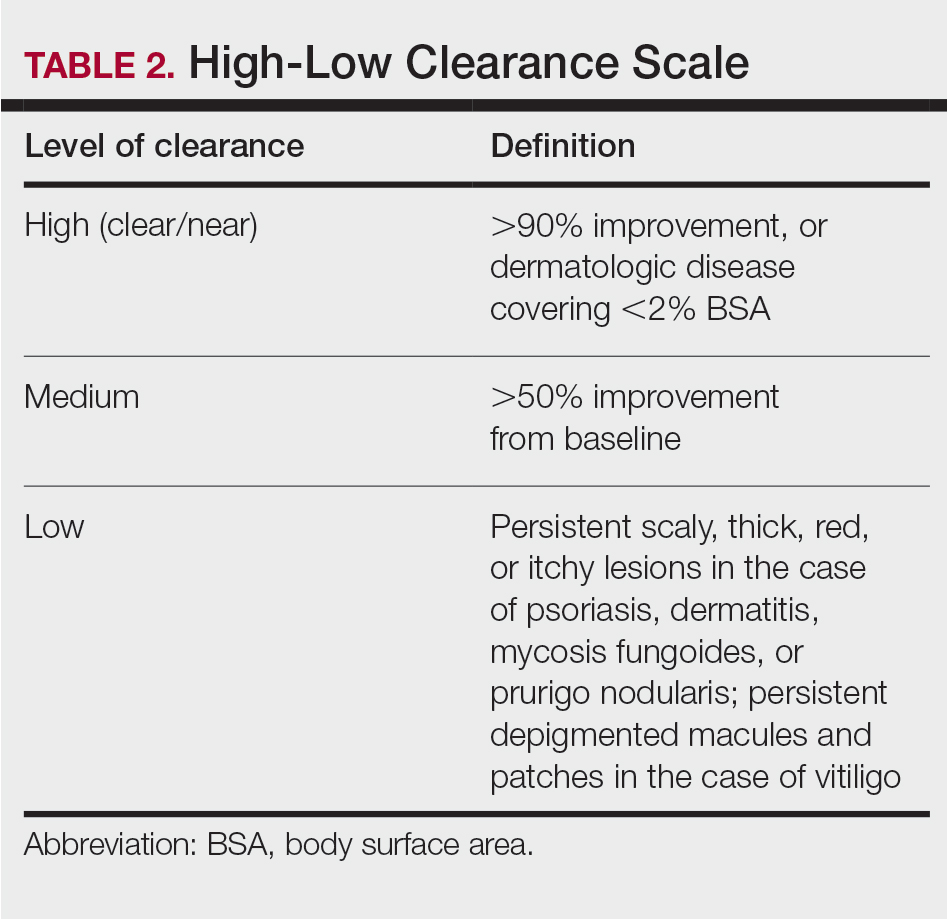
Fifteen (78.9%) patients reported at least slight to great improvement in their global IBS symptoms, and 4 (21.1%) reported no improvement (Table 2), with a mean (SD) improvement score of 5.1 (3.3)(P<.00001).
Abdominal Pain
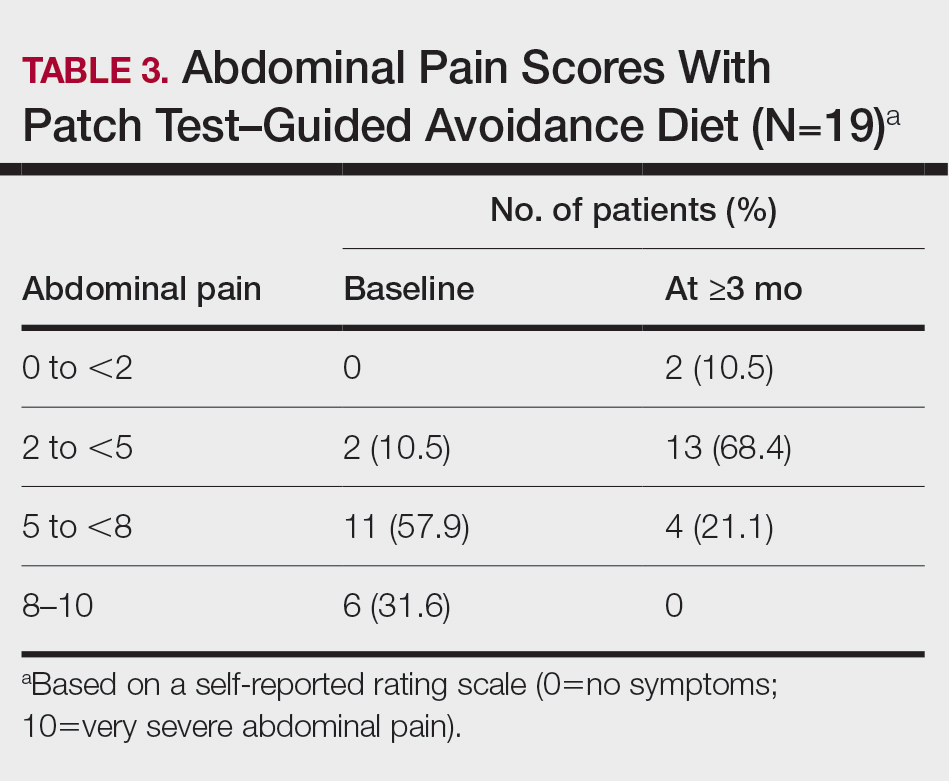
All 19 patients reported mild to marked abdominal pain at baseline. The mean (SD) baseline pain score was 6.6 (1.9). The mean (SD) pain score was 3.4 (1.8)(P<.00001) after an average patch test–guided dietary avoidance of 4.5 (3.0) months (Table 3).
Comment
Despite intense research interest and a growing number of new medications for IBS approved by the US Food and Drug Administration, there remains a large void in the search for cost-effective and efficacious approaches for IBS evaluation and treatment. In addition to major disturbances in quality of life,14,15 the cost to society in direct medical expenses and indirect costs associated with loss of productivity and work absenteeism is considerable; estimates range from $21 billion or more annually.16
Food Hypersensitivities Triggering IBS
This study further evaluated a role for skin patch testing to identify delayed-type (type IV) food hypersensitivities that trigger IBS symptoms and differed from the prior investigations9,10 in that the symptoms used to define IBS were updated from the Rome III17 to the newer Rome IV2 criteria. The data presented here show moderate to great improvement in global IBS symptoms in 58% (11/19) of patients, which is in line with a 2018 report of 40 study participants for whom follow-up at 3 or more months was available,9 providing additional support for a role for type IV food allergies in causing the same gastrointestinal tract symptoms that define IBS. The distinction between food-related studies, including this one, that implicate food allergies9,10 and prior studies that did not support a role for food allergies in IBS pathogenesis8 can be accounted for by the type of allergy investigated. Conclusions that IBS flares after food ingestion were attributable to intolerance rather than true allergy were based on results investigating only the humoral arm and failed to consider the cell-mediated arm of the immune system. As such, foods that appear to trigger IBS symptoms on an allergic basis in our study are recognized in the literature12 as type IV allergens that elicit cell-mediated immunologic responses rather than more widely recognized type I allergens, such as peanuts and shellfish, that elicit immediate-type hypersensitivity responses. Although any type IV food allergen(s) could be responsible, a pattern emerged in this study and the study published in 2018.9 Namely, some foods stood out as more frequently inducing patch test reactions, with the 3 most common being carmine, cinnamon bark oil, and sodium bisulfite (eTable). The sample size is relatively small, but the results raise the question of whether these foods are the most likely to trigger IBS symptoms in the general population. If so, is it the result of a higher innate sensitizing potential and/or a higher frequency of exposure in commonly eaten foods? Larger randomized clinical trials are needed.
Immune Response and IBS
There is mounting evidence that the immune system may play a role in the pathophysiology of IBS.18 Both lymphocyte infiltration of the myenteric plexus and an increase in intestinal mucosal T lymphocytes have been observed, and it is generally accepted that the mucosal immune system seems to be activated, at least in a subset of patients with IBS.19 Irritable bowel syndrome associations with quiescent inflammatory bowel disease or postinfectious gastroenteritis provide 2 potential causes for the inflammation, but most IBS patients have had neither.20 The mucosal lining of the intestine and immune system have vast exposure to intraluminal allergens in transit, and it is hypothesized that the same delayed-type hypersensitivity response elicited in the skin by patch testing is elicited in the intestine, resulting in the inflammation that triggers IBS symptoms.10 The results here add to the growing body of evidence that ingestion of type IV food allergens by previously sensitized individuals could, in fact, be the primary source of the inflammation observed in a large subpopulation of individuals who carry a diagnosis of IBS.
Food Allergens in Patch Testing
Many of the food allergens used in this study are commonly found in various nonfood products that may contact the skin. For example, many flavorings are used as fragrances, and many preservatives, binders, thickeners, emulsifiers, and stabilizers serve the same role in moisturizers, cosmetics, and topical medications. Likewise, nickel sulfate hexahydrate, ubiquitous in foods that arise from the earth, often is found in metal in jewelry, clothing components, and cell phones. All are potential sensitizers. Thus, the question may arise whether the causal relationship between the food allergens identified by patch testing and IBS symptoms might be more of a systemic effect akin to systemic contact dermatitis as sometimes follows ingestion of an allergen to which an individual has been topically sensitized, rather than the proposed localized immunologic response in the intestinal lining. We were unaware of patient history of allergic contact dermatitis to any of the patch test allergens in this study, but the dermatologist author here (M.S.) has unpublished experience with 2 other patients with IBS who have benefited from low-nickel diets after having had positive patch tests to nickel sulfate hexahydrate and who, in retrospect, did report a history of earring dermatitis. Future investigations using pre– and post–food challenge histologic assessments of the intestinal mucosa in patients who benefit from patch test–guided food avoidance diets should help to better define the mechanism.
Because IBS has not been traditionally associated with structural or biochemical abnormalities detectable with current routine diagnostic tools, it has long been viewed as a functional disorder. The findings published more recently,9,10 in addition to this study’s results, would negate this functional classification in the subset of patients with IBS symptoms who experience sustained relief of their symptoms by patch test–directed food avoidance. The underlying delayed-type hypersensitivity pathogenesis of the IBS-like symptoms in these individuals would mandate an organic classification, aptly named allergic contact enteritis.10
Follow-up Data
The mean (SD) follow-up duration for this study and the 2018 report9 was 4.5 (3.0) months and 7.6 (3.9) months, respectively. The placebo effect is a concern for disorders such as IBS in which primarily subjective outcome measures are available,21 and in a retrospective analysis of 25 randomized, placebo-controlled IBS clinical trials, Spiller22 concluded the optimum length of such trials to be more than 3 months, which these studies exceed. Although not blinded or placebo controlled, the length of follow-up in the 2018 report9 and here enhances the validity of the results.
Limitation
The retrospective manner in which the self-assessments were reported in this study introduces the potential for recall bias, a variable that could affect results. The presence and direction of bias by any given individual cannot be known, making it difficult to determine any effect it may have had. Further investigation should include daily assessments and refine the primary study end points to include both abdominal pain and the defecation considerations that define IBS.
Conclusion
Food patch testing has the potential to offer a safe, cost-effective approach to the evaluation and management of IBS symptoms. Randomized clinical trials are needed to further investigate the validity of the proof-of-concept results to date. For patients who benefit from a patch test–guided avoidance diet, invasive and costly endoscopic, radiologic, and laboratory testing and pharmacologic management could be averted. Symptomatic relief could be attained simply by avoiding the implicated foods, essentially doing more by doing less.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:1-24.
- Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99.
- Barbara G, De Giorgio R, Stanghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(suppl 2):1-9
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778-1783.
- Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979.
- O’Mahony L, McCarthy J, Kelly
P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. - Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defecation in the irritable bowel syndrome (IBS): patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415-421.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Shin GH, Smith MS, Toro B, et al. Utility of food patch testing in the evaluation and management of irritable bowel syndrome. Skin. 2018;2:1-15.
- Stierstorfer MB, Sha CT. Food patch testing for irritable bowel syndrome. J Am Acad Dermatol. 2013;68:377-384.
- Marks JG, Belsito DV, DeLeo MD, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol. 1998;38:911-918.
- Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. BC Decker; 2008.
- DeGroot AC. Patch Testing. acdegroot Publishing; 2008.
- Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660.
- Halder SL, Lock GR, Talley NJ, et al. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case–control study. Aliment Pharmacol Ther. 2004;19:233-242.
- International Foundation for Gastrointestinal Disorders. About IBS. statistics. Accessed July 20, 2021. https://www.aboutibs.org/facts-about-ibs/statistics.html
- Rome Foundation. Guidelines—Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:307-312.
- Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;192(suppl):102-105.
- Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? a systemic review. Neurogastroenterol Motil. 2006;18:595-607.
- Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease–irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48-53.
- Hrobiartsson A, Gotzsche PC. Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594-1602.
- Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S-97S.
Irritable bowel syndrome (IBS) is one of the most common disorders managed by primary care physicians and gastroenterologists.1 Characterized by abdominal pain coinciding with altered stool form and/or frequency as defined by the Rome IV diagnostic criteria,2 symptoms range from mild to debilitating and may remarkably impair quality of life and work productivity.1
The cause of IBS is poorly understood. Proposed pathophysiologic factors include impaired mucosal function, microbial imbalance, visceral hypersensitivity, psychologic dysfunction, genetic factors, neurotransmitter imbalance, postinfectious gastroenteritis, inflammation, and food intolerance, any or all of which may lead to the development and maintenance of IBS symptoms.3 More recent observations of inflammation in the intestinal lining4,5 and proinflammatory peripherally circulating cytokines6 challenge its traditional classification as a functional disorder.
The cause of this inflammation is of intense interest, with speculation that the bacterial microbiota, bile acids, association with postinfectious gastroenteritis and inflammatory bowel disease cases, and/or foods may contribute. Although approximately 50% of individuals with IBS report that foods aggravate their symptoms,7 studies investigating type I antibody–mediated immediate hypersensitivity have largely failed to demonstrate a substantial link, prompting many authorities to regard these associations as food “intolerances” rather than true allergies. Based on this body of literature, a large 2010 consensus report on all aspects of food allergies advises against food allergy testing for IBS.8
In contrast, by utilizing type IV food allergen skin patch testing, 2 proof-of-concept studies9,10 investigated a different allergic mechanism in IBS, namely cell-mediated delayed-type hypersensitivity. Because many foods and food additives are known to cause allergic contact dermatitis,11 it was hypothesized that these foods may elicit a similar delayed-type hypersensitivity response in the intestinal lining in previously sensitized individuals. By following a patch test–guided food avoidance diet, a large subpopulation of patients with IBS experienced partial or complete IBS symptom relief.9,10 Our study further investigates a role for food-related delayed-type hypersensitivities in the pathogenesis of IBS.
Methods
Patient Selection
This study was conducted in a secondary care community-based setting. All patients were self-referred over an 18-month period ending in October 2019, had physician-diagnosed IBS, and/or met the Rome IV criteria for IBS and presented expressly for the food patch testing on a fee-for-service basis. Subtype of IBS was determined on presentation by the self-reported historically predominant symptom. Duration of IBS symptoms was self-reported and was rounded to the nearest year for purposes of data collection.
Exclusion criteria included pregnancy, known allergy to adhesive tape or any of the food allergens used in the study, severe skin rash, symptoms that had a known cause other than IBS, or active treatment with systemic immunosuppressive medications.
Patch Testing
Skin patch testing was initiated using an extensive panel of 117 type IV food allergens (eTable)11 identified in the literature,12 most of which utilized standard compounded formulations13 or were available from reputable patch test manufacturers (Brial Allergen GmbH; Chemotechnique Diagnostics). This panel was not approved by the US Food and Drug Administration. The freeze-dried vegetable formulations were taken from the 2018 report.9 Standard skin patch test procedure protocols12 were used, affixing the patches to the upper aspect of the back.
Following patch test application on day 1, two follow-up visits occurred on day 3 and either day 4 or day 5. On day 3, patches were removed, and the initial results were read by a board-certified dermatologist according to a standard grading system.14 Interpretation of patch tests included no reaction, questionable reaction consisting of macular erythema, weak reaction consisting of erythema and slight edema, or strong reaction consisting of erythema and marked edema. On day 4 or day 5, the final patch test reading was performed, and patients were informed of their results. Patients were advised to avoid ingestion of all foods that elicited a questionable or positive patch test response for at least 3 months, and information about the foods and their avoidance also was distributed and reviewed.
Food Avoidance Questionnaire
Patients with questionable or positive patch tests at 72 or 96 hours were advised of their eligibility to participate in an institutional review board–approved food avoidance questionnaire study investigating the utility of patch test–guided food avoidance on IBS symptoms. The questionnaire assessed the following: (1) baseline average abdominal pain prior to patch test–guided avoidance diet (0=no symptoms; 10=very severe); (2) average abdominal pain since initiation of patch test–guided avoidance diet (0=no symptoms; 10=very severe); (3) degree of improvement in overall IBS symptoms by the end of the food avoidance period (0=no improvement; 10=great improvement); (4) compliance with the avoidance diet for the duration of the avoidance period (completely, partially, not at all, or not sure).
Questionnaires and informed consent were mailed to patients via the US Postal Service 3 months after completing the patch testing. The questionnaire and consent were to be completed and returned after dietary avoidance of the identified allergens for at least 3 months. Patients were not compensated for participation in the study.
Statistical Analysis
Statistical analysis of data collected from study questionnaires was performed with Microsoft Excel. Mean abdominal pain and mean global improvement scores were reported along with 1 SD of the mean. For comparison of mean abdominal pain and improvement in global IBS symptoms from baseline to after 3 months of identified allergen avoidance, a Mann-Whitney U test was performed, with P<.05 being considered statistically significant.
Results
Thirty-seven consecutive patients underwent the testing and were eligible for the study. Nineteen patients were included in the study by virtue of completing and returning their posttest food avoidance questionnaire and informed consent. Eighteen patients were White and 1 was Asian. Subcategories of IBS were diarrhea predominant (9 [47.4%]), constipation predominant (3 [15.8%]), mixed type (5 [26.3%]), and undetermined type (2 [10.5%]). Questionnaire answers were reported after a mean (SD) duration of patch test–directed food avoidance of 4.5 (3.0) months (Table 1).
Overall Improvement
Fifteen (78.9%) patients reported at least slight to great improvement in their global IBS symptoms, and 4 (21.1%) reported no improvement (Table 2), with a mean (SD) improvement score of 5.1 (3.3)(P<.00001).
Abdominal Pain
All 19 patients reported mild to marked abdominal pain at baseline. The mean (SD) baseline pain score was 6.6 (1.9). The mean (SD) pain score was 3.4 (1.8)(P<.00001) after an average patch test–guided dietary avoidance of 4.5 (3.0) months (Table 3).
Comment
Despite intense research interest and a growing number of new medications for IBS approved by the US Food and Drug Administration, there remains a large void in the search for cost-effective and efficacious approaches for IBS evaluation and treatment. In addition to major disturbances in quality of life,14,15 the cost to society in direct medical expenses and indirect costs associated with loss of productivity and work absenteeism is considerable; estimates range from $21 billion or more annually.16
Food Hypersensitivities Triggering IBS
This study further evaluated a role for skin patch testing to identify delayed-type (type IV) food hypersensitivities that trigger IBS symptoms and differed from the prior investigations9,10 in that the symptoms used to define IBS were updated from the Rome III17 to the newer Rome IV2 criteria. The data presented here show moderate to great improvement in global IBS symptoms in 58% (11/19) of patients, which is in line with a 2018 report of 40 study participants for whom follow-up at 3 or more months was available,9 providing additional support for a role for type IV food allergies in causing the same gastrointestinal tract symptoms that define IBS. The distinction between food-related studies, including this one, that implicate food allergies9,10 and prior studies that did not support a role for food allergies in IBS pathogenesis8 can be accounted for by the type of allergy investigated. Conclusions that IBS flares after food ingestion were attributable to intolerance rather than true allergy were based on results investigating only the humoral arm and failed to consider the cell-mediated arm of the immune system. As such, foods that appear to trigger IBS symptoms on an allergic basis in our study are recognized in the literature12 as type IV allergens that elicit cell-mediated immunologic responses rather than more widely recognized type I allergens, such as peanuts and shellfish, that elicit immediate-type hypersensitivity responses. Although any type IV food allergen(s) could be responsible, a pattern emerged in this study and the study published in 2018.9 Namely, some foods stood out as more frequently inducing patch test reactions, with the 3 most common being carmine, cinnamon bark oil, and sodium bisulfite (eTable). The sample size is relatively small, but the results raise the question of whether these foods are the most likely to trigger IBS symptoms in the general population. If so, is it the result of a higher innate sensitizing potential and/or a higher frequency of exposure in commonly eaten foods? Larger randomized clinical trials are needed.
Immune Response and IBS
There is mounting evidence that the immune system may play a role in the pathophysiology of IBS.18 Both lymphocyte infiltration of the myenteric plexus and an increase in intestinal mucosal T lymphocytes have been observed, and it is generally accepted that the mucosal immune system seems to be activated, at least in a subset of patients with IBS.19 Irritable bowel syndrome associations with quiescent inflammatory bowel disease or postinfectious gastroenteritis provide 2 potential causes for the inflammation, but most IBS patients have had neither.20 The mucosal lining of the intestine and immune system have vast exposure to intraluminal allergens in transit, and it is hypothesized that the same delayed-type hypersensitivity response elicited in the skin by patch testing is elicited in the intestine, resulting in the inflammation that triggers IBS symptoms.10 The results here add to the growing body of evidence that ingestion of type IV food allergens by previously sensitized individuals could, in fact, be the primary source of the inflammation observed in a large subpopulation of individuals who carry a diagnosis of IBS.
Food Allergens in Patch Testing
Many of the food allergens used in this study are commonly found in various nonfood products that may contact the skin. For example, many flavorings are used as fragrances, and many preservatives, binders, thickeners, emulsifiers, and stabilizers serve the same role in moisturizers, cosmetics, and topical medications. Likewise, nickel sulfate hexahydrate, ubiquitous in foods that arise from the earth, often is found in metal in jewelry, clothing components, and cell phones. All are potential sensitizers. Thus, the question may arise whether the causal relationship between the food allergens identified by patch testing and IBS symptoms might be more of a systemic effect akin to systemic contact dermatitis as sometimes follows ingestion of an allergen to which an individual has been topically sensitized, rather than the proposed localized immunologic response in the intestinal lining. We were unaware of patient history of allergic contact dermatitis to any of the patch test allergens in this study, but the dermatologist author here (M.S.) has unpublished experience with 2 other patients with IBS who have benefited from low-nickel diets after having had positive patch tests to nickel sulfate hexahydrate and who, in retrospect, did report a history of earring dermatitis. Future investigations using pre– and post–food challenge histologic assessments of the intestinal mucosa in patients who benefit from patch test–guided food avoidance diets should help to better define the mechanism.
Because IBS has not been traditionally associated with structural or biochemical abnormalities detectable with current routine diagnostic tools, it has long been viewed as a functional disorder. The findings published more recently,9,10 in addition to this study’s results, would negate this functional classification in the subset of patients with IBS symptoms who experience sustained relief of their symptoms by patch test–directed food avoidance. The underlying delayed-type hypersensitivity pathogenesis of the IBS-like symptoms in these individuals would mandate an organic classification, aptly named allergic contact enteritis.10
Follow-up Data
The mean (SD) follow-up duration for this study and the 2018 report9 was 4.5 (3.0) months and 7.6 (3.9) months, respectively. The placebo effect is a concern for disorders such as IBS in which primarily subjective outcome measures are available,21 and in a retrospective analysis of 25 randomized, placebo-controlled IBS clinical trials, Spiller22 concluded the optimum length of such trials to be more than 3 months, which these studies exceed. Although not blinded or placebo controlled, the length of follow-up in the 2018 report9 and here enhances the validity of the results.
Limitation
The retrospective manner in which the self-assessments were reported in this study introduces the potential for recall bias, a variable that could affect results. The presence and direction of bias by any given individual cannot be known, making it difficult to determine any effect it may have had. Further investigation should include daily assessments and refine the primary study end points to include both abdominal pain and the defecation considerations that define IBS.
Conclusion
Food patch testing has the potential to offer a safe, cost-effective approach to the evaluation and management of IBS symptoms. Randomized clinical trials are needed to further investigate the validity of the proof-of-concept results to date. For patients who benefit from a patch test–guided avoidance diet, invasive and costly endoscopic, radiologic, and laboratory testing and pharmacologic management could be averted. Symptomatic relief could be attained simply by avoiding the implicated foods, essentially doing more by doing less.
Irritable bowel syndrome (IBS) is one of the most common disorders managed by primary care physicians and gastroenterologists.1 Characterized by abdominal pain coinciding with altered stool form and/or frequency as defined by the Rome IV diagnostic criteria,2 symptoms range from mild to debilitating and may remarkably impair quality of life and work productivity.1
The cause of IBS is poorly understood. Proposed pathophysiologic factors include impaired mucosal function, microbial imbalance, visceral hypersensitivity, psychologic dysfunction, genetic factors, neurotransmitter imbalance, postinfectious gastroenteritis, inflammation, and food intolerance, any or all of which may lead to the development and maintenance of IBS symptoms.3 More recent observations of inflammation in the intestinal lining4,5 and proinflammatory peripherally circulating cytokines6 challenge its traditional classification as a functional disorder.
The cause of this inflammation is of intense interest, with speculation that the bacterial microbiota, bile acids, association with postinfectious gastroenteritis and inflammatory bowel disease cases, and/or foods may contribute. Although approximately 50% of individuals with IBS report that foods aggravate their symptoms,7 studies investigating type I antibody–mediated immediate hypersensitivity have largely failed to demonstrate a substantial link, prompting many authorities to regard these associations as food “intolerances” rather than true allergies. Based on this body of literature, a large 2010 consensus report on all aspects of food allergies advises against food allergy testing for IBS.8
In contrast, by utilizing type IV food allergen skin patch testing, 2 proof-of-concept studies9,10 investigated a different allergic mechanism in IBS, namely cell-mediated delayed-type hypersensitivity. Because many foods and food additives are known to cause allergic contact dermatitis,11 it was hypothesized that these foods may elicit a similar delayed-type hypersensitivity response in the intestinal lining in previously sensitized individuals. By following a patch test–guided food avoidance diet, a large subpopulation of patients with IBS experienced partial or complete IBS symptom relief.9,10 Our study further investigates a role for food-related delayed-type hypersensitivities in the pathogenesis of IBS.
Methods
Patient Selection
This study was conducted in a secondary care community-based setting. All patients were self-referred over an 18-month period ending in October 2019, had physician-diagnosed IBS, and/or met the Rome IV criteria for IBS and presented expressly for the food patch testing on a fee-for-service basis. Subtype of IBS was determined on presentation by the self-reported historically predominant symptom. Duration of IBS symptoms was self-reported and was rounded to the nearest year for purposes of data collection.
Exclusion criteria included pregnancy, known allergy to adhesive tape or any of the food allergens used in the study, severe skin rash, symptoms that had a known cause other than IBS, or active treatment with systemic immunosuppressive medications.
Patch Testing
Skin patch testing was initiated using an extensive panel of 117 type IV food allergens (eTable)11 identified in the literature,12 most of which utilized standard compounded formulations13 or were available from reputable patch test manufacturers (Brial Allergen GmbH; Chemotechnique Diagnostics). This panel was not approved by the US Food and Drug Administration. The freeze-dried vegetable formulations were taken from the 2018 report.9 Standard skin patch test procedure protocols12 were used, affixing the patches to the upper aspect of the back.
Following patch test application on day 1, two follow-up visits occurred on day 3 and either day 4 or day 5. On day 3, patches were removed, and the initial results were read by a board-certified dermatologist according to a standard grading system.14 Interpretation of patch tests included no reaction, questionable reaction consisting of macular erythema, weak reaction consisting of erythema and slight edema, or strong reaction consisting of erythema and marked edema. On day 4 or day 5, the final patch test reading was performed, and patients were informed of their results. Patients were advised to avoid ingestion of all foods that elicited a questionable or positive patch test response for at least 3 months, and information about the foods and their avoidance also was distributed and reviewed.
Food Avoidance Questionnaire
Patients with questionable or positive patch tests at 72 or 96 hours were advised of their eligibility to participate in an institutional review board–approved food avoidance questionnaire study investigating the utility of patch test–guided food avoidance on IBS symptoms. The questionnaire assessed the following: (1) baseline average abdominal pain prior to patch test–guided avoidance diet (0=no symptoms; 10=very severe); (2) average abdominal pain since initiation of patch test–guided avoidance diet (0=no symptoms; 10=very severe); (3) degree of improvement in overall IBS symptoms by the end of the food avoidance period (0=no improvement; 10=great improvement); (4) compliance with the avoidance diet for the duration of the avoidance period (completely, partially, not at all, or not sure).
Questionnaires and informed consent were mailed to patients via the US Postal Service 3 months after completing the patch testing. The questionnaire and consent were to be completed and returned after dietary avoidance of the identified allergens for at least 3 months. Patients were not compensated for participation in the study.
Statistical Analysis
Statistical analysis of data collected from study questionnaires was performed with Microsoft Excel. Mean abdominal pain and mean global improvement scores were reported along with 1 SD of the mean. For comparison of mean abdominal pain and improvement in global IBS symptoms from baseline to after 3 months of identified allergen avoidance, a Mann-Whitney U test was performed, with P<.05 being considered statistically significant.
Results
Thirty-seven consecutive patients underwent the testing and were eligible for the study. Nineteen patients were included in the study by virtue of completing and returning their posttest food avoidance questionnaire and informed consent. Eighteen patients were White and 1 was Asian. Subcategories of IBS were diarrhea predominant (9 [47.4%]), constipation predominant (3 [15.8%]), mixed type (5 [26.3%]), and undetermined type (2 [10.5%]). Questionnaire answers were reported after a mean (SD) duration of patch test–directed food avoidance of 4.5 (3.0) months (Table 1).
Overall Improvement
Fifteen (78.9%) patients reported at least slight to great improvement in their global IBS symptoms, and 4 (21.1%) reported no improvement (Table 2), with a mean (SD) improvement score of 5.1 (3.3)(P<.00001).
Abdominal Pain
All 19 patients reported mild to marked abdominal pain at baseline. The mean (SD) baseline pain score was 6.6 (1.9). The mean (SD) pain score was 3.4 (1.8)(P<.00001) after an average patch test–guided dietary avoidance of 4.5 (3.0) months (Table 3).
Comment
Despite intense research interest and a growing number of new medications for IBS approved by the US Food and Drug Administration, there remains a large void in the search for cost-effective and efficacious approaches for IBS evaluation and treatment. In addition to major disturbances in quality of life,14,15 the cost to society in direct medical expenses and indirect costs associated with loss of productivity and work absenteeism is considerable; estimates range from $21 billion or more annually.16
Food Hypersensitivities Triggering IBS
This study further evaluated a role for skin patch testing to identify delayed-type (type IV) food hypersensitivities that trigger IBS symptoms and differed from the prior investigations9,10 in that the symptoms used to define IBS were updated from the Rome III17 to the newer Rome IV2 criteria. The data presented here show moderate to great improvement in global IBS symptoms in 58% (11/19) of patients, which is in line with a 2018 report of 40 study participants for whom follow-up at 3 or more months was available,9 providing additional support for a role for type IV food allergies in causing the same gastrointestinal tract symptoms that define IBS. The distinction between food-related studies, including this one, that implicate food allergies9,10 and prior studies that did not support a role for food allergies in IBS pathogenesis8 can be accounted for by the type of allergy investigated. Conclusions that IBS flares after food ingestion were attributable to intolerance rather than true allergy were based on results investigating only the humoral arm and failed to consider the cell-mediated arm of the immune system. As such, foods that appear to trigger IBS symptoms on an allergic basis in our study are recognized in the literature12 as type IV allergens that elicit cell-mediated immunologic responses rather than more widely recognized type I allergens, such as peanuts and shellfish, that elicit immediate-type hypersensitivity responses. Although any type IV food allergen(s) could be responsible, a pattern emerged in this study and the study published in 2018.9 Namely, some foods stood out as more frequently inducing patch test reactions, with the 3 most common being carmine, cinnamon bark oil, and sodium bisulfite (eTable). The sample size is relatively small, but the results raise the question of whether these foods are the most likely to trigger IBS symptoms in the general population. If so, is it the result of a higher innate sensitizing potential and/or a higher frequency of exposure in commonly eaten foods? Larger randomized clinical trials are needed.
Immune Response and IBS
There is mounting evidence that the immune system may play a role in the pathophysiology of IBS.18 Both lymphocyte infiltration of the myenteric plexus and an increase in intestinal mucosal T lymphocytes have been observed, and it is generally accepted that the mucosal immune system seems to be activated, at least in a subset of patients with IBS.19 Irritable bowel syndrome associations with quiescent inflammatory bowel disease or postinfectious gastroenteritis provide 2 potential causes for the inflammation, but most IBS patients have had neither.20 The mucosal lining of the intestine and immune system have vast exposure to intraluminal allergens in transit, and it is hypothesized that the same delayed-type hypersensitivity response elicited in the skin by patch testing is elicited in the intestine, resulting in the inflammation that triggers IBS symptoms.10 The results here add to the growing body of evidence that ingestion of type IV food allergens by previously sensitized individuals could, in fact, be the primary source of the inflammation observed in a large subpopulation of individuals who carry a diagnosis of IBS.
Food Allergens in Patch Testing
Many of the food allergens used in this study are commonly found in various nonfood products that may contact the skin. For example, many flavorings are used as fragrances, and many preservatives, binders, thickeners, emulsifiers, and stabilizers serve the same role in moisturizers, cosmetics, and topical medications. Likewise, nickel sulfate hexahydrate, ubiquitous in foods that arise from the earth, often is found in metal in jewelry, clothing components, and cell phones. All are potential sensitizers. Thus, the question may arise whether the causal relationship between the food allergens identified by patch testing and IBS symptoms might be more of a systemic effect akin to systemic contact dermatitis as sometimes follows ingestion of an allergen to which an individual has been topically sensitized, rather than the proposed localized immunologic response in the intestinal lining. We were unaware of patient history of allergic contact dermatitis to any of the patch test allergens in this study, but the dermatologist author here (M.S.) has unpublished experience with 2 other patients with IBS who have benefited from low-nickel diets after having had positive patch tests to nickel sulfate hexahydrate and who, in retrospect, did report a history of earring dermatitis. Future investigations using pre– and post–food challenge histologic assessments of the intestinal mucosa in patients who benefit from patch test–guided food avoidance diets should help to better define the mechanism.
Because IBS has not been traditionally associated with structural or biochemical abnormalities detectable with current routine diagnostic tools, it has long been viewed as a functional disorder. The findings published more recently,9,10 in addition to this study’s results, would negate this functional classification in the subset of patients with IBS symptoms who experience sustained relief of their symptoms by patch test–directed food avoidance. The underlying delayed-type hypersensitivity pathogenesis of the IBS-like symptoms in these individuals would mandate an organic classification, aptly named allergic contact enteritis.10
Follow-up Data
The mean (SD) follow-up duration for this study and the 2018 report9 was 4.5 (3.0) months and 7.6 (3.9) months, respectively. The placebo effect is a concern for disorders such as IBS in which primarily subjective outcome measures are available,21 and in a retrospective analysis of 25 randomized, placebo-controlled IBS clinical trials, Spiller22 concluded the optimum length of such trials to be more than 3 months, which these studies exceed. Although not blinded or placebo controlled, the length of follow-up in the 2018 report9 and here enhances the validity of the results.
Limitation
The retrospective manner in which the self-assessments were reported in this study introduces the potential for recall bias, a variable that could affect results. The presence and direction of bias by any given individual cannot be known, making it difficult to determine any effect it may have had. Further investigation should include daily assessments and refine the primary study end points to include both abdominal pain and the defecation considerations that define IBS.
Conclusion
Food patch testing has the potential to offer a safe, cost-effective approach to the evaluation and management of IBS symptoms. Randomized clinical trials are needed to further investigate the validity of the proof-of-concept results to date. For patients who benefit from a patch test–guided avoidance diet, invasive and costly endoscopic, radiologic, and laboratory testing and pharmacologic management could be averted. Symptomatic relief could be attained simply by avoiding the implicated foods, essentially doing more by doing less.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:1-24.
- Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99.
- Barbara G, De Giorgio R, Stanghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(suppl 2):1-9
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778-1783.
- Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979.
- O’Mahony L, McCarthy J, Kelly
P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. - Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defecation in the irritable bowel syndrome (IBS): patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415-421.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Shin GH, Smith MS, Toro B, et al. Utility of food patch testing in the evaluation and management of irritable bowel syndrome. Skin. 2018;2:1-15.
- Stierstorfer MB, Sha CT. Food patch testing for irritable bowel syndrome. J Am Acad Dermatol. 2013;68:377-384.
- Marks JG, Belsito DV, DeLeo MD, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol. 1998;38:911-918.
- Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. BC Decker; 2008.
- DeGroot AC. Patch Testing. acdegroot Publishing; 2008.
- Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660.
- Halder SL, Lock GR, Talley NJ, et al. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case–control study. Aliment Pharmacol Ther. 2004;19:233-242.
- International Foundation for Gastrointestinal Disorders. About IBS. statistics. Accessed July 20, 2021. https://www.aboutibs.org/facts-about-ibs/statistics.html
- Rome Foundation. Guidelines—Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:307-312.
- Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;192(suppl):102-105.
- Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? a systemic review. Neurogastroenterol Motil. 2006;18:595-607.
- Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease–irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48-53.
- Hrobiartsson A, Gotzsche PC. Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594-1602.
- Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S-97S.
- Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:1-24.
- Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6:99.
- Barbara G, De Giorgio R, Stanghellini V, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20(suppl 2):1-9
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778-1783.
- Tornblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123:1972-1979.
- O’Mahony L, McCarthy J, Kelly
P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. - Ragnarsson G, Bodemar G. Pain is temporally related to eating but not to defecation in the irritable bowel syndrome (IBS): patients’ description of diarrhea, constipation and symptom variation during a prospective 6-week study. Eur J Gastroenterol Hepatol. 1998;10:415-421.
- Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 suppl):S1-S58.
- Shin GH, Smith MS, Toro B, et al. Utility of food patch testing in the evaluation and management of irritable bowel syndrome. Skin. 2018;2:1-15.
- Stierstorfer MB, Sha CT. Food patch testing for irritable bowel syndrome. J Am Acad Dermatol. 2013;68:377-384.
- Marks JG, Belsito DV, DeLeo MD, et al. North American Contact Dermatitis Group patch test results for the detection of delayed-type hypersensitivity to topical allergens. J Am Acad Dermatol. 1998;38:911-918.
- Rietschel RL, Fowler JF Jr. Fisher’s Contact Dermatitis. BC Decker; 2008.
- DeGroot AC. Patch Testing. acdegroot Publishing; 2008.
- Gralnek IM, Hays RD, Kilbourne A, et al. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654-660.
- Halder SL, Lock GR, Talley NJ, et al. Impact of functional gastrointestinal disorders on health-related quality of life: a population-based case–control study. Aliment Pharmacol Ther. 2004;19:233-242.
- International Foundation for Gastrointestinal Disorders. About IBS. statistics. Accessed July 20, 2021. https://www.aboutibs.org/facts-about-ibs/statistics.html
- Rome Foundation. Guidelines—Rome III diagnostic criteria for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:307-312.
- Collins SM. Is the irritable gut an inflamed gut? Scand J Gastroenterol. 1992;192(suppl):102-105.
- Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? a systemic review. Neurogastroenterol Motil. 2006;18:595-607.
- Grover M, Herfarth H, Drossman DA. The functional-organic dichotomy: postinfectious irritable bowel syndrome and inflammatory bowel disease–irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:48-53.
- Hrobiartsson A, Gotzsche PC. Is the placebo powerless? an analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594-1602.
- Spiller RC. Problems and challenges in the design of irritable bowel syndrome clinical trials: experience from published trials. Am J Med. 1999;107:91S-97S.
Practice Points
- Recent observations of inflammation in irritable bowel syndrome (IBS) challenge its traditional classification as a functional disorder.
- Delayed-type food hypersensitivities, as detectable by skin patch testing, to type IV food allergens are one plausible cause for intestinal inflammation.
- Patch test–directed food avoidance improves IBS symptoms in some patients and offers a new approach to the evaluation and management of this condition.
- Dermatologists and other health care practitioners with expertise in patch testing are uniquely positioned to utilize these skills to help patients with IBS.
Isolated Scrotal Granular Parakeratosis: An Atypical Clinical Presentation
To the Editor:
Granular parakeratosis is a rare condition with an unclear etiology that results from a myriad of factors, including exposure to irritants, friction, moisture, and heat. The diagnosis is made based on a distinct histologic reaction pattern that may be protective against the triggers. We present a case of isolated scrotal granular parakeratosis in a patient with compensatory hyperhidrosis after endoscopic thoracic sympathectomy.
A 52-year-old man presented with a 5-year history of a recurrent rash affecting the scrotum. He experienced monthly flares that were exacerbated by inguinal hyperhidrosis. His symptoms included a burning sensation and pruritus followed by superficial desquamation, with gradual yet temporary improvement. His medical history was remarkable for primary axillary and palmoplantar hyperhidrosis, with compensatory inguinal hyperhidrosis after endoscopic thoracic sympathectomy 8 years prior to presentation.
Physical examination revealed a well-demarcated, scaly, erythematous plaque affecting the scrotal skin with sparing of the median raphe, penis, and inguinal folds (Figure 1). There were no other lesions noted in the axillary region or other skin folds.
Prior treatments prescribed by other providers included topical pimecrolimus, antifungal creams, topical corticosteroids, zinc oxide ointment, and daily application of an over-the-counter medicated powder with no resolution.
A punch biopsy performed at the current presentation showed psoriasiform hyperplasia of the epidermis with only a focally diminished granular layer. There was overlying thick parakeratosis and retention of keratohyalin granules (Figure 2). Grocott-Gomori methenamine- silver staining was negative for fungal elements in the sections examined. Clinical history, morphology of the eruption, and histologic features were consistent with granular parakeratosis.
Since the first reported incident of granular parakeratosis of the axilla in 1991,1 granular parakeratosis has been reported in other intertriginous areas, including the inframammary folds, inguinal folds, genitalia, perianal skin, and beneath the abdominal pannus.2 One case study in 1998 reported a patient with isolated involvement of the inguinal region3; however, this presentation is rare.4 This condition has been reported in both sexes and all age groups, including children.5
Granular parakeratosis classically presents as erythematous to brown hyperkeratotic papules that coalesce into plaques.6 It is thought to be a reactive inflammatory condition secondary to aggravating factors such as exposure to heat,7 moisture, and friction; skin occlusion; repeated washing; irritation from external agents; antiperspirants; and use of depilatory creams.8 Histopathology is characteristic and consists of retained nuclei and keratohyalin granules within the stratum corneum, beneath which there is a retained stratum granulosum. Epidermal changes may be varied and include atrophy or hyperplasia.
Murine models have postulated that granular parakeratosis may result from a deficiency in caspase 14, a protease vital to the formation of a well-functioning skin barrier.9 A cornified envelope often is noted in granular parakeratotic cells with no defects in desmosomes and cell membranes, suggesting that the pathogenesis lies within processing of profilaggrin to filaggrin, resulting in a failure to degrade keratohyalin granules and aggregation of keratin filaments.10 Granular parakeratosis is not known to be associated with other medical conditions, but it has been observed in patients receiving chemotherapy for breast11 and ovarian12 carcinomas. In infants with atopic dermatitis, granular parakeratosis was reported in 5 out of 7 cases.6 In our patient with secondary inguinal hyperhidrosis after thoracic sympathectomy, granular parakeratosis may be reactive to excess sweating and friction in the scrotal area.
Granular parakeratosis follows a waxing and waning pattern that may spontaneously resolve without any treatment; it also can follow a protracted course, as in a case with associated facial papules that persisted for 20 years.13 Topical corticosteroids alone or in combination with topical antifungal agents have been used for the treatment of granular parakeratosis with the goal of accelerating resolution.2,14 However, the efficacy of these therapeutic interventions is limited, and no controlled trials are underway. Topical vitamin D analogues15,16 and topical retinoids17 also have been reported with successful outcomes. Spontaneous resolution also has been observed in 2 different cases after previously being unresponsive to topical treatment.18,19 Treatment with Clostridium botulinum toxin A resulted in complete remission of the disease observed at 6-month follow-up. The pharmacologic action of the neurotoxin disrupts the stimulation of eccrine sweat glands, resulting in decreased sweating, a known exacerbating factor of granular parakeratosis.20
In summary, our case represents a unique clinical presentation of granular parakeratosis with classic histopathologic features. A high index of suspicion and a biopsy are vital to arriving at the correct diagnosis.
- Northcutt AD, Nelson DM, Tschen JA. Axillary granular parakeratosis. J Am Acad Dermatol. 1991;24:541-544.
- Burford C. Granular parakeratosis of multiple intertriginous areas. Australas J Dermatol. 2008;49:35-38.
- Mehregan DA, Thomas JE, Mehregan DR. Intertriginous granular parakeratosis. J Am Acad Dermatol. 1998;39:495-496.
- Leclerc-Mercier S, Prost-Squarcioni C, Hamel-Teillac D, et al. A case of congenital granular parakeratosis. Am J Dermatopathol. 2011;33:531-533.
- Scheinfeld NS, Mones J. Granular parakeratosis: pathologic and clinical correlation of 18 cases of granular parakeratosis. J Am Acad Dermatol. 2005;52:863-867.
- Akkaya AD, Oram Y, Aydin O. Infantile granular parakeratosis: cytologic examination of superficial scrapings as an aid to diagnosis. Pediatr Dermatol. 2015;32:392-396.
- Rodríguez G. Axillary granular parakeratosis [in Spanish]. Biomedica. 2002;22:519-523.
- Samrao A, Reis M, Niedt G, et al. Granular parakeratosis: response to calcipotriene and brief review of current therapeutic options. Skinmed. 2010;8:357-359.
- Hoste E, Denecker G, Gilbert B, et al. Caspase-14-deficient mice are more prone to the development of parakeratosis. J Invest Dermatol. 2013;133:742-750.
- Metze D, Rutten A. Granular parakeratosis—a unique acquired disorder of keratinization. J Cutan Pathol. 1999;26:339-352.
- Wallace CA, Pichardo RO, Yosipovitch G, et al. Granular parakeratosis: a case report and literature review. J Cutan Pathol. 2003;30:332-335.
- Jaconelli L, Doebelin B, Kanitakis J, et al. Granular parakeratosis in a patient treated with liposomal doxorubicin for ovarian carcinoma. J Am Acad Dermatol. 2008;58(5 suppl 1):S84-S87.
- Reddy IS, Swarnalata G, Mody T. Intertriginous granular parakeratosis persisting for 20 years. Indian J Dermatol Venereol Leprol. 2008;74:405-407.
- Dearden C, al-Nakib W, Andries K, et al. Drug resistant rhinoviruses from the nose of experimentally treated volunteers. Arch Virol. 1989;109:71-81.
- Patel U, Patel T, Skinner RB Jr. Resolution of granular parakeratosis with topical calcitriol. Arch Dermatol. 2011;147:997-998.
- Contreras ME, Gottfried LC, Bang RH, et al. Axillary intertriginous granular parakeratosis responsive to topical calcipotriene and ammonium lactate. Int J Dermatol. 2003;42:382-383.
- Brown SK, Heilman ER. Granular parakeratosis: resolution with topical tretinoin. J Am Acad Dermatol. 2002;47(5 suppl):S279-S280.
- Compton AK, Jackson JM. Isotretinoin as a treatment for axillary granular parakeratosis. Cutis. 2007;80:55-56.
- Webster CG, Resnik KS, Webster GF. Axillary granular parakeratosis: response to isotretinoin. J Am Acad Dermatol. 1997; 37:789-790.
- Ravitskiy L, Heymann WR. Botulinum toxin-induced resolution of axillary granular parakeratosis. Skinmed. 2005;4:118-120.
To the Editor:
Granular parakeratosis is a rare condition with an unclear etiology that results from a myriad of factors, including exposure to irritants, friction, moisture, and heat. The diagnosis is made based on a distinct histologic reaction pattern that may be protective against the triggers. We present a case of isolated scrotal granular parakeratosis in a patient with compensatory hyperhidrosis after endoscopic thoracic sympathectomy.
A 52-year-old man presented with a 5-year history of a recurrent rash affecting the scrotum. He experienced monthly flares that were exacerbated by inguinal hyperhidrosis. His symptoms included a burning sensation and pruritus followed by superficial desquamation, with gradual yet temporary improvement. His medical history was remarkable for primary axillary and palmoplantar hyperhidrosis, with compensatory inguinal hyperhidrosis after endoscopic thoracic sympathectomy 8 years prior to presentation.
Physical examination revealed a well-demarcated, scaly, erythematous plaque affecting the scrotal skin with sparing of the median raphe, penis, and inguinal folds (Figure 1). There were no other lesions noted in the axillary region or other skin folds.
Prior treatments prescribed by other providers included topical pimecrolimus, antifungal creams, topical corticosteroids, zinc oxide ointment, and daily application of an over-the-counter medicated powder with no resolution.
A punch biopsy performed at the current presentation showed psoriasiform hyperplasia of the epidermis with only a focally diminished granular layer. There was overlying thick parakeratosis and retention of keratohyalin granules (Figure 2). Grocott-Gomori methenamine- silver staining was negative for fungal elements in the sections examined. Clinical history, morphology of the eruption, and histologic features were consistent with granular parakeratosis.
Since the first reported incident of granular parakeratosis of the axilla in 1991,1 granular parakeratosis has been reported in other intertriginous areas, including the inframammary folds, inguinal folds, genitalia, perianal skin, and beneath the abdominal pannus.2 One case study in 1998 reported a patient with isolated involvement of the inguinal region3; however, this presentation is rare.4 This condition has been reported in both sexes and all age groups, including children.5
Granular parakeratosis classically presents as erythematous to brown hyperkeratotic papules that coalesce into plaques.6 It is thought to be a reactive inflammatory condition secondary to aggravating factors such as exposure to heat,7 moisture, and friction; skin occlusion; repeated washing; irritation from external agents; antiperspirants; and use of depilatory creams.8 Histopathology is characteristic and consists of retained nuclei and keratohyalin granules within the stratum corneum, beneath which there is a retained stratum granulosum. Epidermal changes may be varied and include atrophy or hyperplasia.
Murine models have postulated that granular parakeratosis may result from a deficiency in caspase 14, a protease vital to the formation of a well-functioning skin barrier.9 A cornified envelope often is noted in granular parakeratotic cells with no defects in desmosomes and cell membranes, suggesting that the pathogenesis lies within processing of profilaggrin to filaggrin, resulting in a failure to degrade keratohyalin granules and aggregation of keratin filaments.10 Granular parakeratosis is not known to be associated with other medical conditions, but it has been observed in patients receiving chemotherapy for breast11 and ovarian12 carcinomas. In infants with atopic dermatitis, granular parakeratosis was reported in 5 out of 7 cases.6 In our patient with secondary inguinal hyperhidrosis after thoracic sympathectomy, granular parakeratosis may be reactive to excess sweating and friction in the scrotal area.
Granular parakeratosis follows a waxing and waning pattern that may spontaneously resolve without any treatment; it also can follow a protracted course, as in a case with associated facial papules that persisted for 20 years.13 Topical corticosteroids alone or in combination with topical antifungal agents have been used for the treatment of granular parakeratosis with the goal of accelerating resolution.2,14 However, the efficacy of these therapeutic interventions is limited, and no controlled trials are underway. Topical vitamin D analogues15,16 and topical retinoids17 also have been reported with successful outcomes. Spontaneous resolution also has been observed in 2 different cases after previously being unresponsive to topical treatment.18,19 Treatment with Clostridium botulinum toxin A resulted in complete remission of the disease observed at 6-month follow-up. The pharmacologic action of the neurotoxin disrupts the stimulation of eccrine sweat glands, resulting in decreased sweating, a known exacerbating factor of granular parakeratosis.20
In summary, our case represents a unique clinical presentation of granular parakeratosis with classic histopathologic features. A high index of suspicion and a biopsy are vital to arriving at the correct diagnosis.
To the Editor:
Granular parakeratosis is a rare condition with an unclear etiology that results from a myriad of factors, including exposure to irritants, friction, moisture, and heat. The diagnosis is made based on a distinct histologic reaction pattern that may be protective against the triggers. We present a case of isolated scrotal granular parakeratosis in a patient with compensatory hyperhidrosis after endoscopic thoracic sympathectomy.
A 52-year-old man presented with a 5-year history of a recurrent rash affecting the scrotum. He experienced monthly flares that were exacerbated by inguinal hyperhidrosis. His symptoms included a burning sensation and pruritus followed by superficial desquamation, with gradual yet temporary improvement. His medical history was remarkable for primary axillary and palmoplantar hyperhidrosis, with compensatory inguinal hyperhidrosis after endoscopic thoracic sympathectomy 8 years prior to presentation.
Physical examination revealed a well-demarcated, scaly, erythematous plaque affecting the scrotal skin with sparing of the median raphe, penis, and inguinal folds (Figure 1). There were no other lesions noted in the axillary region or other skin folds.
Prior treatments prescribed by other providers included topical pimecrolimus, antifungal creams, topical corticosteroids, zinc oxide ointment, and daily application of an over-the-counter medicated powder with no resolution.
A punch biopsy performed at the current presentation showed psoriasiform hyperplasia of the epidermis with only a focally diminished granular layer. There was overlying thick parakeratosis and retention of keratohyalin granules (Figure 2). Grocott-Gomori methenamine- silver staining was negative for fungal elements in the sections examined. Clinical history, morphology of the eruption, and histologic features were consistent with granular parakeratosis.
Since the first reported incident of granular parakeratosis of the axilla in 1991,1 granular parakeratosis has been reported in other intertriginous areas, including the inframammary folds, inguinal folds, genitalia, perianal skin, and beneath the abdominal pannus.2 One case study in 1998 reported a patient with isolated involvement of the inguinal region3; however, this presentation is rare.4 This condition has been reported in both sexes and all age groups, including children.5
Granular parakeratosis classically presents as erythematous to brown hyperkeratotic papules that coalesce into plaques.6 It is thought to be a reactive inflammatory condition secondary to aggravating factors such as exposure to heat,7 moisture, and friction; skin occlusion; repeated washing; irritation from external agents; antiperspirants; and use of depilatory creams.8 Histopathology is characteristic and consists of retained nuclei and keratohyalin granules within the stratum corneum, beneath which there is a retained stratum granulosum. Epidermal changes may be varied and include atrophy or hyperplasia.
Murine models have postulated that granular parakeratosis may result from a deficiency in caspase 14, a protease vital to the formation of a well-functioning skin barrier.9 A cornified envelope often is noted in granular parakeratotic cells with no defects in desmosomes and cell membranes, suggesting that the pathogenesis lies within processing of profilaggrin to filaggrin, resulting in a failure to degrade keratohyalin granules and aggregation of keratin filaments.10 Granular parakeratosis is not known to be associated with other medical conditions, but it has been observed in patients receiving chemotherapy for breast11 and ovarian12 carcinomas. In infants with atopic dermatitis, granular parakeratosis was reported in 5 out of 7 cases.6 In our patient with secondary inguinal hyperhidrosis after thoracic sympathectomy, granular parakeratosis may be reactive to excess sweating and friction in the scrotal area.
Granular parakeratosis follows a waxing and waning pattern that may spontaneously resolve without any treatment; it also can follow a protracted course, as in a case with associated facial papules that persisted for 20 years.13 Topical corticosteroids alone or in combination with topical antifungal agents have been used for the treatment of granular parakeratosis with the goal of accelerating resolution.2,14 However, the efficacy of these therapeutic interventions is limited, and no controlled trials are underway. Topical vitamin D analogues15,16 and topical retinoids17 also have been reported with successful outcomes. Spontaneous resolution also has been observed in 2 different cases after previously being unresponsive to topical treatment.18,19 Treatment with Clostridium botulinum toxin A resulted in complete remission of the disease observed at 6-month follow-up. The pharmacologic action of the neurotoxin disrupts the stimulation of eccrine sweat glands, resulting in decreased sweating, a known exacerbating factor of granular parakeratosis.20
In summary, our case represents a unique clinical presentation of granular parakeratosis with classic histopathologic features. A high index of suspicion and a biopsy are vital to arriving at the correct diagnosis.
- Northcutt AD, Nelson DM, Tschen JA. Axillary granular parakeratosis. J Am Acad Dermatol. 1991;24:541-544.
- Burford C. Granular parakeratosis of multiple intertriginous areas. Australas J Dermatol. 2008;49:35-38.
- Mehregan DA, Thomas JE, Mehregan DR. Intertriginous granular parakeratosis. J Am Acad Dermatol. 1998;39:495-496.
- Leclerc-Mercier S, Prost-Squarcioni C, Hamel-Teillac D, et al. A case of congenital granular parakeratosis. Am J Dermatopathol. 2011;33:531-533.
- Scheinfeld NS, Mones J. Granular parakeratosis: pathologic and clinical correlation of 18 cases of granular parakeratosis. J Am Acad Dermatol. 2005;52:863-867.
- Akkaya AD, Oram Y, Aydin O. Infantile granular parakeratosis: cytologic examination of superficial scrapings as an aid to diagnosis. Pediatr Dermatol. 2015;32:392-396.
- Rodríguez G. Axillary granular parakeratosis [in Spanish]. Biomedica. 2002;22:519-523.
- Samrao A, Reis M, Niedt G, et al. Granular parakeratosis: response to calcipotriene and brief review of current therapeutic options. Skinmed. 2010;8:357-359.
- Hoste E, Denecker G, Gilbert B, et al. Caspase-14-deficient mice are more prone to the development of parakeratosis. J Invest Dermatol. 2013;133:742-750.
- Metze D, Rutten A. Granular parakeratosis—a unique acquired disorder of keratinization. J Cutan Pathol. 1999;26:339-352.
- Wallace CA, Pichardo RO, Yosipovitch G, et al. Granular parakeratosis: a case report and literature review. J Cutan Pathol. 2003;30:332-335.
- Jaconelli L, Doebelin B, Kanitakis J, et al. Granular parakeratosis in a patient treated with liposomal doxorubicin for ovarian carcinoma. J Am Acad Dermatol. 2008;58(5 suppl 1):S84-S87.
- Reddy IS, Swarnalata G, Mody T. Intertriginous granular parakeratosis persisting for 20 years. Indian J Dermatol Venereol Leprol. 2008;74:405-407.
- Dearden C, al-Nakib W, Andries K, et al. Drug resistant rhinoviruses from the nose of experimentally treated volunteers. Arch Virol. 1989;109:71-81.
- Patel U, Patel T, Skinner RB Jr. Resolution of granular parakeratosis with topical calcitriol. Arch Dermatol. 2011;147:997-998.
- Contreras ME, Gottfried LC, Bang RH, et al. Axillary intertriginous granular parakeratosis responsive to topical calcipotriene and ammonium lactate. Int J Dermatol. 2003;42:382-383.
- Brown SK, Heilman ER. Granular parakeratosis: resolution with topical tretinoin. J Am Acad Dermatol. 2002;47(5 suppl):S279-S280.
- Compton AK, Jackson JM. Isotretinoin as a treatment for axillary granular parakeratosis. Cutis. 2007;80:55-56.
- Webster CG, Resnik KS, Webster GF. Axillary granular parakeratosis: response to isotretinoin. J Am Acad Dermatol. 1997; 37:789-790.
- Ravitskiy L, Heymann WR. Botulinum toxin-induced resolution of axillary granular parakeratosis. Skinmed. 2005;4:118-120.
- Northcutt AD, Nelson DM, Tschen JA. Axillary granular parakeratosis. J Am Acad Dermatol. 1991;24:541-544.
- Burford C. Granular parakeratosis of multiple intertriginous areas. Australas J Dermatol. 2008;49:35-38.
- Mehregan DA, Thomas JE, Mehregan DR. Intertriginous granular parakeratosis. J Am Acad Dermatol. 1998;39:495-496.
- Leclerc-Mercier S, Prost-Squarcioni C, Hamel-Teillac D, et al. A case of congenital granular parakeratosis. Am J Dermatopathol. 2011;33:531-533.
- Scheinfeld NS, Mones J. Granular parakeratosis: pathologic and clinical correlation of 18 cases of granular parakeratosis. J Am Acad Dermatol. 2005;52:863-867.
- Akkaya AD, Oram Y, Aydin O. Infantile granular parakeratosis: cytologic examination of superficial scrapings as an aid to diagnosis. Pediatr Dermatol. 2015;32:392-396.
- Rodríguez G. Axillary granular parakeratosis [in Spanish]. Biomedica. 2002;22:519-523.
- Samrao A, Reis M, Niedt G, et al. Granular parakeratosis: response to calcipotriene and brief review of current therapeutic options. Skinmed. 2010;8:357-359.
- Hoste E, Denecker G, Gilbert B, et al. Caspase-14-deficient mice are more prone to the development of parakeratosis. J Invest Dermatol. 2013;133:742-750.
- Metze D, Rutten A. Granular parakeratosis—a unique acquired disorder of keratinization. J Cutan Pathol. 1999;26:339-352.
- Wallace CA, Pichardo RO, Yosipovitch G, et al. Granular parakeratosis: a case report and literature review. J Cutan Pathol. 2003;30:332-335.
- Jaconelli L, Doebelin B, Kanitakis J, et al. Granular parakeratosis in a patient treated with liposomal doxorubicin for ovarian carcinoma. J Am Acad Dermatol. 2008;58(5 suppl 1):S84-S87.
- Reddy IS, Swarnalata G, Mody T. Intertriginous granular parakeratosis persisting for 20 years. Indian J Dermatol Venereol Leprol. 2008;74:405-407.
- Dearden C, al-Nakib W, Andries K, et al. Drug resistant rhinoviruses from the nose of experimentally treated volunteers. Arch Virol. 1989;109:71-81.
- Patel U, Patel T, Skinner RB Jr. Resolution of granular parakeratosis with topical calcitriol. Arch Dermatol. 2011;147:997-998.
- Contreras ME, Gottfried LC, Bang RH, et al. Axillary intertriginous granular parakeratosis responsive to topical calcipotriene and ammonium lactate. Int J Dermatol. 2003;42:382-383.
- Brown SK, Heilman ER. Granular parakeratosis: resolution with topical tretinoin. J Am Acad Dermatol. 2002;47(5 suppl):S279-S280.
- Compton AK, Jackson JM. Isotretinoin as a treatment for axillary granular parakeratosis. Cutis. 2007;80:55-56.
- Webster CG, Resnik KS, Webster GF. Axillary granular parakeratosis: response to isotretinoin. J Am Acad Dermatol. 1997; 37:789-790.
- Ravitskiy L, Heymann WR. Botulinum toxin-induced resolution of axillary granular parakeratosis. Skinmed. 2005;4:118-120.
Practice Points
- Granular parakeratosis can occur in response to triggers such as irritants, friction, hyperhidrosis, and heat.
- Granular parakeratosis can have an atypical presentation; therefore, a high index of suspicion and punch biopsy are vital to arrive at the correct diagnosis.
- Classic histopathology demonstrates retained nuclei and keratohyalin granules within the stratum corneum beneath which there is a retained stratum granulosum.
Fulminant Hemorrhagic Bullae of the Upper Extremities Arising in the Setting of IV Placement During Severe COVID-19 Infection: Observations From a Major Consultative Practice
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.
Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.
Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.
Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
Practice Points
- Hemorrhagic bullae are an uncommon cutaneous manifestation of COVID-19 infection in hospitalized individuals.
- Although there is no reported treatment for COVID-19–associated hemorrhagic bullae, we recommend supportive care and management of underlying etiology.
Pink, Scaly, Annular Plaques in Concentric Rings Localized to Vitiliginous Patches
The Diagnosis: Tinea Pseudoimbricata
Tinea pseudoimbricata and tinea indecisiva are synonyms describing cases of tinea corporis that manifest in scaly plaques in concentric rings evocative of those present in tinea imbricata. However, in contrast to tinea imbricata, cases of tinea pseudoimbricata are caused by dermatophytes other than Trichophyton concentricum. 1 Tinea pseudoimbricata usually presents in association with immunosuppression, either systemic or local, and can be produced by application of topical medications such as corticosteroids.2 Mask-Bull et al3 reported the case of a 21-year-old man in the United States with no history of immunosuppressive conditions who presented with scaly erythematous annular plaques on the lateral neck that resolved with 2 pulsed doses of terbinafine. Potassium hydroxide preparation and fungal culture were both consistent with Trichophyton tonsurans.3
Trichophyton concentricum is an anthropophilic species of dermatophyte endemic to areas within the South Pacific, Southeast Asia, and Central and South America. Infection with T concentricum produces tinea imbricata, which presents with concentric, scaly, annular rings. Cutaneous lesions of tinea imbricata caused by T concentricum have a more generalized distribution and more densely grouped, concentric circles than the cutaneous findings seen in patients with tinea pseudoimbricata.4 Affected patients typically demonstrate negative delayed-type hypersensitivity to T concentricum cytoplasmic antigen and T-lymphocyte hyporeactivity, which may contribute to the development of sequential waves of scaling observed in tinea imbricata.5
Trichophyton rubrum, the most common cause of tinea corporis, has been reported to cause some cases of tinea pseudoimbricata (indecisiva).1,2 It utilizes keratinases such as subtilisins (Sub3 and Sub4), leucine aminopeptidases (Lap1 and Lap2), and dipeptidyl peptidases (DppIV and DppV) to invade the skin. Once inside, mannans, glycoprotein constituents of the cell wall, are released and bind to the cell surface of mononuclear phagocytes, subsequently moving into the cell by phagocytosis, thereafter interfering with RNA synthesis that is necessary for presentation of antigens to appropriate T cells and allowing for initiation of chronic infection.6,7 The cytotoxic response to superficial dermatophyte infection is triggered by major histocompatibility complex class I molecule activation of CD8+ cells.6,8
Our case is of interest given the localization of the superficial dermatophyte infection to only vitiliginous skin. This distribution and appearance while undergoing narrowband UVB (NB-UVB) treatment is rare. We postulate that our patient likely represents a case of locus minoris resistentiae, a phenomenon in which an area of skin exhibits a compromised immune microenvironment that predisposes it to disease.9
In vitiligo, NB-UVB modulates the immune response by increasing IL-10, thereby promoting regulatory T-cell differentiation with suppression of autoreactive T cells and induction of direct T-lymphocyte apoptosis.10,11 Although the mechanism accounting for our patient’s presentation is unknown, we suspect NB-UVB–induced immunosuppression enabled persistence of the dermatophyte infection. The localization of the infection to the vitiliginous patches may result from the greater penetration of the UV light relative to the surrounding, normally pigmented skin. This relative difference in UV penetration would be expected to result in increased immunosuppression in the vitiliginous lesions and enhanced susceptibility to the fungal organisms.
Erythema annulare centrifugum is characterized by annular lesions with a trailing scale instead of the concentric rings seen in tinea pseudoimbricata. Erythema marginatum is seen in acute rheumatic fever and presents with a transient nonpruritic rash, usually on the trunk or extremities. Erythema migrans presents with fewer lesions that are less circinate in shape, and the patient often has a history of a tick bite. Tinea imbricata is caused by T concentricum, while tinea pseudoimbricata is caused by T tonsurans and other dermatophytes.
With the increasing use of immunosuppressant drugs, the prevalence of tinea pseudoimbricata is hypothesized to increase.1 The presence of tinea pseudoimbricata should alert dermatologists to the possible overuse of topical corticosteroids, and other forms of immunosuppression also should be considered.
- Lim SP, Smith AG. “Tinea pseudoimbricata”: tinea corporis in a renal transplant recipient mimicking the concentric rings of tinea imbricata. Clin Exp Dermatol. 2003;28:332-333.
- Batta K, Ramlogan D, Smith AG, et al. ‘Tinea indecisiva’ may mimic the concentric rings of tinea imbricata. Br J Dermatol. 2002;147:384.
- Mask-Bull L, Patel R, Tarbox MB. America’s first case of tinea pseudoimbricata. Am J Dermatol Venereol. 2015;4:15-17.
- Meena M, Mittal A. Tinea pseudo-imbricata. J Assoc Physicians India. 2018;66:79.
- Hay RJ, Reid S, Talwat E, et al. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581-586.
- Dahl MV. Suppression of immunity and inflammation by products produced by dermatophytes. J Am Acad Dermatol. 1993;28(5 pt 1):S19-S23.
- Blutfield MS, Lohre JM, Pawich DA, et al. The immunologic response to Trichophyton rubrum in lower extremity fungal infections. J Fungi (Basel). 2015;1:130-137.
- De Hoog S, Monod M, Dawson T, et al. Skin fungi from colonization to infection [published online July 2017]. Microbiol Spectr. doi:10.1128/ microbiolspec.FUNK-0049-2016
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267-1275.
- Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, et al. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28:1610-1622.
The Diagnosis: Tinea Pseudoimbricata
Tinea pseudoimbricata and tinea indecisiva are synonyms describing cases of tinea corporis that manifest in scaly plaques in concentric rings evocative of those present in tinea imbricata. However, in contrast to tinea imbricata, cases of tinea pseudoimbricata are caused by dermatophytes other than Trichophyton concentricum. 1 Tinea pseudoimbricata usually presents in association with immunosuppression, either systemic or local, and can be produced by application of topical medications such as corticosteroids.2 Mask-Bull et al3 reported the case of a 21-year-old man in the United States with no history of immunosuppressive conditions who presented with scaly erythematous annular plaques on the lateral neck that resolved with 2 pulsed doses of terbinafine. Potassium hydroxide preparation and fungal culture were both consistent with Trichophyton tonsurans.3
Trichophyton concentricum is an anthropophilic species of dermatophyte endemic to areas within the South Pacific, Southeast Asia, and Central and South America. Infection with T concentricum produces tinea imbricata, which presents with concentric, scaly, annular rings. Cutaneous lesions of tinea imbricata caused by T concentricum have a more generalized distribution and more densely grouped, concentric circles than the cutaneous findings seen in patients with tinea pseudoimbricata.4 Affected patients typically demonstrate negative delayed-type hypersensitivity to T concentricum cytoplasmic antigen and T-lymphocyte hyporeactivity, which may contribute to the development of sequential waves of scaling observed in tinea imbricata.5
Trichophyton rubrum, the most common cause of tinea corporis, has been reported to cause some cases of tinea pseudoimbricata (indecisiva).1,2 It utilizes keratinases such as subtilisins (Sub3 and Sub4), leucine aminopeptidases (Lap1 and Lap2), and dipeptidyl peptidases (DppIV and DppV) to invade the skin. Once inside, mannans, glycoprotein constituents of the cell wall, are released and bind to the cell surface of mononuclear phagocytes, subsequently moving into the cell by phagocytosis, thereafter interfering with RNA synthesis that is necessary for presentation of antigens to appropriate T cells and allowing for initiation of chronic infection.6,7 The cytotoxic response to superficial dermatophyte infection is triggered by major histocompatibility complex class I molecule activation of CD8+ cells.6,8
Our case is of interest given the localization of the superficial dermatophyte infection to only vitiliginous skin. This distribution and appearance while undergoing narrowband UVB (NB-UVB) treatment is rare. We postulate that our patient likely represents a case of locus minoris resistentiae, a phenomenon in which an area of skin exhibits a compromised immune microenvironment that predisposes it to disease.9
In vitiligo, NB-UVB modulates the immune response by increasing IL-10, thereby promoting regulatory T-cell differentiation with suppression of autoreactive T cells and induction of direct T-lymphocyte apoptosis.10,11 Although the mechanism accounting for our patient’s presentation is unknown, we suspect NB-UVB–induced immunosuppression enabled persistence of the dermatophyte infection. The localization of the infection to the vitiliginous patches may result from the greater penetration of the UV light relative to the surrounding, normally pigmented skin. This relative difference in UV penetration would be expected to result in increased immunosuppression in the vitiliginous lesions and enhanced susceptibility to the fungal organisms.
Erythema annulare centrifugum is characterized by annular lesions with a trailing scale instead of the concentric rings seen in tinea pseudoimbricata. Erythema marginatum is seen in acute rheumatic fever and presents with a transient nonpruritic rash, usually on the trunk or extremities. Erythema migrans presents with fewer lesions that are less circinate in shape, and the patient often has a history of a tick bite. Tinea imbricata is caused by T concentricum, while tinea pseudoimbricata is caused by T tonsurans and other dermatophytes.
With the increasing use of immunosuppressant drugs, the prevalence of tinea pseudoimbricata is hypothesized to increase.1 The presence of tinea pseudoimbricata should alert dermatologists to the possible overuse of topical corticosteroids, and other forms of immunosuppression also should be considered.
The Diagnosis: Tinea Pseudoimbricata
Tinea pseudoimbricata and tinea indecisiva are synonyms describing cases of tinea corporis that manifest in scaly plaques in concentric rings evocative of those present in tinea imbricata. However, in contrast to tinea imbricata, cases of tinea pseudoimbricata are caused by dermatophytes other than Trichophyton concentricum. 1 Tinea pseudoimbricata usually presents in association with immunosuppression, either systemic or local, and can be produced by application of topical medications such as corticosteroids.2 Mask-Bull et al3 reported the case of a 21-year-old man in the United States with no history of immunosuppressive conditions who presented with scaly erythematous annular plaques on the lateral neck that resolved with 2 pulsed doses of terbinafine. Potassium hydroxide preparation and fungal culture were both consistent with Trichophyton tonsurans.3
Trichophyton concentricum is an anthropophilic species of dermatophyte endemic to areas within the South Pacific, Southeast Asia, and Central and South America. Infection with T concentricum produces tinea imbricata, which presents with concentric, scaly, annular rings. Cutaneous lesions of tinea imbricata caused by T concentricum have a more generalized distribution and more densely grouped, concentric circles than the cutaneous findings seen in patients with tinea pseudoimbricata.4 Affected patients typically demonstrate negative delayed-type hypersensitivity to T concentricum cytoplasmic antigen and T-lymphocyte hyporeactivity, which may contribute to the development of sequential waves of scaling observed in tinea imbricata.5
Trichophyton rubrum, the most common cause of tinea corporis, has been reported to cause some cases of tinea pseudoimbricata (indecisiva).1,2 It utilizes keratinases such as subtilisins (Sub3 and Sub4), leucine aminopeptidases (Lap1 and Lap2), and dipeptidyl peptidases (DppIV and DppV) to invade the skin. Once inside, mannans, glycoprotein constituents of the cell wall, are released and bind to the cell surface of mononuclear phagocytes, subsequently moving into the cell by phagocytosis, thereafter interfering with RNA synthesis that is necessary for presentation of antigens to appropriate T cells and allowing for initiation of chronic infection.6,7 The cytotoxic response to superficial dermatophyte infection is triggered by major histocompatibility complex class I molecule activation of CD8+ cells.6,8
Our case is of interest given the localization of the superficial dermatophyte infection to only vitiliginous skin. This distribution and appearance while undergoing narrowband UVB (NB-UVB) treatment is rare. We postulate that our patient likely represents a case of locus minoris resistentiae, a phenomenon in which an area of skin exhibits a compromised immune microenvironment that predisposes it to disease.9
In vitiligo, NB-UVB modulates the immune response by increasing IL-10, thereby promoting regulatory T-cell differentiation with suppression of autoreactive T cells and induction of direct T-lymphocyte apoptosis.10,11 Although the mechanism accounting for our patient’s presentation is unknown, we suspect NB-UVB–induced immunosuppression enabled persistence of the dermatophyte infection. The localization of the infection to the vitiliginous patches may result from the greater penetration of the UV light relative to the surrounding, normally pigmented skin. This relative difference in UV penetration would be expected to result in increased immunosuppression in the vitiliginous lesions and enhanced susceptibility to the fungal organisms.
Erythema annulare centrifugum is characterized by annular lesions with a trailing scale instead of the concentric rings seen in tinea pseudoimbricata. Erythema marginatum is seen in acute rheumatic fever and presents with a transient nonpruritic rash, usually on the trunk or extremities. Erythema migrans presents with fewer lesions that are less circinate in shape, and the patient often has a history of a tick bite. Tinea imbricata is caused by T concentricum, while tinea pseudoimbricata is caused by T tonsurans and other dermatophytes.
With the increasing use of immunosuppressant drugs, the prevalence of tinea pseudoimbricata is hypothesized to increase.1 The presence of tinea pseudoimbricata should alert dermatologists to the possible overuse of topical corticosteroids, and other forms of immunosuppression also should be considered.
- Lim SP, Smith AG. “Tinea pseudoimbricata”: tinea corporis in a renal transplant recipient mimicking the concentric rings of tinea imbricata. Clin Exp Dermatol. 2003;28:332-333.
- Batta K, Ramlogan D, Smith AG, et al. ‘Tinea indecisiva’ may mimic the concentric rings of tinea imbricata. Br J Dermatol. 2002;147:384.
- Mask-Bull L, Patel R, Tarbox MB. America’s first case of tinea pseudoimbricata. Am J Dermatol Venereol. 2015;4:15-17.
- Meena M, Mittal A. Tinea pseudo-imbricata. J Assoc Physicians India. 2018;66:79.
- Hay RJ, Reid S, Talwat E, et al. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581-586.
- Dahl MV. Suppression of immunity and inflammation by products produced by dermatophytes. J Am Acad Dermatol. 1993;28(5 pt 1):S19-S23.
- Blutfield MS, Lohre JM, Pawich DA, et al. The immunologic response to Trichophyton rubrum in lower extremity fungal infections. J Fungi (Basel). 2015;1:130-137.
- De Hoog S, Monod M, Dawson T, et al. Skin fungi from colonization to infection [published online July 2017]. Microbiol Spectr. doi:10.1128/ microbiolspec.FUNK-0049-2016
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267-1275.
- Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, et al. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28:1610-1622.
- Lim SP, Smith AG. “Tinea pseudoimbricata”: tinea corporis in a renal transplant recipient mimicking the concentric rings of tinea imbricata. Clin Exp Dermatol. 2003;28:332-333.
- Batta K, Ramlogan D, Smith AG, et al. ‘Tinea indecisiva’ may mimic the concentric rings of tinea imbricata. Br J Dermatol. 2002;147:384.
- Mask-Bull L, Patel R, Tarbox MB. America’s first case of tinea pseudoimbricata. Am J Dermatol Venereol. 2015;4:15-17.
- Meena M, Mittal A. Tinea pseudo-imbricata. J Assoc Physicians India. 2018;66:79.
- Hay RJ, Reid S, Talwat E, et al. Immune responses of patients with tinea imbricata. Br J Dermatol. 1983;108:581-586.
- Dahl MV. Suppression of immunity and inflammation by products produced by dermatophytes. J Am Acad Dermatol. 1993;28(5 pt 1):S19-S23.
- Blutfield MS, Lohre JM, Pawich DA, et al. The immunologic response to Trichophyton rubrum in lower extremity fungal infections. J Fungi (Basel). 2015;1:130-137.
- De Hoog S, Monod M, Dawson T, et al. Skin fungi from colonization to infection [published online July 2017]. Microbiol Spectr. doi:10.1128/ microbiolspec.FUNK-0049-2016
- Lo Schiavo A, Ruocco E, Russo T, et al. Locus minoris resistentiae: an old but still valid way of thinking in medicine. Clin Dermatol. 2014;32:553-556.
- Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases—multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267-1275.
- Yazdani Abyaneh M, Griffith RD, Falto-Aizpurua L, et al. Narrowband ultraviolet B phototherapy in combination with other therapies for vitiligo: mechanisms and efficacies. J Eur Acad Dermatol Venereol. 2014;28:1610-1622.
A 64-year-old man presented with generalized vitiligo. In addition to extensive depigmented macules, physical examination revealed the presence of onychomycosis and tinea corporis confirmed by microscopic examination of potassium hydroxide–treated superficial skin scrapings. Vitiligo treatment was postponed, and a 3-month course of oral terbinafine and naftifine cream was undertaken for the dermatophyte infections. Subsequent examination revealed that the patient’s tinea corporis had improved, though there were localized areas of persistence. Given the patient’s eagerness to treat his vitiligo, narrowband UVB phototherapy was started along with tolnaftate cream 1% for treatment of the residual tinea corporis. After 2 months of narrowband UVB, partial repigmentation of the vitiligo was observed; however, he had developed extensive pink, scaly, annular plaques in concentric rings within residual vitiliginous patches on the lower extremities (top). Repeat examination of potassium hydroxide–treated skin scrapings revealed numerous hyphae (bottom). A fungal culture identified Trichophyton rubrum.
Persistent Panniculitis in Dermatomyositis
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.
The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.
The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.
The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
Practice Points
- Clinical panniculitis is a rare subcutaneous manifestation of dermatomyositis (DM) that dermatologists must consider when evaluating patients with this condition.
- Panniculitis can precede, occur simultaneously with, or develop up to 5 years after onset of DM.
- Many patients suffer from treatment-resistant panniculitis in DM, suggesting that therapeutic management of this condition may require long-term and more aggressive treatment modalities.
Formaldehyde-Induced Contact Dermatitis From an N95 Respirator Mask
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19
Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19
Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19
Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
Practice Points
- Prolonged wearing of N95 respirator masks has been associated with causing or complicating a number of facial inflammatory dermatoses.
- Consider the possibility of contact dermatitis secondary to formaldehyde exposure in individuals wearing N95 masks for prolonged periods.
- Information on the chemical components of N95 masks would be useful for clinicians tasked with evaluating patients with facial inflammatory dermatoses.
Phototherapy: Safe and Effective for Challenging Skin Conditions in Older Adults
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated
Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).
Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.
Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.
Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.
In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated
Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).
Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.
Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.
Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.
In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated
Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).
Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.
Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.
Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.
In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
Practice Points
- With appropriate nursing care, phototherapy can be safe and effective for a variety of conditions in elderly patients.
- Compared to younger patients, elderly patients may need more sessions to achieve comparable clearance rates.
- The increased prevalence of photosensitizing medications in the elderly population will require careful adjustments in dosing.
Exophytic Tumor on the Buttock
The Diagnosis: Hidradenocarcinoma
An excisional biopsy revealed a neoplasm in the dermis with focal invasion into the adjacent soft tissue (Figure 1). The tumor consisted of sheets of cells with cytoplasmic vacuoles and ductal differentiation (Figure 2), as well as cells with mild atypia, mild pleomorphism, rare mitotic figures, and abundant pale cytoplasm. Immunohistochemical staining was positive for cytokeratin (CK) 5, CK7, CK20, CK AE1/AE3, and p63 (Figure 3). The culmination of features including the large tumor size, immunohistochemical staining pattern, and mild pleomorphism with focal invasion into the soft tissue supported the diagnosis of hidradenocarcinoma.
Hidradenocarcinoma is an exceedingly rare malignant tumor of eccrine and/or apocrine origin.1 It accounts for less than 0.001% of all tumors and 1 of 13,000 skin biopsies.2 It usually arises in the head and neck region and most commonly affects older adults aged 50 to 70 years.3 The size of hidradenocarcinomas can vary; however, they typically are large, often growing to be greater than 5 cm in diameter.2 It tends to be an aggressive tumor that generally spreads to regional lymph nodes and distant viscera.4 Although it most commonly arises de novo, it may occasionally derive from a benign hidradenoma.1 The diagnosis of hidradenocarcinoma is made based on the tumor’s morphologic and pathologic characteristics. Histologically, it is characterized by an infiltrative and invasive proliferation of lobules made of large clear cells with atypical mitotic figures and nuclear pleomorphism as well as immunohistochemical features displaying various positive markers, such as carcinoembryonic antigen, epithelial membrane antigen, S-100 protein, and CKs AE1/AE3 and 5/6.2 Invasion of the adjacent soft tissue can be present and helps to confirm the diagnosis.
The differential diagnosis for hidradenocarcinoma primarily is the benign hidradenoma, which is similar both clinically and histologically with a few important differences. Hidradenocarcinomas often are larger and ulcerated. Histologically, they usually are more pleomorphic with the presence of mitotic figures in clear cells and tend to invade locally into the surrounding soft tissue. Other similar lesions such as spiradenoma, Merkel cell carcinoma, lymphangioma, cutaneous Crohn disease, tumors metastatic to the skin, and metastatic clear cell carcinomas originating from other organs also are included in the differential diagnosis.2
Spiradenomas are dermal tumors originating from the sweat glands. They typically present as bluish, painful, solitary nodules on the ventral surfaces of the upper body, though multiple nodules also are reported.5 Spiradenomas manifest as a central constellation of pale large cells surrounded by small, dark, basaloid cells containing hyperchromatic nuclei. The microscopic appearance of the blue basaloid cells contrasts with the clear cells seen in hidradenoma.5
Merkel cell carcinoma is a cutaneous neuroendocrine tumor affecting elderly or immunosuppressed individuals. It arises in sun-exposed areas and often is associated with Merkel cell polyomavirus infection. The histologic features display small and round cells that stain positive for CK8, CK18, CK19, and CK20 but stain negative for CK7, a marker that often is positive in hidradenocarcinoma.6
Lymphangioma, particularly cavernous lymphangioma, may resemble the gross appearance of hidradenoma/ hidradenocarcinoma. It usually presents as irregular clear blue papules and nodules in the skin and subcutaneous tissue.7 The key histopathologic finding in this tumor is the endothelium-lined channels that stain positive for D2-40, a lymphatic endothelium marker.7,8
Cutaneous Crohn disease is classified as noncaseating granulomatous skin lesions that are noncontinuous with the gastrointestinal tract.9 Clinical presentations in addition to skin edema include erythematous plaques, ulcerations, and erosions. Histopathology reveals sterile noncaseating granulomas made of Langerhans giant cells, epithelioid histocytes, and plasma cells.9
Metastatic clear cell carcinomas, such as renal cell carcinoma, can be differentiated by a history of primary carcinoma, demonstration of histologic vascular stroma, and other features related to metastatic clear cell carcinoma.2
There are no well-established therapeutic guidelines for hidradenocarcinoma. Wide local excision with margins greater than 2 cm is the preferred initial treatment and often is performed in conjunction with sentinel lymph node biopsy. External beam radiotherapy and adjunctive chemotherapy have been used for tumors that could not be surgically cleared. However, the efficacy of these treatments has not been well established.2 Targeted therapies recently have emerged as an alternative treatment choice for hidradenocarcinoma due to the utilization of immunohistochemical and genomic testing. The discovery of specific gene mutations or the expression of hormonal receptors in this tumor have paved the way for targeting HER2-expressing hidradenocarcinomas with trastuzumab and those expressing estrogen receptor with the estrogen receptor inhibitor tamoxifen.1 Epidermal growth factor receptor inhibitors and PI3K/Akt/mTOR (phosphatidylinositol-3-kinase/AKT/mammalian target of rapamycin) pathway inhibitors also have been used to target various signal transduction pathways.2
Wide excision with 2.5-cm margins was performed on our patient, and a positron emission tomography– computed tomography scan revealed no metastatic disease. She declined sentinel lymph node biopsy and additional treatment. Due to the risk for recurrence, she was monitored closely with skin examinations and positron emission tomography–computed tomography every 3 months for the first year and every 6 months thereafter. Thus far, she has had no evidence of local or regional recurrence.
- Miller DH, Peterson JL, Buskirk SJ, et al. Management of metastatic apocrine hidradenocarcinoma with chemotherapy and radiation. Rare Tumors. 2015;7:6082.
- Soni A, Bansal N, Kaushal V, et al. Current management approach to hidradenocarcinoma: a comprehensive review of the literature. Ecancermedicalscience. 2015;9:517.
- Jinnah AH, Emory CL, Mai NH, et al. Hidradenocarcinoma presenting as soft tissue mass: case report with cytomorphologic description, histologic correlation, and differential diagnosis. Diagn Cytopathol. 2016;44:438-441.
- Khan BM, Mansha MA, Ali N, et al. Hidradenocarcinoma: five years of local and systemic control of a rare sweat gland neoplasm with nodal metastasis. Cureus. 2018;10:E2884.
- Miceli A, Ferrer-Bruker SJ. Spiradenoma. StatPearls. StatPearls Publishing; 2019.
- Banks PD, Sandhu S, Gyorki DE, et al. Recent insights and advances in the management of Merkel cell carcinoma. J Oncol Pract. 2016; 12:637-646.
- Flanagan BP, Helwig EB. Cutaneous lymphangioma. Arch Dermatol. 1977;113:24-30.
- Kalof AN, Cooper K. D2-40 immunohistochemistry—so far! Adv Anat Pathol. 2009;16:62-64.
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574.
The Diagnosis: Hidradenocarcinoma
An excisional biopsy revealed a neoplasm in the dermis with focal invasion into the adjacent soft tissue (Figure 1). The tumor consisted of sheets of cells with cytoplasmic vacuoles and ductal differentiation (Figure 2), as well as cells with mild atypia, mild pleomorphism, rare mitotic figures, and abundant pale cytoplasm. Immunohistochemical staining was positive for cytokeratin (CK) 5, CK7, CK20, CK AE1/AE3, and p63 (Figure 3). The culmination of features including the large tumor size, immunohistochemical staining pattern, and mild pleomorphism with focal invasion into the soft tissue supported the diagnosis of hidradenocarcinoma.
Hidradenocarcinoma is an exceedingly rare malignant tumor of eccrine and/or apocrine origin.1 It accounts for less than 0.001% of all tumors and 1 of 13,000 skin biopsies.2 It usually arises in the head and neck region and most commonly affects older adults aged 50 to 70 years.3 The size of hidradenocarcinomas can vary; however, they typically are large, often growing to be greater than 5 cm in diameter.2 It tends to be an aggressive tumor that generally spreads to regional lymph nodes and distant viscera.4 Although it most commonly arises de novo, it may occasionally derive from a benign hidradenoma.1 The diagnosis of hidradenocarcinoma is made based on the tumor’s morphologic and pathologic characteristics. Histologically, it is characterized by an infiltrative and invasive proliferation of lobules made of large clear cells with atypical mitotic figures and nuclear pleomorphism as well as immunohistochemical features displaying various positive markers, such as carcinoembryonic antigen, epithelial membrane antigen, S-100 protein, and CKs AE1/AE3 and 5/6.2 Invasion of the adjacent soft tissue can be present and helps to confirm the diagnosis.
The differential diagnosis for hidradenocarcinoma primarily is the benign hidradenoma, which is similar both clinically and histologically with a few important differences. Hidradenocarcinomas often are larger and ulcerated. Histologically, they usually are more pleomorphic with the presence of mitotic figures in clear cells and tend to invade locally into the surrounding soft tissue. Other similar lesions such as spiradenoma, Merkel cell carcinoma, lymphangioma, cutaneous Crohn disease, tumors metastatic to the skin, and metastatic clear cell carcinomas originating from other organs also are included in the differential diagnosis.2
Spiradenomas are dermal tumors originating from the sweat glands. They typically present as bluish, painful, solitary nodules on the ventral surfaces of the upper body, though multiple nodules also are reported.5 Spiradenomas manifest as a central constellation of pale large cells surrounded by small, dark, basaloid cells containing hyperchromatic nuclei. The microscopic appearance of the blue basaloid cells contrasts with the clear cells seen in hidradenoma.5
Merkel cell carcinoma is a cutaneous neuroendocrine tumor affecting elderly or immunosuppressed individuals. It arises in sun-exposed areas and often is associated with Merkel cell polyomavirus infection. The histologic features display small and round cells that stain positive for CK8, CK18, CK19, and CK20 but stain negative for CK7, a marker that often is positive in hidradenocarcinoma.6
Lymphangioma, particularly cavernous lymphangioma, may resemble the gross appearance of hidradenoma/ hidradenocarcinoma. It usually presents as irregular clear blue papules and nodules in the skin and subcutaneous tissue.7 The key histopathologic finding in this tumor is the endothelium-lined channels that stain positive for D2-40, a lymphatic endothelium marker.7,8
Cutaneous Crohn disease is classified as noncaseating granulomatous skin lesions that are noncontinuous with the gastrointestinal tract.9 Clinical presentations in addition to skin edema include erythematous plaques, ulcerations, and erosions. Histopathology reveals sterile noncaseating granulomas made of Langerhans giant cells, epithelioid histocytes, and plasma cells.9
Metastatic clear cell carcinomas, such as renal cell carcinoma, can be differentiated by a history of primary carcinoma, demonstration of histologic vascular stroma, and other features related to metastatic clear cell carcinoma.2
There are no well-established therapeutic guidelines for hidradenocarcinoma. Wide local excision with margins greater than 2 cm is the preferred initial treatment and often is performed in conjunction with sentinel lymph node biopsy. External beam radiotherapy and adjunctive chemotherapy have been used for tumors that could not be surgically cleared. However, the efficacy of these treatments has not been well established.2 Targeted therapies recently have emerged as an alternative treatment choice for hidradenocarcinoma due to the utilization of immunohistochemical and genomic testing. The discovery of specific gene mutations or the expression of hormonal receptors in this tumor have paved the way for targeting HER2-expressing hidradenocarcinomas with trastuzumab and those expressing estrogen receptor with the estrogen receptor inhibitor tamoxifen.1 Epidermal growth factor receptor inhibitors and PI3K/Akt/mTOR (phosphatidylinositol-3-kinase/AKT/mammalian target of rapamycin) pathway inhibitors also have been used to target various signal transduction pathways.2
Wide excision with 2.5-cm margins was performed on our patient, and a positron emission tomography– computed tomography scan revealed no metastatic disease. She declined sentinel lymph node biopsy and additional treatment. Due to the risk for recurrence, she was monitored closely with skin examinations and positron emission tomography–computed tomography every 3 months for the first year and every 6 months thereafter. Thus far, she has had no evidence of local or regional recurrence.
The Diagnosis: Hidradenocarcinoma
An excisional biopsy revealed a neoplasm in the dermis with focal invasion into the adjacent soft tissue (Figure 1). The tumor consisted of sheets of cells with cytoplasmic vacuoles and ductal differentiation (Figure 2), as well as cells with mild atypia, mild pleomorphism, rare mitotic figures, and abundant pale cytoplasm. Immunohistochemical staining was positive for cytokeratin (CK) 5, CK7, CK20, CK AE1/AE3, and p63 (Figure 3). The culmination of features including the large tumor size, immunohistochemical staining pattern, and mild pleomorphism with focal invasion into the soft tissue supported the diagnosis of hidradenocarcinoma.
Hidradenocarcinoma is an exceedingly rare malignant tumor of eccrine and/or apocrine origin.1 It accounts for less than 0.001% of all tumors and 1 of 13,000 skin biopsies.2 It usually arises in the head and neck region and most commonly affects older adults aged 50 to 70 years.3 The size of hidradenocarcinomas can vary; however, they typically are large, often growing to be greater than 5 cm in diameter.2 It tends to be an aggressive tumor that generally spreads to regional lymph nodes and distant viscera.4 Although it most commonly arises de novo, it may occasionally derive from a benign hidradenoma.1 The diagnosis of hidradenocarcinoma is made based on the tumor’s morphologic and pathologic characteristics. Histologically, it is characterized by an infiltrative and invasive proliferation of lobules made of large clear cells with atypical mitotic figures and nuclear pleomorphism as well as immunohistochemical features displaying various positive markers, such as carcinoembryonic antigen, epithelial membrane antigen, S-100 protein, and CKs AE1/AE3 and 5/6.2 Invasion of the adjacent soft tissue can be present and helps to confirm the diagnosis.
The differential diagnosis for hidradenocarcinoma primarily is the benign hidradenoma, which is similar both clinically and histologically with a few important differences. Hidradenocarcinomas often are larger and ulcerated. Histologically, they usually are more pleomorphic with the presence of mitotic figures in clear cells and tend to invade locally into the surrounding soft tissue. Other similar lesions such as spiradenoma, Merkel cell carcinoma, lymphangioma, cutaneous Crohn disease, tumors metastatic to the skin, and metastatic clear cell carcinomas originating from other organs also are included in the differential diagnosis.2
Spiradenomas are dermal tumors originating from the sweat glands. They typically present as bluish, painful, solitary nodules on the ventral surfaces of the upper body, though multiple nodules also are reported.5 Spiradenomas manifest as a central constellation of pale large cells surrounded by small, dark, basaloid cells containing hyperchromatic nuclei. The microscopic appearance of the blue basaloid cells contrasts with the clear cells seen in hidradenoma.5
Merkel cell carcinoma is a cutaneous neuroendocrine tumor affecting elderly or immunosuppressed individuals. It arises in sun-exposed areas and often is associated with Merkel cell polyomavirus infection. The histologic features display small and round cells that stain positive for CK8, CK18, CK19, and CK20 but stain negative for CK7, a marker that often is positive in hidradenocarcinoma.6
Lymphangioma, particularly cavernous lymphangioma, may resemble the gross appearance of hidradenoma/ hidradenocarcinoma. It usually presents as irregular clear blue papules and nodules in the skin and subcutaneous tissue.7 The key histopathologic finding in this tumor is the endothelium-lined channels that stain positive for D2-40, a lymphatic endothelium marker.7,8
Cutaneous Crohn disease is classified as noncaseating granulomatous skin lesions that are noncontinuous with the gastrointestinal tract.9 Clinical presentations in addition to skin edema include erythematous plaques, ulcerations, and erosions. Histopathology reveals sterile noncaseating granulomas made of Langerhans giant cells, epithelioid histocytes, and plasma cells.9
Metastatic clear cell carcinomas, such as renal cell carcinoma, can be differentiated by a history of primary carcinoma, demonstration of histologic vascular stroma, and other features related to metastatic clear cell carcinoma.2
There are no well-established therapeutic guidelines for hidradenocarcinoma. Wide local excision with margins greater than 2 cm is the preferred initial treatment and often is performed in conjunction with sentinel lymph node biopsy. External beam radiotherapy and adjunctive chemotherapy have been used for tumors that could not be surgically cleared. However, the efficacy of these treatments has not been well established.2 Targeted therapies recently have emerged as an alternative treatment choice for hidradenocarcinoma due to the utilization of immunohistochemical and genomic testing. The discovery of specific gene mutations or the expression of hormonal receptors in this tumor have paved the way for targeting HER2-expressing hidradenocarcinomas with trastuzumab and those expressing estrogen receptor with the estrogen receptor inhibitor tamoxifen.1 Epidermal growth factor receptor inhibitors and PI3K/Akt/mTOR (phosphatidylinositol-3-kinase/AKT/mammalian target of rapamycin) pathway inhibitors also have been used to target various signal transduction pathways.2
Wide excision with 2.5-cm margins was performed on our patient, and a positron emission tomography– computed tomography scan revealed no metastatic disease. She declined sentinel lymph node biopsy and additional treatment. Due to the risk for recurrence, she was monitored closely with skin examinations and positron emission tomography–computed tomography every 3 months for the first year and every 6 months thereafter. Thus far, she has had no evidence of local or regional recurrence.
- Miller DH, Peterson JL, Buskirk SJ, et al. Management of metastatic apocrine hidradenocarcinoma with chemotherapy and radiation. Rare Tumors. 2015;7:6082.
- Soni A, Bansal N, Kaushal V, et al. Current management approach to hidradenocarcinoma: a comprehensive review of the literature. Ecancermedicalscience. 2015;9:517.
- Jinnah AH, Emory CL, Mai NH, et al. Hidradenocarcinoma presenting as soft tissue mass: case report with cytomorphologic description, histologic correlation, and differential diagnosis. Diagn Cytopathol. 2016;44:438-441.
- Khan BM, Mansha MA, Ali N, et al. Hidradenocarcinoma: five years of local and systemic control of a rare sweat gland neoplasm with nodal metastasis. Cureus. 2018;10:E2884.
- Miceli A, Ferrer-Bruker SJ. Spiradenoma. StatPearls. StatPearls Publishing; 2019.
- Banks PD, Sandhu S, Gyorki DE, et al. Recent insights and advances in the management of Merkel cell carcinoma. J Oncol Pract. 2016; 12:637-646.
- Flanagan BP, Helwig EB. Cutaneous lymphangioma. Arch Dermatol. 1977;113:24-30.
- Kalof AN, Cooper K. D2-40 immunohistochemistry—so far! Adv Anat Pathol. 2009;16:62-64.
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574.
- Miller DH, Peterson JL, Buskirk SJ, et al. Management of metastatic apocrine hidradenocarcinoma with chemotherapy and radiation. Rare Tumors. 2015;7:6082.
- Soni A, Bansal N, Kaushal V, et al. Current management approach to hidradenocarcinoma: a comprehensive review of the literature. Ecancermedicalscience. 2015;9:517.
- Jinnah AH, Emory CL, Mai NH, et al. Hidradenocarcinoma presenting as soft tissue mass: case report with cytomorphologic description, histologic correlation, and differential diagnosis. Diagn Cytopathol. 2016;44:438-441.
- Khan BM, Mansha MA, Ali N, et al. Hidradenocarcinoma: five years of local and systemic control of a rare sweat gland neoplasm with nodal metastasis. Cureus. 2018;10:E2884.
- Miceli A, Ferrer-Bruker SJ. Spiradenoma. StatPearls. StatPearls Publishing; 2019.
- Banks PD, Sandhu S, Gyorki DE, et al. Recent insights and advances in the management of Merkel cell carcinoma. J Oncol Pract. 2016; 12:637-646.
- Flanagan BP, Helwig EB. Cutaneous lymphangioma. Arch Dermatol. 1977;113:24-30.
- Kalof AN, Cooper K. D2-40 immunohistochemistry—so far! Adv Anat Pathol. 2009;16:62-64.
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574.
A 20-year-old woman with no notable medical history presented to the dermatology clinic with an enlarging mass on the right buttock that had been growing over the course of several years. The mass progressed from a small, mildly tender nodule to a 10×10-cm, hyperpigmented, exophytic tumor. There were no other abnormal findings on physical examination, and the patient denied any systemic symptoms.