User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Sudden-Onset Blistering Rash
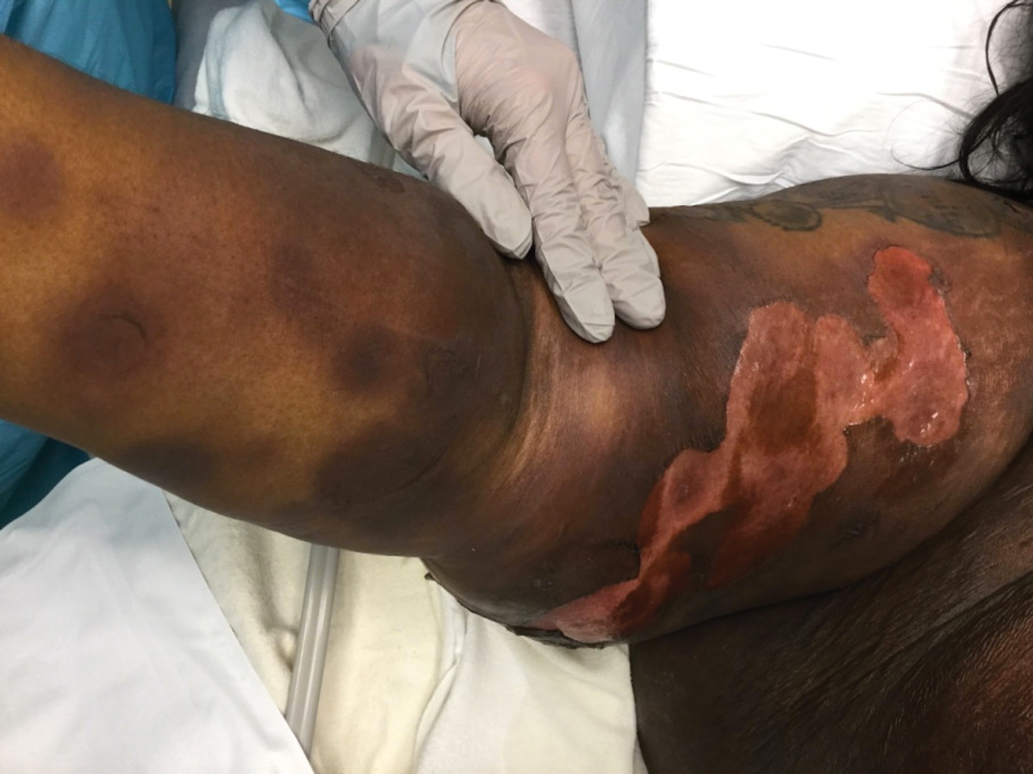
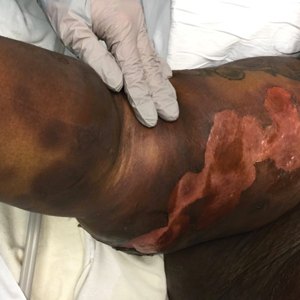
The Diagnosis: Generalized Bullous Fixed Drug Eruption
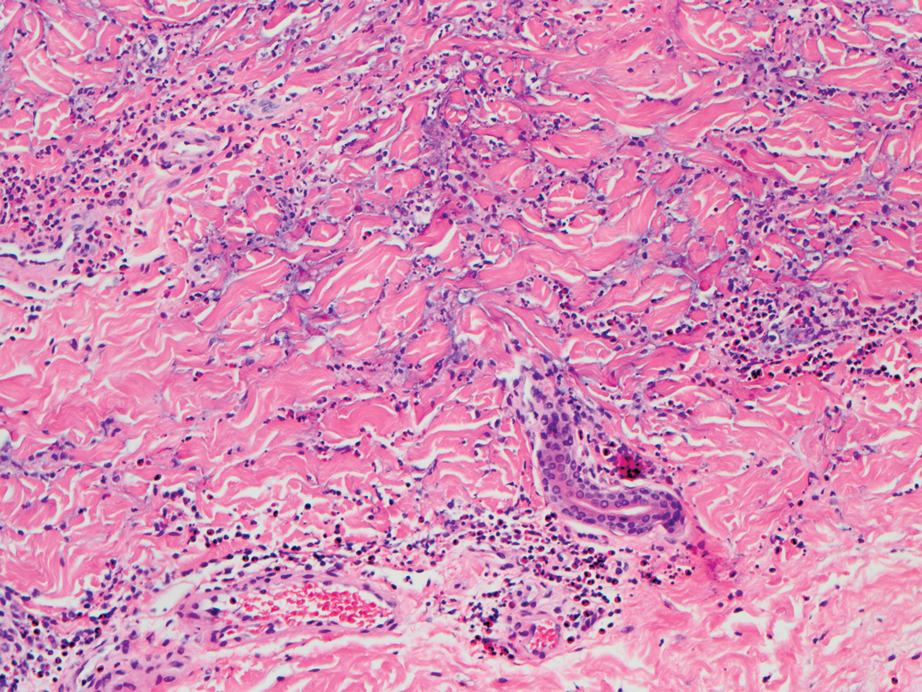
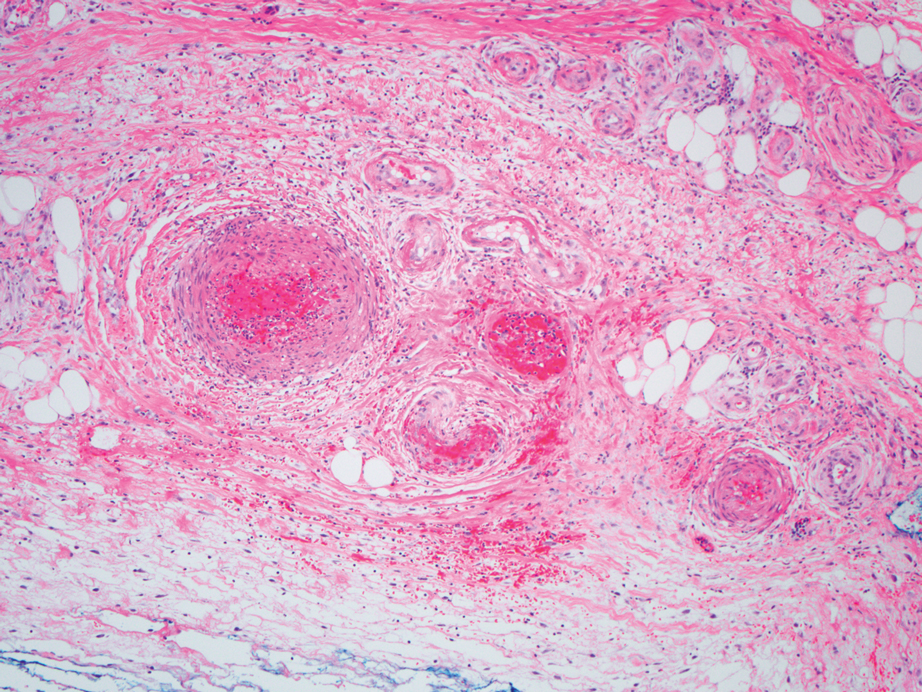
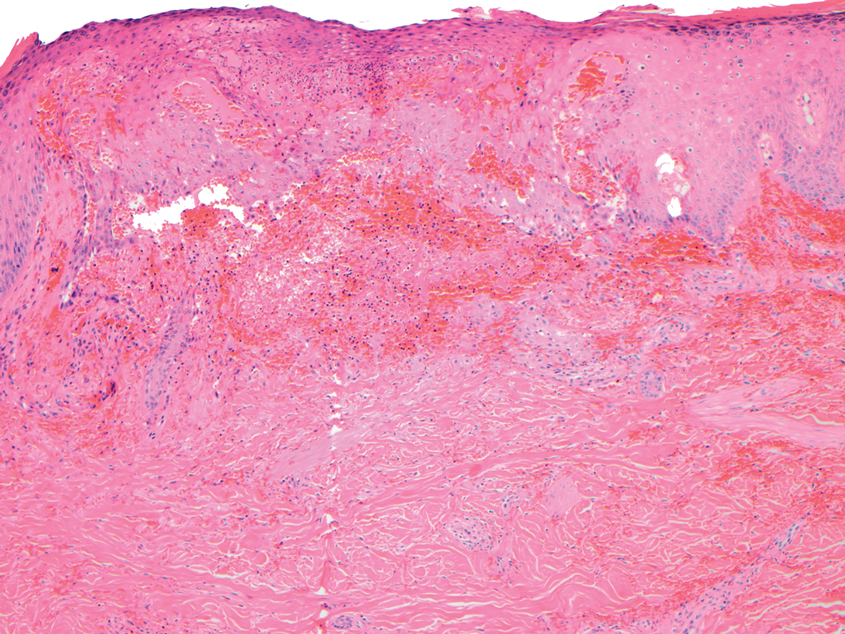
A punch biopsy from the left thigh revealed a vacuolar interface dermatitis with full-thickness necrosis of the epidermis and a patchy lichenoid inflammatory cell infiltrate in the superficial dermis consistent with a generalized bullous fixed drug eruption (GBFDE). The patient received supportive care and methylprednisolone with improvement of symptoms.
Generalized bullous fixed drug eruption is a rare, potentially life-threatening form of a fixed drug eruption (FDE), a cutaneous drug reaction that occurs in response to a causative medication. It typically presents with welldemarcated, dusky, erythematous patches or plaques that recur in the same sites with repeat exposure.1 The pathogenesis of FDE has been hypothesized to involve epidermal CD8+ T cells, which are activated by drug exposure and release cytotoxic molecules including Fas, Fas ligand, perforin, and granzyme B, resulting in lysis of the surrounding keratinocytes.1-3 Common eliciting drugs include nonsteroidal anti-inflammatory drugs, antibacterial agents (particularly trimethoprim-sulfamethoxazole), barbiturates, acetaminophen, and antimalarials.1 In addition to the findings seen in FDE, GBFDE is characterized by widespread bullous skin lesions.1-4 Typical histologic patterns seen in GBFDE are dispersed epidermal apoptotic keratinocytes, prominent dermal eosinophilic and lymphocytic infiltrates, and dermal melanophages.3 Discontinuing the causative agent and diligent prevention of re-exposure are the most important steps in management, as additional exposures can increase the number of lesions and overall severity. Symptoms typically resolve 7 to 14 days after drug discontinuation, often with postinflammatory hyperpigmentation.3
Generalized bullous fixed drug eruption presents a diagnostic challenge, as it sometimes involves the oral mucosa and can exhibit the Nikolsky sign. Thus, it often is confused with Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN).1,4 Stevens-Johnson syndrome and TEN are severe cutaneous drug eruptions that also can present with diffuse bullous skin lesions. Stevens-Johnson syndrome and TEN are thought to be a spectrum of the same disease that initially presents with dusky red macules that can coalesce, develop central blistering, and lead to skin detachment.5 Stevens-Johnson syndrome is defined as skin detachment of less than 10% body surface area (BSA); TEN is defined as skin detachment of more than 30% BSA. Stevens-Johnson syndrome/TEN overlap syndrome includes skin detachment of 10% to 30% BSA.5
Causative medications overlap substantially with GBFDE and include anticonvulsants, sulfa-containing drugs, antibiotics, nonsteroidal anti-inflammatory drugs, and uric acid–lowering agents. The histology of SJS/TEN also is quite similar to GBFDE, and these entities may be indistinguishable without clinical information.5 Lee et al1 found that absence of grouped necrotic keratinocytes (fire flag sign), deep inflammatory infiltrates, notable pigment incontinence, and higher eosinophil counts appear to be more common in GBFDE than SJS/TEN. Constitutional symptoms and mucosal involvement also were more frequent in SJS/TEN.
The timing of clinical presentation and medical history can be useful in differentiating between SJS/TEN and GBFDE. In SJS/TEN, drug exposure typically occurs 1 to 3 weeks before onset of symptoms vs 30 minutes to 24 hours in GBFDE.3 Additionally, a history of similar eruption in the same location is pathognomonic for GBFDE. Although GBFDE has been thought to have a better prognosis than SJS/TEN, more recent data suggest mortality rates may be similar.3 A case-control study found a mortality rate of 22% (13/58) in patients with GBFDE compared to 28% (n=170) in SJS/TEN patients.4
Erythema multiforme (EM) is an uncommon immunemediated disorder that typically presents as targetoid lesions with central epidermal necrosis in an acral distribution. Erythema multiforme can arise from a variety of factors, but up to 90% of cases are due to infection, most commonly herpes simplex virus; medications account for less than 10% of cases.6 Previously, EM has been thought to be on the same disease spectrum as SJS and TEN. It is now clear that EM is a separate entity with similar mucosal erosions but different cutaneous findings,6 mainly typical target lesions that differ from the atypical targets seen in SJS.
Staphylococcal scalded skin syndrome is a blistering skin disorder associated with local Staphylococcus aureus infection. It most commonly is seen in children and rarely occurs in adults who are not on dialysis. Some Staphylococcus strains produce exfoliative toxins A and B, which are serine proteases that target and cleave desmoglein 1, a mediator of keratinocyte adhesion. Staphylococcal scalded skin syndrome initially presents with erythema accentuated in the skin folds that becomes generalized. The disruption of keratinocyte adhesion leads to bullae formation in areas of erythema and diffuse sheetlike desquamation. Pathology reveals subcorneal rather than subepidermal blistering, which is seen in GBFDE and SJS/TEN. Treatment involves antistaphylococcal antibiotics and supportive care. With proper treatment, most cases resolve within 2 to 3 weeks.7
Mycoplasma pneumoniae–induced rash and mucositis presents with prominent mucositis and can have cutaneous findings of sparse vesiculobullous or targetoid eruption.8Mycoplasma pneumoniae typically infects the lungs and is a leading cause of community-acquired pneumonia. However, a subset of patients can have extrapulmonary disease presenting as mucocutaneous eruptions, which is preceded by an approximately weeklong prodrome of fever, cough, and malaise.7Mycoplasma pneumoniae–induced rash and mucositis also affect children and young patients and is more common in males.8
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15. doi:10.1016/j.dsi.2012.02.002
- Cho Y-T, Lin J-W, Chen Y-C, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Mitre V, Applebaum DS, Albahrani Y, et al. Generalized bullous fixed drug eruption imitating toxic epidermal necrolysis: a case report and literature review. Dermatol Online J. 2017;23: 13030/qt25v009gs.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with StevensJohnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732. doi:10.1111/bjd.12133
- Cho Y-T, Chu C-Y. Treatments for severe cutaneous adverse reactions [published online December 27, 2017]. J Immunol Res. doi:10.1155/2017/1503709
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae–induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245. doi:10.1016/j .jaad.2014.06.026
The Diagnosis: Generalized Bullous Fixed Drug Eruption
A punch biopsy from the left thigh revealed a vacuolar interface dermatitis with full-thickness necrosis of the epidermis and a patchy lichenoid inflammatory cell infiltrate in the superficial dermis consistent with a generalized bullous fixed drug eruption (GBFDE). The patient received supportive care and methylprednisolone with improvement of symptoms.
Generalized bullous fixed drug eruption is a rare, potentially life-threatening form of a fixed drug eruption (FDE), a cutaneous drug reaction that occurs in response to a causative medication. It typically presents with welldemarcated, dusky, erythematous patches or plaques that recur in the same sites with repeat exposure.1 The pathogenesis of FDE has been hypothesized to involve epidermal CD8+ T cells, which are activated by drug exposure and release cytotoxic molecules including Fas, Fas ligand, perforin, and granzyme B, resulting in lysis of the surrounding keratinocytes.1-3 Common eliciting drugs include nonsteroidal anti-inflammatory drugs, antibacterial agents (particularly trimethoprim-sulfamethoxazole), barbiturates, acetaminophen, and antimalarials.1 In addition to the findings seen in FDE, GBFDE is characterized by widespread bullous skin lesions.1-4 Typical histologic patterns seen in GBFDE are dispersed epidermal apoptotic keratinocytes, prominent dermal eosinophilic and lymphocytic infiltrates, and dermal melanophages.3 Discontinuing the causative agent and diligent prevention of re-exposure are the most important steps in management, as additional exposures can increase the number of lesions and overall severity. Symptoms typically resolve 7 to 14 days after drug discontinuation, often with postinflammatory hyperpigmentation.3
Generalized bullous fixed drug eruption presents a diagnostic challenge, as it sometimes involves the oral mucosa and can exhibit the Nikolsky sign. Thus, it often is confused with Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN).1,4 Stevens-Johnson syndrome and TEN are severe cutaneous drug eruptions that also can present with diffuse bullous skin lesions. Stevens-Johnson syndrome and TEN are thought to be a spectrum of the same disease that initially presents with dusky red macules that can coalesce, develop central blistering, and lead to skin detachment.5 Stevens-Johnson syndrome is defined as skin detachment of less than 10% body surface area (BSA); TEN is defined as skin detachment of more than 30% BSA. Stevens-Johnson syndrome/TEN overlap syndrome includes skin detachment of 10% to 30% BSA.5
Causative medications overlap substantially with GBFDE and include anticonvulsants, sulfa-containing drugs, antibiotics, nonsteroidal anti-inflammatory drugs, and uric acid–lowering agents. The histology of SJS/TEN also is quite similar to GBFDE, and these entities may be indistinguishable without clinical information.5 Lee et al1 found that absence of grouped necrotic keratinocytes (fire flag sign), deep inflammatory infiltrates, notable pigment incontinence, and higher eosinophil counts appear to be more common in GBFDE than SJS/TEN. Constitutional symptoms and mucosal involvement also were more frequent in SJS/TEN.
The timing of clinical presentation and medical history can be useful in differentiating between SJS/TEN and GBFDE. In SJS/TEN, drug exposure typically occurs 1 to 3 weeks before onset of symptoms vs 30 minutes to 24 hours in GBFDE.3 Additionally, a history of similar eruption in the same location is pathognomonic for GBFDE. Although GBFDE has been thought to have a better prognosis than SJS/TEN, more recent data suggest mortality rates may be similar.3 A case-control study found a mortality rate of 22% (13/58) in patients with GBFDE compared to 28% (n=170) in SJS/TEN patients.4
Erythema multiforme (EM) is an uncommon immunemediated disorder that typically presents as targetoid lesions with central epidermal necrosis in an acral distribution. Erythema multiforme can arise from a variety of factors, but up to 90% of cases are due to infection, most commonly herpes simplex virus; medications account for less than 10% of cases.6 Previously, EM has been thought to be on the same disease spectrum as SJS and TEN. It is now clear that EM is a separate entity with similar mucosal erosions but different cutaneous findings,6 mainly typical target lesions that differ from the atypical targets seen in SJS.
Staphylococcal scalded skin syndrome is a blistering skin disorder associated with local Staphylococcus aureus infection. It most commonly is seen in children and rarely occurs in adults who are not on dialysis. Some Staphylococcus strains produce exfoliative toxins A and B, which are serine proteases that target and cleave desmoglein 1, a mediator of keratinocyte adhesion. Staphylococcal scalded skin syndrome initially presents with erythema accentuated in the skin folds that becomes generalized. The disruption of keratinocyte adhesion leads to bullae formation in areas of erythema and diffuse sheetlike desquamation. Pathology reveals subcorneal rather than subepidermal blistering, which is seen in GBFDE and SJS/TEN. Treatment involves antistaphylococcal antibiotics and supportive care. With proper treatment, most cases resolve within 2 to 3 weeks.7
Mycoplasma pneumoniae–induced rash and mucositis presents with prominent mucositis and can have cutaneous findings of sparse vesiculobullous or targetoid eruption.8Mycoplasma pneumoniae typically infects the lungs and is a leading cause of community-acquired pneumonia. However, a subset of patients can have extrapulmonary disease presenting as mucocutaneous eruptions, which is preceded by an approximately weeklong prodrome of fever, cough, and malaise.7Mycoplasma pneumoniae–induced rash and mucositis also affect children and young patients and is more common in males.8
The Diagnosis: Generalized Bullous Fixed Drug Eruption
A punch biopsy from the left thigh revealed a vacuolar interface dermatitis with full-thickness necrosis of the epidermis and a patchy lichenoid inflammatory cell infiltrate in the superficial dermis consistent with a generalized bullous fixed drug eruption (GBFDE). The patient received supportive care and methylprednisolone with improvement of symptoms.
Generalized bullous fixed drug eruption is a rare, potentially life-threatening form of a fixed drug eruption (FDE), a cutaneous drug reaction that occurs in response to a causative medication. It typically presents with welldemarcated, dusky, erythematous patches or plaques that recur in the same sites with repeat exposure.1 The pathogenesis of FDE has been hypothesized to involve epidermal CD8+ T cells, which are activated by drug exposure and release cytotoxic molecules including Fas, Fas ligand, perforin, and granzyme B, resulting in lysis of the surrounding keratinocytes.1-3 Common eliciting drugs include nonsteroidal anti-inflammatory drugs, antibacterial agents (particularly trimethoprim-sulfamethoxazole), barbiturates, acetaminophen, and antimalarials.1 In addition to the findings seen in FDE, GBFDE is characterized by widespread bullous skin lesions.1-4 Typical histologic patterns seen in GBFDE are dispersed epidermal apoptotic keratinocytes, prominent dermal eosinophilic and lymphocytic infiltrates, and dermal melanophages.3 Discontinuing the causative agent and diligent prevention of re-exposure are the most important steps in management, as additional exposures can increase the number of lesions and overall severity. Symptoms typically resolve 7 to 14 days after drug discontinuation, often with postinflammatory hyperpigmentation.3
Generalized bullous fixed drug eruption presents a diagnostic challenge, as it sometimes involves the oral mucosa and can exhibit the Nikolsky sign. Thus, it often is confused with Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN).1,4 Stevens-Johnson syndrome and TEN are severe cutaneous drug eruptions that also can present with diffuse bullous skin lesions. Stevens-Johnson syndrome and TEN are thought to be a spectrum of the same disease that initially presents with dusky red macules that can coalesce, develop central blistering, and lead to skin detachment.5 Stevens-Johnson syndrome is defined as skin detachment of less than 10% body surface area (BSA); TEN is defined as skin detachment of more than 30% BSA. Stevens-Johnson syndrome/TEN overlap syndrome includes skin detachment of 10% to 30% BSA.5
Causative medications overlap substantially with GBFDE and include anticonvulsants, sulfa-containing drugs, antibiotics, nonsteroidal anti-inflammatory drugs, and uric acid–lowering agents. The histology of SJS/TEN also is quite similar to GBFDE, and these entities may be indistinguishable without clinical information.5 Lee et al1 found that absence of grouped necrotic keratinocytes (fire flag sign), deep inflammatory infiltrates, notable pigment incontinence, and higher eosinophil counts appear to be more common in GBFDE than SJS/TEN. Constitutional symptoms and mucosal involvement also were more frequent in SJS/TEN.
The timing of clinical presentation and medical history can be useful in differentiating between SJS/TEN and GBFDE. In SJS/TEN, drug exposure typically occurs 1 to 3 weeks before onset of symptoms vs 30 minutes to 24 hours in GBFDE.3 Additionally, a history of similar eruption in the same location is pathognomonic for GBFDE. Although GBFDE has been thought to have a better prognosis than SJS/TEN, more recent data suggest mortality rates may be similar.3 A case-control study found a mortality rate of 22% (13/58) in patients with GBFDE compared to 28% (n=170) in SJS/TEN patients.4
Erythema multiforme (EM) is an uncommon immunemediated disorder that typically presents as targetoid lesions with central epidermal necrosis in an acral distribution. Erythema multiforme can arise from a variety of factors, but up to 90% of cases are due to infection, most commonly herpes simplex virus; medications account for less than 10% of cases.6 Previously, EM has been thought to be on the same disease spectrum as SJS and TEN. It is now clear that EM is a separate entity with similar mucosal erosions but different cutaneous findings,6 mainly typical target lesions that differ from the atypical targets seen in SJS.
Staphylococcal scalded skin syndrome is a blistering skin disorder associated with local Staphylococcus aureus infection. It most commonly is seen in children and rarely occurs in adults who are not on dialysis. Some Staphylococcus strains produce exfoliative toxins A and B, which are serine proteases that target and cleave desmoglein 1, a mediator of keratinocyte adhesion. Staphylococcal scalded skin syndrome initially presents with erythema accentuated in the skin folds that becomes generalized. The disruption of keratinocyte adhesion leads to bullae formation in areas of erythema and diffuse sheetlike desquamation. Pathology reveals subcorneal rather than subepidermal blistering, which is seen in GBFDE and SJS/TEN. Treatment involves antistaphylococcal antibiotics and supportive care. With proper treatment, most cases resolve within 2 to 3 weeks.7
Mycoplasma pneumoniae–induced rash and mucositis presents with prominent mucositis and can have cutaneous findings of sparse vesiculobullous or targetoid eruption.8Mycoplasma pneumoniae typically infects the lungs and is a leading cause of community-acquired pneumonia. However, a subset of patients can have extrapulmonary disease presenting as mucocutaneous eruptions, which is preceded by an approximately weeklong prodrome of fever, cough, and malaise.7Mycoplasma pneumoniae–induced rash and mucositis also affect children and young patients and is more common in males.8
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15. doi:10.1016/j.dsi.2012.02.002
- Cho Y-T, Lin J-W, Chen Y-C, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Mitre V, Applebaum DS, Albahrani Y, et al. Generalized bullous fixed drug eruption imitating toxic epidermal necrolysis: a case report and literature review. Dermatol Online J. 2017;23: 13030/qt25v009gs.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with StevensJohnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732. doi:10.1111/bjd.12133
- Cho Y-T, Chu C-Y. Treatments for severe cutaneous adverse reactions [published online December 27, 2017]. J Immunol Res. doi:10.1155/2017/1503709
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae–induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245. doi:10.1016/j .jaad.2014.06.026
- Lee CH, Chen YC, Cho YT, et al. Fixed-drug eruption: a retrospective study in a single referral center in northern Taiwan. Dermatologica Sinica. 2012;30:11-15. doi:10.1016/j.dsi.2012.02.002
- Cho Y-T, Lin J-W, Chen Y-C, et al. Generalized bullous fixed drug eruption is distinct from Stevens-Johnson syndrome/toxic epidermal necrolysis by immunohistopathological features. J Am Acad Dermatol. 2014;70:539-548. doi:10.1016/j.jaad.2013.11.015
- Mitre V, Applebaum DS, Albahrani Y, et al. Generalized bullous fixed drug eruption imitating toxic epidermal necrolysis: a case report and literature review. Dermatol Online J. 2017;23: 13030/qt25v009gs.
- Lipowicz S, Sekula P, Ingen-Housz-Oro S, et al. Prognosis of generalized bullous fixed drug eruption: comparison with StevensJohnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2013;168:726-732. doi:10.1111/bjd.12133
- Cho Y-T, Chu C-Y. Treatments for severe cutaneous adverse reactions [published online December 27, 2017]. J Immunol Res. doi:10.1155/2017/1503709
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Canavan TN, Mathes EF, Frieden I, et al. Mycoplasma pneumoniae–induced rash and mucositis as a syndrome distinct from Stevens-Johnson syndrome and erythema multiforme: a systematic review. J Am Acad Dermatol. 2015;72:239-245. doi:10.1016/j .jaad.2014.06.026
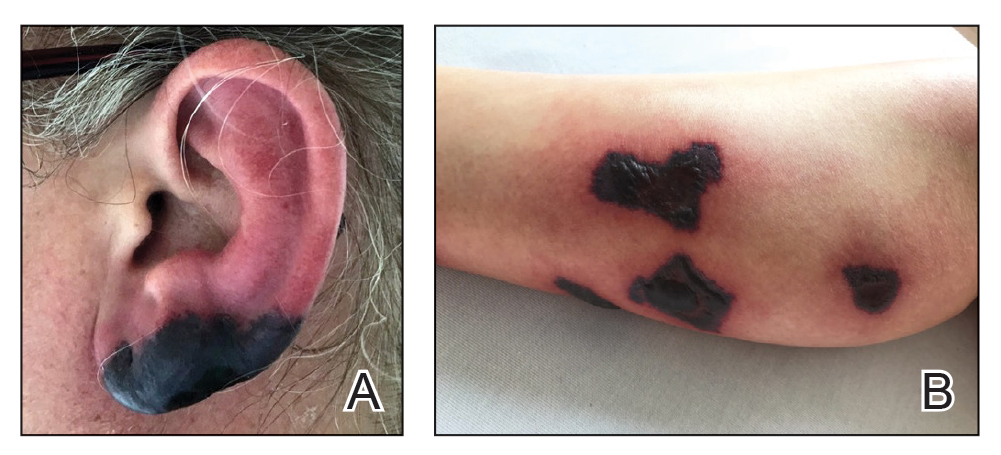
A 45-year-old woman presented with a diffuse rash 2 days after receiving ondansetron. She developed blisters on the arms, legs, trunk, and face 2 hours after exposure. There was no oral or vaginal involvement. She reported a history of leg blisters after prior exposure to ondansetron that were not as severe or numerous as the current episode. Physical examination revealed innumerable coalescing, ovoid and circular, dusky patches, some with central flaccid bullae, along with large areas of denuded skin on the trunk, arms, legs, and face. There were erosions on the lower eyelids without conjunctival or other mucosal involvement.
Cutaneous Chaetomium globosum Infection in a Vedolizumab-Treated Patient
To the Editor:
Broader availability and utilization of novel biologic treatments has heralded the emergence of unusual infections, including skin and soft tissue infections. These unusual infections may not be seen in clinical trials due to their overall rare incidence. In modern society, exposure to unusual pathogens can occur in locations far from their natural habitat.1 Tissue culture remains the gold standard, as histopathology and smears may not identify the organisms. Tissue culture of these less-common pathogens is challenging and may require multiple samples and specialized laboratory evaluations.2 In some cases, a skin biopsy with histopathologic examination is an efficient means to confirm or exclude a dermatologic manifestation of an inflammatory disease. This information can quickly change the course of treatment, especially for those on immunosuppressive medications.3 We report a case of unusual cutaneous infection with Chaetomium globosum in a patient concomitantly treated with vedolizumab, a gut-specific integrin inhibitor, alongside traditional immunosuppressive therapy.
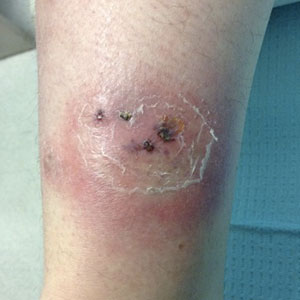
A 33-year-old woman with Crohn disease on vedolizumab and mercaptopurine was referred to the dermatology clinic with firm, tender, erythematous lesions on the legs of 1 month’s duration (Figure, A). She had a history of inflammatory bowel disease with perianal fistula, sacroiliitis, uveitis, guttate psoriasis, and erythema nodosum. She denied recent medication changes, foreign travel, swimming in freshwater or a hot tub, chills, fever, malaise, night sweats, and weight loss. Physical examination revealed several tender, indurated, erythematous plaques across the legs, ranging in size from 4 to 12 cm. The plaques had central hyperpigmentation, atrophy, and scant scale without ulceration, drainage, or pustules. The largest plaque demonstrated a well-defined area of central fluctuance. Prednisone (60 mg) with taper was initiated for presumed recurrence of erythema nodosum with close follow-up.
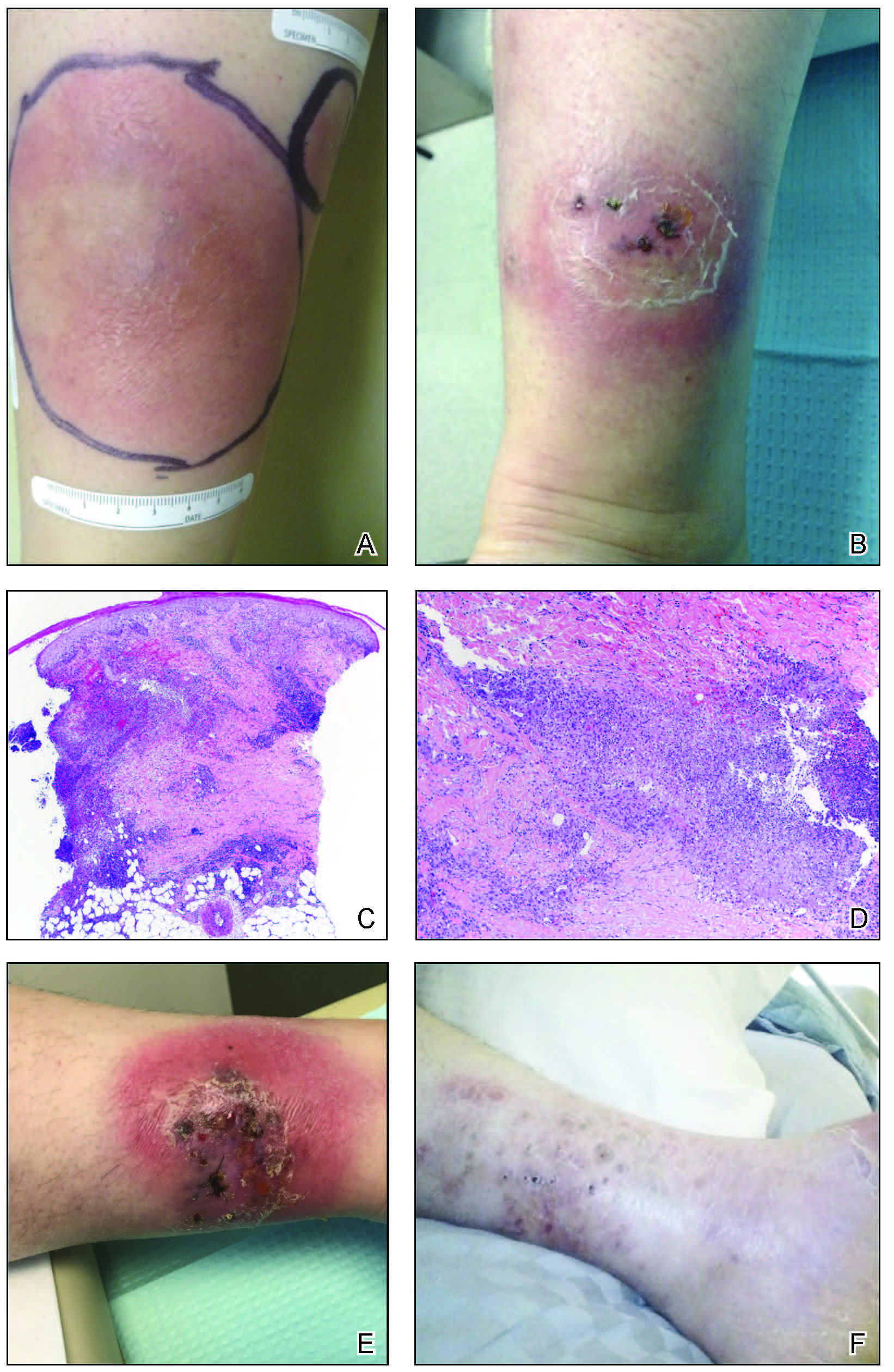
Five weeks later, most indurated plaques healed, leaving depressed scars; however, at 10 mg of prednisone she developed 2 additional nodules on the shin that, unlike earlier plaques, developed a central pustule and drained. The prednisone dose was increased to control the new areas and tapered thereafter to 20 mg daily. Despite the overall improvement, 2 plaques remained on the left side of the shin. Initially, erythema nodosum recurrence was considered, given the setting of inflammatory bowel disease and recent more classic presentation4; however, the disease progression and lack of response to standard treatment suggested an alternate pathology. Further history revealed that the patient had a pedicure 3 weeks prior to initial symptom onset. A swab was sent for routine bacterial culture at an outside clinic; no infectious agents were identified.
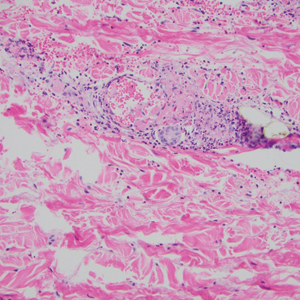
Three weeks later, the patient's condition had worsened again with increased edema, pain with standing, and more drainage (Figure, B). She did not report fevers or joint swelling. A punch biopsy was performed for tissue culture and histopathologic evaluation, which revealed granulomatous and suppurative inflammation and excluded erythema nodosum. Special stains for organisms were negative (Figure, C and D). Two weeks later, tissue culture began growing an unspecified mold. Mercaptopurine and prednisone were immediately discontinued. The patient remained on vedolizumab, started itraconazole (200 mg), and was referred to an infectious disease (ID) specialist. The sample was eventually identified as C globosum (Figure, E) at a specialized facility (University of Texas, San Antonio). Despite several weeks of itraconazole therapy, the patient developed edema surrounding the knee. Upon evaluation by orthopedics, the patient was diagnosed with reactive arthritis in the left knee and ankle. The knee fluid was drained, and cultures were negative. At recommendation of the ID physician, the itraconazole dosage was doubled given the limited clinical response. After several weeks at the increased dosage, she began to experience slow improvement (Figure, F). Because Chaetomium species infections are rare and have limited response to many antifungal agents,5 no standard treatment protocol was available. Initial recommendations for treatment were for 1 year, based on the experience and expertise of the ID physician. Treatment with itraconazole was continued for 10 months, at which point the patient chose to discontinue therapy prior to her follow-up appointments. The patient had no evidence of infection recurrence 2 months after discontinuing therapy.
In the expanding landscape of targeted biologic therapies for chronic inflammatory disease, physicians of various specialties are increasingly encountering unanticipated cutaneous eruptions and infections. Chaetomium is a dematiaceous mold found primarily in soil, water, decaying plants, paper, or dung. Based on its habitat, populations at risk for infection with Chaetomium species include farmers (plant and animal husbandry), children who play on the ground, and people with inadequate foot protection.1,2Chaetomium globosum has been identified in indoor environments, such as moldy rugs and mattresses. In one report, it was cultured from the environmental air in a bone marrow transplant patient’s room after the patient presented with delayed infection.6 Although human infection is uncommon, clinical isolation of Chaetomium species has occurred mainly in superficial samples from the skin, hair, nails, eyes, and respiratory tract.1 It been reported as a causative agent of onychomycosis in several immunocompetent patients7,8 but rarely is a cause of deep-skin infection. Chaetomium is thought to cause superficial infections, as it uses extracellular keratinases1 to degrade protective keratin structures, such as human nails. Infections in the brain, blood, and lymph nodes also have been noted but are quite rare. Deep skin infections present as painful papules and nodules to nonhealing ulcers that develop into inflammatory granulomas on the extremities.3 Local edema and yellow-brown crust often is present and fevers have been reported. Hyphae may be identified in skin biopsy.8 We posit that our patient may have been exposed to Chaetomium during her pedicure, as recirculating baths in nail salons have been a reported site of other infectious organisms, such as atypical mycobacteria.9
Vedolizumab is a humanized IgG1 monoclonal antibody used in the treatment of ulcerative colitis and Crohn disease. It targets the α4β7 integrin, a specific modulator of gut-trafficking lymphocytes. In vedolizumab’s clinical trial for Crohn disease, there was no increased incidence of life-threatening, severe infection.10,11 Often, new biologic treatments are used with known immunosuppressive medications. Mercaptopurine and prednisone are implicated in infections; however, recovery from the immune suppression usually is seen at 1 month after discontinuation.12 Our patient continued to worsen for several weeks and required increased dosing of itraconazole, despite stopping both prednisone and mercaptopurine. It opens the question as to whether vedolizumab played a role in the recalcitrant disease.
This case illustrates the importance of a high index of suspicion for unusual infections in the setting of biologic therapy. An infectious etiology of a cutaneous eruption in an immunosuppressed patient should always be included in the differential diagnosis and actively pursued early on; tissue culture may shorten the treatment course and decrease severity of the disease. Although a direct link between the mechanism of action of vedolizumab and cutaneous infection is not clear, given the rare incidence of this infection, a report of such a case is important to the practicing clinician.
- de Hoog GS, Ahmed SA, Najafzadeh MJ, et al. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Negl Trop Dis. 2013;7:e2229. doi:10.1371/journal.pntd.0002229
- Zhang H, Ran Y, Li D, et al. Clavispora lusitaniae and Chaetomium atrobrunneum as rare agents of cutaneous infection. Mycopathologia. 2010;169:373-380. doi:10.1007/s11046-009-9266-9
- Schieffelin JS, Garcia-Diaz JB, Loss GE, et al. Phaeohyphomycosis fungal infections in solid organ transplant recipients: clinical presentation, pathology, and treatment. Transpl Infect Dis Off J Transplant Soc. 2014;16:270-278. doi:10.1111/tid.12197
- Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87:281-293. doi:10.1097/MD.0b013e318187cc9c
- Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1995;14:613-618.
- Teixeira ABA, Trabasso P, Moretti-Branchini ML, et al. Phaeohyphomycosis caused by Chaetomium globosum in an allogeneic bone marrow transplant recipient. Mycopathologia. 2003;156:309-312.
- Falcón CS, Falcón MDMS, Ceballos JD, et al. Onychomycosis by Chaetomium spp. Mycoses. 2009;52:77-79. doi:10.1111/j.14390507.2008.01519.x
- Kim DM, Lee MH, Suh MK, et al. Onychomycosis caused by Chaetomium globosum. Ann Dermatol. 2013;25:232-236. doi:10.5021/ad.2013.25.2.232
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227-1236. doi:10.1111/apt.13215
- Colombel J-F, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. doi:10.1136/gutjnl-2015-311079
- Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085.
To the Editor:
Broader availability and utilization of novel biologic treatments has heralded the emergence of unusual infections, including skin and soft tissue infections. These unusual infections may not be seen in clinical trials due to their overall rare incidence. In modern society, exposure to unusual pathogens can occur in locations far from their natural habitat.1 Tissue culture remains the gold standard, as histopathology and smears may not identify the organisms. Tissue culture of these less-common pathogens is challenging and may require multiple samples and specialized laboratory evaluations.2 In some cases, a skin biopsy with histopathologic examination is an efficient means to confirm or exclude a dermatologic manifestation of an inflammatory disease. This information can quickly change the course of treatment, especially for those on immunosuppressive medications.3 We report a case of unusual cutaneous infection with Chaetomium globosum in a patient concomitantly treated with vedolizumab, a gut-specific integrin inhibitor, alongside traditional immunosuppressive therapy.
A 33-year-old woman with Crohn disease on vedolizumab and mercaptopurine was referred to the dermatology clinic with firm, tender, erythematous lesions on the legs of 1 month’s duration (Figure, A). She had a history of inflammatory bowel disease with perianal fistula, sacroiliitis, uveitis, guttate psoriasis, and erythema nodosum. She denied recent medication changes, foreign travel, swimming in freshwater or a hot tub, chills, fever, malaise, night sweats, and weight loss. Physical examination revealed several tender, indurated, erythematous plaques across the legs, ranging in size from 4 to 12 cm. The plaques had central hyperpigmentation, atrophy, and scant scale without ulceration, drainage, or pustules. The largest plaque demonstrated a well-defined area of central fluctuance. Prednisone (60 mg) with taper was initiated for presumed recurrence of erythema nodosum with close follow-up.

Five weeks later, most indurated plaques healed, leaving depressed scars; however, at 10 mg of prednisone she developed 2 additional nodules on the shin that, unlike earlier plaques, developed a central pustule and drained. The prednisone dose was increased to control the new areas and tapered thereafter to 20 mg daily. Despite the overall improvement, 2 plaques remained on the left side of the shin. Initially, erythema nodosum recurrence was considered, given the setting of inflammatory bowel disease and recent more classic presentation4; however, the disease progression and lack of response to standard treatment suggested an alternate pathology. Further history revealed that the patient had a pedicure 3 weeks prior to initial symptom onset. A swab was sent for routine bacterial culture at an outside clinic; no infectious agents were identified.
Three weeks later, the patient's condition had worsened again with increased edema, pain with standing, and more drainage (Figure, B). She did not report fevers or joint swelling. A punch biopsy was performed for tissue culture and histopathologic evaluation, which revealed granulomatous and suppurative inflammation and excluded erythema nodosum. Special stains for organisms were negative (Figure, C and D). Two weeks later, tissue culture began growing an unspecified mold. Mercaptopurine and prednisone were immediately discontinued. The patient remained on vedolizumab, started itraconazole (200 mg), and was referred to an infectious disease (ID) specialist. The sample was eventually identified as C globosum (Figure, E) at a specialized facility (University of Texas, San Antonio). Despite several weeks of itraconazole therapy, the patient developed edema surrounding the knee. Upon evaluation by orthopedics, the patient was diagnosed with reactive arthritis in the left knee and ankle. The knee fluid was drained, and cultures were negative. At recommendation of the ID physician, the itraconazole dosage was doubled given the limited clinical response. After several weeks at the increased dosage, she began to experience slow improvement (Figure, F). Because Chaetomium species infections are rare and have limited response to many antifungal agents,5 no standard treatment protocol was available. Initial recommendations for treatment were for 1 year, based on the experience and expertise of the ID physician. Treatment with itraconazole was continued for 10 months, at which point the patient chose to discontinue therapy prior to her follow-up appointments. The patient had no evidence of infection recurrence 2 months after discontinuing therapy.
In the expanding landscape of targeted biologic therapies for chronic inflammatory disease, physicians of various specialties are increasingly encountering unanticipated cutaneous eruptions and infections. Chaetomium is a dematiaceous mold found primarily in soil, water, decaying plants, paper, or dung. Based on its habitat, populations at risk for infection with Chaetomium species include farmers (plant and animal husbandry), children who play on the ground, and people with inadequate foot protection.1,2Chaetomium globosum has been identified in indoor environments, such as moldy rugs and mattresses. In one report, it was cultured from the environmental air in a bone marrow transplant patient’s room after the patient presented with delayed infection.6 Although human infection is uncommon, clinical isolation of Chaetomium species has occurred mainly in superficial samples from the skin, hair, nails, eyes, and respiratory tract.1 It been reported as a causative agent of onychomycosis in several immunocompetent patients7,8 but rarely is a cause of deep-skin infection. Chaetomium is thought to cause superficial infections, as it uses extracellular keratinases1 to degrade protective keratin structures, such as human nails. Infections in the brain, blood, and lymph nodes also have been noted but are quite rare. Deep skin infections present as painful papules and nodules to nonhealing ulcers that develop into inflammatory granulomas on the extremities.3 Local edema and yellow-brown crust often is present and fevers have been reported. Hyphae may be identified in skin biopsy.8 We posit that our patient may have been exposed to Chaetomium during her pedicure, as recirculating baths in nail salons have been a reported site of other infectious organisms, such as atypical mycobacteria.9
Vedolizumab is a humanized IgG1 monoclonal antibody used in the treatment of ulcerative colitis and Crohn disease. It targets the α4β7 integrin, a specific modulator of gut-trafficking lymphocytes. In vedolizumab’s clinical trial for Crohn disease, there was no increased incidence of life-threatening, severe infection.10,11 Often, new biologic treatments are used with known immunosuppressive medications. Mercaptopurine and prednisone are implicated in infections; however, recovery from the immune suppression usually is seen at 1 month after discontinuation.12 Our patient continued to worsen for several weeks and required increased dosing of itraconazole, despite stopping both prednisone and mercaptopurine. It opens the question as to whether vedolizumab played a role in the recalcitrant disease.
This case illustrates the importance of a high index of suspicion for unusual infections in the setting of biologic therapy. An infectious etiology of a cutaneous eruption in an immunosuppressed patient should always be included in the differential diagnosis and actively pursued early on; tissue culture may shorten the treatment course and decrease severity of the disease. Although a direct link between the mechanism of action of vedolizumab and cutaneous infection is not clear, given the rare incidence of this infection, a report of such a case is important to the practicing clinician.
To the Editor:
Broader availability and utilization of novel biologic treatments has heralded the emergence of unusual infections, including skin and soft tissue infections. These unusual infections may not be seen in clinical trials due to their overall rare incidence. In modern society, exposure to unusual pathogens can occur in locations far from their natural habitat.1 Tissue culture remains the gold standard, as histopathology and smears may not identify the organisms. Tissue culture of these less-common pathogens is challenging and may require multiple samples and specialized laboratory evaluations.2 In some cases, a skin biopsy with histopathologic examination is an efficient means to confirm or exclude a dermatologic manifestation of an inflammatory disease. This information can quickly change the course of treatment, especially for those on immunosuppressive medications.3 We report a case of unusual cutaneous infection with Chaetomium globosum in a patient concomitantly treated with vedolizumab, a gut-specific integrin inhibitor, alongside traditional immunosuppressive therapy.
A 33-year-old woman with Crohn disease on vedolizumab and mercaptopurine was referred to the dermatology clinic with firm, tender, erythematous lesions on the legs of 1 month’s duration (Figure, A). She had a history of inflammatory bowel disease with perianal fistula, sacroiliitis, uveitis, guttate psoriasis, and erythema nodosum. She denied recent medication changes, foreign travel, swimming in freshwater or a hot tub, chills, fever, malaise, night sweats, and weight loss. Physical examination revealed several tender, indurated, erythematous plaques across the legs, ranging in size from 4 to 12 cm. The plaques had central hyperpigmentation, atrophy, and scant scale without ulceration, drainage, or pustules. The largest plaque demonstrated a well-defined area of central fluctuance. Prednisone (60 mg) with taper was initiated for presumed recurrence of erythema nodosum with close follow-up.

Five weeks later, most indurated plaques healed, leaving depressed scars; however, at 10 mg of prednisone she developed 2 additional nodules on the shin that, unlike earlier plaques, developed a central pustule and drained. The prednisone dose was increased to control the new areas and tapered thereafter to 20 mg daily. Despite the overall improvement, 2 plaques remained on the left side of the shin. Initially, erythema nodosum recurrence was considered, given the setting of inflammatory bowel disease and recent more classic presentation4; however, the disease progression and lack of response to standard treatment suggested an alternate pathology. Further history revealed that the patient had a pedicure 3 weeks prior to initial symptom onset. A swab was sent for routine bacterial culture at an outside clinic; no infectious agents were identified.
Three weeks later, the patient's condition had worsened again with increased edema, pain with standing, and more drainage (Figure, B). She did not report fevers or joint swelling. A punch biopsy was performed for tissue culture and histopathologic evaluation, which revealed granulomatous and suppurative inflammation and excluded erythema nodosum. Special stains for organisms were negative (Figure, C and D). Two weeks later, tissue culture began growing an unspecified mold. Mercaptopurine and prednisone were immediately discontinued. The patient remained on vedolizumab, started itraconazole (200 mg), and was referred to an infectious disease (ID) specialist. The sample was eventually identified as C globosum (Figure, E) at a specialized facility (University of Texas, San Antonio). Despite several weeks of itraconazole therapy, the patient developed edema surrounding the knee. Upon evaluation by orthopedics, the patient was diagnosed with reactive arthritis in the left knee and ankle. The knee fluid was drained, and cultures were negative. At recommendation of the ID physician, the itraconazole dosage was doubled given the limited clinical response. After several weeks at the increased dosage, she began to experience slow improvement (Figure, F). Because Chaetomium species infections are rare and have limited response to many antifungal agents,5 no standard treatment protocol was available. Initial recommendations for treatment were for 1 year, based on the experience and expertise of the ID physician. Treatment with itraconazole was continued for 10 months, at which point the patient chose to discontinue therapy prior to her follow-up appointments. The patient had no evidence of infection recurrence 2 months after discontinuing therapy.
In the expanding landscape of targeted biologic therapies for chronic inflammatory disease, physicians of various specialties are increasingly encountering unanticipated cutaneous eruptions and infections. Chaetomium is a dematiaceous mold found primarily in soil, water, decaying plants, paper, or dung. Based on its habitat, populations at risk for infection with Chaetomium species include farmers (plant and animal husbandry), children who play on the ground, and people with inadequate foot protection.1,2Chaetomium globosum has been identified in indoor environments, such as moldy rugs and mattresses. In one report, it was cultured from the environmental air in a bone marrow transplant patient’s room after the patient presented with delayed infection.6 Although human infection is uncommon, clinical isolation of Chaetomium species has occurred mainly in superficial samples from the skin, hair, nails, eyes, and respiratory tract.1 It been reported as a causative agent of onychomycosis in several immunocompetent patients7,8 but rarely is a cause of deep-skin infection. Chaetomium is thought to cause superficial infections, as it uses extracellular keratinases1 to degrade protective keratin structures, such as human nails. Infections in the brain, blood, and lymph nodes also have been noted but are quite rare. Deep skin infections present as painful papules and nodules to nonhealing ulcers that develop into inflammatory granulomas on the extremities.3 Local edema and yellow-brown crust often is present and fevers have been reported. Hyphae may be identified in skin biopsy.8 We posit that our patient may have been exposed to Chaetomium during her pedicure, as recirculating baths in nail salons have been a reported site of other infectious organisms, such as atypical mycobacteria.9
Vedolizumab is a humanized IgG1 monoclonal antibody used in the treatment of ulcerative colitis and Crohn disease. It targets the α4β7 integrin, a specific modulator of gut-trafficking lymphocytes. In vedolizumab’s clinical trial for Crohn disease, there was no increased incidence of life-threatening, severe infection.10,11 Often, new biologic treatments are used with known immunosuppressive medications. Mercaptopurine and prednisone are implicated in infections; however, recovery from the immune suppression usually is seen at 1 month after discontinuation.12 Our patient continued to worsen for several weeks and required increased dosing of itraconazole, despite stopping both prednisone and mercaptopurine. It opens the question as to whether vedolizumab played a role in the recalcitrant disease.
This case illustrates the importance of a high index of suspicion for unusual infections in the setting of biologic therapy. An infectious etiology of a cutaneous eruption in an immunosuppressed patient should always be included in the differential diagnosis and actively pursued early on; tissue culture may shorten the treatment course and decrease severity of the disease. Although a direct link between the mechanism of action of vedolizumab and cutaneous infection is not clear, given the rare incidence of this infection, a report of such a case is important to the practicing clinician.
- de Hoog GS, Ahmed SA, Najafzadeh MJ, et al. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Negl Trop Dis. 2013;7:e2229. doi:10.1371/journal.pntd.0002229
- Zhang H, Ran Y, Li D, et al. Clavispora lusitaniae and Chaetomium atrobrunneum as rare agents of cutaneous infection. Mycopathologia. 2010;169:373-380. doi:10.1007/s11046-009-9266-9
- Schieffelin JS, Garcia-Diaz JB, Loss GE, et al. Phaeohyphomycosis fungal infections in solid organ transplant recipients: clinical presentation, pathology, and treatment. Transpl Infect Dis Off J Transplant Soc. 2014;16:270-278. doi:10.1111/tid.12197
- Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87:281-293. doi:10.1097/MD.0b013e318187cc9c
- Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1995;14:613-618.
- Teixeira ABA, Trabasso P, Moretti-Branchini ML, et al. Phaeohyphomycosis caused by Chaetomium globosum in an allogeneic bone marrow transplant recipient. Mycopathologia. 2003;156:309-312.
- Falcón CS, Falcón MDMS, Ceballos JD, et al. Onychomycosis by Chaetomium spp. Mycoses. 2009;52:77-79. doi:10.1111/j.14390507.2008.01519.x
- Kim DM, Lee MH, Suh MK, et al. Onychomycosis caused by Chaetomium globosum. Ann Dermatol. 2013;25:232-236. doi:10.5021/ad.2013.25.2.232
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227-1236. doi:10.1111/apt.13215
- Colombel J-F, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. doi:10.1136/gutjnl-2015-311079
- Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085.
- de Hoog GS, Ahmed SA, Najafzadeh MJ, et al. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Negl Trop Dis. 2013;7:e2229. doi:10.1371/journal.pntd.0002229
- Zhang H, Ran Y, Li D, et al. Clavispora lusitaniae and Chaetomium atrobrunneum as rare agents of cutaneous infection. Mycopathologia. 2010;169:373-380. doi:10.1007/s11046-009-9266-9
- Schieffelin JS, Garcia-Diaz JB, Loss GE, et al. Phaeohyphomycosis fungal infections in solid organ transplant recipients: clinical presentation, pathology, and treatment. Transpl Infect Dis Off J Transplant Soc. 2014;16:270-278. doi:10.1111/tid.12197
- Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87:281-293. doi:10.1097/MD.0b013e318187cc9c
- Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1995;14:613-618.
- Teixeira ABA, Trabasso P, Moretti-Branchini ML, et al. Phaeohyphomycosis caused by Chaetomium globosum in an allogeneic bone marrow transplant recipient. Mycopathologia. 2003;156:309-312.
- Falcón CS, Falcón MDMS, Ceballos JD, et al. Onychomycosis by Chaetomium spp. Mycoses. 2009;52:77-79. doi:10.1111/j.14390507.2008.01519.x
- Kim DM, Lee MH, Suh MK, et al. Onychomycosis caused by Chaetomium globosum. Ann Dermatol. 2013;25:232-236. doi:10.5021/ad.2013.25.2.232
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227-1236. doi:10.1111/apt.13215
- Colombel J-F, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. doi:10.1136/gutjnl-2015-311079
- Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085.
Practice Points
- Tissue culture remains the gold standard for deep fungal infections.
- Physicians must maintain a high index of suspicion for alternate diagnoses when a disease progresses along an unexpected course.
- Biologic medications may have low-incidence side effects that emerge in postmarket use.
Verrucous Carcinoma in a Wounded Military Amputee
To the Editor:
Verrucous carcinoma is a rare, well-differentiated, locally aggressive squamous cell carcinoma first described by Ackerman in 1948.1 There are 4 main clinicopathologic types: oral florid papillomatosis or Ackerman tumor, giant condyloma acuminatum or Buschke-Lowenstein tumor, plantar verrucous carcinoma, and cutaneous verrucous carcinoma.2,3 Historically, most patients are older white men. The lesion commonly occurs in sites of inflammation4 or chronic irritation/trauma. Clinically, patients present with a slowly enlarging, exophytic, verrucous plaque violating the skin, fascia, and occasionally bone. Although these lesions have little tendency to metastasize, substantial morbidity can be seen due to local invasion. Despite surgical excision, recurrence is not uncommon and is associated with a poor prognosis and higher infiltrative potential.5
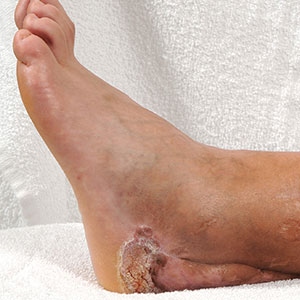
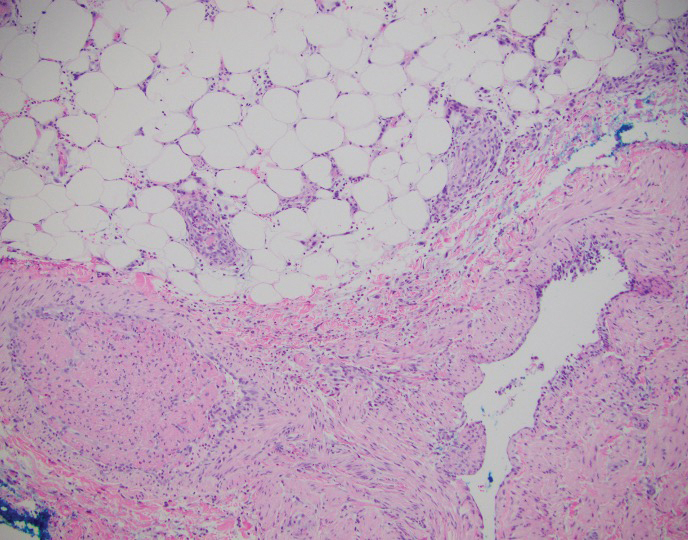
A 45-year-old male veteran initially presented to our dermatology clinic with a 4-cm, macerated, verrucous plaque on the left lateral ankle in the area of a skin graft placed during a prior limb salvage surgery (Figure 1). The patient experienced a traumatic blast injury while deployed 7 years prior with a subsequent right-sided below-the-knee amputation and left lower limb salvage. The lesion was clinically diagnosed as verruca vulgaris and treated with daily salicylic acid. Six weeks after the initial presentation, the lesion remained largely unchanged. A biopsy subsequently was obtained to confirm the diagnosis. At that time, the histopathology was consistent with verruca vulgaris without evidence of carcinoma. Due to the persistence of the lesion, lack of improvement with topical treatment, and overall size, the patient opted for surgical excision.
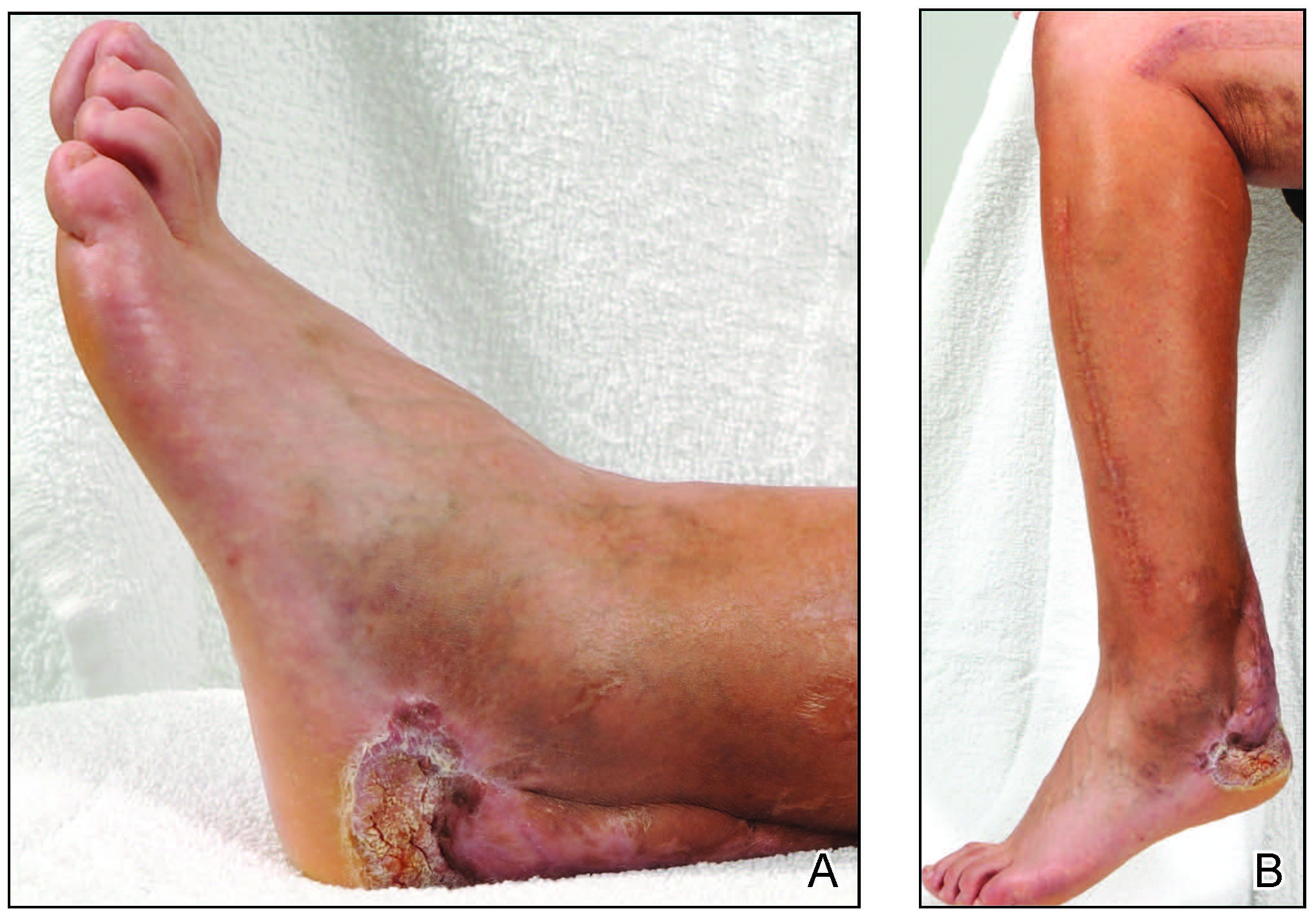
A year later, the lesion was excised again by orthopedic surgery, and the tissue was submitted for histopathologic evaluation, which was suggestive of a verrucous neoplasm with some disagreement on whether it was consistent with verrucous hyperplasia or verrucous carcinoma. Following excision, the patient sustained a nonhealing chronic ulcer that required wound care for a total of 6 months. The lesion recurred a year later and was surgically excised a third time. A split-thickness skin graft was utilized to repair the defect. Histopathology again was consistent with verrucous carcinoma. With a fourth and final recurrence of the verrucous plaque 6 months later, the patient elected to undergo a left-sided below-the-knee amputation.
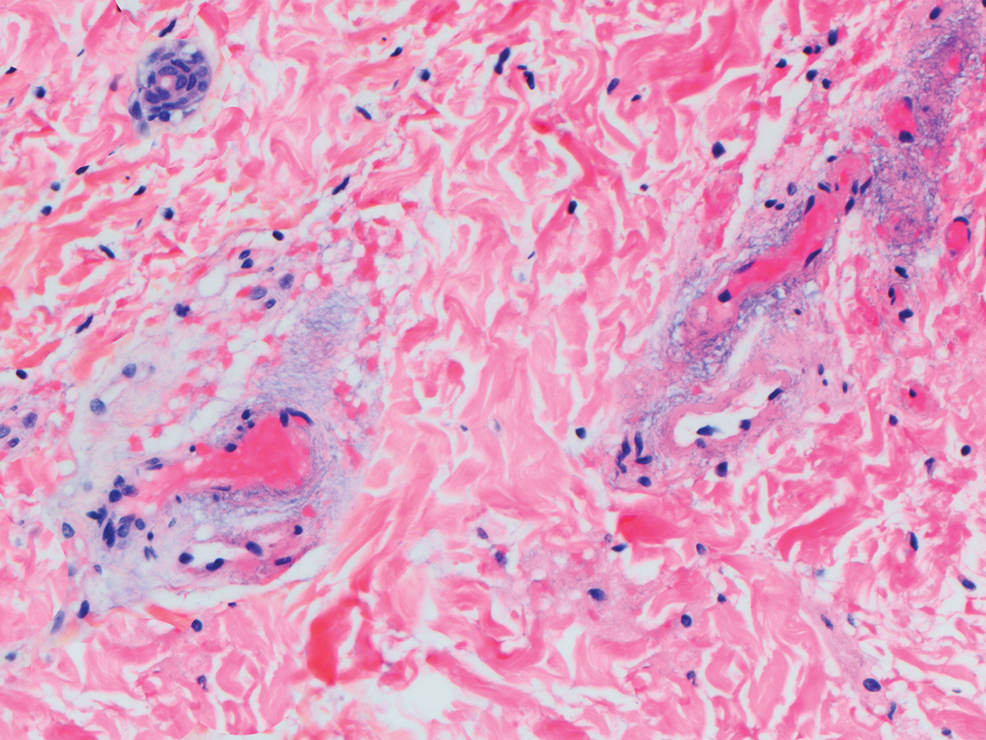
Verrucous carcinoma can represent a diagnostic dilemma, as histologic sections may mimic benign entities. The features of a well-differentiated squamous epithelium with hyperkeratosis, papillomatosis, and acanthosis can be mistaken for verruca vulgaris, keratoacanthoma, and pseudoepitheliomatous hyperplasia,6 which are characteristic of verrucous hyperplasia. Accurate diagnosis can be difficult with a superficial biopsy because of the mature appearance of the epithelium,7 prompting the need for multiple and deeper biopsies8 to include sampling of the base of the hyperplastic epithelium in which the characteristic bulbous pushing growth pattern of the rete ridges is visualized. Precise histologic diagnosis can be further confounded by external mechanical factors, such as pressure, which can distort the classic histopathology.7 The histopathologic features leading to the diagnosis of verrucous carcinoma in our specimen were minimal squamous atypia present in a predominantly exophytic squamous proliferation with human papillomavirus cytopathic effect and focal endophytic pushing borders by rounded bulbous rete ridges into the mid and deep dermis (Figure 2).

Diagnostic uncertainty can delay surgical excision and lead to progression of verrucous carcinoma. Unfortunately, even with appropriate surgical intervention, recurrence has been documented; therefore, close clinical follow-up is recommended. The tumor spreads by local invasion and may follow the path of least resistance.4 In our patient, the frequent tissue manipulation may have facilitated aggressive infiltration of the tumor, ultimately resulting in the loss of his remaining leg. Therefore, it is important for clinicians to recognize that verrucous carcinoma, especially one that develops on a refractory ulcer or scar tissue, may be a complex malignant neoplasm that requires extensive treatment at onset to prevent the amputation of a limb.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Yoshitasu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Schwartz R. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-14.
- Bernstein SC, Lim KK, Brodland DG, et al. The many faces of squamous cell carcinoma. Dermatol Surg. 1996;22:243-254.
- Costache M, Tatiana D, Mitrache L, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Shenoy A, Waghmare R, Kavishwar V, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Klima M, Kurtis B, Jordan P. Verrucous carcinoma of skin. J Cutan Pathol.1980;7:88-98.
- Pleat J, Sacks L, Rigby H. Cutaneous verrucous carcinoma. Br J Plast Surg. 2001;54:554-555.
To the Editor:
Verrucous carcinoma is a rare, well-differentiated, locally aggressive squamous cell carcinoma first described by Ackerman in 1948.1 There are 4 main clinicopathologic types: oral florid papillomatosis or Ackerman tumor, giant condyloma acuminatum or Buschke-Lowenstein tumor, plantar verrucous carcinoma, and cutaneous verrucous carcinoma.2,3 Historically, most patients are older white men. The lesion commonly occurs in sites of inflammation4 or chronic irritation/trauma. Clinically, patients present with a slowly enlarging, exophytic, verrucous plaque violating the skin, fascia, and occasionally bone. Although these lesions have little tendency to metastasize, substantial morbidity can be seen due to local invasion. Despite surgical excision, recurrence is not uncommon and is associated with a poor prognosis and higher infiltrative potential.5
A 45-year-old male veteran initially presented to our dermatology clinic with a 4-cm, macerated, verrucous plaque on the left lateral ankle in the area of a skin graft placed during a prior limb salvage surgery (Figure 1). The patient experienced a traumatic blast injury while deployed 7 years prior with a subsequent right-sided below-the-knee amputation and left lower limb salvage. The lesion was clinically diagnosed as verruca vulgaris and treated with daily salicylic acid. Six weeks after the initial presentation, the lesion remained largely unchanged. A biopsy subsequently was obtained to confirm the diagnosis. At that time, the histopathology was consistent with verruca vulgaris without evidence of carcinoma. Due to the persistence of the lesion, lack of improvement with topical treatment, and overall size, the patient opted for surgical excision.

A year later, the lesion was excised again by orthopedic surgery, and the tissue was submitted for histopathologic evaluation, which was suggestive of a verrucous neoplasm with some disagreement on whether it was consistent with verrucous hyperplasia or verrucous carcinoma. Following excision, the patient sustained a nonhealing chronic ulcer that required wound care for a total of 6 months. The lesion recurred a year later and was surgically excised a third time. A split-thickness skin graft was utilized to repair the defect. Histopathology again was consistent with verrucous carcinoma. With a fourth and final recurrence of the verrucous plaque 6 months later, the patient elected to undergo a left-sided below-the-knee amputation.
Verrucous carcinoma can represent a diagnostic dilemma, as histologic sections may mimic benign entities. The features of a well-differentiated squamous epithelium with hyperkeratosis, papillomatosis, and acanthosis can be mistaken for verruca vulgaris, keratoacanthoma, and pseudoepitheliomatous hyperplasia,6 which are characteristic of verrucous hyperplasia. Accurate diagnosis can be difficult with a superficial biopsy because of the mature appearance of the epithelium,7 prompting the need for multiple and deeper biopsies8 to include sampling of the base of the hyperplastic epithelium in which the characteristic bulbous pushing growth pattern of the rete ridges is visualized. Precise histologic diagnosis can be further confounded by external mechanical factors, such as pressure, which can distort the classic histopathology.7 The histopathologic features leading to the diagnosis of verrucous carcinoma in our specimen were minimal squamous atypia present in a predominantly exophytic squamous proliferation with human papillomavirus cytopathic effect and focal endophytic pushing borders by rounded bulbous rete ridges into the mid and deep dermis (Figure 2).

Diagnostic uncertainty can delay surgical excision and lead to progression of verrucous carcinoma. Unfortunately, even with appropriate surgical intervention, recurrence has been documented; therefore, close clinical follow-up is recommended. The tumor spreads by local invasion and may follow the path of least resistance.4 In our patient, the frequent tissue manipulation may have facilitated aggressive infiltration of the tumor, ultimately resulting in the loss of his remaining leg. Therefore, it is important for clinicians to recognize that verrucous carcinoma, especially one that develops on a refractory ulcer or scar tissue, may be a complex malignant neoplasm that requires extensive treatment at onset to prevent the amputation of a limb.
To the Editor:
Verrucous carcinoma is a rare, well-differentiated, locally aggressive squamous cell carcinoma first described by Ackerman in 1948.1 There are 4 main clinicopathologic types: oral florid papillomatosis or Ackerman tumor, giant condyloma acuminatum or Buschke-Lowenstein tumor, plantar verrucous carcinoma, and cutaneous verrucous carcinoma.2,3 Historically, most patients are older white men. The lesion commonly occurs in sites of inflammation4 or chronic irritation/trauma. Clinically, patients present with a slowly enlarging, exophytic, verrucous plaque violating the skin, fascia, and occasionally bone. Although these lesions have little tendency to metastasize, substantial morbidity can be seen due to local invasion. Despite surgical excision, recurrence is not uncommon and is associated with a poor prognosis and higher infiltrative potential.5
A 45-year-old male veteran initially presented to our dermatology clinic with a 4-cm, macerated, verrucous plaque on the left lateral ankle in the area of a skin graft placed during a prior limb salvage surgery (Figure 1). The patient experienced a traumatic blast injury while deployed 7 years prior with a subsequent right-sided below-the-knee amputation and left lower limb salvage. The lesion was clinically diagnosed as verruca vulgaris and treated with daily salicylic acid. Six weeks after the initial presentation, the lesion remained largely unchanged. A biopsy subsequently was obtained to confirm the diagnosis. At that time, the histopathology was consistent with verruca vulgaris without evidence of carcinoma. Due to the persistence of the lesion, lack of improvement with topical treatment, and overall size, the patient opted for surgical excision.

A year later, the lesion was excised again by orthopedic surgery, and the tissue was submitted for histopathologic evaluation, which was suggestive of a verrucous neoplasm with some disagreement on whether it was consistent with verrucous hyperplasia or verrucous carcinoma. Following excision, the patient sustained a nonhealing chronic ulcer that required wound care for a total of 6 months. The lesion recurred a year later and was surgically excised a third time. A split-thickness skin graft was utilized to repair the defect. Histopathology again was consistent with verrucous carcinoma. With a fourth and final recurrence of the verrucous plaque 6 months later, the patient elected to undergo a left-sided below-the-knee amputation.
Verrucous carcinoma can represent a diagnostic dilemma, as histologic sections may mimic benign entities. The features of a well-differentiated squamous epithelium with hyperkeratosis, papillomatosis, and acanthosis can be mistaken for verruca vulgaris, keratoacanthoma, and pseudoepitheliomatous hyperplasia,6 which are characteristic of verrucous hyperplasia. Accurate diagnosis can be difficult with a superficial biopsy because of the mature appearance of the epithelium,7 prompting the need for multiple and deeper biopsies8 to include sampling of the base of the hyperplastic epithelium in which the characteristic bulbous pushing growth pattern of the rete ridges is visualized. Precise histologic diagnosis can be further confounded by external mechanical factors, such as pressure, which can distort the classic histopathology.7 The histopathologic features leading to the diagnosis of verrucous carcinoma in our specimen were minimal squamous atypia present in a predominantly exophytic squamous proliferation with human papillomavirus cytopathic effect and focal endophytic pushing borders by rounded bulbous rete ridges into the mid and deep dermis (Figure 2).

Diagnostic uncertainty can delay surgical excision and lead to progression of verrucous carcinoma. Unfortunately, even with appropriate surgical intervention, recurrence has been documented; therefore, close clinical follow-up is recommended. The tumor spreads by local invasion and may follow the path of least resistance.4 In our patient, the frequent tissue manipulation may have facilitated aggressive infiltration of the tumor, ultimately resulting in the loss of his remaining leg. Therefore, it is important for clinicians to recognize that verrucous carcinoma, especially one that develops on a refractory ulcer or scar tissue, may be a complex malignant neoplasm that requires extensive treatment at onset to prevent the amputation of a limb.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Yoshitasu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Schwartz R. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-14.
- Bernstein SC, Lim KK, Brodland DG, et al. The many faces of squamous cell carcinoma. Dermatol Surg. 1996;22:243-254.
- Costache M, Tatiana D, Mitrache L, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Shenoy A, Waghmare R, Kavishwar V, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Klima M, Kurtis B, Jordan P. Verrucous carcinoma of skin. J Cutan Pathol.1980;7:88-98.
- Pleat J, Sacks L, Rigby H. Cutaneous verrucous carcinoma. Br J Plast Surg. 2001;54:554-555.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Yoshitasu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Schwartz R. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-14.
- Bernstein SC, Lim KK, Brodland DG, et al. The many faces of squamous cell carcinoma. Dermatol Surg. 1996;22:243-254.
- Costache M, Tatiana D, Mitrache L, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383-388.
- Shenoy A, Waghmare R, Kavishwar V, et al. Carcinoma cuniculatum of foot. Foot. 2011;21:207-208.
- Klima M, Kurtis B, Jordan P. Verrucous carcinoma of skin. J Cutan Pathol.1980;7:88-98.
- Pleat J, Sacks L, Rigby H. Cutaneous verrucous carcinoma. Br J Plast Surg. 2001;54:554-555.
Practice Points
- Verrucous carcinoma is a rare, well-differentiated, locally aggressive squamous cell carcinoma that commonly occurs in sites of inflammation or chronic irritation.
- Histologically, verrucous carcinoma can be mistaken for other entities including verruca vulgaris, keratoacanthoma, and pseudoepitheliomatous hyperplasia, often delaying the appropriate diagnosis and treatment.
Business Education in Dermatology Residency: A Survey of Program Directors
Globally, the United States has the highest per-capita cost of health care; total costs are expected to account for approximately 20% of the nation’s gross domestic product by 2025.1 These rising costs have prompted residency programs and medical schools to incorporate business education into their curricula.2-5 Although medical training is demanding—with little room to add curricular components—these business-focused curricula have consistently received positive feedback from residents.5,6
In dermatology, more than 50% of residents opt to join a private practice upon graduation.7 In the United States, there also is an upward trend of practice acquisition and consolidation by private equity firms. Therefore, dermatology trainees are uniquely positioned to benefit from business education to make well-informed decisions about joining or starting a practice.Furthermore, whether in a private or academic setting, knowledge of foundational economics, business strategy, finance, marketing, and health care policy can equip dermatologists to more effectively advocate for local and national policies that benefit their patient population.7
We conducted a survey of dermatology program directors (PDs) to determine the availability of and perceptions regarding business education during residency training.
Materials and Methods
Institutional review board (Vanderbilt University Medical Center, Nashville, Tennessee) approval was obtained. The survey was distributed weekly during a 5-week period from July 2020 to August 2020 through the Research Electronic Data Capture survey application (www.project-redcap.org). Program director email addresses were obtained through the Accreditation Council for Graduate Medical Education (ACGME) program list. A PD was included in the survey if they were employed by an accredited US osteopathic or allopathic program and their email address was provided in the ACGME program list or on their program’s faculty web page; a PD was excluded if an email address was not provided in the ACGME program list or on their program’s faculty web page.
The 8-part questionnaire was designed to assess the following characteristics: details about the respondent’s residency program (institutional affiliation, number of residents), the respondent’s professional background (number of years as a PD, business training experience), resources for business education provided by the program, the respondent’s opinion about business education for residents, and the respondent’s perception of the most important topics to include in a dermatology curriculum’s business education component, which included economics/finance, health care policy/government, management, marketing, negotiation, private equity involvement in health care, business strategy, supply chain/operations, and technology/product development. Responses were kept anonymous. Categorical and continuous variables were analyzed with medians and proportions.
Results
Of the 139 surveys distributed, 35 were completed and returned (response rate, 25.2%). Most programs were university-affiliated (71.4%) or community-affiliated (22.9%). The median number of residents was 12. The respondents had a median of 5 years’ experience in their role. Most respondents (65.7%) had no business training, although 20.0% had completed undergraduate business coursework, and 8.6% had attended formal seminars on business topics; 5.7% were self-taught on business topics.
Business Education Availability
Approximately half (51.4%) of programs offered business training to residents, primarily through seminars or lectures (94.4%) and take-home modules (16.7%). None of the programs offered a formal gap year during which residents could pursue a professional business degree. Most respondents thought business education during residency was important (82.8%) and that programs should implement more training (57.1%). When asked whether residents were competent to handle business aspects of dermatology upon graduation, most respondents disagreed somewhat (22.9%) or were neutral (40.0%).
Topics for Business Education
The most important topics identified for inclusion in a business curriculum were economics or finance (68.6%), management (68.6%), and health care policy or government (57.1%). Other identified topics included negotiation (40.0%), private equity involvement in health care (40.0%), strategy (11.4%), supply chain or operations (11.4%), marketing (2.9%), and technology (2.9%).
Comment
Residency programs and medical schools in the United States have started to integrate formal business training into their curricula; however, the state of business training in dermatology has not been characterized. Overall, this survey revealed largely positive perceptions about business education and identified a demand for more resources.
Whereas most PDs identified business education as important, only one half (51.4%) of the representative programs offered structured training. Notably, most PDs did not agree that graduating residents were competent to handle the business demands of dermatology practice. These responses highlight a gap in the demand and resources available for business training.
Identifying Curricular Resources
During an already demanding residency, additional curricular components need to be beneficial and worthwhile. To avoid significant disruption, business training could take place in the form of online lectures or take-home modules. Most programs represented in the survey responses had an academic affiliation and therefore commonly have access to an affiliated graduate business school and/or hospital administrators who have clinical and business training.
Community dermatologists who own or run their own practice also are uniquely positioned to provide residents with practical, dermatology-specific business education. Programs can utilize their institutional and local colleagues to aid in curricular design and implementation. In addition, a potential long-term solution to obtaining resources for business education is to coordinate with a national dermatology organization to create standardized modules that are available to all residency programs.
Key Curriculum Topics
Our survey identified the most important topics to include in a business curriculum for dermatology residents. Economics and finance, management, and health care policy would be valuable to a trainee regardless of whether they ultimately choose a career in academia or private practice. A thorough understanding of complex health care policy reinforces knowledge about insurance and regional and national regulations, which could ultimately benefit patient care. As an example, the American Academy of Dermatology outlines several advocacy priorities such as Medicare reimbursement policies, access to dermatologic care through public and private insurance, medication access and pricing, and preservation of private practice in the setting of market consolidation. Having a better understanding of health care policy and business could better equip dermatologists to lead these often business-driven advocacy efforts to ultimately improve patient care and advance the specialty.8
Limitations
There were notable limitations to this survey, primarily related to its design. With a 25% response rate, there was the potential for response and selection biases; therefore, these results might not be generalizable to all programs. In addition, views held by PDs might not be consistent with those of other members of the dermatology community; for example, surveying residents, other faculty members, and dermatologists in private practice would have provided a more comprehensive characterization of the topic.
Conclusion
This study assessed residency program directors’ perceptions of business education in dermatology training. There appears to be an imbalance between the perceived importance of such education and the resources that are available to provide it. More attention is needed to address this gap to ensure that dermatologists are prepared to manage a rapidly changing health care environment. Results of this survey should encourage efforts to establish (1) a standardized, dermatology-specific business curriculum and (2) a plan to make that curriculum accessible to trainees and other members of the dermatology community.
- Branning G, Vater M. Healthcare spending: plenty of blame to go around. Am Health Drug Benefits. 2016;9:445-447.
- Bayard M, Peeples CR, Holt J, et al. An interactive approach to teaching practice management to family practice residents. Fam Med. 2003;35:622-624.
- Chan S. Management education during radiology residency: development of an educational practice. Acad Radiol. 2004;11:1308-1317.
- Ninan D, Patel D. Career and leadership education in anesthesia residency training. Cureus. 2018;10:e2546.
- Yu-Chin R. Teaching administration and management within psychiatric residency training. Acad Psychiatry. 2002;26:245-252.
- Winkelman JW, Brugnara C. Management training for pathology residents. II. experience with a focused curriculum. Am J Clin Pathol. 1994;101:564-568.
- Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021.
- Academy advocacy priorities. American Academy of Dermatology website. Accessed August 11, 2021. www.aad.org/member/advocacy/priorities
Globally, the United States has the highest per-capita cost of health care; total costs are expected to account for approximately 20% of the nation’s gross domestic product by 2025.1 These rising costs have prompted residency programs and medical schools to incorporate business education into their curricula.2-5 Although medical training is demanding—with little room to add curricular components—these business-focused curricula have consistently received positive feedback from residents.5,6
In dermatology, more than 50% of residents opt to join a private practice upon graduation.7 In the United States, there also is an upward trend of practice acquisition and consolidation by private equity firms. Therefore, dermatology trainees are uniquely positioned to benefit from business education to make well-informed decisions about joining or starting a practice.Furthermore, whether in a private or academic setting, knowledge of foundational economics, business strategy, finance, marketing, and health care policy can equip dermatologists to more effectively advocate for local and national policies that benefit their patient population.7
We conducted a survey of dermatology program directors (PDs) to determine the availability of and perceptions regarding business education during residency training.
Materials and Methods
Institutional review board (Vanderbilt University Medical Center, Nashville, Tennessee) approval was obtained. The survey was distributed weekly during a 5-week period from July 2020 to August 2020 through the Research Electronic Data Capture survey application (www.project-redcap.org). Program director email addresses were obtained through the Accreditation Council for Graduate Medical Education (ACGME) program list. A PD was included in the survey if they were employed by an accredited US osteopathic or allopathic program and their email address was provided in the ACGME program list or on their program’s faculty web page; a PD was excluded if an email address was not provided in the ACGME program list or on their program’s faculty web page.
The 8-part questionnaire was designed to assess the following characteristics: details about the respondent’s residency program (institutional affiliation, number of residents), the respondent’s professional background (number of years as a PD, business training experience), resources for business education provided by the program, the respondent’s opinion about business education for residents, and the respondent’s perception of the most important topics to include in a dermatology curriculum’s business education component, which included economics/finance, health care policy/government, management, marketing, negotiation, private equity involvement in health care, business strategy, supply chain/operations, and technology/product development. Responses were kept anonymous. Categorical and continuous variables were analyzed with medians and proportions.
Results
Of the 139 surveys distributed, 35 were completed and returned (response rate, 25.2%). Most programs were university-affiliated (71.4%) or community-affiliated (22.9%). The median number of residents was 12. The respondents had a median of 5 years’ experience in their role. Most respondents (65.7%) had no business training, although 20.0% had completed undergraduate business coursework, and 8.6% had attended formal seminars on business topics; 5.7% were self-taught on business topics.
Business Education Availability
Approximately half (51.4%) of programs offered business training to residents, primarily through seminars or lectures (94.4%) and take-home modules (16.7%). None of the programs offered a formal gap year during which residents could pursue a professional business degree. Most respondents thought business education during residency was important (82.8%) and that programs should implement more training (57.1%). When asked whether residents were competent to handle business aspects of dermatology upon graduation, most respondents disagreed somewhat (22.9%) or were neutral (40.0%).
Topics for Business Education
The most important topics identified for inclusion in a business curriculum were economics or finance (68.6%), management (68.6%), and health care policy or government (57.1%). Other identified topics included negotiation (40.0%), private equity involvement in health care (40.0%), strategy (11.4%), supply chain or operations (11.4%), marketing (2.9%), and technology (2.9%).
Comment
Residency programs and medical schools in the United States have started to integrate formal business training into their curricula; however, the state of business training in dermatology has not been characterized. Overall, this survey revealed largely positive perceptions about business education and identified a demand for more resources.
Whereas most PDs identified business education as important, only one half (51.4%) of the representative programs offered structured training. Notably, most PDs did not agree that graduating residents were competent to handle the business demands of dermatology practice. These responses highlight a gap in the demand and resources available for business training.
Identifying Curricular Resources
During an already demanding residency, additional curricular components need to be beneficial and worthwhile. To avoid significant disruption, business training could take place in the form of online lectures or take-home modules. Most programs represented in the survey responses had an academic affiliation and therefore commonly have access to an affiliated graduate business school and/or hospital administrators who have clinical and business training.
Community dermatologists who own or run their own practice also are uniquely positioned to provide residents with practical, dermatology-specific business education. Programs can utilize their institutional and local colleagues to aid in curricular design and implementation. In addition, a potential long-term solution to obtaining resources for business education is to coordinate with a national dermatology organization to create standardized modules that are available to all residency programs.
Key Curriculum Topics
Our survey identified the most important topics to include in a business curriculum for dermatology residents. Economics and finance, management, and health care policy would be valuable to a trainee regardless of whether they ultimately choose a career in academia or private practice. A thorough understanding of complex health care policy reinforces knowledge about insurance and regional and national regulations, which could ultimately benefit patient care. As an example, the American Academy of Dermatology outlines several advocacy priorities such as Medicare reimbursement policies, access to dermatologic care through public and private insurance, medication access and pricing, and preservation of private practice in the setting of market consolidation. Having a better understanding of health care policy and business could better equip dermatologists to lead these often business-driven advocacy efforts to ultimately improve patient care and advance the specialty.8
Limitations
There were notable limitations to this survey, primarily related to its design. With a 25% response rate, there was the potential for response and selection biases; therefore, these results might not be generalizable to all programs. In addition, views held by PDs might not be consistent with those of other members of the dermatology community; for example, surveying residents, other faculty members, and dermatologists in private practice would have provided a more comprehensive characterization of the topic.
Conclusion
This study assessed residency program directors’ perceptions of business education in dermatology training. There appears to be an imbalance between the perceived importance of such education and the resources that are available to provide it. More attention is needed to address this gap to ensure that dermatologists are prepared to manage a rapidly changing health care environment. Results of this survey should encourage efforts to establish (1) a standardized, dermatology-specific business curriculum and (2) a plan to make that curriculum accessible to trainees and other members of the dermatology community.
Globally, the United States has the highest per-capita cost of health care; total costs are expected to account for approximately 20% of the nation’s gross domestic product by 2025.1 These rising costs have prompted residency programs and medical schools to incorporate business education into their curricula.2-5 Although medical training is demanding—with little room to add curricular components—these business-focused curricula have consistently received positive feedback from residents.5,6
In dermatology, more than 50% of residents opt to join a private practice upon graduation.7 In the United States, there also is an upward trend of practice acquisition and consolidation by private equity firms. Therefore, dermatology trainees are uniquely positioned to benefit from business education to make well-informed decisions about joining or starting a practice.Furthermore, whether in a private or academic setting, knowledge of foundational economics, business strategy, finance, marketing, and health care policy can equip dermatologists to more effectively advocate for local and national policies that benefit their patient population.7
We conducted a survey of dermatology program directors (PDs) to determine the availability of and perceptions regarding business education during residency training.
Materials and Methods
Institutional review board (Vanderbilt University Medical Center, Nashville, Tennessee) approval was obtained. The survey was distributed weekly during a 5-week period from July 2020 to August 2020 through the Research Electronic Data Capture survey application (www.project-redcap.org). Program director email addresses were obtained through the Accreditation Council for Graduate Medical Education (ACGME) program list. A PD was included in the survey if they were employed by an accredited US osteopathic or allopathic program and their email address was provided in the ACGME program list or on their program’s faculty web page; a PD was excluded if an email address was not provided in the ACGME program list or on their program’s faculty web page.
The 8-part questionnaire was designed to assess the following characteristics: details about the respondent’s residency program (institutional affiliation, number of residents), the respondent’s professional background (number of years as a PD, business training experience), resources for business education provided by the program, the respondent’s opinion about business education for residents, and the respondent’s perception of the most important topics to include in a dermatology curriculum’s business education component, which included economics/finance, health care policy/government, management, marketing, negotiation, private equity involvement in health care, business strategy, supply chain/operations, and technology/product development. Responses were kept anonymous. Categorical and continuous variables were analyzed with medians and proportions.
Results
Of the 139 surveys distributed, 35 were completed and returned (response rate, 25.2%). Most programs were university-affiliated (71.4%) or community-affiliated (22.9%). The median number of residents was 12. The respondents had a median of 5 years’ experience in their role. Most respondents (65.7%) had no business training, although 20.0% had completed undergraduate business coursework, and 8.6% had attended formal seminars on business topics; 5.7% were self-taught on business topics.
Business Education Availability
Approximately half (51.4%) of programs offered business training to residents, primarily through seminars or lectures (94.4%) and take-home modules (16.7%). None of the programs offered a formal gap year during which residents could pursue a professional business degree. Most respondents thought business education during residency was important (82.8%) and that programs should implement more training (57.1%). When asked whether residents were competent to handle business aspects of dermatology upon graduation, most respondents disagreed somewhat (22.9%) or were neutral (40.0%).
Topics for Business Education
The most important topics identified for inclusion in a business curriculum were economics or finance (68.6%), management (68.6%), and health care policy or government (57.1%). Other identified topics included negotiation (40.0%), private equity involvement in health care (40.0%), strategy (11.4%), supply chain or operations (11.4%), marketing (2.9%), and technology (2.9%).
Comment
Residency programs and medical schools in the United States have started to integrate formal business training into their curricula; however, the state of business training in dermatology has not been characterized. Overall, this survey revealed largely positive perceptions about business education and identified a demand for more resources.
Whereas most PDs identified business education as important, only one half (51.4%) of the representative programs offered structured training. Notably, most PDs did not agree that graduating residents were competent to handle the business demands of dermatology practice. These responses highlight a gap in the demand and resources available for business training.
Identifying Curricular Resources
During an already demanding residency, additional curricular components need to be beneficial and worthwhile. To avoid significant disruption, business training could take place in the form of online lectures or take-home modules. Most programs represented in the survey responses had an academic affiliation and therefore commonly have access to an affiliated graduate business school and/or hospital administrators who have clinical and business training.
Community dermatologists who own or run their own practice also are uniquely positioned to provide residents with practical, dermatology-specific business education. Programs can utilize their institutional and local colleagues to aid in curricular design and implementation. In addition, a potential long-term solution to obtaining resources for business education is to coordinate with a national dermatology organization to create standardized modules that are available to all residency programs.
Key Curriculum Topics
Our survey identified the most important topics to include in a business curriculum for dermatology residents. Economics and finance, management, and health care policy would be valuable to a trainee regardless of whether they ultimately choose a career in academia or private practice. A thorough understanding of complex health care policy reinforces knowledge about insurance and regional and national regulations, which could ultimately benefit patient care. As an example, the American Academy of Dermatology outlines several advocacy priorities such as Medicare reimbursement policies, access to dermatologic care through public and private insurance, medication access and pricing, and preservation of private practice in the setting of market consolidation. Having a better understanding of health care policy and business could better equip dermatologists to lead these often business-driven advocacy efforts to ultimately improve patient care and advance the specialty.8
Limitations
There were notable limitations to this survey, primarily related to its design. With a 25% response rate, there was the potential for response and selection biases; therefore, these results might not be generalizable to all programs. In addition, views held by PDs might not be consistent with those of other members of the dermatology community; for example, surveying residents, other faculty members, and dermatologists in private practice would have provided a more comprehensive characterization of the topic.
Conclusion
This study assessed residency program directors’ perceptions of business education in dermatology training. There appears to be an imbalance between the perceived importance of such education and the resources that are available to provide it. More attention is needed to address this gap to ensure that dermatologists are prepared to manage a rapidly changing health care environment. Results of this survey should encourage efforts to establish (1) a standardized, dermatology-specific business curriculum and (2) a plan to make that curriculum accessible to trainees and other members of the dermatology community.
- Branning G, Vater M. Healthcare spending: plenty of blame to go around. Am Health Drug Benefits. 2016;9:445-447.
- Bayard M, Peeples CR, Holt J, et al. An interactive approach to teaching practice management to family practice residents. Fam Med. 2003;35:622-624.
- Chan S. Management education during radiology residency: development of an educational practice. Acad Radiol. 2004;11:1308-1317.
- Ninan D, Patel D. Career and leadership education in anesthesia residency training. Cureus. 2018;10:e2546.
- Yu-Chin R. Teaching administration and management within psychiatric residency training. Acad Psychiatry. 2002;26:245-252.
- Winkelman JW, Brugnara C. Management training for pathology residents. II. experience with a focused curriculum. Am J Clin Pathol. 1994;101:564-568.
- Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021.
- Academy advocacy priorities. American Academy of Dermatology website. Accessed August 11, 2021. www.aad.org/member/advocacy/priorities
- Branning G, Vater M. Healthcare spending: plenty of blame to go around. Am Health Drug Benefits. 2016;9:445-447.
- Bayard M, Peeples CR, Holt J, et al. An interactive approach to teaching practice management to family practice residents. Fam Med. 2003;35:622-624.
- Chan S. Management education during radiology residency: development of an educational practice. Acad Radiol. 2004;11:1308-1317.
- Ninan D, Patel D. Career and leadership education in anesthesia residency training. Cureus. 2018;10:e2546.
- Yu-Chin R. Teaching administration and management within psychiatric residency training. Acad Psychiatry. 2002;26:245-252.
- Winkelman JW, Brugnara C. Management training for pathology residents. II. experience with a focused curriculum. Am J Clin Pathol. 1994;101:564-568.
- Tan S, Seiger K, Renehan P, et al. Trends in private equity acquisition of dermatology practices in the United States. JAMA Dermatol. 2019;155:1013-1021.
- Academy advocacy priorities. American Academy of Dermatology website. Accessed August 11, 2021. www.aad.org/member/advocacy/priorities
Practice Points
- In our survey of dermatology program directors, most felt inclusion of business education in residency training was important.
- Approximately half of the dermatology programs that responded to our survey offer business training to their residents.
- Economics and finance, management, and health care policy were the most important topics identified to include in a business curriculum for dermatology residents
Translating the 2019 AAD-NPF Guidelines of Care for Psoriasis With Attention to Comorbidities
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.

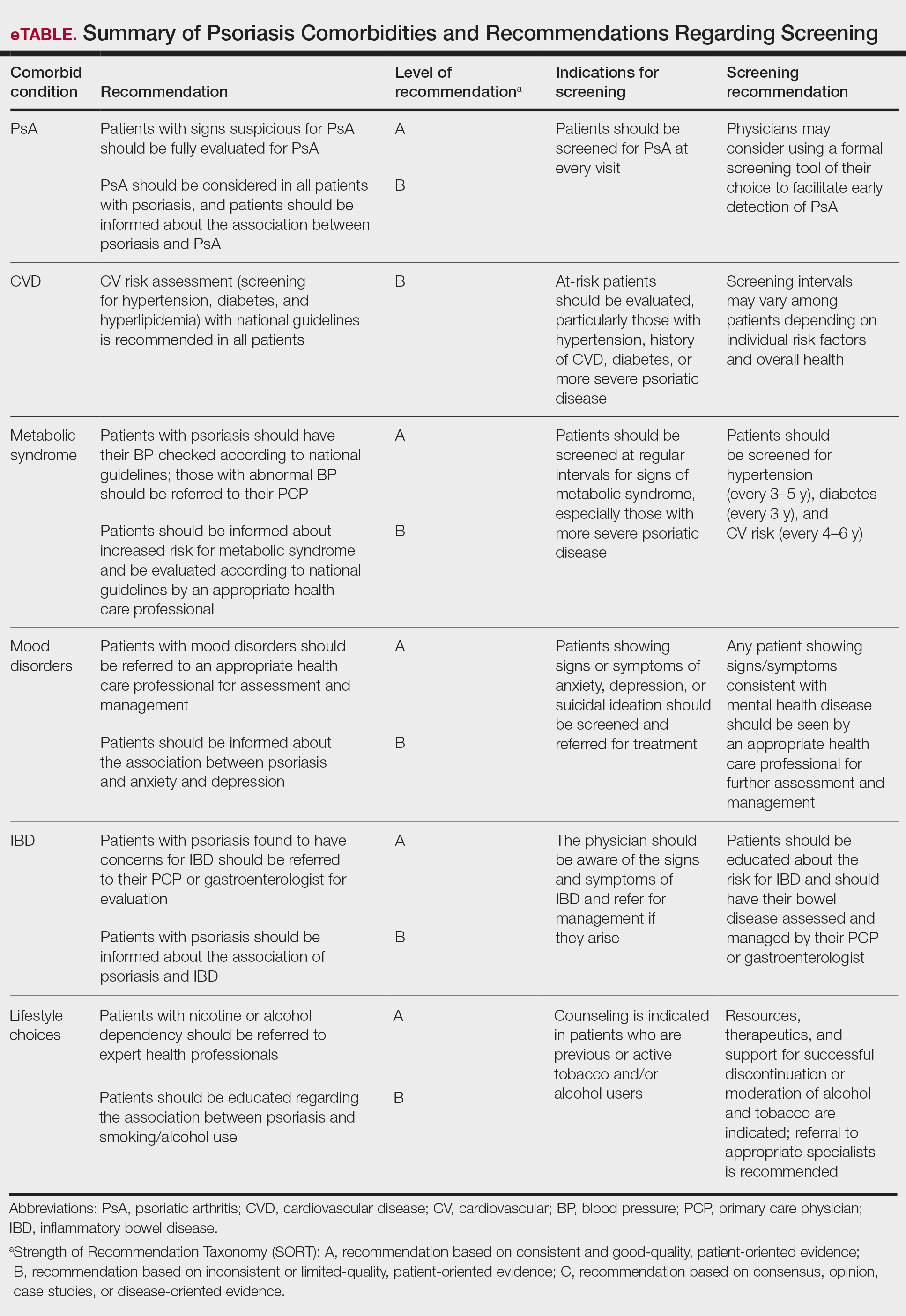
Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Practice Points
- Educating patients about psoriasis and its extracutaneous manifestations, available treatment options, and the impact of lifestyle choices is advised to maximize their patient’s disease awareness and to promote a collaborative physician-patient partnership.
- Physicians are strongly recommended to screen patients with psoriasis for the presence of disease comorbidities to ensure comprehensive management of their disease.
- Managing psoriasis as a multisystem inflammatory disorder requires the combined effort of dermatologists and other specialists to prevent and treat disease comorbidities and enhance patients’ quality of life.
Psoriatic Arthritis Diagnosis and Management in the Era of Telehealth
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
Update on Biologics for Psoriasis in Clinical Practice
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Practice Points
- Choosing a biologic best fit for each patient’s individual health profile can reduce psoriasis disease burden.
- Clinicians should educate psoriasis patients that biologics are safe for most comorbidities, and conditions such as obesity have been associated with poorer therapeutic response.
- It is important to discuss possible side effects of biologics with patients and reassure them that mild side effects are the most common during therapy.
Anecdote Increases Patient Willingness to Take a Biologic Medication for Psoriasis
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).
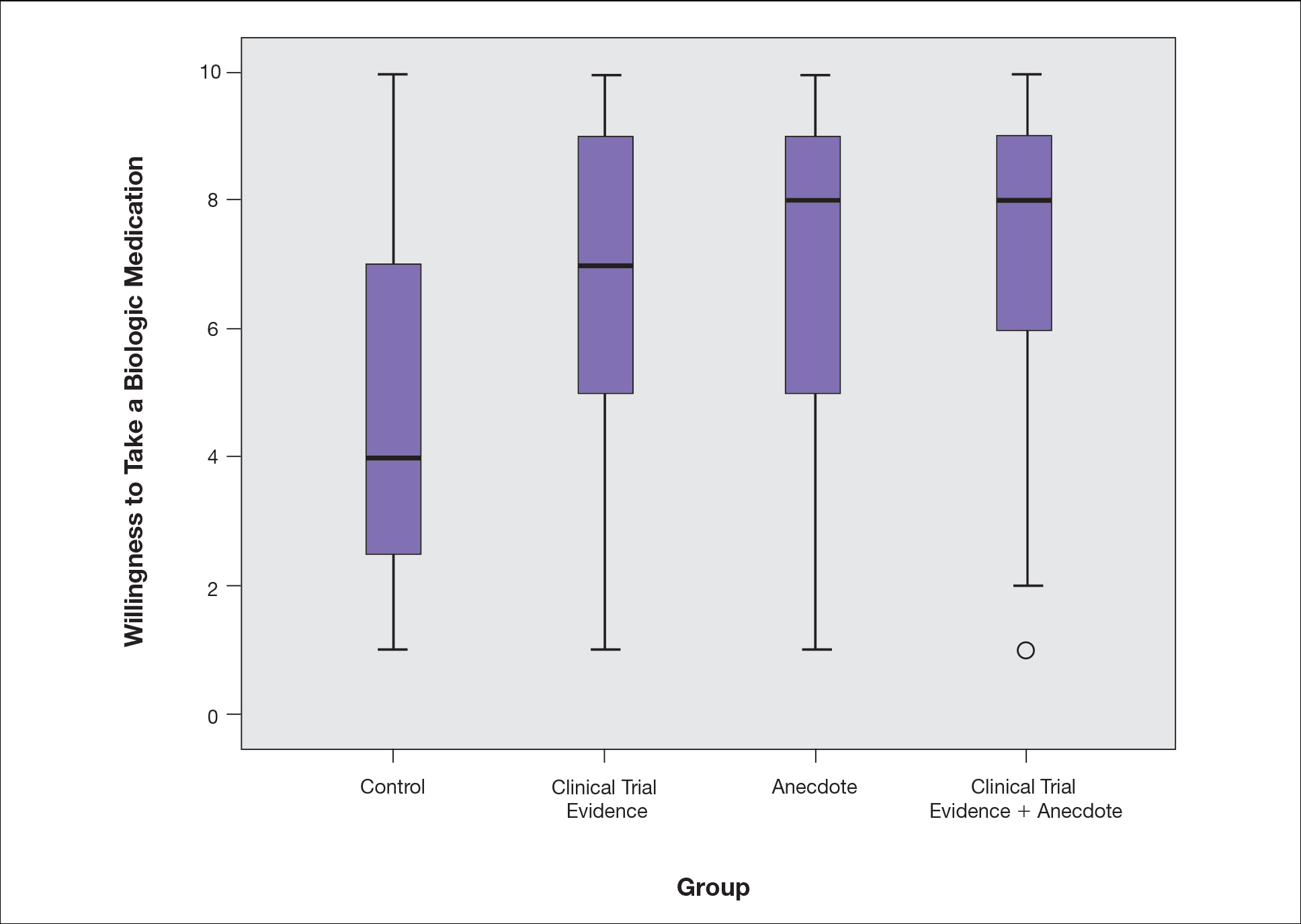
Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.
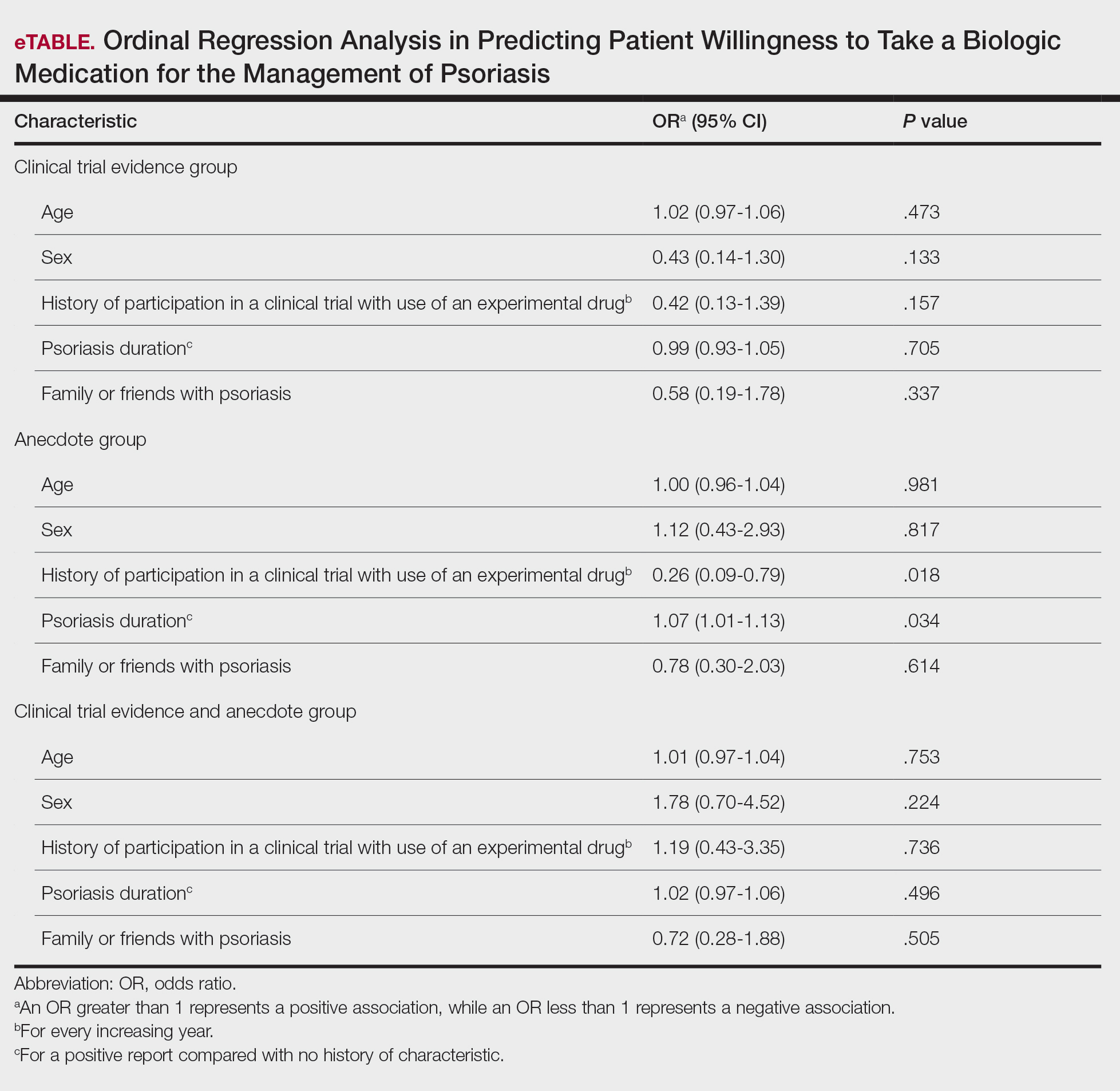
Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
Practice Points
- Patients often are apprehensive to start biologic medications for their psoriasis.
- Clinical trial evidence of a biologic medication’s efficacy and safety as well as anecdotes of patient experiences appear to be important factors for patients when considering taking a medication.
- The use of an anecdote—alone or in combination with clinical trial evidence—to help patients overcome fears of starting a biologic medication for their psoriasis may be an effective way to improve patients’ willingness to take treatment.
Ulcerated and Verrucous Plaque on the Chest
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
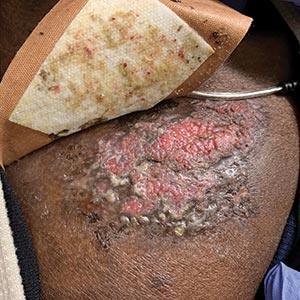
A 36-year-old man presented to an emergency department in the southwestern United States with a cough, fatigue, and worsening back pain associated with night sweats of 1 month’s duration. He experienced a 9.07-kg weight loss, as well as development of a rough, nontender, nonpruritic rash along the left upper chest over the prior month. The patient was born in West Africa and reported that he had moved to the southwestern United States from the eastern United States approximately 6 years prior to presentation. Physical examination on admission revealed a 5×3-cm, purple-brown, verrucous plaque with a central pink cobblestone appearance and ulceration. Chest radiography was notable for perihilar adenopathy with no focal infiltrates or cavitary lesions. Computed tomography and magnetic resonance imaging of the chest were notable for miliary nodules throughout the lungs; extensive lytic spine lesions of cervical, thoracic, and lumbar vertebral bodies and left twelfth rib; and a left paraspinal thoracic epidural soft tissue phlegmon. Initial laboratory investigations revealed peripheral eosinophilia without absolute leukocytosis and a microcytic anemia.
Bullous Retiform Purpura on the Ears and Legs
The Diagnosis: Levamisole-Induced Vasculopathy
Biopsy of one of the bullous retiform purpura on the leg (Figure 1) revealed a combined leukocytoclastic vasculitis and thrombotic vasculopathy (quiz images). Periodic acid-Schiff and Gram stains, with adequate controls, were negative for pathogenic fungal and bacterial organisms. Although this reaction pattern has an extensive differential, in this clinical setting with associated cocaine-positive urine toxicologic analysis, perinuclear antineutrophil cytoplasmic antibodies (p-ANCA), and leukopenia, the histopathologic findings were consistent with levamisole-induced vasculopathy (LIV).1,2 Although not specific, leukocytoclastic vasculitis and thrombotic vasculopathy have been reported as the classic histopathologic findings of LIV. In addition, interstitial and perivascular neovascularization have been reported as a potential histopathologic finding associated with this entity but was not seen in our case.3
Levamisole is an anthelminthic agent used to adulterate cocaine, a practice first noted in 2003 with increasing incidence.1 Both levamisole and cocaine stimulate the sympathetic nervous system by increasing dopamine in the euphoric areas of the brain.1,3 By combining the 2 substances, preparation costs are reduced and stimulant effects are enhanced. It is estimated that 69% to 80% of cocaine in the United States is contaminated with levamisole.2,4,5 The constellation of findings seen in patients abusing levamisole-contaminated cocaine include agranulocytosis; p-ANCA; and a tender, vasculitic, retiform purpura presentation. The most common sites for the purpura include the cheeks and ears. The purpura can progress to bullous lesions, as seen in our patient, followed by necrosis.4,6 Recurrent use of levamisole-contaminated cocaine is associated with recurrent agranulocytosis and classic skin findings, which is suggestive of a causal relationship.6
Serologic testing for levamisole exposure presents a challenge. The half-life of levamisole is relatively short (estimated at 5.6 hours) and is found in urine samples approximately 3% of the time.1,3,6 The volatile diagnostic characteristics of levamisole make concrete laboratory confirmation difficult. Although a skin biopsy can be helpful to rule out other causes of vasculitislike presentations, it is not specific for LIV. Therefore, clinical suspicion for LIV should remain high in patients who present with the cutaneous findings described as well as agranulocytosis, positive p-ANCA, and a history of cocaine use with a skin biopsy showing leukocytoclastic vasculitis and thrombotic vasculopathy.
The differential diagnosis for LIV with retiform bullous lesions includes several other vasculitides and vesiculobullous diseases. Eosinophilic granulomatosis with polyangiitis (EGPA) is a multisystem vasculitis that is characterized by eosinophilia, asthma, and rhinosinusitis. Eosinophilic granulomatosis with polyangiitis primarily affects small and medium arteries in the skin and respiratory tract and occurs in 3 stages: prodromal, eosinophilic, and vasculitic. These stages are characterized by mild asthma or rhinitis, eosinophilia with multiorgan infiltration, and vasculitis with extravascular granulomatosis, respectively. Diagnosis often is clinical based on these findings and laboratory evaluation. Eosinophilic granulomatosis with polyangiitis presents with positive p-ANCA in 40% to 60% of patients.7 The vasculitis stage of EGPA presents with cutaneous findings in 60% of cases, including palpable purpura, infiltrated papules and plaques, urticaria, necrotizing lesions, and rarely vesicles and bullae.8 Classic histopathologic features include leukocytoclastic or eosinophilic vasculitis, an eosinophilic infiltrate, granuloma formation, and eosinophilic granule deposition onto collagen fibrils (otherwise known as flame figures)(Figure 2). Biopsy of these lesions with the aforementioned findings, in constellation with the described systemic signs and symptoms, can aid in diagnosis of EGPA.
Polyarteritis nodosa (PAN) is a vasculitis that can be either multisystem or limited to one organ. Classic PAN affects the small- to medium-sized vessels. When there is multisystem involvement, it most often affects the skin, gastrointestinal tract, and kidneys. It presents with subcutaneous or dermal nodules, necrotic lesions, livedo reticularis, hypertension, abdominal pain, and an acute abdomen.9 When PAN is in its limited form, it most commonly occurs in the skin. The cutaneous manifestations of skin-limited PAN are identical to classic PAN, most commonly occurring on the legs and arms and less often on the trunk, head, and neck.10 To aid in diagnosis, biopsies of cutaneous lesions are beneficial. Dermatopathologic examination of PAN reveals fibrinoid necrosis of small and medium vessels with a perivascular mononuclear inflammatory infiltrate (Figure 3). Cutaneous PAN rarely progresses to multisystem classic PAN and carries a more favorable prognosis.
Microvascular occlusion syndromes can result in clinical presentations that resemble LIV. Idiopathic thrombocytopenic purpura is a hematologic autoimmune condition resulting in destruction of platelets and subsequent thrombocytopenia. Idiopathic thrombocytopenic purpura can be either primary or secondary to infections, drugs, malignancy, or other autoimmune conditions. Clinically, it presents as mucosal or cutaneous bleeding, epistaxis, hematochezia, or hematuria and can result in substantial hemorrhage. On the skin, it can appear as petechiae and ecchymoses in dependent areas and rarely hemorrhagic bullae of the skin and mucous membranes in cases of severe thrombocytopenia.11,12 Biopsies of these lesions will show notable extravasation of red blood cells with incipient hemorrhagic bullae formation (Figure 4). Recognition of hemorrhagic bullae as a presentation of idiopathic thrombocytopenic purpura is critical to identifying severe underlying disease.
Beyond other vasculitides and microvascular occlusion syndromes, vessel-invasive microorganisms can result in similar histopathologic and clinical presentations to LIV. Ecthyma gangrenosum (EG) is a septic vasculitis, often caused by Pseudomonas aeruginosa, usually affecting immunocompromised patients. Ecthyma gangrenosum presents with vesiculobullous lesions with erythematous violaceous borders that develop into hemorrhagic bullae with necrotic centers.13 Biopsy of EG will show vascular occlusion and basophilic granular material within or around vessels, suggestive of bacterial sepsis (Figure 5). The detection of an infectious agent on histopathology allows one to easily distinguish between EG and LIV.
- Bajaj S, Hibler B, Rossi A. Painful violaceous purpura on a 44-year-old woman. Am J Med. 2016;129:E5-E7.
- Munoz-Vahos CH, Herrera-Uribe S, Arbelaez-Cortes A, et al. Clinical profile of levamisole-adulterated cocaine-induced vasculitis/vasculopathy. J Clin Rheumatol. 2019;25:E16-E26.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Gillis JA, Green P, Williams J. Levamisole-induced vasculopathy: staging and management. J Plast Reconstr Aesthet Surg. 2014;67:E29-E31.
- Farhat EK, Muirhead TT, Chafins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;146:1320-1321.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia-a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2010;65:722-725.
- Negbenebor NA, Khalifian S, Foreman RK, et al. A 92-year-old male with eosinophilic asthma presenting with recurrent palpable purpuric plaques. Dermatopathology (Basel). 2018;5:44-48.
- Sherman S, Gal N, Didkovsky E, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) relapsing as bullous eruption. Acta Derm Venereol. 2017;97:406-407.
- Braungart S, Campbell A, Besarovic S. Atypical Henoch-Schonlein purpura? consider polyarteritis nodosa! BMJ Case Rep. 2014. doi:10.1136/bcr-2013-201764
- Alquorain NAA, Aljabr ASH, Alghamdi NJ. Cutaneous polyarteritis nodosa treated with pentoxifylline and clobetasol propionate: a case report. Saudi J Med Sci. 2018;6:104-107.
- Helms AE, Schaffer RI. Idiopathic thrombocytopenic purpura with black oral mucosal lesions. Cutis. 2007;79:456-458.
- Lountzis N, Maroon M, Tyler W. Mucocutaneous hemorrhagic bullae in idiopathic thrombocytopenic purpura. J Am Acad Dermatol. 2009;60:AB124.
- Llamas-Velasco M, Alegeria V, Santos-Briz A, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
The Diagnosis: Levamisole-Induced Vasculopathy
Biopsy of one of the bullous retiform purpura on the leg (Figure 1) revealed a combined leukocytoclastic vasculitis and thrombotic vasculopathy (quiz images). Periodic acid-Schiff and Gram stains, with adequate controls, were negative for pathogenic fungal and bacterial organisms. Although this reaction pattern has an extensive differential, in this clinical setting with associated cocaine-positive urine toxicologic analysis, perinuclear antineutrophil cytoplasmic antibodies (p-ANCA), and leukopenia, the histopathologic findings were consistent with levamisole-induced vasculopathy (LIV).1,2 Although not specific, leukocytoclastic vasculitis and thrombotic vasculopathy have been reported as the classic histopathologic findings of LIV. In addition, interstitial and perivascular neovascularization have been reported as a potential histopathologic finding associated with this entity but was not seen in our case.3
Levamisole is an anthelminthic agent used to adulterate cocaine, a practice first noted in 2003 with increasing incidence.1 Both levamisole and cocaine stimulate the sympathetic nervous system by increasing dopamine in the euphoric areas of the brain.1,3 By combining the 2 substances, preparation costs are reduced and stimulant effects are enhanced. It is estimated that 69% to 80% of cocaine in the United States is contaminated with levamisole.2,4,5 The constellation of findings seen in patients abusing levamisole-contaminated cocaine include agranulocytosis; p-ANCA; and a tender, vasculitic, retiform purpura presentation. The most common sites for the purpura include the cheeks and ears. The purpura can progress to bullous lesions, as seen in our patient, followed by necrosis.4,6 Recurrent use of levamisole-contaminated cocaine is associated with recurrent agranulocytosis and classic skin findings, which is suggestive of a causal relationship.6
Serologic testing for levamisole exposure presents a challenge. The half-life of levamisole is relatively short (estimated at 5.6 hours) and is found in urine samples approximately 3% of the time.1,3,6 The volatile diagnostic characteristics of levamisole make concrete laboratory confirmation difficult. Although a skin biopsy can be helpful to rule out other causes of vasculitislike presentations, it is not specific for LIV. Therefore, clinical suspicion for LIV should remain high in patients who present with the cutaneous findings described as well as agranulocytosis, positive p-ANCA, and a history of cocaine use with a skin biopsy showing leukocytoclastic vasculitis and thrombotic vasculopathy.
The differential diagnosis for LIV with retiform bullous lesions includes several other vasculitides and vesiculobullous diseases. Eosinophilic granulomatosis with polyangiitis (EGPA) is a multisystem vasculitis that is characterized by eosinophilia, asthma, and rhinosinusitis. Eosinophilic granulomatosis with polyangiitis primarily affects small and medium arteries in the skin and respiratory tract and occurs in 3 stages: prodromal, eosinophilic, and vasculitic. These stages are characterized by mild asthma or rhinitis, eosinophilia with multiorgan infiltration, and vasculitis with extravascular granulomatosis, respectively. Diagnosis often is clinical based on these findings and laboratory evaluation. Eosinophilic granulomatosis with polyangiitis presents with positive p-ANCA in 40% to 60% of patients.7 The vasculitis stage of EGPA presents with cutaneous findings in 60% of cases, including palpable purpura, infiltrated papules and plaques, urticaria, necrotizing lesions, and rarely vesicles and bullae.8 Classic histopathologic features include leukocytoclastic or eosinophilic vasculitis, an eosinophilic infiltrate, granuloma formation, and eosinophilic granule deposition onto collagen fibrils (otherwise known as flame figures)(Figure 2). Biopsy of these lesions with the aforementioned findings, in constellation with the described systemic signs and symptoms, can aid in diagnosis of EGPA.
Polyarteritis nodosa (PAN) is a vasculitis that can be either multisystem or limited to one organ. Classic PAN affects the small- to medium-sized vessels. When there is multisystem involvement, it most often affects the skin, gastrointestinal tract, and kidneys. It presents with subcutaneous or dermal nodules, necrotic lesions, livedo reticularis, hypertension, abdominal pain, and an acute abdomen.9 When PAN is in its limited form, it most commonly occurs in the skin. The cutaneous manifestations of skin-limited PAN are identical to classic PAN, most commonly occurring on the legs and arms and less often on the trunk, head, and neck.10 To aid in diagnosis, biopsies of cutaneous lesions are beneficial. Dermatopathologic examination of PAN reveals fibrinoid necrosis of small and medium vessels with a perivascular mononuclear inflammatory infiltrate (Figure 3). Cutaneous PAN rarely progresses to multisystem classic PAN and carries a more favorable prognosis.
Microvascular occlusion syndromes can result in clinical presentations that resemble LIV. Idiopathic thrombocytopenic purpura is a hematologic autoimmune condition resulting in destruction of platelets and subsequent thrombocytopenia. Idiopathic thrombocytopenic purpura can be either primary or secondary to infections, drugs, malignancy, or other autoimmune conditions. Clinically, it presents as mucosal or cutaneous bleeding, epistaxis, hematochezia, or hematuria and can result in substantial hemorrhage. On the skin, it can appear as petechiae and ecchymoses in dependent areas and rarely hemorrhagic bullae of the skin and mucous membranes in cases of severe thrombocytopenia.11,12 Biopsies of these lesions will show notable extravasation of red blood cells with incipient hemorrhagic bullae formation (Figure 4). Recognition of hemorrhagic bullae as a presentation of idiopathic thrombocytopenic purpura is critical to identifying severe underlying disease.
Beyond other vasculitides and microvascular occlusion syndromes, vessel-invasive microorganisms can result in similar histopathologic and clinical presentations to LIV. Ecthyma gangrenosum (EG) is a septic vasculitis, often caused by Pseudomonas aeruginosa, usually affecting immunocompromised patients. Ecthyma gangrenosum presents with vesiculobullous lesions with erythematous violaceous borders that develop into hemorrhagic bullae with necrotic centers.13 Biopsy of EG will show vascular occlusion and basophilic granular material within or around vessels, suggestive of bacterial sepsis (Figure 5). The detection of an infectious agent on histopathology allows one to easily distinguish between EG and LIV.
The Diagnosis: Levamisole-Induced Vasculopathy
Biopsy of one of the bullous retiform purpura on the leg (Figure 1) revealed a combined leukocytoclastic vasculitis and thrombotic vasculopathy (quiz images). Periodic acid-Schiff and Gram stains, with adequate controls, were negative for pathogenic fungal and bacterial organisms. Although this reaction pattern has an extensive differential, in this clinical setting with associated cocaine-positive urine toxicologic analysis, perinuclear antineutrophil cytoplasmic antibodies (p-ANCA), and leukopenia, the histopathologic findings were consistent with levamisole-induced vasculopathy (LIV).1,2 Although not specific, leukocytoclastic vasculitis and thrombotic vasculopathy have been reported as the classic histopathologic findings of LIV. In addition, interstitial and perivascular neovascularization have been reported as a potential histopathologic finding associated with this entity but was not seen in our case.3
Levamisole is an anthelminthic agent used to adulterate cocaine, a practice first noted in 2003 with increasing incidence.1 Both levamisole and cocaine stimulate the sympathetic nervous system by increasing dopamine in the euphoric areas of the brain.1,3 By combining the 2 substances, preparation costs are reduced and stimulant effects are enhanced. It is estimated that 69% to 80% of cocaine in the United States is contaminated with levamisole.2,4,5 The constellation of findings seen in patients abusing levamisole-contaminated cocaine include agranulocytosis; p-ANCA; and a tender, vasculitic, retiform purpura presentation. The most common sites for the purpura include the cheeks and ears. The purpura can progress to bullous lesions, as seen in our patient, followed by necrosis.4,6 Recurrent use of levamisole-contaminated cocaine is associated with recurrent agranulocytosis and classic skin findings, which is suggestive of a causal relationship.6
Serologic testing for levamisole exposure presents a challenge. The half-life of levamisole is relatively short (estimated at 5.6 hours) and is found in urine samples approximately 3% of the time.1,3,6 The volatile diagnostic characteristics of levamisole make concrete laboratory confirmation difficult. Although a skin biopsy can be helpful to rule out other causes of vasculitislike presentations, it is not specific for LIV. Therefore, clinical suspicion for LIV should remain high in patients who present with the cutaneous findings described as well as agranulocytosis, positive p-ANCA, and a history of cocaine use with a skin biopsy showing leukocytoclastic vasculitis and thrombotic vasculopathy.
The differential diagnosis for LIV with retiform bullous lesions includes several other vasculitides and vesiculobullous diseases. Eosinophilic granulomatosis with polyangiitis (EGPA) is a multisystem vasculitis that is characterized by eosinophilia, asthma, and rhinosinusitis. Eosinophilic granulomatosis with polyangiitis primarily affects small and medium arteries in the skin and respiratory tract and occurs in 3 stages: prodromal, eosinophilic, and vasculitic. These stages are characterized by mild asthma or rhinitis, eosinophilia with multiorgan infiltration, and vasculitis with extravascular granulomatosis, respectively. Diagnosis often is clinical based on these findings and laboratory evaluation. Eosinophilic granulomatosis with polyangiitis presents with positive p-ANCA in 40% to 60% of patients.7 The vasculitis stage of EGPA presents with cutaneous findings in 60% of cases, including palpable purpura, infiltrated papules and plaques, urticaria, necrotizing lesions, and rarely vesicles and bullae.8 Classic histopathologic features include leukocytoclastic or eosinophilic vasculitis, an eosinophilic infiltrate, granuloma formation, and eosinophilic granule deposition onto collagen fibrils (otherwise known as flame figures)(Figure 2). Biopsy of these lesions with the aforementioned findings, in constellation with the described systemic signs and symptoms, can aid in diagnosis of EGPA.
Polyarteritis nodosa (PAN) is a vasculitis that can be either multisystem or limited to one organ. Classic PAN affects the small- to medium-sized vessels. When there is multisystem involvement, it most often affects the skin, gastrointestinal tract, and kidneys. It presents with subcutaneous or dermal nodules, necrotic lesions, livedo reticularis, hypertension, abdominal pain, and an acute abdomen.9 When PAN is in its limited form, it most commonly occurs in the skin. The cutaneous manifestations of skin-limited PAN are identical to classic PAN, most commonly occurring on the legs and arms and less often on the trunk, head, and neck.10 To aid in diagnosis, biopsies of cutaneous lesions are beneficial. Dermatopathologic examination of PAN reveals fibrinoid necrosis of small and medium vessels with a perivascular mononuclear inflammatory infiltrate (Figure 3). Cutaneous PAN rarely progresses to multisystem classic PAN and carries a more favorable prognosis.
Microvascular occlusion syndromes can result in clinical presentations that resemble LIV. Idiopathic thrombocytopenic purpura is a hematologic autoimmune condition resulting in destruction of platelets and subsequent thrombocytopenia. Idiopathic thrombocytopenic purpura can be either primary or secondary to infections, drugs, malignancy, or other autoimmune conditions. Clinically, it presents as mucosal or cutaneous bleeding, epistaxis, hematochezia, or hematuria and can result in substantial hemorrhage. On the skin, it can appear as petechiae and ecchymoses in dependent areas and rarely hemorrhagic bullae of the skin and mucous membranes in cases of severe thrombocytopenia.11,12 Biopsies of these lesions will show notable extravasation of red blood cells with incipient hemorrhagic bullae formation (Figure 4). Recognition of hemorrhagic bullae as a presentation of idiopathic thrombocytopenic purpura is critical to identifying severe underlying disease.
Beyond other vasculitides and microvascular occlusion syndromes, vessel-invasive microorganisms can result in similar histopathologic and clinical presentations to LIV. Ecthyma gangrenosum (EG) is a septic vasculitis, often caused by Pseudomonas aeruginosa, usually affecting immunocompromised patients. Ecthyma gangrenosum presents with vesiculobullous lesions with erythematous violaceous borders that develop into hemorrhagic bullae with necrotic centers.13 Biopsy of EG will show vascular occlusion and basophilic granular material within or around vessels, suggestive of bacterial sepsis (Figure 5). The detection of an infectious agent on histopathology allows one to easily distinguish between EG and LIV.
- Bajaj S, Hibler B, Rossi A. Painful violaceous purpura on a 44-year-old woman. Am J Med. 2016;129:E5-E7.
- Munoz-Vahos CH, Herrera-Uribe S, Arbelaez-Cortes A, et al. Clinical profile of levamisole-adulterated cocaine-induced vasculitis/vasculopathy. J Clin Rheumatol. 2019;25:E16-E26.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Gillis JA, Green P, Williams J. Levamisole-induced vasculopathy: staging and management. J Plast Reconstr Aesthet Surg. 2014;67:E29-E31.
- Farhat EK, Muirhead TT, Chafins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;146:1320-1321.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia-a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2010;65:722-725.
- Negbenebor NA, Khalifian S, Foreman RK, et al. A 92-year-old male with eosinophilic asthma presenting with recurrent palpable purpuric plaques. Dermatopathology (Basel). 2018;5:44-48.
- Sherman S, Gal N, Didkovsky E, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) relapsing as bullous eruption. Acta Derm Venereol. 2017;97:406-407.
- Braungart S, Campbell A, Besarovic S. Atypical Henoch-Schonlein purpura? consider polyarteritis nodosa! BMJ Case Rep. 2014. doi:10.1136/bcr-2013-201764
- Alquorain NAA, Aljabr ASH, Alghamdi NJ. Cutaneous polyarteritis nodosa treated with pentoxifylline and clobetasol propionate: a case report. Saudi J Med Sci. 2018;6:104-107.
- Helms AE, Schaffer RI. Idiopathic thrombocytopenic purpura with black oral mucosal lesions. Cutis. 2007;79:456-458.
- Lountzis N, Maroon M, Tyler W. Mucocutaneous hemorrhagic bullae in idiopathic thrombocytopenic purpura. J Am Acad Dermatol. 2009;60:AB124.
- Llamas-Velasco M, Alegeria V, Santos-Briz A, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Bajaj S, Hibler B, Rossi A. Painful violaceous purpura on a 44-year-old woman. Am J Med. 2016;129:E5-E7.
- Munoz-Vahos CH, Herrera-Uribe S, Arbelaez-Cortes A, et al. Clinical profile of levamisole-adulterated cocaine-induced vasculitis/vasculopathy. J Clin Rheumatol. 2019;25:E16-E26.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Gillis JA, Green P, Williams J. Levamisole-induced vasculopathy: staging and management. J Plast Reconstr Aesthet Surg. 2014;67:E29-E31.
- Farhat EK, Muirhead TT, Chafins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;146:1320-1321.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia-a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2010;65:722-725.
- Negbenebor NA, Khalifian S, Foreman RK, et al. A 92-year-old male with eosinophilic asthma presenting with recurrent palpable purpuric plaques. Dermatopathology (Basel). 2018;5:44-48.
- Sherman S, Gal N, Didkovsky E, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) relapsing as bullous eruption. Acta Derm Venereol. 2017;97:406-407.
- Braungart S, Campbell A, Besarovic S. Atypical Henoch-Schonlein purpura? consider polyarteritis nodosa! BMJ Case Rep. 2014. doi:10.1136/bcr-2013-201764
- Alquorain NAA, Aljabr ASH, Alghamdi NJ. Cutaneous polyarteritis nodosa treated with pentoxifylline and clobetasol propionate: a case report. Saudi J Med Sci. 2018;6:104-107.
- Helms AE, Schaffer RI. Idiopathic thrombocytopenic purpura with black oral mucosal lesions. Cutis. 2007;79:456-458.
- Lountzis N, Maroon M, Tyler W. Mucocutaneous hemorrhagic bullae in idiopathic thrombocytopenic purpura. J Am Acad Dermatol. 2009;60:AB124.
- Llamas-Velasco M, Alegeria V, Santos-Briz A, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
A 40-year-old woman presented with a progressive painful rash on the ears and legs of 2 weeks’ duration. She described the rash as initially red and nonpainful; it started on the right leg and progressed to the left leg, eventually involving the earlobes 4 days prior to presentation. Physical examination revealed edematous purpura of the earlobes and bullous retiform purpura on the lower extremities. Laboratory studies revealed leukopenia (3.6×103 /cm2 [reference range, 4.0–10.5×103 /cm2 ]) and elevated antineutrophil cytoplasmic antibodies (1:320 titer [reference range, <1:40]) in a perinuclear pattern (perinuclear antineutrophil cytoplasmic antibodies). Urine toxicology screening was positive for cocaine and opiates. A punch biopsy of a bullous retiform purpura on the right thigh was obtained for standard hematoxylin and eosin staining.