User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Chlorthalidone, HCTZ equally effective in hypertension: DCP published
The Diuretic Comparison Project (DCP) trial, showing no difference in reduction of clinical events between the thiazide diuretics chlorthalidone and hydrochlorothiazide when used for the treatment of hypertension, has now been published.
The trial was first presented at the 2022 annual scientific sessions of the American Heart Association.
In the current paper, published online in the New England Journal of Medicine, the authors, led by Areef Ishani, MD, Minneapolis Veterans Affairs Health Care System, explained that early studies suggested that chlorthalidone was superior to hydrochlorothiazide in patients with hypertension, but more recent observational studies have shown that the two drugs reduced cardiovascular events at a similar rate. Chlorthalidone may be associated with an increased risk of adverse events, including hypokalemia.
They noted that, in 2020, Part D Medicare expenditures showed that approximately 1.5 million persons received prescriptions for chlorthalidone, compared with 11.5 million who received prescriptions for hydrochlorothiazide, despite guidelines that recommended chlorthalidone as the preferred agent. The discrepancy between guideline recommendation and real-world use is possibly related to the belief that chlorthalidone has a greater risk for adverse effects without clear evidence for differences in cardiovascular outcomes, the authors suggested.
They conducted the current study to directly compare the effect of the two agents on cardiovascular outcomes in patients with hypertension.
The pragmatic DCP trial was carried out within the VA Healthcare System, and randomly assigned 13,523 patients (mean age, 72.5 years) with hypertension who were receiving hydrochlorothiazide at baseline (25 or 50 mg per day) to continue hydrochlorothiazide at their baseline dose or to switch to chlorthalidone (12.5 or 25 mg per day).
The mean baseline systolic blood pressure was 139 mm Hg in both trial groups and did not change substantially during the trial.
Over a median follow-up of 2.4 years, there was no difference in the primary outcome – a composite of MI, stroke, hospitalization for heart failure, urgent coronary revascularization for unstable angina, and non–cancer-related death – between the chlorthalidone group (10.4%) and the hydrochlorothiazide group (10.0%), giving a hazard ratio of 1.04 (95% CI, 0.94-1.16; P = .45).
In addition, there were no treatment differences between the two groups in any primary outcome component. Hypokalemia and potassium supplement use were more common in the chlorthalidone group than in the hydrochlorothiazide group.
‘Importance lies in the design’
In an accompanying editorial, Julie R. Ingelfinger, MD, deputy editor of the New England Journal of Medicine, said the results are not surprising and may not change clinical practice. But she suggested that the importance of the trial lies in its design, which shows that a high-quality pragmatic comparative effectiveness trial can be accomplished in a cost-effective manner within a health care system with little disruption in patient care.
Dr. Ingelfinger pointed out several limitations of the trial. These include a lower-than-expected occurrence of primary outcome events, and the stipulation that patients were eligible to participate only if they continued to have hypertension while receiving hydrochlorothiazide.
In addition, 95% of the participants were receiving 25 mg of hydrochlorothiazide and only 5% were receiving 50 mg, which limited comparisons of the dose generally used in practice. Also, only approximately 13% of the patients were receiving hydrochlorothiazide alone for the treatment of hypertension at baseline.
She noted that the DCP is the first head-to-head comparison of hydrochlorothiazide and chlorthalidone in a randomized, prospective outcome trial.
“Without an apparent difference in the hazard ratios for the primary outcome in the two groups over the median follow-up of 2.4 years, results suggest that chlorthalidone therapy remains a good choice for hypertension despite the secondary observation that hypokalemia was more common with chlorthalidone than with hydrochlorothiazide,” Dr. Ingelfinger said.
“Although a subgroup analysis suggested that chlorthalidone was better than hydrochlorothiazide for participants with a history of myocardial infarction or stroke, that result may have been by chance,” she added.
As clinicians generally prefer using hydrochlorothiazide, she suggested that these DCP results will not provide any impetus for change.
“Furthermore, combined therapy and polypills may alter therapy beyond the results of this well-done, highly anticipated trial. Thus, its major effect may be as a model for other pragmatic study programs, which are greatly needed,” she concluded.
This study was supported by the Veterans Affairs Cooperative Studies Program through a grant to the Diuretic Comparison Project. Dr. Ishani reported no relevant financial relationships. Dr. Ingelfinger reported book royalties from Springer and from St. Martin’s Press, outside the submitted work, and that she is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
The Diuretic Comparison Project (DCP) trial, showing no difference in reduction of clinical events between the thiazide diuretics chlorthalidone and hydrochlorothiazide when used for the treatment of hypertension, has now been published.
The trial was first presented at the 2022 annual scientific sessions of the American Heart Association.
In the current paper, published online in the New England Journal of Medicine, the authors, led by Areef Ishani, MD, Minneapolis Veterans Affairs Health Care System, explained that early studies suggested that chlorthalidone was superior to hydrochlorothiazide in patients with hypertension, but more recent observational studies have shown that the two drugs reduced cardiovascular events at a similar rate. Chlorthalidone may be associated with an increased risk of adverse events, including hypokalemia.
They noted that, in 2020, Part D Medicare expenditures showed that approximately 1.5 million persons received prescriptions for chlorthalidone, compared with 11.5 million who received prescriptions for hydrochlorothiazide, despite guidelines that recommended chlorthalidone as the preferred agent. The discrepancy between guideline recommendation and real-world use is possibly related to the belief that chlorthalidone has a greater risk for adverse effects without clear evidence for differences in cardiovascular outcomes, the authors suggested.
They conducted the current study to directly compare the effect of the two agents on cardiovascular outcomes in patients with hypertension.
The pragmatic DCP trial was carried out within the VA Healthcare System, and randomly assigned 13,523 patients (mean age, 72.5 years) with hypertension who were receiving hydrochlorothiazide at baseline (25 or 50 mg per day) to continue hydrochlorothiazide at their baseline dose or to switch to chlorthalidone (12.5 or 25 mg per day).
The mean baseline systolic blood pressure was 139 mm Hg in both trial groups and did not change substantially during the trial.
Over a median follow-up of 2.4 years, there was no difference in the primary outcome – a composite of MI, stroke, hospitalization for heart failure, urgent coronary revascularization for unstable angina, and non–cancer-related death – between the chlorthalidone group (10.4%) and the hydrochlorothiazide group (10.0%), giving a hazard ratio of 1.04 (95% CI, 0.94-1.16; P = .45).
In addition, there were no treatment differences between the two groups in any primary outcome component. Hypokalemia and potassium supplement use were more common in the chlorthalidone group than in the hydrochlorothiazide group.
‘Importance lies in the design’
In an accompanying editorial, Julie R. Ingelfinger, MD, deputy editor of the New England Journal of Medicine, said the results are not surprising and may not change clinical practice. But she suggested that the importance of the trial lies in its design, which shows that a high-quality pragmatic comparative effectiveness trial can be accomplished in a cost-effective manner within a health care system with little disruption in patient care.
Dr. Ingelfinger pointed out several limitations of the trial. These include a lower-than-expected occurrence of primary outcome events, and the stipulation that patients were eligible to participate only if they continued to have hypertension while receiving hydrochlorothiazide.
In addition, 95% of the participants were receiving 25 mg of hydrochlorothiazide and only 5% were receiving 50 mg, which limited comparisons of the dose generally used in practice. Also, only approximately 13% of the patients were receiving hydrochlorothiazide alone for the treatment of hypertension at baseline.
She noted that the DCP is the first head-to-head comparison of hydrochlorothiazide and chlorthalidone in a randomized, prospective outcome trial.
“Without an apparent difference in the hazard ratios for the primary outcome in the two groups over the median follow-up of 2.4 years, results suggest that chlorthalidone therapy remains a good choice for hypertension despite the secondary observation that hypokalemia was more common with chlorthalidone than with hydrochlorothiazide,” Dr. Ingelfinger said.
“Although a subgroup analysis suggested that chlorthalidone was better than hydrochlorothiazide for participants with a history of myocardial infarction or stroke, that result may have been by chance,” she added.
As clinicians generally prefer using hydrochlorothiazide, she suggested that these DCP results will not provide any impetus for change.
“Furthermore, combined therapy and polypills may alter therapy beyond the results of this well-done, highly anticipated trial. Thus, its major effect may be as a model for other pragmatic study programs, which are greatly needed,” she concluded.
This study was supported by the Veterans Affairs Cooperative Studies Program through a grant to the Diuretic Comparison Project. Dr. Ishani reported no relevant financial relationships. Dr. Ingelfinger reported book royalties from Springer and from St. Martin’s Press, outside the submitted work, and that she is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
The Diuretic Comparison Project (DCP) trial, showing no difference in reduction of clinical events between the thiazide diuretics chlorthalidone and hydrochlorothiazide when used for the treatment of hypertension, has now been published.
The trial was first presented at the 2022 annual scientific sessions of the American Heart Association.
In the current paper, published online in the New England Journal of Medicine, the authors, led by Areef Ishani, MD, Minneapolis Veterans Affairs Health Care System, explained that early studies suggested that chlorthalidone was superior to hydrochlorothiazide in patients with hypertension, but more recent observational studies have shown that the two drugs reduced cardiovascular events at a similar rate. Chlorthalidone may be associated with an increased risk of adverse events, including hypokalemia.
They noted that, in 2020, Part D Medicare expenditures showed that approximately 1.5 million persons received prescriptions for chlorthalidone, compared with 11.5 million who received prescriptions for hydrochlorothiazide, despite guidelines that recommended chlorthalidone as the preferred agent. The discrepancy between guideline recommendation and real-world use is possibly related to the belief that chlorthalidone has a greater risk for adverse effects without clear evidence for differences in cardiovascular outcomes, the authors suggested.
They conducted the current study to directly compare the effect of the two agents on cardiovascular outcomes in patients with hypertension.
The pragmatic DCP trial was carried out within the VA Healthcare System, and randomly assigned 13,523 patients (mean age, 72.5 years) with hypertension who were receiving hydrochlorothiazide at baseline (25 or 50 mg per day) to continue hydrochlorothiazide at their baseline dose or to switch to chlorthalidone (12.5 or 25 mg per day).
The mean baseline systolic blood pressure was 139 mm Hg in both trial groups and did not change substantially during the trial.
Over a median follow-up of 2.4 years, there was no difference in the primary outcome – a composite of MI, stroke, hospitalization for heart failure, urgent coronary revascularization for unstable angina, and non–cancer-related death – between the chlorthalidone group (10.4%) and the hydrochlorothiazide group (10.0%), giving a hazard ratio of 1.04 (95% CI, 0.94-1.16; P = .45).
In addition, there were no treatment differences between the two groups in any primary outcome component. Hypokalemia and potassium supplement use were more common in the chlorthalidone group than in the hydrochlorothiazide group.
‘Importance lies in the design’
In an accompanying editorial, Julie R. Ingelfinger, MD, deputy editor of the New England Journal of Medicine, said the results are not surprising and may not change clinical practice. But she suggested that the importance of the trial lies in its design, which shows that a high-quality pragmatic comparative effectiveness trial can be accomplished in a cost-effective manner within a health care system with little disruption in patient care.
Dr. Ingelfinger pointed out several limitations of the trial. These include a lower-than-expected occurrence of primary outcome events, and the stipulation that patients were eligible to participate only if they continued to have hypertension while receiving hydrochlorothiazide.
In addition, 95% of the participants were receiving 25 mg of hydrochlorothiazide and only 5% were receiving 50 mg, which limited comparisons of the dose generally used in practice. Also, only approximately 13% of the patients were receiving hydrochlorothiazide alone for the treatment of hypertension at baseline.
She noted that the DCP is the first head-to-head comparison of hydrochlorothiazide and chlorthalidone in a randomized, prospective outcome trial.
“Without an apparent difference in the hazard ratios for the primary outcome in the two groups over the median follow-up of 2.4 years, results suggest that chlorthalidone therapy remains a good choice for hypertension despite the secondary observation that hypokalemia was more common with chlorthalidone than with hydrochlorothiazide,” Dr. Ingelfinger said.
“Although a subgroup analysis suggested that chlorthalidone was better than hydrochlorothiazide for participants with a history of myocardial infarction or stroke, that result may have been by chance,” she added.
As clinicians generally prefer using hydrochlorothiazide, she suggested that these DCP results will not provide any impetus for change.
“Furthermore, combined therapy and polypills may alter therapy beyond the results of this well-done, highly anticipated trial. Thus, its major effect may be as a model for other pragmatic study programs, which are greatly needed,” she concluded.
This study was supported by the Veterans Affairs Cooperative Studies Program through a grant to the Diuretic Comparison Project. Dr. Ishani reported no relevant financial relationships. Dr. Ingelfinger reported book royalties from Springer and from St. Martin’s Press, outside the submitted work, and that she is employed by the New England Journal of Medicine as deputy editor.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Nitroglycerin’s safety and value examined
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
He has stable angina, having chest pain with exercise. He uses sublingual nitroglycerin (SL NTG prn) about three times a month. His blood pressure is 140/70 mm Hg. His pulse is 60 beats per minute. His current medications are lisinopril, atorvastatin, aspirin, and SL NTG tablets as needed.
What would you recommend?
A. No sildenafil; refer to urologist for other ED options.
B. Okay to use sildenafil if greater than 6 hours from NTG use.
C. Recommend tadalafil.
Is coprescribing nitrates and phosphodiesterase inhibitors safe?
The FDA warns against the use of phosphodiesterase inhibitors in patients taking nitrates. Combining nitrates with phosphodiesterase type 5 (PDE5) inhibitors is contraindicated because of a synergistic blood pressure lowering effect.1 This warning/contraindication was based on theoretical concerns, as well as concern that of the first 130 deaths reported in patients who took sildenafil, 16 of the patients also were taking nitrates.2
Parker and colleagues studied the safety of giving IV nitroglycerin to patients with coronary artery disease (CAD) who have taken sildenafil.3 The study was a randomized, placebo-controlled, crossover trial. Participants received sildenafil 100 mg or placebo, then received intravenous NTG. Patients who received sildenafil had a 4-6 mm Hg systolic BP drop compared with those who took the placebo. There was no difference in severe events between the sildenafil and placebo groups. The blood levels of nitroglycerin in this study were very likely much higher than the levels that occur with SL NTG.
A recent study by Holt et al. looked at overall cardiovascular outcomes with coprescribing nitrates and phosphodiesterase inhibitors.4 The study was a case crossover design, using a nationwide Danish health registry over the period of 2000-2018. In 2000, the rate of coprescribing of phosphodiesterase inhibitors in ischemic heart disease patients on nitrates was .9 per 100 persons/year and rose to 19.5 prescriptions per 100 persons/year in 2018. During this same time, no statistically significant association was found between the coprescription of nitrates with PDE5 inhibitors and the risk for MI, cardiac arrest, syncope, stroke, or an adverse drug event.
Does nitroglycerin response help determine cause of chest pain?
Nitroglycerin response has long been used as a clinical indicator on whether a patient’s chest pain is cardiac or not. Eric A. Shry, MD, and his colleagues looked at the usefulness of nitroglycerin response in the treatment of chest pain as a predictor of ischemic chest pain in an emergency department setting.5
The study was a retrospective review of 223 patients who presented to the emergency department over a 5-month period with ongoing chest pain. They looked at patients who had ongoing chest pain in the emergency department, received nitroglycerin, and did not receive any therapy other than aspirin within 10 minutes of receiving nitroglycerin. Response to the drug was compared with the final diagnosis of cardiac versus noncardiac chest pain.
Of the patients with a final determination of cardiac chest pain, 88% had a nitroglycerin response, whereas 92% of the patients with noncardiac chest pain had a nitroglycerin response (P = .50).
Deborah B. Diercks, MD, and her colleagues looked at improvement in chest pain scores in the emergency department in patients treated with nitroglycerin and whether it correlated with a cardiac etiology of chest pain.6 The study was a prospective, observational study of 664 patients in an urban tertiary care emergency department over a 16-month period. An 11-point numeric chest pain scale was assessed and recorded by research assistants before and 5 minutes after receiving nitroglycerin. The scale ranged from 0 (no pain) to 10 (worst pain imaginable).
A final diagnosis of a cardiac etiology for chest pain was found in 18% of the patients in the study. Of the patients who had cardiac-related chest pain, 20% had no reduction in pain with nitroglycerin, compared with 19% of the patients without cardiac-related chest pain.
A complete or significant reduction in chest pain occurred with nitroglycerin in 31% of patients with cardiac chest pain and 27% of the patients without cardiac chest pain (P = .76).
Nitroglycerin response does not appear to be helpful in distinguishing cardiac from noncardiac chest pain, but a study by His and colleagues offers an interesting twist.7
The authors of this research studied 118 patients looking to see if the side effect of headache with nitroglycerin was more common in patients who did not have CAD than in those who did. All the patients had a varying degree of relief of chest pain with NTG administration within 10 minutes. In patients with normal coronary arteries or minimal CAD, 73% had headache caused by NTG, whereas in patients with obstructive CAD, only 23% had headache after NTG use.
Take-home messages
- Short acting nitroglycerin may not be a contraindication for phosphodiesterase inhibitor use.
- More data are still needed.
- Nitroglycerin response does not help distinguish chest pain from CAD from noncardiac causes.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at imnews@mdedge.com.
References
1. Schwartz BG, Kloner RA. Drug interactions with phosphodiesterase-5 inhibitors used for the treatment of erectile dysfunction or pulmonary hypertension. Circulation. 2010;122:88-95.
2. Kloner RA, Zusman RM. Cardiovascular effects of sildenafil citrate and recommendations for its use. Am J Cardiol. 1999 Sep 9;84(5B):11N-17N.
3. Parker JD et al. Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double-blind, placebo-controlled, randomized, crossover trial. Crit Care Med. 2007;35:1863-8.
4. Holt A et al. Adverse events associated with coprescription of phosphodiesterase type inhibitors and oral organic nitrates in male patients with ischemic heart disease. Ann Intern Med. 2022 Jun;175(6):774-82.
5. Shry EA et al. Usefulness of the response to sublingual nitroglycerin as a predictor of ischemic chest pain in the emergency department. Am J Cardiol. 2002 Dec 1;90(11):1264-6.
6. Diercks DB et al. Changes in the numeric descriptive scale for pain after sublingual nitroglycerin do not predict cardiac etiology of chest pain. Ann Emerg Med. 2005 Jun;45(6):581-5.
7. His DH et al. Headache response to glyceryl trinitrate in patients with and without obstructive coronary artery disease. Heart 2005;91:1164-6.
Intermittent fasting can lead to type 2 diabetes remission
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
In a small randomized controlled trial of patients with type 2 diabetes in China, close to half of those who followed a novel intermittent fasting program for 3 months had diabetes remission (A1c less than 6.5% without taking antidiabetic drugs) that persisted for 1 year.
Importantly, “this study was performed under real-life conditions, and the intervention was delivered by trained nurses in primary care rather than by specialized staff at a research institute, making it a more practical and achievable way to manage” type 2 diabetes, the authors report.
Moreover, 65% of the patients in the intervention group who achieved diabetes remission had had diabetes for more than 6 years, which “suggests the possibility of remission for patients with longer duration” of diabetes, they note.
In addition, antidiabetic medication costs decreased by 77%, compared with baseline, in patients in the intermittent-fasting intervention group.
Although intermittent fasting has been studied for weight loss, it had not been investigated for effectiveness for diabetes remission.
These findings suggest that intermittent fasting “could be a paradigm shift in the management goals in diabetes care,” Xiao Yang and colleagues conclude in their study, published online in The Journal of Clinical Endocrinology & Metabolism.
“Type 2 diabetes is not necessarily a permanent, lifelong disease,” senior author Dongbo Liu, PhD, from the Hunan Agricultural University, Changsha, China, added in a press release from The Endocrine Society.
“Diabetes remission is possible if patients lose weight by changing their diet and exercise habits,” Dr. Liu said.
‘Excellent outcome’
Invited to comment, Amy E. Rothberg, MD, PhD, who was not involved with the research, agreed that the study indicates that intermittent fasting works for diabetes remission.
“We know that diabetes remission is possible with calorie restriction and subsequent weight loss, and intermittent fasting is just one of the many [dietary] approaches that may be suitable, appealing, and sustainable to some individuals, and usually results in calorie restriction and therefore weight loss,” she said.
The most studied types of intermittent fasting diets are alternate-day fasting, the 5:2 diet, and time-restricted consumption, Dr. Rothberg told this news organization.
This study presented a novel type of intermittent fasting, she noted. The intervention consisted of 6 cycles (3 months) of 5 fasting days followed by 10 ad libitum days, and then 3 months of follow-up (with no fasting days).
After 3 months of the intervention plus 3 months of follow-up, 47% of the 36 patients in the intervention group achieved diabetes remission (with a mean A1c of 5.66%), compared with only 2.8% of the 36 patients in the control group.
At 12 months, 44% of patients in the intervention group had sustained diabetes remission (with a mean A1c of 6.33%).
This was “an excellent outcome,” said Dr. Rothberg, professor of nutritional sciences, School of Public Health, University of Michigan, Ann Arbor, and a co-author of an international consensus statement that defined diabetes remission.
On average, patients in the intermittent fasting group lost 5.93 kg (13.0 lb) in 3 months, which was sustained over 12 months. “The large amount of weight reduction is key to continuing to achieve diabetes remission,” she noted.
This contrasted with an average weight loss of just 0.27 kg (0.6 lb) in the control group.
Participants who were prescribed fewer antidiabetic medications were more likely to achieve diabetes remission. The researchers acknowledge that the study was not blinded, and they did not record physical activity (although participants were encouraged to maintain their usual physical activity).
This was a small study, Dr. Rothberg acknowledged. The researchers did not specify which specific antidiabetic drugs patients were taking, and they did not determine waist or hip circumference or assess lipids.
The diet was culturally sensitive, appropriate, and feasible in this Chinese population and would not be generalizable to non-Asians.
Nevertheless, a similar approach could be used in any population if the diet is tailored to the individual, according to Dr. Rothberg. Importantly, patients would need to receive guidance from a dietician to make sure their diet comprises all the necessary micronutrients, vitamins, and minerals on fasting days, and they would need to maintain a relatively balanced diet and not gorge themselves on feast days.
“I think we should campaign widely about lifestyle approaches to achieve diabetes remission,” she urged.
72 patients with diabetes for an average of 6.6 years
“Despite a widespread public consensus that [type 2 diabetes] is irreversible and requires drug treatment escalation, there is some evidence of the possibility of remission,” Dr. Yang and colleagues write in their article.
They aimed to evaluate the effectiveness of intermittent fasting for diabetes remission and the durability of diabetes remission at 1 year.
Diabetes remission was defined having a stable A1c less than 6.5% for at least 3 months after discontinuing all antidiabetic medications, confirmed in at least annual A1c measurements (according to a 2021 consensus statement initiated by the American Diabetes Association).
Between 2019 and 2020, the researchers enrolled 72 participants aged 38-72 years who had had type 2 diabetes (duration 1 to 11 years) and a body mass index (BMI) of 19.1-30.4 kg/m2. Patients were randomized 1:1 to the intermittent fasting group or control group.
Baseline characteristics were similar in both groups. Patients were a mean age of 53 years and roughly 60% were men. They had a mean BMI of 24 kg/m2, a mean duration of diabetes of 6.6 years, and a mean A1c of 7.6%, and they were taking an average of 1.8 glucose-lowering medications.
On fasting days, patients in the intervention group received a Chinese Medical Nutrition Therapy kit that provided approximately 840 kcal/day (46% carbohydrates, 46% fat, 8% protein). The kit included a breakfast of a fruit and vegetable gruel, lunch of a solid beverage plus a nutritional rice composite, and dinner of a solid beverage and a meal replacement biscuit, which participants reconstituted by mixing with boiling water. They were allowed to consume noncaloric beverages.
On nonfasting days, patients chose foods ad libitum based on the 2017 Dietary Guidelines for Diabetes in China, which recommend approximately 50%-65% of total energy intake from carbohydrates, 15%-20% from protein, and 20%-30% from fat, and had greater than or equal to 5 g fiber per serving.
Patients in the control group chose foods ad libitum from the dietary guidelines during the entire study.
The study received funding from the National Natural Science Foundation of China. The authors have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
DELIVER subanalysis ‘seals deal’ for dapagliflozin in HF
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
A prespecified analysis of a large global trial of patients with symptomatic heart failure with mildly reduced and preserved ejection fraction “seals the deal” on the efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors to manage and improve their symptoms.
The prespecified analysis of the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) trial included 5,795 patients with mildly reduced and preserved ejection fraction who completed the Kansas City Cardiomyopathy Questionnaire (KCCQ) after taking the SGLT2 inhibitor dapagliflozin or placebo. The results were published online in the Journal of the American College of Cardiology.
“We’ve known from studies prior to DELIVER that SGLT2 inhibitors have been shown to improve health status, patient symptoms and quality of life among those that are living with heart failure and mildly reduced [HFmrEF] and preserved [HFpEF] ejection fraction,” lead author Mikhail N. Kosiborod, MD, vice president for research at Saint Luke’s Health System, and codirector of the St. Luke’s Michael and Marly Haverty Cardiometabolic Center of Excellence at St. Luke’s Mid America Heart Institute, Kansas City, Mo., said in an interview. “But the picture was incomplete for a number of different reasons, partly because the previous studies were either relatively modest in size, geographically limited, or suggested potential attenuation of these benefits in patients with completely normal ejection fraction.”
Specifically, the study authors noted the EMPEROR-Preserved trial of the SGLT2 inhibitor empagliflozin showed improvement in health status vs. placebo across the range of EF except in those with normal EF of 65% or greater. The PRESERVED-HF trial of dapagliflozin demonstrated a more robust response than EMPEROR-Preserved or DELIVER, but PRESERVED-HF patients were recruited only in the United States and had more debilitating HF symptoms at baseline.
“Because of the results of the DELIVER trial and because of how large, extensive, and inclusive the trial was, it really seals the deal on the value of SGLT2 inhibitors in patients with heart failure,” said Dr. Kosiborod, who is also a professor of medicine at the University of Missouri–Kansas City.
The DELIVER analysis found that the effects of dapagliflozin on reducing cardiovascular death and worsening HF were greatest in patients who had the most debilitating symptoms at baseline, measured as KCCQ total symptom score (TSS) as 63 or less, the lowest of three tertiles used in the analysis. At baseline, these patients had the highest rates of CV death or worsening HF than those in the other two tertiles: KCCQ-TSS of 63-84, and greater than 84.
Compared with placebo, treated patients in the lowest KCCQ-TSS quartile had a 30% reduction in risk for the primary composite outcome, which consisted of time to first CV death or HF event (hazard ratio, 0.70; 95% confidence interval, 0.58-0.84; P < .001). In the second tertile, the relative risk reduction was 19% (HR, 0.81; 95% CI, 0.65-1.01; P < .006), and the highest quartile showed no significant difference between treatment and placebo (HR, 1.07; 95% CI, 0.83-1.37; P < .62).
“The most important take home message is that the SGLT2 inhibitor dapagliflozin significantly improved patient symptoms as measured by the Kansas City Cardiomyopathy Questionnaire symptom score,” Dr. Kosiborod said. “It improved those symptoms within 1 month and those benefits were sustained out to 8 months.”
DELIVER patients also showed improvement in all other key KCCQ domains across the board, he added. “In addition, dapagliflozin also improved the proportion of patients who had small, moderate, and large improvements in a responder analysis. So really, by every measure that we had, dapagliflozin had a significant beneficial effect.”
The DELIVER results taken collectively with the EMPEROR-Preserved and PRESERVED-HF trials cinch the deal for SGLT2 inhibitors, Dr. Kosiborod said. “They deliver on the triple goal of care in patients with heart failure. They reduce the risk of cardiovascular death and worsening heart failure and they improve patient symptoms, function and quality of life – and they accomplish that across the entire continuum of heart failure regardless of ejection fraction, regardless of whether patients are hospitalized or in an ambulatory setting, regardless of age or background therapies or other comorbidities.”
He added: “It’s a pretty historic development because we haven’t had that before.”
AstraZeneca funded the DELIVER trial. Dr. Kosiborod disclosed financial relationships with Alnylam, Amgen, Applied Therapeutics, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Sanofi, Pharmacosmos and Vifor Pharma.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Metabolic syndrome may promote gout in young men
Metabolic syndrome is associated with a significantly increased risk for gout in young men, but the risk can be mitigated by improvement in individual components of the syndrome, based on data from a pair of population-based studies totaling more than 4 million individuals.
Gout remains the most common type of inflammatory arthritis in men, and the rate has been rising among younger adults, Yeonghee Eun, MD, PhD, of Sungkyunkwan University, Seoul, South Korea, and colleagues wrote. An increasing body of evidence suggests a link between gout and metabolic syndrome (MetS), but large studies have been lacking, especially in younger adults.
In a study published in Frontiers in Medicine, the researchers reviewed data from 3,569,104 men aged 20-39 years who underwent a health checkup between 2009 and 2012 in South Korea, based on the Korean National Health Insurance Service. The primary outcome of incident gout was identified using claims data. The mean age of the participants was 31.5 years.
Over a mean follow-up of 7.4 years, the incidence of gout was 3.36 per 1,000 person-years. The risk of developing gout was more than twice as high among individuals who met MetS criteria than in those who did not (adjusted hazard ratio, 2.44).
MetS was defined as the presence of at least two of the following components: hypertriglyceridemia, abdominal obesity, reduced HDL cholesterol, elevated blood pressure, and elevated fasting glucose.
Overall, individuals with all five MetS components had a fivefold increase in gout risk, compared with people who did not have MetS (aHR, 5.24). In an analysis of each component of MetS, hypertriglyceridemia and abdominal obesity showed the strongest association with gout (aHRs of 2.08 and 2.33, respectively).
The impact of MetS on risk of incident gout was greater in younger participants, which suggests that the management of MetS in young people should be emphasized, the researchers said.
In a further analysis of body mass index subgroups, MetS had the greatest impact on gout risk for individuals who were underweight (aHR, 3.82). “In particular, in the underweight group, the risk of gout increased 10-fold when abdominal obesity was present,” the researchers said.
The study was limited by several factors including potential selection bias and potential overestimation of gout incidence because of the use of diagnostic codes, the researchers noted. Other limitations included lack of control for nutritional or dietary risk factors and the inability to include cases that occurred after the study period.
However, findings were strengthened by the large number of participants with MetS who were underweight or normal weight, the researchers wrote. More research on the mechanism of action is needed, but the data suggest that MetS is a key risk factor in the development of gout in young men.
In a second study, published in Arthritis & Rheumatology, Dr. Eun and colleagues examined associations between MetS changes and incident gout in young men. Although previous studies have shown that changes in MetS status can alter the risk of cardiovascular events, atrial fibrillation, end-stage renal disease, and all-cause mortality, the impact of these changes on gout has not been well studied, they said. The researchers used the same study cohort, the National Health Insurance Service database in South Korea. They reviewed data from 1,293,166 individuals aged 20-39 years. Of these, 18,473 were diagnosed with gout for an incidence rate 3.36/1,000 person-years. The researchers compared gout incidence for men who met criteria for MetS at three health checkups and those without MetS.
Overall, patients with MetS at all three checkups had a nearly fourfold higher risk of gout than those who never had MetS, with an adjusted hazard ratio of 3.82, the researchers wrote. The development of MetS over the study period more than doubled the risk of gout, but recovery from MetS reduced incident gout risk by approximately 50% (aHR, 0.52).
In findings similar to the Frontiers in Medicine study, the greatest associations with gout were noted for changes in elevated triglycerides and changes in abdominal obesity; aHRs for development and recovery for elevated triglycerides were 1.74 and 0.56, respectively, and for abdominal obesity, 1.94 and 0.69, respectively.
More research is needed to explore the mechanism by which both abdominal obesity and elevated triglycerides drive the development of gout, the researchers wrote in their discussion.
Also similar to the Frontiers study, the associations among changes in MetS and incident gout were greater for the youngest participants (in their 20s) and in the underweight or normal weight BMI groups.
Limitations of the second study included possible selection bias because of the study population of workplace employees who participated in regular health checks and the lack of data on women or on men aged 40 years and older, the researchers noted. Other limitations included possible misclassification of MetS because of varying health checkup results and drug claims, and lack of data on serum urate, which prevented assessment of hyperuricemia as a cause of gout.
However, the results were strengthened by the large sample size and suggest that MetS is a modifiable risk factor for gout, the researchers concluded.
Neither of the studies received outside funding. The researchers had no financial conflicts to disclose.
Metabolic syndrome is associated with a significantly increased risk for gout in young men, but the risk can be mitigated by improvement in individual components of the syndrome, based on data from a pair of population-based studies totaling more than 4 million individuals.
Gout remains the most common type of inflammatory arthritis in men, and the rate has been rising among younger adults, Yeonghee Eun, MD, PhD, of Sungkyunkwan University, Seoul, South Korea, and colleagues wrote. An increasing body of evidence suggests a link between gout and metabolic syndrome (MetS), but large studies have been lacking, especially in younger adults.
In a study published in Frontiers in Medicine, the researchers reviewed data from 3,569,104 men aged 20-39 years who underwent a health checkup between 2009 and 2012 in South Korea, based on the Korean National Health Insurance Service. The primary outcome of incident gout was identified using claims data. The mean age of the participants was 31.5 years.
Over a mean follow-up of 7.4 years, the incidence of gout was 3.36 per 1,000 person-years. The risk of developing gout was more than twice as high among individuals who met MetS criteria than in those who did not (adjusted hazard ratio, 2.44).
MetS was defined as the presence of at least two of the following components: hypertriglyceridemia, abdominal obesity, reduced HDL cholesterol, elevated blood pressure, and elevated fasting glucose.
Overall, individuals with all five MetS components had a fivefold increase in gout risk, compared with people who did not have MetS (aHR, 5.24). In an analysis of each component of MetS, hypertriglyceridemia and abdominal obesity showed the strongest association with gout (aHRs of 2.08 and 2.33, respectively).
The impact of MetS on risk of incident gout was greater in younger participants, which suggests that the management of MetS in young people should be emphasized, the researchers said.
In a further analysis of body mass index subgroups, MetS had the greatest impact on gout risk for individuals who were underweight (aHR, 3.82). “In particular, in the underweight group, the risk of gout increased 10-fold when abdominal obesity was present,” the researchers said.
The study was limited by several factors including potential selection bias and potential overestimation of gout incidence because of the use of diagnostic codes, the researchers noted. Other limitations included lack of control for nutritional or dietary risk factors and the inability to include cases that occurred after the study period.
However, findings were strengthened by the large number of participants with MetS who were underweight or normal weight, the researchers wrote. More research on the mechanism of action is needed, but the data suggest that MetS is a key risk factor in the development of gout in young men.
In a second study, published in Arthritis & Rheumatology, Dr. Eun and colleagues examined associations between MetS changes and incident gout in young men. Although previous studies have shown that changes in MetS status can alter the risk of cardiovascular events, atrial fibrillation, end-stage renal disease, and all-cause mortality, the impact of these changes on gout has not been well studied, they said. The researchers used the same study cohort, the National Health Insurance Service database in South Korea. They reviewed data from 1,293,166 individuals aged 20-39 years. Of these, 18,473 were diagnosed with gout for an incidence rate 3.36/1,000 person-years. The researchers compared gout incidence for men who met criteria for MetS at three health checkups and those without MetS.
Overall, patients with MetS at all three checkups had a nearly fourfold higher risk of gout than those who never had MetS, with an adjusted hazard ratio of 3.82, the researchers wrote. The development of MetS over the study period more than doubled the risk of gout, but recovery from MetS reduced incident gout risk by approximately 50% (aHR, 0.52).
In findings similar to the Frontiers in Medicine study, the greatest associations with gout were noted for changes in elevated triglycerides and changes in abdominal obesity; aHRs for development and recovery for elevated triglycerides were 1.74 and 0.56, respectively, and for abdominal obesity, 1.94 and 0.69, respectively.
More research is needed to explore the mechanism by which both abdominal obesity and elevated triglycerides drive the development of gout, the researchers wrote in their discussion.
Also similar to the Frontiers study, the associations among changes in MetS and incident gout were greater for the youngest participants (in their 20s) and in the underweight or normal weight BMI groups.
Limitations of the second study included possible selection bias because of the study population of workplace employees who participated in regular health checks and the lack of data on women or on men aged 40 years and older, the researchers noted. Other limitations included possible misclassification of MetS because of varying health checkup results and drug claims, and lack of data on serum urate, which prevented assessment of hyperuricemia as a cause of gout.
However, the results were strengthened by the large sample size and suggest that MetS is a modifiable risk factor for gout, the researchers concluded.
Neither of the studies received outside funding. The researchers had no financial conflicts to disclose.
Metabolic syndrome is associated with a significantly increased risk for gout in young men, but the risk can be mitigated by improvement in individual components of the syndrome, based on data from a pair of population-based studies totaling more than 4 million individuals.
Gout remains the most common type of inflammatory arthritis in men, and the rate has been rising among younger adults, Yeonghee Eun, MD, PhD, of Sungkyunkwan University, Seoul, South Korea, and colleagues wrote. An increasing body of evidence suggests a link between gout and metabolic syndrome (MetS), but large studies have been lacking, especially in younger adults.
In a study published in Frontiers in Medicine, the researchers reviewed data from 3,569,104 men aged 20-39 years who underwent a health checkup between 2009 and 2012 in South Korea, based on the Korean National Health Insurance Service. The primary outcome of incident gout was identified using claims data. The mean age of the participants was 31.5 years.
Over a mean follow-up of 7.4 years, the incidence of gout was 3.36 per 1,000 person-years. The risk of developing gout was more than twice as high among individuals who met MetS criteria than in those who did not (adjusted hazard ratio, 2.44).
MetS was defined as the presence of at least two of the following components: hypertriglyceridemia, abdominal obesity, reduced HDL cholesterol, elevated blood pressure, and elevated fasting glucose.
Overall, individuals with all five MetS components had a fivefold increase in gout risk, compared with people who did not have MetS (aHR, 5.24). In an analysis of each component of MetS, hypertriglyceridemia and abdominal obesity showed the strongest association with gout (aHRs of 2.08 and 2.33, respectively).
The impact of MetS on risk of incident gout was greater in younger participants, which suggests that the management of MetS in young people should be emphasized, the researchers said.
In a further analysis of body mass index subgroups, MetS had the greatest impact on gout risk for individuals who were underweight (aHR, 3.82). “In particular, in the underweight group, the risk of gout increased 10-fold when abdominal obesity was present,” the researchers said.
The study was limited by several factors including potential selection bias and potential overestimation of gout incidence because of the use of diagnostic codes, the researchers noted. Other limitations included lack of control for nutritional or dietary risk factors and the inability to include cases that occurred after the study period.
However, findings were strengthened by the large number of participants with MetS who were underweight or normal weight, the researchers wrote. More research on the mechanism of action is needed, but the data suggest that MetS is a key risk factor in the development of gout in young men.
In a second study, published in Arthritis & Rheumatology, Dr. Eun and colleagues examined associations between MetS changes and incident gout in young men. Although previous studies have shown that changes in MetS status can alter the risk of cardiovascular events, atrial fibrillation, end-stage renal disease, and all-cause mortality, the impact of these changes on gout has not been well studied, they said. The researchers used the same study cohort, the National Health Insurance Service database in South Korea. They reviewed data from 1,293,166 individuals aged 20-39 years. Of these, 18,473 were diagnosed with gout for an incidence rate 3.36/1,000 person-years. The researchers compared gout incidence for men who met criteria for MetS at three health checkups and those without MetS.
Overall, patients with MetS at all three checkups had a nearly fourfold higher risk of gout than those who never had MetS, with an adjusted hazard ratio of 3.82, the researchers wrote. The development of MetS over the study period more than doubled the risk of gout, but recovery from MetS reduced incident gout risk by approximately 50% (aHR, 0.52).
In findings similar to the Frontiers in Medicine study, the greatest associations with gout were noted for changes in elevated triglycerides and changes in abdominal obesity; aHRs for development and recovery for elevated triglycerides were 1.74 and 0.56, respectively, and for abdominal obesity, 1.94 and 0.69, respectively.
More research is needed to explore the mechanism by which both abdominal obesity and elevated triglycerides drive the development of gout, the researchers wrote in their discussion.
Also similar to the Frontiers study, the associations among changes in MetS and incident gout were greater for the youngest participants (in their 20s) and in the underweight or normal weight BMI groups.
Limitations of the second study included possible selection bias because of the study population of workplace employees who participated in regular health checks and the lack of data on women or on men aged 40 years and older, the researchers noted. Other limitations included possible misclassification of MetS because of varying health checkup results and drug claims, and lack of data on serum urate, which prevented assessment of hyperuricemia as a cause of gout.
However, the results were strengthened by the large sample size and suggest that MetS is a modifiable risk factor for gout, the researchers concluded.
Neither of the studies received outside funding. The researchers had no financial conflicts to disclose.
FROM FRONTIERS IN MEDICINE AND ARTHRITIS & RHEUMATOLOGY
RA risk raised by work-related inhaled agents
Exposure to inhaled agents in the workplace could be putting people at risk of developing rheumatoid arthritis, according to research published in Annals of the Rheumatic Diseases.
In an analysis of data from the long-running Swedish Epidemiological Investigation of RA (EIRA) population-based cohort study, there was a 21% increased risk of RA and a 25% increased risk of anti–citrullinated protein antibody (ACPA)–positive RA associated with exposure to any occupationally inhaled agent.
“We have investigated a number of occupational airborne exposures and found that exposure for those agents infer a high risk for RA,” Lars Klareskog, MD, PhD, senior professor of rheumatology at the Karolinska Institute and Karolinska University Hospital (Solna) in Stockholm, said in an interview.
Dr. Klareskog, who is one of the lead authors of the published work, added that the risk is particularly high in individuals who had a genetic susceptibility and in those who smoked.
“The importance of this is that it further demonstrates that exposures to the lung may trigger immune reactions associated with the major subset of rheumatoid arthritis,” Dr. Klareskog said. “Second, it shows that those exposed to these agents should be very keen to not smoke.” “These findings further implicate the respiratory tract mucosa in ACPA-positive RA pathogenesis,” agreed Vanessa L. Kronzer, MD, of the Mayo Clinic in Rochester, Minn., and Jeffrey A. Sparks, MD, MMSc, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
They also “impress the need for public policy initiatives related to occupational inhalants to prevent RA,” they suggested in an editorial.
Multiple occupational inhalable exposures assessed
In the analysis, the researchers assessed exposure to 32 inhalable agents in people with (n = 4,033) and without RA (n = 6,485). The list of agents considered included detergents, diesel engine exhaust, fine particulate matter, solvents, and agricultural chemicals.
A total of 17 agents showed a positive association with an increased risk of ACPA-positive RA. Dr. Kronzer and Dr. Sparks noted that breathing in insecticides and fungicides at work was associated with the highest odds ratios for having ACPA-positive RA (both 2.38).
“Importantly, both the number and duration of exposures exhibited a dose-response effect on RA risk,” the editorialists said.
They also picked out that there was “a gene-environment interaction for RA risk for certain inhalants,” including diesel engine exhaust, asbestos, carbon monoxide, and quartz dust.
Smoking amplified the risk for ACPA-positive RA associated with certain agents, such as detergents, and adding in genetic susceptibility for a third exposure increased the risk still further.
A key message is that there are many agents that can affect the airways and increase the risk of RA rather than there being a specific one, Dr. Klareskog said.
“On one hand, it’s a message of public health,” he said. Many public health authorities are aware of the potential risks of inhaled agents on the lung, “but this just adds another dimension that it’s bad also for rheumatoid arthritis.” Thus, greater efforts to help protect people from being exposed at work may be needed.
From the individual’s perspective, “if you have RA or other immune diseases in your family, then you may know that you’re at increased risk,” Dr. Klareskog said. The message here is perhaps to “be aware, [protect yourself], and stop smoking.”
The EIRA study was supported by funding from the Swedish Research Foundation for Health, Working Life, and Welfare, the Swedish Research Council, the AFA foundation, Region Stockholm, King Gustaf V’s 80-year foundation, and the Swedish Rheumatic Foundation. Dr. Klareskog and coauthors had no competing interests to disclose. Dr. Kronzer and Dr. Sparks had no disclosures of relevance to their comments.
Exposure to inhaled agents in the workplace could be putting people at risk of developing rheumatoid arthritis, according to research published in Annals of the Rheumatic Diseases.
In an analysis of data from the long-running Swedish Epidemiological Investigation of RA (EIRA) population-based cohort study, there was a 21% increased risk of RA and a 25% increased risk of anti–citrullinated protein antibody (ACPA)–positive RA associated with exposure to any occupationally inhaled agent.
“We have investigated a number of occupational airborne exposures and found that exposure for those agents infer a high risk for RA,” Lars Klareskog, MD, PhD, senior professor of rheumatology at the Karolinska Institute and Karolinska University Hospital (Solna) in Stockholm, said in an interview.
Dr. Klareskog, who is one of the lead authors of the published work, added that the risk is particularly high in individuals who had a genetic susceptibility and in those who smoked.
“The importance of this is that it further demonstrates that exposures to the lung may trigger immune reactions associated with the major subset of rheumatoid arthritis,” Dr. Klareskog said. “Second, it shows that those exposed to these agents should be very keen to not smoke.” “These findings further implicate the respiratory tract mucosa in ACPA-positive RA pathogenesis,” agreed Vanessa L. Kronzer, MD, of the Mayo Clinic in Rochester, Minn., and Jeffrey A. Sparks, MD, MMSc, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
They also “impress the need for public policy initiatives related to occupational inhalants to prevent RA,” they suggested in an editorial.
Multiple occupational inhalable exposures assessed
In the analysis, the researchers assessed exposure to 32 inhalable agents in people with (n = 4,033) and without RA (n = 6,485). The list of agents considered included detergents, diesel engine exhaust, fine particulate matter, solvents, and agricultural chemicals.
A total of 17 agents showed a positive association with an increased risk of ACPA-positive RA. Dr. Kronzer and Dr. Sparks noted that breathing in insecticides and fungicides at work was associated with the highest odds ratios for having ACPA-positive RA (both 2.38).
“Importantly, both the number and duration of exposures exhibited a dose-response effect on RA risk,” the editorialists said.
They also picked out that there was “a gene-environment interaction for RA risk for certain inhalants,” including diesel engine exhaust, asbestos, carbon monoxide, and quartz dust.
Smoking amplified the risk for ACPA-positive RA associated with certain agents, such as detergents, and adding in genetic susceptibility for a third exposure increased the risk still further.
A key message is that there are many agents that can affect the airways and increase the risk of RA rather than there being a specific one, Dr. Klareskog said.
“On one hand, it’s a message of public health,” he said. Many public health authorities are aware of the potential risks of inhaled agents on the lung, “but this just adds another dimension that it’s bad also for rheumatoid arthritis.” Thus, greater efforts to help protect people from being exposed at work may be needed.
From the individual’s perspective, “if you have RA or other immune diseases in your family, then you may know that you’re at increased risk,” Dr. Klareskog said. The message here is perhaps to “be aware, [protect yourself], and stop smoking.”
The EIRA study was supported by funding from the Swedish Research Foundation for Health, Working Life, and Welfare, the Swedish Research Council, the AFA foundation, Region Stockholm, King Gustaf V’s 80-year foundation, and the Swedish Rheumatic Foundation. Dr. Klareskog and coauthors had no competing interests to disclose. Dr. Kronzer and Dr. Sparks had no disclosures of relevance to their comments.
Exposure to inhaled agents in the workplace could be putting people at risk of developing rheumatoid arthritis, according to research published in Annals of the Rheumatic Diseases.
In an analysis of data from the long-running Swedish Epidemiological Investigation of RA (EIRA) population-based cohort study, there was a 21% increased risk of RA and a 25% increased risk of anti–citrullinated protein antibody (ACPA)–positive RA associated with exposure to any occupationally inhaled agent.
“We have investigated a number of occupational airborne exposures and found that exposure for those agents infer a high risk for RA,” Lars Klareskog, MD, PhD, senior professor of rheumatology at the Karolinska Institute and Karolinska University Hospital (Solna) in Stockholm, said in an interview.
Dr. Klareskog, who is one of the lead authors of the published work, added that the risk is particularly high in individuals who had a genetic susceptibility and in those who smoked.
“The importance of this is that it further demonstrates that exposures to the lung may trigger immune reactions associated with the major subset of rheumatoid arthritis,” Dr. Klareskog said. “Second, it shows that those exposed to these agents should be very keen to not smoke.” “These findings further implicate the respiratory tract mucosa in ACPA-positive RA pathogenesis,” agreed Vanessa L. Kronzer, MD, of the Mayo Clinic in Rochester, Minn., and Jeffrey A. Sparks, MD, MMSc, of Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
They also “impress the need for public policy initiatives related to occupational inhalants to prevent RA,” they suggested in an editorial.
Multiple occupational inhalable exposures assessed
In the analysis, the researchers assessed exposure to 32 inhalable agents in people with (n = 4,033) and without RA (n = 6,485). The list of agents considered included detergents, diesel engine exhaust, fine particulate matter, solvents, and agricultural chemicals.
A total of 17 agents showed a positive association with an increased risk of ACPA-positive RA. Dr. Kronzer and Dr. Sparks noted that breathing in insecticides and fungicides at work was associated with the highest odds ratios for having ACPA-positive RA (both 2.38).
“Importantly, both the number and duration of exposures exhibited a dose-response effect on RA risk,” the editorialists said.
They also picked out that there was “a gene-environment interaction for RA risk for certain inhalants,” including diesel engine exhaust, asbestos, carbon monoxide, and quartz dust.
Smoking amplified the risk for ACPA-positive RA associated with certain agents, such as detergents, and adding in genetic susceptibility for a third exposure increased the risk still further.
A key message is that there are many agents that can affect the airways and increase the risk of RA rather than there being a specific one, Dr. Klareskog said.
“On one hand, it’s a message of public health,” he said. Many public health authorities are aware of the potential risks of inhaled agents on the lung, “but this just adds another dimension that it’s bad also for rheumatoid arthritis.” Thus, greater efforts to help protect people from being exposed at work may be needed.
From the individual’s perspective, “if you have RA or other immune diseases in your family, then you may know that you’re at increased risk,” Dr. Klareskog said. The message here is perhaps to “be aware, [protect yourself], and stop smoking.”
The EIRA study was supported by funding from the Swedish Research Foundation for Health, Working Life, and Welfare, the Swedish Research Council, the AFA foundation, Region Stockholm, King Gustaf V’s 80-year foundation, and the Swedish Rheumatic Foundation. Dr. Klareskog and coauthors had no competing interests to disclose. Dr. Kronzer and Dr. Sparks had no disclosures of relevance to their comments.
FROM ANNALS OF THE RHEUMATIC DISEASES
Can nanotechnology help cure IBD?
Finding a cure for inflammatory bowel disease is a big goal. But the key to achieving it might be to think small.
University of Wisconsin–Madison researchers are developing nanoparticles – particles measuring between 1 and 100 nanometers (one-billionth of a meter) – designed to treat IBD, including Crohn’s disease and ulcerative colitis. (For context: A sheet of paper is about 100,000 nanometers thick.)
Described in a paper in Science Advances, these , a compatible compound commonly used in medicine.
The nanoparticles – the researchers call them “backpacks” – can be attached to probiotics, which deliver them to the gut.
“Due to the colonizing property of probiotics in colon tissues, the nanoparticles could be delivered to colon tissues by probiotics and released slowly,” says study author Quanyin Hu, PhD, a biomedical engineer and assistant professor at the University of Wisconsin–Madison School of Pharmacy.
This helps give the nanoparticles time to bring the ROS level back down to normal. But that’s only part of the IBD treatment the researchers envision.
The technology builds on a previous development from Dr. Hu and his team – a protective probiotic shell coating. The coating, which is about 330 nanometers thick, helps probiotics survive long enough to establish and multiply in the gut.
“The harsh environment of gastric acid and bile salt would kill most probiotics,” Dr. Hu says. “Moreover, antibiotics usually used in inflammatory bowel disease treatment also harm probiotic growth.”
Early results are promising, he says. Mice with IBD that received the full treatment – combining the ROS-targeting nanoparticles with the coated probiotics – had fewer IBD symptoms, like less weight loss and colon shortening, than those treated with the encapsulated probiotics alone.
By attacking the disease on multiple fronts – reducing the ROS and improving the balance of gut microbiota – a healthy gut environment could be restored, Dr. Hu says. In other words: “[It] might be possible to finally cure inflammatory bowel disease.”
Nanotechnology offers all kinds of unique advantages over traditional IBD treatments, he says. Nanoparticles can be designed to target specific tissues, like colon tissues. And, compared with small molecules, they can circulate throughout the body longer, so they have more time to build up and do their job.
The next steps will be to test the treatment in large animals and “to develop a stable formulation that can be stored for a long time and produced in a scalable and economical manner,” Dr. Hu says.
Current IBD treatments “can only relieve symptoms,” not cure the disease, he says.
“This study is our first try to fundamentally treat inflammatory bowel disease by recovering a healthy microenvironment in the intestines, and our preliminary data demonstrated that this strategy is delivering promises to pave a new treatment strategy for IBD,” Dr. Hu says.
A version of this article first appeared on WebMD.com.
Finding a cure for inflammatory bowel disease is a big goal. But the key to achieving it might be to think small.
University of Wisconsin–Madison researchers are developing nanoparticles – particles measuring between 1 and 100 nanometers (one-billionth of a meter) – designed to treat IBD, including Crohn’s disease and ulcerative colitis. (For context: A sheet of paper is about 100,000 nanometers thick.)
Described in a paper in Science Advances, these , a compatible compound commonly used in medicine.
The nanoparticles – the researchers call them “backpacks” – can be attached to probiotics, which deliver them to the gut.
“Due to the colonizing property of probiotics in colon tissues, the nanoparticles could be delivered to colon tissues by probiotics and released slowly,” says study author Quanyin Hu, PhD, a biomedical engineer and assistant professor at the University of Wisconsin–Madison School of Pharmacy.
This helps give the nanoparticles time to bring the ROS level back down to normal. But that’s only part of the IBD treatment the researchers envision.
The technology builds on a previous development from Dr. Hu and his team – a protective probiotic shell coating. The coating, which is about 330 nanometers thick, helps probiotics survive long enough to establish and multiply in the gut.
“The harsh environment of gastric acid and bile salt would kill most probiotics,” Dr. Hu says. “Moreover, antibiotics usually used in inflammatory bowel disease treatment also harm probiotic growth.”
Early results are promising, he says. Mice with IBD that received the full treatment – combining the ROS-targeting nanoparticles with the coated probiotics – had fewer IBD symptoms, like less weight loss and colon shortening, than those treated with the encapsulated probiotics alone.
By attacking the disease on multiple fronts – reducing the ROS and improving the balance of gut microbiota – a healthy gut environment could be restored, Dr. Hu says. In other words: “[It] might be possible to finally cure inflammatory bowel disease.”
Nanotechnology offers all kinds of unique advantages over traditional IBD treatments, he says. Nanoparticles can be designed to target specific tissues, like colon tissues. And, compared with small molecules, they can circulate throughout the body longer, so they have more time to build up and do their job.
The next steps will be to test the treatment in large animals and “to develop a stable formulation that can be stored for a long time and produced in a scalable and economical manner,” Dr. Hu says.
Current IBD treatments “can only relieve symptoms,” not cure the disease, he says.
“This study is our first try to fundamentally treat inflammatory bowel disease by recovering a healthy microenvironment in the intestines, and our preliminary data demonstrated that this strategy is delivering promises to pave a new treatment strategy for IBD,” Dr. Hu says.
A version of this article first appeared on WebMD.com.
Finding a cure for inflammatory bowel disease is a big goal. But the key to achieving it might be to think small.
University of Wisconsin–Madison researchers are developing nanoparticles – particles measuring between 1 and 100 nanometers (one-billionth of a meter) – designed to treat IBD, including Crohn’s disease and ulcerative colitis. (For context: A sheet of paper is about 100,000 nanometers thick.)
Described in a paper in Science Advances, these , a compatible compound commonly used in medicine.
The nanoparticles – the researchers call them “backpacks” – can be attached to probiotics, which deliver them to the gut.
“Due to the colonizing property of probiotics in colon tissues, the nanoparticles could be delivered to colon tissues by probiotics and released slowly,” says study author Quanyin Hu, PhD, a biomedical engineer and assistant professor at the University of Wisconsin–Madison School of Pharmacy.
This helps give the nanoparticles time to bring the ROS level back down to normal. But that’s only part of the IBD treatment the researchers envision.
The technology builds on a previous development from Dr. Hu and his team – a protective probiotic shell coating. The coating, which is about 330 nanometers thick, helps probiotics survive long enough to establish and multiply in the gut.
“The harsh environment of gastric acid and bile salt would kill most probiotics,” Dr. Hu says. “Moreover, antibiotics usually used in inflammatory bowel disease treatment also harm probiotic growth.”
Early results are promising, he says. Mice with IBD that received the full treatment – combining the ROS-targeting nanoparticles with the coated probiotics – had fewer IBD symptoms, like less weight loss and colon shortening, than those treated with the encapsulated probiotics alone.
By attacking the disease on multiple fronts – reducing the ROS and improving the balance of gut microbiota – a healthy gut environment could be restored, Dr. Hu says. In other words: “[It] might be possible to finally cure inflammatory bowel disease.”
Nanotechnology offers all kinds of unique advantages over traditional IBD treatments, he says. Nanoparticles can be designed to target specific tissues, like colon tissues. And, compared with small molecules, they can circulate throughout the body longer, so they have more time to build up and do their job.
The next steps will be to test the treatment in large animals and “to develop a stable formulation that can be stored for a long time and produced in a scalable and economical manner,” Dr. Hu says.
Current IBD treatments “can only relieve symptoms,” not cure the disease, he says.
“This study is our first try to fundamentally treat inflammatory bowel disease by recovering a healthy microenvironment in the intestines, and our preliminary data demonstrated that this strategy is delivering promises to pave a new treatment strategy for IBD,” Dr. Hu says.
A version of this article first appeared on WebMD.com.
FROM SCIENCE ADVANCES
HIV vaccine trial makes pivotal leap toward making ‘super antibodies’
The announcement comes from the journal Science, which published phase 1 results of a small clinical trial for a vaccine technology that aims to cause the body to create a rare kind of cell.
“At the most general level, the trial results show that one can design vaccines that induce antibodies with prespecified genetic features, and this may herald a new era of precision vaccines,” William Schief, PhD, a researcher at the Scripps Research Institute and study coauthor, told the American Association for the Advancement of Science.
The study was the first to test the approach in humans and was effective in 97% – or 35 of 36 – participants. The vaccine technology is called “germline targeting.” Trial results show that “one can design a vaccine that elicits made-to-order antibodies in humans,” Dr. Schief said in a news release.
In addition to possibly being a breakthrough for the treatment of HIV, the vaccine technology could also impact the development of treatments for flu, hepatitis C, and coronaviruses, study authors wrote.
There is no cure for HIV, but there are treatments to manage how the disease progresses. HIV attacks the body’s immune system, destroys white blood cells, and increases susceptibility to other infections, AAAS summarized. More than 1 million people in the United States and 38 million people worldwide have HIV.
Previous HIV vaccine attempts were not able to cause the production of specialized cells known as “broadly neutralizing antibodies,” CNN reported.
“Call them super antibodies, if you want,” University of Minnesota HIV researcher Timothy Schacker, MD, who was not involved in the research, told CNN. “The hope is that if you can induce this kind of immunity in people, you can protect them from some of these viruses that we’ve had a very hard time designing vaccines for that are effective. So this is an important step forward.”
Study authors said this is just the first step in the multiphase vaccine design, which so far is a theory. Further study is needed to see if the next steps also work in humans, and then if all the steps can be linked together and can be effective against HIV.
A version of this article first appeared on WebMD.com.
The announcement comes from the journal Science, which published phase 1 results of a small clinical trial for a vaccine technology that aims to cause the body to create a rare kind of cell.
“At the most general level, the trial results show that one can design vaccines that induce antibodies with prespecified genetic features, and this may herald a new era of precision vaccines,” William Schief, PhD, a researcher at the Scripps Research Institute and study coauthor, told the American Association for the Advancement of Science.
The study was the first to test the approach in humans and was effective in 97% – or 35 of 36 – participants. The vaccine technology is called “germline targeting.” Trial results show that “one can design a vaccine that elicits made-to-order antibodies in humans,” Dr. Schief said in a news release.
In addition to possibly being a breakthrough for the treatment of HIV, the vaccine technology could also impact the development of treatments for flu, hepatitis C, and coronaviruses, study authors wrote.
There is no cure for HIV, but there are treatments to manage how the disease progresses. HIV attacks the body’s immune system, destroys white blood cells, and increases susceptibility to other infections, AAAS summarized. More than 1 million people in the United States and 38 million people worldwide have HIV.
Previous HIV vaccine attempts were not able to cause the production of specialized cells known as “broadly neutralizing antibodies,” CNN reported.
“Call them super antibodies, if you want,” University of Minnesota HIV researcher Timothy Schacker, MD, who was not involved in the research, told CNN. “The hope is that if you can induce this kind of immunity in people, you can protect them from some of these viruses that we’ve had a very hard time designing vaccines for that are effective. So this is an important step forward.”
Study authors said this is just the first step in the multiphase vaccine design, which so far is a theory. Further study is needed to see if the next steps also work in humans, and then if all the steps can be linked together and can be effective against HIV.
A version of this article first appeared on WebMD.com.
The announcement comes from the journal Science, which published phase 1 results of a small clinical trial for a vaccine technology that aims to cause the body to create a rare kind of cell.
“At the most general level, the trial results show that one can design vaccines that induce antibodies with prespecified genetic features, and this may herald a new era of precision vaccines,” William Schief, PhD, a researcher at the Scripps Research Institute and study coauthor, told the American Association for the Advancement of Science.
The study was the first to test the approach in humans and was effective in 97% – or 35 of 36 – participants. The vaccine technology is called “germline targeting.” Trial results show that “one can design a vaccine that elicits made-to-order antibodies in humans,” Dr. Schief said in a news release.
In addition to possibly being a breakthrough for the treatment of HIV, the vaccine technology could also impact the development of treatments for flu, hepatitis C, and coronaviruses, study authors wrote.
There is no cure for HIV, but there are treatments to manage how the disease progresses. HIV attacks the body’s immune system, destroys white blood cells, and increases susceptibility to other infections, AAAS summarized. More than 1 million people in the United States and 38 million people worldwide have HIV.
Previous HIV vaccine attempts were not able to cause the production of specialized cells known as “broadly neutralizing antibodies,” CNN reported.
“Call them super antibodies, if you want,” University of Minnesota HIV researcher Timothy Schacker, MD, who was not involved in the research, told CNN. “The hope is that if you can induce this kind of immunity in people, you can protect them from some of these viruses that we’ve had a very hard time designing vaccines for that are effective. So this is an important step forward.”
Study authors said this is just the first step in the multiphase vaccine design, which so far is a theory. Further study is needed to see if the next steps also work in humans, and then if all the steps can be linked together and can be effective against HIV.
A version of this article first appeared on WebMD.com.
FROM SCIENCE
The new obesity breakthrough drugs
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.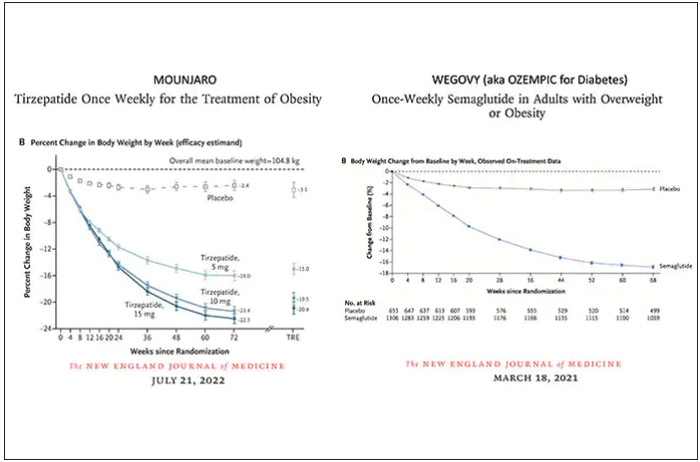
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction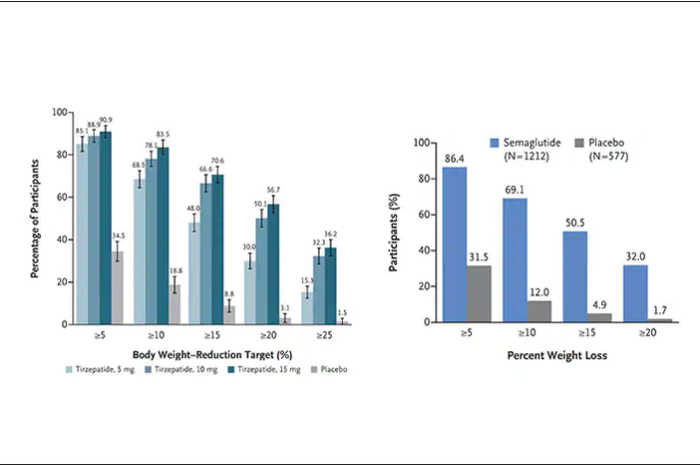
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.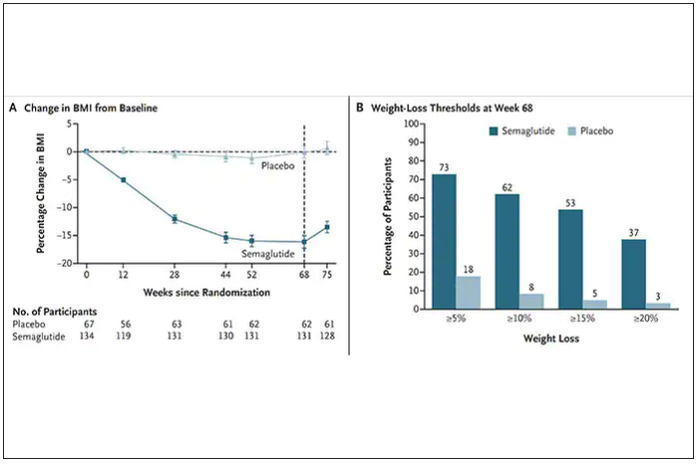
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.
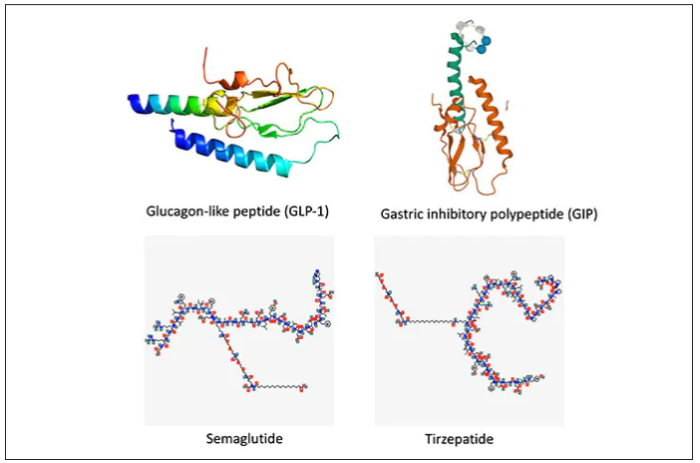
These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.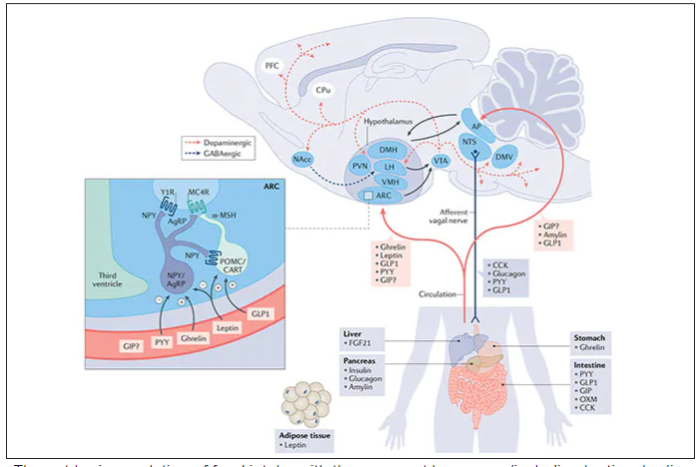
Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.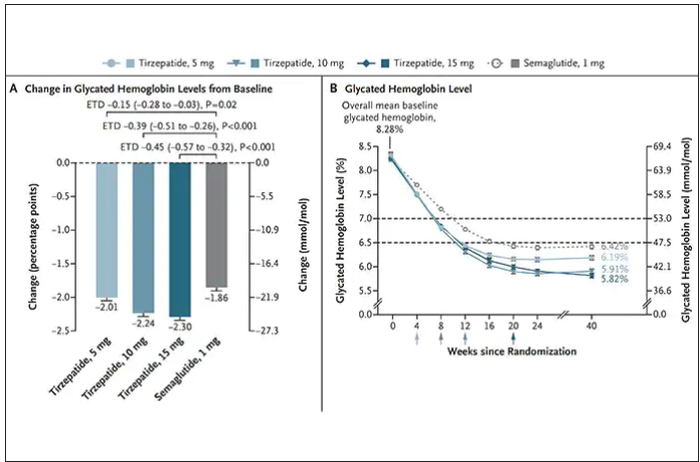
While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.
Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.

These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.
Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.
While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.

Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
This article was originally published December 10 on Medscape editor-in-chief Eric Topol’s Substack ”Ground Truths.”
– achieving a substantial amount of weight loss without serious side effects. Many attempts to get there now fill a graveyard of failed drugs, such as fen-phen in the 1990s when a single small study of this drug combination in 121 people unleashed millions of prescriptions, some leading to serious heart valve lesions that resulted in withdrawal of the drug in 1995. The drug rimonabant, an endocannabinoid receptor blocker (think of blocking the munchies after marijuana) looked encouraging in randomized trials. However, subsequently, in a trial that I led of nearly 19,000 participants in 42 countries around the world, there was a significant excess of depression, neuropsychiatric side-effects and suicidal ideation which spelled the end of that drug’s life.
In the United States, where there had not been an antiobesity drug approved by the Food and Drug Administration since 2014, Wegovy (semaglutide), a once-weekly injection was approved in June 2021. The same drug, at a lower dose, is known as Ozempic (as in O-O-O, Ozempic, the ubiquitous commercial that you undoubtedly hear and see on TV) and had already been approved in January 2020 for improving glucose regulation in diabetes. The next drug on fast track at FDA to be imminently approved is tirzepatide (Mounjaro) following its approval for diabetes in May 2022. It is noteworthy that the discovery of these drugs for weight loss was serendipitous: they were being developed for improving glucose regulation and unexpectedly were found to achieve significant weight reduction.
Both semaglutide and tirzepatide underwent randomized, placebo-controlled trials for obesity, with marked reduction of weight as shown below. Tirzepatide at dose of 10-15 mg per week achieved greater than 20% body weight reduction. Semaglutide at a dose of 2.4 mg achieved about 17% reduction. These per cent changes in body weight are 7-9 fold more than seen with placebo (2%-3% reduction). Note: these levels of percent body-weight reduction resemble what is typically achieved with the different types of bariatric surgery, such as gastric bypass.
Another way to present the data for the two trials is shown here, with an edge for tirzepatide at high (10-15 mg) doses, extending to greater than 25% body-weight reduction
The results with semaglutide were extended to teens in a randomized trial (as shown below), and a similar trial with tirzepatide is in progress.
How do these drugs work?
These are peptides in the class of incretins, mimicking gut hormones that are secreted after food intake which stimulate insulin secretion.

These two drugs have in common long half-lives (about 5 days), which affords once-weekly dosing, but have different mechanisms of action. Semaglutide activates (an agonist) the glucagonlike peptide–1 receptor, while tirzepatide is in a new class of dual agonists: It activates (mimics) both the GLP-1 receptor and GIP receptors (Gastric inhibit polypeptide is also known as glucose-dependent insulinotropic polypeptide.) The potency of activation for tirzepatide is fivefold more for GIPR than GLP1. As seen below, there are body wide effects that include the brain, liver, pancreas, stomach, intestine, skeletal muscle and fat tissue. While their mode of action is somewhat different, their clinical effects are overlapping, which include enhancing satiety, delaying gastric emptying, increasing insulin and its sensitivity, decreasing glucagon, and, of course, reducing high glucose levels. The overlap extends to side effects of nausea, vomiting, abdominal pain, constipation and diarrhea. Yet only 4%-6% of participants discontinued the drug in these trials, mostly owing to these GI side effects (and 1%-2% in the placebo group discontinued the study drug for the same reasons).
In randomized trials among people with type 2 diabetes, the drugs achieved hemoglobin A1c reduction of at least an absolute 2 percentage points which led to their FDA approvals (For semaglutide in January 2020, and for tirzepatide in May 2022). The edge that tirzepatide has exhibited for weight-loss reduction may be related to its dual agonist role, but the enhancement via GIP receptor activation is not fully resolved (as seen below with GIP? designation). The Amgen drug in development (AMG-133) has a marked weight loss effect but inhibits GIP rather than mimics it, clouding our precise understanding of the mechanism.
Nevertheless, when the two drugs were directly compared in a randomized trial for improving glucose regulation, tirzepatide was superior to semaglutide, as shown below. Of note, both drugs achieved very favorable effects on lipids, reducing triglycerides and LDL cholesterol and raising HDL cholesterol, along with reduction of blood pressure, an outgrowth of the indirect effect of weight reduction and direct metabolic effects of the drugs.
While there has been a concern about other side effects besides the GI ones noted above, review of all the trials to date in these classes of medication do not reinforce a risk of acute pancreatitis. Other rare side effects that have been noted with these drugs include allergic reactions, gallstones (which can occur with a large amount of weight loss), and potential of medullary thyroid cancer (so far only documented in rats, not people), which is why they are contraindicated in people with Type 2 multiple endocrine neoplasia syndrome.
How they are given and practical considerations
For semaglutide, which has FDA approval, the indication is a body mass index of 30 kg/m2 or greater than 27 and a weight-related medical condition (such as hypertension, hypercholesterolemia, or diabetes). To reduce the GI side effects, which mainly occur in the early dose escalation period, semaglutide is given in increasing doses by a prefilled pen by self-injection under the skin (abdomen, thigh, or arm) starting at 0.25 mg for a month and gradual increases each month reaching the maximum dose of 2.4 mg at month 5. The FDA label for dosing of tirzepatide has not been provided yet but in the weight loss trial there was a similar dose escalation from 2.5 mg up to 15 mg by month 5. The escalation is essential to reduce the frequent GI side effects, such as seen below in the tirzepatide trial.

Semaglutide is very expensive, about $1,500 per month, and not covered by Medicare. There are manufacturer starter coupons from Novo Nordisk, but that is just for the first month. These drugs have to be taken for a year to 18 months to have their full effect and without changes in lifestyle that are durable, it is likely that weight will be regained after stopping them.
What does this mean?
More than 650 million adults and 340 million children aged 5-18 are obese. The global obesity epidemic has been relentless, worsening each year, and a driver of “diabesity,” the combined dual epidemic. We now have a breakthrough class of drugs that can achieve profound weight loss equivalent to bariatric surgery, along with the side benefits of reducing cardiovascular risk factors (hypertension and hyperlipidemia), improving glucose regulation, reversing fatty liver, and the many detrimental long-term effects of obesity such as osteoarthritis and various cancers. That, in itself, is remarkable. Revolutionary.
But the downsides are also obvious. Self-injections, even though they are once a week, are not palatable for many. We have seen far more of these injectables in recent years such as the proprotein convertase subtilisin/kexin type 9 inhibitors for hypercholesterolemia or the tumor necrosis factor blockers for autoimmune conditions. That still will not make them a popular item for such an enormous population of potential users.
That brings me to Rybelsus, the oral form of semaglutide, which is approved for glucose regulation improvement but not obesity. It effects for weight loss have been modest, compared with Wegovy (5 to 8 pounds for the 7- and 14-mg dose, respectively). But the potential for the very high efficacy of an injectable to be achievable via a pill represents an important path going forward—it could help markedly reduce the cost and uptake.
The problem of discontinuation of the drugs is big, since there are limited data and the likelihood is that the weight will be regained unless there are substantial changes in lifestyle. We know how hard it is to durably achieve such changes, along with the undesirability (and uncertainty with respect to unknown side effects) of having to take injectable drugs for many years, no less the cost of doing that.
The cost of these drugs will clearly and profoundly exacerbate inequities, since they are eminently affordable by the rich, but the need is extreme among the indigent. We’ve already seen celebrities take Wegovy for weight loss who are not obese, a window into how these drugs can and will be used without supportive data. As one physician recently observed, “Other than Viagra and Botox, I’ve seen no other medication so quickly become part of modern culture’s social vernacular.” Already there are concerns that such use is preventing access to the drugs for those who qualify and need them.
There are multiple agents in the class under development which should help increase competition and reduce cost, but they will remain expensive. There is private insurance reimbursement, often with a significant copay, for people who tightly fit the inclusion criteria. Eventual coverage by Medicare will markedly expand their use, and we can expect cost-effectiveness studies to be published showing how much saving there is for the drugs compared with bariatric surgery or not achieving the weight loss. But that doesn’t change the cost at the societal level. Even as we’ve seen with generics, which will ultimately be available, the alleviation of the cost problem isn’t what we’d hoped.
This is not unlike the recent triumphs of gene therapy, as in $3.5 million for a cure of hemophilia that just got FDA approval, but instead of a rare disease we are talking about the most common medical condition in the world. We finally get across the long sought after (what many would qualify as miraculous) goal line, but the economics collide with the uptake and real benefit.
These concerns can’t be put aside in the health inequity-laden world we live in, that will unquestionably be exacerbated. However, we cannot miss that this represents one of the most important, biggest medical breakthroughs in history. This may signify the end or marked reduction in the need for bariatric surgery. These drugs will likely become some of the most prescribed of all medications in the upcoming years. While there are many drawbacks, we shouldn’t miss such an extraordinary advance in medicine – the first real, potent and safe treatment of obesity.
Thanks for reading Ground Truths. I hope you will share these posts and subscribe, to be sure you don’t miss them.
Dr. Topol is director, Scripps Translational Science Institute; executive vice president and professor of molecular medicine at The Scripps Research Institute and senior consultant, division of cardiovascular diseases, at the Scripps Clinic, both in La Jolla, Calif. He disclosed relevant financial relationships with Dexcom, Illumina, Molecular Stethoscope, Walgreens, Quest Diagnostics, MyoKardia, and National Institutes of Health. A version of this article first appeared on Medscape.com.
U.S. sees most flu hospitalizations in a decade
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.








