User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
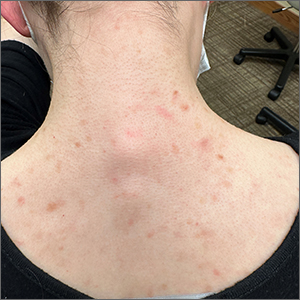
Incidental skin finding
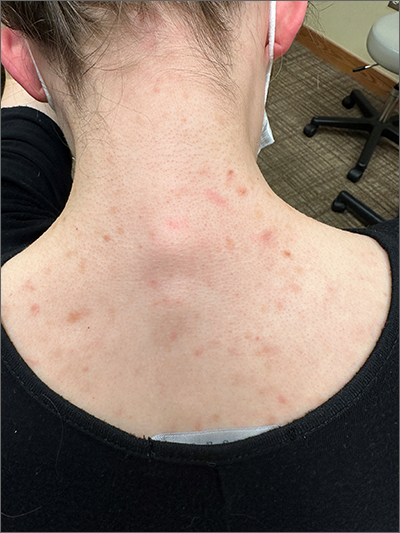
This patient was given a diagnosis of cutaneous mastocytosis. The condition was previously known as urticaria pigmentosa, but in 2016 the World Health Organization reclassified the disease to better suit its pathophysiology as a myeloid cell disorder.1
As the name suggests, this condition—which involves lesions in a sporadic, truncal distribution—involves overactivation of mastocytes at the tissue level from various stimuli, resulting in histocyte degranulation and hyperpigmentation. The exact cause is unknown. What is known is that it is often associated with other allergic or immunologic conditions and is thought to be related to mutations in the gene for CD-117’s receptor for tyrosine kinase.1 Incidence is similar to that of asthma, in that it occurs more often in younger patients; a majority of affected individuals will grow out of the disease by adolescence.1
Although most patients do not experience severe symptomatology, it is still important to differentiate cutaneous vs systemic mastocytosis. If a patient presents with inexplicable systemic symptoms of malaise, vague abdominal pain, heartburn, or flushing, the physician should consider systemic mastocytosis, idiopathic anaphylaxis, or hereditary alpha-tryptasemia.2
The test of choice is a serum tryptase test; levels will be elevated with systemic mastocytosis. Consider obtaining a skin biopsy if lesions are ambiguous or nondistinct.
There is no definitive cure for systemic or cutaneous mastocytosis, so treatment is directed at symptoms. Start by advising patients to avoid triggers and to refrain from scratching the affected areas. Topical antihistamines and oral nonsedating antihistamines can be helpful. If symptoms are more severe, refer the patient to an allergist/immunologist or to a hematologist for further medical management.2
The patient in this case had no systemic symptoms, so she was advised to continue taking oral loratadine 10 mg/d, which had been helpful, and to avoid rubbing her skin.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Murtaza Rizvi, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. doi: 10.1182/blood-2016-03-643544.
2. Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137:35-45. doi: 10.1016/j.jaci.2015.08.034

This patient was given a diagnosis of cutaneous mastocytosis. The condition was previously known as urticaria pigmentosa, but in 2016 the World Health Organization reclassified the disease to better suit its pathophysiology as a myeloid cell disorder.1
As the name suggests, this condition—which involves lesions in a sporadic, truncal distribution—involves overactivation of mastocytes at the tissue level from various stimuli, resulting in histocyte degranulation and hyperpigmentation. The exact cause is unknown. What is known is that it is often associated with other allergic or immunologic conditions and is thought to be related to mutations in the gene for CD-117’s receptor for tyrosine kinase.1 Incidence is similar to that of asthma, in that it occurs more often in younger patients; a majority of affected individuals will grow out of the disease by adolescence.1
Although most patients do not experience severe symptomatology, it is still important to differentiate cutaneous vs systemic mastocytosis. If a patient presents with inexplicable systemic symptoms of malaise, vague abdominal pain, heartburn, or flushing, the physician should consider systemic mastocytosis, idiopathic anaphylaxis, or hereditary alpha-tryptasemia.2
The test of choice is a serum tryptase test; levels will be elevated with systemic mastocytosis. Consider obtaining a skin biopsy if lesions are ambiguous or nondistinct.
There is no definitive cure for systemic or cutaneous mastocytosis, so treatment is directed at symptoms. Start by advising patients to avoid triggers and to refrain from scratching the affected areas. Topical antihistamines and oral nonsedating antihistamines can be helpful. If symptoms are more severe, refer the patient to an allergist/immunologist or to a hematologist for further medical management.2
The patient in this case had no systemic symptoms, so she was advised to continue taking oral loratadine 10 mg/d, which had been helpful, and to avoid rubbing her skin.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Murtaza Rizvi, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

This patient was given a diagnosis of cutaneous mastocytosis. The condition was previously known as urticaria pigmentosa, but in 2016 the World Health Organization reclassified the disease to better suit its pathophysiology as a myeloid cell disorder.1
As the name suggests, this condition—which involves lesions in a sporadic, truncal distribution—involves overactivation of mastocytes at the tissue level from various stimuli, resulting in histocyte degranulation and hyperpigmentation. The exact cause is unknown. What is known is that it is often associated with other allergic or immunologic conditions and is thought to be related to mutations in the gene for CD-117’s receptor for tyrosine kinase.1 Incidence is similar to that of asthma, in that it occurs more often in younger patients; a majority of affected individuals will grow out of the disease by adolescence.1
Although most patients do not experience severe symptomatology, it is still important to differentiate cutaneous vs systemic mastocytosis. If a patient presents with inexplicable systemic symptoms of malaise, vague abdominal pain, heartburn, or flushing, the physician should consider systemic mastocytosis, idiopathic anaphylaxis, or hereditary alpha-tryptasemia.2
The test of choice is a serum tryptase test; levels will be elevated with systemic mastocytosis. Consider obtaining a skin biopsy if lesions are ambiguous or nondistinct.
There is no definitive cure for systemic or cutaneous mastocytosis, so treatment is directed at symptoms. Start by advising patients to avoid triggers and to refrain from scratching the affected areas. Topical antihistamines and oral nonsedating antihistamines can be helpful. If symptoms are more severe, refer the patient to an allergist/immunologist or to a hematologist for further medical management.2
The patient in this case had no systemic symptoms, so she was advised to continue taking oral loratadine 10 mg/d, which had been helpful, and to avoid rubbing her skin.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Murtaza Rizvi, MD, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. doi: 10.1182/blood-2016-03-643544.
2. Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137:35-45. doi: 10.1016/j.jaci.2015.08.034
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405. doi: 10.1182/blood-2016-03-643544.
2. Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol 2016; 137:35-45. doi: 10.1016/j.jaci.2015.08.034
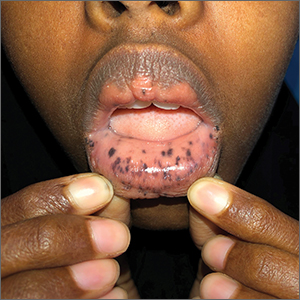
Macules and abdominal pain
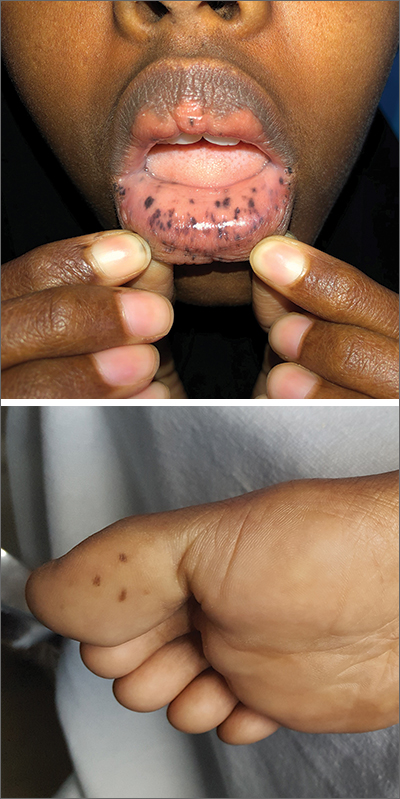
This patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in her stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing. The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
It’s recommended that polyps be removed when technically feasible.3 Pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
This patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. A colonoscopy was normal, and the health care team explained that she would require follow-up and surveillance of her condition due to the high risk of future cancers.
This case was adapted from: Warsame MO, McMichael JR. Chronic abdominal pain and diarrhea. J Fam Pract. 2020;69:365,366,368.
Photos courtesy of Mohamed Omar Warsame, MBBS, and Josette R. McMichael, MD
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.

This patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in her stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing. The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
It’s recommended that polyps be removed when technically feasible.3 Pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
This patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. A colonoscopy was normal, and the health care team explained that she would require follow-up and surveillance of her condition due to the high risk of future cancers.
This case was adapted from: Warsame MO, McMichael JR. Chronic abdominal pain and diarrhea. J Fam Pract. 2020;69:365,366,368.
Photos courtesy of Mohamed Omar Warsame, MBBS, and Josette R. McMichael, MD

This patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in her stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing. The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
It’s recommended that polyps be removed when technically feasible.3 Pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
This patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. A colonoscopy was normal, and the health care team explained that she would require follow-up and surveillance of her condition due to the high risk of future cancers.
This case was adapted from: Warsame MO, McMichael JR. Chronic abdominal pain and diarrhea. J Fam Pract. 2020;69:365,366,368.
Photos courtesy of Mohamed Omar Warsame, MBBS, and Josette R. McMichael, MD
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
Bite-sized bouts of exercise: Why they are valuable and what they are missing
Short bursts of activity are approximately as effective for general health as longer sessions, especially for those who are mainly sedentary, according to several recently published studies.
If your fitness goals are greater, and you want to build muscle strength and endurance, compete in a 5K, or just look better in your swimsuit, you will need to do more. But for basic health, it appears that short bursts can help, the new research papers and experts suggest.
“Whether you accumulate activity in many short bouts versus one extended bout, the general health benefits tend to be similar,” Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, said in an interview.
Current public health recommendations from the Centers for Disease Control and Prevention suggest doing at least 150 minutes of moderate intensity physical activity per week for health benefits, but this activity can be accumulated in any way over the week, she noted. Previous versions of the CDC guidelines on exercise suggested that physical activity bouts should be at least 10 minutes each, but the latest version of the guidelines acknowledges that bursts of less than 10 minutes may be beneficial.
However, “the activity or fitness level at which someone starts and the specific health goals matter,” Dr. Paluch continued. “Short bouts may be particularly beneficial for those least active to get moving more to improve their general wellness.”
The current federal physical activity guidelines are still worth striving for, and patients can work their way to this goal, accumulating 150 or more minutes in a way that works best for them, she added.
“There is a lack of research directly comparing individuals who consistently accumulate their activity in many short bouts versus single bouts over an extended period of time,” Dr. Paluch noted. From a public health perspective, since both short and long bouts have health benefits, the best physical activity is what fits into your life and helps build a lifelong habit.
The benefits of exercise for cardiovascular health are well documented. A review from Circulation published in 2003 summarized the benefits of regular physical activity on measures of cardiovascular health including reduction in body weight, blood pressure, and bad cholesterol, while increasing insulin sensitivity, good cholesterol, and muscular strength and function. In that review, author Jonathan N. Myers, PhD, now of Stanford (Calif.) University, noted that “one need not be a marathon runner or an elite athlete to derive significant benefits from physical activity.” In fact, “the greatest gains in terms of mortality are achieved when an individual goes from being sedentary to becoming moderately active.”
A recent large, population-based study showed the value of short bursts of exercise for those previously sedentary. In this study, published in Nature Medicine, a team in Australia used wearable fitness trackers to measure the health benefits of what researchers have named “vigorous intermittent lifestyle physical activity” or VILPA.
Some examples of VILPA include power walking on the way to work, climbing stairs, or even running around with your kids on the playground.
Specifically, individuals who engaged in the median VILPA frequency of three bursts of vigorous activity lasting 1-2 minutes showed a 38%-40% reduction in all-cause mortality risk and cancer mortality risk, and a 48%-49% reduction in cardiovascular mortality risk.
The researchers repeated their analysis for a group of 62,344 adults from the UK Biobank who reported regular vigorous physical activity (VPA). They found similar effects on mortality, based on 1,552 deaths reported.
These results suggest that VILPA may be a reasonable physical activity target, especially for people not able or willing to exercise more formally or intensely, the researchers noted.
“We have known for a long time that leisure-time exercise often reaches vigorous intensity and has many health benefits, but we understand less about the health potential of daily movement, especially activities done as part of daily living that reach vigorous intensity,” lead author Emmanuel Stamatakis, PhD, professor of physical activity, lifestyle and population health at the University of Sydney’s Charles Perkins Centre, said in an interview.
“As long as the heart rate goes up for a minute or 2 it will likely be vigorous activity,” Dr. Stamatakis said in an interview. “It is also important that clinicians effectively communicate how patients can know that they are reaching vigorous intensity,” he said.
Signs of vigorous intensity include increased heart rate and getting out of breath after about 20-40 seconds from the start of the VILPA burst. After about a minute of VILPA, the person doing it should be too out of breath to speak more than a few words comfortably, he said.
Data support value of any and all exercise
The Nature Medicine study supports other recent research showing the value of short, intense bursts of physical activity. A pair of recent studies also used fitness trackers to measure activity in adults and assess the benefits on outcomes including death and heart disease.
One of these studies, which was published in the European Heart Journal, also used fitness trackers to measure physical activity at moderate and vigorous levels. The researchers found that individuals who performed at least 20% of their physical activity at a moderate to high level, such as by doing brisk walking in lieu of strolling had a significantly lower risk of heart disease than those whose daily activity included less than 20% at a moderate or intense level.
In another study from the European Heart Journal, researchers found that short bursts of vigorous physical activity of 2 minutes or less adding up to 15-20 minutes per week was enough to reduce mortality by as much as 40%.
Plus, a meta-analysis published in the Lancet showed a decrease in all-cause mortality with an increase in the number of daily steps, although the impact of stepping rate on mortality was inconsistent.
“Many studies have investigated the health benefits of physical activity, but not the importance of these difficult-to-capture VILPA bouts that accrue during the course of normal activities of daily living,” Lee Stoner, PhD, an exercise physiologist and director of the Cardiometabolic Lab at the University of North Carolina at Chapel Hill, said in an interview.
Dr. Stoner, who was not involved in the Nature Medicine study, said he was not surprised by the overall finding that doing short bursts of activity impacted mortality and cardiovascular disease, but was slightly surprised by the strength of the evidence.
“The referent group in the Nature Medicine study were those accruing no VILPA”, likely meaning they were very inactive,” Dr. Stoner said and added that he thinks this demonstrates the value of VILPA.
Even without immediately meeting the specific numbers recommended by the CDC, “any physical activity is better than none, especially if vigorous, and VILPA can be built into normal daily routines,” Dr. Stoner added.
What’s missing in short bursts?
Short bursts of activity do have their limits when it comes to overall fitness, said Dr. Stoner.
“Endurance will not be improved as much through short bursts, because such activities are unlikely to be as effective at empowering the mitochondria – the batteries keeping our cells running, including skeletal muscle cells,” he said. “Additionally, the vigorous bouts are unlikely to be as effective at improving muscular strength and endurance. For this, it is recommended that we engage each muscle group in strengthening exercises two times per week.”
However, Dr. Stoner agreed that prescribing short bursts of intense activity as part of daily living may be a great way to get people started with exercise.
“The key is to remove barriers to physical activity pursuit, then focusing on long-term routine rather than short-term gain,” he said. “Individuals are better served if they focus on goals other than weight loss, for which physical activity or exercise may not be the solution. Rather, being physically active can improve vigor, make daily activities simpler, and improve cognitive abilities,” and any physical activity is one of the most effective solutions for regulating blood glucose levels and improving cardiovascular risk factors.
Make it routine – and fun
To benefit from physical activity, cultivating and sustaining a long-term routine is key, said Dr. Stoner, whose research has focused on sedentary behavior and cardiovascular disease. Whatever the activity is, shorter bursts, or longer bouts or both, it is essential that individuals figure out activities that they enjoy if they want to create sustained behavior, and thus health change, Gabriel Zieff, MA, a doctoral candidate in Dr. Stoner’s Cardiometabolic Lab, who conducts studies on exercise, noted in an interview.
“We exercise enthusiasts and researchers are often hyperfocused on whether this duration or that duration is better, whether this intensity or that intensity is better,” but at the end of the day, it is the enjoyment factor that often predicts sustained behavior change, and should be part of discussions with patients to help reduce sedentary behavior and promote activity, Mr. Zieff said.
Short bouts can encourage hesitant exercisers
“To best support health, clinicians should consider taking a few seconds to ask patients about their physical activity levels,” said Dr. Paluch, who was the lead author on the Lancet meta-analysis of daily steps. In that study, Dr. Paluch and colleagues found that taking more steps each day was associated with a progressively lower risk of all-cause mortality. However, that study did not measure step rate.
Clinicians can emphasize that health benefits do not require an hour-long exercise routine and special equipment, and moving more, even in shorts bursts of activity can have meaningful associations with health, particularly for those who are less active, she said.
The recent studies on short bursts of activity agree that “some physical activity is better than none and adults should move more throughout the day in whatever way makes sense to them and fits best into their lives,” said Dr. Paluch. “For example, opting for the stairs instead of the elevator, a brisk walk to the bus stop, a short game of hide and seek with the children or grandchildren – anything that gets your body moving more, even if briefly. Making simple lifestyle changes is often easier in small bites. In time, this can grow into long-term habits, ultimately leading to an overall active lifestyle that supports living healthier for longer.”
The Nature Medicine study was supported by the Australian National Health and Medical Research Council. Several coauthors were supported by the Wellcome Trust, the National Institute for Health Research Oxford Biomedical Research Centre, Novo Nordisk, the British Heart Foundation Centre of Research Excellence, the Alan Turing Institute, the British Heart Foundation, and Health Data Research UK, an initiative funded by UK Research and Innovation. Dr. Paluch and Dr. Stoner had no financial conflicts to disclose.
Short bursts of activity are approximately as effective for general health as longer sessions, especially for those who are mainly sedentary, according to several recently published studies.
If your fitness goals are greater, and you want to build muscle strength and endurance, compete in a 5K, or just look better in your swimsuit, you will need to do more. But for basic health, it appears that short bursts can help, the new research papers and experts suggest.
“Whether you accumulate activity in many short bouts versus one extended bout, the general health benefits tend to be similar,” Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, said in an interview.
Current public health recommendations from the Centers for Disease Control and Prevention suggest doing at least 150 minutes of moderate intensity physical activity per week for health benefits, but this activity can be accumulated in any way over the week, she noted. Previous versions of the CDC guidelines on exercise suggested that physical activity bouts should be at least 10 minutes each, but the latest version of the guidelines acknowledges that bursts of less than 10 minutes may be beneficial.
However, “the activity or fitness level at which someone starts and the specific health goals matter,” Dr. Paluch continued. “Short bouts may be particularly beneficial for those least active to get moving more to improve their general wellness.”
The current federal physical activity guidelines are still worth striving for, and patients can work their way to this goal, accumulating 150 or more minutes in a way that works best for them, she added.
“There is a lack of research directly comparing individuals who consistently accumulate their activity in many short bouts versus single bouts over an extended period of time,” Dr. Paluch noted. From a public health perspective, since both short and long bouts have health benefits, the best physical activity is what fits into your life and helps build a lifelong habit.
The benefits of exercise for cardiovascular health are well documented. A review from Circulation published in 2003 summarized the benefits of regular physical activity on measures of cardiovascular health including reduction in body weight, blood pressure, and bad cholesterol, while increasing insulin sensitivity, good cholesterol, and muscular strength and function. In that review, author Jonathan N. Myers, PhD, now of Stanford (Calif.) University, noted that “one need not be a marathon runner or an elite athlete to derive significant benefits from physical activity.” In fact, “the greatest gains in terms of mortality are achieved when an individual goes from being sedentary to becoming moderately active.”
A recent large, population-based study showed the value of short bursts of exercise for those previously sedentary. In this study, published in Nature Medicine, a team in Australia used wearable fitness trackers to measure the health benefits of what researchers have named “vigorous intermittent lifestyle physical activity” or VILPA.
Some examples of VILPA include power walking on the way to work, climbing stairs, or even running around with your kids on the playground.
Specifically, individuals who engaged in the median VILPA frequency of three bursts of vigorous activity lasting 1-2 minutes showed a 38%-40% reduction in all-cause mortality risk and cancer mortality risk, and a 48%-49% reduction in cardiovascular mortality risk.
The researchers repeated their analysis for a group of 62,344 adults from the UK Biobank who reported regular vigorous physical activity (VPA). They found similar effects on mortality, based on 1,552 deaths reported.
These results suggest that VILPA may be a reasonable physical activity target, especially for people not able or willing to exercise more formally or intensely, the researchers noted.
“We have known for a long time that leisure-time exercise often reaches vigorous intensity and has many health benefits, but we understand less about the health potential of daily movement, especially activities done as part of daily living that reach vigorous intensity,” lead author Emmanuel Stamatakis, PhD, professor of physical activity, lifestyle and population health at the University of Sydney’s Charles Perkins Centre, said in an interview.
“As long as the heart rate goes up for a minute or 2 it will likely be vigorous activity,” Dr. Stamatakis said in an interview. “It is also important that clinicians effectively communicate how patients can know that they are reaching vigorous intensity,” he said.
Signs of vigorous intensity include increased heart rate and getting out of breath after about 20-40 seconds from the start of the VILPA burst. After about a minute of VILPA, the person doing it should be too out of breath to speak more than a few words comfortably, he said.
Data support value of any and all exercise
The Nature Medicine study supports other recent research showing the value of short, intense bursts of physical activity. A pair of recent studies also used fitness trackers to measure activity in adults and assess the benefits on outcomes including death and heart disease.
One of these studies, which was published in the European Heart Journal, also used fitness trackers to measure physical activity at moderate and vigorous levels. The researchers found that individuals who performed at least 20% of their physical activity at a moderate to high level, such as by doing brisk walking in lieu of strolling had a significantly lower risk of heart disease than those whose daily activity included less than 20% at a moderate or intense level.
In another study from the European Heart Journal, researchers found that short bursts of vigorous physical activity of 2 minutes or less adding up to 15-20 minutes per week was enough to reduce mortality by as much as 40%.
Plus, a meta-analysis published in the Lancet showed a decrease in all-cause mortality with an increase in the number of daily steps, although the impact of stepping rate on mortality was inconsistent.
“Many studies have investigated the health benefits of physical activity, but not the importance of these difficult-to-capture VILPA bouts that accrue during the course of normal activities of daily living,” Lee Stoner, PhD, an exercise physiologist and director of the Cardiometabolic Lab at the University of North Carolina at Chapel Hill, said in an interview.
Dr. Stoner, who was not involved in the Nature Medicine study, said he was not surprised by the overall finding that doing short bursts of activity impacted mortality and cardiovascular disease, but was slightly surprised by the strength of the evidence.
“The referent group in the Nature Medicine study were those accruing no VILPA”, likely meaning they were very inactive,” Dr. Stoner said and added that he thinks this demonstrates the value of VILPA.
Even without immediately meeting the specific numbers recommended by the CDC, “any physical activity is better than none, especially if vigorous, and VILPA can be built into normal daily routines,” Dr. Stoner added.
What’s missing in short bursts?
Short bursts of activity do have their limits when it comes to overall fitness, said Dr. Stoner.
“Endurance will not be improved as much through short bursts, because such activities are unlikely to be as effective at empowering the mitochondria – the batteries keeping our cells running, including skeletal muscle cells,” he said. “Additionally, the vigorous bouts are unlikely to be as effective at improving muscular strength and endurance. For this, it is recommended that we engage each muscle group in strengthening exercises two times per week.”
However, Dr. Stoner agreed that prescribing short bursts of intense activity as part of daily living may be a great way to get people started with exercise.
“The key is to remove barriers to physical activity pursuit, then focusing on long-term routine rather than short-term gain,” he said. “Individuals are better served if they focus on goals other than weight loss, for which physical activity or exercise may not be the solution. Rather, being physically active can improve vigor, make daily activities simpler, and improve cognitive abilities,” and any physical activity is one of the most effective solutions for regulating blood glucose levels and improving cardiovascular risk factors.
Make it routine – and fun
To benefit from physical activity, cultivating and sustaining a long-term routine is key, said Dr. Stoner, whose research has focused on sedentary behavior and cardiovascular disease. Whatever the activity is, shorter bursts, or longer bouts or both, it is essential that individuals figure out activities that they enjoy if they want to create sustained behavior, and thus health change, Gabriel Zieff, MA, a doctoral candidate in Dr. Stoner’s Cardiometabolic Lab, who conducts studies on exercise, noted in an interview.
“We exercise enthusiasts and researchers are often hyperfocused on whether this duration or that duration is better, whether this intensity or that intensity is better,” but at the end of the day, it is the enjoyment factor that often predicts sustained behavior change, and should be part of discussions with patients to help reduce sedentary behavior and promote activity, Mr. Zieff said.
Short bouts can encourage hesitant exercisers
“To best support health, clinicians should consider taking a few seconds to ask patients about their physical activity levels,” said Dr. Paluch, who was the lead author on the Lancet meta-analysis of daily steps. In that study, Dr. Paluch and colleagues found that taking more steps each day was associated with a progressively lower risk of all-cause mortality. However, that study did not measure step rate.
Clinicians can emphasize that health benefits do not require an hour-long exercise routine and special equipment, and moving more, even in shorts bursts of activity can have meaningful associations with health, particularly for those who are less active, she said.
The recent studies on short bursts of activity agree that “some physical activity is better than none and adults should move more throughout the day in whatever way makes sense to them and fits best into their lives,” said Dr. Paluch. “For example, opting for the stairs instead of the elevator, a brisk walk to the bus stop, a short game of hide and seek with the children or grandchildren – anything that gets your body moving more, even if briefly. Making simple lifestyle changes is often easier in small bites. In time, this can grow into long-term habits, ultimately leading to an overall active lifestyle that supports living healthier for longer.”
The Nature Medicine study was supported by the Australian National Health and Medical Research Council. Several coauthors were supported by the Wellcome Trust, the National Institute for Health Research Oxford Biomedical Research Centre, Novo Nordisk, the British Heart Foundation Centre of Research Excellence, the Alan Turing Institute, the British Heart Foundation, and Health Data Research UK, an initiative funded by UK Research and Innovation. Dr. Paluch and Dr. Stoner had no financial conflicts to disclose.
Short bursts of activity are approximately as effective for general health as longer sessions, especially for those who are mainly sedentary, according to several recently published studies.
If your fitness goals are greater, and you want to build muscle strength and endurance, compete in a 5K, or just look better in your swimsuit, you will need to do more. But for basic health, it appears that short bursts can help, the new research papers and experts suggest.
“Whether you accumulate activity in many short bouts versus one extended bout, the general health benefits tend to be similar,” Amanda Paluch, PhD, a physical activity epidemiologist at the University of Massachusetts, Amherst, said in an interview.
Current public health recommendations from the Centers for Disease Control and Prevention suggest doing at least 150 minutes of moderate intensity physical activity per week for health benefits, but this activity can be accumulated in any way over the week, she noted. Previous versions of the CDC guidelines on exercise suggested that physical activity bouts should be at least 10 minutes each, but the latest version of the guidelines acknowledges that bursts of less than 10 minutes may be beneficial.
However, “the activity or fitness level at which someone starts and the specific health goals matter,” Dr. Paluch continued. “Short bouts may be particularly beneficial for those least active to get moving more to improve their general wellness.”
The current federal physical activity guidelines are still worth striving for, and patients can work their way to this goal, accumulating 150 or more minutes in a way that works best for them, she added.
“There is a lack of research directly comparing individuals who consistently accumulate their activity in many short bouts versus single bouts over an extended period of time,” Dr. Paluch noted. From a public health perspective, since both short and long bouts have health benefits, the best physical activity is what fits into your life and helps build a lifelong habit.
The benefits of exercise for cardiovascular health are well documented. A review from Circulation published in 2003 summarized the benefits of regular physical activity on measures of cardiovascular health including reduction in body weight, blood pressure, and bad cholesterol, while increasing insulin sensitivity, good cholesterol, and muscular strength and function. In that review, author Jonathan N. Myers, PhD, now of Stanford (Calif.) University, noted that “one need not be a marathon runner or an elite athlete to derive significant benefits from physical activity.” In fact, “the greatest gains in terms of mortality are achieved when an individual goes from being sedentary to becoming moderately active.”
A recent large, population-based study showed the value of short bursts of exercise for those previously sedentary. In this study, published in Nature Medicine, a team in Australia used wearable fitness trackers to measure the health benefits of what researchers have named “vigorous intermittent lifestyle physical activity” or VILPA.
Some examples of VILPA include power walking on the way to work, climbing stairs, or even running around with your kids on the playground.
Specifically, individuals who engaged in the median VILPA frequency of three bursts of vigorous activity lasting 1-2 minutes showed a 38%-40% reduction in all-cause mortality risk and cancer mortality risk, and a 48%-49% reduction in cardiovascular mortality risk.
The researchers repeated their analysis for a group of 62,344 adults from the UK Biobank who reported regular vigorous physical activity (VPA). They found similar effects on mortality, based on 1,552 deaths reported.
These results suggest that VILPA may be a reasonable physical activity target, especially for people not able or willing to exercise more formally or intensely, the researchers noted.
“We have known for a long time that leisure-time exercise often reaches vigorous intensity and has many health benefits, but we understand less about the health potential of daily movement, especially activities done as part of daily living that reach vigorous intensity,” lead author Emmanuel Stamatakis, PhD, professor of physical activity, lifestyle and population health at the University of Sydney’s Charles Perkins Centre, said in an interview.
“As long as the heart rate goes up for a minute or 2 it will likely be vigorous activity,” Dr. Stamatakis said in an interview. “It is also important that clinicians effectively communicate how patients can know that they are reaching vigorous intensity,” he said.
Signs of vigorous intensity include increased heart rate and getting out of breath after about 20-40 seconds from the start of the VILPA burst. After about a minute of VILPA, the person doing it should be too out of breath to speak more than a few words comfortably, he said.
Data support value of any and all exercise
The Nature Medicine study supports other recent research showing the value of short, intense bursts of physical activity. A pair of recent studies also used fitness trackers to measure activity in adults and assess the benefits on outcomes including death and heart disease.
One of these studies, which was published in the European Heart Journal, also used fitness trackers to measure physical activity at moderate and vigorous levels. The researchers found that individuals who performed at least 20% of their physical activity at a moderate to high level, such as by doing brisk walking in lieu of strolling had a significantly lower risk of heart disease than those whose daily activity included less than 20% at a moderate or intense level.
In another study from the European Heart Journal, researchers found that short bursts of vigorous physical activity of 2 minutes or less adding up to 15-20 minutes per week was enough to reduce mortality by as much as 40%.
Plus, a meta-analysis published in the Lancet showed a decrease in all-cause mortality with an increase in the number of daily steps, although the impact of stepping rate on mortality was inconsistent.
“Many studies have investigated the health benefits of physical activity, but not the importance of these difficult-to-capture VILPA bouts that accrue during the course of normal activities of daily living,” Lee Stoner, PhD, an exercise physiologist and director of the Cardiometabolic Lab at the University of North Carolina at Chapel Hill, said in an interview.
Dr. Stoner, who was not involved in the Nature Medicine study, said he was not surprised by the overall finding that doing short bursts of activity impacted mortality and cardiovascular disease, but was slightly surprised by the strength of the evidence.
“The referent group in the Nature Medicine study were those accruing no VILPA”, likely meaning they were very inactive,” Dr. Stoner said and added that he thinks this demonstrates the value of VILPA.
Even without immediately meeting the specific numbers recommended by the CDC, “any physical activity is better than none, especially if vigorous, and VILPA can be built into normal daily routines,” Dr. Stoner added.
What’s missing in short bursts?
Short bursts of activity do have their limits when it comes to overall fitness, said Dr. Stoner.
“Endurance will not be improved as much through short bursts, because such activities are unlikely to be as effective at empowering the mitochondria – the batteries keeping our cells running, including skeletal muscle cells,” he said. “Additionally, the vigorous bouts are unlikely to be as effective at improving muscular strength and endurance. For this, it is recommended that we engage each muscle group in strengthening exercises two times per week.”
However, Dr. Stoner agreed that prescribing short bursts of intense activity as part of daily living may be a great way to get people started with exercise.
“The key is to remove barriers to physical activity pursuit, then focusing on long-term routine rather than short-term gain,” he said. “Individuals are better served if they focus on goals other than weight loss, for which physical activity or exercise may not be the solution. Rather, being physically active can improve vigor, make daily activities simpler, and improve cognitive abilities,” and any physical activity is one of the most effective solutions for regulating blood glucose levels and improving cardiovascular risk factors.
Make it routine – and fun
To benefit from physical activity, cultivating and sustaining a long-term routine is key, said Dr. Stoner, whose research has focused on sedentary behavior and cardiovascular disease. Whatever the activity is, shorter bursts, or longer bouts or both, it is essential that individuals figure out activities that they enjoy if they want to create sustained behavior, and thus health change, Gabriel Zieff, MA, a doctoral candidate in Dr. Stoner’s Cardiometabolic Lab, who conducts studies on exercise, noted in an interview.
“We exercise enthusiasts and researchers are often hyperfocused on whether this duration or that duration is better, whether this intensity or that intensity is better,” but at the end of the day, it is the enjoyment factor that often predicts sustained behavior change, and should be part of discussions with patients to help reduce sedentary behavior and promote activity, Mr. Zieff said.
Short bouts can encourage hesitant exercisers
“To best support health, clinicians should consider taking a few seconds to ask patients about their physical activity levels,” said Dr. Paluch, who was the lead author on the Lancet meta-analysis of daily steps. In that study, Dr. Paluch and colleagues found that taking more steps each day was associated with a progressively lower risk of all-cause mortality. However, that study did not measure step rate.
Clinicians can emphasize that health benefits do not require an hour-long exercise routine and special equipment, and moving more, even in shorts bursts of activity can have meaningful associations with health, particularly for those who are less active, she said.
The recent studies on short bursts of activity agree that “some physical activity is better than none and adults should move more throughout the day in whatever way makes sense to them and fits best into their lives,” said Dr. Paluch. “For example, opting for the stairs instead of the elevator, a brisk walk to the bus stop, a short game of hide and seek with the children or grandchildren – anything that gets your body moving more, even if briefly. Making simple lifestyle changes is often easier in small bites. In time, this can grow into long-term habits, ultimately leading to an overall active lifestyle that supports living healthier for longer.”
The Nature Medicine study was supported by the Australian National Health and Medical Research Council. Several coauthors were supported by the Wellcome Trust, the National Institute for Health Research Oxford Biomedical Research Centre, Novo Nordisk, the British Heart Foundation Centre of Research Excellence, the Alan Turing Institute, the British Heart Foundation, and Health Data Research UK, an initiative funded by UK Research and Innovation. Dr. Paluch and Dr. Stoner had no financial conflicts to disclose.
Fitbit figures: More steps per day cut type 2 diabetes risk
The protective effect of daily step count on type 2 diabetes risk remained after adjusting for smoking and sedentary time.
Taking more steps per day was also associated with less risk of developing type 2 diabetes in different subgroups of physical activity intensity.
“Our data shows the importance of moving your body every day to lower your risk of [type 2] diabetes,” said the lead author of the research, Andrew S. Perry, MD. The findings were published online in the Journal of Clinical Endocrinology & Metabolism.
Despite low baseline risk, benefit from increased physical activity
The study was conducted in more than 5,000 participants in the National Institutes of Health’s All of Us research program who had a median age of 51 and were generally overweight (median BMI 27.8 kg/m2). Three quarters were women and 89% were White.
It used an innovative approach in a real-world population, said Dr. Perry, of Vanderbilt University Medical Center in Nashville, Tenn.
The individuals in this cohort had relatively few risk factors, so it was not surprising that the incidence of type 2 diabetes overall was low (2%), the researchers note. “Yet, despite being low risk, we still detected a signal of benefit from increased” physical activity, Dr. Perry and colleagues write.
The individuals had a median of 16 very active minutes/day, which corresponds to 112 very active minutes/week (ie, less than the guideline-recommended 150 minutes of physical activity/week).
“These results indicate that amounts of physical activity are correlated with lower risk of [type 2] diabetes, regardless of the intensity level, and even at amounts less than current guidelines recommend,” the researchers summarize.
Physical activity tracked over close to 4 years
Prior studies of the relationship between physical activity and type 2 diabetes risk relied primarily on questionnaires that asked people about physical activity at one point in time.
The researchers aimed to examine this association over time, in a contemporary cohort of Fitbit users who participated in the All of Us program.
From 12,781 participants with Fitbit data between 2010 and 2021, they identified 5,677 individuals who were at least 18 years old and had linked electronic health record data, no diabetes at baseline, at least 15 days of Fitbit data in the initial monitoring period, and at least 180 days of follow-up.
The Fitbit counts steps, and it also uses an algorithm to quantify physical activity intensity as lightly active (1.5-3 metabolic equivalent task (METs), fairly active (3-6 METs), and very active (> 6 METs).
During a median 3.8-year follow-up, participants made a median of 7,924 steps/day and were “fairly active” for a median of 16 minutes/day.
They found 97 new cases of type 2 diabetes over a follow-up of 4 years in the dataset.
The predicted cumulative incidence of type 2 diabetes at 5 years was 0.8% for individuals who walked 13,245 steps/day (90th percentile) vs. 2.3% for those who walked 4,301 steps/day (10th percentile).
“We hope to study more diverse populations in future studies to confirm the generalizability of these findings,” Dr. Perry said.
This study received funding from the National Heart, Lung, and Blood Institute. Dr. Perry reports no relevant financial relationships. Disclosures for the other authors are listed with the original article.
A version of this article first appeared on Medscape.com.
The protective effect of daily step count on type 2 diabetes risk remained after adjusting for smoking and sedentary time.
Taking more steps per day was also associated with less risk of developing type 2 diabetes in different subgroups of physical activity intensity.
“Our data shows the importance of moving your body every day to lower your risk of [type 2] diabetes,” said the lead author of the research, Andrew S. Perry, MD. The findings were published online in the Journal of Clinical Endocrinology & Metabolism.
Despite low baseline risk, benefit from increased physical activity
The study was conducted in more than 5,000 participants in the National Institutes of Health’s All of Us research program who had a median age of 51 and were generally overweight (median BMI 27.8 kg/m2). Three quarters were women and 89% were White.
It used an innovative approach in a real-world population, said Dr. Perry, of Vanderbilt University Medical Center in Nashville, Tenn.
The individuals in this cohort had relatively few risk factors, so it was not surprising that the incidence of type 2 diabetes overall was low (2%), the researchers note. “Yet, despite being low risk, we still detected a signal of benefit from increased” physical activity, Dr. Perry and colleagues write.
The individuals had a median of 16 very active minutes/day, which corresponds to 112 very active minutes/week (ie, less than the guideline-recommended 150 minutes of physical activity/week).
“These results indicate that amounts of physical activity are correlated with lower risk of [type 2] diabetes, regardless of the intensity level, and even at amounts less than current guidelines recommend,” the researchers summarize.
Physical activity tracked over close to 4 years
Prior studies of the relationship between physical activity and type 2 diabetes risk relied primarily on questionnaires that asked people about physical activity at one point in time.
The researchers aimed to examine this association over time, in a contemporary cohort of Fitbit users who participated in the All of Us program.
From 12,781 participants with Fitbit data between 2010 and 2021, they identified 5,677 individuals who were at least 18 years old and had linked electronic health record data, no diabetes at baseline, at least 15 days of Fitbit data in the initial monitoring period, and at least 180 days of follow-up.
The Fitbit counts steps, and it also uses an algorithm to quantify physical activity intensity as lightly active (1.5-3 metabolic equivalent task (METs), fairly active (3-6 METs), and very active (> 6 METs).
During a median 3.8-year follow-up, participants made a median of 7,924 steps/day and were “fairly active” for a median of 16 minutes/day.
They found 97 new cases of type 2 diabetes over a follow-up of 4 years in the dataset.
The predicted cumulative incidence of type 2 diabetes at 5 years was 0.8% for individuals who walked 13,245 steps/day (90th percentile) vs. 2.3% for those who walked 4,301 steps/day (10th percentile).
“We hope to study more diverse populations in future studies to confirm the generalizability of these findings,” Dr. Perry said.
This study received funding from the National Heart, Lung, and Blood Institute. Dr. Perry reports no relevant financial relationships. Disclosures for the other authors are listed with the original article.
A version of this article first appeared on Medscape.com.
The protective effect of daily step count on type 2 diabetes risk remained after adjusting for smoking and sedentary time.
Taking more steps per day was also associated with less risk of developing type 2 diabetes in different subgroups of physical activity intensity.
“Our data shows the importance of moving your body every day to lower your risk of [type 2] diabetes,” said the lead author of the research, Andrew S. Perry, MD. The findings were published online in the Journal of Clinical Endocrinology & Metabolism.
Despite low baseline risk, benefit from increased physical activity
The study was conducted in more than 5,000 participants in the National Institutes of Health’s All of Us research program who had a median age of 51 and were generally overweight (median BMI 27.8 kg/m2). Three quarters were women and 89% were White.
It used an innovative approach in a real-world population, said Dr. Perry, of Vanderbilt University Medical Center in Nashville, Tenn.
The individuals in this cohort had relatively few risk factors, so it was not surprising that the incidence of type 2 diabetes overall was low (2%), the researchers note. “Yet, despite being low risk, we still detected a signal of benefit from increased” physical activity, Dr. Perry and colleagues write.
The individuals had a median of 16 very active minutes/day, which corresponds to 112 very active minutes/week (ie, less than the guideline-recommended 150 minutes of physical activity/week).
“These results indicate that amounts of physical activity are correlated with lower risk of [type 2] diabetes, regardless of the intensity level, and even at amounts less than current guidelines recommend,” the researchers summarize.
Physical activity tracked over close to 4 years
Prior studies of the relationship between physical activity and type 2 diabetes risk relied primarily on questionnaires that asked people about physical activity at one point in time.
The researchers aimed to examine this association over time, in a contemporary cohort of Fitbit users who participated in the All of Us program.
From 12,781 participants with Fitbit data between 2010 and 2021, they identified 5,677 individuals who were at least 18 years old and had linked electronic health record data, no diabetes at baseline, at least 15 days of Fitbit data in the initial monitoring period, and at least 180 days of follow-up.
The Fitbit counts steps, and it also uses an algorithm to quantify physical activity intensity as lightly active (1.5-3 metabolic equivalent task (METs), fairly active (3-6 METs), and very active (> 6 METs).
During a median 3.8-year follow-up, participants made a median of 7,924 steps/day and were “fairly active” for a median of 16 minutes/day.
They found 97 new cases of type 2 diabetes over a follow-up of 4 years in the dataset.
The predicted cumulative incidence of type 2 diabetes at 5 years was 0.8% for individuals who walked 13,245 steps/day (90th percentile) vs. 2.3% for those who walked 4,301 steps/day (10th percentile).
“We hope to study more diverse populations in future studies to confirm the generalizability of these findings,” Dr. Perry said.
This study received funding from the National Heart, Lung, and Blood Institute. Dr. Perry reports no relevant financial relationships. Disclosures for the other authors are listed with the original article.
A version of this article first appeared on Medscape.com.
Rise of ‘alarming’ subvariants of COVID ‘worrisome’ for winter
It’s a story perhaps more appropriate for Halloween than for the festive holiday season, given its scary implications.
Not too dire so far, until the researchers’ other findings are considered.
The BQ.1, BQ1.1, XBB, and XBB.1 subvariants are the most resistant to neutralizing antibodies, researcher Qian Wang, PhD, and colleagues wrote in a study published online in the journal Cell. This means people have no or “markedly reduced” protection against infection from these four strains, even if they’ve already had COVID-19 or are vaccinated and boosted multiple times, including with a bivalent vaccine.
On top of that, all available monoclonal antibody treatments are mostly or completely ineffective against these subvariants.
What does that mean for the immediate future? The findings are definitely “worrisome,” said Eric Topol, MD, founder and director of the Scripps Translational Research Institute in La Jolla, Calif.
But evidence from other countries, specifically Singapore and France, show that at least two of these variants turned out not to be as damaging as expected, likely because of high numbers of people vaccinated or who survived previous infections, he said.
Still, there is little to celebrate in the new findings, except that COVID-19 vaccinations and prior infections can still reduce the risk for serious outcomes such as hospitalization and death, the researchers wrote.
In fact, Centers for Disease Control and Prevention data released on Dec. 16 shows that people who have received four shots of the original COVID-19 vaccines as well as the bivalent booster were 57% less likely to visit an urgent care clinic or emergency room, regardless of age.
It comes at a time when BQ.1 and BQ.1.1 account for about 70% of the circulating variants, data show. In addition, hospitalizations are up 18% over the past 2 weeks and COVID-19 deaths are up 50% nationwide, The New York Times reported.
Globally, in many places, an “immunity wall” that has been built, Dr. Topol said. That may not be the case in the United States.
“The problem in the United States, making it harder to predict, is that we have a very low rate of recent boosters, in the past 6 months, especially in seniors,” he said. For example, only 36% of Americans aged 65 years and older, the group with highest risk, have received an updated bivalent booster.
An evolving virus
The subvariants are successfully replacing BA.5, which reigned as one of the most common Omicron variants over the past year. The latest CDC data show that BA.5 now accounts for only about 10% of the circulating virus. The researchers wrote: “This rapid replacement of virus strains is raising the specter of yet another wave of infections in the coming months.”
BQ.1 and BQ.1.1 evolved directly from BA.5 – adding more and some novel mutations to the SARS-CoV-2 virus. XBB and XBB.1 are the “offspring” of a combination of two other strains, known as BJ.1 and BA.2.75.
The story sounds familiar to the researchers. “The rapid rise of these subvariants and their extensive array of spike mutations are reminiscent of the appearance of the first Omicron variant last year, thus raising concerns that they may further compromise the efficacy of current COVID-19 vaccines and monoclonal antibody therapeutics,” they wrote. “We now report findings that indicate that such concerns are, sadly, justified, especially so for the XBB and XBB.1 subvariants.”
To figure out how effective existing antibodies could be against these newer subvariants, Dr. Wang and colleagues used blood samples from five groups of people. They tested serum from people who had three doses of the original COVID-19 vaccine, four doses of the original vaccine, those who received a bivalent booster, people who experienced a breakthrough infection with the BA.2 Omicron variant, and those who had a breakthrough with a BA.4 or BA.5 variant.
Adding the new subvariants to these serum samples revealed that the existing antibodies in the blood were ineffective at wiping out or neutralizing BQ.1, BQ.1.1, XBB, and XBB.1.
The BQ.1 subvariant was six times more resistant to antibodies than BA.5, its parent strain, and XBB.1 was 63 times more resistant compared with its predecessor, BA.2.
This shift in the ability of vaccines to stop the subvariants “is particularly concerning,” the researchers wrote.
Wiping out treatments too
Dr. Wang and colleagues also tested how well a panel of 23 different monoclonal antibody drugs might work against the four subvariants. The therapies all worked well against the original Omicron variant and included some approved for use through the Food and Drug Administration emergency use authorization (EUA) program at the time of the study.
They found that 19 of these 23 monoclonal antibodies lost effectiveness “greatly or completely” against XBB and XBB.1, for example.
This is not the first time that monoclonal antibody therapies have gone from effective to ineffective. Previous variants have come out that no longer responded to treatment with bamlanivimab, etesevimab, imdevimab, casirivimab, tixagevimab, cilgavimab, and sotrovimab. Bebtelovimab now joins this list and is no longer available from Eli Lilly under EUA because of this lack of effectiveness.
The lack of an effective monoclonal antibody treatment “poses a serious problem for millions of immunocompromised individuals who do not respond robustly to COVID-19 vaccines,” the researchers wrote, adding that “the urgent need to develop active monoclonal antibodies for clinical use is obvious.”
A limitation of the study is that the work is done in blood samples. The effectiveness of COVID-19 vaccination against the BQ and XBB subvariants should be evaluated in people in clinical studies, the authors noted.
Also, the current study looked at how well antibodies could neutralize the viral strains, but future research, they added, should look at how well “cellular immunity” or other aspects of the immune system might protect people.
Going forward, the challenge remains to develop vaccines and treatments that offer broad protection as the coronavirus continues to evolve.
In an alarming ending, the researchers wrote: “We have collectively chased after SARS-CoV-2 variants for over 2 years, and yet, the virus continues to evolve and evade.”
A version of this article first appeared on Medscape.com.
It’s a story perhaps more appropriate for Halloween than for the festive holiday season, given its scary implications.
Not too dire so far, until the researchers’ other findings are considered.
The BQ.1, BQ1.1, XBB, and XBB.1 subvariants are the most resistant to neutralizing antibodies, researcher Qian Wang, PhD, and colleagues wrote in a study published online in the journal Cell. This means people have no or “markedly reduced” protection against infection from these four strains, even if they’ve already had COVID-19 or are vaccinated and boosted multiple times, including with a bivalent vaccine.
On top of that, all available monoclonal antibody treatments are mostly or completely ineffective against these subvariants.
What does that mean for the immediate future? The findings are definitely “worrisome,” said Eric Topol, MD, founder and director of the Scripps Translational Research Institute in La Jolla, Calif.
But evidence from other countries, specifically Singapore and France, show that at least two of these variants turned out not to be as damaging as expected, likely because of high numbers of people vaccinated or who survived previous infections, he said.
Still, there is little to celebrate in the new findings, except that COVID-19 vaccinations and prior infections can still reduce the risk for serious outcomes such as hospitalization and death, the researchers wrote.
In fact, Centers for Disease Control and Prevention data released on Dec. 16 shows that people who have received four shots of the original COVID-19 vaccines as well as the bivalent booster were 57% less likely to visit an urgent care clinic or emergency room, regardless of age.
It comes at a time when BQ.1 and BQ.1.1 account for about 70% of the circulating variants, data show. In addition, hospitalizations are up 18% over the past 2 weeks and COVID-19 deaths are up 50% nationwide, The New York Times reported.
Globally, in many places, an “immunity wall” that has been built, Dr. Topol said. That may not be the case in the United States.
“The problem in the United States, making it harder to predict, is that we have a very low rate of recent boosters, in the past 6 months, especially in seniors,” he said. For example, only 36% of Americans aged 65 years and older, the group with highest risk, have received an updated bivalent booster.
An evolving virus
The subvariants are successfully replacing BA.5, which reigned as one of the most common Omicron variants over the past year. The latest CDC data show that BA.5 now accounts for only about 10% of the circulating virus. The researchers wrote: “This rapid replacement of virus strains is raising the specter of yet another wave of infections in the coming months.”
BQ.1 and BQ.1.1 evolved directly from BA.5 – adding more and some novel mutations to the SARS-CoV-2 virus. XBB and XBB.1 are the “offspring” of a combination of two other strains, known as BJ.1 and BA.2.75.
The story sounds familiar to the researchers. “The rapid rise of these subvariants and their extensive array of spike mutations are reminiscent of the appearance of the first Omicron variant last year, thus raising concerns that they may further compromise the efficacy of current COVID-19 vaccines and monoclonal antibody therapeutics,” they wrote. “We now report findings that indicate that such concerns are, sadly, justified, especially so for the XBB and XBB.1 subvariants.”
To figure out how effective existing antibodies could be against these newer subvariants, Dr. Wang and colleagues used blood samples from five groups of people. They tested serum from people who had three doses of the original COVID-19 vaccine, four doses of the original vaccine, those who received a bivalent booster, people who experienced a breakthrough infection with the BA.2 Omicron variant, and those who had a breakthrough with a BA.4 or BA.5 variant.
Adding the new subvariants to these serum samples revealed that the existing antibodies in the blood were ineffective at wiping out or neutralizing BQ.1, BQ.1.1, XBB, and XBB.1.
The BQ.1 subvariant was six times more resistant to antibodies than BA.5, its parent strain, and XBB.1 was 63 times more resistant compared with its predecessor, BA.2.
This shift in the ability of vaccines to stop the subvariants “is particularly concerning,” the researchers wrote.
Wiping out treatments too
Dr. Wang and colleagues also tested how well a panel of 23 different monoclonal antibody drugs might work against the four subvariants. The therapies all worked well against the original Omicron variant and included some approved for use through the Food and Drug Administration emergency use authorization (EUA) program at the time of the study.
They found that 19 of these 23 monoclonal antibodies lost effectiveness “greatly or completely” against XBB and XBB.1, for example.
This is not the first time that monoclonal antibody therapies have gone from effective to ineffective. Previous variants have come out that no longer responded to treatment with bamlanivimab, etesevimab, imdevimab, casirivimab, tixagevimab, cilgavimab, and sotrovimab. Bebtelovimab now joins this list and is no longer available from Eli Lilly under EUA because of this lack of effectiveness.
The lack of an effective monoclonal antibody treatment “poses a serious problem for millions of immunocompromised individuals who do not respond robustly to COVID-19 vaccines,” the researchers wrote, adding that “the urgent need to develop active monoclonal antibodies for clinical use is obvious.”
A limitation of the study is that the work is done in blood samples. The effectiveness of COVID-19 vaccination against the BQ and XBB subvariants should be evaluated in people in clinical studies, the authors noted.
Also, the current study looked at how well antibodies could neutralize the viral strains, but future research, they added, should look at how well “cellular immunity” or other aspects of the immune system might protect people.
Going forward, the challenge remains to develop vaccines and treatments that offer broad protection as the coronavirus continues to evolve.
In an alarming ending, the researchers wrote: “We have collectively chased after SARS-CoV-2 variants for over 2 years, and yet, the virus continues to evolve and evade.”
A version of this article first appeared on Medscape.com.
It’s a story perhaps more appropriate for Halloween than for the festive holiday season, given its scary implications.
Not too dire so far, until the researchers’ other findings are considered.
The BQ.1, BQ1.1, XBB, and XBB.1 subvariants are the most resistant to neutralizing antibodies, researcher Qian Wang, PhD, and colleagues wrote in a study published online in the journal Cell. This means people have no or “markedly reduced” protection against infection from these four strains, even if they’ve already had COVID-19 or are vaccinated and boosted multiple times, including with a bivalent vaccine.
On top of that, all available monoclonal antibody treatments are mostly or completely ineffective against these subvariants.
What does that mean for the immediate future? The findings are definitely “worrisome,” said Eric Topol, MD, founder and director of the Scripps Translational Research Institute in La Jolla, Calif.
But evidence from other countries, specifically Singapore and France, show that at least two of these variants turned out not to be as damaging as expected, likely because of high numbers of people vaccinated or who survived previous infections, he said.
Still, there is little to celebrate in the new findings, except that COVID-19 vaccinations and prior infections can still reduce the risk for serious outcomes such as hospitalization and death, the researchers wrote.
In fact, Centers for Disease Control and Prevention data released on Dec. 16 shows that people who have received four shots of the original COVID-19 vaccines as well as the bivalent booster were 57% less likely to visit an urgent care clinic or emergency room, regardless of age.
It comes at a time when BQ.1 and BQ.1.1 account for about 70% of the circulating variants, data show. In addition, hospitalizations are up 18% over the past 2 weeks and COVID-19 deaths are up 50% nationwide, The New York Times reported.
Globally, in many places, an “immunity wall” that has been built, Dr. Topol said. That may not be the case in the United States.
“The problem in the United States, making it harder to predict, is that we have a very low rate of recent boosters, in the past 6 months, especially in seniors,” he said. For example, only 36% of Americans aged 65 years and older, the group with highest risk, have received an updated bivalent booster.
An evolving virus
The subvariants are successfully replacing BA.5, which reigned as one of the most common Omicron variants over the past year. The latest CDC data show that BA.5 now accounts for only about 10% of the circulating virus. The researchers wrote: “This rapid replacement of virus strains is raising the specter of yet another wave of infections in the coming months.”
BQ.1 and BQ.1.1 evolved directly from BA.5 – adding more and some novel mutations to the SARS-CoV-2 virus. XBB and XBB.1 are the “offspring” of a combination of two other strains, known as BJ.1 and BA.2.75.
The story sounds familiar to the researchers. “The rapid rise of these subvariants and their extensive array of spike mutations are reminiscent of the appearance of the first Omicron variant last year, thus raising concerns that they may further compromise the efficacy of current COVID-19 vaccines and monoclonal antibody therapeutics,” they wrote. “We now report findings that indicate that such concerns are, sadly, justified, especially so for the XBB and XBB.1 subvariants.”
To figure out how effective existing antibodies could be against these newer subvariants, Dr. Wang and colleagues used blood samples from five groups of people. They tested serum from people who had three doses of the original COVID-19 vaccine, four doses of the original vaccine, those who received a bivalent booster, people who experienced a breakthrough infection with the BA.2 Omicron variant, and those who had a breakthrough with a BA.4 or BA.5 variant.
Adding the new subvariants to these serum samples revealed that the existing antibodies in the blood were ineffective at wiping out or neutralizing BQ.1, BQ.1.1, XBB, and XBB.1.
The BQ.1 subvariant was six times more resistant to antibodies than BA.5, its parent strain, and XBB.1 was 63 times more resistant compared with its predecessor, BA.2.
This shift in the ability of vaccines to stop the subvariants “is particularly concerning,” the researchers wrote.
Wiping out treatments too
Dr. Wang and colleagues also tested how well a panel of 23 different monoclonal antibody drugs might work against the four subvariants. The therapies all worked well against the original Omicron variant and included some approved for use through the Food and Drug Administration emergency use authorization (EUA) program at the time of the study.
They found that 19 of these 23 monoclonal antibodies lost effectiveness “greatly or completely” against XBB and XBB.1, for example.
This is not the first time that monoclonal antibody therapies have gone from effective to ineffective. Previous variants have come out that no longer responded to treatment with bamlanivimab, etesevimab, imdevimab, casirivimab, tixagevimab, cilgavimab, and sotrovimab. Bebtelovimab now joins this list and is no longer available from Eli Lilly under EUA because of this lack of effectiveness.
The lack of an effective monoclonal antibody treatment “poses a serious problem for millions of immunocompromised individuals who do not respond robustly to COVID-19 vaccines,” the researchers wrote, adding that “the urgent need to develop active monoclonal antibodies for clinical use is obvious.”
A limitation of the study is that the work is done in blood samples. The effectiveness of COVID-19 vaccination against the BQ and XBB subvariants should be evaluated in people in clinical studies, the authors noted.
Also, the current study looked at how well antibodies could neutralize the viral strains, but future research, they added, should look at how well “cellular immunity” or other aspects of the immune system might protect people.
Going forward, the challenge remains to develop vaccines and treatments that offer broad protection as the coronavirus continues to evolve.
In an alarming ending, the researchers wrote: “We have collectively chased after SARS-CoV-2 variants for over 2 years, and yet, the virus continues to evolve and evade.”
A version of this article first appeared on Medscape.com.
FROM CELL
Flu hospitalizations drop amid signs of an early peak
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.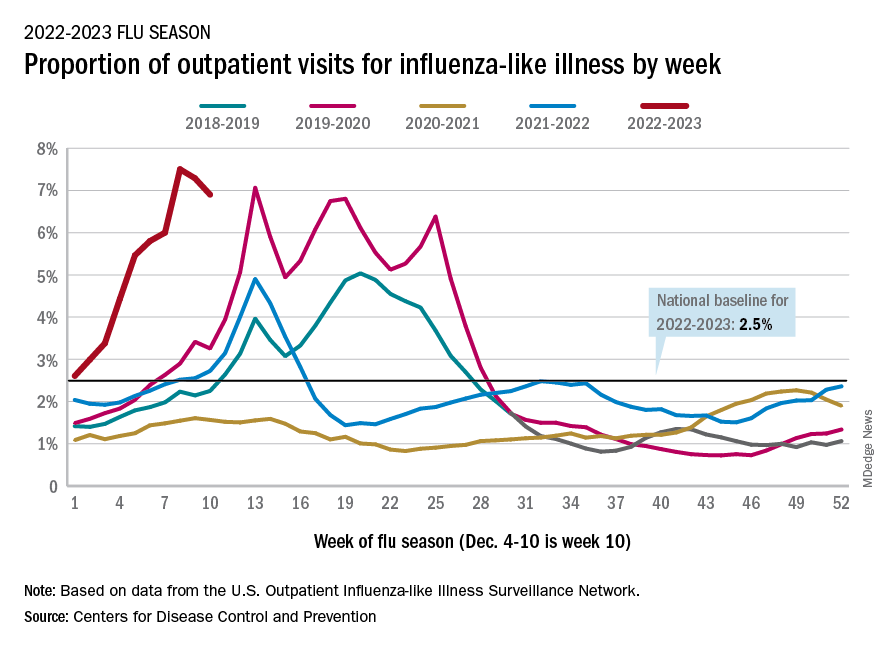
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
Guidance updated for congenital hypothyroidism screening, management
Congenital hypothyroidism is one of the most common preventable causes of intellectual disabilities worldwide, but newborn screening has not been established in all countries.
Additionally, screening alone is not enough to prevent adverse outcomes in children, write authors of a technical report published online in Pediatrics (Jan. 2023;151[1]:e2022060420).
Susan R. Rose, MD, with the division of endocrinology at Cincinnati Children’s Hospital Medical Center in Ohio, led the work group that updated guidance for screening and management of congenital hypothyroidism. The group worked in conjunction with the American Academy of Pediatrics Section on Endocrinology, the AAP Council on Genetics, the Pediatric Endocrine Society, and the American Thyroid Association.
In addition to screening, timely diagnosis, effective treatment, and follow-up are important.
Tests don’t always tell the full story with congenital hypothyroidism.
“Physicians need to consider hypothyroidism in the face of clinical symptoms, even if newborn screening thyroid test results are normal,” the authors write.
They add that newborn screening for congenital hypothyroidism followed by prompt levothyroxine therapy can prevent severe intellectual disability, psychomotor dysfunction, and impaired growth.
Incidence of congenital hypothyroidism ranges from approximately 1 in 2,000 to 1 in 4,000 newborn infants in countries that have newborn screening data, according to the report.
Following are highlights of the guidance:
Clinical signs
Symptoms and signs include large posterior fontanelle, lethargy, large tongue, prolonged jaundice, umbilical hernia, constipation, and/or hypothermia. With these signs, measuring serum thyroid-stimulating hormone (TSH) and free thyroxine (FT4) is indicated, regardless of screening results.
Newborn screening in first days
Population screening is cost effective when performed by state or other public health laboratories working with hospitals or birthing centers in their area, the authors write.
Multidisciplinary teams are best able to conduct comprehensive care when cases are detected.
The screening includes a dried blood spot from a heel stick on an approved paper card using appropriate collection methods. The blood spots are then sent to the laboratory. The preferred age for collecting the specimen is 48-72 hours of age.
That timing may be difficult, the authors note, as 90% of infants in the United States and Europe are discharged before 48 hours, but taking the specimen before discharge is important to avoid missing the early diagnosis.
“However, collection of the NBS [newborn screening] specimen before 48 hours of age, and particularly before 24 hours of age, necessitates the use of age-specific TSH reference ranges or repeat screening, particularly to avoid false-positive results,” the authors note.
If a newborn infant is transferred to another hospital, communication about the screening is critical.
Testing strategies
Three test strategies are used for screening: a primary TSH – reflex T4 measurement; primary T4 – reflex TSH measurement; and combined T4 and TSH measurement.
“All three test strategies detect moderate to severe primary congenital hypothyroidism with similar accuracy,” the authors write.
Most newborn screening programs in the United States and worldwide use a primary TSH test strategy.
Multiple births, same-sex twins
The incidence of congenital hypothyroidism appears to be higher with multiple births (1:876 in twin births and 1:575 in higher-order multiple births in one study). Another study showed the incidence of congenital hypothyroidism in same-sex twins to be 1 in 593, compared with 1 in 3,060 in different-sex twins.
“Most twin pairs (> 95%) are discordant for congenital hypothyroidism,” the authors write. “However, in monozygotic twins who share placental circulation, blood from a euthyroid fetal twin with normal thyroid hormone levels may cross to a fetal twin with congenital hypothyroidism, temporarily correcting the hypothyroidism and preventing its detection by newborn screening at 24-72 hours of life. Thus, all monozygotic twins, or same-sex twins for whom zygosity is unknown, should undergo repeat newborn screening around 2 weeks of age.”
Down syndrome
Congenital hypothyroidism incidence in infants with trisomy 21 (Down syndrome) is high and ranges from 1% to 12% in various reports. The infants tend to have lower T4 concentrations and higher TSH concentrations than do infants without trisomy. Down syndrome is associated with other comorbidities, including congenital heart disease, “that may further increase the risk of abnormal newborn screening results because of acute illness or excess iodine exposure,” the authors write.
Even infants with Down syndrome who don’t have congenital hypothyroidism are still at significant risk of developing primary hypothyroidism in their first year (approximately 7% in one prospective study).
“Therefore, in these infants, a second newborn screening should be performed at 2-4 weeks of life and serum TSH should be measured at 6 and 12 months of life,” the authors say.
Communication with primary care provider
Direct communication between the newborn screening program and the primary care physician is important for appropriate follow-up. Consulting a pediatric endocrinologist can speed diagnosis and management.
Serum confirmation after abnormal screening
The next step if any child’s screening results suggest congenital hypothyroidism is to perform a physical exam (for goiter, lingual thyroid gland, and/or physical signs of hypothyroidism) and to measure the concentrations of TSH and FT4 (or total T4) in the blood.
For confirmation of abnormal screening results, the authors say, measurement of FT4 is preferred over measuring total T4.
Interpreting serum confirmation
Some interpretations are clear cut: “Elevated TSH with low FT4 on the confirmatory serum testing indicates overt primary hypothyroidism,” the authors write.
But there are various other outcomes with more controversy.
Elevated TSH and normal FT4, for instance, is known as hyperthyrotropinemia or subclinical hypothyroidism and represents a mild primary thyroid abnormality.
In this scenario, there is controversy regarding the need for L-T4 therapy because there are few and conflicting studies regarding how mild congenital hypothyroidism affects cognitive development.
“[E]xpert opinion suggests that persistent TSH elevation > 10 mIU/L is an indication to initiate L-T4 treatment,” the authors write.
Normal TSH and low T4 is seen in patients with central hypothyroidism, prematurity, low birth weight, acute illness, or thyroxine-binding globulin deficiency.
“The concept that central hypothyroidism is usually mild appears unfounded: A study from the Netherlands found that mean pretreatment serum FT4 levels in central congenital hypothyroidism were similar to those of patients with moderately severe primary congenital hypothyroidism. Therefore, L-T4 treatment of central congenital hypothyroidism is indicated.”
Imaging
Routine thyroid imaging is controversial for patients with congenital hypothyroidism. In most cases, it won’t alter clinical management before age 3 years.
Thyroid ultrasonography can find thyroid tissue without radiation exposure and can be performed at any time after a congenital hypothyroidism diagnosis.
“Ultrasonography has lower sensitivity than scintigraphy for detecting ectopic thyroid tissue, the most common cause of congenital hypothyroidism, although its sensitivity is improved by the use of color Doppler,” the authors write.
Infants with normal thyroid imaging at birth may have transient hypothyroidism. In these patients, reevaluation of thyroid hormone therapy after 3 years of age to assess for persistent hypothyroidism may be beneficial.
Treatment
Congenital hypothyroidism is treated with enteral L-T4 at a starting dose of 10-15 mcg/kg per day, given once a day.
L-T4 tablets are the treatment of choice and generic tablets are fine for most children, the authors write, adding that a brand name formulation may be more consistent and better for children with severe congenital hypothyroidism.
An oral solution of L-T4 has been approved by the U.S. Food and Drug Administration for use in children.
“[H]owever, limited experience with its use showed that dosing may not be equivalent to dosing with tablet formulations,” the guidance states.
The goal of initial L-T4 therapy is to normalize serum FT4 and TSH levels as quickly as possible. The outlook is poorer for infants whose hypothyroidism is detected later in life, who receive inadequate doses of L-T4, or who have more severe forms.
Age-specific TSH reference ranges vary by laboratory, but recent studies indicate the top limit of normal TSH in infants in the first 3 months of life is 4.1-4.8 mIU/L.
“[T]herefore, TSH values above 5 mIU/L generally are abnormal if observed after 3 months of age. Whether overtreatment (defined by elevated serum FT4) is harmful remains unclear and evidence is conflicting,” the authors write.
Monitoring
In the near-term follow-up, close laboratory monitoring is necessary during L-T4 treatment to maintain blood TSH and FT4 in the target ranges. Studies support measuring those levels every 1-2 months in the first 6 months of life for children with congenital hypothyroidism, every 2-3 months in the second 6 months, and then every 3-4 months between 1 and 3 years of age.
In long-term follow-up, attention to behavioral and cognitive development is important, because children with congenital hypothyroidism may be at higher risk for neurocognitive and socioemotional dysfunction compared with their peers, even with adequate treatment of congenital hypothyroidism. Hearing deficits are reported in about 10% of children with congenital hypothyroidism.
Developmental outcomes
When L-T4 therapy is maintained and TSH and FT4 are within target range, growth and adult height are generally normal in children with congenital hypothyroidism.
In contrast, the neurodevelopmental prognosis is less certain when treatment starts late.
“[I]nfants with severe congenital hypothyroidism and intrauterine hypothyroidism (as indicated by retarded skeletal maturation at birth) may have low-to-normal intelligence,” the report states. “Similarly, although more than 80% of infants given L-T4 replacement therapy before 3 months of age have an intelligence [quotient] greater than 85, 77% of these infants show signs of cognitive impairment in arithmetic ability, speech, or fine motor coordination later in life.”
If a child is properly treated for congenital hypothyroidism but growth or development is abnormal, testing for other illness, hearing deficit, or other hormone deficiency is needed, the report states.
The authors report no relevant financial relationships.
Congenital hypothyroidism is one of the most common preventable causes of intellectual disabilities worldwide, but newborn screening has not been established in all countries.
Additionally, screening alone is not enough to prevent adverse outcomes in children, write authors of a technical report published online in Pediatrics (Jan. 2023;151[1]:e2022060420).
Susan R. Rose, MD, with the division of endocrinology at Cincinnati Children’s Hospital Medical Center in Ohio, led the work group that updated guidance for screening and management of congenital hypothyroidism. The group worked in conjunction with the American Academy of Pediatrics Section on Endocrinology, the AAP Council on Genetics, the Pediatric Endocrine Society, and the American Thyroid Association.
In addition to screening, timely diagnosis, effective treatment, and follow-up are important.
Tests don’t always tell the full story with congenital hypothyroidism.
“Physicians need to consider hypothyroidism in the face of clinical symptoms, even if newborn screening thyroid test results are normal,” the authors write.
They add that newborn screening for congenital hypothyroidism followed by prompt levothyroxine therapy can prevent severe intellectual disability, psychomotor dysfunction, and impaired growth.
Incidence of congenital hypothyroidism ranges from approximately 1 in 2,000 to 1 in 4,000 newborn infants in countries that have newborn screening data, according to the report.
Following are highlights of the guidance:
Clinical signs
Symptoms and signs include large posterior fontanelle, lethargy, large tongue, prolonged jaundice, umbilical hernia, constipation, and/or hypothermia. With these signs, measuring serum thyroid-stimulating hormone (TSH) and free thyroxine (FT4) is indicated, regardless of screening results.
Newborn screening in first days
Population screening is cost effective when performed by state or other public health laboratories working with hospitals or birthing centers in their area, the authors write.
Multidisciplinary teams are best able to conduct comprehensive care when cases are detected.
The screening includes a dried blood spot from a heel stick on an approved paper card using appropriate collection methods. The blood spots are then sent to the laboratory. The preferred age for collecting the specimen is 48-72 hours of age.
That timing may be difficult, the authors note, as 90% of infants in the United States and Europe are discharged before 48 hours, but taking the specimen before discharge is important to avoid missing the early diagnosis.
“However, collection of the NBS [newborn screening] specimen before 48 hours of age, and particularly before 24 hours of age, necessitates the use of age-specific TSH reference ranges or repeat screening, particularly to avoid false-positive results,” the authors note.
If a newborn infant is transferred to another hospital, communication about the screening is critical.
Testing strategies
Three test strategies are used for screening: a primary TSH – reflex T4 measurement; primary T4 – reflex TSH measurement; and combined T4 and TSH measurement.
“All three test strategies detect moderate to severe primary congenital hypothyroidism with similar accuracy,” the authors write.
Most newborn screening programs in the United States and worldwide use a primary TSH test strategy.
Multiple births, same-sex twins
The incidence of congenital hypothyroidism appears to be higher with multiple births (1:876 in twin births and 1:575 in higher-order multiple births in one study). Another study showed the incidence of congenital hypothyroidism in same-sex twins to be 1 in 593, compared with 1 in 3,060 in different-sex twins.
“Most twin pairs (> 95%) are discordant for congenital hypothyroidism,” the authors write. “However, in monozygotic twins who share placental circulation, blood from a euthyroid fetal twin with normal thyroid hormone levels may cross to a fetal twin with congenital hypothyroidism, temporarily correcting the hypothyroidism and preventing its detection by newborn screening at 24-72 hours of life. Thus, all monozygotic twins, or same-sex twins for whom zygosity is unknown, should undergo repeat newborn screening around 2 weeks of age.”
Down syndrome
Congenital hypothyroidism incidence in infants with trisomy 21 (Down syndrome) is high and ranges from 1% to 12% in various reports. The infants tend to have lower T4 concentrations and higher TSH concentrations than do infants without trisomy. Down syndrome is associated with other comorbidities, including congenital heart disease, “that may further increase the risk of abnormal newborn screening results because of acute illness or excess iodine exposure,” the authors write.
Even infants with Down syndrome who don’t have congenital hypothyroidism are still at significant risk of developing primary hypothyroidism in their first year (approximately 7% in one prospective study).
“Therefore, in these infants, a second newborn screening should be performed at 2-4 weeks of life and serum TSH should be measured at 6 and 12 months of life,” the authors say.
Communication with primary care provider
Direct communication between the newborn screening program and the primary care physician is important for appropriate follow-up. Consulting a pediatric endocrinologist can speed diagnosis and management.
Serum confirmation after abnormal screening
The next step if any child’s screening results suggest congenital hypothyroidism is to perform a physical exam (for goiter, lingual thyroid gland, and/or physical signs of hypothyroidism) and to measure the concentrations of TSH and FT4 (or total T4) in the blood.
For confirmation of abnormal screening results, the authors say, measurement of FT4 is preferred over measuring total T4.
Interpreting serum confirmation
Some interpretations are clear cut: “Elevated TSH with low FT4 on the confirmatory serum testing indicates overt primary hypothyroidism,” the authors write.
But there are various other outcomes with more controversy.
Elevated TSH and normal FT4, for instance, is known as hyperthyrotropinemia or subclinical hypothyroidism and represents a mild primary thyroid abnormality.
In this scenario, there is controversy regarding the need for L-T4 therapy because there are few and conflicting studies regarding how mild congenital hypothyroidism affects cognitive development.
“[E]xpert opinion suggests that persistent TSH elevation > 10 mIU/L is an indication to initiate L-T4 treatment,” the authors write.
Normal TSH and low T4 is seen in patients with central hypothyroidism, prematurity, low birth weight, acute illness, or thyroxine-binding globulin deficiency.
“The concept that central hypothyroidism is usually mild appears unfounded: A study from the Netherlands found that mean pretreatment serum FT4 levels in central congenital hypothyroidism were similar to those of patients with moderately severe primary congenital hypothyroidism. Therefore, L-T4 treatment of central congenital hypothyroidism is indicated.”
Imaging
Routine thyroid imaging is controversial for patients with congenital hypothyroidism. In most cases, it won’t alter clinical management before age 3 years.
Thyroid ultrasonography can find thyroid tissue without radiation exposure and can be performed at any time after a congenital hypothyroidism diagnosis.
“Ultrasonography has lower sensitivity than scintigraphy for detecting ectopic thyroid tissue, the most common cause of congenital hypothyroidism, although its sensitivity is improved by the use of color Doppler,” the authors write.
Infants with normal thyroid imaging at birth may have transient hypothyroidism. In these patients, reevaluation of thyroid hormone therapy after 3 years of age to assess for persistent hypothyroidism may be beneficial.
Treatment
Congenital hypothyroidism is treated with enteral L-T4 at a starting dose of 10-15 mcg/kg per day, given once a day.
L-T4 tablets are the treatment of choice and generic tablets are fine for most children, the authors write, adding that a brand name formulation may be more consistent and better for children with severe congenital hypothyroidism.
An oral solution of L-T4 has been approved by the U.S. Food and Drug Administration for use in children.
“[H]owever, limited experience with its use showed that dosing may not be equivalent to dosing with tablet formulations,” the guidance states.
The goal of initial L-T4 therapy is to normalize serum FT4 and TSH levels as quickly as possible. The outlook is poorer for infants whose hypothyroidism is detected later in life, who receive inadequate doses of L-T4, or who have more severe forms.
Age-specific TSH reference ranges vary by laboratory, but recent studies indicate the top limit of normal TSH in infants in the first 3 months of life is 4.1-4.8 mIU/L.
“[T]herefore, TSH values above 5 mIU/L generally are abnormal if observed after 3 months of age. Whether overtreatment (defined by elevated serum FT4) is harmful remains unclear and evidence is conflicting,” the authors write.
Monitoring
In the near-term follow-up, close laboratory monitoring is necessary during L-T4 treatment to maintain blood TSH and FT4 in the target ranges. Studies support measuring those levels every 1-2 months in the first 6 months of life for children with congenital hypothyroidism, every 2-3 months in the second 6 months, and then every 3-4 months between 1 and 3 years of age.
In long-term follow-up, attention to behavioral and cognitive development is important, because children with congenital hypothyroidism may be at higher risk for neurocognitive and socioemotional dysfunction compared with their peers, even with adequate treatment of congenital hypothyroidism. Hearing deficits are reported in about 10% of children with congenital hypothyroidism.
Developmental outcomes
When L-T4 therapy is maintained and TSH and FT4 are within target range, growth and adult height are generally normal in children with congenital hypothyroidism.
In contrast, the neurodevelopmental prognosis is less certain when treatment starts late.
“[I]nfants with severe congenital hypothyroidism and intrauterine hypothyroidism (as indicated by retarded skeletal maturation at birth) may have low-to-normal intelligence,” the report states. “Similarly, although more than 80% of infants given L-T4 replacement therapy before 3 months of age have an intelligence [quotient] greater than 85, 77% of these infants show signs of cognitive impairment in arithmetic ability, speech, or fine motor coordination later in life.”
If a child is properly treated for congenital hypothyroidism but growth or development is abnormal, testing for other illness, hearing deficit, or other hormone deficiency is needed, the report states.
The authors report no relevant financial relationships.
Congenital hypothyroidism is one of the most common preventable causes of intellectual disabilities worldwide, but newborn screening has not been established in all countries.
Additionally, screening alone is not enough to prevent adverse outcomes in children, write authors of a technical report published online in Pediatrics (Jan. 2023;151[1]:e2022060420).
Susan R. Rose, MD, with the division of endocrinology at Cincinnati Children’s Hospital Medical Center in Ohio, led the work group that updated guidance for screening and management of congenital hypothyroidism. The group worked in conjunction with the American Academy of Pediatrics Section on Endocrinology, the AAP Council on Genetics, the Pediatric Endocrine Society, and the American Thyroid Association.
In addition to screening, timely diagnosis, effective treatment, and follow-up are important.
Tests don’t always tell the full story with congenital hypothyroidism.
“Physicians need to consider hypothyroidism in the face of clinical symptoms, even if newborn screening thyroid test results are normal,” the authors write.
They add that newborn screening for congenital hypothyroidism followed by prompt levothyroxine therapy can prevent severe intellectual disability, psychomotor dysfunction, and impaired growth.
Incidence of congenital hypothyroidism ranges from approximately 1 in 2,000 to 1 in 4,000 newborn infants in countries that have newborn screening data, according to the report.
Following are highlights of the guidance:
Clinical signs
Symptoms and signs include large posterior fontanelle, lethargy, large tongue, prolonged jaundice, umbilical hernia, constipation, and/or hypothermia. With these signs, measuring serum thyroid-stimulating hormone (TSH) and free thyroxine (FT4) is indicated, regardless of screening results.
Newborn screening in first days
Population screening is cost effective when performed by state or other public health laboratories working with hospitals or birthing centers in their area, the authors write.
Multidisciplinary teams are best able to conduct comprehensive care when cases are detected.
The screening includes a dried blood spot from a heel stick on an approved paper card using appropriate collection methods. The blood spots are then sent to the laboratory. The preferred age for collecting the specimen is 48-72 hours of age.
That timing may be difficult, the authors note, as 90% of infants in the United States and Europe are discharged before 48 hours, but taking the specimen before discharge is important to avoid missing the early diagnosis.
“However, collection of the NBS [newborn screening] specimen before 48 hours of age, and particularly before 24 hours of age, necessitates the use of age-specific TSH reference ranges or repeat screening, particularly to avoid false-positive results,” the authors note.
If a newborn infant is transferred to another hospital, communication about the screening is critical.
Testing strategies
Three test strategies are used for screening: a primary TSH – reflex T4 measurement; primary T4 – reflex TSH measurement; and combined T4 and TSH measurement.
“All three test strategies detect moderate to severe primary congenital hypothyroidism with similar accuracy,” the authors write.
Most newborn screening programs in the United States and worldwide use a primary TSH test strategy.
Multiple births, same-sex twins
The incidence of congenital hypothyroidism appears to be higher with multiple births (1:876 in twin births and 1:575 in higher-order multiple births in one study). Another study showed the incidence of congenital hypothyroidism in same-sex twins to be 1 in 593, compared with 1 in 3,060 in different-sex twins.
“Most twin pairs (> 95%) are discordant for congenital hypothyroidism,” the authors write. “However, in monozygotic twins who share placental circulation, blood from a euthyroid fetal twin with normal thyroid hormone levels may cross to a fetal twin with congenital hypothyroidism, temporarily correcting the hypothyroidism and preventing its detection by newborn screening at 24-72 hours of life. Thus, all monozygotic twins, or same-sex twins for whom zygosity is unknown, should undergo repeat newborn screening around 2 weeks of age.”
Down syndrome
Congenital hypothyroidism incidence in infants with trisomy 21 (Down syndrome) is high and ranges from 1% to 12% in various reports. The infants tend to have lower T4 concentrations and higher TSH concentrations than do infants without trisomy. Down syndrome is associated with other comorbidities, including congenital heart disease, “that may further increase the risk of abnormal newborn screening results because of acute illness or excess iodine exposure,” the authors write.
Even infants with Down syndrome who don’t have congenital hypothyroidism are still at significant risk of developing primary hypothyroidism in their first year (approximately 7% in one prospective study).
“Therefore, in these infants, a second newborn screening should be performed at 2-4 weeks of life and serum TSH should be measured at 6 and 12 months of life,” the authors say.
Communication with primary care provider
Direct communication between the newborn screening program and the primary care physician is important for appropriate follow-up. Consulting a pediatric endocrinologist can speed diagnosis and management.
Serum confirmation after abnormal screening
The next step if any child’s screening results suggest congenital hypothyroidism is to perform a physical exam (for goiter, lingual thyroid gland, and/or physical signs of hypothyroidism) and to measure the concentrations of TSH and FT4 (or total T4) in the blood.
For confirmation of abnormal screening results, the authors say, measurement of FT4 is preferred over measuring total T4.
Interpreting serum confirmation
Some interpretations are clear cut: “Elevated TSH with low FT4 on the confirmatory serum testing indicates overt primary hypothyroidism,” the authors write.
But there are various other outcomes with more controversy.
Elevated TSH and normal FT4, for instance, is known as hyperthyrotropinemia or subclinical hypothyroidism and represents a mild primary thyroid abnormality.
In this scenario, there is controversy regarding the need for L-T4 therapy because there are few and conflicting studies regarding how mild congenital hypothyroidism affects cognitive development.
“[E]xpert opinion suggests that persistent TSH elevation > 10 mIU/L is an indication to initiate L-T4 treatment,” the authors write.
Normal TSH and low T4 is seen in patients with central hypothyroidism, prematurity, low birth weight, acute illness, or thyroxine-binding globulin deficiency.
“The concept that central hypothyroidism is usually mild appears unfounded: A study from the Netherlands found that mean pretreatment serum FT4 levels in central congenital hypothyroidism were similar to those of patients with moderately severe primary congenital hypothyroidism. Therefore, L-T4 treatment of central congenital hypothyroidism is indicated.”
Imaging
Routine thyroid imaging is controversial for patients with congenital hypothyroidism. In most cases, it won’t alter clinical management before age 3 years.
Thyroid ultrasonography can find thyroid tissue without radiation exposure and can be performed at any time after a congenital hypothyroidism diagnosis.
“Ultrasonography has lower sensitivity than scintigraphy for detecting ectopic thyroid tissue, the most common cause of congenital hypothyroidism, although its sensitivity is improved by the use of color Doppler,” the authors write.
Infants with normal thyroid imaging at birth may have transient hypothyroidism. In these patients, reevaluation of thyroid hormone therapy after 3 years of age to assess for persistent hypothyroidism may be beneficial.
Treatment
Congenital hypothyroidism is treated with enteral L-T4 at a starting dose of 10-15 mcg/kg per day, given once a day.
L-T4 tablets are the treatment of choice and generic tablets are fine for most children, the authors write, adding that a brand name formulation may be more consistent and better for children with severe congenital hypothyroidism.
An oral solution of L-T4 has been approved by the U.S. Food and Drug Administration for use in children.
“[H]owever, limited experience with its use showed that dosing may not be equivalent to dosing with tablet formulations,” the guidance states.
The goal of initial L-T4 therapy is to normalize serum FT4 and TSH levels as quickly as possible. The outlook is poorer for infants whose hypothyroidism is detected later in life, who receive inadequate doses of L-T4, or who have more severe forms.
Age-specific TSH reference ranges vary by laboratory, but recent studies indicate the top limit of normal TSH in infants in the first 3 months of life is 4.1-4.8 mIU/L.
“[T]herefore, TSH values above 5 mIU/L generally are abnormal if observed after 3 months of age. Whether overtreatment (defined by elevated serum FT4) is harmful remains unclear and evidence is conflicting,” the authors write.
Monitoring
In the near-term follow-up, close laboratory monitoring is necessary during L-T4 treatment to maintain blood TSH and FT4 in the target ranges. Studies support measuring those levels every 1-2 months in the first 6 months of life for children with congenital hypothyroidism, every 2-3 months in the second 6 months, and then every 3-4 months between 1 and 3 years of age.
In long-term follow-up, attention to behavioral and cognitive development is important, because children with congenital hypothyroidism may be at higher risk for neurocognitive and socioemotional dysfunction compared with their peers, even with adequate treatment of congenital hypothyroidism. Hearing deficits are reported in about 10% of children with congenital hypothyroidism.
Developmental outcomes
When L-T4 therapy is maintained and TSH and FT4 are within target range, growth and adult height are generally normal in children with congenital hypothyroidism.
In contrast, the neurodevelopmental prognosis is less certain when treatment starts late.
“[I]nfants with severe congenital hypothyroidism and intrauterine hypothyroidism (as indicated by retarded skeletal maturation at birth) may have low-to-normal intelligence,” the report states. “Similarly, although more than 80% of infants given L-T4 replacement therapy before 3 months of age have an intelligence [quotient] greater than 85, 77% of these infants show signs of cognitive impairment in arithmetic ability, speech, or fine motor coordination later in life.”
If a child is properly treated for congenital hypothyroidism but growth or development is abnormal, testing for other illness, hearing deficit, or other hormone deficiency is needed, the report states.
The authors report no relevant financial relationships.
FROM PEDIATRICS
New pediatrics growth charts better reflect severe obesity
The U.S. Centers for Disease Control and Prevention has issued extended growth charts to help doctors and researchers better understand patterns of development for the most overweight children and adolescents.
In 2017-2018, more than 4.5 million U.S. youth met the criteria for severe obesity – defined as 120% of the 95th percentile, or 35 kg/m2 or greater – according to the CDC.
The new growth charts will not replace the current charts but extend beyond the 97th percentile for body mass index. Formerly, data were extrapolated for anything over the 95th percentile based on evidence from 1963 to 1980, when obesity rates were lower.
The extended growth charts are based on data collected between 1988 and 2015 from young children and adolescents with obesity.
Experts said the expanded charts will allow researchers and clinicians to track the effects of interventions for obesity whether they involve an increase in physical activity, a decrease in consumption, or other interventions. The corresponding z-score charts also are provided.
Physicians should still use the CDC’s BMI-for-age growth charts from 2000 for pediatric patients with BMIs under the 95th percentile. The agency said it does not intend to update those charts.
The definitions of overweight, obesity, and severe obesity remain unchanged.
The U.S. Centers for Disease Control and Prevention has issued extended growth charts to help doctors and researchers better understand patterns of development for the most overweight children and adolescents.
In 2017-2018, more than 4.5 million U.S. youth met the criteria for severe obesity – defined as 120% of the 95th percentile, or 35 kg/m2 or greater – according to the CDC.
The new growth charts will not replace the current charts but extend beyond the 97th percentile for body mass index. Formerly, data were extrapolated for anything over the 95th percentile based on evidence from 1963 to 1980, when obesity rates were lower.
The extended growth charts are based on data collected between 1988 and 2015 from young children and adolescents with obesity.
Experts said the expanded charts will allow researchers and clinicians to track the effects of interventions for obesity whether they involve an increase in physical activity, a decrease in consumption, or other interventions. The corresponding z-score charts also are provided.
Physicians should still use the CDC’s BMI-for-age growth charts from 2000 for pediatric patients with BMIs under the 95th percentile. The agency said it does not intend to update those charts.
The definitions of overweight, obesity, and severe obesity remain unchanged.
The U.S. Centers for Disease Control and Prevention has issued extended growth charts to help doctors and researchers better understand patterns of development for the most overweight children and adolescents.
In 2017-2018, more than 4.5 million U.S. youth met the criteria for severe obesity – defined as 120% of the 95th percentile, or 35 kg/m2 or greater – according to the CDC.
The new growth charts will not replace the current charts but extend beyond the 97th percentile for body mass index. Formerly, data were extrapolated for anything over the 95th percentile based on evidence from 1963 to 1980, when obesity rates were lower.
The extended growth charts are based on data collected between 1988 and 2015 from young children and adolescents with obesity.
Experts said the expanded charts will allow researchers and clinicians to track the effects of interventions for obesity whether they involve an increase in physical activity, a decrease in consumption, or other interventions. The corresponding z-score charts also are provided.
Physicians should still use the CDC’s BMI-for-age growth charts from 2000 for pediatric patients with BMIs under the 95th percentile. The agency said it does not intend to update those charts.
The definitions of overweight, obesity, and severe obesity remain unchanged.
New test that detects 14 cancers focuses on sugars, not DNA
The leader in this field is the Galleri test (from GRAIL) which is already in clinical use in some health care networks across the United States. That test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA (cfDNA) in a blood sample.
The new test, under development by Swedish biotechnology company Elypta AB, has a different premise. It can detect 14 cancer types based on the analysis of glycosaminoglycans, which are a diverse group of polysaccharides that are altered by the presence of tumors. Using plasma and urine samples, the method had a 41.6%-62.3% sensitivity for detecting stage I cancer at 95% specificity.
In comparison, say the authors, other assays have reported 39%-73% sensitivity to stage I cancers, but these estimates are usually limited to 12 cancer types that are considered “high-signal,” and the assays perform poorly in cancers that emit little cfDNA, such as genitourinary and brain malignancies.
“The main advantage of glycosaminoglycans appears to be that they change in the blood and urine at the earliest stages of cancer,” said study author Francesco Gatto, PhD, founder and chief scientific officer at Elypta. “Consequently, this method showed an impressive detection rate in stage I compared to other emerging methods.”
The study was published online in Proceedings of the National Academy of Sciences.
Combine tests?
Dr. Gatto commented that he “could envision that one day we may be able to combine these methods.”
“The same blood specimen could be used to test both glycosaminoglycans and genomic biomarkers,” said Dr. Gatto. “This strategy could hopefully detect even more cancers than with either method alone, and the resulting performance may well be sufficient as a one-stop-shop screening program.”
So how does the new test from Elypta compare with the Galleri test?
“Galleri and similar methods mostly focused on information coming from molecules of DNA naturally floating in the blood,” explained Dr. Gatto. “It makes sense to conduct research there because cancers typically start with events in the DNA.”
He noted that the current study explored a new layer of information, molecules called glycosaminoglycans, that participate in the metabolism of cancer.
“This method detected many cancers that the previous methods missed, and a substantial proportion of these were at stage I,” said Dr. Gatto. “Cancer is a complex disease, so the most layers of information we can probe noninvasively, say with a blood test, the more likely we can catch more cancers at its earliest stage.”
Other platforms typically rely on sequencing and detecting cancer-derived fractions of cfDNA, but these methods have challenges that can interfere with their usage. For example, some cancer types do not shed sufficient cfDNA and it cannot be accurately measured.
“An advantage on focusing on glycosaminoglycans is that the method does not require next-generation sequencing or similarly complex assays because glycosaminoglycans are informative with less than 10 simultaneous measurements as opposed to Galleri that looks at over 1 million DNA methylation sites,” he said.
“This makes the assay behind the test much cheaper and robust – we estimated a 5-10 times lower cost difference,” Dr. Gatto said.
Prospective and comparative data needed
In a comment, Eric Klein, MD, emeritus chair of the Glickman Urological and Kidney Institute at the Cleveland Clinic explained that the “only accurate way to know how a test will perform in an intended-use population is to actually test it in that population. It’s not possible to extrapolate results directly from a case-control study.”
Cancers shed many different biologic markers into body fluids, but which of these signals will be best to serve as the basis of an MCED (multi-cancer detection test) that has clinical utility in a screening population has yet to be determined, he noted. “And it’s possible that no single test will be optimum for every clinical situation.”
“The results of this study appear promising, but it is not possible to claim superiority of one test over another based on individual case-control studies because of uncontrolled differences in the selected populations,” Dr. Klein continued. “The only scientifically accurate way to do this is to perform different tests on the same patient samples in a head-to-head comparison.”
There is only one study that he is aware of that has done this recently, in which multiple different assays looking at various signals in cell-free DNA were directly compared on the same samples (Cancer cell. 2022;40:1537-49.e12). “A targeted methylation assay that is the basis for Galleri was best for the lowest limit of detection and for predicting cancer site of origin,” said Dr. Klein.
Another expert agreed that a direct head-to-head study is needed to compare assays. “Based on this data, you cannot say that this method is better than the other one because that requires a comparative study,” said Fred Hirsch, MD, PhD, executive director of the Center for Thoracic Oncology, Tisch Cancer Institute at Mount Sinai, New York.
Metabolomics is interesting, and the data are encouraging, he continued. “But this is a multicancer early detection test and metabolism changes may vary from cancer type to cancer type. I’m not sure that the metabolism of lung cancer is the same as that of a gynecologic cancer.”
Dr. Hirsch also pointed out that there could also be confounding factors. “They have excluded inflammatory disease, but there can be other variables such as smoking,” he said. “Overall it gives some interesting perspectives but I would like to see more prospective validation and studies in specific disease groups, and eventually comparative studies with other methodologies.”
Study details
The authors evaluated if plasma and urine free GAGomes (free glycosaminoglycan profiles) deviated from baseline physiological levels in 14 cancer types and could serve as metabolic cancer biomarkers. They also then validated using free GAGomes for MCED in an external population with 2,064 samples obtained from 1,260 patients with cancer and healthy individuals.
In an in vivo cancer progression model, they observed widespread cancer-specific changes in biofluidic free GAGomes and then developed three machine-learning models based on urine (nurine = 220 cancer vs. 360 healthy) and plasma (nplasma = 517 cancer vs. 425 healthy) free GAGomes that were able to detect any cancer with an area under the receiver operating characteristic curve of 0.83-0.93 (with up to 62% sensitivity to stage I disease at 95% specificity).
To assess if altered GAGome features associated with cancer suggested more aggressive tumor biology, they correlated each score with overall survival. The median follow-up time was 17 months in the plasma cohort (n = 370 across 13 cancer types), 15 months in the urine cohort (n = 162 across 4 cancer types), and 15 months in the combined cohort (n = 152 across 4 cancer types).
They found that all three scores independently predicted overall survival in a multivariable analysis (hazard ratio, 1.29; P = .0009 for plasma; HR, 1.79; P = .0009 for urine; HR, 1.91; P = .0004 for combined) after adjusting for cancer type, age, sex, and stage IV or high-grade disease.
These findings showed an association of free GAGome alterations with aggressive cancer phenotypes and suggested that scores below the 95% specificity cutoff might have a better prognosis, the authors comment.
In addition, other analyses showed that free GAGomes predicted the putative cancer location with 89% accuracy. And finally, to confirm whether the free GAGome MCED scores could be used for screening, a validation analysis was conducted using a typical “screening population,” which requires at least 99% specificity. The combined free GAGomes were able to predict a poor prognosis of any cancer type within 18 months and with 43% sensitivity (21% in stage I; n = 121 and 49 cases).
Dr. Gatto believes that these results, as well as those from other studies looking at glycosaminoglycans as cancer biomarkers, will lead to the next steps of development. “But I speculate that this test could be most useful to assess in a cheap, practical, and noninvasive manner if a person at increased risk of cancer should be selected for cancer screening as part of established or emerging screening programs.”
The study was sponsored by Elypta. Dr. Gatto is listed as an inventor in patent applications related to the biomarkers described in this study and later assigned to Elypta, and is a shareholder and employed at Elypta. Dr. Hirsch reports no relevant financial relationships. Dr. Klein is a consultant for GRAIL and an investigator for CCGA and Pathfinder.
A version of this article first appeared on Medscape.com.
The leader in this field is the Galleri test (from GRAIL) which is already in clinical use in some health care networks across the United States. That test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA (cfDNA) in a blood sample.
The new test, under development by Swedish biotechnology company Elypta AB, has a different premise. It can detect 14 cancer types based on the analysis of glycosaminoglycans, which are a diverse group of polysaccharides that are altered by the presence of tumors. Using plasma and urine samples, the method had a 41.6%-62.3% sensitivity for detecting stage I cancer at 95% specificity.
In comparison, say the authors, other assays have reported 39%-73% sensitivity to stage I cancers, but these estimates are usually limited to 12 cancer types that are considered “high-signal,” and the assays perform poorly in cancers that emit little cfDNA, such as genitourinary and brain malignancies.
“The main advantage of glycosaminoglycans appears to be that they change in the blood and urine at the earliest stages of cancer,” said study author Francesco Gatto, PhD, founder and chief scientific officer at Elypta. “Consequently, this method showed an impressive detection rate in stage I compared to other emerging methods.”
The study was published online in Proceedings of the National Academy of Sciences.
Combine tests?
Dr. Gatto commented that he “could envision that one day we may be able to combine these methods.”
“The same blood specimen could be used to test both glycosaminoglycans and genomic biomarkers,” said Dr. Gatto. “This strategy could hopefully detect even more cancers than with either method alone, and the resulting performance may well be sufficient as a one-stop-shop screening program.”
So how does the new test from Elypta compare with the Galleri test?
“Galleri and similar methods mostly focused on information coming from molecules of DNA naturally floating in the blood,” explained Dr. Gatto. “It makes sense to conduct research there because cancers typically start with events in the DNA.”
He noted that the current study explored a new layer of information, molecules called glycosaminoglycans, that participate in the metabolism of cancer.
“This method detected many cancers that the previous methods missed, and a substantial proportion of these were at stage I,” said Dr. Gatto. “Cancer is a complex disease, so the most layers of information we can probe noninvasively, say with a blood test, the more likely we can catch more cancers at its earliest stage.”
Other platforms typically rely on sequencing and detecting cancer-derived fractions of cfDNA, but these methods have challenges that can interfere with their usage. For example, some cancer types do not shed sufficient cfDNA and it cannot be accurately measured.
“An advantage on focusing on glycosaminoglycans is that the method does not require next-generation sequencing or similarly complex assays because glycosaminoglycans are informative with less than 10 simultaneous measurements as opposed to Galleri that looks at over 1 million DNA methylation sites,” he said.
“This makes the assay behind the test much cheaper and robust – we estimated a 5-10 times lower cost difference,” Dr. Gatto said.
Prospective and comparative data needed
In a comment, Eric Klein, MD, emeritus chair of the Glickman Urological and Kidney Institute at the Cleveland Clinic explained that the “only accurate way to know how a test will perform in an intended-use population is to actually test it in that population. It’s not possible to extrapolate results directly from a case-control study.”
Cancers shed many different biologic markers into body fluids, but which of these signals will be best to serve as the basis of an MCED (multi-cancer detection test) that has clinical utility in a screening population has yet to be determined, he noted. “And it’s possible that no single test will be optimum for every clinical situation.”
“The results of this study appear promising, but it is not possible to claim superiority of one test over another based on individual case-control studies because of uncontrolled differences in the selected populations,” Dr. Klein continued. “The only scientifically accurate way to do this is to perform different tests on the same patient samples in a head-to-head comparison.”
There is only one study that he is aware of that has done this recently, in which multiple different assays looking at various signals in cell-free DNA were directly compared on the same samples (Cancer cell. 2022;40:1537-49.e12). “A targeted methylation assay that is the basis for Galleri was best for the lowest limit of detection and for predicting cancer site of origin,” said Dr. Klein.
Another expert agreed that a direct head-to-head study is needed to compare assays. “Based on this data, you cannot say that this method is better than the other one because that requires a comparative study,” said Fred Hirsch, MD, PhD, executive director of the Center for Thoracic Oncology, Tisch Cancer Institute at Mount Sinai, New York.
Metabolomics is interesting, and the data are encouraging, he continued. “But this is a multicancer early detection test and metabolism changes may vary from cancer type to cancer type. I’m not sure that the metabolism of lung cancer is the same as that of a gynecologic cancer.”
Dr. Hirsch also pointed out that there could also be confounding factors. “They have excluded inflammatory disease, but there can be other variables such as smoking,” he said. “Overall it gives some interesting perspectives but I would like to see more prospective validation and studies in specific disease groups, and eventually comparative studies with other methodologies.”
Study details
The authors evaluated if plasma and urine free GAGomes (free glycosaminoglycan profiles) deviated from baseline physiological levels in 14 cancer types and could serve as metabolic cancer biomarkers. They also then validated using free GAGomes for MCED in an external population with 2,064 samples obtained from 1,260 patients with cancer and healthy individuals.
In an in vivo cancer progression model, they observed widespread cancer-specific changes in biofluidic free GAGomes and then developed three machine-learning models based on urine (nurine = 220 cancer vs. 360 healthy) and plasma (nplasma = 517 cancer vs. 425 healthy) free GAGomes that were able to detect any cancer with an area under the receiver operating characteristic curve of 0.83-0.93 (with up to 62% sensitivity to stage I disease at 95% specificity).
To assess if altered GAGome features associated with cancer suggested more aggressive tumor biology, they correlated each score with overall survival. The median follow-up time was 17 months in the plasma cohort (n = 370 across 13 cancer types), 15 months in the urine cohort (n = 162 across 4 cancer types), and 15 months in the combined cohort (n = 152 across 4 cancer types).
They found that all three scores independently predicted overall survival in a multivariable analysis (hazard ratio, 1.29; P = .0009 for plasma; HR, 1.79; P = .0009 for urine; HR, 1.91; P = .0004 for combined) after adjusting for cancer type, age, sex, and stage IV or high-grade disease.
These findings showed an association of free GAGome alterations with aggressive cancer phenotypes and suggested that scores below the 95% specificity cutoff might have a better prognosis, the authors comment.
In addition, other analyses showed that free GAGomes predicted the putative cancer location with 89% accuracy. And finally, to confirm whether the free GAGome MCED scores could be used for screening, a validation analysis was conducted using a typical “screening population,” which requires at least 99% specificity. The combined free GAGomes were able to predict a poor prognosis of any cancer type within 18 months and with 43% sensitivity (21% in stage I; n = 121 and 49 cases).
Dr. Gatto believes that these results, as well as those from other studies looking at glycosaminoglycans as cancer biomarkers, will lead to the next steps of development. “But I speculate that this test could be most useful to assess in a cheap, practical, and noninvasive manner if a person at increased risk of cancer should be selected for cancer screening as part of established or emerging screening programs.”
The study was sponsored by Elypta. Dr. Gatto is listed as an inventor in patent applications related to the biomarkers described in this study and later assigned to Elypta, and is a shareholder and employed at Elypta. Dr. Hirsch reports no relevant financial relationships. Dr. Klein is a consultant for GRAIL and an investigator for CCGA and Pathfinder.
A version of this article first appeared on Medscape.com.
The leader in this field is the Galleri test (from GRAIL) which is already in clinical use in some health care networks across the United States. That test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA (cfDNA) in a blood sample.
The new test, under development by Swedish biotechnology company Elypta AB, has a different premise. It can detect 14 cancer types based on the analysis of glycosaminoglycans, which are a diverse group of polysaccharides that are altered by the presence of tumors. Using plasma and urine samples, the method had a 41.6%-62.3% sensitivity for detecting stage I cancer at 95% specificity.
In comparison, say the authors, other assays have reported 39%-73% sensitivity to stage I cancers, but these estimates are usually limited to 12 cancer types that are considered “high-signal,” and the assays perform poorly in cancers that emit little cfDNA, such as genitourinary and brain malignancies.
“The main advantage of glycosaminoglycans appears to be that they change in the blood and urine at the earliest stages of cancer,” said study author Francesco Gatto, PhD, founder and chief scientific officer at Elypta. “Consequently, this method showed an impressive detection rate in stage I compared to other emerging methods.”
The study was published online in Proceedings of the National Academy of Sciences.
Combine tests?
Dr. Gatto commented that he “could envision that one day we may be able to combine these methods.”
“The same blood specimen could be used to test both glycosaminoglycans and genomic biomarkers,” said Dr. Gatto. “This strategy could hopefully detect even more cancers than with either method alone, and the resulting performance may well be sufficient as a one-stop-shop screening program.”
So how does the new test from Elypta compare with the Galleri test?
“Galleri and similar methods mostly focused on information coming from molecules of DNA naturally floating in the blood,” explained Dr. Gatto. “It makes sense to conduct research there because cancers typically start with events in the DNA.”
He noted that the current study explored a new layer of information, molecules called glycosaminoglycans, that participate in the metabolism of cancer.
“This method detected many cancers that the previous methods missed, and a substantial proportion of these were at stage I,” said Dr. Gatto. “Cancer is a complex disease, so the most layers of information we can probe noninvasively, say with a blood test, the more likely we can catch more cancers at its earliest stage.”
Other platforms typically rely on sequencing and detecting cancer-derived fractions of cfDNA, but these methods have challenges that can interfere with their usage. For example, some cancer types do not shed sufficient cfDNA and it cannot be accurately measured.
“An advantage on focusing on glycosaminoglycans is that the method does not require next-generation sequencing or similarly complex assays because glycosaminoglycans are informative with less than 10 simultaneous measurements as opposed to Galleri that looks at over 1 million DNA methylation sites,” he said.
“This makes the assay behind the test much cheaper and robust – we estimated a 5-10 times lower cost difference,” Dr. Gatto said.
Prospective and comparative data needed
In a comment, Eric Klein, MD, emeritus chair of the Glickman Urological and Kidney Institute at the Cleveland Clinic explained that the “only accurate way to know how a test will perform in an intended-use population is to actually test it in that population. It’s not possible to extrapolate results directly from a case-control study.”
Cancers shed many different biologic markers into body fluids, but which of these signals will be best to serve as the basis of an MCED (multi-cancer detection test) that has clinical utility in a screening population has yet to be determined, he noted. “And it’s possible that no single test will be optimum for every clinical situation.”
“The results of this study appear promising, but it is not possible to claim superiority of one test over another based on individual case-control studies because of uncontrolled differences in the selected populations,” Dr. Klein continued. “The only scientifically accurate way to do this is to perform different tests on the same patient samples in a head-to-head comparison.”
There is only one study that he is aware of that has done this recently, in which multiple different assays looking at various signals in cell-free DNA were directly compared on the same samples (Cancer cell. 2022;40:1537-49.e12). “A targeted methylation assay that is the basis for Galleri was best for the lowest limit of detection and for predicting cancer site of origin,” said Dr. Klein.
Another expert agreed that a direct head-to-head study is needed to compare assays. “Based on this data, you cannot say that this method is better than the other one because that requires a comparative study,” said Fred Hirsch, MD, PhD, executive director of the Center for Thoracic Oncology, Tisch Cancer Institute at Mount Sinai, New York.
Metabolomics is interesting, and the data are encouraging, he continued. “But this is a multicancer early detection test and metabolism changes may vary from cancer type to cancer type. I’m not sure that the metabolism of lung cancer is the same as that of a gynecologic cancer.”
Dr. Hirsch also pointed out that there could also be confounding factors. “They have excluded inflammatory disease, but there can be other variables such as smoking,” he said. “Overall it gives some interesting perspectives but I would like to see more prospective validation and studies in specific disease groups, and eventually comparative studies with other methodologies.”
Study details
The authors evaluated if plasma and urine free GAGomes (free glycosaminoglycan profiles) deviated from baseline physiological levels in 14 cancer types and could serve as metabolic cancer biomarkers. They also then validated using free GAGomes for MCED in an external population with 2,064 samples obtained from 1,260 patients with cancer and healthy individuals.
In an in vivo cancer progression model, they observed widespread cancer-specific changes in biofluidic free GAGomes and then developed three machine-learning models based on urine (nurine = 220 cancer vs. 360 healthy) and plasma (nplasma = 517 cancer vs. 425 healthy) free GAGomes that were able to detect any cancer with an area under the receiver operating characteristic curve of 0.83-0.93 (with up to 62% sensitivity to stage I disease at 95% specificity).
To assess if altered GAGome features associated with cancer suggested more aggressive tumor biology, they correlated each score with overall survival. The median follow-up time was 17 months in the plasma cohort (n = 370 across 13 cancer types), 15 months in the urine cohort (n = 162 across 4 cancer types), and 15 months in the combined cohort (n = 152 across 4 cancer types).
They found that all three scores independently predicted overall survival in a multivariable analysis (hazard ratio, 1.29; P = .0009 for plasma; HR, 1.79; P = .0009 for urine; HR, 1.91; P = .0004 for combined) after adjusting for cancer type, age, sex, and stage IV or high-grade disease.
These findings showed an association of free GAGome alterations with aggressive cancer phenotypes and suggested that scores below the 95% specificity cutoff might have a better prognosis, the authors comment.
In addition, other analyses showed that free GAGomes predicted the putative cancer location with 89% accuracy. And finally, to confirm whether the free GAGome MCED scores could be used for screening, a validation analysis was conducted using a typical “screening population,” which requires at least 99% specificity. The combined free GAGomes were able to predict a poor prognosis of any cancer type within 18 months and with 43% sensitivity (21% in stage I; n = 121 and 49 cases).
Dr. Gatto believes that these results, as well as those from other studies looking at glycosaminoglycans as cancer biomarkers, will lead to the next steps of development. “But I speculate that this test could be most useful to assess in a cheap, practical, and noninvasive manner if a person at increased risk of cancer should be selected for cancer screening as part of established or emerging screening programs.”
The study was sponsored by Elypta. Dr. Gatto is listed as an inventor in patent applications related to the biomarkers described in this study and later assigned to Elypta, and is a shareholder and employed at Elypta. Dr. Hirsch reports no relevant financial relationships. Dr. Klein is a consultant for GRAIL and an investigator for CCGA and Pathfinder.
A version of this article first appeared on Medscape.com.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Not all children with type 2 diabetes have obesity
Obesity is not a universal phenotype in children with type 2 diabetes (T2D), a global systematic review and meta-analysis reported. In fact, the study found, as many as one in four children with T2D do not have obesity and some have normal reference-range body mass measurements. Further studies should consider other mechanisms beyond obesity in the genesis of pediatric diabetes, the authors of the international analysis concluded, writing for JAMA Network Open.
“We were aware that some children and adolescents with T2D did not have obesity, but we didn’t know the scale of obesity in T2D, or what variables may impact the occurrence of diabetes in this group,” endocrinologist M. Constantine Samaan, MD, MSc, associate professor of pediatrics at McMaster University in Hamilton, Ont., told this news organization. “So, the analysis did help us understand the body mass distribution of this group in more detail.”
The international investigators included in their meta-analysis 53 articles with 8,942 participants from multiple world regions and races/ethnicities. The overall prevalence of obesity in pediatric patients with T2D was 75.27% (95% confidence interval [CI], 70.47%-79.78%). The prevalence of obesity at time of diagnosis in 4,688 participants was 77.24% (95% CI, 70.55%-83.34%). Male participants had higher odds of obesity than females: odds ratio, 2.10 (95% CI, 1.33-3.31) – although girls are generally more likely to develop T2D. The highest prevalence of obesity occurred in Whites at 89.86% (95% CI, 71.50%-99.74%), while prevalence was lowest in Asian participants at 64.50% (95% CI, 53.28%-74.99%).
The authors noted that childhood obesity affects approximately 340 million children worldwide and is a major driver of pediatric T2D, an aggressive disease with a high treatment failure rate. Understanding the contribution of body mass to the evolution of insulin resistance, glucose intolerance, and T2D with its attendant comorbidities and complications, such as nonalcoholic fatty liver disease, remains crucial for developing personalized interventions.
Known risk factors for T2D include interactions between genetics and the environment, including lifestyle factors such as diet and low physical activity levels, Dr. Samaan noted. Certain ethnic groups have higher T2D risks, as do babies exposed in the womb to maternal obesity or diabetes, he said. “And there are likely many other factors that contribute to the risk of T2D, though these remain to be defined.”
Is “lean” T2D in children without obesity likely then to be hereditary, more severe, and harder to control with lifestyle modification? “That’s a great question, but the answer is we don’t know,” Dr. Samaan said.
Commenting on the study but not involved in it, Timothy J. Joos, MD, a pediatrician in Seattle affiliated with the Swedish Medical Center, said the findings raise the question of how many pediatric T2D patients are being missed because they don’t meet current screening criteria. “In nonobese T2D pediatric patients, genetics (and by proxy family history) obviously play a heavier role. In my practice, I often get parents asking me to screen their skinny teenager for diabetes because of diabetes in a family member. In the past I would begrudgingly comply with a smirk on my face. Now the smirk will be gone.”
Dr. Joos said it would be interesting to see what percentage of these T2D patients without obesity (body mass index < 95th percentile) would still meet the criteria for being overweight (BMI > 85th percentile) as this is the primary criterion for screening according to the American Diabetes Association guidelines.
Current guidelines generally look for elevated body mass measures as a main screening indication, Dr. Samaan’s group noted. But in their view, while factors such as ethnicity and in utero exposure to diabetes are already used in combination with BMI-based measures to justify screening, more sophisticated prediabetes and diabetes prediction models are needed to support a more comprehensive screening approach.
“Because being overweight is the initial criterion, children with multiple other criteria are not being screened,” Dr. Joos said. He agreed that more research is needed to sort out the other risk factors for pediatric T2D without obesity so these patients may be detected earlier.
New models may need to incorporate lifestyle factors, hormones, puberty, growth, and sex as well, the authors wrote. Markers of insulin resistance, insulin production capacity, and other markers are needed to refine the identification of those who should be screened.
Dr. Samaan’s group is planning to study the findings in more detail to clarify the effect of body mass on the comorbidities and complications of pediatric T2D.
In addition to the study limitation of significant interstudy heterogeneity, the authors acknowledged varying degrees of glycemic control and dyslipidemia among participants.
No specific funding was provided for this review and meta-analysis. The authors disclosed no conflicts of interest. Dr. Joos disclosed no competing interests with regard to his comments.
Obesity is not a universal phenotype in children with type 2 diabetes (T2D), a global systematic review and meta-analysis reported. In fact, the study found, as many as one in four children with T2D do not have obesity and some have normal reference-range body mass measurements. Further studies should consider other mechanisms beyond obesity in the genesis of pediatric diabetes, the authors of the international analysis concluded, writing for JAMA Network Open.
“We were aware that some children and adolescents with T2D did not have obesity, but we didn’t know the scale of obesity in T2D, or what variables may impact the occurrence of diabetes in this group,” endocrinologist M. Constantine Samaan, MD, MSc, associate professor of pediatrics at McMaster University in Hamilton, Ont., told this news organization. “So, the analysis did help us understand the body mass distribution of this group in more detail.”
The international investigators included in their meta-analysis 53 articles with 8,942 participants from multiple world regions and races/ethnicities. The overall prevalence of obesity in pediatric patients with T2D was 75.27% (95% confidence interval [CI], 70.47%-79.78%). The prevalence of obesity at time of diagnosis in 4,688 participants was 77.24% (95% CI, 70.55%-83.34%). Male participants had higher odds of obesity than females: odds ratio, 2.10 (95% CI, 1.33-3.31) – although girls are generally more likely to develop T2D. The highest prevalence of obesity occurred in Whites at 89.86% (95% CI, 71.50%-99.74%), while prevalence was lowest in Asian participants at 64.50% (95% CI, 53.28%-74.99%).
The authors noted that childhood obesity affects approximately 340 million children worldwide and is a major driver of pediatric T2D, an aggressive disease with a high treatment failure rate. Understanding the contribution of body mass to the evolution of insulin resistance, glucose intolerance, and T2D with its attendant comorbidities and complications, such as nonalcoholic fatty liver disease, remains crucial for developing personalized interventions.
Known risk factors for T2D include interactions between genetics and the environment, including lifestyle factors such as diet and low physical activity levels, Dr. Samaan noted. Certain ethnic groups have higher T2D risks, as do babies exposed in the womb to maternal obesity or diabetes, he said. “And there are likely many other factors that contribute to the risk of T2D, though these remain to be defined.”
Is “lean” T2D in children without obesity likely then to be hereditary, more severe, and harder to control with lifestyle modification? “That’s a great question, but the answer is we don’t know,” Dr. Samaan said.
Commenting on the study but not involved in it, Timothy J. Joos, MD, a pediatrician in Seattle affiliated with the Swedish Medical Center, said the findings raise the question of how many pediatric T2D patients are being missed because they don’t meet current screening criteria. “In nonobese T2D pediatric patients, genetics (and by proxy family history) obviously play a heavier role. In my practice, I often get parents asking me to screen their skinny teenager for diabetes because of diabetes in a family member. In the past I would begrudgingly comply with a smirk on my face. Now the smirk will be gone.”
Dr. Joos said it would be interesting to see what percentage of these T2D patients without obesity (body mass index < 95th percentile) would still meet the criteria for being overweight (BMI > 85th percentile) as this is the primary criterion for screening according to the American Diabetes Association guidelines.
Current guidelines generally look for elevated body mass measures as a main screening indication, Dr. Samaan’s group noted. But in their view, while factors such as ethnicity and in utero exposure to diabetes are already used in combination with BMI-based measures to justify screening, more sophisticated prediabetes and diabetes prediction models are needed to support a more comprehensive screening approach.
“Because being overweight is the initial criterion, children with multiple other criteria are not being screened,” Dr. Joos said. He agreed that more research is needed to sort out the other risk factors for pediatric T2D without obesity so these patients may be detected earlier.
New models may need to incorporate lifestyle factors, hormones, puberty, growth, and sex as well, the authors wrote. Markers of insulin resistance, insulin production capacity, and other markers are needed to refine the identification of those who should be screened.
Dr. Samaan’s group is planning to study the findings in more detail to clarify the effect of body mass on the comorbidities and complications of pediatric T2D.
In addition to the study limitation of significant interstudy heterogeneity, the authors acknowledged varying degrees of glycemic control and dyslipidemia among participants.
No specific funding was provided for this review and meta-analysis. The authors disclosed no conflicts of interest. Dr. Joos disclosed no competing interests with regard to his comments.
Obesity is not a universal phenotype in children with type 2 diabetes (T2D), a global systematic review and meta-analysis reported. In fact, the study found, as many as one in four children with T2D do not have obesity and some have normal reference-range body mass measurements. Further studies should consider other mechanisms beyond obesity in the genesis of pediatric diabetes, the authors of the international analysis concluded, writing for JAMA Network Open.
“We were aware that some children and adolescents with T2D did not have obesity, but we didn’t know the scale of obesity in T2D, or what variables may impact the occurrence of diabetes in this group,” endocrinologist M. Constantine Samaan, MD, MSc, associate professor of pediatrics at McMaster University in Hamilton, Ont., told this news organization. “So, the analysis did help us understand the body mass distribution of this group in more detail.”
The international investigators included in their meta-analysis 53 articles with 8,942 participants from multiple world regions and races/ethnicities. The overall prevalence of obesity in pediatric patients with T2D was 75.27% (95% confidence interval [CI], 70.47%-79.78%). The prevalence of obesity at time of diagnosis in 4,688 participants was 77.24% (95% CI, 70.55%-83.34%). Male participants had higher odds of obesity than females: odds ratio, 2.10 (95% CI, 1.33-3.31) – although girls are generally more likely to develop T2D. The highest prevalence of obesity occurred in Whites at 89.86% (95% CI, 71.50%-99.74%), while prevalence was lowest in Asian participants at 64.50% (95% CI, 53.28%-74.99%).
The authors noted that childhood obesity affects approximately 340 million children worldwide and is a major driver of pediatric T2D, an aggressive disease with a high treatment failure rate. Understanding the contribution of body mass to the evolution of insulin resistance, glucose intolerance, and T2D with its attendant comorbidities and complications, such as nonalcoholic fatty liver disease, remains crucial for developing personalized interventions.
Known risk factors for T2D include interactions between genetics and the environment, including lifestyle factors such as diet and low physical activity levels, Dr. Samaan noted. Certain ethnic groups have higher T2D risks, as do babies exposed in the womb to maternal obesity or diabetes, he said. “And there are likely many other factors that contribute to the risk of T2D, though these remain to be defined.”
Is “lean” T2D in children without obesity likely then to be hereditary, more severe, and harder to control with lifestyle modification? “That’s a great question, but the answer is we don’t know,” Dr. Samaan said.
Commenting on the study but not involved in it, Timothy J. Joos, MD, a pediatrician in Seattle affiliated with the Swedish Medical Center, said the findings raise the question of how many pediatric T2D patients are being missed because they don’t meet current screening criteria. “In nonobese T2D pediatric patients, genetics (and by proxy family history) obviously play a heavier role. In my practice, I often get parents asking me to screen their skinny teenager for diabetes because of diabetes in a family member. In the past I would begrudgingly comply with a smirk on my face. Now the smirk will be gone.”
Dr. Joos said it would be interesting to see what percentage of these T2D patients without obesity (body mass index < 95th percentile) would still meet the criteria for being overweight (BMI > 85th percentile) as this is the primary criterion for screening according to the American Diabetes Association guidelines.
Current guidelines generally look for elevated body mass measures as a main screening indication, Dr. Samaan’s group noted. But in their view, while factors such as ethnicity and in utero exposure to diabetes are already used in combination with BMI-based measures to justify screening, more sophisticated prediabetes and diabetes prediction models are needed to support a more comprehensive screening approach.
“Because being overweight is the initial criterion, children with multiple other criteria are not being screened,” Dr. Joos said. He agreed that more research is needed to sort out the other risk factors for pediatric T2D without obesity so these patients may be detected earlier.
New models may need to incorporate lifestyle factors, hormones, puberty, growth, and sex as well, the authors wrote. Markers of insulin resistance, insulin production capacity, and other markers are needed to refine the identification of those who should be screened.
Dr. Samaan’s group is planning to study the findings in more detail to clarify the effect of body mass on the comorbidities and complications of pediatric T2D.
In addition to the study limitation of significant interstudy heterogeneity, the authors acknowledged varying degrees of glycemic control and dyslipidemia among participants.
No specific funding was provided for this review and meta-analysis. The authors disclosed no conflicts of interest. Dr. Joos disclosed no competing interests with regard to his comments.
FROM JAMA NETWORK OPEN