User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Black HFrEF patients get more empagliflozin benefit in EMPEROR analyses
CHICAGO – Black patients with heart failure with reduced ejection fraction (HFrEF) may receive more benefit from treatment with a sodium-glucose cotransporter-2 (SGLT2) inhibitor than do White patients, according to a new report.
A secondary analysis of data collected from the pivotal trials that assessed the SGLT2 inhibitor empagliflozin in patients with HFrEF, EMPEROR-Reduced, and in patients with heart failure with preserved ejection fraction (HFpEF), EMPEROR-Preserved, was presented by Subodh Verma, MD, PhD, at the American Heart Association scientific sessions.
The “hypothesis-generating” analysis of data from EMPEROR-Reduced showed “a suggestion of a greater benefit of empagliflozin” in Black, compared with White patients, for the study’s primary endpoint (cardiovascular death or hospitalization for heart failure) as well as for first and total hospitalizations for heart failure, he reported.
However, a similar but separate analysis that compared Black and White patients with heart failure who received treatment with a second agent, dapagliflozin, from the same SGLT2-inhibitor class did not show any suggestion of heterogeneity in the drug’s effect based on race.
Race-linked heterogeneity in empagliflozin’s effect
In EMPEROR-Reduced, which randomized 3,730 patients with heart failure and a left ventricular ejection fraction of 40% or less, treatment of White patients with empagliflozin (Jardiance) produced a nonsignificant 16% relative reduction in the rate of the primary endpoint, compared with placebo, during a median 16-month follow-up.
By contrast, among Black patients, treatment with empagliflozin produced a significant 56% reduction in the primary endpoint, compared with placebo-treated patients, a significant heterogeneity (P = .02) in effect between the two race subgroups, said Dr. Verma, a cardiac surgeon and professor at the University of Toronto.
The analysis he reported used combined data from EMPEROR-Reduced and the companion trial EMPEROR-Preserved, which randomized 5,988 patients with heart failure and a left ventricular ejection fraction greater than 40% to treatment with either empagliflozin or placebo and followed them for a median of 26 months.
To assess the effects of the randomized treatments in the two racial subgroups, Dr. Verma and associates used pooled data from both trials, but only from the 3,502 patients enrolled in the Americas, which included 3,024 White patients and 478 Black patients. Analysis of the patients in this subgroup who were randomized to placebo showed a significantly excess rate of the primary outcome among Blacks, who tallied 49% more of the primary outcome events during follow-up than did White patients, Dr. Verma reported. The absolute rate of the primary outcome without empagliflozin treatment was 13.15 events/100 patient-years of follow-up in White patients and 20.83 events/100 patient-years in Black patients.
The impact of empagliflozin was not statistically heterogeneous in the total pool of patients that included both those with HFrEF and those with HFpEF. The drug reduced the primary outcome incidence by a significant 20% in White patients, and by a significant 44% among Black patients.
But this point-estimate difference in efficacy, when coupled with the underlying difference in risk for an event between the two racial groups, meant that the number-needed-to-treat to prevent one primary outcome event was 42 among White patients and 12 among Black patients.
Race-linked treatment responses only in HFrEF
This suggestion of an imbalance in treatment efficacy was especially apparent among patients with HFrEF. In addition to the heterogeneity for the primary outcome, the Black and White subgroups also showed significantly divergent results for the outcomes of first hospitalization for heart failure, with a nonsignificant 21% relative reduction with empagliflozin treatment in Whites but a significant 65% relative cut in this endpoint with empagliflozin in Blacks, and for total hospitalizations for heart failure, which showed a similar level of significant heterogeneity between the two race subgroups.
In contrast, the patients with HFpEF showed no signal at all for heterogeneous outcome rates between Black and White subgroups.
One other study outcome, change in symptom burden measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), also showed suggestion of a race-based imbalance. The adjusted mean difference from baseline in the KCCQ clinical summary score was 1.50 points higher with empagliflozin treatment, compared with placebo among all White patients (those with HFrEF and those with HFpEF), and compared with a 5.25-point increase with empagliflozin over placebo among all Black patients with heart failure in the pooled American EMPEROR dataset, a difference between White and Black patients that just missed significance (P = .06). Again, this difference was especially notable and significant among the patients with HFrEF, where the adjusted mean difference in KCCQ was a 0.77-point increase in White patients and a 6.71-point increase among Black patients (P = .043),
These results also appeared in a report published simultaneously with Dr. Verma’s talk.
But two other analyses that assessed a possible race-based difference in empagliflozin’s effect on renal protection and on functional status showed no suggestion of heterogeneity.
Dr. Verma stressed caution about the limitations of these analyses because they involved a relatively small number of Black patients, and were possibly subject to unadjusted confounding from differences in baseline characteristics between the Black and White patients.
Black patients also had a number-needed-to-treat advantage with dapagliflozin
The finding that Black patients with heart failure potentially get more bang for the buck from treatment with an SGLT2 inhibitor by having a lower number needed to treat also showed up in a separate report at the meeting that assessed the treatment effect from dapagliflozin (Farxiga) in Black and White patients in a pooled analysis of the DAPA-HF pivotal trial of patients with HFrEF and the DELIVER pivotal trial of patients with HFpEF. The pooled cohort included a total of 11,007, but for the analysis by race the investigators also limited their focus to patients from the Americas with 2,626 White patients and 381 Black patients.
Assessment of the effect of dapagliflozin on the primary outcome of cardiovascular death or hospitalization for heart failure among all patients, both those with HFrEF and those with HFpEF, again showed that event rates among patients treated with placebo were significantly higher in Black, compared with White patients, and this led to a difference in the number needed to treat to prevent one primary outcome event of 12 in Blacks and 17 in Whites, Jawad H. Butt, MD said in a talk at the meeting.
Although treatment with dapagliflozin reduced the rate of the primary outcome in this subgroup of patients from the DAPA-HF trial and the DELIVER trial by similar rates in Black and White patients, event rates were higher in the Black patients resulting in “greater benefit in absolute terms” for Black patients, explained Dr. Butt, a cardiologist at Rigshospitalet in Copenhagen.
But in contrast to the empagliflozin findings reported by Dr. Verma, the combined data from the dapagliflozin trials showed no suggestion of heterogeneity in the beneficial effect of dapagliflozin based on left ventricular ejection fraction. In the Black patients, for example, the relative benefit from dapagliflozin on the primary outcome was consistent across the full spectrum of patients with HFrEF and HFpEF.
EMPEROR-Reduced and EMPEROR-Preserved were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). The DAPA-HF and DELIVER trials were sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Verma has received honoraria, research support, or both from AstraZeneca, Boehringer Ingelheim, and Lilly, and from numerous other companies. Dr. Butt has been a consultant to and received travel grants from AstraZeneca, honoraria from Novartis, and has been an adviser to Bayer.
CHICAGO – Black patients with heart failure with reduced ejection fraction (HFrEF) may receive more benefit from treatment with a sodium-glucose cotransporter-2 (SGLT2) inhibitor than do White patients, according to a new report.
A secondary analysis of data collected from the pivotal trials that assessed the SGLT2 inhibitor empagliflozin in patients with HFrEF, EMPEROR-Reduced, and in patients with heart failure with preserved ejection fraction (HFpEF), EMPEROR-Preserved, was presented by Subodh Verma, MD, PhD, at the American Heart Association scientific sessions.
The “hypothesis-generating” analysis of data from EMPEROR-Reduced showed “a suggestion of a greater benefit of empagliflozin” in Black, compared with White patients, for the study’s primary endpoint (cardiovascular death or hospitalization for heart failure) as well as for first and total hospitalizations for heart failure, he reported.
However, a similar but separate analysis that compared Black and White patients with heart failure who received treatment with a second agent, dapagliflozin, from the same SGLT2-inhibitor class did not show any suggestion of heterogeneity in the drug’s effect based on race.
Race-linked heterogeneity in empagliflozin’s effect
In EMPEROR-Reduced, which randomized 3,730 patients with heart failure and a left ventricular ejection fraction of 40% or less, treatment of White patients with empagliflozin (Jardiance) produced a nonsignificant 16% relative reduction in the rate of the primary endpoint, compared with placebo, during a median 16-month follow-up.
By contrast, among Black patients, treatment with empagliflozin produced a significant 56% reduction in the primary endpoint, compared with placebo-treated patients, a significant heterogeneity (P = .02) in effect between the two race subgroups, said Dr. Verma, a cardiac surgeon and professor at the University of Toronto.
The analysis he reported used combined data from EMPEROR-Reduced and the companion trial EMPEROR-Preserved, which randomized 5,988 patients with heart failure and a left ventricular ejection fraction greater than 40% to treatment with either empagliflozin or placebo and followed them for a median of 26 months.
To assess the effects of the randomized treatments in the two racial subgroups, Dr. Verma and associates used pooled data from both trials, but only from the 3,502 patients enrolled in the Americas, which included 3,024 White patients and 478 Black patients. Analysis of the patients in this subgroup who were randomized to placebo showed a significantly excess rate of the primary outcome among Blacks, who tallied 49% more of the primary outcome events during follow-up than did White patients, Dr. Verma reported. The absolute rate of the primary outcome without empagliflozin treatment was 13.15 events/100 patient-years of follow-up in White patients and 20.83 events/100 patient-years in Black patients.
The impact of empagliflozin was not statistically heterogeneous in the total pool of patients that included both those with HFrEF and those with HFpEF. The drug reduced the primary outcome incidence by a significant 20% in White patients, and by a significant 44% among Black patients.
But this point-estimate difference in efficacy, when coupled with the underlying difference in risk for an event between the two racial groups, meant that the number-needed-to-treat to prevent one primary outcome event was 42 among White patients and 12 among Black patients.
Race-linked treatment responses only in HFrEF
This suggestion of an imbalance in treatment efficacy was especially apparent among patients with HFrEF. In addition to the heterogeneity for the primary outcome, the Black and White subgroups also showed significantly divergent results for the outcomes of first hospitalization for heart failure, with a nonsignificant 21% relative reduction with empagliflozin treatment in Whites but a significant 65% relative cut in this endpoint with empagliflozin in Blacks, and for total hospitalizations for heart failure, which showed a similar level of significant heterogeneity between the two race subgroups.
In contrast, the patients with HFpEF showed no signal at all for heterogeneous outcome rates between Black and White subgroups.
One other study outcome, change in symptom burden measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), also showed suggestion of a race-based imbalance. The adjusted mean difference from baseline in the KCCQ clinical summary score was 1.50 points higher with empagliflozin treatment, compared with placebo among all White patients (those with HFrEF and those with HFpEF), and compared with a 5.25-point increase with empagliflozin over placebo among all Black patients with heart failure in the pooled American EMPEROR dataset, a difference between White and Black patients that just missed significance (P = .06). Again, this difference was especially notable and significant among the patients with HFrEF, where the adjusted mean difference in KCCQ was a 0.77-point increase in White patients and a 6.71-point increase among Black patients (P = .043),
These results also appeared in a report published simultaneously with Dr. Verma’s talk.
But two other analyses that assessed a possible race-based difference in empagliflozin’s effect on renal protection and on functional status showed no suggestion of heterogeneity.
Dr. Verma stressed caution about the limitations of these analyses because they involved a relatively small number of Black patients, and were possibly subject to unadjusted confounding from differences in baseline characteristics between the Black and White patients.
Black patients also had a number-needed-to-treat advantage with dapagliflozin
The finding that Black patients with heart failure potentially get more bang for the buck from treatment with an SGLT2 inhibitor by having a lower number needed to treat also showed up in a separate report at the meeting that assessed the treatment effect from dapagliflozin (Farxiga) in Black and White patients in a pooled analysis of the DAPA-HF pivotal trial of patients with HFrEF and the DELIVER pivotal trial of patients with HFpEF. The pooled cohort included a total of 11,007, but for the analysis by race the investigators also limited their focus to patients from the Americas with 2,626 White patients and 381 Black patients.
Assessment of the effect of dapagliflozin on the primary outcome of cardiovascular death or hospitalization for heart failure among all patients, both those with HFrEF and those with HFpEF, again showed that event rates among patients treated with placebo were significantly higher in Black, compared with White patients, and this led to a difference in the number needed to treat to prevent one primary outcome event of 12 in Blacks and 17 in Whites, Jawad H. Butt, MD said in a talk at the meeting.
Although treatment with dapagliflozin reduced the rate of the primary outcome in this subgroup of patients from the DAPA-HF trial and the DELIVER trial by similar rates in Black and White patients, event rates were higher in the Black patients resulting in “greater benefit in absolute terms” for Black patients, explained Dr. Butt, a cardiologist at Rigshospitalet in Copenhagen.
But in contrast to the empagliflozin findings reported by Dr. Verma, the combined data from the dapagliflozin trials showed no suggestion of heterogeneity in the beneficial effect of dapagliflozin based on left ventricular ejection fraction. In the Black patients, for example, the relative benefit from dapagliflozin on the primary outcome was consistent across the full spectrum of patients with HFrEF and HFpEF.
EMPEROR-Reduced and EMPEROR-Preserved were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). The DAPA-HF and DELIVER trials were sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Verma has received honoraria, research support, or both from AstraZeneca, Boehringer Ingelheim, and Lilly, and from numerous other companies. Dr. Butt has been a consultant to and received travel grants from AstraZeneca, honoraria from Novartis, and has been an adviser to Bayer.
CHICAGO – Black patients with heart failure with reduced ejection fraction (HFrEF) may receive more benefit from treatment with a sodium-glucose cotransporter-2 (SGLT2) inhibitor than do White patients, according to a new report.
A secondary analysis of data collected from the pivotal trials that assessed the SGLT2 inhibitor empagliflozin in patients with HFrEF, EMPEROR-Reduced, and in patients with heart failure with preserved ejection fraction (HFpEF), EMPEROR-Preserved, was presented by Subodh Verma, MD, PhD, at the American Heart Association scientific sessions.
The “hypothesis-generating” analysis of data from EMPEROR-Reduced showed “a suggestion of a greater benefit of empagliflozin” in Black, compared with White patients, for the study’s primary endpoint (cardiovascular death or hospitalization for heart failure) as well as for first and total hospitalizations for heart failure, he reported.
However, a similar but separate analysis that compared Black and White patients with heart failure who received treatment with a second agent, dapagliflozin, from the same SGLT2-inhibitor class did not show any suggestion of heterogeneity in the drug’s effect based on race.
Race-linked heterogeneity in empagliflozin’s effect
In EMPEROR-Reduced, which randomized 3,730 patients with heart failure and a left ventricular ejection fraction of 40% or less, treatment of White patients with empagliflozin (Jardiance) produced a nonsignificant 16% relative reduction in the rate of the primary endpoint, compared with placebo, during a median 16-month follow-up.
By contrast, among Black patients, treatment with empagliflozin produced a significant 56% reduction in the primary endpoint, compared with placebo-treated patients, a significant heterogeneity (P = .02) in effect between the two race subgroups, said Dr. Verma, a cardiac surgeon and professor at the University of Toronto.
The analysis he reported used combined data from EMPEROR-Reduced and the companion trial EMPEROR-Preserved, which randomized 5,988 patients with heart failure and a left ventricular ejection fraction greater than 40% to treatment with either empagliflozin or placebo and followed them for a median of 26 months.
To assess the effects of the randomized treatments in the two racial subgroups, Dr. Verma and associates used pooled data from both trials, but only from the 3,502 patients enrolled in the Americas, which included 3,024 White patients and 478 Black patients. Analysis of the patients in this subgroup who were randomized to placebo showed a significantly excess rate of the primary outcome among Blacks, who tallied 49% more of the primary outcome events during follow-up than did White patients, Dr. Verma reported. The absolute rate of the primary outcome without empagliflozin treatment was 13.15 events/100 patient-years of follow-up in White patients and 20.83 events/100 patient-years in Black patients.
The impact of empagliflozin was not statistically heterogeneous in the total pool of patients that included both those with HFrEF and those with HFpEF. The drug reduced the primary outcome incidence by a significant 20% in White patients, and by a significant 44% among Black patients.
But this point-estimate difference in efficacy, when coupled with the underlying difference in risk for an event between the two racial groups, meant that the number-needed-to-treat to prevent one primary outcome event was 42 among White patients and 12 among Black patients.
Race-linked treatment responses only in HFrEF
This suggestion of an imbalance in treatment efficacy was especially apparent among patients with HFrEF. In addition to the heterogeneity for the primary outcome, the Black and White subgroups also showed significantly divergent results for the outcomes of first hospitalization for heart failure, with a nonsignificant 21% relative reduction with empagliflozin treatment in Whites but a significant 65% relative cut in this endpoint with empagliflozin in Blacks, and for total hospitalizations for heart failure, which showed a similar level of significant heterogeneity between the two race subgroups.
In contrast, the patients with HFpEF showed no signal at all for heterogeneous outcome rates between Black and White subgroups.
One other study outcome, change in symptom burden measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), also showed suggestion of a race-based imbalance. The adjusted mean difference from baseline in the KCCQ clinical summary score was 1.50 points higher with empagliflozin treatment, compared with placebo among all White patients (those with HFrEF and those with HFpEF), and compared with a 5.25-point increase with empagliflozin over placebo among all Black patients with heart failure in the pooled American EMPEROR dataset, a difference between White and Black patients that just missed significance (P = .06). Again, this difference was especially notable and significant among the patients with HFrEF, where the adjusted mean difference in KCCQ was a 0.77-point increase in White patients and a 6.71-point increase among Black patients (P = .043),
These results also appeared in a report published simultaneously with Dr. Verma’s talk.
But two other analyses that assessed a possible race-based difference in empagliflozin’s effect on renal protection and on functional status showed no suggestion of heterogeneity.
Dr. Verma stressed caution about the limitations of these analyses because they involved a relatively small number of Black patients, and were possibly subject to unadjusted confounding from differences in baseline characteristics between the Black and White patients.
Black patients also had a number-needed-to-treat advantage with dapagliflozin
The finding that Black patients with heart failure potentially get more bang for the buck from treatment with an SGLT2 inhibitor by having a lower number needed to treat also showed up in a separate report at the meeting that assessed the treatment effect from dapagliflozin (Farxiga) in Black and White patients in a pooled analysis of the DAPA-HF pivotal trial of patients with HFrEF and the DELIVER pivotal trial of patients with HFpEF. The pooled cohort included a total of 11,007, but for the analysis by race the investigators also limited their focus to patients from the Americas with 2,626 White patients and 381 Black patients.
Assessment of the effect of dapagliflozin on the primary outcome of cardiovascular death or hospitalization for heart failure among all patients, both those with HFrEF and those with HFpEF, again showed that event rates among patients treated with placebo were significantly higher in Black, compared with White patients, and this led to a difference in the number needed to treat to prevent one primary outcome event of 12 in Blacks and 17 in Whites, Jawad H. Butt, MD said in a talk at the meeting.
Although treatment with dapagliflozin reduced the rate of the primary outcome in this subgroup of patients from the DAPA-HF trial and the DELIVER trial by similar rates in Black and White patients, event rates were higher in the Black patients resulting in “greater benefit in absolute terms” for Black patients, explained Dr. Butt, a cardiologist at Rigshospitalet in Copenhagen.
But in contrast to the empagliflozin findings reported by Dr. Verma, the combined data from the dapagliflozin trials showed no suggestion of heterogeneity in the beneficial effect of dapagliflozin based on left ventricular ejection fraction. In the Black patients, for example, the relative benefit from dapagliflozin on the primary outcome was consistent across the full spectrum of patients with HFrEF and HFpEF.
EMPEROR-Reduced and EMPEROR-Preserved were sponsored by Boehringer Ingelheim and Lilly, the companies that jointly market empagliflozin (Jardiance). The DAPA-HF and DELIVER trials were sponsored by AstraZeneca, the company that markets dapagliflozin (Farxiga). Dr. Verma has received honoraria, research support, or both from AstraZeneca, Boehringer Ingelheim, and Lilly, and from numerous other companies. Dr. Butt has been a consultant to and received travel grants from AstraZeneca, honoraria from Novartis, and has been an adviser to Bayer.
AT AHA 2022
Children and COVID: New-case counts offer dueling narratives
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.
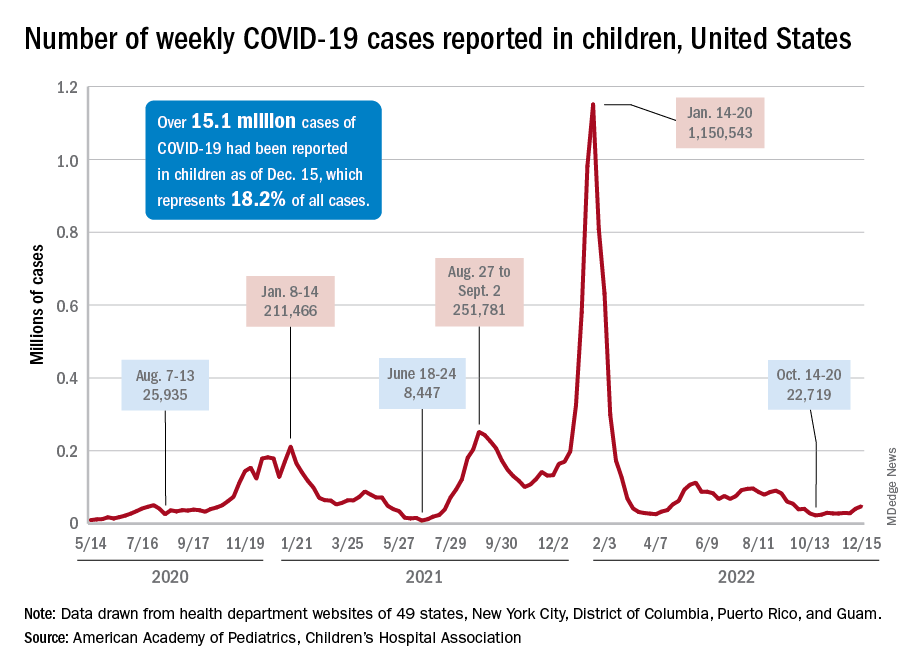
and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.

and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
New COVID-19 cases in children jumped by 66% during the first 2 weeks of December after an 8-week steady period lasting through October and November, according to the American Academy of Pediatrics and the Children’s Hospital Association.

and totaling less than 29,000 for the week of Nov. 25 to Dec. 1. That increase of almost 19,000 cases is the largest over a 2-week period since late July, the AAP and CHA said in their weekly COVID report based on data collected from state and territorial health department websites.
[This publication has been following the AAP/CHA report since the summer of 2020 and continues to share the data for the sake of consistency, but it must be noted that a number of states are no longer updating their public COVID dashboards. As a result, there is now a considerable discrepancy between the AAP/CHA weekly figures and those reported by the Centers for Disease Control and Prevention, which has no such limitations on state data.]
The situation involving new cases over the last 2 weeks is quite different from the CDC’s perspective. The agency does not publish a weekly count, instead offering cumulative cases, which stood at almost 16.1 million as of Dec. 14. Calculating a 2-week total puts the new-case count for Dec. 1-14 at 113,572 among children aged 0-17 years. That is higher than the AAP/CHA count (88,629) for roughly the same period, but it is actually lower than the CDC’s figure (161,832) for the last 2 weeks of November.
The CDC data, in other words, suggest that new cases have gone down in the last 2 weeks, while the AAP and CHA, with their somewhat limited perspective, announced that new cases have gone up.
One COVID-related measure from the CDC that is not contradicted by other sources is hospitalization rates, which had climbed from 0.16 new admissions in children aged 0-17 years with confirmed COVID per 100,000 population on Oct. 22 to 0.29 per 100,000 on Dec. 9. Visits to the emergency department with diagnosed COVID, meanwhile, have been fairly steady so far through December in children, according to the CDC.
High lipoprotein(a) levels plus hypertension add to CVD risk
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
High levels of lipoprotein(a) increase the risk for incident cardiovascular disease (CVD) for hypertensive individuals but not for those without hypertension, a new MESA analysis suggests.
There are ways to test for statistical interaction, “in this case, multiplicative interaction between Lp(a) and hypertension, which suggests that Lp(a) is actually modifying the effect between blood pressure and cardiovascular disease. It’s not simply additive,” senior author Michael D. Shapiro, DO, Wake Forest University, Winston-Salem, N.C., told this news organization.
“So that’s new and I don’t think anybody’s looked at that before.”
Although Lp(a) is recognized as an independent cause of atherosclerotic CVD (ASCVD), the significance of Lp(a) in hypertension has been “virtually untapped,” he noted. A recent prospective study reported that elevated CVD risk was present only in individuals with Lp(a) ≥ 30 mg/dL and hypertension but it included only Chinese participants with stable coronary artery disease.
The current analysis, published online in the journal Hypertension, included 6,674 participants in the ongoing Multi-Ethnic Study of Atherosclerosis (MESA), all free of baseline ASCVD, who were recruited from six communities in the United States and had measured baseline Lp(a), blood pressure, and CVD events data over follow-up from 2000 to 2018.
Participants were stratified into four groups based on the presence or absence of hypertension (defined as 140/90 mm Hg or higher or the use of antihypertensive drugs) and an Lp(a) threshold of 50 mg/dL, as recommended by the American College of Cardiology/American Heart Association cholesterol guideline for consideration as an ASCVD risk-enhancing factor.
Slightly more than half of participants were female (52.8%), 38.6% were White, 27.5% were African American, 22.1% were Hispanic, and 11.9% were Chinese American.
According to the researchers, 809 participants had a CVD event over an average follow-up of 13.9 years, including 7.7% of group 1 with Lp(a) < 50 mg/dL and no hypertension, 8.0% of group 2 with Lp(a) ≥ 50 mg/dL and no hypertension, 16.2% of group 3 with Lp(a) < 50 mg/dL and hypertension, and 18.8% of group 4 with Lp(a) ≥ 50 mg/dL and hypertension.
When compared with group 1 in a fully adjusted Cox proportional model, participants with elevated Lp(a) and no hypertension (group 2) did not have an increased risk of CVD events (hazard ratio [HR], 1.09; 95% confidence interval [CI], 0.79-1.50).
CVD risk, however, was significantly higher in group 3 with normal Lp(a) and hypertension (HR, 1.66; 95% CI, 1.39-1.98) and group 4 with elevated Lp(a) and hypertension (HR, 2.07, 95% CI, 1.63-2.62).
Among all participants with hypertension (groups 3 and 4), Lp(a) was associated with a significant increase in CVD risk (HR, 1.24, 95% CI, 1.01-1.53).
“What I think is interesting here is that in the absence of hypertension, we didn’t really see an increased risk despite having an elevated Lp(a),” said Dr. Shapiro. “What it may indicate is that really for Lp(a) to be associated with risk, there may already need to be some kind of arterial damage that allows the Lp(a) to have its atherogenic impact.
“In other words, in individuals who have totally normal arterial walls, potentially, maybe that is protective enough against Lp(a) that in the absence of any other injurious factor, maybe it’s not an issue,” he said. “That’s a big hypothesis-generating [statement], but hypertension is certainly one of those risk factors that’s known to cause endothelial injury and endothelial dysfunction.”
Dr. Shapiro pointed out that when first measured in MESA, Lp(a) was measured in 4,600 participants who were not on statins, which is important because statins can increase Lp(a) levels.
“When you look just at those participants, those 4,600, you actually do see a relationship between Lp(a) and cardiovascular disease,” he said. “When you look at the whole population, including the 17% who are baseline populations, even when you adjust for statin therapy, we fail to see that, at least in the long-term follow up.”
Nevertheless, he cautioned that hypertension is just one of many traditional cardiovascular risk factors that could affect the relationship between Lp(a) and CVD risk. “I don’t want to suggest that we believe there’s something specifically magical about hypertension and Lp(a). If we chose, say, diabetes or smoking or another traditional risk factor, we may or may not have seen kind of similar results.”
When the investigators stratified the analyses by sex and race/ethnicity, they found that Lp(a) was not associated with CVD risk, regardless of hypertension status. In Black participants, however, greater CVD risk was seen when both elevated Lp(a) and hypertension were present (HR, 2.07, 95% CI, 1.34-3.21; P = .001).
Asked whether the results support one-time universal screening for Lp(a), which is almost exclusively genetically determined, Dr. Shapiro said he supports screening but that this was a secondary analysis and its numbers were modest. He added that median Lp(a) level is higher in African Americans than any other racial/ethnic group but the “most recent data has clarified that, per any absolute level of Lp(a), it appears to confer the same absolute risk in any racial or ethnic group.”
The authors acknowledge that differential loss to follow-up could have resulted in selection bias in the study and that there were relatively few CVD events in group 2, which may have limited the ability to detect differences in groups without hypertension, particularly in the subgroup analyses. Other limitations are the potential for residual confounding and participants may have developed hypertension during follow-up, resulting in misclassification bias.
Further research is needed to better understand the mechanistic link between Lp(a), hypertension, and CVD, Dr. Shapiro said. Further insights also should be provided by the ongoing phase 3 Lp(a) HORIZON trial evaluating the effect of Lp(a) lowering with the investigational antisense drug, pelacarsen, on cardiovascular events in 8,324 patients with established CVD and elevated Lp(a). The study is expected to be completed in May 2025.
The study was supported by contracts from the National Heart, Lung, and Blood Institute and by grants from the National Center for Advanced Translational Sciences. Dr. Shapiro reports participating in scientific advisory boards with Amgen, Novartis, and Novo Nordisk, and consulting for Regeneron.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION
Systematic review supports preferred drugs for HIV in youths
A systematic review of observational studies and clinical trials found dolutegravir and raltegravir to be safe and effective for treating teens and children living with HIV.
Effectiveness was higher across dolutegravir studies, the authors reported. After 12 months of treatment and observation, viral suppression levels were greater than 70% in most studies assessing dolutegravir. Viral suppression with raltegravir after 12 months varied between 42% and 83%.
“Our findings support the use of these two integrase inhibitors as part of WHO-recommended regimens for treating HIV,” said lead study author Claire Townsend, PhD, an epidemiologist and consultant to the World Health Organization HIV department in Geneva. “They were in line with what has been reported in adults and provide reassurance for the continued use of these two drugs in children and adolescents.”
The study was published in the Journal of the International AIDS Society.
Tracking outcomes for WHO guidelines
Integrase inhibitors, including dolutegravir and raltegravir, have become leading first- and second-line treatments in patients with HIV, largely owing to their effectiveness and fewer side effects, compared with other antiretroviral treatments.
Monitoring short- and long-term health outcomes of these widely used drugs is critical, the authors wrote. This is especially the case for dolutegravir, which has recently been approved in pediatric formulations. The review supported the development of the 2021 WHO consolidated HIV guidelines.
Dr. Townsend and colleagues searched the literature and screened trial registries for relevant studies conducted from January 2009 to March 2021. Among more than 4,000 published papers and abstracts, they identified 19 studies that met their review criteria relating to dolutegravir or raltegravir in children or adolescents aged 0-19 years who are living with HIV, including two studies that reported data on both agents.
Data on dolutegravir were extracted from 11 studies that included 2,330 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 9 cohort studies. Data on raltegravir were extracted from 10 studies that included 649 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 8 cohort studies.
The median follow-up in the dolutegravir studies was 6-36 months. Six studies recruited participants from Europe, three studies were based in sub-Saharan Africa, and two studies included persons from multiple geographic regions.
Across all studies, grade 3/4 adverse events were reported in 0%-50% of cases. Of these adverse events, very few were drug related, and no deaths were attributed to either dolutegravir or raltegravir.
However, Dr. Townsend cautioned that future research is needed to fill in evidence gaps “on longer-term safety and effectiveness of dolutegravir and raltegravir in children and adolescents,” including “research into adverse outcomes such as weight gain, potential metabolic changes, and neuropsychiatric adverse events, which have been reported in adults.”
The researchers noted that the small sample size of many of the studies contributed to variability in the findings and that most studies were observational, providing important real-world data but making their results less robust compared with data from randomized controlled studies with large sample sizes. They also noted that there was a high risk of bias (4 studies) and unclear risk of bias (5 studies) among the 15 observational studies included in their analysis.
“This research is particularly important because it supports the WHO recommendation that dolutegravir, which has a particularly high barrier of resistance to the HIV virus, be synchronized in adults and children as the preferred first-line and second-line treatment against HIV,” said Natella Rakhmanina, MD, PhD, director of HIV Services & Special Immunology at the Children’s National Hospital in Washington, D.C. Dr. Rakhmanina was not associated with the study.
Dr. Rakhmanina agreed that the safety profile of both drugs is “very good.” The lack of serious adverse events was meaningful, she highlighted, because “good tolerability is very important, particularly in children” as it means that drug compliance and viral suppression are achievable.
Two authors reported their authorship on two studies included in the review, as well as grant funding from ViiV Healthcare/GlaxoSmithKline, the marketing authorization holder for dolutegravir.
A version of this article first appeared on Medscape.com.
A systematic review of observational studies and clinical trials found dolutegravir and raltegravir to be safe and effective for treating teens and children living with HIV.
Effectiveness was higher across dolutegravir studies, the authors reported. After 12 months of treatment and observation, viral suppression levels were greater than 70% in most studies assessing dolutegravir. Viral suppression with raltegravir after 12 months varied between 42% and 83%.
“Our findings support the use of these two integrase inhibitors as part of WHO-recommended regimens for treating HIV,” said lead study author Claire Townsend, PhD, an epidemiologist and consultant to the World Health Organization HIV department in Geneva. “They were in line with what has been reported in adults and provide reassurance for the continued use of these two drugs in children and adolescents.”
The study was published in the Journal of the International AIDS Society.
Tracking outcomes for WHO guidelines
Integrase inhibitors, including dolutegravir and raltegravir, have become leading first- and second-line treatments in patients with HIV, largely owing to their effectiveness and fewer side effects, compared with other antiretroviral treatments.
Monitoring short- and long-term health outcomes of these widely used drugs is critical, the authors wrote. This is especially the case for dolutegravir, which has recently been approved in pediatric formulations. The review supported the development of the 2021 WHO consolidated HIV guidelines.
Dr. Townsend and colleagues searched the literature and screened trial registries for relevant studies conducted from January 2009 to March 2021. Among more than 4,000 published papers and abstracts, they identified 19 studies that met their review criteria relating to dolutegravir or raltegravir in children or adolescents aged 0-19 years who are living with HIV, including two studies that reported data on both agents.
Data on dolutegravir were extracted from 11 studies that included 2,330 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 9 cohort studies. Data on raltegravir were extracted from 10 studies that included 649 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 8 cohort studies.
The median follow-up in the dolutegravir studies was 6-36 months. Six studies recruited participants from Europe, three studies were based in sub-Saharan Africa, and two studies included persons from multiple geographic regions.
Across all studies, grade 3/4 adverse events were reported in 0%-50% of cases. Of these adverse events, very few were drug related, and no deaths were attributed to either dolutegravir or raltegravir.
However, Dr. Townsend cautioned that future research is needed to fill in evidence gaps “on longer-term safety and effectiveness of dolutegravir and raltegravir in children and adolescents,” including “research into adverse outcomes such as weight gain, potential metabolic changes, and neuropsychiatric adverse events, which have been reported in adults.”
The researchers noted that the small sample size of many of the studies contributed to variability in the findings and that most studies were observational, providing important real-world data but making their results less robust compared with data from randomized controlled studies with large sample sizes. They also noted that there was a high risk of bias (4 studies) and unclear risk of bias (5 studies) among the 15 observational studies included in their analysis.
“This research is particularly important because it supports the WHO recommendation that dolutegravir, which has a particularly high barrier of resistance to the HIV virus, be synchronized in adults and children as the preferred first-line and second-line treatment against HIV,” said Natella Rakhmanina, MD, PhD, director of HIV Services & Special Immunology at the Children’s National Hospital in Washington, D.C. Dr. Rakhmanina was not associated with the study.
Dr. Rakhmanina agreed that the safety profile of both drugs is “very good.” The lack of serious adverse events was meaningful, she highlighted, because “good tolerability is very important, particularly in children” as it means that drug compliance and viral suppression are achievable.
Two authors reported their authorship on two studies included in the review, as well as grant funding from ViiV Healthcare/GlaxoSmithKline, the marketing authorization holder for dolutegravir.
A version of this article first appeared on Medscape.com.
A systematic review of observational studies and clinical trials found dolutegravir and raltegravir to be safe and effective for treating teens and children living with HIV.
Effectiveness was higher across dolutegravir studies, the authors reported. After 12 months of treatment and observation, viral suppression levels were greater than 70% in most studies assessing dolutegravir. Viral suppression with raltegravir after 12 months varied between 42% and 83%.
“Our findings support the use of these two integrase inhibitors as part of WHO-recommended regimens for treating HIV,” said lead study author Claire Townsend, PhD, an epidemiologist and consultant to the World Health Organization HIV department in Geneva. “They were in line with what has been reported in adults and provide reassurance for the continued use of these two drugs in children and adolescents.”
The study was published in the Journal of the International AIDS Society.
Tracking outcomes for WHO guidelines
Integrase inhibitors, including dolutegravir and raltegravir, have become leading first- and second-line treatments in patients with HIV, largely owing to their effectiveness and fewer side effects, compared with other antiretroviral treatments.
Monitoring short- and long-term health outcomes of these widely used drugs is critical, the authors wrote. This is especially the case for dolutegravir, which has recently been approved in pediatric formulations. The review supported the development of the 2021 WHO consolidated HIV guidelines.
Dr. Townsend and colleagues searched the literature and screened trial registries for relevant studies conducted from January 2009 to March 2021. Among more than 4,000 published papers and abstracts, they identified 19 studies that met their review criteria relating to dolutegravir or raltegravir in children or adolescents aged 0-19 years who are living with HIV, including two studies that reported data on both agents.
Data on dolutegravir were extracted from 11 studies that included 2,330 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 9 cohort studies. Data on raltegravir were extracted from 10 studies that included 649 children and adolescents in 1 randomized controlled trial, 1 single-arm trial, and 8 cohort studies.
The median follow-up in the dolutegravir studies was 6-36 months. Six studies recruited participants from Europe, three studies were based in sub-Saharan Africa, and two studies included persons from multiple geographic regions.
Across all studies, grade 3/4 adverse events were reported in 0%-50% of cases. Of these adverse events, very few were drug related, and no deaths were attributed to either dolutegravir or raltegravir.
However, Dr. Townsend cautioned that future research is needed to fill in evidence gaps “on longer-term safety and effectiveness of dolutegravir and raltegravir in children and adolescents,” including “research into adverse outcomes such as weight gain, potential metabolic changes, and neuropsychiatric adverse events, which have been reported in adults.”
The researchers noted that the small sample size of many of the studies contributed to variability in the findings and that most studies were observational, providing important real-world data but making their results less robust compared with data from randomized controlled studies with large sample sizes. They also noted that there was a high risk of bias (4 studies) and unclear risk of bias (5 studies) among the 15 observational studies included in their analysis.
“This research is particularly important because it supports the WHO recommendation that dolutegravir, which has a particularly high barrier of resistance to the HIV virus, be synchronized in adults and children as the preferred first-line and second-line treatment against HIV,” said Natella Rakhmanina, MD, PhD, director of HIV Services & Special Immunology at the Children’s National Hospital in Washington, D.C. Dr. Rakhmanina was not associated with the study.
Dr. Rakhmanina agreed that the safety profile of both drugs is “very good.” The lack of serious adverse events was meaningful, she highlighted, because “good tolerability is very important, particularly in children” as it means that drug compliance and viral suppression are achievable.
Two authors reported their authorship on two studies included in the review, as well as grant funding from ViiV Healthcare/GlaxoSmithKline, the marketing authorization holder for dolutegravir.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE INTERNATIONAL AIDS SOCIETY
Dispatching volunteer responders may not increase AED use in OHCA
Dispatching trained volunteer responders via smartphones to retrieve automated external defibrillators for patients in out-of-hospital cardiac arrest (OHCA) did not significantly increase bystander AED use in a randomized clinical trial in Sweden.
Most patients in OHCA can be saved if cardiopulmonary resuscitation and defibrillation are initiated within minutes, but despite the “substantial” public availability of AEDs and widespread CPR training among the Swedish public, use rates of both are low, Mattias Ringh, MD, PhD, of Karolinska Institutet in Stockholm, and colleagues wrote.
A previous study by the team showed that dispatching volunteer responders via a smartphone app significantly increased bystander CPR. The current study, called the Swedish AED and Mobile Bystander Activation (SAMBA) trial, aimed to see whether dispatching volunteer responders to collect a nearby AED would increase bystander AED use. A control group of volunteer responders was instructed to go straight to the scene and start CPR.
“The results showed that the volunteer responders were first to provide treatment with both CPR and AEDs in a large proportion of cases in both groups, thereby creating a ‘statistical’ dilutional effect,” Dr. Ringh said in an interview. In effect, the control arm also became an active arm.
“But if we agree that treatment with AEDs and CPR is saving lives, then dispatching volunteer responders is doing just that, although we could not fully measure the effect in our study,” he added.
The study was published online in JAMA Cardiology.
No significant differences
The SAMBA trial assessed outcomes of the smartphone dispatch system (Heartrunner), which is triggered at emergency dispatch centers in response to suspected OHCAs at the same time that an ambulance with advanced life support equipment is dispatched.
The volunteer responder system locates a maximum of 30 volunteer responders within a 1.3-km radius from the suspected out-of-hospital cardiac arrest, the researchers explained in their report. Volunteer responders are requested via their smartphone application to accept or decline the alert. If they accept an alert, the volunteer responders receive map-aided route directions to the location of the suspected arrest.
In patients allocated to intervention in this study, four of five of all volunteer responders who accepted the alert received instructions to collect the nearest available AED and then go directly to the patient with suspected out-of-hospital cardiac arrest, the authors noted. Route directions to the scene of the cardiac arrest and the AED were displayed on their smartphones. One of the 5 volunteer responders, closest to the arrest, was dispatched to go directly to initiate CPR.
In patients allocated to the control group, all volunteer responders who accepted the alert were instructed to go directly to the patient with suspected out-of-hospital cardiac arrest to perform CPR. No route directions to or locations of AEDs were displayed.
The study was conducted in Stockholm and in Västra Götaland from 2018 to 2020. At the start of the study, there were 3,123 AEDs and 24,493 volunteer responders in Stockholm and 3,195 AEDs and 19,117 volunteer responders in Västra Götaland.
Post-randomization exclusions included patients without OHCA, those with OHCAs not treated by emergency medical services, and those with OHCAs witnessed by EMS.
The primary outcome was overall bystander AED attachment before the arrival of EMS, including those attached by the volunteer responders but also by lay volunteers who did not use the smartphone app.
Volunteer responders were activated for 947 individuals with OHCA; 461 patients were randomized to the intervention group and 486 to the control group. In both groups, the patients’ median age was 73 and about 65% were men.
Attachment of the AED before the arrival of EMS or first responders occurred in 61 patients (13.2%) in the intervention group versus 46 (9.5%) in the control group (P = .08). However, the majority of all AEDs were attached by lay volunteers who were not volunteer responders using the smartphone app (37 in the intervention arm vs. 28 in the control arm), the researchers noted.
No significant differences were seen in secondary outcomes, which included bystander CPR (69% vs. 71.6%, respectively) and defibrillation before EMS arrival (3.7% vs. 3.9%) between groups.
Among the volunteer responders using the app, crossover was 11% and compliance to instructions was 31%. Overall, volunteer responders attached 38% of all bystander-attached AEDs and provided 45% of all bystander defibrillations and 43% of all bystander CPR.
Going forward, Dr. Ringh and colleagues will be further analyzing the results to understand how to better optimize the logistical challenges involved with smartphone dispatch to OHCA patients. “In the longer term, investigating the impact on survival is also warranted,” he concluded.
U.S. in worse shape
In a comment, Christopher Calandrella, DO, chair of emergency medicine at Long Island Jewish Forest Hills,, New York, part of Northwell Health, said: “Significant data are available to support the importance of prompt initiation of CPR and defibrillation for OHCA, and although this study did not demonstrate a meaningful increase in use of AEDs with the trial system, layperson CPR was initiated in approximately 70% of cases in the cohort as a whole. Because of this, I believe it is evident that patients still benefit from a system that encourages bystanders to provide aid prior to the arrival of EMS.”
Nevertheless, he noted, “despite the training of volunteers in applying an AED, overall, only a small percentage of patients in either group had placement and use of the device. While the reasons likely are multifactorial, it may be in part due to the significant stress and anxiety associated with OHCA.”
Additional research would be helpful, he said. “Future studies focusing on more rural areas with lower population density and limited availability of AEDs may be beneficial. Expanding the research outside of Europe to other countries would be useful. Next-phase trials looking at 30-day survival in these patients would also be important.”
Currently in the United States, research is underway to evaluate the use of smartphones to improve in-hospital cardiac arrests, he added, “but no nationwide programs are in place for OHCA.”
Similarly, Kevin G. Volpp, MD, PhD, and Benjamin S. Abella, MD, MPhil, both of the University of Pennsylvania, Philadelphia, wrote in a related editorial: “It is sobering to recognize that, in the U.S., it may be nearly impossible to even test an idea like this, given the lack of a supporting data infrastructure.”
Although there is an app in the United States to link OHCA events to bystander response, they noted, less than half of eligible 911 centers have linked to it.
“Furthermore, the bystander CPR rate in the U.S. is less than 35%, only about half of the Swedish rate, indicating far fewer people are trained in CPR and comfortable performing it in the U.S.,” they wrote. “A wealthy country like the U.S. should be able to develop a far more effective approach to preventing millions of ... families from having a loved one die of OHCA in the decade to come.”
The study was funded by unrestricted grant from the Swedish Heart-Lung Foundation and Stockholm County. The authors, editorialists, and Dr. Calandrella disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dispatching trained volunteer responders via smartphones to retrieve automated external defibrillators for patients in out-of-hospital cardiac arrest (OHCA) did not significantly increase bystander AED use in a randomized clinical trial in Sweden.
Most patients in OHCA can be saved if cardiopulmonary resuscitation and defibrillation are initiated within minutes, but despite the “substantial” public availability of AEDs and widespread CPR training among the Swedish public, use rates of both are low, Mattias Ringh, MD, PhD, of Karolinska Institutet in Stockholm, and colleagues wrote.
A previous study by the team showed that dispatching volunteer responders via a smartphone app significantly increased bystander CPR. The current study, called the Swedish AED and Mobile Bystander Activation (SAMBA) trial, aimed to see whether dispatching volunteer responders to collect a nearby AED would increase bystander AED use. A control group of volunteer responders was instructed to go straight to the scene and start CPR.
“The results showed that the volunteer responders were first to provide treatment with both CPR and AEDs in a large proportion of cases in both groups, thereby creating a ‘statistical’ dilutional effect,” Dr. Ringh said in an interview. In effect, the control arm also became an active arm.
“But if we agree that treatment with AEDs and CPR is saving lives, then dispatching volunteer responders is doing just that, although we could not fully measure the effect in our study,” he added.
The study was published online in JAMA Cardiology.
No significant differences
The SAMBA trial assessed outcomes of the smartphone dispatch system (Heartrunner), which is triggered at emergency dispatch centers in response to suspected OHCAs at the same time that an ambulance with advanced life support equipment is dispatched.
The volunteer responder system locates a maximum of 30 volunteer responders within a 1.3-km radius from the suspected out-of-hospital cardiac arrest, the researchers explained in their report. Volunteer responders are requested via their smartphone application to accept or decline the alert. If they accept an alert, the volunteer responders receive map-aided route directions to the location of the suspected arrest.
In patients allocated to intervention in this study, four of five of all volunteer responders who accepted the alert received instructions to collect the nearest available AED and then go directly to the patient with suspected out-of-hospital cardiac arrest, the authors noted. Route directions to the scene of the cardiac arrest and the AED were displayed on their smartphones. One of the 5 volunteer responders, closest to the arrest, was dispatched to go directly to initiate CPR.
In patients allocated to the control group, all volunteer responders who accepted the alert were instructed to go directly to the patient with suspected out-of-hospital cardiac arrest to perform CPR. No route directions to or locations of AEDs were displayed.
The study was conducted in Stockholm and in Västra Götaland from 2018 to 2020. At the start of the study, there were 3,123 AEDs and 24,493 volunteer responders in Stockholm and 3,195 AEDs and 19,117 volunteer responders in Västra Götaland.
Post-randomization exclusions included patients without OHCA, those with OHCAs not treated by emergency medical services, and those with OHCAs witnessed by EMS.
The primary outcome was overall bystander AED attachment before the arrival of EMS, including those attached by the volunteer responders but also by lay volunteers who did not use the smartphone app.
Volunteer responders were activated for 947 individuals with OHCA; 461 patients were randomized to the intervention group and 486 to the control group. In both groups, the patients’ median age was 73 and about 65% were men.
Attachment of the AED before the arrival of EMS or first responders occurred in 61 patients (13.2%) in the intervention group versus 46 (9.5%) in the control group (P = .08). However, the majority of all AEDs were attached by lay volunteers who were not volunteer responders using the smartphone app (37 in the intervention arm vs. 28 in the control arm), the researchers noted.
No significant differences were seen in secondary outcomes, which included bystander CPR (69% vs. 71.6%, respectively) and defibrillation before EMS arrival (3.7% vs. 3.9%) between groups.
Among the volunteer responders using the app, crossover was 11% and compliance to instructions was 31%. Overall, volunteer responders attached 38% of all bystander-attached AEDs and provided 45% of all bystander defibrillations and 43% of all bystander CPR.
Going forward, Dr. Ringh and colleagues will be further analyzing the results to understand how to better optimize the logistical challenges involved with smartphone dispatch to OHCA patients. “In the longer term, investigating the impact on survival is also warranted,” he concluded.
U.S. in worse shape
In a comment, Christopher Calandrella, DO, chair of emergency medicine at Long Island Jewish Forest Hills,, New York, part of Northwell Health, said: “Significant data are available to support the importance of prompt initiation of CPR and defibrillation for OHCA, and although this study did not demonstrate a meaningful increase in use of AEDs with the trial system, layperson CPR was initiated in approximately 70% of cases in the cohort as a whole. Because of this, I believe it is evident that patients still benefit from a system that encourages bystanders to provide aid prior to the arrival of EMS.”
Nevertheless, he noted, “despite the training of volunteers in applying an AED, overall, only a small percentage of patients in either group had placement and use of the device. While the reasons likely are multifactorial, it may be in part due to the significant stress and anxiety associated with OHCA.”
Additional research would be helpful, he said. “Future studies focusing on more rural areas with lower population density and limited availability of AEDs may be beneficial. Expanding the research outside of Europe to other countries would be useful. Next-phase trials looking at 30-day survival in these patients would also be important.”
Currently in the United States, research is underway to evaluate the use of smartphones to improve in-hospital cardiac arrests, he added, “but no nationwide programs are in place for OHCA.”
Similarly, Kevin G. Volpp, MD, PhD, and Benjamin S. Abella, MD, MPhil, both of the University of Pennsylvania, Philadelphia, wrote in a related editorial: “It is sobering to recognize that, in the U.S., it may be nearly impossible to even test an idea like this, given the lack of a supporting data infrastructure.”
Although there is an app in the United States to link OHCA events to bystander response, they noted, less than half of eligible 911 centers have linked to it.
“Furthermore, the bystander CPR rate in the U.S. is less than 35%, only about half of the Swedish rate, indicating far fewer people are trained in CPR and comfortable performing it in the U.S.,” they wrote. “A wealthy country like the U.S. should be able to develop a far more effective approach to preventing millions of ... families from having a loved one die of OHCA in the decade to come.”
The study was funded by unrestricted grant from the Swedish Heart-Lung Foundation and Stockholm County. The authors, editorialists, and Dr. Calandrella disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dispatching trained volunteer responders via smartphones to retrieve automated external defibrillators for patients in out-of-hospital cardiac arrest (OHCA) did not significantly increase bystander AED use in a randomized clinical trial in Sweden.
Most patients in OHCA can be saved if cardiopulmonary resuscitation and defibrillation are initiated within minutes, but despite the “substantial” public availability of AEDs and widespread CPR training among the Swedish public, use rates of both are low, Mattias Ringh, MD, PhD, of Karolinska Institutet in Stockholm, and colleagues wrote.
A previous study by the team showed that dispatching volunteer responders via a smartphone app significantly increased bystander CPR. The current study, called the Swedish AED and Mobile Bystander Activation (SAMBA) trial, aimed to see whether dispatching volunteer responders to collect a nearby AED would increase bystander AED use. A control group of volunteer responders was instructed to go straight to the scene and start CPR.
“The results showed that the volunteer responders were first to provide treatment with both CPR and AEDs in a large proportion of cases in both groups, thereby creating a ‘statistical’ dilutional effect,” Dr. Ringh said in an interview. In effect, the control arm also became an active arm.
“But if we agree that treatment with AEDs and CPR is saving lives, then dispatching volunteer responders is doing just that, although we could not fully measure the effect in our study,” he added.
The study was published online in JAMA Cardiology.
No significant differences
The SAMBA trial assessed outcomes of the smartphone dispatch system (Heartrunner), which is triggered at emergency dispatch centers in response to suspected OHCAs at the same time that an ambulance with advanced life support equipment is dispatched.
The volunteer responder system locates a maximum of 30 volunteer responders within a 1.3-km radius from the suspected out-of-hospital cardiac arrest, the researchers explained in their report. Volunteer responders are requested via their smartphone application to accept or decline the alert. If they accept an alert, the volunteer responders receive map-aided route directions to the location of the suspected arrest.
In patients allocated to intervention in this study, four of five of all volunteer responders who accepted the alert received instructions to collect the nearest available AED and then go directly to the patient with suspected out-of-hospital cardiac arrest, the authors noted. Route directions to the scene of the cardiac arrest and the AED were displayed on their smartphones. One of the 5 volunteer responders, closest to the arrest, was dispatched to go directly to initiate CPR.
In patients allocated to the control group, all volunteer responders who accepted the alert were instructed to go directly to the patient with suspected out-of-hospital cardiac arrest to perform CPR. No route directions to or locations of AEDs were displayed.
The study was conducted in Stockholm and in Västra Götaland from 2018 to 2020. At the start of the study, there were 3,123 AEDs and 24,493 volunteer responders in Stockholm and 3,195 AEDs and 19,117 volunteer responders in Västra Götaland.
Post-randomization exclusions included patients without OHCA, those with OHCAs not treated by emergency medical services, and those with OHCAs witnessed by EMS.
The primary outcome was overall bystander AED attachment before the arrival of EMS, including those attached by the volunteer responders but also by lay volunteers who did not use the smartphone app.
Volunteer responders were activated for 947 individuals with OHCA; 461 patients were randomized to the intervention group and 486 to the control group. In both groups, the patients’ median age was 73 and about 65% were men.
Attachment of the AED before the arrival of EMS or first responders occurred in 61 patients (13.2%) in the intervention group versus 46 (9.5%) in the control group (P = .08). However, the majority of all AEDs were attached by lay volunteers who were not volunteer responders using the smartphone app (37 in the intervention arm vs. 28 in the control arm), the researchers noted.
No significant differences were seen in secondary outcomes, which included bystander CPR (69% vs. 71.6%, respectively) and defibrillation before EMS arrival (3.7% vs. 3.9%) between groups.
Among the volunteer responders using the app, crossover was 11% and compliance to instructions was 31%. Overall, volunteer responders attached 38% of all bystander-attached AEDs and provided 45% of all bystander defibrillations and 43% of all bystander CPR.
Going forward, Dr. Ringh and colleagues will be further analyzing the results to understand how to better optimize the logistical challenges involved with smartphone dispatch to OHCA patients. “In the longer term, investigating the impact on survival is also warranted,” he concluded.
U.S. in worse shape
In a comment, Christopher Calandrella, DO, chair of emergency medicine at Long Island Jewish Forest Hills,, New York, part of Northwell Health, said: “Significant data are available to support the importance of prompt initiation of CPR and defibrillation for OHCA, and although this study did not demonstrate a meaningful increase in use of AEDs with the trial system, layperson CPR was initiated in approximately 70% of cases in the cohort as a whole. Because of this, I believe it is evident that patients still benefit from a system that encourages bystanders to provide aid prior to the arrival of EMS.”
Nevertheless, he noted, “despite the training of volunteers in applying an AED, overall, only a small percentage of patients in either group had placement and use of the device. While the reasons likely are multifactorial, it may be in part due to the significant stress and anxiety associated with OHCA.”
Additional research would be helpful, he said. “Future studies focusing on more rural areas with lower population density and limited availability of AEDs may be beneficial. Expanding the research outside of Europe to other countries would be useful. Next-phase trials looking at 30-day survival in these patients would also be important.”
Currently in the United States, research is underway to evaluate the use of smartphones to improve in-hospital cardiac arrests, he added, “but no nationwide programs are in place for OHCA.”
Similarly, Kevin G. Volpp, MD, PhD, and Benjamin S. Abella, MD, MPhil, both of the University of Pennsylvania, Philadelphia, wrote in a related editorial: “It is sobering to recognize that, in the U.S., it may be nearly impossible to even test an idea like this, given the lack of a supporting data infrastructure.”
Although there is an app in the United States to link OHCA events to bystander response, they noted, less than half of eligible 911 centers have linked to it.
“Furthermore, the bystander CPR rate in the U.S. is less than 35%, only about half of the Swedish rate, indicating far fewer people are trained in CPR and comfortable performing it in the U.S.,” they wrote. “A wealthy country like the U.S. should be able to develop a far more effective approach to preventing millions of ... families from having a loved one die of OHCA in the decade to come.”
The study was funded by unrestricted grant from the Swedish Heart-Lung Foundation and Stockholm County. The authors, editorialists, and Dr. Calandrella disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Dubious diagnosis: Is there a better way to define ‘prediabetes’?
and subsequent complications, and therefore merit more intensive intervention.
“Prediabetes” is the term coined to refer to either “impaired fasting glucose (IFG)” or “impaired glucose tolerance (IGT),” both denoting levels of elevated glycemia that don’t meet the thresholds for diabetes. It’s a heterogeneous group overall, and despite its name, not everyone with prediabetes will progress to develop type 2 diabetes.
There have been major increases in prediabetes in the United States and globally over the past 2 decades, epidemiologist Elizabeth Selvin, PhD, said at the recent IDF World Diabetes Congress 2022.
She noted that the concept of “prediabetes” has been controversial, previously dubbed a “dubious diagnosis” and a “boon for Pharma” in a 2019 Science article.
Others have said it’s “not a medical condition” and that it’s “an artificial category with virtually zero clinical relevance” in a press statement issued for a 2014 BMJ article.
“I don’t agree with these statements entirely but I think they speak to the confusion and tremendous controversy around the concept of prediabetes ... I think instead of calling prediabetes a ‘dubious diagnosis’ we should think of it as an opportunity,” said Dr. Selvin, of Johns Hopkins University Bloomberg School of Public Health, Baltimore.
She proposes trying to home in on those with highest risk of developing type 2 diabetes, which she suggests could be achieved by using a combination of elevated fasting glucose and an elevated A1c, although she stresses that this isn’t in any official guidance.
With the appropriate definition, people who are truly at risk for progression to type 2 diabetes can be identified so that lifestyle factors and cardiovascular risk can be addressed, and weight loss efforts implemented.
“Prevention of weight gain is ... important. That message often gets lost. Even if we can’t get people to lose weight, preventing [further] weight gain is important,” she noted.
Asked to comment, Sue Kirkman, MD, told this news organization, “The term prediabetes – or IFG or IGT or any of the ‘intermediate’ terms – is pragmatic in a way. It helps clinicians and patients understand that they are in a higher-risk category and might need intervention and likely need ongoing monitoring. But like many other risk factors [such as] blood pressure, [high] BMI, etc., the risk is not dichotomous but a continuum.
“People at the low end of the ‘intermediate’ range are not going to have much more risk compared to people who are ‘normal,’ while those at the high end of the range have very high risk,” said Dr. Kirkman, of the University of North Carolina, Chapel Hill, and a coauthor of the American Diabetes Association’s diabetes and prediabetes classifications.
“So we lose information if we just lump everyone into a single category. For individual patients, we definitely need better ways to estimate and communicate their potential risk.”
Currently five definitions for prediabetes: Home in on risk
The problem, Dr. Selvin explained, is that currently there are five official definitions for “prediabetes” using cutoffs for hemoglobin A1c, fasting glucose, or an oral glucose tolerance test.
Each one identifies different numbers of people with differing risk levels, ranging from a prevalence of 4.3% of the middle-aged adult population with the International Expert Committee’s definition of A1c 6.0%-6.4% to 43.5% with the American Diabetes Association’s 100-125 mg/dL fasting glucose.
“That’s an enormous difference. No wonder people are confused about who has prediabetes and what we should do about it,” Dr. Selvin said, adding that the concern about overdiagnosing “prediabetes” is even greater for older populations, in whom “it’s incredibly common to have mildly elevated glucose.”
Hence her proposal of what she sees as an evidence-based, “really easy solution” that clinicians can use now to better identify which patients with “intermediate hyperglycemia” to be most concerned about: Use a combination of fasting glucose above 100 mg/dL and an A1c greater than 5.7%.
“If you have both fasting glucose and hemoglobin A1c, you can use them together ... This is not codified in any guidelines. You won’t see this mentioned anywhere. The guidelines are silent on what to do when some people have an elevated fasting glucose but not an elevated A1c ... but I think a simple message is that if people have both an elevated fasting glucose and an elevated A1c, that’s a very high-risk group,” she said.
On the other hand, Dr. Kirkman pointed out, “most discrepancies are near the margins, as in one test is slightly elevated and one isn’t, so those people probably are at low risk.
“It may be that both being elevated means higher risk because they have more hyperglycemia ... so it seems reasonable, but only if it changes what you tell people.”
For example, Dr. Kirkman said, “I’d tell someone with A1c of 5.8% and fasting glucose of 99 mg/dL the same thing I’d tell someone with that A1c and a glucose of 104 mg/dL – that their risk is still pretty low – and I’d recommend healthy lifestyle and weight loss if overweight either way.”
However, she also said, “Certainly people with higher glucose or A1c are at much higher risk, and same for those with both.”
Tie “prediabetes” definition to risk, as cardiology scores do?
Dr. Selvin also believes that risk-based definitions of prediabetes are needed. Ideally, these would incorporate demographics and clinical factors such as age and body mass index. Other biomarkers could potentially be developed and validated for inclusion in the definition, such as C-reactive protein (CRP), lipids, or even genetic/proteomic information.
Moreover, she thinks that the definition should be tied to clinical decision-making, as is the pooled cohort equation in cardiology.
“I think we could do something very similar in prediabetes,” she suggested, adding that even simply incorporating age and BMI into the definition could help further stratify the risk level until other predictors are validated.
Dr. Kirkman said, “The concept of risk scores a la cardiology is interesting, although we’d have to make them simple and also validate them against some outcome.”
Regarding the age issue, Dr. Kirkman noted that although age wasn’t a predictor of progression to type 2 diabetes in the placebo arm of the landmark Diabetes Prevention Program (DPP) trial, “I do agree that it’s a problem that many older folks have the label of prediabetes because of a mildly elevated A1c and we know that most will never get diabetes.”
And, she noted, in the DPP people with prediabetes who had a BMI over 35 kg/m2 did have significantly higher progression rates than those with lower BMI, while women with a history of gestational diabetes mellitus are also known to be at particularly high risk.
Whom should we throw the kitchen sink at?
Some of this discussion, Dr. Kirkman said, “is really a philosophical one, especially when you consider that lifestyle intervention has benefits for almost everyone on many short- and long-term outcomes.”
“The question is probably whom we should ‘throw the kitchen sink at,’ who should get more scalable advice that might apply to everyone regardless of glycemic levels, and whether there’s some more intermediate group that needs more of a [National Diabetes Prevention Program] approach.”
Dr. Selvin’s group is now working on gathering data to inform development of a risk-based prediabetes definition. “We have a whole research effort in this area. I hope that with some really strong data on risk in prediabetes, that can help to solve the heterogeneity issue. I’m focused on bringing evidence to bear to change the guidelines.”
In the meantime, she told this news organization, “I think there are things we can do now to provide more guidance. I get a lot of feedback from people saying things like ‘my physician told me I have prediabetes but now I don’t’ or ‘I saw in my labs that my blood sugar is elevated but my doctor never said anything.’ That’s a communications issue where we can do a better job.”
The meeting was sponsored by the International Diabetes Federation.
Dr. Selvin is deputy editor of Diabetes Care and on the editorial board of Diabetologia. She receives funding from the NIH and the Foundation for the NIH, and royalties from UpToDate for sections related to screening, diagnosis, and laboratory testing for diabetes. Dr. Kirkman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and subsequent complications, and therefore merit more intensive intervention.
“Prediabetes” is the term coined to refer to either “impaired fasting glucose (IFG)” or “impaired glucose tolerance (IGT),” both denoting levels of elevated glycemia that don’t meet the thresholds for diabetes. It’s a heterogeneous group overall, and despite its name, not everyone with prediabetes will progress to develop type 2 diabetes.
There have been major increases in prediabetes in the United States and globally over the past 2 decades, epidemiologist Elizabeth Selvin, PhD, said at the recent IDF World Diabetes Congress 2022.
She noted that the concept of “prediabetes” has been controversial, previously dubbed a “dubious diagnosis” and a “boon for Pharma” in a 2019 Science article.
Others have said it’s “not a medical condition” and that it’s “an artificial category with virtually zero clinical relevance” in a press statement issued for a 2014 BMJ article.
“I don’t agree with these statements entirely but I think they speak to the confusion and tremendous controversy around the concept of prediabetes ... I think instead of calling prediabetes a ‘dubious diagnosis’ we should think of it as an opportunity,” said Dr. Selvin, of Johns Hopkins University Bloomberg School of Public Health, Baltimore.
She proposes trying to home in on those with highest risk of developing type 2 diabetes, which she suggests could be achieved by using a combination of elevated fasting glucose and an elevated A1c, although she stresses that this isn’t in any official guidance.
With the appropriate definition, people who are truly at risk for progression to type 2 diabetes can be identified so that lifestyle factors and cardiovascular risk can be addressed, and weight loss efforts implemented.
“Prevention of weight gain is ... important. That message often gets lost. Even if we can’t get people to lose weight, preventing [further] weight gain is important,” she noted.
Asked to comment, Sue Kirkman, MD, told this news organization, “The term prediabetes – or IFG or IGT or any of the ‘intermediate’ terms – is pragmatic in a way. It helps clinicians and patients understand that they are in a higher-risk category and might need intervention and likely need ongoing monitoring. But like many other risk factors [such as] blood pressure, [high] BMI, etc., the risk is not dichotomous but a continuum.
“People at the low end of the ‘intermediate’ range are not going to have much more risk compared to people who are ‘normal,’ while those at the high end of the range have very high risk,” said Dr. Kirkman, of the University of North Carolina, Chapel Hill, and a coauthor of the American Diabetes Association’s diabetes and prediabetes classifications.
“So we lose information if we just lump everyone into a single category. For individual patients, we definitely need better ways to estimate and communicate their potential risk.”
Currently five definitions for prediabetes: Home in on risk
The problem, Dr. Selvin explained, is that currently there are five official definitions for “prediabetes” using cutoffs for hemoglobin A1c, fasting glucose, or an oral glucose tolerance test.
Each one identifies different numbers of people with differing risk levels, ranging from a prevalence of 4.3% of the middle-aged adult population with the International Expert Committee’s definition of A1c 6.0%-6.4% to 43.5% with the American Diabetes Association’s 100-125 mg/dL fasting glucose.
“That’s an enormous difference. No wonder people are confused about who has prediabetes and what we should do about it,” Dr. Selvin said, adding that the concern about overdiagnosing “prediabetes” is even greater for older populations, in whom “it’s incredibly common to have mildly elevated glucose.”
Hence her proposal of what she sees as an evidence-based, “really easy solution” that clinicians can use now to better identify which patients with “intermediate hyperglycemia” to be most concerned about: Use a combination of fasting glucose above 100 mg/dL and an A1c greater than 5.7%.
“If you have both fasting glucose and hemoglobin A1c, you can use them together ... This is not codified in any guidelines. You won’t see this mentioned anywhere. The guidelines are silent on what to do when some people have an elevated fasting glucose but not an elevated A1c ... but I think a simple message is that if people have both an elevated fasting glucose and an elevated A1c, that’s a very high-risk group,” she said.
On the other hand, Dr. Kirkman pointed out, “most discrepancies are near the margins, as in one test is slightly elevated and one isn’t, so those people probably are at low risk.
“It may be that both being elevated means higher risk because they have more hyperglycemia ... so it seems reasonable, but only if it changes what you tell people.”
For example, Dr. Kirkman said, “I’d tell someone with A1c of 5.8% and fasting glucose of 99 mg/dL the same thing I’d tell someone with that A1c and a glucose of 104 mg/dL – that their risk is still pretty low – and I’d recommend healthy lifestyle and weight loss if overweight either way.”
However, she also said, “Certainly people with higher glucose or A1c are at much higher risk, and same for those with both.”
Tie “prediabetes” definition to risk, as cardiology scores do?
Dr. Selvin also believes that risk-based definitions of prediabetes are needed. Ideally, these would incorporate demographics and clinical factors such as age and body mass index. Other biomarkers could potentially be developed and validated for inclusion in the definition, such as C-reactive protein (CRP), lipids, or even genetic/proteomic information.
Moreover, she thinks that the definition should be tied to clinical decision-making, as is the pooled cohort equation in cardiology.
“I think we could do something very similar in prediabetes,” she suggested, adding that even simply incorporating age and BMI into the definition could help further stratify the risk level until other predictors are validated.
Dr. Kirkman said, “The concept of risk scores a la cardiology is interesting, although we’d have to make them simple and also validate them against some outcome.”
Regarding the age issue, Dr. Kirkman noted that although age wasn’t a predictor of progression to type 2 diabetes in the placebo arm of the landmark Diabetes Prevention Program (DPP) trial, “I do agree that it’s a problem that many older folks have the label of prediabetes because of a mildly elevated A1c and we know that most will never get diabetes.”
And, she noted, in the DPP people with prediabetes who had a BMI over 35 kg/m2 did have significantly higher progression rates than those with lower BMI, while women with a history of gestational diabetes mellitus are also known to be at particularly high risk.
Whom should we throw the kitchen sink at?
Some of this discussion, Dr. Kirkman said, “is really a philosophical one, especially when you consider that lifestyle intervention has benefits for almost everyone on many short- and long-term outcomes.”
“The question is probably whom we should ‘throw the kitchen sink at,’ who should get more scalable advice that might apply to everyone regardless of glycemic levels, and whether there’s some more intermediate group that needs more of a [National Diabetes Prevention Program] approach.”
Dr. Selvin’s group is now working on gathering data to inform development of a risk-based prediabetes definition. “We have a whole research effort in this area. I hope that with some really strong data on risk in prediabetes, that can help to solve the heterogeneity issue. I’m focused on bringing evidence to bear to change the guidelines.”
In the meantime, she told this news organization, “I think there are things we can do now to provide more guidance. I get a lot of feedback from people saying things like ‘my physician told me I have prediabetes but now I don’t’ or ‘I saw in my labs that my blood sugar is elevated but my doctor never said anything.’ That’s a communications issue where we can do a better job.”
The meeting was sponsored by the International Diabetes Federation.
Dr. Selvin is deputy editor of Diabetes Care and on the editorial board of Diabetologia. She receives funding from the NIH and the Foundation for the NIH, and royalties from UpToDate for sections related to screening, diagnosis, and laboratory testing for diabetes. Dr. Kirkman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and subsequent complications, and therefore merit more intensive intervention.
“Prediabetes” is the term coined to refer to either “impaired fasting glucose (IFG)” or “impaired glucose tolerance (IGT),” both denoting levels of elevated glycemia that don’t meet the thresholds for diabetes. It’s a heterogeneous group overall, and despite its name, not everyone with prediabetes will progress to develop type 2 diabetes.
There have been major increases in prediabetes in the United States and globally over the past 2 decades, epidemiologist Elizabeth Selvin, PhD, said at the recent IDF World Diabetes Congress 2022.
She noted that the concept of “prediabetes” has been controversial, previously dubbed a “dubious diagnosis” and a “boon for Pharma” in a 2019 Science article.
Others have said it’s “not a medical condition” and that it’s “an artificial category with virtually zero clinical relevance” in a press statement issued for a 2014 BMJ article.
“I don’t agree with these statements entirely but I think they speak to the confusion and tremendous controversy around the concept of prediabetes ... I think instead of calling prediabetes a ‘dubious diagnosis’ we should think of it as an opportunity,” said Dr. Selvin, of Johns Hopkins University Bloomberg School of Public Health, Baltimore.
She proposes trying to home in on those with highest risk of developing type 2 diabetes, which she suggests could be achieved by using a combination of elevated fasting glucose and an elevated A1c, although she stresses that this isn’t in any official guidance.
With the appropriate definition, people who are truly at risk for progression to type 2 diabetes can be identified so that lifestyle factors and cardiovascular risk can be addressed, and weight loss efforts implemented.
“Prevention of weight gain is ... important. That message often gets lost. Even if we can’t get people to lose weight, preventing [further] weight gain is important,” she noted.
Asked to comment, Sue Kirkman, MD, told this news organization, “The term prediabetes – or IFG or IGT or any of the ‘intermediate’ terms – is pragmatic in a way. It helps clinicians and patients understand that they are in a higher-risk category and might need intervention and likely need ongoing monitoring. But like many other risk factors [such as] blood pressure, [high] BMI, etc., the risk is not dichotomous but a continuum.
“People at the low end of the ‘intermediate’ range are not going to have much more risk compared to people who are ‘normal,’ while those at the high end of the range have very high risk,” said Dr. Kirkman, of the University of North Carolina, Chapel Hill, and a coauthor of the American Diabetes Association’s diabetes and prediabetes classifications.
“So we lose information if we just lump everyone into a single category. For individual patients, we definitely need better ways to estimate and communicate their potential risk.”
Currently five definitions for prediabetes: Home in on risk
The problem, Dr. Selvin explained, is that currently there are five official definitions for “prediabetes” using cutoffs for hemoglobin A1c, fasting glucose, or an oral glucose tolerance test.
Each one identifies different numbers of people with differing risk levels, ranging from a prevalence of 4.3% of the middle-aged adult population with the International Expert Committee’s definition of A1c 6.0%-6.4% to 43.5% with the American Diabetes Association’s 100-125 mg/dL fasting glucose.
“That’s an enormous difference. No wonder people are confused about who has prediabetes and what we should do about it,” Dr. Selvin said, adding that the concern about overdiagnosing “prediabetes” is even greater for older populations, in whom “it’s incredibly common to have mildly elevated glucose.”
Hence her proposal of what she sees as an evidence-based, “really easy solution” that clinicians can use now to better identify which patients with “intermediate hyperglycemia” to be most concerned about: Use a combination of fasting glucose above 100 mg/dL and an A1c greater than 5.7%.
“If you have both fasting glucose and hemoglobin A1c, you can use them together ... This is not codified in any guidelines. You won’t see this mentioned anywhere. The guidelines are silent on what to do when some people have an elevated fasting glucose but not an elevated A1c ... but I think a simple message is that if people have both an elevated fasting glucose and an elevated A1c, that’s a very high-risk group,” she said.
On the other hand, Dr. Kirkman pointed out, “most discrepancies are near the margins, as in one test is slightly elevated and one isn’t, so those people probably are at low risk.
“It may be that both being elevated means higher risk because they have more hyperglycemia ... so it seems reasonable, but only if it changes what you tell people.”
For example, Dr. Kirkman said, “I’d tell someone with A1c of 5.8% and fasting glucose of 99 mg/dL the same thing I’d tell someone with that A1c and a glucose of 104 mg/dL – that their risk is still pretty low – and I’d recommend healthy lifestyle and weight loss if overweight either way.”
However, she also said, “Certainly people with higher glucose or A1c are at much higher risk, and same for those with both.”
Tie “prediabetes” definition to risk, as cardiology scores do?
Dr. Selvin also believes that risk-based definitions of prediabetes are needed. Ideally, these would incorporate demographics and clinical factors such as age and body mass index. Other biomarkers could potentially be developed and validated for inclusion in the definition, such as C-reactive protein (CRP), lipids, or even genetic/proteomic information.
Moreover, she thinks that the definition should be tied to clinical decision-making, as is the pooled cohort equation in cardiology.
“I think we could do something very similar in prediabetes,” she suggested, adding that even simply incorporating age and BMI into the definition could help further stratify the risk level until other predictors are validated.
Dr. Kirkman said, “The concept of risk scores a la cardiology is interesting, although we’d have to make them simple and also validate them against some outcome.”
Regarding the age issue, Dr. Kirkman noted that although age wasn’t a predictor of progression to type 2 diabetes in the placebo arm of the landmark Diabetes Prevention Program (DPP) trial, “I do agree that it’s a problem that many older folks have the label of prediabetes because of a mildly elevated A1c and we know that most will never get diabetes.”
And, she noted, in the DPP people with prediabetes who had a BMI over 35 kg/m2 did have significantly higher progression rates than those with lower BMI, while women with a history of gestational diabetes mellitus are also known to be at particularly high risk.
Whom should we throw the kitchen sink at?
Some of this discussion, Dr. Kirkman said, “is really a philosophical one, especially when you consider that lifestyle intervention has benefits for almost everyone on many short- and long-term outcomes.”
“The question is probably whom we should ‘throw the kitchen sink at,’ who should get more scalable advice that might apply to everyone regardless of glycemic levels, and whether there’s some more intermediate group that needs more of a [National Diabetes Prevention Program] approach.”
Dr. Selvin’s group is now working on gathering data to inform development of a risk-based prediabetes definition. “We have a whole research effort in this area. I hope that with some really strong data on risk in prediabetes, that can help to solve the heterogeneity issue. I’m focused on bringing evidence to bear to change the guidelines.”
In the meantime, she told this news organization, “I think there are things we can do now to provide more guidance. I get a lot of feedback from people saying things like ‘my physician told me I have prediabetes but now I don’t’ or ‘I saw in my labs that my blood sugar is elevated but my doctor never said anything.’ That’s a communications issue where we can do a better job.”
The meeting was sponsored by the International Diabetes Federation.
Dr. Selvin is deputy editor of Diabetes Care and on the editorial board of Diabetologia. She receives funding from the NIH and the Foundation for the NIH, and royalties from UpToDate for sections related to screening, diagnosis, and laboratory testing for diabetes. Dr. Kirkman reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT IDF WORLD DIABETES CONGRESS 2022
Scientists use mRNA technology for universal flu vaccine
Two years ago, when the first COVID-19 vaccines were administered, marked a game-changing moment in the fight against the pandemic. But it also was a significant moment for messenger RNA (mRNA) technology, which up until then had shown promise but had never quite broken through.
It’s the latest advance in a new age of vaccinology, where vaccines are easier and faster to produce, as well as more flexible and customizable.
“It’s all about covering the different flavors of flu in a way the current vaccines cannot do,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, who is not involved with the UPenn research. “The mRNA platform is attractive here given its scalability and modularity, where you can mix and match different mRNAs.”
A recent paper, published in Science, reports successful animal tests of the experimental vaccine, which, like the Pfizer-BioNTech and Moderna COVID vaccines, relies on mRNA. But the idea is not to replace the annual flu shot. It’s to develop a primer that could be administered in childhood, readying the body’s B cells and T cells to react quickly if faced with a flu virus.
It’s all part of a National Institutes of Health–funded effort to develop a universal flu vaccine, with hopes of heading off future flu pandemics. Annual shots protect against flu subtypes known to spread in humans. But many subtypes circulate in animals, like birds and pigs, and occasionally jump to humans, causing pandemics.
“The current vaccines provide very little protection against these other subtypes,” says lead study author Scott Hensley, PhD, a professor of microbiology at UPenn. “We set out to make a vaccine that would provide some level of immunity against essentially every influenza subtype we know about.”
That’s 20 subtypes altogether. The unique properties of mRNA vaccines make immune responses against all those antigens possible, Dr. Hensley says.
Old-school vaccines introduce a weakened or dead bacteria or virus into the body, but mRNA vaccines use mRNA encoded with a protein from the virus. That’s the “spike” protein for COVID, and for the experimental vaccine, it’s hemagglutinin, the major protein found on the surface of all flu viruses.
Mice and ferrets that had never been exposed to the flu were given the vaccine and produced high levels of antibodies against all 20 flu subtypes. Vaccinated mice exposed to the exact strains in the vaccine stayed pretty healthy, while those exposed to strains not found in the vaccine got sick but recovered quickly and survived. Unvaccinated mice exposed to the flu strain died.
The vaccine seems to be able to “induce broad immunity against all the different influenza subtypes,” Dr. Hensley says, preventing severe illness if not infection overall.
Still, whether it could truly stave off a pandemic that hasn’t happened yet is hard to say, Dr. Levy cautions.
“We are going to need to better learn the molecular rules by which these vaccines protect,” he says.
But the UPenn team is forging ahead, with plans to test their vaccine in human adults in 2023 to determine safety, dosing, and antibody response.
A version of this article first appeared on WebMD.com.
Two years ago, when the first COVID-19 vaccines were administered, marked a game-changing moment in the fight against the pandemic. But it also was a significant moment for messenger RNA (mRNA) technology, which up until then had shown promise but had never quite broken through.
It’s the latest advance in a new age of vaccinology, where vaccines are easier and faster to produce, as well as more flexible and customizable.
“It’s all about covering the different flavors of flu in a way the current vaccines cannot do,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, who is not involved with the UPenn research. “The mRNA platform is attractive here given its scalability and modularity, where you can mix and match different mRNAs.”
A recent paper, published in Science, reports successful animal tests of the experimental vaccine, which, like the Pfizer-BioNTech and Moderna COVID vaccines, relies on mRNA. But the idea is not to replace the annual flu shot. It’s to develop a primer that could be administered in childhood, readying the body’s B cells and T cells to react quickly if faced with a flu virus.
It’s all part of a National Institutes of Health–funded effort to develop a universal flu vaccine, with hopes of heading off future flu pandemics. Annual shots protect against flu subtypes known to spread in humans. But many subtypes circulate in animals, like birds and pigs, and occasionally jump to humans, causing pandemics.
“The current vaccines provide very little protection against these other subtypes,” says lead study author Scott Hensley, PhD, a professor of microbiology at UPenn. “We set out to make a vaccine that would provide some level of immunity against essentially every influenza subtype we know about.”
That’s 20 subtypes altogether. The unique properties of mRNA vaccines make immune responses against all those antigens possible, Dr. Hensley says.
Old-school vaccines introduce a weakened or dead bacteria or virus into the body, but mRNA vaccines use mRNA encoded with a protein from the virus. That’s the “spike” protein for COVID, and for the experimental vaccine, it’s hemagglutinin, the major protein found on the surface of all flu viruses.
Mice and ferrets that had never been exposed to the flu were given the vaccine and produced high levels of antibodies against all 20 flu subtypes. Vaccinated mice exposed to the exact strains in the vaccine stayed pretty healthy, while those exposed to strains not found in the vaccine got sick but recovered quickly and survived. Unvaccinated mice exposed to the flu strain died.
The vaccine seems to be able to “induce broad immunity against all the different influenza subtypes,” Dr. Hensley says, preventing severe illness if not infection overall.
Still, whether it could truly stave off a pandemic that hasn’t happened yet is hard to say, Dr. Levy cautions.
“We are going to need to better learn the molecular rules by which these vaccines protect,” he says.
But the UPenn team is forging ahead, with plans to test their vaccine in human adults in 2023 to determine safety, dosing, and antibody response.
A version of this article first appeared on WebMD.com.
Two years ago, when the first COVID-19 vaccines were administered, marked a game-changing moment in the fight against the pandemic. But it also was a significant moment for messenger RNA (mRNA) technology, which up until then had shown promise but had never quite broken through.
It’s the latest advance in a new age of vaccinology, where vaccines are easier and faster to produce, as well as more flexible and customizable.
“It’s all about covering the different flavors of flu in a way the current vaccines cannot do,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital, who is not involved with the UPenn research. “The mRNA platform is attractive here given its scalability and modularity, where you can mix and match different mRNAs.”
A recent paper, published in Science, reports successful animal tests of the experimental vaccine, which, like the Pfizer-BioNTech and Moderna COVID vaccines, relies on mRNA. But the idea is not to replace the annual flu shot. It’s to develop a primer that could be administered in childhood, readying the body’s B cells and T cells to react quickly if faced with a flu virus.
It’s all part of a National Institutes of Health–funded effort to develop a universal flu vaccine, with hopes of heading off future flu pandemics. Annual shots protect against flu subtypes known to spread in humans. But many subtypes circulate in animals, like birds and pigs, and occasionally jump to humans, causing pandemics.
“The current vaccines provide very little protection against these other subtypes,” says lead study author Scott Hensley, PhD, a professor of microbiology at UPenn. “We set out to make a vaccine that would provide some level of immunity against essentially every influenza subtype we know about.”
That’s 20 subtypes altogether. The unique properties of mRNA vaccines make immune responses against all those antigens possible, Dr. Hensley says.
Old-school vaccines introduce a weakened or dead bacteria or virus into the body, but mRNA vaccines use mRNA encoded with a protein from the virus. That’s the “spike” protein for COVID, and for the experimental vaccine, it’s hemagglutinin, the major protein found on the surface of all flu viruses.
Mice and ferrets that had never been exposed to the flu were given the vaccine and produced high levels of antibodies against all 20 flu subtypes. Vaccinated mice exposed to the exact strains in the vaccine stayed pretty healthy, while those exposed to strains not found in the vaccine got sick but recovered quickly and survived. Unvaccinated mice exposed to the flu strain died.
The vaccine seems to be able to “induce broad immunity against all the different influenza subtypes,” Dr. Hensley says, preventing severe illness if not infection overall.
Still, whether it could truly stave off a pandemic that hasn’t happened yet is hard to say, Dr. Levy cautions.
“We are going to need to better learn the molecular rules by which these vaccines protect,” he says.
But the UPenn team is forging ahead, with plans to test their vaccine in human adults in 2023 to determine safety, dosing, and antibody response.
A version of this article first appeared on WebMD.com.
FROM SCIENCE
COVID booster shot poll: People ‘don’t think they need one’
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
Now, a new poll shows why so few people are willing to roll up their sleeves again.
The most common reasons people give for not getting the latest booster shot is that they “don’t think they need one” (44%) and they “don’t think the benefits are worth it” (37%), according to poll results from the Kaiser Family Foundation.
The data comes amid announcements by the Centers for Disease Control and Prevention that boosters reduced COVID-19 hospitalizations by up to 57% for U.S. adults and by up to 84% for people age 65 and older. Those figures are just the latest in a mountain of research reporting the public health benefits of COVID-19 vaccines.
Despite all of the statistical data, health officials’ recent vaccination campaigns have proven far from compelling.
So far, just 15% of people age 12 and older have gotten the latest booster, and 36% of people age 65 and older have gotten it, the CDC’s vaccination trackershows.
Since the start of the pandemic, 1.1 million people in the U.S. have died from COVID-19, with the number of deaths currently rising by 400 per day, The New York Times COVID tracker shows.
Many experts continue to note the need for everyone to get booster shots regularly, but some advocate that perhaps a change in strategy is in order.
“What the administration should do is push for vaccinating people in high-risk groups, including those who are older, those who are immunocompromised and those who have comorbidities,” Paul Offitt, MD, director of the Vaccine Education Center at Children’s Hospital of Philadelphia, told CNN.
Federal regulators have announced they will meet Jan. 26 with a panel of vaccine advisors to examine the current recommended vaccination schedule as well as look at the effectiveness and composition of current vaccines and boosters, with an eye toward the make-up of next-generation shots.
Vaccines are the “best available protection” against hospitalization and death caused by COVID-19, said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement announcing the planned meeting.
“Since the initial authorizations of these vaccines, we have learned that protection wanes over time, especially as the virus rapidly mutates and new variants and subvariants emerge,” he said. “Therefore, it’s important to continue discussions about the optimal composition of COVID-19 vaccines for primary and booster vaccination, as well as the optimal interval for booster vaccination.”
A version of this article first appeared on WebMD.com.
‘The Whale’: Is this new movie fat-phobic or fat-friendly?
“I could relate to many, many, many of the experiences and emotions that Charlie, which is Brendan Fraser’s character, was portraying,” Patricia Nece recalls after watching a preview copy of the new film “The Whale.”
Much of the movie “rang true and hit home for me as things that I, too, had experienced,” Ms. Nece, the board of directors’ chair of the Obesity Action Coalition (OAC) and a person living with obesity, shares with this news organization.
In theaters as of December 9, The Whale chronicles the experience of a 600-lb, middle-aged man named Charlie. Throughout the film, Charlie seeks to rebuild his relationship with his estranged teenage daughter. Charlie had left his daughter and family to pursue a relationship with a man, who eventually died. As he navigates the pain surrounding his partner’s death and his lack of community, Charlie turns to food for comfort.
When the movie premiered at the Venice Film Festival, Mr. Fraser received a 6-minute standing ovation. However, activists criticized the movie for casting Fraser over an actor with obesity as well as its depiction of people with obesity.
Representatives from the National Association to Advance Fat Acceptance contend that casting an actor without obesity only contributes to ongoing bias against people of size. “Medical weight stigma and other socio-political determinants of health for people of all sizes cause far more harm to fat people than body fat does. Bias endangers fat people’s health. Anti-obesity organizations, such as those consulted with for this movie, contribute to stigma rather than reducing it as they claim,” NAAFA wrote in a statement to this news organization.
And they added that though the fat suit used in the movie may be superior to previous ones, it is still not an accurate depiction: “The creators of The Whale consider its CGI-generated fat suit to be superior to tactile fat suits, but we don’t. The issue with fat suits in Hollywood is not that they aren’t realistic enough. The issue is that they are used rather than using performers who actually live in bodies like the ones being depicted. If there is a 600-pound character in a movie, there should be a 600-pound human in that role. Rather than concentrate on the hype around the fake fat body created for The Whale, we want to see Hollywood create more opportunities for fat people across the size spectrum, both in front of the camera and behind the scenes.”
Prosthetics vs. reality?
Ms. Nece says she understands the controversy surrounding the use of fat suits but believes that it was not done in poor taste.
“OAC got involved with the movie after Brendan was already chosen for the part, and we never would have gotten involved with it had the prosthetics or fat suit been used to ridicule or make fun of people with obesity, which is usually the case,” she explains.
“But we knew from the start that that was never the intent of anyone involved with The Whale. And I think that’s shown by the fact that Brendan and Darren Aronofsky, the director, reached out to people who live with obesity on a daily basis to find out and learn more about it and to educate themselves about it,” Ms. Nece continues.
In a Daily Mail article, Mr. Fraser credited his son Griffin, who is autistic and obese, with helping him understand the struggles that people with obesity face.
Rachel Goldman, PhD, a clinical psychologist in private practice in New York and a professor in the psychology department at New York University, notes that there are other considerations that played into casting. “I know there was some pushback in terms of could, a say 600-lb individual, even be able to go to be on set every day and do this kind of work, and the answer is we don’t know.”
“I’m sure Darren chose Brendan for many reasons above and beyond just his body. I think that’s very important to keep in mind that just as much as representation is very important, I think it is also about finding the right person for the right role,” adds Dr. Goldman, who served as a consultant to the film.
Fat suits, extreme weight gains all to play a role
About 42% of adults in the United States have obesity, according to the 2017-2020 National Health and Nutrition Examination Survey, but that reality is not reflected in films or television.
A study of 1018 major television characters found that 24% of men and 14% of women had either overweight or obesity – far below the national average. And when characters with obesity are portrayed, actors often wear prosthetics, like Gwyneth Paltrow in Shallow Hal or Eddie Murphy in the Nutty Professor.
But unlike Mr. Fraser, some actors gain weight quickly instead.
This practice is unhealthy, says Jaime Almandoz, MD, an associate professor at the University of Texas Southwestern Medical Center, Dallas, and a nonsurgical weight management expert. In interviews, actors have shared how they increased calorie intake by drinking two milkshakes per day, going to fast food places regularly, or, in Mark Walhberg’s case, consuming 7,000 calories per day to gain 30 pounds for his role as boxer-turned-priest in the movie Father Stu.
This method provides their bodies with excess calories they are unable to burn off. “Then the amount of sugar and fat that streams into the blood as a result creates problems both directly and indirectly as your body tries to store it. It basically ends up using overflow warehouses for fat storage, like the liver for example, so we can create a condition called fatty liver, or in the muscle and other places, and this excess sugar and fat in the bloodstream cause several factors that are both insulin resistance causing,” Dr. Almandoz explains.
Though gaining weight helps the actor understand the character’s life experience, it may also be risky.
“To have an actor deliberately put his own health at risk and gain a certain amount of weight and whatever that might entail, one – that’s not necessarily the safest thing for that actor – but two, it’s also important to highlight the authentic experience of someone who has dealt with this chronic disease as well,” says Disha Narang, MD, a quadruple-board certified endocrinologist, obesity medicine, and culinary medicine specialist at Northwestern Medicine Lake Forest Hospital, Chicago.
These extreme fluctuations in weight may create problems. “It is typically not something we recommend because there could be metabolic damages as well as health concerns when patients are trying to gain weight quickly, just as we don’t want patients to lose weight quickly,” says Kurt Hong, MD, PhD, board-certified in internal medicine and clinical nutrition at the University of Southern California, Los Angeles.
Dr. Hong notes that it may be difficult for individuals to experience sudden weight gain because the body works hard to maintain a state of homeostasis.
“Similarly, to someone trying to gain weight you overeat, initially your body will try to again, maybe enhance its metabolic efficiency to hold the body stable,” Dr. Hong adds.
Dietary choices that may contribute to insulin resistance or promote high blood sugar can contribute to inflammation and a number of other adverse health outcomes, notes Dr. Almandoz. “The things that actors need to do in order to gain this magnitude of weight and they want to do it in the most time-effective manner is often not helpful for our bodies, it can be very problematic, the same thing goes for weight loss when actors need to lose significant amounts of weight for roles,” says Dr. Almandoz.
And Dr. Hong explained that for patients trying to lose weight, they may cut calories, but the body will try to compensate by slowing down the metabolism to keep their weight the same.
‘Your own worst bully’
In “The Whale,” Charlie appears to suffer from internalized weight bias, which is common to many people living with obesity, Ms. Nece says.
“Internalized weight bias is when the person of size takes all that negativity and turns it on themselves. The easiest way to describe that is to tell you that I became my own worst bully because I started believing all the negative things people said to me about my weight,” Ms. Nece adds.
Her hope is that the film will bring attention to the harm that this bias creates, especially when it derives from other people. “There’s no telling whether it will, but what Charlie experiences in bias and stigma from others clearly happens. It’s realistic. Those of us in large bodies have experienced what he is experiencing, so some people have said the movie is fat-phobic, but I see it as I can relate to those experiences because I have them too, so they are very realistic.”
Ms. Nece notes that it is important for clinicians to understand that obesity is a multifaceted and sensitive topic. “For those medical professionals who do not already know that obesity is complex, I hope the film will begin to open their eyes to the many different facets involved in obesity and their patients with obesity, I hope it will help them empathize and show compassion to their patients with obesity,” she concludes.
A version of this article first appeared on Medscape.com.
“I could relate to many, many, many of the experiences and emotions that Charlie, which is Brendan Fraser’s character, was portraying,” Patricia Nece recalls after watching a preview copy of the new film “The Whale.”
Much of the movie “rang true and hit home for me as things that I, too, had experienced,” Ms. Nece, the board of directors’ chair of the Obesity Action Coalition (OAC) and a person living with obesity, shares with this news organization.
In theaters as of December 9, The Whale chronicles the experience of a 600-lb, middle-aged man named Charlie. Throughout the film, Charlie seeks to rebuild his relationship with his estranged teenage daughter. Charlie had left his daughter and family to pursue a relationship with a man, who eventually died. As he navigates the pain surrounding his partner’s death and his lack of community, Charlie turns to food for comfort.
When the movie premiered at the Venice Film Festival, Mr. Fraser received a 6-minute standing ovation. However, activists criticized the movie for casting Fraser over an actor with obesity as well as its depiction of people with obesity.
Representatives from the National Association to Advance Fat Acceptance contend that casting an actor without obesity only contributes to ongoing bias against people of size. “Medical weight stigma and other socio-political determinants of health for people of all sizes cause far more harm to fat people than body fat does. Bias endangers fat people’s health. Anti-obesity organizations, such as those consulted with for this movie, contribute to stigma rather than reducing it as they claim,” NAAFA wrote in a statement to this news organization.
And they added that though the fat suit used in the movie may be superior to previous ones, it is still not an accurate depiction: “The creators of The Whale consider its CGI-generated fat suit to be superior to tactile fat suits, but we don’t. The issue with fat suits in Hollywood is not that they aren’t realistic enough. The issue is that they are used rather than using performers who actually live in bodies like the ones being depicted. If there is a 600-pound character in a movie, there should be a 600-pound human in that role. Rather than concentrate on the hype around the fake fat body created for The Whale, we want to see Hollywood create more opportunities for fat people across the size spectrum, both in front of the camera and behind the scenes.”
Prosthetics vs. reality?
Ms. Nece says she understands the controversy surrounding the use of fat suits but believes that it was not done in poor taste.
“OAC got involved with the movie after Brendan was already chosen for the part, and we never would have gotten involved with it had the prosthetics or fat suit been used to ridicule or make fun of people with obesity, which is usually the case,” she explains.
“But we knew from the start that that was never the intent of anyone involved with The Whale. And I think that’s shown by the fact that Brendan and Darren Aronofsky, the director, reached out to people who live with obesity on a daily basis to find out and learn more about it and to educate themselves about it,” Ms. Nece continues.
In a Daily Mail article, Mr. Fraser credited his son Griffin, who is autistic and obese, with helping him understand the struggles that people with obesity face.
Rachel Goldman, PhD, a clinical psychologist in private practice in New York and a professor in the psychology department at New York University, notes that there are other considerations that played into casting. “I know there was some pushback in terms of could, a say 600-lb individual, even be able to go to be on set every day and do this kind of work, and the answer is we don’t know.”
“I’m sure Darren chose Brendan for many reasons above and beyond just his body. I think that’s very important to keep in mind that just as much as representation is very important, I think it is also about finding the right person for the right role,” adds Dr. Goldman, who served as a consultant to the film.
Fat suits, extreme weight gains all to play a role
About 42% of adults in the United States have obesity, according to the 2017-2020 National Health and Nutrition Examination Survey, but that reality is not reflected in films or television.
A study of 1018 major television characters found that 24% of men and 14% of women had either overweight or obesity – far below the national average. And when characters with obesity are portrayed, actors often wear prosthetics, like Gwyneth Paltrow in Shallow Hal or Eddie Murphy in the Nutty Professor.
But unlike Mr. Fraser, some actors gain weight quickly instead.
This practice is unhealthy, says Jaime Almandoz, MD, an associate professor at the University of Texas Southwestern Medical Center, Dallas, and a nonsurgical weight management expert. In interviews, actors have shared how they increased calorie intake by drinking two milkshakes per day, going to fast food places regularly, or, in Mark Walhberg’s case, consuming 7,000 calories per day to gain 30 pounds for his role as boxer-turned-priest in the movie Father Stu.
This method provides their bodies with excess calories they are unable to burn off. “Then the amount of sugar and fat that streams into the blood as a result creates problems both directly and indirectly as your body tries to store it. It basically ends up using overflow warehouses for fat storage, like the liver for example, so we can create a condition called fatty liver, or in the muscle and other places, and this excess sugar and fat in the bloodstream cause several factors that are both insulin resistance causing,” Dr. Almandoz explains.
Though gaining weight helps the actor understand the character’s life experience, it may also be risky.
“To have an actor deliberately put his own health at risk and gain a certain amount of weight and whatever that might entail, one – that’s not necessarily the safest thing for that actor – but two, it’s also important to highlight the authentic experience of someone who has dealt with this chronic disease as well,” says Disha Narang, MD, a quadruple-board certified endocrinologist, obesity medicine, and culinary medicine specialist at Northwestern Medicine Lake Forest Hospital, Chicago.
These extreme fluctuations in weight may create problems. “It is typically not something we recommend because there could be metabolic damages as well as health concerns when patients are trying to gain weight quickly, just as we don’t want patients to lose weight quickly,” says Kurt Hong, MD, PhD, board-certified in internal medicine and clinical nutrition at the University of Southern California, Los Angeles.
Dr. Hong notes that it may be difficult for individuals to experience sudden weight gain because the body works hard to maintain a state of homeostasis.
“Similarly, to someone trying to gain weight you overeat, initially your body will try to again, maybe enhance its metabolic efficiency to hold the body stable,” Dr. Hong adds.
Dietary choices that may contribute to insulin resistance or promote high blood sugar can contribute to inflammation and a number of other adverse health outcomes, notes Dr. Almandoz. “The things that actors need to do in order to gain this magnitude of weight and they want to do it in the most time-effective manner is often not helpful for our bodies, it can be very problematic, the same thing goes for weight loss when actors need to lose significant amounts of weight for roles,” says Dr. Almandoz.
And Dr. Hong explained that for patients trying to lose weight, they may cut calories, but the body will try to compensate by slowing down the metabolism to keep their weight the same.
‘Your own worst bully’
In “The Whale,” Charlie appears to suffer from internalized weight bias, which is common to many people living with obesity, Ms. Nece says.
“Internalized weight bias is when the person of size takes all that negativity and turns it on themselves. The easiest way to describe that is to tell you that I became my own worst bully because I started believing all the negative things people said to me about my weight,” Ms. Nece adds.
Her hope is that the film will bring attention to the harm that this bias creates, especially when it derives from other people. “There’s no telling whether it will, but what Charlie experiences in bias and stigma from others clearly happens. It’s realistic. Those of us in large bodies have experienced what he is experiencing, so some people have said the movie is fat-phobic, but I see it as I can relate to those experiences because I have them too, so they are very realistic.”
Ms. Nece notes that it is important for clinicians to understand that obesity is a multifaceted and sensitive topic. “For those medical professionals who do not already know that obesity is complex, I hope the film will begin to open their eyes to the many different facets involved in obesity and their patients with obesity, I hope it will help them empathize and show compassion to their patients with obesity,” she concludes.
A version of this article first appeared on Medscape.com.
“I could relate to many, many, many of the experiences and emotions that Charlie, which is Brendan Fraser’s character, was portraying,” Patricia Nece recalls after watching a preview copy of the new film “The Whale.”
Much of the movie “rang true and hit home for me as things that I, too, had experienced,” Ms. Nece, the board of directors’ chair of the Obesity Action Coalition (OAC) and a person living with obesity, shares with this news organization.
In theaters as of December 9, The Whale chronicles the experience of a 600-lb, middle-aged man named Charlie. Throughout the film, Charlie seeks to rebuild his relationship with his estranged teenage daughter. Charlie had left his daughter and family to pursue a relationship with a man, who eventually died. As he navigates the pain surrounding his partner’s death and his lack of community, Charlie turns to food for comfort.
When the movie premiered at the Venice Film Festival, Mr. Fraser received a 6-minute standing ovation. However, activists criticized the movie for casting Fraser over an actor with obesity as well as its depiction of people with obesity.
Representatives from the National Association to Advance Fat Acceptance contend that casting an actor without obesity only contributes to ongoing bias against people of size. “Medical weight stigma and other socio-political determinants of health for people of all sizes cause far more harm to fat people than body fat does. Bias endangers fat people’s health. Anti-obesity organizations, such as those consulted with for this movie, contribute to stigma rather than reducing it as they claim,” NAAFA wrote in a statement to this news organization.
And they added that though the fat suit used in the movie may be superior to previous ones, it is still not an accurate depiction: “The creators of The Whale consider its CGI-generated fat suit to be superior to tactile fat suits, but we don’t. The issue with fat suits in Hollywood is not that they aren’t realistic enough. The issue is that they are used rather than using performers who actually live in bodies like the ones being depicted. If there is a 600-pound character in a movie, there should be a 600-pound human in that role. Rather than concentrate on the hype around the fake fat body created for The Whale, we want to see Hollywood create more opportunities for fat people across the size spectrum, both in front of the camera and behind the scenes.”
Prosthetics vs. reality?
Ms. Nece says she understands the controversy surrounding the use of fat suits but believes that it was not done in poor taste.
“OAC got involved with the movie after Brendan was already chosen for the part, and we never would have gotten involved with it had the prosthetics or fat suit been used to ridicule or make fun of people with obesity, which is usually the case,” she explains.
“But we knew from the start that that was never the intent of anyone involved with The Whale. And I think that’s shown by the fact that Brendan and Darren Aronofsky, the director, reached out to people who live with obesity on a daily basis to find out and learn more about it and to educate themselves about it,” Ms. Nece continues.
In a Daily Mail article, Mr. Fraser credited his son Griffin, who is autistic and obese, with helping him understand the struggles that people with obesity face.
Rachel Goldman, PhD, a clinical psychologist in private practice in New York and a professor in the psychology department at New York University, notes that there are other considerations that played into casting. “I know there was some pushback in terms of could, a say 600-lb individual, even be able to go to be on set every day and do this kind of work, and the answer is we don’t know.”
“I’m sure Darren chose Brendan for many reasons above and beyond just his body. I think that’s very important to keep in mind that just as much as representation is very important, I think it is also about finding the right person for the right role,” adds Dr. Goldman, who served as a consultant to the film.
Fat suits, extreme weight gains all to play a role
About 42% of adults in the United States have obesity, according to the 2017-2020 National Health and Nutrition Examination Survey, but that reality is not reflected in films or television.
A study of 1018 major television characters found that 24% of men and 14% of women had either overweight or obesity – far below the national average. And when characters with obesity are portrayed, actors often wear prosthetics, like Gwyneth Paltrow in Shallow Hal or Eddie Murphy in the Nutty Professor.
But unlike Mr. Fraser, some actors gain weight quickly instead.
This practice is unhealthy, says Jaime Almandoz, MD, an associate professor at the University of Texas Southwestern Medical Center, Dallas, and a nonsurgical weight management expert. In interviews, actors have shared how they increased calorie intake by drinking two milkshakes per day, going to fast food places regularly, or, in Mark Walhberg’s case, consuming 7,000 calories per day to gain 30 pounds for his role as boxer-turned-priest in the movie Father Stu.
This method provides their bodies with excess calories they are unable to burn off. “Then the amount of sugar and fat that streams into the blood as a result creates problems both directly and indirectly as your body tries to store it. It basically ends up using overflow warehouses for fat storage, like the liver for example, so we can create a condition called fatty liver, or in the muscle and other places, and this excess sugar and fat in the bloodstream cause several factors that are both insulin resistance causing,” Dr. Almandoz explains.
Though gaining weight helps the actor understand the character’s life experience, it may also be risky.
“To have an actor deliberately put his own health at risk and gain a certain amount of weight and whatever that might entail, one – that’s not necessarily the safest thing for that actor – but two, it’s also important to highlight the authentic experience of someone who has dealt with this chronic disease as well,” says Disha Narang, MD, a quadruple-board certified endocrinologist, obesity medicine, and culinary medicine specialist at Northwestern Medicine Lake Forest Hospital, Chicago.
These extreme fluctuations in weight may create problems. “It is typically not something we recommend because there could be metabolic damages as well as health concerns when patients are trying to gain weight quickly, just as we don’t want patients to lose weight quickly,” says Kurt Hong, MD, PhD, board-certified in internal medicine and clinical nutrition at the University of Southern California, Los Angeles.
Dr. Hong notes that it may be difficult for individuals to experience sudden weight gain because the body works hard to maintain a state of homeostasis.
“Similarly, to someone trying to gain weight you overeat, initially your body will try to again, maybe enhance its metabolic efficiency to hold the body stable,” Dr. Hong adds.
Dietary choices that may contribute to insulin resistance or promote high blood sugar can contribute to inflammation and a number of other adverse health outcomes, notes Dr. Almandoz. “The things that actors need to do in order to gain this magnitude of weight and they want to do it in the most time-effective manner is often not helpful for our bodies, it can be very problematic, the same thing goes for weight loss when actors need to lose significant amounts of weight for roles,” says Dr. Almandoz.
And Dr. Hong explained that for patients trying to lose weight, they may cut calories, but the body will try to compensate by slowing down the metabolism to keep their weight the same.
‘Your own worst bully’
In “The Whale,” Charlie appears to suffer from internalized weight bias, which is common to many people living with obesity, Ms. Nece says.
“Internalized weight bias is when the person of size takes all that negativity and turns it on themselves. The easiest way to describe that is to tell you that I became my own worst bully because I started believing all the negative things people said to me about my weight,” Ms. Nece adds.
Her hope is that the film will bring attention to the harm that this bias creates, especially when it derives from other people. “There’s no telling whether it will, but what Charlie experiences in bias and stigma from others clearly happens. It’s realistic. Those of us in large bodies have experienced what he is experiencing, so some people have said the movie is fat-phobic, but I see it as I can relate to those experiences because I have them too, so they are very realistic.”
Ms. Nece notes that it is important for clinicians to understand that obesity is a multifaceted and sensitive topic. “For those medical professionals who do not already know that obesity is complex, I hope the film will begin to open their eyes to the many different facets involved in obesity and their patients with obesity, I hope it will help them empathize and show compassion to their patients with obesity,” she concludes.
A version of this article first appeared on Medscape.com.
New AHA statement on managing ACS in older adults
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.
Age-related changes in general and cardiovascular health likely require modifications in how acute coronary syndrome (ACS) is diagnosed and managed in adults aged 75 and older, the American Heart Association says in a new scientific statement.
The statement outlines a framework to integrate geriatric risks into the management of ACS, including the diagnostic approach, pharmacotherapy, revascularization strategies, prevention of adverse events, and transition care planning.
The 31-page statement was published online in the AHA journal Circulation (2022 Dec 12. doi: 10.1161/CIR.0000000000001112). It updates a 2007 AHA statement on treatment of ACS in the elderly.
Complex patient group
Adults aged 75 and older make up roughly 30%-40% of all hospitalized patients with ACS and the majority of ACS-related deaths occur in this group, the writing group notes.
“Older patients have more pronounced anatomical changes and more severe functional impairment, and they are more likely to have additional health conditions,” writing group chair Abdulla A. Damluji, MD, PhD, director of the Inova Center of Outcomes Research in Fairfax, Va., notes in a news release.
“These include frailty, other chronic disorders (treated with multiple medications), physical dysfunction, cognitive decline and/or urinary incontinence – and these are not regularly studied in the context of ACS,” Dr. Damluji explained.
The writing group notes that the presence of one or more geriatric syndromes may substantially affect ACS clinical presentation, clinical course and prognosis, therapeutic decision-making, and response to treatment.
“It is therefore fundamental that clinicians caring for older patients with ACS be alert to the presence of geriatric syndromes and be able to integrate them into the care plan when appropriate,” they say.
They recommend a holistic, individualized, and patient-centered approach to ACS care in the elderly, taking into consideration coexisting and overlapping health issues.
Considerations for clinical care
The AHA statement offers several “considerations for clinical practice” with regard to ACS diagnosis and management in elderly adults. They include:
- ACS presentations without chest pain, such as shortness of breath, syncope, or sudden confusion, are more common in older adults.
- Many older adults have persistent elevations in cardiac troponin levels from myocardial fibrosis and kidney disease that diminish the positive predictive value of high-sensitivity cardiac troponin (hs-cTn) assays for identifying acute and chronic myocardial injury. For this reason, evaluating patterns of rise and fall is essential.
- Age-related changes in metabolism, weight, and muscle mass may require different choices in anticoagulant medications to lower bleeding risk.
- Clopidogrel (Plavix) is the preferred P2Y12 inhibitor because of a significantly lower bleeding profile than ticagrelor (Brilinta) or prasugrel (Effient). For patients with ST-segment myocardial infarction (STEMI) or complex anatomy, the use of ticagrelor is “reasonable.”
- Poor kidney function can increase the risk for contrast-induced acute kidney injury.
- Although the risks are greater, percutaneous coronary intervention or bypass surgery are beneficial in select older adults with ACS.
- Post-MI care should include cardiac rehabilitation tailored to address each patient’s circumstances and personal goals of care.
- For patients with cognitive difficulties and limited mobility, consider simplified medication plans with fewer doses per day and 90-day supplies to prevent the need for frequent refills.
- Patient care plans should be individualized, with input from a multidisciplinary team that may include cardiologists, surgeons, geriatricians, primary care clinicians, nutritionists, social workers, and family members.
- Determine a priori goals of care in older patients to help avoid an unwanted or futile intervention.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Cardiovascular Diseases in Older Populations Committee of the Council on Clinical Cardiology; the Council on Cardiovascular and Stroke Nursing; the Council on Cardiovascular Radiology and Intervention; and the Council on Lifestyle and Cardiometabolic Health.
A version of this article first appeared on Medscape.com.






