User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
FDA approves Wegovy (semaglutide) for obesity in teens 12 and up
The Food and Drug Administration has approved semaglutide 2.4 mg (Wegovy), a once-weekly subcutaneous injection, for the additional indication of treating obesity in adolescents aged 12 years and older.
This is defined as those with an initial body mass index at or above the 95th percentile for age and sex (based on CDC growth charts). Semaglutide must be administered along with lifestyle intervention of a reduced calorie meal plan and increased physical activity.
When Wegovy was approved for use in adults with obesity in June 2021, it was labeled a “game changer.”
The new approval is based on the results of the STEP TEENS phase 3 trial of once-weekly 2.4 mg of semaglutide in adolescents 12- to <18 years old with obesity, the drug’s manufacturer, Novo Nordisk, announced in a press release.
In STEP TEENS, reported at Obesity Week 2022 in November, and simultaneously published in the New England Journal of Medicine, adolescents with obesity treated with semaglutide for 68 weeks had a 16.1% reduction in BMI compared with a 0.6% increase in BMI in those receiving placebo. Both groups also received lifestyle intervention. Mean weight loss was 15.3 kg (33.7 pounds) among teens on semaglutide, while those on placebo gained 2.4 kg (5.3 pounds).
At the time, Claudia K. Fox, MD, MPH, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota – who was not involved with the research – told this news organization the results were “mind-blowing ... we are getting close to bariatric surgery results” in these adolescent patients with obesity.
Semaglutide is a GLP-1 agonist, as is a related agent, also from Novo Nordisk, liraglutide (Saxenda), a daily subcutaneous injection, which was approved for use in adolescents aged 12 and older in December 2020. Wegovy is the first weekly subcutaneous injection approved for use in adolescents.
Other agents approved for obesity in those older than 12 in the United States include the combination phentermine and topiramate extended-release capsules (Qsymia) in June 2022, and orlistat (Alli). Phentermine is approved for those aged 16 and older.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved semaglutide 2.4 mg (Wegovy), a once-weekly subcutaneous injection, for the additional indication of treating obesity in adolescents aged 12 years and older.
This is defined as those with an initial body mass index at or above the 95th percentile for age and sex (based on CDC growth charts). Semaglutide must be administered along with lifestyle intervention of a reduced calorie meal plan and increased physical activity.
When Wegovy was approved for use in adults with obesity in June 2021, it was labeled a “game changer.”
The new approval is based on the results of the STEP TEENS phase 3 trial of once-weekly 2.4 mg of semaglutide in adolescents 12- to <18 years old with obesity, the drug’s manufacturer, Novo Nordisk, announced in a press release.
In STEP TEENS, reported at Obesity Week 2022 in November, and simultaneously published in the New England Journal of Medicine, adolescents with obesity treated with semaglutide for 68 weeks had a 16.1% reduction in BMI compared with a 0.6% increase in BMI in those receiving placebo. Both groups also received lifestyle intervention. Mean weight loss was 15.3 kg (33.7 pounds) among teens on semaglutide, while those on placebo gained 2.4 kg (5.3 pounds).
At the time, Claudia K. Fox, MD, MPH, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota – who was not involved with the research – told this news organization the results were “mind-blowing ... we are getting close to bariatric surgery results” in these adolescent patients with obesity.
Semaglutide is a GLP-1 agonist, as is a related agent, also from Novo Nordisk, liraglutide (Saxenda), a daily subcutaneous injection, which was approved for use in adolescents aged 12 and older in December 2020. Wegovy is the first weekly subcutaneous injection approved for use in adolescents.
Other agents approved for obesity in those older than 12 in the United States include the combination phentermine and topiramate extended-release capsules (Qsymia) in June 2022, and orlistat (Alli). Phentermine is approved for those aged 16 and older.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved semaglutide 2.4 mg (Wegovy), a once-weekly subcutaneous injection, for the additional indication of treating obesity in adolescents aged 12 years and older.
This is defined as those with an initial body mass index at or above the 95th percentile for age and sex (based on CDC growth charts). Semaglutide must be administered along with lifestyle intervention of a reduced calorie meal plan and increased physical activity.
When Wegovy was approved for use in adults with obesity in June 2021, it was labeled a “game changer.”
The new approval is based on the results of the STEP TEENS phase 3 trial of once-weekly 2.4 mg of semaglutide in adolescents 12- to <18 years old with obesity, the drug’s manufacturer, Novo Nordisk, announced in a press release.
In STEP TEENS, reported at Obesity Week 2022 in November, and simultaneously published in the New England Journal of Medicine, adolescents with obesity treated with semaglutide for 68 weeks had a 16.1% reduction in BMI compared with a 0.6% increase in BMI in those receiving placebo. Both groups also received lifestyle intervention. Mean weight loss was 15.3 kg (33.7 pounds) among teens on semaglutide, while those on placebo gained 2.4 kg (5.3 pounds).
At the time, Claudia K. Fox, MD, MPH, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota – who was not involved with the research – told this news organization the results were “mind-blowing ... we are getting close to bariatric surgery results” in these adolescent patients with obesity.
Semaglutide is a GLP-1 agonist, as is a related agent, also from Novo Nordisk, liraglutide (Saxenda), a daily subcutaneous injection, which was approved for use in adolescents aged 12 and older in December 2020. Wegovy is the first weekly subcutaneous injection approved for use in adolescents.
Other agents approved for obesity in those older than 12 in the United States include the combination phentermine and topiramate extended-release capsules (Qsymia) in June 2022, and orlistat (Alli). Phentermine is approved for those aged 16 and older.
A version of this article first appeared on Medscape.com.
Study of beliefs about what causes cancer sparks debate
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
Topical treatment options for acne continue to expand
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
AT MOAS 2022
Nursing exam failure rates spark review of test results
Nursing oversight groups in the United States and Canada are holding the line on testing standards as more would-be nurses fail entry exams. As a result, pressure is growing to make tests easier to pass given widespread nursing shortages, and some critics wonder whether the exams accurately assess students’ true abilities.
When it comes to training more nurses to keep up with growing demand, the U.S. organization that oversees the main licensing exams for nurses decided earlier this month not to change the passing standards for entry-level tests. Meanwhile,
A similar scenario also is unfolding in Quebec, where the agency overseeing nurse licensing exams announced last month it is holding the line on its passing rates despite an outcry from nurses after more than half of those taking the exam in September failed. Quebec’s commissioner for professional admissions is investigating dozens of complaints from nurses about the failure rate. Nurses who failed the test can sign up to retake it in March.
Joseph Oujeil, DESS, DEF, has been teaching in Canada for 4 years, now at two Quebec nursing schools. “This is surprising and very shocking to our students as well as nurses from outside Quebec who were [completing] an integration program to adjust their practice to Quebec guidelines,” Mr. Oujeil told this news organization. Students from outside the province failed the licensing exam at a higher rate than their Quebec-native peers, he explained.
Quebec’s professional Order of Nurses of Quebec (OIIQ) responded to the nurses’ outcry in a press release last month, saying that the pandemic may be partly to blame for the lower passing rate because it made it more difficult to access internships, labs, and face-to-face teaching. Some students weren’t able to demonstrate their ability to practice during the exam as in previous years, OIIQ reported.
Mr. Oujeil agreed. “I’m sure the pandemic has an impact on the situation as well as some students did less training in hospitals” because of restrictions caused by the pandemic, he said. But students also told Mr. Oujeil some questions seemed ambiguous.
OIIQ stated in its release that it doesn’t want to lower the standard. The goal is to protect the public “by and with nurses,” to “ensure the competence and integrity of nurses in Quebec,” and “promote quality nursing practice,” the release noted.
Similarly in the United States, the National Council of State Boards of Nursing (NCSBN) announced Dec. 8 that it would uphold the current passing standards for its entry-level NCLEX tests for registered nurses and practical nurses. NCSBN analyzes the passing standard every 3 years “to keep the test plan and passing standard current,” a press release explains.
NCLEX pass rates have dropped from about 73% for all candidates and 88% for first-time U.S.-educated candidates to 69% and 82% respectively in 2021, the last full year for which results are available, NCSBN spokesperson Dawn Kappel told this news organization.
Over the past 3 years, including during the pandemic, the board decided “that the current passing standard is appropriate as a measure of safe and effective entry-level nurse practice,” after reviewing national nurse surveys and the findings of panels of nurses representing NCSBN’s geographic areas in the United States and Canada, board president Jay Douglas, MSM, RN, CSAC, said in a press release.
Still, NCSBN is not blind to the larger issues facing nursing, Ms. Kappel told this news organization. “There is a huge nursing shortage in the U.S. and Canada. We want as many nurses in the workforce as possible, but we want to ensure safe practice,” she said.
“Everyone has access to the same test, regardless of which state, province, or country they take it in,” she said. Some international students may not perform as well as U.S.-educated students because of their command of English and the nursing education standards in their home countries, Ms. Kappel added.
“Obviously COVID and the challenge of education in general” affected the results, she said.
Mr. Oujeil, the nursing school professor, said he is frustrated by the test results because the majority of students who failed maintained good grades and passed all of their trainings. Yet they scored just below the passing rate of 55%. He said students are proposing the passing rate be lowered to 50%. The current test doesn’t reflect what students are learning in the classroom or during clinical trainings, Mr. Oujeil added. “I don’t know of any students who scored more than 60%.”
He said he understands that the mission of the OIIQ is protecting the population, but he doesn’t believe lowering the passing rate to 50% will put the population at risk – and it will help offset the staffing shortage.
“I’m especially frustrated by those who were doing integration programs – mothers and fathers with children with family lives and financial responsibilities. Many of them are good, hard workers and were shocked they should have to pass the exam another time.”
Since 2018, the pass rate on the first attempt at the Quebec test has generally been between 71% and 96%, compared with 51.4% during the exam in September, according to the OIIQ press release. Meanwhile, graduates from 30 of the 55 schools and universities in Quebec teaching nurses performed above the average on the recent professional exam, OIIQ reported.
The professional licensing organization pointed out that nursing candidates have three attempts to pass their exam. “In order to better prepare for their next attempt, all those who failed received an individual response detailing the difficulties encountered. The OIIQ offers all the tools necessary to pass the exam; a detailed guide and preparatory workshops are available online.
“In the run up to the next exams, we will continue to support students by working with educational institutions to provide the optimal conditions for passing the exam. This exam is usually successful and we are convinced that the return to face-to-face teaching, as well as support for students, will be factors of success,” OIIQ President Luc Mathieu said in a press release.
Nursing candidates “who have not passed the exam will be put to work in the network, with the possibility of practicing under the supervision of a nurse. ... In addition, we will contact health establishments in order to support them in their supervision activities” of the candidates, he said.
A version of this article first appeared on Medscape.com.
Nursing oversight groups in the United States and Canada are holding the line on testing standards as more would-be nurses fail entry exams. As a result, pressure is growing to make tests easier to pass given widespread nursing shortages, and some critics wonder whether the exams accurately assess students’ true abilities.
When it comes to training more nurses to keep up with growing demand, the U.S. organization that oversees the main licensing exams for nurses decided earlier this month not to change the passing standards for entry-level tests. Meanwhile,
A similar scenario also is unfolding in Quebec, where the agency overseeing nurse licensing exams announced last month it is holding the line on its passing rates despite an outcry from nurses after more than half of those taking the exam in September failed. Quebec’s commissioner for professional admissions is investigating dozens of complaints from nurses about the failure rate. Nurses who failed the test can sign up to retake it in March.
Joseph Oujeil, DESS, DEF, has been teaching in Canada for 4 years, now at two Quebec nursing schools. “This is surprising and very shocking to our students as well as nurses from outside Quebec who were [completing] an integration program to adjust their practice to Quebec guidelines,” Mr. Oujeil told this news organization. Students from outside the province failed the licensing exam at a higher rate than their Quebec-native peers, he explained.
Quebec’s professional Order of Nurses of Quebec (OIIQ) responded to the nurses’ outcry in a press release last month, saying that the pandemic may be partly to blame for the lower passing rate because it made it more difficult to access internships, labs, and face-to-face teaching. Some students weren’t able to demonstrate their ability to practice during the exam as in previous years, OIIQ reported.
Mr. Oujeil agreed. “I’m sure the pandemic has an impact on the situation as well as some students did less training in hospitals” because of restrictions caused by the pandemic, he said. But students also told Mr. Oujeil some questions seemed ambiguous.
OIIQ stated in its release that it doesn’t want to lower the standard. The goal is to protect the public “by and with nurses,” to “ensure the competence and integrity of nurses in Quebec,” and “promote quality nursing practice,” the release noted.
Similarly in the United States, the National Council of State Boards of Nursing (NCSBN) announced Dec. 8 that it would uphold the current passing standards for its entry-level NCLEX tests for registered nurses and practical nurses. NCSBN analyzes the passing standard every 3 years “to keep the test plan and passing standard current,” a press release explains.
NCLEX pass rates have dropped from about 73% for all candidates and 88% for first-time U.S.-educated candidates to 69% and 82% respectively in 2021, the last full year for which results are available, NCSBN spokesperson Dawn Kappel told this news organization.
Over the past 3 years, including during the pandemic, the board decided “that the current passing standard is appropriate as a measure of safe and effective entry-level nurse practice,” after reviewing national nurse surveys and the findings of panels of nurses representing NCSBN’s geographic areas in the United States and Canada, board president Jay Douglas, MSM, RN, CSAC, said in a press release.
Still, NCSBN is not blind to the larger issues facing nursing, Ms. Kappel told this news organization. “There is a huge nursing shortage in the U.S. and Canada. We want as many nurses in the workforce as possible, but we want to ensure safe practice,” she said.
“Everyone has access to the same test, regardless of which state, province, or country they take it in,” she said. Some international students may not perform as well as U.S.-educated students because of their command of English and the nursing education standards in their home countries, Ms. Kappel added.
“Obviously COVID and the challenge of education in general” affected the results, she said.
Mr. Oujeil, the nursing school professor, said he is frustrated by the test results because the majority of students who failed maintained good grades and passed all of their trainings. Yet they scored just below the passing rate of 55%. He said students are proposing the passing rate be lowered to 50%. The current test doesn’t reflect what students are learning in the classroom or during clinical trainings, Mr. Oujeil added. “I don’t know of any students who scored more than 60%.”
He said he understands that the mission of the OIIQ is protecting the population, but he doesn’t believe lowering the passing rate to 50% will put the population at risk – and it will help offset the staffing shortage.
“I’m especially frustrated by those who were doing integration programs – mothers and fathers with children with family lives and financial responsibilities. Many of them are good, hard workers and were shocked they should have to pass the exam another time.”
Since 2018, the pass rate on the first attempt at the Quebec test has generally been between 71% and 96%, compared with 51.4% during the exam in September, according to the OIIQ press release. Meanwhile, graduates from 30 of the 55 schools and universities in Quebec teaching nurses performed above the average on the recent professional exam, OIIQ reported.
The professional licensing organization pointed out that nursing candidates have three attempts to pass their exam. “In order to better prepare for their next attempt, all those who failed received an individual response detailing the difficulties encountered. The OIIQ offers all the tools necessary to pass the exam; a detailed guide and preparatory workshops are available online.
“In the run up to the next exams, we will continue to support students by working with educational institutions to provide the optimal conditions for passing the exam. This exam is usually successful and we are convinced that the return to face-to-face teaching, as well as support for students, will be factors of success,” OIIQ President Luc Mathieu said in a press release.
Nursing candidates “who have not passed the exam will be put to work in the network, with the possibility of practicing under the supervision of a nurse. ... In addition, we will contact health establishments in order to support them in their supervision activities” of the candidates, he said.
A version of this article first appeared on Medscape.com.
Nursing oversight groups in the United States and Canada are holding the line on testing standards as more would-be nurses fail entry exams. As a result, pressure is growing to make tests easier to pass given widespread nursing shortages, and some critics wonder whether the exams accurately assess students’ true abilities.
When it comes to training more nurses to keep up with growing demand, the U.S. organization that oversees the main licensing exams for nurses decided earlier this month not to change the passing standards for entry-level tests. Meanwhile,
A similar scenario also is unfolding in Quebec, where the agency overseeing nurse licensing exams announced last month it is holding the line on its passing rates despite an outcry from nurses after more than half of those taking the exam in September failed. Quebec’s commissioner for professional admissions is investigating dozens of complaints from nurses about the failure rate. Nurses who failed the test can sign up to retake it in March.
Joseph Oujeil, DESS, DEF, has been teaching in Canada for 4 years, now at two Quebec nursing schools. “This is surprising and very shocking to our students as well as nurses from outside Quebec who were [completing] an integration program to adjust their practice to Quebec guidelines,” Mr. Oujeil told this news organization. Students from outside the province failed the licensing exam at a higher rate than their Quebec-native peers, he explained.
Quebec’s professional Order of Nurses of Quebec (OIIQ) responded to the nurses’ outcry in a press release last month, saying that the pandemic may be partly to blame for the lower passing rate because it made it more difficult to access internships, labs, and face-to-face teaching. Some students weren’t able to demonstrate their ability to practice during the exam as in previous years, OIIQ reported.
Mr. Oujeil agreed. “I’m sure the pandemic has an impact on the situation as well as some students did less training in hospitals” because of restrictions caused by the pandemic, he said. But students also told Mr. Oujeil some questions seemed ambiguous.
OIIQ stated in its release that it doesn’t want to lower the standard. The goal is to protect the public “by and with nurses,” to “ensure the competence and integrity of nurses in Quebec,” and “promote quality nursing practice,” the release noted.
Similarly in the United States, the National Council of State Boards of Nursing (NCSBN) announced Dec. 8 that it would uphold the current passing standards for its entry-level NCLEX tests for registered nurses and practical nurses. NCSBN analyzes the passing standard every 3 years “to keep the test plan and passing standard current,” a press release explains.
NCLEX pass rates have dropped from about 73% for all candidates and 88% for first-time U.S.-educated candidates to 69% and 82% respectively in 2021, the last full year for which results are available, NCSBN spokesperson Dawn Kappel told this news organization.
Over the past 3 years, including during the pandemic, the board decided “that the current passing standard is appropriate as a measure of safe and effective entry-level nurse practice,” after reviewing national nurse surveys and the findings of panels of nurses representing NCSBN’s geographic areas in the United States and Canada, board president Jay Douglas, MSM, RN, CSAC, said in a press release.
Still, NCSBN is not blind to the larger issues facing nursing, Ms. Kappel told this news organization. “There is a huge nursing shortage in the U.S. and Canada. We want as many nurses in the workforce as possible, but we want to ensure safe practice,” she said.
“Everyone has access to the same test, regardless of which state, province, or country they take it in,” she said. Some international students may not perform as well as U.S.-educated students because of their command of English and the nursing education standards in their home countries, Ms. Kappel added.
“Obviously COVID and the challenge of education in general” affected the results, she said.
Mr. Oujeil, the nursing school professor, said he is frustrated by the test results because the majority of students who failed maintained good grades and passed all of their trainings. Yet they scored just below the passing rate of 55%. He said students are proposing the passing rate be lowered to 50%. The current test doesn’t reflect what students are learning in the classroom or during clinical trainings, Mr. Oujeil added. “I don’t know of any students who scored more than 60%.”
He said he understands that the mission of the OIIQ is protecting the population, but he doesn’t believe lowering the passing rate to 50% will put the population at risk – and it will help offset the staffing shortage.
“I’m especially frustrated by those who were doing integration programs – mothers and fathers with children with family lives and financial responsibilities. Many of them are good, hard workers and were shocked they should have to pass the exam another time.”
Since 2018, the pass rate on the first attempt at the Quebec test has generally been between 71% and 96%, compared with 51.4% during the exam in September, according to the OIIQ press release. Meanwhile, graduates from 30 of the 55 schools and universities in Quebec teaching nurses performed above the average on the recent professional exam, OIIQ reported.
The professional licensing organization pointed out that nursing candidates have three attempts to pass their exam. “In order to better prepare for their next attempt, all those who failed received an individual response detailing the difficulties encountered. The OIIQ offers all the tools necessary to pass the exam; a detailed guide and preparatory workshops are available online.
“In the run up to the next exams, we will continue to support students by working with educational institutions to provide the optimal conditions for passing the exam. This exam is usually successful and we are convinced that the return to face-to-face teaching, as well as support for students, will be factors of success,” OIIQ President Luc Mathieu said in a press release.
Nursing candidates “who have not passed the exam will be put to work in the network, with the possibility of practicing under the supervision of a nurse. ... In addition, we will contact health establishments in order to support them in their supervision activities” of the candidates, he said.
A version of this article first appeared on Medscape.com.
New treatments aim to tame vitiligo
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Vitiligo, an autoimmune condition that results in patches of skin depigmentation, occurs in 0.5% to 2% of the population. The average age of onset is 20 years, with 25% of cases occurring before age 10, and 70%-80% of cases by age 30 years, which means a long-term effect on quality of life, especially for younger patients, said Dr. Rosmarin, vice chair of education and research and director of the clinical trials unit at Tufts University, Boston.
Studies have shown that 95% of 15- to 17-year-olds with vitiligo are bothered by it, as are approximately 50% of children aged 6-14 years, he said. Although patients with more extensive lesions on the face, arms, legs, and hands report worse quality of life, they report that uncontrolled progression of vitiligo is more concerning than the presence of lesions in exposed areas, he noted.
The current strategy for getting vitiligo under control is a two-step process, said Dr. Rosmarin. First, improve the skin environment by suppressing the overactive immune system, then encourage repigmentation and “nudge the melanocytes to return,” he said.
Topical ruxolitinib, a Janus kinase (JAK) inhibitor, is the latest tool for dermatologists to help give the melanocytes that nudge. In July 2022, the Food and Drug Administration approved ruxolitinib cream for treating nonsegmental vitiligo in patients 12 years of age and older – the first treatment approved to repigment patients with vitiligo.
Vitiligo is driven in part by interferon (IFN)-gamma signaling through JAK 1 and 2, and ruxolitinib acts as an inhibitor, Dr. Rosmarin said.
In the TRuE-V1 and TRuE-V2 studies presented at the 2022 European Academy of Dermatology and Venereology meeting in Milan, adolescents and adults with vitiligo who were randomized to 1.5% ruxolitinib cream twice daily showed significant improvement over those randomized to the vehicle by 24 weeks, at which time all patients could continue with ruxolitinib through 52 weeks, he said.
Dr. Rosmarin presented 52-week data from the TRuE-V1 and TRuE-V2 studies at the 2022 American Academy of Dermatology meeting in Boston. He was the lead author of the studies that were subsequently published in the New England Journal of Medicine.
In the two studies, 52.6% and 48% of the patients in the ruxolitinib groups achieved the primary outcome of at least 75% improvement on the Facial Vitiligo Area Scoring Index (F-VASI75) by 52 weeks, compared with 26.8% and 29.6% of patients on the vehicle, respectively.
In addition, at 52 weeks, 53.2% and 49.2% of patients treated with ruxolitinib in the two studies achieved 50% improvement on the Total Vitiligo Area Scoring Index (T-VASI50), a clinician assessment of affected body surface area and level of depigmentation, compared with 31.7% and 22.2% of those on vehicle, respectively.
Patient satisfaction was high with ruxolitinib, Dr. Rosmarin said. In the TRuE-V1 and TRuE-V2 studies, 39.9% and 32.8% of patients, respectively, achieved a successful treatment response based on the patient-reported Vitiligo Noticeability Scale (VNS) by week 52, versus 19.5% and 13.6% of those on vehicle.
Ruxolitinib cream was well tolerated, with “no clinically significant application site reactions or serious treatment-related adverse events,” he noted. The most common treatment-related adverse events across the TRuE-V1 and TRuE-V2 studies were acne at the application site (affecting about 6% of patients) and pruritus at the application site about (affecting 5%), said Dr. Rosmarin.
JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, have shown effectiveness for vitiligo, which supports the potential role of the IFN-gamma-chemokine signaling axis in the pathogenesis of the disease, said Dr. Rosmarin. However, more studies are required to determine the ideal dosage of JAK inhibitors for the treatment of vitiligo, and to identify other inflammatory pathways that may be implicated in the pathogenesis of this condition.
Ruxolitinib’s success has been consistent across subgroups of age, gender, race, geographic region, and Fitzpatrick skin phototype. Notably, ruxolitinib was effective among the adolescent population, with approximately 60% achieving T-VASI50 and success based on VNS in TRuE-V1 and TRuE-V2.
An oral version of ruxolitinib is in clinical trials, which “makes a lot of sense,” Dr. Rosmarin said. “Patients don’t always have localized disease,” and such patients may benefit from an oral therapy. Topicals may have the advantage in terms of safety, but questions of maintenance remain, he said. Oral treatments may be useful for patients with large body surface areas affected, and those with unstable or progressive disease, he added.
Areas for additional research include combination therapy with ruxolitinib and phototherapy, and an anti-IL 15 therapy in the pipeline has the potential to drive vitiligo into remission, Dr. Rosmarin said. In a study known as REVEAL that is still recruiting patients, researchers will test the efficacy of an IL-15 inhibitor known as AMG 714 to induce facial repigmentation in adults with vitiligo.
Dr. Rosmarin disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb Company, Celgene, Concert Pharmaceuticals, CSL Behring, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Janssen, Kyowa Kirin, Merck, Novartis, Pfizer, Regeneron, Revolo, Sanofi, Sun, UCB, and Viela Bio.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Vitiligo, an autoimmune condition that results in patches of skin depigmentation, occurs in 0.5% to 2% of the population. The average age of onset is 20 years, with 25% of cases occurring before age 10, and 70%-80% of cases by age 30 years, which means a long-term effect on quality of life, especially for younger patients, said Dr. Rosmarin, vice chair of education and research and director of the clinical trials unit at Tufts University, Boston.
Studies have shown that 95% of 15- to 17-year-olds with vitiligo are bothered by it, as are approximately 50% of children aged 6-14 years, he said. Although patients with more extensive lesions on the face, arms, legs, and hands report worse quality of life, they report that uncontrolled progression of vitiligo is more concerning than the presence of lesions in exposed areas, he noted.
The current strategy for getting vitiligo under control is a two-step process, said Dr. Rosmarin. First, improve the skin environment by suppressing the overactive immune system, then encourage repigmentation and “nudge the melanocytes to return,” he said.
Topical ruxolitinib, a Janus kinase (JAK) inhibitor, is the latest tool for dermatologists to help give the melanocytes that nudge. In July 2022, the Food and Drug Administration approved ruxolitinib cream for treating nonsegmental vitiligo in patients 12 years of age and older – the first treatment approved to repigment patients with vitiligo.
Vitiligo is driven in part by interferon (IFN)-gamma signaling through JAK 1 and 2, and ruxolitinib acts as an inhibitor, Dr. Rosmarin said.
In the TRuE-V1 and TRuE-V2 studies presented at the 2022 European Academy of Dermatology and Venereology meeting in Milan, adolescents and adults with vitiligo who were randomized to 1.5% ruxolitinib cream twice daily showed significant improvement over those randomized to the vehicle by 24 weeks, at which time all patients could continue with ruxolitinib through 52 weeks, he said.
Dr. Rosmarin presented 52-week data from the TRuE-V1 and TRuE-V2 studies at the 2022 American Academy of Dermatology meeting in Boston. He was the lead author of the studies that were subsequently published in the New England Journal of Medicine.
In the two studies, 52.6% and 48% of the patients in the ruxolitinib groups achieved the primary outcome of at least 75% improvement on the Facial Vitiligo Area Scoring Index (F-VASI75) by 52 weeks, compared with 26.8% and 29.6% of patients on the vehicle, respectively.
In addition, at 52 weeks, 53.2% and 49.2% of patients treated with ruxolitinib in the two studies achieved 50% improvement on the Total Vitiligo Area Scoring Index (T-VASI50), a clinician assessment of affected body surface area and level of depigmentation, compared with 31.7% and 22.2% of those on vehicle, respectively.
Patient satisfaction was high with ruxolitinib, Dr. Rosmarin said. In the TRuE-V1 and TRuE-V2 studies, 39.9% and 32.8% of patients, respectively, achieved a successful treatment response based on the patient-reported Vitiligo Noticeability Scale (VNS) by week 52, versus 19.5% and 13.6% of those on vehicle.
Ruxolitinib cream was well tolerated, with “no clinically significant application site reactions or serious treatment-related adverse events,” he noted. The most common treatment-related adverse events across the TRuE-V1 and TRuE-V2 studies were acne at the application site (affecting about 6% of patients) and pruritus at the application site about (affecting 5%), said Dr. Rosmarin.
JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, have shown effectiveness for vitiligo, which supports the potential role of the IFN-gamma-chemokine signaling axis in the pathogenesis of the disease, said Dr. Rosmarin. However, more studies are required to determine the ideal dosage of JAK inhibitors for the treatment of vitiligo, and to identify other inflammatory pathways that may be implicated in the pathogenesis of this condition.
Ruxolitinib’s success has been consistent across subgroups of age, gender, race, geographic region, and Fitzpatrick skin phototype. Notably, ruxolitinib was effective among the adolescent population, with approximately 60% achieving T-VASI50 and success based on VNS in TRuE-V1 and TRuE-V2.
An oral version of ruxolitinib is in clinical trials, which “makes a lot of sense,” Dr. Rosmarin said. “Patients don’t always have localized disease,” and such patients may benefit from an oral therapy. Topicals may have the advantage in terms of safety, but questions of maintenance remain, he said. Oral treatments may be useful for patients with large body surface areas affected, and those with unstable or progressive disease, he added.
Areas for additional research include combination therapy with ruxolitinib and phototherapy, and an anti-IL 15 therapy in the pipeline has the potential to drive vitiligo into remission, Dr. Rosmarin said. In a study known as REVEAL that is still recruiting patients, researchers will test the efficacy of an IL-15 inhibitor known as AMG 714 to induce facial repigmentation in adults with vitiligo.
Dr. Rosmarin disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb Company, Celgene, Concert Pharmaceuticals, CSL Behring, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Janssen, Kyowa Kirin, Merck, Novartis, Pfizer, Regeneron, Revolo, Sanofi, Sun, UCB, and Viela Bio.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Vitiligo, an autoimmune condition that results in patches of skin depigmentation, occurs in 0.5% to 2% of the population. The average age of onset is 20 years, with 25% of cases occurring before age 10, and 70%-80% of cases by age 30 years, which means a long-term effect on quality of life, especially for younger patients, said Dr. Rosmarin, vice chair of education and research and director of the clinical trials unit at Tufts University, Boston.
Studies have shown that 95% of 15- to 17-year-olds with vitiligo are bothered by it, as are approximately 50% of children aged 6-14 years, he said. Although patients with more extensive lesions on the face, arms, legs, and hands report worse quality of life, they report that uncontrolled progression of vitiligo is more concerning than the presence of lesions in exposed areas, he noted.
The current strategy for getting vitiligo under control is a two-step process, said Dr. Rosmarin. First, improve the skin environment by suppressing the overactive immune system, then encourage repigmentation and “nudge the melanocytes to return,” he said.
Topical ruxolitinib, a Janus kinase (JAK) inhibitor, is the latest tool for dermatologists to help give the melanocytes that nudge. In July 2022, the Food and Drug Administration approved ruxolitinib cream for treating nonsegmental vitiligo in patients 12 years of age and older – the first treatment approved to repigment patients with vitiligo.
Vitiligo is driven in part by interferon (IFN)-gamma signaling through JAK 1 and 2, and ruxolitinib acts as an inhibitor, Dr. Rosmarin said.
In the TRuE-V1 and TRuE-V2 studies presented at the 2022 European Academy of Dermatology and Venereology meeting in Milan, adolescents and adults with vitiligo who were randomized to 1.5% ruxolitinib cream twice daily showed significant improvement over those randomized to the vehicle by 24 weeks, at which time all patients could continue with ruxolitinib through 52 weeks, he said.
Dr. Rosmarin presented 52-week data from the TRuE-V1 and TRuE-V2 studies at the 2022 American Academy of Dermatology meeting in Boston. He was the lead author of the studies that were subsequently published in the New England Journal of Medicine.
In the two studies, 52.6% and 48% of the patients in the ruxolitinib groups achieved the primary outcome of at least 75% improvement on the Facial Vitiligo Area Scoring Index (F-VASI75) by 52 weeks, compared with 26.8% and 29.6% of patients on the vehicle, respectively.
In addition, at 52 weeks, 53.2% and 49.2% of patients treated with ruxolitinib in the two studies achieved 50% improvement on the Total Vitiligo Area Scoring Index (T-VASI50), a clinician assessment of affected body surface area and level of depigmentation, compared with 31.7% and 22.2% of those on vehicle, respectively.
Patient satisfaction was high with ruxolitinib, Dr. Rosmarin said. In the TRuE-V1 and TRuE-V2 studies, 39.9% and 32.8% of patients, respectively, achieved a successful treatment response based on the patient-reported Vitiligo Noticeability Scale (VNS) by week 52, versus 19.5% and 13.6% of those on vehicle.
Ruxolitinib cream was well tolerated, with “no clinically significant application site reactions or serious treatment-related adverse events,” he noted. The most common treatment-related adverse events across the TRuE-V1 and TRuE-V2 studies were acne at the application site (affecting about 6% of patients) and pruritus at the application site about (affecting 5%), said Dr. Rosmarin.
JAK inhibitors, including ruxolitinib, baricitinib, and tofacitinib, have shown effectiveness for vitiligo, which supports the potential role of the IFN-gamma-chemokine signaling axis in the pathogenesis of the disease, said Dr. Rosmarin. However, more studies are required to determine the ideal dosage of JAK inhibitors for the treatment of vitiligo, and to identify other inflammatory pathways that may be implicated in the pathogenesis of this condition.
Ruxolitinib’s success has been consistent across subgroups of age, gender, race, geographic region, and Fitzpatrick skin phototype. Notably, ruxolitinib was effective among the adolescent population, with approximately 60% achieving T-VASI50 and success based on VNS in TRuE-V1 and TRuE-V2.
An oral version of ruxolitinib is in clinical trials, which “makes a lot of sense,” Dr. Rosmarin said. “Patients don’t always have localized disease,” and such patients may benefit from an oral therapy. Topicals may have the advantage in terms of safety, but questions of maintenance remain, he said. Oral treatments may be useful for patients with large body surface areas affected, and those with unstable or progressive disease, he added.
Areas for additional research include combination therapy with ruxolitinib and phototherapy, and an anti-IL 15 therapy in the pipeline has the potential to drive vitiligo into remission, Dr. Rosmarin said. In a study known as REVEAL that is still recruiting patients, researchers will test the efficacy of an IL-15 inhibitor known as AMG 714 to induce facial repigmentation in adults with vitiligo.
Dr. Rosmarin disclosed ties with AbbVie, Abcuro, AltruBio, Amgen, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol Myers Squibb Company, Celgene, Concert Pharmaceuticals, CSL Behring, Dermavant, Dermira, Eli Lilly, Galderma, Incyte, Janssen, Kyowa Kirin, Merck, Novartis, Pfizer, Regeneron, Revolo, Sanofi, Sun, UCB, and Viela Bio.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
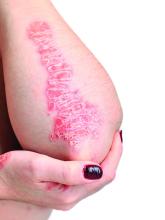
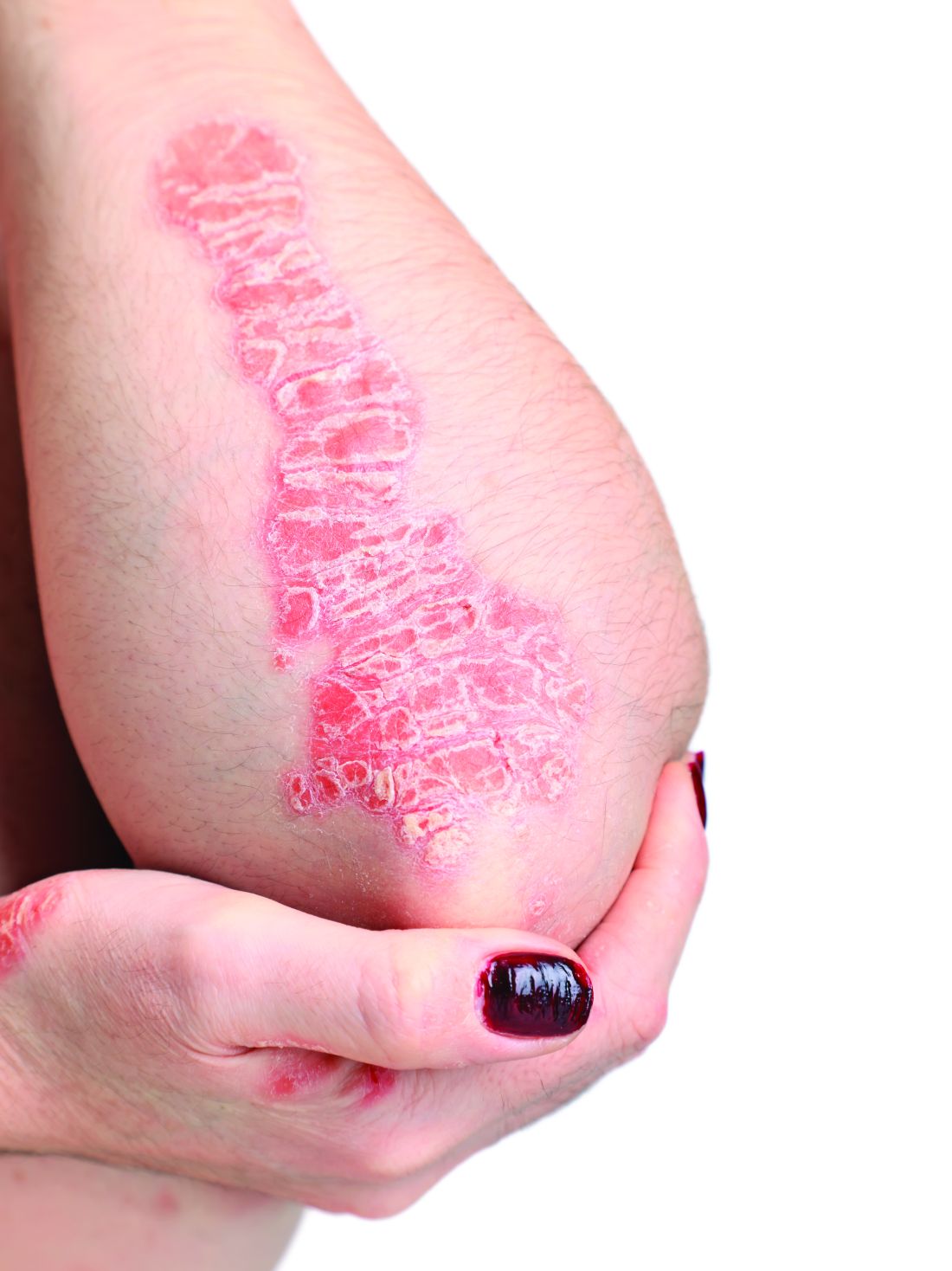
Topical psoriasis treatments
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – ,” said Linda Stein Gold, MD, in a presentation at Medscape Live’s annual Las Vegas Dermatology Seminar.
However, when using topical treatments, combination therapy is generally more effective than monotherapy for psoriasis, especially for plaque psoriasis, said Dr. Stein Gold, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit.
Two combination products, calcipotriene/betamethasone (CAL/BDP) and tazarotene/halobetasol lotion, each offer a complimentary mechanism of action that minimizes side effects, with decreased irritation and less atrophy, she said. Calcipotriene/betamethasone (CAL/BDP) is available as a cream or foam, Dr. Stein Gold noted. The cream is engineered for rapid onset, as well as enhanced penetration, she said. CAL/BDP foam also is designed for enhanced penetration, and has been shown to have long-term maintenance efficacy, she said.
The currently available CAL/BDP cream is made using a patented technology known as “PAD,” in which the internal oil of the cream vehicle is stabilized by encapsulation in “a robust aqueous film,” Dr. Stein Gold said, noting that the greater solubility enhances skin penetration. The creation of “a robust oil droplet” addresses the problems associated with the surfactants present in many cream vehicles, namely irritation and impedance of skin penetration of the cream, she said.
In an 8-week study published in 2021, researchers compared CAL/BDP cream with PAD technology to CAL/BDP topical suspension in adults with mild to moderate psoriasis.
Patients randomized to treatment with CAL/BDP cream were significantly more likely to achieve the primary endpoint of Physician Global Assessment (PGA) treatment success than those randomized to the topical solution or vehicle (37.4%, 22.8%, and 3.7%, respectively).
Get proactive to maintain results
With topical psoriasis treatment, a proactive strategy helps maintain results over time, Dr. Stein Gold said. As an example, she cited a study published in 2021. In that study, known as PSO-LONG, which evaluated topical CAL/BDP foam, proactive management with the CAL/BDP foam formulation, “reduced the risk of experiencing relapse by 43%,” compared with reactive management (treatment with the vehicle foam), she said. Patients in the proactive-management group experienced an average of 41 more days in remission, compared with those in the reactive management group over a 1-year period.
Dr. Stein Gold also highlighted the value of tazarotene/halobetasol lotion for psoriasis, which she described as having synergistic efficacy,
She shared data presented at the 2021 Maui Dermatology meeting showing treatment success by 8 weeks with halobetasol/tazarotene with significantly reduced mean scores on measures of itching, dryness, and burning/stinging, compared with those on vehicle.
What’s new and approved
Joining the current topical treatment options for psoriasis is tapinarof, a small molecule that works by down-regulating Th17 cytokines, said Dr. Stein Gold. Tapinarof is Food and Drug Administration approved for treating psoriasis and is being studied in clinical trials for atopic dermatitis, she noted.
Dr. Stein Gold reviewed data from the PSOARING program published in the New England Journal of Medicine that served as a foundation for the FDA approval of tapinarof 1% cream. In the PSOARING 1 and 2 studies, patients with PSORIASIS showed significant improvement compared with vehicle over 12 weeks for the primary endpoint of Physicians’ Global Assessment scores of 0 or 1 (clear or almost clear). In the two studies, 60.7% and 56.9% of patients randomized to tapinarof met the patient-reported outcome of a minimum 4-point improvement in peak pruritus on the numerical rating scale (NRS) from baseline vs. 43.2% and 29.7% of placebo patients in the two studies, respectively.
In PSOARING 1 and 2, folliculitis (mostly mild or moderate), contact dermatitis, headache, pruritus, and dermatitis were the most common treatment-emergent adverse events, occurring in 1% or more of patients. Adverse event profiles for tapinarof are similar to those seen in previous studies, and a long-term extension showed a consistent safety profile, Dr. Stein Gold said.
Another recently approved topical treatment for psoriasis, a cream formulation of roflumilast, a phosphodiesterase (PDE)-4 inhibitor, has shown efficacy for treating plaque psoriasis, she said.
Patients with psoriasis in the DERMIS 1 and DERMIS 2 phase 3 studies randomized to 0.3% roflumilast cream showed significant improvement compared with those randomized to vehicle in terms of Investigator Global Assessment scores of clear or almost clear with an improvement of at least 2 grades from baseline.
Roflumilast foam also has shown success in improving scalp and body psoriasis, but this vehicle and indication has not yet been approved, Dr. Stein Gold said.
Dr. Stein Gold disclosed serving as a consultant or adviser for companies including AbbVie, Amgen, Arcutis, Bristol Myers Squibb, Dermavant, EPI Health, Galderma, Janssen, Incyte, Ortho Dermatologics, Pfizer, Regeneron, Sanofi; UCB, and serving as a speaker or member of speakers’ bureau for Amgen, AbbVie, Incyte, Pfizer, Regeneron, Sanofi, and Sun Research. She also disclosed receiving funding from AbbVie Amgen, Arcutis, Dermata, Dermavant, Galderma, Incyte, Ortho Dermatologics, Pfizer, and UCB.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Does vitamin D deficiency cause obesity or vice versa?
A recent study found that people with obesity have lower blood levels of vitamin D than people of healthy weight. This association of obesity with low vitamin D levels has led to much speculation on whether low vitamin D levels cause obesity or whether obesity causes low vitamin D levels. The interest in this topic is piqued by the possibility that if vitamin D deficiency causes obesity, perhaps treatment could be as simple as providing vitamin D supplementation to enhance weight loss.
What is known about vitamin D’s role in the body?
It’s well known that vitamin D is essential for bone health as well as balancing the minerals calcium and phosphorus, but what is its role in weight management? Approximately 80%-90% of vitamin D in the body is from the skin synthesis of cholecalciferol through ultraviolet B radiation from sun exposure. The normal range of 25-hydroxy vitamin D is measured as nanograms per milliliter (ng/mL). Most experts recommend a level between 20 and 40 ng/mL, but this has been a controversial topic of never-ending debate in the medical literature.
Vitamin D levels and obesity
This has been noted for many years without identifying the underlying reasons beyond the sequestering of vitamin D in adipose tissue, although I’ll discuss other possibilities.
The inverse correlation between vitamin D and obesity has been seen in other diseases, such as cardiovascular disease, hypertension, prediabetes, and insulin resistance, as well as in sarcopenia and aging. Most studies emphasized the correlation between increasing adiposity with vitamin D deficiency in all ethnic and age groups. The causes and potential direct consequences of the vitamin D deficiency state in obesity are not well understood.
Vitamin D and adipose tissue
It’s been proposed that low vitamin D status in obesity might be due to increased vitamin D clearance from serum and enhanced storage of vitamin D by adipose tissue.
In adipose tissue, vitamin D exerts a variety of effects on inflammation, innate immunity, metabolism, and differentiation and apoptosis in many cell types. There is a stronger association between 25(OH)D and visceral fat as compared to subcutaneous adipose tissue, which suggests an influence of inflammation and components of the metabolic syndrome on vitamin D metabolism.
Because vitamin D has anti-inflammatory properties, it’s possible that low vitamin D status contributes to adipose tissue inflammation, a key link between obesity and its associated metabolic complications in obesity. A higher storage of vitamin D in adipose tissue, if accompanied by a parallel increase in the local synthesis of 1,25(OH)2D3 and action, may conceivably modulate adipocyte function as well as the activity of adipose tissue macrophages and hence the level of adipose tissue inflammation. In addition, it seems likely that 1,25(OH)2D3 also regulates the function of macrophages and other immune cell populations within adipose tissue.
It’s well known that vitamin D is stored in body fat, leading to the assumption that this was important in the evolution of vertebrates, including humans, who lived at latitudes where vitamin D could not be made in the winter and vitamin D stores had to be mobilized to maintain vitamin D sufficiency.
What is vitamin D’s role in obesity?
The main question that has eluded an answer so far is this one: Is vitamin D deficiency only a coincidental finding in obesity due to sequestration of the vitamin in fat, or does it have a role in the development of obesity and its complications, such as cardiovascular disease, type 2 diabetes, and hypertension?
Low vitamin D usually leads to impaired calcium absorption in the intestine and a lower calcium level, and eventually enhanced bone turnover and impaired bone mineral density (BMD).
However, it is known that in obesity there is greater BMD than in those who are lean. This leads to the conclusion that because there is a lack of vitamin D deficiency effects on bone in those with obesity, there is not really a vitamin D deficiency, and it may be that the sequestration in adipose tissue leads to a permanent supply that can maintain bone health.
An alternative explanation is that there is greater skeletal loading in obesity, and elevations in hormones such as estrogen and leptin could compensate for the vitamin D deficiency, leading to greater BMD in obesity.
Several other potential mechanisms besides sequestration of vitamin D in adipose tissue have been identified for low vitamin D and obesity. These include impaired hepatic 25-hydroxylation in nonalcoholic fatty liver disease, less sunlight exposure due to lower mobility and different clothing habits in people with obesity vs. their lean counterparts, and adverse dietary habits. For example, people with higher BMIs spend less time exercising outdoors and are more sedentary in general than their lean counterparts. Therefore, they are less likely to get sun exposure because of a decrease in time spent outdoors. Those with higher BMIs also tend to cover their bodies, showing less skin when outdoors than their leaner counterparts, and thus there is likely to be less conversion to vitamin D via skin and sun exposure in people with obesity.
Some studies suggest that an increased level of parathyroid hormone due to vitamin D deficiency promotes lipogenesis because of greater calcium influx in adipocytes. Another hypothesis is that the active form of vitamin D, 1,25(OH) D, inhibits adipogenesis through actions modulated by vitamin D receptors. These studies are promising, but prospective randomized trials are needed to determine whether vitamin D supplementation is a treatment option in preventing obesity. So far, vitamin D supplementation shows inconsistent results.
To conclude, there is a high prevalence of vitamin D deficiency in obesity, most likely because of dilution and sequestration in greater volumes of fat, blood muscle, and liver in obesity. Low vitamin D levels can’t be ruled out as a cause of obesity because of the research showing some interesting findings in vitamin D receptors in adipose tissue. Vitamin D deficiency in obesity doesn’t seem to affect bone mass but could have deleterious effects on other organ systems.
Weight loss improves obesity and complications, including the risk for cardiovascular disease and type 2 diabetes as well as vitamin D deficiency.
What do the guidelines say?
Treatment of vitamin D deficiency requires higher doses in obesity to achieve the same serum concentration compared with lean persons. Maintenance doses should not differ between those with obesity and lean persons.
The association of vitamin D and obesity remains elusive. Studies need to focus on vitamin D, vitamin D receptors, and actions of vitamin D in adipose tissue to investigate this relationship further.
In the meantime, media attention remains focused on the potential treatment and prevention of obesity with the mighty, all-purpose vitamin D, even though there is scant evidence.
Dr. Apovian is codirector at the Center for Weight Management and Wellness at Brigham and Women’s Hospital and professor of medicine in the division of endocrinology, diabetes and hypertension at Harvard University, Boston. She disclosed conflicts of interest with Abbott, Allergan, Altimmune, Bariatrix Nutrition, Cowen and Company, Curavit, Rhythm Pharma, Jazz, Nutrisystem, Roman, Novo Nordisk, EnteroMedics, Gelesis Srl, Zafgen, Xeno, L-Nutra, Tivity, and Real Appeal.
A version of this article first appeared on Medscape.com.
A recent study found that people with obesity have lower blood levels of vitamin D than people of healthy weight. This association of obesity with low vitamin D levels has led to much speculation on whether low vitamin D levels cause obesity or whether obesity causes low vitamin D levels. The interest in this topic is piqued by the possibility that if vitamin D deficiency causes obesity, perhaps treatment could be as simple as providing vitamin D supplementation to enhance weight loss.
What is known about vitamin D’s role in the body?
It’s well known that vitamin D is essential for bone health as well as balancing the minerals calcium and phosphorus, but what is its role in weight management? Approximately 80%-90% of vitamin D in the body is from the skin synthesis of cholecalciferol through ultraviolet B radiation from sun exposure. The normal range of 25-hydroxy vitamin D is measured as nanograms per milliliter (ng/mL). Most experts recommend a level between 20 and 40 ng/mL, but this has been a controversial topic of never-ending debate in the medical literature.
Vitamin D levels and obesity
This has been noted for many years without identifying the underlying reasons beyond the sequestering of vitamin D in adipose tissue, although I’ll discuss other possibilities.
The inverse correlation between vitamin D and obesity has been seen in other diseases, such as cardiovascular disease, hypertension, prediabetes, and insulin resistance, as well as in sarcopenia and aging. Most studies emphasized the correlation between increasing adiposity with vitamin D deficiency in all ethnic and age groups. The causes and potential direct consequences of the vitamin D deficiency state in obesity are not well understood.
Vitamin D and adipose tissue
It’s been proposed that low vitamin D status in obesity might be due to increased vitamin D clearance from serum and enhanced storage of vitamin D by adipose tissue.
In adipose tissue, vitamin D exerts a variety of effects on inflammation, innate immunity, metabolism, and differentiation and apoptosis in many cell types. There is a stronger association between 25(OH)D and visceral fat as compared to subcutaneous adipose tissue, which suggests an influence of inflammation and components of the metabolic syndrome on vitamin D metabolism.
Because vitamin D has anti-inflammatory properties, it’s possible that low vitamin D status contributes to adipose tissue inflammation, a key link between obesity and its associated metabolic complications in obesity. A higher storage of vitamin D in adipose tissue, if accompanied by a parallel increase in the local synthesis of 1,25(OH)2D3 and action, may conceivably modulate adipocyte function as well as the activity of adipose tissue macrophages and hence the level of adipose tissue inflammation. In addition, it seems likely that 1,25(OH)2D3 also regulates the function of macrophages and other immune cell populations within adipose tissue.
It’s well known that vitamin D is stored in body fat, leading to the assumption that this was important in the evolution of vertebrates, including humans, who lived at latitudes where vitamin D could not be made in the winter and vitamin D stores had to be mobilized to maintain vitamin D sufficiency.
What is vitamin D’s role in obesity?
The main question that has eluded an answer so far is this one: Is vitamin D deficiency only a coincidental finding in obesity due to sequestration of the vitamin in fat, or does it have a role in the development of obesity and its complications, such as cardiovascular disease, type 2 diabetes, and hypertension?
Low vitamin D usually leads to impaired calcium absorption in the intestine and a lower calcium level, and eventually enhanced bone turnover and impaired bone mineral density (BMD).
However, it is known that in obesity there is greater BMD than in those who are lean. This leads to the conclusion that because there is a lack of vitamin D deficiency effects on bone in those with obesity, there is not really a vitamin D deficiency, and it may be that the sequestration in adipose tissue leads to a permanent supply that can maintain bone health.
An alternative explanation is that there is greater skeletal loading in obesity, and elevations in hormones such as estrogen and leptin could compensate for the vitamin D deficiency, leading to greater BMD in obesity.
Several other potential mechanisms besides sequestration of vitamin D in adipose tissue have been identified for low vitamin D and obesity. These include impaired hepatic 25-hydroxylation in nonalcoholic fatty liver disease, less sunlight exposure due to lower mobility and different clothing habits in people with obesity vs. their lean counterparts, and adverse dietary habits. For example, people with higher BMIs spend less time exercising outdoors and are more sedentary in general than their lean counterparts. Therefore, they are less likely to get sun exposure because of a decrease in time spent outdoors. Those with higher BMIs also tend to cover their bodies, showing less skin when outdoors than their leaner counterparts, and thus there is likely to be less conversion to vitamin D via skin and sun exposure in people with obesity.
Some studies suggest that an increased level of parathyroid hormone due to vitamin D deficiency promotes lipogenesis because of greater calcium influx in adipocytes. Another hypothesis is that the active form of vitamin D, 1,25(OH) D, inhibits adipogenesis through actions modulated by vitamin D receptors. These studies are promising, but prospective randomized trials are needed to determine whether vitamin D supplementation is a treatment option in preventing obesity. So far, vitamin D supplementation shows inconsistent results.
To conclude, there is a high prevalence of vitamin D deficiency in obesity, most likely because of dilution and sequestration in greater volumes of fat, blood muscle, and liver in obesity. Low vitamin D levels can’t be ruled out as a cause of obesity because of the research showing some interesting findings in vitamin D receptors in adipose tissue. Vitamin D deficiency in obesity doesn’t seem to affect bone mass but could have deleterious effects on other organ systems.
Weight loss improves obesity and complications, including the risk for cardiovascular disease and type 2 diabetes as well as vitamin D deficiency.
What do the guidelines say?
Treatment of vitamin D deficiency requires higher doses in obesity to achieve the same serum concentration compared with lean persons. Maintenance doses should not differ between those with obesity and lean persons.
The association of vitamin D and obesity remains elusive. Studies need to focus on vitamin D, vitamin D receptors, and actions of vitamin D in adipose tissue to investigate this relationship further.
In the meantime, media attention remains focused on the potential treatment and prevention of obesity with the mighty, all-purpose vitamin D, even though there is scant evidence.
Dr. Apovian is codirector at the Center for Weight Management and Wellness at Brigham and Women’s Hospital and professor of medicine in the division of endocrinology, diabetes and hypertension at Harvard University, Boston. She disclosed conflicts of interest with Abbott, Allergan, Altimmune, Bariatrix Nutrition, Cowen and Company, Curavit, Rhythm Pharma, Jazz, Nutrisystem, Roman, Novo Nordisk, EnteroMedics, Gelesis Srl, Zafgen, Xeno, L-Nutra, Tivity, and Real Appeal.
A version of this article first appeared on Medscape.com.
A recent study found that people with obesity have lower blood levels of vitamin D than people of healthy weight. This association of obesity with low vitamin D levels has led to much speculation on whether low vitamin D levels cause obesity or whether obesity causes low vitamin D levels. The interest in this topic is piqued by the possibility that if vitamin D deficiency causes obesity, perhaps treatment could be as simple as providing vitamin D supplementation to enhance weight loss.
What is known about vitamin D’s role in the body?
It’s well known that vitamin D is essential for bone health as well as balancing the minerals calcium and phosphorus, but what is its role in weight management? Approximately 80%-90% of vitamin D in the body is from the skin synthesis of cholecalciferol through ultraviolet B radiation from sun exposure. The normal range of 25-hydroxy vitamin D is measured as nanograms per milliliter (ng/mL). Most experts recommend a level between 20 and 40 ng/mL, but this has been a controversial topic of never-ending debate in the medical literature.
Vitamin D levels and obesity
This has been noted for many years without identifying the underlying reasons beyond the sequestering of vitamin D in adipose tissue, although I’ll discuss other possibilities.
The inverse correlation between vitamin D and obesity has been seen in other diseases, such as cardiovascular disease, hypertension, prediabetes, and insulin resistance, as well as in sarcopenia and aging. Most studies emphasized the correlation between increasing adiposity with vitamin D deficiency in all ethnic and age groups. The causes and potential direct consequences of the vitamin D deficiency state in obesity are not well understood.
Vitamin D and adipose tissue
It’s been proposed that low vitamin D status in obesity might be due to increased vitamin D clearance from serum and enhanced storage of vitamin D by adipose tissue.
In adipose tissue, vitamin D exerts a variety of effects on inflammation, innate immunity, metabolism, and differentiation and apoptosis in many cell types. There is a stronger association between 25(OH)D and visceral fat as compared to subcutaneous adipose tissue, which suggests an influence of inflammation and components of the metabolic syndrome on vitamin D metabolism.
Because vitamin D has anti-inflammatory properties, it’s possible that low vitamin D status contributes to adipose tissue inflammation, a key link between obesity and its associated metabolic complications in obesity. A higher storage of vitamin D in adipose tissue, if accompanied by a parallel increase in the local synthesis of 1,25(OH)2D3 and action, may conceivably modulate adipocyte function as well as the activity of adipose tissue macrophages and hence the level of adipose tissue inflammation. In addition, it seems likely that 1,25(OH)2D3 also regulates the function of macrophages and other immune cell populations within adipose tissue.
It’s well known that vitamin D is stored in body fat, leading to the assumption that this was important in the evolution of vertebrates, including humans, who lived at latitudes where vitamin D could not be made in the winter and vitamin D stores had to be mobilized to maintain vitamin D sufficiency.
What is vitamin D’s role in obesity?
The main question that has eluded an answer so far is this one: Is vitamin D deficiency only a coincidental finding in obesity due to sequestration of the vitamin in fat, or does it have a role in the development of obesity and its complications, such as cardiovascular disease, type 2 diabetes, and hypertension?
Low vitamin D usually leads to impaired calcium absorption in the intestine and a lower calcium level, and eventually enhanced bone turnover and impaired bone mineral density (BMD).
However, it is known that in obesity there is greater BMD than in those who are lean. This leads to the conclusion that because there is a lack of vitamin D deficiency effects on bone in those with obesity, there is not really a vitamin D deficiency, and it may be that the sequestration in adipose tissue leads to a permanent supply that can maintain bone health.
An alternative explanation is that there is greater skeletal loading in obesity, and elevations in hormones such as estrogen and leptin could compensate for the vitamin D deficiency, leading to greater BMD in obesity.
Several other potential mechanisms besides sequestration of vitamin D in adipose tissue have been identified for low vitamin D and obesity. These include impaired hepatic 25-hydroxylation in nonalcoholic fatty liver disease, less sunlight exposure due to lower mobility and different clothing habits in people with obesity vs. their lean counterparts, and adverse dietary habits. For example, people with higher BMIs spend less time exercising outdoors and are more sedentary in general than their lean counterparts. Therefore, they are less likely to get sun exposure because of a decrease in time spent outdoors. Those with higher BMIs also tend to cover their bodies, showing less skin when outdoors than their leaner counterparts, and thus there is likely to be less conversion to vitamin D via skin and sun exposure in people with obesity.
Some studies suggest that an increased level of parathyroid hormone due to vitamin D deficiency promotes lipogenesis because of greater calcium influx in adipocytes. Another hypothesis is that the active form of vitamin D, 1,25(OH) D, inhibits adipogenesis through actions modulated by vitamin D receptors. These studies are promising, but prospective randomized trials are needed to determine whether vitamin D supplementation is a treatment option in preventing obesity. So far, vitamin D supplementation shows inconsistent results.
To conclude, there is a high prevalence of vitamin D deficiency in obesity, most likely because of dilution and sequestration in greater volumes of fat, blood muscle, and liver in obesity. Low vitamin D levels can’t be ruled out as a cause of obesity because of the research showing some interesting findings in vitamin D receptors in adipose tissue. Vitamin D deficiency in obesity doesn’t seem to affect bone mass but could have deleterious effects on other organ systems.
Weight loss improves obesity and complications, including the risk for cardiovascular disease and type 2 diabetes as well as vitamin D deficiency.
What do the guidelines say?
Treatment of vitamin D deficiency requires higher doses in obesity to achieve the same serum concentration compared with lean persons. Maintenance doses should not differ between those with obesity and lean persons.
The association of vitamin D and obesity remains elusive. Studies need to focus on vitamin D, vitamin D receptors, and actions of vitamin D in adipose tissue to investigate this relationship further.
In the meantime, media attention remains focused on the potential treatment and prevention of obesity with the mighty, all-purpose vitamin D, even though there is scant evidence.
Dr. Apovian is codirector at the Center for Weight Management and Wellness at Brigham and Women’s Hospital and professor of medicine in the division of endocrinology, diabetes and hypertension at Harvard University, Boston. She disclosed conflicts of interest with Abbott, Allergan, Altimmune, Bariatrix Nutrition, Cowen and Company, Curavit, Rhythm Pharma, Jazz, Nutrisystem, Roman, Novo Nordisk, EnteroMedics, Gelesis Srl, Zafgen, Xeno, L-Nutra, Tivity, and Real Appeal.
A version of this article first appeared on Medscape.com.
Lupin recalls quinapril tablets because of potential carcinogen
Lupin Pharmaceuticals is recalling four lots of quinapril tablets because of unacceptable levels of the nitrosamine impurity, N-nitroso-quinapril, a potential carcinogen.
Nitrosamines “may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time,” the company says in a recall notice posted on the Food and Drug Administration website.
Lupin says it “has received no reports of illness that appear to relate to this issue.”
Quinapril is an ACE inhibitor used to treat hypertension. Lupin stopped marketing quinapril tablets in September 2022.
The recalled product – quinapril tablets USP 20 mg and 40 mg – are packaged in 90-count bottles and were distributed nationwide to U.S. wholesalers, drug chains, mail order pharmacies, and supermarkets between March 15, 2021, and Sept. 1, 2022.
Lupin is notifying customers to immediately stop distribution of the recalled product and is arranging for the affected product lots to be returned to the company.
Questions regarding this recall should be directed to Inmar Rx Solutions at (877) 538-8445 Monday to Friday between 9:00 a.m. to 5:00 p.m. EST.
Patients and physicians are also advised to report any adverse events or side effects related to the affected products to MedWatch, the FDA’s Safety Information and Adverse Event Reporting program.
Pfizer recalled several lots of quinapril owing to the presence of the same impurity in March 2022and again in April.
A version of this article first appeared on Medscape.com.
Lupin Pharmaceuticals is recalling four lots of quinapril tablets because of unacceptable levels of the nitrosamine impurity, N-nitroso-quinapril, a potential carcinogen.
Nitrosamines “may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time,” the company says in a recall notice posted on the Food and Drug Administration website.
Lupin says it “has received no reports of illness that appear to relate to this issue.”
Quinapril is an ACE inhibitor used to treat hypertension. Lupin stopped marketing quinapril tablets in September 2022.
The recalled product – quinapril tablets USP 20 mg and 40 mg – are packaged in 90-count bottles and were distributed nationwide to U.S. wholesalers, drug chains, mail order pharmacies, and supermarkets between March 15, 2021, and Sept. 1, 2022.
Lupin is notifying customers to immediately stop distribution of the recalled product and is arranging for the affected product lots to be returned to the company.
Questions regarding this recall should be directed to Inmar Rx Solutions at (877) 538-8445 Monday to Friday between 9:00 a.m. to 5:00 p.m. EST.
Patients and physicians are also advised to report any adverse events or side effects related to the affected products to MedWatch, the FDA’s Safety Information and Adverse Event Reporting program.
Pfizer recalled several lots of quinapril owing to the presence of the same impurity in March 2022and again in April.
A version of this article first appeared on Medscape.com.
Lupin Pharmaceuticals is recalling four lots of quinapril tablets because of unacceptable levels of the nitrosamine impurity, N-nitroso-quinapril, a potential carcinogen.
Nitrosamines “may increase the risk of cancer if people are exposed to them above acceptable levels over long periods of time,” the company says in a recall notice posted on the Food and Drug Administration website.
Lupin says it “has received no reports of illness that appear to relate to this issue.”
Quinapril is an ACE inhibitor used to treat hypertension. Lupin stopped marketing quinapril tablets in September 2022.
The recalled product – quinapril tablets USP 20 mg and 40 mg – are packaged in 90-count bottles and were distributed nationwide to U.S. wholesalers, drug chains, mail order pharmacies, and supermarkets between March 15, 2021, and Sept. 1, 2022.
Lupin is notifying customers to immediately stop distribution of the recalled product and is arranging for the affected product lots to be returned to the company.
Questions regarding this recall should be directed to Inmar Rx Solutions at (877) 538-8445 Monday to Friday between 9:00 a.m. to 5:00 p.m. EST.
Patients and physicians are also advised to report any adverse events or side effects related to the affected products to MedWatch, the FDA’s Safety Information and Adverse Event Reporting program.
Pfizer recalled several lots of quinapril owing to the presence of the same impurity in March 2022and again in April.
A version of this article first appeared on Medscape.com.
Kiwifruit found effective for constipation
Kiwifruit can increase the frequency of bowel movements for people with constipation, according to researchers in New Zealand.
“In addition to improved measures of constipation status, there was a significant improvement in stool consistency, reduction in constipation, indigestion/reflux, and abdominal pain resulting in an improved overall level of GI comfort,” write Richard Gearry, MD, PhD, of the University of Otago, New Zealand, and colleagues.
Dr. Gearry and colleagues describe their international multicenter controlled trial of kiwifruit as a constipation treatment in the American Journal of Gastroenterology.
Although constipation is common, previous researchers have reported a high rate of dissatisfaction with pharmaceutical treatments.
Other components of kiwifruit, such as raphides, may alter mucin production, leading to improved laxation.
Several previous studies have suggested that regular consumption of fresh, green kiwifruit may be effective as a treatment for constipation. However, these studies have typically been small with nonstandardized endpoints, Dr. Gearry and colleagues note. In their article, they did not comment on studies of other fresh fruits or vegetables as constipation treatments.
The researchers set out to test the benefits of the fruit in a more rigorous trial. They recruited adults in New Zealand, Italy, and Japan who had either functional constipation or constipation-predominant irritable bowel syndrome (IBS-C), as well as healthy people as controls. The primary difference between functional constipation and IBS-C is that people with IBS-C experience abdominal pain along with constipation, they write.
Between June 12, 2014, and June 17, 2017, 184 participants were enrolled. Participants included 136 women and 48 men, a proportion consistent with the gender prevalence of constipation. The mean age was 30.5 years in Japan, 36.9 years in Italy, and 44.8 years in New Zealand, and the mean body mass index was 20.6 in Japan, 23.0 in Italy, and 25.4 in New Zealand.
For 2 weeks following recruitment, the participants became accustomed to recording their bowel movements with respect to frequency, completeness, spontaneity, stool form, laxative use, and degree of straining.
Participants were then randomly assigned to consume either two ripe green kiwifruit (Actinidia chinensis var. deliciosa “Hayward”) without the skins, or 7.5 g of psyllium per day for 4 weeks. Psyllium is considered a first-line treatment for both the constipation conditions with which participants were diagnosed. The fiber content of psyllium is similar to that of kiwifruit.
After 4 weeks, treatments were stopped for 4 weeks as a washout period, after which patients were switched to the other treatment for another 4 weeks.
The researchers provided them with two bisacodyl (5-g suppositories) as a pharmacologic rescue therapy.
Overall, 169 participants completed the study, and compliance with diary completion was more than 80%.
At the end of the 4-week treatment periods, the people with functional constipation who ate kiwifruit demonstrated an average increase of 1.53 bowel movements per week, a statistically significant increase (P < .0001). Those with IBS-C demonstrated an increase of 1.73 (P = .0001). Both groups reported significantly improved gastrointestinal comfort on the gastrointestinal symptom rating scale.
The frequency of bowel movements in the healthy participants did not increase.
Among those participants who took psyllium, only those with IBS-C experienced a statistically significant increase in the frequency of their bowel movements or a decrease in their gastrointestinal symptoms. Their bowel movements increased by an average of 1.87 per week (P = .0051).
The participants who ate kiwifruit reported softening of stool consistency, reduction in straining, and improvement in quality of life compared with baseline, and these improvements in straining and stool consistency were better than was reported by those who took psyllium.
“Taken in conjunction with previous clinical trials of green kiwifruit and the emerging physiological data from functional studies, consumption of two green kiwifruit can be safely recommended as an effective treatment for constipation in those with functional gastrointestinal disorders that will also provide improvements in symptoms of GI comfort,” the researchers conclude.
Two of the researchers were employed by Zespri International, a cooperative of kiwifruit growers, which partially funded the study and approved its design. The New Zealand study center trial was jointly funded by a grant from the New Zealand government.
A version of this article first appeared on Medscape.com.
Kiwifruit can increase the frequency of bowel movements for people with constipation, according to researchers in New Zealand.
“In addition to improved measures of constipation status, there was a significant improvement in stool consistency, reduction in constipation, indigestion/reflux, and abdominal pain resulting in an improved overall level of GI comfort,” write Richard Gearry, MD, PhD, of the University of Otago, New Zealand, and colleagues.
Dr. Gearry and colleagues describe their international multicenter controlled trial of kiwifruit as a constipation treatment in the American Journal of Gastroenterology.
Although constipation is common, previous researchers have reported a high rate of dissatisfaction with pharmaceutical treatments.
Other components of kiwifruit, such as raphides, may alter mucin production, leading to improved laxation.
Several previous studies have suggested that regular consumption of fresh, green kiwifruit may be effective as a treatment for constipation. However, these studies have typically been small with nonstandardized endpoints, Dr. Gearry and colleagues note. In their article, they did not comment on studies of other fresh fruits or vegetables as constipation treatments.
The researchers set out to test the benefits of the fruit in a more rigorous trial. They recruited adults in New Zealand, Italy, and Japan who had either functional constipation or constipation-predominant irritable bowel syndrome (IBS-C), as well as healthy people as controls. The primary difference between functional constipation and IBS-C is that people with IBS-C experience abdominal pain along with constipation, they write.
Between June 12, 2014, and June 17, 2017, 184 participants were enrolled. Participants included 136 women and 48 men, a proportion consistent with the gender prevalence of constipation. The mean age was 30.5 years in Japan, 36.9 years in Italy, and 44.8 years in New Zealand, and the mean body mass index was 20.6 in Japan, 23.0 in Italy, and 25.4 in New Zealand.
For 2 weeks following recruitment, the participants became accustomed to recording their bowel movements with respect to frequency, completeness, spontaneity, stool form, laxative use, and degree of straining.
Participants were then randomly assigned to consume either two ripe green kiwifruit (Actinidia chinensis var. deliciosa “Hayward”) without the skins, or 7.5 g of psyllium per day for 4 weeks. Psyllium is considered a first-line treatment for both the constipation conditions with which participants were diagnosed. The fiber content of psyllium is similar to that of kiwifruit.
After 4 weeks, treatments were stopped for 4 weeks as a washout period, after which patients were switched to the other treatment for another 4 weeks.
The researchers provided them with two bisacodyl (5-g suppositories) as a pharmacologic rescue therapy.
Overall, 169 participants completed the study, and compliance with diary completion was more than 80%.
At the end of the 4-week treatment periods, the people with functional constipation who ate kiwifruit demonstrated an average increase of 1.53 bowel movements per week, a statistically significant increase (P < .0001). Those with IBS-C demonstrated an increase of 1.73 (P = .0001). Both groups reported significantly improved gastrointestinal comfort on the gastrointestinal symptom rating scale.
The frequency of bowel movements in the healthy participants did not increase.
Among those participants who took psyllium, only those with IBS-C experienced a statistically significant increase in the frequency of their bowel movements or a decrease in their gastrointestinal symptoms. Their bowel movements increased by an average of 1.87 per week (P = .0051).
The participants who ate kiwifruit reported softening of stool consistency, reduction in straining, and improvement in quality of life compared with baseline, and these improvements in straining and stool consistency were better than was reported by those who took psyllium.
“Taken in conjunction with previous clinical trials of green kiwifruit and the emerging physiological data from functional studies, consumption of two green kiwifruit can be safely recommended as an effective treatment for constipation in those with functional gastrointestinal disorders that will also provide improvements in symptoms of GI comfort,” the researchers conclude.
Two of the researchers were employed by Zespri International, a cooperative of kiwifruit growers, which partially funded the study and approved its design. The New Zealand study center trial was jointly funded by a grant from the New Zealand government.
A version of this article first appeared on Medscape.com.
Kiwifruit can increase the frequency of bowel movements for people with constipation, according to researchers in New Zealand.
“In addition to improved measures of constipation status, there was a significant improvement in stool consistency, reduction in constipation, indigestion/reflux, and abdominal pain resulting in an improved overall level of GI comfort,” write Richard Gearry, MD, PhD, of the University of Otago, New Zealand, and colleagues.
Dr. Gearry and colleagues describe their international multicenter controlled trial of kiwifruit as a constipation treatment in the American Journal of Gastroenterology.
Although constipation is common, previous researchers have reported a high rate of dissatisfaction with pharmaceutical treatments.
Other components of kiwifruit, such as raphides, may alter mucin production, leading to improved laxation.
Several previous studies have suggested that regular consumption of fresh, green kiwifruit may be effective as a treatment for constipation. However, these studies have typically been small with nonstandardized endpoints, Dr. Gearry and colleagues note. In their article, they did not comment on studies of other fresh fruits or vegetables as constipation treatments.
The researchers set out to test the benefits of the fruit in a more rigorous trial. They recruited adults in New Zealand, Italy, and Japan who had either functional constipation or constipation-predominant irritable bowel syndrome (IBS-C), as well as healthy people as controls. The primary difference between functional constipation and IBS-C is that people with IBS-C experience abdominal pain along with constipation, they write.
Between June 12, 2014, and June 17, 2017, 184 participants were enrolled. Participants included 136 women and 48 men, a proportion consistent with the gender prevalence of constipation. The mean age was 30.5 years in Japan, 36.9 years in Italy, and 44.8 years in New Zealand, and the mean body mass index was 20.6 in Japan, 23.0 in Italy, and 25.4 in New Zealand.
For 2 weeks following recruitment, the participants became accustomed to recording their bowel movements with respect to frequency, completeness, spontaneity, stool form, laxative use, and degree of straining.
Participants were then randomly assigned to consume either two ripe green kiwifruit (Actinidia chinensis var. deliciosa “Hayward”) without the skins, or 7.5 g of psyllium per day for 4 weeks. Psyllium is considered a first-line treatment for both the constipation conditions with which participants were diagnosed. The fiber content of psyllium is similar to that of kiwifruit.
After 4 weeks, treatments were stopped for 4 weeks as a washout period, after which patients were switched to the other treatment for another 4 weeks.
The researchers provided them with two bisacodyl (5-g suppositories) as a pharmacologic rescue therapy.
Overall, 169 participants completed the study, and compliance with diary completion was more than 80%.
At the end of the 4-week treatment periods, the people with functional constipation who ate kiwifruit demonstrated an average increase of 1.53 bowel movements per week, a statistically significant increase (P < .0001). Those with IBS-C demonstrated an increase of 1.73 (P = .0001). Both groups reported significantly improved gastrointestinal comfort on the gastrointestinal symptom rating scale.
The frequency of bowel movements in the healthy participants did not increase.
Among those participants who took psyllium, only those with IBS-C experienced a statistically significant increase in the frequency of their bowel movements or a decrease in their gastrointestinal symptoms. Their bowel movements increased by an average of 1.87 per week (P = .0051).
The participants who ate kiwifruit reported softening of stool consistency, reduction in straining, and improvement in quality of life compared with baseline, and these improvements in straining and stool consistency were better than was reported by those who took psyllium.
“Taken in conjunction with previous clinical trials of green kiwifruit and the emerging physiological data from functional studies, consumption of two green kiwifruit can be safely recommended as an effective treatment for constipation in those with functional gastrointestinal disorders that will also provide improvements in symptoms of GI comfort,” the researchers conclude.
Two of the researchers were employed by Zespri International, a cooperative of kiwifruit growers, which partially funded the study and approved its design. The New Zealand study center trial was jointly funded by a grant from the New Zealand government.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY
Study evaluates features of alopecia areata in Hispanic/Latinx patients
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY