User login
Screening for tuberculosis: Updated recommendations
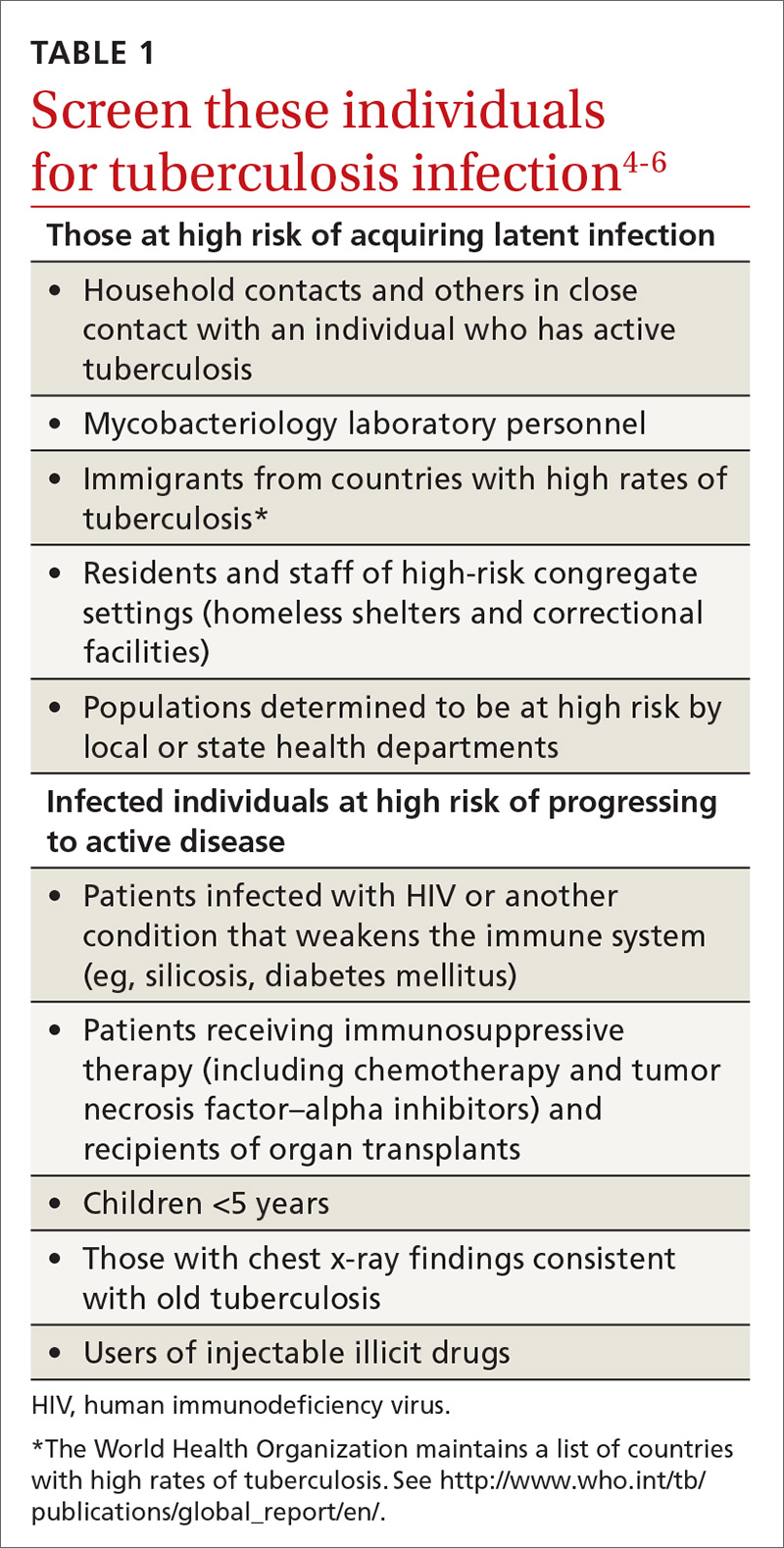
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
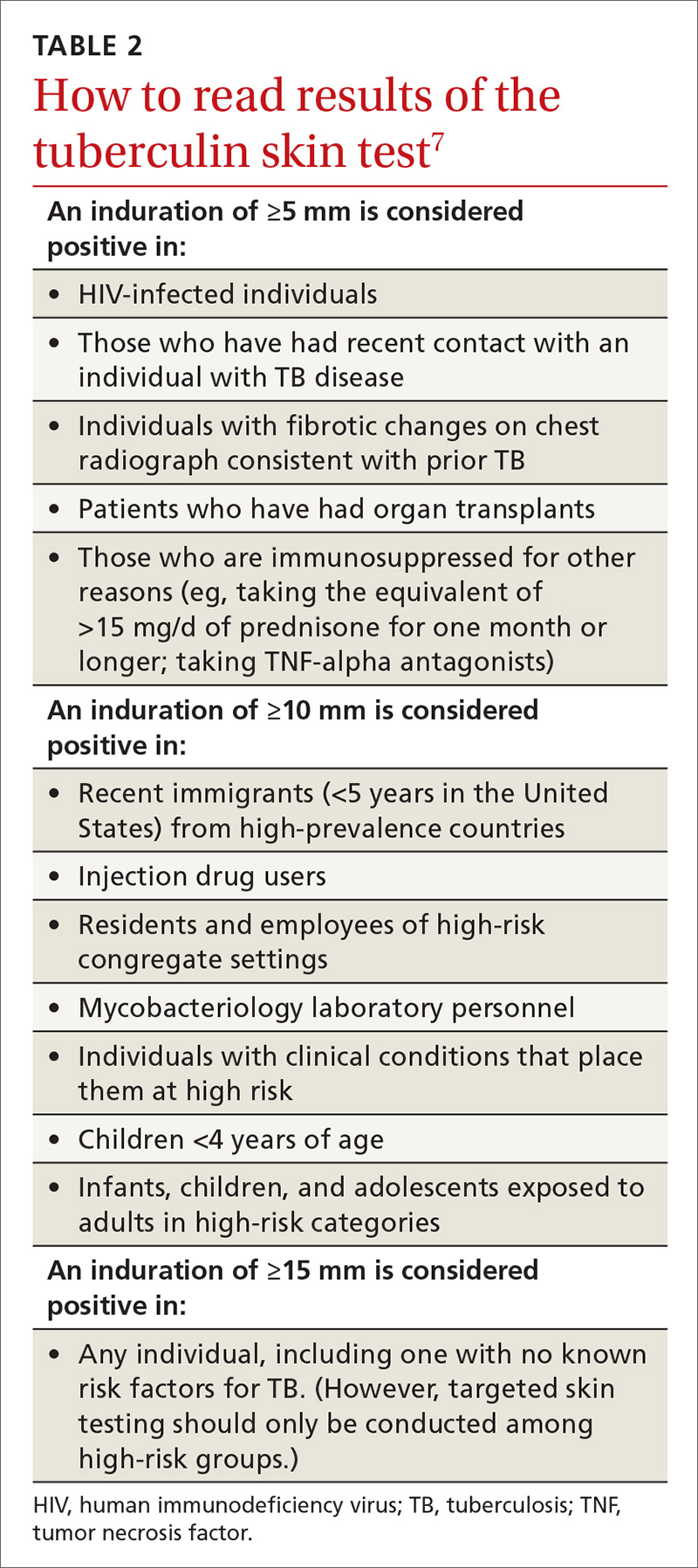
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
Tuberculosis (TB) remains a significant public health problem worldwide with an estimated 10.4 million new cases and 1.7 million deaths having occurred in 2016.1 In that same year, there were 9287 new cases in the United States—the lowest number of TB cases on record.2
TB appears in one of 2 forms: active disease, which causes symptoms, morbidity, and mortality and is a source of transmission to others; and latent TB infection (LTBI), which is asymptomatic and noninfectious but can progress to active disease. The estimated prevalence of LTBI worldwide is 23%,3 although in the United States it is only about 5%.4 The proportion of those with LTBI who will develop active disease is estimated at 5% to 10% and is highly variable depending on risks.4
In the United States, about two-thirds of active TB cases occur among those who are foreign born, whose rate of active disease is 14.6/100,000.2 Five countries account for more than half of foreign-born cases: Mexico, the Philippines, India, Vietnam, and China.2
Who should be tested?
A major public health strategy for controlling TB in the United States is targeted screening for LTBI and treatment to prevent progression to active disease. The US Preventive Services Task Force (USPSTF) recommends screening for LTBI in adults age 18 and older who are at high risk of TB infection.4 This is consistent with recommendations from the Centers for Disease Control and Prevention (CDC), although the CDC also recommends testing infants and children
Two types of testing are available for TB screening: the TB skin test (TST) and the interferon-gamma release assay (IGRA). There are 2 IGRA test options: T-SPOT. TB (Oxford Immunotec) and QuantiFERON-TB Gold (Qiagen). The TST and IGRA each has advantages and disadvantages. The TST must be placed intradermally and read correctly, and the patient must return for the interpretation 48 to 72 hours after placement. Test interpretation depends on the patient’s risk category, with either a 5-mm, 10-mm, or 15-mm induration being classified as a positive result (TABLE 27).
IGRA is a blood test that needs to be processed within a limited time frame and is more expensive than the TST. The USPSTF lists the sensitivity and specificity of each option as follows: TST, using a 10-mm cutoff, 79%, 97%; T-SPOT, 90%, 95%; QuantiFERON-TB Gold In-Tube, 80%, 97%.4
Which test to use?
Recently the CDC, the American Thoracic Society, and the Infectious Diseases Society of America jointly published revised recommendations on TB testing:8
- For children younger than 5 years, TST is the preferred option, although IGRA is acceptable in children older than 3 years of age.
- For individuals at high risk of infection but not at high risk of disease progression, IGRA is recommended if they have received a bacille Calmette-Guerin vaccine or are unlikely to return for TST interpretation.
- For others at high risk of infection but not at high risk of disease progression, IGRA is preferred but TST is acceptable.
- For those who have both a high risk of infection and a high risk of disease progression, evidence is insufficient to recommend one test over another; either type is acceptable.
- For those with neither high risk of infection nor high risk of disease progression, testing is not recommended. However, it may be required by law or for credentialing of some kind (eg, for some health professionals or those who work in schools or nursing homes). If this is the case, IGRA is suggested as the preferred test. If the test result is positive, performing a second test is advised (either TST or an alternative type of IGRA). Consider the individual to be infected only if the second test result is also positive.
If a TB screening result is positive, confirm or rule out active TB by asking about symptoms (cough, fever, weight loss) and performing a chest x-ray. If the radiograph shows signs of active TB, collect 3 sputum samples by induction for analysis by smear microscopy, culture, and, possibly, nucleic acid amplification and rifampin susceptibility testing. Consider consulting your local public health department for advice on, or assistance with, sample collection. Report LTBI to the local health department and seek advice on the appropriate tests and treatments.
Expanded treatment selections
With LTBI there are now 4 treatment options for patients and physicians to consider:9 isoniazid given daily or twice weekly for either 6 or 9 months; isoniazid and rifapentine given once weekly for 3 months; or rifampin given daily for 4 months. Factors influencing treatment selection include a patient’s age, concomitant conditions, and the likelihood of bacterial resistance. Free treatment for LTBI may be available; again, check with your local health department.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
1. WHO. Global tuberculosis report 2017. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed November 8, 2017.
2. Schmit KM, Wansaula Z, Pratt R, et al. Tuberculosis—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:289-294.
3. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016;13(10):e1002152. Available at: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1002152. Accessed November 10, 2017.
4. USPSTF. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:962-969.
5. CDC. Tuberculosis. Who should be tested. Available at: https://www.cdc.gov/tb/topic/testing/whobetested.htm. Accessed November 8, 2017.
6. CDC. Latent tuberculosis infection: a guide for primary health care providers. Targeted testing for tuberculosis. Available at: https://www.cdc.gov/tb/publications/ltbi/targetedtesting.htm#identifyingTBDisease. Accessed November 8, 2017.
7. CDC. TB elimination. Tuberculin skin testing. Available at: https://www.cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Accessed November 8, 2017.
8. Lewinsohn DM, Leonard MK, LoBue PA, el al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of tuberculosis in adults and children. Clin Infect Dis. 2017;64:111-115.
9. CDC. Treatment regimens for latent TB infection (LTBI). Available at: https://www.cdc.gov/tb/topic/treatment/ltbi.htm. Accessed November 8, 2017.
From The Journal of Family Practice | 2017;66(12):755-757.
CDC Updates Guidance on Infants With Possible Zika Infection
Infants with possible prenatal exposure to Zika who test positive for the virus should receive an in-depth ophthalmologic exam, intensified hearing testing, and a thorough neurologic evaluation with brain imaging within one month of birth, according to new interim guidance from the Centers for Disease Control and Prevention (CDC).
The new clinical management guidelines, published in the October 20 issue of Morbidity and Mortality Weekly Report, supersede the CDC guidance issued in August 2016. The update was the product of a forum on the diagnosis, evaluation, and management of Zika in infants that the centers convened with the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Practicing clinicians and federal agency representatives reviewed the evolving body of knowledge on how best to care for these infants. Since Zika emerged as a public health concern, clinicians have reported postnatal onset of some symptoms, including eye abnormalities, incident microcephaly in infants with a normal head circumference at birth, EEG abnormalities, and diaphragmatic paralysis.
Infants With Clinical Findings Consistent With Zika Syndrome
Infants with clinical findings consistent with congenital Zika syndrome who are born to mothers with possible Zika virus exposure in pregnancy should be tested for Zika virus with serum and urine tests. If those tests are negative, and there is no other apparent cause of the symptoms, they should have a CSF sample tested for Zika RNA and IgM Zika antibodies.
By one month, these infants should undergo a head ultrasound and a detailed ophthalmologic exam. The eye exam should pick up any anomalies of the anterior and posterior eye, including microphthalmia, coloboma, intraocular calcifications, optic nerve hypoplasia and atrophy, and macular scarring with focal pigmentary retinal mottling.
By one month, these infants also should undergo auditory brainstem response (ABR) audiometry, especially if the initial newborn hearing screen was done by otoacoustic emissions alone. Zika syndrome can include sensorineural hearing loss, although late-onset hearing loss has not been seen. Therefore, the follow-up ABR previously recommended at four to six months is no longer deemed necessary.
A comprehensive neurologic exam also is recommended. Seizures are sometimes part of Zika syndrome, but infants can also have subclinical EEG abnormalities. Advanced neuroimaging can identify obvious and subtle brain abnormalities, such as cortical thinning, corpus callosum abnormalities, calcifications at the junction of white and gray matter, and ventricular enlargement.
As infants grow, clinicians should be alert for signs of increased intracranial pressure that could signal postnatal hydrocephalus. Diaphragmatic paralysis also has been seen; it manifests as respiratory distress. Dysphagia that interferes with feeding can develop as well.
“The follow-up care of [these infants] requires a multidisciplinary team and an established medical home to facilitate the coordination of care and ensure that abnormal findings are addressed,” said Dr. Adebanjo and colleagues.
Asymptomatic Infants of Mothers With Possible Infection
Infants without clinical findings born to mothers with laboratory evidence of possible Zika virus infection during pregnancy should have the same early head ultrasound, hearing, and eye exams as those with clinical findings. All of these infants also should be tested for Zika virus just as those with clinical findings should be.
If tests are positive, these infants should have all the investigations and follow-up recommended for babies with clinical findings. If laboratory testing is negative, and clinical findings are normal, Zika infection is highly unlikely, and the infants can receive routine care. Clinicians and parents should be on the lookout, however, for new symptoms that might suggest postnatal Zika syndrome.
Asymptomatic Infants Whose Mothers Had Unconfirmed Zika Exposure
Infants without clinical findings consistent with congenital Zika syndrome born to mothers with possible Zika virus exposure in pregnancy, but without laboratory evidence of possible Zika virus infection during pregnancy, constitute a large group. Some women, for example, are never tested during pregnancy, and others have false negative test results. “Because the latter issues are not easily discerned, all mothers with possible exposure to Zika virus during pregnancy who do not have laboratory evidence of possible Zika virus infection, including those who tested negative with currently available technology, should be considered in this group,” said Dr. Adebanjo and colleagues.
The CDC do not recommend further Zika evaluation for these infants unless additional testing confirms maternal infection. For older infants, parents and clinicians should decide together whether further evaluations would be helpful. “If findings consistent with congenital Zika syndrome are identified at any time, referrals to the appropriate specialists should be made, and subsequent evaluation should follow recommendations for infants with clinical findings consistent with congenital Zika syndrome,” said the authors.
The CDC also reiterated their special recommendations for infants who had a prenatal diagnosis of Zika infection. For now, these recommendations remain unchanged, although “as more data become available, understanding of the diagnostic role of prenatal ultrasound and amniocentesis … will improve, and guidance will be updated.”
The optimal timing for a Zika diagnostic ultrasound is uncertain. The CDC recommend that serial ultrasounds be performed every three to four weeks for women with laboratory-confirmed prenatal Zika exposure. Women with possible exposure need only routine ultrasound screenings.
While Zika RNA has been identified in amniotic fluid, there is no consensus on the value of amniocentesis as a prenatal diagnostic tool. Investigations of serial amniocentesis suggest that viral shedding into the amniotic fluid might be transient. If amniocentesis is performed for other reasons, Zika nucleic acid testing can be incorporated.
A shared decision-making process about screening is key, said Dr. Adebanjo and colleagues. “For example, serial ultrasound examinations might be inconvenient, unpleasant, and expensive, and might prompt unnecessary interventions; amniocentesis carries additional known risks such as fetal loss. These potential harms of prenatal screening for congenital Zika syndrome might outweigh the clinical benefits for some patients; therefore, these decisions should be individualized.”
—Michele G. Sullivan
Suggested Reading
Adebanjo T, Godfred-Cato S, Viens L, et al. Update: Interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017;66(41):1089-1099.
Infants with possible prenatal exposure to Zika who test positive for the virus should receive an in-depth ophthalmologic exam, intensified hearing testing, and a thorough neurologic evaluation with brain imaging within one month of birth, according to new interim guidance from the Centers for Disease Control and Prevention (CDC).
The new clinical management guidelines, published in the October 20 issue of Morbidity and Mortality Weekly Report, supersede the CDC guidance issued in August 2016. The update was the product of a forum on the diagnosis, evaluation, and management of Zika in infants that the centers convened with the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Practicing clinicians and federal agency representatives reviewed the evolving body of knowledge on how best to care for these infants. Since Zika emerged as a public health concern, clinicians have reported postnatal onset of some symptoms, including eye abnormalities, incident microcephaly in infants with a normal head circumference at birth, EEG abnormalities, and diaphragmatic paralysis.
Infants With Clinical Findings Consistent With Zika Syndrome
Infants with clinical findings consistent with congenital Zika syndrome who are born to mothers with possible Zika virus exposure in pregnancy should be tested for Zika virus with serum and urine tests. If those tests are negative, and there is no other apparent cause of the symptoms, they should have a CSF sample tested for Zika RNA and IgM Zika antibodies.
By one month, these infants should undergo a head ultrasound and a detailed ophthalmologic exam. The eye exam should pick up any anomalies of the anterior and posterior eye, including microphthalmia, coloboma, intraocular calcifications, optic nerve hypoplasia and atrophy, and macular scarring with focal pigmentary retinal mottling.
By one month, these infants also should undergo auditory brainstem response (ABR) audiometry, especially if the initial newborn hearing screen was done by otoacoustic emissions alone. Zika syndrome can include sensorineural hearing loss, although late-onset hearing loss has not been seen. Therefore, the follow-up ABR previously recommended at four to six months is no longer deemed necessary.
A comprehensive neurologic exam also is recommended. Seizures are sometimes part of Zika syndrome, but infants can also have subclinical EEG abnormalities. Advanced neuroimaging can identify obvious and subtle brain abnormalities, such as cortical thinning, corpus callosum abnormalities, calcifications at the junction of white and gray matter, and ventricular enlargement.
As infants grow, clinicians should be alert for signs of increased intracranial pressure that could signal postnatal hydrocephalus. Diaphragmatic paralysis also has been seen; it manifests as respiratory distress. Dysphagia that interferes with feeding can develop as well.
“The follow-up care of [these infants] requires a multidisciplinary team and an established medical home to facilitate the coordination of care and ensure that abnormal findings are addressed,” said Dr. Adebanjo and colleagues.
Asymptomatic Infants of Mothers With Possible Infection
Infants without clinical findings born to mothers with laboratory evidence of possible Zika virus infection during pregnancy should have the same early head ultrasound, hearing, and eye exams as those with clinical findings. All of these infants also should be tested for Zika virus just as those with clinical findings should be.
If tests are positive, these infants should have all the investigations and follow-up recommended for babies with clinical findings. If laboratory testing is negative, and clinical findings are normal, Zika infection is highly unlikely, and the infants can receive routine care. Clinicians and parents should be on the lookout, however, for new symptoms that might suggest postnatal Zika syndrome.
Asymptomatic Infants Whose Mothers Had Unconfirmed Zika Exposure
Infants without clinical findings consistent with congenital Zika syndrome born to mothers with possible Zika virus exposure in pregnancy, but without laboratory evidence of possible Zika virus infection during pregnancy, constitute a large group. Some women, for example, are never tested during pregnancy, and others have false negative test results. “Because the latter issues are not easily discerned, all mothers with possible exposure to Zika virus during pregnancy who do not have laboratory evidence of possible Zika virus infection, including those who tested negative with currently available technology, should be considered in this group,” said Dr. Adebanjo and colleagues.
The CDC do not recommend further Zika evaluation for these infants unless additional testing confirms maternal infection. For older infants, parents and clinicians should decide together whether further evaluations would be helpful. “If findings consistent with congenital Zika syndrome are identified at any time, referrals to the appropriate specialists should be made, and subsequent evaluation should follow recommendations for infants with clinical findings consistent with congenital Zika syndrome,” said the authors.
The CDC also reiterated their special recommendations for infants who had a prenatal diagnosis of Zika infection. For now, these recommendations remain unchanged, although “as more data become available, understanding of the diagnostic role of prenatal ultrasound and amniocentesis … will improve, and guidance will be updated.”
The optimal timing for a Zika diagnostic ultrasound is uncertain. The CDC recommend that serial ultrasounds be performed every three to four weeks for women with laboratory-confirmed prenatal Zika exposure. Women with possible exposure need only routine ultrasound screenings.
While Zika RNA has been identified in amniotic fluid, there is no consensus on the value of amniocentesis as a prenatal diagnostic tool. Investigations of serial amniocentesis suggest that viral shedding into the amniotic fluid might be transient. If amniocentesis is performed for other reasons, Zika nucleic acid testing can be incorporated.
A shared decision-making process about screening is key, said Dr. Adebanjo and colleagues. “For example, serial ultrasound examinations might be inconvenient, unpleasant, and expensive, and might prompt unnecessary interventions; amniocentesis carries additional known risks such as fetal loss. These potential harms of prenatal screening for congenital Zika syndrome might outweigh the clinical benefits for some patients; therefore, these decisions should be individualized.”
—Michele G. Sullivan
Suggested Reading
Adebanjo T, Godfred-Cato S, Viens L, et al. Update: Interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017;66(41):1089-1099.
Infants with possible prenatal exposure to Zika who test positive for the virus should receive an in-depth ophthalmologic exam, intensified hearing testing, and a thorough neurologic evaluation with brain imaging within one month of birth, according to new interim guidance from the Centers for Disease Control and Prevention (CDC).
The new clinical management guidelines, published in the October 20 issue of Morbidity and Mortality Weekly Report, supersede the CDC guidance issued in August 2016. The update was the product of a forum on the diagnosis, evaluation, and management of Zika in infants that the centers convened with the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. Practicing clinicians and federal agency representatives reviewed the evolving body of knowledge on how best to care for these infants. Since Zika emerged as a public health concern, clinicians have reported postnatal onset of some symptoms, including eye abnormalities, incident microcephaly in infants with a normal head circumference at birth, EEG abnormalities, and diaphragmatic paralysis.
Infants With Clinical Findings Consistent With Zika Syndrome
Infants with clinical findings consistent with congenital Zika syndrome who are born to mothers with possible Zika virus exposure in pregnancy should be tested for Zika virus with serum and urine tests. If those tests are negative, and there is no other apparent cause of the symptoms, they should have a CSF sample tested for Zika RNA and IgM Zika antibodies.
By one month, these infants should undergo a head ultrasound and a detailed ophthalmologic exam. The eye exam should pick up any anomalies of the anterior and posterior eye, including microphthalmia, coloboma, intraocular calcifications, optic nerve hypoplasia and atrophy, and macular scarring with focal pigmentary retinal mottling.
By one month, these infants also should undergo auditory brainstem response (ABR) audiometry, especially if the initial newborn hearing screen was done by otoacoustic emissions alone. Zika syndrome can include sensorineural hearing loss, although late-onset hearing loss has not been seen. Therefore, the follow-up ABR previously recommended at four to six months is no longer deemed necessary.
A comprehensive neurologic exam also is recommended. Seizures are sometimes part of Zika syndrome, but infants can also have subclinical EEG abnormalities. Advanced neuroimaging can identify obvious and subtle brain abnormalities, such as cortical thinning, corpus callosum abnormalities, calcifications at the junction of white and gray matter, and ventricular enlargement.
As infants grow, clinicians should be alert for signs of increased intracranial pressure that could signal postnatal hydrocephalus. Diaphragmatic paralysis also has been seen; it manifests as respiratory distress. Dysphagia that interferes with feeding can develop as well.
“The follow-up care of [these infants] requires a multidisciplinary team and an established medical home to facilitate the coordination of care and ensure that abnormal findings are addressed,” said Dr. Adebanjo and colleagues.
Asymptomatic Infants of Mothers With Possible Infection
Infants without clinical findings born to mothers with laboratory evidence of possible Zika virus infection during pregnancy should have the same early head ultrasound, hearing, and eye exams as those with clinical findings. All of these infants also should be tested for Zika virus just as those with clinical findings should be.
If tests are positive, these infants should have all the investigations and follow-up recommended for babies with clinical findings. If laboratory testing is negative, and clinical findings are normal, Zika infection is highly unlikely, and the infants can receive routine care. Clinicians and parents should be on the lookout, however, for new symptoms that might suggest postnatal Zika syndrome.
Asymptomatic Infants Whose Mothers Had Unconfirmed Zika Exposure
Infants without clinical findings consistent with congenital Zika syndrome born to mothers with possible Zika virus exposure in pregnancy, but without laboratory evidence of possible Zika virus infection during pregnancy, constitute a large group. Some women, for example, are never tested during pregnancy, and others have false negative test results. “Because the latter issues are not easily discerned, all mothers with possible exposure to Zika virus during pregnancy who do not have laboratory evidence of possible Zika virus infection, including those who tested negative with currently available technology, should be considered in this group,” said Dr. Adebanjo and colleagues.
The CDC do not recommend further Zika evaluation for these infants unless additional testing confirms maternal infection. For older infants, parents and clinicians should decide together whether further evaluations would be helpful. “If findings consistent with congenital Zika syndrome are identified at any time, referrals to the appropriate specialists should be made, and subsequent evaluation should follow recommendations for infants with clinical findings consistent with congenital Zika syndrome,” said the authors.
The CDC also reiterated their special recommendations for infants who had a prenatal diagnosis of Zika infection. For now, these recommendations remain unchanged, although “as more data become available, understanding of the diagnostic role of prenatal ultrasound and amniocentesis … will improve, and guidance will be updated.”
The optimal timing for a Zika diagnostic ultrasound is uncertain. The CDC recommend that serial ultrasounds be performed every three to four weeks for women with laboratory-confirmed prenatal Zika exposure. Women with possible exposure need only routine ultrasound screenings.
While Zika RNA has been identified in amniotic fluid, there is no consensus on the value of amniocentesis as a prenatal diagnostic tool. Investigations of serial amniocentesis suggest that viral shedding into the amniotic fluid might be transient. If amniocentesis is performed for other reasons, Zika nucleic acid testing can be incorporated.
A shared decision-making process about screening is key, said Dr. Adebanjo and colleagues. “For example, serial ultrasound examinations might be inconvenient, unpleasant, and expensive, and might prompt unnecessary interventions; amniocentesis carries additional known risks such as fetal loss. These potential harms of prenatal screening for congenital Zika syndrome might outweigh the clinical benefits for some patients; therefore, these decisions should be individualized.”
—Michele G. Sullivan
Suggested Reading
Adebanjo T, Godfred-Cato S, Viens L, et al. Update: Interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR Morb Mortal Wkly Rep. 2017;66(41):1089-1099.
VIDEO: U.S. hypertension guidelines reset threshold to 130/80 mm Hg
ANAHEIM, CALIF. – Thirty million Americans became hypertensive overnight on Nov. 13 with the introduction of new high blood pressure guidelines from the American College of Cardiology and American Heart Association.
That happened by resetting the definition of adult hypertension from the long-standing threshold of 140/90 mm Hg to a blood pressure at or above 130/80 mm Hg, a change that jumps the U.S. adult prevalence of hypertension from roughly 32% to 46%. Nearly half of all U.S. adults now have hypertension, bringing the total national hypertensive population to a staggering 103 million.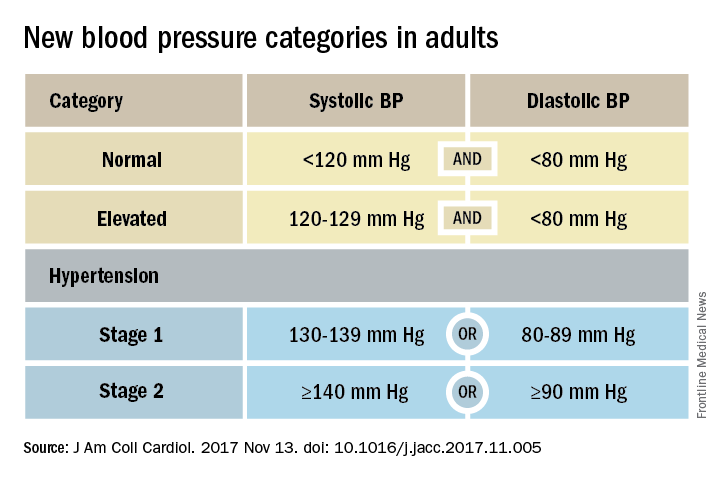
Goal is to transform care
But the new guidelines (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.005) for preventing, detecting, evaluating, and managing adult hypertension do lots more than just shake up the epidemiology of high blood pressure. With 106 total recommendations, the guidelines seek to transform every aspect of blood pressure in American medical practice, starting with how it’s measured and stretching to redefine applications of medical systems to try to ensure that every person with a blood pressure that truly falls outside the redefined limits gets a comprehensive package of interventions.
Many of these are “seismic changes,” said Lawrence J. Appel, MD. He particularly cited as seismic the new classification of stage 1 hypertension as a pressure at or above 130/80 mm Hg, the emphasis on using some form of out-of-office blood pressure measurement to confirm a diagnosis, the use of risk assessment when deciding whether to treat certain patients with drugs, and the same blood pressure goal of less than 130/80 mm Hg for all hypertensives, regardless of age, as long as they remain ambulatory and community dwelling.
One goal for all adults
“The systolic blood pressure goal for older people has gone from 140 mm Hg to 150 mm Hg and now to 130 mm Hg in the space of 2-3 years,” commented Dr. Appel, professor of epidemiology at Johns Hopkins University in Baltimore and not involved in the guideline-writing process.
In fact, the guidelines simplified the treatment goal all around, to less than 130/80 mm Hg for patients with diabetes, those with chronic kidney disease, and the elderly; that goal remains the same for all adults.
“It will be clearer and easier now that everyone should be less than 130/80 mm Hg. You won’t need to remember a second target,” said Sandra J. Taler, MD, a nephrologist and professor of medicine at the Mayo Clinic in Rochester, Minn., and a member of the guidelines task force.
“Some people may be upset that we changed the rules on them. They had normal blood pressure yesterday, and today it’s high. But it’s a good awakening, especially for using lifestyle interventions,” Dr. Taler said in an interview.
Preferred intervention: Lifestyle, not drugs
Lifestyle optimization is repeatedly cited as the cornerstone of intervention for everyone, including those with elevated blood pressure with a systolic pressure of 120-129 mm Hg, and as the only endorsed intervention for patients with hypertension of 130-139 mm Hg but below a 10% risk for a cardiovascular disease event during the next 10 years on the American College of Cardiology’s online risk calculator. The guidelines list six lifestyle goals: weight loss, following a DASH diet, reducing sodium, enhancing potassium, 90-150 min/wk of physical activity, and moderate alcohol intake.
Team-based care essential
The guidelines also put unprecedented emphasis on using a team-based management approach, which means having nurses, nurse practitioners, pharmacists, dietitians, and other clinicians, allowing for more frequent and focused care. Dr. Whelton and others cited in particular the VA Health System and Kaiser-Permanente as operating team-based and system-driven blood pressure management programs that have resulted in control rates for more than 90% of hypertensive patients. The team-based approach is also a key in the Target:BP program that the American Heart Association and American Medical Association founded. Target:BP will be instrumental in promoting implementation of the new guidelines, Dr. Carey said. Another systems recommendation is that every patient with hypertension should have a “clear, detailed, and current evidence-based plan of care.”
“Using nurse practitioners, physician assistants, and pharmacists has been shown to improve blood pressure levels,” and health systems that use this approach have had “great success,” commented Donald M. Lloyd-Jones, MD, professor and chairman of preventive medicine at Northwestern University in Chicago and not part of the guidelines task force. Some systems have used this approach to achieve high levels of blood pressure control. Now that financial penalties and incentives from payers also exist to push for higher levels of blood pressure control, the alignment of financial and health incentives should result in big changes, Dr. Lloyd-Jones predicted in a video interview.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Thirty million Americans became hypertensive overnight on Nov. 13 with the introduction of new high blood pressure guidelines from the American College of Cardiology and American Heart Association.
That happened by resetting the definition of adult hypertension from the long-standing threshold of 140/90 mm Hg to a blood pressure at or above 130/80 mm Hg, a change that jumps the U.S. adult prevalence of hypertension from roughly 32% to 46%. Nearly half of all U.S. adults now have hypertension, bringing the total national hypertensive population to a staggering 103 million.
Goal is to transform care
But the new guidelines (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.005) for preventing, detecting, evaluating, and managing adult hypertension do lots more than just shake up the epidemiology of high blood pressure. With 106 total recommendations, the guidelines seek to transform every aspect of blood pressure in American medical practice, starting with how it’s measured and stretching to redefine applications of medical systems to try to ensure that every person with a blood pressure that truly falls outside the redefined limits gets a comprehensive package of interventions.
Many of these are “seismic changes,” said Lawrence J. Appel, MD. He particularly cited as seismic the new classification of stage 1 hypertension as a pressure at or above 130/80 mm Hg, the emphasis on using some form of out-of-office blood pressure measurement to confirm a diagnosis, the use of risk assessment when deciding whether to treat certain patients with drugs, and the same blood pressure goal of less than 130/80 mm Hg for all hypertensives, regardless of age, as long as they remain ambulatory and community dwelling.
One goal for all adults
“The systolic blood pressure goal for older people has gone from 140 mm Hg to 150 mm Hg and now to 130 mm Hg in the space of 2-3 years,” commented Dr. Appel, professor of epidemiology at Johns Hopkins University in Baltimore and not involved in the guideline-writing process.
In fact, the guidelines simplified the treatment goal all around, to less than 130/80 mm Hg for patients with diabetes, those with chronic kidney disease, and the elderly; that goal remains the same for all adults.
“It will be clearer and easier now that everyone should be less than 130/80 mm Hg. You won’t need to remember a second target,” said Sandra J. Taler, MD, a nephrologist and professor of medicine at the Mayo Clinic in Rochester, Minn., and a member of the guidelines task force.
“Some people may be upset that we changed the rules on them. They had normal blood pressure yesterday, and today it’s high. But it’s a good awakening, especially for using lifestyle interventions,” Dr. Taler said in an interview.
Preferred intervention: Lifestyle, not drugs
Lifestyle optimization is repeatedly cited as the cornerstone of intervention for everyone, including those with elevated blood pressure with a systolic pressure of 120-129 mm Hg, and as the only endorsed intervention for patients with hypertension of 130-139 mm Hg but below a 10% risk for a cardiovascular disease event during the next 10 years on the American College of Cardiology’s online risk calculator. The guidelines list six lifestyle goals: weight loss, following a DASH diet, reducing sodium, enhancing potassium, 90-150 min/wk of physical activity, and moderate alcohol intake.
Team-based care essential
The guidelines also put unprecedented emphasis on using a team-based management approach, which means having nurses, nurse practitioners, pharmacists, dietitians, and other clinicians, allowing for more frequent and focused care. Dr. Whelton and others cited in particular the VA Health System and Kaiser-Permanente as operating team-based and system-driven blood pressure management programs that have resulted in control rates for more than 90% of hypertensive patients. The team-based approach is also a key in the Target:BP program that the American Heart Association and American Medical Association founded. Target:BP will be instrumental in promoting implementation of the new guidelines, Dr. Carey said. Another systems recommendation is that every patient with hypertension should have a “clear, detailed, and current evidence-based plan of care.”
“Using nurse practitioners, physician assistants, and pharmacists has been shown to improve blood pressure levels,” and health systems that use this approach have had “great success,” commented Donald M. Lloyd-Jones, MD, professor and chairman of preventive medicine at Northwestern University in Chicago and not part of the guidelines task force. Some systems have used this approach to achieve high levels of blood pressure control. Now that financial penalties and incentives from payers also exist to push for higher levels of blood pressure control, the alignment of financial and health incentives should result in big changes, Dr. Lloyd-Jones predicted in a video interview.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
ANAHEIM, CALIF. – Thirty million Americans became hypertensive overnight on Nov. 13 with the introduction of new high blood pressure guidelines from the American College of Cardiology and American Heart Association.
That happened by resetting the definition of adult hypertension from the long-standing threshold of 140/90 mm Hg to a blood pressure at or above 130/80 mm Hg, a change that jumps the U.S. adult prevalence of hypertension from roughly 32% to 46%. Nearly half of all U.S. adults now have hypertension, bringing the total national hypertensive population to a staggering 103 million.
Goal is to transform care
But the new guidelines (J Am Coll Cardiol. 2017 Nov 13. doi: 10.1016/j.jacc.2017.11.005) for preventing, detecting, evaluating, and managing adult hypertension do lots more than just shake up the epidemiology of high blood pressure. With 106 total recommendations, the guidelines seek to transform every aspect of blood pressure in American medical practice, starting with how it’s measured and stretching to redefine applications of medical systems to try to ensure that every person with a blood pressure that truly falls outside the redefined limits gets a comprehensive package of interventions.
Many of these are “seismic changes,” said Lawrence J. Appel, MD. He particularly cited as seismic the new classification of stage 1 hypertension as a pressure at or above 130/80 mm Hg, the emphasis on using some form of out-of-office blood pressure measurement to confirm a diagnosis, the use of risk assessment when deciding whether to treat certain patients with drugs, and the same blood pressure goal of less than 130/80 mm Hg for all hypertensives, regardless of age, as long as they remain ambulatory and community dwelling.
One goal for all adults
“The systolic blood pressure goal for older people has gone from 140 mm Hg to 150 mm Hg and now to 130 mm Hg in the space of 2-3 years,” commented Dr. Appel, professor of epidemiology at Johns Hopkins University in Baltimore and not involved in the guideline-writing process.
In fact, the guidelines simplified the treatment goal all around, to less than 130/80 mm Hg for patients with diabetes, those with chronic kidney disease, and the elderly; that goal remains the same for all adults.
“It will be clearer and easier now that everyone should be less than 130/80 mm Hg. You won’t need to remember a second target,” said Sandra J. Taler, MD, a nephrologist and professor of medicine at the Mayo Clinic in Rochester, Minn., and a member of the guidelines task force.
“Some people may be upset that we changed the rules on them. They had normal blood pressure yesterday, and today it’s high. But it’s a good awakening, especially for using lifestyle interventions,” Dr. Taler said in an interview.
Preferred intervention: Lifestyle, not drugs
Lifestyle optimization is repeatedly cited as the cornerstone of intervention for everyone, including those with elevated blood pressure with a systolic pressure of 120-129 mm Hg, and as the only endorsed intervention for patients with hypertension of 130-139 mm Hg but below a 10% risk for a cardiovascular disease event during the next 10 years on the American College of Cardiology’s online risk calculator. The guidelines list six lifestyle goals: weight loss, following a DASH diet, reducing sodium, enhancing potassium, 90-150 min/wk of physical activity, and moderate alcohol intake.
Team-based care essential
The guidelines also put unprecedented emphasis on using a team-based management approach, which means having nurses, nurse practitioners, pharmacists, dietitians, and other clinicians, allowing for more frequent and focused care. Dr. Whelton and others cited in particular the VA Health System and Kaiser-Permanente as operating team-based and system-driven blood pressure management programs that have resulted in control rates for more than 90% of hypertensive patients. The team-based approach is also a key in the Target:BP program that the American Heart Association and American Medical Association founded. Target:BP will be instrumental in promoting implementation of the new guidelines, Dr. Carey said. Another systems recommendation is that every patient with hypertension should have a “clear, detailed, and current evidence-based plan of care.”
“Using nurse practitioners, physician assistants, and pharmacists has been shown to improve blood pressure levels,” and health systems that use this approach have had “great success,” commented Donald M. Lloyd-Jones, MD, professor and chairman of preventive medicine at Northwestern University in Chicago and not part of the guidelines task force. Some systems have used this approach to achieve high levels of blood pressure control. Now that financial penalties and incentives from payers also exist to push for higher levels of blood pressure control, the alignment of financial and health incentives should result in big changes, Dr. Lloyd-Jones predicted in a video interview.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
VIDEO: New PsA guideline expected in 2018
SAN DIEGO – For the first time, a forthcoming evidence-based guideline for the management of psoriatic arthritis recommends tumor necrosis factor inhibitor biologics as first-line therapy.
“Guidelines that have been around for the last several years have been skirting around the fact that there’s really no evidence that methotrexate works for PsA,” Dafna D. Gladman, MD, said during a press briefing at the annual meeting of the American College of Rheumatology. “So it’s refreshing and reassuring that when you do an appropriate, evidence-based approach, you finally find the truth in front of you, and you have TNF inhibitors as the first-line treatment. Obviously, they’re not for everybody. There are patients in whom we cannot use TNF inhibitors, either because they don’t like needles, or because they have contraindications to getting these particular needles, but at least we have a recommendation for the use of these drugs as a first-line treatment.”
“At first, I wasn’t a big fan of the idea of the GRADE guidelines because the number of questions blows up so fast, [but] it really makes you focus on what the most common [clinical] settings are,” said another core oversight team member, Alexis Ogdie, MD, a rheumatologist at the University of Pennsylvania, Philadelphia. “These guidelines also reveal the major gap of no head-to-head studies. I think we’ve known that, but this really called that out as important. When we’re making a treatment decision between [drugs] A and B, we need those studies to be able to better understand how to treat our patients, rather than using the data from one trial to make a decision. ... For my patients, I’m excited that I can now use a TNF inhibitor as a first-line agent. When we have patients come in with very severe disease, occasionally they also have severe psoriasis, so we’ve been able to use TNF inhibitors as first-line treatment in some of our patients in Pennsylvania. This differs state by state. But the exciting thing is that they get better so fast and you don’t have to tell them to wait 12 weeks for methotrexate to work.”
The ACR/NPF guideline is currently under peer review and is expected to be published in Arthritis & Rheumatology, Arthritis Care & Research, and the Journal of Psoriasis and Psoriatic Arthritis in the spring or summer of 2018. It focuses on common PsA patients, not exceptional cases. It includes recommendations on the management of patients with active PsA that is defined by the patients’ self-report and judged by the examining clinician to be caused by PsA, based on the on the presence of at least one of the following: actively inflamed joints; dactylitis; enthesitis; axial disease; active skin and/or nail involvement; and/or extra-articular manifestations such as uveitis or inflammatory bowel disease. Authors of the guideline considered cost as one of many possible factors affecting the use of the recommendations, but explicit cost-effectiveness analyses were not conducted. Also, since the NPF and the American Academy of Dermatology are concurrently developing a psoriasis treatment guideline, the treatment of skin psoriasis was not included in the guideline.
According to the guideline’s principal investigator Jasvinder Singh, MD, professor of medicine and epidemiology at the University of Alabama at Birmingham, the guideline will include 80 recommendations, 75 (94%) that are rated as “conditional,” and 5 (6%) that are rated as “strong,” based on the quality of evidence in the existing medical literature. “Most of our treatment guidelines rely on very low-to-moderate quality evidence, which means that there needs to be an active discussion between the physician and the patient with regard to which treatment to choose,” said Dr. Singh, who is also a staff rheumatologist at the Birmingham Veterans Affairs Medical Center and who led development of the 2012 and 2015 ACR treatment guidelines for RA. “When you’re not choosing the preferred treatment, there are defined specific recommendations under which that second treatment may be preferred over the first treatment.”
During a separate session at the meeting, Dr. Singh unveiled a few of the draft recommendations. One calls for using a treat-to-target strategy over not using one. In the setting of immunizing patients who are receiving a biologic, another recommendation calls for clinicians to start the indicated biologic and administer killed vaccines (as indicated) in patients with active PsA rather than delaying the biologic to give the killed vaccines. In addition, delaying the start of the indicated biologic is recommended over not delaying in order to administer a live attenuated vaccine in patients with active PsA. When patients continue to have with active PsA despite being on a TNF inhibitor, the draft guideline recommends switching to a different TNF inhibitor rather than an IL-17 inhibitor, an IL-12/IL-23 inhibitor, abatacept (Orencia), tofacitinib (Xeljanz), or adding methotrexate. If PsA is still active, the guideline recommends switching to an IL-17 inhibitor instead of an IL-12/IL-23 inhibitor, abatacept, or tofacitinib. If PsA is still active, the guideline recommends switching to an IL-12/IL-23 inhibitor over abatacept or tofacitinib.
The guideline also includes suggestions for nonpharmacologic treatments, including recommending low-impact exercise over high-impact exercise, occupational therapy, physical therapy, and weight loss. It also includes a strong recommendation to provide smoking cessation advice to patients.
Dr. Singh acknowledged significant research gaps in the current PsA medical literature, including no head-to-head comparisons of treatments. He said that the field also could benefit from specific studies for enthesitis, axial disease, and arthritis mutilans; randomized trials of nonpharmacologic interventions; more trials of monotherapy vs. combination therapy; vaccination trials for live attenuated vaccines; trials and registry studies of patients with common comorbidities, and studies of NSAIDs and glucocorticoids, to define their role.
Possible topics for future PsA guidelines, he continued, include treatment options for patients for whom biologic medication is not an option; use of therapies in pregnancy and conception; incorporation of high-quality cost or cost-effectiveness analysis into recommendations; and the role of other comorbidities, such as fibromyalgia, hepatitis, depression/anxiety, malignancy, and cardiovascular disease.
“Evidence-based medicine needs to be practiced, even in situations where it’s difficult to get a drug,” Dr. Gladman said. “One of the things we hope will happen in the near future is that companies will start doing head-to-head studies, to help us support evidence-based recommendations in the future.”
None of the speakers reported having relevant financial disclosures.
SAN DIEGO – For the first time, a forthcoming evidence-based guideline for the management of psoriatic arthritis recommends tumor necrosis factor inhibitor biologics as first-line therapy.
“Guidelines that have been around for the last several years have been skirting around the fact that there’s really no evidence that methotrexate works for PsA,” Dafna D. Gladman, MD, said during a press briefing at the annual meeting of the American College of Rheumatology. “So it’s refreshing and reassuring that when you do an appropriate, evidence-based approach, you finally find the truth in front of you, and you have TNF inhibitors as the first-line treatment. Obviously, they’re not for everybody. There are patients in whom we cannot use TNF inhibitors, either because they don’t like needles, or because they have contraindications to getting these particular needles, but at least we have a recommendation for the use of these drugs as a first-line treatment.”
“At first, I wasn’t a big fan of the idea of the GRADE guidelines because the number of questions blows up so fast, [but] it really makes you focus on what the most common [clinical] settings are,” said another core oversight team member, Alexis Ogdie, MD, a rheumatologist at the University of Pennsylvania, Philadelphia. “These guidelines also reveal the major gap of no head-to-head studies. I think we’ve known that, but this really called that out as important. When we’re making a treatment decision between [drugs] A and B, we need those studies to be able to better understand how to treat our patients, rather than using the data from one trial to make a decision. ... For my patients, I’m excited that I can now use a TNF inhibitor as a first-line agent. When we have patients come in with very severe disease, occasionally they also have severe psoriasis, so we’ve been able to use TNF inhibitors as first-line treatment in some of our patients in Pennsylvania. This differs state by state. But the exciting thing is that they get better so fast and you don’t have to tell them to wait 12 weeks for methotrexate to work.”
The ACR/NPF guideline is currently under peer review and is expected to be published in Arthritis & Rheumatology, Arthritis Care & Research, and the Journal of Psoriasis and Psoriatic Arthritis in the spring or summer of 2018. It focuses on common PsA patients, not exceptional cases. It includes recommendations on the management of patients with active PsA that is defined by the patients’ self-report and judged by the examining clinician to be caused by PsA, based on the on the presence of at least one of the following: actively inflamed joints; dactylitis; enthesitis; axial disease; active skin and/or nail involvement; and/or extra-articular manifestations such as uveitis or inflammatory bowel disease. Authors of the guideline considered cost as one of many possible factors affecting the use of the recommendations, but explicit cost-effectiveness analyses were not conducted. Also, since the NPF and the American Academy of Dermatology are concurrently developing a psoriasis treatment guideline, the treatment of skin psoriasis was not included in the guideline.
According to the guideline’s principal investigator Jasvinder Singh, MD, professor of medicine and epidemiology at the University of Alabama at Birmingham, the guideline will include 80 recommendations, 75 (94%) that are rated as “conditional,” and 5 (6%) that are rated as “strong,” based on the quality of evidence in the existing medical literature. “Most of our treatment guidelines rely on very low-to-moderate quality evidence, which means that there needs to be an active discussion between the physician and the patient with regard to which treatment to choose,” said Dr. Singh, who is also a staff rheumatologist at the Birmingham Veterans Affairs Medical Center and who led development of the 2012 and 2015 ACR treatment guidelines for RA. “When you’re not choosing the preferred treatment, there are defined specific recommendations under which that second treatment may be preferred over the first treatment.”
During a separate session at the meeting, Dr. Singh unveiled a few of the draft recommendations. One calls for using a treat-to-target strategy over not using one. In the setting of immunizing patients who are receiving a biologic, another recommendation calls for clinicians to start the indicated biologic and administer killed vaccines (as indicated) in patients with active PsA rather than delaying the biologic to give the killed vaccines. In addition, delaying the start of the indicated biologic is recommended over not delaying in order to administer a live attenuated vaccine in patients with active PsA. When patients continue to have with active PsA despite being on a TNF inhibitor, the draft guideline recommends switching to a different TNF inhibitor rather than an IL-17 inhibitor, an IL-12/IL-23 inhibitor, abatacept (Orencia), tofacitinib (Xeljanz), or adding methotrexate. If PsA is still active, the guideline recommends switching to an IL-17 inhibitor instead of an IL-12/IL-23 inhibitor, abatacept, or tofacitinib. If PsA is still active, the guideline recommends switching to an IL-12/IL-23 inhibitor over abatacept or tofacitinib.
The guideline also includes suggestions for nonpharmacologic treatments, including recommending low-impact exercise over high-impact exercise, occupational therapy, physical therapy, and weight loss. It also includes a strong recommendation to provide smoking cessation advice to patients.
Dr. Singh acknowledged significant research gaps in the current PsA medical literature, including no head-to-head comparisons of treatments. He said that the field also could benefit from specific studies for enthesitis, axial disease, and arthritis mutilans; randomized trials of nonpharmacologic interventions; more trials of monotherapy vs. combination therapy; vaccination trials for live attenuated vaccines; trials and registry studies of patients with common comorbidities, and studies of NSAIDs and glucocorticoids, to define their role.
Possible topics for future PsA guidelines, he continued, include treatment options for patients for whom biologic medication is not an option; use of therapies in pregnancy and conception; incorporation of high-quality cost or cost-effectiveness analysis into recommendations; and the role of other comorbidities, such as fibromyalgia, hepatitis, depression/anxiety, malignancy, and cardiovascular disease.
“Evidence-based medicine needs to be practiced, even in situations where it’s difficult to get a drug,” Dr. Gladman said. “One of the things we hope will happen in the near future is that companies will start doing head-to-head studies, to help us support evidence-based recommendations in the future.”
None of the speakers reported having relevant financial disclosures.
SAN DIEGO – For the first time, a forthcoming evidence-based guideline for the management of psoriatic arthritis recommends tumor necrosis factor inhibitor biologics as first-line therapy.
“Guidelines that have been around for the last several years have been skirting around the fact that there’s really no evidence that methotrexate works for PsA,” Dafna D. Gladman, MD, said during a press briefing at the annual meeting of the American College of Rheumatology. “So it’s refreshing and reassuring that when you do an appropriate, evidence-based approach, you finally find the truth in front of you, and you have TNF inhibitors as the first-line treatment. Obviously, they’re not for everybody. There are patients in whom we cannot use TNF inhibitors, either because they don’t like needles, or because they have contraindications to getting these particular needles, but at least we have a recommendation for the use of these drugs as a first-line treatment.”
“At first, I wasn’t a big fan of the idea of the GRADE guidelines because the number of questions blows up so fast, [but] it really makes you focus on what the most common [clinical] settings are,” said another core oversight team member, Alexis Ogdie, MD, a rheumatologist at the University of Pennsylvania, Philadelphia. “These guidelines also reveal the major gap of no head-to-head studies. I think we’ve known that, but this really called that out as important. When we’re making a treatment decision between [drugs] A and B, we need those studies to be able to better understand how to treat our patients, rather than using the data from one trial to make a decision. ... For my patients, I’m excited that I can now use a TNF inhibitor as a first-line agent. When we have patients come in with very severe disease, occasionally they also have severe psoriasis, so we’ve been able to use TNF inhibitors as first-line treatment in some of our patients in Pennsylvania. This differs state by state. But the exciting thing is that they get better so fast and you don’t have to tell them to wait 12 weeks for methotrexate to work.”
The ACR/NPF guideline is currently under peer review and is expected to be published in Arthritis & Rheumatology, Arthritis Care & Research, and the Journal of Psoriasis and Psoriatic Arthritis in the spring or summer of 2018. It focuses on common PsA patients, not exceptional cases. It includes recommendations on the management of patients with active PsA that is defined by the patients’ self-report and judged by the examining clinician to be caused by PsA, based on the on the presence of at least one of the following: actively inflamed joints; dactylitis; enthesitis; axial disease; active skin and/or nail involvement; and/or extra-articular manifestations such as uveitis or inflammatory bowel disease. Authors of the guideline considered cost as one of many possible factors affecting the use of the recommendations, but explicit cost-effectiveness analyses were not conducted. Also, since the NPF and the American Academy of Dermatology are concurrently developing a psoriasis treatment guideline, the treatment of skin psoriasis was not included in the guideline.
According to the guideline’s principal investigator Jasvinder Singh, MD, professor of medicine and epidemiology at the University of Alabama at Birmingham, the guideline will include 80 recommendations, 75 (94%) that are rated as “conditional,” and 5 (6%) that are rated as “strong,” based on the quality of evidence in the existing medical literature. “Most of our treatment guidelines rely on very low-to-moderate quality evidence, which means that there needs to be an active discussion between the physician and the patient with regard to which treatment to choose,” said Dr. Singh, who is also a staff rheumatologist at the Birmingham Veterans Affairs Medical Center and who led development of the 2012 and 2015 ACR treatment guidelines for RA. “When you’re not choosing the preferred treatment, there are defined specific recommendations under which that second treatment may be preferred over the first treatment.”
During a separate session at the meeting, Dr. Singh unveiled a few of the draft recommendations. One calls for using a treat-to-target strategy over not using one. In the setting of immunizing patients who are receiving a biologic, another recommendation calls for clinicians to start the indicated biologic and administer killed vaccines (as indicated) in patients with active PsA rather than delaying the biologic to give the killed vaccines. In addition, delaying the start of the indicated biologic is recommended over not delaying in order to administer a live attenuated vaccine in patients with active PsA. When patients continue to have with active PsA despite being on a TNF inhibitor, the draft guideline recommends switching to a different TNF inhibitor rather than an IL-17 inhibitor, an IL-12/IL-23 inhibitor, abatacept (Orencia), tofacitinib (Xeljanz), or adding methotrexate. If PsA is still active, the guideline recommends switching to an IL-17 inhibitor instead of an IL-12/IL-23 inhibitor, abatacept, or tofacitinib. If PsA is still active, the guideline recommends switching to an IL-12/IL-23 inhibitor over abatacept or tofacitinib.
The guideline also includes suggestions for nonpharmacologic treatments, including recommending low-impact exercise over high-impact exercise, occupational therapy, physical therapy, and weight loss. It also includes a strong recommendation to provide smoking cessation advice to patients.
Dr. Singh acknowledged significant research gaps in the current PsA medical literature, including no head-to-head comparisons of treatments. He said that the field also could benefit from specific studies for enthesitis, axial disease, and arthritis mutilans; randomized trials of nonpharmacologic interventions; more trials of monotherapy vs. combination therapy; vaccination trials for live attenuated vaccines; trials and registry studies of patients with common comorbidities, and studies of NSAIDs and glucocorticoids, to define their role.
Possible topics for future PsA guidelines, he continued, include treatment options for patients for whom biologic medication is not an option; use of therapies in pregnancy and conception; incorporation of high-quality cost or cost-effectiveness analysis into recommendations; and the role of other comorbidities, such as fibromyalgia, hepatitis, depression/anxiety, malignancy, and cardiovascular disease.
“Evidence-based medicine needs to be practiced, even in situations where it’s difficult to get a drug,” Dr. Gladman said. “One of the things we hope will happen in the near future is that companies will start doing head-to-head studies, to help us support evidence-based recommendations in the future.”
None of the speakers reported having relevant financial disclosures.
AT ACR 2017
ACOG: VBAC is safe for many women
Women and their , according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Trial of labor after cesarean delivery (TOLAC) results in a successful birth in 60%-80% of cases, sparing mothers from major abdominal surgery and reducing the risk of hemorrhage, thromboses, and infection, the authors of the practice bulletin wrote. “The preponderance of evidence suggests that most women with one previous cesarean delivery with a low-transverse incision are candidates for and should be counseled about and offered TOLAC,” they said (Obstet Gynecol. 2017 Nov;130[5]:e217-33. doi: 10.1097/AOG.0000000000002398).
Rates of cesarean delivery in the United States jumped from 5% to nearly 32% between 1970 and 2016. Although rates of VBAC rose between the mid-1980s and the mid-1990s, cases of uterine rupture and other complications spurred fears of malpractice litigation and reversed this trend. VBAC rates were more than 28% in 1996 but fell to 8.5% by 2006, according to the practice bulletin.
To reduce the risk of uterine rupture, avoid misoprostol for cervical ripening and labor induction in women with a prior cesarean delivery, ACOG recommended.
“No evidence suggests that epidural analgesia is a causal risk factor for unsuccessful TOLAC,” the authors added. “Therefore, epidural analgesia for labor may be used as part of TOLAC, and adequate pain relief may encourage more women to choose TOLAC.”
Women with two prior low-transverse cesareans also are potential candidates for TOLAC, depending on other predictors of successful VBAC. Factors that reduce the chances of a successful TOLAC include advanced maternal age, high body mass index, high birth weight, gestational age of more than 40 weeks at delivery, and preeclampsia at the time of delivery, according to the practice bulletin.
To reduce the risk of adverse outcomes of complications, TOLAC should not occur at home and should only occur at level I facilities (or higher) that can perform an emergency cesarean delivery if the mother or fetus is in jeopardy.
The practice bulletin recommends continuous fetal heart rate monitoring during TOLAC and notes several additional categories of TOLAC candidates. Obstetricians and patients should discuss the potential risks and benefits of both TOLAC and elective repeat cesarean delivery, and that discussion should be documented in the medical record, ACOG recommended.
Women and their , according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Trial of labor after cesarean delivery (TOLAC) results in a successful birth in 60%-80% of cases, sparing mothers from major abdominal surgery and reducing the risk of hemorrhage, thromboses, and infection, the authors of the practice bulletin wrote. “The preponderance of evidence suggests that most women with one previous cesarean delivery with a low-transverse incision are candidates for and should be counseled about and offered TOLAC,” they said (Obstet Gynecol. 2017 Nov;130[5]:e217-33. doi: 10.1097/AOG.0000000000002398).
Rates of cesarean delivery in the United States jumped from 5% to nearly 32% between 1970 and 2016. Although rates of VBAC rose between the mid-1980s and the mid-1990s, cases of uterine rupture and other complications spurred fears of malpractice litigation and reversed this trend. VBAC rates were more than 28% in 1996 but fell to 8.5% by 2006, according to the practice bulletin.
To reduce the risk of uterine rupture, avoid misoprostol for cervical ripening and labor induction in women with a prior cesarean delivery, ACOG recommended.
“No evidence suggests that epidural analgesia is a causal risk factor for unsuccessful TOLAC,” the authors added. “Therefore, epidural analgesia for labor may be used as part of TOLAC, and adequate pain relief may encourage more women to choose TOLAC.”
Women with two prior low-transverse cesareans also are potential candidates for TOLAC, depending on other predictors of successful VBAC. Factors that reduce the chances of a successful TOLAC include advanced maternal age, high body mass index, high birth weight, gestational age of more than 40 weeks at delivery, and preeclampsia at the time of delivery, according to the practice bulletin.
To reduce the risk of adverse outcomes of complications, TOLAC should not occur at home and should only occur at level I facilities (or higher) that can perform an emergency cesarean delivery if the mother or fetus is in jeopardy.
The practice bulletin recommends continuous fetal heart rate monitoring during TOLAC and notes several additional categories of TOLAC candidates. Obstetricians and patients should discuss the potential risks and benefits of both TOLAC and elective repeat cesarean delivery, and that discussion should be documented in the medical record, ACOG recommended.
Women and their , according to an updated practice bulletin from the American College of Obstetricians and Gynecologists.
Trial of labor after cesarean delivery (TOLAC) results in a successful birth in 60%-80% of cases, sparing mothers from major abdominal surgery and reducing the risk of hemorrhage, thromboses, and infection, the authors of the practice bulletin wrote. “The preponderance of evidence suggests that most women with one previous cesarean delivery with a low-transverse incision are candidates for and should be counseled about and offered TOLAC,” they said (Obstet Gynecol. 2017 Nov;130[5]:e217-33. doi: 10.1097/AOG.0000000000002398).
Rates of cesarean delivery in the United States jumped from 5% to nearly 32% between 1970 and 2016. Although rates of VBAC rose between the mid-1980s and the mid-1990s, cases of uterine rupture and other complications spurred fears of malpractice litigation and reversed this trend. VBAC rates were more than 28% in 1996 but fell to 8.5% by 2006, according to the practice bulletin.
To reduce the risk of uterine rupture, avoid misoprostol for cervical ripening and labor induction in women with a prior cesarean delivery, ACOG recommended.
“No evidence suggests that epidural analgesia is a causal risk factor for unsuccessful TOLAC,” the authors added. “Therefore, epidural analgesia for labor may be used as part of TOLAC, and adequate pain relief may encourage more women to choose TOLAC.”
Women with two prior low-transverse cesareans also are potential candidates for TOLAC, depending on other predictors of successful VBAC. Factors that reduce the chances of a successful TOLAC include advanced maternal age, high body mass index, high birth weight, gestational age of more than 40 weeks at delivery, and preeclampsia at the time of delivery, according to the practice bulletin.
To reduce the risk of adverse outcomes of complications, TOLAC should not occur at home and should only occur at level I facilities (or higher) that can perform an emergency cesarean delivery if the mother or fetus is in jeopardy.
The practice bulletin recommends continuous fetal heart rate monitoring during TOLAC and notes several additional categories of TOLAC candidates. Obstetricians and patients should discuss the potential risks and benefits of both TOLAC and elective repeat cesarean delivery, and that discussion should be documented in the medical record, ACOG recommended.
FROM OBSTETRICS & GYNECOLOGY
Statement Offers Guidance for Management of Brain AVMs
Recent studies have helped clarify the natural history risks of intracranial hemorrhage (ICH) and seizure in patients with unruptured brain arteriovenous malformations (AVMs). In addition, researchers have completed the first randomized trial of interventional therapy versus conservative management for unruptured brain AVMs. Nevertheless, data to guide treatment decisions remain limited, and “considerable uncertainties … face physicians managing patients with ruptured and unruptured brain AVMs,” according to a scientific statement for healthcare professionals from the American Heart Association (AHA) and American Stroke Association (ASA). Optimal management of unruptured brain AVMs is a subject of debate, and discussion of treatment options should include careful consideration of natural history risks, the relative risks of intervention strategies, and patients’ life expectancy, the authors said.
The scientific statement on the management of brain AVMs was published in the August issue of Stroke. It updates the American Heart Association’s 2001 statement on this topic. The report reviews epidemiology, diagnosis, natural history, treatment, and management of ruptured and unruptured brain AVMs, as well as areas requiring more evidence. The American Academy of Neurology affirmed the value of the statement as an educational tool for neurologists. The Society of NeuroInterventional Surgery endorsed it, and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Cerebrovascular Section affirmed its educational benefit.
An Uncommon Lesion
Brain AVMs are uncommon, may present with spontaneous ICH, seizures, or headache, and typically present in young adults. Neurologists may identify incidental asymptomatic AVMs when patients undergo brain imaging for other reasons. The primary goal of treatment is prevention of hemorrhagic stroke.
Colin P. Derdeyn, MD, Professor of Neurology and Radiology at the University of Iowa in Iowa City and chair of the group that wrote the scientific statement, and colleagues searched all English-language articles on brain AVMs in humans that had been published following the 2001 statement. “Advances … include the accumulation of new data related to epidemiology, biology, imaging, outcomes with treatment, and introduction of new embolic agents,” the authors said. “Most notable of these data are the results of the first randomized trial of intervention for unruptured brain AVMs, the ARUBA trial (A Randomized Trial of Unruptured Brain Arteriovenous Malformations).”
ARUBA enrolled 226 adult patients with unruptured brain AVMs between 2007 and 2013. Patients were randomized to receive medical management alone or medical management with interventional therapy (eg, resection, embolization, or stereotactic radiosurgery alone or in combination).
A preplanned interim analysis found that after a mean follow-up of 33 months, the risk of stroke or death was more than three times higher in the intervention group (30.7%) than in the medical management group (10.1%). The analysis included data from 224 participants at 39 sites worldwide. On the recommendation of the ARUBA Data and Safety Monitoring Board, the National Institute of Neurological Disorders and Stroke stopped enrollment and stated that “under the experimental conditions in this trial, the interim analysis … shows that medical management is superior to intervention in patients with unruptured brain AVMs.”
Although ARUBA and an observational study by Al-Shahi Salman et al support a conservative approach to management, the studies had relatively short follow-up, considering the long duration of hemorrhage risk for patients with untreated brain AVMs. Furthermore, complication rates in the treatment arms in ARUBA were much higher than expected, the authors said. “The primary end points of ARUBA [ie, stroke or death] in the intervention group for Spetzler-Martin (SM) grade I (14.3%), II (43.3%), and III (57.1%) AVMs are higher than would have been expected in contemporary series, particularly for surgery or radiosurgery performed alone,” the authors said.
Additional studies are needed to better define long-term risks of stroke and seizure and to identify specific predictors of hemorrhagic stroke. “Long-term follow-up of participants in the ARUBA trial will be valuable for determining whether the superiority of conservative management over intervention observed in that study persists in the long term,” they said. “Further randomized controlled trials are justified to investigate the reproducibility of the findings of ARUBA and to investigate whether the balance of risk between conservative management and intervention is different in specific groups (eg, patients with SM grade I brain AVM).”
Recommendations for Management
Neurologists can inform patients about natural history risks, which are reliably quantified over approximately 10 years for ICH and approximately five years for epileptic seizure, the authors said. The annual risk of a first-ever ICH from an unruptured brain AVM is approximately 1%. The five-year risk of developing a first seizure is approximately 8%, and the five-year risk of developing epilepsy after a first seizure is approximately 58%.
Discussion of treatment options should consider natural history risks “weighed carefully against the relative risks of different intervention strategies and life expectancy,” the authors said. The SM grading scale is useful for predicting the risk of surgical resection.
For the management of patients with ruptured brain AVMs, the authors recommend that the evaluation of underlying brain AVMs and management of an initial hemorrhage follow the AHA and ASA’s 2015 spontaneous ICH management guidelines. The annual risk of recurrent ICH from a ruptured brain AVM is approximately 5%, and increasing age, deep venous drainage, arterial aneurysms, and female sex may increase the risk.
—Jake Remaly
Suggested Reading
Al-Shahi Salman R, White PM, Counsell CE, et al. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA. 2014;311(16):1661-1669.
Derdeyn CP, Zipfel GJ, Albuquerque FC, et al. Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(8):e200-e224.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060.
Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614-621.
Recent studies have helped clarify the natural history risks of intracranial hemorrhage (ICH) and seizure in patients with unruptured brain arteriovenous malformations (AVMs). In addition, researchers have completed the first randomized trial of interventional therapy versus conservative management for unruptured brain AVMs. Nevertheless, data to guide treatment decisions remain limited, and “considerable uncertainties … face physicians managing patients with ruptured and unruptured brain AVMs,” according to a scientific statement for healthcare professionals from the American Heart Association (AHA) and American Stroke Association (ASA). Optimal management of unruptured brain AVMs is a subject of debate, and discussion of treatment options should include careful consideration of natural history risks, the relative risks of intervention strategies, and patients’ life expectancy, the authors said.
The scientific statement on the management of brain AVMs was published in the August issue of Stroke. It updates the American Heart Association’s 2001 statement on this topic. The report reviews epidemiology, diagnosis, natural history, treatment, and management of ruptured and unruptured brain AVMs, as well as areas requiring more evidence. The American Academy of Neurology affirmed the value of the statement as an educational tool for neurologists. The Society of NeuroInterventional Surgery endorsed it, and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Cerebrovascular Section affirmed its educational benefit.
An Uncommon Lesion
Brain AVMs are uncommon, may present with spontaneous ICH, seizures, or headache, and typically present in young adults. Neurologists may identify incidental asymptomatic AVMs when patients undergo brain imaging for other reasons. The primary goal of treatment is prevention of hemorrhagic stroke.
Colin P. Derdeyn, MD, Professor of Neurology and Radiology at the University of Iowa in Iowa City and chair of the group that wrote the scientific statement, and colleagues searched all English-language articles on brain AVMs in humans that had been published following the 2001 statement. “Advances … include the accumulation of new data related to epidemiology, biology, imaging, outcomes with treatment, and introduction of new embolic agents,” the authors said. “Most notable of these data are the results of the first randomized trial of intervention for unruptured brain AVMs, the ARUBA trial (A Randomized Trial of Unruptured Brain Arteriovenous Malformations).”
ARUBA enrolled 226 adult patients with unruptured brain AVMs between 2007 and 2013. Patients were randomized to receive medical management alone or medical management with interventional therapy (eg, resection, embolization, or stereotactic radiosurgery alone or in combination).
A preplanned interim analysis found that after a mean follow-up of 33 months, the risk of stroke or death was more than three times higher in the intervention group (30.7%) than in the medical management group (10.1%). The analysis included data from 224 participants at 39 sites worldwide. On the recommendation of the ARUBA Data and Safety Monitoring Board, the National Institute of Neurological Disorders and Stroke stopped enrollment and stated that “under the experimental conditions in this trial, the interim analysis … shows that medical management is superior to intervention in patients with unruptured brain AVMs.”
Although ARUBA and an observational study by Al-Shahi Salman et al support a conservative approach to management, the studies had relatively short follow-up, considering the long duration of hemorrhage risk for patients with untreated brain AVMs. Furthermore, complication rates in the treatment arms in ARUBA were much higher than expected, the authors said. “The primary end points of ARUBA [ie, stroke or death] in the intervention group for Spetzler-Martin (SM) grade I (14.3%), II (43.3%), and III (57.1%) AVMs are higher than would have been expected in contemporary series, particularly for surgery or radiosurgery performed alone,” the authors said.
Additional studies are needed to better define long-term risks of stroke and seizure and to identify specific predictors of hemorrhagic stroke. “Long-term follow-up of participants in the ARUBA trial will be valuable for determining whether the superiority of conservative management over intervention observed in that study persists in the long term,” they said. “Further randomized controlled trials are justified to investigate the reproducibility of the findings of ARUBA and to investigate whether the balance of risk between conservative management and intervention is different in specific groups (eg, patients with SM grade I brain AVM).”
Recommendations for Management
Neurologists can inform patients about natural history risks, which are reliably quantified over approximately 10 years for ICH and approximately five years for epileptic seizure, the authors said. The annual risk of a first-ever ICH from an unruptured brain AVM is approximately 1%. The five-year risk of developing a first seizure is approximately 8%, and the five-year risk of developing epilepsy after a first seizure is approximately 58%.
Discussion of treatment options should consider natural history risks “weighed carefully against the relative risks of different intervention strategies and life expectancy,” the authors said. The SM grading scale is useful for predicting the risk of surgical resection.
For the management of patients with ruptured brain AVMs, the authors recommend that the evaluation of underlying brain AVMs and management of an initial hemorrhage follow the AHA and ASA’s 2015 spontaneous ICH management guidelines. The annual risk of recurrent ICH from a ruptured brain AVM is approximately 5%, and increasing age, deep venous drainage, arterial aneurysms, and female sex may increase the risk.
—Jake Remaly
Suggested Reading
Al-Shahi Salman R, White PM, Counsell CE, et al. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA. 2014;311(16):1661-1669.
Derdeyn CP, Zipfel GJ, Albuquerque FC, et al. Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(8):e200-e224.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060.
Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614-621.
Recent studies have helped clarify the natural history risks of intracranial hemorrhage (ICH) and seizure in patients with unruptured brain arteriovenous malformations (AVMs). In addition, researchers have completed the first randomized trial of interventional therapy versus conservative management for unruptured brain AVMs. Nevertheless, data to guide treatment decisions remain limited, and “considerable uncertainties … face physicians managing patients with ruptured and unruptured brain AVMs,” according to a scientific statement for healthcare professionals from the American Heart Association (AHA) and American Stroke Association (ASA). Optimal management of unruptured brain AVMs is a subject of debate, and discussion of treatment options should include careful consideration of natural history risks, the relative risks of intervention strategies, and patients’ life expectancy, the authors said.
The scientific statement on the management of brain AVMs was published in the August issue of Stroke. It updates the American Heart Association’s 2001 statement on this topic. The report reviews epidemiology, diagnosis, natural history, treatment, and management of ruptured and unruptured brain AVMs, as well as areas requiring more evidence. The American Academy of Neurology affirmed the value of the statement as an educational tool for neurologists. The Society of NeuroInterventional Surgery endorsed it, and the American Association of Neurological Surgeons/Congress of Neurological Surgeons Joint Cerebrovascular Section affirmed its educational benefit.
An Uncommon Lesion
Brain AVMs are uncommon, may present with spontaneous ICH, seizures, or headache, and typically present in young adults. Neurologists may identify incidental asymptomatic AVMs when patients undergo brain imaging for other reasons. The primary goal of treatment is prevention of hemorrhagic stroke.
Colin P. Derdeyn, MD, Professor of Neurology and Radiology at the University of Iowa in Iowa City and chair of the group that wrote the scientific statement, and colleagues searched all English-language articles on brain AVMs in humans that had been published following the 2001 statement. “Advances … include the accumulation of new data related to epidemiology, biology, imaging, outcomes with treatment, and introduction of new embolic agents,” the authors said. “Most notable of these data are the results of the first randomized trial of intervention for unruptured brain AVMs, the ARUBA trial (A Randomized Trial of Unruptured Brain Arteriovenous Malformations).”
ARUBA enrolled 226 adult patients with unruptured brain AVMs between 2007 and 2013. Patients were randomized to receive medical management alone or medical management with interventional therapy (eg, resection, embolization, or stereotactic radiosurgery alone or in combination).
A preplanned interim analysis found that after a mean follow-up of 33 months, the risk of stroke or death was more than three times higher in the intervention group (30.7%) than in the medical management group (10.1%). The analysis included data from 224 participants at 39 sites worldwide. On the recommendation of the ARUBA Data and Safety Monitoring Board, the National Institute of Neurological Disorders and Stroke stopped enrollment and stated that “under the experimental conditions in this trial, the interim analysis … shows that medical management is superior to intervention in patients with unruptured brain AVMs.”
Although ARUBA and an observational study by Al-Shahi Salman et al support a conservative approach to management, the studies had relatively short follow-up, considering the long duration of hemorrhage risk for patients with untreated brain AVMs. Furthermore, complication rates in the treatment arms in ARUBA were much higher than expected, the authors said. “The primary end points of ARUBA [ie, stroke or death] in the intervention group for Spetzler-Martin (SM) grade I (14.3%), II (43.3%), and III (57.1%) AVMs are higher than would have been expected in contemporary series, particularly for surgery or radiosurgery performed alone,” the authors said.
Additional studies are needed to better define long-term risks of stroke and seizure and to identify specific predictors of hemorrhagic stroke. “Long-term follow-up of participants in the ARUBA trial will be valuable for determining whether the superiority of conservative management over intervention observed in that study persists in the long term,” they said. “Further randomized controlled trials are justified to investigate the reproducibility of the findings of ARUBA and to investigate whether the balance of risk between conservative management and intervention is different in specific groups (eg, patients with SM grade I brain AVM).”
Recommendations for Management
Neurologists can inform patients about natural history risks, which are reliably quantified over approximately 10 years for ICH and approximately five years for epileptic seizure, the authors said. The annual risk of a first-ever ICH from an unruptured brain AVM is approximately 1%. The five-year risk of developing a first seizure is approximately 8%, and the five-year risk of developing epilepsy after a first seizure is approximately 58%.
Discussion of treatment options should consider natural history risks “weighed carefully against the relative risks of different intervention strategies and life expectancy,” the authors said. The SM grading scale is useful for predicting the risk of surgical resection.
For the management of patients with ruptured brain AVMs, the authors recommend that the evaluation of underlying brain AVMs and management of an initial hemorrhage follow the AHA and ASA’s 2015 spontaneous ICH management guidelines. The annual risk of recurrent ICH from a ruptured brain AVM is approximately 5%, and increasing age, deep venous drainage, arterial aneurysms, and female sex may increase the risk.
—Jake Remaly
Suggested Reading
Al-Shahi Salman R, White PM, Counsell CE, et al. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA. 2014;311(16):1661-1669.
Derdeyn CP, Zipfel GJ, Albuquerque FC, et al. Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48(8):e200-e224.
Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032-2060.
Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014;383(9917):614-621.
ACOG advises against vaginal seeding
The practice of vaginal seeding should not be performed outside of an approved research protocol until adequate data on safety and potential benefits are available, according to a new policy statement from the American College of Obstetricians and Gynecologists.
Vaginal seeding is “the practice of inoculating a cotton gauze or a cotton swab with vaginal fluids to transfer the vaginal flora to the mouth, nose, or skin of a newborn infant,” according to ACOG.
Data from several studies have suggested babies delivered by cesarean may lack the immunologic and metabolic benefits of vaginally delivered babies because of the unique properties of vaginal fluid, and a proof-of-concept study showed changes in newborns’ microbiome profiles when they received transfers of vaginal fluid soon after a cesarean delivery. However, the impact of the fluid transfer (vaginal seeding) remains unknown, according to the ACOG committee opinion (Obstet Gynecol. 2017;130:e274-8).
Additional safety concerns include the potential transfer of pathogens from mother to neonate from undiagnosed maternal conditions such as gonorrhea, human papillomavirus, group A streptococci, and others, the committee noted.
Women who wish to perform neonatal seeding themselves should be educated about the risks and tested for infectious diseases and pathogenic bacteria, the committee emphasized. Additionally, ACOG urged ob.gyns. to document the discussion in the medical record. The infant’s physician should also be made aware of the procedure because of the potential for neonatal infection.
The research on vaginal seeding currently consists of one pilot study, with an outcome measure of neonatal microbiota. No studies of other clinical outcomes have been completed.
“The paucity of data on this subject supports the need for additional research on the safety and benefit of vaginal seeding,” the ACOG Committee on Obstetric Practice wrote.
In the meantime, ACOG recommends exclusive breastfeeding in the first 6 months, noting that there are mixed data on associations between breastfeeding and the development of asthma and atopic disease in childhood.
The practice of vaginal seeding should not be performed outside of an approved research protocol until adequate data on safety and potential benefits are available, according to a new policy statement from the American College of Obstetricians and Gynecologists.
Vaginal seeding is “the practice of inoculating a cotton gauze or a cotton swab with vaginal fluids to transfer the vaginal flora to the mouth, nose, or skin of a newborn infant,” according to ACOG.
Data from several studies have suggested babies delivered by cesarean may lack the immunologic and metabolic benefits of vaginally delivered babies because of the unique properties of vaginal fluid, and a proof-of-concept study showed changes in newborns’ microbiome profiles when they received transfers of vaginal fluid soon after a cesarean delivery. However, the impact of the fluid transfer (vaginal seeding) remains unknown, according to the ACOG committee opinion (Obstet Gynecol. 2017;130:e274-8).
Additional safety concerns include the potential transfer of pathogens from mother to neonate from undiagnosed maternal conditions such as gonorrhea, human papillomavirus, group A streptococci, and others, the committee noted.
Women who wish to perform neonatal seeding themselves should be educated about the risks and tested for infectious diseases and pathogenic bacteria, the committee emphasized. Additionally, ACOG urged ob.gyns. to document the discussion in the medical record. The infant’s physician should also be made aware of the procedure because of the potential for neonatal infection.
The research on vaginal seeding currently consists of one pilot study, with an outcome measure of neonatal microbiota. No studies of other clinical outcomes have been completed.
“The paucity of data on this subject supports the need for additional research on the safety and benefit of vaginal seeding,” the ACOG Committee on Obstetric Practice wrote.
In the meantime, ACOG recommends exclusive breastfeeding in the first 6 months, noting that there are mixed data on associations between breastfeeding and the development of asthma and atopic disease in childhood.
The practice of vaginal seeding should not be performed outside of an approved research protocol until adequate data on safety and potential benefits are available, according to a new policy statement from the American College of Obstetricians and Gynecologists.
Vaginal seeding is “the practice of inoculating a cotton gauze or a cotton swab with vaginal fluids to transfer the vaginal flora to the mouth, nose, or skin of a newborn infant,” according to ACOG.
Data from several studies have suggested babies delivered by cesarean may lack the immunologic and metabolic benefits of vaginally delivered babies because of the unique properties of vaginal fluid, and a proof-of-concept study showed changes in newborns’ microbiome profiles when they received transfers of vaginal fluid soon after a cesarean delivery. However, the impact of the fluid transfer (vaginal seeding) remains unknown, according to the ACOG committee opinion (Obstet Gynecol. 2017;130:e274-8).
Additional safety concerns include the potential transfer of pathogens from mother to neonate from undiagnosed maternal conditions such as gonorrhea, human papillomavirus, group A streptococci, and others, the committee noted.
Women who wish to perform neonatal seeding themselves should be educated about the risks and tested for infectious diseases and pathogenic bacteria, the committee emphasized. Additionally, ACOG urged ob.gyns. to document the discussion in the medical record. The infant’s physician should also be made aware of the procedure because of the potential for neonatal infection.
The research on vaginal seeding currently consists of one pilot study, with an outcome measure of neonatal microbiota. No studies of other clinical outcomes have been completed.
“The paucity of data on this subject supports the need for additional research on the safety and benefit of vaginal seeding,” the ACOG Committee on Obstetric Practice wrote.
In the meantime, ACOG recommends exclusive breastfeeding in the first 6 months, noting that there are mixed data on associations between breastfeeding and the development of asthma and atopic disease in childhood.
FROM OBSTETRICS & GYNECOLOGY
New appropriate use criteria reframe severe aortic stenosis
New appropriate use criteria (AUC) for severe aortic stenosis (AS) run the full gamut of clinical scenarios and treatment options.
“Cardiology is seeing a radical change in the management of aortic stenosis. This new document incorporates all therapies currently available in the world,” said Vinod H. Thourani, MD, who served on the AUC’s writing group on behalf of the American College of Cardiology. “The AUC highlights state-of-the-art therapy for aortic stenosis and, even more importantly, helps clarify the right indications for surgical and transcatheter valve replacement.”
Surgical risk is assessed based on the Society of Thoracic Surgeons Predicted Risk of Mortality score plus additional anatomic and functional considerations that should be assessed by a multidisciplinary heart team. The AUC repeatedly emphasizes this team’s importance. “Multiple comorbidities can change the pathway of treating AS, and this determination is best made by a heart team that at least includes a noninvasive cardiologist, an interventional cardiologist, and a cardiac surgeon,” Dr. Thourani said. “That’s how patients get the best care.”
Historically, aortic stenosis typically was managed medically or with balloon aortic valvuloplasty (BAV) or open aortic valve replacement, Dr. Thourani said. However, BAV is less common now, and indications for surgical or transcatheter aortic valve replacement (SAVR or TAVR) are expanding. Balloon aortic valvuloplasty sometimes does provide palliative treatment or serve as a bridge to a decision, the AUC states. For example, for a high-risk patient with severe aortic stenosis and severe secondary mitral regurgitation, BAV can help the heart team decide whether TAVR alone will improve mitral regurgitation or whether a double valve procedure is preferable.
Regardless of risk score, the AUC considers a wait-and-see approach as potentially appropriate for patients with asymptomatic high-grade AS whose left ventricular ejection fraction is at least 50%, peak aortic valve velocity is 4.0-4.9 m/sec, and exercise stress test is normal and with no predictors of symptom onset or rapid progression. Asymptomatic patients who are likely to become symptomatic but who have a low risk of sudden death are candidates for intervention (rated “appropriate”) or medical management (“may be appropriate”). In contrast, a positive stress test in an otherwise asymptomatic patient merits consideration of SAVR or TAVR regardless of surgical risk. The recommendations for asymptomatic patients reflect a lack of head-to-head trials in this population, Dr. Thourani said. “We don’t have good randomized data to show one therapy is better than another.”
Symptomatic, high-gradient, severe AS with associated coronary artery disease merits consideration of SAVR with coronary artery bypass graft or, in some cases, TAVR with percutaneous coronary intervention, according to the AUC. Less evidence supports SAVR with PCI. “Optimal management of coronary artery disease in patients with AS is a complex decision process requiring clinical, anatomical, and technical considerations that is best achieved with close collaboration between heart team members,” the authors stress.
The document covers other valvular and structural heart conditions that commonly accompany severe AS, such as symptomatic AS with bicuspid aortic valve and ascending aortic dilation. “Although there remains an increasing prevalence of transcatheter valve usage in bicuspid aortic valve, the standard of care remains surgical therapy, especially in patients who have a dilated aorta,” Dr. Thourani said.
For the first time, the AUC also addresses failing aortic valve prostheses, presenting six relevant clinical scenarios. The AUC consistently recommends SAVR, although the use of TAVR has “dramatically increased” in these patients, Dr. Thourani said. “Long-term data are still pending, but TAVR appears to be a less morbid procedure, when done appropriately.”
The societies involved in creating the AUC statement were the American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Valve Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
Dr. Thourani disclosed ties to Edwards Lifesciences, St. Jude Medical, Abbott, Boston Scientific, and Medtronic.
New appropriate use criteria (AUC) for severe aortic stenosis (AS) run the full gamut of clinical scenarios and treatment options.
“Cardiology is seeing a radical change in the management of aortic stenosis. This new document incorporates all therapies currently available in the world,” said Vinod H. Thourani, MD, who served on the AUC’s writing group on behalf of the American College of Cardiology. “The AUC highlights state-of-the-art therapy for aortic stenosis and, even more importantly, helps clarify the right indications for surgical and transcatheter valve replacement.”
Surgical risk is assessed based on the Society of Thoracic Surgeons Predicted Risk of Mortality score plus additional anatomic and functional considerations that should be assessed by a multidisciplinary heart team. The AUC repeatedly emphasizes this team’s importance. “Multiple comorbidities can change the pathway of treating AS, and this determination is best made by a heart team that at least includes a noninvasive cardiologist, an interventional cardiologist, and a cardiac surgeon,” Dr. Thourani said. “That’s how patients get the best care.”
Historically, aortic stenosis typically was managed medically or with balloon aortic valvuloplasty (BAV) or open aortic valve replacement, Dr. Thourani said. However, BAV is less common now, and indications for surgical or transcatheter aortic valve replacement (SAVR or TAVR) are expanding. Balloon aortic valvuloplasty sometimes does provide palliative treatment or serve as a bridge to a decision, the AUC states. For example, for a high-risk patient with severe aortic stenosis and severe secondary mitral regurgitation, BAV can help the heart team decide whether TAVR alone will improve mitral regurgitation or whether a double valve procedure is preferable.
Regardless of risk score, the AUC considers a wait-and-see approach as potentially appropriate for patients with asymptomatic high-grade AS whose left ventricular ejection fraction is at least 50%, peak aortic valve velocity is 4.0-4.9 m/sec, and exercise stress test is normal and with no predictors of symptom onset or rapid progression. Asymptomatic patients who are likely to become symptomatic but who have a low risk of sudden death are candidates for intervention (rated “appropriate”) or medical management (“may be appropriate”). In contrast, a positive stress test in an otherwise asymptomatic patient merits consideration of SAVR or TAVR regardless of surgical risk. The recommendations for asymptomatic patients reflect a lack of head-to-head trials in this population, Dr. Thourani said. “We don’t have good randomized data to show one therapy is better than another.”
Symptomatic, high-gradient, severe AS with associated coronary artery disease merits consideration of SAVR with coronary artery bypass graft or, in some cases, TAVR with percutaneous coronary intervention, according to the AUC. Less evidence supports SAVR with PCI. “Optimal management of coronary artery disease in patients with AS is a complex decision process requiring clinical, anatomical, and technical considerations that is best achieved with close collaboration between heart team members,” the authors stress.
The document covers other valvular and structural heart conditions that commonly accompany severe AS, such as symptomatic AS with bicuspid aortic valve and ascending aortic dilation. “Although there remains an increasing prevalence of transcatheter valve usage in bicuspid aortic valve, the standard of care remains surgical therapy, especially in patients who have a dilated aorta,” Dr. Thourani said.
For the first time, the AUC also addresses failing aortic valve prostheses, presenting six relevant clinical scenarios. The AUC consistently recommends SAVR, although the use of TAVR has “dramatically increased” in these patients, Dr. Thourani said. “Long-term data are still pending, but TAVR appears to be a less morbid procedure, when done appropriately.”
The societies involved in creating the AUC statement were the American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Valve Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
Dr. Thourani disclosed ties to Edwards Lifesciences, St. Jude Medical, Abbott, Boston Scientific, and Medtronic.
New appropriate use criteria (AUC) for severe aortic stenosis (AS) run the full gamut of clinical scenarios and treatment options.
“Cardiology is seeing a radical change in the management of aortic stenosis. This new document incorporates all therapies currently available in the world,” said Vinod H. Thourani, MD, who served on the AUC’s writing group on behalf of the American College of Cardiology. “The AUC highlights state-of-the-art therapy for aortic stenosis and, even more importantly, helps clarify the right indications for surgical and transcatheter valve replacement.”
Surgical risk is assessed based on the Society of Thoracic Surgeons Predicted Risk of Mortality score plus additional anatomic and functional considerations that should be assessed by a multidisciplinary heart team. The AUC repeatedly emphasizes this team’s importance. “Multiple comorbidities can change the pathway of treating AS, and this determination is best made by a heart team that at least includes a noninvasive cardiologist, an interventional cardiologist, and a cardiac surgeon,” Dr. Thourani said. “That’s how patients get the best care.”
Historically, aortic stenosis typically was managed medically or with balloon aortic valvuloplasty (BAV) or open aortic valve replacement, Dr. Thourani said. However, BAV is less common now, and indications for surgical or transcatheter aortic valve replacement (SAVR or TAVR) are expanding. Balloon aortic valvuloplasty sometimes does provide palliative treatment or serve as a bridge to a decision, the AUC states. For example, for a high-risk patient with severe aortic stenosis and severe secondary mitral regurgitation, BAV can help the heart team decide whether TAVR alone will improve mitral regurgitation or whether a double valve procedure is preferable.
Regardless of risk score, the AUC considers a wait-and-see approach as potentially appropriate for patients with asymptomatic high-grade AS whose left ventricular ejection fraction is at least 50%, peak aortic valve velocity is 4.0-4.9 m/sec, and exercise stress test is normal and with no predictors of symptom onset or rapid progression. Asymptomatic patients who are likely to become symptomatic but who have a low risk of sudden death are candidates for intervention (rated “appropriate”) or medical management (“may be appropriate”). In contrast, a positive stress test in an otherwise asymptomatic patient merits consideration of SAVR or TAVR regardless of surgical risk. The recommendations for asymptomatic patients reflect a lack of head-to-head trials in this population, Dr. Thourani said. “We don’t have good randomized data to show one therapy is better than another.”
Symptomatic, high-gradient, severe AS with associated coronary artery disease merits consideration of SAVR with coronary artery bypass graft or, in some cases, TAVR with percutaneous coronary intervention, according to the AUC. Less evidence supports SAVR with PCI. “Optimal management of coronary artery disease in patients with AS is a complex decision process requiring clinical, anatomical, and technical considerations that is best achieved with close collaboration between heart team members,” the authors stress.
The document covers other valvular and structural heart conditions that commonly accompany severe AS, such as symptomatic AS with bicuspid aortic valve and ascending aortic dilation. “Although there remains an increasing prevalence of transcatheter valve usage in bicuspid aortic valve, the standard of care remains surgical therapy, especially in patients who have a dilated aorta,” Dr. Thourani said.
For the first time, the AUC also addresses failing aortic valve prostheses, presenting six relevant clinical scenarios. The AUC consistently recommends SAVR, although the use of TAVR has “dramatically increased” in these patients, Dr. Thourani said. “Long-term data are still pending, but TAVR appears to be a less morbid procedure, when done appropriately.”
The societies involved in creating the AUC statement were the American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Valve Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons.
Dr. Thourani disclosed ties to Edwards Lifesciences, St. Jude Medical, Abbott, Boston Scientific, and Medtronic.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
CDC: Zika-exposed newborns need intensified eye, hearing, and neurological testing
Infants with possible prenatal Zika exposure who test positive for the virus should receive an in-depth ophthalmologic exam, intensified hearing testing, and a thorough neurological evaluation with brain imaging within 1 month of birth, according to new interim guidance set forth by the Centers for Disease Control and Prevention.
The new clinical management guidelines, published in the Oct. 20 issue of the Morbidity and Mortality Weekly Report, supersede the most recent CDC guidance, issued in August 2016. The agency deemed the update necessary after a recent convocation sponsored by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. The meeting drew dozens of practicing clinicians and federal agency representatives, who reviewed the ever-evolving body of knowledge on how to best manage the care of these infants. Since Zika emerged as a public health threat, clinicians have reported postnatal onset of some symptoms, including eye abnormalities, a developing microcephaly in infants with a normal head circumference at birth, EEG abnormalities, and diaphragmatic paralysis.
The guidance focuses on three groups: infants with clinical findings of Zika syndrome born to mothers with possible Zika exposure during pregnancy; infants without clinical findings of Zika syndrome whose mothers had lab-confirmed Zika exposure; and infants without symptoms whose mothers might have been exposed, but who did not have laboratory-confirmed infection (MMWR. 2017 Oct 20;66[41]:1089-120).
Infants with clinical findings consistent with Zika syndrome and mothers with possible prenatal Zika exposure
These infants should be tested for Zika virus with serum and urine tests. If those are negative and there is no other apparent cause of the symptoms, they should have a cerebrospinal fluid sample tested for Zika RNA and IgM Zika antibodies.
By 1 month, these infants need a head ultrasound and a detailed ophthalmologic exam. The eye exam should pick up any anomalies of the anterior and posterior eye, including microphthalmia, coloboma, intraocular calcifications, optic nerve hypoplasia and atrophy, and macular scarring with focal pigmentary retinal mottling.
A comprehensive neurological exam also is part of the recommendation. Seizures are sometimes part of Zika syndrome, but infants can also have subclinical EEG abnormalities. Advanced neuroimaging can identify both obvious and subtle brain abnormalities: cortical thinning, corpus callosum abnormalities, calcifications at the white/gray matter junction, and ventricular enlargement are possible findings.
As infants grow, clinicians should be alert for signs of increased intracranial pressure that could signal postnatal hydrocephalus. Diaphragmatic paralysis also has been seen; this manifests by respiratory distress. Dysphagia that interferes with feeding can develop as well.
The complicated clinical picture calls for a team approach, Dr. Adebanjo said. “The follow-up care of [these infants] requires a multidisciplinary team and an established medical home to facilitate the coordination of care and ensure that abnormal findings are addressed.”
Infants without clinical findings, whose mothers have lab-confirmed Zika exposure
Initially, these infants should have the same early head ultrasound, hearing, and eye exams as those who display clinical findings. All of these infants also should be tested for Zika virus in the same way as those with clinical findings.
If tests return a positive result, they should have all the investigations and follow-ups recommended for babies with clinical findings. If lab testing is negative, and clinical findings are normal, Zika infection is highly unlikely and they can receive routine care, although clinicians and parents should be on the lookout for any new symptoms that might suggest postnatal Zika syndrome.
Infants without clinical findings, whose mothers had possible, but unconfirmed, Zika exposure
This is a varied and large group, which includes women who were never tested during pregnancy, as well as those who could have had a false negative test. “Because the latter issue is not easily discerned, all mothers with possible exposure to Zika virus infection, including those who tested negative with currently available technology, should be considered in this group,” Dr. Adebanjo said.
CDC does not recommend further Zika evaluation for these infants unless additional testing confirms maternal infection. For older infants, parents and clinicians should decide together whether any further evaluations would be helpful. But, Dr. Adebanjo said, “If findings consistent with congenital Zika syndrome are identified at any time, referrals to appropriate specialties should be made, and subsequent evaluation should follow recommendations for infants with clinical findings consistent with congenital Zika.”
CDC also reiterated its special recommendations for infants who had a prenatal diagnosis of Zika infection. For now, these remain unchanged from 2016, although “as more data become available, understanding of the diagnostic role of prenatal ultrasound and amniocentesis will improve and guidance will be updated.”
No one has yet identified the optimal timing for a Zika diagnostic ultrasound. CDC recommends serial ultrasounds be done every 3-4 weeks for women with lab-confirmed prenatal Zika exposure. Women with possible exposure need only routine ultrasound screenings.
While Zika RNA has been identified in amniotic fluid, there is no consensus on the value of amniocentesis as a prenatal diagnostic tool. Investigations of serial amniocentesis suggests that viral shedding into the amniotic fluid might be transient. If the procedure is done for other reasons, Zika nucleic acid testing can be incorporated.
A shared decision-making process is key when making screening decisions that should be individually weighed, Dr. Adebanjo said. “For example, serial ultrasounds might be inconvenient, unpleasant, and expensive, and might prompt unnecessary interventions; amniocentesis carries additional known risks such as fetal loss. These potential harms of prenatal screening for congenital Zika syndrome might outweigh the clinical benefits for some patients. Therefore, these decisions should be individualized.”
Neither Dr. Adebanjo nor any of the coauthors had any financial disclosures.
Infants with possible prenatal Zika exposure who test positive for the virus should receive an in-depth ophthalmologic exam, intensified hearing testing, and a thorough neurological evaluation with brain imaging within 1 month of birth, according to new interim guidance set forth by the Centers for Disease Control and Prevention.
The new clinical management guidelines, published in the Oct. 20 issue of the Morbidity and Mortality Weekly Report, supersede the most recent CDC guidance, issued in August 2016. The agency deemed the update necessary after a recent convocation sponsored by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. The meeting drew dozens of practicing clinicians and federal agency representatives, who reviewed the ever-evolving body of knowledge on how to best manage the care of these infants. Since Zika emerged as a public health threat, clinicians have reported postnatal onset of some symptoms, including eye abnormalities, a developing microcephaly in infants with a normal head circumference at birth, EEG abnormalities, and diaphragmatic paralysis.
The guidance focuses on three groups: infants with clinical findings of Zika syndrome born to mothers with possible Zika exposure during pregnancy; infants without clinical findings of Zika syndrome whose mothers had lab-confirmed Zika exposure; and infants without symptoms whose mothers might have been exposed, but who did not have laboratory-confirmed infection (MMWR. 2017 Oct 20;66[41]:1089-120).
Infants with clinical findings consistent with Zika syndrome and mothers with possible prenatal Zika exposure
These infants should be tested for Zika virus with serum and urine tests. If those are negative and there is no other apparent cause of the symptoms, they should have a cerebrospinal fluid sample tested for Zika RNA and IgM Zika antibodies.
By 1 month, these infants need a head ultrasound and a detailed ophthalmologic exam. The eye exam should pick up any anomalies of the anterior and posterior eye, including microphthalmia, coloboma, intraocular calcifications, optic nerve hypoplasia and atrophy, and macular scarring with focal pigmentary retinal mottling.
A comprehensive neurological exam also is part of the recommendation. Seizures are sometimes part of Zika syndrome, but infants can also have subclinical EEG abnormalities. Advanced neuroimaging can identify both obvious and subtle brain abnormalities: cortical thinning, corpus callosum abnormalities, calcifications at the white/gray matter junction, and ventricular enlargement are possible findings.
As infants grow, clinicians should be alert for signs of increased intracranial pressure that could signal postnatal hydrocephalus. Diaphragmatic paralysis also has been seen; this manifests by respiratory distress. Dysphagia that interferes with feeding can develop as well.
The complicated clinical picture calls for a team approach, Dr. Adebanjo said. “The follow-up care of [these infants] requires a multidisciplinary team and an established medical home to facilitate the coordination of care and ensure that abnormal findings are addressed.”
Infants without clinical findings, whose mothers have lab-confirmed Zika exposure
Initially, these infants should have the same early head ultrasound, hearing, and eye exams as those who display clinical findings. All of these infants also should be tested for Zika virus in the same way as those with clinical findings.
If tests return a positive result, they should have all the investigations and follow-ups recommended for babies with clinical findings. If lab testing is negative, and clinical findings are normal, Zika infection is highly unlikely and they can receive routine care, although clinicians and parents should be on the lookout for any new symptoms that might suggest postnatal Zika syndrome.
Infants without clinical findings, whose mothers had possible, but unconfirmed, Zika exposure
This is a varied and large group, which includes women who were never tested during pregnancy, as well as those who could have had a false negative test. “Because the latter issue is not easily discerned, all mothers with possible exposure to Zika virus infection, including those who tested negative with currently available technology, should be considered in this group,” Dr. Adebanjo said.
CDC does not recommend further Zika evaluation for these infants unless additional testing confirms maternal infection. For older infants, parents and clinicians should decide together whether any further evaluations would be helpful. But, Dr. Adebanjo said, “If findings consistent with congenital Zika syndrome are identified at any time, referrals to appropriate specialties should be made, and subsequent evaluation should follow recommendations for infants with clinical findings consistent with congenital Zika.”
CDC also reiterated its special recommendations for infants who had a prenatal diagnosis of Zika infection. For now, these remain unchanged from 2016, although “as more data become available, understanding of the diagnostic role of prenatal ultrasound and amniocentesis will improve and guidance will be updated.”
No one has yet identified the optimal timing for a Zika diagnostic ultrasound. CDC recommends serial ultrasounds be done every 3-4 weeks for women with lab-confirmed prenatal Zika exposure. Women with possible exposure need only routine ultrasound screenings.
While Zika RNA has been identified in amniotic fluid, there is no consensus on the value of amniocentesis as a prenatal diagnostic tool. Investigations of serial amniocentesis suggests that viral shedding into the amniotic fluid might be transient. If the procedure is done for other reasons, Zika nucleic acid testing can be incorporated.
A shared decision-making process is key when making screening decisions that should be individually weighed, Dr. Adebanjo said. “For example, serial ultrasounds might be inconvenient, unpleasant, and expensive, and might prompt unnecessary interventions; amniocentesis carries additional known risks such as fetal loss. These potential harms of prenatal screening for congenital Zika syndrome might outweigh the clinical benefits for some patients. Therefore, these decisions should be individualized.”
Neither Dr. Adebanjo nor any of the coauthors had any financial disclosures.
Infants with possible prenatal Zika exposure who test positive for the virus should receive an in-depth ophthalmologic exam, intensified hearing testing, and a thorough neurological evaluation with brain imaging within 1 month of birth, according to new interim guidance set forth by the Centers for Disease Control and Prevention.
The new clinical management guidelines, published in the Oct. 20 issue of the Morbidity and Mortality Weekly Report, supersede the most recent CDC guidance, issued in August 2016. The agency deemed the update necessary after a recent convocation sponsored by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. The meeting drew dozens of practicing clinicians and federal agency representatives, who reviewed the ever-evolving body of knowledge on how to best manage the care of these infants. Since Zika emerged as a public health threat, clinicians have reported postnatal onset of some symptoms, including eye abnormalities, a developing microcephaly in infants with a normal head circumference at birth, EEG abnormalities, and diaphragmatic paralysis.
The guidance focuses on three groups: infants with clinical findings of Zika syndrome born to mothers with possible Zika exposure during pregnancy; infants without clinical findings of Zika syndrome whose mothers had lab-confirmed Zika exposure; and infants without symptoms whose mothers might have been exposed, but who did not have laboratory-confirmed infection (MMWR. 2017 Oct 20;66[41]:1089-120).
Infants with clinical findings consistent with Zika syndrome and mothers with possible prenatal Zika exposure
These infants should be tested for Zika virus with serum and urine tests. If those are negative and there is no other apparent cause of the symptoms, they should have a cerebrospinal fluid sample tested for Zika RNA and IgM Zika antibodies.
By 1 month, these infants need a head ultrasound and a detailed ophthalmologic exam. The eye exam should pick up any anomalies of the anterior and posterior eye, including microphthalmia, coloboma, intraocular calcifications, optic nerve hypoplasia and atrophy, and macular scarring with focal pigmentary retinal mottling.
A comprehensive neurological exam also is part of the recommendation. Seizures are sometimes part of Zika syndrome, but infants can also have subclinical EEG abnormalities. Advanced neuroimaging can identify both obvious and subtle brain abnormalities: cortical thinning, corpus callosum abnormalities, calcifications at the white/gray matter junction, and ventricular enlargement are possible findings.
As infants grow, clinicians should be alert for signs of increased intracranial pressure that could signal postnatal hydrocephalus. Diaphragmatic paralysis also has been seen; this manifests by respiratory distress. Dysphagia that interferes with feeding can develop as well.
The complicated clinical picture calls for a team approach, Dr. Adebanjo said. “The follow-up care of [these infants] requires a multidisciplinary team and an established medical home to facilitate the coordination of care and ensure that abnormal findings are addressed.”
Infants without clinical findings, whose mothers have lab-confirmed Zika exposure
Initially, these infants should have the same early head ultrasound, hearing, and eye exams as those who display clinical findings. All of these infants also should be tested for Zika virus in the same way as those with clinical findings.
If tests return a positive result, they should have all the investigations and follow-ups recommended for babies with clinical findings. If lab testing is negative, and clinical findings are normal, Zika infection is highly unlikely and they can receive routine care, although clinicians and parents should be on the lookout for any new symptoms that might suggest postnatal Zika syndrome.
Infants without clinical findings, whose mothers had possible, but unconfirmed, Zika exposure
This is a varied and large group, which includes women who were never tested during pregnancy, as well as those who could have had a false negative test. “Because the latter issue is not easily discerned, all mothers with possible exposure to Zika virus infection, including those who tested negative with currently available technology, should be considered in this group,” Dr. Adebanjo said.
CDC does not recommend further Zika evaluation for these infants unless additional testing confirms maternal infection. For older infants, parents and clinicians should decide together whether any further evaluations would be helpful. But, Dr. Adebanjo said, “If findings consistent with congenital Zika syndrome are identified at any time, referrals to appropriate specialties should be made, and subsequent evaluation should follow recommendations for infants with clinical findings consistent with congenital Zika.”
CDC also reiterated its special recommendations for infants who had a prenatal diagnosis of Zika infection. For now, these remain unchanged from 2016, although “as more data become available, understanding of the diagnostic role of prenatal ultrasound and amniocentesis will improve and guidance will be updated.”
No one has yet identified the optimal timing for a Zika diagnostic ultrasound. CDC recommends serial ultrasounds be done every 3-4 weeks for women with lab-confirmed prenatal Zika exposure. Women with possible exposure need only routine ultrasound screenings.
While Zika RNA has been identified in amniotic fluid, there is no consensus on the value of amniocentesis as a prenatal diagnostic tool. Investigations of serial amniocentesis suggests that viral shedding into the amniotic fluid might be transient. If the procedure is done for other reasons, Zika nucleic acid testing can be incorporated.
A shared decision-making process is key when making screening decisions that should be individually weighed, Dr. Adebanjo said. “For example, serial ultrasounds might be inconvenient, unpleasant, and expensive, and might prompt unnecessary interventions; amniocentesis carries additional known risks such as fetal loss. These potential harms of prenatal screening for congenital Zika syndrome might outweigh the clinical benefits for some patients. Therefore, these decisions should be individualized.”
Neither Dr. Adebanjo nor any of the coauthors had any financial disclosures.
FROM MMWR
British Society of Rheumatology issues first U.K. lupus guideline
From diagnosing, assessing, and managing common manifestations of nonrenal lupus, such as skin rashes and arthritis, through dealing with less common but potentially more serious problems such as kidney disease, the guideline aims to help everyone involved in the management of patients with SLE to give the best, evidenced-based care.
U.K.-specific guidance
The British Society for Rheumatology’s (BSR) guideline is the first to specifically cover lupus management in the United Kingdom, and it builds on existing European League Against Rheumatism (EULAR) guidance published almost a decade ago (Ann Rheum Dis. 2008;67:195-205), and more recent EULAR/European Renal Association–European Dialysis and Transplant Association (Ann Rheum Dis. 2012;71:1771-82) and American College of Rheumatology (Arthritis Care Res. 2012;64:797-808) recommendations on managing lupus nephritis (LN).
The BSR’s full guideline provides a summary of the EULAR/ERA-EDTA’s LN guidelines, and the degree to which their new guidelines concur.
As with all BSR guidelines, the recommendations have been developed by a multidisciplinary team. This included academic and consultant rheumatologists and nephrologists, rheumatology trainees, a primary care physician, a clinical nurse specialist, a patient representative, and a lay member. This should make the guideline relevant to anyone who may come across someone with SLE, including primary care physicians, dermatologists, and emergency medicine practitioners.
“These recommendations are based on the literature review covering the diagnosis, assessment, monitoring, and treatment of mild, moderate, and severe lupus, including neuropsychiatric disease,” the guideline authors stated. They noted that the reason they looked only at nonrenal disease was because the EULAR/ERA-EDTA recommendations for LN have been published close to the time that work was started on the guideline. Each of the recommendations the multidisciplinary team devised was carefully graded and the degree to which members of the team agreed with each recommendation was calculated.
Diagnosis recommendations
One of the key recommendations regarding the diagnosis of SLE is that a combination of clinical features and at least one relevant immunologic irregularity needs to be present. Blood tests, including serologic marker tests, should be performed if there is clinical suspicion of lupus.
Another recommendation on diagnosis is that if antinuclear antibodies are absent, then it is unlikely that the patient has lupus. This is because around 95% of SLE patients will test positive for antinuclear antibodies. Antiphospholipid antibodies should be tested in all patients with lupus at baseline, according to the guideline.
Monitoring recommendations
“Patients with SLE should be monitored on a regular basis for disease manifestations, drug toxicities, and comorbidities,” Dr. Gordon and her associates advised in one of the recommendations on monitoring patients. In another, they wrote that those with active disease need reviewing at least every 1-3 months, which should include evaluation of patients’ blood pressure, urine, renal function, anti-dsDNA antibodies, complement, a full blood count, and liver function tests.
It is also important to monitor patients for the presence of antiphospholipid antibodies, which are associated with thrombotic events, and it is always important to be on the lookout for comorbidities such as atherosclerotic disease and manage modifiable risk factors such as hypertension. The guideline does not go into detail about managing all of the potential complications of lupus, however, as these are covered by other national guidelines.
Treatment recommendations
Guidance on treatment is separated into how to treat patients with mild, moderate, and severe SLE. The guideline does not cover topical or systemic treatment for isolated cutaneous lupus, nor does it look at how to manage pediatric patients, the authors noted. General guidance is given on how to treat patients, and specific dosing regimens are beyond the scope of the guidelines.
The recommendations encourage the use of a variety of treatments to try to ensure less reliance on the use of steroids to control symptoms. The guideline authors noted that only hydroxychloroquine, corticosteroids, and belimumab are currently licensed treatments for lupus in the United Kingdom.
For mild disease, the disease-modifying antirheumatic drugs hydroxychloroquine and methotrexate are suggested, as are nonsteroidal anti-inflammatory drugs. If prednisolone is used, then it should be in low doses (7.5 mg or less per day). Patients should be encouraged to use sunscreen and sun avoidance to protect them against ultraviolet-induced skin lesions.
For moderate disease, higher steroid doses may be needed and immunosuppressives might be warranted, and in refractory cases, monoclonal antibody treatment may be necessary.
For severe disease, thorough investigation is essential to exclude other possible causes of any renal or neuropsychiatric manifestations. Immunosuppressive treatment is recommended, with biologic therapies considered on a case-by-case basis. Intravenous immunoglobulin and plasmapheresis may also be an option in certain patients.
Key standards of care
As general standards of care, Dr. Gordon and her coauthors wrote that “lupus patients should be referred to a physician with experience in managing lupus who can confirm the diagnosis, assess the level of disease activity, and provide advice on treatment and monitoring of the disease, its complications, and side effects of therapy.”
Furthermore, a multidisciplinary team approach is needed, and if a treatment is not helping get patients into a state of low disease activity within an expected time frame, “it is important to consider adherence to treatment and adjusting the therapy to reduce the accumulation of chronic damage.” It is also recommended that patients receive personalized advice, written information, and education about the disease and its drug treatment.
No specific funding was received from any organizations in the public, commercial, or not-for-profit sectors to carry out the work described in the guideline.
Dr. Gordon has undertaken consultancies and received honoraria from Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, MedImmune, Merck Serono, Parexel, Roche, and UCB. She has been a member of the speakers bureau for GlaxoSmithKline, UCB, and Eli Lilly. She also has received research grant support from Aspreva/Vifor Pharma in the past and currently receives it from UCB.
From diagnosing, assessing, and managing common manifestations of nonrenal lupus, such as skin rashes and arthritis, through dealing with less common but potentially more serious problems such as kidney disease, the guideline aims to help everyone involved in the management of patients with SLE to give the best, evidenced-based care.
U.K.-specific guidance
The British Society for Rheumatology’s (BSR) guideline is the first to specifically cover lupus management in the United Kingdom, and it builds on existing European League Against Rheumatism (EULAR) guidance published almost a decade ago (Ann Rheum Dis. 2008;67:195-205), and more recent EULAR/European Renal Association–European Dialysis and Transplant Association (Ann Rheum Dis. 2012;71:1771-82) and American College of Rheumatology (Arthritis Care Res. 2012;64:797-808) recommendations on managing lupus nephritis (LN).
The BSR’s full guideline provides a summary of the EULAR/ERA-EDTA’s LN guidelines, and the degree to which their new guidelines concur.
As with all BSR guidelines, the recommendations have been developed by a multidisciplinary team. This included academic and consultant rheumatologists and nephrologists, rheumatology trainees, a primary care physician, a clinical nurse specialist, a patient representative, and a lay member. This should make the guideline relevant to anyone who may come across someone with SLE, including primary care physicians, dermatologists, and emergency medicine practitioners.
“These recommendations are based on the literature review covering the diagnosis, assessment, monitoring, and treatment of mild, moderate, and severe lupus, including neuropsychiatric disease,” the guideline authors stated. They noted that the reason they looked only at nonrenal disease was because the EULAR/ERA-EDTA recommendations for LN have been published close to the time that work was started on the guideline. Each of the recommendations the multidisciplinary team devised was carefully graded and the degree to which members of the team agreed with each recommendation was calculated.
Diagnosis recommendations
One of the key recommendations regarding the diagnosis of SLE is that a combination of clinical features and at least one relevant immunologic irregularity needs to be present. Blood tests, including serologic marker tests, should be performed if there is clinical suspicion of lupus.
Another recommendation on diagnosis is that if antinuclear antibodies are absent, then it is unlikely that the patient has lupus. This is because around 95% of SLE patients will test positive for antinuclear antibodies. Antiphospholipid antibodies should be tested in all patients with lupus at baseline, according to the guideline.
Monitoring recommendations
“Patients with SLE should be monitored on a regular basis for disease manifestations, drug toxicities, and comorbidities,” Dr. Gordon and her associates advised in one of the recommendations on monitoring patients. In another, they wrote that those with active disease need reviewing at least every 1-3 months, which should include evaluation of patients’ blood pressure, urine, renal function, anti-dsDNA antibodies, complement, a full blood count, and liver function tests.
It is also important to monitor patients for the presence of antiphospholipid antibodies, which are associated with thrombotic events, and it is always important to be on the lookout for comorbidities such as atherosclerotic disease and manage modifiable risk factors such as hypertension. The guideline does not go into detail about managing all of the potential complications of lupus, however, as these are covered by other national guidelines.
Treatment recommendations
Guidance on treatment is separated into how to treat patients with mild, moderate, and severe SLE. The guideline does not cover topical or systemic treatment for isolated cutaneous lupus, nor does it look at how to manage pediatric patients, the authors noted. General guidance is given on how to treat patients, and specific dosing regimens are beyond the scope of the guidelines.
The recommendations encourage the use of a variety of treatments to try to ensure less reliance on the use of steroids to control symptoms. The guideline authors noted that only hydroxychloroquine, corticosteroids, and belimumab are currently licensed treatments for lupus in the United Kingdom.
For mild disease, the disease-modifying antirheumatic drugs hydroxychloroquine and methotrexate are suggested, as are nonsteroidal anti-inflammatory drugs. If prednisolone is used, then it should be in low doses (7.5 mg or less per day). Patients should be encouraged to use sunscreen and sun avoidance to protect them against ultraviolet-induced skin lesions.
For moderate disease, higher steroid doses may be needed and immunosuppressives might be warranted, and in refractory cases, monoclonal antibody treatment may be necessary.
For severe disease, thorough investigation is essential to exclude other possible causes of any renal or neuropsychiatric manifestations. Immunosuppressive treatment is recommended, with biologic therapies considered on a case-by-case basis. Intravenous immunoglobulin and plasmapheresis may also be an option in certain patients.
Key standards of care
As general standards of care, Dr. Gordon and her coauthors wrote that “lupus patients should be referred to a physician with experience in managing lupus who can confirm the diagnosis, assess the level of disease activity, and provide advice on treatment and monitoring of the disease, its complications, and side effects of therapy.”
Furthermore, a multidisciplinary team approach is needed, and if a treatment is not helping get patients into a state of low disease activity within an expected time frame, “it is important to consider adherence to treatment and adjusting the therapy to reduce the accumulation of chronic damage.” It is also recommended that patients receive personalized advice, written information, and education about the disease and its drug treatment.
No specific funding was received from any organizations in the public, commercial, or not-for-profit sectors to carry out the work described in the guideline.
Dr. Gordon has undertaken consultancies and received honoraria from Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, MedImmune, Merck Serono, Parexel, Roche, and UCB. She has been a member of the speakers bureau for GlaxoSmithKline, UCB, and Eli Lilly. She also has received research grant support from Aspreva/Vifor Pharma in the past and currently receives it from UCB.
From diagnosing, assessing, and managing common manifestations of nonrenal lupus, such as skin rashes and arthritis, through dealing with less common but potentially more serious problems such as kidney disease, the guideline aims to help everyone involved in the management of patients with SLE to give the best, evidenced-based care.
U.K.-specific guidance
The British Society for Rheumatology’s (BSR) guideline is the first to specifically cover lupus management in the United Kingdom, and it builds on existing European League Against Rheumatism (EULAR) guidance published almost a decade ago (Ann Rheum Dis. 2008;67:195-205), and more recent EULAR/European Renal Association–European Dialysis and Transplant Association (Ann Rheum Dis. 2012;71:1771-82) and American College of Rheumatology (Arthritis Care Res. 2012;64:797-808) recommendations on managing lupus nephritis (LN).
The BSR’s full guideline provides a summary of the EULAR/ERA-EDTA’s LN guidelines, and the degree to which their new guidelines concur.
As with all BSR guidelines, the recommendations have been developed by a multidisciplinary team. This included academic and consultant rheumatologists and nephrologists, rheumatology trainees, a primary care physician, a clinical nurse specialist, a patient representative, and a lay member. This should make the guideline relevant to anyone who may come across someone with SLE, including primary care physicians, dermatologists, and emergency medicine practitioners.
“These recommendations are based on the literature review covering the diagnosis, assessment, monitoring, and treatment of mild, moderate, and severe lupus, including neuropsychiatric disease,” the guideline authors stated. They noted that the reason they looked only at nonrenal disease was because the EULAR/ERA-EDTA recommendations for LN have been published close to the time that work was started on the guideline. Each of the recommendations the multidisciplinary team devised was carefully graded and the degree to which members of the team agreed with each recommendation was calculated.
Diagnosis recommendations
One of the key recommendations regarding the diagnosis of SLE is that a combination of clinical features and at least one relevant immunologic irregularity needs to be present. Blood tests, including serologic marker tests, should be performed if there is clinical suspicion of lupus.
Another recommendation on diagnosis is that if antinuclear antibodies are absent, then it is unlikely that the patient has lupus. This is because around 95% of SLE patients will test positive for antinuclear antibodies. Antiphospholipid antibodies should be tested in all patients with lupus at baseline, according to the guideline.
Monitoring recommendations
“Patients with SLE should be monitored on a regular basis for disease manifestations, drug toxicities, and comorbidities,” Dr. Gordon and her associates advised in one of the recommendations on monitoring patients. In another, they wrote that those with active disease need reviewing at least every 1-3 months, which should include evaluation of patients’ blood pressure, urine, renal function, anti-dsDNA antibodies, complement, a full blood count, and liver function tests.
It is also important to monitor patients for the presence of antiphospholipid antibodies, which are associated with thrombotic events, and it is always important to be on the lookout for comorbidities such as atherosclerotic disease and manage modifiable risk factors such as hypertension. The guideline does not go into detail about managing all of the potential complications of lupus, however, as these are covered by other national guidelines.
Treatment recommendations
Guidance on treatment is separated into how to treat patients with mild, moderate, and severe SLE. The guideline does not cover topical or systemic treatment for isolated cutaneous lupus, nor does it look at how to manage pediatric patients, the authors noted. General guidance is given on how to treat patients, and specific dosing regimens are beyond the scope of the guidelines.
The recommendations encourage the use of a variety of treatments to try to ensure less reliance on the use of steroids to control symptoms. The guideline authors noted that only hydroxychloroquine, corticosteroids, and belimumab are currently licensed treatments for lupus in the United Kingdom.
For mild disease, the disease-modifying antirheumatic drugs hydroxychloroquine and methotrexate are suggested, as are nonsteroidal anti-inflammatory drugs. If prednisolone is used, then it should be in low doses (7.5 mg or less per day). Patients should be encouraged to use sunscreen and sun avoidance to protect them against ultraviolet-induced skin lesions.
For moderate disease, higher steroid doses may be needed and immunosuppressives might be warranted, and in refractory cases, monoclonal antibody treatment may be necessary.
For severe disease, thorough investigation is essential to exclude other possible causes of any renal or neuropsychiatric manifestations. Immunosuppressive treatment is recommended, with biologic therapies considered on a case-by-case basis. Intravenous immunoglobulin and plasmapheresis may also be an option in certain patients.
Key standards of care
As general standards of care, Dr. Gordon and her coauthors wrote that “lupus patients should be referred to a physician with experience in managing lupus who can confirm the diagnosis, assess the level of disease activity, and provide advice on treatment and monitoring of the disease, its complications, and side effects of therapy.”
Furthermore, a multidisciplinary team approach is needed, and if a treatment is not helping get patients into a state of low disease activity within an expected time frame, “it is important to consider adherence to treatment and adjusting the therapy to reduce the accumulation of chronic damage.” It is also recommended that patients receive personalized advice, written information, and education about the disease and its drug treatment.
No specific funding was received from any organizations in the public, commercial, or not-for-profit sectors to carry out the work described in the guideline.
Dr. Gordon has undertaken consultancies and received honoraria from Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, MedImmune, Merck Serono, Parexel, Roche, and UCB. She has been a member of the speakers bureau for GlaxoSmithKline, UCB, and Eli Lilly. She also has received research grant support from Aspreva/Vifor Pharma in the past and currently receives it from UCB.
FROM RHEUMATOLOGY