User login
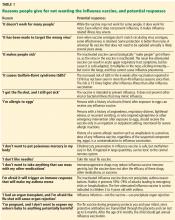
How to respond to flu vaccine doubters
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
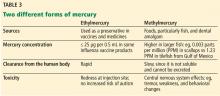
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
The benefits of influenza vaccination are clear to those in the medical community. Yet misinformation and unfounded fears continue to discourage some people from getting a flu shot. During the 2018–2019 influenza season, only 45% of US adults and 63% of children were vaccinated.1
‘IT DOESN’T WORK FOR MANY PEOPLE’
Multiple studies have shown that the flu vaccine prevents millions of flu cases and flu-related doctor’s visits each year. During the 2016–2017 flu season, flu vaccine prevented an estimated 5.3 million influenza cases, 2.6 million influenza-associated medical visits, and 85,000 influenza-associated hospitalizations.2
Several viral and host factors affect vaccine effectiveness. In seasons when the vaccine viruses have matched circulating strains, flu vaccine has been shown to reduce the following:
- The risk of having to go to the doctor with flu by 40% to 60%
- Children’s risk of flu-related death and intensive care unit (ICU) admission by 74%
- The risk in adults of flu-associated hospitalizations by 40% and ICU admission by 82%
- The rate of cardiac events in people with heart disease
- Hospitalizations in people with diabetes or underlying chronic lung disease.3
In people hospitalized with influenza despite receiving the flu vaccine for the season, studies have shown that receiving the flu vaccine shortens the average duration of hospitalization, reduces the chance of ICU admission by 59%, shortens the duration of ICU stay by 4 days, and reduces deaths.3
‘IT TARGETS THE WRONG VIRUS’
Selecting an effective influenza vaccine is a challenge. Every year, the World Health Organization and the CDC decide on the influenza strains expected to circulate in the upcoming flu season in the Northern Hemisphere, based on data for circulating strains in the Southern Hemisphere. This decision takes place about 7 months before the expected onset of the flu season. Flu viruses may mutate between the time the decision is made and the time the vaccine is administered (as well as after the flu season starts). Also, vaccine production in eggs needs time, which is why this decision must be made several months ahead of the flu season.
Vaccine effectiveness varies by virus serotype. Vaccines are typically less effective against influenza A H3N2 viruses than against influenza A H1N1 and influenza B viruses. Effectiveness also varies from season to season depending on how close the vaccine serotypes match the circulating serotypes, but some effectiveness is retained even in seasons when some of the serotypes don’t match circulating viruses. For example, in the 2017–2018 season, when the influenza A H3N2 vaccine serotype did not match the circulating serotype, the overall effectiveness in preventing medically attended, laboratory-confirmed influenza virus infection was 36%.5
A universal flu vaccine that does not need to be updated annually is the ultimate solution, but according to the National Institute of Allergy and Infectious Diseases, such a vaccine is likely several years away.6
‘IT MAKES PEOPLE SICK’
Pain at the injection site of a flu shot occurs in 10% to 65% of people, lasts less than 2 days, and does not usually interfere with daily activities.7
Systemic symptoms such as fever, malaise, and myalgia may occur in people who have had no previous exposure to the influenza virus antigens in the vaccine, particularly in children. In adults, the frequency of systemic symptoms after the flu shot is similar to that with placebo.
The Vaccine Adverse Event Reporting System, which has been capturing data since 1990, shows that the influenza vaccine accounted for 5.7% of people who developed malaise after receiving any vaccine.8
The injectable inactivated influenza vaccine cannot biologically cause an influenza virus-related illness, since the inactivated vaccine viruses can elicit a protective immune response but cannot replicate. The nasal live-attenuated flu vaccine can in theory cause acute illness in the person receiving it, but because it is cold-adapted, it multiplies only in the colder environment of the nasal epithelium, not in the lower airways where the temperature is higher. Consequently, the vaccine virus triggers immunity by multiplying in the nose, but doesn’t infect the lungs.
From 10% to 50% of people who receive the nasal live-attenuated vaccine develop runny nose, wheezing, headache, vomiting, muscle aches, fever, sore throat, or cough shortly after receiving the vaccine, but these symptoms are usually mild and short-lived.
The most common reactions people have to flu vaccines are considerably less severe than the symptoms caused by actual flu illness.
While influenza illness results in natural immunity to the specific viral serotype causing it, this illness results in hospitalization in 2% and is fatal in 0.16% of people. Influenza vaccine results in immunity to the serotypes included in the vaccine, and multiple studies have not found a causal relationship between vaccination and death.9
‘IT CAUSES GUILLAIN-BARRÉ SYNDROME’
In the United States, 3,000 to 6,000 people per year develop Guillain-Barré syndrome, or 1 to 2 of every 100,000, which translates to 80 to 160 cases per week.10 While the exact cause of Guillain-Barré syndrome is unknown, about two-thirds of people have an acute diarrheal or respiratory illness within 3 months before the onset of symptoms. In 1976, the estimated attributable risk of influenza vaccine-related Guillain-Barré syndrome in the US adult population was 1 case per 100,000 in the 6 weeks after vaccination.11 Studies in subsequent influenza seasons have not shown similar findings.12 In fact, one study showed that the risk of developing Guillain-Barré syndrome was 15 times higher after influenza illness than after influenza vaccination.13
Since 5% to 15% of the US population develop symptomatic influenza annually,14 the decision to vaccinate with respect to the risk of Guillain-Barré syndrome should be obvious: vaccinate. The correct question to ask before influenza vaccination should be, “Have you previously developed Guillain-Barré syndrome within 6 weeks after receiving the flu vaccine?” If the answer is yes, the CDC considers this a caution, not a contraindication against receiving the influenza vaccine, since the benefit may still outweigh the risk.
‘I GOT THE FLU SHOT AND STILL GOT SICK’
The flu vaccine does not prevent illnesses caused by other viruses or bacteria that can make people sick during flu season. Influenza, the common cold, and streptococcal pharyngitis can have similar symptoms that make it difficult for patients—and, frequently, even healthcare providers—to distinguish between these illnesses with certainty.
One study suggested that influenza vaccine recipients had an increased risk of virologically confirmed noninfluenza respiratory viral infections,15 citing the phenomenon of virus interference that was described in the 1940s16 as a potential explanation. In essence, people protected against influenza by the vaccine may lack temporary nonspecific immunity against other respiratory viruses. However, these findings have not been replicated in subsequent studies.17
Viral gastroenteritis, mistakenly called “stomach flu,” is also not prevented by influenza vaccination.
‘I’M ALLERGIC TO EGGS’
The prevalence of egg allergy in US children is 0.5% to 2.5%.18 Most outgrow it by school age, but in one-third, the allergy persists into adulthood.
In general, people who can eat lightly cooked eggs (eg, scrambled eggs) without a reaction are unlikely to be allergic. On the other hand, the fact that egg-allergic people may tolerate egg included in baked products does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reaction to eggs and egg-containing foods, in addition to skin or blood testing for immunoglobulin E directed against egg proteins.19
Most currently available influenza vaccines are prepared by propagation of virus in embryonated eggs and so may contain trace amounts of egg proteins such as ovalbumin, with the exception of the inactivated quadrivalent recombinant influenza vaccine (Flublok) and the inactivated quadrivalent cell culture-based vaccine (Flucelvax).
The ACIP recommends that persons with a history of urticaria (hives) after exposure to eggs should receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Persons who report having angioedema, respiratory distress, lightheadedness, or recurrent vomiting, or who required epinephrine or another emergency medical intervention after exposure to eggs, should receive the influenza vaccine in an inpatient or outpatient medical setting under the supervision of a healthcare provider who is able to recognize and manage severe allergic reactions.
A history of severe allergic reaction such as anaphylaxis to a previous dose of any influenza vaccine, regardless of the vaccine component (including eggs) suspected of being responsible for the reaction, is a contraindication to influenza vaccination. The ACIP recommends that vaccine providers consider observing patients for 15 minutes after administration of any vaccine (regardless of history of egg allergy) to decrease the risk of injury should syncope occur.20
‘I DON’T WANT TO PUT POISONOUS MERCURY IN MY BODY’
A process of biomagnification of methylmercury occurs when humans eat large fish that have eaten smaller fish. Thus, larger fish such as shark can be hazardous for women who are or may become pregnant, for nursing mothers, and for young children, while smaller fish such as herring are relatively safe.
As a precautionary measure, thimerosal was taken out of childhood vaccines in the United States in 2001. Thimerosal-free influenza vaccine formulations include the nasal live-attenuated flu vaccine, the inactivated quadrivalent recombinant influenza vaccine, and the inactivated quadrivalent cell culture-based vaccine.
‘I DON’T LIKE NEEDLES’
At least 10% of US adults have aichmophobia, the fear of sharp objects including needles.22 Vasovagal syncope is the most common manifestation. Behavioral therapy, topical anesthetics, and systemic anxiolytics have variable efficacy in treating needle phobia. For those who are absolutely averse to needles, the nasal flu vaccine is an appropriate alternative.
‘I DON’T WANT TO TAKE ANYTHING THAT CAN MESS WITH MY OTHER MEDICATIONS’
Some immunosuppressive medications may decrease influenza vaccine immunogenicity. Concomitant administration of the inactivated influenza vaccine with other vaccines is safe and does not alter immunogenicity of other vaccines.1 The live-attenuated influenza vaccine is contraindicated in children and adolescents taking aspirin or other salicylates due to the risk of Reye syndrome.
‘I’M AFRAID IT WILL TRIGGER AN IMMUNE RESPONSE THAT WILL MAKE MY ASTHMA WORSE’
A recent systematic review and meta-analysis showed that the inactivated influenza vaccine is not associated with asthma exacerbation.23 However, the nasal live-attenuated influenza vaccine is contraindicated in children 2 to 4 years old who have asthma and should be used with caution in persons with asthma 5 years old and older. In the systematic review, influenza vaccine prevented 59% to 78% of asthma attacks leading to emergency visits or hospitalization.23 In other immune-mediated diseases such as rheumatoid arthritis, influenza vaccine does not precipitate exacerbations.24
‘I HAD AN ORGAN TRANSPLANT, AND I’M AFRAID THE FLU SHOT WILL CAUSE ORGAN REJECTION’
A study of 51,730 kidney transplant recipients found that receipt of the inactivated influenza vaccine in the first year after transplant was associated with a lower risk of subsequent allograft loss (adjusted hazard ratio 0.77; 95% confidence interval 0.69–0.85; P < .001) and death (adjusted hazard ratio 0.82; 95% confidence interval 0.76–0.89; P < .001).25 In the same study, although acute rejection in the first year was not associated with influenza vaccination, influenza infection in the first year was associated with rejection (odds ratio 1.58; 95% confidence interval 1.10–2.26; P < 0.001), but not with graft loss or death. Solid organ transplant recipients should receive the inactivated influenza vaccine starting 3 months after transplant.26
Influenza vaccination has not been shown to precipitate graft-vs-host disease in hematopoietic stem cell transplant recipients. These patients should also receive the inactivated influenza vaccine starting 3 to 6 months after transplant.27
The nasal live-attenuated influenza vaccine is contraindicated in these immunocompromised patients.
‘I’M PREGNANT, AND I DON’T WANT TO EXPOSE MY UNBORN BABY TO ANYTHING POTENTIALLY HARMFUL’
The morbidity and mortality risk from influenza is high in children under 2 years old because of low immunogenicity to flu vaccine. This is particularly true in children younger than 6 months, but the vaccine is not recommended in this population. The best way to protect infants is for all household members to be vaccinated against the flu.
Equally important, morbidity and mortality risk from influenza is much higher in pregnant women than in the general population. Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants. A recently published study showed that 18% of infants who developed influenza required hospitalization.28 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively. Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.29 A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.30
Healthcare providers should try to understand the public’s misconceptions31 about seasonal influenza and influenza vaccines in order to best address them.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Flu vaccination coverage, United States, 2018–19 influenza season. www.cdc.gov/flu/fluvaxview/coverage-1819estimates.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Immunogenicity, efficacy, and effectiveness of influenza vaccines. www.cdc.gov/flu/professionals/acip/immunogenicity.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). What are the benefits of flu vaccination? www.cdc.gov/flu/prevent/vaccine-benefits.htm. Accessed November 13, 2019.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2019–20 influenza season. MMWR Recomm Rep 2019; 68(3):1–21. doi:10.15585/mmwr.rr6803a1
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Centers for Disease Control and Prevention (CDC). Seasonal influenza vaccine safety: a summary for clinicians. www.cdc.gov/flu/professionals/vaccination/vaccine_safety.htm. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). About the Vaccine Adverse Event Reporting System (VAERS). https://wonder.cdc.gov/vaers.html. Accessed November 13, 2019.
- Miller ER, Moro PL, Cano M, Shimabukuro TT. Deaths following vaccination: what does the evidence show? Vaccine 2015; 33(29):3288–3292. doi:10.1016/j.vaccine.2015.05.023
- Centers for Disease Control and Prevention (CDC). Guillain-Barré syndrome and flu vaccine. www.cdc.gov/flu/prevent/guillainbarre.htm. Accessed November 13, 2019.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, et al. Guillain-Barre syndrome following vaccination in the national influenza immunization program, United States, 1976–1977. Am J Epidemiol 1979; 110(2):105–123. doi:10.1093/oxfordjournals.aje.a112795
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57(2):197–204. doi:10.1093/cid/cit222
- Kwong JC, Vasa PP, Campitelli MA, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis 2013; 13(9):769–776. doi:10.1016/S1473-3099(13)70104-X
- Centers for Disease Control and Prevention (CDC). Disease burden of influenza. www.cdc.gov/flu/about/burden/index.html. Accessed November 13, 2019.
- Cowling BJ, Fang VJ, Nishiura H, et al. Increased risk of noninfluenza respiratory virus infections associated with receipt of inactivated influenza vaccine. Clin Infect Dis 2012; 54(12):1778–1783. doi:10.1093/cid/cis307
- Henle W, Henle G. Interference of inactive virus with the propagation of virus of influenza. Science 1943; 98(2534):87–89. doi:10.1126/science.98.2534.87
- Sundaram ME, McClure DL, VanWormer JJ, Friedrich TC, Meece JK, Belongia EA. Influenza vaccination is not associated with detection of noninfluenza respiratory viruses in seasonal studies of influenza vaccine effectiveness. Clin Infect Dis 2013; 57(6):789–793. doi:10.1093/cid/cit379
- Caubet JC, Wang J. Current understanding of egg allergy. Pediatr Clin North Am 2011; 58(2):427–443. doi:10.1016/j.pcl.2011.02.014
- Erlewyn-Lajeunesse M, Brathwaite N, Lucas JS, Warner JO. Recommendations for the administration of influenza vaccine in children allergic to egg. BMJ 2009; 339:b3680. doi:10.1136/bmj.b3680
- Ezeanolue E, Harriman K, Hunter P, Kroger A, Pellegrini C. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf. Accessed November 13, 2019.
- Centers for Disease Control and Prevention (CDC). Thimerosal in vaccines. www.cdc.gov/vaccinesafety/concerns/thimerosal/index.html. Accessed November 13, 2019.
- Hamilton JG. Needle phobia: a neglected diagnosis. J Fam Pract 1995; 41(2):169–175. pmid:7636457
- Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65(8):1388–1395. doi:10.1093/cid/cix524
- Fomin I, Caspi D, Levy V, et al. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis 2006; 65(2):191–194. doi:10.1136/ard.2005.036434
- Hurst FP, Lee JJ, Jindal RM, Agodoa LY, Abbott KC. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol 2011; 6(5):1192–1197. doi:10.2215/CJN.05430610
- Chong PP, Handler L, Weber DJ. A systematic review of safety and immunogenicity of influenza vaccination strategies in solid organ transplant recipients. Clin Infect Dis 2018; 66(11):1802–1811. doi:10.1093/cid/cix1081
- Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant 2008; 42(10):637–641. doi:10.1038/bmt.2008.264
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Centers for Disease Control and Prevention (CDC). Misconceptions about seasonal flu and flu vaccines. www.cdc.gov/flu/prevent/misconceptions.htm. Accessed November 13, 2019.
Influenza update 2018–2019: 100 years after the great pandemic
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
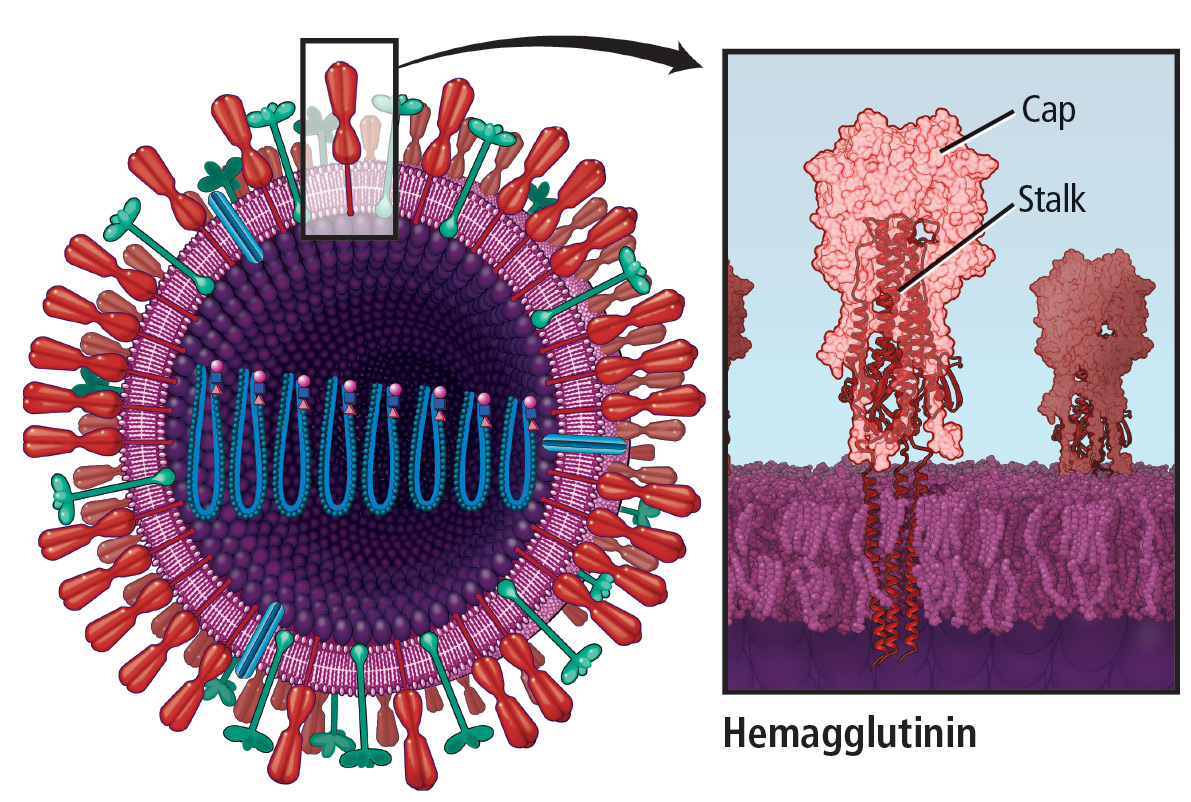
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
This centennial year update focuses primarily on immunization, but also reviews epidemiology, transmission, and treatment.
EPIDEMIOLOGY
2017–2018 was a bad season
The 2017–2018 influenza epidemic was memorable, dominated by influenza A(H3N2) viruses with morbidity and mortality rates approaching pandemic numbers. It lasted 19 weeks, killed more people than any other epidemic since 2010, particularly children, and was associated with 30,453 hospitalizations—almost twice the previous season high in some parts of the United States.2
Regrettably, 171 unvaccinated children died during 2017–2018, accounting for almost 80% of deaths.2 The mean age of the children who died was 7.1 years; 51% had at least 1 underlying medical condition placing them at risk for influenza-related complications, and 57% died after hospitalization.2
Recent estimates of the incidence of symptomatic influenza among all ages ranged from 3% to 11%, which is slightly lower than historical estimates. The rates were higher for children under age 18 than for adults.3 Interestingly, influenza A(H3N2) accounted for 50% of cases of non-mumps viral parotitis during the 2014–2015 influenza season in the United States.4
Influenza C exists but is rare
Influenza A and B account for almost all influenza-related outpatient visits and hospitalizations. Surveillance data from May 2013 through December 2016 showed that influenza C accounts for 0.5% of influenza-related outpatient visits and hospitalizations, particularly affecting children ages 6 to 24 months. Medical comorbidities and copathogens were seen in all patients requiring intensive care and in most hospitalizations.5 Diagnostic tests for influenza C are not widely available.
Dogs and cats: Factories for new flu strains?
While pigs and birds are the major reservoirs of influenza viral genetic diversity from which infection is transmitted to humans, dogs and cats have recently emerged as possible sources of novel reassortant influenza A.6 With their frequent close contact with humans, our pets may prove to pose a significant threat.
Obesity a risk factor for influenza
Obesity emerged as a risk factor for severe influenza in the 2009 pandemic. Recent data also showed that obesity increases the duration of influenza A virus shedding, thus increasing duration of contagiousness.7
Influenza a cardiovascular risk factor
Previous data showed that influenza was a risk factor for cardiovascular events. Two recent epidemiologic studies from the United Kingdom showed that laboratory-confirmed influenza was associated with higher rates of myocardial infarction and stroke for up to 4 weeks.8,9
Which strain is the biggest threat?
Predicting which emerging influenza serotype may cause the next pandemic is difficult, but influenza A(H7N9), which had not infected humans until 2013 but has since infected about 1,600 people in China and killed 37% of them, appears to have the greatest potential.10
National influenza surveillance programs and influenza-related social media applications have been developed and may get a boost from technology. A smartphone equipped with a temperature sensor can instantly detect one’s temperature with great precision. A 2018 study suggested that a smartphone-driven thermometry application correlated well with national influenza-like illness activity and improved its forecast in real time and up to 3 weeks in advance.11
TRANSMISSION
Humidity may not block transmission
Animal studies have suggested that humidity in the air interferes with transmission of airborne influenza virus, partially from biologic inactivation. But when a recent study used humidity-controlled chambers to investigate the stability of the 2009 influenza A(H1N1) virus in suspended aerosols and stationary droplets, the virus remained infectious in aerosols across a wide range of relative humidities, challenging the common belief that humidity destabilizes respiratory viruses in aerosols.12
One sick passenger may not infect the whole plane
Transmission of respiratory viruses on airplane flights has long been considered a potential avenue for spreading influenza. However, a recent study that monitored movements of individuals on 10 transcontinental US flights and simulated inflight transmission based on these data showed a low probability of direct transmission, except for passengers seated in close proximity to an infectious passenger.13
WHAT’S IN THE NEW FLU SHOT?
The 2018–2019 quadrivalent vaccine for the Northern Hemisphere14 contains the following strains:
- A/Michigan/45/2015 A(H1N1)pdm09-like virus
- A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus
- B/Colorado/06/2017-like virus (Victoria lineage)
- B/Phuket/3073/2013-like virus (Yamagata lineage).
The A(H3N2) (Singapore) and B/Victoria lineage components are new this year. The A(H3N2) strain was the main cause of the 2018 influenza epidemic in the Southern Hemisphere.
The quadrivalent live-attenuated vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, has made a comeback and is recommended for the 2018–2019 season in people for whom it is appropriate based on age and comorbidities.15 Although it was effective against influenza B and A(H3N2) viruses, it was less effective against the influenza A(H1N1)pdm09-like viruses during the 2013–2014 and 2015–2016 seasons.
A/Slovenia/2903/2015, the new A(H1N1)pdm09-like virus included in the 2018–2019 quadrivalent live-attenuated vaccine, is significantly more immunogenic than its predecessor, A/Bolivia/559/2013, but its clinical effectiveness remains to be seen.
PROMOTING VACCINATION
How effective is it?
Influenza vaccine effectiveness in the 2017–2018 influenza season was 36% overall, 67% against A(H1N1), 42% against influenza B, and 25% against A(H3N2).16 It is estimated that influenza vaccine prevents 300 to 4,000 deaths annually in the United States alone.17
A 2018 Cochrane review17 concluded that vaccination reduced the incidence of influenza by about half, with 2.3% of the population contracting the flu without vaccination compared with 0.9% with vaccination (risk ratio 0.41, 95% confidence interval 0.36–0.47). The same review found that 71 healthy adults need to be vaccinated to prevent 1 from experiencing influenza, and 29 to prevent 1 influenza-like illness.
Several recent studies showed that influenza vaccine effectiveness varied based on age and influenza serotype, with higher effectiveness in people ages 5 to 17 and ages 18 to 64 than in those age 65 and older.18–20 A mathematical model of influenza transmission and vaccination in the United States determined that even relatively low-efficacy influenza vaccines can be very useful if optimally distributed across age groups.21
Vaccination rates are low, and ‘antivaxxers’ are on the rise
Although the influenza vaccine is recommended in the United States for all people age 6 months and older regardless of the state of their health, vaccination rates remain low. In 2016, only 37% of employed adults were vaccinated. The highest rate was for government employees (45%), followed by private employees (36%), followed by the self-employed (30%).22
A national goal is to immunize 80% of all Americans and 90% of at-risk populations (which include children and the elderly).23 The number of US hospitals that require their employees to be vaccinated increased from 37.1% in 2013 to 61.4% in 2017.24 Regrettably, as of March 2018, 14 lawsuits addressing religious objections to hospital influenza vaccination mandates have been filed.25
Despite hundreds of studies demonstrating the efficacy, safety, and cost savings of influenza vaccination, the antivaccine movement has been growing in the United States and worldwide.26 All US states except West Virginia, Mississippi, and California allow nonmedical exemptions from vaccination based on religious or personal belief.27 Several US metropolitan areas represent “hot spots” for these exemptions.28 This may render such areas vulnerable to vaccine-preventable diseases, including influenza.
Herd immunity: We’re all in this together
Some argue that the potential adverse effects and the cost of vaccination outweigh the benefits, but the protective benefits of herd immunity are significant for those with comorbidities or compromised immunity.
Educating the public about herd immunity and local influenza vaccination uptake increases people’s willingness to be vaccinated.29 A key educational point is that at least 70% of a community needs to be vaccinated to prevent community outbreaks; this protects everyone, including those who do not mount a protective antibody response to influenza vaccination and those who are not vaccinated.
DOES ANNUAL VACCINATION BLUNT ITS EFFECTIVENESS?
Some studies from the 1970s and 1980s raised concern over a possible negative effect of annual influenza vaccination on vaccine effectiveness. The “antigenic distance hypothesis” holds that vaccine effectiveness is influenced by antigenic similarity between the previous season’s vaccine serotypes and the epidemic serotypes, as well as the antigenic similarity between the serotypes of the current and previous seasons.
A meta-analysis of studies from 2010 through 2015 showed significant inconsistencies in repeat vaccination effects within and between seasons and serotypes. It also showed that vaccine effectiveness may be influenced by more than 1 previous season, particularly for influenza A(H3N2), in which repeated vaccination can blunt the hemagglutinin antibody response.30
A study from Japan showed that people who needed medical attention for influenza in the previous season were at lower risk of a similar event in the current season.31 Prior-season influenza vaccination reduced current-season vaccine effectiveness only in those who did not have medically attended influenza in the prior season. This suggests that infection is more immunogenic than vaccination, but only against the serotype causing the infection and not the other serotypes included in the vaccine.
An Australian study showed that annual influenza vaccination did not decrease vaccine effectiveness against influenza-associated hospitalization. Rather, effectiveness increased by about 15% in those vaccinated in both current and previous seasons compared with those vaccinated in either season alone.32
European investigators showed that repeated seasonal influenza vaccination in the elderly prevented the need for hospitalization due to influenza A(H3N2) and B, but not A(H1N1)pdm09.33
VACCINATION IN SPECIAL POPULATIONS
High-dose vaccine for older adults
The high-dose influenza vaccine has been licensed since 2009 for use in the United States for people ages 65 and older.
Recent studies confirmed that high-dose vaccine is more effective than standard-dose vaccine in veterans34 and US Medicare beneficiaries.35
The high-dose vaccine is rapidly becoming the primary vaccine given to people ages 65 and older in retail pharmacies, where vaccination begins earlier in the season than in providers’ offices.36 Some studies have shown that the standard-dose vaccine wanes in effectiveness toward the end of the influenza season (particularly if the season is long) if it is given very early. It remains to be seen whether the same applies to the high-dose influenza vaccine.
Some advocate twice-annual influenza vaccination, particularly for older adults living in tropical and subtropical areas, where influenza seasons are more prolonged. However, a recently published study observed reductions in influenza-specific hemagglutination inhibition and cell-mediated immunity after twice-annual vaccination.37
Vaccination is beneficial during pregnancy
Many studies have shown the value of influenza vaccination during pregnancy for both mothers and their infants.
One recently published study showed that 18% of infants who developed influenza required hospitalization.38 In that study, prenatal and postpartum maternal influenza vaccination decreased the odds of influenza in infants by 61% and 53%, respectively.
Another study showed that vaccine effectiveness did not vary by gestational age at vaccination.39
Some studies have shown that influenza virus infection can increase susceptibility to certain bacterial infections. A post hoc analysis of an influenza vaccination study in pregnant women suggested that the vaccine was also associated with decreased rates of pertussis in these women.40
Factors that make vaccination less effective
Several factors including age-related frailty and iatrogenic and disease-related immunosuppression can affect vaccine effectiveness.
Frailty. A recent study showed that vaccine effectiveness was 77.6% in nonfrail older adults but only 58.7% in frail older adults.41
Immunosuppression. Temporary discontinuation of methotrexate for 2 weeks after influenza vaccination in patients with rheumatoid arthritis improves vaccine immunogenicity without precipitating disease flare.42 Solid-organ and hematopoietic stem cell transplant recipients who received influenza vaccine were less likely to develop pneumonia and require intensive care unit admission.43
The high-dose influenza vaccine is more immunogenic than the standard-dose vaccine in solid-organ transplant recipients.44
Statins are widely prescribed and have recently been associated with reduced influenza vaccine effectiveness against medically attended acute respiratory illness, but their benefits in preventing cardiovascular events outweigh this risk.45
FUTURE VACCINE CONSIDERATIONS
Moving away from eggs
During the annual egg-based production process, which takes several months, the influenza vaccine acquires antigenic changes that allow replication in eggs, particularly in the hemagglutinin protein, which mediates receptor binding. This process of egg adaptation may cause antigenic changes that decrease vaccine effectiveness against circulating viruses.
The cell-based baculovirus influenza vaccine grown in dog kidney cells has higher antigenic content and is not subject to the limitations of egg-based vaccine, although it still requires annual updates. A recombinant influenza vaccine reduces the probability of influenza-like illness by 30% compared with the egg-based influenza vaccine, but also still requires annual updates.46 The market share of these non-egg-based vaccines is small, and thus their effectiveness has yet to be demonstrated.
The US Department of Defense administered the cell-based influenza vaccine to about one-third of Armed Forces personnel, their families, and retirees in the 2017–2018 influenza seasons, and data on its effectiveness are expected in the near future.47
A universal vaccine would be ideal
The quest continues for a universal influenza vaccine, one that remains protective for several years and does not require annual updates.48 Such a vaccine would protect against seasonal epidemic influenza drift variants and pandemic strains. More people could likely be persuaded to be vaccinated once rather than every year.
An ideal universal vaccine would be suitable for all age groups, at least 75% effective against symptomatic influenza virus infection, protective against all influenza A viruses (influenza A, not B, causes pandemics and seasonal epidemics), and durable through multiple influenza seasons.51
Research and production of such a vaccine are expected to require funding of about $1 billion over the next 5 years.
Boosting effectiveness
Estimates of influenza vaccine effectiveness range from 40% to 60% in years when the vaccine viruses closely match the circulating viruses, and variably lower when they do not match. The efficacy of most other vaccines given to prevent other infections is much higher.
New technologies to improve influenza vaccine effectiveness are needed, particularly for influenza A(H3N2) viruses, which are rapidly evolving and are highly susceptible to egg-adaptive mutations in the manufacturing process.
In one study, a nanoparticle vaccine formulated with a saponin-based adjuvant induced hemagglutination inhibition responses that were even greater than those induced by the high-dose vaccine.52
Immunoglobulin A (IgA) may be a more effective vaccine target than traditional influenza vaccines that target IgG, since different parts of IgA may engage the influenza virus simultaneously.53
Vaccines can be developed more quickly than in the past. The timeline from viral sequencing to human studies with deoxyribonucleic acid plasmid vaccines decreased from 20 months in 2003 for the severe acquired respiratory syndrome coronavirus to 11 months in 2006 for influenza A/Indonesia/2006 (H5), to 4 months in 2009 for influenza A/California/2009 (H1), to 3.5 months in 2016 for Zika virus.54 This is because it is possible today to sequence a virus and insert the genetic material into a vaccine platform without ever having to grow the virus.
TREATMENT
Numerous studies have found anti-influenza medications to be effective. Nevertheless, in an analysis of the 2011–2016 influenza seasons, only 15% of high-risk patients were prescribed anti-influenza medications within 2 days of symptom onset, including 37% in those with laboratory-confirmed influenza.55 Fever was associated with an increased rate of antiviral treatment, but 25% of high-risk outpatients were afebrile. Empiric treatment of 4 high-risk outpatients with acute respiratory illness was needed to treat 1 patient with influenza.55
Treatment with a neuraminidase inhibitor within 2 days of illness has recently been shown to improve survival and shorten duration of viral shedding in patients with avian influenza A(H7N9) infection.56 Antiviral treatment within 2 days of illness is associated with improved outcomes in transplant recipients57 and with a lower risk of otitis media in children.58
Appropriate anti-influenza treatment is as important as avoiding unnecessary antibiotics. Regrettably, as many as one-third of patients with laboratory-confirmed influenza are prescribed antibiotics.59
The US Food and Drug Administration warns against fraudulent unapproved over-the-counter influenza products.60
Baloxavir marboxil
Baloxavir marboxil is a new anti-influenza medication approved in Japan in February 2018 and anticipated to be available in the United States sometime in 2019.
This prodrug is hydrolyzed in vivo to the active metabolite, which selectively inhibits cap-dependent endonuclease enzyme, a key enzyme in initiation of messenger ribonucleic acid synthesis required for influenza viral replication.61
In a double-blind phase 3 trial, the median time to alleviation of influenza symptoms is 26.5 hours shorter with baloxavir marboxil than with placebo. One tablet was as effective as 5 days of the neuraminidase inhibitor oseltamivir and was associated with greater reduction in viral load 1 day after initiation, and similar side effects.62 Of concern is the emergence of nucleic acid substitutions conferring resistance to baloxavir; this occurred in 2.2% and 9.7% of baloxavir recipients in the phase 2 and 3 trials, respectively.
CLOSING THE GAPS
Several gaps in the management of influenza persist since the 1918 pandemic.1 These include gaps in epidemiology, prevention, diagnosis, treatment, and prognosis.
- Global networks wider than current ones are needed to address this global disease and to prioritize coordination efforts.
- Establishing and strengthening clinical capacity is needed in limited resource settings. New technologies are needed to expedite vaccine development and to achieve progress toward a universal vaccine.
- Current diagnostic tests do not distinguish between seasonal and novel influenza A viruses of zoonotic origin, which are expected to cause the next pandemic.
- Current antivirals have been shown to shorten duration of illness in outpatients with uncomplicated influenza, but the benefit in hospitalized patients has been less well established.
- In 2007, resistance of seasonal influenza A(H1N1) to oseltamivir became widespread. In 2009, pandemic influenza A(H1N1), which is highly susceptible to oseltamivir, replaced the seasonal virus and remains the predominantly circulating A(H1N1) strain.
- A small-molecule fragment, N-cyclohexyaltaurine, binds to the conserved hemagglutinin receptor-binding site in a manner that mimics the binding mode of the natural receptor sialic acid. This can serve as a template to guide the development of novel broad-spectrum small-molecule anti-influenza drugs.63
- Biomarkers that can accurately predict development of severe disease in patients with influenza are needed.
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
- Uyeki TM, Fowler RA, Fischer WA. Gaps in the clinical management of influenza: a century since the 1918 pandemic. JAMA 2018; 320(8):755–756. doi:10.1001/jama.2018.8113
- Garten R, Blanton L, Elal AI, et al. Update: influenza activity in the United States during the 2017–18 season and composition of the 2018–19 influenza vaccine. MMWR Morb Mortal Wkly Rep 2018; 67(22):634–642. doi:10.15585/mmwr.mm6722a4
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis 2018; 66(10):1511–1518. doi:10.1093/cid/cix1060
- Elbadawi LI, Talley P, Rolfes MA, et al. Non-mumps viral parotitis during the 2014–2015 influenza season in the United States. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy137
- Thielen BK, Friedlander H, Bistodeau S, et al. Detection of influenza C viruses among outpatients and patients hospitalized for severe acute respiratory infection, Minnesota, 2013–2016. Clin Infect Dis 2018; 66(7):1092–1098. doi:10.1093/cid/cix931
- Chena Y, Trovãob NS, Wang G, et al. Emergence and evolution of novel reassortant influenza A viruses in canines in southern China. MBio 2018; 9(3):e00909–e00918. doi:10.1128/mBio.00909-18
- Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis 2018. Epub ahead of print. doi:10.1093/infdis/jiy370
- Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J 2018; 51(3):1701794. doi:10.1183/13993003.01794-2017
- Blackburn R, Zhao H, Pebody R, Hayward A, Warren-Gash C. Laboratory-confirmed respiratory infections as predictors of hospital admission for myocardial infarction and stroke: time-series analysis of English data for 2004–2015. Clin Infect Dis 2018; 67(1):8–17. doi:10.1093/cid/cix1144
- Newsweek; Andrew S. What is disease X? Deadly bird flu virus could be next pandemic. www.newsweek.com/disease-x-bird-flu-deaths-pandemic-what-h7n9-979723. Accessed October 3, 2018.
- Miller AC, Singh I, Koehler E, Polgreen PM. A smartphone-driven thermometer application for real-time population- and individual-level influenza surveillance. Clin Infect Dis 2018; 67(3):388–397. doi:10.1093/cid/ciy073
- Kormuth KA, Lin K, Prussin AJ 2nd, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity, J Infect Dis 2018; 218(5):739–747. doi:10.1093/infdis/jiy221
- Hertzberg VS, Weiss H, Elon L, et. al. Behaviors, movements, and transmission of droplet-mediated respiratory diseases during transcontinental airline flights. Proc Natl Acad Sci U S A 2018; 115(14):3623–3627. doi:10.1073/pnas.1711611115
- Grohskopf LA, Sokolow LZ, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 influenza season. MMWR Recomm Rep 2018; 67(3):1–20. doi:10.15585/mmwr.rr6703a1
- Grohskopf LA, Sokolow LZ, Fry AM, Walter EB, Jernigan DB. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4)—United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep 2018; 67(22):643–645. doi:10.15585/mmwr.mm6722a5
- Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017–18 seasonal influenza vaccine effectiveness—United States, February 2018. MMWR Morb Mortal Wkly Rep 2018; 67(6):180–185. doi:10.15585/mmwr.mm6706a2
- Demicheli V, Jefferson T, Ferroni E, Rivetti A, Di Pietrantonj C. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2018; 2:CD001269. doi:10.1002/14651858.CD001269.pub6
- Flannery B, Smith C, Garten RJ, et al. Influence of birth cohort on effectiveness of 2015–2016 influenza vaccine against medically attended illness due to 2009 pandemic influenza A(H1N1) virus in the United States. J Infect Dis 2018; 218(2):189–196. doi:10.1093/infdis/jix634
- Rondy M, El Omeiri N, Thompson MG, Leveque A, Moren A, Sullivan SG. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J Infect 2017; 75(5):381–394. doi:10.1016/j.jinf.2017.09.010
- Stein Y, Mandelboim M, Sefty H, et al; Israeli Influenza Surveillance Network (IISN). Seasonal influenza vaccine effectiveness in preventing laboratory-confirmed influenza in primary care in Israel, 2016–2017 season: insights into novel age-specific analysis. Clin Infect Dis 2018; 66(9):1383–1391. doi:10.1093/cid/cix1013
- Sah P, Medlock J, Fitzpatrick MC, Singer BH, Galvani AP. Optimizing the impact of low-efficacy influenza vaccines. Proc Natl Acad Sci U S A 2018; 115(20):5151–5156. doi:10.1073/pnas.1802479115
- QuickStats: percentage of currently employed adults aged ≥ 18 years who received influenza vaccine in the past 12 months, by employment category—national health interview survey, United States, 2012 and 2016. MMWR Morb Mortal Wkly Rep 2018; 67(16):480. doi:10.15585/mmwr.mm6716a8
- Healthy People.gov. Immunization and infectious diseases. IID-12. Increase the percentage of children and adults who are vaccinated annually against seasonal influenza. www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases/objectives. Accessed October 3, 2018.
- Greene MT, Fowler KE, Ratz D, Krein SL, Bradley SF, Saint S. Changes in influenza vaccination requirements for health care personnel in US hospitals. JAMA Network Open 2018; 1(2):e180143. doi:10.1001/jamanetworkopen.2018.0143
- Opel DJ, Sonne JA, Mello MM. Vaccination without litigation—addressing religious objections to hospital influenza-vaccination mandates. N Engl J Med 2018; 378(9):785–788. doi:10.1056/NEJMp1716147
- Horowitz J. Italy loosens vaccine law just as children return to school. New York Times Sept. 20, 2018. www.nytimes.com/2018/09/20/world/europe/italy-vaccines-five-star-movement.html.
- National Conference of State Legislature. States with religious and philosophical exemptions from school immunization requirements. www.ncsl.org/research/health/school-immunization-exemption-state-laws.aspx. Accessed October 3, 2018.
- Olive JK, Hotez PJ, Damania A, Nolan MS. The state of the antivaccine movement in the United States: a focused examination of nonmedical exemptions in states and counties. PLoS Med 2018; 15(6):e1002578. doi:10.1371/journal.pmed.1002578
- Logan J, Nederhoff D, Koch B, et al. ‘What have you HEARD about the HERD?’ Does education about local influenza vaccination coverage and herd immunity affect willingness to vaccinate? Vaccine 2018; 36(28):4118–4125. doi:10.1016/j.vaccine.2018.05.037
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16(7):1–14. doi:10.1080/14760584.2017.1334554
- Saito N, Komori K, Suzuki M, et al. Negative impact of prior influenza vaccination on current influenza vaccination among people infected and not infected in prior season: a test-negative case-control study in Japan. Vaccine 2017; 35(4):687–693. doi:10.1016/j.vaccine.2016.11.024
- Cheng AC, Macartney KK, Waterer GW, Kotsimbos T, Kelly PM, Blyth CC; Influenza Complications Alert Network (FluCAN) Investigators. Repeated vaccination does not appear to impact upon influenza vaccine effectiveness against hospitalization with confirmed influenza. Clin Infect Dis 2017; 64(11):1564–1572. doi:10.1093/cid/cix209
- Rondy M, Launay O, Castilla J, et al; InNHOVE/I-MOVE+working group. Repeated seasonal influenza vaccination among elderly in Europe: effects on laboratory confirmed hospitalised influenza. Vaccine 2017; 35(34):4298–4306. doi:10.1016/j.vaccine.2017.06.088
- Young-Xu Y, van Aalst R, Mahmud SM, et al. Relative vaccine effectiveness of high-dose versus standard-dose influenza vaccines among Veterans Health Administration patients. J Infect Dis 2018; 217(11):1718–1727. doi:10.1093/infdis/jiy088
- Shay DK, Chillarige Y, Kelman J, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccines among US Medicare beneficiaries in preventing postinfluenza deaths during 2012–2013 and 2013–2014. J Infect Dis 2017; 215(4):510–517. doi:10.1093/infdis/jiw641
- Madaras-Kelly K, Remington R, Hruza H, Xu D. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in preventing postinfluenza deaths. J Infect Dis 2018; 218(2):336–337. doi:10.1093/infdis/jix645
- Tam YH, Valkenburg SA, Perera RAPM, et al. Immune responses to twice-annual influenza vaccination in older adults in Hong Kong. Clin Infect Dis 2018; 66(6):904–912. doi:10.1093/cid/cix900
- Ohfuji S, Deguchi M, Tachibana D, et al; Osaka Pregnant Women Influenza Study Group. Protective effect of maternal influenza vaccination on influenza in their infants: a prospective cohort study. J Infect Dis 2018; 217(6):878–886. doi:10.1093/infdis/jix629
- Katz J, Englund JA, Steinhoff MC, et al. Impact of timing of influenza vaccination in pregnancy on transplacental antibody transfer, influenza incidence, and birth outcomes: a randomized trial in rural Nepal. Clin Infect Dis 2018; 67(3):334–340. doi:10.1093/cid/ciy090
- Nunes MC, Cutland CL, Madhi SA. Influenza vaccination during pregnancy and protection against pertussis. N Engl J Med 2018; 378(13):1257–1258. doi:10.1056/NEJMc1705208
- Andrew MK, Shinde V, Ye L, et al; Serious Outcomes Surveillance Network of the Public Health Agency of Canada/Canadian Institutes of Health Research Influenza Research Network (PCIRN) and the Toronto Invasive Bacterial Diseases Network (TIBDN). The importance of frailty in the assessment of influenza vaccine effectiveness against influenza-related hospitalization in elderly people. J Infect Dis 2017; 216(4):405–414. doi:10.1093/infdis/jix282
- Park JK, Lee YJ, Shin K, et al. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis 2018; 77(6):898–904. doi:10.1136/annrheumdis-2018-213222
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Natori Y, Shiotsuka M, Slomovic J, et al. A double-blind, randomized trial of high-dose vs standard-dose influenza vaccine in adult solid-organ transplant recipients. Clin Infect Dis 2018; 66(11):1698–1704. doi:10.1093/cid/cix1082
- Omer SB, Phadke VK, Bednarczyk BA, Chamberlain AT, Brosseau JL, Orenstein WA. Impact of statins on influenza vaccine effectiveness against medically attended acute respiratory illness. J Infect Dis 2016; 213(8):1216–1223. doi:10.1093/infdis/jiv457
- Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of recombinant influenza vaccine in adults 50 years of age or older. N Engl J Med 2017; 376(25):2427–2436. doi:10.1056/NEJMoa1608862
- STAT; Branswell H. How the US military might help answer a critical question about the flu vaccine. www.statnews.com/2018/03/02/flu-vaccine-egg-production-data. Accessed October 3, 2018.
- Paules CI, Sullivan SG, Subbarao K, Fauci AS. Chasing seasonal influenza—the need for a universal influenza vaccine. N Engl J Med 2018; 378(1):7–9. doi:10.1056/NEJMp1714916
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129-1134. pmid:16392727
- Wei WI, Brunger AT, Skehel JJ, Wiley DC. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol 1990; 212(4):737–761. doi:10.1016/0022-2836(90)90234-D
- Erbelding EJ, Post DJ, Stemmy EJ, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases, J Infect Dis 2018; 218(3):347–354. doi:10.1093/infdis/jiy103
- Shinde V, Fries L, Wu Y, et al. Improved titers against influenza drift variants with a nanoparticle vaccine. N Engl J Med 2018; 378(24):2346–2348. doi:10.1056/NEJMc1803554
- Maurer MA, Meyer L, Bianchi M, et al. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep 2018; 23(1):90–99. doi:10.1016/j.celrep.2018.03.027
- Graham BS, Mascola JR, Fauci AS. Novel vaccine technologies: essential components of an adequate response to emerging viral diseases. JAMA 2018; 319(14):1431–1432. doi:10.1001/jama.2018.0345
- Stewart RJ, Flannery B, Chung JR, et al. Influenza antiviral prescribing for outpatients with an acute respiratory illness and at high risk for influenza-associated complications during 5 influenza seasons—United States, 2011–2016. Clin Infect Dis 2018; 66(7):1035–1041. doi:10.1093/cid/cix922
- Zheng S, Tang L, Gao H, et al. Benefit of early initiation of neuraminidase inhibitor treatment to hospitalized patients with avian influenza A(H7N9) virus. Clin Infect Dis 2018; 66(7):1054–1060. doi:10.1093/cid/cix930
- Kumar D, Ferreira VH, Blumberg E, et al. A five-year prospective multi-center evaluation of influenza infection in transplant recipients. Clin Infect Dis 2018. Epub ahead of print. doi:10.1093/cid/ciy294
- Malosh RE, Martin ET, Heikkinen T, Brooks WA, Whitley RJ, Monto AS. Efficacy and safety of oseltamivir in children: systematic review and individual patient data meta-analysis of randomized controlled trials. Clin Infect Dis 2018; 66(10):1492–1500. doi:10.1093/cid/cix1040
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open 2018; 1(2):e180243. doi:10.1001/jamanetworkopen.2018.0243
- US Food and Drug Administration. FDA warns of fraudulent and unapproved flu products. www.fda.gov/newsevents/newsroom/pressannouncements/ucm599223.htm. Accessed October 3, 2018.
- Portsmouth S, Kawaguchi K, Arai M, Tsuchiya K, Uehara T. Cap-dependent endonuclease inhibitor S-033188 for the treatment of influenza: results from a phase 3, randomized, double-blind, placebo- and active-controlled study in otherwise healthy adolescents and adults with seasonal influenza. Open Forum Infect Dis 2017; 4(suppl 1):S734. doi:10.1093/ofid/ofx180.001
- Hayden FG, Sugaya N, Hirotsu N, et al; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379(10):913–923. doi:10.1056/NEJMoa1716197
- Kadam RU, Wilson IA. A small-molecule fragment that emulates binding of receptor and broadly neutralizing antibodies to influenza A hemagglutinin. Proc Natl Acad Sci U S A 2018; 115(16):4240–4245. doi:10.1073/pnas.1801999115
KEY POINTS
- Influenza A(H7N9) is a prime candidate to cause the next influenza pandemic.
- Influenza vaccine prevents 300 to 4,000 deaths in the United States every year.
- The 2018–2019 quadrivalent influenza vaccine contains updated A(H3N2) and B/Victoria lineage components different from those in the 2017–2018 Northern Hemisphere vaccine.
- The live-attenuated influenza vaccine, which was not recommended during the 2016–2017 and 2017–2018 influenza seasons, is recommended for the 2018–2019 influenza season.
- Influenza vaccine is recommended any time during pregnancy and is associated with lower infant mortality rates.
- Overall influenza vaccination rates remain below the 80% target for all Americans and 90% for at-risk populations.
Influenza: Still more important than Zika virus
The mass media and the medical literature have been saturated in the last few years by concerns about a variety of emerging viral epidemics such as Ebola and Zika. We must always remember that influenza will continue to affect many more patients worldwide.
The Cleveland Clinic Journal of Medicine periodically publishes updates on influenza, a topic befitting the large proportion of internists and internal medicine subspecialists who regularly read the Journal. This series began in 1975 with an article by Steven R. Mostow, MD,1 which followed three pandemics that changed the world’s attitude about influenza.
A lot has changed since then, including another pandemic in 2009–2010. Here, I review recent information relevant to daily practice.
NO REASON FOR COMPLACENCY
The relatively mild 2015–2016 influenza season is no reason for complacency this season.
Influenza activity in 2015–2016 was milder than in most seasons in the last decade.2 Activity peaked in mid-March and resulted in fewer outpatient visits, hospitalizations, and deaths than in previous seasons. Influenza A (H1N1)pdm09 has remained the predominant circulating virus since 2009. Although the overall rate of influenza-related hospitalization was less than half that in previous years, the hospitalization rate of middle-aged adults was relatively high (16.8 per 100,000 population). Importantly, 92% of adults with influenza illness that required hospitalization had at least one underlying medical condition, alerting us as healthcare providers that there is plenty of room for improvement in preventing such hospitalizations.
We should remain vigilant. We should put forth our best efforts in vaccinating all individuals above the age of 6 months and in diagnosing influenza early in the course of the illness in order to prescribe antiviral therapy within 48 hours of onset of symptoms. These actions not only shorten the illness and prevent hospitalization and secondary bacterial infection, but also reduce contagion and thus reduce overall healthcare costs.
School closure as a measure to halt epidemics has been lately called into question,3 since there are not enough data to support doing this routinely. School closure in Western Kentucky during the 2013 influenza epidemic did not reduce transmission but caused additional economic and social difficulties for certain households.4
STUDIES REINFORCE EARLIER DATA THAT INFLUENZA VACCINE WORKS
In the several decades since influenza vaccine became available, hundreds of studies have demonstrated the value of the “flu shot.” A few recent papers that support these well-established data:
- In adults who sought medical care for acute respiratory illness, influenza vaccine was 58.4% effective in preventing laboratory-confirmed influenza illness in adults age 50 and older.5
- In the same age group, influenza vaccine was 56.8% effective in preventing laboratory-confirmed influenza hospitalizations.6
- Influenza vaccination in patients with heart failure reduced all-cause hospitalizations, particularly cardiovascular hospitalizations (30% reduction) and hospitalizations for respiratory infections (16% reduction).7 This effect lasted up to 4 months after influenza vaccination.
- Patients who were hospitalized with community-acquired, laboratory-confirmed influenza pneumonia were 43% less likely to have received the influenza vaccine than patients hospitalized with community-acquired pneumonia due to other pathogens.8
INFLUENZA VACCINE IS EVEN MORE VALUABLE DURING PREGNANCY
Influenza vaccination during pregnancy prevented one in five preterm deliveries in a developing country9 and reduced the risk of stillbirth by 50% in Australia.10
An interesting collateral benefit was demonstrated in a survey conducted in Minnesota, where children of mothers who self-reported prenatal influenza vaccination were more likely to complete their routine childhood vaccination series.11
ADDITIONAL BENEFITS OF INFLUENZA VACCINATION
A recently appreciated benefit is that influenza vaccine induces cross-reactive protective immune responses (“heterologous immunity”) to viral strains not included in the vaccine, even in immunosuppressed individuals such as kidney transplant recipients.12 Interestingly, patients were more likely to seroconvert for a cross-reactive “heterologous” antigen if they also seroconverted for the vaccine-specific “homologous” antigen.
In a study in mice, an influenza vaccine with an adjuvant protected mice not only from influenza virus challenge, but also from a Staphylococcus aureus superinfection challenge.13 This novel idea suggests that influenza vaccine protects not only against influenza virus infection, but also against a potentially fatal secondary bacterial infection. This has significant implications for curbing antibacterial use, with an expected reduction in antimicrobial resistance.
Another important benefit of influenza vaccination was recently demonstrated when ferrets were intranasally inoculated with the highly pathogenic influenza A(H5N1) strain and then received either influenza vaccine or prophylactic oseltamivir. Ferrets that received the vaccine were less likely to develop severe meningoencephalitis.14 Since influenza A(H5N1) is much more virulent than the current circulating influenza strains, and since it may be the cause of the next pandemic, preventing such a serious complication of influenza would be lifesaving.
SAFETY OF INFLUENZA VACCINATION
Hundreds of studies involving thousands of people have established the safety of influenza vaccination.
Issues related to Guillain-Barré syndrome have long been put to rest. A large retrospective study found no evidence of increased risk of Guillain-Barré syndrome following vaccination of any kind, including influenza vaccination.15
Local reactions after vaccination are transient and do not interfere with the ability to perform daily activities.
In this era of utilization review, it is reassuring to know that giving influenza vaccine to hospitalized surgical patients was not associated with an increased rate of postdischarge fever or other clinical concern for infection requiring emergency room visits or rehospitalization.16
WHY INFLUENZA VACCINE MAY NOT PREVENT ALL CASES OF INFLUENZA
Whether neutralizing antibodies to influenza virus hemagglutinin antigen should be the main immune correlate of protection for influenza vaccines remains in question. Although prepandemic avian influenza vaccines are poorly immunogenic in inducing neutralizing antibodies, they confer considerable protection. A recent study showed that antibody-dependent cell-mediated cytotoxicity to hemagglutinin antigen in an avian influenza vaccine was a better predictor of protective capacity than neutralizing antibodies.17
Patterns of immunity induced by the live-attenuated influenza vaccine and the inactivated influenza vaccine are different.18 In fact, no single cytokine or chemokine measurement predicts protection.
Even though adults age 50 and older mount statistically significant humoral and cell-mediated immune responses to the inactivated vaccine, two-thirds do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H1N1), and one-fifth do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H3N2).19 While age had some negative effect on vaccine responsiveness, prevaccination titers were much better at predicting postvaccination antibody levels.
ONGOING DEBATE OVER LIVE-ATTENUATED INFLUENZA VACCINE
Several studies had shown that the live-attenuated influenza vaccine, given intranasally, was not only more protective in vaccinated children, but also provided herd protection in unvaccinated contacts. However, a recently published study conducted in Canadian Hutterite children showed that the live-attenuated vaccine did not result in herd immunity when compared to the inactivated influenza vaccine.20
On June 22, 2016, the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended against the use of the live-attenuated vaccine for the 2016–2017 season,21 based on data showing negligible protection conferred by the live-attenuated influenza vaccine in the three preceding influenza seasons.
This decision created significant debate among experts in the field. It is unclear why the live-attenuated influenza vaccine was much less protective in the last three seasons than in prior seasons. Recommending against its use in the United States will essentially eliminate any possibility of reassessing its efficacy in this country. Of note, the quadrivalent live-attenuated influenza vaccine had recently replaced the previous trivalent live-attenuated vaccine, which may have introduced some “competition” among the vaccine strains to infect enough cells to allow viral replication and subsequent immune response. Another potential explanation is that consistent annual vaccination may have resulted in a cumulative immunity that could hamper response to subsequent doses.
COMPOSITION OF THE 2016–2017 INFLUENZA VACCINE
The 2016–2017 quadrivalent inactivated influenza vaccine will contain22:
- A/California/7/2009 (H1N1)pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus (B/Victoria lineage)
- B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This represents a change in the A (H3N2) component compared with the 2015–2016 vaccine.
Influenza vaccine manufacturers estimated they would produce 170 million doses for distribution in the United States for the upcoming influenza season. The previously mentioned recommendation against the use of the live-attenuated vaccine, which accounts for approximately 8% of the influenza vaccine supply, may affect vaccine uptake, particularly in children.
NEW ANTI-INFLUENZA AGENTS AND UPDATE ON EXISTING AGENTS
Neuraminidase inhibitors are the only class of antiviral drugs currently recommended for prevention and treatment of influenza. The three products currently available in the United States are oseltamivir, zanamivir, and peramivir. Oseltamivir is administered orally, and the first generic version was approved by the US Food and Drug Administration on August 3, 2016. Zanamivir is administered by oral inhalation. Both oseltamivir and zanamivir are approved for treatment and prevention of influenza. Peramivir is administered intravenously as a single dose and is approved only for the treatment of acute influenza, not prevention.
Unfortunately, the influenza vaccination rate during pregnancy in the United States remains only around 50%.23 Physicians’ recommendations are strongly associated with vaccine uptake, particularly when they emphasize protective effect on the newborn. Influenza during pregnancy carries higher mortality than in the general population, with collateral fetal loss.
Early initiation of antiviral therapy is particularly imperative during pregnancy. A recent study showed that starting antiviral therapy within 2 days of onset of illness in pregnant women hospitalized with severe influenza reduced length of stay by 5.6 days compared with those in whom therapy was started more than 2 days after illness onset.24
A single dose of laninamivir octanoate, a long-acting neuraminidase inhibitor currently approved in Japan for treating influenza, was recently shown to be effective as postexposure prophylaxis.25 This option may be convenient for people who prefer not to take a daily medication for several days, or in an outbreak in a healthcare facility.
- Mostow SR. Current perspectives of influenza. Cleve Clin J Med 1975; 42:63–70.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity — United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Sasaki A, Hoen AG, Al Ozonoff A, et al. Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerg Infect Dis 2009; 15:1841–1843.
- Russell ES, Zheteyeva Y, Gao H, et al. Reactive school closure during increased influenza-like Illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infect Dis (Summer 2016) 3 (3): first published online May 25, 2016. doi:10.1093/ofid/ofw113.
- Chen Q, Griffin MR, Nian H, et al. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥ 50 years. J Infect Dis 2015, 211:1045–1050.
- Havers FP, Sokolow L, Shay DK, et al. Case-control study of vaccine effectiveness in preventing laboratory-confirmed influenza hospitalizations in older adults, United States, 2010–11. Clin Infect Dis 2016. [Epub ahead of print.].
- Influenza vaccination linked to fewer CV, respiratory hospitalizations in patients with HF. Helio Cardiology Today, May 25, 2016. www.healio.com/cardiology/hf-transplantation/news/online/%7B5292db4f-fc81-43f2-a28d-c124f2a6331b%7D/influenza-vaccination-linked-to-fewer-cv-respiratory-hospitalizations-in-patients-with-hf. Accessed October 6, 2016.
- Grijalva CG, Zhu Y, Williams DJ, et al. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA 2015, 314:1488–1497.
- Olsen SJ, Mirza SA, Vonglokham P, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis 2016; 63:487–494.
- Regan AK, Moore HC, de Klerk N, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016; 62:1221–1227.
- Fuchs EL. Self-reported prenatal influenza vaccination and early childhood vaccine series completion. Prev Med 2016; 88:8–12.
- Kumar D, Ferreira VH, Campbell P, Hoschler K, Humar A. Heterologous immune responses to influenza vaccine in kidney transplant recipients. Am J Transplant 2016; accepted manuscript online: 12 Jul 2016; doi: 10.1111/ajt.13960. [Epub ahead of print.]
- Zurli V, Gallotta M, Taccone M, et al. Positive contribution of adjuvanted influenza vaccines to the resolution of bacterial superinfections. J Infect Dis 2016; 213:1876–1885.
- Siegers JY, van den Brand JM, Leijten LM, et al. Vaccination is more effective than prophylactic oseltamivir in preventing CNS invasion by H5N1 virus via the olfactory nerve. J Infect Dis 2016; 214:516–524.
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57:197–204.
- Tartof SY, Qian L, Rieg G, et al. Safety of seasonal Influenza vaccination in hospitalized surgical patients: a cohort study. Ann Intern Med 2016; 213:1876–1885.
- Zhong W, Liu F, Wilson JR, et al. Antibody-dependent cell-mediated cytotoxicity to hemagglutinin of influenza A viruses after influenza vaccination in humans. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw102.
- Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw108.
- Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis 2015; 2 doi:10.1093/ofid/ofv052.
- Loeb M, Russell ML, Manning V, et al. Live attenuated versus inactivated influenza vaccine in Hutterite children: a cluster randomized blinded trial. Ann Intern Med published online August 16, 2016. doi:10.7326/M16-0513.
- CDC Newsroom. ACIP votes down use of LAIV for 2016-2017 flu season. June 22, 2016. www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed October 6, 2016.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Goodman K, Mossad SB, Taksler GB, et al. Impact of video education on influenza vaccination in pregnancy. J Reprod Med 2015; 60:471–479.
- Oboho IK, Reed C, Gargiullo P, et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507–515.
- Kashiwagi S, Watanabe A, Ikematsu H, Uemori M, Awamura S, for the Laninamivir Prophylaxis Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis 2016; 63:330–337.
The mass media and the medical literature have been saturated in the last few years by concerns about a variety of emerging viral epidemics such as Ebola and Zika. We must always remember that influenza will continue to affect many more patients worldwide.
The Cleveland Clinic Journal of Medicine periodically publishes updates on influenza, a topic befitting the large proportion of internists and internal medicine subspecialists who regularly read the Journal. This series began in 1975 with an article by Steven R. Mostow, MD,1 which followed three pandemics that changed the world’s attitude about influenza.
A lot has changed since then, including another pandemic in 2009–2010. Here, I review recent information relevant to daily practice.
NO REASON FOR COMPLACENCY
The relatively mild 2015–2016 influenza season is no reason for complacency this season.
Influenza activity in 2015–2016 was milder than in most seasons in the last decade.2 Activity peaked in mid-March and resulted in fewer outpatient visits, hospitalizations, and deaths than in previous seasons. Influenza A (H1N1)pdm09 has remained the predominant circulating virus since 2009. Although the overall rate of influenza-related hospitalization was less than half that in previous years, the hospitalization rate of middle-aged adults was relatively high (16.8 per 100,000 population). Importantly, 92% of adults with influenza illness that required hospitalization had at least one underlying medical condition, alerting us as healthcare providers that there is plenty of room for improvement in preventing such hospitalizations.
We should remain vigilant. We should put forth our best efforts in vaccinating all individuals above the age of 6 months and in diagnosing influenza early in the course of the illness in order to prescribe antiviral therapy within 48 hours of onset of symptoms. These actions not only shorten the illness and prevent hospitalization and secondary bacterial infection, but also reduce contagion and thus reduce overall healthcare costs.
School closure as a measure to halt epidemics has been lately called into question,3 since there are not enough data to support doing this routinely. School closure in Western Kentucky during the 2013 influenza epidemic did not reduce transmission but caused additional economic and social difficulties for certain households.4
STUDIES REINFORCE EARLIER DATA THAT INFLUENZA VACCINE WORKS
In the several decades since influenza vaccine became available, hundreds of studies have demonstrated the value of the “flu shot.” A few recent papers that support these well-established data:
- In adults who sought medical care for acute respiratory illness, influenza vaccine was 58.4% effective in preventing laboratory-confirmed influenza illness in adults age 50 and older.5
- In the same age group, influenza vaccine was 56.8% effective in preventing laboratory-confirmed influenza hospitalizations.6
- Influenza vaccination in patients with heart failure reduced all-cause hospitalizations, particularly cardiovascular hospitalizations (30% reduction) and hospitalizations for respiratory infections (16% reduction).7 This effect lasted up to 4 months after influenza vaccination.
- Patients who were hospitalized with community-acquired, laboratory-confirmed influenza pneumonia were 43% less likely to have received the influenza vaccine than patients hospitalized with community-acquired pneumonia due to other pathogens.8
INFLUENZA VACCINE IS EVEN MORE VALUABLE DURING PREGNANCY
Influenza vaccination during pregnancy prevented one in five preterm deliveries in a developing country9 and reduced the risk of stillbirth by 50% in Australia.10
An interesting collateral benefit was demonstrated in a survey conducted in Minnesota, where children of mothers who self-reported prenatal influenza vaccination were more likely to complete their routine childhood vaccination series.11
ADDITIONAL BENEFITS OF INFLUENZA VACCINATION
A recently appreciated benefit is that influenza vaccine induces cross-reactive protective immune responses (“heterologous immunity”) to viral strains not included in the vaccine, even in immunosuppressed individuals such as kidney transplant recipients.12 Interestingly, patients were more likely to seroconvert for a cross-reactive “heterologous” antigen if they also seroconverted for the vaccine-specific “homologous” antigen.
In a study in mice, an influenza vaccine with an adjuvant protected mice not only from influenza virus challenge, but also from a Staphylococcus aureus superinfection challenge.13 This novel idea suggests that influenza vaccine protects not only against influenza virus infection, but also against a potentially fatal secondary bacterial infection. This has significant implications for curbing antibacterial use, with an expected reduction in antimicrobial resistance.
Another important benefit of influenza vaccination was recently demonstrated when ferrets were intranasally inoculated with the highly pathogenic influenza A(H5N1) strain and then received either influenza vaccine or prophylactic oseltamivir. Ferrets that received the vaccine were less likely to develop severe meningoencephalitis.14 Since influenza A(H5N1) is much more virulent than the current circulating influenza strains, and since it may be the cause of the next pandemic, preventing such a serious complication of influenza would be lifesaving.
SAFETY OF INFLUENZA VACCINATION
Hundreds of studies involving thousands of people have established the safety of influenza vaccination.
Issues related to Guillain-Barré syndrome have long been put to rest. A large retrospective study found no evidence of increased risk of Guillain-Barré syndrome following vaccination of any kind, including influenza vaccination.15
Local reactions after vaccination are transient and do not interfere with the ability to perform daily activities.
In this era of utilization review, it is reassuring to know that giving influenza vaccine to hospitalized surgical patients was not associated with an increased rate of postdischarge fever or other clinical concern for infection requiring emergency room visits or rehospitalization.16
WHY INFLUENZA VACCINE MAY NOT PREVENT ALL CASES OF INFLUENZA
Whether neutralizing antibodies to influenza virus hemagglutinin antigen should be the main immune correlate of protection for influenza vaccines remains in question. Although prepandemic avian influenza vaccines are poorly immunogenic in inducing neutralizing antibodies, they confer considerable protection. A recent study showed that antibody-dependent cell-mediated cytotoxicity to hemagglutinin antigen in an avian influenza vaccine was a better predictor of protective capacity than neutralizing antibodies.17
Patterns of immunity induced by the live-attenuated influenza vaccine and the inactivated influenza vaccine are different.18 In fact, no single cytokine or chemokine measurement predicts protection.
Even though adults age 50 and older mount statistically significant humoral and cell-mediated immune responses to the inactivated vaccine, two-thirds do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H1N1), and one-fifth do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H3N2).19 While age had some negative effect on vaccine responsiveness, prevaccination titers were much better at predicting postvaccination antibody levels.
ONGOING DEBATE OVER LIVE-ATTENUATED INFLUENZA VACCINE
Several studies had shown that the live-attenuated influenza vaccine, given intranasally, was not only more protective in vaccinated children, but also provided herd protection in unvaccinated contacts. However, a recently published study conducted in Canadian Hutterite children showed that the live-attenuated vaccine did not result in herd immunity when compared to the inactivated influenza vaccine.20
On June 22, 2016, the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended against the use of the live-attenuated vaccine for the 2016–2017 season,21 based on data showing negligible protection conferred by the live-attenuated influenza vaccine in the three preceding influenza seasons.
This decision created significant debate among experts in the field. It is unclear why the live-attenuated influenza vaccine was much less protective in the last three seasons than in prior seasons. Recommending against its use in the United States will essentially eliminate any possibility of reassessing its efficacy in this country. Of note, the quadrivalent live-attenuated influenza vaccine had recently replaced the previous trivalent live-attenuated vaccine, which may have introduced some “competition” among the vaccine strains to infect enough cells to allow viral replication and subsequent immune response. Another potential explanation is that consistent annual vaccination may have resulted in a cumulative immunity that could hamper response to subsequent doses.
COMPOSITION OF THE 2016–2017 INFLUENZA VACCINE
The 2016–2017 quadrivalent inactivated influenza vaccine will contain22:
- A/California/7/2009 (H1N1)pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus (B/Victoria lineage)
- B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This represents a change in the A (H3N2) component compared with the 2015–2016 vaccine.
Influenza vaccine manufacturers estimated they would produce 170 million doses for distribution in the United States for the upcoming influenza season. The previously mentioned recommendation against the use of the live-attenuated vaccine, which accounts for approximately 8% of the influenza vaccine supply, may affect vaccine uptake, particularly in children.
NEW ANTI-INFLUENZA AGENTS AND UPDATE ON EXISTING AGENTS
Neuraminidase inhibitors are the only class of antiviral drugs currently recommended for prevention and treatment of influenza. The three products currently available in the United States are oseltamivir, zanamivir, and peramivir. Oseltamivir is administered orally, and the first generic version was approved by the US Food and Drug Administration on August 3, 2016. Zanamivir is administered by oral inhalation. Both oseltamivir and zanamivir are approved for treatment and prevention of influenza. Peramivir is administered intravenously as a single dose and is approved only for the treatment of acute influenza, not prevention.
Unfortunately, the influenza vaccination rate during pregnancy in the United States remains only around 50%.23 Physicians’ recommendations are strongly associated with vaccine uptake, particularly when they emphasize protective effect on the newborn. Influenza during pregnancy carries higher mortality than in the general population, with collateral fetal loss.
Early initiation of antiviral therapy is particularly imperative during pregnancy. A recent study showed that starting antiviral therapy within 2 days of onset of illness in pregnant women hospitalized with severe influenza reduced length of stay by 5.6 days compared with those in whom therapy was started more than 2 days after illness onset.24
A single dose of laninamivir octanoate, a long-acting neuraminidase inhibitor currently approved in Japan for treating influenza, was recently shown to be effective as postexposure prophylaxis.25 This option may be convenient for people who prefer not to take a daily medication for several days, or in an outbreak in a healthcare facility.
The mass media and the medical literature have been saturated in the last few years by concerns about a variety of emerging viral epidemics such as Ebola and Zika. We must always remember that influenza will continue to affect many more patients worldwide.
The Cleveland Clinic Journal of Medicine periodically publishes updates on influenza, a topic befitting the large proportion of internists and internal medicine subspecialists who regularly read the Journal. This series began in 1975 with an article by Steven R. Mostow, MD,1 which followed three pandemics that changed the world’s attitude about influenza.
A lot has changed since then, including another pandemic in 2009–2010. Here, I review recent information relevant to daily practice.
NO REASON FOR COMPLACENCY
The relatively mild 2015–2016 influenza season is no reason for complacency this season.
Influenza activity in 2015–2016 was milder than in most seasons in the last decade.2 Activity peaked in mid-March and resulted in fewer outpatient visits, hospitalizations, and deaths than in previous seasons. Influenza A (H1N1)pdm09 has remained the predominant circulating virus since 2009. Although the overall rate of influenza-related hospitalization was less than half that in previous years, the hospitalization rate of middle-aged adults was relatively high (16.8 per 100,000 population). Importantly, 92% of adults with influenza illness that required hospitalization had at least one underlying medical condition, alerting us as healthcare providers that there is plenty of room for improvement in preventing such hospitalizations.
We should remain vigilant. We should put forth our best efforts in vaccinating all individuals above the age of 6 months and in diagnosing influenza early in the course of the illness in order to prescribe antiviral therapy within 48 hours of onset of symptoms. These actions not only shorten the illness and prevent hospitalization and secondary bacterial infection, but also reduce contagion and thus reduce overall healthcare costs.
School closure as a measure to halt epidemics has been lately called into question,3 since there are not enough data to support doing this routinely. School closure in Western Kentucky during the 2013 influenza epidemic did not reduce transmission but caused additional economic and social difficulties for certain households.4
STUDIES REINFORCE EARLIER DATA THAT INFLUENZA VACCINE WORKS
In the several decades since influenza vaccine became available, hundreds of studies have demonstrated the value of the “flu shot.” A few recent papers that support these well-established data:
- In adults who sought medical care for acute respiratory illness, influenza vaccine was 58.4% effective in preventing laboratory-confirmed influenza illness in adults age 50 and older.5
- In the same age group, influenza vaccine was 56.8% effective in preventing laboratory-confirmed influenza hospitalizations.6
- Influenza vaccination in patients with heart failure reduced all-cause hospitalizations, particularly cardiovascular hospitalizations (30% reduction) and hospitalizations for respiratory infections (16% reduction).7 This effect lasted up to 4 months after influenza vaccination.
- Patients who were hospitalized with community-acquired, laboratory-confirmed influenza pneumonia were 43% less likely to have received the influenza vaccine than patients hospitalized with community-acquired pneumonia due to other pathogens.8
INFLUENZA VACCINE IS EVEN MORE VALUABLE DURING PREGNANCY
Influenza vaccination during pregnancy prevented one in five preterm deliveries in a developing country9 and reduced the risk of stillbirth by 50% in Australia.10
An interesting collateral benefit was demonstrated in a survey conducted in Minnesota, where children of mothers who self-reported prenatal influenza vaccination were more likely to complete their routine childhood vaccination series.11
ADDITIONAL BENEFITS OF INFLUENZA VACCINATION
A recently appreciated benefit is that influenza vaccine induces cross-reactive protective immune responses (“heterologous immunity”) to viral strains not included in the vaccine, even in immunosuppressed individuals such as kidney transplant recipients.12 Interestingly, patients were more likely to seroconvert for a cross-reactive “heterologous” antigen if they also seroconverted for the vaccine-specific “homologous” antigen.
In a study in mice, an influenza vaccine with an adjuvant protected mice not only from influenza virus challenge, but also from a Staphylococcus aureus superinfection challenge.13 This novel idea suggests that influenza vaccine protects not only against influenza virus infection, but also against a potentially fatal secondary bacterial infection. This has significant implications for curbing antibacterial use, with an expected reduction in antimicrobial resistance.
Another important benefit of influenza vaccination was recently demonstrated when ferrets were intranasally inoculated with the highly pathogenic influenza A(H5N1) strain and then received either influenza vaccine or prophylactic oseltamivir. Ferrets that received the vaccine were less likely to develop severe meningoencephalitis.14 Since influenza A(H5N1) is much more virulent than the current circulating influenza strains, and since it may be the cause of the next pandemic, preventing such a serious complication of influenza would be lifesaving.
SAFETY OF INFLUENZA VACCINATION
Hundreds of studies involving thousands of people have established the safety of influenza vaccination.
Issues related to Guillain-Barré syndrome have long been put to rest. A large retrospective study found no evidence of increased risk of Guillain-Barré syndrome following vaccination of any kind, including influenza vaccination.15
Local reactions after vaccination are transient and do not interfere with the ability to perform daily activities.
In this era of utilization review, it is reassuring to know that giving influenza vaccine to hospitalized surgical patients was not associated with an increased rate of postdischarge fever or other clinical concern for infection requiring emergency room visits or rehospitalization.16
WHY INFLUENZA VACCINE MAY NOT PREVENT ALL CASES OF INFLUENZA
Whether neutralizing antibodies to influenza virus hemagglutinin antigen should be the main immune correlate of protection for influenza vaccines remains in question. Although prepandemic avian influenza vaccines are poorly immunogenic in inducing neutralizing antibodies, they confer considerable protection. A recent study showed that antibody-dependent cell-mediated cytotoxicity to hemagglutinin antigen in an avian influenza vaccine was a better predictor of protective capacity than neutralizing antibodies.17
Patterns of immunity induced by the live-attenuated influenza vaccine and the inactivated influenza vaccine are different.18 In fact, no single cytokine or chemokine measurement predicts protection.
Even though adults age 50 and older mount statistically significant humoral and cell-mediated immune responses to the inactivated vaccine, two-thirds do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H1N1), and one-fifth do not reach hemagglutination inhibition antibody titers of 40 or higher for influenza A(H3N2).19 While age had some negative effect on vaccine responsiveness, prevaccination titers were much better at predicting postvaccination antibody levels.
ONGOING DEBATE OVER LIVE-ATTENUATED INFLUENZA VACCINE
Several studies had shown that the live-attenuated influenza vaccine, given intranasally, was not only more protective in vaccinated children, but also provided herd protection in unvaccinated contacts. However, a recently published study conducted in Canadian Hutterite children showed that the live-attenuated vaccine did not result in herd immunity when compared to the inactivated influenza vaccine.20
On June 22, 2016, the US Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended against the use of the live-attenuated vaccine for the 2016–2017 season,21 based on data showing negligible protection conferred by the live-attenuated influenza vaccine in the three preceding influenza seasons.
This decision created significant debate among experts in the field. It is unclear why the live-attenuated influenza vaccine was much less protective in the last three seasons than in prior seasons. Recommending against its use in the United States will essentially eliminate any possibility of reassessing its efficacy in this country. Of note, the quadrivalent live-attenuated influenza vaccine had recently replaced the previous trivalent live-attenuated vaccine, which may have introduced some “competition” among the vaccine strains to infect enough cells to allow viral replication and subsequent immune response. Another potential explanation is that consistent annual vaccination may have resulted in a cumulative immunity that could hamper response to subsequent doses.
COMPOSITION OF THE 2016–2017 INFLUENZA VACCINE
The 2016–2017 quadrivalent inactivated influenza vaccine will contain22:
- A/California/7/2009 (H1N1)pdm09-like virus
- A/Hong Kong/4801/2014 (H3N2)-like virus
- B/Brisbane/60/2008-like virus (B/Victoria lineage)
- B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This represents a change in the A (H3N2) component compared with the 2015–2016 vaccine.
Influenza vaccine manufacturers estimated they would produce 170 million doses for distribution in the United States for the upcoming influenza season. The previously mentioned recommendation against the use of the live-attenuated vaccine, which accounts for approximately 8% of the influenza vaccine supply, may affect vaccine uptake, particularly in children.
NEW ANTI-INFLUENZA AGENTS AND UPDATE ON EXISTING AGENTS
Neuraminidase inhibitors are the only class of antiviral drugs currently recommended for prevention and treatment of influenza. The three products currently available in the United States are oseltamivir, zanamivir, and peramivir. Oseltamivir is administered orally, and the first generic version was approved by the US Food and Drug Administration on August 3, 2016. Zanamivir is administered by oral inhalation. Both oseltamivir and zanamivir are approved for treatment and prevention of influenza. Peramivir is administered intravenously as a single dose and is approved only for the treatment of acute influenza, not prevention.
Unfortunately, the influenza vaccination rate during pregnancy in the United States remains only around 50%.23 Physicians’ recommendations are strongly associated with vaccine uptake, particularly when they emphasize protective effect on the newborn. Influenza during pregnancy carries higher mortality than in the general population, with collateral fetal loss.
Early initiation of antiviral therapy is particularly imperative during pregnancy. A recent study showed that starting antiviral therapy within 2 days of onset of illness in pregnant women hospitalized with severe influenza reduced length of stay by 5.6 days compared with those in whom therapy was started more than 2 days after illness onset.24
A single dose of laninamivir octanoate, a long-acting neuraminidase inhibitor currently approved in Japan for treating influenza, was recently shown to be effective as postexposure prophylaxis.25 This option may be convenient for people who prefer not to take a daily medication for several days, or in an outbreak in a healthcare facility.
- Mostow SR. Current perspectives of influenza. Cleve Clin J Med 1975; 42:63–70.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity — United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Sasaki A, Hoen AG, Al Ozonoff A, et al. Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerg Infect Dis 2009; 15:1841–1843.
- Russell ES, Zheteyeva Y, Gao H, et al. Reactive school closure during increased influenza-like Illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infect Dis (Summer 2016) 3 (3): first published online May 25, 2016. doi:10.1093/ofid/ofw113.
- Chen Q, Griffin MR, Nian H, et al. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥ 50 years. J Infect Dis 2015, 211:1045–1050.
- Havers FP, Sokolow L, Shay DK, et al. Case-control study of vaccine effectiveness in preventing laboratory-confirmed influenza hospitalizations in older adults, United States, 2010–11. Clin Infect Dis 2016. [Epub ahead of print.].
- Influenza vaccination linked to fewer CV, respiratory hospitalizations in patients with HF. Helio Cardiology Today, May 25, 2016. www.healio.com/cardiology/hf-transplantation/news/online/%7B5292db4f-fc81-43f2-a28d-c124f2a6331b%7D/influenza-vaccination-linked-to-fewer-cv-respiratory-hospitalizations-in-patients-with-hf. Accessed October 6, 2016.
- Grijalva CG, Zhu Y, Williams DJ, et al. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA 2015, 314:1488–1497.
- Olsen SJ, Mirza SA, Vonglokham P, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis 2016; 63:487–494.
- Regan AK, Moore HC, de Klerk N, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016; 62:1221–1227.
- Fuchs EL. Self-reported prenatal influenza vaccination and early childhood vaccine series completion. Prev Med 2016; 88:8–12.
- Kumar D, Ferreira VH, Campbell P, Hoschler K, Humar A. Heterologous immune responses to influenza vaccine in kidney transplant recipients. Am J Transplant 2016; accepted manuscript online: 12 Jul 2016; doi: 10.1111/ajt.13960. [Epub ahead of print.]
- Zurli V, Gallotta M, Taccone M, et al. Positive contribution of adjuvanted influenza vaccines to the resolution of bacterial superinfections. J Infect Dis 2016; 213:1876–1885.
- Siegers JY, van den Brand JM, Leijten LM, et al. Vaccination is more effective than prophylactic oseltamivir in preventing CNS invasion by H5N1 virus via the olfactory nerve. J Infect Dis 2016; 214:516–524.
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57:197–204.
- Tartof SY, Qian L, Rieg G, et al. Safety of seasonal Influenza vaccination in hospitalized surgical patients: a cohort study. Ann Intern Med 2016; 213:1876–1885.
- Zhong W, Liu F, Wilson JR, et al. Antibody-dependent cell-mediated cytotoxicity to hemagglutinin of influenza A viruses after influenza vaccination in humans. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw102.
- Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw108.
- Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis 2015; 2 doi:10.1093/ofid/ofv052.
- Loeb M, Russell ML, Manning V, et al. Live attenuated versus inactivated influenza vaccine in Hutterite children: a cluster randomized blinded trial. Ann Intern Med published online August 16, 2016. doi:10.7326/M16-0513.
- CDC Newsroom. ACIP votes down use of LAIV for 2016-2017 flu season. June 22, 2016. www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed October 6, 2016.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Goodman K, Mossad SB, Taksler GB, et al. Impact of video education on influenza vaccination in pregnancy. J Reprod Med 2015; 60:471–479.
- Oboho IK, Reed C, Gargiullo P, et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507–515.
- Kashiwagi S, Watanabe A, Ikematsu H, Uemori M, Awamura S, for the Laninamivir Prophylaxis Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis 2016; 63:330–337.
- Mostow SR. Current perspectives of influenza. Cleve Clin J Med 1975; 42:63–70.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity — United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Sasaki A, Hoen AG, Al Ozonoff A, et al. Evidence-based tool for triggering school closures during influenza outbreaks, Japan. Emerg Infect Dis 2009; 15:1841–1843.
- Russell ES, Zheteyeva Y, Gao H, et al. Reactive school closure during increased influenza-like Illness (ILI) activity in Western Kentucky, 2013: a field evaluation of effect on ILI incidence and economic and social consequences for families. Open Forum Infect Dis (Summer 2016) 3 (3): first published online May 25, 2016. doi:10.1093/ofid/ofw113.
- Chen Q, Griffin MR, Nian H, et al. Influenza vaccine prevents medically attended influenza-associated acute respiratory illness in adults aged ≥ 50 years. J Infect Dis 2015, 211:1045–1050.
- Havers FP, Sokolow L, Shay DK, et al. Case-control study of vaccine effectiveness in preventing laboratory-confirmed influenza hospitalizations in older adults, United States, 2010–11. Clin Infect Dis 2016. [Epub ahead of print.].
- Influenza vaccination linked to fewer CV, respiratory hospitalizations in patients with HF. Helio Cardiology Today, May 25, 2016. www.healio.com/cardiology/hf-transplantation/news/online/%7B5292db4f-fc81-43f2-a28d-c124f2a6331b%7D/influenza-vaccination-linked-to-fewer-cv-respiratory-hospitalizations-in-patients-with-hf. Accessed October 6, 2016.
- Grijalva CG, Zhu Y, Williams DJ, et al. Association between hospitalization with community-acquired laboratory-confirmed influenza pneumonia and prior receipt of influenza vaccination. JAMA 2015, 314:1488–1497.
- Olsen SJ, Mirza SA, Vonglokham P, et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin Infect Dis 2016; 63:487–494.
- Regan AK, Moore HC, de Klerk N, et al. Seasonal trivalent influenza vaccination during pregnancy and the incidence of stillbirth: population-based retrospective cohort study. Clin Infect Dis 2016; 62:1221–1227.
- Fuchs EL. Self-reported prenatal influenza vaccination and early childhood vaccine series completion. Prev Med 2016; 88:8–12.
- Kumar D, Ferreira VH, Campbell P, Hoschler K, Humar A. Heterologous immune responses to influenza vaccine in kidney transplant recipients. Am J Transplant 2016; accepted manuscript online: 12 Jul 2016; doi: 10.1111/ajt.13960. [Epub ahead of print.]
- Zurli V, Gallotta M, Taccone M, et al. Positive contribution of adjuvanted influenza vaccines to the resolution of bacterial superinfections. J Infect Dis 2016; 213:1876–1885.
- Siegers JY, van den Brand JM, Leijten LM, et al. Vaccination is more effective than prophylactic oseltamivir in preventing CNS invasion by H5N1 virus via the olfactory nerve. J Infect Dis 2016; 214:516–524.
- Baxter R, Bakshi N, Fireman B, et al. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis 2013; 57:197–204.
- Tartof SY, Qian L, Rieg G, et al. Safety of seasonal Influenza vaccination in hospitalized surgical patients: a cohort study. Ann Intern Med 2016; 213:1876–1885.
- Zhong W, Liu F, Wilson JR, et al. Antibody-dependent cell-mediated cytotoxicity to hemagglutinin of influenza A viruses after influenza vaccination in humans. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw102.
- Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, attenuated influenza vaccine. Open Forum Infect Dis 2016; 3: doi:10.1093/ofid/ofw108.
- Reber AJ, Kim JH, Biber R, et al. Preexisting immunity, more than aging, influences influenza vaccine responses. Open Forum Infect Dis 2015; 2 doi:10.1093/ofid/ofv052.
- Loeb M, Russell ML, Manning V, et al. Live attenuated versus inactivated influenza vaccine in Hutterite children: a cluster randomized blinded trial. Ann Intern Med published online August 16, 2016. doi:10.7326/M16-0513.
- CDC Newsroom. ACIP votes down use of LAIV for 2016-2017 flu season. June 22, 2016. www.cdc.gov/media/releases/2016/s0622-laiv-flu.html. Accessed October 6, 2016.
- Davlin SL, Blanton L, Kniss K, et al. Influenza activity—United States, 2015–16 season and composition of the 2016–17 influenza vaccine. MMWR Morb Mortal Wkly Rep 2016; 65:567–575.
- Goodman K, Mossad SB, Taksler GB, et al. Impact of video education on influenza vaccination in pregnancy. J Reprod Med 2015; 60:471–479.
- Oboho IK, Reed C, Gargiullo P, et al. Benefit of early initiation of influenza antiviral treatment to pregnant women hospitalized with laboratory-confirmed influenza. J Infect Dis 2016; 214:507–515.
- Kashiwagi S, Watanabe A, Ikematsu H, Uemori M, Awamura S, for the Laninamivir Prophylaxis Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate as post-exposure prophylaxis for influenza. Clin Infect Dis 2016; 63:330–337.
KEY POINTS
- Influenza vaccine remains the most effective way to prevent influenza. Healthcare providers should continue to vaccinate all people older than 6 months.
- For the 2016–2017 influenza season, only the inactivated influenza vaccine, not the live-attenuated vaccine, is recommended, regardless of age group or underlying disease.
- Early initiation of a neuraminidase inhibitor is advised for an influenza-like illness while awaiting a confirmatory diagnostic test.
2012–2013 Influenza update: Hitting a rapidly moving target
Despite our success in reducing the number of deaths from influenza in the last half-century, we must remain vigilant, since influenza still can kill.1,2 Gene mutations and reassortment among different strains of influenza viruses pose a significant public health threat, especially in an increasingly mobile world.3,4
In this article, we will present an update on influenza to better prepare primary care providers to prevent and treat this ongoing threat.
H3N2v: SWINE FLU DÉJÀ VU?
Outbreaks of swine flu at state and county fairs in 2012 are unprecedented and have raised concerns.
From 1990 to 2010, human infections with swine-origin influenza viruses were sporadic, and the US Centers for Disease Control and Prevention (CDC) confirmed a total of only 27 cases during this period.5 However, the number has been increasing since 2011: as of August 31, 2012, a total of 309 cases had been reported.6
Analysis of viral RNA in clinical respiratory specimens from 12 cases in 2011 revealed a variant strain, called H3N2v, which is a hybrid containing genetic material from swine H3N2 and the 2009 human pandemic virus H1N1pdm09. The M gene in this new variant came from the human virus, while the other seven came from the swine virus when a host was infected with both viruses simultaneously (Figure 1). As a result of this genetic reassortment, this variant virus is genetically and antigenically different from seasonal H3N2.
Epidemiologic data showed that children under 10 years of age are especially susceptible to this new variant because they lack immunity, whereas adolescents and adults may have some immunity from cross-reacting antibodies.7 Most infected people had been exposed to swine in agriculture, including county and state fairs. So far, evidence suggests only limited human-to-human transmission.8 The clinical diagnosis of H3N2v infection relies on the epidemiologic link to exposure to pigs in the week before the onset of illness, since the symptoms are indistinguishable from those of seasonal influenza A or B infections.
In suspected cases, the clinician should notify the local or state public health department and arrange for a special test to be performed on respiratory specimens: the CDC Flu Real-Time Reverse Transcriptase Polymerase Chain Reaction Dx Panel. The reason is that a negative rapid influenza diagnostic test does not rule out influenza infection, and a positive immunofluorescence assay (direct fluorescent antibody staining) cannot specifically detect H3N2v.7
The current seasonal influenza vaccine will not protect against H3N2v. The isolates tested to date were susceptible to the neuraminidase inhibitor drugs oseltamivir (Tamiflu) and zanamivir (Relenza) but resistant to amantadine (Symmetrel) and rimantadine (Flumadine).9
Whether H3N2v will become a significant problem during the upcoming flu season largely depends on the extent of human-to-human transmission. We need to closely follow updates on this virus.
H5N1: THE LOOMING THREAT OF A BIRD FLU PANDEMIC
Since 2003, influenza A H5N1, a highly pathogenic avian virus, has broken out in Asia, Africa, and the Middle East, killing more than 100 million birds. It also has crossed the species barrier to infect humans, with an unusually high death rate.10
As of August 10, 2012, the World Health Organization had reported 608 confirmed cases of this virus infecting humans and 359 associated deaths.11 Most infected patients had a history of close contact with diseased poultry, but limited, nonsustained human-to-human transmission can occur during very close, unprotected contact with a severely ill patient.12
Molecular studies of this virus revealed further insights into its pathogenesis. Some of the viruses isolated from humans have had mutations that allow them to bind to human-type receptors.13 Amino acid substitutions in the polymerase basic protein 2 (PB2) gene are associated with mammalian adaptation, virulence in mice, and viral replication at temperatures present in the upper respiratory tract.14 Furthermore, higher plasma levels of macrophage- and neutrophil-attractant chemokines and both inflammatory and anti-inflammatory cytokines (interleukin 6, interleukin 10, and interferon gamma) have been observed in patients with H5N1 infection, especially in fatal cases.15 A recent study found that H5N1 causes significant perturbations in the host’s protein synthesis machinery as early as 1 hour after infection, suggesting that this virus gains an early advantage in replication by using the host’s proteome.16 The effects of unrestrained viral infection and inflammatory responses induced by H5N1 infection certainly contributed to the primary pathologic process and to death in human fulminant viral pneumonia. The up-regulation of inflammatory cytokines in these infections contributes to the development of sepsis syndrome, acute respiratory distress syndrome, and an increased risk of death, particularly in pregnant women.
Most experts predict that pandemic influenza is probably inevitable.17 If avian H5N1 and a human influenza virus swap genes in a host such as swine, the new hybrid virus will contain genetic material from both strains and will have surface antigens that the human immune system does not recognize. This could lead to a devastating avian flu pandemic with a very high death rate.18
An inactivated whole-virus H5N1 vaccine has been developed by the US government to prevent H5N1 infection.19 For treatment, the neuraminidase inhibitor oseltamivir is the drug of choice.10 Oseltamivir resistance remains uncommon. 20 Fortunately, zanamivir is still active against oseltamivir-resistant variants that have N1 neuraminidase mutations.21
THE 2009 H1N1 PANDEMIC KILLED MORE PEOPLE THAN WE THOUGHT
The fourth flu pandemic of the last 100 years occurred in 2009. (The other three were in 1918, 1957, and 1968.) It was caused by a novel strain, H1N1 of swine origin.22 This 2009 pandemic strain had six genes from the North American swine flu virus and two genes from the Eurasian swine flu virus. The pandemic affected more children and young people (who completely lacked prior immunity to this virus), while older people, who had cross-reacting antibodies, were less affected.
Worldwide, 18,500 people were reported initially to have died in this pandemic from April 2009 to August 2010.23 However, a recent modeling study estimated the number of respiratory and cardiovascular deaths associated with this pandemic at 283,500—about 15 times higher.24
AN AUSTRALIAN OUTBREAK OF OSELTAMIVIR-RESISTANT H1N1
Many strains of influenza A virus are resistant to amantadine and rimantadine, owing to amino acid substitutions in the M2 protein.25 In contrast, resistance to the neuraminidase inhibitors oseltamivir and zanamivir has been reported only occasionally.26
Until recently, most oseltamivir-resistant viruses were isolated from immunocompromised hosts treated with oseltamivir.27–29 All the resistant viral isolates contained an amino acid substitution of histidine (H) to tyrosine (Y) at position 275 of the viral neuraminidase.30 In general, transmission of these oseltamivir-resistant strains has been limited and unsustained, but it can occur in settings of close contact, such as hospitals, school camps, or long train rides.31–35 Oseltamivir-resistant strains were detected in fewer than 1% of isolates from the community during the 2010–2011 influenza season in the Northern Hemisphere and most countries in the Southern Hemisphere during the 2011 flu season.36,37
However, an outbreak of oseltamivir-resistant H1N1 occurred in Australia between June and August 2011.38 In that outbreak, the isolates from only 15% of the 191 people infected with this virus, designated H1N1pdm09, carried the H257Y neuraminidase substitution.39 Further, only 1 of the 191 patients had received oseltamivir before. More importantly, genetic analysis suggested that the infection spread from a single source.
This was the first reported sustained community transmission of oseltamivir-resistant H1N1 in a community previously unexposed to this drug. As such, it is a warning sign of the potential for a widespread outbreak of this virus. In the event of such an outbreak, inhaled zanamivir would be the only effective treatment available.
THIS SEASON’S TRIVALENT INACTIVATED VACCINE
The trivalent inactivated influenza vaccine for the 2012–2013 season contains three inactivated viruses40:
- Influenza A/California/7/2009(H1N1)-like
- Influenza A/Victoria/361/2011(H3N2)-like
- Influenza B/Wisconsin/1/2010-like (Yamagata lineage).
The influenza A H3N2 and influenza B antigens are different from those in the 2011–2012 vaccine.41 The H1N1 strain is derived from H1N1pdm09, which had been contained in the 2011–2012 seasonal vaccine. This vaccine will not protect against H3N2v or H5N1.
LATEST RECOMMENDATIONS ON VACCINATION
Since 2010, the Advisory Committee on Immunization Practices (ACIP) has recommended annual flu shots for all people older than 6 months in the United States.42
Vaccination should be done before the onset of influenza activity in the community as soon as vaccine is available for the season. However, one should continue offering vaccination throughout the influenza season as long as influenza viruses are circulating in the community.
Children ages 6 months through 8 years not previously vaccinated against influenza should receive two doses of influenza vaccine at least 4 weeks apart for an optimal immune response. The US-licensed Afluria vaccine (CSL Biotherapies, King of Prussia, PA), a trivalent inactivated vaccine, is not recommended for children under 9 years of age because of concern about febrile seizures.43,44
There is no contraindication to giving inactivated trivalent influenza vaccine to immunosuppressed people.
The live-attenuated influenza vaccine is indicated only for healthy, nonpregnant people age 2 through 49 years and not for people who care for severely immunosuppressed patients who require a protective environment.
For indications for and details about the different available influenza vaccines, see the ACIP’s current recommendations (www.cdc.gov/mmwr/pdf/wk/mm6132.pdf).40
Updated recommendations for people allergic to eggs
All currently available influenza vaccines are made by growing the virus in chicken eggs. Therefore, severe allergic and anaphylactic reactions can occur in people with egg allergy. The ACIP recommends that if people experienced only hives after egg exposure, they should still receive the trivalent inactivated vaccine. Recently, the ACIP reviewed data from the Vaccine Adverse Event Reporting System45 and issued the following recommendations for the 2012–2013 influenza season40:
- In people who are allergic to eggs, only trivalent inactivated vaccine should be used, not the live-attenuated vaccine, because of lack of data for use of the latter in this group.
- Vaccine should be given by providers who are familiar with the signs of egg allergy.
- Patients with a history of egg allergy who have experienced only hives after exposure to eggs should be observed for a minimum of 30 minutes after vaccination.
- Patients who experience lightheadedness, respiratory distress, angioedema, or recurrent emesis or who require epinephrine or emergency medical attention after egg exposure should be referred before vaccination to a physician who has expertise in managing allergic conditions.
- Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs or egg-containing foods, plus skin or blood testing for immunoglobulin E antibodies to egg proteins.
A high-dose vaccine is available for people 65 years and older
The rates of hospitalization and death due to seasonal flu in elderly people have increased significantly in the last 20 years despite rising rates of vaccination.46–48 This is largely due to lower serologic response rates and vaccine efficacy in older adults with weaker immune systems.
Several studies have shown that the development of protective antibody titers depends on the dose of antigen.49–53 A randomized, controlled clinical trial compared the immunogenicity of a high-dose vaccine and a standard-dose vaccine in older adults and found that the level of antibody response was significantly higher with the high-dose vaccine, and that the rate of adverse reactions was the same.54
In December 2009, the US Food and Drug Administration (FDA) licensed a new trivalent inactivated influenza vaccine with high doses of hemagglutinin antigens for adults over the age of 65.55 Postlicensure safety surveillance in 2010 revealed no serious safety concerns.56
At present, the ACIP expresses no preference for standard-dose or high-dose vaccine for adults 65 years of age and older.40 Importantly, if only the standard-dose vaccine is at hand, the opportunity for influenza vaccination should not be missed with the intention of giving high-dose vaccine at a later date.
A NEW QUADRIVALENT LIVE-ATTENUATED INFLUENZA VACCINE FOR THE 2013–2014 SEASON
In February 2012, the FDA approved the first quadrivalent live-attenuated influenza vaccine, which is expected to replace the currently available trivalent live-attenuated influenza vaccine in the 2013–2014 flu season. The quadrivalent vaccine will include both lineages of the circulating influenza B viruses (the Victoria and Yamagata lineages). The reasons for this inclusion is the difficulty in predicting which of these viruses will predominate in any given season, and the limited cross-resistance by immunization against one of the lineages.
A recent analysis57 estimated that such a vaccine is likely to further reduce influenza cases, related hospitalizations, and deaths compared with the current trivalent vaccine. Like the current trivalent live-attenuated vaccine, the quadrivalent vaccine is inhaled.
EVOLVING VACCINATION POLICY IN HEALTH CARE WORKERS
Starting in January 2013, the Centers for Medicare and Medicaid Services will require hospitals to report how many of their health care workers are vaccinated. These rates will be publicly reported as a measure of hospital quality. This has fueled the ongoing debate about mandating influenza vaccination for health care workers. Studies have shown that the most important factors in increasing influenza vaccination rates among health care workers are requiring vaccination as a condition for employment and making vaccination available on-site, for more than 1 day, at no cost to the worker.58
As an alternative, some institutions have implemented a “shot-or-mask” policy whereby a health care worker who elects not to be vaccinated because of medical or religious reasons would be asked to wear a mask during all faceto-face encounters with patients.
NEW ANTIVIRAL DRUGS ON THE HORIZON
The emergence of oseltamivir-resistant strains in recent years caused a great deal of concern in public health regarding the potential for outbreaks of drug-resistant influenza.34,35,59–61
A recent Asian randomized clinical trial reported the efficacy of a long-acting neuraminidase inhibitor, laninamivir octanoate, in the treatment of seasonal influenza.62 This study showed that a single inhalation of this drug is effective in treating seasonal influenza, including that caused by oseltamivir-resistant strains in adults. Laninamivir is currently approved in Japan.
CHALLENGES IN PREVENTING AND TREATING INFLUENZA
Despite the great advances that we have made in preventing and treating influenza in the last half-century, we still face many challenges. Each year in the United States, influenza infection results in an estimated 31 million outpatient visits, 226,000 hospital admissions, and 36,000 deaths.42
Antigenic drift and shift. Influenza viruses circulating among animals and humans vary genetically from season to season and within seasons. As a result of this changing viral antigenicity, the virus can evade the human immune system, causing widespread outbreaks.
One of the unique and most remarkable features of influenza virus is the antigenic variation: antigenic drift and antigenic shift. Antigenic drift is the relatively minor antigenic changes that occur frequently within an influenza subtype, typically resulting from genetic mutation of viral RNA coding for hemagglutinin or neuraminidase. This causes annual regional epidemics. In contrast, antigenic shift is the result of genetic material reassortment: the emerging of new viral RNA from different strains of different species. This often leads to global pandemics.
Therefore, it is challenging to accurately predict the antigenic makeup of influenza viruses for each season and to include new emerging antigens in the vaccine production, as we are facing a moving target. We prepare influenza vaccines each season based on past experience.63
Vaccination rates have hit a plateau of 60% to 70% in adults since the 1990s, in spite of greater vaccine supply and recommendations that all adults, regardless of underlying disease, be vaccinated annually.64 Similarly, only 51% of children age 6 months to 17 years were vaccinated in the 2010–2011 season.65 And vaccination rates are even lower in low-income populations.66,67
The emergence of oseltamivir-resistant strains in recent years, not only in people exposed to oseltamivir but also in those who haven’t been exposed to this drug, with sustained transmission, certainly raises the possibility of a more difficult epidemic to control.38
Global travel, global infection. Our last H1N1 pandemic in 2009 was an example of how easily the influenza virus can spread rapidly in today’s highly mobile global society.22
What we must do
As primary health care providers, we must closely monitor the community outbreak and the emergence of drug-resistant strains and strongly recommend vaccination for all patients older than 6 months, since timely vaccination is the cornerstone of influenza prevention. Although many have questioned the efficacy of influenza vaccination, a recent meta-analysis showed a 59% pooled efficacy of the trivalent inactivated vaccine in adults age 18 to 65 years in preventing virologically confirmed influenza, and 83% pooled efficacy of the live-attenuated influenza vaccine in children age 6 months to 7 years.68 Novel approaches for vaccination reminders, such as text messaging69 in addition to traditional mail or telephone reminders, can improve vaccination compliance in today’s highly mobile world that is more than ever connected.
With the lessons learned from four pandemics in the last century, updated recommendations for prevention, and adequate vaccine supply, we should be ready to face the challenge of another flu season.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–1062.
- Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol 2004; 2:909–914.
- Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012; 18:834–837.
- Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012; 422:151–160.
- Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/flu/swineflu/h3n2v-outbreak.htm. Accessed September 27, 2012.
- Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count — United States, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:619–621.
- Centers for Disease Control and Prevention (CDC). Update: Influenza A (H3N2)v transmission and guidelines — five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–1744.
- Centers for Disease Control and Prevention (CDC). Interim information for clinicians about human infections with H3N2v virus. http://www.cdc.gov/flu/swineflu/h3n2v-clinician.htm. Accessed September 27, 2012.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus; Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273.
- World Health Organization (WHO). http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed September 27, 2012.
- Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352:333–340.
- Yamada S, Suzuki Y, Suzuki T, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006; 444:378–382.
- Hatta M, Hatta Y, Kim JH, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 2007; 3:1374–1379.
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207.
- Cheung CY, Chan EY, Krasnoselsky A, et al. H5N1 virus causes significant perturbations in host proteome very early in influenza virus-infected primary human monocyte-derived macrophages. J Infect Dis 2012; 206:640–645.
- Gordon S. Avian influenza: a wake-up call from birds to humans. Cleve Clin J Med 2004; 71:273–274.
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129–1234.
- Ehrlich HJ, Müller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005; 353:2667–2672.
- Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005; 437:1108.
- Ison MG, Lee N. Influenza 2010–2011: lessons from the 2009 pandemic. Cleve Clin J Med 2010; 77:812–820.
- World Health Organization (WHO). Pandemic (H1N1) 2009 — update 112. http://www.who.int/csr/don/2010_08_06/en/index.html. Accessed September 27, 2012.
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12:687–695.
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA 2006; 295:891–894.
- Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther 2012; 17:159–173.
- Graitcer SB, Gubareva L, Kamimoto L, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis 2011; 17:255–257.
- Hurt AC, Deng YM, Ernest J, et al. Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010. Euro Surveill 2011; 16:19770.
- Meijer A, Jonges M, Abbink F, et al. Oseltamivir-resistant pandemic A(H1N1) 2009 influenza viruses detected through enhanced surveillance in the Netherlands, 2009–2010. Antiviral Res 2011; 92:81–89.
- Gubareva LV, Kaiser L, Hayden FG. IInfluenza virus neuraminidase inhibitors. Lancet 2000; 355:827–835.
- Wolfe C, Greenwald I, Chen L. Pandemic (H1N1) 2009 and oseltamivir resistance in hematology/oncology patients. Emerg Infect Dis 2010; 16:1809–1811.
- Moore C, Galiano M, Lackenby A, et al. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 2011; 203:18–24.
- Chen LF, Dailey NJ, Rao AK, et al. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients — North Carolina, 2009. J Infect Dis 2011; 203:838–846.
- Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis — North Carolina, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:969–972.
- Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P; Vietnam H1N1 Investigation Team. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362:86–87.
- Lackenby A, Moran Gilad J, Pebody R, et al. Continued emergence and changing epidemiology of oseltamivir-resistant influenza A(H1N1)2009 virus, United Kingdom, winter 2010/11. Euro Surveill 2011; 16:19784.
- World Health Organization (WHO). Summary of influenza antiviral susceptibility surveillance findings, September 2010 – March 2011. http://www.who.int/influenza/gisrs_laboratory/updates/antiviral_susceptibility/en/index.html. Accessed September 27, 2012.
- Hurt AC, Hardie K, Wilson NJ, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza. N Engl J Med 2011; 365:2541–2542.
- Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–157.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. MMWR Morb Mortal Wkly Rep 2012; 61:613–618.
- Food and Drug Administration (FDA). Summary minutes: vaccines and related biological products advisory committee. February 28–29, 2012. Silver Spring, MD. http://www.fda.gov/downloads/Advisory-Committees/CommitteesMeetingMaterials/BloodVaccinesandOther-Biologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM296193.pdf. Accessed September 28, 2012.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Centers for Disease Control and Prevention (CDC). Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep 2010; 59:989–992.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128–1132.
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices: Update on influenza vaccine safety monitoring. June 20–21, 2012. Atlanta, GA. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed September 28, 2012.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad Med J 1973; 49:152–158.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis 1972; 125:656–664.
- Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine 1993; 11:3–9.
- Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–7663.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food and Drug Administration. Vaccines, Blood & Biologics. December 23,2009 approval letter—Fluzone high-dose. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm195481.htm. Accessed October 1, 2012.
- Moro PL, Arana J, Cano M, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54:1608–1614.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among health-care personnel — United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1073–1077.
- Meijer A, Lackenby A, Hungnes O, et al; European Influenza Surveillance Scheme. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis 2009; 15:552–560.
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360:953–956.
- World Health Organization (WHO). Influenza A virus resistance to oseltamivir. http://www.who.int/influenza/patient_care/antivirals/oseltamivir_summary/en/. Accessed September 28, 2012.
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175.
- Deyde VM, Gubareva LV. Influenza genome analysis using pyro-sequencing method: current applications for a moving target. Expert Rev Mol Diagn 2009; 9:493–509.
- Schuchat A, Katz JM. Protecting adults from influenza: tis the season to learn from the pandemic. J Infect Dis 2012; 206:803–805.
- Centers for Disease Control and Prevention (CDC). Final state-level influenza vaccination coverage estimates for the 2010–11 season — United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. http://www.cdc.gov/flu/professionals/vaccination/coverage_1011estimates.htm. Accessed September 28, 2012.
- Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J 2011; 30:100–106.
- Lee BY, Brown ST, Bailey RR, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Aff (Millwood) 2011; 30:1141–1150.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44.
- Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012; 307:1702–1708.
Despite our success in reducing the number of deaths from influenza in the last half-century, we must remain vigilant, since influenza still can kill.1,2 Gene mutations and reassortment among different strains of influenza viruses pose a significant public health threat, especially in an increasingly mobile world.3,4
In this article, we will present an update on influenza to better prepare primary care providers to prevent and treat this ongoing threat.
H3N2v: SWINE FLU DÉJÀ VU?
Outbreaks of swine flu at state and county fairs in 2012 are unprecedented and have raised concerns.
From 1990 to 2010, human infections with swine-origin influenza viruses were sporadic, and the US Centers for Disease Control and Prevention (CDC) confirmed a total of only 27 cases during this period.5 However, the number has been increasing since 2011: as of August 31, 2012, a total of 309 cases had been reported.6
Analysis of viral RNA in clinical respiratory specimens from 12 cases in 2011 revealed a variant strain, called H3N2v, which is a hybrid containing genetic material from swine H3N2 and the 2009 human pandemic virus H1N1pdm09. The M gene in this new variant came from the human virus, while the other seven came from the swine virus when a host was infected with both viruses simultaneously (Figure 1). As a result of this genetic reassortment, this variant virus is genetically and antigenically different from seasonal H3N2.
Epidemiologic data showed that children under 10 years of age are especially susceptible to this new variant because they lack immunity, whereas adolescents and adults may have some immunity from cross-reacting antibodies.7 Most infected people had been exposed to swine in agriculture, including county and state fairs. So far, evidence suggests only limited human-to-human transmission.8 The clinical diagnosis of H3N2v infection relies on the epidemiologic link to exposure to pigs in the week before the onset of illness, since the symptoms are indistinguishable from those of seasonal influenza A or B infections.
In suspected cases, the clinician should notify the local or state public health department and arrange for a special test to be performed on respiratory specimens: the CDC Flu Real-Time Reverse Transcriptase Polymerase Chain Reaction Dx Panel. The reason is that a negative rapid influenza diagnostic test does not rule out influenza infection, and a positive immunofluorescence assay (direct fluorescent antibody staining) cannot specifically detect H3N2v.7
The current seasonal influenza vaccine will not protect against H3N2v. The isolates tested to date were susceptible to the neuraminidase inhibitor drugs oseltamivir (Tamiflu) and zanamivir (Relenza) but resistant to amantadine (Symmetrel) and rimantadine (Flumadine).9
Whether H3N2v will become a significant problem during the upcoming flu season largely depends on the extent of human-to-human transmission. We need to closely follow updates on this virus.
H5N1: THE LOOMING THREAT OF A BIRD FLU PANDEMIC
Since 2003, influenza A H5N1, a highly pathogenic avian virus, has broken out in Asia, Africa, and the Middle East, killing more than 100 million birds. It also has crossed the species barrier to infect humans, with an unusually high death rate.10
As of August 10, 2012, the World Health Organization had reported 608 confirmed cases of this virus infecting humans and 359 associated deaths.11 Most infected patients had a history of close contact with diseased poultry, but limited, nonsustained human-to-human transmission can occur during very close, unprotected contact with a severely ill patient.12
Molecular studies of this virus revealed further insights into its pathogenesis. Some of the viruses isolated from humans have had mutations that allow them to bind to human-type receptors.13 Amino acid substitutions in the polymerase basic protein 2 (PB2) gene are associated with mammalian adaptation, virulence in mice, and viral replication at temperatures present in the upper respiratory tract.14 Furthermore, higher plasma levels of macrophage- and neutrophil-attractant chemokines and both inflammatory and anti-inflammatory cytokines (interleukin 6, interleukin 10, and interferon gamma) have been observed in patients with H5N1 infection, especially in fatal cases.15 A recent study found that H5N1 causes significant perturbations in the host’s protein synthesis machinery as early as 1 hour after infection, suggesting that this virus gains an early advantage in replication by using the host’s proteome.16 The effects of unrestrained viral infection and inflammatory responses induced by H5N1 infection certainly contributed to the primary pathologic process and to death in human fulminant viral pneumonia. The up-regulation of inflammatory cytokines in these infections contributes to the development of sepsis syndrome, acute respiratory distress syndrome, and an increased risk of death, particularly in pregnant women.
Most experts predict that pandemic influenza is probably inevitable.17 If avian H5N1 and a human influenza virus swap genes in a host such as swine, the new hybrid virus will contain genetic material from both strains and will have surface antigens that the human immune system does not recognize. This could lead to a devastating avian flu pandemic with a very high death rate.18
An inactivated whole-virus H5N1 vaccine has been developed by the US government to prevent H5N1 infection.19 For treatment, the neuraminidase inhibitor oseltamivir is the drug of choice.10 Oseltamivir resistance remains uncommon. 20 Fortunately, zanamivir is still active against oseltamivir-resistant variants that have N1 neuraminidase mutations.21
THE 2009 H1N1 PANDEMIC KILLED MORE PEOPLE THAN WE THOUGHT
The fourth flu pandemic of the last 100 years occurred in 2009. (The other three were in 1918, 1957, and 1968.) It was caused by a novel strain, H1N1 of swine origin.22 This 2009 pandemic strain had six genes from the North American swine flu virus and two genes from the Eurasian swine flu virus. The pandemic affected more children and young people (who completely lacked prior immunity to this virus), while older people, who had cross-reacting antibodies, were less affected.
Worldwide, 18,500 people were reported initially to have died in this pandemic from April 2009 to August 2010.23 However, a recent modeling study estimated the number of respiratory and cardiovascular deaths associated with this pandemic at 283,500—about 15 times higher.24
AN AUSTRALIAN OUTBREAK OF OSELTAMIVIR-RESISTANT H1N1
Many strains of influenza A virus are resistant to amantadine and rimantadine, owing to amino acid substitutions in the M2 protein.25 In contrast, resistance to the neuraminidase inhibitors oseltamivir and zanamivir has been reported only occasionally.26
Until recently, most oseltamivir-resistant viruses were isolated from immunocompromised hosts treated with oseltamivir.27–29 All the resistant viral isolates contained an amino acid substitution of histidine (H) to tyrosine (Y) at position 275 of the viral neuraminidase.30 In general, transmission of these oseltamivir-resistant strains has been limited and unsustained, but it can occur in settings of close contact, such as hospitals, school camps, or long train rides.31–35 Oseltamivir-resistant strains were detected in fewer than 1% of isolates from the community during the 2010–2011 influenza season in the Northern Hemisphere and most countries in the Southern Hemisphere during the 2011 flu season.36,37
However, an outbreak of oseltamivir-resistant H1N1 occurred in Australia between June and August 2011.38 In that outbreak, the isolates from only 15% of the 191 people infected with this virus, designated H1N1pdm09, carried the H257Y neuraminidase substitution.39 Further, only 1 of the 191 patients had received oseltamivir before. More importantly, genetic analysis suggested that the infection spread from a single source.
This was the first reported sustained community transmission of oseltamivir-resistant H1N1 in a community previously unexposed to this drug. As such, it is a warning sign of the potential for a widespread outbreak of this virus. In the event of such an outbreak, inhaled zanamivir would be the only effective treatment available.
THIS SEASON’S TRIVALENT INACTIVATED VACCINE
The trivalent inactivated influenza vaccine for the 2012–2013 season contains three inactivated viruses40:
- Influenza A/California/7/2009(H1N1)-like
- Influenza A/Victoria/361/2011(H3N2)-like
- Influenza B/Wisconsin/1/2010-like (Yamagata lineage).
The influenza A H3N2 and influenza B antigens are different from those in the 2011–2012 vaccine.41 The H1N1 strain is derived from H1N1pdm09, which had been contained in the 2011–2012 seasonal vaccine. This vaccine will not protect against H3N2v or H5N1.
LATEST RECOMMENDATIONS ON VACCINATION
Since 2010, the Advisory Committee on Immunization Practices (ACIP) has recommended annual flu shots for all people older than 6 months in the United States.42
Vaccination should be done before the onset of influenza activity in the community as soon as vaccine is available for the season. However, one should continue offering vaccination throughout the influenza season as long as influenza viruses are circulating in the community.
Children ages 6 months through 8 years not previously vaccinated against influenza should receive two doses of influenza vaccine at least 4 weeks apart for an optimal immune response. The US-licensed Afluria vaccine (CSL Biotherapies, King of Prussia, PA), a trivalent inactivated vaccine, is not recommended for children under 9 years of age because of concern about febrile seizures.43,44
There is no contraindication to giving inactivated trivalent influenza vaccine to immunosuppressed people.
The live-attenuated influenza vaccine is indicated only for healthy, nonpregnant people age 2 through 49 years and not for people who care for severely immunosuppressed patients who require a protective environment.
For indications for and details about the different available influenza vaccines, see the ACIP’s current recommendations (www.cdc.gov/mmwr/pdf/wk/mm6132.pdf).40
Updated recommendations for people allergic to eggs
All currently available influenza vaccines are made by growing the virus in chicken eggs. Therefore, severe allergic and anaphylactic reactions can occur in people with egg allergy. The ACIP recommends that if people experienced only hives after egg exposure, they should still receive the trivalent inactivated vaccine. Recently, the ACIP reviewed data from the Vaccine Adverse Event Reporting System45 and issued the following recommendations for the 2012–2013 influenza season40:
- In people who are allergic to eggs, only trivalent inactivated vaccine should be used, not the live-attenuated vaccine, because of lack of data for use of the latter in this group.
- Vaccine should be given by providers who are familiar with the signs of egg allergy.
- Patients with a history of egg allergy who have experienced only hives after exposure to eggs should be observed for a minimum of 30 minutes after vaccination.
- Patients who experience lightheadedness, respiratory distress, angioedema, or recurrent emesis or who require epinephrine or emergency medical attention after egg exposure should be referred before vaccination to a physician who has expertise in managing allergic conditions.
- Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs or egg-containing foods, plus skin or blood testing for immunoglobulin E antibodies to egg proteins.
A high-dose vaccine is available for people 65 years and older
The rates of hospitalization and death due to seasonal flu in elderly people have increased significantly in the last 20 years despite rising rates of vaccination.46–48 This is largely due to lower serologic response rates and vaccine efficacy in older adults with weaker immune systems.
Several studies have shown that the development of protective antibody titers depends on the dose of antigen.49–53 A randomized, controlled clinical trial compared the immunogenicity of a high-dose vaccine and a standard-dose vaccine in older adults and found that the level of antibody response was significantly higher with the high-dose vaccine, and that the rate of adverse reactions was the same.54
In December 2009, the US Food and Drug Administration (FDA) licensed a new trivalent inactivated influenza vaccine with high doses of hemagglutinin antigens for adults over the age of 65.55 Postlicensure safety surveillance in 2010 revealed no serious safety concerns.56
At present, the ACIP expresses no preference for standard-dose or high-dose vaccine for adults 65 years of age and older.40 Importantly, if only the standard-dose vaccine is at hand, the opportunity for influenza vaccination should not be missed with the intention of giving high-dose vaccine at a later date.
A NEW QUADRIVALENT LIVE-ATTENUATED INFLUENZA VACCINE FOR THE 2013–2014 SEASON
In February 2012, the FDA approved the first quadrivalent live-attenuated influenza vaccine, which is expected to replace the currently available trivalent live-attenuated influenza vaccine in the 2013–2014 flu season. The quadrivalent vaccine will include both lineages of the circulating influenza B viruses (the Victoria and Yamagata lineages). The reasons for this inclusion is the difficulty in predicting which of these viruses will predominate in any given season, and the limited cross-resistance by immunization against one of the lineages.
A recent analysis57 estimated that such a vaccine is likely to further reduce influenza cases, related hospitalizations, and deaths compared with the current trivalent vaccine. Like the current trivalent live-attenuated vaccine, the quadrivalent vaccine is inhaled.
EVOLVING VACCINATION POLICY IN HEALTH CARE WORKERS
Starting in January 2013, the Centers for Medicare and Medicaid Services will require hospitals to report how many of their health care workers are vaccinated. These rates will be publicly reported as a measure of hospital quality. This has fueled the ongoing debate about mandating influenza vaccination for health care workers. Studies have shown that the most important factors in increasing influenza vaccination rates among health care workers are requiring vaccination as a condition for employment and making vaccination available on-site, for more than 1 day, at no cost to the worker.58
As an alternative, some institutions have implemented a “shot-or-mask” policy whereby a health care worker who elects not to be vaccinated because of medical or religious reasons would be asked to wear a mask during all faceto-face encounters with patients.
NEW ANTIVIRAL DRUGS ON THE HORIZON
The emergence of oseltamivir-resistant strains in recent years caused a great deal of concern in public health regarding the potential for outbreaks of drug-resistant influenza.34,35,59–61
A recent Asian randomized clinical trial reported the efficacy of a long-acting neuraminidase inhibitor, laninamivir octanoate, in the treatment of seasonal influenza.62 This study showed that a single inhalation of this drug is effective in treating seasonal influenza, including that caused by oseltamivir-resistant strains in adults. Laninamivir is currently approved in Japan.
CHALLENGES IN PREVENTING AND TREATING INFLUENZA
Despite the great advances that we have made in preventing and treating influenza in the last half-century, we still face many challenges. Each year in the United States, influenza infection results in an estimated 31 million outpatient visits, 226,000 hospital admissions, and 36,000 deaths.42
Antigenic drift and shift. Influenza viruses circulating among animals and humans vary genetically from season to season and within seasons. As a result of this changing viral antigenicity, the virus can evade the human immune system, causing widespread outbreaks.
One of the unique and most remarkable features of influenza virus is the antigenic variation: antigenic drift and antigenic shift. Antigenic drift is the relatively minor antigenic changes that occur frequently within an influenza subtype, typically resulting from genetic mutation of viral RNA coding for hemagglutinin or neuraminidase. This causes annual regional epidemics. In contrast, antigenic shift is the result of genetic material reassortment: the emerging of new viral RNA from different strains of different species. This often leads to global pandemics.
Therefore, it is challenging to accurately predict the antigenic makeup of influenza viruses for each season and to include new emerging antigens in the vaccine production, as we are facing a moving target. We prepare influenza vaccines each season based on past experience.63
Vaccination rates have hit a plateau of 60% to 70% in adults since the 1990s, in spite of greater vaccine supply and recommendations that all adults, regardless of underlying disease, be vaccinated annually.64 Similarly, only 51% of children age 6 months to 17 years were vaccinated in the 2010–2011 season.65 And vaccination rates are even lower in low-income populations.66,67
The emergence of oseltamivir-resistant strains in recent years, not only in people exposed to oseltamivir but also in those who haven’t been exposed to this drug, with sustained transmission, certainly raises the possibility of a more difficult epidemic to control.38
Global travel, global infection. Our last H1N1 pandemic in 2009 was an example of how easily the influenza virus can spread rapidly in today’s highly mobile global society.22
What we must do
As primary health care providers, we must closely monitor the community outbreak and the emergence of drug-resistant strains and strongly recommend vaccination for all patients older than 6 months, since timely vaccination is the cornerstone of influenza prevention. Although many have questioned the efficacy of influenza vaccination, a recent meta-analysis showed a 59% pooled efficacy of the trivalent inactivated vaccine in adults age 18 to 65 years in preventing virologically confirmed influenza, and 83% pooled efficacy of the live-attenuated influenza vaccine in children age 6 months to 7 years.68 Novel approaches for vaccination reminders, such as text messaging69 in addition to traditional mail or telephone reminders, can improve vaccination compliance in today’s highly mobile world that is more than ever connected.
With the lessons learned from four pandemics in the last century, updated recommendations for prevention, and adequate vaccine supply, we should be ready to face the challenge of another flu season.
Despite our success in reducing the number of deaths from influenza in the last half-century, we must remain vigilant, since influenza still can kill.1,2 Gene mutations and reassortment among different strains of influenza viruses pose a significant public health threat, especially in an increasingly mobile world.3,4
In this article, we will present an update on influenza to better prepare primary care providers to prevent and treat this ongoing threat.
H3N2v: SWINE FLU DÉJÀ VU?
Outbreaks of swine flu at state and county fairs in 2012 are unprecedented and have raised concerns.
From 1990 to 2010, human infections with swine-origin influenza viruses were sporadic, and the US Centers for Disease Control and Prevention (CDC) confirmed a total of only 27 cases during this period.5 However, the number has been increasing since 2011: as of August 31, 2012, a total of 309 cases had been reported.6
Analysis of viral RNA in clinical respiratory specimens from 12 cases in 2011 revealed a variant strain, called H3N2v, which is a hybrid containing genetic material from swine H3N2 and the 2009 human pandemic virus H1N1pdm09. The M gene in this new variant came from the human virus, while the other seven came from the swine virus when a host was infected with both viruses simultaneously (Figure 1). As a result of this genetic reassortment, this variant virus is genetically and antigenically different from seasonal H3N2.
Epidemiologic data showed that children under 10 years of age are especially susceptible to this new variant because they lack immunity, whereas adolescents and adults may have some immunity from cross-reacting antibodies.7 Most infected people had been exposed to swine in agriculture, including county and state fairs. So far, evidence suggests only limited human-to-human transmission.8 The clinical diagnosis of H3N2v infection relies on the epidemiologic link to exposure to pigs in the week before the onset of illness, since the symptoms are indistinguishable from those of seasonal influenza A or B infections.
In suspected cases, the clinician should notify the local or state public health department and arrange for a special test to be performed on respiratory specimens: the CDC Flu Real-Time Reverse Transcriptase Polymerase Chain Reaction Dx Panel. The reason is that a negative rapid influenza diagnostic test does not rule out influenza infection, and a positive immunofluorescence assay (direct fluorescent antibody staining) cannot specifically detect H3N2v.7
The current seasonal influenza vaccine will not protect against H3N2v. The isolates tested to date were susceptible to the neuraminidase inhibitor drugs oseltamivir (Tamiflu) and zanamivir (Relenza) but resistant to amantadine (Symmetrel) and rimantadine (Flumadine).9
Whether H3N2v will become a significant problem during the upcoming flu season largely depends on the extent of human-to-human transmission. We need to closely follow updates on this virus.
H5N1: THE LOOMING THREAT OF A BIRD FLU PANDEMIC
Since 2003, influenza A H5N1, a highly pathogenic avian virus, has broken out in Asia, Africa, and the Middle East, killing more than 100 million birds. It also has crossed the species barrier to infect humans, with an unusually high death rate.10
As of August 10, 2012, the World Health Organization had reported 608 confirmed cases of this virus infecting humans and 359 associated deaths.11 Most infected patients had a history of close contact with diseased poultry, but limited, nonsustained human-to-human transmission can occur during very close, unprotected contact with a severely ill patient.12
Molecular studies of this virus revealed further insights into its pathogenesis. Some of the viruses isolated from humans have had mutations that allow them to bind to human-type receptors.13 Amino acid substitutions in the polymerase basic protein 2 (PB2) gene are associated with mammalian adaptation, virulence in mice, and viral replication at temperatures present in the upper respiratory tract.14 Furthermore, higher plasma levels of macrophage- and neutrophil-attractant chemokines and both inflammatory and anti-inflammatory cytokines (interleukin 6, interleukin 10, and interferon gamma) have been observed in patients with H5N1 infection, especially in fatal cases.15 A recent study found that H5N1 causes significant perturbations in the host’s protein synthesis machinery as early as 1 hour after infection, suggesting that this virus gains an early advantage in replication by using the host’s proteome.16 The effects of unrestrained viral infection and inflammatory responses induced by H5N1 infection certainly contributed to the primary pathologic process and to death in human fulminant viral pneumonia. The up-regulation of inflammatory cytokines in these infections contributes to the development of sepsis syndrome, acute respiratory distress syndrome, and an increased risk of death, particularly in pregnant women.
Most experts predict that pandemic influenza is probably inevitable.17 If avian H5N1 and a human influenza virus swap genes in a host such as swine, the new hybrid virus will contain genetic material from both strains and will have surface antigens that the human immune system does not recognize. This could lead to a devastating avian flu pandemic with a very high death rate.18
An inactivated whole-virus H5N1 vaccine has been developed by the US government to prevent H5N1 infection.19 For treatment, the neuraminidase inhibitor oseltamivir is the drug of choice.10 Oseltamivir resistance remains uncommon. 20 Fortunately, zanamivir is still active against oseltamivir-resistant variants that have N1 neuraminidase mutations.21
THE 2009 H1N1 PANDEMIC KILLED MORE PEOPLE THAN WE THOUGHT
The fourth flu pandemic of the last 100 years occurred in 2009. (The other three were in 1918, 1957, and 1968.) It was caused by a novel strain, H1N1 of swine origin.22 This 2009 pandemic strain had six genes from the North American swine flu virus and two genes from the Eurasian swine flu virus. The pandemic affected more children and young people (who completely lacked prior immunity to this virus), while older people, who had cross-reacting antibodies, were less affected.
Worldwide, 18,500 people were reported initially to have died in this pandemic from April 2009 to August 2010.23 However, a recent modeling study estimated the number of respiratory and cardiovascular deaths associated with this pandemic at 283,500—about 15 times higher.24
AN AUSTRALIAN OUTBREAK OF OSELTAMIVIR-RESISTANT H1N1
Many strains of influenza A virus are resistant to amantadine and rimantadine, owing to amino acid substitutions in the M2 protein.25 In contrast, resistance to the neuraminidase inhibitors oseltamivir and zanamivir has been reported only occasionally.26
Until recently, most oseltamivir-resistant viruses were isolated from immunocompromised hosts treated with oseltamivir.27–29 All the resistant viral isolates contained an amino acid substitution of histidine (H) to tyrosine (Y) at position 275 of the viral neuraminidase.30 In general, transmission of these oseltamivir-resistant strains has been limited and unsustained, but it can occur in settings of close contact, such as hospitals, school camps, or long train rides.31–35 Oseltamivir-resistant strains were detected in fewer than 1% of isolates from the community during the 2010–2011 influenza season in the Northern Hemisphere and most countries in the Southern Hemisphere during the 2011 flu season.36,37
However, an outbreak of oseltamivir-resistant H1N1 occurred in Australia between June and August 2011.38 In that outbreak, the isolates from only 15% of the 191 people infected with this virus, designated H1N1pdm09, carried the H257Y neuraminidase substitution.39 Further, only 1 of the 191 patients had received oseltamivir before. More importantly, genetic analysis suggested that the infection spread from a single source.
This was the first reported sustained community transmission of oseltamivir-resistant H1N1 in a community previously unexposed to this drug. As such, it is a warning sign of the potential for a widespread outbreak of this virus. In the event of such an outbreak, inhaled zanamivir would be the only effective treatment available.
THIS SEASON’S TRIVALENT INACTIVATED VACCINE
The trivalent inactivated influenza vaccine for the 2012–2013 season contains three inactivated viruses40:
- Influenza A/California/7/2009(H1N1)-like
- Influenza A/Victoria/361/2011(H3N2)-like
- Influenza B/Wisconsin/1/2010-like (Yamagata lineage).
The influenza A H3N2 and influenza B antigens are different from those in the 2011–2012 vaccine.41 The H1N1 strain is derived from H1N1pdm09, which had been contained in the 2011–2012 seasonal vaccine. This vaccine will not protect against H3N2v or H5N1.
LATEST RECOMMENDATIONS ON VACCINATION
Since 2010, the Advisory Committee on Immunization Practices (ACIP) has recommended annual flu shots for all people older than 6 months in the United States.42
Vaccination should be done before the onset of influenza activity in the community as soon as vaccine is available for the season. However, one should continue offering vaccination throughout the influenza season as long as influenza viruses are circulating in the community.
Children ages 6 months through 8 years not previously vaccinated against influenza should receive two doses of influenza vaccine at least 4 weeks apart for an optimal immune response. The US-licensed Afluria vaccine (CSL Biotherapies, King of Prussia, PA), a trivalent inactivated vaccine, is not recommended for children under 9 years of age because of concern about febrile seizures.43,44
There is no contraindication to giving inactivated trivalent influenza vaccine to immunosuppressed people.
The live-attenuated influenza vaccine is indicated only for healthy, nonpregnant people age 2 through 49 years and not for people who care for severely immunosuppressed patients who require a protective environment.
For indications for and details about the different available influenza vaccines, see the ACIP’s current recommendations (www.cdc.gov/mmwr/pdf/wk/mm6132.pdf).40
Updated recommendations for people allergic to eggs
All currently available influenza vaccines are made by growing the virus in chicken eggs. Therefore, severe allergic and anaphylactic reactions can occur in people with egg allergy. The ACIP recommends that if people experienced only hives after egg exposure, they should still receive the trivalent inactivated vaccine. Recently, the ACIP reviewed data from the Vaccine Adverse Event Reporting System45 and issued the following recommendations for the 2012–2013 influenza season40:
- In people who are allergic to eggs, only trivalent inactivated vaccine should be used, not the live-attenuated vaccine, because of lack of data for use of the latter in this group.
- Vaccine should be given by providers who are familiar with the signs of egg allergy.
- Patients with a history of egg allergy who have experienced only hives after exposure to eggs should be observed for a minimum of 30 minutes after vaccination.
- Patients who experience lightheadedness, respiratory distress, angioedema, or recurrent emesis or who require epinephrine or emergency medical attention after egg exposure should be referred before vaccination to a physician who has expertise in managing allergic conditions.
- Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs or egg-containing foods, plus skin or blood testing for immunoglobulin E antibodies to egg proteins.
A high-dose vaccine is available for people 65 years and older
The rates of hospitalization and death due to seasonal flu in elderly people have increased significantly in the last 20 years despite rising rates of vaccination.46–48 This is largely due to lower serologic response rates and vaccine efficacy in older adults with weaker immune systems.
Several studies have shown that the development of protective antibody titers depends on the dose of antigen.49–53 A randomized, controlled clinical trial compared the immunogenicity of a high-dose vaccine and a standard-dose vaccine in older adults and found that the level of antibody response was significantly higher with the high-dose vaccine, and that the rate of adverse reactions was the same.54
In December 2009, the US Food and Drug Administration (FDA) licensed a new trivalent inactivated influenza vaccine with high doses of hemagglutinin antigens for adults over the age of 65.55 Postlicensure safety surveillance in 2010 revealed no serious safety concerns.56
At present, the ACIP expresses no preference for standard-dose or high-dose vaccine for adults 65 years of age and older.40 Importantly, if only the standard-dose vaccine is at hand, the opportunity for influenza vaccination should not be missed with the intention of giving high-dose vaccine at a later date.
A NEW QUADRIVALENT LIVE-ATTENUATED INFLUENZA VACCINE FOR THE 2013–2014 SEASON
In February 2012, the FDA approved the first quadrivalent live-attenuated influenza vaccine, which is expected to replace the currently available trivalent live-attenuated influenza vaccine in the 2013–2014 flu season. The quadrivalent vaccine will include both lineages of the circulating influenza B viruses (the Victoria and Yamagata lineages). The reasons for this inclusion is the difficulty in predicting which of these viruses will predominate in any given season, and the limited cross-resistance by immunization against one of the lineages.
A recent analysis57 estimated that such a vaccine is likely to further reduce influenza cases, related hospitalizations, and deaths compared with the current trivalent vaccine. Like the current trivalent live-attenuated vaccine, the quadrivalent vaccine is inhaled.
EVOLVING VACCINATION POLICY IN HEALTH CARE WORKERS
Starting in January 2013, the Centers for Medicare and Medicaid Services will require hospitals to report how many of their health care workers are vaccinated. These rates will be publicly reported as a measure of hospital quality. This has fueled the ongoing debate about mandating influenza vaccination for health care workers. Studies have shown that the most important factors in increasing influenza vaccination rates among health care workers are requiring vaccination as a condition for employment and making vaccination available on-site, for more than 1 day, at no cost to the worker.58
As an alternative, some institutions have implemented a “shot-or-mask” policy whereby a health care worker who elects not to be vaccinated because of medical or religious reasons would be asked to wear a mask during all faceto-face encounters with patients.
NEW ANTIVIRAL DRUGS ON THE HORIZON
The emergence of oseltamivir-resistant strains in recent years caused a great deal of concern in public health regarding the potential for outbreaks of drug-resistant influenza.34,35,59–61
A recent Asian randomized clinical trial reported the efficacy of a long-acting neuraminidase inhibitor, laninamivir octanoate, in the treatment of seasonal influenza.62 This study showed that a single inhalation of this drug is effective in treating seasonal influenza, including that caused by oseltamivir-resistant strains in adults. Laninamivir is currently approved in Japan.
CHALLENGES IN PREVENTING AND TREATING INFLUENZA
Despite the great advances that we have made in preventing and treating influenza in the last half-century, we still face many challenges. Each year in the United States, influenza infection results in an estimated 31 million outpatient visits, 226,000 hospital admissions, and 36,000 deaths.42
Antigenic drift and shift. Influenza viruses circulating among animals and humans vary genetically from season to season and within seasons. As a result of this changing viral antigenicity, the virus can evade the human immune system, causing widespread outbreaks.
One of the unique and most remarkable features of influenza virus is the antigenic variation: antigenic drift and antigenic shift. Antigenic drift is the relatively minor antigenic changes that occur frequently within an influenza subtype, typically resulting from genetic mutation of viral RNA coding for hemagglutinin or neuraminidase. This causes annual regional epidemics. In contrast, antigenic shift is the result of genetic material reassortment: the emerging of new viral RNA from different strains of different species. This often leads to global pandemics.
Therefore, it is challenging to accurately predict the antigenic makeup of influenza viruses for each season and to include new emerging antigens in the vaccine production, as we are facing a moving target. We prepare influenza vaccines each season based on past experience.63
Vaccination rates have hit a plateau of 60% to 70% in adults since the 1990s, in spite of greater vaccine supply and recommendations that all adults, regardless of underlying disease, be vaccinated annually.64 Similarly, only 51% of children age 6 months to 17 years were vaccinated in the 2010–2011 season.65 And vaccination rates are even lower in low-income populations.66,67
The emergence of oseltamivir-resistant strains in recent years, not only in people exposed to oseltamivir but also in those who haven’t been exposed to this drug, with sustained transmission, certainly raises the possibility of a more difficult epidemic to control.38
Global travel, global infection. Our last H1N1 pandemic in 2009 was an example of how easily the influenza virus can spread rapidly in today’s highly mobile global society.22
What we must do
As primary health care providers, we must closely monitor the community outbreak and the emergence of drug-resistant strains and strongly recommend vaccination for all patients older than 6 months, since timely vaccination is the cornerstone of influenza prevention. Although many have questioned the efficacy of influenza vaccination, a recent meta-analysis showed a 59% pooled efficacy of the trivalent inactivated vaccine in adults age 18 to 65 years in preventing virologically confirmed influenza, and 83% pooled efficacy of the live-attenuated influenza vaccine in children age 6 months to 7 years.68 Novel approaches for vaccination reminders, such as text messaging69 in addition to traditional mail or telephone reminders, can improve vaccination compliance in today’s highly mobile world that is more than ever connected.
With the lessons learned from four pandemics in the last century, updated recommendations for prevention, and adequate vaccine supply, we should be ready to face the challenge of another flu season.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–1062.
- Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol 2004; 2:909–914.
- Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012; 18:834–837.
- Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012; 422:151–160.
- Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/flu/swineflu/h3n2v-outbreak.htm. Accessed September 27, 2012.
- Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count — United States, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:619–621.
- Centers for Disease Control and Prevention (CDC). Update: Influenza A (H3N2)v transmission and guidelines — five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–1744.
- Centers for Disease Control and Prevention (CDC). Interim information for clinicians about human infections with H3N2v virus. http://www.cdc.gov/flu/swineflu/h3n2v-clinician.htm. Accessed September 27, 2012.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus; Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273.
- World Health Organization (WHO). http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed September 27, 2012.
- Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352:333–340.
- Yamada S, Suzuki Y, Suzuki T, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006; 444:378–382.
- Hatta M, Hatta Y, Kim JH, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 2007; 3:1374–1379.
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207.
- Cheung CY, Chan EY, Krasnoselsky A, et al. H5N1 virus causes significant perturbations in host proteome very early in influenza virus-infected primary human monocyte-derived macrophages. J Infect Dis 2012; 206:640–645.
- Gordon S. Avian influenza: a wake-up call from birds to humans. Cleve Clin J Med 2004; 71:273–274.
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129–1234.
- Ehrlich HJ, Müller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005; 353:2667–2672.
- Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005; 437:1108.
- Ison MG, Lee N. Influenza 2010–2011: lessons from the 2009 pandemic. Cleve Clin J Med 2010; 77:812–820.
- World Health Organization (WHO). Pandemic (H1N1) 2009 — update 112. http://www.who.int/csr/don/2010_08_06/en/index.html. Accessed September 27, 2012.
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12:687–695.
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA 2006; 295:891–894.
- Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther 2012; 17:159–173.
- Graitcer SB, Gubareva L, Kamimoto L, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis 2011; 17:255–257.
- Hurt AC, Deng YM, Ernest J, et al. Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010. Euro Surveill 2011; 16:19770.
- Meijer A, Jonges M, Abbink F, et al. Oseltamivir-resistant pandemic A(H1N1) 2009 influenza viruses detected through enhanced surveillance in the Netherlands, 2009–2010. Antiviral Res 2011; 92:81–89.
- Gubareva LV, Kaiser L, Hayden FG. IInfluenza virus neuraminidase inhibitors. Lancet 2000; 355:827–835.
- Wolfe C, Greenwald I, Chen L. Pandemic (H1N1) 2009 and oseltamivir resistance in hematology/oncology patients. Emerg Infect Dis 2010; 16:1809–1811.
- Moore C, Galiano M, Lackenby A, et al. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 2011; 203:18–24.
- Chen LF, Dailey NJ, Rao AK, et al. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients — North Carolina, 2009. J Infect Dis 2011; 203:838–846.
- Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis — North Carolina, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:969–972.
- Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P; Vietnam H1N1 Investigation Team. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362:86–87.
- Lackenby A, Moran Gilad J, Pebody R, et al. Continued emergence and changing epidemiology of oseltamivir-resistant influenza A(H1N1)2009 virus, United Kingdom, winter 2010/11. Euro Surveill 2011; 16:19784.
- World Health Organization (WHO). Summary of influenza antiviral susceptibility surveillance findings, September 2010 – March 2011. http://www.who.int/influenza/gisrs_laboratory/updates/antiviral_susceptibility/en/index.html. Accessed September 27, 2012.
- Hurt AC, Hardie K, Wilson NJ, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza. N Engl J Med 2011; 365:2541–2542.
- Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–157.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. MMWR Morb Mortal Wkly Rep 2012; 61:613–618.
- Food and Drug Administration (FDA). Summary minutes: vaccines and related biological products advisory committee. February 28–29, 2012. Silver Spring, MD. http://www.fda.gov/downloads/Advisory-Committees/CommitteesMeetingMaterials/BloodVaccinesandOther-Biologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM296193.pdf. Accessed September 28, 2012.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Centers for Disease Control and Prevention (CDC). Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep 2010; 59:989–992.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128–1132.
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices: Update on influenza vaccine safety monitoring. June 20–21, 2012. Atlanta, GA. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed September 28, 2012.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad Med J 1973; 49:152–158.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis 1972; 125:656–664.
- Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine 1993; 11:3–9.
- Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–7663.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food and Drug Administration. Vaccines, Blood & Biologics. December 23,2009 approval letter—Fluzone high-dose. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm195481.htm. Accessed October 1, 2012.
- Moro PL, Arana J, Cano M, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54:1608–1614.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among health-care personnel — United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1073–1077.
- Meijer A, Lackenby A, Hungnes O, et al; European Influenza Surveillance Scheme. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis 2009; 15:552–560.
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360:953–956.
- World Health Organization (WHO). Influenza A virus resistance to oseltamivir. http://www.who.int/influenza/patient_care/antivirals/oseltamivir_summary/en/. Accessed September 28, 2012.
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175.
- Deyde VM, Gubareva LV. Influenza genome analysis using pyro-sequencing method: current applications for a moving target. Expert Rev Mol Diagn 2009; 9:493–509.
- Schuchat A, Katz JM. Protecting adults from influenza: tis the season to learn from the pandemic. J Infect Dis 2012; 206:803–805.
- Centers for Disease Control and Prevention (CDC). Final state-level influenza vaccination coverage estimates for the 2010–11 season — United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. http://www.cdc.gov/flu/professionals/vaccination/coverage_1011estimates.htm. Accessed September 28, 2012.
- Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J 2011; 30:100–106.
- Lee BY, Brown ST, Bailey RR, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Aff (Millwood) 2011; 30:1141–1150.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44.
- Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012; 307:1702–1708.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–1062.
- Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol 2004; 2:909–914.
- Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012; 18:834–837.
- Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012; 422:151–160.
- Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/flu/swineflu/h3n2v-outbreak.htm. Accessed September 27, 2012.
- Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count — United States, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:619–621.
- Centers for Disease Control and Prevention (CDC). Update: Influenza A (H3N2)v transmission and guidelines — five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–1744.
- Centers for Disease Control and Prevention (CDC). Interim information for clinicians about human infections with H3N2v virus. http://www.cdc.gov/flu/swineflu/h3n2v-clinician.htm. Accessed September 27, 2012.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus; Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273.
- World Health Organization (WHO). http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed September 27, 2012.
- Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352:333–340.
- Yamada S, Suzuki Y, Suzuki T, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006; 444:378–382.
- Hatta M, Hatta Y, Kim JH, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 2007; 3:1374–1379.
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207.
- Cheung CY, Chan EY, Krasnoselsky A, et al. H5N1 virus causes significant perturbations in host proteome very early in influenza virus-infected primary human monocyte-derived macrophages. J Infect Dis 2012; 206:640–645.
- Gordon S. Avian influenza: a wake-up call from birds to humans. Cleve Clin J Med 2004; 71:273–274.
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129–1234.
- Ehrlich HJ, Müller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005; 353:2667–2672.
- Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005; 437:1108.
- Ison MG, Lee N. Influenza 2010–2011: lessons from the 2009 pandemic. Cleve Clin J Med 2010; 77:812–820.
- World Health Organization (WHO). Pandemic (H1N1) 2009 — update 112. http://www.who.int/csr/don/2010_08_06/en/index.html. Accessed September 27, 2012.
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12:687–695.
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA 2006; 295:891–894.
- Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther 2012; 17:159–173.
- Graitcer SB, Gubareva L, Kamimoto L, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis 2011; 17:255–257.
- Hurt AC, Deng YM, Ernest J, et al. Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010. Euro Surveill 2011; 16:19770.
- Meijer A, Jonges M, Abbink F, et al. Oseltamivir-resistant pandemic A(H1N1) 2009 influenza viruses detected through enhanced surveillance in the Netherlands, 2009–2010. Antiviral Res 2011; 92:81–89.
- Gubareva LV, Kaiser L, Hayden FG. IInfluenza virus neuraminidase inhibitors. Lancet 2000; 355:827–835.
- Wolfe C, Greenwald I, Chen L. Pandemic (H1N1) 2009 and oseltamivir resistance in hematology/oncology patients. Emerg Infect Dis 2010; 16:1809–1811.
- Moore C, Galiano M, Lackenby A, et al. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 2011; 203:18–24.
- Chen LF, Dailey NJ, Rao AK, et al. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients — North Carolina, 2009. J Infect Dis 2011; 203:838–846.
- Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis — North Carolina, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:969–972.
- Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P; Vietnam H1N1 Investigation Team. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362:86–87.
- Lackenby A, Moran Gilad J, Pebody R, et al. Continued emergence and changing epidemiology of oseltamivir-resistant influenza A(H1N1)2009 virus, United Kingdom, winter 2010/11. Euro Surveill 2011; 16:19784.
- World Health Organization (WHO). Summary of influenza antiviral susceptibility surveillance findings, September 2010 – March 2011. http://www.who.int/influenza/gisrs_laboratory/updates/antiviral_susceptibility/en/index.html. Accessed September 27, 2012.
- Hurt AC, Hardie K, Wilson NJ, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza. N Engl J Med 2011; 365:2541–2542.
- Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–157.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. MMWR Morb Mortal Wkly Rep 2012; 61:613–618.
- Food and Drug Administration (FDA). Summary minutes: vaccines and related biological products advisory committee. February 28–29, 2012. Silver Spring, MD. http://www.fda.gov/downloads/Advisory-Committees/CommitteesMeetingMaterials/BloodVaccinesandOther-Biologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM296193.pdf. Accessed September 28, 2012.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Centers for Disease Control and Prevention (CDC). Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep 2010; 59:989–992.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128–1132.
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices: Update on influenza vaccine safety monitoring. June 20–21, 2012. Atlanta, GA. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed September 28, 2012.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad Med J 1973; 49:152–158.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis 1972; 125:656–664.
- Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine 1993; 11:3–9.
- Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–7663.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food and Drug Administration. Vaccines, Blood & Biologics. December 23,2009 approval letter—Fluzone high-dose. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm195481.htm. Accessed October 1, 2012.
- Moro PL, Arana J, Cano M, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54:1608–1614.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among health-care personnel — United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1073–1077.
- Meijer A, Lackenby A, Hungnes O, et al; European Influenza Surveillance Scheme. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis 2009; 15:552–560.
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360:953–956.
- World Health Organization (WHO). Influenza A virus resistance to oseltamivir. http://www.who.int/influenza/patient_care/antivirals/oseltamivir_summary/en/. Accessed September 28, 2012.
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175.
- Deyde VM, Gubareva LV. Influenza genome analysis using pyro-sequencing method: current applications for a moving target. Expert Rev Mol Diagn 2009; 9:493–509.
- Schuchat A, Katz JM. Protecting adults from influenza: tis the season to learn from the pandemic. J Infect Dis 2012; 206:803–805.
- Centers for Disease Control and Prevention (CDC). Final state-level influenza vaccination coverage estimates for the 2010–11 season — United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. http://www.cdc.gov/flu/professionals/vaccination/coverage_1011estimates.htm. Accessed September 28, 2012.
- Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J 2011; 30:100–106.
- Lee BY, Brown ST, Bailey RR, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Aff (Millwood) 2011; 30:1141–1150.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44.
- Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012; 307:1702–1708.
KEY POINTS
- A recent outbreak of swine flu in children exposed to pigs at agricultural fairs is unprecedented. Seasonal influenza vaccine does not protect against this strain, designated H3N2v. The neuraminidase inhibitors oseltamivir (Tamiflu) and zanamivir (Relenza) are the drugs of choice for treatment.
- A highly lethal bird flu, designated H5N1, is still a pandemic threat. In the event of an outbreak, an inactivated whole-virus vaccine is available.
- A community outbreak of oseltamivir-resistant H1N1 in Australia sounded an alarm for a potential drug-resistant flu epidemic. Inhaled zanamivir would be the only effective therapy available in the event of such an epidemic.
- An emerging new antiviral drug is effective against oseltamivir-resistant influenza.
The resurgence of swine-origin influenza A (H1N1)
Editor's note: This paper was posted online prior to publication in print. To expedite publication, the paper was peer-reviewed by a CCJM physician editor.
The unexpected and well-publicized appearance of swine-origin influenza A (H1N1) virus (S-OIV, informally known as swine flu) has both physicians and the general public on edge. The health care system is mobilizing while the world watches to see if S-OIV will become a pandemic or will just fade away, like the swine flu outbreak of 1976.
In this update, written in mid-May 2009, I try to provide an overview of our current understanding of S-OIV, its diagnosis, treatment, and prevention, knowing that the information about the outbreak is being updated almost daily. To stay abreast of the latest developments, physicians should also consult Web sites of the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).
IS IT REALLY ‘SWINE’?
An unexpected surge in influenza A cases toward the end of the 2008–2009 influenza season occurring in and around Mexico City alerted health authorities to a type of influenza virus infection that does not commonly affect humans.
In most years, the annual influenza epidemics in the Northern Hemisphere wane by the end of April. S-OIV infection first appeared in Mexico in April 2009 and shortly after in California and Texas.
In the first few days, the specific viral genetic origin of the epidemic was unclear. But genetic analysis of the virus isolated from a patient in California found that this virus was a recent reassortant of previous triple-reassortants of viruses from pigs, humans, and birds, called triple-reassortant swine influenza A (H1) viruses, which have been circulating in pigs for about a decade, and a Eurasian swine influenza virus.1
Through the years, only a few influenza viruses have been successfully transmitted from birds to humans and then to swine.2 It is interesting that exposure to pigs is not a risk factor for infection with the current S-OIV, unlike in prior cases of swine influenza reported in the literature.3,4 Total reported cases of swine influenza in humans numbered only 50 from 1958 to 2005 and 11 from December 2005 through February 2009, but more cases must have occurred that were not readily identified.
The Veterinary Services of Canada announced on May 2, 2009, that a pig farm in Alberta had been infected with the current type of S-OIV. The infection was introduced to the farm by a carpenter who developed symptoms of influenza after a short stay in Mexico. It is reassuring to learn that, so far, the S-OIV causing illness in these pigs has not been transmitted to people living on that farm. The failure of the S-OIV to transmit back to people suggests that it did not come into the human population directly from swine.
AN EPIDEMIC IN MOTION
As of this writing, 2,532 cases of S-OIV have been confirmed in the United States by the CDC in 44 states, and 3 people have died, for a case-fatality rate of 0.11%. Simultaneously, the WHO reported 4,694 confirmed cases in 30 countries, with 53 deaths (a case-fatality rate of 1.1%), and with 48 of the deaths outside the United States occurring in Mexico.
It is unclear which direction this epidemic will take over the next several months. What happens in the annual influenza season in the Southern Hemisphere, which is just starting, and the early features of influenza activity in the Northern Hemisphere starting in September 2009 will indicate how this epidemic will materialize and the prospects of it’s progressing to an influenza pandemic.
While most adults today have some immunity against previously circulating H1 variants, it is not known if cross-reacting antibodies would provide any protection against the current S-OIV. An animal model showed that mice immunized against the neuraminidase of a human influenza A (H1N1) virus were partially protected from lethal challenge with H5N1 virus.5 In that same study, some humans also had serum antibodies that can inhibit sialidase activity of avian H5N1 viruses.
A remnant of the 1918 pandemic?
The two mechanisms by which pandemic influenza occurred in the 20th century were direct transmission of a novel virus and reassortment of avian and human viruses. In the 1918 pandemic, an influenza A (H1N1) virus closely related to avian viruses adapted to replicate efficiently in humans. Reassortment of an avian influenza A (H2N2) virus and a human influenza A (H1N1) virus resulted in the 1957 pandemic, and reassortment of an avian influenza A H3 virus and a human influenza A (H2N2) virus resulted in the 1968 pandemic.6 One could thus consider the current S-OIV epidemic as genetically a remnant or continuation of the 1918 pandemic, but so far it is less deadly.1
What should we be looking for?
Several characteristic features were seen in prior pandemics that we should be looking for in the next few months to better understand the pandemic potential of the current S-OIV epidemic.7
While the severity of prior pandemics varied significantly, they were all heralded by an antigenic shift in viral subtype. Young adults and previously healthy people were disproportionately affected and had a higher-than-expected death rate. This may be explained by partial protection in older people due to antigen recycling. Secondary bacterial pneumonia is believed to have been a significant cause of death in the 1918 pandemic,8 and bacterial pharyngeal carriage rates are higher in younger people.
Pandemic waves smoldered, lasting 2 to 5 years, but the pattern of deaths varied significantly in different parts of the world. For example, in 1968, most deaths in North America occurred during the first pandemic season, whereas most deaths in Europe and Asia occurred during the second pandemic season.9 This may be explained by geographic variation in preexisting immunity, intrapandemic antigenic drift, viral adaptation, demographic differences, or seasonality.
Of importance, influenza viruses that caused prior pandemics were highly transmissible between humans.
CLINICAL FEATURES OF THE CURRENT OUTBREAK
The current S-OIV epidemic in the United States is affecting mainly younger people: 60% of people affected have been 18 years of age or younger.10,11 It is unclear if this is due to transmission patterns or to possible immunity in older patients. Efficient human-to-human transmission within the United States is occurring, since only 18% of patients had recently traveled to Mexico. School outbreaks accounted for 16% of cases so far.
Patients have symptoms similar to those of seasonal influenza, with few exceptions. The most frequently reported symptoms are cough, fever, fatigue, headache, sore throat, runny nose, chills, and muscle aches, all occurring in 80% or more of patients. Almost all patients fit the CDC definition for influenza-like illness, consisting of subjective fever plus cough or sore throat.
Nausea, abdominal pain, and diarrhea, which are not common symptoms of seasonal influenza, have been reported in approximately 50% of patients with S-OIV. The spectrum of illness ranges from self-limited to severe, with 2% of patients developing pneumonia and 9% requiring hospitalization.
Continued analysis of the case-fatality rate highlights that people ages 20 to 29 are disproportionately represented among the fatalities.
A PCR test has been developed
Since clinical findings identify patients with influenza-like illness but cannot confirm or exclude the diagnosis of influenza,12 a specific diagnostic real-time reverse-transcriptase polymerase chain reaction (RT-PCR) test has been developed, and the CDC is currently distributing it to state health departments.
An interim case definition
An interim case definition for the purpose of epidemiologic investigation of cases of S-OIV infection includes acute fever (temperature ≥ 100°F, 37.8°C) and acute respiratory illness (rhinorrhea, sore throat, or cough), plus:
- For a confirmed case, S-OIV infection confirmed by RT-PCR or viral culture
- For a probable case, laboratory-confirmed influenza A, but negative for H1 and H3 by RT-PCR
- For a suspected case, onset of above illness within 7 days of close contact with a confirmed case of S-OIV infection; or travel within 7 days to a community within the United States or internationally where there are one or more confirmed cases of S-OIV infection; or residing in such a community.
In practice, is it seasonal flu or swine flu?
In clinical practice in United States, in the springtime, a person with influenza-like illness and microbiologically confirmed seasonal influenza B obviously would not raise any concern about the ongoing S-OIV epidemic. Sporadic cases of seasonal influenza A are still occurring, but these are the ones that create a diagnostic dilemma, since very few laboratories currently have the ability to differentiate between influenza A H1 and H3. Since S-OIV has been reported in almost all states in the United States, one can argue that most cases of influenza A currently being identified should be considered suspected S-OIV.
PREVENTIVE MEASURES
In response to this ongoing outbreak, the WHO raised its epidemic alert level from 4 to 5, one level shy of declaring a pandemic. Several measures have been implemented in an attempt to halt this outbreak, the most important of which is the rapid dissemination of information to health professionals,13 with the Internet playing a central role.14
The world is better prepared for a pandemic now than at any time in history. Seed virus for vaccine development has been provided to various governments and pharmaceutical manufacturers. Stockpiles of antiviral agents are being mobilized and distributed to various locations, and dispensing plans are being reviewed for potential execution. The US Food and Drug Administration (FDA) issued emergency-use authorizations for mass deployment of the strategic stockpile of oseltamivir (Tamiflu), including for children younger than 1 year, and of zanamivir (Relenza) for the treatment and prophylaxis of S-OIV infection. It also authorized the use of disposable N95 respiratory masks by the general public, as well as the RT-PCR diagnostic test.
General advice for healthy people in the community
- Maintain a distance of at least 1 meter from a person with influenza-like illness.
- Wear a mask while providing care for a person with influenza-like illness.
- Avoid touching your eyes, nose, or mouth, since these are potential portals of entry for the virus. This may be a difficult recommendation to follow, since it requires constant vigilance of a common human behavior.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with respiratory secretions from a person, including your child, with influenza-like illness.
- If possible, reduce the time spent in close contact with people with influenza-like illness and in crowded settings.
- If possible, open windows in your living space to improve airflow.
While the CDC has recommended avoiding nonessential travel to Mexico at the current time, the WHO is not recommending any travel restrictions, since the outbreak has already spread to many parts of the world and all continents.
There is no limitation on handling or consuming pork meat or other well-processed swine products.
Recommendations for school dismissal and social-distancing interventions are evolving. During the 1918 pandemic, nonpharmaceutical interventions were associated with a significant reduction in deaths,15 but it is unclear how much additional benefit these measures would add to effective immunization, antiviral treatment for patients, and chemoprophylaxis for their contacts.
General advice for people with influenza-like illness
- Stay home for 7 days after the onset of symptoms or 48 hours after symptoms resolve, whichever is longer.
- Maintain a distance of at least 1 meter from all people.
- Cover your mouth and nose with tissues when coughing or sneezing, and dispose of the tissues immediately after use.
- Avoid touching your eyes, nose, and mouth.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with your respiratory secretions during coughing or sneezing. Adding virucidal agents or antiseptics to hand-washing is not likely to have an incremental effect.16
- If possible, open windows in your living space to improve airflow.
- If possible, when you are in close contact with other people, wear a mask to help contain your respiratory secretions.
Masks
The designs and standards of masks vary from country to country. Masks have been shown to reduce the transmission of influenza in health care settings,16 but the benefit in the community has not been established. Advice on proper use of a mask:
- Cover your mouth and nose with the mask and tie it securely to minimize gaps.
- Avoid touching the mask while it is on your face.
- Wash your hands with soap and water or an alcohol-based hand rub for 20 to 30 seconds after removing the mask.
- If the mask becomes damp, replace it with a new one.
- Avoid reusing single-use masks, and dispose of them immediately after removing.
VACCINE DEVELOPMENT
The most difficult question about vaccine development for S-OIV at this time is whether to prepare it as a separate product or try to incorporate it in the seasonal influenza vaccine.
The problem is that the seasonal influenza vaccine for the Southern Hemisphere has already been made and distributed, and vaccination programs are already well under way. Although flu season in the Northern Hemisphere is not expected before September or October 2009, vaccine production and distribution take several months, leaving little time to observe which direction the S-OIV epidemic will take before making this decision.
Vaccine distribution also raises difficult questions, since a limited amount will be available initially and rationing to the most vulnerable people will be necessary. While health care workers are more likely to be exposed to people infected with S-OIV compared with the general population, mandating their immunization may pose other moral dilemmas.17
The current global capacity for production of seasonal influenza vaccine is approximately 400 million doses.18 Since the process of vaccine production takes at least 4 to 6 months, measures have been proposed to speed up the production of pandemic vaccine or immunogenicity; these include recombinant technology, reverse genetics, and the use of adjuvants. In April 2007, the FDA approved the first H5 subviron vaccine for people ages 19 to 64.
This topic brings back memories of the 1976 swine influenza immunization program, in which the rate of Guillain-Barré syndrome was 5 to 10 times the background rate, resulting in a halt in vaccine production.
Why this syndrome occurred is not known, but it is suspected to be due to cross-reacting antibodies against peripheral-nerve antigen that developed after the vaccine was given. Data since then have shown no association between vaccination and Guillain-Barré syndrome. 19 On the other hand, influenza viruses were found to trigger Guillain-Barré syndrome only infrequently, except during major outbreaks, in which they may play a significant role.20
TREATMENT
Antiviral drugs
Tests of current S-OIV isolates showed them to be susceptible to the neuraminidase inhibitors, ie, oseltamivir and zanamivir, but resistant to the adamantanes, ie, amantadine (Symmetrel) and rimantadine (Flumadine).21 All isolates contained the S31N mutation in the M2 protein, which confers resistance against the adamantanes and which has been detected in most influenza A (H3N2) isolates in the United States since 2006. Fortunately, the H274Y mutation in N1—which confers resistance to oseltamivir but not to zanamivir and which has been detected in almost all seasonal influenza A (H1N1) isolates since the early weeks of the current influenza season— has not been detected in any of the current S-OIV isolates.
Patients who are otherwise healthy who present with an uncomplicated febrile illness due to S-OIV do not require antiviral treatment. Either oseltamivir or zanamivir is recommended for treatment of patients hospitalized for management of confirmed, probable, or suspected infection with S-OIV, or for those at high risk of influenza-related complications, defined similarly to seasonal influenza (Table 1).
The duration of shedding of S-OIV is unknown, but starting an antiviral agent early in the course of illness is expected to reduce contagiousness. Extrapolating from data in seasonal influenza, infected persons are assumed to be shedding virus from 1 day prior to illness onset until resolution of symptoms, usually 7 days, and up to 10 days in younger children.
Oseltamivir accounts for the lion’s share of the stockpile of antiviral drugs against pandemic influenza. However, with mass utilization, antiviral resistance to a single agent may develop. A mathematical model showed that adding a smaller stockpile of a second agent, such as zanamivir, to be used either in combination with or sequential to oseltamivir, can effectively prevent or at least delay the development of resistance.22
Other potential measures for management
Since secondary bacterial pneumonia is expected to play a significant role in influenza-related death during the next pandemic, stockpiling antibacterial agents may also be prudent.8 The death rate in methicillin-resistant Staphylococcus aureus pneumonia secondary to seasonal influenza is 50%, further complicating the choice of stockpiling for antibacterial agents.
A meta-analysis of 11 studies involving 1,703 patients during the 1918 pandemic showed that those who received influenza-convalescent human blood products were less likely to die than those who did not.23 Anti-influenza drugs and advanced techniques to care for critically ill patients were not available at that time, so extrapolating these data to the current era may not be appropriate.
The cost of vaccine and antiviral drugs is an expected limitation to mass implementation during a pandemic, particularly in developing countries. Certain inexpensive generic drugs that have been shown to have some activity against influenza, such statins, fibrates, and chloroquine, deserve further attention.24
PUTING THE CURRENT EPIDEMIC IN PERSPECTIVE
In summary, the world is now better prepared, vaccine is in development, and antiviral treatment is available. For more information, readers are directed to go to www.cdc.gov/h1n1flu/ or www.who.int/csr/don/2009_05_11/en/index.html.
- Belshe RB. Implications of the emergence of a novel H1 influenza virus. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903995.
- Ducatez MF, Webster RG, Webby RJ. Animal influenza epidemiology. Vaccine 2008; 26(suppl 4):D67–D69.
- Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis 2007; 44:1084–1088.
- Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903812.
- Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med 2007; 4( 2):e59. doi:10.1371/journal. pmed.0040059.
- Belshe RB. The origins of pandemic influenza—lessons from the 1918 virus. N Engl J Med 2005; 353:2209–2211.
- Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics—implications for policy. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0903906.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Viboud C, Grais RF, Lafont BAP, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis 2005; 192:233–248.
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903810.
- US Centers for Disease Control and PreventionC. Swine-origin influenza A (H1N1) virus infections in a school—New York City, April 2009. MMWR 2009; 58(Dispatch):1–3.
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005: 293:987–997.
- Baden LR, Drazen JM, Kritek PA, Curfman GD, Morrissey S, Campion EW. H1N1 influenza A disease—information for health professionals. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903992.
- Brownstein JS, Freifeld CC, Madoff LC. Influenza A (H1N1) virus, 2009—online monitoring. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0904012.
- Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic. JAMA 2007; 298:644–654.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336( 7635):77–80.
- Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med 2009; 360:1981–1988.
- Sahni R, Mossad SB. Controlling pandemic influenza through vaccination programs. Future Virol 2009; 4:271–276.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- US Centers for Disease Control and Prevention. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR May 1, 2009; 58( 16):433–435.
- Wu JT, Leung GM, Lipsitch M, Cooper BS, Riley S. Hedging against antiviral resistance during the next influenza pandemic using small stockpiles of an alternative chemotherapy. PLoS Med 2009;e1000085. doi:10.1371/journal.pmed.1000085.
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006; 145:599–609.
- Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis 2008; 8:571–576.
Editor's note: This paper was posted online prior to publication in print. To expedite publication, the paper was peer-reviewed by a CCJM physician editor.
The unexpected and well-publicized appearance of swine-origin influenza A (H1N1) virus (S-OIV, informally known as swine flu) has both physicians and the general public on edge. The health care system is mobilizing while the world watches to see if S-OIV will become a pandemic or will just fade away, like the swine flu outbreak of 1976.
In this update, written in mid-May 2009, I try to provide an overview of our current understanding of S-OIV, its diagnosis, treatment, and prevention, knowing that the information about the outbreak is being updated almost daily. To stay abreast of the latest developments, physicians should also consult Web sites of the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).
IS IT REALLY ‘SWINE’?
An unexpected surge in influenza A cases toward the end of the 2008–2009 influenza season occurring in and around Mexico City alerted health authorities to a type of influenza virus infection that does not commonly affect humans.
In most years, the annual influenza epidemics in the Northern Hemisphere wane by the end of April. S-OIV infection first appeared in Mexico in April 2009 and shortly after in California and Texas.
In the first few days, the specific viral genetic origin of the epidemic was unclear. But genetic analysis of the virus isolated from a patient in California found that this virus was a recent reassortant of previous triple-reassortants of viruses from pigs, humans, and birds, called triple-reassortant swine influenza A (H1) viruses, which have been circulating in pigs for about a decade, and a Eurasian swine influenza virus.1
Through the years, only a few influenza viruses have been successfully transmitted from birds to humans and then to swine.2 It is interesting that exposure to pigs is not a risk factor for infection with the current S-OIV, unlike in prior cases of swine influenza reported in the literature.3,4 Total reported cases of swine influenza in humans numbered only 50 from 1958 to 2005 and 11 from December 2005 through February 2009, but more cases must have occurred that were not readily identified.
The Veterinary Services of Canada announced on May 2, 2009, that a pig farm in Alberta had been infected with the current type of S-OIV. The infection was introduced to the farm by a carpenter who developed symptoms of influenza after a short stay in Mexico. It is reassuring to learn that, so far, the S-OIV causing illness in these pigs has not been transmitted to people living on that farm. The failure of the S-OIV to transmit back to people suggests that it did not come into the human population directly from swine.
AN EPIDEMIC IN MOTION
As of this writing, 2,532 cases of S-OIV have been confirmed in the United States by the CDC in 44 states, and 3 people have died, for a case-fatality rate of 0.11%. Simultaneously, the WHO reported 4,694 confirmed cases in 30 countries, with 53 deaths (a case-fatality rate of 1.1%), and with 48 of the deaths outside the United States occurring in Mexico.
It is unclear which direction this epidemic will take over the next several months. What happens in the annual influenza season in the Southern Hemisphere, which is just starting, and the early features of influenza activity in the Northern Hemisphere starting in September 2009 will indicate how this epidemic will materialize and the prospects of it’s progressing to an influenza pandemic.
While most adults today have some immunity against previously circulating H1 variants, it is not known if cross-reacting antibodies would provide any protection against the current S-OIV. An animal model showed that mice immunized against the neuraminidase of a human influenza A (H1N1) virus were partially protected from lethal challenge with H5N1 virus.5 In that same study, some humans also had serum antibodies that can inhibit sialidase activity of avian H5N1 viruses.
A remnant of the 1918 pandemic?
The two mechanisms by which pandemic influenza occurred in the 20th century were direct transmission of a novel virus and reassortment of avian and human viruses. In the 1918 pandemic, an influenza A (H1N1) virus closely related to avian viruses adapted to replicate efficiently in humans. Reassortment of an avian influenza A (H2N2) virus and a human influenza A (H1N1) virus resulted in the 1957 pandemic, and reassortment of an avian influenza A H3 virus and a human influenza A (H2N2) virus resulted in the 1968 pandemic.6 One could thus consider the current S-OIV epidemic as genetically a remnant or continuation of the 1918 pandemic, but so far it is less deadly.1
What should we be looking for?
Several characteristic features were seen in prior pandemics that we should be looking for in the next few months to better understand the pandemic potential of the current S-OIV epidemic.7
While the severity of prior pandemics varied significantly, they were all heralded by an antigenic shift in viral subtype. Young adults and previously healthy people were disproportionately affected and had a higher-than-expected death rate. This may be explained by partial protection in older people due to antigen recycling. Secondary bacterial pneumonia is believed to have been a significant cause of death in the 1918 pandemic,8 and bacterial pharyngeal carriage rates are higher in younger people.
Pandemic waves smoldered, lasting 2 to 5 years, but the pattern of deaths varied significantly in different parts of the world. For example, in 1968, most deaths in North America occurred during the first pandemic season, whereas most deaths in Europe and Asia occurred during the second pandemic season.9 This may be explained by geographic variation in preexisting immunity, intrapandemic antigenic drift, viral adaptation, demographic differences, or seasonality.
Of importance, influenza viruses that caused prior pandemics were highly transmissible between humans.
CLINICAL FEATURES OF THE CURRENT OUTBREAK
The current S-OIV epidemic in the United States is affecting mainly younger people: 60% of people affected have been 18 years of age or younger.10,11 It is unclear if this is due to transmission patterns or to possible immunity in older patients. Efficient human-to-human transmission within the United States is occurring, since only 18% of patients had recently traveled to Mexico. School outbreaks accounted for 16% of cases so far.
Patients have symptoms similar to those of seasonal influenza, with few exceptions. The most frequently reported symptoms are cough, fever, fatigue, headache, sore throat, runny nose, chills, and muscle aches, all occurring in 80% or more of patients. Almost all patients fit the CDC definition for influenza-like illness, consisting of subjective fever plus cough or sore throat.
Nausea, abdominal pain, and diarrhea, which are not common symptoms of seasonal influenza, have been reported in approximately 50% of patients with S-OIV. The spectrum of illness ranges from self-limited to severe, with 2% of patients developing pneumonia and 9% requiring hospitalization.
Continued analysis of the case-fatality rate highlights that people ages 20 to 29 are disproportionately represented among the fatalities.
A PCR test has been developed
Since clinical findings identify patients with influenza-like illness but cannot confirm or exclude the diagnosis of influenza,12 a specific diagnostic real-time reverse-transcriptase polymerase chain reaction (RT-PCR) test has been developed, and the CDC is currently distributing it to state health departments.
An interim case definition
An interim case definition for the purpose of epidemiologic investigation of cases of S-OIV infection includes acute fever (temperature ≥ 100°F, 37.8°C) and acute respiratory illness (rhinorrhea, sore throat, or cough), plus:
- For a confirmed case, S-OIV infection confirmed by RT-PCR or viral culture
- For a probable case, laboratory-confirmed influenza A, but negative for H1 and H3 by RT-PCR
- For a suspected case, onset of above illness within 7 days of close contact with a confirmed case of S-OIV infection; or travel within 7 days to a community within the United States or internationally where there are one or more confirmed cases of S-OIV infection; or residing in such a community.
In practice, is it seasonal flu or swine flu?
In clinical practice in United States, in the springtime, a person with influenza-like illness and microbiologically confirmed seasonal influenza B obviously would not raise any concern about the ongoing S-OIV epidemic. Sporadic cases of seasonal influenza A are still occurring, but these are the ones that create a diagnostic dilemma, since very few laboratories currently have the ability to differentiate between influenza A H1 and H3. Since S-OIV has been reported in almost all states in the United States, one can argue that most cases of influenza A currently being identified should be considered suspected S-OIV.
PREVENTIVE MEASURES
In response to this ongoing outbreak, the WHO raised its epidemic alert level from 4 to 5, one level shy of declaring a pandemic. Several measures have been implemented in an attempt to halt this outbreak, the most important of which is the rapid dissemination of information to health professionals,13 with the Internet playing a central role.14
The world is better prepared for a pandemic now than at any time in history. Seed virus for vaccine development has been provided to various governments and pharmaceutical manufacturers. Stockpiles of antiviral agents are being mobilized and distributed to various locations, and dispensing plans are being reviewed for potential execution. The US Food and Drug Administration (FDA) issued emergency-use authorizations for mass deployment of the strategic stockpile of oseltamivir (Tamiflu), including for children younger than 1 year, and of zanamivir (Relenza) for the treatment and prophylaxis of S-OIV infection. It also authorized the use of disposable N95 respiratory masks by the general public, as well as the RT-PCR diagnostic test.
General advice for healthy people in the community
- Maintain a distance of at least 1 meter from a person with influenza-like illness.
- Wear a mask while providing care for a person with influenza-like illness.
- Avoid touching your eyes, nose, or mouth, since these are potential portals of entry for the virus. This may be a difficult recommendation to follow, since it requires constant vigilance of a common human behavior.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with respiratory secretions from a person, including your child, with influenza-like illness.
- If possible, reduce the time spent in close contact with people with influenza-like illness and in crowded settings.
- If possible, open windows in your living space to improve airflow.
While the CDC has recommended avoiding nonessential travel to Mexico at the current time, the WHO is not recommending any travel restrictions, since the outbreak has already spread to many parts of the world and all continents.
There is no limitation on handling or consuming pork meat or other well-processed swine products.
Recommendations for school dismissal and social-distancing interventions are evolving. During the 1918 pandemic, nonpharmaceutical interventions were associated with a significant reduction in deaths,15 but it is unclear how much additional benefit these measures would add to effective immunization, antiviral treatment for patients, and chemoprophylaxis for their contacts.
General advice for people with influenza-like illness
- Stay home for 7 days after the onset of symptoms or 48 hours after symptoms resolve, whichever is longer.
- Maintain a distance of at least 1 meter from all people.
- Cover your mouth and nose with tissues when coughing or sneezing, and dispose of the tissues immediately after use.
- Avoid touching your eyes, nose, and mouth.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with your respiratory secretions during coughing or sneezing. Adding virucidal agents or antiseptics to hand-washing is not likely to have an incremental effect.16
- If possible, open windows in your living space to improve airflow.
- If possible, when you are in close contact with other people, wear a mask to help contain your respiratory secretions.
Masks
The designs and standards of masks vary from country to country. Masks have been shown to reduce the transmission of influenza in health care settings,16 but the benefit in the community has not been established. Advice on proper use of a mask:
- Cover your mouth and nose with the mask and tie it securely to minimize gaps.
- Avoid touching the mask while it is on your face.
- Wash your hands with soap and water or an alcohol-based hand rub for 20 to 30 seconds after removing the mask.
- If the mask becomes damp, replace it with a new one.
- Avoid reusing single-use masks, and dispose of them immediately after removing.
VACCINE DEVELOPMENT
The most difficult question about vaccine development for S-OIV at this time is whether to prepare it as a separate product or try to incorporate it in the seasonal influenza vaccine.
The problem is that the seasonal influenza vaccine for the Southern Hemisphere has already been made and distributed, and vaccination programs are already well under way. Although flu season in the Northern Hemisphere is not expected before September or October 2009, vaccine production and distribution take several months, leaving little time to observe which direction the S-OIV epidemic will take before making this decision.
Vaccine distribution also raises difficult questions, since a limited amount will be available initially and rationing to the most vulnerable people will be necessary. While health care workers are more likely to be exposed to people infected with S-OIV compared with the general population, mandating their immunization may pose other moral dilemmas.17
The current global capacity for production of seasonal influenza vaccine is approximately 400 million doses.18 Since the process of vaccine production takes at least 4 to 6 months, measures have been proposed to speed up the production of pandemic vaccine or immunogenicity; these include recombinant technology, reverse genetics, and the use of adjuvants. In April 2007, the FDA approved the first H5 subviron vaccine for people ages 19 to 64.
This topic brings back memories of the 1976 swine influenza immunization program, in which the rate of Guillain-Barré syndrome was 5 to 10 times the background rate, resulting in a halt in vaccine production.
Why this syndrome occurred is not known, but it is suspected to be due to cross-reacting antibodies against peripheral-nerve antigen that developed after the vaccine was given. Data since then have shown no association between vaccination and Guillain-Barré syndrome. 19 On the other hand, influenza viruses were found to trigger Guillain-Barré syndrome only infrequently, except during major outbreaks, in which they may play a significant role.20
TREATMENT
Antiviral drugs
Tests of current S-OIV isolates showed them to be susceptible to the neuraminidase inhibitors, ie, oseltamivir and zanamivir, but resistant to the adamantanes, ie, amantadine (Symmetrel) and rimantadine (Flumadine).21 All isolates contained the S31N mutation in the M2 protein, which confers resistance against the adamantanes and which has been detected in most influenza A (H3N2) isolates in the United States since 2006. Fortunately, the H274Y mutation in N1—which confers resistance to oseltamivir but not to zanamivir and which has been detected in almost all seasonal influenza A (H1N1) isolates since the early weeks of the current influenza season— has not been detected in any of the current S-OIV isolates.
Patients who are otherwise healthy who present with an uncomplicated febrile illness due to S-OIV do not require antiviral treatment. Either oseltamivir or zanamivir is recommended for treatment of patients hospitalized for management of confirmed, probable, or suspected infection with S-OIV, or for those at high risk of influenza-related complications, defined similarly to seasonal influenza (Table 1).
The duration of shedding of S-OIV is unknown, but starting an antiviral agent early in the course of illness is expected to reduce contagiousness. Extrapolating from data in seasonal influenza, infected persons are assumed to be shedding virus from 1 day prior to illness onset until resolution of symptoms, usually 7 days, and up to 10 days in younger children.
Oseltamivir accounts for the lion’s share of the stockpile of antiviral drugs against pandemic influenza. However, with mass utilization, antiviral resistance to a single agent may develop. A mathematical model showed that adding a smaller stockpile of a second agent, such as zanamivir, to be used either in combination with or sequential to oseltamivir, can effectively prevent or at least delay the development of resistance.22
Other potential measures for management
Since secondary bacterial pneumonia is expected to play a significant role in influenza-related death during the next pandemic, stockpiling antibacterial agents may also be prudent.8 The death rate in methicillin-resistant Staphylococcus aureus pneumonia secondary to seasonal influenza is 50%, further complicating the choice of stockpiling for antibacterial agents.
A meta-analysis of 11 studies involving 1,703 patients during the 1918 pandemic showed that those who received influenza-convalescent human blood products were less likely to die than those who did not.23 Anti-influenza drugs and advanced techniques to care for critically ill patients were not available at that time, so extrapolating these data to the current era may not be appropriate.
The cost of vaccine and antiviral drugs is an expected limitation to mass implementation during a pandemic, particularly in developing countries. Certain inexpensive generic drugs that have been shown to have some activity against influenza, such statins, fibrates, and chloroquine, deserve further attention.24
PUTING THE CURRENT EPIDEMIC IN PERSPECTIVE
In summary, the world is now better prepared, vaccine is in development, and antiviral treatment is available. For more information, readers are directed to go to www.cdc.gov/h1n1flu/ or www.who.int/csr/don/2009_05_11/en/index.html.
Editor's note: This paper was posted online prior to publication in print. To expedite publication, the paper was peer-reviewed by a CCJM physician editor.
The unexpected and well-publicized appearance of swine-origin influenza A (H1N1) virus (S-OIV, informally known as swine flu) has both physicians and the general public on edge. The health care system is mobilizing while the world watches to see if S-OIV will become a pandemic or will just fade away, like the swine flu outbreak of 1976.
In this update, written in mid-May 2009, I try to provide an overview of our current understanding of S-OIV, its diagnosis, treatment, and prevention, knowing that the information about the outbreak is being updated almost daily. To stay abreast of the latest developments, physicians should also consult Web sites of the US Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).
IS IT REALLY ‘SWINE’?
An unexpected surge in influenza A cases toward the end of the 2008–2009 influenza season occurring in and around Mexico City alerted health authorities to a type of influenza virus infection that does not commonly affect humans.
In most years, the annual influenza epidemics in the Northern Hemisphere wane by the end of April. S-OIV infection first appeared in Mexico in April 2009 and shortly after in California and Texas.
In the first few days, the specific viral genetic origin of the epidemic was unclear. But genetic analysis of the virus isolated from a patient in California found that this virus was a recent reassortant of previous triple-reassortants of viruses from pigs, humans, and birds, called triple-reassortant swine influenza A (H1) viruses, which have been circulating in pigs for about a decade, and a Eurasian swine influenza virus.1
Through the years, only a few influenza viruses have been successfully transmitted from birds to humans and then to swine.2 It is interesting that exposure to pigs is not a risk factor for infection with the current S-OIV, unlike in prior cases of swine influenza reported in the literature.3,4 Total reported cases of swine influenza in humans numbered only 50 from 1958 to 2005 and 11 from December 2005 through February 2009, but more cases must have occurred that were not readily identified.
The Veterinary Services of Canada announced on May 2, 2009, that a pig farm in Alberta had been infected with the current type of S-OIV. The infection was introduced to the farm by a carpenter who developed symptoms of influenza after a short stay in Mexico. It is reassuring to learn that, so far, the S-OIV causing illness in these pigs has not been transmitted to people living on that farm. The failure of the S-OIV to transmit back to people suggests that it did not come into the human population directly from swine.
AN EPIDEMIC IN MOTION
As of this writing, 2,532 cases of S-OIV have been confirmed in the United States by the CDC in 44 states, and 3 people have died, for a case-fatality rate of 0.11%. Simultaneously, the WHO reported 4,694 confirmed cases in 30 countries, with 53 deaths (a case-fatality rate of 1.1%), and with 48 of the deaths outside the United States occurring in Mexico.
It is unclear which direction this epidemic will take over the next several months. What happens in the annual influenza season in the Southern Hemisphere, which is just starting, and the early features of influenza activity in the Northern Hemisphere starting in September 2009 will indicate how this epidemic will materialize and the prospects of it’s progressing to an influenza pandemic.
While most adults today have some immunity against previously circulating H1 variants, it is not known if cross-reacting antibodies would provide any protection against the current S-OIV. An animal model showed that mice immunized against the neuraminidase of a human influenza A (H1N1) virus were partially protected from lethal challenge with H5N1 virus.5 In that same study, some humans also had serum antibodies that can inhibit sialidase activity of avian H5N1 viruses.
A remnant of the 1918 pandemic?
The two mechanisms by which pandemic influenza occurred in the 20th century were direct transmission of a novel virus and reassortment of avian and human viruses. In the 1918 pandemic, an influenza A (H1N1) virus closely related to avian viruses adapted to replicate efficiently in humans. Reassortment of an avian influenza A (H2N2) virus and a human influenza A (H1N1) virus resulted in the 1957 pandemic, and reassortment of an avian influenza A H3 virus and a human influenza A (H2N2) virus resulted in the 1968 pandemic.6 One could thus consider the current S-OIV epidemic as genetically a remnant or continuation of the 1918 pandemic, but so far it is less deadly.1
What should we be looking for?
Several characteristic features were seen in prior pandemics that we should be looking for in the next few months to better understand the pandemic potential of the current S-OIV epidemic.7
While the severity of prior pandemics varied significantly, they were all heralded by an antigenic shift in viral subtype. Young adults and previously healthy people were disproportionately affected and had a higher-than-expected death rate. This may be explained by partial protection in older people due to antigen recycling. Secondary bacterial pneumonia is believed to have been a significant cause of death in the 1918 pandemic,8 and bacterial pharyngeal carriage rates are higher in younger people.
Pandemic waves smoldered, lasting 2 to 5 years, but the pattern of deaths varied significantly in different parts of the world. For example, in 1968, most deaths in North America occurred during the first pandemic season, whereas most deaths in Europe and Asia occurred during the second pandemic season.9 This may be explained by geographic variation in preexisting immunity, intrapandemic antigenic drift, viral adaptation, demographic differences, or seasonality.
Of importance, influenza viruses that caused prior pandemics were highly transmissible between humans.
CLINICAL FEATURES OF THE CURRENT OUTBREAK
The current S-OIV epidemic in the United States is affecting mainly younger people: 60% of people affected have been 18 years of age or younger.10,11 It is unclear if this is due to transmission patterns or to possible immunity in older patients. Efficient human-to-human transmission within the United States is occurring, since only 18% of patients had recently traveled to Mexico. School outbreaks accounted for 16% of cases so far.
Patients have symptoms similar to those of seasonal influenza, with few exceptions. The most frequently reported symptoms are cough, fever, fatigue, headache, sore throat, runny nose, chills, and muscle aches, all occurring in 80% or more of patients. Almost all patients fit the CDC definition for influenza-like illness, consisting of subjective fever plus cough or sore throat.
Nausea, abdominal pain, and diarrhea, which are not common symptoms of seasonal influenza, have been reported in approximately 50% of patients with S-OIV. The spectrum of illness ranges from self-limited to severe, with 2% of patients developing pneumonia and 9% requiring hospitalization.
Continued analysis of the case-fatality rate highlights that people ages 20 to 29 are disproportionately represented among the fatalities.
A PCR test has been developed
Since clinical findings identify patients with influenza-like illness but cannot confirm or exclude the diagnosis of influenza,12 a specific diagnostic real-time reverse-transcriptase polymerase chain reaction (RT-PCR) test has been developed, and the CDC is currently distributing it to state health departments.
An interim case definition
An interim case definition for the purpose of epidemiologic investigation of cases of S-OIV infection includes acute fever (temperature ≥ 100°F, 37.8°C) and acute respiratory illness (rhinorrhea, sore throat, or cough), plus:
- For a confirmed case, S-OIV infection confirmed by RT-PCR or viral culture
- For a probable case, laboratory-confirmed influenza A, but negative for H1 and H3 by RT-PCR
- For a suspected case, onset of above illness within 7 days of close contact with a confirmed case of S-OIV infection; or travel within 7 days to a community within the United States or internationally where there are one or more confirmed cases of S-OIV infection; or residing in such a community.
In practice, is it seasonal flu or swine flu?
In clinical practice in United States, in the springtime, a person with influenza-like illness and microbiologically confirmed seasonal influenza B obviously would not raise any concern about the ongoing S-OIV epidemic. Sporadic cases of seasonal influenza A are still occurring, but these are the ones that create a diagnostic dilemma, since very few laboratories currently have the ability to differentiate between influenza A H1 and H3. Since S-OIV has been reported in almost all states in the United States, one can argue that most cases of influenza A currently being identified should be considered suspected S-OIV.
PREVENTIVE MEASURES
In response to this ongoing outbreak, the WHO raised its epidemic alert level from 4 to 5, one level shy of declaring a pandemic. Several measures have been implemented in an attempt to halt this outbreak, the most important of which is the rapid dissemination of information to health professionals,13 with the Internet playing a central role.14
The world is better prepared for a pandemic now than at any time in history. Seed virus for vaccine development has been provided to various governments and pharmaceutical manufacturers. Stockpiles of antiviral agents are being mobilized and distributed to various locations, and dispensing plans are being reviewed for potential execution. The US Food and Drug Administration (FDA) issued emergency-use authorizations for mass deployment of the strategic stockpile of oseltamivir (Tamiflu), including for children younger than 1 year, and of zanamivir (Relenza) for the treatment and prophylaxis of S-OIV infection. It also authorized the use of disposable N95 respiratory masks by the general public, as well as the RT-PCR diagnostic test.
General advice for healthy people in the community
- Maintain a distance of at least 1 meter from a person with influenza-like illness.
- Wear a mask while providing care for a person with influenza-like illness.
- Avoid touching your eyes, nose, or mouth, since these are potential portals of entry for the virus. This may be a difficult recommendation to follow, since it requires constant vigilance of a common human behavior.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with respiratory secretions from a person, including your child, with influenza-like illness.
- If possible, reduce the time spent in close contact with people with influenza-like illness and in crowded settings.
- If possible, open windows in your living space to improve airflow.
While the CDC has recommended avoiding nonessential travel to Mexico at the current time, the WHO is not recommending any travel restrictions, since the outbreak has already spread to many parts of the world and all continents.
There is no limitation on handling or consuming pork meat or other well-processed swine products.
Recommendations for school dismissal and social-distancing interventions are evolving. During the 1918 pandemic, nonpharmaceutical interventions were associated with a significant reduction in deaths,15 but it is unclear how much additional benefit these measures would add to effective immunization, antiviral treatment for patients, and chemoprophylaxis for their contacts.
General advice for people with influenza-like illness
- Stay home for 7 days after the onset of symptoms or 48 hours after symptoms resolve, whichever is longer.
- Maintain a distance of at least 1 meter from all people.
- Cover your mouth and nose with tissues when coughing or sneezing, and dispose of the tissues immediately after use.
- Avoid touching your eyes, nose, and mouth.
- Wash your hands often with either soap and water or an alcohol-based hand rub for 20 to 30 seconds, particularly after touching your eyes, nose, or mouth or after contact with your respiratory secretions during coughing or sneezing. Adding virucidal agents or antiseptics to hand-washing is not likely to have an incremental effect.16
- If possible, open windows in your living space to improve airflow.
- If possible, when you are in close contact with other people, wear a mask to help contain your respiratory secretions.
Masks
The designs and standards of masks vary from country to country. Masks have been shown to reduce the transmission of influenza in health care settings,16 but the benefit in the community has not been established. Advice on proper use of a mask:
- Cover your mouth and nose with the mask and tie it securely to minimize gaps.
- Avoid touching the mask while it is on your face.
- Wash your hands with soap and water or an alcohol-based hand rub for 20 to 30 seconds after removing the mask.
- If the mask becomes damp, replace it with a new one.
- Avoid reusing single-use masks, and dispose of them immediately after removing.
VACCINE DEVELOPMENT
The most difficult question about vaccine development for S-OIV at this time is whether to prepare it as a separate product or try to incorporate it in the seasonal influenza vaccine.
The problem is that the seasonal influenza vaccine for the Southern Hemisphere has already been made and distributed, and vaccination programs are already well under way. Although flu season in the Northern Hemisphere is not expected before September or October 2009, vaccine production and distribution take several months, leaving little time to observe which direction the S-OIV epidemic will take before making this decision.
Vaccine distribution also raises difficult questions, since a limited amount will be available initially and rationing to the most vulnerable people will be necessary. While health care workers are more likely to be exposed to people infected with S-OIV compared with the general population, mandating their immunization may pose other moral dilemmas.17
The current global capacity for production of seasonal influenza vaccine is approximately 400 million doses.18 Since the process of vaccine production takes at least 4 to 6 months, measures have been proposed to speed up the production of pandemic vaccine or immunogenicity; these include recombinant technology, reverse genetics, and the use of adjuvants. In April 2007, the FDA approved the first H5 subviron vaccine for people ages 19 to 64.
This topic brings back memories of the 1976 swine influenza immunization program, in which the rate of Guillain-Barré syndrome was 5 to 10 times the background rate, resulting in a halt in vaccine production.
Why this syndrome occurred is not known, but it is suspected to be due to cross-reacting antibodies against peripheral-nerve antigen that developed after the vaccine was given. Data since then have shown no association between vaccination and Guillain-Barré syndrome. 19 On the other hand, influenza viruses were found to trigger Guillain-Barré syndrome only infrequently, except during major outbreaks, in which they may play a significant role.20
TREATMENT
Antiviral drugs
Tests of current S-OIV isolates showed them to be susceptible to the neuraminidase inhibitors, ie, oseltamivir and zanamivir, but resistant to the adamantanes, ie, amantadine (Symmetrel) and rimantadine (Flumadine).21 All isolates contained the S31N mutation in the M2 protein, which confers resistance against the adamantanes and which has been detected in most influenza A (H3N2) isolates in the United States since 2006. Fortunately, the H274Y mutation in N1—which confers resistance to oseltamivir but not to zanamivir and which has been detected in almost all seasonal influenza A (H1N1) isolates since the early weeks of the current influenza season— has not been detected in any of the current S-OIV isolates.
Patients who are otherwise healthy who present with an uncomplicated febrile illness due to S-OIV do not require antiviral treatment. Either oseltamivir or zanamivir is recommended for treatment of patients hospitalized for management of confirmed, probable, or suspected infection with S-OIV, or for those at high risk of influenza-related complications, defined similarly to seasonal influenza (Table 1).
The duration of shedding of S-OIV is unknown, but starting an antiviral agent early in the course of illness is expected to reduce contagiousness. Extrapolating from data in seasonal influenza, infected persons are assumed to be shedding virus from 1 day prior to illness onset until resolution of symptoms, usually 7 days, and up to 10 days in younger children.
Oseltamivir accounts for the lion’s share of the stockpile of antiviral drugs against pandemic influenza. However, with mass utilization, antiviral resistance to a single agent may develop. A mathematical model showed that adding a smaller stockpile of a second agent, such as zanamivir, to be used either in combination with or sequential to oseltamivir, can effectively prevent or at least delay the development of resistance.22
Other potential measures for management
Since secondary bacterial pneumonia is expected to play a significant role in influenza-related death during the next pandemic, stockpiling antibacterial agents may also be prudent.8 The death rate in methicillin-resistant Staphylococcus aureus pneumonia secondary to seasonal influenza is 50%, further complicating the choice of stockpiling for antibacterial agents.
A meta-analysis of 11 studies involving 1,703 patients during the 1918 pandemic showed that those who received influenza-convalescent human blood products were less likely to die than those who did not.23 Anti-influenza drugs and advanced techniques to care for critically ill patients were not available at that time, so extrapolating these data to the current era may not be appropriate.
The cost of vaccine and antiviral drugs is an expected limitation to mass implementation during a pandemic, particularly in developing countries. Certain inexpensive generic drugs that have been shown to have some activity against influenza, such statins, fibrates, and chloroquine, deserve further attention.24
PUTING THE CURRENT EPIDEMIC IN PERSPECTIVE
In summary, the world is now better prepared, vaccine is in development, and antiviral treatment is available. For more information, readers are directed to go to www.cdc.gov/h1n1flu/ or www.who.int/csr/don/2009_05_11/en/index.html.
- Belshe RB. Implications of the emergence of a novel H1 influenza virus. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903995.
- Ducatez MF, Webster RG, Webby RJ. Animal influenza epidemiology. Vaccine 2008; 26(suppl 4):D67–D69.
- Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis 2007; 44:1084–1088.
- Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903812.
- Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med 2007; 4( 2):e59. doi:10.1371/journal. pmed.0040059.
- Belshe RB. The origins of pandemic influenza—lessons from the 1918 virus. N Engl J Med 2005; 353:2209–2211.
- Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics—implications for policy. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0903906.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Viboud C, Grais RF, Lafont BAP, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis 2005; 192:233–248.
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903810.
- US Centers for Disease Control and PreventionC. Swine-origin influenza A (H1N1) virus infections in a school—New York City, April 2009. MMWR 2009; 58(Dispatch):1–3.
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005: 293:987–997.
- Baden LR, Drazen JM, Kritek PA, Curfman GD, Morrissey S, Campion EW. H1N1 influenza A disease—information for health professionals. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903992.
- Brownstein JS, Freifeld CC, Madoff LC. Influenza A (H1N1) virus, 2009—online monitoring. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0904012.
- Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic. JAMA 2007; 298:644–654.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336( 7635):77–80.
- Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med 2009; 360:1981–1988.
- Sahni R, Mossad SB. Controlling pandemic influenza through vaccination programs. Future Virol 2009; 4:271–276.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- US Centers for Disease Control and Prevention. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR May 1, 2009; 58( 16):433–435.
- Wu JT, Leung GM, Lipsitch M, Cooper BS, Riley S. Hedging against antiviral resistance during the next influenza pandemic using small stockpiles of an alternative chemotherapy. PLoS Med 2009;e1000085. doi:10.1371/journal.pmed.1000085.
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006; 145:599–609.
- Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis 2008; 8:571–576.
- Belshe RB. Implications of the emergence of a novel H1 influenza virus. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903995.
- Ducatez MF, Webster RG, Webby RJ. Animal influenza epidemiology. Vaccine 2008; 26(suppl 4):D67–D69.
- Myers KP, Olsen CW, Gray GC. Cases of swine influenza in humans: a review of the literature. Clin Infect Dis 2007; 44:1084–1088.
- Shinde V, Bridges CB, Uyeki TM, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903812.
- Sandbulte MR, Jimenez GS, Boon AC, Smith LR, Treanor JJ, Webby RJ. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med 2007; 4( 2):e59. doi:10.1371/journal. pmed.0040059.
- Belshe RB. The origins of pandemic influenza—lessons from the 1918 virus. N Engl J Med 2005; 353:2209–2211.
- Miller MA, Viboud C, Balinska M, Simonsen L. The signature features of influenza pandemics—implications for policy. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0903906.
- Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008; 198:962–970.
- Viboud C, Grais RF, Lafont BAP, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis 2005; 192:233–248.
- Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009 May 7; doi:10.1056/NEJMoa0903810.
- US Centers for Disease Control and PreventionC. Swine-origin influenza A (H1N1) virus infections in a school—New York City, April 2009. MMWR 2009; 58(Dispatch):1–3.
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza? JAMA 2005: 293:987–997.
- Baden LR, Drazen JM, Kritek PA, Curfman GD, Morrissey S, Campion EW. H1N1 influenza A disease—information for health professionals. N Engl J Med 2009 May 7; doi:10.1056/NEJMe0903992.
- Brownstein JS, Freifeld CC, Madoff LC. Influenza A (H1N1) virus, 2009—online monitoring. N Engl J Med 2009 May 7; doi:10.1056/NEJMp0904012.
- Markel H, Lipman HB, Navarro JA, et al. Nonpharmaceutical interventions implemented by US cities during the 1918–1919 influenza pandemic. JAMA 2007; 298:644–654.
- Jefferson T, Foxlee R, Del Mar C, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ 2008; 336( 7635):77–80.
- Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med 2009; 360:1981–1988.
- Sahni R, Mossad SB. Controlling pandemic influenza through vaccination programs. Future Virol 2009; 4:271–276.
- Hughes RA, Charlton J, Latinovic R, Gulliford MC. No association between immunization and Guillain-Barré syndrome in the United Kingdom, 1992 to 2000. Arch Intern Med 2006; 166:1301–1304.
- Sivadon-Tardy V, Orlikowski D, Porcher R, et al. Guillain-Barré syndrome and influenza virus infection. Clin Infect Dis 2009; 48:48–56.
- US Centers for Disease Control and Prevention. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR May 1, 2009; 58( 16):433–435.
- Wu JT, Leung GM, Lipsitch M, Cooper BS, Riley S. Hedging against antiviral resistance during the next influenza pandemic using small stockpiles of an alternative chemotherapy. PLoS Med 2009;e1000085. doi:10.1371/journal.pmed.1000085.
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med 2006; 145:599–609.
- Fedson DS. Confronting an influenza pandemic with inexpensive generic agents: can it be done? Lancet Infect Dis 2008; 8:571–576.
KEY POINTS
- What happens in the annual influenza season in the Southern Hemisphere will indicate the prospects of S-OIV progressing to a pandemic.
- Oseltamivir (Tamiflu) and zanamivir (Relenza) are active against S-OIV and are recommended for hospitalized patients or people at higher risk of influenza-related complications.
- Otherwise-healthy patients who present with an uncomplicated febrile illness due to S-OIV do not require antiviral treatment.
- Hand-washing is the most important preventive measure.
- Vaccine development may take 4 to 6 months. The most difficult question about vaccine development for S-OIV is whether to prepare it as a separate product or incorporate it in the seasonal influenza vaccine.