User login
On February 1, 2016, the World Health Organization declared Zika virus a public health emergency of international concern due to clusters of microcephaly and neurologic manifestations in areas of Zika virus transmission.1 On February 8, the US Centers for Disease Control and Prevention (CDC) elevated its response to level 1, its highest.2
Case reports and guidelines have been published to help clinicians better understand the epidemiology, risk, and pathogenesis of Zika virus infection, but much is still unknown. Clinicians must be ready to address the concerns of international travelers and must also consider Zika virus in the differential diagnosis of fever in the returned traveler.
FLAVIVIRUSES: DENGUE, WEST NILE … ZIKA
Zika virus, a single-stranded RNA arthropod-borne virus (arbovirus), is transmitted by mosquitoes. It is a member of the flavivirus family, which consists of over 70 viruses including some well known for causing diseases in humans, such as dengue, yellow fever, Japanese encephalitis, and West Nile virus.3
Phylogenetically, Zika virus is most similar to and included in a clade with Spondweni virus, which, like Zika, originated in Africa.4 Genomic analysis has revealed an African and an Asian lineage. The Asian lineage is responsible for the current epidemic in the Pacific and the Western Hemisphere.4–6
OUT OF AFRICA AND ASIA
Zika virus is named after a forested area in present-day Uganda, where it was first isolated in a febrile rhesus monkey that was being used to study yellow fever.7 Further studies in the 1950s confirmed its transmission to humans, as 6% of the sera tested in Ugandans showed evidence of specific antibodies to the virus.8 In 1978, antibody prevalence studies showed that up to 40% of Nigerians had Zika virus-neutralizing antibodies.9 Over the next 38 years, scattered case reports and seroprevalence studies showed infections occurring throughout Africa and Asia.9–11
In 2007, the first case of Zika virus transmission outside of Asia and Africa occurred on Yap Island in the Federated States of Micronesia.10–12 No further transmission in the Pacific was noted for 6 years until an outbreak occurred in French Polynesia in 2013.13–15 The first time Zika virus was found in the Western Hemisphere was in January 2014, when an outbreak occurred on Chile’s Easter Island.16 Genomic analysis of the Zika virus isolated on Easter Island indicated it was most closely related to isolates from French Polynesia.16 In 2014, additional cases of Zika virus infection were reported in New Caledonia and the Cook Islands.13,14
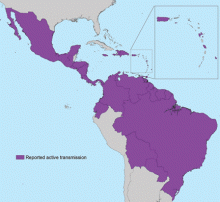
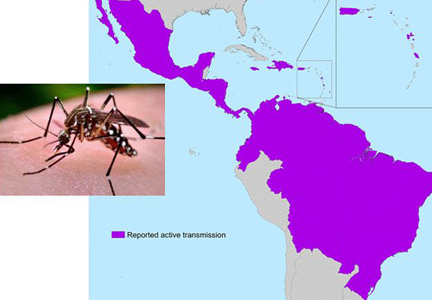
In May 2015, the World Health Organization issued an epidemiologic alert in response to dramatic increases in the spread of Zika virus in Brazil.17 From Brazil, Zika virus has rapidly spread to most countries in South and Central America and the Caribbean (Figure 1).2,5,6
TRANSMITTED BY MOSQUITO
The Aedes (Stegomyia) genus of mosquitoes is a well-known source of transmission for several arboviruses, including yellow fever, dengue, chikungunya, and now Zika virus.18,19 Zika virus was originally isolated in Uganda from Aedes africanus mosquitoes.7,20 Subsequently, other species of Aedes mosquitoes have been shown to transmit Zika virus, with Aedes aegypti being the most important human vector.7,8,19–21
Another species, Aedes albopictus has been identified as a human vector in Gabon and is also suspected of being a vector in the Brazilian outbreak.22 Spread of A albopictus from Asia to Europe, the Mediterranean region, and the Americas, including 32 states in the United States, has increased the fear of potential spread of Zika virus infection to a more expansive geographic range.13,18,19 Local transmission may become established if local mosquitoes become infected when infected travelers return from endemic areas.23
OTHER ROUTES OF TRANSMISSION
While mosquito-borne transmission is the most common route of infection with Zika virus, human-to-human transmission has been documented. Potential routes of transmission include sexual intercourse, blood transfusions, and vertical (mother-to-child) transmission.
Sexual transmission. Replicative Zika virus particles were identified in the semen of a patient who presented with hematospermia in French Polynesia.24
Previously, there was a report of Zika virus being sexually transmitted from a US man who had returned from Senegal to his spouse, who had not traveled to a Zika virus-endemic region. Both patients became ill following vaginal intercourse, with the onset of the wife’s illness occurring 5 days after the onset of the husband’s illness. The husband was noted to have hematospermia.25 Neutralization testing for both patients confirmed infection with Zika virus.25
The first reported case of sexual transmission in the current outbreak in the United States occurred in a traveler returning to Texas from Venezuela.26 The CDC is currently investigating several other potential cases and an additional two laboratory-confirmed cases. All cases were in symptomatic male travelers who had condomless vaginal intercourse with their female partners after return from Zika virus-endemic areas.27
Blood transfusions. Several arboviruses are known to be transmitted via blood.
In French Polynesia, Zika virus RNA was present in 3% of blood donors.28,29 These blood donors had been screened and were asymptomatic at the time of donation. Twenty-six percent of donors who had Zika RNA reported an illness compatible with Zika virus infection in the 3 to 10 days before donation.28
Brazil has reported two cases of Zika virus infection through blood transfusion.30
In May 2015, the European Centers for Disease Control recommended that travelers to affected areas defer blood donation for 28 days.31 The Association of American Blood Banks has also recommended that travelers self-defer donating blood for 28 days after travel to an endemic area.32 Most recently the US Food and Drug Administration recommended a 4-week deferral for travelers to Zika virus-endemic areas and after resolution of symptoms for those who have had Zika virus infection.33 Additional guidance for donors who have had sexual contact with Zika virus-infected persons and areas with active transmission of Zika virus is also available.33
Vertical transmission. Perinatal and transplacental transmission have also been documented.34,35 The extent and frequency of the clinical manifestations of these infections are still being elucidated in light of reports of association with fetal abnormalities.
Although Zika virus has been detected in breast milk, no cases of transmission through breastfeeding have been reported. Currently, women are advised to continue to breastfeed in areas of known Zika virus transmission.34,36,37
IS USUALLY ASYMPTOMATIC OR CAUSES MILD SYMPTOMS
Most Zika virus infections are asymptomatic, as illustrated by reports from the Yap Island outbreak, where only 19% of those with immunoglobulin M (IgM) antibodies to Zika virus had symptoms.12 The illness in symptomatic patients is often mild and self-limited, and most manifestations resolve by 7 days.12,25,38,39
Initial descriptions in the 1950s and 1960s of the clinical features of Zika virus infection in Africa included fever and headache as the most prominent symptoms.38,40 Description of the outbreak on Yap in 2007 characterized the predominant symptoms as rash, fever, arthralgia/arthritis, and nonpurulent conjunctivitis in 31 patients,12 and the current CDC case definition includes at least two of these four symptoms.41 The arthralgia and arthritis are usually of the small joints of the hands and feet and can persist for as long as a month.25,42 The rash can be pruritic.15,33,42,43
Less commonly reported manifestations of Zika virus infection include malaise, stomachaches, dizziness, anorexia, retro-orbital pain, aphthous ulcers, hematospermia, and prostatitis.14,15,24,25,44,45
The initial reports from eight patients in the outbreak in Brazil noted rash and joint pain as the most common manifestations. The maculopapular rash was present in all patients and the joint pain was characterized as severe, with the hands, ankles, elbows, knees, and wrists most consistently described.43
The clinical presentation is similar to those of dengue and chikungunya virus infections, confounding diagnosis, as these viruses may be cocirculating in the same geographic regions (and indeed are transmitted by the same mosquito vectors).11,12,15 The conjunctivitis present in Zika virus infections can also be present in chikungunya but is much less commonly a clinical feature of dengue.15,46,47 See Table 1 for the differential diagnosis of Zika virus infection.
Severe manifestations requiring hospitalization or resulting in death are thought to be uncommon, although neurologic and fetal complications have recently been described.12,29,43,48,49
CLINICAL ASSOCIATIONS
Primary infection with Zika virus is relatively benign. The greatest and most recent concerns are related to postinfectious complications and those that may occur in pregnant women.
Guillain-Barré syndrome
During the Zika virus outbreak in French Polynesia in 2013–2014, the incidence of Guillain-Barré syndrome was multiplied by a factor of 20.50 Prior to the first hospitalization of a patient with Zika virus infection and associated Guillain-Barré syndrome in French Polynesia, there had been no reported hospitalizations for Zika virus infection.50
This same association is now being seen in the recent outbreak in the Americas.50 In July 2015, Brazilian health officials in the State of Bahia reported 76 patients with neurologic syndromes, of whom 55% had Guillain-Barré syndrome.51 A history consistent with Zika virus infection was found in 62%.48
In January 2016, El Salvador also reported an unusual increase in Guillain-Barré syndrome cases since early December 2015.51 Between December 1, 2015, and January 6, 2016, there were 46 Guillain-Barré syndrome cases reported, compared with a baseline of 14 cases per month.51
Other countries where Zika virus infection is endemic are also currently investigating similar trends.51
Microcephaly
On November 17, 2015, the Pan American Health Organization issued an epidemiologic alert because of increased reports of microcephaly in the Pernambuco State of Brazil. Whereas there are typically about 10 cases per year, there had been 141 in the previous 11 months.51 Other states in Brazil such as Paraiba and Rio Grande del Norte also reported increases in the diagnosis of microcephaly. A physician alert published in Brazil described two infants from the Paraiba state who were diagnosed with fetal microcephaly.35 Testing for Zika virus by polymerase chain reaction (PCR) was negative in the maternal blood, but PCR of amniotic fluid was positive in both infants.35
In January 2016, the Brazil Ministry of Health reported that Zika virus had been detected by real-time PCR (RT-PCR) in four infants with congenital malformations in Rio Grande del Norte. Two of these cases were miscarriages and two were infants who died within 24 hours of birth. Immunohistochemistry of tissues from these infants was positive for Zika virus.
A February 2016 case report describes a European woman who developed Zika virus infection at 13 weeks gestation while working in Northeast Brazil and upon return to Europe elected to terminate the pregnancy after ultrasonography showed cerebral calcifications with microcephaly. The infant was found to have a very small brain, hypoplasia of the brainstem and spinal cord with degeneration of spinal tracts, complete absence of cerebral gyri, and severe dilatation of lateral ventricles as well as calcifications throughout the cerebral cortex.49 No genetic abnormalities or evidence of other etiologies was found, and large amounts of Zika virus RNA were found in the brain.
The CDC also recently reported confirmation of Zika virus infection from fetal tissues of two miscarriages (fetal loss at 11 and 13 weeks) and two fetal deaths (36 and 38 weeks) received from the state of Rio Grande do Norte in Brazil.52 All four mothers reported clinical signs of fever and rash during their first trimester of pregnancy.52 Additional testing for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, and human immunodeficiency virus were all negative in the mothers who had miscarriages.52
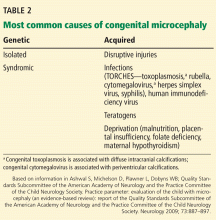
Of critical note, the causality of Zika virus and microcephaly remains under investigation. See Table 2 for other causes of microcephaly.53
Macular atrophy
In January 2016, a case series of three infants with microcephaly and macular atrophy was reported.54 These infants were tested for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, syphilis, and human immunodeficiency virus (HIV), and all the results were negative. The detection of Zika virus fulfilled the Brazilian Ministry of Health’s definition of vertical transmission of Zika virus, and laboratory diagnostic tests for Zika virus were not performed. In this series, one mother reported an illness with rash and arthralgias during the first trimester.54
LABORATORY DIAGNOSTIC METHODS
The diagnosis of Zika virus infection is challenging. The low viremia at initial presentation and cross-reactivity of serologic testing with other flaviviruses, especially dengue, can contribute to misdiagnosis.40,50
In the first 7 days of Zika virus infection, the diagnosis is based on detection of viral RNA in serum by RT-PCR.12,55,56 RT-PCR is very specific for Zika virus and is an important tool in differentiating between Zika virus and other flaviviruses often present in areas where Zika virus is circulating.12,56 After 3 to 4 days, viremia may decrease to levels that may be below the assay’s level of detection.40–42,45
While Zika virus RNA may be undetectable in the serum, other samples such as saliva, urine, and semen may be positive for longer.28,42,57 For example, urine samples were positive by RT-PCR up to 7 days beyond blood RT-PCR in the outbreak in New Caledonia.42 A recent report found semen remaining positive on RT-PCR for 62 days after the onset of confirmed Zika virus illness in a traveler returning to the United Kingdom from the Cook Islands in 2014.58
Because RT-PCR of blood is only useful early in infection, the current diagnostic guidelines recommend testing an acute-phase serum sample for Zika virus IgM collected as early as possible after the onset of illness and repeated 2 to 3 weeks after the initial set. These IgM antibodies typically develop toward the end of the first week of illness and are expected to be present for up to 12 weeks, based on experience with other flaviviruses.41 Cross-reactivity with other flaviviruses circulating in the area can occur and has been problematic in areas where dengue is circulating.12,41,45,56 IgM-positive specimens should be further tested, by plaque-reduction neutralization, to confirm the presence of Zika virus-specific neutralizing antibodies. Results can be difficult to interpret, especially in those who have been previously infected or vaccinated against other flaviviruses.12,41
If amniocentesis is done, these specimens should be tested by RT-PCR. However, the sensitivity of PCR in amniotic fluid is currently unknown.41
In infants with findings of cerebral calcifications and microcephaly, IgM serologies with RT-PCR are also recommended and should be drawn within 2 days of birth. Specimens should be drawn concurrently as it is not known which test is most reliable in infants.23 Additionally, placenta and umbilical cord samples should be collected for immunohistochemical staining at specialized laboratories.36
In the United States, providers should contact their state health departments to determine where tests can be run reliably. Refined diagnostic assays are in development at the time of this publication and are likely to be made available through CDC’s Laboratory Response Network.
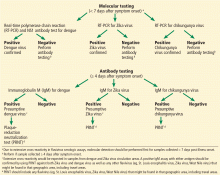
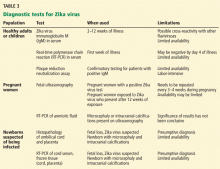
See Figure 2 and Table 3 for a summary of diagnostic tests.
IMPLICATIONS, RECOMMENDATIONS
Pregnant women
The CDC now recommends that asymptomatic pregnant women who returned from travel to a Zika virus-endemic zone in the last 2 to 12 weeks be offered serologic testing.41 This includes women who may be living in an area with ongoing Zika virus transmission; however, these women should also have testing at the initiation of prenatal care and then follow-up testing in the middle of the second trimester. Of importance, these results may be difficult to interpret due to potential cross-reactivity between Zika virus and other flaviviruses, and false-positive results in recipients of yellow fever and Japanese encephalitis vaccines.41,59
If a pregnant woman with a positive travel history is symptomatic, testing should be offered during the first week of illness. After day 4 of the illness, testing should include both RT-PCR and IgM serology.41,59
A screening ultrasound scan is recommended for any pregnant woman who has traveled to a Zika virus-affected area to determine if microcephaly or cerebral or intracranial calcifications are present. Those women with confirmed Zika virus infection should continue to have monthly screening ultrasounds, while those who are negative for Zika virus should have another ultrasound at the end of the second trimester or the beginning of the third trimester to ensure that no abnormalities had developed.41,59
At present, pregnant women and women of childbearing age who may become pregnant are advised by the CDC to postpone travel to affected areas until more information becomes available about mother-to-child transmission.59
Algorithms for the care of pregnant women and women of childbearing age who may have been exposed to Zika virus are available from the CDC41 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e2.htm.
Male partners of pregnant women
Since the length of time that Zika virus remains viable in semen is not known, men who have traveled to Zika virus-endemic areas and who have pregnant partners should refrain from having sex or use a condom with every sexual encounter through the duration of the pregnancy.60
Guidelines for prevention of sexual transmission of Zika virus are available from the CDC59 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e1er.htm.
Infants with possible congenital Zika virus infection
Zika virus testing is recommended for any infant born with microcephaly or intracranial calcifications or whose mother has positive or inconclusive testing if the mother had visited an endemic area during her pregnancy.
Zika virus testing in infants consists of serologic IgM determination and RT-PCR for both dengue and Zika virus drawn concurrently in the first 2 days of life.36 Umbilical cord blood can be used. In addition, if cerebrospinal fluid is being collected for other reasons, it can also be tested for Zika virus. The placenta and umbilical cord should be saved for immunohistochemistry testing for Zika virus.61
An infant who tests positive or inconclusive for Zika virus, regardless of the presence of microcephaly or intracranial calcifications, should have a complete physical examination specifically evaluating growth parameters, estimated gestational age, and signs of neurologic disease, skin rashes, hepatosplenomegaly, or any dysmorphic features. Additional evaluation includes an ophthalmologic examination in the first month of life to evaluate for macular atrophy.36 An ultrasound scan of the head should be completed if it has not been done. Hearing is screened in all newborns, and hearing testing should be repeated at 6 months of age.36
Infants with microcephaly or intracranial calcifications should also have consultations with specialists in genetics, neurology, and pediatric infectious diseases.61 These infants should have blood work including complete blood cell counts and liver function testing that includes alanine aminotransferase, aspartate aminotransferase, and bilirubin levels.36
All infants with possible congenital Zika virus infection should be followed long-term with close attention to developmental milestones and growth parameters including occipital frontal head circumference measurements.61,62
Infants without microcephaly or calcifications whose mothers had negative Zika virus test results or were not tested for Zika virus should have routine care.37
Guidelines for the care of infants with Zika virus infection are available from the CDC36 at www.cdc.gov/mmwr/volumes/65/wr/mm6503e3.htm.
TREATMENT
There is no treatment for Zika virus infection, and care is supportive. Most infections are mild and self-limited.12,15 Avoidance of aspirin and other nonsteroidal anti-inflammatory drugs that may affect platelets is important until dengue infection has been ruled out.
PREVENTION
There is currently no vaccine to prevent Zika virus infection. Woman who are pregnant should avoid travel to any area where Zika virus transmission is occurring.41,59 The CDC advises pregnant women and women of childbearing age who may become pregnant to postpone travel to Zika virus-affected areas.59 Patients can find travel alerts for specific areas at wwwnc.cdc.gov/travel/notices/alert/zika-virus-south-america.
Avoiding mosquito bites is the best way to prevent the spread of Zika virus. Aedes aegypti and A albopictus, the most common vectors of Zika virus, can bite at night but are known more for being aggressive daytime biters.63 Travelers should apply an Environmental Protection Agency-registered insect repellent as directed, wear long-sleeved shirts and long pants, use permethrin-treated clothing and gear, and stay in places with screens or air conditioning. Any containers with standing water should be eliminated as they are breeding areas for mosquitoes. It is also important that symptomatic people in the first week of illness use mosquito precautions to prevent the spread of Zika virus.
Patient handouts and posters for mosquito bite prevention can be found at www.cdc.gov/zika/fs-posters/index.html.
WATCH FOR UPDATES
Many questions remain regarding the epidemiology of this infection and its relationship to neurologic and pregnancy complications. However, due to its rapid spread across the Western hemisphere and its potential for significant complications, much is being done at the local and international levels to better understand the virus and halt its spread. More information will continue to be available as results from ongoing studies are conducted and potential associations are investigated. Until more is known, providers should familiarize themselves with the latest guidelines in order to better counsel their patients who may live in or travel to Zika virus endemic areas. We advise clinicians to follow the CDC’s web site, www.cdc.gov/zika/.
- World Health Organization. Zika virus fact sheet. www.who.int/mediacentre/factsheets/zika/en/. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Areas with Zika. www.cdc.gov/zika/geo/index.html. Accessed February 24, 2016.
- Rice CM. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, Chanock RM, editors. Fields Virology, 3rd ed. Philadelphia: Lippincott-Raven, 1996:961–1034.
- Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol 1998; 72:73–83.
- Haddow AD, Schuh AJ, Yasuda CY, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 2012; 6:e1477.
- Faye O, Freire CC, Iamarino A, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 2014; 8:e2636.
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–520.
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46:521–534.
- Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979; 83:213–219.
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009; 15:1347–1350.
- Heang V, Yasuda CY, Sovann L, et al. Zika virus infection, Cambodia, 2010. Emerg Infect Dis 2012; 18:349–351.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–2543.
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infec 2014; 20:O595–O596.
- Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20:1085–1086.
- Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 2014; 44:302–307.
- Tognarelli J, Ulloa S, Villagra E, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol Nov 26 2015 [Epub ahead of print].
- Pan American Health Organization/World Health Organization, Regional Office for the Americas. Zika virus infection. 7 May 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=30075=en. Accessed February 24, 2016.
- Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 2016; 347:601–604.
- Marcondes CB, Ximenes MF. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop. Dec 22 2015. pii: S0037-86822015005003102. [Epub ahead of print]
- Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg 1958; 52:263–268.
- Diallo D, Sall AA, Diagne CT, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One 2014; 9:e109442.
- Grard G, Caron M, Mombo IM, et al. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 2014; 8:e2681.
- Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR 2016; 65:55–58.
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015; 21:359–361.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–882.
- Smith J, Woldai S, Chung W. Health advisory: sexual transmission of Zika virus. Dallas Country Department of Health and Human Services, February 2, 2016. http://walnuthillobgyn.com/wp-content/uploads/2012/05/zika-transmission.pdf. Accessed February 24, 2016.
- Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Early release February 26, 2016. www.cdc.gov/mmwr/volumes/65/wr/mm6508e2er.htm Accessed February 29, 2016.
- Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 2014; 19(14). pii: 20761. Erratum in Euro Surveill 2014; 19(15). pii/20771.
- Marano G, Pupella S, Vaglio S, Liumbruno GM, Grazzini G. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus 2015; Nov 5:1–6. doi: 10.2450/2015.0066-15. [Epub ahead of print]
- European Centre for Disease Prevention and Control. Epidemiological update: complications potentially linked to Zika virus outbreak, Brazil and French Polynesia. November 27, 2015. http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?ID=1332&List=8db7286c-fe2d-476c-9133-18ff4cb1b568&Source=http%3A%2F%2Fecdc%2Eeuropa%2Eeu%2Fen%2Fpress%2Fepidemiological%5Fupdates%2FPages%2Fepidemiological%5Fupdates%2Easpx. Accessed February 24, 2016
- European Centre for Disease Prevention and Control. Rapid risk assessment. Zika virus infection outbreak, Brazil and the Pacific region 25 May 2015. http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-Zika%20virus-south-america-Brazil-2015.pdf. Accessed February 24, 2016
- Regan DM, Markowitz MA. Association Bulletin #16-03. Re: Zika, dengue, and chikungunya viruses. American Association of Blood Banks, February 1, 2016. www.aabb.org/programs/publications/bulletins/Documents/ab16-03.pdf. Accessed February 24, 2016.
- US Food and Drug Administration (FDA). Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. February, 2016. www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Accessed February 24, 2016.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014; 19(13). pii: 20751.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016; 47:6–7.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Fleming-Dutra K, Nelson J, Fischer M, Staples J, Mateusz P, et al. Update: interim guidelines for health care providers caring for infants and children with possible Zika virus infection—United States, February 2016. MMWR 2016; 65:1–6.
- Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg Jul 1964; 58:335–338.
- Olson JG, Ksiazek TG, Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 1981; 75:389–393.
- Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg 1956; 50:442–448.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR 2016; 65:122–127.
- Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis 2015; 21:84–86.
- Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–572.
- Alera MT, Hermann L, Tac-An IA, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis 2015; 21:722–724.
- Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–1239.
- Centers for Disease Control and Prevention. Chikungunya virus. Clinical evaluation & disease. www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Clinical guidance. Dengue virus. www.cdc.gov/dengue/clinicalLab/clinical.html. Accessed February 24, 2016.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Increase in microcephaly in the northeast of Brazil. November 17, 2015. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32636&lang=en. Accessed February 24, 2016.
- Rubin EJ, Greene MF, Baden LR. Zika virus and microcephaly. N Engl J Med 2016; Feb 10 [Epub ahead of print].
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barré syndrome—case report, French Polynesia, December 2013. Euro Surveill 2014; 19(9). pii: 20720.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1, 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed February 24, 2016.
- Martines R, Bhatnagar J, Keating M, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMRW 2016; 65:159–160.
- Ashwal S, Michelson D, Plawner L, Dobyns WB; Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Practice parameter: evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2009; 73:887–897.
- Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016; 387:228.
- Centers for Disease Control and Prevention. Updated diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. http://stacks.cdc.gov/view/cdc/37594. Accessed February 24, 2016.
- Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 2013; 10:311.
- Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol 2015; 68:53–55.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen [letter]. Emerg Infect Dis 2016 May. http://wwwnc.cdc.gov/eid/article/22/5/16-0107_article. Accessed February 24, 2016.
- Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR 2016; 65:30–33.
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR 2016; 65:120–121.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Centers for Disease Control and Prevention. Zika virus clinical evaluation and disease. www.cdc.gov/zika/hc-providers/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Zika virus. Transmission & risks. www.cdc.gov/zika/transmission/index.html. Accessed February 29, 2016.
On February 1, 2016, the World Health Organization declared Zika virus a public health emergency of international concern due to clusters of microcephaly and neurologic manifestations in areas of Zika virus transmission.1 On February 8, the US Centers for Disease Control and Prevention (CDC) elevated its response to level 1, its highest.2
Case reports and guidelines have been published to help clinicians better understand the epidemiology, risk, and pathogenesis of Zika virus infection, but much is still unknown. Clinicians must be ready to address the concerns of international travelers and must also consider Zika virus in the differential diagnosis of fever in the returned traveler.
FLAVIVIRUSES: DENGUE, WEST NILE … ZIKA
Zika virus, a single-stranded RNA arthropod-borne virus (arbovirus), is transmitted by mosquitoes. It is a member of the flavivirus family, which consists of over 70 viruses including some well known for causing diseases in humans, such as dengue, yellow fever, Japanese encephalitis, and West Nile virus.3
Phylogenetically, Zika virus is most similar to and included in a clade with Spondweni virus, which, like Zika, originated in Africa.4 Genomic analysis has revealed an African and an Asian lineage. The Asian lineage is responsible for the current epidemic in the Pacific and the Western Hemisphere.4–6
OUT OF AFRICA AND ASIA
Zika virus is named after a forested area in present-day Uganda, where it was first isolated in a febrile rhesus monkey that was being used to study yellow fever.7 Further studies in the 1950s confirmed its transmission to humans, as 6% of the sera tested in Ugandans showed evidence of specific antibodies to the virus.8 In 1978, antibody prevalence studies showed that up to 40% of Nigerians had Zika virus-neutralizing antibodies.9 Over the next 38 years, scattered case reports and seroprevalence studies showed infections occurring throughout Africa and Asia.9–11
In 2007, the first case of Zika virus transmission outside of Asia and Africa occurred on Yap Island in the Federated States of Micronesia.10–12 No further transmission in the Pacific was noted for 6 years until an outbreak occurred in French Polynesia in 2013.13–15 The first time Zika virus was found in the Western Hemisphere was in January 2014, when an outbreak occurred on Chile’s Easter Island.16 Genomic analysis of the Zika virus isolated on Easter Island indicated it was most closely related to isolates from French Polynesia.16 In 2014, additional cases of Zika virus infection were reported in New Caledonia and the Cook Islands.13,14
In May 2015, the World Health Organization issued an epidemiologic alert in response to dramatic increases in the spread of Zika virus in Brazil.17 From Brazil, Zika virus has rapidly spread to most countries in South and Central America and the Caribbean (Figure 1).2,5,6
TRANSMITTED BY MOSQUITO
The Aedes (Stegomyia) genus of mosquitoes is a well-known source of transmission for several arboviruses, including yellow fever, dengue, chikungunya, and now Zika virus.18,19 Zika virus was originally isolated in Uganda from Aedes africanus mosquitoes.7,20 Subsequently, other species of Aedes mosquitoes have been shown to transmit Zika virus, with Aedes aegypti being the most important human vector.7,8,19–21
Another species, Aedes albopictus has been identified as a human vector in Gabon and is also suspected of being a vector in the Brazilian outbreak.22 Spread of A albopictus from Asia to Europe, the Mediterranean region, and the Americas, including 32 states in the United States, has increased the fear of potential spread of Zika virus infection to a more expansive geographic range.13,18,19 Local transmission may become established if local mosquitoes become infected when infected travelers return from endemic areas.23
OTHER ROUTES OF TRANSMISSION
While mosquito-borne transmission is the most common route of infection with Zika virus, human-to-human transmission has been documented. Potential routes of transmission include sexual intercourse, blood transfusions, and vertical (mother-to-child) transmission.
Sexual transmission. Replicative Zika virus particles were identified in the semen of a patient who presented with hematospermia in French Polynesia.24
Previously, there was a report of Zika virus being sexually transmitted from a US man who had returned from Senegal to his spouse, who had not traveled to a Zika virus-endemic region. Both patients became ill following vaginal intercourse, with the onset of the wife’s illness occurring 5 days after the onset of the husband’s illness. The husband was noted to have hematospermia.25 Neutralization testing for both patients confirmed infection with Zika virus.25
The first reported case of sexual transmission in the current outbreak in the United States occurred in a traveler returning to Texas from Venezuela.26 The CDC is currently investigating several other potential cases and an additional two laboratory-confirmed cases. All cases were in symptomatic male travelers who had condomless vaginal intercourse with their female partners after return from Zika virus-endemic areas.27
Blood transfusions. Several arboviruses are known to be transmitted via blood.
In French Polynesia, Zika virus RNA was present in 3% of blood donors.28,29 These blood donors had been screened and were asymptomatic at the time of donation. Twenty-six percent of donors who had Zika RNA reported an illness compatible with Zika virus infection in the 3 to 10 days before donation.28
Brazil has reported two cases of Zika virus infection through blood transfusion.30
In May 2015, the European Centers for Disease Control recommended that travelers to affected areas defer blood donation for 28 days.31 The Association of American Blood Banks has also recommended that travelers self-defer donating blood for 28 days after travel to an endemic area.32 Most recently the US Food and Drug Administration recommended a 4-week deferral for travelers to Zika virus-endemic areas and after resolution of symptoms for those who have had Zika virus infection.33 Additional guidance for donors who have had sexual contact with Zika virus-infected persons and areas with active transmission of Zika virus is also available.33
Vertical transmission. Perinatal and transplacental transmission have also been documented.34,35 The extent and frequency of the clinical manifestations of these infections are still being elucidated in light of reports of association with fetal abnormalities.
Although Zika virus has been detected in breast milk, no cases of transmission through breastfeeding have been reported. Currently, women are advised to continue to breastfeed in areas of known Zika virus transmission.34,36,37
IS USUALLY ASYMPTOMATIC OR CAUSES MILD SYMPTOMS
Most Zika virus infections are asymptomatic, as illustrated by reports from the Yap Island outbreak, where only 19% of those with immunoglobulin M (IgM) antibodies to Zika virus had symptoms.12 The illness in symptomatic patients is often mild and self-limited, and most manifestations resolve by 7 days.12,25,38,39
Initial descriptions in the 1950s and 1960s of the clinical features of Zika virus infection in Africa included fever and headache as the most prominent symptoms.38,40 Description of the outbreak on Yap in 2007 characterized the predominant symptoms as rash, fever, arthralgia/arthritis, and nonpurulent conjunctivitis in 31 patients,12 and the current CDC case definition includes at least two of these four symptoms.41 The arthralgia and arthritis are usually of the small joints of the hands and feet and can persist for as long as a month.25,42 The rash can be pruritic.15,33,42,43
Less commonly reported manifestations of Zika virus infection include malaise, stomachaches, dizziness, anorexia, retro-orbital pain, aphthous ulcers, hematospermia, and prostatitis.14,15,24,25,44,45
The initial reports from eight patients in the outbreak in Brazil noted rash and joint pain as the most common manifestations. The maculopapular rash was present in all patients and the joint pain was characterized as severe, with the hands, ankles, elbows, knees, and wrists most consistently described.43
The clinical presentation is similar to those of dengue and chikungunya virus infections, confounding diagnosis, as these viruses may be cocirculating in the same geographic regions (and indeed are transmitted by the same mosquito vectors).11,12,15 The conjunctivitis present in Zika virus infections can also be present in chikungunya but is much less commonly a clinical feature of dengue.15,46,47 See Table 1 for the differential diagnosis of Zika virus infection.
Severe manifestations requiring hospitalization or resulting in death are thought to be uncommon, although neurologic and fetal complications have recently been described.12,29,43,48,49
CLINICAL ASSOCIATIONS
Primary infection with Zika virus is relatively benign. The greatest and most recent concerns are related to postinfectious complications and those that may occur in pregnant women.
Guillain-Barré syndrome
During the Zika virus outbreak in French Polynesia in 2013–2014, the incidence of Guillain-Barré syndrome was multiplied by a factor of 20.50 Prior to the first hospitalization of a patient with Zika virus infection and associated Guillain-Barré syndrome in French Polynesia, there had been no reported hospitalizations for Zika virus infection.50
This same association is now being seen in the recent outbreak in the Americas.50 In July 2015, Brazilian health officials in the State of Bahia reported 76 patients with neurologic syndromes, of whom 55% had Guillain-Barré syndrome.51 A history consistent with Zika virus infection was found in 62%.48
In January 2016, El Salvador also reported an unusual increase in Guillain-Barré syndrome cases since early December 2015.51 Between December 1, 2015, and January 6, 2016, there were 46 Guillain-Barré syndrome cases reported, compared with a baseline of 14 cases per month.51
Other countries where Zika virus infection is endemic are also currently investigating similar trends.51
Microcephaly
On November 17, 2015, the Pan American Health Organization issued an epidemiologic alert because of increased reports of microcephaly in the Pernambuco State of Brazil. Whereas there are typically about 10 cases per year, there had been 141 in the previous 11 months.51 Other states in Brazil such as Paraiba and Rio Grande del Norte also reported increases in the diagnosis of microcephaly. A physician alert published in Brazil described two infants from the Paraiba state who were diagnosed with fetal microcephaly.35 Testing for Zika virus by polymerase chain reaction (PCR) was negative in the maternal blood, but PCR of amniotic fluid was positive in both infants.35
In January 2016, the Brazil Ministry of Health reported that Zika virus had been detected by real-time PCR (RT-PCR) in four infants with congenital malformations in Rio Grande del Norte. Two of these cases were miscarriages and two were infants who died within 24 hours of birth. Immunohistochemistry of tissues from these infants was positive for Zika virus.
A February 2016 case report describes a European woman who developed Zika virus infection at 13 weeks gestation while working in Northeast Brazil and upon return to Europe elected to terminate the pregnancy after ultrasonography showed cerebral calcifications with microcephaly. The infant was found to have a very small brain, hypoplasia of the brainstem and spinal cord with degeneration of spinal tracts, complete absence of cerebral gyri, and severe dilatation of lateral ventricles as well as calcifications throughout the cerebral cortex.49 No genetic abnormalities or evidence of other etiologies was found, and large amounts of Zika virus RNA were found in the brain.
The CDC also recently reported confirmation of Zika virus infection from fetal tissues of two miscarriages (fetal loss at 11 and 13 weeks) and two fetal deaths (36 and 38 weeks) received from the state of Rio Grande do Norte in Brazil.52 All four mothers reported clinical signs of fever and rash during their first trimester of pregnancy.52 Additional testing for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, and human immunodeficiency virus were all negative in the mothers who had miscarriages.52
Of critical note, the causality of Zika virus and microcephaly remains under investigation. See Table 2 for other causes of microcephaly.53
Macular atrophy
In January 2016, a case series of three infants with microcephaly and macular atrophy was reported.54 These infants were tested for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, syphilis, and human immunodeficiency virus (HIV), and all the results were negative. The detection of Zika virus fulfilled the Brazilian Ministry of Health’s definition of vertical transmission of Zika virus, and laboratory diagnostic tests for Zika virus were not performed. In this series, one mother reported an illness with rash and arthralgias during the first trimester.54
LABORATORY DIAGNOSTIC METHODS
The diagnosis of Zika virus infection is challenging. The low viremia at initial presentation and cross-reactivity of serologic testing with other flaviviruses, especially dengue, can contribute to misdiagnosis.40,50
In the first 7 days of Zika virus infection, the diagnosis is based on detection of viral RNA in serum by RT-PCR.12,55,56 RT-PCR is very specific for Zika virus and is an important tool in differentiating between Zika virus and other flaviviruses often present in areas where Zika virus is circulating.12,56 After 3 to 4 days, viremia may decrease to levels that may be below the assay’s level of detection.40–42,45
While Zika virus RNA may be undetectable in the serum, other samples such as saliva, urine, and semen may be positive for longer.28,42,57 For example, urine samples were positive by RT-PCR up to 7 days beyond blood RT-PCR in the outbreak in New Caledonia.42 A recent report found semen remaining positive on RT-PCR for 62 days after the onset of confirmed Zika virus illness in a traveler returning to the United Kingdom from the Cook Islands in 2014.58
Because RT-PCR of blood is only useful early in infection, the current diagnostic guidelines recommend testing an acute-phase serum sample for Zika virus IgM collected as early as possible after the onset of illness and repeated 2 to 3 weeks after the initial set. These IgM antibodies typically develop toward the end of the first week of illness and are expected to be present for up to 12 weeks, based on experience with other flaviviruses.41 Cross-reactivity with other flaviviruses circulating in the area can occur and has been problematic in areas where dengue is circulating.12,41,45,56 IgM-positive specimens should be further tested, by plaque-reduction neutralization, to confirm the presence of Zika virus-specific neutralizing antibodies. Results can be difficult to interpret, especially in those who have been previously infected or vaccinated against other flaviviruses.12,41
If amniocentesis is done, these specimens should be tested by RT-PCR. However, the sensitivity of PCR in amniotic fluid is currently unknown.41
In infants with findings of cerebral calcifications and microcephaly, IgM serologies with RT-PCR are also recommended and should be drawn within 2 days of birth. Specimens should be drawn concurrently as it is not known which test is most reliable in infants.23 Additionally, placenta and umbilical cord samples should be collected for immunohistochemical staining at specialized laboratories.36
In the United States, providers should contact their state health departments to determine where tests can be run reliably. Refined diagnostic assays are in development at the time of this publication and are likely to be made available through CDC’s Laboratory Response Network.
See Figure 2 and Table 3 for a summary of diagnostic tests.
IMPLICATIONS, RECOMMENDATIONS
Pregnant women
The CDC now recommends that asymptomatic pregnant women who returned from travel to a Zika virus-endemic zone in the last 2 to 12 weeks be offered serologic testing.41 This includes women who may be living in an area with ongoing Zika virus transmission; however, these women should also have testing at the initiation of prenatal care and then follow-up testing in the middle of the second trimester. Of importance, these results may be difficult to interpret due to potential cross-reactivity between Zika virus and other flaviviruses, and false-positive results in recipients of yellow fever and Japanese encephalitis vaccines.41,59
If a pregnant woman with a positive travel history is symptomatic, testing should be offered during the first week of illness. After day 4 of the illness, testing should include both RT-PCR and IgM serology.41,59
A screening ultrasound scan is recommended for any pregnant woman who has traveled to a Zika virus-affected area to determine if microcephaly or cerebral or intracranial calcifications are present. Those women with confirmed Zika virus infection should continue to have monthly screening ultrasounds, while those who are negative for Zika virus should have another ultrasound at the end of the second trimester or the beginning of the third trimester to ensure that no abnormalities had developed.41,59
At present, pregnant women and women of childbearing age who may become pregnant are advised by the CDC to postpone travel to affected areas until more information becomes available about mother-to-child transmission.59
Algorithms for the care of pregnant women and women of childbearing age who may have been exposed to Zika virus are available from the CDC41 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e2.htm.
Male partners of pregnant women
Since the length of time that Zika virus remains viable in semen is not known, men who have traveled to Zika virus-endemic areas and who have pregnant partners should refrain from having sex or use a condom with every sexual encounter through the duration of the pregnancy.60
Guidelines for prevention of sexual transmission of Zika virus are available from the CDC59 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e1er.htm.
Infants with possible congenital Zika virus infection
Zika virus testing is recommended for any infant born with microcephaly or intracranial calcifications or whose mother has positive or inconclusive testing if the mother had visited an endemic area during her pregnancy.
Zika virus testing in infants consists of serologic IgM determination and RT-PCR for both dengue and Zika virus drawn concurrently in the first 2 days of life.36 Umbilical cord blood can be used. In addition, if cerebrospinal fluid is being collected for other reasons, it can also be tested for Zika virus. The placenta and umbilical cord should be saved for immunohistochemistry testing for Zika virus.61
An infant who tests positive or inconclusive for Zika virus, regardless of the presence of microcephaly or intracranial calcifications, should have a complete physical examination specifically evaluating growth parameters, estimated gestational age, and signs of neurologic disease, skin rashes, hepatosplenomegaly, or any dysmorphic features. Additional evaluation includes an ophthalmologic examination in the first month of life to evaluate for macular atrophy.36 An ultrasound scan of the head should be completed if it has not been done. Hearing is screened in all newborns, and hearing testing should be repeated at 6 months of age.36
Infants with microcephaly or intracranial calcifications should also have consultations with specialists in genetics, neurology, and pediatric infectious diseases.61 These infants should have blood work including complete blood cell counts and liver function testing that includes alanine aminotransferase, aspartate aminotransferase, and bilirubin levels.36
All infants with possible congenital Zika virus infection should be followed long-term with close attention to developmental milestones and growth parameters including occipital frontal head circumference measurements.61,62
Infants without microcephaly or calcifications whose mothers had negative Zika virus test results or were not tested for Zika virus should have routine care.37
Guidelines for the care of infants with Zika virus infection are available from the CDC36 at www.cdc.gov/mmwr/volumes/65/wr/mm6503e3.htm.
TREATMENT
There is no treatment for Zika virus infection, and care is supportive. Most infections are mild and self-limited.12,15 Avoidance of aspirin and other nonsteroidal anti-inflammatory drugs that may affect platelets is important until dengue infection has been ruled out.
PREVENTION
There is currently no vaccine to prevent Zika virus infection. Woman who are pregnant should avoid travel to any area where Zika virus transmission is occurring.41,59 The CDC advises pregnant women and women of childbearing age who may become pregnant to postpone travel to Zika virus-affected areas.59 Patients can find travel alerts for specific areas at wwwnc.cdc.gov/travel/notices/alert/zika-virus-south-america.
Avoiding mosquito bites is the best way to prevent the spread of Zika virus. Aedes aegypti and A albopictus, the most common vectors of Zika virus, can bite at night but are known more for being aggressive daytime biters.63 Travelers should apply an Environmental Protection Agency-registered insect repellent as directed, wear long-sleeved shirts and long pants, use permethrin-treated clothing and gear, and stay in places with screens or air conditioning. Any containers with standing water should be eliminated as they are breeding areas for mosquitoes. It is also important that symptomatic people in the first week of illness use mosquito precautions to prevent the spread of Zika virus.
Patient handouts and posters for mosquito bite prevention can be found at www.cdc.gov/zika/fs-posters/index.html.
WATCH FOR UPDATES
Many questions remain regarding the epidemiology of this infection and its relationship to neurologic and pregnancy complications. However, due to its rapid spread across the Western hemisphere and its potential for significant complications, much is being done at the local and international levels to better understand the virus and halt its spread. More information will continue to be available as results from ongoing studies are conducted and potential associations are investigated. Until more is known, providers should familiarize themselves with the latest guidelines in order to better counsel their patients who may live in or travel to Zika virus endemic areas. We advise clinicians to follow the CDC’s web site, www.cdc.gov/zika/.
On February 1, 2016, the World Health Organization declared Zika virus a public health emergency of international concern due to clusters of microcephaly and neurologic manifestations in areas of Zika virus transmission.1 On February 8, the US Centers for Disease Control and Prevention (CDC) elevated its response to level 1, its highest.2
Case reports and guidelines have been published to help clinicians better understand the epidemiology, risk, and pathogenesis of Zika virus infection, but much is still unknown. Clinicians must be ready to address the concerns of international travelers and must also consider Zika virus in the differential diagnosis of fever in the returned traveler.
FLAVIVIRUSES: DENGUE, WEST NILE … ZIKA
Zika virus, a single-stranded RNA arthropod-borne virus (arbovirus), is transmitted by mosquitoes. It is a member of the flavivirus family, which consists of over 70 viruses including some well known for causing diseases in humans, such as dengue, yellow fever, Japanese encephalitis, and West Nile virus.3
Phylogenetically, Zika virus is most similar to and included in a clade with Spondweni virus, which, like Zika, originated in Africa.4 Genomic analysis has revealed an African and an Asian lineage. The Asian lineage is responsible for the current epidemic in the Pacific and the Western Hemisphere.4–6
OUT OF AFRICA AND ASIA
Zika virus is named after a forested area in present-day Uganda, where it was first isolated in a febrile rhesus monkey that was being used to study yellow fever.7 Further studies in the 1950s confirmed its transmission to humans, as 6% of the sera tested in Ugandans showed evidence of specific antibodies to the virus.8 In 1978, antibody prevalence studies showed that up to 40% of Nigerians had Zika virus-neutralizing antibodies.9 Over the next 38 years, scattered case reports and seroprevalence studies showed infections occurring throughout Africa and Asia.9–11
In 2007, the first case of Zika virus transmission outside of Asia and Africa occurred on Yap Island in the Federated States of Micronesia.10–12 No further transmission in the Pacific was noted for 6 years until an outbreak occurred in French Polynesia in 2013.13–15 The first time Zika virus was found in the Western Hemisphere was in January 2014, when an outbreak occurred on Chile’s Easter Island.16 Genomic analysis of the Zika virus isolated on Easter Island indicated it was most closely related to isolates from French Polynesia.16 In 2014, additional cases of Zika virus infection were reported in New Caledonia and the Cook Islands.13,14
In May 2015, the World Health Organization issued an epidemiologic alert in response to dramatic increases in the spread of Zika virus in Brazil.17 From Brazil, Zika virus has rapidly spread to most countries in South and Central America and the Caribbean (Figure 1).2,5,6
TRANSMITTED BY MOSQUITO
The Aedes (Stegomyia) genus of mosquitoes is a well-known source of transmission for several arboviruses, including yellow fever, dengue, chikungunya, and now Zika virus.18,19 Zika virus was originally isolated in Uganda from Aedes africanus mosquitoes.7,20 Subsequently, other species of Aedes mosquitoes have been shown to transmit Zika virus, with Aedes aegypti being the most important human vector.7,8,19–21
Another species, Aedes albopictus has been identified as a human vector in Gabon and is also suspected of being a vector in the Brazilian outbreak.22 Spread of A albopictus from Asia to Europe, the Mediterranean region, and the Americas, including 32 states in the United States, has increased the fear of potential spread of Zika virus infection to a more expansive geographic range.13,18,19 Local transmission may become established if local mosquitoes become infected when infected travelers return from endemic areas.23
OTHER ROUTES OF TRANSMISSION
While mosquito-borne transmission is the most common route of infection with Zika virus, human-to-human transmission has been documented. Potential routes of transmission include sexual intercourse, blood transfusions, and vertical (mother-to-child) transmission.
Sexual transmission. Replicative Zika virus particles were identified in the semen of a patient who presented with hematospermia in French Polynesia.24
Previously, there was a report of Zika virus being sexually transmitted from a US man who had returned from Senegal to his spouse, who had not traveled to a Zika virus-endemic region. Both patients became ill following vaginal intercourse, with the onset of the wife’s illness occurring 5 days after the onset of the husband’s illness. The husband was noted to have hematospermia.25 Neutralization testing for both patients confirmed infection with Zika virus.25
The first reported case of sexual transmission in the current outbreak in the United States occurred in a traveler returning to Texas from Venezuela.26 The CDC is currently investigating several other potential cases and an additional two laboratory-confirmed cases. All cases were in symptomatic male travelers who had condomless vaginal intercourse with their female partners after return from Zika virus-endemic areas.27
Blood transfusions. Several arboviruses are known to be transmitted via blood.
In French Polynesia, Zika virus RNA was present in 3% of blood donors.28,29 These blood donors had been screened and were asymptomatic at the time of donation. Twenty-six percent of donors who had Zika RNA reported an illness compatible with Zika virus infection in the 3 to 10 days before donation.28
Brazil has reported two cases of Zika virus infection through blood transfusion.30
In May 2015, the European Centers for Disease Control recommended that travelers to affected areas defer blood donation for 28 days.31 The Association of American Blood Banks has also recommended that travelers self-defer donating blood for 28 days after travel to an endemic area.32 Most recently the US Food and Drug Administration recommended a 4-week deferral for travelers to Zika virus-endemic areas and after resolution of symptoms for those who have had Zika virus infection.33 Additional guidance for donors who have had sexual contact with Zika virus-infected persons and areas with active transmission of Zika virus is also available.33
Vertical transmission. Perinatal and transplacental transmission have also been documented.34,35 The extent and frequency of the clinical manifestations of these infections are still being elucidated in light of reports of association with fetal abnormalities.
Although Zika virus has been detected in breast milk, no cases of transmission through breastfeeding have been reported. Currently, women are advised to continue to breastfeed in areas of known Zika virus transmission.34,36,37
IS USUALLY ASYMPTOMATIC OR CAUSES MILD SYMPTOMS
Most Zika virus infections are asymptomatic, as illustrated by reports from the Yap Island outbreak, where only 19% of those with immunoglobulin M (IgM) antibodies to Zika virus had symptoms.12 The illness in symptomatic patients is often mild and self-limited, and most manifestations resolve by 7 days.12,25,38,39
Initial descriptions in the 1950s and 1960s of the clinical features of Zika virus infection in Africa included fever and headache as the most prominent symptoms.38,40 Description of the outbreak on Yap in 2007 characterized the predominant symptoms as rash, fever, arthralgia/arthritis, and nonpurulent conjunctivitis in 31 patients,12 and the current CDC case definition includes at least two of these four symptoms.41 The arthralgia and arthritis are usually of the small joints of the hands and feet and can persist for as long as a month.25,42 The rash can be pruritic.15,33,42,43
Less commonly reported manifestations of Zika virus infection include malaise, stomachaches, dizziness, anorexia, retro-orbital pain, aphthous ulcers, hematospermia, and prostatitis.14,15,24,25,44,45
The initial reports from eight patients in the outbreak in Brazil noted rash and joint pain as the most common manifestations. The maculopapular rash was present in all patients and the joint pain was characterized as severe, with the hands, ankles, elbows, knees, and wrists most consistently described.43
The clinical presentation is similar to those of dengue and chikungunya virus infections, confounding diagnosis, as these viruses may be cocirculating in the same geographic regions (and indeed are transmitted by the same mosquito vectors).11,12,15 The conjunctivitis present in Zika virus infections can also be present in chikungunya but is much less commonly a clinical feature of dengue.15,46,47 See Table 1 for the differential diagnosis of Zika virus infection.
Severe manifestations requiring hospitalization or resulting in death are thought to be uncommon, although neurologic and fetal complications have recently been described.12,29,43,48,49
CLINICAL ASSOCIATIONS
Primary infection with Zika virus is relatively benign. The greatest and most recent concerns are related to postinfectious complications and those that may occur in pregnant women.
Guillain-Barré syndrome
During the Zika virus outbreak in French Polynesia in 2013–2014, the incidence of Guillain-Barré syndrome was multiplied by a factor of 20.50 Prior to the first hospitalization of a patient with Zika virus infection and associated Guillain-Barré syndrome in French Polynesia, there had been no reported hospitalizations for Zika virus infection.50
This same association is now being seen in the recent outbreak in the Americas.50 In July 2015, Brazilian health officials in the State of Bahia reported 76 patients with neurologic syndromes, of whom 55% had Guillain-Barré syndrome.51 A history consistent with Zika virus infection was found in 62%.48
In January 2016, El Salvador also reported an unusual increase in Guillain-Barré syndrome cases since early December 2015.51 Between December 1, 2015, and January 6, 2016, there were 46 Guillain-Barré syndrome cases reported, compared with a baseline of 14 cases per month.51
Other countries where Zika virus infection is endemic are also currently investigating similar trends.51
Microcephaly
On November 17, 2015, the Pan American Health Organization issued an epidemiologic alert because of increased reports of microcephaly in the Pernambuco State of Brazil. Whereas there are typically about 10 cases per year, there had been 141 in the previous 11 months.51 Other states in Brazil such as Paraiba and Rio Grande del Norte also reported increases in the diagnosis of microcephaly. A physician alert published in Brazil described two infants from the Paraiba state who were diagnosed with fetal microcephaly.35 Testing for Zika virus by polymerase chain reaction (PCR) was negative in the maternal blood, but PCR of amniotic fluid was positive in both infants.35
In January 2016, the Brazil Ministry of Health reported that Zika virus had been detected by real-time PCR (RT-PCR) in four infants with congenital malformations in Rio Grande del Norte. Two of these cases were miscarriages and two were infants who died within 24 hours of birth. Immunohistochemistry of tissues from these infants was positive for Zika virus.
A February 2016 case report describes a European woman who developed Zika virus infection at 13 weeks gestation while working in Northeast Brazil and upon return to Europe elected to terminate the pregnancy after ultrasonography showed cerebral calcifications with microcephaly. The infant was found to have a very small brain, hypoplasia of the brainstem and spinal cord with degeneration of spinal tracts, complete absence of cerebral gyri, and severe dilatation of lateral ventricles as well as calcifications throughout the cerebral cortex.49 No genetic abnormalities or evidence of other etiologies was found, and large amounts of Zika virus RNA were found in the brain.
The CDC also recently reported confirmation of Zika virus infection from fetal tissues of two miscarriages (fetal loss at 11 and 13 weeks) and two fetal deaths (36 and 38 weeks) received from the state of Rio Grande do Norte in Brazil.52 All four mothers reported clinical signs of fever and rash during their first trimester of pregnancy.52 Additional testing for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, and human immunodeficiency virus were all negative in the mothers who had miscarriages.52
Of critical note, the causality of Zika virus and microcephaly remains under investigation. See Table 2 for other causes of microcephaly.53
Macular atrophy
In January 2016, a case series of three infants with microcephaly and macular atrophy was reported.54 These infants were tested for toxoplasmosis, rubella, cytomegalovirus, herpes simplex, syphilis, and human immunodeficiency virus (HIV), and all the results were negative. The detection of Zika virus fulfilled the Brazilian Ministry of Health’s definition of vertical transmission of Zika virus, and laboratory diagnostic tests for Zika virus were not performed. In this series, one mother reported an illness with rash and arthralgias during the first trimester.54
LABORATORY DIAGNOSTIC METHODS
The diagnosis of Zika virus infection is challenging. The low viremia at initial presentation and cross-reactivity of serologic testing with other flaviviruses, especially dengue, can contribute to misdiagnosis.40,50
In the first 7 days of Zika virus infection, the diagnosis is based on detection of viral RNA in serum by RT-PCR.12,55,56 RT-PCR is very specific for Zika virus and is an important tool in differentiating between Zika virus and other flaviviruses often present in areas where Zika virus is circulating.12,56 After 3 to 4 days, viremia may decrease to levels that may be below the assay’s level of detection.40–42,45
While Zika virus RNA may be undetectable in the serum, other samples such as saliva, urine, and semen may be positive for longer.28,42,57 For example, urine samples were positive by RT-PCR up to 7 days beyond blood RT-PCR in the outbreak in New Caledonia.42 A recent report found semen remaining positive on RT-PCR for 62 days after the onset of confirmed Zika virus illness in a traveler returning to the United Kingdom from the Cook Islands in 2014.58
Because RT-PCR of blood is only useful early in infection, the current diagnostic guidelines recommend testing an acute-phase serum sample for Zika virus IgM collected as early as possible after the onset of illness and repeated 2 to 3 weeks after the initial set. These IgM antibodies typically develop toward the end of the first week of illness and are expected to be present for up to 12 weeks, based on experience with other flaviviruses.41 Cross-reactivity with other flaviviruses circulating in the area can occur and has been problematic in areas where dengue is circulating.12,41,45,56 IgM-positive specimens should be further tested, by plaque-reduction neutralization, to confirm the presence of Zika virus-specific neutralizing antibodies. Results can be difficult to interpret, especially in those who have been previously infected or vaccinated against other flaviviruses.12,41
If amniocentesis is done, these specimens should be tested by RT-PCR. However, the sensitivity of PCR in amniotic fluid is currently unknown.41
In infants with findings of cerebral calcifications and microcephaly, IgM serologies with RT-PCR are also recommended and should be drawn within 2 days of birth. Specimens should be drawn concurrently as it is not known which test is most reliable in infants.23 Additionally, placenta and umbilical cord samples should be collected for immunohistochemical staining at specialized laboratories.36
In the United States, providers should contact their state health departments to determine where tests can be run reliably. Refined diagnostic assays are in development at the time of this publication and are likely to be made available through CDC’s Laboratory Response Network.
See Figure 2 and Table 3 for a summary of diagnostic tests.
IMPLICATIONS, RECOMMENDATIONS
Pregnant women
The CDC now recommends that asymptomatic pregnant women who returned from travel to a Zika virus-endemic zone in the last 2 to 12 weeks be offered serologic testing.41 This includes women who may be living in an area with ongoing Zika virus transmission; however, these women should also have testing at the initiation of prenatal care and then follow-up testing in the middle of the second trimester. Of importance, these results may be difficult to interpret due to potential cross-reactivity between Zika virus and other flaviviruses, and false-positive results in recipients of yellow fever and Japanese encephalitis vaccines.41,59
If a pregnant woman with a positive travel history is symptomatic, testing should be offered during the first week of illness. After day 4 of the illness, testing should include both RT-PCR and IgM serology.41,59
A screening ultrasound scan is recommended for any pregnant woman who has traveled to a Zika virus-affected area to determine if microcephaly or cerebral or intracranial calcifications are present. Those women with confirmed Zika virus infection should continue to have monthly screening ultrasounds, while those who are negative for Zika virus should have another ultrasound at the end of the second trimester or the beginning of the third trimester to ensure that no abnormalities had developed.41,59
At present, pregnant women and women of childbearing age who may become pregnant are advised by the CDC to postpone travel to affected areas until more information becomes available about mother-to-child transmission.59
Algorithms for the care of pregnant women and women of childbearing age who may have been exposed to Zika virus are available from the CDC41 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e2.htm.
Male partners of pregnant women
Since the length of time that Zika virus remains viable in semen is not known, men who have traveled to Zika virus-endemic areas and who have pregnant partners should refrain from having sex or use a condom with every sexual encounter through the duration of the pregnancy.60
Guidelines for prevention of sexual transmission of Zika virus are available from the CDC59 at www.cdc.gov/mmwr/volumes/65/wr/mm6505e1er.htm.
Infants with possible congenital Zika virus infection
Zika virus testing is recommended for any infant born with microcephaly or intracranial calcifications or whose mother has positive or inconclusive testing if the mother had visited an endemic area during her pregnancy.
Zika virus testing in infants consists of serologic IgM determination and RT-PCR for both dengue and Zika virus drawn concurrently in the first 2 days of life.36 Umbilical cord blood can be used. In addition, if cerebrospinal fluid is being collected for other reasons, it can also be tested for Zika virus. The placenta and umbilical cord should be saved for immunohistochemistry testing for Zika virus.61
An infant who tests positive or inconclusive for Zika virus, regardless of the presence of microcephaly or intracranial calcifications, should have a complete physical examination specifically evaluating growth parameters, estimated gestational age, and signs of neurologic disease, skin rashes, hepatosplenomegaly, or any dysmorphic features. Additional evaluation includes an ophthalmologic examination in the first month of life to evaluate for macular atrophy.36 An ultrasound scan of the head should be completed if it has not been done. Hearing is screened in all newborns, and hearing testing should be repeated at 6 months of age.36
Infants with microcephaly or intracranial calcifications should also have consultations with specialists in genetics, neurology, and pediatric infectious diseases.61 These infants should have blood work including complete blood cell counts and liver function testing that includes alanine aminotransferase, aspartate aminotransferase, and bilirubin levels.36
All infants with possible congenital Zika virus infection should be followed long-term with close attention to developmental milestones and growth parameters including occipital frontal head circumference measurements.61,62
Infants without microcephaly or calcifications whose mothers had negative Zika virus test results or were not tested for Zika virus should have routine care.37
Guidelines for the care of infants with Zika virus infection are available from the CDC36 at www.cdc.gov/mmwr/volumes/65/wr/mm6503e3.htm.
TREATMENT
There is no treatment for Zika virus infection, and care is supportive. Most infections are mild and self-limited.12,15 Avoidance of aspirin and other nonsteroidal anti-inflammatory drugs that may affect platelets is important until dengue infection has been ruled out.
PREVENTION
There is currently no vaccine to prevent Zika virus infection. Woman who are pregnant should avoid travel to any area where Zika virus transmission is occurring.41,59 The CDC advises pregnant women and women of childbearing age who may become pregnant to postpone travel to Zika virus-affected areas.59 Patients can find travel alerts for specific areas at wwwnc.cdc.gov/travel/notices/alert/zika-virus-south-america.
Avoiding mosquito bites is the best way to prevent the spread of Zika virus. Aedes aegypti and A albopictus, the most common vectors of Zika virus, can bite at night but are known more for being aggressive daytime biters.63 Travelers should apply an Environmental Protection Agency-registered insect repellent as directed, wear long-sleeved shirts and long pants, use permethrin-treated clothing and gear, and stay in places with screens or air conditioning. Any containers with standing water should be eliminated as they are breeding areas for mosquitoes. It is also important that symptomatic people in the first week of illness use mosquito precautions to prevent the spread of Zika virus.
Patient handouts and posters for mosquito bite prevention can be found at www.cdc.gov/zika/fs-posters/index.html.
WATCH FOR UPDATES
Many questions remain regarding the epidemiology of this infection and its relationship to neurologic and pregnancy complications. However, due to its rapid spread across the Western hemisphere and its potential for significant complications, much is being done at the local and international levels to better understand the virus and halt its spread. More information will continue to be available as results from ongoing studies are conducted and potential associations are investigated. Until more is known, providers should familiarize themselves with the latest guidelines in order to better counsel their patients who may live in or travel to Zika virus endemic areas. We advise clinicians to follow the CDC’s web site, www.cdc.gov/zika/.
- World Health Organization. Zika virus fact sheet. www.who.int/mediacentre/factsheets/zika/en/. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Areas with Zika. www.cdc.gov/zika/geo/index.html. Accessed February 24, 2016.
- Rice CM. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, Chanock RM, editors. Fields Virology, 3rd ed. Philadelphia: Lippincott-Raven, 1996:961–1034.
- Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol 1998; 72:73–83.
- Haddow AD, Schuh AJ, Yasuda CY, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 2012; 6:e1477.
- Faye O, Freire CC, Iamarino A, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 2014; 8:e2636.
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–520.
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46:521–534.
- Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979; 83:213–219.
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009; 15:1347–1350.
- Heang V, Yasuda CY, Sovann L, et al. Zika virus infection, Cambodia, 2010. Emerg Infect Dis 2012; 18:349–351.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–2543.
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infec 2014; 20:O595–O596.
- Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20:1085–1086.
- Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 2014; 44:302–307.
- Tognarelli J, Ulloa S, Villagra E, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol Nov 26 2015 [Epub ahead of print].
- Pan American Health Organization/World Health Organization, Regional Office for the Americas. Zika virus infection. 7 May 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=30075=en. Accessed February 24, 2016.
- Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 2016; 347:601–604.
- Marcondes CB, Ximenes MF. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop. Dec 22 2015. pii: S0037-86822015005003102. [Epub ahead of print]
- Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg 1958; 52:263–268.
- Diallo D, Sall AA, Diagne CT, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One 2014; 9:e109442.
- Grard G, Caron M, Mombo IM, et al. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 2014; 8:e2681.
- Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR 2016; 65:55–58.
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015; 21:359–361.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–882.
- Smith J, Woldai S, Chung W. Health advisory: sexual transmission of Zika virus. Dallas Country Department of Health and Human Services, February 2, 2016. http://walnuthillobgyn.com/wp-content/uploads/2012/05/zika-transmission.pdf. Accessed February 24, 2016.
- Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Early release February 26, 2016. www.cdc.gov/mmwr/volumes/65/wr/mm6508e2er.htm Accessed February 29, 2016.
- Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 2014; 19(14). pii: 20761. Erratum in Euro Surveill 2014; 19(15). pii/20771.
- Marano G, Pupella S, Vaglio S, Liumbruno GM, Grazzini G. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus 2015; Nov 5:1–6. doi: 10.2450/2015.0066-15. [Epub ahead of print]
- European Centre for Disease Prevention and Control. Epidemiological update: complications potentially linked to Zika virus outbreak, Brazil and French Polynesia. November 27, 2015. http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?ID=1332&List=8db7286c-fe2d-476c-9133-18ff4cb1b568&Source=http%3A%2F%2Fecdc%2Eeuropa%2Eeu%2Fen%2Fpress%2Fepidemiological%5Fupdates%2FPages%2Fepidemiological%5Fupdates%2Easpx. Accessed February 24, 2016
- European Centre for Disease Prevention and Control. Rapid risk assessment. Zika virus infection outbreak, Brazil and the Pacific region 25 May 2015. http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-Zika%20virus-south-america-Brazil-2015.pdf. Accessed February 24, 2016
- Regan DM, Markowitz MA. Association Bulletin #16-03. Re: Zika, dengue, and chikungunya viruses. American Association of Blood Banks, February 1, 2016. www.aabb.org/programs/publications/bulletins/Documents/ab16-03.pdf. Accessed February 24, 2016.
- US Food and Drug Administration (FDA). Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. February, 2016. www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Accessed February 24, 2016.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014; 19(13). pii: 20751.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016; 47:6–7.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Fleming-Dutra K, Nelson J, Fischer M, Staples J, Mateusz P, et al. Update: interim guidelines for health care providers caring for infants and children with possible Zika virus infection—United States, February 2016. MMWR 2016; 65:1–6.
- Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg Jul 1964; 58:335–338.
- Olson JG, Ksiazek TG, Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 1981; 75:389–393.
- Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg 1956; 50:442–448.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR 2016; 65:122–127.
- Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis 2015; 21:84–86.
- Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–572.
- Alera MT, Hermann L, Tac-An IA, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis 2015; 21:722–724.
- Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–1239.
- Centers for Disease Control and Prevention. Chikungunya virus. Clinical evaluation & disease. www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Clinical guidance. Dengue virus. www.cdc.gov/dengue/clinicalLab/clinical.html. Accessed February 24, 2016.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Increase in microcephaly in the northeast of Brazil. November 17, 2015. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32636&lang=en. Accessed February 24, 2016.
- Rubin EJ, Greene MF, Baden LR. Zika virus and microcephaly. N Engl J Med 2016; Feb 10 [Epub ahead of print].
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barré syndrome—case report, French Polynesia, December 2013. Euro Surveill 2014; 19(9). pii: 20720.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1, 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed February 24, 2016.
- Martines R, Bhatnagar J, Keating M, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMRW 2016; 65:159–160.
- Ashwal S, Michelson D, Plawner L, Dobyns WB; Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Practice parameter: evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2009; 73:887–897.
- Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016; 387:228.
- Centers for Disease Control and Prevention. Updated diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. http://stacks.cdc.gov/view/cdc/37594. Accessed February 24, 2016.
- Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 2013; 10:311.
- Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol 2015; 68:53–55.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen [letter]. Emerg Infect Dis 2016 May. http://wwwnc.cdc.gov/eid/article/22/5/16-0107_article. Accessed February 24, 2016.
- Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR 2016; 65:30–33.
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR 2016; 65:120–121.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Centers for Disease Control and Prevention. Zika virus clinical evaluation and disease. www.cdc.gov/zika/hc-providers/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Zika virus. Transmission & risks. www.cdc.gov/zika/transmission/index.html. Accessed February 29, 2016.
- World Health Organization. Zika virus fact sheet. www.who.int/mediacentre/factsheets/zika/en/. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Areas with Zika. www.cdc.gov/zika/geo/index.html. Accessed February 24, 2016.
- Rice CM. Flaviviruses. In: Fields BN, Knipe DM, Howley PM, Chanock RM, editors. Fields Virology, 3rd ed. Philadelphia: Lippincott-Raven, 1996:961–1034.
- Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol 1998; 72:73–83.
- Haddow AD, Schuh AJ, Yasuda CY, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Negl Trop Dis 2012; 6:e1477.
- Faye O, Freire CC, Iamarino A, et al. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis 2014; 8:e2636.
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952; 46:509–520.
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952; 46:521–534.
- Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. J Hyg (Lond) 1979; 83:213–219.
- Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009; 15:1347–1350.
- Heang V, Yasuda CY, Sovann L, et al. Zika virus infection, Cambodia, 2010. Emerg Infect Dis 2012; 18:349–351.
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–2543.
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infec 2014; 20:O595–O596.
- Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South Pacific, 2013. Emerg Infect Dis 2014; 20:1085–1086.
- Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect 2014; 44:302–307.
- Tognarelli J, Ulloa S, Villagra E, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol Nov 26 2015 [Epub ahead of print].
- Pan American Health Organization/World Health Organization, Regional Office for the Americas. Zika virus infection. 7 May 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=30075=en. Accessed February 24, 2016.
- Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 2016; 347:601–604.
- Marcondes CB, Ximenes MF. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop. Dec 22 2015. pii: S0037-86822015005003102. [Epub ahead of print]
- Weinbren MP, Williams MC. Zika virus: further isolations in the Zika area, and some studies on the strains isolated. Trans R Soc Trop Med Hyg 1958; 52:263–268.
- Diallo D, Sall AA, Diagne CT, et al. Zika virus emergence in mosquitoes in southeastern Senegal, 2011. PLoS One 2014; 9:e109442.
- Grard G, Caron M, Mombo IM, et al. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 2014; 8:e2681.
- Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas—region of the Americas, May 2015–January 2016. MMWR 2016; 65:55–58.
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis 2015; 21:359–361.
- Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–882.
- Smith J, Woldai S, Chung W. Health advisory: sexual transmission of Zika virus. Dallas Country Department of Health and Human Services, February 2, 2016. http://walnuthillobgyn.com/wp-content/uploads/2012/05/zika-transmission.pdf. Accessed February 24, 2016.
- Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Early release February 26, 2016. www.cdc.gov/mmwr/volumes/65/wr/mm6508e2er.htm Accessed February 29, 2016.
- Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 2014; 19(14). pii: 20761. Erratum in Euro Surveill 2014; 19(15). pii/20771.
- Marano G, Pupella S, Vaglio S, Liumbruno GM, Grazzini G. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus 2015; Nov 5:1–6. doi: 10.2450/2015.0066-15. [Epub ahead of print]
- European Centre for Disease Prevention and Control. Epidemiological update: complications potentially linked to Zika virus outbreak, Brazil and French Polynesia. November 27, 2015. http://ecdc.europa.eu/en/press/news/_layouts/forms/News_DispForm.aspx?ID=1332&List=8db7286c-fe2d-476c-9133-18ff4cb1b568&Source=http%3A%2F%2Fecdc%2Eeuropa%2Eeu%2Fen%2Fpress%2Fepidemiological%5Fupdates%2FPages%2Fepidemiological%5Fupdates%2Easpx. Accessed February 24, 2016
- European Centre for Disease Prevention and Control. Rapid risk assessment. Zika virus infection outbreak, Brazil and the Pacific region 25 May 2015. http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-Zika%20virus-south-america-Brazil-2015.pdf. Accessed February 24, 2016
- Regan DM, Markowitz MA. Association Bulletin #16-03. Re: Zika, dengue, and chikungunya viruses. American Association of Blood Banks, February 1, 2016. www.aabb.org/programs/publications/bulletins/Documents/ab16-03.pdf. Accessed February 24, 2016.
- US Food and Drug Administration (FDA). Recommendations for donor screening, deferral, and product management to reduce the risk of transfusion-transmission of Zika virus. Guidance for industry. February, 2016. www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Blood/UCM486360.pdf. Accessed February 24, 2016.
- Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014; 19(13). pii: 20751.
- Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol 2016; 47:6–7.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Fleming-Dutra K, Nelson J, Fischer M, Staples J, Mateusz P, et al. Update: interim guidelines for health care providers caring for infants and children with possible Zika virus infection—United States, February 2016. MMWR 2016; 65:1–6.
- Simpson DI. Zika virus infection in man. Trans R Soc Trop Med Hyg Jul 1964; 58:335–338.
- Olson JG, Ksiazek TG, Suhandiman, Triwibowo. Zika virus, a cause of fever in Central Java, Indonesia. Trans R Soc Trop Med Hyg 1981; 75:389–393.
- Bearcroft WG. Zika virus infection experimentally induced in a human volunteer. Trans R Soc Trop Med Hyg 1956; 50:442–448.
- Oduyebo T, Petersen EE, Rasmussen SA, et al. Update: interim guidelines for health care providers caring for pregnant women and women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR 2016; 65:122–127.
- Gourinat AC, O’Connor O, Calvez E, Goarant C, Dupont-Rouzeyrol M. Detection of Zika virus in urine. Emerg Infect Dis 2015; 21:84–86.
- Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–572.
- Alera MT, Hermann L, Tac-An IA, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis 2015; 21:722–724.
- Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–1239.
- Centers for Disease Control and Prevention. Chikungunya virus. Clinical evaluation & disease. www.cdc.gov/chikungunya/hc/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Clinical guidance. Dengue virus. www.cdc.gov/dengue/clinicalLab/clinical.html. Accessed February 24, 2016.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Increase in microcephaly in the northeast of Brazil. November 17, 2015. http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32636&lang=en. Accessed February 24, 2016.
- Rubin EJ, Greene MF, Baden LR. Zika virus and microcephaly. N Engl J Med 2016; Feb 10 [Epub ahead of print].
- Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barré syndrome—case report, French Polynesia, December 2013. Euro Surveill 2014; 19(9). pii: 20720.
- Pan American Health Organization/World Health Organization. Epidemiological alert. Neurological syndrome, congenital malformations, and Zika virus infection. Implications for public health in the Americas. December 1, 2015. www.paho.org/hq/index.php?option=com_docman&task=doc_view&Itemid=270&gid=32405&lang=en. Accessed February 24, 2016.
- Martines R, Bhatnagar J, Keating M, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMRW 2016; 65:159–160.
- Ashwal S, Michelson D, Plawner L, Dobyns WB; Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Practice parameter: evaluation of the child with microcephaly (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 2009; 73:887–897.
- Ventura CV, Maia M, Bravo-Filho V, Góis AL, Belfort R Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet 2016; 387:228.
- Centers for Disease Control and Prevention. Updated diagnostic testing for Zika, chikungunya, and dengue viruses in US Public Health Laboratories. http://stacks.cdc.gov/view/cdc/37594. Accessed February 24, 2016.
- Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA. Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 2013; 10:311.
- Musso D, Roche C, Nhan TX, Robin E, Teissier A, Cao-Lormeau VM. Detection of Zika virus in saliva. J Clin Virol 2015; 68:53–55.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen [letter]. Emerg Infect Dis 2016 May. http://wwwnc.cdc.gov/eid/article/22/5/16-0107_article. Accessed February 24, 2016.
- Petersen EE, Staples JE, Meaney-Delman D, et al. Interim guidelines for pregnant women during a Zika virus outbreak—United States, 2016. MMWR 2016; 65:30–33.
- Oster AM, Brooks JT, Stryker JE, et al. Interim guidelines for prevention of sexual transmission of Zika virus—United States, 2016. MMWR 2016; 65:120–121.
- Staples JE, Dziuban EJ, Fischer M, et al. Interim guidelines for the evaluation and testing of infants with possible congenital Zika virus infection—United States, 2016. MMWR 2016; 65:63–67.
- Centers for Disease Control and Prevention. Zika virus clinical evaluation and disease. www.cdc.gov/zika/hc-providers/clinicalevaluation.html. Accessed February 24, 2016.
- Centers for Disease Control and Prevention. Zika virus. Transmission & risks. www.cdc.gov/zika/transmission/index.html. Accessed February 29, 2016.
KEY POINTS
- Zika virus infection is spread by the bite of infected mosquitoes and also through sexual contact, blood transfusions, and vertical transmission.
- Most Zika virus infections are asymptomatic, and symptomatic cases are often mild and self-limited, with rash, fever, joint pain, and nonpurulent conjunctivitis the most common symptoms.
- Polymerase chain reaction testing can detect viral RNA in the blood, but only in the first few days after the onset of symptoms. Immunoglobulin M against the virus becomes detectable at approximately 1 week and persists for about 12 weeks, but cross-reactivity with other viruses is a problem with serologic testing.
- As yet, there is no vaccine and no specific treatment.
- Pregnant women and women who may become pregnant are advised to defer travel to areas where Zika virus is endemic.