User login
Why do primary care physicians in nontropical parts of the world need to be on the lookout for tropical diseases such as dengue?
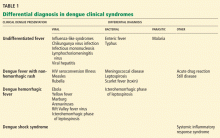
First, more people are traveling than ever before, and second, more people are traveling to parts of the world where dengue and other tropical diseases are endemic. Thus, dengue should now be included in the differential diagnosis of fever in anyone returning from travel to a part of the world where dengue is endemic (Table 1).1
The number of cases of dengue in returning US travelers has been increasing steadily over the past 25 years.2,3 Dengue is a more common cause of febrile illness than malaria in US travelers returning from all tropical and subtropical regions except Africa.4
Moreover, dengue may be gaining a permanent foothold in the United States, and in areas of the country where mosquitoes that can transmit the virus are found, primary care physicians are the first line of defense in public health. Specifically, to prevent the virus from becoming established locally, primary care physicians need to quickly identify and report cases to public health authorities, who can promptly follow up and initiate prevention measures.5
Underscoring the importance of dengue, this infection was added in 2009 to the list of nationally notifiable infectious diseases in the United States.6
A COMMON INFECTION WORLDWIDE
Dengue causes up to 100 million new infections, 500,000 hospitalizations, and 25,000 deaths every year in the 2.5 billion people who live in subtropical and tropical areas of the world.7 It is transmitted by mosquitoes of the genus Aedes (which, unlike most other mosquitoes, often bite in the daytime), and it is the most common arboviral infection (ie, transmitted mainly by arthropods) worldwide.
The dengue virus belongs to the family of flaviviruses, which includes West Nile virus, St. Louis encephalitis, and yellow fever. It has four closely related but antigenically distinct serotypes, designated DENV-1, DENV-2, DENV-3, and DENV-4. Infection with one serotype induces lifetime homotypic immunity but only short-lived heterotypic immunity to the other dengue serotypes.8 Hence, a person can be infected over time with each of the four serotypes. The first infection is termed the primary infection; subsequent infection with any of the remaining three serotypes is termed a secondary infection.
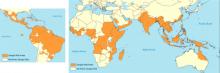
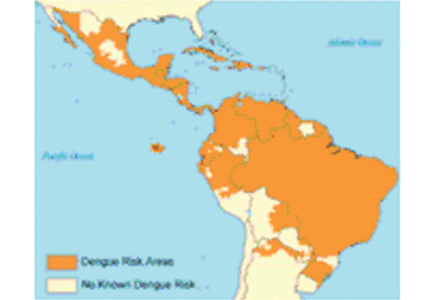
Dengue virus transmission has been expanding since the end of World War II in Asia and since the 1980s in the Americas following the end of many regional vector-control programs.9 Although dengue is known to occur in tropical Africa, its epidemiology is less well defined on that continent (Figure 1).10
Explosive epidemics of dengue occur when there are enough mosquitoes and a susceptible population across a broad age range, ie, both children and adults.11,12 Transmission can be halted with vigorous vector-control programs, or it slows and stops when the pool of susceptible people is exhausted.13–15
On the other hand, hyperendemic transmission occurs in areas in which multiple virus serotypes continuously circulate in a large pool of susceptible people. In these areas, dengue seroprevalence increases with age, and most adults are immune.
Anyone of any age who travels from a nonendemic area to an epidemic or hyperendemic area is at risk of infection.16
DENGUE IN THE UNITED STATES
Reported cases of dengue in South and Central America, the Caribbean, and Mexico, common destinations for US travelers, have increased more than fourfold since the 1980s. There were a total of approximately 1 million cases in the 10-year period ending in 1989, compared with more than 4.5 million in the 8-year period from 2000 to 2007.11
The geographic proximity of these areas to the continental United States and the large numbers of US residents travelling to these areas have raised concern that dengue could emerge in the continental United States in areas where potential vectors exist (see below).17 Furthermore, several US territories and former territories where tourism is an economic mainstay, including the Commonwealth of Puerto Rico,18 the US Virgin Islands,19 American Samoa, and other smaller Pacific island jurisdictions such as Palau,20 have reported dengue virus circulation.
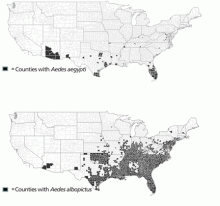
Adding to the concern that dengue could gain a persistent foothold in the United States, competent dengue vectors are found here. Two mosquito vectors, Aedes aegypti21 and Aedes albopictus,22,23 live in some areas of the southwestern and southeastern United States (Figure 2), with Aedes aegypti being the more competent transmitter. Both vectors may be abundant in warmer months. This raises a concern that a returning dengue-infected traveler could initiate an outbreak of autochthonous transmission.24
Notably, endemic dengue transmission has occurred in the past in the United States, and the virus is circulating here again at low levels. From 1946 to 1980, no cases of dengue were acquired in the continental United States. However, since 1980 there have been seven outbreaks of laboratory-confirmed, locally acquired dengue along the Texas-Mexico border.25–27 More recently, local transmission emerged in Key West, Florida,28,29 the first outbreak since 1945 of dengue in the continental United States not to occur near the Texas-Mexico border. And in early 2011, nonsustained but locally acquired transmission was confirmed in Hawaii after a transmission-free decade.30
THE CLINICAL SPECTRUM OF DENGUE VIRUS INFECTION
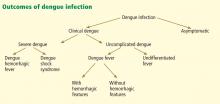
Most primary and secondary dengue infections are asymptomatic.8 The common forms of clinically apparent disease include self-limited, undifferentiated fever and classic dengue fever (Figure 3). Severe disease, manifesting as either dengue hemorrhagic fever or dengue shock syndrome, is a rare outcome of dengue virus infection, estimated to occur in 1% of cases worldwide.31 However, the true proportion of severe infection among all dengue cases seen in travelers is difficult to assess reliably.
Asymptomatic infection
Clinical dengue disease is relatively uncommon, as between 60% and 80% of infections are asymptomatic, particularly in children and adults who never have been infected before.32
In the recent outbreak in Key West, 28 symptomatic cases of locally acquired dengue were detected. The US Centers for Disease Control and Prevention (CDC) conducted a serologic survey of 240 healthy, randomly selected residents of Key West and found evidence of recent infection in 5.4% of those tested.28 Based on this finding, the CDC estimated that 1,000 people had been infected, of whom more than 90% had no symptoms. However, no attempt was made to differentiate primary from secondary infection in this serosurvey. (Previous infection with one dengue serotype places some individuals at risk for severe dengue if infected with a different serotype in the future.)
Uncomplicated dengue infection
Undifferentiated fever33 and classic dengue fever are the most common manifestations of clinical dengue infection. Also known as breakbone fever, classic dengue fever is a fever-arthralgia-rash syndrome.1
The onset is acute, with a high fever (though rarely greater than 40.5°C [104.9°F]) 3 to 14 days (usually 5 to 9 days) after the patient was bitten by an Aedes mosquito. Therefore, a febrile illness beginning more than 2 weeks after returning from travel to an endemic area is unlikely to be dengue, and another diagnosis should be sought.
A prodrome of headache, backache, fatigue, chills, anorexia, and occasionally a rash may precede the onset of fever by about 12 hours.
With fever comes a severe frontal headache, associated retro-orbital pain with eye movement, and conjunctival injection.
Some patients develop a bright, erythematous, maculopapular eruption 2 to 6 days into the illness that appears first on the trunk and then spreads to the face and extremities, with characteristic islands of unaffected skin throughout the involved area.34
Severe back or groin pain occurs in about 60% of adult patients.35
Anorexia, nausea, and vomiting are common.
Patients remain febrile for about 5 days, although some experience a biphasic (saddleback) fever that declines after 2 to 3 days, only to recur in about 24 hours.
In some patients, relative bradycardia is seen 2 to 3 days after fever onset.
Lymphadenopathy, sore throat, diarrhea or constipation, cutaneous hypesthesia, dysuria, dysgeusia, hepatitis, aseptic meningitis, and encephalopathy with delirium have been reported. Splenomegaly is rare.
In classic dengue fever, initial neutropenia and lymphopenia with subsequent lymphocytosis and monocytosis are often noted.
Mild hepatitis can be seen; aspartate aminotransferase and alanine aminotransferase levels can be two to three times the upper limit of normal.
Hemorrhagic manifestations, eg, petechiae, gingival bleeding, and epistaxis, may be seen in patients with mild thrombocytopenia even if they have no evidence of hemoconcentration or evidence of vascular instability.18
Although clinical dengue infection is usually self-limiting, acute symptoms can be incapacitating and can require hospitalization,36 and convalescence may take several months because of ongoing asthenia or depression.37 Furthermore, certain critical findings should alert the clinician to possible impending severe dengue and should lead to hospitalization for further observation until evolution to severe dengue has been ruled out (Table 2).
Severe dengue: Hemorrhagic fever and shock syndrome
In a very small subset of patients, dengue infection develops into a severe, potentially lifethreatening illness. Fortunately, this has rarely been reported in travelers.38
Dengue hemorrhagic fever and dengue shock syndrome arise just as fever is subsiding. They constitute a spectrum of severe illness (Figure 4). Dengue hemorrhagic fever is poorly understood because its hemorrhagic manifestations are not of themselves diagnostic of the condition, as petechiae, epistaxis, and gingival bleeding may be seen in classic dengue fever (Figure 3) without progression to more severe illness.
The differentiating characteristic of severe dengue, in addition to hemorrhagic manifestations, is objective evidence of plasma leakage.39 Impending shock is suggested by the new onset of severe abdominal pain, restlessness, hepatomegaly, hypothermia, and diaphoresis.
The mechanisms causing the severe hemorrhagic manifestations characteristic of dengue hemorrhagic fever and the sudden onset of vascular permeability underlying dengue shock syndrome are not understood.40 Many hypotheses have been generated and risk factors identified from observational and retrospective analyses. These include T-cell immune-pathologic responses involving receptors, antibodies, and cytokines,41 as well as specific host-genetic characteristics,42,43 age,44,45 sex,46 comorbid conditions,47–49 dengue virus virulence factors,50 sequence of dengue infection, and infection parity.51 One hypothesis is that antibody-dependent enhancement of virus occurs during infection with a second dengue serotype after infection with a different serotype in the past, and that this may be the root cause of dengue hemorrhagic fever and dengue shock syndrome. However, this has not been proven.40
DIAGNOSTIC TESTS FOR VIRUS, ANTIGENIC FRAGMENTS, ANTIBODIES
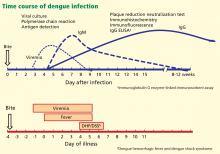
The appropriate test to confirm dengue virus infection is based on the natural history of the infection (Figure 4) coupled with the exposure risk in the returned traveler. These tests include isolation of the virus using cell culture, identification of antigenic fragments (test not available in the United States), and serologic tests for specific immunoglobulin M (IgM) and IgG antibodies using enzyme-linked immunosorbent assay (ELISA) or neutralization assays.52 During primary infection, viremia and antigenemia usually parallel fever, but when a person is later infected with a different dengue serotype, the period of viremia may be as short as 2 to 3 days, with antigens persisting in the serum for several more days.40 Virus isolation is not routinely available but is both sensitive and specific for the diagnosis of dengue virus infection during the viremic period.
If polymerase chain reaction testing, dengue antigen capture ELISA, or virus isolation testing is not available, the ideal confirmatory procedure is to test for dengue IgM (looking for conversion from negative to positive), IgG (looking for a fourfold rise in antibody), or both, in paired serum samples collected 2 weeks apart, with the initial sample collected less than 5 days after the onset of symptoms. A presumptive diagnosis can be made if a single blood sample collected more than 7 days after symptom onset is found to have dengue IgM antibody. A single blood sample for IgM collected earlier than 7 days after the onset of illness may give a false-negative result (Figure 4) in infected persons.
DIFFERENTIAL DIAGNOSES: INFECTIOUS AND NONINFECTIOUS
The differential diagnosis of uncomplicated dengue in a traveler returning from an endemic area includes viral, bacterial, and protozoal infections as well as noninfectious conditions (Table 1).53
Although most dengue virus infections are self-limiting, the clinical presentation may be severe enough to warrant hospitalization so that potentially life-threatening conditions can be systematically dismissed from the differential diagnosis.
Infections that can be rapidly fatal, such as malaria and enteric fever, need to be considered in patients who have traveled to endemic areas who present with undifferentiated fever. In cases of fever and maculopapular eruption, the differential diagnosis should include other causes of rash illness, such as measles and rubella. If hemorrhagic features are present, potentially fatal conditions need to be considered, including the classic viral hemorrhagic fevers caused by the Ebola and Marburg viruses, meningococcemia, the icterohemorrhagic form of leptospirosis, or other causes of bacterial sepsis. Other nonfatal infections should also be considered.
TREATMENT IS SUPPORTIVE
There is no antiviral treatment for dengue across the spectrum of disease presentations. Treatment is supportive and based on clinical presentation.
Acetaminophen (Tylenol) can be used to control fever, but aspirin and nonsteroidal anti-inflammatory drugs should not be used because they can make bleeding worse. Corticosteroids do not improve the outcome in severe dengue.2
Scrupulous attention to fluid and electrolyte balance is critical in severe dengue cases. Proper support and fluid resuscitation, including blood transfusion if needed, result in rapid recovery from dengue hemorrhagic fever with or without shock.
Suspected, probable, or confirmed cases of dengue should be reported to the local health department on the basis of published criteria (Table 3).
ADVICE TO TRAVELERS: DON’T GET BITTEN
There is currently no commercially available dengue vaccine, although several are under development.54 Therefore, pretravel counseling on how to avoid mosquito bites when traveling to dengue-endemic areas is the key dengue prevention strategy. Proactive prevention strategies include use of insect repellents such as those containing diethyltoluamide (DEET) or permethrin55 and elimination of outdoor locations where mosquitoes lay eggs, such as flower planter dishes, to reduce local mosquito breeding.56
Patients who have had a previous dengue infection should be counseled about the possible increased risk of severe disease if infected with a second dengue serotype.
Acknowledgment: The author thanks Chester G. Moore, PhD, of Colorado State University for assistance in creating Figure 2, based on data contained in the CDC, ArboNET, and Exotic/Invasive databases.
- Leggat PA. Assessment of febrile illness in the returned traveller. Aust Fam Physician 2007; 36:328–332.
- Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med 2005; 353:924–932.
- Mohammed HP, Ramos MM, Rivera A, et al. Travel-associated dengue infections in the United States, 1996 to 2005. J Travel Med 2010; 17:8–14.
- Centers for Disease Control and Prevention (CDC). Travel-associated dengue surveillance—United States, 2006–2008. MMWR Morb Mortal Wkly Rep 2010; 59:715–719.
- Ang KT, Rohani I, Look CH. Role of primary care providers in dengue prevention and control in the community. Med J Malaysia 2010; 65:58–62.
- Centers for Disease Control and Prevention. Notice to readers: Changes to the national notifiable infectious disease list and data presentation—January 2010. MMWR Morb Mortal Wkly Rep 2010; 59:11. www.cdc.gov/mmwr/preview/mmwrhtml/mm5901a7.htm. Accessed May 29, 2012.
- Guzman A, Istúriz RE. Update on the global spread of dengue. Int J Antimicrob Agents 2010; 36:(suppl 1):S40–S42.
- Midgley CM, Bajwa-Joseph M, Vasanawathana S, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol 2011; 85:410–421.
- Gubler DJ. Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp 2006; 277:3–16.
- Franco L, Di Caro A, Carletti F, et al. Recent expansion of dengue virus serotype 3 in West Africa. Euro Surveill 2010; 15:19490.
- San Martín JL, Brathwaite O, Zambrano B, et al. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg 2010; 82:128–135.
- Ramos MM, Mohammed H, Zielinski-Gutierrez E, et al; Dengue Serosurvey Working Group. Epidemic dengue and dengue hemorrhagic fever at the Texas-Mexico border: results of a household-based seroepidemiologic survey, December 2005. Am J Trop Med Hyg 2008; 78:364–369.
- Ong DQ, Sitaram N, Rajakulendran M, et al. Knowledge and practice of household mosquito breeding control measures between a dengue hotspot and non-hotspot in Singapore. Ann Acad Med Singapore 2010; 39:146–149.
- Vazquez-Prokopec GM, Chaves LF, Ritchie SA, Davis J, Kitron U. Unforeseen costs of cutting mosquito surveillance budgets. PLoS Negl Trop Dis 2010; 4:e858.
- Ballenger-Browning KK, Elder JP. Multi-modal Aedes aegypti mosquito reduction interventions and dengue fever prevention. Trop Med Int Health 2009; 14:1542–1551.
- Courtney M, Shetty AK. Imported dengue fever: an important reemerging disease. Pediatr Emerg Care 2009; 25:769–772.
- Morens DM, Fauci AS. Dengue and hemorrhagic fever: a potential threat to public health in the United States. JAMA 2008; 299:214–216.
- Gregory CJ, Santiago LM, Argüello DF, Hunsperger E, Tomashek KM. Clinical and laboratory features that differentiate dengue from other febrile illnesses in an endemic area—Puerto Rico, 2007–2008. Am J Trop Med Hyg 2010; 82:922–929.
- Mohammed H, Ramos M, Armstrong J, et al. An outbreak of dengue fever in St. Croix (US Virgin Islands), 2005. PLoS One 2010; 5):e13729.
- Li DS, Liu W, Guigon A, Mostyn C, Grant R, Aaskov J. Rapid displacement of dengue virus type 1 by type 4, Pacific region, 2007–2009. Emerg Infect Dis 2010; 16:123–125.
- Hayden MH, Uejio CK, Walker K, et al. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, US/Sonora, MX border. Ecohealth 2010; 7:64–77.
- Knudsen AB. The significance of the introduction of Aedes albopictus into the southeastern United States with implications for the Caribbean, and perspectives of the Pan American Health Organization. J Am Mosq Control Assoc 1986; 2:420–423.
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol 2004; 18:215–227.
- Franco C, Hynes NA, Bouri N, Henderson DA. The dengue threat to the United States. Biosecur Bioterror 2010; 8:273–276.
- Centers for Disease Control and Prevention (CDC). Dengue hemorrhagic fever—US-Mexico border, 2005. MMWR Morb Mortal Wkly Rep 2007; 56:785–789.
- Brunkard JM, Robles López JL, Ramirez J, et al. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg Infect Dis 2007; 13:1477–1483.
- Hafkin B, Kaplan JE, Reed C, et al. Reintroduction of dengue fever into the continental United States. I. Dengue surveillance in Texas, 1980. Am J Trop Med Hyg 1982; 31:1222–1228.
- Centers for Disease Control and Prevention (CDC). Locally acquired Dengue—Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep 2010; 59:577–581.
- Gill J, Stark LM, Clark GG. Dengue surveillance in Florida, 1997–98. Emerg Infect Dis 2000; 6:30–35.
- Department of Health. DOH investigates cases of dengue fever on Oahu and asks public & community to be vigilant. http://hawaii.gov/health/about/pr/2011/11-028.pdf. Accessed May 29, 2012.
- Wilder-Smith A, Earnest A, Tan SB, Ooi EE, Gubler DJ. Lack of association of dengue activity with haze. Epidemiol Infect 2010; 138:962–967.
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol 2008; 62:71–92.
- Chrispal A, Boorugu H, Gopinath KG, et al. Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors—an experience from a tertiary care hospital in South India. Trop Doct 2010; 40:230–234.
- World Health Organization; the Special Programme for Research and Training in Tropical Diseases (TDR). Dengue: guidelines for diagnosis, treatment, prevention and control. www.who.int/rpc/guidelines/9789241547871/en/. Accessed May 29, 2012.
- Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health 2008; 13:1328–1340.
- Streit JA, Yang M, Cavanaugh JE, Polgreen PM. Upward trend in dengue incidence among hospitalized patients, United States. Emerg Infect Dis 2011; 17:914–916.
- Jelinek T. Dengue fever in international travelers. Clin Infect Dis 2000; 31:144–147.
- Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ 2002; 324:1563–1566.
- Gibbons RV. Dengue conundrums. Int J Antimicrob Agents 2010; 36(suppl 1):S36–S39.
- Halstead SB. Dengue. Lancet 2007; 370:1644–1652.
- Halstead SB. Antibodies determine virulence in dengue. Ann N Y Acad Sci 2009; 1171(suppl 1):E48–E56.
- Coffey LL, Mertens E, Brehin AC, et al. Human genetic determinants of dengue virus susceptibility. Microbes Infect 2009; 11:143–156.
- García G, Sierra B, Pérez AB, et al. Asymptomatic dengue infection in a Cuban population confirms the protective role of the RR variant of the FcgammaRIIa polymorphism. Am J Trop Med Hyg 2010; 82:1153–1156.
- Braga C, Luna CF, Martelli CM, et al. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop 2010; 113:234–240.
- Jain A, Chaturvedi UC. Dengue in infants: an overview. FEMS Immunol Med Microbiol 2010; 59:119–130.
- Almas A, Parkash O, Akhter J. Clinical factors associated with mortality in dengue infection at a tertiary care center. Southeast Asian J Trop Med Public Health 2010; 41:333–340.
- Diaz-Quijano FA, Villar-Centeno LA, Martinez-Vega RA. Predictors of spontaneous bleeding in patients with acute febrile syndrome from a dengue endemic area. J Clin Virol 2010; 49:11–15.
- Marón GM, Clará AW, Diddle JW, et al. Association between nutritional status and severity of dengue infection in children in El Salvador. Am J Trop Med Hyg 2010; 82:324–329.
- Sierra B, Perez AB, Vogt K, et al. Secondary heterologous dengue infection risk: disequilibrium between immune regulation and inflammation? Cell Immunol 2010; 262:134–140.
- Brien JD, Austin SK, Sukupolvi-Petty S, et al. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J Virol 2010; 84:10630–10643.
- Humayoun MA, Waseem T, Jawa AA, Hashmi MS, Akram J. Multiple dengue serotypes and high frequency of dengue hemorrhagic fever at two tertiary care hospitals in Lahore during the 2008 dengue virus outbreak in Punjab, Pakistan. Int J Infect Dis 2010; 14(suppl 3):e54–e59.
- Guzman MG, Halstead SB, Artsob H, et al. Dengue: a continuing global threat. Nature Rev Microbiol 2010; 8:S7–S16.
- Crowell CS, Stamos JK. Evaluation of fever after international travel. Pediatr Ann 2011; 40:39–44.
- Durbin AP, Whitehead SS. Dengue vaccine candidates in development. Curr Top Microbiol Immunol 2010; 338:129–143.
- Chen LH, Wilson ME. Dengue and chikungunya infections in travelers. Curr Opin Infect Dis 2010; 23:438–444.
- Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis 2010; 4:e646.
Why do primary care physicians in nontropical parts of the world need to be on the lookout for tropical diseases such as dengue?
First, more people are traveling than ever before, and second, more people are traveling to parts of the world where dengue and other tropical diseases are endemic. Thus, dengue should now be included in the differential diagnosis of fever in anyone returning from travel to a part of the world where dengue is endemic (Table 1).1
The number of cases of dengue in returning US travelers has been increasing steadily over the past 25 years.2,3 Dengue is a more common cause of febrile illness than malaria in US travelers returning from all tropical and subtropical regions except Africa.4
Moreover, dengue may be gaining a permanent foothold in the United States, and in areas of the country where mosquitoes that can transmit the virus are found, primary care physicians are the first line of defense in public health. Specifically, to prevent the virus from becoming established locally, primary care physicians need to quickly identify and report cases to public health authorities, who can promptly follow up and initiate prevention measures.5
Underscoring the importance of dengue, this infection was added in 2009 to the list of nationally notifiable infectious diseases in the United States.6
A COMMON INFECTION WORLDWIDE
Dengue causes up to 100 million new infections, 500,000 hospitalizations, and 25,000 deaths every year in the 2.5 billion people who live in subtropical and tropical areas of the world.7 It is transmitted by mosquitoes of the genus Aedes (which, unlike most other mosquitoes, often bite in the daytime), and it is the most common arboviral infection (ie, transmitted mainly by arthropods) worldwide.
The dengue virus belongs to the family of flaviviruses, which includes West Nile virus, St. Louis encephalitis, and yellow fever. It has four closely related but antigenically distinct serotypes, designated DENV-1, DENV-2, DENV-3, and DENV-4. Infection with one serotype induces lifetime homotypic immunity but only short-lived heterotypic immunity to the other dengue serotypes.8 Hence, a person can be infected over time with each of the four serotypes. The first infection is termed the primary infection; subsequent infection with any of the remaining three serotypes is termed a secondary infection.
Dengue virus transmission has been expanding since the end of World War II in Asia and since the 1980s in the Americas following the end of many regional vector-control programs.9 Although dengue is known to occur in tropical Africa, its epidemiology is less well defined on that continent (Figure 1).10
Explosive epidemics of dengue occur when there are enough mosquitoes and a susceptible population across a broad age range, ie, both children and adults.11,12 Transmission can be halted with vigorous vector-control programs, or it slows and stops when the pool of susceptible people is exhausted.13–15
On the other hand, hyperendemic transmission occurs in areas in which multiple virus serotypes continuously circulate in a large pool of susceptible people. In these areas, dengue seroprevalence increases with age, and most adults are immune.
Anyone of any age who travels from a nonendemic area to an epidemic or hyperendemic area is at risk of infection.16
DENGUE IN THE UNITED STATES
Reported cases of dengue in South and Central America, the Caribbean, and Mexico, common destinations for US travelers, have increased more than fourfold since the 1980s. There were a total of approximately 1 million cases in the 10-year period ending in 1989, compared with more than 4.5 million in the 8-year period from 2000 to 2007.11
The geographic proximity of these areas to the continental United States and the large numbers of US residents travelling to these areas have raised concern that dengue could emerge in the continental United States in areas where potential vectors exist (see below).17 Furthermore, several US territories and former territories where tourism is an economic mainstay, including the Commonwealth of Puerto Rico,18 the US Virgin Islands,19 American Samoa, and other smaller Pacific island jurisdictions such as Palau,20 have reported dengue virus circulation.
Adding to the concern that dengue could gain a persistent foothold in the United States, competent dengue vectors are found here. Two mosquito vectors, Aedes aegypti21 and Aedes albopictus,22,23 live in some areas of the southwestern and southeastern United States (Figure 2), with Aedes aegypti being the more competent transmitter. Both vectors may be abundant in warmer months. This raises a concern that a returning dengue-infected traveler could initiate an outbreak of autochthonous transmission.24
Notably, endemic dengue transmission has occurred in the past in the United States, and the virus is circulating here again at low levels. From 1946 to 1980, no cases of dengue were acquired in the continental United States. However, since 1980 there have been seven outbreaks of laboratory-confirmed, locally acquired dengue along the Texas-Mexico border.25–27 More recently, local transmission emerged in Key West, Florida,28,29 the first outbreak since 1945 of dengue in the continental United States not to occur near the Texas-Mexico border. And in early 2011, nonsustained but locally acquired transmission was confirmed in Hawaii after a transmission-free decade.30
THE CLINICAL SPECTRUM OF DENGUE VIRUS INFECTION
Most primary and secondary dengue infections are asymptomatic.8 The common forms of clinically apparent disease include self-limited, undifferentiated fever and classic dengue fever (Figure 3). Severe disease, manifesting as either dengue hemorrhagic fever or dengue shock syndrome, is a rare outcome of dengue virus infection, estimated to occur in 1% of cases worldwide.31 However, the true proportion of severe infection among all dengue cases seen in travelers is difficult to assess reliably.
Asymptomatic infection
Clinical dengue disease is relatively uncommon, as between 60% and 80% of infections are asymptomatic, particularly in children and adults who never have been infected before.32
In the recent outbreak in Key West, 28 symptomatic cases of locally acquired dengue were detected. The US Centers for Disease Control and Prevention (CDC) conducted a serologic survey of 240 healthy, randomly selected residents of Key West and found evidence of recent infection in 5.4% of those tested.28 Based on this finding, the CDC estimated that 1,000 people had been infected, of whom more than 90% had no symptoms. However, no attempt was made to differentiate primary from secondary infection in this serosurvey. (Previous infection with one dengue serotype places some individuals at risk for severe dengue if infected with a different serotype in the future.)
Uncomplicated dengue infection
Undifferentiated fever33 and classic dengue fever are the most common manifestations of clinical dengue infection. Also known as breakbone fever, classic dengue fever is a fever-arthralgia-rash syndrome.1
The onset is acute, with a high fever (though rarely greater than 40.5°C [104.9°F]) 3 to 14 days (usually 5 to 9 days) after the patient was bitten by an Aedes mosquito. Therefore, a febrile illness beginning more than 2 weeks after returning from travel to an endemic area is unlikely to be dengue, and another diagnosis should be sought.
A prodrome of headache, backache, fatigue, chills, anorexia, and occasionally a rash may precede the onset of fever by about 12 hours.
With fever comes a severe frontal headache, associated retro-orbital pain with eye movement, and conjunctival injection.
Some patients develop a bright, erythematous, maculopapular eruption 2 to 6 days into the illness that appears first on the trunk and then spreads to the face and extremities, with characteristic islands of unaffected skin throughout the involved area.34
Severe back or groin pain occurs in about 60% of adult patients.35
Anorexia, nausea, and vomiting are common.
Patients remain febrile for about 5 days, although some experience a biphasic (saddleback) fever that declines after 2 to 3 days, only to recur in about 24 hours.
In some patients, relative bradycardia is seen 2 to 3 days after fever onset.
Lymphadenopathy, sore throat, diarrhea or constipation, cutaneous hypesthesia, dysuria, dysgeusia, hepatitis, aseptic meningitis, and encephalopathy with delirium have been reported. Splenomegaly is rare.
In classic dengue fever, initial neutropenia and lymphopenia with subsequent lymphocytosis and monocytosis are often noted.
Mild hepatitis can be seen; aspartate aminotransferase and alanine aminotransferase levels can be two to three times the upper limit of normal.
Hemorrhagic manifestations, eg, petechiae, gingival bleeding, and epistaxis, may be seen in patients with mild thrombocytopenia even if they have no evidence of hemoconcentration or evidence of vascular instability.18
Although clinical dengue infection is usually self-limiting, acute symptoms can be incapacitating and can require hospitalization,36 and convalescence may take several months because of ongoing asthenia or depression.37 Furthermore, certain critical findings should alert the clinician to possible impending severe dengue and should lead to hospitalization for further observation until evolution to severe dengue has been ruled out (Table 2).
Severe dengue: Hemorrhagic fever and shock syndrome
In a very small subset of patients, dengue infection develops into a severe, potentially lifethreatening illness. Fortunately, this has rarely been reported in travelers.38
Dengue hemorrhagic fever and dengue shock syndrome arise just as fever is subsiding. They constitute a spectrum of severe illness (Figure 4). Dengue hemorrhagic fever is poorly understood because its hemorrhagic manifestations are not of themselves diagnostic of the condition, as petechiae, epistaxis, and gingival bleeding may be seen in classic dengue fever (Figure 3) without progression to more severe illness.
The differentiating characteristic of severe dengue, in addition to hemorrhagic manifestations, is objective evidence of plasma leakage.39 Impending shock is suggested by the new onset of severe abdominal pain, restlessness, hepatomegaly, hypothermia, and diaphoresis.
The mechanisms causing the severe hemorrhagic manifestations characteristic of dengue hemorrhagic fever and the sudden onset of vascular permeability underlying dengue shock syndrome are not understood.40 Many hypotheses have been generated and risk factors identified from observational and retrospective analyses. These include T-cell immune-pathologic responses involving receptors, antibodies, and cytokines,41 as well as specific host-genetic characteristics,42,43 age,44,45 sex,46 comorbid conditions,47–49 dengue virus virulence factors,50 sequence of dengue infection, and infection parity.51 One hypothesis is that antibody-dependent enhancement of virus occurs during infection with a second dengue serotype after infection with a different serotype in the past, and that this may be the root cause of dengue hemorrhagic fever and dengue shock syndrome. However, this has not been proven.40
DIAGNOSTIC TESTS FOR VIRUS, ANTIGENIC FRAGMENTS, ANTIBODIES
The appropriate test to confirm dengue virus infection is based on the natural history of the infection (Figure 4) coupled with the exposure risk in the returned traveler. These tests include isolation of the virus using cell culture, identification of antigenic fragments (test not available in the United States), and serologic tests for specific immunoglobulin M (IgM) and IgG antibodies using enzyme-linked immunosorbent assay (ELISA) or neutralization assays.52 During primary infection, viremia and antigenemia usually parallel fever, but when a person is later infected with a different dengue serotype, the period of viremia may be as short as 2 to 3 days, with antigens persisting in the serum for several more days.40 Virus isolation is not routinely available but is both sensitive and specific for the diagnosis of dengue virus infection during the viremic period.
If polymerase chain reaction testing, dengue antigen capture ELISA, or virus isolation testing is not available, the ideal confirmatory procedure is to test for dengue IgM (looking for conversion from negative to positive), IgG (looking for a fourfold rise in antibody), or both, in paired serum samples collected 2 weeks apart, with the initial sample collected less than 5 days after the onset of symptoms. A presumptive diagnosis can be made if a single blood sample collected more than 7 days after symptom onset is found to have dengue IgM antibody. A single blood sample for IgM collected earlier than 7 days after the onset of illness may give a false-negative result (Figure 4) in infected persons.
DIFFERENTIAL DIAGNOSES: INFECTIOUS AND NONINFECTIOUS
The differential diagnosis of uncomplicated dengue in a traveler returning from an endemic area includes viral, bacterial, and protozoal infections as well as noninfectious conditions (Table 1).53
Although most dengue virus infections are self-limiting, the clinical presentation may be severe enough to warrant hospitalization so that potentially life-threatening conditions can be systematically dismissed from the differential diagnosis.
Infections that can be rapidly fatal, such as malaria and enteric fever, need to be considered in patients who have traveled to endemic areas who present with undifferentiated fever. In cases of fever and maculopapular eruption, the differential diagnosis should include other causes of rash illness, such as measles and rubella. If hemorrhagic features are present, potentially fatal conditions need to be considered, including the classic viral hemorrhagic fevers caused by the Ebola and Marburg viruses, meningococcemia, the icterohemorrhagic form of leptospirosis, or other causes of bacterial sepsis. Other nonfatal infections should also be considered.
TREATMENT IS SUPPORTIVE
There is no antiviral treatment for dengue across the spectrum of disease presentations. Treatment is supportive and based on clinical presentation.
Acetaminophen (Tylenol) can be used to control fever, but aspirin and nonsteroidal anti-inflammatory drugs should not be used because they can make bleeding worse. Corticosteroids do not improve the outcome in severe dengue.2
Scrupulous attention to fluid and electrolyte balance is critical in severe dengue cases. Proper support and fluid resuscitation, including blood transfusion if needed, result in rapid recovery from dengue hemorrhagic fever with or without shock.
Suspected, probable, or confirmed cases of dengue should be reported to the local health department on the basis of published criteria (Table 3).
ADVICE TO TRAVELERS: DON’T GET BITTEN
There is currently no commercially available dengue vaccine, although several are under development.54 Therefore, pretravel counseling on how to avoid mosquito bites when traveling to dengue-endemic areas is the key dengue prevention strategy. Proactive prevention strategies include use of insect repellents such as those containing diethyltoluamide (DEET) or permethrin55 and elimination of outdoor locations where mosquitoes lay eggs, such as flower planter dishes, to reduce local mosquito breeding.56
Patients who have had a previous dengue infection should be counseled about the possible increased risk of severe disease if infected with a second dengue serotype.
Acknowledgment: The author thanks Chester G. Moore, PhD, of Colorado State University for assistance in creating Figure 2, based on data contained in the CDC, ArboNET, and Exotic/Invasive databases.
Why do primary care physicians in nontropical parts of the world need to be on the lookout for tropical diseases such as dengue?
First, more people are traveling than ever before, and second, more people are traveling to parts of the world where dengue and other tropical diseases are endemic. Thus, dengue should now be included in the differential diagnosis of fever in anyone returning from travel to a part of the world where dengue is endemic (Table 1).1
The number of cases of dengue in returning US travelers has been increasing steadily over the past 25 years.2,3 Dengue is a more common cause of febrile illness than malaria in US travelers returning from all tropical and subtropical regions except Africa.4
Moreover, dengue may be gaining a permanent foothold in the United States, and in areas of the country where mosquitoes that can transmit the virus are found, primary care physicians are the first line of defense in public health. Specifically, to prevent the virus from becoming established locally, primary care physicians need to quickly identify and report cases to public health authorities, who can promptly follow up and initiate prevention measures.5
Underscoring the importance of dengue, this infection was added in 2009 to the list of nationally notifiable infectious diseases in the United States.6
A COMMON INFECTION WORLDWIDE
Dengue causes up to 100 million new infections, 500,000 hospitalizations, and 25,000 deaths every year in the 2.5 billion people who live in subtropical and tropical areas of the world.7 It is transmitted by mosquitoes of the genus Aedes (which, unlike most other mosquitoes, often bite in the daytime), and it is the most common arboviral infection (ie, transmitted mainly by arthropods) worldwide.
The dengue virus belongs to the family of flaviviruses, which includes West Nile virus, St. Louis encephalitis, and yellow fever. It has four closely related but antigenically distinct serotypes, designated DENV-1, DENV-2, DENV-3, and DENV-4. Infection with one serotype induces lifetime homotypic immunity but only short-lived heterotypic immunity to the other dengue serotypes.8 Hence, a person can be infected over time with each of the four serotypes. The first infection is termed the primary infection; subsequent infection with any of the remaining three serotypes is termed a secondary infection.
Dengue virus transmission has been expanding since the end of World War II in Asia and since the 1980s in the Americas following the end of many regional vector-control programs.9 Although dengue is known to occur in tropical Africa, its epidemiology is less well defined on that continent (Figure 1).10
Explosive epidemics of dengue occur when there are enough mosquitoes and a susceptible population across a broad age range, ie, both children and adults.11,12 Transmission can be halted with vigorous vector-control programs, or it slows and stops when the pool of susceptible people is exhausted.13–15
On the other hand, hyperendemic transmission occurs in areas in which multiple virus serotypes continuously circulate in a large pool of susceptible people. In these areas, dengue seroprevalence increases with age, and most adults are immune.
Anyone of any age who travels from a nonendemic area to an epidemic or hyperendemic area is at risk of infection.16
DENGUE IN THE UNITED STATES
Reported cases of dengue in South and Central America, the Caribbean, and Mexico, common destinations for US travelers, have increased more than fourfold since the 1980s. There were a total of approximately 1 million cases in the 10-year period ending in 1989, compared with more than 4.5 million in the 8-year period from 2000 to 2007.11
The geographic proximity of these areas to the continental United States and the large numbers of US residents travelling to these areas have raised concern that dengue could emerge in the continental United States in areas where potential vectors exist (see below).17 Furthermore, several US territories and former territories where tourism is an economic mainstay, including the Commonwealth of Puerto Rico,18 the US Virgin Islands,19 American Samoa, and other smaller Pacific island jurisdictions such as Palau,20 have reported dengue virus circulation.
Adding to the concern that dengue could gain a persistent foothold in the United States, competent dengue vectors are found here. Two mosquito vectors, Aedes aegypti21 and Aedes albopictus,22,23 live in some areas of the southwestern and southeastern United States (Figure 2), with Aedes aegypti being the more competent transmitter. Both vectors may be abundant in warmer months. This raises a concern that a returning dengue-infected traveler could initiate an outbreak of autochthonous transmission.24
Notably, endemic dengue transmission has occurred in the past in the United States, and the virus is circulating here again at low levels. From 1946 to 1980, no cases of dengue were acquired in the continental United States. However, since 1980 there have been seven outbreaks of laboratory-confirmed, locally acquired dengue along the Texas-Mexico border.25–27 More recently, local transmission emerged in Key West, Florida,28,29 the first outbreak since 1945 of dengue in the continental United States not to occur near the Texas-Mexico border. And in early 2011, nonsustained but locally acquired transmission was confirmed in Hawaii after a transmission-free decade.30
THE CLINICAL SPECTRUM OF DENGUE VIRUS INFECTION
Most primary and secondary dengue infections are asymptomatic.8 The common forms of clinically apparent disease include self-limited, undifferentiated fever and classic dengue fever (Figure 3). Severe disease, manifesting as either dengue hemorrhagic fever or dengue shock syndrome, is a rare outcome of dengue virus infection, estimated to occur in 1% of cases worldwide.31 However, the true proportion of severe infection among all dengue cases seen in travelers is difficult to assess reliably.
Asymptomatic infection
Clinical dengue disease is relatively uncommon, as between 60% and 80% of infections are asymptomatic, particularly in children and adults who never have been infected before.32
In the recent outbreak in Key West, 28 symptomatic cases of locally acquired dengue were detected. The US Centers for Disease Control and Prevention (CDC) conducted a serologic survey of 240 healthy, randomly selected residents of Key West and found evidence of recent infection in 5.4% of those tested.28 Based on this finding, the CDC estimated that 1,000 people had been infected, of whom more than 90% had no symptoms. However, no attempt was made to differentiate primary from secondary infection in this serosurvey. (Previous infection with one dengue serotype places some individuals at risk for severe dengue if infected with a different serotype in the future.)
Uncomplicated dengue infection
Undifferentiated fever33 and classic dengue fever are the most common manifestations of clinical dengue infection. Also known as breakbone fever, classic dengue fever is a fever-arthralgia-rash syndrome.1
The onset is acute, with a high fever (though rarely greater than 40.5°C [104.9°F]) 3 to 14 days (usually 5 to 9 days) after the patient was bitten by an Aedes mosquito. Therefore, a febrile illness beginning more than 2 weeks after returning from travel to an endemic area is unlikely to be dengue, and another diagnosis should be sought.
A prodrome of headache, backache, fatigue, chills, anorexia, and occasionally a rash may precede the onset of fever by about 12 hours.
With fever comes a severe frontal headache, associated retro-orbital pain with eye movement, and conjunctival injection.
Some patients develop a bright, erythematous, maculopapular eruption 2 to 6 days into the illness that appears first on the trunk and then spreads to the face and extremities, with characteristic islands of unaffected skin throughout the involved area.34
Severe back or groin pain occurs in about 60% of adult patients.35
Anorexia, nausea, and vomiting are common.
Patients remain febrile for about 5 days, although some experience a biphasic (saddleback) fever that declines after 2 to 3 days, only to recur in about 24 hours.
In some patients, relative bradycardia is seen 2 to 3 days after fever onset.
Lymphadenopathy, sore throat, diarrhea or constipation, cutaneous hypesthesia, dysuria, dysgeusia, hepatitis, aseptic meningitis, and encephalopathy with delirium have been reported. Splenomegaly is rare.
In classic dengue fever, initial neutropenia and lymphopenia with subsequent lymphocytosis and monocytosis are often noted.
Mild hepatitis can be seen; aspartate aminotransferase and alanine aminotransferase levels can be two to three times the upper limit of normal.
Hemorrhagic manifestations, eg, petechiae, gingival bleeding, and epistaxis, may be seen in patients with mild thrombocytopenia even if they have no evidence of hemoconcentration or evidence of vascular instability.18
Although clinical dengue infection is usually self-limiting, acute symptoms can be incapacitating and can require hospitalization,36 and convalescence may take several months because of ongoing asthenia or depression.37 Furthermore, certain critical findings should alert the clinician to possible impending severe dengue and should lead to hospitalization for further observation until evolution to severe dengue has been ruled out (Table 2).
Severe dengue: Hemorrhagic fever and shock syndrome
In a very small subset of patients, dengue infection develops into a severe, potentially lifethreatening illness. Fortunately, this has rarely been reported in travelers.38
Dengue hemorrhagic fever and dengue shock syndrome arise just as fever is subsiding. They constitute a spectrum of severe illness (Figure 4). Dengue hemorrhagic fever is poorly understood because its hemorrhagic manifestations are not of themselves diagnostic of the condition, as petechiae, epistaxis, and gingival bleeding may be seen in classic dengue fever (Figure 3) without progression to more severe illness.
The differentiating characteristic of severe dengue, in addition to hemorrhagic manifestations, is objective evidence of plasma leakage.39 Impending shock is suggested by the new onset of severe abdominal pain, restlessness, hepatomegaly, hypothermia, and diaphoresis.
The mechanisms causing the severe hemorrhagic manifestations characteristic of dengue hemorrhagic fever and the sudden onset of vascular permeability underlying dengue shock syndrome are not understood.40 Many hypotheses have been generated and risk factors identified from observational and retrospective analyses. These include T-cell immune-pathologic responses involving receptors, antibodies, and cytokines,41 as well as specific host-genetic characteristics,42,43 age,44,45 sex,46 comorbid conditions,47–49 dengue virus virulence factors,50 sequence of dengue infection, and infection parity.51 One hypothesis is that antibody-dependent enhancement of virus occurs during infection with a second dengue serotype after infection with a different serotype in the past, and that this may be the root cause of dengue hemorrhagic fever and dengue shock syndrome. However, this has not been proven.40
DIAGNOSTIC TESTS FOR VIRUS, ANTIGENIC FRAGMENTS, ANTIBODIES
The appropriate test to confirm dengue virus infection is based on the natural history of the infection (Figure 4) coupled with the exposure risk in the returned traveler. These tests include isolation of the virus using cell culture, identification of antigenic fragments (test not available in the United States), and serologic tests for specific immunoglobulin M (IgM) and IgG antibodies using enzyme-linked immunosorbent assay (ELISA) or neutralization assays.52 During primary infection, viremia and antigenemia usually parallel fever, but when a person is later infected with a different dengue serotype, the period of viremia may be as short as 2 to 3 days, with antigens persisting in the serum for several more days.40 Virus isolation is not routinely available but is both sensitive and specific for the diagnosis of dengue virus infection during the viremic period.
If polymerase chain reaction testing, dengue antigen capture ELISA, or virus isolation testing is not available, the ideal confirmatory procedure is to test for dengue IgM (looking for conversion from negative to positive), IgG (looking for a fourfold rise in antibody), or both, in paired serum samples collected 2 weeks apart, with the initial sample collected less than 5 days after the onset of symptoms. A presumptive diagnosis can be made if a single blood sample collected more than 7 days after symptom onset is found to have dengue IgM antibody. A single blood sample for IgM collected earlier than 7 days after the onset of illness may give a false-negative result (Figure 4) in infected persons.
DIFFERENTIAL DIAGNOSES: INFECTIOUS AND NONINFECTIOUS
The differential diagnosis of uncomplicated dengue in a traveler returning from an endemic area includes viral, bacterial, and protozoal infections as well as noninfectious conditions (Table 1).53
Although most dengue virus infections are self-limiting, the clinical presentation may be severe enough to warrant hospitalization so that potentially life-threatening conditions can be systematically dismissed from the differential diagnosis.
Infections that can be rapidly fatal, such as malaria and enteric fever, need to be considered in patients who have traveled to endemic areas who present with undifferentiated fever. In cases of fever and maculopapular eruption, the differential diagnosis should include other causes of rash illness, such as measles and rubella. If hemorrhagic features are present, potentially fatal conditions need to be considered, including the classic viral hemorrhagic fevers caused by the Ebola and Marburg viruses, meningococcemia, the icterohemorrhagic form of leptospirosis, or other causes of bacterial sepsis. Other nonfatal infections should also be considered.
TREATMENT IS SUPPORTIVE
There is no antiviral treatment for dengue across the spectrum of disease presentations. Treatment is supportive and based on clinical presentation.
Acetaminophen (Tylenol) can be used to control fever, but aspirin and nonsteroidal anti-inflammatory drugs should not be used because they can make bleeding worse. Corticosteroids do not improve the outcome in severe dengue.2
Scrupulous attention to fluid and electrolyte balance is critical in severe dengue cases. Proper support and fluid resuscitation, including blood transfusion if needed, result in rapid recovery from dengue hemorrhagic fever with or without shock.
Suspected, probable, or confirmed cases of dengue should be reported to the local health department on the basis of published criteria (Table 3).
ADVICE TO TRAVELERS: DON’T GET BITTEN
There is currently no commercially available dengue vaccine, although several are under development.54 Therefore, pretravel counseling on how to avoid mosquito bites when traveling to dengue-endemic areas is the key dengue prevention strategy. Proactive prevention strategies include use of insect repellents such as those containing diethyltoluamide (DEET) or permethrin55 and elimination of outdoor locations where mosquitoes lay eggs, such as flower planter dishes, to reduce local mosquito breeding.56
Patients who have had a previous dengue infection should be counseled about the possible increased risk of severe disease if infected with a second dengue serotype.
Acknowledgment: The author thanks Chester G. Moore, PhD, of Colorado State University for assistance in creating Figure 2, based on data contained in the CDC, ArboNET, and Exotic/Invasive databases.
- Leggat PA. Assessment of febrile illness in the returned traveller. Aust Fam Physician 2007; 36:328–332.
- Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med 2005; 353:924–932.
- Mohammed HP, Ramos MM, Rivera A, et al. Travel-associated dengue infections in the United States, 1996 to 2005. J Travel Med 2010; 17:8–14.
- Centers for Disease Control and Prevention (CDC). Travel-associated dengue surveillance—United States, 2006–2008. MMWR Morb Mortal Wkly Rep 2010; 59:715–719.
- Ang KT, Rohani I, Look CH. Role of primary care providers in dengue prevention and control in the community. Med J Malaysia 2010; 65:58–62.
- Centers for Disease Control and Prevention. Notice to readers: Changes to the national notifiable infectious disease list and data presentation—January 2010. MMWR Morb Mortal Wkly Rep 2010; 59:11. www.cdc.gov/mmwr/preview/mmwrhtml/mm5901a7.htm. Accessed May 29, 2012.
- Guzman A, Istúriz RE. Update on the global spread of dengue. Int J Antimicrob Agents 2010; 36:(suppl 1):S40–S42.
- Midgley CM, Bajwa-Joseph M, Vasanawathana S, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol 2011; 85:410–421.
- Gubler DJ. Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp 2006; 277:3–16.
- Franco L, Di Caro A, Carletti F, et al. Recent expansion of dengue virus serotype 3 in West Africa. Euro Surveill 2010; 15:19490.
- San Martín JL, Brathwaite O, Zambrano B, et al. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg 2010; 82:128–135.
- Ramos MM, Mohammed H, Zielinski-Gutierrez E, et al; Dengue Serosurvey Working Group. Epidemic dengue and dengue hemorrhagic fever at the Texas-Mexico border: results of a household-based seroepidemiologic survey, December 2005. Am J Trop Med Hyg 2008; 78:364–369.
- Ong DQ, Sitaram N, Rajakulendran M, et al. Knowledge and practice of household mosquito breeding control measures between a dengue hotspot and non-hotspot in Singapore. Ann Acad Med Singapore 2010; 39:146–149.
- Vazquez-Prokopec GM, Chaves LF, Ritchie SA, Davis J, Kitron U. Unforeseen costs of cutting mosquito surveillance budgets. PLoS Negl Trop Dis 2010; 4:e858.
- Ballenger-Browning KK, Elder JP. Multi-modal Aedes aegypti mosquito reduction interventions and dengue fever prevention. Trop Med Int Health 2009; 14:1542–1551.
- Courtney M, Shetty AK. Imported dengue fever: an important reemerging disease. Pediatr Emerg Care 2009; 25:769–772.
- Morens DM, Fauci AS. Dengue and hemorrhagic fever: a potential threat to public health in the United States. JAMA 2008; 299:214–216.
- Gregory CJ, Santiago LM, Argüello DF, Hunsperger E, Tomashek KM. Clinical and laboratory features that differentiate dengue from other febrile illnesses in an endemic area—Puerto Rico, 2007–2008. Am J Trop Med Hyg 2010; 82:922–929.
- Mohammed H, Ramos M, Armstrong J, et al. An outbreak of dengue fever in St. Croix (US Virgin Islands), 2005. PLoS One 2010; 5):e13729.
- Li DS, Liu W, Guigon A, Mostyn C, Grant R, Aaskov J. Rapid displacement of dengue virus type 1 by type 4, Pacific region, 2007–2009. Emerg Infect Dis 2010; 16:123–125.
- Hayden MH, Uejio CK, Walker K, et al. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, US/Sonora, MX border. Ecohealth 2010; 7:64–77.
- Knudsen AB. The significance of the introduction of Aedes albopictus into the southeastern United States with implications for the Caribbean, and perspectives of the Pan American Health Organization. J Am Mosq Control Assoc 1986; 2:420–423.
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol 2004; 18:215–227.
- Franco C, Hynes NA, Bouri N, Henderson DA. The dengue threat to the United States. Biosecur Bioterror 2010; 8:273–276.
- Centers for Disease Control and Prevention (CDC). Dengue hemorrhagic fever—US-Mexico border, 2005. MMWR Morb Mortal Wkly Rep 2007; 56:785–789.
- Brunkard JM, Robles López JL, Ramirez J, et al. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg Infect Dis 2007; 13:1477–1483.
- Hafkin B, Kaplan JE, Reed C, et al. Reintroduction of dengue fever into the continental United States. I. Dengue surveillance in Texas, 1980. Am J Trop Med Hyg 1982; 31:1222–1228.
- Centers for Disease Control and Prevention (CDC). Locally acquired Dengue—Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep 2010; 59:577–581.
- Gill J, Stark LM, Clark GG. Dengue surveillance in Florida, 1997–98. Emerg Infect Dis 2000; 6:30–35.
- Department of Health. DOH investigates cases of dengue fever on Oahu and asks public & community to be vigilant. http://hawaii.gov/health/about/pr/2011/11-028.pdf. Accessed May 29, 2012.
- Wilder-Smith A, Earnest A, Tan SB, Ooi EE, Gubler DJ. Lack of association of dengue activity with haze. Epidemiol Infect 2010; 138:962–967.
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol 2008; 62:71–92.
- Chrispal A, Boorugu H, Gopinath KG, et al. Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors—an experience from a tertiary care hospital in South India. Trop Doct 2010; 40:230–234.
- World Health Organization; the Special Programme for Research and Training in Tropical Diseases (TDR). Dengue: guidelines for diagnosis, treatment, prevention and control. www.who.int/rpc/guidelines/9789241547871/en/. Accessed May 29, 2012.
- Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health 2008; 13:1328–1340.
- Streit JA, Yang M, Cavanaugh JE, Polgreen PM. Upward trend in dengue incidence among hospitalized patients, United States. Emerg Infect Dis 2011; 17:914–916.
- Jelinek T. Dengue fever in international travelers. Clin Infect Dis 2000; 31:144–147.
- Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ 2002; 324:1563–1566.
- Gibbons RV. Dengue conundrums. Int J Antimicrob Agents 2010; 36(suppl 1):S36–S39.
- Halstead SB. Dengue. Lancet 2007; 370:1644–1652.
- Halstead SB. Antibodies determine virulence in dengue. Ann N Y Acad Sci 2009; 1171(suppl 1):E48–E56.
- Coffey LL, Mertens E, Brehin AC, et al. Human genetic determinants of dengue virus susceptibility. Microbes Infect 2009; 11:143–156.
- García G, Sierra B, Pérez AB, et al. Asymptomatic dengue infection in a Cuban population confirms the protective role of the RR variant of the FcgammaRIIa polymorphism. Am J Trop Med Hyg 2010; 82:1153–1156.
- Braga C, Luna CF, Martelli CM, et al. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop 2010; 113:234–240.
- Jain A, Chaturvedi UC. Dengue in infants: an overview. FEMS Immunol Med Microbiol 2010; 59:119–130.
- Almas A, Parkash O, Akhter J. Clinical factors associated with mortality in dengue infection at a tertiary care center. Southeast Asian J Trop Med Public Health 2010; 41:333–340.
- Diaz-Quijano FA, Villar-Centeno LA, Martinez-Vega RA. Predictors of spontaneous bleeding in patients with acute febrile syndrome from a dengue endemic area. J Clin Virol 2010; 49:11–15.
- Marón GM, Clará AW, Diddle JW, et al. Association between nutritional status and severity of dengue infection in children in El Salvador. Am J Trop Med Hyg 2010; 82:324–329.
- Sierra B, Perez AB, Vogt K, et al. Secondary heterologous dengue infection risk: disequilibrium between immune regulation and inflammation? Cell Immunol 2010; 262:134–140.
- Brien JD, Austin SK, Sukupolvi-Petty S, et al. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J Virol 2010; 84:10630–10643.
- Humayoun MA, Waseem T, Jawa AA, Hashmi MS, Akram J. Multiple dengue serotypes and high frequency of dengue hemorrhagic fever at two tertiary care hospitals in Lahore during the 2008 dengue virus outbreak in Punjab, Pakistan. Int J Infect Dis 2010; 14(suppl 3):e54–e59.
- Guzman MG, Halstead SB, Artsob H, et al. Dengue: a continuing global threat. Nature Rev Microbiol 2010; 8:S7–S16.
- Crowell CS, Stamos JK. Evaluation of fever after international travel. Pediatr Ann 2011; 40:39–44.
- Durbin AP, Whitehead SS. Dengue vaccine candidates in development. Curr Top Microbiol Immunol 2010; 338:129–143.
- Chen LH, Wilson ME. Dengue and chikungunya infections in travelers. Curr Opin Infect Dis 2010; 23:438–444.
- Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis 2010; 4:e646.
- Leggat PA. Assessment of febrile illness in the returned traveller. Aust Fam Physician 2007; 36:328–332.
- Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med 2005; 353:924–932.
- Mohammed HP, Ramos MM, Rivera A, et al. Travel-associated dengue infections in the United States, 1996 to 2005. J Travel Med 2010; 17:8–14.
- Centers for Disease Control and Prevention (CDC). Travel-associated dengue surveillance—United States, 2006–2008. MMWR Morb Mortal Wkly Rep 2010; 59:715–719.
- Ang KT, Rohani I, Look CH. Role of primary care providers in dengue prevention and control in the community. Med J Malaysia 2010; 65:58–62.
- Centers for Disease Control and Prevention. Notice to readers: Changes to the national notifiable infectious disease list and data presentation—January 2010. MMWR Morb Mortal Wkly Rep 2010; 59:11. www.cdc.gov/mmwr/preview/mmwrhtml/mm5901a7.htm. Accessed May 29, 2012.
- Guzman A, Istúriz RE. Update on the global spread of dengue. Int J Antimicrob Agents 2010; 36:(suppl 1):S40–S42.
- Midgley CM, Bajwa-Joseph M, Vasanawathana S, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol 2011; 85:410–421.
- Gubler DJ. Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp 2006; 277:3–16.
- Franco L, Di Caro A, Carletti F, et al. Recent expansion of dengue virus serotype 3 in West Africa. Euro Surveill 2010; 15:19490.
- San Martín JL, Brathwaite O, Zambrano B, et al. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg 2010; 82:128–135.
- Ramos MM, Mohammed H, Zielinski-Gutierrez E, et al; Dengue Serosurvey Working Group. Epidemic dengue and dengue hemorrhagic fever at the Texas-Mexico border: results of a household-based seroepidemiologic survey, December 2005. Am J Trop Med Hyg 2008; 78:364–369.
- Ong DQ, Sitaram N, Rajakulendran M, et al. Knowledge and practice of household mosquito breeding control measures between a dengue hotspot and non-hotspot in Singapore. Ann Acad Med Singapore 2010; 39:146–149.
- Vazquez-Prokopec GM, Chaves LF, Ritchie SA, Davis J, Kitron U. Unforeseen costs of cutting mosquito surveillance budgets. PLoS Negl Trop Dis 2010; 4:e858.
- Ballenger-Browning KK, Elder JP. Multi-modal Aedes aegypti mosquito reduction interventions and dengue fever prevention. Trop Med Int Health 2009; 14:1542–1551.
- Courtney M, Shetty AK. Imported dengue fever: an important reemerging disease. Pediatr Emerg Care 2009; 25:769–772.
- Morens DM, Fauci AS. Dengue and hemorrhagic fever: a potential threat to public health in the United States. JAMA 2008; 299:214–216.
- Gregory CJ, Santiago LM, Argüello DF, Hunsperger E, Tomashek KM. Clinical and laboratory features that differentiate dengue from other febrile illnesses in an endemic area—Puerto Rico, 2007–2008. Am J Trop Med Hyg 2010; 82:922–929.
- Mohammed H, Ramos M, Armstrong J, et al. An outbreak of dengue fever in St. Croix (US Virgin Islands), 2005. PLoS One 2010; 5):e13729.
- Li DS, Liu W, Guigon A, Mostyn C, Grant R, Aaskov J. Rapid displacement of dengue virus type 1 by type 4, Pacific region, 2007–2009. Emerg Infect Dis 2010; 16:123–125.
- Hayden MH, Uejio CK, Walker K, et al. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, US/Sonora, MX border. Ecohealth 2010; 7:64–77.
- Knudsen AB. The significance of the introduction of Aedes albopictus into the southeastern United States with implications for the Caribbean, and perspectives of the Pan American Health Organization. J Am Mosq Control Assoc 1986; 2:420–423.
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol 2004; 18:215–227.
- Franco C, Hynes NA, Bouri N, Henderson DA. The dengue threat to the United States. Biosecur Bioterror 2010; 8:273–276.
- Centers for Disease Control and Prevention (CDC). Dengue hemorrhagic fever—US-Mexico border, 2005. MMWR Morb Mortal Wkly Rep 2007; 56:785–789.
- Brunkard JM, Robles López JL, Ramirez J, et al. Dengue fever seroprevalence and risk factors, Texas-Mexico border, 2004. Emerg Infect Dis 2007; 13:1477–1483.
- Hafkin B, Kaplan JE, Reed C, et al. Reintroduction of dengue fever into the continental United States. I. Dengue surveillance in Texas, 1980. Am J Trop Med Hyg 1982; 31:1222–1228.
- Centers for Disease Control and Prevention (CDC). Locally acquired Dengue—Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep 2010; 59:577–581.
- Gill J, Stark LM, Clark GG. Dengue surveillance in Florida, 1997–98. Emerg Infect Dis 2000; 6:30–35.
- Department of Health. DOH investigates cases of dengue fever on Oahu and asks public & community to be vigilant. http://hawaii.gov/health/about/pr/2011/11-028.pdf. Accessed May 29, 2012.
- Wilder-Smith A, Earnest A, Tan SB, Ooi EE, Gubler DJ. Lack of association of dengue activity with haze. Epidemiol Infect 2010; 138:962–967.
- Kyle JL, Harris E. Global spread and persistence of dengue. Annu Rev Microbiol 2008; 62:71–92.
- Chrispal A, Boorugu H, Gopinath KG, et al. Acute undifferentiated febrile illness in adult hospitalized patients: the disease spectrum and diagnostic predictors—an experience from a tertiary care hospital in South India. Trop Doct 2010; 40:230–234.
- World Health Organization; the Special Programme for Research and Training in Tropical Diseases (TDR). Dengue: guidelines for diagnosis, treatment, prevention and control. www.who.int/rpc/guidelines/9789241547871/en/. Accessed May 29, 2012.
- Potts JA, Rothman AL. Clinical and laboratory features that distinguish dengue from other febrile illnesses in endemic populations. Trop Med Int Health 2008; 13:1328–1340.
- Streit JA, Yang M, Cavanaugh JE, Polgreen PM. Upward trend in dengue incidence among hospitalized patients, United States. Emerg Infect Dis 2011; 17:914–916.
- Jelinek T. Dengue fever in international travelers. Clin Infect Dis 2000; 31:144–147.
- Gibbons RV, Vaughn DW. Dengue: an escalating problem. BMJ 2002; 324:1563–1566.
- Gibbons RV. Dengue conundrums. Int J Antimicrob Agents 2010; 36(suppl 1):S36–S39.
- Halstead SB. Dengue. Lancet 2007; 370:1644–1652.
- Halstead SB. Antibodies determine virulence in dengue. Ann N Y Acad Sci 2009; 1171(suppl 1):E48–E56.
- Coffey LL, Mertens E, Brehin AC, et al. Human genetic determinants of dengue virus susceptibility. Microbes Infect 2009; 11:143–156.
- García G, Sierra B, Pérez AB, et al. Asymptomatic dengue infection in a Cuban population confirms the protective role of the RR variant of the FcgammaRIIa polymorphism. Am J Trop Med Hyg 2010; 82:1153–1156.
- Braga C, Luna CF, Martelli CM, et al. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop 2010; 113:234–240.
- Jain A, Chaturvedi UC. Dengue in infants: an overview. FEMS Immunol Med Microbiol 2010; 59:119–130.
- Almas A, Parkash O, Akhter J. Clinical factors associated with mortality in dengue infection at a tertiary care center. Southeast Asian J Trop Med Public Health 2010; 41:333–340.
- Diaz-Quijano FA, Villar-Centeno LA, Martinez-Vega RA. Predictors of spontaneous bleeding in patients with acute febrile syndrome from a dengue endemic area. J Clin Virol 2010; 49:11–15.
- Marón GM, Clará AW, Diddle JW, et al. Association between nutritional status and severity of dengue infection in children in El Salvador. Am J Trop Med Hyg 2010; 82:324–329.
- Sierra B, Perez AB, Vogt K, et al. Secondary heterologous dengue infection risk: disequilibrium between immune regulation and inflammation? Cell Immunol 2010; 262:134–140.
- Brien JD, Austin SK, Sukupolvi-Petty S, et al. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J Virol 2010; 84:10630–10643.
- Humayoun MA, Waseem T, Jawa AA, Hashmi MS, Akram J. Multiple dengue serotypes and high frequency of dengue hemorrhagic fever at two tertiary care hospitals in Lahore during the 2008 dengue virus outbreak in Punjab, Pakistan. Int J Infect Dis 2010; 14(suppl 3):e54–e59.
- Guzman MG, Halstead SB, Artsob H, et al. Dengue: a continuing global threat. Nature Rev Microbiol 2010; 8:S7–S16.
- Crowell CS, Stamos JK. Evaluation of fever after international travel. Pediatr Ann 2011; 40:39–44.
- Durbin AP, Whitehead SS. Dengue vaccine candidates in development. Curr Top Microbiol Immunol 2010; 338:129–143.
- Chen LH, Wilson ME. Dengue and chikungunya infections in travelers. Curr Opin Infect Dis 2010; 23:438–444.
- Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis 2010; 4:e646.
KEY POINTS
- Dengue results from infection with one of four distinct serotypes: DENV-1, DENV-2, DENV-3, and DENV-4.
- The most common outcome after infection by the bite of an Aedes mosquito (which bites in the daytime) is asymptomatic infection, a flulike illness, or classic self-limited dengue fever. Severe, life-threatening disease with hemorrhagic manifestations or shock is rare.
- Obtaining a history of recent travel to a dengue-endemic area is a key in evaluating a person presenting with undifferentiated fever or a fever-rash-arthralgia syndrome.
- Diagnostic testing is based on the natural history of infection; antibody levels begin to rise as levels of viremia begin to decline.
- Risk factors help predict who will develop severe dengue after primary or secondary infection.