User login
What’s the best VTE treatment for patients with cancer?
EVIDENCE SUMMARY
No head-to-head studies or umbrella meta-analyses assess all the main treatments for VTE against each other.
Long-term LMWH decreases VTE recurrence compared with VKA
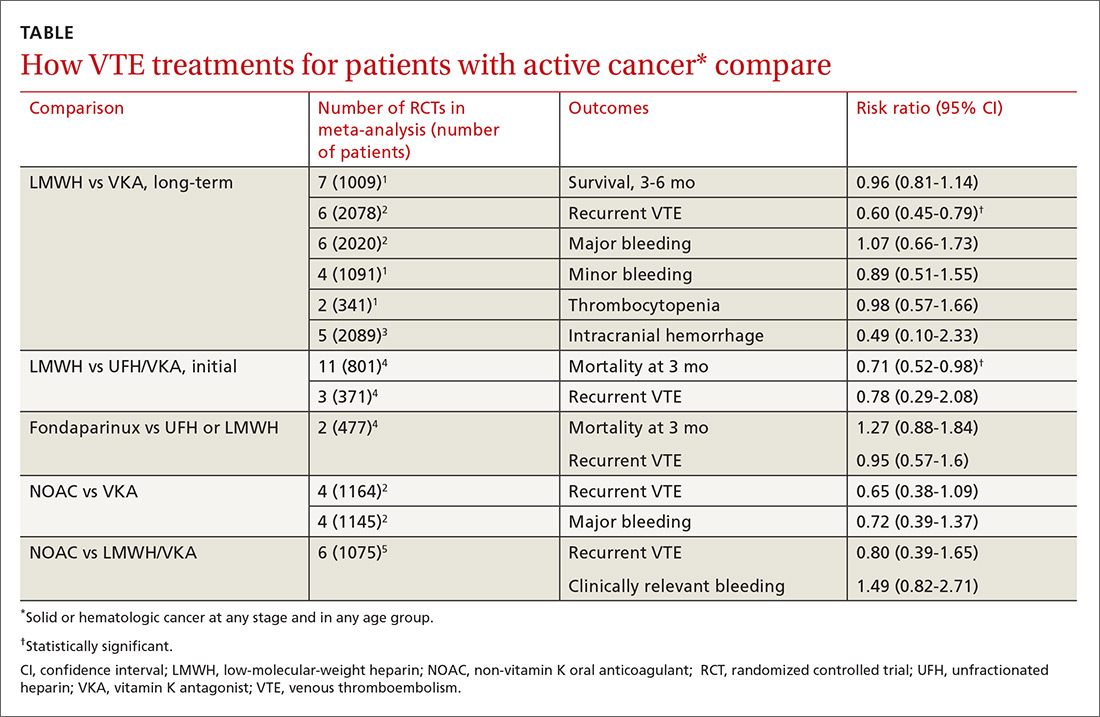
Two meta-analyses of RCTs evaluating LMWH and VKA for long-term treatment (3-12 months) of confirmed VTE in patients with cancer found that LMWH didn’t change mortality, but reduced the rate of VTE recurrence compared with VKA (40% relative reduction).1,2 The comparison showed no differences in major or minor bleeding or thrombocytopenia between LMWH and VKA (TABLE1-5).
The studies included patients with any solid or hematologic cancer at any stage and from any age group, including children. Overall, the mean age of patients was in the mid 60s; approximately 50% were male when specified. Investigators rated the evidence quality as moderate for VTE, but low for the other outcomes.1
The most recent meta-analysis of the same RCTs comparing LMWH with VKA evaluated intracranial hemorrhage rates and found no difference.3
Initial therapy with LMWH: A look at mortality
A meta-analysis of RCTs that compared LMWH with UFH/VKA for initial treatment of confirmed VTE in adult cancer patients (any type or stage of cancer, mean ages not specified) found that LMWH reduced mortality by 30%, but didn’t affect VTE recurrence or major bleeding.4
The control groups received UFH for 5 to 10 days and then continued with VKA, whereas the experimental groups received different types of LMWH (reviparin, nadroparin, tinzaparin, enoxaparin) initially and for 3 months thereafter. Investigators rated all studies low quality because of imprecision and publication bias favoring LMWH.
Fondaparinux shows no advantage for initial therapy
The same meta-analysis compared initial treatment with fondaparinux and initial therapy with enoxaparin or UFH transitioning to warfarin.4 It found no differences in any outcomes at 3 months. Investigators rated both studies as low quality for recurrent VTE and moderate for mortality and bleeding.
Continue to: Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA
Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA: No differences
A meta-analysis of RCTs comparing NOACs (dabigatran, edoxaban, apixaban, rivaroxaban) with VKA for 6 months found no differences in recurrent VTE or major bleeding.2
A second meta-analysis of RCTs that compared NOACs (rivaroxaban, dabigatran, apixaban) with control (LMWH followed by VKA) in adult cancer patients (mean ages, 54-66 years; 50%-60% men) reported no difference in the composite outcome of recurrent VTE or VTE-related death nor clinically significant bleeding over 1 to 36 months (most RCTs ran 3-12 months).5 Separate comparisons for rivaroxaban and dabigatran found no difference in the composite outcome, and rivaroxaban also produced no difference in clinically-significant bleeding.
RECOMMENDATIONS
The 2016 CHEST guidelines recommend LMWH as first-line treatment for VTE in patients with cancer and indicate no preference between NOACs and VKA for second-line treatment.6
1. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(7):CD006650.
2. Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582-589.
3. Rojas-Hernandez CM, Oo TH, García-Perdomo HA. Risk of intracranial hemorrhage associated with therapeutic anticoagulation for venous thromboembolism in cancer patients: a systematic review and meta-analysis. J Thromb Thrombolysis. 2017;43:233-240.
4. Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(6):CD006649.
5. Sardar P, Chatterjee S, Herzog E, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22:460-468.
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.
EVIDENCE SUMMARY
No head-to-head studies or umbrella meta-analyses assess all the main treatments for VTE against each other.
Long-term LMWH decreases VTE recurrence compared with VKA
Two meta-analyses of RCTs evaluating LMWH and VKA for long-term treatment (3-12 months) of confirmed VTE in patients with cancer found that LMWH didn’t change mortality, but reduced the rate of VTE recurrence compared with VKA (40% relative reduction).1,2 The comparison showed no differences in major or minor bleeding or thrombocytopenia between LMWH and VKA (TABLE1-5).
The studies included patients with any solid or hematologic cancer at any stage and from any age group, including children. Overall, the mean age of patients was in the mid 60s; approximately 50% were male when specified. Investigators rated the evidence quality as moderate for VTE, but low for the other outcomes.1
The most recent meta-analysis of the same RCTs comparing LMWH with VKA evaluated intracranial hemorrhage rates and found no difference.3
Initial therapy with LMWH: A look at mortality
A meta-analysis of RCTs that compared LMWH with UFH/VKA for initial treatment of confirmed VTE in adult cancer patients (any type or stage of cancer, mean ages not specified) found that LMWH reduced mortality by 30%, but didn’t affect VTE recurrence or major bleeding.4
The control groups received UFH for 5 to 10 days and then continued with VKA, whereas the experimental groups received different types of LMWH (reviparin, nadroparin, tinzaparin, enoxaparin) initially and for 3 months thereafter. Investigators rated all studies low quality because of imprecision and publication bias favoring LMWH.
Fondaparinux shows no advantage for initial therapy
The same meta-analysis compared initial treatment with fondaparinux and initial therapy with enoxaparin or UFH transitioning to warfarin.4 It found no differences in any outcomes at 3 months. Investigators rated both studies as low quality for recurrent VTE and moderate for mortality and bleeding.
Continue to: Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA
Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA: No differences
A meta-analysis of RCTs comparing NOACs (dabigatran, edoxaban, apixaban, rivaroxaban) with VKA for 6 months found no differences in recurrent VTE or major bleeding.2
A second meta-analysis of RCTs that compared NOACs (rivaroxaban, dabigatran, apixaban) with control (LMWH followed by VKA) in adult cancer patients (mean ages, 54-66 years; 50%-60% men) reported no difference in the composite outcome of recurrent VTE or VTE-related death nor clinically significant bleeding over 1 to 36 months (most RCTs ran 3-12 months).5 Separate comparisons for rivaroxaban and dabigatran found no difference in the composite outcome, and rivaroxaban also produced no difference in clinically-significant bleeding.
RECOMMENDATIONS
The 2016 CHEST guidelines recommend LMWH as first-line treatment for VTE in patients with cancer and indicate no preference between NOACs and VKA for second-line treatment.6
EVIDENCE SUMMARY
No head-to-head studies or umbrella meta-analyses assess all the main treatments for VTE against each other.
Long-term LMWH decreases VTE recurrence compared with VKA
Two meta-analyses of RCTs evaluating LMWH and VKA for long-term treatment (3-12 months) of confirmed VTE in patients with cancer found that LMWH didn’t change mortality, but reduced the rate of VTE recurrence compared with VKA (40% relative reduction).1,2 The comparison showed no differences in major or minor bleeding or thrombocytopenia between LMWH and VKA (TABLE1-5).
The studies included patients with any solid or hematologic cancer at any stage and from any age group, including children. Overall, the mean age of patients was in the mid 60s; approximately 50% were male when specified. Investigators rated the evidence quality as moderate for VTE, but low for the other outcomes.1
The most recent meta-analysis of the same RCTs comparing LMWH with VKA evaluated intracranial hemorrhage rates and found no difference.3
Initial therapy with LMWH: A look at mortality
A meta-analysis of RCTs that compared LMWH with UFH/VKA for initial treatment of confirmed VTE in adult cancer patients (any type or stage of cancer, mean ages not specified) found that LMWH reduced mortality by 30%, but didn’t affect VTE recurrence or major bleeding.4
The control groups received UFH for 5 to 10 days and then continued with VKA, whereas the experimental groups received different types of LMWH (reviparin, nadroparin, tinzaparin, enoxaparin) initially and for 3 months thereafter. Investigators rated all studies low quality because of imprecision and publication bias favoring LMWH.
Fondaparinux shows no advantage for initial therapy
The same meta-analysis compared initial treatment with fondaparinux and initial therapy with enoxaparin or UFH transitioning to warfarin.4 It found no differences in any outcomes at 3 months. Investigators rated both studies as low quality for recurrent VTE and moderate for mortality and bleeding.
Continue to: Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA
Non-vitamin K oral anticoagulants vs LMWH/VKA or VKA: No differences
A meta-analysis of RCTs comparing NOACs (dabigatran, edoxaban, apixaban, rivaroxaban) with VKA for 6 months found no differences in recurrent VTE or major bleeding.2
A second meta-analysis of RCTs that compared NOACs (rivaroxaban, dabigatran, apixaban) with control (LMWH followed by VKA) in adult cancer patients (mean ages, 54-66 years; 50%-60% men) reported no difference in the composite outcome of recurrent VTE or VTE-related death nor clinically significant bleeding over 1 to 36 months (most RCTs ran 3-12 months).5 Separate comparisons for rivaroxaban and dabigatran found no difference in the composite outcome, and rivaroxaban also produced no difference in clinically-significant bleeding.
RECOMMENDATIONS
The 2016 CHEST guidelines recommend LMWH as first-line treatment for VTE in patients with cancer and indicate no preference between NOACs and VKA for second-line treatment.6
1. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(7):CD006650.
2. Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582-589.
3. Rojas-Hernandez CM, Oo TH, García-Perdomo HA. Risk of intracranial hemorrhage associated with therapeutic anticoagulation for venous thromboembolism in cancer patients: a systematic review and meta-analysis. J Thromb Thrombolysis. 2017;43:233-240.
4. Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(6):CD006649.
5. Sardar P, Chatterjee S, Herzog E, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22:460-468.
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.
1. Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(7):CD006650.
2. Posch F, Königsbrügge O, Zielinski C, et al. Treatment of venous thromboembolism in patients with cancer: A network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136:582-589.
3. Rojas-Hernandez CM, Oo TH, García-Perdomo HA. Risk of intracranial hemorrhage associated with therapeutic anticoagulation for venous thromboembolism in cancer patients: a systematic review and meta-analysis. J Thromb Thrombolysis. 2017;43:233-240.
4. Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;(6):CD006649.
5. Sardar P, Chatterjee S, Herzog E, et al. New oral anticoagulants in patients with cancer: current state of evidence. Am J Ther. 2015;22:460-468.
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315-352.
EVIDENCE-BASED ANSWER:
No head-to-head studies directly compare all the main treatments for venous thromboembolism (VTE) in cancer patients. Long-term treatment (3-12 months) with low-molecular-weight heparin (LMWH) reduces recurrence of VTE by 40% compared with vitamin K antagonists (VKA), but doesn’t change rates of mortality, major or minor bleeding, or intracranial hemorrhage in patients with solid or hematologic cancer at any stage or in any age group. Initial treatment with LMWH reduces mortality by 30% compared with unfractionated heparin (UFH) for 5 to 10 days followed by warfarin, but doesn’t alter recurrent VTE or bleeding. Non-vitamin K oral anticoagulants (NOACs) have risks of recurrent VTE or VTE-related death (composite outcome) and clinically significant bleeding comparable to VKA or LMWH/VKA (strength of recommendation [SOR]: B, meta-analyses of randomized controlled trials [RCTs], mostly of low quality).
How effectively do ACE inhibitors and ARBs prevent migraines?
EVIDENCE SUMMARY
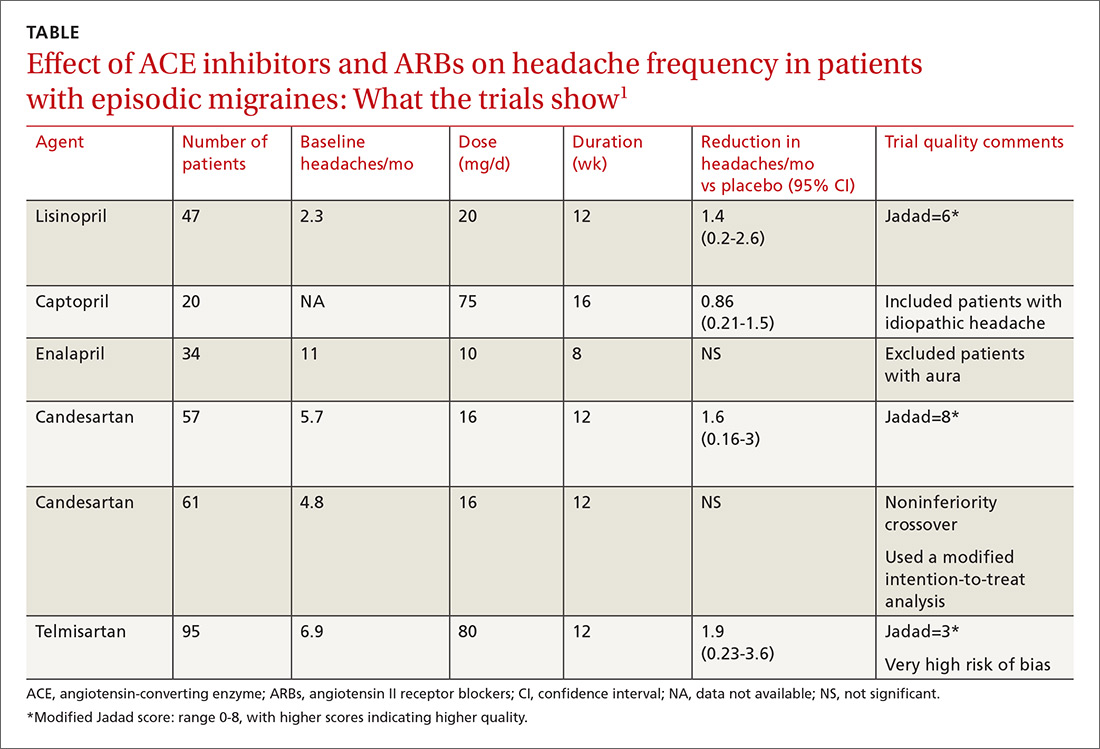
A network meta-analysis of 179 placebo-controlled trials of medications to treat migraine1 headache identified 3 trials involving ACE inhibitors and 3 involving ARBs (TABLE1). The authors of the meta-analysis gave 2 trials (one of lisinopril and one of candesartan) relatively high scores for methodologic quality.
Lisinopril reduces hours, days with headache and days with migraine
The first, a placebo-controlled lisinopril crossover trial, included 60 patients, 19 to 59 years of age, who experienced migraines with or without auras 2 to 6 times per month.2 Thirty patients received lisinopril 10 mg once daily for 1 week followed by 20 mg once daily (using 10-mg tablets) for 11 weeks. The other 30 patients received a similarly titrated placebo for 12 weeks. After a 2-week washout period, the groups were given the other therapy. Patients took triptan medications and analgesics as needed. Primary outcomes, extracted from headache diaries, included the number of hours and days with headache (of any type) and number of days with migraine specifically.
Out of the initial 60 participants, 47 completed the study. Using intention-to-treat analysis, lisinopril therapy resulted in fewer hours with headache (162 vs 138, a 15% difference; 95% confidence interval [CI], 0-30), fewer days with headache (25 vs 21, a 16% difference; 95% CI, 5-27), and fewer days with migraine (19 vs 15, a 22% change; 95% CI, 11-33), compared with placebo. Three patients discontinued lisinopril because of adverse events. Mean blood pressure reduction with lisinopril was 7 mm Hg systolic and 5 mm Hg diastolic more than placebo (P<.0001 for both comparisons).
Candesartan also decreases headaches and migraine
The other study given a high methodologic quality score by the network-meta-analysis authors was a placebo-controlled candesartan crossover trial.3 It enrolled 60 patients, 18 to 65 years of age, who experienced migraines with or without auras 2 to 6 times per month.
Thirty patients received 16 mg candesartan daily for 12 weeks, followed by a 4-week washout period before taking a placebo tablet daily for 12 weeks. The other 30 received placebo followed by candesartan. Patients took triptan medications and analgesics as needed. The primary outcome measure was days with headache, recorded by patients using daily diaries. Three patients didn’t complete the study.
Using intention-to-treat analysis, the mean number of days with headache was 18.5 with placebo and 13.6 with candesartan (P=.001). Secondary end points that also favored candesartan were hours with migraine (92 vs 59; P<.001), hours with headache (139 vs 95; P<.001), days with migraine (13 vs 9; P<.001), and days of sick leave (3.9 vs 1.4; P=.01). Adverse events, including dizziness, were similar with candesartan and placebo. Mean blood pressure reduction with candesartan was 11 mm Hg systolic and 7 mm Hg diastolic over placebo (P<.001 for both comparisons).
Continue to: Overall both drugs have a significant effect on number of headaches
Overall both drugs have a significant effect on number of headaches
Among all ACE inhibitor and ARB trials in the review, a network meta-analysis (designed to compare interventions never studied head-to-head) could be performed only on candesartan, which had a small effect size on headache frequency relative to placebo (2 trials, 118 patients; standardized mean difference [SMD]= −0.33; 95% CI, −0.59 to −0.7).1 (An SMD of 0.2 is considered small, 0.6 moderate, and 1.2 large). Combining data from all ACE inhibitor and ARB trials together in a standard meta-analysis yielded a large effect size on number of headaches per month compared with placebo (6 trials, 351 patients; SMD= −1.12; 95% CI, −1.97 to −0.27).1
RECOMMENDATIONS
In 2012, the American Academy of Neurology and the American Headache Society published guidelines on pharmacologic treatment for episodic migraine prevention in adults.4 The guidelines stated that lisinopril and candesartan were “possibly effective” for migraine prevention (level C recommendation based on a single lower-quality randomized clinical trial). They further advised clinicians to be “mindful of comorbid and coexistent conditions in patients with migraine to maximize potential treatment efficacy.”
1. Jackson JL, Cogbil E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PloS One. 2015;10:e0130733.
2. Schrader H, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomized, placebo controlled, crossover study. BMJ. 2001;322:19-22.
3. Tronvik E, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA. 2003;289:65-69.
4. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
EVIDENCE SUMMARY
A network meta-analysis of 179 placebo-controlled trials of medications to treat migraine1 headache identified 3 trials involving ACE inhibitors and 3 involving ARBs (TABLE1). The authors of the meta-analysis gave 2 trials (one of lisinopril and one of candesartan) relatively high scores for methodologic quality.
Lisinopril reduces hours, days with headache and days with migraine
The first, a placebo-controlled lisinopril crossover trial, included 60 patients, 19 to 59 years of age, who experienced migraines with or without auras 2 to 6 times per month.2 Thirty patients received lisinopril 10 mg once daily for 1 week followed by 20 mg once daily (using 10-mg tablets) for 11 weeks. The other 30 patients received a similarly titrated placebo for 12 weeks. After a 2-week washout period, the groups were given the other therapy. Patients took triptan medications and analgesics as needed. Primary outcomes, extracted from headache diaries, included the number of hours and days with headache (of any type) and number of days with migraine specifically.
Out of the initial 60 participants, 47 completed the study. Using intention-to-treat analysis, lisinopril therapy resulted in fewer hours with headache (162 vs 138, a 15% difference; 95% confidence interval [CI], 0-30), fewer days with headache (25 vs 21, a 16% difference; 95% CI, 5-27), and fewer days with migraine (19 vs 15, a 22% change; 95% CI, 11-33), compared with placebo. Three patients discontinued lisinopril because of adverse events. Mean blood pressure reduction with lisinopril was 7 mm Hg systolic and 5 mm Hg diastolic more than placebo (P<.0001 for both comparisons).
Candesartan also decreases headaches and migraine
The other study given a high methodologic quality score by the network-meta-analysis authors was a placebo-controlled candesartan crossover trial.3 It enrolled 60 patients, 18 to 65 years of age, who experienced migraines with or without auras 2 to 6 times per month.
Thirty patients received 16 mg candesartan daily for 12 weeks, followed by a 4-week washout period before taking a placebo tablet daily for 12 weeks. The other 30 received placebo followed by candesartan. Patients took triptan medications and analgesics as needed. The primary outcome measure was days with headache, recorded by patients using daily diaries. Three patients didn’t complete the study.
Using intention-to-treat analysis, the mean number of days with headache was 18.5 with placebo and 13.6 with candesartan (P=.001). Secondary end points that also favored candesartan were hours with migraine (92 vs 59; P<.001), hours with headache (139 vs 95; P<.001), days with migraine (13 vs 9; P<.001), and days of sick leave (3.9 vs 1.4; P=.01). Adverse events, including dizziness, were similar with candesartan and placebo. Mean blood pressure reduction with candesartan was 11 mm Hg systolic and 7 mm Hg diastolic over placebo (P<.001 for both comparisons).
Continue to: Overall both drugs have a significant effect on number of headaches
Overall both drugs have a significant effect on number of headaches
Among all ACE inhibitor and ARB trials in the review, a network meta-analysis (designed to compare interventions never studied head-to-head) could be performed only on candesartan, which had a small effect size on headache frequency relative to placebo (2 trials, 118 patients; standardized mean difference [SMD]= −0.33; 95% CI, −0.59 to −0.7).1 (An SMD of 0.2 is considered small, 0.6 moderate, and 1.2 large). Combining data from all ACE inhibitor and ARB trials together in a standard meta-analysis yielded a large effect size on number of headaches per month compared with placebo (6 trials, 351 patients; SMD= −1.12; 95% CI, −1.97 to −0.27).1
RECOMMENDATIONS
In 2012, the American Academy of Neurology and the American Headache Society published guidelines on pharmacologic treatment for episodic migraine prevention in adults.4 The guidelines stated that lisinopril and candesartan were “possibly effective” for migraine prevention (level C recommendation based on a single lower-quality randomized clinical trial). They further advised clinicians to be “mindful of comorbid and coexistent conditions in patients with migraine to maximize potential treatment efficacy.”
EVIDENCE SUMMARY
A network meta-analysis of 179 placebo-controlled trials of medications to treat migraine1 headache identified 3 trials involving ACE inhibitors and 3 involving ARBs (TABLE1). The authors of the meta-analysis gave 2 trials (one of lisinopril and one of candesartan) relatively high scores for methodologic quality.
Lisinopril reduces hours, days with headache and days with migraine
The first, a placebo-controlled lisinopril crossover trial, included 60 patients, 19 to 59 years of age, who experienced migraines with or without auras 2 to 6 times per month.2 Thirty patients received lisinopril 10 mg once daily for 1 week followed by 20 mg once daily (using 10-mg tablets) for 11 weeks. The other 30 patients received a similarly titrated placebo for 12 weeks. After a 2-week washout period, the groups were given the other therapy. Patients took triptan medications and analgesics as needed. Primary outcomes, extracted from headache diaries, included the number of hours and days with headache (of any type) and number of days with migraine specifically.
Out of the initial 60 participants, 47 completed the study. Using intention-to-treat analysis, lisinopril therapy resulted in fewer hours with headache (162 vs 138, a 15% difference; 95% confidence interval [CI], 0-30), fewer days with headache (25 vs 21, a 16% difference; 95% CI, 5-27), and fewer days with migraine (19 vs 15, a 22% change; 95% CI, 11-33), compared with placebo. Three patients discontinued lisinopril because of adverse events. Mean blood pressure reduction with lisinopril was 7 mm Hg systolic and 5 mm Hg diastolic more than placebo (P<.0001 for both comparisons).
Candesartan also decreases headaches and migraine
The other study given a high methodologic quality score by the network-meta-analysis authors was a placebo-controlled candesartan crossover trial.3 It enrolled 60 patients, 18 to 65 years of age, who experienced migraines with or without auras 2 to 6 times per month.
Thirty patients received 16 mg candesartan daily for 12 weeks, followed by a 4-week washout period before taking a placebo tablet daily for 12 weeks. The other 30 received placebo followed by candesartan. Patients took triptan medications and analgesics as needed. The primary outcome measure was days with headache, recorded by patients using daily diaries. Three patients didn’t complete the study.
Using intention-to-treat analysis, the mean number of days with headache was 18.5 with placebo and 13.6 with candesartan (P=.001). Secondary end points that also favored candesartan were hours with migraine (92 vs 59; P<.001), hours with headache (139 vs 95; P<.001), days with migraine (13 vs 9; P<.001), and days of sick leave (3.9 vs 1.4; P=.01). Adverse events, including dizziness, were similar with candesartan and placebo. Mean blood pressure reduction with candesartan was 11 mm Hg systolic and 7 mm Hg diastolic over placebo (P<.001 for both comparisons).
Continue to: Overall both drugs have a significant effect on number of headaches
Overall both drugs have a significant effect on number of headaches
Among all ACE inhibitor and ARB trials in the review, a network meta-analysis (designed to compare interventions never studied head-to-head) could be performed only on candesartan, which had a small effect size on headache frequency relative to placebo (2 trials, 118 patients; standardized mean difference [SMD]= −0.33; 95% CI, −0.59 to −0.7).1 (An SMD of 0.2 is considered small, 0.6 moderate, and 1.2 large). Combining data from all ACE inhibitor and ARB trials together in a standard meta-analysis yielded a large effect size on number of headaches per month compared with placebo (6 trials, 351 patients; SMD= −1.12; 95% CI, −1.97 to −0.27).1
RECOMMENDATIONS
In 2012, the American Academy of Neurology and the American Headache Society published guidelines on pharmacologic treatment for episodic migraine prevention in adults.4 The guidelines stated that lisinopril and candesartan were “possibly effective” for migraine prevention (level C recommendation based on a single lower-quality randomized clinical trial). They further advised clinicians to be “mindful of comorbid and coexistent conditions in patients with migraine to maximize potential treatment efficacy.”
1. Jackson JL, Cogbil E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PloS One. 2015;10:e0130733.
2. Schrader H, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomized, placebo controlled, crossover study. BMJ. 2001;322:19-22.
3. Tronvik E, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA. 2003;289:65-69.
4. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
1. Jackson JL, Cogbil E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PloS One. 2015;10:e0130733.
2. Schrader H, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomized, placebo controlled, crossover study. BMJ. 2001;322:19-22.
3. Tronvik E, Stovner LJ, Helde G, et al. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA. 2003;289:65-69.
4. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
EVIDENCE-BASED ANSWER:
The angiotensin-converting enzyme (ACE) inhibitor lisinopril reduces the number of migraines by about 1.5 per month in patients experiencing 2 to 6 migraines monthly (strength of recommendation [SOR]: B, small crossover trial); the angiotensin II receptor blocker (ARB) candesartan may produce a similar reduction (SOR: C, conflicting crossover trials).
Considered as a group, ACE inhibitors and ARBs have a moderate to large effect on the frequency of migraine headaches (SOR: B, meta-analysis of small clinical trials), although only lisinopril and candesartan show fair to good evidence of efficacy.
Providers may consider lisinopril or candesartan for migraine prevention, taking into account their effect on other medical conditions (SOR: C, expert opinion).
Do statins alter the risk or progression of dementia?
EVIDENCE SUMMARY
A 2016 Cochrane systematic review identified 2 double-blind RCTs that evaluated statins for preventing cognitive decline and dementia in patients with either risk factors or a history of vascular disease.1 The authors couldn’t perform a meta-analysis because of heterogeneity.
Statins don’t prevent dementia
The first RCT found that 5804 patients (70-82 years old with pre-existing vascular disease or increased risk because of smoking, hypertension, or diabetes) manifested equivalent cognitive decline at 3.5 years after random assignment to pravastatin 40 mg/d or placebo.2 Investigators measured cognition with the Mini-Mental State Exam (MMSE), which scores cognitive function on a scale of 0 to 30, with higher numbers indicating better function (mean difference [MD] at follow-up=0.06 points; 95% confidence interval [CI], −0.04 to 0.16).
A second RCT evaluated simvastatin 40 mg/d or placebo for as long as 5 years in 20,536 patients 40 to 80 years of age with a history of coronary artery disease or diabetes.3 The study excluded patients with dementia at baseline. The odds of developing dementia didn’t differ between groups (odds ratio=1.0; 95% CI, 0.61-1.65).
Both studies were originally designed to measure cardiovascular outcomes. The authors rated both as high quality with a low risk of bias.
A contrast to earlier, lower-quality studies
These results contrast with an earlier meta-analysis based on one of the previously described RCTs and lower-quality evidence (16 cohort studies and 3 case-control studies) that found using statins to be associated with lower relative risk (RR) of dementia than not using a statin (all-type dementia RR=0.82; 95% CI, 0.69-0.97; Alzheimer’s disease RR=0.70; 95% CI, 0.60-0.83).3,4
The total patient population was more than 2 million and varied widely. Duration of statin use and type of statin (simvastatin, atorvastatin, fluvastatin, pravastatin, rosuvastatin) also varied. The authors noted potential bias in results for 2 reasons: Cross-sectional studies included patients with impaired cognition who were less likely to be prescribed statins, and statin use was determined by patient self-report.
Statins don’t treat dementia
A Cochrane review that included 4 RCTs with 1154 patients, 50 to 90 years old, assessed the effect of ≥6 months of statin therapy (atorvastatin 80 mg/d or simvastatin 40-80 mg/d) on the course of Alzheimer’s disease and vascular dementia.5 Most patients had mild to moderate dementia and most were also taking an anticholinesterase inhibitor.
Continue to: All studies reported...
All studies reported outcomes using the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), scored 0 to 70, with lower numbers indicating better function, and the MMSE. Results of statin use were equivalent to placebo (ADAS-Cog MD= −0.26; 95% CI, −1.05 to 0.52; MMSE MD= −0.32; 95% CI, −0.71 to 0.06).
But do they slow its progression?
In contrast, a case-control study of 6431 patients with mild-to-moderate Alzheimer’s disease concluded that statin use was associated with slower progression of AD.6 Using cholinesterase inhibitor discontinuation as a proxy for worsening dementia, researchers noted that patients with early statin exposure (719 patients) had a lower rate of cholinesterase discontinuation than patients who didn’t receive early statin therapy (RR=0.85; 95% CI, 0.76-0.95).
A 2016 systematic review attempted to identify randomized clinical trials evaluating the effects of statin withdrawal in dementia.7 None were found.
RECOMMENDATIONS
Based primarily on post-marketing surveillance data, the US Food and Drug Administration (FDA) has warned that memory loss and confusion are occasionally associated with statin use from within one day to several years of initiation.8 The FDA indicated that such symptoms are rare, not associated with dementia or clinically significant cognitive decline, and resolve with discontinuation of the medication.
1. McGuinness B, Craig D, Bullock R, et al. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;(1):CD003160.
2. Trompet S, van Vliet P, de Craen AJ, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257:85-90.
3. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7-22.
4. Wong WB, Lin VW, Boudreau D, et al. Statins in the prevention of dementia and Alzheimer’s disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf. 2013;22:345-358.
5. McGuinness B, Craig D, Bullock R, et al. Statins for the treatment of dementia. Cochrane Database Syst Rev. 2014;(7):CD007514.
6. Lin FC, Chuang YS, Hsieh HM, et al. Early statin use and the progression of Alzheimer disease: a total population-based case-control study. Medicine. 2015;94:e2143.
7. McGuinness B, Cardwell CR, Passmore P. Statin withdrawal in people with dementia. Cochrane Database Syst Rev. 2016;(9):CD012050.
8. US Food and Drug Administration. FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. Available at: www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Accessed August 24, 2018.
EVIDENCE SUMMARY
A 2016 Cochrane systematic review identified 2 double-blind RCTs that evaluated statins for preventing cognitive decline and dementia in patients with either risk factors or a history of vascular disease.1 The authors couldn’t perform a meta-analysis because of heterogeneity.
Statins don’t prevent dementia
The first RCT found that 5804 patients (70-82 years old with pre-existing vascular disease or increased risk because of smoking, hypertension, or diabetes) manifested equivalent cognitive decline at 3.5 years after random assignment to pravastatin 40 mg/d or placebo.2 Investigators measured cognition with the Mini-Mental State Exam (MMSE), which scores cognitive function on a scale of 0 to 30, with higher numbers indicating better function (mean difference [MD] at follow-up=0.06 points; 95% confidence interval [CI], −0.04 to 0.16).
A second RCT evaluated simvastatin 40 mg/d or placebo for as long as 5 years in 20,536 patients 40 to 80 years of age with a history of coronary artery disease or diabetes.3 The study excluded patients with dementia at baseline. The odds of developing dementia didn’t differ between groups (odds ratio=1.0; 95% CI, 0.61-1.65).
Both studies were originally designed to measure cardiovascular outcomes. The authors rated both as high quality with a low risk of bias.
A contrast to earlier, lower-quality studies
These results contrast with an earlier meta-analysis based on one of the previously described RCTs and lower-quality evidence (16 cohort studies and 3 case-control studies) that found using statins to be associated with lower relative risk (RR) of dementia than not using a statin (all-type dementia RR=0.82; 95% CI, 0.69-0.97; Alzheimer’s disease RR=0.70; 95% CI, 0.60-0.83).3,4
The total patient population was more than 2 million and varied widely. Duration of statin use and type of statin (simvastatin, atorvastatin, fluvastatin, pravastatin, rosuvastatin) also varied. The authors noted potential bias in results for 2 reasons: Cross-sectional studies included patients with impaired cognition who were less likely to be prescribed statins, and statin use was determined by patient self-report.
Statins don’t treat dementia
A Cochrane review that included 4 RCTs with 1154 patients, 50 to 90 years old, assessed the effect of ≥6 months of statin therapy (atorvastatin 80 mg/d or simvastatin 40-80 mg/d) on the course of Alzheimer’s disease and vascular dementia.5 Most patients had mild to moderate dementia and most were also taking an anticholinesterase inhibitor.
Continue to: All studies reported...
All studies reported outcomes using the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), scored 0 to 70, with lower numbers indicating better function, and the MMSE. Results of statin use were equivalent to placebo (ADAS-Cog MD= −0.26; 95% CI, −1.05 to 0.52; MMSE MD= −0.32; 95% CI, −0.71 to 0.06).
But do they slow its progression?
In contrast, a case-control study of 6431 patients with mild-to-moderate Alzheimer’s disease concluded that statin use was associated with slower progression of AD.6 Using cholinesterase inhibitor discontinuation as a proxy for worsening dementia, researchers noted that patients with early statin exposure (719 patients) had a lower rate of cholinesterase discontinuation than patients who didn’t receive early statin therapy (RR=0.85; 95% CI, 0.76-0.95).
A 2016 systematic review attempted to identify randomized clinical trials evaluating the effects of statin withdrawal in dementia.7 None were found.
RECOMMENDATIONS
Based primarily on post-marketing surveillance data, the US Food and Drug Administration (FDA) has warned that memory loss and confusion are occasionally associated with statin use from within one day to several years of initiation.8 The FDA indicated that such symptoms are rare, not associated with dementia or clinically significant cognitive decline, and resolve with discontinuation of the medication.
EVIDENCE SUMMARY
A 2016 Cochrane systematic review identified 2 double-blind RCTs that evaluated statins for preventing cognitive decline and dementia in patients with either risk factors or a history of vascular disease.1 The authors couldn’t perform a meta-analysis because of heterogeneity.
Statins don’t prevent dementia
The first RCT found that 5804 patients (70-82 years old with pre-existing vascular disease or increased risk because of smoking, hypertension, or diabetes) manifested equivalent cognitive decline at 3.5 years after random assignment to pravastatin 40 mg/d or placebo.2 Investigators measured cognition with the Mini-Mental State Exam (MMSE), which scores cognitive function on a scale of 0 to 30, with higher numbers indicating better function (mean difference [MD] at follow-up=0.06 points; 95% confidence interval [CI], −0.04 to 0.16).
A second RCT evaluated simvastatin 40 mg/d or placebo for as long as 5 years in 20,536 patients 40 to 80 years of age with a history of coronary artery disease or diabetes.3 The study excluded patients with dementia at baseline. The odds of developing dementia didn’t differ between groups (odds ratio=1.0; 95% CI, 0.61-1.65).
Both studies were originally designed to measure cardiovascular outcomes. The authors rated both as high quality with a low risk of bias.
A contrast to earlier, lower-quality studies
These results contrast with an earlier meta-analysis based on one of the previously described RCTs and lower-quality evidence (16 cohort studies and 3 case-control studies) that found using statins to be associated with lower relative risk (RR) of dementia than not using a statin (all-type dementia RR=0.82; 95% CI, 0.69-0.97; Alzheimer’s disease RR=0.70; 95% CI, 0.60-0.83).3,4
The total patient population was more than 2 million and varied widely. Duration of statin use and type of statin (simvastatin, atorvastatin, fluvastatin, pravastatin, rosuvastatin) also varied. The authors noted potential bias in results for 2 reasons: Cross-sectional studies included patients with impaired cognition who were less likely to be prescribed statins, and statin use was determined by patient self-report.
Statins don’t treat dementia
A Cochrane review that included 4 RCTs with 1154 patients, 50 to 90 years old, assessed the effect of ≥6 months of statin therapy (atorvastatin 80 mg/d or simvastatin 40-80 mg/d) on the course of Alzheimer’s disease and vascular dementia.5 Most patients had mild to moderate dementia and most were also taking an anticholinesterase inhibitor.
Continue to: All studies reported...
All studies reported outcomes using the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), scored 0 to 70, with lower numbers indicating better function, and the MMSE. Results of statin use were equivalent to placebo (ADAS-Cog MD= −0.26; 95% CI, −1.05 to 0.52; MMSE MD= −0.32; 95% CI, −0.71 to 0.06).
But do they slow its progression?
In contrast, a case-control study of 6431 patients with mild-to-moderate Alzheimer’s disease concluded that statin use was associated with slower progression of AD.6 Using cholinesterase inhibitor discontinuation as a proxy for worsening dementia, researchers noted that patients with early statin exposure (719 patients) had a lower rate of cholinesterase discontinuation than patients who didn’t receive early statin therapy (RR=0.85; 95% CI, 0.76-0.95).
A 2016 systematic review attempted to identify randomized clinical trials evaluating the effects of statin withdrawal in dementia.7 None were found.
RECOMMENDATIONS
Based primarily on post-marketing surveillance data, the US Food and Drug Administration (FDA) has warned that memory loss and confusion are occasionally associated with statin use from within one day to several years of initiation.8 The FDA indicated that such symptoms are rare, not associated with dementia or clinically significant cognitive decline, and resolve with discontinuation of the medication.
1. McGuinness B, Craig D, Bullock R, et al. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;(1):CD003160.
2. Trompet S, van Vliet P, de Craen AJ, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257:85-90.
3. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7-22.
4. Wong WB, Lin VW, Boudreau D, et al. Statins in the prevention of dementia and Alzheimer’s disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf. 2013;22:345-358.
5. McGuinness B, Craig D, Bullock R, et al. Statins for the treatment of dementia. Cochrane Database Syst Rev. 2014;(7):CD007514.
6. Lin FC, Chuang YS, Hsieh HM, et al. Early statin use and the progression of Alzheimer disease: a total population-based case-control study. Medicine. 2015;94:e2143.
7. McGuinness B, Cardwell CR, Passmore P. Statin withdrawal in people with dementia. Cochrane Database Syst Rev. 2016;(9):CD012050.
8. US Food and Drug Administration. FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. Available at: www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Accessed August 24, 2018.
1. McGuinness B, Craig D, Bullock R, et al. Statins for the prevention of dementia. Cochrane Database Syst Rev. 2016;(1):CD003160.
2. Trompet S, van Vliet P, de Craen AJ, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257:85-90.
3. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7-22.
4. Wong WB, Lin VW, Boudreau D, et al. Statins in the prevention of dementia and Alzheimer’s disease: a meta-analysis of observational studies and an assessment of confounding. Pharmacoepidemiol Drug Saf. 2013;22:345-358.
5. McGuinness B, Craig D, Bullock R, et al. Statins for the treatment of dementia. Cochrane Database Syst Rev. 2014;(7):CD007514.
6. Lin FC, Chuang YS, Hsieh HM, et al. Early statin use and the progression of Alzheimer disease: a total population-based case-control study. Medicine. 2015;94:e2143.
7. McGuinness B, Cardwell CR, Passmore P. Statin withdrawal in people with dementia. Cochrane Database Syst Rev. 2016;(9):CD012050.
8. US Food and Drug Administration. FDA Drug Safety Communication: Important safety label changes to cholesterol-lowering statin drugs. Available at: www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Accessed August 24, 2018.
EVIDENCE-BASED SUMMARY:
Neither moderate- nor high-intensity statin therapy (with simvastatin or atorvastatin, respectively) improves existing mild to moderately severe Alzheimer’s or vascular dementia (SOR: A, RCTs).
Although statin use is associated with a mild, rare, reversible delirium, it isn’t linked to permanent cognitive decline (SOR: C, expert opinion).
What’s the best secondary treatment for patients who fail initial triple therapy for H pylori?
EVIDENCE SUMMARY
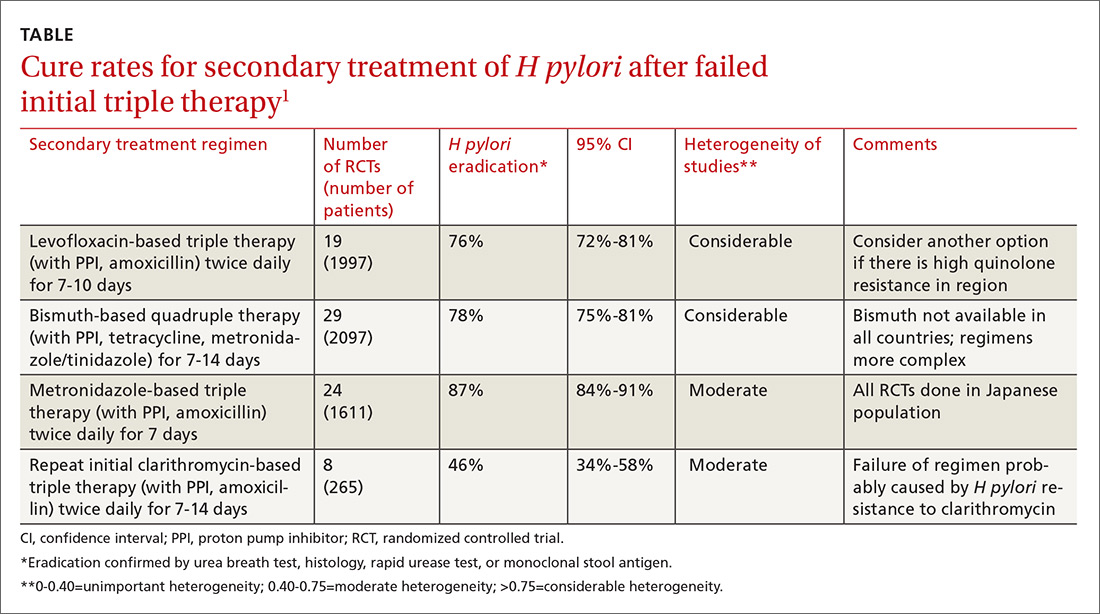
A meta-analysis of RCTs evaluating levofloxacin-based triple therapy as a secondary treatment regimen for patients with H pylori infection who had failed initial clarithromycin-based triple therapy found cure rates averaging 76% (TABLE).1 Most of the regimens comprised levofloxacin (500 mg), amoxicillin (1 g), and a PPI (40 mg), all twice daily for 7 to 10 days. Ten-day regimens produced better cure rates than 7-day regimens (84% vs 69%; comparison statistic not supplied).
The meta-analysis also included RCTs evaluating bismuth-based quadruple therapy as secondary treatment, which found cure rates averaging 78%.1 The regimens varied, comprising bismuth salts (120-600 mg, 2-4 times daily), metronidazole (250-500 mg, 2-4 times daily), tetracycline (250-500 mg, 2-4 times daily), and a PPI (40 mg twice daily). Longer duration of therapy produced higher cure rates (7 days=76%; 95% confidence interval [CI], 0.72-0.80 in 29 RCTs with 2097 patients; 10 days=77%; 95% CI, 0.60-0.93 in 2 RCTs with 142 patients; 14 days=82%; 95% CI, 0.76-0.88 in 12 RCTs with 831 patients).
Repeating the original clarithromycin-based triple therapy (8 RCTs, 265 patients) produced low cure rates (46%).1
Metronidazole-based therapy has high cure rate in a homogeneous population
A meta-analysis of 24 RCTs (1611 patients) that evaluated metronidazole-based triple therapy (mostly composed of amoxicillin 750 mg, metronidazole 250 mg, and any of a number of PPIs, all dosed at 40 mg) twice daily for 7 days found cure rates averaging 87% in an exclusively Japanese study population.1
Comparable cure rates for levofloxacin- and bismuth-based therapy
Six RCTs with a total of 1057 patients compared cure rates for levofloxacin-based triple therapy with bismuth-based quadruple therapy and found no difference.1
Two earlier meta-analyses not included in the previously described study, comprising 8 RCTs with a total of 613 patients, produced conflicting results. The larger study (15 RCTs, 1462 patients) found no difference in cure rates.2 The smaller study (7 RCTs, 787 patients) favored quadruple therapy.3
Continue to: Two secondary antibiotic regimens show similar cure rates
Two secondary antibiotic regimens show similar cure rates
A meta-analysis of 4 RCTs (total 460 patients) that compared susceptibility-guided antibiotic secondary treatment (SGT) with empiric antibiotic secondary treatment found no difference in cure rates, although the largest single RCT (172 patients) favored SGT.4
RECOMMENDATIONS
The Maastricht IV/Florence Consensus Report (a periodically updated European study group evaluating Helicobacter management) includes expert opinion-based guidelines for H pylori treatment that recommend using antibiotic susceptibility to select treatment regimens in the event of 2 treatment failures.5 The report also notes that bismuth-based quadruple therapy may not be available in all countries and has a more complex dosing regimen, and that local resistance to levofloxacin must be taken into account when prescribing levofloxacin-based triple therapy.
1. Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy failure (for Helicobacter pylori eradication). Expert Opin Pharmacother. 2013;14:843-861.
2. Di Caro S, Fini L, Daoud Y, et al. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastro. 2012;18:5669-5678.
3. Wu C, Chen X, Liu J, et al. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter. 2011;16:131-138.
4. Lopez-Gongora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015;70:2447-2455.
5. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
EVIDENCE SUMMARY
A meta-analysis of RCTs evaluating levofloxacin-based triple therapy as a secondary treatment regimen for patients with H pylori infection who had failed initial clarithromycin-based triple therapy found cure rates averaging 76% (TABLE).1 Most of the regimens comprised levofloxacin (500 mg), amoxicillin (1 g), and a PPI (40 mg), all twice daily for 7 to 10 days. Ten-day regimens produced better cure rates than 7-day regimens (84% vs 69%; comparison statistic not supplied).
The meta-analysis also included RCTs evaluating bismuth-based quadruple therapy as secondary treatment, which found cure rates averaging 78%.1 The regimens varied, comprising bismuth salts (120-600 mg, 2-4 times daily), metronidazole (250-500 mg, 2-4 times daily), tetracycline (250-500 mg, 2-4 times daily), and a PPI (40 mg twice daily). Longer duration of therapy produced higher cure rates (7 days=76%; 95% confidence interval [CI], 0.72-0.80 in 29 RCTs with 2097 patients; 10 days=77%; 95% CI, 0.60-0.93 in 2 RCTs with 142 patients; 14 days=82%; 95% CI, 0.76-0.88 in 12 RCTs with 831 patients).
Repeating the original clarithromycin-based triple therapy (8 RCTs, 265 patients) produced low cure rates (46%).1
Metronidazole-based therapy has high cure rate in a homogeneous population
A meta-analysis of 24 RCTs (1611 patients) that evaluated metronidazole-based triple therapy (mostly composed of amoxicillin 750 mg, metronidazole 250 mg, and any of a number of PPIs, all dosed at 40 mg) twice daily for 7 days found cure rates averaging 87% in an exclusively Japanese study population.1
Comparable cure rates for levofloxacin- and bismuth-based therapy
Six RCTs with a total of 1057 patients compared cure rates for levofloxacin-based triple therapy with bismuth-based quadruple therapy and found no difference.1
Two earlier meta-analyses not included in the previously described study, comprising 8 RCTs with a total of 613 patients, produced conflicting results. The larger study (15 RCTs, 1462 patients) found no difference in cure rates.2 The smaller study (7 RCTs, 787 patients) favored quadruple therapy.3
Continue to: Two secondary antibiotic regimens show similar cure rates
Two secondary antibiotic regimens show similar cure rates
A meta-analysis of 4 RCTs (total 460 patients) that compared susceptibility-guided antibiotic secondary treatment (SGT) with empiric antibiotic secondary treatment found no difference in cure rates, although the largest single RCT (172 patients) favored SGT.4
RECOMMENDATIONS
The Maastricht IV/Florence Consensus Report (a periodically updated European study group evaluating Helicobacter management) includes expert opinion-based guidelines for H pylori treatment that recommend using antibiotic susceptibility to select treatment regimens in the event of 2 treatment failures.5 The report also notes that bismuth-based quadruple therapy may not be available in all countries and has a more complex dosing regimen, and that local resistance to levofloxacin must be taken into account when prescribing levofloxacin-based triple therapy.
EVIDENCE SUMMARY
A meta-analysis of RCTs evaluating levofloxacin-based triple therapy as a secondary treatment regimen for patients with H pylori infection who had failed initial clarithromycin-based triple therapy found cure rates averaging 76% (TABLE).1 Most of the regimens comprised levofloxacin (500 mg), amoxicillin (1 g), and a PPI (40 mg), all twice daily for 7 to 10 days. Ten-day regimens produced better cure rates than 7-day regimens (84% vs 69%; comparison statistic not supplied).
The meta-analysis also included RCTs evaluating bismuth-based quadruple therapy as secondary treatment, which found cure rates averaging 78%.1 The regimens varied, comprising bismuth salts (120-600 mg, 2-4 times daily), metronidazole (250-500 mg, 2-4 times daily), tetracycline (250-500 mg, 2-4 times daily), and a PPI (40 mg twice daily). Longer duration of therapy produced higher cure rates (7 days=76%; 95% confidence interval [CI], 0.72-0.80 in 29 RCTs with 2097 patients; 10 days=77%; 95% CI, 0.60-0.93 in 2 RCTs with 142 patients; 14 days=82%; 95% CI, 0.76-0.88 in 12 RCTs with 831 patients).
Repeating the original clarithromycin-based triple therapy (8 RCTs, 265 patients) produced low cure rates (46%).1
Metronidazole-based therapy has high cure rate in a homogeneous population
A meta-analysis of 24 RCTs (1611 patients) that evaluated metronidazole-based triple therapy (mostly composed of amoxicillin 750 mg, metronidazole 250 mg, and any of a number of PPIs, all dosed at 40 mg) twice daily for 7 days found cure rates averaging 87% in an exclusively Japanese study population.1
Comparable cure rates for levofloxacin- and bismuth-based therapy
Six RCTs with a total of 1057 patients compared cure rates for levofloxacin-based triple therapy with bismuth-based quadruple therapy and found no difference.1
Two earlier meta-analyses not included in the previously described study, comprising 8 RCTs with a total of 613 patients, produced conflicting results. The larger study (15 RCTs, 1462 patients) found no difference in cure rates.2 The smaller study (7 RCTs, 787 patients) favored quadruple therapy.3
Continue to: Two secondary antibiotic regimens show similar cure rates
Two secondary antibiotic regimens show similar cure rates
A meta-analysis of 4 RCTs (total 460 patients) that compared susceptibility-guided antibiotic secondary treatment (SGT) with empiric antibiotic secondary treatment found no difference in cure rates, although the largest single RCT (172 patients) favored SGT.4
RECOMMENDATIONS
The Maastricht IV/Florence Consensus Report (a periodically updated European study group evaluating Helicobacter management) includes expert opinion-based guidelines for H pylori treatment that recommend using antibiotic susceptibility to select treatment regimens in the event of 2 treatment failures.5 The report also notes that bismuth-based quadruple therapy may not be available in all countries and has a more complex dosing regimen, and that local resistance to levofloxacin must be taken into account when prescribing levofloxacin-based triple therapy.
1. Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy failure (for Helicobacter pylori eradication). Expert Opin Pharmacother. 2013;14:843-861.
2. Di Caro S, Fini L, Daoud Y, et al. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastro. 2012;18:5669-5678.
3. Wu C, Chen X, Liu J, et al. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter. 2011;16:131-138.
4. Lopez-Gongora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015;70:2447-2455.
5. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
1. Marin AC, McNicholl AG, Gisbert JP. A review of rescue regimens after clarithromycin-containing triple therapy failure (for Helicobacter pylori eradication). Expert Opin Pharmacother. 2013;14:843-861.
2. Di Caro S, Fini L, Daoud Y, et al. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastro. 2012;18:5669-5678.
3. Wu C, Chen X, Liu J, et al. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter. 2011;16:131-138.
4. Lopez-Gongora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 2015;70:2447-2455.
5. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646-664.
EVIDENCE-BASED ANSWER:
Treating patients with Helicobacter pylori infection who have failed clarithromycin-based triple therapy with either levofloxacin-based triple therapy (with amoxicillin and a proton pump inhibitor [PPI]) or a bismuth-based quadruple therapy produces cure rates of 75% to 81%. Ten-day regimens produce higher cure rates than 7-day regimens. Repeating the initial clarithromycin-based triple therapy cures fewer than half of patients (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs]).
Treating with a metronidazole-based triple therapy (with amoxicillin and a PPI) also produces high (87%) cure rates (SOR: A, meta-analyses of RCTs in exclusively Japanese populations).
Selecting a secondary treatment regimen based on H pylori antibiotic susceptibility testing probably doesn’t improve cure rates over empiric antibiotic treatment (SOR: B, meta-analyses of RCTs with conflicting results). However, after 2 treatment failures it may be necessary (SOR: C, expert opinion-based guidelines).
Bismuth-based quadruple therapy has a more complex dosing regimen, and bismuth isn’t available in some countries. Rising rates of H pylori resistance to levofloxacin in certain areas could make levofloxacin-based triple therapy less effective in the future (SOR: C, expert opinion-based guidelines).
How effective and safe is fecal microbial transplant in preventing C difficile recurrence?
EVIDENCE SUMMARY
An open-label RCT enrolled 41 immunocompetent older adults who had relapsed CDI after at least one course of antibiotic therapy.1 Investigators randomized patients to 3 treatment groups:
- vancomycin therapy, bowel lavage (with 4 L nasogastric polyethylene glycol solution), and nasogastric-infused fresh donor feces;
- vancomycin with nasogastric bowel lavage without donor feces; or
- vancomycin alone.
Researchers defined cure as the absence of diarrhea or 3 negative stool samples (if patients continued to have persistent diarrhea) at 10 weeks without relapse.
Thirteen of 16 patients (81%) in the donor feces infusion group were cured with the first infusion. Two of the 3 remaining patients were cured after a second donor transplant. FMT produced higher total cure rates than those of vancomycin (94% vs 27%; P<.001; number needed to treat [NNT]=2). Bowel lavage had no effect on outcome.
FMT cures more patients than vancomycin alone
An open-label RCT of 39 patients compared healthy-donor, fresh FMT given via colonoscopy with vancomycin alone for recurrent CDIs.2 Researchers recruited immunocompetent adults who had recurrent CDIs after at least one course of vancomycin or metronidazole.
Patients in the treatment group received a short course of vancomycin, followed by bowel cleansing and fecal transplant via colonoscopy. Clinicians repeated the fecal transplant every 3 days until resolution for patients with pseudomembranous colitis. Patients in the control group were treated with vancomycin for at least 3 weeks. Researchers defined cure as the absence of diarrhea or 2 negative stool samples (if patients continued to have diarrhea) at 10 weeks without relapse.
Thirteen of 20 patients in the FMT group (65%) achieved cure after the first fecal infusion. The 7 remaining patients received multiple infusions; 5 were cured. Overall, FMT cured more patients than vancomycin alone (90% vs 26%; odds ratio=25.2; 99.9% confidence interval [CI], 1.26-502; NNT=2).
Continue to: Fresh and frozen stool are equally effective
Fresh and frozen stool are equally effective
A randomized, double-blind noninferiority trial compared the effectiveness of frozen and thawed FMT with that of fresh FMT in 219 patients ≥18 years of age with recurrent or refractory CDIs.3 Researchers prescribed suppressive antibiotics, which were discontinued within 24 to 48 hours of FMT, and then administered 50 mL of either fresh or frozen stool by enema. They repeated the FMT with the same donor stool if symptoms didn’t improve within 4 days. Any patient still unresponsive was offered repeat FMT or antibiotic therapy.
Researchers defined a 15% difference in outcome as a clinically important effect. Intention-to-treat analysis yielded no significant difference in clinical resolution between groups (75% frozen vs 70.3% fresh; P=.01 for noninferiority).
Nasogastric delivery works as well as colonoscopy
An open-label RCT (not included in the reviews described previously) evaluated the effectiveness of colonoscopically administered FMT compared with that of nasogastric administration in 20 patients with recurrent or refractory CDIs.4 Patients had experienced either a minimum of 3 episodes of mild-to-moderate CDI with a failed 6- to 8-week taper of vancomycin or 2 episodes of severe CDI resulting in hospitalization. Researchers offered patients from both groups a second FMT if symptoms didn’t improve with the initial administration.
Eight patients in the colonoscopy group and 6 in the nasogastric group were cured (all symptoms resolved) after the first FMT. One patient in the nasogastric group refused subsequent administration. All 5 remaining participants chose to have subsequent nasogastric administration (80% cure rate). Both methods of administering FMT produced comparable cure rates (80% in the initial nasogastric group vs 100% in the initial colonoscopy group; P=.53).
Continue to: A third of patients suffer adverse effects, but serious harms are rare
A third of patients suffer adverse effects, but serious harms are rare
A systematic review analyzed 50 trials (16 case series, 9 case reports, 4 RCTs, 21 unreported type; 1089 FMT-treated patients) for adverse effects of FMT.5 Most patients (831) had CDIs, 235 had inflammatory bowel disease, and 106 had both conditions. Donor screening tests for FMT included viral screenings (hepatitis A, B, and C; Epstein-Barr virus; human immunodeficiency virus; Treponema pallidum; and cytomegalovirus), stool tests for C difficile toxin, and routine bacterial culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), ova, and parasites.
Overall, 28.5% of patients receiving FMT experienced adverse events. Upper gastrointestinal (GI) administration resulted in more total adverse events than did lower GI delivery (43.6% vs 20.6%; P value not given), mostly abdominal discomfort. However, upper GI delivery was associated with fewer serious adverse events than was lower GI delivery (2% vs 6%; P value not given). FMT possibly or probably produced serious infections in 0.7% of patients, and there was one colonoscopy-associated death caused by aspiration (0.1% mortality).
RECOMMENDATIONS
Guidelines published by the American College of Gastroenterology in 2013 listed FMT as a treatment option for patients who have had 3 episodes of CDI and vancomycin therapy (based on moderate quality evidence).6
1. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415.
2. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835-843.
3. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA. 2016;315:142-149.
4. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515-1522.
5. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11:e0161174.
6. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
EVIDENCE SUMMARY
An open-label RCT enrolled 41 immunocompetent older adults who had relapsed CDI after at least one course of antibiotic therapy.1 Investigators randomized patients to 3 treatment groups:
- vancomycin therapy, bowel lavage (with 4 L nasogastric polyethylene glycol solution), and nasogastric-infused fresh donor feces;
- vancomycin with nasogastric bowel lavage without donor feces; or
- vancomycin alone.
Researchers defined cure as the absence of diarrhea or 3 negative stool samples (if patients continued to have persistent diarrhea) at 10 weeks without relapse.
Thirteen of 16 patients (81%) in the donor feces infusion group were cured with the first infusion. Two of the 3 remaining patients were cured after a second donor transplant. FMT produced higher total cure rates than those of vancomycin (94% vs 27%; P<.001; number needed to treat [NNT]=2). Bowel lavage had no effect on outcome.
FMT cures more patients than vancomycin alone
An open-label RCT of 39 patients compared healthy-donor, fresh FMT given via colonoscopy with vancomycin alone for recurrent CDIs.2 Researchers recruited immunocompetent adults who had recurrent CDIs after at least one course of vancomycin or metronidazole.
Patients in the treatment group received a short course of vancomycin, followed by bowel cleansing and fecal transplant via colonoscopy. Clinicians repeated the fecal transplant every 3 days until resolution for patients with pseudomembranous colitis. Patients in the control group were treated with vancomycin for at least 3 weeks. Researchers defined cure as the absence of diarrhea or 2 negative stool samples (if patients continued to have diarrhea) at 10 weeks without relapse.
Thirteen of 20 patients in the FMT group (65%) achieved cure after the first fecal infusion. The 7 remaining patients received multiple infusions; 5 were cured. Overall, FMT cured more patients than vancomycin alone (90% vs 26%; odds ratio=25.2; 99.9% confidence interval [CI], 1.26-502; NNT=2).
Continue to: Fresh and frozen stool are equally effective
Fresh and frozen stool are equally effective
A randomized, double-blind noninferiority trial compared the effectiveness of frozen and thawed FMT with that of fresh FMT in 219 patients ≥18 years of age with recurrent or refractory CDIs.3 Researchers prescribed suppressive antibiotics, which were discontinued within 24 to 48 hours of FMT, and then administered 50 mL of either fresh or frozen stool by enema. They repeated the FMT with the same donor stool if symptoms didn’t improve within 4 days. Any patient still unresponsive was offered repeat FMT or antibiotic therapy.
Researchers defined a 15% difference in outcome as a clinically important effect. Intention-to-treat analysis yielded no significant difference in clinical resolution between groups (75% frozen vs 70.3% fresh; P=.01 for noninferiority).
Nasogastric delivery works as well as colonoscopy
An open-label RCT (not included in the reviews described previously) evaluated the effectiveness of colonoscopically administered FMT compared with that of nasogastric administration in 20 patients with recurrent or refractory CDIs.4 Patients had experienced either a minimum of 3 episodes of mild-to-moderate CDI with a failed 6- to 8-week taper of vancomycin or 2 episodes of severe CDI resulting in hospitalization. Researchers offered patients from both groups a second FMT if symptoms didn’t improve with the initial administration.
Eight patients in the colonoscopy group and 6 in the nasogastric group were cured (all symptoms resolved) after the first FMT. One patient in the nasogastric group refused subsequent administration. All 5 remaining participants chose to have subsequent nasogastric administration (80% cure rate). Both methods of administering FMT produced comparable cure rates (80% in the initial nasogastric group vs 100% in the initial colonoscopy group; P=.53).
Continue to: A third of patients suffer adverse effects, but serious harms are rare
A third of patients suffer adverse effects, but serious harms are rare
A systematic review analyzed 50 trials (16 case series, 9 case reports, 4 RCTs, 21 unreported type; 1089 FMT-treated patients) for adverse effects of FMT.5 Most patients (831) had CDIs, 235 had inflammatory bowel disease, and 106 had both conditions. Donor screening tests for FMT included viral screenings (hepatitis A, B, and C; Epstein-Barr virus; human immunodeficiency virus; Treponema pallidum; and cytomegalovirus), stool tests for C difficile toxin, and routine bacterial culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), ova, and parasites.
Overall, 28.5% of patients receiving FMT experienced adverse events. Upper gastrointestinal (GI) administration resulted in more total adverse events than did lower GI delivery (43.6% vs 20.6%; P value not given), mostly abdominal discomfort. However, upper GI delivery was associated with fewer serious adverse events than was lower GI delivery (2% vs 6%; P value not given). FMT possibly or probably produced serious infections in 0.7% of patients, and there was one colonoscopy-associated death caused by aspiration (0.1% mortality).
RECOMMENDATIONS
Guidelines published by the American College of Gastroenterology in 2013 listed FMT as a treatment option for patients who have had 3 episodes of CDI and vancomycin therapy (based on moderate quality evidence).6
EVIDENCE SUMMARY
An open-label RCT enrolled 41 immunocompetent older adults who had relapsed CDI after at least one course of antibiotic therapy.1 Investigators randomized patients to 3 treatment groups:
- vancomycin therapy, bowel lavage (with 4 L nasogastric polyethylene glycol solution), and nasogastric-infused fresh donor feces;
- vancomycin with nasogastric bowel lavage without donor feces; or
- vancomycin alone.
Researchers defined cure as the absence of diarrhea or 3 negative stool samples (if patients continued to have persistent diarrhea) at 10 weeks without relapse.
Thirteen of 16 patients (81%) in the donor feces infusion group were cured with the first infusion. Two of the 3 remaining patients were cured after a second donor transplant. FMT produced higher total cure rates than those of vancomycin (94% vs 27%; P<.001; number needed to treat [NNT]=2). Bowel lavage had no effect on outcome.
FMT cures more patients than vancomycin alone
An open-label RCT of 39 patients compared healthy-donor, fresh FMT given via colonoscopy with vancomycin alone for recurrent CDIs.2 Researchers recruited immunocompetent adults who had recurrent CDIs after at least one course of vancomycin or metronidazole.
Patients in the treatment group received a short course of vancomycin, followed by bowel cleansing and fecal transplant via colonoscopy. Clinicians repeated the fecal transplant every 3 days until resolution for patients with pseudomembranous colitis. Patients in the control group were treated with vancomycin for at least 3 weeks. Researchers defined cure as the absence of diarrhea or 2 negative stool samples (if patients continued to have diarrhea) at 10 weeks without relapse.
Thirteen of 20 patients in the FMT group (65%) achieved cure after the first fecal infusion. The 7 remaining patients received multiple infusions; 5 were cured. Overall, FMT cured more patients than vancomycin alone (90% vs 26%; odds ratio=25.2; 99.9% confidence interval [CI], 1.26-502; NNT=2).
Continue to: Fresh and frozen stool are equally effective
Fresh and frozen stool are equally effective
A randomized, double-blind noninferiority trial compared the effectiveness of frozen and thawed FMT with that of fresh FMT in 219 patients ≥18 years of age with recurrent or refractory CDIs.3 Researchers prescribed suppressive antibiotics, which were discontinued within 24 to 48 hours of FMT, and then administered 50 mL of either fresh or frozen stool by enema. They repeated the FMT with the same donor stool if symptoms didn’t improve within 4 days. Any patient still unresponsive was offered repeat FMT or antibiotic therapy.
Researchers defined a 15% difference in outcome as a clinically important effect. Intention-to-treat analysis yielded no significant difference in clinical resolution between groups (75% frozen vs 70.3% fresh; P=.01 for noninferiority).
Nasogastric delivery works as well as colonoscopy
An open-label RCT (not included in the reviews described previously) evaluated the effectiveness of colonoscopically administered FMT compared with that of nasogastric administration in 20 patients with recurrent or refractory CDIs.4 Patients had experienced either a minimum of 3 episodes of mild-to-moderate CDI with a failed 6- to 8-week taper of vancomycin or 2 episodes of severe CDI resulting in hospitalization. Researchers offered patients from both groups a second FMT if symptoms didn’t improve with the initial administration.
Eight patients in the colonoscopy group and 6 in the nasogastric group were cured (all symptoms resolved) after the first FMT. One patient in the nasogastric group refused subsequent administration. All 5 remaining participants chose to have subsequent nasogastric administration (80% cure rate). Both methods of administering FMT produced comparable cure rates (80% in the initial nasogastric group vs 100% in the initial colonoscopy group; P=.53).
Continue to: A third of patients suffer adverse effects, but serious harms are rare
A third of patients suffer adverse effects, but serious harms are rare
A systematic review analyzed 50 trials (16 case series, 9 case reports, 4 RCTs, 21 unreported type; 1089 FMT-treated patients) for adverse effects of FMT.5 Most patients (831) had CDIs, 235 had inflammatory bowel disease, and 106 had both conditions. Donor screening tests for FMT included viral screenings (hepatitis A, B, and C; Epstein-Barr virus; human immunodeficiency virus; Treponema pallidum; and cytomegalovirus), stool tests for C difficile toxin, and routine bacterial culture for enteric pathogens (Escherichia coli, Salmonella, Shigella, Yersinia, Campylobacter), ova, and parasites.
Overall, 28.5% of patients receiving FMT experienced adverse events. Upper gastrointestinal (GI) administration resulted in more total adverse events than did lower GI delivery (43.6% vs 20.6%; P value not given), mostly abdominal discomfort. However, upper GI delivery was associated with fewer serious adverse events than was lower GI delivery (2% vs 6%; P value not given). FMT possibly or probably produced serious infections in 0.7% of patients, and there was one colonoscopy-associated death caused by aspiration (0.1% mortality).
RECOMMENDATIONS
Guidelines published by the American College of Gastroenterology in 2013 listed FMT as a treatment option for patients who have had 3 episodes of CDI and vancomycin therapy (based on moderate quality evidence).6
1. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415.
2. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835-843.
3. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA. 2016;315:142-149.
4. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515-1522.
5. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11:e0161174.
6. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
1. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407-415.
2. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835-843.
3. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection. JAMA. 2016;315:142-149.
4. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis. 2014;58:1515-1522.
5. Wang S, Xu M, Wang W, et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016;11:e0161174.
6. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
EVIDENCE-BASED ANSWER:
Fecal microbial transplant (FMT) is reasonably safe and effective. In patients who have had multiple Clostridium difficile infections (CDIs), FMT results in a 65% to 80% cure rate with one treatment and 90% to 95% cure rate with repeated treatments compared with a 25% to 27% cure rate for antibiotics (strength of recommendation [SOR]: B, small open-label randomized controlled trials [RCTs]).
Fresh and frozen donor feces, administered by either nasogastric tube or colonoscope, produce equal results (SOR B, RCTs).
FMT has an overall adverse event rate of 30%, primarily involving abdominal discomfort, but also, rarely, severe infections (0.7%) and death (0.1%) (SOR: B, systematic review not limited to RCTs).
How well do POLST forms assure that patients get the end-of-life care they requested?
EVIDENCE SUMMARY
The POLST form offers choices within 4 treatment areas: “attempt CPR” or “allow natural death” if the patient is in cardiopulmonary arrest; “comfort,” “limited,” or “full” medical interventions if pulse or breathing is present; choices of additional orders, including intravenous fluids, feeding tubes, and antibiotics; and additional written orders. Most POLST studies used cross-sectional and retrospective cohort designs and assessed whether CPR was attempted. Fewer studies also evaluated adherence to orders in the other treatment areas.
Community settings: Patients with POLST more likely to die out of hospital
The largest study of POLST use in community settings evaluated deaths in Oregon over one year.1 It found that patients who indicated “do not attempt CPR” on a POLST form were 6 times more likely to die a natural, out-of-hospital death than those who had no POLST form (TABLE1-10).
A West Virginia study found that patients with POLST forms had 30% higher out-of-hospital death rates than those with traditional advanced directives and no POLST.2 In a Wisconsin study, no decedents who indicated DNR on their POLST forms received CPR.3
One study that evaluated the consistency of actual medical interventions with POLST orders in all 4 treatment areas found it to be good in most areas (“feeding tubes,” “attempting CPR.” “antibiotics,” and “IV fluids”) except “additional written orders.4
Skilled nursing facilities: Generally high adherence to POLST orders
The largest study to evaluate the consistency of treatments with POLST orders among nursing home residents found high adherence overall (94%).5 Caregivers performed CPR on none of 299 residents who selected “DNR.” However, they did not administer CPR to 6 of 7 who chose “attempt CPR” and administered antibiotics to 32% of patients who specified “no antibiotics” on their POLST forms.5
A second study of nursing home residents who selected “comfort measures only” also found high consistency for attempting CPR, intensive care admission, and ventilator support, although physicians hospitalized 2% of patients to extend life.6 Similarly, treatments matched POLST orders well overall in a Washington state study, although one patient got a feeding tube against orders.7
POLST adherence is good, but can EMS workers find the form?
A study comparing emergency medical services (EMS) management with POLST orders in an Oregon registry found good consistency.8 EMS providers didn’t attempt or halted CPR in most patients with DNR orders who were found in cardiac arrest and initiated CPR in most patients who chose “attempt CPR.” EMS providers initiated CPR in the field on 11 patients (22%) with a DNR order but discontinued resuscitation en route to the hospital.
In a smaller study, EMS providers never located paper POLST forms at the scene in most cases.9
Hospice: POLST orders prevent unwanted Tx, except maybe antibiotics
A study evaluating management in hospice programs in 3 states found that care providers followed POLST orders for limited treatment in 98% of cases.10 No patients received unwanted CPR, intubation, or feeding tubes. POLST orders didn’t predict whether patients were treated with antibiotics, however.
1. Fromme EK, Zive D, Schmidt TA, et al. Association between physician orders for life-sustaining treatment for scope of treatment and in-hospital death in Oregon. J Am Geriatr Soc. 2014;62:1246-1251.
2. Pedraza SL, Culp S, Falkenstine EC, et al. POST forms more than advance directives associated with out-of-hospital death: insights from a state registry. J Pain Symptom Manage. 2016; 51:240-246.
3. Hammes B, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliative Med. 2012;15:77-85.
4. Lee MA, Brummel-Smith K, Meyer J, et al. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. J Am Geriatr Soc. 2000;48:1219-1225.
5. Hickman SE, Nelson CA, Moss AH, et al. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc. 2011;59:2091-2099.
6. Tolle SW, Tilden VP, Nelson CA, et al. A prospective study of the efficacy of the physician order form for life sustaining treatment. J Am Ger Soc.1998;46:1097-1102.
7. Meyers J, Moore C, McGrory A, et al. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Geron Nursing. 2004;30:37-46.
8. Richardson DK, Fromme E, Zive D, et al. Concordance of out-of-hospital and emergency department cardiac arrest resuscitation with documented end-of-life choices in Oregon. Ann Emerg Med. 2014;63:375-383.
9. Schmidt T, Olszewski EA, Zive D, et al. The Oregon physician orders for life-sustaining treatment registry: a preliminary study of emergency medical services utilization. J Emerg Med. 2013;44:796-805.
10. Hickman SE, Nelson CA, Moss AH, et al. Use of the physician orders for life-sustaining treatment (POLST) paradigm program in the hospice setting. J Palliat Med. 2009;12:133-141.
EVIDENCE SUMMARY
The POLST form offers choices within 4 treatment areas: “attempt CPR” or “allow natural death” if the patient is in cardiopulmonary arrest; “comfort,” “limited,” or “full” medical interventions if pulse or breathing is present; choices of additional orders, including intravenous fluids, feeding tubes, and antibiotics; and additional written orders. Most POLST studies used cross-sectional and retrospective cohort designs and assessed whether CPR was attempted. Fewer studies also evaluated adherence to orders in the other treatment areas.
Community settings: Patients with POLST more likely to die out of hospital
The largest study of POLST use in community settings evaluated deaths in Oregon over one year.1 It found that patients who indicated “do not attempt CPR” on a POLST form were 6 times more likely to die a natural, out-of-hospital death than those who had no POLST form (TABLE1-10).
A West Virginia study found that patients with POLST forms had 30% higher out-of-hospital death rates than those with traditional advanced directives and no POLST.2 In a Wisconsin study, no decedents who indicated DNR on their POLST forms received CPR.3
One study that evaluated the consistency of actual medical interventions with POLST orders in all 4 treatment areas found it to be good in most areas (“feeding tubes,” “attempting CPR.” “antibiotics,” and “IV fluids”) except “additional written orders.4
Skilled nursing facilities: Generally high adherence to POLST orders
The largest study to evaluate the consistency of treatments with POLST orders among nursing home residents found high adherence overall (94%).5 Caregivers performed CPR on none of 299 residents who selected “DNR.” However, they did not administer CPR to 6 of 7 who chose “attempt CPR” and administered antibiotics to 32% of patients who specified “no antibiotics” on their POLST forms.5
A second study of nursing home residents who selected “comfort measures only” also found high consistency for attempting CPR, intensive care admission, and ventilator support, although physicians hospitalized 2% of patients to extend life.6 Similarly, treatments matched POLST orders well overall in a Washington state study, although one patient got a feeding tube against orders.7
POLST adherence is good, but can EMS workers find the form?
A study comparing emergency medical services (EMS) management with POLST orders in an Oregon registry found good consistency.8 EMS providers didn’t attempt or halted CPR in most patients with DNR orders who were found in cardiac arrest and initiated CPR in most patients who chose “attempt CPR.” EMS providers initiated CPR in the field on 11 patients (22%) with a DNR order but discontinued resuscitation en route to the hospital.
In a smaller study, EMS providers never located paper POLST forms at the scene in most cases.9
Hospice: POLST orders prevent unwanted Tx, except maybe antibiotics
A study evaluating management in hospice programs in 3 states found that care providers followed POLST orders for limited treatment in 98% of cases.10 No patients received unwanted CPR, intubation, or feeding tubes. POLST orders didn’t predict whether patients were treated with antibiotics, however.
EVIDENCE SUMMARY
The POLST form offers choices within 4 treatment areas: “attempt CPR” or “allow natural death” if the patient is in cardiopulmonary arrest; “comfort,” “limited,” or “full” medical interventions if pulse or breathing is present; choices of additional orders, including intravenous fluids, feeding tubes, and antibiotics; and additional written orders. Most POLST studies used cross-sectional and retrospective cohort designs and assessed whether CPR was attempted. Fewer studies also evaluated adherence to orders in the other treatment areas.
Community settings: Patients with POLST more likely to die out of hospital
The largest study of POLST use in community settings evaluated deaths in Oregon over one year.1 It found that patients who indicated “do not attempt CPR” on a POLST form were 6 times more likely to die a natural, out-of-hospital death than those who had no POLST form (TABLE1-10).
A West Virginia study found that patients with POLST forms had 30% higher out-of-hospital death rates than those with traditional advanced directives and no POLST.2 In a Wisconsin study, no decedents who indicated DNR on their POLST forms received CPR.3
One study that evaluated the consistency of actual medical interventions with POLST orders in all 4 treatment areas found it to be good in most areas (“feeding tubes,” “attempting CPR.” “antibiotics,” and “IV fluids”) except “additional written orders.4
Skilled nursing facilities: Generally high adherence to POLST orders
The largest study to evaluate the consistency of treatments with POLST orders among nursing home residents found high adherence overall (94%).5 Caregivers performed CPR on none of 299 residents who selected “DNR.” However, they did not administer CPR to 6 of 7 who chose “attempt CPR” and administered antibiotics to 32% of patients who specified “no antibiotics” on their POLST forms.5
A second study of nursing home residents who selected “comfort measures only” also found high consistency for attempting CPR, intensive care admission, and ventilator support, although physicians hospitalized 2% of patients to extend life.6 Similarly, treatments matched POLST orders well overall in a Washington state study, although one patient got a feeding tube against orders.7
POLST adherence is good, but can EMS workers find the form?
A study comparing emergency medical services (EMS) management with POLST orders in an Oregon registry found good consistency.8 EMS providers didn’t attempt or halted CPR in most patients with DNR orders who were found in cardiac arrest and initiated CPR in most patients who chose “attempt CPR.” EMS providers initiated CPR in the field on 11 patients (22%) with a DNR order but discontinued resuscitation en route to the hospital.
In a smaller study, EMS providers never located paper POLST forms at the scene in most cases.9
Hospice: POLST orders prevent unwanted Tx, except maybe antibiotics
A study evaluating management in hospice programs in 3 states found that care providers followed POLST orders for limited treatment in 98% of cases.10 No patients received unwanted CPR, intubation, or feeding tubes. POLST orders didn’t predict whether patients were treated with antibiotics, however.
1. Fromme EK, Zive D, Schmidt TA, et al. Association between physician orders for life-sustaining treatment for scope of treatment and in-hospital death in Oregon. J Am Geriatr Soc. 2014;62:1246-1251.
2. Pedraza SL, Culp S, Falkenstine EC, et al. POST forms more than advance directives associated with out-of-hospital death: insights from a state registry. J Pain Symptom Manage. 2016; 51:240-246.
3. Hammes B, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliative Med. 2012;15:77-85.
4. Lee MA, Brummel-Smith K, Meyer J, et al. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. J Am Geriatr Soc. 2000;48:1219-1225.
5. Hickman SE, Nelson CA, Moss AH, et al. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc. 2011;59:2091-2099.
6. Tolle SW, Tilden VP, Nelson CA, et al. A prospective study of the efficacy of the physician order form for life sustaining treatment. J Am Ger Soc.1998;46:1097-1102.
7. Meyers J, Moore C, McGrory A, et al. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Geron Nursing. 2004;30:37-46.
8. Richardson DK, Fromme E, Zive D, et al. Concordance of out-of-hospital and emergency department cardiac arrest resuscitation with documented end-of-life choices in Oregon. Ann Emerg Med. 2014;63:375-383.
9. Schmidt T, Olszewski EA, Zive D, et al. The Oregon physician orders for life-sustaining treatment registry: a preliminary study of emergency medical services utilization. J Emerg Med. 2013;44:796-805.
10. Hickman SE, Nelson CA, Moss AH, et al. Use of the physician orders for life-sustaining treatment (POLST) paradigm program in the hospice setting. J Palliat Med. 2009;12:133-141.
1. Fromme EK, Zive D, Schmidt TA, et al. Association between physician orders for life-sustaining treatment for scope of treatment and in-hospital death in Oregon. J Am Geriatr Soc. 2014;62:1246-1251.
2. Pedraza SL, Culp S, Falkenstine EC, et al. POST forms more than advance directives associated with out-of-hospital death: insights from a state registry. J Pain Symptom Manage. 2016; 51:240-246.
3. Hammes B, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliative Med. 2012;15:77-85.
4. Lee MA, Brummel-Smith K, Meyer J, et al. Physician orders for life-sustaining treatment (POLST): outcomes in a PACE program. J Am Geriatr Soc. 2000;48:1219-1225.
5. Hickman SE, Nelson CA, Moss AH, et al. The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc. 2011;59:2091-2099.
6. Tolle SW, Tilden VP, Nelson CA, et al. A prospective study of the efficacy of the physician order form for life sustaining treatment. J Am Ger Soc.1998;46:1097-1102.
7. Meyers J, Moore C, McGrory A, et al. Physician orders for life-sustaining treatment form: honoring end-of-life directives for nursing home residents. J Geron Nursing. 2004;30:37-46.
8. Richardson DK, Fromme E, Zive D, et al. Concordance of out-of-hospital and emergency department cardiac arrest resuscitation with documented end-of-life choices in Oregon. Ann Emerg Med. 2014;63:375-383.
9. Schmidt T, Olszewski EA, Zive D, et al. The Oregon physician orders for life-sustaining treatment registry: a preliminary study of emergency medical services utilization. J Emerg Med. 2013;44:796-805.
10. Hickman SE, Nelson CA, Moss AH, et al. Use of the physician orders for life-sustaining treatment (POLST) paradigm program in the hospice setting. J Palliat Med. 2009;12:133-141.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Quite well, for cardiopulmonary resuscitation (CPR). Most patients (91%-100%) who select “do not resuscitate” (DNR) on their physician’s orders for life-sustaining treatment (POLST) forms are allowed a natural death without attempted CPR across a variety of settings (community, skilled nursing facilities, emergency medical services, and hospice). Few patients (6%) who select “comfort measures only” die in the hospital, whereas more (22%) who choose “limited interventions,” and still more (34%) without a POLST form, die in the hospital (strength of recommendation [SOR]: B, large, consistent cross-sectional and cohort studies).
Most patients (84%) who select “attempt resuscitation” receive resuscitation for out-of-hospital cardiac arrest in emergency services settings (SOR: B, small retrospective cohort study).
POLST orders declining other services (intravenous fluids, intensive care, intubation, feeding tubes) are carried out in most (84%-100%) cases. POLST orders regarding antibiotic treatments are less effectively implemented (SOR: B, moderate-sized retrospective chart review).
Which interventions are effective in managing parental vaccine refusal?
EVIDENCE SUMMARY
A systematic review analyzed 30 predominantly US studies with more than 8000 patients published between 1990 and 2012 (4 RCTs, 7 nonrandomized clinical trials, 13 before/after intervention trials, and 6 evaluation studies) to evaluate interventions that decreased parental vaccine refusal and hesitancy.1 Interventions included: change in state law, changes in state and school policies, and family-centered education initiatives.
Four studies that evaluated the impact of state laws concerning personal exemption (in addition to religious exemption) consistently found that total nonmedical exemption rates were higher in states that allowed personal exemptions. One nationwide survey found that total nonmedical exemption rates were 2.54 times higher (95% confidence interval [CI], 1.68-3.83) in states that allowed personal exemption than in states where only religious nonmedical exemption was allowed.
Fifteen studies evaluated the impact of educational initiatives on parental attitude towards vaccination; 8 of them reported statistically significant changes. None of the studies demonstrated a change in vaccination rates, however. Citing the generally low quality of the studies, the review authors concluded that they didn’t have convincing evidence that educational interventions reduced vaccine hesitancy.
Herd immunity is an iffy motivator
A systematic review analyzed 29 studies from western nations (17 qualitative and 12 quantitative, 4650 patients) regarding willingness to immunize children for the benefit of the community.2 Of the 17 qualitative studies, only 2 (164 patients) identified benefit to others as a motivating factor in parents’ decisions to immunize their children. In the 12 quantitative studies, a wide range of parents (1% to 60%) rated the concept of benefit to others as a reason for immunization. Overall, approximately one-third of parents listed herd immunity as a motivating reason. The authors concluded that the high heterogeneity of the studies made it unclear whether herd immunity was a motivating factor in childhood immunizations.
Multifaceted interventions, education, and tailored approaches may all work
A systematic review of international studies published between 2007 and 2013 investigated interventions to increase uptake of routinely recommended immunizations in groups with vaccine hesitancy and reduced use.3 Authors identified 189 articles (trial types and number of patients not given) that provided outcome measures.
Interventions that resulted in at least a 25% increase in vaccine uptake were primarily multifaceted, including elements of: targeting undervaccinated populations, improving access or convenience, educational initiatives, and mandates. Interventions that produced a greater than 20% increase in knowledge were generally educational interventions embedded in routine processes such as clinic visits.
The authors noted wide variation between studies in effect size, settings, and target populations. They concluded that interventions tailored to specific populations and concerns were likely to work best.
Corrective information doesn’t help with the most worried parents
A subsequent RCT tested whether correcting the myth that the flu vaccine can give people the flu would reduce belief in the misconception, increase perceptions that the flu vaccine is safe, and increase vaccination intent.4 Respondents to a national online poll of 1000 people received one of 3 interventions: correctional education (information debunking the myth), risk education (information about the risks of influenza infection), or no additional education.
Corrective information about the flu vaccine reduced the false belief that the vaccine can cause the flu by 15% to 20% and that the flu vaccine is unsafe by 5% to 10% (data from graphs; P<.05 for both effects). However, corrective information actually decreased parental intention to vaccinate among the group most concerned about the adverse effects of the vaccine (data from graph and text: +5% in the low-concern group vs −18% in the high-concern group; P<.05).
A presumptive approach works—but at a cost
A subsequent observational study videotaped 111 patient-provider vaccine discussions.5 Researchers categorized the initiation of the vaccine discussion as presumptive (eg, “We have to do some shots.”) or participatory (eg, “What do you want to do about shots?”). Using a presumptive style was more likely to result in acceptance of all recommended vaccines by the end of the visit (90% vs 17%; P<.05), but it decreased the chance of a highly rated visit experience (63% vs 95%; P<.05).
RECOMMENDATIONS
The 2015 Centers for Disease Control and Prevention (CDC) Pink Book recommends a combination of strategies, aimed at both providers and the public, for increasing and maintaining high immunization rates. The Pink Book advises providers to be ready to address vaccine safety concerns raised by parents.6
In a 2012 guideline, the CDC encouraged providers to listen attentively, be ready with scientific information and reliable resources, and use appropriate anecdotes in communicating with vaccine-hesitant parents.7 The guideline recommended against excluding families who refuse vaccination from the practice.
1. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-42304.
2. Quadri-Sheriff M, Hendrix K, Downs S, et al. The role of herd immunity in parents’ decision to vaccinate children: a systematic review. Pediatrics. 2012;130:522-530.
3. Jarrett C, Wilson R, O’Leary M, et al. Strategies for addressing vaccine hesitancy: a systematic review. Vaccine. 2015;33:4180-4190.
4. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464.
5. Opel DJ, Mangione-Smith R, Robinson JD, et al. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health. 2015;105:1998-2004.
6. Centers for Disease Control and Prevention. Immunization Strategies for Healthcare Practices and Providers. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/strat.html. Accessed May 11, 2016.
7. Centers for Disease Control and Prevention. Provider Resources for Vaccine Conversations with Parents. Available at: http://www.cdc.gov/vaccines/hcp/conversations/about-vacc-conversations.html. Accessed May 11, 2016.
EVIDENCE SUMMARY
A systematic review analyzed 30 predominantly US studies with more than 8000 patients published between 1990 and 2012 (4 RCTs, 7 nonrandomized clinical trials, 13 before/after intervention trials, and 6 evaluation studies) to evaluate interventions that decreased parental vaccine refusal and hesitancy.1 Interventions included: change in state law, changes in state and school policies, and family-centered education initiatives.
Four studies that evaluated the impact of state laws concerning personal exemption (in addition to religious exemption) consistently found that total nonmedical exemption rates were higher in states that allowed personal exemptions. One nationwide survey found that total nonmedical exemption rates were 2.54 times higher (95% confidence interval [CI], 1.68-3.83) in states that allowed personal exemption than in states where only religious nonmedical exemption was allowed.
Fifteen studies evaluated the impact of educational initiatives on parental attitude towards vaccination; 8 of them reported statistically significant changes. None of the studies demonstrated a change in vaccination rates, however. Citing the generally low quality of the studies, the review authors concluded that they didn’t have convincing evidence that educational interventions reduced vaccine hesitancy.
Herd immunity is an iffy motivator
A systematic review analyzed 29 studies from western nations (17 qualitative and 12 quantitative, 4650 patients) regarding willingness to immunize children for the benefit of the community.2 Of the 17 qualitative studies, only 2 (164 patients) identified benefit to others as a motivating factor in parents’ decisions to immunize their children. In the 12 quantitative studies, a wide range of parents (1% to 60%) rated the concept of benefit to others as a reason for immunization. Overall, approximately one-third of parents listed herd immunity as a motivating reason. The authors concluded that the high heterogeneity of the studies made it unclear whether herd immunity was a motivating factor in childhood immunizations.
Multifaceted interventions, education, and tailored approaches may all work
A systematic review of international studies published between 2007 and 2013 investigated interventions to increase uptake of routinely recommended immunizations in groups with vaccine hesitancy and reduced use.3 Authors identified 189 articles (trial types and number of patients not given) that provided outcome measures.
Interventions that resulted in at least a 25% increase in vaccine uptake were primarily multifaceted, including elements of: targeting undervaccinated populations, improving access or convenience, educational initiatives, and mandates. Interventions that produced a greater than 20% increase in knowledge were generally educational interventions embedded in routine processes such as clinic visits.
The authors noted wide variation between studies in effect size, settings, and target populations. They concluded that interventions tailored to specific populations and concerns were likely to work best.
Corrective information doesn’t help with the most worried parents
A subsequent RCT tested whether correcting the myth that the flu vaccine can give people the flu would reduce belief in the misconception, increase perceptions that the flu vaccine is safe, and increase vaccination intent.4 Respondents to a national online poll of 1000 people received one of 3 interventions: correctional education (information debunking the myth), risk education (information about the risks of influenza infection), or no additional education.
Corrective information about the flu vaccine reduced the false belief that the vaccine can cause the flu by 15% to 20% and that the flu vaccine is unsafe by 5% to 10% (data from graphs; P<.05 for both effects). However, corrective information actually decreased parental intention to vaccinate among the group most concerned about the adverse effects of the vaccine (data from graph and text: +5% in the low-concern group vs −18% in the high-concern group; P<.05).
A presumptive approach works—but at a cost
A subsequent observational study videotaped 111 patient-provider vaccine discussions.5 Researchers categorized the initiation of the vaccine discussion as presumptive (eg, “We have to do some shots.”) or participatory (eg, “What do you want to do about shots?”). Using a presumptive style was more likely to result in acceptance of all recommended vaccines by the end of the visit (90% vs 17%; P<.05), but it decreased the chance of a highly rated visit experience (63% vs 95%; P<.05).
RECOMMENDATIONS
The 2015 Centers for Disease Control and Prevention (CDC) Pink Book recommends a combination of strategies, aimed at both providers and the public, for increasing and maintaining high immunization rates. The Pink Book advises providers to be ready to address vaccine safety concerns raised by parents.6
In a 2012 guideline, the CDC encouraged providers to listen attentively, be ready with scientific information and reliable resources, and use appropriate anecdotes in communicating with vaccine-hesitant parents.7 The guideline recommended against excluding families who refuse vaccination from the practice.
EVIDENCE SUMMARY
A systematic review analyzed 30 predominantly US studies with more than 8000 patients published between 1990 and 2012 (4 RCTs, 7 nonrandomized clinical trials, 13 before/after intervention trials, and 6 evaluation studies) to evaluate interventions that decreased parental vaccine refusal and hesitancy.1 Interventions included: change in state law, changes in state and school policies, and family-centered education initiatives.
Four studies that evaluated the impact of state laws concerning personal exemption (in addition to religious exemption) consistently found that total nonmedical exemption rates were higher in states that allowed personal exemptions. One nationwide survey found that total nonmedical exemption rates were 2.54 times higher (95% confidence interval [CI], 1.68-3.83) in states that allowed personal exemption than in states where only religious nonmedical exemption was allowed.
Fifteen studies evaluated the impact of educational initiatives on parental attitude towards vaccination; 8 of them reported statistically significant changes. None of the studies demonstrated a change in vaccination rates, however. Citing the generally low quality of the studies, the review authors concluded that they didn’t have convincing evidence that educational interventions reduced vaccine hesitancy.
Herd immunity is an iffy motivator
A systematic review analyzed 29 studies from western nations (17 qualitative and 12 quantitative, 4650 patients) regarding willingness to immunize children for the benefit of the community.2 Of the 17 qualitative studies, only 2 (164 patients) identified benefit to others as a motivating factor in parents’ decisions to immunize their children. In the 12 quantitative studies, a wide range of parents (1% to 60%) rated the concept of benefit to others as a reason for immunization. Overall, approximately one-third of parents listed herd immunity as a motivating reason. The authors concluded that the high heterogeneity of the studies made it unclear whether herd immunity was a motivating factor in childhood immunizations.
Multifaceted interventions, education, and tailored approaches may all work
A systematic review of international studies published between 2007 and 2013 investigated interventions to increase uptake of routinely recommended immunizations in groups with vaccine hesitancy and reduced use.3 Authors identified 189 articles (trial types and number of patients not given) that provided outcome measures.
Interventions that resulted in at least a 25% increase in vaccine uptake were primarily multifaceted, including elements of: targeting undervaccinated populations, improving access or convenience, educational initiatives, and mandates. Interventions that produced a greater than 20% increase in knowledge were generally educational interventions embedded in routine processes such as clinic visits.
The authors noted wide variation between studies in effect size, settings, and target populations. They concluded that interventions tailored to specific populations and concerns were likely to work best.
Corrective information doesn’t help with the most worried parents
A subsequent RCT tested whether correcting the myth that the flu vaccine can give people the flu would reduce belief in the misconception, increase perceptions that the flu vaccine is safe, and increase vaccination intent.4 Respondents to a national online poll of 1000 people received one of 3 interventions: correctional education (information debunking the myth), risk education (information about the risks of influenza infection), or no additional education.
Corrective information about the flu vaccine reduced the false belief that the vaccine can cause the flu by 15% to 20% and that the flu vaccine is unsafe by 5% to 10% (data from graphs; P<.05 for both effects). However, corrective information actually decreased parental intention to vaccinate among the group most concerned about the adverse effects of the vaccine (data from graph and text: +5% in the low-concern group vs −18% in the high-concern group; P<.05).
A presumptive approach works—but at a cost
A subsequent observational study videotaped 111 patient-provider vaccine discussions.5 Researchers categorized the initiation of the vaccine discussion as presumptive (eg, “We have to do some shots.”) or participatory (eg, “What do you want to do about shots?”). Using a presumptive style was more likely to result in acceptance of all recommended vaccines by the end of the visit (90% vs 17%; P<.05), but it decreased the chance of a highly rated visit experience (63% vs 95%; P<.05).
RECOMMENDATIONS
The 2015 Centers for Disease Control and Prevention (CDC) Pink Book recommends a combination of strategies, aimed at both providers and the public, for increasing and maintaining high immunization rates. The Pink Book advises providers to be ready to address vaccine safety concerns raised by parents.6
In a 2012 guideline, the CDC encouraged providers to listen attentively, be ready with scientific information and reliable resources, and use appropriate anecdotes in communicating with vaccine-hesitant parents.7 The guideline recommended against excluding families who refuse vaccination from the practice.
1. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-42304.
2. Quadri-Sheriff M, Hendrix K, Downs S, et al. The role of herd immunity in parents’ decision to vaccinate children: a systematic review. Pediatrics. 2012;130:522-530.
3. Jarrett C, Wilson R, O’Leary M, et al. Strategies for addressing vaccine hesitancy: a systematic review. Vaccine. 2015;33:4180-4190.
4. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464.
5. Opel DJ, Mangione-Smith R, Robinson JD, et al. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health. 2015;105:1998-2004.
6. Centers for Disease Control and Prevention. Immunization Strategies for Healthcare Practices and Providers. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/strat.html. Accessed May 11, 2016.
7. Centers for Disease Control and Prevention. Provider Resources for Vaccine Conversations with Parents. Available at: http://www.cdc.gov/vaccines/hcp/conversations/about-vacc-conversations.html. Accessed May 11, 2016.
1. Sadaf A, Richards JL, Glanz J, et al. A systematic review of interventions for reducing parental vaccine refusal and vaccine hesitancy. Vaccine. 2013;31:4293-42304.
2. Quadri-Sheriff M, Hendrix K, Downs S, et al. The role of herd immunity in parents’ decision to vaccinate children: a systematic review. Pediatrics. 2012;130:522-530.
3. Jarrett C, Wilson R, O’Leary M, et al. Strategies for addressing vaccine hesitancy: a systematic review. Vaccine. 2015;33:4180-4190.
4. Nyhan B, Reifler J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine. 2015;33:459-464.
5. Opel DJ, Mangione-Smith R, Robinson JD, et al. The influence of provider communication behaviors on parental vaccine acceptance and visit experience. Am J Public Health. 2015;105:1998-2004.
6. Centers for Disease Control and Prevention. Immunization Strategies for Healthcare Practices and Providers. Available at: http://www.cdc.gov/vaccines/pubs/pinkbook/strat.html. Accessed May 11, 2016.
7. Centers for Disease Control and Prevention. Provider Resources for Vaccine Conversations with Parents. Available at: http://www.cdc.gov/vaccines/hcp/conversations/about-vacc-conversations.html. Accessed May 11, 2016.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
It’s unclear whether educational initiatives alone alter vaccine refusal. Although about a third of parents cite herd immunity as motivation for vaccination, its efficacy in addressing vaccine hesitancy isn’t clear (strength of recommendation [SOR]: B, systematic reviews not limited to randomized controlled trials [RCTs]).
Multifaceted interventions (encompassing improved access to vaccines, immunization mandates, and patient education) may produce a ≥25% increase in vaccine uptake in groups with vaccine hesitancy and low utilization (SOR: B, extrapolated from a meta-analysis across diverse cultures).
Correcting false information about influenza vaccination improves perceptions about the vaccine, but may decrease intention to vaccinate in parents who already have strong concerns about safety (SOR: C, low-quality RCT).
Discussions about vaccines that are more paternalistic (presumptive rather than participatory) are associated with higher vaccination rates, but lower visit satisfaction (SOR: C, observational study).
Providers should thoroughly address patient concerns about safety and encourage vaccine use (SOR: C, expert opinion).
What effects—if any—does marijuana use during pregnancy have on the fetus or child?
EVIDENCE SUMMARY
A large systematic review of prospective and retrospective cohort studies found little or no effect of maternal marijuana use on birth weight, stillbirths, preterm births, or congenital anomalies (TABLE1-8). Some studies found lower birth weights and some found higher birth weights. The authors couldn’t perform a meta-analysis because of heterogeneity, but estimated a clinically insignificant difference of 100 g. Most studies were limited by failure to account for concurrent maternal tobacco smoking.
Moreover, all studies used interview data to determine maternal prenatal marijuana use, which can be subject to large recall bias. A multicenter prospective study of 585 pregnant women that compared interview data with serum screening to identify tetrahydrocannabinol (THC) found poor correlation between history and laboratory validation, for example.1 Only 31% of pregnant women with positive THC testing self-reported marijuana use (31% sensitivity), and only 43% of women who reported marijuana use had a positive THC screen (43% specificity). Most studies didn’t quantify marijuana use well and didn’t associate use with trimester of exposure.
The authors also point out that marijuana potency has increased substantially since the 1980s when many of the studies were done (THC content was 3.2% in 1983 and 13% in 2008); prenatal marijuana use in the present day may expose the fetus to larger amounts of THC.1
A 2016 retrospective cohort study of 56 mothers who reported prenatal marijuana use found no differences in preterm birth, low birth weight, or Apgar scores.2
Neurodevelopmental effects on infants, long-term effects on children, teens
Three prospective cohort studies evaluated neurodevelopmental outcomes in neonates and infants, and 2 studies continued to follow children into adolescence.1,3 All found essentially no differences associated with prenatal marijuana at birth, throughout infancy, and through age 3 years. The studies had the same limitations as those described previously (potential recall bias for identifying which children were exposed to marijuana prenatally and poorly quantified marijuana use not well-associated with trimester of exposure).
The Ottawa Prenatal Prospective Study (OPPS) examined 140 low-risk pregnancies in white women of higher socioeconomic status who used marijuana during pregnancy.1,3-7 Investigators considered: socioeconomic status, standard demographics, obstetric history, and use of other drugs, tobacco, and alcohol. Using a standardized newborn assessment scale, they found subtle behavioral differences at one week but not 9 days. Investigators evaluated children again at 3 years of age, school entry (5 or 6 years), and 9 to 12 years.
The Maternal Health Practices and Child Development study (MHPCD) of 564 high-risk pregnancies in predominantly minority women of low socioeconomic status followed infants from birth through 14 years of age.1,3-5,7,8 It found some small differences in outcomes among children exposed to marijuana prenatally. Of note, when investigators evaluated marijuana use at age 14 years, they compared adolescent self-report history with urine THC testing (specificity 78%).
The MHPCD study was limited because, compared with the nonusing group, mothers who used marijuana were also 20% to 25% more likely to be single and poor, to live in poorer quality homes, and to use alcohol, tobacco, and other drugs. Investigators used statistical modeling to account for these environmental differences and estimated that 10% of the difference in outcomes was attributable to prenatal marijuana exposure.
The Generation R study (Gen R) enrolled 220 lower-risk pregnancies in multiethnic European women of higher socioeconomic status, followed children to 3 years of age, and found no marijuana-associated differences in any parameter.1,3,4 The final assessment included only 51 children.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) recommends screening all women for tobacco, alcohol, and drug use (including marijuana) during early pregnancy.9 Women who report marijuana use should be counseled regarding potential adverse consequences to fetal health and be encouraged to discontinue use.
ACOG says that insufficient data exist to evaluate the effects of marijuana use on infants during lactation and breastfeeding and recommends against it.
The American Society of Addiction Medicine also recommends screening pregnant women for drug use and making appropriate referrals for substance use treatment.10
1. Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol. 2015;213:761-778.
2. Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215:506.e1-e7.
3. Warner TD, Roussos-Ross D, Behnke M. It’s not your mother’s marijuana: effects on maternal-fetal health and the developing child. Clinical Perinatology. 2014;41:877-894.
4. Huizink AC. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;52:45-52.
5. Goldschmidt L, Richardson GA, Willford J, et al. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47:254-263.
6. Fried PA. The Ottawa Prenatal Prospective Study (OPPS): methodological issues and findings—it’s easy to throw the baby out with the bath water. Life Sci. 1995;56:2159-2168.
7. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
8. Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313-1322.
9. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion No. 637: Marijuana use during pregnancy and lactation. Obstet Gynecol. 2015;126:234-238.
10. American Society of Addiction Medicine. Public policy statement on women, alcohol and other drugs, and pregnancy. Chevy Chase MD: American Society of Addiction Medicine; 2011. Available at: http://www.asam.org/docs/default-source/public-policy-statements/1womenandpregnancy_7-11.pdf. Accessed July 5, 2016.
EVIDENCE SUMMARY
A large systematic review of prospective and retrospective cohort studies found little or no effect of maternal marijuana use on birth weight, stillbirths, preterm births, or congenital anomalies (TABLE1-8). Some studies found lower birth weights and some found higher birth weights. The authors couldn’t perform a meta-analysis because of heterogeneity, but estimated a clinically insignificant difference of 100 g. Most studies were limited by failure to account for concurrent maternal tobacco smoking.
Moreover, all studies used interview data to determine maternal prenatal marijuana use, which can be subject to large recall bias. A multicenter prospective study of 585 pregnant women that compared interview data with serum screening to identify tetrahydrocannabinol (THC) found poor correlation between history and laboratory validation, for example.1 Only 31% of pregnant women with positive THC testing self-reported marijuana use (31% sensitivity), and only 43% of women who reported marijuana use had a positive THC screen (43% specificity). Most studies didn’t quantify marijuana use well and didn’t associate use with trimester of exposure.
The authors also point out that marijuana potency has increased substantially since the 1980s when many of the studies were done (THC content was 3.2% in 1983 and 13% in 2008); prenatal marijuana use in the present day may expose the fetus to larger amounts of THC.1
A 2016 retrospective cohort study of 56 mothers who reported prenatal marijuana use found no differences in preterm birth, low birth weight, or Apgar scores.2
Neurodevelopmental effects on infants, long-term effects on children, teens
Three prospective cohort studies evaluated neurodevelopmental outcomes in neonates and infants, and 2 studies continued to follow children into adolescence.1,3 All found essentially no differences associated with prenatal marijuana at birth, throughout infancy, and through age 3 years. The studies had the same limitations as those described previously (potential recall bias for identifying which children were exposed to marijuana prenatally and poorly quantified marijuana use not well-associated with trimester of exposure).
The Ottawa Prenatal Prospective Study (OPPS) examined 140 low-risk pregnancies in white women of higher socioeconomic status who used marijuana during pregnancy.1,3-7 Investigators considered: socioeconomic status, standard demographics, obstetric history, and use of other drugs, tobacco, and alcohol. Using a standardized newborn assessment scale, they found subtle behavioral differences at one week but not 9 days. Investigators evaluated children again at 3 years of age, school entry (5 or 6 years), and 9 to 12 years.
The Maternal Health Practices and Child Development study (MHPCD) of 564 high-risk pregnancies in predominantly minority women of low socioeconomic status followed infants from birth through 14 years of age.1,3-5,7,8 It found some small differences in outcomes among children exposed to marijuana prenatally. Of note, when investigators evaluated marijuana use at age 14 years, they compared adolescent self-report history with urine THC testing (specificity 78%).
The MHPCD study was limited because, compared with the nonusing group, mothers who used marijuana were also 20% to 25% more likely to be single and poor, to live in poorer quality homes, and to use alcohol, tobacco, and other drugs. Investigators used statistical modeling to account for these environmental differences and estimated that 10% of the difference in outcomes was attributable to prenatal marijuana exposure.
The Generation R study (Gen R) enrolled 220 lower-risk pregnancies in multiethnic European women of higher socioeconomic status, followed children to 3 years of age, and found no marijuana-associated differences in any parameter.1,3,4 The final assessment included only 51 children.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) recommends screening all women for tobacco, alcohol, and drug use (including marijuana) during early pregnancy.9 Women who report marijuana use should be counseled regarding potential adverse consequences to fetal health and be encouraged to discontinue use.
ACOG says that insufficient data exist to evaluate the effects of marijuana use on infants during lactation and breastfeeding and recommends against it.
The American Society of Addiction Medicine also recommends screening pregnant women for drug use and making appropriate referrals for substance use treatment.10
EVIDENCE SUMMARY
A large systematic review of prospective and retrospective cohort studies found little or no effect of maternal marijuana use on birth weight, stillbirths, preterm births, or congenital anomalies (TABLE1-8). Some studies found lower birth weights and some found higher birth weights. The authors couldn’t perform a meta-analysis because of heterogeneity, but estimated a clinically insignificant difference of 100 g. Most studies were limited by failure to account for concurrent maternal tobacco smoking.
Moreover, all studies used interview data to determine maternal prenatal marijuana use, which can be subject to large recall bias. A multicenter prospective study of 585 pregnant women that compared interview data with serum screening to identify tetrahydrocannabinol (THC) found poor correlation between history and laboratory validation, for example.1 Only 31% of pregnant women with positive THC testing self-reported marijuana use (31% sensitivity), and only 43% of women who reported marijuana use had a positive THC screen (43% specificity). Most studies didn’t quantify marijuana use well and didn’t associate use with trimester of exposure.
The authors also point out that marijuana potency has increased substantially since the 1980s when many of the studies were done (THC content was 3.2% in 1983 and 13% in 2008); prenatal marijuana use in the present day may expose the fetus to larger amounts of THC.1
A 2016 retrospective cohort study of 56 mothers who reported prenatal marijuana use found no differences in preterm birth, low birth weight, or Apgar scores.2
Neurodevelopmental effects on infants, long-term effects on children, teens
Three prospective cohort studies evaluated neurodevelopmental outcomes in neonates and infants, and 2 studies continued to follow children into adolescence.1,3 All found essentially no differences associated with prenatal marijuana at birth, throughout infancy, and through age 3 years. The studies had the same limitations as those described previously (potential recall bias for identifying which children were exposed to marijuana prenatally and poorly quantified marijuana use not well-associated with trimester of exposure).
The Ottawa Prenatal Prospective Study (OPPS) examined 140 low-risk pregnancies in white women of higher socioeconomic status who used marijuana during pregnancy.1,3-7 Investigators considered: socioeconomic status, standard demographics, obstetric history, and use of other drugs, tobacco, and alcohol. Using a standardized newborn assessment scale, they found subtle behavioral differences at one week but not 9 days. Investigators evaluated children again at 3 years of age, school entry (5 or 6 years), and 9 to 12 years.
The Maternal Health Practices and Child Development study (MHPCD) of 564 high-risk pregnancies in predominantly minority women of low socioeconomic status followed infants from birth through 14 years of age.1,3-5,7,8 It found some small differences in outcomes among children exposed to marijuana prenatally. Of note, when investigators evaluated marijuana use at age 14 years, they compared adolescent self-report history with urine THC testing (specificity 78%).
The MHPCD study was limited because, compared with the nonusing group, mothers who used marijuana were also 20% to 25% more likely to be single and poor, to live in poorer quality homes, and to use alcohol, tobacco, and other drugs. Investigators used statistical modeling to account for these environmental differences and estimated that 10% of the difference in outcomes was attributable to prenatal marijuana exposure.
The Generation R study (Gen R) enrolled 220 lower-risk pregnancies in multiethnic European women of higher socioeconomic status, followed children to 3 years of age, and found no marijuana-associated differences in any parameter.1,3,4 The final assessment included only 51 children.
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) recommends screening all women for tobacco, alcohol, and drug use (including marijuana) during early pregnancy.9 Women who report marijuana use should be counseled regarding potential adverse consequences to fetal health and be encouraged to discontinue use.
ACOG says that insufficient data exist to evaluate the effects of marijuana use on infants during lactation and breastfeeding and recommends against it.
The American Society of Addiction Medicine also recommends screening pregnant women for drug use and making appropriate referrals for substance use treatment.10
1. Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol. 2015;213:761-778.
2. Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215:506.e1-e7.
3. Warner TD, Roussos-Ross D, Behnke M. It’s not your mother’s marijuana: effects on maternal-fetal health and the developing child. Clinical Perinatology. 2014;41:877-894.
4. Huizink AC. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;52:45-52.
5. Goldschmidt L, Richardson GA, Willford J, et al. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47:254-263.
6. Fried PA. The Ottawa Prenatal Prospective Study (OPPS): methodological issues and findings—it’s easy to throw the baby out with the bath water. Life Sci. 1995;56:2159-2168.
7. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
8. Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313-1322.
9. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion No. 637: Marijuana use during pregnancy and lactation. Obstet Gynecol. 2015;126:234-238.
10. American Society of Addiction Medicine. Public policy statement on women, alcohol and other drugs, and pregnancy. Chevy Chase MD: American Society of Addiction Medicine; 2011. Available at: http://www.asam.org/docs/default-source/public-policy-statements/1womenandpregnancy_7-11.pdf. Accessed July 5, 2016.
1. Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol. 2015;213:761-778.
2. Chabarria KC, Racusin DA, Antony KM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215:506.e1-e7.
3. Warner TD, Roussos-Ross D, Behnke M. It’s not your mother’s marijuana: effects on maternal-fetal health and the developing child. Clinical Perinatology. 2014;41:877-894.
4. Huizink AC. Prenatal cannabis exposure and infant outcomes: overview of studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2014;52:45-52.
5. Goldschmidt L, Richardson GA, Willford J, et al. Prenatal marijuana exposure and intelligence test performance at age 6. J Am Acad Child Adolesc Psychiatry. 2008;47:254-263.
6. Fried PA. The Ottawa Prenatal Prospective Study (OPPS): methodological issues and findings—it’s easy to throw the baby out with the bath water. Life Sci. 1995;56:2159-2168.
7. Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325-336.
8. Day NL, Goldschmidt L, Thomas CA. Prenatal marijuana exposure contributes to the prediction of marijuana use at age 14. Addiction. 2006;101:1313-1322.
9. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Committee Opinion No. 637: Marijuana use during pregnancy and lactation. Obstet Gynecol. 2015;126:234-238.
10. American Society of Addiction Medicine. Public policy statement on women, alcohol and other drugs, and pregnancy. Chevy Chase MD: American Society of Addiction Medicine; 2011. Available at: http://www.asam.org/docs/default-source/public-policy-statements/1womenandpregnancy_7-11.pdf. Accessed July 5, 2016.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
The effects are unclear. Marijuana use during pregnancy is associated with clinically unimportant lower birth weights (growth differences of approximately 100 g), but no differences in preterm births or congenital anomalies (strength of recommendation [SOR]: B, prospective and retrospective cohort studies with methodologic flaws).
Similarly, prenatal marijuana use isn’t associated with differences in neurodevelopmental outcomes (behavior problems, intellect, visual perception, language, or sustained attention and memory tasks) at birth, in the neonatal period, or in childhood through age 3 years. However, it may be associated with minimally lower verbal/quantitative IQ scores (1%) at age 6 years and increased impulsivity and hyperactivity (1%) at 10 years. Prenatal use isn’t linked to increased substance use at age 14 years (SOR: B, conflicting long-term prospective and retrospective cohort studies with methodologic flaws).
Do pedometers increase activity and improve health outcomes?
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
EVIDENCE SUMMARY
A systematic review and meta-analysis identified 26 studies evaluating activity and health outcomes with the use of pedometers.1 The studies included 8 RCTs and 18 observational studies with 2767 patients (mean body mass index [BMI]: 30 kg/m2; mean age: 49 years; 85% women). The studies ranged from 3 to 104 weeks. From the RCT data, patients using pedometers had an increase of 2491 steps per day (about one mile) more than control group patients (8 trials, n=305; 95% confidence interval [CI], 1098-3885 steps/day; P<.001).
Across all of the observational studies, pedometer users had a 26.9% increase from their baseline physical activity (P=.001). When data from all of the studies were combined, the researchers found a decrease from baseline BMI (18 studies, n=562; mean difference [MD]=0.38 kg/m2; 95% CI, 0.05-0.72; P=.03) and a decrease in systolic BP (12 studies, n=468; MD=3.8 mm Hg; 95% CI, 1.7-5.9 mm Hg; P<.001). No statistically significant change was noted in cholesterol or fasting glucose levels. Weaknesses of this review include the heterogeneity of the interventions, relatively small study sizes, and short study durations.
A systematic review and meta-analysis of 11 RCTs (N=1258) evaluated pedometer effects in overweight patients with type 2 diabetes.2 (One RCT was included in the above meta-analysis.) Studies ran from 6 to 48 weeks, and mean enrollment BMI (where reported) was 30 kg/m2 or more in at least one treatment arm. Compared to controls, patients using pedometers had greater reductions in weight (weighted mean difference [WMD]= -0.65 kg; 95% CI, -1.12 to -0.17 kg) and BMI (WMD= -0.15 kg/m2; 95% CI, -0.29 to -0.02 kg/m2). The effect persisted in the subset of studies in which the intervention and control groups both received dietary counseling (WMD weight= -0.86 kg; 95% CI, -1.45 to -0.27 kg; WMD BMI= -0.30 kg/m2; 95% CI, -0.50 to -0.10 kg/m2). Study quality was low to moderate, and 5 studies used per-protocol analysis instead of intention-to-treat analysis.
Pedometer use benefits patients with musculoskeletal diseases, too
A systematic review and meta-analysis examined the use of pedometers in patients with musculoskeletal diseases.3 It included 7 RCTs lasting 4 weeks to one year with 484 adults, 40 to 82 years of age, with musculoskeletal disorders (eg, back pain, knee pain, hip pain). (One RCT was also included in the diabetes meta-analysis.) Pedometer use resulted in a mean increase in physical activity of 1950 steps per day above baseline (range=818-2829 steps/day; P<.05). The authors noted that 4 of the 7 studies also demonstrated significant improvement in pain scores and physical function. BMI data were not tracked in this review.
Pedometers increase walking in older patients
A RCT compared the effects of pedometer-based activity prescriptions with standard time-based activity prescriptions in 330 patients ≥65 years of age with baseline low activity levels.4 All patients received an initial physician visit followed by 3 telephone counseling sessions encouraging increased activity. The pedometer group was counseled on increasing steps (without specific targets), while the standard activity prescription group received time-related activity goals.
At one year, “leisure walking” had increased more for the pedometer group than for the standard group (mean 50 minutes/week vs 28 minutes/week; P=.03), although both groups equally increased their amount of “total activity.”
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
1. Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296-2304.
2. Cai X, Qiu SH, Yin H, et al. Pedometer intervention and weight loss in overweight and obese adults with type 2 diabetes: a meta-analysis. Diabet Med. 2016;33:1035-1044.
3. Mansi S, Milosavljevic S, Baxter GD, et al. A systematic review of studies using pedometers as an intervention for musculoskeletal diseases. BMC Musculoskeletal Disorders. 2014;15:231.
4. Kolt GS, Schofield GM, Kerse N, et al. Healthy Steps trial: pedometer-based advice and physical activity for low-active older adults. Ann Fam Med. 2012;10:206-212.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes. In overweight and obese patients, exercise interventions using a pedometer increase steps by about a mile per day over the same interventions without access to pedometer information (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]) and are associated with a modest 4 mm Hg reduction in systolic blood pressure (BP) over baseline (SOR: B, meta-analysis of RCTs and cohort studies). In overweight patients with diabetes, pedometer use with nutritional counseling is associated with 0.86 kg greater weight loss than nutritional counseling alone (SOR: B, meta-analysis of lower quality RCTs).
Pedometers increase activity in patients with various musculoskeletal conditions and may help reduce pain (SOR: B, meta-analysis of RCTs with heterogeneous outcomes). In low-activity elderly patients, pedometers do not appear to increase total activity when added to an exercise program, but they do appear to increase walking (SOR: B, RCT).
There is no evidence concerning the impact of pedometers on cardiovascular outcomes.
Does breastfeeding affect the risk of childhood obesity?
EVIDENCE SUMMARY
A systematic review and meta-analysis of prospective cohort studies evaluating infant risk factors for childhood obesity found that breastfeeding was associated with a lower risk of obesity.1 The authors identified 10 trials (primarily from the United States and Europe) with more than 76,000 infants that compared the effect of some breastfeeding in the first year to no breastfeeding. Follow-up ranged from 2 to 14 years (median 6 years).
Having ever breastfed decreased the odds of future overweight (BMI >85th percentile) or obesity (BMI >95th percentile) by 15% (adjusted odds ratio [AOR]=0.85; 95% confidence interval [CI], 0.74-0.99).
Subsequent studies suggest increased risk with formula feeding
Three large, prospective, longitudinal cohort studies have been published since the meta-analysis. One, which followed 43,367 term infants in Japan, found that formula feeding before 6 months was associated with increased risk of obesity compared with continuous breastfeeding for 6 months.2 Researchers evaluated weight at 7 years and adjusted for child and maternal factors associated with weight gain (AOR for obesity, formula-fed infants=1.8; 95% CI, 1.3-2.6).
A similar prospective longitudinal cohort study of 2868 infants in Australia analyzed maternal breastfeeding diaries and followed children’s weight to age 20 years.3 Introducing a milk other than breast milk before 6 months of age was linked to increased risk of obesity at age 20 (odds ratio [OR]=1.5; 95% CI, 1.1-1.9).
Finally, in a prospective cohort of 568 children in India, 17% of children who breastfed for fewer than 6 months were above the 90th percentile for weight at age 5 years, compared with 10% of children who were breastfed for at least 18 months.4 The result didn’t reach statistical significance, however (P=.08).
Interventions that increase breastfeeding don’t seem to have an impact
An RCT of an intervention to promote breastfeeding didn’t find any effect on subsequent obesity rates. Researchers in Belarus randomized 17,046 mother-infant pairs to breastfeeding promotion, modeled on the UNICEF Baby-Friendly Hospital Initiative, or usual care. The intervention increased the prevalence of exclusive breastfeeding (at 3 months, 43% vs 6%; at 6 months, 7% vs 0.6%; P values not given).
When researchers evaluated 13,879 children at 11 or 12 years by intention-to-treat analysis, however, they found no difference in mean BMI between the children whose mothers received the intervention and those whose mothers didn’t (BMI difference=0.16; 95% CI, -0.02 to 0.35).5
Introduction of solid foods: Later is better
A systematic review investigated the association between the timing of introducing complementary (solid) foods and childhood obesity in 23 primarily cross-sectional and cohort studies (17 from the United States, Canada, and Europe) with more than 33,000 patients. Follow-up ranged from 4 to 19 years.
Eight of the 21 studies that used BMI as an outcome found that early introduction of complementary foods was associated with a higher childhood BMI. In the largest study (a cohort of 17,561 infants), introducing complementary foods before 3 months was associated with higher risk of obesity at age 5 years than introducing them thereafter (OR=1.3; 95% CI, 1.1-1.6).6 Introduction of solids after 4 months was not associated with childhood obesity.
A systematic review of 10 primarily cross-sectional and cohort studies with more than 3000 infants evaluated associations between the types of complementary foods given and the development of childhood obesity.7 Six of the 10 studies were from Europe and none were from the United States. Follow-up ages ranged from 4 to 11 years.
Outcomes were heterogeneous, and no meta-analysis could be performed. The authors cited 3 studies (total 1174 infants) that found various positive associations between total caloric intake during complementary feeding and childhood obesity. No consistent evidence pointed to increased risk from specific foods or food groups.
Scheduled feeding is linked to rapid infant weight gain
A cohort study evaluated the baseline data of an Australian RCT (on an intervention to promote proper nutrition) in 612 infants, mean age 4.3 months.8 Researchers looked at the relationship between feeding on demand vs scheduled feeding (assessed by parental report) and weight gain in infancy. “Rapid weight gain” was defined as >0.67 change in weight-for-age Z-score between birth and enrollment.
Scheduled feeding was associated with rapid weight gain at a higher rate than feeding on demand (OR=2.3; 95% CI, 1.1-4.6). This study didn’t use childhood obesity as an outcome.
1. Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019-1026.
2. Yamakawa M, Yorifuji T, Inoue S, et al. Breastfeeding and obesity among schoolchildren: a national longitudinal survey in Japan. JAMA Pediatr. 2013;167:919-925.
3. Oddy WH, Mari TA, Huang RC, et al. Early infant feeding and adiposity risk: from infancy to adulthood. Ann Nutr Metab. 2014;64:262-270.
4. Caleyachetty A, Krishnaveni GV, Veena SR, et al. Breast-feeding duration, age of starting solids, and high BMI risk and adiposity in Indian children. Matern Child Nutr. 2013;9:199-216.
5. Martin RM, Patel, R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
6. Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:1295-1306.
7. Pearce J, Langley-Evans. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:477-485.
8. Mihrshahi S, Battistutta D, Magarey A, et al. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99.
EVIDENCE SUMMARY
A systematic review and meta-analysis of prospective cohort studies evaluating infant risk factors for childhood obesity found that breastfeeding was associated with a lower risk of obesity.1 The authors identified 10 trials (primarily from the United States and Europe) with more than 76,000 infants that compared the effect of some breastfeeding in the first year to no breastfeeding. Follow-up ranged from 2 to 14 years (median 6 years).
Having ever breastfed decreased the odds of future overweight (BMI >85th percentile) or obesity (BMI >95th percentile) by 15% (adjusted odds ratio [AOR]=0.85; 95% confidence interval [CI], 0.74-0.99).
Subsequent studies suggest increased risk with formula feeding
Three large, prospective, longitudinal cohort studies have been published since the meta-analysis. One, which followed 43,367 term infants in Japan, found that formula feeding before 6 months was associated with increased risk of obesity compared with continuous breastfeeding for 6 months.2 Researchers evaluated weight at 7 years and adjusted for child and maternal factors associated with weight gain (AOR for obesity, formula-fed infants=1.8; 95% CI, 1.3-2.6).
A similar prospective longitudinal cohort study of 2868 infants in Australia analyzed maternal breastfeeding diaries and followed children’s weight to age 20 years.3 Introducing a milk other than breast milk before 6 months of age was linked to increased risk of obesity at age 20 (odds ratio [OR]=1.5; 95% CI, 1.1-1.9).
Finally, in a prospective cohort of 568 children in India, 17% of children who breastfed for fewer than 6 months were above the 90th percentile for weight at age 5 years, compared with 10% of children who were breastfed for at least 18 months.4 The result didn’t reach statistical significance, however (P=.08).
Interventions that increase breastfeeding don’t seem to have an impact
An RCT of an intervention to promote breastfeeding didn’t find any effect on subsequent obesity rates. Researchers in Belarus randomized 17,046 mother-infant pairs to breastfeeding promotion, modeled on the UNICEF Baby-Friendly Hospital Initiative, or usual care. The intervention increased the prevalence of exclusive breastfeeding (at 3 months, 43% vs 6%; at 6 months, 7% vs 0.6%; P values not given).
When researchers evaluated 13,879 children at 11 or 12 years by intention-to-treat analysis, however, they found no difference in mean BMI between the children whose mothers received the intervention and those whose mothers didn’t (BMI difference=0.16; 95% CI, -0.02 to 0.35).5
Introduction of solid foods: Later is better
A systematic review investigated the association between the timing of introducing complementary (solid) foods and childhood obesity in 23 primarily cross-sectional and cohort studies (17 from the United States, Canada, and Europe) with more than 33,000 patients. Follow-up ranged from 4 to 19 years.
Eight of the 21 studies that used BMI as an outcome found that early introduction of complementary foods was associated with a higher childhood BMI. In the largest study (a cohort of 17,561 infants), introducing complementary foods before 3 months was associated with higher risk of obesity at age 5 years than introducing them thereafter (OR=1.3; 95% CI, 1.1-1.6).6 Introduction of solids after 4 months was not associated with childhood obesity.
A systematic review of 10 primarily cross-sectional and cohort studies with more than 3000 infants evaluated associations between the types of complementary foods given and the development of childhood obesity.7 Six of the 10 studies were from Europe and none were from the United States. Follow-up ages ranged from 4 to 11 years.
Outcomes were heterogeneous, and no meta-analysis could be performed. The authors cited 3 studies (total 1174 infants) that found various positive associations between total caloric intake during complementary feeding and childhood obesity. No consistent evidence pointed to increased risk from specific foods or food groups.
Scheduled feeding is linked to rapid infant weight gain
A cohort study evaluated the baseline data of an Australian RCT (on an intervention to promote proper nutrition) in 612 infants, mean age 4.3 months.8 Researchers looked at the relationship between feeding on demand vs scheduled feeding (assessed by parental report) and weight gain in infancy. “Rapid weight gain” was defined as >0.67 change in weight-for-age Z-score between birth and enrollment.
Scheduled feeding was associated with rapid weight gain at a higher rate than feeding on demand (OR=2.3; 95% CI, 1.1-4.6). This study didn’t use childhood obesity as an outcome.
EVIDENCE SUMMARY
A systematic review and meta-analysis of prospective cohort studies evaluating infant risk factors for childhood obesity found that breastfeeding was associated with a lower risk of obesity.1 The authors identified 10 trials (primarily from the United States and Europe) with more than 76,000 infants that compared the effect of some breastfeeding in the first year to no breastfeeding. Follow-up ranged from 2 to 14 years (median 6 years).
Having ever breastfed decreased the odds of future overweight (BMI >85th percentile) or obesity (BMI >95th percentile) by 15% (adjusted odds ratio [AOR]=0.85; 95% confidence interval [CI], 0.74-0.99).
Subsequent studies suggest increased risk with formula feeding
Three large, prospective, longitudinal cohort studies have been published since the meta-analysis. One, which followed 43,367 term infants in Japan, found that formula feeding before 6 months was associated with increased risk of obesity compared with continuous breastfeeding for 6 months.2 Researchers evaluated weight at 7 years and adjusted for child and maternal factors associated with weight gain (AOR for obesity, formula-fed infants=1.8; 95% CI, 1.3-2.6).
A similar prospective longitudinal cohort study of 2868 infants in Australia analyzed maternal breastfeeding diaries and followed children’s weight to age 20 years.3 Introducing a milk other than breast milk before 6 months of age was linked to increased risk of obesity at age 20 (odds ratio [OR]=1.5; 95% CI, 1.1-1.9).
Finally, in a prospective cohort of 568 children in India, 17% of children who breastfed for fewer than 6 months were above the 90th percentile for weight at age 5 years, compared with 10% of children who were breastfed for at least 18 months.4 The result didn’t reach statistical significance, however (P=.08).
Interventions that increase breastfeeding don’t seem to have an impact
An RCT of an intervention to promote breastfeeding didn’t find any effect on subsequent obesity rates. Researchers in Belarus randomized 17,046 mother-infant pairs to breastfeeding promotion, modeled on the UNICEF Baby-Friendly Hospital Initiative, or usual care. The intervention increased the prevalence of exclusive breastfeeding (at 3 months, 43% vs 6%; at 6 months, 7% vs 0.6%; P values not given).
When researchers evaluated 13,879 children at 11 or 12 years by intention-to-treat analysis, however, they found no difference in mean BMI between the children whose mothers received the intervention and those whose mothers didn’t (BMI difference=0.16; 95% CI, -0.02 to 0.35).5
Introduction of solid foods: Later is better
A systematic review investigated the association between the timing of introducing complementary (solid) foods and childhood obesity in 23 primarily cross-sectional and cohort studies (17 from the United States, Canada, and Europe) with more than 33,000 patients. Follow-up ranged from 4 to 19 years.
Eight of the 21 studies that used BMI as an outcome found that early introduction of complementary foods was associated with a higher childhood BMI. In the largest study (a cohort of 17,561 infants), introducing complementary foods before 3 months was associated with higher risk of obesity at age 5 years than introducing them thereafter (OR=1.3; 95% CI, 1.1-1.6).6 Introduction of solids after 4 months was not associated with childhood obesity.
A systematic review of 10 primarily cross-sectional and cohort studies with more than 3000 infants evaluated associations between the types of complementary foods given and the development of childhood obesity.7 Six of the 10 studies were from Europe and none were from the United States. Follow-up ages ranged from 4 to 11 years.
Outcomes were heterogeneous, and no meta-analysis could be performed. The authors cited 3 studies (total 1174 infants) that found various positive associations between total caloric intake during complementary feeding and childhood obesity. No consistent evidence pointed to increased risk from specific foods or food groups.
Scheduled feeding is linked to rapid infant weight gain
A cohort study evaluated the baseline data of an Australian RCT (on an intervention to promote proper nutrition) in 612 infants, mean age 4.3 months.8 Researchers looked at the relationship between feeding on demand vs scheduled feeding (assessed by parental report) and weight gain in infancy. “Rapid weight gain” was defined as >0.67 change in weight-for-age Z-score between birth and enrollment.
Scheduled feeding was associated with rapid weight gain at a higher rate than feeding on demand (OR=2.3; 95% CI, 1.1-4.6). This study didn’t use childhood obesity as an outcome.
1. Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019-1026.
2. Yamakawa M, Yorifuji T, Inoue S, et al. Breastfeeding and obesity among schoolchildren: a national longitudinal survey in Japan. JAMA Pediatr. 2013;167:919-925.
3. Oddy WH, Mari TA, Huang RC, et al. Early infant feeding and adiposity risk: from infancy to adulthood. Ann Nutr Metab. 2014;64:262-270.
4. Caleyachetty A, Krishnaveni GV, Veena SR, et al. Breast-feeding duration, age of starting solids, and high BMI risk and adiposity in Indian children. Matern Child Nutr. 2013;9:199-216.
5. Martin RM, Patel, R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
6. Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:1295-1306.
7. Pearce J, Langley-Evans. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:477-485.
8. Mihrshahi S, Battistutta D, Magarey A, et al. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99.
1. Weng SF, Redsell SA, Swift JA, et al. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019-1026.
2. Yamakawa M, Yorifuji T, Inoue S, et al. Breastfeeding and obesity among schoolchildren: a national longitudinal survey in Japan. JAMA Pediatr. 2013;167:919-925.
3. Oddy WH, Mari TA, Huang RC, et al. Early infant feeding and adiposity risk: from infancy to adulthood. Ann Nutr Metab. 2014;64:262-270.
4. Caleyachetty A, Krishnaveni GV, Veena SR, et al. Breast-feeding duration, age of starting solids, and high BMI risk and adiposity in Indian children. Matern Child Nutr. 2013;9:199-216.
5. Martin RM, Patel, R, Kramer MS, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005-1013.
6. Pearce J, Taylor MA, Langley-Evans SC. Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:1295-1306.
7. Pearce J, Langley-Evans. The types of food introduced during complementary feeding and risk of childhood obesity: a systematic review. Int J Obes (Lond). 2013;37:477-485.
8. Mihrshahi S, Battistutta D, Magarey A, et al. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE-BASED ANSWER:
Yes. Ever having breastfed during the first year of life is associated with a 15% lower risk of overweight or obesity over the next 2 to 14 years compared with never having breastfed. Breastfeeding exclusively for 6 months is associated with a 30% to 50% reduction in risk (strength of recommendation [SOR]: B, meta-analysis of cohort studies and subsequent cohort studies). However, interventions that increase breastfeeding rates during the first 3 to 6 months of life don’t appear to alter body mass index (BMI) at 11 to 12 years of age (SOR: B, randomized clinical trial [RCT]).
Introducing complementary (solid) foods before 3 months is associated with a 30% greater risk of childhood obesity than later introduction; starting solid foods after 4 months isn’t linked to increased obesity. High caloric density of complementary feedings may be associated with greater childhood obesity (SOR: C, systematic reviews of heterogeneous cohort studies).
Scheduled feeding doubles the risk of rapid infant weight gain compared with on-demand feeding, although it’s unclear whether a direct relationship exists between rapid infant weight gain and childhood obesity (SOR: B, cohort study).