User login
Does concurrent use of clopidogrel and PPIs increase CV risk in patients with ACS?
EVIDENCE SUMMARY
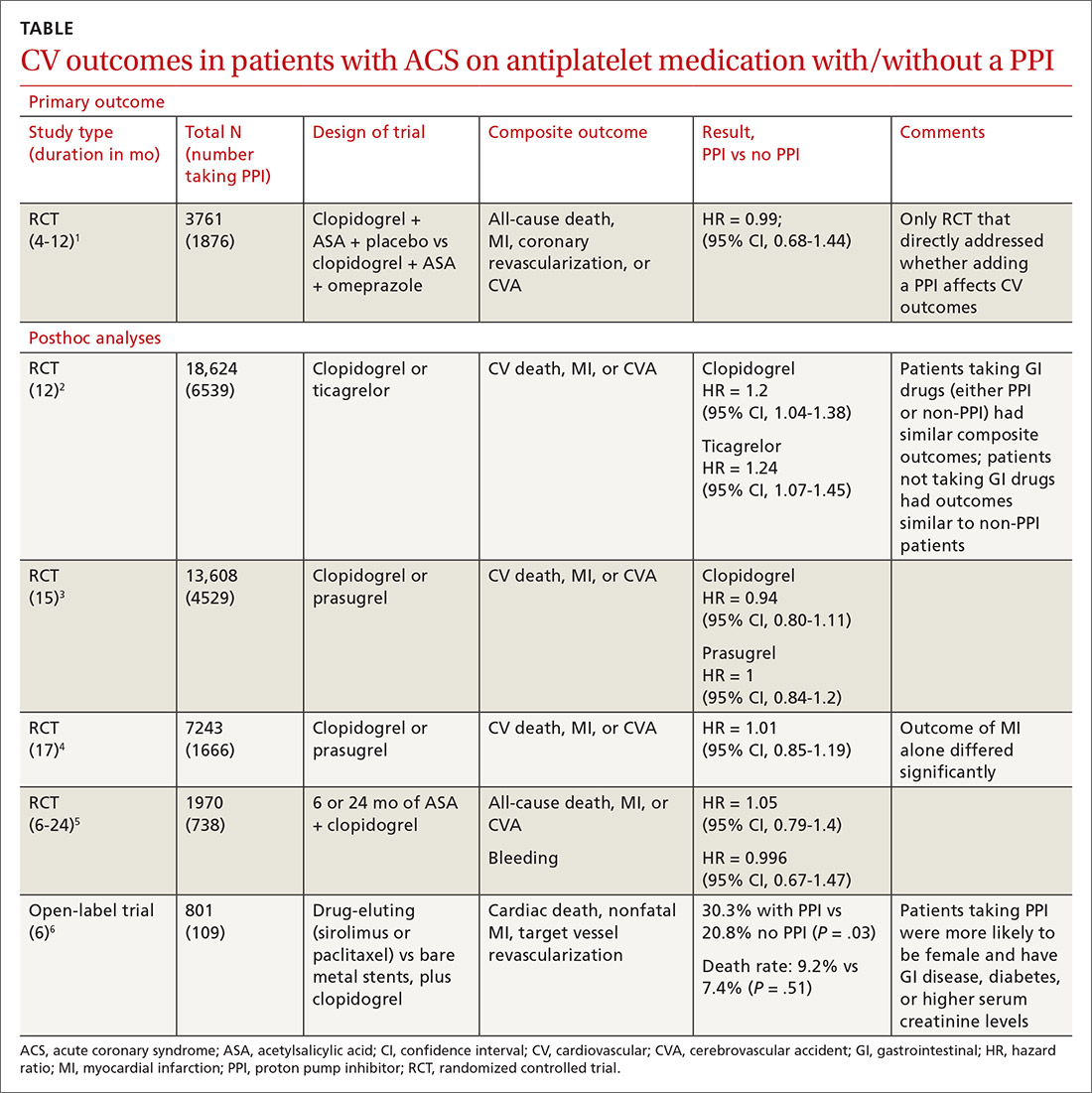
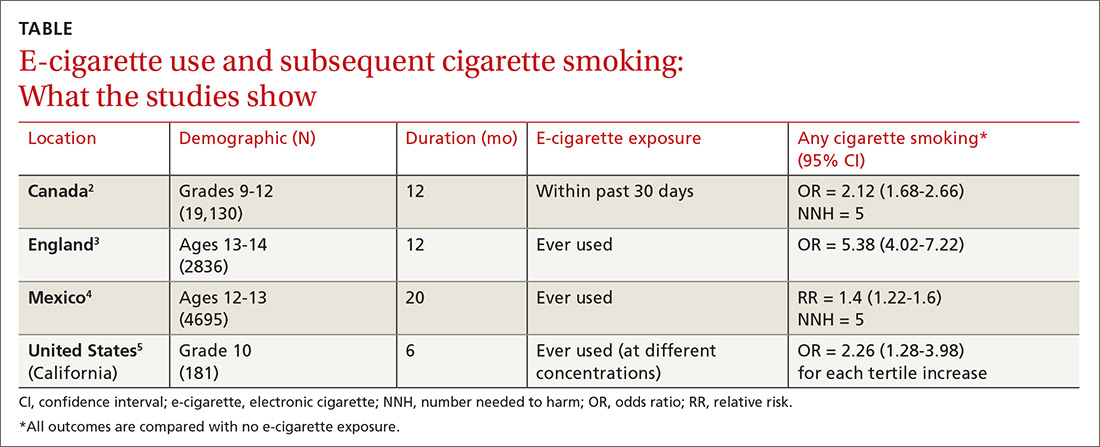
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
EVIDENCE SUMMARY
A double-blind, double-dummy, placebo-controlled RCT comparing a combination of clopidogrel, aspirin, and omeprazole with clopidogrel, aspirin, and placebo found no increase in composite CV outcomes with the PPI (TABLE).1 Using a PPI did, however, significantly reduce gastrointestinal (GI) bleeding (hazard ratio [HR] = 0.13; 95% confidence interval [CI], 0.03-0.56).Although several meta-analyses have been conducted, they all rely on this single RCT that directly addresses the question, plus post-hoc analyses of other RCTs.
Four of 5 analyses find little or no difference in CV outcomes with a PPI
Four of 5 posthoc analyses (which weren’t themselves randomized) of RCTs found unclear or no differences in composite CV outcomes with concurrent use of a PPI and antiplatelet therapy, after multivariate adjustment for differences in populations taking or not taking a PPI.
Posthoc analysis of the largest study found worse CV outcomes for both clopidogrel and ticagrelor with concomitant PPI use.2 However, patients on any GI drugs (PPI or non-PPI) had composite outcomes similar to patients on a PPI (PPI vs non-PPI GI treatment: HR = 0.98; 95% CI, 0.79-1.23), and patients not taking GI drugs had fewer composite outcomes compared with patients on a PPI (clopidogrel vs no GI therapy: HR = 1.29; 95% CI, 1.12-1.49; ticagrelor vs no GI therapy: HR = 1.30; 95% CI, 1.14-1.49). Researchers postulated that because the rate of composite outcomes increased equally for patients on any GI drug, the higher rate of CV adverse events with a PPI might have been related to GI disease rather than PPI use.
A similar posthoc analysis found no differences with or without PPI use among patients with ACS undergoing planned percutaneous coronary intervention (PCI) and assigned to clopidogrel or prasugrel.3 Researchers performed multivariate adjustment for differences in age, gender, ethnicity, and initial presence of unstable angina/non-ST-elevation MI.
A smaller study also found no significant differences in composite CV outcomes in patients using PPIs.4 Patients did have higher rates of MI (HR = 0.62; 95% CI, 0.42-0.91), but they were more likely to be older and have a previous diagnosis of non-ST-elevation MI, higher incidence of previous coronary artery bypass graft surgery, and history of peptic ulcer disease.
The fourth posthoc analysis of an RCT found that concomitant PPI use (91% of patients on lansoprazole) didn’t alter outcomes among patients undergoing PCI and receiving dual antiplatelet therapy with clopidogrel and aspirin.5 Researchers used a multivariate adjustment for differences in age, gender, and renal function and found no difference in outcomes during the 6-month or 24-month period. PPI prescription was at physician discretion. Researchers didn’t assess for dose-dependent effects of PPI.
A fifth, flawed study finds more adverse events with PPIs
A posthoc analysis of a smaller, open-label trial found increased major adverse cardiac events with PPI use among patients taking clopidogrel after PCI.6 Researchers didn’t adjust for differences in populations at baseline, however, and patients taking PPIs were more likely to be female or older and have diabetes, GI disease, or higher serum creatinine levels.
Continue to: Editor's takeaway
Editor’s takeaway
The best evidence (a large RCT) found that adding a PPI to antiplatelet therapy didn’t alter CV outcomes in patients with ACS, but it did reduce GI bleeds. Hopefully this will give providers the confidence to use PPIs, if clinically indicated, in patients taking antiplatelet therapy with clopidogrel or prasugrel.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
1. Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909-1917.
2. Goodman SG, Clare R, Pieper KS, et al. Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978-986.
3. O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989-997.
4. Nicolau JC, Bhatt DL, Roe MT, et al. Concomitant proton-pump inhibitor use, platelet activity, and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel and managed without revascularization: insights from the Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes trial. Am Heart J. 2015;170:683-694.e3.
5. Gargiulo G, Costa F, Ariotti S, et al. Impact of proton pump inhibitors on clinical outcomes in patients treated with a 6- or 24-month dual-antiplatelet therapy duration: insights from the PROlonging Dual-antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY trial. Am Heart J. 2016;174:95-102.
6. Burkard T, Kaiser CA, Brunner-La Rocca H, et al. Combined clopidogrel and proton pump inhibitor therapy is associated with higher cardiovascular event rates after percutaneous coronary intervention: a report from the BASKET trial. J Intern Med. 2012;271:257-263.
EVIDENCE-BASED ANSWER:
No. Adding a proton pump inhibitor (PPI) in patients taking antiplatelet medications such as clopidogrel for acute coronary syndrome (ACS) doesn’t increase the composite risk of cardiovascular (CV) events: CV death, myocardial infarction (MI), and cerebrovascular accident (CVA) (strength of recommendation: B, randomized, controlled trial [RCT] and prepon-derance of posthoc analyses of large RCTs).
Does vitamin D supplementation reduce asthma exacerbations?
EVIDENCE SUMMARY
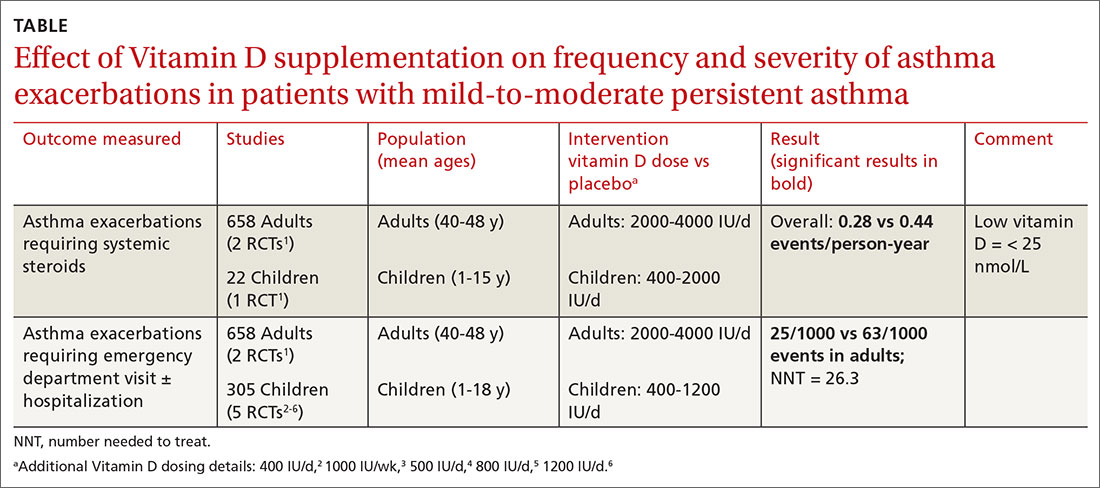
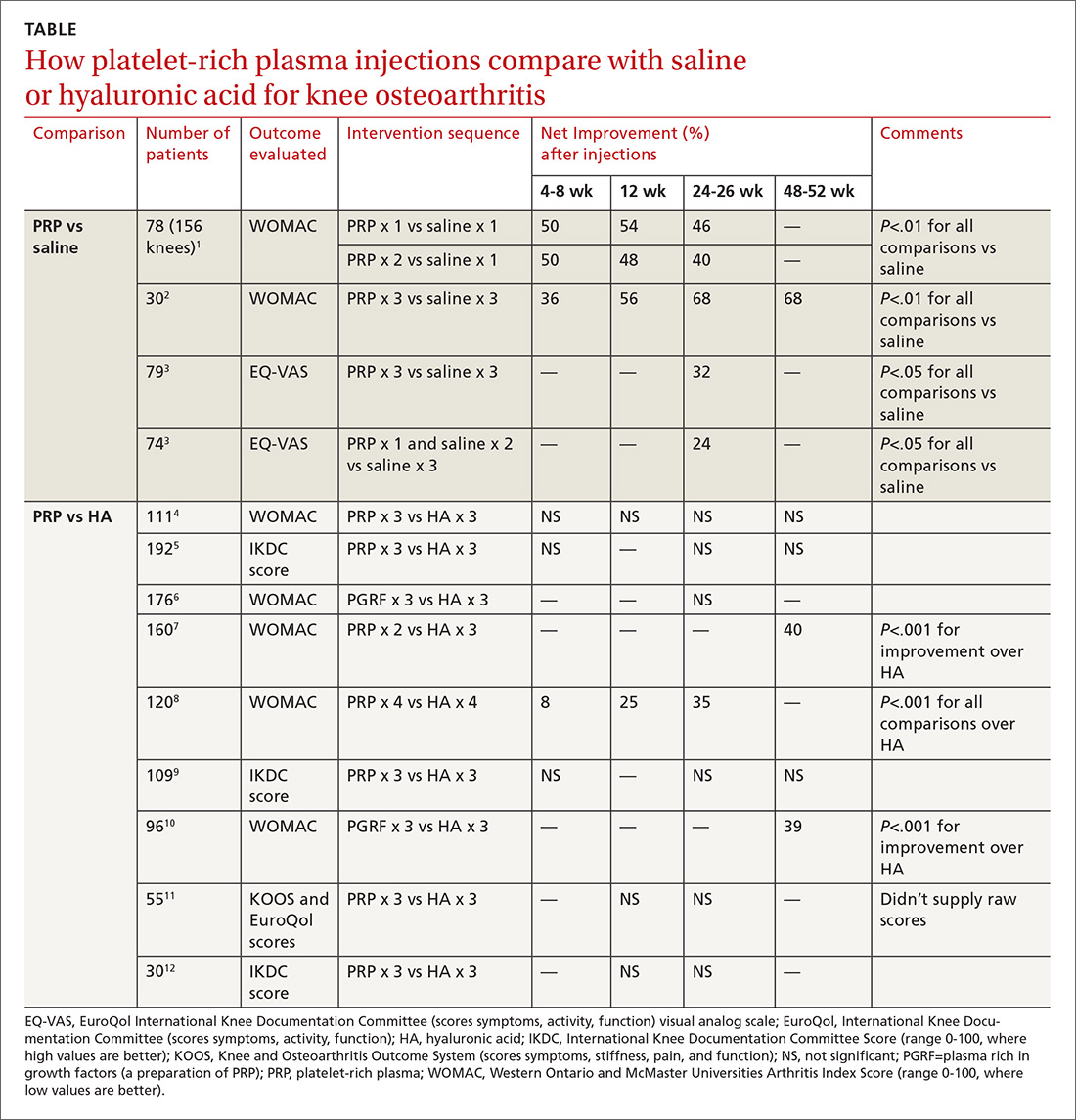
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
EVIDENCE SUMMARY
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
EVIDENCE SUMMARY
A Cochrane systematic review of vitamin D for managing asthma performed meta-analyses on RCTs that evaluated several outcomes.1 The review found improvement in the primary outcome of asthma exacerbations requiring systemic steroids, mainly in adult patients, and in the secondary outcomes of emergency department visits or hospitalization, in a mix of adults and children (TABLE1-6).
Most participants had mild-to-moderate asthma; trials lasted 4 to 12 months. Vitamin D dosage regimens varied, with a median daily dose of 900 IU/d (range, 400-4000 IU/d). Six RCTs were rated high-quality, and 1 had unclear risk of bias.
Supplementation reduced exacerbations in patients with low vitamin D levels
A subsequent (2017) systematic review and meta-analysis evaluating the primary outcome of exacerbations requiring steroids7 included another study8 (in addition to the 6 RCTs in the Cochrane review).
When researchers reanalyzed individual participant data from the trials in the Cochrane review, plus the additional RCT, to include baseline vitamin D levels, they found that vitamin D supplementation reduced exacerbations overall (NNT = 7.7) and in patients with low baseline vitamin D levels (25[OH] vitamin D < 25 nmol/L; 92 participants in 3 RCTs; NNT = 4.3) but not in patients with higher baseline levels (764 participants in 6 RCTs). Vitamin D supplementation reduced the asthma exacerbation rate in patients with low baseline vitamin D levels (0.19 vs 0.42 events per participant-year; P = .046).
Smaller benefit found on ED visits and hospitalizations
The Cochrane review, with 2 RCTs with adults (n = 658)1 and 5 RCTs with children (n = 305),2-6 evaluated whether Vitamin D reduced the need for emergency department visits and hospitalization with asthma exacerbations; they found a smaller benefit (NNT = 26.3).
Effects on FEV1, daily asthma symptoms, and serious adverse effects
Several RCTs included in the 2017 meta-analysis found no effect of vitamin D supplementation on FEV1, daily asthma symptoms (evaluated with the standardized Asthma Control Test Score), or reported serious adverse events.2-6,9,10 No deaths occurred in any trial.
Additional findings in children from lower-quality studies
A 2015 systematic review and meta-analysis of RCTs evaluating vitamin D supplementation for children with asthma found11:
- moderate-quality evidence for decreased emergency department visits (1 RCT from India, 100 children ages 3 to 14 years, decrease not specified; P = .015);
- low-quality evidence for reduced exacerbations (6 RCTs [3 RCTs also in Cochrane review], 507 children ages 3 to 17 years; risk ratio = 0.41; 95% confidence interval, 0.27-0.63); and
- low-quality evidence for reduced standardized asthma symptom scores (6 RCTs [2 RCTs also in Cochrane review], 231 children ages 3 to 17 years; amount of reduction not listed; P = .01).
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
No published guidelines discuss using vitamin D in managing asthma. An American Academy of Family Physicians (AAFP) summary of the Cochrane systematic review recommends that family physicians await further studies and updated guidelines before recommending vitamin D for patients with asthma.12 The AAFP also points out that the Endocrine Society has recommended vitamin D supplementation for adults (1500-2000 IU/d) and children (at least 1000 IU/d) at risk for deficiency.
Editor's takeaway
In the meta-analyses highlighted here, researchers evaluated asthma patients with a wide range of ages, baseline vitamin D levels, and vitamin D supplementation protocols. Although vitamin D reduced asthma exacerbations requiring steroids overall, the effect was driven by 3 studies of patients with low baseline vitamin D levels. As a result, disentangling who might benefit the most remains a challenge. The conservative course for now is to manage asthma according to current guidelines and supplement vitamin D in patients at risk for, or with known, deficiency.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
, , , . Vitamin D for the management of asthma. Cochrane Database Syst Rev. 2016;9:CD011511.
2. Jensen M, Mailhot G, Alos N, et al. Vitamin D intervention in preschoolers with viral-induced asthma (DIVA): a pilot randomised controlled trial. Trials. 2016;26:17:353.
, , , et al. Correlation of vitamin D with Foxp3 induction and steroid-sparing effect of immunotherapy in asthmatic children. Ann Allergy Asthma Immunol. 2012;109:329-335.
, , , et al. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol. 2011;127:1294-1296.
, , , et al. Improved control of childhood asthma with low-dose, short-term vitamin D supplementation: a randomized, double-blind, placebo-controlled trial. Allergy. 2016;71:1001-1009.
, , , et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. Am J Clin Nutr. 2010;91:1255-1260.
7. Joliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta-analysis of individual participant data. Lancet 2017;5:881-890.
8. Kerley CP, Hutchinson K, Cormical L, et al. Vitamin D3 for uncontrolled childhood asthma: a pilot study. Pediatr Allergy Immunol. 2016;27:404-412.
, , , et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014;311:2083-2091.
, , , et al. Double-blind multi-centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid-treated asthma (ViDiAs). Thorax. 2015:70:451-457.
11. Riverin B, Maguire J, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta-analysis. PLOS One. 2015;10:e0136841.
EVIDENCE-BASED ANSWER:
Yes, to some extent it does, and primarily in patients with low vitamin D levels. Supplementation reduces asthma exacerbations requiring systemic steroids by 30% overall in adults and children with mild-to-moderate asthma (number needed to treat [NNT] = 7.7). The outcome is driven by the effect in patients with vitamin D levels < 25 nmol/L (NNT = 4.3), however; supplementation doesn’t decrease exacerbations in patients with higher levels. Supplementation also reduces, by a smaller amount (NNT = 26.3), the odds of exacerbations requiring emergency department care or hospitalization (strength of recommendation [SOR]: A, meta-analysis of randomized controlled trials [RCTs]).
In children, vitamin D supplementation may also reduce exacerbations and improve symptom scores (SOR: C, low-quality RCTs).
Vitamin D doesn’t improve forced expiratory volume in 1 second (FEV1) or standardized asthma control test scores. Also, it isn’t associated with serious adverse effects (SOR: A, meta-analysis of RCTs).
Do prophylactic antipyretics reduce vaccination-associated symptoms in children?
EVIDENCE SUMMARY
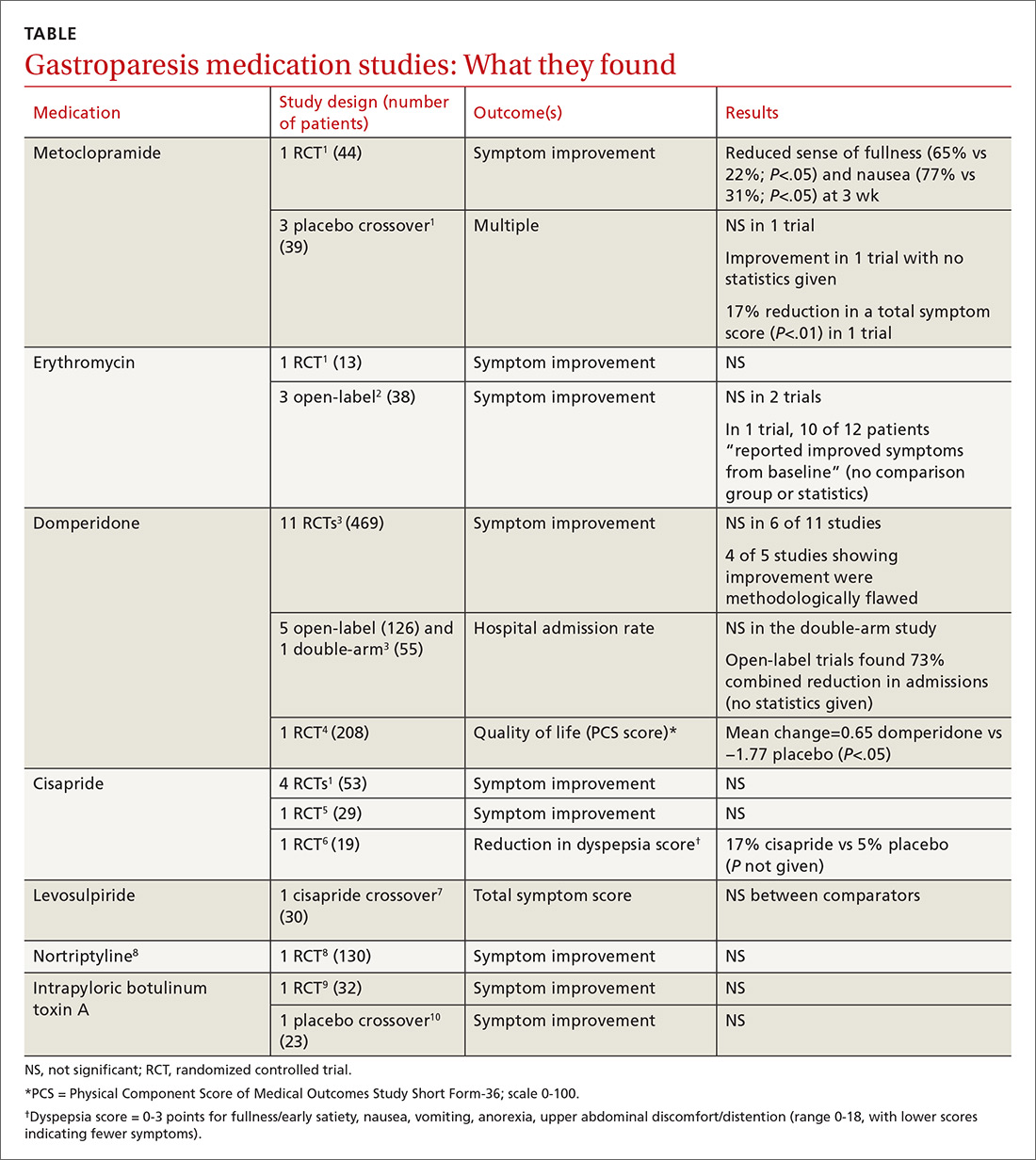
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
EVIDENCE SUMMARY
A systematic review of 13 RCTs (5077 patients) compared the effects of a prophylactic antipyretic (acetaminophen or ibuprofen, doses and schedules not described) with placebo in healthy children 6 years or younger undergoing routine childhood immunizations.1 Trials examined various schedules and combinations of vaccines. Researchers defined febrile reactions as a temperature of 38°C or higher and categorized pain as: none, mild (reaction to touch over vaccine site), moderate (protesting to limb movement), or severe (resisting limb movement).
Acetaminophen works better than ibuprofen for both fever and pain
Acetaminophen prophylaxis resulted in fewer febrile reactions in the first 24 to 48 hours after vaccine administration than placebo following both primary (odds ratio [OR] = 0.35; 95% confidence interval [CI], 0.26-0.48) and booster vaccinations (OR = 0.60; 95% CI, 0.39-0.93). Acetaminophen also reduced pain of all grades (primary vaccination: OR = 0.57; 95% CI, 0.47-0.7; booster vaccination: OR = 0.64; 95% CI, 0.48-0.84).
In contrast, ibuprofen prophylaxis had no effect on early febrile reactions for either primary or booster vaccinations. It reduced pain of all grades after primary vaccination (OR = 0.66; 95% CI, 0.49-0.88) but not after boosters (OR = 1.03; 95% CI, 0.59-1.81).
Reduced antibody response doesn’t affect seroprotective levels
Acetaminophen also generally reduced the antibody response compared with placebo (assessed using the geometric mean concentration [GMC], a statistical technique for comparing values that change logarithmically).1 GMC results are difficult to interpret clinically, however, and they differed by vaccine, antigen, and primary or booster vaccination status.
Nevertheless, patients mounted seroprotective antibody levels with or without acetaminophen prophylaxis, and the nasopharyngeal carriage rates of Streptococcus pneumoniae and Haemophilus influenzae didn’t change. Researchers didn’t publish the antibody responses to ibuprofen, nor did they track actual infection rates.
How do antipyretics work with newer combination vaccines?
A subsequent trial evaluated the immune response in 908 children receiving newer combination vaccines (DTaP/HBV/IPV/Hib and PCV13) who were randomized to 5 groups: acetaminophen 15 mg/kg at vaccination and 6 to 8 hours later; acetaminophen 15 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg/dose at vaccination with a second dose 6 to 8 hours later; ibuprofen 10 mg/kg starting 6 to 8 hours after vaccination with a second dose 6 to 8 hours later; and placebo.2
Patients received age-appropriate vaccination and their assigned antipyretic (or placebo) at 2, 3, 4 and 12 months of age. Researchers measured the immune response at 5 and 13 months of age.
Continue to: Overall, 5% to 10% of the prophylaxis group...
Overall, 5% to 10% of the prophylaxis group had fever on Day 1 or 2 after vaccination, compared with 10% to 20% of the placebo group (no P value given). Antipyretic use produced lower antibody GMC responses for antipertussis and antitetanus vaccines at 5 months but not at 13 months. Patients achieved the prespecified effective antibody levels at both 5 and 13 months, regardless of intervention.
Antipyretics don’t affect immune response with inactivated flu vaccine
A 2017 RCT investigated the effect of either prophylactic acetaminophen (15 mg/kg every 4 to 6 hours for 24 hours) or ibuprofen (10 mg/kg every 4 to 6 hours for 24 hours) on immune response in children receiving inactivated influenza vaccine.3 Researchers randomized 142 children into 3 treatment groups (acetaminophen, 59 children; ibuprofen, 24 children; placebo, 59 children). They defined seroconversion as a hemagglutinin inhibition assay titer of 1:40 postvaccination (if baseline titer was less than 1:10) or a 4-fold rise (if the baseline titer was ≥ 1:10).
All interventions resulted in similar seroconversion rates for all A or B influenza strains investigated. Vaccine protection-level responses ranged from 9% for B/Phuket to 100% for A/Switzerland. The trial didn’t report febrile reactions or infection rates.
RECOMMENDATIONS
In 2017, the Advisory Committee on Immunization Practices (ACIP) issued guidelines generally discouraging the use of antipyretics at the time of vaccination, but allowing their use later for local discomfort or fever that might arise after vaccination. The guidelines also noted that antipyretics at the time of vaccination didn’t reduce the risk of febrile seizures.4
Editor’s takeaway
Although ACIP doesn’t encourage giving antipyretics with vaccines, moderate-quality evidence suggests that prophylactic acetaminophen reduces fever and pain after immunizations by a reasonable amount without an apparent clinical downside.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
1. Das RR, Panigrahi I, Naik SS. The effect of prophylactic antipyretic administration on post-vaccination adverse reactions and antibody response in children: a systematic review. PLoS One. 2014;9:e106629.
2. Wysocki J, Center, KJ, Brzostek J, et al. A randomized study of fever prophylaxis and the immunogenicity of routine pediatric vaccinations. Vaccine. 2017;35:1926-1935.
3. Walter EB, Hornok CP, Grohskopf L, et al. The effect of antipyretics on immune response and fever following receipt of inactivated influenza vaccine in young children. Vaccine. 2017;35:6664–6671.
4. Kroger AT, Duchin J, Vázquez M. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2017.
EVIDENCE-BASED ANSWER:
Yes for acetaminophen, not so much for ibuprofen. Prophylactic acetaminophen reduces the odds of febrile reactions in the first 48 hours after vaccination by 40% to 65% and pain of all grades by 36% to 43%. In contrast, prophylactic ibuprofen reduces pain of all grades by 34% only after primary vaccination and doesn’t alter pain after boosters. Nor does it alter early febrile reactions (strength of recommendation [SOR]: B, meta-analysis of randomized clinical trials [RCTs] with moderate-to-high risk of bias).
Prophylactic administration of acetaminophen or ibuprofen is associated with a reduction in antibody response to the primary vaccine series and to influenza vaccine, but antibody responses still achieve seroprotective levels (SOR: C, bench research).
Does screening by primary care providers effectively detect melanoma and other skin cancers?
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
EVIDENCE SUMMARY
No trials have directly assessed skin cancer morbidity associated with physician visual skin screening. A 2018 ecologic cohort study found no difference in melanoma mortality in a population undergoing a national screening program, although screening was associated with 41% more diagnoses of skin cancer.1 A 2012 cohort study found a reduction in melanoma mortality over 7 years associated with a population-based visual skin cancer screening program compared with similar populations that didn’t undergo specific screening.2 At 12-year follow-up, however, there was no longer a difference in mortality.
Primary care visual screening doesn’t decrease melanoma mortality
German researchers trained 1673 non-dermatologists (64% of general practitioners, obstetrician-gynecologists, and urologists in that region of Germany) and 116 dermatologists (98% in the region) to recognize skin cancer through whole-body visual inspection.1 They recruited and screened 360,000 adults (19% of the population older than 20 years; 74% women) and followed age- and sex-adjusted melanoma mortality over the next 10 years. Non-dermatologists performed most screening exams (77%); 37% of screened positive patients were lost to follow-up.
Melanoma mortality ultimately didn’t change in the screened region, compared with populations in other European countries without national screening programs. Screening detected approximately half of melanoma cases (585/1169) in the region and was associated with 41% greater detection of skin cancers compared with other countries.
Researchers recorded age-adjusted increases in incidence per 100,000 of melanoma from 14.2 (95% confidence interval [CI], 13.3-15.1) to 18 (95% CI, 16.6-19.4), melanoma in situ from 5.8 (95% CI, 5.2-6.4) to 8.5 (95% CI, 7.5-9.5), squamous cell carcinoma from 11.2 (95% CI, 10.6-11.8) to 12.9 (95% CI, 12.0-13.8), and basal cell carcinoma from 60.5 (95% CI, 59.0-62.1) to 78.4 (95% CI, 75.9-80.8).
Visual screening by primary care providers vs screening by dermatologists
A cohort study of 16,383 Australian adults found that visual screening by primary care physicians detected melanoma over 3 years with a sensitivity of 40.2% (95% CIs not supplied) and specificity of 86.1% (95% CI, 85.6-86.6%; positive predictive value = 1.4%).3
A second cohort study, enrolling 7436 adults, that evaluated visual screening by dermatologists and plastic surgeons over 2 years found a sensitivity for melanoma of 49% (95% CI, 34.4-63.7%) and a specificity of 97.6% (95% CI, 97.2-97.9%) with a positive predictive value of 11.9% (95% CI, 7.8-17.2%).4
Visual screening more often detects thinner melanomas
A 3-year case-control study (3762 cases, 3824 controls) that examined the association between visual skin screening by a physician (type of physician not specified) and thickness of melanomas detected found that thin melanomas (≤ 0.75 mm) were more common among screened patients compared with unscreened patients (odds ratio [OR] = 1.38; 95% CI, 1.22-1.56) and thicker melanomas (≥ 0.75 mm) were less common (OR = 0.86; 95% CI, 0.75-0.98).5
Continue to: A systematic review...
A systematic review of 8 observational cohort studies with a total of 200,000 patients found a consistent linear increase in melanoma mortality with increasing tumor thickness.6 The largest study (68,495 patients), which compared melanoma mortality for thinner (< 1 mm) and thicker lesions, reported risk ratios of 2.89 for lesion thicknesses of 1.01 to 2 mm (95% CI, 2.62-3.18); 4.69 for thicknesses of 2.01 to 4 mm (95% CI, 4.24-5.02); and 5.71 for thicknesses > 4 mm (95% CI, 5.10-6.39).
The downside of visual screening: False-positives
The 2012 cohort study, which reported outcomes from 16,000 biopsies performed following visual screening exams, found that 28 biopsies were performed for each diagnosis of melanoma and 9 to 10 biopsies for each basal cell carcinoma.2 Diagnosis rates (number of skin biopsies performed for each case of cancer diagnosed) were equal in men and women for both types of cancer. However, researchers observed more biopsies for each diagnosis of squamous cell carcinoma in women than men (56 vs 28 biopsies per case).
Younger patients underwent more biopsies than older patients for each diagnosis of skin cancer. Women 20 to 34 years of age underwent more biopsies than women 65 years or older for each diagnosis of melanoma (19 additional excisions) and basal cell carcinoma (134 additional excisions). Women 35 to 49 years of age underwent 565 more biopsies for each diagnosis of squamous cell carcinoma than women 65 years or older. Similar patterns applied to men 20 to 34 years of age compared with men 65 years or older (24 additional biopsies per melanoma, 109 per basal cell carcinoma, and 898 per squamous cell carcinoma).
RECOMMENDATIONS
The US Preventive Services Task Force recommendations, based on a systematic review of mostly cohort studies, state that the current evidence is insufficient to assess the balance of benefits and harms of clinician visual skin cancer screening.7,8
The American Academy of Dermatology states that skin cancer screening can save lives and supports research on the benefits and harms of screening in the primary care setting.9
Continue to: Editor's Takeaway
Editor’s Takeaway
Skin cancer screening by primary care physicians is associated with increased detection of skin cancers, including melanomas—even though we have no confirmation that it changes melanoma mortality. It is unclear what the appropriate rate of false-positive screening tests should be, but wider adoption of noninvasive diagnostic techniques such as dermoscopy might reduce unwarranted biopsies.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
1. Kaiser M, Schiller J, Schreckenberger C. The effectiveness of a population-based skin cancer screening program: evidence from Germany. Eur J Health Econ. 2018:19:355-367.
2. Waldmann A, Nolte S, Weinstock MA, et al. Skin cancer screening participation and impact on melanoma incidence in Germany—an observational study on incidence trends in regions with and without population-based screening. Br J Cancer. 2012;106:970-974.
3. Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006:54:105-114.
4. Fritschi L, Dye SA, Katris P. Validity of melanoma diagnosis in a community-based screening program. Am J Epidemiol. 2006:164:385-390.
5. Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010:126:450-458.
6. Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for Skin Cancer in Adults: An Updated Systematic Evidence Review for the US Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality; 2016. Evidence Synthesis 137.
7. Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012:148:903-910.
8. US Preventive Services Task Force. Screening for Skin Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;316:429-435.
9. Torres A. AAD statement on USPSTF recommendation on skin cancer screening. July 2016. https://www.aad.org/media/news-releases/aad-statement-on-uspstf 26. Accessed May 2018.
EVIDENCE-BASED ANSWER:
Possibly. No trials have directly assessed detection of melanoma and other skin cancers by primary care providers.
Training a group comprised largely of primary care physicians to perform skin cancer screening was associated with a 41% increase in skin cancer diagnoses but no change in melanoma mortality.
Visual screening for melanoma by primary care physicians is 40% sensitive and 86% specific (compared with 49% and 98%, respectively, for dermatologists and plastic surgeons).
Melanomas found by visual screening are 38% more likely to be thin (≤ 0.75 mm) than melanomas discovered without screening, which correlates with improved outcomes.
Visual skin cancer screening overall is associated with false-positive rates as follows: 28 biopsies for each melanoma detected, 9 to 10 biopsies for each basal cell carcinoma, and 28 to 56 biopsies for squamous cell carcinoma. False-positive rates are higher for women—as much as double the rate for men—and younger patients—as much as 20-fold the rate for older patients (strength of recommendations for all foregoing statements: B, cohort studies).
Do group visits improve HbA1c more than individual visits in patients with T2DM?
EVIDENCE SUMMARY
A 2012 systematic review of 21 RCTs examined the effect of group-based diabetes education on HbA1c in 2833 adults with T2DM.1 Intervention groups participated in at least 1 group session lasting an hour led by a health professional or team (eg, physician, nurse, diabetes educator); controls received usual care. Most trials involved 6 to 20 hours of group-based education delivered over 1 to 10 months, although some trials continued the intervention for as long as 24 months. The mean HbA1c at baseline across all patients was 8.23%.
Professional-led group visitsimprove HbA1c
Group education resulted in a significant reduction in HbA1c compared with controls at 6 months (13 trials; 1883 patients; mean difference [MD]=−0.44%; 95% confidence interval [CI], −0.69 to −0.19), 12 months (11 studies; 1503 patients; MD=−0.46%; 95% CI, −0.74 to −0.18), and 24 months (3 studies; 397 patients; MD=−0.87%; 95% CI, −1.25 to −0.49). The trials had high heterogeneity, except for the 3 trials with a 24-month end-point (I2 = 0). Most studies had a moderate or high risk of bias.
A larger 2017 meta-analysis enrolling 8533 adults with T2DM came to similar conclusions, although it included a small number of nonrandomized trials (40 RCTs, 3 cluster RCTs, and 4 controlled clinical trials).2 Thirteen of the RCTs overlapped with the previously described systematic review.1 Interventions had to include at least 1 group session with 4 or more adult patients lasting at least 1 hour. In most studies, interventions continued between 4 and 12 months, although some ran 60 months. Controls received usual care. The mean HbA1c at baseline across all patients was 8.3%.
Group-based education compared with controls reduced HbA1c at 6 to 10 months (30 trials, N not given; MD=−0.3%; 95% CI, −0.48 to −0.15), 12 to 14 months (27 trials, N not given; MD=−0.3%; 95% CI, −0.49 to −0.17), and 36 to 48 months (5 trials, N not given; MD=−0.9%; 95% CI, −1.52 to −0.34). In a subgroup analysis, peer-led group visits had no effect (5 trials, 1066 patients; MD=−0.02%; 95% CI, −0.12 to 0.16).
Patients on oral agents alone showed a larger benefit than patients using insulin (38 trials, 5871 patients; −0.81 vs −0.19; P < .0001). Authors of the meta-analysis classified most studies as having a moderate to high risk of bias, with only 4 having low risk.
Duration of intervention: Longer is better for HbA1c values
Another systematic review analyzed 13 RCTs with 4652 patients 16 years and older with T2DM or type 1 diabetes to assess the effect of group visits on HbA1c.3 The review excluded studies that didn’t include a health care provider who could prescribe, diagnose, assess, and refer patients when appropriate.
Most interventions ran 3 to 12 months, although one lasted 36 months. (Two RCTs overlapped with the 2012 review, and 2 others with the 2017 review.) Group medical visits resulted in a significant decrease in HbA1c at the end of the intervention period (MD=−0.46%; 95% CI, −0.80 to −0.13) compared with controls. A meta-regression analysis suggested that ongoing treatment (for as long as 3 years) decreased HbA1c more than a shorter treatment duration (by 0.25% per year of treatment), whereas the frequency of treatments didn’t alter the effect. Overall, the trials were heterogenous and most had a high risk of bias.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2015 National Institute for Health and Care Excellence guideline for the management of T2DM in adults calls group education programs “the preferred option” for diabetes education, suggesting that clinicians reserve individual education for patients unable or unwilling to participate in group programs.4
The 2017 diabetes self-management education and support policy endorsed by the American Diabetes Association recommends using interprofessional teams and “creative solutions” to increase patient engagement and endorses group meetings as an effective option for patients who choose them.5
Editor’s takeaway
Moderate-quality evidence demonstrates that group visits can significantly reduce HbA1c levels. We should consider them for our patients with diabetes who are willing to attend group sessions.
1. Steinsbekk A, Rygg LO, Lisulo M, et al. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. a systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213.
2. Odgers-Jewell K, Ball LE, Kelly JT, et al. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med. 2017;34:1027-1039.
3. Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185:e635–e644.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline [NG 28]. December 2015. Updated May 2017. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#individualised-care. Accessed January 24, 2020.
5. Beck J, Greenwood DA, Blanton L. et al. 2017 National standards for diabetes self-management, education and support. Diabetes Care. 2017; 40:1409–1419.
EVIDENCE SUMMARY
A 2012 systematic review of 21 RCTs examined the effect of group-based diabetes education on HbA1c in 2833 adults with T2DM.1 Intervention groups participated in at least 1 group session lasting an hour led by a health professional or team (eg, physician, nurse, diabetes educator); controls received usual care. Most trials involved 6 to 20 hours of group-based education delivered over 1 to 10 months, although some trials continued the intervention for as long as 24 months. The mean HbA1c at baseline across all patients was 8.23%.
Professional-led group visitsimprove HbA1c
Group education resulted in a significant reduction in HbA1c compared with controls at 6 months (13 trials; 1883 patients; mean difference [MD]=−0.44%; 95% confidence interval [CI], −0.69 to −0.19), 12 months (11 studies; 1503 patients; MD=−0.46%; 95% CI, −0.74 to −0.18), and 24 months (3 studies; 397 patients; MD=−0.87%; 95% CI, −1.25 to −0.49). The trials had high heterogeneity, except for the 3 trials with a 24-month end-point (I2 = 0). Most studies had a moderate or high risk of bias.
A larger 2017 meta-analysis enrolling 8533 adults with T2DM came to similar conclusions, although it included a small number of nonrandomized trials (40 RCTs, 3 cluster RCTs, and 4 controlled clinical trials).2 Thirteen of the RCTs overlapped with the previously described systematic review.1 Interventions had to include at least 1 group session with 4 or more adult patients lasting at least 1 hour. In most studies, interventions continued between 4 and 12 months, although some ran 60 months. Controls received usual care. The mean HbA1c at baseline across all patients was 8.3%.
Group-based education compared with controls reduced HbA1c at 6 to 10 months (30 trials, N not given; MD=−0.3%; 95% CI, −0.48 to −0.15), 12 to 14 months (27 trials, N not given; MD=−0.3%; 95% CI, −0.49 to −0.17), and 36 to 48 months (5 trials, N not given; MD=−0.9%; 95% CI, −1.52 to −0.34). In a subgroup analysis, peer-led group visits had no effect (5 trials, 1066 patients; MD=−0.02%; 95% CI, −0.12 to 0.16).
Patients on oral agents alone showed a larger benefit than patients using insulin (38 trials, 5871 patients; −0.81 vs −0.19; P < .0001). Authors of the meta-analysis classified most studies as having a moderate to high risk of bias, with only 4 having low risk.
Duration of intervention: Longer is better for HbA1c values
Another systematic review analyzed 13 RCTs with 4652 patients 16 years and older with T2DM or type 1 diabetes to assess the effect of group visits on HbA1c.3 The review excluded studies that didn’t include a health care provider who could prescribe, diagnose, assess, and refer patients when appropriate.
Most interventions ran 3 to 12 months, although one lasted 36 months. (Two RCTs overlapped with the 2012 review, and 2 others with the 2017 review.) Group medical visits resulted in a significant decrease in HbA1c at the end of the intervention period (MD=−0.46%; 95% CI, −0.80 to −0.13) compared with controls. A meta-regression analysis suggested that ongoing treatment (for as long as 3 years) decreased HbA1c more than a shorter treatment duration (by 0.25% per year of treatment), whereas the frequency of treatments didn’t alter the effect. Overall, the trials were heterogenous and most had a high risk of bias.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2015 National Institute for Health and Care Excellence guideline for the management of T2DM in adults calls group education programs “the preferred option” for diabetes education, suggesting that clinicians reserve individual education for patients unable or unwilling to participate in group programs.4
The 2017 diabetes self-management education and support policy endorsed by the American Diabetes Association recommends using interprofessional teams and “creative solutions” to increase patient engagement and endorses group meetings as an effective option for patients who choose them.5
Editor’s takeaway
Moderate-quality evidence demonstrates that group visits can significantly reduce HbA1c levels. We should consider them for our patients with diabetes who are willing to attend group sessions.
EVIDENCE SUMMARY
A 2012 systematic review of 21 RCTs examined the effect of group-based diabetes education on HbA1c in 2833 adults with T2DM.1 Intervention groups participated in at least 1 group session lasting an hour led by a health professional or team (eg, physician, nurse, diabetes educator); controls received usual care. Most trials involved 6 to 20 hours of group-based education delivered over 1 to 10 months, although some trials continued the intervention for as long as 24 months. The mean HbA1c at baseline across all patients was 8.23%.
Professional-led group visitsimprove HbA1c
Group education resulted in a significant reduction in HbA1c compared with controls at 6 months (13 trials; 1883 patients; mean difference [MD]=−0.44%; 95% confidence interval [CI], −0.69 to −0.19), 12 months (11 studies; 1503 patients; MD=−0.46%; 95% CI, −0.74 to −0.18), and 24 months (3 studies; 397 patients; MD=−0.87%; 95% CI, −1.25 to −0.49). The trials had high heterogeneity, except for the 3 trials with a 24-month end-point (I2 = 0). Most studies had a moderate or high risk of bias.
A larger 2017 meta-analysis enrolling 8533 adults with T2DM came to similar conclusions, although it included a small number of nonrandomized trials (40 RCTs, 3 cluster RCTs, and 4 controlled clinical trials).2 Thirteen of the RCTs overlapped with the previously described systematic review.1 Interventions had to include at least 1 group session with 4 or more adult patients lasting at least 1 hour. In most studies, interventions continued between 4 and 12 months, although some ran 60 months. Controls received usual care. The mean HbA1c at baseline across all patients was 8.3%.
Group-based education compared with controls reduced HbA1c at 6 to 10 months (30 trials, N not given; MD=−0.3%; 95% CI, −0.48 to −0.15), 12 to 14 months (27 trials, N not given; MD=−0.3%; 95% CI, −0.49 to −0.17), and 36 to 48 months (5 trials, N not given; MD=−0.9%; 95% CI, −1.52 to −0.34). In a subgroup analysis, peer-led group visits had no effect (5 trials, 1066 patients; MD=−0.02%; 95% CI, −0.12 to 0.16).
Patients on oral agents alone showed a larger benefit than patients using insulin (38 trials, 5871 patients; −0.81 vs −0.19; P < .0001). Authors of the meta-analysis classified most studies as having a moderate to high risk of bias, with only 4 having low risk.
Duration of intervention: Longer is better for HbA1c values
Another systematic review analyzed 13 RCTs with 4652 patients 16 years and older with T2DM or type 1 diabetes to assess the effect of group visits on HbA1c.3 The review excluded studies that didn’t include a health care provider who could prescribe, diagnose, assess, and refer patients when appropriate.
Most interventions ran 3 to 12 months, although one lasted 36 months. (Two RCTs overlapped with the 2012 review, and 2 others with the 2017 review.) Group medical visits resulted in a significant decrease in HbA1c at the end of the intervention period (MD=−0.46%; 95% CI, −0.80 to −0.13) compared with controls. A meta-regression analysis suggested that ongoing treatment (for as long as 3 years) decreased HbA1c more than a shorter treatment duration (by 0.25% per year of treatment), whereas the frequency of treatments didn’t alter the effect. Overall, the trials were heterogenous and most had a high risk of bias.
Continue to: RECOMMENDATIONS
RECOMMENDATIONS
The 2015 National Institute for Health and Care Excellence guideline for the management of T2DM in adults calls group education programs “the preferred option” for diabetes education, suggesting that clinicians reserve individual education for patients unable or unwilling to participate in group programs.4
The 2017 diabetes self-management education and support policy endorsed by the American Diabetes Association recommends using interprofessional teams and “creative solutions” to increase patient engagement and endorses group meetings as an effective option for patients who choose them.5
Editor’s takeaway
Moderate-quality evidence demonstrates that group visits can significantly reduce HbA1c levels. We should consider them for our patients with diabetes who are willing to attend group sessions.
1. Steinsbekk A, Rygg LO, Lisulo M, et al. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. a systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213.
2. Odgers-Jewell K, Ball LE, Kelly JT, et al. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med. 2017;34:1027-1039.
3. Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185:e635–e644.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline [NG 28]. December 2015. Updated May 2017. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#individualised-care. Accessed January 24, 2020.
5. Beck J, Greenwood DA, Blanton L. et al. 2017 National standards for diabetes self-management, education and support. Diabetes Care. 2017; 40:1409–1419.
1. Steinsbekk A, Rygg LO, Lisulo M, et al. Group based diabetes self-management education compared to routine treatment for people with type 2 diabetes mellitus. a systematic review with meta-analysis. BMC Health Serv Res. 2012;12:213.
2. Odgers-Jewell K, Ball LE, Kelly JT, et al. Effectiveness of group-based self-management education for individuals with Type 2 diabetes: a systematic review with meta-analyses and meta-regression. Diabet Med. 2017;34:1027-1039.
3. Housden L, Wong ST, Dawes M. Effectiveness of group medical visits for improving diabetes care: a systematic review and meta-analysis. CMAJ. 2013;185:e635–e644.
4. National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline [NG 28]. December 2015. Updated May 2017. https://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#individualised-care. Accessed January 24, 2020.
5. Beck J, Greenwood DA, Blanton L. et al. 2017 National standards for diabetes self-management, education and support. Diabetes Care. 2017; 40:1409–1419.
EVIDENCE-BASED ANSWER:
Yes. In patients with type 2 diabetes mellitus (T2DM), group visits led by health professionals or teams improved glycosylated hemoglobin (HbA1c) by 0.3% to 0.9% over usual care (strength of recommendation [SOR]: B, meta-analyses of randomized clinical trials [RCTs] with moderate to high risk of bias).
Patients taking oral antidiabetic agents alone appear to benefit more than patients on insulin. Peer-led group visits likely have no effect (SOR: B, subgroup analysis within a meta-analysis).
Treatment durations as long as 3 years are associated with larger decreases in HbA1c (by 0.25% per year) than treatment lasting less than a year (SOR: B, meta-analysis of RCTs involving patents with type 1 diabetes and T2DM).
Patients with T2DM should be offered group visits for diabetes education when available (SOR: C, expert opinion).
Does using e-cigarettes increase cigarette smoking in adolescents?
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
EVIDENCE SUMMARY
A meta-analysis of 9 prospective cohort studies (total 17,389 patients) at least 6 months in duration evaluated the association between e-cigarette exposure and subsequent cigarette smoking in adolescents and young adults.1 It found that smoking was more prevalent in ever-users of e-cigarettes than nonusers at 1 year (23.3% vs 7.2%; odds ratio [OR] = 3.5; 95% confidence interval [CI], 2.38-5.16). The association was even stronger among recent users (within 30 days) of e-cigarettes compared with nonusers (21.5% vs 4.6%; OR = 4.28; 95% CI, 2.52-7.27). The mean age of approximately 80% of participants was 20 years or younger.
Further studies also support a link between e-cigarette and cigarette use
Four subsequent cohort studies also found links between e-cigarette exposure and any level of cigarette smoking (TABLE).2-5 A Canadian study of high school students reported a positive association between recent e-cigarette use (within the previous 30 days) and subsequent daily cigarette usage (OR = 1.79; 95% CI, 1.41-2.28).2 A British study that documented the largest association uniquely validated smoking status with carbon monoxide testing.3 A study of Mexican adolescents found that adolescents who tried e-cigarettes were more likely to smoke cigarettes and also reported an association between e-cigarette use and marijuana use (relative risk [RR] = 1.93; 95% CI, 1.14–3.28).4 A California study that evaluated e-cigarette nicotine level and subsequent cigarette smoking found a dose-dependent response, suggesting an association between nicotine concentration and subsequent uptake of cigarettes.5
RECOMMENDATIONS
A policy statement from The American Academy of Pediatrics Section on Tobacco Control states that youth who use e-cigarettes are more likely to use cigarettes and other tobacco products.6 It recommends that physicians screen patients for use of electronic nicotine delivery systems (ENDS), counsel about immediate and long-term harms and the importance of not using ENDS, and offer current users tobacco cessation counseling (with Food and Drug Administration-approved tobacco dependence treatment).
Editor’s takeaway
While these cohort studies don’t definitively prove causation, they provide the best quality evidence that we are likely to see in support of counseling adolescents against using e-cigarettes, educating them about harms, and offering tobacco cessation measures when appropriate.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
1. Soneji S, Barrington-Trimis JL, Willis TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults, a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797.
2. Hammond D, Reid JL, Cole AG, et al. Electronic cigarette use and smoking initiation among youth: a longitudinal cohort study. CMAJ. 2017;189:E1328-E1336.
3. Conner M, Grogan S, Simms-Ellis R, et al. Do electronic cigarettes increase cigarette smoking in UK adolescents? Evidence from a 12-month prospective study. Tob Control. 2018;27:365-372.
4. Lozano P, Barrientos-Gutierrez I, Arillo-Santillan E, et al. A longitudinal study of electronic cigarette use and onset of conventional cigarette smoking and marijuana use among Mexican adolescents. Drug Alcohol Depend. 2017;180:427-430.
5. Goldenson NI, Leventhal AM, Stone MD, et al. Associations of electronic cigarette nicotine concentration with subsequent cigarette smoking and vaping levels in adolescents. JAMA Pediatr. 2017;171:1192-1199.
6. Walley SC, Jenssen BP; Section on Tobacco Control. Electronic nicotine delivery systems. Pediatrics. 2015;136:1018-1026.
EVIDENCE-BASED ANSWER:
Probably. Electronic cigarette (e-cigarette) use by adolescents is associated with a 2- to 4-fold increase in cigarette smoking over the next year (strength of recommendation: A, meta-analysis and subsequent prospective cohort studies).
How safe and effective is ondansetron for nausea and vomiting in pregnancy?
EVIDENCE SUMMARY
Efficacy. A 2014 double-blind RCT compared ondansetron with pyridoxine plus doxylamine (standard care) for outpatient treatment of nausea and vomiting in pregnancy.1 The 36 patients had an average gestational age of 8 weeks and received either 4 mg oral ondansetron plus placebo or 25 mg pyridoxine plus 12.5 mg doxylamine 3 times daily for 5 days. Nausea and vomiting severity was measured using 2 separate 10-cm visual analog scales (VAS) with scores ranging from 0 to 10 (worst nausea or vomiting imaginable). Researchers determined that a VAS score reduction of 2.5 cm was clinically significant.
Patients treated with ondansetron described greater improvements in nausea (mean VAS change −5.1 cm vs −2 cm; P = .019) and vomiting (mean VAS change −4.1 cm vs −1.7 cm; P = .049). No patient required hospitalization. The researchers didn’t report on adverse effects or birth outcomes. The study was limited by the small sample size and a high rate (17%) of patients with missing data or who were lost to follow-up.
IV ondansetron vs metoclopramide: Similar efficacy, fewer adverse effects
A 2014 double-blind RCT compared IV ondansetron with IV metoclopramide (standard care) for treating hyperemesis gravidarum.2 The 160 patients had an average gestational age of 9.5 weeks and intractable nausea and vomiting severe enough to cause dehydration, metabolic disturbance, and hospitalization. Patients received either 4 mg ondansetron or 10 mg metoclopramide IV every 8 hours for 24 hours. The primary outcomes were number of episodes of vomiting over 24 hours and self-reported sense of well-being rated on a 10-point scale.
No differences were found between the ondansetron- and metoclopramide-treated groups in terms of vomiting over 24 hours (median episodes 1 and 1; P = .38) or sense of well-being (mean scores 8.7 vs 8.3; P = .13). Patients treated with ondansetron were less likely to have persistent ketonuria at 24 hours (relative risk [RR] = 0.3; 95% confidence interval [CI], 0.1-0.8; number needed to treat [NNT] = 6). They also were less likely to feel drowsy (RR = 0.3; 95% CI, 0.1–0.8; NNT = 6) or complain of dry mouth (RR = 0.4; 95% CI, 0.1-0.9; NNT = 8). The study didn’t report birth outcomes or adverse fetal effects.
Oral ondansetron outperforms oral metoclopramide in small study
A 2013 double-blind RCT compared ondansetron with metoclopramide (standard care) for controlling severe nausea and vomiting.3 The 83 patients, with an average gestational age of 8.7 weeks, had more than 3 vomiting episodes daily, weight loss, and ketonuria. They received either 4 mg oral ondansetron or 10 mg oral metoclopramide for 2 weeks as follows: 3 times daily for 1 week, then twice daily for 3 days, then once daily for 4 days. Patients rated nausea severity using a 10-cm VAS from 0 to 10 (severe nausea) and recorded the number of vomiting episodes.
Women treated with ondansetron had significantly lower VAS scores on Days 3 and 4 of treatment (5.4 vs 6, P = .024 on Day 3; 4.1 vs 5.7, P = .023 on Day 4). They also had fewer episodes of vomiting on Days 2, 3, and 4 (3.7 vs 6, P = .006 on Day 2; 3.2 vs 5.3, P = .006 on Day 3; and 3.3 vs 5, P = .013 on Day 4). The study was limited by the small sample size.
Safety. A 2016 systematic review examining the risk of birth defects associated with ondansetron exposure in pregnancy found 8 reports: 5 birth registries, 2 case-control studies, and 1 prospective cohort study.4 Investigators compared rates of major malformations—cleft lips, cleft palates, neural tube defects, cardiac defects, and hypospadias—in 5101 women exposed to ondansetron in the first trimester with birth defect rates in more than 3.1 million nonexposed women.
Continue to: No study demonstrated...
No study demonstrated an increased rate of major malformations associated with ondansetron exposure except for 2 disease registry studies with nearly 2.4 million patients that reported a slight increase in the risk of cardiac defects (odds ratio [OR] = 2; 95% CI, 1.3-3.1; OR = 1.6, 95% CI, 1-2.1). Comparisons of other birth defect rates associated with ondansetron exposure were inconsistent, with studies showing small increases, decreases, or no difference in rates between exposed and nonexposed women.
Exposure vs nonexposure: No difference in adverse outcomes
A 2013 retrospective cohort study looked at 608,385 pregnancies among women in Denmark, of whom 1970 (0.3%) had been exposed to ondansetron.5 The study found that exposure to ondansetron compared with nonexposure was associated with a lower risk for spontaneous abortion between 7 and 12 weeks’ gestation (1.1% vs 3.7%; hazard ratio [HR] = 0.5; 95% CI, 0.3-0.9).
No significant differences between ondansetron exposure and nonexposure were found for the following adverse outcomes: spontaneous abortion between 13 and 22 weeks’ gestation (1% vs 2.1%; HR = 0.6; 95% CI, 0.3-1.2); stillbirth (0.3% vs 0.4%; HR = 0.4; 95% CI, 0.1-1.7); any major birth defect (2.9% in both exposed and nonexposed women; OR = 1.12; 95% CI, 0.69-1.82); preterm delivery (6.2% vs 5.2%; OR = 0.9; 95% CI, 0.7-1.3), low birth weight infant (4.1% vs 3.7%; OR = 0.8; 95% CI, 0.5-1.1); and small-for-gestational-age infant (10.4% vs 9.2%; OR = 1.1; 95% CI, 0.9-1.4).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) states that insufficient data exist regarding the safety of ondansetron for the fetus.6 ACOG recommends individualizing the use of ondansetron before 10 weeks of pregnancy after weighing the risks and benefits. ACOG also recommends adding ondansetron as third-line treatment for nausea and vomiting unresponsive to first- and second-line treatments.
EDITOR'S TAKEAWAY
Higher-quality studies showed ondansetron to be an effective treatment for hyperemesis gravidarum. Lower-quality studies raised some concerns about adverse fetal effects. Although the adverse effects were rare and the quality of the evidence was lower, the cautionary principle suggests that ondansetron should be a second-line option.
1. Oliveira LG, Capp SM, You WB, et al. Ondansetron compared with doxylamine and pyridoxine for treatment of nausea in pregnancy: a randomized controlled trial. Obstet Gynecol. 2014;124:735-742.
2. Abas MN, Tan PC, Azmi N, et al. Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2014;123:1272-1279.
3. Kashifard M, Basirat Z, Kashifard M, et al. Ondansetrone or metoclopromide? Which is more effective in severe nausea and vomiting of pregnancy? A randomized trial double-blind study. Clin Exp Obstet Gynecol. 2013;40:127-130.
4. Carstairs SD. Ondansetron use in pregnancy and birth defects: a systematic review. Obstet Gynecol. 2016;127:878-883.
5. Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013;368:814-823.
6. American College of Obstetricians and Gynecologists, Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131:e15-e30.
EVIDENCE SUMMARY
Efficacy. A 2014 double-blind RCT compared ondansetron with pyridoxine plus doxylamine (standard care) for outpatient treatment of nausea and vomiting in pregnancy.1 The 36 patients had an average gestational age of 8 weeks and received either 4 mg oral ondansetron plus placebo or 25 mg pyridoxine plus 12.5 mg doxylamine 3 times daily for 5 days. Nausea and vomiting severity was measured using 2 separate 10-cm visual analog scales (VAS) with scores ranging from 0 to 10 (worst nausea or vomiting imaginable). Researchers determined that a VAS score reduction of 2.5 cm was clinically significant.
Patients treated with ondansetron described greater improvements in nausea (mean VAS change −5.1 cm vs −2 cm; P = .019) and vomiting (mean VAS change −4.1 cm vs −1.7 cm; P = .049). No patient required hospitalization. The researchers didn’t report on adverse effects or birth outcomes. The study was limited by the small sample size and a high rate (17%) of patients with missing data or who were lost to follow-up.
IV ondansetron vs metoclopramide: Similar efficacy, fewer adverse effects
A 2014 double-blind RCT compared IV ondansetron with IV metoclopramide (standard care) for treating hyperemesis gravidarum.2 The 160 patients had an average gestational age of 9.5 weeks and intractable nausea and vomiting severe enough to cause dehydration, metabolic disturbance, and hospitalization. Patients received either 4 mg ondansetron or 10 mg metoclopramide IV every 8 hours for 24 hours. The primary outcomes were number of episodes of vomiting over 24 hours and self-reported sense of well-being rated on a 10-point scale.
No differences were found between the ondansetron- and metoclopramide-treated groups in terms of vomiting over 24 hours (median episodes 1 and 1; P = .38) or sense of well-being (mean scores 8.7 vs 8.3; P = .13). Patients treated with ondansetron were less likely to have persistent ketonuria at 24 hours (relative risk [RR] = 0.3; 95% confidence interval [CI], 0.1-0.8; number needed to treat [NNT] = 6). They also were less likely to feel drowsy (RR = 0.3; 95% CI, 0.1–0.8; NNT = 6) or complain of dry mouth (RR = 0.4; 95% CI, 0.1-0.9; NNT = 8). The study didn’t report birth outcomes or adverse fetal effects.
Oral ondansetron outperforms oral metoclopramide in small study
A 2013 double-blind RCT compared ondansetron with metoclopramide (standard care) for controlling severe nausea and vomiting.3 The 83 patients, with an average gestational age of 8.7 weeks, had more than 3 vomiting episodes daily, weight loss, and ketonuria. They received either 4 mg oral ondansetron or 10 mg oral metoclopramide for 2 weeks as follows: 3 times daily for 1 week, then twice daily for 3 days, then once daily for 4 days. Patients rated nausea severity using a 10-cm VAS from 0 to 10 (severe nausea) and recorded the number of vomiting episodes.
Women treated with ondansetron had significantly lower VAS scores on Days 3 and 4 of treatment (5.4 vs 6, P = .024 on Day 3; 4.1 vs 5.7, P = .023 on Day 4). They also had fewer episodes of vomiting on Days 2, 3, and 4 (3.7 vs 6, P = .006 on Day 2; 3.2 vs 5.3, P = .006 on Day 3; and 3.3 vs 5, P = .013 on Day 4). The study was limited by the small sample size.
Safety. A 2016 systematic review examining the risk of birth defects associated with ondansetron exposure in pregnancy found 8 reports: 5 birth registries, 2 case-control studies, and 1 prospective cohort study.4 Investigators compared rates of major malformations—cleft lips, cleft palates, neural tube defects, cardiac defects, and hypospadias—in 5101 women exposed to ondansetron in the first trimester with birth defect rates in more than 3.1 million nonexposed women.
Continue to: No study demonstrated...
No study demonstrated an increased rate of major malformations associated with ondansetron exposure except for 2 disease registry studies with nearly 2.4 million patients that reported a slight increase in the risk of cardiac defects (odds ratio [OR] = 2; 95% CI, 1.3-3.1; OR = 1.6, 95% CI, 1-2.1). Comparisons of other birth defect rates associated with ondansetron exposure were inconsistent, with studies showing small increases, decreases, or no difference in rates between exposed and nonexposed women.
Exposure vs nonexposure: No difference in adverse outcomes
A 2013 retrospective cohort study looked at 608,385 pregnancies among women in Denmark, of whom 1970 (0.3%) had been exposed to ondansetron.5 The study found that exposure to ondansetron compared with nonexposure was associated with a lower risk for spontaneous abortion between 7 and 12 weeks’ gestation (1.1% vs 3.7%; hazard ratio [HR] = 0.5; 95% CI, 0.3-0.9).
No significant differences between ondansetron exposure and nonexposure were found for the following adverse outcomes: spontaneous abortion between 13 and 22 weeks’ gestation (1% vs 2.1%; HR = 0.6; 95% CI, 0.3-1.2); stillbirth (0.3% vs 0.4%; HR = 0.4; 95% CI, 0.1-1.7); any major birth defect (2.9% in both exposed and nonexposed women; OR = 1.12; 95% CI, 0.69-1.82); preterm delivery (6.2% vs 5.2%; OR = 0.9; 95% CI, 0.7-1.3), low birth weight infant (4.1% vs 3.7%; OR = 0.8; 95% CI, 0.5-1.1); and small-for-gestational-age infant (10.4% vs 9.2%; OR = 1.1; 95% CI, 0.9-1.4).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) states that insufficient data exist regarding the safety of ondansetron for the fetus.6 ACOG recommends individualizing the use of ondansetron before 10 weeks of pregnancy after weighing the risks and benefits. ACOG also recommends adding ondansetron as third-line treatment for nausea and vomiting unresponsive to first- and second-line treatments.
EDITOR'S TAKEAWAY
Higher-quality studies showed ondansetron to be an effective treatment for hyperemesis gravidarum. Lower-quality studies raised some concerns about adverse fetal effects. Although the adverse effects were rare and the quality of the evidence was lower, the cautionary principle suggests that ondansetron should be a second-line option.
EVIDENCE SUMMARY
Efficacy. A 2014 double-blind RCT compared ondansetron with pyridoxine plus doxylamine (standard care) for outpatient treatment of nausea and vomiting in pregnancy.1 The 36 patients had an average gestational age of 8 weeks and received either 4 mg oral ondansetron plus placebo or 25 mg pyridoxine plus 12.5 mg doxylamine 3 times daily for 5 days. Nausea and vomiting severity was measured using 2 separate 10-cm visual analog scales (VAS) with scores ranging from 0 to 10 (worst nausea or vomiting imaginable). Researchers determined that a VAS score reduction of 2.5 cm was clinically significant.
Patients treated with ondansetron described greater improvements in nausea (mean VAS change −5.1 cm vs −2 cm; P = .019) and vomiting (mean VAS change −4.1 cm vs −1.7 cm; P = .049). No patient required hospitalization. The researchers didn’t report on adverse effects or birth outcomes. The study was limited by the small sample size and a high rate (17%) of patients with missing data or who were lost to follow-up.
IV ondansetron vs metoclopramide: Similar efficacy, fewer adverse effects
A 2014 double-blind RCT compared IV ondansetron with IV metoclopramide (standard care) for treating hyperemesis gravidarum.2 The 160 patients had an average gestational age of 9.5 weeks and intractable nausea and vomiting severe enough to cause dehydration, metabolic disturbance, and hospitalization. Patients received either 4 mg ondansetron or 10 mg metoclopramide IV every 8 hours for 24 hours. The primary outcomes were number of episodes of vomiting over 24 hours and self-reported sense of well-being rated on a 10-point scale.
No differences were found between the ondansetron- and metoclopramide-treated groups in terms of vomiting over 24 hours (median episodes 1 and 1; P = .38) or sense of well-being (mean scores 8.7 vs 8.3; P = .13). Patients treated with ondansetron were less likely to have persistent ketonuria at 24 hours (relative risk [RR] = 0.3; 95% confidence interval [CI], 0.1-0.8; number needed to treat [NNT] = 6). They also were less likely to feel drowsy (RR = 0.3; 95% CI, 0.1–0.8; NNT = 6) or complain of dry mouth (RR = 0.4; 95% CI, 0.1-0.9; NNT = 8). The study didn’t report birth outcomes or adverse fetal effects.
Oral ondansetron outperforms oral metoclopramide in small study
A 2013 double-blind RCT compared ondansetron with metoclopramide (standard care) for controlling severe nausea and vomiting.3 The 83 patients, with an average gestational age of 8.7 weeks, had more than 3 vomiting episodes daily, weight loss, and ketonuria. They received either 4 mg oral ondansetron or 10 mg oral metoclopramide for 2 weeks as follows: 3 times daily for 1 week, then twice daily for 3 days, then once daily for 4 days. Patients rated nausea severity using a 10-cm VAS from 0 to 10 (severe nausea) and recorded the number of vomiting episodes.
Women treated with ondansetron had significantly lower VAS scores on Days 3 and 4 of treatment (5.4 vs 6, P = .024 on Day 3; 4.1 vs 5.7, P = .023 on Day 4). They also had fewer episodes of vomiting on Days 2, 3, and 4 (3.7 vs 6, P = .006 on Day 2; 3.2 vs 5.3, P = .006 on Day 3; and 3.3 vs 5, P = .013 on Day 4). The study was limited by the small sample size.
Safety. A 2016 systematic review examining the risk of birth defects associated with ondansetron exposure in pregnancy found 8 reports: 5 birth registries, 2 case-control studies, and 1 prospective cohort study.4 Investigators compared rates of major malformations—cleft lips, cleft palates, neural tube defects, cardiac defects, and hypospadias—in 5101 women exposed to ondansetron in the first trimester with birth defect rates in more than 3.1 million nonexposed women.
Continue to: No study demonstrated...
No study demonstrated an increased rate of major malformations associated with ondansetron exposure except for 2 disease registry studies with nearly 2.4 million patients that reported a slight increase in the risk of cardiac defects (odds ratio [OR] = 2; 95% CI, 1.3-3.1; OR = 1.6, 95% CI, 1-2.1). Comparisons of other birth defect rates associated with ondansetron exposure were inconsistent, with studies showing small increases, decreases, or no difference in rates between exposed and nonexposed women.
Exposure vs nonexposure: No difference in adverse outcomes
A 2013 retrospective cohort study looked at 608,385 pregnancies among women in Denmark, of whom 1970 (0.3%) had been exposed to ondansetron.5 The study found that exposure to ondansetron compared with nonexposure was associated with a lower risk for spontaneous abortion between 7 and 12 weeks’ gestation (1.1% vs 3.7%; hazard ratio [HR] = 0.5; 95% CI, 0.3-0.9).
No significant differences between ondansetron exposure and nonexposure were found for the following adverse outcomes: spontaneous abortion between 13 and 22 weeks’ gestation (1% vs 2.1%; HR = 0.6; 95% CI, 0.3-1.2); stillbirth (0.3% vs 0.4%; HR = 0.4; 95% CI, 0.1-1.7); any major birth defect (2.9% in both exposed and nonexposed women; OR = 1.12; 95% CI, 0.69-1.82); preterm delivery (6.2% vs 5.2%; OR = 0.9; 95% CI, 0.7-1.3), low birth weight infant (4.1% vs 3.7%; OR = 0.8; 95% CI, 0.5-1.1); and small-for-gestational-age infant (10.4% vs 9.2%; OR = 1.1; 95% CI, 0.9-1.4).
RECOMMENDATIONS
The American College of Obstetricians and Gynecologists (ACOG) states that insufficient data exist regarding the safety of ondansetron for the fetus.6 ACOG recommends individualizing the use of ondansetron before 10 weeks of pregnancy after weighing the risks and benefits. ACOG also recommends adding ondansetron as third-line treatment for nausea and vomiting unresponsive to first- and second-line treatments.
EDITOR'S TAKEAWAY
Higher-quality studies showed ondansetron to be an effective treatment for hyperemesis gravidarum. Lower-quality studies raised some concerns about adverse fetal effects. Although the adverse effects were rare and the quality of the evidence was lower, the cautionary principle suggests that ondansetron should be a second-line option.
1. Oliveira LG, Capp SM, You WB, et al. Ondansetron compared with doxylamine and pyridoxine for treatment of nausea in pregnancy: a randomized controlled trial. Obstet Gynecol. 2014;124:735-742.
2. Abas MN, Tan PC, Azmi N, et al. Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2014;123:1272-1279.
3. Kashifard M, Basirat Z, Kashifard M, et al. Ondansetrone or metoclopromide? Which is more effective in severe nausea and vomiting of pregnancy? A randomized trial double-blind study. Clin Exp Obstet Gynecol. 2013;40:127-130.
4. Carstairs SD. Ondansetron use in pregnancy and birth defects: a systematic review. Obstet Gynecol. 2016;127:878-883.
5. Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013;368:814-823.
6. American College of Obstetricians and Gynecologists, Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131:e15-e30.
1. Oliveira LG, Capp SM, You WB, et al. Ondansetron compared with doxylamine and pyridoxine for treatment of nausea in pregnancy: a randomized controlled trial. Obstet Gynecol. 2014;124:735-742.
2. Abas MN, Tan PC, Azmi N, et al. Ondansetron compared with metoclopramide for hyperemesis gravidarum: a randomized controlled trial. Obstet Gynecol. 2014;123:1272-1279.
3. Kashifard M, Basirat Z, Kashifard M, et al. Ondansetrone or metoclopromide? Which is more effective in severe nausea and vomiting of pregnancy? A randomized trial double-blind study. Clin Exp Obstet Gynecol. 2013;40:127-130.
4. Carstairs SD. Ondansetron use in pregnancy and birth defects: a systematic review. Obstet Gynecol. 2016;127:878-883.
5. Pasternak B, Svanström H, Hviid A. Ondansetron in pregnancy and risk of adverse fetal outcomes. N Engl J Med. 2013;368:814-823.
6. American College of Obstetricians and Gynecologists, Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea and vomiting of pregnancy. Obstet Gynecol. 2018;131:e15-e30.
EVIDENCE-BASED ANSWER:
Oral ondansetron is more effective than a combination of pyridoxine and doxylamine for outpatient treatment of nausea and vomiting in pregnancy (strength of recommendation [SOR]: B, randomized controlled trial [RCT]).
For moderate to severe nausea and vomiting, intravenous (IV) ondansetron is at least as effective as IV metoclopramide and may cause fewer adverse reactions (SOR: B, RCTs).
Disease registry, case-control, and cohort studies report a slight increase in the risk of cardiac defects with ondansetron use in first-trimester pregnancies, but no major or other birth defects are associated with ondansetron exposure (SOR: B, a systematic review of observational trials and a single retrospective cohort study).
A specialty society guideline recommends weighing the risks and benefits of ondansetron use before 10 weeks’ gestational age and suggests reserving ondansetron for patients who have persistent nausea and vomiting unresponsive to first- and second-line treatments (SOR: C, expert opinion).
Is intra-articular platelet-rich plasma injection an effective treatment for knee OA?
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).
The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).
The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
EVIDENCE SUMMARY
PRP vs placebo. Three RCTs compared PRP with saline placebo injections and 2 found that PRP improved the Western Ontario and McMaster Universities Arthritis Index (WOMAC, a standardized scale assessing knee pain, function, and stiffness) by 40% to 70%; the third found 24% to 32% improvements in the EuroQol visual analog scale (EQ-VAS) scores at 6 months1-3 (TABLE1-12).
The first 2 studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity (baseline WOMAC scores about 50).1,2 Investigators in the first RCT injected PRP once in one subgroup and twice in another subgroup, compared with a single injection of saline in a third subgroup.1 They gave 3 weekly injections of PRP or saline in the second RCT.2
The third study enrolled mainly patients with early osteoarthritis (mean age early 50s, slightly more women). Investigators injected PRP 3 times in one subgroup and once (plus 2 saline injections) in another, compared with 3 saline injections, and evaluated patients at baseline and 6 months.3
PRP vs HA. Nine RCTs compared PRP with HA injections. Six studies (673 patients) found no significant difference; 3 studies (376 patients) found that PRP improved standardized knee assessment scores by 35% to 40% at 24-48 weeks.7,8,10 All studies enrolled patients (mean age early 60s, approximately 50% women) with clinically and radiographically evaluated knee OA of mostly moderate severity. In 7 RCTs, 4-6,9-12 investigators injected PRP or HA weekly for 3 weeks, in one RCT8 they gave 4 weekly injections, and in one7they gave 2 PRP injections separated by 4 weeks.
Three RCTs used the International Knee Documentation Committee (IKDC) score, considered the most reliable standardized scoring system, which quantifies subjective symptoms (pain, stiffness, swelling, giving way), activity (climbing stairs, rising from a chair, squatting, jumping), and function pre- and postintervention.5,9,12 All 3 studies using the IKDC found no difference between PRP and HA injections. Most RCTs used the WOMAC standardized scale, scoring 5 items for pain, 2 for stiffness, and 17 for function.1,2,4,6-8.10
Risk for bias
A systematic review13 that evaluated methodologic quality of the 3 studies comparing PRP with placebo rated 21,3 at high risk of bias and one2 at moderate risk. Another meta-analysis14 performed a quality assessment including 4 of the 9 RCTs,8-10,12 comparing PRP with HA and concluded that 3 had a high risk of bias; the fourth RCT had a moderate risk. No independent quality assessments of the other RCTs were available.4-7,11
RECOMMENDATIONS
The American Academy of Orthopaedic Surgeons doesn’t recommend for or against PRP injections because of insufficient evidence and strongly recommends against HA injections based on multiple RCTs of moderate quality that found no difference between HA and placebo.15
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
1. Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356-364.
2. Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee arthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44:884-891.
3. Gorelli G, Gormelli CA, Ataoglu B, et al. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2015;25:958-965.
4. Cole BJ, Karas V, Hussey K, et al. Hyaluronic acid versus platelet-rich plasma: a prospective double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2016;45:339-346.
5. Filardo G, Di Matteo B, Di Martino A, et al. Platelet-rich intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43:1575-1582.
6. Sanchez M, Fiz N, Azofra J, et al. A randomized clinical trial evaluating plasma rich in growth factors (PGRF-endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy: J Arth and Related Surg. 2012;28:1070-1078.
7. Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Knee osteoarthritis injection choices: platelet-rich plasma (PRP) versus hyaluronic acid (a one-year randomized clinical trial). Clin Med Insights: Arth Musc Dis. 2015;8:1-8.
8. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40:2822-2827.
9. Filardo G, Kon E, Di Martino B, et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskeletal Disorders. 2012;13:229-236.
10. Vaquerizo V, Plasencia MA, Arribas I, et al. Comparison of intra-articular injections of plasma rich in growth factors (PGRF-endoret) versus durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: a randomized controlled trial. Arthroscopy: J Arth and Related Surg. 2013;29:1635-1643.
11. Montanez-Heredia E, Irizar S, Huertas PJ, et al. Intra-articular injections of platelet-rich plasma versus hyaluronic acid in the treatment of osteoarthritis knee pain: a randomized clinical trial in the context of the Spanish national health care system. Intl J Molec Sci. 2016;17:1064-1077.
12. Li M, Zhang C, Ai Z, et al. Therapeutic effectiveness of intra-knee articular injections of platelet-rich plasma on knee articular cartilage degeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2011 25:1192-11966. (Article published in Chinese with abstract in English.)
13. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Ortho Surg Res. 2017;12:16.
14. Laudy ABM, Bakker EWP, Rekers M, et al. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672.
15. American Academy of Orthopaedic Surgeons. Clinical practice guideline on the treatment of osteoarthritis of the knee, 2nd ed. www.aaos.org/cc_files/aaosorg/research/guidelines/treatmentofosteoarthritisofthekneeguideline.pdf. Published May 2013. Accessed February 22, 2019.
EVIDENCE-BASED ANSWER:
Probably not, based on the balance of evidence. While low-quality evidence may suggest potential benefit, the balance of evidence suggests it is no better than placebo.
Compared with saline placebo, platelet-rich plasma (PRP) injections may improve standardized scores for knee osteoarthritis (OA) pain, function, and stiffness by 24% to 70% for periods of 6 to 52 weeks in patients with early to moderate OA (strength of recommendation [SOR]: B, small randomized controlled trials [RCTs] with methodologic flaws).
Compared with hyaluronic acid (HA), PRP probably improves scores by a similar amount for periods of 8 to 52 weeks (SOR: B, multiple RCTs with conflicting results favoring no difference). However, since HA alone likely doesn’t improve scores more than placebo (SOR: B, RCTs of moderate quality), if both HA and PRP are about the same, then both are not better than placebo.
Does amniotomy shorten spontaneous labor or improve outcomes?
EVIDENCE SUMMARY
A meta-analysis of 15 RCTs (5583 women) compared intentional artificial rupture of the amniotic membranes during labor (amniotomy) with intention to preserve the membranes (no amniotomy). The study found no differences in any of the measured primary outcomes: length of first stage of labor, cesarean section, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes.1
Investigators included 9 trials with both nulliparous and multiparous women and 6 trials with only nulliparous women. Thirteen trials compared amniotomy with intention to preserve the membranes, and 2 trials performed amniotomy in the control group if the membranes were intact at full cervical dilation.
Amniotomy doesn’t affect first-stage labor or cesarean risk
Five trials (1127 women) reported no difference in length of the first stage of labor between the amniotomy and no amniotomy groups (mean difference [MD]= −20 minutes; 95% confidence interval [CI], −96 to 55). Subgroups of primiparous and multiparous women showed no difference (MD= −58 minutes; 95% CI, −153 to 37 and MD= +23 minutes; 95% CI, −51 to 97, respectively).
Nine trials (5021 women) reported no significant difference in cesarean section risk overall or when compared by parity, multiparous vs primiparous (risk ratio [RR]= 1.27; 95% CI, 0.99-1.63). One trial (84 women) found no difference in maternal satisfaction scores with childbirth experience. Six trials (3598 women) that reported risk of low Apgar score (<4 at 1 minute or <7 at 5 minutes) found no difference overall (RR=0.53; 95% CI, 0.28-1.00), or when compared by parity (multiparous vs primiparous).
Investigators reported that the included trials varied in quality and described the following limitations: inconsistent or unspecified timing of amniotomy during labor, proportion of women in the control group undergoing amniotomy, and ≥30% of women not getting the allocated treatment in all but one of the trials.
Secondary outcomes: Amniotomy reduces oxytocin use
Eight trials (4264 women) evaluated oxytocin augmentation and found that amniotomy decreased its use in multiparous (RR=0.43; 95% CI, 0.30-0.60), but not primiparous, women.
Eight trials (1927 women) reported length of second stage of labor as a secondary outcome, with no difference overall (MD= −1.33 minutes; 95% CI, −2.92 to 0.26). Amniotomy produced a statistical but not clinically significant shortening in subanalysis of primiparous women (MD= −5.43 minutes; 95% CI, −9.98 to −0.89) but not multiparous women.
Continue to: Three trials...
Three trials (1695 women) evaluated dysfunctional labor, defined as no progress in cervical dilation in 2 hours or ineffective uterine contractions. Amniotomy reduced dysfunctional labor in both primiparous (RR=0.49; 95% CI, 0.33-0.73) and multiparous women (RR=0.44; 95% CI, 0.31-0.62).
No differences found in other maternal and fetal outcomes
Investigators reported no differences in other secondary maternal outcomes: instrumental vaginal birth (10 trials, 5121 women); pain relief (8 trials, 3475 women); postpartum hemorrhage (2 trials, 1822 women); serious maternal morbidity or death (3 trials, 1740 women); umbilical cord prolapse (2 trials, 1615 women); and cesarean section for fetal distress, prolonged labor, or antepartum hemorrhage (1 RCT, 690 women).
Investigators also found no differences in secondary fetal outcomes: serious neonatal morbidity or perinatal death (8 trials, 3397 women); neonatal admission to neonatal intensive care (5 trials, 2686 women); abnormal fetal heart rate tracing in first stage of labor (4 trials, 1284 women); meconium aspiration (2 trials, 1615 women); and fetal acidosis (2 trials, 1014 women). Similarly, 1 RCT (39 women) that compared amniotomy with intent to preserve membranes in spontaneous labors that became prolonged found no difference in cesarean section, maternal satisfaction, or Apgar scores.
A few studies claim shorter labor with amniotomy
However, a later Iranian RCT (300 women) reported that early amniotomy shortened labor (labor duration: 7.5 ± 0.7 hours with amniotomy vs 9.9 ± 1.0 hours without amniotomy; P<.001) and reduced the risk of dystocia (RR=0.81; 95% CI, 0.59-0.90) and cesarean section (RR=0.82; 95% CI, 0.66-0.90).2
A similar Nigerian RCT (214 women) and an Indian RCT (144 women) both claimed that amniotomy also shortened labor (4.7 ± 0.9 hours vs 5.9 ± 1.3, and 3.9 ± 2 hours vs 6.1 ± 2.8 hours, respectively).3,4 In neither trial, however, did investigators explain how the difference was significant when the duration of labor times overlapped within the margin of error.
1. Smyth RMD, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev. 2013;(6):CD006167.
2. Ghafarzadeh M, Moeininasab S, Namdari M. Effect of early amniotomy on dystocia risk and cesarean delivery in nulliparous women: a randomized clinical trial. Arch Gynecol Obstet. 2015;292:321-325.
3. Onah LN, Dim CC, Nwagha UI, et al. Effect of early amniotomy on the outcome of spontaneous labour: a randomized controlled trial of pregnant women in Enugu, South-east Nigeria. Afr Health Sci. 2015;15:1097-1103.
4. Vadivelu M, Rathore S, Benjamin SJ, et al. Randomized controlled trial of the effect of amniotomy on the duration of spontaneous labor. Int J Gynaecol Obstet. 2017;138:152-157.
EVIDENCE SUMMARY
A meta-analysis of 15 RCTs (5583 women) compared intentional artificial rupture of the amniotic membranes during labor (amniotomy) with intention to preserve the membranes (no amniotomy). The study found no differences in any of the measured primary outcomes: length of first stage of labor, cesarean section, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes.1
Investigators included 9 trials with both nulliparous and multiparous women and 6 trials with only nulliparous women. Thirteen trials compared amniotomy with intention to preserve the membranes, and 2 trials performed amniotomy in the control group if the membranes were intact at full cervical dilation.
Amniotomy doesn’t affect first-stage labor or cesarean risk
Five trials (1127 women) reported no difference in length of the first stage of labor between the amniotomy and no amniotomy groups (mean difference [MD]= −20 minutes; 95% confidence interval [CI], −96 to 55). Subgroups of primiparous and multiparous women showed no difference (MD= −58 minutes; 95% CI, −153 to 37 and MD= +23 minutes; 95% CI, −51 to 97, respectively).
Nine trials (5021 women) reported no significant difference in cesarean section risk overall or when compared by parity, multiparous vs primiparous (risk ratio [RR]= 1.27; 95% CI, 0.99-1.63). One trial (84 women) found no difference in maternal satisfaction scores with childbirth experience. Six trials (3598 women) that reported risk of low Apgar score (<4 at 1 minute or <7 at 5 minutes) found no difference overall (RR=0.53; 95% CI, 0.28-1.00), or when compared by parity (multiparous vs primiparous).
Investigators reported that the included trials varied in quality and described the following limitations: inconsistent or unspecified timing of amniotomy during labor, proportion of women in the control group undergoing amniotomy, and ≥30% of women not getting the allocated treatment in all but one of the trials.
Secondary outcomes: Amniotomy reduces oxytocin use
Eight trials (4264 women) evaluated oxytocin augmentation and found that amniotomy decreased its use in multiparous (RR=0.43; 95% CI, 0.30-0.60), but not primiparous, women.
Eight trials (1927 women) reported length of second stage of labor as a secondary outcome, with no difference overall (MD= −1.33 minutes; 95% CI, −2.92 to 0.26). Amniotomy produced a statistical but not clinically significant shortening in subanalysis of primiparous women (MD= −5.43 minutes; 95% CI, −9.98 to −0.89) but not multiparous women.
Continue to: Three trials...
Three trials (1695 women) evaluated dysfunctional labor, defined as no progress in cervical dilation in 2 hours or ineffective uterine contractions. Amniotomy reduced dysfunctional labor in both primiparous (RR=0.49; 95% CI, 0.33-0.73) and multiparous women (RR=0.44; 95% CI, 0.31-0.62).
No differences found in other maternal and fetal outcomes
Investigators reported no differences in other secondary maternal outcomes: instrumental vaginal birth (10 trials, 5121 women); pain relief (8 trials, 3475 women); postpartum hemorrhage (2 trials, 1822 women); serious maternal morbidity or death (3 trials, 1740 women); umbilical cord prolapse (2 trials, 1615 women); and cesarean section for fetal distress, prolonged labor, or antepartum hemorrhage (1 RCT, 690 women).
Investigators also found no differences in secondary fetal outcomes: serious neonatal morbidity or perinatal death (8 trials, 3397 women); neonatal admission to neonatal intensive care (5 trials, 2686 women); abnormal fetal heart rate tracing in first stage of labor (4 trials, 1284 women); meconium aspiration (2 trials, 1615 women); and fetal acidosis (2 trials, 1014 women). Similarly, 1 RCT (39 women) that compared amniotomy with intent to preserve membranes in spontaneous labors that became prolonged found no difference in cesarean section, maternal satisfaction, or Apgar scores.
A few studies claim shorter labor with amniotomy
However, a later Iranian RCT (300 women) reported that early amniotomy shortened labor (labor duration: 7.5 ± 0.7 hours with amniotomy vs 9.9 ± 1.0 hours without amniotomy; P<.001) and reduced the risk of dystocia (RR=0.81; 95% CI, 0.59-0.90) and cesarean section (RR=0.82; 95% CI, 0.66-0.90).2
A similar Nigerian RCT (214 women) and an Indian RCT (144 women) both claimed that amniotomy also shortened labor (4.7 ± 0.9 hours vs 5.9 ± 1.3, and 3.9 ± 2 hours vs 6.1 ± 2.8 hours, respectively).3,4 In neither trial, however, did investigators explain how the difference was significant when the duration of labor times overlapped within the margin of error.
EVIDENCE SUMMARY
A meta-analysis of 15 RCTs (5583 women) compared intentional artificial rupture of the amniotic membranes during labor (amniotomy) with intention to preserve the membranes (no amniotomy). The study found no differences in any of the measured primary outcomes: length of first stage of labor, cesarean section, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes.1
Investigators included 9 trials with both nulliparous and multiparous women and 6 trials with only nulliparous women. Thirteen trials compared amniotomy with intention to preserve the membranes, and 2 trials performed amniotomy in the control group if the membranes were intact at full cervical dilation.
Amniotomy doesn’t affect first-stage labor or cesarean risk
Five trials (1127 women) reported no difference in length of the first stage of labor between the amniotomy and no amniotomy groups (mean difference [MD]= −20 minutes; 95% confidence interval [CI], −96 to 55). Subgroups of primiparous and multiparous women showed no difference (MD= −58 minutes; 95% CI, −153 to 37 and MD= +23 minutes; 95% CI, −51 to 97, respectively).
Nine trials (5021 women) reported no significant difference in cesarean section risk overall or when compared by parity, multiparous vs primiparous (risk ratio [RR]= 1.27; 95% CI, 0.99-1.63). One trial (84 women) found no difference in maternal satisfaction scores with childbirth experience. Six trials (3598 women) that reported risk of low Apgar score (<4 at 1 minute or <7 at 5 minutes) found no difference overall (RR=0.53; 95% CI, 0.28-1.00), or when compared by parity (multiparous vs primiparous).
Investigators reported that the included trials varied in quality and described the following limitations: inconsistent or unspecified timing of amniotomy during labor, proportion of women in the control group undergoing amniotomy, and ≥30% of women not getting the allocated treatment in all but one of the trials.
Secondary outcomes: Amniotomy reduces oxytocin use
Eight trials (4264 women) evaluated oxytocin augmentation and found that amniotomy decreased its use in multiparous (RR=0.43; 95% CI, 0.30-0.60), but not primiparous, women.
Eight trials (1927 women) reported length of second stage of labor as a secondary outcome, with no difference overall (MD= −1.33 minutes; 95% CI, −2.92 to 0.26). Amniotomy produced a statistical but not clinically significant shortening in subanalysis of primiparous women (MD= −5.43 minutes; 95% CI, −9.98 to −0.89) but not multiparous women.
Continue to: Three trials...
Three trials (1695 women) evaluated dysfunctional labor, defined as no progress in cervical dilation in 2 hours or ineffective uterine contractions. Amniotomy reduced dysfunctional labor in both primiparous (RR=0.49; 95% CI, 0.33-0.73) and multiparous women (RR=0.44; 95% CI, 0.31-0.62).
No differences found in other maternal and fetal outcomes
Investigators reported no differences in other secondary maternal outcomes: instrumental vaginal birth (10 trials, 5121 women); pain relief (8 trials, 3475 women); postpartum hemorrhage (2 trials, 1822 women); serious maternal morbidity or death (3 trials, 1740 women); umbilical cord prolapse (2 trials, 1615 women); and cesarean section for fetal distress, prolonged labor, or antepartum hemorrhage (1 RCT, 690 women).
Investigators also found no differences in secondary fetal outcomes: serious neonatal morbidity or perinatal death (8 trials, 3397 women); neonatal admission to neonatal intensive care (5 trials, 2686 women); abnormal fetal heart rate tracing in first stage of labor (4 trials, 1284 women); meconium aspiration (2 trials, 1615 women); and fetal acidosis (2 trials, 1014 women). Similarly, 1 RCT (39 women) that compared amniotomy with intent to preserve membranes in spontaneous labors that became prolonged found no difference in cesarean section, maternal satisfaction, or Apgar scores.
A few studies claim shorter labor with amniotomy
However, a later Iranian RCT (300 women) reported that early amniotomy shortened labor (labor duration: 7.5 ± 0.7 hours with amniotomy vs 9.9 ± 1.0 hours without amniotomy; P<.001) and reduced the risk of dystocia (RR=0.81; 95% CI, 0.59-0.90) and cesarean section (RR=0.82; 95% CI, 0.66-0.90).2
A similar Nigerian RCT (214 women) and an Indian RCT (144 women) both claimed that amniotomy also shortened labor (4.7 ± 0.9 hours vs 5.9 ± 1.3, and 3.9 ± 2 hours vs 6.1 ± 2.8 hours, respectively).3,4 In neither trial, however, did investigators explain how the difference was significant when the duration of labor times overlapped within the margin of error.
1. Smyth RMD, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev. 2013;(6):CD006167.
2. Ghafarzadeh M, Moeininasab S, Namdari M. Effect of early amniotomy on dystocia risk and cesarean delivery in nulliparous women: a randomized clinical trial. Arch Gynecol Obstet. 2015;292:321-325.
3. Onah LN, Dim CC, Nwagha UI, et al. Effect of early amniotomy on the outcome of spontaneous labour: a randomized controlled trial of pregnant women in Enugu, South-east Nigeria. Afr Health Sci. 2015;15:1097-1103.
4. Vadivelu M, Rathore S, Benjamin SJ, et al. Randomized controlled trial of the effect of amniotomy on the duration of spontaneous labor. Int J Gynaecol Obstet. 2017;138:152-157.
1. Smyth RMD, Markham C, Dowswell T. Amniotomy for shortening spontaneous labour. Cochrane Database Syst Rev. 2013;(6):CD006167.
2. Ghafarzadeh M, Moeininasab S, Namdari M. Effect of early amniotomy on dystocia risk and cesarean delivery in nulliparous women: a randomized clinical trial. Arch Gynecol Obstet. 2015;292:321-325.
3. Onah LN, Dim CC, Nwagha UI, et al. Effect of early amniotomy on the outcome of spontaneous labour: a randomized controlled trial of pregnant women in Enugu, South-east Nigeria. Afr Health Sci. 2015;15:1097-1103.
4. Vadivelu M, Rathore S, Benjamin SJ, et al. Randomized controlled trial of the effect of amniotomy on the duration of spontaneous labor. Int J Gynaecol Obstet. 2017;138:152-157.
EVIDENCE-BASED ANSWER:
No. Amniotomy neither shortens spontaneous labor nor improves any of the following outcomes: length of first stage of labor, cesarean section rate, maternal satisfaction with childbirth, or Apgar score <7 at 5 minutes (strength of recommendation [SOR]: A, large meta-analyses of randomized controlled trials [RCTs] and a single RCT with conflicting results).
Amniotomy does result in about a 55% reduction of pitocin use in multiparous women, a small (5 minutes) decrease in the duration of second-stage labor in primiparous women, and about a 50% overall reduction in dysfunctional labor—ie, no progress in cervical dilation in 2 hours or ineffective uterine contractions (SOR: A, large meta-analyses of RCTs and a single RCT with conflicting results).
Amniotomy doesn’t improve other maternal outcomes—instrumented vaginal birth; pain relief; postpartum hemorrhage; serious morbidity or death; umbilical cord prolapse; cesarean section for fetal distress, prolonged labor, antepartum hemorrhage—nor fetal outcomes—serious neonatal morbidity or perinatal death; neonatal admission to intensive care; abnormal fetal heart rate tracing in first-stage labor; meconium aspiration; or fetal acidosis (SOR: A, large meta-analyses of RCTs and a single RCT with conflicting results).
What medical therapies work for gastroparesis?
EVIDENCE SUMMARY
Metoclopramide. One systematic review that looked at the efficacy of metoclopramide for gastroparesis identified one small RCT and 3 smaller placebo-controlled crossover trials.1 The RCT (using 10 mg of metoclopramide after meals and at bedtime) found consistent improvement in the sense of fullness over 3 weeks of therapy, with reduction of nausea at one and 3 weeks, but not at 2 weeks. Vomiting, anorexia, and early satiety didn’t improve. The crossover trials had inconsistent results. The largest one, with only 16 patients, didn’t find an improvement in symptoms.
Erythromycin. Two systematic reviews looked at the efficacy of erythromycin, primarily identifying studies 20- to 30-years old. The first systematic review identified only one small (single-blind) RCT in which erythromycin treatment didn’t change symptoms.1 A second review identified 3 trials described as “open label,” all with fewer than 14 subjects and all lasting a month or less.2 Erythromycin improved patient symptoms in only 1 of the 3, and this trial (like the others) had significant methodologic flaws. The authors of the second review concluded that “the true efficacy of erythromycin in relieving symptoms … remains to be determined.”
Domperidone. A systematic review and one subsequent RCT evaluated domperidone. The systematic review identified 11 randomized, placebo-controlled trials (469 patients).3 Six studies found no impact on patient symptoms, while 5 reported a positive effect. The review also identified 6 trials that evaluated domperidone treatment on hospitalization rates. Open-label (single-arm, unblinded) trials tended to find a reduction in hospitalizations with domperidone, an effect not seen in the one double-arm study that evaluated this outcome.
The review authors noted that given the small size and low methodologic quality of most studies “it is not surprising … that there continues to be controversy about the efficacy of this drug” for symptoms of gastroparesis.
One subsequent RCT, using domperidone 20 mg 4 times daily for 4 weeks, found a 2% improvement over placebo in the physical component of a multifaceted quality-of-life measure.4 The improvement was statistically significant, but of unclear clinical importance.
Cisapride. One systematic review and 2 subsequent RCTs evaluated the clinical effects of cisapride. The systematic review included 4 small RCTs (53 patients) that didn’t individually find changes in patient symptoms.
In one subsequent RCT, comparing 10 mg cisapride 3 times daily to placebo for 2 weeks, cisapride yielded no significant change in symptoms.5 The other RCT compared oral cisapride 10 mg 3 times daily to placebo for one year. Cisapride treatment produced a 17% reduction in symptoms (P<.002 vs baseline), and placebo produced a 5% reduction (P=NS vs baseline). The study didn’t state if the difference between the 2 outcomes was statistically significant.6
Continue to: Levosulpiride
Levosulpiride. One crossover study compared 25 mg levosulpiride with 10 mg cisapride (both given orally 3 times a day) on gastroparesis symptoms and gastric emptying. Each medication was given for one month (washout duration not given). The study found similar efficacy between levosulpiride and cisapride in terms of improvement in gastric emptying rates and total symptom scores.7 No studies compare levosulpiride to placebo.
Nortriptyline. A multicenter, parallel-group, double-blind RCT comparing 75 mg/d nortriptyline for 15 weeks with placebo in adult patients with moderate to severe symptoms of idiopathic gastroparesis for at least 6 months found that nortriptyline didn’t improve symptoms.8
Botulinum toxin A. An RCT comparing a single injection of 200 units intrapyloric botulinum toxin A with placebo in adult patients with severe gastroparesis symptoms found that botulinum toxin A didn’t result in symptomatic improvement.9 A crossover trial comparing 100 units monthly intrapyloric botulinum toxin A for 3 months with placebo in patients with gastroparesis found that neither symptoms nor rate of gastric emptying changed with the toxin.10
RECOMMENDATIONS
The 2013 guidelines from the American College of Gastroenterology list metoclopramide as the first-line agent for gastroparesis requiring medical therapy, followed by domperidone and then erythromycin (all based on “moderate quality evidence”). Antiemetic agents are also recommended for symptom control.11
1. Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422-427.
2. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98:259-263.
3. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726-733.
4. Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699-1706.
5. Dutta U, Padhy AK, Ahuja V, et al. Double blind controlled trial of effect of cisapride on gastric emptying in diabetics. Trop Gastroenterol. 1999;20:116-119.
6. Braden B, Enghofer M, Schaub M, et al. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Aliment Pharmacol Ther. 2002;16:1341-1346.
7. Mansi C, Borro P, Giacomini M, et al. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther. 2000;14:561-569.
8. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640-2649.
9. Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416-423.
10. Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251-1258.
11. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-38.
EVIDENCE SUMMARY
Metoclopramide. One systematic review that looked at the efficacy of metoclopramide for gastroparesis identified one small RCT and 3 smaller placebo-controlled crossover trials.1 The RCT (using 10 mg of metoclopramide after meals and at bedtime) found consistent improvement in the sense of fullness over 3 weeks of therapy, with reduction of nausea at one and 3 weeks, but not at 2 weeks. Vomiting, anorexia, and early satiety didn’t improve. The crossover trials had inconsistent results. The largest one, with only 16 patients, didn’t find an improvement in symptoms.
Erythromycin. Two systematic reviews looked at the efficacy of erythromycin, primarily identifying studies 20- to 30-years old. The first systematic review identified only one small (single-blind) RCT in which erythromycin treatment didn’t change symptoms.1 A second review identified 3 trials described as “open label,” all with fewer than 14 subjects and all lasting a month or less.2 Erythromycin improved patient symptoms in only 1 of the 3, and this trial (like the others) had significant methodologic flaws. The authors of the second review concluded that “the true efficacy of erythromycin in relieving symptoms … remains to be determined.”
Domperidone. A systematic review and one subsequent RCT evaluated domperidone. The systematic review identified 11 randomized, placebo-controlled trials (469 patients).3 Six studies found no impact on patient symptoms, while 5 reported a positive effect. The review also identified 6 trials that evaluated domperidone treatment on hospitalization rates. Open-label (single-arm, unblinded) trials tended to find a reduction in hospitalizations with domperidone, an effect not seen in the one double-arm study that evaluated this outcome.
The review authors noted that given the small size and low methodologic quality of most studies “it is not surprising … that there continues to be controversy about the efficacy of this drug” for symptoms of gastroparesis.
One subsequent RCT, using domperidone 20 mg 4 times daily for 4 weeks, found a 2% improvement over placebo in the physical component of a multifaceted quality-of-life measure.4 The improvement was statistically significant, but of unclear clinical importance.
Cisapride. One systematic review and 2 subsequent RCTs evaluated the clinical effects of cisapride. The systematic review included 4 small RCTs (53 patients) that didn’t individually find changes in patient symptoms.
In one subsequent RCT, comparing 10 mg cisapride 3 times daily to placebo for 2 weeks, cisapride yielded no significant change in symptoms.5 The other RCT compared oral cisapride 10 mg 3 times daily to placebo for one year. Cisapride treatment produced a 17% reduction in symptoms (P<.002 vs baseline), and placebo produced a 5% reduction (P=NS vs baseline). The study didn’t state if the difference between the 2 outcomes was statistically significant.6
Continue to: Levosulpiride
Levosulpiride. One crossover study compared 25 mg levosulpiride with 10 mg cisapride (both given orally 3 times a day) on gastroparesis symptoms and gastric emptying. Each medication was given for one month (washout duration not given). The study found similar efficacy between levosulpiride and cisapride in terms of improvement in gastric emptying rates and total symptom scores.7 No studies compare levosulpiride to placebo.
Nortriptyline. A multicenter, parallel-group, double-blind RCT comparing 75 mg/d nortriptyline for 15 weeks with placebo in adult patients with moderate to severe symptoms of idiopathic gastroparesis for at least 6 months found that nortriptyline didn’t improve symptoms.8
Botulinum toxin A. An RCT comparing a single injection of 200 units intrapyloric botulinum toxin A with placebo in adult patients with severe gastroparesis symptoms found that botulinum toxin A didn’t result in symptomatic improvement.9 A crossover trial comparing 100 units monthly intrapyloric botulinum toxin A for 3 months with placebo in patients with gastroparesis found that neither symptoms nor rate of gastric emptying changed with the toxin.10
RECOMMENDATIONS
The 2013 guidelines from the American College of Gastroenterology list metoclopramide as the first-line agent for gastroparesis requiring medical therapy, followed by domperidone and then erythromycin (all based on “moderate quality evidence”). Antiemetic agents are also recommended for symptom control.11
EVIDENCE SUMMARY
Metoclopramide. One systematic review that looked at the efficacy of metoclopramide for gastroparesis identified one small RCT and 3 smaller placebo-controlled crossover trials.1 The RCT (using 10 mg of metoclopramide after meals and at bedtime) found consistent improvement in the sense of fullness over 3 weeks of therapy, with reduction of nausea at one and 3 weeks, but not at 2 weeks. Vomiting, anorexia, and early satiety didn’t improve. The crossover trials had inconsistent results. The largest one, with only 16 patients, didn’t find an improvement in symptoms.
Erythromycin. Two systematic reviews looked at the efficacy of erythromycin, primarily identifying studies 20- to 30-years old. The first systematic review identified only one small (single-blind) RCT in which erythromycin treatment didn’t change symptoms.1 A second review identified 3 trials described as “open label,” all with fewer than 14 subjects and all lasting a month or less.2 Erythromycin improved patient symptoms in only 1 of the 3, and this trial (like the others) had significant methodologic flaws. The authors of the second review concluded that “the true efficacy of erythromycin in relieving symptoms … remains to be determined.”
Domperidone. A systematic review and one subsequent RCT evaluated domperidone. The systematic review identified 11 randomized, placebo-controlled trials (469 patients).3 Six studies found no impact on patient symptoms, while 5 reported a positive effect. The review also identified 6 trials that evaluated domperidone treatment on hospitalization rates. Open-label (single-arm, unblinded) trials tended to find a reduction in hospitalizations with domperidone, an effect not seen in the one double-arm study that evaluated this outcome.
The review authors noted that given the small size and low methodologic quality of most studies “it is not surprising … that there continues to be controversy about the efficacy of this drug” for symptoms of gastroparesis.
One subsequent RCT, using domperidone 20 mg 4 times daily for 4 weeks, found a 2% improvement over placebo in the physical component of a multifaceted quality-of-life measure.4 The improvement was statistically significant, but of unclear clinical importance.
Cisapride. One systematic review and 2 subsequent RCTs evaluated the clinical effects of cisapride. The systematic review included 4 small RCTs (53 patients) that didn’t individually find changes in patient symptoms.
In one subsequent RCT, comparing 10 mg cisapride 3 times daily to placebo for 2 weeks, cisapride yielded no significant change in symptoms.5 The other RCT compared oral cisapride 10 mg 3 times daily to placebo for one year. Cisapride treatment produced a 17% reduction in symptoms (P<.002 vs baseline), and placebo produced a 5% reduction (P=NS vs baseline). The study didn’t state if the difference between the 2 outcomes was statistically significant.6
Continue to: Levosulpiride
Levosulpiride. One crossover study compared 25 mg levosulpiride with 10 mg cisapride (both given orally 3 times a day) on gastroparesis symptoms and gastric emptying. Each medication was given for one month (washout duration not given). The study found similar efficacy between levosulpiride and cisapride in terms of improvement in gastric emptying rates and total symptom scores.7 No studies compare levosulpiride to placebo.
Nortriptyline. A multicenter, parallel-group, double-blind RCT comparing 75 mg/d nortriptyline for 15 weeks with placebo in adult patients with moderate to severe symptoms of idiopathic gastroparesis for at least 6 months found that nortriptyline didn’t improve symptoms.8
Botulinum toxin A. An RCT comparing a single injection of 200 units intrapyloric botulinum toxin A with placebo in adult patients with severe gastroparesis symptoms found that botulinum toxin A didn’t result in symptomatic improvement.9 A crossover trial comparing 100 units monthly intrapyloric botulinum toxin A for 3 months with placebo in patients with gastroparesis found that neither symptoms nor rate of gastric emptying changed with the toxin.10
RECOMMENDATIONS
The 2013 guidelines from the American College of Gastroenterology list metoclopramide as the first-line agent for gastroparesis requiring medical therapy, followed by domperidone and then erythromycin (all based on “moderate quality evidence”). Antiemetic agents are also recommended for symptom control.11
1. Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422-427.
2. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98:259-263.
3. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726-733.
4. Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699-1706.
5. Dutta U, Padhy AK, Ahuja V, et al. Double blind controlled trial of effect of cisapride on gastric emptying in diabetics. Trop Gastroenterol. 1999;20:116-119.
6. Braden B, Enghofer M, Schaub M, et al. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Aliment Pharmacol Ther. 2002;16:1341-1346.
7. Mansi C, Borro P, Giacomini M, et al. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther. 2000;14:561-569.
8. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640-2649.
9. Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416-423.
10. Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251-1258.
11. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-38.
1. Sturm A, Holtmann G, Goebell H, et al. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422-427.
2. Maganti K, Onyemere K, Jones MP. Oral erythromycin and symptomatic relief of gastroparesis: a systematic review. Am J Gastroenterol. 2003;98:259-263.
3. Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726-733.
4. Farup CE, Leidy NK, Murray M, et al. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699-1706.
5. Dutta U, Padhy AK, Ahuja V, et al. Double blind controlled trial of effect of cisapride on gastric emptying in diabetics. Trop Gastroenterol. 1999;20:116-119.
6. Braden B, Enghofer M, Schaub M, et al. Long-term cisapride treatment improves diabetic gastroparesis but not glycaemic control. Aliment Pharmacol Ther. 2002;16:1341-1346.
7. Mansi C, Borro P, Giacomini M, et al. Comparative effects of levosulpiride and cisapride on gastric emptying and symptoms in patients with functional dyspepsia and gastroparesis. Aliment Pharmacol Ther. 2000;14:561-569.
8. Parkman HP, Van Natta ML, Abell TL, et al. Effect of nortriptyline on symptoms of idiopathic gastroparesis: the NORIG randomized clinical trial. JAMA. 2013;310:2640-2649.
9. Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416-423.
10. Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251-1258.
11. Camilleri M, Parkman HP, Shafi MA, et al. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18-38.
EVIDENCE-BASED ANSWER:
It’s unclear if there are any highly effective medications for gastroparesis (TABLE1-10). Metoclopramide improves the sense of fullness by about 40% for as long as 3 weeks, may improve nausea, and doesn’t affect vomiting or anorexia (strength of recommendation [SOR]: B, small randomized controlled trial [RCT]).
Whether or not erythromycin has an effect on symptoms is unclear (SOR: C, conflicting trials and expert opinion).
Domperidone may improve quality of life (by 2%) for as long as a year, but its effect on symptoms is also unclear (SOR: C, small RCTs).
Cisapride may not be effective for symptom relief (SOR: C, small conflicting RCTs), and levosulpiride is likely similar to cisapride (SOR: C, single small crossover trial).
Nortriptyline (SOR: B, single RCT) and intrapyloric botulinum toxin A (SOR: B, small RCT and crossover trial) aren’t effective for symptom relief.