User login
Idiopathic Livedo Racemosa Presenting With Splenomegaly and Diffuse Lymphadenopathy
Sneddon syndrome (SS) was first described in 1965 in patients with persistent livedo racemosa and neurological events.1 Because the other manifestations of SS are nonspecific (eg, hypertension, cardiac valvulopathy, arterial and venous occlusion), the diagnosis often is delayed. Many patients who experience prodromal neurologic symptoms such as headaches, depression, anxiety, dizziness, and neuropathy often present to a physician prior to developing ischemic brain manifestations2 but seldom receive the correct diagnosis. Onset of cerebral occlusive events typically occurs in patients younger than 45 years and may present as a transient ischemic attack, stroke, or intracranial hemorrhage.3 The disease is more prevalent in females than males (2:1 ratio). The exact pathogenesis of SS is still unknown, and although it has been thought of as a separate entity from systemic lupus erythematosus and other antiphospholipid disorders, it has been postulated that an immunological dysfunction damages vessel walls leading to thrombosis.
Cutaneous findings associated with SS involve small- to medium-sized dermal-subdermal arteries. Histopathology in some patients demonstrates proliferation of the endothelium and fibrin deposits with subsequent obliteration of involved arteries.4 In many patients including our patient, histopathologic examination of involved skin fails to show specific abnormalities.1 Zelger et al5 reported the sequence of histopathologic skin events in a series of antiphospholipid-negative SS patients. The authors reported that only small arteries at the dermis-subcutis junction were involved and a progression of endothelial dysfunction was observed. The authors believed there were several nonspecific stages prior to fibrin occlusion of involved arteries.5 Stage I involved loosening of endothelial cells with nonspecific perivascular lymphocytic infiltration with perivascular inflammation and lymphocytic infiltration representing the prime mover of the disease.5,6 This stage is thought to be short lived, thus the reason why it has gone undetected for many years in SS patients. Stages II to IV progress through fibrin deposition and occlusion.5 Histological features of stages I to II have not been reported because of late diagnosis of SS. Stage I patients typically present with an average duration of symptoms of 6 months with few neurologic symptoms, the most common being paresthesia of the legs.5
Case Report
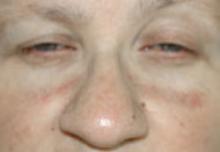
A 37-year-old woman with epigastric tenderness on the left side and splenomegaly seen on computed tomography was referred by a hematologist for evaluation of a reticular rash on the left side of the flank of 9 months’ duration with a presumed diagnosis of focal melanoderma. Her medical history was remarkable for a congenital ventricular septal defect and coarctation of the aorta, as well as endometriosis, myalgia, and joint stiffness that had all developed over the last year. Her medical history also was remarkable for nephrolithiasis, irritable bowel syndrome, and chronic sinusitis, as well as psychiatric depression and anxiety disorders. She recently had been diagnosed with moderate hypertension and had experienced difficulty getting pregnant for the last several years with 3 consecutive miscarriages in the first trimester. Neurologic symptoms included neuropathy involving the feet, intermittent paresthesia of the legs, and a history of chronic migraine headaches for several months.
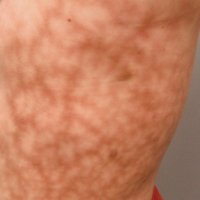
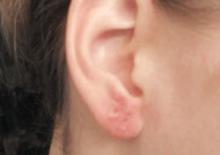
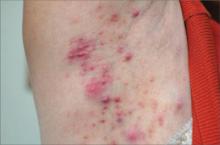
Dermatologic examination revealed a slightly overweight woman with a 25×30-cm dusky, erythematous, irregular, netlike pattern on the left side of the upper and lower trunk (Figure 1). Extensive livedo racemosa was not altered by changes in temperature and had been unchanged for more than 9 months. There were no signs of pruritus or ulcerations, and areas of livedo racemosa were slightly tender to palpation.
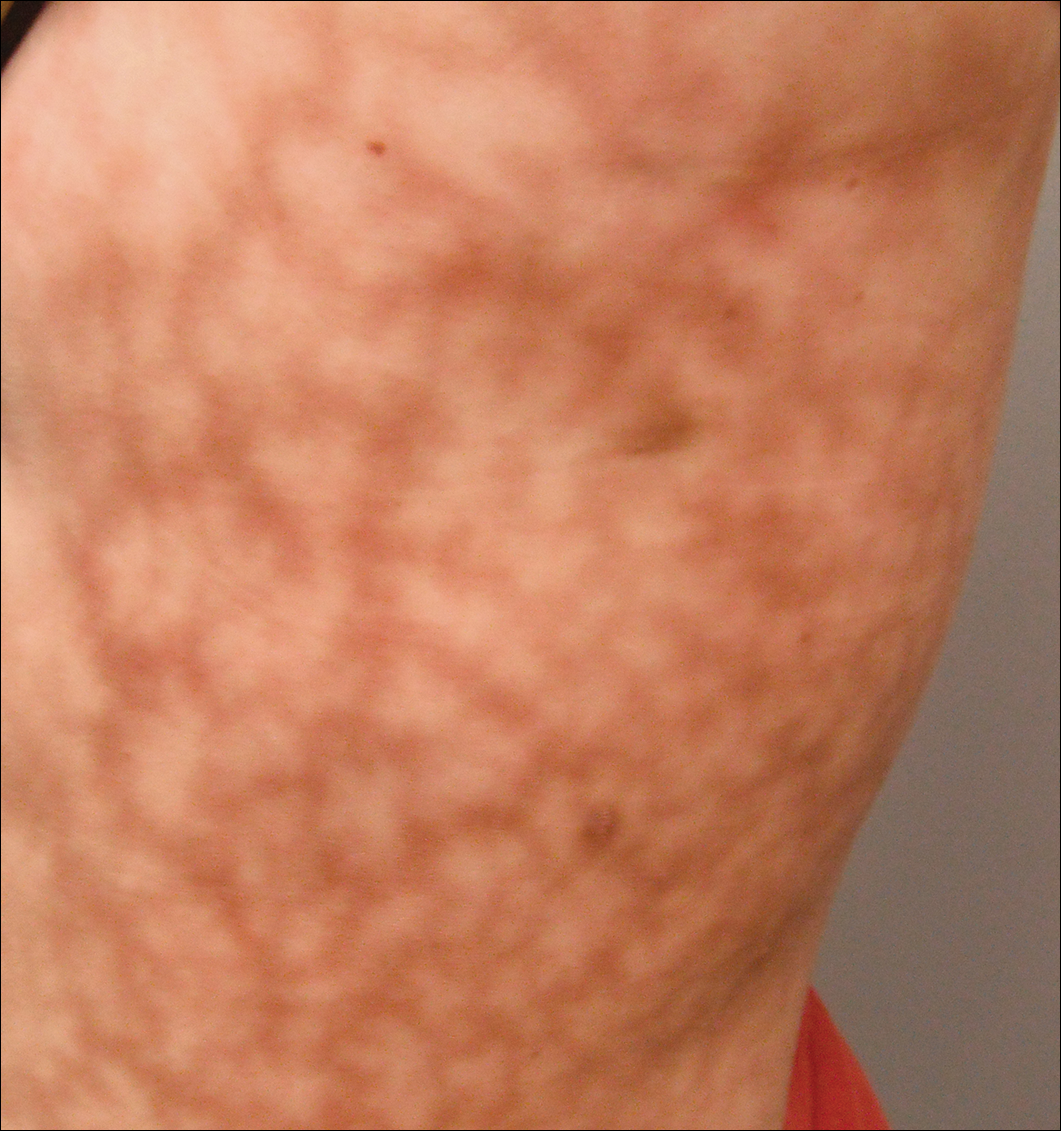
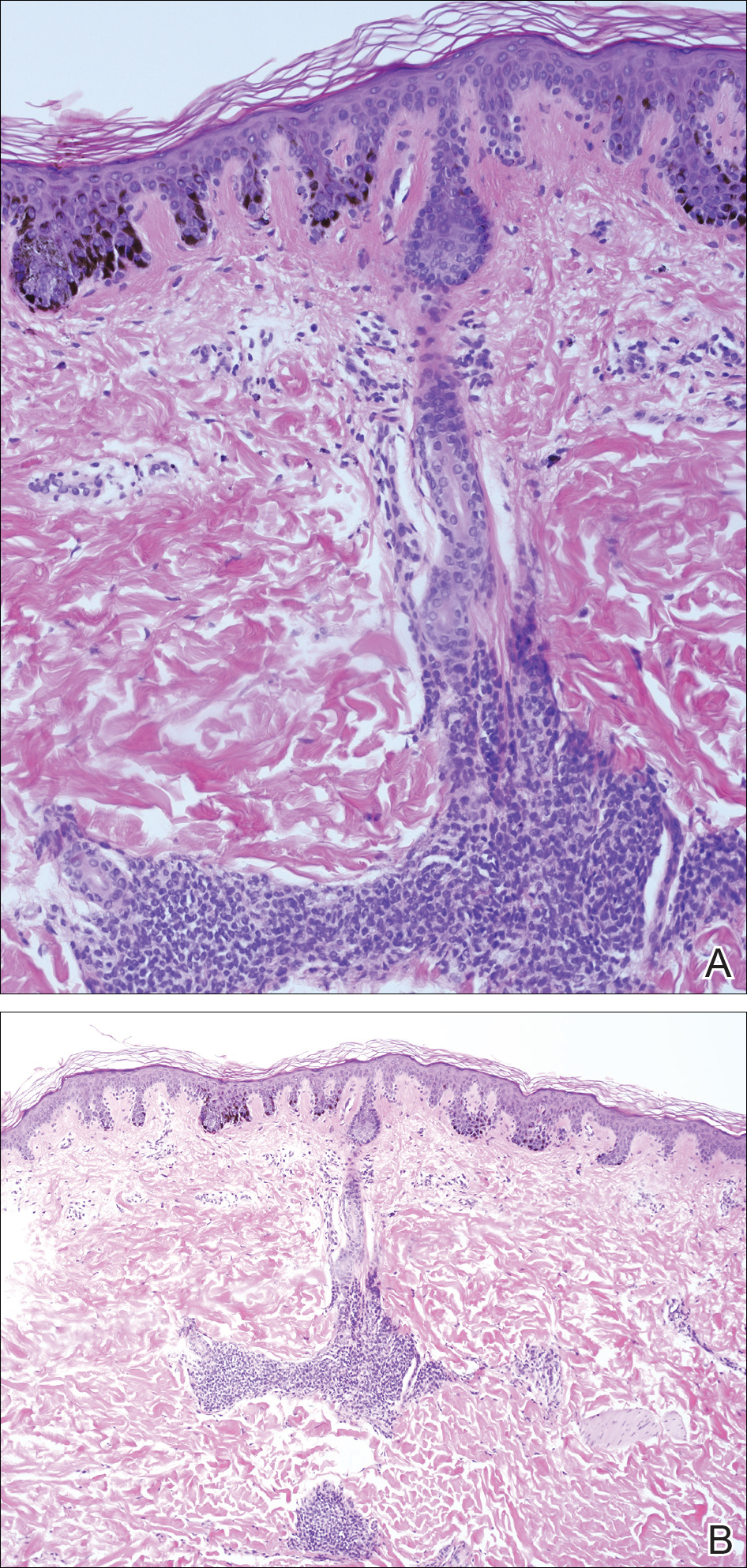
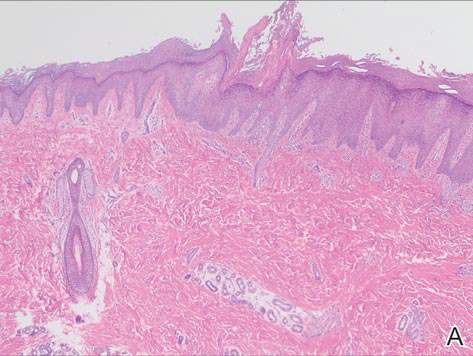
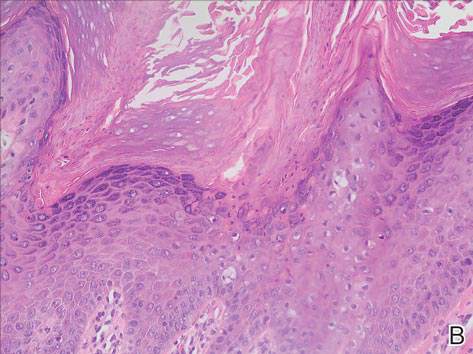
We performed 2 sets of three 4-mm biopsies. The first set targeted areas within the violaceous pattern, while the second set targeted areas of normal tissue between the mottled areas. All 6 specimens demonstrated superficial perivascular lymphocytic infiltrate with no evidence of vasculitis or connective tissue disease. The vessels showed no microthrombi or surrounding fibrosis. No eosinophils were identified within the epidermis. There was no evidence of increased dermal mucin. Both the superficial and deep vascular plexuses were unremarkable and showed no evidence of damage to the walls (Figure 2).
To rule out other possible causes of livedo racemosa, complete blood cell count, comprehensive metabolic panel, coagulation profile, lipase test, urinalysis, serologic testing, and immunologic workup were performed. Lipase was within reference range. The complete blood cell count revealed mild anemia, while the rest of the values were within reference range. An immunologic workup included Sjögren syndrome antigen A, Sjögren syndrome antigen B, anticardiolipin antibodies, and antinuclear antibody, which were all negative. Family history was remarkable for first-degree relatives with systemic lupus erythematosus and Crohn disease.
Computed tomography revealed enlargement of the spleen, as well as periaortic, portacaval, and porta hepatis lymphadenopathy. Based on the laboratory findings and clinical presentation as well as the patient’s medical history, the diagnosis of exclusion was idiopathic livedo racemosa with unknown progression to full-blown SS. The patient did not meet the current diagnostic criteria for SS, and her immunologic studies failed to confirm any present antibodies, but involvement of the reticuloendothelial system pointed to production of antibodies that were not yet detectable on laboratory testing.
Comment
More than 50 years after the first case of SS was diagnosed, better laboratory workup is available and more information is known about the pathophysiology. Sneddon syndrome is a rare disorder, affecting only approximately 4 patients per million each year worldwide. Seronegative antiphospholipid antibody syndrome (SNAPS) describes patients with clinical presentations of antiphospholipid syndrome (APS) without detectable serological markers.7 Antiphospholipid-negative SS, which was seen in our patient, would be categorized under SNAPS. A PubMed search of articles indexed for MEDLINE using the terms livedo racemosa, Sneddon syndrome, and SNAPS and splenomegaly revealed there currently are no known cases of SNAPS that have been reported with splenomegaly and lymphadenopathy. Our patient presented with the following clinical features of SS: livedo racemosa, history of miscarriage, psychiatric disturbances, and hypertension. Surprisingly, biopsies from affected skin did not show any fibrin deposition or microthrombi but did reveal perivascular lymphocytic infiltrations. Magnetic resonance imaging did not show any pathological lesions or vascular changes.
Sneddon syndrome and APS share a common pathway to occlusive arteriolopathy for which 4 stages have been described by Zelger et al.5 Stage I involves a nonspecific Langerhans cell infiltrate with polymorphonuclear leukocytes. The tunica media and elastic lamina usually are unaltered at this early stage, while the surrounding connective tissue may appear edematous.5 This early stage of histopathology has not been evaluated in SS patients, primarily because of delay of diagnosis. Late stages III and IV will show fibrin deposition and shrinkage of affected vessels.7
A PubMed search using the terms Sneddon syndrome, lymphadenopathy and livedo racemosa, and Sneddon syndrome and lymphadenopathy revealed that splenomegaly and lymphadenopathy have not been reported in patients with SS. In patients with antiphospholipid-negative SS, one can assume that antibodies to other phospholipids not tested must exist because of striking similarities between APS and antiphospholipid-negative SS.8 Although our patient did not test positive for any of these antibodies, she did present with lymphadenopathy and splenic enlargement, leading us to believe that involvement of the reticuloendothelial system may be a feature of SS that has not been previously reported. Further studies are required to name specific antigens responsible for clinical manifestations in SS.
Currently, no single diagnostic test for SS exists, thus delaying both diagnosis and initiation of treatment. Histopathologic examination may be helpful, but in many cases it is nonspecific, as are serologic markers. Neuroradiological confirmation of involvement usually is the confirmatory feature in many patients with late-stage diagnosis.2 A diagnostic schematic for SS, which was first described by Daoud et al,2 illustrates classification of symptoms and aids in diagnosis. A working diagnosis of idiopathic livedo racemosa is made after ruling out other causes of SS in a patient with nonspecific biopsy findings and negative magnetic resonance imaging results with prodromal symptoms. The prognosis for such patients progressing to full SS is unknown with or without management using anticoagulant therapy.
Conclusion
Early diagnosis of livedo racemosa and SS is essential, as prevention of cerebrovascular accidents, myocardial infarction, and other thromboembolic diseases can be minimized by attacking risk factors such as smoking, taking oral contraceptive pills, becoming pregnant,9 and by initiating either antiplatelet or anticoagulation treatments. These treatments have been shown to delay the development of neurovascular damage and early-onset dementia. We present this case to demonstrate the variability of early-presenting symptoms in idiopathic livedo racemosa. Recognizing some of the early manifestations can lead to early diagnosis and initiation of treatment.
- Sneddon IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Daoud MS, Wilmoth GJ, Su WP, et al. Sneddon syndrome. Semin Dermatol. 1995;14:166-172.
- Besnier R, Francès C, Ankri A, et al. Factor V Leiden mutation in Sneddon syndrome. Lupus. 2003;12:406-408.
- K aragülle AT, Karadağ D, Erden A, et al. Sneddon’s syndrome: MR imaging findings. Eur Radiol. 2002;12:144-146.
- Zelg er B, Sepp N, Schmid KW, et al. Life-history of cutaneous vascular-lesions in Sneddon’s syndrome. Hum Pathol. 1992;23:668-675.
- Ayoub N, Esposito G, Barete S, et al. Protein Z deficiency in antiphospholipid-negative Sneddon’s syndrome. Stroke. 2004;35:1329-1332.
- Duva l A, Darnige L, Glowacki F, et al. Livedo, dementia, thrombocytopenia, and endotheliitis without antiphospholipid antibodies: seronegative antiphospholipid-like syndrome. J Am Acad Dermatol. 2009;61:1076-1078.
- Kala shnikova LA, Nasonov EL, Kushekbaeva AE, et al. Anticardiolipin antibodies in Sneddon’s syndrome. Neurology. 1990;40:464-467.
- Wohl rab J, Fischer M, Wolter M, et al. Diagnostic impact and sensitivity of skin biopsies in Sneddon’s syndrome. a report of 15 cases. Br J Dermatol. 2001;145:285-288.
Sneddon syndrome (SS) was first described in 1965 in patients with persistent livedo racemosa and neurological events.1 Because the other manifestations of SS are nonspecific (eg, hypertension, cardiac valvulopathy, arterial and venous occlusion), the diagnosis often is delayed. Many patients who experience prodromal neurologic symptoms such as headaches, depression, anxiety, dizziness, and neuropathy often present to a physician prior to developing ischemic brain manifestations2 but seldom receive the correct diagnosis. Onset of cerebral occlusive events typically occurs in patients younger than 45 years and may present as a transient ischemic attack, stroke, or intracranial hemorrhage.3 The disease is more prevalent in females than males (2:1 ratio). The exact pathogenesis of SS is still unknown, and although it has been thought of as a separate entity from systemic lupus erythematosus and other antiphospholipid disorders, it has been postulated that an immunological dysfunction damages vessel walls leading to thrombosis.
Cutaneous findings associated with SS involve small- to medium-sized dermal-subdermal arteries. Histopathology in some patients demonstrates proliferation of the endothelium and fibrin deposits with subsequent obliteration of involved arteries.4 In many patients including our patient, histopathologic examination of involved skin fails to show specific abnormalities.1 Zelger et al5 reported the sequence of histopathologic skin events in a series of antiphospholipid-negative SS patients. The authors reported that only small arteries at the dermis-subcutis junction were involved and a progression of endothelial dysfunction was observed. The authors believed there were several nonspecific stages prior to fibrin occlusion of involved arteries.5 Stage I involved loosening of endothelial cells with nonspecific perivascular lymphocytic infiltration with perivascular inflammation and lymphocytic infiltration representing the prime mover of the disease.5,6 This stage is thought to be short lived, thus the reason why it has gone undetected for many years in SS patients. Stages II to IV progress through fibrin deposition and occlusion.5 Histological features of stages I to II have not been reported because of late diagnosis of SS. Stage I patients typically present with an average duration of symptoms of 6 months with few neurologic symptoms, the most common being paresthesia of the legs.5
Case Report
A 37-year-old woman with epigastric tenderness on the left side and splenomegaly seen on computed tomography was referred by a hematologist for evaluation of a reticular rash on the left side of the flank of 9 months’ duration with a presumed diagnosis of focal melanoderma. Her medical history was remarkable for a congenital ventricular septal defect and coarctation of the aorta, as well as endometriosis, myalgia, and joint stiffness that had all developed over the last year. Her medical history also was remarkable for nephrolithiasis, irritable bowel syndrome, and chronic sinusitis, as well as psychiatric depression and anxiety disorders. She recently had been diagnosed with moderate hypertension and had experienced difficulty getting pregnant for the last several years with 3 consecutive miscarriages in the first trimester. Neurologic symptoms included neuropathy involving the feet, intermittent paresthesia of the legs, and a history of chronic migraine headaches for several months.
Dermatologic examination revealed a slightly overweight woman with a 25×30-cm dusky, erythematous, irregular, netlike pattern on the left side of the upper and lower trunk (Figure 1). Extensive livedo racemosa was not altered by changes in temperature and had been unchanged for more than 9 months. There were no signs of pruritus or ulcerations, and areas of livedo racemosa were slightly tender to palpation.

We performed 2 sets of three 4-mm biopsies. The first set targeted areas within the violaceous pattern, while the second set targeted areas of normal tissue between the mottled areas. All 6 specimens demonstrated superficial perivascular lymphocytic infiltrate with no evidence of vasculitis or connective tissue disease. The vessels showed no microthrombi or surrounding fibrosis. No eosinophils were identified within the epidermis. There was no evidence of increased dermal mucin. Both the superficial and deep vascular plexuses were unremarkable and showed no evidence of damage to the walls (Figure 2).

To rule out other possible causes of livedo racemosa, complete blood cell count, comprehensive metabolic panel, coagulation profile, lipase test, urinalysis, serologic testing, and immunologic workup were performed. Lipase was within reference range. The complete blood cell count revealed mild anemia, while the rest of the values were within reference range. An immunologic workup included Sjögren syndrome antigen A, Sjögren syndrome antigen B, anticardiolipin antibodies, and antinuclear antibody, which were all negative. Family history was remarkable for first-degree relatives with systemic lupus erythematosus and Crohn disease.
Computed tomography revealed enlargement of the spleen, as well as periaortic, portacaval, and porta hepatis lymphadenopathy. Based on the laboratory findings and clinical presentation as well as the patient’s medical history, the diagnosis of exclusion was idiopathic livedo racemosa with unknown progression to full-blown SS. The patient did not meet the current diagnostic criteria for SS, and her immunologic studies failed to confirm any present antibodies, but involvement of the reticuloendothelial system pointed to production of antibodies that were not yet detectable on laboratory testing.
Comment
More than 50 years after the first case of SS was diagnosed, better laboratory workup is available and more information is known about the pathophysiology. Sneddon syndrome is a rare disorder, affecting only approximately 4 patients per million each year worldwide. Seronegative antiphospholipid antibody syndrome (SNAPS) describes patients with clinical presentations of antiphospholipid syndrome (APS) without detectable serological markers.7 Antiphospholipid-negative SS, which was seen in our patient, would be categorized under SNAPS. A PubMed search of articles indexed for MEDLINE using the terms livedo racemosa, Sneddon syndrome, and SNAPS and splenomegaly revealed there currently are no known cases of SNAPS that have been reported with splenomegaly and lymphadenopathy. Our patient presented with the following clinical features of SS: livedo racemosa, history of miscarriage, psychiatric disturbances, and hypertension. Surprisingly, biopsies from affected skin did not show any fibrin deposition or microthrombi but did reveal perivascular lymphocytic infiltrations. Magnetic resonance imaging did not show any pathological lesions or vascular changes.
Sneddon syndrome and APS share a common pathway to occlusive arteriolopathy for which 4 stages have been described by Zelger et al.5 Stage I involves a nonspecific Langerhans cell infiltrate with polymorphonuclear leukocytes. The tunica media and elastic lamina usually are unaltered at this early stage, while the surrounding connective tissue may appear edematous.5 This early stage of histopathology has not been evaluated in SS patients, primarily because of delay of diagnosis. Late stages III and IV will show fibrin deposition and shrinkage of affected vessels.7
A PubMed search using the terms Sneddon syndrome, lymphadenopathy and livedo racemosa, and Sneddon syndrome and lymphadenopathy revealed that splenomegaly and lymphadenopathy have not been reported in patients with SS. In patients with antiphospholipid-negative SS, one can assume that antibodies to other phospholipids not tested must exist because of striking similarities between APS and antiphospholipid-negative SS.8 Although our patient did not test positive for any of these antibodies, she did present with lymphadenopathy and splenic enlargement, leading us to believe that involvement of the reticuloendothelial system may be a feature of SS that has not been previously reported. Further studies are required to name specific antigens responsible for clinical manifestations in SS.
Currently, no single diagnostic test for SS exists, thus delaying both diagnosis and initiation of treatment. Histopathologic examination may be helpful, but in many cases it is nonspecific, as are serologic markers. Neuroradiological confirmation of involvement usually is the confirmatory feature in many patients with late-stage diagnosis.2 A diagnostic schematic for SS, which was first described by Daoud et al,2 illustrates classification of symptoms and aids in diagnosis. A working diagnosis of idiopathic livedo racemosa is made after ruling out other causes of SS in a patient with nonspecific biopsy findings and negative magnetic resonance imaging results with prodromal symptoms. The prognosis for such patients progressing to full SS is unknown with or without management using anticoagulant therapy.
Conclusion
Early diagnosis of livedo racemosa and SS is essential, as prevention of cerebrovascular accidents, myocardial infarction, and other thromboembolic diseases can be minimized by attacking risk factors such as smoking, taking oral contraceptive pills, becoming pregnant,9 and by initiating either antiplatelet or anticoagulation treatments. These treatments have been shown to delay the development of neurovascular damage and early-onset dementia. We present this case to demonstrate the variability of early-presenting symptoms in idiopathic livedo racemosa. Recognizing some of the early manifestations can lead to early diagnosis and initiation of treatment.
Sneddon syndrome (SS) was first described in 1965 in patients with persistent livedo racemosa and neurological events.1 Because the other manifestations of SS are nonspecific (eg, hypertension, cardiac valvulopathy, arterial and venous occlusion), the diagnosis often is delayed. Many patients who experience prodromal neurologic symptoms such as headaches, depression, anxiety, dizziness, and neuropathy often present to a physician prior to developing ischemic brain manifestations2 but seldom receive the correct diagnosis. Onset of cerebral occlusive events typically occurs in patients younger than 45 years and may present as a transient ischemic attack, stroke, or intracranial hemorrhage.3 The disease is more prevalent in females than males (2:1 ratio). The exact pathogenesis of SS is still unknown, and although it has been thought of as a separate entity from systemic lupus erythematosus and other antiphospholipid disorders, it has been postulated that an immunological dysfunction damages vessel walls leading to thrombosis.
Cutaneous findings associated with SS involve small- to medium-sized dermal-subdermal arteries. Histopathology in some patients demonstrates proliferation of the endothelium and fibrin deposits with subsequent obliteration of involved arteries.4 In many patients including our patient, histopathologic examination of involved skin fails to show specific abnormalities.1 Zelger et al5 reported the sequence of histopathologic skin events in a series of antiphospholipid-negative SS patients. The authors reported that only small arteries at the dermis-subcutis junction were involved and a progression of endothelial dysfunction was observed. The authors believed there were several nonspecific stages prior to fibrin occlusion of involved arteries.5 Stage I involved loosening of endothelial cells with nonspecific perivascular lymphocytic infiltration with perivascular inflammation and lymphocytic infiltration representing the prime mover of the disease.5,6 This stage is thought to be short lived, thus the reason why it has gone undetected for many years in SS patients. Stages II to IV progress through fibrin deposition and occlusion.5 Histological features of stages I to II have not been reported because of late diagnosis of SS. Stage I patients typically present with an average duration of symptoms of 6 months with few neurologic symptoms, the most common being paresthesia of the legs.5
Case Report
A 37-year-old woman with epigastric tenderness on the left side and splenomegaly seen on computed tomography was referred by a hematologist for evaluation of a reticular rash on the left side of the flank of 9 months’ duration with a presumed diagnosis of focal melanoderma. Her medical history was remarkable for a congenital ventricular septal defect and coarctation of the aorta, as well as endometriosis, myalgia, and joint stiffness that had all developed over the last year. Her medical history also was remarkable for nephrolithiasis, irritable bowel syndrome, and chronic sinusitis, as well as psychiatric depression and anxiety disorders. She recently had been diagnosed with moderate hypertension and had experienced difficulty getting pregnant for the last several years with 3 consecutive miscarriages in the first trimester. Neurologic symptoms included neuropathy involving the feet, intermittent paresthesia of the legs, and a history of chronic migraine headaches for several months.
Dermatologic examination revealed a slightly overweight woman with a 25×30-cm dusky, erythematous, irregular, netlike pattern on the left side of the upper and lower trunk (Figure 1). Extensive livedo racemosa was not altered by changes in temperature and had been unchanged for more than 9 months. There were no signs of pruritus or ulcerations, and areas of livedo racemosa were slightly tender to palpation.

We performed 2 sets of three 4-mm biopsies. The first set targeted areas within the violaceous pattern, while the second set targeted areas of normal tissue between the mottled areas. All 6 specimens demonstrated superficial perivascular lymphocytic infiltrate with no evidence of vasculitis or connective tissue disease. The vessels showed no microthrombi or surrounding fibrosis. No eosinophils were identified within the epidermis. There was no evidence of increased dermal mucin. Both the superficial and deep vascular plexuses were unremarkable and showed no evidence of damage to the walls (Figure 2).

To rule out other possible causes of livedo racemosa, complete blood cell count, comprehensive metabolic panel, coagulation profile, lipase test, urinalysis, serologic testing, and immunologic workup were performed. Lipase was within reference range. The complete blood cell count revealed mild anemia, while the rest of the values were within reference range. An immunologic workup included Sjögren syndrome antigen A, Sjögren syndrome antigen B, anticardiolipin antibodies, and antinuclear antibody, which were all negative. Family history was remarkable for first-degree relatives with systemic lupus erythematosus and Crohn disease.
Computed tomography revealed enlargement of the spleen, as well as periaortic, portacaval, and porta hepatis lymphadenopathy. Based on the laboratory findings and clinical presentation as well as the patient’s medical history, the diagnosis of exclusion was idiopathic livedo racemosa with unknown progression to full-blown SS. The patient did not meet the current diagnostic criteria for SS, and her immunologic studies failed to confirm any present antibodies, but involvement of the reticuloendothelial system pointed to production of antibodies that were not yet detectable on laboratory testing.
Comment
More than 50 years after the first case of SS was diagnosed, better laboratory workup is available and more information is known about the pathophysiology. Sneddon syndrome is a rare disorder, affecting only approximately 4 patients per million each year worldwide. Seronegative antiphospholipid antibody syndrome (SNAPS) describes patients with clinical presentations of antiphospholipid syndrome (APS) without detectable serological markers.7 Antiphospholipid-negative SS, which was seen in our patient, would be categorized under SNAPS. A PubMed search of articles indexed for MEDLINE using the terms livedo racemosa, Sneddon syndrome, and SNAPS and splenomegaly revealed there currently are no known cases of SNAPS that have been reported with splenomegaly and lymphadenopathy. Our patient presented with the following clinical features of SS: livedo racemosa, history of miscarriage, psychiatric disturbances, and hypertension. Surprisingly, biopsies from affected skin did not show any fibrin deposition or microthrombi but did reveal perivascular lymphocytic infiltrations. Magnetic resonance imaging did not show any pathological lesions or vascular changes.
Sneddon syndrome and APS share a common pathway to occlusive arteriolopathy for which 4 stages have been described by Zelger et al.5 Stage I involves a nonspecific Langerhans cell infiltrate with polymorphonuclear leukocytes. The tunica media and elastic lamina usually are unaltered at this early stage, while the surrounding connective tissue may appear edematous.5 This early stage of histopathology has not been evaluated in SS patients, primarily because of delay of diagnosis. Late stages III and IV will show fibrin deposition and shrinkage of affected vessels.7
A PubMed search using the terms Sneddon syndrome, lymphadenopathy and livedo racemosa, and Sneddon syndrome and lymphadenopathy revealed that splenomegaly and lymphadenopathy have not been reported in patients with SS. In patients with antiphospholipid-negative SS, one can assume that antibodies to other phospholipids not tested must exist because of striking similarities between APS and antiphospholipid-negative SS.8 Although our patient did not test positive for any of these antibodies, she did present with lymphadenopathy and splenic enlargement, leading us to believe that involvement of the reticuloendothelial system may be a feature of SS that has not been previously reported. Further studies are required to name specific antigens responsible for clinical manifestations in SS.
Currently, no single diagnostic test for SS exists, thus delaying both diagnosis and initiation of treatment. Histopathologic examination may be helpful, but in many cases it is nonspecific, as are serologic markers. Neuroradiological confirmation of involvement usually is the confirmatory feature in many patients with late-stage diagnosis.2 A diagnostic schematic for SS, which was first described by Daoud et al,2 illustrates classification of symptoms and aids in diagnosis. A working diagnosis of idiopathic livedo racemosa is made after ruling out other causes of SS in a patient with nonspecific biopsy findings and negative magnetic resonance imaging results with prodromal symptoms. The prognosis for such patients progressing to full SS is unknown with or without management using anticoagulant therapy.
Conclusion
Early diagnosis of livedo racemosa and SS is essential, as prevention of cerebrovascular accidents, myocardial infarction, and other thromboembolic diseases can be minimized by attacking risk factors such as smoking, taking oral contraceptive pills, becoming pregnant,9 and by initiating either antiplatelet or anticoagulation treatments. These treatments have been shown to delay the development of neurovascular damage and early-onset dementia. We present this case to demonstrate the variability of early-presenting symptoms in idiopathic livedo racemosa. Recognizing some of the early manifestations can lead to early diagnosis and initiation of treatment.
- Sneddon IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Daoud MS, Wilmoth GJ, Su WP, et al. Sneddon syndrome. Semin Dermatol. 1995;14:166-172.
- Besnier R, Francès C, Ankri A, et al. Factor V Leiden mutation in Sneddon syndrome. Lupus. 2003;12:406-408.
- K aragülle AT, Karadağ D, Erden A, et al. Sneddon’s syndrome: MR imaging findings. Eur Radiol. 2002;12:144-146.
- Zelg er B, Sepp N, Schmid KW, et al. Life-history of cutaneous vascular-lesions in Sneddon’s syndrome. Hum Pathol. 1992;23:668-675.
- Ayoub N, Esposito G, Barete S, et al. Protein Z deficiency in antiphospholipid-negative Sneddon’s syndrome. Stroke. 2004;35:1329-1332.
- Duva l A, Darnige L, Glowacki F, et al. Livedo, dementia, thrombocytopenia, and endotheliitis without antiphospholipid antibodies: seronegative antiphospholipid-like syndrome. J Am Acad Dermatol. 2009;61:1076-1078.
- Kala shnikova LA, Nasonov EL, Kushekbaeva AE, et al. Anticardiolipin antibodies in Sneddon’s syndrome. Neurology. 1990;40:464-467.
- Wohl rab J, Fischer M, Wolter M, et al. Diagnostic impact and sensitivity of skin biopsies in Sneddon’s syndrome. a report of 15 cases. Br J Dermatol. 2001;145:285-288.
- Sneddon IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180-185.
- Daoud MS, Wilmoth GJ, Su WP, et al. Sneddon syndrome. Semin Dermatol. 1995;14:166-172.
- Besnier R, Francès C, Ankri A, et al. Factor V Leiden mutation in Sneddon syndrome. Lupus. 2003;12:406-408.
- K aragülle AT, Karadağ D, Erden A, et al. Sneddon’s syndrome: MR imaging findings. Eur Radiol. 2002;12:144-146.
- Zelg er B, Sepp N, Schmid KW, et al. Life-history of cutaneous vascular-lesions in Sneddon’s syndrome. Hum Pathol. 1992;23:668-675.
- Ayoub N, Esposito G, Barete S, et al. Protein Z deficiency in antiphospholipid-negative Sneddon’s syndrome. Stroke. 2004;35:1329-1332.
- Duva l A, Darnige L, Glowacki F, et al. Livedo, dementia, thrombocytopenia, and endotheliitis without antiphospholipid antibodies: seronegative antiphospholipid-like syndrome. J Am Acad Dermatol. 2009;61:1076-1078.
- Kala shnikova LA, Nasonov EL, Kushekbaeva AE, et al. Anticardiolipin antibodies in Sneddon’s syndrome. Neurology. 1990;40:464-467.
- Wohl rab J, Fischer M, Wolter M, et al. Diagnostic impact and sensitivity of skin biopsies in Sneddon’s syndrome. a report of 15 cases. Br J Dermatol. 2001;145:285-288.
Practice Points
- The classic physical diagnostic finding of Sneddon syndrome (SS) is livedo racemosa.
- Early identification and treatment of SS can prevent serious morbidity due to stroke, myocardial infarction, and other thrombotic events.
- Preventive care in SS should include antiplatelet therapy or anticoagulants and smoking cessation along with avoidance of birth control pills.
Nevus Spilus: Is the Presence of Hair Associated With an Increased Risk for Melanoma?
The term nevus spilus (NS), also known as speckled lentiginous nevus, was first used in the 19th century to describe lesions with background café au lait–like lentiginous melanocytic hyperplasia speckled with small, 1- to 3-mm, darker foci. The dark spots reflect lentigines; junctional, compound, and intradermal nevus cell nests; and more rarely Spitz and blue nevi. Both macular and papular subtypes have been described.1 This birthmark is quite common, occurring in 1.3% to 2.3% of the adult population worldwide.2 Hypertrichosis has been described in NS.3-9 Two subsequent cases of malignant melanoma in hairy NS suggested that lesions may be particularly prone to malignant degeneration.4,8 We report an additional case of hairy NS that was not associated with melanoma and consider whether dermatologists should warn their patients about this association.
Case Report
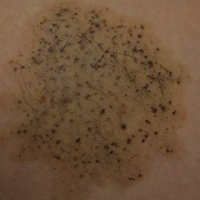
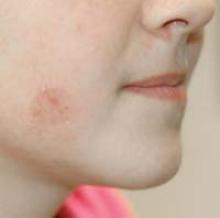
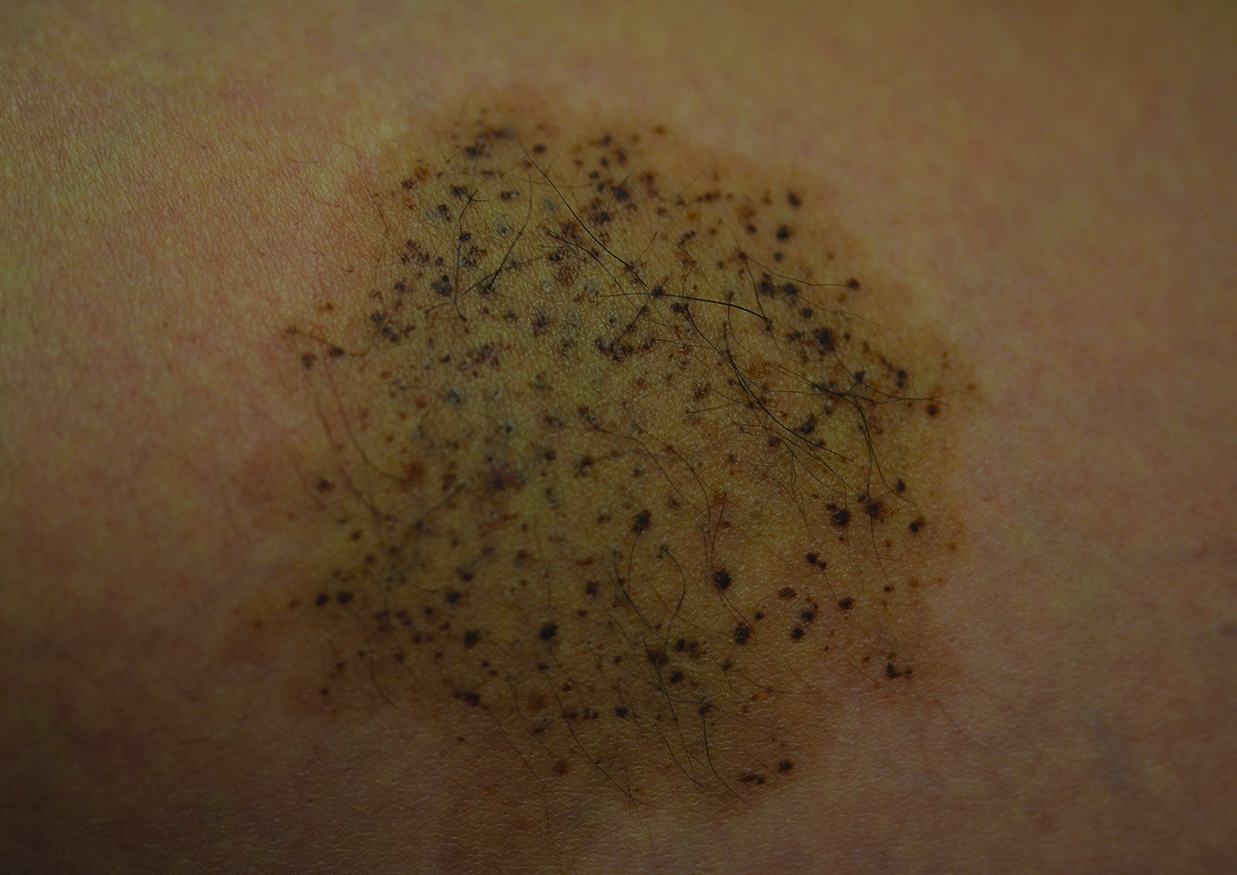
A 26-year-old woman presented with a stable 7×8-cm, tan-brown, macular, pigmented birthmark studded with darker 1- to 2-mm, irregular, brown-black and blue, confettilike macules on the left proximal lateral thigh that had been present since birth (Figure 1). Dark terminal hairs were present, arising from both the darker and lighter pigmented areas but not the surrounding normal skin.
A 4-mm punch biopsy from one of the dark blue macules demonstrated uniform lentiginous melanocytic hyperplasia and nevus cell nests adjacent to the sweat glands extending into the mid dermis (Figure 2). No clinical evidence of malignant degeneration was present.
Comment
The risk for melanoma is increased in classic nonspeckled congenital nevi and the risk correlates with the size of the lesion and most probably the number of nevus cells in the lesion that increase the risk for a random mutation.8,10,11 It is likely that NS with or without hair presages a small increased risk for melanoma,6,9,12 which is not surprising because NS is a subtype of congenital melanocytic nevus (CMN), a condition that is present at birth and results from a proliferation of melanocytes.6 Nevus spilus, however, appears to have a notably lower risk for malignant degeneration than other classic CMN of the same size. The following support for this hypothesis is offered: First, CMN have nevus cells broadly filling the dermis that extend more deeply into the dermis than NS (Figure 2A).10 In our estimation, CMN have at least 100 times the number of nevus cells per square centimeter compared to NS. The potential for malignant degeneration of any one melanocyte is greater when more are present. Second, although some NS lesions evolve, classic CMN are universally more proliferative than NS.10,13 The involved skin in CMN thickens over time with increased numbers of melanocytes and marked overgrowth of adjacent tissue. Melanocytes in a proliferative phase may be more likely to undergo malignant degeneration.10
A PubMed search of articles indexed for MEDLINE using the search term nevus spilus and melanoma yielded 2 cases4,8 of melanoma arising among 15 cases of hairy NS in the literature, which led to the suggestion that the presence of hair could be associated with an increased risk for malignant degeneration in NS (Table). This apparent high incidence of melanoma most likely reflects referral/publication bias rather than a statistically significant association. In fact, the clinical lesion most clinically similar to hairy NS is Becker nevus, with tan macules demonstrating lentiginous melanocytic hyperplasia associated with numerous coarse terminal hairs. There is no indication that Becker nevi have a considerable premalignant potential, though one case of melanoma arising in a Becker nevus has been reported.9 There is no evidence to suggest that classic CMN with hypertrichosis has a greater premalignant potential than similar lesions without hypertrichosis.

We noticed the presence of hair in our patient’s lesion only after reports in the literature caused us to look for this phenomenon.9 This occurrence may actually be quite common. We do not recommend prophylactic excision of NS and believe the risk for malignant degeneration is low in NS with or without hair, though larger NS (>4 cm), especially giant, zosteriform, or segmental lesions, may have a greater risk.1,6,9,10 It is prudent for physicians to carefully examine NS and sample suspicious foci, especially when patients describe a lesion as changing.
- Vidaurri-de la Cruz H, Happle R. Two distinct types of speckled lentiginous nevi characterized by macular versus papular speckles. Dermatology. 2006;212:53-58.
- Ly L, Christie M, Swain S, et al. Melanoma(s) arising in large segmental speckled lentiginous nevi: a case series. J Am Acad Dermatol. 2011;64:1190-1193.
- Prose NS, Heilman E, Felman YM, et al. Multiple benign juvenile melanoma. J Am Acad Dermatol. 1983;9:236-242.
- Grinspan D, Casala A, Abulafia J, et al. Melanoma on dysplastic nevus spilus. Int J Dermatol. 1997;36:499-502 .
- Langenbach N, Pfau A, Landthaler M, et al. Naevi spili, café-au-lait spots and melanocytic naevi aggregated alongside Blaschko’s lines, with a review of segmental melanocytic lesions. Acta Derm Venereol. 1998;78:378-380.
- Schaffer JV, Orlow SJ, Lazova R, et al. Speckled lentiginous nevus: within the spectrum of congenital melanocytic nevi. Arch Dermatol. 2001;137:172-178.
- Saraswat A, Dogra S, Bansali A, et al. Phakomatosis pigmentokeratotica associated with hypophosphataemic vitamin D–resistant rickets: improvement in phosphate homeostasis after partial laser ablation. Br J Dermatol. 2003;148:1074-1076.
- Zeren-Bilgin
i , Gür S, Aydın O, et al. Melanoma arising in a hairy nevus spilus. Int J Dermatol. 2006;45:1362-1364. - Singh S, Jain N, Khanna N, et al. Hairy nevus spilus: a case series. Pediatr Dermatol. 2013;30:100-104.
- Price HN, Schaffer JV. Congenital melanocytic nevi—when to worry and how to treat: facts and controversies. Clin Dermatol. 2010;28:293-302.
- Alikhan Ali, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? J Am Acad Dermatol. 2012;67:495.e1-495.e17.
- Haenssle HA, Kaune KM, Buhl T, et al. Melanoma arising in segmental nevus spilus: detection by sequential digital dermatoscopy. J Am Acad Dermatol. 2009;61:337-341.
- Cohen LM. Nevus spilus: congenital or acquired? Arch Dermatol. 2001;137:215-216.
The term nevus spilus (NS), also known as speckled lentiginous nevus, was first used in the 19th century to describe lesions with background café au lait–like lentiginous melanocytic hyperplasia speckled with small, 1- to 3-mm, darker foci. The dark spots reflect lentigines; junctional, compound, and intradermal nevus cell nests; and more rarely Spitz and blue nevi. Both macular and papular subtypes have been described.1 This birthmark is quite common, occurring in 1.3% to 2.3% of the adult population worldwide.2 Hypertrichosis has been described in NS.3-9 Two subsequent cases of malignant melanoma in hairy NS suggested that lesions may be particularly prone to malignant degeneration.4,8 We report an additional case of hairy NS that was not associated with melanoma and consider whether dermatologists should warn their patients about this association.
Case Report
A 26-year-old woman presented with a stable 7×8-cm, tan-brown, macular, pigmented birthmark studded with darker 1- to 2-mm, irregular, brown-black and blue, confettilike macules on the left proximal lateral thigh that had been present since birth (Figure 1). Dark terminal hairs were present, arising from both the darker and lighter pigmented areas but not the surrounding normal skin.

A 4-mm punch biopsy from one of the dark blue macules demonstrated uniform lentiginous melanocytic hyperplasia and nevus cell nests adjacent to the sweat glands extending into the mid dermis (Figure 2). No clinical evidence of malignant degeneration was present.

Comment
The risk for melanoma is increased in classic nonspeckled congenital nevi and the risk correlates with the size of the lesion and most probably the number of nevus cells in the lesion that increase the risk for a random mutation.8,10,11 It is likely that NS with or without hair presages a small increased risk for melanoma,6,9,12 which is not surprising because NS is a subtype of congenital melanocytic nevus (CMN), a condition that is present at birth and results from a proliferation of melanocytes.6 Nevus spilus, however, appears to have a notably lower risk for malignant degeneration than other classic CMN of the same size. The following support for this hypothesis is offered: First, CMN have nevus cells broadly filling the dermis that extend more deeply into the dermis than NS (Figure 2A).10 In our estimation, CMN have at least 100 times the number of nevus cells per square centimeter compared to NS. The potential for malignant degeneration of any one melanocyte is greater when more are present. Second, although some NS lesions evolve, classic CMN are universally more proliferative than NS.10,13 The involved skin in CMN thickens over time with increased numbers of melanocytes and marked overgrowth of adjacent tissue. Melanocytes in a proliferative phase may be more likely to undergo malignant degeneration.10
A PubMed search of articles indexed for MEDLINE using the search term nevus spilus and melanoma yielded 2 cases4,8 of melanoma arising among 15 cases of hairy NS in the literature, which led to the suggestion that the presence of hair could be associated with an increased risk for malignant degeneration in NS (Table). This apparent high incidence of melanoma most likely reflects referral/publication bias rather than a statistically significant association. In fact, the clinical lesion most clinically similar to hairy NS is Becker nevus, with tan macules demonstrating lentiginous melanocytic hyperplasia associated with numerous coarse terminal hairs. There is no indication that Becker nevi have a considerable premalignant potential, though one case of melanoma arising in a Becker nevus has been reported.9 There is no evidence to suggest that classic CMN with hypertrichosis has a greater premalignant potential than similar lesions without hypertrichosis.

We noticed the presence of hair in our patient’s lesion only after reports in the literature caused us to look for this phenomenon.9 This occurrence may actually be quite common. We do not recommend prophylactic excision of NS and believe the risk for malignant degeneration is low in NS with or without hair, though larger NS (>4 cm), especially giant, zosteriform, or segmental lesions, may have a greater risk.1,6,9,10 It is prudent for physicians to carefully examine NS and sample suspicious foci, especially when patients describe a lesion as changing.
The term nevus spilus (NS), also known as speckled lentiginous nevus, was first used in the 19th century to describe lesions with background café au lait–like lentiginous melanocytic hyperplasia speckled with small, 1- to 3-mm, darker foci. The dark spots reflect lentigines; junctional, compound, and intradermal nevus cell nests; and more rarely Spitz and blue nevi. Both macular and papular subtypes have been described.1 This birthmark is quite common, occurring in 1.3% to 2.3% of the adult population worldwide.2 Hypertrichosis has been described in NS.3-9 Two subsequent cases of malignant melanoma in hairy NS suggested that lesions may be particularly prone to malignant degeneration.4,8 We report an additional case of hairy NS that was not associated with melanoma and consider whether dermatologists should warn their patients about this association.
Case Report
A 26-year-old woman presented with a stable 7×8-cm, tan-brown, macular, pigmented birthmark studded with darker 1- to 2-mm, irregular, brown-black and blue, confettilike macules on the left proximal lateral thigh that had been present since birth (Figure 1). Dark terminal hairs were present, arising from both the darker and lighter pigmented areas but not the surrounding normal skin.

A 4-mm punch biopsy from one of the dark blue macules demonstrated uniform lentiginous melanocytic hyperplasia and nevus cell nests adjacent to the sweat glands extending into the mid dermis (Figure 2). No clinical evidence of malignant degeneration was present.

Comment
The risk for melanoma is increased in classic nonspeckled congenital nevi and the risk correlates with the size of the lesion and most probably the number of nevus cells in the lesion that increase the risk for a random mutation.8,10,11 It is likely that NS with or without hair presages a small increased risk for melanoma,6,9,12 which is not surprising because NS is a subtype of congenital melanocytic nevus (CMN), a condition that is present at birth and results from a proliferation of melanocytes.6 Nevus spilus, however, appears to have a notably lower risk for malignant degeneration than other classic CMN of the same size. The following support for this hypothesis is offered: First, CMN have nevus cells broadly filling the dermis that extend more deeply into the dermis than NS (Figure 2A).10 In our estimation, CMN have at least 100 times the number of nevus cells per square centimeter compared to NS. The potential for malignant degeneration of any one melanocyte is greater when more are present. Second, although some NS lesions evolve, classic CMN are universally more proliferative than NS.10,13 The involved skin in CMN thickens over time with increased numbers of melanocytes and marked overgrowth of adjacent tissue. Melanocytes in a proliferative phase may be more likely to undergo malignant degeneration.10
A PubMed search of articles indexed for MEDLINE using the search term nevus spilus and melanoma yielded 2 cases4,8 of melanoma arising among 15 cases of hairy NS in the literature, which led to the suggestion that the presence of hair could be associated with an increased risk for malignant degeneration in NS (Table). This apparent high incidence of melanoma most likely reflects referral/publication bias rather than a statistically significant association. In fact, the clinical lesion most clinically similar to hairy NS is Becker nevus, with tan macules demonstrating lentiginous melanocytic hyperplasia associated with numerous coarse terminal hairs. There is no indication that Becker nevi have a considerable premalignant potential, though one case of melanoma arising in a Becker nevus has been reported.9 There is no evidence to suggest that classic CMN with hypertrichosis has a greater premalignant potential than similar lesions without hypertrichosis.

We noticed the presence of hair in our patient’s lesion only after reports in the literature caused us to look for this phenomenon.9 This occurrence may actually be quite common. We do not recommend prophylactic excision of NS and believe the risk for malignant degeneration is low in NS with or without hair, though larger NS (>4 cm), especially giant, zosteriform, or segmental lesions, may have a greater risk.1,6,9,10 It is prudent for physicians to carefully examine NS and sample suspicious foci, especially when patients describe a lesion as changing.
- Vidaurri-de la Cruz H, Happle R. Two distinct types of speckled lentiginous nevi characterized by macular versus papular speckles. Dermatology. 2006;212:53-58.
- Ly L, Christie M, Swain S, et al. Melanoma(s) arising in large segmental speckled lentiginous nevi: a case series. J Am Acad Dermatol. 2011;64:1190-1193.
- Prose NS, Heilman E, Felman YM, et al. Multiple benign juvenile melanoma. J Am Acad Dermatol. 1983;9:236-242.
- Grinspan D, Casala A, Abulafia J, et al. Melanoma on dysplastic nevus spilus. Int J Dermatol. 1997;36:499-502 .
- Langenbach N, Pfau A, Landthaler M, et al. Naevi spili, café-au-lait spots and melanocytic naevi aggregated alongside Blaschko’s lines, with a review of segmental melanocytic lesions. Acta Derm Venereol. 1998;78:378-380.
- Schaffer JV, Orlow SJ, Lazova R, et al. Speckled lentiginous nevus: within the spectrum of congenital melanocytic nevi. Arch Dermatol. 2001;137:172-178.
- Saraswat A, Dogra S, Bansali A, et al. Phakomatosis pigmentokeratotica associated with hypophosphataemic vitamin D–resistant rickets: improvement in phosphate homeostasis after partial laser ablation. Br J Dermatol. 2003;148:1074-1076.
- Zeren-Bilgin
i , Gür S, Aydın O, et al. Melanoma arising in a hairy nevus spilus. Int J Dermatol. 2006;45:1362-1364. - Singh S, Jain N, Khanna N, et al. Hairy nevus spilus: a case series. Pediatr Dermatol. 2013;30:100-104.
- Price HN, Schaffer JV. Congenital melanocytic nevi—when to worry and how to treat: facts and controversies. Clin Dermatol. 2010;28:293-302.
- Alikhan Ali, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? J Am Acad Dermatol. 2012;67:495.e1-495.e17.
- Haenssle HA, Kaune KM, Buhl T, et al. Melanoma arising in segmental nevus spilus: detection by sequential digital dermatoscopy. J Am Acad Dermatol. 2009;61:337-341.
- Cohen LM. Nevus spilus: congenital or acquired? Arch Dermatol. 2001;137:215-216.
- Vidaurri-de la Cruz H, Happle R. Two distinct types of speckled lentiginous nevi characterized by macular versus papular speckles. Dermatology. 2006;212:53-58.
- Ly L, Christie M, Swain S, et al. Melanoma(s) arising in large segmental speckled lentiginous nevi: a case series. J Am Acad Dermatol. 2011;64:1190-1193.
- Prose NS, Heilman E, Felman YM, et al. Multiple benign juvenile melanoma. J Am Acad Dermatol. 1983;9:236-242.
- Grinspan D, Casala A, Abulafia J, et al. Melanoma on dysplastic nevus spilus. Int J Dermatol. 1997;36:499-502 .
- Langenbach N, Pfau A, Landthaler M, et al. Naevi spili, café-au-lait spots and melanocytic naevi aggregated alongside Blaschko’s lines, with a review of segmental melanocytic lesions. Acta Derm Venereol. 1998;78:378-380.
- Schaffer JV, Orlow SJ, Lazova R, et al. Speckled lentiginous nevus: within the spectrum of congenital melanocytic nevi. Arch Dermatol. 2001;137:172-178.
- Saraswat A, Dogra S, Bansali A, et al. Phakomatosis pigmentokeratotica associated with hypophosphataemic vitamin D–resistant rickets: improvement in phosphate homeostasis after partial laser ablation. Br J Dermatol. 2003;148:1074-1076.
- Zeren-Bilgin
i , Gür S, Aydın O, et al. Melanoma arising in a hairy nevus spilus. Int J Dermatol. 2006;45:1362-1364. - Singh S, Jain N, Khanna N, et al. Hairy nevus spilus: a case series. Pediatr Dermatol. 2013;30:100-104.
- Price HN, Schaffer JV. Congenital melanocytic nevi—when to worry and how to treat: facts and controversies. Clin Dermatol. 2010;28:293-302.
- Alikhan Ali, Ibrahimi OA, Eisen DB. Congenital melanocytic nevi: where are we now? J Am Acad Dermatol. 2012;67:495.e1-495.e17.
- Haenssle HA, Kaune KM, Buhl T, et al. Melanoma arising in segmental nevus spilus: detection by sequential digital dermatoscopy. J Am Acad Dermatol. 2009;61:337-341.
- Cohen LM. Nevus spilus: congenital or acquired? Arch Dermatol. 2001;137:215-216.
Practice Points
- Nevus spilus (NS) appears as a café au lait macule studded with darker brown “moles.”
- Although melanoma has been described in NS, it is rare.
- There is no evidence that hairy NS are predisposed to melanoma.
Diagnosing Porokeratosis of Mibelli Every Time: A Novel Biopsy Technique to Maximize Histopathologic Confirmation
Porokeratosis of Mibelli (PM) is a lesion characterized by a surrounding cornoid lamella with variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) in the center of the lesion that typically presents in infancy to early childhood.1 We report a case of PM in which a prior biopsy from the center of the lesion demonstrated papulosquamous dermatitis. We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM.
Case Report
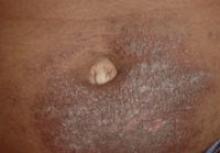
A 3-year-old girl presented with an erythematous, hypopigmented, scaling plaque on the posterior aspect of the left ankle surrounded by a hard rim. The plaque was first noted at 12 months of age and had slowly enlarged as the patient grew. Six months prior, a biopsy from the center of the lesion performed at another facility demonstrated a papulosquamous dermatitis.
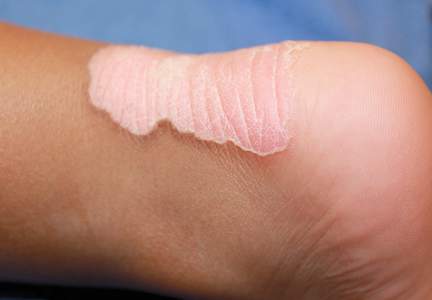
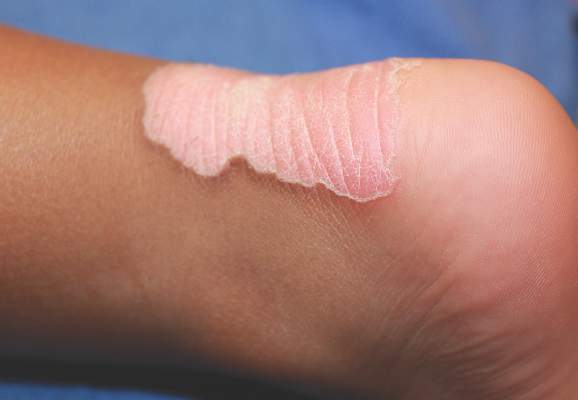
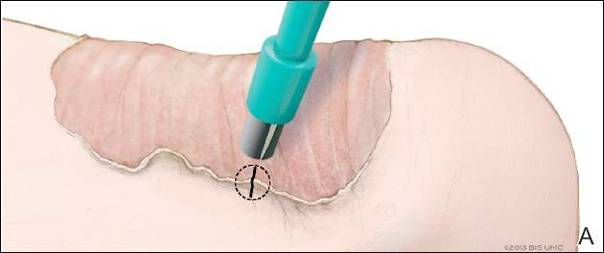
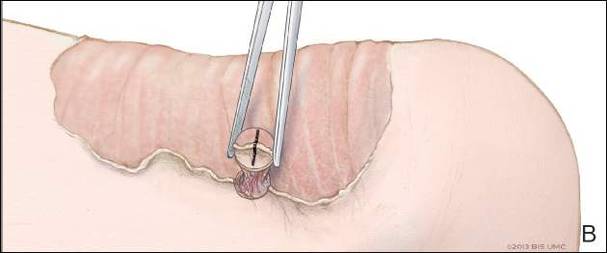
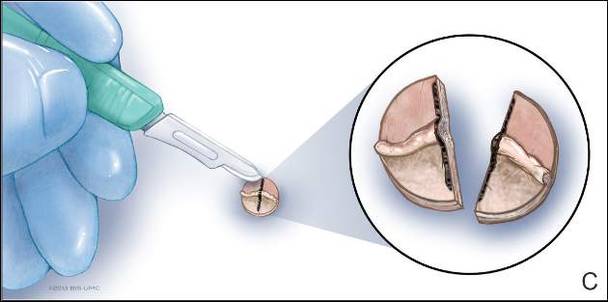
Physical examination revealed a lesion that was 4.2-cm long, 2.2-cm wide at the superior pole, and 3.5-cm wide at the inferior pole (Figure 1). A line was drawn with a skin marker perpendicular to the rim of the lesion (Figure 2A) and a 6-mm punch biopsy was performed, centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). The tissue was then bisected at the bedside along the skin marker line with a #15 blade (Figure 2C) and submitted in formalin for histologic processing. Histologic examination revealed an invagination of the epidermis producing a tier of parakeratotic cells with its apex pointed away from the center of the lesion. Dyskeratotic cells were noted at the base of the parakeratosis (Figure 3). Verrucous hyperplasia was present in the central portion of the specimen adjacent to the cornoid lamella. Based on these histopathologic findings, the correct diagnosis of PM was made.
Comment
Porokeratosis of Mibelli is a rare condition that typically presents in infancy to early childhood.1 It may appear as small keratotic papules or larger plaques that reach several centimeters in diameter.2 There is a 7.5% risk for malignant transformation (eg, basal cell carcinoma, squamous cell carcinoma, Bowen disease).3 Variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) typically are present in the center of the lesion. In our case, a biopsy from the center of the plaque demonstrated verrucous hyperplasia. The incorrect diagnosis of PM as psoriasis also has been reported.4
We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM. First, draw a line perpendicular to the rim of the lesion to mark the biopsy site (Figure 2A). Second, perform a punch biopsy centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). Third, section the biopsied tissue with a #15 blade along the perpendicular line at the bedside (Figure 2C). The surgical pathology requisition should mention that the specimen has been transected and the cut edges should be placed down in the cassette, ensuring that the cornoid lamella will be present in cross-section on the slides.
If the punch biopsy specimen is not bisected, it can be difficult to orient it in the pathology laboratory, especially if the cornoid lamellae are not prominent. Furthermore, the technician processing the tissue may not be aware of the importance of sectioning the specimen perpendicular to the cornoid lamella. Following this procedure, diagnosis can be confirmed in virtually every case of PM.
- Richard G, Irvine A, Traupe H, et al. Ichthyosis and disorders of other conification. In: Schachner L, Hansen R, Krafchik B, et al, eds. Pediatric Dermatology. Philadelphia, PA: Elsevier Health Sciences; 2011:640-643.
- Pierson D, Bandel C, Ehrig, et al. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Rapini R, et al, eds. Dermatology. 1st ed. Vol 2. Edinburgh, Scotland: Elsevier; 2003:1707-1709.
- Cort DF, Abdel-Aziz AH. Epithelioma arising in porokeratosis of Mibelli. Br J Plast Surg. 1972;25:318-328.
- De Simone C, Paradisi A, Massi G, et al. Giant verrucous porokeratosis of Mibelli mimicking psoriasis in a patient with psoriasis. J Am Acad Dermatol. 2007;57:665-668.
Porokeratosis of Mibelli (PM) is a lesion characterized by a surrounding cornoid lamella with variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) in the center of the lesion that typically presents in infancy to early childhood.1 We report a case of PM in which a prior biopsy from the center of the lesion demonstrated papulosquamous dermatitis. We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM.
Case Report
A 3-year-old girl presented with an erythematous, hypopigmented, scaling plaque on the posterior aspect of the left ankle surrounded by a hard rim. The plaque was first noted at 12 months of age and had slowly enlarged as the patient grew. Six months prior, a biopsy from the center of the lesion performed at another facility demonstrated a papulosquamous dermatitis.
Physical examination revealed a lesion that was 4.2-cm long, 2.2-cm wide at the superior pole, and 3.5-cm wide at the inferior pole (Figure 1). A line was drawn with a skin marker perpendicular to the rim of the lesion (Figure 2A) and a 6-mm punch biopsy was performed, centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). The tissue was then bisected at the bedside along the skin marker line with a #15 blade (Figure 2C) and submitted in formalin for histologic processing. Histologic examination revealed an invagination of the epidermis producing a tier of parakeratotic cells with its apex pointed away from the center of the lesion. Dyskeratotic cells were noted at the base of the parakeratosis (Figure 3). Verrucous hyperplasia was present in the central portion of the specimen adjacent to the cornoid lamella. Based on these histopathologic findings, the correct diagnosis of PM was made.






Comment
Porokeratosis of Mibelli is a rare condition that typically presents in infancy to early childhood.1 It may appear as small keratotic papules or larger plaques that reach several centimeters in diameter.2 There is a 7.5% risk for malignant transformation (eg, basal cell carcinoma, squamous cell carcinoma, Bowen disease).3 Variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) typically are present in the center of the lesion. In our case, a biopsy from the center of the plaque demonstrated verrucous hyperplasia. The incorrect diagnosis of PM as psoriasis also has been reported.4
We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM. First, draw a line perpendicular to the rim of the lesion to mark the biopsy site (Figure 2A). Second, perform a punch biopsy centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). Third, section the biopsied tissue with a #15 blade along the perpendicular line at the bedside (Figure 2C). The surgical pathology requisition should mention that the specimen has been transected and the cut edges should be placed down in the cassette, ensuring that the cornoid lamella will be present in cross-section on the slides.
If the punch biopsy specimen is not bisected, it can be difficult to orient it in the pathology laboratory, especially if the cornoid lamellae are not prominent. Furthermore, the technician processing the tissue may not be aware of the importance of sectioning the specimen perpendicular to the cornoid lamella. Following this procedure, diagnosis can be confirmed in virtually every case of PM.
Porokeratosis of Mibelli (PM) is a lesion characterized by a surrounding cornoid lamella with variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) in the center of the lesion that typically presents in infancy to early childhood.1 We report a case of PM in which a prior biopsy from the center of the lesion demonstrated papulosquamous dermatitis. We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM.
Case Report
A 3-year-old girl presented with an erythematous, hypopigmented, scaling plaque on the posterior aspect of the left ankle surrounded by a hard rim. The plaque was first noted at 12 months of age and had slowly enlarged as the patient grew. Six months prior, a biopsy from the center of the lesion performed at another facility demonstrated a papulosquamous dermatitis.
Physical examination revealed a lesion that was 4.2-cm long, 2.2-cm wide at the superior pole, and 3.5-cm wide at the inferior pole (Figure 1). A line was drawn with a skin marker perpendicular to the rim of the lesion (Figure 2A) and a 6-mm punch biopsy was performed, centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). The tissue was then bisected at the bedside along the skin marker line with a #15 blade (Figure 2C) and submitted in formalin for histologic processing. Histologic examination revealed an invagination of the epidermis producing a tier of parakeratotic cells with its apex pointed away from the center of the lesion. Dyskeratotic cells were noted at the base of the parakeratosis (Figure 3). Verrucous hyperplasia was present in the central portion of the specimen adjacent to the cornoid lamella. Based on these histopathologic findings, the correct diagnosis of PM was made.






Comment
Porokeratosis of Mibelli is a rare condition that typically presents in infancy to early childhood.1 It may appear as small keratotic papules or larger plaques that reach several centimeters in diameter.2 There is a 7.5% risk for malignant transformation (eg, basal cell carcinoma, squamous cell carcinoma, Bowen disease).3 Variable nonspecific findings (eg, atrophy, acanthosis, verrucous hyperplasia) typically are present in the center of the lesion. In our case, a biopsy from the center of the plaque demonstrated verrucous hyperplasia. The incorrect diagnosis of PM as psoriasis also has been reported.4
We propose a 3-step technique to ensure proper orientation of a punch biopsy in cases of suspected PM. First, draw a line perpendicular to the rim of the lesion to mark the biopsy site (Figure 2A). Second, perform a punch biopsy centered at the intersection of the drawn line and the cornoid lamella (Figure 2B). Third, section the biopsied tissue with a #15 blade along the perpendicular line at the bedside (Figure 2C). The surgical pathology requisition should mention that the specimen has been transected and the cut edges should be placed down in the cassette, ensuring that the cornoid lamella will be present in cross-section on the slides.
If the punch biopsy specimen is not bisected, it can be difficult to orient it in the pathology laboratory, especially if the cornoid lamellae are not prominent. Furthermore, the technician processing the tissue may not be aware of the importance of sectioning the specimen perpendicular to the cornoid lamella. Following this procedure, diagnosis can be confirmed in virtually every case of PM.
- Richard G, Irvine A, Traupe H, et al. Ichthyosis and disorders of other conification. In: Schachner L, Hansen R, Krafchik B, et al, eds. Pediatric Dermatology. Philadelphia, PA: Elsevier Health Sciences; 2011:640-643.
- Pierson D, Bandel C, Ehrig, et al. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Rapini R, et al, eds. Dermatology. 1st ed. Vol 2. Edinburgh, Scotland: Elsevier; 2003:1707-1709.
- Cort DF, Abdel-Aziz AH. Epithelioma arising in porokeratosis of Mibelli. Br J Plast Surg. 1972;25:318-328.
- De Simone C, Paradisi A, Massi G, et al. Giant verrucous porokeratosis of Mibelli mimicking psoriasis in a patient with psoriasis. J Am Acad Dermatol. 2007;57:665-668.
- Richard G, Irvine A, Traupe H, et al. Ichthyosis and disorders of other conification. In: Schachner L, Hansen R, Krafchik B, et al, eds. Pediatric Dermatology. Philadelphia, PA: Elsevier Health Sciences; 2011:640-643.
- Pierson D, Bandel C, Ehrig, et al. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Rapini R, et al, eds. Dermatology. 1st ed. Vol 2. Edinburgh, Scotland: Elsevier; 2003:1707-1709.
- Cort DF, Abdel-Aziz AH. Epithelioma arising in porokeratosis of Mibelli. Br J Plast Surg. 1972;25:318-328.
- De Simone C, Paradisi A, Massi G, et al. Giant verrucous porokeratosis of Mibelli mimicking psoriasis in a patient with psoriasis. J Am Acad Dermatol. 2007;57:665-668.
Practice Points
- A biopsy from the center of a plaque of porokeratosis will produce nonspecific findings.
- Bisecting the punch specimen at the bedside along a line drawn perpendicular to the cornoid lamella guarantees proper orientation of the specimen.
The Clinical Learning Environment Review as a Model for Impactful Self-directed Quality Control Initiatives in Clinical Practice
As part of its Next Accreditation System, the Accreditation Council for Graduate Medical Education (ACGME) has introduced the Clinical Learning Environment Review (CLER) program, designed to assess the learning environment of institutions that have ACGME residency and fellowship programs.1 The CLER program emphasizes the responsibility of these hospitals, multispecialty groups, and other organizations to focus on quality and safety in the health care environment of resident learning and patient care. The expectation is that emphasis on quality of care in a residency training program will influence these physicians’ approach to quality of care after graduation.2,3 The Department of Dermatology at the University of Mississippi Medical Center (UMMC)(Jackson, Mississippi) saw CLER as an opportunity to demonstrate leadership in the patient safety movement.
CLER Program at UMMC
As a model CLER program at our institution, our project at the outset concentrated resident efforts on the focus areas specified by the ACGME (Table 1). We also were aware that our ACGME committee would need to answer questions during CLER site visits (Table 2). Because the data generated would not be used for accreditation decisions, there was no concern that exposing errors would jeopardize our postgraduate training certification.

The first 15 minutes of monthly faculty meetings were devoted to the presentation of a resident project, called a QA/QI (quality assurance/quality improvement) moment, that addressed ACGME focus areas 1, 2, 3, or 6 (Table 1). (Transitions in care [focus area 4] and work hours and fatigue [focus area 5] generally are less important issues in a predominantly outpatient specialty such as dermatology.) The residents were encouraged to identify areas where patient harm could occur due to poorly designed systems and to report situations in which patients actually were harmed.

Each project had to be approved by the department chairperson based on the following 4 requirements: First, the initiative must have the potential to notably impact patient safety and reduce harm. Second, residents with faculty support had to design methods to assess the identified problem. Third, participants had to design (to the best of their abilities) cost-effective and achievable interventions in a manner that would not produce unintended consequences. Fourth, residents were asked to devise a system to close the loop, ensuring that the effort put into the process was not wasted.
Findings From the CLER Program
The CLER program generates data on program and institutional attributes that have a salutatory effect on quality and safety, specifically involving 6 focus areas highlighted in Table 1. Putting residents at the center of efforts to improve the quality of care in our department proved critical to improving patient safety.
Involving residents in a series of QA/QI initiatives was logical because they rotate with faculty members. They also are in a position to view inconsistencies and to work to establish consistent patterns of patient care. In addition, our busy faculty members are charged with a variety of other clinical, educational, and administrative duties complicated by requirements in the design of a new residency training program. Faculty and residents working together were able to find problem areas in our department and devise solutions to improve those problems.
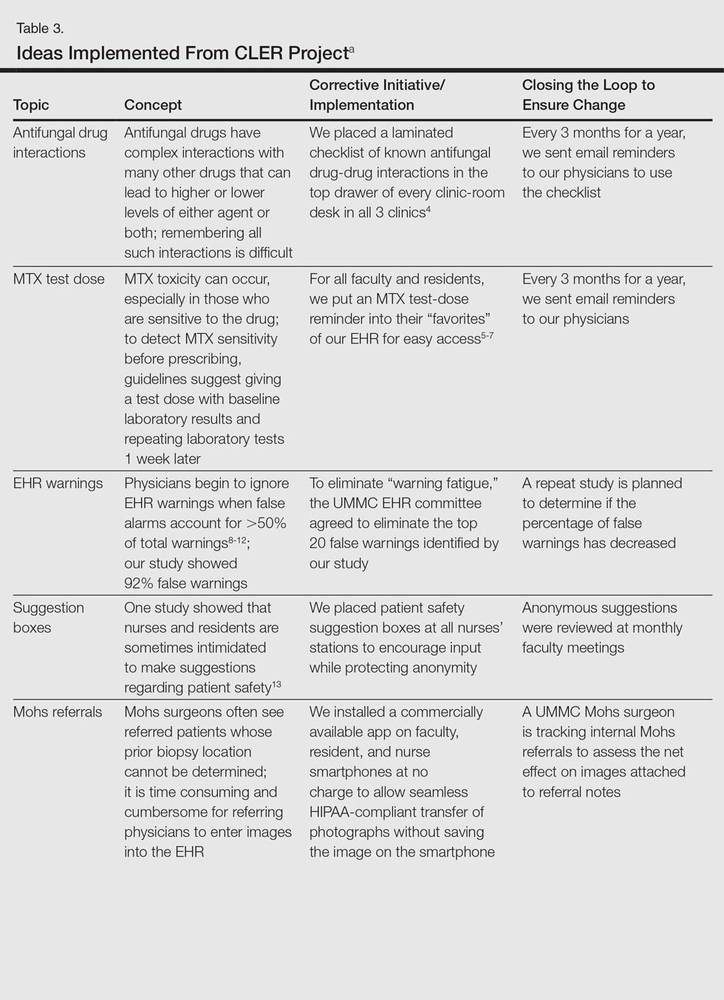
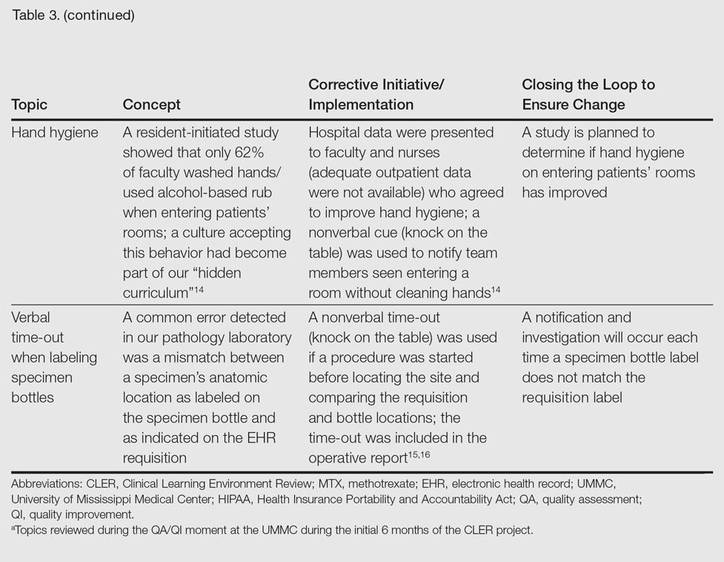
The CLER program involved a series of steps. Residents were charged with identifying errors (QA) and then devising a system to prevent similar errors from being repeated (QI)(Table 3). Efforts focused on preventing needless harm in our department. Initiatives developed by residents, who are closest to patients, have advantages over safety programs developed by the hospital’s administration. Residents became passionate about error prevention when they determined that their efforts could make a difference to patients.
Forward Thinking for Dermatology Practices
Perhaps there are lessons here that could apply to safety promotion in the practicing dermatologist’s office. The American Board of Dermatology, within the framework established by the American Board of Medical Specialties, requires physicians seeking recertification to participate in preapproved practice assessment QI exercises twice every 10 years.17 Six programs sponsored by the American Academy of Dermatology have now been approved in the areas of melanoma, biopsy follow-up measure, psoriasis, chronic urticaria, venous insufficiency, and laser- and light-based therapy for rejuvenation.18 An additional program has been approved for dermatopathologists through the American Society of Dermatopathology.19 None of these programs match the topics chosen by our residents in consultation with faculty to meet safety gaps identified in clinics at UMMC. Perhaps the next generation of performance improvement continuing medical education programs could include a pilot program for part 4 of Maintenance of Certification credit that is nonpunitive, patient focused, and allows dermatologists to design specific error-prevention solutions tailored to their individual practice in the same way residency programs are taking up this task.
- Nasca TJ, Philibert I, Brigham T, et al. The Next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Philibert I, Gonzalez del Rey JA, Lannon C, et al. Quality improvement skills for pediatric residents: from lecture to implementation and sustainability. Acad Pediatr. 2014;14:40-46.
- Vidyarthi AR, Green AL, Rosenbluth G, et al. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89:460-468.
- Brodell RT, Elewski B. Antifungal drug interactions. avoidance requires more than memorization. Postgrad Med. 2000;107:41-43.
- Kerr IG, Jolivet J, Collin JM, et al. Test dose for predicting high-dose methotrexate infusions. Clin Pharmacol Ther. 1983;33:44-51.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Saporito FC, Menter MA. Methotrexate and psoriasis in the era of new biologic agents. J Am Acad Dermatol. 2004;50:301-309.
- Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138-147.
- Hunter KM. Implementation of an electronic medication administration record and bedside verification system. Online J Nurs Inform (OJNI). 2011;15:672.
- Nanji KC, Slight SP, Seger DL, et al. Overrides of medication-related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21:487-491.
- Schedlbauer A, Prasad V, Mulvaney C, et al. What evidence supports the use of computerized alerts and prompts to improve clinicians’ prescribing behavior? J Am Med Inform Assoc. 2009;16:531-538.
- Lee EK, Mejia AF, Senior T, et al. Improving patient safety through medical alert management: an automated decision tool to reduce alert fatigue. AMIA Annu Symp Proc. 2010;2010:417-421.
- Brenner AB. Physician and nurse relationships, a key to patient safety. J Ky Med Assoc. 2007;105:165-169.
- Rush JL, Flowers RH, Casamiquela KM, et al. Research letter: the knock: an adjunct to education opening the door to improved outpatient hand hygiene. J Am Acad Dermatol. In press.
- Lee SL. The extended surgical time-out: does it improve quality and prevent wrong-site surgery? Perm J. 2010;14:19-23.
- Altpeter T, Luckhardt K, Lewis JN, et al. Expanded surgical time out: a key to real-time data collection and quality improvement. J Am Coll Surg. 2007;204:527-532.
- MOC requirements. American Board of Dermatology Web site. https://www.abderm.org/diplomates/fulfilling-moc-requirements/moc-requirements.aspx#PI. Accessed January 18, 2016.
- How AAD develops measures. American Academy of Dermatology Web site. https://www.aad.org/practice-tools/quality-care/quality-measures. Accessed January 20, 2016.
- Quality assurance programs. The American Society of Dermatopathology Web site. http://www.asdp.org/education/quality-assurance-programs. Accessed January 20, 2016.
As part of its Next Accreditation System, the Accreditation Council for Graduate Medical Education (ACGME) has introduced the Clinical Learning Environment Review (CLER) program, designed to assess the learning environment of institutions that have ACGME residency and fellowship programs.1 The CLER program emphasizes the responsibility of these hospitals, multispecialty groups, and other organizations to focus on quality and safety in the health care environment of resident learning and patient care. The expectation is that emphasis on quality of care in a residency training program will influence these physicians’ approach to quality of care after graduation.2,3 The Department of Dermatology at the University of Mississippi Medical Center (UMMC)(Jackson, Mississippi) saw CLER as an opportunity to demonstrate leadership in the patient safety movement.
CLER Program at UMMC
As a model CLER program at our institution, our project at the outset concentrated resident efforts on the focus areas specified by the ACGME (Table 1). We also were aware that our ACGME committee would need to answer questions during CLER site visits (Table 2). Because the data generated would not be used for accreditation decisions, there was no concern that exposing errors would jeopardize our postgraduate training certification.

The first 15 minutes of monthly faculty meetings were devoted to the presentation of a resident project, called a QA/QI (quality assurance/quality improvement) moment, that addressed ACGME focus areas 1, 2, 3, or 6 (Table 1). (Transitions in care [focus area 4] and work hours and fatigue [focus area 5] generally are less important issues in a predominantly outpatient specialty such as dermatology.) The residents were encouraged to identify areas where patient harm could occur due to poorly designed systems and to report situations in which patients actually were harmed.

Each project had to be approved by the department chairperson based on the following 4 requirements: First, the initiative must have the potential to notably impact patient safety and reduce harm. Second, residents with faculty support had to design methods to assess the identified problem. Third, participants had to design (to the best of their abilities) cost-effective and achievable interventions in a manner that would not produce unintended consequences. Fourth, residents were asked to devise a system to close the loop, ensuring that the effort put into the process was not wasted.
Findings From the CLER Program
The CLER program generates data on program and institutional attributes that have a salutatory effect on quality and safety, specifically involving 6 focus areas highlighted in Table 1. Putting residents at the center of efforts to improve the quality of care in our department proved critical to improving patient safety.
Involving residents in a series of QA/QI initiatives was logical because they rotate with faculty members. They also are in a position to view inconsistencies and to work to establish consistent patterns of patient care. In addition, our busy faculty members are charged with a variety of other clinical, educational, and administrative duties complicated by requirements in the design of a new residency training program. Faculty and residents working together were able to find problem areas in our department and devise solutions to improve those problems.
The CLER program involved a series of steps. Residents were charged with identifying errors (QA) and then devising a system to prevent similar errors from being repeated (QI)(Table 3). Efforts focused on preventing needless harm in our department. Initiatives developed by residents, who are closest to patients, have advantages over safety programs developed by the hospital’s administration. Residents became passionate about error prevention when they determined that their efforts could make a difference to patients.


Forward Thinking for Dermatology Practices
Perhaps there are lessons here that could apply to safety promotion in the practicing dermatologist’s office. The American Board of Dermatology, within the framework established by the American Board of Medical Specialties, requires physicians seeking recertification to participate in preapproved practice assessment QI exercises twice every 10 years.17 Six programs sponsored by the American Academy of Dermatology have now been approved in the areas of melanoma, biopsy follow-up measure, psoriasis, chronic urticaria, venous insufficiency, and laser- and light-based therapy for rejuvenation.18 An additional program has been approved for dermatopathologists through the American Society of Dermatopathology.19 None of these programs match the topics chosen by our residents in consultation with faculty to meet safety gaps identified in clinics at UMMC. Perhaps the next generation of performance improvement continuing medical education programs could include a pilot program for part 4 of Maintenance of Certification credit that is nonpunitive, patient focused, and allows dermatologists to design specific error-prevention solutions tailored to their individual practice in the same way residency programs are taking up this task.
As part of its Next Accreditation System, the Accreditation Council for Graduate Medical Education (ACGME) has introduced the Clinical Learning Environment Review (CLER) program, designed to assess the learning environment of institutions that have ACGME residency and fellowship programs.1 The CLER program emphasizes the responsibility of these hospitals, multispecialty groups, and other organizations to focus on quality and safety in the health care environment of resident learning and patient care. The expectation is that emphasis on quality of care in a residency training program will influence these physicians’ approach to quality of care after graduation.2,3 The Department of Dermatology at the University of Mississippi Medical Center (UMMC)(Jackson, Mississippi) saw CLER as an opportunity to demonstrate leadership in the patient safety movement.
CLER Program at UMMC
As a model CLER program at our institution, our project at the outset concentrated resident efforts on the focus areas specified by the ACGME (Table 1). We also were aware that our ACGME committee would need to answer questions during CLER site visits (Table 2). Because the data generated would not be used for accreditation decisions, there was no concern that exposing errors would jeopardize our postgraduate training certification.

The first 15 minutes of monthly faculty meetings were devoted to the presentation of a resident project, called a QA/QI (quality assurance/quality improvement) moment, that addressed ACGME focus areas 1, 2, 3, or 6 (Table 1). (Transitions in care [focus area 4] and work hours and fatigue [focus area 5] generally are less important issues in a predominantly outpatient specialty such as dermatology.) The residents were encouraged to identify areas where patient harm could occur due to poorly designed systems and to report situations in which patients actually were harmed.

Each project had to be approved by the department chairperson based on the following 4 requirements: First, the initiative must have the potential to notably impact patient safety and reduce harm. Second, residents with faculty support had to design methods to assess the identified problem. Third, participants had to design (to the best of their abilities) cost-effective and achievable interventions in a manner that would not produce unintended consequences. Fourth, residents were asked to devise a system to close the loop, ensuring that the effort put into the process was not wasted.
Findings From the CLER Program
The CLER program generates data on program and institutional attributes that have a salutatory effect on quality and safety, specifically involving 6 focus areas highlighted in Table 1. Putting residents at the center of efforts to improve the quality of care in our department proved critical to improving patient safety.
Involving residents in a series of QA/QI initiatives was logical because they rotate with faculty members. They also are in a position to view inconsistencies and to work to establish consistent patterns of patient care. In addition, our busy faculty members are charged with a variety of other clinical, educational, and administrative duties complicated by requirements in the design of a new residency training program. Faculty and residents working together were able to find problem areas in our department and devise solutions to improve those problems.
The CLER program involved a series of steps. Residents were charged with identifying errors (QA) and then devising a system to prevent similar errors from being repeated (QI)(Table 3). Efforts focused on preventing needless harm in our department. Initiatives developed by residents, who are closest to patients, have advantages over safety programs developed by the hospital’s administration. Residents became passionate about error prevention when they determined that their efforts could make a difference to patients.


Forward Thinking for Dermatology Practices
Perhaps there are lessons here that could apply to safety promotion in the practicing dermatologist’s office. The American Board of Dermatology, within the framework established by the American Board of Medical Specialties, requires physicians seeking recertification to participate in preapproved practice assessment QI exercises twice every 10 years.17 Six programs sponsored by the American Academy of Dermatology have now been approved in the areas of melanoma, biopsy follow-up measure, psoriasis, chronic urticaria, venous insufficiency, and laser- and light-based therapy for rejuvenation.18 An additional program has been approved for dermatopathologists through the American Society of Dermatopathology.19 None of these programs match the topics chosen by our residents in consultation with faculty to meet safety gaps identified in clinics at UMMC. Perhaps the next generation of performance improvement continuing medical education programs could include a pilot program for part 4 of Maintenance of Certification credit that is nonpunitive, patient focused, and allows dermatologists to design specific error-prevention solutions tailored to their individual practice in the same way residency programs are taking up this task.
- Nasca TJ, Philibert I, Brigham T, et al. The Next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Philibert I, Gonzalez del Rey JA, Lannon C, et al. Quality improvement skills for pediatric residents: from lecture to implementation and sustainability. Acad Pediatr. 2014;14:40-46.
- Vidyarthi AR, Green AL, Rosenbluth G, et al. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89:460-468.
- Brodell RT, Elewski B. Antifungal drug interactions. avoidance requires more than memorization. Postgrad Med. 2000;107:41-43.
- Kerr IG, Jolivet J, Collin JM, et al. Test dose for predicting high-dose methotrexate infusions. Clin Pharmacol Ther. 1983;33:44-51.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Saporito FC, Menter MA. Methotrexate and psoriasis in the era of new biologic agents. J Am Acad Dermatol. 2004;50:301-309.
- Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138-147.
- Hunter KM. Implementation of an electronic medication administration record and bedside verification system. Online J Nurs Inform (OJNI). 2011;15:672.
- Nanji KC, Slight SP, Seger DL, et al. Overrides of medication-related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21:487-491.
- Schedlbauer A, Prasad V, Mulvaney C, et al. What evidence supports the use of computerized alerts and prompts to improve clinicians’ prescribing behavior? J Am Med Inform Assoc. 2009;16:531-538.
- Lee EK, Mejia AF, Senior T, et al. Improving patient safety through medical alert management: an automated decision tool to reduce alert fatigue. AMIA Annu Symp Proc. 2010;2010:417-421.
- Brenner AB. Physician and nurse relationships, a key to patient safety. J Ky Med Assoc. 2007;105:165-169.
- Rush JL, Flowers RH, Casamiquela KM, et al. Research letter: the knock: an adjunct to education opening the door to improved outpatient hand hygiene. J Am Acad Dermatol. In press.
- Lee SL. The extended surgical time-out: does it improve quality and prevent wrong-site surgery? Perm J. 2010;14:19-23.
- Altpeter T, Luckhardt K, Lewis JN, et al. Expanded surgical time out: a key to real-time data collection and quality improvement. J Am Coll Surg. 2007;204:527-532.
- MOC requirements. American Board of Dermatology Web site. https://www.abderm.org/diplomates/fulfilling-moc-requirements/moc-requirements.aspx#PI. Accessed January 18, 2016.
- How AAD develops measures. American Academy of Dermatology Web site. https://www.aad.org/practice-tools/quality-care/quality-measures. Accessed January 20, 2016.
- Quality assurance programs. The American Society of Dermatopathology Web site. http://www.asdp.org/education/quality-assurance-programs. Accessed January 20, 2016.
- Nasca TJ, Philibert I, Brigham T, et al. The Next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Philibert I, Gonzalez del Rey JA, Lannon C, et al. Quality improvement skills for pediatric residents: from lecture to implementation and sustainability. Acad Pediatr. 2014;14:40-46.
- Vidyarthi AR, Green AL, Rosenbluth G, et al. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89:460-468.
- Brodell RT, Elewski B. Antifungal drug interactions. avoidance requires more than memorization. Postgrad Med. 2000;107:41-43.
- Kerr IG, Jolivet J, Collin JM, et al. Test dose for predicting high-dose methotrexate infusions. Clin Pharmacol Ther. 1983;33:44-51.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Saporito FC, Menter MA. Methotrexate and psoriasis in the era of new biologic agents. J Am Acad Dermatol. 2004;50:301-309.
- Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138-147.
- Hunter KM. Implementation of an electronic medication administration record and bedside verification system. Online J Nurs Inform (OJNI). 2011;15:672.
- Nanji KC, Slight SP, Seger DL, et al. Overrides of medication-related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21:487-491.
- Schedlbauer A, Prasad V, Mulvaney C, et al. What evidence supports the use of computerized alerts and prompts to improve clinicians’ prescribing behavior? J Am Med Inform Assoc. 2009;16:531-538.
- Lee EK, Mejia AF, Senior T, et al. Improving patient safety through medical alert management: an automated decision tool to reduce alert fatigue. AMIA Annu Symp Proc. 2010;2010:417-421.
- Brenner AB. Physician and nurse relationships, a key to patient safety. J Ky Med Assoc. 2007;105:165-169.
- Rush JL, Flowers RH, Casamiquela KM, et al. Research letter: the knock: an adjunct to education opening the door to improved outpatient hand hygiene. J Am Acad Dermatol. In press.
- Lee SL. The extended surgical time-out: does it improve quality and prevent wrong-site surgery? Perm J. 2010;14:19-23.
- Altpeter T, Luckhardt K, Lewis JN, et al. Expanded surgical time out: a key to real-time data collection and quality improvement. J Am Coll Surg. 2007;204:527-532.
- MOC requirements. American Board of Dermatology Web site. https://www.abderm.org/diplomates/fulfilling-moc-requirements/moc-requirements.aspx#PI. Accessed January 18, 2016.
- How AAD develops measures. American Academy of Dermatology Web site. https://www.aad.org/practice-tools/quality-care/quality-measures. Accessed January 20, 2016.
- Quality assurance programs. The American Society of Dermatopathology Web site. http://www.asdp.org/education/quality-assurance-programs. Accessed January 20, 2016.
Practice Points
- The Clinical Learning Environment Review mobilizes residency and fellowship training programs in the movement to improve the quality of patient care.
- Quality assessment/quality improvement (QA/QI) projects enhance communication between residents and faculty and promote systems that improve patient safety.
- Emphasis on resident-initiated QA/QI impacts quality of care in clinical practice long after graduation.
Recurrent vesicular rash over the sacrum
A 35-year-old woman sought care at our dermatology clinic with the self-diagnosis of “recurrent shingles,” noting that she’d had a rash over her sacrum on and off for the past 10 years. She said that the tender blisters typically appeared in this area 3 to 4 times per year (FIGURE) and that their onset was occasionally associated with stress. The rash tended to resolve—without treatment—within 5 to 7 days. The patient had no other medical problems or symptoms. Physical examination revealed 3 groups of vesicular lesions, each on an erythematous base, located bilaterally over the gluteal cleft.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Recurrent herpes simplex virus-2
While the presentation of herpes zoster (shingles) and herpes simplex virus (HSV) is similar—grouped vesicles on an erythematous base—recurrent shingles is rare in immunocompetent patients. Also, the herpes zoster rash is generally unilateral and is not common on the buttocks.1,2
Herpes simplex virus-1 (HSV-1) generally occurs around the mouth. Herpes simplex virus-2 (HSV-2) is generally a genital rash. (Our patient was not aware that she’d had a genital primary HSV-2 infection.) That said, non-genital recurrences in the sacral region and lower extremities occur in up to 60% of patients whose primary genital HSV-2 infection also involved non-genital sites.3
HSV-2 infects an estimated 5% to 25% of adults in western nations.4 In 2012, approximately 417 million people ages 15 to 49 were living with HSV-2 worldwide, including 19 million who were newly infected.5
A dormant infection that is reactivated. Following a genital primary infection, HSV-2 lies dormant in the sacral nerve root ganglia, which innervate both the genitals and sacrum. Reactivation can thus result in recurrences anywhere over the sacral dermatome.6 The sacral area is the most common non-genital site for recurrent HSV-2.3 Reactivation of HSV-2 is more common and more severe in patients with human immunodeficiency virus infection.7
Neurologic complications in some patients with genital herpes (eg, sacral radiculopathy, hyperesthesia) reinforce the hypothesis that genital herpes can infect ganglia that are also associated with sacral nerves.8 Contrary to popular belief, sacral HSV-2 is not commonly contracted from toilet seats.
How to differentiate herpes simplex from herpes zoster
As noted earlier, the location of the vesicles and unilateral nature of herpes zoster are useful in differentiating HSV from herpes zoster.
Tzanck preparation can’t be used to differentiate HSV and herpes zoster because it will demonstrate multinucleated giant cells in both cases. When necessary, viral culture can be used to distinguish the 2 conditions, although HSV often takes 24 to 72 hours to grow and herpes zoster may take up to 2 weeks.9,10 Increasingly, polymerase chain reaction is being used for this purpose.
Antivirals are used for both genital and non-genital recurrences
The mainstay of treatment for HSV is antiviral therapy with acyclovir. Famciclovir and valacyclovir can be used as well (TABLE).11 These antivirals inhibit viral DNA replication, shorten duration of symptoms, increase lesion healing, and decrease viral shedding time.12 They are generally safe; the main adverse effects of oral therapy are nausea, vomiting, and diarrhea.
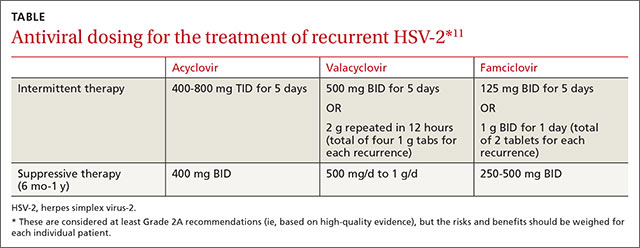
In general, non-genital recurrences of HSV are treated the same as genital recurrences.13 Dosing during prodromal symptoms, or at the first sign of a recurrence is recommended for maximum efficacy.13 Suppressive therapy can be effective in patients who experience frequent recurrences and is generally recommended for 6 months to a year or longer.
Patients should also be warned that because of increased genital viral shedding during sacral recurrences, they should avoid sexual contact during outbreaks.14
Our patient began taking oral valacyclovir 1 g daily and had no recurrences over the next year.
CORRESPONDENCE
Robert T. Brodell, MD, Department of Dermatology, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; rbrodell@umc.edu
1. Donahue JG, Choo PW, Manson JE, et al. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605-1609.
2. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9-20.
3. Benedetti JK, Zeh J, Selke S, et al. Frequency and reactivation of nongenital lesions among patients with genital herpes simplex virus. Am J Med. 1995;98:237-242.
4. Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186 Suppl 1:S3-S28.
5. Looker KJ, Magaret AS, Turner KME, et al. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE. 2015;10:e114989.
6. Corey L, Adams HG, Brown ZA, et al. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958-972.
7. Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135:1393-1397.
8. Ooi C, Zawar V. Hyperaesthesia following genital herpes: a case report. Dermatol Res Pract. 2011;2011:903595.
9. Domeika M, Bashmakova M, Savicheva A, et al; Eastern European Network for Sexual and Reproductive Health (EE SRH Network). Guidelines for the laboratory diagnosis of genital herpes in eastern European countries. Euro Surveill. 2010;15(44). pii:19703.
10. Solomon AR, Rasmussen JE, Weiss JS. A comparison of the Tzanck smear and viral isolation in varicella and herpes zoster. Arch Dermatol. 1986;122:282-285.
11. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
12. Goldberg LH, Kaufman R, Conant MA, et al. Oral acyclovir for episodic treatment of recurrent genital herpes. Efficacy and safety. J Am Acad Dermatol. 1986;15:256-264.
13. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s color atlas and synopsis of clinical dermatology. 5th ed. Chicago, IL: McGraw-Hill:2005;800-803.
14. Kerkering K, Gardella C, Selke S, et al. Isolation of herpes simplex virus from the genital tract during symptomatic recurrence on the buttocks. Obstet Gynecol. 2006;108:947-952.
A 35-year-old woman sought care at our dermatology clinic with the self-diagnosis of “recurrent shingles,” noting that she’d had a rash over her sacrum on and off for the past 10 years. She said that the tender blisters typically appeared in this area 3 to 4 times per year (FIGURE) and that their onset was occasionally associated with stress. The rash tended to resolve—without treatment—within 5 to 7 days. The patient had no other medical problems or symptoms. Physical examination revealed 3 groups of vesicular lesions, each on an erythematous base, located bilaterally over the gluteal cleft.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Recurrent herpes simplex virus-2
While the presentation of herpes zoster (shingles) and herpes simplex virus (HSV) is similar—grouped vesicles on an erythematous base—recurrent shingles is rare in immunocompetent patients. Also, the herpes zoster rash is generally unilateral and is not common on the buttocks.1,2
Herpes simplex virus-1 (HSV-1) generally occurs around the mouth. Herpes simplex virus-2 (HSV-2) is generally a genital rash. (Our patient was not aware that she’d had a genital primary HSV-2 infection.) That said, non-genital recurrences in the sacral region and lower extremities occur in up to 60% of patients whose primary genital HSV-2 infection also involved non-genital sites.3
HSV-2 infects an estimated 5% to 25% of adults in western nations.4 In 2012, approximately 417 million people ages 15 to 49 were living with HSV-2 worldwide, including 19 million who were newly infected.5
A dormant infection that is reactivated. Following a genital primary infection, HSV-2 lies dormant in the sacral nerve root ganglia, which innervate both the genitals and sacrum. Reactivation can thus result in recurrences anywhere over the sacral dermatome.6 The sacral area is the most common non-genital site for recurrent HSV-2.3 Reactivation of HSV-2 is more common and more severe in patients with human immunodeficiency virus infection.7
Neurologic complications in some patients with genital herpes (eg, sacral radiculopathy, hyperesthesia) reinforce the hypothesis that genital herpes can infect ganglia that are also associated with sacral nerves.8 Contrary to popular belief, sacral HSV-2 is not commonly contracted from toilet seats.
How to differentiate herpes simplex from herpes zoster
As noted earlier, the location of the vesicles and unilateral nature of herpes zoster are useful in differentiating HSV from herpes zoster.
Tzanck preparation can’t be used to differentiate HSV and herpes zoster because it will demonstrate multinucleated giant cells in both cases. When necessary, viral culture can be used to distinguish the 2 conditions, although HSV often takes 24 to 72 hours to grow and herpes zoster may take up to 2 weeks.9,10 Increasingly, polymerase chain reaction is being used for this purpose.
Antivirals are used for both genital and non-genital recurrences
The mainstay of treatment for HSV is antiviral therapy with acyclovir. Famciclovir and valacyclovir can be used as well (TABLE).11 These antivirals inhibit viral DNA replication, shorten duration of symptoms, increase lesion healing, and decrease viral shedding time.12 They are generally safe; the main adverse effects of oral therapy are nausea, vomiting, and diarrhea.

In general, non-genital recurrences of HSV are treated the same as genital recurrences.13 Dosing during prodromal symptoms, or at the first sign of a recurrence is recommended for maximum efficacy.13 Suppressive therapy can be effective in patients who experience frequent recurrences and is generally recommended for 6 months to a year or longer.
Patients should also be warned that because of increased genital viral shedding during sacral recurrences, they should avoid sexual contact during outbreaks.14
Our patient began taking oral valacyclovir 1 g daily and had no recurrences over the next year.
CORRESPONDENCE
Robert T. Brodell, MD, Department of Dermatology, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; rbrodell@umc.edu
A 35-year-old woman sought care at our dermatology clinic with the self-diagnosis of “recurrent shingles,” noting that she’d had a rash over her sacrum on and off for the past 10 years. She said that the tender blisters typically appeared in this area 3 to 4 times per year (FIGURE) and that their onset was occasionally associated with stress. The rash tended to resolve—without treatment—within 5 to 7 days. The patient had no other medical problems or symptoms. Physical examination revealed 3 groups of vesicular lesions, each on an erythematous base, located bilaterally over the gluteal cleft.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Recurrent herpes simplex virus-2
While the presentation of herpes zoster (shingles) and herpes simplex virus (HSV) is similar—grouped vesicles on an erythematous base—recurrent shingles is rare in immunocompetent patients. Also, the herpes zoster rash is generally unilateral and is not common on the buttocks.1,2
Herpes simplex virus-1 (HSV-1) generally occurs around the mouth. Herpes simplex virus-2 (HSV-2) is generally a genital rash. (Our patient was not aware that she’d had a genital primary HSV-2 infection.) That said, non-genital recurrences in the sacral region and lower extremities occur in up to 60% of patients whose primary genital HSV-2 infection also involved non-genital sites.3
HSV-2 infects an estimated 5% to 25% of adults in western nations.4 In 2012, approximately 417 million people ages 15 to 49 were living with HSV-2 worldwide, including 19 million who were newly infected.5
A dormant infection that is reactivated. Following a genital primary infection, HSV-2 lies dormant in the sacral nerve root ganglia, which innervate both the genitals and sacrum. Reactivation can thus result in recurrences anywhere over the sacral dermatome.6 The sacral area is the most common non-genital site for recurrent HSV-2.3 Reactivation of HSV-2 is more common and more severe in patients with human immunodeficiency virus infection.7
Neurologic complications in some patients with genital herpes (eg, sacral radiculopathy, hyperesthesia) reinforce the hypothesis that genital herpes can infect ganglia that are also associated with sacral nerves.8 Contrary to popular belief, sacral HSV-2 is not commonly contracted from toilet seats.
How to differentiate herpes simplex from herpes zoster
As noted earlier, the location of the vesicles and unilateral nature of herpes zoster are useful in differentiating HSV from herpes zoster.
Tzanck preparation can’t be used to differentiate HSV and herpes zoster because it will demonstrate multinucleated giant cells in both cases. When necessary, viral culture can be used to distinguish the 2 conditions, although HSV often takes 24 to 72 hours to grow and herpes zoster may take up to 2 weeks.9,10 Increasingly, polymerase chain reaction is being used for this purpose.
Antivirals are used for both genital and non-genital recurrences
The mainstay of treatment for HSV is antiviral therapy with acyclovir. Famciclovir and valacyclovir can be used as well (TABLE).11 These antivirals inhibit viral DNA replication, shorten duration of symptoms, increase lesion healing, and decrease viral shedding time.12 They are generally safe; the main adverse effects of oral therapy are nausea, vomiting, and diarrhea.

In general, non-genital recurrences of HSV are treated the same as genital recurrences.13 Dosing during prodromal symptoms, or at the first sign of a recurrence is recommended for maximum efficacy.13 Suppressive therapy can be effective in patients who experience frequent recurrences and is generally recommended for 6 months to a year or longer.
Patients should also be warned that because of increased genital viral shedding during sacral recurrences, they should avoid sexual contact during outbreaks.14
Our patient began taking oral valacyclovir 1 g daily and had no recurrences over the next year.
CORRESPONDENCE
Robert T. Brodell, MD, Department of Dermatology, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; rbrodell@umc.edu
1. Donahue JG, Choo PW, Manson JE, et al. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605-1609.
2. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9-20.
3. Benedetti JK, Zeh J, Selke S, et al. Frequency and reactivation of nongenital lesions among patients with genital herpes simplex virus. Am J Med. 1995;98:237-242.
4. Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186 Suppl 1:S3-S28.
5. Looker KJ, Magaret AS, Turner KME, et al. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE. 2015;10:e114989.
6. Corey L, Adams HG, Brown ZA, et al. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958-972.
7. Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135:1393-1397.
8. Ooi C, Zawar V. Hyperaesthesia following genital herpes: a case report. Dermatol Res Pract. 2011;2011:903595.
9. Domeika M, Bashmakova M, Savicheva A, et al; Eastern European Network for Sexual and Reproductive Health (EE SRH Network). Guidelines for the laboratory diagnosis of genital herpes in eastern European countries. Euro Surveill. 2010;15(44). pii:19703.
10. Solomon AR, Rasmussen JE, Weiss JS. A comparison of the Tzanck smear and viral isolation in varicella and herpes zoster. Arch Dermatol. 1986;122:282-285.
11. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
12. Goldberg LH, Kaufman R, Conant MA, et al. Oral acyclovir for episodic treatment of recurrent genital herpes. Efficacy and safety. J Am Acad Dermatol. 1986;15:256-264.
13. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s color atlas and synopsis of clinical dermatology. 5th ed. Chicago, IL: McGraw-Hill:2005;800-803.
14. Kerkering K, Gardella C, Selke S, et al. Isolation of herpes simplex virus from the genital tract during symptomatic recurrence on the buttocks. Obstet Gynecol. 2006;108:947-952.
1. Donahue JG, Choo PW, Manson JE, et al. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605-1609.
2. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9-20.
3. Benedetti JK, Zeh J, Selke S, et al. Frequency and reactivation of nongenital lesions among patients with genital herpes simplex virus. Am J Med. 1995;98:237-242.
4. Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J Infect Dis. 2002;186 Suppl 1:S3-S28.
5. Looker KJ, Magaret AS, Turner KME, et al. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE. 2015;10:e114989.
6. Corey L, Adams HG, Brown ZA, et al. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958-972.
7. Severson JL, Tyring SK. Relation between herpes simplex viruses and human immunodeficiency virus infections. Arch Dermatol. 1999;135:1393-1397.
8. Ooi C, Zawar V. Hyperaesthesia following genital herpes: a case report. Dermatol Res Pract. 2011;2011:903595.
9. Domeika M, Bashmakova M, Savicheva A, et al; Eastern European Network for Sexual and Reproductive Health (EE SRH Network). Guidelines for the laboratory diagnosis of genital herpes in eastern European countries. Euro Surveill. 2010;15(44). pii:19703.
10. Solomon AR, Rasmussen JE, Weiss JS. A comparison of the Tzanck smear and viral isolation in varicella and herpes zoster. Arch Dermatol. 1986;122:282-285.
11. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
12. Goldberg LH, Kaufman R, Conant MA, et al. Oral acyclovir for episodic treatment of recurrent genital herpes. Efficacy and safety. J Am Acad Dermatol. 1986;15:256-264.
13. Wolff K, Johnson RA, Suurmond D. Fitzpatrick’s color atlas and synopsis of clinical dermatology. 5th ed. Chicago, IL: McGraw-Hill:2005;800-803.
14. Kerkering K, Gardella C, Selke S, et al. Isolation of herpes simplex virus from the genital tract during symptomatic recurrence on the buttocks. Obstet Gynecol. 2006;108:947-952.
Pruritic rash
CASE 1: A 13-year-old Caucasian girl sought treatment for a pruritic, scaling patch on her right cheek. The patient had a 10-year history of atopic eczema, with rashes primarily in flexural areas, and a history of skin reactions to earrings and rivets in her blue jeans. She had gone 3 years without a rash on her earlobes and periumbilical areas by scrupulously avoiding contact with metal.
The 2 x 2-cm patch of pruritic, scaling, lichenified skin with focal excoriation that brought her in on this day had been on her right cheek for the past 3 months ( FIGURE 1 ). It had not responded to hydrocortisone 2.5% cream and desonide 0.05% cream when she applied it twice daily, nor had it responded to an intralesional injection of 2 cc of 2.5 mg/cc triamcinolone acetonide.
CASE 2: A 13-year-old African American girl with a history of atopic dermatitis went to her doctor for a rash beneath the umbilicus. She’d had the rash, which she said was extremely itchy, for 6 weeks; over the previous 10 days, it had become more widespread. A 6 x 5-cm scaling, lichenified, hyperpigmented plaque was present in the infra-umbilical region. She also had a papular rash on the dorsal hands and axillae.
CASE 3: A 15-year-old Caucasian girl sought care for a pruritic, erythematous rash circumscribing her left wrist in an 8-mm diameter band. She’d had the rash for 6 weeks.
CASE 4: A 58-year-old Caucasian woman presented with a pruritic, erythematous, scaling rash on both upper cheeks just below her lower eyelids. She told the physician that she had a similar pruritic rash on her earlobes when she wore costume jewelry.
CASE 5: A 50-year-old woman went to her doctor for a pruritic, erythematous patch on the anterior and posterior sides of her ear-lobes, bilaterally. She told the physician that her ears had been pierced 2 weeks earlier.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU MANAGE THIS CONDITION?
Diagnosis: Nickel dermatitis
Each of these 5 patients had allergic contact dermatitis caused by nickel. The cheek dermatitis was produced by contact with the circular “menu” button on the patient’s cell phone (Case 1/ FIGURE 1 ), the periumbilical rash by the rivet behind a blue jeans button (Case 2/ FIGURE 2 ), the wrist dermatitis by a bracelet (Case 3/ FIGURE 3 ), the rash on the upper cheeks by eyeglass frames (Case 4/ FIGURE 4 ), and the earlobe dermatitis by earrings (Case 5/ FIGURE 5 ). The presence of nickel in each object was confirmed with a positive dimethylglyoxime (DMG) test.
What you’ll see. Besides an erythematous, pruritic, scaling rash, other findings can include vesicles and bullae that break and form crusts at sites of contact. Extreme pruritus is also commonly seen and prompts chronic rubbing and scratching, resulting in the development of lichenification and hyper-pigmentation.
FIGURE 1
Scaling patch on cheek
FIGURE 2
Rash beneath umbilicus
The cause: The rivet on the patient’s blue jeans.
FIGURE 3
… around the wrist
The cause: The bracelet (shown), which would normally sit where the erythematous band is located.
FIGURE 4
… on the cheeks
The cause: Eyeglasses.
FIGURE 5
… on the earlobes
The cause: Nickel in pierced earrings.
Nickel dermatitis is increasingly common
Nickel is a leading cause of allergic contact dermatitis and is responsible for more cases than all other metals combined.1,2 The incidence of nickel dermatitis has been increasing in the United States for the last 15 years, annually affecting an estimated 14% to 20% of women and 2% to 4% of men.3,4 The higher percentage in women is related to nickel exposure associated with ear piercing and nickel-plated jewelry. In fact, the highest risk for nickel allergy is in young females with pierced ears.2,5,6 The number of affected males, however, is increasing, as earrings and body piercing gain popularity in this group.3
Certain occupations with high exposure to nickel, such as cashiers, hairdressers, metal workers, domestic cleaners, food handlers, bar workers, and painters, are also at risk for acquiring nickel dermatitis.7 Patients with atopic eczema are also at increased risk.8,9
Sweating may increase the severity of the dermatitis. Sodium chloride in the sweat causes corrosion of the metal and increases nickel exposure.10 Nickel release is therefore common in areas of the body that tend to be sweaty—for example, the hands, especially around the fingers, where inexpensive rings containing nickel are worn, or on the hands of individuals who carry metal key rings.
Consider oral intake of nickel, too. Another far less common, but important, nickel allergy presentation is systemic contact dermatitis from oral intake of nickel. Nuts, legumes, and chocolate can cause a flare-up reaction in a previously positive patch test site or previous site of nickel dermatitis.11 Patients can also develop a dyshidrotic eczema on the hands. Itching and general symptoms, such as headache, nausea, and malaise, have also been reported after the oral nickel exposure of nickel-sensitive individuals.12 Dietary intervention studies indicate that it is possible to reduce the activity of dermatitis in these patients by maintaining a diet low in nickel.13-16
Finally, severe local reactions to nickel and other contact allergens can lead to auto-eczematization, in which a papular or papulosquamous eruption and pruritus appear distant from the site of exposure.
Is it atopic eczema or contact dermatitis?
It can be difficult to distinguish a flare in atopic eczema from chronic allergic contact dermatitis in individuals who may display both processes concurrently. The primary clue to the diagnosis is the peculiar localization of the rash, as evidenced by the cases we have described.
Patch testing is not required for diagnosis
Nickel contact dermatitis is often suspected when patients present with an acute or sub-acute eruption characterized by erythema, vesicles, and scaling in a distribution corresponding to a metal contact allergen. Nickel allergy is one of the few types of allergic contact dermatitis where the history of exposure along with the signs and symptoms are so distinctive that patch testing is often not required.
Amelioration of the rash associated with the withdrawal of the contactant serves as adequate confirmation of the diagnosis in many cases. A DMG test is a simple, inexpensive way to determine whether the object in question contains nickel. It can be used in the physician’s office or by the patient at home. Therefore, in some clinical situations, as in the presented cases, it is appropriate to make a presumptive diagnosis of nickel dermatitis, confirm the presence of nickel with a DMG test, remove the off ending metal item, treat with topical anti-inflammatory medications, and confirm the diagnosis by monitoring the patient’s response.
When patients do not respond to withdrawal of the suspected allergen and anti-inflammatory treatment, when multiple allergens are suspected, or when a definitive diagnosis is required for legal purposes, patch testing with nickel can confirm the diagnosis. In some cases, when the distribution of the rash is not distinctive, patch test screening may elicit a positive test to nickel that prompts the physician to investigate the source of the exposure.
Tx: Remove the item, apply topical steroids
Acute episodes of nickel dermatitis are treated with topical steroid creams to break the scratch-itch cycle (which potentiates the reaction) and to reduce the inflammation. This tactic is futile, however, if the allergen remains in contact with the skin. The source of the nickel must be identified and eliminated by the patient, as was done by the 5 patients we cared for.
Clothing accessories containing nickel, such as buckles, zippers, buttons, and metal clips, must be eliminated, as well as other sources of nickel: jewelry, watches, eyeglasses, and cell phones. The situation is complicated by the fact that many patients have underlying atopic eczema/irritant dermatitis, and patients may have more than 1 form of allergic contact dermatitis. For instance, self-treatment with neomycin-containing topical antibiotics may lead to superimposed allergic contact dermatitis from this agent.
Easy preventive steps. Routine prophylactic measures should focus on eliminating exposure to nickel from all identifiable sources. You can suggest that your patient with nickel dermatitis:
- Use a clear plastic cover over the nickel-containing parts of a cell phone.
- Apply a clear coat of nail polish to the buttons and rivets on pants; this can prevent nickel release and will last through at least 2 wash and dry cycles.17 (Tucking shirts in to prevent buttons and belt buckles from touching the skin is generally ineffective; not only is the shirt unlikely to stay in place all day, but perspiration and friction can also cause problems.)
- Use other barrier coatings, such as Nickel Guard and Beauty Secrets Hardener, which may be effective in preventing contact dermatitis.18
- Replace rivets on pants with a plastic button, or cover the rivet with a sew-on denim patch. This offers a more permanent approach to eliminating nickel exposure.
- Use plastic covers for earring studs.
- Replace metal eyeglass frames with ones made out of plastic.
- Choose “hypoallergenic” or nickel-free jewelry. Of note, though: Nickel may be present in jewelry labeled hypoallergenic. The patient can perform a DMG test to verify its nickel content.
In patients who continue to react in the absence of nickel jewelry, co-sensitization to gold must be considered, as many patients do react to multiple metals. Gold is a more common allergen than previously reported and is statistically linked to allergic reactions to nickel and cobalt metals.19 Unlike nickel dermatitis, the clinical relevance of a positive gold patch test is harder to ascertain because rashing distant from areas in direct contact with gold jewelry often occurs. In fact, in some cases of eyelid dermatitis induced by contact allergy to gold, titanium dioxide particles on the skin from cosmetics and sunscreen are thought to adsorb gold particles and carry them to the eyelids.
Disclosure
Dr. Brodell reports that he receives grants/research support from Amgen Inc., Doak Dermatologics, Galderma, and OrthoNeutrogena. He serves as a consultant for, or is on the speakers bureau of, 3M/Graceway Pharmaceuticals, Allergan, CollagGenex Pharmaceuticals, Connetics Corp., Dermik/BenzaClin, Galderma, Genentech, Inc., Genentech/ Raptiva, GlaxoSmithKline, Janssen, MDsConnect.net, Medicis, Novartis Pharmaceuticals Corp., Pedinol Pharmacal, Inc., Roerig-Pfizer, Sandoz/Novartis, sanofi-aventis, Sirius Laboratories, Stiefel, Westwood-Squibb, and Upjohn. Ms. Uhlenhake and Dr. Nedorost reported no potential conflict of interest relevant to this article.
CORRESPONDENCE
Robert Brodell, MD, 2660 East Market Street, Warren, OH 44483; rtb@neoucom.edu
1. Arnold HL, Odom RB, James WD. Andrews’ Diseases of the Skin: Clinical Dermatology. 8th ed. Philadelphia, Pa: W.B. Saunders Co.; 1990.
2. Thyssen JP, Linneberg A, Menne T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
3. Rietschel RL, Fowler JF, Warshaw EM, et al. Detection of nickel sensitivity has increased in North American patch-test patients. Dermatitis. 2008;19:16-19.
4. Belsito DV. Allergic contact dermatitis. In: Freedberg IM, Eisen AZ, Wolff K, et al. Fitzpatrick’s dermatology in general medicine. 5th ed. New York, NY: McGraw-Hill; 1999:1447-1461.
5. McDonagh AJG, Wright AL, Cork MJ, et al. Nickel sensitivity: the influence of ear piercing and atopy. Br J Dermatol. 1992;126:16-18
6. Larsson-Stymne B, Widstrom L. Ear piercing: a cause of nickel allergy in school girls? Contact Dermatitis. 1985;13:289-293.
7. Shah M, Lewis F, Gawkrodger DJ. Nickel as an occupational allergen. A survey of 368 nickel-sensitive subjects. Arch Dermatol. 1998;134:1231-1236.
8. De Groot AC. The frequency of contact allergy in atopic patients with dermatitis. Contact Dermatitis. 1990;22:273-277.
9. Cronin E, McFadden JP. Patients with atopic eczema do become sensitized to contact allergens. Contact Dermatitis. 1993;28:225-228.
10. Suchoski JP. Allergic contact dermatitis. J Assoc Milit Dermatol. 1983;9:65-8.
11. Veien NK, Hattel T, Justesen O, Norholm A. Diagnostic procedures for eczema patients. Contact Dermatitis. 1987;17:35-40.
12. Jensen CS, Menne T, Lisby S, et al. Experimental systemic contact dermatitis from nickel: a dose-response study. Contact Dermatitis. 2003;49:124-132.
13. Kaaber K, Veien NK, Tjell JC. Low nickel diet in the treatment of patients with chronic nickel dermatitis. Br J Dermatol. 1978;98:197-200.
14. Veien NK, Hattel T, Justesen O, et al. Dietary treatment of nickel dermatitis. Acta Derm Venereol. 1985;65:138-142.
15. Veien NK, Hattel T, Laurberg G. Low nickel diet: an open, prospective trial. J Am Acad Dermatol. 1993;29:1002-1007.
16. Antico A, Soana R. Chronic allergic-like dermatopathies in nickel-sensitive patients. Results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
17. Suneja T, Flanagan KH, Glaser DA. Blue-jean button nickel: prevalence and prevention of its release from buttons. Dermatitis. 2007;18:208-211.
18. Sprigle AM, Marks JG, Jr. Anderson BE. Prevention of nickel release with barrier coatings. Dermatitis. 2008;19:28-31.
19. Fowler J, Jr, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
CASE 1: A 13-year-old Caucasian girl sought treatment for a pruritic, scaling patch on her right cheek. The patient had a 10-year history of atopic eczema, with rashes primarily in flexural areas, and a history of skin reactions to earrings and rivets in her blue jeans. She had gone 3 years without a rash on her earlobes and periumbilical areas by scrupulously avoiding contact with metal.
The 2 x 2-cm patch of pruritic, scaling, lichenified skin with focal excoriation that brought her in on this day had been on her right cheek for the past 3 months ( FIGURE 1 ). It had not responded to hydrocortisone 2.5% cream and desonide 0.05% cream when she applied it twice daily, nor had it responded to an intralesional injection of 2 cc of 2.5 mg/cc triamcinolone acetonide.
CASE 2: A 13-year-old African American girl with a history of atopic dermatitis went to her doctor for a rash beneath the umbilicus. She’d had the rash, which she said was extremely itchy, for 6 weeks; over the previous 10 days, it had become more widespread. A 6 x 5-cm scaling, lichenified, hyperpigmented plaque was present in the infra-umbilical region. She also had a papular rash on the dorsal hands and axillae.
CASE 3: A 15-year-old Caucasian girl sought care for a pruritic, erythematous rash circumscribing her left wrist in an 8-mm diameter band. She’d had the rash for 6 weeks.
CASE 4: A 58-year-old Caucasian woman presented with a pruritic, erythematous, scaling rash on both upper cheeks just below her lower eyelids. She told the physician that she had a similar pruritic rash on her earlobes when she wore costume jewelry.
CASE 5: A 50-year-old woman went to her doctor for a pruritic, erythematous patch on the anterior and posterior sides of her ear-lobes, bilaterally. She told the physician that her ears had been pierced 2 weeks earlier.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU MANAGE THIS CONDITION?
Diagnosis: Nickel dermatitis
Each of these 5 patients had allergic contact dermatitis caused by nickel. The cheek dermatitis was produced by contact with the circular “menu” button on the patient’s cell phone (Case 1/ FIGURE 1 ), the periumbilical rash by the rivet behind a blue jeans button (Case 2/ FIGURE 2 ), the wrist dermatitis by a bracelet (Case 3/ FIGURE 3 ), the rash on the upper cheeks by eyeglass frames (Case 4/ FIGURE 4 ), and the earlobe dermatitis by earrings (Case 5/ FIGURE 5 ). The presence of nickel in each object was confirmed with a positive dimethylglyoxime (DMG) test.
What you’ll see. Besides an erythematous, pruritic, scaling rash, other findings can include vesicles and bullae that break and form crusts at sites of contact. Extreme pruritus is also commonly seen and prompts chronic rubbing and scratching, resulting in the development of lichenification and hyper-pigmentation.
FIGURE 1
Scaling patch on cheek
FIGURE 2
Rash beneath umbilicus
The cause: The rivet on the patient’s blue jeans.
FIGURE 3
… around the wrist
The cause: The bracelet (shown), which would normally sit where the erythematous band is located.
FIGURE 4
… on the cheeks
The cause: Eyeglasses.
FIGURE 5
… on the earlobes
The cause: Nickel in pierced earrings.
Nickel dermatitis is increasingly common
Nickel is a leading cause of allergic contact dermatitis and is responsible for more cases than all other metals combined.1,2 The incidence of nickel dermatitis has been increasing in the United States for the last 15 years, annually affecting an estimated 14% to 20% of women and 2% to 4% of men.3,4 The higher percentage in women is related to nickel exposure associated with ear piercing and nickel-plated jewelry. In fact, the highest risk for nickel allergy is in young females with pierced ears.2,5,6 The number of affected males, however, is increasing, as earrings and body piercing gain popularity in this group.3
Certain occupations with high exposure to nickel, such as cashiers, hairdressers, metal workers, domestic cleaners, food handlers, bar workers, and painters, are also at risk for acquiring nickel dermatitis.7 Patients with atopic eczema are also at increased risk.8,9
Sweating may increase the severity of the dermatitis. Sodium chloride in the sweat causes corrosion of the metal and increases nickel exposure.10 Nickel release is therefore common in areas of the body that tend to be sweaty—for example, the hands, especially around the fingers, where inexpensive rings containing nickel are worn, or on the hands of individuals who carry metal key rings.
Consider oral intake of nickel, too. Another far less common, but important, nickel allergy presentation is systemic contact dermatitis from oral intake of nickel. Nuts, legumes, and chocolate can cause a flare-up reaction in a previously positive patch test site or previous site of nickel dermatitis.11 Patients can also develop a dyshidrotic eczema on the hands. Itching and general symptoms, such as headache, nausea, and malaise, have also been reported after the oral nickel exposure of nickel-sensitive individuals.12 Dietary intervention studies indicate that it is possible to reduce the activity of dermatitis in these patients by maintaining a diet low in nickel.13-16
Finally, severe local reactions to nickel and other contact allergens can lead to auto-eczematization, in which a papular or papulosquamous eruption and pruritus appear distant from the site of exposure.
Is it atopic eczema or contact dermatitis?
It can be difficult to distinguish a flare in atopic eczema from chronic allergic contact dermatitis in individuals who may display both processes concurrently. The primary clue to the diagnosis is the peculiar localization of the rash, as evidenced by the cases we have described.
Patch testing is not required for diagnosis
Nickel contact dermatitis is often suspected when patients present with an acute or sub-acute eruption characterized by erythema, vesicles, and scaling in a distribution corresponding to a metal contact allergen. Nickel allergy is one of the few types of allergic contact dermatitis where the history of exposure along with the signs and symptoms are so distinctive that patch testing is often not required.
Amelioration of the rash associated with the withdrawal of the contactant serves as adequate confirmation of the diagnosis in many cases. A DMG test is a simple, inexpensive way to determine whether the object in question contains nickel. It can be used in the physician’s office or by the patient at home. Therefore, in some clinical situations, as in the presented cases, it is appropriate to make a presumptive diagnosis of nickel dermatitis, confirm the presence of nickel with a DMG test, remove the off ending metal item, treat with topical anti-inflammatory medications, and confirm the diagnosis by monitoring the patient’s response.
When patients do not respond to withdrawal of the suspected allergen and anti-inflammatory treatment, when multiple allergens are suspected, or when a definitive diagnosis is required for legal purposes, patch testing with nickel can confirm the diagnosis. In some cases, when the distribution of the rash is not distinctive, patch test screening may elicit a positive test to nickel that prompts the physician to investigate the source of the exposure.
Tx: Remove the item, apply topical steroids
Acute episodes of nickel dermatitis are treated with topical steroid creams to break the scratch-itch cycle (which potentiates the reaction) and to reduce the inflammation. This tactic is futile, however, if the allergen remains in contact with the skin. The source of the nickel must be identified and eliminated by the patient, as was done by the 5 patients we cared for.
Clothing accessories containing nickel, such as buckles, zippers, buttons, and metal clips, must be eliminated, as well as other sources of nickel: jewelry, watches, eyeglasses, and cell phones. The situation is complicated by the fact that many patients have underlying atopic eczema/irritant dermatitis, and patients may have more than 1 form of allergic contact dermatitis. For instance, self-treatment with neomycin-containing topical antibiotics may lead to superimposed allergic contact dermatitis from this agent.
Easy preventive steps. Routine prophylactic measures should focus on eliminating exposure to nickel from all identifiable sources. You can suggest that your patient with nickel dermatitis:
- Use a clear plastic cover over the nickel-containing parts of a cell phone.
- Apply a clear coat of nail polish to the buttons and rivets on pants; this can prevent nickel release and will last through at least 2 wash and dry cycles.17 (Tucking shirts in to prevent buttons and belt buckles from touching the skin is generally ineffective; not only is the shirt unlikely to stay in place all day, but perspiration and friction can also cause problems.)
- Use other barrier coatings, such as Nickel Guard and Beauty Secrets Hardener, which may be effective in preventing contact dermatitis.18
- Replace rivets on pants with a plastic button, or cover the rivet with a sew-on denim patch. This offers a more permanent approach to eliminating nickel exposure.
- Use plastic covers for earring studs.
- Replace metal eyeglass frames with ones made out of plastic.
- Choose “hypoallergenic” or nickel-free jewelry. Of note, though: Nickel may be present in jewelry labeled hypoallergenic. The patient can perform a DMG test to verify its nickel content.
In patients who continue to react in the absence of nickel jewelry, co-sensitization to gold must be considered, as many patients do react to multiple metals. Gold is a more common allergen than previously reported and is statistically linked to allergic reactions to nickel and cobalt metals.19 Unlike nickel dermatitis, the clinical relevance of a positive gold patch test is harder to ascertain because rashing distant from areas in direct contact with gold jewelry often occurs. In fact, in some cases of eyelid dermatitis induced by contact allergy to gold, titanium dioxide particles on the skin from cosmetics and sunscreen are thought to adsorb gold particles and carry them to the eyelids.
Disclosure
Dr. Brodell reports that he receives grants/research support from Amgen Inc., Doak Dermatologics, Galderma, and OrthoNeutrogena. He serves as a consultant for, or is on the speakers bureau of, 3M/Graceway Pharmaceuticals, Allergan, CollagGenex Pharmaceuticals, Connetics Corp., Dermik/BenzaClin, Galderma, Genentech, Inc., Genentech/ Raptiva, GlaxoSmithKline, Janssen, MDsConnect.net, Medicis, Novartis Pharmaceuticals Corp., Pedinol Pharmacal, Inc., Roerig-Pfizer, Sandoz/Novartis, sanofi-aventis, Sirius Laboratories, Stiefel, Westwood-Squibb, and Upjohn. Ms. Uhlenhake and Dr. Nedorost reported no potential conflict of interest relevant to this article.
CORRESPONDENCE
Robert Brodell, MD, 2660 East Market Street, Warren, OH 44483; rtb@neoucom.edu
CASE 1: A 13-year-old Caucasian girl sought treatment for a pruritic, scaling patch on her right cheek. The patient had a 10-year history of atopic eczema, with rashes primarily in flexural areas, and a history of skin reactions to earrings and rivets in her blue jeans. She had gone 3 years without a rash on her earlobes and periumbilical areas by scrupulously avoiding contact with metal.
The 2 x 2-cm patch of pruritic, scaling, lichenified skin with focal excoriation that brought her in on this day had been on her right cheek for the past 3 months ( FIGURE 1 ). It had not responded to hydrocortisone 2.5% cream and desonide 0.05% cream when she applied it twice daily, nor had it responded to an intralesional injection of 2 cc of 2.5 mg/cc triamcinolone acetonide.
CASE 2: A 13-year-old African American girl with a history of atopic dermatitis went to her doctor for a rash beneath the umbilicus. She’d had the rash, which she said was extremely itchy, for 6 weeks; over the previous 10 days, it had become more widespread. A 6 x 5-cm scaling, lichenified, hyperpigmented plaque was present in the infra-umbilical region. She also had a papular rash on the dorsal hands and axillae.
CASE 3: A 15-year-old Caucasian girl sought care for a pruritic, erythematous rash circumscribing her left wrist in an 8-mm diameter band. She’d had the rash for 6 weeks.
CASE 4: A 58-year-old Caucasian woman presented with a pruritic, erythematous, scaling rash on both upper cheeks just below her lower eyelids. She told the physician that she had a similar pruritic rash on her earlobes when she wore costume jewelry.
CASE 5: A 50-year-old woman went to her doctor for a pruritic, erythematous patch on the anterior and posterior sides of her ear-lobes, bilaterally. She told the physician that her ears had been pierced 2 weeks earlier.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU MANAGE THIS CONDITION?
Diagnosis: Nickel dermatitis
Each of these 5 patients had allergic contact dermatitis caused by nickel. The cheek dermatitis was produced by contact with the circular “menu” button on the patient’s cell phone (Case 1/ FIGURE 1 ), the periumbilical rash by the rivet behind a blue jeans button (Case 2/ FIGURE 2 ), the wrist dermatitis by a bracelet (Case 3/ FIGURE 3 ), the rash on the upper cheeks by eyeglass frames (Case 4/ FIGURE 4 ), and the earlobe dermatitis by earrings (Case 5/ FIGURE 5 ). The presence of nickel in each object was confirmed with a positive dimethylglyoxime (DMG) test.
What you’ll see. Besides an erythematous, pruritic, scaling rash, other findings can include vesicles and bullae that break and form crusts at sites of contact. Extreme pruritus is also commonly seen and prompts chronic rubbing and scratching, resulting in the development of lichenification and hyper-pigmentation.
FIGURE 1
Scaling patch on cheek
FIGURE 2
Rash beneath umbilicus
The cause: The rivet on the patient’s blue jeans.
FIGURE 3
… around the wrist
The cause: The bracelet (shown), which would normally sit where the erythematous band is located.
FIGURE 4
… on the cheeks
The cause: Eyeglasses.
FIGURE 5
… on the earlobes
The cause: Nickel in pierced earrings.
Nickel dermatitis is increasingly common
Nickel is a leading cause of allergic contact dermatitis and is responsible for more cases than all other metals combined.1,2 The incidence of nickel dermatitis has been increasing in the United States for the last 15 years, annually affecting an estimated 14% to 20% of women and 2% to 4% of men.3,4 The higher percentage in women is related to nickel exposure associated with ear piercing and nickel-plated jewelry. In fact, the highest risk for nickel allergy is in young females with pierced ears.2,5,6 The number of affected males, however, is increasing, as earrings and body piercing gain popularity in this group.3
Certain occupations with high exposure to nickel, such as cashiers, hairdressers, metal workers, domestic cleaners, food handlers, bar workers, and painters, are also at risk for acquiring nickel dermatitis.7 Patients with atopic eczema are also at increased risk.8,9
Sweating may increase the severity of the dermatitis. Sodium chloride in the sweat causes corrosion of the metal and increases nickel exposure.10 Nickel release is therefore common in areas of the body that tend to be sweaty—for example, the hands, especially around the fingers, where inexpensive rings containing nickel are worn, or on the hands of individuals who carry metal key rings.
Consider oral intake of nickel, too. Another far less common, but important, nickel allergy presentation is systemic contact dermatitis from oral intake of nickel. Nuts, legumes, and chocolate can cause a flare-up reaction in a previously positive patch test site or previous site of nickel dermatitis.11 Patients can also develop a dyshidrotic eczema on the hands. Itching and general symptoms, such as headache, nausea, and malaise, have also been reported after the oral nickel exposure of nickel-sensitive individuals.12 Dietary intervention studies indicate that it is possible to reduce the activity of dermatitis in these patients by maintaining a diet low in nickel.13-16
Finally, severe local reactions to nickel and other contact allergens can lead to auto-eczematization, in which a papular or papulosquamous eruption and pruritus appear distant from the site of exposure.
Is it atopic eczema or contact dermatitis?
It can be difficult to distinguish a flare in atopic eczema from chronic allergic contact dermatitis in individuals who may display both processes concurrently. The primary clue to the diagnosis is the peculiar localization of the rash, as evidenced by the cases we have described.
Patch testing is not required for diagnosis
Nickel contact dermatitis is often suspected when patients present with an acute or sub-acute eruption characterized by erythema, vesicles, and scaling in a distribution corresponding to a metal contact allergen. Nickel allergy is one of the few types of allergic contact dermatitis where the history of exposure along with the signs and symptoms are so distinctive that patch testing is often not required.
Amelioration of the rash associated with the withdrawal of the contactant serves as adequate confirmation of the diagnosis in many cases. A DMG test is a simple, inexpensive way to determine whether the object in question contains nickel. It can be used in the physician’s office or by the patient at home. Therefore, in some clinical situations, as in the presented cases, it is appropriate to make a presumptive diagnosis of nickel dermatitis, confirm the presence of nickel with a DMG test, remove the off ending metal item, treat with topical anti-inflammatory medications, and confirm the diagnosis by monitoring the patient’s response.
When patients do not respond to withdrawal of the suspected allergen and anti-inflammatory treatment, when multiple allergens are suspected, or when a definitive diagnosis is required for legal purposes, patch testing with nickel can confirm the diagnosis. In some cases, when the distribution of the rash is not distinctive, patch test screening may elicit a positive test to nickel that prompts the physician to investigate the source of the exposure.
Tx: Remove the item, apply topical steroids
Acute episodes of nickel dermatitis are treated with topical steroid creams to break the scratch-itch cycle (which potentiates the reaction) and to reduce the inflammation. This tactic is futile, however, if the allergen remains in contact with the skin. The source of the nickel must be identified and eliminated by the patient, as was done by the 5 patients we cared for.
Clothing accessories containing nickel, such as buckles, zippers, buttons, and metal clips, must be eliminated, as well as other sources of nickel: jewelry, watches, eyeglasses, and cell phones. The situation is complicated by the fact that many patients have underlying atopic eczema/irritant dermatitis, and patients may have more than 1 form of allergic contact dermatitis. For instance, self-treatment with neomycin-containing topical antibiotics may lead to superimposed allergic contact dermatitis from this agent.
Easy preventive steps. Routine prophylactic measures should focus on eliminating exposure to nickel from all identifiable sources. You can suggest that your patient with nickel dermatitis:
- Use a clear plastic cover over the nickel-containing parts of a cell phone.
- Apply a clear coat of nail polish to the buttons and rivets on pants; this can prevent nickel release and will last through at least 2 wash and dry cycles.17 (Tucking shirts in to prevent buttons and belt buckles from touching the skin is generally ineffective; not only is the shirt unlikely to stay in place all day, but perspiration and friction can also cause problems.)
- Use other barrier coatings, such as Nickel Guard and Beauty Secrets Hardener, which may be effective in preventing contact dermatitis.18
- Replace rivets on pants with a plastic button, or cover the rivet with a sew-on denim patch. This offers a more permanent approach to eliminating nickel exposure.
- Use plastic covers for earring studs.
- Replace metal eyeglass frames with ones made out of plastic.
- Choose “hypoallergenic” or nickel-free jewelry. Of note, though: Nickel may be present in jewelry labeled hypoallergenic. The patient can perform a DMG test to verify its nickel content.
In patients who continue to react in the absence of nickel jewelry, co-sensitization to gold must be considered, as many patients do react to multiple metals. Gold is a more common allergen than previously reported and is statistically linked to allergic reactions to nickel and cobalt metals.19 Unlike nickel dermatitis, the clinical relevance of a positive gold patch test is harder to ascertain because rashing distant from areas in direct contact with gold jewelry often occurs. In fact, in some cases of eyelid dermatitis induced by contact allergy to gold, titanium dioxide particles on the skin from cosmetics and sunscreen are thought to adsorb gold particles and carry them to the eyelids.
Disclosure
Dr. Brodell reports that he receives grants/research support from Amgen Inc., Doak Dermatologics, Galderma, and OrthoNeutrogena. He serves as a consultant for, or is on the speakers bureau of, 3M/Graceway Pharmaceuticals, Allergan, CollagGenex Pharmaceuticals, Connetics Corp., Dermik/BenzaClin, Galderma, Genentech, Inc., Genentech/ Raptiva, GlaxoSmithKline, Janssen, MDsConnect.net, Medicis, Novartis Pharmaceuticals Corp., Pedinol Pharmacal, Inc., Roerig-Pfizer, Sandoz/Novartis, sanofi-aventis, Sirius Laboratories, Stiefel, Westwood-Squibb, and Upjohn. Ms. Uhlenhake and Dr. Nedorost reported no potential conflict of interest relevant to this article.
CORRESPONDENCE
Robert Brodell, MD, 2660 East Market Street, Warren, OH 44483; rtb@neoucom.edu
1. Arnold HL, Odom RB, James WD. Andrews’ Diseases of the Skin: Clinical Dermatology. 8th ed. Philadelphia, Pa: W.B. Saunders Co.; 1990.
2. Thyssen JP, Linneberg A, Menne T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
3. Rietschel RL, Fowler JF, Warshaw EM, et al. Detection of nickel sensitivity has increased in North American patch-test patients. Dermatitis. 2008;19:16-19.
4. Belsito DV. Allergic contact dermatitis. In: Freedberg IM, Eisen AZ, Wolff K, et al. Fitzpatrick’s dermatology in general medicine. 5th ed. New York, NY: McGraw-Hill; 1999:1447-1461.
5. McDonagh AJG, Wright AL, Cork MJ, et al. Nickel sensitivity: the influence of ear piercing and atopy. Br J Dermatol. 1992;126:16-18
6. Larsson-Stymne B, Widstrom L. Ear piercing: a cause of nickel allergy in school girls? Contact Dermatitis. 1985;13:289-293.
7. Shah M, Lewis F, Gawkrodger DJ. Nickel as an occupational allergen. A survey of 368 nickel-sensitive subjects. Arch Dermatol. 1998;134:1231-1236.
8. De Groot AC. The frequency of contact allergy in atopic patients with dermatitis. Contact Dermatitis. 1990;22:273-277.
9. Cronin E, McFadden JP. Patients with atopic eczema do become sensitized to contact allergens. Contact Dermatitis. 1993;28:225-228.
10. Suchoski JP. Allergic contact dermatitis. J Assoc Milit Dermatol. 1983;9:65-8.
11. Veien NK, Hattel T, Justesen O, Norholm A. Diagnostic procedures for eczema patients. Contact Dermatitis. 1987;17:35-40.
12. Jensen CS, Menne T, Lisby S, et al. Experimental systemic contact dermatitis from nickel: a dose-response study. Contact Dermatitis. 2003;49:124-132.
13. Kaaber K, Veien NK, Tjell JC. Low nickel diet in the treatment of patients with chronic nickel dermatitis. Br J Dermatol. 1978;98:197-200.
14. Veien NK, Hattel T, Justesen O, et al. Dietary treatment of nickel dermatitis. Acta Derm Venereol. 1985;65:138-142.
15. Veien NK, Hattel T, Laurberg G. Low nickel diet: an open, prospective trial. J Am Acad Dermatol. 1993;29:1002-1007.
16. Antico A, Soana R. Chronic allergic-like dermatopathies in nickel-sensitive patients. Results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
17. Suneja T, Flanagan KH, Glaser DA. Blue-jean button nickel: prevalence and prevention of its release from buttons. Dermatitis. 2007;18:208-211.
18. Sprigle AM, Marks JG, Jr. Anderson BE. Prevention of nickel release with barrier coatings. Dermatitis. 2008;19:28-31.
19. Fowler J, Jr, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
1. Arnold HL, Odom RB, James WD. Andrews’ Diseases of the Skin: Clinical Dermatology. 8th ed. Philadelphia, Pa: W.B. Saunders Co.; 1990.
2. Thyssen JP, Linneberg A, Menne T, et al. The epidemiology of contact allergy in the general population—prevalence and main findings. Contact Dermatitis. 2007;57:287-299.
3. Rietschel RL, Fowler JF, Warshaw EM, et al. Detection of nickel sensitivity has increased in North American patch-test patients. Dermatitis. 2008;19:16-19.
4. Belsito DV. Allergic contact dermatitis. In: Freedberg IM, Eisen AZ, Wolff K, et al. Fitzpatrick’s dermatology in general medicine. 5th ed. New York, NY: McGraw-Hill; 1999:1447-1461.
5. McDonagh AJG, Wright AL, Cork MJ, et al. Nickel sensitivity: the influence of ear piercing and atopy. Br J Dermatol. 1992;126:16-18
6. Larsson-Stymne B, Widstrom L. Ear piercing: a cause of nickel allergy in school girls? Contact Dermatitis. 1985;13:289-293.
7. Shah M, Lewis F, Gawkrodger DJ. Nickel as an occupational allergen. A survey of 368 nickel-sensitive subjects. Arch Dermatol. 1998;134:1231-1236.
8. De Groot AC. The frequency of contact allergy in atopic patients with dermatitis. Contact Dermatitis. 1990;22:273-277.
9. Cronin E, McFadden JP. Patients with atopic eczema do become sensitized to contact allergens. Contact Dermatitis. 1993;28:225-228.
10. Suchoski JP. Allergic contact dermatitis. J Assoc Milit Dermatol. 1983;9:65-8.
11. Veien NK, Hattel T, Justesen O, Norholm A. Diagnostic procedures for eczema patients. Contact Dermatitis. 1987;17:35-40.
12. Jensen CS, Menne T, Lisby S, et al. Experimental systemic contact dermatitis from nickel: a dose-response study. Contact Dermatitis. 2003;49:124-132.
13. Kaaber K, Veien NK, Tjell JC. Low nickel diet in the treatment of patients with chronic nickel dermatitis. Br J Dermatol. 1978;98:197-200.
14. Veien NK, Hattel T, Justesen O, et al. Dietary treatment of nickel dermatitis. Acta Derm Venereol. 1985;65:138-142.
15. Veien NK, Hattel T, Laurberg G. Low nickel diet: an open, prospective trial. J Am Acad Dermatol. 1993;29:1002-1007.
16. Antico A, Soana R. Chronic allergic-like dermatopathies in nickel-sensitive patients. Results of dietary restrictions and challenge with nickel salts. Allergy Asthma Proc. 1999;20:235-242.
17. Suneja T, Flanagan KH, Glaser DA. Blue-jean button nickel: prevalence and prevention of its release from buttons. Dermatitis. 2007;18:208-211.
18. Sprigle AM, Marks JG, Jr. Anderson BE. Prevention of nickel release with barrier coatings. Dermatitis. 2008;19:28-31.
19. Fowler J, Jr, Taylor J, Storrs F, et al. Gold allergy in North America. Am J Contact Dermat. 2001;12:3-5.
Bilateral axillary pustules
A healthy 29-year-old woman presented to the clinic with pustules and boil-like lesions in the axillary areas, on the left forearm, and on the right thigh (FIGURE). She indicated that she’d had the lesions for 4 weeks. She said that she had not had any recent infections, and there was no evidence of immune compromise in her history.
We incised a pustule in the right axilla and expressed the pus. We sent the sample out for bacterial culture and sensitivity testing.
We immediately considered bacterial folliculitis and hidradenitis suppurativa as part of the differential. The acute nature of the process led us to favor a diagnosis of bacterial folliculitis.
FIGURE
Papules and papulopustules on right axilla
Would you favor this diagnosis?
What would you choose for initial empiric antibiotic treatment?
Diagnosis: MRSA folliculitis
We began empiric systemic antibiotic therapy with doxycycline 100 mg daily and topical treatment with clindamycin 1% lotion twice a day. The bacterial culture came back positive for methicillin-resistant Staphylococcus aureus (MRSA) and the organism proved to be tetracycline sensitive.
Staphylococci species have long been the most common causes of skin and soft tissue infections.1 A recent study in 11 major US cities identified MRSA as the most common cause of acute purulent skin and soft tissue infections in patients presenting to the emergency department.2 This rate, however, varied from 15% to 74% in various cities.2 Today, clinicians must consider MRSA as a potential causative agent whenever they treat a patient with a skin and soft tissue infection.
Community-acquired MRSA: Don’t expect typical risk factors
Community-acquired MRSA (CA-MRSA) cases differ from hospital-acquired MRSA (HA-MRSA) cases demographically, microbiologically, and clinically. CA-MRSA infections typically occur in young, healthy individuals without traditional MRSA risk factors such as recent hospitalization, residence in a long-term care facility, or prior antibiotic use.3,4
CA-MRSA outbreaks have been associated with participation in team sports, living in prison, dormitory, or group home settings, IV drug use, sharing personal items, and men who have sex with men.4,5 However, the prevalence of MRSA infections in patients without any recognized risk factors, like our patient, is increasing.6
CA-MRSA is genetically distinct from HA-MRSA in that it contains the Panton-Valentine leukocidin, an important virulence factor, and carries the type IV or type V SCCmec cassette, which resides at a different methicillin-resistant locus.7,8 CA-MRSA also does not demonstrate the multi-drug resistance typical of HA-MRSA, and these infections tend to be susceptible to most non-beta-lactam antibiotics.6
Is it MRSA or another pathogen?
When considering the differential diagnosis of MRSA folliculitis, consider methicillin-susceptible staphylococci species as equally likely pathogens. Gram-negative bacteria, including Pseudomonas aeruginosa, Malassezia furfur, and Candida species are less common, but notable causes of folliculitis. Other causes include eosinophilic folliculitis, non-bacterial/irritant folliculitis, pseudofolliculitis (chronic in-grown hairs), hidradenitis suppurativa, and acne vulgaris.
Use a low threshold for obtaining cultures CA-MRSA sometimes presents as an edematous, tender abscess, an expanding cellulitis, or both.9,10 These presentations, however, are neither sensitive nor specific. Cutaneous infection may also present as erythematous papules, nodules, pustules, crusted plaques, and infrequently suppurative folliculitis.3,10 Therefore, the clinician should have a low threshold to perform bacterial culture and sensitivity studies whenever a skin and soft tissue infection is suspected.
The potential complications of CA-MRSA infection, including pneumonia, sepsis, and endocarditis, can be avoided or minimized with early diagnosis and initiation of appropriate treatment.4,11,12
Consider community factors
In the past, folliculitis and other localized skin infections were traditionally caused by methicillin-susceptible Staphylococcus aureus and treated with beta-lactam antibiotics. In communities with high prevalence of CA-MRSA skin and soft tissue infections, beta-lactam antibiotics like cephalexin or dicloxacillin may no longer be appropriate.2,5 Therefore, let the prevalence of CA-MRSA in your community help guide your initial antibiotic choice while recognizing that empiric antibiotic therapy may be adjusted after cultures are available.
Good initial choices for CA-MRSA skin and soft tissue infections are trim-ethoprim/sulfamethoxazole with or without rifampin, clindamycin, gentamicin, and tetracycline,2,4,5,7 but inducible clindamycin resistance has been reported.13 if your community has a high prevalence of CA-MRSA, you may decide to begin empiric therapy that provides MRSA coverage.2 In addition, incision and drainage is necessary for adequate treatment of furuncles, carbuncles, and abscesses.5 Healthy, afebrile, immunocompetent patients without cellulitis may not require systemic antibiotics to clear local infections.2,4
Take steps to prevent spread of the disease
Early diagnosis of CA-MRSA infection is important to prevent the spread of this highly virulent disease in the community. It’s important to cover the infected area with sterile gauze and instruct the patient to avoid direct skin-to-skin contact with others, not share sports equipment or personal items, and decontaminate linens and surfaces.4,6
Stress the importance of frequent hand washing and using alcohol-based hand rubs to reduce transmission. Adjuvant topical therapy may be useful: intra-nasal mupirocin 2% ointment to prevent colonization and a persistent carrier state is prudent. Bathing with antimicrobial povidone-iodine or chlorhexidine gluconate is often recommended as an adjunctive treatment for skin and soft tissue infections, colonization, or both.14
Mupirocin 2% for our patient
We instructed our patient to continue the initial medications and to apply mupirocin 2% ointment to the nares twice a day for 1 week to eliminate the possibility of a staphylococcal carrier state.
At follow-up 2 weeks later, only a few excoriated papules and some post-inflammatory erythema remained. The topical clindamycin lotion and the doxycycline were continued for an additional 30 days, at which time the patient was clear and treatment was discontinued.
1. Elston DM. Epidemiology and prevention of skin and soft tissue infections. Cutis 2004;73:S3-7.
2. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, et al. Methicillin-resistant S. aureus. infections among patients in the emergency department. N Engl J Med 2006;355:666-674.
3. Cohen PR, Kurzrock R. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: An emerging clinical problem. J Am Acad Dermatol 2004;50:277-280.
4. Romero DV, Treston J, O’Sullivan AL. Hand-to-hand combat: Preventing MRSA infection. Adv Skin Wound Care 2006;19:328-333.
5. Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infec Dis 2005;11:928-930.
6. Cohen PR, Grossman ME. Management of cutaneous lesions associated with an emerging epidemic: Community-acquired methicillin-resistant Staphylococcus aureus skin infections. J Am Acad Dermatol 2004;51:132-135.
7. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976-2984.
8. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978-984.
9. Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: A retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol 2004;50:854-858.
10. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infection presenting as a periumbilical folliculitis. Cutis. 2006;77:229-231.
11. Bahrain M, Vasiliades M, Wolff M, Younus F. Five cases of bacterial endocarditis after furunculosis and the ongoing saga of community-acquired methicillin-resistant Staphylococcus aureus infections. Scand J Infect Dis 2006;38:702-707.
12. Herold B, Immergluck L, Maranan M, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998;279:593-598.
13. Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis 2003;27:1257-1260.
14. Boyce JM. MRSA patient: proven methods to treat colonization and infection. J Hosp Infect 2001;48:S9-14.
A healthy 29-year-old woman presented to the clinic with pustules and boil-like lesions in the axillary areas, on the left forearm, and on the right thigh (FIGURE). She indicated that she’d had the lesions for 4 weeks. She said that she had not had any recent infections, and there was no evidence of immune compromise in her history.
We incised a pustule in the right axilla and expressed the pus. We sent the sample out for bacterial culture and sensitivity testing.
We immediately considered bacterial folliculitis and hidradenitis suppurativa as part of the differential. The acute nature of the process led us to favor a diagnosis of bacterial folliculitis.
FIGURE
Papules and papulopustules on right axilla
Would you favor this diagnosis?
What would you choose for initial empiric antibiotic treatment?
Diagnosis: MRSA folliculitis
We began empiric systemic antibiotic therapy with doxycycline 100 mg daily and topical treatment with clindamycin 1% lotion twice a day. The bacterial culture came back positive for methicillin-resistant Staphylococcus aureus (MRSA) and the organism proved to be tetracycline sensitive.
Staphylococci species have long been the most common causes of skin and soft tissue infections.1 A recent study in 11 major US cities identified MRSA as the most common cause of acute purulent skin and soft tissue infections in patients presenting to the emergency department.2 This rate, however, varied from 15% to 74% in various cities.2 Today, clinicians must consider MRSA as a potential causative agent whenever they treat a patient with a skin and soft tissue infection.
Community-acquired MRSA: Don’t expect typical risk factors
Community-acquired MRSA (CA-MRSA) cases differ from hospital-acquired MRSA (HA-MRSA) cases demographically, microbiologically, and clinically. CA-MRSA infections typically occur in young, healthy individuals without traditional MRSA risk factors such as recent hospitalization, residence in a long-term care facility, or prior antibiotic use.3,4
CA-MRSA outbreaks have been associated with participation in team sports, living in prison, dormitory, or group home settings, IV drug use, sharing personal items, and men who have sex with men.4,5 However, the prevalence of MRSA infections in patients without any recognized risk factors, like our patient, is increasing.6
CA-MRSA is genetically distinct from HA-MRSA in that it contains the Panton-Valentine leukocidin, an important virulence factor, and carries the type IV or type V SCCmec cassette, which resides at a different methicillin-resistant locus.7,8 CA-MRSA also does not demonstrate the multi-drug resistance typical of HA-MRSA, and these infections tend to be susceptible to most non-beta-lactam antibiotics.6
Is it MRSA or another pathogen?
When considering the differential diagnosis of MRSA folliculitis, consider methicillin-susceptible staphylococci species as equally likely pathogens. Gram-negative bacteria, including Pseudomonas aeruginosa, Malassezia furfur, and Candida species are less common, but notable causes of folliculitis. Other causes include eosinophilic folliculitis, non-bacterial/irritant folliculitis, pseudofolliculitis (chronic in-grown hairs), hidradenitis suppurativa, and acne vulgaris.
Use a low threshold for obtaining cultures CA-MRSA sometimes presents as an edematous, tender abscess, an expanding cellulitis, or both.9,10 These presentations, however, are neither sensitive nor specific. Cutaneous infection may also present as erythematous papules, nodules, pustules, crusted plaques, and infrequently suppurative folliculitis.3,10 Therefore, the clinician should have a low threshold to perform bacterial culture and sensitivity studies whenever a skin and soft tissue infection is suspected.
The potential complications of CA-MRSA infection, including pneumonia, sepsis, and endocarditis, can be avoided or minimized with early diagnosis and initiation of appropriate treatment.4,11,12
Consider community factors
In the past, folliculitis and other localized skin infections were traditionally caused by methicillin-susceptible Staphylococcus aureus and treated with beta-lactam antibiotics. In communities with high prevalence of CA-MRSA skin and soft tissue infections, beta-lactam antibiotics like cephalexin or dicloxacillin may no longer be appropriate.2,5 Therefore, let the prevalence of CA-MRSA in your community help guide your initial antibiotic choice while recognizing that empiric antibiotic therapy may be adjusted after cultures are available.
Good initial choices for CA-MRSA skin and soft tissue infections are trim-ethoprim/sulfamethoxazole with or without rifampin, clindamycin, gentamicin, and tetracycline,2,4,5,7 but inducible clindamycin resistance has been reported.13 if your community has a high prevalence of CA-MRSA, you may decide to begin empiric therapy that provides MRSA coverage.2 In addition, incision and drainage is necessary for adequate treatment of furuncles, carbuncles, and abscesses.5 Healthy, afebrile, immunocompetent patients without cellulitis may not require systemic antibiotics to clear local infections.2,4
Take steps to prevent spread of the disease
Early diagnosis of CA-MRSA infection is important to prevent the spread of this highly virulent disease in the community. It’s important to cover the infected area with sterile gauze and instruct the patient to avoid direct skin-to-skin contact with others, not share sports equipment or personal items, and decontaminate linens and surfaces.4,6
Stress the importance of frequent hand washing and using alcohol-based hand rubs to reduce transmission. Adjuvant topical therapy may be useful: intra-nasal mupirocin 2% ointment to prevent colonization and a persistent carrier state is prudent. Bathing with antimicrobial povidone-iodine or chlorhexidine gluconate is often recommended as an adjunctive treatment for skin and soft tissue infections, colonization, or both.14
Mupirocin 2% for our patient
We instructed our patient to continue the initial medications and to apply mupirocin 2% ointment to the nares twice a day for 1 week to eliminate the possibility of a staphylococcal carrier state.
At follow-up 2 weeks later, only a few excoriated papules and some post-inflammatory erythema remained. The topical clindamycin lotion and the doxycycline were continued for an additional 30 days, at which time the patient was clear and treatment was discontinued.
A healthy 29-year-old woman presented to the clinic with pustules and boil-like lesions in the axillary areas, on the left forearm, and on the right thigh (FIGURE). She indicated that she’d had the lesions for 4 weeks. She said that she had not had any recent infections, and there was no evidence of immune compromise in her history.
We incised a pustule in the right axilla and expressed the pus. We sent the sample out for bacterial culture and sensitivity testing.
We immediately considered bacterial folliculitis and hidradenitis suppurativa as part of the differential. The acute nature of the process led us to favor a diagnosis of bacterial folliculitis.
FIGURE
Papules and papulopustules on right axilla
Would you favor this diagnosis?
What would you choose for initial empiric antibiotic treatment?
Diagnosis: MRSA folliculitis
We began empiric systemic antibiotic therapy with doxycycline 100 mg daily and topical treatment with clindamycin 1% lotion twice a day. The bacterial culture came back positive for methicillin-resistant Staphylococcus aureus (MRSA) and the organism proved to be tetracycline sensitive.
Staphylococci species have long been the most common causes of skin and soft tissue infections.1 A recent study in 11 major US cities identified MRSA as the most common cause of acute purulent skin and soft tissue infections in patients presenting to the emergency department.2 This rate, however, varied from 15% to 74% in various cities.2 Today, clinicians must consider MRSA as a potential causative agent whenever they treat a patient with a skin and soft tissue infection.
Community-acquired MRSA: Don’t expect typical risk factors
Community-acquired MRSA (CA-MRSA) cases differ from hospital-acquired MRSA (HA-MRSA) cases demographically, microbiologically, and clinically. CA-MRSA infections typically occur in young, healthy individuals without traditional MRSA risk factors such as recent hospitalization, residence in a long-term care facility, or prior antibiotic use.3,4
CA-MRSA outbreaks have been associated with participation in team sports, living in prison, dormitory, or group home settings, IV drug use, sharing personal items, and men who have sex with men.4,5 However, the prevalence of MRSA infections in patients without any recognized risk factors, like our patient, is increasing.6
CA-MRSA is genetically distinct from HA-MRSA in that it contains the Panton-Valentine leukocidin, an important virulence factor, and carries the type IV or type V SCCmec cassette, which resides at a different methicillin-resistant locus.7,8 CA-MRSA also does not demonstrate the multi-drug resistance typical of HA-MRSA, and these infections tend to be susceptible to most non-beta-lactam antibiotics.6
Is it MRSA or another pathogen?
When considering the differential diagnosis of MRSA folliculitis, consider methicillin-susceptible staphylococci species as equally likely pathogens. Gram-negative bacteria, including Pseudomonas aeruginosa, Malassezia furfur, and Candida species are less common, but notable causes of folliculitis. Other causes include eosinophilic folliculitis, non-bacterial/irritant folliculitis, pseudofolliculitis (chronic in-grown hairs), hidradenitis suppurativa, and acne vulgaris.
Use a low threshold for obtaining cultures CA-MRSA sometimes presents as an edematous, tender abscess, an expanding cellulitis, or both.9,10 These presentations, however, are neither sensitive nor specific. Cutaneous infection may also present as erythematous papules, nodules, pustules, crusted plaques, and infrequently suppurative folliculitis.3,10 Therefore, the clinician should have a low threshold to perform bacterial culture and sensitivity studies whenever a skin and soft tissue infection is suspected.
The potential complications of CA-MRSA infection, including pneumonia, sepsis, and endocarditis, can be avoided or minimized with early diagnosis and initiation of appropriate treatment.4,11,12
Consider community factors
In the past, folliculitis and other localized skin infections were traditionally caused by methicillin-susceptible Staphylococcus aureus and treated with beta-lactam antibiotics. In communities with high prevalence of CA-MRSA skin and soft tissue infections, beta-lactam antibiotics like cephalexin or dicloxacillin may no longer be appropriate.2,5 Therefore, let the prevalence of CA-MRSA in your community help guide your initial antibiotic choice while recognizing that empiric antibiotic therapy may be adjusted after cultures are available.
Good initial choices for CA-MRSA skin and soft tissue infections are trim-ethoprim/sulfamethoxazole with or without rifampin, clindamycin, gentamicin, and tetracycline,2,4,5,7 but inducible clindamycin resistance has been reported.13 if your community has a high prevalence of CA-MRSA, you may decide to begin empiric therapy that provides MRSA coverage.2 In addition, incision and drainage is necessary for adequate treatment of furuncles, carbuncles, and abscesses.5 Healthy, afebrile, immunocompetent patients without cellulitis may not require systemic antibiotics to clear local infections.2,4
Take steps to prevent spread of the disease
Early diagnosis of CA-MRSA infection is important to prevent the spread of this highly virulent disease in the community. It’s important to cover the infected area with sterile gauze and instruct the patient to avoid direct skin-to-skin contact with others, not share sports equipment or personal items, and decontaminate linens and surfaces.4,6
Stress the importance of frequent hand washing and using alcohol-based hand rubs to reduce transmission. Adjuvant topical therapy may be useful: intra-nasal mupirocin 2% ointment to prevent colonization and a persistent carrier state is prudent. Bathing with antimicrobial povidone-iodine or chlorhexidine gluconate is often recommended as an adjunctive treatment for skin and soft tissue infections, colonization, or both.14
Mupirocin 2% for our patient
We instructed our patient to continue the initial medications and to apply mupirocin 2% ointment to the nares twice a day for 1 week to eliminate the possibility of a staphylococcal carrier state.
At follow-up 2 weeks later, only a few excoriated papules and some post-inflammatory erythema remained. The topical clindamycin lotion and the doxycycline were continued for an additional 30 days, at which time the patient was clear and treatment was discontinued.
1. Elston DM. Epidemiology and prevention of skin and soft tissue infections. Cutis 2004;73:S3-7.
2. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, et al. Methicillin-resistant S. aureus. infections among patients in the emergency department. N Engl J Med 2006;355:666-674.
3. Cohen PR, Kurzrock R. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: An emerging clinical problem. J Am Acad Dermatol 2004;50:277-280.
4. Romero DV, Treston J, O’Sullivan AL. Hand-to-hand combat: Preventing MRSA infection. Adv Skin Wound Care 2006;19:328-333.
5. Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infec Dis 2005;11:928-930.
6. Cohen PR, Grossman ME. Management of cutaneous lesions associated with an emerging epidemic: Community-acquired methicillin-resistant Staphylococcus aureus skin infections. J Am Acad Dermatol 2004;51:132-135.
7. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976-2984.
8. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978-984.
9. Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: A retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol 2004;50:854-858.
10. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infection presenting as a periumbilical folliculitis. Cutis. 2006;77:229-231.
11. Bahrain M, Vasiliades M, Wolff M, Younus F. Five cases of bacterial endocarditis after furunculosis and the ongoing saga of community-acquired methicillin-resistant Staphylococcus aureus infections. Scand J Infect Dis 2006;38:702-707.
12. Herold B, Immergluck L, Maranan M, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998;279:593-598.
13. Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis 2003;27:1257-1260.
14. Boyce JM. MRSA patient: proven methods to treat colonization and infection. J Hosp Infect 2001;48:S9-14.
1. Elston DM. Epidemiology and prevention of skin and soft tissue infections. Cutis 2004;73:S3-7.
2. Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, et al. Methicillin-resistant S. aureus. infections among patients in the emergency department. N Engl J Med 2006;355:666-674.
3. Cohen PR, Kurzrock R. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: An emerging clinical problem. J Am Acad Dermatol 2004;50:277-280.
4. Romero DV, Treston J, O’Sullivan AL. Hand-to-hand combat: Preventing MRSA infection. Adv Skin Wound Care 2006;19:328-333.
5. Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infec Dis 2005;11:928-930.
6. Cohen PR, Grossman ME. Management of cutaneous lesions associated with an emerging epidemic: Community-acquired methicillin-resistant Staphylococcus aureus skin infections. J Am Acad Dermatol 2004;51:132-135.
7. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003;290:2976-2984.
8. Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 2003;9:978-984.
9. Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: A retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol 2004;50:854-858.
10. Cohen PR. Community-acquired methicillin-resistant Staphylococcus aureus skin infection presenting as a periumbilical folliculitis. Cutis. 2006;77:229-231.
11. Bahrain M, Vasiliades M, Wolff M, Younus F. Five cases of bacterial endocarditis after furunculosis and the ongoing saga of community-acquired methicillin-resistant Staphylococcus aureus infections. Scand J Infect Dis 2006;38:702-707.
12. Herold B, Immergluck L, Maranan M, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998;279:593-598.
13. Siberry GK, Tekle T, Carroll K, Dick J. Failure of clindamycin treatment of methicillin-resistant Staphylococcus aureus expressing inducible clindamycin resistance in vitro. Clin Infect Dis 2003;27:1257-1260.
14. Boyce JM. MRSA patient: proven methods to treat colonization and infection. J Hosp Infect 2001;48:S9-14.