User login
Frailty Tools are Not Yet Ready for Prime Time in High-Risk Identification
In this issue of the Journal of Hospital Medicine, McAlister et al.1 compared the ability of the Clinical Frailty Scale (CFS) and the Hospital Frailty Risk Score (HFRS) to predict 30-day readmission or death. The authors prospectively assessed adult patients aged ≥18 years without cognitive impairment being discharged back to the community after medical admissions. They demonstrated only modest overlap in frailty designation between HFRS and CFS and concluded that CFS is better than HFRS for predicting the outcomes of interest.
Before a prediction rule is widely adopted for use in routine practice, robust external validation is needed.2 Factors such as the prevalence of disease in a population, the clinical competencies of a health system, the socioeconomic status, and the ethnicity of the population can all affect how well a clinical rule performs, but may not become apparent until a prospective validation in a different population is attempted.
In developing the HFRS, Gilbert et al. aimed to create a low-cost, highly generalizable method of identifying frailty using International Classification of Diseases (ICD) 10 billing codes.3 The derivation and validation cohorts for HFRS included older adults aged >75 years in the United Kingdom, many of whom had cognitive impairment. Therefore, it is not surprising that the tool behaved very differently in the younger Canadian cohort described by McAlister et al. where persons with cognitive impairment were excluded. That the HFRS had less predictability in the Canadian cohort may simply indicate that it performs better in an older population with cognitive vulnerabilities; given the frailty constructs of the CFS, it may provide less insights in older populations.
We applaud the efforts to find a way to better identify high-risk groups of adults. We also appreciate the increasing attention to function and other frailty-related domains in risk prediction models. Nevertheless, we recommend caution in using any of the many existing frailty indices4 in risk prediction tools unless it is clear what domains of frailty are most relevant for the predicted outcome and what population is the subject of interest.
One of the challenges of choosing an appropriate frailty tool is that different tools are measuring different domains or constructs of frailty. Most consider frailty either as a physical phenotype5 or as a more multifaceted construct with impairments in physical and mental health, function, and social interaction.6 There is often poor overlap between those individuals identified as frail by different measures, highlighting that they are in fact identifying different people within the population studied and have different predictive abilities.
An ideal frailty tool for clinical use would allow clinicians to identify high-risk patients relative to specific outcome(s) in real time prior to discharge from hospital or prior to a sentinel event in the community. CFS can be calculated at the bedside, but HFRS calculation can only be done retrospectively when medical records are coded for claims after discharge. This makes HFRS more suited to research or post hoc quality measure work and CFS more suited to clinical use as the authors describe.
Although using a frailty indicator to help determine those at high risk of early readmission is an important objective, the presence of frailty accounts for only part of a person’s risk for readmission or other untoward events. Reasons for readmissions are complex and often heavily weighted on a lack of social and community supports. A deeper understanding of the reasons for readmission is needed to establish whether readmission of these complex patients has more to do with frailty or other drivers such as poor transitions of care.
The prevalence of frailty will continue to increase as our population ages. Definitions of frailty vary, but there is a broad agreement that frailty, regardless of how it is constructed, increases with age, results in multisystem changes, and leads to increased healthcare utilization and costs. Preventing the development of frailty, identifying frailty, and developing interventions to address frailty in and out of the hospital setting are all vital. We welcome further research regarding the biopsychosocial constructs of frailty, how they overlap with the frailty phenotype, and how these constructs inform both our understanding of frailty and the use of frailty tools.
Disclosures
The authors have no conflicts of interest to report.
1. McAlister FA, Lin M, Bakal JA. Prevalence and Postdischarge Outcomes Associated with Frailty in Medical Inpatients: Impact of Different Frailty Definitions. J Hosp Med. 2019;14(7):407-410. doi: 10.12788/jhm.3174 PubMed
2. Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313(13):793-799. doi: 10.1056/NEJM198509263131306. PubMed
3. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8. PubMed
4. de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 0.1016/j.arr.2010.09.001. PubMed
5. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3);M146-M156. PubMed
6. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10-12. doi: 10.1093/ageing/aft160. PubMed
In this issue of the Journal of Hospital Medicine, McAlister et al.1 compared the ability of the Clinical Frailty Scale (CFS) and the Hospital Frailty Risk Score (HFRS) to predict 30-day readmission or death. The authors prospectively assessed adult patients aged ≥18 years without cognitive impairment being discharged back to the community after medical admissions. They demonstrated only modest overlap in frailty designation between HFRS and CFS and concluded that CFS is better than HFRS for predicting the outcomes of interest.
Before a prediction rule is widely adopted for use in routine practice, robust external validation is needed.2 Factors such as the prevalence of disease in a population, the clinical competencies of a health system, the socioeconomic status, and the ethnicity of the population can all affect how well a clinical rule performs, but may not become apparent until a prospective validation in a different population is attempted.
In developing the HFRS, Gilbert et al. aimed to create a low-cost, highly generalizable method of identifying frailty using International Classification of Diseases (ICD) 10 billing codes.3 The derivation and validation cohorts for HFRS included older adults aged >75 years in the United Kingdom, many of whom had cognitive impairment. Therefore, it is not surprising that the tool behaved very differently in the younger Canadian cohort described by McAlister et al. where persons with cognitive impairment were excluded. That the HFRS had less predictability in the Canadian cohort may simply indicate that it performs better in an older population with cognitive vulnerabilities; given the frailty constructs of the CFS, it may provide less insights in older populations.
We applaud the efforts to find a way to better identify high-risk groups of adults. We also appreciate the increasing attention to function and other frailty-related domains in risk prediction models. Nevertheless, we recommend caution in using any of the many existing frailty indices4 in risk prediction tools unless it is clear what domains of frailty are most relevant for the predicted outcome and what population is the subject of interest.
One of the challenges of choosing an appropriate frailty tool is that different tools are measuring different domains or constructs of frailty. Most consider frailty either as a physical phenotype5 or as a more multifaceted construct with impairments in physical and mental health, function, and social interaction.6 There is often poor overlap between those individuals identified as frail by different measures, highlighting that they are in fact identifying different people within the population studied and have different predictive abilities.
An ideal frailty tool for clinical use would allow clinicians to identify high-risk patients relative to specific outcome(s) in real time prior to discharge from hospital or prior to a sentinel event in the community. CFS can be calculated at the bedside, but HFRS calculation can only be done retrospectively when medical records are coded for claims after discharge. This makes HFRS more suited to research or post hoc quality measure work and CFS more suited to clinical use as the authors describe.
Although using a frailty indicator to help determine those at high risk of early readmission is an important objective, the presence of frailty accounts for only part of a person’s risk for readmission or other untoward events. Reasons for readmissions are complex and often heavily weighted on a lack of social and community supports. A deeper understanding of the reasons for readmission is needed to establish whether readmission of these complex patients has more to do with frailty or other drivers such as poor transitions of care.
The prevalence of frailty will continue to increase as our population ages. Definitions of frailty vary, but there is a broad agreement that frailty, regardless of how it is constructed, increases with age, results in multisystem changes, and leads to increased healthcare utilization and costs. Preventing the development of frailty, identifying frailty, and developing interventions to address frailty in and out of the hospital setting are all vital. We welcome further research regarding the biopsychosocial constructs of frailty, how they overlap with the frailty phenotype, and how these constructs inform both our understanding of frailty and the use of frailty tools.
Disclosures
The authors have no conflicts of interest to report.
In this issue of the Journal of Hospital Medicine, McAlister et al.1 compared the ability of the Clinical Frailty Scale (CFS) and the Hospital Frailty Risk Score (HFRS) to predict 30-day readmission or death. The authors prospectively assessed adult patients aged ≥18 years without cognitive impairment being discharged back to the community after medical admissions. They demonstrated only modest overlap in frailty designation between HFRS and CFS and concluded that CFS is better than HFRS for predicting the outcomes of interest.
Before a prediction rule is widely adopted for use in routine practice, robust external validation is needed.2 Factors such as the prevalence of disease in a population, the clinical competencies of a health system, the socioeconomic status, and the ethnicity of the population can all affect how well a clinical rule performs, but may not become apparent until a prospective validation in a different population is attempted.
In developing the HFRS, Gilbert et al. aimed to create a low-cost, highly generalizable method of identifying frailty using International Classification of Diseases (ICD) 10 billing codes.3 The derivation and validation cohorts for HFRS included older adults aged >75 years in the United Kingdom, many of whom had cognitive impairment. Therefore, it is not surprising that the tool behaved very differently in the younger Canadian cohort described by McAlister et al. where persons with cognitive impairment were excluded. That the HFRS had less predictability in the Canadian cohort may simply indicate that it performs better in an older population with cognitive vulnerabilities; given the frailty constructs of the CFS, it may provide less insights in older populations.
We applaud the efforts to find a way to better identify high-risk groups of adults. We also appreciate the increasing attention to function and other frailty-related domains in risk prediction models. Nevertheless, we recommend caution in using any of the many existing frailty indices4 in risk prediction tools unless it is clear what domains of frailty are most relevant for the predicted outcome and what population is the subject of interest.
One of the challenges of choosing an appropriate frailty tool is that different tools are measuring different domains or constructs of frailty. Most consider frailty either as a physical phenotype5 or as a more multifaceted construct with impairments in physical and mental health, function, and social interaction.6 There is often poor overlap between those individuals identified as frail by different measures, highlighting that they are in fact identifying different people within the population studied and have different predictive abilities.
An ideal frailty tool for clinical use would allow clinicians to identify high-risk patients relative to specific outcome(s) in real time prior to discharge from hospital or prior to a sentinel event in the community. CFS can be calculated at the bedside, but HFRS calculation can only be done retrospectively when medical records are coded for claims after discharge. This makes HFRS more suited to research or post hoc quality measure work and CFS more suited to clinical use as the authors describe.
Although using a frailty indicator to help determine those at high risk of early readmission is an important objective, the presence of frailty accounts for only part of a person’s risk for readmission or other untoward events. Reasons for readmissions are complex and often heavily weighted on a lack of social and community supports. A deeper understanding of the reasons for readmission is needed to establish whether readmission of these complex patients has more to do with frailty or other drivers such as poor transitions of care.
The prevalence of frailty will continue to increase as our population ages. Definitions of frailty vary, but there is a broad agreement that frailty, regardless of how it is constructed, increases with age, results in multisystem changes, and leads to increased healthcare utilization and costs. Preventing the development of frailty, identifying frailty, and developing interventions to address frailty in and out of the hospital setting are all vital. We welcome further research regarding the biopsychosocial constructs of frailty, how they overlap with the frailty phenotype, and how these constructs inform both our understanding of frailty and the use of frailty tools.
Disclosures
The authors have no conflicts of interest to report.
1. McAlister FA, Lin M, Bakal JA. Prevalence and Postdischarge Outcomes Associated with Frailty in Medical Inpatients: Impact of Different Frailty Definitions. J Hosp Med. 2019;14(7):407-410. doi: 10.12788/jhm.3174 PubMed
2. Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313(13):793-799. doi: 10.1056/NEJM198509263131306. PubMed
3. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8. PubMed
4. de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 0.1016/j.arr.2010.09.001. PubMed
5. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3);M146-M156. PubMed
6. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10-12. doi: 10.1093/ageing/aft160. PubMed
1. McAlister FA, Lin M, Bakal JA. Prevalence and Postdischarge Outcomes Associated with Frailty in Medical Inpatients: Impact of Different Frailty Definitions. J Hosp Med. 2019;14(7):407-410. doi: 10.12788/jhm.3174 PubMed
2. Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313(13):793-799. doi: 10.1056/NEJM198509263131306. PubMed
3. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8. PubMed
4. de Vries NM, Staal JB, van Ravensberg CD, et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 0.1016/j.arr.2010.09.001. PubMed
5. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3);M146-M156. PubMed
6. Cesari M, Gambassi G, van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43(1):10-12. doi: 10.1093/ageing/aft160. PubMed
© 2019 Society of Hospital Medicine
Association of Weekend Admission and Weekend Discharge with Length of Stay and 30-Day Readmission in Children’s Hospitals
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
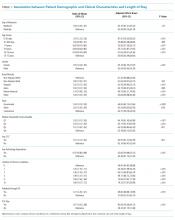
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
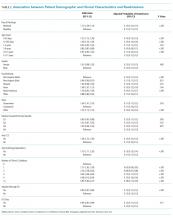
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
© 2018 Society of Hospital Medicine
Instability After Reverse Total Shoulder Arthroplasty: Which Patients Dislocate?
Risk factors for dislocation after reverse total shoulder arthroplasty (RTSA) are not clearly defined. Prosthetic dislocation can result in poor patient satisfaction, worse functional outcomes, and return to the operating room.1-3 As a result, identification of modifiable risk factors for complications represents an important research initiative for shoulder surgeons.
There is a paucity of literature devoted to the study of dislocation after RTSA. Chalmers and colleagues4 found a 2.9% (11/385) incidence of early dislocation within 3 months after index surgery—an improvement over the 15.8% reported for early instability over the period 2004–2006.5 As prosthesis design has improved and surgeons have become more comfortable with the RTSA prosthesis, surgical indications have expanded,6,7 and dislocation rates appear to have decreased. Although the most common indication for RTSA continues to be cuff tear arthropathy (CTA),6 there has been increased use in rheumatoid arthritis8-10; proximal humerus fractures, especially in cases of poor bone quality and unreliable fixation of tuberosities11-13; and failed previous shoulder reconstruction.14,15 As RTSA is performed more often, limiting the complications will become more important for both patient care and economics.
We conducted a study to analyze dislocation rates at our institution and to identify both modifiable and nonmodifiable risk factors for dislocation after RTSA. By identifying risk factors for dislocation, we will be able to implement additional perioperative clinical measures to reduce the incidence of dislocation.
Materials and Methods
This retrospective study of dislocation after RTSA was conducted at the Rothman Institute of Orthopedics and Methodist Hospital (Thomas Jefferson University Hospitals, Philadelphia, PA). After obtaining Institutional Review Board approval for the study, we searched our institution’s electronic database of shoulder arthroplasties to identify all RTSAs performed at our 2 large-volume urban institutions between September 27, 2010 and December 31, 2013. For the record search, International Classification of Diseases, Ninth Revision (ICD-9) codes were used (Table 1).
The medical records of each patient were used to identify independent variables that could be associated with dislocation rate. Demographic variables included sex, age, and race. Preoperative clinical data included body mass index (BMI), etiology of shoulder disease leading to RTSA, individual comorbidities, and Charlson Comorbidity Index (CCI)16 modified to be used with ICD-9 codes.17 In addition, prior shoulder surgery history and arthroplasty type (primary or revision) were determined. Postoperative considerations were time to dislocation, mechanism of dislocation, and intervention(s) needed for dislocation. Although the institutional database did not include operative variables such as prosthesis type and surgical approach, all 6 surgeons in this study were using a standard deltopectoral approach in beach-chair position with a Grammont style prosthesis for RTSA cases.
Descriptive statistics for RTSA patients and the dislocation subpopulation were compiled. Bivariate analysis was used to evaluate which of the previously described variables influenced dislocation rates. Last, multivariate logistic regression analysis was performed to evaluate which factors were independent predictors of dislocation. We included demographic variables (age, sex, ethnicity), clinical variables (BMI, individual comorbidities, CCI), and surgical variables (primary vs revision, diagnosis at time of surgery). All statistical analyses were performed with Excel 2013 (Microsoft) and SPSS Statistics Version 20.0 (SPSS Inc.).
Results
From the database, we identified 487 patients who underwent 510 RTSAs during the study period. These surgeries were performed by 6 shoulder and elbow fellowship–trained surgeons. Of the 510 RTSAs, 393 (77.1%) were primary cases, and 117 (22.9%) were revision cases.
Of the 510 shoulders that underwent RTSA, 15 (2.9%; 14 patients) dislocated. Of these 15 cases, 5 were primary (1.3% of all primary cases) and 10 were revision (8.5% of all revision cases). Mean time from index surgery to diagnosis of dislocation was 58.2 days (range, 0-319 days). One dislocation occurred immediately after surgery, 2 after falls, 4 from patient-identified low-energy mechanisms of injury, and 8 without known inciting events. Nine dislocations (60%) did not have a subscapularis repair (7 were irreparable, 2 underwent subscapularis peel without repair), and the other 6 were repaired primarily (Table 2).
Male patients accounted for 32.2% of the study population but 60.0% of the dislocations (P = .019) (Table 3).
Multivariate logistic regression analysis revealed revision arthroplasty (OR = 7.515; P = .042) and increased BMI (OR = 1.09; P = .047) to be independent risk factors for dislocation after RTSA. Analysis also found a diagnosis of primary CTA to be independently associated with lower risk of dislocation after RTSA (OR = 0.025; P = .008). Last, the previously described risk factor of male sex was found not to be a significant independent risk factor, though it did trend positively (OR = 3.011; P = .071).
Discussion
With more RTSAs being performed, evaluation of their common complications becomes increasingly important.18 We found a 3.0% rate of dislocation after RTSA, which is consistent with the most recently reported incidence4 and falls within the previously described range of 0% to 8.6%.19-26 Of the clinical risk factors identified in this study, those previously described were prior surgery, subscapularis insufficiency, higher BMI, and male sex.4 However, our finding of lower risk of dislocation after RTSA for primary rotator cuff pathology was not previously described. Although Chalmers and colleagues4 did not report this lower risk, 3 (27.3%) of their 11 patients with dislocation had primary CTA, compared with 1 (6.7%) of 15 patients in the present study.4 Our literature review did not identify any studies that independently reported the dislocation rate in patients who underwent RTSA for rotator cuff failure.
The risk factors of subscapularis irreparability and revision surgery suggest the importance of the soft-tissue envelope and bony anatomy in dislocation prevention. Previous analyses have suggested implant malpositioning,27,28 poor subscapularis quality,29 and inadequate muscle tensioning5,30-32 as risk factors for RTSA. Patients with an irreparable subscapularis tendon have often had multiple surgeries with compromise to the muscle/soft-tissue envelope or bony anatomy of the shoulder. A biomechanical study by Gutiérrez and colleagues31 found the compressive forces of the soft tissue at the glenohumeral joint to be the most important contributor to stability in the RTSA prosthesis. In clinical studies, the role of the subscapularis in preventing instability after RTSA remains unclear. Edwards and colleagues29 prospectively compared dislocation rates in patients with reparable and irreparable subscapularis tendons during RTSA and found a higher rate of dislocation in the irreparable subscapularis group. Of note, patients in the irreparable subscapularis group also had more complex diagnoses, including proximal humeral nonunion, fixed glenohumeral dislocation, and failed prior arthroplasty. Clark and colleagues33 retrospectively analyzed subscapularis repair in 2 RTSA groups and found no appreciable effect on complication rate, dislocation events, range-of-motion gains, or pain relief.
Our finding that higher BMI is an independent risk factor was previously described.4 The association is unclear but could be related to implant positioning, difficulty in intraoperative assessment of muscle tensioning, or body habitus that may generate a lever arm for impingement and dislocation when the arm is in adduction. Last, our finding that male sex is a risk factor for dislocation approached significance, and this relationship was previously reported.4 This could be attributable to a higher rate of activity or of indolent infection in male patients.34,35Besides studying risk factors for dislocation after RTSA, we investigated treatment. None of our patients were treated successfully and definitively with closed reduction in the clinic. This finding diverges from findings in studies by Teusink and colleagues2 and Chalmers and colleagues,4who respectively reported 62% and 44% rates of success with closed reduction. Our cohort of 14 patients with 15 dislocations required a total of 17 trips to the operating room after dislocation. This significantly higher rate of return to the operating room suggests that dislocation after RTSA may be a more costly and morbid problem than has been previously described.
This study had several weaknesses. Despite its large consecutive series of patients, the study was retrospective, and several variables that would be documented and controlled in a prospective study could not be measured here. Specifically, neither preoperative physical examination nor patient-specific assessments of pain or function were consistently obtained. Similarly, postoperative patient-specific instruments of outcomes evaluation were not obtained consistently, so results of patients with dislocation could not be compared with those of a control group. In addition, preoperative and postoperative radiographs were not consistently present in our electronic medical records, so the influence of preoperative bony anatomy, intraoperative limb lengthening, and any implant malpositioning could not be determined. Furthermore, operative details, such as reparability of the subscapularis, were not fully available for the control group and could not be included in statistical analysis. In addition, that the known dislocation risk factor of male sex4 was identified here but was not significant in multivariate regression analysis suggests that this study may not have been adequately powered to identify a significant difference in dislocation rate between the sexes. Last, though our results suggested associations between the aforementioned variables and dislocation after RTSA, a truly causative relationship could not be confirmed with this study design or analysis. Therefore, our study findings are hypothesis-generating and may indicate a benefit to greater deltoid tensioning, use of retentive liners, or more conservative rehabilitation protocols for high-risk patients.
Conclusion
Dislocation after RTSA is an uncommon complication that often requires a return to the operating room. This study identified a modifiable risk factor (higher BMI) and 3 nonmodifiable risk factors (male sex, subscapularis insufficiency, revision surgery) for dislocation after RTSA. In contrast, patients who undergo RTSA for primary rotator cuff pathology are unlikely to dislocate after surgery. This low risk of dislocation after RTSA for primary cuff pathology was not previously described. Patients in the higher risk category may benefit from preoperative lifestyle modification, intraoperative techniques for increasing stability, and more conservative therapy after surgery. In addition, unlike previous investigations, this study did not find closed reduction in the clinic alone to be successful in definitively treating this patient population.
Am J Orthop. 2016;45(7):E444-E450. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
2. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(4):621-627.
3. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
4. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
5. Gallo RA, Gamradt SC, Mattern CJ, et al; Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584-590.
6. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
7. Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br. 2012;94(5):577-583.
8. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
9. Hedtmann A, Werner A. Shoulder arthroplasty in rheumatoid arthritis [in German]. Orthopade. 2007;36(11):1050-1061.
10. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
11. Acevedo DC, Vanbeek C, Lazarus MD, Williams GR, Abboud JA. Reverse shoulder arthroplasty for proximal humeral fractures: update on indications, technique, and results. J Shoulder Elbow Surg. 2014;23(2):279-289.
12. Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516-520.
13. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
14. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522.
15. Valenti P, Kilinc AS, Sauzières P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
18. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
19. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
20. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
21. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
24. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
25. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
26. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
27. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535-539.
28. Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652-658.
29. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892-896.
30. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
31. Gutiérrez S, Keller TS, Levy JC, Lee WE 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670-676.
32. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
33. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
34. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472(9):2809-2815.
35. Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21(11):1534-1541.
Risk factors for dislocation after reverse total shoulder arthroplasty (RTSA) are not clearly defined. Prosthetic dislocation can result in poor patient satisfaction, worse functional outcomes, and return to the operating room.1-3 As a result, identification of modifiable risk factors for complications represents an important research initiative for shoulder surgeons.
There is a paucity of literature devoted to the study of dislocation after RTSA. Chalmers and colleagues4 found a 2.9% (11/385) incidence of early dislocation within 3 months after index surgery—an improvement over the 15.8% reported for early instability over the period 2004–2006.5 As prosthesis design has improved and surgeons have become more comfortable with the RTSA prosthesis, surgical indications have expanded,6,7 and dislocation rates appear to have decreased. Although the most common indication for RTSA continues to be cuff tear arthropathy (CTA),6 there has been increased use in rheumatoid arthritis8-10; proximal humerus fractures, especially in cases of poor bone quality and unreliable fixation of tuberosities11-13; and failed previous shoulder reconstruction.14,15 As RTSA is performed more often, limiting the complications will become more important for both patient care and economics.
We conducted a study to analyze dislocation rates at our institution and to identify both modifiable and nonmodifiable risk factors for dislocation after RTSA. By identifying risk factors for dislocation, we will be able to implement additional perioperative clinical measures to reduce the incidence of dislocation.
Materials and Methods
This retrospective study of dislocation after RTSA was conducted at the Rothman Institute of Orthopedics and Methodist Hospital (Thomas Jefferson University Hospitals, Philadelphia, PA). After obtaining Institutional Review Board approval for the study, we searched our institution’s electronic database of shoulder arthroplasties to identify all RTSAs performed at our 2 large-volume urban institutions between September 27, 2010 and December 31, 2013. For the record search, International Classification of Diseases, Ninth Revision (ICD-9) codes were used (Table 1).
The medical records of each patient were used to identify independent variables that could be associated with dislocation rate. Demographic variables included sex, age, and race. Preoperative clinical data included body mass index (BMI), etiology of shoulder disease leading to RTSA, individual comorbidities, and Charlson Comorbidity Index (CCI)16 modified to be used with ICD-9 codes.17 In addition, prior shoulder surgery history and arthroplasty type (primary or revision) were determined. Postoperative considerations were time to dislocation, mechanism of dislocation, and intervention(s) needed for dislocation. Although the institutional database did not include operative variables such as prosthesis type and surgical approach, all 6 surgeons in this study were using a standard deltopectoral approach in beach-chair position with a Grammont style prosthesis for RTSA cases.
Descriptive statistics for RTSA patients and the dislocation subpopulation were compiled. Bivariate analysis was used to evaluate which of the previously described variables influenced dislocation rates. Last, multivariate logistic regression analysis was performed to evaluate which factors were independent predictors of dislocation. We included demographic variables (age, sex, ethnicity), clinical variables (BMI, individual comorbidities, CCI), and surgical variables (primary vs revision, diagnosis at time of surgery). All statistical analyses were performed with Excel 2013 (Microsoft) and SPSS Statistics Version 20.0 (SPSS Inc.).
Results
From the database, we identified 487 patients who underwent 510 RTSAs during the study period. These surgeries were performed by 6 shoulder and elbow fellowship–trained surgeons. Of the 510 RTSAs, 393 (77.1%) were primary cases, and 117 (22.9%) were revision cases.
Of the 510 shoulders that underwent RTSA, 15 (2.9%; 14 patients) dislocated. Of these 15 cases, 5 were primary (1.3% of all primary cases) and 10 were revision (8.5% of all revision cases). Mean time from index surgery to diagnosis of dislocation was 58.2 days (range, 0-319 days). One dislocation occurred immediately after surgery, 2 after falls, 4 from patient-identified low-energy mechanisms of injury, and 8 without known inciting events. Nine dislocations (60%) did not have a subscapularis repair (7 were irreparable, 2 underwent subscapularis peel without repair), and the other 6 were repaired primarily (Table 2).
Male patients accounted for 32.2% of the study population but 60.0% of the dislocations (P = .019) (Table 3).
Multivariate logistic regression analysis revealed revision arthroplasty (OR = 7.515; P = .042) and increased BMI (OR = 1.09; P = .047) to be independent risk factors for dislocation after RTSA. Analysis also found a diagnosis of primary CTA to be independently associated with lower risk of dislocation after RTSA (OR = 0.025; P = .008). Last, the previously described risk factor of male sex was found not to be a significant independent risk factor, though it did trend positively (OR = 3.011; P = .071).
Discussion
With more RTSAs being performed, evaluation of their common complications becomes increasingly important.18 We found a 3.0% rate of dislocation after RTSA, which is consistent with the most recently reported incidence4 and falls within the previously described range of 0% to 8.6%.19-26 Of the clinical risk factors identified in this study, those previously described were prior surgery, subscapularis insufficiency, higher BMI, and male sex.4 However, our finding of lower risk of dislocation after RTSA for primary rotator cuff pathology was not previously described. Although Chalmers and colleagues4 did not report this lower risk, 3 (27.3%) of their 11 patients with dislocation had primary CTA, compared with 1 (6.7%) of 15 patients in the present study.4 Our literature review did not identify any studies that independently reported the dislocation rate in patients who underwent RTSA for rotator cuff failure.
The risk factors of subscapularis irreparability and revision surgery suggest the importance of the soft-tissue envelope and bony anatomy in dislocation prevention. Previous analyses have suggested implant malpositioning,27,28 poor subscapularis quality,29 and inadequate muscle tensioning5,30-32 as risk factors for RTSA. Patients with an irreparable subscapularis tendon have often had multiple surgeries with compromise to the muscle/soft-tissue envelope or bony anatomy of the shoulder. A biomechanical study by Gutiérrez and colleagues31 found the compressive forces of the soft tissue at the glenohumeral joint to be the most important contributor to stability in the RTSA prosthesis. In clinical studies, the role of the subscapularis in preventing instability after RTSA remains unclear. Edwards and colleagues29 prospectively compared dislocation rates in patients with reparable and irreparable subscapularis tendons during RTSA and found a higher rate of dislocation in the irreparable subscapularis group. Of note, patients in the irreparable subscapularis group also had more complex diagnoses, including proximal humeral nonunion, fixed glenohumeral dislocation, and failed prior arthroplasty. Clark and colleagues33 retrospectively analyzed subscapularis repair in 2 RTSA groups and found no appreciable effect on complication rate, dislocation events, range-of-motion gains, or pain relief.
Our finding that higher BMI is an independent risk factor was previously described.4 The association is unclear but could be related to implant positioning, difficulty in intraoperative assessment of muscle tensioning, or body habitus that may generate a lever arm for impingement and dislocation when the arm is in adduction. Last, our finding that male sex is a risk factor for dislocation approached significance, and this relationship was previously reported.4 This could be attributable to a higher rate of activity or of indolent infection in male patients.34,35Besides studying risk factors for dislocation after RTSA, we investigated treatment. None of our patients were treated successfully and definitively with closed reduction in the clinic. This finding diverges from findings in studies by Teusink and colleagues2 and Chalmers and colleagues,4who respectively reported 62% and 44% rates of success with closed reduction. Our cohort of 14 patients with 15 dislocations required a total of 17 trips to the operating room after dislocation. This significantly higher rate of return to the operating room suggests that dislocation after RTSA may be a more costly and morbid problem than has been previously described.
This study had several weaknesses. Despite its large consecutive series of patients, the study was retrospective, and several variables that would be documented and controlled in a prospective study could not be measured here. Specifically, neither preoperative physical examination nor patient-specific assessments of pain or function were consistently obtained. Similarly, postoperative patient-specific instruments of outcomes evaluation were not obtained consistently, so results of patients with dislocation could not be compared with those of a control group. In addition, preoperative and postoperative radiographs were not consistently present in our electronic medical records, so the influence of preoperative bony anatomy, intraoperative limb lengthening, and any implant malpositioning could not be determined. Furthermore, operative details, such as reparability of the subscapularis, were not fully available for the control group and could not be included in statistical analysis. In addition, that the known dislocation risk factor of male sex4 was identified here but was not significant in multivariate regression analysis suggests that this study may not have been adequately powered to identify a significant difference in dislocation rate between the sexes. Last, though our results suggested associations between the aforementioned variables and dislocation after RTSA, a truly causative relationship could not be confirmed with this study design or analysis. Therefore, our study findings are hypothesis-generating and may indicate a benefit to greater deltoid tensioning, use of retentive liners, or more conservative rehabilitation protocols for high-risk patients.
Conclusion
Dislocation after RTSA is an uncommon complication that often requires a return to the operating room. This study identified a modifiable risk factor (higher BMI) and 3 nonmodifiable risk factors (male sex, subscapularis insufficiency, revision surgery) for dislocation after RTSA. In contrast, patients who undergo RTSA for primary rotator cuff pathology are unlikely to dislocate after surgery. This low risk of dislocation after RTSA for primary cuff pathology was not previously described. Patients in the higher risk category may benefit from preoperative lifestyle modification, intraoperative techniques for increasing stability, and more conservative therapy after surgery. In addition, unlike previous investigations, this study did not find closed reduction in the clinic alone to be successful in definitively treating this patient population.
Am J Orthop. 2016;45(7):E444-E450. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Risk factors for dislocation after reverse total shoulder arthroplasty (RTSA) are not clearly defined. Prosthetic dislocation can result in poor patient satisfaction, worse functional outcomes, and return to the operating room.1-3 As a result, identification of modifiable risk factors for complications represents an important research initiative for shoulder surgeons.
There is a paucity of literature devoted to the study of dislocation after RTSA. Chalmers and colleagues4 found a 2.9% (11/385) incidence of early dislocation within 3 months after index surgery—an improvement over the 15.8% reported for early instability over the period 2004–2006.5 As prosthesis design has improved and surgeons have become more comfortable with the RTSA prosthesis, surgical indications have expanded,6,7 and dislocation rates appear to have decreased. Although the most common indication for RTSA continues to be cuff tear arthropathy (CTA),6 there has been increased use in rheumatoid arthritis8-10; proximal humerus fractures, especially in cases of poor bone quality and unreliable fixation of tuberosities11-13; and failed previous shoulder reconstruction.14,15 As RTSA is performed more often, limiting the complications will become more important for both patient care and economics.
We conducted a study to analyze dislocation rates at our institution and to identify both modifiable and nonmodifiable risk factors for dislocation after RTSA. By identifying risk factors for dislocation, we will be able to implement additional perioperative clinical measures to reduce the incidence of dislocation.
Materials and Methods
This retrospective study of dislocation after RTSA was conducted at the Rothman Institute of Orthopedics and Methodist Hospital (Thomas Jefferson University Hospitals, Philadelphia, PA). After obtaining Institutional Review Board approval for the study, we searched our institution’s electronic database of shoulder arthroplasties to identify all RTSAs performed at our 2 large-volume urban institutions between September 27, 2010 and December 31, 2013. For the record search, International Classification of Diseases, Ninth Revision (ICD-9) codes were used (Table 1).
The medical records of each patient were used to identify independent variables that could be associated with dislocation rate. Demographic variables included sex, age, and race. Preoperative clinical data included body mass index (BMI), etiology of shoulder disease leading to RTSA, individual comorbidities, and Charlson Comorbidity Index (CCI)16 modified to be used with ICD-9 codes.17 In addition, prior shoulder surgery history and arthroplasty type (primary or revision) were determined. Postoperative considerations were time to dislocation, mechanism of dislocation, and intervention(s) needed for dislocation. Although the institutional database did not include operative variables such as prosthesis type and surgical approach, all 6 surgeons in this study were using a standard deltopectoral approach in beach-chair position with a Grammont style prosthesis for RTSA cases.
Descriptive statistics for RTSA patients and the dislocation subpopulation were compiled. Bivariate analysis was used to evaluate which of the previously described variables influenced dislocation rates. Last, multivariate logistic regression analysis was performed to evaluate which factors were independent predictors of dislocation. We included demographic variables (age, sex, ethnicity), clinical variables (BMI, individual comorbidities, CCI), and surgical variables (primary vs revision, diagnosis at time of surgery). All statistical analyses were performed with Excel 2013 (Microsoft) and SPSS Statistics Version 20.0 (SPSS Inc.).
Results
From the database, we identified 487 patients who underwent 510 RTSAs during the study period. These surgeries were performed by 6 shoulder and elbow fellowship–trained surgeons. Of the 510 RTSAs, 393 (77.1%) were primary cases, and 117 (22.9%) were revision cases.
Of the 510 shoulders that underwent RTSA, 15 (2.9%; 14 patients) dislocated. Of these 15 cases, 5 were primary (1.3% of all primary cases) and 10 were revision (8.5% of all revision cases). Mean time from index surgery to diagnosis of dislocation was 58.2 days (range, 0-319 days). One dislocation occurred immediately after surgery, 2 after falls, 4 from patient-identified low-energy mechanisms of injury, and 8 without known inciting events. Nine dislocations (60%) did not have a subscapularis repair (7 were irreparable, 2 underwent subscapularis peel without repair), and the other 6 were repaired primarily (Table 2).
Male patients accounted for 32.2% of the study population but 60.0% of the dislocations (P = .019) (Table 3).
Multivariate logistic regression analysis revealed revision arthroplasty (OR = 7.515; P = .042) and increased BMI (OR = 1.09; P = .047) to be independent risk factors for dislocation after RTSA. Analysis also found a diagnosis of primary CTA to be independently associated with lower risk of dislocation after RTSA (OR = 0.025; P = .008). Last, the previously described risk factor of male sex was found not to be a significant independent risk factor, though it did trend positively (OR = 3.011; P = .071).
Discussion
With more RTSAs being performed, evaluation of their common complications becomes increasingly important.18 We found a 3.0% rate of dislocation after RTSA, which is consistent with the most recently reported incidence4 and falls within the previously described range of 0% to 8.6%.19-26 Of the clinical risk factors identified in this study, those previously described were prior surgery, subscapularis insufficiency, higher BMI, and male sex.4 However, our finding of lower risk of dislocation after RTSA for primary rotator cuff pathology was not previously described. Although Chalmers and colleagues4 did not report this lower risk, 3 (27.3%) of their 11 patients with dislocation had primary CTA, compared with 1 (6.7%) of 15 patients in the present study.4 Our literature review did not identify any studies that independently reported the dislocation rate in patients who underwent RTSA for rotator cuff failure.
The risk factors of subscapularis irreparability and revision surgery suggest the importance of the soft-tissue envelope and bony anatomy in dislocation prevention. Previous analyses have suggested implant malpositioning,27,28 poor subscapularis quality,29 and inadequate muscle tensioning5,30-32 as risk factors for RTSA. Patients with an irreparable subscapularis tendon have often had multiple surgeries with compromise to the muscle/soft-tissue envelope or bony anatomy of the shoulder. A biomechanical study by Gutiérrez and colleagues31 found the compressive forces of the soft tissue at the glenohumeral joint to be the most important contributor to stability in the RTSA prosthesis. In clinical studies, the role of the subscapularis in preventing instability after RTSA remains unclear. Edwards and colleagues29 prospectively compared dislocation rates in patients with reparable and irreparable subscapularis tendons during RTSA and found a higher rate of dislocation in the irreparable subscapularis group. Of note, patients in the irreparable subscapularis group also had more complex diagnoses, including proximal humeral nonunion, fixed glenohumeral dislocation, and failed prior arthroplasty. Clark and colleagues33 retrospectively analyzed subscapularis repair in 2 RTSA groups and found no appreciable effect on complication rate, dislocation events, range-of-motion gains, or pain relief.
Our finding that higher BMI is an independent risk factor was previously described.4 The association is unclear but could be related to implant positioning, difficulty in intraoperative assessment of muscle tensioning, or body habitus that may generate a lever arm for impingement and dislocation when the arm is in adduction. Last, our finding that male sex is a risk factor for dislocation approached significance, and this relationship was previously reported.4 This could be attributable to a higher rate of activity or of indolent infection in male patients.34,35Besides studying risk factors for dislocation after RTSA, we investigated treatment. None of our patients were treated successfully and definitively with closed reduction in the clinic. This finding diverges from findings in studies by Teusink and colleagues2 and Chalmers and colleagues,4who respectively reported 62% and 44% rates of success with closed reduction. Our cohort of 14 patients with 15 dislocations required a total of 17 trips to the operating room after dislocation. This significantly higher rate of return to the operating room suggests that dislocation after RTSA may be a more costly and morbid problem than has been previously described.
This study had several weaknesses. Despite its large consecutive series of patients, the study was retrospective, and several variables that would be documented and controlled in a prospective study could not be measured here. Specifically, neither preoperative physical examination nor patient-specific assessments of pain or function were consistently obtained. Similarly, postoperative patient-specific instruments of outcomes evaluation were not obtained consistently, so results of patients with dislocation could not be compared with those of a control group. In addition, preoperative and postoperative radiographs were not consistently present in our electronic medical records, so the influence of preoperative bony anatomy, intraoperative limb lengthening, and any implant malpositioning could not be determined. Furthermore, operative details, such as reparability of the subscapularis, were not fully available for the control group and could not be included in statistical analysis. In addition, that the known dislocation risk factor of male sex4 was identified here but was not significant in multivariate regression analysis suggests that this study may not have been adequately powered to identify a significant difference in dislocation rate between the sexes. Last, though our results suggested associations between the aforementioned variables and dislocation after RTSA, a truly causative relationship could not be confirmed with this study design or analysis. Therefore, our study findings are hypothesis-generating and may indicate a benefit to greater deltoid tensioning, use of retentive liners, or more conservative rehabilitation protocols for high-risk patients.
Conclusion
Dislocation after RTSA is an uncommon complication that often requires a return to the operating room. This study identified a modifiable risk factor (higher BMI) and 3 nonmodifiable risk factors (male sex, subscapularis insufficiency, revision surgery) for dislocation after RTSA. In contrast, patients who undergo RTSA for primary rotator cuff pathology are unlikely to dislocate after surgery. This low risk of dislocation after RTSA for primary cuff pathology was not previously described. Patients in the higher risk category may benefit from preoperative lifestyle modification, intraoperative techniques for increasing stability, and more conservative therapy after surgery. In addition, unlike previous investigations, this study did not find closed reduction in the clinic alone to be successful in definitively treating this patient population.
Am J Orthop. 2016;45(7):E444-E450. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
2. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(4):621-627.
3. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
4. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
5. Gallo RA, Gamradt SC, Mattern CJ, et al; Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584-590.
6. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
7. Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br. 2012;94(5):577-583.
8. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
9. Hedtmann A, Werner A. Shoulder arthroplasty in rheumatoid arthritis [in German]. Orthopade. 2007;36(11):1050-1061.
10. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
11. Acevedo DC, Vanbeek C, Lazarus MD, Williams GR, Abboud JA. Reverse shoulder arthroplasty for proximal humeral fractures: update on indications, technique, and results. J Shoulder Elbow Surg. 2014;23(2):279-289.
12. Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516-520.
13. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
14. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522.
15. Valenti P, Kilinc AS, Sauzières P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
18. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
19. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
20. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
21. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
24. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
25. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
26. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
27. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535-539.
28. Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652-658.
29. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892-896.
30. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
31. Gutiérrez S, Keller TS, Levy JC, Lee WE 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670-676.
32. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
33. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
34. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472(9):2809-2815.
35. Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21(11):1534-1541.
1. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
2. Teusink MJ, Pappou IP, Schwartz DG, Cottrell BJ, Frankle MA. Results of closed management of acute dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2015;24(4):621-627.
3. Fink Barnes LA, Grantham WJ, Meadows MC, Bigliani LU, Levine WN, Ahmad CS. Sports activity after reverse total shoulder arthroplasty with minimum 2-year follow-up. Am J Orthop. 2015;44(2):68-72.
4. Chalmers PN, Rahman Z, Romeo AA, Nicholson GP. Early dislocation after reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(5):737-744.
5. Gallo RA, Gamradt SC, Mattern CJ, et al; Sports Medicine and Shoulder Service at the Hospital for Special Surgery, New York, NY. Instability after reverse total shoulder replacement. J Shoulder Elbow Surg. 2011;20(4):584-590.
6. Walch G, Bacle G, Lädermann A, Nové-Josserand L, Smithers CJ. Do the indications, results, and complications of reverse shoulder arthroplasty change with surgeon’s experience? J Shoulder Elbow Surg. 2012;21(11):1470-1477.
7. Smith CD, Guyver P, Bunker TD. Indications for reverse shoulder replacement: a systematic review. J Bone Joint Surg Br. 2012;94(5):577-583.
8. Young AA, Smith MM, Bacle G, Moraga C, Walch G. Early results of reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am. 2011;93(20):1915-1923.
9. Hedtmann A, Werner A. Shoulder arthroplasty in rheumatoid arthritis [in German]. Orthopade. 2007;36(11):1050-1061.
10. Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22.
11. Acevedo DC, Vanbeek C, Lazarus MD, Williams GR, Abboud JA. Reverse shoulder arthroplasty for proximal humeral fractures: update on indications, technique, and results. J Shoulder Elbow Surg. 2014;23(2):279-289.
12. Bufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. J Bone Joint Surg Br. 2007;89(4):516-520.
13. Cuff DJ, Pupello DR. Comparison of hemiarthroplasty and reverse shoulder arthroplasty for the treatment of proximal humeral fractures in elderly patients. J Bone Joint Surg Am. 2013;95(22):2050-2055.
14. Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(4):514-522.
15. Valenti P, Kilinc AS, Sauzières P, Katz D. Results of 30 reverse shoulder prostheses for revision of failed hemi- or total shoulder arthroplasty. Eur J Orthop Surg Traumatol. 2014;24(8):1375-1382.
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383.
17. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613-619.
18. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254.
19. Boileau P, Watkinson D, Hatzidakis AM, Hovorka I. Neer Award 2005: the Grammont reverse shoulder prosthesis: results in cuff tear arthritis, fracture sequelae, and revision arthroplasty. J Shoulder Elbow Surg. 2006;15(5):527-540.
20. Cuff D, Pupello D, Virani N, Levy J, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency. J Bone Joint Surg Am. 2008;90(6):1244-1251.
21. Frankle M, Siegal S, Pupello D, Saleem A, Mighell M, Vasey M. The reverse shoulder prosthesis for glenohumeral arthritis associated with severe rotator cuff deficiency. A minimum two-year follow-up study of sixty patients. J Bone Joint Surg Am. 2005;87(8):1697-1705.
22. Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty. Survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747.
23. Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544-2556.
24. Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86(3):388-395.
25. Wall B, Nové-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89(7):1476-1485.
26. Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. J Bone Joint Surg Am. 2005;87(7):1476-1486.
27. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535-539.
28. Stephenson DR, Oh JH, McGarry MH, Rick Hatch GF 3rd, Lee TQ. Effect of humeral component version on impingement in reverse total shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):652-658.
29. Edwards TB, Williams MD, Labriola JE, Elkousy HA, Gartsman GM, O’Connor DP. Subscapularis insufficiency and the risk of shoulder dislocation after reverse shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):892-896.
30. Affonso J, Nicholson GP, Frankle MA, et al. Complications of the reverse prosthesis: prevention and treatment. Instr Course Lect. 2012;61:157-168.
31. Gutiérrez S, Keller TS, Levy JC, Lee WE 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res. 2008;466(3):670-676.
32. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg. 2005;14(1 suppl S):147S-161S.
33. Clark JC, Ritchie J, Song FS, et al. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. J Shoulder Elbow Surg. 2012;21(1):36-41.
34. Richards J, Inacio MC, Beckett M, et al. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472(9):2809-2815.
35. Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21(11):1534-1541.
Diagnosis and Management of Vestibular Migraine
From the Department of Neurootology, National Hospital of Neurology and Neurosurgery, London (Dr. Tsang, Miss Anwer) and the Ear Institute, University College London, and Guy’s and St Thomas’ NHS Foundation Trust, London, UK (Dr. Murdin).
Abstract
- Objective: To review the clinical manifestations, diagnosis, and management of vestibular migraine (VM).
- Methods: Review of the literature.
- Results: Apart from headache, other symptoms of VM include unsteadiness, imbalance, and spontaneous as well as visual vertigo. Acute vestibular symptoms that qualify for VM must be of at least moderate or severe intensity which lasts within a time window of 5 minutes to 72 hours. The interindividual temporal association of headache and vertigo is highly variable in VM patients Grossly normal peripheral vestibular function and audiometry both during and between attacks distinguishes VM from its mimics. Treatment options for VM are mainly based on expert opinion and include lifestyle modifications, acute and prophylactic migraine pharmacotherapy, and vestibular rehabilitation therapy.
- Conclusion: Despite a lack of diagnostic biomarkers for VM, a meticulous workup is important to exclude alternative mimics. More longitudinal and treatment studies are required to help elucidate the prognosis and optimal management of this condition.
The coexistence of migraine and vestibular symptoms has been mentioned in the headache literature for many years [1–3]. It was first addressed by Kayan and Hood in 1984, who found that dizziness and vertigo occurred in 54% of migraine patients compared with 30% of patients with tension-type headache [1]. The frequent coexistence of migraine and vertigo led researchers to hypothesize that their co-occurence could be due to more than mere chance. As per Lempert and Neuhauser’s evaluation, there is a lifetime prevalence of 16% for migraine and 7% for vertigo, with a 1.1 % chance of vertigo and migraine occurring together by chance alone [4]. In a study looking at the point prevalence of vertigo or dizziness among those presenting for a routine appointment at a headache center, an astounding 72.8% of those with severe headaches had vestibular symptoms [5].
Most epidemiologic studies of what we call vestibular migraine (VM) were based on presentations to specialist clinics and were performed in an era during which no established diagnostic criteria existed. Despite this, most neurootologists would consider VM to be one of the most common causes of spontaneous recurrent vertigo [6]. Neuhauser et al reported that VM was diagnosed in 7% of a group of 200 specialist clinic patients with dizziness and 9% of a group of 200 clinic patients who had migraine [2]. In a population-based study in Germany, the lifetime prevalence of VM according to the Neuhauser criteria was estimated to be 0.98% and the 12-month prevalence 0.89% [7]. The condition has a 3:1 female predilection [8].
VM has only recently been recognised as a separate migraine entity by the International Headache Society (IHS), appearing in the appendix of their International Classification of Headache Disorders (ICHD)–3 beta. The previous ICHD recognised vertigo as a migrainous symptom only within the framework of basilar migraine. The nomenclature used in the literature to describe this entity has been inconsistent and therefore confusing, including terms such as migraine-associated vertigo [9], migraine-related dizziness [3] or vertigo [10],migrainous vertigo [2], benign recurrent vertigo [11], and migraine-related vestibulopathy [12]. For the most part, these terms refer to the co-experience of migraine and vertigo or dizziness, with only a few terms having a more specific meaning of how the 2 symptoms relate temporally. Neuhauser and colleagues developed criteria in 2001 to classify migraineurs for whom vestibular symptoms are an integral part of migraine symptomatology, using the term migrainous vertigo [2]. Others preferred the terms migraine-associated dizziness or migraine-related dizziness [3] over migrainous vertigo because they felt the symptoms of vestibular dysfunction related to migraine are varied and may include gait instability and spatial disorientation but not necessarily with vertigo. To best avoid confounding nonvestibular dizziness or motion sickness associated with migraine, VM has been the preferred term because it emphasises the particular vestibular manifestation of migraine.
The lack of a universally accepted definition for this complex entity has contributed to delayed diagnosis and and treatment for those with this disorder. In this article, we will review the clinical manifestation, diagnosis and management of VM, with a focus on assisting in the differentiation between other potential diagnoses.
Pathophysiology of VM
A clear pathophysiology of VM has not been elucidated. Although predominantly a sporadic disease, there have been reported cases of familial occurrence with an auto-somal dominant inheritance [11,13]. Bahmad and colleagues mapped the first locus for familial VM to 5q35 within a 4-generation family [13]. On the contrary, a larger study conducted by Lee et al found VM to be to genetically heterogeneous with a subset linking to chromosome 22q12 [14]. Genetic defects of voltage-gated calcium channels are identified as causal factors for familial hemiplegic migraine and episodic ataxia type 2. Both these disease entities present with vertigo and migraine headaches suggesting a defective gene within the same chromosomal region could indicate a direct genetic link to VM. However, no such gene has been identified.
General consensus is that the action of spreading cortical depression as it reaches the somatosensory cortex in the posterior insula and temporoparietal junction elucidates migraine aura in patients with short attacks. However, due to the heterogeneity of VM, canal paresis and complex conditional nystagmus during acute stages are not explained through cortical spreading. Eggers et al suggests that vertigo symptoms occur as ictal sensation rather than the spreading of sensory or motor cortical depression [15]. However, due to discrepancies within the literature it is apparent that further research needs to be conducted to fully understand the pathophysiology of VM.
Clinical Manifestations of VM
Symptoms
As many as 80% to 90% of patients with VM report unsteadiness or balance problems, of which 50% to 60% typically report episodic spontaneous vertigo [16], either internal vertigo (a false sensation of self-motion) or external vertigo (a false sensation that the visual surround is spinning or flowing) [17]. The duration of episodes is highly variable, whereby approximately 30% of patients have episodes lasting minutes, 30% have attacks lasting hours, 30% have attacks over several days, while the remaining 10% have attacks lasting seconds only [18]. It may be difficult to distinguish if vestibular symptoms lasting seconds are related to their head motion intolerance, also known as head motion–induced vertigo [17], which is another frequent symptom in VM. Head motion–induced vertigo bears many similarities to motion sickness.
The interindividual temporal association of headache and vertigo is highly variable in VM patients and is a reason many patients find this diagnostic construct difficult to accept. Approximately 30% of adult patients eventually diagnosed with VM initially present without headaches [8]. Vertigo is only regularly associated with headache in 25% to 50% of VM patients [2,7]. A minority of patients report headache and vertigo never occurring together [2]. A temporal pattern, presenting as aura, occurs only in approximately 10% of cases [19]; therefore, vestibular episodes of VM should not be regarded as migraine auras [18]. Patients typically have migraine manifesting earlier in life with the vestibular symptoms following [13,20], whereby the mean age at onset of migraine and diagnosis of VM are approximately 22 and 35 years, respectively [2]. Consistently across studies that measure quality of life scores, VM patients report higher subjective levels of disability compared to patients with other vestibular illnesses, despite having less objective abnormalities [21]. Approximately 85% of VM patients experienced vestibular symptoms for at least 1 year before consulting neurootology services [21]. It could be argued that hypersensitivity of percept to vestibular symptoms reflect the general finding of augmented perceptions to various external stimuli underlying migraine [22,23].
Another prominent feature of VM is that patients report a syndrome of visually-induced dizziness termed visual vertigo (VV). This is a heterogeneous syndrome with strabismic, peripheral, and/or central vestibular aetiologies [24]. Patients with VV complain of discomfort, postural destabilisation, dizziness, imbalance and spatial disorientation in challenging visual environments. Examples of such environments include walking down supermarket aisles, observing moving objects (eg, disco lights, people walking, moving traffic) or moving surroundings during travelling, and the movement of the eyes in general [24–26]. Most patients report more than one visual trigger [24]. Visual vertigo can often be difficult to distinguish from oscillopsia in patients with bilateral vestibular failure. What is most surprising is that patients with VV have a similar handicap level yet report much more vestibular symptoms compared with patients with bilateral vestibular failure [25]. Postural reactions triggered by external visual motion are destabilising with respect to the earth-vertical and are normally suppressed by central re-weighting of sensory postural cues [24]. Surprisingly, premorbid levels of anxiety and childhood motion sickness do not appear to have a correlation with VV [25]. Even in normal subjects, certain complex visual stimuli can induce transient motion sickness–like symptoms, as shown in experimental visually induced self-vection [27]. The Situational Characteristics Questionnaire (SVQ) is a 19-question, symptom-based questionnaire that has been shown to be useful in quantifying features of VV and may be useful in gauging improvement following physical therapies [25,26].
Early in the disease course, hearing loss should prompt an alternative diagnosis. However, late onset cochlear symptoms have been reported in VM. A study found that after 9 years of follow-up, the number of patients with cochlear symptoms more than doubled [28].
Clinical Examination Findings
The importance of the clinical examination is to rule out peripheral vestibular dysfunction and perform positional testing to look for benign paroxysmal positional vertigo (BPPV) or central positional nystagmus. Nonetheless, positional nystagmus has been reported in up to 28% of cases, including definite central-type positional nystagmus reported in as many as 18% [28].
Audiometric Findings and Auditory Brainstem Responses
Normal audiometry both during and between attacks is one of the key clinical features that distinguishes VM from Meniere’s disease [29]. Auditory brainstem response (ABR) results are typically normal in about 65% of patients [29]. Abnormal ABR results are typically nonspecific, such as mild elongation of wave I, III and V latencies and less commonly, prolongation of the inter-peak latencies.
Findings on Vestibular Function Testing
Whilst there are some reported abnormalities in vestibular function testing in VM patients, such findings need to be interpreted with caution due to the small number of subjects, as well as the variation in case definition and cut-off values. Most importantly, very few papers studied patients in the acute phase, and in some studies it was not even specified. The majority of studies report that VM patients interictally have grossly normal peripheral vestibular function with occasional minor irregularities. Profound interictal abnormalities such as complete canal paresis are usually indicative of other diagnoses. In between acute attacks, patients with VM typically have normal gaze, saccadic parameters, ocular pursuit gains and optokinetic nystagmus (OKN) gains on electronystagmography (ENG) or videonystagmography (VNG) [3]. A minority had a low amplitude (< 4 degrees per second) persistent positional nystagmus. On rotation testing of the vestibo-ocular reflex there is reduction of the mean gains compared to headache-free controls. Most reports in the literature do support that the majority of VM patients have grossly normal bithermal caloric testing, although abnormalities including higher slow phase velocities and canal paresis (usually partial) are reported [29–31]. The observation that the artificial vestibular stimulation caused by the caloric test was followed by a migraine attack within 24 hours in 49% of patients with migraine is very interesting [30], and it remains to be tested whether this phenomenon has the potential to be of assistance in the diagnosis of VM. Both VM patients and migraineurs without vertigo have similar subtle cVEMP (Cervical vestibular-evoked myogenic potentials) abnormalities, namely decreased global amplitude and absence of habituation [31]. On computerized dynamic posturography (CDP), a test of sway, VM patients typically demonstrate a surface-dependent pattern based on their SOT analysis [3], suggesting that VM patients may have a substantial vestibulo-spinal abnormality leading to difficulties integrating multiple conflicting sensory inputs [32].
Diagnostic Criteria
Separating VM into 2 diagnostic entities seems particularly useful: definite VM and the more sensitive but less specific category of probable VM. The sensitivity and specificity of the proposed criteria still need to be determined. Although some authors criticize the probable diagnostic entity for its heterogeneity, about 50% of patients initially diagnosed with probable VM ultimately progress to definite VM [12,33]. Definite vestibular migraine appears in the ICHD-3 beta but only in the appendix section for “new disorders that need further research for validation.” However, probable VM will not be included until further evidence of its utility has been accumulated.
The diagnosis is particularly challenging when headache is not a regular accompaniment of the vertiginous attacks. A patient diary may help link migrainous and vertigo symptoms. When headache is not a prominent feature of the attacks, the clinician will have to put migrainous triggers or symptoms such as photophobia or scintillating scotomas in the context of vertigo symptoms to aid with the diagnosis. One needs to be pedantic about differentiating the qualifying symptom of phonophobia, which is defined as a sound-induced discomfort that is often transient and bilateral from the uncomfortable distorted loud sound perception, which occurs with a recruiting sensorineural hearing loss, and is often persistent and unilateral [18]. Response to migraine treatment is not sufficiently specific to be included in the diagnostic criteria. High placebo response rates from migraine trials [34] suggest that placebo effects can likewise be expected in the treatment of VM. Despite these challenges, acceptance of the diagnostic entity of VM seems to be gaining momentum. In a follow-up study over 9 years, the diagnosis remained consistent in 85% of patients [33].
Benign Paroxysmal Vertigo of Childhood and Vestibular Migraine in Children
VM can present at any age, however, the ICHD specifically recognises an early vertiginous entity regarded as a precursor syndrome of migraine in otherwise healthy children called benign paroxysmal vertigo of childhood. This diagnosis requires 5 episodes of severe vertigo, occurring without warning and resolving spontaneously after minutes to hours [35]. In between episodes, neurological examination, audiometry, vestibular functions and EEG must be normal. A unilateral throbbing headache may occur during attacks but it is not a mandatory criterion. It is unclear whether these two conditions in children are the same entity, however it is important to note that the classification of VM does not involve any age limit [18].
Basilar-type Migraine
The term basilar migraine should be restricted to patients who fulfill the ICHD diagnostic criteria [35] given it is a clinically distinct entity from VM. Less than 10% of VM patients further fulfill the ICHD criteria for basilar migraine [2,18]. More than 60% of basilar-type migraine patients have vertigo and there are many overlapping clinical manifestations with VM. This diagnosis requires at least 2 symptoms from aura in the posterior circulation territory, whereas most patients with VM have vestibular symptoms only [35]. Moreover, in basilar migraine the duration of vertigo should correspond to the length of an aura, that is, between 5 and 60 minutes [35]. Further studies are required to further elucidate and delineate these 2 conditions.
Other Important Diagnostic Considerations
Meniere’s Disease
An important differential diagnosis of VM is the early presentation of Meniere’s disease (MD). Although fluctuating hearing loss, aural fullness and episodic vertigo are important symptoms in the recent updated diagnostic criteria for definite MD [36,37], these symptoms have been reported in patients with migraine [38]. Moreover, minor abnormalities in cVEMPs and arguably in caloric testing can be found in VM patients, as previously mentioned. Predominantly, the distinction can be made considering that a more sustained, albeit occasionally fluctuating, hearing loss would occur in MD, which can progress to severe hearing loss within a few years. However, the diagnosis can be difficult considering that audiometric and vestibular function abnormalities as well as the typical cochlear symptoms are often absent in the early stages of the MD. Nonetheless, preclinical labelling of patients with episodic vertigo without hearing loss as “vestibular MD” is unhelpful as this population may be overrepresented by actual migraineurs. Studies of patients with so-called benign recurrent vertigo or recurrent vestibulopathy are likely to be heterogeneous entities, with perhaps cases later evolving into VM or MD.
Coexisting migraine and MD is often challenging both in terms of diagnosis and management. Many studies have shown an increased prevalence of migraine in MD patients compared to controls [39,40], an asso-ciation suggested by Prosper Ménière himself in 1861 [41]. A study by Radtke et al found that the lifetime prevalence of migraine with and without aura was over 2 times higher in definite MD patients of both sexes compared to age-matched controls (56% versus 25%) [39]. Interestingly, 45% of the patients with MD always experienced at least 1 migrainous symptom (migrainous headache, photophobia, aura symptoms) with their Meniere attacks [39]. This may be at least partly due to the triggering effect of vestibular symptoms on migraineurs [30]. Migraine may even influence the disease course of MD as indicated by a retrospective case control study which found that definite MD patients who have concomitant ICHD criteria for migraine [35] had a significantly earlier onset of MD symptoms (mean age, 37.2 versus 49.3 years) and a much greater susceptibility to simultaneous bilateral, but not sequential, hearing loss as compared to MD patients without migraine (56% versus 4%) [42]. There were no significant differences in the severity of hearing loss between the 2 groups even when controlling for time to evaluation [42]. A family history of episodic vertigo was seen in 39% of MD patients with migraine, which is significantly higher than the 2% seen in MD patients, suggesting a possible genetic basis for this association [42]. The nature of the association between migraine and MD is not well elucidated, however, some authors propose that migraine leads to isolated microvascular ischaemic damage of the inner ear, presumably through small arterial vasospasm [40,42].
In summary, when the criteria for MD are met together with documented audiometric abnormalities, MD should be diagnosed, even if migraine symptoms occur during the vestibular attacks [18]. Only patients who experience 2 different types of attacks, one fulfilling the criteria for VM and the other for MD, should be labelled as Meniere’s disease/migraine overlap syndrome. It is hoped that future revisions of diagnostic criteria will include this overlap entity.
Migraine and Benign Paroxysmal Positional Vertigo
VM patients can experience brief positional dizziness and therefore VM may mimic BPPV. It is therefore important to perform positional testing to look for nystagmus typical for BPPV. Certainly the positional characteristics are distinct from BPPV with regard to the duration of attacks (often as long as the head position is maintained in VM rather than seconds in BPPV). BPPV may also produce attacks of vertigo that can act as triggers for migraine headaches. In these patients, treatment of the BPPV will reduce headache frequency [30].
Transient Ischemic Attacks
Transient ischemic attack (TIA) is a cerebrovascular disease with temporary neurological symptoms [43] and is differentiated from VM mainly from the characteristics of reported symptoms. Being a vascular phenomenon, one would expect TIA symptoms to have a sudden onset, with a brief duration of symptoms (typically short minutes), followed by a rapid improvement to baseline, as well as correspond to a vascular territory. The other important message is that stereotyped, frequently recurrent symptoms are less likely to be TIAs, with the exception of capsular warning syndrome [44] and limb shaking TIAs [43] described elsewhere.
Migraine and Motion Sickness
In an individual patient it may be difficult to differentiate between motion sickness and acute attacks of VM induced by motion stimuli. The distinction may be helped by observing nausea and dizziness improving after cessation of motion which points more towards motion sickness, as oppose to the persistent vertigo after the motion stimulus has ended, thus pointing more towards VM.
Episodic Ataxia Type 2
Of the various episodic ataxias, episodic ataxia type 2 would be the most important subtype in the differential diagnosis of VM given it presents with episodic vertigo and is the most frequently occurring subtype. It is a rare autosomal dominant inherited neurological disorder resulting from mutations of the calcium channel gene CACNA1A [45]. The clinical manifestations include recurrent disabling attacks of imbalance, vertigo and ataxia, which can be provoked by physical exertion or emotional stress. Patients may have downbeat nystagmus interictally. A slow progression of cerebellar signs accompanied by atrophy of midline cerebellar structures and a response to acetazolamide or 4-aminopyridine can help distinguish it from VM.
Migraine, Dizziness, and Comorbid Psychiatric Disorders
Particularly in patients with protracted symptoms, it is difficult to tease out the difference between the symptoms of migraine and dizziness from the symptoms of certain psychiatric disorders given their bidirectional associations. Migraine is a risk factor for first-onset major depression [46] and panic disorder [47]. Patients with VM have very high rates (30%–65%) of coexisting psychiatric illness, especially anxiety and depression, with frequencies higher than that associated with other migraine or vestibular disorders [48,49]. Vestibular migraine patients who have a positive history of psychiatric disorders have a comparatively higher risk of developing somatoform dizziness [48]. The unpredictability of recurrent vestibular symptoms could be a factor leading to elevated distress in VM patients. It is not uncommon to see a premature diagnosis of psychogenic dizziness to be given to patients without objective abnormalities. On the contrary, a diagnosis of psychogenic dizziness can rarely be made with certainty due to multiple reasons. Disabling vertigo leading to physical symptoms and avoidance of social activities can easily be misconstrued to have panic disorder with or without agoraphobia. Moreover, dizziness is the second most common symptom of a panic attack after palpitations [50].
Unfortunately, there are no objective tests that can reliably discriminate vestibular syndromes from psychiatric syndromes in patients with dizziness. The SVQ is not specific enough to differentiate symptoms of VV from the space and motion discomfort symptoms often found in agoraphobic patients [25]. Experimentally, agoraphobia patients may have a more surface-dependent strategy rather than a visual-dependent strategy on CDP [51]. It is unclear whether the vestibular system is causally linked to emotion processing pathways.
Chronic Subjective Dizziness
Chronic subjective dizziness is an entity characterised by chronic unsteadiness or nonvertiginous dizziness accompanied by hypersensitivity to motion stimuli and poor tolerance for complex visual stimuli lasting for 3 months or more without objective abnormalities [52]. These vestibular symptoms are often difficult to distinguish from symptoms of VM. This condition is thought to be a spatial sensory analog of allodynia experienced by some chronic migraine headache sufferers [8].
Dizziness Due to Side Effects of Migraine Prophylactic Medications
Dizziness is often listed as a side effect in the product information of various medications including those used for migraine prophylaxis. It is important to take an accurate history of the suspected offending drug in terms of its temporal relationship to vestibular symptoms. Tricyclic antidepressants (TCAs) can cause drowsiness, lightheadedness, fatigue and blurred vision [53]. Beta-blockers can cause orthostatic hypotension [53]. All the above effects could be confused with vestibular symptoms.
Treatment of Vestibular Migraine
Current treatment options for VM are mainly limited to expert opinion rather than inferred from randomized controlled trials (RCTs) [54]. Below we have offered our consensus on how VM should be managed, with concepts based on the guidelines of treatment for typical migraine [55]. Avoidance of migraine triggers should always be the first avenue of treatment. In addition, any vestibular disorder that is triggering migraine attacks should be identified and treated in its own right. Pharmacotherapy can be abortive for acute episodes and prophylactic.
Lifestyle Advice
The key first task in management is the correct diagnosis and educating the patient about the condition. A thorough explanation of the migraine origin of the attacks can address patients fear and expectations. Nonpharmaceutical approaches in the treatment of VM should not be neglected, even though only a very small proportion of patients may derive a benefit. Advice on dietary manipulation is routinely given; however, its efficacy in VM is questionable. Dietary advice includes healthy eating at regular intervals to prevent skipped meals as well as avoidance of excess caffeine and rich foods. A retrospective study found that lifestyle intervention alone resulted in 13 of 81 patients experiencing significant relief from vestibular symptoms with migraine. The remaining cohort of patients required a multifaceted approach including pharmacotherapy to achieve similar benefit [56].
Acute Abortive Treatments
Oral antiemetics are commonly prescribed for motion sickness and acute migraine, however there is no evidence supporting their effectiveness in VM (Table 2). Patients should be counselled about avoiding overuse of antiemetics given their risk of causing extrapyramidal side effects [53].
Simple analgesics, such as paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs), have been found to be helpful in acute VM attacks in observational studies. Bikhazi performed a survey of patients presenting to a headache clinic with vestibular symptoms and found that simple analgesics were valued by patients as effective symptomatic treatment, but were not considered as effective as triptans [59]. Doses of simple analgesics are listed in Table 2. Soluble formulations are preferable due to faster absorption and speed of onset. Opioids should be avoided in acute attacks of VM given the risk of developing opioid overuse headache [55].
Migraine Prophylaxis in Vestibular Migraine
TCAs remain a popular choice of migraine prophylaxis amongst neurootologists because of its additional effects on comorbid affective symptoms. We recommend that the starting dose of either amitriptyline or nortriptyline should be between 5 to 10 mg daily at night, slowly uptitrated to response over several weeks up to a maximum of 100 mg at night. Interval electrocardiography should be performed to monitor for prolongation of the QTc interval. A retrospective chart review found 46% of VM patients (by Neuhauser criteria) reported a reduction in dizziness after nortriptyline administration up to 75 mg daily [62]. However, the current evidence is limited to observational studies [59,62–64].
The evidence for beta-blockers is limited in VM but anecdotally has been useful for patients with frequent episodic migraine [59,63,64]. Recommended starting and maintenance doses are listed in Table 3. Furthermore, propranolol can be used in patients with depression [65,66]. Heart rate and electrocardiography should be monitored during dose escalation. Beta-blockers should be avoided in asthmatics. Commonly reported adverse events include cold, extremities reduced exercise tolerance and dizziness [53].
Flunarizine, a calcium channel blocker widely used in migraine [67,68] and vestibular conditions [69], was recently studied in a RCT of 12 weeks' duration for prophylaxis of migrainous vertigo (Neuhauser criteria) in 48 patients [70]. Although flunarizine 10 mg daily did not result in improved headache frequency and severity compared to the control arm, there was a significant improvement in vertigo severity. The most commonly reported side effects of flunarizine are weight gain and somnolence, both of which are minimal or infrequent. Verapamil is another calcium channel blocker that may be helpful but has major limiting adverse effects are bradycardia, constipation and peripheral edema [53].
Pizotifen, a serotonin antagonist, is one of the most well tolerated prophylaxis agents from our experience, however some patients do not adhere to treatment due to drowsiness or weight gain, as evidenced in retrospective case studies [64].
Topiramate with an average daily dose of 100 mg has reported positive results in a prospective observational study of ten patients with VM with auditory symptoms [71]. Nine of 10 patients reported no symptoms after follow-up period of up to sixteen months. The recommended dose is listed in Table 3. Common side effects include distal paresthesias, reduced ability to concentrate and drowsiness [53]. Sodium valproate has been anecdotally effective [59] and is usually well tolerated especially when starting at a low dose of 200 mg at night, slowly titrated to 1200 mg in 2 divided doses. Liver function and full blood evaluation should be monitored on a periodic basis [53].
Third-line medications have only been used anecdotally and should be reserved for extenuating cases (Table 3).
Vestibular Rehabilitation
Vestibular rehabilitation therapy (VRT) has been shown to alleviate significantly ongoing balance and dizziness symptoms in patients with various vestibular disorders [73,74] and improving confidence with balance in elderly patients [75,76]. However, the value of VRT is not as well established in VM. Anecdotally, patients with VM report persistent significant symptoms at the end of a standard VRT period, in contrast to other nonmigrainous patients who appear to be accomplishing their treatment goals faster. However, recent studies [21,73,77] are suggesting that customised VRT may play a useful role in VM, especially since it appears to target issues of anxiety, visual dependence or loss of confidence in balance. Small retrospective case series found that VRT reduced disability scores, and gait and balance function in over 85% of patients with migraine and vestibular symptoms [73,76,77]. An Australian VRT study (21) has recently assessed the efficacy of a 9-week customised VRT in 20 patients with VM compared to 16 patients with vestibular symptoms but without migraine. The customized VRT program consisted of habituation, gaze stability, static tilt, balance and gait exercises. A pictorial exercise instruction sheet for home use would describe these exercises of approximately 15 minutes duration consisting of 4 to 6 exercises to be performed 3 times a day, every day for 9 weeks. Interestingly, both groups benefitted equally from VRT. Compliance with VRT was comparable between the two groups. Commonly reported reasons for non-attendance in VM patients included a recent acute attack of VM, anxiety related to using public transport, and commitment issues related to occupation. This study also suggested that VM patients required more customized and intensive therapy as 15% of VM patients required additional appointments outside the study timeline.
Given that visual dependency has been shown to be reduced with short-term graded optokinetic stimulation exposure in healthy subjects [78], there has been interest using this intervention in conjunction with customized VRT to promote desensitization to visual stimuli as a treatment for VM patients with VV. Most promisingly is the finding that a subgroup of patients with a history of migraine improved significantly more than other vestibular patients with respect to VV symptoms.
There has been controversy surrounding whether patients should avoid medications when undergoing VRT. The protagonists of this view suggest that medications that affect the central nervous system (CNS) may modulate the rate of central compensation. In the aforementioned study by Vitkovic and colleagues [21], the same degree of improvement was seen in the VM group regardless of medication regimen. A study by Whitney and colleagues [73] found that migraine related vestibulopathy patients taking prophylaxis demonstrated better subjective and objective balance scores at baseline and after therapy. Further research is required to clarify the role of CNS-acting medication and their administration around VRT sessions.
Physical therapists dealing with VM patients may face additional challenges in encouraging exercise compliance and providing emotional support. Although more time consuming for the therapist, this is important in the face of high rates of comorbid affective disorders and head motion intolerance. Supervised VRT is believed to implicitly improve psychological status through increasing confidence, providing reassurance, and emphasizing positive effects of VRT, particularly when the patient feels their symptoms have been made worse by it.
Cognitive Behavioral Therapy
Cognitive behavioral therapy (CBT) has been shown to be helpful as part of the holistic treatment of various disorders including post-concussive syndrome and depression in neurology patients [79,80]. Among patients suffering from dizziness, a small study comparing explicit CBT combined with VRT versus waiting-list controls demonstrated improvements in patients’ coping ability, function, symptoms, and care satisfaction [81]. However, to our knowledge there are no studies directly evaluating the benefits of CBT specifically in VM patients. Despite this, it is our practice to request CBT for VM patients who report disabling anxiety or depressive symptoms.
Prognosis
Although migraine in general can improve in later life, this is less certain with VM given the lack of good quality longitudinal studies. Recently Radtke and colleagues published their long-term (median, 9 years) follow-up study of 61 definite VM cases (28). They found that 87% of patients had recurrent vertigo at follow-up. The frequency of vertigo was reduced in 56%, increased in 29%, and unchanged in 16% of patients. The impact of vertigo was graded as severe in 21%, moderate in 43%, and mild in 36% of patients. However, they found that concomitant cochlear symptoms with vertigo had increased from 15% at study inception to 49% at follow-up and secondly, 18% of patients had developed mild bilateral low-frequency sensorineural hearing loss. Therefore, one major criticism of the study is whether some of the patients had MD as their eventual diagnosis rather than definite VM. On the contrary, the authors conclude that these changes represent new vestibulo-cochlear dysfunction as a result of VM disease progression. Due to these reasons, the prognosis of VM patients is unclear. It is our practice to ensure patients do receive delayed follow-up to allow consideration of other neurotological diagnoses.
Conclusion
Given the large heterogeneity in presentation and objective testing, VM as a diagnostic construct has remained quite controversial, though increasingly more accepted. The more we study this common vestibular condition, the more we are realising that the complex relationship between migraine and dizziness extend beyond VM to encompass other vestibular disorders such as MD and anxiety. The lack of a physiological biomarker contributes to its diagnostic difficulties, but a meticulous workup is important to exclude alternative vestibular diagnoses. More longitudinal studies and RCTs are required to help both understand the prognosis and management of VM patients.
Corresponding author: Benjamin K-T Tsang, MBBS, FRACP, The Prince Charles Hospital, Rode Road, Chermside, Queensland 4032, Australia, benjamim.tsang@health.qld.gov.au.
Financial disclosures: None.
1. Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain 1984;107(Pt 4):1123–42.
2. Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56:436–41.
3. Furman JM, Sparto PJ, Soso M, Marcus D. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res 2005;15:327–32.
4. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol 2009;256:333–8.
5. Calhoun AH, Ford S, Pruitt AP, Fisher KG. The point prevalence of dizziness or vertigo in migraine--and factors that influence presentation. Headache 2011;51:1388–92.
6. Bisdorff A. Migraine and dizziness. Curr Opin Neurol 2014;27:105–10.
7. Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology 2006;67:1028–33.
8. Sargent EW. The challenge of vestibular migraine. Curr Opin Otolaryngol Head Neck Surg 2013;21:473–9.
9. Cha YH. Migraine-associated vertigo: diagnosis and treatment. Sem Neurol 2010;30:167–74.
10. Cherian N. Vertigo as a migraine phenomenon. Curr Neurol Neurosci Rep 2013;13:343.
11. Oh AK, Lee H, Jen JC, et al. Familial benign recurrent vertigo. Am J Med Genet 2001;100:287–91.
12. Cass SP, Furman JM, Ankerstjerne K, et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol 1997;106:182–9.
13. Bahmad F Jr, DePalma SR, Merchant SN, et al. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol 2009;118:670–6.
14. Lee H, Jen JC, Wang H, et al. A genome-wide linkage scan of familial benign recurrent vertigo: linkage to 22q12 with evidence of heterogeneity. Hum Molec Genet 2006;15:251–8.
15. Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res 2014;24:387–95.
16. Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms--identifying clinical features that predict “vestibular migraine”. Headache 2011;51:1393–7.
17. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1-13.
18. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167-72.
19. Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999;246:883–92.
20. Eggers SD, Staab JP, Neff BA, et al. Investigation of the coherence of definite and probable vestibular migraine as distinct clinical entities. Otol Neurotol 2011;32:1144–51.
21. Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol 2013;260:3039–48.
22. Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache 2004;44:1019–23.
23. Morrison DP. Abnormal perceptual experiences in migraine. Cephalalgia 1990;10:273–7.
24. Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psych 1995;59:472–6.
25. Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain 2001;124(Pt 8):1646–56.
26. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res 2006;16:223-31.
27. Dobie TG, May JG, Gutierrez C, Heller SS. The transfer of adaptation between actual and simulated rotary stimulation. Aviat Space Environ Med 1990;61:1085–91.
28. Radtke A, von Brevern M, Neuhauser H, et al. Vestibular migraine: long-term follow-up of clinical symptoms and vestibulo-cochlear findings. Neurology 2012;79:1607–14.
29. Bayazit Y, Yilmaz M, Mumbuc S, Kanlikama M. Assessment of migraine-related cochleovestibular symptoms. Revue Laryngol Otol Rhinol 2001;122:85–8.
30. Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology 2009;73:638–42.
31. Roceanu A, Allena M, De Pasqua V, et al. Abnormalities of the vestibulo-collic reflex are similar in migraineurs with and without vertigo. Cephalalgia 2008;28:988–90.
32. Hong HR, Shim DB, Kim TS, et al. Results of caloric and sensory organization testing of dynamic posturography in migrainous vertigo: comparison with Meniere’s disease and vestibular neuritis. Acta Otolaryngol 2013;133:1236–41.
33. Radtke A, Neuhauser H, von Brevern M, et al. Vestibular migraine--validity of clinical diagnostic criteria. Cephalalgia 2011;31:906-13.
34. Rothner AD, Wasiewski W, Winner P, et al. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 2006;46:101-9.
35. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd ed (beta version). Cephalalgia 2013;33:629–808.
36. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere’s disease. J Vestib Res 2015;25:1–7.
37. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation. Otolaryngol Head Neck Surg 1995;113:181–5.
38. Baloh RW. Neurotology of migraine. Headache 1997;37:615-21.
39. Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology 2002;59:1700–4.
40. Lee H, Lopez I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurol 2000;57:1631–4.
41. Ménière P. Pathologie auriculaire: memoires sur une lésion de l’oreille interne donnant lieu à des symptoms de congestion cérébrale apoplectiforme. Gaz Med Paris 1861;16:597–601.
42. Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Meniere’s disease. Acta Otolaryngol 2007;127:1241–5.
43. Kim JS. Symptoms of transient ischemic attack. Front Neurol Neurosci 2014;33:82–102.
44. Paul NL, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology 2012;79:1356–62.
45. Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics 2007;4:267–73.
46. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54:308–13.
47. Breslau N, Schultz LR, Stewart WF, et al. Headache types and panic disorder: directionality and specificity. Neurology 2001;56:350–4.
48. Best C, Eckhardt-Henn A, Tschan R, Dieterich M. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes. Results of a prospective longitudinal study over one year. J Neurol 2009;256:58–65.
49. Eckhardt-Henn A, Best C, Bense S, et al. Psychiatric comorbidity in different organic vertigo syndromes. J Neurol 2008;255:420–8.
50. Segui J, Salvador-Carulla L, Garcia L, et al. Semiology and subtyping of panic disorders. Act Psychiatr Scand 1998;97:272–7.
51. Jacob RG, Furman JM, Durrant JD, Turner SM. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med 1997;59:323–30.
52. Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am 2009;42:71–7, ix.
53. Australian medicines handbook : AMH. Adelaide, S.Aust.: Australian Medicines Handbook; 2015. p. v.
54. Maldonado Fernandez M, Birdi JS, Irving GJ, et al. Pharmacological agents for the prevention of vestibular migraine. Cochrane Database Syst Rev 2015;6:CD010600.
55. British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication overuse headache. 3rd ed. 2010.
56. Reploeg MD, Goebel JA. Migraine-associated dizziness: patient characteristics and management options. Otol Neurotol 2002;23:364–71.
57. Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo-controlled trial. Neurology 2003;60:882–3.
58. Roberto G, Raschi E, Piccinni C, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia 2015;35:118–31.
59. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol 1997;18:350–4.
60. MedicinesComplete. London: Pharmaceutical Press. Available at www.medicinescomplete.com.
61. Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000;60:1259–87.
62. Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol 2012;33:121–7.
63. Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006;116:1782–6.
64. Waterston J. Chronic migrainous vertigo. J Clin Neurosci 2004;11:384–8.
65. de Bock GH, Eelhart J, van Marwijk HW, et al. A postmarketing study of flunarizine in migraine and vertigo. Pharm World Sci 1997;19:269–74.
66. Verspeelt J, De Locht P, Amery WK. Postmarketing study of the use of flunarizine in vestibular vertigo and in migraine. Eur J Clin Pharmacol 1996;51:15–22.
67. Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. J Cardiovasc Pharmacol 1991;18 Suppl 8:S21–6.
68. Lucetti C, Nuti A, Pavese N, et al. Flunarizine in migraine prophylaxis: predictive factors for a positive response. Cephalalgia 1998;18:349–52.
69. Schmidt R, Oestreich W. Flunarizine in the treatment of vestibular vertigo: experimental and clinical data. J Cardiovasc Pharmacol 1991;18 Suppl 8:S27–30.
70. Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol 2014;271:2931–6.
71. Carmona S, Settecase N. Use of topiramate (topamax) in a subgroup of migraine-vertigo patients with auditory symptoms. Ann N Y Acad Sci 2005;1039:517–20.
72. Bisdorff AR. Treatment of migraine related vertigo with lamotrigine an observational study. Bull Soc Sci Med Grand Duche Luxemb 2004:103–8.
73. Whitney SL, Rossi MM. Efficacy of vestibular rehabilitation. Otolaryngol Clin North Am 2000;33:659–72.
74. Enticott JC, Vitkovic JJ, Reid B, et al. Vestibular rehabilitation in individuals with inner-ear dysfunction: a pilot study. Audiol Neurootol 2008;13:19–28.
75. Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol Ser A Biol Sci Med Sci 1998;53:M287–94.
76. Wrisley DM, Whitney SL, Furman JM. Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol 2002;23:483–7.
77. Gottshall KR, Moore RJ, Hoffer ME. Vestibular rehabilitation for migraine-associated dizziness. Int Tinnitus J 2005;11:81–4.
78. Pavlou M, Quinn C, Murray K, et al. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture 2011;33:113–8.
79. Leddy JJ, Sandhu H, Sodhi V, et al. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54.
80. Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res 2015;78:411–9.
81. Andersson G, Asmundson GJ, Denev J, et al. A controlled trial of cognitive-behavior therapy combined with vestibular rehabilitation in the treatment of dizziness. Behav Res Ther 2006;44:1265–73.
From the Department of Neurootology, National Hospital of Neurology and Neurosurgery, London (Dr. Tsang, Miss Anwer) and the Ear Institute, University College London, and Guy’s and St Thomas’ NHS Foundation Trust, London, UK (Dr. Murdin).
Abstract
- Objective: To review the clinical manifestations, diagnosis, and management of vestibular migraine (VM).
- Methods: Review of the literature.
- Results: Apart from headache, other symptoms of VM include unsteadiness, imbalance, and spontaneous as well as visual vertigo. Acute vestibular symptoms that qualify for VM must be of at least moderate or severe intensity which lasts within a time window of 5 minutes to 72 hours. The interindividual temporal association of headache and vertigo is highly variable in VM patients Grossly normal peripheral vestibular function and audiometry both during and between attacks distinguishes VM from its mimics. Treatment options for VM are mainly based on expert opinion and include lifestyle modifications, acute and prophylactic migraine pharmacotherapy, and vestibular rehabilitation therapy.
- Conclusion: Despite a lack of diagnostic biomarkers for VM, a meticulous workup is important to exclude alternative mimics. More longitudinal and treatment studies are required to help elucidate the prognosis and optimal management of this condition.
The coexistence of migraine and vestibular symptoms has been mentioned in the headache literature for many years [1–3]. It was first addressed by Kayan and Hood in 1984, who found that dizziness and vertigo occurred in 54% of migraine patients compared with 30% of patients with tension-type headache [1]. The frequent coexistence of migraine and vertigo led researchers to hypothesize that their co-occurence could be due to more than mere chance. As per Lempert and Neuhauser’s evaluation, there is a lifetime prevalence of 16% for migraine and 7% for vertigo, with a 1.1 % chance of vertigo and migraine occurring together by chance alone [4]. In a study looking at the point prevalence of vertigo or dizziness among those presenting for a routine appointment at a headache center, an astounding 72.8% of those with severe headaches had vestibular symptoms [5].
Most epidemiologic studies of what we call vestibular migraine (VM) were based on presentations to specialist clinics and were performed in an era during which no established diagnostic criteria existed. Despite this, most neurootologists would consider VM to be one of the most common causes of spontaneous recurrent vertigo [6]. Neuhauser et al reported that VM was diagnosed in 7% of a group of 200 specialist clinic patients with dizziness and 9% of a group of 200 clinic patients who had migraine [2]. In a population-based study in Germany, the lifetime prevalence of VM according to the Neuhauser criteria was estimated to be 0.98% and the 12-month prevalence 0.89% [7]. The condition has a 3:1 female predilection [8].
VM has only recently been recognised as a separate migraine entity by the International Headache Society (IHS), appearing in the appendix of their International Classification of Headache Disorders (ICHD)–3 beta. The previous ICHD recognised vertigo as a migrainous symptom only within the framework of basilar migraine. The nomenclature used in the literature to describe this entity has been inconsistent and therefore confusing, including terms such as migraine-associated vertigo [9], migraine-related dizziness [3] or vertigo [10],migrainous vertigo [2], benign recurrent vertigo [11], and migraine-related vestibulopathy [12]. For the most part, these terms refer to the co-experience of migraine and vertigo or dizziness, with only a few terms having a more specific meaning of how the 2 symptoms relate temporally. Neuhauser and colleagues developed criteria in 2001 to classify migraineurs for whom vestibular symptoms are an integral part of migraine symptomatology, using the term migrainous vertigo [2]. Others preferred the terms migraine-associated dizziness or migraine-related dizziness [3] over migrainous vertigo because they felt the symptoms of vestibular dysfunction related to migraine are varied and may include gait instability and spatial disorientation but not necessarily with vertigo. To best avoid confounding nonvestibular dizziness or motion sickness associated with migraine, VM has been the preferred term because it emphasises the particular vestibular manifestation of migraine.
The lack of a universally accepted definition for this complex entity has contributed to delayed diagnosis and and treatment for those with this disorder. In this article, we will review the clinical manifestation, diagnosis and management of VM, with a focus on assisting in the differentiation between other potential diagnoses.
Pathophysiology of VM
A clear pathophysiology of VM has not been elucidated. Although predominantly a sporadic disease, there have been reported cases of familial occurrence with an auto-somal dominant inheritance [11,13]. Bahmad and colleagues mapped the first locus for familial VM to 5q35 within a 4-generation family [13]. On the contrary, a larger study conducted by Lee et al found VM to be to genetically heterogeneous with a subset linking to chromosome 22q12 [14]. Genetic defects of voltage-gated calcium channels are identified as causal factors for familial hemiplegic migraine and episodic ataxia type 2. Both these disease entities present with vertigo and migraine headaches suggesting a defective gene within the same chromosomal region could indicate a direct genetic link to VM. However, no such gene has been identified.
General consensus is that the action of spreading cortical depression as it reaches the somatosensory cortex in the posterior insula and temporoparietal junction elucidates migraine aura in patients with short attacks. However, due to the heterogeneity of VM, canal paresis and complex conditional nystagmus during acute stages are not explained through cortical spreading. Eggers et al suggests that vertigo symptoms occur as ictal sensation rather than the spreading of sensory or motor cortical depression [15]. However, due to discrepancies within the literature it is apparent that further research needs to be conducted to fully understand the pathophysiology of VM.
Clinical Manifestations of VM
Symptoms
As many as 80% to 90% of patients with VM report unsteadiness or balance problems, of which 50% to 60% typically report episodic spontaneous vertigo [16], either internal vertigo (a false sensation of self-motion) or external vertigo (a false sensation that the visual surround is spinning or flowing) [17]. The duration of episodes is highly variable, whereby approximately 30% of patients have episodes lasting minutes, 30% have attacks lasting hours, 30% have attacks over several days, while the remaining 10% have attacks lasting seconds only [18]. It may be difficult to distinguish if vestibular symptoms lasting seconds are related to their head motion intolerance, also known as head motion–induced vertigo [17], which is another frequent symptom in VM. Head motion–induced vertigo bears many similarities to motion sickness.
The interindividual temporal association of headache and vertigo is highly variable in VM patients and is a reason many patients find this diagnostic construct difficult to accept. Approximately 30% of adult patients eventually diagnosed with VM initially present without headaches [8]. Vertigo is only regularly associated with headache in 25% to 50% of VM patients [2,7]. A minority of patients report headache and vertigo never occurring together [2]. A temporal pattern, presenting as aura, occurs only in approximately 10% of cases [19]; therefore, vestibular episodes of VM should not be regarded as migraine auras [18]. Patients typically have migraine manifesting earlier in life with the vestibular symptoms following [13,20], whereby the mean age at onset of migraine and diagnosis of VM are approximately 22 and 35 years, respectively [2]. Consistently across studies that measure quality of life scores, VM patients report higher subjective levels of disability compared to patients with other vestibular illnesses, despite having less objective abnormalities [21]. Approximately 85% of VM patients experienced vestibular symptoms for at least 1 year before consulting neurootology services [21]. It could be argued that hypersensitivity of percept to vestibular symptoms reflect the general finding of augmented perceptions to various external stimuli underlying migraine [22,23].
Another prominent feature of VM is that patients report a syndrome of visually-induced dizziness termed visual vertigo (VV). This is a heterogeneous syndrome with strabismic, peripheral, and/or central vestibular aetiologies [24]. Patients with VV complain of discomfort, postural destabilisation, dizziness, imbalance and spatial disorientation in challenging visual environments. Examples of such environments include walking down supermarket aisles, observing moving objects (eg, disco lights, people walking, moving traffic) or moving surroundings during travelling, and the movement of the eyes in general [24–26]. Most patients report more than one visual trigger [24]. Visual vertigo can often be difficult to distinguish from oscillopsia in patients with bilateral vestibular failure. What is most surprising is that patients with VV have a similar handicap level yet report much more vestibular symptoms compared with patients with bilateral vestibular failure [25]. Postural reactions triggered by external visual motion are destabilising with respect to the earth-vertical and are normally suppressed by central re-weighting of sensory postural cues [24]. Surprisingly, premorbid levels of anxiety and childhood motion sickness do not appear to have a correlation with VV [25]. Even in normal subjects, certain complex visual stimuli can induce transient motion sickness–like symptoms, as shown in experimental visually induced self-vection [27]. The Situational Characteristics Questionnaire (SVQ) is a 19-question, symptom-based questionnaire that has been shown to be useful in quantifying features of VV and may be useful in gauging improvement following physical therapies [25,26].
Early in the disease course, hearing loss should prompt an alternative diagnosis. However, late onset cochlear symptoms have been reported in VM. A study found that after 9 years of follow-up, the number of patients with cochlear symptoms more than doubled [28].
Clinical Examination Findings
The importance of the clinical examination is to rule out peripheral vestibular dysfunction and perform positional testing to look for benign paroxysmal positional vertigo (BPPV) or central positional nystagmus. Nonetheless, positional nystagmus has been reported in up to 28% of cases, including definite central-type positional nystagmus reported in as many as 18% [28].
Audiometric Findings and Auditory Brainstem Responses
Normal audiometry both during and between attacks is one of the key clinical features that distinguishes VM from Meniere’s disease [29]. Auditory brainstem response (ABR) results are typically normal in about 65% of patients [29]. Abnormal ABR results are typically nonspecific, such as mild elongation of wave I, III and V latencies and less commonly, prolongation of the inter-peak latencies.
Findings on Vestibular Function Testing
Whilst there are some reported abnormalities in vestibular function testing in VM patients, such findings need to be interpreted with caution due to the small number of subjects, as well as the variation in case definition and cut-off values. Most importantly, very few papers studied patients in the acute phase, and in some studies it was not even specified. The majority of studies report that VM patients interictally have grossly normal peripheral vestibular function with occasional minor irregularities. Profound interictal abnormalities such as complete canal paresis are usually indicative of other diagnoses. In between acute attacks, patients with VM typically have normal gaze, saccadic parameters, ocular pursuit gains and optokinetic nystagmus (OKN) gains on electronystagmography (ENG) or videonystagmography (VNG) [3]. A minority had a low amplitude (< 4 degrees per second) persistent positional nystagmus. On rotation testing of the vestibo-ocular reflex there is reduction of the mean gains compared to headache-free controls. Most reports in the literature do support that the majority of VM patients have grossly normal bithermal caloric testing, although abnormalities including higher slow phase velocities and canal paresis (usually partial) are reported [29–31]. The observation that the artificial vestibular stimulation caused by the caloric test was followed by a migraine attack within 24 hours in 49% of patients with migraine is very interesting [30], and it remains to be tested whether this phenomenon has the potential to be of assistance in the diagnosis of VM. Both VM patients and migraineurs without vertigo have similar subtle cVEMP (Cervical vestibular-evoked myogenic potentials) abnormalities, namely decreased global amplitude and absence of habituation [31]. On computerized dynamic posturography (CDP), a test of sway, VM patients typically demonstrate a surface-dependent pattern based on their SOT analysis [3], suggesting that VM patients may have a substantial vestibulo-spinal abnormality leading to difficulties integrating multiple conflicting sensory inputs [32].
Diagnostic Criteria
Separating VM into 2 diagnostic entities seems particularly useful: definite VM and the more sensitive but less specific category of probable VM. The sensitivity and specificity of the proposed criteria still need to be determined. Although some authors criticize the probable diagnostic entity for its heterogeneity, about 50% of patients initially diagnosed with probable VM ultimately progress to definite VM [12,33]. Definite vestibular migraine appears in the ICHD-3 beta but only in the appendix section for “new disorders that need further research for validation.” However, probable VM will not be included until further evidence of its utility has been accumulated.
The diagnosis is particularly challenging when headache is not a regular accompaniment of the vertiginous attacks. A patient diary may help link migrainous and vertigo symptoms. When headache is not a prominent feature of the attacks, the clinician will have to put migrainous triggers or symptoms such as photophobia or scintillating scotomas in the context of vertigo symptoms to aid with the diagnosis. One needs to be pedantic about differentiating the qualifying symptom of phonophobia, which is defined as a sound-induced discomfort that is often transient and bilateral from the uncomfortable distorted loud sound perception, which occurs with a recruiting sensorineural hearing loss, and is often persistent and unilateral [18]. Response to migraine treatment is not sufficiently specific to be included in the diagnostic criteria. High placebo response rates from migraine trials [34] suggest that placebo effects can likewise be expected in the treatment of VM. Despite these challenges, acceptance of the diagnostic entity of VM seems to be gaining momentum. In a follow-up study over 9 years, the diagnosis remained consistent in 85% of patients [33].
Benign Paroxysmal Vertigo of Childhood and Vestibular Migraine in Children
VM can present at any age, however, the ICHD specifically recognises an early vertiginous entity regarded as a precursor syndrome of migraine in otherwise healthy children called benign paroxysmal vertigo of childhood. This diagnosis requires 5 episodes of severe vertigo, occurring without warning and resolving spontaneously after minutes to hours [35]. In between episodes, neurological examination, audiometry, vestibular functions and EEG must be normal. A unilateral throbbing headache may occur during attacks but it is not a mandatory criterion. It is unclear whether these two conditions in children are the same entity, however it is important to note that the classification of VM does not involve any age limit [18].
Basilar-type Migraine
The term basilar migraine should be restricted to patients who fulfill the ICHD diagnostic criteria [35] given it is a clinically distinct entity from VM. Less than 10% of VM patients further fulfill the ICHD criteria for basilar migraine [2,18]. More than 60% of basilar-type migraine patients have vertigo and there are many overlapping clinical manifestations with VM. This diagnosis requires at least 2 symptoms from aura in the posterior circulation territory, whereas most patients with VM have vestibular symptoms only [35]. Moreover, in basilar migraine the duration of vertigo should correspond to the length of an aura, that is, between 5 and 60 minutes [35]. Further studies are required to further elucidate and delineate these 2 conditions.
Other Important Diagnostic Considerations
Meniere’s Disease
An important differential diagnosis of VM is the early presentation of Meniere’s disease (MD). Although fluctuating hearing loss, aural fullness and episodic vertigo are important symptoms in the recent updated diagnostic criteria for definite MD [36,37], these symptoms have been reported in patients with migraine [38]. Moreover, minor abnormalities in cVEMPs and arguably in caloric testing can be found in VM patients, as previously mentioned. Predominantly, the distinction can be made considering that a more sustained, albeit occasionally fluctuating, hearing loss would occur in MD, which can progress to severe hearing loss within a few years. However, the diagnosis can be difficult considering that audiometric and vestibular function abnormalities as well as the typical cochlear symptoms are often absent in the early stages of the MD. Nonetheless, preclinical labelling of patients with episodic vertigo without hearing loss as “vestibular MD” is unhelpful as this population may be overrepresented by actual migraineurs. Studies of patients with so-called benign recurrent vertigo or recurrent vestibulopathy are likely to be heterogeneous entities, with perhaps cases later evolving into VM or MD.
Coexisting migraine and MD is often challenging both in terms of diagnosis and management. Many studies have shown an increased prevalence of migraine in MD patients compared to controls [39,40], an asso-ciation suggested by Prosper Ménière himself in 1861 [41]. A study by Radtke et al found that the lifetime prevalence of migraine with and without aura was over 2 times higher in definite MD patients of both sexes compared to age-matched controls (56% versus 25%) [39]. Interestingly, 45% of the patients with MD always experienced at least 1 migrainous symptom (migrainous headache, photophobia, aura symptoms) with their Meniere attacks [39]. This may be at least partly due to the triggering effect of vestibular symptoms on migraineurs [30]. Migraine may even influence the disease course of MD as indicated by a retrospective case control study which found that definite MD patients who have concomitant ICHD criteria for migraine [35] had a significantly earlier onset of MD symptoms (mean age, 37.2 versus 49.3 years) and a much greater susceptibility to simultaneous bilateral, but not sequential, hearing loss as compared to MD patients without migraine (56% versus 4%) [42]. There were no significant differences in the severity of hearing loss between the 2 groups even when controlling for time to evaluation [42]. A family history of episodic vertigo was seen in 39% of MD patients with migraine, which is significantly higher than the 2% seen in MD patients, suggesting a possible genetic basis for this association [42]. The nature of the association between migraine and MD is not well elucidated, however, some authors propose that migraine leads to isolated microvascular ischaemic damage of the inner ear, presumably through small arterial vasospasm [40,42].
In summary, when the criteria for MD are met together with documented audiometric abnormalities, MD should be diagnosed, even if migraine symptoms occur during the vestibular attacks [18]. Only patients who experience 2 different types of attacks, one fulfilling the criteria for VM and the other for MD, should be labelled as Meniere’s disease/migraine overlap syndrome. It is hoped that future revisions of diagnostic criteria will include this overlap entity.
Migraine and Benign Paroxysmal Positional Vertigo
VM patients can experience brief positional dizziness and therefore VM may mimic BPPV. It is therefore important to perform positional testing to look for nystagmus typical for BPPV. Certainly the positional characteristics are distinct from BPPV with regard to the duration of attacks (often as long as the head position is maintained in VM rather than seconds in BPPV). BPPV may also produce attacks of vertigo that can act as triggers for migraine headaches. In these patients, treatment of the BPPV will reduce headache frequency [30].
Transient Ischemic Attacks
Transient ischemic attack (TIA) is a cerebrovascular disease with temporary neurological symptoms [43] and is differentiated from VM mainly from the characteristics of reported symptoms. Being a vascular phenomenon, one would expect TIA symptoms to have a sudden onset, with a brief duration of symptoms (typically short minutes), followed by a rapid improvement to baseline, as well as correspond to a vascular territory. The other important message is that stereotyped, frequently recurrent symptoms are less likely to be TIAs, with the exception of capsular warning syndrome [44] and limb shaking TIAs [43] described elsewhere.
Migraine and Motion Sickness
In an individual patient it may be difficult to differentiate between motion sickness and acute attacks of VM induced by motion stimuli. The distinction may be helped by observing nausea and dizziness improving after cessation of motion which points more towards motion sickness, as oppose to the persistent vertigo after the motion stimulus has ended, thus pointing more towards VM.
Episodic Ataxia Type 2
Of the various episodic ataxias, episodic ataxia type 2 would be the most important subtype in the differential diagnosis of VM given it presents with episodic vertigo and is the most frequently occurring subtype. It is a rare autosomal dominant inherited neurological disorder resulting from mutations of the calcium channel gene CACNA1A [45]. The clinical manifestations include recurrent disabling attacks of imbalance, vertigo and ataxia, which can be provoked by physical exertion or emotional stress. Patients may have downbeat nystagmus interictally. A slow progression of cerebellar signs accompanied by atrophy of midline cerebellar structures and a response to acetazolamide or 4-aminopyridine can help distinguish it from VM.
Migraine, Dizziness, and Comorbid Psychiatric Disorders
Particularly in patients with protracted symptoms, it is difficult to tease out the difference between the symptoms of migraine and dizziness from the symptoms of certain psychiatric disorders given their bidirectional associations. Migraine is a risk factor for first-onset major depression [46] and panic disorder [47]. Patients with VM have very high rates (30%–65%) of coexisting psychiatric illness, especially anxiety and depression, with frequencies higher than that associated with other migraine or vestibular disorders [48,49]. Vestibular migraine patients who have a positive history of psychiatric disorders have a comparatively higher risk of developing somatoform dizziness [48]. The unpredictability of recurrent vestibular symptoms could be a factor leading to elevated distress in VM patients. It is not uncommon to see a premature diagnosis of psychogenic dizziness to be given to patients without objective abnormalities. On the contrary, a diagnosis of psychogenic dizziness can rarely be made with certainty due to multiple reasons. Disabling vertigo leading to physical symptoms and avoidance of social activities can easily be misconstrued to have panic disorder with or without agoraphobia. Moreover, dizziness is the second most common symptom of a panic attack after palpitations [50].
Unfortunately, there are no objective tests that can reliably discriminate vestibular syndromes from psychiatric syndromes in patients with dizziness. The SVQ is not specific enough to differentiate symptoms of VV from the space and motion discomfort symptoms often found in agoraphobic patients [25]. Experimentally, agoraphobia patients may have a more surface-dependent strategy rather than a visual-dependent strategy on CDP [51]. It is unclear whether the vestibular system is causally linked to emotion processing pathways.
Chronic Subjective Dizziness
Chronic subjective dizziness is an entity characterised by chronic unsteadiness or nonvertiginous dizziness accompanied by hypersensitivity to motion stimuli and poor tolerance for complex visual stimuli lasting for 3 months or more without objective abnormalities [52]. These vestibular symptoms are often difficult to distinguish from symptoms of VM. This condition is thought to be a spatial sensory analog of allodynia experienced by some chronic migraine headache sufferers [8].
Dizziness Due to Side Effects of Migraine Prophylactic Medications
Dizziness is often listed as a side effect in the product information of various medications including those used for migraine prophylaxis. It is important to take an accurate history of the suspected offending drug in terms of its temporal relationship to vestibular symptoms. Tricyclic antidepressants (TCAs) can cause drowsiness, lightheadedness, fatigue and blurred vision [53]. Beta-blockers can cause orthostatic hypotension [53]. All the above effects could be confused with vestibular symptoms.
Treatment of Vestibular Migraine
Current treatment options for VM are mainly limited to expert opinion rather than inferred from randomized controlled trials (RCTs) [54]. Below we have offered our consensus on how VM should be managed, with concepts based on the guidelines of treatment for typical migraine [55]. Avoidance of migraine triggers should always be the first avenue of treatment. In addition, any vestibular disorder that is triggering migraine attacks should be identified and treated in its own right. Pharmacotherapy can be abortive for acute episodes and prophylactic.
Lifestyle Advice
The key first task in management is the correct diagnosis and educating the patient about the condition. A thorough explanation of the migraine origin of the attacks can address patients fear and expectations. Nonpharmaceutical approaches in the treatment of VM should not be neglected, even though only a very small proportion of patients may derive a benefit. Advice on dietary manipulation is routinely given; however, its efficacy in VM is questionable. Dietary advice includes healthy eating at regular intervals to prevent skipped meals as well as avoidance of excess caffeine and rich foods. A retrospective study found that lifestyle intervention alone resulted in 13 of 81 patients experiencing significant relief from vestibular symptoms with migraine. The remaining cohort of patients required a multifaceted approach including pharmacotherapy to achieve similar benefit [56].
Acute Abortive Treatments
Oral antiemetics are commonly prescribed for motion sickness and acute migraine, however there is no evidence supporting their effectiveness in VM (Table 2). Patients should be counselled about avoiding overuse of antiemetics given their risk of causing extrapyramidal side effects [53].
Simple analgesics, such as paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs), have been found to be helpful in acute VM attacks in observational studies. Bikhazi performed a survey of patients presenting to a headache clinic with vestibular symptoms and found that simple analgesics were valued by patients as effective symptomatic treatment, but were not considered as effective as triptans [59]. Doses of simple analgesics are listed in Table 2. Soluble formulations are preferable due to faster absorption and speed of onset. Opioids should be avoided in acute attacks of VM given the risk of developing opioid overuse headache [55].
Migraine Prophylaxis in Vestibular Migraine
TCAs remain a popular choice of migraine prophylaxis amongst neurootologists because of its additional effects on comorbid affective symptoms. We recommend that the starting dose of either amitriptyline or nortriptyline should be between 5 to 10 mg daily at night, slowly uptitrated to response over several weeks up to a maximum of 100 mg at night. Interval electrocardiography should be performed to monitor for prolongation of the QTc interval. A retrospective chart review found 46% of VM patients (by Neuhauser criteria) reported a reduction in dizziness after nortriptyline administration up to 75 mg daily [62]. However, the current evidence is limited to observational studies [59,62–64].
The evidence for beta-blockers is limited in VM but anecdotally has been useful for patients with frequent episodic migraine [59,63,64]. Recommended starting and maintenance doses are listed in Table 3. Furthermore, propranolol can be used in patients with depression [65,66]. Heart rate and electrocardiography should be monitored during dose escalation. Beta-blockers should be avoided in asthmatics. Commonly reported adverse events include cold, extremities reduced exercise tolerance and dizziness [53].
Flunarizine, a calcium channel blocker widely used in migraine [67,68] and vestibular conditions [69], was recently studied in a RCT of 12 weeks' duration for prophylaxis of migrainous vertigo (Neuhauser criteria) in 48 patients [70]. Although flunarizine 10 mg daily did not result in improved headache frequency and severity compared to the control arm, there was a significant improvement in vertigo severity. The most commonly reported side effects of flunarizine are weight gain and somnolence, both of which are minimal or infrequent. Verapamil is another calcium channel blocker that may be helpful but has major limiting adverse effects are bradycardia, constipation and peripheral edema [53].
Pizotifen, a serotonin antagonist, is one of the most well tolerated prophylaxis agents from our experience, however some patients do not adhere to treatment due to drowsiness or weight gain, as evidenced in retrospective case studies [64].
Topiramate with an average daily dose of 100 mg has reported positive results in a prospective observational study of ten patients with VM with auditory symptoms [71]. Nine of 10 patients reported no symptoms after follow-up period of up to sixteen months. The recommended dose is listed in Table 3. Common side effects include distal paresthesias, reduced ability to concentrate and drowsiness [53]. Sodium valproate has been anecdotally effective [59] and is usually well tolerated especially when starting at a low dose of 200 mg at night, slowly titrated to 1200 mg in 2 divided doses. Liver function and full blood evaluation should be monitored on a periodic basis [53].
Third-line medications have only been used anecdotally and should be reserved for extenuating cases (Table 3).
Vestibular Rehabilitation
Vestibular rehabilitation therapy (VRT) has been shown to alleviate significantly ongoing balance and dizziness symptoms in patients with various vestibular disorders [73,74] and improving confidence with balance in elderly patients [75,76]. However, the value of VRT is not as well established in VM. Anecdotally, patients with VM report persistent significant symptoms at the end of a standard VRT period, in contrast to other nonmigrainous patients who appear to be accomplishing their treatment goals faster. However, recent studies [21,73,77] are suggesting that customised VRT may play a useful role in VM, especially since it appears to target issues of anxiety, visual dependence or loss of confidence in balance. Small retrospective case series found that VRT reduced disability scores, and gait and balance function in over 85% of patients with migraine and vestibular symptoms [73,76,77]. An Australian VRT study (21) has recently assessed the efficacy of a 9-week customised VRT in 20 patients with VM compared to 16 patients with vestibular symptoms but without migraine. The customized VRT program consisted of habituation, gaze stability, static tilt, balance and gait exercises. A pictorial exercise instruction sheet for home use would describe these exercises of approximately 15 minutes duration consisting of 4 to 6 exercises to be performed 3 times a day, every day for 9 weeks. Interestingly, both groups benefitted equally from VRT. Compliance with VRT was comparable between the two groups. Commonly reported reasons for non-attendance in VM patients included a recent acute attack of VM, anxiety related to using public transport, and commitment issues related to occupation. This study also suggested that VM patients required more customized and intensive therapy as 15% of VM patients required additional appointments outside the study timeline.
Given that visual dependency has been shown to be reduced with short-term graded optokinetic stimulation exposure in healthy subjects [78], there has been interest using this intervention in conjunction with customized VRT to promote desensitization to visual stimuli as a treatment for VM patients with VV. Most promisingly is the finding that a subgroup of patients with a history of migraine improved significantly more than other vestibular patients with respect to VV symptoms.
There has been controversy surrounding whether patients should avoid medications when undergoing VRT. The protagonists of this view suggest that medications that affect the central nervous system (CNS) may modulate the rate of central compensation. In the aforementioned study by Vitkovic and colleagues [21], the same degree of improvement was seen in the VM group regardless of medication regimen. A study by Whitney and colleagues [73] found that migraine related vestibulopathy patients taking prophylaxis demonstrated better subjective and objective balance scores at baseline and after therapy. Further research is required to clarify the role of CNS-acting medication and their administration around VRT sessions.
Physical therapists dealing with VM patients may face additional challenges in encouraging exercise compliance and providing emotional support. Although more time consuming for the therapist, this is important in the face of high rates of comorbid affective disorders and head motion intolerance. Supervised VRT is believed to implicitly improve psychological status through increasing confidence, providing reassurance, and emphasizing positive effects of VRT, particularly when the patient feels their symptoms have been made worse by it.
Cognitive Behavioral Therapy
Cognitive behavioral therapy (CBT) has been shown to be helpful as part of the holistic treatment of various disorders including post-concussive syndrome and depression in neurology patients [79,80]. Among patients suffering from dizziness, a small study comparing explicit CBT combined with VRT versus waiting-list controls demonstrated improvements in patients’ coping ability, function, symptoms, and care satisfaction [81]. However, to our knowledge there are no studies directly evaluating the benefits of CBT specifically in VM patients. Despite this, it is our practice to request CBT for VM patients who report disabling anxiety or depressive symptoms.
Prognosis
Although migraine in general can improve in later life, this is less certain with VM given the lack of good quality longitudinal studies. Recently Radtke and colleagues published their long-term (median, 9 years) follow-up study of 61 definite VM cases (28). They found that 87% of patients had recurrent vertigo at follow-up. The frequency of vertigo was reduced in 56%, increased in 29%, and unchanged in 16% of patients. The impact of vertigo was graded as severe in 21%, moderate in 43%, and mild in 36% of patients. However, they found that concomitant cochlear symptoms with vertigo had increased from 15% at study inception to 49% at follow-up and secondly, 18% of patients had developed mild bilateral low-frequency sensorineural hearing loss. Therefore, one major criticism of the study is whether some of the patients had MD as their eventual diagnosis rather than definite VM. On the contrary, the authors conclude that these changes represent new vestibulo-cochlear dysfunction as a result of VM disease progression. Due to these reasons, the prognosis of VM patients is unclear. It is our practice to ensure patients do receive delayed follow-up to allow consideration of other neurotological diagnoses.
Conclusion
Given the large heterogeneity in presentation and objective testing, VM as a diagnostic construct has remained quite controversial, though increasingly more accepted. The more we study this common vestibular condition, the more we are realising that the complex relationship between migraine and dizziness extend beyond VM to encompass other vestibular disorders such as MD and anxiety. The lack of a physiological biomarker contributes to its diagnostic difficulties, but a meticulous workup is important to exclude alternative vestibular diagnoses. More longitudinal studies and RCTs are required to help both understand the prognosis and management of VM patients.
Corresponding author: Benjamin K-T Tsang, MBBS, FRACP, The Prince Charles Hospital, Rode Road, Chermside, Queensland 4032, Australia, benjamim.tsang@health.qld.gov.au.
Financial disclosures: None.
From the Department of Neurootology, National Hospital of Neurology and Neurosurgery, London (Dr. Tsang, Miss Anwer) and the Ear Institute, University College London, and Guy’s and St Thomas’ NHS Foundation Trust, London, UK (Dr. Murdin).
Abstract
- Objective: To review the clinical manifestations, diagnosis, and management of vestibular migraine (VM).
- Methods: Review of the literature.
- Results: Apart from headache, other symptoms of VM include unsteadiness, imbalance, and spontaneous as well as visual vertigo. Acute vestibular symptoms that qualify for VM must be of at least moderate or severe intensity which lasts within a time window of 5 minutes to 72 hours. The interindividual temporal association of headache and vertigo is highly variable in VM patients Grossly normal peripheral vestibular function and audiometry both during and between attacks distinguishes VM from its mimics. Treatment options for VM are mainly based on expert opinion and include lifestyle modifications, acute and prophylactic migraine pharmacotherapy, and vestibular rehabilitation therapy.
- Conclusion: Despite a lack of diagnostic biomarkers for VM, a meticulous workup is important to exclude alternative mimics. More longitudinal and treatment studies are required to help elucidate the prognosis and optimal management of this condition.
The coexistence of migraine and vestibular symptoms has been mentioned in the headache literature for many years [1–3]. It was first addressed by Kayan and Hood in 1984, who found that dizziness and vertigo occurred in 54% of migraine patients compared with 30% of patients with tension-type headache [1]. The frequent coexistence of migraine and vertigo led researchers to hypothesize that their co-occurence could be due to more than mere chance. As per Lempert and Neuhauser’s evaluation, there is a lifetime prevalence of 16% for migraine and 7% for vertigo, with a 1.1 % chance of vertigo and migraine occurring together by chance alone [4]. In a study looking at the point prevalence of vertigo or dizziness among those presenting for a routine appointment at a headache center, an astounding 72.8% of those with severe headaches had vestibular symptoms [5].
Most epidemiologic studies of what we call vestibular migraine (VM) were based on presentations to specialist clinics and were performed in an era during which no established diagnostic criteria existed. Despite this, most neurootologists would consider VM to be one of the most common causes of spontaneous recurrent vertigo [6]. Neuhauser et al reported that VM was diagnosed in 7% of a group of 200 specialist clinic patients with dizziness and 9% of a group of 200 clinic patients who had migraine [2]. In a population-based study in Germany, the lifetime prevalence of VM according to the Neuhauser criteria was estimated to be 0.98% and the 12-month prevalence 0.89% [7]. The condition has a 3:1 female predilection [8].
VM has only recently been recognised as a separate migraine entity by the International Headache Society (IHS), appearing in the appendix of their International Classification of Headache Disorders (ICHD)–3 beta. The previous ICHD recognised vertigo as a migrainous symptom only within the framework of basilar migraine. The nomenclature used in the literature to describe this entity has been inconsistent and therefore confusing, including terms such as migraine-associated vertigo [9], migraine-related dizziness [3] or vertigo [10],migrainous vertigo [2], benign recurrent vertigo [11], and migraine-related vestibulopathy [12]. For the most part, these terms refer to the co-experience of migraine and vertigo or dizziness, with only a few terms having a more specific meaning of how the 2 symptoms relate temporally. Neuhauser and colleagues developed criteria in 2001 to classify migraineurs for whom vestibular symptoms are an integral part of migraine symptomatology, using the term migrainous vertigo [2]. Others preferred the terms migraine-associated dizziness or migraine-related dizziness [3] over migrainous vertigo because they felt the symptoms of vestibular dysfunction related to migraine are varied and may include gait instability and spatial disorientation but not necessarily with vertigo. To best avoid confounding nonvestibular dizziness or motion sickness associated with migraine, VM has been the preferred term because it emphasises the particular vestibular manifestation of migraine.
The lack of a universally accepted definition for this complex entity has contributed to delayed diagnosis and and treatment for those with this disorder. In this article, we will review the clinical manifestation, diagnosis and management of VM, with a focus on assisting in the differentiation between other potential diagnoses.
Pathophysiology of VM
A clear pathophysiology of VM has not been elucidated. Although predominantly a sporadic disease, there have been reported cases of familial occurrence with an auto-somal dominant inheritance [11,13]. Bahmad and colleagues mapped the first locus for familial VM to 5q35 within a 4-generation family [13]. On the contrary, a larger study conducted by Lee et al found VM to be to genetically heterogeneous with a subset linking to chromosome 22q12 [14]. Genetic defects of voltage-gated calcium channels are identified as causal factors for familial hemiplegic migraine and episodic ataxia type 2. Both these disease entities present with vertigo and migraine headaches suggesting a defective gene within the same chromosomal region could indicate a direct genetic link to VM. However, no such gene has been identified.
General consensus is that the action of spreading cortical depression as it reaches the somatosensory cortex in the posterior insula and temporoparietal junction elucidates migraine aura in patients with short attacks. However, due to the heterogeneity of VM, canal paresis and complex conditional nystagmus during acute stages are not explained through cortical spreading. Eggers et al suggests that vertigo symptoms occur as ictal sensation rather than the spreading of sensory or motor cortical depression [15]. However, due to discrepancies within the literature it is apparent that further research needs to be conducted to fully understand the pathophysiology of VM.
Clinical Manifestations of VM
Symptoms
As many as 80% to 90% of patients with VM report unsteadiness or balance problems, of which 50% to 60% typically report episodic spontaneous vertigo [16], either internal vertigo (a false sensation of self-motion) or external vertigo (a false sensation that the visual surround is spinning or flowing) [17]. The duration of episodes is highly variable, whereby approximately 30% of patients have episodes lasting minutes, 30% have attacks lasting hours, 30% have attacks over several days, while the remaining 10% have attacks lasting seconds only [18]. It may be difficult to distinguish if vestibular symptoms lasting seconds are related to their head motion intolerance, also known as head motion–induced vertigo [17], which is another frequent symptom in VM. Head motion–induced vertigo bears many similarities to motion sickness.
The interindividual temporal association of headache and vertigo is highly variable in VM patients and is a reason many patients find this diagnostic construct difficult to accept. Approximately 30% of adult patients eventually diagnosed with VM initially present without headaches [8]. Vertigo is only regularly associated with headache in 25% to 50% of VM patients [2,7]. A minority of patients report headache and vertigo never occurring together [2]. A temporal pattern, presenting as aura, occurs only in approximately 10% of cases [19]; therefore, vestibular episodes of VM should not be regarded as migraine auras [18]. Patients typically have migraine manifesting earlier in life with the vestibular symptoms following [13,20], whereby the mean age at onset of migraine and diagnosis of VM are approximately 22 and 35 years, respectively [2]. Consistently across studies that measure quality of life scores, VM patients report higher subjective levels of disability compared to patients with other vestibular illnesses, despite having less objective abnormalities [21]. Approximately 85% of VM patients experienced vestibular symptoms for at least 1 year before consulting neurootology services [21]. It could be argued that hypersensitivity of percept to vestibular symptoms reflect the general finding of augmented perceptions to various external stimuli underlying migraine [22,23].
Another prominent feature of VM is that patients report a syndrome of visually-induced dizziness termed visual vertigo (VV). This is a heterogeneous syndrome with strabismic, peripheral, and/or central vestibular aetiologies [24]. Patients with VV complain of discomfort, postural destabilisation, dizziness, imbalance and spatial disorientation in challenging visual environments. Examples of such environments include walking down supermarket aisles, observing moving objects (eg, disco lights, people walking, moving traffic) or moving surroundings during travelling, and the movement of the eyes in general [24–26]. Most patients report more than one visual trigger [24]. Visual vertigo can often be difficult to distinguish from oscillopsia in patients with bilateral vestibular failure. What is most surprising is that patients with VV have a similar handicap level yet report much more vestibular symptoms compared with patients with bilateral vestibular failure [25]. Postural reactions triggered by external visual motion are destabilising with respect to the earth-vertical and are normally suppressed by central re-weighting of sensory postural cues [24]. Surprisingly, premorbid levels of anxiety and childhood motion sickness do not appear to have a correlation with VV [25]. Even in normal subjects, certain complex visual stimuli can induce transient motion sickness–like symptoms, as shown in experimental visually induced self-vection [27]. The Situational Characteristics Questionnaire (SVQ) is a 19-question, symptom-based questionnaire that has been shown to be useful in quantifying features of VV and may be useful in gauging improvement following physical therapies [25,26].
Early in the disease course, hearing loss should prompt an alternative diagnosis. However, late onset cochlear symptoms have been reported in VM. A study found that after 9 years of follow-up, the number of patients with cochlear symptoms more than doubled [28].
Clinical Examination Findings
The importance of the clinical examination is to rule out peripheral vestibular dysfunction and perform positional testing to look for benign paroxysmal positional vertigo (BPPV) or central positional nystagmus. Nonetheless, positional nystagmus has been reported in up to 28% of cases, including definite central-type positional nystagmus reported in as many as 18% [28].
Audiometric Findings and Auditory Brainstem Responses
Normal audiometry both during and between attacks is one of the key clinical features that distinguishes VM from Meniere’s disease [29]. Auditory brainstem response (ABR) results are typically normal in about 65% of patients [29]. Abnormal ABR results are typically nonspecific, such as mild elongation of wave I, III and V latencies and less commonly, prolongation of the inter-peak latencies.
Findings on Vestibular Function Testing
Whilst there are some reported abnormalities in vestibular function testing in VM patients, such findings need to be interpreted with caution due to the small number of subjects, as well as the variation in case definition and cut-off values. Most importantly, very few papers studied patients in the acute phase, and in some studies it was not even specified. The majority of studies report that VM patients interictally have grossly normal peripheral vestibular function with occasional minor irregularities. Profound interictal abnormalities such as complete canal paresis are usually indicative of other diagnoses. In between acute attacks, patients with VM typically have normal gaze, saccadic parameters, ocular pursuit gains and optokinetic nystagmus (OKN) gains on electronystagmography (ENG) or videonystagmography (VNG) [3]. A minority had a low amplitude (< 4 degrees per second) persistent positional nystagmus. On rotation testing of the vestibo-ocular reflex there is reduction of the mean gains compared to headache-free controls. Most reports in the literature do support that the majority of VM patients have grossly normal bithermal caloric testing, although abnormalities including higher slow phase velocities and canal paresis (usually partial) are reported [29–31]. The observation that the artificial vestibular stimulation caused by the caloric test was followed by a migraine attack within 24 hours in 49% of patients with migraine is very interesting [30], and it remains to be tested whether this phenomenon has the potential to be of assistance in the diagnosis of VM. Both VM patients and migraineurs without vertigo have similar subtle cVEMP (Cervical vestibular-evoked myogenic potentials) abnormalities, namely decreased global amplitude and absence of habituation [31]. On computerized dynamic posturography (CDP), a test of sway, VM patients typically demonstrate a surface-dependent pattern based on their SOT analysis [3], suggesting that VM patients may have a substantial vestibulo-spinal abnormality leading to difficulties integrating multiple conflicting sensory inputs [32].
Diagnostic Criteria
Separating VM into 2 diagnostic entities seems particularly useful: definite VM and the more sensitive but less specific category of probable VM. The sensitivity and specificity of the proposed criteria still need to be determined. Although some authors criticize the probable diagnostic entity for its heterogeneity, about 50% of patients initially diagnosed with probable VM ultimately progress to definite VM [12,33]. Definite vestibular migraine appears in the ICHD-3 beta but only in the appendix section for “new disorders that need further research for validation.” However, probable VM will not be included until further evidence of its utility has been accumulated.
The diagnosis is particularly challenging when headache is not a regular accompaniment of the vertiginous attacks. A patient diary may help link migrainous and vertigo symptoms. When headache is not a prominent feature of the attacks, the clinician will have to put migrainous triggers or symptoms such as photophobia or scintillating scotomas in the context of vertigo symptoms to aid with the diagnosis. One needs to be pedantic about differentiating the qualifying symptom of phonophobia, which is defined as a sound-induced discomfort that is often transient and bilateral from the uncomfortable distorted loud sound perception, which occurs with a recruiting sensorineural hearing loss, and is often persistent and unilateral [18]. Response to migraine treatment is not sufficiently specific to be included in the diagnostic criteria. High placebo response rates from migraine trials [34] suggest that placebo effects can likewise be expected in the treatment of VM. Despite these challenges, acceptance of the diagnostic entity of VM seems to be gaining momentum. In a follow-up study over 9 years, the diagnosis remained consistent in 85% of patients [33].
Benign Paroxysmal Vertigo of Childhood and Vestibular Migraine in Children
VM can present at any age, however, the ICHD specifically recognises an early vertiginous entity regarded as a precursor syndrome of migraine in otherwise healthy children called benign paroxysmal vertigo of childhood. This diagnosis requires 5 episodes of severe vertigo, occurring without warning and resolving spontaneously after minutes to hours [35]. In between episodes, neurological examination, audiometry, vestibular functions and EEG must be normal. A unilateral throbbing headache may occur during attacks but it is not a mandatory criterion. It is unclear whether these two conditions in children are the same entity, however it is important to note that the classification of VM does not involve any age limit [18].
Basilar-type Migraine
The term basilar migraine should be restricted to patients who fulfill the ICHD diagnostic criteria [35] given it is a clinically distinct entity from VM. Less than 10% of VM patients further fulfill the ICHD criteria for basilar migraine [2,18]. More than 60% of basilar-type migraine patients have vertigo and there are many overlapping clinical manifestations with VM. This diagnosis requires at least 2 symptoms from aura in the posterior circulation territory, whereas most patients with VM have vestibular symptoms only [35]. Moreover, in basilar migraine the duration of vertigo should correspond to the length of an aura, that is, between 5 and 60 minutes [35]. Further studies are required to further elucidate and delineate these 2 conditions.
Other Important Diagnostic Considerations
Meniere’s Disease
An important differential diagnosis of VM is the early presentation of Meniere’s disease (MD). Although fluctuating hearing loss, aural fullness and episodic vertigo are important symptoms in the recent updated diagnostic criteria for definite MD [36,37], these symptoms have been reported in patients with migraine [38]. Moreover, minor abnormalities in cVEMPs and arguably in caloric testing can be found in VM patients, as previously mentioned. Predominantly, the distinction can be made considering that a more sustained, albeit occasionally fluctuating, hearing loss would occur in MD, which can progress to severe hearing loss within a few years. However, the diagnosis can be difficult considering that audiometric and vestibular function abnormalities as well as the typical cochlear symptoms are often absent in the early stages of the MD. Nonetheless, preclinical labelling of patients with episodic vertigo without hearing loss as “vestibular MD” is unhelpful as this population may be overrepresented by actual migraineurs. Studies of patients with so-called benign recurrent vertigo or recurrent vestibulopathy are likely to be heterogeneous entities, with perhaps cases later evolving into VM or MD.
Coexisting migraine and MD is often challenging both in terms of diagnosis and management. Many studies have shown an increased prevalence of migraine in MD patients compared to controls [39,40], an asso-ciation suggested by Prosper Ménière himself in 1861 [41]. A study by Radtke et al found that the lifetime prevalence of migraine with and without aura was over 2 times higher in definite MD patients of both sexes compared to age-matched controls (56% versus 25%) [39]. Interestingly, 45% of the patients with MD always experienced at least 1 migrainous symptom (migrainous headache, photophobia, aura symptoms) with their Meniere attacks [39]. This may be at least partly due to the triggering effect of vestibular symptoms on migraineurs [30]. Migraine may even influence the disease course of MD as indicated by a retrospective case control study which found that definite MD patients who have concomitant ICHD criteria for migraine [35] had a significantly earlier onset of MD symptoms (mean age, 37.2 versus 49.3 years) and a much greater susceptibility to simultaneous bilateral, but not sequential, hearing loss as compared to MD patients without migraine (56% versus 4%) [42]. There were no significant differences in the severity of hearing loss between the 2 groups even when controlling for time to evaluation [42]. A family history of episodic vertigo was seen in 39% of MD patients with migraine, which is significantly higher than the 2% seen in MD patients, suggesting a possible genetic basis for this association [42]. The nature of the association between migraine and MD is not well elucidated, however, some authors propose that migraine leads to isolated microvascular ischaemic damage of the inner ear, presumably through small arterial vasospasm [40,42].
In summary, when the criteria for MD are met together with documented audiometric abnormalities, MD should be diagnosed, even if migraine symptoms occur during the vestibular attacks [18]. Only patients who experience 2 different types of attacks, one fulfilling the criteria for VM and the other for MD, should be labelled as Meniere’s disease/migraine overlap syndrome. It is hoped that future revisions of diagnostic criteria will include this overlap entity.
Migraine and Benign Paroxysmal Positional Vertigo
VM patients can experience brief positional dizziness and therefore VM may mimic BPPV. It is therefore important to perform positional testing to look for nystagmus typical for BPPV. Certainly the positional characteristics are distinct from BPPV with regard to the duration of attacks (often as long as the head position is maintained in VM rather than seconds in BPPV). BPPV may also produce attacks of vertigo that can act as triggers for migraine headaches. In these patients, treatment of the BPPV will reduce headache frequency [30].
Transient Ischemic Attacks
Transient ischemic attack (TIA) is a cerebrovascular disease with temporary neurological symptoms [43] and is differentiated from VM mainly from the characteristics of reported symptoms. Being a vascular phenomenon, one would expect TIA symptoms to have a sudden onset, with a brief duration of symptoms (typically short minutes), followed by a rapid improvement to baseline, as well as correspond to a vascular territory. The other important message is that stereotyped, frequently recurrent symptoms are less likely to be TIAs, with the exception of capsular warning syndrome [44] and limb shaking TIAs [43] described elsewhere.
Migraine and Motion Sickness
In an individual patient it may be difficult to differentiate between motion sickness and acute attacks of VM induced by motion stimuli. The distinction may be helped by observing nausea and dizziness improving after cessation of motion which points more towards motion sickness, as oppose to the persistent vertigo after the motion stimulus has ended, thus pointing more towards VM.
Episodic Ataxia Type 2
Of the various episodic ataxias, episodic ataxia type 2 would be the most important subtype in the differential diagnosis of VM given it presents with episodic vertigo and is the most frequently occurring subtype. It is a rare autosomal dominant inherited neurological disorder resulting from mutations of the calcium channel gene CACNA1A [45]. The clinical manifestations include recurrent disabling attacks of imbalance, vertigo and ataxia, which can be provoked by physical exertion or emotional stress. Patients may have downbeat nystagmus interictally. A slow progression of cerebellar signs accompanied by atrophy of midline cerebellar structures and a response to acetazolamide or 4-aminopyridine can help distinguish it from VM.
Migraine, Dizziness, and Comorbid Psychiatric Disorders
Particularly in patients with protracted symptoms, it is difficult to tease out the difference between the symptoms of migraine and dizziness from the symptoms of certain psychiatric disorders given their bidirectional associations. Migraine is a risk factor for first-onset major depression [46] and panic disorder [47]. Patients with VM have very high rates (30%–65%) of coexisting psychiatric illness, especially anxiety and depression, with frequencies higher than that associated with other migraine or vestibular disorders [48,49]. Vestibular migraine patients who have a positive history of psychiatric disorders have a comparatively higher risk of developing somatoform dizziness [48]. The unpredictability of recurrent vestibular symptoms could be a factor leading to elevated distress in VM patients. It is not uncommon to see a premature diagnosis of psychogenic dizziness to be given to patients without objective abnormalities. On the contrary, a diagnosis of psychogenic dizziness can rarely be made with certainty due to multiple reasons. Disabling vertigo leading to physical symptoms and avoidance of social activities can easily be misconstrued to have panic disorder with or without agoraphobia. Moreover, dizziness is the second most common symptom of a panic attack after palpitations [50].
Unfortunately, there are no objective tests that can reliably discriminate vestibular syndromes from psychiatric syndromes in patients with dizziness. The SVQ is not specific enough to differentiate symptoms of VV from the space and motion discomfort symptoms often found in agoraphobic patients [25]. Experimentally, agoraphobia patients may have a more surface-dependent strategy rather than a visual-dependent strategy on CDP [51]. It is unclear whether the vestibular system is causally linked to emotion processing pathways.
Chronic Subjective Dizziness
Chronic subjective dizziness is an entity characterised by chronic unsteadiness or nonvertiginous dizziness accompanied by hypersensitivity to motion stimuli and poor tolerance for complex visual stimuli lasting for 3 months or more without objective abnormalities [52]. These vestibular symptoms are often difficult to distinguish from symptoms of VM. This condition is thought to be a spatial sensory analog of allodynia experienced by some chronic migraine headache sufferers [8].
Dizziness Due to Side Effects of Migraine Prophylactic Medications
Dizziness is often listed as a side effect in the product information of various medications including those used for migraine prophylaxis. It is important to take an accurate history of the suspected offending drug in terms of its temporal relationship to vestibular symptoms. Tricyclic antidepressants (TCAs) can cause drowsiness, lightheadedness, fatigue and blurred vision [53]. Beta-blockers can cause orthostatic hypotension [53]. All the above effects could be confused with vestibular symptoms.
Treatment of Vestibular Migraine
Current treatment options for VM are mainly limited to expert opinion rather than inferred from randomized controlled trials (RCTs) [54]. Below we have offered our consensus on how VM should be managed, with concepts based on the guidelines of treatment for typical migraine [55]. Avoidance of migraine triggers should always be the first avenue of treatment. In addition, any vestibular disorder that is triggering migraine attacks should be identified and treated in its own right. Pharmacotherapy can be abortive for acute episodes and prophylactic.
Lifestyle Advice
The key first task in management is the correct diagnosis and educating the patient about the condition. A thorough explanation of the migraine origin of the attacks can address patients fear and expectations. Nonpharmaceutical approaches in the treatment of VM should not be neglected, even though only a very small proportion of patients may derive a benefit. Advice on dietary manipulation is routinely given; however, its efficacy in VM is questionable. Dietary advice includes healthy eating at regular intervals to prevent skipped meals as well as avoidance of excess caffeine and rich foods. A retrospective study found that lifestyle intervention alone resulted in 13 of 81 patients experiencing significant relief from vestibular symptoms with migraine. The remaining cohort of patients required a multifaceted approach including pharmacotherapy to achieve similar benefit [56].
Acute Abortive Treatments
Oral antiemetics are commonly prescribed for motion sickness and acute migraine, however there is no evidence supporting their effectiveness in VM (Table 2). Patients should be counselled about avoiding overuse of antiemetics given their risk of causing extrapyramidal side effects [53].
Simple analgesics, such as paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs), have been found to be helpful in acute VM attacks in observational studies. Bikhazi performed a survey of patients presenting to a headache clinic with vestibular symptoms and found that simple analgesics were valued by patients as effective symptomatic treatment, but were not considered as effective as triptans [59]. Doses of simple analgesics are listed in Table 2. Soluble formulations are preferable due to faster absorption and speed of onset. Opioids should be avoided in acute attacks of VM given the risk of developing opioid overuse headache [55].
Migraine Prophylaxis in Vestibular Migraine
TCAs remain a popular choice of migraine prophylaxis amongst neurootologists because of its additional effects on comorbid affective symptoms. We recommend that the starting dose of either amitriptyline or nortriptyline should be between 5 to 10 mg daily at night, slowly uptitrated to response over several weeks up to a maximum of 100 mg at night. Interval electrocardiography should be performed to monitor for prolongation of the QTc interval. A retrospective chart review found 46% of VM patients (by Neuhauser criteria) reported a reduction in dizziness after nortriptyline administration up to 75 mg daily [62]. However, the current evidence is limited to observational studies [59,62–64].
The evidence for beta-blockers is limited in VM but anecdotally has been useful for patients with frequent episodic migraine [59,63,64]. Recommended starting and maintenance doses are listed in Table 3. Furthermore, propranolol can be used in patients with depression [65,66]. Heart rate and electrocardiography should be monitored during dose escalation. Beta-blockers should be avoided in asthmatics. Commonly reported adverse events include cold, extremities reduced exercise tolerance and dizziness [53].
Flunarizine, a calcium channel blocker widely used in migraine [67,68] and vestibular conditions [69], was recently studied in a RCT of 12 weeks' duration for prophylaxis of migrainous vertigo (Neuhauser criteria) in 48 patients [70]. Although flunarizine 10 mg daily did not result in improved headache frequency and severity compared to the control arm, there was a significant improvement in vertigo severity. The most commonly reported side effects of flunarizine are weight gain and somnolence, both of which are minimal or infrequent. Verapamil is another calcium channel blocker that may be helpful but has major limiting adverse effects are bradycardia, constipation and peripheral edema [53].
Pizotifen, a serotonin antagonist, is one of the most well tolerated prophylaxis agents from our experience, however some patients do not adhere to treatment due to drowsiness or weight gain, as evidenced in retrospective case studies [64].
Topiramate with an average daily dose of 100 mg has reported positive results in a prospective observational study of ten patients with VM with auditory symptoms [71]. Nine of 10 patients reported no symptoms after follow-up period of up to sixteen months. The recommended dose is listed in Table 3. Common side effects include distal paresthesias, reduced ability to concentrate and drowsiness [53]. Sodium valproate has been anecdotally effective [59] and is usually well tolerated especially when starting at a low dose of 200 mg at night, slowly titrated to 1200 mg in 2 divided doses. Liver function and full blood evaluation should be monitored on a periodic basis [53].
Third-line medications have only been used anecdotally and should be reserved for extenuating cases (Table 3).
Vestibular Rehabilitation
Vestibular rehabilitation therapy (VRT) has been shown to alleviate significantly ongoing balance and dizziness symptoms in patients with various vestibular disorders [73,74] and improving confidence with balance in elderly patients [75,76]. However, the value of VRT is not as well established in VM. Anecdotally, patients with VM report persistent significant symptoms at the end of a standard VRT period, in contrast to other nonmigrainous patients who appear to be accomplishing their treatment goals faster. However, recent studies [21,73,77] are suggesting that customised VRT may play a useful role in VM, especially since it appears to target issues of anxiety, visual dependence or loss of confidence in balance. Small retrospective case series found that VRT reduced disability scores, and gait and balance function in over 85% of patients with migraine and vestibular symptoms [73,76,77]. An Australian VRT study (21) has recently assessed the efficacy of a 9-week customised VRT in 20 patients with VM compared to 16 patients with vestibular symptoms but without migraine. The customized VRT program consisted of habituation, gaze stability, static tilt, balance and gait exercises. A pictorial exercise instruction sheet for home use would describe these exercises of approximately 15 minutes duration consisting of 4 to 6 exercises to be performed 3 times a day, every day for 9 weeks. Interestingly, both groups benefitted equally from VRT. Compliance with VRT was comparable between the two groups. Commonly reported reasons for non-attendance in VM patients included a recent acute attack of VM, anxiety related to using public transport, and commitment issues related to occupation. This study also suggested that VM patients required more customized and intensive therapy as 15% of VM patients required additional appointments outside the study timeline.
Given that visual dependency has been shown to be reduced with short-term graded optokinetic stimulation exposure in healthy subjects [78], there has been interest using this intervention in conjunction with customized VRT to promote desensitization to visual stimuli as a treatment for VM patients with VV. Most promisingly is the finding that a subgroup of patients with a history of migraine improved significantly more than other vestibular patients with respect to VV symptoms.
There has been controversy surrounding whether patients should avoid medications when undergoing VRT. The protagonists of this view suggest that medications that affect the central nervous system (CNS) may modulate the rate of central compensation. In the aforementioned study by Vitkovic and colleagues [21], the same degree of improvement was seen in the VM group regardless of medication regimen. A study by Whitney and colleagues [73] found that migraine related vestibulopathy patients taking prophylaxis demonstrated better subjective and objective balance scores at baseline and after therapy. Further research is required to clarify the role of CNS-acting medication and their administration around VRT sessions.
Physical therapists dealing with VM patients may face additional challenges in encouraging exercise compliance and providing emotional support. Although more time consuming for the therapist, this is important in the face of high rates of comorbid affective disorders and head motion intolerance. Supervised VRT is believed to implicitly improve psychological status through increasing confidence, providing reassurance, and emphasizing positive effects of VRT, particularly when the patient feels their symptoms have been made worse by it.
Cognitive Behavioral Therapy
Cognitive behavioral therapy (CBT) has been shown to be helpful as part of the holistic treatment of various disorders including post-concussive syndrome and depression in neurology patients [79,80]. Among patients suffering from dizziness, a small study comparing explicit CBT combined with VRT versus waiting-list controls demonstrated improvements in patients’ coping ability, function, symptoms, and care satisfaction [81]. However, to our knowledge there are no studies directly evaluating the benefits of CBT specifically in VM patients. Despite this, it is our practice to request CBT for VM patients who report disabling anxiety or depressive symptoms.
Prognosis
Although migraine in general can improve in later life, this is less certain with VM given the lack of good quality longitudinal studies. Recently Radtke and colleagues published their long-term (median, 9 years) follow-up study of 61 definite VM cases (28). They found that 87% of patients had recurrent vertigo at follow-up. The frequency of vertigo was reduced in 56%, increased in 29%, and unchanged in 16% of patients. The impact of vertigo was graded as severe in 21%, moderate in 43%, and mild in 36% of patients. However, they found that concomitant cochlear symptoms with vertigo had increased from 15% at study inception to 49% at follow-up and secondly, 18% of patients had developed mild bilateral low-frequency sensorineural hearing loss. Therefore, one major criticism of the study is whether some of the patients had MD as their eventual diagnosis rather than definite VM. On the contrary, the authors conclude that these changes represent new vestibulo-cochlear dysfunction as a result of VM disease progression. Due to these reasons, the prognosis of VM patients is unclear. It is our practice to ensure patients do receive delayed follow-up to allow consideration of other neurotological diagnoses.
Conclusion
Given the large heterogeneity in presentation and objective testing, VM as a diagnostic construct has remained quite controversial, though increasingly more accepted. The more we study this common vestibular condition, the more we are realising that the complex relationship between migraine and dizziness extend beyond VM to encompass other vestibular disorders such as MD and anxiety. The lack of a physiological biomarker contributes to its diagnostic difficulties, but a meticulous workup is important to exclude alternative vestibular diagnoses. More longitudinal studies and RCTs are required to help both understand the prognosis and management of VM patients.
Corresponding author: Benjamin K-T Tsang, MBBS, FRACP, The Prince Charles Hospital, Rode Road, Chermside, Queensland 4032, Australia, benjamim.tsang@health.qld.gov.au.
Financial disclosures: None.
1. Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain 1984;107(Pt 4):1123–42.
2. Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56:436–41.
3. Furman JM, Sparto PJ, Soso M, Marcus D. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res 2005;15:327–32.
4. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol 2009;256:333–8.
5. Calhoun AH, Ford S, Pruitt AP, Fisher KG. The point prevalence of dizziness or vertigo in migraine--and factors that influence presentation. Headache 2011;51:1388–92.
6. Bisdorff A. Migraine and dizziness. Curr Opin Neurol 2014;27:105–10.
7. Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology 2006;67:1028–33.
8. Sargent EW. The challenge of vestibular migraine. Curr Opin Otolaryngol Head Neck Surg 2013;21:473–9.
9. Cha YH. Migraine-associated vertigo: diagnosis and treatment. Sem Neurol 2010;30:167–74.
10. Cherian N. Vertigo as a migraine phenomenon. Curr Neurol Neurosci Rep 2013;13:343.
11. Oh AK, Lee H, Jen JC, et al. Familial benign recurrent vertigo. Am J Med Genet 2001;100:287–91.
12. Cass SP, Furman JM, Ankerstjerne K, et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol 1997;106:182–9.
13. Bahmad F Jr, DePalma SR, Merchant SN, et al. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol 2009;118:670–6.
14. Lee H, Jen JC, Wang H, et al. A genome-wide linkage scan of familial benign recurrent vertigo: linkage to 22q12 with evidence of heterogeneity. Hum Molec Genet 2006;15:251–8.
15. Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res 2014;24:387–95.
16. Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms--identifying clinical features that predict “vestibular migraine”. Headache 2011;51:1393–7.
17. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1-13.
18. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167-72.
19. Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999;246:883–92.
20. Eggers SD, Staab JP, Neff BA, et al. Investigation of the coherence of definite and probable vestibular migraine as distinct clinical entities. Otol Neurotol 2011;32:1144–51.
21. Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol 2013;260:3039–48.
22. Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache 2004;44:1019–23.
23. Morrison DP. Abnormal perceptual experiences in migraine. Cephalalgia 1990;10:273–7.
24. Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psych 1995;59:472–6.
25. Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain 2001;124(Pt 8):1646–56.
26. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res 2006;16:223-31.
27. Dobie TG, May JG, Gutierrez C, Heller SS. The transfer of adaptation between actual and simulated rotary stimulation. Aviat Space Environ Med 1990;61:1085–91.
28. Radtke A, von Brevern M, Neuhauser H, et al. Vestibular migraine: long-term follow-up of clinical symptoms and vestibulo-cochlear findings. Neurology 2012;79:1607–14.
29. Bayazit Y, Yilmaz M, Mumbuc S, Kanlikama M. Assessment of migraine-related cochleovestibular symptoms. Revue Laryngol Otol Rhinol 2001;122:85–8.
30. Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology 2009;73:638–42.
31. Roceanu A, Allena M, De Pasqua V, et al. Abnormalities of the vestibulo-collic reflex are similar in migraineurs with and without vertigo. Cephalalgia 2008;28:988–90.
32. Hong HR, Shim DB, Kim TS, et al. Results of caloric and sensory organization testing of dynamic posturography in migrainous vertigo: comparison with Meniere’s disease and vestibular neuritis. Acta Otolaryngol 2013;133:1236–41.
33. Radtke A, Neuhauser H, von Brevern M, et al. Vestibular migraine--validity of clinical diagnostic criteria. Cephalalgia 2011;31:906-13.
34. Rothner AD, Wasiewski W, Winner P, et al. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 2006;46:101-9.
35. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd ed (beta version). Cephalalgia 2013;33:629–808.
36. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere’s disease. J Vestib Res 2015;25:1–7.
37. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation. Otolaryngol Head Neck Surg 1995;113:181–5.
38. Baloh RW. Neurotology of migraine. Headache 1997;37:615-21.
39. Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology 2002;59:1700–4.
40. Lee H, Lopez I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurol 2000;57:1631–4.
41. Ménière P. Pathologie auriculaire: memoires sur une lésion de l’oreille interne donnant lieu à des symptoms de congestion cérébrale apoplectiforme. Gaz Med Paris 1861;16:597–601.
42. Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Meniere’s disease. Acta Otolaryngol 2007;127:1241–5.
43. Kim JS. Symptoms of transient ischemic attack. Front Neurol Neurosci 2014;33:82–102.
44. Paul NL, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology 2012;79:1356–62.
45. Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics 2007;4:267–73.
46. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54:308–13.
47. Breslau N, Schultz LR, Stewart WF, et al. Headache types and panic disorder: directionality and specificity. Neurology 2001;56:350–4.
48. Best C, Eckhardt-Henn A, Tschan R, Dieterich M. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes. Results of a prospective longitudinal study over one year. J Neurol 2009;256:58–65.
49. Eckhardt-Henn A, Best C, Bense S, et al. Psychiatric comorbidity in different organic vertigo syndromes. J Neurol 2008;255:420–8.
50. Segui J, Salvador-Carulla L, Garcia L, et al. Semiology and subtyping of panic disorders. Act Psychiatr Scand 1998;97:272–7.
51. Jacob RG, Furman JM, Durrant JD, Turner SM. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med 1997;59:323–30.
52. Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am 2009;42:71–7, ix.
53. Australian medicines handbook : AMH. Adelaide, S.Aust.: Australian Medicines Handbook; 2015. p. v.
54. Maldonado Fernandez M, Birdi JS, Irving GJ, et al. Pharmacological agents for the prevention of vestibular migraine. Cochrane Database Syst Rev 2015;6:CD010600.
55. British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication overuse headache. 3rd ed. 2010.
56. Reploeg MD, Goebel JA. Migraine-associated dizziness: patient characteristics and management options. Otol Neurotol 2002;23:364–71.
57. Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo-controlled trial. Neurology 2003;60:882–3.
58. Roberto G, Raschi E, Piccinni C, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia 2015;35:118–31.
59. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol 1997;18:350–4.
60. MedicinesComplete. London: Pharmaceutical Press. Available at www.medicinescomplete.com.
61. Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000;60:1259–87.
62. Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol 2012;33:121–7.
63. Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006;116:1782–6.
64. Waterston J. Chronic migrainous vertigo. J Clin Neurosci 2004;11:384–8.
65. de Bock GH, Eelhart J, van Marwijk HW, et al. A postmarketing study of flunarizine in migraine and vertigo. Pharm World Sci 1997;19:269–74.
66. Verspeelt J, De Locht P, Amery WK. Postmarketing study of the use of flunarizine in vestibular vertigo and in migraine. Eur J Clin Pharmacol 1996;51:15–22.
67. Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. J Cardiovasc Pharmacol 1991;18 Suppl 8:S21–6.
68. Lucetti C, Nuti A, Pavese N, et al. Flunarizine in migraine prophylaxis: predictive factors for a positive response. Cephalalgia 1998;18:349–52.
69. Schmidt R, Oestreich W. Flunarizine in the treatment of vestibular vertigo: experimental and clinical data. J Cardiovasc Pharmacol 1991;18 Suppl 8:S27–30.
70. Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol 2014;271:2931–6.
71. Carmona S, Settecase N. Use of topiramate (topamax) in a subgroup of migraine-vertigo patients with auditory symptoms. Ann N Y Acad Sci 2005;1039:517–20.
72. Bisdorff AR. Treatment of migraine related vertigo with lamotrigine an observational study. Bull Soc Sci Med Grand Duche Luxemb 2004:103–8.
73. Whitney SL, Rossi MM. Efficacy of vestibular rehabilitation. Otolaryngol Clin North Am 2000;33:659–72.
74. Enticott JC, Vitkovic JJ, Reid B, et al. Vestibular rehabilitation in individuals with inner-ear dysfunction: a pilot study. Audiol Neurootol 2008;13:19–28.
75. Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol Ser A Biol Sci Med Sci 1998;53:M287–94.
76. Wrisley DM, Whitney SL, Furman JM. Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol 2002;23:483–7.
77. Gottshall KR, Moore RJ, Hoffer ME. Vestibular rehabilitation for migraine-associated dizziness. Int Tinnitus J 2005;11:81–4.
78. Pavlou M, Quinn C, Murray K, et al. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture 2011;33:113–8.
79. Leddy JJ, Sandhu H, Sodhi V, et al. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54.
80. Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res 2015;78:411–9.
81. Andersson G, Asmundson GJ, Denev J, et al. A controlled trial of cognitive-behavior therapy combined with vestibular rehabilitation in the treatment of dizziness. Behav Res Ther 2006;44:1265–73.
1. Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain 1984;107(Pt 4):1123–42.
2. Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56:436–41.
3. Furman JM, Sparto PJ, Soso M, Marcus D. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res 2005;15:327–32.
4. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol 2009;256:333–8.
5. Calhoun AH, Ford S, Pruitt AP, Fisher KG. The point prevalence of dizziness or vertigo in migraine--and factors that influence presentation. Headache 2011;51:1388–92.
6. Bisdorff A. Migraine and dizziness. Curr Opin Neurol 2014;27:105–10.
7. Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology 2006;67:1028–33.
8. Sargent EW. The challenge of vestibular migraine. Curr Opin Otolaryngol Head Neck Surg 2013;21:473–9.
9. Cha YH. Migraine-associated vertigo: diagnosis and treatment. Sem Neurol 2010;30:167–74.
10. Cherian N. Vertigo as a migraine phenomenon. Curr Neurol Neurosci Rep 2013;13:343.
11. Oh AK, Lee H, Jen JC, et al. Familial benign recurrent vertigo. Am J Med Genet 2001;100:287–91.
12. Cass SP, Furman JM, Ankerstjerne K, et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol 1997;106:182–9.
13. Bahmad F Jr, DePalma SR, Merchant SN, et al. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol 2009;118:670–6.
14. Lee H, Jen JC, Wang H, et al. A genome-wide linkage scan of familial benign recurrent vertigo: linkage to 22q12 with evidence of heterogeneity. Hum Molec Genet 2006;15:251–8.
15. Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res 2014;24:387–95.
16. Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms--identifying clinical features that predict “vestibular migraine”. Headache 2011;51:1393–7.
17. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1-13.
18. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167-72.
19. Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999;246:883–92.
20. Eggers SD, Staab JP, Neff BA, et al. Investigation of the coherence of definite and probable vestibular migraine as distinct clinical entities. Otol Neurotol 2011;32:1144–51.
21. Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol 2013;260:3039–48.
22. Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache 2004;44:1019–23.
23. Morrison DP. Abnormal perceptual experiences in migraine. Cephalalgia 1990;10:273–7.
24. Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psych 1995;59:472–6.
25. Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain 2001;124(Pt 8):1646–56.
26. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res 2006;16:223-31.
27. Dobie TG, May JG, Gutierrez C, Heller SS. The transfer of adaptation between actual and simulated rotary stimulation. Aviat Space Environ Med 1990;61:1085–91.
28. Radtke A, von Brevern M, Neuhauser H, et al. Vestibular migraine: long-term follow-up of clinical symptoms and vestibulo-cochlear findings. Neurology 2012;79:1607–14.
29. Bayazit Y, Yilmaz M, Mumbuc S, Kanlikama M. Assessment of migraine-related cochleovestibular symptoms. Revue Laryngol Otol Rhinol 2001;122:85–8.
30. Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology 2009;73:638–42.
31. Roceanu A, Allena M, De Pasqua V, et al. Abnormalities of the vestibulo-collic reflex are similar in migraineurs with and without vertigo. Cephalalgia 2008;28:988–90.
32. Hong HR, Shim DB, Kim TS, et al. Results of caloric and sensory organization testing of dynamic posturography in migrainous vertigo: comparison with Meniere’s disease and vestibular neuritis. Acta Otolaryngol 2013;133:1236–41.
33. Radtke A, Neuhauser H, von Brevern M, et al. Vestibular migraine--validity of clinical diagnostic criteria. Cephalalgia 2011;31:906-13.
34. Rothner AD, Wasiewski W, Winner P, et al. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 2006;46:101-9.
35. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd ed (beta version). Cephalalgia 2013;33:629–808.
36. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere’s disease. J Vestib Res 2015;25:1–7.
37. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation. Otolaryngol Head Neck Surg 1995;113:181–5.
38. Baloh RW. Neurotology of migraine. Headache 1997;37:615-21.
39. Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology 2002;59:1700–4.
40. Lee H, Lopez I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurol 2000;57:1631–4.
41. Ménière P. Pathologie auriculaire: memoires sur une lésion de l’oreille interne donnant lieu à des symptoms de congestion cérébrale apoplectiforme. Gaz Med Paris 1861;16:597–601.
42. Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Meniere’s disease. Acta Otolaryngol 2007;127:1241–5.
43. Kim JS. Symptoms of transient ischemic attack. Front Neurol Neurosci 2014;33:82–102.
44. Paul NL, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology 2012;79:1356–62.
45. Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics 2007;4:267–73.
46. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54:308–13.
47. Breslau N, Schultz LR, Stewart WF, et al. Headache types and panic disorder: directionality and specificity. Neurology 2001;56:350–4.
48. Best C, Eckhardt-Henn A, Tschan R, Dieterich M. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes. Results of a prospective longitudinal study over one year. J Neurol 2009;256:58–65.
49. Eckhardt-Henn A, Best C, Bense S, et al. Psychiatric comorbidity in different organic vertigo syndromes. J Neurol 2008;255:420–8.
50. Segui J, Salvador-Carulla L, Garcia L, et al. Semiology and subtyping of panic disorders. Act Psychiatr Scand 1998;97:272–7.
51. Jacob RG, Furman JM, Durrant JD, Turner SM. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med 1997;59:323–30.
52. Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am 2009;42:71–7, ix.
53. Australian medicines handbook : AMH. Adelaide, S.Aust.: Australian Medicines Handbook; 2015. p. v.
54. Maldonado Fernandez M, Birdi JS, Irving GJ, et al. Pharmacological agents for the prevention of vestibular migraine. Cochrane Database Syst Rev 2015;6:CD010600.
55. British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication overuse headache. 3rd ed. 2010.
56. Reploeg MD, Goebel JA. Migraine-associated dizziness: patient characteristics and management options. Otol Neurotol 2002;23:364–71.
57. Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo-controlled trial. Neurology 2003;60:882–3.
58. Roberto G, Raschi E, Piccinni C, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia 2015;35:118–31.
59. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol 1997;18:350–4.
60. MedicinesComplete. London: Pharmaceutical Press. Available at www.medicinescomplete.com.
61. Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000;60:1259–87.
62. Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol 2012;33:121–7.
63. Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006;116:1782–6.
64. Waterston J. Chronic migrainous vertigo. J Clin Neurosci 2004;11:384–8.
65. de Bock GH, Eelhart J, van Marwijk HW, et al. A postmarketing study of flunarizine in migraine and vertigo. Pharm World Sci 1997;19:269–74.
66. Verspeelt J, De Locht P, Amery WK. Postmarketing study of the use of flunarizine in vestibular vertigo and in migraine. Eur J Clin Pharmacol 1996;51:15–22.
67. Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. J Cardiovasc Pharmacol 1991;18 Suppl 8:S21–6.
68. Lucetti C, Nuti A, Pavese N, et al. Flunarizine in migraine prophylaxis: predictive factors for a positive response. Cephalalgia 1998;18:349–52.
69. Schmidt R, Oestreich W. Flunarizine in the treatment of vestibular vertigo: experimental and clinical data. J Cardiovasc Pharmacol 1991;18 Suppl 8:S27–30.
70. Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol 2014;271:2931–6.
71. Carmona S, Settecase N. Use of topiramate (topamax) in a subgroup of migraine-vertigo patients with auditory symptoms. Ann N Y Acad Sci 2005;1039:517–20.
72. Bisdorff AR. Treatment of migraine related vertigo with lamotrigine an observational study. Bull Soc Sci Med Grand Duche Luxemb 2004:103–8.
73. Whitney SL, Rossi MM. Efficacy of vestibular rehabilitation. Otolaryngol Clin North Am 2000;33:659–72.
74. Enticott JC, Vitkovic JJ, Reid B, et al. Vestibular rehabilitation in individuals with inner-ear dysfunction: a pilot study. Audiol Neurootol 2008;13:19–28.
75. Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol Ser A Biol Sci Med Sci 1998;53:M287–94.
76. Wrisley DM, Whitney SL, Furman JM. Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol 2002;23:483–7.
77. Gottshall KR, Moore RJ, Hoffer ME. Vestibular rehabilitation for migraine-associated dizziness. Int Tinnitus J 2005;11:81–4.
78. Pavlou M, Quinn C, Murray K, et al. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture 2011;33:113–8.
79. Leddy JJ, Sandhu H, Sodhi V, et al. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54.
80. Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res 2015;78:411–9.
81. Andersson G, Asmundson GJ, Denev J, et al. A controlled trial of cognitive-behavior therapy combined with vestibular rehabilitation in the treatment of dizziness. Behav Res Ther 2006;44:1265–73.
Measuring the quality of palliative care and supportive oncology: principles and practice
Palliative care quality indicators should be part of oncology performance assessment initiatives. Palliative care programs should also include initiatives to address the overall quality of palliative care issues, such as pain management, in the settings where the programs are located.1 Measuring quality facilitates justifying palliative care initiatives and documenting their impact, targeting quality improvement efforts, monitoring care for deficiencies, and evaluating providers (Table 1). However, measurement in this field is often not straightforward. Potential challenges include defining the population to measure and data sources, collection and analysis, as well as choosing among many potentially relevant issues and quality measures. This article describes an approach to quality measurement in palliative care, beginning with a description of key frameworks to guide the measurement approach. The article also reviews key steps in designing a quality measurement program, which include defining the quality problem and population to measure and choosing domains and specific measures. Finally, the article addresses other key considerations, such as considering unintended consequences and using data for quality improvement.
Frameworks for evaluating quality
The Donabedian framework of structure (stable elements of the health care system), process (what health care services are provided), and outcome (end results for the patient and family) can be
applied to relevant domains to guide evaluation design (Table 2).2-8 Key structural elements may include characteristics of programs (eg, palliative clinic availability), providers (eg, multidisciplinary members of the palliative care team), and tools (eg, do-not-resuscitate policies). Processes may include technical aspects of care, such as appropriate prescribing and interpersonal aspects of care (eg, coordination among providers). Outcomes may include patient quality of life or symptoms, perceptions of care, or caregiver outcomes such as burden. Outcomes may also be categorized as overuse (eg, use of chemotherapy at the end of life compared to national benchmarks), underuse (eg, lower rates of hospice care or use of antinausea drugs), or appropriateness of care (eg, accurately documenting patients’ preferences for care).
Palliative care quality indicators should be part of oncology performance assessment initiatives. Palliative care programs should also include initiatives to address the overall quality of palliative care issues, such as pain management, in the settings where the programs are located.1 Measuring quality facilitates justifying palliative care initiatives and documenting their impact, targeting quality improvement efforts, monitoring care for deficiencies, and evaluating providers (Table 1). However, measurement in this field is often not straightforward. Potential challenges include defining the population to measure and data sources, collection and analysis, as well as choosing among many potentially relevant issues and quality measures. This article describes an approach to quality measurement in palliative care, beginning with a description of key frameworks to guide the measurement approach. The article also reviews key steps in designing a quality measurement program, which include defining the quality problem and population to measure and choosing domains and specific measures. Finally, the article addresses other key considerations, such as considering unintended consequences and using data for quality improvement.
Frameworks for evaluating quality
The Donabedian framework of structure (stable elements of the health care system), process (what health care services are provided), and outcome (end results for the patient and family) can be
applied to relevant domains to guide evaluation design (Table 2).2-8 Key structural elements may include characteristics of programs (eg, palliative clinic availability), providers (eg, multidisciplinary members of the palliative care team), and tools (eg, do-not-resuscitate policies). Processes may include technical aspects of care, such as appropriate prescribing and interpersonal aspects of care (eg, coordination among providers). Outcomes may include patient quality of life or symptoms, perceptions of care, or caregiver outcomes such as burden. Outcomes may also be categorized as overuse (eg, use of chemotherapy at the end of life compared to national benchmarks), underuse (eg, lower rates of hospice care or use of antinausea drugs), or appropriateness of care (eg, accurately documenting patients’ preferences for care).
Palliative care quality indicators should be part of oncology performance assessment initiatives. Palliative care programs should also include initiatives to address the overall quality of palliative care issues, such as pain management, in the settings where the programs are located.1 Measuring quality facilitates justifying palliative care initiatives and documenting their impact, targeting quality improvement efforts, monitoring care for deficiencies, and evaluating providers (Table 1). However, measurement in this field is often not straightforward. Potential challenges include defining the population to measure and data sources, collection and analysis, as well as choosing among many potentially relevant issues and quality measures. This article describes an approach to quality measurement in palliative care, beginning with a description of key frameworks to guide the measurement approach. The article also reviews key steps in designing a quality measurement program, which include defining the quality problem and population to measure and choosing domains and specific measures. Finally, the article addresses other key considerations, such as considering unintended consequences and using data for quality improvement.
Frameworks for evaluating quality
The Donabedian framework of structure (stable elements of the health care system), process (what health care services are provided), and outcome (end results for the patient and family) can be
applied to relevant domains to guide evaluation design (Table 2).2-8 Key structural elements may include characteristics of programs (eg, palliative clinic availability), providers (eg, multidisciplinary members of the palliative care team), and tools (eg, do-not-resuscitate policies). Processes may include technical aspects of care, such as appropriate prescribing and interpersonal aspects of care (eg, coordination among providers). Outcomes may include patient quality of life or symptoms, perceptions of care, or caregiver outcomes such as burden. Outcomes may also be categorized as overuse (eg, use of chemotherapy at the end of life compared to national benchmarks), underuse (eg, lower rates of hospice care or use of antinausea drugs), or appropriateness of care (eg, accurately documenting patients’ preferences for care).