User login
From the Department of Neurootology, National Hospital of Neurology and Neurosurgery, London (Dr. Tsang, Miss Anwer) and the Ear Institute, University College London, and Guy’s and St Thomas’ NHS Foundation Trust, London, UK (Dr. Murdin).
Abstract
- Objective: To review the clinical manifestations, diagnosis, and management of vestibular migraine (VM).
- Methods: Review of the literature.
- Results: Apart from headache, other symptoms of VM include unsteadiness, imbalance, and spontaneous as well as visual vertigo. Acute vestibular symptoms that qualify for VM must be of at least moderate or severe intensity which lasts within a time window of 5 minutes to 72 hours. The interindividual temporal association of headache and vertigo is highly variable in VM patients Grossly normal peripheral vestibular function and audiometry both during and between attacks distinguishes VM from its mimics. Treatment options for VM are mainly based on expert opinion and include lifestyle modifications, acute and prophylactic migraine pharmacotherapy, and vestibular rehabilitation therapy.
- Conclusion: Despite a lack of diagnostic biomarkers for VM, a meticulous workup is important to exclude alternative mimics. More longitudinal and treatment studies are required to help elucidate the prognosis and optimal management of this condition.
The coexistence of migraine and vestibular symptoms has been mentioned in the headache literature for many years [1–3]. It was first addressed by Kayan and Hood in 1984, who found that dizziness and vertigo occurred in 54% of migraine patients compared with 30% of patients with tension-type headache [1]. The frequent coexistence of migraine and vertigo led researchers to hypothesize that their co-occurence could be due to more than mere chance. As per Lempert and Neuhauser’s evaluation, there is a lifetime prevalence of 16% for migraine and 7% for vertigo, with a 1.1 % chance of vertigo and migraine occurring together by chance alone [4]. In a study looking at the point prevalence of vertigo or dizziness among those presenting for a routine appointment at a headache center, an astounding 72.8% of those with severe headaches had vestibular symptoms [5].
Most epidemiologic studies of what we call vestibular migraine (VM) were based on presentations to specialist clinics and were performed in an era during which no established diagnostic criteria existed. Despite this, most neurootologists would consider VM to be one of the most common causes of spontaneous recurrent vertigo [6]. Neuhauser et al reported that VM was diagnosed in 7% of a group of 200 specialist clinic patients with dizziness and 9% of a group of 200 clinic patients who had migraine [2]. In a population-based study in Germany, the lifetime prevalence of VM according to the Neuhauser criteria was estimated to be 0.98% and the 12-month prevalence 0.89% [7]. The condition has a 3:1 female predilection [8].
VM has only recently been recognised as a separate migraine entity by the International Headache Society (IHS), appearing in the appendix of their International Classification of Headache Disorders (ICHD)–3 beta. The previous ICHD recognised vertigo as a migrainous symptom only within the framework of basilar migraine. The nomenclature used in the literature to describe this entity has been inconsistent and therefore confusing, including terms such as migraine-associated vertigo [9], migraine-related dizziness [3] or vertigo [10],migrainous vertigo [2], benign recurrent vertigo [11], and migraine-related vestibulopathy [12]. For the most part, these terms refer to the co-experience of migraine and vertigo or dizziness, with only a few terms having a more specific meaning of how the 2 symptoms relate temporally. Neuhauser and colleagues developed criteria in 2001 to classify migraineurs for whom vestibular symptoms are an integral part of migraine symptomatology, using the term migrainous vertigo [2]. Others preferred the terms migraine-associated dizziness or migraine-related dizziness [3] over migrainous vertigo because they felt the symptoms of vestibular dysfunction related to migraine are varied and may include gait instability and spatial disorientation but not necessarily with vertigo. To best avoid confounding nonvestibular dizziness or motion sickness associated with migraine, VM has been the preferred term because it emphasises the particular vestibular manifestation of migraine.
The lack of a universally accepted definition for this complex entity has contributed to delayed diagnosis and and treatment for those with this disorder. In this article, we will review the clinical manifestation, diagnosis and management of VM, with a focus on assisting in the differentiation between other potential diagnoses.
Pathophysiology of VM
A clear pathophysiology of VM has not been elucidated. Although predominantly a sporadic disease, there have been reported cases of familial occurrence with an auto-somal dominant inheritance [11,13]. Bahmad and colleagues mapped the first locus for familial VM to 5q35 within a 4-generation family [13]. On the contrary, a larger study conducted by Lee et al found VM to be to genetically heterogeneous with a subset linking to chromosome 22q12 [14]. Genetic defects of voltage-gated calcium channels are identified as causal factors for familial hemiplegic migraine and episodic ataxia type 2. Both these disease entities present with vertigo and migraine headaches suggesting a defective gene within the same chromosomal region could indicate a direct genetic link to VM. However, no such gene has been identified.
General consensus is that the action of spreading cortical depression as it reaches the somatosensory cortex in the posterior insula and temporoparietal junction elucidates migraine aura in patients with short attacks. However, due to the heterogeneity of VM, canal paresis and complex conditional nystagmus during acute stages are not explained through cortical spreading. Eggers et al suggests that vertigo symptoms occur as ictal sensation rather than the spreading of sensory or motor cortical depression [15]. However, due to discrepancies within the literature it is apparent that further research needs to be conducted to fully understand the pathophysiology of VM.
Clinical Manifestations of VM
Symptoms
As many as 80% to 90% of patients with VM report unsteadiness or balance problems, of which 50% to 60% typically report episodic spontaneous vertigo [16], either internal vertigo (a false sensation of self-motion) or external vertigo (a false sensation that the visual surround is spinning or flowing) [17]. The duration of episodes is highly variable, whereby approximately 30% of patients have episodes lasting minutes, 30% have attacks lasting hours, 30% have attacks over several days, while the remaining 10% have attacks lasting seconds only [18]. It may be difficult to distinguish if vestibular symptoms lasting seconds are related to their head motion intolerance, also known as head motion–induced vertigo [17], which is another frequent symptom in VM. Head motion–induced vertigo bears many similarities to motion sickness.
The interindividual temporal association of headache and vertigo is highly variable in VM patients and is a reason many patients find this diagnostic construct difficult to accept. Approximately 30% of adult patients eventually diagnosed with VM initially present without headaches [8]. Vertigo is only regularly associated with headache in 25% to 50% of VM patients [2,7]. A minority of patients report headache and vertigo never occurring together [2]. A temporal pattern, presenting as aura, occurs only in approximately 10% of cases [19]; therefore, vestibular episodes of VM should not be regarded as migraine auras [18]. Patients typically have migraine manifesting earlier in life with the vestibular symptoms following [13,20], whereby the mean age at onset of migraine and diagnosis of VM are approximately 22 and 35 years, respectively [2]. Consistently across studies that measure quality of life scores, VM patients report higher subjective levels of disability compared to patients with other vestibular illnesses, despite having less objective abnormalities [21]. Approximately 85% of VM patients experienced vestibular symptoms for at least 1 year before consulting neurootology services [21]. It could be argued that hypersensitivity of percept to vestibular symptoms reflect the general finding of augmented perceptions to various external stimuli underlying migraine [22,23].
Another prominent feature of VM is that patients report a syndrome of visually-induced dizziness termed visual vertigo (VV). This is a heterogeneous syndrome with strabismic, peripheral, and/or central vestibular aetiologies [24]. Patients with VV complain of discomfort, postural destabilisation, dizziness, imbalance and spatial disorientation in challenging visual environments. Examples of such environments include walking down supermarket aisles, observing moving objects (eg, disco lights, people walking, moving traffic) or moving surroundings during travelling, and the movement of the eyes in general [24–26]. Most patients report more than one visual trigger [24]. Visual vertigo can often be difficult to distinguish from oscillopsia in patients with bilateral vestibular failure. What is most surprising is that patients with VV have a similar handicap level yet report much more vestibular symptoms compared with patients with bilateral vestibular failure [25]. Postural reactions triggered by external visual motion are destabilising with respect to the earth-vertical and are normally suppressed by central re-weighting of sensory postural cues [24]. Surprisingly, premorbid levels of anxiety and childhood motion sickness do not appear to have a correlation with VV [25]. Even in normal subjects, certain complex visual stimuli can induce transient motion sickness–like symptoms, as shown in experimental visually induced self-vection [27]. The Situational Characteristics Questionnaire (SVQ) is a 19-question, symptom-based questionnaire that has been shown to be useful in quantifying features of VV and may be useful in gauging improvement following physical therapies [25,26].
Early in the disease course, hearing loss should prompt an alternative diagnosis. However, late onset cochlear symptoms have been reported in VM. A study found that after 9 years of follow-up, the number of patients with cochlear symptoms more than doubled [28].
Clinical Examination Findings
The importance of the clinical examination is to rule out peripheral vestibular dysfunction and perform positional testing to look for benign paroxysmal positional vertigo (BPPV) or central positional nystagmus. Nonetheless, positional nystagmus has been reported in up to 28% of cases, including definite central-type positional nystagmus reported in as many as 18% [28].
Audiometric Findings and Auditory Brainstem Responses
Normal audiometry both during and between attacks is one of the key clinical features that distinguishes VM from Meniere’s disease [29]. Auditory brainstem response (ABR) results are typically normal in about 65% of patients [29]. Abnormal ABR results are typically nonspecific, such as mild elongation of wave I, III and V latencies and less commonly, prolongation of the inter-peak latencies.
Findings on Vestibular Function Testing
Whilst there are some reported abnormalities in vestibular function testing in VM patients, such findings need to be interpreted with caution due to the small number of subjects, as well as the variation in case definition and cut-off values. Most importantly, very few papers studied patients in the acute phase, and in some studies it was not even specified. The majority of studies report that VM patients interictally have grossly normal peripheral vestibular function with occasional minor irregularities. Profound interictal abnormalities such as complete canal paresis are usually indicative of other diagnoses. In between acute attacks, patients with VM typically have normal gaze, saccadic parameters, ocular pursuit gains and optokinetic nystagmus (OKN) gains on electronystagmography (ENG) or videonystagmography (VNG) [3]. A minority had a low amplitude (< 4 degrees per second) persistent positional nystagmus. On rotation testing of the vestibo-ocular reflex there is reduction of the mean gains compared to headache-free controls. Most reports in the literature do support that the majority of VM patients have grossly normal bithermal caloric testing, although abnormalities including higher slow phase velocities and canal paresis (usually partial) are reported [29–31]. The observation that the artificial vestibular stimulation caused by the caloric test was followed by a migraine attack within 24 hours in 49% of patients with migraine is very interesting [30], and it remains to be tested whether this phenomenon has the potential to be of assistance in the diagnosis of VM. Both VM patients and migraineurs without vertigo have similar subtle cVEMP (Cervical vestibular-evoked myogenic potentials) abnormalities, namely decreased global amplitude and absence of habituation [31]. On computerized dynamic posturography (CDP), a test of sway, VM patients typically demonstrate a surface-dependent pattern based on their SOT analysis [3], suggesting that VM patients may have a substantial vestibulo-spinal abnormality leading to difficulties integrating multiple conflicting sensory inputs [32].
Diagnostic Criteria
Separating VM into 2 diagnostic entities seems particularly useful: definite VM and the more sensitive but less specific category of probable VM. The sensitivity and specificity of the proposed criteria still need to be determined. Although some authors criticize the probable diagnostic entity for its heterogeneity, about 50% of patients initially diagnosed with probable VM ultimately progress to definite VM [12,33]. Definite vestibular migraine appears in the ICHD-3 beta but only in the appendix section for “new disorders that need further research for validation.” However, probable VM will not be included until further evidence of its utility has been accumulated.
The diagnosis is particularly challenging when headache is not a regular accompaniment of the vertiginous attacks. A patient diary may help link migrainous and vertigo symptoms. When headache is not a prominent feature of the attacks, the clinician will have to put migrainous triggers or symptoms such as photophobia or scintillating scotomas in the context of vertigo symptoms to aid with the diagnosis. One needs to be pedantic about differentiating the qualifying symptom of phonophobia, which is defined as a sound-induced discomfort that is often transient and bilateral from the uncomfortable distorted loud sound perception, which occurs with a recruiting sensorineural hearing loss, and is often persistent and unilateral [18]. Response to migraine treatment is not sufficiently specific to be included in the diagnostic criteria. High placebo response rates from migraine trials [34] suggest that placebo effects can likewise be expected in the treatment of VM. Despite these challenges, acceptance of the diagnostic entity of VM seems to be gaining momentum. In a follow-up study over 9 years, the diagnosis remained consistent in 85% of patients [33].
Benign Paroxysmal Vertigo of Childhood and Vestibular Migraine in Children
VM can present at any age, however, the ICHD specifically recognises an early vertiginous entity regarded as a precursor syndrome of migraine in otherwise healthy children called benign paroxysmal vertigo of childhood. This diagnosis requires 5 episodes of severe vertigo, occurring without warning and resolving spontaneously after minutes to hours [35]. In between episodes, neurological examination, audiometry, vestibular functions and EEG must be normal. A unilateral throbbing headache may occur during attacks but it is not a mandatory criterion. It is unclear whether these two conditions in children are the same entity, however it is important to note that the classification of VM does not involve any age limit [18].
Basilar-type Migraine
The term basilar migraine should be restricted to patients who fulfill the ICHD diagnostic criteria [35] given it is a clinically distinct entity from VM. Less than 10% of VM patients further fulfill the ICHD criteria for basilar migraine [2,18]. More than 60% of basilar-type migraine patients have vertigo and there are many overlapping clinical manifestations with VM. This diagnosis requires at least 2 symptoms from aura in the posterior circulation territory, whereas most patients with VM have vestibular symptoms only [35]. Moreover, in basilar migraine the duration of vertigo should correspond to the length of an aura, that is, between 5 and 60 minutes [35]. Further studies are required to further elucidate and delineate these 2 conditions.
Other Important Diagnostic Considerations
Meniere’s Disease
An important differential diagnosis of VM is the early presentation of Meniere’s disease (MD). Although fluctuating hearing loss, aural fullness and episodic vertigo are important symptoms in the recent updated diagnostic criteria for definite MD [36,37], these symptoms have been reported in patients with migraine [38]. Moreover, minor abnormalities in cVEMPs and arguably in caloric testing can be found in VM patients, as previously mentioned. Predominantly, the distinction can be made considering that a more sustained, albeit occasionally fluctuating, hearing loss would occur in MD, which can progress to severe hearing loss within a few years. However, the diagnosis can be difficult considering that audiometric and vestibular function abnormalities as well as the typical cochlear symptoms are often absent in the early stages of the MD. Nonetheless, preclinical labelling of patients with episodic vertigo without hearing loss as “vestibular MD” is unhelpful as this population may be overrepresented by actual migraineurs. Studies of patients with so-called benign recurrent vertigo or recurrent vestibulopathy are likely to be heterogeneous entities, with perhaps cases later evolving into VM or MD.
Coexisting migraine and MD is often challenging both in terms of diagnosis and management. Many studies have shown an increased prevalence of migraine in MD patients compared to controls [39,40], an asso-ciation suggested by Prosper Ménière himself in 1861 [41]. A study by Radtke et al found that the lifetime prevalence of migraine with and without aura was over 2 times higher in definite MD patients of both sexes compared to age-matched controls (56% versus 25%) [39]. Interestingly, 45% of the patients with MD always experienced at least 1 migrainous symptom (migrainous headache, photophobia, aura symptoms) with their Meniere attacks [39]. This may be at least partly due to the triggering effect of vestibular symptoms on migraineurs [30]. Migraine may even influence the disease course of MD as indicated by a retrospective case control study which found that definite MD patients who have concomitant ICHD criteria for migraine [35] had a significantly earlier onset of MD symptoms (mean age, 37.2 versus 49.3 years) and a much greater susceptibility to simultaneous bilateral, but not sequential, hearing loss as compared to MD patients without migraine (56% versus 4%) [42]. There were no significant differences in the severity of hearing loss between the 2 groups even when controlling for time to evaluation [42]. A family history of episodic vertigo was seen in 39% of MD patients with migraine, which is significantly higher than the 2% seen in MD patients, suggesting a possible genetic basis for this association [42]. The nature of the association between migraine and MD is not well elucidated, however, some authors propose that migraine leads to isolated microvascular ischaemic damage of the inner ear, presumably through small arterial vasospasm [40,42].
In summary, when the criteria for MD are met together with documented audiometric abnormalities, MD should be diagnosed, even if migraine symptoms occur during the vestibular attacks [18]. Only patients who experience 2 different types of attacks, one fulfilling the criteria for VM and the other for MD, should be labelled as Meniere’s disease/migraine overlap syndrome. It is hoped that future revisions of diagnostic criteria will include this overlap entity.
Migraine and Benign Paroxysmal Positional Vertigo
VM patients can experience brief positional dizziness and therefore VM may mimic BPPV. It is therefore important to perform positional testing to look for nystagmus typical for BPPV. Certainly the positional characteristics are distinct from BPPV with regard to the duration of attacks (often as long as the head position is maintained in VM rather than seconds in BPPV). BPPV may also produce attacks of vertigo that can act as triggers for migraine headaches. In these patients, treatment of the BPPV will reduce headache frequency [30].
Transient Ischemic Attacks
Transient ischemic attack (TIA) is a cerebrovascular disease with temporary neurological symptoms [43] and is differentiated from VM mainly from the characteristics of reported symptoms. Being a vascular phenomenon, one would expect TIA symptoms to have a sudden onset, with a brief duration of symptoms (typically short minutes), followed by a rapid improvement to baseline, as well as correspond to a vascular territory. The other important message is that stereotyped, frequently recurrent symptoms are less likely to be TIAs, with the exception of capsular warning syndrome [44] and limb shaking TIAs [43] described elsewhere.
Migraine and Motion Sickness
In an individual patient it may be difficult to differentiate between motion sickness and acute attacks of VM induced by motion stimuli. The distinction may be helped by observing nausea and dizziness improving after cessation of motion which points more towards motion sickness, as oppose to the persistent vertigo after the motion stimulus has ended, thus pointing more towards VM.
Episodic Ataxia Type 2
Of the various episodic ataxias, episodic ataxia type 2 would be the most important subtype in the differential diagnosis of VM given it presents with episodic vertigo and is the most frequently occurring subtype. It is a rare autosomal dominant inherited neurological disorder resulting from mutations of the calcium channel gene CACNA1A [45]. The clinical manifestations include recurrent disabling attacks of imbalance, vertigo and ataxia, which can be provoked by physical exertion or emotional stress. Patients may have downbeat nystagmus interictally. A slow progression of cerebellar signs accompanied by atrophy of midline cerebellar structures and a response to acetazolamide or 4-aminopyridine can help distinguish it from VM.
Migraine, Dizziness, and Comorbid Psychiatric Disorders
Particularly in patients with protracted symptoms, it is difficult to tease out the difference between the symptoms of migraine and dizziness from the symptoms of certain psychiatric disorders given their bidirectional associations. Migraine is a risk factor for first-onset major depression [46] and panic disorder [47]. Patients with VM have very high rates (30%–65%) of coexisting psychiatric illness, especially anxiety and depression, with frequencies higher than that associated with other migraine or vestibular disorders [48,49]. Vestibular migraine patients who have a positive history of psychiatric disorders have a comparatively higher risk of developing somatoform dizziness [48]. The unpredictability of recurrent vestibular symptoms could be a factor leading to elevated distress in VM patients. It is not uncommon to see a premature diagnosis of psychogenic dizziness to be given to patients without objective abnormalities. On the contrary, a diagnosis of psychogenic dizziness can rarely be made with certainty due to multiple reasons. Disabling vertigo leading to physical symptoms and avoidance of social activities can easily be misconstrued to have panic disorder with or without agoraphobia. Moreover, dizziness is the second most common symptom of a panic attack after palpitations [50].
Unfortunately, there are no objective tests that can reliably discriminate vestibular syndromes from psychiatric syndromes in patients with dizziness. The SVQ is not specific enough to differentiate symptoms of VV from the space and motion discomfort symptoms often found in agoraphobic patients [25]. Experimentally, agoraphobia patients may have a more surface-dependent strategy rather than a visual-dependent strategy on CDP [51]. It is unclear whether the vestibular system is causally linked to emotion processing pathways.
Chronic Subjective Dizziness
Chronic subjective dizziness is an entity characterised by chronic unsteadiness or nonvertiginous dizziness accompanied by hypersensitivity to motion stimuli and poor tolerance for complex visual stimuli lasting for 3 months or more without objective abnormalities [52]. These vestibular symptoms are often difficult to distinguish from symptoms of VM. This condition is thought to be a spatial sensory analog of allodynia experienced by some chronic migraine headache sufferers [8].
Dizziness Due to Side Effects of Migraine Prophylactic Medications
Dizziness is often listed as a side effect in the product information of various medications including those used for migraine prophylaxis. It is important to take an accurate history of the suspected offending drug in terms of its temporal relationship to vestibular symptoms. Tricyclic antidepressants (TCAs) can cause drowsiness, lightheadedness, fatigue and blurred vision [53]. Beta-blockers can cause orthostatic hypotension [53]. All the above effects could be confused with vestibular symptoms.
Treatment of Vestibular Migraine
Current treatment options for VM are mainly limited to expert opinion rather than inferred from randomized controlled trials (RCTs) [54]. Below we have offered our consensus on how VM should be managed, with concepts based on the guidelines of treatment for typical migraine [55]. Avoidance of migraine triggers should always be the first avenue of treatment. In addition, any vestibular disorder that is triggering migraine attacks should be identified and treated in its own right. Pharmacotherapy can be abortive for acute episodes and prophylactic.
Lifestyle Advice
The key first task in management is the correct diagnosis and educating the patient about the condition. A thorough explanation of the migraine origin of the attacks can address patients fear and expectations. Nonpharmaceutical approaches in the treatment of VM should not be neglected, even though only a very small proportion of patients may derive a benefit. Advice on dietary manipulation is routinely given; however, its efficacy in VM is questionable. Dietary advice includes healthy eating at regular intervals to prevent skipped meals as well as avoidance of excess caffeine and rich foods. A retrospective study found that lifestyle intervention alone resulted in 13 of 81 patients experiencing significant relief from vestibular symptoms with migraine. The remaining cohort of patients required a multifaceted approach including pharmacotherapy to achieve similar benefit [56].
Acute Abortive Treatments
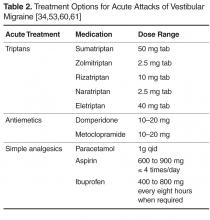
Oral antiemetics are commonly prescribed for motion sickness and acute migraine, however there is no evidence supporting their effectiveness in VM (Table 2). Patients should be counselled about avoiding overuse of antiemetics given their risk of causing extrapyramidal side effects [53].
Simple analgesics, such as paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs), have been found to be helpful in acute VM attacks in observational studies. Bikhazi performed a survey of patients presenting to a headache clinic with vestibular symptoms and found that simple analgesics were valued by patients as effective symptomatic treatment, but were not considered as effective as triptans [59]. Doses of simple analgesics are listed in Table 2. Soluble formulations are preferable due to faster absorption and speed of onset. Opioids should be avoided in acute attacks of VM given the risk of developing opioid overuse headache [55].
Migraine Prophylaxis in Vestibular Migraine
TCAs remain a popular choice of migraine prophylaxis amongst neurootologists because of its additional effects on comorbid affective symptoms. We recommend that the starting dose of either amitriptyline or nortriptyline should be between 5 to 10 mg daily at night, slowly uptitrated to response over several weeks up to a maximum of 100 mg at night. Interval electrocardiography should be performed to monitor for prolongation of the QTc interval. A retrospective chart review found 46% of VM patients (by Neuhauser criteria) reported a reduction in dizziness after nortriptyline administration up to 75 mg daily [62]. However, the current evidence is limited to observational studies [59,62–64].
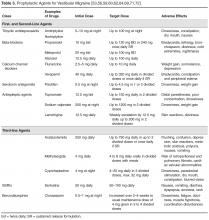
The evidence for beta-blockers is limited in VM but anecdotally has been useful for patients with frequent episodic migraine [59,63,64]. Recommended starting and maintenance doses are listed in Table 3. Furthermore, propranolol can be used in patients with depression [65,66]. Heart rate and electrocardiography should be monitored during dose escalation. Beta-blockers should be avoided in asthmatics. Commonly reported adverse events include cold, extremities reduced exercise tolerance and dizziness [53].
Flunarizine, a calcium channel blocker widely used in migraine [67,68] and vestibular conditions [69], was recently studied in a RCT of 12 weeks' duration for prophylaxis of migrainous vertigo (Neuhauser criteria) in 48 patients [70]. Although flunarizine 10 mg daily did not result in improved headache frequency and severity compared to the control arm, there was a significant improvement in vertigo severity. The most commonly reported side effects of flunarizine are weight gain and somnolence, both of which are minimal or infrequent. Verapamil is another calcium channel blocker that may be helpful but has major limiting adverse effects are bradycardia, constipation and peripheral edema [53].
Pizotifen, a serotonin antagonist, is one of the most well tolerated prophylaxis agents from our experience, however some patients do not adhere to treatment due to drowsiness or weight gain, as evidenced in retrospective case studies [64].
Topiramate with an average daily dose of 100 mg has reported positive results in a prospective observational study of ten patients with VM with auditory symptoms [71]. Nine of 10 patients reported no symptoms after follow-up period of up to sixteen months. The recommended dose is listed in Table 3. Common side effects include distal paresthesias, reduced ability to concentrate and drowsiness [53]. Sodium valproate has been anecdotally effective [59] and is usually well tolerated especially when starting at a low dose of 200 mg at night, slowly titrated to 1200 mg in 2 divided doses. Liver function and full blood evaluation should be monitored on a periodic basis [53].
Third-line medications have only been used anecdotally and should be reserved for extenuating cases (Table 3).
Vestibular Rehabilitation
Vestibular rehabilitation therapy (VRT) has been shown to alleviate significantly ongoing balance and dizziness symptoms in patients with various vestibular disorders [73,74] and improving confidence with balance in elderly patients [75,76]. However, the value of VRT is not as well established in VM. Anecdotally, patients with VM report persistent significant symptoms at the end of a standard VRT period, in contrast to other nonmigrainous patients who appear to be accomplishing their treatment goals faster. However, recent studies [21,73,77] are suggesting that customised VRT may play a useful role in VM, especially since it appears to target issues of anxiety, visual dependence or loss of confidence in balance. Small retrospective case series found that VRT reduced disability scores, and gait and balance function in over 85% of patients with migraine and vestibular symptoms [73,76,77]. An Australian VRT study (21) has recently assessed the efficacy of a 9-week customised VRT in 20 patients with VM compared to 16 patients with vestibular symptoms but without migraine. The customized VRT program consisted of habituation, gaze stability, static tilt, balance and gait exercises. A pictorial exercise instruction sheet for home use would describe these exercises of approximately 15 minutes duration consisting of 4 to 6 exercises to be performed 3 times a day, every day for 9 weeks. Interestingly, both groups benefitted equally from VRT. Compliance with VRT was comparable between the two groups. Commonly reported reasons for non-attendance in VM patients included a recent acute attack of VM, anxiety related to using public transport, and commitment issues related to occupation. This study also suggested that VM patients required more customized and intensive therapy as 15% of VM patients required additional appointments outside the study timeline.
Given that visual dependency has been shown to be reduced with short-term graded optokinetic stimulation exposure in healthy subjects [78], there has been interest using this intervention in conjunction with customized VRT to promote desensitization to visual stimuli as a treatment for VM patients with VV. Most promisingly is the finding that a subgroup of patients with a history of migraine improved significantly more than other vestibular patients with respect to VV symptoms.
There has been controversy surrounding whether patients should avoid medications when undergoing VRT. The protagonists of this view suggest that medications that affect the central nervous system (CNS) may modulate the rate of central compensation. In the aforementioned study by Vitkovic and colleagues [21], the same degree of improvement was seen in the VM group regardless of medication regimen. A study by Whitney and colleagues [73] found that migraine related vestibulopathy patients taking prophylaxis demonstrated better subjective and objective balance scores at baseline and after therapy. Further research is required to clarify the role of CNS-acting medication and their administration around VRT sessions.
Physical therapists dealing with VM patients may face additional challenges in encouraging exercise compliance and providing emotional support. Although more time consuming for the therapist, this is important in the face of high rates of comorbid affective disorders and head motion intolerance. Supervised VRT is believed to implicitly improve psychological status through increasing confidence, providing reassurance, and emphasizing positive effects of VRT, particularly when the patient feels their symptoms have been made worse by it.
Cognitive Behavioral Therapy
Cognitive behavioral therapy (CBT) has been shown to be helpful as part of the holistic treatment of various disorders including post-concussive syndrome and depression in neurology patients [79,80]. Among patients suffering from dizziness, a small study comparing explicit CBT combined with VRT versus waiting-list controls demonstrated improvements in patients’ coping ability, function, symptoms, and care satisfaction [81]. However, to our knowledge there are no studies directly evaluating the benefits of CBT specifically in VM patients. Despite this, it is our practice to request CBT for VM patients who report disabling anxiety or depressive symptoms.
Prognosis
Although migraine in general can improve in later life, this is less certain with VM given the lack of good quality longitudinal studies. Recently Radtke and colleagues published their long-term (median, 9 years) follow-up study of 61 definite VM cases (28). They found that 87% of patients had recurrent vertigo at follow-up. The frequency of vertigo was reduced in 56%, increased in 29%, and unchanged in 16% of patients. The impact of vertigo was graded as severe in 21%, moderate in 43%, and mild in 36% of patients. However, they found that concomitant cochlear symptoms with vertigo had increased from 15% at study inception to 49% at follow-up and secondly, 18% of patients had developed mild bilateral low-frequency sensorineural hearing loss. Therefore, one major criticism of the study is whether some of the patients had MD as their eventual diagnosis rather than definite VM. On the contrary, the authors conclude that these changes represent new vestibulo-cochlear dysfunction as a result of VM disease progression. Due to these reasons, the prognosis of VM patients is unclear. It is our practice to ensure patients do receive delayed follow-up to allow consideration of other neurotological diagnoses.
Conclusion
Given the large heterogeneity in presentation and objective testing, VM as a diagnostic construct has remained quite controversial, though increasingly more accepted. The more we study this common vestibular condition, the more we are realising that the complex relationship between migraine and dizziness extend beyond VM to encompass other vestibular disorders such as MD and anxiety. The lack of a physiological biomarker contributes to its diagnostic difficulties, but a meticulous workup is important to exclude alternative vestibular diagnoses. More longitudinal studies and RCTs are required to help both understand the prognosis and management of VM patients.
Corresponding author: Benjamin K-T Tsang, MBBS, FRACP, The Prince Charles Hospital, Rode Road, Chermside, Queensland 4032, Australia, benjamim.tsang@health.qld.gov.au.
Financial disclosures: None.
1. Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain 1984;107(Pt 4):1123–42.
2. Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56:436–41.
3. Furman JM, Sparto PJ, Soso M, Marcus D. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res 2005;15:327–32.
4. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol 2009;256:333–8.
5. Calhoun AH, Ford S, Pruitt AP, Fisher KG. The point prevalence of dizziness or vertigo in migraine--and factors that influence presentation. Headache 2011;51:1388–92.
6. Bisdorff A. Migraine and dizziness. Curr Opin Neurol 2014;27:105–10.
7. Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology 2006;67:1028–33.
8. Sargent EW. The challenge of vestibular migraine. Curr Opin Otolaryngol Head Neck Surg 2013;21:473–9.
9. Cha YH. Migraine-associated vertigo: diagnosis and treatment. Sem Neurol 2010;30:167–74.
10. Cherian N. Vertigo as a migraine phenomenon. Curr Neurol Neurosci Rep 2013;13:343.
11. Oh AK, Lee H, Jen JC, et al. Familial benign recurrent vertigo. Am J Med Genet 2001;100:287–91.
12. Cass SP, Furman JM, Ankerstjerne K, et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol 1997;106:182–9.
13. Bahmad F Jr, DePalma SR, Merchant SN, et al. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol 2009;118:670–6.
14. Lee H, Jen JC, Wang H, et al. A genome-wide linkage scan of familial benign recurrent vertigo: linkage to 22q12 with evidence of heterogeneity. Hum Molec Genet 2006;15:251–8.
15. Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res 2014;24:387–95.
16. Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms--identifying clinical features that predict “vestibular migraine”. Headache 2011;51:1393–7.
17. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1-13.
18. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167-72.
19. Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999;246:883–92.
20. Eggers SD, Staab JP, Neff BA, et al. Investigation of the coherence of definite and probable vestibular migraine as distinct clinical entities. Otol Neurotol 2011;32:1144–51.
21. Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol 2013;260:3039–48.
22. Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache 2004;44:1019–23.
23. Morrison DP. Abnormal perceptual experiences in migraine. Cephalalgia 1990;10:273–7.
24. Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psych 1995;59:472–6.
25. Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain 2001;124(Pt 8):1646–56.
26. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res 2006;16:223-31.
27. Dobie TG, May JG, Gutierrez C, Heller SS. The transfer of adaptation between actual and simulated rotary stimulation. Aviat Space Environ Med 1990;61:1085–91.
28. Radtke A, von Brevern M, Neuhauser H, et al. Vestibular migraine: long-term follow-up of clinical symptoms and vestibulo-cochlear findings. Neurology 2012;79:1607–14.
29. Bayazit Y, Yilmaz M, Mumbuc S, Kanlikama M. Assessment of migraine-related cochleovestibular symptoms. Revue Laryngol Otol Rhinol 2001;122:85–8.
30. Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology 2009;73:638–42.
31. Roceanu A, Allena M, De Pasqua V, et al. Abnormalities of the vestibulo-collic reflex are similar in migraineurs with and without vertigo. Cephalalgia 2008;28:988–90.
32. Hong HR, Shim DB, Kim TS, et al. Results of caloric and sensory organization testing of dynamic posturography in migrainous vertigo: comparison with Meniere’s disease and vestibular neuritis. Acta Otolaryngol 2013;133:1236–41.
33. Radtke A, Neuhauser H, von Brevern M, et al. Vestibular migraine--validity of clinical diagnostic criteria. Cephalalgia 2011;31:906-13.
34. Rothner AD, Wasiewski W, Winner P, et al. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 2006;46:101-9.
35. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd ed (beta version). Cephalalgia 2013;33:629–808.
36. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere’s disease. J Vestib Res 2015;25:1–7.
37. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation. Otolaryngol Head Neck Surg 1995;113:181–5.
38. Baloh RW. Neurotology of migraine. Headache 1997;37:615-21.
39. Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology 2002;59:1700–4.
40. Lee H, Lopez I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurol 2000;57:1631–4.
41. Ménière P. Pathologie auriculaire: memoires sur une lésion de l’oreille interne donnant lieu à des symptoms de congestion cérébrale apoplectiforme. Gaz Med Paris 1861;16:597–601.
42. Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Meniere’s disease. Acta Otolaryngol 2007;127:1241–5.
43. Kim JS. Symptoms of transient ischemic attack. Front Neurol Neurosci 2014;33:82–102.
44. Paul NL, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology 2012;79:1356–62.
45. Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics 2007;4:267–73.
46. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54:308–13.
47. Breslau N, Schultz LR, Stewart WF, et al. Headache types and panic disorder: directionality and specificity. Neurology 2001;56:350–4.
48. Best C, Eckhardt-Henn A, Tschan R, Dieterich M. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes. Results of a prospective longitudinal study over one year. J Neurol 2009;256:58–65.
49. Eckhardt-Henn A, Best C, Bense S, et al. Psychiatric comorbidity in different organic vertigo syndromes. J Neurol 2008;255:420–8.
50. Segui J, Salvador-Carulla L, Garcia L, et al. Semiology and subtyping of panic disorders. Act Psychiatr Scand 1998;97:272–7.
51. Jacob RG, Furman JM, Durrant JD, Turner SM. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med 1997;59:323–30.
52. Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am 2009;42:71–7, ix.
53. Australian medicines handbook : AMH. Adelaide, S.Aust.: Australian Medicines Handbook; 2015. p. v.
54. Maldonado Fernandez M, Birdi JS, Irving GJ, et al. Pharmacological agents for the prevention of vestibular migraine. Cochrane Database Syst Rev 2015;6:CD010600.
55. British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication overuse headache. 3rd ed. 2010.
56. Reploeg MD, Goebel JA. Migraine-associated dizziness: patient characteristics and management options. Otol Neurotol 2002;23:364–71.
57. Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo-controlled trial. Neurology 2003;60:882–3.
58. Roberto G, Raschi E, Piccinni C, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia 2015;35:118–31.
59. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol 1997;18:350–4.
60. MedicinesComplete. London: Pharmaceutical Press. Available at www.medicinescomplete.com.
61. Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000;60:1259–87.
62. Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol 2012;33:121–7.
63. Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006;116:1782–6.
64. Waterston J. Chronic migrainous vertigo. J Clin Neurosci 2004;11:384–8.
65. de Bock GH, Eelhart J, van Marwijk HW, et al. A postmarketing study of flunarizine in migraine and vertigo. Pharm World Sci 1997;19:269–74.
66. Verspeelt J, De Locht P, Amery WK. Postmarketing study of the use of flunarizine in vestibular vertigo and in migraine. Eur J Clin Pharmacol 1996;51:15–22.
67. Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. J Cardiovasc Pharmacol 1991;18 Suppl 8:S21–6.
68. Lucetti C, Nuti A, Pavese N, et al. Flunarizine in migraine prophylaxis: predictive factors for a positive response. Cephalalgia 1998;18:349–52.
69. Schmidt R, Oestreich W. Flunarizine in the treatment of vestibular vertigo: experimental and clinical data. J Cardiovasc Pharmacol 1991;18 Suppl 8:S27–30.
70. Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol 2014;271:2931–6.
71. Carmona S, Settecase N. Use of topiramate (topamax) in a subgroup of migraine-vertigo patients with auditory symptoms. Ann N Y Acad Sci 2005;1039:517–20.
72. Bisdorff AR. Treatment of migraine related vertigo with lamotrigine an observational study. Bull Soc Sci Med Grand Duche Luxemb 2004:103–8.
73. Whitney SL, Rossi MM. Efficacy of vestibular rehabilitation. Otolaryngol Clin North Am 2000;33:659–72.
74. Enticott JC, Vitkovic JJ, Reid B, et al. Vestibular rehabilitation in individuals with inner-ear dysfunction: a pilot study. Audiol Neurootol 2008;13:19–28.
75. Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol Ser A Biol Sci Med Sci 1998;53:M287–94.
76. Wrisley DM, Whitney SL, Furman JM. Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol 2002;23:483–7.
77. Gottshall KR, Moore RJ, Hoffer ME. Vestibular rehabilitation for migraine-associated dizziness. Int Tinnitus J 2005;11:81–4.
78. Pavlou M, Quinn C, Murray K, et al. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture 2011;33:113–8.
79. Leddy JJ, Sandhu H, Sodhi V, et al. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54.
80. Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res 2015;78:411–9.
81. Andersson G, Asmundson GJ, Denev J, et al. A controlled trial of cognitive-behavior therapy combined with vestibular rehabilitation in the treatment of dizziness. Behav Res Ther 2006;44:1265–73.
From the Department of Neurootology, National Hospital of Neurology and Neurosurgery, London (Dr. Tsang, Miss Anwer) and the Ear Institute, University College London, and Guy’s and St Thomas’ NHS Foundation Trust, London, UK (Dr. Murdin).
Abstract
- Objective: To review the clinical manifestations, diagnosis, and management of vestibular migraine (VM).
- Methods: Review of the literature.
- Results: Apart from headache, other symptoms of VM include unsteadiness, imbalance, and spontaneous as well as visual vertigo. Acute vestibular symptoms that qualify for VM must be of at least moderate or severe intensity which lasts within a time window of 5 minutes to 72 hours. The interindividual temporal association of headache and vertigo is highly variable in VM patients Grossly normal peripheral vestibular function and audiometry both during and between attacks distinguishes VM from its mimics. Treatment options for VM are mainly based on expert opinion and include lifestyle modifications, acute and prophylactic migraine pharmacotherapy, and vestibular rehabilitation therapy.
- Conclusion: Despite a lack of diagnostic biomarkers for VM, a meticulous workup is important to exclude alternative mimics. More longitudinal and treatment studies are required to help elucidate the prognosis and optimal management of this condition.
The coexistence of migraine and vestibular symptoms has been mentioned in the headache literature for many years [1–3]. It was first addressed by Kayan and Hood in 1984, who found that dizziness and vertigo occurred in 54% of migraine patients compared with 30% of patients with tension-type headache [1]. The frequent coexistence of migraine and vertigo led researchers to hypothesize that their co-occurence could be due to more than mere chance. As per Lempert and Neuhauser’s evaluation, there is a lifetime prevalence of 16% for migraine and 7% for vertigo, with a 1.1 % chance of vertigo and migraine occurring together by chance alone [4]. In a study looking at the point prevalence of vertigo or dizziness among those presenting for a routine appointment at a headache center, an astounding 72.8% of those with severe headaches had vestibular symptoms [5].
Most epidemiologic studies of what we call vestibular migraine (VM) were based on presentations to specialist clinics and were performed in an era during which no established diagnostic criteria existed. Despite this, most neurootologists would consider VM to be one of the most common causes of spontaneous recurrent vertigo [6]. Neuhauser et al reported that VM was diagnosed in 7% of a group of 200 specialist clinic patients with dizziness and 9% of a group of 200 clinic patients who had migraine [2]. In a population-based study in Germany, the lifetime prevalence of VM according to the Neuhauser criteria was estimated to be 0.98% and the 12-month prevalence 0.89% [7]. The condition has a 3:1 female predilection [8].
VM has only recently been recognised as a separate migraine entity by the International Headache Society (IHS), appearing in the appendix of their International Classification of Headache Disorders (ICHD)–3 beta. The previous ICHD recognised vertigo as a migrainous symptom only within the framework of basilar migraine. The nomenclature used in the literature to describe this entity has been inconsistent and therefore confusing, including terms such as migraine-associated vertigo [9], migraine-related dizziness [3] or vertigo [10],migrainous vertigo [2], benign recurrent vertigo [11], and migraine-related vestibulopathy [12]. For the most part, these terms refer to the co-experience of migraine and vertigo or dizziness, with only a few terms having a more specific meaning of how the 2 symptoms relate temporally. Neuhauser and colleagues developed criteria in 2001 to classify migraineurs for whom vestibular symptoms are an integral part of migraine symptomatology, using the term migrainous vertigo [2]. Others preferred the terms migraine-associated dizziness or migraine-related dizziness [3] over migrainous vertigo because they felt the symptoms of vestibular dysfunction related to migraine are varied and may include gait instability and spatial disorientation but not necessarily with vertigo. To best avoid confounding nonvestibular dizziness or motion sickness associated with migraine, VM has been the preferred term because it emphasises the particular vestibular manifestation of migraine.
The lack of a universally accepted definition for this complex entity has contributed to delayed diagnosis and and treatment for those with this disorder. In this article, we will review the clinical manifestation, diagnosis and management of VM, with a focus on assisting in the differentiation between other potential diagnoses.
Pathophysiology of VM
A clear pathophysiology of VM has not been elucidated. Although predominantly a sporadic disease, there have been reported cases of familial occurrence with an auto-somal dominant inheritance [11,13]. Bahmad and colleagues mapped the first locus for familial VM to 5q35 within a 4-generation family [13]. On the contrary, a larger study conducted by Lee et al found VM to be to genetically heterogeneous with a subset linking to chromosome 22q12 [14]. Genetic defects of voltage-gated calcium channels are identified as causal factors for familial hemiplegic migraine and episodic ataxia type 2. Both these disease entities present with vertigo and migraine headaches suggesting a defective gene within the same chromosomal region could indicate a direct genetic link to VM. However, no such gene has been identified.
General consensus is that the action of spreading cortical depression as it reaches the somatosensory cortex in the posterior insula and temporoparietal junction elucidates migraine aura in patients with short attacks. However, due to the heterogeneity of VM, canal paresis and complex conditional nystagmus during acute stages are not explained through cortical spreading. Eggers et al suggests that vertigo symptoms occur as ictal sensation rather than the spreading of sensory or motor cortical depression [15]. However, due to discrepancies within the literature it is apparent that further research needs to be conducted to fully understand the pathophysiology of VM.
Clinical Manifestations of VM
Symptoms
As many as 80% to 90% of patients with VM report unsteadiness or balance problems, of which 50% to 60% typically report episodic spontaneous vertigo [16], either internal vertigo (a false sensation of self-motion) or external vertigo (a false sensation that the visual surround is spinning or flowing) [17]. The duration of episodes is highly variable, whereby approximately 30% of patients have episodes lasting minutes, 30% have attacks lasting hours, 30% have attacks over several days, while the remaining 10% have attacks lasting seconds only [18]. It may be difficult to distinguish if vestibular symptoms lasting seconds are related to their head motion intolerance, also known as head motion–induced vertigo [17], which is another frequent symptom in VM. Head motion–induced vertigo bears many similarities to motion sickness.
The interindividual temporal association of headache and vertigo is highly variable in VM patients and is a reason many patients find this diagnostic construct difficult to accept. Approximately 30% of adult patients eventually diagnosed with VM initially present without headaches [8]. Vertigo is only regularly associated with headache in 25% to 50% of VM patients [2,7]. A minority of patients report headache and vertigo never occurring together [2]. A temporal pattern, presenting as aura, occurs only in approximately 10% of cases [19]; therefore, vestibular episodes of VM should not be regarded as migraine auras [18]. Patients typically have migraine manifesting earlier in life with the vestibular symptoms following [13,20], whereby the mean age at onset of migraine and diagnosis of VM are approximately 22 and 35 years, respectively [2]. Consistently across studies that measure quality of life scores, VM patients report higher subjective levels of disability compared to patients with other vestibular illnesses, despite having less objective abnormalities [21]. Approximately 85% of VM patients experienced vestibular symptoms for at least 1 year before consulting neurootology services [21]. It could be argued that hypersensitivity of percept to vestibular symptoms reflect the general finding of augmented perceptions to various external stimuli underlying migraine [22,23].
Another prominent feature of VM is that patients report a syndrome of visually-induced dizziness termed visual vertigo (VV). This is a heterogeneous syndrome with strabismic, peripheral, and/or central vestibular aetiologies [24]. Patients with VV complain of discomfort, postural destabilisation, dizziness, imbalance and spatial disorientation in challenging visual environments. Examples of such environments include walking down supermarket aisles, observing moving objects (eg, disco lights, people walking, moving traffic) or moving surroundings during travelling, and the movement of the eyes in general [24–26]. Most patients report more than one visual trigger [24]. Visual vertigo can often be difficult to distinguish from oscillopsia in patients with bilateral vestibular failure. What is most surprising is that patients with VV have a similar handicap level yet report much more vestibular symptoms compared with patients with bilateral vestibular failure [25]. Postural reactions triggered by external visual motion are destabilising with respect to the earth-vertical and are normally suppressed by central re-weighting of sensory postural cues [24]. Surprisingly, premorbid levels of anxiety and childhood motion sickness do not appear to have a correlation with VV [25]. Even in normal subjects, certain complex visual stimuli can induce transient motion sickness–like symptoms, as shown in experimental visually induced self-vection [27]. The Situational Characteristics Questionnaire (SVQ) is a 19-question, symptom-based questionnaire that has been shown to be useful in quantifying features of VV and may be useful in gauging improvement following physical therapies [25,26].
Early in the disease course, hearing loss should prompt an alternative diagnosis. However, late onset cochlear symptoms have been reported in VM. A study found that after 9 years of follow-up, the number of patients with cochlear symptoms more than doubled [28].
Clinical Examination Findings
The importance of the clinical examination is to rule out peripheral vestibular dysfunction and perform positional testing to look for benign paroxysmal positional vertigo (BPPV) or central positional nystagmus. Nonetheless, positional nystagmus has been reported in up to 28% of cases, including definite central-type positional nystagmus reported in as many as 18% [28].
Audiometric Findings and Auditory Brainstem Responses
Normal audiometry both during and between attacks is one of the key clinical features that distinguishes VM from Meniere’s disease [29]. Auditory brainstem response (ABR) results are typically normal in about 65% of patients [29]. Abnormal ABR results are typically nonspecific, such as mild elongation of wave I, III and V latencies and less commonly, prolongation of the inter-peak latencies.
Findings on Vestibular Function Testing
Whilst there are some reported abnormalities in vestibular function testing in VM patients, such findings need to be interpreted with caution due to the small number of subjects, as well as the variation in case definition and cut-off values. Most importantly, very few papers studied patients in the acute phase, and in some studies it was not even specified. The majority of studies report that VM patients interictally have grossly normal peripheral vestibular function with occasional minor irregularities. Profound interictal abnormalities such as complete canal paresis are usually indicative of other diagnoses. In between acute attacks, patients with VM typically have normal gaze, saccadic parameters, ocular pursuit gains and optokinetic nystagmus (OKN) gains on electronystagmography (ENG) or videonystagmography (VNG) [3]. A minority had a low amplitude (< 4 degrees per second) persistent positional nystagmus. On rotation testing of the vestibo-ocular reflex there is reduction of the mean gains compared to headache-free controls. Most reports in the literature do support that the majority of VM patients have grossly normal bithermal caloric testing, although abnormalities including higher slow phase velocities and canal paresis (usually partial) are reported [29–31]. The observation that the artificial vestibular stimulation caused by the caloric test was followed by a migraine attack within 24 hours in 49% of patients with migraine is very interesting [30], and it remains to be tested whether this phenomenon has the potential to be of assistance in the diagnosis of VM. Both VM patients and migraineurs without vertigo have similar subtle cVEMP (Cervical vestibular-evoked myogenic potentials) abnormalities, namely decreased global amplitude and absence of habituation [31]. On computerized dynamic posturography (CDP), a test of sway, VM patients typically demonstrate a surface-dependent pattern based on their SOT analysis [3], suggesting that VM patients may have a substantial vestibulo-spinal abnormality leading to difficulties integrating multiple conflicting sensory inputs [32].
Diagnostic Criteria
Separating VM into 2 diagnostic entities seems particularly useful: definite VM and the more sensitive but less specific category of probable VM. The sensitivity and specificity of the proposed criteria still need to be determined. Although some authors criticize the probable diagnostic entity for its heterogeneity, about 50% of patients initially diagnosed with probable VM ultimately progress to definite VM [12,33]. Definite vestibular migraine appears in the ICHD-3 beta but only in the appendix section for “new disorders that need further research for validation.” However, probable VM will not be included until further evidence of its utility has been accumulated.
The diagnosis is particularly challenging when headache is not a regular accompaniment of the vertiginous attacks. A patient diary may help link migrainous and vertigo symptoms. When headache is not a prominent feature of the attacks, the clinician will have to put migrainous triggers or symptoms such as photophobia or scintillating scotomas in the context of vertigo symptoms to aid with the diagnosis. One needs to be pedantic about differentiating the qualifying symptom of phonophobia, which is defined as a sound-induced discomfort that is often transient and bilateral from the uncomfortable distorted loud sound perception, which occurs with a recruiting sensorineural hearing loss, and is often persistent and unilateral [18]. Response to migraine treatment is not sufficiently specific to be included in the diagnostic criteria. High placebo response rates from migraine trials [34] suggest that placebo effects can likewise be expected in the treatment of VM. Despite these challenges, acceptance of the diagnostic entity of VM seems to be gaining momentum. In a follow-up study over 9 years, the diagnosis remained consistent in 85% of patients [33].
Benign Paroxysmal Vertigo of Childhood and Vestibular Migraine in Children
VM can present at any age, however, the ICHD specifically recognises an early vertiginous entity regarded as a precursor syndrome of migraine in otherwise healthy children called benign paroxysmal vertigo of childhood. This diagnosis requires 5 episodes of severe vertigo, occurring without warning and resolving spontaneously after minutes to hours [35]. In between episodes, neurological examination, audiometry, vestibular functions and EEG must be normal. A unilateral throbbing headache may occur during attacks but it is not a mandatory criterion. It is unclear whether these two conditions in children are the same entity, however it is important to note that the classification of VM does not involve any age limit [18].
Basilar-type Migraine
The term basilar migraine should be restricted to patients who fulfill the ICHD diagnostic criteria [35] given it is a clinically distinct entity from VM. Less than 10% of VM patients further fulfill the ICHD criteria for basilar migraine [2,18]. More than 60% of basilar-type migraine patients have vertigo and there are many overlapping clinical manifestations with VM. This diagnosis requires at least 2 symptoms from aura in the posterior circulation territory, whereas most patients with VM have vestibular symptoms only [35]. Moreover, in basilar migraine the duration of vertigo should correspond to the length of an aura, that is, between 5 and 60 minutes [35]. Further studies are required to further elucidate and delineate these 2 conditions.
Other Important Diagnostic Considerations
Meniere’s Disease
An important differential diagnosis of VM is the early presentation of Meniere’s disease (MD). Although fluctuating hearing loss, aural fullness and episodic vertigo are important symptoms in the recent updated diagnostic criteria for definite MD [36,37], these symptoms have been reported in patients with migraine [38]. Moreover, minor abnormalities in cVEMPs and arguably in caloric testing can be found in VM patients, as previously mentioned. Predominantly, the distinction can be made considering that a more sustained, albeit occasionally fluctuating, hearing loss would occur in MD, which can progress to severe hearing loss within a few years. However, the diagnosis can be difficult considering that audiometric and vestibular function abnormalities as well as the typical cochlear symptoms are often absent in the early stages of the MD. Nonetheless, preclinical labelling of patients with episodic vertigo without hearing loss as “vestibular MD” is unhelpful as this population may be overrepresented by actual migraineurs. Studies of patients with so-called benign recurrent vertigo or recurrent vestibulopathy are likely to be heterogeneous entities, with perhaps cases later evolving into VM or MD.
Coexisting migraine and MD is often challenging both in terms of diagnosis and management. Many studies have shown an increased prevalence of migraine in MD patients compared to controls [39,40], an asso-ciation suggested by Prosper Ménière himself in 1861 [41]. A study by Radtke et al found that the lifetime prevalence of migraine with and without aura was over 2 times higher in definite MD patients of both sexes compared to age-matched controls (56% versus 25%) [39]. Interestingly, 45% of the patients with MD always experienced at least 1 migrainous symptom (migrainous headache, photophobia, aura symptoms) with their Meniere attacks [39]. This may be at least partly due to the triggering effect of vestibular symptoms on migraineurs [30]. Migraine may even influence the disease course of MD as indicated by a retrospective case control study which found that definite MD patients who have concomitant ICHD criteria for migraine [35] had a significantly earlier onset of MD symptoms (mean age, 37.2 versus 49.3 years) and a much greater susceptibility to simultaneous bilateral, but not sequential, hearing loss as compared to MD patients without migraine (56% versus 4%) [42]. There were no significant differences in the severity of hearing loss between the 2 groups even when controlling for time to evaluation [42]. A family history of episodic vertigo was seen in 39% of MD patients with migraine, which is significantly higher than the 2% seen in MD patients, suggesting a possible genetic basis for this association [42]. The nature of the association between migraine and MD is not well elucidated, however, some authors propose that migraine leads to isolated microvascular ischaemic damage of the inner ear, presumably through small arterial vasospasm [40,42].
In summary, when the criteria for MD are met together with documented audiometric abnormalities, MD should be diagnosed, even if migraine symptoms occur during the vestibular attacks [18]. Only patients who experience 2 different types of attacks, one fulfilling the criteria for VM and the other for MD, should be labelled as Meniere’s disease/migraine overlap syndrome. It is hoped that future revisions of diagnostic criteria will include this overlap entity.
Migraine and Benign Paroxysmal Positional Vertigo
VM patients can experience brief positional dizziness and therefore VM may mimic BPPV. It is therefore important to perform positional testing to look for nystagmus typical for BPPV. Certainly the positional characteristics are distinct from BPPV with regard to the duration of attacks (often as long as the head position is maintained in VM rather than seconds in BPPV). BPPV may also produce attacks of vertigo that can act as triggers for migraine headaches. In these patients, treatment of the BPPV will reduce headache frequency [30].
Transient Ischemic Attacks
Transient ischemic attack (TIA) is a cerebrovascular disease with temporary neurological symptoms [43] and is differentiated from VM mainly from the characteristics of reported symptoms. Being a vascular phenomenon, one would expect TIA symptoms to have a sudden onset, with a brief duration of symptoms (typically short minutes), followed by a rapid improvement to baseline, as well as correspond to a vascular territory. The other important message is that stereotyped, frequently recurrent symptoms are less likely to be TIAs, with the exception of capsular warning syndrome [44] and limb shaking TIAs [43] described elsewhere.
Migraine and Motion Sickness
In an individual patient it may be difficult to differentiate between motion sickness and acute attacks of VM induced by motion stimuli. The distinction may be helped by observing nausea and dizziness improving after cessation of motion which points more towards motion sickness, as oppose to the persistent vertigo after the motion stimulus has ended, thus pointing more towards VM.
Episodic Ataxia Type 2
Of the various episodic ataxias, episodic ataxia type 2 would be the most important subtype in the differential diagnosis of VM given it presents with episodic vertigo and is the most frequently occurring subtype. It is a rare autosomal dominant inherited neurological disorder resulting from mutations of the calcium channel gene CACNA1A [45]. The clinical manifestations include recurrent disabling attacks of imbalance, vertigo and ataxia, which can be provoked by physical exertion or emotional stress. Patients may have downbeat nystagmus interictally. A slow progression of cerebellar signs accompanied by atrophy of midline cerebellar structures and a response to acetazolamide or 4-aminopyridine can help distinguish it from VM.
Migraine, Dizziness, and Comorbid Psychiatric Disorders
Particularly in patients with protracted symptoms, it is difficult to tease out the difference between the symptoms of migraine and dizziness from the symptoms of certain psychiatric disorders given their bidirectional associations. Migraine is a risk factor for first-onset major depression [46] and panic disorder [47]. Patients with VM have very high rates (30%–65%) of coexisting psychiatric illness, especially anxiety and depression, with frequencies higher than that associated with other migraine or vestibular disorders [48,49]. Vestibular migraine patients who have a positive history of psychiatric disorders have a comparatively higher risk of developing somatoform dizziness [48]. The unpredictability of recurrent vestibular symptoms could be a factor leading to elevated distress in VM patients. It is not uncommon to see a premature diagnosis of psychogenic dizziness to be given to patients without objective abnormalities. On the contrary, a diagnosis of psychogenic dizziness can rarely be made with certainty due to multiple reasons. Disabling vertigo leading to physical symptoms and avoidance of social activities can easily be misconstrued to have panic disorder with or without agoraphobia. Moreover, dizziness is the second most common symptom of a panic attack after palpitations [50].
Unfortunately, there are no objective tests that can reliably discriminate vestibular syndromes from psychiatric syndromes in patients with dizziness. The SVQ is not specific enough to differentiate symptoms of VV from the space and motion discomfort symptoms often found in agoraphobic patients [25]. Experimentally, agoraphobia patients may have a more surface-dependent strategy rather than a visual-dependent strategy on CDP [51]. It is unclear whether the vestibular system is causally linked to emotion processing pathways.
Chronic Subjective Dizziness
Chronic subjective dizziness is an entity characterised by chronic unsteadiness or nonvertiginous dizziness accompanied by hypersensitivity to motion stimuli and poor tolerance for complex visual stimuli lasting for 3 months or more without objective abnormalities [52]. These vestibular symptoms are often difficult to distinguish from symptoms of VM. This condition is thought to be a spatial sensory analog of allodynia experienced by some chronic migraine headache sufferers [8].
Dizziness Due to Side Effects of Migraine Prophylactic Medications
Dizziness is often listed as a side effect in the product information of various medications including those used for migraine prophylaxis. It is important to take an accurate history of the suspected offending drug in terms of its temporal relationship to vestibular symptoms. Tricyclic antidepressants (TCAs) can cause drowsiness, lightheadedness, fatigue and blurred vision [53]. Beta-blockers can cause orthostatic hypotension [53]. All the above effects could be confused with vestibular symptoms.
Treatment of Vestibular Migraine
Current treatment options for VM are mainly limited to expert opinion rather than inferred from randomized controlled trials (RCTs) [54]. Below we have offered our consensus on how VM should be managed, with concepts based on the guidelines of treatment for typical migraine [55]. Avoidance of migraine triggers should always be the first avenue of treatment. In addition, any vestibular disorder that is triggering migraine attacks should be identified and treated in its own right. Pharmacotherapy can be abortive for acute episodes and prophylactic.
Lifestyle Advice
The key first task in management is the correct diagnosis and educating the patient about the condition. A thorough explanation of the migraine origin of the attacks can address patients fear and expectations. Nonpharmaceutical approaches in the treatment of VM should not be neglected, even though only a very small proportion of patients may derive a benefit. Advice on dietary manipulation is routinely given; however, its efficacy in VM is questionable. Dietary advice includes healthy eating at regular intervals to prevent skipped meals as well as avoidance of excess caffeine and rich foods. A retrospective study found that lifestyle intervention alone resulted in 13 of 81 patients experiencing significant relief from vestibular symptoms with migraine. The remaining cohort of patients required a multifaceted approach including pharmacotherapy to achieve similar benefit [56].
Acute Abortive Treatments
Oral antiemetics are commonly prescribed for motion sickness and acute migraine, however there is no evidence supporting their effectiveness in VM (Table 2). Patients should be counselled about avoiding overuse of antiemetics given their risk of causing extrapyramidal side effects [53].
Simple analgesics, such as paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs), have been found to be helpful in acute VM attacks in observational studies. Bikhazi performed a survey of patients presenting to a headache clinic with vestibular symptoms and found that simple analgesics were valued by patients as effective symptomatic treatment, but were not considered as effective as triptans [59]. Doses of simple analgesics are listed in Table 2. Soluble formulations are preferable due to faster absorption and speed of onset. Opioids should be avoided in acute attacks of VM given the risk of developing opioid overuse headache [55].
Migraine Prophylaxis in Vestibular Migraine
TCAs remain a popular choice of migraine prophylaxis amongst neurootologists because of its additional effects on comorbid affective symptoms. We recommend that the starting dose of either amitriptyline or nortriptyline should be between 5 to 10 mg daily at night, slowly uptitrated to response over several weeks up to a maximum of 100 mg at night. Interval electrocardiography should be performed to monitor for prolongation of the QTc interval. A retrospective chart review found 46% of VM patients (by Neuhauser criteria) reported a reduction in dizziness after nortriptyline administration up to 75 mg daily [62]. However, the current evidence is limited to observational studies [59,62–64].
The evidence for beta-blockers is limited in VM but anecdotally has been useful for patients with frequent episodic migraine [59,63,64]. Recommended starting and maintenance doses are listed in Table 3. Furthermore, propranolol can be used in patients with depression [65,66]. Heart rate and electrocardiography should be monitored during dose escalation. Beta-blockers should be avoided in asthmatics. Commonly reported adverse events include cold, extremities reduced exercise tolerance and dizziness [53].
Flunarizine, a calcium channel blocker widely used in migraine [67,68] and vestibular conditions [69], was recently studied in a RCT of 12 weeks' duration for prophylaxis of migrainous vertigo (Neuhauser criteria) in 48 patients [70]. Although flunarizine 10 mg daily did not result in improved headache frequency and severity compared to the control arm, there was a significant improvement in vertigo severity. The most commonly reported side effects of flunarizine are weight gain and somnolence, both of which are minimal or infrequent. Verapamil is another calcium channel blocker that may be helpful but has major limiting adverse effects are bradycardia, constipation and peripheral edema [53].
Pizotifen, a serotonin antagonist, is one of the most well tolerated prophylaxis agents from our experience, however some patients do not adhere to treatment due to drowsiness or weight gain, as evidenced in retrospective case studies [64].
Topiramate with an average daily dose of 100 mg has reported positive results in a prospective observational study of ten patients with VM with auditory symptoms [71]. Nine of 10 patients reported no symptoms after follow-up period of up to sixteen months. The recommended dose is listed in Table 3. Common side effects include distal paresthesias, reduced ability to concentrate and drowsiness [53]. Sodium valproate has been anecdotally effective [59] and is usually well tolerated especially when starting at a low dose of 200 mg at night, slowly titrated to 1200 mg in 2 divided doses. Liver function and full blood evaluation should be monitored on a periodic basis [53].
Third-line medications have only been used anecdotally and should be reserved for extenuating cases (Table 3).
Vestibular Rehabilitation
Vestibular rehabilitation therapy (VRT) has been shown to alleviate significantly ongoing balance and dizziness symptoms in patients with various vestibular disorders [73,74] and improving confidence with balance in elderly patients [75,76]. However, the value of VRT is not as well established in VM. Anecdotally, patients with VM report persistent significant symptoms at the end of a standard VRT period, in contrast to other nonmigrainous patients who appear to be accomplishing their treatment goals faster. However, recent studies [21,73,77] are suggesting that customised VRT may play a useful role in VM, especially since it appears to target issues of anxiety, visual dependence or loss of confidence in balance. Small retrospective case series found that VRT reduced disability scores, and gait and balance function in over 85% of patients with migraine and vestibular symptoms [73,76,77]. An Australian VRT study (21) has recently assessed the efficacy of a 9-week customised VRT in 20 patients with VM compared to 16 patients with vestibular symptoms but without migraine. The customized VRT program consisted of habituation, gaze stability, static tilt, balance and gait exercises. A pictorial exercise instruction sheet for home use would describe these exercises of approximately 15 minutes duration consisting of 4 to 6 exercises to be performed 3 times a day, every day for 9 weeks. Interestingly, both groups benefitted equally from VRT. Compliance with VRT was comparable between the two groups. Commonly reported reasons for non-attendance in VM patients included a recent acute attack of VM, anxiety related to using public transport, and commitment issues related to occupation. This study also suggested that VM patients required more customized and intensive therapy as 15% of VM patients required additional appointments outside the study timeline.
Given that visual dependency has been shown to be reduced with short-term graded optokinetic stimulation exposure in healthy subjects [78], there has been interest using this intervention in conjunction with customized VRT to promote desensitization to visual stimuli as a treatment for VM patients with VV. Most promisingly is the finding that a subgroup of patients with a history of migraine improved significantly more than other vestibular patients with respect to VV symptoms.
There has been controversy surrounding whether patients should avoid medications when undergoing VRT. The protagonists of this view suggest that medications that affect the central nervous system (CNS) may modulate the rate of central compensation. In the aforementioned study by Vitkovic and colleagues [21], the same degree of improvement was seen in the VM group regardless of medication regimen. A study by Whitney and colleagues [73] found that migraine related vestibulopathy patients taking prophylaxis demonstrated better subjective and objective balance scores at baseline and after therapy. Further research is required to clarify the role of CNS-acting medication and their administration around VRT sessions.
Physical therapists dealing with VM patients may face additional challenges in encouraging exercise compliance and providing emotional support. Although more time consuming for the therapist, this is important in the face of high rates of comorbid affective disorders and head motion intolerance. Supervised VRT is believed to implicitly improve psychological status through increasing confidence, providing reassurance, and emphasizing positive effects of VRT, particularly when the patient feels their symptoms have been made worse by it.
Cognitive Behavioral Therapy
Cognitive behavioral therapy (CBT) has been shown to be helpful as part of the holistic treatment of various disorders including post-concussive syndrome and depression in neurology patients [79,80]. Among patients suffering from dizziness, a small study comparing explicit CBT combined with VRT versus waiting-list controls demonstrated improvements in patients’ coping ability, function, symptoms, and care satisfaction [81]. However, to our knowledge there are no studies directly evaluating the benefits of CBT specifically in VM patients. Despite this, it is our practice to request CBT for VM patients who report disabling anxiety or depressive symptoms.
Prognosis
Although migraine in general can improve in later life, this is less certain with VM given the lack of good quality longitudinal studies. Recently Radtke and colleagues published their long-term (median, 9 years) follow-up study of 61 definite VM cases (28). They found that 87% of patients had recurrent vertigo at follow-up. The frequency of vertigo was reduced in 56%, increased in 29%, and unchanged in 16% of patients. The impact of vertigo was graded as severe in 21%, moderate in 43%, and mild in 36% of patients. However, they found that concomitant cochlear symptoms with vertigo had increased from 15% at study inception to 49% at follow-up and secondly, 18% of patients had developed mild bilateral low-frequency sensorineural hearing loss. Therefore, one major criticism of the study is whether some of the patients had MD as their eventual diagnosis rather than definite VM. On the contrary, the authors conclude that these changes represent new vestibulo-cochlear dysfunction as a result of VM disease progression. Due to these reasons, the prognosis of VM patients is unclear. It is our practice to ensure patients do receive delayed follow-up to allow consideration of other neurotological diagnoses.
Conclusion
Given the large heterogeneity in presentation and objective testing, VM as a diagnostic construct has remained quite controversial, though increasingly more accepted. The more we study this common vestibular condition, the more we are realising that the complex relationship between migraine and dizziness extend beyond VM to encompass other vestibular disorders such as MD and anxiety. The lack of a physiological biomarker contributes to its diagnostic difficulties, but a meticulous workup is important to exclude alternative vestibular diagnoses. More longitudinal studies and RCTs are required to help both understand the prognosis and management of VM patients.
Corresponding author: Benjamin K-T Tsang, MBBS, FRACP, The Prince Charles Hospital, Rode Road, Chermside, Queensland 4032, Australia, benjamim.tsang@health.qld.gov.au.
Financial disclosures: None.
From the Department of Neurootology, National Hospital of Neurology and Neurosurgery, London (Dr. Tsang, Miss Anwer) and the Ear Institute, University College London, and Guy’s and St Thomas’ NHS Foundation Trust, London, UK (Dr. Murdin).
Abstract
- Objective: To review the clinical manifestations, diagnosis, and management of vestibular migraine (VM).
- Methods: Review of the literature.
- Results: Apart from headache, other symptoms of VM include unsteadiness, imbalance, and spontaneous as well as visual vertigo. Acute vestibular symptoms that qualify for VM must be of at least moderate or severe intensity which lasts within a time window of 5 minutes to 72 hours. The interindividual temporal association of headache and vertigo is highly variable in VM patients Grossly normal peripheral vestibular function and audiometry both during and between attacks distinguishes VM from its mimics. Treatment options for VM are mainly based on expert opinion and include lifestyle modifications, acute and prophylactic migraine pharmacotherapy, and vestibular rehabilitation therapy.
- Conclusion: Despite a lack of diagnostic biomarkers for VM, a meticulous workup is important to exclude alternative mimics. More longitudinal and treatment studies are required to help elucidate the prognosis and optimal management of this condition.
The coexistence of migraine and vestibular symptoms has been mentioned in the headache literature for many years [1–3]. It was first addressed by Kayan and Hood in 1984, who found that dizziness and vertigo occurred in 54% of migraine patients compared with 30% of patients with tension-type headache [1]. The frequent coexistence of migraine and vertigo led researchers to hypothesize that their co-occurence could be due to more than mere chance. As per Lempert and Neuhauser’s evaluation, there is a lifetime prevalence of 16% for migraine and 7% for vertigo, with a 1.1 % chance of vertigo and migraine occurring together by chance alone [4]. In a study looking at the point prevalence of vertigo or dizziness among those presenting for a routine appointment at a headache center, an astounding 72.8% of those with severe headaches had vestibular symptoms [5].
Most epidemiologic studies of what we call vestibular migraine (VM) were based on presentations to specialist clinics and were performed in an era during which no established diagnostic criteria existed. Despite this, most neurootologists would consider VM to be one of the most common causes of spontaneous recurrent vertigo [6]. Neuhauser et al reported that VM was diagnosed in 7% of a group of 200 specialist clinic patients with dizziness and 9% of a group of 200 clinic patients who had migraine [2]. In a population-based study in Germany, the lifetime prevalence of VM according to the Neuhauser criteria was estimated to be 0.98% and the 12-month prevalence 0.89% [7]. The condition has a 3:1 female predilection [8].
VM has only recently been recognised as a separate migraine entity by the International Headache Society (IHS), appearing in the appendix of their International Classification of Headache Disorders (ICHD)–3 beta. The previous ICHD recognised vertigo as a migrainous symptom only within the framework of basilar migraine. The nomenclature used in the literature to describe this entity has been inconsistent and therefore confusing, including terms such as migraine-associated vertigo [9], migraine-related dizziness [3] or vertigo [10],migrainous vertigo [2], benign recurrent vertigo [11], and migraine-related vestibulopathy [12]. For the most part, these terms refer to the co-experience of migraine and vertigo or dizziness, with only a few terms having a more specific meaning of how the 2 symptoms relate temporally. Neuhauser and colleagues developed criteria in 2001 to classify migraineurs for whom vestibular symptoms are an integral part of migraine symptomatology, using the term migrainous vertigo [2]. Others preferred the terms migraine-associated dizziness or migraine-related dizziness [3] over migrainous vertigo because they felt the symptoms of vestibular dysfunction related to migraine are varied and may include gait instability and spatial disorientation but not necessarily with vertigo. To best avoid confounding nonvestibular dizziness or motion sickness associated with migraine, VM has been the preferred term because it emphasises the particular vestibular manifestation of migraine.
The lack of a universally accepted definition for this complex entity has contributed to delayed diagnosis and and treatment for those with this disorder. In this article, we will review the clinical manifestation, diagnosis and management of VM, with a focus on assisting in the differentiation between other potential diagnoses.
Pathophysiology of VM
A clear pathophysiology of VM has not been elucidated. Although predominantly a sporadic disease, there have been reported cases of familial occurrence with an auto-somal dominant inheritance [11,13]. Bahmad and colleagues mapped the first locus for familial VM to 5q35 within a 4-generation family [13]. On the contrary, a larger study conducted by Lee et al found VM to be to genetically heterogeneous with a subset linking to chromosome 22q12 [14]. Genetic defects of voltage-gated calcium channels are identified as causal factors for familial hemiplegic migraine and episodic ataxia type 2. Both these disease entities present with vertigo and migraine headaches suggesting a defective gene within the same chromosomal region could indicate a direct genetic link to VM. However, no such gene has been identified.
General consensus is that the action of spreading cortical depression as it reaches the somatosensory cortex in the posterior insula and temporoparietal junction elucidates migraine aura in patients with short attacks. However, due to the heterogeneity of VM, canal paresis and complex conditional nystagmus during acute stages are not explained through cortical spreading. Eggers et al suggests that vertigo symptoms occur as ictal sensation rather than the spreading of sensory or motor cortical depression [15]. However, due to discrepancies within the literature it is apparent that further research needs to be conducted to fully understand the pathophysiology of VM.
Clinical Manifestations of VM
Symptoms
As many as 80% to 90% of patients with VM report unsteadiness or balance problems, of which 50% to 60% typically report episodic spontaneous vertigo [16], either internal vertigo (a false sensation of self-motion) or external vertigo (a false sensation that the visual surround is spinning or flowing) [17]. The duration of episodes is highly variable, whereby approximately 30% of patients have episodes lasting minutes, 30% have attacks lasting hours, 30% have attacks over several days, while the remaining 10% have attacks lasting seconds only [18]. It may be difficult to distinguish if vestibular symptoms lasting seconds are related to their head motion intolerance, also known as head motion–induced vertigo [17], which is another frequent symptom in VM. Head motion–induced vertigo bears many similarities to motion sickness.
The interindividual temporal association of headache and vertigo is highly variable in VM patients and is a reason many patients find this diagnostic construct difficult to accept. Approximately 30% of adult patients eventually diagnosed with VM initially present without headaches [8]. Vertigo is only regularly associated with headache in 25% to 50% of VM patients [2,7]. A minority of patients report headache and vertigo never occurring together [2]. A temporal pattern, presenting as aura, occurs only in approximately 10% of cases [19]; therefore, vestibular episodes of VM should not be regarded as migraine auras [18]. Patients typically have migraine manifesting earlier in life with the vestibular symptoms following [13,20], whereby the mean age at onset of migraine and diagnosis of VM are approximately 22 and 35 years, respectively [2]. Consistently across studies that measure quality of life scores, VM patients report higher subjective levels of disability compared to patients with other vestibular illnesses, despite having less objective abnormalities [21]. Approximately 85% of VM patients experienced vestibular symptoms for at least 1 year before consulting neurootology services [21]. It could be argued that hypersensitivity of percept to vestibular symptoms reflect the general finding of augmented perceptions to various external stimuli underlying migraine [22,23].
Another prominent feature of VM is that patients report a syndrome of visually-induced dizziness termed visual vertigo (VV). This is a heterogeneous syndrome with strabismic, peripheral, and/or central vestibular aetiologies [24]. Patients with VV complain of discomfort, postural destabilisation, dizziness, imbalance and spatial disorientation in challenging visual environments. Examples of such environments include walking down supermarket aisles, observing moving objects (eg, disco lights, people walking, moving traffic) or moving surroundings during travelling, and the movement of the eyes in general [24–26]. Most patients report more than one visual trigger [24]. Visual vertigo can often be difficult to distinguish from oscillopsia in patients with bilateral vestibular failure. What is most surprising is that patients with VV have a similar handicap level yet report much more vestibular symptoms compared with patients with bilateral vestibular failure [25]. Postural reactions triggered by external visual motion are destabilising with respect to the earth-vertical and are normally suppressed by central re-weighting of sensory postural cues [24]. Surprisingly, premorbid levels of anxiety and childhood motion sickness do not appear to have a correlation with VV [25]. Even in normal subjects, certain complex visual stimuli can induce transient motion sickness–like symptoms, as shown in experimental visually induced self-vection [27]. The Situational Characteristics Questionnaire (SVQ) is a 19-question, symptom-based questionnaire that has been shown to be useful in quantifying features of VV and may be useful in gauging improvement following physical therapies [25,26].
Early in the disease course, hearing loss should prompt an alternative diagnosis. However, late onset cochlear symptoms have been reported in VM. A study found that after 9 years of follow-up, the number of patients with cochlear symptoms more than doubled [28].
Clinical Examination Findings
The importance of the clinical examination is to rule out peripheral vestibular dysfunction and perform positional testing to look for benign paroxysmal positional vertigo (BPPV) or central positional nystagmus. Nonetheless, positional nystagmus has been reported in up to 28% of cases, including definite central-type positional nystagmus reported in as many as 18% [28].
Audiometric Findings and Auditory Brainstem Responses
Normal audiometry both during and between attacks is one of the key clinical features that distinguishes VM from Meniere’s disease [29]. Auditory brainstem response (ABR) results are typically normal in about 65% of patients [29]. Abnormal ABR results are typically nonspecific, such as mild elongation of wave I, III and V latencies and less commonly, prolongation of the inter-peak latencies.
Findings on Vestibular Function Testing
Whilst there are some reported abnormalities in vestibular function testing in VM patients, such findings need to be interpreted with caution due to the small number of subjects, as well as the variation in case definition and cut-off values. Most importantly, very few papers studied patients in the acute phase, and in some studies it was not even specified. The majority of studies report that VM patients interictally have grossly normal peripheral vestibular function with occasional minor irregularities. Profound interictal abnormalities such as complete canal paresis are usually indicative of other diagnoses. In between acute attacks, patients with VM typically have normal gaze, saccadic parameters, ocular pursuit gains and optokinetic nystagmus (OKN) gains on electronystagmography (ENG) or videonystagmography (VNG) [3]. A minority had a low amplitude (< 4 degrees per second) persistent positional nystagmus. On rotation testing of the vestibo-ocular reflex there is reduction of the mean gains compared to headache-free controls. Most reports in the literature do support that the majority of VM patients have grossly normal bithermal caloric testing, although abnormalities including higher slow phase velocities and canal paresis (usually partial) are reported [29–31]. The observation that the artificial vestibular stimulation caused by the caloric test was followed by a migraine attack within 24 hours in 49% of patients with migraine is very interesting [30], and it remains to be tested whether this phenomenon has the potential to be of assistance in the diagnosis of VM. Both VM patients and migraineurs without vertigo have similar subtle cVEMP (Cervical vestibular-evoked myogenic potentials) abnormalities, namely decreased global amplitude and absence of habituation [31]. On computerized dynamic posturography (CDP), a test of sway, VM patients typically demonstrate a surface-dependent pattern based on their SOT analysis [3], suggesting that VM patients may have a substantial vestibulo-spinal abnormality leading to difficulties integrating multiple conflicting sensory inputs [32].
Diagnostic Criteria
Separating VM into 2 diagnostic entities seems particularly useful: definite VM and the more sensitive but less specific category of probable VM. The sensitivity and specificity of the proposed criteria still need to be determined. Although some authors criticize the probable diagnostic entity for its heterogeneity, about 50% of patients initially diagnosed with probable VM ultimately progress to definite VM [12,33]. Definite vestibular migraine appears in the ICHD-3 beta but only in the appendix section for “new disorders that need further research for validation.” However, probable VM will not be included until further evidence of its utility has been accumulated.
The diagnosis is particularly challenging when headache is not a regular accompaniment of the vertiginous attacks. A patient diary may help link migrainous and vertigo symptoms. When headache is not a prominent feature of the attacks, the clinician will have to put migrainous triggers or symptoms such as photophobia or scintillating scotomas in the context of vertigo symptoms to aid with the diagnosis. One needs to be pedantic about differentiating the qualifying symptom of phonophobia, which is defined as a sound-induced discomfort that is often transient and bilateral from the uncomfortable distorted loud sound perception, which occurs with a recruiting sensorineural hearing loss, and is often persistent and unilateral [18]. Response to migraine treatment is not sufficiently specific to be included in the diagnostic criteria. High placebo response rates from migraine trials [34] suggest that placebo effects can likewise be expected in the treatment of VM. Despite these challenges, acceptance of the diagnostic entity of VM seems to be gaining momentum. In a follow-up study over 9 years, the diagnosis remained consistent in 85% of patients [33].
Benign Paroxysmal Vertigo of Childhood and Vestibular Migraine in Children
VM can present at any age, however, the ICHD specifically recognises an early vertiginous entity regarded as a precursor syndrome of migraine in otherwise healthy children called benign paroxysmal vertigo of childhood. This diagnosis requires 5 episodes of severe vertigo, occurring without warning and resolving spontaneously after minutes to hours [35]. In between episodes, neurological examination, audiometry, vestibular functions and EEG must be normal. A unilateral throbbing headache may occur during attacks but it is not a mandatory criterion. It is unclear whether these two conditions in children are the same entity, however it is important to note that the classification of VM does not involve any age limit [18].
Basilar-type Migraine
The term basilar migraine should be restricted to patients who fulfill the ICHD diagnostic criteria [35] given it is a clinically distinct entity from VM. Less than 10% of VM patients further fulfill the ICHD criteria for basilar migraine [2,18]. More than 60% of basilar-type migraine patients have vertigo and there are many overlapping clinical manifestations with VM. This diagnosis requires at least 2 symptoms from aura in the posterior circulation territory, whereas most patients with VM have vestibular symptoms only [35]. Moreover, in basilar migraine the duration of vertigo should correspond to the length of an aura, that is, between 5 and 60 minutes [35]. Further studies are required to further elucidate and delineate these 2 conditions.
Other Important Diagnostic Considerations
Meniere’s Disease
An important differential diagnosis of VM is the early presentation of Meniere’s disease (MD). Although fluctuating hearing loss, aural fullness and episodic vertigo are important symptoms in the recent updated diagnostic criteria for definite MD [36,37], these symptoms have been reported in patients with migraine [38]. Moreover, minor abnormalities in cVEMPs and arguably in caloric testing can be found in VM patients, as previously mentioned. Predominantly, the distinction can be made considering that a more sustained, albeit occasionally fluctuating, hearing loss would occur in MD, which can progress to severe hearing loss within a few years. However, the diagnosis can be difficult considering that audiometric and vestibular function abnormalities as well as the typical cochlear symptoms are often absent in the early stages of the MD. Nonetheless, preclinical labelling of patients with episodic vertigo without hearing loss as “vestibular MD” is unhelpful as this population may be overrepresented by actual migraineurs. Studies of patients with so-called benign recurrent vertigo or recurrent vestibulopathy are likely to be heterogeneous entities, with perhaps cases later evolving into VM or MD.
Coexisting migraine and MD is often challenging both in terms of diagnosis and management. Many studies have shown an increased prevalence of migraine in MD patients compared to controls [39,40], an asso-ciation suggested by Prosper Ménière himself in 1861 [41]. A study by Radtke et al found that the lifetime prevalence of migraine with and without aura was over 2 times higher in definite MD patients of both sexes compared to age-matched controls (56% versus 25%) [39]. Interestingly, 45% of the patients with MD always experienced at least 1 migrainous symptom (migrainous headache, photophobia, aura symptoms) with their Meniere attacks [39]. This may be at least partly due to the triggering effect of vestibular symptoms on migraineurs [30]. Migraine may even influence the disease course of MD as indicated by a retrospective case control study which found that definite MD patients who have concomitant ICHD criteria for migraine [35] had a significantly earlier onset of MD symptoms (mean age, 37.2 versus 49.3 years) and a much greater susceptibility to simultaneous bilateral, but not sequential, hearing loss as compared to MD patients without migraine (56% versus 4%) [42]. There were no significant differences in the severity of hearing loss between the 2 groups even when controlling for time to evaluation [42]. A family history of episodic vertigo was seen in 39% of MD patients with migraine, which is significantly higher than the 2% seen in MD patients, suggesting a possible genetic basis for this association [42]. The nature of the association between migraine and MD is not well elucidated, however, some authors propose that migraine leads to isolated microvascular ischaemic damage of the inner ear, presumably through small arterial vasospasm [40,42].
In summary, when the criteria for MD are met together with documented audiometric abnormalities, MD should be diagnosed, even if migraine symptoms occur during the vestibular attacks [18]. Only patients who experience 2 different types of attacks, one fulfilling the criteria for VM and the other for MD, should be labelled as Meniere’s disease/migraine overlap syndrome. It is hoped that future revisions of diagnostic criteria will include this overlap entity.
Migraine and Benign Paroxysmal Positional Vertigo
VM patients can experience brief positional dizziness and therefore VM may mimic BPPV. It is therefore important to perform positional testing to look for nystagmus typical for BPPV. Certainly the positional characteristics are distinct from BPPV with regard to the duration of attacks (often as long as the head position is maintained in VM rather than seconds in BPPV). BPPV may also produce attacks of vertigo that can act as triggers for migraine headaches. In these patients, treatment of the BPPV will reduce headache frequency [30].
Transient Ischemic Attacks
Transient ischemic attack (TIA) is a cerebrovascular disease with temporary neurological symptoms [43] and is differentiated from VM mainly from the characteristics of reported symptoms. Being a vascular phenomenon, one would expect TIA symptoms to have a sudden onset, with a brief duration of symptoms (typically short minutes), followed by a rapid improvement to baseline, as well as correspond to a vascular territory. The other important message is that stereotyped, frequently recurrent symptoms are less likely to be TIAs, with the exception of capsular warning syndrome [44] and limb shaking TIAs [43] described elsewhere.
Migraine and Motion Sickness
In an individual patient it may be difficult to differentiate between motion sickness and acute attacks of VM induced by motion stimuli. The distinction may be helped by observing nausea and dizziness improving after cessation of motion which points more towards motion sickness, as oppose to the persistent vertigo after the motion stimulus has ended, thus pointing more towards VM.
Episodic Ataxia Type 2
Of the various episodic ataxias, episodic ataxia type 2 would be the most important subtype in the differential diagnosis of VM given it presents with episodic vertigo and is the most frequently occurring subtype. It is a rare autosomal dominant inherited neurological disorder resulting from mutations of the calcium channel gene CACNA1A [45]. The clinical manifestations include recurrent disabling attacks of imbalance, vertigo and ataxia, which can be provoked by physical exertion or emotional stress. Patients may have downbeat nystagmus interictally. A slow progression of cerebellar signs accompanied by atrophy of midline cerebellar structures and a response to acetazolamide or 4-aminopyridine can help distinguish it from VM.
Migraine, Dizziness, and Comorbid Psychiatric Disorders
Particularly in patients with protracted symptoms, it is difficult to tease out the difference between the symptoms of migraine and dizziness from the symptoms of certain psychiatric disorders given their bidirectional associations. Migraine is a risk factor for first-onset major depression [46] and panic disorder [47]. Patients with VM have very high rates (30%–65%) of coexisting psychiatric illness, especially anxiety and depression, with frequencies higher than that associated with other migraine or vestibular disorders [48,49]. Vestibular migraine patients who have a positive history of psychiatric disorders have a comparatively higher risk of developing somatoform dizziness [48]. The unpredictability of recurrent vestibular symptoms could be a factor leading to elevated distress in VM patients. It is not uncommon to see a premature diagnosis of psychogenic dizziness to be given to patients without objective abnormalities. On the contrary, a diagnosis of psychogenic dizziness can rarely be made with certainty due to multiple reasons. Disabling vertigo leading to physical symptoms and avoidance of social activities can easily be misconstrued to have panic disorder with or without agoraphobia. Moreover, dizziness is the second most common symptom of a panic attack after palpitations [50].
Unfortunately, there are no objective tests that can reliably discriminate vestibular syndromes from psychiatric syndromes in patients with dizziness. The SVQ is not specific enough to differentiate symptoms of VV from the space and motion discomfort symptoms often found in agoraphobic patients [25]. Experimentally, agoraphobia patients may have a more surface-dependent strategy rather than a visual-dependent strategy on CDP [51]. It is unclear whether the vestibular system is causally linked to emotion processing pathways.
Chronic Subjective Dizziness
Chronic subjective dizziness is an entity characterised by chronic unsteadiness or nonvertiginous dizziness accompanied by hypersensitivity to motion stimuli and poor tolerance for complex visual stimuli lasting for 3 months or more without objective abnormalities [52]. These vestibular symptoms are often difficult to distinguish from symptoms of VM. This condition is thought to be a spatial sensory analog of allodynia experienced by some chronic migraine headache sufferers [8].
Dizziness Due to Side Effects of Migraine Prophylactic Medications
Dizziness is often listed as a side effect in the product information of various medications including those used for migraine prophylaxis. It is important to take an accurate history of the suspected offending drug in terms of its temporal relationship to vestibular symptoms. Tricyclic antidepressants (TCAs) can cause drowsiness, lightheadedness, fatigue and blurred vision [53]. Beta-blockers can cause orthostatic hypotension [53]. All the above effects could be confused with vestibular symptoms.
Treatment of Vestibular Migraine
Current treatment options for VM are mainly limited to expert opinion rather than inferred from randomized controlled trials (RCTs) [54]. Below we have offered our consensus on how VM should be managed, with concepts based on the guidelines of treatment for typical migraine [55]. Avoidance of migraine triggers should always be the first avenue of treatment. In addition, any vestibular disorder that is triggering migraine attacks should be identified and treated in its own right. Pharmacotherapy can be abortive for acute episodes and prophylactic.
Lifestyle Advice
The key first task in management is the correct diagnosis and educating the patient about the condition. A thorough explanation of the migraine origin of the attacks can address patients fear and expectations. Nonpharmaceutical approaches in the treatment of VM should not be neglected, even though only a very small proportion of patients may derive a benefit. Advice on dietary manipulation is routinely given; however, its efficacy in VM is questionable. Dietary advice includes healthy eating at regular intervals to prevent skipped meals as well as avoidance of excess caffeine and rich foods. A retrospective study found that lifestyle intervention alone resulted in 13 of 81 patients experiencing significant relief from vestibular symptoms with migraine. The remaining cohort of patients required a multifaceted approach including pharmacotherapy to achieve similar benefit [56].
Acute Abortive Treatments
Oral antiemetics are commonly prescribed for motion sickness and acute migraine, however there is no evidence supporting their effectiveness in VM (Table 2). Patients should be counselled about avoiding overuse of antiemetics given their risk of causing extrapyramidal side effects [53].
Simple analgesics, such as paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs), have been found to be helpful in acute VM attacks in observational studies. Bikhazi performed a survey of patients presenting to a headache clinic with vestibular symptoms and found that simple analgesics were valued by patients as effective symptomatic treatment, but were not considered as effective as triptans [59]. Doses of simple analgesics are listed in Table 2. Soluble formulations are preferable due to faster absorption and speed of onset. Opioids should be avoided in acute attacks of VM given the risk of developing opioid overuse headache [55].
Migraine Prophylaxis in Vestibular Migraine
TCAs remain a popular choice of migraine prophylaxis amongst neurootologists because of its additional effects on comorbid affective symptoms. We recommend that the starting dose of either amitriptyline or nortriptyline should be between 5 to 10 mg daily at night, slowly uptitrated to response over several weeks up to a maximum of 100 mg at night. Interval electrocardiography should be performed to monitor for prolongation of the QTc interval. A retrospective chart review found 46% of VM patients (by Neuhauser criteria) reported a reduction in dizziness after nortriptyline administration up to 75 mg daily [62]. However, the current evidence is limited to observational studies [59,62–64].
The evidence for beta-blockers is limited in VM but anecdotally has been useful for patients with frequent episodic migraine [59,63,64]. Recommended starting and maintenance doses are listed in Table 3. Furthermore, propranolol can be used in patients with depression [65,66]. Heart rate and electrocardiography should be monitored during dose escalation. Beta-blockers should be avoided in asthmatics. Commonly reported adverse events include cold, extremities reduced exercise tolerance and dizziness [53].
Flunarizine, a calcium channel blocker widely used in migraine [67,68] and vestibular conditions [69], was recently studied in a RCT of 12 weeks' duration for prophylaxis of migrainous vertigo (Neuhauser criteria) in 48 patients [70]. Although flunarizine 10 mg daily did not result in improved headache frequency and severity compared to the control arm, there was a significant improvement in vertigo severity. The most commonly reported side effects of flunarizine are weight gain and somnolence, both of which are minimal or infrequent. Verapamil is another calcium channel blocker that may be helpful but has major limiting adverse effects are bradycardia, constipation and peripheral edema [53].
Pizotifen, a serotonin antagonist, is one of the most well tolerated prophylaxis agents from our experience, however some patients do not adhere to treatment due to drowsiness or weight gain, as evidenced in retrospective case studies [64].
Topiramate with an average daily dose of 100 mg has reported positive results in a prospective observational study of ten patients with VM with auditory symptoms [71]. Nine of 10 patients reported no symptoms after follow-up period of up to sixteen months. The recommended dose is listed in Table 3. Common side effects include distal paresthesias, reduced ability to concentrate and drowsiness [53]. Sodium valproate has been anecdotally effective [59] and is usually well tolerated especially when starting at a low dose of 200 mg at night, slowly titrated to 1200 mg in 2 divided doses. Liver function and full blood evaluation should be monitored on a periodic basis [53].
Third-line medications have only been used anecdotally and should be reserved for extenuating cases (Table 3).
Vestibular Rehabilitation
Vestibular rehabilitation therapy (VRT) has been shown to alleviate significantly ongoing balance and dizziness symptoms in patients with various vestibular disorders [73,74] and improving confidence with balance in elderly patients [75,76]. However, the value of VRT is not as well established in VM. Anecdotally, patients with VM report persistent significant symptoms at the end of a standard VRT period, in contrast to other nonmigrainous patients who appear to be accomplishing their treatment goals faster. However, recent studies [21,73,77] are suggesting that customised VRT may play a useful role in VM, especially since it appears to target issues of anxiety, visual dependence or loss of confidence in balance. Small retrospective case series found that VRT reduced disability scores, and gait and balance function in over 85% of patients with migraine and vestibular symptoms [73,76,77]. An Australian VRT study (21) has recently assessed the efficacy of a 9-week customised VRT in 20 patients with VM compared to 16 patients with vestibular symptoms but without migraine. The customized VRT program consisted of habituation, gaze stability, static tilt, balance and gait exercises. A pictorial exercise instruction sheet for home use would describe these exercises of approximately 15 minutes duration consisting of 4 to 6 exercises to be performed 3 times a day, every day for 9 weeks. Interestingly, both groups benefitted equally from VRT. Compliance with VRT was comparable between the two groups. Commonly reported reasons for non-attendance in VM patients included a recent acute attack of VM, anxiety related to using public transport, and commitment issues related to occupation. This study also suggested that VM patients required more customized and intensive therapy as 15% of VM patients required additional appointments outside the study timeline.
Given that visual dependency has been shown to be reduced with short-term graded optokinetic stimulation exposure in healthy subjects [78], there has been interest using this intervention in conjunction with customized VRT to promote desensitization to visual stimuli as a treatment for VM patients with VV. Most promisingly is the finding that a subgroup of patients with a history of migraine improved significantly more than other vestibular patients with respect to VV symptoms.
There has been controversy surrounding whether patients should avoid medications when undergoing VRT. The protagonists of this view suggest that medications that affect the central nervous system (CNS) may modulate the rate of central compensation. In the aforementioned study by Vitkovic and colleagues [21], the same degree of improvement was seen in the VM group regardless of medication regimen. A study by Whitney and colleagues [73] found that migraine related vestibulopathy patients taking prophylaxis demonstrated better subjective and objective balance scores at baseline and after therapy. Further research is required to clarify the role of CNS-acting medication and their administration around VRT sessions.
Physical therapists dealing with VM patients may face additional challenges in encouraging exercise compliance and providing emotional support. Although more time consuming for the therapist, this is important in the face of high rates of comorbid affective disorders and head motion intolerance. Supervised VRT is believed to implicitly improve psychological status through increasing confidence, providing reassurance, and emphasizing positive effects of VRT, particularly when the patient feels their symptoms have been made worse by it.
Cognitive Behavioral Therapy
Cognitive behavioral therapy (CBT) has been shown to be helpful as part of the holistic treatment of various disorders including post-concussive syndrome and depression in neurology patients [79,80]. Among patients suffering from dizziness, a small study comparing explicit CBT combined with VRT versus waiting-list controls demonstrated improvements in patients’ coping ability, function, symptoms, and care satisfaction [81]. However, to our knowledge there are no studies directly evaluating the benefits of CBT specifically in VM patients. Despite this, it is our practice to request CBT for VM patients who report disabling anxiety or depressive symptoms.
Prognosis
Although migraine in general can improve in later life, this is less certain with VM given the lack of good quality longitudinal studies. Recently Radtke and colleagues published their long-term (median, 9 years) follow-up study of 61 definite VM cases (28). They found that 87% of patients had recurrent vertigo at follow-up. The frequency of vertigo was reduced in 56%, increased in 29%, and unchanged in 16% of patients. The impact of vertigo was graded as severe in 21%, moderate in 43%, and mild in 36% of patients. However, they found that concomitant cochlear symptoms with vertigo had increased from 15% at study inception to 49% at follow-up and secondly, 18% of patients had developed mild bilateral low-frequency sensorineural hearing loss. Therefore, one major criticism of the study is whether some of the patients had MD as their eventual diagnosis rather than definite VM. On the contrary, the authors conclude that these changes represent new vestibulo-cochlear dysfunction as a result of VM disease progression. Due to these reasons, the prognosis of VM patients is unclear. It is our practice to ensure patients do receive delayed follow-up to allow consideration of other neurotological diagnoses.
Conclusion
Given the large heterogeneity in presentation and objective testing, VM as a diagnostic construct has remained quite controversial, though increasingly more accepted. The more we study this common vestibular condition, the more we are realising that the complex relationship between migraine and dizziness extend beyond VM to encompass other vestibular disorders such as MD and anxiety. The lack of a physiological biomarker contributes to its diagnostic difficulties, but a meticulous workup is important to exclude alternative vestibular diagnoses. More longitudinal studies and RCTs are required to help both understand the prognosis and management of VM patients.
Corresponding author: Benjamin K-T Tsang, MBBS, FRACP, The Prince Charles Hospital, Rode Road, Chermside, Queensland 4032, Australia, benjamim.tsang@health.qld.gov.au.
Financial disclosures: None.
1. Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain 1984;107(Pt 4):1123–42.
2. Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56:436–41.
3. Furman JM, Sparto PJ, Soso M, Marcus D. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res 2005;15:327–32.
4. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol 2009;256:333–8.
5. Calhoun AH, Ford S, Pruitt AP, Fisher KG. The point prevalence of dizziness or vertigo in migraine--and factors that influence presentation. Headache 2011;51:1388–92.
6. Bisdorff A. Migraine and dizziness. Curr Opin Neurol 2014;27:105–10.
7. Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology 2006;67:1028–33.
8. Sargent EW. The challenge of vestibular migraine. Curr Opin Otolaryngol Head Neck Surg 2013;21:473–9.
9. Cha YH. Migraine-associated vertigo: diagnosis and treatment. Sem Neurol 2010;30:167–74.
10. Cherian N. Vertigo as a migraine phenomenon. Curr Neurol Neurosci Rep 2013;13:343.
11. Oh AK, Lee H, Jen JC, et al. Familial benign recurrent vertigo. Am J Med Genet 2001;100:287–91.
12. Cass SP, Furman JM, Ankerstjerne K, et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol 1997;106:182–9.
13. Bahmad F Jr, DePalma SR, Merchant SN, et al. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol 2009;118:670–6.
14. Lee H, Jen JC, Wang H, et al. A genome-wide linkage scan of familial benign recurrent vertigo: linkage to 22q12 with evidence of heterogeneity. Hum Molec Genet 2006;15:251–8.
15. Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res 2014;24:387–95.
16. Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms--identifying clinical features that predict “vestibular migraine”. Headache 2011;51:1393–7.
17. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1-13.
18. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167-72.
19. Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999;246:883–92.
20. Eggers SD, Staab JP, Neff BA, et al. Investigation of the coherence of definite and probable vestibular migraine as distinct clinical entities. Otol Neurotol 2011;32:1144–51.
21. Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol 2013;260:3039–48.
22. Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache 2004;44:1019–23.
23. Morrison DP. Abnormal perceptual experiences in migraine. Cephalalgia 1990;10:273–7.
24. Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psych 1995;59:472–6.
25. Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain 2001;124(Pt 8):1646–56.
26. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res 2006;16:223-31.
27. Dobie TG, May JG, Gutierrez C, Heller SS. The transfer of adaptation between actual and simulated rotary stimulation. Aviat Space Environ Med 1990;61:1085–91.
28. Radtke A, von Brevern M, Neuhauser H, et al. Vestibular migraine: long-term follow-up of clinical symptoms and vestibulo-cochlear findings. Neurology 2012;79:1607–14.
29. Bayazit Y, Yilmaz M, Mumbuc S, Kanlikama M. Assessment of migraine-related cochleovestibular symptoms. Revue Laryngol Otol Rhinol 2001;122:85–8.
30. Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology 2009;73:638–42.
31. Roceanu A, Allena M, De Pasqua V, et al. Abnormalities of the vestibulo-collic reflex are similar in migraineurs with and without vertigo. Cephalalgia 2008;28:988–90.
32. Hong HR, Shim DB, Kim TS, et al. Results of caloric and sensory organization testing of dynamic posturography in migrainous vertigo: comparison with Meniere’s disease and vestibular neuritis. Acta Otolaryngol 2013;133:1236–41.
33. Radtke A, Neuhauser H, von Brevern M, et al. Vestibular migraine--validity of clinical diagnostic criteria. Cephalalgia 2011;31:906-13.
34. Rothner AD, Wasiewski W, Winner P, et al. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 2006;46:101-9.
35. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd ed (beta version). Cephalalgia 2013;33:629–808.
36. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere’s disease. J Vestib Res 2015;25:1–7.
37. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation. Otolaryngol Head Neck Surg 1995;113:181–5.
38. Baloh RW. Neurotology of migraine. Headache 1997;37:615-21.
39. Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology 2002;59:1700–4.
40. Lee H, Lopez I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurol 2000;57:1631–4.
41. Ménière P. Pathologie auriculaire: memoires sur une lésion de l’oreille interne donnant lieu à des symptoms de congestion cérébrale apoplectiforme. Gaz Med Paris 1861;16:597–601.
42. Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Meniere’s disease. Acta Otolaryngol 2007;127:1241–5.
43. Kim JS. Symptoms of transient ischemic attack. Front Neurol Neurosci 2014;33:82–102.
44. Paul NL, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology 2012;79:1356–62.
45. Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics 2007;4:267–73.
46. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54:308–13.
47. Breslau N, Schultz LR, Stewart WF, et al. Headache types and panic disorder: directionality and specificity. Neurology 2001;56:350–4.
48. Best C, Eckhardt-Henn A, Tschan R, Dieterich M. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes. Results of a prospective longitudinal study over one year. J Neurol 2009;256:58–65.
49. Eckhardt-Henn A, Best C, Bense S, et al. Psychiatric comorbidity in different organic vertigo syndromes. J Neurol 2008;255:420–8.
50. Segui J, Salvador-Carulla L, Garcia L, et al. Semiology and subtyping of panic disorders. Act Psychiatr Scand 1998;97:272–7.
51. Jacob RG, Furman JM, Durrant JD, Turner SM. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med 1997;59:323–30.
52. Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am 2009;42:71–7, ix.
53. Australian medicines handbook : AMH. Adelaide, S.Aust.: Australian Medicines Handbook; 2015. p. v.
54. Maldonado Fernandez M, Birdi JS, Irving GJ, et al. Pharmacological agents for the prevention of vestibular migraine. Cochrane Database Syst Rev 2015;6:CD010600.
55. British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication overuse headache. 3rd ed. 2010.
56. Reploeg MD, Goebel JA. Migraine-associated dizziness: patient characteristics and management options. Otol Neurotol 2002;23:364–71.
57. Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo-controlled trial. Neurology 2003;60:882–3.
58. Roberto G, Raschi E, Piccinni C, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia 2015;35:118–31.
59. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol 1997;18:350–4.
60. MedicinesComplete. London: Pharmaceutical Press. Available at www.medicinescomplete.com.
61. Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000;60:1259–87.
62. Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol 2012;33:121–7.
63. Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006;116:1782–6.
64. Waterston J. Chronic migrainous vertigo. J Clin Neurosci 2004;11:384–8.
65. de Bock GH, Eelhart J, van Marwijk HW, et al. A postmarketing study of flunarizine in migraine and vertigo. Pharm World Sci 1997;19:269–74.
66. Verspeelt J, De Locht P, Amery WK. Postmarketing study of the use of flunarizine in vestibular vertigo and in migraine. Eur J Clin Pharmacol 1996;51:15–22.
67. Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. J Cardiovasc Pharmacol 1991;18 Suppl 8:S21–6.
68. Lucetti C, Nuti A, Pavese N, et al. Flunarizine in migraine prophylaxis: predictive factors for a positive response. Cephalalgia 1998;18:349–52.
69. Schmidt R, Oestreich W. Flunarizine in the treatment of vestibular vertigo: experimental and clinical data. J Cardiovasc Pharmacol 1991;18 Suppl 8:S27–30.
70. Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol 2014;271:2931–6.
71. Carmona S, Settecase N. Use of topiramate (topamax) in a subgroup of migraine-vertigo patients with auditory symptoms. Ann N Y Acad Sci 2005;1039:517–20.
72. Bisdorff AR. Treatment of migraine related vertigo with lamotrigine an observational study. Bull Soc Sci Med Grand Duche Luxemb 2004:103–8.
73. Whitney SL, Rossi MM. Efficacy of vestibular rehabilitation. Otolaryngol Clin North Am 2000;33:659–72.
74. Enticott JC, Vitkovic JJ, Reid B, et al. Vestibular rehabilitation in individuals with inner-ear dysfunction: a pilot study. Audiol Neurootol 2008;13:19–28.
75. Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol Ser A Biol Sci Med Sci 1998;53:M287–94.
76. Wrisley DM, Whitney SL, Furman JM. Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol 2002;23:483–7.
77. Gottshall KR, Moore RJ, Hoffer ME. Vestibular rehabilitation for migraine-associated dizziness. Int Tinnitus J 2005;11:81–4.
78. Pavlou M, Quinn C, Murray K, et al. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture 2011;33:113–8.
79. Leddy JJ, Sandhu H, Sodhi V, et al. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54.
80. Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res 2015;78:411–9.
81. Andersson G, Asmundson GJ, Denev J, et al. A controlled trial of cognitive-behavior therapy combined with vestibular rehabilitation in the treatment of dizziness. Behav Res Ther 2006;44:1265–73.
1. Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain 1984;107(Pt 4):1123–42.
2. Neuhauser H, Leopold M, von Brevern M, et al. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001;56:436–41.
3. Furman JM, Sparto PJ, Soso M, Marcus D. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res 2005;15:327–32.
4. Lempert T, Neuhauser H. Epidemiology of vertigo, migraine and vestibular migraine. J Neurol 2009;256:333–8.
5. Calhoun AH, Ford S, Pruitt AP, Fisher KG. The point prevalence of dizziness or vertigo in migraine--and factors that influence presentation. Headache 2011;51:1388–92.
6. Bisdorff A. Migraine and dizziness. Curr Opin Neurol 2014;27:105–10.
7. Neuhauser HK, Radtke A, von Brevern M, et al. Migrainous vertigo: prevalence and impact on quality of life. Neurology 2006;67:1028–33.
8. Sargent EW. The challenge of vestibular migraine. Curr Opin Otolaryngol Head Neck Surg 2013;21:473–9.
9. Cha YH. Migraine-associated vertigo: diagnosis and treatment. Sem Neurol 2010;30:167–74.
10. Cherian N. Vertigo as a migraine phenomenon. Curr Neurol Neurosci Rep 2013;13:343.
11. Oh AK, Lee H, Jen JC, et al. Familial benign recurrent vertigo. Am J Med Genet 2001;100:287–91.
12. Cass SP, Furman JM, Ankerstjerne K, et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol 1997;106:182–9.
13. Bahmad F Jr, DePalma SR, Merchant SN, et al. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol 2009;118:670–6.
14. Lee H, Jen JC, Wang H, et al. A genome-wide linkage scan of familial benign recurrent vertigo: linkage to 22q12 with evidence of heterogeneity. Hum Molec Genet 2006;15:251–8.
15. Eggers SD, Neff BA, Shepard NT, Staab JP. Comorbidities in vestibular migraine. J Vestib Res 2014;24:387–95.
16. Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms--identifying clinical features that predict “vestibular migraine”. Headache 2011;51:1393–7.
17. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1-13.
18. Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res 2012;22:167-72.
19. Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol 1999;246:883–92.
20. Eggers SD, Staab JP, Neff BA, et al. Investigation of the coherence of definite and probable vestibular migraine as distinct clinical entities. Otol Neurotol 2011;32:1144–51.
21. Vitkovic J, Winoto A, Rance G, et al. Vestibular rehabilitation outcomes in patients with and without vestibular migraine. J Neurol 2013;260:3039–48.
22. Kelman L. Osmophobia and taste abnormality in migraineurs: a tertiary care study. Headache 2004;44:1019–23.
23. Morrison DP. Abnormal perceptual experiences in migraine. Cephalalgia 1990;10:273–7.
24. Bronstein AM. Visual vertigo syndrome: clinical and posturography findings. J Neurol Neurosurg Psych 1995;59:472–6.
25. Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain 2001;124(Pt 8):1646–56.
26. Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res 2006;16:223-31.
27. Dobie TG, May JG, Gutierrez C, Heller SS. The transfer of adaptation between actual and simulated rotary stimulation. Aviat Space Environ Med 1990;61:1085–91.
28. Radtke A, von Brevern M, Neuhauser H, et al. Vestibular migraine: long-term follow-up of clinical symptoms and vestibulo-cochlear findings. Neurology 2012;79:1607–14.
29. Bayazit Y, Yilmaz M, Mumbuc S, Kanlikama M. Assessment of migraine-related cochleovestibular symptoms. Revue Laryngol Otol Rhinol 2001;122:85–8.
30. Murdin L, Davies RA, Bronstein AM. Vertigo as a migraine trigger. Neurology 2009;73:638–42.
31. Roceanu A, Allena M, De Pasqua V, et al. Abnormalities of the vestibulo-collic reflex are similar in migraineurs with and without vertigo. Cephalalgia 2008;28:988–90.
32. Hong HR, Shim DB, Kim TS, et al. Results of caloric and sensory organization testing of dynamic posturography in migrainous vertigo: comparison with Meniere’s disease and vestibular neuritis. Acta Otolaryngol 2013;133:1236–41.
33. Radtke A, Neuhauser H, von Brevern M, et al. Vestibular migraine--validity of clinical diagnostic criteria. Cephalalgia 2011;31:906-13.
34. Rothner AD, Wasiewski W, Winner P, et al. Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 2006;46:101-9.
35. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd ed (beta version). Cephalalgia 2013;33:629–808.
36. Lopez-Escamez JA, Carey J, Chung WH, et al. Diagnostic criteria for Meniere’s disease. J Vestib Res 2015;25:1–7.
37. Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Meniere’s disease. American Academy of Otolaryngology-Head and Neck Foundation. Otolaryngol Head Neck Surg 1995;113:181–5.
38. Baloh RW. Neurotology of migraine. Headache 1997;37:615-21.
39. Radtke A, Lempert T, Gresty MA, et al. Migraine and Meniere’s disease: is there a link? Neurology 2002;59:1700–4.
40. Lee H, Lopez I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurol 2000;57:1631–4.
41. Ménière P. Pathologie auriculaire: memoires sur une lésion de l’oreille interne donnant lieu à des symptoms de congestion cérébrale apoplectiforme. Gaz Med Paris 1861;16:597–601.
42. Cha YH, Brodsky J, Ishiyama G, et al. The relevance of migraine in patients with Meniere’s disease. Acta Otolaryngol 2007;127:1241–5.
43. Kim JS. Symptoms of transient ischemic attack. Front Neurol Neurosci 2014;33:82–102.
44. Paul NL, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology 2012;79:1356–62.
45. Strupp M, Zwergal A, Brandt T. Episodic ataxia type 2. Neurotherapeutics 2007;4:267–73.
46. Breslau N, Schultz LR, Stewart WF, et al. Headache and major depression: is the association specific to migraine? Neurology 2000;54:308–13.
47. Breslau N, Schultz LR, Stewart WF, et al. Headache types and panic disorder: directionality and specificity. Neurology 2001;56:350–4.
48. Best C, Eckhardt-Henn A, Tschan R, Dieterich M. Psychiatric morbidity and comorbidity in different vestibular vertigo syndromes. Results of a prospective longitudinal study over one year. J Neurol 2009;256:58–65.
49. Eckhardt-Henn A, Best C, Bense S, et al. Psychiatric comorbidity in different organic vertigo syndromes. J Neurol 2008;255:420–8.
50. Segui J, Salvador-Carulla L, Garcia L, et al. Semiology and subtyping of panic disorders. Act Psychiatr Scand 1998;97:272–7.
51. Jacob RG, Furman JM, Durrant JD, Turner SM. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med 1997;59:323–30.
52. Ruckenstein MJ, Staab JP. Chronic subjective dizziness. Otolaryngol Clin North Am 2009;42:71–7, ix.
53. Australian medicines handbook : AMH. Adelaide, S.Aust.: Australian Medicines Handbook; 2015. p. v.
54. Maldonado Fernandez M, Birdi JS, Irving GJ, et al. Pharmacological agents for the prevention of vestibular migraine. Cochrane Database Syst Rev 2015;6:CD010600.
55. British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension-type headache, cluster headache and medication overuse headache. 3rd ed. 2010.
56. Reploeg MD, Goebel JA. Migraine-associated dizziness: patient characteristics and management options. Otol Neurotol 2002;23:364–71.
57. Neuhauser H, Radtke A, von Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo-controlled trial. Neurology 2003;60:882–3.
58. Roberto G, Raschi E, Piccinni C, et al. Adverse cardiovascular events associated with triptans and ergotamines for treatment of migraine: systematic review of observational studies. Cephalalgia 2015;35:118–31.
59. Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol 1997;18:350–4.
60. MedicinesComplete. London: Pharmaceutical Press. Available at www.medicinescomplete.com.
61. Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs 2000;60:1259–87.
62. Mikulec AA, Faraji F, Kinsella LJ. Evaluation of the efficacy of caffeine cessation, nortriptyline, and topiramate therapy in vestibular migraine and complex dizziness of unknown etiology. Am J Otolaryngol 2012;33:121–7.
63. Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope 2006;116:1782–6.
64. Waterston J. Chronic migrainous vertigo. J Clin Neurosci 2004;11:384–8.
65. de Bock GH, Eelhart J, van Marwijk HW, et al. A postmarketing study of flunarizine in migraine and vertigo. Pharm World Sci 1997;19:269–74.
66. Verspeelt J, De Locht P, Amery WK. Postmarketing study of the use of flunarizine in vestibular vertigo and in migraine. Eur J Clin Pharmacol 1996;51:15–22.
67. Schmidt R, Oestreich W. Flunarizine in migraine prophylaxis: the clinical experience. J Cardiovasc Pharmacol 1991;18 Suppl 8:S21–6.
68. Lucetti C, Nuti A, Pavese N, et al. Flunarizine in migraine prophylaxis: predictive factors for a positive response. Cephalalgia 1998;18:349–52.
69. Schmidt R, Oestreich W. Flunarizine in the treatment of vestibular vertigo: experimental and clinical data. J Cardiovasc Pharmacol 1991;18 Suppl 8:S27–30.
70. Lepcha A, Amalanathan S, Augustine AM, et al. Flunarizine in the prophylaxis of migrainous vertigo: a randomized controlled trial. Eur Arch Otorhinolaryngol 2014;271:2931–6.
71. Carmona S, Settecase N. Use of topiramate (topamax) in a subgroup of migraine-vertigo patients with auditory symptoms. Ann N Y Acad Sci 2005;1039:517–20.
72. Bisdorff AR. Treatment of migraine related vertigo with lamotrigine an observational study. Bull Soc Sci Med Grand Duche Luxemb 2004:103–8.
73. Whitney SL, Rossi MM. Efficacy of vestibular rehabilitation. Otolaryngol Clin North Am 2000;33:659–72.
74. Enticott JC, Vitkovic JJ, Reid B, et al. Vestibular rehabilitation in individuals with inner-ear dysfunction: a pilot study. Audiol Neurootol 2008;13:19–28.
75. Myers AM, Fletcher PC, Myers AH, Sherk W. Discriminative and evaluative properties of the activities-specific balance confidence (ABC) scale. J Gerontol Ser A Biol Sci Med Sci 1998;53:M287–94.
76. Wrisley DM, Whitney SL, Furman JM. Vestibular rehabilitation outcomes in patients with a history of migraine. Otol Neurotol 2002;23:483–7.
77. Gottshall KR, Moore RJ, Hoffer ME. Vestibular rehabilitation for migraine-associated dizziness. Int Tinnitus J 2005;11:81–4.
78. Pavlou M, Quinn C, Murray K, et al. The effect of repeated visual motion stimuli on visual dependence and postural control in normal subjects. Gait Posture 2011;33:113–8.
79. Leddy JJ, Sandhu H, Sodhi V, et al. Rehabilitation of concussion and post-concussion syndrome. Sports Health 2012;4:147–54.
80. Fernie BA, Kollmann J, Brown RG. Cognitive behavioural interventions for depression in chronic neurological conditions: a systematic review. J Psychosom Res 2015;78:411–9.
81. Andersson G, Asmundson GJ, Denev J, et al. A controlled trial of cognitive-behavior therapy combined with vestibular rehabilitation in the treatment of dizziness. Behav Res Ther 2006;44:1265–73.