User login
Clear Coverage Preference for Humira Over Biosimilars Seen in Most Medicare Part D Plans
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.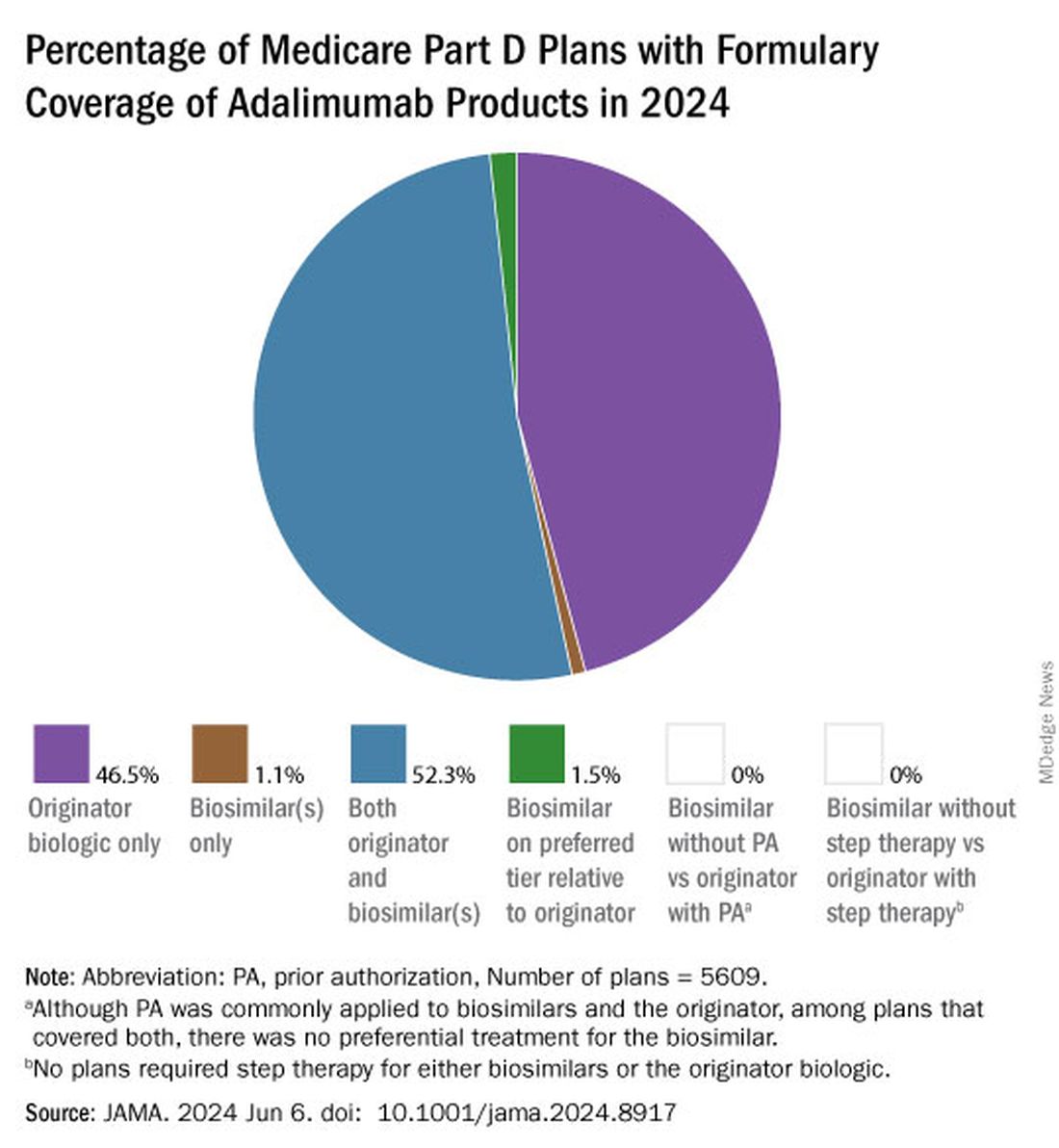
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
FROM JAMA
Spondyloarthritis Screening Study Finds ‘High Burden of Need’ in Patients With Inflammatory Bowel Disease
More than 40% of patients with inflammatory bowel disease (IBD) screened positive for joint pain symptomatic of spondyloarthritis (SpA), according to a new study.
Of these patients, 75% did not have any history of arthritis.
“What we know is that a substantial proportion of patients with IBD do report musculoskeletal symptoms, and inflammatory back pain stands out as being one of the more frequent symptoms reported,” said Reem Jan, MBBS, a rheumatologist at the University of Chicago Medicine. She presented the study findings during the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN) in Cleveland.
“Yet a minority of these patients are evaluated by rheumatologists. So that suggests there’s a high burden of need in the IBD population to have this joint pain evaluated and addressed,” she said during her presentation.
She presented preliminary data from an ongoing project to better understand the prevalence of inflammatory arthritis in IBD — estimates range from 17% to 39%— and the risk factors for developing arthritis in this patient population.
Study Details
Researchers enrolled patients from outpatient gastroenterology clinics or procedure units at NYU Langone Health, New York City; Brigham and Women’s Hospital, Boston; University of Colorado Anschutz Medical Campus, Aurora, Colorado; Mayo Clinic, Rochester, Minnesota; University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago; and Icahn School of Medicine at Mount Sinai, New York City. Additional patients were recruited from Mercy Health, a community health system in Ohio.
Upon entry into the study, participants completed a survey documenting their history with joint pain. The survey combined questions from the DETAIL and the IBIS questionnaires.
Between January 2021 and December 2022, 669 patients joined the study. In total, 41% of patients (n = 275) screened positive.
“What really stood out to us was that of all the positive screens, only about a quarter of those patients were known to have SpA,” Dr. Jan said during her presentation. “[This] means 75% of the patients who screened positive were not known to have any type of arthritic disease.”
In addition, only 24% (n = 65) of all patients who screened positive — including those with a SpA diagnosis — had seen a rheumatologist in the previous year.
Among these patients, inflammatory back pain was the most commonly reported symptom, followed by painful swelling of peripheral joints and heel pain.
Excluding patients with a SpA diagnosis, researchers also investigated which characteristics were associated with a higher likelihood of screening positive in the questionnaire. The analysis, including 588 patients, identified the following risk factors:
- Female sex: Odds ratio (OR), 2.0; 95% CI, 1.4-2.9
- Older age: OR, 1.02; 95% CI, 1.01-1.4
- History of smoking: OR, 1.7; 95% CI, 1.1-2.6
- History of prior IBD-related surgery: OR, 1.60; 95% CI, 1.1-2.5
- History of biologic or small molecule therapy: OR, 2.3; 95% CI, 1.4-4.0
Future Directions
Commenting on the study, Mark Hwang, MD, a rheumatologist at UTHealth Houston, noted that it was “very interesting to see the fairly large, positive rates” of joint pain in patients with IBD, which certainly have clinical implications. However, it is not yet known if any of these patients went on to be diagnosed with SpA.
Jan noted that potential next steps include a follow-up analysis of patients who screened positive to see how many went on to see a rheumatologist and which patients were ultimately diagnosed with SpA or other inflammatory arthritis conditions.
These findings are a first step, Dr. Hwang said, and will likely “help further establish some of the validity of these questionnaires by testing in different patient populations,” he noted.
The ultimate goal is to “develop really good strategies to risk stratify IBD patients with the greatest need of rheumatologist consultation,” Dr. Jan said. “We certainly don’t want to see all these patients, so how can we figure out who really needs to be seen?”
Funding information was not available for this study. Dr. Hwang is conducting two clinical trials for psoriatic arthritis sponsored by Janssen and Eli Lilly. Dr. Jan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
More than 40% of patients with inflammatory bowel disease (IBD) screened positive for joint pain symptomatic of spondyloarthritis (SpA), according to a new study.
Of these patients, 75% did not have any history of arthritis.
“What we know is that a substantial proportion of patients with IBD do report musculoskeletal symptoms, and inflammatory back pain stands out as being one of the more frequent symptoms reported,” said Reem Jan, MBBS, a rheumatologist at the University of Chicago Medicine. She presented the study findings during the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN) in Cleveland.
“Yet a minority of these patients are evaluated by rheumatologists. So that suggests there’s a high burden of need in the IBD population to have this joint pain evaluated and addressed,” she said during her presentation.
She presented preliminary data from an ongoing project to better understand the prevalence of inflammatory arthritis in IBD — estimates range from 17% to 39%— and the risk factors for developing arthritis in this patient population.
Study Details
Researchers enrolled patients from outpatient gastroenterology clinics or procedure units at NYU Langone Health, New York City; Brigham and Women’s Hospital, Boston; University of Colorado Anschutz Medical Campus, Aurora, Colorado; Mayo Clinic, Rochester, Minnesota; University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago; and Icahn School of Medicine at Mount Sinai, New York City. Additional patients were recruited from Mercy Health, a community health system in Ohio.
Upon entry into the study, participants completed a survey documenting their history with joint pain. The survey combined questions from the DETAIL and the IBIS questionnaires.
Between January 2021 and December 2022, 669 patients joined the study. In total, 41% of patients (n = 275) screened positive.
“What really stood out to us was that of all the positive screens, only about a quarter of those patients were known to have SpA,” Dr. Jan said during her presentation. “[This] means 75% of the patients who screened positive were not known to have any type of arthritic disease.”
In addition, only 24% (n = 65) of all patients who screened positive — including those with a SpA diagnosis — had seen a rheumatologist in the previous year.
Among these patients, inflammatory back pain was the most commonly reported symptom, followed by painful swelling of peripheral joints and heel pain.
Excluding patients with a SpA diagnosis, researchers also investigated which characteristics were associated with a higher likelihood of screening positive in the questionnaire. The analysis, including 588 patients, identified the following risk factors:
- Female sex: Odds ratio (OR), 2.0; 95% CI, 1.4-2.9
- Older age: OR, 1.02; 95% CI, 1.01-1.4
- History of smoking: OR, 1.7; 95% CI, 1.1-2.6
- History of prior IBD-related surgery: OR, 1.60; 95% CI, 1.1-2.5
- History of biologic or small molecule therapy: OR, 2.3; 95% CI, 1.4-4.0
Future Directions
Commenting on the study, Mark Hwang, MD, a rheumatologist at UTHealth Houston, noted that it was “very interesting to see the fairly large, positive rates” of joint pain in patients with IBD, which certainly have clinical implications. However, it is not yet known if any of these patients went on to be diagnosed with SpA.
Jan noted that potential next steps include a follow-up analysis of patients who screened positive to see how many went on to see a rheumatologist and which patients were ultimately diagnosed with SpA or other inflammatory arthritis conditions.
These findings are a first step, Dr. Hwang said, and will likely “help further establish some of the validity of these questionnaires by testing in different patient populations,” he noted.
The ultimate goal is to “develop really good strategies to risk stratify IBD patients with the greatest need of rheumatologist consultation,” Dr. Jan said. “We certainly don’t want to see all these patients, so how can we figure out who really needs to be seen?”
Funding information was not available for this study. Dr. Hwang is conducting two clinical trials for psoriatic arthritis sponsored by Janssen and Eli Lilly. Dr. Jan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
More than 40% of patients with inflammatory bowel disease (IBD) screened positive for joint pain symptomatic of spondyloarthritis (SpA), according to a new study.
Of these patients, 75% did not have any history of arthritis.
“What we know is that a substantial proportion of patients with IBD do report musculoskeletal symptoms, and inflammatory back pain stands out as being one of the more frequent symptoms reported,” said Reem Jan, MBBS, a rheumatologist at the University of Chicago Medicine. She presented the study findings during the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN) in Cleveland.
“Yet a minority of these patients are evaluated by rheumatologists. So that suggests there’s a high burden of need in the IBD population to have this joint pain evaluated and addressed,” she said during her presentation.
She presented preliminary data from an ongoing project to better understand the prevalence of inflammatory arthritis in IBD — estimates range from 17% to 39%— and the risk factors for developing arthritis in this patient population.
Study Details
Researchers enrolled patients from outpatient gastroenterology clinics or procedure units at NYU Langone Health, New York City; Brigham and Women’s Hospital, Boston; University of Colorado Anschutz Medical Campus, Aurora, Colorado; Mayo Clinic, Rochester, Minnesota; University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago; and Icahn School of Medicine at Mount Sinai, New York City. Additional patients were recruited from Mercy Health, a community health system in Ohio.
Upon entry into the study, participants completed a survey documenting their history with joint pain. The survey combined questions from the DETAIL and the IBIS questionnaires.
Between January 2021 and December 2022, 669 patients joined the study. In total, 41% of patients (n = 275) screened positive.
“What really stood out to us was that of all the positive screens, only about a quarter of those patients were known to have SpA,” Dr. Jan said during her presentation. “[This] means 75% of the patients who screened positive were not known to have any type of arthritic disease.”
In addition, only 24% (n = 65) of all patients who screened positive — including those with a SpA diagnosis — had seen a rheumatologist in the previous year.
Among these patients, inflammatory back pain was the most commonly reported symptom, followed by painful swelling of peripheral joints and heel pain.
Excluding patients with a SpA diagnosis, researchers also investigated which characteristics were associated with a higher likelihood of screening positive in the questionnaire. The analysis, including 588 patients, identified the following risk factors:
- Female sex: Odds ratio (OR), 2.0; 95% CI, 1.4-2.9
- Older age: OR, 1.02; 95% CI, 1.01-1.4
- History of smoking: OR, 1.7; 95% CI, 1.1-2.6
- History of prior IBD-related surgery: OR, 1.60; 95% CI, 1.1-2.5
- History of biologic or small molecule therapy: OR, 2.3; 95% CI, 1.4-4.0
Future Directions
Commenting on the study, Mark Hwang, MD, a rheumatologist at UTHealth Houston, noted that it was “very interesting to see the fairly large, positive rates” of joint pain in patients with IBD, which certainly have clinical implications. However, it is not yet known if any of these patients went on to be diagnosed with SpA.
Jan noted that potential next steps include a follow-up analysis of patients who screened positive to see how many went on to see a rheumatologist and which patients were ultimately diagnosed with SpA or other inflammatory arthritis conditions.
These findings are a first step, Dr. Hwang said, and will likely “help further establish some of the validity of these questionnaires by testing in different patient populations,” he noted.
The ultimate goal is to “develop really good strategies to risk stratify IBD patients with the greatest need of rheumatologist consultation,” Dr. Jan said. “We certainly don’t want to see all these patients, so how can we figure out who really needs to be seen?”
Funding information was not available for this study. Dr. Hwang is conducting two clinical trials for psoriatic arthritis sponsored by Janssen and Eli Lilly. Dr. Jan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SPARTAN 2024
Semaglutide Improves Taste Sensitivity in Women With Obesity
The glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Ozempic, Wegovy) enhances taste sensitivity, changes brain responses to sweet tastes and may even alter expression of genes in the tongue associated with taste bud development, according to new research presented at the annual meeting of the Endocrine Society, held in Boston.
“Some studies have reported that individuals living with obesity often perceive tastes as less intense,” noted Mojca Jensterle Sever, PhD, of the University Medical Centre in Ljubljana, Slovenia, who presented the work. Research also suggests that “populations prone to obesity have an inherently elevated desire for sweet and energy-dense foods,” she continued.
Studies in animal models have also previously shown that GLP-1 plays an important role in taste sensitivity, but it was not known if this hormone also influenced human taste perception.
In this proof-of-concept study, researchers randomly assigned 30 women with polycystic ovary syndrome (PCOS) to either 1 mg of semaglutide, administered once a week, or placebo for 16 weeks. Participants were on average 34 years old with a body mass index (BMI) of 36.4. Participants with PCOS were selected with the “aim to reduce variability in taste perception across different phases of the menstrual cycle,” Dr. Sever said.
Prior to the intervention, researchers tested participants’ taste sensitivity using 16 taste strips infused with four different concentrations of sweet, sour, salty, and bitter substances. Participants were asked to identify the taste of each strip. Every correct answer counted as one point, with a possible total of 16 points overall. Tongue biopsies were conducted for gene expression analysis.
Researchers also used functional MRI (fMRI) to evaluate brain responses to a series of calorie-dense, low-calorie, and non-food visual cues as well as to sweet taste stimulus. A sweet solution was administered on the tongue 30 minutes before and after participants consumed a standardized meal: a high-protein enriched nutritional drink.
These tests were repeated after 16 weeks.
The semaglutide group also exhibited decreased activation of the putamen (a structure in the brain involved with the brain’s reward system) on fMRI in response to calorie-dense cues. In response to sweet taste stimulus, those taking semaglutide showed increased activation of angular gyrus on MRI compared with the placebo group. The angular gyrus is part of the brain’s parietal lobe and is involved with language, memory, reasoning, and attention.
Lastly, researchers identified differential mRNA expression in the genes EYA, PRMT8, CRLF1, and CYP1B1, which are associated with taste bud development, renewal, and differentiation.
The findings are “fascinating, because we think about all of the factors that this new class of agents are able to improve, but taste is often not something that we look at, though there have been very strong associations,” said Gitanjali Srivastava, MD, of Vanderbilt University, Nashville, Tennessee, who moderated the session.
“Is it possible that another mechanism of action for this class of agents is perhaps indirectly altering our taste perception,” she posited, and, because of that, “we have an altered sense of satiety and hunger?”
Dr. Sever noted Dr. Several limitations to the study, including that only specific tastes were evaluated in a controlled study environment, “which may not reflect everyday experience,” she said. Taste perception can also vary widely from person to person, and changes in mRNA expression do not necessarily reflect changes in protein levels or activity.
“Our study should be seen and interpreted as a proof-of-concept study,” Dr. Sever added, with additional research needed to explore the relationship between semaglutide and taste perception.
Dr. Srivastava consults for Novo Nordisk, Eli Lilly, and Rhythm Pharmaceuticals. She has received research grant support from Eli Lilly. Dr. Sever reports no relevant financial relationships.
A version of this article appeared on Medscape.com .
The glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Ozempic, Wegovy) enhances taste sensitivity, changes brain responses to sweet tastes and may even alter expression of genes in the tongue associated with taste bud development, according to new research presented at the annual meeting of the Endocrine Society, held in Boston.
“Some studies have reported that individuals living with obesity often perceive tastes as less intense,” noted Mojca Jensterle Sever, PhD, of the University Medical Centre in Ljubljana, Slovenia, who presented the work. Research also suggests that “populations prone to obesity have an inherently elevated desire for sweet and energy-dense foods,” she continued.
Studies in animal models have also previously shown that GLP-1 plays an important role in taste sensitivity, but it was not known if this hormone also influenced human taste perception.
In this proof-of-concept study, researchers randomly assigned 30 women with polycystic ovary syndrome (PCOS) to either 1 mg of semaglutide, administered once a week, or placebo for 16 weeks. Participants were on average 34 years old with a body mass index (BMI) of 36.4. Participants with PCOS were selected with the “aim to reduce variability in taste perception across different phases of the menstrual cycle,” Dr. Sever said.
Prior to the intervention, researchers tested participants’ taste sensitivity using 16 taste strips infused with four different concentrations of sweet, sour, salty, and bitter substances. Participants were asked to identify the taste of each strip. Every correct answer counted as one point, with a possible total of 16 points overall. Tongue biopsies were conducted for gene expression analysis.
Researchers also used functional MRI (fMRI) to evaluate brain responses to a series of calorie-dense, low-calorie, and non-food visual cues as well as to sweet taste stimulus. A sweet solution was administered on the tongue 30 minutes before and after participants consumed a standardized meal: a high-protein enriched nutritional drink.
These tests were repeated after 16 weeks.
The semaglutide group also exhibited decreased activation of the putamen (a structure in the brain involved with the brain’s reward system) on fMRI in response to calorie-dense cues. In response to sweet taste stimulus, those taking semaglutide showed increased activation of angular gyrus on MRI compared with the placebo group. The angular gyrus is part of the brain’s parietal lobe and is involved with language, memory, reasoning, and attention.
Lastly, researchers identified differential mRNA expression in the genes EYA, PRMT8, CRLF1, and CYP1B1, which are associated with taste bud development, renewal, and differentiation.
The findings are “fascinating, because we think about all of the factors that this new class of agents are able to improve, but taste is often not something that we look at, though there have been very strong associations,” said Gitanjali Srivastava, MD, of Vanderbilt University, Nashville, Tennessee, who moderated the session.
“Is it possible that another mechanism of action for this class of agents is perhaps indirectly altering our taste perception,” she posited, and, because of that, “we have an altered sense of satiety and hunger?”
Dr. Sever noted Dr. Several limitations to the study, including that only specific tastes were evaluated in a controlled study environment, “which may not reflect everyday experience,” she said. Taste perception can also vary widely from person to person, and changes in mRNA expression do not necessarily reflect changes in protein levels or activity.
“Our study should be seen and interpreted as a proof-of-concept study,” Dr. Sever added, with additional research needed to explore the relationship between semaglutide and taste perception.
Dr. Srivastava consults for Novo Nordisk, Eli Lilly, and Rhythm Pharmaceuticals. She has received research grant support from Eli Lilly. Dr. Sever reports no relevant financial relationships.
A version of this article appeared on Medscape.com .
The glucagon-like peptide-1 (GLP-1) receptor agonist semaglutide (Ozempic, Wegovy) enhances taste sensitivity, changes brain responses to sweet tastes and may even alter expression of genes in the tongue associated with taste bud development, according to new research presented at the annual meeting of the Endocrine Society, held in Boston.
“Some studies have reported that individuals living with obesity often perceive tastes as less intense,” noted Mojca Jensterle Sever, PhD, of the University Medical Centre in Ljubljana, Slovenia, who presented the work. Research also suggests that “populations prone to obesity have an inherently elevated desire for sweet and energy-dense foods,” she continued.
Studies in animal models have also previously shown that GLP-1 plays an important role in taste sensitivity, but it was not known if this hormone also influenced human taste perception.
In this proof-of-concept study, researchers randomly assigned 30 women with polycystic ovary syndrome (PCOS) to either 1 mg of semaglutide, administered once a week, or placebo for 16 weeks. Participants were on average 34 years old with a body mass index (BMI) of 36.4. Participants with PCOS were selected with the “aim to reduce variability in taste perception across different phases of the menstrual cycle,” Dr. Sever said.
Prior to the intervention, researchers tested participants’ taste sensitivity using 16 taste strips infused with four different concentrations of sweet, sour, salty, and bitter substances. Participants were asked to identify the taste of each strip. Every correct answer counted as one point, with a possible total of 16 points overall. Tongue biopsies were conducted for gene expression analysis.
Researchers also used functional MRI (fMRI) to evaluate brain responses to a series of calorie-dense, low-calorie, and non-food visual cues as well as to sweet taste stimulus. A sweet solution was administered on the tongue 30 minutes before and after participants consumed a standardized meal: a high-protein enriched nutritional drink.
These tests were repeated after 16 weeks.
The semaglutide group also exhibited decreased activation of the putamen (a structure in the brain involved with the brain’s reward system) on fMRI in response to calorie-dense cues. In response to sweet taste stimulus, those taking semaglutide showed increased activation of angular gyrus on MRI compared with the placebo group. The angular gyrus is part of the brain’s parietal lobe and is involved with language, memory, reasoning, and attention.
Lastly, researchers identified differential mRNA expression in the genes EYA, PRMT8, CRLF1, and CYP1B1, which are associated with taste bud development, renewal, and differentiation.
The findings are “fascinating, because we think about all of the factors that this new class of agents are able to improve, but taste is often not something that we look at, though there have been very strong associations,” said Gitanjali Srivastava, MD, of Vanderbilt University, Nashville, Tennessee, who moderated the session.
“Is it possible that another mechanism of action for this class of agents is perhaps indirectly altering our taste perception,” she posited, and, because of that, “we have an altered sense of satiety and hunger?”
Dr. Sever noted Dr. Several limitations to the study, including that only specific tastes were evaluated in a controlled study environment, “which may not reflect everyday experience,” she said. Taste perception can also vary widely from person to person, and changes in mRNA expression do not necessarily reflect changes in protein levels or activity.
“Our study should be seen and interpreted as a proof-of-concept study,” Dr. Sever added, with additional research needed to explore the relationship between semaglutide and taste perception.
Dr. Srivastava consults for Novo Nordisk, Eli Lilly, and Rhythm Pharmaceuticals. She has received research grant support from Eli Lilly. Dr. Sever reports no relevant financial relationships.
A version of this article appeared on Medscape.com .
Rheumatologists Deserve Better Pay, Say Respondents to Compensation Survey
While rheumatologists reported small pay gains this year, more than half said the specialty was underpaid.
In the Medscape Rheumatologist Compensation Report 2024, 53% said that they did not feel fairly paid given their work demands. Rheumatologist respondents reported earning an average of $286,000 annually, ranking them as the seventh lowest earners out of a total of 29 specialties surveyed. Orthopedics was the highest earning specialty, with $558,000 in annual income, and diabetes & endocrinology was the lowest earning specialty, with $256,000 in annual compensation.
In last year’s report, a rheumatologist’s average income was $281,000.
This new report was compiled from an online survey including more than 7000 physicians from 29 specialties, of whom 1% of respondents were rheumatologists. Most respondents (58%) were women, and 39% were men. The survey was available from October 2, 2023, to January 16, 2024.
Rheumatologists reported a 2% increase in pay compared with that cited in the previous year’s report. Physical medicine and rehabilitation had the largest bump in pay at 11%. A total of 29% of rheumatologists said their pay had increased from that in the previous year, and 18% reported fewer earnings. About half (53%) reported that their income remained the same.
When asked about physician pay in the United States, 61% of rheumatologists said most physicians were underpaid, 34% said physicians were paid fairly, and only 4% said most physicians were overpaid.
“Most physicians who take care of chronic illnesses in long-term patients are underpaid. Not all doctors are,” said one survey respondent.
Another 41% of rheumatologists said they supplemented income with additional work, including other medical-related work (30%), nonmedical-related work (5%), adding more hours to their primary job (5%), and medical moonlighting (4%). (Respondents could choose more than one option in the survey.) This is slightly lower than last year’s survey, where 46% of rheumatologist respondents said they took on additional work.
About three out of four rheumatologists said that other medical businesses or competing physician practices did not affect their income, and only 5% said these competitors considerably affected income.
Rheumatologists listed being good at their job/diagnosing (36%) as the most rewarding part of their profession, followed by gratitude from/relationships with patients (26%) and making the world a better place/helping others (19%). Difficulties with insurance and receiving fair reimbursement (22%), dealing with difficult patients (20%), having many rules and regulations (18%), and working with an electronic health record system (15%) were the most commonly reported challenges for rheumatologists.
A version of this article appeared on Medscape.com.
While rheumatologists reported small pay gains this year, more than half said the specialty was underpaid.
In the Medscape Rheumatologist Compensation Report 2024, 53% said that they did not feel fairly paid given their work demands. Rheumatologist respondents reported earning an average of $286,000 annually, ranking them as the seventh lowest earners out of a total of 29 specialties surveyed. Orthopedics was the highest earning specialty, with $558,000 in annual income, and diabetes & endocrinology was the lowest earning specialty, with $256,000 in annual compensation.
In last year’s report, a rheumatologist’s average income was $281,000.
This new report was compiled from an online survey including more than 7000 physicians from 29 specialties, of whom 1% of respondents were rheumatologists. Most respondents (58%) were women, and 39% were men. The survey was available from October 2, 2023, to January 16, 2024.
Rheumatologists reported a 2% increase in pay compared with that cited in the previous year’s report. Physical medicine and rehabilitation had the largest bump in pay at 11%. A total of 29% of rheumatologists said their pay had increased from that in the previous year, and 18% reported fewer earnings. About half (53%) reported that their income remained the same.
When asked about physician pay in the United States, 61% of rheumatologists said most physicians were underpaid, 34% said physicians were paid fairly, and only 4% said most physicians were overpaid.
“Most physicians who take care of chronic illnesses in long-term patients are underpaid. Not all doctors are,” said one survey respondent.
Another 41% of rheumatologists said they supplemented income with additional work, including other medical-related work (30%), nonmedical-related work (5%), adding more hours to their primary job (5%), and medical moonlighting (4%). (Respondents could choose more than one option in the survey.) This is slightly lower than last year’s survey, where 46% of rheumatologist respondents said they took on additional work.
About three out of four rheumatologists said that other medical businesses or competing physician practices did not affect their income, and only 5% said these competitors considerably affected income.
Rheumatologists listed being good at their job/diagnosing (36%) as the most rewarding part of their profession, followed by gratitude from/relationships with patients (26%) and making the world a better place/helping others (19%). Difficulties with insurance and receiving fair reimbursement (22%), dealing with difficult patients (20%), having many rules and regulations (18%), and working with an electronic health record system (15%) were the most commonly reported challenges for rheumatologists.
A version of this article appeared on Medscape.com.
While rheumatologists reported small pay gains this year, more than half said the specialty was underpaid.
In the Medscape Rheumatologist Compensation Report 2024, 53% said that they did not feel fairly paid given their work demands. Rheumatologist respondents reported earning an average of $286,000 annually, ranking them as the seventh lowest earners out of a total of 29 specialties surveyed. Orthopedics was the highest earning specialty, with $558,000 in annual income, and diabetes & endocrinology was the lowest earning specialty, with $256,000 in annual compensation.
In last year’s report, a rheumatologist’s average income was $281,000.
This new report was compiled from an online survey including more than 7000 physicians from 29 specialties, of whom 1% of respondents were rheumatologists. Most respondents (58%) were women, and 39% were men. The survey was available from October 2, 2023, to January 16, 2024.
Rheumatologists reported a 2% increase in pay compared with that cited in the previous year’s report. Physical medicine and rehabilitation had the largest bump in pay at 11%. A total of 29% of rheumatologists said their pay had increased from that in the previous year, and 18% reported fewer earnings. About half (53%) reported that their income remained the same.
When asked about physician pay in the United States, 61% of rheumatologists said most physicians were underpaid, 34% said physicians were paid fairly, and only 4% said most physicians were overpaid.
“Most physicians who take care of chronic illnesses in long-term patients are underpaid. Not all doctors are,” said one survey respondent.
Another 41% of rheumatologists said they supplemented income with additional work, including other medical-related work (30%), nonmedical-related work (5%), adding more hours to their primary job (5%), and medical moonlighting (4%). (Respondents could choose more than one option in the survey.) This is slightly lower than last year’s survey, where 46% of rheumatologist respondents said they took on additional work.
About three out of four rheumatologists said that other medical businesses or competing physician practices did not affect their income, and only 5% said these competitors considerably affected income.
Rheumatologists listed being good at their job/diagnosing (36%) as the most rewarding part of their profession, followed by gratitude from/relationships with patients (26%) and making the world a better place/helping others (19%). Difficulties with insurance and receiving fair reimbursement (22%), dealing with difficult patients (20%), having many rules and regulations (18%), and working with an electronic health record system (15%) were the most commonly reported challenges for rheumatologists.
A version of this article appeared on Medscape.com.
More Women Report First Hip Fracture in Their 60s
TOPLINE:
Women with low bone density are more likely to report their first fragility hip fracture in their 60s rather than at older ages.
METHODOLOGY:
- Researchers used hip fracture data from the National Health and Nutrition Examination Survey for 2009-2010, 2013-2014, and 2017-2018.
- They included women older than 60 years with a bone mineral density T score ≤ −1 at the femur neck, measured by dual-energy x-ray absorptiometry.
- Fragility fractures are defined as a self-reported hip fracture resulting from a fall from standing height or less.
TAKEAWAY:
- The number of women in their 60s who reported their first hip fracture grew by 50% from 2009 to 2018.
- The opposite was true for women in their 70s and 80s who reported fewer first hip fractures over the study period.
- Reported fragility hip fractures in women overall decreased by half from 2009 to 2018.
- The prevalence of women with osteoporosis (T score ≤ −2.5) grew from 18.1% to 21.3% over 10 years.
IN PRACTICE:
The decrease in fractures overall and in women older than 70 years “may be due to increasing awareness and utilization of measures to decrease falls such as exercise, nutrition, health education, and environmental modifications targeted toward the elderly population,” the authors wrote. The findings also underscore the importance of earlier bone health awareness in primary care to curb the rising trend in younger women, they added.
SOURCE:
The study was led by Avica Atri, MD, of Albert Einstein Medical Center in Philadelphia. She presented the findings at ENDO 2024: The Endocrine Society Annual Meeting.
LIMITATIONS:
The study was retrospective in nature and included self-reported health data.
DISCLOSURES:
The study received no commercial funding. The authors have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
Women with low bone density are more likely to report their first fragility hip fracture in their 60s rather than at older ages.
METHODOLOGY:
- Researchers used hip fracture data from the National Health and Nutrition Examination Survey for 2009-2010, 2013-2014, and 2017-2018.
- They included women older than 60 years with a bone mineral density T score ≤ −1 at the femur neck, measured by dual-energy x-ray absorptiometry.
- Fragility fractures are defined as a self-reported hip fracture resulting from a fall from standing height or less.
TAKEAWAY:
- The number of women in their 60s who reported their first hip fracture grew by 50% from 2009 to 2018.
- The opposite was true for women in their 70s and 80s who reported fewer first hip fractures over the study period.
- Reported fragility hip fractures in women overall decreased by half from 2009 to 2018.
- The prevalence of women with osteoporosis (T score ≤ −2.5) grew from 18.1% to 21.3% over 10 years.
IN PRACTICE:
The decrease in fractures overall and in women older than 70 years “may be due to increasing awareness and utilization of measures to decrease falls such as exercise, nutrition, health education, and environmental modifications targeted toward the elderly population,” the authors wrote. The findings also underscore the importance of earlier bone health awareness in primary care to curb the rising trend in younger women, they added.
SOURCE:
The study was led by Avica Atri, MD, of Albert Einstein Medical Center in Philadelphia. She presented the findings at ENDO 2024: The Endocrine Society Annual Meeting.
LIMITATIONS:
The study was retrospective in nature and included self-reported health data.
DISCLOSURES:
The study received no commercial funding. The authors have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
Women with low bone density are more likely to report their first fragility hip fracture in their 60s rather than at older ages.
METHODOLOGY:
- Researchers used hip fracture data from the National Health and Nutrition Examination Survey for 2009-2010, 2013-2014, and 2017-2018.
- They included women older than 60 years with a bone mineral density T score ≤ −1 at the femur neck, measured by dual-energy x-ray absorptiometry.
- Fragility fractures are defined as a self-reported hip fracture resulting from a fall from standing height or less.
TAKEAWAY:
- The number of women in their 60s who reported their first hip fracture grew by 50% from 2009 to 2018.
- The opposite was true for women in their 70s and 80s who reported fewer first hip fractures over the study period.
- Reported fragility hip fractures in women overall decreased by half from 2009 to 2018.
- The prevalence of women with osteoporosis (T score ≤ −2.5) grew from 18.1% to 21.3% over 10 years.
IN PRACTICE:
The decrease in fractures overall and in women older than 70 years “may be due to increasing awareness and utilization of measures to decrease falls such as exercise, nutrition, health education, and environmental modifications targeted toward the elderly population,” the authors wrote. The findings also underscore the importance of earlier bone health awareness in primary care to curb the rising trend in younger women, they added.
SOURCE:
The study was led by Avica Atri, MD, of Albert Einstein Medical Center in Philadelphia. She presented the findings at ENDO 2024: The Endocrine Society Annual Meeting.
LIMITATIONS:
The study was retrospective in nature and included self-reported health data.
DISCLOSURES:
The study received no commercial funding. The authors have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
High NSAID Use in Patients With Axial Spondyloarthritis May Not Raise Risk for Hypertension
TOPLINE:
Patients with axial spondyloarthritis (axSpA) who reported high nonsteroidal anti-inflammatory drug (NSAID) use did not have a higher risk for hypertension than those who reported low NSAID use.
METHODOLOGY:
- NSAIDs are first-line therapy for axSpA and are associated with a high risk for hypertension in the general population, but it’s unknown whether NSAID use increases the risk for hypertension in patients with axSpA, who are already at higher risk for cardiovascular disease and hypertension than the general population
- This study used the DESIR cohort, a multicenter cohort of patients with recent-onset axSpA in France, including 631 individuals aged 18-50 years who did not have hypertension at baseline and had 6 years of follow-up.
- NSAID use was evaluated at each follow-up visit, using the Assessment of Spondyloarthritis International Society NSAID index.
- A score ≥ 50 was categorized as high use, and a score < 50 was considered low use.
- The primary outcome was hypertension, defined by the use of antihypertensive medication, self-reported hypertension, and/or systolic blood pressure (BP) ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg on at least two visits.
TAKEAWAY:
- A total of 39% of patients were categorized as high NSAID users.
- Over 6 years of follow-up, 70 patients (11%) developed hypertension.
- There was no significant association between high NSAID use and the risk for hypertension.
IN PRACTICE:
The study is too preliminary to have practice application.
SOURCE:
The research was led and presented by Jose Meade-Aguilar, MD, of Boston University School of Medicine, at the Spondyloarthritis Research and Treatment Network (SPARTAN) 2024 annual meeting in Cleveland.
LIMITATIONS:
The study had a low number of hypertension events, which could be due to the younger age of participants and earlier disease stage. The study was observational, so residual or unmeasured confounding is possible.
DISCLOSURES:
The DESIR cohort study is financially supported by unrestricted grants from both the French Society for Rheumatology and Pfizer France. One coauthor reported receiving research grants and/or consultancy fees from AbbVie, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, UCB, and Sanofi. Another coauthor reported receiving research grants from UCB and consulting fees from Eli Lilly, Novartis, Pfizer, and UCB. The remaining authors had no financial, relational, or commercial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with axial spondyloarthritis (axSpA) who reported high nonsteroidal anti-inflammatory drug (NSAID) use did not have a higher risk for hypertension than those who reported low NSAID use.
METHODOLOGY:
- NSAIDs are first-line therapy for axSpA and are associated with a high risk for hypertension in the general population, but it’s unknown whether NSAID use increases the risk for hypertension in patients with axSpA, who are already at higher risk for cardiovascular disease and hypertension than the general population
- This study used the DESIR cohort, a multicenter cohort of patients with recent-onset axSpA in France, including 631 individuals aged 18-50 years who did not have hypertension at baseline and had 6 years of follow-up.
- NSAID use was evaluated at each follow-up visit, using the Assessment of Spondyloarthritis International Society NSAID index.
- A score ≥ 50 was categorized as high use, and a score < 50 was considered low use.
- The primary outcome was hypertension, defined by the use of antihypertensive medication, self-reported hypertension, and/or systolic blood pressure (BP) ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg on at least two visits.
TAKEAWAY:
- A total of 39% of patients were categorized as high NSAID users.
- Over 6 years of follow-up, 70 patients (11%) developed hypertension.
- There was no significant association between high NSAID use and the risk for hypertension.
IN PRACTICE:
The study is too preliminary to have practice application.
SOURCE:
The research was led and presented by Jose Meade-Aguilar, MD, of Boston University School of Medicine, at the Spondyloarthritis Research and Treatment Network (SPARTAN) 2024 annual meeting in Cleveland.
LIMITATIONS:
The study had a low number of hypertension events, which could be due to the younger age of participants and earlier disease stage. The study was observational, so residual or unmeasured confounding is possible.
DISCLOSURES:
The DESIR cohort study is financially supported by unrestricted grants from both the French Society for Rheumatology and Pfizer France. One coauthor reported receiving research grants and/or consultancy fees from AbbVie, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, UCB, and Sanofi. Another coauthor reported receiving research grants from UCB and consulting fees from Eli Lilly, Novartis, Pfizer, and UCB. The remaining authors had no financial, relational, or commercial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with axial spondyloarthritis (axSpA) who reported high nonsteroidal anti-inflammatory drug (NSAID) use did not have a higher risk for hypertension than those who reported low NSAID use.
METHODOLOGY:
- NSAIDs are first-line therapy for axSpA and are associated with a high risk for hypertension in the general population, but it’s unknown whether NSAID use increases the risk for hypertension in patients with axSpA, who are already at higher risk for cardiovascular disease and hypertension than the general population
- This study used the DESIR cohort, a multicenter cohort of patients with recent-onset axSpA in France, including 631 individuals aged 18-50 years who did not have hypertension at baseline and had 6 years of follow-up.
- NSAID use was evaluated at each follow-up visit, using the Assessment of Spondyloarthritis International Society NSAID index.
- A score ≥ 50 was categorized as high use, and a score < 50 was considered low use.
- The primary outcome was hypertension, defined by the use of antihypertensive medication, self-reported hypertension, and/or systolic blood pressure (BP) ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg on at least two visits.
TAKEAWAY:
- A total of 39% of patients were categorized as high NSAID users.
- Over 6 years of follow-up, 70 patients (11%) developed hypertension.
- There was no significant association between high NSAID use and the risk for hypertension.
IN PRACTICE:
The study is too preliminary to have practice application.
SOURCE:
The research was led and presented by Jose Meade-Aguilar, MD, of Boston University School of Medicine, at the Spondyloarthritis Research and Treatment Network (SPARTAN) 2024 annual meeting in Cleveland.
LIMITATIONS:
The study had a low number of hypertension events, which could be due to the younger age of participants and earlier disease stage. The study was observational, so residual or unmeasured confounding is possible.
DISCLOSURES:
The DESIR cohort study is financially supported by unrestricted grants from both the French Society for Rheumatology and Pfizer France. One coauthor reported receiving research grants and/or consultancy fees from AbbVie, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, UCB, and Sanofi. Another coauthor reported receiving research grants from UCB and consulting fees from Eli Lilly, Novartis, Pfizer, and UCB. The remaining authors had no financial, relational, or commercial conflicts to disclose.
A version of this article appeared on Medscape.com.
Key Risk Factors for Hydroxychloroquine Retinopathy Described in Large Study
Older patients prescribed hydroxychloroquine (HCQ) have a higher risk of developing retinal damage from taking the medication, according to a new analysis.
In addition to known risk factors such as a higher weight-based HCQ dose and higher cumulative dose, researchers also found that female sex, chronic kidney disease stage III, and tamoxifen use were associated with HCQ retinopathy.
The findings provide “evidence for other key risk factors for hydroxychloroquine retinopathy beyond hydroxychloroquine exposure itself,” wrote April M. Jorge, MD, of the Division of Rheumatology, Allergy, and Immunology at Massachusetts General Hospital, Boston, and colleagues.
“It is the largest cohort study to date looking specifically at the association of [HCQ] retinopathy with risk factors,” Christina Weng, MD, MBA, professor of ophthalmology at Baylor College of Medicine, Houston, said in an interview. She was not involved with the research. Some of the associations, such as tamoxifen use, “have been suggested before in smaller studies, but never on this scale,” she said.
“It’s provided reinforcement of findings that we have seen from prior research and also some new glimpses into strengthening some associations that were identified, but not yet fully understood, in prior work,” she continued.
Study Details
Researchers identified patients in the Kaiser Permanente Northern California (KPNC), Oakland, California, health system who began taking HCQ between July 1, 1997, and December 14, 2014. To be included, patients needed to have at least 5 years of continuous enrollment in the KPNC system and at least one prescription for HCQ after more than 5 years of starting the drug. Patients were followed from HCQ initiation to their last retinopathy screening study, up to December 31, 2020.
The study was published May 9 in JAMA Network Open.
Of the 4677 users followed for the study, 83% were women, and the average age starting HCQ was 52. Most patients were White (58.1%), while 13.7% were Asian, 10.5% were Black, and 17.7% were Hispanic.
More than 60% of patients had an initial dose > 5 mg/kg/d, though the mean initial dose of HCQ was 4.4 mg/kg/d. After 5 years, only 34.4% of patients were using a daily dose over 5 mg/kg.
Of the entire cohort, 125 patients (2.7%) developed HCQ retinopathy. As expected, cumulative HCQ exposure was associated with a higher retinopathy risk: For every 100 g of HCQ cumulative exposure, risk rose by 64% (hazard ratio [HR], 1.64; 95% CI, 1.44-1.87).
Age was a significant risk factor for retinal damage from HCQ use. Individuals who began taking the drug at 65 years or older were nearly six times more likely to develop retinopathy than those who started HCQ when they were younger than 45. In people aged 55-64 years, this risk was nearly four times higher, and individuals aged 45-54 years when starting the drug were 2.5 times more likely to have retinal damage than those younger than 45.
Other risk factors were female sex (HR, 3.83; 95% CI, 1.86-7.89), chronic kidney disease stage III (HR, 1.95; 95% CI, 1.25-3.04), and tamoxifen use (HR, 3.43; 95% CI, 1.08-10.89), although only 17 patients were taking tamoxifen during the study.
Researchers also found that the type of HCQ retinopathy varied by race. Of the 125 cases in the cohort, 102 had a parafoveal pattern, and 23 had a pericentral pattern. Asian individuals were 15 times more likely, and Black individuals were more than 5 times more likely to develop this pericentral type than were White patients.
This association in Asian patients has also been found in previous studies, Dr. Weng said, and many eye practices now screen their Asian patients with a 30-2 Humphrey visual field — rather than the more commonly used 10-2 — to examine areas farther outside the center.
This study also found this association in Black patients, though only five Black patients developed HCQ retinopathy over the study period.
“More studies and larger studies will be very helpful in strengthening or dispelling some of the associations that have been seen here,” Dr. Weng said.
‘More Room for Personalized Medicine’
The team found a “relatively linear” relationship between HCQ dose and retinopathy risk, with higher daily doses correlating with higher incidence. While 2016 guidelines from the American Academy of Ophthalmology advise using < 5 mg/kg, “what we found is it’s not that straightforward [where there’s] just this one cutoff,” Dr. Jorge told this news organization. “It does seem like the higher the dose of medication per bodyweight and the longer duration of use, there is a higher risk of retinopathy.”
These findings leave “a bit more room for personalized medicine” with patients, she explained. “For an elderly female patient with CKD, aiming for a dose < 5 mg/kg might be more appropriate; however, a young male patient without any additional risk factors may be able to exceed 5 mg/kg and continue to have a low risk for HCQ retinopathy,” she said.
“For anyone, I think it really is more of an individual risk-benefit evaluation,” rather than strict cutoffs, she continued.
“Guidelines are just that: They’re guidelines,” added Dr. Weng, “and treatment plans should be tailored to each individual patient.”
As the study authors also discussed, “the goal is to treat the patient with the lowest dose that is still effective and also be mindful of the duration that a patient is left at higher doses,” Dr. Weng said. “But ultimately, we need to control these diseases, which can cause damage across multiple organ systems in the body. While it’s important to be aware of the potential retinopathy adverse events, we also don’t want physicians to feel restricted in their use of this very effective drug.”
The work of three coauthors on the current study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). Dr. Jorge’s work on the study was supported by an award from the Rheumatology Research Foundation and a grant from NIAMS. Dr. Jorge reported clinical trial agreements with Bristol Myers Squibb and Cabaletta Bio outside of this study. Dr. Weng has served as a consultant for Allergan/AbbVie, Alcon, Apellis Pharmaceuticals, Alimera Sciences, DORC, Novartis, Genentech, Regeneron, RegenxBio, Iveric Bio, and EyePoint Pharmaceuticals. Dr. Weng disclosed financial relationships with Springer Publishers (royalties) and DRCR Retina Network, Alimera Sciences, and Applied Genetic Technologies Corporation (research).
A version of this article appeared on Medscape.com.
Older patients prescribed hydroxychloroquine (HCQ) have a higher risk of developing retinal damage from taking the medication, according to a new analysis.
In addition to known risk factors such as a higher weight-based HCQ dose and higher cumulative dose, researchers also found that female sex, chronic kidney disease stage III, and tamoxifen use were associated with HCQ retinopathy.
The findings provide “evidence for other key risk factors for hydroxychloroquine retinopathy beyond hydroxychloroquine exposure itself,” wrote April M. Jorge, MD, of the Division of Rheumatology, Allergy, and Immunology at Massachusetts General Hospital, Boston, and colleagues.
“It is the largest cohort study to date looking specifically at the association of [HCQ] retinopathy with risk factors,” Christina Weng, MD, MBA, professor of ophthalmology at Baylor College of Medicine, Houston, said in an interview. She was not involved with the research. Some of the associations, such as tamoxifen use, “have been suggested before in smaller studies, but never on this scale,” she said.
“It’s provided reinforcement of findings that we have seen from prior research and also some new glimpses into strengthening some associations that were identified, but not yet fully understood, in prior work,” she continued.
Study Details
Researchers identified patients in the Kaiser Permanente Northern California (KPNC), Oakland, California, health system who began taking HCQ between July 1, 1997, and December 14, 2014. To be included, patients needed to have at least 5 years of continuous enrollment in the KPNC system and at least one prescription for HCQ after more than 5 years of starting the drug. Patients were followed from HCQ initiation to their last retinopathy screening study, up to December 31, 2020.
The study was published May 9 in JAMA Network Open.
Of the 4677 users followed for the study, 83% were women, and the average age starting HCQ was 52. Most patients were White (58.1%), while 13.7% were Asian, 10.5% were Black, and 17.7% were Hispanic.
More than 60% of patients had an initial dose > 5 mg/kg/d, though the mean initial dose of HCQ was 4.4 mg/kg/d. After 5 years, only 34.4% of patients were using a daily dose over 5 mg/kg.
Of the entire cohort, 125 patients (2.7%) developed HCQ retinopathy. As expected, cumulative HCQ exposure was associated with a higher retinopathy risk: For every 100 g of HCQ cumulative exposure, risk rose by 64% (hazard ratio [HR], 1.64; 95% CI, 1.44-1.87).
Age was a significant risk factor for retinal damage from HCQ use. Individuals who began taking the drug at 65 years or older were nearly six times more likely to develop retinopathy than those who started HCQ when they were younger than 45. In people aged 55-64 years, this risk was nearly four times higher, and individuals aged 45-54 years when starting the drug were 2.5 times more likely to have retinal damage than those younger than 45.
Other risk factors were female sex (HR, 3.83; 95% CI, 1.86-7.89), chronic kidney disease stage III (HR, 1.95; 95% CI, 1.25-3.04), and tamoxifen use (HR, 3.43; 95% CI, 1.08-10.89), although only 17 patients were taking tamoxifen during the study.
Researchers also found that the type of HCQ retinopathy varied by race. Of the 125 cases in the cohort, 102 had a parafoveal pattern, and 23 had a pericentral pattern. Asian individuals were 15 times more likely, and Black individuals were more than 5 times more likely to develop this pericentral type than were White patients.
This association in Asian patients has also been found in previous studies, Dr. Weng said, and many eye practices now screen their Asian patients with a 30-2 Humphrey visual field — rather than the more commonly used 10-2 — to examine areas farther outside the center.
This study also found this association in Black patients, though only five Black patients developed HCQ retinopathy over the study period.
“More studies and larger studies will be very helpful in strengthening or dispelling some of the associations that have been seen here,” Dr. Weng said.
‘More Room for Personalized Medicine’
The team found a “relatively linear” relationship between HCQ dose and retinopathy risk, with higher daily doses correlating with higher incidence. While 2016 guidelines from the American Academy of Ophthalmology advise using < 5 mg/kg, “what we found is it’s not that straightforward [where there’s] just this one cutoff,” Dr. Jorge told this news organization. “It does seem like the higher the dose of medication per bodyweight and the longer duration of use, there is a higher risk of retinopathy.”
These findings leave “a bit more room for personalized medicine” with patients, she explained. “For an elderly female patient with CKD, aiming for a dose < 5 mg/kg might be more appropriate; however, a young male patient without any additional risk factors may be able to exceed 5 mg/kg and continue to have a low risk for HCQ retinopathy,” she said.
“For anyone, I think it really is more of an individual risk-benefit evaluation,” rather than strict cutoffs, she continued.
“Guidelines are just that: They’re guidelines,” added Dr. Weng, “and treatment plans should be tailored to each individual patient.”
As the study authors also discussed, “the goal is to treat the patient with the lowest dose that is still effective and also be mindful of the duration that a patient is left at higher doses,” Dr. Weng said. “But ultimately, we need to control these diseases, which can cause damage across multiple organ systems in the body. While it’s important to be aware of the potential retinopathy adverse events, we also don’t want physicians to feel restricted in their use of this very effective drug.”
The work of three coauthors on the current study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). Dr. Jorge’s work on the study was supported by an award from the Rheumatology Research Foundation and a grant from NIAMS. Dr. Jorge reported clinical trial agreements with Bristol Myers Squibb and Cabaletta Bio outside of this study. Dr. Weng has served as a consultant for Allergan/AbbVie, Alcon, Apellis Pharmaceuticals, Alimera Sciences, DORC, Novartis, Genentech, Regeneron, RegenxBio, Iveric Bio, and EyePoint Pharmaceuticals. Dr. Weng disclosed financial relationships with Springer Publishers (royalties) and DRCR Retina Network, Alimera Sciences, and Applied Genetic Technologies Corporation (research).
A version of this article appeared on Medscape.com.
Older patients prescribed hydroxychloroquine (HCQ) have a higher risk of developing retinal damage from taking the medication, according to a new analysis.
In addition to known risk factors such as a higher weight-based HCQ dose and higher cumulative dose, researchers also found that female sex, chronic kidney disease stage III, and tamoxifen use were associated with HCQ retinopathy.
The findings provide “evidence for other key risk factors for hydroxychloroquine retinopathy beyond hydroxychloroquine exposure itself,” wrote April M. Jorge, MD, of the Division of Rheumatology, Allergy, and Immunology at Massachusetts General Hospital, Boston, and colleagues.
“It is the largest cohort study to date looking specifically at the association of [HCQ] retinopathy with risk factors,” Christina Weng, MD, MBA, professor of ophthalmology at Baylor College of Medicine, Houston, said in an interview. She was not involved with the research. Some of the associations, such as tamoxifen use, “have been suggested before in smaller studies, but never on this scale,” she said.
“It’s provided reinforcement of findings that we have seen from prior research and also some new glimpses into strengthening some associations that were identified, but not yet fully understood, in prior work,” she continued.
Study Details
Researchers identified patients in the Kaiser Permanente Northern California (KPNC), Oakland, California, health system who began taking HCQ between July 1, 1997, and December 14, 2014. To be included, patients needed to have at least 5 years of continuous enrollment in the KPNC system and at least one prescription for HCQ after more than 5 years of starting the drug. Patients were followed from HCQ initiation to their last retinopathy screening study, up to December 31, 2020.
The study was published May 9 in JAMA Network Open.
Of the 4677 users followed for the study, 83% were women, and the average age starting HCQ was 52. Most patients were White (58.1%), while 13.7% were Asian, 10.5% were Black, and 17.7% were Hispanic.
More than 60% of patients had an initial dose > 5 mg/kg/d, though the mean initial dose of HCQ was 4.4 mg/kg/d. After 5 years, only 34.4% of patients were using a daily dose over 5 mg/kg.
Of the entire cohort, 125 patients (2.7%) developed HCQ retinopathy. As expected, cumulative HCQ exposure was associated with a higher retinopathy risk: For every 100 g of HCQ cumulative exposure, risk rose by 64% (hazard ratio [HR], 1.64; 95% CI, 1.44-1.87).
Age was a significant risk factor for retinal damage from HCQ use. Individuals who began taking the drug at 65 years or older were nearly six times more likely to develop retinopathy than those who started HCQ when they were younger than 45. In people aged 55-64 years, this risk was nearly four times higher, and individuals aged 45-54 years when starting the drug were 2.5 times more likely to have retinal damage than those younger than 45.
Other risk factors were female sex (HR, 3.83; 95% CI, 1.86-7.89), chronic kidney disease stage III (HR, 1.95; 95% CI, 1.25-3.04), and tamoxifen use (HR, 3.43; 95% CI, 1.08-10.89), although only 17 patients were taking tamoxifen during the study.
Researchers also found that the type of HCQ retinopathy varied by race. Of the 125 cases in the cohort, 102 had a parafoveal pattern, and 23 had a pericentral pattern. Asian individuals were 15 times more likely, and Black individuals were more than 5 times more likely to develop this pericentral type than were White patients.
This association in Asian patients has also been found in previous studies, Dr. Weng said, and many eye practices now screen their Asian patients with a 30-2 Humphrey visual field — rather than the more commonly used 10-2 — to examine areas farther outside the center.
This study also found this association in Black patients, though only five Black patients developed HCQ retinopathy over the study period.
“More studies and larger studies will be very helpful in strengthening or dispelling some of the associations that have been seen here,” Dr. Weng said.
‘More Room for Personalized Medicine’
The team found a “relatively linear” relationship between HCQ dose and retinopathy risk, with higher daily doses correlating with higher incidence. While 2016 guidelines from the American Academy of Ophthalmology advise using < 5 mg/kg, “what we found is it’s not that straightforward [where there’s] just this one cutoff,” Dr. Jorge told this news organization. “It does seem like the higher the dose of medication per bodyweight and the longer duration of use, there is a higher risk of retinopathy.”
These findings leave “a bit more room for personalized medicine” with patients, she explained. “For an elderly female patient with CKD, aiming for a dose < 5 mg/kg might be more appropriate; however, a young male patient without any additional risk factors may be able to exceed 5 mg/kg and continue to have a low risk for HCQ retinopathy,” she said.
“For anyone, I think it really is more of an individual risk-benefit evaluation,” rather than strict cutoffs, she continued.
“Guidelines are just that: They’re guidelines,” added Dr. Weng, “and treatment plans should be tailored to each individual patient.”
As the study authors also discussed, “the goal is to treat the patient with the lowest dose that is still effective and also be mindful of the duration that a patient is left at higher doses,” Dr. Weng said. “But ultimately, we need to control these diseases, which can cause damage across multiple organ systems in the body. While it’s important to be aware of the potential retinopathy adverse events, we also don’t want physicians to feel restricted in their use of this very effective drug.”
The work of three coauthors on the current study was supported by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). Dr. Jorge’s work on the study was supported by an award from the Rheumatology Research Foundation and a grant from NIAMS. Dr. Jorge reported clinical trial agreements with Bristol Myers Squibb and Cabaletta Bio outside of this study. Dr. Weng has served as a consultant for Allergan/AbbVie, Alcon, Apellis Pharmaceuticals, Alimera Sciences, DORC, Novartis, Genentech, Regeneron, RegenxBio, Iveric Bio, and EyePoint Pharmaceuticals. Dr. Weng disclosed financial relationships with Springer Publishers (royalties) and DRCR Retina Network, Alimera Sciences, and Applied Genetic Technologies Corporation (research).
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Belimumab Autoinjector Approved for Pediatric Lupus
The US Food and Drug Administration (FDA) has approved Benlysta (belimumab) autoinjector for patients aged 5 years or older with active systemic lupus erythematosus (SLE) on standard therapy. This is the first time that children with SLE can receive this treatment at home, according to a GSK press release.
Prior to this approval, pediatric patients aged 5 years or older could receive belimumab only intravenously via a 1-hour infusion in a hospital or clinic setting.
“Going to the doctor’s office once every 4 weeks can be a logistical hurdle for some children and their caregivers, so having the option to administer Benlysta in the comfort of their home provides much-needed flexibility,” Mary Crimmings, the interim CEO and senior vice president for marketing and communications at the Lupus Foundation of America, said in a statement.
An estimated 5000-10,000 children in the United States are living with SLE.
Belimumab is a B-lymphocyte stimulator–specific inhibitor approved for the treatment of active SLE and active lupus nephritis in patients aged 5 years or older receiving standard therapy. This approval of the subcutaneous administration of belimumab applies only to pediatric patients with SLE.
The 200-mg injection can be administered once every week for children who weigh ≥ 40 kg and should be given once every 2 weeks for children weighing between 15 and 40 kg.
The autoinjector “will be available immediately” for caregivers, the company announcement said.
“Patients are our top priority, and we are always working to innovate solutions that can improve lives and address unmet needs,” Court Horncastle, senior vice president and head of US specialty at GSK, said in the press release. “This approval for an at-home treatment is the first and only of its kind for children with lupus and is a testament to our continued commitment to the lupus community.”
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved Benlysta (belimumab) autoinjector for patients aged 5 years or older with active systemic lupus erythematosus (SLE) on standard therapy. This is the first time that children with SLE can receive this treatment at home, according to a GSK press release.
Prior to this approval, pediatric patients aged 5 years or older could receive belimumab only intravenously via a 1-hour infusion in a hospital or clinic setting.
“Going to the doctor’s office once every 4 weeks can be a logistical hurdle for some children and their caregivers, so having the option to administer Benlysta in the comfort of their home provides much-needed flexibility,” Mary Crimmings, the interim CEO and senior vice president for marketing and communications at the Lupus Foundation of America, said in a statement.
An estimated 5000-10,000 children in the United States are living with SLE.
Belimumab is a B-lymphocyte stimulator–specific inhibitor approved for the treatment of active SLE and active lupus nephritis in patients aged 5 years or older receiving standard therapy. This approval of the subcutaneous administration of belimumab applies only to pediatric patients with SLE.
The 200-mg injection can be administered once every week for children who weigh ≥ 40 kg and should be given once every 2 weeks for children weighing between 15 and 40 kg.
The autoinjector “will be available immediately” for caregivers, the company announcement said.
“Patients are our top priority, and we are always working to innovate solutions that can improve lives and address unmet needs,” Court Horncastle, senior vice president and head of US specialty at GSK, said in the press release. “This approval for an at-home treatment is the first and only of its kind for children with lupus and is a testament to our continued commitment to the lupus community.”
A version of this article appeared on Medscape.com.
The US Food and Drug Administration (FDA) has approved Benlysta (belimumab) autoinjector for patients aged 5 years or older with active systemic lupus erythematosus (SLE) on standard therapy. This is the first time that children with SLE can receive this treatment at home, according to a GSK press release.
Prior to this approval, pediatric patients aged 5 years or older could receive belimumab only intravenously via a 1-hour infusion in a hospital or clinic setting.
“Going to the doctor’s office once every 4 weeks can be a logistical hurdle for some children and their caregivers, so having the option to administer Benlysta in the comfort of their home provides much-needed flexibility,” Mary Crimmings, the interim CEO and senior vice president for marketing and communications at the Lupus Foundation of America, said in a statement.
An estimated 5000-10,000 children in the United States are living with SLE.
Belimumab is a B-lymphocyte stimulator–specific inhibitor approved for the treatment of active SLE and active lupus nephritis in patients aged 5 years or older receiving standard therapy. This approval of the subcutaneous administration of belimumab applies only to pediatric patients with SLE.
The 200-mg injection can be administered once every week for children who weigh ≥ 40 kg and should be given once every 2 weeks for children weighing between 15 and 40 kg.
The autoinjector “will be available immediately” for caregivers, the company announcement said.
“Patients are our top priority, and we are always working to innovate solutions that can improve lives and address unmet needs,” Court Horncastle, senior vice president and head of US specialty at GSK, said in the press release. “This approval for an at-home treatment is the first and only of its kind for children with lupus and is a testament to our continued commitment to the lupus community.”
A version of this article appeared on Medscape.com.
Diagnosing Giant Cell Arteritis Using Ultrasound First Proves Accurate, Avoids Biopsy in Many Cases
Temporal artery ultrasound alone was sufficient to accurately diagnose giant cell arteritis (GCA) in over half of patients in a new prospective study.
The findings provide further evidence that “[ultrasound] of temporal arteries could really take the place of traditional temporal artery biopsy (TAB)” in patients with high clinical suspicion of GCA, lead author Guillaume Denis, MD, of the Centre Hospitalier de Rochefort in Rochefort, France, told this news organization.
The European Alliance of Associations for Rheumatology (EULAR) already recommends ultrasound as a first-line diagnostic tool for patients with suspected large vessel vasculitis, and the 2022 American College of Rheumatology (ACR)/EULAR classification criteria for GCA weighs positive TAB or temporal artery halo sign on ultrasound equally.
Guidelines from the ACR and the Vasculitis Foundation still recommend TAB over ultrasound.
“In general, rheumatologists and radiologists in the US are less experienced in using ultrasound to diagnose temporal artery involvement in GCA compared to their counterparts in Europe,” the 2021 guidelines stated. “In centers with appropriate training and expertise in using temporal artery ultrasound, ultrasound may be a useful and complementary tool for diagnosing GCA.”
Methodology
In the study, researchers recruited 165 individuals with high clinical suspicion of GCA from August 2016 through February 2020 at six French hospitals. Only patients older than 50 years of age and with biologic inflammatory syndrome with C-reactive protein elevation (≥ 6 mg/L) qualified for the study. Patients also needed to have at least one of these factors:
- Clinical signs of GCA (abnormal temporal arteries, scalp hyperesthesia, jaw claudication, or vision loss)
- General signs of GCA (headache, fever, or impaired general condition)
- Large-vessel vasculitis visible on imaging (CT angiography [CTA], MR angiography [MRA], and/or PET/CT)
All participants underwent a color Doppler ultrasound of the temporal artery, performed less than 1 week after the initiation of corticosteroid therapy. (Previous research demonstrated that corticosteroids can change the hallmark halo sign of vasculitis detectable via ultrasound as early as 1 week after initiation of therapy, the authors noted.) In this study, the time between consultation with a specialist and ultrasound was less than 1 day.
“Patients with halo signs detected around the lumen of both temporal arteries (that is, bilateral temporal halo sign) were considered as ultrasound-positive,” Guillaume Denis, MD, and colleagues explained. “Patients with no halo sign, or bilateral halo signs in the axillary arteries, or a unilateral halo sign in the temporal artery were considered as ultrasound-negative.”
The findings were published in Annals of Internal Medicine on May 7.
Results
In total, 73 participants (44%) had positive ultrasounds and were diagnosed with GCA. These patients also underwent a second ultrasound a month later to document if the halo sign remained unchanged, reduced, or disappeared.
The remaining 92 patients with negative ultrasound results underwent TAB, which was conducted on average 4.5 days after the ultrasound. A total of 28 patients (30%) had a positive TAB result. Physicians diagnosed 35 TAB-negative patients with GCA using clinical, imaging, and biologic data, and 29 patients received alternative diagnoses. These other diagnoses included polymyalgia rheumatica, infectious diseases, cancer, and other systemic inflammatory rheumatic diseases.
All patients diagnosed with GCA via ultrasound had their diagnoses reconfirmed at 1 month and for up to 2 years of follow-up.
“In summary, our study showed that the use of temporal artery ultrasound may be an efficient way to make the diagnosis of GCA in patients with high clinical suspicion and to reduce imaging costs and the need for biopsy, thereby limiting complications and the need for a surgeon,” the authors concluded.
Qualifications and Limitations
While over half of patients ultimately diagnosed with GCA were diagnosed using ultrasound, that percentage was “a bit lower than expected,” said Mark Matza, MD, MBA, the co-clinical director of rheumatology at Massachusetts General Hospital in Boston. By comparison, one systematic review calculated ultrasound’s pooled sensitivity at 88% and pooled specificity at 96% for the diagnosis of GCA.
“In this [current] study, 30% of patients who had negative ultrasound were then found to have positive biopsy, indicating that ultrasound missed a substantial portion of patients who were ultimately diagnosed with GCA,” he continued.
Ultrasound is “very operator dependent,” he added, and there has been “variability in test performance of ultrasound.”
The authors acknowledged that techniques for ultrasound of the temporal arteries have also evolved over the study period, and thus, findings may not have been consistent.
However, about one in four patients with GCA were diagnosed after having both negative ultrasound and TAB results.
“One of the things that this paper shows is that even the gold standard of temporal artery biopsy isn’t 100% either,” noted Minna Kohler, MD, who directs the rheumatology musculoskeletal ultrasound program at Massachusetts General Hospital. “That’s why clinically, there is an increasing emphasis on using multimodality imaging to assist in the diagnosis of GCA along with a physician’s clinical intuition,” she said.
While ultrasound can visualize axillary, subclavian, and carotid arteries, other imaging modalities such as CTA, MRA, and PET/CT are better to fully assess supra-aortic and aortic vessels, she continued. However, “this imaging is more expensive and takes more time to coordinate, schedule, whereas ultrasound of temporal and axillary arteries can easily be done within the clinic with an immediate answer.”
This study was supported by a grant from “Recherche CH-CHU Poitou-Charentes 2014.” Dr. Denis disclosed relationships with Leo Pharma, Janssen, Novartis, Takeda, and Sanofi. Dr. Matza reported honoraria from the Ultrasound School of North American Rheumatologists. Kohler had no relevant disclosures.
A version of this article appeared on Medscape.com.
Temporal artery ultrasound alone was sufficient to accurately diagnose giant cell arteritis (GCA) in over half of patients in a new prospective study.
The findings provide further evidence that “[ultrasound] of temporal arteries could really take the place of traditional temporal artery biopsy (TAB)” in patients with high clinical suspicion of GCA, lead author Guillaume Denis, MD, of the Centre Hospitalier de Rochefort in Rochefort, France, told this news organization.
The European Alliance of Associations for Rheumatology (EULAR) already recommends ultrasound as a first-line diagnostic tool for patients with suspected large vessel vasculitis, and the 2022 American College of Rheumatology (ACR)/EULAR classification criteria for GCA weighs positive TAB or temporal artery halo sign on ultrasound equally.
Guidelines from the ACR and the Vasculitis Foundation still recommend TAB over ultrasound.
“In general, rheumatologists and radiologists in the US are less experienced in using ultrasound to diagnose temporal artery involvement in GCA compared to their counterparts in Europe,” the 2021 guidelines stated. “In centers with appropriate training and expertise in using temporal artery ultrasound, ultrasound may be a useful and complementary tool for diagnosing GCA.”
Methodology
In the study, researchers recruited 165 individuals with high clinical suspicion of GCA from August 2016 through February 2020 at six French hospitals. Only patients older than 50 years of age and with biologic inflammatory syndrome with C-reactive protein elevation (≥ 6 mg/L) qualified for the study. Patients also needed to have at least one of these factors:
- Clinical signs of GCA (abnormal temporal arteries, scalp hyperesthesia, jaw claudication, or vision loss)
- General signs of GCA (headache, fever, or impaired general condition)
- Large-vessel vasculitis visible on imaging (CT angiography [CTA], MR angiography [MRA], and/or PET/CT)
All participants underwent a color Doppler ultrasound of the temporal artery, performed less than 1 week after the initiation of corticosteroid therapy. (Previous research demonstrated that corticosteroids can change the hallmark halo sign of vasculitis detectable via ultrasound as early as 1 week after initiation of therapy, the authors noted.) In this study, the time between consultation with a specialist and ultrasound was less than 1 day.
“Patients with halo signs detected around the lumen of both temporal arteries (that is, bilateral temporal halo sign) were considered as ultrasound-positive,” Guillaume Denis, MD, and colleagues explained. “Patients with no halo sign, or bilateral halo signs in the axillary arteries, or a unilateral halo sign in the temporal artery were considered as ultrasound-negative.”
The findings were published in Annals of Internal Medicine on May 7.
Results
In total, 73 participants (44%) had positive ultrasounds and were diagnosed with GCA. These patients also underwent a second ultrasound a month later to document if the halo sign remained unchanged, reduced, or disappeared.
The remaining 92 patients with negative ultrasound results underwent TAB, which was conducted on average 4.5 days after the ultrasound. A total of 28 patients (30%) had a positive TAB result. Physicians diagnosed 35 TAB-negative patients with GCA using clinical, imaging, and biologic data, and 29 patients received alternative diagnoses. These other diagnoses included polymyalgia rheumatica, infectious diseases, cancer, and other systemic inflammatory rheumatic diseases.
All patients diagnosed with GCA via ultrasound had their diagnoses reconfirmed at 1 month and for up to 2 years of follow-up.
“In summary, our study showed that the use of temporal artery ultrasound may be an efficient way to make the diagnosis of GCA in patients with high clinical suspicion and to reduce imaging costs and the need for biopsy, thereby limiting complications and the need for a surgeon,” the authors concluded.
Qualifications and Limitations
While over half of patients ultimately diagnosed with GCA were diagnosed using ultrasound, that percentage was “a bit lower than expected,” said Mark Matza, MD, MBA, the co-clinical director of rheumatology at Massachusetts General Hospital in Boston. By comparison, one systematic review calculated ultrasound’s pooled sensitivity at 88% and pooled specificity at 96% for the diagnosis of GCA.
“In this [current] study, 30% of patients who had negative ultrasound were then found to have positive biopsy, indicating that ultrasound missed a substantial portion of patients who were ultimately diagnosed with GCA,” he continued.
Ultrasound is “very operator dependent,” he added, and there has been “variability in test performance of ultrasound.”
The authors acknowledged that techniques for ultrasound of the temporal arteries have also evolved over the study period, and thus, findings may not have been consistent.
However, about one in four patients with GCA were diagnosed after having both negative ultrasound and TAB results.
“One of the things that this paper shows is that even the gold standard of temporal artery biopsy isn’t 100% either,” noted Minna Kohler, MD, who directs the rheumatology musculoskeletal ultrasound program at Massachusetts General Hospital. “That’s why clinically, there is an increasing emphasis on using multimodality imaging to assist in the diagnosis of GCA along with a physician’s clinical intuition,” she said.
While ultrasound can visualize axillary, subclavian, and carotid arteries, other imaging modalities such as CTA, MRA, and PET/CT are better to fully assess supra-aortic and aortic vessels, she continued. However, “this imaging is more expensive and takes more time to coordinate, schedule, whereas ultrasound of temporal and axillary arteries can easily be done within the clinic with an immediate answer.”
This study was supported by a grant from “Recherche CH-CHU Poitou-Charentes 2014.” Dr. Denis disclosed relationships with Leo Pharma, Janssen, Novartis, Takeda, and Sanofi. Dr. Matza reported honoraria from the Ultrasound School of North American Rheumatologists. Kohler had no relevant disclosures.
A version of this article appeared on Medscape.com.
Temporal artery ultrasound alone was sufficient to accurately diagnose giant cell arteritis (GCA) in over half of patients in a new prospective study.
The findings provide further evidence that “[ultrasound] of temporal arteries could really take the place of traditional temporal artery biopsy (TAB)” in patients with high clinical suspicion of GCA, lead author Guillaume Denis, MD, of the Centre Hospitalier de Rochefort in Rochefort, France, told this news organization.
The European Alliance of Associations for Rheumatology (EULAR) already recommends ultrasound as a first-line diagnostic tool for patients with suspected large vessel vasculitis, and the 2022 American College of Rheumatology (ACR)/EULAR classification criteria for GCA weighs positive TAB or temporal artery halo sign on ultrasound equally.
Guidelines from the ACR and the Vasculitis Foundation still recommend TAB over ultrasound.
“In general, rheumatologists and radiologists in the US are less experienced in using ultrasound to diagnose temporal artery involvement in GCA compared to their counterparts in Europe,” the 2021 guidelines stated. “In centers with appropriate training and expertise in using temporal artery ultrasound, ultrasound may be a useful and complementary tool for diagnosing GCA.”
Methodology
In the study, researchers recruited 165 individuals with high clinical suspicion of GCA from August 2016 through February 2020 at six French hospitals. Only patients older than 50 years of age and with biologic inflammatory syndrome with C-reactive protein elevation (≥ 6 mg/L) qualified for the study. Patients also needed to have at least one of these factors:
- Clinical signs of GCA (abnormal temporal arteries, scalp hyperesthesia, jaw claudication, or vision loss)
- General signs of GCA (headache, fever, or impaired general condition)
- Large-vessel vasculitis visible on imaging (CT angiography [CTA], MR angiography [MRA], and/or PET/CT)
All participants underwent a color Doppler ultrasound of the temporal artery, performed less than 1 week after the initiation of corticosteroid therapy. (Previous research demonstrated that corticosteroids can change the hallmark halo sign of vasculitis detectable via ultrasound as early as 1 week after initiation of therapy, the authors noted.) In this study, the time between consultation with a specialist and ultrasound was less than 1 day.
“Patients with halo signs detected around the lumen of both temporal arteries (that is, bilateral temporal halo sign) were considered as ultrasound-positive,” Guillaume Denis, MD, and colleagues explained. “Patients with no halo sign, or bilateral halo signs in the axillary arteries, or a unilateral halo sign in the temporal artery were considered as ultrasound-negative.”
The findings were published in Annals of Internal Medicine on May 7.
Results
In total, 73 participants (44%) had positive ultrasounds and were diagnosed with GCA. These patients also underwent a second ultrasound a month later to document if the halo sign remained unchanged, reduced, or disappeared.
The remaining 92 patients with negative ultrasound results underwent TAB, which was conducted on average 4.5 days after the ultrasound. A total of 28 patients (30%) had a positive TAB result. Physicians diagnosed 35 TAB-negative patients with GCA using clinical, imaging, and biologic data, and 29 patients received alternative diagnoses. These other diagnoses included polymyalgia rheumatica, infectious diseases, cancer, and other systemic inflammatory rheumatic diseases.
All patients diagnosed with GCA via ultrasound had their diagnoses reconfirmed at 1 month and for up to 2 years of follow-up.
“In summary, our study showed that the use of temporal artery ultrasound may be an efficient way to make the diagnosis of GCA in patients with high clinical suspicion and to reduce imaging costs and the need for biopsy, thereby limiting complications and the need for a surgeon,” the authors concluded.
Qualifications and Limitations
While over half of patients ultimately diagnosed with GCA were diagnosed using ultrasound, that percentage was “a bit lower than expected,” said Mark Matza, MD, MBA, the co-clinical director of rheumatology at Massachusetts General Hospital in Boston. By comparison, one systematic review calculated ultrasound’s pooled sensitivity at 88% and pooled specificity at 96% for the diagnosis of GCA.
“In this [current] study, 30% of patients who had negative ultrasound were then found to have positive biopsy, indicating that ultrasound missed a substantial portion of patients who were ultimately diagnosed with GCA,” he continued.
Ultrasound is “very operator dependent,” he added, and there has been “variability in test performance of ultrasound.”
The authors acknowledged that techniques for ultrasound of the temporal arteries have also evolved over the study period, and thus, findings may not have been consistent.
However, about one in four patients with GCA were diagnosed after having both negative ultrasound and TAB results.
“One of the things that this paper shows is that even the gold standard of temporal artery biopsy isn’t 100% either,” noted Minna Kohler, MD, who directs the rheumatology musculoskeletal ultrasound program at Massachusetts General Hospital. “That’s why clinically, there is an increasing emphasis on using multimodality imaging to assist in the diagnosis of GCA along with a physician’s clinical intuition,” she said.
While ultrasound can visualize axillary, subclavian, and carotid arteries, other imaging modalities such as CTA, MRA, and PET/CT are better to fully assess supra-aortic and aortic vessels, she continued. However, “this imaging is more expensive and takes more time to coordinate, schedule, whereas ultrasound of temporal and axillary arteries can easily be done within the clinic with an immediate answer.”
This study was supported by a grant from “Recherche CH-CHU Poitou-Charentes 2014.” Dr. Denis disclosed relationships with Leo Pharma, Janssen, Novartis, Takeda, and Sanofi. Dr. Matza reported honoraria from the Ultrasound School of North American Rheumatologists. Kohler had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Blood Biomarkers Predict Knee Osteoarthritis Years in Advance
A small number of blood biomarkers can identify patients who will develop knee osteoarthritis (OA) up to 8 years before signs of the disease are detectable via X-ray, according to new research.
The study “provides more evidence for a pre-radiographic phase of disease,” wrote Virginia Byers Dr. Kraus, MD, PhD, a professor of medicine, pathology, and orthopedic surgery at Duke University School of Medicine in Durham, North Carolina, and colleagues. The results also “provide valuable information for understanding the molecular events of early disease that could inform strategies to develop disease-modifying drugs for preclinical OA,” they continued.
In the study, published in Science Advances, researchers analyzed blood samples from a population-based, longitudinal study of women in London that assessed participants annually for osteoporosis and OA. They selected individuals at low risk for radiographic knee OA, who did not have traditional risk factors for knee OA such as a history of major knee injury, knee surgery, or OA of the hand or opposite knee.
The researchers analyzed serum of 100 women who went on to develop radiographic knee OA and 100 controls who were matched by age and body mass index (BMI). Participants were, on average, aged 54 years with a BMI of 26 and all were White. They analyzed serum peptides via mass spectrometry and used machine learning to select which out of the 115 identified peptides were most predictive of OA.
Ultimately, the team zeroed in on six peptides, corresponding to six proteins, that could most accurately distinguish women who went on to develop radiographic signs of OA from controls (area under the receiver operating characteristic curve, 0.77) up to 8 years before x-rays detected these changes.
“The value of our study is a panel that, in the absence of clinical factors indicative of high-risk knee OA, has the potential to discriminate individuals at risk for incident radiographic knee OA from those not at risk,” the authors wrote.
In earlier work, a similar group of biomarkers could accurately diagnose knee OA as well as predict the progression of the disease. More than half (58%) of biomarkers that predicted incident OA also predicted OA progression.
“Even for the ones that didn’t overlap with OA progression, they all pointed to the same sort of disease process, which is an unresolved acute phase response type of biological process,” Dr. Kraus told this news organization.
Commenting on the study, Andrew Grose, MD, an orthopedic trauma surgeon at the Hospital for Special Surgery in New York City, noted that the methods and conclusions seemed sound but cautioned that the study only looked for radiographic evidence of OA, and not symptomatic OA.
“Clinically relevant OA is correlated with what you see on an x-ray, but the x-ray is definitely not the whole story,” he said in an interview with this news organization.
To be clinically relevant, patients must also have symptoms, such as pain and stiffness, and interfere with daily life. But what shows up on an x-ray is not necessarily indicative of what patients are experiencing, he said. Solely focusing on radiographic findings could lead to overdiagnosis and overtreatment of OA, he said.
The study population was also small, and only included White women, he added, so further validation is necessary. Dr. Kraus and colleagues also acknowledged these limitations.
“Further validation will be needed in independent and larger cohorts, preferability prospectively collected and including male participants and the combination of incident radiographic and symptomatic OA,” they wrote. They noted that while this current study included only women, the biomarkers were not associated with sex in previous studies that used larger and mixed-sex cohorts.
“If they did more studies showing that this [test] was able to predict clinically relevant OA, then I think you could have a meaningful conversation with a patient in a primary care doctor’s office,” Dr. Grose added. “Until that time, just the fact that it predicts an x-ray finding is a little bit of a red herring.”
This work was supported by grants from the National Institutes of Health. Dr. Kraus is an inventor on a patent related to OA progression biomarkers. Dr. Grose had no relevant disclosures.
A version of this article appeared on Medscape.com.
A small number of blood biomarkers can identify patients who will develop knee osteoarthritis (OA) up to 8 years before signs of the disease are detectable via X-ray, according to new research.
The study “provides more evidence for a pre-radiographic phase of disease,” wrote Virginia Byers Dr. Kraus, MD, PhD, a professor of medicine, pathology, and orthopedic surgery at Duke University School of Medicine in Durham, North Carolina, and colleagues. The results also “provide valuable information for understanding the molecular events of early disease that could inform strategies to develop disease-modifying drugs for preclinical OA,” they continued.
In the study, published in Science Advances, researchers analyzed blood samples from a population-based, longitudinal study of women in London that assessed participants annually for osteoporosis and OA. They selected individuals at low risk for radiographic knee OA, who did not have traditional risk factors for knee OA such as a history of major knee injury, knee surgery, or OA of the hand or opposite knee.
The researchers analyzed serum of 100 women who went on to develop radiographic knee OA and 100 controls who were matched by age and body mass index (BMI). Participants were, on average, aged 54 years with a BMI of 26 and all were White. They analyzed serum peptides via mass spectrometry and used machine learning to select which out of the 115 identified peptides were most predictive of OA.
Ultimately, the team zeroed in on six peptides, corresponding to six proteins, that could most accurately distinguish women who went on to develop radiographic signs of OA from controls (area under the receiver operating characteristic curve, 0.77) up to 8 years before x-rays detected these changes.
“The value of our study is a panel that, in the absence of clinical factors indicative of high-risk knee OA, has the potential to discriminate individuals at risk for incident radiographic knee OA from those not at risk,” the authors wrote.
In earlier work, a similar group of biomarkers could accurately diagnose knee OA as well as predict the progression of the disease. More than half (58%) of biomarkers that predicted incident OA also predicted OA progression.
“Even for the ones that didn’t overlap with OA progression, they all pointed to the same sort of disease process, which is an unresolved acute phase response type of biological process,” Dr. Kraus told this news organization.
Commenting on the study, Andrew Grose, MD, an orthopedic trauma surgeon at the Hospital for Special Surgery in New York City, noted that the methods and conclusions seemed sound but cautioned that the study only looked for radiographic evidence of OA, and not symptomatic OA.
“Clinically relevant OA is correlated with what you see on an x-ray, but the x-ray is definitely not the whole story,” he said in an interview with this news organization.
To be clinically relevant, patients must also have symptoms, such as pain and stiffness, and interfere with daily life. But what shows up on an x-ray is not necessarily indicative of what patients are experiencing, he said. Solely focusing on radiographic findings could lead to overdiagnosis and overtreatment of OA, he said.
The study population was also small, and only included White women, he added, so further validation is necessary. Dr. Kraus and colleagues also acknowledged these limitations.
“Further validation will be needed in independent and larger cohorts, preferability prospectively collected and including male participants and the combination of incident radiographic and symptomatic OA,” they wrote. They noted that while this current study included only women, the biomarkers were not associated with sex in previous studies that used larger and mixed-sex cohorts.
“If they did more studies showing that this [test] was able to predict clinically relevant OA, then I think you could have a meaningful conversation with a patient in a primary care doctor’s office,” Dr. Grose added. “Until that time, just the fact that it predicts an x-ray finding is a little bit of a red herring.”
This work was supported by grants from the National Institutes of Health. Dr. Kraus is an inventor on a patent related to OA progression biomarkers. Dr. Grose had no relevant disclosures.
A version of this article appeared on Medscape.com.
A small number of blood biomarkers can identify patients who will develop knee osteoarthritis (OA) up to 8 years before signs of the disease are detectable via X-ray, according to new research.
The study “provides more evidence for a pre-radiographic phase of disease,” wrote Virginia Byers Dr. Kraus, MD, PhD, a professor of medicine, pathology, and orthopedic surgery at Duke University School of Medicine in Durham, North Carolina, and colleagues. The results also “provide valuable information for understanding the molecular events of early disease that could inform strategies to develop disease-modifying drugs for preclinical OA,” they continued.
In the study, published in Science Advances, researchers analyzed blood samples from a population-based, longitudinal study of women in London that assessed participants annually for osteoporosis and OA. They selected individuals at low risk for radiographic knee OA, who did not have traditional risk factors for knee OA such as a history of major knee injury, knee surgery, or OA of the hand or opposite knee.
The researchers analyzed serum of 100 women who went on to develop radiographic knee OA and 100 controls who were matched by age and body mass index (BMI). Participants were, on average, aged 54 years with a BMI of 26 and all were White. They analyzed serum peptides via mass spectrometry and used machine learning to select which out of the 115 identified peptides were most predictive of OA.
Ultimately, the team zeroed in on six peptides, corresponding to six proteins, that could most accurately distinguish women who went on to develop radiographic signs of OA from controls (area under the receiver operating characteristic curve, 0.77) up to 8 years before x-rays detected these changes.
“The value of our study is a panel that, in the absence of clinical factors indicative of high-risk knee OA, has the potential to discriminate individuals at risk for incident radiographic knee OA from those not at risk,” the authors wrote.
In earlier work, a similar group of biomarkers could accurately diagnose knee OA as well as predict the progression of the disease. More than half (58%) of biomarkers that predicted incident OA also predicted OA progression.
“Even for the ones that didn’t overlap with OA progression, they all pointed to the same sort of disease process, which is an unresolved acute phase response type of biological process,” Dr. Kraus told this news organization.
Commenting on the study, Andrew Grose, MD, an orthopedic trauma surgeon at the Hospital for Special Surgery in New York City, noted that the methods and conclusions seemed sound but cautioned that the study only looked for radiographic evidence of OA, and not symptomatic OA.
“Clinically relevant OA is correlated with what you see on an x-ray, but the x-ray is definitely not the whole story,” he said in an interview with this news organization.
To be clinically relevant, patients must also have symptoms, such as pain and stiffness, and interfere with daily life. But what shows up on an x-ray is not necessarily indicative of what patients are experiencing, he said. Solely focusing on radiographic findings could lead to overdiagnosis and overtreatment of OA, he said.
The study population was also small, and only included White women, he added, so further validation is necessary. Dr. Kraus and colleagues also acknowledged these limitations.
“Further validation will be needed in independent and larger cohorts, preferability prospectively collected and including male participants and the combination of incident radiographic and symptomatic OA,” they wrote. They noted that while this current study included only women, the biomarkers were not associated with sex in previous studies that used larger and mixed-sex cohorts.
“If they did more studies showing that this [test] was able to predict clinically relevant OA, then I think you could have a meaningful conversation with a patient in a primary care doctor’s office,” Dr. Grose added. “Until that time, just the fact that it predicts an x-ray finding is a little bit of a red herring.”
This work was supported by grants from the National Institutes of Health. Dr. Kraus is an inventor on a patent related to OA progression biomarkers. Dr. Grose had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SCIENCE ADVANCES