User login
Tofacitinib may have possible protective effect against ILD in RA
Patients with rheumatoid arthritis treated with tofacitinib (Xeljanz) were 69% less likely to develop interstitial lung disease (ILD), compared with those treated with adalimumab (Humira), according to a new retrospective study.
About 10% of RA patients develop ILD, but data on how different biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) may affect the risk of developing ILD is lacking, the authors wrote. Identifying treatments that may have protective effects could be useful when prescribing treatments for patients with RA who are at higher risk for ILD, first author Matthew C. Baker, MD, clinical chief in the division of immunology and rheumatology at Stanford (Calif.) University, said in an interview.
In the analysis, published in JAMA Network Open researchers used the Optum Clinformatics Data Mart to identify claims data for patients with RA who were taking b/tsDMARDs from December 2003 to December 2019. Patients were excluded if they had a preexisting diagnosis of ILD or if they had less than 1 year of continuous enrollment in the data set.
The researchers identified 28,559 patients with RA who were treated with adalimumab (13,326), abatacept (Orencia; 5,676), rituximab (Rituxan; 5,444), tocilizumab (Actemra; 2,548), and tofacitinib (1,565). More than three-fourths of patients were female (78%), and their average age was 55.6 years old. During the study period, 276 developed ILD. An adjusted model showed a 69% lower incidence of ILD in patients treated with tofacitinib, compared with those treated with adalimumab (adjusted hazard ratio, 0.31; 95% confidence interval, 0.12-0.78; P = .009). An additional sensitivity analysis also showed a similar reduction in ILD risk in those taking tofacitinib, compared with adalimumab (aHR, 0.32; 95% CI, 0.13-0.82; P < .001). There was no significant difference in risk of developing ILD in the abatacept, rituximab, or tocilizumab groups, compared with the adalimumab group.
“Patients who generally looked similar with RA, but were given different treatments, had different risks of developing ILD,” Dr. Baker said. “Based on what we found, most of the biologic therapies had similar rates of developing ILD, but the JAK inhibitor tofacitinib had a reduced risk.” Additional research is necessary to see if tofacitinib shows the same benefit in prospective studies, he said.
“Even though this wasn’t a clinical trial, it suggested that one of the medications that we use to treat RA could potentially prevent the development of ILD,” Elizabeth Volkmann, MD, codirector of the Connective Tissue Disease-Related Interstitial Lung Disease Program at the University of California, Los Angeles, told this news organization. She was not involved with the study.
With retrospective studies, it is difficult to account for all confounding factors, even with adjusted models, she said. For example, the authors did not have data on patients’ history of smoking, a known risk factor for ILD that could have affected which treatment was selected, they acknowledged. The tofacitinib group was also smaller than other treatment groups, which “may have contributed to a small number of events,” the authors wrote. “However, the follow-up time was similar across all groups, and we used Cox proportional hazard models to investigate the association between time-to-event and use of treatment while controlling for the other baseline characteristics.”
Both Dr. Baker and Dr. Volkmann agreed that future research could also investigate whether tofacitinib prevents the progression of ILD in patients with RA who already have the lung condition. “That’s never been looked at before,” Dr. Volkmann said.
Dr. Baker and a coauthor received support for this work from grants from the National Institutes of Health. Dr. Baker and Dr. Volkmann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with rheumatoid arthritis treated with tofacitinib (Xeljanz) were 69% less likely to develop interstitial lung disease (ILD), compared with those treated with adalimumab (Humira), according to a new retrospective study.
About 10% of RA patients develop ILD, but data on how different biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) may affect the risk of developing ILD is lacking, the authors wrote. Identifying treatments that may have protective effects could be useful when prescribing treatments for patients with RA who are at higher risk for ILD, first author Matthew C. Baker, MD, clinical chief in the division of immunology and rheumatology at Stanford (Calif.) University, said in an interview.
In the analysis, published in JAMA Network Open researchers used the Optum Clinformatics Data Mart to identify claims data for patients with RA who were taking b/tsDMARDs from December 2003 to December 2019. Patients were excluded if they had a preexisting diagnosis of ILD or if they had less than 1 year of continuous enrollment in the data set.
The researchers identified 28,559 patients with RA who were treated with adalimumab (13,326), abatacept (Orencia; 5,676), rituximab (Rituxan; 5,444), tocilizumab (Actemra; 2,548), and tofacitinib (1,565). More than three-fourths of patients were female (78%), and their average age was 55.6 years old. During the study period, 276 developed ILD. An adjusted model showed a 69% lower incidence of ILD in patients treated with tofacitinib, compared with those treated with adalimumab (adjusted hazard ratio, 0.31; 95% confidence interval, 0.12-0.78; P = .009). An additional sensitivity analysis also showed a similar reduction in ILD risk in those taking tofacitinib, compared with adalimumab (aHR, 0.32; 95% CI, 0.13-0.82; P < .001). There was no significant difference in risk of developing ILD in the abatacept, rituximab, or tocilizumab groups, compared with the adalimumab group.
“Patients who generally looked similar with RA, but were given different treatments, had different risks of developing ILD,” Dr. Baker said. “Based on what we found, most of the biologic therapies had similar rates of developing ILD, but the JAK inhibitor tofacitinib had a reduced risk.” Additional research is necessary to see if tofacitinib shows the same benefit in prospective studies, he said.
“Even though this wasn’t a clinical trial, it suggested that one of the medications that we use to treat RA could potentially prevent the development of ILD,” Elizabeth Volkmann, MD, codirector of the Connective Tissue Disease-Related Interstitial Lung Disease Program at the University of California, Los Angeles, told this news organization. She was not involved with the study.
With retrospective studies, it is difficult to account for all confounding factors, even with adjusted models, she said. For example, the authors did not have data on patients’ history of smoking, a known risk factor for ILD that could have affected which treatment was selected, they acknowledged. The tofacitinib group was also smaller than other treatment groups, which “may have contributed to a small number of events,” the authors wrote. “However, the follow-up time was similar across all groups, and we used Cox proportional hazard models to investigate the association between time-to-event and use of treatment while controlling for the other baseline characteristics.”
Both Dr. Baker and Dr. Volkmann agreed that future research could also investigate whether tofacitinib prevents the progression of ILD in patients with RA who already have the lung condition. “That’s never been looked at before,” Dr. Volkmann said.
Dr. Baker and a coauthor received support for this work from grants from the National Institutes of Health. Dr. Baker and Dr. Volkmann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with rheumatoid arthritis treated with tofacitinib (Xeljanz) were 69% less likely to develop interstitial lung disease (ILD), compared with those treated with adalimumab (Humira), according to a new retrospective study.
About 10% of RA patients develop ILD, but data on how different biologic and targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) may affect the risk of developing ILD is lacking, the authors wrote. Identifying treatments that may have protective effects could be useful when prescribing treatments for patients with RA who are at higher risk for ILD, first author Matthew C. Baker, MD, clinical chief in the division of immunology and rheumatology at Stanford (Calif.) University, said in an interview.
In the analysis, published in JAMA Network Open researchers used the Optum Clinformatics Data Mart to identify claims data for patients with RA who were taking b/tsDMARDs from December 2003 to December 2019. Patients were excluded if they had a preexisting diagnosis of ILD or if they had less than 1 year of continuous enrollment in the data set.
The researchers identified 28,559 patients with RA who were treated with adalimumab (13,326), abatacept (Orencia; 5,676), rituximab (Rituxan; 5,444), tocilizumab (Actemra; 2,548), and tofacitinib (1,565). More than three-fourths of patients were female (78%), and their average age was 55.6 years old. During the study period, 276 developed ILD. An adjusted model showed a 69% lower incidence of ILD in patients treated with tofacitinib, compared with those treated with adalimumab (adjusted hazard ratio, 0.31; 95% confidence interval, 0.12-0.78; P = .009). An additional sensitivity analysis also showed a similar reduction in ILD risk in those taking tofacitinib, compared with adalimumab (aHR, 0.32; 95% CI, 0.13-0.82; P < .001). There was no significant difference in risk of developing ILD in the abatacept, rituximab, or tocilizumab groups, compared with the adalimumab group.
“Patients who generally looked similar with RA, but were given different treatments, had different risks of developing ILD,” Dr. Baker said. “Based on what we found, most of the biologic therapies had similar rates of developing ILD, but the JAK inhibitor tofacitinib had a reduced risk.” Additional research is necessary to see if tofacitinib shows the same benefit in prospective studies, he said.
“Even though this wasn’t a clinical trial, it suggested that one of the medications that we use to treat RA could potentially prevent the development of ILD,” Elizabeth Volkmann, MD, codirector of the Connective Tissue Disease-Related Interstitial Lung Disease Program at the University of California, Los Angeles, told this news organization. She was not involved with the study.
With retrospective studies, it is difficult to account for all confounding factors, even with adjusted models, she said. For example, the authors did not have data on patients’ history of smoking, a known risk factor for ILD that could have affected which treatment was selected, they acknowledged. The tofacitinib group was also smaller than other treatment groups, which “may have contributed to a small number of events,” the authors wrote. “However, the follow-up time was similar across all groups, and we used Cox proportional hazard models to investigate the association between time-to-event and use of treatment while controlling for the other baseline characteristics.”
Both Dr. Baker and Dr. Volkmann agreed that future research could also investigate whether tofacitinib prevents the progression of ILD in patients with RA who already have the lung condition. “That’s never been looked at before,” Dr. Volkmann said.
Dr. Baker and a coauthor received support for this work from grants from the National Institutes of Health. Dr. Baker and Dr. Volkmann report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Marathon running does not increase arthritis risk: Survey
Runners who had undergone knee or hip surgery or had a previous hip or knee injury that prevented running were most likely to have arthritis, researchers found. Family history of arthritis, higher body mass index (BMI), and older age were also associated with increased risk of the condition.
The study was presented at the American Academy of Orthopaedic Surgeons 2023 Annual Meeting.
It has generally been thought that running may increase risk of osteoarthritis because it puts more load on joints than walking or standing, noted Grace Hsiao-Wei Lo, MD, an assistant professor of immunology, allergy, and rheumatology at the Baylor College of Medicine, Houston, who was not involved with the work. Research in this area has yielded mixed results: A 2017 analysis of multiple studies found that competitive runners did have higher rates of arthritis than recreational runners, while another study conducted by Dr. Lo found that runners did not have an increased risk of knee osteoarthritis, compared with nonrunners. A 2018 study showed that marathon runners had lower instances of arthritis, compared with the general population.
In this new study, researchers surveyed 3,804 runners who participated in the 2019 or 2021 Chicago Marathon about their running history, average mileage per week, and average running pace. The survey also asked about known risk factors for osteoarthritis, including BMI, family history of arthritis, and past knee and hip injuries that prevented running.
Runners, on average, were about 44 years old and ran 27.9 miles per week. The largest proportion of respondents had completed 2-5 marathons (37.3%), around 21% of respondents had finished 6-10 marathons, and 17% were running their first marathon. Study participants had an average of 15 years of running experience, 1,892 reported a previous hip or knee injury, and 413 had undergone knee or hip surgery. Overall, 36.4% reported experiencing hip or knee pain in the past year, and 7.3% had been diagnosed with arthritis.
Researchers found that there was no association between the risk of osteoarthritis and weekly mileage, years spent running, number of marathons completed, or running pace. Respondents who had undergone knee or hip surgery had the highest risk of osteoarthritis (odds ratio, 5.85; P < .0001), followed by those with a history of knee or hip injuries that prevented running (OR, 5.04; P < .0001). Other identified risk factors were family history of arthritis (OR, 3.47; P < .0001), BMI (OR, 1.10; P < .0001), and older age (OR, 1.08; P < .0001).
The news should be encouraging for runners, said Matthew Hartwell, MD, an orthopedic surgeon at the University of California, San Francisco, who led the research. If someone does not have injuries or surgeries that keep them from running, “you can still continue to run,” he said. “There may not necessarily be this dose-response relationship where the more you run, the more you break down your knee or your hip.”
Still, 24.2% of runners reported that their physician had advised them to reduce their mileage or stop running altogether. Most runners (94.2%) said they planned to run another marathon.
“The results of this study are consistent with the experiences of many lifelong runners and observations of sports medicine professionals that osteoarthritis is not an inevitable consequence of distance running,” said Brett Toresdahl, MD, a sports medicine physician at the Hospital for Special Surgery in New York, who was not involved with the study.
Still, he emphasized that more research is necessary to understand whether running contributes to the risk of developing osteoarthritis. The participants in the study were current marathoners, he noted, so it is likely they have healthy joints that can tolerate running longer distances. “If there is a subset of people who have joints that are negatively affected by running, they wouldn’t likely be registering for a marathon,” he said in an email interview.
Dr. Lo added that comparing these marathoners to a group who did not run would help assess whether running can be harmful to joints. “To be fair, this is a challenging subject to study,” she said. “Osteoarthritis has a long natural history, and so it is difficult to evaluate this kind of question over many years of running and many years of evaluation of arthritis.”
While the research does not answer the question of whether running can lead to osteoarthritis, it helps show the need for long-term research on how running affects joints over time as well as one’s general health, Dr. Toresdahl noted. “I would not be surprised if future longitudinal research will come to the same conclusion that running for the majority of patients is a net benefit for overall health and at least net neutral for joint health when done in moderation,” he said.
Dr. Hartwell, Dr. Lo, and Dr. Toresdahl report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Runners who had undergone knee or hip surgery or had a previous hip or knee injury that prevented running were most likely to have arthritis, researchers found. Family history of arthritis, higher body mass index (BMI), and older age were also associated with increased risk of the condition.
The study was presented at the American Academy of Orthopaedic Surgeons 2023 Annual Meeting.
It has generally been thought that running may increase risk of osteoarthritis because it puts more load on joints than walking or standing, noted Grace Hsiao-Wei Lo, MD, an assistant professor of immunology, allergy, and rheumatology at the Baylor College of Medicine, Houston, who was not involved with the work. Research in this area has yielded mixed results: A 2017 analysis of multiple studies found that competitive runners did have higher rates of arthritis than recreational runners, while another study conducted by Dr. Lo found that runners did not have an increased risk of knee osteoarthritis, compared with nonrunners. A 2018 study showed that marathon runners had lower instances of arthritis, compared with the general population.
In this new study, researchers surveyed 3,804 runners who participated in the 2019 or 2021 Chicago Marathon about their running history, average mileage per week, and average running pace. The survey also asked about known risk factors for osteoarthritis, including BMI, family history of arthritis, and past knee and hip injuries that prevented running.
Runners, on average, were about 44 years old and ran 27.9 miles per week. The largest proportion of respondents had completed 2-5 marathons (37.3%), around 21% of respondents had finished 6-10 marathons, and 17% were running their first marathon. Study participants had an average of 15 years of running experience, 1,892 reported a previous hip or knee injury, and 413 had undergone knee or hip surgery. Overall, 36.4% reported experiencing hip or knee pain in the past year, and 7.3% had been diagnosed with arthritis.
Researchers found that there was no association between the risk of osteoarthritis and weekly mileage, years spent running, number of marathons completed, or running pace. Respondents who had undergone knee or hip surgery had the highest risk of osteoarthritis (odds ratio, 5.85; P < .0001), followed by those with a history of knee or hip injuries that prevented running (OR, 5.04; P < .0001). Other identified risk factors were family history of arthritis (OR, 3.47; P < .0001), BMI (OR, 1.10; P < .0001), and older age (OR, 1.08; P < .0001).
The news should be encouraging for runners, said Matthew Hartwell, MD, an orthopedic surgeon at the University of California, San Francisco, who led the research. If someone does not have injuries or surgeries that keep them from running, “you can still continue to run,” he said. “There may not necessarily be this dose-response relationship where the more you run, the more you break down your knee or your hip.”
Still, 24.2% of runners reported that their physician had advised them to reduce their mileage or stop running altogether. Most runners (94.2%) said they planned to run another marathon.
“The results of this study are consistent with the experiences of many lifelong runners and observations of sports medicine professionals that osteoarthritis is not an inevitable consequence of distance running,” said Brett Toresdahl, MD, a sports medicine physician at the Hospital for Special Surgery in New York, who was not involved with the study.
Still, he emphasized that more research is necessary to understand whether running contributes to the risk of developing osteoarthritis. The participants in the study were current marathoners, he noted, so it is likely they have healthy joints that can tolerate running longer distances. “If there is a subset of people who have joints that are negatively affected by running, they wouldn’t likely be registering for a marathon,” he said in an email interview.
Dr. Lo added that comparing these marathoners to a group who did not run would help assess whether running can be harmful to joints. “To be fair, this is a challenging subject to study,” she said. “Osteoarthritis has a long natural history, and so it is difficult to evaluate this kind of question over many years of running and many years of evaluation of arthritis.”
While the research does not answer the question of whether running can lead to osteoarthritis, it helps show the need for long-term research on how running affects joints over time as well as one’s general health, Dr. Toresdahl noted. “I would not be surprised if future longitudinal research will come to the same conclusion that running for the majority of patients is a net benefit for overall health and at least net neutral for joint health when done in moderation,” he said.
Dr. Hartwell, Dr. Lo, and Dr. Toresdahl report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Runners who had undergone knee or hip surgery or had a previous hip or knee injury that prevented running were most likely to have arthritis, researchers found. Family history of arthritis, higher body mass index (BMI), and older age were also associated with increased risk of the condition.
The study was presented at the American Academy of Orthopaedic Surgeons 2023 Annual Meeting.
It has generally been thought that running may increase risk of osteoarthritis because it puts more load on joints than walking or standing, noted Grace Hsiao-Wei Lo, MD, an assistant professor of immunology, allergy, and rheumatology at the Baylor College of Medicine, Houston, who was not involved with the work. Research in this area has yielded mixed results: A 2017 analysis of multiple studies found that competitive runners did have higher rates of arthritis than recreational runners, while another study conducted by Dr. Lo found that runners did not have an increased risk of knee osteoarthritis, compared with nonrunners. A 2018 study showed that marathon runners had lower instances of arthritis, compared with the general population.
In this new study, researchers surveyed 3,804 runners who participated in the 2019 or 2021 Chicago Marathon about their running history, average mileage per week, and average running pace. The survey also asked about known risk factors for osteoarthritis, including BMI, family history of arthritis, and past knee and hip injuries that prevented running.
Runners, on average, were about 44 years old and ran 27.9 miles per week. The largest proportion of respondents had completed 2-5 marathons (37.3%), around 21% of respondents had finished 6-10 marathons, and 17% were running their first marathon. Study participants had an average of 15 years of running experience, 1,892 reported a previous hip or knee injury, and 413 had undergone knee or hip surgery. Overall, 36.4% reported experiencing hip or knee pain in the past year, and 7.3% had been diagnosed with arthritis.
Researchers found that there was no association between the risk of osteoarthritis and weekly mileage, years spent running, number of marathons completed, or running pace. Respondents who had undergone knee or hip surgery had the highest risk of osteoarthritis (odds ratio, 5.85; P < .0001), followed by those with a history of knee or hip injuries that prevented running (OR, 5.04; P < .0001). Other identified risk factors were family history of arthritis (OR, 3.47; P < .0001), BMI (OR, 1.10; P < .0001), and older age (OR, 1.08; P < .0001).
The news should be encouraging for runners, said Matthew Hartwell, MD, an orthopedic surgeon at the University of California, San Francisco, who led the research. If someone does not have injuries or surgeries that keep them from running, “you can still continue to run,” he said. “There may not necessarily be this dose-response relationship where the more you run, the more you break down your knee or your hip.”
Still, 24.2% of runners reported that their physician had advised them to reduce their mileage or stop running altogether. Most runners (94.2%) said they planned to run another marathon.
“The results of this study are consistent with the experiences of many lifelong runners and observations of sports medicine professionals that osteoarthritis is not an inevitable consequence of distance running,” said Brett Toresdahl, MD, a sports medicine physician at the Hospital for Special Surgery in New York, who was not involved with the study.
Still, he emphasized that more research is necessary to understand whether running contributes to the risk of developing osteoarthritis. The participants in the study were current marathoners, he noted, so it is likely they have healthy joints that can tolerate running longer distances. “If there is a subset of people who have joints that are negatively affected by running, they wouldn’t likely be registering for a marathon,” he said in an email interview.
Dr. Lo added that comparing these marathoners to a group who did not run would help assess whether running can be harmful to joints. “To be fair, this is a challenging subject to study,” she said. “Osteoarthritis has a long natural history, and so it is difficult to evaluate this kind of question over many years of running and many years of evaluation of arthritis.”
While the research does not answer the question of whether running can lead to osteoarthritis, it helps show the need for long-term research on how running affects joints over time as well as one’s general health, Dr. Toresdahl noted. “I would not be surprised if future longitudinal research will come to the same conclusion that running for the majority of patients is a net benefit for overall health and at least net neutral for joint health when done in moderation,” he said.
Dr. Hartwell, Dr. Lo, and Dr. Toresdahl report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAOS 2023
Guidelines: Don’t delay total joint arthroplasty for additional nonoperative therapies
Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative therapies should proceed directly to surgery, according to new guidelines from the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“One of the reasons for creating this guideline was that many patients have been subjected to delays for surgery after completing nonoperative therapy, despite persistent moderate to severe pain, loss of function, and moderate to severe radiographic OA or ON,” said coauthors Susan M. Goodman, MD, a rheumatologist at Hospital for Special Surgery in New York, and Charles Hannon, MD, an orthopedic surgeon at Washington University in St. Louis, in an email interview with this news organization. “This guideline supports surgery being performed in an expeditious fashion after the decision has been made to proceed with surgery by both the physician and patient through a shared decision-making process,” they said.
The guidelines also state that obesity by itself should not be a reason to delay TJA. “We could not find a rationale for a strict cut off for weight/body mass index (BMI). Our literature review revealed that though many adverse events were, in fact, increased in patients with morbid obesity, there is also an increase in adverse events for those who had bariatric surgery prior to their arthroplasty,” they added, noting that patients need to be made aware of the increased risk for adverse events in patients with obesity. Though the guidelines do not pose any BMI cutoffs, they state that weight loss should be “strongly encouraged.” These new recommendations are conditional, and all had a “low” to “very low” certainty of evidence; however, there was high consensus on the recommendations from the expert panel.
The guidelines also recommended:
- Delaying TJA to achieve smoking and nicotine cessation or reduction.
- Delaying TJA to improve glycemic control in patients with diabetes, although the group did not recommend any specific measure or threshold.
- Not delaying TJA in patients with a severe deformity, bone loss, or a neuropathic joint.
The new guidelines formalize what many surgeons have already been doing for the past few years, said Arjun Saxena, MD, MBA, an orthopedic surgeon in Philadelphia who was not involved with the guidelines. “A lot of total joint programs have really focused on patient optimization, including smoking cessation, glycemic control, and weight loss prior to surgery,” he said.
Most importantly, the guidelines put an emphasis on how the decision to proceed with TJA should be a shared decision between a physician and patient, he added. Some insurance companies with prior authorization policies may require a patient to try additional nonoperative therapies before approving surgery, creating barriers to care, he said. “Hopefully [these new recommendations] will help third parties understand that joint replacement is a big decision – most doctors aren’t going to recommend that unless it’s necessary or something that is going to help patients,” he said. “I understand that there is a certain need for preauthorization, but just having strict guidelines isn’t appropriate. You really need to look at the whole picture,” he added.
The full manuscript has been submitted for review and is expected to be jointly published in American College of Rheumatology and the American Association of Hip and Knee Surgeons journals later this year.
Dr. Saxena consults for the orthopedic implant company Corin.
A version of this article originally appeared on Medscape.com.
Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative therapies should proceed directly to surgery, according to new guidelines from the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“One of the reasons for creating this guideline was that many patients have been subjected to delays for surgery after completing nonoperative therapy, despite persistent moderate to severe pain, loss of function, and moderate to severe radiographic OA or ON,” said coauthors Susan M. Goodman, MD, a rheumatologist at Hospital for Special Surgery in New York, and Charles Hannon, MD, an orthopedic surgeon at Washington University in St. Louis, in an email interview with this news organization. “This guideline supports surgery being performed in an expeditious fashion after the decision has been made to proceed with surgery by both the physician and patient through a shared decision-making process,” they said.
The guidelines also state that obesity by itself should not be a reason to delay TJA. “We could not find a rationale for a strict cut off for weight/body mass index (BMI). Our literature review revealed that though many adverse events were, in fact, increased in patients with morbid obesity, there is also an increase in adverse events for those who had bariatric surgery prior to their arthroplasty,” they added, noting that patients need to be made aware of the increased risk for adverse events in patients with obesity. Though the guidelines do not pose any BMI cutoffs, they state that weight loss should be “strongly encouraged.” These new recommendations are conditional, and all had a “low” to “very low” certainty of evidence; however, there was high consensus on the recommendations from the expert panel.
The guidelines also recommended:
- Delaying TJA to achieve smoking and nicotine cessation or reduction.
- Delaying TJA to improve glycemic control in patients with diabetes, although the group did not recommend any specific measure or threshold.
- Not delaying TJA in patients with a severe deformity, bone loss, or a neuropathic joint.
The new guidelines formalize what many surgeons have already been doing for the past few years, said Arjun Saxena, MD, MBA, an orthopedic surgeon in Philadelphia who was not involved with the guidelines. “A lot of total joint programs have really focused on patient optimization, including smoking cessation, glycemic control, and weight loss prior to surgery,” he said.
Most importantly, the guidelines put an emphasis on how the decision to proceed with TJA should be a shared decision between a physician and patient, he added. Some insurance companies with prior authorization policies may require a patient to try additional nonoperative therapies before approving surgery, creating barriers to care, he said. “Hopefully [these new recommendations] will help third parties understand that joint replacement is a big decision – most doctors aren’t going to recommend that unless it’s necessary or something that is going to help patients,” he said. “I understand that there is a certain need for preauthorization, but just having strict guidelines isn’t appropriate. You really need to look at the whole picture,” he added.
The full manuscript has been submitted for review and is expected to be jointly published in American College of Rheumatology and the American Association of Hip and Knee Surgeons journals later this year.
Dr. Saxena consults for the orthopedic implant company Corin.
A version of this article originally appeared on Medscape.com.
Patients with moderate to severe osteoarthritis (OA) or osteonecrosis (ON) eligible for total joint arthroplasty (TJA) who have failed one or more nonoperative therapies should proceed directly to surgery, according to new guidelines from the American College of Rheumatology and the American Association of Hip and Knee Surgeons.
“One of the reasons for creating this guideline was that many patients have been subjected to delays for surgery after completing nonoperative therapy, despite persistent moderate to severe pain, loss of function, and moderate to severe radiographic OA or ON,” said coauthors Susan M. Goodman, MD, a rheumatologist at Hospital for Special Surgery in New York, and Charles Hannon, MD, an orthopedic surgeon at Washington University in St. Louis, in an email interview with this news organization. “This guideline supports surgery being performed in an expeditious fashion after the decision has been made to proceed with surgery by both the physician and patient through a shared decision-making process,” they said.
The guidelines also state that obesity by itself should not be a reason to delay TJA. “We could not find a rationale for a strict cut off for weight/body mass index (BMI). Our literature review revealed that though many adverse events were, in fact, increased in patients with morbid obesity, there is also an increase in adverse events for those who had bariatric surgery prior to their arthroplasty,” they added, noting that patients need to be made aware of the increased risk for adverse events in patients with obesity. Though the guidelines do not pose any BMI cutoffs, they state that weight loss should be “strongly encouraged.” These new recommendations are conditional, and all had a “low” to “very low” certainty of evidence; however, there was high consensus on the recommendations from the expert panel.
The guidelines also recommended:
- Delaying TJA to achieve smoking and nicotine cessation or reduction.
- Delaying TJA to improve glycemic control in patients with diabetes, although the group did not recommend any specific measure or threshold.
- Not delaying TJA in patients with a severe deformity, bone loss, or a neuropathic joint.
The new guidelines formalize what many surgeons have already been doing for the past few years, said Arjun Saxena, MD, MBA, an orthopedic surgeon in Philadelphia who was not involved with the guidelines. “A lot of total joint programs have really focused on patient optimization, including smoking cessation, glycemic control, and weight loss prior to surgery,” he said.
Most importantly, the guidelines put an emphasis on how the decision to proceed with TJA should be a shared decision between a physician and patient, he added. Some insurance companies with prior authorization policies may require a patient to try additional nonoperative therapies before approving surgery, creating barriers to care, he said. “Hopefully [these new recommendations] will help third parties understand that joint replacement is a big decision – most doctors aren’t going to recommend that unless it’s necessary or something that is going to help patients,” he said. “I understand that there is a certain need for preauthorization, but just having strict guidelines isn’t appropriate. You really need to look at the whole picture,” he added.
The full manuscript has been submitted for review and is expected to be jointly published in American College of Rheumatology and the American Association of Hip and Knee Surgeons journals later this year.
Dr. Saxena consults for the orthopedic implant company Corin.
A version of this article originally appeared on Medscape.com.
CDC recommends screening all adults for hepatitis B
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
This is the first update to HBV screening guidelines since 2008, the agency said.
“Risk-based testing alone has not identified most persons living with chronic HBV infection and is considered inefficient for providers to implement,” the authors wrote in the new guidance, published in the CDC’s Morbidity and Mortality Weekly Report. “Universal screening of adults for HBV infection is cost-effective, compared with risk-based screening and averts liver disease and death. Although a curative treatment is not yet available, early diagnosis and treatment of chronic HBV infections reduces the risk for cirrhosis, liver cancer, and death.”
Howard Lee, MD, an assistant professor in the section of gastroenterology and hepatology at Baylor College of Medicine in Houston, agreed that risk-based screening has not been effective. A universal screening approach “is the way to go,” he said. With this new screening approach, patients can get tested without having to admit that they may be at risk for a chronic disease like HIV and HBV, which can be stigmatizing, said Dr. Lee, who was not involved with making these recommendations.
An estimated 580,000 to 2.4 million individuals are living with HBV infection in the United States, and two-thirds may be unaware they are infected, according to the CDC. The virus spreads through contact with blood, semen, and other body fluids of an infected person.
The guidance now recommends using the triple panel (HBsAg, anti-HBs, total anti-HBc) for initial screening.
“It can help identify persons who have an active HBV infection and could be linked to care; have resolved infection and might be susceptible to reactivation (for example, immunosuppressed persons); are susceptible and need vaccination; or are vaccinated,” the authors wrote.
Patients with previous HBV infection can have the infection reactivated with immunosuppressive treatments, Dr. Lee said, which is why detecting prior infection via the triple panel screening is important.
Women who are pregnant should be screened, ideally, in the first trimester of each pregnancy, regardless of vaccination status or testing history. If they have already received timely triple panel screening for hepatitis B and have no new HBV exposures, pregnant women only need HBsAg screening, the guidelines state.
The guidelines also specify that higher risk groups, specifically those incarcerated or formerly incarcerated, adults with current or past hepatitis C virus infection, and those with current or past sexually transmitted infections and multiple sex partners.
People who are susceptible for infection, refuse vaccination and are at higher risk for HBV should be screened periodically, but how often they should be screened should be based on shared decision-making between the provider and patient as well as individual risk and immune status.
Additional research into the optimal frequency of periodic testing is necessary, the authors say.
“Along with vaccination strategies, universal screening of adults and appropriate testing of persons at increased risk for HBV infection will improve health outcomes, reduce the prevalence of HBV infection in the United States, and advance viral hepatitis elimination goals,” the authors wrote.
The new recommendations now contrast with the 2020 screening guidelines issued by the U.S. Preventive Services Task Force (USPSTF) that recommend risk-based screening for hepatitis B.
“When that recommendation was published, the Task Force was aligned with several other organizations, including the CDC, in supporting screening for hepatitis B in high-risk populations — and importantly, we’re all still aligned in making sure that people get the care that they need,” said Michael Barry, MD, chair of the USPSTF, in an emailed statement. “The evidence on clinical preventive services is always changing, and the Task Force aims to keep all recommendations current, updating each recommendation approximately every 5 years.”
“In the meantime, we always encourage clinicians to use their judgment as they provide care for their patients — including those who may benefit from screening for hepatitis B — and to decide together with each patient which preventive services can best help them live a long and healthy life,” Dr. Barry said.
The American Association for the Study of Liver Diseases is currently updating their HBV screening recommendations, Dr. Lee said, and he expects other professional societies to follow the CDC recommendations.
“It’s not uncommon that we see the CDC or societies making recommendations and the USPSTF following along, so hopefully that’s the case for hepatitis B as well,” he said.
The authors reported no potential conflicts of interest.
A version of this article originally appeared on Medscape.com.
Biologics show signs of delaying arthritis in psoriasis patients
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
FROM LANCET RHEUMATOLOGY
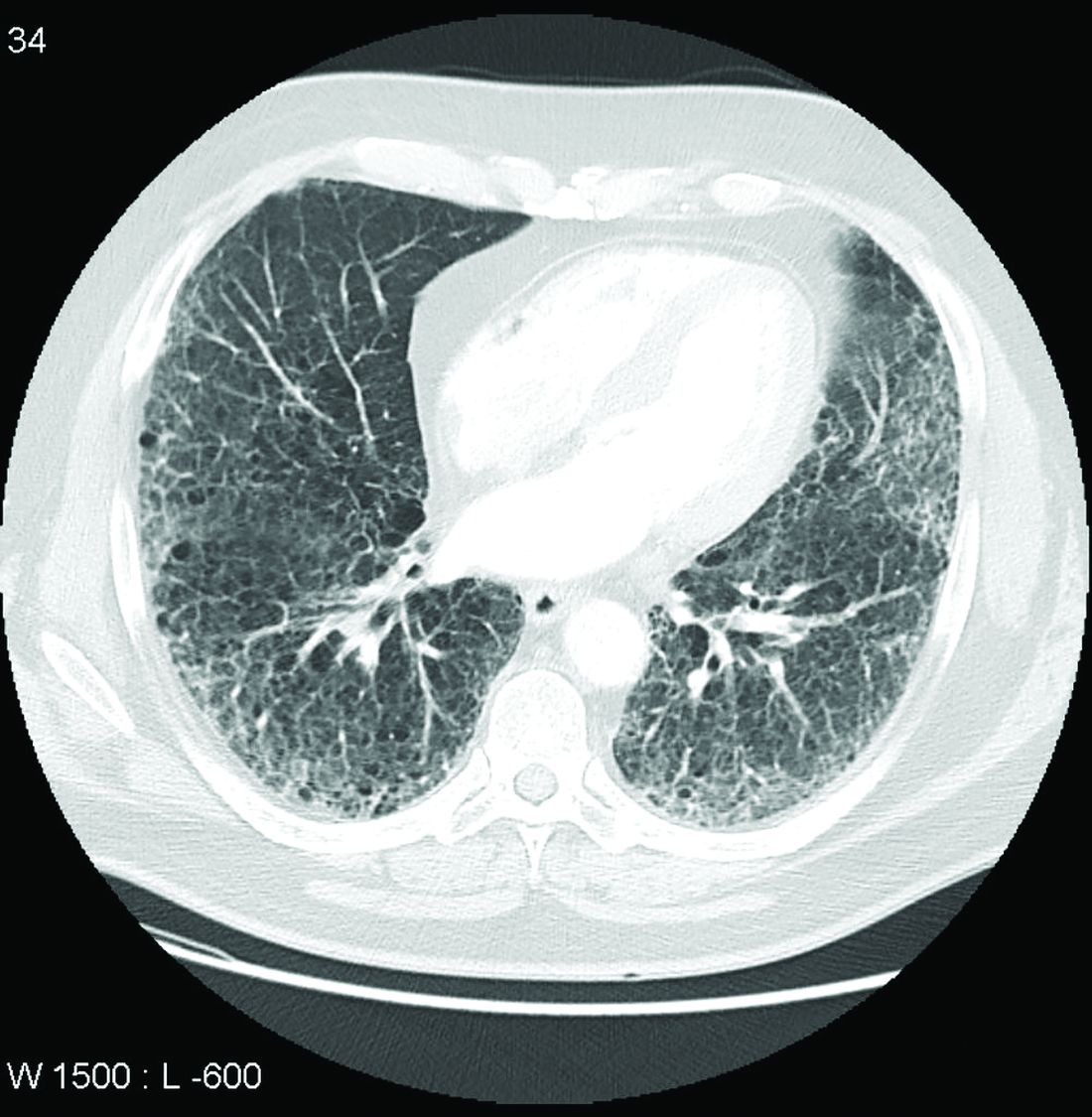
Call it preclinical or subclinical, ILD in RA needs to be tracked
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More clinical guidance is needed for monitoring interstitial lung disease (ILD) in patients with rheumatoid arthritis, according to a new commentary.
Though ILD is a leading cause of death among patients with RA, these patients are not routinely screened for ILD, the authors say, and there are currently no guidelines on how to monitor ILD progression in patients with RA.
“ILD associated with rheumatoid arthritis is a disease for which there’s been very little research done, so it’s an area of rheumatology where there are many unknowns,” lead author Elizabeth R. Volkmann, MD, who codirects the connective tissue disease–related interstitial lung disease (CTD-ILD) program at University of California, Los Angeles, told this news organization.
The commentary was published in The Lancet Rheumatology.
Defining disease
One of the major unknowns is how to define the disease, she said. RA patients sometimes undergo imaging for other medical reasons, and interstitial lung abnormalities are incidentally detected. These patients can be classified as having “preclinical” or “subclinical” ILD, as they do not yet have symptoms; however, there is no consensus as to what these terms mean, the commentary authors write. “The other problem that we have with these terms is that it sometimes creates the perception that this is a nonworrisome feature of rheumatoid arthritis,” Dr. Volkmann said, although the condition should be followed closely.
“We know we can detect imaging features of ILD in people who may not yet have symptoms, and we need to know when to define a clinically important informality that requires follow-up or treatment,” added John M. Davis III, MD, a rheumatologist at the Mayo Clinic, Rochester, Minn. He was not involved with the work.
Dr. Volkmann proposed eliminating the prefixes “pre” and “sub” when referring to ILD. “In other connective tissue diseases, like systemic sclerosis, for example, we can use the term ‘limited’ or ‘extensive’ ILD, based on the extent of involvement of the ILD on high-resolution computed tomography (HRCT) imaging,” she said. “This could potentially be something that is applied to how we classify patients with RA-ILD.”
Tracking ILD progression
Once ILD is identified, monitoring its progression poses challenges, as respiratory symptoms may be difficult to detect. RA patients may already be avoiding exercise because of joint pain, so they may not notice shortness of breath during physical activity, noted Jessica K. Gordon, MD, of the Hospital for Special Surgery, New York, in an interview with this news organization. She was not involved with the commentary. Cough is a potential symptom of ILD, but cough can also be the result of allergies, postnasal drip, or reflux, she said. Making the distinction between “preclinical” and symptomatic disease can be “complicated,” she added; “you may have to really dig.”
Additionally, there has been little research on the outcomes of patients with preclinical or subclinical ILD and clinical ILD, the commentary authors write. “It is therefore conceivable that some patients with rheumatoid arthritis diagnosed with preclinical or subclinical ILD could potentially have worse outcomes if both the rheumatoid arthritis and ILD are not monitored closely,” they note.
To better track RA-associated ILD for patients with and those without symptoms, the authors advocate for monitoring patients using pulmonary testing and CT scanning, as well as evaluating symptoms. How often these assessments should be conducted depends on the individual, they note. In her own practice, Dr. Volkmann sees patients every 3 months to evaluate their symptoms and conduct pulmonary function tests (PFTs). For patients early in the course of ILD, she orders HRCT imaging once per year.
For Dr. Davis, the frequency of follow-up depends on the severity of ILD. “For minimally symptomatic patients without compromised lung function, we would generally follow annually. For patients with symptomatic ILD on stable therapy, we may monitor every 6 months. For patients with active/progressive ILD, we would generally be following at least every 1-3 months,” he said.
Screening and future research
While there is no evidence to recommend screening patients for ILD using CT, there are certain risk factors for ILD in RA patients, including a history of smoking, male sex, and high RA disease activity despite antirheumatic treatment, Dr. Volkmann said. In both of their practices, Dr. Davis and Dr. Volkmann screen with RA via HRCT and PFTs for ILD for patients with known risk factors that predispose them to the lung condition and/or for patients who report respiratory symptoms.
“We still don’t have an algorithm [for screening patients], and that is a desperate need in this field,” added Joshua J. Solomon, MD, a pulmonologist at National Jewish Health, Denver, whose research focuses on RA-associated ILD. While recommendations state that all patients with scleroderma should be screened with CT, ILD incidence is lower among patients with RA, and thus these screening recommendations need to be narrowed, he said. But more research is needed to better fine tune recommendations, he said; “The only thing you can do is give some expert consensus until there are good data.”
Dr. Volkmann has received consulting and speaking fees from Boehringer Ingelheim and institutional support for performing studies on systemic sclerosis for Kadmon, Forbius, Boehringer Ingelheim, Horizon, and Prometheus. Dr. Gordon, Dr. Davis, and Dr. Solomon report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Posttransplant NASH patients fare worse with older donor livers
All-cause mortality was twice as high and death from an infectious cause was more than three times as high for patients with NASH who received liver grafts from octogenarian donors than for those who received a liver from someone younger than 50.
“The findings from this study implicate a critical need to investigate donor age as an important risk factor for poorer host and graft survival,” wrote the authors, led by David Lee, MD, of the University of Maryland, Baltimore.
“Given the possibility of infectious graft complications, post–liver transplant follow-ups may need to be more comprehensive and frequent in these individuals who receive grafts from older donors,” they add.
The study was published online in Digestive and Liver Disease.
Donor age trends
Dr. Lee and colleagues pulled data from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research database, a national database of deidentified donor and recipient transplant data. The analysis excluded recipients younger than 18, those with a living donor, those who had hepatocellular carcinoma prior to transplant, and those who had been diagnosed with additional liver disorders apart from NASH.
The team identified 8,88 recipients with NASH who received a liver transplant from 2005–2019. They stratified recipients by donor age. The 5,187 patients who received livers from donors who were younger than 50 served as the reference group. The remainder were placed into four cohorts – 1,842 whose donors were in their 50s, 1,290 whose donors were in their 60s, 504 whose donors were in their 70s, and 65 whose donors were in their 80s.
The researchers found that in comparison with the reference group, the average age of recipients in each donor-age cohort was progressively older. Two donor-age cohorts had significantly higher proportions of recipients with diabetes than the 46.5% in the reference group – the sexagenarian cohort (51.7%) and the octogenarian group (66.2%).
The median follow-up time ranged from 2.35–3.61 years across all age groups.
The researchers found that for all donor-age groups excluding donors in their 60s, recipients had higher risk of all-cause mortality after transplant than the reference group. Recipients with donors in their 50s had a 16% greater risk for death (P = .01), and recipients with donors in their 70s had a 20% greater risk (P = .05). For recipients with octogenarian donors, the adjusted hazard ratio for all-cause mortality was 2.01 (P < .001).
Only recipients in the octogenarian donor cohort were at increased risk of graft failure, compared with the reference group (aHR, 3.72; P = .002).
As donor age increased, the recipient’s risk of dying from sepsis and infectious causes rose, compared with the reference group. Recipients’ likelihood of sepsis death increased by 71% (P = .001) with donors in their 50s, 73% (P = .003) with donors in their 60s, and 76% (P = .03) with donors in their 70s. For recipients with octogenarian donors, the risk more than tripled (aHR, 3.58; P = .007). Likewise, recipients with donors in their 70s were 73% more likely to die from infectious causes. That risk nearly quadrupled among those with donors in their 80s.
Recipient factors at play?
While the study found a relationship between liver donor age and recipient outcomes, it is not clear whether any other recipient factors may have contributed to the higher risk of all-cause mortality, sad Nancy Reau, MD, chief of the hepatology section at Rush University Medical Center, Chicago. The researchers did not parse out whether younger recipients did better with older organs than older recipients or whether older recipients fared worse with younger organs, she said in an interview.
“I wasn’t convinced that they had demonstrated that the recipient may not have played a role in that,” said Dr. Reau, who wasn’t involved with the study.
The analysis only a found an increased risk of graft failure among recipients who received organs from octogenarian donors, so factors other than liver transplant may have contributed to all-cause mortality, she noted.
The UNOS database has some limitations, noted Timothy Pruett, MD, who directs the liver transplant program at the University of Minnesota, Minneapolis. Because the database pulls information from transplantation centers across the country, it can be difficult to standardize specific patient variables in the data.
While it’s clear that a patient died, it’s less certain whether an infection was the cause of death and whether that infection was somehow associated with the liver, noted Dr. Pruett, who wasn’t involved in the research. For example, a patient could have had broken a hip, gone to the hospital, and contracted pneumonia, which led to their death.
“There’s just not much granularity in the database, and we can’t overextrapolate what we see,” Dr. Pruett said in an interview.
Knowledge gaps
Dr. Lee agreed that more research is needed to understand what may be driving higher mortality rates among patients who receive older organs. “There are still a lot of gaps in knowledge with respect to why.”
Dr. Reau said she is curious as to whether certain comorbidities, such as previous infection, diabetes, or obesity, could predict worse outcomes for recipients with older organs.
“We would love to give all of our patients younger organs, but if that leads to even more disparity in need [compared] to availability and the alternative is not surviving, I think you have to place [this work] into context,” she said.
The study findings shouldn’t be used to deter patients with NASH from considering older organs, Dr. Reau said. More insight as to which populations might want to be choosier owing to an elevated risk would be beneficial, she added.
Dr. Lee, Dr. Pruett, and Dr. Reau reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
All-cause mortality was twice as high and death from an infectious cause was more than three times as high for patients with NASH who received liver grafts from octogenarian donors than for those who received a liver from someone younger than 50.
“The findings from this study implicate a critical need to investigate donor age as an important risk factor for poorer host and graft survival,” wrote the authors, led by David Lee, MD, of the University of Maryland, Baltimore.
“Given the possibility of infectious graft complications, post–liver transplant follow-ups may need to be more comprehensive and frequent in these individuals who receive grafts from older donors,” they add.
The study was published online in Digestive and Liver Disease.
Donor age trends
Dr. Lee and colleagues pulled data from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research database, a national database of deidentified donor and recipient transplant data. The analysis excluded recipients younger than 18, those with a living donor, those who had hepatocellular carcinoma prior to transplant, and those who had been diagnosed with additional liver disorders apart from NASH.
The team identified 8,88 recipients with NASH who received a liver transplant from 2005–2019. They stratified recipients by donor age. The 5,187 patients who received livers from donors who were younger than 50 served as the reference group. The remainder were placed into four cohorts – 1,842 whose donors were in their 50s, 1,290 whose donors were in their 60s, 504 whose donors were in their 70s, and 65 whose donors were in their 80s.
The researchers found that in comparison with the reference group, the average age of recipients in each donor-age cohort was progressively older. Two donor-age cohorts had significantly higher proportions of recipients with diabetes than the 46.5% in the reference group – the sexagenarian cohort (51.7%) and the octogenarian group (66.2%).
The median follow-up time ranged from 2.35–3.61 years across all age groups.
The researchers found that for all donor-age groups excluding donors in their 60s, recipients had higher risk of all-cause mortality after transplant than the reference group. Recipients with donors in their 50s had a 16% greater risk for death (P = .01), and recipients with donors in their 70s had a 20% greater risk (P = .05). For recipients with octogenarian donors, the adjusted hazard ratio for all-cause mortality was 2.01 (P < .001).
Only recipients in the octogenarian donor cohort were at increased risk of graft failure, compared with the reference group (aHR, 3.72; P = .002).
As donor age increased, the recipient’s risk of dying from sepsis and infectious causes rose, compared with the reference group. Recipients’ likelihood of sepsis death increased by 71% (P = .001) with donors in their 50s, 73% (P = .003) with donors in their 60s, and 76% (P = .03) with donors in their 70s. For recipients with octogenarian donors, the risk more than tripled (aHR, 3.58; P = .007). Likewise, recipients with donors in their 70s were 73% more likely to die from infectious causes. That risk nearly quadrupled among those with donors in their 80s.
Recipient factors at play?
While the study found a relationship between liver donor age and recipient outcomes, it is not clear whether any other recipient factors may have contributed to the higher risk of all-cause mortality, sad Nancy Reau, MD, chief of the hepatology section at Rush University Medical Center, Chicago. The researchers did not parse out whether younger recipients did better with older organs than older recipients or whether older recipients fared worse with younger organs, she said in an interview.
“I wasn’t convinced that they had demonstrated that the recipient may not have played a role in that,” said Dr. Reau, who wasn’t involved with the study.
The analysis only a found an increased risk of graft failure among recipients who received organs from octogenarian donors, so factors other than liver transplant may have contributed to all-cause mortality, she noted.
The UNOS database has some limitations, noted Timothy Pruett, MD, who directs the liver transplant program at the University of Minnesota, Minneapolis. Because the database pulls information from transplantation centers across the country, it can be difficult to standardize specific patient variables in the data.
While it’s clear that a patient died, it’s less certain whether an infection was the cause of death and whether that infection was somehow associated with the liver, noted Dr. Pruett, who wasn’t involved in the research. For example, a patient could have had broken a hip, gone to the hospital, and contracted pneumonia, which led to their death.
“There’s just not much granularity in the database, and we can’t overextrapolate what we see,” Dr. Pruett said in an interview.
Knowledge gaps
Dr. Lee agreed that more research is needed to understand what may be driving higher mortality rates among patients who receive older organs. “There are still a lot of gaps in knowledge with respect to why.”
Dr. Reau said she is curious as to whether certain comorbidities, such as previous infection, diabetes, or obesity, could predict worse outcomes for recipients with older organs.
“We would love to give all of our patients younger organs, but if that leads to even more disparity in need [compared] to availability and the alternative is not surviving, I think you have to place [this work] into context,” she said.
The study findings shouldn’t be used to deter patients with NASH from considering older organs, Dr. Reau said. More insight as to which populations might want to be choosier owing to an elevated risk would be beneficial, she added.
Dr. Lee, Dr. Pruett, and Dr. Reau reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
All-cause mortality was twice as high and death from an infectious cause was more than three times as high for patients with NASH who received liver grafts from octogenarian donors than for those who received a liver from someone younger than 50.
“The findings from this study implicate a critical need to investigate donor age as an important risk factor for poorer host and graft survival,” wrote the authors, led by David Lee, MD, of the University of Maryland, Baltimore.
“Given the possibility of infectious graft complications, post–liver transplant follow-ups may need to be more comprehensive and frequent in these individuals who receive grafts from older donors,” they add.
The study was published online in Digestive and Liver Disease.
Donor age trends
Dr. Lee and colleagues pulled data from the United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research database, a national database of deidentified donor and recipient transplant data. The analysis excluded recipients younger than 18, those with a living donor, those who had hepatocellular carcinoma prior to transplant, and those who had been diagnosed with additional liver disorders apart from NASH.
The team identified 8,88 recipients with NASH who received a liver transplant from 2005–2019. They stratified recipients by donor age. The 5,187 patients who received livers from donors who were younger than 50 served as the reference group. The remainder were placed into four cohorts – 1,842 whose donors were in their 50s, 1,290 whose donors were in their 60s, 504 whose donors were in their 70s, and 65 whose donors were in their 80s.
The researchers found that in comparison with the reference group, the average age of recipients in each donor-age cohort was progressively older. Two donor-age cohorts had significantly higher proportions of recipients with diabetes than the 46.5% in the reference group – the sexagenarian cohort (51.7%) and the octogenarian group (66.2%).
The median follow-up time ranged from 2.35–3.61 years across all age groups.
The researchers found that for all donor-age groups excluding donors in their 60s, recipients had higher risk of all-cause mortality after transplant than the reference group. Recipients with donors in their 50s had a 16% greater risk for death (P = .01), and recipients with donors in their 70s had a 20% greater risk (P = .05). For recipients with octogenarian donors, the adjusted hazard ratio for all-cause mortality was 2.01 (P < .001).
Only recipients in the octogenarian donor cohort were at increased risk of graft failure, compared with the reference group (aHR, 3.72; P = .002).
As donor age increased, the recipient’s risk of dying from sepsis and infectious causes rose, compared with the reference group. Recipients’ likelihood of sepsis death increased by 71% (P = .001) with donors in their 50s, 73% (P = .003) with donors in their 60s, and 76% (P = .03) with donors in their 70s. For recipients with octogenarian donors, the risk more than tripled (aHR, 3.58; P = .007). Likewise, recipients with donors in their 70s were 73% more likely to die from infectious causes. That risk nearly quadrupled among those with donors in their 80s.
Recipient factors at play?
While the study found a relationship between liver donor age and recipient outcomes, it is not clear whether any other recipient factors may have contributed to the higher risk of all-cause mortality, sad Nancy Reau, MD, chief of the hepatology section at Rush University Medical Center, Chicago. The researchers did not parse out whether younger recipients did better with older organs than older recipients or whether older recipients fared worse with younger organs, she said in an interview.
“I wasn’t convinced that they had demonstrated that the recipient may not have played a role in that,” said Dr. Reau, who wasn’t involved with the study.
The analysis only a found an increased risk of graft failure among recipients who received organs from octogenarian donors, so factors other than liver transplant may have contributed to all-cause mortality, she noted.
The UNOS database has some limitations, noted Timothy Pruett, MD, who directs the liver transplant program at the University of Minnesota, Minneapolis. Because the database pulls information from transplantation centers across the country, it can be difficult to standardize specific patient variables in the data.
While it’s clear that a patient died, it’s less certain whether an infection was the cause of death and whether that infection was somehow associated with the liver, noted Dr. Pruett, who wasn’t involved in the research. For example, a patient could have had broken a hip, gone to the hospital, and contracted pneumonia, which led to their death.
“There’s just not much granularity in the database, and we can’t overextrapolate what we see,” Dr. Pruett said in an interview.
Knowledge gaps
Dr. Lee agreed that more research is needed to understand what may be driving higher mortality rates among patients who receive older organs. “There are still a lot of gaps in knowledge with respect to why.”
Dr. Reau said she is curious as to whether certain comorbidities, such as previous infection, diabetes, or obesity, could predict worse outcomes for recipients with older organs.
“We would love to give all of our patients younger organs, but if that leads to even more disparity in need [compared] to availability and the alternative is not surviving, I think you have to place [this work] into context,” she said.
The study findings shouldn’t be used to deter patients with NASH from considering older organs, Dr. Reau said. More insight as to which populations might want to be choosier owing to an elevated risk would be beneficial, she added.
Dr. Lee, Dr. Pruett, and Dr. Reau reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIGESTIVE AND LIVER DISEASE
FDA approves first biologic treatment for polymyalgia rheumatica
The Food and Drug Administration approved sarilumab (Kevzara) on March 1 for the treatment of polymyalgia rheumatica (PMR) in adults who have had an inadequate response to corticosteroids or could not tolerate a corticosteroid taper, joint developers Sanofi and Regeneron announced. The drug is the first and only FDA-approved biologic treatment for this inflammatory rheumatic disease.
The FDA previously approved sarilumab, an interleukin-6 receptor antagonist, in May 2017 for the treatment of moderate to severe active rheumatoid arthritis in adults who do not respond well or have an intolerance to disease-modifying antirheumatic drugs (DMARDs), like methotrexate.
The FDA approval for this new indication was based on results from the multicenter, phase 3 SAPHYR trial in patients with corticosteroid-resistant, active PMR. In the randomized, double-blind, placebo-controlled study, 59 participants received 200 mg of sarilumab plus a 14-week taper of corticosteroid treatment and 58 participants received placebo every 2 weeks along with a 52-week taper of corticosteroid treatment.
After 1 year, 28% of sarilumab patients achieved sustained remission, compared with 10% of the placebo group (P = .0193). This news organization previously reported these trial results in November when they were presented at the 2022 annual meeting of the American College of Rheumatology.
The most common adverse events in the sarilumab group were neutropenia (15%), leukopenia (7%), constipation (7%), pruritic rash (5%), myalgia (7%), fatigue (5%), and injection-site pruritus (5%). Two patients had serious adverse reactions of neutropenia, which resolved after discontinuing treatment.
“Polymyalgia rheumatica can be an incapacitating disease, causing painful disease flares in multiple parts of the bodies that leave people fatigued and unable to fully perform everyday activities. Corticosteroids have been the primary treatment to date, but many patients do not adequately respond to steroids or cannot be tapered off steroids, which puts such patients at risk of complications from long-term steroid therapy,” George D. Yancopolous, MD, PhD, president and chief scientific officer at Regeneron, said in the announcement. “With the approval of Kevzara for polymyalgia rheumatica, patients now have an FDA-approved treatment to help offer relief from the disabling symptoms of this disease and long-term dependence on steroids.”
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration approved sarilumab (Kevzara) on March 1 for the treatment of polymyalgia rheumatica (PMR) in adults who have had an inadequate response to corticosteroids or could not tolerate a corticosteroid taper, joint developers Sanofi and Regeneron announced. The drug is the first and only FDA-approved biologic treatment for this inflammatory rheumatic disease.
The FDA previously approved sarilumab, an interleukin-6 receptor antagonist, in May 2017 for the treatment of moderate to severe active rheumatoid arthritis in adults who do not respond well or have an intolerance to disease-modifying antirheumatic drugs (DMARDs), like methotrexate.
The FDA approval for this new indication was based on results from the multicenter, phase 3 SAPHYR trial in patients with corticosteroid-resistant, active PMR. In the randomized, double-blind, placebo-controlled study, 59 participants received 200 mg of sarilumab plus a 14-week taper of corticosteroid treatment and 58 participants received placebo every 2 weeks along with a 52-week taper of corticosteroid treatment.
After 1 year, 28% of sarilumab patients achieved sustained remission, compared with 10% of the placebo group (P = .0193). This news organization previously reported these trial results in November when they were presented at the 2022 annual meeting of the American College of Rheumatology.
The most common adverse events in the sarilumab group were neutropenia (15%), leukopenia (7%), constipation (7%), pruritic rash (5%), myalgia (7%), fatigue (5%), and injection-site pruritus (5%). Two patients had serious adverse reactions of neutropenia, which resolved after discontinuing treatment.
“Polymyalgia rheumatica can be an incapacitating disease, causing painful disease flares in multiple parts of the bodies that leave people fatigued and unable to fully perform everyday activities. Corticosteroids have been the primary treatment to date, but many patients do not adequately respond to steroids or cannot be tapered off steroids, which puts such patients at risk of complications from long-term steroid therapy,” George D. Yancopolous, MD, PhD, president and chief scientific officer at Regeneron, said in the announcement. “With the approval of Kevzara for polymyalgia rheumatica, patients now have an FDA-approved treatment to help offer relief from the disabling symptoms of this disease and long-term dependence on steroids.”
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration approved sarilumab (Kevzara) on March 1 for the treatment of polymyalgia rheumatica (PMR) in adults who have had an inadequate response to corticosteroids or could not tolerate a corticosteroid taper, joint developers Sanofi and Regeneron announced. The drug is the first and only FDA-approved biologic treatment for this inflammatory rheumatic disease.
The FDA previously approved sarilumab, an interleukin-6 receptor antagonist, in May 2017 for the treatment of moderate to severe active rheumatoid arthritis in adults who do not respond well or have an intolerance to disease-modifying antirheumatic drugs (DMARDs), like methotrexate.
The FDA approval for this new indication was based on results from the multicenter, phase 3 SAPHYR trial in patients with corticosteroid-resistant, active PMR. In the randomized, double-blind, placebo-controlled study, 59 participants received 200 mg of sarilumab plus a 14-week taper of corticosteroid treatment and 58 participants received placebo every 2 weeks along with a 52-week taper of corticosteroid treatment.
After 1 year, 28% of sarilumab patients achieved sustained remission, compared with 10% of the placebo group (P = .0193). This news organization previously reported these trial results in November when they were presented at the 2022 annual meeting of the American College of Rheumatology.
The most common adverse events in the sarilumab group were neutropenia (15%), leukopenia (7%), constipation (7%), pruritic rash (5%), myalgia (7%), fatigue (5%), and injection-site pruritus (5%). Two patients had serious adverse reactions of neutropenia, which resolved after discontinuing treatment.
“Polymyalgia rheumatica can be an incapacitating disease, causing painful disease flares in multiple parts of the bodies that leave people fatigued and unable to fully perform everyday activities. Corticosteroids have been the primary treatment to date, but many patients do not adequately respond to steroids or cannot be tapered off steroids, which puts such patients at risk of complications from long-term steroid therapy,” George D. Yancopolous, MD, PhD, president and chief scientific officer at Regeneron, said in the announcement. “With the approval of Kevzara for polymyalgia rheumatica, patients now have an FDA-approved treatment to help offer relief from the disabling symptoms of this disease and long-term dependence on steroids.”
A version of this article originally appeared on Medscape.com.
What’s holding back physicians from prescribing biosimilars? Four specialties weigh in
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
FDA broadens warning on potentially contaminated eye products
The announcement released Wednesday adds to a previous warning issued earlier this month for EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears because of potential bacterial contamination. All three products are manufactured by the same company, Global Pharma Healthcare, based in Tamilnadu, India.
The FDA has faulted the company for multiple violations, including “lack of appropriate microbial testing” and “lack of proper controls concerning tamper-evident packaging,” and has banned imports to the United States.
The updated warning from the FDA did not give additional information about the over-the-counter eye ointment beyond potential bacterial contamination.
On Feb. 1, the CDC issued an alert about an outbreak of a drug-resistant strain of bacteria, Pseudomonas aeruginosa, linked to artificial tear products. To date, 58 patients across 13 states have been identified, and the most commonly reported artificial tear brand was EzriCare Artificial Tears. Five patients had permanent vision loss, and one patient died.
A version of this article first appeared on Medscape.com.
The announcement released Wednesday adds to a previous warning issued earlier this month for EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears because of potential bacterial contamination. All three products are manufactured by the same company, Global Pharma Healthcare, based in Tamilnadu, India.
The FDA has faulted the company for multiple violations, including “lack of appropriate microbial testing” and “lack of proper controls concerning tamper-evident packaging,” and has banned imports to the United States.
The updated warning from the FDA did not give additional information about the over-the-counter eye ointment beyond potential bacterial contamination.
On Feb. 1, the CDC issued an alert about an outbreak of a drug-resistant strain of bacteria, Pseudomonas aeruginosa, linked to artificial tear products. To date, 58 patients across 13 states have been identified, and the most commonly reported artificial tear brand was EzriCare Artificial Tears. Five patients had permanent vision loss, and one patient died.
A version of this article first appeared on Medscape.com.
The announcement released Wednesday adds to a previous warning issued earlier this month for EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears because of potential bacterial contamination. All three products are manufactured by the same company, Global Pharma Healthcare, based in Tamilnadu, India.
The FDA has faulted the company for multiple violations, including “lack of appropriate microbial testing” and “lack of proper controls concerning tamper-evident packaging,” and has banned imports to the United States.
The updated warning from the FDA did not give additional information about the over-the-counter eye ointment beyond potential bacterial contamination.
On Feb. 1, the CDC issued an alert about an outbreak of a drug-resistant strain of bacteria, Pseudomonas aeruginosa, linked to artificial tear products. To date, 58 patients across 13 states have been identified, and the most commonly reported artificial tear brand was EzriCare Artificial Tears. Five patients had permanent vision loss, and one patient died.
A version of this article first appeared on Medscape.com.