User login
Stearic acid
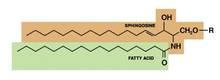
Stearic acid, a waxlike fatty acid also known as octadecanoic acid, is an important component of stratum corneum lipids. Stearic acid is also found in cocoa butter, shea butter, and other vegetable fats, as well as animal tallow. As an FDA-approved ingredient in several cosmetic products, it is used as a surfactant and emulsifying agent for fragrance and as the base for other fatty acid ingredients that are synthesized into emollients and lubricants. Stearic acid is used most often to thicken and retain the shape of soaps (indirectly, through saponification of triglycerides composed of stearic acid esters), and it is also used in shampoos, shaving creams, and detergents.
There is limited evidence for the potential of exogenously produced stearic acid to play a significant role as a topical dermatologic therapeutic agent. Stearic acid is thought to be associated with behenyltrimethylammonium chloride through salt bridges, and the combination is believed to have the capacity to build bilayer vesicles with the aid of hinokitiol (beta-thujaplicin), a natural monoterpenoid found in the wood of trees in the Cupressaceae family that has been shown to exert topical inhibitory activity against Chlamydia trachomatis (Antimicrob. Agents Chemother. 2005;49:2519-21). These vesicles, used to enhance the skin permeation of hinokitiol, were tested in hairless mice and appear to have the potential to promote hair growth (Drug Dev. Ind. Pharm. 2010;36:556-62).
In 2000, Khalil et al. studied the effects of cream formulations on chemically induced burns in mice based on reports that the ingredients, docosanol or stearic acid, were associated with antiviral and anti-inflammatory activity. Burns were engendered by painting murine abdomens with a chloroform solution of phenol. Investigators then topically applied the test formulations 0.5, 3, and 6 hours after injury. They found that the docosanol- and stearic acid–containing creams significantly mitigated the severity and progression of skin lesions compared with untreated sites, yielding, respectively, 76% and 57% declines in mean lesion scores (Contact Dermatitis 2000;43:79-81).
In 2001, Fluhr et al. studied the effects of the free fatty acid pool on stratum corneum (SC) acidification and function by topically applying two phospholipase inhibitors – bromphenacylbromide and 1-hexadecyl-3-trifluoroethylglycero-sn-2-phosphomethanol – for 3 days to murine skin. This raised skin pH and yielded permeability barrier abnormality, altered SC integrity, and reduced SC cohesion. All malfunctions were normalized, including SC pH, with the coapplication of either palmitic, stearic, or linoleic acids along with the inhibiting agents (J. Invest. Dermatol. 2001;117:44-51).
In 2010, Mukherjee et al. evaluated a recently marketed mild, moisturizing body wash containing stearic acid and emollient soybean oil to ascertain the location and amount of stearic acid deposited in the SC after in vivo usage of the product. They conducted clinical cleansing studies for 1 and 5 consecutive days using the soybean product or petroleum jelly. The deuterated variant of stearic acid replaced the free stearic acid in the soybean formulation. The researchers detected deuterated stearic acid in all 10 consecutive layers of SC, with a total stearic acid level measured at 0.33 mcg/cm2 after five washes with the soybean oil product. They concluded that the estimated total fatty acid delivered to the skin from cleansing, probably incorporated into the SC lipid phase, is comparable to the fatty acid amount in an SC layer (J. Cosmet. Dermatol. 2010;9:202-10).
Stearic acid is incorporated into several over-the-counter products, including formulations by Aveda (Green Science Firming Face Cream), Yves Rocher (Les Plaisirs Nature), Kiss My Face (with alpha hydroxy acid), Valeant Pharmaceuticals’ Kinerase line (including Clear Skin Regulating Mask), Buster’s Skin Care for Men (peptide complex organic face moisturizer), and Dermalogica (Soothing Shaving Cream with Daily Defense Block), among others.
Conclusion
While stearic acid is an important component in stratum corneum lipids and a widely used ingredient in skin care products, there is a dearth of data on its significance, if any, in the topical dermatologic armamentarium beyond its primary activity as a surfactant and emulsifying agent. Specifically, it remains to be seen whether stearic acid can be replenished in the stratum corneum through topical treatment. Much more research is needed in this area to assess the potential of stearic acid as a therapeutic agent.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. She has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever. E-mail sknews@frontlinemedcom.com to contact Dr. Baumann or to suggest topics for a future column.
Stearic acid, a waxlike fatty acid also known as octadecanoic acid, is an important component of stratum corneum lipids. Stearic acid is also found in cocoa butter, shea butter, and other vegetable fats, as well as animal tallow. As an FDA-approved ingredient in several cosmetic products, it is used as a surfactant and emulsifying agent for fragrance and as the base for other fatty acid ingredients that are synthesized into emollients and lubricants. Stearic acid is used most often to thicken and retain the shape of soaps (indirectly, through saponification of triglycerides composed of stearic acid esters), and it is also used in shampoos, shaving creams, and detergents.
There is limited evidence for the potential of exogenously produced stearic acid to play a significant role as a topical dermatologic therapeutic agent. Stearic acid is thought to be associated with behenyltrimethylammonium chloride through salt bridges, and the combination is believed to have the capacity to build bilayer vesicles with the aid of hinokitiol (beta-thujaplicin), a natural monoterpenoid found in the wood of trees in the Cupressaceae family that has been shown to exert topical inhibitory activity against Chlamydia trachomatis (Antimicrob. Agents Chemother. 2005;49:2519-21). These vesicles, used to enhance the skin permeation of hinokitiol, were tested in hairless mice and appear to have the potential to promote hair growth (Drug Dev. Ind. Pharm. 2010;36:556-62).
In 2000, Khalil et al. studied the effects of cream formulations on chemically induced burns in mice based on reports that the ingredients, docosanol or stearic acid, were associated with antiviral and anti-inflammatory activity. Burns were engendered by painting murine abdomens with a chloroform solution of phenol. Investigators then topically applied the test formulations 0.5, 3, and 6 hours after injury. They found that the docosanol- and stearic acid–containing creams significantly mitigated the severity and progression of skin lesions compared with untreated sites, yielding, respectively, 76% and 57% declines in mean lesion scores (Contact Dermatitis 2000;43:79-81).
In 2001, Fluhr et al. studied the effects of the free fatty acid pool on stratum corneum (SC) acidification and function by topically applying two phospholipase inhibitors – bromphenacylbromide and 1-hexadecyl-3-trifluoroethylglycero-sn-2-phosphomethanol – for 3 days to murine skin. This raised skin pH and yielded permeability barrier abnormality, altered SC integrity, and reduced SC cohesion. All malfunctions were normalized, including SC pH, with the coapplication of either palmitic, stearic, or linoleic acids along with the inhibiting agents (J. Invest. Dermatol. 2001;117:44-51).
In 2010, Mukherjee et al. evaluated a recently marketed mild, moisturizing body wash containing stearic acid and emollient soybean oil to ascertain the location and amount of stearic acid deposited in the SC after in vivo usage of the product. They conducted clinical cleansing studies for 1 and 5 consecutive days using the soybean product or petroleum jelly. The deuterated variant of stearic acid replaced the free stearic acid in the soybean formulation. The researchers detected deuterated stearic acid in all 10 consecutive layers of SC, with a total stearic acid level measured at 0.33 mcg/cm2 after five washes with the soybean oil product. They concluded that the estimated total fatty acid delivered to the skin from cleansing, probably incorporated into the SC lipid phase, is comparable to the fatty acid amount in an SC layer (J. Cosmet. Dermatol. 2010;9:202-10).
Stearic acid is incorporated into several over-the-counter products, including formulations by Aveda (Green Science Firming Face Cream), Yves Rocher (Les Plaisirs Nature), Kiss My Face (with alpha hydroxy acid), Valeant Pharmaceuticals’ Kinerase line (including Clear Skin Regulating Mask), Buster’s Skin Care for Men (peptide complex organic face moisturizer), and Dermalogica (Soothing Shaving Cream with Daily Defense Block), among others.
Conclusion
While stearic acid is an important component in stratum corneum lipids and a widely used ingredient in skin care products, there is a dearth of data on its significance, if any, in the topical dermatologic armamentarium beyond its primary activity as a surfactant and emulsifying agent. Specifically, it remains to be seen whether stearic acid can be replenished in the stratum corneum through topical treatment. Much more research is needed in this area to assess the potential of stearic acid as a therapeutic agent.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. She has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever. E-mail sknews@frontlinemedcom.com to contact Dr. Baumann or to suggest topics for a future column.
Stearic acid, a waxlike fatty acid also known as octadecanoic acid, is an important component of stratum corneum lipids. Stearic acid is also found in cocoa butter, shea butter, and other vegetable fats, as well as animal tallow. As an FDA-approved ingredient in several cosmetic products, it is used as a surfactant and emulsifying agent for fragrance and as the base for other fatty acid ingredients that are synthesized into emollients and lubricants. Stearic acid is used most often to thicken and retain the shape of soaps (indirectly, through saponification of triglycerides composed of stearic acid esters), and it is also used in shampoos, shaving creams, and detergents.
There is limited evidence for the potential of exogenously produced stearic acid to play a significant role as a topical dermatologic therapeutic agent. Stearic acid is thought to be associated with behenyltrimethylammonium chloride through salt bridges, and the combination is believed to have the capacity to build bilayer vesicles with the aid of hinokitiol (beta-thujaplicin), a natural monoterpenoid found in the wood of trees in the Cupressaceae family that has been shown to exert topical inhibitory activity against Chlamydia trachomatis (Antimicrob. Agents Chemother. 2005;49:2519-21). These vesicles, used to enhance the skin permeation of hinokitiol, were tested in hairless mice and appear to have the potential to promote hair growth (Drug Dev. Ind. Pharm. 2010;36:556-62).
In 2000, Khalil et al. studied the effects of cream formulations on chemically induced burns in mice based on reports that the ingredients, docosanol or stearic acid, were associated with antiviral and anti-inflammatory activity. Burns were engendered by painting murine abdomens with a chloroform solution of phenol. Investigators then topically applied the test formulations 0.5, 3, and 6 hours after injury. They found that the docosanol- and stearic acid–containing creams significantly mitigated the severity and progression of skin lesions compared with untreated sites, yielding, respectively, 76% and 57% declines in mean lesion scores (Contact Dermatitis 2000;43:79-81).
In 2001, Fluhr et al. studied the effects of the free fatty acid pool on stratum corneum (SC) acidification and function by topically applying two phospholipase inhibitors – bromphenacylbromide and 1-hexadecyl-3-trifluoroethylglycero-sn-2-phosphomethanol – for 3 days to murine skin. This raised skin pH and yielded permeability barrier abnormality, altered SC integrity, and reduced SC cohesion. All malfunctions were normalized, including SC pH, with the coapplication of either palmitic, stearic, or linoleic acids along with the inhibiting agents (J. Invest. Dermatol. 2001;117:44-51).
In 2010, Mukherjee et al. evaluated a recently marketed mild, moisturizing body wash containing stearic acid and emollient soybean oil to ascertain the location and amount of stearic acid deposited in the SC after in vivo usage of the product. They conducted clinical cleansing studies for 1 and 5 consecutive days using the soybean product or petroleum jelly. The deuterated variant of stearic acid replaced the free stearic acid in the soybean formulation. The researchers detected deuterated stearic acid in all 10 consecutive layers of SC, with a total stearic acid level measured at 0.33 mcg/cm2 after five washes with the soybean oil product. They concluded that the estimated total fatty acid delivered to the skin from cleansing, probably incorporated into the SC lipid phase, is comparable to the fatty acid amount in an SC layer (J. Cosmet. Dermatol. 2010;9:202-10).
Stearic acid is incorporated into several over-the-counter products, including formulations by Aveda (Green Science Firming Face Cream), Yves Rocher (Les Plaisirs Nature), Kiss My Face (with alpha hydroxy acid), Valeant Pharmaceuticals’ Kinerase line (including Clear Skin Regulating Mask), Buster’s Skin Care for Men (peptide complex organic face moisturizer), and Dermalogica (Soothing Shaving Cream with Daily Defense Block), among others.
Conclusion
While stearic acid is an important component in stratum corneum lipids and a widely used ingredient in skin care products, there is a dearth of data on its significance, if any, in the topical dermatologic armamentarium beyond its primary activity as a surfactant and emulsifying agent. Specifically, it remains to be seen whether stearic acid can be replenished in the stratum corneum through topical treatment. Much more research is needed in this area to assess the potential of stearic acid as a therapeutic agent.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, April 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. She has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever. E-mail sknews@frontlinemedcom.com to contact Dr. Baumann or to suggest topics for a future column.
Mulberry
Often in this column, several species within a family might be discussed in relation to a broad range of health benefits. Licorice and mushrooms are good examples. In this case, and in this column, the focus will be on several species within a family that are thought to confer the same type of dermatologic benefit. The Morus genus within the Moraceae family appears to include several species that display skin-lightening properties.
Tyrosinase is the enzyme that controls the production of melanin. Suppressing tyrosinase activity to achieve skin lightening is a well-established method in dermatologic practice. The desire for products with fewer side effects than the mainstay, hydroquinone, or natural products such as kojic acid or arbutin, has led to investigations of several species in the Moraceae family. Notably, several Moraceae trees have been found to exhibit antioxidant activity (Int. J. Mol. Sci. 2012;13:2472-80; Biol. Pharm. Bull. 2002;25:1045-8; Biosci. Biotechnol. Biochem. 2010;74:2385-95; J. Pharm. Pharmacol. 2004;56:1291-8). The focus here, though, will be on the skin-lightening activity of various parts of Morus (commonly known as mulberry) trees.
In 2013, Singh et al. assessed the effects of mulberry, kiwi, and Sophora extracts on melanogenesis and melanin transfer in human melanocytes and in cocultures with phototype-matched normal adult epidermal keratinocytes. The extracts were evaluated against isobutylmethylxanthine, hydroquinone, vitamin C, and niacinamide. The investigators found that compared with unstimulated control, mulberry, kiwi, and Sophora extracts significantly reduced melanogenesis in normal adult epidermal melanocytes and human melanoma cells. Melanin transfer also was lowered, as was filopodia expression on melanocytes. The authors concluded that the test compounds compared well with standard-bearing depigmenting agents and warrant consideration as topical agents for diminishing hyperpigmentation (Exp. Dermatol. 2013;22:67-9).
Encouraging results in melasma treatment
A randomized, single-blind, placebo-controlled trial of 50 Filipino patients (49 women, 1 man) to examine the safety and efficacy of 75% Morus alba (white mulberry) extract oil was conducted by Alvin et al. in 2011. Patients were evaluated at weeks 4 and 8. The Melasma Area and Severity Index (MASI) score, Mexameter score, and Melasma Quality of Life (MelasQOL) score were measured, with the mulberry extract group performing significantly better than the placebo group according to all metrics.
The 25 patients treated with mulberry extract showed improvement in the MASI score, from 4.076 at baseline to 2.884 at week 8 (mean difference, 1.19); the mean difference for the placebo group was 0.06. The mean Mexameter reading revealed a significant difference, with a slight increase for the mulberry group (indicating lighter pigmentation), and the placebo group scored a slightly higher value. In addition, the MelasQOL score for the mulberry group improved markedly from baseline to week 8 (58.84 to 44.16), whereas the placebo group score improved only slightly, from 57.44 at baseline to 54.28 at week 8.
Adverse events were rare, with mild itching in 4 patients reported from the mulberry group, and 12 cases of either itching or erythema reported by the placebo group.
The investigators concluded that 75% mulberry extract oil objectively diminishes the hyperpigmentation of melasma in skin types III-V, although they recommend additional research with a larger sample size and longer treatment duration and follow-up (J. Drugs Dermatol. 2011;10:1025-31).
Paper mulberry
The bark of paper mulberry (Broussonetia papyrifera, also known as Morus papyrifera) is composed of extremely strong fibers used to produce high-quality paper and cloth. In China, the leaves, stem, leaf juice, roots, fruits, and bark have all been found to impart various health benefits, with the stem and leaf juice used to treat skin disorders and insect bites (Phytother. Res. 2012;26:1-10).
In one study, a 0.4% concentration of paper mulberry extract was demonstrated to suppress tyrosinase activity by 50% compared with 5.5% hydroquinone and 10% kojic acid. Notably, paper mulberry is not considered a significant irritant even at 1% concentration (J. Drugs Dermatol. 2009;8:s5-9).
White mulberry
In 2002, Lee et al. investigated the in vitro effects of an 85% methanol extract of dried white mulberry leaves on melanin biosynthesis. They found that one of the primary bioactive constituents, mulberroside F (moracin M-6, 3’-di-O-beta-D-glucopyranoside), inhibited the tyrosinase activity that converts dopa to dopachrome in the melanin synthesis process and also suppressed the melanin formation of melan-a cells. In addition, the mulberry extract inhibited tyrosinase activity more potently than did kojic acid (Biol. Pharm. Bull. 2002;25:1045-8).
The following year, a different team found that the young twigs of white mulberry also suppressed tyrosinase activity as well as melanin production in B-16 melanoma cells. In vivo, the extracts decreased melanin synthesis in a guinea pig model without displaying toxicity (J. Cosmet. Sci. 2003;54:133-42).
In 2006, Wang et al. investigated 25 traditional Chinese herbal medicines potentially useful in dermatology, particularly for skin whitening, and found that white mulberry was one of four species to potently inhibit tyrosinase activity, and more strongly than arbutin did (J. Ethnopharmacol. 2006;106:353-9).
Chinese mulberry/shimaguwa
In 2012, Zheng et al. isolated constituents from the roots of Chinese mulberry and found that several ingredients, including oxyresveratrol, moracenin D, sanggenon T, and kuwanon O, displayed more potent tyrosinase inhibition than kojic acid did. They concluded that Chinese mulberry is a good natural source of tyrosinase inhibitors and is potentially useful in cosmetic skin-lightening products as well as in foods as antibrowning agents (Fitoterapia 2012;83:1008-13).
Conclusion
Mulberry is actively used within the dermatologic armamentarium as one of the many options for skin lightening. A significant body of evidence has emerged over the past 15 years to establish the antityrosinase activity of various mulberry species, particularly white mulberry and paper mulberry.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Often in this column, several species within a family might be discussed in relation to a broad range of health benefits. Licorice and mushrooms are good examples. In this case, and in this column, the focus will be on several species within a family that are thought to confer the same type of dermatologic benefit. The Morus genus within the Moraceae family appears to include several species that display skin-lightening properties.
Tyrosinase is the enzyme that controls the production of melanin. Suppressing tyrosinase activity to achieve skin lightening is a well-established method in dermatologic practice. The desire for products with fewer side effects than the mainstay, hydroquinone, or natural products such as kojic acid or arbutin, has led to investigations of several species in the Moraceae family. Notably, several Moraceae trees have been found to exhibit antioxidant activity (Int. J. Mol. Sci. 2012;13:2472-80; Biol. Pharm. Bull. 2002;25:1045-8; Biosci. Biotechnol. Biochem. 2010;74:2385-95; J. Pharm. Pharmacol. 2004;56:1291-8). The focus here, though, will be on the skin-lightening activity of various parts of Morus (commonly known as mulberry) trees.
In 2013, Singh et al. assessed the effects of mulberry, kiwi, and Sophora extracts on melanogenesis and melanin transfer in human melanocytes and in cocultures with phototype-matched normal adult epidermal keratinocytes. The extracts were evaluated against isobutylmethylxanthine, hydroquinone, vitamin C, and niacinamide. The investigators found that compared with unstimulated control, mulberry, kiwi, and Sophora extracts significantly reduced melanogenesis in normal adult epidermal melanocytes and human melanoma cells. Melanin transfer also was lowered, as was filopodia expression on melanocytes. The authors concluded that the test compounds compared well with standard-bearing depigmenting agents and warrant consideration as topical agents for diminishing hyperpigmentation (Exp. Dermatol. 2013;22:67-9).
Encouraging results in melasma treatment
A randomized, single-blind, placebo-controlled trial of 50 Filipino patients (49 women, 1 man) to examine the safety and efficacy of 75% Morus alba (white mulberry) extract oil was conducted by Alvin et al. in 2011. Patients were evaluated at weeks 4 and 8. The Melasma Area and Severity Index (MASI) score, Mexameter score, and Melasma Quality of Life (MelasQOL) score were measured, with the mulberry extract group performing significantly better than the placebo group according to all metrics.
The 25 patients treated with mulberry extract showed improvement in the MASI score, from 4.076 at baseline to 2.884 at week 8 (mean difference, 1.19); the mean difference for the placebo group was 0.06. The mean Mexameter reading revealed a significant difference, with a slight increase for the mulberry group (indicating lighter pigmentation), and the placebo group scored a slightly higher value. In addition, the MelasQOL score for the mulberry group improved markedly from baseline to week 8 (58.84 to 44.16), whereas the placebo group score improved only slightly, from 57.44 at baseline to 54.28 at week 8.
Adverse events were rare, with mild itching in 4 patients reported from the mulberry group, and 12 cases of either itching or erythema reported by the placebo group.
The investigators concluded that 75% mulberry extract oil objectively diminishes the hyperpigmentation of melasma in skin types III-V, although they recommend additional research with a larger sample size and longer treatment duration and follow-up (J. Drugs Dermatol. 2011;10:1025-31).
Paper mulberry
The bark of paper mulberry (Broussonetia papyrifera, also known as Morus papyrifera) is composed of extremely strong fibers used to produce high-quality paper and cloth. In China, the leaves, stem, leaf juice, roots, fruits, and bark have all been found to impart various health benefits, with the stem and leaf juice used to treat skin disorders and insect bites (Phytother. Res. 2012;26:1-10).
In one study, a 0.4% concentration of paper mulberry extract was demonstrated to suppress tyrosinase activity by 50% compared with 5.5% hydroquinone and 10% kojic acid. Notably, paper mulberry is not considered a significant irritant even at 1% concentration (J. Drugs Dermatol. 2009;8:s5-9).
White mulberry
In 2002, Lee et al. investigated the in vitro effects of an 85% methanol extract of dried white mulberry leaves on melanin biosynthesis. They found that one of the primary bioactive constituents, mulberroside F (moracin M-6, 3’-di-O-beta-D-glucopyranoside), inhibited the tyrosinase activity that converts dopa to dopachrome in the melanin synthesis process and also suppressed the melanin formation of melan-a cells. In addition, the mulberry extract inhibited tyrosinase activity more potently than did kojic acid (Biol. Pharm. Bull. 2002;25:1045-8).
The following year, a different team found that the young twigs of white mulberry also suppressed tyrosinase activity as well as melanin production in B-16 melanoma cells. In vivo, the extracts decreased melanin synthesis in a guinea pig model without displaying toxicity (J. Cosmet. Sci. 2003;54:133-42).
In 2006, Wang et al. investigated 25 traditional Chinese herbal medicines potentially useful in dermatology, particularly for skin whitening, and found that white mulberry was one of four species to potently inhibit tyrosinase activity, and more strongly than arbutin did (J. Ethnopharmacol. 2006;106:353-9).
Chinese mulberry/shimaguwa
In 2012, Zheng et al. isolated constituents from the roots of Chinese mulberry and found that several ingredients, including oxyresveratrol, moracenin D, sanggenon T, and kuwanon O, displayed more potent tyrosinase inhibition than kojic acid did. They concluded that Chinese mulberry is a good natural source of tyrosinase inhibitors and is potentially useful in cosmetic skin-lightening products as well as in foods as antibrowning agents (Fitoterapia 2012;83:1008-13).
Conclusion
Mulberry is actively used within the dermatologic armamentarium as one of the many options for skin lightening. A significant body of evidence has emerged over the past 15 years to establish the antityrosinase activity of various mulberry species, particularly white mulberry and paper mulberry.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Often in this column, several species within a family might be discussed in relation to a broad range of health benefits. Licorice and mushrooms are good examples. In this case, and in this column, the focus will be on several species within a family that are thought to confer the same type of dermatologic benefit. The Morus genus within the Moraceae family appears to include several species that display skin-lightening properties.
Tyrosinase is the enzyme that controls the production of melanin. Suppressing tyrosinase activity to achieve skin lightening is a well-established method in dermatologic practice. The desire for products with fewer side effects than the mainstay, hydroquinone, or natural products such as kojic acid or arbutin, has led to investigations of several species in the Moraceae family. Notably, several Moraceae trees have been found to exhibit antioxidant activity (Int. J. Mol. Sci. 2012;13:2472-80; Biol. Pharm. Bull. 2002;25:1045-8; Biosci. Biotechnol. Biochem. 2010;74:2385-95; J. Pharm. Pharmacol. 2004;56:1291-8). The focus here, though, will be on the skin-lightening activity of various parts of Morus (commonly known as mulberry) trees.
In 2013, Singh et al. assessed the effects of mulberry, kiwi, and Sophora extracts on melanogenesis and melanin transfer in human melanocytes and in cocultures with phototype-matched normal adult epidermal keratinocytes. The extracts were evaluated against isobutylmethylxanthine, hydroquinone, vitamin C, and niacinamide. The investigators found that compared with unstimulated control, mulberry, kiwi, and Sophora extracts significantly reduced melanogenesis in normal adult epidermal melanocytes and human melanoma cells. Melanin transfer also was lowered, as was filopodia expression on melanocytes. The authors concluded that the test compounds compared well with standard-bearing depigmenting agents and warrant consideration as topical agents for diminishing hyperpigmentation (Exp. Dermatol. 2013;22:67-9).
Encouraging results in melasma treatment
A randomized, single-blind, placebo-controlled trial of 50 Filipino patients (49 women, 1 man) to examine the safety and efficacy of 75% Morus alba (white mulberry) extract oil was conducted by Alvin et al. in 2011. Patients were evaluated at weeks 4 and 8. The Melasma Area and Severity Index (MASI) score, Mexameter score, and Melasma Quality of Life (MelasQOL) score were measured, with the mulberry extract group performing significantly better than the placebo group according to all metrics.
The 25 patients treated with mulberry extract showed improvement in the MASI score, from 4.076 at baseline to 2.884 at week 8 (mean difference, 1.19); the mean difference for the placebo group was 0.06. The mean Mexameter reading revealed a significant difference, with a slight increase for the mulberry group (indicating lighter pigmentation), and the placebo group scored a slightly higher value. In addition, the MelasQOL score for the mulberry group improved markedly from baseline to week 8 (58.84 to 44.16), whereas the placebo group score improved only slightly, from 57.44 at baseline to 54.28 at week 8.
Adverse events were rare, with mild itching in 4 patients reported from the mulberry group, and 12 cases of either itching or erythema reported by the placebo group.
The investigators concluded that 75% mulberry extract oil objectively diminishes the hyperpigmentation of melasma in skin types III-V, although they recommend additional research with a larger sample size and longer treatment duration and follow-up (J. Drugs Dermatol. 2011;10:1025-31).
Paper mulberry
The bark of paper mulberry (Broussonetia papyrifera, also known as Morus papyrifera) is composed of extremely strong fibers used to produce high-quality paper and cloth. In China, the leaves, stem, leaf juice, roots, fruits, and bark have all been found to impart various health benefits, with the stem and leaf juice used to treat skin disorders and insect bites (Phytother. Res. 2012;26:1-10).
In one study, a 0.4% concentration of paper mulberry extract was demonstrated to suppress tyrosinase activity by 50% compared with 5.5% hydroquinone and 10% kojic acid. Notably, paper mulberry is not considered a significant irritant even at 1% concentration (J. Drugs Dermatol. 2009;8:s5-9).
White mulberry
In 2002, Lee et al. investigated the in vitro effects of an 85% methanol extract of dried white mulberry leaves on melanin biosynthesis. They found that one of the primary bioactive constituents, mulberroside F (moracin M-6, 3’-di-O-beta-D-glucopyranoside), inhibited the tyrosinase activity that converts dopa to dopachrome in the melanin synthesis process and also suppressed the melanin formation of melan-a cells. In addition, the mulberry extract inhibited tyrosinase activity more potently than did kojic acid (Biol. Pharm. Bull. 2002;25:1045-8).
The following year, a different team found that the young twigs of white mulberry also suppressed tyrosinase activity as well as melanin production in B-16 melanoma cells. In vivo, the extracts decreased melanin synthesis in a guinea pig model without displaying toxicity (J. Cosmet. Sci. 2003;54:133-42).
In 2006, Wang et al. investigated 25 traditional Chinese herbal medicines potentially useful in dermatology, particularly for skin whitening, and found that white mulberry was one of four species to potently inhibit tyrosinase activity, and more strongly than arbutin did (J. Ethnopharmacol. 2006;106:353-9).
Chinese mulberry/shimaguwa
In 2012, Zheng et al. isolated constituents from the roots of Chinese mulberry and found that several ingredients, including oxyresveratrol, moracenin D, sanggenon T, and kuwanon O, displayed more potent tyrosinase inhibition than kojic acid did. They concluded that Chinese mulberry is a good natural source of tyrosinase inhibitors and is potentially useful in cosmetic skin-lightening products as well as in foods as antibrowning agents (Fitoterapia 2012;83:1008-13).
Conclusion
Mulberry is actively used within the dermatologic armamentarium as one of the many options for skin lightening. A significant body of evidence has emerged over the past 15 years to establish the antityrosinase activity of various mulberry species, particularly white mulberry and paper mulberry.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She founded the cosmetic dermatology center at the University of Miami in 1997. Dr. Baumann wrote the textbook "Cosmetic Dermatology: Principles and Practice" (McGraw-Hill, 2009), and a book for consumers, "The Skin Type Solution" (Bantam, 2006). She has contributed to the Cosmeceutical Critique column in Skin & Allergy News since January 2001 and joined the editorial advisory board in 2004. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever.
Tamanu oil
Calophyllum inophyllum is a large, nondeciduous tree native to a wide swath of regions including Central and East Africa; a north-to-south swath of India; Southeast Asia; Polynesia; the Philippines; and Australia. A member of the mangosteen family (Clusiaceae, also known as Guttiferae), C. inophyllum is now cultivated in much of the tropical world.
The oil derived from this abundant plant is known by a wide variety of names, including Alexandrian laurel, beach mahogany, beauty leaf, beach calophyllum, dilo, and kamani. But perhaps it is best known by the French Polynesian name: tamanu. Tamanu oil has been used for hundreds of years in cuisine and to treat various medical conditions. Ocular burn and cutaneous wound healing are some distinct conditions for which the oil of C. inophyllum has a long history of use in traditional folk medicine (Oncol. Rep. 2012;28:1096-102; Int. J. Cosmet. Sci. 2002;24:341-8).
Wound healing and eye protection
The C. inophyllum components to which wound-healing activity have been attributed include calophyllolide and inophyllum, as well as various polyphenols, many of which exert antioxidant effects (Int. J. Cosmet. Sci. 2002;24:341-8).
Modern research buttresses the use of tamanu oil for corneal protection from burns. In 2007, Said et al. explored the anti-UV activity of tamanu oil for eye protection. They found that the botanical oil displayed a significant capacity to absorb UV radiation, even at low concentrations (1/10,000, v/v), with a sun protection factor ranging from 18 to 22. Concentrations of C. inophyllum oil of up to 1% were not cytotoxic to human conjunctival epithelial cells, with the agent acting against oxidative stress and DNA damage. In light of the apparent antioxidant and cytoprotective effects of C. inophyllum oil in the study, the researchers concluded that the oil has potential as a natural UV filter in ophthalmic formulations (Eur. J. Pharm. Sci. 2007;30:203-10).
In 2009, Said et al. performed in vitro, in vivo, and ex vivo studies to assess the effects of different rinsing and healing protocols for alkali-induced ocular burn and inflammation in rabbits. The researchers used NaOH to induce corneal reactions in the rabbits, followed by rinses with NaCl 0.9% or controlled-ionization marine formula combined with N-acetylcysteine or vegetable oils (from C. inophyllum and Aleurites moluccana). The investigators assessed corneal epithelium regeneration and inflammatory cell infiltration using in vivo confocal microscopy and ex vivo histological cuts. They found that the combination of controlled-ionization marine solution with 10% C. inophyllum oil and 90% A. moluccana oil promoted corneal epithelium regeneration while reducing inflammatory cells, suggesting its viability as ocular burn therapy (Ophthalmologica 2009;223:52-9).
Other medical benefits
A wide range of health benefits have been ascribed to tamanu oil, and the ingredient has been found in an increasing number of topical products. It is thought to impart anti-inflammatory, antioxidant, antibacterial, antiviral, and photoprotective activity.
In 2011, Ayyanar et al. concluded a 4-year study intended to ascertain the herbs used in traditional medicine practiced by the Kani tribes in the Tirunelveli hills of Western Ghats, India. The researchers identified 90 species of plants used traditionally as ethnomedicinal treatments, with 65 different indications reported, particularly dermatologic conditions and gastrointestinal illnesses. Based on their study, they identified 16 species, including C. inophyllum, for additional ethnopharmacological investigation as potential sources of new drug agents (J. Ethnopharmacol. 2011;134:851-64).
In 2004, Yimdjo et al. investigated the chemical constituents of the root bark and nut of C. inophyllum, resulting in the isolation of several compounds and the discovery of antibacterial activity against several microbes (Phytochemistry 2004;65:2789-95).
C. inophyllum leaf extracts from the islands of French Polynesia have also been touted for several constituents that hold promise as anti-HIV-1 agents, including inophyllum B and P (Anal. Chim. Acta 2008;624:147-53). In addition, quantitative high-performance liquid chromatography (HPLC) analysis of callus cultures of C. inophyllum has revealed the anti-HIV activity of the dipyranocoumarins inophyllum B and P (J. Biotechnol. 2007;130:346-53).
Tamanu oil also has demonstrated potential use for humans and domestic animals as an insect repellent, specifically against the stable fly, Stomoxys calcitrans (J. Med. Entomol. 2010;47:575-80; Pest Manag. Sci. 2010;66:1191-8).
In 2012, Tsai et al. investigated the anti-inflammatory properties of an acetone extract of C. inophyllum leaves using lipopolysaccharide (LPS)-induced RAW 264.7 cells to assess the impact of the extract on nitric oxide (NO) expression and inducible nitric oxide synthase (iNOS). They found that C. inophyllum significantly inhibited, in dose-dependent fashion, the LPS-induced synthesis of NO, in addition to the expression of iNOS, cyclooxygenase (COX)-2, and nuclear factor–kappa B (NF-kappaB). The researchers concluded that the C. inophyllum extract exhibits anti-inflammatory activity and has potential application to inflammatory conditions in human beings (Oncol. Rep. 2012;28:1096-102).
Cancer
Recent work suggests the anti-cancer potential of C. inophyllum. In a study just over a decade ago, Itogawa et al. examined the potential inhibitory effects of C. inophyllum 4-phenylcoumarin isolates on Epstein-Barr virus early antigen (EBV-EA) activation caused by 12-O-tetradecanoylphorbol-13-acetate in Raji cells. All 10 of the isolates displayed inhibitory activity against EBV and no cytotoxicity. The strongest compound tested was calocoumarin-A (5), which also demonstrated a significant capacity to suppress murine skin tumor promotion in a two-stage cancer model. The investigators concluded that some 4-phenylcoumarin constituents of C. inophyllum warrant further study as possible antitumor agents (Cancer Lett. 2001;169:15-19).
C. inophyllum was one of 155 extracts from 93 plant species found on peninsular Malaysia during a screening by Ong et al. in 2009 for in vitro photocytotoxic activity using human leukemia cells (cell line HL60). Further, C. inophyllum was among the 29 plants to lower the in vitro cell viability by more than 50% after exposure to 9.6 J/cm2 of a broad-spectrum light when tested at a concentration of 20 mcg/mL (J. Photochem. Photobiol. B 2009;96:216-22). In addition, Li et al. isolated one new friedelane-type triterpene and seven previously discovered triterpenoids from the stems and leaves of C. inophyllum, and ascertained that they exhibited growth inhibitory activity against human leukemia HL-60 cells (Fitoterapia 2010;81:586-9).
In 2008, Xiao et al. isolated a new prenylated xanthone (caloxanthone) as well as two previously known xanthones from the ethanolic extract of C. inophyllum twigs and reported that two of the constituents (including the new xanthone) demonstrated cytotoxicity against myelogenous leukemia (cell line K562) (J. Asian Nat. Prod. Res. 2008;10:993-7).
C. inophyllum is known to contain an abundance of phytosterols – primarily stigmasterol and beta-sitosterol, which are steroids associated with several healthy benefits (stigmasterol is a potent antioxidant) – as well as delta-tocotrienol, a form of vitamin E that acts as an antioxidant and is associated with anticancer activity, particularly against murine melanoma (Phytochemistry 2005;66:1825-31; J. Nutr. 1997;127:668-74).
Conclusion
Tamanu oil certainly isn’t a passing fad for the numerous traditional societies in the mainly eastern and southern hemispheres who have used the botanical for medicinal and culinary purposes for centuries. As an ingredient in skin care products, though, more research is needed. While modern studies are promising, randomized, placebo-controlled clinical trials are necessary to establish a potential role of C. inophyllum in the large array of topical dermatologic formulations.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever. To respond to this column, or to suggest topics for future columns, write to her at sknews@frontlinemedcom.com.
Calophyllum inophyllum is a large, nondeciduous tree native to a wide swath of regions including Central and East Africa; a north-to-south swath of India; Southeast Asia; Polynesia; the Philippines; and Australia. A member of the mangosteen family (Clusiaceae, also known as Guttiferae), C. inophyllum is now cultivated in much of the tropical world.
The oil derived from this abundant plant is known by a wide variety of names, including Alexandrian laurel, beach mahogany, beauty leaf, beach calophyllum, dilo, and kamani. But perhaps it is best known by the French Polynesian name: tamanu. Tamanu oil has been used for hundreds of years in cuisine and to treat various medical conditions. Ocular burn and cutaneous wound healing are some distinct conditions for which the oil of C. inophyllum has a long history of use in traditional folk medicine (Oncol. Rep. 2012;28:1096-102; Int. J. Cosmet. Sci. 2002;24:341-8).
Wound healing and eye protection
The C. inophyllum components to which wound-healing activity have been attributed include calophyllolide and inophyllum, as well as various polyphenols, many of which exert antioxidant effects (Int. J. Cosmet. Sci. 2002;24:341-8).
Modern research buttresses the use of tamanu oil for corneal protection from burns. In 2007, Said et al. explored the anti-UV activity of tamanu oil for eye protection. They found that the botanical oil displayed a significant capacity to absorb UV radiation, even at low concentrations (1/10,000, v/v), with a sun protection factor ranging from 18 to 22. Concentrations of C. inophyllum oil of up to 1% were not cytotoxic to human conjunctival epithelial cells, with the agent acting against oxidative stress and DNA damage. In light of the apparent antioxidant and cytoprotective effects of C. inophyllum oil in the study, the researchers concluded that the oil has potential as a natural UV filter in ophthalmic formulations (Eur. J. Pharm. Sci. 2007;30:203-10).
In 2009, Said et al. performed in vitro, in vivo, and ex vivo studies to assess the effects of different rinsing and healing protocols for alkali-induced ocular burn and inflammation in rabbits. The researchers used NaOH to induce corneal reactions in the rabbits, followed by rinses with NaCl 0.9% or controlled-ionization marine formula combined with N-acetylcysteine or vegetable oils (from C. inophyllum and Aleurites moluccana). The investigators assessed corneal epithelium regeneration and inflammatory cell infiltration using in vivo confocal microscopy and ex vivo histological cuts. They found that the combination of controlled-ionization marine solution with 10% C. inophyllum oil and 90% A. moluccana oil promoted corneal epithelium regeneration while reducing inflammatory cells, suggesting its viability as ocular burn therapy (Ophthalmologica 2009;223:52-9).
Other medical benefits
A wide range of health benefits have been ascribed to tamanu oil, and the ingredient has been found in an increasing number of topical products. It is thought to impart anti-inflammatory, antioxidant, antibacterial, antiviral, and photoprotective activity.
In 2011, Ayyanar et al. concluded a 4-year study intended to ascertain the herbs used in traditional medicine practiced by the Kani tribes in the Tirunelveli hills of Western Ghats, India. The researchers identified 90 species of plants used traditionally as ethnomedicinal treatments, with 65 different indications reported, particularly dermatologic conditions and gastrointestinal illnesses. Based on their study, they identified 16 species, including C. inophyllum, for additional ethnopharmacological investigation as potential sources of new drug agents (J. Ethnopharmacol. 2011;134:851-64).
In 2004, Yimdjo et al. investigated the chemical constituents of the root bark and nut of C. inophyllum, resulting in the isolation of several compounds and the discovery of antibacterial activity against several microbes (Phytochemistry 2004;65:2789-95).
C. inophyllum leaf extracts from the islands of French Polynesia have also been touted for several constituents that hold promise as anti-HIV-1 agents, including inophyllum B and P (Anal. Chim. Acta 2008;624:147-53). In addition, quantitative high-performance liquid chromatography (HPLC) analysis of callus cultures of C. inophyllum has revealed the anti-HIV activity of the dipyranocoumarins inophyllum B and P (J. Biotechnol. 2007;130:346-53).
Tamanu oil also has demonstrated potential use for humans and domestic animals as an insect repellent, specifically against the stable fly, Stomoxys calcitrans (J. Med. Entomol. 2010;47:575-80; Pest Manag. Sci. 2010;66:1191-8).
In 2012, Tsai et al. investigated the anti-inflammatory properties of an acetone extract of C. inophyllum leaves using lipopolysaccharide (LPS)-induced RAW 264.7 cells to assess the impact of the extract on nitric oxide (NO) expression and inducible nitric oxide synthase (iNOS). They found that C. inophyllum significantly inhibited, in dose-dependent fashion, the LPS-induced synthesis of NO, in addition to the expression of iNOS, cyclooxygenase (COX)-2, and nuclear factor–kappa B (NF-kappaB). The researchers concluded that the C. inophyllum extract exhibits anti-inflammatory activity and has potential application to inflammatory conditions in human beings (Oncol. Rep. 2012;28:1096-102).
Cancer
Recent work suggests the anti-cancer potential of C. inophyllum. In a study just over a decade ago, Itogawa et al. examined the potential inhibitory effects of C. inophyllum 4-phenylcoumarin isolates on Epstein-Barr virus early antigen (EBV-EA) activation caused by 12-O-tetradecanoylphorbol-13-acetate in Raji cells. All 10 of the isolates displayed inhibitory activity against EBV and no cytotoxicity. The strongest compound tested was calocoumarin-A (5), which also demonstrated a significant capacity to suppress murine skin tumor promotion in a two-stage cancer model. The investigators concluded that some 4-phenylcoumarin constituents of C. inophyllum warrant further study as possible antitumor agents (Cancer Lett. 2001;169:15-19).
C. inophyllum was one of 155 extracts from 93 plant species found on peninsular Malaysia during a screening by Ong et al. in 2009 for in vitro photocytotoxic activity using human leukemia cells (cell line HL60). Further, C. inophyllum was among the 29 plants to lower the in vitro cell viability by more than 50% after exposure to 9.6 J/cm2 of a broad-spectrum light when tested at a concentration of 20 mcg/mL (J. Photochem. Photobiol. B 2009;96:216-22). In addition, Li et al. isolated one new friedelane-type triterpene and seven previously discovered triterpenoids from the stems and leaves of C. inophyllum, and ascertained that they exhibited growth inhibitory activity against human leukemia HL-60 cells (Fitoterapia 2010;81:586-9).
In 2008, Xiao et al. isolated a new prenylated xanthone (caloxanthone) as well as two previously known xanthones from the ethanolic extract of C. inophyllum twigs and reported that two of the constituents (including the new xanthone) demonstrated cytotoxicity against myelogenous leukemia (cell line K562) (J. Asian Nat. Prod. Res. 2008;10:993-7).
C. inophyllum is known to contain an abundance of phytosterols – primarily stigmasterol and beta-sitosterol, which are steroids associated with several healthy benefits (stigmasterol is a potent antioxidant) – as well as delta-tocotrienol, a form of vitamin E that acts as an antioxidant and is associated with anticancer activity, particularly against murine melanoma (Phytochemistry 2005;66:1825-31; J. Nutr. 1997;127:668-74).
Conclusion
Tamanu oil certainly isn’t a passing fad for the numerous traditional societies in the mainly eastern and southern hemispheres who have used the botanical for medicinal and culinary purposes for centuries. As an ingredient in skin care products, though, more research is needed. While modern studies are promising, randomized, placebo-controlled clinical trials are necessary to establish a potential role of C. inophyllum in the large array of topical dermatologic formulations.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever. To respond to this column, or to suggest topics for future columns, write to her at sknews@frontlinemedcom.com.
Calophyllum inophyllum is a large, nondeciduous tree native to a wide swath of regions including Central and East Africa; a north-to-south swath of India; Southeast Asia; Polynesia; the Philippines; and Australia. A member of the mangosteen family (Clusiaceae, also known as Guttiferae), C. inophyllum is now cultivated in much of the tropical world.
The oil derived from this abundant plant is known by a wide variety of names, including Alexandrian laurel, beach mahogany, beauty leaf, beach calophyllum, dilo, and kamani. But perhaps it is best known by the French Polynesian name: tamanu. Tamanu oil has been used for hundreds of years in cuisine and to treat various medical conditions. Ocular burn and cutaneous wound healing are some distinct conditions for which the oil of C. inophyllum has a long history of use in traditional folk medicine (Oncol. Rep. 2012;28:1096-102; Int. J. Cosmet. Sci. 2002;24:341-8).
Wound healing and eye protection
The C. inophyllum components to which wound-healing activity have been attributed include calophyllolide and inophyllum, as well as various polyphenols, many of which exert antioxidant effects (Int. J. Cosmet. Sci. 2002;24:341-8).
Modern research buttresses the use of tamanu oil for corneal protection from burns. In 2007, Said et al. explored the anti-UV activity of tamanu oil for eye protection. They found that the botanical oil displayed a significant capacity to absorb UV radiation, even at low concentrations (1/10,000, v/v), with a sun protection factor ranging from 18 to 22. Concentrations of C. inophyllum oil of up to 1% were not cytotoxic to human conjunctival epithelial cells, with the agent acting against oxidative stress and DNA damage. In light of the apparent antioxidant and cytoprotective effects of C. inophyllum oil in the study, the researchers concluded that the oil has potential as a natural UV filter in ophthalmic formulations (Eur. J. Pharm. Sci. 2007;30:203-10).
In 2009, Said et al. performed in vitro, in vivo, and ex vivo studies to assess the effects of different rinsing and healing protocols for alkali-induced ocular burn and inflammation in rabbits. The researchers used NaOH to induce corneal reactions in the rabbits, followed by rinses with NaCl 0.9% or controlled-ionization marine formula combined with N-acetylcysteine or vegetable oils (from C. inophyllum and Aleurites moluccana). The investigators assessed corneal epithelium regeneration and inflammatory cell infiltration using in vivo confocal microscopy and ex vivo histological cuts. They found that the combination of controlled-ionization marine solution with 10% C. inophyllum oil and 90% A. moluccana oil promoted corneal epithelium regeneration while reducing inflammatory cells, suggesting its viability as ocular burn therapy (Ophthalmologica 2009;223:52-9).
Other medical benefits
A wide range of health benefits have been ascribed to tamanu oil, and the ingredient has been found in an increasing number of topical products. It is thought to impart anti-inflammatory, antioxidant, antibacterial, antiviral, and photoprotective activity.
In 2011, Ayyanar et al. concluded a 4-year study intended to ascertain the herbs used in traditional medicine practiced by the Kani tribes in the Tirunelveli hills of Western Ghats, India. The researchers identified 90 species of plants used traditionally as ethnomedicinal treatments, with 65 different indications reported, particularly dermatologic conditions and gastrointestinal illnesses. Based on their study, they identified 16 species, including C. inophyllum, for additional ethnopharmacological investigation as potential sources of new drug agents (J. Ethnopharmacol. 2011;134:851-64).
In 2004, Yimdjo et al. investigated the chemical constituents of the root bark and nut of C. inophyllum, resulting in the isolation of several compounds and the discovery of antibacterial activity against several microbes (Phytochemistry 2004;65:2789-95).
C. inophyllum leaf extracts from the islands of French Polynesia have also been touted for several constituents that hold promise as anti-HIV-1 agents, including inophyllum B and P (Anal. Chim. Acta 2008;624:147-53). In addition, quantitative high-performance liquid chromatography (HPLC) analysis of callus cultures of C. inophyllum has revealed the anti-HIV activity of the dipyranocoumarins inophyllum B and P (J. Biotechnol. 2007;130:346-53).
Tamanu oil also has demonstrated potential use for humans and domestic animals as an insect repellent, specifically against the stable fly, Stomoxys calcitrans (J. Med. Entomol. 2010;47:575-80; Pest Manag. Sci. 2010;66:1191-8).
In 2012, Tsai et al. investigated the anti-inflammatory properties of an acetone extract of C. inophyllum leaves using lipopolysaccharide (LPS)-induced RAW 264.7 cells to assess the impact of the extract on nitric oxide (NO) expression and inducible nitric oxide synthase (iNOS). They found that C. inophyllum significantly inhibited, in dose-dependent fashion, the LPS-induced synthesis of NO, in addition to the expression of iNOS, cyclooxygenase (COX)-2, and nuclear factor–kappa B (NF-kappaB). The researchers concluded that the C. inophyllum extract exhibits anti-inflammatory activity and has potential application to inflammatory conditions in human beings (Oncol. Rep. 2012;28:1096-102).
Cancer
Recent work suggests the anti-cancer potential of C. inophyllum. In a study just over a decade ago, Itogawa et al. examined the potential inhibitory effects of C. inophyllum 4-phenylcoumarin isolates on Epstein-Barr virus early antigen (EBV-EA) activation caused by 12-O-tetradecanoylphorbol-13-acetate in Raji cells. All 10 of the isolates displayed inhibitory activity against EBV and no cytotoxicity. The strongest compound tested was calocoumarin-A (5), which also demonstrated a significant capacity to suppress murine skin tumor promotion in a two-stage cancer model. The investigators concluded that some 4-phenylcoumarin constituents of C. inophyllum warrant further study as possible antitumor agents (Cancer Lett. 2001;169:15-19).
C. inophyllum was one of 155 extracts from 93 plant species found on peninsular Malaysia during a screening by Ong et al. in 2009 for in vitro photocytotoxic activity using human leukemia cells (cell line HL60). Further, C. inophyllum was among the 29 plants to lower the in vitro cell viability by more than 50% after exposure to 9.6 J/cm2 of a broad-spectrum light when tested at a concentration of 20 mcg/mL (J. Photochem. Photobiol. B 2009;96:216-22). In addition, Li et al. isolated one new friedelane-type triterpene and seven previously discovered triterpenoids from the stems and leaves of C. inophyllum, and ascertained that they exhibited growth inhibitory activity against human leukemia HL-60 cells (Fitoterapia 2010;81:586-9).
In 2008, Xiao et al. isolated a new prenylated xanthone (caloxanthone) as well as two previously known xanthones from the ethanolic extract of C. inophyllum twigs and reported that two of the constituents (including the new xanthone) demonstrated cytotoxicity against myelogenous leukemia (cell line K562) (J. Asian Nat. Prod. Res. 2008;10:993-7).
C. inophyllum is known to contain an abundance of phytosterols – primarily stigmasterol and beta-sitosterol, which are steroids associated with several healthy benefits (stigmasterol is a potent antioxidant) – as well as delta-tocotrienol, a form of vitamin E that acts as an antioxidant and is associated with anticancer activity, particularly against murine melanoma (Phytochemistry 2005;66:1825-31; J. Nutr. 1997;127:668-74).
Conclusion
Tamanu oil certainly isn’t a passing fad for the numerous traditional societies in the mainly eastern and southern hemispheres who have used the botanical for medicinal and culinary purposes for centuries. As an ingredient in skin care products, though, more research is needed. While modern studies are promising, randomized, placebo-controlled clinical trials are necessary to establish a potential role of C. inophyllum in the large array of topical dermatologic formulations.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in Miami Beach. She has received funding for clinical grants from Allergan, Aveeno, Avon Products, Galderma, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Stiefel, Topix Pharmaceuticals, and Unilever. To respond to this column, or to suggest topics for future columns, write to her at sknews@frontlinemedcom.com.
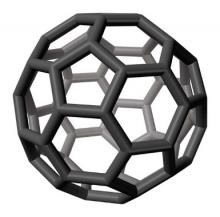
Fullerenes
Discovered in nature in 1985 (Science 1992;257:215-7), fullerenes are novel, classically engineered chemical compounds composed only of carbon atoms. The stable form, a third carbon allotrope, is a molecule made up of 60 carbon atoms in a structure resembling a soccer ball or geodesic dome, although some fullerenes are ellipsoid or tube shaped. In fact, fullerene C(60), the first of the group of compounds to be discovered, is also known as buckminsterfullerene, based on Buckminster Fuller’s geodesic dome. The carbon nanotube structure, composed of thin carbon filaments (1-3 mcm in length and 1-3 nm in diameter) and possessing a wide range of mechanical characteristics, was discovered in 1991 (J. Nanosci. Nanotechnol. 2006;6:591-9).
The potential applications of C(60) and fullerene derivatives have been extensively studied, and recent data suggest that fullerenes exhibit potent antioxidant activity, even acting as a "free radical sponge" (Bioorg. Med. Chem. Lett. 2006;16:1590-5; Biomaterials 2008;29:3561-73). Therefore, fullerenes may be appropriate active ingredients for various skin care products, particularly rejuvenation products (Recent Pat. Biotechnol. 2011;5:67-73; Recent. Pat. Biotechnol. 2009;3:118-23).
Early studies
In one of the early studies of topical applications of fullerenes (C60), Nelson et al. examined the potential acute and subchronic toxic effects of fullerenes (200 mcg) applied in benzene on mouse skin. After 72 hours, they observed no effect on either DNA synthesis or ornithine decarboxylase activity. A skin tumor initiation model using 7,12-dimethylbenzanthracene (DMBA) failed to show benign or malignant skin tumor formation, but promotion using 12-O-tetradecanoyl-phorbol-13-acetate (TPA) resulted in benign skin tumors. The investigators concluded that fullerenes applied in benzene at a likely industrial exposure level did not cause acute toxic effects in mice (Toxicol. Ind. Health. 1993;9:623-30).
In 1997, Tabata et al., noted that fullerene C(60) efficiently generates singlet oxygen when irradiated with light, and found that polyethylene glycol (PEG)-modified C60 exhibits potential as an agent for photodynamic tumor therapy (Jpn. J. Cancer Res. 1997;88:1108-16).
Antiviral capacity
Fullerenes exhibit unique chemical and physical properties, including photodynamic characteristics and a hydrophobic spheroid and radical sponge quality. They also display antiviral activity, particularly in relation to HIV, and their potential commercial applications include use in patent-pending anticancer drug delivery systems employing photodynamic therapy (PDT), HIV drugs, and antiaging cosmetics (Int. J. Nanomedicine 2007;2:639-49).
Anti-inflammatory activity
An anti-inflammatory role has also been identified for fullerenes. Ryan et al. noted that preincubation with C(60) resulted in significant suppression of IgE-dependent mediator release in human mast cells and peripheral blood basophils. IgE-induced increases in cytoplasmic reactive oxygen species (ROS) levels was also hindered by preincubation with fullerenes. Using a mast cell–dependent anaphylaxis model, Ryan and colleagues also found that fullerenes prevented the in vivo release of histamine and fall in core body temperature. They concluded that fullerenes might suggest an innovative approach to managing mast cell–dependent conditions such as asthma, heart disease, inflammatory arthritis, and multiple sclerosis (J. Immunol. 2007;179(1):665-72).
Antimicrobial potential
In 2005, Tegos et al. compared the antimicrobial activity of six C(60) compounds functionalized with one, two, or three hydrophilic or cationic groups combined with white light against gram-positive and negative bacteria, and fungi. They found that following 10 minutes of incubation, bis- and tris-cationic fullerenes actively eliminated all tested microbes while leaving mammalian cells comparatively intact. The investigators also noted that the fullerene compounds were significantly more effective than a widely used antimicrobial photosensitizer, toluidine blue O. They concluded that the compounds warrant consideration as photosensitizers for antimicrobial use given their high selectivity and efficacy (Chem. Biol. 2005;12(10):1127-35).
Potent antioxidant
In 2006, Xiao et al. reported on their development of the Radical Sponge, a fullerene entrapped in polyvinylpyrrolidone to yield a water-soluble derivative with a mean particle diameter of about 688 nm and reactive oxygen species (ROS) scavenging abilities. The researchers repeatedly irradiated human skin keratinocytes (HaCaT) with visible light (400-2,000 nm) in the presence or absence of Radical Sponge, with no photocytotoxicity apparent in the Radical Sponge–exposed cells. In addition, the water-soluble fullerene derivative displayed a cytoprotective effect (10-40 mcM doses) against UVA exposure (30 J/cm2) when it was administered prior to exposure and rinsed out immediately before the irradiation, more so than when administered only during or after irradiation. The researchers concluded that this finding suggested more of a preventive as opposed to therapeutic effect conferred by Radical Sponge against UVA damage (Bioorg. Med. Chem. Lett. 2006;16:1590-5).
In a subsequent study, the researchers compared the Radical Sponge with two whitening agents and found the fullerene to have imparted stronger antimelanogenic activity, possibly due to down-regulation of the tyrosinase expression promoted by UVA-induced ROS generation (Arch. Dermatol. Res. 2007;299(5-6):245-57).
More recently, Kato et al. demonstrated the antioxidant activity of C(60) incorporated into liposomes, with persistent scavenging of hydroxyl radicals and cytoprotection of keratinocytes against UVA- and UVB-induced damage ascribed to the fullerene component (J. Nanosci. Nanotechnol. 2011;11(5):3814-23).
Safety
The first study establishing the safety of highly purified fullerenes (HPFs) as an additive in cosmeceuticals was conducted in 2009. Aoshima et al. performed in vivo tests in animals and in vitro examinations using human epidermal keratinocytes and fibroblasts. No primary or cumulative skin irritation, sensitization, photosensitization, or contact phototoxicity was observed. In the patch test on human skin, no reaction was noted. HPFs were deemed to be "minimally irritating" after the eye-irritation test in rabbits. The investigators concluded, based on their findings and in light of previously published data, that HPFs are safe for human skin as ingredients in cosmetic skin care formulations (J. Toxicol. Sci. 2009;34(5):555-62).
Hair regrowth
In 2009, Zhou et al. used shaved mice and SKH-1 hairless mice to study whether fullerene-based compounds could elicit hair growth. Fullerenes were found to significantly increase the hair growth rate compared with a placebo vehicle. Significantly increased numbers of hair follicles were also observed in SKH-1 hairless mice treated topically or subdermally with fullerenes. Cultured human skin treated with fullerenes also showed augmented hair growth. The data suggested implications for hair loss due to alopecia, chemotherapy, or other chemical reactions (Nanomedicine 2009;5:202-7).
Antiacne properties
In 2011, Inui et al. conducted an open trial of effects of fullerene gel on acne. Subjects used the gel twice daily, and significant reductions in the mean number of inflammatory lesions were noted at 4 and 8 weeks of treatment. In addition, the researchers conducted in vitro assays of sebum production in hamster sebocytes and found that 75 mcM polyvinylpyrrolidone-fullerene suppressed sebum production, suggesting that topical fullerenes inhibit acne by reducing neutrophil infiltration and sebum production (Nanomedicine 2011;7:238-41).
Water solubility and photodynamic activity
In 2007, Mroz et al. compared the photodynamic activity of six fullerenes functionalized to become soluble with 1, 2, or 3 hydrophilic or 1, 2, or 3 cationic groups in three mouse cancer cell lines (J774, LLC, and CT26) incubated for 24 hours with fullerenes and illuminated with white light. They found that some functionalized fullerenes (particularly monopyrrolidinium fullerene) induced apoptosis in all cancer lines (Free Radic. Biol. Med. 2007;43:711-9). In a subsequent study, Mroz et al. found that some fullerenes can be functionalized to photoinactivate pathogenic malignant cancer cells and/or microbial cells in a mechanism that involves superoxide anion and singlet oxygen. The researchers suggested that fullerenes have the potential to supersede photosensitizers in current clinical use in PDT for some conditions (Photochem. Photobiol. Sci. 2007;6:1139-49).
In 2009, Yin et al. showed that three different functionalized water-soluble fullerenes can intercept and protect cells against all of the primary physiologically important ROS and can efficiently suppress lipid peroxidation in vitro. The findings suggest the potential of fullerene derivatives as effective cytoprotective therapeutic agents (Biomaterials. 2009;30:611-21), the researchers wrote.
Conclusion
The discovery of the fullerene family has stimulated widespread research in chemistry and biology for a broad range of therapeutic applications. Anti-inflammatory, antioxidant, and antiviral activities have been linked to these compounds. In addition, fullerenes appear to be effective in drug and gene delivery and as an adjunct in acne therapy. The compounds have shown potential as photoprotectants as well as photosensitizers, which may be useful in photodynamic therapy. More research is needed, but the potential applications of fullerenes in dermatology are promising, particularly as potent antioxidants conferring skin protection.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.
Discovered in nature in 1985 (Science 1992;257:215-7), fullerenes are novel, classically engineered chemical compounds composed only of carbon atoms. The stable form, a third carbon allotrope, is a molecule made up of 60 carbon atoms in a structure resembling a soccer ball or geodesic dome, although some fullerenes are ellipsoid or tube shaped. In fact, fullerene C(60), the first of the group of compounds to be discovered, is also known as buckminsterfullerene, based on Buckminster Fuller’s geodesic dome. The carbon nanotube structure, composed of thin carbon filaments (1-3 mcm in length and 1-3 nm in diameter) and possessing a wide range of mechanical characteristics, was discovered in 1991 (J. Nanosci. Nanotechnol. 2006;6:591-9).
The potential applications of C(60) and fullerene derivatives have been extensively studied, and recent data suggest that fullerenes exhibit potent antioxidant activity, even acting as a "free radical sponge" (Bioorg. Med. Chem. Lett. 2006;16:1590-5; Biomaterials 2008;29:3561-73). Therefore, fullerenes may be appropriate active ingredients for various skin care products, particularly rejuvenation products (Recent Pat. Biotechnol. 2011;5:67-73; Recent. Pat. Biotechnol. 2009;3:118-23).
Early studies
In one of the early studies of topical applications of fullerenes (C60), Nelson et al. examined the potential acute and subchronic toxic effects of fullerenes (200 mcg) applied in benzene on mouse skin. After 72 hours, they observed no effect on either DNA synthesis or ornithine decarboxylase activity. A skin tumor initiation model using 7,12-dimethylbenzanthracene (DMBA) failed to show benign or malignant skin tumor formation, but promotion using 12-O-tetradecanoyl-phorbol-13-acetate (TPA) resulted in benign skin tumors. The investigators concluded that fullerenes applied in benzene at a likely industrial exposure level did not cause acute toxic effects in mice (Toxicol. Ind. Health. 1993;9:623-30).
In 1997, Tabata et al., noted that fullerene C(60) efficiently generates singlet oxygen when irradiated with light, and found that polyethylene glycol (PEG)-modified C60 exhibits potential as an agent for photodynamic tumor therapy (Jpn. J. Cancer Res. 1997;88:1108-16).
Antiviral capacity
Fullerenes exhibit unique chemical and physical properties, including photodynamic characteristics and a hydrophobic spheroid and radical sponge quality. They also display antiviral activity, particularly in relation to HIV, and their potential commercial applications include use in patent-pending anticancer drug delivery systems employing photodynamic therapy (PDT), HIV drugs, and antiaging cosmetics (Int. J. Nanomedicine 2007;2:639-49).
Anti-inflammatory activity
An anti-inflammatory role has also been identified for fullerenes. Ryan et al. noted that preincubation with C(60) resulted in significant suppression of IgE-dependent mediator release in human mast cells and peripheral blood basophils. IgE-induced increases in cytoplasmic reactive oxygen species (ROS) levels was also hindered by preincubation with fullerenes. Using a mast cell–dependent anaphylaxis model, Ryan and colleagues also found that fullerenes prevented the in vivo release of histamine and fall in core body temperature. They concluded that fullerenes might suggest an innovative approach to managing mast cell–dependent conditions such as asthma, heart disease, inflammatory arthritis, and multiple sclerosis (J. Immunol. 2007;179(1):665-72).
Antimicrobial potential
In 2005, Tegos et al. compared the antimicrobial activity of six C(60) compounds functionalized with one, two, or three hydrophilic or cationic groups combined with white light against gram-positive and negative bacteria, and fungi. They found that following 10 minutes of incubation, bis- and tris-cationic fullerenes actively eliminated all tested microbes while leaving mammalian cells comparatively intact. The investigators also noted that the fullerene compounds were significantly more effective than a widely used antimicrobial photosensitizer, toluidine blue O. They concluded that the compounds warrant consideration as photosensitizers for antimicrobial use given their high selectivity and efficacy (Chem. Biol. 2005;12(10):1127-35).
Potent antioxidant
In 2006, Xiao et al. reported on their development of the Radical Sponge, a fullerene entrapped in polyvinylpyrrolidone to yield a water-soluble derivative with a mean particle diameter of about 688 nm and reactive oxygen species (ROS) scavenging abilities. The researchers repeatedly irradiated human skin keratinocytes (HaCaT) with visible light (400-2,000 nm) in the presence or absence of Radical Sponge, with no photocytotoxicity apparent in the Radical Sponge–exposed cells. In addition, the water-soluble fullerene derivative displayed a cytoprotective effect (10-40 mcM doses) against UVA exposure (30 J/cm2) when it was administered prior to exposure and rinsed out immediately before the irradiation, more so than when administered only during or after irradiation. The researchers concluded that this finding suggested more of a preventive as opposed to therapeutic effect conferred by Radical Sponge against UVA damage (Bioorg. Med. Chem. Lett. 2006;16:1590-5).
In a subsequent study, the researchers compared the Radical Sponge with two whitening agents and found the fullerene to have imparted stronger antimelanogenic activity, possibly due to down-regulation of the tyrosinase expression promoted by UVA-induced ROS generation (Arch. Dermatol. Res. 2007;299(5-6):245-57).
More recently, Kato et al. demonstrated the antioxidant activity of C(60) incorporated into liposomes, with persistent scavenging of hydroxyl radicals and cytoprotection of keratinocytes against UVA- and UVB-induced damage ascribed to the fullerene component (J. Nanosci. Nanotechnol. 2011;11(5):3814-23).
Safety
The first study establishing the safety of highly purified fullerenes (HPFs) as an additive in cosmeceuticals was conducted in 2009. Aoshima et al. performed in vivo tests in animals and in vitro examinations using human epidermal keratinocytes and fibroblasts. No primary or cumulative skin irritation, sensitization, photosensitization, or contact phototoxicity was observed. In the patch test on human skin, no reaction was noted. HPFs were deemed to be "minimally irritating" after the eye-irritation test in rabbits. The investigators concluded, based on their findings and in light of previously published data, that HPFs are safe for human skin as ingredients in cosmetic skin care formulations (J. Toxicol. Sci. 2009;34(5):555-62).
Hair regrowth
In 2009, Zhou et al. used shaved mice and SKH-1 hairless mice to study whether fullerene-based compounds could elicit hair growth. Fullerenes were found to significantly increase the hair growth rate compared with a placebo vehicle. Significantly increased numbers of hair follicles were also observed in SKH-1 hairless mice treated topically or subdermally with fullerenes. Cultured human skin treated with fullerenes also showed augmented hair growth. The data suggested implications for hair loss due to alopecia, chemotherapy, or other chemical reactions (Nanomedicine 2009;5:202-7).
Antiacne properties
In 2011, Inui et al. conducted an open trial of effects of fullerene gel on acne. Subjects used the gel twice daily, and significant reductions in the mean number of inflammatory lesions were noted at 4 and 8 weeks of treatment. In addition, the researchers conducted in vitro assays of sebum production in hamster sebocytes and found that 75 mcM polyvinylpyrrolidone-fullerene suppressed sebum production, suggesting that topical fullerenes inhibit acne by reducing neutrophil infiltration and sebum production (Nanomedicine 2011;7:238-41).
Water solubility and photodynamic activity
In 2007, Mroz et al. compared the photodynamic activity of six fullerenes functionalized to become soluble with 1, 2, or 3 hydrophilic or 1, 2, or 3 cationic groups in three mouse cancer cell lines (J774, LLC, and CT26) incubated for 24 hours with fullerenes and illuminated with white light. They found that some functionalized fullerenes (particularly monopyrrolidinium fullerene) induced apoptosis in all cancer lines (Free Radic. Biol. Med. 2007;43:711-9). In a subsequent study, Mroz et al. found that some fullerenes can be functionalized to photoinactivate pathogenic malignant cancer cells and/or microbial cells in a mechanism that involves superoxide anion and singlet oxygen. The researchers suggested that fullerenes have the potential to supersede photosensitizers in current clinical use in PDT for some conditions (Photochem. Photobiol. Sci. 2007;6:1139-49).
In 2009, Yin et al. showed that three different functionalized water-soluble fullerenes can intercept and protect cells against all of the primary physiologically important ROS and can efficiently suppress lipid peroxidation in vitro. The findings suggest the potential of fullerene derivatives as effective cytoprotective therapeutic agents (Biomaterials. 2009;30:611-21), the researchers wrote.
Conclusion
The discovery of the fullerene family has stimulated widespread research in chemistry and biology for a broad range of therapeutic applications. Anti-inflammatory, antioxidant, and antiviral activities have been linked to these compounds. In addition, fullerenes appear to be effective in drug and gene delivery and as an adjunct in acne therapy. The compounds have shown potential as photoprotectants as well as photosensitizers, which may be useful in photodynamic therapy. More research is needed, but the potential applications of fullerenes in dermatology are promising, particularly as potent antioxidants conferring skin protection.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.
Discovered in nature in 1985 (Science 1992;257:215-7), fullerenes are novel, classically engineered chemical compounds composed only of carbon atoms. The stable form, a third carbon allotrope, is a molecule made up of 60 carbon atoms in a structure resembling a soccer ball or geodesic dome, although some fullerenes are ellipsoid or tube shaped. In fact, fullerene C(60), the first of the group of compounds to be discovered, is also known as buckminsterfullerene, based on Buckminster Fuller’s geodesic dome. The carbon nanotube structure, composed of thin carbon filaments (1-3 mcm in length and 1-3 nm in diameter) and possessing a wide range of mechanical characteristics, was discovered in 1991 (J. Nanosci. Nanotechnol. 2006;6:591-9).
The potential applications of C(60) and fullerene derivatives have been extensively studied, and recent data suggest that fullerenes exhibit potent antioxidant activity, even acting as a "free radical sponge" (Bioorg. Med. Chem. Lett. 2006;16:1590-5; Biomaterials 2008;29:3561-73). Therefore, fullerenes may be appropriate active ingredients for various skin care products, particularly rejuvenation products (Recent Pat. Biotechnol. 2011;5:67-73; Recent. Pat. Biotechnol. 2009;3:118-23).
Early studies
In one of the early studies of topical applications of fullerenes (C60), Nelson et al. examined the potential acute and subchronic toxic effects of fullerenes (200 mcg) applied in benzene on mouse skin. After 72 hours, they observed no effect on either DNA synthesis or ornithine decarboxylase activity. A skin tumor initiation model using 7,12-dimethylbenzanthracene (DMBA) failed to show benign or malignant skin tumor formation, but promotion using 12-O-tetradecanoyl-phorbol-13-acetate (TPA) resulted in benign skin tumors. The investigators concluded that fullerenes applied in benzene at a likely industrial exposure level did not cause acute toxic effects in mice (Toxicol. Ind. Health. 1993;9:623-30).
In 1997, Tabata et al., noted that fullerene C(60) efficiently generates singlet oxygen when irradiated with light, and found that polyethylene glycol (PEG)-modified C60 exhibits potential as an agent for photodynamic tumor therapy (Jpn. J. Cancer Res. 1997;88:1108-16).
Antiviral capacity
Fullerenes exhibit unique chemical and physical properties, including photodynamic characteristics and a hydrophobic spheroid and radical sponge quality. They also display antiviral activity, particularly in relation to HIV, and their potential commercial applications include use in patent-pending anticancer drug delivery systems employing photodynamic therapy (PDT), HIV drugs, and antiaging cosmetics (Int. J. Nanomedicine 2007;2:639-49).
Anti-inflammatory activity
An anti-inflammatory role has also been identified for fullerenes. Ryan et al. noted that preincubation with C(60) resulted in significant suppression of IgE-dependent mediator release in human mast cells and peripheral blood basophils. IgE-induced increases in cytoplasmic reactive oxygen species (ROS) levels was also hindered by preincubation with fullerenes. Using a mast cell–dependent anaphylaxis model, Ryan and colleagues also found that fullerenes prevented the in vivo release of histamine and fall in core body temperature. They concluded that fullerenes might suggest an innovative approach to managing mast cell–dependent conditions such as asthma, heart disease, inflammatory arthritis, and multiple sclerosis (J. Immunol. 2007;179(1):665-72).
Antimicrobial potential
In 2005, Tegos et al. compared the antimicrobial activity of six C(60) compounds functionalized with one, two, or three hydrophilic or cationic groups combined with white light against gram-positive and negative bacteria, and fungi. They found that following 10 minutes of incubation, bis- and tris-cationic fullerenes actively eliminated all tested microbes while leaving mammalian cells comparatively intact. The investigators also noted that the fullerene compounds were significantly more effective than a widely used antimicrobial photosensitizer, toluidine blue O. They concluded that the compounds warrant consideration as photosensitizers for antimicrobial use given their high selectivity and efficacy (Chem. Biol. 2005;12(10):1127-35).
Potent antioxidant
In 2006, Xiao et al. reported on their development of the Radical Sponge, a fullerene entrapped in polyvinylpyrrolidone to yield a water-soluble derivative with a mean particle diameter of about 688 nm and reactive oxygen species (ROS) scavenging abilities. The researchers repeatedly irradiated human skin keratinocytes (HaCaT) with visible light (400-2,000 nm) in the presence or absence of Radical Sponge, with no photocytotoxicity apparent in the Radical Sponge–exposed cells. In addition, the water-soluble fullerene derivative displayed a cytoprotective effect (10-40 mcM doses) against UVA exposure (30 J/cm2) when it was administered prior to exposure and rinsed out immediately before the irradiation, more so than when administered only during or after irradiation. The researchers concluded that this finding suggested more of a preventive as opposed to therapeutic effect conferred by Radical Sponge against UVA damage (Bioorg. Med. Chem. Lett. 2006;16:1590-5).
In a subsequent study, the researchers compared the Radical Sponge with two whitening agents and found the fullerene to have imparted stronger antimelanogenic activity, possibly due to down-regulation of the tyrosinase expression promoted by UVA-induced ROS generation (Arch. Dermatol. Res. 2007;299(5-6):245-57).
More recently, Kato et al. demonstrated the antioxidant activity of C(60) incorporated into liposomes, with persistent scavenging of hydroxyl radicals and cytoprotection of keratinocytes against UVA- and UVB-induced damage ascribed to the fullerene component (J. Nanosci. Nanotechnol. 2011;11(5):3814-23).
Safety
The first study establishing the safety of highly purified fullerenes (HPFs) as an additive in cosmeceuticals was conducted in 2009. Aoshima et al. performed in vivo tests in animals and in vitro examinations using human epidermal keratinocytes and fibroblasts. No primary or cumulative skin irritation, sensitization, photosensitization, or contact phototoxicity was observed. In the patch test on human skin, no reaction was noted. HPFs were deemed to be "minimally irritating" after the eye-irritation test in rabbits. The investigators concluded, based on their findings and in light of previously published data, that HPFs are safe for human skin as ingredients in cosmetic skin care formulations (J. Toxicol. Sci. 2009;34(5):555-62).
Hair regrowth
In 2009, Zhou et al. used shaved mice and SKH-1 hairless mice to study whether fullerene-based compounds could elicit hair growth. Fullerenes were found to significantly increase the hair growth rate compared with a placebo vehicle. Significantly increased numbers of hair follicles were also observed in SKH-1 hairless mice treated topically or subdermally with fullerenes. Cultured human skin treated with fullerenes also showed augmented hair growth. The data suggested implications for hair loss due to alopecia, chemotherapy, or other chemical reactions (Nanomedicine 2009;5:202-7).
Antiacne properties
In 2011, Inui et al. conducted an open trial of effects of fullerene gel on acne. Subjects used the gel twice daily, and significant reductions in the mean number of inflammatory lesions were noted at 4 and 8 weeks of treatment. In addition, the researchers conducted in vitro assays of sebum production in hamster sebocytes and found that 75 mcM polyvinylpyrrolidone-fullerene suppressed sebum production, suggesting that topical fullerenes inhibit acne by reducing neutrophil infiltration and sebum production (Nanomedicine 2011;7:238-41).
Water solubility and photodynamic activity
In 2007, Mroz et al. compared the photodynamic activity of six fullerenes functionalized to become soluble with 1, 2, or 3 hydrophilic or 1, 2, or 3 cationic groups in three mouse cancer cell lines (J774, LLC, and CT26) incubated for 24 hours with fullerenes and illuminated with white light. They found that some functionalized fullerenes (particularly monopyrrolidinium fullerene) induced apoptosis in all cancer lines (Free Radic. Biol. Med. 2007;43:711-9). In a subsequent study, Mroz et al. found that some fullerenes can be functionalized to photoinactivate pathogenic malignant cancer cells and/or microbial cells in a mechanism that involves superoxide anion and singlet oxygen. The researchers suggested that fullerenes have the potential to supersede photosensitizers in current clinical use in PDT for some conditions (Photochem. Photobiol. Sci. 2007;6:1139-49).
In 2009, Yin et al. showed that three different functionalized water-soluble fullerenes can intercept and protect cells against all of the primary physiologically important ROS and can efficiently suppress lipid peroxidation in vitro. The findings suggest the potential of fullerene derivatives as effective cytoprotective therapeutic agents (Biomaterials. 2009;30:611-21), the researchers wrote.
Conclusion
The discovery of the fullerene family has stimulated widespread research in chemistry and biology for a broad range of therapeutic applications. Anti-inflammatory, antioxidant, and antiviral activities have been linked to these compounds. In addition, fullerenes appear to be effective in drug and gene delivery and as an adjunct in acne therapy. The compounds have shown potential as photoprotectants as well as photosensitizers, which may be useful in photodynamic therapy. More research is needed, but the potential applications of fullerenes in dermatology are promising, particularly as potent antioxidants conferring skin protection.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.
Ceramides
Structured in lamellar sheets, the primary lipids of the epidermis – ceramides, cholesterol, and free fatty acids – play a crucial role in the barrier function of the skin. Ceramides have come to be known as a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell signaling in addition to their role in barrier homeostasis and water retention. In fact, ceramides are known to play a critical role in cell proliferation, differentiation, and apoptosis (Food Chem. Toxicol. 2009;47:681-6). Significantly, they cannot be replenished or obtained through natural sources, but synthetic ceramides, studied since the 1950s, are increasingly sophisticated and useful.
This column will review some key aspects of natural human ceramides as well as topically applied synthetic versions (also known as pseudoceramides), which are thought to ameliorate the structure and function of ceramide-depleted skin.
Ceramide structure and function
Lipids in the stratum corneum (SC) play an important role in the barrier function of the skin. The intercellular lipids of the SC are thought to be composed of approximately equal proportions of ceramides (J. Invest. Dermatol. 1987;88:2s-6s), cholesterol, and fatty acids (Am. J. Clin. Dermatol. 2003;4:107-29). Ceramides are not found in significant supply in lower levels of the epidermis, such as the stratum granulosum or basal layer. This implies that terminal differentiation is an important component of the natural production of ceramides, of which there are at least nine classes in the SC. Ceramide 1 was first identified in 1982. In addition to ceramides 1 to 9, there are two protein-bound ceramides classified as ceramides A and B, which are covalently bound to cornified envelope proteins, such as involucrin (Bouwstra JA, Pilgrim K, Ponec M. Structure of the skin barrier, in "Skin Barrier," Elias PM, Feingold KR, Eds. New York: Taylor & Francis, 2006, p. 65) .
Ceramides are named based on the polarity and composition of the molecule. As suggested above, the foundational ceramide structure is a fatty acid covalently bound to a sphingoid base. The various classes of ceramides are grouped according to the arrangements of sphingosine (S), phytosphingosine (P), or 6-hydroxysphingosine (H) bases, to which an alpha-hydroxy (A) or nonhydroxy (N) fatty acid is attached, in addition to the presence or absence of a discrete omega-esterified linoleic acid residue (J. Lipid. Res. 2004;45:923-32).
Ceramide 1 is unique in that it is nonpolar, and it contains linoleic acid. The special function of ceramide 1 in the SC is typically ascribed to its unique structure, which is thought to allow it to act as a molecular rivet, binding the multiple bilayers of the SC (J. Invest. Dermatol. 1987;88:2s-6s). This would explain the stacking of lipid bilayers in lamellar sheets observed in the barrier. Ceramides 1, 4, and 7 exhibit critical functions in terms of epidermal integrity by serving as the primary storage areas for linoleic acid, an essential fatty acid with significant roles in the epidermal lipid barrier (J. Invest. Dermatol. 1980;74:230-3). Although all epidermal ceramides are produced from a lamellar body–derived glucosylceramide precursor, sphingomyelin-derived ceramides (ceramides 2 and 5) are essential for maintaining the integrity of the SC (J. Lipid. Res. 2000;41:2071-82). It is worth noting that because an alkaline pH suppresses beta-glucocerebrosidase and acid sphingomyelinase activity (J. Invest. Dermatol. 2005;125:510-20), alkaline soaps can exacerbate poor barrier formation.
Exposure to UVB radiation and cytokines has been associated with an increase in the regulatory enzyme for ceramide synthesis, serine palmitoyltransferase, and it has been determined that in response to UVB exposure, the epidermis upregulates sphingolipid synthesis at the mRNA and protein levels (J. Lipid. Res. 1998;39:2031-8).
Synthetic ceramides
Skin conditions such as atopic dermatitis (AD), psoriasis, contact dermatitis, and some genetic disorders have been associated with depleted ceramide levels (Am. J. Clin. Dermatol. 2005;6:215-23), but these diseases can be ameliorated through the use of exogenous ceramides or their analogues (topical ceramide replacement therapy) (Curr. Med. Chem. 2010;17:2301-24; J. Dermatol. Sci. 2008;51:37-43; Am. J. Clin. Dermatol. 2005;6:215-23). Notably, the activities of enzymes in the SC, particularly ceramidase, sphingomyelin deacylase, and glucosylceramide deacylase, have been shown to be elevated in epidermal AD (Am. J. Clin. Dermatol. 2005;6:215-23).
Synthetic ceramides, or pseudoceramides, contain hydroxyl groups, two alkyl groups, and an amide bond – the same key structural components as natural ceramides. Consequently, various synthetic ceramides have been reported to form the multilamellar structure observed in the intercellular spaces of the SC (J. Lipid. Res. 1996;37:361-7).
Coderch et al., in a review of ceramides and skin function, endorsed the potential of topical therapy for several skin conditions using complete lipid mixtures and some ceramide supplementation, as well as the topical delivery of lipid precursors (Am. J. Clin. Dermatol. 2003;4:107-29). And, in fact, the topical application of synthetic ceramides has been shown to speed up the repair of impaired SC (J. Clin. Invest. 1994;94:89-96; Dermatology 2005;211:128-34). Recent reports by Tokudome et al. also indicate that the application of sphingomyelin-based liposomes effectively augments the levels of various ceramides in cultured human skin models (Skin Pharmacol. Physiol. 2011;24:218-23; J. Liposome Res. 2010;20:49-54).
In 2005, de Jager et al. used small-angle and wide-angle x-ray diffraction to show that lipid mixtures prepared with well-defined synthetic ceramides exhibit organization and lipid-phase behavior that are very similar to those of lamellar and lateral SC lipids, and can be used to further elucidate the molecular structure and roles of individual ceramides (J. Lipid. Res. 2005;46:2649-56).
In light of the uncertainty regarding the metabolic impact of pseudoceramides, in 2008, Uchida et al. compared the effects of two chemically unrelated, commercially available products to exogenous cell-permeant or natural ceramide on cell growth and apoptosis thresholds. Using cultured human keratinocytes, the investigators found that the commercial ceramides did not suppress keratinocyte growth or increase cell toxicity, as did the cell-permeant. The investigators suggested that these findings buttress the preclinical studies indicating that these pseudoceramides are safe for topical application (J. Dermatol. Sci. 2008;51:37-43).
Kang et al. recently conducted studies of synthetic ceramide derivatives of PC-9S (N-ethanol-2-mirystyl-3-oxostearamide), which, itself, has been shown to be effective in atopic and psoriatic patients. Both studies, conducted in NC/Nga mice, demonstrated that the topical application of the derivative K6PC-9 or the derivative K6PC-9p reduced skin inflammation and AD symptoms. According to the authors, K6PC-9 warrants consideration as a topical agent for AD, and K6PC-9p warrants consideration as a treatment for inflammatory skin diseases in general (Int. Immunopharmacol. 2007;7:1589-97; Exp. Dermatol. 2008;17:958-64).
Subsequently, Kang et al. studied the effects of another ceramide derivative of PC-9S, K112PC-5 (2-acetyl-N-(1,3-dihydroxyisopropyl)tetradecanamide), on macrophage and T-lymphocyte function in primary macrophages and splenocytes, respectively. The researchers also studied the impact of topically applied K112PC-5 on skin inflammation and AD in NC/Nga mice. Among several findings, the investigators noted that K112PC-5 suppressed AD induced by extracts of dust mites, Dermatophagoides pteronyssinus and Dermatophagoides farinae, with the pseudoceramide exhibiting in vitro and in vivo anti-inflammatory activity. They concluded that K112PC-5 is another synthetic ceramide derivative with potential as a topical agent for the treatment of AD (Arch. Pharm. Res. 2008;31:1004-9).
In 2009, Morita et al. studied the potential adverse effects of the synthetic pseudoceramide SLE66, which has demonstrated the capacity to improve xerosis, pruritus, and scaling of human skin. They found that the tested product failed to provoke cutaneous irritation or sensitization in animal and human studies. In addition, they did not observe any phototoxicity or photosensitization, and they established 1,000 mg/kg/day (the highest level tested) as the no-observed-adverse-effect (NOAEL) for systemic toxicity after oral administration or topical application (Food Chem. Toxicol. 2009;47:669-73).
Conclusion
Ceramides are among the primary lipid constituents, along with cholesterol and fatty acids, of the lamellar sheets found in the intercellular spaces of the SC. Together, these lipids maintain the water permeability barrier role of the skin. Ceramides also play an important role in cell signaling. Research over the last several decades, particularly the last 20 years, indicates that topically applied synthetic ceramide agents can effectively compensate for diminished ceramide levels associated with various skin conditions.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at sknews@elsevier.com.
Structured in lamellar sheets, the primary lipids of the epidermis – ceramides, cholesterol, and free fatty acids – play a crucial role in the barrier function of the skin. Ceramides have come to be known as a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell signaling in addition to their role in barrier homeostasis and water retention. In fact, ceramides are known to play a critical role in cell proliferation, differentiation, and apoptosis (Food Chem. Toxicol. 2009;47:681-6). Significantly, they cannot be replenished or obtained through natural sources, but synthetic ceramides, studied since the 1950s, are increasingly sophisticated and useful.
This column will review some key aspects of natural human ceramides as well as topically applied synthetic versions (also known as pseudoceramides), which are thought to ameliorate the structure and function of ceramide-depleted skin.
Ceramide structure and function
Lipids in the stratum corneum (SC) play an important role in the barrier function of the skin. The intercellular lipids of the SC are thought to be composed of approximately equal proportions of ceramides (J. Invest. Dermatol. 1987;88:2s-6s), cholesterol, and fatty acids (Am. J. Clin. Dermatol. 2003;4:107-29). Ceramides are not found in significant supply in lower levels of the epidermis, such as the stratum granulosum or basal layer. This implies that terminal differentiation is an important component of the natural production of ceramides, of which there are at least nine classes in the SC. Ceramide 1 was first identified in 1982. In addition to ceramides 1 to 9, there are two protein-bound ceramides classified as ceramides A and B, which are covalently bound to cornified envelope proteins, such as involucrin (Bouwstra JA, Pilgrim K, Ponec M. Structure of the skin barrier, in "Skin Barrier," Elias PM, Feingold KR, Eds. New York: Taylor & Francis, 2006, p. 65) .
Ceramides are named based on the polarity and composition of the molecule. As suggested above, the foundational ceramide structure is a fatty acid covalently bound to a sphingoid base. The various classes of ceramides are grouped according to the arrangements of sphingosine (S), phytosphingosine (P), or 6-hydroxysphingosine (H) bases, to which an alpha-hydroxy (A) or nonhydroxy (N) fatty acid is attached, in addition to the presence or absence of a discrete omega-esterified linoleic acid residue (J. Lipid. Res. 2004;45:923-32).
Ceramide 1 is unique in that it is nonpolar, and it contains linoleic acid. The special function of ceramide 1 in the SC is typically ascribed to its unique structure, which is thought to allow it to act as a molecular rivet, binding the multiple bilayers of the SC (J. Invest. Dermatol. 1987;88:2s-6s). This would explain the stacking of lipid bilayers in lamellar sheets observed in the barrier. Ceramides 1, 4, and 7 exhibit critical functions in terms of epidermal integrity by serving as the primary storage areas for linoleic acid, an essential fatty acid with significant roles in the epidermal lipid barrier (J. Invest. Dermatol. 1980;74:230-3). Although all epidermal ceramides are produced from a lamellar body–derived glucosylceramide precursor, sphingomyelin-derived ceramides (ceramides 2 and 5) are essential for maintaining the integrity of the SC (J. Lipid. Res. 2000;41:2071-82). It is worth noting that because an alkaline pH suppresses beta-glucocerebrosidase and acid sphingomyelinase activity (J. Invest. Dermatol. 2005;125:510-20), alkaline soaps can exacerbate poor barrier formation.
Exposure to UVB radiation and cytokines has been associated with an increase in the regulatory enzyme for ceramide synthesis, serine palmitoyltransferase, and it has been determined that in response to UVB exposure, the epidermis upregulates sphingolipid synthesis at the mRNA and protein levels (J. Lipid. Res. 1998;39:2031-8).
Synthetic ceramides
Skin conditions such as atopic dermatitis (AD), psoriasis, contact dermatitis, and some genetic disorders have been associated with depleted ceramide levels (Am. J. Clin. Dermatol. 2005;6:215-23), but these diseases can be ameliorated through the use of exogenous ceramides or their analogues (topical ceramide replacement therapy) (Curr. Med. Chem. 2010;17:2301-24; J. Dermatol. Sci. 2008;51:37-43; Am. J. Clin. Dermatol. 2005;6:215-23). Notably, the activities of enzymes in the SC, particularly ceramidase, sphingomyelin deacylase, and glucosylceramide deacylase, have been shown to be elevated in epidermal AD (Am. J. Clin. Dermatol. 2005;6:215-23).
Synthetic ceramides, or pseudoceramides, contain hydroxyl groups, two alkyl groups, and an amide bond – the same key structural components as natural ceramides. Consequently, various synthetic ceramides have been reported to form the multilamellar structure observed in the intercellular spaces of the SC (J. Lipid. Res. 1996;37:361-7).
Coderch et al., in a review of ceramides and skin function, endorsed the potential of topical therapy for several skin conditions using complete lipid mixtures and some ceramide supplementation, as well as the topical delivery of lipid precursors (Am. J. Clin. Dermatol. 2003;4:107-29). And, in fact, the topical application of synthetic ceramides has been shown to speed up the repair of impaired SC (J. Clin. Invest. 1994;94:89-96; Dermatology 2005;211:128-34). Recent reports by Tokudome et al. also indicate that the application of sphingomyelin-based liposomes effectively augments the levels of various ceramides in cultured human skin models (Skin Pharmacol. Physiol. 2011;24:218-23; J. Liposome Res. 2010;20:49-54).
In 2005, de Jager et al. used small-angle and wide-angle x-ray diffraction to show that lipid mixtures prepared with well-defined synthetic ceramides exhibit organization and lipid-phase behavior that are very similar to those of lamellar and lateral SC lipids, and can be used to further elucidate the molecular structure and roles of individual ceramides (J. Lipid. Res. 2005;46:2649-56).
In light of the uncertainty regarding the metabolic impact of pseudoceramides, in 2008, Uchida et al. compared the effects of two chemically unrelated, commercially available products to exogenous cell-permeant or natural ceramide on cell growth and apoptosis thresholds. Using cultured human keratinocytes, the investigators found that the commercial ceramides did not suppress keratinocyte growth or increase cell toxicity, as did the cell-permeant. The investigators suggested that these findings buttress the preclinical studies indicating that these pseudoceramides are safe for topical application (J. Dermatol. Sci. 2008;51:37-43).
Kang et al. recently conducted studies of synthetic ceramide derivatives of PC-9S (N-ethanol-2-mirystyl-3-oxostearamide), which, itself, has been shown to be effective in atopic and psoriatic patients. Both studies, conducted in NC/Nga mice, demonstrated that the topical application of the derivative K6PC-9 or the derivative K6PC-9p reduced skin inflammation and AD symptoms. According to the authors, K6PC-9 warrants consideration as a topical agent for AD, and K6PC-9p warrants consideration as a treatment for inflammatory skin diseases in general (Int. Immunopharmacol. 2007;7:1589-97; Exp. Dermatol. 2008;17:958-64).
Subsequently, Kang et al. studied the effects of another ceramide derivative of PC-9S, K112PC-5 (2-acetyl-N-(1,3-dihydroxyisopropyl)tetradecanamide), on macrophage and T-lymphocyte function in primary macrophages and splenocytes, respectively. The researchers also studied the impact of topically applied K112PC-5 on skin inflammation and AD in NC/Nga mice. Among several findings, the investigators noted that K112PC-5 suppressed AD induced by extracts of dust mites, Dermatophagoides pteronyssinus and Dermatophagoides farinae, with the pseudoceramide exhibiting in vitro and in vivo anti-inflammatory activity. They concluded that K112PC-5 is another synthetic ceramide derivative with potential as a topical agent for the treatment of AD (Arch. Pharm. Res. 2008;31:1004-9).
In 2009, Morita et al. studied the potential adverse effects of the synthetic pseudoceramide SLE66, which has demonstrated the capacity to improve xerosis, pruritus, and scaling of human skin. They found that the tested product failed to provoke cutaneous irritation or sensitization in animal and human studies. In addition, they did not observe any phototoxicity or photosensitization, and they established 1,000 mg/kg/day (the highest level tested) as the no-observed-adverse-effect (NOAEL) for systemic toxicity after oral administration or topical application (Food Chem. Toxicol. 2009;47:669-73).
Conclusion
Ceramides are among the primary lipid constituents, along with cholesterol and fatty acids, of the lamellar sheets found in the intercellular spaces of the SC. Together, these lipids maintain the water permeability barrier role of the skin. Ceramides also play an important role in cell signaling. Research over the last several decades, particularly the last 20 years, indicates that topically applied synthetic ceramide agents can effectively compensate for diminished ceramide levels associated with various skin conditions.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at sknews@elsevier.com.
Structured in lamellar sheets, the primary lipids of the epidermis – ceramides, cholesterol, and free fatty acids – play a crucial role in the barrier function of the skin. Ceramides have come to be known as a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell signaling in addition to their role in barrier homeostasis and water retention. In fact, ceramides are known to play a critical role in cell proliferation, differentiation, and apoptosis (Food Chem. Toxicol. 2009;47:681-6). Significantly, they cannot be replenished or obtained through natural sources, but synthetic ceramides, studied since the 1950s, are increasingly sophisticated and useful.
This column will review some key aspects of natural human ceramides as well as topically applied synthetic versions (also known as pseudoceramides), which are thought to ameliorate the structure and function of ceramide-depleted skin.
Ceramide structure and function
Lipids in the stratum corneum (SC) play an important role in the barrier function of the skin. The intercellular lipids of the SC are thought to be composed of approximately equal proportions of ceramides (J. Invest. Dermatol. 1987;88:2s-6s), cholesterol, and fatty acids (Am. J. Clin. Dermatol. 2003;4:107-29). Ceramides are not found in significant supply in lower levels of the epidermis, such as the stratum granulosum or basal layer. This implies that terminal differentiation is an important component of the natural production of ceramides, of which there are at least nine classes in the SC. Ceramide 1 was first identified in 1982. In addition to ceramides 1 to 9, there are two protein-bound ceramides classified as ceramides A and B, which are covalently bound to cornified envelope proteins, such as involucrin (Bouwstra JA, Pilgrim K, Ponec M. Structure of the skin barrier, in "Skin Barrier," Elias PM, Feingold KR, Eds. New York: Taylor & Francis, 2006, p. 65) .
Ceramides are named based on the polarity and composition of the molecule. As suggested above, the foundational ceramide structure is a fatty acid covalently bound to a sphingoid base. The various classes of ceramides are grouped according to the arrangements of sphingosine (S), phytosphingosine (P), or 6-hydroxysphingosine (H) bases, to which an alpha-hydroxy (A) or nonhydroxy (N) fatty acid is attached, in addition to the presence or absence of a discrete omega-esterified linoleic acid residue (J. Lipid. Res. 2004;45:923-32).
Ceramide 1 is unique in that it is nonpolar, and it contains linoleic acid. The special function of ceramide 1 in the SC is typically ascribed to its unique structure, which is thought to allow it to act as a molecular rivet, binding the multiple bilayers of the SC (J. Invest. Dermatol. 1987;88:2s-6s). This would explain the stacking of lipid bilayers in lamellar sheets observed in the barrier. Ceramides 1, 4, and 7 exhibit critical functions in terms of epidermal integrity by serving as the primary storage areas for linoleic acid, an essential fatty acid with significant roles in the epidermal lipid barrier (J. Invest. Dermatol. 1980;74:230-3). Although all epidermal ceramides are produced from a lamellar body–derived glucosylceramide precursor, sphingomyelin-derived ceramides (ceramides 2 and 5) are essential for maintaining the integrity of the SC (J. Lipid. Res. 2000;41:2071-82). It is worth noting that because an alkaline pH suppresses beta-glucocerebrosidase and acid sphingomyelinase activity (J. Invest. Dermatol. 2005;125:510-20), alkaline soaps can exacerbate poor barrier formation.
Exposure to UVB radiation and cytokines has been associated with an increase in the regulatory enzyme for ceramide synthesis, serine palmitoyltransferase, and it has been determined that in response to UVB exposure, the epidermis upregulates sphingolipid synthesis at the mRNA and protein levels (J. Lipid. Res. 1998;39:2031-8).
Synthetic ceramides
Skin conditions such as atopic dermatitis (AD), psoriasis, contact dermatitis, and some genetic disorders have been associated with depleted ceramide levels (Am. J. Clin. Dermatol. 2005;6:215-23), but these diseases can be ameliorated through the use of exogenous ceramides or their analogues (topical ceramide replacement therapy) (Curr. Med. Chem. 2010;17:2301-24; J. Dermatol. Sci. 2008;51:37-43; Am. J. Clin. Dermatol. 2005;6:215-23). Notably, the activities of enzymes in the SC, particularly ceramidase, sphingomyelin deacylase, and glucosylceramide deacylase, have been shown to be elevated in epidermal AD (Am. J. Clin. Dermatol. 2005;6:215-23).
Synthetic ceramides, or pseudoceramides, contain hydroxyl groups, two alkyl groups, and an amide bond – the same key structural components as natural ceramides. Consequently, various synthetic ceramides have been reported to form the multilamellar structure observed in the intercellular spaces of the SC (J. Lipid. Res. 1996;37:361-7).
Coderch et al., in a review of ceramides and skin function, endorsed the potential of topical therapy for several skin conditions using complete lipid mixtures and some ceramide supplementation, as well as the topical delivery of lipid precursors (Am. J. Clin. Dermatol. 2003;4:107-29). And, in fact, the topical application of synthetic ceramides has been shown to speed up the repair of impaired SC (J. Clin. Invest. 1994;94:89-96; Dermatology 2005;211:128-34). Recent reports by Tokudome et al. also indicate that the application of sphingomyelin-based liposomes effectively augments the levels of various ceramides in cultured human skin models (Skin Pharmacol. Physiol. 2011;24:218-23; J. Liposome Res. 2010;20:49-54).
In 2005, de Jager et al. used small-angle and wide-angle x-ray diffraction to show that lipid mixtures prepared with well-defined synthetic ceramides exhibit organization and lipid-phase behavior that are very similar to those of lamellar and lateral SC lipids, and can be used to further elucidate the molecular structure and roles of individual ceramides (J. Lipid. Res. 2005;46:2649-56).
In light of the uncertainty regarding the metabolic impact of pseudoceramides, in 2008, Uchida et al. compared the effects of two chemically unrelated, commercially available products to exogenous cell-permeant or natural ceramide on cell growth and apoptosis thresholds. Using cultured human keratinocytes, the investigators found that the commercial ceramides did not suppress keratinocyte growth or increase cell toxicity, as did the cell-permeant. The investigators suggested that these findings buttress the preclinical studies indicating that these pseudoceramides are safe for topical application (J. Dermatol. Sci. 2008;51:37-43).
Kang et al. recently conducted studies of synthetic ceramide derivatives of PC-9S (N-ethanol-2-mirystyl-3-oxostearamide), which, itself, has been shown to be effective in atopic and psoriatic patients. Both studies, conducted in NC/Nga mice, demonstrated that the topical application of the derivative K6PC-9 or the derivative K6PC-9p reduced skin inflammation and AD symptoms. According to the authors, K6PC-9 warrants consideration as a topical agent for AD, and K6PC-9p warrants consideration as a treatment for inflammatory skin diseases in general (Int. Immunopharmacol. 2007;7:1589-97; Exp. Dermatol. 2008;17:958-64).
Subsequently, Kang et al. studied the effects of another ceramide derivative of PC-9S, K112PC-5 (2-acetyl-N-(1,3-dihydroxyisopropyl)tetradecanamide), on macrophage and T-lymphocyte function in primary macrophages and splenocytes, respectively. The researchers also studied the impact of topically applied K112PC-5 on skin inflammation and AD in NC/Nga mice. Among several findings, the investigators noted that K112PC-5 suppressed AD induced by extracts of dust mites, Dermatophagoides pteronyssinus and Dermatophagoides farinae, with the pseudoceramide exhibiting in vitro and in vivo anti-inflammatory activity. They concluded that K112PC-5 is another synthetic ceramide derivative with potential as a topical agent for the treatment of AD (Arch. Pharm. Res. 2008;31:1004-9).
In 2009, Morita et al. studied the potential adverse effects of the synthetic pseudoceramide SLE66, which has demonstrated the capacity to improve xerosis, pruritus, and scaling of human skin. They found that the tested product failed to provoke cutaneous irritation or sensitization in animal and human studies. In addition, they did not observe any phototoxicity or photosensitization, and they established 1,000 mg/kg/day (the highest level tested) as the no-observed-adverse-effect (NOAEL) for systemic toxicity after oral administration or topical application (Food Chem. Toxicol. 2009;47:669-73).
Conclusion
Ceramides are among the primary lipid constituents, along with cholesterol and fatty acids, of the lamellar sheets found in the intercellular spaces of the SC. Together, these lipids maintain the water permeability barrier role of the skin. Ceramides also play an important role in cell signaling. Research over the last several decades, particularly the last 20 years, indicates that topically applied synthetic ceramide agents can effectively compensate for diminished ceramide levels associated with various skin conditions.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at sknews@elsevier.com.
Retinyl palmitate
Retinyl palmitate, a storage and ester form of retinol (vitamin A) and the prevailing type of vitamin A found naturally in the skin (Toxicol. Ind. Health 2005;21:167-75), has become increasingly popular during the past 2 decades. It is widely used in more than 600 skin care products, including cosmetics and sunscreens, and, with FDA approval, over-the-counter and prescription drugs (Photodermatol. Photoimmunol. Photomed. 2011;27:58-67). It was also the subject of a controversial summer 2010 report by the Environmental Working Group (EWG) in which the organization warned of possible photocarcinogenicity associated with retinyl palmitate (RP)-containing sunscreens.
Although vitamin A storage in the epidermis takes the form of retinyl esters and retinols, they act differently when exposed to UV light. The retinols display UVB-resistant and UVB-sensitive characteristics not exhibited by retinyl esters such as RP (Dermatology 1999;199:302-7). The EWG used "vitamin A" and "retinyl palmitate" interchangeably in their criticisms and follow-ups, which is misleading. The vitamin A family of drugs includes retinyl esters, retinol, tretinoin, adapalene, tazarotene, and oral isotretinoin (Accutane), in addition to four carotenoids, including beta-carotene, many of which have been shown to prevent or protect against cancer (Br. J. Cancer 1988;57:428-33; Cancer Epidemiol. Biomarkers Prev. 1997;6:949-56; J. Invest. Dermatol. 1981;76:178-80; Arch. Dermatol. Res. 1981;270:453-62). That does not mean that RP prevents cancer just because oral retinol, beta-carotene, or tretinoin have been shown to do so, for example. In fact, the study that the EWG refers to shows evidence that RP may lead to skin tumors in mice.
In response to the EWG report, Wang et al. acknowledged that of the eight in vitro studies published by the Food and Drug Administration from 2002 to 2009, four revealed that reactive oxygen species were produced by RP after UVA exposure (J. Am. Acad. Dermatol. 2010;63:903-6; Photodermatol. Photoimmunol. Photomed. 2011;27:58-67; Toxicol. Ind. Health 2007;23:625-31; Toxicol. Lett. 2006;163:30-43; Int. J. Environ. Res. Public Health 2006;3:185-90; Chem. Res. Toxicol. 2005;18:129-38). However, they questioned the relevance of these results in the context of the convoluted mechanisms of the antioxidant setting in human skin. They also contended that the National Toxicology Program (NTP) study on which the EWG based its report failed to prove that the combination of RP and UV results in photocarcinogenesis and, in fact, was rife with reasons for skepticism (J. Am. Acad. Dermatol. 2010;63:903-6; Photodermatol. Photoimmunol. Photomed. 2011;27:58-67). The EWG offered its own counterarguments and stood by its report. Rather than wade further into the debate that occurred in 2010 and found its way into the pages of the Journal of the American Academy of Dermatology (2010;63:903-6), let’s review what is known about RP.
What else do we know about RP?
In 1997, Duell et al. showed that unoccluded retinol is more effective at penetrating human skin in vivo than RP or retinoic acid (J. Invest. Dermatol. 1997;109:301-5).
In 2003, Antille et al. used an in vitro model to evaluate the photoprotective activity of RP, and then applied topical RP on the back of hairless mice before exposing them to UVB. They also applied topical RP or a sunscreen on the buttocks of human volunteers before exposing them to four minimal erythema doses of UVB. The investigators found that RP was as efficient in vitro as the commercial filter octylmethoxycinnamate in preventing UVB-induced fluorescence or photobleaching of fluorescent markers. Topical RP also significantly suppressed the formation of thymine dimers in mouse epidermis and human skin. In the volunteers, topical RP was as efficient as an SPF (sun protection factor) 20 sunscreen in preventing sunburn erythema (J. Invest. Dermatol. 2003;121:1163-7).
In 2005, Yan et al. studied the phototoxicity of RP, anhydroretinol (AR), and 5,6-epoxyretinyl palmitate (5,6-epoxy-RP) in human skin Jurkat T cells with and without light irradiation. Irradiation of cells in the absence of a retinoid rendered little damage, but the presence of RP, 5,6-epoxy-RP, or AR (50, 100, 150, and 200 micromol/L) yielded DNA fragmentation, with cell death occurring at retinoid concentrations of 100 micromol/L or greater. The investigators concluded that DNA damage and cytotoxicity are engendered by RP and its photodecomposition products in association with UVA and visible light exposure. They also determined that UVA irradiation of these retinoids produces free radicals that spur DNA strand cleavage (Toxicol. Ind. Health 2005;21:167-75).
RP accounts for most of the retinyl esters endogenously formed in skin. In 2006, Yan et al., noting that exogenous RP accumulates via topically applied cosmetic and skin care formulations, investigated the time course for buildup and disappearance of RP and retinol in the stratified layers of skin from female SKH-1 mice singly or repeatedly dosed with topical creams containing 0.5% or 2% RP. The researchers observed that within 24 hours of application, RP quickly diffused into the stratum corneum and epidermal skin layers. RP and retinol levels were lowest in the dermis, intermediate in the stratum corneum, and highest in the epidermis. In separated skin layers and intact skin, RP and retinol levels declined over time, but for 18 days, RP levels remained higher than control values. The investigators concluded that topically applied RP changed the normal physiological levels of RP and retinol in the skin of mice (Toxicol. Ind. Health 2006;22:181-91).
Having previously shown that irradiation of RP with UVA leads to the formation of photodecomposition products, synthesis of reactive oxygen species, and lipid peroxidation induction, Xia et al. demonstrated comparable results, identifying RP as a photosensitizer following irradiation with UVB light (Int. J. Environ. Res. Public Health 2006;3:185-90).
Recommendations
In light of the controversy swirling around RP and the appropriate concern it has engendered, in addition to the weight of evidence as well as experience from personal observation, I advise patients to avoid daytime use of products with RP high on the ingredient list. I add that it poses real risks while offering minimal benefits. Such patients should be using retinol or tretinoin. I recommend the use of retinoids at night, to avoid the photosensitizing action induced by UVA or UVB on retinoids left on the skin.
Conclusion
Retinyl palmitate does not penetrate very well into the skin. Consequently, for over-the-counter topical formulations, I recommend retinol instead. Because of the slow penetration of RP into the skin, the RP that remains on the skin will undergo photoreaction more than a substance that is rapidly absorbed. When exposed to light, RP on the skin may undergo metabolism and/or photoreaction to generate reactive oxygen species. These reactive oxygen species or free radicals can theoretically lead to increased skin cancer. That said, sufficient evidence to establish a causal link between RP and skin cancer has not been produced. Nor, I’m afraid, are there any good reasons to recommend the use of RP. More research on this subject is needed and will likely emerge in a timely fashion.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at dermnews@frontlinemedcom.com. This column, "Cosmeceutical Critique," appears regularly in Skin & Allergy News.
Retinyl palmitate, a storage and ester form of retinol (vitamin A) and the prevailing type of vitamin A found naturally in the skin (Toxicol. Ind. Health 2005;21:167-75), has become increasingly popular during the past 2 decades. It is widely used in more than 600 skin care products, including cosmetics and sunscreens, and, with FDA approval, over-the-counter and prescription drugs (Photodermatol. Photoimmunol. Photomed. 2011;27:58-67). It was also the subject of a controversial summer 2010 report by the Environmental Working Group (EWG) in which the organization warned of possible photocarcinogenicity associated with retinyl palmitate (RP)-containing sunscreens.
Although vitamin A storage in the epidermis takes the form of retinyl esters and retinols, they act differently when exposed to UV light. The retinols display UVB-resistant and UVB-sensitive characteristics not exhibited by retinyl esters such as RP (Dermatology 1999;199:302-7). The EWG used "vitamin A" and "retinyl palmitate" interchangeably in their criticisms and follow-ups, which is misleading. The vitamin A family of drugs includes retinyl esters, retinol, tretinoin, adapalene, tazarotene, and oral isotretinoin (Accutane), in addition to four carotenoids, including beta-carotene, many of which have been shown to prevent or protect against cancer (Br. J. Cancer 1988;57:428-33; Cancer Epidemiol. Biomarkers Prev. 1997;6:949-56; J. Invest. Dermatol. 1981;76:178-80; Arch. Dermatol. Res. 1981;270:453-62). That does not mean that RP prevents cancer just because oral retinol, beta-carotene, or tretinoin have been shown to do so, for example. In fact, the study that the EWG refers to shows evidence that RP may lead to skin tumors in mice.
In response to the EWG report, Wang et al. acknowledged that of the eight in vitro studies published by the Food and Drug Administration from 2002 to 2009, four revealed that reactive oxygen species were produced by RP after UVA exposure (J. Am. Acad. Dermatol. 2010;63:903-6; Photodermatol. Photoimmunol. Photomed. 2011;27:58-67; Toxicol. Ind. Health 2007;23:625-31; Toxicol. Lett. 2006;163:30-43; Int. J. Environ. Res. Public Health 2006;3:185-90; Chem. Res. Toxicol. 2005;18:129-38). However, they questioned the relevance of these results in the context of the convoluted mechanisms of the antioxidant setting in human skin. They also contended that the National Toxicology Program (NTP) study on which the EWG based its report failed to prove that the combination of RP and UV results in photocarcinogenesis and, in fact, was rife with reasons for skepticism (J. Am. Acad. Dermatol. 2010;63:903-6; Photodermatol. Photoimmunol. Photomed. 2011;27:58-67). The EWG offered its own counterarguments and stood by its report. Rather than wade further into the debate that occurred in 2010 and found its way into the pages of the Journal of the American Academy of Dermatology (2010;63:903-6), let’s review what is known about RP.
What else do we know about RP?
In 1997, Duell et al. showed that unoccluded retinol is more effective at penetrating human skin in vivo than RP or retinoic acid (J. Invest. Dermatol. 1997;109:301-5).
In 2003, Antille et al. used an in vitro model to evaluate the photoprotective activity of RP, and then applied topical RP on the back of hairless mice before exposing them to UVB. They also applied topical RP or a sunscreen on the buttocks of human volunteers before exposing them to four minimal erythema doses of UVB. The investigators found that RP was as efficient in vitro as the commercial filter octylmethoxycinnamate in preventing UVB-induced fluorescence or photobleaching of fluorescent markers. Topical RP also significantly suppressed the formation of thymine dimers in mouse epidermis and human skin. In the volunteers, topical RP was as efficient as an SPF (sun protection factor) 20 sunscreen in preventing sunburn erythema (J. Invest. Dermatol. 2003;121:1163-7).
In 2005, Yan et al. studied the phototoxicity of RP, anhydroretinol (AR), and 5,6-epoxyretinyl palmitate (5,6-epoxy-RP) in human skin Jurkat T cells with and without light irradiation. Irradiation of cells in the absence of a retinoid rendered little damage, but the presence of RP, 5,6-epoxy-RP, or AR (50, 100, 150, and 200 micromol/L) yielded DNA fragmentation, with cell death occurring at retinoid concentrations of 100 micromol/L or greater. The investigators concluded that DNA damage and cytotoxicity are engendered by RP and its photodecomposition products in association with UVA and visible light exposure. They also determined that UVA irradiation of these retinoids produces free radicals that spur DNA strand cleavage (Toxicol. Ind. Health 2005;21:167-75).
RP accounts for most of the retinyl esters endogenously formed in skin. In 2006, Yan et al., noting that exogenous RP accumulates via topically applied cosmetic and skin care formulations, investigated the time course for buildup and disappearance of RP and retinol in the stratified layers of skin from female SKH-1 mice singly or repeatedly dosed with topical creams containing 0.5% or 2% RP. The researchers observed that within 24 hours of application, RP quickly diffused into the stratum corneum and epidermal skin layers. RP and retinol levels were lowest in the dermis, intermediate in the stratum corneum, and highest in the epidermis. In separated skin layers and intact skin, RP and retinol levels declined over time, but for 18 days, RP levels remained higher than control values. The investigators concluded that topically applied RP changed the normal physiological levels of RP and retinol in the skin of mice (Toxicol. Ind. Health 2006;22:181-91).
Having previously shown that irradiation of RP with UVA leads to the formation of photodecomposition products, synthesis of reactive oxygen species, and lipid peroxidation induction, Xia et al. demonstrated comparable results, identifying RP as a photosensitizer following irradiation with UVB light (Int. J. Environ. Res. Public Health 2006;3:185-90).
Recommendations
In light of the controversy swirling around RP and the appropriate concern it has engendered, in addition to the weight of evidence as well as experience from personal observation, I advise patients to avoid daytime use of products with RP high on the ingredient list. I add that it poses real risks while offering minimal benefits. Such patients should be using retinol or tretinoin. I recommend the use of retinoids at night, to avoid the photosensitizing action induced by UVA or UVB on retinoids left on the skin.
Conclusion
Retinyl palmitate does not penetrate very well into the skin. Consequently, for over-the-counter topical formulations, I recommend retinol instead. Because of the slow penetration of RP into the skin, the RP that remains on the skin will undergo photoreaction more than a substance that is rapidly absorbed. When exposed to light, RP on the skin may undergo metabolism and/or photoreaction to generate reactive oxygen species. These reactive oxygen species or free radicals can theoretically lead to increased skin cancer. That said, sufficient evidence to establish a causal link between RP and skin cancer has not been produced. Nor, I’m afraid, are there any good reasons to recommend the use of RP. More research on this subject is needed and will likely emerge in a timely fashion.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at dermnews@frontlinemedcom.com. This column, "Cosmeceutical Critique," appears regularly in Skin & Allergy News.
Retinyl palmitate, a storage and ester form of retinol (vitamin A) and the prevailing type of vitamin A found naturally in the skin (Toxicol. Ind. Health 2005;21:167-75), has become increasingly popular during the past 2 decades. It is widely used in more than 600 skin care products, including cosmetics and sunscreens, and, with FDA approval, over-the-counter and prescription drugs (Photodermatol. Photoimmunol. Photomed. 2011;27:58-67). It was also the subject of a controversial summer 2010 report by the Environmental Working Group (EWG) in which the organization warned of possible photocarcinogenicity associated with retinyl palmitate (RP)-containing sunscreens.
Although vitamin A storage in the epidermis takes the form of retinyl esters and retinols, they act differently when exposed to UV light. The retinols display UVB-resistant and UVB-sensitive characteristics not exhibited by retinyl esters such as RP (Dermatology 1999;199:302-7). The EWG used "vitamin A" and "retinyl palmitate" interchangeably in their criticisms and follow-ups, which is misleading. The vitamin A family of drugs includes retinyl esters, retinol, tretinoin, adapalene, tazarotene, and oral isotretinoin (Accutane), in addition to four carotenoids, including beta-carotene, many of which have been shown to prevent or protect against cancer (Br. J. Cancer 1988;57:428-33; Cancer Epidemiol. Biomarkers Prev. 1997;6:949-56; J. Invest. Dermatol. 1981;76:178-80; Arch. Dermatol. Res. 1981;270:453-62). That does not mean that RP prevents cancer just because oral retinol, beta-carotene, or tretinoin have been shown to do so, for example. In fact, the study that the EWG refers to shows evidence that RP may lead to skin tumors in mice.
In response to the EWG report, Wang et al. acknowledged that of the eight in vitro studies published by the Food and Drug Administration from 2002 to 2009, four revealed that reactive oxygen species were produced by RP after UVA exposure (J. Am. Acad. Dermatol. 2010;63:903-6; Photodermatol. Photoimmunol. Photomed. 2011;27:58-67; Toxicol. Ind. Health 2007;23:625-31; Toxicol. Lett. 2006;163:30-43; Int. J. Environ. Res. Public Health 2006;3:185-90; Chem. Res. Toxicol. 2005;18:129-38). However, they questioned the relevance of these results in the context of the convoluted mechanisms of the antioxidant setting in human skin. They also contended that the National Toxicology Program (NTP) study on which the EWG based its report failed to prove that the combination of RP and UV results in photocarcinogenesis and, in fact, was rife with reasons for skepticism (J. Am. Acad. Dermatol. 2010;63:903-6; Photodermatol. Photoimmunol. Photomed. 2011;27:58-67). The EWG offered its own counterarguments and stood by its report. Rather than wade further into the debate that occurred in 2010 and found its way into the pages of the Journal of the American Academy of Dermatology (2010;63:903-6), let’s review what is known about RP.
What else do we know about RP?
In 1997, Duell et al. showed that unoccluded retinol is more effective at penetrating human skin in vivo than RP or retinoic acid (J. Invest. Dermatol. 1997;109:301-5).
In 2003, Antille et al. used an in vitro model to evaluate the photoprotective activity of RP, and then applied topical RP on the back of hairless mice before exposing them to UVB. They also applied topical RP or a sunscreen on the buttocks of human volunteers before exposing them to four minimal erythema doses of UVB. The investigators found that RP was as efficient in vitro as the commercial filter octylmethoxycinnamate in preventing UVB-induced fluorescence or photobleaching of fluorescent markers. Topical RP also significantly suppressed the formation of thymine dimers in mouse epidermis and human skin. In the volunteers, topical RP was as efficient as an SPF (sun protection factor) 20 sunscreen in preventing sunburn erythema (J. Invest. Dermatol. 2003;121:1163-7).
In 2005, Yan et al. studied the phototoxicity of RP, anhydroretinol (AR), and 5,6-epoxyretinyl palmitate (5,6-epoxy-RP) in human skin Jurkat T cells with and without light irradiation. Irradiation of cells in the absence of a retinoid rendered little damage, but the presence of RP, 5,6-epoxy-RP, or AR (50, 100, 150, and 200 micromol/L) yielded DNA fragmentation, with cell death occurring at retinoid concentrations of 100 micromol/L or greater. The investigators concluded that DNA damage and cytotoxicity are engendered by RP and its photodecomposition products in association with UVA and visible light exposure. They also determined that UVA irradiation of these retinoids produces free radicals that spur DNA strand cleavage (Toxicol. Ind. Health 2005;21:167-75).
RP accounts for most of the retinyl esters endogenously formed in skin. In 2006, Yan et al., noting that exogenous RP accumulates via topically applied cosmetic and skin care formulations, investigated the time course for buildup and disappearance of RP and retinol in the stratified layers of skin from female SKH-1 mice singly or repeatedly dosed with topical creams containing 0.5% or 2% RP. The researchers observed that within 24 hours of application, RP quickly diffused into the stratum corneum and epidermal skin layers. RP and retinol levels were lowest in the dermis, intermediate in the stratum corneum, and highest in the epidermis. In separated skin layers and intact skin, RP and retinol levels declined over time, but for 18 days, RP levels remained higher than control values. The investigators concluded that topically applied RP changed the normal physiological levels of RP and retinol in the skin of mice (Toxicol. Ind. Health 2006;22:181-91).
Having previously shown that irradiation of RP with UVA leads to the formation of photodecomposition products, synthesis of reactive oxygen species, and lipid peroxidation induction, Xia et al. demonstrated comparable results, identifying RP as a photosensitizer following irradiation with UVB light (Int. J. Environ. Res. Public Health 2006;3:185-90).
Recommendations
In light of the controversy swirling around RP and the appropriate concern it has engendered, in addition to the weight of evidence as well as experience from personal observation, I advise patients to avoid daytime use of products with RP high on the ingredient list. I add that it poses real risks while offering minimal benefits. Such patients should be using retinol or tretinoin. I recommend the use of retinoids at night, to avoid the photosensitizing action induced by UVA or UVB on retinoids left on the skin.
Conclusion
Retinyl palmitate does not penetrate very well into the skin. Consequently, for over-the-counter topical formulations, I recommend retinol instead. Because of the slow penetration of RP into the skin, the RP that remains on the skin will undergo photoreaction more than a substance that is rapidly absorbed. When exposed to light, RP on the skin may undergo metabolism and/or photoreaction to generate reactive oxygen species. These reactive oxygen species or free radicals can theoretically lead to increased skin cancer. That said, sufficient evidence to establish a causal link between RP and skin cancer has not been produced. Nor, I’m afraid, are there any good reasons to recommend the use of RP. More research on this subject is needed and will likely emerge in a timely fashion.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to her at dermnews@frontlinemedcom.com. This column, "Cosmeceutical Critique," appears regularly in Skin & Allergy News.
Melissa Officinalis
Used in foods, some traditional medicines, herbal tea, herbal toothpastes, and aromatherapy, Melissa officinalis (lemon balm) is a perennial herb in the Lamiaceae (mint) family found in southern Europe and the Mediterranean area. The medicinal use of lemon balm dates back at least 2,000 years (Ann. N. Y. Acad. Sci. 1965;130:474-82). Lower abdominal distress and nervous conditions are some of the ailments treated with lemon balm in folk medicine; herpes lesions are a modern indication (Nat. Prod. Res. 2008;22:1433-40). The essential oil and phenylpropanoid derivatives are thought to be the two primary groups of active constituents in lemon balm (Phytochemistry. 2011;72:572-8).
The main individual components of M. officinalis essential oil have been identified as the monoterpenaldehydes citral a, citral b, and citronellal (Phytomedicine. 2008;15:734-40). The chief phenolic compounds are rosmarinic acid, which is an ester of caffeic acid and 3,4-dihydroxyphenyllactic acid, as well as caffeic acid, which is isolated from the fresh leaves and stems (J. Nat. Prod. 2009;72:1512-5Phytochemistry. 2011;72:572-8). Six flavonoids, including luteolin and apigenin, have also been isolated from the leaves of lemon balm (Acta. Pol. Pharm. 2002;59:139-43; J. Nat. Prod. 2007;70:1889-94). Given the presence of such ingredients known to exhibit antioxidant properties, it is not surprising that such a capacity is considered one of the main medicinal benefits of M. officinalis. Indeed, lemon balm is reputed to display significant antioxidant, anxiolytic (Med. J. Nutrition. Metab. 2011;4:211-8; Phytomedicine. 2010;17:397-403; Psychosom. Med. 2004;66:607-13), and antiviral (particularly antiherpetic) activity (Proc. Soc. Exp. Biol. Med. 1964;117:431-4; Virol. J. 2011;8:188). M. officinalis is also a component, with two other herbs, in a mixture (Ob-X) recently shown to lower body weight gain and adipose tissue mass in genetically obese mice (Pharm. Biol. 2011;49:614-9).
Antioxidant Activity
In a 2009 study, investigators examined the antioxidant potential of three plants (M. officinalis, Matricaria recutita (German chamomile), and Cymbopogon citrus [lemon grass]) used in Brazil to treat neurologic conditions. M. officinalis was found to deliver the greatest reduction in thiobarbituric acid reactive species (TBARS) and the most salient antioxidant effect as evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. The investigators concluded that M. officinalis warrants consideration as a treatment for oxidative stress–associated neurologic diseases (Neurochem. Res. 2009;34:973-83).
Additional evidence of its antioxidant activity is emerging. In early 2012, Martins et al. reported on their study in which an aqueous extract of M. officinalis significantly mitigated manganese-induced brain oxidative stress in mice. They found that the extract attenuated oxidative damage (TBARS) and reduced total thiol levels, and concluded that their findings show the potent antioxidant activity of M. officinalis (Brain. Res. Bull. 2012;87;74-9). In addition, a recent study found that lemon balm infusion in a tea, after 30 days of daily consumption, significantly lowered oxidative stress and DNA damage in radiology staff exposed to low doses of radiation at work (Toxicol. Ind. Health. 2011;27:205-12).
Antiviral Activity
In 2006, Gaby reported on various natural substances, used in the diet or topically, that exert activity against herpes simplex lesions and prevent recurrences, serving as effective alternatives to acyclovir and its attendant side effects. He cited lemon balm as having exhibited antiviral properties in two studies in the 1990s (Altern. Med. Rev. 2006;11:93-101).
In 1994, 116 patients with acute herpes simplex applied a standardized lemon balm cream (containing 1% Lo-701) or a placebo cream two to four times daily in a randomized, double-blind trial over a 5- to 10-day period within 72 hours of symptom onset. While only 19% of the placebo group reported satisfactory healing, 41% of the active treatment group was satisfied (Phytomedicine. 1994;1:25-31). In 1999, a double-blind, placebo-controlled trial randomized 66 patients with a minimum of four herpes simplex episodes per year to treatment (four times daily for 5 days) with the same standardized lemon balm cream or placebo. Symptom scores were significantly lower in the treatment group than the control group by the second day of the protocol, though the trend supporting active treatment over 5 days was not significant (Phytomedicine 1999;6:225-30).
In 2008, Mazzanti et al. evaluated the antiviral activity against herpes simplex virus type 2 (HSV-2) of a hydroalcoholic extract of lemon balm leaves using a cytopathic effect inhibition assay on Vero cells. They found that lemon balm diminished the cytopathic effect of HSV-2 on Vero cells, with a maximum suppression effect with 0.5 mg/mL. The extract, shown through NMR (nuclear magnetic resonance) and HPLC (high-performance liquid chromatography) analysis to contain rosmarinic acid (4.1% w/w), did not prevent the entry of HSV-2 into cells, indicating postpenetration activity by the botanical agent. The investigators concluded that their work supports the use of lemon balm for treating herpes lesions, and justifies its further study in clinical trials (Nat. Prod. Res. 2008;22:1433-40).
Also that year, Schnitzler et al. evaluated the antiviral effect of lemon balm oil on HSV-1 and HSV-2 in vitro on monkey kidney cells. They found that plaque formation was significantly lowered (by 98.8% for HSV-1 and 97.2% for HSV-2) by noncytotoxic lemon balm oil concentrations, with higher concentrations nearly eradicating infections. Using time-on-addition assays, the investigators determined that pretreatment with lemon balm oil significantly suppressed both viruses before infection of cells, suggesting that the oil impacted the virus prior to adsorption, but not after reaching the host cell. They concluded that this implies the capacity for direct antiviral activity. The authors added that the lipophilic nature of lemon balm oil allows for its penetration into the skin, further supporting its suitability as a topical treatment of herpes (Phytomedicine. 2008;15:734-40).
In a more recent in vitro experiment evaluating antiviral activity against HSV-1, Astani et al. compared an aqueous extract of M. officinalis and phenolic extract compounds (caffeic acid, p-coumaric acid, and rosmarinic acid). The lemon balm extract exhibited high virucidal activity against HSV-1, even at concentrations of 1.5 mcg/mL; phenolic compounds showed similar results only at concentrations 100 times greater. Further, lemon balm extract and rosmarinic acid dose-dependently suppressed HSV-1 attachment to host cells. The researchers concluded that rosmarinic acid was the primary constituent responsible for the antiviral activity displayed by lemon balm, but noted that M. officinalis extract, which imparted virucidal activity against HSV-1 in vitro with low toxicity, has a greater selectivity index against HSV than that of its constituents alone (Chemotherapy. 2012;58:70-7).
In 2008, Geuenich et al. investigated several species of the Lamiaceae family (including lemon balm) for their potency in suppressing HIV-1 infection. The aqueous extracts from the leaves of lemon balm (as well as peppermint and sage) dose-dependently displayed substantial activity against HIV-1 infection in T-cell lines, primary macrophages, and in ex vivo tonsil histocultures. The investigators also found that exposure of extracts to free virions strongly and quickly suppressed infections, though no antiviral effect was seen in exposure to surface-bound virions or target cells alone. Noting the antiherpetic activity of these Lamiaceae family extracts, the investigators suggested that the development of virucidal topical microbicides using such ingredients is warranted (Retrovirology. 2008;5:27).
Hypopigmentary Potential
A potential hypopigmentary application of lemon balm also may be emerging. In 2011, Fujita et al. isolated 16-hydroxy-9-oxo-10E,12E,14E-octadecatrienoic acid (also called Corchorifatty acid B [CFAB]) from the ethanol extracts of the aerial parts of M. officinalis, and found that it suppresses pigmentation in human melanocytes and murine melanoma B16 cells, probably by promoting accelerated degradation of tyrosinase in B16 cells. Further, they noted that the mechanism of action of CFAB is markedly different from those of many other hypopigmentary agents, which facilitate tyrosinase degradation in proteasomes or lysosomes. That is, the reductions in tyrosinase caused by CFAB are thought to take place in post–Golgi complex areas, not in proteasomal or lysosomal ones (Exp. Dermatol. 2011;20(5):420-4).
Conclusions
Like many botanical ingredients studied and harnessed in our modern pharmacopeia, lemon balm has a history of use in traditional medicine. Recent studies suggest antioxidant, anxiolytic, and, especially, antiviral properties, notably in the treatment of herpes viruses. More research is necessary, however, to establish a broader role for M. officinalis in the dermatologic armamentarium.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to Dr. Baumann at sknews@elsevier.com.
Used in foods, some traditional medicines, herbal tea, herbal toothpastes, and aromatherapy, Melissa officinalis (lemon balm) is a perennial herb in the Lamiaceae (mint) family found in southern Europe and the Mediterranean area. The medicinal use of lemon balm dates back at least 2,000 years (Ann. N. Y. Acad. Sci. 1965;130:474-82). Lower abdominal distress and nervous conditions are some of the ailments treated with lemon balm in folk medicine; herpes lesions are a modern indication (Nat. Prod. Res. 2008;22:1433-40). The essential oil and phenylpropanoid derivatives are thought to be the two primary groups of active constituents in lemon balm (Phytochemistry. 2011;72:572-8).
The main individual components of M. officinalis essential oil have been identified as the monoterpenaldehydes citral a, citral b, and citronellal (Phytomedicine. 2008;15:734-40). The chief phenolic compounds are rosmarinic acid, which is an ester of caffeic acid and 3,4-dihydroxyphenyllactic acid, as well as caffeic acid, which is isolated from the fresh leaves and stems (J. Nat. Prod. 2009;72:1512-5Phytochemistry. 2011;72:572-8). Six flavonoids, including luteolin and apigenin, have also been isolated from the leaves of lemon balm (Acta. Pol. Pharm. 2002;59:139-43; J. Nat. Prod. 2007;70:1889-94). Given the presence of such ingredients known to exhibit antioxidant properties, it is not surprising that such a capacity is considered one of the main medicinal benefits of M. officinalis. Indeed, lemon balm is reputed to display significant antioxidant, anxiolytic (Med. J. Nutrition. Metab. 2011;4:211-8; Phytomedicine. 2010;17:397-403; Psychosom. Med. 2004;66:607-13), and antiviral (particularly antiherpetic) activity (Proc. Soc. Exp. Biol. Med. 1964;117:431-4; Virol. J. 2011;8:188). M. officinalis is also a component, with two other herbs, in a mixture (Ob-X) recently shown to lower body weight gain and adipose tissue mass in genetically obese mice (Pharm. Biol. 2011;49:614-9).
Antioxidant Activity
In a 2009 study, investigators examined the antioxidant potential of three plants (M. officinalis, Matricaria recutita (German chamomile), and Cymbopogon citrus [lemon grass]) used in Brazil to treat neurologic conditions. M. officinalis was found to deliver the greatest reduction in thiobarbituric acid reactive species (TBARS) and the most salient antioxidant effect as evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. The investigators concluded that M. officinalis warrants consideration as a treatment for oxidative stress–associated neurologic diseases (Neurochem. Res. 2009;34:973-83).
Additional evidence of its antioxidant activity is emerging. In early 2012, Martins et al. reported on their study in which an aqueous extract of M. officinalis significantly mitigated manganese-induced brain oxidative stress in mice. They found that the extract attenuated oxidative damage (TBARS) and reduced total thiol levels, and concluded that their findings show the potent antioxidant activity of M. officinalis (Brain. Res. Bull. 2012;87;74-9). In addition, a recent study found that lemon balm infusion in a tea, after 30 days of daily consumption, significantly lowered oxidative stress and DNA damage in radiology staff exposed to low doses of radiation at work (Toxicol. Ind. Health. 2011;27:205-12).
Antiviral Activity
In 2006, Gaby reported on various natural substances, used in the diet or topically, that exert activity against herpes simplex lesions and prevent recurrences, serving as effective alternatives to acyclovir and its attendant side effects. He cited lemon balm as having exhibited antiviral properties in two studies in the 1990s (Altern. Med. Rev. 2006;11:93-101).
In 1994, 116 patients with acute herpes simplex applied a standardized lemon balm cream (containing 1% Lo-701) or a placebo cream two to four times daily in a randomized, double-blind trial over a 5- to 10-day period within 72 hours of symptom onset. While only 19% of the placebo group reported satisfactory healing, 41% of the active treatment group was satisfied (Phytomedicine. 1994;1:25-31). In 1999, a double-blind, placebo-controlled trial randomized 66 patients with a minimum of four herpes simplex episodes per year to treatment (four times daily for 5 days) with the same standardized lemon balm cream or placebo. Symptom scores were significantly lower in the treatment group than the control group by the second day of the protocol, though the trend supporting active treatment over 5 days was not significant (Phytomedicine 1999;6:225-30).
In 2008, Mazzanti et al. evaluated the antiviral activity against herpes simplex virus type 2 (HSV-2) of a hydroalcoholic extract of lemon balm leaves using a cytopathic effect inhibition assay on Vero cells. They found that lemon balm diminished the cytopathic effect of HSV-2 on Vero cells, with a maximum suppression effect with 0.5 mg/mL. The extract, shown through NMR (nuclear magnetic resonance) and HPLC (high-performance liquid chromatography) analysis to contain rosmarinic acid (4.1% w/w), did not prevent the entry of HSV-2 into cells, indicating postpenetration activity by the botanical agent. The investigators concluded that their work supports the use of lemon balm for treating herpes lesions, and justifies its further study in clinical trials (Nat. Prod. Res. 2008;22:1433-40).
Also that year, Schnitzler et al. evaluated the antiviral effect of lemon balm oil on HSV-1 and HSV-2 in vitro on monkey kidney cells. They found that plaque formation was significantly lowered (by 98.8% for HSV-1 and 97.2% for HSV-2) by noncytotoxic lemon balm oil concentrations, with higher concentrations nearly eradicating infections. Using time-on-addition assays, the investigators determined that pretreatment with lemon balm oil significantly suppressed both viruses before infection of cells, suggesting that the oil impacted the virus prior to adsorption, but not after reaching the host cell. They concluded that this implies the capacity for direct antiviral activity. The authors added that the lipophilic nature of lemon balm oil allows for its penetration into the skin, further supporting its suitability as a topical treatment of herpes (Phytomedicine. 2008;15:734-40).
In a more recent in vitro experiment evaluating antiviral activity against HSV-1, Astani et al. compared an aqueous extract of M. officinalis and phenolic extract compounds (caffeic acid, p-coumaric acid, and rosmarinic acid). The lemon balm extract exhibited high virucidal activity against HSV-1, even at concentrations of 1.5 mcg/mL; phenolic compounds showed similar results only at concentrations 100 times greater. Further, lemon balm extract and rosmarinic acid dose-dependently suppressed HSV-1 attachment to host cells. The researchers concluded that rosmarinic acid was the primary constituent responsible for the antiviral activity displayed by lemon balm, but noted that M. officinalis extract, which imparted virucidal activity against HSV-1 in vitro with low toxicity, has a greater selectivity index against HSV than that of its constituents alone (Chemotherapy. 2012;58:70-7).
In 2008, Geuenich et al. investigated several species of the Lamiaceae family (including lemon balm) for their potency in suppressing HIV-1 infection. The aqueous extracts from the leaves of lemon balm (as well as peppermint and sage) dose-dependently displayed substantial activity against HIV-1 infection in T-cell lines, primary macrophages, and in ex vivo tonsil histocultures. The investigators also found that exposure of extracts to free virions strongly and quickly suppressed infections, though no antiviral effect was seen in exposure to surface-bound virions or target cells alone. Noting the antiherpetic activity of these Lamiaceae family extracts, the investigators suggested that the development of virucidal topical microbicides using such ingredients is warranted (Retrovirology. 2008;5:27).
Hypopigmentary Potential
A potential hypopigmentary application of lemon balm also may be emerging. In 2011, Fujita et al. isolated 16-hydroxy-9-oxo-10E,12E,14E-octadecatrienoic acid (also called Corchorifatty acid B [CFAB]) from the ethanol extracts of the aerial parts of M. officinalis, and found that it suppresses pigmentation in human melanocytes and murine melanoma B16 cells, probably by promoting accelerated degradation of tyrosinase in B16 cells. Further, they noted that the mechanism of action of CFAB is markedly different from those of many other hypopigmentary agents, which facilitate tyrosinase degradation in proteasomes or lysosomes. That is, the reductions in tyrosinase caused by CFAB are thought to take place in post–Golgi complex areas, not in proteasomal or lysosomal ones (Exp. Dermatol. 2011;20(5):420-4).
Conclusions
Like many botanical ingredients studied and harnessed in our modern pharmacopeia, lemon balm has a history of use in traditional medicine. Recent studies suggest antioxidant, anxiolytic, and, especially, antiviral properties, notably in the treatment of herpes viruses. More research is necessary, however, to establish a broader role for M. officinalis in the dermatologic armamentarium.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to Dr. Baumann at sknews@elsevier.com.
Used in foods, some traditional medicines, herbal tea, herbal toothpastes, and aromatherapy, Melissa officinalis (lemon balm) is a perennial herb in the Lamiaceae (mint) family found in southern Europe and the Mediterranean area. The medicinal use of lemon balm dates back at least 2,000 years (Ann. N. Y. Acad. Sci. 1965;130:474-82). Lower abdominal distress and nervous conditions are some of the ailments treated with lemon balm in folk medicine; herpes lesions are a modern indication (Nat. Prod. Res. 2008;22:1433-40). The essential oil and phenylpropanoid derivatives are thought to be the two primary groups of active constituents in lemon balm (Phytochemistry. 2011;72:572-8).
The main individual components of M. officinalis essential oil have been identified as the monoterpenaldehydes citral a, citral b, and citronellal (Phytomedicine. 2008;15:734-40). The chief phenolic compounds are rosmarinic acid, which is an ester of caffeic acid and 3,4-dihydroxyphenyllactic acid, as well as caffeic acid, which is isolated from the fresh leaves and stems (J. Nat. Prod. 2009;72:1512-5Phytochemistry. 2011;72:572-8). Six flavonoids, including luteolin and apigenin, have also been isolated from the leaves of lemon balm (Acta. Pol. Pharm. 2002;59:139-43; J. Nat. Prod. 2007;70:1889-94). Given the presence of such ingredients known to exhibit antioxidant properties, it is not surprising that such a capacity is considered one of the main medicinal benefits of M. officinalis. Indeed, lemon balm is reputed to display significant antioxidant, anxiolytic (Med. J. Nutrition. Metab. 2011;4:211-8; Phytomedicine. 2010;17:397-403; Psychosom. Med. 2004;66:607-13), and antiviral (particularly antiherpetic) activity (Proc. Soc. Exp. Biol. Med. 1964;117:431-4; Virol. J. 2011;8:188). M. officinalis is also a component, with two other herbs, in a mixture (Ob-X) recently shown to lower body weight gain and adipose tissue mass in genetically obese mice (Pharm. Biol. 2011;49:614-9).
Antioxidant Activity
In a 2009 study, investigators examined the antioxidant potential of three plants (M. officinalis, Matricaria recutita (German chamomile), and Cymbopogon citrus [lemon grass]) used in Brazil to treat neurologic conditions. M. officinalis was found to deliver the greatest reduction in thiobarbituric acid reactive species (TBARS) and the most salient antioxidant effect as evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay. The investigators concluded that M. officinalis warrants consideration as a treatment for oxidative stress–associated neurologic diseases (Neurochem. Res. 2009;34:973-83).
Additional evidence of its antioxidant activity is emerging. In early 2012, Martins et al. reported on their study in which an aqueous extract of M. officinalis significantly mitigated manganese-induced brain oxidative stress in mice. They found that the extract attenuated oxidative damage (TBARS) and reduced total thiol levels, and concluded that their findings show the potent antioxidant activity of M. officinalis (Brain. Res. Bull. 2012;87;74-9). In addition, a recent study found that lemon balm infusion in a tea, after 30 days of daily consumption, significantly lowered oxidative stress and DNA damage in radiology staff exposed to low doses of radiation at work (Toxicol. Ind. Health. 2011;27:205-12).
Antiviral Activity
In 2006, Gaby reported on various natural substances, used in the diet or topically, that exert activity against herpes simplex lesions and prevent recurrences, serving as effective alternatives to acyclovir and its attendant side effects. He cited lemon balm as having exhibited antiviral properties in two studies in the 1990s (Altern. Med. Rev. 2006;11:93-101).
In 1994, 116 patients with acute herpes simplex applied a standardized lemon balm cream (containing 1% Lo-701) or a placebo cream two to four times daily in a randomized, double-blind trial over a 5- to 10-day period within 72 hours of symptom onset. While only 19% of the placebo group reported satisfactory healing, 41% of the active treatment group was satisfied (Phytomedicine. 1994;1:25-31). In 1999, a double-blind, placebo-controlled trial randomized 66 patients with a minimum of four herpes simplex episodes per year to treatment (four times daily for 5 days) with the same standardized lemon balm cream or placebo. Symptom scores were significantly lower in the treatment group than the control group by the second day of the protocol, though the trend supporting active treatment over 5 days was not significant (Phytomedicine 1999;6:225-30).
In 2008, Mazzanti et al. evaluated the antiviral activity against herpes simplex virus type 2 (HSV-2) of a hydroalcoholic extract of lemon balm leaves using a cytopathic effect inhibition assay on Vero cells. They found that lemon balm diminished the cytopathic effect of HSV-2 on Vero cells, with a maximum suppression effect with 0.5 mg/mL. The extract, shown through NMR (nuclear magnetic resonance) and HPLC (high-performance liquid chromatography) analysis to contain rosmarinic acid (4.1% w/w), did not prevent the entry of HSV-2 into cells, indicating postpenetration activity by the botanical agent. The investigators concluded that their work supports the use of lemon balm for treating herpes lesions, and justifies its further study in clinical trials (Nat. Prod. Res. 2008;22:1433-40).
Also that year, Schnitzler et al. evaluated the antiviral effect of lemon balm oil on HSV-1 and HSV-2 in vitro on monkey kidney cells. They found that plaque formation was significantly lowered (by 98.8% for HSV-1 and 97.2% for HSV-2) by noncytotoxic lemon balm oil concentrations, with higher concentrations nearly eradicating infections. Using time-on-addition assays, the investigators determined that pretreatment with lemon balm oil significantly suppressed both viruses before infection of cells, suggesting that the oil impacted the virus prior to adsorption, but not after reaching the host cell. They concluded that this implies the capacity for direct antiviral activity. The authors added that the lipophilic nature of lemon balm oil allows for its penetration into the skin, further supporting its suitability as a topical treatment of herpes (Phytomedicine. 2008;15:734-40).
In a more recent in vitro experiment evaluating antiviral activity against HSV-1, Astani et al. compared an aqueous extract of M. officinalis and phenolic extract compounds (caffeic acid, p-coumaric acid, and rosmarinic acid). The lemon balm extract exhibited high virucidal activity against HSV-1, even at concentrations of 1.5 mcg/mL; phenolic compounds showed similar results only at concentrations 100 times greater. Further, lemon balm extract and rosmarinic acid dose-dependently suppressed HSV-1 attachment to host cells. The researchers concluded that rosmarinic acid was the primary constituent responsible for the antiviral activity displayed by lemon balm, but noted that M. officinalis extract, which imparted virucidal activity against HSV-1 in vitro with low toxicity, has a greater selectivity index against HSV than that of its constituents alone (Chemotherapy. 2012;58:70-7).
In 2008, Geuenich et al. investigated several species of the Lamiaceae family (including lemon balm) for their potency in suppressing HIV-1 infection. The aqueous extracts from the leaves of lemon balm (as well as peppermint and sage) dose-dependently displayed substantial activity against HIV-1 infection in T-cell lines, primary macrophages, and in ex vivo tonsil histocultures. The investigators also found that exposure of extracts to free virions strongly and quickly suppressed infections, though no antiviral effect was seen in exposure to surface-bound virions or target cells alone. Noting the antiherpetic activity of these Lamiaceae family extracts, the investigators suggested that the development of virucidal topical microbicides using such ingredients is warranted (Retrovirology. 2008;5:27).
Hypopigmentary Potential
A potential hypopigmentary application of lemon balm also may be emerging. In 2011, Fujita et al. isolated 16-hydroxy-9-oxo-10E,12E,14E-octadecatrienoic acid (also called Corchorifatty acid B [CFAB]) from the ethanol extracts of the aerial parts of M. officinalis, and found that it suppresses pigmentation in human melanocytes and murine melanoma B16 cells, probably by promoting accelerated degradation of tyrosinase in B16 cells. Further, they noted that the mechanism of action of CFAB is markedly different from those of many other hypopigmentary agents, which facilitate tyrosinase degradation in proteasomes or lysosomes. That is, the reductions in tyrosinase caused by CFAB are thought to take place in post–Golgi complex areas, not in proteasomal or lysosomal ones (Exp. Dermatol. 2011;20(5):420-4).
Conclusions
Like many botanical ingredients studied and harnessed in our modern pharmacopeia, lemon balm has a history of use in traditional medicine. Recent studies suggest antioxidant, anxiolytic, and, especially, antiviral properties, notably in the treatment of herpes viruses. More research is necessary, however, to establish a broader role for M. officinalis in the dermatologic armamentarium.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest. To respond to this column, or to suggest topics for future columns, write to Dr. Baumann at sknews@elsevier.com.
Dill
Dill (Anethum graveolens), an aromatic perennial herb often used as a culinary spice, has been utilized for medical purposes for hundreds of years, at least since medieval times (Wurzbg. Medizinhist. Forsch. 1982;24:411-24). A member of the Umbelliferae (carrot or parsley) family, dill is used in traditional Chinese medicine, and its use in cooking and Uygur medicine is believed to date back to ancient times in China (Evid. Based Complement. Alternat. Med. 2011;2011:659-704).
Based on such past uses, as well as modern research, dill is known for having demonstrated anti-inflammatory, antispasmodic, carminative, aromatic, and galactagogue activity (Medical Herbalism: The Science and Practice of Herbal Medicine. Rochester, Vt.: Healing Arts Press, 2003).
Antimicrobial Activity
In a 2003 study of the essential oil of seeds of dill stored for more than 35 years, Bulgarian researchers tested its antimicrobial activity using various microorganisms. They noted high activity of the essential A. graveolens oil against the mold Aspergillus niger and the yeasts Saccharomyces cerevisiae and Candida albicans (J. Agric. Food. Chem. 2003;51:3854-7). Worth noting from this study is not only the anticandidal properties of dill but its potency even after a long time in storage.
In 2009, Kaur and Arora examined the spices dill, fennel (Foeniculum vulgare), and ajwain (Trachyspermum ammi) for their antibacterial activity. The investigators ascertained antibacterial activity by using agar diffusion assay, minimum inhibitory concentration, and viable cell count studies, and compared effects with those of some standard antibiotics. All three spices in the study exhibited significant activity against a wide range of bacteria, with the exception of Klebsiella pneumoniae and a strain of Pseudomonas aeruginosa. The researchers concluded that the results reveal a scientific basis for the reputed antibacterial effects of these plants and lend credence to their traditional medicinal applications. Further, they suggested that future work may lead to viable antibacterial agents based on these ingredients (BMC Complement. Altern. Med. 2009;9:30).
In 2011, Zeng et al. found that the essential oil produced from dill displays properties effective against vulvovaginal candidiasis in immunosuppressed mice (Evid. Based Complement. Alternat. Med. 2011;659704).
In addition, dill has been found to play an adjuvant role in augmenting the antibacterial activity of nitrofurantoin, which is used to treat urinary tract infections. Researchers used disk-diffusion and agar-dilution methods to determine the effects of essential oils of spearmint (Mentha spicata), dill (A. graveolens), and peppermint (Mentha piperita) and their components on the antibacterial activity of nitrofurantoin against Enterobacter cloacae. They used gas chromatography-mass spectrometry to examine essential oil composition. Dill and spearmint were found to exhibit the most significant effects, with pure carvone and piperitone identified as the most active constituents (Chemotherapy 2007;53:21-5).
Elastogenesis Promotion
In 2006, Cenizo et al. set out to induce elastogenesis in adult dermal fibroblasts by targeting lysyl oxidase (LOX) and lysyl oxidase–like (LOXL) enzymes, which are responsible for elastin cross-linking. LOX and LOXL have been identified as the rate-limiting step in synthesizing mature elastin in adult skin. The expression of LOXL in particular decreases with age. Copious amounts of LOX and LOXL allow the catalysis of immature elastin into desmosine and isodesmosine.
In studying these enzymes, Cenizo and colleagues screened more than 1,000 active ingredients to identify agents that could spur LOXL gene expression in adult dermal fibroblasts. They found that a dill extract was capable of penetrating into the epidermis and dermis in skin engineering and in vitro models, significantly stimulating LOXL gene expression in dermal equivalents (an increase of 64% in mRNA level compared with controls). The researchers also noted increases in elastin detection in dermal equivalents under the dermal-epidermal junction without a corresponding elevation in elastin mRNA. They concluded that LOXL is a suitable target for stimulating elastogenesis, and that dill extract appears to foster such activity (Exp. Dermatol. 2006;15:574-81).
Having shown that dill increases LOX and LOXL expression, along with their respective mRNAs, in 2011, some of the same investigators, this time led by Sohm, assessed the capacity of dill extract to enhance skin elasticity in vitro and in vivo. They reported that skin firmness and elasticity did indeed improve significantly in subjects treated for 56 days with a 1% topical application of dill extract, compared with subjects treated with placebo, based on cutometer measurements, biotribometer measurements, investigator evaluations, subject assessments, and photography. Most volunteers treated with dill extract identified marked enhancements in elasticity, firmness, and jaw line slackness. After 84 days, subjects treated with dill also exhibited significantly reduced mean wrinkle area and length compared with those taking the placebo formulation (Int. J. Cosmet. Sci. 2011;33:157-63). This greater elasticity might be attributed to increased LOX expression stimulated by dill extract application, though this study did not measure LOX or LOXL.
Antioxidant Activity
Recently, investigators compared the radical scavenging and antioxidant activities of the phenolic compounds in six spice plants using spectrophotometric and chromatographic methods. They analyzed onion (Allium cepa), parsley (Petroselinum crispum) roots and leaves, and celery (Apium graveolens) roots and leaves, as well as dill (A. graveolens) leaves, and found that the total amounts of phenolic compounds and radical scavenging activity were greatest in celery leaves and dill extracts (J. Sep. Sci. 2011;34:1261-7).
Combination Therapy
It is worth noting that significant improvement in most measures of photoaged skin was observed after the use of a day and night regimen containing dill extract, blackberry leaf extract, and Zn-Cu(II) bi-mineral complex in patients with mild to moderate photodamage in a small (n = 33), single-center, open-label study led by the author (Baumann LS, Figueras KA, Bell M, Flitter CJ. Unpublished results). This small study supports the notion of dill contributing to cutaneous improvement in combination therapy.
Conclusion
Dill is a culinary spice used worldwide and has a history of traditional use in medicine. Current results suggest reasons for optimism in harnessing the medicinal properties of this plant for various uses in the modern armamentarium, particularly as an antibacterial agent. Much more research is necessary, of course, but recent findings regarding the elastogenesis effects of dill are encouraging and suggest the potential for dermatologic, particularly antiaging, applications.
Dill (Anethum graveolens), an aromatic perennial herb often used as a culinary spice, has been utilized for medical purposes for hundreds of years, at least since medieval times (Wurzbg. Medizinhist. Forsch. 1982;24:411-24). A member of the Umbelliferae (carrot or parsley) family, dill is used in traditional Chinese medicine, and its use in cooking and Uygur medicine is believed to date back to ancient times in China (Evid. Based Complement. Alternat. Med. 2011;2011:659-704).
Based on such past uses, as well as modern research, dill is known for having demonstrated anti-inflammatory, antispasmodic, carminative, aromatic, and galactagogue activity (Medical Herbalism: The Science and Practice of Herbal Medicine. Rochester, Vt.: Healing Arts Press, 2003).
Antimicrobial Activity
In a 2003 study of the essential oil of seeds of dill stored for more than 35 years, Bulgarian researchers tested its antimicrobial activity using various microorganisms. They noted high activity of the essential A. graveolens oil against the mold Aspergillus niger and the yeasts Saccharomyces cerevisiae and Candida albicans (J. Agric. Food. Chem. 2003;51:3854-7). Worth noting from this study is not only the anticandidal properties of dill but its potency even after a long time in storage.
In 2009, Kaur and Arora examined the spices dill, fennel (Foeniculum vulgare), and ajwain (Trachyspermum ammi) for their antibacterial activity. The investigators ascertained antibacterial activity by using agar diffusion assay, minimum inhibitory concentration, and viable cell count studies, and compared effects with those of some standard antibiotics. All three spices in the study exhibited significant activity against a wide range of bacteria, with the exception of Klebsiella pneumoniae and a strain of Pseudomonas aeruginosa. The researchers concluded that the results reveal a scientific basis for the reputed antibacterial effects of these plants and lend credence to their traditional medicinal applications. Further, they suggested that future work may lead to viable antibacterial agents based on these ingredients (BMC Complement. Altern. Med. 2009;9:30).
In 2011, Zeng et al. found that the essential oil produced from dill displays properties effective against vulvovaginal candidiasis in immunosuppressed mice (Evid. Based Complement. Alternat. Med. 2011;659704).
In addition, dill has been found to play an adjuvant role in augmenting the antibacterial activity of nitrofurantoin, which is used to treat urinary tract infections. Researchers used disk-diffusion and agar-dilution methods to determine the effects of essential oils of spearmint (Mentha spicata), dill (A. graveolens), and peppermint (Mentha piperita) and their components on the antibacterial activity of nitrofurantoin against Enterobacter cloacae. They used gas chromatography-mass spectrometry to examine essential oil composition. Dill and spearmint were found to exhibit the most significant effects, with pure carvone and piperitone identified as the most active constituents (Chemotherapy 2007;53:21-5).
Elastogenesis Promotion
In 2006, Cenizo et al. set out to induce elastogenesis in adult dermal fibroblasts by targeting lysyl oxidase (LOX) and lysyl oxidase–like (LOXL) enzymes, which are responsible for elastin cross-linking. LOX and LOXL have been identified as the rate-limiting step in synthesizing mature elastin in adult skin. The expression of LOXL in particular decreases with age. Copious amounts of LOX and LOXL allow the catalysis of immature elastin into desmosine and isodesmosine.
In studying these enzymes, Cenizo and colleagues screened more than 1,000 active ingredients to identify agents that could spur LOXL gene expression in adult dermal fibroblasts. They found that a dill extract was capable of penetrating into the epidermis and dermis in skin engineering and in vitro models, significantly stimulating LOXL gene expression in dermal equivalents (an increase of 64% in mRNA level compared with controls). The researchers also noted increases in elastin detection in dermal equivalents under the dermal-epidermal junction without a corresponding elevation in elastin mRNA. They concluded that LOXL is a suitable target for stimulating elastogenesis, and that dill extract appears to foster such activity (Exp. Dermatol. 2006;15:574-81).
Having shown that dill increases LOX and LOXL expression, along with their respective mRNAs, in 2011, some of the same investigators, this time led by Sohm, assessed the capacity of dill extract to enhance skin elasticity in vitro and in vivo. They reported that skin firmness and elasticity did indeed improve significantly in subjects treated for 56 days with a 1% topical application of dill extract, compared with subjects treated with placebo, based on cutometer measurements, biotribometer measurements, investigator evaluations, subject assessments, and photography. Most volunteers treated with dill extract identified marked enhancements in elasticity, firmness, and jaw line slackness. After 84 days, subjects treated with dill also exhibited significantly reduced mean wrinkle area and length compared with those taking the placebo formulation (Int. J. Cosmet. Sci. 2011;33:157-63). This greater elasticity might be attributed to increased LOX expression stimulated by dill extract application, though this study did not measure LOX or LOXL.
Antioxidant Activity
Recently, investigators compared the radical scavenging and antioxidant activities of the phenolic compounds in six spice plants using spectrophotometric and chromatographic methods. They analyzed onion (Allium cepa), parsley (Petroselinum crispum) roots and leaves, and celery (Apium graveolens) roots and leaves, as well as dill (A. graveolens) leaves, and found that the total amounts of phenolic compounds and radical scavenging activity were greatest in celery leaves and dill extracts (J. Sep. Sci. 2011;34:1261-7).
Combination Therapy
It is worth noting that significant improvement in most measures of photoaged skin was observed after the use of a day and night regimen containing dill extract, blackberry leaf extract, and Zn-Cu(II) bi-mineral complex in patients with mild to moderate photodamage in a small (n = 33), single-center, open-label study led by the author (Baumann LS, Figueras KA, Bell M, Flitter CJ. Unpublished results). This small study supports the notion of dill contributing to cutaneous improvement in combination therapy.
Conclusion
Dill is a culinary spice used worldwide and has a history of traditional use in medicine. Current results suggest reasons for optimism in harnessing the medicinal properties of this plant for various uses in the modern armamentarium, particularly as an antibacterial agent. Much more research is necessary, of course, but recent findings regarding the elastogenesis effects of dill are encouraging and suggest the potential for dermatologic, particularly antiaging, applications.
Dill (Anethum graveolens), an aromatic perennial herb often used as a culinary spice, has been utilized for medical purposes for hundreds of years, at least since medieval times (Wurzbg. Medizinhist. Forsch. 1982;24:411-24). A member of the Umbelliferae (carrot or parsley) family, dill is used in traditional Chinese medicine, and its use in cooking and Uygur medicine is believed to date back to ancient times in China (Evid. Based Complement. Alternat. Med. 2011;2011:659-704).
Based on such past uses, as well as modern research, dill is known for having demonstrated anti-inflammatory, antispasmodic, carminative, aromatic, and galactagogue activity (Medical Herbalism: The Science and Practice of Herbal Medicine. Rochester, Vt.: Healing Arts Press, 2003).
Antimicrobial Activity
In a 2003 study of the essential oil of seeds of dill stored for more than 35 years, Bulgarian researchers tested its antimicrobial activity using various microorganisms. They noted high activity of the essential A. graveolens oil against the mold Aspergillus niger and the yeasts Saccharomyces cerevisiae and Candida albicans (J. Agric. Food. Chem. 2003;51:3854-7). Worth noting from this study is not only the anticandidal properties of dill but its potency even after a long time in storage.
In 2009, Kaur and Arora examined the spices dill, fennel (Foeniculum vulgare), and ajwain (Trachyspermum ammi) for their antibacterial activity. The investigators ascertained antibacterial activity by using agar diffusion assay, minimum inhibitory concentration, and viable cell count studies, and compared effects with those of some standard antibiotics. All three spices in the study exhibited significant activity against a wide range of bacteria, with the exception of Klebsiella pneumoniae and a strain of Pseudomonas aeruginosa. The researchers concluded that the results reveal a scientific basis for the reputed antibacterial effects of these plants and lend credence to their traditional medicinal applications. Further, they suggested that future work may lead to viable antibacterial agents based on these ingredients (BMC Complement. Altern. Med. 2009;9:30).
In 2011, Zeng et al. found that the essential oil produced from dill displays properties effective against vulvovaginal candidiasis in immunosuppressed mice (Evid. Based Complement. Alternat. Med. 2011;659704).
In addition, dill has been found to play an adjuvant role in augmenting the antibacterial activity of nitrofurantoin, which is used to treat urinary tract infections. Researchers used disk-diffusion and agar-dilution methods to determine the effects of essential oils of spearmint (Mentha spicata), dill (A. graveolens), and peppermint (Mentha piperita) and their components on the antibacterial activity of nitrofurantoin against Enterobacter cloacae. They used gas chromatography-mass spectrometry to examine essential oil composition. Dill and spearmint were found to exhibit the most significant effects, with pure carvone and piperitone identified as the most active constituents (Chemotherapy 2007;53:21-5).
Elastogenesis Promotion
In 2006, Cenizo et al. set out to induce elastogenesis in adult dermal fibroblasts by targeting lysyl oxidase (LOX) and lysyl oxidase–like (LOXL) enzymes, which are responsible for elastin cross-linking. LOX and LOXL have been identified as the rate-limiting step in synthesizing mature elastin in adult skin. The expression of LOXL in particular decreases with age. Copious amounts of LOX and LOXL allow the catalysis of immature elastin into desmosine and isodesmosine.
In studying these enzymes, Cenizo and colleagues screened more than 1,000 active ingredients to identify agents that could spur LOXL gene expression in adult dermal fibroblasts. They found that a dill extract was capable of penetrating into the epidermis and dermis in skin engineering and in vitro models, significantly stimulating LOXL gene expression in dermal equivalents (an increase of 64% in mRNA level compared with controls). The researchers also noted increases in elastin detection in dermal equivalents under the dermal-epidermal junction without a corresponding elevation in elastin mRNA. They concluded that LOXL is a suitable target for stimulating elastogenesis, and that dill extract appears to foster such activity (Exp. Dermatol. 2006;15:574-81).
Having shown that dill increases LOX and LOXL expression, along with their respective mRNAs, in 2011, some of the same investigators, this time led by Sohm, assessed the capacity of dill extract to enhance skin elasticity in vitro and in vivo. They reported that skin firmness and elasticity did indeed improve significantly in subjects treated for 56 days with a 1% topical application of dill extract, compared with subjects treated with placebo, based on cutometer measurements, biotribometer measurements, investigator evaluations, subject assessments, and photography. Most volunteers treated with dill extract identified marked enhancements in elasticity, firmness, and jaw line slackness. After 84 days, subjects treated with dill also exhibited significantly reduced mean wrinkle area and length compared with those taking the placebo formulation (Int. J. Cosmet. Sci. 2011;33:157-63). This greater elasticity might be attributed to increased LOX expression stimulated by dill extract application, though this study did not measure LOX or LOXL.
Antioxidant Activity
Recently, investigators compared the radical scavenging and antioxidant activities of the phenolic compounds in six spice plants using spectrophotometric and chromatographic methods. They analyzed onion (Allium cepa), parsley (Petroselinum crispum) roots and leaves, and celery (Apium graveolens) roots and leaves, as well as dill (A. graveolens) leaves, and found that the total amounts of phenolic compounds and radical scavenging activity were greatest in celery leaves and dill extracts (J. Sep. Sci. 2011;34:1261-7).
Combination Therapy
It is worth noting that significant improvement in most measures of photoaged skin was observed after the use of a day and night regimen containing dill extract, blackberry leaf extract, and Zn-Cu(II) bi-mineral complex in patients with mild to moderate photodamage in a small (n = 33), single-center, open-label study led by the author (Baumann LS, Figueras KA, Bell M, Flitter CJ. Unpublished results). This small study supports the notion of dill contributing to cutaneous improvement in combination therapy.
Conclusion
Dill is a culinary spice used worldwide and has a history of traditional use in medicine. Current results suggest reasons for optimism in harnessing the medicinal properties of this plant for various uses in the modern armamentarium, particularly as an antibacterial agent. Much more research is necessary, of course, but recent findings regarding the elastogenesis effects of dill are encouraging and suggest the potential for dermatologic, particularly antiaging, applications.
Cucumber
Cucumis sativus is a member of the Cucurbitaceae family, which also includes pumpkin, zucchini, watermelon, and squash. Found growing wild in the Himalayan region and commonly referred to as cucumber in English, khira in Hindi, and sakusa in Sanskrit, the plant is cultivated throughout India and China, in particular, as well as in Europe and the United States (J. Young. Pharm. 2010;2:365-8).
It is grown as a food crop, with its fruit (the cucumber) found in many cuisines as well as a component in traditional medicine and folk cosmetics (Planta. Med. 2008;74:1785-8). Headache was one traditional indication; the fruit juice was used as a demulcent in antiacne lotions and the seeds were noted for their cooling and diuretic effects (J. Young. Pharm. 2010;2:365-8). In traditional Chinese medicine, the leaves, roots, and stems of the plant have been used to detoxify as well as to treat diarrhea and gonorrhea (Planta. Med. 2008;74:1785-8). Popularly, the application of cucumber slices to ameliorate swelling or dark circles under the eyes has long been accepted throughout the world as an effective treatment.
Antioxidant Activity
In 2002, Villaseñor and coinvestigators assessed the comparative effectiveness of sugar beet roots, cucumber fruits, New Zealand spinach leaves, and turmeric rhizomes against dimethylbenz[a]anthracene-initiated and croton oil–promoted skin tumors in a Swiss Webster albino mouse model using three different protocols. The four species were selected based on prior findings of antioxidant activity and effectiveness in preventing skin tumors induced in laboratory settings. All four displayed antioxidant activity, and all were found to be effective in lowering skin tumor incidence and the number of skin tumors as well as delaying the onset of skin tumor formation, compared with the control, with turmeric exhibiting the greatest potency (Nutr. Cancer. 2002;44:66-70).
Melanin Suppressing Properties
In 2008, Kai and coinvestigators assessed six plant parts of C. sativus to compare their inhibitory effects on melanogenesis. They found that methanol extracts of the leaves and stems suppressed melanin production in cultured B16 mouse melanoma cells. Although they did not alter mushroom tyrosinase activity or crude enzyme lysate activity in these cells, the methanol extracts did reduce tyrosinase expression at the protein level. The researchers suggested that these findings indicate that the depigmenting activity of C. sativus extracts is associated with tyrosinase expression. They also found that lutein, of eight compounds isolated from the leaves, inhibited melanogenesis, significantly lowering tyrosinase expression. The investigators concluded that the leaves, and lutein in particular, of C. sativus effectively suppress tyrosinase expression and warrant consideration as a skin-whitening agent (Planta. Med. 2008;74:1785-8). Tyrosinase-inhibitory activity exhibited by cucumber extracts had also been previously attributed to enzymes found in cucumber skin (J. Agric. Food. Chem. 2003;51:7764-9).
In 2010, Kumar and coinvestigators evaluated the aqueous fruit extract of C. sativus for free radical scavenging and analgesic activities using in vitro and in vivo models. Preliminary phytochemical screening indicated that cucumber contains various classes of compounds known to exert antioxidant as well as analgesic activity, including flavonoids and tannins. The investigators found that the fruit extract showed maximum antioxidant and analgesic effect at 500 mcg/mL and 500 mg/kg, respectively, although the exact constituents of C. sativus fruits responsible for the promising effects were not elucidated by the study (J. Young. Pharm. 2010;2:365-8).
Research on Skin Care Properties
Early in 2011, Akhtar and coinvestigators reported on their efforts to formulate a topical water in oil (without) emulsion of 3% cucumber extracts, and to assess it according to multiple parameters, compared with its base (lacking cucumber ingredients) as a control, in 21 healthy volunteers over 4 weeks. The cucumber formulation demonstrated statistically significant reductions in sebum, as well as a decline in melanin content that was not statistically significant. Transepidermal water loss and erythema were elevated by the test formulation, but these changes were also not statistically significant. While identifying the need for more research, the authors concluded that their findings point to the potential for cucumber extracts to be effective ingredients in skin care agents for medical and cosmetic purposes (African J. Biotechnol. 2011;10:1206-16).
Later in 2011, Nema and coinvestigators subjected the lyophilized juice of C. sativus fruit to 1,1-diphenyl-2-picrylhydrazyl and superoxide radical scavenging assays in reference to butylated hydroxytoluene, and hyaluronoidase and elastase inhibitory assays in reference to oleanolic acid. The cucumber juice, rich in ascorbic acid, was found to exhibit significant free radical scavenging activity as well as potent antihyaluronidase and antielastase activity. The researchers concluded that C. sativus warrants consideration for its potential used as an antiwrinkle ingredient in cosmetic formulations (Arch. Dermatol. Res. 2011;303;247-52).
Cucumber extracts can currently be found in a wide range of over-the-counter skin care creams and eye gels.
Conclusion
Cucumber has captured the popular imagination as an effective, temporary agent for the relief of swollen eyes, or dark circles under the eyes. It has developed an anecdotal reputation as a diuretic that systemically and topically acts against water retention, thus ameliorating burns, dermatitis, and swollen eyes. This folk medicine success or popularity has, perhaps, spurred the inclusion of C. sativa in various skin care products. There is a dearth of research on the dermatologic benefits of the plant, however, as well as its usefulness in skin care products. That said, some emerging evidence regarding its inhibitory effect on melanin production bears watching. Much more research is necessary to determine the appropriate role of cucumber in dermatology.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.
Cucumis sativus is a member of the Cucurbitaceae family, which also includes pumpkin, zucchini, watermelon, and squash. Found growing wild in the Himalayan region and commonly referred to as cucumber in English, khira in Hindi, and sakusa in Sanskrit, the plant is cultivated throughout India and China, in particular, as well as in Europe and the United States (J. Young. Pharm. 2010;2:365-8).
It is grown as a food crop, with its fruit (the cucumber) found in many cuisines as well as a component in traditional medicine and folk cosmetics (Planta. Med. 2008;74:1785-8). Headache was one traditional indication; the fruit juice was used as a demulcent in antiacne lotions and the seeds were noted for their cooling and diuretic effects (J. Young. Pharm. 2010;2:365-8). In traditional Chinese medicine, the leaves, roots, and stems of the plant have been used to detoxify as well as to treat diarrhea and gonorrhea (Planta. Med. 2008;74:1785-8). Popularly, the application of cucumber slices to ameliorate swelling or dark circles under the eyes has long been accepted throughout the world as an effective treatment.
Antioxidant Activity
In 2002, Villaseñor and coinvestigators assessed the comparative effectiveness of sugar beet roots, cucumber fruits, New Zealand spinach leaves, and turmeric rhizomes against dimethylbenz[a]anthracene-initiated and croton oil–promoted skin tumors in a Swiss Webster albino mouse model using three different protocols. The four species were selected based on prior findings of antioxidant activity and effectiveness in preventing skin tumors induced in laboratory settings. All four displayed antioxidant activity, and all were found to be effective in lowering skin tumor incidence and the number of skin tumors as well as delaying the onset of skin tumor formation, compared with the control, with turmeric exhibiting the greatest potency (Nutr. Cancer. 2002;44:66-70).
Melanin Suppressing Properties
In 2008, Kai and coinvestigators assessed six plant parts of C. sativus to compare their inhibitory effects on melanogenesis. They found that methanol extracts of the leaves and stems suppressed melanin production in cultured B16 mouse melanoma cells. Although they did not alter mushroom tyrosinase activity or crude enzyme lysate activity in these cells, the methanol extracts did reduce tyrosinase expression at the protein level. The researchers suggested that these findings indicate that the depigmenting activity of C. sativus extracts is associated with tyrosinase expression. They also found that lutein, of eight compounds isolated from the leaves, inhibited melanogenesis, significantly lowering tyrosinase expression. The investigators concluded that the leaves, and lutein in particular, of C. sativus effectively suppress tyrosinase expression and warrant consideration as a skin-whitening agent (Planta. Med. 2008;74:1785-8). Tyrosinase-inhibitory activity exhibited by cucumber extracts had also been previously attributed to enzymes found in cucumber skin (J. Agric. Food. Chem. 2003;51:7764-9).
In 2010, Kumar and coinvestigators evaluated the aqueous fruit extract of C. sativus for free radical scavenging and analgesic activities using in vitro and in vivo models. Preliminary phytochemical screening indicated that cucumber contains various classes of compounds known to exert antioxidant as well as analgesic activity, including flavonoids and tannins. The investigators found that the fruit extract showed maximum antioxidant and analgesic effect at 500 mcg/mL and 500 mg/kg, respectively, although the exact constituents of C. sativus fruits responsible for the promising effects were not elucidated by the study (J. Young. Pharm. 2010;2:365-8).
Research on Skin Care Properties
Early in 2011, Akhtar and coinvestigators reported on their efforts to formulate a topical water in oil (without) emulsion of 3% cucumber extracts, and to assess it according to multiple parameters, compared with its base (lacking cucumber ingredients) as a control, in 21 healthy volunteers over 4 weeks. The cucumber formulation demonstrated statistically significant reductions in sebum, as well as a decline in melanin content that was not statistically significant. Transepidermal water loss and erythema were elevated by the test formulation, but these changes were also not statistically significant. While identifying the need for more research, the authors concluded that their findings point to the potential for cucumber extracts to be effective ingredients in skin care agents for medical and cosmetic purposes (African J. Biotechnol. 2011;10:1206-16).
Later in 2011, Nema and coinvestigators subjected the lyophilized juice of C. sativus fruit to 1,1-diphenyl-2-picrylhydrazyl and superoxide radical scavenging assays in reference to butylated hydroxytoluene, and hyaluronoidase and elastase inhibitory assays in reference to oleanolic acid. The cucumber juice, rich in ascorbic acid, was found to exhibit significant free radical scavenging activity as well as potent antihyaluronidase and antielastase activity. The researchers concluded that C. sativus warrants consideration for its potential used as an antiwrinkle ingredient in cosmetic formulations (Arch. Dermatol. Res. 2011;303;247-52).
Cucumber extracts can currently be found in a wide range of over-the-counter skin care creams and eye gels.
Conclusion
Cucumber has captured the popular imagination as an effective, temporary agent for the relief of swollen eyes, or dark circles under the eyes. It has developed an anecdotal reputation as a diuretic that systemically and topically acts against water retention, thus ameliorating burns, dermatitis, and swollen eyes. This folk medicine success or popularity has, perhaps, spurred the inclusion of C. sativa in various skin care products. There is a dearth of research on the dermatologic benefits of the plant, however, as well as its usefulness in skin care products. That said, some emerging evidence regarding its inhibitory effect on melanin production bears watching. Much more research is necessary to determine the appropriate role of cucumber in dermatology.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.
Cucumis sativus is a member of the Cucurbitaceae family, which also includes pumpkin, zucchini, watermelon, and squash. Found growing wild in the Himalayan region and commonly referred to as cucumber in English, khira in Hindi, and sakusa in Sanskrit, the plant is cultivated throughout India and China, in particular, as well as in Europe and the United States (J. Young. Pharm. 2010;2:365-8).
It is grown as a food crop, with its fruit (the cucumber) found in many cuisines as well as a component in traditional medicine and folk cosmetics (Planta. Med. 2008;74:1785-8). Headache was one traditional indication; the fruit juice was used as a demulcent in antiacne lotions and the seeds were noted for their cooling and diuretic effects (J. Young. Pharm. 2010;2:365-8). In traditional Chinese medicine, the leaves, roots, and stems of the plant have been used to detoxify as well as to treat diarrhea and gonorrhea (Planta. Med. 2008;74:1785-8). Popularly, the application of cucumber slices to ameliorate swelling or dark circles under the eyes has long been accepted throughout the world as an effective treatment.
Antioxidant Activity
In 2002, Villaseñor and coinvestigators assessed the comparative effectiveness of sugar beet roots, cucumber fruits, New Zealand spinach leaves, and turmeric rhizomes against dimethylbenz[a]anthracene-initiated and croton oil–promoted skin tumors in a Swiss Webster albino mouse model using three different protocols. The four species were selected based on prior findings of antioxidant activity and effectiveness in preventing skin tumors induced in laboratory settings. All four displayed antioxidant activity, and all were found to be effective in lowering skin tumor incidence and the number of skin tumors as well as delaying the onset of skin tumor formation, compared with the control, with turmeric exhibiting the greatest potency (Nutr. Cancer. 2002;44:66-70).
Melanin Suppressing Properties
In 2008, Kai and coinvestigators assessed six plant parts of C. sativus to compare their inhibitory effects on melanogenesis. They found that methanol extracts of the leaves and stems suppressed melanin production in cultured B16 mouse melanoma cells. Although they did not alter mushroom tyrosinase activity or crude enzyme lysate activity in these cells, the methanol extracts did reduce tyrosinase expression at the protein level. The researchers suggested that these findings indicate that the depigmenting activity of C. sativus extracts is associated with tyrosinase expression. They also found that lutein, of eight compounds isolated from the leaves, inhibited melanogenesis, significantly lowering tyrosinase expression. The investigators concluded that the leaves, and lutein in particular, of C. sativus effectively suppress tyrosinase expression and warrant consideration as a skin-whitening agent (Planta. Med. 2008;74:1785-8). Tyrosinase-inhibitory activity exhibited by cucumber extracts had also been previously attributed to enzymes found in cucumber skin (J. Agric. Food. Chem. 2003;51:7764-9).
In 2010, Kumar and coinvestigators evaluated the aqueous fruit extract of C. sativus for free radical scavenging and analgesic activities using in vitro and in vivo models. Preliminary phytochemical screening indicated that cucumber contains various classes of compounds known to exert antioxidant as well as analgesic activity, including flavonoids and tannins. The investigators found that the fruit extract showed maximum antioxidant and analgesic effect at 500 mcg/mL and 500 mg/kg, respectively, although the exact constituents of C. sativus fruits responsible for the promising effects were not elucidated by the study (J. Young. Pharm. 2010;2:365-8).
Research on Skin Care Properties
Early in 2011, Akhtar and coinvestigators reported on their efforts to formulate a topical water in oil (without) emulsion of 3% cucumber extracts, and to assess it according to multiple parameters, compared with its base (lacking cucumber ingredients) as a control, in 21 healthy volunteers over 4 weeks. The cucumber formulation demonstrated statistically significant reductions in sebum, as well as a decline in melanin content that was not statistically significant. Transepidermal water loss and erythema were elevated by the test formulation, but these changes were also not statistically significant. While identifying the need for more research, the authors concluded that their findings point to the potential for cucumber extracts to be effective ingredients in skin care agents for medical and cosmetic purposes (African J. Biotechnol. 2011;10:1206-16).
Later in 2011, Nema and coinvestigators subjected the lyophilized juice of C. sativus fruit to 1,1-diphenyl-2-picrylhydrazyl and superoxide radical scavenging assays in reference to butylated hydroxytoluene, and hyaluronoidase and elastase inhibitory assays in reference to oleanolic acid. The cucumber juice, rich in ascorbic acid, was found to exhibit significant free radical scavenging activity as well as potent antihyaluronidase and antielastase activity. The researchers concluded that C. sativus warrants consideration for its potential used as an antiwrinkle ingredient in cosmetic formulations (Arch. Dermatol. Res. 2011;303;247-52).
Cucumber extracts can currently be found in a wide range of over-the-counter skin care creams and eye gels.
Conclusion
Cucumber has captured the popular imagination as an effective, temporary agent for the relief of swollen eyes, or dark circles under the eyes. It has developed an anecdotal reputation as a diuretic that systemically and topically acts against water retention, thus ameliorating burns, dermatitis, and swollen eyes. This folk medicine success or popularity has, perhaps, spurred the inclusion of C. sativa in various skin care products. There is a dearth of research on the dermatologic benefits of the plant, however, as well as its usefulness in skin care products. That said, some emerging evidence regarding its inhibitory effect on melanin production bears watching. Much more research is necessary to determine the appropriate role of cucumber in dermatology.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.
Probiotics
Probiotics are live microorganisms that impart health benefits to the host when present or administered at appropriate levels (J. Appl. Bacteriol. 1989;66:365-78). Intestinal microflora are better understood and more frequently used than cutaneous microbiota, but researchers have recently applied the concepts underlying the efficacy of intestinal probiotics to investigate potential benefits in the dermatologic realm. This work has also been spurred by the flurry of investigations on probiotics for the treatment of atopic dermatitis (AD).
In one of the earlier studies of possible uses of probiotics for the skin, Ouwehand et al. identified strains that adhere to keratin. However, the tested microbes (Propionibacteria, selected because they are among the normal microbiota of the skin) were not found to inhibit cutaneous pathogen adhesion to human keratin. Nevertheless, the researchers concluded that additional study was warranted to identify strains that adhere in vivo and exhibit activity against potential skin pathogens (Lett. Appl. Microbiol. 2003;36:327-31).
Clearly, some probiotic strains have been shown to exhibit strong immunomodulatory activity at the cutaneous level (Eur. J. Dermatol. 2010;20:731-7). In addition, some topical skin products now contain probiotic strains as active ingredients, including Clinique Medical, which incorporates lactobacillus cultures to yield molecules that help ameliorate skin barrier effects from laser treatments or chemical peels (Facial Plast. Surg. 2009;25:285-9). Probiotics have also been used successively as adjuvant therapy to treat suppurative inflammatory conditions (i.e., boils and abscesses) in the maxilla-facial region (Stomatologiia (Mosk) 2009;88:50-2).
This column will consider some of the most current AD studies as well as other recent research. History is not to be disregarded, though. After all, the notion of topical probiotics conferring cutaneous benefits against acne and seborrhea was considered in 1912 (Gut Pathog. 2011;3:1). Current findings may, indeed, bear this out.
Atopic Dermatitis
The safety of probiotic, as well as prebiotic, treatments for AD in children was established in a long-term, randomized, double-blind trial (Clin. Dermatol. 2010;28:57-61; Pediatrics 2008;122:8-1). But questions remain regarding efficacy, with conflicting data emerging from recent studies. Given increasing interest in the use of probiotics for the treatment of AD, Boyle et al. conducted an extensive literature search up to 2008 and found that 12 trials, including 781 subjects (all children), met their inclusion criteria, with probiotics not emerging as an effective treatment for eczema (Cochrane Database Syst. Rev. 2008;CD006135).
In 2008, Betsi et al. reviewed the results of 13 relevant randomized, placebo-controlled trials, 10 of which assessed probiotics as treatment and 3 for prevention of AD. Overall, they found that probiotics, particularly Lactobacillus rhamnosus GG, appear to be effective for preventing AD, lowering its severity in half of the trials assessed though inflammatory markers were not significantly affected. The authors called for more research to determine the usefulness of probiotics in AD treatment or prevention (Am. J. Clin. Dermatol. 2008;9:93-103).
In a 2010 analysis of systematic reviews indexed between August 2007 and August 2008 covering disease prevention and atopic eczema treatment, Williams and Grindlay found two independent systematic reviews suggesting that ingestion of probiotics by mothers during pregnancy might lower the incidence of subsequent eczema. However, they noted that a review of 13 studies of probiotics for treating established eczema revealed no convincing support for its clinical use, a stance that was buttressed by a later Cochrane Review (Clin. Exp. Dermatol. 2010;35:223-7).
In 2010, Gerasimov et al. conducted a randomized, double-blind, placebo-controlled, prospective study of 90 children (1-3 years old) with moderate to severe AD treated with a mixture of L. acidophilus DDS-1 and Bifidobacterium lactis UABLA-12 with fructo-oligosaccharide (5 billion colony-forming units twice daily for 8 weeks vs. placebo). The researchers found that the use of the probiotic compound correlated with significant clinical improvement in children with AD, and suggested that additional research is necessary to assess the efficacy of probiotics in adults with AD (Am. J. Clin. Dermatol. 2010;11:351-61).
In another recent study, investigators isolated L. plantarum strains from the Korean fermented food kimchi, and found that they hindered the dermatitis promoted by house-dust mites in a mouse model representative of human AD (J. Appl. Microbiol. 2011;110:1195-202). Overall, clinical trials conducted to test the use of probiotics to treat AD over the last 15 years have yielded conflicting results, though it appears that there is not enough evidence to warrant support for such therapy (Clin. Rev. Allergy Immunol. 2011;41:267-71).
Mechanism of Action
Presently, the mechanism of action whereby skin benefits are manifested through the oral ingestion of probiotics is thought to be a downstream result of the boost in systemic immune response, especially T-cell subsets such as Th1 cells that may ultimately enhance immune responses in organs beyond the digestive tract (Clin. Plast. Surg. 2012;39:59-64; Clin. Dermatol. 2008;26:4-11).
In discussing an alternative to antibacterial products for imbalances in cutaneous microorganisms that result in mild acne, xerosis, or AD, Simmering and Breves suggest that "prebiotic actives rebalance the skin microflora while probiotic approaches predominantly consist of applying an inactivated microbial biomass of beneficial bacteria" (Hautarzt. 2009;60:809-14).
Cutaneous Immune Homeostasis
In a 2009 randomized, double-blind, placebo-controlled clinical trial with 54 volunteers, Guéniche et al. set out to ascertain whether the probiotic bacterium L. johnsonii (La1) could influence cutaneous immune homeostasis in humans after solar-simulated UV exposure (twice 1.5 MED [minimal erythema dose]). They showed that La1 consumption contributed to hastening the recovery of allostimulatory function in epidermal cells (Dermatoendocrinol. 2009;1:275-9).
Previously, in 2008, Peguet-Navarro et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 54 healthy volunteers to study the potential impact of oral supplementation with La1 on skin immune status after UV exposure. Subjects received either La1 or placebo during the 6 weeks prior to solar-simulated UV exposure. The investigators found on day 4 after exposure that the allostimulatory capacity of epidermal cells had completely recovered in the La1 group correlating with the normalization of epidermal CD1a expression. They concluded that ingested probiotic bacteria hasten the recovery of skin immune homeostasis after UV-provoked immunosuppression (Eur. J. Dermatol. 2008;18:504-11).
Anti-inflammatory Activity
In 2010, Guéniche et al. demonstrated that B. longum can reduce skin inflammation mediated by substance P (Exp. Dermatol. 2010;19:e1-8). In a separate study, some of the same investigators found that L. paracasei appears to have the capacity to confer benefits related to barrier function and skin reactivity, also blunting the effects of substance P–induced skin inflammation (Eur. J. Dermatol. 2010;20:731-7).
Antiphotoaging Activity
In 2010, Bouilly-Gauthier et al. evaluated the effects of a dietary supplement combining L. johnsonii (La1), which is thought to protect the skin immune system after UV exposure, and nutritional doses of carotenoids on early UV-induced skin damage. They performed three clinical trials using various UV sources (non–extreme UV with a high UVA irradiance, extreme simulated solar radiation, and natural sunlight) in 139 healthy women over age 18 with skin type II-IV. The investigators found over the 10 weeks of the study that the combination of probiotic (La1) and nutritional doses of carotenoids lowered early UV-induced skin damage caused by simulated or natural sun exposure. Further study of the possible long-term effects against UV exposure and photoaging is warranted, they concluded (Br. J. Dermatol. 2010;163:536-43).
Topical Uses
In a study more than a decade ago, Di Marzio et al. showed that the topical application of a cream containing Streptococcus thermophilus, an organism found in most yogurts, raised the production of ceramides, which is notable given the anti-inflammatory activity and antimicrobial activity of some ceramides against Proprionibacterium acnes (J. Invest. Dermatol. 1999;113:98-106;Gut Pathog. 2011;3:1). Two recent in vitro studies have also revealed that probiotics can have antibacterial activity against P. acnes (Int. J. Cosmet. Sci. 2010;32:139-42; J. Microbiol. 2009;47:101-9). The prospects for efficacy of topically applied probiotics in the prevention and treatment of pro-inflammatory immune reactions are considered, by some, to be promising, however (Hautarzt. 2009;60:795-801). And, in fact, Guéniche et al. have found that the topical application of Vitreoscilla filiformis demonstrated efficacy against seborrheic dermatitis and AD (J. Eur. Acad. Dermatol. Venereol. 2008;22:1014-5; Eur. J. Dermatol. 2006;16:380-4).
Conclusion
While still controversial, the findings of probiotics’ effects in the treatment of atopic dermatitis remain compelling. Even more interesting, though, is the current work that suggests additional potential applications of probiotics in the dermatologic armamentarium. The work is in its early stages, but results warrant additional research, at the very least, if not cause for optimism over the prospect of more supportive evidence.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.
Probiotics are live microorganisms that impart health benefits to the host when present or administered at appropriate levels (J. Appl. Bacteriol. 1989;66:365-78). Intestinal microflora are better understood and more frequently used than cutaneous microbiota, but researchers have recently applied the concepts underlying the efficacy of intestinal probiotics to investigate potential benefits in the dermatologic realm. This work has also been spurred by the flurry of investigations on probiotics for the treatment of atopic dermatitis (AD).
In one of the earlier studies of possible uses of probiotics for the skin, Ouwehand et al. identified strains that adhere to keratin. However, the tested microbes (Propionibacteria, selected because they are among the normal microbiota of the skin) were not found to inhibit cutaneous pathogen adhesion to human keratin. Nevertheless, the researchers concluded that additional study was warranted to identify strains that adhere in vivo and exhibit activity against potential skin pathogens (Lett. Appl. Microbiol. 2003;36:327-31).
Clearly, some probiotic strains have been shown to exhibit strong immunomodulatory activity at the cutaneous level (Eur. J. Dermatol. 2010;20:731-7). In addition, some topical skin products now contain probiotic strains as active ingredients, including Clinique Medical, which incorporates lactobacillus cultures to yield molecules that help ameliorate skin barrier effects from laser treatments or chemical peels (Facial Plast. Surg. 2009;25:285-9). Probiotics have also been used successively as adjuvant therapy to treat suppurative inflammatory conditions (i.e., boils and abscesses) in the maxilla-facial region (Stomatologiia (Mosk) 2009;88:50-2).
This column will consider some of the most current AD studies as well as other recent research. History is not to be disregarded, though. After all, the notion of topical probiotics conferring cutaneous benefits against acne and seborrhea was considered in 1912 (Gut Pathog. 2011;3:1). Current findings may, indeed, bear this out.
Atopic Dermatitis
The safety of probiotic, as well as prebiotic, treatments for AD in children was established in a long-term, randomized, double-blind trial (Clin. Dermatol. 2010;28:57-61; Pediatrics 2008;122:8-1). But questions remain regarding efficacy, with conflicting data emerging from recent studies. Given increasing interest in the use of probiotics for the treatment of AD, Boyle et al. conducted an extensive literature search up to 2008 and found that 12 trials, including 781 subjects (all children), met their inclusion criteria, with probiotics not emerging as an effective treatment for eczema (Cochrane Database Syst. Rev. 2008;CD006135).
In 2008, Betsi et al. reviewed the results of 13 relevant randomized, placebo-controlled trials, 10 of which assessed probiotics as treatment and 3 for prevention of AD. Overall, they found that probiotics, particularly Lactobacillus rhamnosus GG, appear to be effective for preventing AD, lowering its severity in half of the trials assessed though inflammatory markers were not significantly affected. The authors called for more research to determine the usefulness of probiotics in AD treatment or prevention (Am. J. Clin. Dermatol. 2008;9:93-103).
In a 2010 analysis of systematic reviews indexed between August 2007 and August 2008 covering disease prevention and atopic eczema treatment, Williams and Grindlay found two independent systematic reviews suggesting that ingestion of probiotics by mothers during pregnancy might lower the incidence of subsequent eczema. However, they noted that a review of 13 studies of probiotics for treating established eczema revealed no convincing support for its clinical use, a stance that was buttressed by a later Cochrane Review (Clin. Exp. Dermatol. 2010;35:223-7).
In 2010, Gerasimov et al. conducted a randomized, double-blind, placebo-controlled, prospective study of 90 children (1-3 years old) with moderate to severe AD treated with a mixture of L. acidophilus DDS-1 and Bifidobacterium lactis UABLA-12 with fructo-oligosaccharide (5 billion colony-forming units twice daily for 8 weeks vs. placebo). The researchers found that the use of the probiotic compound correlated with significant clinical improvement in children with AD, and suggested that additional research is necessary to assess the efficacy of probiotics in adults with AD (Am. J. Clin. Dermatol. 2010;11:351-61).
In another recent study, investigators isolated L. plantarum strains from the Korean fermented food kimchi, and found that they hindered the dermatitis promoted by house-dust mites in a mouse model representative of human AD (J. Appl. Microbiol. 2011;110:1195-202). Overall, clinical trials conducted to test the use of probiotics to treat AD over the last 15 years have yielded conflicting results, though it appears that there is not enough evidence to warrant support for such therapy (Clin. Rev. Allergy Immunol. 2011;41:267-71).
Mechanism of Action
Presently, the mechanism of action whereby skin benefits are manifested through the oral ingestion of probiotics is thought to be a downstream result of the boost in systemic immune response, especially T-cell subsets such as Th1 cells that may ultimately enhance immune responses in organs beyond the digestive tract (Clin. Plast. Surg. 2012;39:59-64; Clin. Dermatol. 2008;26:4-11).
In discussing an alternative to antibacterial products for imbalances in cutaneous microorganisms that result in mild acne, xerosis, or AD, Simmering and Breves suggest that "prebiotic actives rebalance the skin microflora while probiotic approaches predominantly consist of applying an inactivated microbial biomass of beneficial bacteria" (Hautarzt. 2009;60:809-14).
Cutaneous Immune Homeostasis
In a 2009 randomized, double-blind, placebo-controlled clinical trial with 54 volunteers, Guéniche et al. set out to ascertain whether the probiotic bacterium L. johnsonii (La1) could influence cutaneous immune homeostasis in humans after solar-simulated UV exposure (twice 1.5 MED [minimal erythema dose]). They showed that La1 consumption contributed to hastening the recovery of allostimulatory function in epidermal cells (Dermatoendocrinol. 2009;1:275-9).
Previously, in 2008, Peguet-Navarro et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 54 healthy volunteers to study the potential impact of oral supplementation with La1 on skin immune status after UV exposure. Subjects received either La1 or placebo during the 6 weeks prior to solar-simulated UV exposure. The investigators found on day 4 after exposure that the allostimulatory capacity of epidermal cells had completely recovered in the La1 group correlating with the normalization of epidermal CD1a expression. They concluded that ingested probiotic bacteria hasten the recovery of skin immune homeostasis after UV-provoked immunosuppression (Eur. J. Dermatol. 2008;18:504-11).
Anti-inflammatory Activity
In 2010, Guéniche et al. demonstrated that B. longum can reduce skin inflammation mediated by substance P (Exp. Dermatol. 2010;19:e1-8). In a separate study, some of the same investigators found that L. paracasei appears to have the capacity to confer benefits related to barrier function and skin reactivity, also blunting the effects of substance P–induced skin inflammation (Eur. J. Dermatol. 2010;20:731-7).
Antiphotoaging Activity
In 2010, Bouilly-Gauthier et al. evaluated the effects of a dietary supplement combining L. johnsonii (La1), which is thought to protect the skin immune system after UV exposure, and nutritional doses of carotenoids on early UV-induced skin damage. They performed three clinical trials using various UV sources (non–extreme UV with a high UVA irradiance, extreme simulated solar radiation, and natural sunlight) in 139 healthy women over age 18 with skin type II-IV. The investigators found over the 10 weeks of the study that the combination of probiotic (La1) and nutritional doses of carotenoids lowered early UV-induced skin damage caused by simulated or natural sun exposure. Further study of the possible long-term effects against UV exposure and photoaging is warranted, they concluded (Br. J. Dermatol. 2010;163:536-43).
Topical Uses
In a study more than a decade ago, Di Marzio et al. showed that the topical application of a cream containing Streptococcus thermophilus, an organism found in most yogurts, raised the production of ceramides, which is notable given the anti-inflammatory activity and antimicrobial activity of some ceramides against Proprionibacterium acnes (J. Invest. Dermatol. 1999;113:98-106;Gut Pathog. 2011;3:1). Two recent in vitro studies have also revealed that probiotics can have antibacterial activity against P. acnes (Int. J. Cosmet. Sci. 2010;32:139-42; J. Microbiol. 2009;47:101-9). The prospects for efficacy of topically applied probiotics in the prevention and treatment of pro-inflammatory immune reactions are considered, by some, to be promising, however (Hautarzt. 2009;60:795-801). And, in fact, Guéniche et al. have found that the topical application of Vitreoscilla filiformis demonstrated efficacy against seborrheic dermatitis and AD (J. Eur. Acad. Dermatol. Venereol. 2008;22:1014-5; Eur. J. Dermatol. 2006;16:380-4).
Conclusion
While still controversial, the findings of probiotics’ effects in the treatment of atopic dermatitis remain compelling. Even more interesting, though, is the current work that suggests additional potential applications of probiotics in the dermatologic armamentarium. The work is in its early stages, but results warrant additional research, at the very least, if not cause for optimism over the prospect of more supportive evidence.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.
Probiotics are live microorganisms that impart health benefits to the host when present or administered at appropriate levels (J. Appl. Bacteriol. 1989;66:365-78). Intestinal microflora are better understood and more frequently used than cutaneous microbiota, but researchers have recently applied the concepts underlying the efficacy of intestinal probiotics to investigate potential benefits in the dermatologic realm. This work has also been spurred by the flurry of investigations on probiotics for the treatment of atopic dermatitis (AD).
In one of the earlier studies of possible uses of probiotics for the skin, Ouwehand et al. identified strains that adhere to keratin. However, the tested microbes (Propionibacteria, selected because they are among the normal microbiota of the skin) were not found to inhibit cutaneous pathogen adhesion to human keratin. Nevertheless, the researchers concluded that additional study was warranted to identify strains that adhere in vivo and exhibit activity against potential skin pathogens (Lett. Appl. Microbiol. 2003;36:327-31).
Clearly, some probiotic strains have been shown to exhibit strong immunomodulatory activity at the cutaneous level (Eur. J. Dermatol. 2010;20:731-7). In addition, some topical skin products now contain probiotic strains as active ingredients, including Clinique Medical, which incorporates lactobacillus cultures to yield molecules that help ameliorate skin barrier effects from laser treatments or chemical peels (Facial Plast. Surg. 2009;25:285-9). Probiotics have also been used successively as adjuvant therapy to treat suppurative inflammatory conditions (i.e., boils and abscesses) in the maxilla-facial region (Stomatologiia (Mosk) 2009;88:50-2).
This column will consider some of the most current AD studies as well as other recent research. History is not to be disregarded, though. After all, the notion of topical probiotics conferring cutaneous benefits against acne and seborrhea was considered in 1912 (Gut Pathog. 2011;3:1). Current findings may, indeed, bear this out.
Atopic Dermatitis
The safety of probiotic, as well as prebiotic, treatments for AD in children was established in a long-term, randomized, double-blind trial (Clin. Dermatol. 2010;28:57-61; Pediatrics 2008;122:8-1). But questions remain regarding efficacy, with conflicting data emerging from recent studies. Given increasing interest in the use of probiotics for the treatment of AD, Boyle et al. conducted an extensive literature search up to 2008 and found that 12 trials, including 781 subjects (all children), met their inclusion criteria, with probiotics not emerging as an effective treatment for eczema (Cochrane Database Syst. Rev. 2008;CD006135).
In 2008, Betsi et al. reviewed the results of 13 relevant randomized, placebo-controlled trials, 10 of which assessed probiotics as treatment and 3 for prevention of AD. Overall, they found that probiotics, particularly Lactobacillus rhamnosus GG, appear to be effective for preventing AD, lowering its severity in half of the trials assessed though inflammatory markers were not significantly affected. The authors called for more research to determine the usefulness of probiotics in AD treatment or prevention (Am. J. Clin. Dermatol. 2008;9:93-103).
In a 2010 analysis of systematic reviews indexed between August 2007 and August 2008 covering disease prevention and atopic eczema treatment, Williams and Grindlay found two independent systematic reviews suggesting that ingestion of probiotics by mothers during pregnancy might lower the incidence of subsequent eczema. However, they noted that a review of 13 studies of probiotics for treating established eczema revealed no convincing support for its clinical use, a stance that was buttressed by a later Cochrane Review (Clin. Exp. Dermatol. 2010;35:223-7).
In 2010, Gerasimov et al. conducted a randomized, double-blind, placebo-controlled, prospective study of 90 children (1-3 years old) with moderate to severe AD treated with a mixture of L. acidophilus DDS-1 and Bifidobacterium lactis UABLA-12 with fructo-oligosaccharide (5 billion colony-forming units twice daily for 8 weeks vs. placebo). The researchers found that the use of the probiotic compound correlated with significant clinical improvement in children with AD, and suggested that additional research is necessary to assess the efficacy of probiotics in adults with AD (Am. J. Clin. Dermatol. 2010;11:351-61).
In another recent study, investigators isolated L. plantarum strains from the Korean fermented food kimchi, and found that they hindered the dermatitis promoted by house-dust mites in a mouse model representative of human AD (J. Appl. Microbiol. 2011;110:1195-202). Overall, clinical trials conducted to test the use of probiotics to treat AD over the last 15 years have yielded conflicting results, though it appears that there is not enough evidence to warrant support for such therapy (Clin. Rev. Allergy Immunol. 2011;41:267-71).
Mechanism of Action
Presently, the mechanism of action whereby skin benefits are manifested through the oral ingestion of probiotics is thought to be a downstream result of the boost in systemic immune response, especially T-cell subsets such as Th1 cells that may ultimately enhance immune responses in organs beyond the digestive tract (Clin. Plast. Surg. 2012;39:59-64; Clin. Dermatol. 2008;26:4-11).
In discussing an alternative to antibacterial products for imbalances in cutaneous microorganisms that result in mild acne, xerosis, or AD, Simmering and Breves suggest that "prebiotic actives rebalance the skin microflora while probiotic approaches predominantly consist of applying an inactivated microbial biomass of beneficial bacteria" (Hautarzt. 2009;60:809-14).
Cutaneous Immune Homeostasis
In a 2009 randomized, double-blind, placebo-controlled clinical trial with 54 volunteers, Guéniche et al. set out to ascertain whether the probiotic bacterium L. johnsonii (La1) could influence cutaneous immune homeostasis in humans after solar-simulated UV exposure (twice 1.5 MED [minimal erythema dose]). They showed that La1 consumption contributed to hastening the recovery of allostimulatory function in epidermal cells (Dermatoendocrinol. 2009;1:275-9).
Previously, in 2008, Peguet-Navarro et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 54 healthy volunteers to study the potential impact of oral supplementation with La1 on skin immune status after UV exposure. Subjects received either La1 or placebo during the 6 weeks prior to solar-simulated UV exposure. The investigators found on day 4 after exposure that the allostimulatory capacity of epidermal cells had completely recovered in the La1 group correlating with the normalization of epidermal CD1a expression. They concluded that ingested probiotic bacteria hasten the recovery of skin immune homeostasis after UV-provoked immunosuppression (Eur. J. Dermatol. 2008;18:504-11).
Anti-inflammatory Activity
In 2010, Guéniche et al. demonstrated that B. longum can reduce skin inflammation mediated by substance P (Exp. Dermatol. 2010;19:e1-8). In a separate study, some of the same investigators found that L. paracasei appears to have the capacity to confer benefits related to barrier function and skin reactivity, also blunting the effects of substance P–induced skin inflammation (Eur. J. Dermatol. 2010;20:731-7).
Antiphotoaging Activity
In 2010, Bouilly-Gauthier et al. evaluated the effects of a dietary supplement combining L. johnsonii (La1), which is thought to protect the skin immune system after UV exposure, and nutritional doses of carotenoids on early UV-induced skin damage. They performed three clinical trials using various UV sources (non–extreme UV with a high UVA irradiance, extreme simulated solar radiation, and natural sunlight) in 139 healthy women over age 18 with skin type II-IV. The investigators found over the 10 weeks of the study that the combination of probiotic (La1) and nutritional doses of carotenoids lowered early UV-induced skin damage caused by simulated or natural sun exposure. Further study of the possible long-term effects against UV exposure and photoaging is warranted, they concluded (Br. J. Dermatol. 2010;163:536-43).
Topical Uses
In a study more than a decade ago, Di Marzio et al. showed that the topical application of a cream containing Streptococcus thermophilus, an organism found in most yogurts, raised the production of ceramides, which is notable given the anti-inflammatory activity and antimicrobial activity of some ceramides against Proprionibacterium acnes (J. Invest. Dermatol. 1999;113:98-106;Gut Pathog. 2011;3:1). Two recent in vitro studies have also revealed that probiotics can have antibacterial activity against P. acnes (Int. J. Cosmet. Sci. 2010;32:139-42; J. Microbiol. 2009;47:101-9). The prospects for efficacy of topically applied probiotics in the prevention and treatment of pro-inflammatory immune reactions are considered, by some, to be promising, however (Hautarzt. 2009;60:795-801). And, in fact, Guéniche et al. have found that the topical application of Vitreoscilla filiformis demonstrated efficacy against seborrheic dermatitis and AD (J. Eur. Acad. Dermatol. Venereol. 2008;22:1014-5; Eur. J. Dermatol. 2006;16:380-4).
Conclusion
While still controversial, the findings of probiotics’ effects in the treatment of atopic dermatitis remain compelling. Even more interesting, though, is the current work that suggests additional potential applications of probiotics in the dermatologic armamentarium. The work is in its early stages, but results warrant additional research, at the very least, if not cause for optimism over the prospect of more supportive evidence.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.