User login
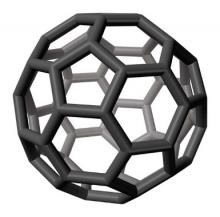
Discovered in nature in 1985 (Science 1992;257:215-7), fullerenes are novel, classically engineered chemical compounds composed only of carbon atoms. The stable form, a third carbon allotrope, is a molecule made up of 60 carbon atoms in a structure resembling a soccer ball or geodesic dome, although some fullerenes are ellipsoid or tube shaped. In fact, fullerene C(60), the first of the group of compounds to be discovered, is also known as buckminsterfullerene, based on Buckminster Fuller’s geodesic dome. The carbon nanotube structure, composed of thin carbon filaments (1-3 mcm in length and 1-3 nm in diameter) and possessing a wide range of mechanical characteristics, was discovered in 1991 (J. Nanosci. Nanotechnol. 2006;6:591-9).
The potential applications of C(60) and fullerene derivatives have been extensively studied, and recent data suggest that fullerenes exhibit potent antioxidant activity, even acting as a "free radical sponge" (Bioorg. Med. Chem. Lett. 2006;16:1590-5; Biomaterials 2008;29:3561-73). Therefore, fullerenes may be appropriate active ingredients for various skin care products, particularly rejuvenation products (Recent Pat. Biotechnol. 2011;5:67-73; Recent. Pat. Biotechnol. 2009;3:118-23).
Early studies
In one of the early studies of topical applications of fullerenes (C60), Nelson et al. examined the potential acute and subchronic toxic effects of fullerenes (200 mcg) applied in benzene on mouse skin. After 72 hours, they observed no effect on either DNA synthesis or ornithine decarboxylase activity. A skin tumor initiation model using 7,12-dimethylbenzanthracene (DMBA) failed to show benign or malignant skin tumor formation, but promotion using 12-O-tetradecanoyl-phorbol-13-acetate (TPA) resulted in benign skin tumors. The investigators concluded that fullerenes applied in benzene at a likely industrial exposure level did not cause acute toxic effects in mice (Toxicol. Ind. Health. 1993;9:623-30).
In 1997, Tabata et al., noted that fullerene C(60) efficiently generates singlet oxygen when irradiated with light, and found that polyethylene glycol (PEG)-modified C60 exhibits potential as an agent for photodynamic tumor therapy (Jpn. J. Cancer Res. 1997;88:1108-16).
Antiviral capacity
Fullerenes exhibit unique chemical and physical properties, including photodynamic characteristics and a hydrophobic spheroid and radical sponge quality. They also display antiviral activity, particularly in relation to HIV, and their potential commercial applications include use in patent-pending anticancer drug delivery systems employing photodynamic therapy (PDT), HIV drugs, and antiaging cosmetics (Int. J. Nanomedicine 2007;2:639-49).
Anti-inflammatory activity
An anti-inflammatory role has also been identified for fullerenes. Ryan et al. noted that preincubation with C(60) resulted in significant suppression of IgE-dependent mediator release in human mast cells and peripheral blood basophils. IgE-induced increases in cytoplasmic reactive oxygen species (ROS) levels was also hindered by preincubation with fullerenes. Using a mast cell–dependent anaphylaxis model, Ryan and colleagues also found that fullerenes prevented the in vivo release of histamine and fall in core body temperature. They concluded that fullerenes might suggest an innovative approach to managing mast cell–dependent conditions such as asthma, heart disease, inflammatory arthritis, and multiple sclerosis (J. Immunol. 2007;179(1):665-72).
Antimicrobial potential
In 2005, Tegos et al. compared the antimicrobial activity of six C(60) compounds functionalized with one, two, or three hydrophilic or cationic groups combined with white light against gram-positive and negative bacteria, and fungi. They found that following 10 minutes of incubation, bis- and tris-cationic fullerenes actively eliminated all tested microbes while leaving mammalian cells comparatively intact. The investigators also noted that the fullerene compounds were significantly more effective than a widely used antimicrobial photosensitizer, toluidine blue O. They concluded that the compounds warrant consideration as photosensitizers for antimicrobial use given their high selectivity and efficacy (Chem. Biol. 2005;12(10):1127-35).
Potent antioxidant
In 2006, Xiao et al. reported on their development of the Radical Sponge, a fullerene entrapped in polyvinylpyrrolidone to yield a water-soluble derivative with a mean particle diameter of about 688 nm and reactive oxygen species (ROS) scavenging abilities. The researchers repeatedly irradiated human skin keratinocytes (HaCaT) with visible light (400-2,000 nm) in the presence or absence of Radical Sponge, with no photocytotoxicity apparent in the Radical Sponge–exposed cells. In addition, the water-soluble fullerene derivative displayed a cytoprotective effect (10-40 mcM doses) against UVA exposure (30 J/cm2) when it was administered prior to exposure and rinsed out immediately before the irradiation, more so than when administered only during or after irradiation. The researchers concluded that this finding suggested more of a preventive as opposed to therapeutic effect conferred by Radical Sponge against UVA damage (Bioorg. Med. Chem. Lett. 2006;16:1590-5).
In a subsequent study, the researchers compared the Radical Sponge with two whitening agents and found the fullerene to have imparted stronger antimelanogenic activity, possibly due to down-regulation of the tyrosinase expression promoted by UVA-induced ROS generation (Arch. Dermatol. Res. 2007;299(5-6):245-57).
More recently, Kato et al. demonstrated the antioxidant activity of C(60) incorporated into liposomes, with persistent scavenging of hydroxyl radicals and cytoprotection of keratinocytes against UVA- and UVB-induced damage ascribed to the fullerene component (J. Nanosci. Nanotechnol. 2011;11(5):3814-23).
Safety
The first study establishing the safety of highly purified fullerenes (HPFs) as an additive in cosmeceuticals was conducted in 2009. Aoshima et al. performed in vivo tests in animals and in vitro examinations using human epidermal keratinocytes and fibroblasts. No primary or cumulative skin irritation, sensitization, photosensitization, or contact phototoxicity was observed. In the patch test on human skin, no reaction was noted. HPFs were deemed to be "minimally irritating" after the eye-irritation test in rabbits. The investigators concluded, based on their findings and in light of previously published data, that HPFs are safe for human skin as ingredients in cosmetic skin care formulations (J. Toxicol. Sci. 2009;34(5):555-62).
Hair regrowth
In 2009, Zhou et al. used shaved mice and SKH-1 hairless mice to study whether fullerene-based compounds could elicit hair growth. Fullerenes were found to significantly increase the hair growth rate compared with a placebo vehicle. Significantly increased numbers of hair follicles were also observed in SKH-1 hairless mice treated topically or subdermally with fullerenes. Cultured human skin treated with fullerenes also showed augmented hair growth. The data suggested implications for hair loss due to alopecia, chemotherapy, or other chemical reactions (Nanomedicine 2009;5:202-7).
Antiacne properties
In 2011, Inui et al. conducted an open trial of effects of fullerene gel on acne. Subjects used the gel twice daily, and significant reductions in the mean number of inflammatory lesions were noted at 4 and 8 weeks of treatment. In addition, the researchers conducted in vitro assays of sebum production in hamster sebocytes and found that 75 mcM polyvinylpyrrolidone-fullerene suppressed sebum production, suggesting that topical fullerenes inhibit acne by reducing neutrophil infiltration and sebum production (Nanomedicine 2011;7:238-41).
Water solubility and photodynamic activity
In 2007, Mroz et al. compared the photodynamic activity of six fullerenes functionalized to become soluble with 1, 2, or 3 hydrophilic or 1, 2, or 3 cationic groups in three mouse cancer cell lines (J774, LLC, and CT26) incubated for 24 hours with fullerenes and illuminated with white light. They found that some functionalized fullerenes (particularly monopyrrolidinium fullerene) induced apoptosis in all cancer lines (Free Radic. Biol. Med. 2007;43:711-9). In a subsequent study, Mroz et al. found that some fullerenes can be functionalized to photoinactivate pathogenic malignant cancer cells and/or microbial cells in a mechanism that involves superoxide anion and singlet oxygen. The researchers suggested that fullerenes have the potential to supersede photosensitizers in current clinical use in PDT for some conditions (Photochem. Photobiol. Sci. 2007;6:1139-49).
In 2009, Yin et al. showed that three different functionalized water-soluble fullerenes can intercept and protect cells against all of the primary physiologically important ROS and can efficiently suppress lipid peroxidation in vitro. The findings suggest the potential of fullerene derivatives as effective cytoprotective therapeutic agents (Biomaterials. 2009;30:611-21), the researchers wrote.
Conclusion
The discovery of the fullerene family has stimulated widespread research in chemistry and biology for a broad range of therapeutic applications. Anti-inflammatory, antioxidant, and antiviral activities have been linked to these compounds. In addition, fullerenes appear to be effective in drug and gene delivery and as an adjunct in acne therapy. The compounds have shown potential as photoprotectants as well as photosensitizers, which may be useful in photodynamic therapy. More research is needed, but the potential applications of fullerenes in dermatology are promising, particularly as potent antioxidants conferring skin protection.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.
Discovered in nature in 1985 (Science 1992;257:215-7), fullerenes are novel, classically engineered chemical compounds composed only of carbon atoms. The stable form, a third carbon allotrope, is a molecule made up of 60 carbon atoms in a structure resembling a soccer ball or geodesic dome, although some fullerenes are ellipsoid or tube shaped. In fact, fullerene C(60), the first of the group of compounds to be discovered, is also known as buckminsterfullerene, based on Buckminster Fuller’s geodesic dome. The carbon nanotube structure, composed of thin carbon filaments (1-3 mcm in length and 1-3 nm in diameter) and possessing a wide range of mechanical characteristics, was discovered in 1991 (J. Nanosci. Nanotechnol. 2006;6:591-9).
The potential applications of C(60) and fullerene derivatives have been extensively studied, and recent data suggest that fullerenes exhibit potent antioxidant activity, even acting as a "free radical sponge" (Bioorg. Med. Chem. Lett. 2006;16:1590-5; Biomaterials 2008;29:3561-73). Therefore, fullerenes may be appropriate active ingredients for various skin care products, particularly rejuvenation products (Recent Pat. Biotechnol. 2011;5:67-73; Recent. Pat. Biotechnol. 2009;3:118-23).
Early studies
In one of the early studies of topical applications of fullerenes (C60), Nelson et al. examined the potential acute and subchronic toxic effects of fullerenes (200 mcg) applied in benzene on mouse skin. After 72 hours, they observed no effect on either DNA synthesis or ornithine decarboxylase activity. A skin tumor initiation model using 7,12-dimethylbenzanthracene (DMBA) failed to show benign or malignant skin tumor formation, but promotion using 12-O-tetradecanoyl-phorbol-13-acetate (TPA) resulted in benign skin tumors. The investigators concluded that fullerenes applied in benzene at a likely industrial exposure level did not cause acute toxic effects in mice (Toxicol. Ind. Health. 1993;9:623-30).
In 1997, Tabata et al., noted that fullerene C(60) efficiently generates singlet oxygen when irradiated with light, and found that polyethylene glycol (PEG)-modified C60 exhibits potential as an agent for photodynamic tumor therapy (Jpn. J. Cancer Res. 1997;88:1108-16).
Antiviral capacity
Fullerenes exhibit unique chemical and physical properties, including photodynamic characteristics and a hydrophobic spheroid and radical sponge quality. They also display antiviral activity, particularly in relation to HIV, and their potential commercial applications include use in patent-pending anticancer drug delivery systems employing photodynamic therapy (PDT), HIV drugs, and antiaging cosmetics (Int. J. Nanomedicine 2007;2:639-49).
Anti-inflammatory activity
An anti-inflammatory role has also been identified for fullerenes. Ryan et al. noted that preincubation with C(60) resulted in significant suppression of IgE-dependent mediator release in human mast cells and peripheral blood basophils. IgE-induced increases in cytoplasmic reactive oxygen species (ROS) levels was also hindered by preincubation with fullerenes. Using a mast cell–dependent anaphylaxis model, Ryan and colleagues also found that fullerenes prevented the in vivo release of histamine and fall in core body temperature. They concluded that fullerenes might suggest an innovative approach to managing mast cell–dependent conditions such as asthma, heart disease, inflammatory arthritis, and multiple sclerosis (J. Immunol. 2007;179(1):665-72).
Antimicrobial potential
In 2005, Tegos et al. compared the antimicrobial activity of six C(60) compounds functionalized with one, two, or three hydrophilic or cationic groups combined with white light against gram-positive and negative bacteria, and fungi. They found that following 10 minutes of incubation, bis- and tris-cationic fullerenes actively eliminated all tested microbes while leaving mammalian cells comparatively intact. The investigators also noted that the fullerene compounds were significantly more effective than a widely used antimicrobial photosensitizer, toluidine blue O. They concluded that the compounds warrant consideration as photosensitizers for antimicrobial use given their high selectivity and efficacy (Chem. Biol. 2005;12(10):1127-35).
Potent antioxidant
In 2006, Xiao et al. reported on their development of the Radical Sponge, a fullerene entrapped in polyvinylpyrrolidone to yield a water-soluble derivative with a mean particle diameter of about 688 nm and reactive oxygen species (ROS) scavenging abilities. The researchers repeatedly irradiated human skin keratinocytes (HaCaT) with visible light (400-2,000 nm) in the presence or absence of Radical Sponge, with no photocytotoxicity apparent in the Radical Sponge–exposed cells. In addition, the water-soluble fullerene derivative displayed a cytoprotective effect (10-40 mcM doses) against UVA exposure (30 J/cm2) when it was administered prior to exposure and rinsed out immediately before the irradiation, more so than when administered only during or after irradiation. The researchers concluded that this finding suggested more of a preventive as opposed to therapeutic effect conferred by Radical Sponge against UVA damage (Bioorg. Med. Chem. Lett. 2006;16:1590-5).
In a subsequent study, the researchers compared the Radical Sponge with two whitening agents and found the fullerene to have imparted stronger antimelanogenic activity, possibly due to down-regulation of the tyrosinase expression promoted by UVA-induced ROS generation (Arch. Dermatol. Res. 2007;299(5-6):245-57).
More recently, Kato et al. demonstrated the antioxidant activity of C(60) incorporated into liposomes, with persistent scavenging of hydroxyl radicals and cytoprotection of keratinocytes against UVA- and UVB-induced damage ascribed to the fullerene component (J. Nanosci. Nanotechnol. 2011;11(5):3814-23).
Safety
The first study establishing the safety of highly purified fullerenes (HPFs) as an additive in cosmeceuticals was conducted in 2009. Aoshima et al. performed in vivo tests in animals and in vitro examinations using human epidermal keratinocytes and fibroblasts. No primary or cumulative skin irritation, sensitization, photosensitization, or contact phototoxicity was observed. In the patch test on human skin, no reaction was noted. HPFs were deemed to be "minimally irritating" after the eye-irritation test in rabbits. The investigators concluded, based on their findings and in light of previously published data, that HPFs are safe for human skin as ingredients in cosmetic skin care formulations (J. Toxicol. Sci. 2009;34(5):555-62).
Hair regrowth
In 2009, Zhou et al. used shaved mice and SKH-1 hairless mice to study whether fullerene-based compounds could elicit hair growth. Fullerenes were found to significantly increase the hair growth rate compared with a placebo vehicle. Significantly increased numbers of hair follicles were also observed in SKH-1 hairless mice treated topically or subdermally with fullerenes. Cultured human skin treated with fullerenes also showed augmented hair growth. The data suggested implications for hair loss due to alopecia, chemotherapy, or other chemical reactions (Nanomedicine 2009;5:202-7).
Antiacne properties
In 2011, Inui et al. conducted an open trial of effects of fullerene gel on acne. Subjects used the gel twice daily, and significant reductions in the mean number of inflammatory lesions were noted at 4 and 8 weeks of treatment. In addition, the researchers conducted in vitro assays of sebum production in hamster sebocytes and found that 75 mcM polyvinylpyrrolidone-fullerene suppressed sebum production, suggesting that topical fullerenes inhibit acne by reducing neutrophil infiltration and sebum production (Nanomedicine 2011;7:238-41).
Water solubility and photodynamic activity
In 2007, Mroz et al. compared the photodynamic activity of six fullerenes functionalized to become soluble with 1, 2, or 3 hydrophilic or 1, 2, or 3 cationic groups in three mouse cancer cell lines (J774, LLC, and CT26) incubated for 24 hours with fullerenes and illuminated with white light. They found that some functionalized fullerenes (particularly monopyrrolidinium fullerene) induced apoptosis in all cancer lines (Free Radic. Biol. Med. 2007;43:711-9). In a subsequent study, Mroz et al. found that some fullerenes can be functionalized to photoinactivate pathogenic malignant cancer cells and/or microbial cells in a mechanism that involves superoxide anion and singlet oxygen. The researchers suggested that fullerenes have the potential to supersede photosensitizers in current clinical use in PDT for some conditions (Photochem. Photobiol. Sci. 2007;6:1139-49).
In 2009, Yin et al. showed that three different functionalized water-soluble fullerenes can intercept and protect cells against all of the primary physiologically important ROS and can efficiently suppress lipid peroxidation in vitro. The findings suggest the potential of fullerene derivatives as effective cytoprotective therapeutic agents (Biomaterials. 2009;30:611-21), the researchers wrote.
Conclusion
The discovery of the fullerene family has stimulated widespread research in chemistry and biology for a broad range of therapeutic applications. Anti-inflammatory, antioxidant, and antiviral activities have been linked to these compounds. In addition, fullerenes appear to be effective in drug and gene delivery and as an adjunct in acne therapy. The compounds have shown potential as photoprotectants as well as photosensitizers, which may be useful in photodynamic therapy. More research is needed, but the potential applications of fullerenes in dermatology are promising, particularly as potent antioxidants conferring skin protection.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.
Discovered in nature in 1985 (Science 1992;257:215-7), fullerenes are novel, classically engineered chemical compounds composed only of carbon atoms. The stable form, a third carbon allotrope, is a molecule made up of 60 carbon atoms in a structure resembling a soccer ball or geodesic dome, although some fullerenes are ellipsoid or tube shaped. In fact, fullerene C(60), the first of the group of compounds to be discovered, is also known as buckminsterfullerene, based on Buckminster Fuller’s geodesic dome. The carbon nanotube structure, composed of thin carbon filaments (1-3 mcm in length and 1-3 nm in diameter) and possessing a wide range of mechanical characteristics, was discovered in 1991 (J. Nanosci. Nanotechnol. 2006;6:591-9).
The potential applications of C(60) and fullerene derivatives have been extensively studied, and recent data suggest that fullerenes exhibit potent antioxidant activity, even acting as a "free radical sponge" (Bioorg. Med. Chem. Lett. 2006;16:1590-5; Biomaterials 2008;29:3561-73). Therefore, fullerenes may be appropriate active ingredients for various skin care products, particularly rejuvenation products (Recent Pat. Biotechnol. 2011;5:67-73; Recent. Pat. Biotechnol. 2009;3:118-23).
Early studies
In one of the early studies of topical applications of fullerenes (C60), Nelson et al. examined the potential acute and subchronic toxic effects of fullerenes (200 mcg) applied in benzene on mouse skin. After 72 hours, they observed no effect on either DNA synthesis or ornithine decarboxylase activity. A skin tumor initiation model using 7,12-dimethylbenzanthracene (DMBA) failed to show benign or malignant skin tumor formation, but promotion using 12-O-tetradecanoyl-phorbol-13-acetate (TPA) resulted in benign skin tumors. The investigators concluded that fullerenes applied in benzene at a likely industrial exposure level did not cause acute toxic effects in mice (Toxicol. Ind. Health. 1993;9:623-30).
In 1997, Tabata et al., noted that fullerene C(60) efficiently generates singlet oxygen when irradiated with light, and found that polyethylene glycol (PEG)-modified C60 exhibits potential as an agent for photodynamic tumor therapy (Jpn. J. Cancer Res. 1997;88:1108-16).
Antiviral capacity
Fullerenes exhibit unique chemical and physical properties, including photodynamic characteristics and a hydrophobic spheroid and radical sponge quality. They also display antiviral activity, particularly in relation to HIV, and their potential commercial applications include use in patent-pending anticancer drug delivery systems employing photodynamic therapy (PDT), HIV drugs, and antiaging cosmetics (Int. J. Nanomedicine 2007;2:639-49).
Anti-inflammatory activity
An anti-inflammatory role has also been identified for fullerenes. Ryan et al. noted that preincubation with C(60) resulted in significant suppression of IgE-dependent mediator release in human mast cells and peripheral blood basophils. IgE-induced increases in cytoplasmic reactive oxygen species (ROS) levels was also hindered by preincubation with fullerenes. Using a mast cell–dependent anaphylaxis model, Ryan and colleagues also found that fullerenes prevented the in vivo release of histamine and fall in core body temperature. They concluded that fullerenes might suggest an innovative approach to managing mast cell–dependent conditions such as asthma, heart disease, inflammatory arthritis, and multiple sclerosis (J. Immunol. 2007;179(1):665-72).
Antimicrobial potential
In 2005, Tegos et al. compared the antimicrobial activity of six C(60) compounds functionalized with one, two, or three hydrophilic or cationic groups combined with white light against gram-positive and negative bacteria, and fungi. They found that following 10 minutes of incubation, bis- and tris-cationic fullerenes actively eliminated all tested microbes while leaving mammalian cells comparatively intact. The investigators also noted that the fullerene compounds were significantly more effective than a widely used antimicrobial photosensitizer, toluidine blue O. They concluded that the compounds warrant consideration as photosensitizers for antimicrobial use given their high selectivity and efficacy (Chem. Biol. 2005;12(10):1127-35).
Potent antioxidant
In 2006, Xiao et al. reported on their development of the Radical Sponge, a fullerene entrapped in polyvinylpyrrolidone to yield a water-soluble derivative with a mean particle diameter of about 688 nm and reactive oxygen species (ROS) scavenging abilities. The researchers repeatedly irradiated human skin keratinocytes (HaCaT) with visible light (400-2,000 nm) in the presence or absence of Radical Sponge, with no photocytotoxicity apparent in the Radical Sponge–exposed cells. In addition, the water-soluble fullerene derivative displayed a cytoprotective effect (10-40 mcM doses) against UVA exposure (30 J/cm2) when it was administered prior to exposure and rinsed out immediately before the irradiation, more so than when administered only during or after irradiation. The researchers concluded that this finding suggested more of a preventive as opposed to therapeutic effect conferred by Radical Sponge against UVA damage (Bioorg. Med. Chem. Lett. 2006;16:1590-5).
In a subsequent study, the researchers compared the Radical Sponge with two whitening agents and found the fullerene to have imparted stronger antimelanogenic activity, possibly due to down-regulation of the tyrosinase expression promoted by UVA-induced ROS generation (Arch. Dermatol. Res. 2007;299(5-6):245-57).
More recently, Kato et al. demonstrated the antioxidant activity of C(60) incorporated into liposomes, with persistent scavenging of hydroxyl radicals and cytoprotection of keratinocytes against UVA- and UVB-induced damage ascribed to the fullerene component (J. Nanosci. Nanotechnol. 2011;11(5):3814-23).
Safety
The first study establishing the safety of highly purified fullerenes (HPFs) as an additive in cosmeceuticals was conducted in 2009. Aoshima et al. performed in vivo tests in animals and in vitro examinations using human epidermal keratinocytes and fibroblasts. No primary or cumulative skin irritation, sensitization, photosensitization, or contact phototoxicity was observed. In the patch test on human skin, no reaction was noted. HPFs were deemed to be "minimally irritating" after the eye-irritation test in rabbits. The investigators concluded, based on their findings and in light of previously published data, that HPFs are safe for human skin as ingredients in cosmetic skin care formulations (J. Toxicol. Sci. 2009;34(5):555-62).
Hair regrowth
In 2009, Zhou et al. used shaved mice and SKH-1 hairless mice to study whether fullerene-based compounds could elicit hair growth. Fullerenes were found to significantly increase the hair growth rate compared with a placebo vehicle. Significantly increased numbers of hair follicles were also observed in SKH-1 hairless mice treated topically or subdermally with fullerenes. Cultured human skin treated with fullerenes also showed augmented hair growth. The data suggested implications for hair loss due to alopecia, chemotherapy, or other chemical reactions (Nanomedicine 2009;5:202-7).
Antiacne properties
In 2011, Inui et al. conducted an open trial of effects of fullerene gel on acne. Subjects used the gel twice daily, and significant reductions in the mean number of inflammatory lesions were noted at 4 and 8 weeks of treatment. In addition, the researchers conducted in vitro assays of sebum production in hamster sebocytes and found that 75 mcM polyvinylpyrrolidone-fullerene suppressed sebum production, suggesting that topical fullerenes inhibit acne by reducing neutrophil infiltration and sebum production (Nanomedicine 2011;7:238-41).
Water solubility and photodynamic activity
In 2007, Mroz et al. compared the photodynamic activity of six fullerenes functionalized to become soluble with 1, 2, or 3 hydrophilic or 1, 2, or 3 cationic groups in three mouse cancer cell lines (J774, LLC, and CT26) incubated for 24 hours with fullerenes and illuminated with white light. They found that some functionalized fullerenes (particularly monopyrrolidinium fullerene) induced apoptosis in all cancer lines (Free Radic. Biol. Med. 2007;43:711-9). In a subsequent study, Mroz et al. found that some fullerenes can be functionalized to photoinactivate pathogenic malignant cancer cells and/or microbial cells in a mechanism that involves superoxide anion and singlet oxygen. The researchers suggested that fullerenes have the potential to supersede photosensitizers in current clinical use in PDT for some conditions (Photochem. Photobiol. Sci. 2007;6:1139-49).
In 2009, Yin et al. showed that three different functionalized water-soluble fullerenes can intercept and protect cells against all of the primary physiologically important ROS and can efficiently suppress lipid peroxidation in vitro. The findings suggest the potential of fullerene derivatives as effective cytoprotective therapeutic agents (Biomaterials. 2009;30:611-21), the researchers wrote.
Conclusion
The discovery of the fullerene family has stimulated widespread research in chemistry and biology for a broad range of therapeutic applications. Anti-inflammatory, antioxidant, and antiviral activities have been linked to these compounds. In addition, fullerenes appear to be effective in drug and gene delivery and as an adjunct in acne therapy. The compounds have shown potential as photoprotectants as well as photosensitizers, which may be useful in photodynamic therapy. More research is needed, but the potential applications of fullerenes in dermatology are promising, particularly as potent antioxidants conferring skin protection.
Dr. Baumann is in private practice in Miami Beach. She did not disclose any conflicts of interest.